User login
Disaster medical response: Maximizing your effectiveness
In the aftermath of Hurricane Katrina, physicians and other health professionals volunteered for deployment to the affected area to provide medical services. The frustrating reality most of them encountered was the incapacity of those in charge to use the number of professional volunteers expressing interest.
Lesson: Build the infrastructure to support professional volunteerism
Untrained volunteers, though well intentioned, are often not that helpful. The immediate needs of a disaster area relate to public health and other safety issues. Until a proper infrastructure is re-established, general medical services cannot be provided. Physician services are most effectively provided in collaboration with, or as part of, an organized local response agency.
First things first
In addition to immediate loss of life and injuries caused by a disaster—natural or man-made (eg, war, terrorism)—mass disruption of the local infrastructure and relocation of a large segment of the population pose ongoing threats to health. The most crucial services to re-establish include adequate clean water, sanitation, food supplies, vector control (eg, insects and rodents), shelter, and immunizations. Also essential is establishing surveillance systems to rapidly assess needs and to detect disease trends.
Tasks that can wait
Contrary to what is commonly believed and stated in the press, rapid burial or cremation of cadavers is not an immediate need. Bodies almost never pose a serious public health threat. Moreover, rapid disposal of bodies can deprive families of knowing what happened to their relatives and cause psychological harm as well as legal and economic hardships.
Epidemics can occur but they usually result from respiratory or gastrointestinal pathogens caused by poor sanitation, inadequate water supplies, and overcrowding in inadequate shelters. Public health surveillance systems are important for detecting, tracking, and controlling such outbreaks.
Physicians as volunteers
Volunteer physicians are most effective following a disaster if they understand the importance of re-establishing the needed infrastructure, and if they arrive on scene as part of an organized response, having been trained in disaster medicine and public health. Disaster Medicine is becoming a recognized field of medicine with its own set of skills and an evolving literature base and training materials and courses.
After the immediate post-disaster period, it often takes a prolonged period of time to re-establish basic medical services. During this phase, volunteers continue to be needed but are harder to recruit. Mental health professionals are especially useful to assist with the posttraumatic stress and grief issues common after disasters.
If you would like to become part of organized disaster response team, you have several options.
If volunteering for deployment to other regions is not something you’re likely to do, but you live in an area vulnerable to, say, tornadoes or earthquakes, there is plenty you can do to prepare for disaster.
The AMA offers 3 courses in basic disaster response: Core Disaster Life Support (CDLS); Basic Disaster Life Support (BDLS); and Advanced Disaster Life Support (ADLS). Details are available at the AMA website: www.ama-assn.org/ama/pub/category/12606.html.
The CDC offers web-based training materials in the medical and public health response to an array of natural and man-made disasters (available on the Web at www.phppo.cdc.gov/phtn/default.asp).
You can also assist your local health department in planning for the most likely disasters in your area.
The Medical Reserve Corps
The Medical Reserve Corps (MRC) is a program started by the federal government after the terrorist attacks of September 11, 2001. It is part of the Citizen Corps, which is one component of the USA Freedom Corps (www.usafreedomcorps.gov). The purpose of the MRC is to organize local groups of medical and public health professionals to prepare for and respond to local and national emergency needs.
The Office of the Surgeon General coordinates the MRC program. This coordinating function includes recognizing and listing MRCs, offering technical assistance, serving as a clearinghouse of information for local MRCs, and offering training. Physicians interested in joining a local MRC can check on the MRC home page (www.medicalreservecorps.gov) to see if one has been organized their area. If no MRC exists in your area, you can help start one with the approval of the local Citizen Corps Council (www.citizencorps.gov/councils).
Since the MRC is a federal program—albeit relying on local organization and initiative—it is not clear how well local MRC units are fitting into the local, state, and national disaster relief infrastructure. Reportedly at least 20 MRC units assisted with relief efforts in Louisiana after Katrina. The MRC is intended to serve as a local resource and to augment the public health workforce should mass immunization or antibiotic distribution be needed.
Disaster Medical Assistance Teams
Disaster Medical Assistance Teams (DMATs) are part of the National Disaster Medical System, under the auspices of the Department of Homeland Security. The role of these teams is to provide medical care in a disaster area.
As stated in DMAT promotional material, “DMATs deploy to disaster sites with sufficient supplies and equipment to sustain themselves for a period of 72 hours while providing medical care at a fixed or temporary medical care site.” In incidents with large numbers of casualties, DMATs responsibilities include “triaging patients, providing high-quality medical care despite the adverse and austere environment often found at a disaster site, and preparing patients for evacuation.” DMATs may also provide primary medical care or may augment overloaded local health care staffs.
Under those unusual circumstances when victims of a disaster are evacuated to another location for their medical care, “DMATs may be activated to support patient reception and disposition of patients to hospitals. DMATs are designed to be a rapid-response element to supplement local medical care until other Federal or contract resources can be mobilized, or the situation is resolved.”
DMATs are organized by a local sponsor—a medical center, local public health agency, or a nonprofit organization. The responsibilities of the sponsor include recruiting DMAT team members, training, and organizing the dispatch of team members if called upon. Members of DMATs become temporary federal employees when deployed; this provides them liability protection through the Federal Tort Claims Act. In addition, professional licenses of federal employees are recognized by states, freeing DMAT team members from state licensing concerns.
To become a member of a DMAT, you must fill out a Federal Job Application form, be interviewed, and accepted as a team member. The NDMS has 10 regional offices (detailed at www.oep-ndms.dhhs.gov/region_1.html) where information can be found about existing DMAT teams and how to form a team. The DMAT home page is www.oep-ndms.dhhs.gov/dmat.html.
Search-and-rescue teams
Local fire departments and law enforcement departments frequently have search-and-rescue teams that can be called on to respond to disasters throughout the country. When these teams are deployed, they should take along medical personnel to attend to the needs of the responders. The medical professional should be prepared to screen responders and provide medical clearance before they deploy, provide urgent care medical services to responders, and ensure that measures are taken to prevent illness among team members.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
In the aftermath of Hurricane Katrina, physicians and other health professionals volunteered for deployment to the affected area to provide medical services. The frustrating reality most of them encountered was the incapacity of those in charge to use the number of professional volunteers expressing interest.
Lesson: Build the infrastructure to support professional volunteerism
Untrained volunteers, though well intentioned, are often not that helpful. The immediate needs of a disaster area relate to public health and other safety issues. Until a proper infrastructure is re-established, general medical services cannot be provided. Physician services are most effectively provided in collaboration with, or as part of, an organized local response agency.
First things first
In addition to immediate loss of life and injuries caused by a disaster—natural or man-made (eg, war, terrorism)—mass disruption of the local infrastructure and relocation of a large segment of the population pose ongoing threats to health. The most crucial services to re-establish include adequate clean water, sanitation, food supplies, vector control (eg, insects and rodents), shelter, and immunizations. Also essential is establishing surveillance systems to rapidly assess needs and to detect disease trends.
Tasks that can wait
Contrary to what is commonly believed and stated in the press, rapid burial or cremation of cadavers is not an immediate need. Bodies almost never pose a serious public health threat. Moreover, rapid disposal of bodies can deprive families of knowing what happened to their relatives and cause psychological harm as well as legal and economic hardships.
Epidemics can occur but they usually result from respiratory or gastrointestinal pathogens caused by poor sanitation, inadequate water supplies, and overcrowding in inadequate shelters. Public health surveillance systems are important for detecting, tracking, and controlling such outbreaks.
Physicians as volunteers
Volunteer physicians are most effective following a disaster if they understand the importance of re-establishing the needed infrastructure, and if they arrive on scene as part of an organized response, having been trained in disaster medicine and public health. Disaster Medicine is becoming a recognized field of medicine with its own set of skills and an evolving literature base and training materials and courses.
After the immediate post-disaster period, it often takes a prolonged period of time to re-establish basic medical services. During this phase, volunteers continue to be needed but are harder to recruit. Mental health professionals are especially useful to assist with the posttraumatic stress and grief issues common after disasters.
If you would like to become part of organized disaster response team, you have several options.
If volunteering for deployment to other regions is not something you’re likely to do, but you live in an area vulnerable to, say, tornadoes or earthquakes, there is plenty you can do to prepare for disaster.
The AMA offers 3 courses in basic disaster response: Core Disaster Life Support (CDLS); Basic Disaster Life Support (BDLS); and Advanced Disaster Life Support (ADLS). Details are available at the AMA website: www.ama-assn.org/ama/pub/category/12606.html.
The CDC offers web-based training materials in the medical and public health response to an array of natural and man-made disasters (available on the Web at www.phppo.cdc.gov/phtn/default.asp).
You can also assist your local health department in planning for the most likely disasters in your area.
The Medical Reserve Corps
The Medical Reserve Corps (MRC) is a program started by the federal government after the terrorist attacks of September 11, 2001. It is part of the Citizen Corps, which is one component of the USA Freedom Corps (www.usafreedomcorps.gov). The purpose of the MRC is to organize local groups of medical and public health professionals to prepare for and respond to local and national emergency needs.
The Office of the Surgeon General coordinates the MRC program. This coordinating function includes recognizing and listing MRCs, offering technical assistance, serving as a clearinghouse of information for local MRCs, and offering training. Physicians interested in joining a local MRC can check on the MRC home page (www.medicalreservecorps.gov) to see if one has been organized their area. If no MRC exists in your area, you can help start one with the approval of the local Citizen Corps Council (www.citizencorps.gov/councils).
Since the MRC is a federal program—albeit relying on local organization and initiative—it is not clear how well local MRC units are fitting into the local, state, and national disaster relief infrastructure. Reportedly at least 20 MRC units assisted with relief efforts in Louisiana after Katrina. The MRC is intended to serve as a local resource and to augment the public health workforce should mass immunization or antibiotic distribution be needed.
Disaster Medical Assistance Teams
Disaster Medical Assistance Teams (DMATs) are part of the National Disaster Medical System, under the auspices of the Department of Homeland Security. The role of these teams is to provide medical care in a disaster area.
As stated in DMAT promotional material, “DMATs deploy to disaster sites with sufficient supplies and equipment to sustain themselves for a period of 72 hours while providing medical care at a fixed or temporary medical care site.” In incidents with large numbers of casualties, DMATs responsibilities include “triaging patients, providing high-quality medical care despite the adverse and austere environment often found at a disaster site, and preparing patients for evacuation.” DMATs may also provide primary medical care or may augment overloaded local health care staffs.
Under those unusual circumstances when victims of a disaster are evacuated to another location for their medical care, “DMATs may be activated to support patient reception and disposition of patients to hospitals. DMATs are designed to be a rapid-response element to supplement local medical care until other Federal or contract resources can be mobilized, or the situation is resolved.”
DMATs are organized by a local sponsor—a medical center, local public health agency, or a nonprofit organization. The responsibilities of the sponsor include recruiting DMAT team members, training, and organizing the dispatch of team members if called upon. Members of DMATs become temporary federal employees when deployed; this provides them liability protection through the Federal Tort Claims Act. In addition, professional licenses of federal employees are recognized by states, freeing DMAT team members from state licensing concerns.
To become a member of a DMAT, you must fill out a Federal Job Application form, be interviewed, and accepted as a team member. The NDMS has 10 regional offices (detailed at www.oep-ndms.dhhs.gov/region_1.html) where information can be found about existing DMAT teams and how to form a team. The DMAT home page is www.oep-ndms.dhhs.gov/dmat.html.
Search-and-rescue teams
Local fire departments and law enforcement departments frequently have search-and-rescue teams that can be called on to respond to disasters throughout the country. When these teams are deployed, they should take along medical personnel to attend to the needs of the responders. The medical professional should be prepared to screen responders and provide medical clearance before they deploy, provide urgent care medical services to responders, and ensure that measures are taken to prevent illness among team members.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
In the aftermath of Hurricane Katrina, physicians and other health professionals volunteered for deployment to the affected area to provide medical services. The frustrating reality most of them encountered was the incapacity of those in charge to use the number of professional volunteers expressing interest.
Lesson: Build the infrastructure to support professional volunteerism
Untrained volunteers, though well intentioned, are often not that helpful. The immediate needs of a disaster area relate to public health and other safety issues. Until a proper infrastructure is re-established, general medical services cannot be provided. Physician services are most effectively provided in collaboration with, or as part of, an organized local response agency.
First things first
In addition to immediate loss of life and injuries caused by a disaster—natural or man-made (eg, war, terrorism)—mass disruption of the local infrastructure and relocation of a large segment of the population pose ongoing threats to health. The most crucial services to re-establish include adequate clean water, sanitation, food supplies, vector control (eg, insects and rodents), shelter, and immunizations. Also essential is establishing surveillance systems to rapidly assess needs and to detect disease trends.
Tasks that can wait
Contrary to what is commonly believed and stated in the press, rapid burial or cremation of cadavers is not an immediate need. Bodies almost never pose a serious public health threat. Moreover, rapid disposal of bodies can deprive families of knowing what happened to their relatives and cause psychological harm as well as legal and economic hardships.
Epidemics can occur but they usually result from respiratory or gastrointestinal pathogens caused by poor sanitation, inadequate water supplies, and overcrowding in inadequate shelters. Public health surveillance systems are important for detecting, tracking, and controlling such outbreaks.
Physicians as volunteers
Volunteer physicians are most effective following a disaster if they understand the importance of re-establishing the needed infrastructure, and if they arrive on scene as part of an organized response, having been trained in disaster medicine and public health. Disaster Medicine is becoming a recognized field of medicine with its own set of skills and an evolving literature base and training materials and courses.
After the immediate post-disaster period, it often takes a prolonged period of time to re-establish basic medical services. During this phase, volunteers continue to be needed but are harder to recruit. Mental health professionals are especially useful to assist with the posttraumatic stress and grief issues common after disasters.
If you would like to become part of organized disaster response team, you have several options.
If volunteering for deployment to other regions is not something you’re likely to do, but you live in an area vulnerable to, say, tornadoes or earthquakes, there is plenty you can do to prepare for disaster.
The AMA offers 3 courses in basic disaster response: Core Disaster Life Support (CDLS); Basic Disaster Life Support (BDLS); and Advanced Disaster Life Support (ADLS). Details are available at the AMA website: www.ama-assn.org/ama/pub/category/12606.html.
The CDC offers web-based training materials in the medical and public health response to an array of natural and man-made disasters (available on the Web at www.phppo.cdc.gov/phtn/default.asp).
You can also assist your local health department in planning for the most likely disasters in your area.
The Medical Reserve Corps
The Medical Reserve Corps (MRC) is a program started by the federal government after the terrorist attacks of September 11, 2001. It is part of the Citizen Corps, which is one component of the USA Freedom Corps (www.usafreedomcorps.gov). The purpose of the MRC is to organize local groups of medical and public health professionals to prepare for and respond to local and national emergency needs.
The Office of the Surgeon General coordinates the MRC program. This coordinating function includes recognizing and listing MRCs, offering technical assistance, serving as a clearinghouse of information for local MRCs, and offering training. Physicians interested in joining a local MRC can check on the MRC home page (www.medicalreservecorps.gov) to see if one has been organized their area. If no MRC exists in your area, you can help start one with the approval of the local Citizen Corps Council (www.citizencorps.gov/councils).
Since the MRC is a federal program—albeit relying on local organization and initiative—it is not clear how well local MRC units are fitting into the local, state, and national disaster relief infrastructure. Reportedly at least 20 MRC units assisted with relief efforts in Louisiana after Katrina. The MRC is intended to serve as a local resource and to augment the public health workforce should mass immunization or antibiotic distribution be needed.
Disaster Medical Assistance Teams
Disaster Medical Assistance Teams (DMATs) are part of the National Disaster Medical System, under the auspices of the Department of Homeland Security. The role of these teams is to provide medical care in a disaster area.
As stated in DMAT promotional material, “DMATs deploy to disaster sites with sufficient supplies and equipment to sustain themselves for a period of 72 hours while providing medical care at a fixed or temporary medical care site.” In incidents with large numbers of casualties, DMATs responsibilities include “triaging patients, providing high-quality medical care despite the adverse and austere environment often found at a disaster site, and preparing patients for evacuation.” DMATs may also provide primary medical care or may augment overloaded local health care staffs.
Under those unusual circumstances when victims of a disaster are evacuated to another location for their medical care, “DMATs may be activated to support patient reception and disposition of patients to hospitals. DMATs are designed to be a rapid-response element to supplement local medical care until other Federal or contract resources can be mobilized, or the situation is resolved.”
DMATs are organized by a local sponsor—a medical center, local public health agency, or a nonprofit organization. The responsibilities of the sponsor include recruiting DMAT team members, training, and organizing the dispatch of team members if called upon. Members of DMATs become temporary federal employees when deployed; this provides them liability protection through the Federal Tort Claims Act. In addition, professional licenses of federal employees are recognized by states, freeing DMAT team members from state licensing concerns.
To become a member of a DMAT, you must fill out a Federal Job Application form, be interviewed, and accepted as a team member. The NDMS has 10 regional offices (detailed at www.oep-ndms.dhhs.gov/region_1.html) where information can be found about existing DMAT teams and how to form a team. The DMAT home page is www.oep-ndms.dhhs.gov/dmat.html.
Search-and-rescue teams
Local fire departments and law enforcement departments frequently have search-and-rescue teams that can be called on to respond to disasters throughout the country. When these teams are deployed, they should take along medical personnel to attend to the needs of the responders. The medical professional should be prepared to screen responders and provide medical clearance before they deploy, provide urgent care medical services to responders, and ensure that measures are taken to prevent illness among team members.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
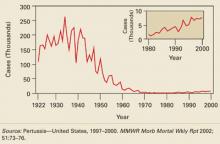
Pandemic influenza: How it would progress and what it would require of you
An influenza pandemic, or world-wide outbreak, advances through 3 periods—interpandemic, pandemic alert, and pandemic—and their respective phases defined by the World Health Organization (TABLE 1). Your responsibilities would be different in each of these periods (TABLE 2), requiring you to stay current on the progression of the disease and changing recommendations coming from the Centers for Disease Control and Prevention (CDC) and state and local public health departments.
A pandemic would be caused by the emergence of a new strain of influenza A. This strain could be the avian strain described in the May 2005 Practice Alert,1 “The growing threat of avian influenza,”or another novel strain.
This column describes the family physician’s role in a pandemic and includes advice on diagnosis, treatment, and prevention of disease transmission. It is based on recent recommendations from the CDC.2
Major differences between pandemic flu and a regular flu season
Vaccine shortage. Unless faster vaccine production methods are developed, there will probably be little to no vaccine initially, and once vaccine production commences the amount produced will not keep up with the need. This will necessitate prioritizing vaccine administration, forcing us to weigh societal infrastructure needs (fire-fighters, health care workers, police, etc) against those of individuals at high risk of complications.
In addition, 2 doses of vaccine 1 month apart will be needed for full protection. (Note: There is an approved provisional plan through the Advisory Committee on Immunization Practices [ACIP] and the National Vaccine Advisory Committee [NVAC] for vaccine prioritization.)
Antiviral shortage. There will also likely be a shortage of antiviral medication. Amantadine (Symmetrel) and rimantadine (Flumadine)—antivirals recommended for use against influenza A—have reduced efficacy against avian influenza, and the same may be true with any other novel strain.
Other antivirals if they are effective and available, will be used to treat acute infections and to prevent infection in those exposed and/or at high risk of complications and will be administered according to a prioritization schedule. Recommendations for prioritization of both vaccine and antivirals will come from ACIP/NVAC and the Secretary of the Department of Health and Human Services. The recommendations will be implemented by the CDC and state and local health departments, but may change as the pandemic evolves, depending on the number of people and age groups infected and the rates of morbidity and mortality.
Complicating factors. A common influenza strain could circulate at the same time as a pandemic strain, complicating the diagnostic and epidemiological picture. Office-based, rapid diagnostic tests cannot distinguish between influenza A strains. Finally, if pandemic flu exhibits the expected high rates of proliferation and mortality seen in past pandemics, our current hospital capacity will be strained and likely exceeded.
TABLE 1
WHO global pandemic phases
| INTERPANDEMIC PERIOD |
| Phase 1 No new influenza virus subtypes have been detected in humans. An influenza virus subtype that has caused human infection may exist in animals but the risk of human infection or disease is considered low. |
| Phase 2 No new influenza virus subtypes have been detected in humans. However, a circulating animal influenza virus subtype poses a substantial risk of human disease. |
| PANDEMIC ALERT PERIOD |
| Phase 3 Human infection with a new sub-type has occurred but no human-to-human spread has occurred, or at most there have been rare instances of spread to a close contact. |
| Phase 4 Small clusters with limited human-to-human transmission are detected, but spread is highly localized, suggesting that the virus is not well adapted to humans. |
| Phase 5 Larger clusters but human-to-human spread is still localized, suggesting the virus is becoming increasingly better adapted to humans but may not yet be fully transmissible. |
| PANDEMIC PERIOD |
| Phase 6 Transmission increases and is sustained in the general population. |
| POSTPANDEMIC PERIOD |
| Return to Phase 1 |
TABLE 2
Family physician responsibilities
| INTERPANDEMIC AND PANDEMIC ALERT PERIODS |
| Become familiar with case definitions |
| Know procedures for screening, infection control, and laboratory testing |
| Know antiviral regimens for Avian and other novel influenza viruses |
| Notify local public health authorities about suspected and confirmed novel influenza cases |
| Collect recommended specimens for diagnosis of novel influenza strains and have them forwarded to designated public health laboratories |
| PANDEMIC PERIOD |
| Regularly review updates on case definitions and recommendations for screening, laboratory testing and treatment |
| Report pandemic influenza cases as requested by the public health department |
| Collect specimens as requested by the public health department for ongoing surveillance and have them forwarded to designated public health laboratories |
| Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department |
Back to basics
Even with a limited supply of vaccine and antiviral medication, useful advice can still be given to individuals and the public to help them protect them and others from infection should a pandemic occur. People should be advised to:
- What hands frequently and thoroughly
- Avoid locations where infection is likely to occur
- Avoid close contact with those who have flu-like symptoms
- Cover coughs and sneezes with tissues, properly dispose of used tissue, and wash hands after handling waste
- Use infection control measures in the home if a household member is ill (TABLE 3)
- Possibly use masks. (No consensus exists on the use of masks by those infected or potentially exposed. Surgical masks may be useful for providers of patient care.)
Physicians can take measures to minimize the chance of spreading the virus in their clinics and to protect themselves and other staff (covered in a previous Practice Alert).3 Infection control guidelines can be implemented in hospitals and other health care facilities, as well as in schools and other high-risk settings.
TABLE 3
Infection control measures for patients cared for at home
| MANAGING THE PATIENT |
| Place the patient in a separate room or separate physically from other household members as much as possible |
| The patient should stay at home while most infectious (5 days after symptom onset) to avoid infecting others. If they have to leave the home they should strictly follow respiratory hygiene |
| Consider having the patient wear a surgical mask |
| ADVICE FOR OTHERS IN THE HOME |
| Non-household members should not enter the home |
| If non-household members need to enter the home they should avoid close contact with the patient |
| Limit the number of household members having contact with the patient Follow hand hygiene after contact with the patient or the patient environment and waste products. This includes hand washing with soap and water or use of an alcohol-based hand rub |
| Consider having direct caregivers wear a surgical mask |
| Wash dishes, utensils, and laundry in warm water and soap |
| Consider antiviral prophylaxis for household members, if it is available |
| Have household members seek care as soon as they develop symptoms of influenza |
Clinical guidelines: Pandemic alert
The recommended clinical approach to a patient suspected of having a novel flu strain will vary depending on the phase of the pandemic.
Through phase 5, in the pandemic alert period, acute febrile respiratory illness will be caused by a novel influenza virus only rarely. Suspect novel influenza only if the patient meets both clinical and epidemiologic criteria. The clinical criteria are fever plus 1 or more of the following: sore throat, cough, dyspnea.
Epidemiologic criteria include travel within the past 10 days to an area affected by highly pathogenic avian influenza out-breaks in poultry or where human cases of novel influenza have been confirmed; and either direct contact with poultry (touching birds or bird feces or surfaces contaminated by bird feces or eating uncooked poultry products) or close contact with a person with confirmed or suspected novel influenza. Occupational exposure through laboratory work with the novel influenza strain would also be considered an epidemiologic criterion, but this occurrence would be rare. Geographic areas affected by avian influenza can be found on the CDC web site (www.cdc.gov/flu/) and World Health Organization web site (www.who.int.en/).
6 Steps to proper management. Once a patient is suspected of having a novel influenza strain, take the following steps.
- Control spread of infection. Consider admitting the patient to a single-patient hospital room. If this is not possible, take precautions to control infection in the home (TABLE 3). Details of hospital infection control precautions can be found on the CDC influenza web site.
- Notify local or state public health departments. Report the suspicious case and ask for advice regarding collecting laboratory specimens and treatment options.
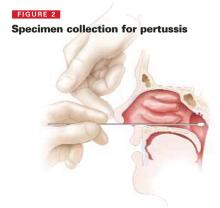
- Obtain clinical specimens requested by the public health department and arrange to have them transported to a designated public health laboratory. These will probably consist of a nasopharyngeal swab, nasal swab, throat swab, and an acute serum specimen (for comparison to a convalescent specimen 2 to 3 weeks later).
- Evaluate alternative diagnoses. Remember that a novel influenza infection can co-infect with a more common organism. Discontinue isolation and antiviral therapy prematurely only if an alternative diagnosis is confirmed with a high-predictive value test, the clinical course is explained by the alternative diagnosis, and the epidemiologic link to the novel influenza strain is not strong.
- Start antiviral treatment.
- Assist the public health department in locating potentially exposed contacts and providing antiviral prophylaxis if recommended.
Clinical guidelines: Pandemic period
During the pandemic period, managing suspected infection differs from the pandemic alert period in several respects.
- Suspected cases need only meet the clinical criteria: fever with sore throat, cough, or dyspnea. These criteria may be modified as the pandemic evolves.
- Hospitalize only those patients with severe complications who cannot be cared for at home.
- Submit clinical specimens to the designated lab only as requested by the public health department. Such monitoring will probably be needed only for a subset of patients to watch the epidemiology of the epidemic or to investigate unusual presentations or failures of preventive therapy.
- Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department.
Pre-pandemic planning
If and when another influenza pandemic will occur is difficult to predict. To be prepared, follow sound public health practices: adhere to office infection control practices, insure that patients and staff are current on all immunizations—influenza and pneumococcal vaccines can probably limit the complications from a novel influenza pandemic—maintain a line of communication with the local public health department, report communicable diseases and suspicious presentations to the public health department, and participate in local emergency planning.
Family physicians who serve in leadership positions in hospitals and other health care facilities can also promote planning for a possible pandemic at these facilities, including how to manage a surge of critically ill patients.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
An influenza pandemic, or world-wide outbreak, advances through 3 periods—interpandemic, pandemic alert, and pandemic—and their respective phases defined by the World Health Organization (TABLE 1). Your responsibilities would be different in each of these periods (TABLE 2), requiring you to stay current on the progression of the disease and changing recommendations coming from the Centers for Disease Control and Prevention (CDC) and state and local public health departments.
A pandemic would be caused by the emergence of a new strain of influenza A. This strain could be the avian strain described in the May 2005 Practice Alert,1 “The growing threat of avian influenza,”or another novel strain.
This column describes the family physician’s role in a pandemic and includes advice on diagnosis, treatment, and prevention of disease transmission. It is based on recent recommendations from the CDC.2
Major differences between pandemic flu and a regular flu season
Vaccine shortage. Unless faster vaccine production methods are developed, there will probably be little to no vaccine initially, and once vaccine production commences the amount produced will not keep up with the need. This will necessitate prioritizing vaccine administration, forcing us to weigh societal infrastructure needs (fire-fighters, health care workers, police, etc) against those of individuals at high risk of complications.
In addition, 2 doses of vaccine 1 month apart will be needed for full protection. (Note: There is an approved provisional plan through the Advisory Committee on Immunization Practices [ACIP] and the National Vaccine Advisory Committee [NVAC] for vaccine prioritization.)
Antiviral shortage. There will also likely be a shortage of antiviral medication. Amantadine (Symmetrel) and rimantadine (Flumadine)—antivirals recommended for use against influenza A—have reduced efficacy against avian influenza, and the same may be true with any other novel strain.
Other antivirals if they are effective and available, will be used to treat acute infections and to prevent infection in those exposed and/or at high risk of complications and will be administered according to a prioritization schedule. Recommendations for prioritization of both vaccine and antivirals will come from ACIP/NVAC and the Secretary of the Department of Health and Human Services. The recommendations will be implemented by the CDC and state and local health departments, but may change as the pandemic evolves, depending on the number of people and age groups infected and the rates of morbidity and mortality.
Complicating factors. A common influenza strain could circulate at the same time as a pandemic strain, complicating the diagnostic and epidemiological picture. Office-based, rapid diagnostic tests cannot distinguish between influenza A strains. Finally, if pandemic flu exhibits the expected high rates of proliferation and mortality seen in past pandemics, our current hospital capacity will be strained and likely exceeded.
TABLE 1
WHO global pandemic phases
| INTERPANDEMIC PERIOD |
| Phase 1 No new influenza virus subtypes have been detected in humans. An influenza virus subtype that has caused human infection may exist in animals but the risk of human infection or disease is considered low. |
| Phase 2 No new influenza virus subtypes have been detected in humans. However, a circulating animal influenza virus subtype poses a substantial risk of human disease. |
| PANDEMIC ALERT PERIOD |
| Phase 3 Human infection with a new sub-type has occurred but no human-to-human spread has occurred, or at most there have been rare instances of spread to a close contact. |
| Phase 4 Small clusters with limited human-to-human transmission are detected, but spread is highly localized, suggesting that the virus is not well adapted to humans. |
| Phase 5 Larger clusters but human-to-human spread is still localized, suggesting the virus is becoming increasingly better adapted to humans but may not yet be fully transmissible. |
| PANDEMIC PERIOD |
| Phase 6 Transmission increases and is sustained in the general population. |
| POSTPANDEMIC PERIOD |
| Return to Phase 1 |
TABLE 2
Family physician responsibilities
| INTERPANDEMIC AND PANDEMIC ALERT PERIODS |
| Become familiar with case definitions |
| Know procedures for screening, infection control, and laboratory testing |
| Know antiviral regimens for Avian and other novel influenza viruses |
| Notify local public health authorities about suspected and confirmed novel influenza cases |
| Collect recommended specimens for diagnosis of novel influenza strains and have them forwarded to designated public health laboratories |
| PANDEMIC PERIOD |
| Regularly review updates on case definitions and recommendations for screening, laboratory testing and treatment |
| Report pandemic influenza cases as requested by the public health department |
| Collect specimens as requested by the public health department for ongoing surveillance and have them forwarded to designated public health laboratories |
| Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department |
Back to basics
Even with a limited supply of vaccine and antiviral medication, useful advice can still be given to individuals and the public to help them protect them and others from infection should a pandemic occur. People should be advised to:
- What hands frequently and thoroughly
- Avoid locations where infection is likely to occur
- Avoid close contact with those who have flu-like symptoms
- Cover coughs and sneezes with tissues, properly dispose of used tissue, and wash hands after handling waste
- Use infection control measures in the home if a household member is ill (TABLE 3)
- Possibly use masks. (No consensus exists on the use of masks by those infected or potentially exposed. Surgical masks may be useful for providers of patient care.)
Physicians can take measures to minimize the chance of spreading the virus in their clinics and to protect themselves and other staff (covered in a previous Practice Alert).3 Infection control guidelines can be implemented in hospitals and other health care facilities, as well as in schools and other high-risk settings.
TABLE 3
Infection control measures for patients cared for at home
| MANAGING THE PATIENT |
| Place the patient in a separate room or separate physically from other household members as much as possible |
| The patient should stay at home while most infectious (5 days after symptom onset) to avoid infecting others. If they have to leave the home they should strictly follow respiratory hygiene |
| Consider having the patient wear a surgical mask |
| ADVICE FOR OTHERS IN THE HOME |
| Non-household members should not enter the home |
| If non-household members need to enter the home they should avoid close contact with the patient |
| Limit the number of household members having contact with the patient Follow hand hygiene after contact with the patient or the patient environment and waste products. This includes hand washing with soap and water or use of an alcohol-based hand rub |
| Consider having direct caregivers wear a surgical mask |
| Wash dishes, utensils, and laundry in warm water and soap |
| Consider antiviral prophylaxis for household members, if it is available |
| Have household members seek care as soon as they develop symptoms of influenza |
Clinical guidelines: Pandemic alert
The recommended clinical approach to a patient suspected of having a novel flu strain will vary depending on the phase of the pandemic.
Through phase 5, in the pandemic alert period, acute febrile respiratory illness will be caused by a novel influenza virus only rarely. Suspect novel influenza only if the patient meets both clinical and epidemiologic criteria. The clinical criteria are fever plus 1 or more of the following: sore throat, cough, dyspnea.
Epidemiologic criteria include travel within the past 10 days to an area affected by highly pathogenic avian influenza out-breaks in poultry or where human cases of novel influenza have been confirmed; and either direct contact with poultry (touching birds or bird feces or surfaces contaminated by bird feces or eating uncooked poultry products) or close contact with a person with confirmed or suspected novel influenza. Occupational exposure through laboratory work with the novel influenza strain would also be considered an epidemiologic criterion, but this occurrence would be rare. Geographic areas affected by avian influenza can be found on the CDC web site (www.cdc.gov/flu/) and World Health Organization web site (www.who.int.en/).
6 Steps to proper management. Once a patient is suspected of having a novel influenza strain, take the following steps.
- Control spread of infection. Consider admitting the patient to a single-patient hospital room. If this is not possible, take precautions to control infection in the home (TABLE 3). Details of hospital infection control precautions can be found on the CDC influenza web site.
- Notify local or state public health departments. Report the suspicious case and ask for advice regarding collecting laboratory specimens and treatment options.
- Obtain clinical specimens requested by the public health department and arrange to have them transported to a designated public health laboratory. These will probably consist of a nasopharyngeal swab, nasal swab, throat swab, and an acute serum specimen (for comparison to a convalescent specimen 2 to 3 weeks later).
- Evaluate alternative diagnoses. Remember that a novel influenza infection can co-infect with a more common organism. Discontinue isolation and antiviral therapy prematurely only if an alternative diagnosis is confirmed with a high-predictive value test, the clinical course is explained by the alternative diagnosis, and the epidemiologic link to the novel influenza strain is not strong.
- Start antiviral treatment.
- Assist the public health department in locating potentially exposed contacts and providing antiviral prophylaxis if recommended.
Clinical guidelines: Pandemic period
During the pandemic period, managing suspected infection differs from the pandemic alert period in several respects.
- Suspected cases need only meet the clinical criteria: fever with sore throat, cough, or dyspnea. These criteria may be modified as the pandemic evolves.
- Hospitalize only those patients with severe complications who cannot be cared for at home.
- Submit clinical specimens to the designated lab only as requested by the public health department. Such monitoring will probably be needed only for a subset of patients to watch the epidemiology of the epidemic or to investigate unusual presentations or failures of preventive therapy.
- Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department.
Pre-pandemic planning
If and when another influenza pandemic will occur is difficult to predict. To be prepared, follow sound public health practices: adhere to office infection control practices, insure that patients and staff are current on all immunizations—influenza and pneumococcal vaccines can probably limit the complications from a novel influenza pandemic—maintain a line of communication with the local public health department, report communicable diseases and suspicious presentations to the public health department, and participate in local emergency planning.
Family physicians who serve in leadership positions in hospitals and other health care facilities can also promote planning for a possible pandemic at these facilities, including how to manage a surge of critically ill patients.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
An influenza pandemic, or world-wide outbreak, advances through 3 periods—interpandemic, pandemic alert, and pandemic—and their respective phases defined by the World Health Organization (TABLE 1). Your responsibilities would be different in each of these periods (TABLE 2), requiring you to stay current on the progression of the disease and changing recommendations coming from the Centers for Disease Control and Prevention (CDC) and state and local public health departments.
A pandemic would be caused by the emergence of a new strain of influenza A. This strain could be the avian strain described in the May 2005 Practice Alert,1 “The growing threat of avian influenza,”or another novel strain.
This column describes the family physician’s role in a pandemic and includes advice on diagnosis, treatment, and prevention of disease transmission. It is based on recent recommendations from the CDC.2
Major differences between pandemic flu and a regular flu season
Vaccine shortage. Unless faster vaccine production methods are developed, there will probably be little to no vaccine initially, and once vaccine production commences the amount produced will not keep up with the need. This will necessitate prioritizing vaccine administration, forcing us to weigh societal infrastructure needs (fire-fighters, health care workers, police, etc) against those of individuals at high risk of complications.
In addition, 2 doses of vaccine 1 month apart will be needed for full protection. (Note: There is an approved provisional plan through the Advisory Committee on Immunization Practices [ACIP] and the National Vaccine Advisory Committee [NVAC] for vaccine prioritization.)
Antiviral shortage. There will also likely be a shortage of antiviral medication. Amantadine (Symmetrel) and rimantadine (Flumadine)—antivirals recommended for use against influenza A—have reduced efficacy against avian influenza, and the same may be true with any other novel strain.
Other antivirals if they are effective and available, will be used to treat acute infections and to prevent infection in those exposed and/or at high risk of complications and will be administered according to a prioritization schedule. Recommendations for prioritization of both vaccine and antivirals will come from ACIP/NVAC and the Secretary of the Department of Health and Human Services. The recommendations will be implemented by the CDC and state and local health departments, but may change as the pandemic evolves, depending on the number of people and age groups infected and the rates of morbidity and mortality.
Complicating factors. A common influenza strain could circulate at the same time as a pandemic strain, complicating the diagnostic and epidemiological picture. Office-based, rapid diagnostic tests cannot distinguish between influenza A strains. Finally, if pandemic flu exhibits the expected high rates of proliferation and mortality seen in past pandemics, our current hospital capacity will be strained and likely exceeded.
TABLE 1
WHO global pandemic phases
| INTERPANDEMIC PERIOD |
| Phase 1 No new influenza virus subtypes have been detected in humans. An influenza virus subtype that has caused human infection may exist in animals but the risk of human infection or disease is considered low. |
| Phase 2 No new influenza virus subtypes have been detected in humans. However, a circulating animal influenza virus subtype poses a substantial risk of human disease. |
| PANDEMIC ALERT PERIOD |
| Phase 3 Human infection with a new sub-type has occurred but no human-to-human spread has occurred, or at most there have been rare instances of spread to a close contact. |
| Phase 4 Small clusters with limited human-to-human transmission are detected, but spread is highly localized, suggesting that the virus is not well adapted to humans. |
| Phase 5 Larger clusters but human-to-human spread is still localized, suggesting the virus is becoming increasingly better adapted to humans but may not yet be fully transmissible. |
| PANDEMIC PERIOD |
| Phase 6 Transmission increases and is sustained in the general population. |
| POSTPANDEMIC PERIOD |
| Return to Phase 1 |
TABLE 2
Family physician responsibilities
| INTERPANDEMIC AND PANDEMIC ALERT PERIODS |
| Become familiar with case definitions |
| Know procedures for screening, infection control, and laboratory testing |
| Know antiviral regimens for Avian and other novel influenza viruses |
| Notify local public health authorities about suspected and confirmed novel influenza cases |
| Collect recommended specimens for diagnosis of novel influenza strains and have them forwarded to designated public health laboratories |
| PANDEMIC PERIOD |
| Regularly review updates on case definitions and recommendations for screening, laboratory testing and treatment |
| Report pandemic influenza cases as requested by the public health department |
| Collect specimens as requested by the public health department for ongoing surveillance and have them forwarded to designated public health laboratories |
| Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department |
Back to basics
Even with a limited supply of vaccine and antiviral medication, useful advice can still be given to individuals and the public to help them protect them and others from infection should a pandemic occur. People should be advised to:
- What hands frequently and thoroughly
- Avoid locations where infection is likely to occur
- Avoid close contact with those who have flu-like symptoms
- Cover coughs and sneezes with tissues, properly dispose of used tissue, and wash hands after handling waste
- Use infection control measures in the home if a household member is ill (TABLE 3)
- Possibly use masks. (No consensus exists on the use of masks by those infected or potentially exposed. Surgical masks may be useful for providers of patient care.)
Physicians can take measures to minimize the chance of spreading the virus in their clinics and to protect themselves and other staff (covered in a previous Practice Alert).3 Infection control guidelines can be implemented in hospitals and other health care facilities, as well as in schools and other high-risk settings.
TABLE 3
Infection control measures for patients cared for at home
| MANAGING THE PATIENT |
| Place the patient in a separate room or separate physically from other household members as much as possible |
| The patient should stay at home while most infectious (5 days after symptom onset) to avoid infecting others. If they have to leave the home they should strictly follow respiratory hygiene |
| Consider having the patient wear a surgical mask |
| ADVICE FOR OTHERS IN THE HOME |
| Non-household members should not enter the home |
| If non-household members need to enter the home they should avoid close contact with the patient |
| Limit the number of household members having contact with the patient Follow hand hygiene after contact with the patient or the patient environment and waste products. This includes hand washing with soap and water or use of an alcohol-based hand rub |
| Consider having direct caregivers wear a surgical mask |
| Wash dishes, utensils, and laundry in warm water and soap |
| Consider antiviral prophylaxis for household members, if it is available |
| Have household members seek care as soon as they develop symptoms of influenza |
Clinical guidelines: Pandemic alert
The recommended clinical approach to a patient suspected of having a novel flu strain will vary depending on the phase of the pandemic.
Through phase 5, in the pandemic alert period, acute febrile respiratory illness will be caused by a novel influenza virus only rarely. Suspect novel influenza only if the patient meets both clinical and epidemiologic criteria. The clinical criteria are fever plus 1 or more of the following: sore throat, cough, dyspnea.
Epidemiologic criteria include travel within the past 10 days to an area affected by highly pathogenic avian influenza out-breaks in poultry or where human cases of novel influenza have been confirmed; and either direct contact with poultry (touching birds or bird feces or surfaces contaminated by bird feces or eating uncooked poultry products) or close contact with a person with confirmed or suspected novel influenza. Occupational exposure through laboratory work with the novel influenza strain would also be considered an epidemiologic criterion, but this occurrence would be rare. Geographic areas affected by avian influenza can be found on the CDC web site (www.cdc.gov/flu/) and World Health Organization web site (www.who.int.en/).
6 Steps to proper management. Once a patient is suspected of having a novel influenza strain, take the following steps.
- Control spread of infection. Consider admitting the patient to a single-patient hospital room. If this is not possible, take precautions to control infection in the home (TABLE 3). Details of hospital infection control precautions can be found on the CDC influenza web site.
- Notify local or state public health departments. Report the suspicious case and ask for advice regarding collecting laboratory specimens and treatment options.
- Obtain clinical specimens requested by the public health department and arrange to have them transported to a designated public health laboratory. These will probably consist of a nasopharyngeal swab, nasal swab, throat swab, and an acute serum specimen (for comparison to a convalescent specimen 2 to 3 weeks later).
- Evaluate alternative diagnoses. Remember that a novel influenza infection can co-infect with a more common organism. Discontinue isolation and antiviral therapy prematurely only if an alternative diagnosis is confirmed with a high-predictive value test, the clinical course is explained by the alternative diagnosis, and the epidemiologic link to the novel influenza strain is not strong.
- Start antiviral treatment.
- Assist the public health department in locating potentially exposed contacts and providing antiviral prophylaxis if recommended.
Clinical guidelines: Pandemic period
During the pandemic period, managing suspected infection differs from the pandemic alert period in several respects.
- Suspected cases need only meet the clinical criteria: fever with sore throat, cough, or dyspnea. These criteria may be modified as the pandemic evolves.
- Hospitalize only those patients with severe complications who cannot be cared for at home.
- Submit clinical specimens to the designated lab only as requested by the public health department. Such monitoring will probably be needed only for a subset of patients to watch the epidemiology of the epidemic or to investigate unusual presentations or failures of preventive therapy.
- Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department.
Pre-pandemic planning
If and when another influenza pandemic will occur is difficult to predict. To be prepared, follow sound public health practices: adhere to office infection control practices, insure that patients and staff are current on all immunizations—influenza and pneumococcal vaccines can probably limit the complications from a novel influenza pandemic—maintain a line of communication with the local public health department, report communicable diseases and suspicious presentations to the public health department, and participate in local emergency planning.
Family physicians who serve in leadership positions in hospitals and other health care facilities can also promote planning for a possible pandemic at these facilities, including how to manage a surge of critically ill patients.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
When, and when not, to use the interferon-gamma TB blood test
Consider the following cases of patients needing testing for tuberculosis:
- A 55-year-old nurse returns to work after 10 years. Her pre-employment evaluation requires a test for tuberculosis.
- A 35-year-old woman has lupus. Her physician is considering placing her on prednisone and wants to know if she has latent TB infection.
- A homeless man presents to the clinic stating he has lost his TB medications that he has taken or the past 2 months. His diagnosis of TB was made in another city; he cannot remember its name. The physician wants to confirm the diagnosis of TB.
- A 5-year-old from Mexico with a history of BCG vaccination presents for a preschool health evaluation.
- A 35-year-old immigrant from Africa is pregnant and presents for prenatal care.
Patients 1 and 2 above are logical candidates for the newer interferon-gamma blood test to detect tuberculosis (TB). Patients 3, 4, and 5 are not; They should be evaluated with the conventional TB skin test.
The advantages and disadvantages of both kinds of testing are described in this review.
Is a better test at hand?
The TB skin test, using an intradermal injection of purified protein derivative, has been used to assist in the detection of active and latent TB for more than a century. However, this test has its problems: difficulty in accurately measuring and interpreting the reaction; low sensitivity in those with depressed cell-mediated immunity and those with early infections; lower specificity in those with a history of bacille Calmette-Guérin (BCG) vaccination and infection with other Mycobacteria; the need for 2 visits with-in 48 to 72 hours for test interpretation; and boosting of immune response caused by the TB skin test itself (see Boosting phenomena).
Those who work in TB control programs have sought better diagnostic tools. Two that are available today are the interferon-gamma blood tests QuantiFERON-TB (QFT) and QuantiFERON-TB GOLD (QFTG). The QFT and QFTG measure the release of interferon-gamma by sensitized lymphocytes when exposed to antigens of Mycobacterium tuberculosis.
The QFTG, licensed in 2004, is an improvement over the earlier QFT, licensed in 2001, which included antigens that M tuberculosis shares with other commonly encountered Mycobacteria. The QFTG is more specific to M tuberculosis, although it does cross-react with several relatively uncommon nontuberculous Mycobacteria. These tests have several advantages over the older skin test (TABLE 1).
In those who have had prior Mycobacteria infections or BCG vaccine, cell-mediated immunity can wane and a TB skin test can therefore be negative. However, the skin test can boost the person’s immunity, and a second skin test can then be positive as a result of the immune boost. This can cause someone who is actually a reactor (someone who reacts positively to a skin test because of prior Mycobacteria infection) to look like a converter (someone who has a negative skin test at a recent point in time followed by a positive skin test, indicated recent infection with Mycobacteria). It is recommended that adults who have not had a skin test within 12 months and who will need repeated skin tests should receive a 2-step skin test on initial evaluation. A 2-step skin test involves an intitial test followed by a second one 1–2 weeks later.
TABLE 1
Advantages/disadvantages of the QFTG
| Advantages of QFTG compared with the TB skin test |
|
| Disadvantages of QFTG |
|
Factors to keep in mind when considering the QFT
In spite of the theoretical advantages of the QFT, research on its use is at an early stage. It can be considered a testing option for persons identified in TABLE 2. It may ultimately prove to be the test of choice for patients who have previously received a BCG vaccine, and in other instances where specificity is the predominant consideration. However, the Centers for Disease Control and Prevention (CDC) does not recommend it over a TB skin test in any situation.
Whether a skin test or QFTG is used, testing is recommended only for those at high risk of having latent TB infection and for those at high risk of developing active TB disease, if infected. Targeted testing along with treatment of active and latent TB remains the basis of TB control activities in the US.
When QFTG is unwarranted. The QFTG appears to be less sensitive than a TB skin test in those with symptoms of active TB, with the exception of those who are HIV-positive.1 In addition, the QFTG is not recommended for use with patients who are being treated for active TB. Current information is inadequate to recommend any QFTG use in children and pregnant women.
Gray areas. While there is some indication that QFTG is more sensitive for detecting TB infection in those exposed to an infectious patient, it is unknown whether it will predict as well as a skin test which patients are at risk of developing active disease.2 Therefore, it is not clear at this time if all those who have a positive QFTG should be considered candidates for treatment of latent TB infection, or if this should be offered only to those who have both a positive QFTG and TB skin test.
Level of risk influences interpretation. The current CDC recommendations, which have not been updated since QFTG was licensed, state that low-risk patients with a positive QFT and negative TB skin test should not receive treatment for latent infection.3 However, clinical judgment and perceived risk should be the basis for deciding on treatment in those at increased risk who have a positive QFT and negative TB skin test.
It is also not clear what effect a recent TB skin test has on QFTG results, and performing a QFTG soon after a TB skin test is not recommended by the US Food and Drug Administration or the manufacturer.
How to interpret QFTG results. If the QFTG result is positive, the patient needs clinical evaluation and a chest x-ray to rule out active disease. The diagnosis and treatment of active and latent TB has been covered in a previous Practice Alert.4 If the QFTG result is negative, no further evaluation is indicated unless symptoms of TB exist. The QFTG can have an indeterminate result, in which case a skin test can be useful.
Weighing the cost. The cost of a QFTG (about $80–$100 per test) needs to be compared with cost of staff time to read and interpret a skin test and to follow up with patients who fail to return for a skin test measurement.
TABLE 2
Recommendations for use of the QFT
| SITUATIONS WHERE QFT IS RECOMMENDED AS A POSSIBLE DIAGNOSTIC TOOL |
| Persons at increased risk for latent TB infection |
|
| Persons at increased risk of TB infection if exposed |
|
| Persons with conditions that cause increased risk of TB disease if infected, including those with: |
|
| Persons at low risk of TB infection who require initial or periodic testing |
|
| SITUATIONS WHERE QFT IS NOT CURRENTLY RECOMMENDED |
|
| SITUATIONS WHERE QFT IS PROMISING BUT FUTURE VALUE IS UNCERTAIN |
|
Conclusion
The QFTG test is relatively new; as more evidence becomes available, its place among the tools available for the diagnosis of latent and active TB will clarify. Check with your state and local public health departments to find out the situations for which they are recommending this new diagnostic tool, as practice varies across the country.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
1. Pai M, Riley LW, Colford JM. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004;4:761-776.
2. Richeldi L, Ewer K, Losi M, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med 2004;170:288-295.
3. Centers for Disease Control and Prevention. Guidelines for using QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Recomm Rep 2003;52(RR-2):15-18.
4. Campos-Outcalt D. Tuberculosis: old problem, new concerns. J Fam Pract 2003;52:792-798.
Consider the following cases of patients needing testing for tuberculosis:
- A 55-year-old nurse returns to work after 10 years. Her pre-employment evaluation requires a test for tuberculosis.
- A 35-year-old woman has lupus. Her physician is considering placing her on prednisone and wants to know if she has latent TB infection.
- A homeless man presents to the clinic stating he has lost his TB medications that he has taken or the past 2 months. His diagnosis of TB was made in another city; he cannot remember its name. The physician wants to confirm the diagnosis of TB.
- A 5-year-old from Mexico with a history of BCG vaccination presents for a preschool health evaluation.
- A 35-year-old immigrant from Africa is pregnant and presents for prenatal care.
Patients 1 and 2 above are logical candidates for the newer interferon-gamma blood test to detect tuberculosis (TB). Patients 3, 4, and 5 are not; They should be evaluated with the conventional TB skin test.
The advantages and disadvantages of both kinds of testing are described in this review.
Is a better test at hand?
The TB skin test, using an intradermal injection of purified protein derivative, has been used to assist in the detection of active and latent TB for more than a century. However, this test has its problems: difficulty in accurately measuring and interpreting the reaction; low sensitivity in those with depressed cell-mediated immunity and those with early infections; lower specificity in those with a history of bacille Calmette-Guérin (BCG) vaccination and infection with other Mycobacteria; the need for 2 visits with-in 48 to 72 hours for test interpretation; and boosting of immune response caused by the TB skin test itself (see Boosting phenomena).
Those who work in TB control programs have sought better diagnostic tools. Two that are available today are the interferon-gamma blood tests QuantiFERON-TB (QFT) and QuantiFERON-TB GOLD (QFTG). The QFT and QFTG measure the release of interferon-gamma by sensitized lymphocytes when exposed to antigens of Mycobacterium tuberculosis.
The QFTG, licensed in 2004, is an improvement over the earlier QFT, licensed in 2001, which included antigens that M tuberculosis shares with other commonly encountered Mycobacteria. The QFTG is more specific to M tuberculosis, although it does cross-react with several relatively uncommon nontuberculous Mycobacteria. These tests have several advantages over the older skin test (TABLE 1).
In those who have had prior Mycobacteria infections or BCG vaccine, cell-mediated immunity can wane and a TB skin test can therefore be negative. However, the skin test can boost the person’s immunity, and a second skin test can then be positive as a result of the immune boost. This can cause someone who is actually a reactor (someone who reacts positively to a skin test because of prior Mycobacteria infection) to look like a converter (someone who has a negative skin test at a recent point in time followed by a positive skin test, indicated recent infection with Mycobacteria). It is recommended that adults who have not had a skin test within 12 months and who will need repeated skin tests should receive a 2-step skin test on initial evaluation. A 2-step skin test involves an intitial test followed by a second one 1–2 weeks later.
TABLE 1
Advantages/disadvantages of the QFTG
| Advantages of QFTG compared with the TB skin test |
|
| Disadvantages of QFTG |
|
Factors to keep in mind when considering the QFT
In spite of the theoretical advantages of the QFT, research on its use is at an early stage. It can be considered a testing option for persons identified in TABLE 2. It may ultimately prove to be the test of choice for patients who have previously received a BCG vaccine, and in other instances where specificity is the predominant consideration. However, the Centers for Disease Control and Prevention (CDC) does not recommend it over a TB skin test in any situation.
Whether a skin test or QFTG is used, testing is recommended only for those at high risk of having latent TB infection and for those at high risk of developing active TB disease, if infected. Targeted testing along with treatment of active and latent TB remains the basis of TB control activities in the US.
When QFTG is unwarranted. The QFTG appears to be less sensitive than a TB skin test in those with symptoms of active TB, with the exception of those who are HIV-positive.1 In addition, the QFTG is not recommended for use with patients who are being treated for active TB. Current information is inadequate to recommend any QFTG use in children and pregnant women.
Gray areas. While there is some indication that QFTG is more sensitive for detecting TB infection in those exposed to an infectious patient, it is unknown whether it will predict as well as a skin test which patients are at risk of developing active disease.2 Therefore, it is not clear at this time if all those who have a positive QFTG should be considered candidates for treatment of latent TB infection, or if this should be offered only to those who have both a positive QFTG and TB skin test.
Level of risk influences interpretation. The current CDC recommendations, which have not been updated since QFTG was licensed, state that low-risk patients with a positive QFT and negative TB skin test should not receive treatment for latent infection.3 However, clinical judgment and perceived risk should be the basis for deciding on treatment in those at increased risk who have a positive QFT and negative TB skin test.
It is also not clear what effect a recent TB skin test has on QFTG results, and performing a QFTG soon after a TB skin test is not recommended by the US Food and Drug Administration or the manufacturer.
How to interpret QFTG results. If the QFTG result is positive, the patient needs clinical evaluation and a chest x-ray to rule out active disease. The diagnosis and treatment of active and latent TB has been covered in a previous Practice Alert.4 If the QFTG result is negative, no further evaluation is indicated unless symptoms of TB exist. The QFTG can have an indeterminate result, in which case a skin test can be useful.
Weighing the cost. The cost of a QFTG (about $80–$100 per test) needs to be compared with cost of staff time to read and interpret a skin test and to follow up with patients who fail to return for a skin test measurement.
TABLE 2
Recommendations for use of the QFT
| SITUATIONS WHERE QFT IS RECOMMENDED AS A POSSIBLE DIAGNOSTIC TOOL |
| Persons at increased risk for latent TB infection |
|
| Persons at increased risk of TB infection if exposed |
|
| Persons with conditions that cause increased risk of TB disease if infected, including those with: |
|
| Persons at low risk of TB infection who require initial or periodic testing |
|
| SITUATIONS WHERE QFT IS NOT CURRENTLY RECOMMENDED |
|
| SITUATIONS WHERE QFT IS PROMISING BUT FUTURE VALUE IS UNCERTAIN |
|
Conclusion
The QFTG test is relatively new; as more evidence becomes available, its place among the tools available for the diagnosis of latent and active TB will clarify. Check with your state and local public health departments to find out the situations for which they are recommending this new diagnostic tool, as practice varies across the country.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
Consider the following cases of patients needing testing for tuberculosis:
- A 55-year-old nurse returns to work after 10 years. Her pre-employment evaluation requires a test for tuberculosis.
- A 35-year-old woman has lupus. Her physician is considering placing her on prednisone and wants to know if she has latent TB infection.
- A homeless man presents to the clinic stating he has lost his TB medications that he has taken or the past 2 months. His diagnosis of TB was made in another city; he cannot remember its name. The physician wants to confirm the diagnosis of TB.
- A 5-year-old from Mexico with a history of BCG vaccination presents for a preschool health evaluation.
- A 35-year-old immigrant from Africa is pregnant and presents for prenatal care.
Patients 1 and 2 above are logical candidates for the newer interferon-gamma blood test to detect tuberculosis (TB). Patients 3, 4, and 5 are not; They should be evaluated with the conventional TB skin test.
The advantages and disadvantages of both kinds of testing are described in this review.
Is a better test at hand?
The TB skin test, using an intradermal injection of purified protein derivative, has been used to assist in the detection of active and latent TB for more than a century. However, this test has its problems: difficulty in accurately measuring and interpreting the reaction; low sensitivity in those with depressed cell-mediated immunity and those with early infections; lower specificity in those with a history of bacille Calmette-Guérin (BCG) vaccination and infection with other Mycobacteria; the need for 2 visits with-in 48 to 72 hours for test interpretation; and boosting of immune response caused by the TB skin test itself (see Boosting phenomena).
Those who work in TB control programs have sought better diagnostic tools. Two that are available today are the interferon-gamma blood tests QuantiFERON-TB (QFT) and QuantiFERON-TB GOLD (QFTG). The QFT and QFTG measure the release of interferon-gamma by sensitized lymphocytes when exposed to antigens of Mycobacterium tuberculosis.
The QFTG, licensed in 2004, is an improvement over the earlier QFT, licensed in 2001, which included antigens that M tuberculosis shares with other commonly encountered Mycobacteria. The QFTG is more specific to M tuberculosis, although it does cross-react with several relatively uncommon nontuberculous Mycobacteria. These tests have several advantages over the older skin test (TABLE 1).
In those who have had prior Mycobacteria infections or BCG vaccine, cell-mediated immunity can wane and a TB skin test can therefore be negative. However, the skin test can boost the person’s immunity, and a second skin test can then be positive as a result of the immune boost. This can cause someone who is actually a reactor (someone who reacts positively to a skin test because of prior Mycobacteria infection) to look like a converter (someone who has a negative skin test at a recent point in time followed by a positive skin test, indicated recent infection with Mycobacteria). It is recommended that adults who have not had a skin test within 12 months and who will need repeated skin tests should receive a 2-step skin test on initial evaluation. A 2-step skin test involves an intitial test followed by a second one 1–2 weeks later.
TABLE 1
Advantages/disadvantages of the QFTG
| Advantages of QFTG compared with the TB skin test |
|
| Disadvantages of QFTG |
|
Factors to keep in mind when considering the QFT
In spite of the theoretical advantages of the QFT, research on its use is at an early stage. It can be considered a testing option for persons identified in TABLE 2. It may ultimately prove to be the test of choice for patients who have previously received a BCG vaccine, and in other instances where specificity is the predominant consideration. However, the Centers for Disease Control and Prevention (CDC) does not recommend it over a TB skin test in any situation.
Whether a skin test or QFTG is used, testing is recommended only for those at high risk of having latent TB infection and for those at high risk of developing active TB disease, if infected. Targeted testing along with treatment of active and latent TB remains the basis of TB control activities in the US.
When QFTG is unwarranted. The QFTG appears to be less sensitive than a TB skin test in those with symptoms of active TB, with the exception of those who are HIV-positive.1 In addition, the QFTG is not recommended for use with patients who are being treated for active TB. Current information is inadequate to recommend any QFTG use in children and pregnant women.
Gray areas. While there is some indication that QFTG is more sensitive for detecting TB infection in those exposed to an infectious patient, it is unknown whether it will predict as well as a skin test which patients are at risk of developing active disease.2 Therefore, it is not clear at this time if all those who have a positive QFTG should be considered candidates for treatment of latent TB infection, or if this should be offered only to those who have both a positive QFTG and TB skin test.
Level of risk influences interpretation. The current CDC recommendations, which have not been updated since QFTG was licensed, state that low-risk patients with a positive QFT and negative TB skin test should not receive treatment for latent infection.3 However, clinical judgment and perceived risk should be the basis for deciding on treatment in those at increased risk who have a positive QFT and negative TB skin test.
It is also not clear what effect a recent TB skin test has on QFTG results, and performing a QFTG soon after a TB skin test is not recommended by the US Food and Drug Administration or the manufacturer.
How to interpret QFTG results. If the QFTG result is positive, the patient needs clinical evaluation and a chest x-ray to rule out active disease. The diagnosis and treatment of active and latent TB has been covered in a previous Practice Alert.4 If the QFTG result is negative, no further evaluation is indicated unless symptoms of TB exist. The QFTG can have an indeterminate result, in which case a skin test can be useful.
Weighing the cost. The cost of a QFTG (about $80–$100 per test) needs to be compared with cost of staff time to read and interpret a skin test and to follow up with patients who fail to return for a skin test measurement.
TABLE 2
Recommendations for use of the QFT
| SITUATIONS WHERE QFT IS RECOMMENDED AS A POSSIBLE DIAGNOSTIC TOOL |
| Persons at increased risk for latent TB infection |
|
| Persons at increased risk of TB infection if exposed |
|
| Persons with conditions that cause increased risk of TB disease if infected, including those with: |
|
| Persons at low risk of TB infection who require initial or periodic testing |
|
| SITUATIONS WHERE QFT IS NOT CURRENTLY RECOMMENDED |
|
| SITUATIONS WHERE QFT IS PROMISING BUT FUTURE VALUE IS UNCERTAIN |
|
Conclusion
The QFTG test is relatively new; as more evidence becomes available, its place among the tools available for the diagnosis of latent and active TB will clarify. Check with your state and local public health departments to find out the situations for which they are recommending this new diagnostic tool, as practice varies across the country.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
1. Pai M, Riley LW, Colford JM. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004;4:761-776.
2. Richeldi L, Ewer K, Losi M, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med 2004;170:288-295.
3. Centers for Disease Control and Prevention. Guidelines for using QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Recomm Rep 2003;52(RR-2):15-18.
4. Campos-Outcalt D. Tuberculosis: old problem, new concerns. J Fam Pract 2003;52:792-798.
1. Pai M, Riley LW, Colford JM. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004;4:761-776.
2. Richeldi L, Ewer K, Losi M, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med 2004;170:288-295.
3. Centers for Disease Control and Prevention. Guidelines for using QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Recomm Rep 2003;52(RR-2):15-18.
4. Campos-Outcalt D. Tuberculosis: old problem, new concerns. J Fam Pract 2003;52:792-798.
Pertussis: A disease re-emerges
The incidence of pertussis in the United States declined dramatically after the introduction of pertussis vaccine in the 1940s. Before that, an average of 160,000 cases of pertussis (150/100,000 population) occurred each year, resulting in 5000 deaths. FIGURE 1 shows how pertussis incidence declined steadily through 3 decades to reach a low of 1010 cases in 1976.
While other vaccine-preventable diseases such as polio, measles, rubella, diphtheria, and tetanus have been eradicated or have declined to only a few cases each year, pertussis has made a slight comeback. The number of cases began increasing in the 1980s and reached a level of 7000 to 8000 cases annually between 1996 and 2000 (see insert in FIGURE 1). There were 11,647 cases in 2003.
While these numbers are small compared with all cases that occurred in the prevaccine era, the increase is cause for public health concern.
FIGURE 1
Number of reported pertussis cases, by year—1922–2000
Unique features of this rebound
The recent rise of pertussis displays several notable trends:
- Disease incidence now ebbs and flows in 3- to 4-year cycles
- The proportion of cases occurring among adolescents and young adults has increased. TABLE 1 shows the age breakdown of reported pertussis cases for 2001. Infants still account for the highest proportion of cases (29%) and the highest attack rates (55 cases per 100,000); but half of reported cases now appear in those age 10 years and older
- Nonimmunized or incompletely immunized infants are usually exposed to the disease by older household members, and not by same-age cohorts
- Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed. The incidence is probably much higher than the reported number of cases would indicate.
TABLE 1
Pertussis-related hospitalizations, complications, and deaths, by age group—United States, 1997–2000
| AGE GROUP | NO. WITH PERTUSSIS | HOSITALIZED NO. (%) | COMPLICATIONS | DEATHS NO. (%) | ||
|---|---|---|---|---|---|---|
| PNEUMONIA* NO. (%) | SEIZURES NO. (%) | ENCEPHALOPTHY NO. (%) | ||||
| < 6 mo | 7203 | 4543 (63.1) | 847 (11.8) | 103 (1.4) | 15 (0.2) | 56 (0.8) |
| 6–11 mo | 1073 | 301 (28.1) | 92 (8.6) | 7 (0.7) | 1 (0.1) | 1 (0.1) |
| 1–4 years | 3137 | 324 (10.3) | 168 (5.4) | 36 (1.2) | 3 (0.1) | 1 (<0.1) |
| 5–9 years | 2756 | 86 (3.1) | 68 (2.5) | 13 (0.5) | 0 | 2 (0.1) |
| 10–19 years | 8273 | 174 (2.1) | 155 (1.9) | 25 (0.3) | 4 (0.1) | 0 |
| ≥ 20 years | 5745 | 202 (3.5) | 147 (2.6) | 32 (0.6) | 3 (0.1) | 2 (<0.1) |
| Total | 28,187† | 5630 (20.0) | 1477 (5.2) | 216‡ (0.8) | 26 (0.1) | 62 (0.2) |
| *Radiographicaly confirmed. | ||||||
| †Excludes 92 (0.3%) persons of unknown age with pertussis. | ||||||
| ‡Excludes one person of unknown age with seizures. | ||||||
| Source: Pertussis—United States, 1997–2000. MMWR Morb Mortal Wkly Rpt 2002; 51:73–76. | ||||||
Why the increase in cases?
Several possible causes could account for the increased incidence in reported pertussis. For one, the efficacy of the vaccine wanes with time after vaccination. Adolescents and young adults are left susceptible because, until recently, no vaccine has been available for persons after their 7th birthday. This, however, has been true for decades and does not explain recent increases.
The Bordetella pertussis bacteria may have genetically drifted to become less susceptible to vaccine-induced antibodies, or the apparent increase in cases could actually be just an increase in case detection.
It is also possible that the recent emphasis on avoiding unnecessary antibiotic use for respiratory infections has had the unanticipated consequence of decreasing previously fortuitous treatment of undiagnosed pertussis among older age groups.
New tool in fight against pertussis now available
Two products with tetanus toxoid—reduced diphtheria toxoid and acellular pertussis vaccines adsorbed (Tdap)—have been licensed for active booster immunization against tetanus, diphtheria, and pertussis as a single dose. BOOSTRIX (GlaxoSmithKline) is for persons aged 10 to 18 years, and ADACEL (Sanofi Pasteur) is for those aged 11 to 64 years.
The Advisory Committee on Immunization Practice of the Centers for Disease Control and Prevention (CDC) has recommended Tdap for universal use among adolescents aged 11 to 12 years, and may in the future recommend its use periodically for all adults.
Rethink your approach to older patients
Clinical presentation of pertussis in an adolescent and adult is nonspecific. After an incubation period of 1 to 3 weeks, pertussis infection appears as a mild respiratory infection or the common cold.
After 1 to 2 weeks, the nonproductive cough can evolve into paroxysms of severe coughing (causing apnea), a posttussive inspiratory whoop, and vomiting. The inspiratory whoop and apnea are usually absent in previously immunized adults and adolescents. The cough gradually diminishes but can persist for up to 3 months.
Suspect pertussis in any adolescent or adult who has had a cough for 2 weeks or longer, even if the paroxysms and post tussive symptoms are absent.
Infants exhibit more severe symptoms and suffer higher rates of complications, including severe apnea, hospitalization, seizures, secondary pneumonia, and death (TABLE 1).
Required laboratory confirmation
For any patient with suspected pertussis, obtain an aspirate of the posterior nasopharyngeal region or swab it for culture. The specimen can be collected by inserting a Dacron nasopharyngeal swab into the nostril to reach the posterior nares and leaving it in place for 10 seconds (FIGURE 2). If the specimen cannot be streaked onto a special enriched culture medium, place it in a special transport medium and refrigerate it until sent to the laboratory. Local or state health departments can often assist with obtaining the transport medium.
Polymerase chain reaction can be used to make a presumptive diagnosis but should be followed by culture confirmation. Direct fluorescent antibody testing of nasopharyngeal specimens and serologic testing for antibodies are not currently recommended as diagnostic tests by the CDC.
FIGURE 2
To collect a Bordella pertussis specimen, a nasopharyngeal swab should be performed in both nares. Insert the swab gently through the nostril toward the posterior nasopharynx; leave it there 15 to 30 seconds, then rotate it and remove. The sample should be put in an appropriate transport medium or immediately onto agar. (Choose shipping conditions based on how long the sample will be in transit; the laboratory will provide specific swab and transport medium requirements). Cotton swabs are not recommended because cotton is harmful to B pertussis. Cultures are usually incubated 10 to 14 days, but results are often available in 7 to 10 days.
Four antibiotics available for treatment of pertussis
The CDC’s recommended medications, doses, and duration of treatment are listed in TABLE 2.
Treatment, if started within the first 2 to 3 weeks, can reduce symptoms and clear B pertussis from the nasopharynx, thus reducing transmission to others. The CDC recommends initiating treatment as soon as pertussis is suspected.
Erythromycin is the agent with the longest history of use and most evidence for effectiveness. Due to its duration of therapy, however, and to the incidence of gastrointestinal side effects, it is less attractive to patients and physicians than other options.
New outcome studies of other macrolides are promising. Most authorities believe azithromycin and clarithromycin are acceptable alternatives and should achieve the same results as erythromycin.
Trimethoprim-sulfamethoxazole can be used for those older than 2 months, for those allergic to, or intolerant of, macrolides. The dose for children is 8 mg/40 mg per day in 2 divided doses for 14 days; for adults it is 320 mg/1200 mg per day in 2 divided doses for 14 days.
TABLE 2
Antibiotic treatment and chemoprophylaxis for pertussis
| AGE | AZITHROMYCIN (5-DAY COURSE) | ERYTHROMYCIN (14-DAY COURSE) | CLARITHROMYCIN (7-DAY COURSE) |
|---|---|---|---|
| <1 month | Recommended in this age group. 10 mg/kg/d in a single dose for 5 days | Not preferred due to association with pyloric stenosis. If used dose is 40–50 mg/kg/d divided in 4 doses for 14 days | Not recommended |
| 1–5 months | 10 mg/kg/d in a single dose for 5 days | 40–50 mg/kg/day divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| ≥ 6 months | 10 mg/kg/d in a single dose on day 1 then 5 mg/kg/d on days 2–5 | 40–50 mg/kg/d divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| Adults | 500 mg single dose on day 1 then 250 mg on days 2–5 | 2 g/d in 4 divided doses for 14 days | 500 mg twice a day for 7 days |
TABLE 3
Important public health functions for physicians in control of pertussis
| Be aware of the local infectious disease epidemiology and know when pertussis is circulating and increasing. |
| Think of the potential for pertussis in adults with a cough of two weeks duration or greater. |
| Collect nasopharyngeal specimens for culture on all patients with suspected pertussis. |
| Begin treatment when pertussis is suspected. |
| Report suspect and confirmed pertussis to the local public health department. |
| When pertussis is highly suspected or confirmed, begin chemoprophylaxis for family members. |
| Insure that respiratory hygiene is practiced in the clinic waiting areas. |
| Implement systems to insure that all patients are vaccinated according to CDC recommendations. |
| Insure that all clinic staff who have been exposed are given chemoprophylaxis and that symptomatic staff are excluded from work until after 5 days of treatment or until 21 days after cough onset if treatment is refused. |
| Collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected. |
Preventing infection in family members and close contacts
Those with pertussis are most infectious in the first 3 weeks of symptoms. The CDC recommends initiating chemoprophylaxis for all household and close contacts of those in whom pertussis is highly suspected or confirmed, regardless of the contacts’ age and vaccination status.
Close contacts are those with direct face-to-face contact with a symptomatic patient, or those who share a confined space for a prolonged period with the patient. Chemoprophylaxis is especially important for high-risk contacts: those under 1 year of age and those with chronic conditions that make them susceptible to complications from pertussis (eg, immune deficiencies, chronic lung disease, or cystic fibrosis).
Chemoprophylaxis has limited benefit if started beyond 3 weeks after exposure. The same antibiotics, doses, and treatment durations are recommended for chemoprophylaxis as for treatment.
Completion of a 4-dose series of pertussis-containing vaccine is also recommended for close contacts. This recommendation has historically pertained only to those before their seventh birthday. With the licensure of Tdap for adolescents and adults, this recommendation may soon include contacts through age 18 and could be expanded to include adults through age 64 in the near future.
Preventing the spread of pertussis in your community
Schools, day care centers, and health care facilities are all potential foci of spread of infection. During outbreaks the local public health department may implement guidelines at schools and day care centers that refer symptomatic staff and students to a physician for evaluation.
If you examine such a patient, perform a nasopharyngeal culture and initiate treatment for those who are symptomatic and for all high-risk contacts. Symptomatic persons should not attend school until either pertussis is ruled out or they have completed 5 days of antibiotic therapy, regardless of their vaccination history. If they refuse treatment, they should be barred from attending school for 21 days from onset of cough.
In health care settings, staff should receive chemoprophylaxis if they have had close exposure to a person with confirmed pertussis, or have had contact with nasal, respiratory, or oral secretions of such a person. Staff members who refuse chemoprophylaxis should be closely observed for symptoms of pertussis; if they become symptomatic, they should be treated and allowed to return to work after 5 days of treatment.
Take-home message
Awareness of local infectious disease epidemiology and knowing when pertussis is circulating and increasing will ensure that you serve the most valuable public health role possible.
Consider pertussis when an adolescent or adult has had a cough for 2 weeks or longer, and collect nasopharyngeal specimens for culture on all patients with suspected pertussis.
Initiate treatment when pertussis is suspected, report suspected and confirmed pertussis to the local public health department, and begin chemoprophylaxis for family members and contacts as indicated.
Implement systems that insure all patients are vaccinated according to CDC recommendations. Institute policies and procedures to insure that respiratory hygiene is practiced in the clinic waiting areas and that staff practice infectious disease precautions and are managed appropriately if they are exposed.
Finally, collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected.
Some of these procedures may become unnecessary in the future if the new pertussis vaccine products for adolescents and adults are successful in turning pertussis into another member of an expanding list of rarely encountered, vaccine-preventable diseases.
Corresponding Author
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The incidence of pertussis in the United States declined dramatically after the introduction of pertussis vaccine in the 1940s. Before that, an average of 160,000 cases of pertussis (150/100,000 population) occurred each year, resulting in 5000 deaths. FIGURE 1 shows how pertussis incidence declined steadily through 3 decades to reach a low of 1010 cases in 1976.
While other vaccine-preventable diseases such as polio, measles, rubella, diphtheria, and tetanus have been eradicated or have declined to only a few cases each year, pertussis has made a slight comeback. The number of cases began increasing in the 1980s and reached a level of 7000 to 8000 cases annually between 1996 and 2000 (see insert in FIGURE 1). There were 11,647 cases in 2003.
While these numbers are small compared with all cases that occurred in the prevaccine era, the increase is cause for public health concern.
FIGURE 1
Number of reported pertussis cases, by year—1922–2000
Unique features of this rebound
The recent rise of pertussis displays several notable trends:
- Disease incidence now ebbs and flows in 3- to 4-year cycles
- The proportion of cases occurring among adolescents and young adults has increased. TABLE 1 shows the age breakdown of reported pertussis cases for 2001. Infants still account for the highest proportion of cases (29%) and the highest attack rates (55 cases per 100,000); but half of reported cases now appear in those age 10 years and older
- Nonimmunized or incompletely immunized infants are usually exposed to the disease by older household members, and not by same-age cohorts
- Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed. The incidence is probably much higher than the reported number of cases would indicate.
TABLE 1
Pertussis-related hospitalizations, complications, and deaths, by age group—United States, 1997–2000
| AGE GROUP | NO. WITH PERTUSSIS | HOSITALIZED NO. (%) | COMPLICATIONS | DEATHS NO. (%) | ||
|---|---|---|---|---|---|---|
| PNEUMONIA* NO. (%) | SEIZURES NO. (%) | ENCEPHALOPTHY NO. (%) | ||||
| < 6 mo | 7203 | 4543 (63.1) | 847 (11.8) | 103 (1.4) | 15 (0.2) | 56 (0.8) |
| 6–11 mo | 1073 | 301 (28.1) | 92 (8.6) | 7 (0.7) | 1 (0.1) | 1 (0.1) |
| 1–4 years | 3137 | 324 (10.3) | 168 (5.4) | 36 (1.2) | 3 (0.1) | 1 (<0.1) |
| 5–9 years | 2756 | 86 (3.1) | 68 (2.5) | 13 (0.5) | 0 | 2 (0.1) |
| 10–19 years | 8273 | 174 (2.1) | 155 (1.9) | 25 (0.3) | 4 (0.1) | 0 |
| ≥ 20 years | 5745 | 202 (3.5) | 147 (2.6) | 32 (0.6) | 3 (0.1) | 2 (<0.1) |
| Total | 28,187† | 5630 (20.0) | 1477 (5.2) | 216‡ (0.8) | 26 (0.1) | 62 (0.2) |
| *Radiographicaly confirmed. | ||||||
| †Excludes 92 (0.3%) persons of unknown age with pertussis. | ||||||
| ‡Excludes one person of unknown age with seizures. | ||||||
| Source: Pertussis—United States, 1997–2000. MMWR Morb Mortal Wkly Rpt 2002; 51:73–76. | ||||||
Why the increase in cases?
Several possible causes could account for the increased incidence in reported pertussis. For one, the efficacy of the vaccine wanes with time after vaccination. Adolescents and young adults are left susceptible because, until recently, no vaccine has been available for persons after their 7th birthday. This, however, has been true for decades and does not explain recent increases.
The Bordetella pertussis bacteria may have genetically drifted to become less susceptible to vaccine-induced antibodies, or the apparent increase in cases could actually be just an increase in case detection.
It is also possible that the recent emphasis on avoiding unnecessary antibiotic use for respiratory infections has had the unanticipated consequence of decreasing previously fortuitous treatment of undiagnosed pertussis among older age groups.
New tool in fight against pertussis now available
Two products with tetanus toxoid—reduced diphtheria toxoid and acellular pertussis vaccines adsorbed (Tdap)—have been licensed for active booster immunization against tetanus, diphtheria, and pertussis as a single dose. BOOSTRIX (GlaxoSmithKline) is for persons aged 10 to 18 years, and ADACEL (Sanofi Pasteur) is for those aged 11 to 64 years.
The Advisory Committee on Immunization Practice of the Centers for Disease Control and Prevention (CDC) has recommended Tdap for universal use among adolescents aged 11 to 12 years, and may in the future recommend its use periodically for all adults.
Rethink your approach to older patients
Clinical presentation of pertussis in an adolescent and adult is nonspecific. After an incubation period of 1 to 3 weeks, pertussis infection appears as a mild respiratory infection or the common cold.
After 1 to 2 weeks, the nonproductive cough can evolve into paroxysms of severe coughing (causing apnea), a posttussive inspiratory whoop, and vomiting. The inspiratory whoop and apnea are usually absent in previously immunized adults and adolescents. The cough gradually diminishes but can persist for up to 3 months.
Suspect pertussis in any adolescent or adult who has had a cough for 2 weeks or longer, even if the paroxysms and post tussive symptoms are absent.
Infants exhibit more severe symptoms and suffer higher rates of complications, including severe apnea, hospitalization, seizures, secondary pneumonia, and death (TABLE 1).
Required laboratory confirmation
For any patient with suspected pertussis, obtain an aspirate of the posterior nasopharyngeal region or swab it for culture. The specimen can be collected by inserting a Dacron nasopharyngeal swab into the nostril to reach the posterior nares and leaving it in place for 10 seconds (FIGURE 2). If the specimen cannot be streaked onto a special enriched culture medium, place it in a special transport medium and refrigerate it until sent to the laboratory. Local or state health departments can often assist with obtaining the transport medium.
Polymerase chain reaction can be used to make a presumptive diagnosis but should be followed by culture confirmation. Direct fluorescent antibody testing of nasopharyngeal specimens and serologic testing for antibodies are not currently recommended as diagnostic tests by the CDC.
FIGURE 2
To collect a Bordella pertussis specimen, a nasopharyngeal swab should be performed in both nares. Insert the swab gently through the nostril toward the posterior nasopharynx; leave it there 15 to 30 seconds, then rotate it and remove. The sample should be put in an appropriate transport medium or immediately onto agar. (Choose shipping conditions based on how long the sample will be in transit; the laboratory will provide specific swab and transport medium requirements). Cotton swabs are not recommended because cotton is harmful to B pertussis. Cultures are usually incubated 10 to 14 days, but results are often available in 7 to 10 days.
Four antibiotics available for treatment of pertussis
The CDC’s recommended medications, doses, and duration of treatment are listed in TABLE 2.
Treatment, if started within the first 2 to 3 weeks, can reduce symptoms and clear B pertussis from the nasopharynx, thus reducing transmission to others. The CDC recommends initiating treatment as soon as pertussis is suspected.
Erythromycin is the agent with the longest history of use and most evidence for effectiveness. Due to its duration of therapy, however, and to the incidence of gastrointestinal side effects, it is less attractive to patients and physicians than other options.
New outcome studies of other macrolides are promising. Most authorities believe azithromycin and clarithromycin are acceptable alternatives and should achieve the same results as erythromycin.
Trimethoprim-sulfamethoxazole can be used for those older than 2 months, for those allergic to, or intolerant of, macrolides. The dose for children is 8 mg/40 mg per day in 2 divided doses for 14 days; for adults it is 320 mg/1200 mg per day in 2 divided doses for 14 days.
TABLE 2
Antibiotic treatment and chemoprophylaxis for pertussis
| AGE | AZITHROMYCIN (5-DAY COURSE) | ERYTHROMYCIN (14-DAY COURSE) | CLARITHROMYCIN (7-DAY COURSE) |
|---|---|---|---|
| <1 month | Recommended in this age group. 10 mg/kg/d in a single dose for 5 days | Not preferred due to association with pyloric stenosis. If used dose is 40–50 mg/kg/d divided in 4 doses for 14 days | Not recommended |
| 1–5 months | 10 mg/kg/d in a single dose for 5 days | 40–50 mg/kg/day divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| ≥ 6 months | 10 mg/kg/d in a single dose on day 1 then 5 mg/kg/d on days 2–5 | 40–50 mg/kg/d divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| Adults | 500 mg single dose on day 1 then 250 mg on days 2–5 | 2 g/d in 4 divided doses for 14 days | 500 mg twice a day for 7 days |
TABLE 3
Important public health functions for physicians in control of pertussis
| Be aware of the local infectious disease epidemiology and know when pertussis is circulating and increasing. |
| Think of the potential for pertussis in adults with a cough of two weeks duration or greater. |
| Collect nasopharyngeal specimens for culture on all patients with suspected pertussis. |
| Begin treatment when pertussis is suspected. |
| Report suspect and confirmed pertussis to the local public health department. |
| When pertussis is highly suspected or confirmed, begin chemoprophylaxis for family members. |
| Insure that respiratory hygiene is practiced in the clinic waiting areas. |
| Implement systems to insure that all patients are vaccinated according to CDC recommendations. |
| Insure that all clinic staff who have been exposed are given chemoprophylaxis and that symptomatic staff are excluded from work until after 5 days of treatment or until 21 days after cough onset if treatment is refused. |
| Collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected. |
Preventing infection in family members and close contacts
Those with pertussis are most infectious in the first 3 weeks of symptoms. The CDC recommends initiating chemoprophylaxis for all household and close contacts of those in whom pertussis is highly suspected or confirmed, regardless of the contacts’ age and vaccination status.
Close contacts are those with direct face-to-face contact with a symptomatic patient, or those who share a confined space for a prolonged period with the patient. Chemoprophylaxis is especially important for high-risk contacts: those under 1 year of age and those with chronic conditions that make them susceptible to complications from pertussis (eg, immune deficiencies, chronic lung disease, or cystic fibrosis).
Chemoprophylaxis has limited benefit if started beyond 3 weeks after exposure. The same antibiotics, doses, and treatment durations are recommended for chemoprophylaxis as for treatment.
Completion of a 4-dose series of pertussis-containing vaccine is also recommended for close contacts. This recommendation has historically pertained only to those before their seventh birthday. With the licensure of Tdap for adolescents and adults, this recommendation may soon include contacts through age 18 and could be expanded to include adults through age 64 in the near future.
Preventing the spread of pertussis in your community
Schools, day care centers, and health care facilities are all potential foci of spread of infection. During outbreaks the local public health department may implement guidelines at schools and day care centers that refer symptomatic staff and students to a physician for evaluation.
If you examine such a patient, perform a nasopharyngeal culture and initiate treatment for those who are symptomatic and for all high-risk contacts. Symptomatic persons should not attend school until either pertussis is ruled out or they have completed 5 days of antibiotic therapy, regardless of their vaccination history. If they refuse treatment, they should be barred from attending school for 21 days from onset of cough.
In health care settings, staff should receive chemoprophylaxis if they have had close exposure to a person with confirmed pertussis, or have had contact with nasal, respiratory, or oral secretions of such a person. Staff members who refuse chemoprophylaxis should be closely observed for symptoms of pertussis; if they become symptomatic, they should be treated and allowed to return to work after 5 days of treatment.
Take-home message
Awareness of local infectious disease epidemiology and knowing when pertussis is circulating and increasing will ensure that you serve the most valuable public health role possible.
Consider pertussis when an adolescent or adult has had a cough for 2 weeks or longer, and collect nasopharyngeal specimens for culture on all patients with suspected pertussis.
Initiate treatment when pertussis is suspected, report suspected and confirmed pertussis to the local public health department, and begin chemoprophylaxis for family members and contacts as indicated.
Implement systems that insure all patients are vaccinated according to CDC recommendations. Institute policies and procedures to insure that respiratory hygiene is practiced in the clinic waiting areas and that staff practice infectious disease precautions and are managed appropriately if they are exposed.
Finally, collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected.
Some of these procedures may become unnecessary in the future if the new pertussis vaccine products for adolescents and adults are successful in turning pertussis into another member of an expanding list of rarely encountered, vaccine-preventable diseases.
Corresponding Author
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The incidence of pertussis in the United States declined dramatically after the introduction of pertussis vaccine in the 1940s. Before that, an average of 160,000 cases of pertussis (150/100,000 population) occurred each year, resulting in 5000 deaths. FIGURE 1 shows how pertussis incidence declined steadily through 3 decades to reach a low of 1010 cases in 1976.
While other vaccine-preventable diseases such as polio, measles, rubella, diphtheria, and tetanus have been eradicated or have declined to only a few cases each year, pertussis has made a slight comeback. The number of cases began increasing in the 1980s and reached a level of 7000 to 8000 cases annually between 1996 and 2000 (see insert in FIGURE 1). There were 11,647 cases in 2003.
While these numbers are small compared with all cases that occurred in the prevaccine era, the increase is cause for public health concern.
FIGURE 1
Number of reported pertussis cases, by year—1922–2000
Unique features of this rebound
The recent rise of pertussis displays several notable trends:
- Disease incidence now ebbs and flows in 3- to 4-year cycles
- The proportion of cases occurring among adolescents and young adults has increased. TABLE 1 shows the age breakdown of reported pertussis cases for 2001. Infants still account for the highest proportion of cases (29%) and the highest attack rates (55 cases per 100,000); but half of reported cases now appear in those age 10 years and older
- Nonimmunized or incompletely immunized infants are usually exposed to the disease by older household members, and not by same-age cohorts
- Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed. The incidence is probably much higher than the reported number of cases would indicate.
TABLE 1
Pertussis-related hospitalizations, complications, and deaths, by age group—United States, 1997–2000
| AGE GROUP | NO. WITH PERTUSSIS | HOSITALIZED NO. (%) | COMPLICATIONS | DEATHS NO. (%) | ||
|---|---|---|---|---|---|---|
| PNEUMONIA* NO. (%) | SEIZURES NO. (%) | ENCEPHALOPTHY NO. (%) | ||||
| < 6 mo | 7203 | 4543 (63.1) | 847 (11.8) | 103 (1.4) | 15 (0.2) | 56 (0.8) |
| 6–11 mo | 1073 | 301 (28.1) | 92 (8.6) | 7 (0.7) | 1 (0.1) | 1 (0.1) |
| 1–4 years | 3137 | 324 (10.3) | 168 (5.4) | 36 (1.2) | 3 (0.1) | 1 (<0.1) |
| 5–9 years | 2756 | 86 (3.1) | 68 (2.5) | 13 (0.5) | 0 | 2 (0.1) |
| 10–19 years | 8273 | 174 (2.1) | 155 (1.9) | 25 (0.3) | 4 (0.1) | 0 |
| ≥ 20 years | 5745 | 202 (3.5) | 147 (2.6) | 32 (0.6) | 3 (0.1) | 2 (<0.1) |
| Total | 28,187† | 5630 (20.0) | 1477 (5.2) | 216‡ (0.8) | 26 (0.1) | 62 (0.2) |
| *Radiographicaly confirmed. | ||||||
| †Excludes 92 (0.3%) persons of unknown age with pertussis. | ||||||
| ‡Excludes one person of unknown age with seizures. | ||||||
| Source: Pertussis—United States, 1997–2000. MMWR Morb Mortal Wkly Rpt 2002; 51:73–76. | ||||||
Why the increase in cases?
Several possible causes could account for the increased incidence in reported pertussis. For one, the efficacy of the vaccine wanes with time after vaccination. Adolescents and young adults are left susceptible because, until recently, no vaccine has been available for persons after their 7th birthday. This, however, has been true for decades and does not explain recent increases.
The Bordetella pertussis bacteria may have genetically drifted to become less susceptible to vaccine-induced antibodies, or the apparent increase in cases could actually be just an increase in case detection.
It is also possible that the recent emphasis on avoiding unnecessary antibiotic use for respiratory infections has had the unanticipated consequence of decreasing previously fortuitous treatment of undiagnosed pertussis among older age groups.
New tool in fight against pertussis now available
Two products with tetanus toxoid—reduced diphtheria toxoid and acellular pertussis vaccines adsorbed (Tdap)—have been licensed for active booster immunization against tetanus, diphtheria, and pertussis as a single dose. BOOSTRIX (GlaxoSmithKline) is for persons aged 10 to 18 years, and ADACEL (Sanofi Pasteur) is for those aged 11 to 64 years.
The Advisory Committee on Immunization Practice of the Centers for Disease Control and Prevention (CDC) has recommended Tdap for universal use among adolescents aged 11 to 12 years, and may in the future recommend its use periodically for all adults.
Rethink your approach to older patients
Clinical presentation of pertussis in an adolescent and adult is nonspecific. After an incubation period of 1 to 3 weeks, pertussis infection appears as a mild respiratory infection or the common cold.
After 1 to 2 weeks, the nonproductive cough can evolve into paroxysms of severe coughing (causing apnea), a posttussive inspiratory whoop, and vomiting. The inspiratory whoop and apnea are usually absent in previously immunized adults and adolescents. The cough gradually diminishes but can persist for up to 3 months.
Suspect pertussis in any adolescent or adult who has had a cough for 2 weeks or longer, even if the paroxysms and post tussive symptoms are absent.
Infants exhibit more severe symptoms and suffer higher rates of complications, including severe apnea, hospitalization, seizures, secondary pneumonia, and death (TABLE 1).
Required laboratory confirmation
For any patient with suspected pertussis, obtain an aspirate of the posterior nasopharyngeal region or swab it for culture. The specimen can be collected by inserting a Dacron nasopharyngeal swab into the nostril to reach the posterior nares and leaving it in place for 10 seconds (FIGURE 2). If the specimen cannot be streaked onto a special enriched culture medium, place it in a special transport medium and refrigerate it until sent to the laboratory. Local or state health departments can often assist with obtaining the transport medium.
Polymerase chain reaction can be used to make a presumptive diagnosis but should be followed by culture confirmation. Direct fluorescent antibody testing of nasopharyngeal specimens and serologic testing for antibodies are not currently recommended as diagnostic tests by the CDC.
FIGURE 2
To collect a Bordella pertussis specimen, a nasopharyngeal swab should be performed in both nares. Insert the swab gently through the nostril toward the posterior nasopharynx; leave it there 15 to 30 seconds, then rotate it and remove. The sample should be put in an appropriate transport medium or immediately onto agar. (Choose shipping conditions based on how long the sample will be in transit; the laboratory will provide specific swab and transport medium requirements). Cotton swabs are not recommended because cotton is harmful to B pertussis. Cultures are usually incubated 10 to 14 days, but results are often available in 7 to 10 days.
Four antibiotics available for treatment of pertussis
The CDC’s recommended medications, doses, and duration of treatment are listed in TABLE 2.
Treatment, if started within the first 2 to 3 weeks, can reduce symptoms and clear B pertussis from the nasopharynx, thus reducing transmission to others. The CDC recommends initiating treatment as soon as pertussis is suspected.
Erythromycin is the agent with the longest history of use and most evidence for effectiveness. Due to its duration of therapy, however, and to the incidence of gastrointestinal side effects, it is less attractive to patients and physicians than other options.
New outcome studies of other macrolides are promising. Most authorities believe azithromycin and clarithromycin are acceptable alternatives and should achieve the same results as erythromycin.
Trimethoprim-sulfamethoxazole can be used for those older than 2 months, for those allergic to, or intolerant of, macrolides. The dose for children is 8 mg/40 mg per day in 2 divided doses for 14 days; for adults it is 320 mg/1200 mg per day in 2 divided doses for 14 days.
TABLE 2
Antibiotic treatment and chemoprophylaxis for pertussis
| AGE | AZITHROMYCIN (5-DAY COURSE) | ERYTHROMYCIN (14-DAY COURSE) | CLARITHROMYCIN (7-DAY COURSE) |
|---|---|---|---|
| <1 month | Recommended in this age group. 10 mg/kg/d in a single dose for 5 days | Not preferred due to association with pyloric stenosis. If used dose is 40–50 mg/kg/d divided in 4 doses for 14 days | Not recommended |
| 1–5 months | 10 mg/kg/d in a single dose for 5 days | 40–50 mg/kg/day divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| ≥ 6 months | 10 mg/kg/d in a single dose on day 1 then 5 mg/kg/d on days 2–5 | 40–50 mg/kg/d divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| Adults | 500 mg single dose on day 1 then 250 mg on days 2–5 | 2 g/d in 4 divided doses for 14 days | 500 mg twice a day for 7 days |
TABLE 3
Important public health functions for physicians in control of pertussis
| Be aware of the local infectious disease epidemiology and know when pertussis is circulating and increasing. |
| Think of the potential for pertussis in adults with a cough of two weeks duration or greater. |
| Collect nasopharyngeal specimens for culture on all patients with suspected pertussis. |
| Begin treatment when pertussis is suspected. |
| Report suspect and confirmed pertussis to the local public health department. |
| When pertussis is highly suspected or confirmed, begin chemoprophylaxis for family members. |
| Insure that respiratory hygiene is practiced in the clinic waiting areas. |
| Implement systems to insure that all patients are vaccinated according to CDC recommendations. |
| Insure that all clinic staff who have been exposed are given chemoprophylaxis and that symptomatic staff are excluded from work until after 5 days of treatment or until 21 days after cough onset if treatment is refused. |
| Collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected. |
Preventing infection in family members and close contacts
Those with pertussis are most infectious in the first 3 weeks of symptoms. The CDC recommends initiating chemoprophylaxis for all household and close contacts of those in whom pertussis is highly suspected or confirmed, regardless of the contacts’ age and vaccination status.
Close contacts are those with direct face-to-face contact with a symptomatic patient, or those who share a confined space for a prolonged period with the patient. Chemoprophylaxis is especially important for high-risk contacts: those under 1 year of age and those with chronic conditions that make them susceptible to complications from pertussis (eg, immune deficiencies, chronic lung disease, or cystic fibrosis).
Chemoprophylaxis has limited benefit if started beyond 3 weeks after exposure. The same antibiotics, doses, and treatment durations are recommended for chemoprophylaxis as for treatment.
Completion of a 4-dose series of pertussis-containing vaccine is also recommended for close contacts. This recommendation has historically pertained only to those before their seventh birthday. With the licensure of Tdap for adolescents and adults, this recommendation may soon include contacts through age 18 and could be expanded to include adults through age 64 in the near future.
Preventing the spread of pertussis in your community
Schools, day care centers, and health care facilities are all potential foci of spread of infection. During outbreaks the local public health department may implement guidelines at schools and day care centers that refer symptomatic staff and students to a physician for evaluation.
If you examine such a patient, perform a nasopharyngeal culture and initiate treatment for those who are symptomatic and for all high-risk contacts. Symptomatic persons should not attend school until either pertussis is ruled out or they have completed 5 days of antibiotic therapy, regardless of their vaccination history. If they refuse treatment, they should be barred from attending school for 21 days from onset of cough.
In health care settings, staff should receive chemoprophylaxis if they have had close exposure to a person with confirmed pertussis, or have had contact with nasal, respiratory, or oral secretions of such a person. Staff members who refuse chemoprophylaxis should be closely observed for symptoms of pertussis; if they become symptomatic, they should be treated and allowed to return to work after 5 days of treatment.
Take-home message
Awareness of local infectious disease epidemiology and knowing when pertussis is circulating and increasing will ensure that you serve the most valuable public health role possible.
Consider pertussis when an adolescent or adult has had a cough for 2 weeks or longer, and collect nasopharyngeal specimens for culture on all patients with suspected pertussis.
Initiate treatment when pertussis is suspected, report suspected and confirmed pertussis to the local public health department, and begin chemoprophylaxis for family members and contacts as indicated.
Implement systems that insure all patients are vaccinated according to CDC recommendations. Institute policies and procedures to insure that respiratory hygiene is practiced in the clinic waiting areas and that staff practice infectious disease precautions and are managed appropriately if they are exposed.
Finally, collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected.
Some of these procedures may become unnecessary in the future if the new pertussis vaccine products for adolescents and adults are successful in turning pertussis into another member of an expanding list of rarely encountered, vaccine-preventable diseases.
Corresponding Author
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
US Preventive Services Task Force: The gold standard of evidence-based prevention
The United States Preventive Services Task Force (USPSTF) was first formed in 1984 to assist physicians in making decisions about which preventive services to offer patients. It consists of a 15-member panel of independent scientists picked for their expertise in primary care, clinical prevention, and evidence-based methodology. The first set of recommendations was published in 1989 as the Guide to Clinical Preventive Services, and was revised in 1996 in the second edition. Recommendations are now published on the USPSTF web site (www.ahrq.gov/ clinic/uspstfix.htm).
The USPSTF uses an explicit set of steps and criteria to judge the effectiveness, harms, costs and benefits of preventive interventions: screening, counseling, and chemoprevention. Topics are suggested by outside partners, including the American Academy of Family Physicians, and are then sent to one of 13 evidence-based practice centers, where an extensive review is conducted of the current scientific literature on the topic. The evidence report is then reviewed by the 15-member USPSTF and a recommendation is made using the rating system described in TABLE 1. The current members of the USPSTF arefound at www.ahrq.gov/clinic/uspstfab.htm# Members. The staff for the task force is provided by the Agency for Health Care Quality and Research (AHRQ), one of the agencies in the Public Health Service of the US Department of Health and Human Services.
In addition to listing the recommendations and the rationales behind them, the USPSTF web site also provides the evidence report and a description of recommendations on that topic made by other organizations, with a discussion of clinical implications of the recommendation. During 2004, the USPSTF made or updated 29 recommendations ( TABLE 2 ). There were 5 A recommendations, 4 B recommendations, no C recommendations, 11 recommendations against an intervention (D recommendation), and 9 instances of insufficient evidence to make a recommendation.
TABLE 1
Standard recommendation language, USPSTF
| RECOMMENDATION: |
| A Language: The USPSTF strongly recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found good evidence that [the service] improves important health outcomes and concludes that benefits substantially outweigh harms.) |
| RECOMMENDATION: |
| B Language: The USPSTF recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found at least fair evidence that [the service] improves important health outcomes and concludes that benefits outweigh harms.) |
| RECOMMENDATION: |
| C Language: The USPSTF makes no recommendation for or against routine provision of [the service]. (The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of the benefits and harms is too close to justify a general recommendation.) |
| RECOMMENDATION: |
| D Language: The USPSTF recommends against routinely providing [the service] to asymptomatic patients. (The USPSTF found at least fair evidence that [the service] is ineffective or that harms outweigh benefits.) |
| RECOMMENDATION: |
| I Language: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing [the service]. (Evidence that [the service] is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined.) |
TABLE 2
USPSTF recommendations made in 2004
| A Recommendation (strongly recommends) |
|
| B Recommendation (recommends) |
|
| D Recommendation (recommends against) |
|
| I Recommendation (insufficient evidence) |
|
Recommendations for 2005
So far in 2005, new recommendations have been added on 3 topics: abdominal aortic aneurisms, glaucoma, and herpes simplex.
Abdominal aortic aneurisms. The recommendations on screening for abdominal aortic aneurisms are contained in TABLE 3. Of special note is the recommendation to screen (using abdominal ultrasound) men over the age of 65 years who have ever smoked.
Glaucoma. The statement that evidence is insufficient to recommend for or against routinely screening for glaucoma reflects the uncertainty about the contribution of screening to improved outcomes, as well as the documented harms of treating elevated intraocular pressure, such as local eye irritation and an increased risk for cataracts.
Herpes simplex. The task force recommends against screening for herpes in pregnant women and asymptomatic adults and adolescents because of a lack of improved outcomes and documented potential harms.
TABLE 3
USPSTF 2005 recommendations for screening for abdominal aortic aneurisms
| The USPSTF recommends one-time screening for abdominal aortic aneurysm (AAA) by ultrasonography in men aged 65 to 75 who have ever smoked. |
| RATING: B RECOMMENDATION |
| Rationale: The USPSTF found good evidence that screening for AAA and surgical repair of large AAAs (5.5 cm or more) in men aged 65 to 75 who have ever smoked (current and former smokers) leads to decreased AAA-specific mortality. There is good evidence that abdominal ultrasonography, performed in a setting with adequate quality assurance (ie, in an accredited facility with credentialed technologists), is an accurate screening test for AAA. There is also good evidence of important harms of screening and early treatment, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. Based on the moderate magnitude of net benefit, the USPSTF concluded that the benefits of screening for AAA in men aged 65 to 75 who have ever smoked outweigh the harms. |
| The USPSTF makes no recommendation for or against screening for AAA in men aged 65 to 75 who have never smoked. |
| RATING: C RECOMMENDATION. |
| Rationale: The USPSTF found good evidence that screening for AAA in men aged 65 to 75 who have never smoked leads to decreased AAA-specific mortality. There is, however, a lower prevalence of large AAAs in men who have never smoked compared with men who have ever smoked; thus, the potential benefit from screening men who have never smoked is small. There is good evidence that screening and early treatment leads to important harms, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. The USPSTF concluded that the balance between the benefits and harms of screening for AAA is too close to make a general recommendation in this population. |
| The USPSTF recommends against routine screening for AAA in women. |
| RATING: D RECOMMENDATION. |
| Rationale: Because of the low prevalence of large AAAs in women, the number of AAA-related deaths that can be prevented by screening this population is small. There is good evidence that screening and early treatment result in important harms, including an increased number of surgeries with associated morbidity and mortality, and psychological harms. The USPSTF concluded that the harms of screening women for AAA outweigh the benefits. |
USPSTF The Gold Standard
The USPSTF offers busy practicing physicians a valuable set of resources to assist in staying current on the ever changing field of clinical prevention and to guide clinical practice. Their recommendations often are at odds with common beliefs. But over time, their methodology and resulting recommendations have become the gold standard for evidence-based prevention practice.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The United States Preventive Services Task Force (USPSTF) was first formed in 1984 to assist physicians in making decisions about which preventive services to offer patients. It consists of a 15-member panel of independent scientists picked for their expertise in primary care, clinical prevention, and evidence-based methodology. The first set of recommendations was published in 1989 as the Guide to Clinical Preventive Services, and was revised in 1996 in the second edition. Recommendations are now published on the USPSTF web site (www.ahrq.gov/ clinic/uspstfix.htm).
The USPSTF uses an explicit set of steps and criteria to judge the effectiveness, harms, costs and benefits of preventive interventions: screening, counseling, and chemoprevention. Topics are suggested by outside partners, including the American Academy of Family Physicians, and are then sent to one of 13 evidence-based practice centers, where an extensive review is conducted of the current scientific literature on the topic. The evidence report is then reviewed by the 15-member USPSTF and a recommendation is made using the rating system described in TABLE 1. The current members of the USPSTF arefound at www.ahrq.gov/clinic/uspstfab.htm# Members. The staff for the task force is provided by the Agency for Health Care Quality and Research (AHRQ), one of the agencies in the Public Health Service of the US Department of Health and Human Services.
In addition to listing the recommendations and the rationales behind them, the USPSTF web site also provides the evidence report and a description of recommendations on that topic made by other organizations, with a discussion of clinical implications of the recommendation. During 2004, the USPSTF made or updated 29 recommendations ( TABLE 2 ). There were 5 A recommendations, 4 B recommendations, no C recommendations, 11 recommendations against an intervention (D recommendation), and 9 instances of insufficient evidence to make a recommendation.
TABLE 1
Standard recommendation language, USPSTF
| RECOMMENDATION: |
| A Language: The USPSTF strongly recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found good evidence that [the service] improves important health outcomes and concludes that benefits substantially outweigh harms.) |
| RECOMMENDATION: |
| B Language: The USPSTF recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found at least fair evidence that [the service] improves important health outcomes and concludes that benefits outweigh harms.) |
| RECOMMENDATION: |
| C Language: The USPSTF makes no recommendation for or against routine provision of [the service]. (The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of the benefits and harms is too close to justify a general recommendation.) |
| RECOMMENDATION: |
| D Language: The USPSTF recommends against routinely providing [the service] to asymptomatic patients. (The USPSTF found at least fair evidence that [the service] is ineffective or that harms outweigh benefits.) |
| RECOMMENDATION: |
| I Language: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing [the service]. (Evidence that [the service] is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined.) |
TABLE 2
USPSTF recommendations made in 2004
| A Recommendation (strongly recommends) |
|
| B Recommendation (recommends) |
|
| D Recommendation (recommends against) |
|
| I Recommendation (insufficient evidence) |
|
Recommendations for 2005
So far in 2005, new recommendations have been added on 3 topics: abdominal aortic aneurisms, glaucoma, and herpes simplex.
Abdominal aortic aneurisms. The recommendations on screening for abdominal aortic aneurisms are contained in TABLE 3. Of special note is the recommendation to screen (using abdominal ultrasound) men over the age of 65 years who have ever smoked.
Glaucoma. The statement that evidence is insufficient to recommend for or against routinely screening for glaucoma reflects the uncertainty about the contribution of screening to improved outcomes, as well as the documented harms of treating elevated intraocular pressure, such as local eye irritation and an increased risk for cataracts.
Herpes simplex. The task force recommends against screening for herpes in pregnant women and asymptomatic adults and adolescents because of a lack of improved outcomes and documented potential harms.
TABLE 3
USPSTF 2005 recommendations for screening for abdominal aortic aneurisms
| The USPSTF recommends one-time screening for abdominal aortic aneurysm (AAA) by ultrasonography in men aged 65 to 75 who have ever smoked. |
| RATING: B RECOMMENDATION |
| Rationale: The USPSTF found good evidence that screening for AAA and surgical repair of large AAAs (5.5 cm or more) in men aged 65 to 75 who have ever smoked (current and former smokers) leads to decreased AAA-specific mortality. There is good evidence that abdominal ultrasonography, performed in a setting with adequate quality assurance (ie, in an accredited facility with credentialed technologists), is an accurate screening test for AAA. There is also good evidence of important harms of screening and early treatment, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. Based on the moderate magnitude of net benefit, the USPSTF concluded that the benefits of screening for AAA in men aged 65 to 75 who have ever smoked outweigh the harms. |
| The USPSTF makes no recommendation for or against screening for AAA in men aged 65 to 75 who have never smoked. |
| RATING: C RECOMMENDATION. |
| Rationale: The USPSTF found good evidence that screening for AAA in men aged 65 to 75 who have never smoked leads to decreased AAA-specific mortality. There is, however, a lower prevalence of large AAAs in men who have never smoked compared with men who have ever smoked; thus, the potential benefit from screening men who have never smoked is small. There is good evidence that screening and early treatment leads to important harms, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. The USPSTF concluded that the balance between the benefits and harms of screening for AAA is too close to make a general recommendation in this population. |
| The USPSTF recommends against routine screening for AAA in women. |
| RATING: D RECOMMENDATION. |
| Rationale: Because of the low prevalence of large AAAs in women, the number of AAA-related deaths that can be prevented by screening this population is small. There is good evidence that screening and early treatment result in important harms, including an increased number of surgeries with associated morbidity and mortality, and psychological harms. The USPSTF concluded that the harms of screening women for AAA outweigh the benefits. |
USPSTF The Gold Standard
The USPSTF offers busy practicing physicians a valuable set of resources to assist in staying current on the ever changing field of clinical prevention and to guide clinical practice. Their recommendations often are at odds with common beliefs. But over time, their methodology and resulting recommendations have become the gold standard for evidence-based prevention practice.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The United States Preventive Services Task Force (USPSTF) was first formed in 1984 to assist physicians in making decisions about which preventive services to offer patients. It consists of a 15-member panel of independent scientists picked for their expertise in primary care, clinical prevention, and evidence-based methodology. The first set of recommendations was published in 1989 as the Guide to Clinical Preventive Services, and was revised in 1996 in the second edition. Recommendations are now published on the USPSTF web site (www.ahrq.gov/ clinic/uspstfix.htm).
The USPSTF uses an explicit set of steps and criteria to judge the effectiveness, harms, costs and benefits of preventive interventions: screening, counseling, and chemoprevention. Topics are suggested by outside partners, including the American Academy of Family Physicians, and are then sent to one of 13 evidence-based practice centers, where an extensive review is conducted of the current scientific literature on the topic. The evidence report is then reviewed by the 15-member USPSTF and a recommendation is made using the rating system described in TABLE 1. The current members of the USPSTF arefound at www.ahrq.gov/clinic/uspstfab.htm# Members. The staff for the task force is provided by the Agency for Health Care Quality and Research (AHRQ), one of the agencies in the Public Health Service of the US Department of Health and Human Services.
In addition to listing the recommendations and the rationales behind them, the USPSTF web site also provides the evidence report and a description of recommendations on that topic made by other organizations, with a discussion of clinical implications of the recommendation. During 2004, the USPSTF made or updated 29 recommendations ( TABLE 2 ). There were 5 A recommendations, 4 B recommendations, no C recommendations, 11 recommendations against an intervention (D recommendation), and 9 instances of insufficient evidence to make a recommendation.
TABLE 1
Standard recommendation language, USPSTF
| RECOMMENDATION: |
| A Language: The USPSTF strongly recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found good evidence that [the service] improves important health outcomes and concludes that benefits substantially outweigh harms.) |
| RECOMMENDATION: |
| B Language: The USPSTF recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found at least fair evidence that [the service] improves important health outcomes and concludes that benefits outweigh harms.) |
| RECOMMENDATION: |
| C Language: The USPSTF makes no recommendation for or against routine provision of [the service]. (The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of the benefits and harms is too close to justify a general recommendation.) |
| RECOMMENDATION: |
| D Language: The USPSTF recommends against routinely providing [the service] to asymptomatic patients. (The USPSTF found at least fair evidence that [the service] is ineffective or that harms outweigh benefits.) |
| RECOMMENDATION: |
| I Language: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing [the service]. (Evidence that [the service] is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined.) |
TABLE 2
USPSTF recommendations made in 2004
| A Recommendation (strongly recommends) |
|
| B Recommendation (recommends) |
|
| D Recommendation (recommends against) |
|
| I Recommendation (insufficient evidence) |
|
Recommendations for 2005
So far in 2005, new recommendations have been added on 3 topics: abdominal aortic aneurisms, glaucoma, and herpes simplex.
Abdominal aortic aneurisms. The recommendations on screening for abdominal aortic aneurisms are contained in TABLE 3. Of special note is the recommendation to screen (using abdominal ultrasound) men over the age of 65 years who have ever smoked.
Glaucoma. The statement that evidence is insufficient to recommend for or against routinely screening for glaucoma reflects the uncertainty about the contribution of screening to improved outcomes, as well as the documented harms of treating elevated intraocular pressure, such as local eye irritation and an increased risk for cataracts.
Herpes simplex. The task force recommends against screening for herpes in pregnant women and asymptomatic adults and adolescents because of a lack of improved outcomes and documented potential harms.
TABLE 3
USPSTF 2005 recommendations for screening for abdominal aortic aneurisms
| The USPSTF recommends one-time screening for abdominal aortic aneurysm (AAA) by ultrasonography in men aged 65 to 75 who have ever smoked. |
| RATING: B RECOMMENDATION |
| Rationale: The USPSTF found good evidence that screening for AAA and surgical repair of large AAAs (5.5 cm or more) in men aged 65 to 75 who have ever smoked (current and former smokers) leads to decreased AAA-specific mortality. There is good evidence that abdominal ultrasonography, performed in a setting with adequate quality assurance (ie, in an accredited facility with credentialed technologists), is an accurate screening test for AAA. There is also good evidence of important harms of screening and early treatment, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. Based on the moderate magnitude of net benefit, the USPSTF concluded that the benefits of screening for AAA in men aged 65 to 75 who have ever smoked outweigh the harms. |
| The USPSTF makes no recommendation for or against screening for AAA in men aged 65 to 75 who have never smoked. |
| RATING: C RECOMMENDATION. |
| Rationale: The USPSTF found good evidence that screening for AAA in men aged 65 to 75 who have never smoked leads to decreased AAA-specific mortality. There is, however, a lower prevalence of large AAAs in men who have never smoked compared with men who have ever smoked; thus, the potential benefit from screening men who have never smoked is small. There is good evidence that screening and early treatment leads to important harms, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. The USPSTF concluded that the balance between the benefits and harms of screening for AAA is too close to make a general recommendation in this population. |
| The USPSTF recommends against routine screening for AAA in women. |
| RATING: D RECOMMENDATION. |
| Rationale: Because of the low prevalence of large AAAs in women, the number of AAA-related deaths that can be prevented by screening this population is small. There is good evidence that screening and early treatment result in important harms, including an increased number of surgeries with associated morbidity and mortality, and psychological harms. The USPSTF concluded that the harms of screening women for AAA outweigh the benefits. |
USPSTF The Gold Standard
The USPSTF offers busy practicing physicians a valuable set of resources to assist in staying current on the ever changing field of clinical prevention and to guide clinical practice. Their recommendations often are at odds with common beliefs. But over time, their methodology and resulting recommendations have become the gold standard for evidence-based prevention practice.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
Meningococcal vaccine: New product, new recommendations
The Centers for Disease Control and Prevention (CDC) now recommends that all adolescents aged 11 to 12 years receive a quadrivalent, conjugate meningococcal vaccine (MCV-4). With time, universal administration of meningococcal vaccine for this age group will make moot the question of whether entering college freshmen should receive meningococcal vaccine. It should lead to a marked reduction in a potentially catastrophic disease and contribute to the continued decline in morbidity and mortality from bacterial meningitis in the United States, which has largely been due to advances in vaccine technology.
Some 1400 to 2800 cases of meningococcal infection occur in the US each year.1 The infection has a 10% to 14% fatality rate,1 and 11% to 19% of survivors are left with serious sequelae such as hearing loss, neurological problems, and mental deficits.
Risk of infection varies by age (FIGURE). At highest risk are those under 1 year, with rates of between 5–15/100,000.
Five serogroups of the bacteria are important causes of disease: A, B, C, Y, and W-135. In the US, most disease is caused by groups B, C, and Y; about a third by each.
FIGURE
Meningococcal disease rates by age and burden of disease (United States, 1992–2002)
New vaccine lasts longer
Fortunately, some meningococcal infections are vaccine-preventable. The new quadrivalent conjugate vaccine (Menactra) has been approved for persons aged 11–55 years. It offers protection against the same serogroups (A, C, Y, and W-135) as the older polysaccharide vaccine (Menomune). However, whereas immunity from the polysaccharide vaccine wanes after about 3 to 5 years, the newer vaccine is expected to provide much longer immunity (though experience with the conjugate product has not accumulated).
Who should receive the vaccine?
Until the cohort of current 11- to 12-year-olds being vaccinated reaches high school and college, the CDC recommends universal administration of the vaccine to those entering high school and to college freshmen living in dormitories. Those for whom the vaccine is recommended who have received the polysaccharide vaccine more than 3 years previously should be revaccinated with conjugate vaccine.
Meningococcal vaccine has also been recommended for those in high-risk groups: those with anatomical or functional asplenia, those with terminal complement component deficiency, laboratory and research personnel with potential exposure to meningococci, and travelers to areas with endemic meningococcal disease.2
Meningococcal vaccine is also used in the military and to control outbreaks, defined as 3 or more cases resulting in a case rate of 10/100,000 in a 3-month period. College freshmen who live in dormitories are at higher risk for meningococcal infection and the recommendation has been to discuss the risks and benefits of vaccine with those in this category.
Caveats
The new vaccine is contraindicated for those with allergy to latex since that substance is used in the vial stopper. Adverse reactions have been mild; redness, pain and swelling at the injection site, headache, and malaise. As with all new products, physicians should be alert for previously unreported adverse reactions and report suspected reactions to the Vaccine Adverse Event Reporting System (www.vaers.org/).
Unresolved issues
These new meningococcal vaccine recommendations leave several issues to be resolved with time.
Will there be enough vaccine? Since new vaccine recommendations for vaccination take time to become universal in actual practice, it is expected that the supply of the vaccine will keep up with demand.
What will be the fate of the old polysaccharide vaccine? The manufacturer intends for the new conjugate vaccine to replace the polysaccharide vaccine, especially if the license can be expanded to include other age groups. Until that occurs, the polysaccharide vaccine is the only product available for those at risk who are aged 2 to 10 years or aged more than 55 years.
Will a booster dose be needed? The full duration of protection from the new vaccine is not currently known but is expected to be at least 10 years. This will protect adolescents and young adults through the highest-risk periods. Whether a booster will eventually be recommended will depend on information gathered in the next several years.
Will the license for the new vaccine be extended to a larger age group? It is anticipated that with time the license for the conjugate vaccine will be expanded to include other age groups, particularly children under age 11.
What about children under age 2? There is currently no meningococcal vaccine proven effective for children in this age group. More than 50% of invasive meningococcal disease in this age group is caused by serogroup B. Neither meningococcal vaccine offers protection against this serogroup.
Chemoprophylaxis of contacts
The universal use of conjugate meningococcal vaccine should lead to a marked decrease in the number of meningococcal infections. However, physicians should keep in mind that close contacts of patients with meningococcal infections should be given one of the antibiotic regimens described in the TABLE within 24 hours of confirming the disease. Close contacts include household members, daycare center cohorts, and those directly exposed to the patients’ oral secretions through kissing, mouth-to-mouth resuscitation, and intubations.
Patients treated for meningococcemia with other than a third-generation cephalosporin should also be treated with one of the regimens in the TABLE since other antibiotics have not been shown to eradicate nasopharyngeal carriage.
An expected result of conjugate vaccine is to decrease the nasopharyngeal carriage of Neisseria meningitidis. It is unknown if close contacts who have been vaccinated will benefit from chemoprophylaxis.
TABLE
Schedule for administering chemoprophylaxis against meningococcal disease
| DRUG | AGE GROUP | DOSAGE | DURATION/ROUTE OF ADMINISTRATION* |
|---|---|---|---|
| Rifampin† | Children <1 mo | 5 mg/kg every 12 h | 2 days |
| Children ≥1 mo | 10 mg/kg every 12 h | 2 days | |
| Adults | 600 mg every 12 h | 2 days | |
| Ciprofloxacin§ | Adults | 500 mg | Single dose |
| Ceftriaxone | Children <15 yrs | 125 mg | Single intramuscular dose |
| Ceftriaxone | Adults | 250 mg | Single intramuscular dose |
| *Oral administration unless indicated otherwise. †Rifampin is not recommended for pregnant women because the drug is teratogenic in laboratory animals. Because the reliability of oral contraceptives may be affected by rifampin therapy, consideration should be given to using alternative contraceptive measures while rifampin is being administered. §Ciprofloxacin is not generally recommended for persons aged <18 years or for pregnant and lactating women because the drug causes cartilage damage for immature laboratory animals. However, ciprofloxacin can be used for chemoprophylaxis of children when no acceptable alternative therapy is available. | |||
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. MMWR 2005; in press.
2. CDC. National Center for Infectious Diseases. Traveler’s Health web site. Health information for international travel: meningococcal disease. Available at: www.cdc.gov/travel/diseases/menin.htm. Map of African meningitis belt available at: www.cdc.gov/ travel/diseases/maps/menin_map.htm. Accessed on February 23, 2005.
The Centers for Disease Control and Prevention (CDC) now recommends that all adolescents aged 11 to 12 years receive a quadrivalent, conjugate meningococcal vaccine (MCV-4). With time, universal administration of meningococcal vaccine for this age group will make moot the question of whether entering college freshmen should receive meningococcal vaccine. It should lead to a marked reduction in a potentially catastrophic disease and contribute to the continued decline in morbidity and mortality from bacterial meningitis in the United States, which has largely been due to advances in vaccine technology.
Some 1400 to 2800 cases of meningococcal infection occur in the US each year.1 The infection has a 10% to 14% fatality rate,1 and 11% to 19% of survivors are left with serious sequelae such as hearing loss, neurological problems, and mental deficits.
Risk of infection varies by age (FIGURE). At highest risk are those under 1 year, with rates of between 5–15/100,000.
Five serogroups of the bacteria are important causes of disease: A, B, C, Y, and W-135. In the US, most disease is caused by groups B, C, and Y; about a third by each.
FIGURE
Meningococcal disease rates by age and burden of disease (United States, 1992–2002)
New vaccine lasts longer
Fortunately, some meningococcal infections are vaccine-preventable. The new quadrivalent conjugate vaccine (Menactra) has been approved for persons aged 11–55 years. It offers protection against the same serogroups (A, C, Y, and W-135) as the older polysaccharide vaccine (Menomune). However, whereas immunity from the polysaccharide vaccine wanes after about 3 to 5 years, the newer vaccine is expected to provide much longer immunity (though experience with the conjugate product has not accumulated).
Who should receive the vaccine?
Until the cohort of current 11- to 12-year-olds being vaccinated reaches high school and college, the CDC recommends universal administration of the vaccine to those entering high school and to college freshmen living in dormitories. Those for whom the vaccine is recommended who have received the polysaccharide vaccine more than 3 years previously should be revaccinated with conjugate vaccine.
Meningococcal vaccine has also been recommended for those in high-risk groups: those with anatomical or functional asplenia, those with terminal complement component deficiency, laboratory and research personnel with potential exposure to meningococci, and travelers to areas with endemic meningococcal disease.2
Meningococcal vaccine is also used in the military and to control outbreaks, defined as 3 or more cases resulting in a case rate of 10/100,000 in a 3-month period. College freshmen who live in dormitories are at higher risk for meningococcal infection and the recommendation has been to discuss the risks and benefits of vaccine with those in this category.
Caveats
The new vaccine is contraindicated for those with allergy to latex since that substance is used in the vial stopper. Adverse reactions have been mild; redness, pain and swelling at the injection site, headache, and malaise. As with all new products, physicians should be alert for previously unreported adverse reactions and report suspected reactions to the Vaccine Adverse Event Reporting System (www.vaers.org/).
Unresolved issues
These new meningococcal vaccine recommendations leave several issues to be resolved with time.
Will there be enough vaccine? Since new vaccine recommendations for vaccination take time to become universal in actual practice, it is expected that the supply of the vaccine will keep up with demand.
What will be the fate of the old polysaccharide vaccine? The manufacturer intends for the new conjugate vaccine to replace the polysaccharide vaccine, especially if the license can be expanded to include other age groups. Until that occurs, the polysaccharide vaccine is the only product available for those at risk who are aged 2 to 10 years or aged more than 55 years.
Will a booster dose be needed? The full duration of protection from the new vaccine is not currently known but is expected to be at least 10 years. This will protect adolescents and young adults through the highest-risk periods. Whether a booster will eventually be recommended will depend on information gathered in the next several years.
Will the license for the new vaccine be extended to a larger age group? It is anticipated that with time the license for the conjugate vaccine will be expanded to include other age groups, particularly children under age 11.
What about children under age 2? There is currently no meningococcal vaccine proven effective for children in this age group. More than 50% of invasive meningococcal disease in this age group is caused by serogroup B. Neither meningococcal vaccine offers protection against this serogroup.
Chemoprophylaxis of contacts
The universal use of conjugate meningococcal vaccine should lead to a marked decrease in the number of meningococcal infections. However, physicians should keep in mind that close contacts of patients with meningococcal infections should be given one of the antibiotic regimens described in the TABLE within 24 hours of confirming the disease. Close contacts include household members, daycare center cohorts, and those directly exposed to the patients’ oral secretions through kissing, mouth-to-mouth resuscitation, and intubations.
Patients treated for meningococcemia with other than a third-generation cephalosporin should also be treated with one of the regimens in the TABLE since other antibiotics have not been shown to eradicate nasopharyngeal carriage.
An expected result of conjugate vaccine is to decrease the nasopharyngeal carriage of Neisseria meningitidis. It is unknown if close contacts who have been vaccinated will benefit from chemoprophylaxis.
TABLE
Schedule for administering chemoprophylaxis against meningococcal disease
| DRUG | AGE GROUP | DOSAGE | DURATION/ROUTE OF ADMINISTRATION* |
|---|---|---|---|
| Rifampin† | Children <1 mo | 5 mg/kg every 12 h | 2 days |
| Children ≥1 mo | 10 mg/kg every 12 h | 2 days | |
| Adults | 600 mg every 12 h | 2 days | |
| Ciprofloxacin§ | Adults | 500 mg | Single dose |
| Ceftriaxone | Children <15 yrs | 125 mg | Single intramuscular dose |
| Ceftriaxone | Adults | 250 mg | Single intramuscular dose |
| *Oral administration unless indicated otherwise. †Rifampin is not recommended for pregnant women because the drug is teratogenic in laboratory animals. Because the reliability of oral contraceptives may be affected by rifampin therapy, consideration should be given to using alternative contraceptive measures while rifampin is being administered. §Ciprofloxacin is not generally recommended for persons aged <18 years or for pregnant and lactating women because the drug causes cartilage damage for immature laboratory animals. However, ciprofloxacin can be used for chemoprophylaxis of children when no acceptable alternative therapy is available. | |||
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected].
The Centers for Disease Control and Prevention (CDC) now recommends that all adolescents aged 11 to 12 years receive a quadrivalent, conjugate meningococcal vaccine (MCV-4). With time, universal administration of meningococcal vaccine for this age group will make moot the question of whether entering college freshmen should receive meningococcal vaccine. It should lead to a marked reduction in a potentially catastrophic disease and contribute to the continued decline in morbidity and mortality from bacterial meningitis in the United States, which has largely been due to advances in vaccine technology.
Some 1400 to 2800 cases of meningococcal infection occur in the US each year.1 The infection has a 10% to 14% fatality rate,1 and 11% to 19% of survivors are left with serious sequelae such as hearing loss, neurological problems, and mental deficits.
Risk of infection varies by age (FIGURE). At highest risk are those under 1 year, with rates of between 5–15/100,000.
Five serogroups of the bacteria are important causes of disease: A, B, C, Y, and W-135. In the US, most disease is caused by groups B, C, and Y; about a third by each.
FIGURE
Meningococcal disease rates by age and burden of disease (United States, 1992–2002)
New vaccine lasts longer
Fortunately, some meningococcal infections are vaccine-preventable. The new quadrivalent conjugate vaccine (Menactra) has been approved for persons aged 11–55 years. It offers protection against the same serogroups (A, C, Y, and W-135) as the older polysaccharide vaccine (Menomune). However, whereas immunity from the polysaccharide vaccine wanes after about 3 to 5 years, the newer vaccine is expected to provide much longer immunity (though experience with the conjugate product has not accumulated).
Who should receive the vaccine?
Until the cohort of current 11- to 12-year-olds being vaccinated reaches high school and college, the CDC recommends universal administration of the vaccine to those entering high school and to college freshmen living in dormitories. Those for whom the vaccine is recommended who have received the polysaccharide vaccine more than 3 years previously should be revaccinated with conjugate vaccine.
Meningococcal vaccine has also been recommended for those in high-risk groups: those with anatomical or functional asplenia, those with terminal complement component deficiency, laboratory and research personnel with potential exposure to meningococci, and travelers to areas with endemic meningococcal disease.2
Meningococcal vaccine is also used in the military and to control outbreaks, defined as 3 or more cases resulting in a case rate of 10/100,000 in a 3-month period. College freshmen who live in dormitories are at higher risk for meningococcal infection and the recommendation has been to discuss the risks and benefits of vaccine with those in this category.
Caveats
The new vaccine is contraindicated for those with allergy to latex since that substance is used in the vial stopper. Adverse reactions have been mild; redness, pain and swelling at the injection site, headache, and malaise. As with all new products, physicians should be alert for previously unreported adverse reactions and report suspected reactions to the Vaccine Adverse Event Reporting System (www.vaers.org/).
Unresolved issues
These new meningococcal vaccine recommendations leave several issues to be resolved with time.
Will there be enough vaccine? Since new vaccine recommendations for vaccination take time to become universal in actual practice, it is expected that the supply of the vaccine will keep up with demand.
What will be the fate of the old polysaccharide vaccine? The manufacturer intends for the new conjugate vaccine to replace the polysaccharide vaccine, especially if the license can be expanded to include other age groups. Until that occurs, the polysaccharide vaccine is the only product available for those at risk who are aged 2 to 10 years or aged more than 55 years.
Will a booster dose be needed? The full duration of protection from the new vaccine is not currently known but is expected to be at least 10 years. This will protect adolescents and young adults through the highest-risk periods. Whether a booster will eventually be recommended will depend on information gathered in the next several years.
Will the license for the new vaccine be extended to a larger age group? It is anticipated that with time the license for the conjugate vaccine will be expanded to include other age groups, particularly children under age 11.
What about children under age 2? There is currently no meningococcal vaccine proven effective for children in this age group. More than 50% of invasive meningococcal disease in this age group is caused by serogroup B. Neither meningococcal vaccine offers protection against this serogroup.
Chemoprophylaxis of contacts
The universal use of conjugate meningococcal vaccine should lead to a marked decrease in the number of meningococcal infections. However, physicians should keep in mind that close contacts of patients with meningococcal infections should be given one of the antibiotic regimens described in the TABLE within 24 hours of confirming the disease. Close contacts include household members, daycare center cohorts, and those directly exposed to the patients’ oral secretions through kissing, mouth-to-mouth resuscitation, and intubations.
Patients treated for meningococcemia with other than a third-generation cephalosporin should also be treated with one of the regimens in the TABLE since other antibiotics have not been shown to eradicate nasopharyngeal carriage.
An expected result of conjugate vaccine is to decrease the nasopharyngeal carriage of Neisseria meningitidis. It is unknown if close contacts who have been vaccinated will benefit from chemoprophylaxis.
TABLE
Schedule for administering chemoprophylaxis against meningococcal disease
| DRUG | AGE GROUP | DOSAGE | DURATION/ROUTE OF ADMINISTRATION* |
|---|---|---|---|
| Rifampin† | Children <1 mo | 5 mg/kg every 12 h | 2 days |
| Children ≥1 mo | 10 mg/kg every 12 h | 2 days | |
| Adults | 600 mg every 12 h | 2 days | |
| Ciprofloxacin§ | Adults | 500 mg | Single dose |
| Ceftriaxone | Children <15 yrs | 125 mg | Single intramuscular dose |
| Ceftriaxone | Adults | 250 mg | Single intramuscular dose |
| *Oral administration unless indicated otherwise. †Rifampin is not recommended for pregnant women because the drug is teratogenic in laboratory animals. Because the reliability of oral contraceptives may be affected by rifampin therapy, consideration should be given to using alternative contraceptive measures while rifampin is being administered. §Ciprofloxacin is not generally recommended for persons aged <18 years or for pregnant and lactating women because the drug causes cartilage damage for immature laboratory animals. However, ciprofloxacin can be used for chemoprophylaxis of children when no acceptable alternative therapy is available. | |||
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. MMWR 2005; in press.
2. CDC. National Center for Infectious Diseases. Traveler’s Health web site. Health information for international travel: meningococcal disease. Available at: www.cdc.gov/travel/diseases/menin.htm. Map of African meningitis belt available at: www.cdc.gov/ travel/diseases/maps/menin_map.htm. Accessed on February 23, 2005.
1. Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. MMWR 2005; in press.
2. CDC. National Center for Infectious Diseases. Traveler’s Health web site. Health information for international travel: meningococcal disease. Available at: www.cdc.gov/travel/diseases/menin.htm. Map of African meningitis belt available at: www.cdc.gov/ travel/diseases/maps/menin_map.htm. Accessed on February 23, 2005.
Non-consented IUD placement reported by Mexican immigrants: A caution for caregivers in the US?
Published reports of non-consented contraceptive practices in Mexico,1-3 including intrauterine device (IUD) placement, have been largely anecdotal and have not been systematically validated. The Family Planning Clinic of the Maricopa County Department of Public Health predominantly serves a Mexican immigrant population. Providers in the clinic have also reported hearing about non-consented IUD placement. To investigate this issue, 466 women between July 1, 2000, and July 1, 2002, were recruited to answer a survey. This sample of convenience represented 29% of new clients during this period. Informed consent was obtained from each participant and no woman refused to participate. The study was IRB-approved. The survey was translated into Spanish and delivered orally by bilingual interviewers.
The mean age of the participants was 27.2 years, and the mean number of prior pregnancies was 2.6. One hundred eighty-eight women (40%) reported receiving gynecologic health care in Mexico from 1 month to 20 years previously (mean of 6.1 years). One hundred four women reported having an IUD placed at some time, including 85 of those who had received care in Mexico (46%). Of these, 23 women reported having an IUD inserted without their knowledge or consent. All 23 reported that the IUD had been placed while receiving care in Mexico, representing 27 % of those who had received an IUD there. Twenty-one of the 23 women said the IUD had been placed immediately after they had given birth, and 2 while seeking family planning services. Sixteen reported that the IUD insertion occurred at a hospital, while 4 said it happened at a clinic; 3 did not respond to this question.
Five of the women realized within days that an IUD had been placed; 3 within weeks, 4 within months, and 9 did not find out for a year or more; 5 did not respond. Three felt a string, 3 said the IUD fell out, 7 reported that a second healthcare worker discovered it, 2 were informed later by the person who placed the IUD, and 4 had adverse symptoms (2 from infection). Of 19 responding, 2 said they had the IUD taken out the same day it was discovered, 4 about a month later, 5 about a year later, 5 one to five years later, 1 five to ten years later, and 2 still had the IUD in place. Sixteen provinces in Mexico were listed as the location where the IUD placement occurred.
A significant percentage of women of reproductive age from Mexico served by this border area family planning clinic reported that they had an IUD placed without their knowledge or consent in Mexico. It is possible that these women were actually informed about the procedure, and just did not fully understand it. We were not able to investigate if this practice occurs in other countries or if it affects women of all socioeconomic classes in Mexico.
It is not clear if the women who did not have the IUD removed chose to keep it as their contraceptive method of choice or because they lacked access to health care to have it removed. It is possible that many of them would have chosen an IUD had the option been presented to them.
Since our sample was one of convenience in a busy public clinic, the possibility for selection bias exists. Therefore these findings are very preliminary and need to be verified in larger, better controlled studies. However, all those who provide healthcare services to women immigrants from Mexico should add the possibility of complications from an unrecognized IUD to the differential diagnosis if patients present with pelvic or abdominal pain, pelvic infections, or infertility.
Corresponding author
Doug Campos-Outcalt, MD, MPA, 4001 North 3rd Street, Phoenix, AZ 85012. E-mail: [email protected].
1. Kirsch JD, Cedeño MA. Informed consent for family planning for poor women in Chiapas, Mexico. Lancet 1999;354 (9176):419-420.
2. Diebel L. Mexico’s Indians target of sterilization ‘sweep’. The Toronto Star Latin America Bureau. Toronto Star, March 26, 2000. [cited 2002, Dec 14]; [6 screens]. Available at: www.thestar.com/thestar/back_issues/fsED20000326/news/20000326NEW01c_FO-DIEBEL.html. Accessed on February 8, 2005.
3. Dirección general de salud maternoinfantil, la mujer adolescente adulta, anciana y su salud. Mexico City: Secretaria de Salud, 1992.
Published reports of non-consented contraceptive practices in Mexico,1-3 including intrauterine device (IUD) placement, have been largely anecdotal and have not been systematically validated. The Family Planning Clinic of the Maricopa County Department of Public Health predominantly serves a Mexican immigrant population. Providers in the clinic have also reported hearing about non-consented IUD placement. To investigate this issue, 466 women between July 1, 2000, and July 1, 2002, were recruited to answer a survey. This sample of convenience represented 29% of new clients during this period. Informed consent was obtained from each participant and no woman refused to participate. The study was IRB-approved. The survey was translated into Spanish and delivered orally by bilingual interviewers.
The mean age of the participants was 27.2 years, and the mean number of prior pregnancies was 2.6. One hundred eighty-eight women (40%) reported receiving gynecologic health care in Mexico from 1 month to 20 years previously (mean of 6.1 years). One hundred four women reported having an IUD placed at some time, including 85 of those who had received care in Mexico (46%). Of these, 23 women reported having an IUD inserted without their knowledge or consent. All 23 reported that the IUD had been placed while receiving care in Mexico, representing 27 % of those who had received an IUD there. Twenty-one of the 23 women said the IUD had been placed immediately after they had given birth, and 2 while seeking family planning services. Sixteen reported that the IUD insertion occurred at a hospital, while 4 said it happened at a clinic; 3 did not respond to this question.
Five of the women realized within days that an IUD had been placed; 3 within weeks, 4 within months, and 9 did not find out for a year or more; 5 did not respond. Three felt a string, 3 said the IUD fell out, 7 reported that a second healthcare worker discovered it, 2 were informed later by the person who placed the IUD, and 4 had adverse symptoms (2 from infection). Of 19 responding, 2 said they had the IUD taken out the same day it was discovered, 4 about a month later, 5 about a year later, 5 one to five years later, 1 five to ten years later, and 2 still had the IUD in place. Sixteen provinces in Mexico were listed as the location where the IUD placement occurred.
A significant percentage of women of reproductive age from Mexico served by this border area family planning clinic reported that they had an IUD placed without their knowledge or consent in Mexico. It is possible that these women were actually informed about the procedure, and just did not fully understand it. We were not able to investigate if this practice occurs in other countries or if it affects women of all socioeconomic classes in Mexico.
It is not clear if the women who did not have the IUD removed chose to keep it as their contraceptive method of choice or because they lacked access to health care to have it removed. It is possible that many of them would have chosen an IUD had the option been presented to them.
Since our sample was one of convenience in a busy public clinic, the possibility for selection bias exists. Therefore these findings are very preliminary and need to be verified in larger, better controlled studies. However, all those who provide healthcare services to women immigrants from Mexico should add the possibility of complications from an unrecognized IUD to the differential diagnosis if patients present with pelvic or abdominal pain, pelvic infections, or infertility.
Corresponding author
Doug Campos-Outcalt, MD, MPA, 4001 North 3rd Street, Phoenix, AZ 85012. E-mail: [email protected].
Published reports of non-consented contraceptive practices in Mexico,1-3 including intrauterine device (IUD) placement, have been largely anecdotal and have not been systematically validated. The Family Planning Clinic of the Maricopa County Department of Public Health predominantly serves a Mexican immigrant population. Providers in the clinic have also reported hearing about non-consented IUD placement. To investigate this issue, 466 women between July 1, 2000, and July 1, 2002, were recruited to answer a survey. This sample of convenience represented 29% of new clients during this period. Informed consent was obtained from each participant and no woman refused to participate. The study was IRB-approved. The survey was translated into Spanish and delivered orally by bilingual interviewers.
The mean age of the participants was 27.2 years, and the mean number of prior pregnancies was 2.6. One hundred eighty-eight women (40%) reported receiving gynecologic health care in Mexico from 1 month to 20 years previously (mean of 6.1 years). One hundred four women reported having an IUD placed at some time, including 85 of those who had received care in Mexico (46%). Of these, 23 women reported having an IUD inserted without their knowledge or consent. All 23 reported that the IUD had been placed while receiving care in Mexico, representing 27 % of those who had received an IUD there. Twenty-one of the 23 women said the IUD had been placed immediately after they had given birth, and 2 while seeking family planning services. Sixteen reported that the IUD insertion occurred at a hospital, while 4 said it happened at a clinic; 3 did not respond to this question.
Five of the women realized within days that an IUD had been placed; 3 within weeks, 4 within months, and 9 did not find out for a year or more; 5 did not respond. Three felt a string, 3 said the IUD fell out, 7 reported that a second healthcare worker discovered it, 2 were informed later by the person who placed the IUD, and 4 had adverse symptoms (2 from infection). Of 19 responding, 2 said they had the IUD taken out the same day it was discovered, 4 about a month later, 5 about a year later, 5 one to five years later, 1 five to ten years later, and 2 still had the IUD in place. Sixteen provinces in Mexico were listed as the location where the IUD placement occurred.
A significant percentage of women of reproductive age from Mexico served by this border area family planning clinic reported that they had an IUD placed without their knowledge or consent in Mexico. It is possible that these women were actually informed about the procedure, and just did not fully understand it. We were not able to investigate if this practice occurs in other countries or if it affects women of all socioeconomic classes in Mexico.
It is not clear if the women who did not have the IUD removed chose to keep it as their contraceptive method of choice or because they lacked access to health care to have it removed. It is possible that many of them would have chosen an IUD had the option been presented to them.
Since our sample was one of convenience in a busy public clinic, the possibility for selection bias exists. Therefore these findings are very preliminary and need to be verified in larger, better controlled studies. However, all those who provide healthcare services to women immigrants from Mexico should add the possibility of complications from an unrecognized IUD to the differential diagnosis if patients present with pelvic or abdominal pain, pelvic infections, or infertility.
Corresponding author
Doug Campos-Outcalt, MD, MPA, 4001 North 3rd Street, Phoenix, AZ 85012. E-mail: [email protected].
1. Kirsch JD, Cedeño MA. Informed consent for family planning for poor women in Chiapas, Mexico. Lancet 1999;354 (9176):419-420.
2. Diebel L. Mexico’s Indians target of sterilization ‘sweep’. The Toronto Star Latin America Bureau. Toronto Star, March 26, 2000. [cited 2002, Dec 14]; [6 screens]. Available at: www.thestar.com/thestar/back_issues/fsED20000326/news/20000326NEW01c_FO-DIEBEL.html. Accessed on February 8, 2005.
3. Dirección general de salud maternoinfantil, la mujer adolescente adulta, anciana y su salud. Mexico City: Secretaria de Salud, 1992.
1. Kirsch JD, Cedeño MA. Informed consent for family planning for poor women in Chiapas, Mexico. Lancet 1999;354 (9176):419-420.
2. Diebel L. Mexico’s Indians target of sterilization ‘sweep’. The Toronto Star Latin America Bureau. Toronto Star, March 26, 2000. [cited 2002, Dec 14]; [6 screens]. Available at: www.thestar.com/thestar/back_issues/fsED20000326/news/20000326NEW01c_FO-DIEBEL.html. Accessed on February 8, 2005.
3. Dirección general de salud maternoinfantil, la mujer adolescente adulta, anciana y su salud. Mexico City: Secretaria de Salud, 1992.
Cause-of-death certification: Not as easy as it seems
Think you know how to fill out a death certificate? It’s often not as easy as it seems. Try the following cases.
Case 1
A 68-year-old woman is admitted to the ICU because of acute chest pain. She has a history of type 2 diabetes, hypertension, obesity, and angina. Over the next 24 hours an acute myocardial infarction is confirmed. Heart failure develops but improves with medical management. The patient then experiences a pulmonary embolus, confirmed by ventilation-perfusion lung scan and blood gases; over the next 2 hours she becomes unresponsive and dies.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: pulmonary embolus due to acute myocardial infarction due to atherosclerotic heart disease.
Question: What should be listed as conditions contributing to death but not directly causing death? Answer: type 2 diabetes, obesity, hypertension, and congestive heart failure.
Case 2
A 78-year-old woman has left hemiparesis from a stroke 2 years earlier. She has been unable to care for herself and has lived in a nursing home. She has had an indwelling urinary catheter for the past 6 months. Because of fever, increased leukocyte count, and pyuria, she is admitted to the hospital and started on 2 antibiotics. Two days later, the blood culture result is positive for Pseudomonas aeruginosa resistant to the antibiotics being administered. Despite a change of antibiotics, hypotension ensues and the patient dies on hospital day 4.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: P aeruginosa sepsis, due to a urinary tract infection due to an indwelling catheter, due to left hemiparesis, due to an old cerebral infarction.
Question: What should be listed as conditions that contributed to the death but that did not directly cause the death? Answer: nothing.
If you were correct on both cases, congratulations. If you were not, this article offers basic advice that will help you provide accurate medical information on death certificates.
Death certificates are important official records used for personal, legal, and public health purposes, yet they are frequently filled out inaccurately. Physicians are responsible for determining the cause and manner of death, yet they are seldom formally trained for this responsibility in medical school or residency. The result is frequent and avoidable errors.
Who is responsible for what?
Registration of deaths is a state responsibility. The National Center for Health Statistics compiles data from all states to produce national vital statistics, and most states use death certificate forms that conform to a recommended national standard. Though funeral directors are responsible for filing the certificate with the state, physicians are responsible for completing the medical portion of the certificate.
With the medical information provided, trained coders classify the cause of death using standardized methodology.
Medical examiners or coroners are responsible for investigating and certifying the cause of any death that is unexpected, unexplained, or resulting from injury, poisoning, or a public health threat.
Physicians are additionally responsible for answering inquiries from the registrar (these inquiries can be reduced by accurately and completely filling in the medical information) and submitting a supplemental report when autopsy findings or other information indicates a cause of death different from that originally reported.
How to complete the medical portion of the death certificate
The Figure is a standard certificate of death. It may vary slightly state to state. Physicians are responsible for items 24 through 49. If the state requires a pronouncing physician (Table 1), the pronouncing and certifying physicians may be different, in which case the pronouncing physician completes items 24 through 31 and the certifying physician items 32 through 49. If the pronouncing physician is also the certifying physician, items 26 through 28 need not be completed. If the death is referred to the coroner or medical examiner, they complete items 24, 25, 29, 30, and 32 through 49.
FIGURE
US standard death certificate
TABLE 1
Definitions
| Immediate cause of death:The final disease or injury causing the death. |
| Intermediate cause of death: A disease or condition that preceded and caused the immediate cause of death. |
| Underlying cause of death: A disease or condition present before, and leading to, the intermediate or immediate cause of death. It can be present for years before the death. |
| Manner of death: The circumstances leading to death—accident, homicide, suicide, unknown or undetermined, and natural causes. |
| Medical examiner: A physician, acting in an official capacity within a particular jurisdiction, charged with the investigation and examination of persons dying suddenly, unexpectedly, or violently, or whose death resulted from, or presents, a potential public health hazard. The medical examiner is not always required to be a specialist in death investigation or pathology. Most systems employing physicians as part time medical examiners encourage them to obtain training for medical examiners such as that offered by the National Association of Medical Examiners. |
| Coroner: A coroner is a public official, appointed or elected, in a particular geographic jurisdiction, whose official duty is to make inquiry into deaths in certain categories. In some jurisdictions, the coroner is a physician, but in many localities, the coroner is not required to be a physician nor be trained in medicine. |
| Pronouncing physician: The one who determines the decedent is legally dead. Not all states require a death to be pronounced by a physician. |
| Certifying physician: The one who certifies the cause of death. |
The most challenging part
Item 32, the Cause of Death, is the most difficult item to complete accurately. It consists of two parts. Part I is a sequential list of conditions leading to the immediate cause of death and the time interval between their onset and the death. Part II is a list of other conditions contributing to the death but not directly causing death. Thinking about the death as a sequence of events and reconstructing this sequence helps classify correctly the various illnesses and conditions the decedent might have had.
Immediate cause of death. Part I, line a, is for the immediate cause of death (see Table 1). This should be a disease, complication, or injury that directly caused the death. A common error is to list a mechanism of death (for example, cardiac arrest) rather than a disease (myocardial infarction).
Specific terms are better than vague ones. For instance,“cerebral infarction” is better than “stroke.” “Escherichia coli sepsis” is better than just “sepsis.”
When cancer is the cause of death, list the primary site, cell type, cancer grade, and specific organ or lobe affected.
Avoid terms without medical meaning, such as old age or senescence.
If additional information is expected from an autopsy, it is acceptable to list the cause of death as pending. But an update to certificate will be required once the additional information is obtained. It is also acceptable to list a cause as unknown. This will not automatically forward the case to the medical examiner.
Intermediate/underlying causes. Lines b, c, and d are for intermediate and underlying causes. Each condition listed should cause the one above it. You should be able to proceed logically from the underlying cause through each intermediate cause by saying the phrase “due to” or “as a consequence of,” moving from the lower line up through line b. There may be several intermediate causes. For example, a death may be due to a pulmonary embolus, as a consequence of hip surgery, resulting from a injury from a fall, resulting from a cerebral infarction. The underlying cause is the cerebral infarction.
Marking time intervals. To the right of lines a through d is space to write the time interval between the condition listed (immediate, intermediate, or underlying cause of death) and the time of death. The more precise the time the better. But it is understood that times must occasionally be estimated, and terms such as “approximately” are acceptable. If the time cannot be estimated, insert the phrase “unknown duration.” Something should be listed on this line next to the immediate, intermediate, and underlying conditions listed. No lines should be left blank.
Other illnesses. Part II is where to list other significant illnesses or conditions that may have contributed to the death but were not the direct causes of it. More than one condition may be listed. Many patients have multiple conditions and there may be uncertainty as to direct and contributing causes of the death. The physician is only expected to make the best judgment possible as to the most likely causes and sequences. Coders referring to international standards and rules will use the information to make a final classification of the underlying cause.
Specific errors to avoid
Table 2 includes some points to remember to avoid making errors when filling out the death certificate medical information. By following these rules, studying the cases provided in the Physicians’ Handbook on Medical Certification and Death, and systematically thinking about the sequence of events that caused the death, physicians can improve on their accuracy when performing the important and under appreciated role of accurately certifying the medical cause of death.
TABLE 2
Important points to remember when completing medical information on a death certificate
| Do not use abbreviations. |
| Do not use numbers for months; spell out the month. |
| Use a 24-hour clock (1600, not 4 P.M.). |
| Do not alter the document or erase any part of it. |
| Print legibly using black ink. |
| Complete all required items, do not leave them blank. If necessary, write “unknown.” |
| Do not delay completing the certification. The burial or other disposition of the remains depends on the correct completion of the certificate and its acceptance by the state or local registrar. |
| Do not complete the medical information if another physician has more knowledge of the circumstances, unless they are unavailable. |
Useful resources
The Physicians’ Handbook on Medical Certification of Death, published by the Centers for Disease Control, National Center for Health Statistics, is available at www.cdc.gov/nchs/data/misc/hb_cod.pdf. It contains instructions on how to complete a death certificate and a series of useful examples that take about a half hour to review.
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
Think you know how to fill out a death certificate? It’s often not as easy as it seems. Try the following cases.
Case 1
A 68-year-old woman is admitted to the ICU because of acute chest pain. She has a history of type 2 diabetes, hypertension, obesity, and angina. Over the next 24 hours an acute myocardial infarction is confirmed. Heart failure develops but improves with medical management. The patient then experiences a pulmonary embolus, confirmed by ventilation-perfusion lung scan and blood gases; over the next 2 hours she becomes unresponsive and dies.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: pulmonary embolus due to acute myocardial infarction due to atherosclerotic heart disease.
Question: What should be listed as conditions contributing to death but not directly causing death? Answer: type 2 diabetes, obesity, hypertension, and congestive heart failure.
Case 2
A 78-year-old woman has left hemiparesis from a stroke 2 years earlier. She has been unable to care for herself and has lived in a nursing home. She has had an indwelling urinary catheter for the past 6 months. Because of fever, increased leukocyte count, and pyuria, she is admitted to the hospital and started on 2 antibiotics. Two days later, the blood culture result is positive for Pseudomonas aeruginosa resistant to the antibiotics being administered. Despite a change of antibiotics, hypotension ensues and the patient dies on hospital day 4.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: P aeruginosa sepsis, due to a urinary tract infection due to an indwelling catheter, due to left hemiparesis, due to an old cerebral infarction.
Question: What should be listed as conditions that contributed to the death but that did not directly cause the death? Answer: nothing.
If you were correct on both cases, congratulations. If you were not, this article offers basic advice that will help you provide accurate medical information on death certificates.
Death certificates are important official records used for personal, legal, and public health purposes, yet they are frequently filled out inaccurately. Physicians are responsible for determining the cause and manner of death, yet they are seldom formally trained for this responsibility in medical school or residency. The result is frequent and avoidable errors.
Who is responsible for what?
Registration of deaths is a state responsibility. The National Center for Health Statistics compiles data from all states to produce national vital statistics, and most states use death certificate forms that conform to a recommended national standard. Though funeral directors are responsible for filing the certificate with the state, physicians are responsible for completing the medical portion of the certificate.
With the medical information provided, trained coders classify the cause of death using standardized methodology.
Medical examiners or coroners are responsible for investigating and certifying the cause of any death that is unexpected, unexplained, or resulting from injury, poisoning, or a public health threat.
Physicians are additionally responsible for answering inquiries from the registrar (these inquiries can be reduced by accurately and completely filling in the medical information) and submitting a supplemental report when autopsy findings or other information indicates a cause of death different from that originally reported.
How to complete the medical portion of the death certificate
The Figure is a standard certificate of death. It may vary slightly state to state. Physicians are responsible for items 24 through 49. If the state requires a pronouncing physician (Table 1), the pronouncing and certifying physicians may be different, in which case the pronouncing physician completes items 24 through 31 and the certifying physician items 32 through 49. If the pronouncing physician is also the certifying physician, items 26 through 28 need not be completed. If the death is referred to the coroner or medical examiner, they complete items 24, 25, 29, 30, and 32 through 49.
FIGURE
US standard death certificate
TABLE 1
Definitions
| Immediate cause of death:The final disease or injury causing the death. |
| Intermediate cause of death: A disease or condition that preceded and caused the immediate cause of death. |
| Underlying cause of death: A disease or condition present before, and leading to, the intermediate or immediate cause of death. It can be present for years before the death. |
| Manner of death: The circumstances leading to death—accident, homicide, suicide, unknown or undetermined, and natural causes. |
| Medical examiner: A physician, acting in an official capacity within a particular jurisdiction, charged with the investigation and examination of persons dying suddenly, unexpectedly, or violently, or whose death resulted from, or presents, a potential public health hazard. The medical examiner is not always required to be a specialist in death investigation or pathology. Most systems employing physicians as part time medical examiners encourage them to obtain training for medical examiners such as that offered by the National Association of Medical Examiners. |
| Coroner: A coroner is a public official, appointed or elected, in a particular geographic jurisdiction, whose official duty is to make inquiry into deaths in certain categories. In some jurisdictions, the coroner is a physician, but in many localities, the coroner is not required to be a physician nor be trained in medicine. |
| Pronouncing physician: The one who determines the decedent is legally dead. Not all states require a death to be pronounced by a physician. |
| Certifying physician: The one who certifies the cause of death. |
The most challenging part
Item 32, the Cause of Death, is the most difficult item to complete accurately. It consists of two parts. Part I is a sequential list of conditions leading to the immediate cause of death and the time interval between their onset and the death. Part II is a list of other conditions contributing to the death but not directly causing death. Thinking about the death as a sequence of events and reconstructing this sequence helps classify correctly the various illnesses and conditions the decedent might have had.
Immediate cause of death. Part I, line a, is for the immediate cause of death (see Table 1). This should be a disease, complication, or injury that directly caused the death. A common error is to list a mechanism of death (for example, cardiac arrest) rather than a disease (myocardial infarction).
Specific terms are better than vague ones. For instance,“cerebral infarction” is better than “stroke.” “Escherichia coli sepsis” is better than just “sepsis.”
When cancer is the cause of death, list the primary site, cell type, cancer grade, and specific organ or lobe affected.
Avoid terms without medical meaning, such as old age or senescence.
If additional information is expected from an autopsy, it is acceptable to list the cause of death as pending. But an update to certificate will be required once the additional information is obtained. It is also acceptable to list a cause as unknown. This will not automatically forward the case to the medical examiner.
Intermediate/underlying causes. Lines b, c, and d are for intermediate and underlying causes. Each condition listed should cause the one above it. You should be able to proceed logically from the underlying cause through each intermediate cause by saying the phrase “due to” or “as a consequence of,” moving from the lower line up through line b. There may be several intermediate causes. For example, a death may be due to a pulmonary embolus, as a consequence of hip surgery, resulting from a injury from a fall, resulting from a cerebral infarction. The underlying cause is the cerebral infarction.
Marking time intervals. To the right of lines a through d is space to write the time interval between the condition listed (immediate, intermediate, or underlying cause of death) and the time of death. The more precise the time the better. But it is understood that times must occasionally be estimated, and terms such as “approximately” are acceptable. If the time cannot be estimated, insert the phrase “unknown duration.” Something should be listed on this line next to the immediate, intermediate, and underlying conditions listed. No lines should be left blank.
Other illnesses. Part II is where to list other significant illnesses or conditions that may have contributed to the death but were not the direct causes of it. More than one condition may be listed. Many patients have multiple conditions and there may be uncertainty as to direct and contributing causes of the death. The physician is only expected to make the best judgment possible as to the most likely causes and sequences. Coders referring to international standards and rules will use the information to make a final classification of the underlying cause.
Specific errors to avoid
Table 2 includes some points to remember to avoid making errors when filling out the death certificate medical information. By following these rules, studying the cases provided in the Physicians’ Handbook on Medical Certification and Death, and systematically thinking about the sequence of events that caused the death, physicians can improve on their accuracy when performing the important and under appreciated role of accurately certifying the medical cause of death.
TABLE 2
Important points to remember when completing medical information on a death certificate
| Do not use abbreviations. |
| Do not use numbers for months; spell out the month. |
| Use a 24-hour clock (1600, not 4 P.M.). |
| Do not alter the document or erase any part of it. |
| Print legibly using black ink. |
| Complete all required items, do not leave them blank. If necessary, write “unknown.” |
| Do not delay completing the certification. The burial or other disposition of the remains depends on the correct completion of the certificate and its acceptance by the state or local registrar. |
| Do not complete the medical information if another physician has more knowledge of the circumstances, unless they are unavailable. |
Useful resources
The Physicians’ Handbook on Medical Certification of Death, published by the Centers for Disease Control, National Center for Health Statistics, is available at www.cdc.gov/nchs/data/misc/hb_cod.pdf. It contains instructions on how to complete a death certificate and a series of useful examples that take about a half hour to review.
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
Think you know how to fill out a death certificate? It’s often not as easy as it seems. Try the following cases.
Case 1
A 68-year-old woman is admitted to the ICU because of acute chest pain. She has a history of type 2 diabetes, hypertension, obesity, and angina. Over the next 24 hours an acute myocardial infarction is confirmed. Heart failure develops but improves with medical management. The patient then experiences a pulmonary embolus, confirmed by ventilation-perfusion lung scan and blood gases; over the next 2 hours she becomes unresponsive and dies.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: pulmonary embolus due to acute myocardial infarction due to atherosclerotic heart disease.
Question: What should be listed as conditions contributing to death but not directly causing death? Answer: type 2 diabetes, obesity, hypertension, and congestive heart failure.
Case 2
A 78-year-old woman has left hemiparesis from a stroke 2 years earlier. She has been unable to care for herself and has lived in a nursing home. She has had an indwelling urinary catheter for the past 6 months. Because of fever, increased leukocyte count, and pyuria, she is admitted to the hospital and started on 2 antibiotics. Two days later, the blood culture result is positive for Pseudomonas aeruginosa resistant to the antibiotics being administered. Despite a change of antibiotics, hypotension ensues and the patient dies on hospital day 4.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: P aeruginosa sepsis, due to a urinary tract infection due to an indwelling catheter, due to left hemiparesis, due to an old cerebral infarction.
Question: What should be listed as conditions that contributed to the death but that did not directly cause the death? Answer: nothing.
If you were correct on both cases, congratulations. If you were not, this article offers basic advice that will help you provide accurate medical information on death certificates.
Death certificates are important official records used for personal, legal, and public health purposes, yet they are frequently filled out inaccurately. Physicians are responsible for determining the cause and manner of death, yet they are seldom formally trained for this responsibility in medical school or residency. The result is frequent and avoidable errors.
Who is responsible for what?
Registration of deaths is a state responsibility. The National Center for Health Statistics compiles data from all states to produce national vital statistics, and most states use death certificate forms that conform to a recommended national standard. Though funeral directors are responsible for filing the certificate with the state, physicians are responsible for completing the medical portion of the certificate.
With the medical information provided, trained coders classify the cause of death using standardized methodology.
Medical examiners or coroners are responsible for investigating and certifying the cause of any death that is unexpected, unexplained, or resulting from injury, poisoning, or a public health threat.
Physicians are additionally responsible for answering inquiries from the registrar (these inquiries can be reduced by accurately and completely filling in the medical information) and submitting a supplemental report when autopsy findings or other information indicates a cause of death different from that originally reported.
How to complete the medical portion of the death certificate
The Figure is a standard certificate of death. It may vary slightly state to state. Physicians are responsible for items 24 through 49. If the state requires a pronouncing physician (Table 1), the pronouncing and certifying physicians may be different, in which case the pronouncing physician completes items 24 through 31 and the certifying physician items 32 through 49. If the pronouncing physician is also the certifying physician, items 26 through 28 need not be completed. If the death is referred to the coroner or medical examiner, they complete items 24, 25, 29, 30, and 32 through 49.
FIGURE
US standard death certificate
TABLE 1
Definitions
| Immediate cause of death:The final disease or injury causing the death. |
| Intermediate cause of death: A disease or condition that preceded and caused the immediate cause of death. |
| Underlying cause of death: A disease or condition present before, and leading to, the intermediate or immediate cause of death. It can be present for years before the death. |
| Manner of death: The circumstances leading to death—accident, homicide, suicide, unknown or undetermined, and natural causes. |
| Medical examiner: A physician, acting in an official capacity within a particular jurisdiction, charged with the investigation and examination of persons dying suddenly, unexpectedly, or violently, or whose death resulted from, or presents, a potential public health hazard. The medical examiner is not always required to be a specialist in death investigation or pathology. Most systems employing physicians as part time medical examiners encourage them to obtain training for medical examiners such as that offered by the National Association of Medical Examiners. |
| Coroner: A coroner is a public official, appointed or elected, in a particular geographic jurisdiction, whose official duty is to make inquiry into deaths in certain categories. In some jurisdictions, the coroner is a physician, but in many localities, the coroner is not required to be a physician nor be trained in medicine. |
| Pronouncing physician: The one who determines the decedent is legally dead. Not all states require a death to be pronounced by a physician. |
| Certifying physician: The one who certifies the cause of death. |
The most challenging part
Item 32, the Cause of Death, is the most difficult item to complete accurately. It consists of two parts. Part I is a sequential list of conditions leading to the immediate cause of death and the time interval between their onset and the death. Part II is a list of other conditions contributing to the death but not directly causing death. Thinking about the death as a sequence of events and reconstructing this sequence helps classify correctly the various illnesses and conditions the decedent might have had.
Immediate cause of death. Part I, line a, is for the immediate cause of death (see Table 1). This should be a disease, complication, or injury that directly caused the death. A common error is to list a mechanism of death (for example, cardiac arrest) rather than a disease (myocardial infarction).
Specific terms are better than vague ones. For instance,“cerebral infarction” is better than “stroke.” “Escherichia coli sepsis” is better than just “sepsis.”
When cancer is the cause of death, list the primary site, cell type, cancer grade, and specific organ or lobe affected.
Avoid terms without medical meaning, such as old age or senescence.
If additional information is expected from an autopsy, it is acceptable to list the cause of death as pending. But an update to certificate will be required once the additional information is obtained. It is also acceptable to list a cause as unknown. This will not automatically forward the case to the medical examiner.
Intermediate/underlying causes. Lines b, c, and d are for intermediate and underlying causes. Each condition listed should cause the one above it. You should be able to proceed logically from the underlying cause through each intermediate cause by saying the phrase “due to” or “as a consequence of,” moving from the lower line up through line b. There may be several intermediate causes. For example, a death may be due to a pulmonary embolus, as a consequence of hip surgery, resulting from a injury from a fall, resulting from a cerebral infarction. The underlying cause is the cerebral infarction.
Marking time intervals. To the right of lines a through d is space to write the time interval between the condition listed (immediate, intermediate, or underlying cause of death) and the time of death. The more precise the time the better. But it is understood that times must occasionally be estimated, and terms such as “approximately” are acceptable. If the time cannot be estimated, insert the phrase “unknown duration.” Something should be listed on this line next to the immediate, intermediate, and underlying conditions listed. No lines should be left blank.
Other illnesses. Part II is where to list other significant illnesses or conditions that may have contributed to the death but were not the direct causes of it. More than one condition may be listed. Many patients have multiple conditions and there may be uncertainty as to direct and contributing causes of the death. The physician is only expected to make the best judgment possible as to the most likely causes and sequences. Coders referring to international standards and rules will use the information to make a final classification of the underlying cause.
Specific errors to avoid
Table 2 includes some points to remember to avoid making errors when filling out the death certificate medical information. By following these rules, studying the cases provided in the Physicians’ Handbook on Medical Certification and Death, and systematically thinking about the sequence of events that caused the death, physicians can improve on their accuracy when performing the important and under appreciated role of accurately certifying the medical cause of death.
TABLE 2
Important points to remember when completing medical information on a death certificate
| Do not use abbreviations. |
| Do not use numbers for months; spell out the month. |
| Use a 24-hour clock (1600, not 4 P.M.). |
| Do not alter the document or erase any part of it. |
| Print legibly using black ink. |
| Complete all required items, do not leave them blank. If necessary, write “unknown.” |
| Do not delay completing the certification. The burial or other disposition of the remains depends on the correct completion of the certificate and its acceptance by the state or local registrar. |
| Do not complete the medical information if another physician has more knowledge of the circumstances, unless they are unavailable. |
Useful resources
The Physicians’ Handbook on Medical Certification of Death, published by the Centers for Disease Control, National Center for Health Statistics, is available at www.cdc.gov/nchs/data/misc/hb_cod.pdf. It contains instructions on how to complete a death certificate and a series of useful examples that take about a half hour to review.
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
Influenza vaccine: New recommendations for infants and children aged 6 to 23 months
For the 2004–2005 influenza season, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends that influenza vaccine be given to all infants and children aged 6 to 23 months.1 It further recommends vaccination for members of households that have children aged <2 years and for out-of-home caregivers for these children.
These changes will make universal coverage more difficult to achieve. Family physicians will need to educate themselves, office staff, and parents and guardians about these recommendations. Be prepared to implement office protocols that identify and notify those who require vaccination, and address the concerns of parents and guardians regarding thimerosal and the addition of yet another vaccine to the child vaccine schedule.
Rationale for change
In 2002, ACIP began to encourage use of influenza vaccine for all children 6 to 23 month old. Before then, it had been recommended only for those in that age group with certain chronic medical conditions.
Still, the coverage level achieved in the 2002 to 2003 flu season for this age group was low—only 4.4 % were fully immunized with 2 doses.2 To increase coverage, the influenza vaccine was included in vaccines offered by the Vaccine for Children program in 2003 and was made part of the universal recommendations for the coming season.
The rationale for the new universal recommendation is the high rate of influenza-related hospitalizations among those aged 6 to 23 months, which varies from year to year and has been documented to be as high as 5/1000.3,4 While hospitalization rates are higher for infants aged 0 to 5 months, influenza vaccine is not approved for use in this age group.
Death from influenza in infants and children is not common. However, in the 2003 to 2004 influenza season, 58 influenza deaths among those aged <2 years were recorded.1 Added benefits from vaccinating infants and children may include decreased rates of otitis media.5,6
Practical aspects of vaccine administration
Some of the most important practical details involved with immunizing 6- to 23-month-olds include the following:
- The age group includes those who have completed 6 months of life (are now in their 7th month) up to the second birthday. Influenza vaccine is 65% to 90% effective in this age group.
- The dose of influenza vaccine for infants and children up to their 3rd birthday is 0.25 mL.
- The vaccine should be administered intramuscularly in the anterolateral thigh with a 1-inch needle.
- Two doses, 1 month apart, are recommended for infants or children aged <9 years receiving influenza vaccine for the first time. (Dose recommendations for all age groups are listed in Table 1.)
- Only 1 vaccine product has been approved for those younger than 4 years: FluZone, produced by Aventis Pasteur.
- The vaccine is made with killed virus and can be given simultaneously with other recommended vaccines.
- The live attenuated influenza vaccine, administered intranasally, is not approved for children before their 5th birthday.
- The vaccine should be stored at 2° to 8°C (35° to 46°F) and should not be frozen.
- Vaccine left over from last year should not be used this year.
- Contraindications to influenza vaccine are listed in Table 2.
TABLE 1
Inactivated influenza vaccine* dosage, by age group
| Age group† | Dose | No of doses | Route‡ |
|---|---|---|---|
| 6–35 mo | 0.25 mL | 1 or 2§ | Intramuscular |
| 3–8 y | 0.50 mL | 1 or 2§ | Intramuscular |
| 9 y | 0.50 mL | 1 | Intramuscular |
| *A 0.5-mL dose contains 15 mg each of A/Fujian/411/2002 (H3H2)-like, A/New Caledonia/20/99 (H1N1)-like, and B/Shanghai/361/2002-like antigens. For the A/Fujian/411/2002 (H3H2)-like antigen, manufacturers may use the antigenically equivalent A/Wyoming/3/2003 (H3N2) virus, and for the B/Shanghai/361/2002-like antigen, manufacturers may use the antigenically equivalent B/Jilin/20/2003 virus or B/Jiangsu/10/2003 virus. Manufacturers include Aventis Pasteur, Inc (FluZone© split virus), and Chiron (Fluvirin™ purified surface antigen vaccine). FluZone is approved by the Food and Drug Administration for use among persons aged 6 months. Fluvirin is approved for use among persons aged 4 months. For further product information, call Aventis Pasteur at 800-822-2463 or Chiron at 800-200-4278. | |||
| † Because of their decreased potential for causing febrile reactions, only split-virus vaccines should be used for children aged <13 years. Whole-virus vaccine is not available in the United States. Split-virus vaccine might be labeled as split, subvirion, or purified surface antigen vaccine. Immunogenicity and side effects of split- and whole-virus vaccines are similar among adult when vaccines are administered in the recommended dosage. | |||
| ‡ For adults and older children, the recommended site of vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. | |||
| § Two doses administered at least 1 month apart are recommended for children aged <9 years who are receiving influenza vaccine for the first time. | |||
| Source: Harper et al 2004.1 | |||
TABLE 2
Contraindications to influenza vaccine
|
The thimerosal controversy
Family physicians are likely to encounter questions from parents or guardians about thimerosal in influenza vaccines. Thimerosal is a preservative containing mercury, which has been used in vaccines for more than 70 years. Because of the increasing number of recommended childhood vaccines and the resulting cumulative exposure to mercury, there has been a concerted effort since 1999 to reduce the content of thimerosal in vaccine products.
Almost all vaccines are now free of thimerosal. However, inactivated influenza vaccine distributed in multidose vials does contain thimerosal, 12.5 μg mercury/0.25 mL dose. Single-dose vials containing inactivated influenza vaccine do not contain thimerosal as a preservative, but this product still contains trace amounts of mercury, <0.5 μg/0.25 mL dose.
No evidence has shown conclusively that mercury-containing vaccines cause serious adverse effects. A recent Institute of Medicine report concludes that the weight of evidence supports a lack of causation between thimerosal and autism.7 Nevertheless, many parents remain concerned about mercury exposure from vaccines. Reassure those who are concerned that the cumulative exposure to mercury from all vaccines has decreased markedly and that influenza vaccine contains only low amounts of thimerosal. Those wishing to decrease this risk even further should be given the option of the single-dose vial product, if available.
Timing vaccination for optimal protection
The influenza season varies year to year but normally occurs between November and March. Vaccination for those at high risk of influenza complications, including those aged 6 to 23 months, should begin in September and October. Those who need 2 doses should receive the second by December if possible.
Because of the length of the influenza season and the fact that the inactivated influenza vaccine is not approved for use during the first 6 months of life, infants will become eligible for the vaccine at different points in the influenza season. Office procedures should be implemented to identify eligible infants as the season progresses and to notify parents or guardians of the opportunity to vaccinate their infant.
If an infant enters the 7th month of life late in the influenza season and vaccine is still available, vaccination should still be considered, not only to offer protection in the current year but also to reduce the number of doses needed the next year. In instances when only 1 dose of a recommended 2-dose schedule is completed, only 1 dose is needed the following year.
Vaccinate close contacts of infants and children
The new ACIP recommendations state that persons who can transmit influenza to those at high risk of complications should also be vaccinated. This includes household contacts of, and those who provide care to, children aged <2 years.
For infants in their first 6 months of life, preventing infection among close contacts is the major preventive intervention available. The Vaccine for Children program now includes influenza vaccine for members of households (those who are aged <18 years) where children aged <2 years live.
Vaccine complications and contraindications
Local reactions including redness, pain, and swelling are common after influenza vaccine administration. Generalized reactions including fever, malaise, and myalgia are less common and can start within 6 to 12 hours and last 1 to 2 days. Contraindications to vaccine administration are few (Table 2).
When chemoprophylaxis is an option
Only 2 options exist for chemoprophylaxis against influenza in children before their 13th birthday: amantadine and rimantadine. The dosage for each is 5 mg/kg/d, up to 150 mg, in 1 or 2 doses. Both are effective only against influenza A and are approved for use only after the 1st birthday. Still, this option should be kept in mind for unvaccinated children who are exposed to influenza.
A chemoprophylactic agent can be started at the same time the vaccine is administered. Since it takes 2 weeks to develop protective levels of antibodies, chemoprophylaxis should be continued for 2 weeks with those who need only 1 vaccine, and until 2 weeks after the 2nd vaccine for those who need 2 doses.
In early October, 1 of the 2 producers of inactivated influenza vaccine for the United States market, Chiron Corporation, had its license suspended by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom, where their plant is located. This will cause a shortage of vaccine, since Chiron was to have supplied about half of the 100 million doses planned for this country.
Chiron, however, was not a producer of vaccine for children aged <4 years. Based on this anticipated shortfall, the ACIP is recommending that the vaccine be given preferentially to those at high risk for influenza complications ( Table 3 ) and that those who are not in one of these categories forgo vaccination. Physicians who will not have an adequate supply of vaccine for their patient population are encouraged to have their priority patients seek vaccine at another location.
TABLE 3
Priority groups for influenza vaccination
| The following priority groups for vaccination with inactivated influenza vaccine this season are considered to be of equal importance. |
|
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
1. Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004; 53(RR-6):1–40. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5306a1.htm. Accessed on October 19, 2004. (Note: Recommendations for prevention of influenza in all age groups are available in this article.)
2. CDC. Childhood Influenza—vaccination coverage—United States, 2002–2003 Influenza season. MMWR Morb Mortal Wkly Rep 2004;53:863-866.
3. Neuzil KM, Wright PF, Mitchel EF, Jr, Griffin MR. Burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr 2000;137:856-864.
4. Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000;342:225-231.
5. Heikkinen T, Ruuskanen O, Waris M, Ziegler T, Arola M, Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child 1991;145:445-448.
6. Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med 1995;149:1113-1117.
7. Institute of Medicine. Immunization Safety Review: Vaccines and Autism. Washington, DC: National Academies Press; 2004.
For the 2004–2005 influenza season, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends that influenza vaccine be given to all infants and children aged 6 to 23 months.1 It further recommends vaccination for members of households that have children aged <2 years and for out-of-home caregivers for these children.
These changes will make universal coverage more difficult to achieve. Family physicians will need to educate themselves, office staff, and parents and guardians about these recommendations. Be prepared to implement office protocols that identify and notify those who require vaccination, and address the concerns of parents and guardians regarding thimerosal and the addition of yet another vaccine to the child vaccine schedule.
Rationale for change
In 2002, ACIP began to encourage use of influenza vaccine for all children 6 to 23 month old. Before then, it had been recommended only for those in that age group with certain chronic medical conditions.
Still, the coverage level achieved in the 2002 to 2003 flu season for this age group was low—only 4.4 % were fully immunized with 2 doses.2 To increase coverage, the influenza vaccine was included in vaccines offered by the Vaccine for Children program in 2003 and was made part of the universal recommendations for the coming season.
The rationale for the new universal recommendation is the high rate of influenza-related hospitalizations among those aged 6 to 23 months, which varies from year to year and has been documented to be as high as 5/1000.3,4 While hospitalization rates are higher for infants aged 0 to 5 months, influenza vaccine is not approved for use in this age group.
Death from influenza in infants and children is not common. However, in the 2003 to 2004 influenza season, 58 influenza deaths among those aged <2 years were recorded.1 Added benefits from vaccinating infants and children may include decreased rates of otitis media.5,6
Practical aspects of vaccine administration
Some of the most important practical details involved with immunizing 6- to 23-month-olds include the following:
- The age group includes those who have completed 6 months of life (are now in their 7th month) up to the second birthday. Influenza vaccine is 65% to 90% effective in this age group.
- The dose of influenza vaccine for infants and children up to their 3rd birthday is 0.25 mL.
- The vaccine should be administered intramuscularly in the anterolateral thigh with a 1-inch needle.
- Two doses, 1 month apart, are recommended for infants or children aged <9 years receiving influenza vaccine for the first time. (Dose recommendations for all age groups are listed in Table 1.)
- Only 1 vaccine product has been approved for those younger than 4 years: FluZone, produced by Aventis Pasteur.
- The vaccine is made with killed virus and can be given simultaneously with other recommended vaccines.
- The live attenuated influenza vaccine, administered intranasally, is not approved for children before their 5th birthday.
- The vaccine should be stored at 2° to 8°C (35° to 46°F) and should not be frozen.
- Vaccine left over from last year should not be used this year.
- Contraindications to influenza vaccine are listed in Table 2.
TABLE 1
Inactivated influenza vaccine* dosage, by age group
| Age group† | Dose | No of doses | Route‡ |
|---|---|---|---|
| 6–35 mo | 0.25 mL | 1 or 2§ | Intramuscular |
| 3–8 y | 0.50 mL | 1 or 2§ | Intramuscular |
| 9 y | 0.50 mL | 1 | Intramuscular |
| *A 0.5-mL dose contains 15 mg each of A/Fujian/411/2002 (H3H2)-like, A/New Caledonia/20/99 (H1N1)-like, and B/Shanghai/361/2002-like antigens. For the A/Fujian/411/2002 (H3H2)-like antigen, manufacturers may use the antigenically equivalent A/Wyoming/3/2003 (H3N2) virus, and for the B/Shanghai/361/2002-like antigen, manufacturers may use the antigenically equivalent B/Jilin/20/2003 virus or B/Jiangsu/10/2003 virus. Manufacturers include Aventis Pasteur, Inc (FluZone© split virus), and Chiron (Fluvirin™ purified surface antigen vaccine). FluZone is approved by the Food and Drug Administration for use among persons aged 6 months. Fluvirin is approved for use among persons aged 4 months. For further product information, call Aventis Pasteur at 800-822-2463 or Chiron at 800-200-4278. | |||
| † Because of their decreased potential for causing febrile reactions, only split-virus vaccines should be used for children aged <13 years. Whole-virus vaccine is not available in the United States. Split-virus vaccine might be labeled as split, subvirion, or purified surface antigen vaccine. Immunogenicity and side effects of split- and whole-virus vaccines are similar among adult when vaccines are administered in the recommended dosage. | |||
| ‡ For adults and older children, the recommended site of vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. | |||
| § Two doses administered at least 1 month apart are recommended for children aged <9 years who are receiving influenza vaccine for the first time. | |||
| Source: Harper et al 2004.1 | |||
TABLE 2
Contraindications to influenza vaccine
|
The thimerosal controversy
Family physicians are likely to encounter questions from parents or guardians about thimerosal in influenza vaccines. Thimerosal is a preservative containing mercury, which has been used in vaccines for more than 70 years. Because of the increasing number of recommended childhood vaccines and the resulting cumulative exposure to mercury, there has been a concerted effort since 1999 to reduce the content of thimerosal in vaccine products.
Almost all vaccines are now free of thimerosal. However, inactivated influenza vaccine distributed in multidose vials does contain thimerosal, 12.5 μg mercury/0.25 mL dose. Single-dose vials containing inactivated influenza vaccine do not contain thimerosal as a preservative, but this product still contains trace amounts of mercury, <0.5 μg/0.25 mL dose.
No evidence has shown conclusively that mercury-containing vaccines cause serious adverse effects. A recent Institute of Medicine report concludes that the weight of evidence supports a lack of causation between thimerosal and autism.7 Nevertheless, many parents remain concerned about mercury exposure from vaccines. Reassure those who are concerned that the cumulative exposure to mercury from all vaccines has decreased markedly and that influenza vaccine contains only low amounts of thimerosal. Those wishing to decrease this risk even further should be given the option of the single-dose vial product, if available.
Timing vaccination for optimal protection
The influenza season varies year to year but normally occurs between November and March. Vaccination for those at high risk of influenza complications, including those aged 6 to 23 months, should begin in September and October. Those who need 2 doses should receive the second by December if possible.
Because of the length of the influenza season and the fact that the inactivated influenza vaccine is not approved for use during the first 6 months of life, infants will become eligible for the vaccine at different points in the influenza season. Office procedures should be implemented to identify eligible infants as the season progresses and to notify parents or guardians of the opportunity to vaccinate their infant.
If an infant enters the 7th month of life late in the influenza season and vaccine is still available, vaccination should still be considered, not only to offer protection in the current year but also to reduce the number of doses needed the next year. In instances when only 1 dose of a recommended 2-dose schedule is completed, only 1 dose is needed the following year.
Vaccinate close contacts of infants and children
The new ACIP recommendations state that persons who can transmit influenza to those at high risk of complications should also be vaccinated. This includes household contacts of, and those who provide care to, children aged <2 years.
For infants in their first 6 months of life, preventing infection among close contacts is the major preventive intervention available. The Vaccine for Children program now includes influenza vaccine for members of households (those who are aged <18 years) where children aged <2 years live.
Vaccine complications and contraindications
Local reactions including redness, pain, and swelling are common after influenza vaccine administration. Generalized reactions including fever, malaise, and myalgia are less common and can start within 6 to 12 hours and last 1 to 2 days. Contraindications to vaccine administration are few (Table 2).
When chemoprophylaxis is an option
Only 2 options exist for chemoprophylaxis against influenza in children before their 13th birthday: amantadine and rimantadine. The dosage for each is 5 mg/kg/d, up to 150 mg, in 1 or 2 doses. Both are effective only against influenza A and are approved for use only after the 1st birthday. Still, this option should be kept in mind for unvaccinated children who are exposed to influenza.
A chemoprophylactic agent can be started at the same time the vaccine is administered. Since it takes 2 weeks to develop protective levels of antibodies, chemoprophylaxis should be continued for 2 weeks with those who need only 1 vaccine, and until 2 weeks after the 2nd vaccine for those who need 2 doses.
In early October, 1 of the 2 producers of inactivated influenza vaccine for the United States market, Chiron Corporation, had its license suspended by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom, where their plant is located. This will cause a shortage of vaccine, since Chiron was to have supplied about half of the 100 million doses planned for this country.
Chiron, however, was not a producer of vaccine for children aged <4 years. Based on this anticipated shortfall, the ACIP is recommending that the vaccine be given preferentially to those at high risk for influenza complications ( Table 3 ) and that those who are not in one of these categories forgo vaccination. Physicians who will not have an adequate supply of vaccine for their patient population are encouraged to have their priority patients seek vaccine at another location.
TABLE 3
Priority groups for influenza vaccination
| The following priority groups for vaccination with inactivated influenza vaccine this season are considered to be of equal importance. |
|
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
For the 2004–2005 influenza season, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends that influenza vaccine be given to all infants and children aged 6 to 23 months.1 It further recommends vaccination for members of households that have children aged <2 years and for out-of-home caregivers for these children.
These changes will make universal coverage more difficult to achieve. Family physicians will need to educate themselves, office staff, and parents and guardians about these recommendations. Be prepared to implement office protocols that identify and notify those who require vaccination, and address the concerns of parents and guardians regarding thimerosal and the addition of yet another vaccine to the child vaccine schedule.
Rationale for change
In 2002, ACIP began to encourage use of influenza vaccine for all children 6 to 23 month old. Before then, it had been recommended only for those in that age group with certain chronic medical conditions.
Still, the coverage level achieved in the 2002 to 2003 flu season for this age group was low—only 4.4 % were fully immunized with 2 doses.2 To increase coverage, the influenza vaccine was included in vaccines offered by the Vaccine for Children program in 2003 and was made part of the universal recommendations for the coming season.
The rationale for the new universal recommendation is the high rate of influenza-related hospitalizations among those aged 6 to 23 months, which varies from year to year and has been documented to be as high as 5/1000.3,4 While hospitalization rates are higher for infants aged 0 to 5 months, influenza vaccine is not approved for use in this age group.
Death from influenza in infants and children is not common. However, in the 2003 to 2004 influenza season, 58 influenza deaths among those aged <2 years were recorded.1 Added benefits from vaccinating infants and children may include decreased rates of otitis media.5,6
Practical aspects of vaccine administration
Some of the most important practical details involved with immunizing 6- to 23-month-olds include the following:
- The age group includes those who have completed 6 months of life (are now in their 7th month) up to the second birthday. Influenza vaccine is 65% to 90% effective in this age group.
- The dose of influenza vaccine for infants and children up to their 3rd birthday is 0.25 mL.
- The vaccine should be administered intramuscularly in the anterolateral thigh with a 1-inch needle.
- Two doses, 1 month apart, are recommended for infants or children aged <9 years receiving influenza vaccine for the first time. (Dose recommendations for all age groups are listed in Table 1.)
- Only 1 vaccine product has been approved for those younger than 4 years: FluZone, produced by Aventis Pasteur.
- The vaccine is made with killed virus and can be given simultaneously with other recommended vaccines.
- The live attenuated influenza vaccine, administered intranasally, is not approved for children before their 5th birthday.
- The vaccine should be stored at 2° to 8°C (35° to 46°F) and should not be frozen.
- Vaccine left over from last year should not be used this year.
- Contraindications to influenza vaccine are listed in Table 2.
TABLE 1
Inactivated influenza vaccine* dosage, by age group
| Age group† | Dose | No of doses | Route‡ |
|---|---|---|---|
| 6–35 mo | 0.25 mL | 1 or 2§ | Intramuscular |
| 3–8 y | 0.50 mL | 1 or 2§ | Intramuscular |
| 9 y | 0.50 mL | 1 | Intramuscular |
| *A 0.5-mL dose contains 15 mg each of A/Fujian/411/2002 (H3H2)-like, A/New Caledonia/20/99 (H1N1)-like, and B/Shanghai/361/2002-like antigens. For the A/Fujian/411/2002 (H3H2)-like antigen, manufacturers may use the antigenically equivalent A/Wyoming/3/2003 (H3N2) virus, and for the B/Shanghai/361/2002-like antigen, manufacturers may use the antigenically equivalent B/Jilin/20/2003 virus or B/Jiangsu/10/2003 virus. Manufacturers include Aventis Pasteur, Inc (FluZone© split virus), and Chiron (Fluvirin™ purified surface antigen vaccine). FluZone is approved by the Food and Drug Administration for use among persons aged 6 months. Fluvirin is approved for use among persons aged 4 months. For further product information, call Aventis Pasteur at 800-822-2463 or Chiron at 800-200-4278. | |||
| † Because of their decreased potential for causing febrile reactions, only split-virus vaccines should be used for children aged <13 years. Whole-virus vaccine is not available in the United States. Split-virus vaccine might be labeled as split, subvirion, or purified surface antigen vaccine. Immunogenicity and side effects of split- and whole-virus vaccines are similar among adult when vaccines are administered in the recommended dosage. | |||
| ‡ For adults and older children, the recommended site of vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. | |||
| § Two doses administered at least 1 month apart are recommended for children aged <9 years who are receiving influenza vaccine for the first time. | |||
| Source: Harper et al 2004.1 | |||
TABLE 2
Contraindications to influenza vaccine
|
The thimerosal controversy
Family physicians are likely to encounter questions from parents or guardians about thimerosal in influenza vaccines. Thimerosal is a preservative containing mercury, which has been used in vaccines for more than 70 years. Because of the increasing number of recommended childhood vaccines and the resulting cumulative exposure to mercury, there has been a concerted effort since 1999 to reduce the content of thimerosal in vaccine products.
Almost all vaccines are now free of thimerosal. However, inactivated influenza vaccine distributed in multidose vials does contain thimerosal, 12.5 μg mercury/0.25 mL dose. Single-dose vials containing inactivated influenza vaccine do not contain thimerosal as a preservative, but this product still contains trace amounts of mercury, <0.5 μg/0.25 mL dose.
No evidence has shown conclusively that mercury-containing vaccines cause serious adverse effects. A recent Institute of Medicine report concludes that the weight of evidence supports a lack of causation between thimerosal and autism.7 Nevertheless, many parents remain concerned about mercury exposure from vaccines. Reassure those who are concerned that the cumulative exposure to mercury from all vaccines has decreased markedly and that influenza vaccine contains only low amounts of thimerosal. Those wishing to decrease this risk even further should be given the option of the single-dose vial product, if available.
Timing vaccination for optimal protection
The influenza season varies year to year but normally occurs between November and March. Vaccination for those at high risk of influenza complications, including those aged 6 to 23 months, should begin in September and October. Those who need 2 doses should receive the second by December if possible.
Because of the length of the influenza season and the fact that the inactivated influenza vaccine is not approved for use during the first 6 months of life, infants will become eligible for the vaccine at different points in the influenza season. Office procedures should be implemented to identify eligible infants as the season progresses and to notify parents or guardians of the opportunity to vaccinate their infant.
If an infant enters the 7th month of life late in the influenza season and vaccine is still available, vaccination should still be considered, not only to offer protection in the current year but also to reduce the number of doses needed the next year. In instances when only 1 dose of a recommended 2-dose schedule is completed, only 1 dose is needed the following year.
Vaccinate close contacts of infants and children
The new ACIP recommendations state that persons who can transmit influenza to those at high risk of complications should also be vaccinated. This includes household contacts of, and those who provide care to, children aged <2 years.
For infants in their first 6 months of life, preventing infection among close contacts is the major preventive intervention available. The Vaccine for Children program now includes influenza vaccine for members of households (those who are aged <18 years) where children aged <2 years live.
Vaccine complications and contraindications
Local reactions including redness, pain, and swelling are common after influenza vaccine administration. Generalized reactions including fever, malaise, and myalgia are less common and can start within 6 to 12 hours and last 1 to 2 days. Contraindications to vaccine administration are few (Table 2).
When chemoprophylaxis is an option
Only 2 options exist for chemoprophylaxis against influenza in children before their 13th birthday: amantadine and rimantadine. The dosage for each is 5 mg/kg/d, up to 150 mg, in 1 or 2 doses. Both are effective only against influenza A and are approved for use only after the 1st birthday. Still, this option should be kept in mind for unvaccinated children who are exposed to influenza.
A chemoprophylactic agent can be started at the same time the vaccine is administered. Since it takes 2 weeks to develop protective levels of antibodies, chemoprophylaxis should be continued for 2 weeks with those who need only 1 vaccine, and until 2 weeks after the 2nd vaccine for those who need 2 doses.
In early October, 1 of the 2 producers of inactivated influenza vaccine for the United States market, Chiron Corporation, had its license suspended by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom, where their plant is located. This will cause a shortage of vaccine, since Chiron was to have supplied about half of the 100 million doses planned for this country.
Chiron, however, was not a producer of vaccine for children aged <4 years. Based on this anticipated shortfall, the ACIP is recommending that the vaccine be given preferentially to those at high risk for influenza complications ( Table 3 ) and that those who are not in one of these categories forgo vaccination. Physicians who will not have an adequate supply of vaccine for their patient population are encouraged to have their priority patients seek vaccine at another location.
TABLE 3
Priority groups for influenza vaccination
| The following priority groups for vaccination with inactivated influenza vaccine this season are considered to be of equal importance. |
|
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
1. Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004; 53(RR-6):1–40. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5306a1.htm. Accessed on October 19, 2004. (Note: Recommendations for prevention of influenza in all age groups are available in this article.)
2. CDC. Childhood Influenza—vaccination coverage—United States, 2002–2003 Influenza season. MMWR Morb Mortal Wkly Rep 2004;53:863-866.
3. Neuzil KM, Wright PF, Mitchel EF, Jr, Griffin MR. Burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr 2000;137:856-864.
4. Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000;342:225-231.
5. Heikkinen T, Ruuskanen O, Waris M, Ziegler T, Arola M, Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child 1991;145:445-448.
6. Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med 1995;149:1113-1117.
7. Institute of Medicine. Immunization Safety Review: Vaccines and Autism. Washington, DC: National Academies Press; 2004.
1. Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004; 53(RR-6):1–40. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5306a1.htm. Accessed on October 19, 2004. (Note: Recommendations for prevention of influenza in all age groups are available in this article.)
2. CDC. Childhood Influenza—vaccination coverage—United States, 2002–2003 Influenza season. MMWR Morb Mortal Wkly Rep 2004;53:863-866.
3. Neuzil KM, Wright PF, Mitchel EF, Jr, Griffin MR. Burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr 2000;137:856-864.
4. Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000;342:225-231.
5. Heikkinen T, Ruuskanen O, Waris M, Ziegler T, Arola M, Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child 1991;145:445-448.
6. Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med 1995;149:1113-1117.
7. Institute of Medicine. Immunization Safety Review: Vaccines and Autism. Washington, DC: National Academies Press; 2004.
How does HIPAA affect public health reporting?
Since the Health Insurance Portability and Accountability Act (HIPAA) privacy rule was put into effect in April 2003, healthcare providers have sometimes been confused about what information they can legally disclose to public health agencies. A clear understanding of permissible disclosure will enable family physicians to continue their important role of providing individual patient information for the critical activities of disease surveillance, outbreak investigation, monitoring causes of death and birth complications, assuring health care services, conducting public health research, and formulating health policy.
HIPAA does not prohibit disclosure for public health purposes
The HIPAA is intended to protect the public from unauthorized access to, use of, and disclosure of individually identifiable health information. It places responsibility on health care providers to avoid using or disclosing protected health information (PHI) unless authorized by the person to whom it pertains, or unless the disclosure or use is required or permitted by regulation or law. Specifically excluded from the requirement for individual authorization are disclosures for public health activities. This means that sharing PHI for public health purposes is permitted as long as the agency to which the information is provided is legally authorized to collect and receive the information (see Lawful recipients of personal health information).
This specific exclusion was allowed because public health authorities have a legitimate need for PHI to ensure public health and safety, and because public health agencies have a track record of protecting the confidentiality of PHI. The HIPAA privacy rule attempts to strike a balance between individual privacy rights and the need for public protection.
Public health agencies included in this category include state, territorial, tribal, and local health departments, as well as federal health agencies such as the Centers for Disease Control and Prevention, the Food and Drug Administration, the National Institutes of Health, the Occupational Safety and Health Administration, the Substance Abuse and Mental Health Services Administration, and others. It also includes individuals and agencies working under a grant of authority from a public health agency.
Lawful disclosure: Examples
It’s instructive to consider how this public health HIPAA exception plays out in the daily practice of medicine. First, some definitions:
Protected Health Information.Individually identifiable health information transmitted electronically or any other way. It includes information about past, present, or anticipated mental or physical health, and the provision of or payment for health care.
Covered entities. These are the entities who must adhere to the HIPAA rules. Included are health care providers, health plans, and health care clearinghouses that transmit any health information in an electronic format
Personal Identifiers. Information that can be used to find the identity of an individual to link them to their PHI.
Scenario 1
A family physician’s patient dies at home. The physician is asked to fill out a death certificate, which contains PHI as defined by the HIPAA privacy rule. Is this permitted without family authorization?
Unauthorized disclosure is permitted. Vital statistics—required information on death and birth certificates—has not been changed by HIPAA. The information required on the death certificate can be provided without authorization.
Scenario 2
A patient is diagnosed with tuberculosis.This is a reportable disease per the state health code. Can the physician report the PHI requested on the disease reporting form?
Unauthorized disclosure is permitted. Each state health authority requires health care providers to report information about individuals who have contracted a disease of public health significance. Reportable disease lists differ by jurisdiction, and physicians should be aware of the diseases reportable in their areas and how the information is to be reported. Individual authorization for release of PHI in these disease reports is not required by HIPAA.
Scenario 3
A physician examines an infant who has unexplained injuries. Child abuse is suspected. Is child abuse reporting exempted from the privacy rule?
Unauthorized disclosure is permitted. Reporting of child abuse and neglect is exempted. This information may even be reported to a non-health agency, such as a child protective service, as long as the reportable information is required by law, and individual authorization is not required.
Scenario 4
A patient suffers what appears to be an adverse reaction to a medication. The FDA adverse event reporting form asks for PHI. Can a physician report PHI in this instance without patient authorization?
Unauthorized disclosure is permitted. Reporting of adverse events or reactions from drugs, food, biological products, and medical devices is still permitted without authorization.
Scenario 5
A patient is newly diagnosed with lung cancer. The state maintains a cancer registry and physicians are required to report PHI about patients with cancer. In this state the cancer registry is maintained by the university under contract with the State Health Department. Is reporting permitted without patient authorization?
Unauthorized disclosure is permitted. Cancer and immunization registry reporting of PHI is still permitted even if the entity responsible for the registry is not a public health agency, as long as it is under the authority of the agency to perform this public health function.
Scenario 6
A patient dies from meningitis and the local health department requests to view the hospital record to investigate cause of death. The cause turns out to be West Nile virus, which is not on the list of reportable diseases. Is the health department permitted to view the record and is authorization required?
Unauthorized disclosure is permitted. The privacy rule exception does not require a law or regulation specifically mandating disclosure. The health care provider can release requested information to a public health authority when the information is for the purpose of controlling disease, injury, or disability. The information released should be the minimum necessary for the stated public health purpose, and the provider can rely on the agency to determine what that information is. In this case, examination of the record is permitted and authorization is not required.
Scenario 7
An auditor from the Vaccine for Children program arrives at the office and requests to see patient records to audit adherence to the rules governing this program. Is the auditor allowed to exam records, and is authorization required?
Unauthorized disclosure is permitted. Patient records can be reviewed by staff of public health agencies authorized by law to collect PHI for program management purposes. No patient authorization is required.
Scenario 8
A local community agency is concerned about the potential health effects of groundwater contamination. They request information about all your patients who have contracted cancer within the past 5 years. What information can you provide them?
PHI disclosure requires patient authorization. This agency, unless under the authority of a public health agency to collect PHI, cannot obtain PHI without patient authorization. However, deidentified information could be provided. Deidentified data are not covered by HIPAA and do not require individual privacy protection or authorization for release. De-identification means removing 18 “identifiers” (Table) or enough information that allows a statistician to conclude that the chance of an individual being identified is remote.
TABLE
Individual identifiers to be removed from reports
| The following 18 identifiers of a person, or of relatives, employers, or household members of a person must be removed, and the covered entity must not have actual knowledge that the information could be used alone or in combination with other information to identify the individual, for the information to be considered de-identified and not protected health information. |
|---|
| • Names |
| • All geographic subdivisions smaller than a state, including county, city, street address, precinct, zip code (first 3 digits OK if geographic unit contains >20,000 persons), and their equivalent geocodes |
| • All elements of dates (except year) directly related to an individual; all ages >89 and all elements of dates (including year) indicative of such age (except for an aggregate into a single category of age >90) |
| • Telephone numbers |
| • Fax numbers |
| • Electronic mail addresses |
| • Social Security numbers |
| • Medical record numbers |
| • Health-plan beneficiary numbers |
| • Account numbers |
| • Certificate and license numbers |
| • Vehicle identifiers and serial numbers, including license plate numbers |
| • Medical device identifiers and serial numbers |
| • Internet universal resource locators (URLs) |
| • Internet protocol (IP) addresses |
| • Biometric identifiers, including fingerprints and voice prints |
| • Full-face photographic images and any comparable images |
| • Any other unique identifying number, characteristic, or code, except that covered identities may, under certain circumstances, assign a code or other means of record identification that allows de-identified information to be re-identified. |
| Source: “HIPAA privacy rule and public health,” Morbidity and Mortality Weekly Report, April 11, 2003; 52:1–12. |
Physician obligations with disclosure
Confirm the legitimacy of a request. Even though physicians can release PHI to public health agencies without a patient’s authorization, they have other obligations to meet. One of these is to ensure that the person or agency requesting PHI is a legitimate public health authority. If the request is made in person, some form of credentials or proof of government status should be provided. If the request is in writing, it should be on official letterhead. A person or agency acting under the authority of a pubic health agency should provide proof of this authority. If physicians have any doubt about the authenticity of a request, they should call the agency being represented and inquire.
Let patients know. The second obligation is to provide information about the disclosure to the individual whose PHI was released, if this information is requested, and to inform patients in statements about privacy practices that PHI information is released to public health agencies when required and permitted by law.
Other exceptions to HIPAA
HIPAA allows the legitimate use of PHI, without authorization, for the purpose of protecting the public under conditions involving law enforcement, court proceedings, worker’s compensation, and national security. These exceptions are outside the scope of this article.
Explain HIPAA to patients
The trend toward electronic medical records and the increasing public concern about privacy led to the enactment of the HIPAA privacy rule. A natural tension exists between individual rights and public protection, and the HIPAA privacy rule attempts to balance these competing concerns. For patients who are concerned about confidentiality, family physicians can explain the purpose of public health exceptions and give reassurance about how public health agencies have a good record of protecting individuals’ identity.
Correspondence
4001 North Third #415, Phoenix, AZ 85012. E-mail: [email protected].
Since the Health Insurance Portability and Accountability Act (HIPAA) privacy rule was put into effect in April 2003, healthcare providers have sometimes been confused about what information they can legally disclose to public health agencies. A clear understanding of permissible disclosure will enable family physicians to continue their important role of providing individual patient information for the critical activities of disease surveillance, outbreak investigation, monitoring causes of death and birth complications, assuring health care services, conducting public health research, and formulating health policy.
HIPAA does not prohibit disclosure for public health purposes
The HIPAA is intended to protect the public from unauthorized access to, use of, and disclosure of individually identifiable health information. It places responsibility on health care providers to avoid using or disclosing protected health information (PHI) unless authorized by the person to whom it pertains, or unless the disclosure or use is required or permitted by regulation or law. Specifically excluded from the requirement for individual authorization are disclosures for public health activities. This means that sharing PHI for public health purposes is permitted as long as the agency to which the information is provided is legally authorized to collect and receive the information (see Lawful recipients of personal health information).
This specific exclusion was allowed because public health authorities have a legitimate need for PHI to ensure public health and safety, and because public health agencies have a track record of protecting the confidentiality of PHI. The HIPAA privacy rule attempts to strike a balance between individual privacy rights and the need for public protection.
Public health agencies included in this category include state, territorial, tribal, and local health departments, as well as federal health agencies such as the Centers for Disease Control and Prevention, the Food and Drug Administration, the National Institutes of Health, the Occupational Safety and Health Administration, the Substance Abuse and Mental Health Services Administration, and others. It also includes individuals and agencies working under a grant of authority from a public health agency.
Lawful disclosure: Examples
It’s instructive to consider how this public health HIPAA exception plays out in the daily practice of medicine. First, some definitions:
Protected Health Information.Individually identifiable health information transmitted electronically or any other way. It includes information about past, present, or anticipated mental or physical health, and the provision of or payment for health care.
Covered entities. These are the entities who must adhere to the HIPAA rules. Included are health care providers, health plans, and health care clearinghouses that transmit any health information in an electronic format
Personal Identifiers. Information that can be used to find the identity of an individual to link them to their PHI.
Scenario 1
A family physician’s patient dies at home. The physician is asked to fill out a death certificate, which contains PHI as defined by the HIPAA privacy rule. Is this permitted without family authorization?
Unauthorized disclosure is permitted. Vital statistics—required information on death and birth certificates—has not been changed by HIPAA. The information required on the death certificate can be provided without authorization.
Scenario 2
A patient is diagnosed with tuberculosis.This is a reportable disease per the state health code. Can the physician report the PHI requested on the disease reporting form?
Unauthorized disclosure is permitted. Each state health authority requires health care providers to report information about individuals who have contracted a disease of public health significance. Reportable disease lists differ by jurisdiction, and physicians should be aware of the diseases reportable in their areas and how the information is to be reported. Individual authorization for release of PHI in these disease reports is not required by HIPAA.
Scenario 3
A physician examines an infant who has unexplained injuries. Child abuse is suspected. Is child abuse reporting exempted from the privacy rule?
Unauthorized disclosure is permitted. Reporting of child abuse and neglect is exempted. This information may even be reported to a non-health agency, such as a child protective service, as long as the reportable information is required by law, and individual authorization is not required.
Scenario 4
A patient suffers what appears to be an adverse reaction to a medication. The FDA adverse event reporting form asks for PHI. Can a physician report PHI in this instance without patient authorization?
Unauthorized disclosure is permitted. Reporting of adverse events or reactions from drugs, food, biological products, and medical devices is still permitted without authorization.
Scenario 5
A patient is newly diagnosed with lung cancer. The state maintains a cancer registry and physicians are required to report PHI about patients with cancer. In this state the cancer registry is maintained by the university under contract with the State Health Department. Is reporting permitted without patient authorization?
Unauthorized disclosure is permitted. Cancer and immunization registry reporting of PHI is still permitted even if the entity responsible for the registry is not a public health agency, as long as it is under the authority of the agency to perform this public health function.
Scenario 6
A patient dies from meningitis and the local health department requests to view the hospital record to investigate cause of death. The cause turns out to be West Nile virus, which is not on the list of reportable diseases. Is the health department permitted to view the record and is authorization required?
Unauthorized disclosure is permitted. The privacy rule exception does not require a law or regulation specifically mandating disclosure. The health care provider can release requested information to a public health authority when the information is for the purpose of controlling disease, injury, or disability. The information released should be the minimum necessary for the stated public health purpose, and the provider can rely on the agency to determine what that information is. In this case, examination of the record is permitted and authorization is not required.
Scenario 7
An auditor from the Vaccine for Children program arrives at the office and requests to see patient records to audit adherence to the rules governing this program. Is the auditor allowed to exam records, and is authorization required?
Unauthorized disclosure is permitted. Patient records can be reviewed by staff of public health agencies authorized by law to collect PHI for program management purposes. No patient authorization is required.
Scenario 8
A local community agency is concerned about the potential health effects of groundwater contamination. They request information about all your patients who have contracted cancer within the past 5 years. What information can you provide them?
PHI disclosure requires patient authorization. This agency, unless under the authority of a public health agency to collect PHI, cannot obtain PHI without patient authorization. However, deidentified information could be provided. Deidentified data are not covered by HIPAA and do not require individual privacy protection or authorization for release. De-identification means removing 18 “identifiers” (Table) or enough information that allows a statistician to conclude that the chance of an individual being identified is remote.
TABLE
Individual identifiers to be removed from reports
| The following 18 identifiers of a person, or of relatives, employers, or household members of a person must be removed, and the covered entity must not have actual knowledge that the information could be used alone or in combination with other information to identify the individual, for the information to be considered de-identified and not protected health information. |
|---|
| • Names |
| • All geographic subdivisions smaller than a state, including county, city, street address, precinct, zip code (first 3 digits OK if geographic unit contains >20,000 persons), and their equivalent geocodes |
| • All elements of dates (except year) directly related to an individual; all ages >89 and all elements of dates (including year) indicative of such age (except for an aggregate into a single category of age >90) |
| • Telephone numbers |
| • Fax numbers |
| • Electronic mail addresses |
| • Social Security numbers |
| • Medical record numbers |
| • Health-plan beneficiary numbers |
| • Account numbers |
| • Certificate and license numbers |
| • Vehicle identifiers and serial numbers, including license plate numbers |
| • Medical device identifiers and serial numbers |
| • Internet universal resource locators (URLs) |
| • Internet protocol (IP) addresses |
| • Biometric identifiers, including fingerprints and voice prints |
| • Full-face photographic images and any comparable images |
| • Any other unique identifying number, characteristic, or code, except that covered identities may, under certain circumstances, assign a code or other means of record identification that allows de-identified information to be re-identified. |
| Source: “HIPAA privacy rule and public health,” Morbidity and Mortality Weekly Report, April 11, 2003; 52:1–12. |
Physician obligations with disclosure
Confirm the legitimacy of a request. Even though physicians can release PHI to public health agencies without a patient’s authorization, they have other obligations to meet. One of these is to ensure that the person or agency requesting PHI is a legitimate public health authority. If the request is made in person, some form of credentials or proof of government status should be provided. If the request is in writing, it should be on official letterhead. A person or agency acting under the authority of a pubic health agency should provide proof of this authority. If physicians have any doubt about the authenticity of a request, they should call the agency being represented and inquire.
Let patients know. The second obligation is to provide information about the disclosure to the individual whose PHI was released, if this information is requested, and to inform patients in statements about privacy practices that PHI information is released to public health agencies when required and permitted by law.
Other exceptions to HIPAA
HIPAA allows the legitimate use of PHI, without authorization, for the purpose of protecting the public under conditions involving law enforcement, court proceedings, worker’s compensation, and national security. These exceptions are outside the scope of this article.
Explain HIPAA to patients
The trend toward electronic medical records and the increasing public concern about privacy led to the enactment of the HIPAA privacy rule. A natural tension exists between individual rights and public protection, and the HIPAA privacy rule attempts to balance these competing concerns. For patients who are concerned about confidentiality, family physicians can explain the purpose of public health exceptions and give reassurance about how public health agencies have a good record of protecting individuals’ identity.
Correspondence
4001 North Third #415, Phoenix, AZ 85012. E-mail: [email protected].
Since the Health Insurance Portability and Accountability Act (HIPAA) privacy rule was put into effect in April 2003, healthcare providers have sometimes been confused about what information they can legally disclose to public health agencies. A clear understanding of permissible disclosure will enable family physicians to continue their important role of providing individual patient information for the critical activities of disease surveillance, outbreak investigation, monitoring causes of death and birth complications, assuring health care services, conducting public health research, and formulating health policy.
HIPAA does not prohibit disclosure for public health purposes
The HIPAA is intended to protect the public from unauthorized access to, use of, and disclosure of individually identifiable health information. It places responsibility on health care providers to avoid using or disclosing protected health information (PHI) unless authorized by the person to whom it pertains, or unless the disclosure or use is required or permitted by regulation or law. Specifically excluded from the requirement for individual authorization are disclosures for public health activities. This means that sharing PHI for public health purposes is permitted as long as the agency to which the information is provided is legally authorized to collect and receive the information (see Lawful recipients of personal health information).
This specific exclusion was allowed because public health authorities have a legitimate need for PHI to ensure public health and safety, and because public health agencies have a track record of protecting the confidentiality of PHI. The HIPAA privacy rule attempts to strike a balance between individual privacy rights and the need for public protection.
Public health agencies included in this category include state, territorial, tribal, and local health departments, as well as federal health agencies such as the Centers for Disease Control and Prevention, the Food and Drug Administration, the National Institutes of Health, the Occupational Safety and Health Administration, the Substance Abuse and Mental Health Services Administration, and others. It also includes individuals and agencies working under a grant of authority from a public health agency.
Lawful disclosure: Examples
It’s instructive to consider how this public health HIPAA exception plays out in the daily practice of medicine. First, some definitions:
Protected Health Information.Individually identifiable health information transmitted electronically or any other way. It includes information about past, present, or anticipated mental or physical health, and the provision of or payment for health care.
Covered entities. These are the entities who must adhere to the HIPAA rules. Included are health care providers, health plans, and health care clearinghouses that transmit any health information in an electronic format
Personal Identifiers. Information that can be used to find the identity of an individual to link them to their PHI.
Scenario 1
A family physician’s patient dies at home. The physician is asked to fill out a death certificate, which contains PHI as defined by the HIPAA privacy rule. Is this permitted without family authorization?
Unauthorized disclosure is permitted. Vital statistics—required information on death and birth certificates—has not been changed by HIPAA. The information required on the death certificate can be provided without authorization.
Scenario 2
A patient is diagnosed with tuberculosis.This is a reportable disease per the state health code. Can the physician report the PHI requested on the disease reporting form?
Unauthorized disclosure is permitted. Each state health authority requires health care providers to report information about individuals who have contracted a disease of public health significance. Reportable disease lists differ by jurisdiction, and physicians should be aware of the diseases reportable in their areas and how the information is to be reported. Individual authorization for release of PHI in these disease reports is not required by HIPAA.
Scenario 3
A physician examines an infant who has unexplained injuries. Child abuse is suspected. Is child abuse reporting exempted from the privacy rule?
Unauthorized disclosure is permitted. Reporting of child abuse and neglect is exempted. This information may even be reported to a non-health agency, such as a child protective service, as long as the reportable information is required by law, and individual authorization is not required.
Scenario 4
A patient suffers what appears to be an adverse reaction to a medication. The FDA adverse event reporting form asks for PHI. Can a physician report PHI in this instance without patient authorization?
Unauthorized disclosure is permitted. Reporting of adverse events or reactions from drugs, food, biological products, and medical devices is still permitted without authorization.
Scenario 5
A patient is newly diagnosed with lung cancer. The state maintains a cancer registry and physicians are required to report PHI about patients with cancer. In this state the cancer registry is maintained by the university under contract with the State Health Department. Is reporting permitted without patient authorization?
Unauthorized disclosure is permitted. Cancer and immunization registry reporting of PHI is still permitted even if the entity responsible for the registry is not a public health agency, as long as it is under the authority of the agency to perform this public health function.
Scenario 6
A patient dies from meningitis and the local health department requests to view the hospital record to investigate cause of death. The cause turns out to be West Nile virus, which is not on the list of reportable diseases. Is the health department permitted to view the record and is authorization required?
Unauthorized disclosure is permitted. The privacy rule exception does not require a law or regulation specifically mandating disclosure. The health care provider can release requested information to a public health authority when the information is for the purpose of controlling disease, injury, or disability. The information released should be the minimum necessary for the stated public health purpose, and the provider can rely on the agency to determine what that information is. In this case, examination of the record is permitted and authorization is not required.
Scenario 7
An auditor from the Vaccine for Children program arrives at the office and requests to see patient records to audit adherence to the rules governing this program. Is the auditor allowed to exam records, and is authorization required?
Unauthorized disclosure is permitted. Patient records can be reviewed by staff of public health agencies authorized by law to collect PHI for program management purposes. No patient authorization is required.
Scenario 8
A local community agency is concerned about the potential health effects of groundwater contamination. They request information about all your patients who have contracted cancer within the past 5 years. What information can you provide them?
PHI disclosure requires patient authorization. This agency, unless under the authority of a public health agency to collect PHI, cannot obtain PHI without patient authorization. However, deidentified information could be provided. Deidentified data are not covered by HIPAA and do not require individual privacy protection or authorization for release. De-identification means removing 18 “identifiers” (Table) or enough information that allows a statistician to conclude that the chance of an individual being identified is remote.
TABLE
Individual identifiers to be removed from reports
| The following 18 identifiers of a person, or of relatives, employers, or household members of a person must be removed, and the covered entity must not have actual knowledge that the information could be used alone or in combination with other information to identify the individual, for the information to be considered de-identified and not protected health information. |
|---|
| • Names |
| • All geographic subdivisions smaller than a state, including county, city, street address, precinct, zip code (first 3 digits OK if geographic unit contains >20,000 persons), and their equivalent geocodes |
| • All elements of dates (except year) directly related to an individual; all ages >89 and all elements of dates (including year) indicative of such age (except for an aggregate into a single category of age >90) |
| • Telephone numbers |
| • Fax numbers |
| • Electronic mail addresses |
| • Social Security numbers |
| • Medical record numbers |
| • Health-plan beneficiary numbers |
| • Account numbers |
| • Certificate and license numbers |
| • Vehicle identifiers and serial numbers, including license plate numbers |
| • Medical device identifiers and serial numbers |
| • Internet universal resource locators (URLs) |
| • Internet protocol (IP) addresses |
| • Biometric identifiers, including fingerprints and voice prints |
| • Full-face photographic images and any comparable images |
| • Any other unique identifying number, characteristic, or code, except that covered identities may, under certain circumstances, assign a code or other means of record identification that allows de-identified information to be re-identified. |
| Source: “HIPAA privacy rule and public health,” Morbidity and Mortality Weekly Report, April 11, 2003; 52:1–12. |
Physician obligations with disclosure
Confirm the legitimacy of a request. Even though physicians can release PHI to public health agencies without a patient’s authorization, they have other obligations to meet. One of these is to ensure that the person or agency requesting PHI is a legitimate public health authority. If the request is made in person, some form of credentials or proof of government status should be provided. If the request is in writing, it should be on official letterhead. A person or agency acting under the authority of a pubic health agency should provide proof of this authority. If physicians have any doubt about the authenticity of a request, they should call the agency being represented and inquire.
Let patients know. The second obligation is to provide information about the disclosure to the individual whose PHI was released, if this information is requested, and to inform patients in statements about privacy practices that PHI information is released to public health agencies when required and permitted by law.
Other exceptions to HIPAA
HIPAA allows the legitimate use of PHI, without authorization, for the purpose of protecting the public under conditions involving law enforcement, court proceedings, worker’s compensation, and national security. These exceptions are outside the scope of this article.
Explain HIPAA to patients
The trend toward electronic medical records and the increasing public concern about privacy led to the enactment of the HIPAA privacy rule. A natural tension exists between individual rights and public protection, and the HIPAA privacy rule attempts to balance these competing concerns. For patients who are concerned about confidentiality, family physicians can explain the purpose of public health exceptions and give reassurance about how public health agencies have a good record of protecting individuals’ identity.
Correspondence
4001 North Third #415, Phoenix, AZ 85012. E-mail: [email protected].