User login
Prior bariatric surgery may lead to IBD
.
“A past history of bariatric surgery, but not recent surgery, was associated with both new-onset” Crohn’s disease and ulcerative colitis, wrote Ryan Ungaro, MD, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “Analyses further classifying recent bariatric surgery as preserved or altered intestinal anatomy showed no significant difference in risk … when compared to patients without a history of bariatric surgery.”
IBD and more specific conditions like ulcerative colitis (UC) and Crohn’s disease (CD) are on the rise. One environmental factor that can increase the likelihood of these disorders is surgery. To investigate how IBD may develop in response to bariatric surgery, Dr. Ungaro and his colleagues compiled descriptive case studies from six (three New York, three European) medical centers, then conducted a case-control study with a sample of patients from a large health claims database (Symphony Health Solutions Integrated Dataverse) to assess the association between prior bariatric surgery and risk of new-onset IBD. Using the information from the Symphony database, a database focusing exclusively on IBD patients was developed.
From the case studies included in the study, Dr. Ungaro and his colleagues identified 15 patients with IBD who had a prior history of bariatric surgery: 10 patients had CD, 4 had UC, and 1 had an unclassified form of IBD. Most of the patients had undergone Roux-en-Y gastroenterostomy. The average time between surgery and diagnosis of IBD was 5.7 years. Most of the cases (67%) were mild or moderate in severity and over half (53.3%) of the cases were mildly active. Only about a quarter of these patients required immunosuppressant therapy.
The database portion of the study also revealed some interesting associations between bariatric surgery and IBD. The researchers identified 8,980 patients with IBD and 43,059 controls. Ultimately, Dr. Ungaro and his colleagues found that any type of bariatric surgery increased the odds of developing IBD (adjusted odds ratio, 1.45; 95% confidence interval, 1.08-1.94). Interestingly, only past history of bariatric surgery was associated with an almost twofold increase in onset of IBD. The study also revealed that bariatric surgeries prior to 2008 were associated with an increase chance of developing IBD.
Bariatric surgery was not limited to increasing the risk of IBD, but also increased the specific risks for CD and UC. A past history of bariatric surgery was heavily associated with an increase of both CD (aOR,1.86; 95% CI,1.10-3.15; P = .066) and UC (aOR, 2.12; 95% CI, 1.26-3.57; P = .015).
The size of the sample and utilization of a large, national database strengthen the results of the study, but the use of retrospective data is a potential limitation. The deidentified nature of the data also makes it difficult to validate cases and determine whether new cases of IBD were definitely incident cases.
Regardless, there is still no clear-cut explanation why bariatric surgery would cause IBD. One of the proposed mechanisms is vitamin D deficiency caused by malabsorption, a common consequence of the Roux-en-Y procedure. Vitamin D deficiency is often linked with an increased risk of developing IBD. Additionally, bariatric surgery may alter bile acid composition, which can have inflammatory effects.
Because no direct mechanism has been identified, Dr. Ungaro stated that further research needs to be done.
“The potential association between prior bariatric surgery and new IBD highlights the need to perform a thorough work-up and have a broad differential diagnosis in postbariatric surgery patients with new gastrointestinal symptoms,” Dr. Ungaro and his colleagues wrote. “However, given the nature of administrative database studies, we cannot draw causative conclusions and further prospective studies are needed to confirm this association and delineate if certain types of bariatric surgeries have differential effects on risk of IBD.”
Multiple authors who worked on this study have worked as consultants for pharmaceutical companies. Dr. Ungaro is supported by a Crohn’s and Colitis Foundation career development award and a KL2 Scholar award.
SOURCE: Ungaro R et al. Aliment Pharmacol Ther. 2018 Mar 7;47[8]:1126-34: doi: 10.1111/apt.14569.
.
“A past history of bariatric surgery, but not recent surgery, was associated with both new-onset” Crohn’s disease and ulcerative colitis, wrote Ryan Ungaro, MD, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “Analyses further classifying recent bariatric surgery as preserved or altered intestinal anatomy showed no significant difference in risk … when compared to patients without a history of bariatric surgery.”
IBD and more specific conditions like ulcerative colitis (UC) and Crohn’s disease (CD) are on the rise. One environmental factor that can increase the likelihood of these disorders is surgery. To investigate how IBD may develop in response to bariatric surgery, Dr. Ungaro and his colleagues compiled descriptive case studies from six (three New York, three European) medical centers, then conducted a case-control study with a sample of patients from a large health claims database (Symphony Health Solutions Integrated Dataverse) to assess the association between prior bariatric surgery and risk of new-onset IBD. Using the information from the Symphony database, a database focusing exclusively on IBD patients was developed.
From the case studies included in the study, Dr. Ungaro and his colleagues identified 15 patients with IBD who had a prior history of bariatric surgery: 10 patients had CD, 4 had UC, and 1 had an unclassified form of IBD. Most of the patients had undergone Roux-en-Y gastroenterostomy. The average time between surgery and diagnosis of IBD was 5.7 years. Most of the cases (67%) were mild or moderate in severity and over half (53.3%) of the cases were mildly active. Only about a quarter of these patients required immunosuppressant therapy.
The database portion of the study also revealed some interesting associations between bariatric surgery and IBD. The researchers identified 8,980 patients with IBD and 43,059 controls. Ultimately, Dr. Ungaro and his colleagues found that any type of bariatric surgery increased the odds of developing IBD (adjusted odds ratio, 1.45; 95% confidence interval, 1.08-1.94). Interestingly, only past history of bariatric surgery was associated with an almost twofold increase in onset of IBD. The study also revealed that bariatric surgeries prior to 2008 were associated with an increase chance of developing IBD.
Bariatric surgery was not limited to increasing the risk of IBD, but also increased the specific risks for CD and UC. A past history of bariatric surgery was heavily associated with an increase of both CD (aOR,1.86; 95% CI,1.10-3.15; P = .066) and UC (aOR, 2.12; 95% CI, 1.26-3.57; P = .015).
The size of the sample and utilization of a large, national database strengthen the results of the study, but the use of retrospective data is a potential limitation. The deidentified nature of the data also makes it difficult to validate cases and determine whether new cases of IBD were definitely incident cases.
Regardless, there is still no clear-cut explanation why bariatric surgery would cause IBD. One of the proposed mechanisms is vitamin D deficiency caused by malabsorption, a common consequence of the Roux-en-Y procedure. Vitamin D deficiency is often linked with an increased risk of developing IBD. Additionally, bariatric surgery may alter bile acid composition, which can have inflammatory effects.
Because no direct mechanism has been identified, Dr. Ungaro stated that further research needs to be done.
“The potential association between prior bariatric surgery and new IBD highlights the need to perform a thorough work-up and have a broad differential diagnosis in postbariatric surgery patients with new gastrointestinal symptoms,” Dr. Ungaro and his colleagues wrote. “However, given the nature of administrative database studies, we cannot draw causative conclusions and further prospective studies are needed to confirm this association and delineate if certain types of bariatric surgeries have differential effects on risk of IBD.”
Multiple authors who worked on this study have worked as consultants for pharmaceutical companies. Dr. Ungaro is supported by a Crohn’s and Colitis Foundation career development award and a KL2 Scholar award.
SOURCE: Ungaro R et al. Aliment Pharmacol Ther. 2018 Mar 7;47[8]:1126-34: doi: 10.1111/apt.14569.
.
“A past history of bariatric surgery, but not recent surgery, was associated with both new-onset” Crohn’s disease and ulcerative colitis, wrote Ryan Ungaro, MD, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “Analyses further classifying recent bariatric surgery as preserved or altered intestinal anatomy showed no significant difference in risk … when compared to patients without a history of bariatric surgery.”
IBD and more specific conditions like ulcerative colitis (UC) and Crohn’s disease (CD) are on the rise. One environmental factor that can increase the likelihood of these disorders is surgery. To investigate how IBD may develop in response to bariatric surgery, Dr. Ungaro and his colleagues compiled descriptive case studies from six (three New York, three European) medical centers, then conducted a case-control study with a sample of patients from a large health claims database (Symphony Health Solutions Integrated Dataverse) to assess the association between prior bariatric surgery and risk of new-onset IBD. Using the information from the Symphony database, a database focusing exclusively on IBD patients was developed.
From the case studies included in the study, Dr. Ungaro and his colleagues identified 15 patients with IBD who had a prior history of bariatric surgery: 10 patients had CD, 4 had UC, and 1 had an unclassified form of IBD. Most of the patients had undergone Roux-en-Y gastroenterostomy. The average time between surgery and diagnosis of IBD was 5.7 years. Most of the cases (67%) were mild or moderate in severity and over half (53.3%) of the cases were mildly active. Only about a quarter of these patients required immunosuppressant therapy.
The database portion of the study also revealed some interesting associations between bariatric surgery and IBD. The researchers identified 8,980 patients with IBD and 43,059 controls. Ultimately, Dr. Ungaro and his colleagues found that any type of bariatric surgery increased the odds of developing IBD (adjusted odds ratio, 1.45; 95% confidence interval, 1.08-1.94). Interestingly, only past history of bariatric surgery was associated with an almost twofold increase in onset of IBD. The study also revealed that bariatric surgeries prior to 2008 were associated with an increase chance of developing IBD.
Bariatric surgery was not limited to increasing the risk of IBD, but also increased the specific risks for CD and UC. A past history of bariatric surgery was heavily associated with an increase of both CD (aOR,1.86; 95% CI,1.10-3.15; P = .066) and UC (aOR, 2.12; 95% CI, 1.26-3.57; P = .015).
The size of the sample and utilization of a large, national database strengthen the results of the study, but the use of retrospective data is a potential limitation. The deidentified nature of the data also makes it difficult to validate cases and determine whether new cases of IBD were definitely incident cases.
Regardless, there is still no clear-cut explanation why bariatric surgery would cause IBD. One of the proposed mechanisms is vitamin D deficiency caused by malabsorption, a common consequence of the Roux-en-Y procedure. Vitamin D deficiency is often linked with an increased risk of developing IBD. Additionally, bariatric surgery may alter bile acid composition, which can have inflammatory effects.
Because no direct mechanism has been identified, Dr. Ungaro stated that further research needs to be done.
“The potential association between prior bariatric surgery and new IBD highlights the need to perform a thorough work-up and have a broad differential diagnosis in postbariatric surgery patients with new gastrointestinal symptoms,” Dr. Ungaro and his colleagues wrote. “However, given the nature of administrative database studies, we cannot draw causative conclusions and further prospective studies are needed to confirm this association and delineate if certain types of bariatric surgeries have differential effects on risk of IBD.”
Multiple authors who worked on this study have worked as consultants for pharmaceutical companies. Dr. Ungaro is supported by a Crohn’s and Colitis Foundation career development award and a KL2 Scholar award.
SOURCE: Ungaro R et al. Aliment Pharmacol Ther. 2018 Mar 7;47[8]:1126-34: doi: 10.1111/apt.14569.
FROM ALIMENTARY PHARMACOLOGY AND THERAPEUTICS
Key clinical point: Bariatric surgery is associated with an increase of bowel disorders.
Major finding: Any type of bariatric surgery increased the odds of developing IBD (aOR 1.45; 95% CI, 1.08-1.94).
Study details: A multicenter case study analysis and a case-control study using a medical claims database from 2008 to 2012.
Disclosures: Multiple authors who worked on this study have worked as consultants for pharmaceutical companies.
Source: Ungaro R et al. Aliment Pharmacol Ther. 2018 Mar 7;47[8]:1126-34. doi: 10.1111/apt.14569.
The Resident and Student Luncheon exposes future hospitalists to professional possibilities
“Trainees can ask hospitalists who are administrative leaders, QI gurus, medical educators, global health hospitalists, pediatricians, and researchers about their day-to-day life and what they love about their careers,” stated Darlene B. Tad-y, MD, SFHM, who is an associate professor and hospitalist at the University of Colorado Hospital, Denver. “It’s a great way for trainees to build their network in hospital medicine in addition to learning about the diverse careers available in our field.”
The luncheon is structured in such a way to maximizes attendees’ exposure and interaction with experienced hospitalists. Brian Kwan, MD, FHM, an associate professor of health science at the University of California, San Diego, and a hospitalist, elaborated on the sessions design.
“Each round table features a speaker that will highlight a different topic, including but not limited to medical education, executive leadership, global and rural health, quality improvement, advocacy, and informatics,” said Dr. Kwan.
But the session is not limited to a one-way presentation. “Conversations are facilitated by members of the SHM Physicians in Training (PIT) Committee,” he said. To maximize their exposure, “attendees will have an opportunity to select one table for the main meal and a different one for dessert, so that they have the opportunity to hear from more than one speaker.”
Dr. Kwan and Dr. Tad-y said they believe that this type of exposure is important in the professional development of medical students and residents.
“We believe it is critical for students and residents to be exposed to hospitalists working at the forefront of our field who inspire and can provide a glimpse at different HM career paths and practices. Invited speakers are selected by the PIT Committee, and the luncheon serves as a launching point for networking and potential mentorship. Engaging residents and students is critical to sustaining our pipeline for future hospitalist leaders,” Dr. Kwan said.
Dr. Tad-y stated that this could be a defining professional moment for many of the attendees. “The resident or student may even meet their next project or career mentor, as well as potential peers or project partners.”
Resident and Student Luncheon
April 9, Monday, 12-1 p.m.
New York/New Orleans Room
“Trainees can ask hospitalists who are administrative leaders, QI gurus, medical educators, global health hospitalists, pediatricians, and researchers about their day-to-day life and what they love about their careers,” stated Darlene B. Tad-y, MD, SFHM, who is an associate professor and hospitalist at the University of Colorado Hospital, Denver. “It’s a great way for trainees to build their network in hospital medicine in addition to learning about the diverse careers available in our field.”
The luncheon is structured in such a way to maximizes attendees’ exposure and interaction with experienced hospitalists. Brian Kwan, MD, FHM, an associate professor of health science at the University of California, San Diego, and a hospitalist, elaborated on the sessions design.
“Each round table features a speaker that will highlight a different topic, including but not limited to medical education, executive leadership, global and rural health, quality improvement, advocacy, and informatics,” said Dr. Kwan.
But the session is not limited to a one-way presentation. “Conversations are facilitated by members of the SHM Physicians in Training (PIT) Committee,” he said. To maximize their exposure, “attendees will have an opportunity to select one table for the main meal and a different one for dessert, so that they have the opportunity to hear from more than one speaker.”
Dr. Kwan and Dr. Tad-y said they believe that this type of exposure is important in the professional development of medical students and residents.
“We believe it is critical for students and residents to be exposed to hospitalists working at the forefront of our field who inspire and can provide a glimpse at different HM career paths and practices. Invited speakers are selected by the PIT Committee, and the luncheon serves as a launching point for networking and potential mentorship. Engaging residents and students is critical to sustaining our pipeline for future hospitalist leaders,” Dr. Kwan said.
Dr. Tad-y stated that this could be a defining professional moment for many of the attendees. “The resident or student may even meet their next project or career mentor, as well as potential peers or project partners.”
Resident and Student Luncheon
April 9, Monday, 12-1 p.m.
New York/New Orleans Room
“Trainees can ask hospitalists who are administrative leaders, QI gurus, medical educators, global health hospitalists, pediatricians, and researchers about their day-to-day life and what they love about their careers,” stated Darlene B. Tad-y, MD, SFHM, who is an associate professor and hospitalist at the University of Colorado Hospital, Denver. “It’s a great way for trainees to build their network in hospital medicine in addition to learning about the diverse careers available in our field.”
The luncheon is structured in such a way to maximizes attendees’ exposure and interaction with experienced hospitalists. Brian Kwan, MD, FHM, an associate professor of health science at the University of California, San Diego, and a hospitalist, elaborated on the sessions design.
“Each round table features a speaker that will highlight a different topic, including but not limited to medical education, executive leadership, global and rural health, quality improvement, advocacy, and informatics,” said Dr. Kwan.
But the session is not limited to a one-way presentation. “Conversations are facilitated by members of the SHM Physicians in Training (PIT) Committee,” he said. To maximize their exposure, “attendees will have an opportunity to select one table for the main meal and a different one for dessert, so that they have the opportunity to hear from more than one speaker.”
Dr. Kwan and Dr. Tad-y said they believe that this type of exposure is important in the professional development of medical students and residents.
“We believe it is critical for students and residents to be exposed to hospitalists working at the forefront of our field who inspire and can provide a glimpse at different HM career paths and practices. Invited speakers are selected by the PIT Committee, and the luncheon serves as a launching point for networking and potential mentorship. Engaging residents and students is critical to sustaining our pipeline for future hospitalist leaders,” Dr. Kwan said.
Dr. Tad-y stated that this could be a defining professional moment for many of the attendees. “The resident or student may even meet their next project or career mentor, as well as potential peers or project partners.”
Resident and Student Luncheon
April 9, Monday, 12-1 p.m.
New York/New Orleans Room
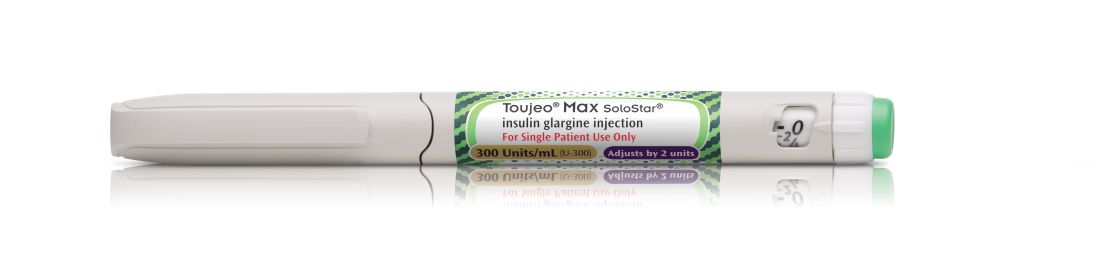
FDA approves highest capacity insulin pen
This will be the highest capacity long-acting insulin pen to be available commercially, according to a company press release.
The Max SoloStar pen holds more than 900 units of insulin glargine and can provide up to 160 units/mL in a single injection, which may reduce the number of injections needed to deliver the necessary dosage to adults. Another benefit of the higher capacity is that the device will require fewer refills and the associated copays, which could potentially lower costs for patients, depending on their insurance coverage.
The dosing instructions for insulin glargine vary depending on whether patients use the older SoloStar or the high capacity Max SoloStar. The SoloStar holds 450 units (1.5mL) of insulin glargine and delivers doses in 1 unit increments, delivering a maximum dose of 80 units per injection. The MaxSoloStar has twice the capacity of the original – 900 units (3 mL) of insulin glargine – and delivers doses in 2 unit increments up to a maximum 160 unit injection. This is recommended for patients who require at least 20 units per day. All insulin glargine injections should be administered subcutaneously in the abdomen, thigh, or deltoid at the same time each day.
The most common side effect of insulin products, such as insulin glargine, is hypoglycemia.
The Max SoloStar will be available in retail pharmacies throughout the United States in the third quarter of 2018.
This will be the highest capacity long-acting insulin pen to be available commercially, according to a company press release.
The Max SoloStar pen holds more than 900 units of insulin glargine and can provide up to 160 units/mL in a single injection, which may reduce the number of injections needed to deliver the necessary dosage to adults. Another benefit of the higher capacity is that the device will require fewer refills and the associated copays, which could potentially lower costs for patients, depending on their insurance coverage.
The dosing instructions for insulin glargine vary depending on whether patients use the older SoloStar or the high capacity Max SoloStar. The SoloStar holds 450 units (1.5mL) of insulin glargine and delivers doses in 1 unit increments, delivering a maximum dose of 80 units per injection. The MaxSoloStar has twice the capacity of the original – 900 units (3 mL) of insulin glargine – and delivers doses in 2 unit increments up to a maximum 160 unit injection. This is recommended for patients who require at least 20 units per day. All insulin glargine injections should be administered subcutaneously in the abdomen, thigh, or deltoid at the same time each day.
The most common side effect of insulin products, such as insulin glargine, is hypoglycemia.
The Max SoloStar will be available in retail pharmacies throughout the United States in the third quarter of 2018.
This will be the highest capacity long-acting insulin pen to be available commercially, according to a company press release.
The Max SoloStar pen holds more than 900 units of insulin glargine and can provide up to 160 units/mL in a single injection, which may reduce the number of injections needed to deliver the necessary dosage to adults. Another benefit of the higher capacity is that the device will require fewer refills and the associated copays, which could potentially lower costs for patients, depending on their insurance coverage.
The dosing instructions for insulin glargine vary depending on whether patients use the older SoloStar or the high capacity Max SoloStar. The SoloStar holds 450 units (1.5mL) of insulin glargine and delivers doses in 1 unit increments, delivering a maximum dose of 80 units per injection. The MaxSoloStar has twice the capacity of the original – 900 units (3 mL) of insulin glargine – and delivers doses in 2 unit increments up to a maximum 160 unit injection. This is recommended for patients who require at least 20 units per day. All insulin glargine injections should be administered subcutaneously in the abdomen, thigh, or deltoid at the same time each day.
The most common side effect of insulin products, such as insulin glargine, is hypoglycemia.
The Max SoloStar will be available in retail pharmacies throughout the United States in the third quarter of 2018.
FDA advisors recommend lofexidine for opioid withdrawal
SILVER SPRING, MD. – Members of the Food and Drug Administration Psychopharmacologic Drugs Advisory Committee voted 11 to 1 to recommend approval of lofexidine as the first nonopioid treatment option for the symptomatic treatment of opioid withdrawal.
Opioid withdrawal symptoms are the largest barrier to discontinuing opioid use, according to Louis Baxter, MD, executive medical director of the Professional Assistance Program in Princeton, N.J., who presented on behalf of U.S. WorldMeds, which plans to market lofexidine as Lucemyra.
Lofexidine, a selective alpha2-adrenergic receptor agonist that regulates norepinephrine release has been approved for management of opioid withdrawal in the United Kingdom since 1992.
The advisory committee voted to recommend lofexidine on the strength of the results of two randomized, double-blind, and placebo controlled phase 3 studies on the safety and efficacy of lofexidine for symptomatic treatment of opioid withdrawal between days 1 through 7. One study randomized 264 patients to lofexidine (134) or placebo (130), with patients in the treatment arm received 3.2 mg of lofexidine on days 1-5, then placebo until day 7. The second study randomized 603 patients to three groups, comparing high dose (3.2 mg/day) and low dose (2.4 mg/day) regimens of lofexidine to placebo; patients in the treatment arms took four smaller doses of lofexidine throughout the day to achieve the cumulative dose.
Researchers enrolled heavy users of short-acting opioids; heroin was the predominant agent. Both studies were conducted in the scenario of abrupt withdrawal, or the most intense withdrawal situation.
Symptomatic benefit was measured using the Short Opiate Withdrawal Scale of Gossop (SOWS-Gossop), a patient reported outcome. Patients were asked to rank their symptoms as none, mild, moderate or severe across measures like feeling sick, stomach cramps, and heart pounding among other symptoms.
Lofexidine increased completion of withdrawal treatment compared to placebo. Patients in the first study had a 5-day completion rate of 53%, compared to 35% for the placebo group. Researchers observed similar results in the 7-day completion rates the second study, with low and high dose completion rates of 42% and 40%, respectively, both of which were much higher than placebo (28%).
Lofexidine also reduced patient withdrawal symptoms, according to SOWS-Gossop scores during peak withdrawal. In the first study, SOWS-Gossop scores were 2-4 points lower in the lofexidine group compared to placebo. Similarly, the scores were significantly better in both lofexidine groups in the second study, compared to placebo, particularly on days 1 to 4. Decreasing withdrawal symptoms during this period is particularly important because this is the most vulnerable window for patient dropout, briefing documents from US WorldMeds.
Several notable adverse events occurred during the study, particularly at higher doses of lofexidine. The risk of bradycardia and hypotension are prominent in patients taking lofexidine, but these are risks associated with this class of drug, according to Mark Pirner, MD, senior medical director at US WorldMeds, who noted that “the lower dose, if that’s what ultimately gets approved [by the FDA], is safe and effective too.”
Development of lofexidine was conducted in collaboration with the National Institute on Drug Abuse and the FDA, according to briefing documents from US WorldMeds.
The Prescription Drug User Fee Act (PDUFA) for lofexidine is May 26; FDA actions on new drug applications often occur at near the PDUFA date.
The FDA is not obligated to follow the recommendations of its advisory committees.
SILVER SPRING, MD. – Members of the Food and Drug Administration Psychopharmacologic Drugs Advisory Committee voted 11 to 1 to recommend approval of lofexidine as the first nonopioid treatment option for the symptomatic treatment of opioid withdrawal.
Opioid withdrawal symptoms are the largest barrier to discontinuing opioid use, according to Louis Baxter, MD, executive medical director of the Professional Assistance Program in Princeton, N.J., who presented on behalf of U.S. WorldMeds, which plans to market lofexidine as Lucemyra.
Lofexidine, a selective alpha2-adrenergic receptor agonist that regulates norepinephrine release has been approved for management of opioid withdrawal in the United Kingdom since 1992.
The advisory committee voted to recommend lofexidine on the strength of the results of two randomized, double-blind, and placebo controlled phase 3 studies on the safety and efficacy of lofexidine for symptomatic treatment of opioid withdrawal between days 1 through 7. One study randomized 264 patients to lofexidine (134) or placebo (130), with patients in the treatment arm received 3.2 mg of lofexidine on days 1-5, then placebo until day 7. The second study randomized 603 patients to three groups, comparing high dose (3.2 mg/day) and low dose (2.4 mg/day) regimens of lofexidine to placebo; patients in the treatment arms took four smaller doses of lofexidine throughout the day to achieve the cumulative dose.
Researchers enrolled heavy users of short-acting opioids; heroin was the predominant agent. Both studies were conducted in the scenario of abrupt withdrawal, or the most intense withdrawal situation.
Symptomatic benefit was measured using the Short Opiate Withdrawal Scale of Gossop (SOWS-Gossop), a patient reported outcome. Patients were asked to rank their symptoms as none, mild, moderate or severe across measures like feeling sick, stomach cramps, and heart pounding among other symptoms.
Lofexidine increased completion of withdrawal treatment compared to placebo. Patients in the first study had a 5-day completion rate of 53%, compared to 35% for the placebo group. Researchers observed similar results in the 7-day completion rates the second study, with low and high dose completion rates of 42% and 40%, respectively, both of which were much higher than placebo (28%).
Lofexidine also reduced patient withdrawal symptoms, according to SOWS-Gossop scores during peak withdrawal. In the first study, SOWS-Gossop scores were 2-4 points lower in the lofexidine group compared to placebo. Similarly, the scores were significantly better in both lofexidine groups in the second study, compared to placebo, particularly on days 1 to 4. Decreasing withdrawal symptoms during this period is particularly important because this is the most vulnerable window for patient dropout, briefing documents from US WorldMeds.
Several notable adverse events occurred during the study, particularly at higher doses of lofexidine. The risk of bradycardia and hypotension are prominent in patients taking lofexidine, but these are risks associated with this class of drug, according to Mark Pirner, MD, senior medical director at US WorldMeds, who noted that “the lower dose, if that’s what ultimately gets approved [by the FDA], is safe and effective too.”
Development of lofexidine was conducted in collaboration with the National Institute on Drug Abuse and the FDA, according to briefing documents from US WorldMeds.
The Prescription Drug User Fee Act (PDUFA) for lofexidine is May 26; FDA actions on new drug applications often occur at near the PDUFA date.
The FDA is not obligated to follow the recommendations of its advisory committees.
SILVER SPRING, MD. – Members of the Food and Drug Administration Psychopharmacologic Drugs Advisory Committee voted 11 to 1 to recommend approval of lofexidine as the first nonopioid treatment option for the symptomatic treatment of opioid withdrawal.
Opioid withdrawal symptoms are the largest barrier to discontinuing opioid use, according to Louis Baxter, MD, executive medical director of the Professional Assistance Program in Princeton, N.J., who presented on behalf of U.S. WorldMeds, which plans to market lofexidine as Lucemyra.
Lofexidine, a selective alpha2-adrenergic receptor agonist that regulates norepinephrine release has been approved for management of opioid withdrawal in the United Kingdom since 1992.
The advisory committee voted to recommend lofexidine on the strength of the results of two randomized, double-blind, and placebo controlled phase 3 studies on the safety and efficacy of lofexidine for symptomatic treatment of opioid withdrawal between days 1 through 7. One study randomized 264 patients to lofexidine (134) or placebo (130), with patients in the treatment arm received 3.2 mg of lofexidine on days 1-5, then placebo until day 7. The second study randomized 603 patients to three groups, comparing high dose (3.2 mg/day) and low dose (2.4 mg/day) regimens of lofexidine to placebo; patients in the treatment arms took four smaller doses of lofexidine throughout the day to achieve the cumulative dose.
Researchers enrolled heavy users of short-acting opioids; heroin was the predominant agent. Both studies were conducted in the scenario of abrupt withdrawal, or the most intense withdrawal situation.
Symptomatic benefit was measured using the Short Opiate Withdrawal Scale of Gossop (SOWS-Gossop), a patient reported outcome. Patients were asked to rank their symptoms as none, mild, moderate or severe across measures like feeling sick, stomach cramps, and heart pounding among other symptoms.
Lofexidine increased completion of withdrawal treatment compared to placebo. Patients in the first study had a 5-day completion rate of 53%, compared to 35% for the placebo group. Researchers observed similar results in the 7-day completion rates the second study, with low and high dose completion rates of 42% and 40%, respectively, both of which were much higher than placebo (28%).
Lofexidine also reduced patient withdrawal symptoms, according to SOWS-Gossop scores during peak withdrawal. In the first study, SOWS-Gossop scores were 2-4 points lower in the lofexidine group compared to placebo. Similarly, the scores were significantly better in both lofexidine groups in the second study, compared to placebo, particularly on days 1 to 4. Decreasing withdrawal symptoms during this period is particularly important because this is the most vulnerable window for patient dropout, briefing documents from US WorldMeds.
Several notable adverse events occurred during the study, particularly at higher doses of lofexidine. The risk of bradycardia and hypotension are prominent in patients taking lofexidine, but these are risks associated with this class of drug, according to Mark Pirner, MD, senior medical director at US WorldMeds, who noted that “the lower dose, if that’s what ultimately gets approved [by the FDA], is safe and effective too.”
Development of lofexidine was conducted in collaboration with the National Institute on Drug Abuse and the FDA, according to briefing documents from US WorldMeds.
The Prescription Drug User Fee Act (PDUFA) for lofexidine is May 26; FDA actions on new drug applications often occur at near the PDUFA date.
The FDA is not obligated to follow the recommendations of its advisory committees.
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
Statin use is uniformly low in adults with dyslipidemia disorders
Only half of adults with familial hypercholesterolemia are currently on statin therapy, with even fewer receiving a high-intensity statin, according to an analysis of data from the National Health and Nutrition Examination Survey.
“We found that 0.47% of the adult U.S. population has definite/probable FH [familial hypercholesterolemia] but observed a large disconnect between screening and treatment rates in adults with definite/ probable FH and adults with severe dyslipidemia,” wrote Emily Bucholz, MD, of Boston’s Children Hospital, and her colleagues in Circulation.
The analysis found that 14 million adults, or almost 7%, in the population had severe dyslipidemia (LDL cholesterol of at least 190 mg/dL), and that 1 million of these adults, 7.2%, had definite or probable FH. Among the adults with FH, awareness and screening rates were above 80%, but those rates were only moderate among the general population. Adults who were likely to have FH were also more likely to be screened and be aware of hyperlipidemia than were those with only severe dyslipidemia.
Despite high rates of awareness, statin use was low across the board. Just over half (52% ) of patients with definite/probable FH were using a statin. The rate of statin use was even lower in patients with severe dyslipidemia, at only 38%. High-intensity statin use also dwindled in both dyslipidemia and FH patient groups (37% and 30%, respectively).
Rates of cholesterol screening, awareness, and lipid-lowering medication were particularly low in younger patients, uninsured patients, and patients without a usual source of care. Among young patients (20-39 years), 62% reported cholesterol screening in the past 5 years and 64% reported being aware of having hypercholesterolemia. Of this group, 13% were on a statin. Uninsured adults fared only slightly better, with nearly a third (29%) undergoing a recent screening and using a statin.
Limitations to the analysis include the self-reported information on cholesterol screening, awareness, treatment rates, and the lack of information concerning statin dosages.
In light of the results of this study, Dr. Bucholz emphasized that statin use among underserved patient groups without access to care needs to be a priority.
“Although rates of statin prescription have been steadily increasing over time, the rate of growth over the past decade has been slow, and there remains a significant gap in treatment rates,” wrote Dr. Bucholz and her colleagues. “Younger and uninsured adults with severe dyslipidemia in addition to those without a usual source of care are significantly less likely to be prescribed statins, highlighting the need for community-based interventions to target these adults with limited access to care.”
Apart from Sarah de Ferranti, MD, who receives research funding and royalties related to pediatric cardiology, no other authors had an relevant financial disclosures to report.
SOURCE: Bucholz EM et al. Circulation. 2018 Mar 23. doi: 10.1161/CIRCULATIONAHA.117.032321.
Only half of adults with familial hypercholesterolemia are currently on statin therapy, with even fewer receiving a high-intensity statin, according to an analysis of data from the National Health and Nutrition Examination Survey.
“We found that 0.47% of the adult U.S. population has definite/probable FH [familial hypercholesterolemia] but observed a large disconnect between screening and treatment rates in adults with definite/ probable FH and adults with severe dyslipidemia,” wrote Emily Bucholz, MD, of Boston’s Children Hospital, and her colleagues in Circulation.
The analysis found that 14 million adults, or almost 7%, in the population had severe dyslipidemia (LDL cholesterol of at least 190 mg/dL), and that 1 million of these adults, 7.2%, had definite or probable FH. Among the adults with FH, awareness and screening rates were above 80%, but those rates were only moderate among the general population. Adults who were likely to have FH were also more likely to be screened and be aware of hyperlipidemia than were those with only severe dyslipidemia.
Despite high rates of awareness, statin use was low across the board. Just over half (52% ) of patients with definite/probable FH were using a statin. The rate of statin use was even lower in patients with severe dyslipidemia, at only 38%. High-intensity statin use also dwindled in both dyslipidemia and FH patient groups (37% and 30%, respectively).
Rates of cholesterol screening, awareness, and lipid-lowering medication were particularly low in younger patients, uninsured patients, and patients without a usual source of care. Among young patients (20-39 years), 62% reported cholesterol screening in the past 5 years and 64% reported being aware of having hypercholesterolemia. Of this group, 13% were on a statin. Uninsured adults fared only slightly better, with nearly a third (29%) undergoing a recent screening and using a statin.
Limitations to the analysis include the self-reported information on cholesterol screening, awareness, treatment rates, and the lack of information concerning statin dosages.
In light of the results of this study, Dr. Bucholz emphasized that statin use among underserved patient groups without access to care needs to be a priority.
“Although rates of statin prescription have been steadily increasing over time, the rate of growth over the past decade has been slow, and there remains a significant gap in treatment rates,” wrote Dr. Bucholz and her colleagues. “Younger and uninsured adults with severe dyslipidemia in addition to those without a usual source of care are significantly less likely to be prescribed statins, highlighting the need for community-based interventions to target these adults with limited access to care.”
Apart from Sarah de Ferranti, MD, who receives research funding and royalties related to pediatric cardiology, no other authors had an relevant financial disclosures to report.
SOURCE: Bucholz EM et al. Circulation. 2018 Mar 23. doi: 10.1161/CIRCULATIONAHA.117.032321.
Only half of adults with familial hypercholesterolemia are currently on statin therapy, with even fewer receiving a high-intensity statin, according to an analysis of data from the National Health and Nutrition Examination Survey.
“We found that 0.47% of the adult U.S. population has definite/probable FH [familial hypercholesterolemia] but observed a large disconnect between screening and treatment rates in adults with definite/ probable FH and adults with severe dyslipidemia,” wrote Emily Bucholz, MD, of Boston’s Children Hospital, and her colleagues in Circulation.
The analysis found that 14 million adults, or almost 7%, in the population had severe dyslipidemia (LDL cholesterol of at least 190 mg/dL), and that 1 million of these adults, 7.2%, had definite or probable FH. Among the adults with FH, awareness and screening rates were above 80%, but those rates were only moderate among the general population. Adults who were likely to have FH were also more likely to be screened and be aware of hyperlipidemia than were those with only severe dyslipidemia.
Despite high rates of awareness, statin use was low across the board. Just over half (52% ) of patients with definite/probable FH were using a statin. The rate of statin use was even lower in patients with severe dyslipidemia, at only 38%. High-intensity statin use also dwindled in both dyslipidemia and FH patient groups (37% and 30%, respectively).
Rates of cholesterol screening, awareness, and lipid-lowering medication were particularly low in younger patients, uninsured patients, and patients without a usual source of care. Among young patients (20-39 years), 62% reported cholesterol screening in the past 5 years and 64% reported being aware of having hypercholesterolemia. Of this group, 13% were on a statin. Uninsured adults fared only slightly better, with nearly a third (29%) undergoing a recent screening and using a statin.
Limitations to the analysis include the self-reported information on cholesterol screening, awareness, treatment rates, and the lack of information concerning statin dosages.
In light of the results of this study, Dr. Bucholz emphasized that statin use among underserved patient groups without access to care needs to be a priority.
“Although rates of statin prescription have been steadily increasing over time, the rate of growth over the past decade has been slow, and there remains a significant gap in treatment rates,” wrote Dr. Bucholz and her colleagues. “Younger and uninsured adults with severe dyslipidemia in addition to those without a usual source of care are significantly less likely to be prescribed statins, highlighting the need for community-based interventions to target these adults with limited access to care.”
Apart from Sarah de Ferranti, MD, who receives research funding and royalties related to pediatric cardiology, no other authors had an relevant financial disclosures to report.
SOURCE: Bucholz EM et al. Circulation. 2018 Mar 23. doi: 10.1161/CIRCULATIONAHA.117.032321.
FROM CIRCULATION
Key clinical point: Statin use is uniformly low across multiple patient groups.
Major finding:
Study details: A cross-sectional analysis of 42,471 patients from the National Health and Nutrition Examination Survey.
Disclosures: Apart from Sarah de Ferranti, MD, who receives research funding and royalties related to pediatric cardiology, no other authors had any relevant financial disclosures to report.
Source: Bucholz EM et al. Circulation. 2018 Mar 23. doi: 10.1161/CIRCULATIONAHA.117.032321.
Axial SpA disease activity remains mostly stable throughout pregnancy
, according to results of a prospective study.
“In the largest prospective study to date exploring disease activity during pregnancy in women with axSpA, we found that the majority experienced stable, low disease activity,” Kristin Ursin, MD, of the Trondheim (Norway) University Hospital and her associates wrote in Rheumatology. “In accordance with two previous studies, we found a small increase in disease activity in the second trimester.”
All women in the study fulfilled the Assessment of SpondyloArthritis International Society criteria for axSpA and had seven clinic visits throughout their pregnancy: one before conception, one at each trimester, another at 6 weeks, and two visits 6 and 12 months after delivery. No differentiation was made between women with radiographic or nonradiographic axSpA. At each visit, patients’ disease activity was determined using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is calculated based on six patient factors scored from 1 to 10, including: fatigue, back pain, peripheral joint pain and swelling, localized tenderness, duration of morning stiffness, and severity of morning stiffness. A disease score of 4 is commonly used to define active disease. Disease activity was also assessed by measuring C-reactive protein (CRP). Women’s function and health was also assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI), scored similarly to the BASDAI, and the RAND-36 questionnaire to assess general health.
The research team found that, across the span of pregnancy, axSpA disease activity was relatively low and consistent throughout the study. But there was a significant relationship between disease activity and time (P = .029) in which disease activity was highest during the second trimester and proved to be significantly higher than 6 weeks after giving birth (mean BASDAI 3.97 vs. 3.46, P = .005). In fact, 45% of women had active disease in their second trimester (BASDAI of 4 or greater). But among women with data from the second trimester and 6 weeks post partum, 42% had a decrease in BASDAI score of 1 or more, and 22% had an equivalent increase. CRP values remained low and stable throughout the study.
Like the BASDAI scores, physical function and well being seemed to correlate with later time points during pregnancy. The lowest level of physical functioning for most women was during the second and third trimesters, and those periods were significantly worse than 6 weeks after giving birth (mean BASFI of 3.2 during the second trimester and 3.6 during the third vs. 2.6 at 6 weeks post partum). Similarly, patients reported overall worse general health during the second and third trimesters, compared with 6 weeks after giving birth, based on RAND-36 scores (mean physical functioning score of 63.1 in the second trimester and 54.5 in the third vs. 71.0 at 6 weeks post partum).
Use of nonbiologic medications remained relatively stable during pregnancy, while biologic disease-modifying antirheumatic drug (DMARD) use (all tumor necrosis factor [TNF] inhibitors) dropped significantly when pregnancy was confirmed. About 20% used NSAIDs during pregnancy, except for during the third trimester when use decreased to 8%. Prednisolone use remained stable at 5%-8%. A total of 11% had used synthetic DMARDs prior to pregnancy, and this rate stayed at 10%-16% during the three trimesters. Overall, 44% had used a biologic DMARD (TNF inhibitor) prior to pregnancy, but this declined sharply to 6% during the first trimester and 1% in the third.
The main limitations of the study included not having data from all of the women for all the time points. In fact, only 22% were included prior to conceiving, which could suggest that women with low disease activity were less likely to visit their rheumatologist, thereby biasing the sample toward an overestimated disease activity at preconception that could have potentially hidden a “more evident deterioration between the preconception visit and the second trimester,” the authors said.
“Future research on pregnancy in women with axSpA should differentiate between subgroups of the disease and aim to include objective assessment of inflammation.”
The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
SOURCE: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
, according to results of a prospective study.
“In the largest prospective study to date exploring disease activity during pregnancy in women with axSpA, we found that the majority experienced stable, low disease activity,” Kristin Ursin, MD, of the Trondheim (Norway) University Hospital and her associates wrote in Rheumatology. “In accordance with two previous studies, we found a small increase in disease activity in the second trimester.”
All women in the study fulfilled the Assessment of SpondyloArthritis International Society criteria for axSpA and had seven clinic visits throughout their pregnancy: one before conception, one at each trimester, another at 6 weeks, and two visits 6 and 12 months after delivery. No differentiation was made between women with radiographic or nonradiographic axSpA. At each visit, patients’ disease activity was determined using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is calculated based on six patient factors scored from 1 to 10, including: fatigue, back pain, peripheral joint pain and swelling, localized tenderness, duration of morning stiffness, and severity of morning stiffness. A disease score of 4 is commonly used to define active disease. Disease activity was also assessed by measuring C-reactive protein (CRP). Women’s function and health was also assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI), scored similarly to the BASDAI, and the RAND-36 questionnaire to assess general health.
The research team found that, across the span of pregnancy, axSpA disease activity was relatively low and consistent throughout the study. But there was a significant relationship between disease activity and time (P = .029) in which disease activity was highest during the second trimester and proved to be significantly higher than 6 weeks after giving birth (mean BASDAI 3.97 vs. 3.46, P = .005). In fact, 45% of women had active disease in their second trimester (BASDAI of 4 or greater). But among women with data from the second trimester and 6 weeks post partum, 42% had a decrease in BASDAI score of 1 or more, and 22% had an equivalent increase. CRP values remained low and stable throughout the study.
Like the BASDAI scores, physical function and well being seemed to correlate with later time points during pregnancy. The lowest level of physical functioning for most women was during the second and third trimesters, and those periods were significantly worse than 6 weeks after giving birth (mean BASFI of 3.2 during the second trimester and 3.6 during the third vs. 2.6 at 6 weeks post partum). Similarly, patients reported overall worse general health during the second and third trimesters, compared with 6 weeks after giving birth, based on RAND-36 scores (mean physical functioning score of 63.1 in the second trimester and 54.5 in the third vs. 71.0 at 6 weeks post partum).
Use of nonbiologic medications remained relatively stable during pregnancy, while biologic disease-modifying antirheumatic drug (DMARD) use (all tumor necrosis factor [TNF] inhibitors) dropped significantly when pregnancy was confirmed. About 20% used NSAIDs during pregnancy, except for during the third trimester when use decreased to 8%. Prednisolone use remained stable at 5%-8%. A total of 11% had used synthetic DMARDs prior to pregnancy, and this rate stayed at 10%-16% during the three trimesters. Overall, 44% had used a biologic DMARD (TNF inhibitor) prior to pregnancy, but this declined sharply to 6% during the first trimester and 1% in the third.
The main limitations of the study included not having data from all of the women for all the time points. In fact, only 22% were included prior to conceiving, which could suggest that women with low disease activity were less likely to visit their rheumatologist, thereby biasing the sample toward an overestimated disease activity at preconception that could have potentially hidden a “more evident deterioration between the preconception visit and the second trimester,” the authors said.
“Future research on pregnancy in women with axSpA should differentiate between subgroups of the disease and aim to include objective assessment of inflammation.”
The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
SOURCE: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
, according to results of a prospective study.
“In the largest prospective study to date exploring disease activity during pregnancy in women with axSpA, we found that the majority experienced stable, low disease activity,” Kristin Ursin, MD, of the Trondheim (Norway) University Hospital and her associates wrote in Rheumatology. “In accordance with two previous studies, we found a small increase in disease activity in the second trimester.”
All women in the study fulfilled the Assessment of SpondyloArthritis International Society criteria for axSpA and had seven clinic visits throughout their pregnancy: one before conception, one at each trimester, another at 6 weeks, and two visits 6 and 12 months after delivery. No differentiation was made between women with radiographic or nonradiographic axSpA. At each visit, patients’ disease activity was determined using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is calculated based on six patient factors scored from 1 to 10, including: fatigue, back pain, peripheral joint pain and swelling, localized tenderness, duration of morning stiffness, and severity of morning stiffness. A disease score of 4 is commonly used to define active disease. Disease activity was also assessed by measuring C-reactive protein (CRP). Women’s function and health was also assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI), scored similarly to the BASDAI, and the RAND-36 questionnaire to assess general health.
The research team found that, across the span of pregnancy, axSpA disease activity was relatively low and consistent throughout the study. But there was a significant relationship between disease activity and time (P = .029) in which disease activity was highest during the second trimester and proved to be significantly higher than 6 weeks after giving birth (mean BASDAI 3.97 vs. 3.46, P = .005). In fact, 45% of women had active disease in their second trimester (BASDAI of 4 or greater). But among women with data from the second trimester and 6 weeks post partum, 42% had a decrease in BASDAI score of 1 or more, and 22% had an equivalent increase. CRP values remained low and stable throughout the study.
Like the BASDAI scores, physical function and well being seemed to correlate with later time points during pregnancy. The lowest level of physical functioning for most women was during the second and third trimesters, and those periods were significantly worse than 6 weeks after giving birth (mean BASFI of 3.2 during the second trimester and 3.6 during the third vs. 2.6 at 6 weeks post partum). Similarly, patients reported overall worse general health during the second and third trimesters, compared with 6 weeks after giving birth, based on RAND-36 scores (mean physical functioning score of 63.1 in the second trimester and 54.5 in the third vs. 71.0 at 6 weeks post partum).
Use of nonbiologic medications remained relatively stable during pregnancy, while biologic disease-modifying antirheumatic drug (DMARD) use (all tumor necrosis factor [TNF] inhibitors) dropped significantly when pregnancy was confirmed. About 20% used NSAIDs during pregnancy, except for during the third trimester when use decreased to 8%. Prednisolone use remained stable at 5%-8%. A total of 11% had used synthetic DMARDs prior to pregnancy, and this rate stayed at 10%-16% during the three trimesters. Overall, 44% had used a biologic DMARD (TNF inhibitor) prior to pregnancy, but this declined sharply to 6% during the first trimester and 1% in the third.
The main limitations of the study included not having data from all of the women for all the time points. In fact, only 22% were included prior to conceiving, which could suggest that women with low disease activity were less likely to visit their rheumatologist, thereby biasing the sample toward an overestimated disease activity at preconception that could have potentially hidden a “more evident deterioration between the preconception visit and the second trimester,” the authors said.
“Future research on pregnancy in women with axSpA should differentiate between subgroups of the disease and aim to include objective assessment of inflammation.”
The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
SOURCE: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
FROM RHEUMATOLOGY
Key clinical point: Higher disease activity and lower physical well being correspond with late stage pregnancy.
Major finding: Disease activity appears to be highest in the second trimester, compared with 6 weeks post partum (mean BASDAI 3.97 vs. 3.46, P = .005).
Study details: A prospective study of 179 pregnancies in 166 Norwegian women with axSpA from a Norwegian health registry between 2006 and 2016.
Disclosures: The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
Source: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
FDA approves IL-23 antagonist for plaque psoriasis
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
FDA updates breast implant–associated lymphoma cases, risk
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
FDA approves subcutaneous immunoglobulin treatment for CIDP
The Food and Drug Administration has approved Hizentra as the first subcutaneously administered human immunoglobulin maintenance therapy for adults with chronic inflammatory demyelinating polyneuropathy (CIDP), according to a statement from its manufacturer, CSL Behring.
Immune globulin subcutaneous (human) 20% liquid (Hizentra) was approved at doses of 0.2 and 0.4 g/kg per week because of the strength of the phase 3 PATH (Polyneuropathy and Treatment with Hizentra) clinical trial and the PATH extension study, which is still ongoing. PATH is the largest and longest running randomized study of patients with CIDP. The clinical trial studied the safety, efficacy, and tolerability of the two different doses of the subcutaneous immunoglobulin in 172 patients.
There were markedly lower rates of CIDP relapse or withdrawal for any reason during Hizentra treatment among patients taking a high dose of 0.4 g/kg weekly (39%; P less than .001) and those taking a low dose of 0.2 g/kg weekly (33%; P less than .007), compared with those among patients taking placebo (63%). The adverse reactions that occurred in 5% or more of patients included local infusion-site reactions, headache, diarrhea, fatigue, and upper respiratory tract infections.
The European Commission also recently granted marketing authorization for Hizentra based on the information from the PATH trial.
Hizentra was initially approved in the United States in 2010 for primary immunodeficiency in patients aged 2 years and older.
Since Hizentra is a self-administered drug, it is important for physicians to teach their patients how to properly inject this treatment without hitting a blood vessel. Apart from issues related to self-administration, the risk of thrombosis is also present, which is not uncommon for immune globulin products.
The Food and Drug Administration has approved Hizentra as the first subcutaneously administered human immunoglobulin maintenance therapy for adults with chronic inflammatory demyelinating polyneuropathy (CIDP), according to a statement from its manufacturer, CSL Behring.
Immune globulin subcutaneous (human) 20% liquid (Hizentra) was approved at doses of 0.2 and 0.4 g/kg per week because of the strength of the phase 3 PATH (Polyneuropathy and Treatment with Hizentra) clinical trial and the PATH extension study, which is still ongoing. PATH is the largest and longest running randomized study of patients with CIDP. The clinical trial studied the safety, efficacy, and tolerability of the two different doses of the subcutaneous immunoglobulin in 172 patients.
There were markedly lower rates of CIDP relapse or withdrawal for any reason during Hizentra treatment among patients taking a high dose of 0.4 g/kg weekly (39%; P less than .001) and those taking a low dose of 0.2 g/kg weekly (33%; P less than .007), compared with those among patients taking placebo (63%). The adverse reactions that occurred in 5% or more of patients included local infusion-site reactions, headache, diarrhea, fatigue, and upper respiratory tract infections.
The European Commission also recently granted marketing authorization for Hizentra based on the information from the PATH trial.
Hizentra was initially approved in the United States in 2010 for primary immunodeficiency in patients aged 2 years and older.
Since Hizentra is a self-administered drug, it is important for physicians to teach their patients how to properly inject this treatment without hitting a blood vessel. Apart from issues related to self-administration, the risk of thrombosis is also present, which is not uncommon for immune globulin products.
The Food and Drug Administration has approved Hizentra as the first subcutaneously administered human immunoglobulin maintenance therapy for adults with chronic inflammatory demyelinating polyneuropathy (CIDP), according to a statement from its manufacturer, CSL Behring.
Immune globulin subcutaneous (human) 20% liquid (Hizentra) was approved at doses of 0.2 and 0.4 g/kg per week because of the strength of the phase 3 PATH (Polyneuropathy and Treatment with Hizentra) clinical trial and the PATH extension study, which is still ongoing. PATH is the largest and longest running randomized study of patients with CIDP. The clinical trial studied the safety, efficacy, and tolerability of the two different doses of the subcutaneous immunoglobulin in 172 patients.
There were markedly lower rates of CIDP relapse or withdrawal for any reason during Hizentra treatment among patients taking a high dose of 0.4 g/kg weekly (39%; P less than .001) and those taking a low dose of 0.2 g/kg weekly (33%; P less than .007), compared with those among patients taking placebo (63%). The adverse reactions that occurred in 5% or more of patients included local infusion-site reactions, headache, diarrhea, fatigue, and upper respiratory tract infections.
The European Commission also recently granted marketing authorization for Hizentra based on the information from the PATH trial.
Hizentra was initially approved in the United States in 2010 for primary immunodeficiency in patients aged 2 years and older.
Since Hizentra is a self-administered drug, it is important for physicians to teach their patients how to properly inject this treatment without hitting a blood vessel. Apart from issues related to self-administration, the risk of thrombosis is also present, which is not uncommon for immune globulin products.
More evidence links increased BMI to higher multiple myeloma risk
A high body mass index in both early and later adulthood increases the risk for developing multiple myeloma (MM), according to a prospective analysis.
“This association did not significantly differ by gender but was nonetheless slightly stronger in men,” wrote Catherine R. Marinac, PhD, of the Dana-Farber Cancer Institute, Boston, and her colleagues. “MM risk was significantly positively associated with weight change and suggestive of a positive association for change in BMI since young adulthood. In contrast, we did not observe statistically significant associations of cumulative average physical activity or walking with MM risk.”
Dr. Marinac and her associates analyzed participants from the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Women’s Health Study (WHS) with a pooled total of 575 MM cases and more than 5 million person-years of follow-up. From all of those databases, a combined baseline total of 49,374 men and 153,260 women were included in the analyses. Participants in all three of the cohorts were predominately white.
Each participant was required to report height and weight on a baseline questionnaire and updated weights on subsequent questionnaires. Using that height and weight information, the researchers calculated BMI. Physical activity also was reported using questionnaires, beginning in 1986 in the HPFS and NHS groups and at baseline for WHS, with all groups providing updates every 2-4 years. The researchers used the physical activity information to calculate the total metabolic equivalent (MET) hours of all activity and of walking per week.
Dr. Marinac and her team identified a total of 205 men from the HPFS cohort and 370 women (325 NHS, 45 WHS) with confirmed diagnoses of MM. The BMIs of those participants ranged from 23.8-25.8 kg/m2 at baseline and from 21.3-23.0 kg/m2 in young adulthood. Across all cohorts, each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17% (hazard ratio, 1.17; 95% confidence interval, 1.05-1.29).
In addition, the MM risk rose almost 30% for every 5 kg/m2 increase in young adult BMI (HR, 1.28; 95% CI, 1.12-1.47). Increased risk was not strictly related to changes in BMI but to incremental weight gain since young adulthood. (pooled HR, 1.04; 95% CI, 1.00-1.08; P = 0.03).
The study confirmed correlations between weight gain and increased MM risk, however, it also had certain limitations. For example, much of the data concerning weight, height, and physical activity were all self-reported. Another limitation is the sociodemographic heterogeneity of the study population.
Despite those limitations, Dr. Marinac emphasized that the study results add to evidence concerning weight gain and MM risk.
“Our findings support the growing body of literature demonstrating that a high BMI both early and later in adulthood is associated with the risk of MM, and suggest that maintaining a healthy body weight throughout life may be an important component to a much-needed MM prevention strategy,” wrote Dr. Marinac, who also is affiliated with the Harvard T.H. Chan School of Public Health, also in Boston.
“Further larger-scale studies aimed at clarifying the influence of obesity timing and duration and at directly evaluating the role of weight loss, ideally conducted in diverse prospective study populations and in [monoclonal gammopathy of undetermined significance] patients, will be important for elaborating the role of weight maintenance in MM prevention and for identifying high risk subgroups of patients that may benefit from weight loss.”
None of the researchers had competing financial interests to disclose.
SOURCE: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
A high body mass index in both early and later adulthood increases the risk for developing multiple myeloma (MM), according to a prospective analysis.
“This association did not significantly differ by gender but was nonetheless slightly stronger in men,” wrote Catherine R. Marinac, PhD, of the Dana-Farber Cancer Institute, Boston, and her colleagues. “MM risk was significantly positively associated with weight change and suggestive of a positive association for change in BMI since young adulthood. In contrast, we did not observe statistically significant associations of cumulative average physical activity or walking with MM risk.”
Dr. Marinac and her associates analyzed participants from the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Women’s Health Study (WHS) with a pooled total of 575 MM cases and more than 5 million person-years of follow-up. From all of those databases, a combined baseline total of 49,374 men and 153,260 women were included in the analyses. Participants in all three of the cohorts were predominately white.
Each participant was required to report height and weight on a baseline questionnaire and updated weights on subsequent questionnaires. Using that height and weight information, the researchers calculated BMI. Physical activity also was reported using questionnaires, beginning in 1986 in the HPFS and NHS groups and at baseline for WHS, with all groups providing updates every 2-4 years. The researchers used the physical activity information to calculate the total metabolic equivalent (MET) hours of all activity and of walking per week.
Dr. Marinac and her team identified a total of 205 men from the HPFS cohort and 370 women (325 NHS, 45 WHS) with confirmed diagnoses of MM. The BMIs of those participants ranged from 23.8-25.8 kg/m2 at baseline and from 21.3-23.0 kg/m2 in young adulthood. Across all cohorts, each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17% (hazard ratio, 1.17; 95% confidence interval, 1.05-1.29).
In addition, the MM risk rose almost 30% for every 5 kg/m2 increase in young adult BMI (HR, 1.28; 95% CI, 1.12-1.47). Increased risk was not strictly related to changes in BMI but to incremental weight gain since young adulthood. (pooled HR, 1.04; 95% CI, 1.00-1.08; P = 0.03).
The study confirmed correlations between weight gain and increased MM risk, however, it also had certain limitations. For example, much of the data concerning weight, height, and physical activity were all self-reported. Another limitation is the sociodemographic heterogeneity of the study population.
Despite those limitations, Dr. Marinac emphasized that the study results add to evidence concerning weight gain and MM risk.
“Our findings support the growing body of literature demonstrating that a high BMI both early and later in adulthood is associated with the risk of MM, and suggest that maintaining a healthy body weight throughout life may be an important component to a much-needed MM prevention strategy,” wrote Dr. Marinac, who also is affiliated with the Harvard T.H. Chan School of Public Health, also in Boston.
“Further larger-scale studies aimed at clarifying the influence of obesity timing and duration and at directly evaluating the role of weight loss, ideally conducted in diverse prospective study populations and in [monoclonal gammopathy of undetermined significance] patients, will be important for elaborating the role of weight maintenance in MM prevention and for identifying high risk subgroups of patients that may benefit from weight loss.”
None of the researchers had competing financial interests to disclose.
SOURCE: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
A high body mass index in both early and later adulthood increases the risk for developing multiple myeloma (MM), according to a prospective analysis.
“This association did not significantly differ by gender but was nonetheless slightly stronger in men,” wrote Catherine R. Marinac, PhD, of the Dana-Farber Cancer Institute, Boston, and her colleagues. “MM risk was significantly positively associated with weight change and suggestive of a positive association for change in BMI since young adulthood. In contrast, we did not observe statistically significant associations of cumulative average physical activity or walking with MM risk.”
Dr. Marinac and her associates analyzed participants from the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Women’s Health Study (WHS) with a pooled total of 575 MM cases and more than 5 million person-years of follow-up. From all of those databases, a combined baseline total of 49,374 men and 153,260 women were included in the analyses. Participants in all three of the cohorts were predominately white.
Each participant was required to report height and weight on a baseline questionnaire and updated weights on subsequent questionnaires. Using that height and weight information, the researchers calculated BMI. Physical activity also was reported using questionnaires, beginning in 1986 in the HPFS and NHS groups and at baseline for WHS, with all groups providing updates every 2-4 years. The researchers used the physical activity information to calculate the total metabolic equivalent (MET) hours of all activity and of walking per week.
Dr. Marinac and her team identified a total of 205 men from the HPFS cohort and 370 women (325 NHS, 45 WHS) with confirmed diagnoses of MM. The BMIs of those participants ranged from 23.8-25.8 kg/m2 at baseline and from 21.3-23.0 kg/m2 in young adulthood. Across all cohorts, each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17% (hazard ratio, 1.17; 95% confidence interval, 1.05-1.29).
In addition, the MM risk rose almost 30% for every 5 kg/m2 increase in young adult BMI (HR, 1.28; 95% CI, 1.12-1.47). Increased risk was not strictly related to changes in BMI but to incremental weight gain since young adulthood. (pooled HR, 1.04; 95% CI, 1.00-1.08; P = 0.03).
The study confirmed correlations between weight gain and increased MM risk, however, it also had certain limitations. For example, much of the data concerning weight, height, and physical activity were all self-reported. Another limitation is the sociodemographic heterogeneity of the study population.
Despite those limitations, Dr. Marinac emphasized that the study results add to evidence concerning weight gain and MM risk.
“Our findings support the growing body of literature demonstrating that a high BMI both early and later in adulthood is associated with the risk of MM, and suggest that maintaining a healthy body weight throughout life may be an important component to a much-needed MM prevention strategy,” wrote Dr. Marinac, who also is affiliated with the Harvard T.H. Chan School of Public Health, also in Boston.
“Further larger-scale studies aimed at clarifying the influence of obesity timing and duration and at directly evaluating the role of weight loss, ideally conducted in diverse prospective study populations and in [monoclonal gammopathy of undetermined significance] patients, will be important for elaborating the role of weight maintenance in MM prevention and for identifying high risk subgroups of patients that may benefit from weight loss.”
None of the researchers had competing financial interests to disclose.
SOURCE: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
FROM BRITISH JOURNAL OF CANCER
Key clinical point: Moderate increases in body mass index (BMI) can dramatically increase the risk of developing multiple myeloma (MM).
Major finding: Each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17%.
Study details: Prospective analysis of 49,374 men and 153,260 women from three databases.
Disclosures: None of the researchers had competing financial interests to disclose.
Source: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.