User login
New, Tried, and True: Moisturizer Advice for Your Patients
Patients often ask their dermatologists for recommendations on over-the-counter moisturizers, said Dr. Joshua Zeichner.
The trouble is that "many dermatologists don’t really know what makes a good moisturizer, and are unfamiliar with the latest advances in moisturizing technology," he said at SDEF Las Vegas Dermatology Seminar.
While the purpose of all moisturizers is simple enough – to boost and maintain the water content of the skin – their formulations and the manner of their activity vary widely. Some work by increasing the expression of aquaporins, the water channels in the skin cell membrane; others by replacing skin lipids, temporarily repairing the skin’s barrier function and improving appearance.
Any high-quality moisturizer will contain a mixture of humectant, emollient, and occlusive ingredients, said Dr. Zeichner, director of cosmetic and clinical research in dermatology at Mount Sinai Medical Center in New York.
Among the novel humectants being incorporated into moisturizers today are natural moisturizing factors (NMF), an umbrella term for the naturally occurring humectants glycerol, urea, and lactic acid, along with the amino acids urocanic acid (UCA) and pyrrolidone carboxylic acid (PCA), which are produced by the breakdown of filaggrin.
Many moisturizers contain ceramides, the major lipids that make up the "mortar" between skin-cell "bricks" in the stratum corneum; moisturizers also may contain ceramide precursors.
"Applying a moisturizer with [the ceramide precursor] pseudoceramide 5 has been shown to lead to an increase in ceramide levels in the stratum corneum," Dr. Zeichner said. "However, it’s unclear whether the ingredient is directly incorporated into those ceramides. Regardless, you can see the clinical improvement."
Generally with all moisturizers, he said, "we need more studies to evaluate the long-term effects to the skin beyond just the immediate effect."
Glyceryl glucoside, a modified glycerin molecule that enhances the activity of aquaporin channels, shows promise as a humectant, Dr. Zeichner said.
He described glyceryl glucoside as a "super humectant" whose effect on skin may last longer than that of other humectants.
Dr. Zeichner also tipped his hat to an old school moisturizer ingredient – colloidal oatmeal.
"There’s a lot of talk about all of these exciting new technologies but you don’t want to forget about tried and true colloidal oatmeal," he said. Oatmeal, which has occlusive, humectant, and emollient properties, "serves as the backbone for many skin brands and works very well. Advances in cosmetic chemistry have made oatmeal formulations much more elegant now – they’re not your grandmother’s oatmeal anymore."
Dr. Zeichner disclosed financial relationships with Allergan, Bayer, Beiersdorf, Galderma, Johnson and Johnson, L’Oreal, Medicis, Onset, Pharmaderm, Procter and Gamble, and Valeant.
SDEF and this news organization are owned by Frontline Medical Communications.
Patients often ask their dermatologists for recommendations on over-the-counter moisturizers, said Dr. Joshua Zeichner.
The trouble is that "many dermatologists don’t really know what makes a good moisturizer, and are unfamiliar with the latest advances in moisturizing technology," he said at SDEF Las Vegas Dermatology Seminar.
While the purpose of all moisturizers is simple enough – to boost and maintain the water content of the skin – their formulations and the manner of their activity vary widely. Some work by increasing the expression of aquaporins, the water channels in the skin cell membrane; others by replacing skin lipids, temporarily repairing the skin’s barrier function and improving appearance.
Any high-quality moisturizer will contain a mixture of humectant, emollient, and occlusive ingredients, said Dr. Zeichner, director of cosmetic and clinical research in dermatology at Mount Sinai Medical Center in New York.
Among the novel humectants being incorporated into moisturizers today are natural moisturizing factors (NMF), an umbrella term for the naturally occurring humectants glycerol, urea, and lactic acid, along with the amino acids urocanic acid (UCA) and pyrrolidone carboxylic acid (PCA), which are produced by the breakdown of filaggrin.
Many moisturizers contain ceramides, the major lipids that make up the "mortar" between skin-cell "bricks" in the stratum corneum; moisturizers also may contain ceramide precursors.
"Applying a moisturizer with [the ceramide precursor] pseudoceramide 5 has been shown to lead to an increase in ceramide levels in the stratum corneum," Dr. Zeichner said. "However, it’s unclear whether the ingredient is directly incorporated into those ceramides. Regardless, you can see the clinical improvement."
Generally with all moisturizers, he said, "we need more studies to evaluate the long-term effects to the skin beyond just the immediate effect."
Glyceryl glucoside, a modified glycerin molecule that enhances the activity of aquaporin channels, shows promise as a humectant, Dr. Zeichner said.
He described glyceryl glucoside as a "super humectant" whose effect on skin may last longer than that of other humectants.
Dr. Zeichner also tipped his hat to an old school moisturizer ingredient – colloidal oatmeal.
"There’s a lot of talk about all of these exciting new technologies but you don’t want to forget about tried and true colloidal oatmeal," he said. Oatmeal, which has occlusive, humectant, and emollient properties, "serves as the backbone for many skin brands and works very well. Advances in cosmetic chemistry have made oatmeal formulations much more elegant now – they’re not your grandmother’s oatmeal anymore."
Dr. Zeichner disclosed financial relationships with Allergan, Bayer, Beiersdorf, Galderma, Johnson and Johnson, L’Oreal, Medicis, Onset, Pharmaderm, Procter and Gamble, and Valeant.
SDEF and this news organization are owned by Frontline Medical Communications.
Patients often ask their dermatologists for recommendations on over-the-counter moisturizers, said Dr. Joshua Zeichner.
The trouble is that "many dermatologists don’t really know what makes a good moisturizer, and are unfamiliar with the latest advances in moisturizing technology," he said at SDEF Las Vegas Dermatology Seminar.
While the purpose of all moisturizers is simple enough – to boost and maintain the water content of the skin – their formulations and the manner of their activity vary widely. Some work by increasing the expression of aquaporins, the water channels in the skin cell membrane; others by replacing skin lipids, temporarily repairing the skin’s barrier function and improving appearance.
Any high-quality moisturizer will contain a mixture of humectant, emollient, and occlusive ingredients, said Dr. Zeichner, director of cosmetic and clinical research in dermatology at Mount Sinai Medical Center in New York.
Among the novel humectants being incorporated into moisturizers today are natural moisturizing factors (NMF), an umbrella term for the naturally occurring humectants glycerol, urea, and lactic acid, along with the amino acids urocanic acid (UCA) and pyrrolidone carboxylic acid (PCA), which are produced by the breakdown of filaggrin.
Many moisturizers contain ceramides, the major lipids that make up the "mortar" between skin-cell "bricks" in the stratum corneum; moisturizers also may contain ceramide precursors.
"Applying a moisturizer with [the ceramide precursor] pseudoceramide 5 has been shown to lead to an increase in ceramide levels in the stratum corneum," Dr. Zeichner said. "However, it’s unclear whether the ingredient is directly incorporated into those ceramides. Regardless, you can see the clinical improvement."
Generally with all moisturizers, he said, "we need more studies to evaluate the long-term effects to the skin beyond just the immediate effect."
Glyceryl glucoside, a modified glycerin molecule that enhances the activity of aquaporin channels, shows promise as a humectant, Dr. Zeichner said.
He described glyceryl glucoside as a "super humectant" whose effect on skin may last longer than that of other humectants.
Dr. Zeichner also tipped his hat to an old school moisturizer ingredient – colloidal oatmeal.
"There’s a lot of talk about all of these exciting new technologies but you don’t want to forget about tried and true colloidal oatmeal," he said. Oatmeal, which has occlusive, humectant, and emollient properties, "serves as the backbone for many skin brands and works very well. Advances in cosmetic chemistry have made oatmeal formulations much more elegant now – they’re not your grandmother’s oatmeal anymore."
Dr. Zeichner disclosed financial relationships with Allergan, Bayer, Beiersdorf, Galderma, Johnson and Johnson, L’Oreal, Medicis, Onset, Pharmaderm, Procter and Gamble, and Valeant.
SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
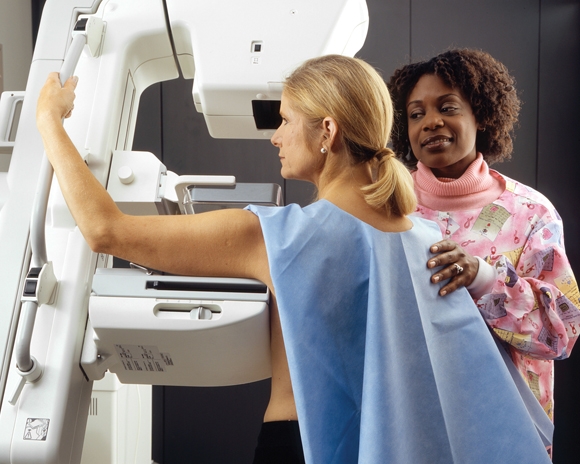
Screening Cuts Breast Cancer Deaths by 20%
Women offered breast cancer screening have a 20% lower relative risk of dying from breast cancer than women not invited to participate in screening, a epidemiologic study in the Lancet from the United Kingdom has found.
The study also found that women invited to screening are also more likely to be overdiagnosed, meaning that they are treated surgically for tumors that, if not for screening, would have caused no illness and remained undetected. The researchers cautioned, however, that their findings on overdiagnosis were based on lower-quality evidence than that used to determine relative risk reduction.
For their research, published online Oct. 29, epidemiologist Dr. Michael Marmot of University College London and his colleagues conducted a meta-analysis of data from 11 randomized controlled trials (Lancet 2012 [http://dx.doi.org/10.1016/S0140-6736(12)61611-0]).
Altogether, the included randomized trials enrolled about 674,000 women in Canada, Sweden, the United States, and the United Kingdom between 1963 and 1991. Women in the studies were invited for mammographic screening at intervals ranging from 12 to 33 months; their ages ranged from 38 to 75 years.
The relative risk of breast cancer mortality for women invited to screening, compared with controls, was 0.80 (95% confidence interval, 0.73-0.89), Dr. Marmot and his colleagues found. This corresponds to one breast cancer death prevented for every 235 women invited to screening, they said, and to one death averted for every 180 women screened.
Dr. Marmot and his colleagues acknowledged that the trials used in their meta-analysis were heterogeneous in design and populations enrolled, and reflected both older screening techniques and breast-cancer treatments; however, they noted, the mortality benefit arrived at was largely in keeping with that seen in other studies, including recent observational studies.
Dr. Marmot’s research group was convened as an independent panel to review evidence for the benefits and harms of breast screening in the United Kingdom, where, as in the United States, Canada and other countries, there have been fierce debates about the benefits and harms of government breast-cancer screening recommendations. None of the panel’s members – who included epidemiologists, clinicians, and statisticians – had previously published on breast cancer screening.
Women in the United Kingdom aged 50-70 years are invited to have screenings once every 3 years. Therefore, the investigators estimated, for every 10,000 U.K. women invited to screening between the ages of 50 and 70 years, some 681 cancers will be found; 129 of them will represent overdiagnosis and 43 deaths from breast cancer will be prevented.
Thus, among women invited to screening, about 11% of the cancers diagnosed during their lifetimes are considered overdiagnosis, the investigators estimated, and about 19% of the cancers diagnosed during the period while women are being screened are overdiagnosis.
Dr. Marmot and his colleagues emphasized, however, that these overdiagnosis figures represent "best estimates from inadequate data." Only 3 of the 11 trials included in the study, along with some observational studies, provided information relevant to overdiagnosis.
In an unsigned editorial accompanying the article, the editors of the Lancet called Dr. Marmot and his colleagues’ report, "the latest and best available systematic review" to date on breast cancer screening (2012 [http://dx.doi.org/10.1016/ S0140-6736(12)61775-9]). The report, they said, "shows that the U.K. breast-screening program extends lives and that, overall, the benefits outweigh the harms. Dissemination of these findings is now imperative in the media, the NHS screening program, and between doctors and their patients."
Dr. Marmot and his colleagues’ study was jointly commissioned by the National Cancer Director of England and Cancer Research UK, and funded by the U.K. Department of Health. None of the panel members declared conflicts of interest.
Women offered breast cancer screening have a 20% lower relative risk of dying from breast cancer than women not invited to participate in screening, a epidemiologic study in the Lancet from the United Kingdom has found.
The study also found that women invited to screening are also more likely to be overdiagnosed, meaning that they are treated surgically for tumors that, if not for screening, would have caused no illness and remained undetected. The researchers cautioned, however, that their findings on overdiagnosis were based on lower-quality evidence than that used to determine relative risk reduction.
For their research, published online Oct. 29, epidemiologist Dr. Michael Marmot of University College London and his colleagues conducted a meta-analysis of data from 11 randomized controlled trials (Lancet 2012 [http://dx.doi.org/10.1016/S0140-6736(12)61611-0]).
Altogether, the included randomized trials enrolled about 674,000 women in Canada, Sweden, the United States, and the United Kingdom between 1963 and 1991. Women in the studies were invited for mammographic screening at intervals ranging from 12 to 33 months; their ages ranged from 38 to 75 years.
The relative risk of breast cancer mortality for women invited to screening, compared with controls, was 0.80 (95% confidence interval, 0.73-0.89), Dr. Marmot and his colleagues found. This corresponds to one breast cancer death prevented for every 235 women invited to screening, they said, and to one death averted for every 180 women screened.
Dr. Marmot and his colleagues acknowledged that the trials used in their meta-analysis were heterogeneous in design and populations enrolled, and reflected both older screening techniques and breast-cancer treatments; however, they noted, the mortality benefit arrived at was largely in keeping with that seen in other studies, including recent observational studies.
Dr. Marmot’s research group was convened as an independent panel to review evidence for the benefits and harms of breast screening in the United Kingdom, where, as in the United States, Canada and other countries, there have been fierce debates about the benefits and harms of government breast-cancer screening recommendations. None of the panel’s members – who included epidemiologists, clinicians, and statisticians – had previously published on breast cancer screening.
Women in the United Kingdom aged 50-70 years are invited to have screenings once every 3 years. Therefore, the investigators estimated, for every 10,000 U.K. women invited to screening between the ages of 50 and 70 years, some 681 cancers will be found; 129 of them will represent overdiagnosis and 43 deaths from breast cancer will be prevented.
Thus, among women invited to screening, about 11% of the cancers diagnosed during their lifetimes are considered overdiagnosis, the investigators estimated, and about 19% of the cancers diagnosed during the period while women are being screened are overdiagnosis.
Dr. Marmot and his colleagues emphasized, however, that these overdiagnosis figures represent "best estimates from inadequate data." Only 3 of the 11 trials included in the study, along with some observational studies, provided information relevant to overdiagnosis.
In an unsigned editorial accompanying the article, the editors of the Lancet called Dr. Marmot and his colleagues’ report, "the latest and best available systematic review" to date on breast cancer screening (2012 [http://dx.doi.org/10.1016/ S0140-6736(12)61775-9]). The report, they said, "shows that the U.K. breast-screening program extends lives and that, overall, the benefits outweigh the harms. Dissemination of these findings is now imperative in the media, the NHS screening program, and between doctors and their patients."
Dr. Marmot and his colleagues’ study was jointly commissioned by the National Cancer Director of England and Cancer Research UK, and funded by the U.K. Department of Health. None of the panel members declared conflicts of interest.
Women offered breast cancer screening have a 20% lower relative risk of dying from breast cancer than women not invited to participate in screening, a epidemiologic study in the Lancet from the United Kingdom has found.
The study also found that women invited to screening are also more likely to be overdiagnosed, meaning that they are treated surgically for tumors that, if not for screening, would have caused no illness and remained undetected. The researchers cautioned, however, that their findings on overdiagnosis were based on lower-quality evidence than that used to determine relative risk reduction.
For their research, published online Oct. 29, epidemiologist Dr. Michael Marmot of University College London and his colleagues conducted a meta-analysis of data from 11 randomized controlled trials (Lancet 2012 [http://dx.doi.org/10.1016/S0140-6736(12)61611-0]).
Altogether, the included randomized trials enrolled about 674,000 women in Canada, Sweden, the United States, and the United Kingdom between 1963 and 1991. Women in the studies were invited for mammographic screening at intervals ranging from 12 to 33 months; their ages ranged from 38 to 75 years.
The relative risk of breast cancer mortality for women invited to screening, compared with controls, was 0.80 (95% confidence interval, 0.73-0.89), Dr. Marmot and his colleagues found. This corresponds to one breast cancer death prevented for every 235 women invited to screening, they said, and to one death averted for every 180 women screened.
Dr. Marmot and his colleagues acknowledged that the trials used in their meta-analysis were heterogeneous in design and populations enrolled, and reflected both older screening techniques and breast-cancer treatments; however, they noted, the mortality benefit arrived at was largely in keeping with that seen in other studies, including recent observational studies.
Dr. Marmot’s research group was convened as an independent panel to review evidence for the benefits and harms of breast screening in the United Kingdom, where, as in the United States, Canada and other countries, there have been fierce debates about the benefits and harms of government breast-cancer screening recommendations. None of the panel’s members – who included epidemiologists, clinicians, and statisticians – had previously published on breast cancer screening.
Women in the United Kingdom aged 50-70 years are invited to have screenings once every 3 years. Therefore, the investigators estimated, for every 10,000 U.K. women invited to screening between the ages of 50 and 70 years, some 681 cancers will be found; 129 of them will represent overdiagnosis and 43 deaths from breast cancer will be prevented.
Thus, among women invited to screening, about 11% of the cancers diagnosed during their lifetimes are considered overdiagnosis, the investigators estimated, and about 19% of the cancers diagnosed during the period while women are being screened are overdiagnosis.
Dr. Marmot and his colleagues emphasized, however, that these overdiagnosis figures represent "best estimates from inadequate data." Only 3 of the 11 trials included in the study, along with some observational studies, provided information relevant to overdiagnosis.
In an unsigned editorial accompanying the article, the editors of the Lancet called Dr. Marmot and his colleagues’ report, "the latest and best available systematic review" to date on breast cancer screening (2012 [http://dx.doi.org/10.1016/ S0140-6736(12)61775-9]). The report, they said, "shows that the U.K. breast-screening program extends lives and that, overall, the benefits outweigh the harms. Dissemination of these findings is now imperative in the media, the NHS screening program, and between doctors and their patients."
Dr. Marmot and his colleagues’ study was jointly commissioned by the National Cancer Director of England and Cancer Research UK, and funded by the U.K. Department of Health. None of the panel members declared conflicts of interest.
FROM THE LANCET
Major Finding: For every 10,000 U.K. women invited to screening between the ages of 50 and 70 years, some 681 cancers will be found; 129 of them will represent overdiagnosis and 43 deaths from breast cancer will be prevented.
Data Source: This was a meta-analysis of data from 11 randomized, controlled trials that enrolled about 674,000 women in Canada, Sweden, the United States, and the United Kingdom between 1963 and 1991.
Disclosures: Dr. Marmot and his colleagues’ study was jointly commissioned by the National Cancer Director of England and Cancer Research UK, and funded by the U.K. Department of Health. None of the panel members declared conflicts of interest.
HCV Rapid Tests Accurate in First-Line Screening
Rapid diagnostic and point-of-care tests for hepatitis C virus are accurate enough to be used in first-line HCV screening worldwide, according to results from the first systematic review of evidence on such tests.
Blood-based point-of-care tests (POCTs) had better sensitivity and specificity overall than did blood-based rapid diagnostic tests (RDTs), Sushmita Shivkumar of McGill University, Montreal, Quebec, and her colleagues reported.
POCTs do not require sample processing or refrigeration, and have a shelf life of 6 months or more. Rapid diagnostic tests, or RDTs, require refrigeration and sample processing. Manufactured by a variety of firms under various marketing names, all the tests can detect HCV infection in under 30 minutes, with many taking less than 5 minutes, according to the investigators, who conducted a meta-analysis of 18 studies evaluating the accuracy of one or more RDT or POCT, compared with standard assays (Ann. Intern. Med. 2012;157:558-66).
Ms. Shivkumar and her colleagues found blood-based POCTs (using serum, plasma, or whole blood) to be the most accurate of the tests evaluated in the included studies, showing 98.9% sensitivity with a 95% confidence interval [CI] of 94.5%-99.8% for whole blood and 96.8%-99.6% for serum or plasma). RDTs of serum or plasma had 98.4% sensitivity (95% CI 88.9%-99.8%). Specificity was highest in POCTs of serum or plasma at 99.7% (95% CI, 99.3%-99.9%), followed by POCTs of whole blood at 99.5% (95% CI, 97.5%-99.9%), RDTs of serum or plasma at 98.6% (95% CI, 94.9%-99.6%). POCTs for oral fluids had a sensitivity of 97.1% (95% CI, 94.7%-98.4%) and specificity of 98.2% (95% CI, 92.2%-99.6%).
POCTs and RDTs cannot distinguish between acute and chronic HCV infections, which is one of the reasons that public health organizations continue to favor conventional assays. The Food and Drug Administration currently approves the use of a single POCT for HCV, and for use only in nontraditional settings; in the United Kingdom and Canada such tests have not yet been approved.
However, by being fast, accurate, and cheaper than conventional tests, the rapid diagnostic and point-of-care tests offer "great potential for expanded first-line screening for hepatitis C infection and demonstrate the utility of blood-based singleton POCTs and of multiplex POCTs designed to provide integrated HIV and HCV screening of at-risk populations," Ms. Shivkumar and her associates wrote in their analysis. "Their rapid turnaround time limits loss to follow-up and facilitates early linkages."
The authors also concluded that their findings showed that the rapid and point-of-care tests could play a "substantial role" in expanded global screening initiatives for HCV.
The blood-based POCTs, besides being the most accurate, have the advantage of not requiring refrigeration, the investigators wrote. This feature is key in developing countries in Africa and Asia, where prevalence of HCV infection is highest.
Ms. Shivkumar and her colleagues noted several weaknesses in their meta-analysis, mostly related to the quality of studies included. Only three of the studies they evaluated were blinded, while four received industry funding; the conventional assays used as reference varied in the studies, and HCV genotype information was not collected in most.
The study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Chronic hepatitis C virus (HCV) infection is a common worldwide problem. It is estimated that 170 million people are afflicted. Unfortunately, the majority of infected people remain undiagnosed. In many parts of the world, patients do not have access to medical personnel or diagnostic testing so that the diagnosis of HCV can be made. For patients that do have access, standard testing for HCV is cumbersome and expensive, adding to the diagnostic burden and diminishing the opportunity for diagnosis. Even in the United States, it is estimated that the majority of people infected with HCV have not been diagnosed. These factors have provided the impetus to develop less cumbersome and inexpensive tests for HCV, with the hope that they could be implemented throughout the world to increase the number of patients diagnosed with HCV.
Although there have been numerous studies examining individual diagnostic tests for HCV, there has not been an exhaustive review of both rapid and point-of-care screening tests for HCV. Ms. Shivkumar and her associates performed a literature review and meta-analysis of 18 studies worldwide that use the tests to screen for HCV in adults. They assessed the studies for diagnostic accuracy variables including sensitivity, specificity, likelihood rates (LRs), and diagnostic odds ratios (DORs) for available rapid and point-of-care diagnostic tests.
The assessment indicates that point-of-care tests of blood (serum, plasma, or whole blood) have the highest accuracy, followed by rapid diagnostic tests of serum or plasma and then by point-of-care diagnostic tests of oral fluids. However, all tests showed excellent sensitivity, specificity, likelihood rates, and diagnostic odds ratios.
Strategies to improve the identification of patients infected with HCV are changing. In fact, the Centers for Disease Control and Prevention recently updated screening guidelines, recommending that screening change from ineffective risk factor–based screening to age-based screening. Testing strategies are also a source of potential improvement. This comprehensive global review suggests that rapid diagnostic and point-of-care tests are useful as initial screening tests for HCV. Although potential biases inherent in retrospective meta-analysis reviews are discussed, the quality of the analysis is excellent and the conclusions are valid.
These diagnostic test results are available rapidly in the field, and thus are available at the point of care. The tests are also relatively inexpensive and are easy to perform. With more effective therapies for HCV in development, broadening the ability to diagnose HCV worldwide is increasingly important. Integration of these tests into the diagnostic algorithm for HCV offers a more effective screening strategy with subsequent diagnosis of more people infected with HCV worldwide.
Steven L. Flamm, M.D., is a professor of gastroenterology, hepatology, and surgery at Northwestern University, Chicago. He has no disclosures.
Chronic hepatitis C virus (HCV) infection is a common worldwide problem. It is estimated that 170 million people are afflicted. Unfortunately, the majority of infected people remain undiagnosed. In many parts of the world, patients do not have access to medical personnel or diagnostic testing so that the diagnosis of HCV can be made. For patients that do have access, standard testing for HCV is cumbersome and expensive, adding to the diagnostic burden and diminishing the opportunity for diagnosis. Even in the United States, it is estimated that the majority of people infected with HCV have not been diagnosed. These factors have provided the impetus to develop less cumbersome and inexpensive tests for HCV, with the hope that they could be implemented throughout the world to increase the number of patients diagnosed with HCV.
Although there have been numerous studies examining individual diagnostic tests for HCV, there has not been an exhaustive review of both rapid and point-of-care screening tests for HCV. Ms. Shivkumar and her associates performed a literature review and meta-analysis of 18 studies worldwide that use the tests to screen for HCV in adults. They assessed the studies for diagnostic accuracy variables including sensitivity, specificity, likelihood rates (LRs), and diagnostic odds ratios (DORs) for available rapid and point-of-care diagnostic tests.
The assessment indicates that point-of-care tests of blood (serum, plasma, or whole blood) have the highest accuracy, followed by rapid diagnostic tests of serum or plasma and then by point-of-care diagnostic tests of oral fluids. However, all tests showed excellent sensitivity, specificity, likelihood rates, and diagnostic odds ratios.
Strategies to improve the identification of patients infected with HCV are changing. In fact, the Centers for Disease Control and Prevention recently updated screening guidelines, recommending that screening change from ineffective risk factor–based screening to age-based screening. Testing strategies are also a source of potential improvement. This comprehensive global review suggests that rapid diagnostic and point-of-care tests are useful as initial screening tests for HCV. Although potential biases inherent in retrospective meta-analysis reviews are discussed, the quality of the analysis is excellent and the conclusions are valid.
These diagnostic test results are available rapidly in the field, and thus are available at the point of care. The tests are also relatively inexpensive and are easy to perform. With more effective therapies for HCV in development, broadening the ability to diagnose HCV worldwide is increasingly important. Integration of these tests into the diagnostic algorithm for HCV offers a more effective screening strategy with subsequent diagnosis of more people infected with HCV worldwide.
Steven L. Flamm, M.D., is a professor of gastroenterology, hepatology, and surgery at Northwestern University, Chicago. He has no disclosures.
Chronic hepatitis C virus (HCV) infection is a common worldwide problem. It is estimated that 170 million people are afflicted. Unfortunately, the majority of infected people remain undiagnosed. In many parts of the world, patients do not have access to medical personnel or diagnostic testing so that the diagnosis of HCV can be made. For patients that do have access, standard testing for HCV is cumbersome and expensive, adding to the diagnostic burden and diminishing the opportunity for diagnosis. Even in the United States, it is estimated that the majority of people infected with HCV have not been diagnosed. These factors have provided the impetus to develop less cumbersome and inexpensive tests for HCV, with the hope that they could be implemented throughout the world to increase the number of patients diagnosed with HCV.
Although there have been numerous studies examining individual diagnostic tests for HCV, there has not been an exhaustive review of both rapid and point-of-care screening tests for HCV. Ms. Shivkumar and her associates performed a literature review and meta-analysis of 18 studies worldwide that use the tests to screen for HCV in adults. They assessed the studies for diagnostic accuracy variables including sensitivity, specificity, likelihood rates (LRs), and diagnostic odds ratios (DORs) for available rapid and point-of-care diagnostic tests.
The assessment indicates that point-of-care tests of blood (serum, plasma, or whole blood) have the highest accuracy, followed by rapid diagnostic tests of serum or plasma and then by point-of-care diagnostic tests of oral fluids. However, all tests showed excellent sensitivity, specificity, likelihood rates, and diagnostic odds ratios.
Strategies to improve the identification of patients infected with HCV are changing. In fact, the Centers for Disease Control and Prevention recently updated screening guidelines, recommending that screening change from ineffective risk factor–based screening to age-based screening. Testing strategies are also a source of potential improvement. This comprehensive global review suggests that rapid diagnostic and point-of-care tests are useful as initial screening tests for HCV. Although potential biases inherent in retrospective meta-analysis reviews are discussed, the quality of the analysis is excellent and the conclusions are valid.
These diagnostic test results are available rapidly in the field, and thus are available at the point of care. The tests are also relatively inexpensive and are easy to perform. With more effective therapies for HCV in development, broadening the ability to diagnose HCV worldwide is increasingly important. Integration of these tests into the diagnostic algorithm for HCV offers a more effective screening strategy with subsequent diagnosis of more people infected with HCV worldwide.
Steven L. Flamm, M.D., is a professor of gastroenterology, hepatology, and surgery at Northwestern University, Chicago. He has no disclosures.
Rapid diagnostic and point-of-care tests for hepatitis C virus are accurate enough to be used in first-line HCV screening worldwide, according to results from the first systematic review of evidence on such tests.
Blood-based point-of-care tests (POCTs) had better sensitivity and specificity overall than did blood-based rapid diagnostic tests (RDTs), Sushmita Shivkumar of McGill University, Montreal, Quebec, and her colleagues reported.
POCTs do not require sample processing or refrigeration, and have a shelf life of 6 months or more. Rapid diagnostic tests, or RDTs, require refrigeration and sample processing. Manufactured by a variety of firms under various marketing names, all the tests can detect HCV infection in under 30 minutes, with many taking less than 5 minutes, according to the investigators, who conducted a meta-analysis of 18 studies evaluating the accuracy of one or more RDT or POCT, compared with standard assays (Ann. Intern. Med. 2012;157:558-66).
Ms. Shivkumar and her colleagues found blood-based POCTs (using serum, plasma, or whole blood) to be the most accurate of the tests evaluated in the included studies, showing 98.9% sensitivity with a 95% confidence interval [CI] of 94.5%-99.8% for whole blood and 96.8%-99.6% for serum or plasma). RDTs of serum or plasma had 98.4% sensitivity (95% CI 88.9%-99.8%). Specificity was highest in POCTs of serum or plasma at 99.7% (95% CI, 99.3%-99.9%), followed by POCTs of whole blood at 99.5% (95% CI, 97.5%-99.9%), RDTs of serum or plasma at 98.6% (95% CI, 94.9%-99.6%). POCTs for oral fluids had a sensitivity of 97.1% (95% CI, 94.7%-98.4%) and specificity of 98.2% (95% CI, 92.2%-99.6%).
POCTs and RDTs cannot distinguish between acute and chronic HCV infections, which is one of the reasons that public health organizations continue to favor conventional assays. The Food and Drug Administration currently approves the use of a single POCT for HCV, and for use only in nontraditional settings; in the United Kingdom and Canada such tests have not yet been approved.
However, by being fast, accurate, and cheaper than conventional tests, the rapid diagnostic and point-of-care tests offer "great potential for expanded first-line screening for hepatitis C infection and demonstrate the utility of blood-based singleton POCTs and of multiplex POCTs designed to provide integrated HIV and HCV screening of at-risk populations," Ms. Shivkumar and her associates wrote in their analysis. "Their rapid turnaround time limits loss to follow-up and facilitates early linkages."
The authors also concluded that their findings showed that the rapid and point-of-care tests could play a "substantial role" in expanded global screening initiatives for HCV.
The blood-based POCTs, besides being the most accurate, have the advantage of not requiring refrigeration, the investigators wrote. This feature is key in developing countries in Africa and Asia, where prevalence of HCV infection is highest.
Ms. Shivkumar and her colleagues noted several weaknesses in their meta-analysis, mostly related to the quality of studies included. Only three of the studies they evaluated were blinded, while four received industry funding; the conventional assays used as reference varied in the studies, and HCV genotype information was not collected in most.
The study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Rapid diagnostic and point-of-care tests for hepatitis C virus are accurate enough to be used in first-line HCV screening worldwide, according to results from the first systematic review of evidence on such tests.
Blood-based point-of-care tests (POCTs) had better sensitivity and specificity overall than did blood-based rapid diagnostic tests (RDTs), Sushmita Shivkumar of McGill University, Montreal, Quebec, and her colleagues reported.
POCTs do not require sample processing or refrigeration, and have a shelf life of 6 months or more. Rapid diagnostic tests, or RDTs, require refrigeration and sample processing. Manufactured by a variety of firms under various marketing names, all the tests can detect HCV infection in under 30 minutes, with many taking less than 5 minutes, according to the investigators, who conducted a meta-analysis of 18 studies evaluating the accuracy of one or more RDT or POCT, compared with standard assays (Ann. Intern. Med. 2012;157:558-66).
Ms. Shivkumar and her colleagues found blood-based POCTs (using serum, plasma, or whole blood) to be the most accurate of the tests evaluated in the included studies, showing 98.9% sensitivity with a 95% confidence interval [CI] of 94.5%-99.8% for whole blood and 96.8%-99.6% for serum or plasma). RDTs of serum or plasma had 98.4% sensitivity (95% CI 88.9%-99.8%). Specificity was highest in POCTs of serum or plasma at 99.7% (95% CI, 99.3%-99.9%), followed by POCTs of whole blood at 99.5% (95% CI, 97.5%-99.9%), RDTs of serum or plasma at 98.6% (95% CI, 94.9%-99.6%). POCTs for oral fluids had a sensitivity of 97.1% (95% CI, 94.7%-98.4%) and specificity of 98.2% (95% CI, 92.2%-99.6%).
POCTs and RDTs cannot distinguish between acute and chronic HCV infections, which is one of the reasons that public health organizations continue to favor conventional assays. The Food and Drug Administration currently approves the use of a single POCT for HCV, and for use only in nontraditional settings; in the United Kingdom and Canada such tests have not yet been approved.
However, by being fast, accurate, and cheaper than conventional tests, the rapid diagnostic and point-of-care tests offer "great potential for expanded first-line screening for hepatitis C infection and demonstrate the utility of blood-based singleton POCTs and of multiplex POCTs designed to provide integrated HIV and HCV screening of at-risk populations," Ms. Shivkumar and her associates wrote in their analysis. "Their rapid turnaround time limits loss to follow-up and facilitates early linkages."
The authors also concluded that their findings showed that the rapid and point-of-care tests could play a "substantial role" in expanded global screening initiatives for HCV.
The blood-based POCTs, besides being the most accurate, have the advantage of not requiring refrigeration, the investigators wrote. This feature is key in developing countries in Africa and Asia, where prevalence of HCV infection is highest.
Ms. Shivkumar and her colleagues noted several weaknesses in their meta-analysis, mostly related to the quality of studies included. Only three of the studies they evaluated were blinded, while four received industry funding; the conventional assays used as reference varied in the studies, and HCV genotype information was not collected in most.
The study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Major Finding: Blood-based point-of-care tests for hepatitis C virus have the highest sensitivity for screening at 98.9%, with specificity ranging from 99.5% for whole blood and 99.7% for serum or plasma.
Data Source: A meta-analysis of 18 studies that evaluated the accuracy of one or more tests.
Disclosures: Ms. Shivkumar and colleagues’ study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Limit HT Use to Menopause Symptoms, Task Force Reaffirms
Combined estrogen and progestin should not be used for the prevention of osteoporosis or other chronic conditions in postmenopausal women, according to recommendations issued by the U.S. Preventive Services Task Force.
Hormone therapy currently has Food and Drug Administration approval for use in the prevention of osteoporosis in postmenopausal women.
The task force, an independent body of volunteer experts that advises the Department of Health and Human Services, issued the recommendations Oct. 22 as an update of its 2005 statement on hormone therapy for prevention of disease in postmenopausal women.
Using the most recent scientific evidence available, including long-term follow-up data from the Women's Health Initiative (WHI) studies of hormone therapy use in postmenopausal women, the task force reached the same conclusions as it had in 2005, advising against combined estrogen and progestin for prevention of chronic conditions, and also against the use of estrogen alone for the prevention of chronic conditions in postmenopausal women who have had a hysterectomy.
The task force emphasized that hormone therapy was still indicated for the management of menopausal symptoms, such as hot flashes or vaginal dryness. It additionally made clear that its recommendation against hormone therapy for disease prevention does not apply to women younger than 50 years of age who have undergone surgical menopause.
Prior to the WHI studies, a series of government-funded trials that began in the 1990s, with follow-up ending in 2010, hormones had been widely used for the prevention of bone disease in postmenopausal women. Both estrogen and combined estrogen and progestin are known to reduce fracture risk.
However, both forms of hormone therapy were shown during the WHI studies to also increase the risk of serious adverse events, to the point where the trials were stopped early. In one randomized, placebo-controlled trial, estrogen alone was associated with a significantly higher risk of stroke, deep vein thrombosis, and gallbladder disease, while combined therapy was associated with an increased risk of stroke, invasive breast cancer, dementia, gallbladder disease, deep vein thrombosis, and pulmonary embolism.
Reproductive endocrinologist Jan L. Shifren of the department of obstetrics and gynecology and reproductive biology at Harvard Medical School and director of the menopause program at Massachusetts General Hospital, both in Boston, said in an interview that the task force’s updated position largely reflected the current consensus of the ob.gyn. community, "which is that HT should not be used to prevent the diseases of aging."
The task force was "very careful to point out that they are not saying HT should not be used for the treatment of vasomotor symptoms or vaginal atrophy. It’s not that hormones aren’t indicated; they’re just not indicated for prevention. They remain an appropriate treatment for otherwise healthy, very symptomatic women at the menopause transition," said Dr. Shifren, who is not a task force member.
FDA-approved indications for hormone therapy in postmenopausal women include treatment of menopausal symptoms and prevention of osteoporosis. A black box warning indicates that estrogen with or without progestin should be prescribed at the lowest effective dose and for the shortest time possible. The task force’s findings were based on the dosages and formulations used in the WHI trials: oral conjugated equine estrogen (0.625 mg/day plus medroxyprogesterone acetate, 2.5 mg/day) or estrogen 0.625 mg/day alone.
Dr. Shifren said that there are some practitioners "who believe that hormone therapy could still be appropriate for the prevention of osteoporosis in people who absolutely cannot tolerate any other therapy. But what I would argue is that it is incredibly rare that there is a patient who can’t tolerate one of the very many other FDA-approved treatments for the prevention of osteoporosis."
The task force members declared no relevant financial conflicts of interest.
Combined estrogen and progestin should not be used for the prevention of osteoporosis or other chronic conditions in postmenopausal women, according to recommendations issued by the U.S. Preventive Services Task Force.
Hormone therapy currently has Food and Drug Administration approval for use in the prevention of osteoporosis in postmenopausal women.
The task force, an independent body of volunteer experts that advises the Department of Health and Human Services, issued the recommendations Oct. 22 as an update of its 2005 statement on hormone therapy for prevention of disease in postmenopausal women.
Using the most recent scientific evidence available, including long-term follow-up data from the Women's Health Initiative (WHI) studies of hormone therapy use in postmenopausal women, the task force reached the same conclusions as it had in 2005, advising against combined estrogen and progestin for prevention of chronic conditions, and also against the use of estrogen alone for the prevention of chronic conditions in postmenopausal women who have had a hysterectomy.
The task force emphasized that hormone therapy was still indicated for the management of menopausal symptoms, such as hot flashes or vaginal dryness. It additionally made clear that its recommendation against hormone therapy for disease prevention does not apply to women younger than 50 years of age who have undergone surgical menopause.
Prior to the WHI studies, a series of government-funded trials that began in the 1990s, with follow-up ending in 2010, hormones had been widely used for the prevention of bone disease in postmenopausal women. Both estrogen and combined estrogen and progestin are known to reduce fracture risk.
However, both forms of hormone therapy were shown during the WHI studies to also increase the risk of serious adverse events, to the point where the trials were stopped early. In one randomized, placebo-controlled trial, estrogen alone was associated with a significantly higher risk of stroke, deep vein thrombosis, and gallbladder disease, while combined therapy was associated with an increased risk of stroke, invasive breast cancer, dementia, gallbladder disease, deep vein thrombosis, and pulmonary embolism.
Reproductive endocrinologist Jan L. Shifren of the department of obstetrics and gynecology and reproductive biology at Harvard Medical School and director of the menopause program at Massachusetts General Hospital, both in Boston, said in an interview that the task force’s updated position largely reflected the current consensus of the ob.gyn. community, "which is that HT should not be used to prevent the diseases of aging."
The task force was "very careful to point out that they are not saying HT should not be used for the treatment of vasomotor symptoms or vaginal atrophy. It’s not that hormones aren’t indicated; they’re just not indicated for prevention. They remain an appropriate treatment for otherwise healthy, very symptomatic women at the menopause transition," said Dr. Shifren, who is not a task force member.
FDA-approved indications for hormone therapy in postmenopausal women include treatment of menopausal symptoms and prevention of osteoporosis. A black box warning indicates that estrogen with or without progestin should be prescribed at the lowest effective dose and for the shortest time possible. The task force’s findings were based on the dosages and formulations used in the WHI trials: oral conjugated equine estrogen (0.625 mg/day plus medroxyprogesterone acetate, 2.5 mg/day) or estrogen 0.625 mg/day alone.
Dr. Shifren said that there are some practitioners "who believe that hormone therapy could still be appropriate for the prevention of osteoporosis in people who absolutely cannot tolerate any other therapy. But what I would argue is that it is incredibly rare that there is a patient who can’t tolerate one of the very many other FDA-approved treatments for the prevention of osteoporosis."
The task force members declared no relevant financial conflicts of interest.
Combined estrogen and progestin should not be used for the prevention of osteoporosis or other chronic conditions in postmenopausal women, according to recommendations issued by the U.S. Preventive Services Task Force.
Hormone therapy currently has Food and Drug Administration approval for use in the prevention of osteoporosis in postmenopausal women.
The task force, an independent body of volunteer experts that advises the Department of Health and Human Services, issued the recommendations Oct. 22 as an update of its 2005 statement on hormone therapy for prevention of disease in postmenopausal women.
Using the most recent scientific evidence available, including long-term follow-up data from the Women's Health Initiative (WHI) studies of hormone therapy use in postmenopausal women, the task force reached the same conclusions as it had in 2005, advising against combined estrogen and progestin for prevention of chronic conditions, and also against the use of estrogen alone for the prevention of chronic conditions in postmenopausal women who have had a hysterectomy.
The task force emphasized that hormone therapy was still indicated for the management of menopausal symptoms, such as hot flashes or vaginal dryness. It additionally made clear that its recommendation against hormone therapy for disease prevention does not apply to women younger than 50 years of age who have undergone surgical menopause.
Prior to the WHI studies, a series of government-funded trials that began in the 1990s, with follow-up ending in 2010, hormones had been widely used for the prevention of bone disease in postmenopausal women. Both estrogen and combined estrogen and progestin are known to reduce fracture risk.
However, both forms of hormone therapy were shown during the WHI studies to also increase the risk of serious adverse events, to the point where the trials were stopped early. In one randomized, placebo-controlled trial, estrogen alone was associated with a significantly higher risk of stroke, deep vein thrombosis, and gallbladder disease, while combined therapy was associated with an increased risk of stroke, invasive breast cancer, dementia, gallbladder disease, deep vein thrombosis, and pulmonary embolism.
Reproductive endocrinologist Jan L. Shifren of the department of obstetrics and gynecology and reproductive biology at Harvard Medical School and director of the menopause program at Massachusetts General Hospital, both in Boston, said in an interview that the task force’s updated position largely reflected the current consensus of the ob.gyn. community, "which is that HT should not be used to prevent the diseases of aging."
The task force was "very careful to point out that they are not saying HT should not be used for the treatment of vasomotor symptoms or vaginal atrophy. It’s not that hormones aren’t indicated; they’re just not indicated for prevention. They remain an appropriate treatment for otherwise healthy, very symptomatic women at the menopause transition," said Dr. Shifren, who is not a task force member.
FDA-approved indications for hormone therapy in postmenopausal women include treatment of menopausal symptoms and prevention of osteoporosis. A black box warning indicates that estrogen with or without progestin should be prescribed at the lowest effective dose and for the shortest time possible. The task force’s findings were based on the dosages and formulations used in the WHI trials: oral conjugated equine estrogen (0.625 mg/day plus medroxyprogesterone acetate, 2.5 mg/day) or estrogen 0.625 mg/day alone.
Dr. Shifren said that there are some practitioners "who believe that hormone therapy could still be appropriate for the prevention of osteoporosis in people who absolutely cannot tolerate any other therapy. But what I would argue is that it is incredibly rare that there is a patient who can’t tolerate one of the very many other FDA-approved treatments for the prevention of osteoporosis."
The task force members declared no relevant financial conflicts of interest.
EULAR's Lupus Nephritis Guidelines Emphasize Early Biopsy
The European League Against Rheumatism has issued its first guidelines on management of lupus nephritis, and they advise renal biopsy at the first sign of kidney involvement, unlike the guidance issued by the American College of Rheumatology, which leaves timing of that testing up to the clinician’s judgment.
Other bright spots in EULAR’s guidelines, which it issued jointly with the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) are their position that mycophenolic acid should be the first choice for immunosuppressive therapy, the precise recommendations for steroid dosage, stratification of treatment plans according to disease severity, and explicit advice on switching therapies after one drug has failed.
The European guidelines were published in the November issue of Annals of the Rheumatic Diseases (2012;71:1771-82) and are intended for rheumatologists, nephrologists, and internists managing adult and pediatric patients with lupus nephritis. The EULAR guidelines differ in several key ways from those issued by the American College of Rheumatology (ACR) in June (Arthritis Care Res. 2012;64:797-808).
The EULAR guidelines are unequivocal in their support for renal biopsy at any sign of renal involvement, including when unexplained renal insufficiency is accompanied by normal urinary findings. The ACR guidelines, by contrast, allow for more physician latitude in determining whether to biopsy.
"This can be an emotional issue because rheumatologists do not do biopsies and may try to avoid them," said the EULAR guidelines’ lead author, rheumatologist Dr. Dimitrios T. Boumpas, who is professor of medicine and director of internal medicine/rheumatology at the University of Crete in Heraklion, Greece, in an interview. "We felt that someone should make a position statement. Biopsy is simply best care. If you’re dealing with something severe like LN [lupus nephritis] and you avoid biopsy, that’s not good medicine."
Dr. Boumpas said the EULAR task force had also moved to put mycophenolic acid in the first position as an initial immunosuppressant treatment for most cases of class III-IV LN. Low-dose intravenous cyclophosphamide in combination with steroids is also recommended for this patient group.
There are no 5-year data for mycophenolic acid as there are for cyclophosphamide, Dr. Boumpas said. But the task force that developed the guidelines weighed data on safety and efficacy and found mycophenolic acid "as the clear first choice, while at the same time recognizing the limitations of the studies," he noted.
The ACR guidelines do not recommend use of azathioprine (AZA) as induction treatment. The EULAR recommendations acknowledge that AZA has been associated with a higher risk of renal flares, and call for its use in certain patients who have no adverse clinical or histological risk factors. Patients treated with AZA need close follow-up. "This is particularly important for countries without access to MPA [mycophenolic acid]," Dr. Boumpas said.
The EULAR guidelines also recommend switching to an alternative agent when patients fail to improve in 3-4 months or do not achieve partial response after 6-12 months, or a complete response after 2 years.
"This is based on evidence from both controlled trials and observational cohort studies, which highlight the fact that immunosuppressive agents, particularly cyclophosphamide, may take up to 2 years to achieve complete renal response," Dr. George K. Bertsias, also of the University of Crete in Heraklion and the first author of the guidelines, said in an interview. "On the other hand, lack of improvement at early time points (3-6 months) is associated with adverse prognosis and should evoke discussions for treatment intensification or switch."
This is a different timetable from that described in the ACR guidelines, which advocate switching after patients fail to respond after 6 months of treatment based on the treating physician’s clinical impression.
For patients not responding to mycophenolic acid or cyclophosphamide, treatment may be switched from mycophenolic acid to cyclophosphamide or from cyclophosphamide to mycophenolic acid, according to the guidelines.
If switching fails, rituximab, a biological agent, may be given either as an add-on treatment or as monotherapy. Although randomized controlled trials have failed to demonstrate the superiority of rituximab over standard treatment in lupus nephritis, "there is culminating evidence from several uncontrolled studies and several groups worldwide that rituximab works in about half of patients with nephritis refractory to conventional immunosuppressive therapy," Dr. Boumpas said. "Since rituximab does not have adverse effects on the gonads – a significant issue in the care of young women with lupus – the committee decided to recommend it as an additional treatment resource."
The EULAR guidelines, in contrast to the ACR guidelines, contain specific dosing advice on steroids, advocating pulse steroids (500-1,000 mg of methylprednisolone daily for three doses) in combination with initial immunosuppressive therapy, followed by daily oral glucocorticoids (0.5-1.0 mg/kg per day), afterward tapering to the minimal amount necessary to control disease.
The EULAR guidelines also contain specific recommendations for patients planning pregnancy. In addition, they cover diagnosis and management of pediatric lupus nephritis, which largely follow adult recommendations. The pediatric recommendations are based on evidence in adults, and on nonrandomized evidence in children.
Work on the recommendations was funded by EULAR and the ERA-EDTA. The authors declared no conflicts of interest.
European Renal Association-European Dialysis and Transplant Association, ERA-EDTA, mycophenolic acid, immunosuppressive therapy, Annals of the Rheumatic Diseases, Dr. Dimitrios T. Boumpas,
The European League Against Rheumatism has issued its first guidelines on management of lupus nephritis, and they advise renal biopsy at the first sign of kidney involvement, unlike the guidance issued by the American College of Rheumatology, which leaves timing of that testing up to the clinician’s judgment.
Other bright spots in EULAR’s guidelines, which it issued jointly with the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) are their position that mycophenolic acid should be the first choice for immunosuppressive therapy, the precise recommendations for steroid dosage, stratification of treatment plans according to disease severity, and explicit advice on switching therapies after one drug has failed.
The European guidelines were published in the November issue of Annals of the Rheumatic Diseases (2012;71:1771-82) and are intended for rheumatologists, nephrologists, and internists managing adult and pediatric patients with lupus nephritis. The EULAR guidelines differ in several key ways from those issued by the American College of Rheumatology (ACR) in June (Arthritis Care Res. 2012;64:797-808).
The EULAR guidelines are unequivocal in their support for renal biopsy at any sign of renal involvement, including when unexplained renal insufficiency is accompanied by normal urinary findings. The ACR guidelines, by contrast, allow for more physician latitude in determining whether to biopsy.
"This can be an emotional issue because rheumatologists do not do biopsies and may try to avoid them," said the EULAR guidelines’ lead author, rheumatologist Dr. Dimitrios T. Boumpas, who is professor of medicine and director of internal medicine/rheumatology at the University of Crete in Heraklion, Greece, in an interview. "We felt that someone should make a position statement. Biopsy is simply best care. If you’re dealing with something severe like LN [lupus nephritis] and you avoid biopsy, that’s not good medicine."
Dr. Boumpas said the EULAR task force had also moved to put mycophenolic acid in the first position as an initial immunosuppressant treatment for most cases of class III-IV LN. Low-dose intravenous cyclophosphamide in combination with steroids is also recommended for this patient group.
There are no 5-year data for mycophenolic acid as there are for cyclophosphamide, Dr. Boumpas said. But the task force that developed the guidelines weighed data on safety and efficacy and found mycophenolic acid "as the clear first choice, while at the same time recognizing the limitations of the studies," he noted.
The ACR guidelines do not recommend use of azathioprine (AZA) as induction treatment. The EULAR recommendations acknowledge that AZA has been associated with a higher risk of renal flares, and call for its use in certain patients who have no adverse clinical or histological risk factors. Patients treated with AZA need close follow-up. "This is particularly important for countries without access to MPA [mycophenolic acid]," Dr. Boumpas said.
The EULAR guidelines also recommend switching to an alternative agent when patients fail to improve in 3-4 months or do not achieve partial response after 6-12 months, or a complete response after 2 years.
"This is based on evidence from both controlled trials and observational cohort studies, which highlight the fact that immunosuppressive agents, particularly cyclophosphamide, may take up to 2 years to achieve complete renal response," Dr. George K. Bertsias, also of the University of Crete in Heraklion and the first author of the guidelines, said in an interview. "On the other hand, lack of improvement at early time points (3-6 months) is associated with adverse prognosis and should evoke discussions for treatment intensification or switch."
This is a different timetable from that described in the ACR guidelines, which advocate switching after patients fail to respond after 6 months of treatment based on the treating physician’s clinical impression.
For patients not responding to mycophenolic acid or cyclophosphamide, treatment may be switched from mycophenolic acid to cyclophosphamide or from cyclophosphamide to mycophenolic acid, according to the guidelines.
If switching fails, rituximab, a biological agent, may be given either as an add-on treatment or as monotherapy. Although randomized controlled trials have failed to demonstrate the superiority of rituximab over standard treatment in lupus nephritis, "there is culminating evidence from several uncontrolled studies and several groups worldwide that rituximab works in about half of patients with nephritis refractory to conventional immunosuppressive therapy," Dr. Boumpas said. "Since rituximab does not have adverse effects on the gonads – a significant issue in the care of young women with lupus – the committee decided to recommend it as an additional treatment resource."
The EULAR guidelines, in contrast to the ACR guidelines, contain specific dosing advice on steroids, advocating pulse steroids (500-1,000 mg of methylprednisolone daily for three doses) in combination with initial immunosuppressive therapy, followed by daily oral glucocorticoids (0.5-1.0 mg/kg per day), afterward tapering to the minimal amount necessary to control disease.
The EULAR guidelines also contain specific recommendations for patients planning pregnancy. In addition, they cover diagnosis and management of pediatric lupus nephritis, which largely follow adult recommendations. The pediatric recommendations are based on evidence in adults, and on nonrandomized evidence in children.
Work on the recommendations was funded by EULAR and the ERA-EDTA. The authors declared no conflicts of interest.
The European League Against Rheumatism has issued its first guidelines on management of lupus nephritis, and they advise renal biopsy at the first sign of kidney involvement, unlike the guidance issued by the American College of Rheumatology, which leaves timing of that testing up to the clinician’s judgment.
Other bright spots in EULAR’s guidelines, which it issued jointly with the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) are their position that mycophenolic acid should be the first choice for immunosuppressive therapy, the precise recommendations for steroid dosage, stratification of treatment plans according to disease severity, and explicit advice on switching therapies after one drug has failed.
The European guidelines were published in the November issue of Annals of the Rheumatic Diseases (2012;71:1771-82) and are intended for rheumatologists, nephrologists, and internists managing adult and pediatric patients with lupus nephritis. The EULAR guidelines differ in several key ways from those issued by the American College of Rheumatology (ACR) in June (Arthritis Care Res. 2012;64:797-808).
The EULAR guidelines are unequivocal in their support for renal biopsy at any sign of renal involvement, including when unexplained renal insufficiency is accompanied by normal urinary findings. The ACR guidelines, by contrast, allow for more physician latitude in determining whether to biopsy.
"This can be an emotional issue because rheumatologists do not do biopsies and may try to avoid them," said the EULAR guidelines’ lead author, rheumatologist Dr. Dimitrios T. Boumpas, who is professor of medicine and director of internal medicine/rheumatology at the University of Crete in Heraklion, Greece, in an interview. "We felt that someone should make a position statement. Biopsy is simply best care. If you’re dealing with something severe like LN [lupus nephritis] and you avoid biopsy, that’s not good medicine."
Dr. Boumpas said the EULAR task force had also moved to put mycophenolic acid in the first position as an initial immunosuppressant treatment for most cases of class III-IV LN. Low-dose intravenous cyclophosphamide in combination with steroids is also recommended for this patient group.
There are no 5-year data for mycophenolic acid as there are for cyclophosphamide, Dr. Boumpas said. But the task force that developed the guidelines weighed data on safety and efficacy and found mycophenolic acid "as the clear first choice, while at the same time recognizing the limitations of the studies," he noted.
The ACR guidelines do not recommend use of azathioprine (AZA) as induction treatment. The EULAR recommendations acknowledge that AZA has been associated with a higher risk of renal flares, and call for its use in certain patients who have no adverse clinical or histological risk factors. Patients treated with AZA need close follow-up. "This is particularly important for countries without access to MPA [mycophenolic acid]," Dr. Boumpas said.
The EULAR guidelines also recommend switching to an alternative agent when patients fail to improve in 3-4 months or do not achieve partial response after 6-12 months, or a complete response after 2 years.
"This is based on evidence from both controlled trials and observational cohort studies, which highlight the fact that immunosuppressive agents, particularly cyclophosphamide, may take up to 2 years to achieve complete renal response," Dr. George K. Bertsias, also of the University of Crete in Heraklion and the first author of the guidelines, said in an interview. "On the other hand, lack of improvement at early time points (3-6 months) is associated with adverse prognosis and should evoke discussions for treatment intensification or switch."
This is a different timetable from that described in the ACR guidelines, which advocate switching after patients fail to respond after 6 months of treatment based on the treating physician’s clinical impression.
For patients not responding to mycophenolic acid or cyclophosphamide, treatment may be switched from mycophenolic acid to cyclophosphamide or from cyclophosphamide to mycophenolic acid, according to the guidelines.
If switching fails, rituximab, a biological agent, may be given either as an add-on treatment or as monotherapy. Although randomized controlled trials have failed to demonstrate the superiority of rituximab over standard treatment in lupus nephritis, "there is culminating evidence from several uncontrolled studies and several groups worldwide that rituximab works in about half of patients with nephritis refractory to conventional immunosuppressive therapy," Dr. Boumpas said. "Since rituximab does not have adverse effects on the gonads – a significant issue in the care of young women with lupus – the committee decided to recommend it as an additional treatment resource."
The EULAR guidelines, in contrast to the ACR guidelines, contain specific dosing advice on steroids, advocating pulse steroids (500-1,000 mg of methylprednisolone daily for three doses) in combination with initial immunosuppressive therapy, followed by daily oral glucocorticoids (0.5-1.0 mg/kg per day), afterward tapering to the minimal amount necessary to control disease.
The EULAR guidelines also contain specific recommendations for patients planning pregnancy. In addition, they cover diagnosis and management of pediatric lupus nephritis, which largely follow adult recommendations. The pediatric recommendations are based on evidence in adults, and on nonrandomized evidence in children.
Work on the recommendations was funded by EULAR and the ERA-EDTA. The authors declared no conflicts of interest.
European Renal Association-European Dialysis and Transplant Association, ERA-EDTA, mycophenolic acid, immunosuppressive therapy, Annals of the Rheumatic Diseases, Dr. Dimitrios T. Boumpas,
European Renal Association-European Dialysis and Transplant Association, ERA-EDTA, mycophenolic acid, immunosuppressive therapy, Annals of the Rheumatic Diseases, Dr. Dimitrios T. Boumpas,
FROM ANNALS OF THE RHEUMATIC DISEASES
Celebrating the Career of a GI Leader and Mentor
When Don W. Powell was a medical student at the University of Alabama at Birmingham, he had the good fortune to work with a man who had just revolutionized gastroenterology.
Dr. Powell’s mentor, Dr. Basil Hirschowitz, is best known for inventing the flexible fiberoptic endoscope, "which made gastroenterology the specialty it is today," Dr. Powell said in an interview. "But he was also very much interested in how the stomach secretes acid."
So, too, was Dr. Powell, who dedicated the first few years of his 4-decade research career, which has been conducted under continuous funding from the National Institutes of Health, to studying processes governing acid production, and eventually the transport of water and electrolytes in the intestine. This, in turn, led him to study the pathophysiology of inflammatory bowel and diarrheal diseases.
At the University of Texas Medical Branch (UTMB) in Galveston, where he is now director of the Institute for Translational Sciences-Clinical Research Center and professor of internal medicine and neuroscience and cell biology, Dr. Powell began focusing on the study of intestinal stromal cells and how they contribute to intestinal repair. This brought him to his current research focus on the complex signaling pathways of cyclooxygenase-2, and its roles both in disease pathogenesis and normal gut function. Recently, his group has discovered an immunologic function in these cells.
"Most of my research has been fairly basic – I didn’t do much clinical research," Dr. Powell said. "Which is kind of ironic, because I now run a huge clinical research department."
In 2001, Dr. Powell was awarded the American Gastroenterological Association’s (AGA’s) Julius Friedenwald Medal, and was the association’s president in 2002. That same year he received the John P. McGovern Award in Oslerian Medicine from UTMB, and in 2003, he was elected a fellow of the American Association for the Advancement of Science. In 2005, Dr. Powell received the AGA Mentors Research Scholar Award, and in 2009, the annual Don W. Powell lecture was established by the AGA Institute. A UTMB professorship was established in his name this year.
To his current and former colleagues, several of whom acknowledge being fuzzy on the particulars of his biochemical research, Dr. Powell is simply "Don," an attentive mentor and talented leader who has become something of a living treasure to UTMB.
"It doesn’t matter if you are a medical student, a resident, or a colleague, he takes time with you. I really mean this. Your needs and your career goals are his focus," said Dr. J. Marc Shabot, professor of medicine at UTMB and a gastroenterology colleague of Dr. Powell’s for more than 20 years, in an interview. "So many times you see people in academia who just don’t make time for others."
As with his own early mentor, Dr. Hirschowitz, Dr. Powell’s influence on gastroenterology is deeply felt through the legions of research physicians he’s trained. Dr. G. Nicholas Verne, now professor and director of the division of gastroenterology and hepatology at UTMB, recalled in an interview how, as a junior investigator 10 years ago, "it was daunting to know if I was on the right track. At an AGA career-development conference, Don spent the whole afternoon with me going over my research."
Dr. QiQi Zhou of the department of gastroenterology at UTMB, calls Dr. Powell a "father type" for his warmth and approachability. Dr. Zhou admits that he would be the kind of father given to constant challenging with pointed questions.
In appreciation of Dr. Powell’s gifts as an educator, and to celebrate his research career, Dr. Verne, Dr. Zhou, Dr. Shabot, and other department colleagues at UTMB have called on more than a dozen of Dr. Powell’s current and former colleagues, students, and collaborators to present on a diverse array of gastroenterology topics in Galveston Oct. 19 and 20, at an event titled the Don Powell Decades. Presenters will touch on topics ranging from C. difficile infections to diverticulitis to gastroenterology in the context of global public health.
"All of the speakers are people I have mentored," said Dr. Powell, who described his approach to mentoring as flexible. "I don’t always mentor people doing the same kind of research I do – you can be just as effective in other areas of research and academic development," he said. "You have to be willing to let people make their way and give them advice when they need it. I really don’t know what the formula is, but it seems to work."
Among the presenters at the conference will be Dr. Tadataka Yamada, president of the global health program at the Bill and Melinda Gates Foundation, who has collaborated with Dr. Powell on some important gastroenterology textbooks, including multiple editions of both "Atlas of Gastroenterology" (Hoboken, N.J.: Wiley-Blackwell, 2003) and "Textbook of Gastroenterology" (Wiley-Blackwell, 2009).
"A textbook is a tremendous amount of work," Dr. Powell said. "You do it because it’s a wonderful way to learn your specialty, and you’re contributing to the education of whole generations of people all over the world."
Dr. Powell says his ongoing clinical practice is another way he keeps grounded. He continues to see a handful of private patients and enjoys it. "I think it’s good for all physician scientists to still do some medicine. It’s the reason you’re doing all the research and administration," he said. "Some end up giving up the clinical world completely, but I think it’s always valuable to have a little reality in your face."
Dr. Shabot praised Dr. Powell as a "superb and incisive" clinician, despite seeing a very limited number of patients these days. He also praised Dr. Powell’s abilities as an administrator. "The word ‘administrator’ has a cold sound to it," Dr. Shabot said. "But Dr. Powell has been passionately committed to the institution."
Dr. Powell was born in Alabama in 1938. He earned his medical degree from the Medical College of Alabama, Birmingham, and completed his residency at Peter Bent Brigham Hospital in Boston and at Yale–New Haven Community Hospital, later receiving an National Institutes of Health fellowship in physiology at Yale. Prior to landing at UTMB in 1991, he served as professor of medicine at the University of North Carolina, Chapel Hill, for 20 years.
One of the first things Dr. Powell helped do upon his arrival at UTMB was to encourage the creation of an alumni society for those who had trained in internal medicine as residents or fellows, or who had served on the faculty.
"This is one of the oldest medical schools west of the Mississippi," he said. "It’s been here since 1891. There’s this really rich history, and people like history and heritage and to know what they belong to. We decided that, with more than 100 years of medical school and thousands of people coming through it, we’re going to make people welcome and make a family out of it."
Now, 21 years later, "we have a well-established alumni society with a mailing list of 1,500 individuals," said Dr. Shabot, who is vice chair for alumni affairs and development for the department. "Dr. Powell believes in the importance of the history of the department and the institution."
Dr. Powell is married to Frances Rourke Powell, a journalist and former actress. He has three children from a previous marriage, four stepchildren, and 10 grandchildren. They will gather along with his ever-growing intellectual family to honor him in Galveston at the Don Powell Decades conference.
"He’s a giant in gastroenterology," Dr. Shabot said of Dr. Powell, "but hopefully not one who can’t take a joke." The conference will begin with a roast.
When Don W. Powell was a medical student at the University of Alabama at Birmingham, he had the good fortune to work with a man who had just revolutionized gastroenterology.
Dr. Powell’s mentor, Dr. Basil Hirschowitz, is best known for inventing the flexible fiberoptic endoscope, "which made gastroenterology the specialty it is today," Dr. Powell said in an interview. "But he was also very much interested in how the stomach secretes acid."
So, too, was Dr. Powell, who dedicated the first few years of his 4-decade research career, which has been conducted under continuous funding from the National Institutes of Health, to studying processes governing acid production, and eventually the transport of water and electrolytes in the intestine. This, in turn, led him to study the pathophysiology of inflammatory bowel and diarrheal diseases.
At the University of Texas Medical Branch (UTMB) in Galveston, where he is now director of the Institute for Translational Sciences-Clinical Research Center and professor of internal medicine and neuroscience and cell biology, Dr. Powell began focusing on the study of intestinal stromal cells and how they contribute to intestinal repair. This brought him to his current research focus on the complex signaling pathways of cyclooxygenase-2, and its roles both in disease pathogenesis and normal gut function. Recently, his group has discovered an immunologic function in these cells.
"Most of my research has been fairly basic – I didn’t do much clinical research," Dr. Powell said. "Which is kind of ironic, because I now run a huge clinical research department."
In 2001, Dr. Powell was awarded the American Gastroenterological Association’s (AGA’s) Julius Friedenwald Medal, and was the association’s president in 2002. That same year he received the John P. McGovern Award in Oslerian Medicine from UTMB, and in 2003, he was elected a fellow of the American Association for the Advancement of Science. In 2005, Dr. Powell received the AGA Mentors Research Scholar Award, and in 2009, the annual Don W. Powell lecture was established by the AGA Institute. A UTMB professorship was established in his name this year.
To his current and former colleagues, several of whom acknowledge being fuzzy on the particulars of his biochemical research, Dr. Powell is simply "Don," an attentive mentor and talented leader who has become something of a living treasure to UTMB.
"It doesn’t matter if you are a medical student, a resident, or a colleague, he takes time with you. I really mean this. Your needs and your career goals are his focus," said Dr. J. Marc Shabot, professor of medicine at UTMB and a gastroenterology colleague of Dr. Powell’s for more than 20 years, in an interview. "So many times you see people in academia who just don’t make time for others."
As with his own early mentor, Dr. Hirschowitz, Dr. Powell’s influence on gastroenterology is deeply felt through the legions of research physicians he’s trained. Dr. G. Nicholas Verne, now professor and director of the division of gastroenterology and hepatology at UTMB, recalled in an interview how, as a junior investigator 10 years ago, "it was daunting to know if I was on the right track. At an AGA career-development conference, Don spent the whole afternoon with me going over my research."
Dr. QiQi Zhou of the department of gastroenterology at UTMB, calls Dr. Powell a "father type" for his warmth and approachability. Dr. Zhou admits that he would be the kind of father given to constant challenging with pointed questions.
In appreciation of Dr. Powell’s gifts as an educator, and to celebrate his research career, Dr. Verne, Dr. Zhou, Dr. Shabot, and other department colleagues at UTMB have called on more than a dozen of Dr. Powell’s current and former colleagues, students, and collaborators to present on a diverse array of gastroenterology topics in Galveston Oct. 19 and 20, at an event titled the Don Powell Decades. Presenters will touch on topics ranging from C. difficile infections to diverticulitis to gastroenterology in the context of global public health.
"All of the speakers are people I have mentored," said Dr. Powell, who described his approach to mentoring as flexible. "I don’t always mentor people doing the same kind of research I do – you can be just as effective in other areas of research and academic development," he said. "You have to be willing to let people make their way and give them advice when they need it. I really don’t know what the formula is, but it seems to work."
Among the presenters at the conference will be Dr. Tadataka Yamada, president of the global health program at the Bill and Melinda Gates Foundation, who has collaborated with Dr. Powell on some important gastroenterology textbooks, including multiple editions of both "Atlas of Gastroenterology" (Hoboken, N.J.: Wiley-Blackwell, 2003) and "Textbook of Gastroenterology" (Wiley-Blackwell, 2009).
"A textbook is a tremendous amount of work," Dr. Powell said. "You do it because it’s a wonderful way to learn your specialty, and you’re contributing to the education of whole generations of people all over the world."
Dr. Powell says his ongoing clinical practice is another way he keeps grounded. He continues to see a handful of private patients and enjoys it. "I think it’s good for all physician scientists to still do some medicine. It’s the reason you’re doing all the research and administration," he said. "Some end up giving up the clinical world completely, but I think it’s always valuable to have a little reality in your face."
Dr. Shabot praised Dr. Powell as a "superb and incisive" clinician, despite seeing a very limited number of patients these days. He also praised Dr. Powell’s abilities as an administrator. "The word ‘administrator’ has a cold sound to it," Dr. Shabot said. "But Dr. Powell has been passionately committed to the institution."
Dr. Powell was born in Alabama in 1938. He earned his medical degree from the Medical College of Alabama, Birmingham, and completed his residency at Peter Bent Brigham Hospital in Boston and at Yale–New Haven Community Hospital, later receiving an National Institutes of Health fellowship in physiology at Yale. Prior to landing at UTMB in 1991, he served as professor of medicine at the University of North Carolina, Chapel Hill, for 20 years.
One of the first things Dr. Powell helped do upon his arrival at UTMB was to encourage the creation of an alumni society for those who had trained in internal medicine as residents or fellows, or who had served on the faculty.
"This is one of the oldest medical schools west of the Mississippi," he said. "It’s been here since 1891. There’s this really rich history, and people like history and heritage and to know what they belong to. We decided that, with more than 100 years of medical school and thousands of people coming through it, we’re going to make people welcome and make a family out of it."
Now, 21 years later, "we have a well-established alumni society with a mailing list of 1,500 individuals," said Dr. Shabot, who is vice chair for alumni affairs and development for the department. "Dr. Powell believes in the importance of the history of the department and the institution."
Dr. Powell is married to Frances Rourke Powell, a journalist and former actress. He has three children from a previous marriage, four stepchildren, and 10 grandchildren. They will gather along with his ever-growing intellectual family to honor him in Galveston at the Don Powell Decades conference.
"He’s a giant in gastroenterology," Dr. Shabot said of Dr. Powell, "but hopefully not one who can’t take a joke." The conference will begin with a roast.
When Don W. Powell was a medical student at the University of Alabama at Birmingham, he had the good fortune to work with a man who had just revolutionized gastroenterology.
Dr. Powell’s mentor, Dr. Basil Hirschowitz, is best known for inventing the flexible fiberoptic endoscope, "which made gastroenterology the specialty it is today," Dr. Powell said in an interview. "But he was also very much interested in how the stomach secretes acid."
So, too, was Dr. Powell, who dedicated the first few years of his 4-decade research career, which has been conducted under continuous funding from the National Institutes of Health, to studying processes governing acid production, and eventually the transport of water and electrolytes in the intestine. This, in turn, led him to study the pathophysiology of inflammatory bowel and diarrheal diseases.
At the University of Texas Medical Branch (UTMB) in Galveston, where he is now director of the Institute for Translational Sciences-Clinical Research Center and professor of internal medicine and neuroscience and cell biology, Dr. Powell began focusing on the study of intestinal stromal cells and how they contribute to intestinal repair. This brought him to his current research focus on the complex signaling pathways of cyclooxygenase-2, and its roles both in disease pathogenesis and normal gut function. Recently, his group has discovered an immunologic function in these cells.
"Most of my research has been fairly basic – I didn’t do much clinical research," Dr. Powell said. "Which is kind of ironic, because I now run a huge clinical research department."
In 2001, Dr. Powell was awarded the American Gastroenterological Association’s (AGA’s) Julius Friedenwald Medal, and was the association’s president in 2002. That same year he received the John P. McGovern Award in Oslerian Medicine from UTMB, and in 2003, he was elected a fellow of the American Association for the Advancement of Science. In 2005, Dr. Powell received the AGA Mentors Research Scholar Award, and in 2009, the annual Don W. Powell lecture was established by the AGA Institute. A UTMB professorship was established in his name this year.
To his current and former colleagues, several of whom acknowledge being fuzzy on the particulars of his biochemical research, Dr. Powell is simply "Don," an attentive mentor and talented leader who has become something of a living treasure to UTMB.
"It doesn’t matter if you are a medical student, a resident, or a colleague, he takes time with you. I really mean this. Your needs and your career goals are his focus," said Dr. J. Marc Shabot, professor of medicine at UTMB and a gastroenterology colleague of Dr. Powell’s for more than 20 years, in an interview. "So many times you see people in academia who just don’t make time for others."
As with his own early mentor, Dr. Hirschowitz, Dr. Powell’s influence on gastroenterology is deeply felt through the legions of research physicians he’s trained. Dr. G. Nicholas Verne, now professor and director of the division of gastroenterology and hepatology at UTMB, recalled in an interview how, as a junior investigator 10 years ago, "it was daunting to know if I was on the right track. At an AGA career-development conference, Don spent the whole afternoon with me going over my research."
Dr. QiQi Zhou of the department of gastroenterology at UTMB, calls Dr. Powell a "father type" for his warmth and approachability. Dr. Zhou admits that he would be the kind of father given to constant challenging with pointed questions.
In appreciation of Dr. Powell’s gifts as an educator, and to celebrate his research career, Dr. Verne, Dr. Zhou, Dr. Shabot, and other department colleagues at UTMB have called on more than a dozen of Dr. Powell’s current and former colleagues, students, and collaborators to present on a diverse array of gastroenterology topics in Galveston Oct. 19 and 20, at an event titled the Don Powell Decades. Presenters will touch on topics ranging from C. difficile infections to diverticulitis to gastroenterology in the context of global public health.
"All of the speakers are people I have mentored," said Dr. Powell, who described his approach to mentoring as flexible. "I don’t always mentor people doing the same kind of research I do – you can be just as effective in other areas of research and academic development," he said. "You have to be willing to let people make their way and give them advice when they need it. I really don’t know what the formula is, but it seems to work."
Among the presenters at the conference will be Dr. Tadataka Yamada, president of the global health program at the Bill and Melinda Gates Foundation, who has collaborated with Dr. Powell on some important gastroenterology textbooks, including multiple editions of both "Atlas of Gastroenterology" (Hoboken, N.J.: Wiley-Blackwell, 2003) and "Textbook of Gastroenterology" (Wiley-Blackwell, 2009).
"A textbook is a tremendous amount of work," Dr. Powell said. "You do it because it’s a wonderful way to learn your specialty, and you’re contributing to the education of whole generations of people all over the world."
Dr. Powell says his ongoing clinical practice is another way he keeps grounded. He continues to see a handful of private patients and enjoys it. "I think it’s good for all physician scientists to still do some medicine. It’s the reason you’re doing all the research and administration," he said. "Some end up giving up the clinical world completely, but I think it’s always valuable to have a little reality in your face."
Dr. Shabot praised Dr. Powell as a "superb and incisive" clinician, despite seeing a very limited number of patients these days. He also praised Dr. Powell’s abilities as an administrator. "The word ‘administrator’ has a cold sound to it," Dr. Shabot said. "But Dr. Powell has been passionately committed to the institution."
Dr. Powell was born in Alabama in 1938. He earned his medical degree from the Medical College of Alabama, Birmingham, and completed his residency at Peter Bent Brigham Hospital in Boston and at Yale–New Haven Community Hospital, later receiving an National Institutes of Health fellowship in physiology at Yale. Prior to landing at UTMB in 1991, he served as professor of medicine at the University of North Carolina, Chapel Hill, for 20 years.
One of the first things Dr. Powell helped do upon his arrival at UTMB was to encourage the creation of an alumni society for those who had trained in internal medicine as residents or fellows, or who had served on the faculty.
"This is one of the oldest medical schools west of the Mississippi," he said. "It’s been here since 1891. There’s this really rich history, and people like history and heritage and to know what they belong to. We decided that, with more than 100 years of medical school and thousands of people coming through it, we’re going to make people welcome and make a family out of it."
Now, 21 years later, "we have a well-established alumni society with a mailing list of 1,500 individuals," said Dr. Shabot, who is vice chair for alumni affairs and development for the department. "Dr. Powell believes in the importance of the history of the department and the institution."
Dr. Powell is married to Frances Rourke Powell, a journalist and former actress. He has three children from a previous marriage, four stepchildren, and 10 grandchildren. They will gather along with his ever-growing intellectual family to honor him in Galveston at the Don Powell Decades conference.
"He’s a giant in gastroenterology," Dr. Shabot said of Dr. Powell, "but hopefully not one who can’t take a joke." The conference will begin with a roast.
Rapid Tests Accurate in First-Line HCV Screening
Rapid diagnostic and point-of-care tests for hepatitis C virus are accurate enough to be used in first-line HCV screening worldwide, according to results from the first systematic review of evidence on such tests.
Blood-based point-of-care tests (POCTs) had better sensitivity and specificity overall than did blood-based rapid diagnostic tests (RDTs), Sushmita Shivkumar of McGill University, Montreal, Quebec, and her colleagues reported Oct. 15 in Annals of Internal Medicine.
POCTs do not require sample processing or refrigeration, and have a shelf life of 6 months or more. Rapid diagnostic tests, or RDTs, require refrigeration and sample processing. Manufactured by a variety of firms under various marketing names, all the tests can detect HCV infection in under 30 minutes, with many taking less than 5 minutes, according to the investigators, who conducted a meta-analysis of 18 studies evaluating the accuracy of one or more RDT or POCT, compared with standard assays (Ann. Intern. Med. 2012;157:558-566).
Ms. Shivkumar and her colleagues found blood-based POCTs (using serum, plasma, or whole blood) to be the most accurate of the tests evaluated in the included studies, showing 98.9% sensitivity with a 95% confidence interval [CI] of 94.5% to 99.8% for whole blood and 96.8% to 99.6% for serum or plasma). RDTs of serum or plasma had 98.4% sensitivity (95% CI 88.9% to 99.8%). Specificity was highest in POCTs of serum or plasma at 99.7% (95% CI, 99.3% to 99.9%), followed by POCTs of whole blood at 99.5% (95% CI, 97.5% to 99.9%), RDTs of serum or plasma at 98.6% (95% CI, 94.9% to 99.6%). POCTs for oral fluids had a sensitivity of 97.1% (95% CI, 94.7% to 98.4%) and specificity of 98.2% (95% CI, 92.2% to 99.6%).
POCTs and RDTs cannot distinguish between acute and chronic HCV infections, which is one of the reasons that public health organizations continue to favor conventional assays. The Food and Drug Administration currently approves the use of a single POCT for HCV, and for use only in nontraditional settings; in the United Kingdom and Canada such tests have not yet been approved.
However, by being fast, accurate, and cheaper than conventional tests, the rapid diagnostic and point-of-care tests offer "great potential for expanded first-line screening for hepatitis C infection and demonstrate the utility of blood-based singleton POCTs and of multiplex POCTs designed to provide integrated HIV and HCV screening of at-risk populations," Ms. Shivkumar and her associates wrote in their analysis. "Their rapid turnaround time limits loss to follow-up and facilitates early linkages."
The authors also concluded that their findings showed that the rapid and point-of-care tests could play a "substantial role" in expanded global screening initiatives for HCV.
The blood-based POCTs, besides being the most accurate, have the advantage of not requiring refrigeration, the investigators wrote. This feature is key in developing countries in Africa and Asia, where prevalence of HCV infection is highest.
Ms. Shivkumar and her colleagues noted several weaknesses in their meta-analysis, mostly related to the quality of studies included. Only three of the studies they evaluated were blinded, while four received industry funding; the conventional assays used as reference varied in the studies, and HCV genotype information was not collected in most.
The study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Chronic hepatitis C virus (HCV) infection is a common worldwide problem. It is estimated that 170 million people are afflicted. Unfortunately, the majority of infected people remain undiagnosed. In many parts of the world, patients do not have access to medical personnel or diagnostic testing so that the diagnosis of HCV can be made. For patients that do have access, standard testing for HCV is cumbersome and expensive, adding to the diagnostic burden and diminishing the opportunity for diagnosis. Even in the United States, it is estimated that the majority of people infected with HCV have not been diagnosed. These factors have provided the impetus to develop less cumbersome and inexpensive tests for HCV, with the hope that they could be implemented throughout the world to increase the number of patients diagnosed with HCV.

|
|
Although there have been numerous studies examining individual diagnostic tests for HCV, there has not been an exhaustive review of both rapid and point-of-care screening tests for HCV. Ms. Shivkumar and her associates performed a literature review and meta-analysis of 18 studies worldwide that use the tests to screen for HCV in adults. They assessed the studies for diagnostic accuracy variables including sensitivity, specificity, likelihood rates (LRs) and diagnostic odds ratios (DORs) for available rapid and point-of-care diagnostic tests.
The assessment indicates that point-of-care tests of blood (serum, plasma, or whole blood) have the highest accuracy, followed by rapid diagnostic tests of serum or plasma and then by point-of-care diagnostic tests of oral fluids. However, all tests showed excellent sensitivity, specificity, likelihood rates and diagnostic odds ratios.
Strategies to improve the identification of patients infected with HCV are changing. In fact, the Centers for Disease Control and Prevention recently updated screening guidelines, recommending that screening change from ineffective risk-factor based screening to age-based screening. Testing strategies are also a source of potential improvement. This comprehensive global review suggests that rapid diagnostic and point-of-care tests are useful as initial screening tests for HCV. Although potential biases inherent in retrospective meta-analysis reviews are discussed, the quality of the analysis is excellent and the conclusions are valid.
These diagnostic test results are available rapidly in the field, and thus are available at the point of care. The tests are also relatively inexpensive and are easy to perform. With more effective therapies for HCV in development, broadening the ability to diagnose HCV worldwide is increasingly important. Integration of these tests into the diagnostic algorithm for HCV offers a more effective screening strategy with subsequent diagnosis of more people infected with HCV worldwide.
Dr. Steven L. Flamm is a professor of gastroenterology, hepatology, and surgery at Northwestern University, Chicago. He has no disclosures.
Chronic hepatitis C virus (HCV) infection is a common worldwide problem. It is estimated that 170 million people are afflicted. Unfortunately, the majority of infected people remain undiagnosed. In many parts of the world, patients do not have access to medical personnel or diagnostic testing so that the diagnosis of HCV can be made. For patients that do have access, standard testing for HCV is cumbersome and expensive, adding to the diagnostic burden and diminishing the opportunity for diagnosis. Even in the United States, it is estimated that the majority of people infected with HCV have not been diagnosed. These factors have provided the impetus to develop less cumbersome and inexpensive tests for HCV, with the hope that they could be implemented throughout the world to increase the number of patients diagnosed with HCV.

|
|
Although there have been numerous studies examining individual diagnostic tests for HCV, there has not been an exhaustive review of both rapid and point-of-care screening tests for HCV. Ms. Shivkumar and her associates performed a literature review and meta-analysis of 18 studies worldwide that use the tests to screen for HCV in adults. They assessed the studies for diagnostic accuracy variables including sensitivity, specificity, likelihood rates (LRs) and diagnostic odds ratios (DORs) for available rapid and point-of-care diagnostic tests.
The assessment indicates that point-of-care tests of blood (serum, plasma, or whole blood) have the highest accuracy, followed by rapid diagnostic tests of serum or plasma and then by point-of-care diagnostic tests of oral fluids. However, all tests showed excellent sensitivity, specificity, likelihood rates and diagnostic odds ratios.
Strategies to improve the identification of patients infected with HCV are changing. In fact, the Centers for Disease Control and Prevention recently updated screening guidelines, recommending that screening change from ineffective risk-factor based screening to age-based screening. Testing strategies are also a source of potential improvement. This comprehensive global review suggests that rapid diagnostic and point-of-care tests are useful as initial screening tests for HCV. Although potential biases inherent in retrospective meta-analysis reviews are discussed, the quality of the analysis is excellent and the conclusions are valid.
These diagnostic test results are available rapidly in the field, and thus are available at the point of care. The tests are also relatively inexpensive and are easy to perform. With more effective therapies for HCV in development, broadening the ability to diagnose HCV worldwide is increasingly important. Integration of these tests into the diagnostic algorithm for HCV offers a more effective screening strategy with subsequent diagnosis of more people infected with HCV worldwide.
Dr. Steven L. Flamm is a professor of gastroenterology, hepatology, and surgery at Northwestern University, Chicago. He has no disclosures.
Chronic hepatitis C virus (HCV) infection is a common worldwide problem. It is estimated that 170 million people are afflicted. Unfortunately, the majority of infected people remain undiagnosed. In many parts of the world, patients do not have access to medical personnel or diagnostic testing so that the diagnosis of HCV can be made. For patients that do have access, standard testing for HCV is cumbersome and expensive, adding to the diagnostic burden and diminishing the opportunity for diagnosis. Even in the United States, it is estimated that the majority of people infected with HCV have not been diagnosed. These factors have provided the impetus to develop less cumbersome and inexpensive tests for HCV, with the hope that they could be implemented throughout the world to increase the number of patients diagnosed with HCV.

|
|
Although there have been numerous studies examining individual diagnostic tests for HCV, there has not been an exhaustive review of both rapid and point-of-care screening tests for HCV. Ms. Shivkumar and her associates performed a literature review and meta-analysis of 18 studies worldwide that use the tests to screen for HCV in adults. They assessed the studies for diagnostic accuracy variables including sensitivity, specificity, likelihood rates (LRs) and diagnostic odds ratios (DORs) for available rapid and point-of-care diagnostic tests.
The assessment indicates that point-of-care tests of blood (serum, plasma, or whole blood) have the highest accuracy, followed by rapid diagnostic tests of serum or plasma and then by point-of-care diagnostic tests of oral fluids. However, all tests showed excellent sensitivity, specificity, likelihood rates and diagnostic odds ratios.
Strategies to improve the identification of patients infected with HCV are changing. In fact, the Centers for Disease Control and Prevention recently updated screening guidelines, recommending that screening change from ineffective risk-factor based screening to age-based screening. Testing strategies are also a source of potential improvement. This comprehensive global review suggests that rapid diagnostic and point-of-care tests are useful as initial screening tests for HCV. Although potential biases inherent in retrospective meta-analysis reviews are discussed, the quality of the analysis is excellent and the conclusions are valid.
These diagnostic test results are available rapidly in the field, and thus are available at the point of care. The tests are also relatively inexpensive and are easy to perform. With more effective therapies for HCV in development, broadening the ability to diagnose HCV worldwide is increasingly important. Integration of these tests into the diagnostic algorithm for HCV offers a more effective screening strategy with subsequent diagnosis of more people infected with HCV worldwide.
Dr. Steven L. Flamm is a professor of gastroenterology, hepatology, and surgery at Northwestern University, Chicago. He has no disclosures.
Rapid diagnostic and point-of-care tests for hepatitis C virus are accurate enough to be used in first-line HCV screening worldwide, according to results from the first systematic review of evidence on such tests.
Blood-based point-of-care tests (POCTs) had better sensitivity and specificity overall than did blood-based rapid diagnostic tests (RDTs), Sushmita Shivkumar of McGill University, Montreal, Quebec, and her colleagues reported Oct. 15 in Annals of Internal Medicine.
POCTs do not require sample processing or refrigeration, and have a shelf life of 6 months or more. Rapid diagnostic tests, or RDTs, require refrigeration and sample processing. Manufactured by a variety of firms under various marketing names, all the tests can detect HCV infection in under 30 minutes, with many taking less than 5 minutes, according to the investigators, who conducted a meta-analysis of 18 studies evaluating the accuracy of one or more RDT or POCT, compared with standard assays (Ann. Intern. Med. 2012;157:558-566).
Ms. Shivkumar and her colleagues found blood-based POCTs (using serum, plasma, or whole blood) to be the most accurate of the tests evaluated in the included studies, showing 98.9% sensitivity with a 95% confidence interval [CI] of 94.5% to 99.8% for whole blood and 96.8% to 99.6% for serum or plasma). RDTs of serum or plasma had 98.4% sensitivity (95% CI 88.9% to 99.8%). Specificity was highest in POCTs of serum or plasma at 99.7% (95% CI, 99.3% to 99.9%), followed by POCTs of whole blood at 99.5% (95% CI, 97.5% to 99.9%), RDTs of serum or plasma at 98.6% (95% CI, 94.9% to 99.6%). POCTs for oral fluids had a sensitivity of 97.1% (95% CI, 94.7% to 98.4%) and specificity of 98.2% (95% CI, 92.2% to 99.6%).
POCTs and RDTs cannot distinguish between acute and chronic HCV infections, which is one of the reasons that public health organizations continue to favor conventional assays. The Food and Drug Administration currently approves the use of a single POCT for HCV, and for use only in nontraditional settings; in the United Kingdom and Canada such tests have not yet been approved.
However, by being fast, accurate, and cheaper than conventional tests, the rapid diagnostic and point-of-care tests offer "great potential for expanded first-line screening for hepatitis C infection and demonstrate the utility of blood-based singleton POCTs and of multiplex POCTs designed to provide integrated HIV and HCV screening of at-risk populations," Ms. Shivkumar and her associates wrote in their analysis. "Their rapid turnaround time limits loss to follow-up and facilitates early linkages."
The authors also concluded that their findings showed that the rapid and point-of-care tests could play a "substantial role" in expanded global screening initiatives for HCV.
The blood-based POCTs, besides being the most accurate, have the advantage of not requiring refrigeration, the investigators wrote. This feature is key in developing countries in Africa and Asia, where prevalence of HCV infection is highest.
Ms. Shivkumar and her colleagues noted several weaknesses in their meta-analysis, mostly related to the quality of studies included. Only three of the studies they evaluated were blinded, while four received industry funding; the conventional assays used as reference varied in the studies, and HCV genotype information was not collected in most.
The study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Rapid diagnostic and point-of-care tests for hepatitis C virus are accurate enough to be used in first-line HCV screening worldwide, according to results from the first systematic review of evidence on such tests.
Blood-based point-of-care tests (POCTs) had better sensitivity and specificity overall than did blood-based rapid diagnostic tests (RDTs), Sushmita Shivkumar of McGill University, Montreal, Quebec, and her colleagues reported Oct. 15 in Annals of Internal Medicine.
POCTs do not require sample processing or refrigeration, and have a shelf life of 6 months or more. Rapid diagnostic tests, or RDTs, require refrigeration and sample processing. Manufactured by a variety of firms under various marketing names, all the tests can detect HCV infection in under 30 minutes, with many taking less than 5 minutes, according to the investigators, who conducted a meta-analysis of 18 studies evaluating the accuracy of one or more RDT or POCT, compared with standard assays (Ann. Intern. Med. 2012;157:558-566).
Ms. Shivkumar and her colleagues found blood-based POCTs (using serum, plasma, or whole blood) to be the most accurate of the tests evaluated in the included studies, showing 98.9% sensitivity with a 95% confidence interval [CI] of 94.5% to 99.8% for whole blood and 96.8% to 99.6% for serum or plasma). RDTs of serum or plasma had 98.4% sensitivity (95% CI 88.9% to 99.8%). Specificity was highest in POCTs of serum or plasma at 99.7% (95% CI, 99.3% to 99.9%), followed by POCTs of whole blood at 99.5% (95% CI, 97.5% to 99.9%), RDTs of serum or plasma at 98.6% (95% CI, 94.9% to 99.6%). POCTs for oral fluids had a sensitivity of 97.1% (95% CI, 94.7% to 98.4%) and specificity of 98.2% (95% CI, 92.2% to 99.6%).
POCTs and RDTs cannot distinguish between acute and chronic HCV infections, which is one of the reasons that public health organizations continue to favor conventional assays. The Food and Drug Administration currently approves the use of a single POCT for HCV, and for use only in nontraditional settings; in the United Kingdom and Canada such tests have not yet been approved.
However, by being fast, accurate, and cheaper than conventional tests, the rapid diagnostic and point-of-care tests offer "great potential for expanded first-line screening for hepatitis C infection and demonstrate the utility of blood-based singleton POCTs and of multiplex POCTs designed to provide integrated HIV and HCV screening of at-risk populations," Ms. Shivkumar and her associates wrote in their analysis. "Their rapid turnaround time limits loss to follow-up and facilitates early linkages."
The authors also concluded that their findings showed that the rapid and point-of-care tests could play a "substantial role" in expanded global screening initiatives for HCV.
The blood-based POCTs, besides being the most accurate, have the advantage of not requiring refrigeration, the investigators wrote. This feature is key in developing countries in Africa and Asia, where prevalence of HCV infection is highest.
Ms. Shivkumar and her colleagues noted several weaknesses in their meta-analysis, mostly related to the quality of studies included. Only three of the studies they evaluated were blinded, while four received industry funding; the conventional assays used as reference varied in the studies, and HCV genotype information was not collected in most.
The study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Major Finding: Blood-based point-of-care tests for hepatitis C virus have the highest sensitivity for screening at 98.9%, with specificity ranging from 99.5% for whole blood and 99.7% for serum or plasma.
Data Source: A meta-analysis of 18 studies that evaluated the accuracy of one or more tests.
Disclosures: Ms. Shivkumar and colleagues’ study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Observation Units Could Reap $3 Billion in Savings for Hospitals
The use of observation units for patients who cannot safely be discharged following emergency room visits is highly cost effective, compared with inpatient admissions, according to a new study, yet only about one-third of U.S. hospitals currently make use of such units.
Observation units are dedicated spaces, most with about 10 beds and located close to or within the emergency department, where patients typically receive care for up to 24 hours. The study, published in the October 2012 issue of Health Affairs (doi:10.1377/hlthaff.2011.0926), found that maintaining an observation unit saved hospitals an average of $1,572 per patient who would have otherwise be admitted to an inpatient service.
"If all hospitals with sufficient emergency department volume created an observation unit and ran it at benchmark levels of efficiency, more than $3 billion in avoidable health care costs could be saved every year," wrote the study’s investigators, led by Dr. Christopher W. Baugh of Harvard Medical School and Brigham and Women’s Hospital, both in Boston.
While use of observation units has risen in recent years, from 0.6% of emergency visits in 2001 to 1.9% in 2008 (PLoS One 2011;6:[9] [doi:10.1371/journal.pone.0024326]), Dr. Baugh said in an e-mail interview that while the Centers for Medicare and Medicaid Services have warmed to observation in recent years, now allowing it for any diagnosis, "in practice, observation services occupy a gray area between the outpatient and inpatient settings. This unique feature of observation care, combined with dynamic payer policy, has created some barriers to its wider adoption," he said.
While most payers increasingly recognize observation care as valuable, "they do not require any specific setting for its delivery," Dr. Baugh said. "As a result, patients in inpatient areas can be classified as observation patients. Many of the cost advantages disappear when it is used there." Payers, he said, "need to show that they recognize the value of observation care by adopting a consistent and fair policy, both for the providers and patients."
For their research, Dr. Baugh and his colleagues conducted a literature review of studies comparing the costs of observation unit care with standard inpatient admission; 16 studies were ultimately included, all comparing observation with inpatient admission at single sites. The investigators also used data from the National Hospital Ambulatory Medical Care survey to estimate the percentage of observation unit visits nationwide that were actually avoided inpatient admissions.
Dr. Baugh and his colleagues used modeling to determine that inflation-adjusted cost savings would be $1,572/observation unit visit (standard deviation, plus or minus $812), compared with an inpatient admission, and that the annual national cost savings would be $3.1 billion (SD, plus or minus $1.9 billion) if observation unit use were universal among hospitals with sufficient emergency department volume.
These savings would result from the avoidance of about 2.4 million (SD, plus or minus 490,000) annual inpatient admissions. For hospitals with the volume to justify an observation unit, annual cost savings would be $4.6 million (SD, plus or minus $2.9 million) from maximum use, resulting from about 3,600 (SD, plus or minus 740) inpatient admissions avoided each year.
The investigators noted several limitations to their study, including that it did not account for the costs of creating an observation unit and that no distinctions were made between units run out of the emergency department and those managed by other departments. Also, chest pain studies made up a substantial portion of the analyzed literature, which, they acknowledged, "may not reflect the true spectrum of observation unit care." They further noted that single-center studies used different costing methods.
They also pointed out some potential indirect benefits from the units not captured in their study, such as fewer complications associated with inpatient hospitalization, a decrease in interdepartmental handoff errors, potential reduction of falls and hospital-acquired infections, and the creation of inpatient capacity.
Dr. Baugh and his colleagues’ study received no outside funding, and none of its authors reported conflicts of interest.
The use of observation units for patients who cannot safely be discharged following emergency room visits is highly cost effective, compared with inpatient admissions, according to a new study, yet only about one-third of U.S. hospitals currently make use of such units.
Observation units are dedicated spaces, most with about 10 beds and located close to or within the emergency department, where patients typically receive care for up to 24 hours. The study, published in the October 2012 issue of Health Affairs (doi:10.1377/hlthaff.2011.0926), found that maintaining an observation unit saved hospitals an average of $1,572 per patient who would have otherwise be admitted to an inpatient service.
"If all hospitals with sufficient emergency department volume created an observation unit and ran it at benchmark levels of efficiency, more than $3 billion in avoidable health care costs could be saved every year," wrote the study’s investigators, led by Dr. Christopher W. Baugh of Harvard Medical School and Brigham and Women’s Hospital, both in Boston.
While use of observation units has risen in recent years, from 0.6% of emergency visits in 2001 to 1.9% in 2008 (PLoS One 2011;6:[9] [doi:10.1371/journal.pone.0024326]), Dr. Baugh said in an e-mail interview that while the Centers for Medicare and Medicaid Services have warmed to observation in recent years, now allowing it for any diagnosis, "in practice, observation services occupy a gray area between the outpatient and inpatient settings. This unique feature of observation care, combined with dynamic payer policy, has created some barriers to its wider adoption," he said.
While most payers increasingly recognize observation care as valuable, "they do not require any specific setting for its delivery," Dr. Baugh said. "As a result, patients in inpatient areas can be classified as observation patients. Many of the cost advantages disappear when it is used there." Payers, he said, "need to show that they recognize the value of observation care by adopting a consistent and fair policy, both for the providers and patients."
For their research, Dr. Baugh and his colleagues conducted a literature review of studies comparing the costs of observation unit care with standard inpatient admission; 16 studies were ultimately included, all comparing observation with inpatient admission at single sites. The investigators also used data from the National Hospital Ambulatory Medical Care survey to estimate the percentage of observation unit visits nationwide that were actually avoided inpatient admissions.
Dr. Baugh and his colleagues used modeling to determine that inflation-adjusted cost savings would be $1,572/observation unit visit (standard deviation, plus or minus $812), compared with an inpatient admission, and that the annual national cost savings would be $3.1 billion (SD, plus or minus $1.9 billion) if observation unit use were universal among hospitals with sufficient emergency department volume.
These savings would result from the avoidance of about 2.4 million (SD, plus or minus 490,000) annual inpatient admissions. For hospitals with the volume to justify an observation unit, annual cost savings would be $4.6 million (SD, plus or minus $2.9 million) from maximum use, resulting from about 3,600 (SD, plus or minus 740) inpatient admissions avoided each year.
The investigators noted several limitations to their study, including that it did not account for the costs of creating an observation unit and that no distinctions were made between units run out of the emergency department and those managed by other departments. Also, chest pain studies made up a substantial portion of the analyzed literature, which, they acknowledged, "may not reflect the true spectrum of observation unit care." They further noted that single-center studies used different costing methods.
They also pointed out some potential indirect benefits from the units not captured in their study, such as fewer complications associated with inpatient hospitalization, a decrease in interdepartmental handoff errors, potential reduction of falls and hospital-acquired infections, and the creation of inpatient capacity.
Dr. Baugh and his colleagues’ study received no outside funding, and none of its authors reported conflicts of interest.
The use of observation units for patients who cannot safely be discharged following emergency room visits is highly cost effective, compared with inpatient admissions, according to a new study, yet only about one-third of U.S. hospitals currently make use of such units.
Observation units are dedicated spaces, most with about 10 beds and located close to or within the emergency department, where patients typically receive care for up to 24 hours. The study, published in the October 2012 issue of Health Affairs (doi:10.1377/hlthaff.2011.0926), found that maintaining an observation unit saved hospitals an average of $1,572 per patient who would have otherwise be admitted to an inpatient service.
"If all hospitals with sufficient emergency department volume created an observation unit and ran it at benchmark levels of efficiency, more than $3 billion in avoidable health care costs could be saved every year," wrote the study’s investigators, led by Dr. Christopher W. Baugh of Harvard Medical School and Brigham and Women’s Hospital, both in Boston.
While use of observation units has risen in recent years, from 0.6% of emergency visits in 2001 to 1.9% in 2008 (PLoS One 2011;6:[9] [doi:10.1371/journal.pone.0024326]), Dr. Baugh said in an e-mail interview that while the Centers for Medicare and Medicaid Services have warmed to observation in recent years, now allowing it for any diagnosis, "in practice, observation services occupy a gray area between the outpatient and inpatient settings. This unique feature of observation care, combined with dynamic payer policy, has created some barriers to its wider adoption," he said.
While most payers increasingly recognize observation care as valuable, "they do not require any specific setting for its delivery," Dr. Baugh said. "As a result, patients in inpatient areas can be classified as observation patients. Many of the cost advantages disappear when it is used there." Payers, he said, "need to show that they recognize the value of observation care by adopting a consistent and fair policy, both for the providers and patients."
For their research, Dr. Baugh and his colleagues conducted a literature review of studies comparing the costs of observation unit care with standard inpatient admission; 16 studies were ultimately included, all comparing observation with inpatient admission at single sites. The investigators also used data from the National Hospital Ambulatory Medical Care survey to estimate the percentage of observation unit visits nationwide that were actually avoided inpatient admissions.
Dr. Baugh and his colleagues used modeling to determine that inflation-adjusted cost savings would be $1,572/observation unit visit (standard deviation, plus or minus $812), compared with an inpatient admission, and that the annual national cost savings would be $3.1 billion (SD, plus or minus $1.9 billion) if observation unit use were universal among hospitals with sufficient emergency department volume.
These savings would result from the avoidance of about 2.4 million (SD, plus or minus 490,000) annual inpatient admissions. For hospitals with the volume to justify an observation unit, annual cost savings would be $4.6 million (SD, plus or minus $2.9 million) from maximum use, resulting from about 3,600 (SD, plus or minus 740) inpatient admissions avoided each year.
The investigators noted several limitations to their study, including that it did not account for the costs of creating an observation unit and that no distinctions were made between units run out of the emergency department and those managed by other departments. Also, chest pain studies made up a substantial portion of the analyzed literature, which, they acknowledged, "may not reflect the true spectrum of observation unit care." They further noted that single-center studies used different costing methods.
They also pointed out some potential indirect benefits from the units not captured in their study, such as fewer complications associated with inpatient hospitalization, a decrease in interdepartmental handoff errors, potential reduction of falls and hospital-acquired infections, and the creation of inpatient capacity.
Dr. Baugh and his colleagues’ study received no outside funding, and none of its authors reported conflicts of interest.
SPECT/CT Before SLN Excision Improves Melanoma Survival
Single-photon emission computed tomography/computed tomography imaging before sentinel lymph node excision was associated with significantly higher disease-free survival rates in melanoma patients, according to the results of a new study published in JAMA Sept. 12.
In addition to less local relapse and a better 4-year progression-free survival, SPECT/CT was associated with the detection of more positive nodes, more sentinel lymph node-excision (SLNE) procedures performed in the head and neck area, and improved detection of positive nodes in obese patients than standard SLNE (JAMA 2012;308:1007-14).
SPECT/CT offers "the preoperative possibility of determining the exact location and visualization of the SLN, especially if the tracer signal is too weak for detection by the handheld gamma probe alone or the SLN is in the immediate vicinity of the remaining tracer depot," Dr. Ingo Stoffels and colleagues wrote. They noted that for 33 patients in the SPECT/CT cohort, the surgical approach was changed based on SPECT/CT findings.
Dr. Stoffels, of the University of Essen-Duisburg, Germany, and colleagues, analyzed a cohort of 403 patients with clinically negative lymph nodes. All patients underwent SLNE with (149) or without (254) preoperative SPECT/CT between 2003 and 2011 at a skin cancer treatment facility where, after 2008, preoperative SPECT/CT became the standard of care.
Dr. Stoffels and colleagues found that SPECT/CT allowed SLNEs in the head and neck more frequently (23.5% for SPECT/CT, compared with 2% for standard care). Also, more SLNs per patient were detected in the SPECT/CT cohort than in the SLNE alone cohort (2.40 vs. 1.87, respectively), and the number of positive SLNs per patient was also higher in the SPECT/CT cohort (0.34 vs. 0.21). The false-negative SLN rate was 6.8% in the SPECT/CT cohort and 23.8% in the SLNE alone cohort.
Importantly, the local relapse rate in the SPECT/CT cohort was lower than in the SLNE alone cohort (6.8% vs. 23.8%, respectively), and 4-year disease-free survival was higher in the SPECT/CT cohort than in the SLNE alone cohort (93.9% vs. 79.2%, respectively). However, overall survival did not differ between the cohorts.
Dr. Stoffels and colleagues also found that SPECT/CT improved detection of positive SLNs among patients with a body mass index of 30 or higher; 5 positive SLNs out of 20 were detected (25%) in 7 obese patients in the SPECT/CT cohort, compared with 4 positive SLNs out of 44 (9.1%) in 24 obese patients in the SLNE alone cohort.
"Our results demonstrate clear advantages of adding the described preoperative SLN imaging by SPECT/CT to the current practice of preoperative lymphoscintigraphy in patients with melanoma," the investigators wrote.
They acknowledged that the temporal separation of the two cohorts was a limitation of the study as it "could lead to a bias for the time-dependent end points."
The investigators received no outside funding for their research. A coauthor, Dr. Dirk Schadendorf, disclosed receiving consultancy fees, board membership, and lecture fees from Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, MSD, Novartis, and Roche.
The study by Dr. Stoffels and colleagues has several limitations worth mentioning, beginning with the inherent limitation in looking at groups undergoing procedures during two different time periods during which there may have been slight variations in surgical technique.
Second, the median follow-up was shorter in the SPECT/CT cohort (11 months), versus the standard lymphoscintigraphy cohort (35 months). Third, there were significant differences in patient characteristics between the two cohorts, such as fewer patients with head and neck primaries in the standard cohort than in the SPECT/CT cohort (6 vs. 32, respectively), and fewer obese patients in the SPECT/CT cohort. Finally, vital blue dye – routinely used by many centers internationally in conjunction with radioactive colloid dye for SLN localization – was not used by the investigators. Therefore, it is difficult to determine how the use of this second dye may have impacted the results.
These limitations aside, there is the strong suggestion from the data that the use of SPECT/CT –particularly in obese patients or in those with head and neck primaries – may help in the identification of sentinel nodes and, therefore, more accurately stage patients with melanoma.
Moreover, this technology may assist in surgical planning, allowing for more directed, and potentially smaller, surgical incisions. Further studies with larger patient numbers will be needed to further corroborate the findings.
Giorgos C. Karakousis, M.D., is an assistant professor of surgery at the Abramson Cancer Center, Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia. He had no relevant conflicts of interest to disclose.
The study by Dr. Stoffels and colleagues has several limitations worth mentioning, beginning with the inherent limitation in looking at groups undergoing procedures during two different time periods during which there may have been slight variations in surgical technique.
Second, the median follow-up was shorter in the SPECT/CT cohort (11 months), versus the standard lymphoscintigraphy cohort (35 months). Third, there were significant differences in patient characteristics between the two cohorts, such as fewer patients with head and neck primaries in the standard cohort than in the SPECT/CT cohort (6 vs. 32, respectively), and fewer obese patients in the SPECT/CT cohort. Finally, vital blue dye – routinely used by many centers internationally in conjunction with radioactive colloid dye for SLN localization – was not used by the investigators. Therefore, it is difficult to determine how the use of this second dye may have impacted the results.
These limitations aside, there is the strong suggestion from the data that the use of SPECT/CT –particularly in obese patients or in those with head and neck primaries – may help in the identification of sentinel nodes and, therefore, more accurately stage patients with melanoma.
Moreover, this technology may assist in surgical planning, allowing for more directed, and potentially smaller, surgical incisions. Further studies with larger patient numbers will be needed to further corroborate the findings.
Giorgos C. Karakousis, M.D., is an assistant professor of surgery at the Abramson Cancer Center, Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia. He had no relevant conflicts of interest to disclose.
The study by Dr. Stoffels and colleagues has several limitations worth mentioning, beginning with the inherent limitation in looking at groups undergoing procedures during two different time periods during which there may have been slight variations in surgical technique.
Second, the median follow-up was shorter in the SPECT/CT cohort (11 months), versus the standard lymphoscintigraphy cohort (35 months). Third, there were significant differences in patient characteristics between the two cohorts, such as fewer patients with head and neck primaries in the standard cohort than in the SPECT/CT cohort (6 vs. 32, respectively), and fewer obese patients in the SPECT/CT cohort. Finally, vital blue dye – routinely used by many centers internationally in conjunction with radioactive colloid dye for SLN localization – was not used by the investigators. Therefore, it is difficult to determine how the use of this second dye may have impacted the results.
These limitations aside, there is the strong suggestion from the data that the use of SPECT/CT –particularly in obese patients or in those with head and neck primaries – may help in the identification of sentinel nodes and, therefore, more accurately stage patients with melanoma.
Moreover, this technology may assist in surgical planning, allowing for more directed, and potentially smaller, surgical incisions. Further studies with larger patient numbers will be needed to further corroborate the findings.
Giorgos C. Karakousis, M.D., is an assistant professor of surgery at the Abramson Cancer Center, Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia. He had no relevant conflicts of interest to disclose.
Single-photon emission computed tomography/computed tomography imaging before sentinel lymph node excision was associated with significantly higher disease-free survival rates in melanoma patients, according to the results of a new study published in JAMA Sept. 12.
In addition to less local relapse and a better 4-year progression-free survival, SPECT/CT was associated with the detection of more positive nodes, more sentinel lymph node-excision (SLNE) procedures performed in the head and neck area, and improved detection of positive nodes in obese patients than standard SLNE (JAMA 2012;308:1007-14).
SPECT/CT offers "the preoperative possibility of determining the exact location and visualization of the SLN, especially if the tracer signal is too weak for detection by the handheld gamma probe alone or the SLN is in the immediate vicinity of the remaining tracer depot," Dr. Ingo Stoffels and colleagues wrote. They noted that for 33 patients in the SPECT/CT cohort, the surgical approach was changed based on SPECT/CT findings.
Dr. Stoffels, of the University of Essen-Duisburg, Germany, and colleagues, analyzed a cohort of 403 patients with clinically negative lymph nodes. All patients underwent SLNE with (149) or without (254) preoperative SPECT/CT between 2003 and 2011 at a skin cancer treatment facility where, after 2008, preoperative SPECT/CT became the standard of care.
Dr. Stoffels and colleagues found that SPECT/CT allowed SLNEs in the head and neck more frequently (23.5% for SPECT/CT, compared with 2% for standard care). Also, more SLNs per patient were detected in the SPECT/CT cohort than in the SLNE alone cohort (2.40 vs. 1.87, respectively), and the number of positive SLNs per patient was also higher in the SPECT/CT cohort (0.34 vs. 0.21). The false-negative SLN rate was 6.8% in the SPECT/CT cohort and 23.8% in the SLNE alone cohort.
Importantly, the local relapse rate in the SPECT/CT cohort was lower than in the SLNE alone cohort (6.8% vs. 23.8%, respectively), and 4-year disease-free survival was higher in the SPECT/CT cohort than in the SLNE alone cohort (93.9% vs. 79.2%, respectively). However, overall survival did not differ between the cohorts.
Dr. Stoffels and colleagues also found that SPECT/CT improved detection of positive SLNs among patients with a body mass index of 30 or higher; 5 positive SLNs out of 20 were detected (25%) in 7 obese patients in the SPECT/CT cohort, compared with 4 positive SLNs out of 44 (9.1%) in 24 obese patients in the SLNE alone cohort.
"Our results demonstrate clear advantages of adding the described preoperative SLN imaging by SPECT/CT to the current practice of preoperative lymphoscintigraphy in patients with melanoma," the investigators wrote.
They acknowledged that the temporal separation of the two cohorts was a limitation of the study as it "could lead to a bias for the time-dependent end points."
The investigators received no outside funding for their research. A coauthor, Dr. Dirk Schadendorf, disclosed receiving consultancy fees, board membership, and lecture fees from Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, MSD, Novartis, and Roche.
Single-photon emission computed tomography/computed tomography imaging before sentinel lymph node excision was associated with significantly higher disease-free survival rates in melanoma patients, according to the results of a new study published in JAMA Sept. 12.
In addition to less local relapse and a better 4-year progression-free survival, SPECT/CT was associated with the detection of more positive nodes, more sentinel lymph node-excision (SLNE) procedures performed in the head and neck area, and improved detection of positive nodes in obese patients than standard SLNE (JAMA 2012;308:1007-14).
SPECT/CT offers "the preoperative possibility of determining the exact location and visualization of the SLN, especially if the tracer signal is too weak for detection by the handheld gamma probe alone or the SLN is in the immediate vicinity of the remaining tracer depot," Dr. Ingo Stoffels and colleagues wrote. They noted that for 33 patients in the SPECT/CT cohort, the surgical approach was changed based on SPECT/CT findings.
Dr. Stoffels, of the University of Essen-Duisburg, Germany, and colleagues, analyzed a cohort of 403 patients with clinically negative lymph nodes. All patients underwent SLNE with (149) or without (254) preoperative SPECT/CT between 2003 and 2011 at a skin cancer treatment facility where, after 2008, preoperative SPECT/CT became the standard of care.
Dr. Stoffels and colleagues found that SPECT/CT allowed SLNEs in the head and neck more frequently (23.5% for SPECT/CT, compared with 2% for standard care). Also, more SLNs per patient were detected in the SPECT/CT cohort than in the SLNE alone cohort (2.40 vs. 1.87, respectively), and the number of positive SLNs per patient was also higher in the SPECT/CT cohort (0.34 vs. 0.21). The false-negative SLN rate was 6.8% in the SPECT/CT cohort and 23.8% in the SLNE alone cohort.
Importantly, the local relapse rate in the SPECT/CT cohort was lower than in the SLNE alone cohort (6.8% vs. 23.8%, respectively), and 4-year disease-free survival was higher in the SPECT/CT cohort than in the SLNE alone cohort (93.9% vs. 79.2%, respectively). However, overall survival did not differ between the cohorts.
Dr. Stoffels and colleagues also found that SPECT/CT improved detection of positive SLNs among patients with a body mass index of 30 or higher; 5 positive SLNs out of 20 were detected (25%) in 7 obese patients in the SPECT/CT cohort, compared with 4 positive SLNs out of 44 (9.1%) in 24 obese patients in the SLNE alone cohort.
"Our results demonstrate clear advantages of adding the described preoperative SLN imaging by SPECT/CT to the current practice of preoperative lymphoscintigraphy in patients with melanoma," the investigators wrote.
They acknowledged that the temporal separation of the two cohorts was a limitation of the study as it "could lead to a bias for the time-dependent end points."
The investigators received no outside funding for their research. A coauthor, Dr. Dirk Schadendorf, disclosed receiving consultancy fees, board membership, and lecture fees from Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, MSD, Novartis, and Roche.
FROM JAMA
Major Finding: The local relapse rate in the SPECT/CT cohort was lower than in the SLNE alone cohort (6.8% vs. 23.8%, respectively).
Data Source: A cohort of 403 patients with clinically negative lymph nodes who underwent SLNE with (149) or without (254) preoperative SPECT/CT between 2003 and 2011.
Disclosures: The investigators received no outside funding for their research. A coauthor, Dr. Dirk Schadendorf, disclosed receiving consultancy fees, board membership, and lecture fees from Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, MSD, Novartis, and Roche.
Hyponatremia Increases Death Risk After Elective Surgery
An observational study of nearly 1 million adults undergoing major surgery has found that those with hyponatremia saw a 44% increased risk of death within 30 days of surgery, compared with subjects without the disorder.
Hyponatremia is already a known negative prognostic factor in heart failure, liver disease, kidney disease, and pneumonia. The new study, published online Sept. 10 in Archives of Internal Medicine (doi:10.1001/archinternmed.2012.3992), marks the first time that hyponatremia has been linked to higher risk of postsurgical mortality. Patients with any degree of hyponatremia before surgery saw a 5.2% risk of death, compared with 1.3% for patients without the disorder, even after the researchers adjusted for potential confounders (adjusted odds ratio 1.44; 95% confidence interval, 1.38-1.50).
Adding to this stark finding was the fact that among patients undergoing elective surgery, the mortality risk associated with hyponatremia was even higher (aOR 1.59; 95% CI 1.50-1.69) and more pronounced still among a subgroup of subjects considered the healthiest preoperative candidates, those with a class 1 or 2 status according to American Society of Anesthesiologists criteria (aOR 1.93; 1.57-2.36).
For their research, investigators Dr. Alexander A. Leung of Brigham and Women’s Hospital, Boston, and his colleagues, identified 964,263 adults undergoing major surgery from more than 200 hospitals between from January 2005 through December 2010 and evaluated their 30-day perioperative outcomes. Presurgery serum sodium levels were available for all patients included in the study.
Hyponatremia, defined as a serum sodium level of less than 135 mEq/L, occurred in 7.8% of all study patients, with 89% of these cases classified as "mild."
Dr. Leung and his colleagues wrote that their findings show that even mild hyponatremia preceding surgery is "not inconsequential and should not be ignored." In addition to the increased mortality risk, the investigators also found the presence of hyponatremia to be associated with significantly increased risk of morbidity, including major coronary events (1.8% vs. 0.7%; aOR 1.21; 95% CI 1.14-1.29), wound infections (7.4% vs. 4.6%; 1.24; 1.20-1.28), and pneumonia (3.7% vs. 1.5%; 1.17; 1.12-1.22).
Also, median length of hospital stay was prolonged by approximately 1 day among subjects with hyponatremia.
Dr. Leung and his colleagues wrote in their analysis that further research was needed to clarify whether hyponatremia caused adverse events or whether it merely indicated the presence of other serious underlying conditions contributing to morbidity and mortality.
The authors stopped short of making explicit clinical recommendations about correcting hyponatremia when it is detected prior to surgery.
Inducing rapid changes to sodium levels in a short period of time "can be potentially disastrous," the investigators wrote. However, "if monitored correction of hyponatremia is found to be safe and beneficial, it would strengthen causal inference and would be transformative to routine care since serum sodium is not presently recognized as an independent and reversible risk factor for perioperative complications."
Until further studies establish that reversing hyponatremia before surgery does in fact reduce risk, "one reasonable approach is to monitor for perioperative complications in all patients at risk and to selectively treat hyponatremia before nonemergency surgical procedures when a reversible cause is found," Dr. Leung and his colleagues wrote.
The investigators noted among the weaknesses of their study its observational design, the potential existence of unmeasured confounders, and a lack of medication data that did not allow them to determine how risk may vary according to different drug exposures.
Dr. Leung and his colleagues’ study was supported in part by Alberta Innovates–Health Solutions and the Canadian Institutes for Health Research. They reported having no relevant conflicts of interest.
The association of preoperative hyponatremia with adverse perioperative outcomes raises a variety of key therapeutic questions. How should preoperative hyponatremia be treated? Can preoperative medical consultation and comanagement attenuate the risk of hyponatremia or improve the serum sodium concentration in a timely and safe manner? Is there a role for vasopressin receptor antagonist therapy in preoperative hyponatremia? Severe cases require an immediate diagnostic evaluation and consideration for postponement of surgery to allow for correction, particularly if the case is elective. Mild hyponatremia, though, is the much more common situation, and, at a minimum, comorbidities require collaboration among specialties to ensure that the patient’s condition is optimized before surgery. In addition, the possibility of undiagnosed comorbidities needs to be considered.
Whether elective surgery should be postponed for the treatment of mild hyponatremia cannot be ascertained from this study, but the diagnosis should contribute to the informed consent process. The challenge lies in determining the next steps. Although the algorithm is relatively straightforward for treating hyponatremia, it is unclear how much this treatment should factor into a decision to proceed with elective surgical procedures. ... An individualized approach considering hyponatremia in the context of the patient’s comorbidities and the planned surgical procedure can be the only guide to the sequence of interventions.
Joseph A. Vassalotti, M.D., and Erin DuPree, M.D., are with Mount Sinai Medical Center, New York. They reported having no relevant disclosures.
The association of preoperative hyponatremia with adverse perioperative outcomes raises a variety of key therapeutic questions. How should preoperative hyponatremia be treated? Can preoperative medical consultation and comanagement attenuate the risk of hyponatremia or improve the serum sodium concentration in a timely and safe manner? Is there a role for vasopressin receptor antagonist therapy in preoperative hyponatremia? Severe cases require an immediate diagnostic evaluation and consideration for postponement of surgery to allow for correction, particularly if the case is elective. Mild hyponatremia, though, is the much more common situation, and, at a minimum, comorbidities require collaboration among specialties to ensure that the patient’s condition is optimized before surgery. In addition, the possibility of undiagnosed comorbidities needs to be considered.
Whether elective surgery should be postponed for the treatment of mild hyponatremia cannot be ascertained from this study, but the diagnosis should contribute to the informed consent process. The challenge lies in determining the next steps. Although the algorithm is relatively straightforward for treating hyponatremia, it is unclear how much this treatment should factor into a decision to proceed with elective surgical procedures. ... An individualized approach considering hyponatremia in the context of the patient’s comorbidities and the planned surgical procedure can be the only guide to the sequence of interventions.
Joseph A. Vassalotti, M.D., and Erin DuPree, M.D., are with Mount Sinai Medical Center, New York. They reported having no relevant disclosures.
The association of preoperative hyponatremia with adverse perioperative outcomes raises a variety of key therapeutic questions. How should preoperative hyponatremia be treated? Can preoperative medical consultation and comanagement attenuate the risk of hyponatremia or improve the serum sodium concentration in a timely and safe manner? Is there a role for vasopressin receptor antagonist therapy in preoperative hyponatremia? Severe cases require an immediate diagnostic evaluation and consideration for postponement of surgery to allow for correction, particularly if the case is elective. Mild hyponatremia, though, is the much more common situation, and, at a minimum, comorbidities require collaboration among specialties to ensure that the patient’s condition is optimized before surgery. In addition, the possibility of undiagnosed comorbidities needs to be considered.
Whether elective surgery should be postponed for the treatment of mild hyponatremia cannot be ascertained from this study, but the diagnosis should contribute to the informed consent process. The challenge lies in determining the next steps. Although the algorithm is relatively straightforward for treating hyponatremia, it is unclear how much this treatment should factor into a decision to proceed with elective surgical procedures. ... An individualized approach considering hyponatremia in the context of the patient’s comorbidities and the planned surgical procedure can be the only guide to the sequence of interventions.
Joseph A. Vassalotti, M.D., and Erin DuPree, M.D., are with Mount Sinai Medical Center, New York. They reported having no relevant disclosures.
An observational study of nearly 1 million adults undergoing major surgery has found that those with hyponatremia saw a 44% increased risk of death within 30 days of surgery, compared with subjects without the disorder.
Hyponatremia is already a known negative prognostic factor in heart failure, liver disease, kidney disease, and pneumonia. The new study, published online Sept. 10 in Archives of Internal Medicine (doi:10.1001/archinternmed.2012.3992), marks the first time that hyponatremia has been linked to higher risk of postsurgical mortality. Patients with any degree of hyponatremia before surgery saw a 5.2% risk of death, compared with 1.3% for patients without the disorder, even after the researchers adjusted for potential confounders (adjusted odds ratio 1.44; 95% confidence interval, 1.38-1.50).
Adding to this stark finding was the fact that among patients undergoing elective surgery, the mortality risk associated with hyponatremia was even higher (aOR 1.59; 95% CI 1.50-1.69) and more pronounced still among a subgroup of subjects considered the healthiest preoperative candidates, those with a class 1 or 2 status according to American Society of Anesthesiologists criteria (aOR 1.93; 1.57-2.36).
For their research, investigators Dr. Alexander A. Leung of Brigham and Women’s Hospital, Boston, and his colleagues, identified 964,263 adults undergoing major surgery from more than 200 hospitals between from January 2005 through December 2010 and evaluated their 30-day perioperative outcomes. Presurgery serum sodium levels were available for all patients included in the study.
Hyponatremia, defined as a serum sodium level of less than 135 mEq/L, occurred in 7.8% of all study patients, with 89% of these cases classified as "mild."
Dr. Leung and his colleagues wrote that their findings show that even mild hyponatremia preceding surgery is "not inconsequential and should not be ignored." In addition to the increased mortality risk, the investigators also found the presence of hyponatremia to be associated with significantly increased risk of morbidity, including major coronary events (1.8% vs. 0.7%; aOR 1.21; 95% CI 1.14-1.29), wound infections (7.4% vs. 4.6%; 1.24; 1.20-1.28), and pneumonia (3.7% vs. 1.5%; 1.17; 1.12-1.22).
Also, median length of hospital stay was prolonged by approximately 1 day among subjects with hyponatremia.
Dr. Leung and his colleagues wrote in their analysis that further research was needed to clarify whether hyponatremia caused adverse events or whether it merely indicated the presence of other serious underlying conditions contributing to morbidity and mortality.
The authors stopped short of making explicit clinical recommendations about correcting hyponatremia when it is detected prior to surgery.
Inducing rapid changes to sodium levels in a short period of time "can be potentially disastrous," the investigators wrote. However, "if monitored correction of hyponatremia is found to be safe and beneficial, it would strengthen causal inference and would be transformative to routine care since serum sodium is not presently recognized as an independent and reversible risk factor for perioperative complications."
Until further studies establish that reversing hyponatremia before surgery does in fact reduce risk, "one reasonable approach is to monitor for perioperative complications in all patients at risk and to selectively treat hyponatremia before nonemergency surgical procedures when a reversible cause is found," Dr. Leung and his colleagues wrote.
The investigators noted among the weaknesses of their study its observational design, the potential existence of unmeasured confounders, and a lack of medication data that did not allow them to determine how risk may vary according to different drug exposures.
Dr. Leung and his colleagues’ study was supported in part by Alberta Innovates–Health Solutions and the Canadian Institutes for Health Research. They reported having no relevant conflicts of interest.
An observational study of nearly 1 million adults undergoing major surgery has found that those with hyponatremia saw a 44% increased risk of death within 30 days of surgery, compared with subjects without the disorder.
Hyponatremia is already a known negative prognostic factor in heart failure, liver disease, kidney disease, and pneumonia. The new study, published online Sept. 10 in Archives of Internal Medicine (doi:10.1001/archinternmed.2012.3992), marks the first time that hyponatremia has been linked to higher risk of postsurgical mortality. Patients with any degree of hyponatremia before surgery saw a 5.2% risk of death, compared with 1.3% for patients without the disorder, even after the researchers adjusted for potential confounders (adjusted odds ratio 1.44; 95% confidence interval, 1.38-1.50).
Adding to this stark finding was the fact that among patients undergoing elective surgery, the mortality risk associated with hyponatremia was even higher (aOR 1.59; 95% CI 1.50-1.69) and more pronounced still among a subgroup of subjects considered the healthiest preoperative candidates, those with a class 1 or 2 status according to American Society of Anesthesiologists criteria (aOR 1.93; 1.57-2.36).
For their research, investigators Dr. Alexander A. Leung of Brigham and Women’s Hospital, Boston, and his colleagues, identified 964,263 adults undergoing major surgery from more than 200 hospitals between from January 2005 through December 2010 and evaluated their 30-day perioperative outcomes. Presurgery serum sodium levels were available for all patients included in the study.
Hyponatremia, defined as a serum sodium level of less than 135 mEq/L, occurred in 7.8% of all study patients, with 89% of these cases classified as "mild."
Dr. Leung and his colleagues wrote that their findings show that even mild hyponatremia preceding surgery is "not inconsequential and should not be ignored." In addition to the increased mortality risk, the investigators also found the presence of hyponatremia to be associated with significantly increased risk of morbidity, including major coronary events (1.8% vs. 0.7%; aOR 1.21; 95% CI 1.14-1.29), wound infections (7.4% vs. 4.6%; 1.24; 1.20-1.28), and pneumonia (3.7% vs. 1.5%; 1.17; 1.12-1.22).
Also, median length of hospital stay was prolonged by approximately 1 day among subjects with hyponatremia.
Dr. Leung and his colleagues wrote in their analysis that further research was needed to clarify whether hyponatremia caused adverse events or whether it merely indicated the presence of other serious underlying conditions contributing to morbidity and mortality.
The authors stopped short of making explicit clinical recommendations about correcting hyponatremia when it is detected prior to surgery.
Inducing rapid changes to sodium levels in a short period of time "can be potentially disastrous," the investigators wrote. However, "if monitored correction of hyponatremia is found to be safe and beneficial, it would strengthen causal inference and would be transformative to routine care since serum sodium is not presently recognized as an independent and reversible risk factor for perioperative complications."
Until further studies establish that reversing hyponatremia before surgery does in fact reduce risk, "one reasonable approach is to monitor for perioperative complications in all patients at risk and to selectively treat hyponatremia before nonemergency surgical procedures when a reversible cause is found," Dr. Leung and his colleagues wrote.
The investigators noted among the weaknesses of their study its observational design, the potential existence of unmeasured confounders, and a lack of medication data that did not allow them to determine how risk may vary according to different drug exposures.
Dr. Leung and his colleagues’ study was supported in part by Alberta Innovates–Health Solutions and the Canadian Institutes for Health Research. They reported having no relevant conflicts of interest.
Major Finding: Patients who had hyponatremia within 90 days prior to major surgery had a 5.2% risk of death within 30 days of the procedure, compared with only 1.3% for patients who did not have evidence of hyponatremia.
Data Source: Results were taken from a national database containing records on 964,263 adults undergoing major surgery from more than 200 U.S. hospitals between 2005 and 2011.
Disclosures: The researchers reported having no relevant conflicts of interest.