User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Risk of cancer in NAFLD is 91% higher than in control subjects
SAN FRANCISCO –
Those are key findings from a community cohort study with up to 21 years of follow-up, which one of the authors, Alina M. Allen, MD, discussed during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD is the most common chronic liver disease in the Western world,” said Dr. Allen, a gastroenterologist at the Mayo Clinic, Rochester, Minn. “It affects one in four adults in the U.S. It is related to fat accumulation in the liver in people who are overweight or obese and can lead to cirrhosis and liver-related mortality. However, the most common cause of death in this population is not liver disease but malignancy and cardiovascular disease. There is a paucity of epidemiologic studies of extrahepatic cancer in NAFLD. It is not clear what types of cancers and how much higher their cancer risk is in reference to the general population.”
In an effort to determine the incidence of cancer diagnoses in NALFD, compared with controls, in a U.S. community, Dr. Allen and her colleagues drew from the Rochester Epidemiology Project to evaluate 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects in Olmstead County, Minn., during 1997-2017. The researchers obtained corresponding Surveillance, Epidemiology, and End Results Program (SEER) rates as a quality check and used a regression model to assess the malignancy risk in NAFLD overall and by cancer type, age, sex, and body mass index. They recorded all new diagnoses of cancers that developed in both groups until 2018, for a total possible follow-up of 21 years, and they reported results in incidence rate ratios, a risk estimate similar to hazard ratios.
The mean age of the study population was 53 years, 53% were female, and the mean follow-up was 8 years with a range from 1 to 21 years. New cancers were identified in 16% of subjects with NALFD and in 12% of control subjects. The overall risk of malignancy was 91% higher in NAFLD subjects, compared with control subjects; there were higher rates in the NAFLD subjects for most types of cancers, but the largest increases were in GI cancers. The greatest malignancy risk was for cancer of the liver (RR, 3.24), followed by cancer of the uterus (RR, 2.39), stomach (RR, 2.34), pancreas (RR, 2.09), and colon (RR, 1.75). “Interestingly, the risk of colon cancer increased only in men but not in women,” Dr. Allen said. “These data provide an important hierarchical overview of the top most important malignancy risks associated with NAFLD that the medical community should be aware of.”
When the researchers looked for differences in age at cancer diagnosis between NAFLD and controls, they found that pancreas cancer occurred at a younger age among subjects with NAFLD. They also observed that colon cancer occurred at a younger age in men with NAFLD, but not in women with the disease. “What was most interesting to us was the assessment of cancer risk in NAFLD versus obesity alone,” Dr. Allen said. “Previous studies from the general population have linked obesity to a higher risk of cancer. Whether the presence of fatty liver disease would impact that risk has not been assessed. We showed that obesity is associated with a higher risk of cancer only in those with NAFLD, not in those without. If validated in independent cohorts, these findings could change our understanding of the relationship between obesity and cancer and the importance of screening for NAFLD – not only to risk-stratify liver disease but also for the risk of extrahepatic malignancy.”
Dr. Allen concluded her presentation by noting that findings from large population-based studies such as the Rochester Epidemiology Project “can offer important epidemiologic data regarding the biggest threats to the health of a community,” she said. “Such data increase awareness, enable appropriate counseling, and could inform screening policies. There is a signal in the fact that the GI cancers are increased [in NAFLD]. It’s an interesting signal that needs to be studied further.”
Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]: Abstract 31.
SAN FRANCISCO –
Those are key findings from a community cohort study with up to 21 years of follow-up, which one of the authors, Alina M. Allen, MD, discussed during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD is the most common chronic liver disease in the Western world,” said Dr. Allen, a gastroenterologist at the Mayo Clinic, Rochester, Minn. “It affects one in four adults in the U.S. It is related to fat accumulation in the liver in people who are overweight or obese and can lead to cirrhosis and liver-related mortality. However, the most common cause of death in this population is not liver disease but malignancy and cardiovascular disease. There is a paucity of epidemiologic studies of extrahepatic cancer in NAFLD. It is not clear what types of cancers and how much higher their cancer risk is in reference to the general population.”
In an effort to determine the incidence of cancer diagnoses in NALFD, compared with controls, in a U.S. community, Dr. Allen and her colleagues drew from the Rochester Epidemiology Project to evaluate 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects in Olmstead County, Minn., during 1997-2017. The researchers obtained corresponding Surveillance, Epidemiology, and End Results Program (SEER) rates as a quality check and used a regression model to assess the malignancy risk in NAFLD overall and by cancer type, age, sex, and body mass index. They recorded all new diagnoses of cancers that developed in both groups until 2018, for a total possible follow-up of 21 years, and they reported results in incidence rate ratios, a risk estimate similar to hazard ratios.
The mean age of the study population was 53 years, 53% were female, and the mean follow-up was 8 years with a range from 1 to 21 years. New cancers were identified in 16% of subjects with NALFD and in 12% of control subjects. The overall risk of malignancy was 91% higher in NAFLD subjects, compared with control subjects; there were higher rates in the NAFLD subjects for most types of cancers, but the largest increases were in GI cancers. The greatest malignancy risk was for cancer of the liver (RR, 3.24), followed by cancer of the uterus (RR, 2.39), stomach (RR, 2.34), pancreas (RR, 2.09), and colon (RR, 1.75). “Interestingly, the risk of colon cancer increased only in men but not in women,” Dr. Allen said. “These data provide an important hierarchical overview of the top most important malignancy risks associated with NAFLD that the medical community should be aware of.”
When the researchers looked for differences in age at cancer diagnosis between NAFLD and controls, they found that pancreas cancer occurred at a younger age among subjects with NAFLD. They also observed that colon cancer occurred at a younger age in men with NAFLD, but not in women with the disease. “What was most interesting to us was the assessment of cancer risk in NAFLD versus obesity alone,” Dr. Allen said. “Previous studies from the general population have linked obesity to a higher risk of cancer. Whether the presence of fatty liver disease would impact that risk has not been assessed. We showed that obesity is associated with a higher risk of cancer only in those with NAFLD, not in those without. If validated in independent cohorts, these findings could change our understanding of the relationship between obesity and cancer and the importance of screening for NAFLD – not only to risk-stratify liver disease but also for the risk of extrahepatic malignancy.”
Dr. Allen concluded her presentation by noting that findings from large population-based studies such as the Rochester Epidemiology Project “can offer important epidemiologic data regarding the biggest threats to the health of a community,” she said. “Such data increase awareness, enable appropriate counseling, and could inform screening policies. There is a signal in the fact that the GI cancers are increased [in NAFLD]. It’s an interesting signal that needs to be studied further.”
Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]: Abstract 31.
SAN FRANCISCO –
Those are key findings from a community cohort study with up to 21 years of follow-up, which one of the authors, Alina M. Allen, MD, discussed during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD is the most common chronic liver disease in the Western world,” said Dr. Allen, a gastroenterologist at the Mayo Clinic, Rochester, Minn. “It affects one in four adults in the U.S. It is related to fat accumulation in the liver in people who are overweight or obese and can lead to cirrhosis and liver-related mortality. However, the most common cause of death in this population is not liver disease but malignancy and cardiovascular disease. There is a paucity of epidemiologic studies of extrahepatic cancer in NAFLD. It is not clear what types of cancers and how much higher their cancer risk is in reference to the general population.”
In an effort to determine the incidence of cancer diagnoses in NALFD, compared with controls, in a U.S. community, Dr. Allen and her colleagues drew from the Rochester Epidemiology Project to evaluate 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects in Olmstead County, Minn., during 1997-2017. The researchers obtained corresponding Surveillance, Epidemiology, and End Results Program (SEER) rates as a quality check and used a regression model to assess the malignancy risk in NAFLD overall and by cancer type, age, sex, and body mass index. They recorded all new diagnoses of cancers that developed in both groups until 2018, for a total possible follow-up of 21 years, and they reported results in incidence rate ratios, a risk estimate similar to hazard ratios.
The mean age of the study population was 53 years, 53% were female, and the mean follow-up was 8 years with a range from 1 to 21 years. New cancers were identified in 16% of subjects with NALFD and in 12% of control subjects. The overall risk of malignancy was 91% higher in NAFLD subjects, compared with control subjects; there were higher rates in the NAFLD subjects for most types of cancers, but the largest increases were in GI cancers. The greatest malignancy risk was for cancer of the liver (RR, 3.24), followed by cancer of the uterus (RR, 2.39), stomach (RR, 2.34), pancreas (RR, 2.09), and colon (RR, 1.75). “Interestingly, the risk of colon cancer increased only in men but not in women,” Dr. Allen said. “These data provide an important hierarchical overview of the top most important malignancy risks associated with NAFLD that the medical community should be aware of.”
When the researchers looked for differences in age at cancer diagnosis between NAFLD and controls, they found that pancreas cancer occurred at a younger age among subjects with NAFLD. They also observed that colon cancer occurred at a younger age in men with NAFLD, but not in women with the disease. “What was most interesting to us was the assessment of cancer risk in NAFLD versus obesity alone,” Dr. Allen said. “Previous studies from the general population have linked obesity to a higher risk of cancer. Whether the presence of fatty liver disease would impact that risk has not been assessed. We showed that obesity is associated with a higher risk of cancer only in those with NAFLD, not in those without. If validated in independent cohorts, these findings could change our understanding of the relationship between obesity and cancer and the importance of screening for NAFLD – not only to risk-stratify liver disease but also for the risk of extrahepatic malignancy.”
Dr. Allen concluded her presentation by noting that findings from large population-based studies such as the Rochester Epidemiology Project “can offer important epidemiologic data regarding the biggest threats to the health of a community,” she said. “Such data increase awareness, enable appropriate counseling, and could inform screening policies. There is a signal in the fact that the GI cancers are increased [in NAFLD]. It’s an interesting signal that needs to be studied further.”
Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]: Abstract 31.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: The risk of cancer in patients with nonalcoholic fatty liver disease is 91% higher than in control subjects.
Major finding: Among subjects with NAFLD, the greatest malignancy risk was for cancer of the liver (risk ratio, 3.24).
Study details: A cohort study of 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects from the Rochester Epidemiology Project.
Disclosures: Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]:Abstract 31
Chronic liver disease independently linked to increased risk of falls
SAN FRANCISCO – .
“We have previously known that having cirrhosis, for example, is associated with the risk of falling, but we didn’t have any data from a nationally representative sample,” lead study author Maria Camila Pérez-Matos, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “What surprised us is that just by having chronic liver disease – any subtype – you’re more likely to fall, and also to have a fracture after you have fallen.”
In an effort to define the association between CLD and fall history and its related injuries, Dr. Pérez-Matos of the division of gastroenterology and hepatology at Beth Israel Deaconess Medical Center, Boston, and her associates examined data from 5,363 subjects aged 60 years and older in the Third National Health and Nutrition Examination Survey, which represents the noninstitutionalized civilian population in the United States. Their outcomes of interest were one or more falls occurring in the previous 12 months and fall-related injuries. The main exposure was definitive CLD, defined by chronic viral hepatitis (hepatitis C RNA or hepatitis B surface antigen), nonalcoholic steatohepatitis (NASH; hepatosteatosis by ultrasound with abnormal transaminases), and alcohol-related liver disease (females consuming more than 7 drinks/week and males consuming 14 drinks/week among, plus having abnormal transaminases). Suspected CLD was defined as having abnormal alanine aminotransferase levels (males greater than 30 IU/L, females greater than 19 IU/L), aspartate aminotransferase levels above 33 IU/L, or alkaline phosphatase levels above 100 IU/L. The researchers used univariate and multivariate logistic regression to examine associations.
The average age of subjects was 70 years, and 59% were female. Of the 5,363 subjects, 340 had definitive CLD. Of these, 234 (69%) had NASH, 85 (25%) had viral hepatitis, and 21 (6%) had alcoholic liver disease. Subjects with definitive CLD were more likely to be female and have diabetes mellitus, a higher body mass index, and physical/functional impairment. Dr. Pérez-Matos and her colleagues found that definitive CLD was associated with a 52% increase in the odds of having a history of falls (odds ratio, 1.52; P = .01). The association remained significant after controlling for age, sex, smoking, race, physical or functional impairment, impaired vision, polypharmacy, and body mass index. The degree of excess falling risk posed by CLD was similar to that of having impaired vision (OR, 1.48; P less than .001).
Of the CLD subtypes, subjects with viral hepatitis had the strongest association with a history of falls (OR, 2.2; P = .001). In addition, definitive CLD was found to have significant association with any physical impairment, even after adjusting for relevant covariates (OR, 1.63; P = .001).
Finally, multivariate logistic regression revealed that both suspected and definitive CLD were associated with injurious falls (OR, 1.40 and OR of 1.67, respectively). “Everyone is interested in preventing falls because of its public health impact, and predictors of falls are relatively limited,” said Elliott B. Tapper, MD, the study’s principal investigator, who is with the division of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “Because chronic liver disease is increasingly common, our data is speaking to a hitherto unknown risk factor: one which if you apply it to other data sets might help figure out why more people are falling. The lesson is, there’s something about chronic liver disease; it’s a sign. If it’s fatty liver disease, it’s a sign that diabetes has taken its toll on the body – its nerves and muscles. There’s something about what’s going on in that person that’s worse than it is for other people. We don’t know cause or effect, but the association is strong and deserves further study, particularly when it comes to determining [which patients] in our clinics are at higher risk and making sure they’re doing physical therapy to prevent falls in the future.”
Dr. Tapper disclosed that she has a career development award from the National Institutes of Health. Dr. Pérez-Matos reported having no monetary conflicts.
Source: Hepatol. 2018;68[S1], Abstract 756.
SAN FRANCISCO – .
“We have previously known that having cirrhosis, for example, is associated with the risk of falling, but we didn’t have any data from a nationally representative sample,” lead study author Maria Camila Pérez-Matos, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “What surprised us is that just by having chronic liver disease – any subtype – you’re more likely to fall, and also to have a fracture after you have fallen.”
In an effort to define the association between CLD and fall history and its related injuries, Dr. Pérez-Matos of the division of gastroenterology and hepatology at Beth Israel Deaconess Medical Center, Boston, and her associates examined data from 5,363 subjects aged 60 years and older in the Third National Health and Nutrition Examination Survey, which represents the noninstitutionalized civilian population in the United States. Their outcomes of interest were one or more falls occurring in the previous 12 months and fall-related injuries. The main exposure was definitive CLD, defined by chronic viral hepatitis (hepatitis C RNA or hepatitis B surface antigen), nonalcoholic steatohepatitis (NASH; hepatosteatosis by ultrasound with abnormal transaminases), and alcohol-related liver disease (females consuming more than 7 drinks/week and males consuming 14 drinks/week among, plus having abnormal transaminases). Suspected CLD was defined as having abnormal alanine aminotransferase levels (males greater than 30 IU/L, females greater than 19 IU/L), aspartate aminotransferase levels above 33 IU/L, or alkaline phosphatase levels above 100 IU/L. The researchers used univariate and multivariate logistic regression to examine associations.
The average age of subjects was 70 years, and 59% were female. Of the 5,363 subjects, 340 had definitive CLD. Of these, 234 (69%) had NASH, 85 (25%) had viral hepatitis, and 21 (6%) had alcoholic liver disease. Subjects with definitive CLD were more likely to be female and have diabetes mellitus, a higher body mass index, and physical/functional impairment. Dr. Pérez-Matos and her colleagues found that definitive CLD was associated with a 52% increase in the odds of having a history of falls (odds ratio, 1.52; P = .01). The association remained significant after controlling for age, sex, smoking, race, physical or functional impairment, impaired vision, polypharmacy, and body mass index. The degree of excess falling risk posed by CLD was similar to that of having impaired vision (OR, 1.48; P less than .001).
Of the CLD subtypes, subjects with viral hepatitis had the strongest association with a history of falls (OR, 2.2; P = .001). In addition, definitive CLD was found to have significant association with any physical impairment, even after adjusting for relevant covariates (OR, 1.63; P = .001).
Finally, multivariate logistic regression revealed that both suspected and definitive CLD were associated with injurious falls (OR, 1.40 and OR of 1.67, respectively). “Everyone is interested in preventing falls because of its public health impact, and predictors of falls are relatively limited,” said Elliott B. Tapper, MD, the study’s principal investigator, who is with the division of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “Because chronic liver disease is increasingly common, our data is speaking to a hitherto unknown risk factor: one which if you apply it to other data sets might help figure out why more people are falling. The lesson is, there’s something about chronic liver disease; it’s a sign. If it’s fatty liver disease, it’s a sign that diabetes has taken its toll on the body – its nerves and muscles. There’s something about what’s going on in that person that’s worse than it is for other people. We don’t know cause or effect, but the association is strong and deserves further study, particularly when it comes to determining [which patients] in our clinics are at higher risk and making sure they’re doing physical therapy to prevent falls in the future.”
Dr. Tapper disclosed that she has a career development award from the National Institutes of Health. Dr. Pérez-Matos reported having no monetary conflicts.
Source: Hepatol. 2018;68[S1], Abstract 756.
SAN FRANCISCO – .
“We have previously known that having cirrhosis, for example, is associated with the risk of falling, but we didn’t have any data from a nationally representative sample,” lead study author Maria Camila Pérez-Matos, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “What surprised us is that just by having chronic liver disease – any subtype – you’re more likely to fall, and also to have a fracture after you have fallen.”
In an effort to define the association between CLD and fall history and its related injuries, Dr. Pérez-Matos of the division of gastroenterology and hepatology at Beth Israel Deaconess Medical Center, Boston, and her associates examined data from 5,363 subjects aged 60 years and older in the Third National Health and Nutrition Examination Survey, which represents the noninstitutionalized civilian population in the United States. Their outcomes of interest were one or more falls occurring in the previous 12 months and fall-related injuries. The main exposure was definitive CLD, defined by chronic viral hepatitis (hepatitis C RNA or hepatitis B surface antigen), nonalcoholic steatohepatitis (NASH; hepatosteatosis by ultrasound with abnormal transaminases), and alcohol-related liver disease (females consuming more than 7 drinks/week and males consuming 14 drinks/week among, plus having abnormal transaminases). Suspected CLD was defined as having abnormal alanine aminotransferase levels (males greater than 30 IU/L, females greater than 19 IU/L), aspartate aminotransferase levels above 33 IU/L, or alkaline phosphatase levels above 100 IU/L. The researchers used univariate and multivariate logistic regression to examine associations.
The average age of subjects was 70 years, and 59% were female. Of the 5,363 subjects, 340 had definitive CLD. Of these, 234 (69%) had NASH, 85 (25%) had viral hepatitis, and 21 (6%) had alcoholic liver disease. Subjects with definitive CLD were more likely to be female and have diabetes mellitus, a higher body mass index, and physical/functional impairment. Dr. Pérez-Matos and her colleagues found that definitive CLD was associated with a 52% increase in the odds of having a history of falls (odds ratio, 1.52; P = .01). The association remained significant after controlling for age, sex, smoking, race, physical or functional impairment, impaired vision, polypharmacy, and body mass index. The degree of excess falling risk posed by CLD was similar to that of having impaired vision (OR, 1.48; P less than .001).
Of the CLD subtypes, subjects with viral hepatitis had the strongest association with a history of falls (OR, 2.2; P = .001). In addition, definitive CLD was found to have significant association with any physical impairment, even after adjusting for relevant covariates (OR, 1.63; P = .001).
Finally, multivariate logistic regression revealed that both suspected and definitive CLD were associated with injurious falls (OR, 1.40 and OR of 1.67, respectively). “Everyone is interested in preventing falls because of its public health impact, and predictors of falls are relatively limited,” said Elliott B. Tapper, MD, the study’s principal investigator, who is with the division of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “Because chronic liver disease is increasingly common, our data is speaking to a hitherto unknown risk factor: one which if you apply it to other data sets might help figure out why more people are falling. The lesson is, there’s something about chronic liver disease; it’s a sign. If it’s fatty liver disease, it’s a sign that diabetes has taken its toll on the body – its nerves and muscles. There’s something about what’s going on in that person that’s worse than it is for other people. We don’t know cause or effect, but the association is strong and deserves further study, particularly when it comes to determining [which patients] in our clinics are at higher risk and making sure they’re doing physical therapy to prevent falls in the future.”
Dr. Tapper disclosed that she has a career development award from the National Institutes of Health. Dr. Pérez-Matos reported having no monetary conflicts.
Source: Hepatol. 2018;68[S1], Abstract 756.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Attention to falls is warranted in chronic liver disease (CLD) patients at all stages of disease.
Major finding: Having definitive CLD was associated with a 52% increase in the odds of having a history of falls (OR 1.52; P = .01).
Study details: A cross-sectional analysis of 5,363 subjects in the Third National Health and Nutrition Examination Survey.
Disclosures: Dr. Tapper disclosed that he has a career development award from the National Institutes of Health. Dr. Perez-Matos reported having no monetary conflicts.
Source: Hepatol. 2018;68[S1]:Abstract 756.
About 13% of liver transplant recipients affected by PTSD
SAN FRANCISCO –
“Serving the emotional and mental health of these patients is just as important as [improving] liver function and their immunosuppression,” study author Meg O’Meara, NP, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases.
Recent data suggests that patients who undergo significant medical events like solid organ transplantation face a risk of developing PTSD, but limited information exists regarding the psychological effects following adult liver transplantation, said Ms. O’Meara, a nurse practitioner in the division of gastroenterology and hepatology at the University of Colorado at Denver, Aurora. In an effort to determine the prevalence of and risk factors for PTSD in adult liver transplant recipients, she and her associates used the PCL-5 to screen 71 patients seen at the university’s posttransplant clinic from Dec. 1, 2017, to May 31, 2018. The PCL-5 is a validated 20-item questionnaire that corresponds to the DSM-5 symptom criteria for PTSD.
The researchers also collected clinical and demographic information including pretransplant disease severity, history of psychiatric disease, duration of transplant hospitalization, and need for rehospitalization. They used a multivariable regression model to evaluate associations between clinical and demographic variables and a positive screen for PTSD.
The median age of the 71 patients was 58 years; 61% were male. A preexisting diagnosis of depression was present in 25 patients (35%), their mean Model for End-Stage Liver Disease (MELD) score at time of transplant was 31, and their median time from transplant was 1,163 days. In all, nine patients (12.7%) tested positive for PTSD on the PCL-5, and all met criteria for a provisional diagnosis of PTSD based on the DSM-5 criteria. This prevalence is about two times higher than the prevalence of PTSD in the general population (6.7%), comparable with that reported for heart transplant recipients (10.8%) and coronary artery disease (9%), but lower than that reported for ICU patients (38%), HIV patients (35.3%), and in those with Crohn’s disease (19.1%).
On multivariable logistic regression, only three factors were found to be significantly associated with the development of PTSD: younger age at transplant (P = .019), history of depression (P = .008), and a history of PTSD (P less than .001). “What I found surprising was that the MELD score at the time of transplant and the number of readmissions or complications around the time of transplant were not predictive of PTSD,” Ms. O’Meara said.
One possible explanation for the finding of younger age being a predictor of PTSD, she continued, is that younger liver transplant patients may lack certain coping skills, compared with their older counterparts. “Maybe patients who have had more years to deal with their disease are more accepting of it and they learn how to use productive tools to manage it.”
In their abstract, the researchers acknowledged certain limitations of the study, including its cross-sectional design, small sample size, and lack of corresponding pretransplant data.
The researchers reported having no financial disclosures.
SAN FRANCISCO –
“Serving the emotional and mental health of these patients is just as important as [improving] liver function and their immunosuppression,” study author Meg O’Meara, NP, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases.
Recent data suggests that patients who undergo significant medical events like solid organ transplantation face a risk of developing PTSD, but limited information exists regarding the psychological effects following adult liver transplantation, said Ms. O’Meara, a nurse practitioner in the division of gastroenterology and hepatology at the University of Colorado at Denver, Aurora. In an effort to determine the prevalence of and risk factors for PTSD in adult liver transplant recipients, she and her associates used the PCL-5 to screen 71 patients seen at the university’s posttransplant clinic from Dec. 1, 2017, to May 31, 2018. The PCL-5 is a validated 20-item questionnaire that corresponds to the DSM-5 symptom criteria for PTSD.
The researchers also collected clinical and demographic information including pretransplant disease severity, history of psychiatric disease, duration of transplant hospitalization, and need for rehospitalization. They used a multivariable regression model to evaluate associations between clinical and demographic variables and a positive screen for PTSD.
The median age of the 71 patients was 58 years; 61% were male. A preexisting diagnosis of depression was present in 25 patients (35%), their mean Model for End-Stage Liver Disease (MELD) score at time of transplant was 31, and their median time from transplant was 1,163 days. In all, nine patients (12.7%) tested positive for PTSD on the PCL-5, and all met criteria for a provisional diagnosis of PTSD based on the DSM-5 criteria. This prevalence is about two times higher than the prevalence of PTSD in the general population (6.7%), comparable with that reported for heart transplant recipients (10.8%) and coronary artery disease (9%), but lower than that reported for ICU patients (38%), HIV patients (35.3%), and in those with Crohn’s disease (19.1%).
On multivariable logistic regression, only three factors were found to be significantly associated with the development of PTSD: younger age at transplant (P = .019), history of depression (P = .008), and a history of PTSD (P less than .001). “What I found surprising was that the MELD score at the time of transplant and the number of readmissions or complications around the time of transplant were not predictive of PTSD,” Ms. O’Meara said.
One possible explanation for the finding of younger age being a predictor of PTSD, she continued, is that younger liver transplant patients may lack certain coping skills, compared with their older counterparts. “Maybe patients who have had more years to deal with their disease are more accepting of it and they learn how to use productive tools to manage it.”
In their abstract, the researchers acknowledged certain limitations of the study, including its cross-sectional design, small sample size, and lack of corresponding pretransplant data.
The researchers reported having no financial disclosures.
SAN FRANCISCO –
“Serving the emotional and mental health of these patients is just as important as [improving] liver function and their immunosuppression,” study author Meg O’Meara, NP, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases.
Recent data suggests that patients who undergo significant medical events like solid organ transplantation face a risk of developing PTSD, but limited information exists regarding the psychological effects following adult liver transplantation, said Ms. O’Meara, a nurse practitioner in the division of gastroenterology and hepatology at the University of Colorado at Denver, Aurora. In an effort to determine the prevalence of and risk factors for PTSD in adult liver transplant recipients, she and her associates used the PCL-5 to screen 71 patients seen at the university’s posttransplant clinic from Dec. 1, 2017, to May 31, 2018. The PCL-5 is a validated 20-item questionnaire that corresponds to the DSM-5 symptom criteria for PTSD.
The researchers also collected clinical and demographic information including pretransplant disease severity, history of psychiatric disease, duration of transplant hospitalization, and need for rehospitalization. They used a multivariable regression model to evaluate associations between clinical and demographic variables and a positive screen for PTSD.
The median age of the 71 patients was 58 years; 61% were male. A preexisting diagnosis of depression was present in 25 patients (35%), their mean Model for End-Stage Liver Disease (MELD) score at time of transplant was 31, and their median time from transplant was 1,163 days. In all, nine patients (12.7%) tested positive for PTSD on the PCL-5, and all met criteria for a provisional diagnosis of PTSD based on the DSM-5 criteria. This prevalence is about two times higher than the prevalence of PTSD in the general population (6.7%), comparable with that reported for heart transplant recipients (10.8%) and coronary artery disease (9%), but lower than that reported for ICU patients (38%), HIV patients (35.3%), and in those with Crohn’s disease (19.1%).
On multivariable logistic regression, only three factors were found to be significantly associated with the development of PTSD: younger age at transplant (P = .019), history of depression (P = .008), and a history of PTSD (P less than .001). “What I found surprising was that the MELD score at the time of transplant and the number of readmissions or complications around the time of transplant were not predictive of PTSD,” Ms. O’Meara said.
One possible explanation for the finding of younger age being a predictor of PTSD, she continued, is that younger liver transplant patients may lack certain coping skills, compared with their older counterparts. “Maybe patients who have had more years to deal with their disease are more accepting of it and they learn how to use productive tools to manage it.”
In their abstract, the researchers acknowledged certain limitations of the study, including its cross-sectional design, small sample size, and lack of corresponding pretransplant data.
The researchers reported having no financial disclosures.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Liver transplant recipients have a higher rate of PTSD than the general population and younger patients may be at a higher risk.
Major finding: In all, nine liver transplant patients (12.7%) tested positive for PTSD, which is about two times higher than the prevalence of PTSD in the general population (6.7%).
Study details: A cross-sectional analysis of 71 liver transplant recipients.
Disclosures: The researchers reported having no financial disclosures.
Brain stimulation device improved fluency in persons who stutter
ATLANTA – After undergoing 10 days of noninvasive magnetic brain stimulation with a novel device, eight of nine persons who stutter experienced improvements in speech fluency.
“This is the first step in what we believe is a major breakthrough in treatment,” lead study author David B. Rosenfield, MD, said in an interview at the annual meeting of the American Neurological Association.
In previously published work, Dr. Rosenfield, Director of the Speech and Language Center at Houston Methodist Neurological Institute, and his colleagues showed significant reduction in functional connectivity between Broca’s and Wernicke’s areas in persons who stutter, compared with normal speakers, by performing resting-state functional MRI of the brain. In the current open-label pilot trial, they tested the hypothesis that using direct noninvasive synchronous bifocal stimulation to potentiate the strength of connectivity between Broca’s and Wernicke’s areas in persons who stutter should improve their fluency.
Researchers enrolled nine persons who stutter who ranged in age from 18 to 80 years. For 40 minutes each consecutive weekday over the course of 2 weeks, the participants wore a compact, portable device known as a Transcranial Rotating Permanent Magnetic Stimulator (TRPMS) to deliver highly focal stimuli to Broca’s and Wernicke’s area locations specified by 10-20 international electroencephalographic electrode sites on the left side. Magnetic stimuli were 100 milliseconds in duration and delivered at 0.2 Hz. Next, a certified speech-language pathologist viewed video recordings of the study subjects both speaking spontaneously and reading a passage aloud on day 1 (before stimulation), day 5 (after stimulation), and day 10 (after stimulation), to assess fluency using the Stuttering Speech Severity Instrument version 4 (SSI-4).
The researchers found that all study participants significantly improved in fluency on either day 5 (P = 0.01) or day 10 (P = 0.02). Only one subject failed to show improvement on day 10 compared with day 1 after showing it on day 5. “This wasn’t meant to be a 10-day treatment that would last forever; this was meant to be a 10-day treatment to see whether the magnetic stimulation would work,” Dr. Rosenfield said. “It might well be that patients need treatment every day, once a week, or once a month. All we can say is that we have an input and an output. We gave them the treatment and they improved. One patient was so happy with it that he begged us to come back for additional treatment. It seems as though it’s a robust therapy.”
Going forward, the researchers plan to study fMRI brain imaging before and following external magnetic treatments to speech and language areas to confirm the efficacy of this therapy in a randomized, double-blind, sham treatment-controlled trial.
The TRPMS device was coinvented by Santosh A. Helekar, MD, PhD, and Henning Voss, PhD, both at Weill Cornell Medical College. The study received funding support from The Houston Methodist Hospital System Physicians Organization. Dr. Rosenfield reported having no financial disclosures. He noted that commercialization of the patented technology underlying the TRPMS device used in this study and in other diseases is currently being advanced by Seraya Medical Systems LLC.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S45-6. Abstract S115.
ATLANTA – After undergoing 10 days of noninvasive magnetic brain stimulation with a novel device, eight of nine persons who stutter experienced improvements in speech fluency.
“This is the first step in what we believe is a major breakthrough in treatment,” lead study author David B. Rosenfield, MD, said in an interview at the annual meeting of the American Neurological Association.
In previously published work, Dr. Rosenfield, Director of the Speech and Language Center at Houston Methodist Neurological Institute, and his colleagues showed significant reduction in functional connectivity between Broca’s and Wernicke’s areas in persons who stutter, compared with normal speakers, by performing resting-state functional MRI of the brain. In the current open-label pilot trial, they tested the hypothesis that using direct noninvasive synchronous bifocal stimulation to potentiate the strength of connectivity between Broca’s and Wernicke’s areas in persons who stutter should improve their fluency.
Researchers enrolled nine persons who stutter who ranged in age from 18 to 80 years. For 40 minutes each consecutive weekday over the course of 2 weeks, the participants wore a compact, portable device known as a Transcranial Rotating Permanent Magnetic Stimulator (TRPMS) to deliver highly focal stimuli to Broca’s and Wernicke’s area locations specified by 10-20 international electroencephalographic electrode sites on the left side. Magnetic stimuli were 100 milliseconds in duration and delivered at 0.2 Hz. Next, a certified speech-language pathologist viewed video recordings of the study subjects both speaking spontaneously and reading a passage aloud on day 1 (before stimulation), day 5 (after stimulation), and day 10 (after stimulation), to assess fluency using the Stuttering Speech Severity Instrument version 4 (SSI-4).
The researchers found that all study participants significantly improved in fluency on either day 5 (P = 0.01) or day 10 (P = 0.02). Only one subject failed to show improvement on day 10 compared with day 1 after showing it on day 5. “This wasn’t meant to be a 10-day treatment that would last forever; this was meant to be a 10-day treatment to see whether the magnetic stimulation would work,” Dr. Rosenfield said. “It might well be that patients need treatment every day, once a week, or once a month. All we can say is that we have an input and an output. We gave them the treatment and they improved. One patient was so happy with it that he begged us to come back for additional treatment. It seems as though it’s a robust therapy.”
Going forward, the researchers plan to study fMRI brain imaging before and following external magnetic treatments to speech and language areas to confirm the efficacy of this therapy in a randomized, double-blind, sham treatment-controlled trial.
The TRPMS device was coinvented by Santosh A. Helekar, MD, PhD, and Henning Voss, PhD, both at Weill Cornell Medical College. The study received funding support from The Houston Methodist Hospital System Physicians Organization. Dr. Rosenfield reported having no financial disclosures. He noted that commercialization of the patented technology underlying the TRPMS device used in this study and in other diseases is currently being advanced by Seraya Medical Systems LLC.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S45-6. Abstract S115.
ATLANTA – After undergoing 10 days of noninvasive magnetic brain stimulation with a novel device, eight of nine persons who stutter experienced improvements in speech fluency.
“This is the first step in what we believe is a major breakthrough in treatment,” lead study author David B. Rosenfield, MD, said in an interview at the annual meeting of the American Neurological Association.
In previously published work, Dr. Rosenfield, Director of the Speech and Language Center at Houston Methodist Neurological Institute, and his colleagues showed significant reduction in functional connectivity between Broca’s and Wernicke’s areas in persons who stutter, compared with normal speakers, by performing resting-state functional MRI of the brain. In the current open-label pilot trial, they tested the hypothesis that using direct noninvasive synchronous bifocal stimulation to potentiate the strength of connectivity between Broca’s and Wernicke’s areas in persons who stutter should improve their fluency.
Researchers enrolled nine persons who stutter who ranged in age from 18 to 80 years. For 40 minutes each consecutive weekday over the course of 2 weeks, the participants wore a compact, portable device known as a Transcranial Rotating Permanent Magnetic Stimulator (TRPMS) to deliver highly focal stimuli to Broca’s and Wernicke’s area locations specified by 10-20 international electroencephalographic electrode sites on the left side. Magnetic stimuli were 100 milliseconds in duration and delivered at 0.2 Hz. Next, a certified speech-language pathologist viewed video recordings of the study subjects both speaking spontaneously and reading a passage aloud on day 1 (before stimulation), day 5 (after stimulation), and day 10 (after stimulation), to assess fluency using the Stuttering Speech Severity Instrument version 4 (SSI-4).
The researchers found that all study participants significantly improved in fluency on either day 5 (P = 0.01) or day 10 (P = 0.02). Only one subject failed to show improvement on day 10 compared with day 1 after showing it on day 5. “This wasn’t meant to be a 10-day treatment that would last forever; this was meant to be a 10-day treatment to see whether the magnetic stimulation would work,” Dr. Rosenfield said. “It might well be that patients need treatment every day, once a week, or once a month. All we can say is that we have an input and an output. We gave them the treatment and they improved. One patient was so happy with it that he begged us to come back for additional treatment. It seems as though it’s a robust therapy.”
Going forward, the researchers plan to study fMRI brain imaging before and following external magnetic treatments to speech and language areas to confirm the efficacy of this therapy in a randomized, double-blind, sham treatment-controlled trial.
The TRPMS device was coinvented by Santosh A. Helekar, MD, PhD, and Henning Voss, PhD, both at Weill Cornell Medical College. The study received funding support from The Houston Methodist Hospital System Physicians Organization. Dr. Rosenfield reported having no financial disclosures. He noted that commercialization of the patented technology underlying the TRPMS device used in this study and in other diseases is currently being advanced by Seraya Medical Systems LLC.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S45-6. Abstract S115.
AT ANA 2018
Key clinical point: .
Major finding: All study participants significantly improved in speech fluency on either day 5 of treatment or on day 10.
Study details: An open-label pilot trial of a novel devices used in nine persons who stutter.
Disclosures: The study received funding support from The Houston Methodist Hospital System Physicians Organization. Dr. Rosenfield reported having no financial disclosures. He noted that commercialization of the patented technology underlying the TRPMS device used in this study and in other diseases is currently being advanced by Seraya Medical Systems LLC.
Source: Ann Neurol. 2018;84[S22]:S45-6. Abstract S115.
High rates of prescription opioid, benzodiazepine use observed in chronic liver disease
SAN FRANCISCO – .
“Middle-aged individuals and those with a background of substance abuse and mental health conditions appear to have highest rates of use and represent populations for which targeted interventions to curb use could be highest yield,” lead study author Monica Konerman, MD, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases.
In an effort to better understand the rates of prescription opioid and benzodiazepine use in chronic liver disease, Dr. Konerman, director of the Michigan Medicine NAFLD Clinic at the University of Michigan, Ann Arbor, and her colleagues drew from the Truven Health Analytics Marketscan databases from 2009 to 2015. They limited the analysis to individuals with drug coverage who had chronic hepatitis C (HCV) without cirrhosis, cirrhosis, congestive heart failure (CHF), or chronic obstructive pulmonary disease (COPD), and examined pharmacy files for outpatient prescriptions.
Dr. Konerman reported data from 210,191 patients with HCV, 79,332 with cirrhosis, 766,840 with CHF, and 1,438,798 with COPD. Their median age was 59 years, and 51% were female. In per person-years, the prevalence of prescription opioid use was 25% among patients with chronic HCV, 53% among patients with cirrhosis, 26% among those with CHF, and 24% among those with COPD. At the same time, in per person-years, the prevalence of benzodiazepine use was 12% among patients with chronic HCV, 21% among patients with cirrhosis, 12% among those with CHF, and 13% among those with COPD. Use of opioids was greatest in adults 40-59 years of age (P less than .001). High-dose opioid use, defined as 100 opioid morphine equivalents per day or greater, occurred in 23% of those with cirrhosis and in 22% of those with HCV.
“The significant increase in rates of use in chronic liver disease, compared to other chronic conditions was remarkable, particularly given that patients with liver disease are at higher risk for adverse consequences of use due to hepatic metabolism of these medications,” Dr. Konerman said.
She went on to acknowledge “inherent limitations to studies that are secondary database analyses that rely on diagnosis codes for categorization of disease with potential for both over and under classification. We also did not capture inpatient prescriptions,” she said.
Dr. Konerman reported having no financial disclosures.
SAN FRANCISCO – .
“Middle-aged individuals and those with a background of substance abuse and mental health conditions appear to have highest rates of use and represent populations for which targeted interventions to curb use could be highest yield,” lead study author Monica Konerman, MD, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases.
In an effort to better understand the rates of prescription opioid and benzodiazepine use in chronic liver disease, Dr. Konerman, director of the Michigan Medicine NAFLD Clinic at the University of Michigan, Ann Arbor, and her colleagues drew from the Truven Health Analytics Marketscan databases from 2009 to 2015. They limited the analysis to individuals with drug coverage who had chronic hepatitis C (HCV) without cirrhosis, cirrhosis, congestive heart failure (CHF), or chronic obstructive pulmonary disease (COPD), and examined pharmacy files for outpatient prescriptions.
Dr. Konerman reported data from 210,191 patients with HCV, 79,332 with cirrhosis, 766,840 with CHF, and 1,438,798 with COPD. Their median age was 59 years, and 51% were female. In per person-years, the prevalence of prescription opioid use was 25% among patients with chronic HCV, 53% among patients with cirrhosis, 26% among those with CHF, and 24% among those with COPD. At the same time, in per person-years, the prevalence of benzodiazepine use was 12% among patients with chronic HCV, 21% among patients with cirrhosis, 12% among those with CHF, and 13% among those with COPD. Use of opioids was greatest in adults 40-59 years of age (P less than .001). High-dose opioid use, defined as 100 opioid morphine equivalents per day or greater, occurred in 23% of those with cirrhosis and in 22% of those with HCV.
“The significant increase in rates of use in chronic liver disease, compared to other chronic conditions was remarkable, particularly given that patients with liver disease are at higher risk for adverse consequences of use due to hepatic metabolism of these medications,” Dr. Konerman said.
She went on to acknowledge “inherent limitations to studies that are secondary database analyses that rely on diagnosis codes for categorization of disease with potential for both over and under classification. We also did not capture inpatient prescriptions,” she said.
Dr. Konerman reported having no financial disclosures.
SAN FRANCISCO – .
“Middle-aged individuals and those with a background of substance abuse and mental health conditions appear to have highest rates of use and represent populations for which targeted interventions to curb use could be highest yield,” lead study author Monica Konerman, MD, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases.
In an effort to better understand the rates of prescription opioid and benzodiazepine use in chronic liver disease, Dr. Konerman, director of the Michigan Medicine NAFLD Clinic at the University of Michigan, Ann Arbor, and her colleagues drew from the Truven Health Analytics Marketscan databases from 2009 to 2015. They limited the analysis to individuals with drug coverage who had chronic hepatitis C (HCV) without cirrhosis, cirrhosis, congestive heart failure (CHF), or chronic obstructive pulmonary disease (COPD), and examined pharmacy files for outpatient prescriptions.
Dr. Konerman reported data from 210,191 patients with HCV, 79,332 with cirrhosis, 766,840 with CHF, and 1,438,798 with COPD. Their median age was 59 years, and 51% were female. In per person-years, the prevalence of prescription opioid use was 25% among patients with chronic HCV, 53% among patients with cirrhosis, 26% among those with CHF, and 24% among those with COPD. At the same time, in per person-years, the prevalence of benzodiazepine use was 12% among patients with chronic HCV, 21% among patients with cirrhosis, 12% among those with CHF, and 13% among those with COPD. Use of opioids was greatest in adults 40-59 years of age (P less than .001). High-dose opioid use, defined as 100 opioid morphine equivalents per day or greater, occurred in 23% of those with cirrhosis and in 22% of those with HCV.
“The significant increase in rates of use in chronic liver disease, compared to other chronic conditions was remarkable, particularly given that patients with liver disease are at higher risk for adverse consequences of use due to hepatic metabolism of these medications,” Dr. Konerman said.
She went on to acknowledge “inherent limitations to studies that are secondary database analyses that rely on diagnosis codes for categorization of disease with potential for both over and under classification. We also did not capture inpatient prescriptions,” she said.
Dr. Konerman reported having no financial disclosures.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: About one-fourth of patients with chronic hepatitis C use prescription opioids.
Major finding: In per person-years, the prevalence of prescription opioid use was 25% among patients with chronic HCV, 53% among patients with cirrhosis, 26% among those with congestive heart failure, and 24% among those with chronic obstructive pulmonary disease.
Study details: An analysis of 210,191 patients who had chronic hepatitis C.
Disclosures: Dr. Konerman reported having no financial disclosures.
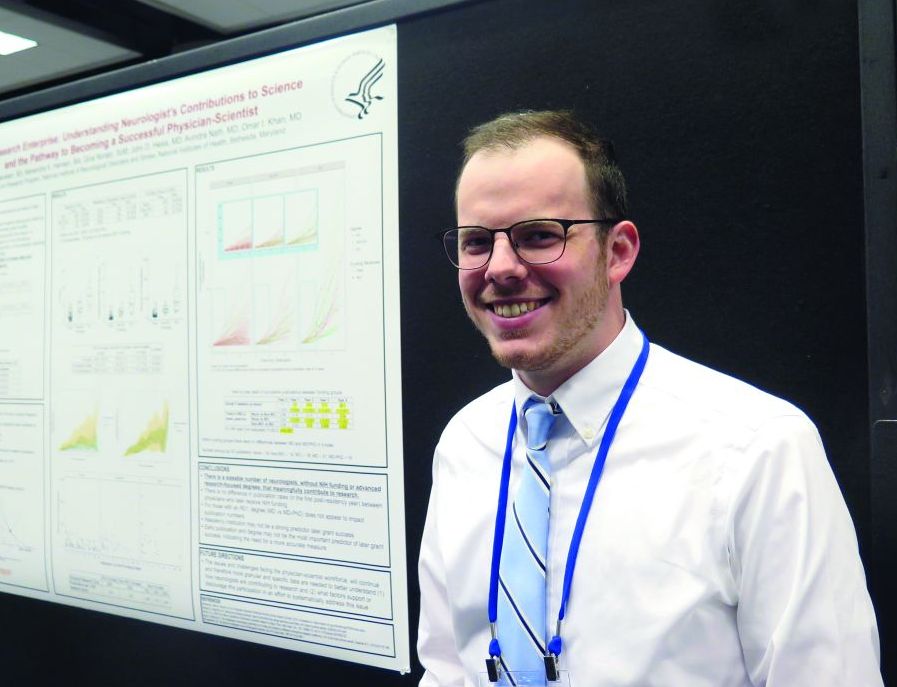
Think research is just for MD-PhDs? Think again
ATLANTA – You don’t have to hold an advanced research degree or secure National Institutes of Health funding in order to contribute to neurology research in a meaningful way.
That’s a key finding from an analysis of 244 neurology residency program graduates.
“Science as a whole is trying to get better,” lead study author Wyatt P. Bensken said in an interview at the annual meeting of the American Neurological Association. “If your goal is to be a clinician, that doesn’t mean you can’t contribute to research. If your goal is to see patients for 80% of your time, that doesn’t mean that other 20% – which is research – disqualifies you from being a physician-scientist.”
In an effort to better understand the current status of the physician-scientist workforce in the neurology field, Mr. Bensken and his colleagues identified neurology residency graduates from the top National Institute of Neurological Disorders and Stroke–funded institutions for 2003, 2004, and 2005 via program websites. Data points collected for each individual included complete NIH and other government funding history, number of post-residency publications by year, and the Hirsch-index, or h-index, which measures an individual’s research publication impact. The researchers conducted data analysis via visualization and ANOVA testing.
Mr. Bensken, a research collaborator with the NINDS who is also a PhD student at Case Western Reserve University in Cleveland, reported that 186 of the 244 neurology residency program graduates had demonstrated interest in research based on their publication activity findings. Specifically, 26 had obtained an R01 grant, 31 were non–R01-funded, and 129 were nonfunded. Of the 26 individuals who had obtained an R01, 15 (58%) were MD-PhDs, from a total of 50 MD‐PhDs in the cohort. In addition, 43 individuals had a K‐series award, with 18 going on to receive R01 funding.
Of those with non‐R01 funding or no funding, a number of individuals performed as well as R01‐funded individuals with respect to post‐residency publication rate and impact factor. However, the publication rate and impact factor were highest in the R01-funded group (6.4 and 28.6, respectively), followed by those in the non‐R01 group (3.0 and 15.9), and those in the nonfunded group (1.2 and 8.0). Further, the publications‐per‐research hour for the three groups revealed varied productivity levels. Specifically, those in the R01-funded group with 80% protected research time produced 3.2 publications per 1,000 research hours, while those in the non–R01-funded group with 40% protected research time produced 3.0 publications per 1,000 research hours. Meanwhile, those without R01 funding overall (those with non-RO1 funding and those without funding) performed at a higher per-hour rate, when estimating 10% or 15% protected time (4.9 and 3.3 publications per 1,000 research hours, respectively).
“I think this reinforces the notion that there are far more neurologists out there who aren’t trained as MD-PhDs, who aren’t receiving R01s, but who are making meaningful contributions,” Mr. Bensken said. “Our ultimate goal is to maximize the potential of everybody in this environment to contribute. If everyone was able to contribute what they could, I think research would be far more successful and far more impactful than it is now.”
The study was funded by the NINDS. Mr. Bensken reported having no financial disclosures.
SOURCE: Bensken WP et al. Ann Neurol. 2018;84[S22]:S72-3, Abstract S176.
ATLANTA – You don’t have to hold an advanced research degree or secure National Institutes of Health funding in order to contribute to neurology research in a meaningful way.
That’s a key finding from an analysis of 244 neurology residency program graduates.
“Science as a whole is trying to get better,” lead study author Wyatt P. Bensken said in an interview at the annual meeting of the American Neurological Association. “If your goal is to be a clinician, that doesn’t mean you can’t contribute to research. If your goal is to see patients for 80% of your time, that doesn’t mean that other 20% – which is research – disqualifies you from being a physician-scientist.”
In an effort to better understand the current status of the physician-scientist workforce in the neurology field, Mr. Bensken and his colleagues identified neurology residency graduates from the top National Institute of Neurological Disorders and Stroke–funded institutions for 2003, 2004, and 2005 via program websites. Data points collected for each individual included complete NIH and other government funding history, number of post-residency publications by year, and the Hirsch-index, or h-index, which measures an individual’s research publication impact. The researchers conducted data analysis via visualization and ANOVA testing.
Mr. Bensken, a research collaborator with the NINDS who is also a PhD student at Case Western Reserve University in Cleveland, reported that 186 of the 244 neurology residency program graduates had demonstrated interest in research based on their publication activity findings. Specifically, 26 had obtained an R01 grant, 31 were non–R01-funded, and 129 were nonfunded. Of the 26 individuals who had obtained an R01, 15 (58%) were MD-PhDs, from a total of 50 MD‐PhDs in the cohort. In addition, 43 individuals had a K‐series award, with 18 going on to receive R01 funding.
Of those with non‐R01 funding or no funding, a number of individuals performed as well as R01‐funded individuals with respect to post‐residency publication rate and impact factor. However, the publication rate and impact factor were highest in the R01-funded group (6.4 and 28.6, respectively), followed by those in the non‐R01 group (3.0 and 15.9), and those in the nonfunded group (1.2 and 8.0). Further, the publications‐per‐research hour for the three groups revealed varied productivity levels. Specifically, those in the R01-funded group with 80% protected research time produced 3.2 publications per 1,000 research hours, while those in the non–R01-funded group with 40% protected research time produced 3.0 publications per 1,000 research hours. Meanwhile, those without R01 funding overall (those with non-RO1 funding and those without funding) performed at a higher per-hour rate, when estimating 10% or 15% protected time (4.9 and 3.3 publications per 1,000 research hours, respectively).
“I think this reinforces the notion that there are far more neurologists out there who aren’t trained as MD-PhDs, who aren’t receiving R01s, but who are making meaningful contributions,” Mr. Bensken said. “Our ultimate goal is to maximize the potential of everybody in this environment to contribute. If everyone was able to contribute what they could, I think research would be far more successful and far more impactful than it is now.”
The study was funded by the NINDS. Mr. Bensken reported having no financial disclosures.
SOURCE: Bensken WP et al. Ann Neurol. 2018;84[S22]:S72-3, Abstract S176.
ATLANTA – You don’t have to hold an advanced research degree or secure National Institutes of Health funding in order to contribute to neurology research in a meaningful way.
That’s a key finding from an analysis of 244 neurology residency program graduates.
“Science as a whole is trying to get better,” lead study author Wyatt P. Bensken said in an interview at the annual meeting of the American Neurological Association. “If your goal is to be a clinician, that doesn’t mean you can’t contribute to research. If your goal is to see patients for 80% of your time, that doesn’t mean that other 20% – which is research – disqualifies you from being a physician-scientist.”
In an effort to better understand the current status of the physician-scientist workforce in the neurology field, Mr. Bensken and his colleagues identified neurology residency graduates from the top National Institute of Neurological Disorders and Stroke–funded institutions for 2003, 2004, and 2005 via program websites. Data points collected for each individual included complete NIH and other government funding history, number of post-residency publications by year, and the Hirsch-index, or h-index, which measures an individual’s research publication impact. The researchers conducted data analysis via visualization and ANOVA testing.
Mr. Bensken, a research collaborator with the NINDS who is also a PhD student at Case Western Reserve University in Cleveland, reported that 186 of the 244 neurology residency program graduates had demonstrated interest in research based on their publication activity findings. Specifically, 26 had obtained an R01 grant, 31 were non–R01-funded, and 129 were nonfunded. Of the 26 individuals who had obtained an R01, 15 (58%) were MD-PhDs, from a total of 50 MD‐PhDs in the cohort. In addition, 43 individuals had a K‐series award, with 18 going on to receive R01 funding.
Of those with non‐R01 funding or no funding, a number of individuals performed as well as R01‐funded individuals with respect to post‐residency publication rate and impact factor. However, the publication rate and impact factor were highest in the R01-funded group (6.4 and 28.6, respectively), followed by those in the non‐R01 group (3.0 and 15.9), and those in the nonfunded group (1.2 and 8.0). Further, the publications‐per‐research hour for the three groups revealed varied productivity levels. Specifically, those in the R01-funded group with 80% protected research time produced 3.2 publications per 1,000 research hours, while those in the non–R01-funded group with 40% protected research time produced 3.0 publications per 1,000 research hours. Meanwhile, those without R01 funding overall (those with non-RO1 funding and those without funding) performed at a higher per-hour rate, when estimating 10% or 15% protected time (4.9 and 3.3 publications per 1,000 research hours, respectively).
“I think this reinforces the notion that there are far more neurologists out there who aren’t trained as MD-PhDs, who aren’t receiving R01s, but who are making meaningful contributions,” Mr. Bensken said. “Our ultimate goal is to maximize the potential of everybody in this environment to contribute. If everyone was able to contribute what they could, I think research would be far more successful and far more impactful than it is now.”
The study was funded by the NINDS. Mr. Bensken reported having no financial disclosures.
SOURCE: Bensken WP et al. Ann Neurol. 2018;84[S22]:S72-3, Abstract S176.
REPORTING FROM ANA 2018
Key clinical point:
Major finding: Those in the R01-funded group with 80% protected research time produced 3.2 publications per 1,000 research hours, while those in the non–R01-funded group with 40% protected research time produced 3.0 publications per 1,000 research hours.
Study details: An analysis of 244 neurology residency program graduates.
Disclosures: The study was funded by the NINDS. Mr. Bensken reported having no financial disclosures.
Source: Bensken WP et al. Ann Neurol. 2018;84[S 22]:S72-3, Abstract S176.
U.S. death rates from chronic liver disease continue to rise
SAN FRANCISCO –
“I believe it’s all related to a big increase in obesity and type 2 diabetes in this country,” lead study author Zobair M. Younossi, MD, MPH, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases. “Those two risk factors drive NAFLD and its progressive type, nonalcoholic steatohepatitis (NASH). That accounts for at least part of the increase in mortality related to liver disease.”
In an effort to evaluate recent mortality trends in chronic liver disease in the United States, Dr. Younossi and his colleagues drew from National Vital Statistics Data during 2007-2016. They used ICD-10 codes to select mortality data for alcoholic liver disease, chronic hepatitis B and C, iron overload, NAFLD, cirrhosis, and hepatocellular carcinoma. NAFLD cases were defined as those having an ICD-10 code for NAFLD/NASH or an ICD-10 code for “cirrhosis of unknown etiology.” Next, the researchers adjusted age-standardized death rates to the 2000 U.S. Census population and used logistic regression and propensity scores to estimate predictors of chronic liver disease-related deaths.
Dr. Younossi, who chairs the department of medicine at Inova Fairfax Medical Campus, in Falls Church, Va., and his colleagues reported findings from 838,809 chronic liver disease–related deaths during the study period. They found that the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population in 2007 to 24.9/100,000 population in 2016, which translated into an annual percentage change of 1.3% for males and 2.5% for females. Chronic liver disease–related deaths increased with age and were highest among those aged 55-64 years, followed by those aged 65-74 years – an average annual percentage change of 3.4% and 3.1% in each group.
Among chronic liver disease–related deaths, the most common diagnostic etiology was NAFLD (34.7%), followed by alcoholic liver disease (28.8%) and chronic hepatitis C (21.1%). Between 2007 and 2016, death rates increased from 7.6 to 9.0 per 100,000 population for NAFLD (an average annual percentage change of 2.1%) and from 6.1 to 7.9 per 100,000 population for alcoholic liver disease (an average annual percentage change of 3.1%). “What surprised me was that, despite highly effective treatment for HCV, we still have a burden of hepatitis C in this country,” Dr. Younossi said. “It’s still the most common cause of liver disease in the U.S. But it seems like hepatitis C–related liver disease is being replaced quickly by liver disease from nonalcoholic steatohepatitis. This transition between hepatitis C as the most important cause of liver disease and liver mortality to NASH or obesity-related NASH is becoming more rapid than I expected.”
On multivariate analysis, three factors were independently associated with an increased risk of death in NAFLD: the presence of type 2 diabetes (odds ratio, 1.78), cardiovascular disease (OR, 1.07), and renal failure (OR, 1.08).
“One important message from this study is that NASH is very common in the U.S. population,” said Dr. Younossi, who is also a professor of medicine at Virginia Commonwealth University, Richmond. “These patients are underrecognized and underdiagnosed because they are asymptomatic. The second message is that there is a subtype of patients with fatty liver disease – even a subtype of NASH – that can progress to cirrhosis and its complications. We have to pay attention to this silent disease to identify patients who are at risk for progressive liver disease and try to address some of the risk issues, such as tight control of diabetes, obesity, and control of hypertension and hyperlipidemia. Short of that, right now we have very few medical treatments such as vitamin E and pioglitazone recommended for a very selected group. In contrast, there are plenty of new medications that are being developed. The first step in tackling this disease is to identify who the patients are with fatty liver disease who are at risk for bad outcomes and make sure they’re linked to care by a knowledgeable caregiver [who] understands the importance of NASH.”
Dr. Younossi acknowledged certain limitations of the study, including the fact that liver disease diagnoses were based on ICD-10 coding. He disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, BMS, AbbVie, Viking, Term Quest Diagnostics, Echosens,and Shionogi. He has also received grant/research support from Gilead, Intercept, and BMS.
Source: Hepatol. 2018;68[S1], Abstract 763.
SAN FRANCISCO –
“I believe it’s all related to a big increase in obesity and type 2 diabetes in this country,” lead study author Zobair M. Younossi, MD, MPH, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases. “Those two risk factors drive NAFLD and its progressive type, nonalcoholic steatohepatitis (NASH). That accounts for at least part of the increase in mortality related to liver disease.”
In an effort to evaluate recent mortality trends in chronic liver disease in the United States, Dr. Younossi and his colleagues drew from National Vital Statistics Data during 2007-2016. They used ICD-10 codes to select mortality data for alcoholic liver disease, chronic hepatitis B and C, iron overload, NAFLD, cirrhosis, and hepatocellular carcinoma. NAFLD cases were defined as those having an ICD-10 code for NAFLD/NASH or an ICD-10 code for “cirrhosis of unknown etiology.” Next, the researchers adjusted age-standardized death rates to the 2000 U.S. Census population and used logistic regression and propensity scores to estimate predictors of chronic liver disease-related deaths.
Dr. Younossi, who chairs the department of medicine at Inova Fairfax Medical Campus, in Falls Church, Va., and his colleagues reported findings from 838,809 chronic liver disease–related deaths during the study period. They found that the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population in 2007 to 24.9/100,000 population in 2016, which translated into an annual percentage change of 1.3% for males and 2.5% for females. Chronic liver disease–related deaths increased with age and were highest among those aged 55-64 years, followed by those aged 65-74 years – an average annual percentage change of 3.4% and 3.1% in each group.
Among chronic liver disease–related deaths, the most common diagnostic etiology was NAFLD (34.7%), followed by alcoholic liver disease (28.8%) and chronic hepatitis C (21.1%). Between 2007 and 2016, death rates increased from 7.6 to 9.0 per 100,000 population for NAFLD (an average annual percentage change of 2.1%) and from 6.1 to 7.9 per 100,000 population for alcoholic liver disease (an average annual percentage change of 3.1%). “What surprised me was that, despite highly effective treatment for HCV, we still have a burden of hepatitis C in this country,” Dr. Younossi said. “It’s still the most common cause of liver disease in the U.S. But it seems like hepatitis C–related liver disease is being replaced quickly by liver disease from nonalcoholic steatohepatitis. This transition between hepatitis C as the most important cause of liver disease and liver mortality to NASH or obesity-related NASH is becoming more rapid than I expected.”
On multivariate analysis, three factors were independently associated with an increased risk of death in NAFLD: the presence of type 2 diabetes (odds ratio, 1.78), cardiovascular disease (OR, 1.07), and renal failure (OR, 1.08).
“One important message from this study is that NASH is very common in the U.S. population,” said Dr. Younossi, who is also a professor of medicine at Virginia Commonwealth University, Richmond. “These patients are underrecognized and underdiagnosed because they are asymptomatic. The second message is that there is a subtype of patients with fatty liver disease – even a subtype of NASH – that can progress to cirrhosis and its complications. We have to pay attention to this silent disease to identify patients who are at risk for progressive liver disease and try to address some of the risk issues, such as tight control of diabetes, obesity, and control of hypertension and hyperlipidemia. Short of that, right now we have very few medical treatments such as vitamin E and pioglitazone recommended for a very selected group. In contrast, there are plenty of new medications that are being developed. The first step in tackling this disease is to identify who the patients are with fatty liver disease who are at risk for bad outcomes and make sure they’re linked to care by a knowledgeable caregiver [who] understands the importance of NASH.”
Dr. Younossi acknowledged certain limitations of the study, including the fact that liver disease diagnoses were based on ICD-10 coding. He disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, BMS, AbbVie, Viking, Term Quest Diagnostics, Echosens,and Shionogi. He has also received grant/research support from Gilead, Intercept, and BMS.
Source: Hepatol. 2018;68[S1], Abstract 763.
SAN FRANCISCO –
“I believe it’s all related to a big increase in obesity and type 2 diabetes in this country,” lead study author Zobair M. Younossi, MD, MPH, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases. “Those two risk factors drive NAFLD and its progressive type, nonalcoholic steatohepatitis (NASH). That accounts for at least part of the increase in mortality related to liver disease.”
In an effort to evaluate recent mortality trends in chronic liver disease in the United States, Dr. Younossi and his colleagues drew from National Vital Statistics Data during 2007-2016. They used ICD-10 codes to select mortality data for alcoholic liver disease, chronic hepatitis B and C, iron overload, NAFLD, cirrhosis, and hepatocellular carcinoma. NAFLD cases were defined as those having an ICD-10 code for NAFLD/NASH or an ICD-10 code for “cirrhosis of unknown etiology.” Next, the researchers adjusted age-standardized death rates to the 2000 U.S. Census population and used logistic regression and propensity scores to estimate predictors of chronic liver disease-related deaths.
Dr. Younossi, who chairs the department of medicine at Inova Fairfax Medical Campus, in Falls Church, Va., and his colleagues reported findings from 838,809 chronic liver disease–related deaths during the study period. They found that the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population in 2007 to 24.9/100,000 population in 2016, which translated into an annual percentage change of 1.3% for males and 2.5% for females. Chronic liver disease–related deaths increased with age and were highest among those aged 55-64 years, followed by those aged 65-74 years – an average annual percentage change of 3.4% and 3.1% in each group.
Among chronic liver disease–related deaths, the most common diagnostic etiology was NAFLD (34.7%), followed by alcoholic liver disease (28.8%) and chronic hepatitis C (21.1%). Between 2007 and 2016, death rates increased from 7.6 to 9.0 per 100,000 population for NAFLD (an average annual percentage change of 2.1%) and from 6.1 to 7.9 per 100,000 population for alcoholic liver disease (an average annual percentage change of 3.1%). “What surprised me was that, despite highly effective treatment for HCV, we still have a burden of hepatitis C in this country,” Dr. Younossi said. “It’s still the most common cause of liver disease in the U.S. But it seems like hepatitis C–related liver disease is being replaced quickly by liver disease from nonalcoholic steatohepatitis. This transition between hepatitis C as the most important cause of liver disease and liver mortality to NASH or obesity-related NASH is becoming more rapid than I expected.”
On multivariate analysis, three factors were independently associated with an increased risk of death in NAFLD: the presence of type 2 diabetes (odds ratio, 1.78), cardiovascular disease (OR, 1.07), and renal failure (OR, 1.08).
“One important message from this study is that NASH is very common in the U.S. population,” said Dr. Younossi, who is also a professor of medicine at Virginia Commonwealth University, Richmond. “These patients are underrecognized and underdiagnosed because they are asymptomatic. The second message is that there is a subtype of patients with fatty liver disease – even a subtype of NASH – that can progress to cirrhosis and its complications. We have to pay attention to this silent disease to identify patients who are at risk for progressive liver disease and try to address some of the risk issues, such as tight control of diabetes, obesity, and control of hypertension and hyperlipidemia. Short of that, right now we have very few medical treatments such as vitamin E and pioglitazone recommended for a very selected group. In contrast, there are plenty of new medications that are being developed. The first step in tackling this disease is to identify who the patients are with fatty liver disease who are at risk for bad outcomes and make sure they’re linked to care by a knowledgeable caregiver [who] understands the importance of NASH.”
Dr. Younossi acknowledged certain limitations of the study, including the fact that liver disease diagnoses were based on ICD-10 coding. He disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, BMS, AbbVie, Viking, Term Quest Diagnostics, Echosens,and Shionogi. He has also received grant/research support from Gilead, Intercept, and BMS.
Source: Hepatol. 2018;68[S1], Abstract 763.
AT THE LIVER MEETING 2018
Key clinical point: Nonalcoholic steatohepatitis is very common in the U.S. population.
Major finding: Between 2007 and 2016, the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population to 24.9/100,000 population.
Study details: An analysis of 838,809 chronic liver disease–related deaths from 2007 to 2016.
Disclosures: Dr. Younossi disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, Bristol-Myers Squibb, AbbVie, Viking, Term Quest Diagnostics, Echosens, and Shionogi. He has also received grant/research support from Gilead, Intercept, and Bristol-Myers Squibb.
Source: Hepatol. 2018;68[S1], Abstract 763.
Robin Williams’ widow recounts ‘terror’ of late husband’s Lewy body dementia
ATLANTA –
“With our medical team’s care, for the next 10 months we chased symptoms, but they were so elusive,” Mr. Williams’ widow, Susan Schneider Williams, said during a keynote address at the annual meeting of the American Neurological Association. “One hallmark of LBD is that symptoms appear and disappear randomly. The game whack-a-mole comes to mind. As soon as you think you are about to figure out a symptom, it disappears, and another one pops up.”
Mr. Williams’ medical team included one general physician, one neurologist, one motor specialist, two psychiatrists, one hypnotherapist, one physical trainer, and assorted alternative specialists. “We had been celebrating our second wedding anniversary when Robin started having gut discomfort,” Ms. Williams recalled. “He was tested for diverticulitis [but] the results came back negative. The pain eventually subsided but what was alarming was Robin’s reaction to it. He had a sudden and sustained spike in fear and anxiety unlike anything I’d seen before. By that point, we’d been by each other’s side long enough that I knew his normal baseline moods, fears, and anxieties. This was totally out of character, and I wondered privately: ‘Is my husband a hypochondriac?’ What I know now is that he was exhibiting a notable hallmark of LBD: new onset anxiety, sustained.” Lewy body disease is characterized by more than 40 symptoms, she continued, “and Robin experienced nearly all of them. He was particularly debilitated by fear, anxiety, delusions, paranoia, and as I came to find out later, hallucinations.”
The medical team continued running all sorts of tests, but everything kept came back negative, except for a very high cortisol count. By the late spring of 2014, however, Mr. Williams was diagnosed with Parkinson’s disease. “I was relieved to find out we finally had an answer, but I could tell, Robin was not buying it,” said Ms. Williams, who is a California-based fine artist, author, and brain health advocate. “The motor specialist said it was early and mild and that he’d be feeling better once he adjusted to the medications, [that] he had another 10 good years.”
In an attempt to treat the Parkinson’s and what was assumed to be depression, his care plan involved adjusting Parkinson’s medications, combined with an antidepressant. His physician also recommended a visit to the Dan Anderson Renewal Center in Minnesota, “for enhanced 12-step work to augment his sobriety,” Ms. Williams said. “The hope was this might help with fear and anxiety. Robin was clean and sober for 8 continuous years when he passed. I watched how he gained spiritually in so many ways from all the work he’d been doing, but his brain biology was going in the exact opposite direction. He tried desperately to join the parts of his heart, mind, and spirit, but his brain was pulling him apart. I felt like I was watching my husband disintegrate before my eyes, and there was nothing anyone could do about it. There came a day when we were getting ready to go to one of our dear friend’s birthday party. I came and saw Robin as he lay on our bed, imprisoned by fear and anxiety. Through tears, he pleaded, ‘I just want to reboot my brain!’ I promised him, ‘I know, honey. I swear we’re going to get to the bottom of this.’ ”
The couple was about a week away from choosing which neurocognitive testing facility to go to for further evaluation when Mr. Williams took his own life in his Paradise Cay, Calif., home on Aug. 11, 2014. “Robin was exhausted from the terror coming from his brain,” Ms. Williams said. “He took [his own life] before it could take any more of him.”
About 3 months later, the underlying cause of death was revealed: diffuse Lewy body dementia, “one of the worst cases they’d ever seen,” she said. “Because Robin’s disease pathway was extreme and unfolded the way it did, it highlights quite strikingly this disease spectrum. He had a perfusion of Lewy bodies, the essential underlying shared biology between Parkinson’s and Lewy body disease, scattered throughout his entire brain and brain stem.” She added that her husband’s prior history of depression from earlier in life “added to the challenge of getting a proper diagnosis. That single symptom of depression was being treated as its own illness, rather than part of the larger neurocognitive disease. It seems that one of the biggest challenges to getting an accurate diagnosis is that LBD symptoms have tremendous crossover with normal human psychology and behavior, mood, cognition and sleep issues. All of us experience fear, stress, anxiety, paranoia, trouble sleeping, mild depression, and other issues from time to time. We would hardly be human if we didn’t. The challenge of LBD is seeing the giant constellation that it is, rather than just a few of its stars.”
In early 2016, Ms. Williams received the “Commitment to Cures Award” from American Brain Foundation, honoring work she’s done raising awareness for Lewy body disease since her husband’s death. “The day I accepted that award and told our story to a room full of neurologists, my path was forever changed,” she said. “The ABF’s mission of connecting donors to researchers and curing brain disease was an alignment with my mission and hope.” She currently serves as vice chair of the ABF’s board of directors.
“From my own research and from the myriad of letters and information that has come to me, I have distilled what I think are the top three overlooked ideas in this disease space,” Ms. Williams said. “1. Diagnosis: The norm seems to be misdiagnosis, switched diagnosis, or no diagnosis at all. 2. Symptoms: They are being treated independently, apart from the neurological disorder. 3. Suicides: If more autopsies were done, more suicides would be attributed to this disease.”
She concluded her address by reflecting on the impact of her husband’s death has had in bringing an international spotlight to LBD. “When I meet individuals who have lost someone they loved to LBD, I see the pain in their eyes, but I hear the determination in their voice as they chart their own course toward making a difference,” Ms. Williams said. “I have been blessed to learn over and over again that I am not alone. I believe that Robin’s death in this battle against these diseases holds a profound purpose. There was tremendous power in what he suffered, and I saw that power up close. I’m here doing all that I can to see that power transformed into something good.”
ATLANTA –
“With our medical team’s care, for the next 10 months we chased symptoms, but they were so elusive,” Mr. Williams’ widow, Susan Schneider Williams, said during a keynote address at the annual meeting of the American Neurological Association. “One hallmark of LBD is that symptoms appear and disappear randomly. The game whack-a-mole comes to mind. As soon as you think you are about to figure out a symptom, it disappears, and another one pops up.”
Mr. Williams’ medical team included one general physician, one neurologist, one motor specialist, two psychiatrists, one hypnotherapist, one physical trainer, and assorted alternative specialists. “We had been celebrating our second wedding anniversary when Robin started having gut discomfort,” Ms. Williams recalled. “He was tested for diverticulitis [but] the results came back negative. The pain eventually subsided but what was alarming was Robin’s reaction to it. He had a sudden and sustained spike in fear and anxiety unlike anything I’d seen before. By that point, we’d been by each other’s side long enough that I knew his normal baseline moods, fears, and anxieties. This was totally out of character, and I wondered privately: ‘Is my husband a hypochondriac?’ What I know now is that he was exhibiting a notable hallmark of LBD: new onset anxiety, sustained.” Lewy body disease is characterized by more than 40 symptoms, she continued, “and Robin experienced nearly all of them. He was particularly debilitated by fear, anxiety, delusions, paranoia, and as I came to find out later, hallucinations.”
The medical team continued running all sorts of tests, but everything kept came back negative, except for a very high cortisol count. By the late spring of 2014, however, Mr. Williams was diagnosed with Parkinson’s disease. “I was relieved to find out we finally had an answer, but I could tell, Robin was not buying it,” said Ms. Williams, who is a California-based fine artist, author, and brain health advocate. “The motor specialist said it was early and mild and that he’d be feeling better once he adjusted to the medications, [that] he had another 10 good years.”
In an attempt to treat the Parkinson’s and what was assumed to be depression, his care plan involved adjusting Parkinson’s medications, combined with an antidepressant. His physician also recommended a visit to the Dan Anderson Renewal Center in Minnesota, “for enhanced 12-step work to augment his sobriety,” Ms. Williams said. “The hope was this might help with fear and anxiety. Robin was clean and sober for 8 continuous years when he passed. I watched how he gained spiritually in so many ways from all the work he’d been doing, but his brain biology was going in the exact opposite direction. He tried desperately to join the parts of his heart, mind, and spirit, but his brain was pulling him apart. I felt like I was watching my husband disintegrate before my eyes, and there was nothing anyone could do about it. There came a day when we were getting ready to go to one of our dear friend’s birthday party. I came and saw Robin as he lay on our bed, imprisoned by fear and anxiety. Through tears, he pleaded, ‘I just want to reboot my brain!’ I promised him, ‘I know, honey. I swear we’re going to get to the bottom of this.’ ”
The couple was about a week away from choosing which neurocognitive testing facility to go to for further evaluation when Mr. Williams took his own life in his Paradise Cay, Calif., home on Aug. 11, 2014. “Robin was exhausted from the terror coming from his brain,” Ms. Williams said. “He took [his own life] before it could take any more of him.”
About 3 months later, the underlying cause of death was revealed: diffuse Lewy body dementia, “one of the worst cases they’d ever seen,” she said. “Because Robin’s disease pathway was extreme and unfolded the way it did, it highlights quite strikingly this disease spectrum. He had a perfusion of Lewy bodies, the essential underlying shared biology between Parkinson’s and Lewy body disease, scattered throughout his entire brain and brain stem.” She added that her husband’s prior history of depression from earlier in life “added to the challenge of getting a proper diagnosis. That single symptom of depression was being treated as its own illness, rather than part of the larger neurocognitive disease. It seems that one of the biggest challenges to getting an accurate diagnosis is that LBD symptoms have tremendous crossover with normal human psychology and behavior, mood, cognition and sleep issues. All of us experience fear, stress, anxiety, paranoia, trouble sleeping, mild depression, and other issues from time to time. We would hardly be human if we didn’t. The challenge of LBD is seeing the giant constellation that it is, rather than just a few of its stars.”
In early 2016, Ms. Williams received the “Commitment to Cures Award” from American Brain Foundation, honoring work she’s done raising awareness for Lewy body disease since her husband’s death. “The day I accepted that award and told our story to a room full of neurologists, my path was forever changed,” she said. “The ABF’s mission of connecting donors to researchers and curing brain disease was an alignment with my mission and hope.” She currently serves as vice chair of the ABF’s board of directors.
“From my own research and from the myriad of letters and information that has come to me, I have distilled what I think are the top three overlooked ideas in this disease space,” Ms. Williams said. “1. Diagnosis: The norm seems to be misdiagnosis, switched diagnosis, or no diagnosis at all. 2. Symptoms: They are being treated independently, apart from the neurological disorder. 3. Suicides: If more autopsies were done, more suicides would be attributed to this disease.”
She concluded her address by reflecting on the impact of her husband’s death has had in bringing an international spotlight to LBD. “When I meet individuals who have lost someone they loved to LBD, I see the pain in their eyes, but I hear the determination in their voice as they chart their own course toward making a difference,” Ms. Williams said. “I have been blessed to learn over and over again that I am not alone. I believe that Robin’s death in this battle against these diseases holds a profound purpose. There was tremendous power in what he suffered, and I saw that power up close. I’m here doing all that I can to see that power transformed into something good.”
ATLANTA –
“With our medical team’s care, for the next 10 months we chased symptoms, but they were so elusive,” Mr. Williams’ widow, Susan Schneider Williams, said during a keynote address at the annual meeting of the American Neurological Association. “One hallmark of LBD is that symptoms appear and disappear randomly. The game whack-a-mole comes to mind. As soon as you think you are about to figure out a symptom, it disappears, and another one pops up.”
Mr. Williams’ medical team included one general physician, one neurologist, one motor specialist, two psychiatrists, one hypnotherapist, one physical trainer, and assorted alternative specialists. “We had been celebrating our second wedding anniversary when Robin started having gut discomfort,” Ms. Williams recalled. “He was tested for diverticulitis [but] the results came back negative. The pain eventually subsided but what was alarming was Robin’s reaction to it. He had a sudden and sustained spike in fear and anxiety unlike anything I’d seen before. By that point, we’d been by each other’s side long enough that I knew his normal baseline moods, fears, and anxieties. This was totally out of character, and I wondered privately: ‘Is my husband a hypochondriac?’ What I know now is that he was exhibiting a notable hallmark of LBD: new onset anxiety, sustained.” Lewy body disease is characterized by more than 40 symptoms, she continued, “and Robin experienced nearly all of them. He was particularly debilitated by fear, anxiety, delusions, paranoia, and as I came to find out later, hallucinations.”
The medical team continued running all sorts of tests, but everything kept came back negative, except for a very high cortisol count. By the late spring of 2014, however, Mr. Williams was diagnosed with Parkinson’s disease. “I was relieved to find out we finally had an answer, but I could tell, Robin was not buying it,” said Ms. Williams, who is a California-based fine artist, author, and brain health advocate. “The motor specialist said it was early and mild and that he’d be feeling better once he adjusted to the medications, [that] he had another 10 good years.”
In an attempt to treat the Parkinson’s and what was assumed to be depression, his care plan involved adjusting Parkinson’s medications, combined with an antidepressant. His physician also recommended a visit to the Dan Anderson Renewal Center in Minnesota, “for enhanced 12-step work to augment his sobriety,” Ms. Williams said. “The hope was this might help with fear and anxiety. Robin was clean and sober for 8 continuous years when he passed. I watched how he gained spiritually in so many ways from all the work he’d been doing, but his brain biology was going in the exact opposite direction. He tried desperately to join the parts of his heart, mind, and spirit, but his brain was pulling him apart. I felt like I was watching my husband disintegrate before my eyes, and there was nothing anyone could do about it. There came a day when we were getting ready to go to one of our dear friend’s birthday party. I came and saw Robin as he lay on our bed, imprisoned by fear and anxiety. Through tears, he pleaded, ‘I just want to reboot my brain!’ I promised him, ‘I know, honey. I swear we’re going to get to the bottom of this.’ ”
The couple was about a week away from choosing which neurocognitive testing facility to go to for further evaluation when Mr. Williams took his own life in his Paradise Cay, Calif., home on Aug. 11, 2014. “Robin was exhausted from the terror coming from his brain,” Ms. Williams said. “He took [his own life] before it could take any more of him.”
About 3 months later, the underlying cause of death was revealed: diffuse Lewy body dementia, “one of the worst cases they’d ever seen,” she said. “Because Robin’s disease pathway was extreme and unfolded the way it did, it highlights quite strikingly this disease spectrum. He had a perfusion of Lewy bodies, the essential underlying shared biology between Parkinson’s and Lewy body disease, scattered throughout his entire brain and brain stem.” She added that her husband’s prior history of depression from earlier in life “added to the challenge of getting a proper diagnosis. That single symptom of depression was being treated as its own illness, rather than part of the larger neurocognitive disease. It seems that one of the biggest challenges to getting an accurate diagnosis is that LBD symptoms have tremendous crossover with normal human psychology and behavior, mood, cognition and sleep issues. All of us experience fear, stress, anxiety, paranoia, trouble sleeping, mild depression, and other issues from time to time. We would hardly be human if we didn’t. The challenge of LBD is seeing the giant constellation that it is, rather than just a few of its stars.”
In early 2016, Ms. Williams received the “Commitment to Cures Award” from American Brain Foundation, honoring work she’s done raising awareness for Lewy body disease since her husband’s death. “The day I accepted that award and told our story to a room full of neurologists, my path was forever changed,” she said. “The ABF’s mission of connecting donors to researchers and curing brain disease was an alignment with my mission and hope.” She currently serves as vice chair of the ABF’s board of directors.
“From my own research and from the myriad of letters and information that has come to me, I have distilled what I think are the top three overlooked ideas in this disease space,” Ms. Williams said. “1. Diagnosis: The norm seems to be misdiagnosis, switched diagnosis, or no diagnosis at all. 2. Symptoms: They are being treated independently, apart from the neurological disorder. 3. Suicides: If more autopsies were done, more suicides would be attributed to this disease.”
She concluded her address by reflecting on the impact of her husband’s death has had in bringing an international spotlight to LBD. “When I meet individuals who have lost someone they loved to LBD, I see the pain in their eyes, but I hear the determination in their voice as they chart their own course toward making a difference,” Ms. Williams said. “I have been blessed to learn over and over again that I am not alone. I believe that Robin’s death in this battle against these diseases holds a profound purpose. There was tremendous power in what he suffered, and I saw that power up close. I’m here doing all that I can to see that power transformed into something good.”
REPORTING FROM ANA 2018
Pneumonia, COPD most common emergency care–sensitive conditions
SAN DIEGO – Emergency care–sensitive conditions – those for which timely access to high-quality emergency care impact morbidity and mortality—account for 14% of all ED visits, results from a large analysis of national data showed.
In previously published work, an eight-member expert panel identified 51 condition groups as emergency care–sensitive conditions (ECSCs), including asthma, cardiac arrest, cerebral infarction, and pneumonia. The purpose of the current study, published in Annals of Emergency Medicine and presented by Anita Vashi, MD, MPH, at the annual meeting of the American College of Emergency Physicians, was to provide the first national estimates of acute care utilization and the demographic characteristics of adults experiencing ECSCs, compare ECSC and non-ECSC ED visits, and assess patient- and hospital-level characteristics predictive of an ECSC-related ED visit.
Using the Nationwide Emergency Department Sample data set, Dr. Vashi, a physician investigator at the Center for Innovation to Implementation at the VA Palo Alto Health Care System, and her colleagues retrospectively evaluated all ED visits for patients aged 18 years and older from 2009 to 2014. The researchers used summary statistics to compare population characteristics across groups and multivariable logistic regression models to assess the odds of an ECSC-related ED visit with patient- and hospital-level characteristics.
Of the 622,725,542 estimated ED visits evaluated during the study period, 86,577,041 (14%) were ECSCs. Among these ECSC visits, 58% of patients were admitted for an average length of 3.2 days and an average charge of $2,240. The most frequent ECSC-related visits were for pneumonia (9%), chronic obstructive pulmonary disease (9%), asthma (7%), heart failure (7%), and sepsis (5%), but varied by age group.
Dr. Vashi and her colleagues found that ECSCs were more common among older adults, males, those who reside in low-income areas, those who reside in the South, and among metropolitan-based hospitals and nontrauma center hospitals. ECSCs also accounted for about 45% of all inpatient admissions.
Multivariate logistic regression analysis revealed that the odds of having an ECSC-related visit was highest among patients aged 65 years and older (odds ratio, 3.84), those on Medicare (OR, 1.37), those who resided in rural counties (OR, 1.21), and those who reside in the Western portion of the United States (OR, 1.11). Significant hospital-related factors related to ECSC visits included trauma centers (OR, 1.09), nonteaching hospitals (OR, 1.04), and EDs located in the wealthiest counties (OR, 1.02).
The researchers also found that 40% of patients who made ECSC-related ED visits were treated and discharged back to the community. “There is evidence of regional variability, suggesting the need for future research,” said Dr. Vashi, who also holds a faculty position in the department of emergency medicine at Stanford (Calif.) University. “We found no consistent relationship between insurance, income, and ED use for ECSC-related conditions. This suggests that ECSCs are not significantly influenced by socioeconomic factor and can serve as a reliable marker for acuity.”
The next steps in this research area, she added, are to create condition-specific measures related to morbidity, mortality, and posthospital events, as well as to analyze regional and hospital variations including correlation across conditions, and to compare performance across conditions and hospitals.
Dr. Vashi reported having no financial disclosures.
Source: Vashi A et al. Ann Emerg Med. 2018 Oct;72;4:S38. doi. 10.1016/j.annemergmed.2018.08.091.
SAN DIEGO – Emergency care–sensitive conditions – those for which timely access to high-quality emergency care impact morbidity and mortality—account for 14% of all ED visits, results from a large analysis of national data showed.
In previously published work, an eight-member expert panel identified 51 condition groups as emergency care–sensitive conditions (ECSCs), including asthma, cardiac arrest, cerebral infarction, and pneumonia. The purpose of the current study, published in Annals of Emergency Medicine and presented by Anita Vashi, MD, MPH, at the annual meeting of the American College of Emergency Physicians, was to provide the first national estimates of acute care utilization and the demographic characteristics of adults experiencing ECSCs, compare ECSC and non-ECSC ED visits, and assess patient- and hospital-level characteristics predictive of an ECSC-related ED visit.
Using the Nationwide Emergency Department Sample data set, Dr. Vashi, a physician investigator at the Center for Innovation to Implementation at the VA Palo Alto Health Care System, and her colleagues retrospectively evaluated all ED visits for patients aged 18 years and older from 2009 to 2014. The researchers used summary statistics to compare population characteristics across groups and multivariable logistic regression models to assess the odds of an ECSC-related ED visit with patient- and hospital-level characteristics.
Of the 622,725,542 estimated ED visits evaluated during the study period, 86,577,041 (14%) were ECSCs. Among these ECSC visits, 58% of patients were admitted for an average length of 3.2 days and an average charge of $2,240. The most frequent ECSC-related visits were for pneumonia (9%), chronic obstructive pulmonary disease (9%), asthma (7%), heart failure (7%), and sepsis (5%), but varied by age group.
Dr. Vashi and her colleagues found that ECSCs were more common among older adults, males, those who reside in low-income areas, those who reside in the South, and among metropolitan-based hospitals and nontrauma center hospitals. ECSCs also accounted for about 45% of all inpatient admissions.
Multivariate logistic regression analysis revealed that the odds of having an ECSC-related visit was highest among patients aged 65 years and older (odds ratio, 3.84), those on Medicare (OR, 1.37), those who resided in rural counties (OR, 1.21), and those who reside in the Western portion of the United States (OR, 1.11). Significant hospital-related factors related to ECSC visits included trauma centers (OR, 1.09), nonteaching hospitals (OR, 1.04), and EDs located in the wealthiest counties (OR, 1.02).
The researchers also found that 40% of patients who made ECSC-related ED visits were treated and discharged back to the community. “There is evidence of regional variability, suggesting the need for future research,” said Dr. Vashi, who also holds a faculty position in the department of emergency medicine at Stanford (Calif.) University. “We found no consistent relationship between insurance, income, and ED use for ECSC-related conditions. This suggests that ECSCs are not significantly influenced by socioeconomic factor and can serve as a reliable marker for acuity.”
The next steps in this research area, she added, are to create condition-specific measures related to morbidity, mortality, and posthospital events, as well as to analyze regional and hospital variations including correlation across conditions, and to compare performance across conditions and hospitals.
Dr. Vashi reported having no financial disclosures.
Source: Vashi A et al. Ann Emerg Med. 2018 Oct;72;4:S38. doi. 10.1016/j.annemergmed.2018.08.091.
SAN DIEGO – Emergency care–sensitive conditions – those for which timely access to high-quality emergency care impact morbidity and mortality—account for 14% of all ED visits, results from a large analysis of national data showed.
In previously published work, an eight-member expert panel identified 51 condition groups as emergency care–sensitive conditions (ECSCs), including asthma, cardiac arrest, cerebral infarction, and pneumonia. The purpose of the current study, published in Annals of Emergency Medicine and presented by Anita Vashi, MD, MPH, at the annual meeting of the American College of Emergency Physicians, was to provide the first national estimates of acute care utilization and the demographic characteristics of adults experiencing ECSCs, compare ECSC and non-ECSC ED visits, and assess patient- and hospital-level characteristics predictive of an ECSC-related ED visit.
Using the Nationwide Emergency Department Sample data set, Dr. Vashi, a physician investigator at the Center for Innovation to Implementation at the VA Palo Alto Health Care System, and her colleagues retrospectively evaluated all ED visits for patients aged 18 years and older from 2009 to 2014. The researchers used summary statistics to compare population characteristics across groups and multivariable logistic regression models to assess the odds of an ECSC-related ED visit with patient- and hospital-level characteristics.
Of the 622,725,542 estimated ED visits evaluated during the study period, 86,577,041 (14%) were ECSCs. Among these ECSC visits, 58% of patients were admitted for an average length of 3.2 days and an average charge of $2,240. The most frequent ECSC-related visits were for pneumonia (9%), chronic obstructive pulmonary disease (9%), asthma (7%), heart failure (7%), and sepsis (5%), but varied by age group.
Dr. Vashi and her colleagues found that ECSCs were more common among older adults, males, those who reside in low-income areas, those who reside in the South, and among metropolitan-based hospitals and nontrauma center hospitals. ECSCs also accounted for about 45% of all inpatient admissions.
Multivariate logistic regression analysis revealed that the odds of having an ECSC-related visit was highest among patients aged 65 years and older (odds ratio, 3.84), those on Medicare (OR, 1.37), those who resided in rural counties (OR, 1.21), and those who reside in the Western portion of the United States (OR, 1.11). Significant hospital-related factors related to ECSC visits included trauma centers (OR, 1.09), nonteaching hospitals (OR, 1.04), and EDs located in the wealthiest counties (OR, 1.02).
The researchers also found that 40% of patients who made ECSC-related ED visits were treated and discharged back to the community. “There is evidence of regional variability, suggesting the need for future research,” said Dr. Vashi, who also holds a faculty position in the department of emergency medicine at Stanford (Calif.) University. “We found no consistent relationship between insurance, income, and ED use for ECSC-related conditions. This suggests that ECSCs are not significantly influenced by socioeconomic factor and can serve as a reliable marker for acuity.”
The next steps in this research area, she added, are to create condition-specific measures related to morbidity, mortality, and posthospital events, as well as to analyze regional and hospital variations including correlation across conditions, and to compare performance across conditions and hospitals.
Dr. Vashi reported having no financial disclosures.
Source: Vashi A et al. Ann Emerg Med. 2018 Oct;72;4:S38. doi. 10.1016/j.annemergmed.2018.08.091.
REPORTING FROM ACEP18
Key clinical point: Emergency care–sensitive conditions (ECSCs) make up a significant proportion of ED visits.
Major finding: The most common ECSC-related visits were for pneumonia (9%), chronic obstructive pulmonary disease (9%), and asthma (7%).
Study details: A retrospective cohort study of more than 86.5 million ECSC-related ED visits.
Disclosures: Dr. Vashi reported having no financial disclosures.
Source: Vashi A et al. Ann Emerg Med. 2018 Oct;72;4:S38. doi. 10.1016/j.annemergmed.2018.08.091.
ED clinicians’ confidence in dealing with ICE mixed, survey finds
.
“More robust training and education may improve provider comfort and ability to appropriately balance patients’ rights with law enforcement,” one of the study authors, Charlotte Roy, MD, said at the annual meeting of the American College of Emergency Physicians.
Immigration and Customs Enforcement (ICE) is a federal government agency responsible for the detainment and deportation of immigrants whom they deem to be in violation of federal immigration law. Typically, hospitals have fallen into the category of “sensitive locations,” meaning they are avoided by ICE for enforcement actions, said Dr. Roy, a third-year resident in the department of emergency medicine at University of Chicago Hospital. However, “we’ve seen an increase in the apprehension and removal of illegal immigrants by 30% in 2017 across all locations,” she said.
In an effort to assess provider comfort, knowledge, and previous training on the subject, Dr. Roy and her colleagues administered a 15-question multiple-choice survey to 128 emergency residents, fellows, and attendings at John H. Stroger Jr. Hospital of Cook County and University of Chicago Hospital. Most of the 128 survey participants were residents (70), followed by 54 fellows and 4 attendings. One of the two hospital ED programs offered formal training on the topic, yet only 44% of respondents from that program reported that they participated in the training.
Examples of questions included “Do you know in what circumstances you are required to give protected health information of patients to immigration officers?” “Do you feel confident in your knowledge of which areas of the emergency department ICE agents could enter?” and “Are you confident you can get real-time help to answer questions regarding interactions with immigration officers?”
Among all respondents, 52% said that they would not feel comfortable interacting with immigration officers in the ED, 12% felt confident knowing which areas of the ED immigration officers could freely enter, and 11% were familiar with the designation of “sensitive locations” within their hospital. In addition, 23% of respondents knew that immigration status is protected under the Health Insurance Portability and Accountability Act of 1996 as protected health information, 16% knew in which circumstances they were required to convey immigrant status, and 51% knew a warrant or a court order is required for an immigration office to enter a patient’s room.
“Our conclusion based on these survey results is that there is very limited comfort and knowledge amongst ED providers regarding interactions with ICE officers in the ED,” Dr. Roy said. One resource she recommended is the National Immigration Law Center, which publishes a guide, “What to Do If Immigration Comes to Your Workplace.” The study’s primary author was Joseph Palter, MD. The researchers reported having no financial disclosures.
Source: Palter J et al. Ann Emerg Med. 2018 Oct;72;4:S117. doi. 10.1016/j.annemergmed.2018.08.303.
.
“More robust training and education may improve provider comfort and ability to appropriately balance patients’ rights with law enforcement,” one of the study authors, Charlotte Roy, MD, said at the annual meeting of the American College of Emergency Physicians.
Immigration and Customs Enforcement (ICE) is a federal government agency responsible for the detainment and deportation of immigrants whom they deem to be in violation of federal immigration law. Typically, hospitals have fallen into the category of “sensitive locations,” meaning they are avoided by ICE for enforcement actions, said Dr. Roy, a third-year resident in the department of emergency medicine at University of Chicago Hospital. However, “we’ve seen an increase in the apprehension and removal of illegal immigrants by 30% in 2017 across all locations,” she said.
In an effort to assess provider comfort, knowledge, and previous training on the subject, Dr. Roy and her colleagues administered a 15-question multiple-choice survey to 128 emergency residents, fellows, and attendings at John H. Stroger Jr. Hospital of Cook County and University of Chicago Hospital. Most of the 128 survey participants were residents (70), followed by 54 fellows and 4 attendings. One of the two hospital ED programs offered formal training on the topic, yet only 44% of respondents from that program reported that they participated in the training.
Examples of questions included “Do you know in what circumstances you are required to give protected health information of patients to immigration officers?” “Do you feel confident in your knowledge of which areas of the emergency department ICE agents could enter?” and “Are you confident you can get real-time help to answer questions regarding interactions with immigration officers?”
Among all respondents, 52% said that they would not feel comfortable interacting with immigration officers in the ED, 12% felt confident knowing which areas of the ED immigration officers could freely enter, and 11% were familiar with the designation of “sensitive locations” within their hospital. In addition, 23% of respondents knew that immigration status is protected under the Health Insurance Portability and Accountability Act of 1996 as protected health information, 16% knew in which circumstances they were required to convey immigrant status, and 51% knew a warrant or a court order is required for an immigration office to enter a patient’s room.
“Our conclusion based on these survey results is that there is very limited comfort and knowledge amongst ED providers regarding interactions with ICE officers in the ED,” Dr. Roy said. One resource she recommended is the National Immigration Law Center, which publishes a guide, “What to Do If Immigration Comes to Your Workplace.” The study’s primary author was Joseph Palter, MD. The researchers reported having no financial disclosures.
Source: Palter J et al. Ann Emerg Med. 2018 Oct;72;4:S117. doi. 10.1016/j.annemergmed.2018.08.303.
.
“More robust training and education may improve provider comfort and ability to appropriately balance patients’ rights with law enforcement,” one of the study authors, Charlotte Roy, MD, said at the annual meeting of the American College of Emergency Physicians.
Immigration and Customs Enforcement (ICE) is a federal government agency responsible for the detainment and deportation of immigrants whom they deem to be in violation of federal immigration law. Typically, hospitals have fallen into the category of “sensitive locations,” meaning they are avoided by ICE for enforcement actions, said Dr. Roy, a third-year resident in the department of emergency medicine at University of Chicago Hospital. However, “we’ve seen an increase in the apprehension and removal of illegal immigrants by 30% in 2017 across all locations,” she said.
In an effort to assess provider comfort, knowledge, and previous training on the subject, Dr. Roy and her colleagues administered a 15-question multiple-choice survey to 128 emergency residents, fellows, and attendings at John H. Stroger Jr. Hospital of Cook County and University of Chicago Hospital. Most of the 128 survey participants were residents (70), followed by 54 fellows and 4 attendings. One of the two hospital ED programs offered formal training on the topic, yet only 44% of respondents from that program reported that they participated in the training.
Examples of questions included “Do you know in what circumstances you are required to give protected health information of patients to immigration officers?” “Do you feel confident in your knowledge of which areas of the emergency department ICE agents could enter?” and “Are you confident you can get real-time help to answer questions regarding interactions with immigration officers?”
Among all respondents, 52% said that they would not feel comfortable interacting with immigration officers in the ED, 12% felt confident knowing which areas of the ED immigration officers could freely enter, and 11% were familiar with the designation of “sensitive locations” within their hospital. In addition, 23% of respondents knew that immigration status is protected under the Health Insurance Portability and Accountability Act of 1996 as protected health information, 16% knew in which circumstances they were required to convey immigrant status, and 51% knew a warrant or a court order is required for an immigration office to enter a patient’s room.
“Our conclusion based on these survey results is that there is very limited comfort and knowledge amongst ED providers regarding interactions with ICE officers in the ED,” Dr. Roy said. One resource she recommended is the National Immigration Law Center, which publishes a guide, “What to Do If Immigration Comes to Your Workplace.” The study’s primary author was Joseph Palter, MD. The researchers reported having no financial disclosures.
Source: Palter J et al. Ann Emerg Med. 2018 Oct;72;4:S117. doi. 10.1016/j.annemergmed.2018.08.303.
AT ACEP18
Key clinical point: Emergency physicians’ knowledge of how to interact with immigration agencies is highly variable.
Major finding: More than half of respondents (52%) said that they would not feel comfortable interacting with immigration officers in the ED.
Study details: A survey of 128 emergency medicine physicians.
Disclosures: The researchers reported having no financial disclosures.
Source: Palter J et al. Ann Emerg Med. 2018 Oct;72;4:S117. doi. 10.1016/j.annemergmed.2018.08.303.