User login
FDA okays dosing software for hemophilia A
The Food and Drug Administration has granted 510(k) clearance to myPKFiT for ADVATE (Antihemophilic Factor, Recombinant), pharmokinetic dosing software used for tailoring prophylaxis regimens for hemophilia A patients.
The software can be used for hemophilia A patients aged 16 years or older who weigh at least 45 kilograms (about 99 pounds) and are treated with ADVATE.
The software is expected to be available in the United States by the end of the first quarter of 2018. A version of the software is already marketed in Europe.
The Food and Drug Administration has granted 510(k) clearance to myPKFiT for ADVATE (Antihemophilic Factor, Recombinant), pharmokinetic dosing software used for tailoring prophylaxis regimens for hemophilia A patients.
The software can be used for hemophilia A patients aged 16 years or older who weigh at least 45 kilograms (about 99 pounds) and are treated with ADVATE.
The software is expected to be available in the United States by the end of the first quarter of 2018. A version of the software is already marketed in Europe.
The Food and Drug Administration has granted 510(k) clearance to myPKFiT for ADVATE (Antihemophilic Factor, Recombinant), pharmokinetic dosing software used for tailoring prophylaxis regimens for hemophilia A patients.
The software can be used for hemophilia A patients aged 16 years or older who weigh at least 45 kilograms (about 99 pounds) and are treated with ADVATE.
The software is expected to be available in the United States by the end of the first quarter of 2018. A version of the software is already marketed in Europe.
FDA approves topical antibiotic for impetigo infections
The Food and Drug Administration has approved in patients aged 2 months or older.
This is the first topical treatment for impetigo to be approved in more than 10 years, according to the press release from the manufacturer, Medimetriks Pharmaceuticals.
Ozenoxacin is a quinolone antimicrobial. The prescribing information is available on the FDA website.
The Food and Drug Administration has approved in patients aged 2 months or older.
This is the first topical treatment for impetigo to be approved in more than 10 years, according to the press release from the manufacturer, Medimetriks Pharmaceuticals.
Ozenoxacin is a quinolone antimicrobial. The prescribing information is available on the FDA website.
The Food and Drug Administration has approved in patients aged 2 months or older.
This is the first topical treatment for impetigo to be approved in more than 10 years, according to the press release from the manufacturer, Medimetriks Pharmaceuticals.
Ozenoxacin is a quinolone antimicrobial. The prescribing information is available on the FDA website.
Adolescents’ use of opioids, cigarettes is down; pot use is up
The positive trends in drug use among adolescents appear to be continuing – overall, according to the 2017 Monitoring the Future survey.
“This year, we have very good news, because ... the pattern of drug use among teenagers in the United States is continuing to go down, and it’s most notable in the case of opioid drugs,” Nora D. Volkow, MD, director of the National Institute on Drug Abuse said in a video interview posted by NIDA. “We are observing some of the lowest rates of opioid use that we have been monitoring through the survey.”
The national survey results of 8th, 10th, and 12th graders, released Dec. 14, found that lifetime, past-year, and past-month misuse of prescription pain medications are at lows that NIDA called historic. Among high school seniors, the survey showed, Vicodin use was at its lowest point since 2002 at 2% – a decrease from its high of 10.5% in 2003. Similarly, the reported use of OxyContin among high school seniors dropped from 5.5% in 2005 to 2.7% this year.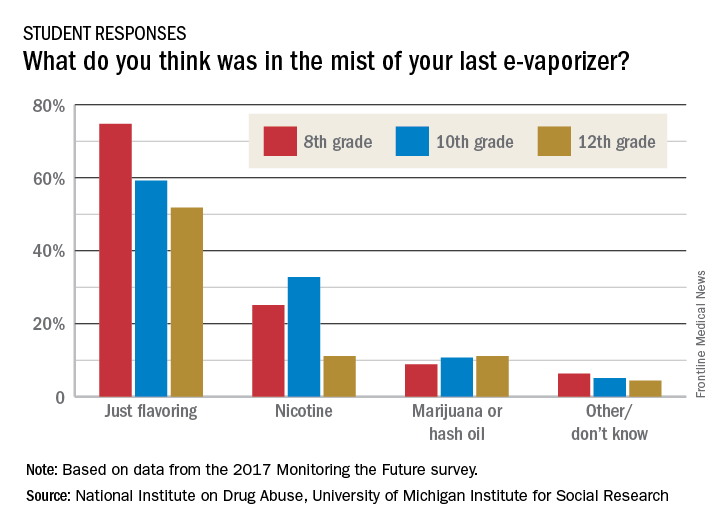
“We’re also seeing continuing decreases in cigarette smoking that are at the lowest levels that we’ve ever seen,” Dr. Volkow said in the interview. Daily cigarette use was 0.6% among 8th graders, 2.2% among 10th graders, and 4.2% among 12th graders in 2017. Those numbers were down from 10.4% and 18.3% among 8th and 10th graders in 1996, and a peak of 24.6 % of 12th graders in 1997.
Twelfth graders also reported lower past-year use of hookah: Use of that substance declined to 10% this year from 13% a year earlier.
As the use of traditional and smokeless tobacco has decreased, however, overall vaping levels held steady in 2017. For the first time, Monitoring the Future reported national, standard estimates of four categories of vaping: nicotine, marijuana, flavoring-only, and any vaping. The reported past-year rates of use under the “any vaping” category in the 2017 survey was 13.3% for 8th graders, 23.9% for 10th graders, and 27.8% for 12th graders. Vaping for flavoring only was the most popular option, and the highest rates were reported by 12th graders – who reported past-month use of 9.7%.
Dr. Volkow said a key concern is that it is unclear that adolescents know what substance they are vaping. Furthermore, adolescents who start vaping any substance are more likely to start to vape nicotine than are those who do not vape. “And we know that when an individual is exposed to nicotine and then gets exposed to another drug, that drug will be more rewarding,” Dr. Volkow said in a teleconference announcing the results.
Richard A. Miech, PhD, MPH, principal investigator of the survey, echoed Dr. Volkow’s concerns.
“These findings emphasize that vaping has progressed well beyond a cigarette alternative,” Dr. Miech said in a statement. “.”
Meanwhile, the past-year use of marijuana among adolescents climbed significantly by 1.3% in 2017, to 24% for all three grades levels combined.
“Historically marijuana use has gone up as adolescents see less risk of harm in using it,” Dr. Miech, of the University of Michigan, Ann Arbor, said in another statement. “We’ve found that the risk adolescents see in marijuana use has been steadily going down for years to the point that it is now at the lowest level we’ve seen in 4 decades.”
Reported overall illicit use of substances such as cocaine, anabolic steroids, and “synthetic marijuana” was relatively low.
“We should take encouragement from this year’s MTF results,” Dr. Volkow wrote on her blog. “Most substance use by middle and high school students is the lowest it has ever been, which suggests that prevention interventions and policies continue to have the desired effect. ...“There is also need to expand on prevention strategies to further reduce drug use – such as use of marijuana.”
The Monitoring the Future survey has been conducted since 1975. The 2017 survey, taken at the beginning of the year, is based on reports from almost 45,000 students in about 380 public and private secondary schools across the country. The survey is designed and conducted by research scientists at the University of Michigan, and is funded by NIDA.
SOURCE: Miech RA et al. 2017 Monitoring the Future
The positive trends in drug use among adolescents appear to be continuing – overall, according to the 2017 Monitoring the Future survey.
“This year, we have very good news, because ... the pattern of drug use among teenagers in the United States is continuing to go down, and it’s most notable in the case of opioid drugs,” Nora D. Volkow, MD, director of the National Institute on Drug Abuse said in a video interview posted by NIDA. “We are observing some of the lowest rates of opioid use that we have been monitoring through the survey.”
The national survey results of 8th, 10th, and 12th graders, released Dec. 14, found that lifetime, past-year, and past-month misuse of prescription pain medications are at lows that NIDA called historic. Among high school seniors, the survey showed, Vicodin use was at its lowest point since 2002 at 2% – a decrease from its high of 10.5% in 2003. Similarly, the reported use of OxyContin among high school seniors dropped from 5.5% in 2005 to 2.7% this year.
“We’re also seeing continuing decreases in cigarette smoking that are at the lowest levels that we’ve ever seen,” Dr. Volkow said in the interview. Daily cigarette use was 0.6% among 8th graders, 2.2% among 10th graders, and 4.2% among 12th graders in 2017. Those numbers were down from 10.4% and 18.3% among 8th and 10th graders in 1996, and a peak of 24.6 % of 12th graders in 1997.
Twelfth graders also reported lower past-year use of hookah: Use of that substance declined to 10% this year from 13% a year earlier.
As the use of traditional and smokeless tobacco has decreased, however, overall vaping levels held steady in 2017. For the first time, Monitoring the Future reported national, standard estimates of four categories of vaping: nicotine, marijuana, flavoring-only, and any vaping. The reported past-year rates of use under the “any vaping” category in the 2017 survey was 13.3% for 8th graders, 23.9% for 10th graders, and 27.8% for 12th graders. Vaping for flavoring only was the most popular option, and the highest rates were reported by 12th graders – who reported past-month use of 9.7%.
Dr. Volkow said a key concern is that it is unclear that adolescents know what substance they are vaping. Furthermore, adolescents who start vaping any substance are more likely to start to vape nicotine than are those who do not vape. “And we know that when an individual is exposed to nicotine and then gets exposed to another drug, that drug will be more rewarding,” Dr. Volkow said in a teleconference announcing the results.
Richard A. Miech, PhD, MPH, principal investigator of the survey, echoed Dr. Volkow’s concerns.
“These findings emphasize that vaping has progressed well beyond a cigarette alternative,” Dr. Miech said in a statement. “.”
Meanwhile, the past-year use of marijuana among adolescents climbed significantly by 1.3% in 2017, to 24% for all three grades levels combined.
“Historically marijuana use has gone up as adolescents see less risk of harm in using it,” Dr. Miech, of the University of Michigan, Ann Arbor, said in another statement. “We’ve found that the risk adolescents see in marijuana use has been steadily going down for years to the point that it is now at the lowest level we’ve seen in 4 decades.”
Reported overall illicit use of substances such as cocaine, anabolic steroids, and “synthetic marijuana” was relatively low.
“We should take encouragement from this year’s MTF results,” Dr. Volkow wrote on her blog. “Most substance use by middle and high school students is the lowest it has ever been, which suggests that prevention interventions and policies continue to have the desired effect. ...“There is also need to expand on prevention strategies to further reduce drug use – such as use of marijuana.”
The Monitoring the Future survey has been conducted since 1975. The 2017 survey, taken at the beginning of the year, is based on reports from almost 45,000 students in about 380 public and private secondary schools across the country. The survey is designed and conducted by research scientists at the University of Michigan, and is funded by NIDA.
SOURCE: Miech RA et al. 2017 Monitoring the Future
The positive trends in drug use among adolescents appear to be continuing – overall, according to the 2017 Monitoring the Future survey.
“This year, we have very good news, because ... the pattern of drug use among teenagers in the United States is continuing to go down, and it’s most notable in the case of opioid drugs,” Nora D. Volkow, MD, director of the National Institute on Drug Abuse said in a video interview posted by NIDA. “We are observing some of the lowest rates of opioid use that we have been monitoring through the survey.”
The national survey results of 8th, 10th, and 12th graders, released Dec. 14, found that lifetime, past-year, and past-month misuse of prescription pain medications are at lows that NIDA called historic. Among high school seniors, the survey showed, Vicodin use was at its lowest point since 2002 at 2% – a decrease from its high of 10.5% in 2003. Similarly, the reported use of OxyContin among high school seniors dropped from 5.5% in 2005 to 2.7% this year.
“We’re also seeing continuing decreases in cigarette smoking that are at the lowest levels that we’ve ever seen,” Dr. Volkow said in the interview. Daily cigarette use was 0.6% among 8th graders, 2.2% among 10th graders, and 4.2% among 12th graders in 2017. Those numbers were down from 10.4% and 18.3% among 8th and 10th graders in 1996, and a peak of 24.6 % of 12th graders in 1997.
Twelfth graders also reported lower past-year use of hookah: Use of that substance declined to 10% this year from 13% a year earlier.
As the use of traditional and smokeless tobacco has decreased, however, overall vaping levels held steady in 2017. For the first time, Monitoring the Future reported national, standard estimates of four categories of vaping: nicotine, marijuana, flavoring-only, and any vaping. The reported past-year rates of use under the “any vaping” category in the 2017 survey was 13.3% for 8th graders, 23.9% for 10th graders, and 27.8% for 12th graders. Vaping for flavoring only was the most popular option, and the highest rates were reported by 12th graders – who reported past-month use of 9.7%.
Dr. Volkow said a key concern is that it is unclear that adolescents know what substance they are vaping. Furthermore, adolescents who start vaping any substance are more likely to start to vape nicotine than are those who do not vape. “And we know that when an individual is exposed to nicotine and then gets exposed to another drug, that drug will be more rewarding,” Dr. Volkow said in a teleconference announcing the results.
Richard A. Miech, PhD, MPH, principal investigator of the survey, echoed Dr. Volkow’s concerns.
“These findings emphasize that vaping has progressed well beyond a cigarette alternative,” Dr. Miech said in a statement. “.”
Meanwhile, the past-year use of marijuana among adolescents climbed significantly by 1.3% in 2017, to 24% for all three grades levels combined.
“Historically marijuana use has gone up as adolescents see less risk of harm in using it,” Dr. Miech, of the University of Michigan, Ann Arbor, said in another statement. “We’ve found that the risk adolescents see in marijuana use has been steadily going down for years to the point that it is now at the lowest level we’ve seen in 4 decades.”
Reported overall illicit use of substances such as cocaine, anabolic steroids, and “synthetic marijuana” was relatively low.
“We should take encouragement from this year’s MTF results,” Dr. Volkow wrote on her blog. “Most substance use by middle and high school students is the lowest it has ever been, which suggests that prevention interventions and policies continue to have the desired effect. ...“There is also need to expand on prevention strategies to further reduce drug use – such as use of marijuana.”
The Monitoring the Future survey has been conducted since 1975. The 2017 survey, taken at the beginning of the year, is based on reports from almost 45,000 students in about 380 public and private secondary schools across the country. The survey is designed and conducted by research scientists at the University of Michigan, and is funded by NIDA.
SOURCE: Miech RA et al. 2017 Monitoring the Future
Key clinical point: Eighth, 10th, and 12th grade students are reporting higher rates of marijuana use.
Major finding: Teen misuse of Vicodin stood at 2% in 2017, a decline from a 10.5% peak in 2003.
Study details: Survey of 43,703 students from 360 public and private schools in the United States.
Disclosures: This survey is sponsored by a grant from the National Institute on Drug Abuse.
Source: Miech RA et al. 2017 Monitoring the Future.
FDA approves premixed, low-volume colon-cleansing solution
in adults preparing to undergo colonoscopy, according to Ferring Pharmaceuticals.
The sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is a relatively low-volume, premixed, cranberry-flavored solution, making it easier to use and more palatable for patients.
Sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is approved with two dosing options: split dose, one dose the evening prior and one dose the morning of the procedure, or the day before dose, which involves taking both doses the day prior to the procedure. Day before dosing is an alternative and should be used when split dosing is not appropriate. After each dose of sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution, clear liquids should be consumed based on the dosing recommendation. The American College of Gastroenterology recommends the split-dose regimen because of its improved cleansing quality of the colon and better tolerability of the liquid volume by patients.
Patients with impaired renal function should exercise caution if using sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution as it may effect renal function. A more comprehensive list of safety information is available at www.clenpiq.com.
To help your patients understand and prepare for a colonoscopy, use AGA’s patient education materials at http://www.gastro.org/patient-care/procedures/colonoscopy.
in adults preparing to undergo colonoscopy, according to Ferring Pharmaceuticals.
The sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is a relatively low-volume, premixed, cranberry-flavored solution, making it easier to use and more palatable for patients.
Sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is approved with two dosing options: split dose, one dose the evening prior and one dose the morning of the procedure, or the day before dose, which involves taking both doses the day prior to the procedure. Day before dosing is an alternative and should be used when split dosing is not appropriate. After each dose of sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution, clear liquids should be consumed based on the dosing recommendation. The American College of Gastroenterology recommends the split-dose regimen because of its improved cleansing quality of the colon and better tolerability of the liquid volume by patients.
Patients with impaired renal function should exercise caution if using sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution as it may effect renal function. A more comprehensive list of safety information is available at www.clenpiq.com.
To help your patients understand and prepare for a colonoscopy, use AGA’s patient education materials at http://www.gastro.org/patient-care/procedures/colonoscopy.
in adults preparing to undergo colonoscopy, according to Ferring Pharmaceuticals.
The sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is a relatively low-volume, premixed, cranberry-flavored solution, making it easier to use and more palatable for patients.
Sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is approved with two dosing options: split dose, one dose the evening prior and one dose the morning of the procedure, or the day before dose, which involves taking both doses the day prior to the procedure. Day before dosing is an alternative and should be used when split dosing is not appropriate. After each dose of sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution, clear liquids should be consumed based on the dosing recommendation. The American College of Gastroenterology recommends the split-dose regimen because of its improved cleansing quality of the colon and better tolerability of the liquid volume by patients.
Patients with impaired renal function should exercise caution if using sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution as it may effect renal function. A more comprehensive list of safety information is available at www.clenpiq.com.
To help your patients understand and prepare for a colonoscopy, use AGA’s patient education materials at http://www.gastro.org/patient-care/procedures/colonoscopy.
A broadly effective meningitis B vaccine has been proved effective in a Danish study
MenB-FHbp, a licensed meningococcal B vaccine that targets factor H-binding protein, a surface-exposed bacterial lipoprotein, has been shown to provide broad immunological protection against 4 primary and 10 additional strains of meningococcal B disease in teens and young adults, suggesting the potential to reduce the number of shots needed to protect this population, according to two phase 3 randomized, controlled, observer-blinded, multicenter trials.
After the third dose of MenB-FHbp, the percentage of patients whose antibody titers of serum bactericidal assays with human complement (hSBA) had increased by a factor of four rose as high as 78.8%-90.2% in adolescents and 78.9%-89.7% in young adults in the modified intention-to-treat population, depending on which strain was tested. This population was made up of randomized patients who had at least one valid and determinate assay. Among participants, 82% of adolescents and 84.5% of young adults had the composite response, which was defined as the proportion of patients who had an hSBA titer response that reached or exceeded the limit of quantitation for all primary test strains combined at 1 month after the third MenB-FHbp vaccine dose was administered.
Using the hSBA responses to the primary strains, a positive predictive value analysis predicted the immune responses to other meningitis strains with H-binding protein. For subfamily A strains among adolescents, the positive predictive values for patient responses were 64.4%-100% after dose 2 and 75.6%-99.6% after dose 3. Among young adults, the positive predictive values after dose 2 and 3 were 61.6%-100% and 72.2%-100%, respectively.
A similar pattern was observed for subfamily B strains.* Among adolescents the positive predictive values for patient responses were 78.9%-100% and 86.4%-99.6% after doses 2 and 3, respectively. Young adults also had high positive predictive values after doses 2 and 3, with values of 70%-100% and 80.5%-98.8%, respectively.
Injection site reactions and systemic events were both observed in response to MenB-FHbp in both adolescents and young adults. The most common injection site reaction was pain, which often occurred occurring after the first dose. Six adolescents and three young adults, one of whom received a saline injection, withdrew from the study because of injection site reactions. The most common systemic events among adolescents were headaches and fatigue. The events that caused patients to withdraw were associated with fever, chills, and mild arthralgia and moder myalgia.
“By demonstrating with positive–predictive value analyses the ability of 4 primary test strains to predict coverage with the use of 10 additional test strains, our findings provide assurance that observed immune responses to the primary strains are representative and indicative of vaccine responses to diverse disease-causing meningococcal B strains,” wrote Lars Ostergaard, MD, of the Aarhus (Denmark) University Hospital and his colleagues.
The studies were supported by Pfizer, and all researchers reported having received various forms of support from that company. Although many of the researchers hold patents, or have patents pending, that are relevant to these studies, they had no other.
SOURCE: Ostergaard L et al. N Engl J Med. 2017 Dec 14;377(24):2350-62.
Correction 12/14/17: An earlier version of this story misstated the findings concerning this subfamily.
MenB-FHbp, a licensed meningococcal B vaccine that targets factor H-binding protein, a surface-exposed bacterial lipoprotein, has been shown to provide broad immunological protection against 4 primary and 10 additional strains of meningococcal B disease in teens and young adults, suggesting the potential to reduce the number of shots needed to protect this population, according to two phase 3 randomized, controlled, observer-blinded, multicenter trials.
After the third dose of MenB-FHbp, the percentage of patients whose antibody titers of serum bactericidal assays with human complement (hSBA) had increased by a factor of four rose as high as 78.8%-90.2% in adolescents and 78.9%-89.7% in young adults in the modified intention-to-treat population, depending on which strain was tested. This population was made up of randomized patients who had at least one valid and determinate assay. Among participants, 82% of adolescents and 84.5% of young adults had the composite response, which was defined as the proportion of patients who had an hSBA titer response that reached or exceeded the limit of quantitation for all primary test strains combined at 1 month after the third MenB-FHbp vaccine dose was administered.
Using the hSBA responses to the primary strains, a positive predictive value analysis predicted the immune responses to other meningitis strains with H-binding protein. For subfamily A strains among adolescents, the positive predictive values for patient responses were 64.4%-100% after dose 2 and 75.6%-99.6% after dose 3. Among young adults, the positive predictive values after dose 2 and 3 were 61.6%-100% and 72.2%-100%, respectively.
A similar pattern was observed for subfamily B strains.* Among adolescents the positive predictive values for patient responses were 78.9%-100% and 86.4%-99.6% after doses 2 and 3, respectively. Young adults also had high positive predictive values after doses 2 and 3, with values of 70%-100% and 80.5%-98.8%, respectively.
Injection site reactions and systemic events were both observed in response to MenB-FHbp in both adolescents and young adults. The most common injection site reaction was pain, which often occurred occurring after the first dose. Six adolescents and three young adults, one of whom received a saline injection, withdrew from the study because of injection site reactions. The most common systemic events among adolescents were headaches and fatigue. The events that caused patients to withdraw were associated with fever, chills, and mild arthralgia and moder myalgia.
“By demonstrating with positive–predictive value analyses the ability of 4 primary test strains to predict coverage with the use of 10 additional test strains, our findings provide assurance that observed immune responses to the primary strains are representative and indicative of vaccine responses to diverse disease-causing meningococcal B strains,” wrote Lars Ostergaard, MD, of the Aarhus (Denmark) University Hospital and his colleagues.
The studies were supported by Pfizer, and all researchers reported having received various forms of support from that company. Although many of the researchers hold patents, or have patents pending, that are relevant to these studies, they had no other.
SOURCE: Ostergaard L et al. N Engl J Med. 2017 Dec 14;377(24):2350-62.
Correction 12/14/17: An earlier version of this story misstated the findings concerning this subfamily.
MenB-FHbp, a licensed meningococcal B vaccine that targets factor H-binding protein, a surface-exposed bacterial lipoprotein, has been shown to provide broad immunological protection against 4 primary and 10 additional strains of meningococcal B disease in teens and young adults, suggesting the potential to reduce the number of shots needed to protect this population, according to two phase 3 randomized, controlled, observer-blinded, multicenter trials.
After the third dose of MenB-FHbp, the percentage of patients whose antibody titers of serum bactericidal assays with human complement (hSBA) had increased by a factor of four rose as high as 78.8%-90.2% in adolescents and 78.9%-89.7% in young adults in the modified intention-to-treat population, depending on which strain was tested. This population was made up of randomized patients who had at least one valid and determinate assay. Among participants, 82% of adolescents and 84.5% of young adults had the composite response, which was defined as the proportion of patients who had an hSBA titer response that reached or exceeded the limit of quantitation for all primary test strains combined at 1 month after the third MenB-FHbp vaccine dose was administered.
Using the hSBA responses to the primary strains, a positive predictive value analysis predicted the immune responses to other meningitis strains with H-binding protein. For subfamily A strains among adolescents, the positive predictive values for patient responses were 64.4%-100% after dose 2 and 75.6%-99.6% after dose 3. Among young adults, the positive predictive values after dose 2 and 3 were 61.6%-100% and 72.2%-100%, respectively.
A similar pattern was observed for subfamily B strains.* Among adolescents the positive predictive values for patient responses were 78.9%-100% and 86.4%-99.6% after doses 2 and 3, respectively. Young adults also had high positive predictive values after doses 2 and 3, with values of 70%-100% and 80.5%-98.8%, respectively.
Injection site reactions and systemic events were both observed in response to MenB-FHbp in both adolescents and young adults. The most common injection site reaction was pain, which often occurred occurring after the first dose. Six adolescents and three young adults, one of whom received a saline injection, withdrew from the study because of injection site reactions. The most common systemic events among adolescents were headaches and fatigue. The events that caused patients to withdraw were associated with fever, chills, and mild arthralgia and moder myalgia.
“By demonstrating with positive–predictive value analyses the ability of 4 primary test strains to predict coverage with the use of 10 additional test strains, our findings provide assurance that observed immune responses to the primary strains are representative and indicative of vaccine responses to diverse disease-causing meningococcal B strains,” wrote Lars Ostergaard, MD, of the Aarhus (Denmark) University Hospital and his colleagues.
The studies were supported by Pfizer, and all researchers reported having received various forms of support from that company. Although many of the researchers hold patents, or have patents pending, that are relevant to these studies, they had no other.
SOURCE: Ostergaard L et al. N Engl J Med. 2017 Dec 14;377(24):2350-62.
Correction 12/14/17: An earlier version of this story misstated the findings concerning this subfamily.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A surface protein–focused vaccine is broadly effective against meningitis B.
Major finding: After the third dose of MenB-FHbp, hSBA titers against each primary strain increased by a factor of four or more in 78.8%-90.2% of adolescents and 78.9%-89.7% of young adults in the modified intention-to-treat population, depending on which strain was tested.
Study details: Two phase 3 randomized, controlled, observer-blinded, multicenter trials of 3,596 adolescents recruited between April 18, 2013, and June 17, 2015, and 3,304 young adults recruited between May 3, 2013, and July 9, 2015.
Disclosures: The studies were supported by Pfizer, and all researchers reported having received various forms of support from that company. Although many of the researchers hold patents, or have patents pending, that are relevant to these studies, they had no other.
Source: Ostergaard L et al. N Engl J Med. 2017 Dec 14;377(24):2350-62.
FDA approves premixed, low-volume colon-cleansing solution
in adults preparing to undergo colonoscopy, according to Ferring Pharmaceuticals.
The sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is a relatively low-volume, premixed, cranberry-flavored solution, making it easier to use and more palatable for patients.
The oral solution is approved with two dosing options: split dose, one dose the evening prior and one dose the morning of the procedure, or the day before dose, which involves taking both doses the day prior to the procedure. Day before dosing is an alternative and should be used when split dosing is not appropriate. After each dose of sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution, clear liquids should be consumed based on the dosing recommendation. The American College of Gastroenterology recommends the split-dose regimen because of its improved cleansing quality of the colon and better tolerability of the liquid volume by patients.
Patients with impaired renal function should exercise caution if using sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution as it may effect renal function. A more comprehensive list of safety information is available at www.clenpiq.com.
in adults preparing to undergo colonoscopy, according to Ferring Pharmaceuticals.
The sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is a relatively low-volume, premixed, cranberry-flavored solution, making it easier to use and more palatable for patients.
The oral solution is approved with two dosing options: split dose, one dose the evening prior and one dose the morning of the procedure, or the day before dose, which involves taking both doses the day prior to the procedure. Day before dosing is an alternative and should be used when split dosing is not appropriate. After each dose of sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution, clear liquids should be consumed based on the dosing recommendation. The American College of Gastroenterology recommends the split-dose regimen because of its improved cleansing quality of the colon and better tolerability of the liquid volume by patients.
Patients with impaired renal function should exercise caution if using sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution as it may effect renal function. A more comprehensive list of safety information is available at www.clenpiq.com.
in adults preparing to undergo colonoscopy, according to Ferring Pharmaceuticals.
The sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution is a relatively low-volume, premixed, cranberry-flavored solution, making it easier to use and more palatable for patients.
The oral solution is approved with two dosing options: split dose, one dose the evening prior and one dose the morning of the procedure, or the day before dose, which involves taking both doses the day prior to the procedure. Day before dosing is an alternative and should be used when split dosing is not appropriate. After each dose of sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution, clear liquids should be consumed based on the dosing recommendation. The American College of Gastroenterology recommends the split-dose regimen because of its improved cleansing quality of the colon and better tolerability of the liquid volume by patients.
Patients with impaired renal function should exercise caution if using sodium picosulfate, magnesium oxide, and anhydrous citric acid oral solution as it may effect renal function. A more comprehensive list of safety information is available at www.clenpiq.com.
Nebulized LAMA for COPD approved
The Food and Drug Administration has given the nod to the first nebulized long-acting muscarinic antagonist (LAMA) treatment for chronic obstructive pulmonary disease (COPD) in the United States.
Glycopyrrolate (Lonhala Magnair) utilizes the eFlow technology system, developed by Pari Pharma. This nebulizing system is portable, virtually silent, and delivers the drug in 2-3 minutes, according to a statement from Sunovion Pharmaceuticals.
The approval of glycopyrrolate is based on the results of the GOLDEN (Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer) trials. The GOLDEN program comprised the GOLDEN-3 and GOLDEN-4 trials, both of which were phase 3, 12-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter safety and efficacy trials, which compared adult glycopyrrolate patients to a placebo group with moderate to severe COPD. At 12 weeks, patients receiving treatment with glycopyrrolate showed clinical and statistically significant improvements in their baseline forced expiratory volume second (FEV1), compared with placebo.
GOLDEN-5, an additional study, followed the same criteria as previous studies, but increased its length to 48 weeks to evaluate the long-term safety and patient tolerability of glycopyrrolate. It also compared treatment of COPD with glycopyrrolate to treatment of COPD with the previously approved LAMA Spiriva (tiotropium bromide), delivered by the Handihaler device. Glycopyrrolate was well tolerated, and the overall treatment emergence of adverse events for glycopyrrolate and tiotropium bromide were similar.
Sunovion expects glycopyrrolate to be available in U.S. pharmacies in early 2018, according to the statement.
The Food and Drug Administration has given the nod to the first nebulized long-acting muscarinic antagonist (LAMA) treatment for chronic obstructive pulmonary disease (COPD) in the United States.
Glycopyrrolate (Lonhala Magnair) utilizes the eFlow technology system, developed by Pari Pharma. This nebulizing system is portable, virtually silent, and delivers the drug in 2-3 minutes, according to a statement from Sunovion Pharmaceuticals.
The approval of glycopyrrolate is based on the results of the GOLDEN (Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer) trials. The GOLDEN program comprised the GOLDEN-3 and GOLDEN-4 trials, both of which were phase 3, 12-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter safety and efficacy trials, which compared adult glycopyrrolate patients to a placebo group with moderate to severe COPD. At 12 weeks, patients receiving treatment with glycopyrrolate showed clinical and statistically significant improvements in their baseline forced expiratory volume second (FEV1), compared with placebo.
GOLDEN-5, an additional study, followed the same criteria as previous studies, but increased its length to 48 weeks to evaluate the long-term safety and patient tolerability of glycopyrrolate. It also compared treatment of COPD with glycopyrrolate to treatment of COPD with the previously approved LAMA Spiriva (tiotropium bromide), delivered by the Handihaler device. Glycopyrrolate was well tolerated, and the overall treatment emergence of adverse events for glycopyrrolate and tiotropium bromide were similar.
Sunovion expects glycopyrrolate to be available in U.S. pharmacies in early 2018, according to the statement.
The Food and Drug Administration has given the nod to the first nebulized long-acting muscarinic antagonist (LAMA) treatment for chronic obstructive pulmonary disease (COPD) in the United States.
Glycopyrrolate (Lonhala Magnair) utilizes the eFlow technology system, developed by Pari Pharma. This nebulizing system is portable, virtually silent, and delivers the drug in 2-3 minutes, according to a statement from Sunovion Pharmaceuticals.
The approval of glycopyrrolate is based on the results of the GOLDEN (Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer) trials. The GOLDEN program comprised the GOLDEN-3 and GOLDEN-4 trials, both of which were phase 3, 12-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter safety and efficacy trials, which compared adult glycopyrrolate patients to a placebo group with moderate to severe COPD. At 12 weeks, patients receiving treatment with glycopyrrolate showed clinical and statistically significant improvements in their baseline forced expiratory volume second (FEV1), compared with placebo.
GOLDEN-5, an additional study, followed the same criteria as previous studies, but increased its length to 48 weeks to evaluate the long-term safety and patient tolerability of glycopyrrolate. It also compared treatment of COPD with glycopyrrolate to treatment of COPD with the previously approved LAMA Spiriva (tiotropium bromide), delivered by the Handihaler device. Glycopyrrolate was well tolerated, and the overall treatment emergence of adverse events for glycopyrrolate and tiotropium bromide were similar.
Sunovion expects glycopyrrolate to be available in U.S. pharmacies in early 2018, according to the statement.
FDA approves first follow-on short-acting insulin product using abbreviated approval pathway
aged 3 years and older with type 1 and type 2 diabetes mellitus, according to a news release from the FDA.
Insulin lispro injections are to be administered subcutaneously by injection, infusion via an insulin pump, or intravenously. Doses should be individualized based on route of administration and patients’ individual metabolic needs. All diabetes patients should regularly monitor their blood sugar levels and insulin regimens should be exclusively modified under medical supervision.
“With today’s approval, we are providing an important short-acting insulin option for patients that meets our standards for safety and effectiveness,” Mary T. Thanh Hai, MD, deputy director of the Office of New Drug Evaluation II in the FDA’s Center for Drug Evaluation and Research, said in a statement.
Admelog, a biosimilar to Eli Lilly’s Humalog, was approved as a follow-on product based on the prior approval of Humalog. This allowed insulin lispro injections to be passed through the abbreviated approval pathway under the Federal Food, Drug, and Cosmetic Act, formally known as the 505(b)(2) pathway. The makers of insulin lispro found it was scientifically justified to use the previous safety and effectiveness data from the approval of Humalog to support the approval of insulin lispro injections. In addition to the Humalog data, the insulin lispro injection data included findings from two phase 3 clinical trials, each of which comprised about 500 diabetes patients. Using this abbreviated pathway can reduce the costs of drug development significantly, allowing new products to be offered to patients at lower prices.
“One of my key policy efforts is increasing competition in the market for prescription drugs and helping facilitate the entry of lower-cost alternatives. This is particularly important for drugs like insulin that are taken by millions of Americans every day for a patient’s lifetime to manage a chronic disease,” FDA Commissioner Scott Gottlieb, MD, said in a statement “In the coming months, we’ll be taking additional policy steps to help to make sure patients continue to benefit from improved access to lower cost, safe and effective alternatives to brand name drugs approved through the agency’s abbreviated pathways.”
Insulin lispro injections are short-acting insulin products that can help improve blood sugar levels in diabetes patients. This can be useful in controlling blood sugar levels after eating. This contrasts with long-acting insulin products, which are intended to control background insulin levels between meals. The blood sugar control needs of type 1 and type 2 diabetes patients are unique. Type 1 patients require both short- and long-term controls, while some type 2 patients may never need a short-acting insulin product. Because of these differences, providing insulin lispro injections as a blood sugar control method may be particularly useful to type 1 diabetes patients who need to control mealtime blood sugar levels.
aged 3 years and older with type 1 and type 2 diabetes mellitus, according to a news release from the FDA.
Insulin lispro injections are to be administered subcutaneously by injection, infusion via an insulin pump, or intravenously. Doses should be individualized based on route of administration and patients’ individual metabolic needs. All diabetes patients should regularly monitor their blood sugar levels and insulin regimens should be exclusively modified under medical supervision.
“With today’s approval, we are providing an important short-acting insulin option for patients that meets our standards for safety and effectiveness,” Mary T. Thanh Hai, MD, deputy director of the Office of New Drug Evaluation II in the FDA’s Center for Drug Evaluation and Research, said in a statement.
Admelog, a biosimilar to Eli Lilly’s Humalog, was approved as a follow-on product based on the prior approval of Humalog. This allowed insulin lispro injections to be passed through the abbreviated approval pathway under the Federal Food, Drug, and Cosmetic Act, formally known as the 505(b)(2) pathway. The makers of insulin lispro found it was scientifically justified to use the previous safety and effectiveness data from the approval of Humalog to support the approval of insulin lispro injections. In addition to the Humalog data, the insulin lispro injection data included findings from two phase 3 clinical trials, each of which comprised about 500 diabetes patients. Using this abbreviated pathway can reduce the costs of drug development significantly, allowing new products to be offered to patients at lower prices.
“One of my key policy efforts is increasing competition in the market for prescription drugs and helping facilitate the entry of lower-cost alternatives. This is particularly important for drugs like insulin that are taken by millions of Americans every day for a patient’s lifetime to manage a chronic disease,” FDA Commissioner Scott Gottlieb, MD, said in a statement “In the coming months, we’ll be taking additional policy steps to help to make sure patients continue to benefit from improved access to lower cost, safe and effective alternatives to brand name drugs approved through the agency’s abbreviated pathways.”
Insulin lispro injections are short-acting insulin products that can help improve blood sugar levels in diabetes patients. This can be useful in controlling blood sugar levels after eating. This contrasts with long-acting insulin products, which are intended to control background insulin levels between meals. The blood sugar control needs of type 1 and type 2 diabetes patients are unique. Type 1 patients require both short- and long-term controls, while some type 2 patients may never need a short-acting insulin product. Because of these differences, providing insulin lispro injections as a blood sugar control method may be particularly useful to type 1 diabetes patients who need to control mealtime blood sugar levels.
aged 3 years and older with type 1 and type 2 diabetes mellitus, according to a news release from the FDA.
Insulin lispro injections are to be administered subcutaneously by injection, infusion via an insulin pump, or intravenously. Doses should be individualized based on route of administration and patients’ individual metabolic needs. All diabetes patients should regularly monitor their blood sugar levels and insulin regimens should be exclusively modified under medical supervision.
“With today’s approval, we are providing an important short-acting insulin option for patients that meets our standards for safety and effectiveness,” Mary T. Thanh Hai, MD, deputy director of the Office of New Drug Evaluation II in the FDA’s Center for Drug Evaluation and Research, said in a statement.
Admelog, a biosimilar to Eli Lilly’s Humalog, was approved as a follow-on product based on the prior approval of Humalog. This allowed insulin lispro injections to be passed through the abbreviated approval pathway under the Federal Food, Drug, and Cosmetic Act, formally known as the 505(b)(2) pathway. The makers of insulin lispro found it was scientifically justified to use the previous safety and effectiveness data from the approval of Humalog to support the approval of insulin lispro injections. In addition to the Humalog data, the insulin lispro injection data included findings from two phase 3 clinical trials, each of which comprised about 500 diabetes patients. Using this abbreviated pathway can reduce the costs of drug development significantly, allowing new products to be offered to patients at lower prices.
“One of my key policy efforts is increasing competition in the market for prescription drugs and helping facilitate the entry of lower-cost alternatives. This is particularly important for drugs like insulin that are taken by millions of Americans every day for a patient’s lifetime to manage a chronic disease,” FDA Commissioner Scott Gottlieb, MD, said in a statement “In the coming months, we’ll be taking additional policy steps to help to make sure patients continue to benefit from improved access to lower cost, safe and effective alternatives to brand name drugs approved through the agency’s abbreviated pathways.”
Insulin lispro injections are short-acting insulin products that can help improve blood sugar levels in diabetes patients. This can be useful in controlling blood sugar levels after eating. This contrasts with long-acting insulin products, which are intended to control background insulin levels between meals. The blood sugar control needs of type 1 and type 2 diabetes patients are unique. Type 1 patients require both short- and long-term controls, while some type 2 patients may never need a short-acting insulin product. Because of these differences, providing insulin lispro injections as a blood sugar control method may be particularly useful to type 1 diabetes patients who need to control mealtime blood sugar levels.
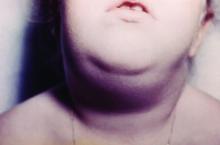
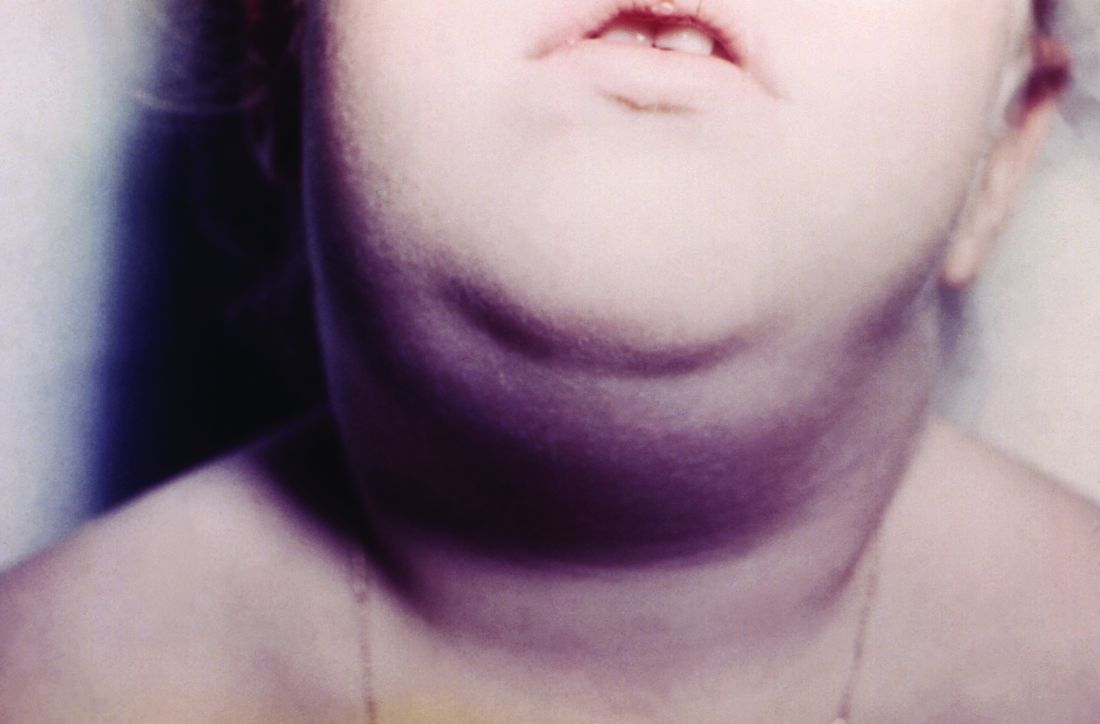
Hawaii experiencing a statewide outbreak of mumps
As of Nov. 30, 2017, 636 cases of mumps had been confirmed in Hawaii, according to the state’s Department of Health (DOH).
The Hawaii DOH originally reported 14 confirmed mumps cases statewide in April 2017, but the number of confirmed cases has increased by more than 4,000% since that time.
In the midst of the outbreak, the Hawaii DOH recommends that all adolescents between the aged 10-19 years old, and adults born in or after 1957, should receive an additional MMR vaccine dose as soon as possible. The outbreak dose is recommended regardless of previous vaccination or documented immunity to mumps. Administering additional doses of vaccine is not an ideal situation, the DOH noted, but said it should not cause any medical complications.
The Hawaii DOH will investigate mumps cases statewide as the outbreak continues.
As of Nov. 30, 2017, 636 cases of mumps had been confirmed in Hawaii, according to the state’s Department of Health (DOH).
The Hawaii DOH originally reported 14 confirmed mumps cases statewide in April 2017, but the number of confirmed cases has increased by more than 4,000% since that time.
In the midst of the outbreak, the Hawaii DOH recommends that all adolescents between the aged 10-19 years old, and adults born in or after 1957, should receive an additional MMR vaccine dose as soon as possible. The outbreak dose is recommended regardless of previous vaccination or documented immunity to mumps. Administering additional doses of vaccine is not an ideal situation, the DOH noted, but said it should not cause any medical complications.
The Hawaii DOH will investigate mumps cases statewide as the outbreak continues.
As of Nov. 30, 2017, 636 cases of mumps had been confirmed in Hawaii, according to the state’s Department of Health (DOH).
The Hawaii DOH originally reported 14 confirmed mumps cases statewide in April 2017, but the number of confirmed cases has increased by more than 4,000% since that time.
In the midst of the outbreak, the Hawaii DOH recommends that all adolescents between the aged 10-19 years old, and adults born in or after 1957, should receive an additional MMR vaccine dose as soon as possible. The outbreak dose is recommended regardless of previous vaccination or documented immunity to mumps. Administering additional doses of vaccine is not an ideal situation, the DOH noted, but said it should not cause any medical complications.
The Hawaii DOH will investigate mumps cases statewide as the outbreak continues.
FDA approves injectable diabetes drug that improves A1c scores
The Food and Drug Administration has approved semaglutide (OZEMPIC) injections for treatment of type 2 diabetes in adults, according to a press release from Novo Nordisk.
Semaglutide is a once-weekly injection of glucagon-like peptide (GLP-1) receptor agonist that, combined with diet and exercise, can improve glycemic control in adults with type 2 diabetes. Weekly injections are administered by health care providers in a prefilled pen subcutaneously in the stomach, abdomen, thigh, or upper arm as a 0.5-mg or 1-mg formulation. It is important that all doses be administered on the same day each week, according to the OZEMPIC package insert.
“The OZEMPIC (semaglutide) approval builds on Novo Nordisk’s commitment to offering health care professionals a range of treatments that effectively addresses the complex needs of diabetes management and fits their patients’ lifestyles,” said Todd Hobbs, vice president and U.S. chief medical officer of Novo Nordisk.
To ensure access to semaglutide, Novo Nordisk is pricing the drug competitively with other GLP-1 receptor agonists and will offer an associated savings card program to reduce copays for insured patients, the company said. Novo Nordisk expects to launch OZEMPIC in the United States in the first quarter of 2018, and is working on contracting solutions with health insurance providers to increase patient access to the drug.
According to the Novo Nordisk statement, clinicians should not consider semaglutide as a first choice option for treating diabetes or as a substitute for insulin in patients with type 1 diabetes and diabetic ketoacidosis. Whether semaglutide can be used by people who have had pancreatitis or is safe in patients under the age of 18 years old remains to be seen.
“Type 2 diabetes is a serious condition that affects more than 28 million people in the U.S., and despite advancements in treatment, some people with type 2 diabetes do not achieve their A1c goals,” said Helena Rodbard, MD, past president of the American Association of Clinical Endocrinologists. “The approval of semaglutide offers health care professionals an important new treatment option to help adults with type 2 diabetes meet their A1c goals.”
The Food and Drug Administration has approved semaglutide (OZEMPIC) injections for treatment of type 2 diabetes in adults, according to a press release from Novo Nordisk.
Semaglutide is a once-weekly injection of glucagon-like peptide (GLP-1) receptor agonist that, combined with diet and exercise, can improve glycemic control in adults with type 2 diabetes. Weekly injections are administered by health care providers in a prefilled pen subcutaneously in the stomach, abdomen, thigh, or upper arm as a 0.5-mg or 1-mg formulation. It is important that all doses be administered on the same day each week, according to the OZEMPIC package insert.
“The OZEMPIC (semaglutide) approval builds on Novo Nordisk’s commitment to offering health care professionals a range of treatments that effectively addresses the complex needs of diabetes management and fits their patients’ lifestyles,” said Todd Hobbs, vice president and U.S. chief medical officer of Novo Nordisk.
To ensure access to semaglutide, Novo Nordisk is pricing the drug competitively with other GLP-1 receptor agonists and will offer an associated savings card program to reduce copays for insured patients, the company said. Novo Nordisk expects to launch OZEMPIC in the United States in the first quarter of 2018, and is working on contracting solutions with health insurance providers to increase patient access to the drug.
According to the Novo Nordisk statement, clinicians should not consider semaglutide as a first choice option for treating diabetes or as a substitute for insulin in patients with type 1 diabetes and diabetic ketoacidosis. Whether semaglutide can be used by people who have had pancreatitis or is safe in patients under the age of 18 years old remains to be seen.
“Type 2 diabetes is a serious condition that affects more than 28 million people in the U.S., and despite advancements in treatment, some people with type 2 diabetes do not achieve their A1c goals,” said Helena Rodbard, MD, past president of the American Association of Clinical Endocrinologists. “The approval of semaglutide offers health care professionals an important new treatment option to help adults with type 2 diabetes meet their A1c goals.”
The Food and Drug Administration has approved semaglutide (OZEMPIC) injections for treatment of type 2 diabetes in adults, according to a press release from Novo Nordisk.
Semaglutide is a once-weekly injection of glucagon-like peptide (GLP-1) receptor agonist that, combined with diet and exercise, can improve glycemic control in adults with type 2 diabetes. Weekly injections are administered by health care providers in a prefilled pen subcutaneously in the stomach, abdomen, thigh, or upper arm as a 0.5-mg or 1-mg formulation. It is important that all doses be administered on the same day each week, according to the OZEMPIC package insert.
“The OZEMPIC (semaglutide) approval builds on Novo Nordisk’s commitment to offering health care professionals a range of treatments that effectively addresses the complex needs of diabetes management and fits their patients’ lifestyles,” said Todd Hobbs, vice president and U.S. chief medical officer of Novo Nordisk.
To ensure access to semaglutide, Novo Nordisk is pricing the drug competitively with other GLP-1 receptor agonists and will offer an associated savings card program to reduce copays for insured patients, the company said. Novo Nordisk expects to launch OZEMPIC in the United States in the first quarter of 2018, and is working on contracting solutions with health insurance providers to increase patient access to the drug.
According to the Novo Nordisk statement, clinicians should not consider semaglutide as a first choice option for treating diabetes or as a substitute for insulin in patients with type 1 diabetes and diabetic ketoacidosis. Whether semaglutide can be used by people who have had pancreatitis or is safe in patients under the age of 18 years old remains to be seen.
“Type 2 diabetes is a serious condition that affects more than 28 million people in the U.S., and despite advancements in treatment, some people with type 2 diabetes do not achieve their A1c goals,” said Helena Rodbard, MD, past president of the American Association of Clinical Endocrinologists. “The approval of semaglutide offers health care professionals an important new treatment option to help adults with type 2 diabetes meet their A1c goals.”