User login
Fever and bilateral ankle pain
A 35-year-old man presents with bilateral ankle pain and swelling. He has had fevers over the past 5 days. Physical examination: temperature, 38° C; pulse, 90; blood pressure, 140/70 mm Hg. Ext: Edema bilateral ankles, ankle joints tender. No other joints are involved. Lab: WBC, 6,000; polys, 4.8; mono, 0.5; lymph, 0.7.
What is the most useful diagnostic test?
A. CRP.
B. ESR.
C. Uric acid.
D. Chest x-ray.
E. Rheumatoid factor.
This patient has acute onset of fevers and bilateral ankle pain and swelling. The acute onset and presence of a fever makes rheumatoid arthritis unlikely. Bilateral ankle arthritis is a very unusual presentation for gout, and would be very unlikely in such a young patient unless there were other risk factors for gout. Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate) will not help make a specific diagnosis.
This patient has Lofgren’s syndrome (acute presentation of sarcoidosis). A chest x-ray would be diagnostic, as the presence of bilateral hilar adenopathy along with the other symptoms would be diagnostic of Lofgren’s syndrome. The patient also has a low peripheral lymphocyte count, which is common with active sarcoidosis.
The combination of bilateral ankle swelling and inflammation is a clue to think about sarcoidosis. Juan Mañá, MD, and his colleagues reviewed the charts of 330 sarcoid patients who presented over a 20-year period.1 A total of 33 patients presented with periarticular ankle inflammation. Interestingly, the majority of these patients presented in the spring (54%). The average age of the patients was 33 years, and about 80% had stage 1 sarcoid on chest radiography (bilateral hilar adenopathy). All 24 patients who were followed up were in remission a year later.
In another study, the same investigators reported on the clinical features and course of Lofgren’s syndrome in 186 patients. Almost all the patients (93%) had erythema nodosum or periarticular ankle inflammation at presentation.2 Half of the patients presented in the spring, and the vast majority (87%) had no respiratory symptoms at the time of presentation. Most of the 133 patients (86%) who were available for follow-up (mean follow-up, 5 years) were in complete remission from sarcoid.
Johan Grunewald, MD, and Anders Eklund, MD, reported on 150 patients with Lofgren’s syndrome.3 In that study, 87 patients had erythema nodosum, and 63 had no erythema nodosum but did have symmetric ankle inflammation. There was an increase in patients presenting in the spring, about 80% had stage 1 sarcoid on chest x-ray, and the majority of the patients who presented with bilateral ankle inflammation and no erythema nodosum were men. They also found that there was a strong association with the presence of HLA-DRB1*0301/DQB1*0201 in patients who developed Lofgren’s syndrome. Resolution of disease was very common (85%) without recurrences.
There are several pearls to emphasize. Think of Lofgren’s syndrome in patients with symmetrical ankle inflammation or erythema nodosum. Order a chest x-ray to make the diagnosis; these patients usually will have no pulmonary symptoms to lead you in that direction. The prognosis is very good for these patients, with the great majority of them having full clinical resolution without recurrences.
Key pearl: Think of Lofgren’s syndrome in patients presenting with bilateral ankle inflammation.
References
1. J Rheumatol. 1996 May;23(5):874-7.
2. Am J Med. 1999 Sep;107(3):240-5.
3. Am J Respir Crit Care Med. 2007 Jan 1;175(1):40-4.
A 35-year-old man presents with bilateral ankle pain and swelling. He has had fevers over the past 5 days. Physical examination: temperature, 38° C; pulse, 90; blood pressure, 140/70 mm Hg. Ext: Edema bilateral ankles, ankle joints tender. No other joints are involved. Lab: WBC, 6,000; polys, 4.8; mono, 0.5; lymph, 0.7.
What is the most useful diagnostic test?
A. CRP.
B. ESR.
C. Uric acid.
D. Chest x-ray.
E. Rheumatoid factor.
This patient has acute onset of fevers and bilateral ankle pain and swelling. The acute onset and presence of a fever makes rheumatoid arthritis unlikely. Bilateral ankle arthritis is a very unusual presentation for gout, and would be very unlikely in such a young patient unless there were other risk factors for gout. Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate) will not help make a specific diagnosis.
This patient has Lofgren’s syndrome (acute presentation of sarcoidosis). A chest x-ray would be diagnostic, as the presence of bilateral hilar adenopathy along with the other symptoms would be diagnostic of Lofgren’s syndrome. The patient also has a low peripheral lymphocyte count, which is common with active sarcoidosis.
The combination of bilateral ankle swelling and inflammation is a clue to think about sarcoidosis. Juan Mañá, MD, and his colleagues reviewed the charts of 330 sarcoid patients who presented over a 20-year period.1 A total of 33 patients presented with periarticular ankle inflammation. Interestingly, the majority of these patients presented in the spring (54%). The average age of the patients was 33 years, and about 80% had stage 1 sarcoid on chest radiography (bilateral hilar adenopathy). All 24 patients who were followed up were in remission a year later.
In another study, the same investigators reported on the clinical features and course of Lofgren’s syndrome in 186 patients. Almost all the patients (93%) had erythema nodosum or periarticular ankle inflammation at presentation.2 Half of the patients presented in the spring, and the vast majority (87%) had no respiratory symptoms at the time of presentation. Most of the 133 patients (86%) who were available for follow-up (mean follow-up, 5 years) were in complete remission from sarcoid.
Johan Grunewald, MD, and Anders Eklund, MD, reported on 150 patients with Lofgren’s syndrome.3 In that study, 87 patients had erythema nodosum, and 63 had no erythema nodosum but did have symmetric ankle inflammation. There was an increase in patients presenting in the spring, about 80% had stage 1 sarcoid on chest x-ray, and the majority of the patients who presented with bilateral ankle inflammation and no erythema nodosum were men. They also found that there was a strong association with the presence of HLA-DRB1*0301/DQB1*0201 in patients who developed Lofgren’s syndrome. Resolution of disease was very common (85%) without recurrences.
There are several pearls to emphasize. Think of Lofgren’s syndrome in patients with symmetrical ankle inflammation or erythema nodosum. Order a chest x-ray to make the diagnosis; these patients usually will have no pulmonary symptoms to lead you in that direction. The prognosis is very good for these patients, with the great majority of them having full clinical resolution without recurrences.
Key pearl: Think of Lofgren’s syndrome in patients presenting with bilateral ankle inflammation.
References
1. J Rheumatol. 1996 May;23(5):874-7.
2. Am J Med. 1999 Sep;107(3):240-5.
3. Am J Respir Crit Care Med. 2007 Jan 1;175(1):40-4.
A 35-year-old man presents with bilateral ankle pain and swelling. He has had fevers over the past 5 days. Physical examination: temperature, 38° C; pulse, 90; blood pressure, 140/70 mm Hg. Ext: Edema bilateral ankles, ankle joints tender. No other joints are involved. Lab: WBC, 6,000; polys, 4.8; mono, 0.5; lymph, 0.7.
What is the most useful diagnostic test?
A. CRP.
B. ESR.
C. Uric acid.
D. Chest x-ray.
E. Rheumatoid factor.
This patient has acute onset of fevers and bilateral ankle pain and swelling. The acute onset and presence of a fever makes rheumatoid arthritis unlikely. Bilateral ankle arthritis is a very unusual presentation for gout, and would be very unlikely in such a young patient unless there were other risk factors for gout. Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate) will not help make a specific diagnosis.
This patient has Lofgren’s syndrome (acute presentation of sarcoidosis). A chest x-ray would be diagnostic, as the presence of bilateral hilar adenopathy along with the other symptoms would be diagnostic of Lofgren’s syndrome. The patient also has a low peripheral lymphocyte count, which is common with active sarcoidosis.
The combination of bilateral ankle swelling and inflammation is a clue to think about sarcoidosis. Juan Mañá, MD, and his colleagues reviewed the charts of 330 sarcoid patients who presented over a 20-year period.1 A total of 33 patients presented with periarticular ankle inflammation. Interestingly, the majority of these patients presented in the spring (54%). The average age of the patients was 33 years, and about 80% had stage 1 sarcoid on chest radiography (bilateral hilar adenopathy). All 24 patients who were followed up were in remission a year later.
In another study, the same investigators reported on the clinical features and course of Lofgren’s syndrome in 186 patients. Almost all the patients (93%) had erythema nodosum or periarticular ankle inflammation at presentation.2 Half of the patients presented in the spring, and the vast majority (87%) had no respiratory symptoms at the time of presentation. Most of the 133 patients (86%) who were available for follow-up (mean follow-up, 5 years) were in complete remission from sarcoid.
Johan Grunewald, MD, and Anders Eklund, MD, reported on 150 patients with Lofgren’s syndrome.3 In that study, 87 patients had erythema nodosum, and 63 had no erythema nodosum but did have symmetric ankle inflammation. There was an increase in patients presenting in the spring, about 80% had stage 1 sarcoid on chest x-ray, and the majority of the patients who presented with bilateral ankle inflammation and no erythema nodosum were men. They also found that there was a strong association with the presence of HLA-DRB1*0301/DQB1*0201 in patients who developed Lofgren’s syndrome. Resolution of disease was very common (85%) without recurrences.
There are several pearls to emphasize. Think of Lofgren’s syndrome in patients with symmetrical ankle inflammation or erythema nodosum. Order a chest x-ray to make the diagnosis; these patients usually will have no pulmonary symptoms to lead you in that direction. The prognosis is very good for these patients, with the great majority of them having full clinical resolution without recurrences.
Key pearl: Think of Lofgren’s syndrome in patients presenting with bilateral ankle inflammation.
References
1. J Rheumatol. 1996 May;23(5):874-7.
2. Am J Med. 1999 Sep;107(3):240-5.
3. Am J Respir Crit Care Med. 2007 Jan 1;175(1):40-4.
Unexplained leukocytosis in a hospitalized patient
A 70-year-old man is evaluated for a persistent leukocytosis. He was hospitalized 10 days ago for a severe exacerbation of chronic obstructive pulmonary disease. He was intubated for 3 days, was diagnosed with a left lower lobe pneumonia, and was treated with antibiotics. His white blood cell count on admission was 20,000 per mcL. It dropped as low as 15,000 on day 6 but is now 25,000, with 23,000 polymorphonuclear leukocytes (10% band forms). He is on oral prednisone 15 mg once daily. Chest x-ray shows no infiltrate. Urinalysis without WBCs.
What is the most likely cause of his leukocytosis?
A) Pulmonary embolus.
B) Lung abscess.
C) Perinephric abscess.
D) Prednisone.
E) Clostridium difficile infection.
The most likely diagnosis in otherwise unexplained leukocytosis in a hospitalized patient is C. difficile.
Anna Wanahita, MD, of the St. John Clinic in Tulsa, Okla., and her colleagues prospectively studied 60 patients admitted to a VA hospital who had unexplained leukocytosis.1 All patients had stool specimens sent for C. difficile toxin; in addition, 26 hospitalized control patients without leukocytosis also had stool sent for C. difficile toxin. For study purposes, leukocytosis was defined as a WBC greater than 15,000 per mcL. Any patient for whom C. difficile toxin was sent because of clinical suspicion and who was positive was excluded from the study results.
Almost 60% of the patients with unexplained leukocytosis (35 of 60) had a positive C. difficile toxin, compared with 12% of the controls (P less than .001). More than half of the patients with a positive C. difficile test had the onset of leukocytosis prior to any symptoms of colitis. Leukocytosis responded to treatment with metronidazole in 83% of the patients with a positive C. difficile toxin, and 75% of the patients who had leukocytosis did not have a positive C. difficile toxin.
In another study, Mamatha Bulusu, and colleagues did a retrospective study of 70 hospitalized patients who had diarrhea and underwent testing for C. difficile.2 They evaluated the pattern of white blood cell counts in patients who were positive and negative for C. difficile toxin. The mean WBC for C. difficile–positive patients was 15,800, compared with 7,700 for the patients who were C. difficile negative (P less than .01). They described three patterns: one in which leukocytosis occurred at the onset of diarrhea; a pattern in which unexplained leukocytosis occurred days prior to diarrhea; and a pattern in which patients treated for infection with leukocytosis had a worsening of their leukocytosis at the onset of diarrheal symptoms. Treatment with metronidazole led to a resolution of leukocytosis in all the C. difficile–positive patients.
Another possibility in this case was WBC elevation because of the patient’s prednisone. Prednisone can increase WBC as early as the first day of therapy.3 The elevation and rapidity of increase are dose related. The important pearl is that steroid-induced leukocytosis involves an increase of polymorphonuclear white blood cells with a rise in monocytes and a decrease in eosinophils and lymphocytes.
Pearl: Think of underlying C. difficile infection in your hospitalized patient with unexplained leukocytosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Med. 2003 Nov;115(7):543-6.
2. Am J Gastroenterol. 2000 Nov;95(11):3137-41.
3. J Clin Invest. 1975 Oct;56(4):808-13.
4. Am J Med. 1981 Nov;71(5):773-8.
A 70-year-old man is evaluated for a persistent leukocytosis. He was hospitalized 10 days ago for a severe exacerbation of chronic obstructive pulmonary disease. He was intubated for 3 days, was diagnosed with a left lower lobe pneumonia, and was treated with antibiotics. His white blood cell count on admission was 20,000 per mcL. It dropped as low as 15,000 on day 6 but is now 25,000, with 23,000 polymorphonuclear leukocytes (10% band forms). He is on oral prednisone 15 mg once daily. Chest x-ray shows no infiltrate. Urinalysis without WBCs.
What is the most likely cause of his leukocytosis?
A) Pulmonary embolus.
B) Lung abscess.
C) Perinephric abscess.
D) Prednisone.
E) Clostridium difficile infection.
The most likely diagnosis in otherwise unexplained leukocytosis in a hospitalized patient is C. difficile.
Anna Wanahita, MD, of the St. John Clinic in Tulsa, Okla., and her colleagues prospectively studied 60 patients admitted to a VA hospital who had unexplained leukocytosis.1 All patients had stool specimens sent for C. difficile toxin; in addition, 26 hospitalized control patients without leukocytosis also had stool sent for C. difficile toxin. For study purposes, leukocytosis was defined as a WBC greater than 15,000 per mcL. Any patient for whom C. difficile toxin was sent because of clinical suspicion and who was positive was excluded from the study results.
Almost 60% of the patients with unexplained leukocytosis (35 of 60) had a positive C. difficile toxin, compared with 12% of the controls (P less than .001). More than half of the patients with a positive C. difficile test had the onset of leukocytosis prior to any symptoms of colitis. Leukocytosis responded to treatment with metronidazole in 83% of the patients with a positive C. difficile toxin, and 75% of the patients who had leukocytosis did not have a positive C. difficile toxin.
In another study, Mamatha Bulusu, and colleagues did a retrospective study of 70 hospitalized patients who had diarrhea and underwent testing for C. difficile.2 They evaluated the pattern of white blood cell counts in patients who were positive and negative for C. difficile toxin. The mean WBC for C. difficile–positive patients was 15,800, compared with 7,700 for the patients who were C. difficile negative (P less than .01). They described three patterns: one in which leukocytosis occurred at the onset of diarrhea; a pattern in which unexplained leukocytosis occurred days prior to diarrhea; and a pattern in which patients treated for infection with leukocytosis had a worsening of their leukocytosis at the onset of diarrheal symptoms. Treatment with metronidazole led to a resolution of leukocytosis in all the C. difficile–positive patients.
Another possibility in this case was WBC elevation because of the patient’s prednisone. Prednisone can increase WBC as early as the first day of therapy.3 The elevation and rapidity of increase are dose related. The important pearl is that steroid-induced leukocytosis involves an increase of polymorphonuclear white blood cells with a rise in monocytes and a decrease in eosinophils and lymphocytes.
Pearl: Think of underlying C. difficile infection in your hospitalized patient with unexplained leukocytosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Med. 2003 Nov;115(7):543-6.
2. Am J Gastroenterol. 2000 Nov;95(11):3137-41.
3. J Clin Invest. 1975 Oct;56(4):808-13.
4. Am J Med. 1981 Nov;71(5):773-8.
A 70-year-old man is evaluated for a persistent leukocytosis. He was hospitalized 10 days ago for a severe exacerbation of chronic obstructive pulmonary disease. He was intubated for 3 days, was diagnosed with a left lower lobe pneumonia, and was treated with antibiotics. His white blood cell count on admission was 20,000 per mcL. It dropped as low as 15,000 on day 6 but is now 25,000, with 23,000 polymorphonuclear leukocytes (10% band forms). He is on oral prednisone 15 mg once daily. Chest x-ray shows no infiltrate. Urinalysis without WBCs.
What is the most likely cause of his leukocytosis?
A) Pulmonary embolus.
B) Lung abscess.
C) Perinephric abscess.
D) Prednisone.
E) Clostridium difficile infection.
The most likely diagnosis in otherwise unexplained leukocytosis in a hospitalized patient is C. difficile.
Anna Wanahita, MD, of the St. John Clinic in Tulsa, Okla., and her colleagues prospectively studied 60 patients admitted to a VA hospital who had unexplained leukocytosis.1 All patients had stool specimens sent for C. difficile toxin; in addition, 26 hospitalized control patients without leukocytosis also had stool sent for C. difficile toxin. For study purposes, leukocytosis was defined as a WBC greater than 15,000 per mcL. Any patient for whom C. difficile toxin was sent because of clinical suspicion and who was positive was excluded from the study results.
Almost 60% of the patients with unexplained leukocytosis (35 of 60) had a positive C. difficile toxin, compared with 12% of the controls (P less than .001). More than half of the patients with a positive C. difficile test had the onset of leukocytosis prior to any symptoms of colitis. Leukocytosis responded to treatment with metronidazole in 83% of the patients with a positive C. difficile toxin, and 75% of the patients who had leukocytosis did not have a positive C. difficile toxin.
In another study, Mamatha Bulusu, and colleagues did a retrospective study of 70 hospitalized patients who had diarrhea and underwent testing for C. difficile.2 They evaluated the pattern of white blood cell counts in patients who were positive and negative for C. difficile toxin. The mean WBC for C. difficile–positive patients was 15,800, compared with 7,700 for the patients who were C. difficile negative (P less than .01). They described three patterns: one in which leukocytosis occurred at the onset of diarrhea; a pattern in which unexplained leukocytosis occurred days prior to diarrhea; and a pattern in which patients treated for infection with leukocytosis had a worsening of their leukocytosis at the onset of diarrheal symptoms. Treatment with metronidazole led to a resolution of leukocytosis in all the C. difficile–positive patients.
Another possibility in this case was WBC elevation because of the patient’s prednisone. Prednisone can increase WBC as early as the first day of therapy.3 The elevation and rapidity of increase are dose related. The important pearl is that steroid-induced leukocytosis involves an increase of polymorphonuclear white blood cells with a rise in monocytes and a decrease in eosinophils and lymphocytes.
Pearl: Think of underlying C. difficile infection in your hospitalized patient with unexplained leukocytosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Med. 2003 Nov;115(7):543-6.
2. Am J Gastroenterol. 2000 Nov;95(11):3137-41.
3. J Clin Invest. 1975 Oct;56(4):808-13.
4. Am J Med. 1981 Nov;71(5):773-8.
Nocturia and sleep apnea
Author’s note: I have been writing “Myth of the Month” columns for the last several years. I will try to continue to write about myths when possible, but I would like to introduce a new column, “Pearl of the Month.” I want to share with you pearls that I have found really helpful in medical practice. Some of these will be new news, while some may be old news that may not be well known.
A 65-year-old man comes to a clinic concerned about frequent nocturia. He is getting up four times a night to urinate, and he has been urinating about every 5 hours during the day. He has been seen twice for this problem and was diagnosed with benign prostatic hyperplasia and started on tamsulosin.
He found a slight improvement when he started on 0.4 mg qhs, reducing his nocturia episodes from four to three. His dose was increased to 0.8 mg qhs, with no improvement in nocturia.
Exam today: BP, 140/94; pulse, 70. Rectal exam: Prostate is twice normal size without nodules. Labs: Na, 140; K, 4.0; glucose, 80; Ca, 9.6.
He is frustrated because he feels tired and sleepy from having to get up so often to urinate every night.
What is the best treatment/advice at this point?
A. Check hemoglobin A1C.
B. Start finasteride.
C. Switch tamsulosin to terazosin.
D. Evaluate for sleep apnea.
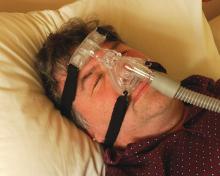
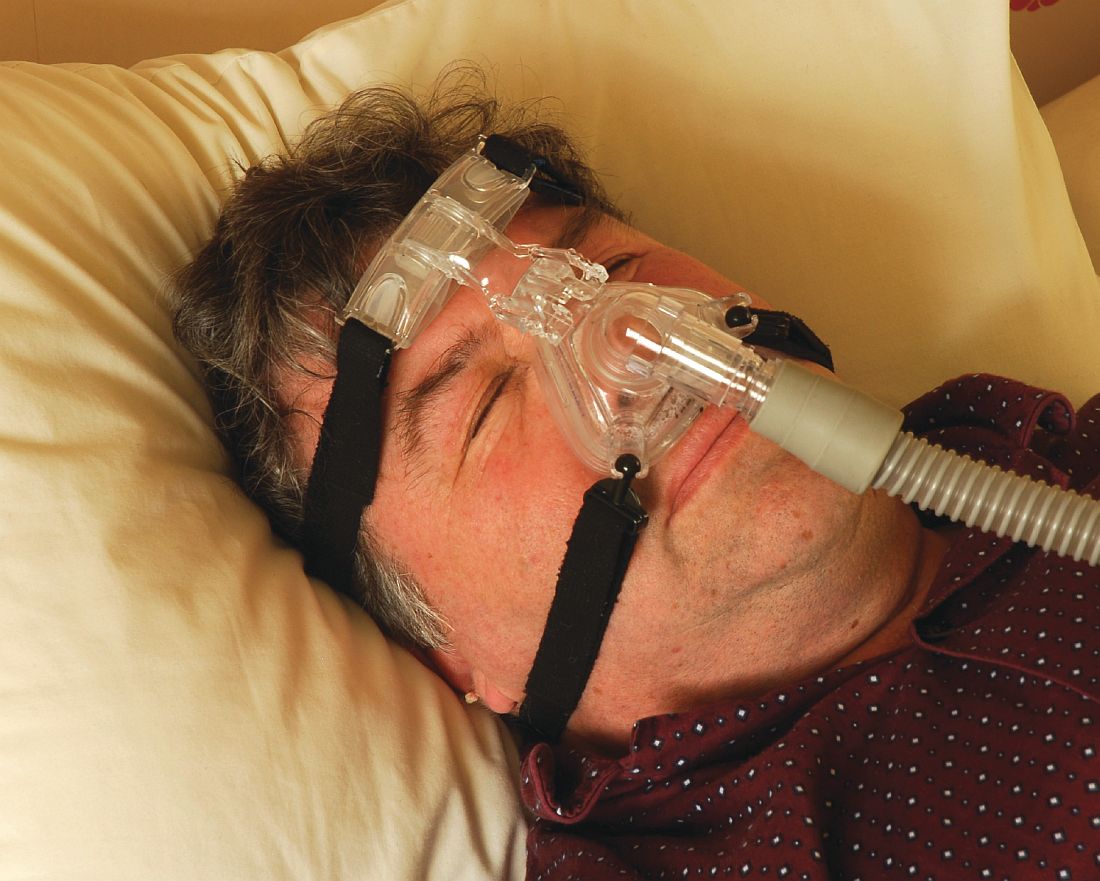
Umpei Yamamoto, MD, of Kyushu University Hospital, Japan, and colleagues studied the prevalence of sleep-disordered breathing among patients who presented to a urology clinic with nocturia and in those who visited a sleep apnea clinic with symptoms of excessive daytime sleepiness.1 Sleep-disordered breathing was found in 91% of the patients from the sleep apnea clinic and 70% of the patients from the urology clinic. The frequency of nocturia was reduced with continuous positive airway pressure (CPAP) in both groups in the patients who had not responded to conventional therapy or nocturia.
The symptom of nocturia as a symptom of sleep apnea might be even more common in women.2 Ozen K. Basoglu, MD, and Mehmet Sezai Tasbakan, MD, of Ege University, Izmir, Turkey, described clinical similarities and differences based on gender in a large group of patients with sleep apnea. Both men and women with sleep apnea had similar rates of excessive daytime sleepiness, snoring, and impaired concentration. Women had more frequent nocturia.
Nocturia especially should be considered a possible clue for the presence of sleep apnea in younger patients who have fewer other reasons to have nocturia. Takahiro Maeda, MD, of Keio University, Tokyo, and colleagues found that men younger than 50 years had more nocturnal urinations the worse their apnea-hypopnea index was.3 Overall in the study, 85% of the patients had a reduction in nighttime urination after CPAP therapy.
Treatment of sleep apnea has been shown in several studies to improve the nocturia that occurs in patients with sleep apnea. Hyoung Keun Park, MD, of Konkuk University, Seoul, and colleagues studied whether surgical intervention with uvulopalatopharyngoplasty (UPPP) reduced nocturia in patients with sleep apnea.4 In the study, there was a 73% success rate in treatment for sleep apnea with the UPPP surgery, and, among those who had successful surgeries, nocturia episodes decreased from 1.9 preoperatively to 0.7 postoperatively (P less than .001).
Minoru Miyazato, MD, PhD, of University of the Ryukyus, Okinawa, Japan, and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.5 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P less than .01).
Pearl: Sleep apnea should be considered in the differential diagnosis of patients with nocturia, and treatment of sleep apnea may decrease nocturia.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Intern Med. 2016;55(8):901-5.
2. Sleep Breath. 2017 Feb 14. doi: 10.1007/s11325-017-1482-9.
3. Can Urol Assoc J. 2016 Jul-Aug;10(7-8):E241-5.
4. Int Neurourol J. 2016 Dec;20(4):329-34.
5. Neurourol Urodyn. 2017 Feb;36(2):376-9.
Author’s note: I have been writing “Myth of the Month” columns for the last several years. I will try to continue to write about myths when possible, but I would like to introduce a new column, “Pearl of the Month.” I want to share with you pearls that I have found really helpful in medical practice. Some of these will be new news, while some may be old news that may not be well known.
A 65-year-old man comes to a clinic concerned about frequent nocturia. He is getting up four times a night to urinate, and he has been urinating about every 5 hours during the day. He has been seen twice for this problem and was diagnosed with benign prostatic hyperplasia and started on tamsulosin.
He found a slight improvement when he started on 0.4 mg qhs, reducing his nocturia episodes from four to three. His dose was increased to 0.8 mg qhs, with no improvement in nocturia.
Exam today: BP, 140/94; pulse, 70. Rectal exam: Prostate is twice normal size without nodules. Labs: Na, 140; K, 4.0; glucose, 80; Ca, 9.6.
He is frustrated because he feels tired and sleepy from having to get up so often to urinate every night.
What is the best treatment/advice at this point?
A. Check hemoglobin A1C.
B. Start finasteride.
C. Switch tamsulosin to terazosin.
D. Evaluate for sleep apnea.
Umpei Yamamoto, MD, of Kyushu University Hospital, Japan, and colleagues studied the prevalence of sleep-disordered breathing among patients who presented to a urology clinic with nocturia and in those who visited a sleep apnea clinic with symptoms of excessive daytime sleepiness.1 Sleep-disordered breathing was found in 91% of the patients from the sleep apnea clinic and 70% of the patients from the urology clinic. The frequency of nocturia was reduced with continuous positive airway pressure (CPAP) in both groups in the patients who had not responded to conventional therapy or nocturia.
The symptom of nocturia as a symptom of sleep apnea might be even more common in women.2 Ozen K. Basoglu, MD, and Mehmet Sezai Tasbakan, MD, of Ege University, Izmir, Turkey, described clinical similarities and differences based on gender in a large group of patients with sleep apnea. Both men and women with sleep apnea had similar rates of excessive daytime sleepiness, snoring, and impaired concentration. Women had more frequent nocturia.
Nocturia especially should be considered a possible clue for the presence of sleep apnea in younger patients who have fewer other reasons to have nocturia. Takahiro Maeda, MD, of Keio University, Tokyo, and colleagues found that men younger than 50 years had more nocturnal urinations the worse their apnea-hypopnea index was.3 Overall in the study, 85% of the patients had a reduction in nighttime urination after CPAP therapy.
Treatment of sleep apnea has been shown in several studies to improve the nocturia that occurs in patients with sleep apnea. Hyoung Keun Park, MD, of Konkuk University, Seoul, and colleagues studied whether surgical intervention with uvulopalatopharyngoplasty (UPPP) reduced nocturia in patients with sleep apnea.4 In the study, there was a 73% success rate in treatment for sleep apnea with the UPPP surgery, and, among those who had successful surgeries, nocturia episodes decreased from 1.9 preoperatively to 0.7 postoperatively (P less than .001).
Minoru Miyazato, MD, PhD, of University of the Ryukyus, Okinawa, Japan, and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.5 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P less than .01).
Pearl: Sleep apnea should be considered in the differential diagnosis of patients with nocturia, and treatment of sleep apnea may decrease nocturia.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Intern Med. 2016;55(8):901-5.
2. Sleep Breath. 2017 Feb 14. doi: 10.1007/s11325-017-1482-9.
3. Can Urol Assoc J. 2016 Jul-Aug;10(7-8):E241-5.
4. Int Neurourol J. 2016 Dec;20(4):329-34.
5. Neurourol Urodyn. 2017 Feb;36(2):376-9.
Author’s note: I have been writing “Myth of the Month” columns for the last several years. I will try to continue to write about myths when possible, but I would like to introduce a new column, “Pearl of the Month.” I want to share with you pearls that I have found really helpful in medical practice. Some of these will be new news, while some may be old news that may not be well known.
A 65-year-old man comes to a clinic concerned about frequent nocturia. He is getting up four times a night to urinate, and he has been urinating about every 5 hours during the day. He has been seen twice for this problem and was diagnosed with benign prostatic hyperplasia and started on tamsulosin.
He found a slight improvement when he started on 0.4 mg qhs, reducing his nocturia episodes from four to three. His dose was increased to 0.8 mg qhs, with no improvement in nocturia.
Exam today: BP, 140/94; pulse, 70. Rectal exam: Prostate is twice normal size without nodules. Labs: Na, 140; K, 4.0; glucose, 80; Ca, 9.6.
He is frustrated because he feels tired and sleepy from having to get up so often to urinate every night.
What is the best treatment/advice at this point?
A. Check hemoglobin A1C.
B. Start finasteride.
C. Switch tamsulosin to terazosin.
D. Evaluate for sleep apnea.
Umpei Yamamoto, MD, of Kyushu University Hospital, Japan, and colleagues studied the prevalence of sleep-disordered breathing among patients who presented to a urology clinic with nocturia and in those who visited a sleep apnea clinic with symptoms of excessive daytime sleepiness.1 Sleep-disordered breathing was found in 91% of the patients from the sleep apnea clinic and 70% of the patients from the urology clinic. The frequency of nocturia was reduced with continuous positive airway pressure (CPAP) in both groups in the patients who had not responded to conventional therapy or nocturia.
The symptom of nocturia as a symptom of sleep apnea might be even more common in women.2 Ozen K. Basoglu, MD, and Mehmet Sezai Tasbakan, MD, of Ege University, Izmir, Turkey, described clinical similarities and differences based on gender in a large group of patients with sleep apnea. Both men and women with sleep apnea had similar rates of excessive daytime sleepiness, snoring, and impaired concentration. Women had more frequent nocturia.
Nocturia especially should be considered a possible clue for the presence of sleep apnea in younger patients who have fewer other reasons to have nocturia. Takahiro Maeda, MD, of Keio University, Tokyo, and colleagues found that men younger than 50 years had more nocturnal urinations the worse their apnea-hypopnea index was.3 Overall in the study, 85% of the patients had a reduction in nighttime urination after CPAP therapy.
Treatment of sleep apnea has been shown in several studies to improve the nocturia that occurs in patients with sleep apnea. Hyoung Keun Park, MD, of Konkuk University, Seoul, and colleagues studied whether surgical intervention with uvulopalatopharyngoplasty (UPPP) reduced nocturia in patients with sleep apnea.4 In the study, there was a 73% success rate in treatment for sleep apnea with the UPPP surgery, and, among those who had successful surgeries, nocturia episodes decreased from 1.9 preoperatively to 0.7 postoperatively (P less than .001).
Minoru Miyazato, MD, PhD, of University of the Ryukyus, Okinawa, Japan, and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.5 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P less than .01).
Pearl: Sleep apnea should be considered in the differential diagnosis of patients with nocturia, and treatment of sleep apnea may decrease nocturia.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Intern Med. 2016;55(8):901-5.
2. Sleep Breath. 2017 Feb 14. doi: 10.1007/s11325-017-1482-9.
3. Can Urol Assoc J. 2016 Jul-Aug;10(7-8):E241-5.
4. Int Neurourol J. 2016 Dec;20(4):329-34.
5. Neurourol Urodyn. 2017 Feb;36(2):376-9.
Double the dose of antihypertensive meds?
A 50-year-old man returns for follow-up of hypertension. He is currently taking 20 mg of lisinopril. His blood pressure readings over the past month are 150/96, 155/98, 160/94, and 162/96. His renal function is normal, and he has been taking his lisinopril regularly.
What do you recommend?
A. Increase his lisinopril to 20 mg twice a day.
B. Switch to valsartan.
C. Add amlodipine.
But is there much benefit in doubling the dose of antihypertensive medications?
H.J. Gomez and colleagues studied the dose response of lisinopril in essential hypertension.2 Patients received very-low-dose (1.25 mg or 5 mg), moderate-dose (20 mg), or high-dose (80 mg) lisinopril. The difference in blood pressure reduction between 20 mg and 80 mg was modest (5 mm/3 mm less in those receiving 80 mg, compared with 20 mg). There was no clinical effect at 1.25 mg of lisinopril, but a relatively flat dose response above 20 mg.
A similar finding was reported by J.R. Benz and colleagues in regard to escalating doses of valsartan.3 The study looked at blood pressure in response to valsartan at doses of 80 mg and 160 mg, and in combination with hydrochlorothiazide. The difference in blood pressure between valsartan 160 mg and 80 mg was 3 mm/0.8 mm. The difference in blood pressure between patients taking 80 mg of valsartan and 25 mg hydrochlorothiazide, compared with those taking 80 mg of valsartan, was 12/6.
In a meta-analysis of 354 randomized trials of fixed-dose blood pressure medications, M.R. Law and colleagues found that cutting the doses in half only reduced effectiveness of lowering BP by 20%.4 The average reduction in systolic BP was 9.1 mm Hg, and reduction in diastolic BP was 5.5mm Hg – which only was reduced to 7.1 mm Hg/4.4 mm Hg when the doses of medications were cut in half. Side effects attributed to beta-blockers, calcium channel blockers, and diuretics were very dose related, whereas the side effects attributed to ACE inhibitors were not.
In another meta-analysis comparing monotherapy vs. combination therapy for lowering blood pressure, adding another drug lowered blood pressure fivefold more than doubling the dose of the initial antihypertensive drug.5
I think the right answer in this case would be to add amlodipine instead of doubling the dose of lisinopril or switching to valsartan as a single agent. The data are striking on how little effect there is in increasing antihypertensive medication doses. Adding another antihypertensive medication should be the standard practice when the first medication started does not achieve the desired goal.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. JAMA. 2003 May 21;289(19):2560-72.
2. Br J Clin Pharm. 1989;28:415-20.
3. J Hum Hypertens. 1998 Dec;12(12):861-6.
4. BMJ. 2003 Jun 28;326(7404):1427.
5. Am J Med. 2009 Mar;122(3):290-300.
A 50-year-old man returns for follow-up of hypertension. He is currently taking 20 mg of lisinopril. His blood pressure readings over the past month are 150/96, 155/98, 160/94, and 162/96. His renal function is normal, and he has been taking his lisinopril regularly.
What do you recommend?
A. Increase his lisinopril to 20 mg twice a day.
B. Switch to valsartan.
C. Add amlodipine.
But is there much benefit in doubling the dose of antihypertensive medications?
H.J. Gomez and colleagues studied the dose response of lisinopril in essential hypertension.2 Patients received very-low-dose (1.25 mg or 5 mg), moderate-dose (20 mg), or high-dose (80 mg) lisinopril. The difference in blood pressure reduction between 20 mg and 80 mg was modest (5 mm/3 mm less in those receiving 80 mg, compared with 20 mg). There was no clinical effect at 1.25 mg of lisinopril, but a relatively flat dose response above 20 mg.
A similar finding was reported by J.R. Benz and colleagues in regard to escalating doses of valsartan.3 The study looked at blood pressure in response to valsartan at doses of 80 mg and 160 mg, and in combination with hydrochlorothiazide. The difference in blood pressure between valsartan 160 mg and 80 mg was 3 mm/0.8 mm. The difference in blood pressure between patients taking 80 mg of valsartan and 25 mg hydrochlorothiazide, compared with those taking 80 mg of valsartan, was 12/6.
In a meta-analysis of 354 randomized trials of fixed-dose blood pressure medications, M.R. Law and colleagues found that cutting the doses in half only reduced effectiveness of lowering BP by 20%.4 The average reduction in systolic BP was 9.1 mm Hg, and reduction in diastolic BP was 5.5mm Hg – which only was reduced to 7.1 mm Hg/4.4 mm Hg when the doses of medications were cut in half. Side effects attributed to beta-blockers, calcium channel blockers, and diuretics were very dose related, whereas the side effects attributed to ACE inhibitors were not.
In another meta-analysis comparing monotherapy vs. combination therapy for lowering blood pressure, adding another drug lowered blood pressure fivefold more than doubling the dose of the initial antihypertensive drug.5
I think the right answer in this case would be to add amlodipine instead of doubling the dose of lisinopril or switching to valsartan as a single agent. The data are striking on how little effect there is in increasing antihypertensive medication doses. Adding another antihypertensive medication should be the standard practice when the first medication started does not achieve the desired goal.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. JAMA. 2003 May 21;289(19):2560-72.
2. Br J Clin Pharm. 1989;28:415-20.
3. J Hum Hypertens. 1998 Dec;12(12):861-6.
4. BMJ. 2003 Jun 28;326(7404):1427.
5. Am J Med. 2009 Mar;122(3):290-300.
A 50-year-old man returns for follow-up of hypertension. He is currently taking 20 mg of lisinopril. His blood pressure readings over the past month are 150/96, 155/98, 160/94, and 162/96. His renal function is normal, and he has been taking his lisinopril regularly.
What do you recommend?
A. Increase his lisinopril to 20 mg twice a day.
B. Switch to valsartan.
C. Add amlodipine.
But is there much benefit in doubling the dose of antihypertensive medications?
H.J. Gomez and colleagues studied the dose response of lisinopril in essential hypertension.2 Patients received very-low-dose (1.25 mg or 5 mg), moderate-dose (20 mg), or high-dose (80 mg) lisinopril. The difference in blood pressure reduction between 20 mg and 80 mg was modest (5 mm/3 mm less in those receiving 80 mg, compared with 20 mg). There was no clinical effect at 1.25 mg of lisinopril, but a relatively flat dose response above 20 mg.
A similar finding was reported by J.R. Benz and colleagues in regard to escalating doses of valsartan.3 The study looked at blood pressure in response to valsartan at doses of 80 mg and 160 mg, and in combination with hydrochlorothiazide. The difference in blood pressure between valsartan 160 mg and 80 mg was 3 mm/0.8 mm. The difference in blood pressure between patients taking 80 mg of valsartan and 25 mg hydrochlorothiazide, compared with those taking 80 mg of valsartan, was 12/6.
In a meta-analysis of 354 randomized trials of fixed-dose blood pressure medications, M.R. Law and colleagues found that cutting the doses in half only reduced effectiveness of lowering BP by 20%.4 The average reduction in systolic BP was 9.1 mm Hg, and reduction in diastolic BP was 5.5mm Hg – which only was reduced to 7.1 mm Hg/4.4 mm Hg when the doses of medications were cut in half. Side effects attributed to beta-blockers, calcium channel blockers, and diuretics were very dose related, whereas the side effects attributed to ACE inhibitors were not.
In another meta-analysis comparing monotherapy vs. combination therapy for lowering blood pressure, adding another drug lowered blood pressure fivefold more than doubling the dose of the initial antihypertensive drug.5
I think the right answer in this case would be to add amlodipine instead of doubling the dose of lisinopril or switching to valsartan as a single agent. The data are striking on how little effect there is in increasing antihypertensive medication doses. Adding another antihypertensive medication should be the standard practice when the first medication started does not achieve the desired goal.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. JAMA. 2003 May 21;289(19):2560-72.
2. Br J Clin Pharm. 1989;28:415-20.
3. J Hum Hypertens. 1998 Dec;12(12):861-6.
4. BMJ. 2003 Jun 28;326(7404):1427.
5. Am J Med. 2009 Mar;122(3):290-300.
Antibiotic prophylaxis for artificial joints
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
Myth of the Month: CT scan before lumbar puncture in suspected meningitis?
A 28-year-old male presents to the emergency department with fever and severe headache. He has had a fever for 24 hours. Headache began that morning. On exam, he has marked nuchal rigidity, with a temperature of 103.5° F. He is fully oriented and has a nonfocal neurologic examination.
What would you do?
A) Lumbar puncture (LP).
B) CT scan, then LP.
C) Antibiotics, CT scan, then LP.
D) MRI, then LP.
E) Antibiotics, MRI, then LP.
The practice of obtaining a head CT scan (or MRI) before performing a lumbar puncture (LP) is commonplace in emergency departments. The concern is that if a lumbar puncture is done in a patient with increased intracranial pressure, then brain herniation could occur. How common is brain herniation, are there useful clinical indicators that make a CT scan not helpful, and does this concern reach myth status?
In a retrospective study well before CT scans were available, 401 patients with brain tumors who had LP were reviewed, and 32% of the patients had papilledema.3 There was only one poor outcome because of the LP. This would be considered a high-risk group for complications, because many of these patients had clear focal neurologic signs, and one-third of them had papilledema.
There have been a number of studies looking at whether there is utility in obtaining a CT scan prior to LP in patients with suspected acute bacterial meningitis.
Dr. N.D. Baker of Brigham and Women’s Hospital, Boston, and colleagues retrospectively reviewed the records of 112 patients who had a routine CT before LP for suspected meningitis.2 Regardless of CT scan result, all patients received a lumbar puncture. Four patients had mass lesions on CT scan, though none of these patients had an increased opening pressure. No patient had an adverse outcome from LP.
Dr. Paul Greig and Dr. D. Goroszeniuk of Horton General Hospital, Banbury, England, reported on a retrospective study of all patients over a 6-month period considered for a LP.4 A total of 64 LPs were considered; 54 of these patients had a CT before LP. No patients had a bad outcome from the lumbar puncture.
The sensitivity and negative predictive value of a normal neurologic exam were very good in this study, leading to the conclusion from the authors that a normal neurologic exam and fundoscopic exam is an accurate predictor of a normal CT scan. The authors were dismayed that only 45% of the patients in the study received a fundoscopic exam.
Rodrigo Hasbun, MD, formerly of Yale University, New Haven, Conn., and his colleagues did a prospective study of 301 patients with suspected meningitis to see if clinical signs and symptoms could help guide utilization of CT scans.5 A total of 235 patients got CT scans, and 24% were abnormal.
The clinical features associated with higher likelihood of an abnormal CT were age greater than 60 years, immunosuppression, known CNS disease, and a seizure within the past week. On exam, altered mental status, aphasia, and focal neurologic findings were associated with higher likelihood of an abnormal CT scan.
Of the 96 patients who did not have any of these features, 93 had a normal CT exam, giving a negative predictive value of 97%.
No patients in the study had herniation from lumbar puncture. The only patients in the study who had brain herniations were two patients who had severe mass effect on CT, and did not receive lumbar punctures.
I think the patient in this case should have a lumbar puncture and does not need any imaging. I think that there is no reason to get neuroimaging in patients with suspected meningitis who are alert and have a nonfocal neurologic exam, and do not have papilledema.
References
1. Br J Radiol. 1999 Mar;72(855):319.
2. J Emerg Med. 1994 Sep-Oct;12(5):597-601.
3. AMA Arch Neurol Psychiatry. 1954 Nov;72(5):568-72.
4. Postgrad Med J. 2006 Mar;82(965):162-5.
5. N Engl J Med. 2001 Dec 13;345(24):1727-33.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 28-year-old male presents to the emergency department with fever and severe headache. He has had a fever for 24 hours. Headache began that morning. On exam, he has marked nuchal rigidity, with a temperature of 103.5° F. He is fully oriented and has a nonfocal neurologic examination.
What would you do?
A) Lumbar puncture (LP).
B) CT scan, then LP.
C) Antibiotics, CT scan, then LP.
D) MRI, then LP.
E) Antibiotics, MRI, then LP.
The practice of obtaining a head CT scan (or MRI) before performing a lumbar puncture (LP) is commonplace in emergency departments. The concern is that if a lumbar puncture is done in a patient with increased intracranial pressure, then brain herniation could occur. How common is brain herniation, are there useful clinical indicators that make a CT scan not helpful, and does this concern reach myth status?
In a retrospective study well before CT scans were available, 401 patients with brain tumors who had LP were reviewed, and 32% of the patients had papilledema.3 There was only one poor outcome because of the LP. This would be considered a high-risk group for complications, because many of these patients had clear focal neurologic signs, and one-third of them had papilledema.
There have been a number of studies looking at whether there is utility in obtaining a CT scan prior to LP in patients with suspected acute bacterial meningitis.
Dr. N.D. Baker of Brigham and Women’s Hospital, Boston, and colleagues retrospectively reviewed the records of 112 patients who had a routine CT before LP for suspected meningitis.2 Regardless of CT scan result, all patients received a lumbar puncture. Four patients had mass lesions on CT scan, though none of these patients had an increased opening pressure. No patient had an adverse outcome from LP.
Dr. Paul Greig and Dr. D. Goroszeniuk of Horton General Hospital, Banbury, England, reported on a retrospective study of all patients over a 6-month period considered for a LP.4 A total of 64 LPs were considered; 54 of these patients had a CT before LP. No patients had a bad outcome from the lumbar puncture.
The sensitivity and negative predictive value of a normal neurologic exam were very good in this study, leading to the conclusion from the authors that a normal neurologic exam and fundoscopic exam is an accurate predictor of a normal CT scan. The authors were dismayed that only 45% of the patients in the study received a fundoscopic exam.
Rodrigo Hasbun, MD, formerly of Yale University, New Haven, Conn., and his colleagues did a prospective study of 301 patients with suspected meningitis to see if clinical signs and symptoms could help guide utilization of CT scans.5 A total of 235 patients got CT scans, and 24% were abnormal.
The clinical features associated with higher likelihood of an abnormal CT were age greater than 60 years, immunosuppression, known CNS disease, and a seizure within the past week. On exam, altered mental status, aphasia, and focal neurologic findings were associated with higher likelihood of an abnormal CT scan.
Of the 96 patients who did not have any of these features, 93 had a normal CT exam, giving a negative predictive value of 97%.
No patients in the study had herniation from lumbar puncture. The only patients in the study who had brain herniations were two patients who had severe mass effect on CT, and did not receive lumbar punctures.
I think the patient in this case should have a lumbar puncture and does not need any imaging. I think that there is no reason to get neuroimaging in patients with suspected meningitis who are alert and have a nonfocal neurologic exam, and do not have papilledema.
References
1. Br J Radiol. 1999 Mar;72(855):319.
2. J Emerg Med. 1994 Sep-Oct;12(5):597-601.
3. AMA Arch Neurol Psychiatry. 1954 Nov;72(5):568-72.
4. Postgrad Med J. 2006 Mar;82(965):162-5.
5. N Engl J Med. 2001 Dec 13;345(24):1727-33.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 28-year-old male presents to the emergency department with fever and severe headache. He has had a fever for 24 hours. Headache began that morning. On exam, he has marked nuchal rigidity, with a temperature of 103.5° F. He is fully oriented and has a nonfocal neurologic examination.
What would you do?
A) Lumbar puncture (LP).
B) CT scan, then LP.
C) Antibiotics, CT scan, then LP.
D) MRI, then LP.
E) Antibiotics, MRI, then LP.
The practice of obtaining a head CT scan (or MRI) before performing a lumbar puncture (LP) is commonplace in emergency departments. The concern is that if a lumbar puncture is done in a patient with increased intracranial pressure, then brain herniation could occur. How common is brain herniation, are there useful clinical indicators that make a CT scan not helpful, and does this concern reach myth status?
In a retrospective study well before CT scans were available, 401 patients with brain tumors who had LP were reviewed, and 32% of the patients had papilledema.3 There was only one poor outcome because of the LP. This would be considered a high-risk group for complications, because many of these patients had clear focal neurologic signs, and one-third of them had papilledema.
There have been a number of studies looking at whether there is utility in obtaining a CT scan prior to LP in patients with suspected acute bacterial meningitis.
Dr. N.D. Baker of Brigham and Women’s Hospital, Boston, and colleagues retrospectively reviewed the records of 112 patients who had a routine CT before LP for suspected meningitis.2 Regardless of CT scan result, all patients received a lumbar puncture. Four patients had mass lesions on CT scan, though none of these patients had an increased opening pressure. No patient had an adverse outcome from LP.
Dr. Paul Greig and Dr. D. Goroszeniuk of Horton General Hospital, Banbury, England, reported on a retrospective study of all patients over a 6-month period considered for a LP.4 A total of 64 LPs were considered; 54 of these patients had a CT before LP. No patients had a bad outcome from the lumbar puncture.
The sensitivity and negative predictive value of a normal neurologic exam were very good in this study, leading to the conclusion from the authors that a normal neurologic exam and fundoscopic exam is an accurate predictor of a normal CT scan. The authors were dismayed that only 45% of the patients in the study received a fundoscopic exam.
Rodrigo Hasbun, MD, formerly of Yale University, New Haven, Conn., and his colleagues did a prospective study of 301 patients with suspected meningitis to see if clinical signs and symptoms could help guide utilization of CT scans.5 A total of 235 patients got CT scans, and 24% were abnormal.
The clinical features associated with higher likelihood of an abnormal CT were age greater than 60 years, immunosuppression, known CNS disease, and a seizure within the past week. On exam, altered mental status, aphasia, and focal neurologic findings were associated with higher likelihood of an abnormal CT scan.
Of the 96 patients who did not have any of these features, 93 had a normal CT exam, giving a negative predictive value of 97%.
No patients in the study had herniation from lumbar puncture. The only patients in the study who had brain herniations were two patients who had severe mass effect on CT, and did not receive lumbar punctures.
I think the patient in this case should have a lumbar puncture and does not need any imaging. I think that there is no reason to get neuroimaging in patients with suspected meningitis who are alert and have a nonfocal neurologic exam, and do not have papilledema.
References
1. Br J Radiol. 1999 Mar;72(855):319.
2. J Emerg Med. 1994 Sep-Oct;12(5):597-601.
3. AMA Arch Neurol Psychiatry. 1954 Nov;72(5):568-72.
4. Postgrad Med J. 2006 Mar;82(965):162-5.
5. N Engl J Med. 2001 Dec 13;345(24):1727-33.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
Myth of the Month: Does nitroglycerin response predict coronary artery disease?
A 55-year-old man presents to the emergency department with substernal chest pain. The pain has occurred off and on over the past 2 hours. He has no family history of coronary artery disease. He has no history of diabetes, hypertension, or cigarette smoking. His most recent total cholesterol was 220 mg/dL (HDL, 40; LDL, 155). Blood pressure is 130/70. An ECG obtained on arrival is unremarkable. When he reached the ED, he received a nitroglycerin tablet with resolution of his pain within 4 minutes.
What is the most accurate statement?
A. The chance of CAD in this man over the next 10 years was 8% before his symptoms and is now greater than 20%.
B. The chance of CAD in this man over the next 10 years was 8% and is still 8%.
C. The chance of CAD in this man over the next 10 years was 15% before his symptoms and is now close to 100%.
D. The chance of CAD in this man over the next 10 years was 15% before his symptoms and is now close to 50%.
For years, giving nitroglycerin to patients who present with chest pain has been considered a good therapy, and the response to the medication has been considered a sign that the pain was likely due to cardiac ischemia. Is there evidence that this is true?
The study was a retrospective review of 223 patients who presented to the ED over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the ED, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Nitroglycerin response was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the ED in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.2 The study was a prospective, observational study of 664 patients in an urban tertiary care ED over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain. Complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Two other studies with similar designs showed similar results. Robert Steele, MD, and his colleagues studied 270 patients in a prospective observational cohort study of patients with chest pain presenting to an urban ED.3 Patients presenting to the ED with active chest pain who received nitroglycerin were enrolled.
The sensitivity in this study for nitroglycerin relief determining cardiac chest pain was 72%, and the specificity was 37%, with a positive likelihood ratio for coronary artery disease if nitroglycerin response of 1.1 (0.96-1.34).
In another prospective, observational cohort study, 459 patients who presented to an ED with chest pain were evaluated for response to nitroglycerin as a marker for ischemic cardiac disease.4 In this study, presence of ischemic cardiac disease was defined as diagnosis in the ED or during a 4-month follow-up period. Nitroglycerin relieved chest pain in 35% of patients who had coronary disease, whereas 41% of patients without coronary disease had a nitroglycerin response. This study had a much lower overall nitroglycerin response rate than any of the other studies.
Katherine Grailey, MD, and Paul Glasziou, MD, PhD, published a meta-analysis of nitroglycerin use for the diagnosis of chest pain, using the above referenced studies. They concluded that in the acute setting, nitroglycerin is not a reliable test of treatment for use in diagnosis of coronary artery disease.5
High response rate for nitroglycerin in the noncoronary artery groups in the studies may be due to a strong placebo effect and/or that nitroglycerin may help with pain caused by esophageal spasm. The lack of specificity in the pain relief response for nitroglycerin makes it not a helpful test. Note that all the studies have been in the acute, ED setting for chest pain. In the case presented at the beginning of the article, the response the patient had to nitroglycerin would not change the probability that he has coronary artery disease.
References
1. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
2. Ann Emerg Med. 2005 Jun;45(6):581-5.
3. CJEM. 2006 May;8(3):164-9.
4. Ann Intern Med. 2003 Dec 16;139(12):979-86.
5. Emerg Med J. 2012 Mar;29(3):173-6.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected] .
A 55-year-old man presents to the emergency department with substernal chest pain. The pain has occurred off and on over the past 2 hours. He has no family history of coronary artery disease. He has no history of diabetes, hypertension, or cigarette smoking. His most recent total cholesterol was 220 mg/dL (HDL, 40; LDL, 155). Blood pressure is 130/70. An ECG obtained on arrival is unremarkable. When he reached the ED, he received a nitroglycerin tablet with resolution of his pain within 4 minutes.
What is the most accurate statement?
A. The chance of CAD in this man over the next 10 years was 8% before his symptoms and is now greater than 20%.
B. The chance of CAD in this man over the next 10 years was 8% and is still 8%.
C. The chance of CAD in this man over the next 10 years was 15% before his symptoms and is now close to 100%.
D. The chance of CAD in this man over the next 10 years was 15% before his symptoms and is now close to 50%.
For years, giving nitroglycerin to patients who present with chest pain has been considered a good therapy, and the response to the medication has been considered a sign that the pain was likely due to cardiac ischemia. Is there evidence that this is true?
The study was a retrospective review of 223 patients who presented to the ED over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the ED, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Nitroglycerin response was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the ED in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.2 The study was a prospective, observational study of 664 patients in an urban tertiary care ED over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain. Complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Two other studies with similar designs showed similar results. Robert Steele, MD, and his colleagues studied 270 patients in a prospective observational cohort study of patients with chest pain presenting to an urban ED.3 Patients presenting to the ED with active chest pain who received nitroglycerin were enrolled.
The sensitivity in this study for nitroglycerin relief determining cardiac chest pain was 72%, and the specificity was 37%, with a positive likelihood ratio for coronary artery disease if nitroglycerin response of 1.1 (0.96-1.34).
In another prospective, observational cohort study, 459 patients who presented to an ED with chest pain were evaluated for response to nitroglycerin as a marker for ischemic cardiac disease.4 In this study, presence of ischemic cardiac disease was defined as diagnosis in the ED or during a 4-month follow-up period. Nitroglycerin relieved chest pain in 35% of patients who had coronary disease, whereas 41% of patients without coronary disease had a nitroglycerin response. This study had a much lower overall nitroglycerin response rate than any of the other studies.
Katherine Grailey, MD, and Paul Glasziou, MD, PhD, published a meta-analysis of nitroglycerin use for the diagnosis of chest pain, using the above referenced studies. They concluded that in the acute setting, nitroglycerin is not a reliable test of treatment for use in diagnosis of coronary artery disease.5
High response rate for nitroglycerin in the noncoronary artery groups in the studies may be due to a strong placebo effect and/or that nitroglycerin may help with pain caused by esophageal spasm. The lack of specificity in the pain relief response for nitroglycerin makes it not a helpful test. Note that all the studies have been in the acute, ED setting for chest pain. In the case presented at the beginning of the article, the response the patient had to nitroglycerin would not change the probability that he has coronary artery disease.
References
1. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
2. Ann Emerg Med. 2005 Jun;45(6):581-5.
3. CJEM. 2006 May;8(3):164-9.
4. Ann Intern Med. 2003 Dec 16;139(12):979-86.
5. Emerg Med J. 2012 Mar;29(3):173-6.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected] .
A 55-year-old man presents to the emergency department with substernal chest pain. The pain has occurred off and on over the past 2 hours. He has no family history of coronary artery disease. He has no history of diabetes, hypertension, or cigarette smoking. His most recent total cholesterol was 220 mg/dL (HDL, 40; LDL, 155). Blood pressure is 130/70. An ECG obtained on arrival is unremarkable. When he reached the ED, he received a nitroglycerin tablet with resolution of his pain within 4 minutes.
What is the most accurate statement?
A. The chance of CAD in this man over the next 10 years was 8% before his symptoms and is now greater than 20%.
B. The chance of CAD in this man over the next 10 years was 8% and is still 8%.
C. The chance of CAD in this man over the next 10 years was 15% before his symptoms and is now close to 100%.
D. The chance of CAD in this man over the next 10 years was 15% before his symptoms and is now close to 50%.
For years, giving nitroglycerin to patients who present with chest pain has been considered a good therapy, and the response to the medication has been considered a sign that the pain was likely due to cardiac ischemia. Is there evidence that this is true?
The study was a retrospective review of 223 patients who presented to the ED over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the ED, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Nitroglycerin response was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the ED in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.2 The study was a prospective, observational study of 664 patients in an urban tertiary care ED over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain. Complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Two other studies with similar designs showed similar results. Robert Steele, MD, and his colleagues studied 270 patients in a prospective observational cohort study of patients with chest pain presenting to an urban ED.3 Patients presenting to the ED with active chest pain who received nitroglycerin were enrolled.
The sensitivity in this study for nitroglycerin relief determining cardiac chest pain was 72%, and the specificity was 37%, with a positive likelihood ratio for coronary artery disease if nitroglycerin response of 1.1 (0.96-1.34).
In another prospective, observational cohort study, 459 patients who presented to an ED with chest pain were evaluated for response to nitroglycerin as a marker for ischemic cardiac disease.4 In this study, presence of ischemic cardiac disease was defined as diagnosis in the ED or during a 4-month follow-up period. Nitroglycerin relieved chest pain in 35% of patients who had coronary disease, whereas 41% of patients without coronary disease had a nitroglycerin response. This study had a much lower overall nitroglycerin response rate than any of the other studies.
Katherine Grailey, MD, and Paul Glasziou, MD, PhD, published a meta-analysis of nitroglycerin use for the diagnosis of chest pain, using the above referenced studies. They concluded that in the acute setting, nitroglycerin is not a reliable test of treatment for use in diagnosis of coronary artery disease.5
High response rate for nitroglycerin in the noncoronary artery groups in the studies may be due to a strong placebo effect and/or that nitroglycerin may help with pain caused by esophageal spasm. The lack of specificity in the pain relief response for nitroglycerin makes it not a helpful test. Note that all the studies have been in the acute, ED setting for chest pain. In the case presented at the beginning of the article, the response the patient had to nitroglycerin would not change the probability that he has coronary artery disease.
References
1. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
2. Ann Emerg Med. 2005 Jun;45(6):581-5.
3. CJEM. 2006 May;8(3):164-9.
4. Ann Intern Med. 2003 Dec 16;139(12):979-86.
5. Emerg Med J. 2012 Mar;29(3):173-6.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected] .
Should I throw out my expired medications?
A 55-year-old patient requests new prescriptions at a routine appointment. She will be traveling internationally next month and wants to replace her emergency medication kit, as the medications in it (ciprofloxacin, loperamide, and oxycodone) have all expired.
What do you do?
A) Replace the prescription for ciprofloxacin.
B) Replace all three medications.
C) Tell the patient that all the meds should still be fine.
This is a common concern brought up by patients. Many patients discard medications when they pass the expiration date on the package. Is this necessary? Is there a health risk to taking expired medications, and is it okay from a therapeutic standpoint to use medications past their expiration date?
The expiration date is not a date that the drug stops being effective or potentially becomes toxic. It is a date, required by law, that the manufacturer can guarantee greater than 90% original potency of the medication. There really isn’t incentive for pharmaceutical companies to extend the expiration dates, as it is profitable for patients to throw away expired medications and replace them with new prescriptions.
The U.S. military purchases a large stockpile of drugs and has the potential for having a great deal of expired medications. To help reduce this problem, the Food and Drug Administration administers the shelf-life extension program (SLEP) for the U.S. military as a testing and evaluation program designed to justify an extension of the shelf life of stockpiled drug products.1
Robbe Lyon, MD, and colleagues reported data from the SLEP.2 A total of 122 drugs were studied representing 3,005 lots, with 88% of these extended at least 1 year past the expiration date, with an average extension of more than 5 years. Several antibiotics were studied, including ciprofloxacin (mean extension, 55 months), amoxicillin (mean extension, 23 months), and doxycycline (mean extension, 50 months).
Lee Cantrell, PharmD, and coinvestigators looked at sealed drugs from a retail pharmacy that were 28-40 years past their expiration date.3 Amazingly, 12 of the 14 compounds tested were in concentrations that were at least 90% of the labeled amount. Among the drugs that were tested that maintained greater than 90% of the labeled amount were acetaminophen, codeine, hydrocodone, and barbiturates. Aspirin and amphetamine were the two drugs that did not have greater than 90% of the labeled amount. The aspirin amounts were very low, about 1% of the listed amount on the package.
The major myth surrounding expired medications is that taking an expired medication could be toxic.
There is one case report of toxicity from the use of expired tetracycline.4 This was a case series of three patients who developed reversible Fanconi syndrome linked to using an expired tetracycline preparation. That preparation of tetracycline is no longer available.
There are no other reports of toxicity due to use of expired medications.5,6 There appears to be no direct risk of toxicity to the patient by using medication past the expiration date.
The risk that isn’t answered is the risk of using medications that may not be effective if potency isn’t guaranteed beyond the expiration date. It appears that most drugs are effective for years past the expiration date.
This isn’t an issue for medications for treatment of chronic conditions, as patients take the medications every day, and the medications will be used up long before they expire. For medications that are used infrequently – analgesics, antihistamines, and medications for traveler’s diarrhea – they appear to be stable for years past expiration, and there is little risk to the patient if the medications are not fully effective.
I think aspirin should not be used past expiration, given the data showing that it breaks down more so than other medications. For the case at the start of this article, I think having the patient use the expired medications should be fine.
References
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 55-year-old patient requests new prescriptions at a routine appointment. She will be traveling internationally next month and wants to replace her emergency medication kit, as the medications in it (ciprofloxacin, loperamide, and oxycodone) have all expired.
What do you do?
A) Replace the prescription for ciprofloxacin.
B) Replace all three medications.
C) Tell the patient that all the meds should still be fine.
This is a common concern brought up by patients. Many patients discard medications when they pass the expiration date on the package. Is this necessary? Is there a health risk to taking expired medications, and is it okay from a therapeutic standpoint to use medications past their expiration date?
The expiration date is not a date that the drug stops being effective or potentially becomes toxic. It is a date, required by law, that the manufacturer can guarantee greater than 90% original potency of the medication. There really isn’t incentive for pharmaceutical companies to extend the expiration dates, as it is profitable for patients to throw away expired medications and replace them with new prescriptions.
The U.S. military purchases a large stockpile of drugs and has the potential for having a great deal of expired medications. To help reduce this problem, the Food and Drug Administration administers the shelf-life extension program (SLEP) for the U.S. military as a testing and evaluation program designed to justify an extension of the shelf life of stockpiled drug products.1
Robbe Lyon, MD, and colleagues reported data from the SLEP.2 A total of 122 drugs were studied representing 3,005 lots, with 88% of these extended at least 1 year past the expiration date, with an average extension of more than 5 years. Several antibiotics were studied, including ciprofloxacin (mean extension, 55 months), amoxicillin (mean extension, 23 months), and doxycycline (mean extension, 50 months).
Lee Cantrell, PharmD, and coinvestigators looked at sealed drugs from a retail pharmacy that were 28-40 years past their expiration date.3 Amazingly, 12 of the 14 compounds tested were in concentrations that were at least 90% of the labeled amount. Among the drugs that were tested that maintained greater than 90% of the labeled amount were acetaminophen, codeine, hydrocodone, and barbiturates. Aspirin and amphetamine were the two drugs that did not have greater than 90% of the labeled amount. The aspirin amounts were very low, about 1% of the listed amount on the package.
The major myth surrounding expired medications is that taking an expired medication could be toxic.
There is one case report of toxicity from the use of expired tetracycline.4 This was a case series of three patients who developed reversible Fanconi syndrome linked to using an expired tetracycline preparation. That preparation of tetracycline is no longer available.
There are no other reports of toxicity due to use of expired medications.5,6 There appears to be no direct risk of toxicity to the patient by using medication past the expiration date.
The risk that isn’t answered is the risk of using medications that may not be effective if potency isn’t guaranteed beyond the expiration date. It appears that most drugs are effective for years past the expiration date.
This isn’t an issue for medications for treatment of chronic conditions, as patients take the medications every day, and the medications will be used up long before they expire. For medications that are used infrequently – analgesics, antihistamines, and medications for traveler’s diarrhea – they appear to be stable for years past expiration, and there is little risk to the patient if the medications are not fully effective.
I think aspirin should not be used past expiration, given the data showing that it breaks down more so than other medications. For the case at the start of this article, I think having the patient use the expired medications should be fine.
References
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 55-year-old patient requests new prescriptions at a routine appointment. She will be traveling internationally next month and wants to replace her emergency medication kit, as the medications in it (ciprofloxacin, loperamide, and oxycodone) have all expired.
What do you do?
A) Replace the prescription for ciprofloxacin.
B) Replace all three medications.
C) Tell the patient that all the meds should still be fine.
This is a common concern brought up by patients. Many patients discard medications when they pass the expiration date on the package. Is this necessary? Is there a health risk to taking expired medications, and is it okay from a therapeutic standpoint to use medications past their expiration date?
The expiration date is not a date that the drug stops being effective or potentially becomes toxic. It is a date, required by law, that the manufacturer can guarantee greater than 90% original potency of the medication. There really isn’t incentive for pharmaceutical companies to extend the expiration dates, as it is profitable for patients to throw away expired medications and replace them with new prescriptions.
The U.S. military purchases a large stockpile of drugs and has the potential for having a great deal of expired medications. To help reduce this problem, the Food and Drug Administration administers the shelf-life extension program (SLEP) for the U.S. military as a testing and evaluation program designed to justify an extension of the shelf life of stockpiled drug products.1
Robbe Lyon, MD, and colleagues reported data from the SLEP.2 A total of 122 drugs were studied representing 3,005 lots, with 88% of these extended at least 1 year past the expiration date, with an average extension of more than 5 years. Several antibiotics were studied, including ciprofloxacin (mean extension, 55 months), amoxicillin (mean extension, 23 months), and doxycycline (mean extension, 50 months).
Lee Cantrell, PharmD, and coinvestigators looked at sealed drugs from a retail pharmacy that were 28-40 years past their expiration date.3 Amazingly, 12 of the 14 compounds tested were in concentrations that were at least 90% of the labeled amount. Among the drugs that were tested that maintained greater than 90% of the labeled amount were acetaminophen, codeine, hydrocodone, and barbiturates. Aspirin and amphetamine were the two drugs that did not have greater than 90% of the labeled amount. The aspirin amounts were very low, about 1% of the listed amount on the package.
The major myth surrounding expired medications is that taking an expired medication could be toxic.
There is one case report of toxicity from the use of expired tetracycline.4 This was a case series of three patients who developed reversible Fanconi syndrome linked to using an expired tetracycline preparation. That preparation of tetracycline is no longer available.
There are no other reports of toxicity due to use of expired medications.5,6 There appears to be no direct risk of toxicity to the patient by using medication past the expiration date.
The risk that isn’t answered is the risk of using medications that may not be effective if potency isn’t guaranteed beyond the expiration date. It appears that most drugs are effective for years past the expiration date.
This isn’t an issue for medications for treatment of chronic conditions, as patients take the medications every day, and the medications will be used up long before they expire. For medications that are used infrequently – analgesics, antihistamines, and medications for traveler’s diarrhea – they appear to be stable for years past expiration, and there is little risk to the patient if the medications are not fully effective.
I think aspirin should not be used past expiration, given the data showing that it breaks down more so than other medications. For the case at the start of this article, I think having the patient use the expired medications should be fine.
References
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
Myth of the Month: Vaccinations in patients with Guillain-Barré syndrome
A 66-year-old woman presents as a new patient for a clinic visit. She has a history of Guillain-Barré syndrome 10 years ago. The last immunization she received was a tetanus-diphtheria 12 years ago.
What do you recommend for her to receive over the next year?
A. Pneumococcal 13/Pneumococcal 23/Tdap/influenza vaccines.
B. Pneumococcal 13/Pneumococcal 23/Tdap vaccines.
C. Influenza vaccine.
D. No vaccines.
Guillain-Barré syndrome (GBS) is a rare, acute, immune-mediated polyneuropathy that has an incidence of about 2 cases per 100,000 people each year.1 Most cases of GBS follow an infectious event (usually an upper respiratory infection or gastrointestinal infection). In 1976, administration of the swine flu vaccine was associated with an up to eightfold increased risk of GBS.2,3 Many patients who have had GBS have been advised not to – or are fearful to – receive influenza vaccine or any vaccine.
Is there good evidence for patients with a history of GBS to avoid influenza vaccines or vaccinations in general?
The initial concern over the increased risk of GBS following the large-scale influenza vaccination in 1976 has not been realized with subsequent influenza vaccines. In a study by Baxter and colleagues, GBS cases from Kaiser Permanente Northern California from 1995 to 2006 were reviewed.4 They looked at whether patients had received influenza vaccine in the 6 weeks prior to GBS, compared with vaccination within the prior 9 months.
The odds ratio for influenza vaccination in the 6 weeks prior to GBS was 1.1 (95% confidence interval, 0.4-3.1). The odds ratio for receiving tetanus diphtheria vaccine in the 6 weeks prior to GBS was 1.4 (95% CI, 0.3-4.5); pneumococcal 23 vaccine, 0.7 (95% CI, 0.1-2.9); and all vaccines combined, 1.3 (95% CI, 0.8-2.3).
Shahed Iqbal, MBBS, et al. looked at the relationship between influenza illness, pneumonia, influenza vaccination, and GBS.5 They found that although influenza vaccine coverage increased from 20% to 36% over the study period, there was not an increase in GBS hospitalizations over the same period. There was a significant correlation between hospitalizations for pneumonia and influenza and GBS hospitalizations in the same month.
In a simulation study, Steven Hawken, PhD, and his colleagues concluded that under typical conditions (influenza incidence greater than 5% and vaccine effectiveness greater than 60%), influenza vaccination reduced GBS risk.6
There are fewer data on vaccination in patients who have previously had GBS, but there is enough evidence to help guide us.
Roger Baxter, MD, and colleagues, using the database in reference 4, looked at outcome of patients with GBS who received vaccinations subsequent to recovery from GBS.7 A total of 279 patient with previous GBS received a total of 989 vaccinations, including 405 trivalent influenza vaccinations. None of the patients with GBS who received vaccinations had a recurrence of GBS.
Krista Kuitwaard, MD, et al. reported identical findings in a survey of patients with a history of GBS or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).8 A total of 245 patients with GBS responded to the survey. A total of 106 GBS patients had received influenza vaccine following their GBS diagnosis (a total of 775 vaccinations in those patients). None of the patients with a history of GBS who received influenza vaccination had a recurrence of their GBS.
The current position of the GBS/CIDP Foundation on vaccination for patients with GBS is as follows: The GBS/CIDP Foundation recommends avoiding immunizations that a GBS patient had received within 6 weeks of developing their initial symptoms.9
I think the current evidence is enough to guide us in this issue. Vaccinations, including influenza vaccine, are likely safe for patients with a history of GBS. The recommendation of the GBS/CIDP foundation is reasonable – to avoid immunizations that appeared to have potentially triggered the initial GBS (ones that had been received within 6 weeks of onset of symptoms).
In the case presented above, I think that choice A – receiving all the recommended immunizations – would be appropriate.
References
1. Neuroepidemiology 2011; 36(2):123-33.
2. Am J Epidemiol. 1979 Aug;110(2):105-23.
3. Clin Infect Dis. 2014 Apr;58(8):1149-55.
4. Clin Infect Dis. 2013 Jul;57(2):197-204.
5. Vaccine. 2015 Apr 21;33(17):2045-9.
6. Emerg Infect Dis. 2015 Feb;21(2):224-31.
7. Clin Infect Dis. 2012 Mar;54(6):800-4.
8. J Peripher Nerv Syst. 2009 Dec;14(4):310-5.
9. GBS/CIDP Foundation International, Position on Flu Shots and Vaccinations.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman presents as a new patient for a clinic visit. She has a history of Guillain-Barré syndrome 10 years ago. The last immunization she received was a tetanus-diphtheria 12 years ago.
What do you recommend for her to receive over the next year?
A. Pneumococcal 13/Pneumococcal 23/Tdap/influenza vaccines.
B. Pneumococcal 13/Pneumococcal 23/Tdap vaccines.
C. Influenza vaccine.
D. No vaccines.
Guillain-Barré syndrome (GBS) is a rare, acute, immune-mediated polyneuropathy that has an incidence of about 2 cases per 100,000 people each year.1 Most cases of GBS follow an infectious event (usually an upper respiratory infection or gastrointestinal infection). In 1976, administration of the swine flu vaccine was associated with an up to eightfold increased risk of GBS.2,3 Many patients who have had GBS have been advised not to – or are fearful to – receive influenza vaccine or any vaccine.
Is there good evidence for patients with a history of GBS to avoid influenza vaccines or vaccinations in general?
The initial concern over the increased risk of GBS following the large-scale influenza vaccination in 1976 has not been realized with subsequent influenza vaccines. In a study by Baxter and colleagues, GBS cases from Kaiser Permanente Northern California from 1995 to 2006 were reviewed.4 They looked at whether patients had received influenza vaccine in the 6 weeks prior to GBS, compared with vaccination within the prior 9 months.
The odds ratio for influenza vaccination in the 6 weeks prior to GBS was 1.1 (95% confidence interval, 0.4-3.1). The odds ratio for receiving tetanus diphtheria vaccine in the 6 weeks prior to GBS was 1.4 (95% CI, 0.3-4.5); pneumococcal 23 vaccine, 0.7 (95% CI, 0.1-2.9); and all vaccines combined, 1.3 (95% CI, 0.8-2.3).
Shahed Iqbal, MBBS, et al. looked at the relationship between influenza illness, pneumonia, influenza vaccination, and GBS.5 They found that although influenza vaccine coverage increased from 20% to 36% over the study period, there was not an increase in GBS hospitalizations over the same period. There was a significant correlation between hospitalizations for pneumonia and influenza and GBS hospitalizations in the same month.
In a simulation study, Steven Hawken, PhD, and his colleagues concluded that under typical conditions (influenza incidence greater than 5% and vaccine effectiveness greater than 60%), influenza vaccination reduced GBS risk.6
There are fewer data on vaccination in patients who have previously had GBS, but there is enough evidence to help guide us.
Roger Baxter, MD, and colleagues, using the database in reference 4, looked at outcome of patients with GBS who received vaccinations subsequent to recovery from GBS.7 A total of 279 patient with previous GBS received a total of 989 vaccinations, including 405 trivalent influenza vaccinations. None of the patients with GBS who received vaccinations had a recurrence of GBS.
Krista Kuitwaard, MD, et al. reported identical findings in a survey of patients with a history of GBS or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).8 A total of 245 patients with GBS responded to the survey. A total of 106 GBS patients had received influenza vaccine following their GBS diagnosis (a total of 775 vaccinations in those patients). None of the patients with a history of GBS who received influenza vaccination had a recurrence of their GBS.
The current position of the GBS/CIDP Foundation on vaccination for patients with GBS is as follows: The GBS/CIDP Foundation recommends avoiding immunizations that a GBS patient had received within 6 weeks of developing their initial symptoms.9
I think the current evidence is enough to guide us in this issue. Vaccinations, including influenza vaccine, are likely safe for patients with a history of GBS. The recommendation of the GBS/CIDP foundation is reasonable – to avoid immunizations that appeared to have potentially triggered the initial GBS (ones that had been received within 6 weeks of onset of symptoms).
In the case presented above, I think that choice A – receiving all the recommended immunizations – would be appropriate.
References
1. Neuroepidemiology 2011; 36(2):123-33.
2. Am J Epidemiol. 1979 Aug;110(2):105-23.
3. Clin Infect Dis. 2014 Apr;58(8):1149-55.
4. Clin Infect Dis. 2013 Jul;57(2):197-204.
5. Vaccine. 2015 Apr 21;33(17):2045-9.
6. Emerg Infect Dis. 2015 Feb;21(2):224-31.
7. Clin Infect Dis. 2012 Mar;54(6):800-4.
8. J Peripher Nerv Syst. 2009 Dec;14(4):310-5.
9. GBS/CIDP Foundation International, Position on Flu Shots and Vaccinations.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman presents as a new patient for a clinic visit. She has a history of Guillain-Barré syndrome 10 years ago. The last immunization she received was a tetanus-diphtheria 12 years ago.
What do you recommend for her to receive over the next year?
A. Pneumococcal 13/Pneumococcal 23/Tdap/influenza vaccines.
B. Pneumococcal 13/Pneumococcal 23/Tdap vaccines.
C. Influenza vaccine.
D. No vaccines.
Guillain-Barré syndrome (GBS) is a rare, acute, immune-mediated polyneuropathy that has an incidence of about 2 cases per 100,000 people each year.1 Most cases of GBS follow an infectious event (usually an upper respiratory infection or gastrointestinal infection). In 1976, administration of the swine flu vaccine was associated with an up to eightfold increased risk of GBS.2,3 Many patients who have had GBS have been advised not to – or are fearful to – receive influenza vaccine or any vaccine.
Is there good evidence for patients with a history of GBS to avoid influenza vaccines or vaccinations in general?
The initial concern over the increased risk of GBS following the large-scale influenza vaccination in 1976 has not been realized with subsequent influenza vaccines. In a study by Baxter and colleagues, GBS cases from Kaiser Permanente Northern California from 1995 to 2006 were reviewed.4 They looked at whether patients had received influenza vaccine in the 6 weeks prior to GBS, compared with vaccination within the prior 9 months.
The odds ratio for influenza vaccination in the 6 weeks prior to GBS was 1.1 (95% confidence interval, 0.4-3.1). The odds ratio for receiving tetanus diphtheria vaccine in the 6 weeks prior to GBS was 1.4 (95% CI, 0.3-4.5); pneumococcal 23 vaccine, 0.7 (95% CI, 0.1-2.9); and all vaccines combined, 1.3 (95% CI, 0.8-2.3).
Shahed Iqbal, MBBS, et al. looked at the relationship between influenza illness, pneumonia, influenza vaccination, and GBS.5 They found that although influenza vaccine coverage increased from 20% to 36% over the study period, there was not an increase in GBS hospitalizations over the same period. There was a significant correlation between hospitalizations for pneumonia and influenza and GBS hospitalizations in the same month.
In a simulation study, Steven Hawken, PhD, and his colleagues concluded that under typical conditions (influenza incidence greater than 5% and vaccine effectiveness greater than 60%), influenza vaccination reduced GBS risk.6
There are fewer data on vaccination in patients who have previously had GBS, but there is enough evidence to help guide us.
Roger Baxter, MD, and colleagues, using the database in reference 4, looked at outcome of patients with GBS who received vaccinations subsequent to recovery from GBS.7 A total of 279 patient with previous GBS received a total of 989 vaccinations, including 405 trivalent influenza vaccinations. None of the patients with GBS who received vaccinations had a recurrence of GBS.
Krista Kuitwaard, MD, et al. reported identical findings in a survey of patients with a history of GBS or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).8 A total of 245 patients with GBS responded to the survey. A total of 106 GBS patients had received influenza vaccine following their GBS diagnosis (a total of 775 vaccinations in those patients). None of the patients with a history of GBS who received influenza vaccination had a recurrence of their GBS.
The current position of the GBS/CIDP Foundation on vaccination for patients with GBS is as follows: The GBS/CIDP Foundation recommends avoiding immunizations that a GBS patient had received within 6 weeks of developing their initial symptoms.9
I think the current evidence is enough to guide us in this issue. Vaccinations, including influenza vaccine, are likely safe for patients with a history of GBS. The recommendation of the GBS/CIDP foundation is reasonable – to avoid immunizations that appeared to have potentially triggered the initial GBS (ones that had been received within 6 weeks of onset of symptoms).
In the case presented above, I think that choice A – receiving all the recommended immunizations – would be appropriate.
References
1. Neuroepidemiology 2011; 36(2):123-33.
2. Am J Epidemiol. 1979 Aug;110(2):105-23.
3. Clin Infect Dis. 2014 Apr;58(8):1149-55.
4. Clin Infect Dis. 2013 Jul;57(2):197-204.
5. Vaccine. 2015 Apr 21;33(17):2045-9.
6. Emerg Infect Dis. 2015 Feb;21(2):224-31.
7. Clin Infect Dis. 2012 Mar;54(6):800-4.
8. J Peripher Nerv Syst. 2009 Dec;14(4):310-5.
9. GBS/CIDP Foundation International, Position on Flu Shots and Vaccinations.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
Ipsilateral arm BP measurements after breast cancer?
A 47-year-old woman with a history of right-sided breast cancer – status after lumpectomy, lymph node dissection, and radiation – comes in to clinic for evaluation. She asks the MA to take precautions on blood pressure measurement.
What precautions should be done?
A. Check BP in left arm only.
B. Do not inflate cuff greater than 180 mm in the right arm.
C. It’s okay to check BP in either arm.
About 10 years ago, a person asked me after a medical myth lecture I had given if I had any information on whether avoiding blood pressure readings in the ipsilateral arm in breast cancer patients was a myth. We both agreed that it sounded like a myth, and I promised to research it.
I found no studies at that time that refuted the advice that breast cancer patients were given to avoid blood pressure measurement, blood draws, and injections in the ipsilateral arm. I found no evidence at that time supporting this practice, just very authoritative statements in medical and nursing journals. Currently, the American Cancer society website recommends against blood pressure checks and blood draws from the ipsilateral arm in breast cancer patients.1
Are there more data now to weigh in on whether this is a myth or not?
The rationale behind this longstanding advice is that women who have had breast surgery, lymph node dissections, or radiation were at higher risk for lymphedema in the ipsilateral arm.
The advice to avoid blood draws and injections was to decrease the risk of infection and subsequent cellulitis that could lead to longstanding lymphedema. The avoidance of blood pressure measurements was, I suppose, to decrease venous pressure that could stimulate edema.
Sarah A. McLaughlin, MD, and her colleagues reported on the precautionary behaviors that patients with breast cancer observed in an attempt to avoid lymphedema.2 They looked at two groups: women who had undergone axillary lymph node biopsy and those who had undergone sentinel node biopsy.
More than 90% of the women who had undergone axillary node dissection avoided blood draws, intravenous lines, and blood pressure measurements on the involved side – with more than 70% in the sentinel node biopsy group avoiding blood pressure measurements on the involved side, and almost 90% avoiding intravenous lines.
In the Physical Activity and Lymphedema trial, Shayna L. Showalter, MD, and her colleagues looked at a number of potential risk factors for arm swelling in patients with a history of breast cancer.3 There was no increased risk of arm swelling in patients who had blood draws or blood pressure checks in the ipsilateral arm. There also was no association with burns, bug bites, hangnails, or cuts in the ipsilateral arm – all risks that would suggest an increased risk of infection in the arm.
Chantal Ferguson and her colleagues reported on a 10-year prospective study looking at lymphedema and risk factors for lymphedema in breast cancer patients.4 Bilateral arm volume measurements were made preoperatively and postoperatively, and at each visit, patients reported on whether they had blood pressure measurements, injections, or blood draws in the ipsilateral arm.
In more than 3,000 measurements, there was no evidence of volume change associated with blood pressure measurements, blood draws, or injections. Risk factors that did increase arm volume were body mass index greater than 25 kg/m2, axillary lymph node dissection, cellulitis, and regional lymph node irradiation.
There just isn’t evidence that these classic behaviors to protect the ipsilateral arm are warranted. Hopefully, patients will have less worry and less stress if they do not have to be so vigilant trying to “protect” their arm.
References
1. American Cancer Society: “Lymphedema: What Every Woman With Breast Cancer Should Know.” Accessed online at www.cancer.org.
2. J Am Coll Surg. 2013 Mar;216(3):380-9.
3. Ann Surg Oncol. 2013 Mar;20(3):842-9.
4. J Clin Oncol. 2016 Mar 1;34(7):691-8.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 47-year-old woman with a history of right-sided breast cancer – status after lumpectomy, lymph node dissection, and radiation – comes in to clinic for evaluation. She asks the MA to take precautions on blood pressure measurement.
What precautions should be done?
A. Check BP in left arm only.
B. Do not inflate cuff greater than 180 mm in the right arm.
C. It’s okay to check BP in either arm.
About 10 years ago, a person asked me after a medical myth lecture I had given if I had any information on whether avoiding blood pressure readings in the ipsilateral arm in breast cancer patients was a myth. We both agreed that it sounded like a myth, and I promised to research it.
I found no studies at that time that refuted the advice that breast cancer patients were given to avoid blood pressure measurement, blood draws, and injections in the ipsilateral arm. I found no evidence at that time supporting this practice, just very authoritative statements in medical and nursing journals. Currently, the American Cancer society website recommends against blood pressure checks and blood draws from the ipsilateral arm in breast cancer patients.1
Are there more data now to weigh in on whether this is a myth or not?
The rationale behind this longstanding advice is that women who have had breast surgery, lymph node dissections, or radiation were at higher risk for lymphedema in the ipsilateral arm.
The advice to avoid blood draws and injections was to decrease the risk of infection and subsequent cellulitis that could lead to longstanding lymphedema. The avoidance of blood pressure measurements was, I suppose, to decrease venous pressure that could stimulate edema.
Sarah A. McLaughlin, MD, and her colleagues reported on the precautionary behaviors that patients with breast cancer observed in an attempt to avoid lymphedema.2 They looked at two groups: women who had undergone axillary lymph node biopsy and those who had undergone sentinel node biopsy.
More than 90% of the women who had undergone axillary node dissection avoided blood draws, intravenous lines, and blood pressure measurements on the involved side – with more than 70% in the sentinel node biopsy group avoiding blood pressure measurements on the involved side, and almost 90% avoiding intravenous lines.
In the Physical Activity and Lymphedema trial, Shayna L. Showalter, MD, and her colleagues looked at a number of potential risk factors for arm swelling in patients with a history of breast cancer.3 There was no increased risk of arm swelling in patients who had blood draws or blood pressure checks in the ipsilateral arm. There also was no association with burns, bug bites, hangnails, or cuts in the ipsilateral arm – all risks that would suggest an increased risk of infection in the arm.
Chantal Ferguson and her colleagues reported on a 10-year prospective study looking at lymphedema and risk factors for lymphedema in breast cancer patients.4 Bilateral arm volume measurements were made preoperatively and postoperatively, and at each visit, patients reported on whether they had blood pressure measurements, injections, or blood draws in the ipsilateral arm.
In more than 3,000 measurements, there was no evidence of volume change associated with blood pressure measurements, blood draws, or injections. Risk factors that did increase arm volume were body mass index greater than 25 kg/m2, axillary lymph node dissection, cellulitis, and regional lymph node irradiation.
There just isn’t evidence that these classic behaviors to protect the ipsilateral arm are warranted. Hopefully, patients will have less worry and less stress if they do not have to be so vigilant trying to “protect” their arm.
References
1. American Cancer Society: “Lymphedema: What Every Woman With Breast Cancer Should Know.” Accessed online at www.cancer.org.
2. J Am Coll Surg. 2013 Mar;216(3):380-9.
3. Ann Surg Oncol. 2013 Mar;20(3):842-9.
4. J Clin Oncol. 2016 Mar 1;34(7):691-8.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 47-year-old woman with a history of right-sided breast cancer – status after lumpectomy, lymph node dissection, and radiation – comes in to clinic for evaluation. She asks the MA to take precautions on blood pressure measurement.
What precautions should be done?
A. Check BP in left arm only.
B. Do not inflate cuff greater than 180 mm in the right arm.
C. It’s okay to check BP in either arm.
About 10 years ago, a person asked me after a medical myth lecture I had given if I had any information on whether avoiding blood pressure readings in the ipsilateral arm in breast cancer patients was a myth. We both agreed that it sounded like a myth, and I promised to research it.
I found no studies at that time that refuted the advice that breast cancer patients were given to avoid blood pressure measurement, blood draws, and injections in the ipsilateral arm. I found no evidence at that time supporting this practice, just very authoritative statements in medical and nursing journals. Currently, the American Cancer society website recommends against blood pressure checks and blood draws from the ipsilateral arm in breast cancer patients.1
Are there more data now to weigh in on whether this is a myth or not?
The rationale behind this longstanding advice is that women who have had breast surgery, lymph node dissections, or radiation were at higher risk for lymphedema in the ipsilateral arm.
The advice to avoid blood draws and injections was to decrease the risk of infection and subsequent cellulitis that could lead to longstanding lymphedema. The avoidance of blood pressure measurements was, I suppose, to decrease venous pressure that could stimulate edema.
Sarah A. McLaughlin, MD, and her colleagues reported on the precautionary behaviors that patients with breast cancer observed in an attempt to avoid lymphedema.2 They looked at two groups: women who had undergone axillary lymph node biopsy and those who had undergone sentinel node biopsy.
More than 90% of the women who had undergone axillary node dissection avoided blood draws, intravenous lines, and blood pressure measurements on the involved side – with more than 70% in the sentinel node biopsy group avoiding blood pressure measurements on the involved side, and almost 90% avoiding intravenous lines.
In the Physical Activity and Lymphedema trial, Shayna L. Showalter, MD, and her colleagues looked at a number of potential risk factors for arm swelling in patients with a history of breast cancer.3 There was no increased risk of arm swelling in patients who had blood draws or blood pressure checks in the ipsilateral arm. There also was no association with burns, bug bites, hangnails, or cuts in the ipsilateral arm – all risks that would suggest an increased risk of infection in the arm.
Chantal Ferguson and her colleagues reported on a 10-year prospective study looking at lymphedema and risk factors for lymphedema in breast cancer patients.4 Bilateral arm volume measurements were made preoperatively and postoperatively, and at each visit, patients reported on whether they had blood pressure measurements, injections, or blood draws in the ipsilateral arm.
In more than 3,000 measurements, there was no evidence of volume change associated with blood pressure measurements, blood draws, or injections. Risk factors that did increase arm volume were body mass index greater than 25 kg/m2, axillary lymph node dissection, cellulitis, and regional lymph node irradiation.
There just isn’t evidence that these classic behaviors to protect the ipsilateral arm are warranted. Hopefully, patients will have less worry and less stress if they do not have to be so vigilant trying to “protect” their arm.
References
1. American Cancer Society: “Lymphedema: What Every Woman With Breast Cancer Should Know.” Accessed online at www.cancer.org.
2. J Am Coll Surg. 2013 Mar;216(3):380-9.
3. Ann Surg Oncol. 2013 Mar;20(3):842-9.
4. J Clin Oncol. 2016 Mar 1;34(7):691-8.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].