User login
FDA advisory committee recommends baricitinib 2 mg to treat rheumatoid arthritis
The Food and Drug Administration Arthritis Advisory Committee has voted to recommend the 2-mg dose of baricitinib, an orally administered Janus kinase (JAK) inhibitor, to treat adults with moderate to severe rheumatoid arthritis who have responded inadequately or poorly to methotrexate but rejected a 4-mg dose of the same drug.
In separate votes on efficacy of the 2-mg and 4-mg doses of baricitinib (14-1 and 15-0, respectively), on their safety (9-6 and 4-11), and on their benefit-risk ratio (10-5 and 5-10), the advisory committee consistently backed the 2-mg dose, but the committee rejected the 4-mg dose despite its effectiveness in improving the symptoms of rheumatoid arthritis. Although the FDA does not always follow the advice of its advisory committees, it generally does.
The New Drug Application was resubmitted by Eli Lilly and Incyte. The proposed doses included a 2-mg once-daily dose and a 4-mg dose for some patients. The original submission was filed in January of 2016 with an indication to treat moderate to severe rheumatoid arthritis, but that application was rejected primarily because of concerns about thrombotic events. Other issues with the original application included inadequate safety exposure for the 2-mg dose of baricitinib, as well as inconsistent findings concerning the efficacy of the higher 4-mg dose.
The resubmission addressed several of the issues noted by the FDA, and changed the indication to treat patients with moderate to severe RA who have had an inadequate response to methotrexate. Along with the different indication, the dosing regimen shifted to 2 mg once daily. For patients who did not adequately respond to disease-modifying antirheumatic drugs (DMARDS) or had an intolerance for one or more of these drugs, a 4-mg dose was recommended; after disease activity had been controlled, a taper to 2 mg once daily could be considered.
Apart from changes in the drug dosage and indication, the resubmission also included accumulated safety data, comparative epidemiologic data concerning venous thromboembolism and pulmonary embolism, and efficacy analyses to support the new dosing recommendations.
“The risk-benefit ratio may be less good here,” stated Donald Miller, PharmD, a professor of pharmacy practice at North Dakota State University, Fargo. “If there is a safety issue, it’s more likely to be a problem with the 4-mg dose.”
Jon Russell, MD, PhD, medical director of Fibromyalgia Research and Consulting, San Antonio, felt that the manufacturer understood that the safety signal indicated that the benefits outweighed the risks with the 4-mg dose of baricitinib.
“The drug is efficacious in resistant rheumatoid arthritis, and rheumatoid arthritis is a devastating disease. Organs are being destroyed, joints as well as organs, and it’s war. We need to make the patient aware that it’s war and then fight it like it is,” he said.
Many of the committee members mentioned that the efficacies of the 2-mg and 4-mg doses were not in question, primarily based on the data from four phase 3 clinical trials.
The studies RA-BEACON (JADW), RA-BUILD (JADX), RA-BEGIN (JADZ), and RA-BEAM (JADV) were all randomized phase 3 trials that evaluated the efficacy of baricitinib in patients with moderate to severe RA.
RA-BEACON and RA-BUILD both had similar designs and compared 2-mg and 4-mg doses of baricitinib with placebo; the trials primarily differed in their patient populations.
RA-BEACON. Investigators looked at 527 randomized patients who had failed treatment with a biologic DMARD with nearly half failing multiple classes of this drug. The primary endpoint for this study was met, with the 4-mg dose showing superior results, compared with placebo, based on American College of Rheumatology (ACR) 20 scores (P less than .0001). As early as week 1 of the trial, both patients receiving 2 mg and those receiving 4 mg of baricitinib showed significant improvement, compared with placebo. By week 4, the 4-mg dose produced as much improvement as the 2-mg dose achieved over nearly 6 months. While the 4-mg dose was considered more effective, the 2-mg dose showed improvement in ACR 20 scores, change in Disease Activity Score 28 C-reactive protein (DAS28-CRP), and change in health assessment questionnaire disability index (HAQ-DI) (P less than .001). Neither the 4-mg nor the 2-mg dose was able to reduce Simple Disease Activity Index (SDAI) scores, a difficult endpoint to achieve, particularly in such a short time frame.
RA-BUILD. The researchers looked at patients who had failed treatment with conventional DMARDs and had not been treated with biologic DMARDS. The investigators looked at 684 randomized patients and saw similar results to RA-BEACON, with both the 2-mg and 4-mg doses displaying significant improvement in ACR 20, change in DAS28-CRP, and change in HAQ-DI, as well as SDAI remission which had been absent in RA-BEACON. Patients taking the 4-mg dose showed improvement in morning joint stiffness duration and severity (P less than .0001), as well as improvements in worst joint pain (P less than .0001) and tiredness (P less than .027).
RA-BEGIN. The investigators took a different approach and compared various drug combinations, including baricitinib 4 mg alone or in combination with oral methotrexate or in patients taking methotrexate who were DMARD-naive. Ultimately, this trial displayed that baricitinib 4 mg alone was superior to methotrexate, according to ACR 20 scores. This held true across all clinical measures at week 24 whether baricitinib was administered alone or in combination with methotrexate. As it had in the previously discussed trials, the 4-mg dose improved all of the previously mentioned test scores, compared with methotrexate (P less than .0001) except for modified Total Sharp Score (mTSS) (P = 0.158). When baricitinib 4 mg was used in conjunction with methotrexate, improvements in test scores, including mTSS, were statistically significant.
RA-BEAM. The researchers compared baricitinib 4 mg with placebo and adalimumab in 1,305 patients. All patients maintained a background level of methotrexate to improve the efficacy of adalimumab. Consistent with previous studies, baricitinib 4 mg outperformed other therapies and placebo in improvement in ACR 20, change in DAS28-CRP, change in HAQ-DI, and SDAI remission, as well as improvements in morning joint stiffness duration and severity, worst joint pain, and worst tiredness (P less than .0001).
Despite the clear efficacy of baricitinib 4 mg, the primary issue of contention was safety and benefit-to-risk ratio. The primary safety concerns were serious infection from opportunistic pathogens, herpes zoster, various malignancies, arterial and venous thrombosis, and laboratory abnormalities including elevated platelet counts and liver test elevations.
The Food and Drug Administration Arthritis Advisory Committee has voted to recommend the 2-mg dose of baricitinib, an orally administered Janus kinase (JAK) inhibitor, to treat adults with moderate to severe rheumatoid arthritis who have responded inadequately or poorly to methotrexate but rejected a 4-mg dose of the same drug.
In separate votes on efficacy of the 2-mg and 4-mg doses of baricitinib (14-1 and 15-0, respectively), on their safety (9-6 and 4-11), and on their benefit-risk ratio (10-5 and 5-10), the advisory committee consistently backed the 2-mg dose, but the committee rejected the 4-mg dose despite its effectiveness in improving the symptoms of rheumatoid arthritis. Although the FDA does not always follow the advice of its advisory committees, it generally does.
The New Drug Application was resubmitted by Eli Lilly and Incyte. The proposed doses included a 2-mg once-daily dose and a 4-mg dose for some patients. The original submission was filed in January of 2016 with an indication to treat moderate to severe rheumatoid arthritis, but that application was rejected primarily because of concerns about thrombotic events. Other issues with the original application included inadequate safety exposure for the 2-mg dose of baricitinib, as well as inconsistent findings concerning the efficacy of the higher 4-mg dose.
The resubmission addressed several of the issues noted by the FDA, and changed the indication to treat patients with moderate to severe RA who have had an inadequate response to methotrexate. Along with the different indication, the dosing regimen shifted to 2 mg once daily. For patients who did not adequately respond to disease-modifying antirheumatic drugs (DMARDS) or had an intolerance for one or more of these drugs, a 4-mg dose was recommended; after disease activity had been controlled, a taper to 2 mg once daily could be considered.
Apart from changes in the drug dosage and indication, the resubmission also included accumulated safety data, comparative epidemiologic data concerning venous thromboembolism and pulmonary embolism, and efficacy analyses to support the new dosing recommendations.
“The risk-benefit ratio may be less good here,” stated Donald Miller, PharmD, a professor of pharmacy practice at North Dakota State University, Fargo. “If there is a safety issue, it’s more likely to be a problem with the 4-mg dose.”
Jon Russell, MD, PhD, medical director of Fibromyalgia Research and Consulting, San Antonio, felt that the manufacturer understood that the safety signal indicated that the benefits outweighed the risks with the 4-mg dose of baricitinib.
“The drug is efficacious in resistant rheumatoid arthritis, and rheumatoid arthritis is a devastating disease. Organs are being destroyed, joints as well as organs, and it’s war. We need to make the patient aware that it’s war and then fight it like it is,” he said.
Many of the committee members mentioned that the efficacies of the 2-mg and 4-mg doses were not in question, primarily based on the data from four phase 3 clinical trials.
The studies RA-BEACON (JADW), RA-BUILD (JADX), RA-BEGIN (JADZ), and RA-BEAM (JADV) were all randomized phase 3 trials that evaluated the efficacy of baricitinib in patients with moderate to severe RA.
RA-BEACON and RA-BUILD both had similar designs and compared 2-mg and 4-mg doses of baricitinib with placebo; the trials primarily differed in their patient populations.
RA-BEACON. Investigators looked at 527 randomized patients who had failed treatment with a biologic DMARD with nearly half failing multiple classes of this drug. The primary endpoint for this study was met, with the 4-mg dose showing superior results, compared with placebo, based on American College of Rheumatology (ACR) 20 scores (P less than .0001). As early as week 1 of the trial, both patients receiving 2 mg and those receiving 4 mg of baricitinib showed significant improvement, compared with placebo. By week 4, the 4-mg dose produced as much improvement as the 2-mg dose achieved over nearly 6 months. While the 4-mg dose was considered more effective, the 2-mg dose showed improvement in ACR 20 scores, change in Disease Activity Score 28 C-reactive protein (DAS28-CRP), and change in health assessment questionnaire disability index (HAQ-DI) (P less than .001). Neither the 4-mg nor the 2-mg dose was able to reduce Simple Disease Activity Index (SDAI) scores, a difficult endpoint to achieve, particularly in such a short time frame.
RA-BUILD. The researchers looked at patients who had failed treatment with conventional DMARDs and had not been treated with biologic DMARDS. The investigators looked at 684 randomized patients and saw similar results to RA-BEACON, with both the 2-mg and 4-mg doses displaying significant improvement in ACR 20, change in DAS28-CRP, and change in HAQ-DI, as well as SDAI remission which had been absent in RA-BEACON. Patients taking the 4-mg dose showed improvement in morning joint stiffness duration and severity (P less than .0001), as well as improvements in worst joint pain (P less than .0001) and tiredness (P less than .027).
RA-BEGIN. The investigators took a different approach and compared various drug combinations, including baricitinib 4 mg alone or in combination with oral methotrexate or in patients taking methotrexate who were DMARD-naive. Ultimately, this trial displayed that baricitinib 4 mg alone was superior to methotrexate, according to ACR 20 scores. This held true across all clinical measures at week 24 whether baricitinib was administered alone or in combination with methotrexate. As it had in the previously discussed trials, the 4-mg dose improved all of the previously mentioned test scores, compared with methotrexate (P less than .0001) except for modified Total Sharp Score (mTSS) (P = 0.158). When baricitinib 4 mg was used in conjunction with methotrexate, improvements in test scores, including mTSS, were statistically significant.
RA-BEAM. The researchers compared baricitinib 4 mg with placebo and adalimumab in 1,305 patients. All patients maintained a background level of methotrexate to improve the efficacy of adalimumab. Consistent with previous studies, baricitinib 4 mg outperformed other therapies and placebo in improvement in ACR 20, change in DAS28-CRP, change in HAQ-DI, and SDAI remission, as well as improvements in morning joint stiffness duration and severity, worst joint pain, and worst tiredness (P less than .0001).
Despite the clear efficacy of baricitinib 4 mg, the primary issue of contention was safety and benefit-to-risk ratio. The primary safety concerns were serious infection from opportunistic pathogens, herpes zoster, various malignancies, arterial and venous thrombosis, and laboratory abnormalities including elevated platelet counts and liver test elevations.
The Food and Drug Administration Arthritis Advisory Committee has voted to recommend the 2-mg dose of baricitinib, an orally administered Janus kinase (JAK) inhibitor, to treat adults with moderate to severe rheumatoid arthritis who have responded inadequately or poorly to methotrexate but rejected a 4-mg dose of the same drug.
In separate votes on efficacy of the 2-mg and 4-mg doses of baricitinib (14-1 and 15-0, respectively), on their safety (9-6 and 4-11), and on their benefit-risk ratio (10-5 and 5-10), the advisory committee consistently backed the 2-mg dose, but the committee rejected the 4-mg dose despite its effectiveness in improving the symptoms of rheumatoid arthritis. Although the FDA does not always follow the advice of its advisory committees, it generally does.
The New Drug Application was resubmitted by Eli Lilly and Incyte. The proposed doses included a 2-mg once-daily dose and a 4-mg dose for some patients. The original submission was filed in January of 2016 with an indication to treat moderate to severe rheumatoid arthritis, but that application was rejected primarily because of concerns about thrombotic events. Other issues with the original application included inadequate safety exposure for the 2-mg dose of baricitinib, as well as inconsistent findings concerning the efficacy of the higher 4-mg dose.
The resubmission addressed several of the issues noted by the FDA, and changed the indication to treat patients with moderate to severe RA who have had an inadequate response to methotrexate. Along with the different indication, the dosing regimen shifted to 2 mg once daily. For patients who did not adequately respond to disease-modifying antirheumatic drugs (DMARDS) or had an intolerance for one or more of these drugs, a 4-mg dose was recommended; after disease activity had been controlled, a taper to 2 mg once daily could be considered.
Apart from changes in the drug dosage and indication, the resubmission also included accumulated safety data, comparative epidemiologic data concerning venous thromboembolism and pulmonary embolism, and efficacy analyses to support the new dosing recommendations.
“The risk-benefit ratio may be less good here,” stated Donald Miller, PharmD, a professor of pharmacy practice at North Dakota State University, Fargo. “If there is a safety issue, it’s more likely to be a problem with the 4-mg dose.”
Jon Russell, MD, PhD, medical director of Fibromyalgia Research and Consulting, San Antonio, felt that the manufacturer understood that the safety signal indicated that the benefits outweighed the risks with the 4-mg dose of baricitinib.
“The drug is efficacious in resistant rheumatoid arthritis, and rheumatoid arthritis is a devastating disease. Organs are being destroyed, joints as well as organs, and it’s war. We need to make the patient aware that it’s war and then fight it like it is,” he said.
Many of the committee members mentioned that the efficacies of the 2-mg and 4-mg doses were not in question, primarily based on the data from four phase 3 clinical trials.
The studies RA-BEACON (JADW), RA-BUILD (JADX), RA-BEGIN (JADZ), and RA-BEAM (JADV) were all randomized phase 3 trials that evaluated the efficacy of baricitinib in patients with moderate to severe RA.
RA-BEACON and RA-BUILD both had similar designs and compared 2-mg and 4-mg doses of baricitinib with placebo; the trials primarily differed in their patient populations.
RA-BEACON. Investigators looked at 527 randomized patients who had failed treatment with a biologic DMARD with nearly half failing multiple classes of this drug. The primary endpoint for this study was met, with the 4-mg dose showing superior results, compared with placebo, based on American College of Rheumatology (ACR) 20 scores (P less than .0001). As early as week 1 of the trial, both patients receiving 2 mg and those receiving 4 mg of baricitinib showed significant improvement, compared with placebo. By week 4, the 4-mg dose produced as much improvement as the 2-mg dose achieved over nearly 6 months. While the 4-mg dose was considered more effective, the 2-mg dose showed improvement in ACR 20 scores, change in Disease Activity Score 28 C-reactive protein (DAS28-CRP), and change in health assessment questionnaire disability index (HAQ-DI) (P less than .001). Neither the 4-mg nor the 2-mg dose was able to reduce Simple Disease Activity Index (SDAI) scores, a difficult endpoint to achieve, particularly in such a short time frame.
RA-BUILD. The researchers looked at patients who had failed treatment with conventional DMARDs and had not been treated with biologic DMARDS. The investigators looked at 684 randomized patients and saw similar results to RA-BEACON, with both the 2-mg and 4-mg doses displaying significant improvement in ACR 20, change in DAS28-CRP, and change in HAQ-DI, as well as SDAI remission which had been absent in RA-BEACON. Patients taking the 4-mg dose showed improvement in morning joint stiffness duration and severity (P less than .0001), as well as improvements in worst joint pain (P less than .0001) and tiredness (P less than .027).
RA-BEGIN. The investigators took a different approach and compared various drug combinations, including baricitinib 4 mg alone or in combination with oral methotrexate or in patients taking methotrexate who were DMARD-naive. Ultimately, this trial displayed that baricitinib 4 mg alone was superior to methotrexate, according to ACR 20 scores. This held true across all clinical measures at week 24 whether baricitinib was administered alone or in combination with methotrexate. As it had in the previously discussed trials, the 4-mg dose improved all of the previously mentioned test scores, compared with methotrexate (P less than .0001) except for modified Total Sharp Score (mTSS) (P = 0.158). When baricitinib 4 mg was used in conjunction with methotrexate, improvements in test scores, including mTSS, were statistically significant.
RA-BEAM. The researchers compared baricitinib 4 mg with placebo and adalimumab in 1,305 patients. All patients maintained a background level of methotrexate to improve the efficacy of adalimumab. Consistent with previous studies, baricitinib 4 mg outperformed other therapies and placebo in improvement in ACR 20, change in DAS28-CRP, change in HAQ-DI, and SDAI remission, as well as improvements in morning joint stiffness duration and severity, worst joint pain, and worst tiredness (P less than .0001).
Despite the clear efficacy of baricitinib 4 mg, the primary issue of contention was safety and benefit-to-risk ratio. The primary safety concerns were serious infection from opportunistic pathogens, herpes zoster, various malignancies, arterial and venous thrombosis, and laboratory abnormalities including elevated platelet counts and liver test elevations.
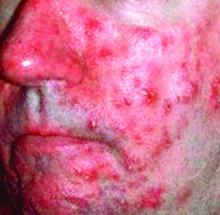
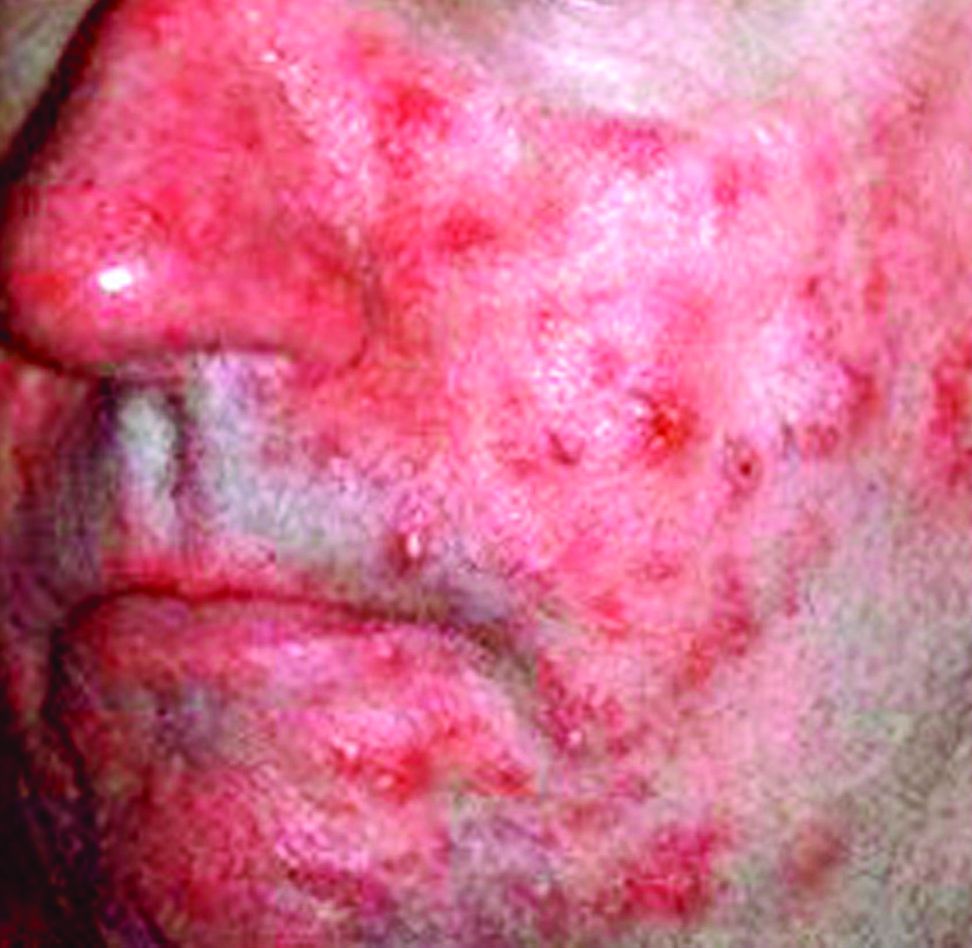
2 in 1: Rosacea-like demodicosis, papulopustular rosacea may be phenotypes of same disease
according to the authors of a retrospective study.
Currently, there is a lack of consensus among physicians and health care organizations on how Demodex mites affect the development of rosacea. Many experts separate rosacea into two camps: PPR not caused by Demodex and rosacea-like demodicosis caused by Demodex.
During each evaluation session, each patient underwent two consecutive standardized skin surface biopsies (SSSBs), a small 1 square centimeter sample of the horny skin layer and the follicular content, on each cheek. The first sample, SSSB1, was a superficial sample, and SSSB2, the second sample, was a deep sample. The sum of the two samples, SSSB1+2, also was noted.
During the same session, patients had Demodex densities (Dds) measured. To avoid any confounding factors that could affect facial skin symptoms, Dr. Forton and Dr. de Maertelaer evaluated a subgroup of 132 patients who had not been treated in the previous 3 months and had no other facial dermatoses, such as acne vulgaris and seborrheic dermatitis.
The study revealed that, among the 242 patients in the primary analysis group, those with persistent erythema had higher Dds than did those without and the differences were statistically significant when comparing SSSB2 (208 D/cm2 vs. 130 D/cm2; P = 0.031) and SSB1+2 (298 D/cm2 vs. 191 D/cm2; P = 0.025), respectively.
This pattern of greater Dds density among patients with persistent erythema, compared with patients without, also held true among the subgroup of 132 patients who had not received any recent dermatological treatments, but the difference was not statistically significant. Nonetheless, patients with follicular scales did have greater SSSBs than did those without follicular scales.
As part of the study, Dr. Forton and Dr. de Maertelaer analyzed the findings of a case report of a 19-year-old woman who had a facial papulopustular eruption that had been present for 1 year. She had two SSSBs taken on each cheek: the right had follicular scales and papulopustules, and the left was clinically normal. This revealed that she had much higher Dds on the affected cheek than on the clinically normal one (108 and 216 D/cm2 vs. 12 and 20 D/cm2). She was subsequently diagnosed with rosacea-like demodicosis.
After treating the areas with acaricidal ointment, the symptoms improved. But 27 months after stopping maintenance treatments, the facial eruptions reappeared, and the papulopustules were larger than during her original consultation, leading to the diagnosis of PPR. This time, the Dds was high on both cheeks but responded to acaricidal treatment, which indicates that her eruptions were caused Demodex mites.
“All our observations, therefore, highlight the nosological confusion that persists between PPR and rosacea-like demodicosis and the need to update the consensus concerning the definition and classification of rosacea. Moreover, they suggest that PPR and rosacea-like demodicosis may be phenotypes of the same disease,” wrote Dr. Forton and Dr. de Maertelaer. “This concept is supported by our case report, with many features indicating that the second presentation was an evolution of the first.”
They cautioned that their research is not definitive but should provide strong evidence that PPR and rosacea-like demodicosis are the same disease.
“While our observations do not prove a causative role of Demodex in rosacea, they nevertheless support the idea that PPR and rosacea-like demodicosis should no longer be considered as two separate entities but, rather, as two phenotypes of the same disease,” they wrote. “As such, the definition of rosacea subtype II (PPR) should be reconsidered and simplified to include all patients with central face papulopustules – with or without persistent erythema – and thus also patients with ‘rosacea-like demodicosis,’ which is a term that should therefore disappear.”
This study received no external funding. Dr. Forton works for Galderma as a consultant. Dr. de Maertelaer had no conflicts of interest to declare.
SOURCE: Forton F et al. J Eur Acad Dermatol Venereol. 2018 Feb 25. doi: 10.1111/jdv.14885.
according to the authors of a retrospective study.
Currently, there is a lack of consensus among physicians and health care organizations on how Demodex mites affect the development of rosacea. Many experts separate rosacea into two camps: PPR not caused by Demodex and rosacea-like demodicosis caused by Demodex.
During each evaluation session, each patient underwent two consecutive standardized skin surface biopsies (SSSBs), a small 1 square centimeter sample of the horny skin layer and the follicular content, on each cheek. The first sample, SSSB1, was a superficial sample, and SSSB2, the second sample, was a deep sample. The sum of the two samples, SSSB1+2, also was noted.
During the same session, patients had Demodex densities (Dds) measured. To avoid any confounding factors that could affect facial skin symptoms, Dr. Forton and Dr. de Maertelaer evaluated a subgroup of 132 patients who had not been treated in the previous 3 months and had no other facial dermatoses, such as acne vulgaris and seborrheic dermatitis.
The study revealed that, among the 242 patients in the primary analysis group, those with persistent erythema had higher Dds than did those without and the differences were statistically significant when comparing SSSB2 (208 D/cm2 vs. 130 D/cm2; P = 0.031) and SSB1+2 (298 D/cm2 vs. 191 D/cm2; P = 0.025), respectively.
This pattern of greater Dds density among patients with persistent erythema, compared with patients without, also held true among the subgroup of 132 patients who had not received any recent dermatological treatments, but the difference was not statistically significant. Nonetheless, patients with follicular scales did have greater SSSBs than did those without follicular scales.
As part of the study, Dr. Forton and Dr. de Maertelaer analyzed the findings of a case report of a 19-year-old woman who had a facial papulopustular eruption that had been present for 1 year. She had two SSSBs taken on each cheek: the right had follicular scales and papulopustules, and the left was clinically normal. This revealed that she had much higher Dds on the affected cheek than on the clinically normal one (108 and 216 D/cm2 vs. 12 and 20 D/cm2). She was subsequently diagnosed with rosacea-like demodicosis.
After treating the areas with acaricidal ointment, the symptoms improved. But 27 months after stopping maintenance treatments, the facial eruptions reappeared, and the papulopustules were larger than during her original consultation, leading to the diagnosis of PPR. This time, the Dds was high on both cheeks but responded to acaricidal treatment, which indicates that her eruptions were caused Demodex mites.
“All our observations, therefore, highlight the nosological confusion that persists between PPR and rosacea-like demodicosis and the need to update the consensus concerning the definition and classification of rosacea. Moreover, they suggest that PPR and rosacea-like demodicosis may be phenotypes of the same disease,” wrote Dr. Forton and Dr. de Maertelaer. “This concept is supported by our case report, with many features indicating that the second presentation was an evolution of the first.”
They cautioned that their research is not definitive but should provide strong evidence that PPR and rosacea-like demodicosis are the same disease.
“While our observations do not prove a causative role of Demodex in rosacea, they nevertheless support the idea that PPR and rosacea-like demodicosis should no longer be considered as two separate entities but, rather, as two phenotypes of the same disease,” they wrote. “As such, the definition of rosacea subtype II (PPR) should be reconsidered and simplified to include all patients with central face papulopustules – with or without persistent erythema – and thus also patients with ‘rosacea-like demodicosis,’ which is a term that should therefore disappear.”
This study received no external funding. Dr. Forton works for Galderma as a consultant. Dr. de Maertelaer had no conflicts of interest to declare.
SOURCE: Forton F et al. J Eur Acad Dermatol Venereol. 2018 Feb 25. doi: 10.1111/jdv.14885.
according to the authors of a retrospective study.
Currently, there is a lack of consensus among physicians and health care organizations on how Demodex mites affect the development of rosacea. Many experts separate rosacea into two camps: PPR not caused by Demodex and rosacea-like demodicosis caused by Demodex.
During each evaluation session, each patient underwent two consecutive standardized skin surface biopsies (SSSBs), a small 1 square centimeter sample of the horny skin layer and the follicular content, on each cheek. The first sample, SSSB1, was a superficial sample, and SSSB2, the second sample, was a deep sample. The sum of the two samples, SSSB1+2, also was noted.
During the same session, patients had Demodex densities (Dds) measured. To avoid any confounding factors that could affect facial skin symptoms, Dr. Forton and Dr. de Maertelaer evaluated a subgroup of 132 patients who had not been treated in the previous 3 months and had no other facial dermatoses, such as acne vulgaris and seborrheic dermatitis.
The study revealed that, among the 242 patients in the primary analysis group, those with persistent erythema had higher Dds than did those without and the differences were statistically significant when comparing SSSB2 (208 D/cm2 vs. 130 D/cm2; P = 0.031) and SSB1+2 (298 D/cm2 vs. 191 D/cm2; P = 0.025), respectively.
This pattern of greater Dds density among patients with persistent erythema, compared with patients without, also held true among the subgroup of 132 patients who had not received any recent dermatological treatments, but the difference was not statistically significant. Nonetheless, patients with follicular scales did have greater SSSBs than did those without follicular scales.
As part of the study, Dr. Forton and Dr. de Maertelaer analyzed the findings of a case report of a 19-year-old woman who had a facial papulopustular eruption that had been present for 1 year. She had two SSSBs taken on each cheek: the right had follicular scales and papulopustules, and the left was clinically normal. This revealed that she had much higher Dds on the affected cheek than on the clinically normal one (108 and 216 D/cm2 vs. 12 and 20 D/cm2). She was subsequently diagnosed with rosacea-like demodicosis.
After treating the areas with acaricidal ointment, the symptoms improved. But 27 months after stopping maintenance treatments, the facial eruptions reappeared, and the papulopustules were larger than during her original consultation, leading to the diagnosis of PPR. This time, the Dds was high on both cheeks but responded to acaricidal treatment, which indicates that her eruptions were caused Demodex mites.
“All our observations, therefore, highlight the nosological confusion that persists between PPR and rosacea-like demodicosis and the need to update the consensus concerning the definition and classification of rosacea. Moreover, they suggest that PPR and rosacea-like demodicosis may be phenotypes of the same disease,” wrote Dr. Forton and Dr. de Maertelaer. “This concept is supported by our case report, with many features indicating that the second presentation was an evolution of the first.”
They cautioned that their research is not definitive but should provide strong evidence that PPR and rosacea-like demodicosis are the same disease.
“While our observations do not prove a causative role of Demodex in rosacea, they nevertheless support the idea that PPR and rosacea-like demodicosis should no longer be considered as two separate entities but, rather, as two phenotypes of the same disease,” they wrote. “As such, the definition of rosacea subtype II (PPR) should be reconsidered and simplified to include all patients with central face papulopustules – with or without persistent erythema – and thus also patients with ‘rosacea-like demodicosis,’ which is a term that should therefore disappear.”
This study received no external funding. Dr. Forton works for Galderma as a consultant. Dr. de Maertelaer had no conflicts of interest to declare.
SOURCE: Forton F et al. J Eur Acad Dermatol Venereol. 2018 Feb 25. doi: 10.1111/jdv.14885.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: Demodex densities were greater in patients with persistent erythema than in those without.
Major finding: Deep tissue samples showed higher mite densities in patients with persistent erythema than in those without (SSSB2: 208 D/cm2 and 130 D/cm2; P = 0.031).
Study details: A retrospective, observational, case-control study of 242 patients with central face papulopustules.
Disclosures: This study received no external funding. Dr. Forton works for Galderma as a consultant. Dr. de Maertelaer has no conflicts of interest to declare.
Source: Forton F et al. J Eur Acad Dermatol Venereol. 2018 Feb 25. doi: 10.1111/jdv.14885.
Anxiety, depression prevalent in children with comorbid autism and ADHD
Children with comorbid autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are at an increased risk of anxiety and mood disorders, a cross-sectional analysis has shown.
“Our study supports that anxiety and mood disorders, although highly prevalent in those with ASD alone, are even more prevalent in individuals who have ADHD,” wrote Eliza Gordon-Lipkin, MD, of the Kennedy Krieger Institute, Baltimore, and her associates. ”The identification of psychiatric conditions in children with ASD is important because these disorders are treatable and affect quality of life.”
The study was published in Pediatrics.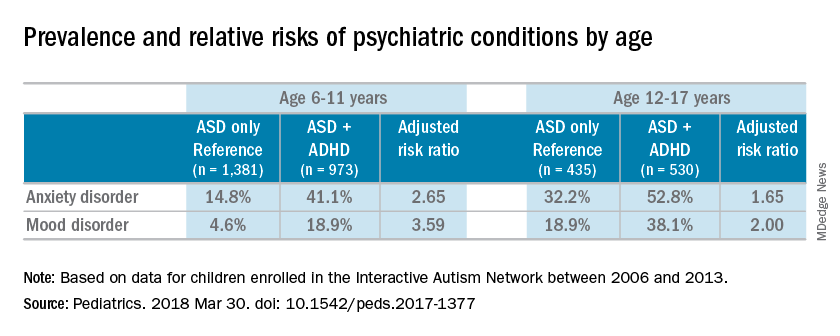
Most of the children were male (83%), white (87%), and non-Hispanic (92%); the mean age of the children was 10 years. Almost half of the children in the study had parent-reported ADHD (45%). Almost one-third of patients were diagnosed with an anxiety disorder (31%), and many also were reported to have been diagnosed with a mood disorder (16%). An increased risk of reported anxiety disorder was found in patients with both ADHD and ASD (adjusted relative risk, 2.20; 95% confidence interval, 1.97-2.46).
The researchers also found an increased risk of mood disorders (aRR, 2.72; 95% CI, 2.28-3.24) among children with comorbid conditions. Those risks increased with age (both P less than .001). An increased prevalence of anxiety and mood disorders was found in adolescents, compared with school-aged children with both ASD and ADHD or ASD alone. But higher relative risk ratios were found for the younger children, compared with the adolescents for those in the ADHD/ASD group and the ASD alone group.
“This suggests that or more likely to exhibit detectable symptoms at an earlier age,” reported Dr. Gordon-Lipkin, also with the department of pediatrics at Johns Hopkins University, in Baltimore.
The research team cited several limitations. For example, patient-reported data might be subject to recall or reporting biases. Also, computer and Internet access was required to complete the IAN questionnaires, which means that the findings could be biased toward people of higher socioeconomic status.
Nevertheless, the researchers wrote, their study is the largest to compare comorbidities in patients with ASD and ADHD, or ASD alone.
Further research is needed to better understand the relationship between ASD and ADHD. “ADHD affects nearly half of the children with ASD. This subgroup of individuals with ASD may represent a distinct clinical phenotype, with different diagnostic and therapeutic implications,” Dr. Gordon-Lipkin and her associates wrote. “Better understanding the differences between children with ASD with and without ADHD is crucial to designing effective interventions.”
None of the study authors had relevant financial disclosures to report. The Interactive Autism Network is funded by the Simons Foundation and the Patient-Centered Outcomes Research Institute.
SOURCE: Gordon-Lipkin E et al. Pediatrics. 2018 Mar 30. doi: 10.1542/ peds.2017-1377.
The work of Gordon-Lipkin et al. is one of the largest studies analyzing the relationships between autism, ADHD, and anxiety and mood disorders. But because of the inherent behavioral and biological complexity of autism, changes in the diagnostic criteria, and the use of parent-reported data, the current study might not reflect what is truly occurring in patients with autism, Christopher J. McDougle, MD, said in an interview.
“There are a number of things to say about [the study]. [One] of the strengths of the paper [is] the sample size,” Dr. McDougle said.“It’s always good to have a big sample size. The downside to having informant-databased information is that it is exactly what it is. This is fine, but the information may be inaccurate.”
In addition to parent-reported data, physicians are dealing with the relatively new diagnostic criteria. The May 2013 update of the Diagnostic and Statistical Manual of Mental Disorders to the DSM-5 brought with it the ability to diagnose ADHD with autism, when just the day before the DSM-5 was released, this differential diagnosis was not listed in the manual, Dr. McDougle said. “If something that important can change with the strike of the clock, it makes me concerned.” He also said listing the differential diagnosis in the diagnostic manual underscored the uncertainty of medicine’s understanding of comorbid autism and ADHD.
“That’s reflective of the field’s lack of knowledge. Sometimes I think we like to portray things as though we understand what’s going on, when I think it’s better to be honest and say we really don’t; we are just doing our best.”
Dr. McDougle is the director of the Lurie Center for Autism at Massachusetts General Hospital and is the Nancy Lurie Marks Professor of Psychiatry at Harvard Medical Center, both in Boston. He treats children, adolescents, and adults with autism spectrum disorder and other neurodevelopmental disorders. He was asked to comment on this study.
The work of Gordon-Lipkin et al. is one of the largest studies analyzing the relationships between autism, ADHD, and anxiety and mood disorders. But because of the inherent behavioral and biological complexity of autism, changes in the diagnostic criteria, and the use of parent-reported data, the current study might not reflect what is truly occurring in patients with autism, Christopher J. McDougle, MD, said in an interview.
“There are a number of things to say about [the study]. [One] of the strengths of the paper [is] the sample size,” Dr. McDougle said.“It’s always good to have a big sample size. The downside to having informant-databased information is that it is exactly what it is. This is fine, but the information may be inaccurate.”
In addition to parent-reported data, physicians are dealing with the relatively new diagnostic criteria. The May 2013 update of the Diagnostic and Statistical Manual of Mental Disorders to the DSM-5 brought with it the ability to diagnose ADHD with autism, when just the day before the DSM-5 was released, this differential diagnosis was not listed in the manual, Dr. McDougle said. “If something that important can change with the strike of the clock, it makes me concerned.” He also said listing the differential diagnosis in the diagnostic manual underscored the uncertainty of medicine’s understanding of comorbid autism and ADHD.
“That’s reflective of the field’s lack of knowledge. Sometimes I think we like to portray things as though we understand what’s going on, when I think it’s better to be honest and say we really don’t; we are just doing our best.”
Dr. McDougle is the director of the Lurie Center for Autism at Massachusetts General Hospital and is the Nancy Lurie Marks Professor of Psychiatry at Harvard Medical Center, both in Boston. He treats children, adolescents, and adults with autism spectrum disorder and other neurodevelopmental disorders. He was asked to comment on this study.
The work of Gordon-Lipkin et al. is one of the largest studies analyzing the relationships between autism, ADHD, and anxiety and mood disorders. But because of the inherent behavioral and biological complexity of autism, changes in the diagnostic criteria, and the use of parent-reported data, the current study might not reflect what is truly occurring in patients with autism, Christopher J. McDougle, MD, said in an interview.
“There are a number of things to say about [the study]. [One] of the strengths of the paper [is] the sample size,” Dr. McDougle said.“It’s always good to have a big sample size. The downside to having informant-databased information is that it is exactly what it is. This is fine, but the information may be inaccurate.”
In addition to parent-reported data, physicians are dealing with the relatively new diagnostic criteria. The May 2013 update of the Diagnostic and Statistical Manual of Mental Disorders to the DSM-5 brought with it the ability to diagnose ADHD with autism, when just the day before the DSM-5 was released, this differential diagnosis was not listed in the manual, Dr. McDougle said. “If something that important can change with the strike of the clock, it makes me concerned.” He also said listing the differential diagnosis in the diagnostic manual underscored the uncertainty of medicine’s understanding of comorbid autism and ADHD.
“That’s reflective of the field’s lack of knowledge. Sometimes I think we like to portray things as though we understand what’s going on, when I think it’s better to be honest and say we really don’t; we are just doing our best.”
Dr. McDougle is the director of the Lurie Center for Autism at Massachusetts General Hospital and is the Nancy Lurie Marks Professor of Psychiatry at Harvard Medical Center, both in Boston. He treats children, adolescents, and adults with autism spectrum disorder and other neurodevelopmental disorders. He was asked to comment on this study.
Children with comorbid autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are at an increased risk of anxiety and mood disorders, a cross-sectional analysis has shown.
“Our study supports that anxiety and mood disorders, although highly prevalent in those with ASD alone, are even more prevalent in individuals who have ADHD,” wrote Eliza Gordon-Lipkin, MD, of the Kennedy Krieger Institute, Baltimore, and her associates. ”The identification of psychiatric conditions in children with ASD is important because these disorders are treatable and affect quality of life.”
The study was published in Pediatrics.
Most of the children were male (83%), white (87%), and non-Hispanic (92%); the mean age of the children was 10 years. Almost half of the children in the study had parent-reported ADHD (45%). Almost one-third of patients were diagnosed with an anxiety disorder (31%), and many also were reported to have been diagnosed with a mood disorder (16%). An increased risk of reported anxiety disorder was found in patients with both ADHD and ASD (adjusted relative risk, 2.20; 95% confidence interval, 1.97-2.46).
The researchers also found an increased risk of mood disorders (aRR, 2.72; 95% CI, 2.28-3.24) among children with comorbid conditions. Those risks increased with age (both P less than .001). An increased prevalence of anxiety and mood disorders was found in adolescents, compared with school-aged children with both ASD and ADHD or ASD alone. But higher relative risk ratios were found for the younger children, compared with the adolescents for those in the ADHD/ASD group and the ASD alone group.
“This suggests that or more likely to exhibit detectable symptoms at an earlier age,” reported Dr. Gordon-Lipkin, also with the department of pediatrics at Johns Hopkins University, in Baltimore.
The research team cited several limitations. For example, patient-reported data might be subject to recall or reporting biases. Also, computer and Internet access was required to complete the IAN questionnaires, which means that the findings could be biased toward people of higher socioeconomic status.
Nevertheless, the researchers wrote, their study is the largest to compare comorbidities in patients with ASD and ADHD, or ASD alone.
Further research is needed to better understand the relationship between ASD and ADHD. “ADHD affects nearly half of the children with ASD. This subgroup of individuals with ASD may represent a distinct clinical phenotype, with different diagnostic and therapeutic implications,” Dr. Gordon-Lipkin and her associates wrote. “Better understanding the differences between children with ASD with and without ADHD is crucial to designing effective interventions.”
None of the study authors had relevant financial disclosures to report. The Interactive Autism Network is funded by the Simons Foundation and the Patient-Centered Outcomes Research Institute.
SOURCE: Gordon-Lipkin E et al. Pediatrics. 2018 Mar 30. doi: 10.1542/ peds.2017-1377.
Children with comorbid autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are at an increased risk of anxiety and mood disorders, a cross-sectional analysis has shown.
“Our study supports that anxiety and mood disorders, although highly prevalent in those with ASD alone, are even more prevalent in individuals who have ADHD,” wrote Eliza Gordon-Lipkin, MD, of the Kennedy Krieger Institute, Baltimore, and her associates. ”The identification of psychiatric conditions in children with ASD is important because these disorders are treatable and affect quality of life.”
The study was published in Pediatrics.
Most of the children were male (83%), white (87%), and non-Hispanic (92%); the mean age of the children was 10 years. Almost half of the children in the study had parent-reported ADHD (45%). Almost one-third of patients were diagnosed with an anxiety disorder (31%), and many also were reported to have been diagnosed with a mood disorder (16%). An increased risk of reported anxiety disorder was found in patients with both ADHD and ASD (adjusted relative risk, 2.20; 95% confidence interval, 1.97-2.46).
The researchers also found an increased risk of mood disorders (aRR, 2.72; 95% CI, 2.28-3.24) among children with comorbid conditions. Those risks increased with age (both P less than .001). An increased prevalence of anxiety and mood disorders was found in adolescents, compared with school-aged children with both ASD and ADHD or ASD alone. But higher relative risk ratios were found for the younger children, compared with the adolescents for those in the ADHD/ASD group and the ASD alone group.
“This suggests that or more likely to exhibit detectable symptoms at an earlier age,” reported Dr. Gordon-Lipkin, also with the department of pediatrics at Johns Hopkins University, in Baltimore.
The research team cited several limitations. For example, patient-reported data might be subject to recall or reporting biases. Also, computer and Internet access was required to complete the IAN questionnaires, which means that the findings could be biased toward people of higher socioeconomic status.
Nevertheless, the researchers wrote, their study is the largest to compare comorbidities in patients with ASD and ADHD, or ASD alone.
Further research is needed to better understand the relationship between ASD and ADHD. “ADHD affects nearly half of the children with ASD. This subgroup of individuals with ASD may represent a distinct clinical phenotype, with different diagnostic and therapeutic implications,” Dr. Gordon-Lipkin and her associates wrote. “Better understanding the differences between children with ASD with and without ADHD is crucial to designing effective interventions.”
None of the study authors had relevant financial disclosures to report. The Interactive Autism Network is funded by the Simons Foundation and the Patient-Centered Outcomes Research Institute.
SOURCE: Gordon-Lipkin E et al. Pediatrics. 2018 Mar 30. doi: 10.1542/ peds.2017-1377.
FROM PEDIATRICS
Key clinical point: “Better understanding the differences between children with ASD with and without ADHD is crucial to designing effective interventions.”
Major finding: Sixteen percent of the children with autistic spectrum disorder had a mood disorder, and 31% had an anxiety disorder.
Study details: A cross-sectional analysis of information on 3,319 patients, obtained between 2006 and 2013 in the Interactive Autism Network (IAN), an online autism research registry that uses parent report information.
Disclosures: None of the study authors reported relevant financial disclosures. The Interactive Autism Network is funded by the Simons Foundation and the Patient-Centered Outcomes Research Institute.
Source: Gordon-Lipkin E et al. Pediatrics. 2018 Mar 30. doi: 10.1542/ peds.2017-1377.
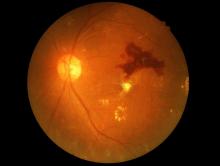
FDA approves marketing for retinal imaging device that uses artificial intelligence
The Food and Drug Administration has permitted the marketing of IDx-DR, a retinal imaging device that uses artificial intelligence (AI) to detect greater than a mild level of diabetic retinopathy in adult patients with diabetes. It also is the first authorized device that provides a screening tool without the need of an eye care specialist, making it ideal for health care providers who do not specialize in eye care.
“Early detection of retinopathy is an important part of managing care for the millions of people with diabetes, yet many patients with diabetes are not adequately screened for diabetic retinopathy.” About 50% of people with diabetes do not have the recommended annual retinopathy screening exam, Malvina Eydelman, MD, director of the division of ophthalmic, and ear, nose, and throat devices at the FDA’s Center for Devices and Radiological Health noted in a press release.
The software behind IDx-DR utilizes an artificial intelligence program to analyze retinal images captured by a retinal camera, the Topcon NW400. After one or more images are taken, they are uploaded to a Cloud server, on which IDx-DR software is installed, where they can be analyzed using an algorithm. In cases in which the images are of sufficient quality, the software provides the doctor with one of two results: “More than mild diabetic retinopathy detected: refer to an eye care professional” or “Negative for more than mild diabetic retinopathy; rescreen in 12 months.”
If a positive result is detected, patients should see an eye care specialist for further diagnostic evaluation and treatment as soon as possible.
The FDA reviewed data obtained from a clinical study prior to approving IDx-DR for marketing. The study looked at retinal images from 900 patients at 10 primary care sites. The aim of the study was to determine how often IDx-DR was correct in identifying mild diabetic retinopathy. The device was accurate nearly 90% of the time, correctly identifying mild diabetic retinopathy 87.4% of the time. It was also able to identify correctly patients who did not have mild diabetic retinopathy 89.5% of the time.
IDx-DR was approved via the FDA’s De Novo premarket review pathway, which offers a way to approve novel, low to moderate risk devices for which there are no similar devices previously approved. It also was granted a Breakthrough Device designation, because of its effectiveness in treating or diagnosing a irreversibly debilitating disease or condition.
Michael Abramoff, MD, PhD, and founder and president of IDx, said in an interview: “The FDA’s authorization to market IDx-DR is a historic moment that will launch a transformation in the way U.S. health care is delivered. Autonomous AI systems have massive potential to improve health care productivity, lower health care costs, and improve accessibility and quality. As the first of its kind to be authorized for commercialization, IDx-DR provides a roadmap for the safe and responsible use of AI in medicine.”
IDx-DR is intended to identify mild diabetic retinopathy and should not be used to detect rapidly progressive cases of diabetic retinopathy, particularly in pregnant women in which the disease can progress quickly. Patients with a history of laser treatment, surgery, or injections in the eye and other types of retinopathy, including radiation retinopathy, should not use IDx-DR.
For more information on other uses of AI in the treatment of diabetic disorders, click here.
The Food and Drug Administration has permitted the marketing of IDx-DR, a retinal imaging device that uses artificial intelligence (AI) to detect greater than a mild level of diabetic retinopathy in adult patients with diabetes. It also is the first authorized device that provides a screening tool without the need of an eye care specialist, making it ideal for health care providers who do not specialize in eye care.
“Early detection of retinopathy is an important part of managing care for the millions of people with diabetes, yet many patients with diabetes are not adequately screened for diabetic retinopathy.” About 50% of people with diabetes do not have the recommended annual retinopathy screening exam, Malvina Eydelman, MD, director of the division of ophthalmic, and ear, nose, and throat devices at the FDA’s Center for Devices and Radiological Health noted in a press release.
The software behind IDx-DR utilizes an artificial intelligence program to analyze retinal images captured by a retinal camera, the Topcon NW400. After one or more images are taken, they are uploaded to a Cloud server, on which IDx-DR software is installed, where they can be analyzed using an algorithm. In cases in which the images are of sufficient quality, the software provides the doctor with one of two results: “More than mild diabetic retinopathy detected: refer to an eye care professional” or “Negative for more than mild diabetic retinopathy; rescreen in 12 months.”
If a positive result is detected, patients should see an eye care specialist for further diagnostic evaluation and treatment as soon as possible.
The FDA reviewed data obtained from a clinical study prior to approving IDx-DR for marketing. The study looked at retinal images from 900 patients at 10 primary care sites. The aim of the study was to determine how often IDx-DR was correct in identifying mild diabetic retinopathy. The device was accurate nearly 90% of the time, correctly identifying mild diabetic retinopathy 87.4% of the time. It was also able to identify correctly patients who did not have mild diabetic retinopathy 89.5% of the time.
IDx-DR was approved via the FDA’s De Novo premarket review pathway, which offers a way to approve novel, low to moderate risk devices for which there are no similar devices previously approved. It also was granted a Breakthrough Device designation, because of its effectiveness in treating or diagnosing a irreversibly debilitating disease or condition.
Michael Abramoff, MD, PhD, and founder and president of IDx, said in an interview: “The FDA’s authorization to market IDx-DR is a historic moment that will launch a transformation in the way U.S. health care is delivered. Autonomous AI systems have massive potential to improve health care productivity, lower health care costs, and improve accessibility and quality. As the first of its kind to be authorized for commercialization, IDx-DR provides a roadmap for the safe and responsible use of AI in medicine.”
IDx-DR is intended to identify mild diabetic retinopathy and should not be used to detect rapidly progressive cases of diabetic retinopathy, particularly in pregnant women in which the disease can progress quickly. Patients with a history of laser treatment, surgery, or injections in the eye and other types of retinopathy, including radiation retinopathy, should not use IDx-DR.
For more information on other uses of AI in the treatment of diabetic disorders, click here.
The Food and Drug Administration has permitted the marketing of IDx-DR, a retinal imaging device that uses artificial intelligence (AI) to detect greater than a mild level of diabetic retinopathy in adult patients with diabetes. It also is the first authorized device that provides a screening tool without the need of an eye care specialist, making it ideal for health care providers who do not specialize in eye care.
“Early detection of retinopathy is an important part of managing care for the millions of people with diabetes, yet many patients with diabetes are not adequately screened for diabetic retinopathy.” About 50% of people with diabetes do not have the recommended annual retinopathy screening exam, Malvina Eydelman, MD, director of the division of ophthalmic, and ear, nose, and throat devices at the FDA’s Center for Devices and Radiological Health noted in a press release.
The software behind IDx-DR utilizes an artificial intelligence program to analyze retinal images captured by a retinal camera, the Topcon NW400. After one or more images are taken, they are uploaded to a Cloud server, on which IDx-DR software is installed, where they can be analyzed using an algorithm. In cases in which the images are of sufficient quality, the software provides the doctor with one of two results: “More than mild diabetic retinopathy detected: refer to an eye care professional” or “Negative for more than mild diabetic retinopathy; rescreen in 12 months.”
If a positive result is detected, patients should see an eye care specialist for further diagnostic evaluation and treatment as soon as possible.
The FDA reviewed data obtained from a clinical study prior to approving IDx-DR for marketing. The study looked at retinal images from 900 patients at 10 primary care sites. The aim of the study was to determine how often IDx-DR was correct in identifying mild diabetic retinopathy. The device was accurate nearly 90% of the time, correctly identifying mild diabetic retinopathy 87.4% of the time. It was also able to identify correctly patients who did not have mild diabetic retinopathy 89.5% of the time.
IDx-DR was approved via the FDA’s De Novo premarket review pathway, which offers a way to approve novel, low to moderate risk devices for which there are no similar devices previously approved. It also was granted a Breakthrough Device designation, because of its effectiveness in treating or diagnosing a irreversibly debilitating disease or condition.
Michael Abramoff, MD, PhD, and founder and president of IDx, said in an interview: “The FDA’s authorization to market IDx-DR is a historic moment that will launch a transformation in the way U.S. health care is delivered. Autonomous AI systems have massive potential to improve health care productivity, lower health care costs, and improve accessibility and quality. As the first of its kind to be authorized for commercialization, IDx-DR provides a roadmap for the safe and responsible use of AI in medicine.”
IDx-DR is intended to identify mild diabetic retinopathy and should not be used to detect rapidly progressive cases of diabetic retinopathy, particularly in pregnant women in which the disease can progress quickly. Patients with a history of laser treatment, surgery, or injections in the eye and other types of retinopathy, including radiation retinopathy, should not use IDx-DR.
For more information on other uses of AI in the treatment of diabetic disorders, click here.
Understanding palliative care: An important part of practicing hospital medicine
according to Brett Hendel-Paterson, MD, FHM, a hospitalist at Region’s Hospital in St. Paul, Minn. and a presenter for this session.
Dr. Hendel-Paterson, Jeffrey L. Greenwald, MD, SFHM, of Massachusetts General Hospital, Boston, and Jeffrey Frank, MD, MBA, of Vituity, will each present on the topic of administering palliative care as a hospitalist and why it is important for hospitalists to better understand this area of medicine.
A common misunderstanding about palliative care is that it is end-of-life care only, a misconception within both the medical and patient community. Most people believe that palliative care is associated with the “angel of death,” as Dr. Greenwald stated. Palliative care does encompass end-of-life care but is also associated with life-limiting illness. Both areas of palliative can be improved with better patient communication and symptom management.
As frontline providers at times of critical illness, and throughout illness, hospitalists are ideally positioned to provide palliative care services, Dr. Greenwald stated during an interview.
With hospitalists in such a prominent role in providing palliative care, Dr. Hendel-Paterson offered a detailed explanation about why the information from this session is important for hospitalists.
“The majority of Americans who die in this country die in hospitals. We see and we know that patients sometimes get more aggressive care leading to greater suffering in their final days,” he said. “As hospitalists, we are expected to be the primary physicians in the hospital caring for patients with a variety of health conditions. We are expected to have a basic expertise and be able to independently manage health conditions. For example, we are expected to be able to diagnose and treat pneumonia without consulting infectious disease or pulmonology specialists for basic care. In the same way, we must be able to communicate well with our patients and families and help lead them through discussions of prognosis and advance care planning. Primary palliative care refers to the skill set that includes communications about serious illness and basic symptom management.”
Dr. Greenwald expanded on Dr. Hendel-Paterson’s point concerning the growing need for hospitalists who are competent in palliative care.
“As the population ages, this issue is going to become more and more important for our field, because there isn’t a sufficient pipeline, current state – or predicted future state – of palliative care providers in hospitals to meet the need. So there’s a gap in the need, and that need is increasing.”
According to Dr. Hendel-Paterson, he and his copresenters “hope that, after this session, participants will better understand primary palliative care, take ownership of end-of-life care of their patients, and will be motivated to increase skills in areas where they are lacking.”
Building on this idea of increasing one’s skills as a hospitalist, he emphasized the importance of understanding palliative care.
“The ability to practice high-quality primary palliative care is essential to being a competent hospitalist.”
Primary Palliative Care – What Every Hospitalist Should Know
Wednesday, 10:00-10:40 a.m.
Crystal Ballroom J1
according to Brett Hendel-Paterson, MD, FHM, a hospitalist at Region’s Hospital in St. Paul, Minn. and a presenter for this session.
Dr. Hendel-Paterson, Jeffrey L. Greenwald, MD, SFHM, of Massachusetts General Hospital, Boston, and Jeffrey Frank, MD, MBA, of Vituity, will each present on the topic of administering palliative care as a hospitalist and why it is important for hospitalists to better understand this area of medicine.
A common misunderstanding about palliative care is that it is end-of-life care only, a misconception within both the medical and patient community. Most people believe that palliative care is associated with the “angel of death,” as Dr. Greenwald stated. Palliative care does encompass end-of-life care but is also associated with life-limiting illness. Both areas of palliative can be improved with better patient communication and symptom management.
As frontline providers at times of critical illness, and throughout illness, hospitalists are ideally positioned to provide palliative care services, Dr. Greenwald stated during an interview.
With hospitalists in such a prominent role in providing palliative care, Dr. Hendel-Paterson offered a detailed explanation about why the information from this session is important for hospitalists.
“The majority of Americans who die in this country die in hospitals. We see and we know that patients sometimes get more aggressive care leading to greater suffering in their final days,” he said. “As hospitalists, we are expected to be the primary physicians in the hospital caring for patients with a variety of health conditions. We are expected to have a basic expertise and be able to independently manage health conditions. For example, we are expected to be able to diagnose and treat pneumonia without consulting infectious disease or pulmonology specialists for basic care. In the same way, we must be able to communicate well with our patients and families and help lead them through discussions of prognosis and advance care planning. Primary palliative care refers to the skill set that includes communications about serious illness and basic symptom management.”
Dr. Greenwald expanded on Dr. Hendel-Paterson’s point concerning the growing need for hospitalists who are competent in palliative care.
“As the population ages, this issue is going to become more and more important for our field, because there isn’t a sufficient pipeline, current state – or predicted future state – of palliative care providers in hospitals to meet the need. So there’s a gap in the need, and that need is increasing.”
According to Dr. Hendel-Paterson, he and his copresenters “hope that, after this session, participants will better understand primary palliative care, take ownership of end-of-life care of their patients, and will be motivated to increase skills in areas where they are lacking.”
Building on this idea of increasing one’s skills as a hospitalist, he emphasized the importance of understanding palliative care.
“The ability to practice high-quality primary palliative care is essential to being a competent hospitalist.”
Primary Palliative Care – What Every Hospitalist Should Know
Wednesday, 10:00-10:40 a.m.
Crystal Ballroom J1
according to Brett Hendel-Paterson, MD, FHM, a hospitalist at Region’s Hospital in St. Paul, Minn. and a presenter for this session.
Dr. Hendel-Paterson, Jeffrey L. Greenwald, MD, SFHM, of Massachusetts General Hospital, Boston, and Jeffrey Frank, MD, MBA, of Vituity, will each present on the topic of administering palliative care as a hospitalist and why it is important for hospitalists to better understand this area of medicine.
A common misunderstanding about palliative care is that it is end-of-life care only, a misconception within both the medical and patient community. Most people believe that palliative care is associated with the “angel of death,” as Dr. Greenwald stated. Palliative care does encompass end-of-life care but is also associated with life-limiting illness. Both areas of palliative can be improved with better patient communication and symptom management.
As frontline providers at times of critical illness, and throughout illness, hospitalists are ideally positioned to provide palliative care services, Dr. Greenwald stated during an interview.
With hospitalists in such a prominent role in providing palliative care, Dr. Hendel-Paterson offered a detailed explanation about why the information from this session is important for hospitalists.
“The majority of Americans who die in this country die in hospitals. We see and we know that patients sometimes get more aggressive care leading to greater suffering in their final days,” he said. “As hospitalists, we are expected to be the primary physicians in the hospital caring for patients with a variety of health conditions. We are expected to have a basic expertise and be able to independently manage health conditions. For example, we are expected to be able to diagnose and treat pneumonia without consulting infectious disease or pulmonology specialists for basic care. In the same way, we must be able to communicate well with our patients and families and help lead them through discussions of prognosis and advance care planning. Primary palliative care refers to the skill set that includes communications about serious illness and basic symptom management.”
Dr. Greenwald expanded on Dr. Hendel-Paterson’s point concerning the growing need for hospitalists who are competent in palliative care.
“As the population ages, this issue is going to become more and more important for our field, because there isn’t a sufficient pipeline, current state – or predicted future state – of palliative care providers in hospitals to meet the need. So there’s a gap in the need, and that need is increasing.”
According to Dr. Hendel-Paterson, he and his copresenters “hope that, after this session, participants will better understand primary palliative care, take ownership of end-of-life care of their patients, and will be motivated to increase skills in areas where they are lacking.”
Building on this idea of increasing one’s skills as a hospitalist, he emphasized the importance of understanding palliative care.
“The ability to practice high-quality primary palliative care is essential to being a competent hospitalist.”
Primary Palliative Care – What Every Hospitalist Should Know
Wednesday, 10:00-10:40 a.m.
Crystal Ballroom J1
Myriad career options for hospitalists
The “Hospitalist Career Options” education session provided future and early-career hospitalists with information about the diversity of potential career tracks within hospital medicine.
“There are so many different things that people do and that’s what so amazing about hospital medicine,” said Dennis Chang, MD, FHM, associate professor in Mount Sinai Hospital’s division of hospital medicine, New York, in his talk on Monday. “You never really know where its going to go, and it’s really a matter of keeping your eye out for opportunities.”
Hospital medicine offers a diverse and interesting career that presents a variety of professional opportunities to those who practice it, Dr. Chang said. He noted that many hospitalists are gravitating toward careers in improving patient safety and quality improvement.
“They are working on the systems that are in the hospital and trying to make them more efficient and safer for patients,” he said.
Keeping with the theme of the talk, Dr. Chang pointed out that there a number of other specialty areas that hospitalists can explore.
“A lot of hospitalists also get into education, educating students and residents,” he said. If teaching is not your desired area of practice, you can also try your hand at “becoming CMO [chief medical officer] of a hospital” or other areas of administrative leadership or “informatics and electronic health records.” Most importantly, there are a variety of professional avenues available within hospital medicine, he added.
Dr. Chang said that the design of the session was intended to help early-career hospitalists navigate their professional path and indicated that it definitely would have provided him with some guidance. “When I was a resident thinking about what I wanted to do after residency, I didn’t necessarily know what hospital medicine was,” he said. “I think I thought it was a cool clinical job, but I didn’t understand that there were so many other things that you could do with it that are not clinical, but still really interesting.”
Dr. Chang emphasized that early-career hospitalists do not need to have a fully formed idea of the professional track they wish to pursue.
“It’s okay if you don’t know what you want to do, just do what you think is interesting and it’s amazing the things you can end up doing,” he said, noting that the best thing for residents and early-career hospitalists is “to get experience and training.”
At the end of the talk, Dr. Chang and his copresenter Daniel Ricotta, MD, offered attendees tips about other events that they might attend to advance their careers. Dr. Chang noted that SHM offers many smaller conferences that offer career development skills such as leadership.
The “Hospitalist Career Options” education session provided future and early-career hospitalists with information about the diversity of potential career tracks within hospital medicine.
“There are so many different things that people do and that’s what so amazing about hospital medicine,” said Dennis Chang, MD, FHM, associate professor in Mount Sinai Hospital’s division of hospital medicine, New York, in his talk on Monday. “You never really know where its going to go, and it’s really a matter of keeping your eye out for opportunities.”
Hospital medicine offers a diverse and interesting career that presents a variety of professional opportunities to those who practice it, Dr. Chang said. He noted that many hospitalists are gravitating toward careers in improving patient safety and quality improvement.
“They are working on the systems that are in the hospital and trying to make them more efficient and safer for patients,” he said.
Keeping with the theme of the talk, Dr. Chang pointed out that there a number of other specialty areas that hospitalists can explore.
“A lot of hospitalists also get into education, educating students and residents,” he said. If teaching is not your desired area of practice, you can also try your hand at “becoming CMO [chief medical officer] of a hospital” or other areas of administrative leadership or “informatics and electronic health records.” Most importantly, there are a variety of professional avenues available within hospital medicine, he added.
Dr. Chang said that the design of the session was intended to help early-career hospitalists navigate their professional path and indicated that it definitely would have provided him with some guidance. “When I was a resident thinking about what I wanted to do after residency, I didn’t necessarily know what hospital medicine was,” he said. “I think I thought it was a cool clinical job, but I didn’t understand that there were so many other things that you could do with it that are not clinical, but still really interesting.”
Dr. Chang emphasized that early-career hospitalists do not need to have a fully formed idea of the professional track they wish to pursue.
“It’s okay if you don’t know what you want to do, just do what you think is interesting and it’s amazing the things you can end up doing,” he said, noting that the best thing for residents and early-career hospitalists is “to get experience and training.”
At the end of the talk, Dr. Chang and his copresenter Daniel Ricotta, MD, offered attendees tips about other events that they might attend to advance their careers. Dr. Chang noted that SHM offers many smaller conferences that offer career development skills such as leadership.
The “Hospitalist Career Options” education session provided future and early-career hospitalists with information about the diversity of potential career tracks within hospital medicine.
“There are so many different things that people do and that’s what so amazing about hospital medicine,” said Dennis Chang, MD, FHM, associate professor in Mount Sinai Hospital’s division of hospital medicine, New York, in his talk on Monday. “You never really know where its going to go, and it’s really a matter of keeping your eye out for opportunities.”
Hospital medicine offers a diverse and interesting career that presents a variety of professional opportunities to those who practice it, Dr. Chang said. He noted that many hospitalists are gravitating toward careers in improving patient safety and quality improvement.
“They are working on the systems that are in the hospital and trying to make them more efficient and safer for patients,” he said.
Keeping with the theme of the talk, Dr. Chang pointed out that there a number of other specialty areas that hospitalists can explore.
“A lot of hospitalists also get into education, educating students and residents,” he said. If teaching is not your desired area of practice, you can also try your hand at “becoming CMO [chief medical officer] of a hospital” or other areas of administrative leadership or “informatics and electronic health records.” Most importantly, there are a variety of professional avenues available within hospital medicine, he added.
Dr. Chang said that the design of the session was intended to help early-career hospitalists navigate their professional path and indicated that it definitely would have provided him with some guidance. “When I was a resident thinking about what I wanted to do after residency, I didn’t necessarily know what hospital medicine was,” he said. “I think I thought it was a cool clinical job, but I didn’t understand that there were so many other things that you could do with it that are not clinical, but still really interesting.”
Dr. Chang emphasized that early-career hospitalists do not need to have a fully formed idea of the professional track they wish to pursue.
“It’s okay if you don’t know what you want to do, just do what you think is interesting and it’s amazing the things you can end up doing,” he said, noting that the best thing for residents and early-career hospitalists is “to get experience and training.”
At the end of the talk, Dr. Chang and his copresenter Daniel Ricotta, MD, offered attendees tips about other events that they might attend to advance their careers. Dr. Chang noted that SHM offers many smaller conferences that offer career development skills such as leadership.
April 2018: Click for Credit
Here are 4 articles in the April issue of Journal of Clinical Outcomes Management.
1. Young Adult HIV Patients May Be At Increased Risk of Hypertension
2. Written Exposure Therapy Rivals Cognitive Processing Therapy For PTSD
3. Large Database Analysis Suggests Safety of Bariatric Surgery in Seniors
4. Who Fares Best After Successful ECT?
Here are 4 articles in the April issue of Journal of Clinical Outcomes Management.
1. Young Adult HIV Patients May Be At Increased Risk of Hypertension
2. Written Exposure Therapy Rivals Cognitive Processing Therapy For PTSD
3. Large Database Analysis Suggests Safety of Bariatric Surgery in Seniors
4. Who Fares Best After Successful ECT?
Here are 4 articles in the April issue of Journal of Clinical Outcomes Management.
1. Young Adult HIV Patients May Be At Increased Risk of Hypertension
2. Written Exposure Therapy Rivals Cognitive Processing Therapy For PTSD
3. Large Database Analysis Suggests Safety of Bariatric Surgery in Seniors
4. Who Fares Best After Successful ECT?
Treating female-to-male transgender adolescents with acne presents unique concerns with depression
according to the results of a case series.
“Acne is a foreseeable adverse effect of testosterone treatment in transgender adolescents, and it may be advisable that, once such treatment has begun, they be monitored for the appearance of acne,” Lucia Campos-Munoz, MD, of the Hospital Clinico San Carlos in Madrid wrote in Pediatric Dermatology. “Even if only mild, treatment should be provided.”
Dr. Campos-Munoz and her colleagues examined five female-to-male transgender patients who were admitted to their clinic from 2016-2017. All five patients presented with testosterone-associated acne. Two patients with severe acne were treated with 20 mg/day of isotretinoin. While one patient tolerated this well and discontinued treatment after 4 months, another patient stopped treatment because of a bout of depression at 3 months. The remaining patients received other treatments, including doxycycline, 0.05 topical tretinoin, and 3% benzoyl peroxide.
This case study highlights the unique role that dermatologists and primary care providers play in treating acne in female-to-male transgender patients. Using the proper pronouns and recognizing that physical examinations of the chest and thorax may be especially embarrassing for these patients are important considerations, according to Dr. Campos-Munoz and her colleagues. Also, neither antiandrogenic agents nor contraceptives can be given because “this would conflict with the masculinization sought.”
Apart from being aware of the patients’ feelings, there are real medical concerns associated with dermatologic treatment of acne in female-to-male transgender patients. One of these risks is depression, which several studies have shown to be associated with severe acne. This is compounded by higher rates of depression and suicidal ideation in transgender adolescents, they said.
An additional concern is the teratogenic effects of isotretinoin in patients with natal female internal genitalia. While these patients may not think they can get pregnant because of testosterone-associated amenorrhea, the potential is still present and pregnancy should be avoided, Dr. Campos-Munoz and her colleagues warned.
No funding or conflicts of interest were disclosed.
SOURCE: Campos-Munoz L et al. Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13448.
according to the results of a case series.
“Acne is a foreseeable adverse effect of testosterone treatment in transgender adolescents, and it may be advisable that, once such treatment has begun, they be monitored for the appearance of acne,” Lucia Campos-Munoz, MD, of the Hospital Clinico San Carlos in Madrid wrote in Pediatric Dermatology. “Even if only mild, treatment should be provided.”
Dr. Campos-Munoz and her colleagues examined five female-to-male transgender patients who were admitted to their clinic from 2016-2017. All five patients presented with testosterone-associated acne. Two patients with severe acne were treated with 20 mg/day of isotretinoin. While one patient tolerated this well and discontinued treatment after 4 months, another patient stopped treatment because of a bout of depression at 3 months. The remaining patients received other treatments, including doxycycline, 0.05 topical tretinoin, and 3% benzoyl peroxide.
This case study highlights the unique role that dermatologists and primary care providers play in treating acne in female-to-male transgender patients. Using the proper pronouns and recognizing that physical examinations of the chest and thorax may be especially embarrassing for these patients are important considerations, according to Dr. Campos-Munoz and her colleagues. Also, neither antiandrogenic agents nor contraceptives can be given because “this would conflict with the masculinization sought.”
Apart from being aware of the patients’ feelings, there are real medical concerns associated with dermatologic treatment of acne in female-to-male transgender patients. One of these risks is depression, which several studies have shown to be associated with severe acne. This is compounded by higher rates of depression and suicidal ideation in transgender adolescents, they said.
An additional concern is the teratogenic effects of isotretinoin in patients with natal female internal genitalia. While these patients may not think they can get pregnant because of testosterone-associated amenorrhea, the potential is still present and pregnancy should be avoided, Dr. Campos-Munoz and her colleagues warned.
No funding or conflicts of interest were disclosed.
SOURCE: Campos-Munoz L et al. Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13448.
according to the results of a case series.
“Acne is a foreseeable adverse effect of testosterone treatment in transgender adolescents, and it may be advisable that, once such treatment has begun, they be monitored for the appearance of acne,” Lucia Campos-Munoz, MD, of the Hospital Clinico San Carlos in Madrid wrote in Pediatric Dermatology. “Even if only mild, treatment should be provided.”
Dr. Campos-Munoz and her colleagues examined five female-to-male transgender patients who were admitted to their clinic from 2016-2017. All five patients presented with testosterone-associated acne. Two patients with severe acne were treated with 20 mg/day of isotretinoin. While one patient tolerated this well and discontinued treatment after 4 months, another patient stopped treatment because of a bout of depression at 3 months. The remaining patients received other treatments, including doxycycline, 0.05 topical tretinoin, and 3% benzoyl peroxide.
This case study highlights the unique role that dermatologists and primary care providers play in treating acne in female-to-male transgender patients. Using the proper pronouns and recognizing that physical examinations of the chest and thorax may be especially embarrassing for these patients are important considerations, according to Dr. Campos-Munoz and her colleagues. Also, neither antiandrogenic agents nor contraceptives can be given because “this would conflict with the masculinization sought.”
Apart from being aware of the patients’ feelings, there are real medical concerns associated with dermatologic treatment of acne in female-to-male transgender patients. One of these risks is depression, which several studies have shown to be associated with severe acne. This is compounded by higher rates of depression and suicidal ideation in transgender adolescents, they said.
An additional concern is the teratogenic effects of isotretinoin in patients with natal female internal genitalia. While these patients may not think they can get pregnant because of testosterone-associated amenorrhea, the potential is still present and pregnancy should be avoided, Dr. Campos-Munoz and her colleagues warned.
No funding or conflicts of interest were disclosed.
SOURCE: Campos-Munoz L et al. Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13448.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: Being transgender and severe acne both are related to higher rates of depression and suicide.
Major finding: In the case study, one of the five patients discontinued use of isotretinoin because of a bout of depression.
Study details: A 2016-2017 case series of five female-to-male transgender adolescents (aged 16-18 years) who had testosterone-associated acne.
Disclosures: No funding or conflicts of interest were disclosed.
Source: Campos-Munoz L et al. Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13448.
Being overweight as a child increases the risk of developing diabetes
a time marked by decreased insulin sensitivity, or by early adulthood, according to data from a study of Danish men.
“This large-scale longitudinal study showed that men who had remission of overweight between 7 and 13 years of age and had subsequently maintained a normal weight in early adulthood had a risk of type 2 diabetes similar to that among men with normal weights at all of these ages,” Lise G. Bjerregaard, PhD, of the Center for Clinical Research and Prevention in Copenhagen and her colleagues wrote in the New England Journal of Medicine.
Studies in adults have shown that lowering body weights and body mass indexes delayed the onset of type 2 diabetes. While correcting body mass index seems to benefit adults, little is known about how being overweight or obese as children and young adults can affect the risk for type 2 diabetes. This is a particularly pressing issue because almost a quarter of children in developed countries are either overweight or obese.
The investigators looked at the heights and weights of 62,565 Danish men during childhood, measured at 7 and 13 years of age, and during early adulthood between 17 and 26 years of age. The height and weight data were obtained from two national health databases. The Copenhagen School Health Record Register (CSHRR) contains information on most of the children in Copenhagen who attended school during 1930-1989. The researchers then corroborated the information from this database and connected it with data from the Danish Conscription Database, which included heights and weights of men born during 1939-1959 that was taken at conscription examinations. In addition, diagnoses of type 2 diabetes were obtained from the Danish National Patient Register.
A little more than 10% (6,710) of the 62,565 men in the study were diagnosed with type 2 diabetes during the course of the 2 million person-years of follow-up. The prevalence of being overweight increased from 5.4% to 8.2% from age 7 years of age to early adulthood.
The risk of developing type 2 diabetes was heavily dependent on how old patients were when they were overweight and at what age they reduced their bodyweight. Being overweight at any age corresponded to an increased risk of type 2 diabetes. Men who were overweight during early adulthood had the highest incidence of type 2 diabetes. An encouraging finding was that men who had been overweight at age 7 years but had reduced their bodyweight by age 13 years and maintained a stable bodyweight as young men had a risk of being diagnosed similar to men who had never been overweight (hazard ratio, 0.96; 95% confidence interval, 0.75-1.21). Similarly, men who had been overweight only at age 13 years or between ages 7 and 13 years had a lower risks of developing type 2 diabetes than did men who had been persistently overweight, but the risks were still higher than in men who had never been overweight (overweight only at 7 and 13 years of age vs. never overweight: HR , 1.47; 95% CI, 1.10-1.9; persistently overweight vs. never overweight: HR, 4.14; 95% CI, 3.57-4.79). Men who had been overweight later in life had a higher risk of type 2 diabetes, compared with men who were only overweight as young adults, but the risk was similar to men who had been overweight at all ages.
The findings from this study are supported by the large sample size and the length of follow-up. Unfortunately, the researchers analyzed exclusively men and no information was available for early-life explanatory factors like pubertal timing and parental socioeconomic class or later-life body mass index.
The findings for this study were reason to be positive, according to, Elvira Isganaitis, MD, an assistant investigator and staff pediatric endocrinologist at the Joslin Diabetes Center in Boston.
“What I found really interesting about this study is that the authors were able to define certain periods over the life course where one’s bodyweight – being obese versus lean – seems to predict the long term risk of type 2 diabetes. And, so, it turned out that certain time periods were potentially more important than others, which is important for public health considerations and prevention. Individuals who were only overweight or obese at age 7 but had a healthy weight by age 13 and older, the risk of developing type 2 diabetes normalized. But, for those individuals in whom the overweight persisted beyond childhood, the risk was a lot stronger,” she said.
“As a pediatric endocrinologist who is really interested in obesity treatment and prevention, it is a really heartening message that our efforts to achieve healthy weight balance in early childhood has the potential to pay dividends over decades,” she added.
The results of the study offer greater insight regarding how being overweight at younger ages can influence the development of type 2 diabetes. When asked how these results from a population with broad access to health care may translate to the U.S. population, Dr. Bjerregaard stated, “The organization of the health care system and access to treatment of type 2 diabetes is not relevant if type 2 diabetes is successfully prevented by early normalization of weight. However, access to health care for the overweight pediatric population may be an important factor determining the likelihood of remission of overweight in contemporary populations who are exposed to more obesogenic environments.”
Dr. Bjerregaard and another researcher both received grants from the European Union Horizon 2020 research and innovation program. All other researchers had no financial conflicts to report. The study was supported by funding from the European Commission Horizon 2020 program as part of the DynaHEALTH project and by the European Research Council.
SOURCE: Bjerregaard L et al. N Engl J Med. 2018 Apr 04. doi: 10.1056/NEJMoa1713231.
a time marked by decreased insulin sensitivity, or by early adulthood, according to data from a study of Danish men.
“This large-scale longitudinal study showed that men who had remission of overweight between 7 and 13 years of age and had subsequently maintained a normal weight in early adulthood had a risk of type 2 diabetes similar to that among men with normal weights at all of these ages,” Lise G. Bjerregaard, PhD, of the Center for Clinical Research and Prevention in Copenhagen and her colleagues wrote in the New England Journal of Medicine.
Studies in adults have shown that lowering body weights and body mass indexes delayed the onset of type 2 diabetes. While correcting body mass index seems to benefit adults, little is known about how being overweight or obese as children and young adults can affect the risk for type 2 diabetes. This is a particularly pressing issue because almost a quarter of children in developed countries are either overweight or obese.
The investigators looked at the heights and weights of 62,565 Danish men during childhood, measured at 7 and 13 years of age, and during early adulthood between 17 and 26 years of age. The height and weight data were obtained from two national health databases. The Copenhagen School Health Record Register (CSHRR) contains information on most of the children in Copenhagen who attended school during 1930-1989. The researchers then corroborated the information from this database and connected it with data from the Danish Conscription Database, which included heights and weights of men born during 1939-1959 that was taken at conscription examinations. In addition, diagnoses of type 2 diabetes were obtained from the Danish National Patient Register.
A little more than 10% (6,710) of the 62,565 men in the study were diagnosed with type 2 diabetes during the course of the 2 million person-years of follow-up. The prevalence of being overweight increased from 5.4% to 8.2% from age 7 years of age to early adulthood.
The risk of developing type 2 diabetes was heavily dependent on how old patients were when they were overweight and at what age they reduced their bodyweight. Being overweight at any age corresponded to an increased risk of type 2 diabetes. Men who were overweight during early adulthood had the highest incidence of type 2 diabetes. An encouraging finding was that men who had been overweight at age 7 years but had reduced their bodyweight by age 13 years and maintained a stable bodyweight as young men had a risk of being diagnosed similar to men who had never been overweight (hazard ratio, 0.96; 95% confidence interval, 0.75-1.21). Similarly, men who had been overweight only at age 13 years or between ages 7 and 13 years had a lower risks of developing type 2 diabetes than did men who had been persistently overweight, but the risks were still higher than in men who had never been overweight (overweight only at 7 and 13 years of age vs. never overweight: HR , 1.47; 95% CI, 1.10-1.9; persistently overweight vs. never overweight: HR, 4.14; 95% CI, 3.57-4.79). Men who had been overweight later in life had a higher risk of type 2 diabetes, compared with men who were only overweight as young adults, but the risk was similar to men who had been overweight at all ages.
The findings from this study are supported by the large sample size and the length of follow-up. Unfortunately, the researchers analyzed exclusively men and no information was available for early-life explanatory factors like pubertal timing and parental socioeconomic class or later-life body mass index.
The findings for this study were reason to be positive, according to, Elvira Isganaitis, MD, an assistant investigator and staff pediatric endocrinologist at the Joslin Diabetes Center in Boston.
“What I found really interesting about this study is that the authors were able to define certain periods over the life course where one’s bodyweight – being obese versus lean – seems to predict the long term risk of type 2 diabetes. And, so, it turned out that certain time periods were potentially more important than others, which is important for public health considerations and prevention. Individuals who were only overweight or obese at age 7 but had a healthy weight by age 13 and older, the risk of developing type 2 diabetes normalized. But, for those individuals in whom the overweight persisted beyond childhood, the risk was a lot stronger,” she said.
“As a pediatric endocrinologist who is really interested in obesity treatment and prevention, it is a really heartening message that our efforts to achieve healthy weight balance in early childhood has the potential to pay dividends over decades,” she added.
The results of the study offer greater insight regarding how being overweight at younger ages can influence the development of type 2 diabetes. When asked how these results from a population with broad access to health care may translate to the U.S. population, Dr. Bjerregaard stated, “The organization of the health care system and access to treatment of type 2 diabetes is not relevant if type 2 diabetes is successfully prevented by early normalization of weight. However, access to health care for the overweight pediatric population may be an important factor determining the likelihood of remission of overweight in contemporary populations who are exposed to more obesogenic environments.”
Dr. Bjerregaard and another researcher both received grants from the European Union Horizon 2020 research and innovation program. All other researchers had no financial conflicts to report. The study was supported by funding from the European Commission Horizon 2020 program as part of the DynaHEALTH project and by the European Research Council.
SOURCE: Bjerregaard L et al. N Engl J Med. 2018 Apr 04. doi: 10.1056/NEJMoa1713231.
a time marked by decreased insulin sensitivity, or by early adulthood, according to data from a study of Danish men.
“This large-scale longitudinal study showed that men who had remission of overweight between 7 and 13 years of age and had subsequently maintained a normal weight in early adulthood had a risk of type 2 diabetes similar to that among men with normal weights at all of these ages,” Lise G. Bjerregaard, PhD, of the Center for Clinical Research and Prevention in Copenhagen and her colleagues wrote in the New England Journal of Medicine.
Studies in adults have shown that lowering body weights and body mass indexes delayed the onset of type 2 diabetes. While correcting body mass index seems to benefit adults, little is known about how being overweight or obese as children and young adults can affect the risk for type 2 diabetes. This is a particularly pressing issue because almost a quarter of children in developed countries are either overweight or obese.
The investigators looked at the heights and weights of 62,565 Danish men during childhood, measured at 7 and 13 years of age, and during early adulthood between 17 and 26 years of age. The height and weight data were obtained from two national health databases. The Copenhagen School Health Record Register (CSHRR) contains information on most of the children in Copenhagen who attended school during 1930-1989. The researchers then corroborated the information from this database and connected it with data from the Danish Conscription Database, which included heights and weights of men born during 1939-1959 that was taken at conscription examinations. In addition, diagnoses of type 2 diabetes were obtained from the Danish National Patient Register.
A little more than 10% (6,710) of the 62,565 men in the study were diagnosed with type 2 diabetes during the course of the 2 million person-years of follow-up. The prevalence of being overweight increased from 5.4% to 8.2% from age 7 years of age to early adulthood.
The risk of developing type 2 diabetes was heavily dependent on how old patients were when they were overweight and at what age they reduced their bodyweight. Being overweight at any age corresponded to an increased risk of type 2 diabetes. Men who were overweight during early adulthood had the highest incidence of type 2 diabetes. An encouraging finding was that men who had been overweight at age 7 years but had reduced their bodyweight by age 13 years and maintained a stable bodyweight as young men had a risk of being diagnosed similar to men who had never been overweight (hazard ratio, 0.96; 95% confidence interval, 0.75-1.21). Similarly, men who had been overweight only at age 13 years or between ages 7 and 13 years had a lower risks of developing type 2 diabetes than did men who had been persistently overweight, but the risks were still higher than in men who had never been overweight (overweight only at 7 and 13 years of age vs. never overweight: HR , 1.47; 95% CI, 1.10-1.9; persistently overweight vs. never overweight: HR, 4.14; 95% CI, 3.57-4.79). Men who had been overweight later in life had a higher risk of type 2 diabetes, compared with men who were only overweight as young adults, but the risk was similar to men who had been overweight at all ages.
The findings from this study are supported by the large sample size and the length of follow-up. Unfortunately, the researchers analyzed exclusively men and no information was available for early-life explanatory factors like pubertal timing and parental socioeconomic class or later-life body mass index.
The findings for this study were reason to be positive, according to, Elvira Isganaitis, MD, an assistant investigator and staff pediatric endocrinologist at the Joslin Diabetes Center in Boston.
“What I found really interesting about this study is that the authors were able to define certain periods over the life course where one’s bodyweight – being obese versus lean – seems to predict the long term risk of type 2 diabetes. And, so, it turned out that certain time periods were potentially more important than others, which is important for public health considerations and prevention. Individuals who were only overweight or obese at age 7 but had a healthy weight by age 13 and older, the risk of developing type 2 diabetes normalized. But, for those individuals in whom the overweight persisted beyond childhood, the risk was a lot stronger,” she said.
“As a pediatric endocrinologist who is really interested in obesity treatment and prevention, it is a really heartening message that our efforts to achieve healthy weight balance in early childhood has the potential to pay dividends over decades,” she added.
The results of the study offer greater insight regarding how being overweight at younger ages can influence the development of type 2 diabetes. When asked how these results from a population with broad access to health care may translate to the U.S. population, Dr. Bjerregaard stated, “The organization of the health care system and access to treatment of type 2 diabetes is not relevant if type 2 diabetes is successfully prevented by early normalization of weight. However, access to health care for the overweight pediatric population may be an important factor determining the likelihood of remission of overweight in contemporary populations who are exposed to more obesogenic environments.”
Dr. Bjerregaard and another researcher both received grants from the European Union Horizon 2020 research and innovation program. All other researchers had no financial conflicts to report. The study was supported by funding from the European Commission Horizon 2020 program as part of the DynaHEALTH project and by the European Research Council.
SOURCE: Bjerregaard L et al. N Engl J Med. 2018 Apr 04. doi: 10.1056/NEJMoa1713231.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Being overweight in childhood was associated with an increased risk of type 2 diabetes in adulthood unless weight is normalized before puberty.
Major finding: Men who had been overweight age 7 years but had reduced their body weight by age 13 years and maintained a stable body weight as young men had a risk of being diagnosed with diabetes similar to that among men who had never been overweight (hazard ratio, 0.96; 95% confidence interval, 0.75-1.21).
Study details: Using information from several databases, researchers looked at the heights and weights of 62,565 Danish men from childhood and young adulthood.
Disclosures: Dr. Bjerregaard and another researcher both received grants from the European Union Horizon 2020 research and innovation program. All other researchers had no financial conflicts to report.
Source: Bjerregaard L et al. N Engl J Med. 2018 Apr 04. doi: 10.1056/NEJMoa1713231.
Unusual antibiotic resistance found in more than 200 bacteria
The Centers for Disease Control and Prevention’s Antibiotic Resistance (AR) Lab Network has detected 221 instances of bacteria with especially rare resistance genes in the United States, according to a Vital Signs report published online and expanded upon in CDC’s MMWR Weekly.
The MMWR Weekly report, “Containment of Novel Multidrug-Resistant Organisms and Resistance Mechanisms,” which goes deeper into the science behind the issue, shows that in 9 months, in all states and Puerto Rico, health department workers in the AR lab network tested 5,776 samples of highly resistant bacteria, according to Anne Schuchat, MD, principal deputy director of the CDC. These bacteria were immediately tested for unusual resistance – “those genes that were highly resistant, or rare, with special resistance that could spread,” she said.
“Of the 5,776, about 1 in 4 of the bacteria had a gene that helped it spread its resistance. And there were 221 instances of an especially rare resistance gene,” she added. This prompted intense screening, revealing “that 1 in 10 tests were also positive. Meaning the unusual resistance may have spread to other patients. And could have continued spreading if left undetected.”
The report looked at carbapenem-resistant Enterobacteriaceae (CRE) and Enterobacteriaceae with extended-spectrum beta-lactamases (ESBL) infection data from the National Healthcare Safety Network from 2006-2015 to calculate changes in the year over year proportions of these infections and how an enhanced detection and control strategy curbs carbapenem resistance.
This strategy includes components such as timely implementation of appropriate infection control measures, conducting a health care and contact investigation with follow-up, and implementing a system to ensure adherence to infection control measures.
“With independent, or single facility approaches to control spread, a dangerous type of unusual resistance in Enterobacteriaceae [ESBL phenotype] decreased by about 2% per year [(risk ratio [RR] = 0.98, P less than .001)].” With a more aggressive approach, using guidance such as CDC’s CRE toolkit, released in 2009, another type of unusual resistance [CRE] in the same bacteria (Enterobacteriaceae) decreased by nearly 15% per year (RR = 0.85, P less than .01).
The difference may be due in part to the more directed response utilized to slow the spread of the “nightmare bacteria,” once it was identified, said Dr. Schuchat.
These results show massive promise even if only partially effective, specifically for CRE.
“CDC estimates show that if only 20% effective, the containment strategy can reduce the number of nightmare bacteria [CRE] cases by 76% over 3 years in one area.”
Due to the nature of antibiotic resistance and its ability to spread, this poses a significant public health threat. Antibiotics are not simply used to treat infections but are a safety net that is used in cancer treatment, surgery, and ICU care, Dr. Schuchat pointed out. The rise of antibiotic resistance is a threat to that safety net and accounts for nearly 2,000,000 antibiotic resistant infections and approximately 23,000 deaths per year.
But aggressive responses to these infections can control their spread. Dr. Schuchat used the analogy of controlling a fire to illustrate the concept.
“Much like a fire, finding and stopping unusual resistance early when it’s just a spark protects people.”
Dr. Schuchat reiterated that simply identifying the issue is only part of the equation.
“Detection is not enough on its own. When there is a fire, somebody needs to put it out. CDC supports more than 500 local staff across the country to combat antibiotic resistance wherever it emerges.”
While the report highlights the strides that have been made in combating antibiotic resistance, Paul Auwaerter, MD, president of the Infectious Diseases Society of America, released a statement highlighting the need to further fund these efforts.
“The report spells out the need to accelerate efforts to curb resistance or face an increasing burden including novel resistance mutations that threaten health,” stated Dr. Auwaerter. “The efforts detailed in the Vital Signs report were made possible through new congressional funding in 2016 to combat antibiotic resistance. We urge Congress to sustain and to grow that investment so that further progress will prepare us to meet the future challenges of antibiotic resistance from a position of strength,” he added.
A fact sheet with a brief summation of the vital signs report is available here.
The Centers for Disease Control and Prevention’s Antibiotic Resistance (AR) Lab Network has detected 221 instances of bacteria with especially rare resistance genes in the United States, according to a Vital Signs report published online and expanded upon in CDC’s MMWR Weekly.
The MMWR Weekly report, “Containment of Novel Multidrug-Resistant Organisms and Resistance Mechanisms,” which goes deeper into the science behind the issue, shows that in 9 months, in all states and Puerto Rico, health department workers in the AR lab network tested 5,776 samples of highly resistant bacteria, according to Anne Schuchat, MD, principal deputy director of the CDC. These bacteria were immediately tested for unusual resistance – “those genes that were highly resistant, or rare, with special resistance that could spread,” she said.
“Of the 5,776, about 1 in 4 of the bacteria had a gene that helped it spread its resistance. And there were 221 instances of an especially rare resistance gene,” she added. This prompted intense screening, revealing “that 1 in 10 tests were also positive. Meaning the unusual resistance may have spread to other patients. And could have continued spreading if left undetected.”
The report looked at carbapenem-resistant Enterobacteriaceae (CRE) and Enterobacteriaceae with extended-spectrum beta-lactamases (ESBL) infection data from the National Healthcare Safety Network from 2006-2015 to calculate changes in the year over year proportions of these infections and how an enhanced detection and control strategy curbs carbapenem resistance.
This strategy includes components such as timely implementation of appropriate infection control measures, conducting a health care and contact investigation with follow-up, and implementing a system to ensure adherence to infection control measures.
“With independent, or single facility approaches to control spread, a dangerous type of unusual resistance in Enterobacteriaceae [ESBL phenotype] decreased by about 2% per year [(risk ratio [RR] = 0.98, P less than .001)].” With a more aggressive approach, using guidance such as CDC’s CRE toolkit, released in 2009, another type of unusual resistance [CRE] in the same bacteria (Enterobacteriaceae) decreased by nearly 15% per year (RR = 0.85, P less than .01).
The difference may be due in part to the more directed response utilized to slow the spread of the “nightmare bacteria,” once it was identified, said Dr. Schuchat.
These results show massive promise even if only partially effective, specifically for CRE.
“CDC estimates show that if only 20% effective, the containment strategy can reduce the number of nightmare bacteria [CRE] cases by 76% over 3 years in one area.”
Due to the nature of antibiotic resistance and its ability to spread, this poses a significant public health threat. Antibiotics are not simply used to treat infections but are a safety net that is used in cancer treatment, surgery, and ICU care, Dr. Schuchat pointed out. The rise of antibiotic resistance is a threat to that safety net and accounts for nearly 2,000,000 antibiotic resistant infections and approximately 23,000 deaths per year.
But aggressive responses to these infections can control their spread. Dr. Schuchat used the analogy of controlling a fire to illustrate the concept.
“Much like a fire, finding and stopping unusual resistance early when it’s just a spark protects people.”
Dr. Schuchat reiterated that simply identifying the issue is only part of the equation.
“Detection is not enough on its own. When there is a fire, somebody needs to put it out. CDC supports more than 500 local staff across the country to combat antibiotic resistance wherever it emerges.”
While the report highlights the strides that have been made in combating antibiotic resistance, Paul Auwaerter, MD, president of the Infectious Diseases Society of America, released a statement highlighting the need to further fund these efforts.
“The report spells out the need to accelerate efforts to curb resistance or face an increasing burden including novel resistance mutations that threaten health,” stated Dr. Auwaerter. “The efforts detailed in the Vital Signs report were made possible through new congressional funding in 2016 to combat antibiotic resistance. We urge Congress to sustain and to grow that investment so that further progress will prepare us to meet the future challenges of antibiotic resistance from a position of strength,” he added.
A fact sheet with a brief summation of the vital signs report is available here.
The Centers for Disease Control and Prevention’s Antibiotic Resistance (AR) Lab Network has detected 221 instances of bacteria with especially rare resistance genes in the United States, according to a Vital Signs report published online and expanded upon in CDC’s MMWR Weekly.
The MMWR Weekly report, “Containment of Novel Multidrug-Resistant Organisms and Resistance Mechanisms,” which goes deeper into the science behind the issue, shows that in 9 months, in all states and Puerto Rico, health department workers in the AR lab network tested 5,776 samples of highly resistant bacteria, according to Anne Schuchat, MD, principal deputy director of the CDC. These bacteria were immediately tested for unusual resistance – “those genes that were highly resistant, or rare, with special resistance that could spread,” she said.
“Of the 5,776, about 1 in 4 of the bacteria had a gene that helped it spread its resistance. And there were 221 instances of an especially rare resistance gene,” she added. This prompted intense screening, revealing “that 1 in 10 tests were also positive. Meaning the unusual resistance may have spread to other patients. And could have continued spreading if left undetected.”
The report looked at carbapenem-resistant Enterobacteriaceae (CRE) and Enterobacteriaceae with extended-spectrum beta-lactamases (ESBL) infection data from the National Healthcare Safety Network from 2006-2015 to calculate changes in the year over year proportions of these infections and how an enhanced detection and control strategy curbs carbapenem resistance.
This strategy includes components such as timely implementation of appropriate infection control measures, conducting a health care and contact investigation with follow-up, and implementing a system to ensure adherence to infection control measures.
“With independent, or single facility approaches to control spread, a dangerous type of unusual resistance in Enterobacteriaceae [ESBL phenotype] decreased by about 2% per year [(risk ratio [RR] = 0.98, P less than .001)].” With a more aggressive approach, using guidance such as CDC’s CRE toolkit, released in 2009, another type of unusual resistance [CRE] in the same bacteria (Enterobacteriaceae) decreased by nearly 15% per year (RR = 0.85, P less than .01).
The difference may be due in part to the more directed response utilized to slow the spread of the “nightmare bacteria,” once it was identified, said Dr. Schuchat.
These results show massive promise even if only partially effective, specifically for CRE.
“CDC estimates show that if only 20% effective, the containment strategy can reduce the number of nightmare bacteria [CRE] cases by 76% over 3 years in one area.”
Due to the nature of antibiotic resistance and its ability to spread, this poses a significant public health threat. Antibiotics are not simply used to treat infections but are a safety net that is used in cancer treatment, surgery, and ICU care, Dr. Schuchat pointed out. The rise of antibiotic resistance is a threat to that safety net and accounts for nearly 2,000,000 antibiotic resistant infections and approximately 23,000 deaths per year.
But aggressive responses to these infections can control their spread. Dr. Schuchat used the analogy of controlling a fire to illustrate the concept.
“Much like a fire, finding and stopping unusual resistance early when it’s just a spark protects people.”
Dr. Schuchat reiterated that simply identifying the issue is only part of the equation.
“Detection is not enough on its own. When there is a fire, somebody needs to put it out. CDC supports more than 500 local staff across the country to combat antibiotic resistance wherever it emerges.”
While the report highlights the strides that have been made in combating antibiotic resistance, Paul Auwaerter, MD, president of the Infectious Diseases Society of America, released a statement highlighting the need to further fund these efforts.
“The report spells out the need to accelerate efforts to curb resistance or face an increasing burden including novel resistance mutations that threaten health,” stated Dr. Auwaerter. “The efforts detailed in the Vital Signs report were made possible through new congressional funding in 2016 to combat antibiotic resistance. We urge Congress to sustain and to grow that investment so that further progress will prepare us to meet the future challenges of antibiotic resistance from a position of strength,” he added.
A fact sheet with a brief summation of the vital signs report is available here.
FROM A CDC TELEBRIEFING