User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
EASI, Other Instruments Recommended to Evaluate Patients With Atopic Dermatitis
recommended.
These include the Eczema Area and Severity Index (EASI), the Validated Investigator Global Assessment for AD (vIGAAD), and the Investigator’s Global Assessment (IGA) multiplied by or measured concurrently with a body surface area (BSA) assessment.
The recommendations are part of a consensus statement based on an updated systematic review conducted by the Harmonizing Outcome Measures for Eczema Clinical Practice (HOME-CP) initiative, whose goal is to identify validated, feasible outcome instruments designed to measure AD in the clinical setting. In the statement, which was published in JAMA Dermatology on May 22, 2024, corresponding author Eric L. Simpson, MD, MCR, professor of dermatology at Oregon Health & Science University, Portland, and coauthors described HOME-CP as “a ‘pick-and-choose’ list of valid and feasible OMIs [outcome measure instruments] that can be incorporated into the practice setting depending on the particular need of that clinic or health system.”
For the effort, the authors implemented a mixed methods design and incorporated systematic reviews and qualitative consensus methods modeled after the HOME core outcome set initiative, which developed a set of consensus-based core outcome sets for clinical trials and clinical practice. In October of 2022, a daylong in-person consensus exercise was held in Montreal, Canada, where attendees met to reach consensus on recommended instruments to measure AD clinical signs in clinical practice, based on an updated systematic review evaluating the validity of clinical signs instruments.
The review included 22 studies describing 16 instruments that assessed AD clinical signs and an additional 12 variants of instruments. The meeting was attended by 34 individuals from 13 countries, including patient and patient advocate research partners, health care professionals, researchers, methodologists, and industry representatives. Consensus was defined as less than 30% disagreement.
Following their daylong consensus exercise, the stakeholders reached consensus on recommendations to use the EASI, the vIGAAD, and an IGA multiplied or measured alongside a BSA measurement to measure the domain of clinical signs of AD in the clinical practice setting. “The use of multiple IGAs, most with insufficient validation, and the diverse methods used to assess BSA prevented participants from making specific recommendations for the exact IGA/BSA instrument,” the authors wrote. “We recommend that clinicians include at least one of the recommended instruments in their clinical practices and in documentation.”
They explained that the ideal method of measuring BSA was difficult to assess “because multiple techniques exist for its measurement, including regional percentages, the Rule of Nines, or the handprint method. Most studies did not report which method was performed, and to our knowledge, no studies have been performed in patients with AD that have formally compared them.”
During the consensus exercise, the authors noted, several clinicians “expressed concern whether the EASI was feasible for universal use in clinical practice given its complexity, long completion time, and documentation/calculation requirements.” But clinicians who commonly perform the EASI in clinical practice said that the time it takes to complete this measure “has dropped substantially and now is not a considerable burden,” they wrote, adding that, “studies have shown that with trained investigators, EASI completion times can be as low as nearly 2 minutes.”
The authors acknowledged certain limitations of their recommendations, including the lack of input from primary care clinicians. “It is unknown whether ClinROMs [clinician-reported outcome measures] for AD clinical signs are used in the primary care setting, especially given the large amount of conditions that are managed simultaneously and the ever-increasing number of primary care documentation requirements,” they wrote.
Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the consensus statement, said that with the advent of new, improved, and more expensive medications for AD, “it is ever more important that [the clinical] assessment is reliable and reproducible.”
Insurers “are understandably less willing to rubber-stamp approval of more expensive medications without a reliable standard by which to justify such decisions,” he added. “This is even more important in a disease state like atopic dermatitis that lacks a reliable biomarker. Therefore, one or several practical, reliable, validated severity metrics will help standardize and improve AD care.”
Dr. Sidbury, who cochaired the 2023 American Academy of Dermatology guidelines of care for the management of AD in adults with phototherapy and systemic therapies, added that the instruments evaluated in the review “can be challenging for anyone,” not just primary care providers. “The EASI isn’t that easy, and while there is a learning curve and it ultimately does, like anything, become more efficient in the gathering, it is unclear if non-AD researchers will be willing to invest the time” to routinely use it, he said.
Dr. Simpson and several coauthors reported receiving grants and personal fees from multiple pharmaceutical companies. Dr. Sidbury reported that he serves as an investigator for Regeneron, Galderma, UCB, Castle, and Pfizer; is a consultant for LEO, Lilly, Arcutis, Dermavant, and Pierre Fabre; and a speaker for Beiersdorf.
A version of this article appeared on Medscape.com .
recommended.
These include the Eczema Area and Severity Index (EASI), the Validated Investigator Global Assessment for AD (vIGAAD), and the Investigator’s Global Assessment (IGA) multiplied by or measured concurrently with a body surface area (BSA) assessment.
The recommendations are part of a consensus statement based on an updated systematic review conducted by the Harmonizing Outcome Measures for Eczema Clinical Practice (HOME-CP) initiative, whose goal is to identify validated, feasible outcome instruments designed to measure AD in the clinical setting. In the statement, which was published in JAMA Dermatology on May 22, 2024, corresponding author Eric L. Simpson, MD, MCR, professor of dermatology at Oregon Health & Science University, Portland, and coauthors described HOME-CP as “a ‘pick-and-choose’ list of valid and feasible OMIs [outcome measure instruments] that can be incorporated into the practice setting depending on the particular need of that clinic or health system.”
For the effort, the authors implemented a mixed methods design and incorporated systematic reviews and qualitative consensus methods modeled after the HOME core outcome set initiative, which developed a set of consensus-based core outcome sets for clinical trials and clinical practice. In October of 2022, a daylong in-person consensus exercise was held in Montreal, Canada, where attendees met to reach consensus on recommended instruments to measure AD clinical signs in clinical practice, based on an updated systematic review evaluating the validity of clinical signs instruments.
The review included 22 studies describing 16 instruments that assessed AD clinical signs and an additional 12 variants of instruments. The meeting was attended by 34 individuals from 13 countries, including patient and patient advocate research partners, health care professionals, researchers, methodologists, and industry representatives. Consensus was defined as less than 30% disagreement.
Following their daylong consensus exercise, the stakeholders reached consensus on recommendations to use the EASI, the vIGAAD, and an IGA multiplied or measured alongside a BSA measurement to measure the domain of clinical signs of AD in the clinical practice setting. “The use of multiple IGAs, most with insufficient validation, and the diverse methods used to assess BSA prevented participants from making specific recommendations for the exact IGA/BSA instrument,” the authors wrote. “We recommend that clinicians include at least one of the recommended instruments in their clinical practices and in documentation.”
They explained that the ideal method of measuring BSA was difficult to assess “because multiple techniques exist for its measurement, including regional percentages, the Rule of Nines, or the handprint method. Most studies did not report which method was performed, and to our knowledge, no studies have been performed in patients with AD that have formally compared them.”
During the consensus exercise, the authors noted, several clinicians “expressed concern whether the EASI was feasible for universal use in clinical practice given its complexity, long completion time, and documentation/calculation requirements.” But clinicians who commonly perform the EASI in clinical practice said that the time it takes to complete this measure “has dropped substantially and now is not a considerable burden,” they wrote, adding that, “studies have shown that with trained investigators, EASI completion times can be as low as nearly 2 minutes.”
The authors acknowledged certain limitations of their recommendations, including the lack of input from primary care clinicians. “It is unknown whether ClinROMs [clinician-reported outcome measures] for AD clinical signs are used in the primary care setting, especially given the large amount of conditions that are managed simultaneously and the ever-increasing number of primary care documentation requirements,” they wrote.
Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the consensus statement, said that with the advent of new, improved, and more expensive medications for AD, “it is ever more important that [the clinical] assessment is reliable and reproducible.”
Insurers “are understandably less willing to rubber-stamp approval of more expensive medications without a reliable standard by which to justify such decisions,” he added. “This is even more important in a disease state like atopic dermatitis that lacks a reliable biomarker. Therefore, one or several practical, reliable, validated severity metrics will help standardize and improve AD care.”
Dr. Sidbury, who cochaired the 2023 American Academy of Dermatology guidelines of care for the management of AD in adults with phototherapy and systemic therapies, added that the instruments evaluated in the review “can be challenging for anyone,” not just primary care providers. “The EASI isn’t that easy, and while there is a learning curve and it ultimately does, like anything, become more efficient in the gathering, it is unclear if non-AD researchers will be willing to invest the time” to routinely use it, he said.
Dr. Simpson and several coauthors reported receiving grants and personal fees from multiple pharmaceutical companies. Dr. Sidbury reported that he serves as an investigator for Regeneron, Galderma, UCB, Castle, and Pfizer; is a consultant for LEO, Lilly, Arcutis, Dermavant, and Pierre Fabre; and a speaker for Beiersdorf.
A version of this article appeared on Medscape.com .
recommended.
These include the Eczema Area and Severity Index (EASI), the Validated Investigator Global Assessment for AD (vIGAAD), and the Investigator’s Global Assessment (IGA) multiplied by or measured concurrently with a body surface area (BSA) assessment.
The recommendations are part of a consensus statement based on an updated systematic review conducted by the Harmonizing Outcome Measures for Eczema Clinical Practice (HOME-CP) initiative, whose goal is to identify validated, feasible outcome instruments designed to measure AD in the clinical setting. In the statement, which was published in JAMA Dermatology on May 22, 2024, corresponding author Eric L. Simpson, MD, MCR, professor of dermatology at Oregon Health & Science University, Portland, and coauthors described HOME-CP as “a ‘pick-and-choose’ list of valid and feasible OMIs [outcome measure instruments] that can be incorporated into the practice setting depending on the particular need of that clinic or health system.”
For the effort, the authors implemented a mixed methods design and incorporated systematic reviews and qualitative consensus methods modeled after the HOME core outcome set initiative, which developed a set of consensus-based core outcome sets for clinical trials and clinical practice. In October of 2022, a daylong in-person consensus exercise was held in Montreal, Canada, where attendees met to reach consensus on recommended instruments to measure AD clinical signs in clinical practice, based on an updated systematic review evaluating the validity of clinical signs instruments.
The review included 22 studies describing 16 instruments that assessed AD clinical signs and an additional 12 variants of instruments. The meeting was attended by 34 individuals from 13 countries, including patient and patient advocate research partners, health care professionals, researchers, methodologists, and industry representatives. Consensus was defined as less than 30% disagreement.
Following their daylong consensus exercise, the stakeholders reached consensus on recommendations to use the EASI, the vIGAAD, and an IGA multiplied or measured alongside a BSA measurement to measure the domain of clinical signs of AD in the clinical practice setting. “The use of multiple IGAs, most with insufficient validation, and the diverse methods used to assess BSA prevented participants from making specific recommendations for the exact IGA/BSA instrument,” the authors wrote. “We recommend that clinicians include at least one of the recommended instruments in their clinical practices and in documentation.”
They explained that the ideal method of measuring BSA was difficult to assess “because multiple techniques exist for its measurement, including regional percentages, the Rule of Nines, or the handprint method. Most studies did not report which method was performed, and to our knowledge, no studies have been performed in patients with AD that have formally compared them.”
During the consensus exercise, the authors noted, several clinicians “expressed concern whether the EASI was feasible for universal use in clinical practice given its complexity, long completion time, and documentation/calculation requirements.” But clinicians who commonly perform the EASI in clinical practice said that the time it takes to complete this measure “has dropped substantially and now is not a considerable burden,” they wrote, adding that, “studies have shown that with trained investigators, EASI completion times can be as low as nearly 2 minutes.”
The authors acknowledged certain limitations of their recommendations, including the lack of input from primary care clinicians. “It is unknown whether ClinROMs [clinician-reported outcome measures] for AD clinical signs are used in the primary care setting, especially given the large amount of conditions that are managed simultaneously and the ever-increasing number of primary care documentation requirements,” they wrote.
Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the consensus statement, said that with the advent of new, improved, and more expensive medications for AD, “it is ever more important that [the clinical] assessment is reliable and reproducible.”
Insurers “are understandably less willing to rubber-stamp approval of more expensive medications without a reliable standard by which to justify such decisions,” he added. “This is even more important in a disease state like atopic dermatitis that lacks a reliable biomarker. Therefore, one or several practical, reliable, validated severity metrics will help standardize and improve AD care.”
Dr. Sidbury, who cochaired the 2023 American Academy of Dermatology guidelines of care for the management of AD in adults with phototherapy and systemic therapies, added that the instruments evaluated in the review “can be challenging for anyone,” not just primary care providers. “The EASI isn’t that easy, and while there is a learning curve and it ultimately does, like anything, become more efficient in the gathering, it is unclear if non-AD researchers will be willing to invest the time” to routinely use it, he said.
Dr. Simpson and several coauthors reported receiving grants and personal fees from multiple pharmaceutical companies. Dr. Sidbury reported that he serves as an investigator for Regeneron, Galderma, UCB, Castle, and Pfizer; is a consultant for LEO, Lilly, Arcutis, Dermavant, and Pierre Fabre; and a speaker for Beiersdorf.
A version of this article appeared on Medscape.com .
FROM JAMA DERMATOLOGY
Recent Evidence for Home Phototherapy Benefits May Improve Access for Patients with Psoriasis
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Supporters of home phototherapy for patients with plaque and guttate psoriasis had plenty to cheer about at the annual meeting of the American Academy of Dermatology (AAD) in March. There, Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania in Philadelphia, presented results from the LITE study, a trial that tested the hypothesis that narrowband ultraviolet B phototherapy of psoriasis at home is noninferior to office treatment, based on outcomes that matter to patients, clinicians, and payers. While smaller studies have drawn similar conclusions,
The co-primary outcomes in the LITE study were a Physician’s Global Assessment (PGA) score of 0/1 (clear, almost clear) and a Dermatology Life Quality Index (DLQI) score of 5 or less (small, no effect on health-related quality of life).
Dr. Gelfand and colleagues at 42 sites in the United States enrolled 783 patients aged 12 years and older who had plaque or guttate psoriasis and were candidates for phototherapy at home or in an office setting. Following 12 weeks of treatment, 25.6% of patients in the office-based phototherapy group achieved a PGA score of 0/1 compared with 32.8% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data). Similarly, 33.6% of patients in the office-based phototherapy group achieved a DLQI score of 5 or less compared with 52.4% of patients in the home-based phototherapy group (P > .0001 for noninferiority, non-response imputation for missing data).
A Safe and Effective Option
“I think that it’s important for physicians, insurance companies, and patients with psoriasis to understand that this is a very safe and effective form of therapy,” Craig A. Elmets, MD, professor of dermatology at The University of Alabama at Birmingham, said in an interview. “For people who are not interested in systemic medications or who have contraindications to systemic medications, phototherapy would be ideal,” added Dr. Elmets, first author of the joint AAD–National Psoriasis Foundation (NPF) guidelines for the management and treatment of psoriasis with phototherapy, published in 2019.
Factors beyond efficacy support the role of home phototherapy, Dr. Gelfand said, including the fact that it costs 10-100 times less than biologics for psoriasis and that office-based phototherapy is not available in 90% of counties in the United States. However, insurance coverage of home phototherapy “is highly variable because until the LITE study, there was no large-scale US data to support its use,” he told this news organization.
“Also, insurance companies are broken up into two parts: Durable medical goods and the medical side such as pharmacy costs, and they are siloed. The durable medical goods side views phototherapy as expensive, while the pharmacy side views it as dirt cheap. This is part of the problem with our health system. A lot of things are siloed and don’t make any sense,” said Dr. Gelfand, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania. By working with the NPF and payers, he added, “we’re hoping ... to transform the way insurance companies think about covering home phototherapy.”
In the meantime, he and Dr. Elmets shared practical ways to optimize access to home phototherapy for psoriasis patients:
Have the discussion. Patients “rarely bring this up as an option,” Dr. Elmets said, so the onus is on clinicians to talk about it. In his view, the ideal candidate “is averse to using systemic agents but whose disease is beyond the point where topical medicines alone will work. One of the advantages of phototherapy is that it doesn’t have immunosuppressive effects.”
Clinicians and patients can learn about the efficacy and safety of phototherapy for psoriasis, including home-based options, on the NPF’s web site and by reading the 2019 joint AAD-NPF guidelines.
Shared decision-making is key. “When a patient comes in, I’ll discuss what their treatment options are and [we] will decide upon a course of action based on their unique needs and preferences [and] if it’s medically appropriate, meaning they have the type of psoriasis likely to respond to phototherapy,” Dr. Gelfand said. A patient with psoriasis mainly on the fingernails or genitals “is not a good candidate for phototherapy. If it’s on the trunk or extremities, that patient would be a good candidate.”
Home phototherapy candidates also must be willing and able to operate a machine and have dedicated space in their dwelling for it (most units are about the size of a door). Patients also have to be reliable, follow directions, and come back in person for follow-up appointments “so we can assess their response to treatment and fine-tune things as necessary and make sure they’re not developing any skin damage,” Dr. Gelfand said.
Educate yourself about existing options. Home phototherapy units from manufacturers such as Daavlin, National Biological Corporation, and SolRx range between $1200 and $6000 in cost, Dr. Gelfand said. He and his colleagues used the Daavlin 7 series in the LITE study. That unit features an integrated dosimetry system that delivers the correct dose of energy based on parameters that the prescribing clinician recommends. Settings are based on the patient’s skin type and how much the prescriber wants to increase the dose for each treatment. “The machine does the rest,” he said. “It knows what dose to give, so they get the same dosing as they would in an office situation.”
Smaller home-based phototherapy units designed to treat the hands and feet are available. So are handheld units to treat the scalp. “These can be a nice option for patients who have a few spots, but if the disease is moderate to severe, then it’s going to be pretty laborious to [use them],” Dr. Elmets said.
Remember that phototherapy is not a cure-all. According to the joint AAD-NPF guidelines, most phototherapy regimens require treatments two to three times per week for 10-14 weeks. Once patients achieve their home phototherapy treatment goal, Dr. Elmets often recommends treatments one to two times per week for maintenance.
“Patients with psoriasis have a lifetime condition,” he noted. “There are certainly cases where people have gone on phototherapy, cleared, and then stopped for a period of time. If they flare up, they can always go back to phototherapy. Usually, people who are on phototherapy use some type of topical agents to touch up areas that are resistant.”
Expect pushback from insurers on coverage. While Medicare and some integrated health plans cover home phototherapy, expect to spend time writing letters or placing phone calls to insurance companies to convince them why they should cover home phototherapy for candidate psoriasis patients. “Usually there’s a lot of letter writing and a long delay in getting approval,” Dr. Elmets said.
Dr. Elmets and Dr. Gelfand reported no relevant financial relationships. The LITE study was funded by the Patient-Centered Outcomes Research Institute. Research partners included the National Psoriasis Foundation and Daavlin, which provided the home phototherapy machines and covered the cost of shipping the devices.
A version of this article appeared on Medscape.com.
Subcutaneous Antifibrinolytic Reduces Bleeding After Mohs Surgery
“Though Mohs micrographic surgery is associated with low bleeding complication rates, around 1% of patients in the literature report postoperative bleeding,” corresponding author Abigail H. Waldman, MD, director of the Mohs and Dermatologic Surgery Center, at Brigham and Women’s Hospital, Boston, and colleagues wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Intravenous tranexamic acid has been used across surgical specialties to reduce perioperative blood loss. Prior studies have shown topical TXA, an antifibrinolytic agent, following MMS may be effective in reducing postoperative bleeding complications, but there are no large cohort studies on injectable TXA utilization in all patients undergoing MMS.”
To improve the understanding of this intervention, the researchers examined the impact of off-label, locally injected TXA on postoperative bleeding outcomes following MMS conducted at Brigham and Women’s Hospital. They evaluated two cohorts: 1843 patients who underwent MMS from January 1, 2019, to December 31, 2019 (the pre-TXA cohort), and 2101 patients who underwent MMS from July 1, 2022, to June 30, 2023 (the TXA cohort), and extracted data, including patient and tumor characteristics, MMS procedure details, antithrombotic medication use, systemic conditions that predispose to bleeding, encounters reporting postoperative bleeding, and interventions required for postoperative bleeding, from electronic medical records. Patients reconstructed by a non-MMS surgeon were excluded from the analysis.
Overall, 2509 cases among 1843 patients and 2818 cases among 2101 were included in the pre-TXA and TXA cohorts, respectively. The researchers found that local subcutaneous injection of TXA reduced the risk for postoperative phone calls or visits for bleeding by 25% (RR [risk ratio], 0.75; 0.57-0.99) and risk for bleeding necessitating a medical visit by 51% (RR, 0.49; 0.32-0.77).
The use of preoperative TXA in several subgroups of patients also was also associated with a reduction in visits for bleeding, including those using alcohol (52% reduction; RR, 0.47; 0.26-0.85), cigarettes (57% reduction; RR, 0.43; 0.23-0.82), oral anticoagulants (61% reduction; RR, 0.39; 0.20-0.77), or antiplatelets (60% reduction; RR, 0.40; 0.20-0.79). The use of TXA was also associated with reduced visits for bleeding in tumors of the head and neck (RR, 0.45; 0.26-0.77) and tumors with a preoperative diameter > 2 cm (RR, 0.37; 0.15-0.90).
Impact of Surgical Repair Type
In other findings, the type of surgical repair was a potential confounder, the authors reported. Grafts and flaps were associated with an increased risk for bleeding across both cohorts (RR, 2.36 [1.5-3.6] and 1.7 [1.1-2.6], respectively) and together comprised 15% of all procedures in the pre-TXA cohort compared with 11.1% in TXA cohort. Two patients in the TXA cohort (0.11%) developed deep vein thrombosis (DVT) 10- and 20-days postoperation, a rate that the authors said is comparable to that of the general population. The two patients had risk factors for hypercoagulability, including advanced cancer and recurrent DVT.
“Overall, local injection of TXA was an effective method for reducing the risk of clinically significant bleeding following MMS,” the researchers concluded. “Perioperative TXA may help to limit the risk of bleeding overall, as well as in populations predisposed to bleeding.” Adverse events with TXA use were rare “and delayed beyond the activity of TXA, indicating a low likelihood of being due to TXA,” they wrote.
“Dermatologists performing MMS may consider incorporating local TXA injection into their regular practice,” they noted, adding that “legal counsel on adverse effects in the setting of off-label pharmaceutical usage may be advised.”
In an interview, Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who was asked to comment on the study, said that postoperative bleeding is one of the most commonly encountered Mohs surgery complications. “Because of increased clinic visits and phone calls, it can also often result in decreased patient satisfaction,” she said.
“This study is particularly notable in that we see that local subcutaneous TXA injection decreased visits for bleeding even in those using oral anticoagulants, antiplatelets, alcohol, and cigarettes. Dermatologic surgery has a very low complication rate, even in patients on anticoagulant and antiplatelet medications, but this study shows that TXA is a fantastic option for Mohs surgeons and patients.”
Neither the study authors nor Dr. Richey reported having financial disclosures.
A version of this article first appeared on Medscape.com.
“Though Mohs micrographic surgery is associated with low bleeding complication rates, around 1% of patients in the literature report postoperative bleeding,” corresponding author Abigail H. Waldman, MD, director of the Mohs and Dermatologic Surgery Center, at Brigham and Women’s Hospital, Boston, and colleagues wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Intravenous tranexamic acid has been used across surgical specialties to reduce perioperative blood loss. Prior studies have shown topical TXA, an antifibrinolytic agent, following MMS may be effective in reducing postoperative bleeding complications, but there are no large cohort studies on injectable TXA utilization in all patients undergoing MMS.”
To improve the understanding of this intervention, the researchers examined the impact of off-label, locally injected TXA on postoperative bleeding outcomes following MMS conducted at Brigham and Women’s Hospital. They evaluated two cohorts: 1843 patients who underwent MMS from January 1, 2019, to December 31, 2019 (the pre-TXA cohort), and 2101 patients who underwent MMS from July 1, 2022, to June 30, 2023 (the TXA cohort), and extracted data, including patient and tumor characteristics, MMS procedure details, antithrombotic medication use, systemic conditions that predispose to bleeding, encounters reporting postoperative bleeding, and interventions required for postoperative bleeding, from electronic medical records. Patients reconstructed by a non-MMS surgeon were excluded from the analysis.
Overall, 2509 cases among 1843 patients and 2818 cases among 2101 were included in the pre-TXA and TXA cohorts, respectively. The researchers found that local subcutaneous injection of TXA reduced the risk for postoperative phone calls or visits for bleeding by 25% (RR [risk ratio], 0.75; 0.57-0.99) and risk for bleeding necessitating a medical visit by 51% (RR, 0.49; 0.32-0.77).
The use of preoperative TXA in several subgroups of patients also was also associated with a reduction in visits for bleeding, including those using alcohol (52% reduction; RR, 0.47; 0.26-0.85), cigarettes (57% reduction; RR, 0.43; 0.23-0.82), oral anticoagulants (61% reduction; RR, 0.39; 0.20-0.77), or antiplatelets (60% reduction; RR, 0.40; 0.20-0.79). The use of TXA was also associated with reduced visits for bleeding in tumors of the head and neck (RR, 0.45; 0.26-0.77) and tumors with a preoperative diameter > 2 cm (RR, 0.37; 0.15-0.90).
Impact of Surgical Repair Type
In other findings, the type of surgical repair was a potential confounder, the authors reported. Grafts and flaps were associated with an increased risk for bleeding across both cohorts (RR, 2.36 [1.5-3.6] and 1.7 [1.1-2.6], respectively) and together comprised 15% of all procedures in the pre-TXA cohort compared with 11.1% in TXA cohort. Two patients in the TXA cohort (0.11%) developed deep vein thrombosis (DVT) 10- and 20-days postoperation, a rate that the authors said is comparable to that of the general population. The two patients had risk factors for hypercoagulability, including advanced cancer and recurrent DVT.
“Overall, local injection of TXA was an effective method for reducing the risk of clinically significant bleeding following MMS,” the researchers concluded. “Perioperative TXA may help to limit the risk of bleeding overall, as well as in populations predisposed to bleeding.” Adverse events with TXA use were rare “and delayed beyond the activity of TXA, indicating a low likelihood of being due to TXA,” they wrote.
“Dermatologists performing MMS may consider incorporating local TXA injection into their regular practice,” they noted, adding that “legal counsel on adverse effects in the setting of off-label pharmaceutical usage may be advised.”
In an interview, Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who was asked to comment on the study, said that postoperative bleeding is one of the most commonly encountered Mohs surgery complications. “Because of increased clinic visits and phone calls, it can also often result in decreased patient satisfaction,” she said.
“This study is particularly notable in that we see that local subcutaneous TXA injection decreased visits for bleeding even in those using oral anticoagulants, antiplatelets, alcohol, and cigarettes. Dermatologic surgery has a very low complication rate, even in patients on anticoagulant and antiplatelet medications, but this study shows that TXA is a fantastic option for Mohs surgeons and patients.”
Neither the study authors nor Dr. Richey reported having financial disclosures.
A version of this article first appeared on Medscape.com.
“Though Mohs micrographic surgery is associated with low bleeding complication rates, around 1% of patients in the literature report postoperative bleeding,” corresponding author Abigail H. Waldman, MD, director of the Mohs and Dermatologic Surgery Center, at Brigham and Women’s Hospital, Boston, and colleagues wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Intravenous tranexamic acid has been used across surgical specialties to reduce perioperative blood loss. Prior studies have shown topical TXA, an antifibrinolytic agent, following MMS may be effective in reducing postoperative bleeding complications, but there are no large cohort studies on injectable TXA utilization in all patients undergoing MMS.”
To improve the understanding of this intervention, the researchers examined the impact of off-label, locally injected TXA on postoperative bleeding outcomes following MMS conducted at Brigham and Women’s Hospital. They evaluated two cohorts: 1843 patients who underwent MMS from January 1, 2019, to December 31, 2019 (the pre-TXA cohort), and 2101 patients who underwent MMS from July 1, 2022, to June 30, 2023 (the TXA cohort), and extracted data, including patient and tumor characteristics, MMS procedure details, antithrombotic medication use, systemic conditions that predispose to bleeding, encounters reporting postoperative bleeding, and interventions required for postoperative bleeding, from electronic medical records. Patients reconstructed by a non-MMS surgeon were excluded from the analysis.
Overall, 2509 cases among 1843 patients and 2818 cases among 2101 were included in the pre-TXA and TXA cohorts, respectively. The researchers found that local subcutaneous injection of TXA reduced the risk for postoperative phone calls or visits for bleeding by 25% (RR [risk ratio], 0.75; 0.57-0.99) and risk for bleeding necessitating a medical visit by 51% (RR, 0.49; 0.32-0.77).
The use of preoperative TXA in several subgroups of patients also was also associated with a reduction in visits for bleeding, including those using alcohol (52% reduction; RR, 0.47; 0.26-0.85), cigarettes (57% reduction; RR, 0.43; 0.23-0.82), oral anticoagulants (61% reduction; RR, 0.39; 0.20-0.77), or antiplatelets (60% reduction; RR, 0.40; 0.20-0.79). The use of TXA was also associated with reduced visits for bleeding in tumors of the head and neck (RR, 0.45; 0.26-0.77) and tumors with a preoperative diameter > 2 cm (RR, 0.37; 0.15-0.90).
Impact of Surgical Repair Type
In other findings, the type of surgical repair was a potential confounder, the authors reported. Grafts and flaps were associated with an increased risk for bleeding across both cohorts (RR, 2.36 [1.5-3.6] and 1.7 [1.1-2.6], respectively) and together comprised 15% of all procedures in the pre-TXA cohort compared with 11.1% in TXA cohort. Two patients in the TXA cohort (0.11%) developed deep vein thrombosis (DVT) 10- and 20-days postoperation, a rate that the authors said is comparable to that of the general population. The two patients had risk factors for hypercoagulability, including advanced cancer and recurrent DVT.
“Overall, local injection of TXA was an effective method for reducing the risk of clinically significant bleeding following MMS,” the researchers concluded. “Perioperative TXA may help to limit the risk of bleeding overall, as well as in populations predisposed to bleeding.” Adverse events with TXA use were rare “and delayed beyond the activity of TXA, indicating a low likelihood of being due to TXA,” they wrote.
“Dermatologists performing MMS may consider incorporating local TXA injection into their regular practice,” they noted, adding that “legal counsel on adverse effects in the setting of off-label pharmaceutical usage may be advised.”
In an interview, Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who was asked to comment on the study, said that postoperative bleeding is one of the most commonly encountered Mohs surgery complications. “Because of increased clinic visits and phone calls, it can also often result in decreased patient satisfaction,” she said.
“This study is particularly notable in that we see that local subcutaneous TXA injection decreased visits for bleeding even in those using oral anticoagulants, antiplatelets, alcohol, and cigarettes. Dermatologic surgery has a very low complication rate, even in patients on anticoagulant and antiplatelet medications, but this study shows that TXA is a fantastic option for Mohs surgeons and patients.”
Neither the study authors nor Dr. Richey reported having financial disclosures.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Treatments for Early HS Range From Topical Therapies to Laser Hair Removal
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
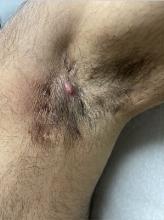
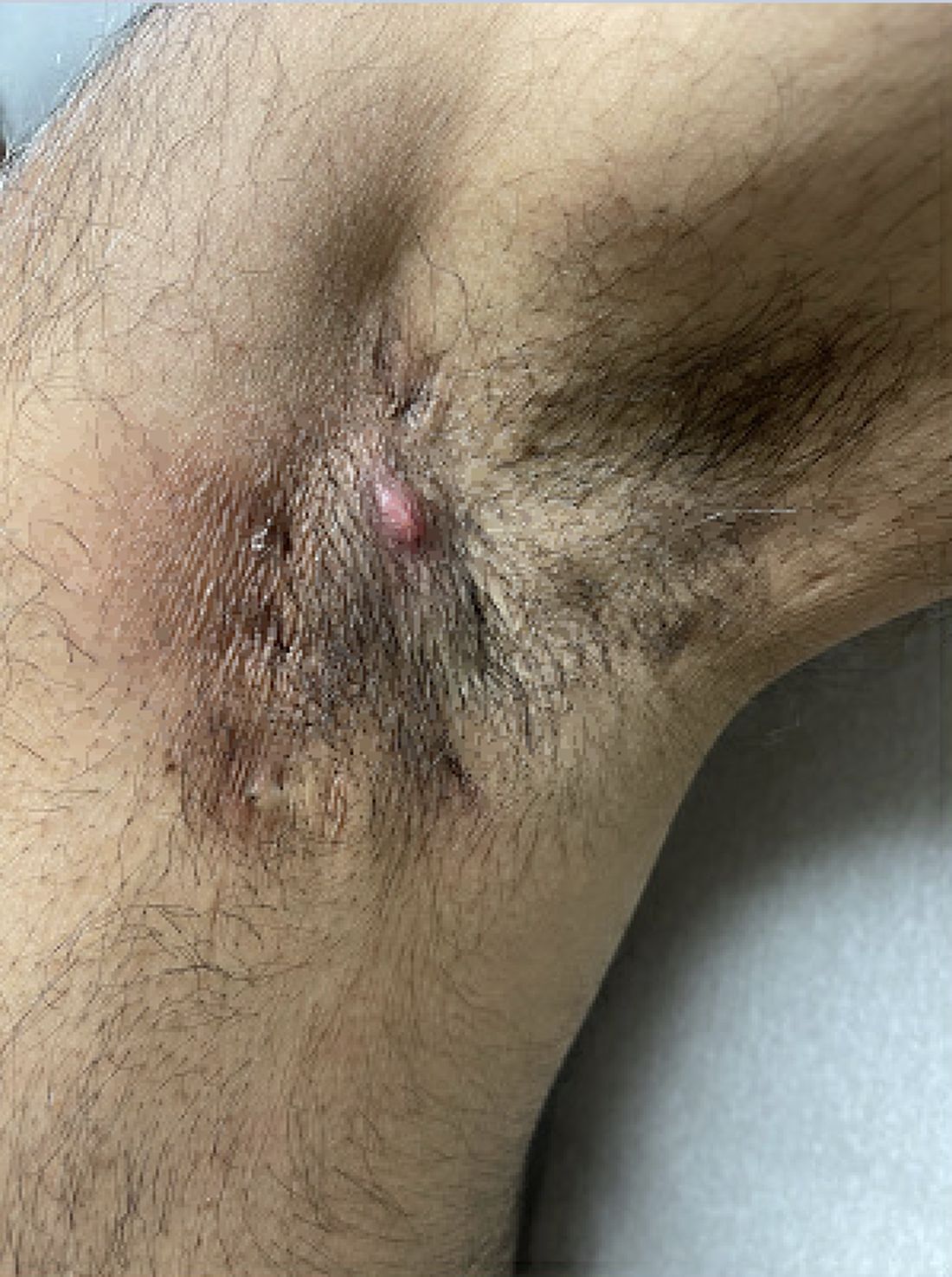
Diagnosing Mild Hidradenitis Suppurativa: Early Stage Can Mimic Other Diseases
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
Pediatric Dermatologists Beat ChatGPT on Board Questions
In an experiment that pitted the wits of results from a small single-center study showed.
“We were relieved to find that the pediatric dermatologists in our study performed better than ChatGPT on both multiple choice and case-based questions; however, the latest iteration of ChatGPT (4.0) was very close,” one of the study’s first authors Charles Huang, a fourth-year medical student at Thomas Jefferson University, Philadelphia, said in an interview. “Something else that was interesting in our data was that the pediatric dermatologists performed much better than ChatGPT on questions related to procedural dermatology/surgical techniques, perhaps indicating that knowledge/reasoning gained through practical experience isn’t easily replicated in AI tools such as ChatGPT.”
For the study, which was published on May 9 in Pediatric Dermatology, Mr. Huang, and co-first author Esther Zhang, BS, a medical student at the University of Pennsylvania, Philadelphia, and coauthors from the Department of Dermatology, Children’s Hospital of Philadelphia, asked five pediatric dermatologists to answer 24 text-based questions including 16 single-answer, multiple-choice questions and two multiple answer questions drawn from the American Board of Dermatology 2021 Certification Sample Test and six free-response case-based questions drawn from the “Photoquiz” section of Pediatric Dermatology between July 2022 and July 2023. The researchers then processed the same set of questions through ChatGPT versions 3.5 and 4.0 and used statistical analysis to compare responses between the pediatric dermatologists and ChatGPT. A 5-point scale adapted from current AI tools was used to score replies to case-based questions.
On average, study participants had 5.6 years of clinical experience. Pediatric dermatologists performed significantly better than ChatGPT version 3.5 on multiple-choice and multiple answer questions (91.4% vs 76.2%, respectively; P = .021) but not significantly better than ChatGPT version 4.0 (90.5%; P = .44). As for replies to case-based questions, the average performance based on the 5-point scale was 3.81 for pediatric dermatologists and 3.53 for ChatGPT overall. The mean scores were significantly greater for pediatric dermatologists than for ChatGPT version 3.5 (P = .039) but not ChatGPT version 4.0 (P = .43).
The researchers acknowledged certain limitations of the analysis, including the evolving nature of AI tools, which may affect the reproducibility of results with subsequent model updates. And, while participating pediatric dermatologists said they were unfamiliar with the questions and cases used in the study, “there is potential for prior exposure through other dermatology board examination review processes,” they wrote.
“AI tools such as ChatGPT and similar large language models can be a valuable tool in your clinical practice, but be aware of potential pitfalls such as patient privacy, medical inaccuracies, [and] intrinsic biases in the tools,” Mr. Huang told this news organization. “As these technologies continue to advance, it is essential for all of us as medical clinicians to gain familiarity and stay abreast of new developments, just as we adapted to electronic health records and the use of the Internet.”
Maria Buethe, MD, PhD, a pediatric dermatology fellow at Rady Children’s Hospital–San Diego, who was asked to comment on the study, said she found it “interesting” that ChatGPT’s version 4.0 started to produce comparable results to clinician responses in some of the tested scenarios.
“The authors propose a set of best practices for pediatric dermatology clinicians using ChatGPT and other AI tools,” said Dr. Buethe, who was senior author of a recent literature review on AI and its application to pediatric dermatology. It was published in SKIN The Journal of Cutaneous Medicine. “One interesting recommended use for AI tools is to utilize it to generate differential diagnosis, which can broaden the list of pathologies previously considered.”
Asked to comment on the study, Erum Ilyas, MD, who practices dermatology in King of Prussia, Pennsylvania, and is a member of the Society for Pediatric Dermatology, said she was not surprised that ChatGPT “can perform fairly well on multiple-choice questions as we find available in testing circumstances,” as presented in the study. “Just as board questions only support testing a base of medical knowledge and facts for clinicians to master, they do not necessarily provide real-life circumstances that apply to caring for patients, which is inherently nuanced.”
In addition, the study “highlights that ChatGPT can be an aid to support thinking through differentials based on data entered by a clinician who understands how to phrase queries, especially if provided with enough data while respecting patient privacy, in the context of fact checking responses,” Dr. Ilyas said. “This underscores the fact that AI tools can be helpful to clinicians in assimilating various data points entered. However, ultimately, the tool is only able to support an output based on the information it has access to.” She added, “ChatGPT cannot be relied on to provide a single diagnosis with the clinician still responsible for making a final diagnosis. The tool is not definitive and cannot assimilate data that is not entered correctly.”
The study was not funded, and the study authors reported having no disclosures. Dr. Buethe and Dr. Ilyas, who were not involved with the study, had no disclosures.
A version of this article appeared on Medscape.com .
In an experiment that pitted the wits of results from a small single-center study showed.
“We were relieved to find that the pediatric dermatologists in our study performed better than ChatGPT on both multiple choice and case-based questions; however, the latest iteration of ChatGPT (4.0) was very close,” one of the study’s first authors Charles Huang, a fourth-year medical student at Thomas Jefferson University, Philadelphia, said in an interview. “Something else that was interesting in our data was that the pediatric dermatologists performed much better than ChatGPT on questions related to procedural dermatology/surgical techniques, perhaps indicating that knowledge/reasoning gained through practical experience isn’t easily replicated in AI tools such as ChatGPT.”
For the study, which was published on May 9 in Pediatric Dermatology, Mr. Huang, and co-first author Esther Zhang, BS, a medical student at the University of Pennsylvania, Philadelphia, and coauthors from the Department of Dermatology, Children’s Hospital of Philadelphia, asked five pediatric dermatologists to answer 24 text-based questions including 16 single-answer, multiple-choice questions and two multiple answer questions drawn from the American Board of Dermatology 2021 Certification Sample Test and six free-response case-based questions drawn from the “Photoquiz” section of Pediatric Dermatology between July 2022 and July 2023. The researchers then processed the same set of questions through ChatGPT versions 3.5 and 4.0 and used statistical analysis to compare responses between the pediatric dermatologists and ChatGPT. A 5-point scale adapted from current AI tools was used to score replies to case-based questions.
On average, study participants had 5.6 years of clinical experience. Pediatric dermatologists performed significantly better than ChatGPT version 3.5 on multiple-choice and multiple answer questions (91.4% vs 76.2%, respectively; P = .021) but not significantly better than ChatGPT version 4.0 (90.5%; P = .44). As for replies to case-based questions, the average performance based on the 5-point scale was 3.81 for pediatric dermatologists and 3.53 for ChatGPT overall. The mean scores were significantly greater for pediatric dermatologists than for ChatGPT version 3.5 (P = .039) but not ChatGPT version 4.0 (P = .43).
The researchers acknowledged certain limitations of the analysis, including the evolving nature of AI tools, which may affect the reproducibility of results with subsequent model updates. And, while participating pediatric dermatologists said they were unfamiliar with the questions and cases used in the study, “there is potential for prior exposure through other dermatology board examination review processes,” they wrote.
“AI tools such as ChatGPT and similar large language models can be a valuable tool in your clinical practice, but be aware of potential pitfalls such as patient privacy, medical inaccuracies, [and] intrinsic biases in the tools,” Mr. Huang told this news organization. “As these technologies continue to advance, it is essential for all of us as medical clinicians to gain familiarity and stay abreast of new developments, just as we adapted to electronic health records and the use of the Internet.”
Maria Buethe, MD, PhD, a pediatric dermatology fellow at Rady Children’s Hospital–San Diego, who was asked to comment on the study, said she found it “interesting” that ChatGPT’s version 4.0 started to produce comparable results to clinician responses in some of the tested scenarios.
“The authors propose a set of best practices for pediatric dermatology clinicians using ChatGPT and other AI tools,” said Dr. Buethe, who was senior author of a recent literature review on AI and its application to pediatric dermatology. It was published in SKIN The Journal of Cutaneous Medicine. “One interesting recommended use for AI tools is to utilize it to generate differential diagnosis, which can broaden the list of pathologies previously considered.”
Asked to comment on the study, Erum Ilyas, MD, who practices dermatology in King of Prussia, Pennsylvania, and is a member of the Society for Pediatric Dermatology, said she was not surprised that ChatGPT “can perform fairly well on multiple-choice questions as we find available in testing circumstances,” as presented in the study. “Just as board questions only support testing a base of medical knowledge and facts for clinicians to master, they do not necessarily provide real-life circumstances that apply to caring for patients, which is inherently nuanced.”
In addition, the study “highlights that ChatGPT can be an aid to support thinking through differentials based on data entered by a clinician who understands how to phrase queries, especially if provided with enough data while respecting patient privacy, in the context of fact checking responses,” Dr. Ilyas said. “This underscores the fact that AI tools can be helpful to clinicians in assimilating various data points entered. However, ultimately, the tool is only able to support an output based on the information it has access to.” She added, “ChatGPT cannot be relied on to provide a single diagnosis with the clinician still responsible for making a final diagnosis. The tool is not definitive and cannot assimilate data that is not entered correctly.”
The study was not funded, and the study authors reported having no disclosures. Dr. Buethe and Dr. Ilyas, who were not involved with the study, had no disclosures.
A version of this article appeared on Medscape.com .
In an experiment that pitted the wits of results from a small single-center study showed.
“We were relieved to find that the pediatric dermatologists in our study performed better than ChatGPT on both multiple choice and case-based questions; however, the latest iteration of ChatGPT (4.0) was very close,” one of the study’s first authors Charles Huang, a fourth-year medical student at Thomas Jefferson University, Philadelphia, said in an interview. “Something else that was interesting in our data was that the pediatric dermatologists performed much better than ChatGPT on questions related to procedural dermatology/surgical techniques, perhaps indicating that knowledge/reasoning gained through practical experience isn’t easily replicated in AI tools such as ChatGPT.”
For the study, which was published on May 9 in Pediatric Dermatology, Mr. Huang, and co-first author Esther Zhang, BS, a medical student at the University of Pennsylvania, Philadelphia, and coauthors from the Department of Dermatology, Children’s Hospital of Philadelphia, asked five pediatric dermatologists to answer 24 text-based questions including 16 single-answer, multiple-choice questions and two multiple answer questions drawn from the American Board of Dermatology 2021 Certification Sample Test and six free-response case-based questions drawn from the “Photoquiz” section of Pediatric Dermatology between July 2022 and July 2023. The researchers then processed the same set of questions through ChatGPT versions 3.5 and 4.0 and used statistical analysis to compare responses between the pediatric dermatologists and ChatGPT. A 5-point scale adapted from current AI tools was used to score replies to case-based questions.
On average, study participants had 5.6 years of clinical experience. Pediatric dermatologists performed significantly better than ChatGPT version 3.5 on multiple-choice and multiple answer questions (91.4% vs 76.2%, respectively; P = .021) but not significantly better than ChatGPT version 4.0 (90.5%; P = .44). As for replies to case-based questions, the average performance based on the 5-point scale was 3.81 for pediatric dermatologists and 3.53 for ChatGPT overall. The mean scores were significantly greater for pediatric dermatologists than for ChatGPT version 3.5 (P = .039) but not ChatGPT version 4.0 (P = .43).
The researchers acknowledged certain limitations of the analysis, including the evolving nature of AI tools, which may affect the reproducibility of results with subsequent model updates. And, while participating pediatric dermatologists said they were unfamiliar with the questions and cases used in the study, “there is potential for prior exposure through other dermatology board examination review processes,” they wrote.
“AI tools such as ChatGPT and similar large language models can be a valuable tool in your clinical practice, but be aware of potential pitfalls such as patient privacy, medical inaccuracies, [and] intrinsic biases in the tools,” Mr. Huang told this news organization. “As these technologies continue to advance, it is essential for all of us as medical clinicians to gain familiarity and stay abreast of new developments, just as we adapted to electronic health records and the use of the Internet.”
Maria Buethe, MD, PhD, a pediatric dermatology fellow at Rady Children’s Hospital–San Diego, who was asked to comment on the study, said she found it “interesting” that ChatGPT’s version 4.0 started to produce comparable results to clinician responses in some of the tested scenarios.
“The authors propose a set of best practices for pediatric dermatology clinicians using ChatGPT and other AI tools,” said Dr. Buethe, who was senior author of a recent literature review on AI and its application to pediatric dermatology. It was published in SKIN The Journal of Cutaneous Medicine. “One interesting recommended use for AI tools is to utilize it to generate differential diagnosis, which can broaden the list of pathologies previously considered.”
Asked to comment on the study, Erum Ilyas, MD, who practices dermatology in King of Prussia, Pennsylvania, and is a member of the Society for Pediatric Dermatology, said she was not surprised that ChatGPT “can perform fairly well on multiple-choice questions as we find available in testing circumstances,” as presented in the study. “Just as board questions only support testing a base of medical knowledge and facts for clinicians to master, they do not necessarily provide real-life circumstances that apply to caring for patients, which is inherently nuanced.”
In addition, the study “highlights that ChatGPT can be an aid to support thinking through differentials based on data entered by a clinician who understands how to phrase queries, especially if provided with enough data while respecting patient privacy, in the context of fact checking responses,” Dr. Ilyas said. “This underscores the fact that AI tools can be helpful to clinicians in assimilating various data points entered. However, ultimately, the tool is only able to support an output based on the information it has access to.” She added, “ChatGPT cannot be relied on to provide a single diagnosis with the clinician still responsible for making a final diagnosis. The tool is not definitive and cannot assimilate data that is not entered correctly.”
The study was not funded, and the study authors reported having no disclosures. Dr. Buethe and Dr. Ilyas, who were not involved with the study, had no disclosures.
A version of this article appeared on Medscape.com .
Survey Spotlights Identification of Dermatologic Adverse Events From Cancer Therapies
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology annual meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Dr. Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
Dr. LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures. Dr. Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.
A version of this article first appeared on Medscape.com.
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology annual meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Dr. Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
Dr. LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures. Dr. Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.
A version of this article first appeared on Medscape.com.
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology annual meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Dr. Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
Dr. LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures. Dr. Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.
A version of this article first appeared on Medscape.com.
FROM AAD 2024
Darker Skin Tones Underrepresented on Skin Cancer Education Websites
“Given the known disparities patients with darker skin tones face in terms of increased skin cancer morbidity and mortality, this lack of representation further disadvantages those patients by not providing them with an adequate representation of how skin cancers manifest on their skin tones,” the study’s first author, Alana Sadur, who recently completed her third year at the George Washington School of Medicine and Health Sciences, Washington, said in an interview. “By not having images to refer to, patients are less likely to self-identify and seek treatment for concerning skin lesions.”
For the study, which was published in Journal of Drugs in Dermatology, Ms. Sadur and coauthors evaluated the inclusivity and representation of skin tones in photos of skin cancer on the following patient-facing websites: CDC.gov, NIH.gov, skincancer.org, americancancerfund.org, mayoclinic.org, and cancer.org. The researchers counted each individual person or image showing skin as a separate representation, and three independent reviewers used the 5-color Pantone swatch as described in a dermatology atlas to categorize representations as “lighter-toned skin” (Pantones A-B or lighter) or “darker-toned skin” (Pantones C-E or darker).
Of the 372 total representations identified on the websites, only 49 (13.2%) showed darker skin tones. Of these, 44.9% depicted Pantone C, 34.7% depicted Pantone D, and 20.4% depicted Pantone E. The researchers also found that only 11% of nonmelanoma skin cancers (NMSC) and 5.8% of melanoma skin cancers (MSC) were shown on darker skin tones, while no cartoon portrayals of NMSC or MSC included darker skin tones.
In findings related to nondisease representations on the websites, darker skin tones were depicted in just 22.7% of stock photos and 26.1% of website front pages.
The study’s senior author, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, emphasized the need for trusted sources like national organizations and federally funded agencies to be purposeful with their selection of images to “ensure all visitors to the site are represented,” he told this news organization.
“This is very important when dealing with skin cancer as a lack of representation could easily be misinterpreted as epidemiological data, meaning this gap could suggest certain individuals do not get skin cancer because photos in those skin tones are not present,” he added. “This doesn’t even begin to touch upon the diversity of individuals in the stock photos or lack thereof, which can perpetuate the lack of diversity in our specialty. We need to do better.”
The authors reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Given the known disparities patients with darker skin tones face in terms of increased skin cancer morbidity and mortality, this lack of representation further disadvantages those patients by not providing them with an adequate representation of how skin cancers manifest on their skin tones,” the study’s first author, Alana Sadur, who recently completed her third year at the George Washington School of Medicine and Health Sciences, Washington, said in an interview. “By not having images to refer to, patients are less likely to self-identify and seek treatment for concerning skin lesions.”
For the study, which was published in Journal of Drugs in Dermatology, Ms. Sadur and coauthors evaluated the inclusivity and representation of skin tones in photos of skin cancer on the following patient-facing websites: CDC.gov, NIH.gov, skincancer.org, americancancerfund.org, mayoclinic.org, and cancer.org. The researchers counted each individual person or image showing skin as a separate representation, and three independent reviewers used the 5-color Pantone swatch as described in a dermatology atlas to categorize representations as “lighter-toned skin” (Pantones A-B or lighter) or “darker-toned skin” (Pantones C-E or darker).
Of the 372 total representations identified on the websites, only 49 (13.2%) showed darker skin tones. Of these, 44.9% depicted Pantone C, 34.7% depicted Pantone D, and 20.4% depicted Pantone E. The researchers also found that only 11% of nonmelanoma skin cancers (NMSC) and 5.8% of melanoma skin cancers (MSC) were shown on darker skin tones, while no cartoon portrayals of NMSC or MSC included darker skin tones.
In findings related to nondisease representations on the websites, darker skin tones were depicted in just 22.7% of stock photos and 26.1% of website front pages.
The study’s senior author, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, emphasized the need for trusted sources like national organizations and federally funded agencies to be purposeful with their selection of images to “ensure all visitors to the site are represented,” he told this news organization.
“This is very important when dealing with skin cancer as a lack of representation could easily be misinterpreted as epidemiological data, meaning this gap could suggest certain individuals do not get skin cancer because photos in those skin tones are not present,” he added. “This doesn’t even begin to touch upon the diversity of individuals in the stock photos or lack thereof, which can perpetuate the lack of diversity in our specialty. We need to do better.”
The authors reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Given the known disparities patients with darker skin tones face in terms of increased skin cancer morbidity and mortality, this lack of representation further disadvantages those patients by not providing them with an adequate representation of how skin cancers manifest on their skin tones,” the study’s first author, Alana Sadur, who recently completed her third year at the George Washington School of Medicine and Health Sciences, Washington, said in an interview. “By not having images to refer to, patients are less likely to self-identify and seek treatment for concerning skin lesions.”
For the study, which was published in Journal of Drugs in Dermatology, Ms. Sadur and coauthors evaluated the inclusivity and representation of skin tones in photos of skin cancer on the following patient-facing websites: CDC.gov, NIH.gov, skincancer.org, americancancerfund.org, mayoclinic.org, and cancer.org. The researchers counted each individual person or image showing skin as a separate representation, and three independent reviewers used the 5-color Pantone swatch as described in a dermatology atlas to categorize representations as “lighter-toned skin” (Pantones A-B or lighter) or “darker-toned skin” (Pantones C-E or darker).
Of the 372 total representations identified on the websites, only 49 (13.2%) showed darker skin tones. Of these, 44.9% depicted Pantone C, 34.7% depicted Pantone D, and 20.4% depicted Pantone E. The researchers also found that only 11% of nonmelanoma skin cancers (NMSC) and 5.8% of melanoma skin cancers (MSC) were shown on darker skin tones, while no cartoon portrayals of NMSC or MSC included darker skin tones.
In findings related to nondisease representations on the websites, darker skin tones were depicted in just 22.7% of stock photos and 26.1% of website front pages.
The study’s senior author, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, emphasized the need for trusted sources like national organizations and federally funded agencies to be purposeful with their selection of images to “ensure all visitors to the site are represented,” he told this news organization.
“This is very important when dealing with skin cancer as a lack of representation could easily be misinterpreted as epidemiological data, meaning this gap could suggest certain individuals do not get skin cancer because photos in those skin tones are not present,” he added. “This doesn’t even begin to touch upon the diversity of individuals in the stock photos or lack thereof, which can perpetuate the lack of diversity in our specialty. We need to do better.”
The authors reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Consider a Four-Step Approach to Shared Decision-Making in Pediatric Dermatology
SAN DIEGO — In the clinical experience of Kelly M. Cordoro, MD, .
“SDM is a cornerstone of person-centered care,” Dr. Cordoro, professor of dermatology and pediatrics at the University of California, San Francisco, said at the Society for Pediatric Dermatology meeting, held in advance of the annual meeting of the American Academy of Dermatology. “We do it all the time. It can be patient-led, clinician-led, or a patient/family dyad approach. If we do it well, it can improve outcomes. Patients report more satisfying interactions with their care team. It brings adolescent patients especially a sense of independence and they adapt faster to their illness.”
Conditions such as acne, psoriasis, and atopic dermatitis have multiple treatment options, often without a single best choice. The ideal treatment depends on disease characteristics (extent, sites affected, symptoms, and natural history), the patient (age, comorbidities, overall disease burden), therapies (safety, efficacy, duration, and adverse events), and preferences (logistics, time, shots vs. pills, etc.). “These factors vary between patients and within the same patient over time, and at each step along the course of the condition, SDM approaches are relevant,” she said.
AHRQ’s Five-Step Approach
The Agency for Healthcare Research and Quality developed a five-step approach to SDM known as SHARE: Seek your patient’s participation; Help your patient explore and compare treatment options; Assess your patient’s values and preferences; Reach a decision with your patient, and Evaluate your patient’s decision. “We do this all the time in practice with adult patients, but may not label it as SDM,” said Dr. Cordoro, chief and fellowship director of pediatric dermatology at UCSF.
“Where it gets a little murkier is in pediatric decision-making, which is a complex type of surrogate decision-making.” In this situation the patient — a minor — does not have full autonomy. The challenge for caregivers is that giving or withholding permission for interventions is a difficult role. “Their job is to protect the patient’s well-being while empowering them toward independence,” she said. “It can be hard for caregivers to understand complex information.” The challenge for clinicians, she continued, is to know when to invite SDM. This requires relational and sharp communication skills. “We must consider our patient’s/family’s health literacy and be sure the information we share is understood,” she said. “What are the social and structural determinants of health that are going to influence decision-making? You want to move into a relationship like this with cultural humility so you can understand what their preferences are and how they’re seeing the problem. Because there’s no universal agreement on the age at which minors should be deemed decision-making competent in health care, the approach is nuanced and depends on each individual patient and family.”
Dr. Cordoro proposed the following four-step approach to SDM to use in pediatric dermatology:
Step 1: Share relevant information about the condition and treatment options in a clear and understandable manner. The average US resident is at the seventh-to eighth-grade level, “so we have to avoid medical jargon and use plain language,” Dr. Cordoro said. Then, use the teach-back approach to assess their understanding. “Ask, ‘What is your understanding of the most important points that we talked about?’ Or, ‘Please share with me what you heard so I’m sure we all understand the plan.’ Using these techniques will reduce the barriers to care such as health literacy.”
Step 2: Solicit and understand patient/patient family perspectives, preferences and priorities. The goal here is to uncover their beliefs, concerns, and assumptions that may influence their decisions. “Be mindful of power asymmetry,” she noted. “Many families still believe the doctor is the boss and they are there to be told what to do. Be clear that the patient has a say. Talk directly to the patient about their interests if developmentally appropriate.”
Step 3: Invite patients/family into a shared decision-making conversation. Consider statements like, “There are many reasonable options here. Let’s work together to come up with the decision that’s right for you.” Or, “Let’s start by exploring your specific goals and concerns. As you think about the options I just talked to you about, what’s important to you?” Or, “Do you want to think about this decision with anyone else?”
Step 4: Check back in frequently. Pause between significant points and check in. “See how they’re doing during the conversation,” she said. “At future appointments, remember to solicit their input on additional decisions.”
In Dr. Cordoro’s opinion, one potential pitfall of SDM is an over-reliance on patient decision aids. “Very few are available in dermatology,” she said. “Some are relevant but none specifically to pediatric dermatology. They are often complex and require a high reading comprehension level. This disadvantages patients and families with low health literacy. Keep it clear and simple. Your patients will appreciate it.”
Dr. Cordoro reported having no relevant disclosures.
SAN DIEGO — In the clinical experience of Kelly M. Cordoro, MD, .
“SDM is a cornerstone of person-centered care,” Dr. Cordoro, professor of dermatology and pediatrics at the University of California, San Francisco, said at the Society for Pediatric Dermatology meeting, held in advance of the annual meeting of the American Academy of Dermatology. “We do it all the time. It can be patient-led, clinician-led, or a patient/family dyad approach. If we do it well, it can improve outcomes. Patients report more satisfying interactions with their care team. It brings adolescent patients especially a sense of independence and they adapt faster to their illness.”
Conditions such as acne, psoriasis, and atopic dermatitis have multiple treatment options, often without a single best choice. The ideal treatment depends on disease characteristics (extent, sites affected, symptoms, and natural history), the patient (age, comorbidities, overall disease burden), therapies (safety, efficacy, duration, and adverse events), and preferences (logistics, time, shots vs. pills, etc.). “These factors vary between patients and within the same patient over time, and at each step along the course of the condition, SDM approaches are relevant,” she said.
AHRQ’s Five-Step Approach
The Agency for Healthcare Research and Quality developed a five-step approach to SDM known as SHARE: Seek your patient’s participation; Help your patient explore and compare treatment options; Assess your patient’s values and preferences; Reach a decision with your patient, and Evaluate your patient’s decision. “We do this all the time in practice with adult patients, but may not label it as SDM,” said Dr. Cordoro, chief and fellowship director of pediatric dermatology at UCSF.
“Where it gets a little murkier is in pediatric decision-making, which is a complex type of surrogate decision-making.” In this situation the patient — a minor — does not have full autonomy. The challenge for caregivers is that giving or withholding permission for interventions is a difficult role. “Their job is to protect the patient’s well-being while empowering them toward independence,” she said. “It can be hard for caregivers to understand complex information.” The challenge for clinicians, she continued, is to know when to invite SDM. This requires relational and sharp communication skills. “We must consider our patient’s/family’s health literacy and be sure the information we share is understood,” she said. “What are the social and structural determinants of health that are going to influence decision-making? You want to move into a relationship like this with cultural humility so you can understand what their preferences are and how they’re seeing the problem. Because there’s no universal agreement on the age at which minors should be deemed decision-making competent in health care, the approach is nuanced and depends on each individual patient and family.”
Dr. Cordoro proposed the following four-step approach to SDM to use in pediatric dermatology:
Step 1: Share relevant information about the condition and treatment options in a clear and understandable manner. The average US resident is at the seventh-to eighth-grade level, “so we have to avoid medical jargon and use plain language,” Dr. Cordoro said. Then, use the teach-back approach to assess their understanding. “Ask, ‘What is your understanding of the most important points that we talked about?’ Or, ‘Please share with me what you heard so I’m sure we all understand the plan.’ Using these techniques will reduce the barriers to care such as health literacy.”
Step 2: Solicit and understand patient/patient family perspectives, preferences and priorities. The goal here is to uncover their beliefs, concerns, and assumptions that may influence their decisions. “Be mindful of power asymmetry,” she noted. “Many families still believe the doctor is the boss and they are there to be told what to do. Be clear that the patient has a say. Talk directly to the patient about their interests if developmentally appropriate.”
Step 3: Invite patients/family into a shared decision-making conversation. Consider statements like, “There are many reasonable options here. Let’s work together to come up with the decision that’s right for you.” Or, “Let’s start by exploring your specific goals and concerns. As you think about the options I just talked to you about, what’s important to you?” Or, “Do you want to think about this decision with anyone else?”
Step 4: Check back in frequently. Pause between significant points and check in. “See how they’re doing during the conversation,” she said. “At future appointments, remember to solicit their input on additional decisions.”
In Dr. Cordoro’s opinion, one potential pitfall of SDM is an over-reliance on patient decision aids. “Very few are available in dermatology,” she said. “Some are relevant but none specifically to pediatric dermatology. They are often complex and require a high reading comprehension level. This disadvantages patients and families with low health literacy. Keep it clear and simple. Your patients will appreciate it.”
Dr. Cordoro reported having no relevant disclosures.
SAN DIEGO — In the clinical experience of Kelly M. Cordoro, MD, .
“SDM is a cornerstone of person-centered care,” Dr. Cordoro, professor of dermatology and pediatrics at the University of California, San Francisco, said at the Society for Pediatric Dermatology meeting, held in advance of the annual meeting of the American Academy of Dermatology. “We do it all the time. It can be patient-led, clinician-led, or a patient/family dyad approach. If we do it well, it can improve outcomes. Patients report more satisfying interactions with their care team. It brings adolescent patients especially a sense of independence and they adapt faster to their illness.”
Conditions such as acne, psoriasis, and atopic dermatitis have multiple treatment options, often without a single best choice. The ideal treatment depends on disease characteristics (extent, sites affected, symptoms, and natural history), the patient (age, comorbidities, overall disease burden), therapies (safety, efficacy, duration, and adverse events), and preferences (logistics, time, shots vs. pills, etc.). “These factors vary between patients and within the same patient over time, and at each step along the course of the condition, SDM approaches are relevant,” she said.
AHRQ’s Five-Step Approach
The Agency for Healthcare Research and Quality developed a five-step approach to SDM known as SHARE: Seek your patient’s participation; Help your patient explore and compare treatment options; Assess your patient’s values and preferences; Reach a decision with your patient, and Evaluate your patient’s decision. “We do this all the time in practice with adult patients, but may not label it as SDM,” said Dr. Cordoro, chief and fellowship director of pediatric dermatology at UCSF.
“Where it gets a little murkier is in pediatric decision-making, which is a complex type of surrogate decision-making.” In this situation the patient — a minor — does not have full autonomy. The challenge for caregivers is that giving or withholding permission for interventions is a difficult role. “Their job is to protect the patient’s well-being while empowering them toward independence,” she said. “It can be hard for caregivers to understand complex information.” The challenge for clinicians, she continued, is to know when to invite SDM. This requires relational and sharp communication skills. “We must consider our patient’s/family’s health literacy and be sure the information we share is understood,” she said. “What are the social and structural determinants of health that are going to influence decision-making? You want to move into a relationship like this with cultural humility so you can understand what their preferences are and how they’re seeing the problem. Because there’s no universal agreement on the age at which minors should be deemed decision-making competent in health care, the approach is nuanced and depends on each individual patient and family.”
Dr. Cordoro proposed the following four-step approach to SDM to use in pediatric dermatology:
Step 1: Share relevant information about the condition and treatment options in a clear and understandable manner. The average US resident is at the seventh-to eighth-grade level, “so we have to avoid medical jargon and use plain language,” Dr. Cordoro said. Then, use the teach-back approach to assess their understanding. “Ask, ‘What is your understanding of the most important points that we talked about?’ Or, ‘Please share with me what you heard so I’m sure we all understand the plan.’ Using these techniques will reduce the barriers to care such as health literacy.”
Step 2: Solicit and understand patient/patient family perspectives, preferences and priorities. The goal here is to uncover their beliefs, concerns, and assumptions that may influence their decisions. “Be mindful of power asymmetry,” she noted. “Many families still believe the doctor is the boss and they are there to be told what to do. Be clear that the patient has a say. Talk directly to the patient about their interests if developmentally appropriate.”
Step 3: Invite patients/family into a shared decision-making conversation. Consider statements like, “There are many reasonable options here. Let’s work together to come up with the decision that’s right for you.” Or, “Let’s start by exploring your specific goals and concerns. As you think about the options I just talked to you about, what’s important to you?” Or, “Do you want to think about this decision with anyone else?”
Step 4: Check back in frequently. Pause between significant points and check in. “See how they’re doing during the conversation,” she said. “At future appointments, remember to solicit their input on additional decisions.”
In Dr. Cordoro’s opinion, one potential pitfall of SDM is an over-reliance on patient decision aids. “Very few are available in dermatology,” she said. “Some are relevant but none specifically to pediatric dermatology. They are often complex and require a high reading comprehension level. This disadvantages patients and families with low health literacy. Keep it clear and simple. Your patients will appreciate it.”
Dr. Cordoro reported having no relevant disclosures.
FROM AAD 2024
FDA Requests More Information for RDEB Rx Under Review
The Food and Drug Administration (RDEB), requesting more information from the manufacturer.
Pz-cel, which comprises autologous, COL7A1 gene–corrected epidermal sheets, is being evaluated for its ability to enable normal type VII collagen expression in a patient’s skin cells and to facilitate wound healing and pain reduction in wounds in patients with RDEB after a one-time application procedure. The cause of RDEB is a defect in the COL7A1 gene that “results in the inability to produce type VII collagen,” a press release from the manufacturer noted.
On April 22, 2024, the manufacturer Abeona Therapeutics announced that following a meeting with the FDA in March and in a subsequent request for information, the agency requires additional information to satisfy certain Chemistry Manufacturing and Controls requirements before the BLA for pz-cel can be approved. According to a press release from the company, the information pertains to validation requirements for certain manufacturing and release testing methods, including some that were observed during the FDA’s pre-licensing inspection.
The complete response letter did not identify any issues related to the clinical efficacy or safety data in the BLA, and the FDA did not request any new clinical trials or clinical data to support approval, according to the company.
The company anticipates completing the BLA resubmission in the third quarter of 2024. The application is supported by clinical efficacy and safety data from the pivotal phase 3 VIITAL study and a phase 1/2a study in patients with RDEB.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (RDEB), requesting more information from the manufacturer.
Pz-cel, which comprises autologous, COL7A1 gene–corrected epidermal sheets, is being evaluated for its ability to enable normal type VII collagen expression in a patient’s skin cells and to facilitate wound healing and pain reduction in wounds in patients with RDEB after a one-time application procedure. The cause of RDEB is a defect in the COL7A1 gene that “results in the inability to produce type VII collagen,” a press release from the manufacturer noted.
On April 22, 2024, the manufacturer Abeona Therapeutics announced that following a meeting with the FDA in March and in a subsequent request for information, the agency requires additional information to satisfy certain Chemistry Manufacturing and Controls requirements before the BLA for pz-cel can be approved. According to a press release from the company, the information pertains to validation requirements for certain manufacturing and release testing methods, including some that were observed during the FDA’s pre-licensing inspection.
The complete response letter did not identify any issues related to the clinical efficacy or safety data in the BLA, and the FDA did not request any new clinical trials or clinical data to support approval, according to the company.
The company anticipates completing the BLA resubmission in the third quarter of 2024. The application is supported by clinical efficacy and safety data from the pivotal phase 3 VIITAL study and a phase 1/2a study in patients with RDEB.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (RDEB), requesting more information from the manufacturer.
Pz-cel, which comprises autologous, COL7A1 gene–corrected epidermal sheets, is being evaluated for its ability to enable normal type VII collagen expression in a patient’s skin cells and to facilitate wound healing and pain reduction in wounds in patients with RDEB after a one-time application procedure. The cause of RDEB is a defect in the COL7A1 gene that “results in the inability to produce type VII collagen,” a press release from the manufacturer noted.
On April 22, 2024, the manufacturer Abeona Therapeutics announced that following a meeting with the FDA in March and in a subsequent request for information, the agency requires additional information to satisfy certain Chemistry Manufacturing and Controls requirements before the BLA for pz-cel can be approved. According to a press release from the company, the information pertains to validation requirements for certain manufacturing and release testing methods, including some that were observed during the FDA’s pre-licensing inspection.
The complete response letter did not identify any issues related to the clinical efficacy or safety data in the BLA, and the FDA did not request any new clinical trials or clinical data to support approval, according to the company.
The company anticipates completing the BLA resubmission in the third quarter of 2024. The application is supported by clinical efficacy and safety data from the pivotal phase 3 VIITAL study and a phase 1/2a study in patients with RDEB.
A version of this article first appeared on Medscape.com.