User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Atopic Dermatitis: Study Compares Prevalence by Gender, Age, and Ethnic Background
than adults from other ethnic backgrounds.
Those are among the key findings from an analysis of nationally representative cross-sectional data that were presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis conference in Chicago.
“In the past few years, there has been a much-needed focus on better understanding disparities in atopic dermatitis,” one of the study authors, Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University, North Chicago, told this news organization after the conference.
“Epidemiology is one of the key ways in which we can query differences in AD at a population level.”
Drawing from the 2021 National Health Interview Survey, the researchers identified 3103 respondents who reported being diagnosed with AD or eczema. They estimated the prevalence rates of AD for the overall population and each subgroup by dividing US frequency estimates by their corresponding US population totals and used multivariable logistic regression to assess the odds of having AD.
More than half of the respondents (1643) were aged between 18 and 64 years, 522 were aged 65 years and older, and 922 were children younger than 18 years. Overall, the prevalence of AD was 7.6% in adults aged 18-64 years and 6.1% in adults aged 65 years and older, for a weighted US estimate of 15.3 and 3.2 million, respectively. The prevalence of AD varied by race/ethnicity and was highest for those from “other single and multiple races” group (12.4%), followed by Black/African American (8.5%), White (7.7%), Asian (6.5%), American Indian/Alaskan Native (4.9%), and Hispanic (4.8%) populations.
In children, race/ethnicity prevalence were highest for those from other single and multiple races (15.2.%), followed by Black/African American (14.2%), American Indian/Alaskan Native (12%), White (10.2%), Hispanic (9.5%), and Asian (9%) populations.
When the researchers combined all age groups, they observed higher prevalence rates of AD among females than among males. However, in an analysis limited to children, the prevalence rates were similar between girls and boys (10.8% vs 10.7%, respectively), for a weighted US estimate of 7.8 million children with AD.
On multiple regression, the odds of having AD were greater among women than among men (odds ratio [OR], 1.4), among adults aged 18-64 years than among those aged 65 years and older (OR, 1.4), among those younger than 18 years than among those aged 65 years and older (OR, 2.0), and among Black/African American individuals than among White individuals (OR, 1.2). Hispanic adults had a lower risk for AD than non-Hispanic White adults (OR, 0.69) as did Asian adults than White adults (OR, 0.82).
“We found AD prevalence rates were higher in children and adult females, Hispanic adults had a lower prevalence of AD than all other adult groups, and there were numerical differences in AD prevalence across racial groups,” Dr. Chovatiya said in the interview. “While there are of course limitations to the use of any nationally representative cross-sectional dataset that requires weighting to project results from a smaller sample to reflect a larger more heterogeneous group, these results are important for us to consider targeted strategies to address AD burden.”
Jonathan I. Silverberg, MD, PhD, professor of dermatology at The George Washington University, Washington, who was asked to comment on the study, said that while the prevalence of AD in children has been well documented in prior research, “this study fills an important gap by showing us that the prevalence does remain high in adults.”
In addition, “it has not shown any evidence of AD decreasing over time; if anything, it might be slightly increasing,” he said. “We’re also seeing differences [in AD] by race and ethnicity. We have seen that demonstrated in children but [has been] less clearly demonstrated in adults.”
Eli Lilly and Company funded the analysis. Dr. Chovatiya and Dr. Silverberg disclosed ties to several pharmaceutical companies, including Eli Lilly.
A version of this article appeared on Medscape.com .
than adults from other ethnic backgrounds.
Those are among the key findings from an analysis of nationally representative cross-sectional data that were presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis conference in Chicago.
“In the past few years, there has been a much-needed focus on better understanding disparities in atopic dermatitis,” one of the study authors, Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University, North Chicago, told this news organization after the conference.
“Epidemiology is one of the key ways in which we can query differences in AD at a population level.”
Drawing from the 2021 National Health Interview Survey, the researchers identified 3103 respondents who reported being diagnosed with AD or eczema. They estimated the prevalence rates of AD for the overall population and each subgroup by dividing US frequency estimates by their corresponding US population totals and used multivariable logistic regression to assess the odds of having AD.
More than half of the respondents (1643) were aged between 18 and 64 years, 522 were aged 65 years and older, and 922 were children younger than 18 years. Overall, the prevalence of AD was 7.6% in adults aged 18-64 years and 6.1% in adults aged 65 years and older, for a weighted US estimate of 15.3 and 3.2 million, respectively. The prevalence of AD varied by race/ethnicity and was highest for those from “other single and multiple races” group (12.4%), followed by Black/African American (8.5%), White (7.7%), Asian (6.5%), American Indian/Alaskan Native (4.9%), and Hispanic (4.8%) populations.
In children, race/ethnicity prevalence were highest for those from other single and multiple races (15.2.%), followed by Black/African American (14.2%), American Indian/Alaskan Native (12%), White (10.2%), Hispanic (9.5%), and Asian (9%) populations.
When the researchers combined all age groups, they observed higher prevalence rates of AD among females than among males. However, in an analysis limited to children, the prevalence rates were similar between girls and boys (10.8% vs 10.7%, respectively), for a weighted US estimate of 7.8 million children with AD.
On multiple regression, the odds of having AD were greater among women than among men (odds ratio [OR], 1.4), among adults aged 18-64 years than among those aged 65 years and older (OR, 1.4), among those younger than 18 years than among those aged 65 years and older (OR, 2.0), and among Black/African American individuals than among White individuals (OR, 1.2). Hispanic adults had a lower risk for AD than non-Hispanic White adults (OR, 0.69) as did Asian adults than White adults (OR, 0.82).
“We found AD prevalence rates were higher in children and adult females, Hispanic adults had a lower prevalence of AD than all other adult groups, and there were numerical differences in AD prevalence across racial groups,” Dr. Chovatiya said in the interview. “While there are of course limitations to the use of any nationally representative cross-sectional dataset that requires weighting to project results from a smaller sample to reflect a larger more heterogeneous group, these results are important for us to consider targeted strategies to address AD burden.”
Jonathan I. Silverberg, MD, PhD, professor of dermatology at The George Washington University, Washington, who was asked to comment on the study, said that while the prevalence of AD in children has been well documented in prior research, “this study fills an important gap by showing us that the prevalence does remain high in adults.”
In addition, “it has not shown any evidence of AD decreasing over time; if anything, it might be slightly increasing,” he said. “We’re also seeing differences [in AD] by race and ethnicity. We have seen that demonstrated in children but [has been] less clearly demonstrated in adults.”
Eli Lilly and Company funded the analysis. Dr. Chovatiya and Dr. Silverberg disclosed ties to several pharmaceutical companies, including Eli Lilly.
A version of this article appeared on Medscape.com .
than adults from other ethnic backgrounds.
Those are among the key findings from an analysis of nationally representative cross-sectional data that were presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis conference in Chicago.
“In the past few years, there has been a much-needed focus on better understanding disparities in atopic dermatitis,” one of the study authors, Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University, North Chicago, told this news organization after the conference.
“Epidemiology is one of the key ways in which we can query differences in AD at a population level.”
Drawing from the 2021 National Health Interview Survey, the researchers identified 3103 respondents who reported being diagnosed with AD or eczema. They estimated the prevalence rates of AD for the overall population and each subgroup by dividing US frequency estimates by their corresponding US population totals and used multivariable logistic regression to assess the odds of having AD.
More than half of the respondents (1643) were aged between 18 and 64 years, 522 were aged 65 years and older, and 922 were children younger than 18 years. Overall, the prevalence of AD was 7.6% in adults aged 18-64 years and 6.1% in adults aged 65 years and older, for a weighted US estimate of 15.3 and 3.2 million, respectively. The prevalence of AD varied by race/ethnicity and was highest for those from “other single and multiple races” group (12.4%), followed by Black/African American (8.5%), White (7.7%), Asian (6.5%), American Indian/Alaskan Native (4.9%), and Hispanic (4.8%) populations.
In children, race/ethnicity prevalence were highest for those from other single and multiple races (15.2.%), followed by Black/African American (14.2%), American Indian/Alaskan Native (12%), White (10.2%), Hispanic (9.5%), and Asian (9%) populations.
When the researchers combined all age groups, they observed higher prevalence rates of AD among females than among males. However, in an analysis limited to children, the prevalence rates were similar between girls and boys (10.8% vs 10.7%, respectively), for a weighted US estimate of 7.8 million children with AD.
On multiple regression, the odds of having AD were greater among women than among men (odds ratio [OR], 1.4), among adults aged 18-64 years than among those aged 65 years and older (OR, 1.4), among those younger than 18 years than among those aged 65 years and older (OR, 2.0), and among Black/African American individuals than among White individuals (OR, 1.2). Hispanic adults had a lower risk for AD than non-Hispanic White adults (OR, 0.69) as did Asian adults than White adults (OR, 0.82).
“We found AD prevalence rates were higher in children and adult females, Hispanic adults had a lower prevalence of AD than all other adult groups, and there were numerical differences in AD prevalence across racial groups,” Dr. Chovatiya said in the interview. “While there are of course limitations to the use of any nationally representative cross-sectional dataset that requires weighting to project results from a smaller sample to reflect a larger more heterogeneous group, these results are important for us to consider targeted strategies to address AD burden.”
Jonathan I. Silverberg, MD, PhD, professor of dermatology at The George Washington University, Washington, who was asked to comment on the study, said that while the prevalence of AD in children has been well documented in prior research, “this study fills an important gap by showing us that the prevalence does remain high in adults.”
In addition, “it has not shown any evidence of AD decreasing over time; if anything, it might be slightly increasing,” he said. “We’re also seeing differences [in AD] by race and ethnicity. We have seen that demonstrated in children but [has been] less clearly demonstrated in adults.”
Eli Lilly and Company funded the analysis. Dr. Chovatiya and Dr. Silverberg disclosed ties to several pharmaceutical companies, including Eli Lilly.
A version of this article appeared on Medscape.com .
Topical Ruxolitinib Effective for AD in Study of Children Ages 2-11 years
) affecting ≥ 35% or more of their body surface area (BSA), results from a small open-label maximum-use trial showed.
When approved for this age group, ruxolitinib cream will provide a topical nonsteroidal option for patients aged 2-11, which will “simplify the treatment regimen,” one of the study investigators, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said in an interview after the Revolutionizing Atopic Dermatitis conference, where the study was presented during a late-breaking abstract session.
A topical formulation of the selective Janus kinase (JAK) 1/JAK2 inhibitor, ruxolitinib cream 1.5% is currently approved by the Food and Drug Administration for the short-term and noncontinuous chronic treatment of mild to moderate AD in non-immunocompromised adult and pediatric patients aged 12 years and older, whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
In previous reports of this trial in children aged 2-11 years with ≥ 35% affected BSA, ruxolitinib cream 1.5% was generally well tolerated, with rapid anti-inflammatory and antipruritic effects and improvements in patient-reported outcomes observed with ≤ 4 weeks of continuous treatment and maintained with as-needed treatment from 4 to 8 weeks.
For the current trial, investigators evaluated data on tolerability, safety, systemic exposure, and clinical and patient-reported outcomes through 52 weeks to determine whether clinical benefits and tolerability observed through 8 weeks were sustained.
Dr. Stein Gold and colleagues reported results from 29 children who received ruxolitinib cream 1.5% from baseline through week 8. Of these, 22 continued into the long-term safety period from week 8 through 52. From baseline through week 8, patients applied a mean of 6.5 g per day of ruxolitinib cream; this dropped to a mean of 3.2 g per day from weeks 8 through 52. The mean steady-state plasma concentration of ruxolitinib throughout the study was 98.2 nM, which is “well below half-maximal concentration of JAK-mediated myelosuppression in adults (281 nM),” the researchers stated in their abstract.
No treatment-related interruptions, discontinuations, or serious adverse events were observed between baseline and week 52. One patient (3.4%) had two treatment-related application site reactions (paresthesia and folliculitis). At weeks 4 and 52, 53.8% of patients achieved treatment success, which was defined as an Investigator Global Assessment of 0/1 with a ≥ 2-grade improvement from baseline. The mean affected BSA decreased from 58.0% at baseline to 11.4% at week 4 and continued to decrease to 2.2% through week 52. “I was surprised that patients could maintain control over the long-term using the medication as needed,” Dr. Stein Gold told this news organization. “I was also pleased to see that there was low systemic exposure even when used on large body surface areas.”
In other findings, the mean total Patient Oriented Eczema Measure score dropped from a baseline of 19.4 to a mean of 4.5 at week 8 and 3.6 at week 52 and the mean total Children’s Dermatology Life Quality Index score fell from a baseline of 15.4 to a mean of 5.3 at week 8 and a mean of 2.1 at week 52. Meanwhile, the mean total Infants’ Dermatology Quality of Life Index score fell from a mean of 12.3 at baseline to a mean of 2.8 at week 8 and a mean of 0.7 at week 52.
Dr. Stein Gold noted certain limitations of the study, including the fact that it did not study children aged younger than 2 years.
The study was funded by Incyte, which markets ruxolitinib cream 1.5% as Opzelura. Dr. Stein Gold disclosed that she has served as an investigator, advisor, and/or speaker for several pharmaceutical companies, including Incyte.
A version of this article appeared on Medscape.com.
) affecting ≥ 35% or more of their body surface area (BSA), results from a small open-label maximum-use trial showed.
When approved for this age group, ruxolitinib cream will provide a topical nonsteroidal option for patients aged 2-11, which will “simplify the treatment regimen,” one of the study investigators, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said in an interview after the Revolutionizing Atopic Dermatitis conference, where the study was presented during a late-breaking abstract session.
A topical formulation of the selective Janus kinase (JAK) 1/JAK2 inhibitor, ruxolitinib cream 1.5% is currently approved by the Food and Drug Administration for the short-term and noncontinuous chronic treatment of mild to moderate AD in non-immunocompromised adult and pediatric patients aged 12 years and older, whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
In previous reports of this trial in children aged 2-11 years with ≥ 35% affected BSA, ruxolitinib cream 1.5% was generally well tolerated, with rapid anti-inflammatory and antipruritic effects and improvements in patient-reported outcomes observed with ≤ 4 weeks of continuous treatment and maintained with as-needed treatment from 4 to 8 weeks.
For the current trial, investigators evaluated data on tolerability, safety, systemic exposure, and clinical and patient-reported outcomes through 52 weeks to determine whether clinical benefits and tolerability observed through 8 weeks were sustained.
Dr. Stein Gold and colleagues reported results from 29 children who received ruxolitinib cream 1.5% from baseline through week 8. Of these, 22 continued into the long-term safety period from week 8 through 52. From baseline through week 8, patients applied a mean of 6.5 g per day of ruxolitinib cream; this dropped to a mean of 3.2 g per day from weeks 8 through 52. The mean steady-state plasma concentration of ruxolitinib throughout the study was 98.2 nM, which is “well below half-maximal concentration of JAK-mediated myelosuppression in adults (281 nM),” the researchers stated in their abstract.
No treatment-related interruptions, discontinuations, or serious adverse events were observed between baseline and week 52. One patient (3.4%) had two treatment-related application site reactions (paresthesia and folliculitis). At weeks 4 and 52, 53.8% of patients achieved treatment success, which was defined as an Investigator Global Assessment of 0/1 with a ≥ 2-grade improvement from baseline. The mean affected BSA decreased from 58.0% at baseline to 11.4% at week 4 and continued to decrease to 2.2% through week 52. “I was surprised that patients could maintain control over the long-term using the medication as needed,” Dr. Stein Gold told this news organization. “I was also pleased to see that there was low systemic exposure even when used on large body surface areas.”
In other findings, the mean total Patient Oriented Eczema Measure score dropped from a baseline of 19.4 to a mean of 4.5 at week 8 and 3.6 at week 52 and the mean total Children’s Dermatology Life Quality Index score fell from a baseline of 15.4 to a mean of 5.3 at week 8 and a mean of 2.1 at week 52. Meanwhile, the mean total Infants’ Dermatology Quality of Life Index score fell from a mean of 12.3 at baseline to a mean of 2.8 at week 8 and a mean of 0.7 at week 52.
Dr. Stein Gold noted certain limitations of the study, including the fact that it did not study children aged younger than 2 years.
The study was funded by Incyte, which markets ruxolitinib cream 1.5% as Opzelura. Dr. Stein Gold disclosed that she has served as an investigator, advisor, and/or speaker for several pharmaceutical companies, including Incyte.
A version of this article appeared on Medscape.com.
) affecting ≥ 35% or more of their body surface area (BSA), results from a small open-label maximum-use trial showed.
When approved for this age group, ruxolitinib cream will provide a topical nonsteroidal option for patients aged 2-11, which will “simplify the treatment regimen,” one of the study investigators, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said in an interview after the Revolutionizing Atopic Dermatitis conference, where the study was presented during a late-breaking abstract session.
A topical formulation of the selective Janus kinase (JAK) 1/JAK2 inhibitor, ruxolitinib cream 1.5% is currently approved by the Food and Drug Administration for the short-term and noncontinuous chronic treatment of mild to moderate AD in non-immunocompromised adult and pediatric patients aged 12 years and older, whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
In previous reports of this trial in children aged 2-11 years with ≥ 35% affected BSA, ruxolitinib cream 1.5% was generally well tolerated, with rapid anti-inflammatory and antipruritic effects and improvements in patient-reported outcomes observed with ≤ 4 weeks of continuous treatment and maintained with as-needed treatment from 4 to 8 weeks.
For the current trial, investigators evaluated data on tolerability, safety, systemic exposure, and clinical and patient-reported outcomes through 52 weeks to determine whether clinical benefits and tolerability observed through 8 weeks were sustained.
Dr. Stein Gold and colleagues reported results from 29 children who received ruxolitinib cream 1.5% from baseline through week 8. Of these, 22 continued into the long-term safety period from week 8 through 52. From baseline through week 8, patients applied a mean of 6.5 g per day of ruxolitinib cream; this dropped to a mean of 3.2 g per day from weeks 8 through 52. The mean steady-state plasma concentration of ruxolitinib throughout the study was 98.2 nM, which is “well below half-maximal concentration of JAK-mediated myelosuppression in adults (281 nM),” the researchers stated in their abstract.
No treatment-related interruptions, discontinuations, or serious adverse events were observed between baseline and week 52. One patient (3.4%) had two treatment-related application site reactions (paresthesia and folliculitis). At weeks 4 and 52, 53.8% of patients achieved treatment success, which was defined as an Investigator Global Assessment of 0/1 with a ≥ 2-grade improvement from baseline. The mean affected BSA decreased from 58.0% at baseline to 11.4% at week 4 and continued to decrease to 2.2% through week 52. “I was surprised that patients could maintain control over the long-term using the medication as needed,” Dr. Stein Gold told this news organization. “I was also pleased to see that there was low systemic exposure even when used on large body surface areas.”
In other findings, the mean total Patient Oriented Eczema Measure score dropped from a baseline of 19.4 to a mean of 4.5 at week 8 and 3.6 at week 52 and the mean total Children’s Dermatology Life Quality Index score fell from a baseline of 15.4 to a mean of 5.3 at week 8 and a mean of 2.1 at week 52. Meanwhile, the mean total Infants’ Dermatology Quality of Life Index score fell from a mean of 12.3 at baseline to a mean of 2.8 at week 8 and a mean of 0.7 at week 52.
Dr. Stein Gold noted certain limitations of the study, including the fact that it did not study children aged younger than 2 years.
The study was funded by Incyte, which markets ruxolitinib cream 1.5% as Opzelura. Dr. Stein Gold disclosed that she has served as an investigator, advisor, and/or speaker for several pharmaceutical companies, including Incyte.
A version of this article appeared on Medscape.com.
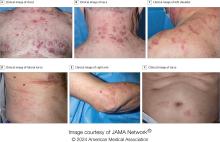
VEXAS Syndrome: Study Highlights Cutaneous Symptoms
Additionally, the most common histologic findings include leukocytoclastic vasculitis, neutrophilic dermatosis, and perivascular dermatitis; different variants in the UBA1 gene are associated with specific skin manifestations.
Those are key findings from a cohort study of 112 patients with VEXAS published online in JAMA Dermatology. The study, conducted by researchers at the National Institutes of Health (NIH) and several other institutions, aimed to define the spectrum of cutaneous manifestations in VEXAS in association with genetic, histologic, and other clinical findings.
First described in 2020, VEXAS syndrome is an adult-onset multisystem disease that can pose a diagnostic challenge to clinicians, the study’s corresponding author, Edward W. Cowen, MD, MHSc, of the dermatology branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), said in an interview. The disease is caused by pathogenic variants in the UBA1 gene, located on the X chromosome. Affected individuals exhibit a wide range of manifestations, including cytopenia/myelodysplasia, multiorgan systemic inflammation, and cutaneous involvement.
“Patients may present to a variety of disease specialists depending on their symptoms and providers may not immediately consider a genetic etiology in an older individual,” Dr. Cowen said in an interview. “Although skin involvement occurs in more than 80% of patients, it is pleomorphic and may resemble a variety of other conditions such as vasculitis and Sweet syndrome.”
To better understand the cutaneous manifestations of VEXAS syndrome, the researchers evaluated data from 112 patients with VEXAS-defining genetic variants in the UBA1 gene between 2019 and 2023. Of the 112 patients, 73 underwent medical record review only, and 39 were prospectively evaluated at NIH. All but one of the patients were men, 94% were White individuals, and their mean age was 64 years. Skin involvement occurred in 83% of cases and was the most common presenting feature of VEXAS in 61% of cases.
Of the 64 histopathologic reports available from 60 patients, the main skin histopathologic findings were leukocytoclastic vasculitis in 23 patients (36%), neutrophilic dermatosis in 22 patients (34%), and perivascular dermatitis in 19 patients (30%). According to Dr. Cowen, one key histologic finding was a distinct pattern of “histiocytoid” dermal neutrophilic inflammation, which was present in 13 of 15 specimens (86%) that underwent central re-review. “This pattern can occasionally also be seen in patients with Sweet syndrome, unrelated to VEXAS, but was a hallmark feature found in the majority of skin biopsies of patients with VEXAS,” he said.
“Together with another pathologic finding, leukocytoclasia, these features can be useful clues to alert the pathologist to a potential diagnosis of VEXAS. This myeloid predominant pattern of skin inflammation was also most strongly associated with the leucine pathogenic variant of the UBA1 gene.” In contrast, cutaneous vasculitis was most strongly associated with the valine pathogenic variant of UBA1. “This is important because the valine variant has been previously independently linked to decreased survival,” he said.
In findings related to pathogenic genetic variants, the researchers observed that the p.Met41Leu variant was most frequently associated with neutrophilic dermal infiltrates in 14 of 17 patients (82%) with this variant and often resembled histiocytoid Sweet syndrome. In addition, the p.Met41Val variant was associated with vasculitic lesions in 11 of 20 patients (55%) with this variant and with a mixed leukocytic infiltrate in 17 of these 20 patients (85%).
Treatment Outcomes
In the realm of therapies, skin manifestations improved in 67 of 73 patients (92%) treated with oral prednisone, while treatment with the interleukin-1 receptor antagonist anakinra improved cutaneous disease in 9 of the 16 (56%) who received it. However, 12 (75%) of those who received anakinra developed severe injection-site reactions, including ulceration in two patients and abscess formation in one patient.
Dr. Cowen noted that VEXAS is associated with high mortality (22% in this cohort), and a high degree of suspicion is required to diagnose patients with VEXAS before significant end organ damage has occurred. “This diagnosis should be considered in all older male patients who present with neutrophilic dermatosis — particularly histiocytoid Sweet syndrome, vasculitis, or leukocytoclasia without vasculitis. Patients who appear to have isolated skin involvement may have cytopenias and acute phase reactants. Therefore, complete blood count with differential and ESR and CRP should be considered to investigate for macrocytosis, cytopenias, and systemic inflammation.”
He acknowledged certain limitations of the study, including the fact that many patients were first evaluated at the NIH after having disease symptoms for many months or years. “It is possible that patients with VEXAS referred to the NIH, either for genetic testing or in person evaluation, represent a population with more aggressive disease.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the study, emphasized the importance of the UBA1 mutation in the diagnosis of this complex syndrome. “Dermatologists should be aware of VEXAS syndrome as the majority of patients present with skin lesions, which can range from urticarial to Sweet syndrome–like to palpable purpura,” Dr. Ko said.
“Chondritis and periorbital edema, sometimes unilateral, are also associated. Histopathologic clues include a predominantly histiocytoid infiltrate,” she noted. In addition, “the prominent myxoid stroma around blood vessels and adnexal structures as a clue to VEXAS syndrome surprised me; I had not read that before.”
The study was supported by the Intramural Research Program of NIAMS. One of the study authors reported personal fees from Alexion, Novartis, and Sobi outside of the submitted work. No other disclosures were reported. Dr. Ko reported having no disclosures.
A version of this article appeared on Medscape.com .
Additionally, the most common histologic findings include leukocytoclastic vasculitis, neutrophilic dermatosis, and perivascular dermatitis; different variants in the UBA1 gene are associated with specific skin manifestations.
Those are key findings from a cohort study of 112 patients with VEXAS published online in JAMA Dermatology. The study, conducted by researchers at the National Institutes of Health (NIH) and several other institutions, aimed to define the spectrum of cutaneous manifestations in VEXAS in association with genetic, histologic, and other clinical findings.
First described in 2020, VEXAS syndrome is an adult-onset multisystem disease that can pose a diagnostic challenge to clinicians, the study’s corresponding author, Edward W. Cowen, MD, MHSc, of the dermatology branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), said in an interview. The disease is caused by pathogenic variants in the UBA1 gene, located on the X chromosome. Affected individuals exhibit a wide range of manifestations, including cytopenia/myelodysplasia, multiorgan systemic inflammation, and cutaneous involvement.
“Patients may present to a variety of disease specialists depending on their symptoms and providers may not immediately consider a genetic etiology in an older individual,” Dr. Cowen said in an interview. “Although skin involvement occurs in more than 80% of patients, it is pleomorphic and may resemble a variety of other conditions such as vasculitis and Sweet syndrome.”
To better understand the cutaneous manifestations of VEXAS syndrome, the researchers evaluated data from 112 patients with VEXAS-defining genetic variants in the UBA1 gene between 2019 and 2023. Of the 112 patients, 73 underwent medical record review only, and 39 were prospectively evaluated at NIH. All but one of the patients were men, 94% were White individuals, and their mean age was 64 years. Skin involvement occurred in 83% of cases and was the most common presenting feature of VEXAS in 61% of cases.
Of the 64 histopathologic reports available from 60 patients, the main skin histopathologic findings were leukocytoclastic vasculitis in 23 patients (36%), neutrophilic dermatosis in 22 patients (34%), and perivascular dermatitis in 19 patients (30%). According to Dr. Cowen, one key histologic finding was a distinct pattern of “histiocytoid” dermal neutrophilic inflammation, which was present in 13 of 15 specimens (86%) that underwent central re-review. “This pattern can occasionally also be seen in patients with Sweet syndrome, unrelated to VEXAS, but was a hallmark feature found in the majority of skin biopsies of patients with VEXAS,” he said.
“Together with another pathologic finding, leukocytoclasia, these features can be useful clues to alert the pathologist to a potential diagnosis of VEXAS. This myeloid predominant pattern of skin inflammation was also most strongly associated with the leucine pathogenic variant of the UBA1 gene.” In contrast, cutaneous vasculitis was most strongly associated with the valine pathogenic variant of UBA1. “This is important because the valine variant has been previously independently linked to decreased survival,” he said.
In findings related to pathogenic genetic variants, the researchers observed that the p.Met41Leu variant was most frequently associated with neutrophilic dermal infiltrates in 14 of 17 patients (82%) with this variant and often resembled histiocytoid Sweet syndrome. In addition, the p.Met41Val variant was associated with vasculitic lesions in 11 of 20 patients (55%) with this variant and with a mixed leukocytic infiltrate in 17 of these 20 patients (85%).
Treatment Outcomes
In the realm of therapies, skin manifestations improved in 67 of 73 patients (92%) treated with oral prednisone, while treatment with the interleukin-1 receptor antagonist anakinra improved cutaneous disease in 9 of the 16 (56%) who received it. However, 12 (75%) of those who received anakinra developed severe injection-site reactions, including ulceration in two patients and abscess formation in one patient.
Dr. Cowen noted that VEXAS is associated with high mortality (22% in this cohort), and a high degree of suspicion is required to diagnose patients with VEXAS before significant end organ damage has occurred. “This diagnosis should be considered in all older male patients who present with neutrophilic dermatosis — particularly histiocytoid Sweet syndrome, vasculitis, or leukocytoclasia without vasculitis. Patients who appear to have isolated skin involvement may have cytopenias and acute phase reactants. Therefore, complete blood count with differential and ESR and CRP should be considered to investigate for macrocytosis, cytopenias, and systemic inflammation.”
He acknowledged certain limitations of the study, including the fact that many patients were first evaluated at the NIH after having disease symptoms for many months or years. “It is possible that patients with VEXAS referred to the NIH, either for genetic testing or in person evaluation, represent a population with more aggressive disease.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the study, emphasized the importance of the UBA1 mutation in the diagnosis of this complex syndrome. “Dermatologists should be aware of VEXAS syndrome as the majority of patients present with skin lesions, which can range from urticarial to Sweet syndrome–like to palpable purpura,” Dr. Ko said.
“Chondritis and periorbital edema, sometimes unilateral, are also associated. Histopathologic clues include a predominantly histiocytoid infiltrate,” she noted. In addition, “the prominent myxoid stroma around blood vessels and adnexal structures as a clue to VEXAS syndrome surprised me; I had not read that before.”
The study was supported by the Intramural Research Program of NIAMS. One of the study authors reported personal fees from Alexion, Novartis, and Sobi outside of the submitted work. No other disclosures were reported. Dr. Ko reported having no disclosures.
A version of this article appeared on Medscape.com .
Additionally, the most common histologic findings include leukocytoclastic vasculitis, neutrophilic dermatosis, and perivascular dermatitis; different variants in the UBA1 gene are associated with specific skin manifestations.
Those are key findings from a cohort study of 112 patients with VEXAS published online in JAMA Dermatology. The study, conducted by researchers at the National Institutes of Health (NIH) and several other institutions, aimed to define the spectrum of cutaneous manifestations in VEXAS in association with genetic, histologic, and other clinical findings.
First described in 2020, VEXAS syndrome is an adult-onset multisystem disease that can pose a diagnostic challenge to clinicians, the study’s corresponding author, Edward W. Cowen, MD, MHSc, of the dermatology branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), said in an interview. The disease is caused by pathogenic variants in the UBA1 gene, located on the X chromosome. Affected individuals exhibit a wide range of manifestations, including cytopenia/myelodysplasia, multiorgan systemic inflammation, and cutaneous involvement.
“Patients may present to a variety of disease specialists depending on their symptoms and providers may not immediately consider a genetic etiology in an older individual,” Dr. Cowen said in an interview. “Although skin involvement occurs in more than 80% of patients, it is pleomorphic and may resemble a variety of other conditions such as vasculitis and Sweet syndrome.”
To better understand the cutaneous manifestations of VEXAS syndrome, the researchers evaluated data from 112 patients with VEXAS-defining genetic variants in the UBA1 gene between 2019 and 2023. Of the 112 patients, 73 underwent medical record review only, and 39 were prospectively evaluated at NIH. All but one of the patients were men, 94% were White individuals, and their mean age was 64 years. Skin involvement occurred in 83% of cases and was the most common presenting feature of VEXAS in 61% of cases.
Of the 64 histopathologic reports available from 60 patients, the main skin histopathologic findings were leukocytoclastic vasculitis in 23 patients (36%), neutrophilic dermatosis in 22 patients (34%), and perivascular dermatitis in 19 patients (30%). According to Dr. Cowen, one key histologic finding was a distinct pattern of “histiocytoid” dermal neutrophilic inflammation, which was present in 13 of 15 specimens (86%) that underwent central re-review. “This pattern can occasionally also be seen in patients with Sweet syndrome, unrelated to VEXAS, but was a hallmark feature found in the majority of skin biopsies of patients with VEXAS,” he said.
“Together with another pathologic finding, leukocytoclasia, these features can be useful clues to alert the pathologist to a potential diagnosis of VEXAS. This myeloid predominant pattern of skin inflammation was also most strongly associated with the leucine pathogenic variant of the UBA1 gene.” In contrast, cutaneous vasculitis was most strongly associated with the valine pathogenic variant of UBA1. “This is important because the valine variant has been previously independently linked to decreased survival,” he said.
In findings related to pathogenic genetic variants, the researchers observed that the p.Met41Leu variant was most frequently associated with neutrophilic dermal infiltrates in 14 of 17 patients (82%) with this variant and often resembled histiocytoid Sweet syndrome. In addition, the p.Met41Val variant was associated with vasculitic lesions in 11 of 20 patients (55%) with this variant and with a mixed leukocytic infiltrate in 17 of these 20 patients (85%).
Treatment Outcomes
In the realm of therapies, skin manifestations improved in 67 of 73 patients (92%) treated with oral prednisone, while treatment with the interleukin-1 receptor antagonist anakinra improved cutaneous disease in 9 of the 16 (56%) who received it. However, 12 (75%) of those who received anakinra developed severe injection-site reactions, including ulceration in two patients and abscess formation in one patient.
Dr. Cowen noted that VEXAS is associated with high mortality (22% in this cohort), and a high degree of suspicion is required to diagnose patients with VEXAS before significant end organ damage has occurred. “This diagnosis should be considered in all older male patients who present with neutrophilic dermatosis — particularly histiocytoid Sweet syndrome, vasculitis, or leukocytoclasia without vasculitis. Patients who appear to have isolated skin involvement may have cytopenias and acute phase reactants. Therefore, complete blood count with differential and ESR and CRP should be considered to investigate for macrocytosis, cytopenias, and systemic inflammation.”
He acknowledged certain limitations of the study, including the fact that many patients were first evaluated at the NIH after having disease symptoms for many months or years. “It is possible that patients with VEXAS referred to the NIH, either for genetic testing or in person evaluation, represent a population with more aggressive disease.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the study, emphasized the importance of the UBA1 mutation in the diagnosis of this complex syndrome. “Dermatologists should be aware of VEXAS syndrome as the majority of patients present with skin lesions, which can range from urticarial to Sweet syndrome–like to palpable purpura,” Dr. Ko said.
“Chondritis and periorbital edema, sometimes unilateral, are also associated. Histopathologic clues include a predominantly histiocytoid infiltrate,” she noted. In addition, “the prominent myxoid stroma around blood vessels and adnexal structures as a clue to VEXAS syndrome surprised me; I had not read that before.”
The study was supported by the Intramural Research Program of NIAMS. One of the study authors reported personal fees from Alexion, Novartis, and Sobi outside of the submitted work. No other disclosures were reported. Dr. Ko reported having no disclosures.
A version of this article appeared on Medscape.com .
FROM JAMA DERMATOLOGY
Study Highlights Melanoma Survival Disparities in Rural vs Urban Settings
, results from an analysis of data from the National Cancer Institute showed.
“Melanoma is currently the fifth most common malignancy in the United States, with approximately 106,000 new cases and 7180 reported deaths occurring in 2021,” the study’s first author, Mitchell Taylor, MD, a dermatology research fellow at the University of Nebraska, Omaha, and colleagues wrote in the abstract, which was presented during a poster session at the annual meeting of the Society for Investigative Dermatology. “Rural areas have been shown to bear a higher melanoma disease burden, yet there is a paucity of national-level studies examining these disparities.”
To characterize the rural population diagnosed with cutaneous melanoma and assess associated disparities in the United States, the researchers queried the NCI’s Surveillance, Epidemiology, and End Results database to identify individuals diagnosed with cutaneous melanoma from 2000 to 2020 (International Classification of Diseases, 3rd Edition, 8720/3 — 8780/3; Primary Site codes C44.0-C44.9). They drew from US Office of Management and Budget terminology to define and categorize rural and urban communities.
Among 391,047 patients included during the study period, binary logistic regression analysis revealed that patients in rural areas had a greater odds of being older, from ages 50 to 75 years (odds ratio [OR], 1.10; P < .001); had annual incomes < $70,000 (OR, 16.80; P < .001); had tumors located on the head and neck (OR, 1.24; P < .001); and presented with regional/distant disease (OR, 1.13; P < .001).
As for disease-specific survival, patients living in rural areas had significantly reduced survival compared with those living in urban areas (a mean of 207.3 vs 216.3 months, respectively; P < .001). Multivariate Cox regression revealed that living in a rural setting was significantly associated with reduced disease-specific survival (hazard ratio [HR], 1.10; P < .001), as was having head and neck tumors (HR, 1.41; P < .001).“Overall, this study underscores a significant decrease in disease-specific survival among rural patients diagnosed with cutaneous melanoma and establishes a significant association between rural living and high-risk primary tumor locations, particularly the head and neck,” the authors concluded.
Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, Colorado, who was asked to comment on the results, said the findings echo the results of a recent study which characterized melanoma rates among non-Hispanic American Indian/Alaska Native individuals from 1999 to 2019.
“I suspect this decreased disease-specific survival highlights the issues our rural-residing patients face with access to dermatology care,” Dr. Kohn told this news organization. “Dermatologists are able to detect thinner melanomas than patients [and] are preferentially concentrated in metropolitan areas. Dermatologists are also the most skilled and knowledgeable to screen, diagnose, and manage melanomas. Having fewer dermatologists in rural areas impedes melanoma care for our rural-residing patients.”
Neither the researchers nor Dr. Kohn reported any relevant disclosures.
A version of this article first appeared on Medscape.com.
, results from an analysis of data from the National Cancer Institute showed.
“Melanoma is currently the fifth most common malignancy in the United States, with approximately 106,000 new cases and 7180 reported deaths occurring in 2021,” the study’s first author, Mitchell Taylor, MD, a dermatology research fellow at the University of Nebraska, Omaha, and colleagues wrote in the abstract, which was presented during a poster session at the annual meeting of the Society for Investigative Dermatology. “Rural areas have been shown to bear a higher melanoma disease burden, yet there is a paucity of national-level studies examining these disparities.”
To characterize the rural population diagnosed with cutaneous melanoma and assess associated disparities in the United States, the researchers queried the NCI’s Surveillance, Epidemiology, and End Results database to identify individuals diagnosed with cutaneous melanoma from 2000 to 2020 (International Classification of Diseases, 3rd Edition, 8720/3 — 8780/3; Primary Site codes C44.0-C44.9). They drew from US Office of Management and Budget terminology to define and categorize rural and urban communities.
Among 391,047 patients included during the study period, binary logistic regression analysis revealed that patients in rural areas had a greater odds of being older, from ages 50 to 75 years (odds ratio [OR], 1.10; P < .001); had annual incomes < $70,000 (OR, 16.80; P < .001); had tumors located on the head and neck (OR, 1.24; P < .001); and presented with regional/distant disease (OR, 1.13; P < .001).
As for disease-specific survival, patients living in rural areas had significantly reduced survival compared with those living in urban areas (a mean of 207.3 vs 216.3 months, respectively; P < .001). Multivariate Cox regression revealed that living in a rural setting was significantly associated with reduced disease-specific survival (hazard ratio [HR], 1.10; P < .001), as was having head and neck tumors (HR, 1.41; P < .001).“Overall, this study underscores a significant decrease in disease-specific survival among rural patients diagnosed with cutaneous melanoma and establishes a significant association between rural living and high-risk primary tumor locations, particularly the head and neck,” the authors concluded.
Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, Colorado, who was asked to comment on the results, said the findings echo the results of a recent study which characterized melanoma rates among non-Hispanic American Indian/Alaska Native individuals from 1999 to 2019.
“I suspect this decreased disease-specific survival highlights the issues our rural-residing patients face with access to dermatology care,” Dr. Kohn told this news organization. “Dermatologists are able to detect thinner melanomas than patients [and] are preferentially concentrated in metropolitan areas. Dermatologists are also the most skilled and knowledgeable to screen, diagnose, and manage melanomas. Having fewer dermatologists in rural areas impedes melanoma care for our rural-residing patients.”
Neither the researchers nor Dr. Kohn reported any relevant disclosures.
A version of this article first appeared on Medscape.com.
, results from an analysis of data from the National Cancer Institute showed.
“Melanoma is currently the fifth most common malignancy in the United States, with approximately 106,000 new cases and 7180 reported deaths occurring in 2021,” the study’s first author, Mitchell Taylor, MD, a dermatology research fellow at the University of Nebraska, Omaha, and colleagues wrote in the abstract, which was presented during a poster session at the annual meeting of the Society for Investigative Dermatology. “Rural areas have been shown to bear a higher melanoma disease burden, yet there is a paucity of national-level studies examining these disparities.”
To characterize the rural population diagnosed with cutaneous melanoma and assess associated disparities in the United States, the researchers queried the NCI’s Surveillance, Epidemiology, and End Results database to identify individuals diagnosed with cutaneous melanoma from 2000 to 2020 (International Classification of Diseases, 3rd Edition, 8720/3 — 8780/3; Primary Site codes C44.0-C44.9). They drew from US Office of Management and Budget terminology to define and categorize rural and urban communities.
Among 391,047 patients included during the study period, binary logistic regression analysis revealed that patients in rural areas had a greater odds of being older, from ages 50 to 75 years (odds ratio [OR], 1.10; P < .001); had annual incomes < $70,000 (OR, 16.80; P < .001); had tumors located on the head and neck (OR, 1.24; P < .001); and presented with regional/distant disease (OR, 1.13; P < .001).
As for disease-specific survival, patients living in rural areas had significantly reduced survival compared with those living in urban areas (a mean of 207.3 vs 216.3 months, respectively; P < .001). Multivariate Cox regression revealed that living in a rural setting was significantly associated with reduced disease-specific survival (hazard ratio [HR], 1.10; P < .001), as was having head and neck tumors (HR, 1.41; P < .001).“Overall, this study underscores a significant decrease in disease-specific survival among rural patients diagnosed with cutaneous melanoma and establishes a significant association between rural living and high-risk primary tumor locations, particularly the head and neck,” the authors concluded.
Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, Colorado, who was asked to comment on the results, said the findings echo the results of a recent study which characterized melanoma rates among non-Hispanic American Indian/Alaska Native individuals from 1999 to 2019.
“I suspect this decreased disease-specific survival highlights the issues our rural-residing patients face with access to dermatology care,” Dr. Kohn told this news organization. “Dermatologists are able to detect thinner melanomas than patients [and] are preferentially concentrated in metropolitan areas. Dermatologists are also the most skilled and knowledgeable to screen, diagnose, and manage melanomas. Having fewer dermatologists in rural areas impedes melanoma care for our rural-residing patients.”
Neither the researchers nor Dr. Kohn reported any relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SID 2024
Features of Merkel Cell in Hispanic Patients Explored
. In addition, the most affected site was the upper limb/shoulder, which differs from what has been reported in previous studies.
Those are key findings from a retrospective study of national cancer data that was presented during a poster session at the annual meeting of the Society for Investigative Dermatology.
“Merkel cell carcinoma is an infrequent and aggressive form of neuroendocrine skin cancer that mainly impacts individuals of White ethnicity, with a general occurrence rate of 0.7 instances per 100,000 person-years,” one of the study authors, Luis J. Borda, MD, chief dermatology resident at Eastern Virginia Medical School, Norfolk, Virginia, told this news organization. The incidence of MCC is increasing among all racial groups, especially in the Hispanic population, he added.
To determine how age, sex, and primary site of MCC differ in White vs non-White Hispanic patients, the researchers evaluated the 22 population-based cancer registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program from 2000 through 2020. They reported categorical variables as counts and percentages and used chi-square test with Yates’s correction to assess the association between categorical variables.
Of the 17,920 MCCs identified by the researchers, 40 (0.22%) were in non-White Hispanic patients. Compared with the White patients with MCC, significantly fewer non-White Hispanic patients were age 70 years or older (50% vs 72.1%, respectively; P < .001), and MCC was more common in female non-White Hispanic patients (23, or 57.5%), while White patients with MCC were predominantly male (11,309, or 63.2%; P < .05). “This suggests that MCC in non-White Hispanic patients may involve different risk factors related to age beyond just cumulative UV exposure and aging-related immunosenescence, which may additionally account for the higher prevalence of females in this cohort, as historically male outdoor occupation has resulted in increased lifetime cumulative UV exposure,” Dr. Borda said.
The head and neck were the most common sites of disease involvement in White patients (41.9% vs 27.5% in non-White Hispanic patients; P = .09), while the upper limb and shoulder were the most common sites of disease involvement in non-White Hispanic patients (37.5% vs 23.8% in White patients; P = .06). This finding “differs from previous studies showing head/neck being the most common site in Hispanics,” Dr. Borda said, adding that this could be a result of White patients not being included in the Hispanic cohort in this study. “Because non-White Hispanic patients have darker skin, they may have proportionally more cases on sun-protected skin, as is described by the present data, suggesting that they are less likely to have UV-driven MCC.”
The study “highlights distinct demographic and clinical characteristics of MCC among non-White Hispanic patients compared to their White counterparts, emphasizing the importance of considering race/ethnicity in understanding the epidemiology of this rare but increasingly prevalent cancer,” Dr. Borda said. He and his co-authors are planning to do further research on the increasing incidence of MCC in non-White Hispanic patients and on staging at diagnosis compared to White patients.
Dr. Borda acknowledged certain limitations of the analysis, including the small sample size in the non-White Hispanic group, the retrospective nature of SEER data, selection bias, and the potential for underreporting. He and his co-authors reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
. In addition, the most affected site was the upper limb/shoulder, which differs from what has been reported in previous studies.
Those are key findings from a retrospective study of national cancer data that was presented during a poster session at the annual meeting of the Society for Investigative Dermatology.
“Merkel cell carcinoma is an infrequent and aggressive form of neuroendocrine skin cancer that mainly impacts individuals of White ethnicity, with a general occurrence rate of 0.7 instances per 100,000 person-years,” one of the study authors, Luis J. Borda, MD, chief dermatology resident at Eastern Virginia Medical School, Norfolk, Virginia, told this news organization. The incidence of MCC is increasing among all racial groups, especially in the Hispanic population, he added.
To determine how age, sex, and primary site of MCC differ in White vs non-White Hispanic patients, the researchers evaluated the 22 population-based cancer registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program from 2000 through 2020. They reported categorical variables as counts and percentages and used chi-square test with Yates’s correction to assess the association between categorical variables.
Of the 17,920 MCCs identified by the researchers, 40 (0.22%) were in non-White Hispanic patients. Compared with the White patients with MCC, significantly fewer non-White Hispanic patients were age 70 years or older (50% vs 72.1%, respectively; P < .001), and MCC was more common in female non-White Hispanic patients (23, or 57.5%), while White patients with MCC were predominantly male (11,309, or 63.2%; P < .05). “This suggests that MCC in non-White Hispanic patients may involve different risk factors related to age beyond just cumulative UV exposure and aging-related immunosenescence, which may additionally account for the higher prevalence of females in this cohort, as historically male outdoor occupation has resulted in increased lifetime cumulative UV exposure,” Dr. Borda said.
The head and neck were the most common sites of disease involvement in White patients (41.9% vs 27.5% in non-White Hispanic patients; P = .09), while the upper limb and shoulder were the most common sites of disease involvement in non-White Hispanic patients (37.5% vs 23.8% in White patients; P = .06). This finding “differs from previous studies showing head/neck being the most common site in Hispanics,” Dr. Borda said, adding that this could be a result of White patients not being included in the Hispanic cohort in this study. “Because non-White Hispanic patients have darker skin, they may have proportionally more cases on sun-protected skin, as is described by the present data, suggesting that they are less likely to have UV-driven MCC.”
The study “highlights distinct demographic and clinical characteristics of MCC among non-White Hispanic patients compared to their White counterparts, emphasizing the importance of considering race/ethnicity in understanding the epidemiology of this rare but increasingly prevalent cancer,” Dr. Borda said. He and his co-authors are planning to do further research on the increasing incidence of MCC in non-White Hispanic patients and on staging at diagnosis compared to White patients.
Dr. Borda acknowledged certain limitations of the analysis, including the small sample size in the non-White Hispanic group, the retrospective nature of SEER data, selection bias, and the potential for underreporting. He and his co-authors reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
. In addition, the most affected site was the upper limb/shoulder, which differs from what has been reported in previous studies.
Those are key findings from a retrospective study of national cancer data that was presented during a poster session at the annual meeting of the Society for Investigative Dermatology.
“Merkel cell carcinoma is an infrequent and aggressive form of neuroendocrine skin cancer that mainly impacts individuals of White ethnicity, with a general occurrence rate of 0.7 instances per 100,000 person-years,” one of the study authors, Luis J. Borda, MD, chief dermatology resident at Eastern Virginia Medical School, Norfolk, Virginia, told this news organization. The incidence of MCC is increasing among all racial groups, especially in the Hispanic population, he added.
To determine how age, sex, and primary site of MCC differ in White vs non-White Hispanic patients, the researchers evaluated the 22 population-based cancer registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program from 2000 through 2020. They reported categorical variables as counts and percentages and used chi-square test with Yates’s correction to assess the association between categorical variables.
Of the 17,920 MCCs identified by the researchers, 40 (0.22%) were in non-White Hispanic patients. Compared with the White patients with MCC, significantly fewer non-White Hispanic patients were age 70 years or older (50% vs 72.1%, respectively; P < .001), and MCC was more common in female non-White Hispanic patients (23, or 57.5%), while White patients with MCC were predominantly male (11,309, or 63.2%; P < .05). “This suggests that MCC in non-White Hispanic patients may involve different risk factors related to age beyond just cumulative UV exposure and aging-related immunosenescence, which may additionally account for the higher prevalence of females in this cohort, as historically male outdoor occupation has resulted in increased lifetime cumulative UV exposure,” Dr. Borda said.
The head and neck were the most common sites of disease involvement in White patients (41.9% vs 27.5% in non-White Hispanic patients; P = .09), while the upper limb and shoulder were the most common sites of disease involvement in non-White Hispanic patients (37.5% vs 23.8% in White patients; P = .06). This finding “differs from previous studies showing head/neck being the most common site in Hispanics,” Dr. Borda said, adding that this could be a result of White patients not being included in the Hispanic cohort in this study. “Because non-White Hispanic patients have darker skin, they may have proportionally more cases on sun-protected skin, as is described by the present data, suggesting that they are less likely to have UV-driven MCC.”
The study “highlights distinct demographic and clinical characteristics of MCC among non-White Hispanic patients compared to their White counterparts, emphasizing the importance of considering race/ethnicity in understanding the epidemiology of this rare but increasingly prevalent cancer,” Dr. Borda said. He and his co-authors are planning to do further research on the increasing incidence of MCC in non-White Hispanic patients and on staging at diagnosis compared to White patients.
Dr. Borda acknowledged certain limitations of the analysis, including the small sample size in the non-White Hispanic group, the retrospective nature of SEER data, selection bias, and the potential for underreporting. He and his co-authors reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
FROM SID 2024
High Sodium Intake Linked to Greater Risk for Eczema
In a study of adults, an increase of 1 g in estimated 24-hour urinary sodium excretion was associated with 11% higher odds of an atopic dermatitis (AD) diagnosis, 16% higher odds of having active AD, and 11% higher odds of increased severity of AD.
Those are key findings from a cross-sectional analysis of data from the United Kingdom.
“Excessive dietary sodium, common in fast food, may be associated with AD,” corresponding author Katrina Abuabara, MD, MA, MSCE, and colleagues wrote in the study, which was published online in JAMA Dermatology. They referred to recent research using sodium MRI, which showed that “the majority of the body’s exchangeable sodium is stored in the skin and that skin sodium is associated with autoimmune and chronic inflammatory conditions, including AD.” And in another study published in 2019, lesional skin sodium was 30-fold greater in patients with AD than in healthy controls.
To investigate whether there is an association between higher levels of sodium consumption and AD prevalence, activity, and severity at the population level, Dr. Abuabara, of the program for clinical research in the Department of Dermatology at the University of California, San Francisco, and coauthors drew from the UK Biobank, a population-based cohort of more than 500,000 individuals aged 37-73 years at the time of recruitment by the National Health Service. The primary exposure was 24-hour urinary sodium excretion, which was calculated by using the INTERSALT equation, a sex-specific estimation that incorporates body mass index; age; and urine concentrations of potassium, sodium, and creatinine. The primary study outcome was AD or active AD based on diagnostic and prescription codes from linked electronic medical records. The researchers used multivariable logistic regression models adjusted for age, sex, race and ethnicity, Townsend deprivation index, and education to measure the association.
Of the 215,832 Biobank participants included in the analysis, 54% were female, their mean age was 57 years, 95% were White, their mean estimated 24-hour urine sodium excretion was 3.01 g/day, and 10,839 (5%) had a diagnosis of AD. The researchers observed that on multivariable logistic regression, a 1-g increase in estimated 24-hour urine sodium excretion was associated with increased odds of AD (adjusted odds ratio [AOR], 1.11; 95% CI, 1.07-1.14), increased odds of active AD (AOR, 1.16; 95% CI, 1.05-1.28), and increased odds of increasing severity of AD (AOR, 1.11; 95% CI, 1.07-1.15).
Validating Results With US Data
To validate the findings, the researchers evaluated a cohort of 13,014 participants from the US-based National Health and Nutrition Examination Survey (NHANES), using pooled data from the 1999-2000, 2001-2002, and 2003-2004 samples. Of the 13,014 participants, 796 reported current AD, and 1493 reported AD in the past year. The mean dietary sodium intake of overall NHANES participants estimated with 24-hour dietary recall questionnaires was 3.45 g, with a mean of 3.47 g for those with current AD and a mean of 3.44 g for those without AD.
The researchers observed that a 1-g/day higher dietary sodium intake was associated with a higher risk for current AD (AOR, 1.22; 95%CI, 1.01-1.47) and a somewhat higher risk for AD in the past year (AOR, 1.14; 95% CI, 0.97-1.35).
“Future work should examine whether variation of sodium intake over time might trigger AD flares and whether it helps to explain heterogeneity in response to new immunomodulatory treatments for AD,” the authors wrote. “Reduced sodium intake was recommended as a treatment for AD more than a century ago, but there have yet to be studies examining the association of dietary sodium reduction with skin sodium concentration or AD severity,” they added. Noting that sodium reduction “has been shown to be a cost-effective intervention for hypertension and other cardiovascular disease outcomes,” they said that their data “support experimental studies of this approach in AD.”
They acknowledged certain limitations of the study, including the fact that a single spot urine sample was used in the UK Biobank cohort, “which only captures dietary intake of the last 24 hours and is not the best measure of usual or long-term intake of sodium.” They also noted that the findings may not be generalizable to other populations and that AD was based on self-report in the NHANES validation cohort.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the results, said the study by Dr. Abuabara and colleagues “gives us another reason to avoid salt, showing that 1 g/day of higher salt intake increases the risk of AD in an adult population and more severe AD.”
He added that, “Now, can you say that reducing salt intake will have a therapeutic effect or clinically relevant impact? No. [That is] certainly worth exploring but at a minimum, gives some more credibility to keeping it bland.”
The study was supported by a grant from the Medical Student in Aging Research Program, the National Institute on Aging, and the National Eczema Association. Dr. Abuabara reported receiving research funding for her institution from Pfizer and Cosmetique Internacional/La Roche-Posay and consulting fees from Target RWE, Sanofi, Nektar, and Amgen. No other disclosures were reported. Dr. Friedman had no relevant disclosures.
A version of this article appeared on Medscape.com.
In a study of adults, an increase of 1 g in estimated 24-hour urinary sodium excretion was associated with 11% higher odds of an atopic dermatitis (AD) diagnosis, 16% higher odds of having active AD, and 11% higher odds of increased severity of AD.
Those are key findings from a cross-sectional analysis of data from the United Kingdom.
“Excessive dietary sodium, common in fast food, may be associated with AD,” corresponding author Katrina Abuabara, MD, MA, MSCE, and colleagues wrote in the study, which was published online in JAMA Dermatology. They referred to recent research using sodium MRI, which showed that “the majority of the body’s exchangeable sodium is stored in the skin and that skin sodium is associated with autoimmune and chronic inflammatory conditions, including AD.” And in another study published in 2019, lesional skin sodium was 30-fold greater in patients with AD than in healthy controls.
To investigate whether there is an association between higher levels of sodium consumption and AD prevalence, activity, and severity at the population level, Dr. Abuabara, of the program for clinical research in the Department of Dermatology at the University of California, San Francisco, and coauthors drew from the UK Biobank, a population-based cohort of more than 500,000 individuals aged 37-73 years at the time of recruitment by the National Health Service. The primary exposure was 24-hour urinary sodium excretion, which was calculated by using the INTERSALT equation, a sex-specific estimation that incorporates body mass index; age; and urine concentrations of potassium, sodium, and creatinine. The primary study outcome was AD or active AD based on diagnostic and prescription codes from linked electronic medical records. The researchers used multivariable logistic regression models adjusted for age, sex, race and ethnicity, Townsend deprivation index, and education to measure the association.
Of the 215,832 Biobank participants included in the analysis, 54% were female, their mean age was 57 years, 95% were White, their mean estimated 24-hour urine sodium excretion was 3.01 g/day, and 10,839 (5%) had a diagnosis of AD. The researchers observed that on multivariable logistic regression, a 1-g increase in estimated 24-hour urine sodium excretion was associated with increased odds of AD (adjusted odds ratio [AOR], 1.11; 95% CI, 1.07-1.14), increased odds of active AD (AOR, 1.16; 95% CI, 1.05-1.28), and increased odds of increasing severity of AD (AOR, 1.11; 95% CI, 1.07-1.15).
Validating Results With US Data
To validate the findings, the researchers evaluated a cohort of 13,014 participants from the US-based National Health and Nutrition Examination Survey (NHANES), using pooled data from the 1999-2000, 2001-2002, and 2003-2004 samples. Of the 13,014 participants, 796 reported current AD, and 1493 reported AD in the past year. The mean dietary sodium intake of overall NHANES participants estimated with 24-hour dietary recall questionnaires was 3.45 g, with a mean of 3.47 g for those with current AD and a mean of 3.44 g for those without AD.
The researchers observed that a 1-g/day higher dietary sodium intake was associated with a higher risk for current AD (AOR, 1.22; 95%CI, 1.01-1.47) and a somewhat higher risk for AD in the past year (AOR, 1.14; 95% CI, 0.97-1.35).
“Future work should examine whether variation of sodium intake over time might trigger AD flares and whether it helps to explain heterogeneity in response to new immunomodulatory treatments for AD,” the authors wrote. “Reduced sodium intake was recommended as a treatment for AD more than a century ago, but there have yet to be studies examining the association of dietary sodium reduction with skin sodium concentration or AD severity,” they added. Noting that sodium reduction “has been shown to be a cost-effective intervention for hypertension and other cardiovascular disease outcomes,” they said that their data “support experimental studies of this approach in AD.”
They acknowledged certain limitations of the study, including the fact that a single spot urine sample was used in the UK Biobank cohort, “which only captures dietary intake of the last 24 hours and is not the best measure of usual or long-term intake of sodium.” They also noted that the findings may not be generalizable to other populations and that AD was based on self-report in the NHANES validation cohort.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the results, said the study by Dr. Abuabara and colleagues “gives us another reason to avoid salt, showing that 1 g/day of higher salt intake increases the risk of AD in an adult population and more severe AD.”
He added that, “Now, can you say that reducing salt intake will have a therapeutic effect or clinically relevant impact? No. [That is] certainly worth exploring but at a minimum, gives some more credibility to keeping it bland.”
The study was supported by a grant from the Medical Student in Aging Research Program, the National Institute on Aging, and the National Eczema Association. Dr. Abuabara reported receiving research funding for her institution from Pfizer and Cosmetique Internacional/La Roche-Posay and consulting fees from Target RWE, Sanofi, Nektar, and Amgen. No other disclosures were reported. Dr. Friedman had no relevant disclosures.
A version of this article appeared on Medscape.com.
In a study of adults, an increase of 1 g in estimated 24-hour urinary sodium excretion was associated with 11% higher odds of an atopic dermatitis (AD) diagnosis, 16% higher odds of having active AD, and 11% higher odds of increased severity of AD.
Those are key findings from a cross-sectional analysis of data from the United Kingdom.
“Excessive dietary sodium, common in fast food, may be associated with AD,” corresponding author Katrina Abuabara, MD, MA, MSCE, and colleagues wrote in the study, which was published online in JAMA Dermatology. They referred to recent research using sodium MRI, which showed that “the majority of the body’s exchangeable sodium is stored in the skin and that skin sodium is associated with autoimmune and chronic inflammatory conditions, including AD.” And in another study published in 2019, lesional skin sodium was 30-fold greater in patients with AD than in healthy controls.
To investigate whether there is an association between higher levels of sodium consumption and AD prevalence, activity, and severity at the population level, Dr. Abuabara, of the program for clinical research in the Department of Dermatology at the University of California, San Francisco, and coauthors drew from the UK Biobank, a population-based cohort of more than 500,000 individuals aged 37-73 years at the time of recruitment by the National Health Service. The primary exposure was 24-hour urinary sodium excretion, which was calculated by using the INTERSALT equation, a sex-specific estimation that incorporates body mass index; age; and urine concentrations of potassium, sodium, and creatinine. The primary study outcome was AD or active AD based on diagnostic and prescription codes from linked electronic medical records. The researchers used multivariable logistic regression models adjusted for age, sex, race and ethnicity, Townsend deprivation index, and education to measure the association.
Of the 215,832 Biobank participants included in the analysis, 54% were female, their mean age was 57 years, 95% were White, their mean estimated 24-hour urine sodium excretion was 3.01 g/day, and 10,839 (5%) had a diagnosis of AD. The researchers observed that on multivariable logistic regression, a 1-g increase in estimated 24-hour urine sodium excretion was associated with increased odds of AD (adjusted odds ratio [AOR], 1.11; 95% CI, 1.07-1.14), increased odds of active AD (AOR, 1.16; 95% CI, 1.05-1.28), and increased odds of increasing severity of AD (AOR, 1.11; 95% CI, 1.07-1.15).
Validating Results With US Data
To validate the findings, the researchers evaluated a cohort of 13,014 participants from the US-based National Health and Nutrition Examination Survey (NHANES), using pooled data from the 1999-2000, 2001-2002, and 2003-2004 samples. Of the 13,014 participants, 796 reported current AD, and 1493 reported AD in the past year. The mean dietary sodium intake of overall NHANES participants estimated with 24-hour dietary recall questionnaires was 3.45 g, with a mean of 3.47 g for those with current AD and a mean of 3.44 g for those without AD.
The researchers observed that a 1-g/day higher dietary sodium intake was associated with a higher risk for current AD (AOR, 1.22; 95%CI, 1.01-1.47) and a somewhat higher risk for AD in the past year (AOR, 1.14; 95% CI, 0.97-1.35).
“Future work should examine whether variation of sodium intake over time might trigger AD flares and whether it helps to explain heterogeneity in response to new immunomodulatory treatments for AD,” the authors wrote. “Reduced sodium intake was recommended as a treatment for AD more than a century ago, but there have yet to be studies examining the association of dietary sodium reduction with skin sodium concentration or AD severity,” they added. Noting that sodium reduction “has been shown to be a cost-effective intervention for hypertension and other cardiovascular disease outcomes,” they said that their data “support experimental studies of this approach in AD.”
They acknowledged certain limitations of the study, including the fact that a single spot urine sample was used in the UK Biobank cohort, “which only captures dietary intake of the last 24 hours and is not the best measure of usual or long-term intake of sodium.” They also noted that the findings may not be generalizable to other populations and that AD was based on self-report in the NHANES validation cohort.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the results, said the study by Dr. Abuabara and colleagues “gives us another reason to avoid salt, showing that 1 g/day of higher salt intake increases the risk of AD in an adult population and more severe AD.”
He added that, “Now, can you say that reducing salt intake will have a therapeutic effect or clinically relevant impact? No. [That is] certainly worth exploring but at a minimum, gives some more credibility to keeping it bland.”
The study was supported by a grant from the Medical Student in Aging Research Program, the National Institute on Aging, and the National Eczema Association. Dr. Abuabara reported receiving research funding for her institution from Pfizer and Cosmetique Internacional/La Roche-Posay and consulting fees from Target RWE, Sanofi, Nektar, and Amgen. No other disclosures were reported. Dr. Friedman had no relevant disclosures.
A version of this article appeared on Medscape.com.
Study Finds Mace Risk Remains High in Patients with Psoriasis, Dyslipidemia
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
FROM SID 2024
Dupilumab Evaluated as Treatment for Pediatric Alopecia Areata
showed.
“We might be opening a new avenue for a safe, long-term treatment for our children with AA,” the study’s lead investigator, Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York City, said in an interview during the annual meeting of the Society for Investigative Dermatology (SID), where the results were presented during a poster session. “I think AA is likely joining the atopic march, which may allow us to adapt some treatments from the atopy world to AA.”
When the original phase 2 and phase 3 trials of dupilumab for patients with moderate to severe AD were being conducted, Dr. Guttman-Yassky, one of the investigators, recalled observing that some patients who also had patch alopecia experienced hair regrowth. “I was scratching my head because, at the time, AA was considered to be only a Th1-driven disease,” she said. “I asked myself, ‘How can this happen?’ I looked in the literature and found many publications linking atopy in general to alopecia areata. The largest of the dermatologic publications showed that eczema and atopy in general are the highest comorbidities in alopecia areata.”
“This and other findings such as IL [interleukin]-13 genetic linkage with AA and high IgE in patients with AA link AA with Th2 immune skewing, particularly in the setting of atopy,” she continued. In addition, she said, in a large biomarker study involving the scalp and blood of patients with AA, “we found increases in Th2 biomarkers that were associated with alopecia severity.”
Case Series of 20 Pediatric Patients
As part of a case series of children with both AD and AA, Dr. Guttman-Yassky and colleagues evaluated hair regrowth using the Severity of Alopecia Tool (SALT) in 20 pediatric patients (mean age, 10.8 years) who were being treated at Mount Sinai. They collected patient demographics, atopic history, immunoglobulin E (IgE) levels, and SALT scores at follow-up visits every 12-16 weeks for more than 72 weeks and performed Spearman correlations between clinical scores, demographics, and IgE levels.
At baseline, the mean SALT score was 54.4, the mean IgE level was 1567.7 IU/mL, and 75% of patients also had a family history of atopy. The mean follow-up was 67.6 weeks. The researchers observed a significant reduction in SALT scores at week 48 compared with baseline (a mean score of 20.4; P < .01) and continued improvement up to at least 72 weeks (P < .01 vs baseline). They also noted that patients who achieved a treatment response at week 24 had baseline IgE levels > 200 IU/mL.
In other findings, baseline IgE positively correlated with improvement in SALT scores at week 36 (P < .05), while baseline SALT scores positively correlated with disease duration (P < .01) and negatively correlated with improvement in SALT scores at weeks 24, 36, and 48 (P < .005). “The robustness of the response surprised me,” Dr. Guttman-Yassky said in the interview. “Dupilumab for AA takes time to work, but once it kicks in, it kicks in. It takes anywhere from 6 to 12 months to see hair regrowth.”
She acknowledged certain limitations of the analysis, including its small sample size and the fact that it was not a standardized trial. “But, based on our data and the adult data, we are very encouraged about the potential of using dupilumab for children with AA,” she said.
Mount Sinai recently announced that the National Institutes of Health awarded a $6.6 million, 5-year grant to Dr. Guttman-Yassky to further investigate dupilumab as a treatment for children with AA. She will lead a multicenter controlled trial of 76 children with alopecia affecting at least 30% of the scalp, who will be randomized 2:1 (dupilumab:placebo) for 48 weeks, followed by 48 weeks of open-label dupilumab for all participants, with 16 weeks of follow-up, for a total of 112 weeks. Participating sites include Mount Sinai, Yale University, Northwestern University, and the University of California, Irvine.
Dr. Guttman-Yassky disclosed that she is a consultant to many pharmaceutical companies, including dupilumab manufacturers Sanofi and Regeneron.
A version of this article appeared on Medscape.com.
showed.
“We might be opening a new avenue for a safe, long-term treatment for our children with AA,” the study’s lead investigator, Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York City, said in an interview during the annual meeting of the Society for Investigative Dermatology (SID), where the results were presented during a poster session. “I think AA is likely joining the atopic march, which may allow us to adapt some treatments from the atopy world to AA.”
When the original phase 2 and phase 3 trials of dupilumab for patients with moderate to severe AD were being conducted, Dr. Guttman-Yassky, one of the investigators, recalled observing that some patients who also had patch alopecia experienced hair regrowth. “I was scratching my head because, at the time, AA was considered to be only a Th1-driven disease,” she said. “I asked myself, ‘How can this happen?’ I looked in the literature and found many publications linking atopy in general to alopecia areata. The largest of the dermatologic publications showed that eczema and atopy in general are the highest comorbidities in alopecia areata.”
“This and other findings such as IL [interleukin]-13 genetic linkage with AA and high IgE in patients with AA link AA with Th2 immune skewing, particularly in the setting of atopy,” she continued. In addition, she said, in a large biomarker study involving the scalp and blood of patients with AA, “we found increases in Th2 biomarkers that were associated with alopecia severity.”
Case Series of 20 Pediatric Patients
As part of a case series of children with both AD and AA, Dr. Guttman-Yassky and colleagues evaluated hair regrowth using the Severity of Alopecia Tool (SALT) in 20 pediatric patients (mean age, 10.8 years) who were being treated at Mount Sinai. They collected patient demographics, atopic history, immunoglobulin E (IgE) levels, and SALT scores at follow-up visits every 12-16 weeks for more than 72 weeks and performed Spearman correlations between clinical scores, demographics, and IgE levels.
At baseline, the mean SALT score was 54.4, the mean IgE level was 1567.7 IU/mL, and 75% of patients also had a family history of atopy. The mean follow-up was 67.6 weeks. The researchers observed a significant reduction in SALT scores at week 48 compared with baseline (a mean score of 20.4; P < .01) and continued improvement up to at least 72 weeks (P < .01 vs baseline). They also noted that patients who achieved a treatment response at week 24 had baseline IgE levels > 200 IU/mL.
In other findings, baseline IgE positively correlated with improvement in SALT scores at week 36 (P < .05), while baseline SALT scores positively correlated with disease duration (P < .01) and negatively correlated with improvement in SALT scores at weeks 24, 36, and 48 (P < .005). “The robustness of the response surprised me,” Dr. Guttman-Yassky said in the interview. “Dupilumab for AA takes time to work, but once it kicks in, it kicks in. It takes anywhere from 6 to 12 months to see hair regrowth.”
She acknowledged certain limitations of the analysis, including its small sample size and the fact that it was not a standardized trial. “But, based on our data and the adult data, we are very encouraged about the potential of using dupilumab for children with AA,” she said.
Mount Sinai recently announced that the National Institutes of Health awarded a $6.6 million, 5-year grant to Dr. Guttman-Yassky to further investigate dupilumab as a treatment for children with AA. She will lead a multicenter controlled trial of 76 children with alopecia affecting at least 30% of the scalp, who will be randomized 2:1 (dupilumab:placebo) for 48 weeks, followed by 48 weeks of open-label dupilumab for all participants, with 16 weeks of follow-up, for a total of 112 weeks. Participating sites include Mount Sinai, Yale University, Northwestern University, and the University of California, Irvine.
Dr. Guttman-Yassky disclosed that she is a consultant to many pharmaceutical companies, including dupilumab manufacturers Sanofi and Regeneron.
A version of this article appeared on Medscape.com.
showed.
“We might be opening a new avenue for a safe, long-term treatment for our children with AA,” the study’s lead investigator, Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York City, said in an interview during the annual meeting of the Society for Investigative Dermatology (SID), where the results were presented during a poster session. “I think AA is likely joining the atopic march, which may allow us to adapt some treatments from the atopy world to AA.”
When the original phase 2 and phase 3 trials of dupilumab for patients with moderate to severe AD were being conducted, Dr. Guttman-Yassky, one of the investigators, recalled observing that some patients who also had patch alopecia experienced hair regrowth. “I was scratching my head because, at the time, AA was considered to be only a Th1-driven disease,” she said. “I asked myself, ‘How can this happen?’ I looked in the literature and found many publications linking atopy in general to alopecia areata. The largest of the dermatologic publications showed that eczema and atopy in general are the highest comorbidities in alopecia areata.”
“This and other findings such as IL [interleukin]-13 genetic linkage with AA and high IgE in patients with AA link AA with Th2 immune skewing, particularly in the setting of atopy,” she continued. In addition, she said, in a large biomarker study involving the scalp and blood of patients with AA, “we found increases in Th2 biomarkers that were associated with alopecia severity.”
Case Series of 20 Pediatric Patients
As part of a case series of children with both AD and AA, Dr. Guttman-Yassky and colleagues evaluated hair regrowth using the Severity of Alopecia Tool (SALT) in 20 pediatric patients (mean age, 10.8 years) who were being treated at Mount Sinai. They collected patient demographics, atopic history, immunoglobulin E (IgE) levels, and SALT scores at follow-up visits every 12-16 weeks for more than 72 weeks and performed Spearman correlations between clinical scores, demographics, and IgE levels.
At baseline, the mean SALT score was 54.4, the mean IgE level was 1567.7 IU/mL, and 75% of patients also had a family history of atopy. The mean follow-up was 67.6 weeks. The researchers observed a significant reduction in SALT scores at week 48 compared with baseline (a mean score of 20.4; P < .01) and continued improvement up to at least 72 weeks (P < .01 vs baseline). They also noted that patients who achieved a treatment response at week 24 had baseline IgE levels > 200 IU/mL.
In other findings, baseline IgE positively correlated with improvement in SALT scores at week 36 (P < .05), while baseline SALT scores positively correlated with disease duration (P < .01) and negatively correlated with improvement in SALT scores at weeks 24, 36, and 48 (P < .005). “The robustness of the response surprised me,” Dr. Guttman-Yassky said in the interview. “Dupilumab for AA takes time to work, but once it kicks in, it kicks in. It takes anywhere from 6 to 12 months to see hair regrowth.”
She acknowledged certain limitations of the analysis, including its small sample size and the fact that it was not a standardized trial. “But, based on our data and the adult data, we are very encouraged about the potential of using dupilumab for children with AA,” she said.
Mount Sinai recently announced that the National Institutes of Health awarded a $6.6 million, 5-year grant to Dr. Guttman-Yassky to further investigate dupilumab as a treatment for children with AA. She will lead a multicenter controlled trial of 76 children with alopecia affecting at least 30% of the scalp, who will be randomized 2:1 (dupilumab:placebo) for 48 weeks, followed by 48 weeks of open-label dupilumab for all participants, with 16 weeks of follow-up, for a total of 112 weeks. Participating sites include Mount Sinai, Yale University, Northwestern University, and the University of California, Irvine.
Dr. Guttman-Yassky disclosed that she is a consultant to many pharmaceutical companies, including dupilumab manufacturers Sanofi and Regeneron.
A version of this article appeared on Medscape.com.
FROM SID 2024
Analysis Finds Minority of Chronic Wounds Treated by Dermatologists
. However, fewer than 8% of chronic wounds were managed by dermatologists during this time.
Those are among key findings from an analysis of National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2019 presented as a late-breaking abstract at the annual meeting of the Society for Investigative Dermatology. “Cutaneous wounds were estimated to account for 28.1 to 96.1 billion dollars in US health care costs in 2014,” one of the study authors, Rithi Chandy, MD, MS, a research fellow at the Center for Dermatology Research at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in an interview following the meeting. “By examining national trends in patient visits and treatment, we may be able to better inform health care utilization for cutaneous wounds.”
Dr. Chandy and colleagues analyzed de-identified patient data from the 2011 to 2019 NAMCS for acute and chronic wound diagnoses, medications prescribed, and physician specialty categories. During the time studied, 5.76 billion patient visits were made, including 45.1 million visits for cutaneous wounds. Of these, the most common diagnoses were open wounds of the thumb without nail damage (7.96%), the lower leg (5.75%), nonpressure chronic ulcers of other parts of the foot (5.08%), and open wounds of the ear (5%).
Among all visits for cutaneous wounds, about one third were chronic cutaneous wounds, with the following descriptions: “Nonpressure chronic ulcer of other part of foot” (17.8%); “nonpressure chronic ulcer of skin, not elsewhere classified” (9.38%); and “ulcer of lower limbs, excluding decubitus, unspecified” (8.72%). “The frequency of patient visits per year during the study period remained stable for both acute and chronic wounds,” Dr. Chandy said. The number of visits for which antimicrobials were used was stable over time for both acute and chronic cutaneous wounds, with the exception of increased use of antivirals for chronic cutaneous wounds, he added.
Specifically, prescriptions were issued in 156 million visits over the time studied, most commonly cephalexin (4.22%), topical silver sulfadiazine (1.59%), topical mupirocin (1.12%), and miscellaneous antibiotics (1.18%).
“Our data shows that topical mupirocin is the most commonly used topical antimicrobial for cutaneous wounds,” Dr. Chandy said. “However, there are reports of emerging bacterial resistance to mupirocin. Our data can inform ongoing efforts to promote antimicrobial stewardship and drug development to provide alternative options that are less likely to induce antimicrobial resistance.”
In findings limited to specialty-specific NAMCS data available from 2011 and from 2013 to 2016, dermatologists managed 3.85% of overall cutaneous wounds, 2.35% of acute wounds, and 7.39% of chronic wounds. By contrast, Dr. Chandy said, 21.1% of chronic wounds were managed by general/family practice physicians, 20.7% by internists, 6.84% by general surgeons, and 5.65% by orthopedic surgeons.
“As dermatologists are experts in the structure and function of the skin and are trained to manage cutaneous disorders including wound healing, we [believe that] dermatologists are equipped with the skill set” for managing wounds, especially for chronic ulcers, he said. The decline in dermatologists who specialize in wound care, he added, “underscores the need for structured dermatology fellowship programs to prepare next-generation dermatologists to address this shortage and ensure dermatology leadership in cutaneous wound healing.”
Dr. Chandy acknowledged certain limitations of the study, including the potential for misclassification of diagnoses or medications prescribed and the fact that the NAMCS database is unable to provide insight into individual patient experiences such as continual cutaneous wound management for the same patient over time.
In the opinion of Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the Nail Division at Weill Cornell Medicine, New York, who was asked to comment on the study, the most interesting finding was that dermatologists cared for a small minority of patients with cutaneous wounds. “It would be interesting to know whether this is due to dermatologist shortages or knowledge gaps on the part of primary care physicians or patients that dermatologists are trained to care for wounds,” Dr. Lipner told this news organization. Other unanswered questions, she noted, “are patient demographics, geographic locations, and comorbidities.”
One of the study authors, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, disclosed that he has received research, speaking and/or consulting support from numerous pharmaceutical companies. No other authors reported having relevant disclosures. Dr. Lipner reported having no disclosures.
A version of this article appeared on Medscape.com .
. However, fewer than 8% of chronic wounds were managed by dermatologists during this time.
Those are among key findings from an analysis of National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2019 presented as a late-breaking abstract at the annual meeting of the Society for Investigative Dermatology. “Cutaneous wounds were estimated to account for 28.1 to 96.1 billion dollars in US health care costs in 2014,” one of the study authors, Rithi Chandy, MD, MS, a research fellow at the Center for Dermatology Research at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in an interview following the meeting. “By examining national trends in patient visits and treatment, we may be able to better inform health care utilization for cutaneous wounds.”
Dr. Chandy and colleagues analyzed de-identified patient data from the 2011 to 2019 NAMCS for acute and chronic wound diagnoses, medications prescribed, and physician specialty categories. During the time studied, 5.76 billion patient visits were made, including 45.1 million visits for cutaneous wounds. Of these, the most common diagnoses were open wounds of the thumb without nail damage (7.96%), the lower leg (5.75%), nonpressure chronic ulcers of other parts of the foot (5.08%), and open wounds of the ear (5%).
Among all visits for cutaneous wounds, about one third were chronic cutaneous wounds, with the following descriptions: “Nonpressure chronic ulcer of other part of foot” (17.8%); “nonpressure chronic ulcer of skin, not elsewhere classified” (9.38%); and “ulcer of lower limbs, excluding decubitus, unspecified” (8.72%). “The frequency of patient visits per year during the study period remained stable for both acute and chronic wounds,” Dr. Chandy said. The number of visits for which antimicrobials were used was stable over time for both acute and chronic cutaneous wounds, with the exception of increased use of antivirals for chronic cutaneous wounds, he added.
Specifically, prescriptions were issued in 156 million visits over the time studied, most commonly cephalexin (4.22%), topical silver sulfadiazine (1.59%), topical mupirocin (1.12%), and miscellaneous antibiotics (1.18%).
“Our data shows that topical mupirocin is the most commonly used topical antimicrobial for cutaneous wounds,” Dr. Chandy said. “However, there are reports of emerging bacterial resistance to mupirocin. Our data can inform ongoing efforts to promote antimicrobial stewardship and drug development to provide alternative options that are less likely to induce antimicrobial resistance.”
In findings limited to specialty-specific NAMCS data available from 2011 and from 2013 to 2016, dermatologists managed 3.85% of overall cutaneous wounds, 2.35% of acute wounds, and 7.39% of chronic wounds. By contrast, Dr. Chandy said, 21.1% of chronic wounds were managed by general/family practice physicians, 20.7% by internists, 6.84% by general surgeons, and 5.65% by orthopedic surgeons.
“As dermatologists are experts in the structure and function of the skin and are trained to manage cutaneous disorders including wound healing, we [believe that] dermatologists are equipped with the skill set” for managing wounds, especially for chronic ulcers, he said. The decline in dermatologists who specialize in wound care, he added, “underscores the need for structured dermatology fellowship programs to prepare next-generation dermatologists to address this shortage and ensure dermatology leadership in cutaneous wound healing.”
Dr. Chandy acknowledged certain limitations of the study, including the potential for misclassification of diagnoses or medications prescribed and the fact that the NAMCS database is unable to provide insight into individual patient experiences such as continual cutaneous wound management for the same patient over time.
In the opinion of Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the Nail Division at Weill Cornell Medicine, New York, who was asked to comment on the study, the most interesting finding was that dermatologists cared for a small minority of patients with cutaneous wounds. “It would be interesting to know whether this is due to dermatologist shortages or knowledge gaps on the part of primary care physicians or patients that dermatologists are trained to care for wounds,” Dr. Lipner told this news organization. Other unanswered questions, she noted, “are patient demographics, geographic locations, and comorbidities.”
One of the study authors, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, disclosed that he has received research, speaking and/or consulting support from numerous pharmaceutical companies. No other authors reported having relevant disclosures. Dr. Lipner reported having no disclosures.
A version of this article appeared on Medscape.com .
. However, fewer than 8% of chronic wounds were managed by dermatologists during this time.
Those are among key findings from an analysis of National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2019 presented as a late-breaking abstract at the annual meeting of the Society for Investigative Dermatology. “Cutaneous wounds were estimated to account for 28.1 to 96.1 billion dollars in US health care costs in 2014,” one of the study authors, Rithi Chandy, MD, MS, a research fellow at the Center for Dermatology Research at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in an interview following the meeting. “By examining national trends in patient visits and treatment, we may be able to better inform health care utilization for cutaneous wounds.”
Dr. Chandy and colleagues analyzed de-identified patient data from the 2011 to 2019 NAMCS for acute and chronic wound diagnoses, medications prescribed, and physician specialty categories. During the time studied, 5.76 billion patient visits were made, including 45.1 million visits for cutaneous wounds. Of these, the most common diagnoses were open wounds of the thumb without nail damage (7.96%), the lower leg (5.75%), nonpressure chronic ulcers of other parts of the foot (5.08%), and open wounds of the ear (5%).
Among all visits for cutaneous wounds, about one third were chronic cutaneous wounds, with the following descriptions: “Nonpressure chronic ulcer of other part of foot” (17.8%); “nonpressure chronic ulcer of skin, not elsewhere classified” (9.38%); and “ulcer of lower limbs, excluding decubitus, unspecified” (8.72%). “The frequency of patient visits per year during the study period remained stable for both acute and chronic wounds,” Dr. Chandy said. The number of visits for which antimicrobials were used was stable over time for both acute and chronic cutaneous wounds, with the exception of increased use of antivirals for chronic cutaneous wounds, he added.
Specifically, prescriptions were issued in 156 million visits over the time studied, most commonly cephalexin (4.22%), topical silver sulfadiazine (1.59%), topical mupirocin (1.12%), and miscellaneous antibiotics (1.18%).
“Our data shows that topical mupirocin is the most commonly used topical antimicrobial for cutaneous wounds,” Dr. Chandy said. “However, there are reports of emerging bacterial resistance to mupirocin. Our data can inform ongoing efforts to promote antimicrobial stewardship and drug development to provide alternative options that are less likely to induce antimicrobial resistance.”
In findings limited to specialty-specific NAMCS data available from 2011 and from 2013 to 2016, dermatologists managed 3.85% of overall cutaneous wounds, 2.35% of acute wounds, and 7.39% of chronic wounds. By contrast, Dr. Chandy said, 21.1% of chronic wounds were managed by general/family practice physicians, 20.7% by internists, 6.84% by general surgeons, and 5.65% by orthopedic surgeons.
“As dermatologists are experts in the structure and function of the skin and are trained to manage cutaneous disorders including wound healing, we [believe that] dermatologists are equipped with the skill set” for managing wounds, especially for chronic ulcers, he said. The decline in dermatologists who specialize in wound care, he added, “underscores the need for structured dermatology fellowship programs to prepare next-generation dermatologists to address this shortage and ensure dermatology leadership in cutaneous wound healing.”
Dr. Chandy acknowledged certain limitations of the study, including the potential for misclassification of diagnoses or medications prescribed and the fact that the NAMCS database is unable to provide insight into individual patient experiences such as continual cutaneous wound management for the same patient over time.
In the opinion of Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the Nail Division at Weill Cornell Medicine, New York, who was asked to comment on the study, the most interesting finding was that dermatologists cared for a small minority of patients with cutaneous wounds. “It would be interesting to know whether this is due to dermatologist shortages or knowledge gaps on the part of primary care physicians or patients that dermatologists are trained to care for wounds,” Dr. Lipner told this news organization. Other unanswered questions, she noted, “are patient demographics, geographic locations, and comorbidities.”
One of the study authors, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, disclosed that he has received research, speaking and/or consulting support from numerous pharmaceutical companies. No other authors reported having relevant disclosures. Dr. Lipner reported having no disclosures.
A version of this article appeared on Medscape.com .
FROM SID 2024
Prenatal Antibiotics May Increase Seborrheic Dermatitis Risk in Babies
, but this association was not as strong for childhood-onset SD.
The findings come from a large analysis of data from the United Kingdom that was presented during a late-breaking abstract session at the annual meeting of the Society for Investigative Dermatology.
SD is a common skin disease “that shares similarities with atopic dermatitis or atopic eczema as both are prevalent inflammatory skin diseases that can present with a chronic relapsing, remitting course,” the study’s corresponding author Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview. “Like atopic dermatitis, the pathophysiology of seborrheic dermatitis is thought to be complex and involves an interplay between genetics, immune dysregulation, and alterations in lipid composition and the skin microbiome, among others.”
In a previous study, she and colleagues showed that exposure to antibiotics both in utero and during the first 90 days of life increases the risk for atopic dermatitis (AD) in children, with risk being highest with exposure to penicillin even among children whose mothers did not have a history of AD.
For the current study, the researchers drew from a large electronic medical records database in the United Kingdom to perform a prospective cohort analysis of mother-child pairs that used proportional hazards models to examine the association between maternal in utero antibiotic exposure and SD in the child. The population included 1,023,140 children with linked maternal data who were followed for a mean of 10.2 years, which amounts to more than 10-million-person years of data. At baseline, the mean age of mothers was 28 years, 3% had SD, 14% had AD, and 51% of the children were male.
In unadjusted analyses, mothers with SD were more likely to receive an antibiotic during pregnancy than were those who did not have SD (odds ratio [OR], 1.42; 95% CI, 1.39-1.46). In addition, maternal in utero exposure to any antibiotic was associated with an increased risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) but less for childhood-onset SD (OR, 1.26; 95% CI, 1.20-1.32). “This effect changed little after adjustment and was still observed if mothers with SD and their babies were excluded,” the authors wrote in their poster abstract.
Any penicillin exposure during pregnancy increased the likelihood of a child having SD (OR, 1.54; 95% CI, 1.50-1.59), with the greater risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) than for childhood-onset SD (OR, 1.25; 95% CI, 1.18-1.32). “The trimester of the in utero penicillin exposure did not seem to affect the association with SD,” the authors wrote. The risk was also increased with cephalosporin exposure but was less for sulfonamides and not for childhood-onset SD.
“We observed that antibiotic exposure in utero was primarily associated with an increased risk of infantile SD regardless of the mother’s history of SD, but this association was not as strong for childhood-onset SD,” Dr. Chiesa Fuxench said. “This would suggest that in utero exposure to antibiotics, particularly penicillin, may have its greatest effect on the colonization of skin microbiota in the newborn period leading to the development of infantile SD. Aside from seeking to improve our understanding of the pathophysiology of SD, our findings also suggest that infantile SD and childhood-onset SD may be separate entities with different risk factors, a hypothesis that needs to be further studied.”
She acknowledged certain limitations of the analysis, including the potential for unrecorded diagnoses of SD or misclassified cases in the database. For example, AD and psoriasis “may appear clinically like SD,” she said, although they performed sensitivity analysis excluding patients with these diagnoses and found similar results. In addition, there is the possibility that not all antibiotic exposures were captured in this database, and data on antibiotic exposure may be missing, she added.
Dr. Chiesa Fuxench disclosed that she received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, Vanda, and Incyte for work related to AD and from Menlo Therapeutics and Galderma for work related to prurigo nodularis. She has served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer and from Beiersdorf for work related to skin cancer and sun protection.
A version of this article appeared on Medscape.com .
, but this association was not as strong for childhood-onset SD.
The findings come from a large analysis of data from the United Kingdom that was presented during a late-breaking abstract session at the annual meeting of the Society for Investigative Dermatology.
SD is a common skin disease “that shares similarities with atopic dermatitis or atopic eczema as both are prevalent inflammatory skin diseases that can present with a chronic relapsing, remitting course,” the study’s corresponding author Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview. “Like atopic dermatitis, the pathophysiology of seborrheic dermatitis is thought to be complex and involves an interplay between genetics, immune dysregulation, and alterations in lipid composition and the skin microbiome, among others.”
In a previous study, she and colleagues showed that exposure to antibiotics both in utero and during the first 90 days of life increases the risk for atopic dermatitis (AD) in children, with risk being highest with exposure to penicillin even among children whose mothers did not have a history of AD.
For the current study, the researchers drew from a large electronic medical records database in the United Kingdom to perform a prospective cohort analysis of mother-child pairs that used proportional hazards models to examine the association between maternal in utero antibiotic exposure and SD in the child. The population included 1,023,140 children with linked maternal data who were followed for a mean of 10.2 years, which amounts to more than 10-million-person years of data. At baseline, the mean age of mothers was 28 years, 3% had SD, 14% had AD, and 51% of the children were male.
In unadjusted analyses, mothers with SD were more likely to receive an antibiotic during pregnancy than were those who did not have SD (odds ratio [OR], 1.42; 95% CI, 1.39-1.46). In addition, maternal in utero exposure to any antibiotic was associated with an increased risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) but less for childhood-onset SD (OR, 1.26; 95% CI, 1.20-1.32). “This effect changed little after adjustment and was still observed if mothers with SD and their babies were excluded,” the authors wrote in their poster abstract.
Any penicillin exposure during pregnancy increased the likelihood of a child having SD (OR, 1.54; 95% CI, 1.50-1.59), with the greater risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) than for childhood-onset SD (OR, 1.25; 95% CI, 1.18-1.32). “The trimester of the in utero penicillin exposure did not seem to affect the association with SD,” the authors wrote. The risk was also increased with cephalosporin exposure but was less for sulfonamides and not for childhood-onset SD.
“We observed that antibiotic exposure in utero was primarily associated with an increased risk of infantile SD regardless of the mother’s history of SD, but this association was not as strong for childhood-onset SD,” Dr. Chiesa Fuxench said. “This would suggest that in utero exposure to antibiotics, particularly penicillin, may have its greatest effect on the colonization of skin microbiota in the newborn period leading to the development of infantile SD. Aside from seeking to improve our understanding of the pathophysiology of SD, our findings also suggest that infantile SD and childhood-onset SD may be separate entities with different risk factors, a hypothesis that needs to be further studied.”
She acknowledged certain limitations of the analysis, including the potential for unrecorded diagnoses of SD or misclassified cases in the database. For example, AD and psoriasis “may appear clinically like SD,” she said, although they performed sensitivity analysis excluding patients with these diagnoses and found similar results. In addition, there is the possibility that not all antibiotic exposures were captured in this database, and data on antibiotic exposure may be missing, she added.
Dr. Chiesa Fuxench disclosed that she received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, Vanda, and Incyte for work related to AD and from Menlo Therapeutics and Galderma for work related to prurigo nodularis. She has served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer and from Beiersdorf for work related to skin cancer and sun protection.
A version of this article appeared on Medscape.com .
, but this association was not as strong for childhood-onset SD.
The findings come from a large analysis of data from the United Kingdom that was presented during a late-breaking abstract session at the annual meeting of the Society for Investigative Dermatology.
SD is a common skin disease “that shares similarities with atopic dermatitis or atopic eczema as both are prevalent inflammatory skin diseases that can present with a chronic relapsing, remitting course,” the study’s corresponding author Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview. “Like atopic dermatitis, the pathophysiology of seborrheic dermatitis is thought to be complex and involves an interplay between genetics, immune dysregulation, and alterations in lipid composition and the skin microbiome, among others.”
In a previous study, she and colleagues showed that exposure to antibiotics both in utero and during the first 90 days of life increases the risk for atopic dermatitis (AD) in children, with risk being highest with exposure to penicillin even among children whose mothers did not have a history of AD.
For the current study, the researchers drew from a large electronic medical records database in the United Kingdom to perform a prospective cohort analysis of mother-child pairs that used proportional hazards models to examine the association between maternal in utero antibiotic exposure and SD in the child. The population included 1,023,140 children with linked maternal data who were followed for a mean of 10.2 years, which amounts to more than 10-million-person years of data. At baseline, the mean age of mothers was 28 years, 3% had SD, 14% had AD, and 51% of the children were male.
In unadjusted analyses, mothers with SD were more likely to receive an antibiotic during pregnancy than were those who did not have SD (odds ratio [OR], 1.42; 95% CI, 1.39-1.46). In addition, maternal in utero exposure to any antibiotic was associated with an increased risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) but less for childhood-onset SD (OR, 1.26; 95% CI, 1.20-1.32). “This effect changed little after adjustment and was still observed if mothers with SD and their babies were excluded,” the authors wrote in their poster abstract.
Any penicillin exposure during pregnancy increased the likelihood of a child having SD (OR, 1.54; 95% CI, 1.50-1.59), with the greater risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) than for childhood-onset SD (OR, 1.25; 95% CI, 1.18-1.32). “The trimester of the in utero penicillin exposure did not seem to affect the association with SD,” the authors wrote. The risk was also increased with cephalosporin exposure but was less for sulfonamides and not for childhood-onset SD.
“We observed that antibiotic exposure in utero was primarily associated with an increased risk of infantile SD regardless of the mother’s history of SD, but this association was not as strong for childhood-onset SD,” Dr. Chiesa Fuxench said. “This would suggest that in utero exposure to antibiotics, particularly penicillin, may have its greatest effect on the colonization of skin microbiota in the newborn period leading to the development of infantile SD. Aside from seeking to improve our understanding of the pathophysiology of SD, our findings also suggest that infantile SD and childhood-onset SD may be separate entities with different risk factors, a hypothesis that needs to be further studied.”
She acknowledged certain limitations of the analysis, including the potential for unrecorded diagnoses of SD or misclassified cases in the database. For example, AD and psoriasis “may appear clinically like SD,” she said, although they performed sensitivity analysis excluding patients with these diagnoses and found similar results. In addition, there is the possibility that not all antibiotic exposures were captured in this database, and data on antibiotic exposure may be missing, she added.
Dr. Chiesa Fuxench disclosed that she received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, Vanda, and Incyte for work related to AD and from Menlo Therapeutics and Galderma for work related to prurigo nodularis. She has served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer and from Beiersdorf for work related to skin cancer and sun protection.
A version of this article appeared on Medscape.com .
FROM SID 2024