User login
AAFP Congress votes to keep Coke alliance
WASHINGTON – Family doctors who do not support the alliance between the American Academy of Family Physicians and the Coca-Cola Company were unable to convince their peers to break up that union.
The final vote on the resolution introduced by the Maryland and Rhode Island chapters was 72-20 in favor of the academy considering a renewal of the relationship in late 2015.
Dr. Robert E. Reneker Jr., a delegate from Michigan, said that he was voting in favor of the relationship based on its financial benefits. However, he added, “the board, our leadership, should understand this: You need to extract us from Coke as soon as it is financially viable and actually actively work to do that. I will vote against this resolution this year, but I am not going to keep giving you a pass on this. Get us out of Coke.”
Delegates voted by consent to accept a number of committee recommendations, including not adopting resolutions on forming a task force to examine proportional representation within the Congress of Delegates; that family medicine physicians be referred to as physicians and not health care providers; and changes to awarding a title of fellow that would recognize a student’s medical school membership toward the 6-year requirement.
Delegates referred for further investigation resolutions examining the language of the oath of AAFP fellows, specifically the clause to placing patient “welfare above all else” and that the AAFP use the International System of Units throughout its journals, publications, and clinical communications and present a similar resolution to the American Medical Association.
WASHINGTON – Family doctors who do not support the alliance between the American Academy of Family Physicians and the Coca-Cola Company were unable to convince their peers to break up that union.
The final vote on the resolution introduced by the Maryland and Rhode Island chapters was 72-20 in favor of the academy considering a renewal of the relationship in late 2015.
Dr. Robert E. Reneker Jr., a delegate from Michigan, said that he was voting in favor of the relationship based on its financial benefits. However, he added, “the board, our leadership, should understand this: You need to extract us from Coke as soon as it is financially viable and actually actively work to do that. I will vote against this resolution this year, but I am not going to keep giving you a pass on this. Get us out of Coke.”
Delegates voted by consent to accept a number of committee recommendations, including not adopting resolutions on forming a task force to examine proportional representation within the Congress of Delegates; that family medicine physicians be referred to as physicians and not health care providers; and changes to awarding a title of fellow that would recognize a student’s medical school membership toward the 6-year requirement.
Delegates referred for further investigation resolutions examining the language of the oath of AAFP fellows, specifically the clause to placing patient “welfare above all else” and that the AAFP use the International System of Units throughout its journals, publications, and clinical communications and present a similar resolution to the American Medical Association.
WASHINGTON – Family doctors who do not support the alliance between the American Academy of Family Physicians and the Coca-Cola Company were unable to convince their peers to break up that union.
The final vote on the resolution introduced by the Maryland and Rhode Island chapters was 72-20 in favor of the academy considering a renewal of the relationship in late 2015.
Dr. Robert E. Reneker Jr., a delegate from Michigan, said that he was voting in favor of the relationship based on its financial benefits. However, he added, “the board, our leadership, should understand this: You need to extract us from Coke as soon as it is financially viable and actually actively work to do that. I will vote against this resolution this year, but I am not going to keep giving you a pass on this. Get us out of Coke.”
Delegates voted by consent to accept a number of committee recommendations, including not adopting resolutions on forming a task force to examine proportional representation within the Congress of Delegates; that family medicine physicians be referred to as physicians and not health care providers; and changes to awarding a title of fellow that would recognize a student’s medical school membership toward the 6-year requirement.
Delegates referred for further investigation resolutions examining the language of the oath of AAFP fellows, specifically the clause to placing patient “welfare above all else” and that the AAFP use the International System of Units throughout its journals, publications, and clinical communications and present a similar resolution to the American Medical Association.
AAFP Congress to push for ending of tobacco sales at pharmacies
WASHINGTON – The American Academy of Family Physicians voiced its support for ending of tobacco sales at pharmacies and for developing end-of-life registries.
Resolutions from the Reference Committee on Health of the Public & Science were adopted by consent without debate Oct. 21 during a session of the full Congress of Delegates, with a number being referred back to the board of directors for further investigation.
During an Oct. 20 session to present the resolutions, all testimony provided by delegates was in support of a resolution introduced by the New York State, Pennsylvania, and Rhode Island chapters directing AAFP to “support ending the sale of tobacco products in all pharmacies and stores that contain a pharmacy department,” and to work with various pharmacy and pharmacist associations to push for the prohibition of sale of tobacco products at pharmacies.
The move comes in the wake of CVS/Caremark announcing that it will charge higher copays at pharmacies that also sell tobacco products, a move that could be designed to drive business to CVS/pharmacies, which no longer sells tobacco products in the retail portion of its stores.
An end-of-life planning resolution offered by the Michigan chapter also received support, though specific concerns were voiced during the Oct. 20 reference committee session.
The resolution directs AAFP to support the implementation of centralized registries, similar to Michigan’s “Peace of Mind Registry” that offers a central repository housing a patient’s end-of-life care preferences. It also calls for support of mechanisms that encourage the uploading of durable powers of attorney, physician order scope of treatment forms, and do-not-resuscitate orders into the registry, advocates for the development of centralized HIPAA registries of an individual’s preferences for end-of-life care, and supports mechanisms that allow for the registries to be readily accessible and routinely used by medical professionals.
Delegates generally supported the resolution during the Oct. 20 session, though some concerns regarding its implementation were raised, including wording that was eventually dropped regarding how public the information would be.
Other concerns included understanding how the registries would be updated as patient preferences changed.
Dr. Dale Ragle, president of the Texas delegation, offered his support of the spirit of the resolution, asked for wording to be included specifically on who could access such registries, similar to wording that is used to determine access to prescription drug–monitoring programs, including patients and their families as well as physicians.
“What happens if the wishes or the conditions of the patient changes?” Dr. Ragle asked. “Perhaps there should be some sort of mechanism in place or some sort of assurances that would be ... reviewed and updated periodically as the conditions may change. Also maybe put wording in there that a physician could override the database ... at the wishes of the patients that they verbally express them ... or if the physician has reliable information that perhaps the patient’s wishes have changed or the condition has changed.”
Florida delegate Dr. Dennis Saver also noted that access when a patient travels outside of his home state also need to be addressed.
Integrating information into electronic health records also was raised as a need from multiple delegates voicing support for the resolution.
Other resolutions adopted relate to addressing disparities and discrimination in immigrant populations and support for the Center for the Study of Tobacco and Society.
One resolution that received strong support from delegates at the reference committee meeting but ultimately was referred to the board for further study was the development of educational materials regarding the recreational use of marijuana, particularly as states begin to legalize it for that purpose.
Dr Elizabeth Snyder, the Oregon delegate, noted that family physicians are “going to have a different kind of counseling that we need to do and even without it becoming legal, we find ourselves with a paucity of useful, valid scientific information that we can use to educate our patients, both young and older, about marijuana use and its risks. We would therefore encourage the academy to help develop high-quality educational materials as it has done for many other substances that people use or medical conditions that we can reliably use in our practices.”
Other resolutions referred back to the board for further investigation addressed a vaccination personal belief exemption policy, support of the One Health Initiative, expansion of venues for peer-reviewed family medicine research, support of the prohibition of sale/distribution of raw or unpasteurized milk or milk products and support of the ban on nontherapeutic antibiotic use in farm animals.
WASHINGTON – The American Academy of Family Physicians voiced its support for ending of tobacco sales at pharmacies and for developing end-of-life registries.
Resolutions from the Reference Committee on Health of the Public & Science were adopted by consent without debate Oct. 21 during a session of the full Congress of Delegates, with a number being referred back to the board of directors for further investigation.
During an Oct. 20 session to present the resolutions, all testimony provided by delegates was in support of a resolution introduced by the New York State, Pennsylvania, and Rhode Island chapters directing AAFP to “support ending the sale of tobacco products in all pharmacies and stores that contain a pharmacy department,” and to work with various pharmacy and pharmacist associations to push for the prohibition of sale of tobacco products at pharmacies.
The move comes in the wake of CVS/Caremark announcing that it will charge higher copays at pharmacies that also sell tobacco products, a move that could be designed to drive business to CVS/pharmacies, which no longer sells tobacco products in the retail portion of its stores.
An end-of-life planning resolution offered by the Michigan chapter also received support, though specific concerns were voiced during the Oct. 20 reference committee session.
The resolution directs AAFP to support the implementation of centralized registries, similar to Michigan’s “Peace of Mind Registry” that offers a central repository housing a patient’s end-of-life care preferences. It also calls for support of mechanisms that encourage the uploading of durable powers of attorney, physician order scope of treatment forms, and do-not-resuscitate orders into the registry, advocates for the development of centralized HIPAA registries of an individual’s preferences for end-of-life care, and supports mechanisms that allow for the registries to be readily accessible and routinely used by medical professionals.
Delegates generally supported the resolution during the Oct. 20 session, though some concerns regarding its implementation were raised, including wording that was eventually dropped regarding how public the information would be.
Other concerns included understanding how the registries would be updated as patient preferences changed.
Dr. Dale Ragle, president of the Texas delegation, offered his support of the spirit of the resolution, asked for wording to be included specifically on who could access such registries, similar to wording that is used to determine access to prescription drug–monitoring programs, including patients and their families as well as physicians.
“What happens if the wishes or the conditions of the patient changes?” Dr. Ragle asked. “Perhaps there should be some sort of mechanism in place or some sort of assurances that would be ... reviewed and updated periodically as the conditions may change. Also maybe put wording in there that a physician could override the database ... at the wishes of the patients that they verbally express them ... or if the physician has reliable information that perhaps the patient’s wishes have changed or the condition has changed.”
Florida delegate Dr. Dennis Saver also noted that access when a patient travels outside of his home state also need to be addressed.
Integrating information into electronic health records also was raised as a need from multiple delegates voicing support for the resolution.
Other resolutions adopted relate to addressing disparities and discrimination in immigrant populations and support for the Center for the Study of Tobacco and Society.
One resolution that received strong support from delegates at the reference committee meeting but ultimately was referred to the board for further study was the development of educational materials regarding the recreational use of marijuana, particularly as states begin to legalize it for that purpose.
Dr Elizabeth Snyder, the Oregon delegate, noted that family physicians are “going to have a different kind of counseling that we need to do and even without it becoming legal, we find ourselves with a paucity of useful, valid scientific information that we can use to educate our patients, both young and older, about marijuana use and its risks. We would therefore encourage the academy to help develop high-quality educational materials as it has done for many other substances that people use or medical conditions that we can reliably use in our practices.”
Other resolutions referred back to the board for further investigation addressed a vaccination personal belief exemption policy, support of the One Health Initiative, expansion of venues for peer-reviewed family medicine research, support of the prohibition of sale/distribution of raw or unpasteurized milk or milk products and support of the ban on nontherapeutic antibiotic use in farm animals.
WASHINGTON – The American Academy of Family Physicians voiced its support for ending of tobacco sales at pharmacies and for developing end-of-life registries.
Resolutions from the Reference Committee on Health of the Public & Science were adopted by consent without debate Oct. 21 during a session of the full Congress of Delegates, with a number being referred back to the board of directors for further investigation.
During an Oct. 20 session to present the resolutions, all testimony provided by delegates was in support of a resolution introduced by the New York State, Pennsylvania, and Rhode Island chapters directing AAFP to “support ending the sale of tobacco products in all pharmacies and stores that contain a pharmacy department,” and to work with various pharmacy and pharmacist associations to push for the prohibition of sale of tobacco products at pharmacies.
The move comes in the wake of CVS/Caremark announcing that it will charge higher copays at pharmacies that also sell tobacco products, a move that could be designed to drive business to CVS/pharmacies, which no longer sells tobacco products in the retail portion of its stores.
An end-of-life planning resolution offered by the Michigan chapter also received support, though specific concerns were voiced during the Oct. 20 reference committee session.
The resolution directs AAFP to support the implementation of centralized registries, similar to Michigan’s “Peace of Mind Registry” that offers a central repository housing a patient’s end-of-life care preferences. It also calls for support of mechanisms that encourage the uploading of durable powers of attorney, physician order scope of treatment forms, and do-not-resuscitate orders into the registry, advocates for the development of centralized HIPAA registries of an individual’s preferences for end-of-life care, and supports mechanisms that allow for the registries to be readily accessible and routinely used by medical professionals.
Delegates generally supported the resolution during the Oct. 20 session, though some concerns regarding its implementation were raised, including wording that was eventually dropped regarding how public the information would be.
Other concerns included understanding how the registries would be updated as patient preferences changed.
Dr. Dale Ragle, president of the Texas delegation, offered his support of the spirit of the resolution, asked for wording to be included specifically on who could access such registries, similar to wording that is used to determine access to prescription drug–monitoring programs, including patients and their families as well as physicians.
“What happens if the wishes or the conditions of the patient changes?” Dr. Ragle asked. “Perhaps there should be some sort of mechanism in place or some sort of assurances that would be ... reviewed and updated periodically as the conditions may change. Also maybe put wording in there that a physician could override the database ... at the wishes of the patients that they verbally express them ... or if the physician has reliable information that perhaps the patient’s wishes have changed or the condition has changed.”
Florida delegate Dr. Dennis Saver also noted that access when a patient travels outside of his home state also need to be addressed.
Integrating information into electronic health records also was raised as a need from multiple delegates voicing support for the resolution.
Other resolutions adopted relate to addressing disparities and discrimination in immigrant populations and support for the Center for the Study of Tobacco and Society.
One resolution that received strong support from delegates at the reference committee meeting but ultimately was referred to the board for further study was the development of educational materials regarding the recreational use of marijuana, particularly as states begin to legalize it for that purpose.
Dr Elizabeth Snyder, the Oregon delegate, noted that family physicians are “going to have a different kind of counseling that we need to do and even without it becoming legal, we find ourselves with a paucity of useful, valid scientific information that we can use to educate our patients, both young and older, about marijuana use and its risks. We would therefore encourage the academy to help develop high-quality educational materials as it has done for many other substances that people use or medical conditions that we can reliably use in our practices.”
Other resolutions referred back to the board for further investigation addressed a vaccination personal belief exemption policy, support of the One Health Initiative, expansion of venues for peer-reviewed family medicine research, support of the prohibition of sale/distribution of raw or unpasteurized milk or milk products and support of the ban on nontherapeutic antibiotic use in farm animals.
AT THE AAFP CONGRESS OF DELEGATES
Physician groups: Fix interoperability before advancing with meaningful use
Physician groups are growing increasingly frustrated with the focus on meaningful use of electronic health records at the expense of creating an interoperable health information technology infrastructure and are calling on the Department of Health & Human Services to step up on interoperability.
In an Oct. 15 letter to HHS Secretary Sylvia Burwell, a number of groups cited the HHS Office of the National Coordinator for Health Information Technology’s finding that less than 14% of physicians are able to electronically transmit health information outside of their organization because of a lack of EHR interoperability and other issues.
“These barriers to data exchange proliferated as a result of a variety of factors [including] strict MU [meaningful use] requirements and deadlines that do not provide sufficient time to focus on achieving interoperability. This dynamic is also in part due to the strict EHR certification requirements that have forced all stakeholders involved to focus on meeting MU measures as opposed to developing more innovative technological solutions that will enhance patient care and safety while growing the marketplace.”
Groups signing the letter include the American Academy of Family Physicians, American Medical Association, Medical Group Management Association, National Rural Health Association and a number of health care systems.
The letter also notes that in addition to interoperability, usability remains an issue that causes disruption in provider workflow and diverts resources away from patient care, noting that “vendors are limited from addressing these concerns as they focus on meeting increasingly complex certification requirements.”
Among its recommendations, the groups asked for HHS to recognize that the vendor community needs time to develop, test, and implement updates to meet new criteria and should be afforded that time “before continuing on with subsequent stages of the MU program. Testing and achievement of specific performance benchmarks should occur before providers are held accountable for any MU requirements.”
The letter comes as an advisory committee to the Office of the National Coordinator (ONC) is making a same-day recommendation that it delays or staggers meaningful use stage 3 to shift focus on achieving meaningful interoperability and addressing other infrastructure issues.
In its October 2014 report to Congress, the ONC acknowledged issues related to interoperability and other issues that are presenting a barrier for health IT to achieve potential.
“Despite progress in establishing standards and services to support health information exchange and interoperability, practice patterns have not changed to the point that health care providers share patient health information electronically across organizational, vendor, and geographic boundaries,” the report states. “Patient electronic health information needs to be available for appropriate use in solving major challenges, such as providing more effective care and informing and accelerating scientific research.”
To that end, ONC released during an Oct. 15 advisory committee meeting some top-level aspects of its 10-year framework on how it will improve interoperability, which is scheduled to be formalized in March 2015.
According to draft materials, the roadmap calls for health care providers to be able to send, receive, find, and use a basic set of essential health information. By 2020, more granular information should be accessible across systems, which would lead to improved quality and reduced costs. By 2024, the interoperability vision, with systems communicating in full, will lead to a learning health system and facilitate ubiquitous precision medicine.
Separately, AMA in an Oct. 14 letter to CMS and ONC criticized the meaningful use program and offered a series of recommendations to fix it before movement to stage 3 of the program. The group wants to see more flexibility in requirements physicians need to meet requirements, expanding hardship exemptions for all stages, improving quality reporting, and addressing physician EHR usability challenges.
“Many of the MU requirements were designed to increase patient choice and quality of care,” the AMA writes. “Unfortunately, many of these requirements, especially those in the latter phases of the MU program, are having the opposite effect. Oftentimes the requirements decrease the efficiency of patient visits.”
AMA also called on CMS and ONC to “study the total cost of compliance with MU to understand the impact this program is having on practice.”
Physician groups are growing increasingly frustrated with the focus on meaningful use of electronic health records at the expense of creating an interoperable health information technology infrastructure and are calling on the Department of Health & Human Services to step up on interoperability.
In an Oct. 15 letter to HHS Secretary Sylvia Burwell, a number of groups cited the HHS Office of the National Coordinator for Health Information Technology’s finding that less than 14% of physicians are able to electronically transmit health information outside of their organization because of a lack of EHR interoperability and other issues.
“These barriers to data exchange proliferated as a result of a variety of factors [including] strict MU [meaningful use] requirements and deadlines that do not provide sufficient time to focus on achieving interoperability. This dynamic is also in part due to the strict EHR certification requirements that have forced all stakeholders involved to focus on meeting MU measures as opposed to developing more innovative technological solutions that will enhance patient care and safety while growing the marketplace.”
Groups signing the letter include the American Academy of Family Physicians, American Medical Association, Medical Group Management Association, National Rural Health Association and a number of health care systems.
The letter also notes that in addition to interoperability, usability remains an issue that causes disruption in provider workflow and diverts resources away from patient care, noting that “vendors are limited from addressing these concerns as they focus on meeting increasingly complex certification requirements.”
Among its recommendations, the groups asked for HHS to recognize that the vendor community needs time to develop, test, and implement updates to meet new criteria and should be afforded that time “before continuing on with subsequent stages of the MU program. Testing and achievement of specific performance benchmarks should occur before providers are held accountable for any MU requirements.”
The letter comes as an advisory committee to the Office of the National Coordinator (ONC) is making a same-day recommendation that it delays or staggers meaningful use stage 3 to shift focus on achieving meaningful interoperability and addressing other infrastructure issues.
In its October 2014 report to Congress, the ONC acknowledged issues related to interoperability and other issues that are presenting a barrier for health IT to achieve potential.
“Despite progress in establishing standards and services to support health information exchange and interoperability, practice patterns have not changed to the point that health care providers share patient health information electronically across organizational, vendor, and geographic boundaries,” the report states. “Patient electronic health information needs to be available for appropriate use in solving major challenges, such as providing more effective care and informing and accelerating scientific research.”
To that end, ONC released during an Oct. 15 advisory committee meeting some top-level aspects of its 10-year framework on how it will improve interoperability, which is scheduled to be formalized in March 2015.
According to draft materials, the roadmap calls for health care providers to be able to send, receive, find, and use a basic set of essential health information. By 2020, more granular information should be accessible across systems, which would lead to improved quality and reduced costs. By 2024, the interoperability vision, with systems communicating in full, will lead to a learning health system and facilitate ubiquitous precision medicine.
Separately, AMA in an Oct. 14 letter to CMS and ONC criticized the meaningful use program and offered a series of recommendations to fix it before movement to stage 3 of the program. The group wants to see more flexibility in requirements physicians need to meet requirements, expanding hardship exemptions for all stages, improving quality reporting, and addressing physician EHR usability challenges.
“Many of the MU requirements were designed to increase patient choice and quality of care,” the AMA writes. “Unfortunately, many of these requirements, especially those in the latter phases of the MU program, are having the opposite effect. Oftentimes the requirements decrease the efficiency of patient visits.”
AMA also called on CMS and ONC to “study the total cost of compliance with MU to understand the impact this program is having on practice.”
Physician groups are growing increasingly frustrated with the focus on meaningful use of electronic health records at the expense of creating an interoperable health information technology infrastructure and are calling on the Department of Health & Human Services to step up on interoperability.
In an Oct. 15 letter to HHS Secretary Sylvia Burwell, a number of groups cited the HHS Office of the National Coordinator for Health Information Technology’s finding that less than 14% of physicians are able to electronically transmit health information outside of their organization because of a lack of EHR interoperability and other issues.
“These barriers to data exchange proliferated as a result of a variety of factors [including] strict MU [meaningful use] requirements and deadlines that do not provide sufficient time to focus on achieving interoperability. This dynamic is also in part due to the strict EHR certification requirements that have forced all stakeholders involved to focus on meeting MU measures as opposed to developing more innovative technological solutions that will enhance patient care and safety while growing the marketplace.”
Groups signing the letter include the American Academy of Family Physicians, American Medical Association, Medical Group Management Association, National Rural Health Association and a number of health care systems.
The letter also notes that in addition to interoperability, usability remains an issue that causes disruption in provider workflow and diverts resources away from patient care, noting that “vendors are limited from addressing these concerns as they focus on meeting increasingly complex certification requirements.”
Among its recommendations, the groups asked for HHS to recognize that the vendor community needs time to develop, test, and implement updates to meet new criteria and should be afforded that time “before continuing on with subsequent stages of the MU program. Testing and achievement of specific performance benchmarks should occur before providers are held accountable for any MU requirements.”
The letter comes as an advisory committee to the Office of the National Coordinator (ONC) is making a same-day recommendation that it delays or staggers meaningful use stage 3 to shift focus on achieving meaningful interoperability and addressing other infrastructure issues.
In its October 2014 report to Congress, the ONC acknowledged issues related to interoperability and other issues that are presenting a barrier for health IT to achieve potential.
“Despite progress in establishing standards and services to support health information exchange and interoperability, practice patterns have not changed to the point that health care providers share patient health information electronically across organizational, vendor, and geographic boundaries,” the report states. “Patient electronic health information needs to be available for appropriate use in solving major challenges, such as providing more effective care and informing and accelerating scientific research.”
To that end, ONC released during an Oct. 15 advisory committee meeting some top-level aspects of its 10-year framework on how it will improve interoperability, which is scheduled to be formalized in March 2015.
According to draft materials, the roadmap calls for health care providers to be able to send, receive, find, and use a basic set of essential health information. By 2020, more granular information should be accessible across systems, which would lead to improved quality and reduced costs. By 2024, the interoperability vision, with systems communicating in full, will lead to a learning health system and facilitate ubiquitous precision medicine.
Separately, AMA in an Oct. 14 letter to CMS and ONC criticized the meaningful use program and offered a series of recommendations to fix it before movement to stage 3 of the program. The group wants to see more flexibility in requirements physicians need to meet requirements, expanding hardship exemptions for all stages, improving quality reporting, and addressing physician EHR usability challenges.
“Many of the MU requirements were designed to increase patient choice and quality of care,” the AMA writes. “Unfortunately, many of these requirements, especially those in the latter phases of the MU program, are having the opposite effect. Oftentimes the requirements decrease the efficiency of patient visits.”
AMA also called on CMS and ONC to “study the total cost of compliance with MU to understand the impact this program is having on practice.”
AAFP Congress: Delegates remain divided on Coca-Cola alliance
WASHINGTON – The controversial alliance between the American Academy of Family Physicians and the Coca-Cola Company took center stage during preliminary discussions Oct. 20 at the AAFP Congress of Delegates.
The congress’s Reference Committee on Organization and Finance addressed a resolution offered by the Maryland and Rhode Island chapters that calls on AAFP to not engage in discussions to renew the alliance when the time comes in November 2015.
Public perception of the partnership dominated those who opposed the renewing alliance as sugary drinks come under public scrutiny for their role in the growing obesity problem, despite positive steps the Coca-Cola Co. has taken to encourage healthier behavior.
A delegate from New York, who admitted to being a Coke drinker, said that the organization “shouldn’t underestimate how negatively that AAFP is looked upon for its link to this company where most of their products are not health choices for their patients. As a group that is trying to promote health, I would say we shouldn’t be linked or taking large amounts of sponsorship from them.”
A renewed relationship with Coca-Cola could bring the AAFP more than $1 million over 2 years, according to the resolution.
Another delegate noted recent evidence suggesting that artificial sweeteners could be contributing to metabolic disorders, making it more difficult to promote diet soft drinks as an alternative.
In support of the alliance, a delegate from Texas noted that many AAFP member benefits, including offering continuing medical education credits and political advocacy, “cost money. You do have to look at this [alliance] with eyes open and sometimes have to look at both sides. ... Sometimes we have to make difficult compromises to support our overall mission.”
Dr. Lori J. Heim, AAFP president from 2009-2010, also spoke in support of the alliance.
Dr. Heim noted that the company has sought out not only AAFP, but also other medical specialty societies representing cardiologists, pediatricians, and sports medicine specialties, as well as representatives from groups representing nutritionists and nurses, to solicit feedback on becoming a better corporate citizen.
And while the changes that have come from this have not gone far enough, Dr. Heim said, there has been progress, such as the smaller cans and clearly displayed calorie information to help patients make better choices.
“This is pretty much unheard of,” said Dr. Heim, who was AAFP president when the alliance was formed. “Have there been monumental changes? No. Have they gone as far as I would have liked them to go? No. Have we seen changes though? Yes.”
And those steps of progress go well beyond the initial intent of the alliance, which Dr. Heim reminded reference committee members was to help promote FamilyDoctor.org.
As for the perception issue, while many delegates felt that the alliance put them in an awkward position to have to defend it to their patients, Dr. Reid Blackwelder suggested the perception issue may be more individualized rather than widespread.
“I will assure you our reputation is strong and our voice is heard,” said Dr. Blackwelder, who completes his term as AAFP president on Oct. 22. “And even though you will hear stories of how folks are having to justify the relationship, as your face and voice this past year, I can promise you I have not done a single media interview or question about this issue. You can argue whether that should or should not be the case but many other issues are where I am being asked for input and not for this one.”
Other resolutions addressed at the reference committee meeting included:
• A resolution from the Tennessee delegation to modify the oath of AAFP Fellows. The resolution would strike a clause about patients and “placing their welfare above all else” and replace it with language that better supports work/life balance.
• A resolution from the Texas delegation that would shift the composition of the AAFP Congress of Delegates away from a senatorial style makeup (two delegates and an alternate from each state chapter) and instead compose the body on a more proportional basis.
WASHINGTON – The controversial alliance between the American Academy of Family Physicians and the Coca-Cola Company took center stage during preliminary discussions Oct. 20 at the AAFP Congress of Delegates.
The congress’s Reference Committee on Organization and Finance addressed a resolution offered by the Maryland and Rhode Island chapters that calls on AAFP to not engage in discussions to renew the alliance when the time comes in November 2015.
Public perception of the partnership dominated those who opposed the renewing alliance as sugary drinks come under public scrutiny for their role in the growing obesity problem, despite positive steps the Coca-Cola Co. has taken to encourage healthier behavior.
A delegate from New York, who admitted to being a Coke drinker, said that the organization “shouldn’t underestimate how negatively that AAFP is looked upon for its link to this company where most of their products are not health choices for their patients. As a group that is trying to promote health, I would say we shouldn’t be linked or taking large amounts of sponsorship from them.”
A renewed relationship with Coca-Cola could bring the AAFP more than $1 million over 2 years, according to the resolution.
Another delegate noted recent evidence suggesting that artificial sweeteners could be contributing to metabolic disorders, making it more difficult to promote diet soft drinks as an alternative.
In support of the alliance, a delegate from Texas noted that many AAFP member benefits, including offering continuing medical education credits and political advocacy, “cost money. You do have to look at this [alliance] with eyes open and sometimes have to look at both sides. ... Sometimes we have to make difficult compromises to support our overall mission.”
Dr. Lori J. Heim, AAFP president from 2009-2010, also spoke in support of the alliance.
Dr. Heim noted that the company has sought out not only AAFP, but also other medical specialty societies representing cardiologists, pediatricians, and sports medicine specialties, as well as representatives from groups representing nutritionists and nurses, to solicit feedback on becoming a better corporate citizen.
And while the changes that have come from this have not gone far enough, Dr. Heim said, there has been progress, such as the smaller cans and clearly displayed calorie information to help patients make better choices.
“This is pretty much unheard of,” said Dr. Heim, who was AAFP president when the alliance was formed. “Have there been monumental changes? No. Have they gone as far as I would have liked them to go? No. Have we seen changes though? Yes.”
And those steps of progress go well beyond the initial intent of the alliance, which Dr. Heim reminded reference committee members was to help promote FamilyDoctor.org.
As for the perception issue, while many delegates felt that the alliance put them in an awkward position to have to defend it to their patients, Dr. Reid Blackwelder suggested the perception issue may be more individualized rather than widespread.
“I will assure you our reputation is strong and our voice is heard,” said Dr. Blackwelder, who completes his term as AAFP president on Oct. 22. “And even though you will hear stories of how folks are having to justify the relationship, as your face and voice this past year, I can promise you I have not done a single media interview or question about this issue. You can argue whether that should or should not be the case but many other issues are where I am being asked for input and not for this one.”
Other resolutions addressed at the reference committee meeting included:
• A resolution from the Tennessee delegation to modify the oath of AAFP Fellows. The resolution would strike a clause about patients and “placing their welfare above all else” and replace it with language that better supports work/life balance.
• A resolution from the Texas delegation that would shift the composition of the AAFP Congress of Delegates away from a senatorial style makeup (two delegates and an alternate from each state chapter) and instead compose the body on a more proportional basis.
WASHINGTON – The controversial alliance between the American Academy of Family Physicians and the Coca-Cola Company took center stage during preliminary discussions Oct. 20 at the AAFP Congress of Delegates.
The congress’s Reference Committee on Organization and Finance addressed a resolution offered by the Maryland and Rhode Island chapters that calls on AAFP to not engage in discussions to renew the alliance when the time comes in November 2015.
Public perception of the partnership dominated those who opposed the renewing alliance as sugary drinks come under public scrutiny for their role in the growing obesity problem, despite positive steps the Coca-Cola Co. has taken to encourage healthier behavior.
A delegate from New York, who admitted to being a Coke drinker, said that the organization “shouldn’t underestimate how negatively that AAFP is looked upon for its link to this company where most of their products are not health choices for their patients. As a group that is trying to promote health, I would say we shouldn’t be linked or taking large amounts of sponsorship from them.”
A renewed relationship with Coca-Cola could bring the AAFP more than $1 million over 2 years, according to the resolution.
Another delegate noted recent evidence suggesting that artificial sweeteners could be contributing to metabolic disorders, making it more difficult to promote diet soft drinks as an alternative.
In support of the alliance, a delegate from Texas noted that many AAFP member benefits, including offering continuing medical education credits and political advocacy, “cost money. You do have to look at this [alliance] with eyes open and sometimes have to look at both sides. ... Sometimes we have to make difficult compromises to support our overall mission.”
Dr. Lori J. Heim, AAFP president from 2009-2010, also spoke in support of the alliance.
Dr. Heim noted that the company has sought out not only AAFP, but also other medical specialty societies representing cardiologists, pediatricians, and sports medicine specialties, as well as representatives from groups representing nutritionists and nurses, to solicit feedback on becoming a better corporate citizen.
And while the changes that have come from this have not gone far enough, Dr. Heim said, there has been progress, such as the smaller cans and clearly displayed calorie information to help patients make better choices.
“This is pretty much unheard of,” said Dr. Heim, who was AAFP president when the alliance was formed. “Have there been monumental changes? No. Have they gone as far as I would have liked them to go? No. Have we seen changes though? Yes.”
And those steps of progress go well beyond the initial intent of the alliance, which Dr. Heim reminded reference committee members was to help promote FamilyDoctor.org.
As for the perception issue, while many delegates felt that the alliance put them in an awkward position to have to defend it to their patients, Dr. Reid Blackwelder suggested the perception issue may be more individualized rather than widespread.
“I will assure you our reputation is strong and our voice is heard,” said Dr. Blackwelder, who completes his term as AAFP president on Oct. 22. “And even though you will hear stories of how folks are having to justify the relationship, as your face and voice this past year, I can promise you I have not done a single media interview or question about this issue. You can argue whether that should or should not be the case but many other issues are where I am being asked for input and not for this one.”
Other resolutions addressed at the reference committee meeting included:
• A resolution from the Tennessee delegation to modify the oath of AAFP Fellows. The resolution would strike a clause about patients and “placing their welfare above all else” and replace it with language that better supports work/life balance.
• A resolution from the Texas delegation that would shift the composition of the AAFP Congress of Delegates away from a senatorial style makeup (two delegates and an alternate from each state chapter) and instead compose the body on a more proportional basis.
AT THE AAFP CONGRESS OF DELEGATES
Comparative effectiveness research results alone don’t change clinical practice
Practice patterns shifted little in the 12 months following the publishing of study results and in the 12 months following incorporation into clinical practice guidelines, based on an analysis of four comparative effectiveness research case studies, funded by the National Pharmaceutical Council (NPC).
The case studies, published in the American Journal of Managed Care (Am. J. Manag. Care 2014;20:e208-e220), looked at practice changes after the 2004 PROVE-IT study on statin therapies, the 2004 Mammography With MRI study on breast cancer surveillance methods in women with BRCA1 or BRCA2 mutations, the 2006 SPORT study comparing standard open diskectomy versus nonoperative treatments for patients with intervertebal disk herniation, and the 2007 COURAGE trial comparing optimal medical therapy and percutaneous coronary intervention versus optimal medical therapy alone.
"In some cases, we might have expected an uptick or change in what was happening," said report author and NPC Director of Comparative Effectiveness Research Jennifer Graff, Pharm.D., speaking in an interview. "For instance, in the PROVE-IT study, we would have expected, based upon the results, that you would have seen many more providers and patients using intensive statin therapy, ... [but it took] 3 years after the study’s publication before you started to see the change in which types of statins were being used."
The report authors developed suggestions on how to get clinicians to more quickly incorporate the results of comparative effectiveness research.
Involve clinicians, payers, policy makers, and patients in the research design "to make sure we are asking the right questions," said Dr. Graff. This approach is now being taken at the Food and Drug Administration to spur drug development and at the Patient-Centered Outcomes Research Institute, a comparative effectiveness research body created as part of the Affordable Care Act.
Perform more confirmatory studies. "One single study probably won’t change the mind of a provider who is seeing many patients and has in their mind what treatments work," Dr. Graff said. "Similarly, we need to fund studies when clinical opinion is shifting."
Align financial incentives with study results. This can be accomplished through value-based insurance design or incentivized provider pay based on outcomes. Bundled payments as well as incentivizing the use of clinical pathways, such as WellPoint’s recently announced program to provide bonus payments in oncology, are examples of such options, Dr. Graff said.
The study was funded by the NPC. Authors Teresa Gibson, Emily Ehrlich, and Amanda Farr reported employment with Truven Health Analytics, which received consulting fees from NPC. Authors Dr. Robert Dubois and Dr. Jennifer Graff are employees of the NPC. The remaining authors reported no financial conflicts of interest.
I disagree with the first sentence of this article. The gist of the article and the interview is that clinicians are guilty of not adopting important new changes in evidence into their practices soon enough.

|
| Dr. Larry Kraiss |
What this report does not make clear is that the four comparative effectiveness research (CER) studies cited in the American Journal Managed of Care paper were specifically chosen for analysis because no subsequent studies had been published to contradict the major findings. The period of analysis extended to the end of 2009, so in the case of the two 2004 studies, the waiting period to see if contradictory results were published lasted as long as 5 years.
To retrospectively criticize practitioners for not responding to the major findings in these studies sooner is logically fallacious because it presumes that we all should have known that no conflicting information was going to later appear. This is hard to swallow by those of us smarting from being duped by the discredited DECREASE trials regarding perioperative beta-blockade usage.
The article also claims that clinician behavior is not influenced by clinical practice guidelines. This generalization is inaccurate because the American Journal of Managed Care article does indicate that clinical practice guidelines do influence practice.
Finally, in the interview, one of the authors bemoans the 3-year lag between the publication of PROVE-IT and more widespread use of statins. In my opinion, this is not an excessively long lag period.
A healthy sense of skepticism regarding the results of individual CER trials, especially randomized controlled trials where generalization can be suspect, is still warranted. Waiting for the appearance of clinical practice guidelines is prudent. This is what happened with PROVE-IT and what should still happen going forward.
Dr. Larry Kraiss is a professor and chief of the Division of Vascular Surgery and medical director of the Noninvasive Vascular Laboratory at the University of Utah School of Medicine and an associate medical editor of Vascular Specialist.
I disagree with the first sentence of this article. The gist of the article and the interview is that clinicians are guilty of not adopting important new changes in evidence into their practices soon enough.

|
| Dr. Larry Kraiss |
What this report does not make clear is that the four comparative effectiveness research (CER) studies cited in the American Journal Managed of Care paper were specifically chosen for analysis because no subsequent studies had been published to contradict the major findings. The period of analysis extended to the end of 2009, so in the case of the two 2004 studies, the waiting period to see if contradictory results were published lasted as long as 5 years.
To retrospectively criticize practitioners for not responding to the major findings in these studies sooner is logically fallacious because it presumes that we all should have known that no conflicting information was going to later appear. This is hard to swallow by those of us smarting from being duped by the discredited DECREASE trials regarding perioperative beta-blockade usage.
The article also claims that clinician behavior is not influenced by clinical practice guidelines. This generalization is inaccurate because the American Journal of Managed Care article does indicate that clinical practice guidelines do influence practice.
Finally, in the interview, one of the authors bemoans the 3-year lag between the publication of PROVE-IT and more widespread use of statins. In my opinion, this is not an excessively long lag period.
A healthy sense of skepticism regarding the results of individual CER trials, especially randomized controlled trials where generalization can be suspect, is still warranted. Waiting for the appearance of clinical practice guidelines is prudent. This is what happened with PROVE-IT and what should still happen going forward.
Dr. Larry Kraiss is a professor and chief of the Division of Vascular Surgery and medical director of the Noninvasive Vascular Laboratory at the University of Utah School of Medicine and an associate medical editor of Vascular Specialist.
I disagree with the first sentence of this article. The gist of the article and the interview is that clinicians are guilty of not adopting important new changes in evidence into their practices soon enough.

|
| Dr. Larry Kraiss |
What this report does not make clear is that the four comparative effectiveness research (CER) studies cited in the American Journal Managed of Care paper were specifically chosen for analysis because no subsequent studies had been published to contradict the major findings. The period of analysis extended to the end of 2009, so in the case of the two 2004 studies, the waiting period to see if contradictory results were published lasted as long as 5 years.
To retrospectively criticize practitioners for not responding to the major findings in these studies sooner is logically fallacious because it presumes that we all should have known that no conflicting information was going to later appear. This is hard to swallow by those of us smarting from being duped by the discredited DECREASE trials regarding perioperative beta-blockade usage.
The article also claims that clinician behavior is not influenced by clinical practice guidelines. This generalization is inaccurate because the American Journal of Managed Care article does indicate that clinical practice guidelines do influence practice.
Finally, in the interview, one of the authors bemoans the 3-year lag between the publication of PROVE-IT and more widespread use of statins. In my opinion, this is not an excessively long lag period.
A healthy sense of skepticism regarding the results of individual CER trials, especially randomized controlled trials where generalization can be suspect, is still warranted. Waiting for the appearance of clinical practice guidelines is prudent. This is what happened with PROVE-IT and what should still happen going forward.
Dr. Larry Kraiss is a professor and chief of the Division of Vascular Surgery and medical director of the Noninvasive Vascular Laboratory at the University of Utah School of Medicine and an associate medical editor of Vascular Specialist.
Practice patterns shifted little in the 12 months following the publishing of study results and in the 12 months following incorporation into clinical practice guidelines, based on an analysis of four comparative effectiveness research case studies, funded by the National Pharmaceutical Council (NPC).
The case studies, published in the American Journal of Managed Care (Am. J. Manag. Care 2014;20:e208-e220), looked at practice changes after the 2004 PROVE-IT study on statin therapies, the 2004 Mammography With MRI study on breast cancer surveillance methods in women with BRCA1 or BRCA2 mutations, the 2006 SPORT study comparing standard open diskectomy versus nonoperative treatments for patients with intervertebal disk herniation, and the 2007 COURAGE trial comparing optimal medical therapy and percutaneous coronary intervention versus optimal medical therapy alone.
"In some cases, we might have expected an uptick or change in what was happening," said report author and NPC Director of Comparative Effectiveness Research Jennifer Graff, Pharm.D., speaking in an interview. "For instance, in the PROVE-IT study, we would have expected, based upon the results, that you would have seen many more providers and patients using intensive statin therapy, ... [but it took] 3 years after the study’s publication before you started to see the change in which types of statins were being used."
The report authors developed suggestions on how to get clinicians to more quickly incorporate the results of comparative effectiveness research.
Involve clinicians, payers, policy makers, and patients in the research design "to make sure we are asking the right questions," said Dr. Graff. This approach is now being taken at the Food and Drug Administration to spur drug development and at the Patient-Centered Outcomes Research Institute, a comparative effectiveness research body created as part of the Affordable Care Act.
Perform more confirmatory studies. "One single study probably won’t change the mind of a provider who is seeing many patients and has in their mind what treatments work," Dr. Graff said. "Similarly, we need to fund studies when clinical opinion is shifting."
Align financial incentives with study results. This can be accomplished through value-based insurance design or incentivized provider pay based on outcomes. Bundled payments as well as incentivizing the use of clinical pathways, such as WellPoint’s recently announced program to provide bonus payments in oncology, are examples of such options, Dr. Graff said.
The study was funded by the NPC. Authors Teresa Gibson, Emily Ehrlich, and Amanda Farr reported employment with Truven Health Analytics, which received consulting fees from NPC. Authors Dr. Robert Dubois and Dr. Jennifer Graff are employees of the NPC. The remaining authors reported no financial conflicts of interest.
Practice patterns shifted little in the 12 months following the publishing of study results and in the 12 months following incorporation into clinical practice guidelines, based on an analysis of four comparative effectiveness research case studies, funded by the National Pharmaceutical Council (NPC).
The case studies, published in the American Journal of Managed Care (Am. J. Manag. Care 2014;20:e208-e220), looked at practice changes after the 2004 PROVE-IT study on statin therapies, the 2004 Mammography With MRI study on breast cancer surveillance methods in women with BRCA1 or BRCA2 mutations, the 2006 SPORT study comparing standard open diskectomy versus nonoperative treatments for patients with intervertebal disk herniation, and the 2007 COURAGE trial comparing optimal medical therapy and percutaneous coronary intervention versus optimal medical therapy alone.
"In some cases, we might have expected an uptick or change in what was happening," said report author and NPC Director of Comparative Effectiveness Research Jennifer Graff, Pharm.D., speaking in an interview. "For instance, in the PROVE-IT study, we would have expected, based upon the results, that you would have seen many more providers and patients using intensive statin therapy, ... [but it took] 3 years after the study’s publication before you started to see the change in which types of statins were being used."
The report authors developed suggestions on how to get clinicians to more quickly incorporate the results of comparative effectiveness research.
Involve clinicians, payers, policy makers, and patients in the research design "to make sure we are asking the right questions," said Dr. Graff. This approach is now being taken at the Food and Drug Administration to spur drug development and at the Patient-Centered Outcomes Research Institute, a comparative effectiveness research body created as part of the Affordable Care Act.
Perform more confirmatory studies. "One single study probably won’t change the mind of a provider who is seeing many patients and has in their mind what treatments work," Dr. Graff said. "Similarly, we need to fund studies when clinical opinion is shifting."
Align financial incentives with study results. This can be accomplished through value-based insurance design or incentivized provider pay based on outcomes. Bundled payments as well as incentivizing the use of clinical pathways, such as WellPoint’s recently announced program to provide bonus payments in oncology, are examples of such options, Dr. Graff said.
The study was funded by the NPC. Authors Teresa Gibson, Emily Ehrlich, and Amanda Farr reported employment with Truven Health Analytics, which received consulting fees from NPC. Authors Dr. Robert Dubois and Dr. Jennifer Graff are employees of the NPC. The remaining authors reported no financial conflicts of interest.
FROM THE AMERICAN JOURNAL OF MANAGED CARE
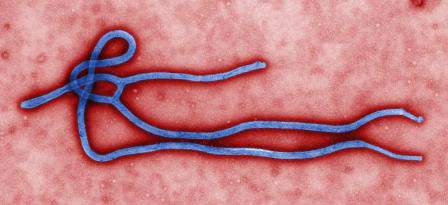
CDC program supplies guidance on Ebola patient management
Drawing upon the experience of Emory Healthcare in treating Ebola patients, the Centers for Disease Control and Prevention has issued a Clinician Outreach and Communication Activity program.
As hospitals in the United States begin to treat Ebola patients, a wide variety of processes including biological waste management, shipping processes, and lab testing are coming under scrutiny. At Emory, patients’ liquid wastes were disinfected with bleach or quaternary disinfecting detergents for more than 5 minutes prior to flushing or disposal. Linens and disposable material exposed to patients were handled as regulated medical waste and placed in leakproof containers. Additionally, Emory’s contractor called for a certification that materials were Ebola-free before transporting the waste to its incinerator.
Because sending a possible Ebola-contaminated specimen to the main hospital laboratory could cause that lab to be shut down for hours to address decontamination, Emory put in place a small point-of-care testing lab dedicated solely to specimens related to patients suspected of having Ebola. The dedicated lab included a chemistry analyzer, hematology analyzer, blood-gas analyzer, automated urinalysis analyzer, coagulation analyzer, and a malaria point-of-care device.
The Centers for Disease Control and Prevention has posted several online resources related to Ebola treatment and is expected to have a new web-based on-demand training video posted on Oct. 14.
Drawing upon the experience of Emory Healthcare in treating Ebola patients, the Centers for Disease Control and Prevention has issued a Clinician Outreach and Communication Activity program.
As hospitals in the United States begin to treat Ebola patients, a wide variety of processes including biological waste management, shipping processes, and lab testing are coming under scrutiny. At Emory, patients’ liquid wastes were disinfected with bleach or quaternary disinfecting detergents for more than 5 minutes prior to flushing or disposal. Linens and disposable material exposed to patients were handled as regulated medical waste and placed in leakproof containers. Additionally, Emory’s contractor called for a certification that materials were Ebola-free before transporting the waste to its incinerator.
Because sending a possible Ebola-contaminated specimen to the main hospital laboratory could cause that lab to be shut down for hours to address decontamination, Emory put in place a small point-of-care testing lab dedicated solely to specimens related to patients suspected of having Ebola. The dedicated lab included a chemistry analyzer, hematology analyzer, blood-gas analyzer, automated urinalysis analyzer, coagulation analyzer, and a malaria point-of-care device.
The Centers for Disease Control and Prevention has posted several online resources related to Ebola treatment and is expected to have a new web-based on-demand training video posted on Oct. 14.
Drawing upon the experience of Emory Healthcare in treating Ebola patients, the Centers for Disease Control and Prevention has issued a Clinician Outreach and Communication Activity program.
As hospitals in the United States begin to treat Ebola patients, a wide variety of processes including biological waste management, shipping processes, and lab testing are coming under scrutiny. At Emory, patients’ liquid wastes were disinfected with bleach or quaternary disinfecting detergents for more than 5 minutes prior to flushing or disposal. Linens and disposable material exposed to patients were handled as regulated medical waste and placed in leakproof containers. Additionally, Emory’s contractor called for a certification that materials were Ebola-free before transporting the waste to its incinerator.
Because sending a possible Ebola-contaminated specimen to the main hospital laboratory could cause that lab to be shut down for hours to address decontamination, Emory put in place a small point-of-care testing lab dedicated solely to specimens related to patients suspected of having Ebola. The dedicated lab included a chemistry analyzer, hematology analyzer, blood-gas analyzer, automated urinalysis analyzer, coagulation analyzer, and a malaria point-of-care device.
The Centers for Disease Control and Prevention has posted several online resources related to Ebola treatment and is expected to have a new web-based on-demand training video posted on Oct. 14.
Specialty drugs: High cost, high value
Although a number of highly specialized medications are entering practice with increasingly higher price tags and additional costs beyond administration, evidence suggests that it is worthwhile for the overall health care system to cover them.
Analysis of 102 traditional and specialty drugs approved by the Food and Drug Administration between 1999 and 2011 showed that, while traditional drugs generally showed little additional benefit when compared with comparator products available at the time of approval, the 58 specialty drugs, in the aggregate, generally showed higher efficacy and cost effectiveness, James Chambers of Tufts University, Boston, and his colleagues wrote (Health Aff. 2014;33:1751-60 [doi:10.1377/hlthaff.2014.0574]).
Mr. Chambers and his colleagues found that 5 of the 15 drugs with the largest additional cost also were among the 15 drugs that showed the largest additional benefit, measured in gains to quality-adjusted life years.
Overall, 14 of the specialty drugs and two traditional drugs offered more than 6 months of quality-adjusted life expectancy.
“Specialty products were often introduced for diseases with substantial unmet health needs, such as cancer or multiple sclerosis,” the authors wrote. “In contrast, many of the traditional drugs in our sample were indicated for conditions … that have been fairly well-managed.” Thus, there was plenty of room for specialty products to improve over existing standards of care while many of the traditional drug products already were coming into a crowded field.
Although specialty drugs are shown to offer improvements to health, payers “have not yet found the formula for managing specialty drugs,” authors noted. “Insurers should continue to experiment with a combination of existing and novel strategies to maximize patient access to specialty drugs while minimizing their cost.”
Although a number of highly specialized medications are entering practice with increasingly higher price tags and additional costs beyond administration, evidence suggests that it is worthwhile for the overall health care system to cover them.
Analysis of 102 traditional and specialty drugs approved by the Food and Drug Administration between 1999 and 2011 showed that, while traditional drugs generally showed little additional benefit when compared with comparator products available at the time of approval, the 58 specialty drugs, in the aggregate, generally showed higher efficacy and cost effectiveness, James Chambers of Tufts University, Boston, and his colleagues wrote (Health Aff. 2014;33:1751-60 [doi:10.1377/hlthaff.2014.0574]).
Mr. Chambers and his colleagues found that 5 of the 15 drugs with the largest additional cost also were among the 15 drugs that showed the largest additional benefit, measured in gains to quality-adjusted life years.
Overall, 14 of the specialty drugs and two traditional drugs offered more than 6 months of quality-adjusted life expectancy.
“Specialty products were often introduced for diseases with substantial unmet health needs, such as cancer or multiple sclerosis,” the authors wrote. “In contrast, many of the traditional drugs in our sample were indicated for conditions … that have been fairly well-managed.” Thus, there was plenty of room for specialty products to improve over existing standards of care while many of the traditional drug products already were coming into a crowded field.
Although specialty drugs are shown to offer improvements to health, payers “have not yet found the formula for managing specialty drugs,” authors noted. “Insurers should continue to experiment with a combination of existing and novel strategies to maximize patient access to specialty drugs while minimizing their cost.”
Although a number of highly specialized medications are entering practice with increasingly higher price tags and additional costs beyond administration, evidence suggests that it is worthwhile for the overall health care system to cover them.
Analysis of 102 traditional and specialty drugs approved by the Food and Drug Administration between 1999 and 2011 showed that, while traditional drugs generally showed little additional benefit when compared with comparator products available at the time of approval, the 58 specialty drugs, in the aggregate, generally showed higher efficacy and cost effectiveness, James Chambers of Tufts University, Boston, and his colleagues wrote (Health Aff. 2014;33:1751-60 [doi:10.1377/hlthaff.2014.0574]).
Mr. Chambers and his colleagues found that 5 of the 15 drugs with the largest additional cost also were among the 15 drugs that showed the largest additional benefit, measured in gains to quality-adjusted life years.
Overall, 14 of the specialty drugs and two traditional drugs offered more than 6 months of quality-adjusted life expectancy.
“Specialty products were often introduced for diseases with substantial unmet health needs, such as cancer or multiple sclerosis,” the authors wrote. “In contrast, many of the traditional drugs in our sample were indicated for conditions … that have been fairly well-managed.” Thus, there was plenty of room for specialty products to improve over existing standards of care while many of the traditional drug products already were coming into a crowded field.
Although specialty drugs are shown to offer improvements to health, payers “have not yet found the formula for managing specialty drugs,” authors noted. “Insurers should continue to experiment with a combination of existing and novel strategies to maximize patient access to specialty drugs while minimizing their cost.”
FROM HEALTH AFFAIRS
Curbing opioid abuse could be a quality of care issue
WASHINGTON – MedPAC advisors think it might be time to address opioid abuse as a quality of care issue.
Commission member Herb Kuhn, president and CEO of the Missouri Hospital Association, suggested the Centers for Medicare & Medicaid Services develop coding that tracks how opioids are used and what conditions they are treating and see of those treatments line up with standards of care.
“You could begin to look at some of the protocols from some of the medical societies in terms of proper prescription or activities here and begin to look at this more as a quality initiative so that, ultimately, that comes into play in terms of payment,” Mr. Kuhn, a former deputy director at CMS, said at the Oct 9 meeting of the Medicare Payment Advisory Commission.
Commissioner Scott Armstrong, president and CEO of Seattle’s Group Health Cooperative, agreed.
“To me, this is much more a quality of care issue,” Mr. Armstrong said. Currently “what’s so difficult here is that all of our interventions are retrospective.”
He noted that within his organization, once opioid prescribing became an issue, every patient prescribed an opioid was identified.
“We began to monitor what percentage of those patients had a proactive care management plan,” he said. “Every patients’ needs are going to be different, but it was really about are we paying attention to the evidence and the kind of use of opioids that should be appropriate given to every individual patient.”
Commissioner Kathy Buto, former vice president of global health policy at Johnson & Johnson and senior health advisor at the Congressional Budget Office, called on CMS to better understand how Medicare can incentivize better pain management efforts.
One challenge that will need to be overcome in Medicare is that in Part D stand alone plans, there is no direct connection between the drug benefit and the rest of the health care services provided, something that also will need to be resolved as the country shifts to an accountable care model.
MedPAC advises Congress on issues affecting Medicare and makes recommendations on payment and quality issues affecting the program. No recommendations were made at this meeting as commissioners still sought seeking further information on how to address opioid abuse.
Commissioners also suggested CMS find incentives to promote the use of tools such as prescription drug monitoring programs more regularly.
WASHINGTON – MedPAC advisors think it might be time to address opioid abuse as a quality of care issue.
Commission member Herb Kuhn, president and CEO of the Missouri Hospital Association, suggested the Centers for Medicare & Medicaid Services develop coding that tracks how opioids are used and what conditions they are treating and see of those treatments line up with standards of care.
“You could begin to look at some of the protocols from some of the medical societies in terms of proper prescription or activities here and begin to look at this more as a quality initiative so that, ultimately, that comes into play in terms of payment,” Mr. Kuhn, a former deputy director at CMS, said at the Oct 9 meeting of the Medicare Payment Advisory Commission.
Commissioner Scott Armstrong, president and CEO of Seattle’s Group Health Cooperative, agreed.
“To me, this is much more a quality of care issue,” Mr. Armstrong said. Currently “what’s so difficult here is that all of our interventions are retrospective.”
He noted that within his organization, once opioid prescribing became an issue, every patient prescribed an opioid was identified.
“We began to monitor what percentage of those patients had a proactive care management plan,” he said. “Every patients’ needs are going to be different, but it was really about are we paying attention to the evidence and the kind of use of opioids that should be appropriate given to every individual patient.”
Commissioner Kathy Buto, former vice president of global health policy at Johnson & Johnson and senior health advisor at the Congressional Budget Office, called on CMS to better understand how Medicare can incentivize better pain management efforts.
One challenge that will need to be overcome in Medicare is that in Part D stand alone plans, there is no direct connection between the drug benefit and the rest of the health care services provided, something that also will need to be resolved as the country shifts to an accountable care model.
MedPAC advises Congress on issues affecting Medicare and makes recommendations on payment and quality issues affecting the program. No recommendations were made at this meeting as commissioners still sought seeking further information on how to address opioid abuse.
Commissioners also suggested CMS find incentives to promote the use of tools such as prescription drug monitoring programs more regularly.
WASHINGTON – MedPAC advisors think it might be time to address opioid abuse as a quality of care issue.
Commission member Herb Kuhn, president and CEO of the Missouri Hospital Association, suggested the Centers for Medicare & Medicaid Services develop coding that tracks how opioids are used and what conditions they are treating and see of those treatments line up with standards of care.
“You could begin to look at some of the protocols from some of the medical societies in terms of proper prescription or activities here and begin to look at this more as a quality initiative so that, ultimately, that comes into play in terms of payment,” Mr. Kuhn, a former deputy director at CMS, said at the Oct 9 meeting of the Medicare Payment Advisory Commission.
Commissioner Scott Armstrong, president and CEO of Seattle’s Group Health Cooperative, agreed.
“To me, this is much more a quality of care issue,” Mr. Armstrong said. Currently “what’s so difficult here is that all of our interventions are retrospective.”
He noted that within his organization, once opioid prescribing became an issue, every patient prescribed an opioid was identified.
“We began to monitor what percentage of those patients had a proactive care management plan,” he said. “Every patients’ needs are going to be different, but it was really about are we paying attention to the evidence and the kind of use of opioids that should be appropriate given to every individual patient.”
Commissioner Kathy Buto, former vice president of global health policy at Johnson & Johnson and senior health advisor at the Congressional Budget Office, called on CMS to better understand how Medicare can incentivize better pain management efforts.
One challenge that will need to be overcome in Medicare is that in Part D stand alone plans, there is no direct connection between the drug benefit and the rest of the health care services provided, something that also will need to be resolved as the country shifts to an accountable care model.
MedPAC advises Congress on issues affecting Medicare and makes recommendations on payment and quality issues affecting the program. No recommendations were made at this meeting as commissioners still sought seeking further information on how to address opioid abuse.
Commissioners also suggested CMS find incentives to promote the use of tools such as prescription drug monitoring programs more regularly.
Specialty drug coupons - a double-edged sword of improved adherence, increased costs
While the availability of coupons can help defray out-of-pocket costs and improve access and adherence to high-cost specialty drugs, it may also result in higher costs to the health system.
Patients used drug coupons to pay for $21.2 million of $35.3 million in out-of-pocket costs, according to an analysis of 265,000 prescriptions worth $911 million in 2013. The coupons generally dropped a patient’s out-of-pocket costs to less than $250 per prescription, a point below which patients were “far less likely” to abandon prescriptions in cases of specialty anti-inflammatory drugs and treatments for multiple sclerosis (Health Affairs 2014;33:1761-9 [doi:10.1377/hlthaff.2014.0497]).
But while the use of coupons demonstrated that the lower cost led to lower prescription abandonment rates, it also allowed patients to circumvent cost-containment measures that pharmacy benefit managers employ.
“We found that drug coupons were extremely effective in lowering patients’ costs to less than $50 per prescription, thus eliminating the incentive to select a preferred drug,” wrote Catherine I. Starner, Pharm.D., senior health outcomes researcher at pharmacy benefit manager Prime Therapeutics, and her colleagues.
To control costs at the health system level, tighter controls could be placed on specialty drugs at the pharmacy benefit manager level, according to Dr. Starner and her colleagues. Such controls could include prior authorization or the use of step therapies,as well as excluding coverage of nonpreferred drugs. The latter strategy, however, could make drug access a problem, especially if physicians are not aware of an insurance plan taking these steps.
Excluding specialty drugs “makes the patient responsible for the entire cost of the drug,” the authors noted. “If prescribers are unaware of such exclusions, or if patients require specific therapies, such exclusions may impose considerable costs on patients” and potentially lead to access issues.
While the availability of coupons can help defray out-of-pocket costs and improve access and adherence to high-cost specialty drugs, it may also result in higher costs to the health system.
Patients used drug coupons to pay for $21.2 million of $35.3 million in out-of-pocket costs, according to an analysis of 265,000 prescriptions worth $911 million in 2013. The coupons generally dropped a patient’s out-of-pocket costs to less than $250 per prescription, a point below which patients were “far less likely” to abandon prescriptions in cases of specialty anti-inflammatory drugs and treatments for multiple sclerosis (Health Affairs 2014;33:1761-9 [doi:10.1377/hlthaff.2014.0497]).
But while the use of coupons demonstrated that the lower cost led to lower prescription abandonment rates, it also allowed patients to circumvent cost-containment measures that pharmacy benefit managers employ.
“We found that drug coupons were extremely effective in lowering patients’ costs to less than $50 per prescription, thus eliminating the incentive to select a preferred drug,” wrote Catherine I. Starner, Pharm.D., senior health outcomes researcher at pharmacy benefit manager Prime Therapeutics, and her colleagues.
To control costs at the health system level, tighter controls could be placed on specialty drugs at the pharmacy benefit manager level, according to Dr. Starner and her colleagues. Such controls could include prior authorization or the use of step therapies,as well as excluding coverage of nonpreferred drugs. The latter strategy, however, could make drug access a problem, especially if physicians are not aware of an insurance plan taking these steps.
Excluding specialty drugs “makes the patient responsible for the entire cost of the drug,” the authors noted. “If prescribers are unaware of such exclusions, or if patients require specific therapies, such exclusions may impose considerable costs on patients” and potentially lead to access issues.
While the availability of coupons can help defray out-of-pocket costs and improve access and adherence to high-cost specialty drugs, it may also result in higher costs to the health system.
Patients used drug coupons to pay for $21.2 million of $35.3 million in out-of-pocket costs, according to an analysis of 265,000 prescriptions worth $911 million in 2013. The coupons generally dropped a patient’s out-of-pocket costs to less than $250 per prescription, a point below which patients were “far less likely” to abandon prescriptions in cases of specialty anti-inflammatory drugs and treatments for multiple sclerosis (Health Affairs 2014;33:1761-9 [doi:10.1377/hlthaff.2014.0497]).
But while the use of coupons demonstrated that the lower cost led to lower prescription abandonment rates, it also allowed patients to circumvent cost-containment measures that pharmacy benefit managers employ.
“We found that drug coupons were extremely effective in lowering patients’ costs to less than $50 per prescription, thus eliminating the incentive to select a preferred drug,” wrote Catherine I. Starner, Pharm.D., senior health outcomes researcher at pharmacy benefit manager Prime Therapeutics, and her colleagues.
To control costs at the health system level, tighter controls could be placed on specialty drugs at the pharmacy benefit manager level, according to Dr. Starner and her colleagues. Such controls could include prior authorization or the use of step therapies,as well as excluding coverage of nonpreferred drugs. The latter strategy, however, could make drug access a problem, especially if physicians are not aware of an insurance plan taking these steps.
Excluding specialty drugs “makes the patient responsible for the entire cost of the drug,” the authors noted. “If prescribers are unaware of such exclusions, or if patients require specific therapies, such exclusions may impose considerable costs on patients” and potentially lead to access issues.
FROM HEALTH AFFAIRS
FDA finalizes medical device cybersecurity guidance
The Food and Drug Administration is calling on device manufacturers that have network-connected devices to consider cybersecurity risks as part of product design.
The agency finalized guidance recommending that manufacturers submit documentation to the FDA about identified risks and the controls in place to mitigate the risks, as well as about how manufacturers plan to update software as risks are discovered.
“There is no such thing as a threat-proof medical device,” Suzanne Schwartz, director of emergency preparedness/operations and medical countermeasures at the FDA’s Center for Devices and Radiological Health, said in a statement. “It is important for medical device manufacturers to remain vigilant about cybersecurity and to appropriately protect patients from those risks.”
While directed at manufacturers, the guidance notes that medical device security “is a shared responsibility” across all stakeholders, including health care facilities, physicians, patients, and manufacturers.
FDA will host a 2-day workshop on Oct. 21-22, 2014, to discuss the guidance and collaborative approaches to medical device cybersecurity.
The Food and Drug Administration is calling on device manufacturers that have network-connected devices to consider cybersecurity risks as part of product design.
The agency finalized guidance recommending that manufacturers submit documentation to the FDA about identified risks and the controls in place to mitigate the risks, as well as about how manufacturers plan to update software as risks are discovered.
“There is no such thing as a threat-proof medical device,” Suzanne Schwartz, director of emergency preparedness/operations and medical countermeasures at the FDA’s Center for Devices and Radiological Health, said in a statement. “It is important for medical device manufacturers to remain vigilant about cybersecurity and to appropriately protect patients from those risks.”
While directed at manufacturers, the guidance notes that medical device security “is a shared responsibility” across all stakeholders, including health care facilities, physicians, patients, and manufacturers.
FDA will host a 2-day workshop on Oct. 21-22, 2014, to discuss the guidance and collaborative approaches to medical device cybersecurity.
The Food and Drug Administration is calling on device manufacturers that have network-connected devices to consider cybersecurity risks as part of product design.
The agency finalized guidance recommending that manufacturers submit documentation to the FDA about identified risks and the controls in place to mitigate the risks, as well as about how manufacturers plan to update software as risks are discovered.
“There is no such thing as a threat-proof medical device,” Suzanne Schwartz, director of emergency preparedness/operations and medical countermeasures at the FDA’s Center for Devices and Radiological Health, said in a statement. “It is important for medical device manufacturers to remain vigilant about cybersecurity and to appropriately protect patients from those risks.”
While directed at manufacturers, the guidance notes that medical device security “is a shared responsibility” across all stakeholders, including health care facilities, physicians, patients, and manufacturers.
FDA will host a 2-day workshop on Oct. 21-22, 2014, to discuss the guidance and collaborative approaches to medical device cybersecurity.