User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Study explores gender differences in pediatric melanoma
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
Ruxolitinib found to benefit adolescents with vitiligo up to one year
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
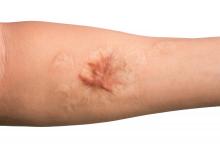
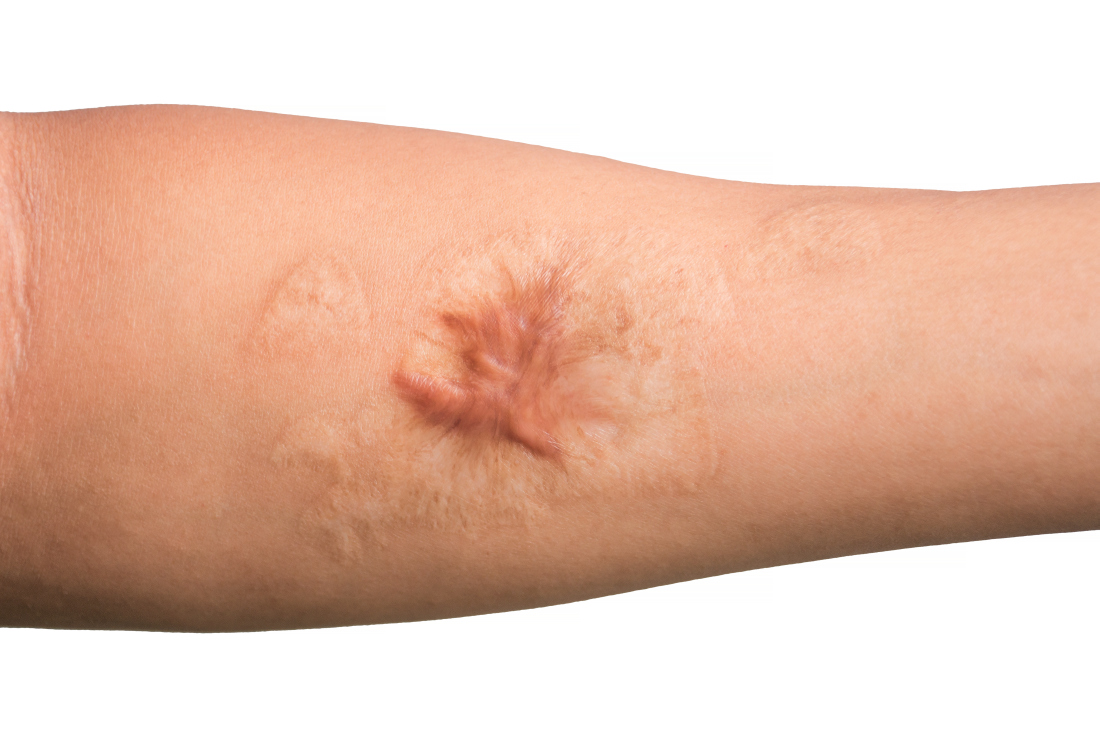
Combo of excision, cryosurgery found to benefit keloid scar outcomes
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
FROM JAMA DERMATOLOGY
Study provides consensus on lab monitoring during isotretinoin therapy
For generally ideally within a month prior to the start of treatment, and a second time at peak dose.
Other tests such as complete blood cell counts and basic metabolic panels as well as specific laboratory tests such as LDL and HDL cholesterol should not be routinely monitored.
Those are key conclusions from a Delphi consensus study that included 22 acne experts from five continents and was published in JAMA Dermatology.
“Our results apply findings from recent literature and are in accordance with recent studies that have recommended against excessive laboratory monitoring,” senior corresponding author Arash Mostaghimi, MD, MPA, MPH, and coauthors wrote. “For instance, several studies in both teenagers and adults have shown that routine complete blood cell count laboratory tests are unnecessary without suspicion of an underlying abnormality and that rare abnormalities, if present, either resolved or remained stable without clinical impact on treatment. Likewise, liver function tests and lipid panels ordered at baseline and after 2 months of therapy were deemed sufficient if the clinical context and results do not suggest potential abnormalities.”
The authors also noted that, while published acne management guidelines exist, such as the American Academy of Dermatology work group guidelines and the National Institute for Health and Care Excellence guideline, “the specific recommendations surrounding laboratory monitoring frequency are nonstandardized and often nonspecific.”
To establish a consensus for isotretinoin laboratory monitoring, Dr. Mostaghimi, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to administer four rounds of electronic surveys to 22 board-certified dermatologists between 2021 and 2022. The primary outcome measured was whether participants could reach consensus on key isotretinoin lab monitoring parameters. Responses that failed to reach a threshold of 70% indicated no consensus.
The surveyed dermatologists had been in practice for a mean of 23.7 years, 54.5% were female, 54.5% practiced in an academic setting, and 63.9% were based in North America. They reached consensus for checking ALT within a month prior to initiation (89.5%) and at peak dose (89.5%), but not checking monthly (76.2%) or after completing treatment (73.7%). They also reached consensus on checking triglycerides within a month prior to initiation (89.5%) and at peak dose (78.9%) but not to check monthly (84.2%) or after completing treatment (73.7%).
Meanwhile, consensus was achieved for not checking complete blood cell count or basic metabolic panel parameters at any point during isotretinoin treatment (all > 70%), as well as not checking gamma-glutamyl transferase (78.9%), bilirubin (81.0%), albumin (72.7%), total protein (72.7%), LDL cholesterol (73.7%), HDL cholesterol (73.7%), or C-reactive protein (77.3%).
“Additional research is required to determine best practices for laboratory measures that did not reach consensus,” the authors wrote. The study results “are intended to guide appropriate clinical decision-making,” they added. “Although our recommendations cannot replace clinical judgment based on the unique circumstances of individual patients, we believe they provide a framework for management of a typical, otherwise healthy patient being treated with isotretinoin for acne. More routine monitoring, or reduced monitoring, should be considered on a case-by-case basis accounting for the unique medical history, circumstances, and baseline abnormalities, if present, of each patient.”
“Practicing dermatologists, including myself, routinely check blood laboratory values during isotretinoin treatment,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “Even though just a small number of U.S.-based and international acne researchers were involved in this Delphi consensus statement, this article still makes us practicing clinicians feel more comfortable in checking fewer lab chemistries and also less frequently checking labs when we use isotretinoin.
“That said, I don’t think most of us are ready, because of legal reasons, to do that infrequent monitoring” during isotretinoin therapy, Dr. Green added. “I think most dermatologists do not routinely perform CBCs anymore, but we still feel obligated to check triglycerides and liver function more frequently” than recommended in the new study.
Dr. Mostaghimi reported receiving grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees and licensing from Concert, personal fees from Bioniz, holds equity and advisory board membership from Hims & Hers and Figure 1, personal fees from Digital Diagnostics, and personal fees from AbbVie outside the submitted work. Other authors reported serving as an adviser, a speaker consultant, investigator, and/or board member, or having received honoraria from different pharmaceutical companies; several authors had no disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For generally ideally within a month prior to the start of treatment, and a second time at peak dose.
Other tests such as complete blood cell counts and basic metabolic panels as well as specific laboratory tests such as LDL and HDL cholesterol should not be routinely monitored.
Those are key conclusions from a Delphi consensus study that included 22 acne experts from five continents and was published in JAMA Dermatology.
“Our results apply findings from recent literature and are in accordance with recent studies that have recommended against excessive laboratory monitoring,” senior corresponding author Arash Mostaghimi, MD, MPA, MPH, and coauthors wrote. “For instance, several studies in both teenagers and adults have shown that routine complete blood cell count laboratory tests are unnecessary without suspicion of an underlying abnormality and that rare abnormalities, if present, either resolved or remained stable without clinical impact on treatment. Likewise, liver function tests and lipid panels ordered at baseline and after 2 months of therapy were deemed sufficient if the clinical context and results do not suggest potential abnormalities.”
The authors also noted that, while published acne management guidelines exist, such as the American Academy of Dermatology work group guidelines and the National Institute for Health and Care Excellence guideline, “the specific recommendations surrounding laboratory monitoring frequency are nonstandardized and often nonspecific.”
To establish a consensus for isotretinoin laboratory monitoring, Dr. Mostaghimi, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to administer four rounds of electronic surveys to 22 board-certified dermatologists between 2021 and 2022. The primary outcome measured was whether participants could reach consensus on key isotretinoin lab monitoring parameters. Responses that failed to reach a threshold of 70% indicated no consensus.
The surveyed dermatologists had been in practice for a mean of 23.7 years, 54.5% were female, 54.5% practiced in an academic setting, and 63.9% were based in North America. They reached consensus for checking ALT within a month prior to initiation (89.5%) and at peak dose (89.5%), but not checking monthly (76.2%) or after completing treatment (73.7%). They also reached consensus on checking triglycerides within a month prior to initiation (89.5%) and at peak dose (78.9%) but not to check monthly (84.2%) or after completing treatment (73.7%).
Meanwhile, consensus was achieved for not checking complete blood cell count or basic metabolic panel parameters at any point during isotretinoin treatment (all > 70%), as well as not checking gamma-glutamyl transferase (78.9%), bilirubin (81.0%), albumin (72.7%), total protein (72.7%), LDL cholesterol (73.7%), HDL cholesterol (73.7%), or C-reactive protein (77.3%).
“Additional research is required to determine best practices for laboratory measures that did not reach consensus,” the authors wrote. The study results “are intended to guide appropriate clinical decision-making,” they added. “Although our recommendations cannot replace clinical judgment based on the unique circumstances of individual patients, we believe they provide a framework for management of a typical, otherwise healthy patient being treated with isotretinoin for acne. More routine monitoring, or reduced monitoring, should be considered on a case-by-case basis accounting for the unique medical history, circumstances, and baseline abnormalities, if present, of each patient.”
“Practicing dermatologists, including myself, routinely check blood laboratory values during isotretinoin treatment,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “Even though just a small number of U.S.-based and international acne researchers were involved in this Delphi consensus statement, this article still makes us practicing clinicians feel more comfortable in checking fewer lab chemistries and also less frequently checking labs when we use isotretinoin.
“That said, I don’t think most of us are ready, because of legal reasons, to do that infrequent monitoring” during isotretinoin therapy, Dr. Green added. “I think most dermatologists do not routinely perform CBCs anymore, but we still feel obligated to check triglycerides and liver function more frequently” than recommended in the new study.
Dr. Mostaghimi reported receiving grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees and licensing from Concert, personal fees from Bioniz, holds equity and advisory board membership from Hims & Hers and Figure 1, personal fees from Digital Diagnostics, and personal fees from AbbVie outside the submitted work. Other authors reported serving as an adviser, a speaker consultant, investigator, and/or board member, or having received honoraria from different pharmaceutical companies; several authors had no disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For generally ideally within a month prior to the start of treatment, and a second time at peak dose.
Other tests such as complete blood cell counts and basic metabolic panels as well as specific laboratory tests such as LDL and HDL cholesterol should not be routinely monitored.
Those are key conclusions from a Delphi consensus study that included 22 acne experts from five continents and was published in JAMA Dermatology.
“Our results apply findings from recent literature and are in accordance with recent studies that have recommended against excessive laboratory monitoring,” senior corresponding author Arash Mostaghimi, MD, MPA, MPH, and coauthors wrote. “For instance, several studies in both teenagers and adults have shown that routine complete blood cell count laboratory tests are unnecessary without suspicion of an underlying abnormality and that rare abnormalities, if present, either resolved or remained stable without clinical impact on treatment. Likewise, liver function tests and lipid panels ordered at baseline and after 2 months of therapy were deemed sufficient if the clinical context and results do not suggest potential abnormalities.”
The authors also noted that, while published acne management guidelines exist, such as the American Academy of Dermatology work group guidelines and the National Institute for Health and Care Excellence guideline, “the specific recommendations surrounding laboratory monitoring frequency are nonstandardized and often nonspecific.”
To establish a consensus for isotretinoin laboratory monitoring, Dr. Mostaghimi, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to administer four rounds of electronic surveys to 22 board-certified dermatologists between 2021 and 2022. The primary outcome measured was whether participants could reach consensus on key isotretinoin lab monitoring parameters. Responses that failed to reach a threshold of 70% indicated no consensus.
The surveyed dermatologists had been in practice for a mean of 23.7 years, 54.5% were female, 54.5% practiced in an academic setting, and 63.9% were based in North America. They reached consensus for checking ALT within a month prior to initiation (89.5%) and at peak dose (89.5%), but not checking monthly (76.2%) or after completing treatment (73.7%). They also reached consensus on checking triglycerides within a month prior to initiation (89.5%) and at peak dose (78.9%) but not to check monthly (84.2%) or after completing treatment (73.7%).
Meanwhile, consensus was achieved for not checking complete blood cell count or basic metabolic panel parameters at any point during isotretinoin treatment (all > 70%), as well as not checking gamma-glutamyl transferase (78.9%), bilirubin (81.0%), albumin (72.7%), total protein (72.7%), LDL cholesterol (73.7%), HDL cholesterol (73.7%), or C-reactive protein (77.3%).
“Additional research is required to determine best practices for laboratory measures that did not reach consensus,” the authors wrote. The study results “are intended to guide appropriate clinical decision-making,” they added. “Although our recommendations cannot replace clinical judgment based on the unique circumstances of individual patients, we believe they provide a framework for management of a typical, otherwise healthy patient being treated with isotretinoin for acne. More routine monitoring, or reduced monitoring, should be considered on a case-by-case basis accounting for the unique medical history, circumstances, and baseline abnormalities, if present, of each patient.”
“Practicing dermatologists, including myself, routinely check blood laboratory values during isotretinoin treatment,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “Even though just a small number of U.S.-based and international acne researchers were involved in this Delphi consensus statement, this article still makes us practicing clinicians feel more comfortable in checking fewer lab chemistries and also less frequently checking labs when we use isotretinoin.
“That said, I don’t think most of us are ready, because of legal reasons, to do that infrequent monitoring” during isotretinoin therapy, Dr. Green added. “I think most dermatologists do not routinely perform CBCs anymore, but we still feel obligated to check triglycerides and liver function more frequently” than recommended in the new study.
Dr. Mostaghimi reported receiving grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees and licensing from Concert, personal fees from Bioniz, holds equity and advisory board membership from Hims & Hers and Figure 1, personal fees from Digital Diagnostics, and personal fees from AbbVie outside the submitted work. Other authors reported serving as an adviser, a speaker consultant, investigator, and/or board member, or having received honoraria from different pharmaceutical companies; several authors had no disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM JAMA DERMATOLOGY
Current monkeypox outbreak marked by unconventional spread, clinical features
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
Using the skin to probe within: the promise of intradermal microdialysis
SAN DIEGO – When Lacy M. Alexander, PhD, began her career as a kinesiology researcher, she focused on the skin as a model of circulation for examining mechanisms of vascular function and dysfunction in diseases, as well as the influence of drug interventions.
“The skin is an accessible circulation; we see many of the same neural and endothelial pathways mediating vasodilation that we see throughout the entire vascular system,” Dr. Alexander, professor of kinesiology at Penn State University’s College of Health and Human Development, University Park, Pa., said during a lecture at the annual meeting of the American College for Sports Medicine.
The main . “This catheter has the ability to perfuse or infuse various substances into a bidirectional membrane,” Dr. Alexander explained. “This enables us to recover various substances into that catheter. We pair this with a measurement of skin blood flow with Doppler probes as well as the ability to control local temperature to either isolate reflex control of the human cutaneous circulation or elicit local changes in temperature to elicit vasodilation and vasoconstriction.”
In a 2005 article on evaluating the microcirculation in vascular disease, microvascular dysfunction is described as “a systemic disease process that occurs in a similar fashion in multiple tissue beds throughout the body”. Therefore, early identification of the mechanism leading to microvascular dysfunction is important, Dr. Alexander said. “We can also monitor the progression of disease and the progression of a given treatment if we have a noninvasive way to do that.”
A question that clinicians often ask Dr. Alexander is, do changes in forearm skin blood flow represent changes in, say, leg skin blood flow, or blood flow in other parts of the body? “The answer to that question is yes; we see similar changes in forearm skin that we see in other regions of the body,” she said. “We tend to use the forearm skin because there’s less UV damage, especially when we’re looking at questions related to human aging.”
Over the years, she and other investigators have used intradermal microdialysis to develop and refine many skin-specific approaches for examining endothelial function, including reactive hyperemia, direct drug delivery, local heating, whole body heating, local cooling, whole body cooling, and spectral analysis. For example, using this approach in the direct drug delivery realm, researchers have discovered that oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolemic middle-aged adults.
In more recent work using intradermal microdialysis, researchers have observed that peripheral microvascular function is impaired in adults with hypertension.
Another study using intradermal microdialysis found that 16 weeks of a sulfhydryl intervention improved endothelial function through nitric oxide and hydrogen sulfide-dependent mechanisms in adults with hypertension.
The technology has also helped to further understanding of nontraditional risk factors of microvascular dysfunction. In one study, researchers found that endothelium-dependent vasodilation is blunted in adults with major depressive disorder due to a reduced functional contribution of nitric oxide.
According to Dr. Alexander, a study being reviewed for publication found that microvascular endothelial function is impaired in women with endometriosis.
Also in 2019, a European Academy of Allergy and Clinical Immunology (EAACI) position paper on the use of intradermal microdialysis in investigations of the pathogenesis of chronic inflammatory skin diseases was published.
The broad focus of Dr. Alexander’s current projects includes examining the roles of arginase in nitric oxide synthase uncoupling in human vasculature with hypercholesterolemia and hypertension; inflammation-induced alteration in vasodilatory signaling with essential hypertension; the role of reactive oxygen species in altering vasoconstriction and vascular remodeling with hypertension, and the effects of common platelet inhibitors on microvascular function in human skin as they relate to basic mechanisms of skin blood flow and functional thermoregulatory outcomes. “The hope is that we can intervene with things like dietary and exercise interventions early on to mitigate this progression to avert cardiovascular disease,” she said.
Dr. Alexander disclosed that she has received research support from the National Heart, Lung, and Blood Institute, the National Dairy Council, the American College of Sports Medicine, and the American Heart Association.
SAN DIEGO – When Lacy M. Alexander, PhD, began her career as a kinesiology researcher, she focused on the skin as a model of circulation for examining mechanisms of vascular function and dysfunction in diseases, as well as the influence of drug interventions.
“The skin is an accessible circulation; we see many of the same neural and endothelial pathways mediating vasodilation that we see throughout the entire vascular system,” Dr. Alexander, professor of kinesiology at Penn State University’s College of Health and Human Development, University Park, Pa., said during a lecture at the annual meeting of the American College for Sports Medicine.
The main . “This catheter has the ability to perfuse or infuse various substances into a bidirectional membrane,” Dr. Alexander explained. “This enables us to recover various substances into that catheter. We pair this with a measurement of skin blood flow with Doppler probes as well as the ability to control local temperature to either isolate reflex control of the human cutaneous circulation or elicit local changes in temperature to elicit vasodilation and vasoconstriction.”
In a 2005 article on evaluating the microcirculation in vascular disease, microvascular dysfunction is described as “a systemic disease process that occurs in a similar fashion in multiple tissue beds throughout the body”. Therefore, early identification of the mechanism leading to microvascular dysfunction is important, Dr. Alexander said. “We can also monitor the progression of disease and the progression of a given treatment if we have a noninvasive way to do that.”
A question that clinicians often ask Dr. Alexander is, do changes in forearm skin blood flow represent changes in, say, leg skin blood flow, or blood flow in other parts of the body? “The answer to that question is yes; we see similar changes in forearm skin that we see in other regions of the body,” she said. “We tend to use the forearm skin because there’s less UV damage, especially when we’re looking at questions related to human aging.”
Over the years, she and other investigators have used intradermal microdialysis to develop and refine many skin-specific approaches for examining endothelial function, including reactive hyperemia, direct drug delivery, local heating, whole body heating, local cooling, whole body cooling, and spectral analysis. For example, using this approach in the direct drug delivery realm, researchers have discovered that oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolemic middle-aged adults.
In more recent work using intradermal microdialysis, researchers have observed that peripheral microvascular function is impaired in adults with hypertension.
Another study using intradermal microdialysis found that 16 weeks of a sulfhydryl intervention improved endothelial function through nitric oxide and hydrogen sulfide-dependent mechanisms in adults with hypertension.
The technology has also helped to further understanding of nontraditional risk factors of microvascular dysfunction. In one study, researchers found that endothelium-dependent vasodilation is blunted in adults with major depressive disorder due to a reduced functional contribution of nitric oxide.
According to Dr. Alexander, a study being reviewed for publication found that microvascular endothelial function is impaired in women with endometriosis.
Also in 2019, a European Academy of Allergy and Clinical Immunology (EAACI) position paper on the use of intradermal microdialysis in investigations of the pathogenesis of chronic inflammatory skin diseases was published.
The broad focus of Dr. Alexander’s current projects includes examining the roles of arginase in nitric oxide synthase uncoupling in human vasculature with hypercholesterolemia and hypertension; inflammation-induced alteration in vasodilatory signaling with essential hypertension; the role of reactive oxygen species in altering vasoconstriction and vascular remodeling with hypertension, and the effects of common platelet inhibitors on microvascular function in human skin as they relate to basic mechanisms of skin blood flow and functional thermoregulatory outcomes. “The hope is that we can intervene with things like dietary and exercise interventions early on to mitigate this progression to avert cardiovascular disease,” she said.
Dr. Alexander disclosed that she has received research support from the National Heart, Lung, and Blood Institute, the National Dairy Council, the American College of Sports Medicine, and the American Heart Association.
SAN DIEGO – When Lacy M. Alexander, PhD, began her career as a kinesiology researcher, she focused on the skin as a model of circulation for examining mechanisms of vascular function and dysfunction in diseases, as well as the influence of drug interventions.
“The skin is an accessible circulation; we see many of the same neural and endothelial pathways mediating vasodilation that we see throughout the entire vascular system,” Dr. Alexander, professor of kinesiology at Penn State University’s College of Health and Human Development, University Park, Pa., said during a lecture at the annual meeting of the American College for Sports Medicine.
The main . “This catheter has the ability to perfuse or infuse various substances into a bidirectional membrane,” Dr. Alexander explained. “This enables us to recover various substances into that catheter. We pair this with a measurement of skin blood flow with Doppler probes as well as the ability to control local temperature to either isolate reflex control of the human cutaneous circulation or elicit local changes in temperature to elicit vasodilation and vasoconstriction.”
In a 2005 article on evaluating the microcirculation in vascular disease, microvascular dysfunction is described as “a systemic disease process that occurs in a similar fashion in multiple tissue beds throughout the body”. Therefore, early identification of the mechanism leading to microvascular dysfunction is important, Dr. Alexander said. “We can also monitor the progression of disease and the progression of a given treatment if we have a noninvasive way to do that.”
A question that clinicians often ask Dr. Alexander is, do changes in forearm skin blood flow represent changes in, say, leg skin blood flow, or blood flow in other parts of the body? “The answer to that question is yes; we see similar changes in forearm skin that we see in other regions of the body,” she said. “We tend to use the forearm skin because there’s less UV damage, especially when we’re looking at questions related to human aging.”
Over the years, she and other investigators have used intradermal microdialysis to develop and refine many skin-specific approaches for examining endothelial function, including reactive hyperemia, direct drug delivery, local heating, whole body heating, local cooling, whole body cooling, and spectral analysis. For example, using this approach in the direct drug delivery realm, researchers have discovered that oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolemic middle-aged adults.
In more recent work using intradermal microdialysis, researchers have observed that peripheral microvascular function is impaired in adults with hypertension.
Another study using intradermal microdialysis found that 16 weeks of a sulfhydryl intervention improved endothelial function through nitric oxide and hydrogen sulfide-dependent mechanisms in adults with hypertension.
The technology has also helped to further understanding of nontraditional risk factors of microvascular dysfunction. In one study, researchers found that endothelium-dependent vasodilation is blunted in adults with major depressive disorder due to a reduced functional contribution of nitric oxide.
According to Dr. Alexander, a study being reviewed for publication found that microvascular endothelial function is impaired in women with endometriosis.
Also in 2019, a European Academy of Allergy and Clinical Immunology (EAACI) position paper on the use of intradermal microdialysis in investigations of the pathogenesis of chronic inflammatory skin diseases was published.
The broad focus of Dr. Alexander’s current projects includes examining the roles of arginase in nitric oxide synthase uncoupling in human vasculature with hypercholesterolemia and hypertension; inflammation-induced alteration in vasodilatory signaling with essential hypertension; the role of reactive oxygen species in altering vasoconstriction and vascular remodeling with hypertension, and the effects of common platelet inhibitors on microvascular function in human skin as they relate to basic mechanisms of skin blood flow and functional thermoregulatory outcomes. “The hope is that we can intervene with things like dietary and exercise interventions early on to mitigate this progression to avert cardiovascular disease,” she said.
Dr. Alexander disclosed that she has received research support from the National Heart, Lung, and Blood Institute, the National Dairy Council, the American College of Sports Medicine, and the American Heart Association.
AT ACSM 2022
FDA approves dupilumab for children with eczema aged 6 months to 5 years
The whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The approval, announced on June 7, 2022, makes dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, the first biologic available in the United States to treat uncontrolled moderate to severe atopic dermatitis in this age group. In this age group, it is administered subcutaneously every 4 weeks. Dupilumab remains the only biologic treatment approved for patients aged 6 years and older for this indication.
Approval was based on data from a 16-week pivotal phase 3 trial that evaluated the efficacy and safety of dupilumab added to standard of care topical corticosteroids (TCS) in children aged 6 months to 5 years with uncontrolled moderate to severe atopic dermatitis. The trial’s principal investigator, Amy S. Paller, MD, professor and chair of dermatology at Northwestern University, Chicago, and colleagues, found that, at 16 weeks, 28% of patients who were treated with dupilumab, added to low-potency TCS, met the primary endpoint of clear or almost clear skin, compared with 4% of those who received low-potency TCS alone (P < .0001).
In addition, patients who received the combined treatment experienced a 70% average improvement in disease severity from baseline, compared with a 20% improvement among those in the TCS-only group (P < .0001). They also experienced a 49% improvement in itch, compared with a 2% improvement among their counterparts in the TCS-only group (P < .0001).
Outside of the United States, the study’s coprimary endpoint was achievement of 75% or greater improvement in overall disease severity. More than half of the patients who received combined treatment (53%) met this endpoint, compared with 11% in the TCS-only group (P < .0001), according to the company.
Safety results were generally consistent with the safety profile of dupilumab in atopic dermatitis for patients aged 6 years and older. The most common adverse events that were more commonly observed with dupilumab included conjunctivitis (5% vs 0% in the placebo group) and herpes viral infections (6% vs. 5% in the placebo group). Among those on dupilumab, ages 6 months to 5 years, hand,foot, and mouth disease was reported in 5% and skin papilloma were reported in 2%, but these cases did not lead to discontinuation of treatment, according to the company release.
A version of this article first appeared on Medscape.com.
The whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The approval, announced on June 7, 2022, makes dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, the first biologic available in the United States to treat uncontrolled moderate to severe atopic dermatitis in this age group. In this age group, it is administered subcutaneously every 4 weeks. Dupilumab remains the only biologic treatment approved for patients aged 6 years and older for this indication.
Approval was based on data from a 16-week pivotal phase 3 trial that evaluated the efficacy and safety of dupilumab added to standard of care topical corticosteroids (TCS) in children aged 6 months to 5 years with uncontrolled moderate to severe atopic dermatitis. The trial’s principal investigator, Amy S. Paller, MD, professor and chair of dermatology at Northwestern University, Chicago, and colleagues, found that, at 16 weeks, 28% of patients who were treated with dupilumab, added to low-potency TCS, met the primary endpoint of clear or almost clear skin, compared with 4% of those who received low-potency TCS alone (P < .0001).
In addition, patients who received the combined treatment experienced a 70% average improvement in disease severity from baseline, compared with a 20% improvement among those in the TCS-only group (P < .0001). They also experienced a 49% improvement in itch, compared with a 2% improvement among their counterparts in the TCS-only group (P < .0001).
Outside of the United States, the study’s coprimary endpoint was achievement of 75% or greater improvement in overall disease severity. More than half of the patients who received combined treatment (53%) met this endpoint, compared with 11% in the TCS-only group (P < .0001), according to the company.
Safety results were generally consistent with the safety profile of dupilumab in atopic dermatitis for patients aged 6 years and older. The most common adverse events that were more commonly observed with dupilumab included conjunctivitis (5% vs 0% in the placebo group) and herpes viral infections (6% vs. 5% in the placebo group). Among those on dupilumab, ages 6 months to 5 years, hand,foot, and mouth disease was reported in 5% and skin papilloma were reported in 2%, but these cases did not lead to discontinuation of treatment, according to the company release.
A version of this article first appeared on Medscape.com.
The whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The approval, announced on June 7, 2022, makes dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, the first biologic available in the United States to treat uncontrolled moderate to severe atopic dermatitis in this age group. In this age group, it is administered subcutaneously every 4 weeks. Dupilumab remains the only biologic treatment approved for patients aged 6 years and older for this indication.
Approval was based on data from a 16-week pivotal phase 3 trial that evaluated the efficacy and safety of dupilumab added to standard of care topical corticosteroids (TCS) in children aged 6 months to 5 years with uncontrolled moderate to severe atopic dermatitis. The trial’s principal investigator, Amy S. Paller, MD, professor and chair of dermatology at Northwestern University, Chicago, and colleagues, found that, at 16 weeks, 28% of patients who were treated with dupilumab, added to low-potency TCS, met the primary endpoint of clear or almost clear skin, compared with 4% of those who received low-potency TCS alone (P < .0001).
In addition, patients who received the combined treatment experienced a 70% average improvement in disease severity from baseline, compared with a 20% improvement among those in the TCS-only group (P < .0001). They also experienced a 49% improvement in itch, compared with a 2% improvement among their counterparts in the TCS-only group (P < .0001).
Outside of the United States, the study’s coprimary endpoint was achievement of 75% or greater improvement in overall disease severity. More than half of the patients who received combined treatment (53%) met this endpoint, compared with 11% in the TCS-only group (P < .0001), according to the company.
Safety results were generally consistent with the safety profile of dupilumab in atopic dermatitis for patients aged 6 years and older. The most common adverse events that were more commonly observed with dupilumab included conjunctivitis (5% vs 0% in the placebo group) and herpes viral infections (6% vs. 5% in the placebo group). Among those on dupilumab, ages 6 months to 5 years, hand,foot, and mouth disease was reported in 5% and skin papilloma were reported in 2%, but these cases did not lead to discontinuation of treatment, according to the company release.
A version of this article first appeared on Medscape.com.
Pending further study, caution recommended in treating vitiligo patients with lasers, IPL
SAN DIEGO – The .
Those are the preliminary conclusions from a systematic review and survey of experts that Albert Wolkerstorfer, MD, presented during a clinical abstract session at the annual conference of the American Society for Laser Medicine and Surgery.
According to Dr. Wolkerstorfer, a dermatologist at Amsterdam University Medical Center, clinicians are reluctant to perform laser/intense pulsed light (IPL) treatments in patients with vitiligo because of the absence of clear guidelines, so he and his colleagues set out to investigate the risks of laser/IPL-induced vitiligo in patients with vitiligo and to seek out international consensus on recommendations from experts. “There is hardly any literature about it and certainly no guidelines,” he pointed out.
Dr. Wolkerstorfer and his colleagues designed three consecutive studies: A systematic review of laser/IPL-induced vitiligo; an international survey among 14 vitiligo experts from 10 countries about the occurrence of laser‐induced vitiligo, and a Delphi technique aimed at establishing a broad consensus about recommendations for safe use of lasers in vitiligo patients. At the time of the meeting, the Delphi process was still being carried out, so he did not discuss that study.
For the systematic review, the researchers found 11,073 unique hits on PubMed, Embase, and CINAHL using the terms “vitiligo,” “depigmentation,” “hypopigmentation,” and “leukoderma.” Only six case reports of laser/IPL-induced vitiligo were included in the final analysis. Of these, three had de novo vitiligo and three had vitiligo/halo nevi. These cases included two that occurred following treatment of port wine stains with the 585-nm laser; one that occurred following treatment of dyspigmentation with IPL; one that occurred following treatment of hypertrichosis with the 1,064-nm laser, one that occurred following treatment of hypertrichosis with the 755-nm laser, and one case that occurred following treatment of melasma with the ablative laser.
For the international survey of 14 experts from 10 countries, respondents said they had 10,670 new face-to-face vitiligo consultations in the past year. They reported that 30 of the vitiligo cases (0.3%) were likely caused by laser/IPL. Of these 30 cases, 18 (60%) had de novo vitiligo.
Of these cases, vitiligo occurred most frequently after laser hair reduction (47%), followed by use of the fractional laser (17%), and the ablative laser (13%). The interval between laser/IPL treatment and onset of vitiligo was 0-4 weeks in 27% of cases and 4-12 weeks in 57% of cases. Direct complications such as blistering, crusting, and erosions occurred in 57% of cases.
“Our conclusion is that laser and IPL-induced vitiligo is a rare phenomenon, and it often affects patients without prior vitiligo, which was really a surprise to us,” Dr. Wolkerstorfer said. “Complications seem to increase the risk,” he added.
“Despite the apparently low risk, we recommend caution [in patients with vitiligo], especially with aggressive laser procedures,” he said. “We recommend using conservative settings, not to treat active vitiligo patients ... and to perform test spots prior to treating large areas.” But he characterized this recommendation as “totally preliminary” pending results of the Delphi technique aimed at building consensus about laser/IPL treatments in vitiligo.
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices in Alexandria, Va., said that as clinicians await results of the study’s Delphi consensus, current use of lasers and IPL in patients with vitiligo “is based on your clinical judgment and whether the vitiligo is active or inactive. If the patient has vitiligo and you’re doing laser hair removal in the armpit, they may get active lesions in that area, but they can cover it. So, they may take that as a ‘win’ with the risk. But if it can erupt in other areas, that’s a risk they must be willing to take.”
Dr. Wolkerstorfer disclosed that he has received grant or research funding from Lumenis, Novartis, and Avita Medical. He is an advisory board member for Incyte. Dr. Onwudiwe reported having no disclosures.
SAN DIEGO – The .
Those are the preliminary conclusions from a systematic review and survey of experts that Albert Wolkerstorfer, MD, presented during a clinical abstract session at the annual conference of the American Society for Laser Medicine and Surgery.
According to Dr. Wolkerstorfer, a dermatologist at Amsterdam University Medical Center, clinicians are reluctant to perform laser/intense pulsed light (IPL) treatments in patients with vitiligo because of the absence of clear guidelines, so he and his colleagues set out to investigate the risks of laser/IPL-induced vitiligo in patients with vitiligo and to seek out international consensus on recommendations from experts. “There is hardly any literature about it and certainly no guidelines,” he pointed out.
Dr. Wolkerstorfer and his colleagues designed three consecutive studies: A systematic review of laser/IPL-induced vitiligo; an international survey among 14 vitiligo experts from 10 countries about the occurrence of laser‐induced vitiligo, and a Delphi technique aimed at establishing a broad consensus about recommendations for safe use of lasers in vitiligo patients. At the time of the meeting, the Delphi process was still being carried out, so he did not discuss that study.
For the systematic review, the researchers found 11,073 unique hits on PubMed, Embase, and CINAHL using the terms “vitiligo,” “depigmentation,” “hypopigmentation,” and “leukoderma.” Only six case reports of laser/IPL-induced vitiligo were included in the final analysis. Of these, three had de novo vitiligo and three had vitiligo/halo nevi. These cases included two that occurred following treatment of port wine stains with the 585-nm laser; one that occurred following treatment of dyspigmentation with IPL; one that occurred following treatment of hypertrichosis with the 1,064-nm laser, one that occurred following treatment of hypertrichosis with the 755-nm laser, and one case that occurred following treatment of melasma with the ablative laser.
For the international survey of 14 experts from 10 countries, respondents said they had 10,670 new face-to-face vitiligo consultations in the past year. They reported that 30 of the vitiligo cases (0.3%) were likely caused by laser/IPL. Of these 30 cases, 18 (60%) had de novo vitiligo.
Of these cases, vitiligo occurred most frequently after laser hair reduction (47%), followed by use of the fractional laser (17%), and the ablative laser (13%). The interval between laser/IPL treatment and onset of vitiligo was 0-4 weeks in 27% of cases and 4-12 weeks in 57% of cases. Direct complications such as blistering, crusting, and erosions occurred in 57% of cases.
“Our conclusion is that laser and IPL-induced vitiligo is a rare phenomenon, and it often affects patients without prior vitiligo, which was really a surprise to us,” Dr. Wolkerstorfer said. “Complications seem to increase the risk,” he added.
“Despite the apparently low risk, we recommend caution [in patients with vitiligo], especially with aggressive laser procedures,” he said. “We recommend using conservative settings, not to treat active vitiligo patients ... and to perform test spots prior to treating large areas.” But he characterized this recommendation as “totally preliminary” pending results of the Delphi technique aimed at building consensus about laser/IPL treatments in vitiligo.
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices in Alexandria, Va., said that as clinicians await results of the study’s Delphi consensus, current use of lasers and IPL in patients with vitiligo “is based on your clinical judgment and whether the vitiligo is active or inactive. If the patient has vitiligo and you’re doing laser hair removal in the armpit, they may get active lesions in that area, but they can cover it. So, they may take that as a ‘win’ with the risk. But if it can erupt in other areas, that’s a risk they must be willing to take.”
Dr. Wolkerstorfer disclosed that he has received grant or research funding from Lumenis, Novartis, and Avita Medical. He is an advisory board member for Incyte. Dr. Onwudiwe reported having no disclosures.
SAN DIEGO – The .
Those are the preliminary conclusions from a systematic review and survey of experts that Albert Wolkerstorfer, MD, presented during a clinical abstract session at the annual conference of the American Society for Laser Medicine and Surgery.
According to Dr. Wolkerstorfer, a dermatologist at Amsterdam University Medical Center, clinicians are reluctant to perform laser/intense pulsed light (IPL) treatments in patients with vitiligo because of the absence of clear guidelines, so he and his colleagues set out to investigate the risks of laser/IPL-induced vitiligo in patients with vitiligo and to seek out international consensus on recommendations from experts. “There is hardly any literature about it and certainly no guidelines,” he pointed out.
Dr. Wolkerstorfer and his colleagues designed three consecutive studies: A systematic review of laser/IPL-induced vitiligo; an international survey among 14 vitiligo experts from 10 countries about the occurrence of laser‐induced vitiligo, and a Delphi technique aimed at establishing a broad consensus about recommendations for safe use of lasers in vitiligo patients. At the time of the meeting, the Delphi process was still being carried out, so he did not discuss that study.
For the systematic review, the researchers found 11,073 unique hits on PubMed, Embase, and CINAHL using the terms “vitiligo,” “depigmentation,” “hypopigmentation,” and “leukoderma.” Only six case reports of laser/IPL-induced vitiligo were included in the final analysis. Of these, three had de novo vitiligo and three had vitiligo/halo nevi. These cases included two that occurred following treatment of port wine stains with the 585-nm laser; one that occurred following treatment of dyspigmentation with IPL; one that occurred following treatment of hypertrichosis with the 1,064-nm laser, one that occurred following treatment of hypertrichosis with the 755-nm laser, and one case that occurred following treatment of melasma with the ablative laser.
For the international survey of 14 experts from 10 countries, respondents said they had 10,670 new face-to-face vitiligo consultations in the past year. They reported that 30 of the vitiligo cases (0.3%) were likely caused by laser/IPL. Of these 30 cases, 18 (60%) had de novo vitiligo.
Of these cases, vitiligo occurred most frequently after laser hair reduction (47%), followed by use of the fractional laser (17%), and the ablative laser (13%). The interval between laser/IPL treatment and onset of vitiligo was 0-4 weeks in 27% of cases and 4-12 weeks in 57% of cases. Direct complications such as blistering, crusting, and erosions occurred in 57% of cases.
“Our conclusion is that laser and IPL-induced vitiligo is a rare phenomenon, and it often affects patients without prior vitiligo, which was really a surprise to us,” Dr. Wolkerstorfer said. “Complications seem to increase the risk,” he added.
“Despite the apparently low risk, we recommend caution [in patients with vitiligo], especially with aggressive laser procedures,” he said. “We recommend using conservative settings, not to treat active vitiligo patients ... and to perform test spots prior to treating large areas.” But he characterized this recommendation as “totally preliminary” pending results of the Delphi technique aimed at building consensus about laser/IPL treatments in vitiligo.
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices in Alexandria, Va., said that as clinicians await results of the study’s Delphi consensus, current use of lasers and IPL in patients with vitiligo “is based on your clinical judgment and whether the vitiligo is active or inactive. If the patient has vitiligo and you’re doing laser hair removal in the armpit, they may get active lesions in that area, but they can cover it. So, they may take that as a ‘win’ with the risk. But if it can erupt in other areas, that’s a risk they must be willing to take.”
Dr. Wolkerstorfer disclosed that he has received grant or research funding from Lumenis, Novartis, and Avita Medical. He is an advisory board member for Incyte. Dr. Onwudiwe reported having no disclosures.
AT ASLMS 2022
What’s ahead for laser-assisted drug delivery?
SAN DIEGO – Twelve years ago, Merete Haedersdal, MD, PhD, and colleagues published data from a swine study, which showed for the first time that the ablative fractional laser can be used to boost the uptake of drugs into the skin.
That discovery paved the way for what are now well-established clinical applications of laser-assisted drug delivery for treating actinic keratoses and scars. According to Dr. Haedersdal, professor of dermatology at the University of Copenhagen, evolving clinical indications for laser-assisted drug delivery include rejuvenation, local anesthesia, melasma, onychomycosis, hyperhidrosis, alopecia, and vitiligo, while emerging indications include treatment of skin cancer with PD-1 inhibitors and combination chemotherapy regimens, and vaccinations.
During a presentation at the annual conference of the American Society for Laser Medicine and Surgery, she said that researchers have much to learn about laser-assisted drug delivery, including biodistribution of the drug being delivered. Pointing out that so far, “what we have been dealing with is primarily looking at the skin as a black box,” she asked, “what happens when we drill the holes and drugs are applied on top of the skin and swim through the tiny channels?”
By using high-performance liquid chromatography (HPLC) and HPLC mass spectrometry to measure drug concentration in the skin, she and her colleagues have observed enhanced uptake of drugs – 4-fold to 40-fold greater – primarily in ex vivo pig skin. “We do know from ex vivo models that it’s much easier to boost the uptake in the skin” when compared with in vivo human use, where much lower drug concentrations are detected, said Dr. Haedersdal, who, along with Emily Wenande, MD, PhD, and R. Rox Anderson, MD, at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston, authored a clinical review, published in 2020, on the basics of laser-assisted drug delivery.
“What we are working on now is visualizing what’s taking place when we apply the holes and the drugs in the skin. This is the key to tailoring laser-assisted uptake to specific dermatologic diseases being treated,” she said. To date, she and her colleagues have examined the interaction with tissue using different devices, including ex vivo confocal microscopy, to view the thermal response to ablative fractional laser and radiofrequency. “We want to take that to the next level and look at the drug biodistribution.”
Efforts are underway to compare the pattern of drug distribution with different modes of delivery, such as comparing ablative fractional laser to intradermal needle injection. “We are also working on pneumatic jet injection, which creates a focal drug distribution,” said Dr. Haedersdal, who is a visiting scientist at the Wellman Center. “In the future, we may take advantage of device-tailored biodistribution, depending on which clinical indication we are treating.”
Another important aspect to consider is drug retention in the skin. In a study presented as an abstract at the meeting, led by Dr. Wenande, she, Dr. Haedersdal, and colleagues used a pig model to evaluate the effect of three vasoregulative interventions on ablative fractional laser-assisted 5-fluororacil concentrations in in vivo skin. The three interventions were brimonidine 0.33% solution, epinephrine 10 mcg/mL gel, and a 595-nm pulsed dye laser (PDL) in designated treatment areas.
“What we learned from that was in the short term – 1-4 hours – the ablative fractional laser enhanced the uptake of 5-FU, but it was very transient,” with a twofold increased concentration of 5-FU, Dr. Haedersdal said. Over 48-72 hours, after PDL, there was “sustained enhancement of drug in the skin by three to four times,” she noted.
The synergy of systemic drugs with ablative fractional laser therapy is also being evaluated. In a mouse study led by Dr. Haedersdal’s colleague, senior researcher Uffe H. Olesen, PhD, the treatment of advanced squamous cell carcinoma tumors with a combination of ablative fractional laser and systemic treatment with PD-1 inhibitors resulted in the clearance of more tumors than with either treatment as monotherapy. “What we want to explore is the laser-induced tumor immune response in keratinocyte cancers,” she added.
“When you shine the laser on the skin, there is a robust increase of neutrophilic granulocytes.” Combining this topical immune-boosting response with systemic delivery of PD-1 inhibitors in a mouse model with basal cell carcinoma, she said, “we learned that, when we compare systemic PD-1 inhibitors alone to the laser alone and then with combination therapy, there was an increased tumor clearance of basal cell carcinomas and also enhanced survival of the mice” with the combination, she said. There were also “enhanced neutrophilic counts and both CD4- and CD8-positive cells were increased,” she added.
Dr. Haedersdal disclosed that she has received grants or research funding from Lutronic, Venus Concept, Leo Pharma, and Mirai Medical.
SAN DIEGO – Twelve years ago, Merete Haedersdal, MD, PhD, and colleagues published data from a swine study, which showed for the first time that the ablative fractional laser can be used to boost the uptake of drugs into the skin.
That discovery paved the way for what are now well-established clinical applications of laser-assisted drug delivery for treating actinic keratoses and scars. According to Dr. Haedersdal, professor of dermatology at the University of Copenhagen, evolving clinical indications for laser-assisted drug delivery include rejuvenation, local anesthesia, melasma, onychomycosis, hyperhidrosis, alopecia, and vitiligo, while emerging indications include treatment of skin cancer with PD-1 inhibitors and combination chemotherapy regimens, and vaccinations.
During a presentation at the annual conference of the American Society for Laser Medicine and Surgery, she said that researchers have much to learn about laser-assisted drug delivery, including biodistribution of the drug being delivered. Pointing out that so far, “what we have been dealing with is primarily looking at the skin as a black box,” she asked, “what happens when we drill the holes and drugs are applied on top of the skin and swim through the tiny channels?”
By using high-performance liquid chromatography (HPLC) and HPLC mass spectrometry to measure drug concentration in the skin, she and her colleagues have observed enhanced uptake of drugs – 4-fold to 40-fold greater – primarily in ex vivo pig skin. “We do know from ex vivo models that it’s much easier to boost the uptake in the skin” when compared with in vivo human use, where much lower drug concentrations are detected, said Dr. Haedersdal, who, along with Emily Wenande, MD, PhD, and R. Rox Anderson, MD, at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston, authored a clinical review, published in 2020, on the basics of laser-assisted drug delivery.
“What we are working on now is visualizing what’s taking place when we apply the holes and the drugs in the skin. This is the key to tailoring laser-assisted uptake to specific dermatologic diseases being treated,” she said. To date, she and her colleagues have examined the interaction with tissue using different devices, including ex vivo confocal microscopy, to view the thermal response to ablative fractional laser and radiofrequency. “We want to take that to the next level and look at the drug biodistribution.”
Efforts are underway to compare the pattern of drug distribution with different modes of delivery, such as comparing ablative fractional laser to intradermal needle injection. “We are also working on pneumatic jet injection, which creates a focal drug distribution,” said Dr. Haedersdal, who is a visiting scientist at the Wellman Center. “In the future, we may take advantage of device-tailored biodistribution, depending on which clinical indication we are treating.”
Another important aspect to consider is drug retention in the skin. In a study presented as an abstract at the meeting, led by Dr. Wenande, she, Dr. Haedersdal, and colleagues used a pig model to evaluate the effect of three vasoregulative interventions on ablative fractional laser-assisted 5-fluororacil concentrations in in vivo skin. The three interventions were brimonidine 0.33% solution, epinephrine 10 mcg/mL gel, and a 595-nm pulsed dye laser (PDL) in designated treatment areas.
“What we learned from that was in the short term – 1-4 hours – the ablative fractional laser enhanced the uptake of 5-FU, but it was very transient,” with a twofold increased concentration of 5-FU, Dr. Haedersdal said. Over 48-72 hours, after PDL, there was “sustained enhancement of drug in the skin by three to four times,” she noted.
The synergy of systemic drugs with ablative fractional laser therapy is also being evaluated. In a mouse study led by Dr. Haedersdal’s colleague, senior researcher Uffe H. Olesen, PhD, the treatment of advanced squamous cell carcinoma tumors with a combination of ablative fractional laser and systemic treatment with PD-1 inhibitors resulted in the clearance of more tumors than with either treatment as monotherapy. “What we want to explore is the laser-induced tumor immune response in keratinocyte cancers,” she added.
“When you shine the laser on the skin, there is a robust increase of neutrophilic granulocytes.” Combining this topical immune-boosting response with systemic delivery of PD-1 inhibitors in a mouse model with basal cell carcinoma, she said, “we learned that, when we compare systemic PD-1 inhibitors alone to the laser alone and then with combination therapy, there was an increased tumor clearance of basal cell carcinomas and also enhanced survival of the mice” with the combination, she said. There were also “enhanced neutrophilic counts and both CD4- and CD8-positive cells were increased,” she added.
Dr. Haedersdal disclosed that she has received grants or research funding from Lutronic, Venus Concept, Leo Pharma, and Mirai Medical.
SAN DIEGO – Twelve years ago, Merete Haedersdal, MD, PhD, and colleagues published data from a swine study, which showed for the first time that the ablative fractional laser can be used to boost the uptake of drugs into the skin.
That discovery paved the way for what are now well-established clinical applications of laser-assisted drug delivery for treating actinic keratoses and scars. According to Dr. Haedersdal, professor of dermatology at the University of Copenhagen, evolving clinical indications for laser-assisted drug delivery include rejuvenation, local anesthesia, melasma, onychomycosis, hyperhidrosis, alopecia, and vitiligo, while emerging indications include treatment of skin cancer with PD-1 inhibitors and combination chemotherapy regimens, and vaccinations.
During a presentation at the annual conference of the American Society for Laser Medicine and Surgery, she said that researchers have much to learn about laser-assisted drug delivery, including biodistribution of the drug being delivered. Pointing out that so far, “what we have been dealing with is primarily looking at the skin as a black box,” she asked, “what happens when we drill the holes and drugs are applied on top of the skin and swim through the tiny channels?”
By using high-performance liquid chromatography (HPLC) and HPLC mass spectrometry to measure drug concentration in the skin, she and her colleagues have observed enhanced uptake of drugs – 4-fold to 40-fold greater – primarily in ex vivo pig skin. “We do know from ex vivo models that it’s much easier to boost the uptake in the skin” when compared with in vivo human use, where much lower drug concentrations are detected, said Dr. Haedersdal, who, along with Emily Wenande, MD, PhD, and R. Rox Anderson, MD, at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston, authored a clinical review, published in 2020, on the basics of laser-assisted drug delivery.
“What we are working on now is visualizing what’s taking place when we apply the holes and the drugs in the skin. This is the key to tailoring laser-assisted uptake to specific dermatologic diseases being treated,” she said. To date, she and her colleagues have examined the interaction with tissue using different devices, including ex vivo confocal microscopy, to view the thermal response to ablative fractional laser and radiofrequency. “We want to take that to the next level and look at the drug biodistribution.”
Efforts are underway to compare the pattern of drug distribution with different modes of delivery, such as comparing ablative fractional laser to intradermal needle injection. “We are also working on pneumatic jet injection, which creates a focal drug distribution,” said Dr. Haedersdal, who is a visiting scientist at the Wellman Center. “In the future, we may take advantage of device-tailored biodistribution, depending on which clinical indication we are treating.”
Another important aspect to consider is drug retention in the skin. In a study presented as an abstract at the meeting, led by Dr. Wenande, she, Dr. Haedersdal, and colleagues used a pig model to evaluate the effect of three vasoregulative interventions on ablative fractional laser-assisted 5-fluororacil concentrations in in vivo skin. The three interventions were brimonidine 0.33% solution, epinephrine 10 mcg/mL gel, and a 595-nm pulsed dye laser (PDL) in designated treatment areas.
“What we learned from that was in the short term – 1-4 hours – the ablative fractional laser enhanced the uptake of 5-FU, but it was very transient,” with a twofold increased concentration of 5-FU, Dr. Haedersdal said. Over 48-72 hours, after PDL, there was “sustained enhancement of drug in the skin by three to four times,” she noted.
The synergy of systemic drugs with ablative fractional laser therapy is also being evaluated. In a mouse study led by Dr. Haedersdal’s colleague, senior researcher Uffe H. Olesen, PhD, the treatment of advanced squamous cell carcinoma tumors with a combination of ablative fractional laser and systemic treatment with PD-1 inhibitors resulted in the clearance of more tumors than with either treatment as monotherapy. “What we want to explore is the laser-induced tumor immune response in keratinocyte cancers,” she added.
“When you shine the laser on the skin, there is a robust increase of neutrophilic granulocytes.” Combining this topical immune-boosting response with systemic delivery of PD-1 inhibitors in a mouse model with basal cell carcinoma, she said, “we learned that, when we compare systemic PD-1 inhibitors alone to the laser alone and then with combination therapy, there was an increased tumor clearance of basal cell carcinomas and also enhanced survival of the mice” with the combination, she said. There were also “enhanced neutrophilic counts and both CD4- and CD8-positive cells were increased,” she added.
Dr. Haedersdal disclosed that she has received grants or research funding from Lutronic, Venus Concept, Leo Pharma, and Mirai Medical.
AT ASLMS 2022
Can lasers be used to measure nerve sensitivity in the skin?
SAN DIEGO – In a 2006 report of complications from laser dermatologic surgery, one of the authors, Dieter Manstein, MD, PhD, who had subjected his forearm to treatment with a fractional laser skin resurfacing prototype device, was included as 1 of the 19 featured cases.
Dr. Manstein, of the Cutaneous Biology Research Center in the department of dermatology at Massachusetts General Hospital, Boston, was exposed to three test spots in the evaluation of the effects of different microscopic thermal zone densities for the prototype device, emitting at 1,450 nm and an energy per MTZ of 3 mJ.
Two years later, hypopigmentation persisted at the test site treated with the highest MTZ density, while two other sites treated with the lower MTZ densities did not show any dyspigmentation. But he noticed something else during the experiment: He felt minimal to no pain as each test site was being treated.
“It took 7 minutes without any cooling or anesthesia,” Dr. Manstein recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “It was not completely painless, but each time the laser was applied, sometimes I felt a little prick, sometimes I felt nothing.” Essentially, he added, “we created cell injury with a focused laser beam without anesthesia,” but this could also indicate that if skin is treated with a fractional laser very slowly, anesthesia is not needed. “Current devices are meant to treat very quickly, but if we [treat] slowly, maybe you could remove lesions painlessly without anesthesia.”
The observation from that experiment also led Dr. Manstein and colleagues to wonder: Could a focused laser beam pattern be used to assess cutaneous innervation? If so, they postulated, perhaps it could be used to not only assess nerve sensitivity of candidates for dermatologic surgery, but as a tool to help diagnose small fiber neuropathies such as diabetic neuropathy, and neuropathies in patients with HIV and sarcoidosis.
The current gold standard for making these diagnoses involves a skin biopsy, immunohistochemical analysis, and nerve fiber quantification, which is not widely available. It also requires strict histologic processing and nerve counting rules. Confocal microscopy of nerve fibers in the cornea is another approach, but is very difficult to perform, “so it would be nice if there was a simple way” to determine nerve fiber density in the skin using a focused laser beam, Dr. Manstein said.
With help from Payal Patel, MD, a dermatology research fellow at MGH, records each subject’s perception of a stimulus, and maps the areas of stimulus response. Current diameters being studied range from 0.076-1.15 mm and depths less than 0.71 mm. “We can focus the laser beam, preset the beam diameter, and very slowly, in a controlled manner, make a rectangular pattern, and after each time, inquire if the subject felt the pulse or not,” Dr. Manstein explained.
“This laser could become a new method for diagnosing nerve fiber neuropathies. If this works well, I think we can miniaturize the device,” he added.
Dr. Manstein disclosed that he is a consultant for Blossom Innovations, R2 Dermatology, and AVAVA. He is also a member of the advisory board for Blossom Innovations.
SAN DIEGO – In a 2006 report of complications from laser dermatologic surgery, one of the authors, Dieter Manstein, MD, PhD, who had subjected his forearm to treatment with a fractional laser skin resurfacing prototype device, was included as 1 of the 19 featured cases.
Dr. Manstein, of the Cutaneous Biology Research Center in the department of dermatology at Massachusetts General Hospital, Boston, was exposed to three test spots in the evaluation of the effects of different microscopic thermal zone densities for the prototype device, emitting at 1,450 nm and an energy per MTZ of 3 mJ.
Two years later, hypopigmentation persisted at the test site treated with the highest MTZ density, while two other sites treated with the lower MTZ densities did not show any dyspigmentation. But he noticed something else during the experiment: He felt minimal to no pain as each test site was being treated.
“It took 7 minutes without any cooling or anesthesia,” Dr. Manstein recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “It was not completely painless, but each time the laser was applied, sometimes I felt a little prick, sometimes I felt nothing.” Essentially, he added, “we created cell injury with a focused laser beam without anesthesia,” but this could also indicate that if skin is treated with a fractional laser very slowly, anesthesia is not needed. “Current devices are meant to treat very quickly, but if we [treat] slowly, maybe you could remove lesions painlessly without anesthesia.”
The observation from that experiment also led Dr. Manstein and colleagues to wonder: Could a focused laser beam pattern be used to assess cutaneous innervation? If so, they postulated, perhaps it could be used to not only assess nerve sensitivity of candidates for dermatologic surgery, but as a tool to help diagnose small fiber neuropathies such as diabetic neuropathy, and neuropathies in patients with HIV and sarcoidosis.
The current gold standard for making these diagnoses involves a skin biopsy, immunohistochemical analysis, and nerve fiber quantification, which is not widely available. It also requires strict histologic processing and nerve counting rules. Confocal microscopy of nerve fibers in the cornea is another approach, but is very difficult to perform, “so it would be nice if there was a simple way” to determine nerve fiber density in the skin using a focused laser beam, Dr. Manstein said.
With help from Payal Patel, MD, a dermatology research fellow at MGH, records each subject’s perception of a stimulus, and maps the areas of stimulus response. Current diameters being studied range from 0.076-1.15 mm and depths less than 0.71 mm. “We can focus the laser beam, preset the beam diameter, and very slowly, in a controlled manner, make a rectangular pattern, and after each time, inquire if the subject felt the pulse or not,” Dr. Manstein explained.
“This laser could become a new method for diagnosing nerve fiber neuropathies. If this works well, I think we can miniaturize the device,” he added.
Dr. Manstein disclosed that he is a consultant for Blossom Innovations, R2 Dermatology, and AVAVA. He is also a member of the advisory board for Blossom Innovations.
SAN DIEGO – In a 2006 report of complications from laser dermatologic surgery, one of the authors, Dieter Manstein, MD, PhD, who had subjected his forearm to treatment with a fractional laser skin resurfacing prototype device, was included as 1 of the 19 featured cases.
Dr. Manstein, of the Cutaneous Biology Research Center in the department of dermatology at Massachusetts General Hospital, Boston, was exposed to three test spots in the evaluation of the effects of different microscopic thermal zone densities for the prototype device, emitting at 1,450 nm and an energy per MTZ of 3 mJ.
Two years later, hypopigmentation persisted at the test site treated with the highest MTZ density, while two other sites treated with the lower MTZ densities did not show any dyspigmentation. But he noticed something else during the experiment: He felt minimal to no pain as each test site was being treated.
“It took 7 minutes without any cooling or anesthesia,” Dr. Manstein recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “It was not completely painless, but each time the laser was applied, sometimes I felt a little prick, sometimes I felt nothing.” Essentially, he added, “we created cell injury with a focused laser beam without anesthesia,” but this could also indicate that if skin is treated with a fractional laser very slowly, anesthesia is not needed. “Current devices are meant to treat very quickly, but if we [treat] slowly, maybe you could remove lesions painlessly without anesthesia.”
The observation from that experiment also led Dr. Manstein and colleagues to wonder: Could a focused laser beam pattern be used to assess cutaneous innervation? If so, they postulated, perhaps it could be used to not only assess nerve sensitivity of candidates for dermatologic surgery, but as a tool to help diagnose small fiber neuropathies such as diabetic neuropathy, and neuropathies in patients with HIV and sarcoidosis.
The current gold standard for making these diagnoses involves a skin biopsy, immunohistochemical analysis, and nerve fiber quantification, which is not widely available. It also requires strict histologic processing and nerve counting rules. Confocal microscopy of nerve fibers in the cornea is another approach, but is very difficult to perform, “so it would be nice if there was a simple way” to determine nerve fiber density in the skin using a focused laser beam, Dr. Manstein said.
With help from Payal Patel, MD, a dermatology research fellow at MGH, records each subject’s perception of a stimulus, and maps the areas of stimulus response. Current diameters being studied range from 0.076-1.15 mm and depths less than 0.71 mm. “We can focus the laser beam, preset the beam diameter, and very slowly, in a controlled manner, make a rectangular pattern, and after each time, inquire if the subject felt the pulse or not,” Dr. Manstein explained.
“This laser could become a new method for diagnosing nerve fiber neuropathies. If this works well, I think we can miniaturize the device,” he added.
Dr. Manstein disclosed that he is a consultant for Blossom Innovations, R2 Dermatology, and AVAVA. He is also a member of the advisory board for Blossom Innovations.
AT ASLMS 2022