User login
Antibiotic-obesity link ‘clinically insignificant’ at age 10 years
NASHVILLE, tenn. – Antibiotic use in the first 2 years of life was associated with a small amount of weight gain by 10 years of age, but the amount is “likely clinically insignificant,” according to new data presented at Obesity Week.
At 10 years of age, children without chronic health conditions who received any antibiotics had an odds ratio of 1.02 for being overweight or obese; for children with complex chronic conditions, the OR was 1.07 (95% confidence intervals, 0.97-1.07 and 0.96-1.19, respectively). The findings were based on data from almost 60,000 children studied in a large, multi-institutional national cohort.
“This is good news,” said first author Sheryl Rifas-Shiman, MPH, discussing the findings during a poster session. She noted that the 10-year data from the longitudinal study are consistent with findings at the 5-year mark that had previously been reported.
The study comes against the background of a recent meta-analysis finding that children given any antibiotics before age 24 months had a higher body mass index z-score (BMI-z) in childhood than children who didn’t take antibiotics. Disruptions that antibiotics can cause in the gut microbiome have been hypothesized to promote overweight and obesity in children.
The present study was conducted using electronic medical record data from 2009-2016 drawn from institutions participating in PCORnet, a national research collaboration and clearinghouse.
The analysis dichotomized the cohort into those who, by 24 months of age, had received any antibiotics and those who received none. Ms. Rifas-Shiman and her colleagues also looked at a categorical count of the number of antibiotic prescriptions, from 0 to 4 or more.
Finally, they broke the type of antibiotics into narrow- or broad-spectrum, she said in an interview during the poster session. In order for exposure to be considered narrow-spectrum only, the analysis was limited to participants who had no broad-spectrum antibiotic exposure during the same time frame.
The study’s multivariable analysis also took into account complex chronic conditions the children may have had.
Fifty-seven percent of children received antibiotics before the age of 24 months. Patients were overall just under half (48%) female, and about half (49%) were white. Black children made up 37% of the cohort, and Hispanic children constituted 12%.
By 10 years of age, 36% of participants were overweight or obese, with BMIs at or above the 85th percentile, according to 2000 Centers for Disease Control growth charts.
There was a suggestion of a dose-response relationship for both narrow- and broad-spectrum antibiotics because only at the higher antibiotic exposures did increased BMI-z reach statistical significance. However, broad-spectrum antibiotics were not more likely than narrow-spectrum antibiotics to be associated with increased BMI-z.
Other multivariable adjustment took into account site clustering and adjusted for sex, race, ethnicity, preterm birth, asthma or infectious disease diagnoses, and corticosteroid exposure, as well as health care visits in the first 2 years of life.
Limitations of the study included the fact that it was observational, raising the potential for undetected confounders. Also, since the study looked at prescription, rather than dispensing data, there could be some exposure misclassification by which children who were classified as having taken antibiotics did not actually take them or did not take the full amount prescribed.
“Antibiotics should always be used judiciously; however, the long-term risk of childhood obesity from antibiotics in infancy appears to be small and clinically insignificant,” wrote Ms. Rifas-Shiman, of the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, both in Boston.
The study was funded by an award from the Patient-Centered Outcomes Research Institute.
SOURCE: Rifas-Shiman et al. Obesity Week 2018, Poster TP-3155.
NASHVILLE, tenn. – Antibiotic use in the first 2 years of life was associated with a small amount of weight gain by 10 years of age, but the amount is “likely clinically insignificant,” according to new data presented at Obesity Week.
At 10 years of age, children without chronic health conditions who received any antibiotics had an odds ratio of 1.02 for being overweight or obese; for children with complex chronic conditions, the OR was 1.07 (95% confidence intervals, 0.97-1.07 and 0.96-1.19, respectively). The findings were based on data from almost 60,000 children studied in a large, multi-institutional national cohort.
“This is good news,” said first author Sheryl Rifas-Shiman, MPH, discussing the findings during a poster session. She noted that the 10-year data from the longitudinal study are consistent with findings at the 5-year mark that had previously been reported.
The study comes against the background of a recent meta-analysis finding that children given any antibiotics before age 24 months had a higher body mass index z-score (BMI-z) in childhood than children who didn’t take antibiotics. Disruptions that antibiotics can cause in the gut microbiome have been hypothesized to promote overweight and obesity in children.
The present study was conducted using electronic medical record data from 2009-2016 drawn from institutions participating in PCORnet, a national research collaboration and clearinghouse.
The analysis dichotomized the cohort into those who, by 24 months of age, had received any antibiotics and those who received none. Ms. Rifas-Shiman and her colleagues also looked at a categorical count of the number of antibiotic prescriptions, from 0 to 4 or more.
Finally, they broke the type of antibiotics into narrow- or broad-spectrum, she said in an interview during the poster session. In order for exposure to be considered narrow-spectrum only, the analysis was limited to participants who had no broad-spectrum antibiotic exposure during the same time frame.
The study’s multivariable analysis also took into account complex chronic conditions the children may have had.
Fifty-seven percent of children received antibiotics before the age of 24 months. Patients were overall just under half (48%) female, and about half (49%) were white. Black children made up 37% of the cohort, and Hispanic children constituted 12%.
By 10 years of age, 36% of participants were overweight or obese, with BMIs at or above the 85th percentile, according to 2000 Centers for Disease Control growth charts.
There was a suggestion of a dose-response relationship for both narrow- and broad-spectrum antibiotics because only at the higher antibiotic exposures did increased BMI-z reach statistical significance. However, broad-spectrum antibiotics were not more likely than narrow-spectrum antibiotics to be associated with increased BMI-z.
Other multivariable adjustment took into account site clustering and adjusted for sex, race, ethnicity, preterm birth, asthma or infectious disease diagnoses, and corticosteroid exposure, as well as health care visits in the first 2 years of life.
Limitations of the study included the fact that it was observational, raising the potential for undetected confounders. Also, since the study looked at prescription, rather than dispensing data, there could be some exposure misclassification by which children who were classified as having taken antibiotics did not actually take them or did not take the full amount prescribed.
“Antibiotics should always be used judiciously; however, the long-term risk of childhood obesity from antibiotics in infancy appears to be small and clinically insignificant,” wrote Ms. Rifas-Shiman, of the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, both in Boston.
The study was funded by an award from the Patient-Centered Outcomes Research Institute.
SOURCE: Rifas-Shiman et al. Obesity Week 2018, Poster TP-3155.
NASHVILLE, tenn. – Antibiotic use in the first 2 years of life was associated with a small amount of weight gain by 10 years of age, but the amount is “likely clinically insignificant,” according to new data presented at Obesity Week.
At 10 years of age, children without chronic health conditions who received any antibiotics had an odds ratio of 1.02 for being overweight or obese; for children with complex chronic conditions, the OR was 1.07 (95% confidence intervals, 0.97-1.07 and 0.96-1.19, respectively). The findings were based on data from almost 60,000 children studied in a large, multi-institutional national cohort.
“This is good news,” said first author Sheryl Rifas-Shiman, MPH, discussing the findings during a poster session. She noted that the 10-year data from the longitudinal study are consistent with findings at the 5-year mark that had previously been reported.
The study comes against the background of a recent meta-analysis finding that children given any antibiotics before age 24 months had a higher body mass index z-score (BMI-z) in childhood than children who didn’t take antibiotics. Disruptions that antibiotics can cause in the gut microbiome have been hypothesized to promote overweight and obesity in children.
The present study was conducted using electronic medical record data from 2009-2016 drawn from institutions participating in PCORnet, a national research collaboration and clearinghouse.
The analysis dichotomized the cohort into those who, by 24 months of age, had received any antibiotics and those who received none. Ms. Rifas-Shiman and her colleagues also looked at a categorical count of the number of antibiotic prescriptions, from 0 to 4 or more.
Finally, they broke the type of antibiotics into narrow- or broad-spectrum, she said in an interview during the poster session. In order for exposure to be considered narrow-spectrum only, the analysis was limited to participants who had no broad-spectrum antibiotic exposure during the same time frame.
The study’s multivariable analysis also took into account complex chronic conditions the children may have had.
Fifty-seven percent of children received antibiotics before the age of 24 months. Patients were overall just under half (48%) female, and about half (49%) were white. Black children made up 37% of the cohort, and Hispanic children constituted 12%.
By 10 years of age, 36% of participants were overweight or obese, with BMIs at or above the 85th percentile, according to 2000 Centers for Disease Control growth charts.
There was a suggestion of a dose-response relationship for both narrow- and broad-spectrum antibiotics because only at the higher antibiotic exposures did increased BMI-z reach statistical significance. However, broad-spectrum antibiotics were not more likely than narrow-spectrum antibiotics to be associated with increased BMI-z.
Other multivariable adjustment took into account site clustering and adjusted for sex, race, ethnicity, preterm birth, asthma or infectious disease diagnoses, and corticosteroid exposure, as well as health care visits in the first 2 years of life.
Limitations of the study included the fact that it was observational, raising the potential for undetected confounders. Also, since the study looked at prescription, rather than dispensing data, there could be some exposure misclassification by which children who were classified as having taken antibiotics did not actually take them or did not take the full amount prescribed.
“Antibiotics should always be used judiciously; however, the long-term risk of childhood obesity from antibiotics in infancy appears to be small and clinically insignificant,” wrote Ms. Rifas-Shiman, of the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, both in Boston.
The study was funded by an award from the Patient-Centered Outcomes Research Institute.
SOURCE: Rifas-Shiman et al. Obesity Week 2018, Poster TP-3155.
REPORTING FROM OBESITY WEEK 2018
Key clinical point: Antibiotic use before the age of 2 years was not associated with clinically significant weight gain at age 10 years.
Major finding: By age 10 years, children given antibiotics without chronic health conditions had an odds ratio of 1.02 for being overweight or obese, and those with chronic health conditions had an OR of 1.07, with 95% confidence intervals for both groups crossing unity.
Study details: Prospective, multisite, national cohort study of 56,727 children.
Disclosures: The study was funded by the Patient-Centered Outcomes Research Institute. Ms. Rifas-Shiman reported that she had no conflicts of interest.
Source: Rifas-Shiman S et al. Obesity Week 2018, poster TP-3155,
Cardiologist: Obesity has basically stopped progress on cardiovascular disease
NASHVILLE – It’s all hands on deck to fight the obesity epidemic, according to a cardiologist who made a plea for collaboration at the opening session at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. said Steven Nissen, MD, because when significant weight loss is achieved, “we can have an amazing effect on cardiovascular death, stroke, myocardial infarction, and these feared complications of obesity.”
From Dr. Nissen’s perspective, though, rates of death from cardiovascular disease have plateaued and are creeping up after decades of marked improvement.
“I am sorry to tell you that these rates are beginning to go up again – because of the obesity epidemic. That’s why we need to work together on this problem,” said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic. “It has basically stopped progress on cardiovascular disease.”
Dr. Nissen drew from the broad literature intertwining obesity and cardiometabolic health to tell a story that went beyond weight loss to focus on outcomes.
For bariatric surgery, the evidence of reduction in cardiovascular risk is looking very good, said Dr. Nissen. “There are just huge changes in the metabolic risk factors. … Clearly we have evidence that if we get people to lose substantial weight, you can get people to normalize major metabolic risk factors.”
Recent data from a multisite, retrospective, matched cohort study of patients with diabetes and severe obesity show the promise of substantial weight loss in reducing risk. The study tracked 5,301 bariatric surgery patients and compared them with 14,934 “well-matched” control participants who did not have bariatric surgery but received usual care for diabetes, said Dr. Nissen.
During 7 years of follow-up, the bariatric surgery group had a hazard ratio for coronary events of 0.64 (95% confidence interval, 0.42-0.99; P less than .001). A post hoc analysis showed an HR of 0.34 for all-cause mortality among the bariatric surgery patients (95% CI, 0.15-0.74; P less than .001; JAMA. 2018;320[15]:1570-82).
“I’ve been practicing in this field for about 40 years,” said Dr. Nissen. “With statins, we get about 25% risk reduction … a 34% risk reduction is just a whopping big reduction.” Background risk was high among this population with diabetes, and this was a cohort study, not a randomized, controlled trial (RCT).
“We need RCTs,” he said. “I hope we can come together – all of us – and do a large, multicenter, global RCT on the effects of bariatric surgery on cardiovascular outcomes. But barring that, these are the best data we are going to have.”
But can these changes be achieved and sustained without surgery?
“Can diet or drug therapy favorably affect atherosclerotic cardiovascular outcomes? To me, this is the holy grail,” Dr. Nissen said.
A major cautionary note was sounded by a European Medicines Agency–mandated cardiovascular outcomes study of sibutramine, said Dr. Nissen. In clinical trials, patients taking sibutramine had seen modest weight loss, with increased HDL cholesterol and decreased triglycerides. However, blood pressure rose by 1-3 mm Hg, and heart rates also climbed by 4-5 beats per minute, changes consistent with sibutramine’s sympathomimetic effects, said Dr. Nissen. The EMA-mandated trial included over 10,000 patients and looked at a composite endpoint of major cardiovascular events, including death, MI, stroke, and resuscitated arrest. Patients were included if they were aged older than 55 years, had a body mass index of greater than 27 kg/m2, and had a history of cardiovascular disease or diabetes with an additional risk factor. Patients who had significant heart rate or blood pressure increases during the study run-in period were excluded.
In the end, patients taking sibutramine had an increased risk for the composite endpoint (11.4% vs. 10.0%; P = .02). The risk for nonfatal stroke and nonfatal MI was also significantly elevated for the sibutramine group (N Engl J Med. 2010; 363:905-17).
Phentermine is a pharmacologic relative of sibutramine, with similar effects on blood pressure and heart rate. Since it was approved prior to the current increased focus on real-world clinical outcomes in drug approvals, phentermine’s cardiovascular outcomes have never been studied by means of a RCT. “Nobody’s going to do this study unless we push for it, but it has to be done,” he said. “Although this drug reduces weight, there is considerable uncertainty whether it increases cardiovascular outcomes.”
Even looking at weight loss alone, pharmacologic treatments show marginal benefit over time, said Dr. Nissen, citing, as an example, recently published outcome data on lorcaserin. Over 40 months of treatment, there was a “complete absence of any benefit for lorcaserin,” compared with placebo, and participants saw an average weight loss of just 1.9 kg by the end of the study period, with no change in cardiovascular outcomes (N Engl J Med. 2018;379:1107-17).
To drive home the point, Dr. Nissen shared a slide entitled “Established Benefits of Weight Loss Drugs on Clinically Important Outcomes.” The slide’s text read, “This slide intentionally left blank.”
“It’s very hard for me to argue in favor of giving any of these drugs,” said Dr. Nissen. “In the absence of established outcome benefits, there are only risks and costs. I know this is not going to be popular with everyone in this audience, but I have to tell you what I really believe here: We have to do better.”
More broadly, “I think we have to demand outcome trials for obesity drugs,” said Dr. Nissen. He noted that such trials are underway for some glucagonlike peptide–1 agonists, “and I applaud them. ... I hope you will participate in those studies, because they are going to give us some answers.”
Calling for renewed efforts to improve the efficacy of lifestyle interventions, Dr. Nissen said, “What we have to do is try. .... You know as well as I that there are some outliers” who will achieve profound weight loss without surgery, and those patients are likely to reap big benefits in risk reduction.
“We’ve got a problem that affects tens of millions of people, and we’ve got to find a societal approach to this. But we share these patients; let’s work together on trying to make them better.”
Dr. Nissen did not report any relevant financial disclosures.
NASHVILLE – It’s all hands on deck to fight the obesity epidemic, according to a cardiologist who made a plea for collaboration at the opening session at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. said Steven Nissen, MD, because when significant weight loss is achieved, “we can have an amazing effect on cardiovascular death, stroke, myocardial infarction, and these feared complications of obesity.”
From Dr. Nissen’s perspective, though, rates of death from cardiovascular disease have plateaued and are creeping up after decades of marked improvement.
“I am sorry to tell you that these rates are beginning to go up again – because of the obesity epidemic. That’s why we need to work together on this problem,” said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic. “It has basically stopped progress on cardiovascular disease.”
Dr. Nissen drew from the broad literature intertwining obesity and cardiometabolic health to tell a story that went beyond weight loss to focus on outcomes.
For bariatric surgery, the evidence of reduction in cardiovascular risk is looking very good, said Dr. Nissen. “There are just huge changes in the metabolic risk factors. … Clearly we have evidence that if we get people to lose substantial weight, you can get people to normalize major metabolic risk factors.”
Recent data from a multisite, retrospective, matched cohort study of patients with diabetes and severe obesity show the promise of substantial weight loss in reducing risk. The study tracked 5,301 bariatric surgery patients and compared them with 14,934 “well-matched” control participants who did not have bariatric surgery but received usual care for diabetes, said Dr. Nissen.
During 7 years of follow-up, the bariatric surgery group had a hazard ratio for coronary events of 0.64 (95% confidence interval, 0.42-0.99; P less than .001). A post hoc analysis showed an HR of 0.34 for all-cause mortality among the bariatric surgery patients (95% CI, 0.15-0.74; P less than .001; JAMA. 2018;320[15]:1570-82).
“I’ve been practicing in this field for about 40 years,” said Dr. Nissen. “With statins, we get about 25% risk reduction … a 34% risk reduction is just a whopping big reduction.” Background risk was high among this population with diabetes, and this was a cohort study, not a randomized, controlled trial (RCT).
“We need RCTs,” he said. “I hope we can come together – all of us – and do a large, multicenter, global RCT on the effects of bariatric surgery on cardiovascular outcomes. But barring that, these are the best data we are going to have.”
But can these changes be achieved and sustained without surgery?
“Can diet or drug therapy favorably affect atherosclerotic cardiovascular outcomes? To me, this is the holy grail,” Dr. Nissen said.
A major cautionary note was sounded by a European Medicines Agency–mandated cardiovascular outcomes study of sibutramine, said Dr. Nissen. In clinical trials, patients taking sibutramine had seen modest weight loss, with increased HDL cholesterol and decreased triglycerides. However, blood pressure rose by 1-3 mm Hg, and heart rates also climbed by 4-5 beats per minute, changes consistent with sibutramine’s sympathomimetic effects, said Dr. Nissen. The EMA-mandated trial included over 10,000 patients and looked at a composite endpoint of major cardiovascular events, including death, MI, stroke, and resuscitated arrest. Patients were included if they were aged older than 55 years, had a body mass index of greater than 27 kg/m2, and had a history of cardiovascular disease or diabetes with an additional risk factor. Patients who had significant heart rate or blood pressure increases during the study run-in period were excluded.
In the end, patients taking sibutramine had an increased risk for the composite endpoint (11.4% vs. 10.0%; P = .02). The risk for nonfatal stroke and nonfatal MI was also significantly elevated for the sibutramine group (N Engl J Med. 2010; 363:905-17).
Phentermine is a pharmacologic relative of sibutramine, with similar effects on blood pressure and heart rate. Since it was approved prior to the current increased focus on real-world clinical outcomes in drug approvals, phentermine’s cardiovascular outcomes have never been studied by means of a RCT. “Nobody’s going to do this study unless we push for it, but it has to be done,” he said. “Although this drug reduces weight, there is considerable uncertainty whether it increases cardiovascular outcomes.”
Even looking at weight loss alone, pharmacologic treatments show marginal benefit over time, said Dr. Nissen, citing, as an example, recently published outcome data on lorcaserin. Over 40 months of treatment, there was a “complete absence of any benefit for lorcaserin,” compared with placebo, and participants saw an average weight loss of just 1.9 kg by the end of the study period, with no change in cardiovascular outcomes (N Engl J Med. 2018;379:1107-17).
To drive home the point, Dr. Nissen shared a slide entitled “Established Benefits of Weight Loss Drugs on Clinically Important Outcomes.” The slide’s text read, “This slide intentionally left blank.”
“It’s very hard for me to argue in favor of giving any of these drugs,” said Dr. Nissen. “In the absence of established outcome benefits, there are only risks and costs. I know this is not going to be popular with everyone in this audience, but I have to tell you what I really believe here: We have to do better.”
More broadly, “I think we have to demand outcome trials for obesity drugs,” said Dr. Nissen. He noted that such trials are underway for some glucagonlike peptide–1 agonists, “and I applaud them. ... I hope you will participate in those studies, because they are going to give us some answers.”
Calling for renewed efforts to improve the efficacy of lifestyle interventions, Dr. Nissen said, “What we have to do is try. .... You know as well as I that there are some outliers” who will achieve profound weight loss without surgery, and those patients are likely to reap big benefits in risk reduction.
“We’ve got a problem that affects tens of millions of people, and we’ve got to find a societal approach to this. But we share these patients; let’s work together on trying to make them better.”
Dr. Nissen did not report any relevant financial disclosures.
NASHVILLE – It’s all hands on deck to fight the obesity epidemic, according to a cardiologist who made a plea for collaboration at the opening session at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. said Steven Nissen, MD, because when significant weight loss is achieved, “we can have an amazing effect on cardiovascular death, stroke, myocardial infarction, and these feared complications of obesity.”
From Dr. Nissen’s perspective, though, rates of death from cardiovascular disease have plateaued and are creeping up after decades of marked improvement.
“I am sorry to tell you that these rates are beginning to go up again – because of the obesity epidemic. That’s why we need to work together on this problem,” said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic. “It has basically stopped progress on cardiovascular disease.”
Dr. Nissen drew from the broad literature intertwining obesity and cardiometabolic health to tell a story that went beyond weight loss to focus on outcomes.
For bariatric surgery, the evidence of reduction in cardiovascular risk is looking very good, said Dr. Nissen. “There are just huge changes in the metabolic risk factors. … Clearly we have evidence that if we get people to lose substantial weight, you can get people to normalize major metabolic risk factors.”
Recent data from a multisite, retrospective, matched cohort study of patients with diabetes and severe obesity show the promise of substantial weight loss in reducing risk. The study tracked 5,301 bariatric surgery patients and compared them with 14,934 “well-matched” control participants who did not have bariatric surgery but received usual care for diabetes, said Dr. Nissen.
During 7 years of follow-up, the bariatric surgery group had a hazard ratio for coronary events of 0.64 (95% confidence interval, 0.42-0.99; P less than .001). A post hoc analysis showed an HR of 0.34 for all-cause mortality among the bariatric surgery patients (95% CI, 0.15-0.74; P less than .001; JAMA. 2018;320[15]:1570-82).
“I’ve been practicing in this field for about 40 years,” said Dr. Nissen. “With statins, we get about 25% risk reduction … a 34% risk reduction is just a whopping big reduction.” Background risk was high among this population with diabetes, and this was a cohort study, not a randomized, controlled trial (RCT).
“We need RCTs,” he said. “I hope we can come together – all of us – and do a large, multicenter, global RCT on the effects of bariatric surgery on cardiovascular outcomes. But barring that, these are the best data we are going to have.”
But can these changes be achieved and sustained without surgery?
“Can diet or drug therapy favorably affect atherosclerotic cardiovascular outcomes? To me, this is the holy grail,” Dr. Nissen said.
A major cautionary note was sounded by a European Medicines Agency–mandated cardiovascular outcomes study of sibutramine, said Dr. Nissen. In clinical trials, patients taking sibutramine had seen modest weight loss, with increased HDL cholesterol and decreased triglycerides. However, blood pressure rose by 1-3 mm Hg, and heart rates also climbed by 4-5 beats per minute, changes consistent with sibutramine’s sympathomimetic effects, said Dr. Nissen. The EMA-mandated trial included over 10,000 patients and looked at a composite endpoint of major cardiovascular events, including death, MI, stroke, and resuscitated arrest. Patients were included if they were aged older than 55 years, had a body mass index of greater than 27 kg/m2, and had a history of cardiovascular disease or diabetes with an additional risk factor. Patients who had significant heart rate or blood pressure increases during the study run-in period were excluded.
In the end, patients taking sibutramine had an increased risk for the composite endpoint (11.4% vs. 10.0%; P = .02). The risk for nonfatal stroke and nonfatal MI was also significantly elevated for the sibutramine group (N Engl J Med. 2010; 363:905-17).
Phentermine is a pharmacologic relative of sibutramine, with similar effects on blood pressure and heart rate. Since it was approved prior to the current increased focus on real-world clinical outcomes in drug approvals, phentermine’s cardiovascular outcomes have never been studied by means of a RCT. “Nobody’s going to do this study unless we push for it, but it has to be done,” he said. “Although this drug reduces weight, there is considerable uncertainty whether it increases cardiovascular outcomes.”
Even looking at weight loss alone, pharmacologic treatments show marginal benefit over time, said Dr. Nissen, citing, as an example, recently published outcome data on lorcaserin. Over 40 months of treatment, there was a “complete absence of any benefit for lorcaserin,” compared with placebo, and participants saw an average weight loss of just 1.9 kg by the end of the study period, with no change in cardiovascular outcomes (N Engl J Med. 2018;379:1107-17).
To drive home the point, Dr. Nissen shared a slide entitled “Established Benefits of Weight Loss Drugs on Clinically Important Outcomes.” The slide’s text read, “This slide intentionally left blank.”
“It’s very hard for me to argue in favor of giving any of these drugs,” said Dr. Nissen. “In the absence of established outcome benefits, there are only risks and costs. I know this is not going to be popular with everyone in this audience, but I have to tell you what I really believe here: We have to do better.”
More broadly, “I think we have to demand outcome trials for obesity drugs,” said Dr. Nissen. He noted that such trials are underway for some glucagonlike peptide–1 agonists, “and I applaud them. ... I hope you will participate in those studies, because they are going to give us some answers.”
Calling for renewed efforts to improve the efficacy of lifestyle interventions, Dr. Nissen said, “What we have to do is try. .... You know as well as I that there are some outliers” who will achieve profound weight loss without surgery, and those patients are likely to reap big benefits in risk reduction.
“We’ve got a problem that affects tens of millions of people, and we’ve got to find a societal approach to this. But we share these patients; let’s work together on trying to make them better.”
Dr. Nissen did not report any relevant financial disclosures.
EXPERT ANALYSIS FROM OBESITY WEEK 2018
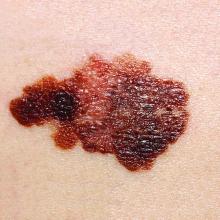
New pregnancy, genetic testing guidance added to AAD’s melanoma guidelines
Pregnancy does not necessarily increase a woman’s risk for melanoma, nor is it clear that becoming pregnant affects melanoma’s disease course, according to current evidence. This guidance is among several updates added to newly released guidelines for managing patients with primary cutaneous melanoma.
also addressed the burgeoning field of genetic testing for cancer in the guidelines, which were published online on Nov. 1. Although there may be a hereditary component to some melanomas, genetic testing may not be appropriate for all patients, and any formal genetic testing should be carried out only after individualized education and counseling, according to the updates.
However, the guidelines make it clear that all patients whose family history includes melanoma should be counseled about their genetic risk.
As with genetic testing, counseling regarding future pregnancies for women with melanoma, or a history of melanoma, should be personalized and account for individual history and melanoma risk, according to the new guidelines. Since evidence is lacking that pregnancy affects the course of melanoma, physicians caring for pregnant women with melanoma should first look at patient and the disease characteristics. The addition of detailed guidance regarding pregnancy reflects research showing that CM is the most common malignancy seen in pregnancy, amounting to nearly one-third of the malignancies that arise in pregnancy. “Although the incidence of CM is generally higher in men, it is higher in younger women than in men, most notably during women’s reproductive years,” wrote Susan M. Swetter, MD, and her guideline coauthors.
“Melanoma is the deadliest form of skin cancer, and we hope these guidelines will help dermatologists and other physicians enhance their delivery of life-saving treatment to patients,” Dr. Swetter said in a press release announcing the guideline updates. Dr. Swetter, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, led the working group that developed the guidelines. “In order to provide the best possible resource for practitioners, we reviewed the latest scientific data and addressed certain topics that weren’t covered in the AAD’s previous melanoma guidelines,” she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. Adjuvant topical therapies or radiation, say the guidelines, can be considered as second-line care, but only in limited situations in which surgery is not feasible. Staged excision techniques, such as Mohs surgery, also may be considered for certain types of melanoma and in certain body areas.
In an interview, Dr. Swetter also said that is critical that the updated guidelines have been harmonized with changes made in the American Joint Committee on Cancer’s 8th edition of its melanoma staging manual. Key points for dermatologists to understand that reporting of Breslow thickness to the nearest 1/10th decimal point (over the nearest 1/100th), such that a melanoma measuring 0.75-0.84 mm in thickness would be reported as 0.8 mm depth and one between 0.95-1.04 mm would be rounded to 1 mm.
The main changes regarding staging of thin (T1) melanoma – that is less than or equal to 1 mm – is that the 0.8 mm thickness is the threshold for a T1a melanoma (now classified as less than 0.8 mm without ulceration), whereas T1b is now 0.8 – 1.0 mm thickness with or w/out ulceration or less than 0.8 mm thickness with ulceration. A T1a melanoma generally is not considered appropriate for staging of the regional lymph nodes with sentinel lymph node biopsy (with exceptions noted in the guideline), whereas a T1b melanoma may be considered for SLNB staging – though rates of SLN positivity remain relatively low in the T1b group.”
Dr. Swetter also emphasized that histologic ulceration of the primary tumor was affirmed as an indicator of worse prognosis; mitotic rate, although removed from T1 staging, is still tracked by pathologists and still seen as an independent predictor of worse prognosis, according to the 8th edition, she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. “Mohs micrographic surgery and other staged excision techniques can provide exhaustive peripheral margin histologic assessment for melanoma in situ, lentigo maligna type and tissue sparing in anatomically constrained sites,” Dr. Swetter said. “Current data are insufficient to recommend Mohs surgery for invasive cutaneous melanoma, in which the use of surgical margins less than 1 cm has not been adequately studied,” she cautioned.
Reinforcing the importance of surgery as the primary treatment for melanoma, Dr. Swetter clarified that “Nonsurgical approaches (imiquimod and traditional forms of radiation therapy) should be considered [only] if surgery is impractical or contraindicated, and only for melanoma in situ, lentigo maligna type, as cure rates are lower.”
In terms of other therapies, the guideline working group found insufficient evidence to recommend electronic brachytherapy for melanoma.
Assessment of novel diagnostic and molecular imaging modalities was not the primary focus of the AAD guidelines, Dr. Swetter pointed out. Looking to the future, though, she added that the hope is “that these prebiopsy modalities can one day reduce unnecessary biopsies from being done” in the clinic.
Other knowledge gaps cited by the working group included several related to pathology, including determination of appropriate margin control in some lesion types, and the quest to reduce inter-reader variability in histopathologic diagnosis of melanocytic tissue samples. However, noted Dr. Swetter and her coauthors, the rapid pace of genomic medicine advances “may make many of the aforementioned issues obsolete” before the next guideline update.
In the interview, Dr. Swetter said that the guidelines reflect evolving thinking about melanoma in the context of a rapidly growing field. “Only in the last year have effective, more tolerable adjuvant therapies been [Food and Drug Administration] approved for patients with resected stage III melanoma, including patients with regional lymph node disease detected via sentinel lymph node biopsy. The hope is that less invasive procedures for melanoma will be performed in the future, and replaced by better drugs and novel techniques.”
Dr. Swetter reported that she had no relevant financial disclosures; several working group members reported multiple financial relationships with pharmaceutical, diagnostic, and imaging companies. Working group members were recused from discussion of guidelines where their particular relationships might pose a conflict of interest.
SOURCE: Swetter S. et al. J Am Acad Dermatol. 2011 Nov;65(5):1032-47.
Pregnancy does not necessarily increase a woman’s risk for melanoma, nor is it clear that becoming pregnant affects melanoma’s disease course, according to current evidence. This guidance is among several updates added to newly released guidelines for managing patients with primary cutaneous melanoma.
also addressed the burgeoning field of genetic testing for cancer in the guidelines, which were published online on Nov. 1. Although there may be a hereditary component to some melanomas, genetic testing may not be appropriate for all patients, and any formal genetic testing should be carried out only after individualized education and counseling, according to the updates.
However, the guidelines make it clear that all patients whose family history includes melanoma should be counseled about their genetic risk.
As with genetic testing, counseling regarding future pregnancies for women with melanoma, or a history of melanoma, should be personalized and account for individual history and melanoma risk, according to the new guidelines. Since evidence is lacking that pregnancy affects the course of melanoma, physicians caring for pregnant women with melanoma should first look at patient and the disease characteristics. The addition of detailed guidance regarding pregnancy reflects research showing that CM is the most common malignancy seen in pregnancy, amounting to nearly one-third of the malignancies that arise in pregnancy. “Although the incidence of CM is generally higher in men, it is higher in younger women than in men, most notably during women’s reproductive years,” wrote Susan M. Swetter, MD, and her guideline coauthors.
“Melanoma is the deadliest form of skin cancer, and we hope these guidelines will help dermatologists and other physicians enhance their delivery of life-saving treatment to patients,” Dr. Swetter said in a press release announcing the guideline updates. Dr. Swetter, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, led the working group that developed the guidelines. “In order to provide the best possible resource for practitioners, we reviewed the latest scientific data and addressed certain topics that weren’t covered in the AAD’s previous melanoma guidelines,” she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. Adjuvant topical therapies or radiation, say the guidelines, can be considered as second-line care, but only in limited situations in which surgery is not feasible. Staged excision techniques, such as Mohs surgery, also may be considered for certain types of melanoma and in certain body areas.
In an interview, Dr. Swetter also said that is critical that the updated guidelines have been harmonized with changes made in the American Joint Committee on Cancer’s 8th edition of its melanoma staging manual. Key points for dermatologists to understand that reporting of Breslow thickness to the nearest 1/10th decimal point (over the nearest 1/100th), such that a melanoma measuring 0.75-0.84 mm in thickness would be reported as 0.8 mm depth and one between 0.95-1.04 mm would be rounded to 1 mm.
The main changes regarding staging of thin (T1) melanoma – that is less than or equal to 1 mm – is that the 0.8 mm thickness is the threshold for a T1a melanoma (now classified as less than 0.8 mm without ulceration), whereas T1b is now 0.8 – 1.0 mm thickness with or w/out ulceration or less than 0.8 mm thickness with ulceration. A T1a melanoma generally is not considered appropriate for staging of the regional lymph nodes with sentinel lymph node biopsy (with exceptions noted in the guideline), whereas a T1b melanoma may be considered for SLNB staging – though rates of SLN positivity remain relatively low in the T1b group.”
Dr. Swetter also emphasized that histologic ulceration of the primary tumor was affirmed as an indicator of worse prognosis; mitotic rate, although removed from T1 staging, is still tracked by pathologists and still seen as an independent predictor of worse prognosis, according to the 8th edition, she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. “Mohs micrographic surgery and other staged excision techniques can provide exhaustive peripheral margin histologic assessment for melanoma in situ, lentigo maligna type and tissue sparing in anatomically constrained sites,” Dr. Swetter said. “Current data are insufficient to recommend Mohs surgery for invasive cutaneous melanoma, in which the use of surgical margins less than 1 cm has not been adequately studied,” she cautioned.
Reinforcing the importance of surgery as the primary treatment for melanoma, Dr. Swetter clarified that “Nonsurgical approaches (imiquimod and traditional forms of radiation therapy) should be considered [only] if surgery is impractical or contraindicated, and only for melanoma in situ, lentigo maligna type, as cure rates are lower.”
In terms of other therapies, the guideline working group found insufficient evidence to recommend electronic brachytherapy for melanoma.
Assessment of novel diagnostic and molecular imaging modalities was not the primary focus of the AAD guidelines, Dr. Swetter pointed out. Looking to the future, though, she added that the hope is “that these prebiopsy modalities can one day reduce unnecessary biopsies from being done” in the clinic.
Other knowledge gaps cited by the working group included several related to pathology, including determination of appropriate margin control in some lesion types, and the quest to reduce inter-reader variability in histopathologic diagnosis of melanocytic tissue samples. However, noted Dr. Swetter and her coauthors, the rapid pace of genomic medicine advances “may make many of the aforementioned issues obsolete” before the next guideline update.
In the interview, Dr. Swetter said that the guidelines reflect evolving thinking about melanoma in the context of a rapidly growing field. “Only in the last year have effective, more tolerable adjuvant therapies been [Food and Drug Administration] approved for patients with resected stage III melanoma, including patients with regional lymph node disease detected via sentinel lymph node biopsy. The hope is that less invasive procedures for melanoma will be performed in the future, and replaced by better drugs and novel techniques.”
Dr. Swetter reported that she had no relevant financial disclosures; several working group members reported multiple financial relationships with pharmaceutical, diagnostic, and imaging companies. Working group members were recused from discussion of guidelines where their particular relationships might pose a conflict of interest.
SOURCE: Swetter S. et al. J Am Acad Dermatol. 2011 Nov;65(5):1032-47.
Pregnancy does not necessarily increase a woman’s risk for melanoma, nor is it clear that becoming pregnant affects melanoma’s disease course, according to current evidence. This guidance is among several updates added to newly released guidelines for managing patients with primary cutaneous melanoma.
also addressed the burgeoning field of genetic testing for cancer in the guidelines, which were published online on Nov. 1. Although there may be a hereditary component to some melanomas, genetic testing may not be appropriate for all patients, and any formal genetic testing should be carried out only after individualized education and counseling, according to the updates.
However, the guidelines make it clear that all patients whose family history includes melanoma should be counseled about their genetic risk.
As with genetic testing, counseling regarding future pregnancies for women with melanoma, or a history of melanoma, should be personalized and account for individual history and melanoma risk, according to the new guidelines. Since evidence is lacking that pregnancy affects the course of melanoma, physicians caring for pregnant women with melanoma should first look at patient and the disease characteristics. The addition of detailed guidance regarding pregnancy reflects research showing that CM is the most common malignancy seen in pregnancy, amounting to nearly one-third of the malignancies that arise in pregnancy. “Although the incidence of CM is generally higher in men, it is higher in younger women than in men, most notably during women’s reproductive years,” wrote Susan M. Swetter, MD, and her guideline coauthors.
“Melanoma is the deadliest form of skin cancer, and we hope these guidelines will help dermatologists and other physicians enhance their delivery of life-saving treatment to patients,” Dr. Swetter said in a press release announcing the guideline updates. Dr. Swetter, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, led the working group that developed the guidelines. “In order to provide the best possible resource for practitioners, we reviewed the latest scientific data and addressed certain topics that weren’t covered in the AAD’s previous melanoma guidelines,” she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. Adjuvant topical therapies or radiation, say the guidelines, can be considered as second-line care, but only in limited situations in which surgery is not feasible. Staged excision techniques, such as Mohs surgery, also may be considered for certain types of melanoma and in certain body areas.
In an interview, Dr. Swetter also said that is critical that the updated guidelines have been harmonized with changes made in the American Joint Committee on Cancer’s 8th edition of its melanoma staging manual. Key points for dermatologists to understand that reporting of Breslow thickness to the nearest 1/10th decimal point (over the nearest 1/100th), such that a melanoma measuring 0.75-0.84 mm in thickness would be reported as 0.8 mm depth and one between 0.95-1.04 mm would be rounded to 1 mm.
The main changes regarding staging of thin (T1) melanoma – that is less than or equal to 1 mm – is that the 0.8 mm thickness is the threshold for a T1a melanoma (now classified as less than 0.8 mm without ulceration), whereas T1b is now 0.8 – 1.0 mm thickness with or w/out ulceration or less than 0.8 mm thickness with ulceration. A T1a melanoma generally is not considered appropriate for staging of the regional lymph nodes with sentinel lymph node biopsy (with exceptions noted in the guideline), whereas a T1b melanoma may be considered for SLNB staging – though rates of SLN positivity remain relatively low in the T1b group.”
Dr. Swetter also emphasized that histologic ulceration of the primary tumor was affirmed as an indicator of worse prognosis; mitotic rate, although removed from T1 staging, is still tracked by pathologists and still seen as an independent predictor of worse prognosis, according to the 8th edition, she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. “Mohs micrographic surgery and other staged excision techniques can provide exhaustive peripheral margin histologic assessment for melanoma in situ, lentigo maligna type and tissue sparing in anatomically constrained sites,” Dr. Swetter said. “Current data are insufficient to recommend Mohs surgery for invasive cutaneous melanoma, in which the use of surgical margins less than 1 cm has not been adequately studied,” she cautioned.
Reinforcing the importance of surgery as the primary treatment for melanoma, Dr. Swetter clarified that “Nonsurgical approaches (imiquimod and traditional forms of radiation therapy) should be considered [only] if surgery is impractical or contraindicated, and only for melanoma in situ, lentigo maligna type, as cure rates are lower.”
In terms of other therapies, the guideline working group found insufficient evidence to recommend electronic brachytherapy for melanoma.
Assessment of novel diagnostic and molecular imaging modalities was not the primary focus of the AAD guidelines, Dr. Swetter pointed out. Looking to the future, though, she added that the hope is “that these prebiopsy modalities can one day reduce unnecessary biopsies from being done” in the clinic.
Other knowledge gaps cited by the working group included several related to pathology, including determination of appropriate margin control in some lesion types, and the quest to reduce inter-reader variability in histopathologic diagnosis of melanocytic tissue samples. However, noted Dr. Swetter and her coauthors, the rapid pace of genomic medicine advances “may make many of the aforementioned issues obsolete” before the next guideline update.
In the interview, Dr. Swetter said that the guidelines reflect evolving thinking about melanoma in the context of a rapidly growing field. “Only in the last year have effective, more tolerable adjuvant therapies been [Food and Drug Administration] approved for patients with resected stage III melanoma, including patients with regional lymph node disease detected via sentinel lymph node biopsy. The hope is that less invasive procedures for melanoma will be performed in the future, and replaced by better drugs and novel techniques.”
Dr. Swetter reported that she had no relevant financial disclosures; several working group members reported multiple financial relationships with pharmaceutical, diagnostic, and imaging companies. Working group members were recused from discussion of guidelines where their particular relationships might pose a conflict of interest.
SOURCE: Swetter S. et al. J Am Acad Dermatol. 2011 Nov;65(5):1032-47.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Propolis plus L-dopa buffers PD symptoms in rat model
NEW YORK – A bee-derived supplement known to have anti-inflammatory properties showed promise in potentiating the effect of levodopa in a rodent model of Parkinson’s disease, with biochemical and behavioral improvements seen that exceeded high-dose levodopa alone.
Levodopa (L-dopa) has long been a keystone in treatment for individuals with Parkinson’s disease (PD). However, its effectiveness wanes with time and long-term use is associated with significant undesirable side effects, including dyskinesias.
A dopamine precursor, L-dopa, once converted to dopamine, replaces the dopamine no longer made by the substantia nigra. Increasingly, neuroinflammation and oxidative stress are felt to play a role in the natural history of PD, said Azza Ali, PhD, who presented the findings at a poster session of the International Conference on Parkinson’s Disease and Movement Disorders. Whether mitigating these effects can alter the disease course for individuals with PD has not been well investigated, she added.
Dr. Ali and her research group at Al-Azhar University, Cairo, Egypt, where she heads the department of pharmacology and toxicology, are investigating several nutritional and supplement strategies to dampen inflammation. Among the substances she and her colleagues are investigating is propolis, a plant-derived product produced by bees and distinct from honey or beeswax.
Propolis has been shown to have antioxidant properties in addition to other reported health benefits.
Dr. Ali and her colleagues used a rodent model for PD: Rats were dosed with rotenone, which is known to induce a histologically verifiable parkinsonian syndrome. The investigators used six groups of rats; one group was the normal control, while the others all had rotenone-induced parkinsonism.
Of the remaining groups, one rotenone-dosed group was given neither L-dopa nor propolis. Two groups were treated with oral L-dopa alone, at 10 or 25 mg/kg per day. Another group was given an oral dose of 300 mg/kg per day of propolis, and the last group received propolis at that dose while also receiving the lower dose of L-dopa.
All groups were subject to behavioral assessments including a swim test, an open field test, a maze test, and other tasks designed to assess motor function and other aspects of behavior. Additionally, Dr. Ali and her collaborators assessed a variety of biochemical parameters that looked at oxidative stress and neuroinflammation in all rodent groups.
For most parameters, the rotenone-dosed rats that showed results most like normal controls were those who received both low-dose L-dopa and propolis. In particular, the rats receiving both L-dopa and propolis outperformed the other rotenone groups in the behavioral open field test and a grid test, where their performance neared the control group.
Levels of interleukin-1 beta fell for all treated rodents, but fell the most for those treated with the combination. Tissue dopamine levels were also lower for the rats who received combination treatment, and acetylcholinesterase and malondialdehyde levels also fell to near normal for rats receiving the combination treatment, but not for the other groups.
“Propolis is efficient in protection from PD development and represents a suitable adjuvant therapy, which can be translated to serious reduction of the long-term therapy side effects of the mainstay drug L-dopa,” wrote Dr. Ali and her coauthors. “Consequently, propolis could be recommended as a disease-modifying therapy of PD as well as a promising adjunct therapy with L-dopa especially when given early in the treatment course.”
NEW YORK – A bee-derived supplement known to have anti-inflammatory properties showed promise in potentiating the effect of levodopa in a rodent model of Parkinson’s disease, with biochemical and behavioral improvements seen that exceeded high-dose levodopa alone.
Levodopa (L-dopa) has long been a keystone in treatment for individuals with Parkinson’s disease (PD). However, its effectiveness wanes with time and long-term use is associated with significant undesirable side effects, including dyskinesias.
A dopamine precursor, L-dopa, once converted to dopamine, replaces the dopamine no longer made by the substantia nigra. Increasingly, neuroinflammation and oxidative stress are felt to play a role in the natural history of PD, said Azza Ali, PhD, who presented the findings at a poster session of the International Conference on Parkinson’s Disease and Movement Disorders. Whether mitigating these effects can alter the disease course for individuals with PD has not been well investigated, she added.
Dr. Ali and her research group at Al-Azhar University, Cairo, Egypt, where she heads the department of pharmacology and toxicology, are investigating several nutritional and supplement strategies to dampen inflammation. Among the substances she and her colleagues are investigating is propolis, a plant-derived product produced by bees and distinct from honey or beeswax.
Propolis has been shown to have antioxidant properties in addition to other reported health benefits.
Dr. Ali and her colleagues used a rodent model for PD: Rats were dosed with rotenone, which is known to induce a histologically verifiable parkinsonian syndrome. The investigators used six groups of rats; one group was the normal control, while the others all had rotenone-induced parkinsonism.
Of the remaining groups, one rotenone-dosed group was given neither L-dopa nor propolis. Two groups were treated with oral L-dopa alone, at 10 or 25 mg/kg per day. Another group was given an oral dose of 300 mg/kg per day of propolis, and the last group received propolis at that dose while also receiving the lower dose of L-dopa.
All groups were subject to behavioral assessments including a swim test, an open field test, a maze test, and other tasks designed to assess motor function and other aspects of behavior. Additionally, Dr. Ali and her collaborators assessed a variety of biochemical parameters that looked at oxidative stress and neuroinflammation in all rodent groups.
For most parameters, the rotenone-dosed rats that showed results most like normal controls were those who received both low-dose L-dopa and propolis. In particular, the rats receiving both L-dopa and propolis outperformed the other rotenone groups in the behavioral open field test and a grid test, where their performance neared the control group.
Levels of interleukin-1 beta fell for all treated rodents, but fell the most for those treated with the combination. Tissue dopamine levels were also lower for the rats who received combination treatment, and acetylcholinesterase and malondialdehyde levels also fell to near normal for rats receiving the combination treatment, but not for the other groups.
“Propolis is efficient in protection from PD development and represents a suitable adjuvant therapy, which can be translated to serious reduction of the long-term therapy side effects of the mainstay drug L-dopa,” wrote Dr. Ali and her coauthors. “Consequently, propolis could be recommended as a disease-modifying therapy of PD as well as a promising adjunct therapy with L-dopa especially when given early in the treatment course.”
NEW YORK – A bee-derived supplement known to have anti-inflammatory properties showed promise in potentiating the effect of levodopa in a rodent model of Parkinson’s disease, with biochemical and behavioral improvements seen that exceeded high-dose levodopa alone.
Levodopa (L-dopa) has long been a keystone in treatment for individuals with Parkinson’s disease (PD). However, its effectiveness wanes with time and long-term use is associated with significant undesirable side effects, including dyskinesias.
A dopamine precursor, L-dopa, once converted to dopamine, replaces the dopamine no longer made by the substantia nigra. Increasingly, neuroinflammation and oxidative stress are felt to play a role in the natural history of PD, said Azza Ali, PhD, who presented the findings at a poster session of the International Conference on Parkinson’s Disease and Movement Disorders. Whether mitigating these effects can alter the disease course for individuals with PD has not been well investigated, she added.
Dr. Ali and her research group at Al-Azhar University, Cairo, Egypt, where she heads the department of pharmacology and toxicology, are investigating several nutritional and supplement strategies to dampen inflammation. Among the substances she and her colleagues are investigating is propolis, a plant-derived product produced by bees and distinct from honey or beeswax.
Propolis has been shown to have antioxidant properties in addition to other reported health benefits.
Dr. Ali and her colleagues used a rodent model for PD: Rats were dosed with rotenone, which is known to induce a histologically verifiable parkinsonian syndrome. The investigators used six groups of rats; one group was the normal control, while the others all had rotenone-induced parkinsonism.
Of the remaining groups, one rotenone-dosed group was given neither L-dopa nor propolis. Two groups were treated with oral L-dopa alone, at 10 or 25 mg/kg per day. Another group was given an oral dose of 300 mg/kg per day of propolis, and the last group received propolis at that dose while also receiving the lower dose of L-dopa.
All groups were subject to behavioral assessments including a swim test, an open field test, a maze test, and other tasks designed to assess motor function and other aspects of behavior. Additionally, Dr. Ali and her collaborators assessed a variety of biochemical parameters that looked at oxidative stress and neuroinflammation in all rodent groups.
For most parameters, the rotenone-dosed rats that showed results most like normal controls were those who received both low-dose L-dopa and propolis. In particular, the rats receiving both L-dopa and propolis outperformed the other rotenone groups in the behavioral open field test and a grid test, where their performance neared the control group.
Levels of interleukin-1 beta fell for all treated rodents, but fell the most for those treated with the combination. Tissue dopamine levels were also lower for the rats who received combination treatment, and acetylcholinesterase and malondialdehyde levels also fell to near normal for rats receiving the combination treatment, but not for the other groups.
“Propolis is efficient in protection from PD development and represents a suitable adjuvant therapy, which can be translated to serious reduction of the long-term therapy side effects of the mainstay drug L-dopa,” wrote Dr. Ali and her coauthors. “Consequently, propolis could be recommended as a disease-modifying therapy of PD as well as a promising adjunct therapy with L-dopa especially when given early in the treatment course.”
REPORTING FROM ICPDMD 2018
Hospitals could reduce maternal mortality with four achievable steps
rate in the United States, according to a new perspective from leading obstetricians published in the New England Journal of Medicine.
The authors, including Kimberlee McKay, MD, president of the American College of Obstetricians and Gynecologists (ACOG), also call for collaboration with family physicians to increase access to obstetric care in rural areas.
The president of the American Academy of Family Physicians (AAFP), John S. Cullen, MD, in a separate statement, welcomed the opportunity for collaboration in addressing the maternal mortality crisis. However, some distance still lies between the finer points of how the two organizations see family physicians helping curb the crisis.
“Women in the United States are more likely to die from childbirth- or pregnancy-related causes than women in any other high-income country, and black women die at a rate three to four times that of white women,” noted Susan Mann, MD, along with her coauthors of the obstetric perspective, calling increasing maternal mortality a “tragedy.”
In an interview, Dr. Cullen concurred, calling the current situation “unconscionable.” One of the primary reasons he sought AAFP leadership, he said, was to bring experiences learned during his 25 years of obstetric practice in rural Alaska to bear on the current crisis.
A set of maternal care bundles created by the Alliance for Innovation on Maternal Health (AIM) provides the framework for the first action recommended by Dr. Mann and her coauthors. The AIM bundles focus on protocols that improve readiness, recognition, response, and reporting in maternal care. The protocols are institution specific. For example, the authors noted, antihypertensive medications should be readily available around the clock, because not all facilities will have a pharmacist in-house at all times, and hypertensive emergencies are among the gravest obstetric complications.
“Although management may vary from institution to institution, each unit can be required to demonstrate readiness to deal with emergencies 24/7,” said Dr. Mann, a physician in private practice in Boston, and her coauthors.
The second recommended action revolves around multidisciplinary staff meetings to perform individual assessments for each woman’s obstetric risk factors. These huddles should include assessing hemorrhage risk by using the California Maternal Quality Care Collaborative guidelines, and briefings with the full care team to “develop shared understandings of the patient and the procedure” before elective or nonurgent cesarean deliveries, said Dr. Mann and her colleagues. Patients should be informed of any safety concerns, and caregivers should use shared decision-making moving forward.
“Approximately 50% of U.S. hospitals provide care for three or fewer deliveries per day, but the need to identify women at risk is equally important for these small obstetrics services,” Dr. Mann and her coauthors noted.
Third, simulation of obstetric emergencies lets all members of the team understand the speed at which decisions must be made and actions taken when minutes, or even seconds, count. Dr. Mann and her coauthors pointed out that logistics problems come out during a well-run simulation, giving such examples as a lag in receiving blood products or a poorly placed hemorrhage cart.
Drawing an analogy to the extensive time pilots spend in flight simulators, Dr. Mann and her coauthors noted that “because severe maternity-related events are rare and often unpredictable, and because members of the care team may not know each other, it is important to train for low-probability but high-risk events.”
Using the Maternal Health Compact will help ensure that lower-resource hospitals have a relationship in place with a facility that can provide a higher level of maternal care when needed, they said.
This fourth action means that there’s a connection that “can be activated by lower-resource hospitals to get immediate consultation in the event of an unexpected obstetrical emergency whose care demands exceed their resources,” said Dr. Mann and her coauthors. When transport is necessary, it too can be facilitated if a compact is already in place.
The final, broader, recommendation from the obstetricians is that ACOG and AAFP collaborate “on an additional year of comprehensive training for family medicine physicians who are considering practicing obstetrics in rural areas.” The fourth year of training for these family physicians could help address widespread shortages of obstetricians and midwives in rural communities, Dr. Mann and her coauthors wrote. They observed that “pregnant women who live in rural areas may need to travel hundreds of miles to obtain routine prenatal care or consultation with an obstetrical specialist.”
Dr. Cullen, whose practice is in Valdez, Alaska, said he happily reciprocates the desire for collaboration. In his inaugural blog post after assuming the AAFP presidency in October, 2018, he extended warm thanks to his obstetrician and perinatologist colleagues. “The relationship between family physicians and obstetricians is vital in rural communities like mine. The training I received has saved lives and prevented catastrophe,” he wrote.
At the same time, Dr. Cullen said that he believes family physicians are the best hope for quality obstetrical care in rural communities. Dr. Cullen’s own training, he said, was structured to involve significant obstetric experience with obstetrician mentorship. “A 3-year residency left me very comfortable with fairly sophisticated obstetric management at graduation,” he added.
In his practice, Dr. Cullen said, a family physician hired a decade ago after a 3-year residency came with excellent obstetric skills, which have been further honed during the succeeding years of rural practice. During a 3-year residency, “family physicians receive training and demonstrate the skills and competencies required to deliver high-quality maternity care in any community, including those in rural settings,” he said in his statement.
“The bigger issue is really the closure of obstetric units in rural hospitals,” said Dr. Cullen. He pointed out that although obstetric services may have left a community, that rural hospital will still have miscarrying patients with hemorrhage, patients in preterm labor, and other obstetric emergencies arriving at the doorstep. These and other instances often “represent true emergencies without possibility of transfer, requiring immediate and effective response,” he wrote.
“We would encourage the authors to ensure an equal focus on improving care at all levels and in all hospitals, as well as relying on transfer as appropriate,” Dr. Cullen said in his statement.
Such mutual support can be bolstered by new technology, wrote Dr. Mann and her coauthors, because “telehealth and collaborations and consultation with clinics and regional hospitals can help increase the availability of maternity care in the United States.”
For Dr. Cullen also, the way forward is together. In his blog, he wrote: “As family physicians, we can only deliver obstetrical care to our patients with the cooperation of obstetricians and perinatologists. Conversely, obstetricians can only improve maternal and infant mortality rates, especially in rural areas, with the help of family physicians.”
SOURCE: Mann S et al. N Engl J Med 2018;379:1689-91.
rate in the United States, according to a new perspective from leading obstetricians published in the New England Journal of Medicine.
The authors, including Kimberlee McKay, MD, president of the American College of Obstetricians and Gynecologists (ACOG), also call for collaboration with family physicians to increase access to obstetric care in rural areas.
The president of the American Academy of Family Physicians (AAFP), John S. Cullen, MD, in a separate statement, welcomed the opportunity for collaboration in addressing the maternal mortality crisis. However, some distance still lies between the finer points of how the two organizations see family physicians helping curb the crisis.
“Women in the United States are more likely to die from childbirth- or pregnancy-related causes than women in any other high-income country, and black women die at a rate three to four times that of white women,” noted Susan Mann, MD, along with her coauthors of the obstetric perspective, calling increasing maternal mortality a “tragedy.”
In an interview, Dr. Cullen concurred, calling the current situation “unconscionable.” One of the primary reasons he sought AAFP leadership, he said, was to bring experiences learned during his 25 years of obstetric practice in rural Alaska to bear on the current crisis.
A set of maternal care bundles created by the Alliance for Innovation on Maternal Health (AIM) provides the framework for the first action recommended by Dr. Mann and her coauthors. The AIM bundles focus on protocols that improve readiness, recognition, response, and reporting in maternal care. The protocols are institution specific. For example, the authors noted, antihypertensive medications should be readily available around the clock, because not all facilities will have a pharmacist in-house at all times, and hypertensive emergencies are among the gravest obstetric complications.
“Although management may vary from institution to institution, each unit can be required to demonstrate readiness to deal with emergencies 24/7,” said Dr. Mann, a physician in private practice in Boston, and her coauthors.
The second recommended action revolves around multidisciplinary staff meetings to perform individual assessments for each woman’s obstetric risk factors. These huddles should include assessing hemorrhage risk by using the California Maternal Quality Care Collaborative guidelines, and briefings with the full care team to “develop shared understandings of the patient and the procedure” before elective or nonurgent cesarean deliveries, said Dr. Mann and her colleagues. Patients should be informed of any safety concerns, and caregivers should use shared decision-making moving forward.
“Approximately 50% of U.S. hospitals provide care for three or fewer deliveries per day, but the need to identify women at risk is equally important for these small obstetrics services,” Dr. Mann and her coauthors noted.
Third, simulation of obstetric emergencies lets all members of the team understand the speed at which decisions must be made and actions taken when minutes, or even seconds, count. Dr. Mann and her coauthors pointed out that logistics problems come out during a well-run simulation, giving such examples as a lag in receiving blood products or a poorly placed hemorrhage cart.
Drawing an analogy to the extensive time pilots spend in flight simulators, Dr. Mann and her coauthors noted that “because severe maternity-related events are rare and often unpredictable, and because members of the care team may not know each other, it is important to train for low-probability but high-risk events.”
Using the Maternal Health Compact will help ensure that lower-resource hospitals have a relationship in place with a facility that can provide a higher level of maternal care when needed, they said.
This fourth action means that there’s a connection that “can be activated by lower-resource hospitals to get immediate consultation in the event of an unexpected obstetrical emergency whose care demands exceed their resources,” said Dr. Mann and her coauthors. When transport is necessary, it too can be facilitated if a compact is already in place.
The final, broader, recommendation from the obstetricians is that ACOG and AAFP collaborate “on an additional year of comprehensive training for family medicine physicians who are considering practicing obstetrics in rural areas.” The fourth year of training for these family physicians could help address widespread shortages of obstetricians and midwives in rural communities, Dr. Mann and her coauthors wrote. They observed that “pregnant women who live in rural areas may need to travel hundreds of miles to obtain routine prenatal care or consultation with an obstetrical specialist.”
Dr. Cullen, whose practice is in Valdez, Alaska, said he happily reciprocates the desire for collaboration. In his inaugural blog post after assuming the AAFP presidency in October, 2018, he extended warm thanks to his obstetrician and perinatologist colleagues. “The relationship between family physicians and obstetricians is vital in rural communities like mine. The training I received has saved lives and prevented catastrophe,” he wrote.
At the same time, Dr. Cullen said that he believes family physicians are the best hope for quality obstetrical care in rural communities. Dr. Cullen’s own training, he said, was structured to involve significant obstetric experience with obstetrician mentorship. “A 3-year residency left me very comfortable with fairly sophisticated obstetric management at graduation,” he added.
In his practice, Dr. Cullen said, a family physician hired a decade ago after a 3-year residency came with excellent obstetric skills, which have been further honed during the succeeding years of rural practice. During a 3-year residency, “family physicians receive training and demonstrate the skills and competencies required to deliver high-quality maternity care in any community, including those in rural settings,” he said in his statement.
“The bigger issue is really the closure of obstetric units in rural hospitals,” said Dr. Cullen. He pointed out that although obstetric services may have left a community, that rural hospital will still have miscarrying patients with hemorrhage, patients in preterm labor, and other obstetric emergencies arriving at the doorstep. These and other instances often “represent true emergencies without possibility of transfer, requiring immediate and effective response,” he wrote.
“We would encourage the authors to ensure an equal focus on improving care at all levels and in all hospitals, as well as relying on transfer as appropriate,” Dr. Cullen said in his statement.
Such mutual support can be bolstered by new technology, wrote Dr. Mann and her coauthors, because “telehealth and collaborations and consultation with clinics and regional hospitals can help increase the availability of maternity care in the United States.”
For Dr. Cullen also, the way forward is together. In his blog, he wrote: “As family physicians, we can only deliver obstetrical care to our patients with the cooperation of obstetricians and perinatologists. Conversely, obstetricians can only improve maternal and infant mortality rates, especially in rural areas, with the help of family physicians.”
SOURCE: Mann S et al. N Engl J Med 2018;379:1689-91.
rate in the United States, according to a new perspective from leading obstetricians published in the New England Journal of Medicine.
The authors, including Kimberlee McKay, MD, president of the American College of Obstetricians and Gynecologists (ACOG), also call for collaboration with family physicians to increase access to obstetric care in rural areas.
The president of the American Academy of Family Physicians (AAFP), John S. Cullen, MD, in a separate statement, welcomed the opportunity for collaboration in addressing the maternal mortality crisis. However, some distance still lies between the finer points of how the two organizations see family physicians helping curb the crisis.
“Women in the United States are more likely to die from childbirth- or pregnancy-related causes than women in any other high-income country, and black women die at a rate three to four times that of white women,” noted Susan Mann, MD, along with her coauthors of the obstetric perspective, calling increasing maternal mortality a “tragedy.”
In an interview, Dr. Cullen concurred, calling the current situation “unconscionable.” One of the primary reasons he sought AAFP leadership, he said, was to bring experiences learned during his 25 years of obstetric practice in rural Alaska to bear on the current crisis.
A set of maternal care bundles created by the Alliance for Innovation on Maternal Health (AIM) provides the framework for the first action recommended by Dr. Mann and her coauthors. The AIM bundles focus on protocols that improve readiness, recognition, response, and reporting in maternal care. The protocols are institution specific. For example, the authors noted, antihypertensive medications should be readily available around the clock, because not all facilities will have a pharmacist in-house at all times, and hypertensive emergencies are among the gravest obstetric complications.
“Although management may vary from institution to institution, each unit can be required to demonstrate readiness to deal with emergencies 24/7,” said Dr. Mann, a physician in private practice in Boston, and her coauthors.
The second recommended action revolves around multidisciplinary staff meetings to perform individual assessments for each woman’s obstetric risk factors. These huddles should include assessing hemorrhage risk by using the California Maternal Quality Care Collaborative guidelines, and briefings with the full care team to “develop shared understandings of the patient and the procedure” before elective or nonurgent cesarean deliveries, said Dr. Mann and her colleagues. Patients should be informed of any safety concerns, and caregivers should use shared decision-making moving forward.
“Approximately 50% of U.S. hospitals provide care for three or fewer deliveries per day, but the need to identify women at risk is equally important for these small obstetrics services,” Dr. Mann and her coauthors noted.
Third, simulation of obstetric emergencies lets all members of the team understand the speed at which decisions must be made and actions taken when minutes, or even seconds, count. Dr. Mann and her coauthors pointed out that logistics problems come out during a well-run simulation, giving such examples as a lag in receiving blood products or a poorly placed hemorrhage cart.
Drawing an analogy to the extensive time pilots spend in flight simulators, Dr. Mann and her coauthors noted that “because severe maternity-related events are rare and often unpredictable, and because members of the care team may not know each other, it is important to train for low-probability but high-risk events.”
Using the Maternal Health Compact will help ensure that lower-resource hospitals have a relationship in place with a facility that can provide a higher level of maternal care when needed, they said.
This fourth action means that there’s a connection that “can be activated by lower-resource hospitals to get immediate consultation in the event of an unexpected obstetrical emergency whose care demands exceed their resources,” said Dr. Mann and her coauthors. When transport is necessary, it too can be facilitated if a compact is already in place.
The final, broader, recommendation from the obstetricians is that ACOG and AAFP collaborate “on an additional year of comprehensive training for family medicine physicians who are considering practicing obstetrics in rural areas.” The fourth year of training for these family physicians could help address widespread shortages of obstetricians and midwives in rural communities, Dr. Mann and her coauthors wrote. They observed that “pregnant women who live in rural areas may need to travel hundreds of miles to obtain routine prenatal care or consultation with an obstetrical specialist.”
Dr. Cullen, whose practice is in Valdez, Alaska, said he happily reciprocates the desire for collaboration. In his inaugural blog post after assuming the AAFP presidency in October, 2018, he extended warm thanks to his obstetrician and perinatologist colleagues. “The relationship between family physicians and obstetricians is vital in rural communities like mine. The training I received has saved lives and prevented catastrophe,” he wrote.
At the same time, Dr. Cullen said that he believes family physicians are the best hope for quality obstetrical care in rural communities. Dr. Cullen’s own training, he said, was structured to involve significant obstetric experience with obstetrician mentorship. “A 3-year residency left me very comfortable with fairly sophisticated obstetric management at graduation,” he added.
In his practice, Dr. Cullen said, a family physician hired a decade ago after a 3-year residency came with excellent obstetric skills, which have been further honed during the succeeding years of rural practice. During a 3-year residency, “family physicians receive training and demonstrate the skills and competencies required to deliver high-quality maternity care in any community, including those in rural settings,” he said in his statement.
“The bigger issue is really the closure of obstetric units in rural hospitals,” said Dr. Cullen. He pointed out that although obstetric services may have left a community, that rural hospital will still have miscarrying patients with hemorrhage, patients in preterm labor, and other obstetric emergencies arriving at the doorstep. These and other instances often “represent true emergencies without possibility of transfer, requiring immediate and effective response,” he wrote.
“We would encourage the authors to ensure an equal focus on improving care at all levels and in all hospitals, as well as relying on transfer as appropriate,” Dr. Cullen said in his statement.
Such mutual support can be bolstered by new technology, wrote Dr. Mann and her coauthors, because “telehealth and collaborations and consultation with clinics and regional hospitals can help increase the availability of maternity care in the United States.”
For Dr. Cullen also, the way forward is together. In his blog, he wrote: “As family physicians, we can only deliver obstetrical care to our patients with the cooperation of obstetricians and perinatologists. Conversely, obstetricians can only improve maternal and infant mortality rates, especially in rural areas, with the help of family physicians.”
SOURCE: Mann S et al. N Engl J Med 2018;379:1689-91.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Estetrol safely limited menopause symptoms in a phase 2b study
Estetrol (Donesta) relieved vasomotor menopausal symptoms without stimulating breast tenderness or raising triglyceride levels in a dose-finding study of the investigational drug presented at the annual meeting of the North American Menopause Society.
Estetrol (E4), an estrogen produced by the fetal liver, is the first native estrogen acting selectively in tissues. Since it crosses the placenta, estetrol is present in maternal urine at about 9 weeks’ gestation. In fetal plasma, it circulates at concentrations about 12 times higher than maternal estetrol levels. The hormone has a half-life of 28-32 hours, longer than the half-life of most other estrogens.
E4 “has been shown to have a remarkably safe profile and I like to describe it as the first natural oral estrogen with the safety profile of a transdermal estrogen,” said Wulf H. Utian, MD, the Arthur H. Bill Professor of Obstetrics & Gynecology at Case Western Reserve University, Cleveland. Estetrol uniquely activates nuclear estrogen receptor–alpha, while antagonizing membrane estrogen receptor-alpha. These properties give E4 selective tissue action, with low breast stimulation meaning less breast tenderness and “low carcinogenic impact.”
Additionally, triglyceride levels are minimally affected, and serum markers for venous thromboembolism generally remain unchanged with E4 exposure, he said.
In a phase 2b dose-finding study, a variety of estetrol doses were compared with placebo to treat vasomotor symptoms in postmenopausal women aged 40-65 years with at least 7 moderate to severe hot flashes daily, or at least 50 moderate to severe hot flashes in the week before randomization. The multicenter, double-blind randomized controlled trial took place in five European countries, with 200 women overall completing the study. The study design excluded women with personal histories of malignancy, thromboembolism, or coagulopathy, and women with diabetes and poor glycemic control. Women with a uterus who had current or past endometrial hyperplasia, polyps, or an abnormal cervical smear were also excluded.
Women who had an intact uterus were included if transvaginal ultrasound showed endometrial thickness of 5 mm or less. Participants were randomized 1:1:1:1 to receive four different E4 doses: 2.5 mg, 5 mg, 10 mg, or 15 mg.
At the highest dose of 15 mg, E4 significantly reduced the frequency of vasomotor symptoms compared with placebo by study week 4 and throughout the 12-week study period (P less than .05).
This high dose also resulted in a 28% reduction in vasomotor symptom severity, compared with placebo by study week 12, with a significant separation from placebo by week 12.
In terms of the number of women who experienced at least a 50% drop in severe vasomotor symptoms, the 15-mg E4 dose also bested placebo (P less than .01). Among the group taking the 15 mg dose, significantly more also saw at least a 75% drop in frequency of severe vasomotor symptom (P less than .001).
Vaginal cytology showed that by week 12 all doses of E4 significantly increased the vaginal maturation index from baseline, a finding that corresponds with less thinning of vaginal tissues (P less than .001, compared with placebo for all doses).
The safety profile of E4 was good, Dr. Utian said. Coagulation markers were unaffected, and most lipid and blood glucose markers were also unchanged. There were “small but potentially beneficial changes in HDL-C [HDL cholesterol levels] and HbA1c values [hemoglobin A1c]” in groups taking the two highest doses of E4.
Also, C-telopeptide of type 1 collagen and osteocalcin values were reduced, “suggesting reduction in bone resorption,” he said.
According to Dr. Utian, those taking the two highest doses also saw a “slight though significant increase” from baseline in sex hormone–binding globulin levels, “indicating that the E4 estrogenic effect was mild and dose dependent.”
Endometrial biopsies showed no hyperplasia. In those taking the 15-mg E4 dose, mean endometrial thickness did increase from a mean 2 mm at baseline to 6 mm at 12 weeks. However, endometrial thickness returned to baseline after progestin therapy, said Dr. Utian. There were no unexpected adverse events during the study.
Dr. Utian reported consultant relationships with Mithra, the maker of estetrol; AMAG; Pharmavite; and Endoceutics. The study was funded by Mithra.
SOURCE: Utian W. NAMS 2018, Friday concurrent session 1.
Estetrol (Donesta) relieved vasomotor menopausal symptoms without stimulating breast tenderness or raising triglyceride levels in a dose-finding study of the investigational drug presented at the annual meeting of the North American Menopause Society.
Estetrol (E4), an estrogen produced by the fetal liver, is the first native estrogen acting selectively in tissues. Since it crosses the placenta, estetrol is present in maternal urine at about 9 weeks’ gestation. In fetal plasma, it circulates at concentrations about 12 times higher than maternal estetrol levels. The hormone has a half-life of 28-32 hours, longer than the half-life of most other estrogens.
E4 “has been shown to have a remarkably safe profile and I like to describe it as the first natural oral estrogen with the safety profile of a transdermal estrogen,” said Wulf H. Utian, MD, the Arthur H. Bill Professor of Obstetrics & Gynecology at Case Western Reserve University, Cleveland. Estetrol uniquely activates nuclear estrogen receptor–alpha, while antagonizing membrane estrogen receptor-alpha. These properties give E4 selective tissue action, with low breast stimulation meaning less breast tenderness and “low carcinogenic impact.”
Additionally, triglyceride levels are minimally affected, and serum markers for venous thromboembolism generally remain unchanged with E4 exposure, he said.
In a phase 2b dose-finding study, a variety of estetrol doses were compared with placebo to treat vasomotor symptoms in postmenopausal women aged 40-65 years with at least 7 moderate to severe hot flashes daily, or at least 50 moderate to severe hot flashes in the week before randomization. The multicenter, double-blind randomized controlled trial took place in five European countries, with 200 women overall completing the study. The study design excluded women with personal histories of malignancy, thromboembolism, or coagulopathy, and women with diabetes and poor glycemic control. Women with a uterus who had current or past endometrial hyperplasia, polyps, or an abnormal cervical smear were also excluded.
Women who had an intact uterus were included if transvaginal ultrasound showed endometrial thickness of 5 mm or less. Participants were randomized 1:1:1:1 to receive four different E4 doses: 2.5 mg, 5 mg, 10 mg, or 15 mg.
At the highest dose of 15 mg, E4 significantly reduced the frequency of vasomotor symptoms compared with placebo by study week 4 and throughout the 12-week study period (P less than .05).
This high dose also resulted in a 28% reduction in vasomotor symptom severity, compared with placebo by study week 12, with a significant separation from placebo by week 12.
In terms of the number of women who experienced at least a 50% drop in severe vasomotor symptoms, the 15-mg E4 dose also bested placebo (P less than .01). Among the group taking the 15 mg dose, significantly more also saw at least a 75% drop in frequency of severe vasomotor symptom (P less than .001).
Vaginal cytology showed that by week 12 all doses of E4 significantly increased the vaginal maturation index from baseline, a finding that corresponds with less thinning of vaginal tissues (P less than .001, compared with placebo for all doses).
The safety profile of E4 was good, Dr. Utian said. Coagulation markers were unaffected, and most lipid and blood glucose markers were also unchanged. There were “small but potentially beneficial changes in HDL-C [HDL cholesterol levels] and HbA1c values [hemoglobin A1c]” in groups taking the two highest doses of E4.
Also, C-telopeptide of type 1 collagen and osteocalcin values were reduced, “suggesting reduction in bone resorption,” he said.
According to Dr. Utian, those taking the two highest doses also saw a “slight though significant increase” from baseline in sex hormone–binding globulin levels, “indicating that the E4 estrogenic effect was mild and dose dependent.”
Endometrial biopsies showed no hyperplasia. In those taking the 15-mg E4 dose, mean endometrial thickness did increase from a mean 2 mm at baseline to 6 mm at 12 weeks. However, endometrial thickness returned to baseline after progestin therapy, said Dr. Utian. There were no unexpected adverse events during the study.
Dr. Utian reported consultant relationships with Mithra, the maker of estetrol; AMAG; Pharmavite; and Endoceutics. The study was funded by Mithra.
SOURCE: Utian W. NAMS 2018, Friday concurrent session 1.
Estetrol (Donesta) relieved vasomotor menopausal symptoms without stimulating breast tenderness or raising triglyceride levels in a dose-finding study of the investigational drug presented at the annual meeting of the North American Menopause Society.
Estetrol (E4), an estrogen produced by the fetal liver, is the first native estrogen acting selectively in tissues. Since it crosses the placenta, estetrol is present in maternal urine at about 9 weeks’ gestation. In fetal plasma, it circulates at concentrations about 12 times higher than maternal estetrol levels. The hormone has a half-life of 28-32 hours, longer than the half-life of most other estrogens.
E4 “has been shown to have a remarkably safe profile and I like to describe it as the first natural oral estrogen with the safety profile of a transdermal estrogen,” said Wulf H. Utian, MD, the Arthur H. Bill Professor of Obstetrics & Gynecology at Case Western Reserve University, Cleveland. Estetrol uniquely activates nuclear estrogen receptor–alpha, while antagonizing membrane estrogen receptor-alpha. These properties give E4 selective tissue action, with low breast stimulation meaning less breast tenderness and “low carcinogenic impact.”
Additionally, triglyceride levels are minimally affected, and serum markers for venous thromboembolism generally remain unchanged with E4 exposure, he said.
In a phase 2b dose-finding study, a variety of estetrol doses were compared with placebo to treat vasomotor symptoms in postmenopausal women aged 40-65 years with at least 7 moderate to severe hot flashes daily, or at least 50 moderate to severe hot flashes in the week before randomization. The multicenter, double-blind randomized controlled trial took place in five European countries, with 200 women overall completing the study. The study design excluded women with personal histories of malignancy, thromboembolism, or coagulopathy, and women with diabetes and poor glycemic control. Women with a uterus who had current or past endometrial hyperplasia, polyps, or an abnormal cervical smear were also excluded.
Women who had an intact uterus were included if transvaginal ultrasound showed endometrial thickness of 5 mm or less. Participants were randomized 1:1:1:1 to receive four different E4 doses: 2.5 mg, 5 mg, 10 mg, or 15 mg.
At the highest dose of 15 mg, E4 significantly reduced the frequency of vasomotor symptoms compared with placebo by study week 4 and throughout the 12-week study period (P less than .05).
This high dose also resulted in a 28% reduction in vasomotor symptom severity, compared with placebo by study week 12, with a significant separation from placebo by week 12.
In terms of the number of women who experienced at least a 50% drop in severe vasomotor symptoms, the 15-mg E4 dose also bested placebo (P less than .01). Among the group taking the 15 mg dose, significantly more also saw at least a 75% drop in frequency of severe vasomotor symptom (P less than .001).
Vaginal cytology showed that by week 12 all doses of E4 significantly increased the vaginal maturation index from baseline, a finding that corresponds with less thinning of vaginal tissues (P less than .001, compared with placebo for all doses).
The safety profile of E4 was good, Dr. Utian said. Coagulation markers were unaffected, and most lipid and blood glucose markers were also unchanged. There were “small but potentially beneficial changes in HDL-C [HDL cholesterol levels] and HbA1c values [hemoglobin A1c]” in groups taking the two highest doses of E4.
Also, C-telopeptide of type 1 collagen and osteocalcin values were reduced, “suggesting reduction in bone resorption,” he said.
According to Dr. Utian, those taking the two highest doses also saw a “slight though significant increase” from baseline in sex hormone–binding globulin levels, “indicating that the E4 estrogenic effect was mild and dose dependent.”
Endometrial biopsies showed no hyperplasia. In those taking the 15-mg E4 dose, mean endometrial thickness did increase from a mean 2 mm at baseline to 6 mm at 12 weeks. However, endometrial thickness returned to baseline after progestin therapy, said Dr. Utian. There were no unexpected adverse events during the study.
Dr. Utian reported consultant relationships with Mithra, the maker of estetrol; AMAG; Pharmavite; and Endoceutics. The study was funded by Mithra.
SOURCE: Utian W. NAMS 2018, Friday concurrent session 1.
FROM NAMS 2018
Key clinical point: Estetrol relieved hot flashes without adversely affecting lipid markers.
Major finding: Estetrol 15 mg reduced vasomotor symptom severity by 28%.
Study details: Randomized, double-blind, placebo-controlled phase 2b dose-finding study of 200 women.
Disclosures: Dr. Utian reported financial relationships with several pharmaceutical companies, including Mithra, the sponsor of the study.
Source: Utian WH. NAMS 2018, Friday concurrent session 1.
Vascular emergencies on the rise, but more patients surviving
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
REPORTING FROM MIDWESTERN VASCULAR 2018
Key clinical point: Rates of endovascular repair for nontraumatic vascular emergencies rose sharply.
Major finding: Endovascular repair rates for nontraumatic vascular emergencies climbed from 24% to 36% of cases from 2005 to 2014 (P for trend, less than .0001).
Study details: A 10-year sample of hospitalizations for nontraumatic vascular emergencies from the U.S. National Inpatient Sample.
Disclosures: Dr. Vogel reported no outside sources of funding and no conflicts of interest.
FDA okays serum AMH assay to determine menopause status
The PicoAMH Elisa diagnostic test measures circulating levels of anti-Müllerian hormone (AMH), a granulosa cell product in ovaries that is present only until menopause. In research settings, AMH levels have been used to predict menopause and to confirm the occurrence of menopause; levels have been shown to track well with antral follicle count (J Clin Endocrinol Metab. 2011 Aug 1;96[8]:2532-9).
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventative care for women experiencing menopausal symptoms,” Courtney H. Lias, PhD, director of the division of chemistry and toxicology devices in the FDA’s Center for Devices and Radiological Health, said in a press release announcing the marketing permission. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventative care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
As Dr. Lias emphasized, the new test is designed to be used along with a thorough clinical assessment and other laboratory tests. Having a reliable test for circulating AMH for clinical use allows measurement of a hormone that, unlike follicle stimulating hormone and luteinizing hormone, does not fluctuate throughout the menstrual cycle for premenopausal women.
JoAnn Pinkerton, MD, executive director of the North American Menopause Society, provided clinical context about the utility of the new assay. “AMH levels appear to provide valuable information about timing of menopause. While not needed for women undergoing a natural menopause at age 51, it will be very helpful for women at risk of early ovarian failure, such as following chemotherapy for cancer or genetic or endocrine reasons,” said Dr. Pinkerton. “Women desiring pregnancy who are skipping periods can be more reassured if their AMH is normal, as studies suggest, that AMH is highly predictive of timing of menopause.”*
In permitting marketing of the PicoAMH Elisa assay, the FDA looked at data drawn from the Study of Women’s Health Across the Nation. For 690 women aged 42-62 years, “the PicoAMH Elisa test performed reasonably well at determining levels of AMH in the blood,” the FDA said in the press release. The test also was able to identify women who had already had their last menstrual period, and to determine women who were at least 5 years away from stopping menstruation, according to the longitudinal study.
The PicoAMH Elisa test will be marketed by Ansh Labs. Since the device’s review went through a de novo premarket pathway designed for novel devices of low to medium risk, there will be an additional set of criteria, called special controls, put in place to monitor the safety and effectiveness of the test.
*This article was updated on 10/26/2018.
The PicoAMH Elisa diagnostic test measures circulating levels of anti-Müllerian hormone (AMH), a granulosa cell product in ovaries that is present only until menopause. In research settings, AMH levels have been used to predict menopause and to confirm the occurrence of menopause; levels have been shown to track well with antral follicle count (J Clin Endocrinol Metab. 2011 Aug 1;96[8]:2532-9).
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventative care for women experiencing menopausal symptoms,” Courtney H. Lias, PhD, director of the division of chemistry and toxicology devices in the FDA’s Center for Devices and Radiological Health, said in a press release announcing the marketing permission. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventative care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
As Dr. Lias emphasized, the new test is designed to be used along with a thorough clinical assessment and other laboratory tests. Having a reliable test for circulating AMH for clinical use allows measurement of a hormone that, unlike follicle stimulating hormone and luteinizing hormone, does not fluctuate throughout the menstrual cycle for premenopausal women.
JoAnn Pinkerton, MD, executive director of the North American Menopause Society, provided clinical context about the utility of the new assay. “AMH levels appear to provide valuable information about timing of menopause. While not needed for women undergoing a natural menopause at age 51, it will be very helpful for women at risk of early ovarian failure, such as following chemotherapy for cancer or genetic or endocrine reasons,” said Dr. Pinkerton. “Women desiring pregnancy who are skipping periods can be more reassured if their AMH is normal, as studies suggest, that AMH is highly predictive of timing of menopause.”*
In permitting marketing of the PicoAMH Elisa assay, the FDA looked at data drawn from the Study of Women’s Health Across the Nation. For 690 women aged 42-62 years, “the PicoAMH Elisa test performed reasonably well at determining levels of AMH in the blood,” the FDA said in the press release. The test also was able to identify women who had already had their last menstrual period, and to determine women who were at least 5 years away from stopping menstruation, according to the longitudinal study.
The PicoAMH Elisa test will be marketed by Ansh Labs. Since the device’s review went through a de novo premarket pathway designed for novel devices of low to medium risk, there will be an additional set of criteria, called special controls, put in place to monitor the safety and effectiveness of the test.
*This article was updated on 10/26/2018.
The PicoAMH Elisa diagnostic test measures circulating levels of anti-Müllerian hormone (AMH), a granulosa cell product in ovaries that is present only until menopause. In research settings, AMH levels have been used to predict menopause and to confirm the occurrence of menopause; levels have been shown to track well with antral follicle count (J Clin Endocrinol Metab. 2011 Aug 1;96[8]:2532-9).
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventative care for women experiencing menopausal symptoms,” Courtney H. Lias, PhD, director of the division of chemistry and toxicology devices in the FDA’s Center for Devices and Radiological Health, said in a press release announcing the marketing permission. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventative care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
As Dr. Lias emphasized, the new test is designed to be used along with a thorough clinical assessment and other laboratory tests. Having a reliable test for circulating AMH for clinical use allows measurement of a hormone that, unlike follicle stimulating hormone and luteinizing hormone, does not fluctuate throughout the menstrual cycle for premenopausal women.
JoAnn Pinkerton, MD, executive director of the North American Menopause Society, provided clinical context about the utility of the new assay. “AMH levels appear to provide valuable information about timing of menopause. While not needed for women undergoing a natural menopause at age 51, it will be very helpful for women at risk of early ovarian failure, such as following chemotherapy for cancer or genetic or endocrine reasons,” said Dr. Pinkerton. “Women desiring pregnancy who are skipping periods can be more reassured if their AMH is normal, as studies suggest, that AMH is highly predictive of timing of menopause.”*
In permitting marketing of the PicoAMH Elisa assay, the FDA looked at data drawn from the Study of Women’s Health Across the Nation. For 690 women aged 42-62 years, “the PicoAMH Elisa test performed reasonably well at determining levels of AMH in the blood,” the FDA said in the press release. The test also was able to identify women who had already had their last menstrual period, and to determine women who were at least 5 years away from stopping menstruation, according to the longitudinal study.
The PicoAMH Elisa test will be marketed by Ansh Labs. Since the device’s review went through a de novo premarket pathway designed for novel devices of low to medium risk, there will be an additional set of criteria, called special controls, put in place to monitor the safety and effectiveness of the test.
*This article was updated on 10/26/2018.
Quadrivalent flu vaccine okayed for 6 months and up
The expanded approval now includes persons aged 6-59 months; the quadrivalent vaccine had previously been approved for ages 5 years and up. A trivalent version of the Afluria influenza vaccine also now is indicated for people aged 6 months and up, according to an Oct. 4 communication from the FDA.
A total of 172 pediatric influenza-related deaths occurred in the United States during the 2017-2018 season, representing a new high in nonpandemic influenza seasons. About half of the pediatric influenza deaths occurred in otherwise healthy children, and about 22% of children who died were fully vaccinated, according to the Centers for Disease Control and Prevention, reporting U.S. data from 2010 to 2016.
“As we enter a new flu season, we are reminded of the enormous impact that influenza can have on public health,” Seqirus’ vice president of medical affairs Gregg Sylvester, MD, said in a press release announcing the extended indication. “Having another option to fight this disease can translate to saved lives and fewer flu-related hospitalizations this season and going forward.”
According to the CDC, the 2018-2019 influenza vaccine has been updated to provide a better match – and more protection against – viruses circulating in this influenza season. Specifically, says the CDC, the influenza B Victoria lineage and the influenza A(H3N2) components were updated.
In addition to providing protection against these two strains of influenza, trivalent vaccines for the 2018-2019 season are recommended to include protection against H1N1 influenza as well. Quadrivalent vaccines protect against a second influenza B lineage.
Most people will receive a quadrivalent vaccine this year, according to the CDC.
The expanded approval now includes persons aged 6-59 months; the quadrivalent vaccine had previously been approved for ages 5 years and up. A trivalent version of the Afluria influenza vaccine also now is indicated for people aged 6 months and up, according to an Oct. 4 communication from the FDA.
A total of 172 pediatric influenza-related deaths occurred in the United States during the 2017-2018 season, representing a new high in nonpandemic influenza seasons. About half of the pediatric influenza deaths occurred in otherwise healthy children, and about 22% of children who died were fully vaccinated, according to the Centers for Disease Control and Prevention, reporting U.S. data from 2010 to 2016.
“As we enter a new flu season, we are reminded of the enormous impact that influenza can have on public health,” Seqirus’ vice president of medical affairs Gregg Sylvester, MD, said in a press release announcing the extended indication. “Having another option to fight this disease can translate to saved lives and fewer flu-related hospitalizations this season and going forward.”
According to the CDC, the 2018-2019 influenza vaccine has been updated to provide a better match – and more protection against – viruses circulating in this influenza season. Specifically, says the CDC, the influenza B Victoria lineage and the influenza A(H3N2) components were updated.
In addition to providing protection against these two strains of influenza, trivalent vaccines for the 2018-2019 season are recommended to include protection against H1N1 influenza as well. Quadrivalent vaccines protect against a second influenza B lineage.
Most people will receive a quadrivalent vaccine this year, according to the CDC.
The expanded approval now includes persons aged 6-59 months; the quadrivalent vaccine had previously been approved for ages 5 years and up. A trivalent version of the Afluria influenza vaccine also now is indicated for people aged 6 months and up, according to an Oct. 4 communication from the FDA.
A total of 172 pediatric influenza-related deaths occurred in the United States during the 2017-2018 season, representing a new high in nonpandemic influenza seasons. About half of the pediatric influenza deaths occurred in otherwise healthy children, and about 22% of children who died were fully vaccinated, according to the Centers for Disease Control and Prevention, reporting U.S. data from 2010 to 2016.
“As we enter a new flu season, we are reminded of the enormous impact that influenza can have on public health,” Seqirus’ vice president of medical affairs Gregg Sylvester, MD, said in a press release announcing the extended indication. “Having another option to fight this disease can translate to saved lives and fewer flu-related hospitalizations this season and going forward.”
According to the CDC, the 2018-2019 influenza vaccine has been updated to provide a better match – and more protection against – viruses circulating in this influenza season. Specifically, says the CDC, the influenza B Victoria lineage and the influenza A(H3N2) components were updated.
In addition to providing protection against these two strains of influenza, trivalent vaccines for the 2018-2019 season are recommended to include protection against H1N1 influenza as well. Quadrivalent vaccines protect against a second influenza B lineage.
Most people will receive a quadrivalent vaccine this year, according to the CDC.
Physiologically functional organoid offers promise for rapid, realistic in vitro drug discovery
NEW YORK – A self-assembling model brain neurovascular unit showed that it emulated in vivo behavior of the human blood-brain barrier under a variety of conditions, including hypoxia and histamine exposure.
Goodwell Nzou, a doctoral student at Wake Forest University, Winston-Salem, N.C., discussed findings published earlier this year in Scientific Reports showing that the three-dimensional brain organoid has promise for rapid in vitro testing of central nervous system drugs.
The model contains all the primary cell types in the human brain cortex, said Mr. Nzou, speaking at the International Conference for Parkinson’s Disease and Movement Disorders. These include human brain microvascular endothelial cells, pericytes, astrocytes, microglia, oligodendrocytes, and neurons. Human endothelial cells enclose the parenchymal cells in the model.
The human neurovascular unit (NVU) organoid model was developed using induced pluripotent stem cells for the microglial, oligodendrocyte, and neuron cell components. Human primary cells were used for the remaining components.
First, Mr. Nzou and his collaborators constructed a four-cell model. By placing the cells in a hanging drop culture environment and culturing for 96 hours, the investigators were able to induce assembly of the organoids. Since the cells had been pretreated with a durable labeling dye, the investigators could confirm anatomically appropriate self-assembly using confocal microscopy. Blood-brain barrier (BBB) tight junctions were confirmed by testing for the tight junction protein ZO-1 via immunofluorescent labeling, said Mr. Nzou.
From this experience, they were able to conduct a staged assembly using all six cell types, yielding a neurovascular unit that was durable, maintaining “very high cell viability for up to 21 days in vitro,” Mr. Nzou said, with both core and outer cells showing good viability.
Mr. Nzou and his colleagues at the Wake Forest Institute for Regenerative Medicine tested the model’s function against several emulated physical states: In one, they flooded the field with histamine, finding that the junctions lost integrity, accurately mimicking the “leaky” tissue state that occurs in vivo with histamine release.
The histamine-treated organoids allowed IgG permeability that was largely absent in the control organoids. “In the control system we did not see much of the IgG going in. We did see a lot more going in after we treated the organoids with histamine,” said Mr. Nzou.
However, IgG is a large molecule, and much CNS drug discovery right now is focused on small molecules, so Mr. Nzou and his colleagues also wanted to see whether the NVU’s BBB integrity would hold up against a small molecule.
Using exposure to a molecule called MPTP, Mr. Nzou and his collaborators compared how much MPTP entered two different types of organoids: One was the six-cell organoid, and the other was made up of neurons only.
The neuron-only organoid would not be expected to prevent influx of MPTP since it lacked the BBB-like composition of the full organoid, explained Mr. Nzou. Once past the BBB, MPTP is converted to an active substance that interferes with adenosine triphosphate (ATP) production . The investigators did see a significant drop in APT production with MPTP exposure in the neuron-only, but not the full, organoid, said Mr. Nzou.
In another trial, they exposed the model to an atmosphere with lowered oxygen tension and saw resultant changes consistent with ischemia. The model “showed normal physiologic responses under hypoxic conditions,” they said. These included increased proinflammatory cytokine production and decreased integrity of the BBB.
The in vitro hypoxia was profound – oxygen exposure was dropped to 1% from normal atmospheric composition of 21%. Still, the organoids maintained good viability despite the hypoxia-induced changes in physiology, making them appropriate candidates for testing such hypoxic conditions as ischemic stroke and conditions that elevate intracranial pressure, Mr. Nzou said.
In addition to drug discovery uses, the model could allow for rapid and safe toxicology research and for accelerated investigation of neurologic diseases, including Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis. The research group, said Mr. Nzou, has largely achieved its model of “forming a better blood-brain barrier–equivalent model through the concerted interactions of all cell types with the endothelial layer,” he said.
NEW YORK – A self-assembling model brain neurovascular unit showed that it emulated in vivo behavior of the human blood-brain barrier under a variety of conditions, including hypoxia and histamine exposure.
Goodwell Nzou, a doctoral student at Wake Forest University, Winston-Salem, N.C., discussed findings published earlier this year in Scientific Reports showing that the three-dimensional brain organoid has promise for rapid in vitro testing of central nervous system drugs.
The model contains all the primary cell types in the human brain cortex, said Mr. Nzou, speaking at the International Conference for Parkinson’s Disease and Movement Disorders. These include human brain microvascular endothelial cells, pericytes, astrocytes, microglia, oligodendrocytes, and neurons. Human endothelial cells enclose the parenchymal cells in the model.
The human neurovascular unit (NVU) organoid model was developed using induced pluripotent stem cells for the microglial, oligodendrocyte, and neuron cell components. Human primary cells were used for the remaining components.
First, Mr. Nzou and his collaborators constructed a four-cell model. By placing the cells in a hanging drop culture environment and culturing for 96 hours, the investigators were able to induce assembly of the organoids. Since the cells had been pretreated with a durable labeling dye, the investigators could confirm anatomically appropriate self-assembly using confocal microscopy. Blood-brain barrier (BBB) tight junctions were confirmed by testing for the tight junction protein ZO-1 via immunofluorescent labeling, said Mr. Nzou.
From this experience, they were able to conduct a staged assembly using all six cell types, yielding a neurovascular unit that was durable, maintaining “very high cell viability for up to 21 days in vitro,” Mr. Nzou said, with both core and outer cells showing good viability.
Mr. Nzou and his colleagues at the Wake Forest Institute for Regenerative Medicine tested the model’s function against several emulated physical states: In one, they flooded the field with histamine, finding that the junctions lost integrity, accurately mimicking the “leaky” tissue state that occurs in vivo with histamine release.
The histamine-treated organoids allowed IgG permeability that was largely absent in the control organoids. “In the control system we did not see much of the IgG going in. We did see a lot more going in after we treated the organoids with histamine,” said Mr. Nzou.
However, IgG is a large molecule, and much CNS drug discovery right now is focused on small molecules, so Mr. Nzou and his colleagues also wanted to see whether the NVU’s BBB integrity would hold up against a small molecule.
Using exposure to a molecule called MPTP, Mr. Nzou and his collaborators compared how much MPTP entered two different types of organoids: One was the six-cell organoid, and the other was made up of neurons only.
The neuron-only organoid would not be expected to prevent influx of MPTP since it lacked the BBB-like composition of the full organoid, explained Mr. Nzou. Once past the BBB, MPTP is converted to an active substance that interferes with adenosine triphosphate (ATP) production . The investigators did see a significant drop in APT production with MPTP exposure in the neuron-only, but not the full, organoid, said Mr. Nzou.
In another trial, they exposed the model to an atmosphere with lowered oxygen tension and saw resultant changes consistent with ischemia. The model “showed normal physiologic responses under hypoxic conditions,” they said. These included increased proinflammatory cytokine production and decreased integrity of the BBB.
The in vitro hypoxia was profound – oxygen exposure was dropped to 1% from normal atmospheric composition of 21%. Still, the organoids maintained good viability despite the hypoxia-induced changes in physiology, making them appropriate candidates for testing such hypoxic conditions as ischemic stroke and conditions that elevate intracranial pressure, Mr. Nzou said.
In addition to drug discovery uses, the model could allow for rapid and safe toxicology research and for accelerated investigation of neurologic diseases, including Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis. The research group, said Mr. Nzou, has largely achieved its model of “forming a better blood-brain barrier–equivalent model through the concerted interactions of all cell types with the endothelial layer,” he said.
NEW YORK – A self-assembling model brain neurovascular unit showed that it emulated in vivo behavior of the human blood-brain barrier under a variety of conditions, including hypoxia and histamine exposure.
Goodwell Nzou, a doctoral student at Wake Forest University, Winston-Salem, N.C., discussed findings published earlier this year in Scientific Reports showing that the three-dimensional brain organoid has promise for rapid in vitro testing of central nervous system drugs.
The model contains all the primary cell types in the human brain cortex, said Mr. Nzou, speaking at the International Conference for Parkinson’s Disease and Movement Disorders. These include human brain microvascular endothelial cells, pericytes, astrocytes, microglia, oligodendrocytes, and neurons. Human endothelial cells enclose the parenchymal cells in the model.
The human neurovascular unit (NVU) organoid model was developed using induced pluripotent stem cells for the microglial, oligodendrocyte, and neuron cell components. Human primary cells were used for the remaining components.
First, Mr. Nzou and his collaborators constructed a four-cell model. By placing the cells in a hanging drop culture environment and culturing for 96 hours, the investigators were able to induce assembly of the organoids. Since the cells had been pretreated with a durable labeling dye, the investigators could confirm anatomically appropriate self-assembly using confocal microscopy. Blood-brain barrier (BBB) tight junctions were confirmed by testing for the tight junction protein ZO-1 via immunofluorescent labeling, said Mr. Nzou.
From this experience, they were able to conduct a staged assembly using all six cell types, yielding a neurovascular unit that was durable, maintaining “very high cell viability for up to 21 days in vitro,” Mr. Nzou said, with both core and outer cells showing good viability.
Mr. Nzou and his colleagues at the Wake Forest Institute for Regenerative Medicine tested the model’s function against several emulated physical states: In one, they flooded the field with histamine, finding that the junctions lost integrity, accurately mimicking the “leaky” tissue state that occurs in vivo with histamine release.
The histamine-treated organoids allowed IgG permeability that was largely absent in the control organoids. “In the control system we did not see much of the IgG going in. We did see a lot more going in after we treated the organoids with histamine,” said Mr. Nzou.
However, IgG is a large molecule, and much CNS drug discovery right now is focused on small molecules, so Mr. Nzou and his colleagues also wanted to see whether the NVU’s BBB integrity would hold up against a small molecule.
Using exposure to a molecule called MPTP, Mr. Nzou and his collaborators compared how much MPTP entered two different types of organoids: One was the six-cell organoid, and the other was made up of neurons only.
The neuron-only organoid would not be expected to prevent influx of MPTP since it lacked the BBB-like composition of the full organoid, explained Mr. Nzou. Once past the BBB, MPTP is converted to an active substance that interferes with adenosine triphosphate (ATP) production . The investigators did see a significant drop in APT production with MPTP exposure in the neuron-only, but not the full, organoid, said Mr. Nzou.
In another trial, they exposed the model to an atmosphere with lowered oxygen tension and saw resultant changes consistent with ischemia. The model “showed normal physiologic responses under hypoxic conditions,” they said. These included increased proinflammatory cytokine production and decreased integrity of the BBB.
The in vitro hypoxia was profound – oxygen exposure was dropped to 1% from normal atmospheric composition of 21%. Still, the organoids maintained good viability despite the hypoxia-induced changes in physiology, making them appropriate candidates for testing such hypoxic conditions as ischemic stroke and conditions that elevate intracranial pressure, Mr. Nzou said.
In addition to drug discovery uses, the model could allow for rapid and safe toxicology research and for accelerated investigation of neurologic diseases, including Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis. The research group, said Mr. Nzou, has largely achieved its model of “forming a better blood-brain barrier–equivalent model through the concerted interactions of all cell types with the endothelial layer,” he said.
REPORTING FROM ICPDMD 2018