User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Skin-picking, hair-pulling disorders: Diagnostic criteria, prevalence, and treatment
INDIANAPOLIS –
And while both body-focused repetitive behavior disorders affect a greater proportion of females than males, “we have no current information that is useful about what hormonal influences may or may not play in terms of picking and pulling behaviors,” Jon E. Grant, MD, JD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said at the annual meeting of the Society for Pediatric Dermatology. “On a cognitive level, affected children and adolescents often have impaired inhibitory control but they are often 1-2 standard deviations above average IQ. They have Type A personalities [and are] very driven young kids. They also do not tolerate any down time or boredom. They need to be doing something all the time.”
According to the DSM-5, the diagnostic criteria for skin picking includes recurrent skin picking that results in skin lesions and is not attributable to another medical condition or substance. It also involves repeated attempts to decrease or stop the behavior and causes clinically significant distress or impairment.
“The other medical condition that we are interested in is the misuse of or dependence upon amphetamines or other prescription-based or illicit stimulants,” Dr. Grant said. “I saw a young man who was using about 600 mg of Ritalin a day, and he was picking all over the place. He did not have a primary skin disorder.”
The lifetime prevalence of skin picking disorder ranges between 1.4% and 5.4% of the general population. However, about 63% of people in a community sample endorsed some form of skin picking, and in a study of 105 college students, almost 40% said they picked their skin and had noticeable tissue damage as a result.
“Skin picking is not the same as self-injury,” Dr. Grant said. “It is also not simply an anxiety disorder. Anxiety will make people who pick worse, so people will say that they pick when they’re under stress. I can give them benzodiazepines and they’re still going to pick.”
Animal and human studies demonstrate that skin picking and hair pulling primarily affect females. “You will encounter young boys that pick and pull, but it largely affects females, and it tends to start around puberty,” he said. “Picking can have an onset after the age of 30, which is quite uncommon.”
From a cognitive standpoint, pathological skin pickers demonstrate impaired inhibitory control, impaired stop signal reaction time, increased rates of negative urgency (a tendency to act impulsively in response to negative emotions), and increased rates of positive urgency (a tendency to act impulsively in response to exciting or pleasurable emotions).
Trichotillomania
The lifetime prevalence of trichotillomania ranges between 0.6% and 3.9%. The onset is typically from ages 10-13 years, and the mean duration of illness is 22 years.
The DSM-5 criteria for trichotillomania are similar to that of skin-picking disorder, “although we don’t really worry about the substance use issue with people who pull their hair,” Dr. Grant said. “It doesn’t seem to have a correlation.” In addition, sometimes, children “will worsen pulling or picking when they have co-occurring ADHD and they’ve been started on a stimulant, even at a typical dose. For kids who have those issues, we prefer to try nonstimulant options for their ADHD such as bupropion or atomoxetine.”
Individuals with trichotillomania also tend to have low self-esteem and increased social anxiety, he added, and about one-third report low or very low quality of life. “When you notice alopecia, particularly in young girls who often have longer hair, up to 20% will eat their hair,” Dr. Grant said. “We don’t know why. It’s not related to vitamin deficiencies; it’s not a pica type of iron deficiency. There seems to be a shame piece about eating one’s own hair, but it’s important to assess that. Ask about constipation or overflow incontinence because they can get a bezoar, which can rupture” and can be fatal.
Skin-picking disorder and trichotillomania co-occur in up to 20% of cases. “When they do it tends to be a more difficult problem,” he said. These patients often come for mental health care because of depression, and most, he added, say “I don’t think I would be depressed if I wasn’t covered with excoriations or missing most of my hair.”
Treatment for both conditions
According to Dr. Grant, the treatment of choice for skin-picking disorder and trichotillomania is a specific psychotherapy known as “habit reversal therapy,” which involves helping the patient gain better self-control. The drawback is that it’s difficult to find someone trained in habit reversal therapy, “who know anything about skin picking and hair pulling,” he said. “That has been a huge challenge in the field.”
In his experience, the medical treatment of choice for skin-picking disorder and trichotillomania is N-acetylcysteine, an over-the-counter amino acid and antioxidant, which has been shown to be helpful at a dose of 2,400 mg per day. “Patients report to me that some of the excoriations clear up a little quicker as they’re taking it,” Dr. Grant said.
There may also be a role for antipsychotic therapy, he said, “but because of the associated weight gain with most antipsychotics we prefer not to use them.”
The opioid antagonist naltrexone has been shown to be effective in the subset of patients with skin-picking or hair-pulling disorders whose parents have a substance use disorder, Dr. Grant said. “The thought is that there’s something addictive about this behavior in some kids. These kids will look forward to picking and find it rewarding and exciting.”
Dr. Grant reported having no relevant financial disclosures.
INDIANAPOLIS –
And while both body-focused repetitive behavior disorders affect a greater proportion of females than males, “we have no current information that is useful about what hormonal influences may or may not play in terms of picking and pulling behaviors,” Jon E. Grant, MD, JD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said at the annual meeting of the Society for Pediatric Dermatology. “On a cognitive level, affected children and adolescents often have impaired inhibitory control but they are often 1-2 standard deviations above average IQ. They have Type A personalities [and are] very driven young kids. They also do not tolerate any down time or boredom. They need to be doing something all the time.”
According to the DSM-5, the diagnostic criteria for skin picking includes recurrent skin picking that results in skin lesions and is not attributable to another medical condition or substance. It also involves repeated attempts to decrease or stop the behavior and causes clinically significant distress or impairment.
“The other medical condition that we are interested in is the misuse of or dependence upon amphetamines or other prescription-based or illicit stimulants,” Dr. Grant said. “I saw a young man who was using about 600 mg of Ritalin a day, and he was picking all over the place. He did not have a primary skin disorder.”
The lifetime prevalence of skin picking disorder ranges between 1.4% and 5.4% of the general population. However, about 63% of people in a community sample endorsed some form of skin picking, and in a study of 105 college students, almost 40% said they picked their skin and had noticeable tissue damage as a result.
“Skin picking is not the same as self-injury,” Dr. Grant said. “It is also not simply an anxiety disorder. Anxiety will make people who pick worse, so people will say that they pick when they’re under stress. I can give them benzodiazepines and they’re still going to pick.”
Animal and human studies demonstrate that skin picking and hair pulling primarily affect females. “You will encounter young boys that pick and pull, but it largely affects females, and it tends to start around puberty,” he said. “Picking can have an onset after the age of 30, which is quite uncommon.”
From a cognitive standpoint, pathological skin pickers demonstrate impaired inhibitory control, impaired stop signal reaction time, increased rates of negative urgency (a tendency to act impulsively in response to negative emotions), and increased rates of positive urgency (a tendency to act impulsively in response to exciting or pleasurable emotions).
Trichotillomania
The lifetime prevalence of trichotillomania ranges between 0.6% and 3.9%. The onset is typically from ages 10-13 years, and the mean duration of illness is 22 years.
The DSM-5 criteria for trichotillomania are similar to that of skin-picking disorder, “although we don’t really worry about the substance use issue with people who pull their hair,” Dr. Grant said. “It doesn’t seem to have a correlation.” In addition, sometimes, children “will worsen pulling or picking when they have co-occurring ADHD and they’ve been started on a stimulant, even at a typical dose. For kids who have those issues, we prefer to try nonstimulant options for their ADHD such as bupropion or atomoxetine.”
Individuals with trichotillomania also tend to have low self-esteem and increased social anxiety, he added, and about one-third report low or very low quality of life. “When you notice alopecia, particularly in young girls who often have longer hair, up to 20% will eat their hair,” Dr. Grant said. “We don’t know why. It’s not related to vitamin deficiencies; it’s not a pica type of iron deficiency. There seems to be a shame piece about eating one’s own hair, but it’s important to assess that. Ask about constipation or overflow incontinence because they can get a bezoar, which can rupture” and can be fatal.
Skin-picking disorder and trichotillomania co-occur in up to 20% of cases. “When they do it tends to be a more difficult problem,” he said. These patients often come for mental health care because of depression, and most, he added, say “I don’t think I would be depressed if I wasn’t covered with excoriations or missing most of my hair.”
Treatment for both conditions
According to Dr. Grant, the treatment of choice for skin-picking disorder and trichotillomania is a specific psychotherapy known as “habit reversal therapy,” which involves helping the patient gain better self-control. The drawback is that it’s difficult to find someone trained in habit reversal therapy, “who know anything about skin picking and hair pulling,” he said. “That has been a huge challenge in the field.”
In his experience, the medical treatment of choice for skin-picking disorder and trichotillomania is N-acetylcysteine, an over-the-counter amino acid and antioxidant, which has been shown to be helpful at a dose of 2,400 mg per day. “Patients report to me that some of the excoriations clear up a little quicker as they’re taking it,” Dr. Grant said.
There may also be a role for antipsychotic therapy, he said, “but because of the associated weight gain with most antipsychotics we prefer not to use them.”
The opioid antagonist naltrexone has been shown to be effective in the subset of patients with skin-picking or hair-pulling disorders whose parents have a substance use disorder, Dr. Grant said. “The thought is that there’s something addictive about this behavior in some kids. These kids will look forward to picking and find it rewarding and exciting.”
Dr. Grant reported having no relevant financial disclosures.
INDIANAPOLIS –
And while both body-focused repetitive behavior disorders affect a greater proportion of females than males, “we have no current information that is useful about what hormonal influences may or may not play in terms of picking and pulling behaviors,” Jon E. Grant, MD, JD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said at the annual meeting of the Society for Pediatric Dermatology. “On a cognitive level, affected children and adolescents often have impaired inhibitory control but they are often 1-2 standard deviations above average IQ. They have Type A personalities [and are] very driven young kids. They also do not tolerate any down time or boredom. They need to be doing something all the time.”
According to the DSM-5, the diagnostic criteria for skin picking includes recurrent skin picking that results in skin lesions and is not attributable to another medical condition or substance. It also involves repeated attempts to decrease or stop the behavior and causes clinically significant distress or impairment.
“The other medical condition that we are interested in is the misuse of or dependence upon amphetamines or other prescription-based or illicit stimulants,” Dr. Grant said. “I saw a young man who was using about 600 mg of Ritalin a day, and he was picking all over the place. He did not have a primary skin disorder.”
The lifetime prevalence of skin picking disorder ranges between 1.4% and 5.4% of the general population. However, about 63% of people in a community sample endorsed some form of skin picking, and in a study of 105 college students, almost 40% said they picked their skin and had noticeable tissue damage as a result.
“Skin picking is not the same as self-injury,” Dr. Grant said. “It is also not simply an anxiety disorder. Anxiety will make people who pick worse, so people will say that they pick when they’re under stress. I can give them benzodiazepines and they’re still going to pick.”
Animal and human studies demonstrate that skin picking and hair pulling primarily affect females. “You will encounter young boys that pick and pull, but it largely affects females, and it tends to start around puberty,” he said. “Picking can have an onset after the age of 30, which is quite uncommon.”
From a cognitive standpoint, pathological skin pickers demonstrate impaired inhibitory control, impaired stop signal reaction time, increased rates of negative urgency (a tendency to act impulsively in response to negative emotions), and increased rates of positive urgency (a tendency to act impulsively in response to exciting or pleasurable emotions).
Trichotillomania
The lifetime prevalence of trichotillomania ranges between 0.6% and 3.9%. The onset is typically from ages 10-13 years, and the mean duration of illness is 22 years.
The DSM-5 criteria for trichotillomania are similar to that of skin-picking disorder, “although we don’t really worry about the substance use issue with people who pull their hair,” Dr. Grant said. “It doesn’t seem to have a correlation.” In addition, sometimes, children “will worsen pulling or picking when they have co-occurring ADHD and they’ve been started on a stimulant, even at a typical dose. For kids who have those issues, we prefer to try nonstimulant options for their ADHD such as bupropion or atomoxetine.”
Individuals with trichotillomania also tend to have low self-esteem and increased social anxiety, he added, and about one-third report low or very low quality of life. “When you notice alopecia, particularly in young girls who often have longer hair, up to 20% will eat their hair,” Dr. Grant said. “We don’t know why. It’s not related to vitamin deficiencies; it’s not a pica type of iron deficiency. There seems to be a shame piece about eating one’s own hair, but it’s important to assess that. Ask about constipation or overflow incontinence because they can get a bezoar, which can rupture” and can be fatal.
Skin-picking disorder and trichotillomania co-occur in up to 20% of cases. “When they do it tends to be a more difficult problem,” he said. These patients often come for mental health care because of depression, and most, he added, say “I don’t think I would be depressed if I wasn’t covered with excoriations or missing most of my hair.”
Treatment for both conditions
According to Dr. Grant, the treatment of choice for skin-picking disorder and trichotillomania is a specific psychotherapy known as “habit reversal therapy,” which involves helping the patient gain better self-control. The drawback is that it’s difficult to find someone trained in habit reversal therapy, “who know anything about skin picking and hair pulling,” he said. “That has been a huge challenge in the field.”
In his experience, the medical treatment of choice for skin-picking disorder and trichotillomania is N-acetylcysteine, an over-the-counter amino acid and antioxidant, which has been shown to be helpful at a dose of 2,400 mg per day. “Patients report to me that some of the excoriations clear up a little quicker as they’re taking it,” Dr. Grant said.
There may also be a role for antipsychotic therapy, he said, “but because of the associated weight gain with most antipsychotics we prefer not to use them.”
The opioid antagonist naltrexone has been shown to be effective in the subset of patients with skin-picking or hair-pulling disorders whose parents have a substance use disorder, Dr. Grant said. “The thought is that there’s something addictive about this behavior in some kids. These kids will look forward to picking and find it rewarding and exciting.”
Dr. Grant reported having no relevant financial disclosures.
AT SPD 2022
Avoiding harm in the diagnosis and treatment of food allergies
INDIANAPOLIS – If there’s one truth that David R. Stukus, MD, has come to realize from his 2 years as director of a food allergy treatment center, it’s that
“When they’re given a diagnosis of food allergy, many families do not receive proper education to help them understand the risk as well as self-management and prognosis,” he said at the annual meeting of the Society for Pediatric Dermatology. “They are left to fend for themselves, which leads to increased anxiety. If they don’t understand what it means to manage their child’s food allergy, they’re going to think that they’re a ticking time bomb,” said Dr. Stukus, director of the Food Allergy Treatment Center and professor of pediatrics in the division of allergy and immunology at Nationwide Children’s Hospital in Columbus, Ohio.
During his presentation, he toured clinicians through best practices to diagnose and treat food allergies and shared cautionary tales of unsupported claims, unnecessary testing, and potential harm to misdiagnosed patients.
While food allergies can be serious and life-threatening, they are also manageable, he continued. It doesn’t mean that children with food allergies can’t go to school, attend baseball games, or participate in activities that any other child would. “Telling someone to adopt a restricted diet is not a benign recommendation,” he said. “That can cause real harm.”
Dr. Stukus defined food allergy as an immunologic response to an allergen that results in reproducible symptoms with every exposure. “Most commonly we’re going to see IgE-mediated food allergies, which often occur within minutes of eating certain foods,” he said.
Food intolerance, on the other hand, is a nonimmunologic response to a food that causes gastrointestinal symptoms with exposure. “This can come and go over time,” he said. “The most common example is lactose intolerance.”
Then there’s food sensitivity, which Dr. Stukus said is not a medical term but a marketing term often applied to a variety of symptoms without evidence to support its use.
“On the Internet you will find many companies marketing food sensitivity tests,” he said. “Gluten-free foods are now a billion-dollar industry. There are no validated tests to diagnose food sensitivity. All the blood tests measure IgG, which is memory antibody. If you eat a food, it is a normal response to produce IgG to it, but these companies will test all these things and when it comes back elevated, they say ‘Aha! This is your food sensitivity and this is why you’re not sleeping well at night.’ ” To illustrate the harm that can come from food allergy tests he discussed a 6-year-old girl who presented to his clinic several years ago with typical symptoms of allergic rhinitis. The parent reported a history of sneezing around dogs, itchy, watery eyes in the spring, recurrent cough, and frequent upper respiratory infections.
The referring physician had ordered an allergy panel, which flagged a long list of foods that the girl was supposedly allergic to, including banana, egg white, cod, and peanut. “This family was told to take all of these foods out of her diet,” Dr. Stukus said. “Interestingly, she had been seen by this physician for evaluation of environmental allergies, but the only ones included in the test were cat, cockroach, dog, and dust mite. They didn’t even include the spring pollen allergies. You want to avoid tests like this.”
Food sensitization is not the same as food allergy, he continued, noting that about 30% of all children will have detectable IgE toward peanuts, milk, egg, and shrimp, but that only about 5% are truly allergic to those foods.
“If we go by IgE testing alone, we’re going to overdiagnose the vast majority of people with food allergies that they don’t actually have,” he said. “Food allergy is diagnosed by the history and then confirmed by testing. With IgE-mediated food allergies we know that milk, egg, wheat, soy, finned fish, shellfish, and peanuts account for more than 90% of all food allergy reactions. Can any food potentially cause a food allergy? Yes, potentially, but we know that most fruits and vegetables and grains are very unlikely to cause an allergy.”
IgE-mediated food allergies are objective, immediate onset, and reproducible with every exposure to the offending food, no matter what form. Typical symptoms include hives, swelling, vomiting, runny nose/congestion, wheezing, hypotension, and anaphylaxis.
“We can also accurately identify infants that are more at risk to develop food allergies,” Dr. Stukus said. Infants with refractory atopic dermatitis often progress from eczema to food allergies to allergic rhinitis and asthma, the so-called “allergic march.” “Family history does have a role as well, but it’s not as significant,” he said. As for diagnostic tools, skin prick testing detects the presence of specific IgE bound to cutaneous mast cells and has a high negative predictive value and a low positive predictive value (around 50%).
With serum-specific IgE testing, levels of IgE for food and/or inhalant allergen can be obtained conveniently through routine venipuncture. Results are reported in ranges from 0.1 kU/L to 100 kU/L, and some are reported as arbitrary classes in levels of severity from 1 to 5.
“I highly discourage anybody from paying attention to arbitrary classes [on these reports],” Dr. Stukus said. “Those are meaningless. The absolute value is all that matters.”
He added that both skin and blood testing have high rates of false positive results. “We really need to use the history to help guide what tests we do; they were never designed to be used as screening tests, yet they’re used as screening tests on a regular basis,” he said. “There is also no indication to do shotgun testing. The reason why is because we see lots of cross reactivity on testing. If we have someone with peanut allergy and we start doing specific IgE testing for all legumes, more often than not we’re going to find detectable IgE, but it’s much less likely that they actually have clinical reactivity to foods like soy and beans.”
Dr. Stukus advises clinicians to consider certain questions before they order an allergen panel, the first being: Do I have the knowledge and experience to properly interpret the results?
“If you don’t know how to interpret the test, you probably shouldn’t order it in the first place,” he said. “If you do have the knowledge to interpret the results, will the results help to determine the diagnosis or change management? If not, why are you testing just to test? There is zero clinical indication to order a food allergy panel.” Dr. Stukus recommended a review of unproven tests for adverse reactions to foods published in 2018 in The Journal of Allergy and Clinical Immunology.
According to Dr. Stukus, potential harms from unproven food allergy tests include cost, unnecessary dietary avoidance, and a delay in diagnosis for the underlying condition. During the COVID-19 pandemic, he observed an increase in the number of patients with orthorexia, which he described as an eating disorder characterized by having an unsafe obsession with healthy food that becomes deeply rooted in the individual’s way of thinking to the point that it interferes with daily life.
“If you take someone who has anxiety at baseline, and then you give them a list of foods that they allegedly can’t eat, that’s going to cause worse anxiety,” he added. “We’re seeing that from the results of these tests.”
Dr. Stukus disclosed that he is a consultant for Before Brands, Kaleo, and Novartis. He is also associate editor of the Annals of Allergy, Asthma and Immunology.
INDIANAPOLIS – If there’s one truth that David R. Stukus, MD, has come to realize from his 2 years as director of a food allergy treatment center, it’s that
“When they’re given a diagnosis of food allergy, many families do not receive proper education to help them understand the risk as well as self-management and prognosis,” he said at the annual meeting of the Society for Pediatric Dermatology. “They are left to fend for themselves, which leads to increased anxiety. If they don’t understand what it means to manage their child’s food allergy, they’re going to think that they’re a ticking time bomb,” said Dr. Stukus, director of the Food Allergy Treatment Center and professor of pediatrics in the division of allergy and immunology at Nationwide Children’s Hospital in Columbus, Ohio.
During his presentation, he toured clinicians through best practices to diagnose and treat food allergies and shared cautionary tales of unsupported claims, unnecessary testing, and potential harm to misdiagnosed patients.
While food allergies can be serious and life-threatening, they are also manageable, he continued. It doesn’t mean that children with food allergies can’t go to school, attend baseball games, or participate in activities that any other child would. “Telling someone to adopt a restricted diet is not a benign recommendation,” he said. “That can cause real harm.”
Dr. Stukus defined food allergy as an immunologic response to an allergen that results in reproducible symptoms with every exposure. “Most commonly we’re going to see IgE-mediated food allergies, which often occur within minutes of eating certain foods,” he said.
Food intolerance, on the other hand, is a nonimmunologic response to a food that causes gastrointestinal symptoms with exposure. “This can come and go over time,” he said. “The most common example is lactose intolerance.”
Then there’s food sensitivity, which Dr. Stukus said is not a medical term but a marketing term often applied to a variety of symptoms without evidence to support its use.
“On the Internet you will find many companies marketing food sensitivity tests,” he said. “Gluten-free foods are now a billion-dollar industry. There are no validated tests to diagnose food sensitivity. All the blood tests measure IgG, which is memory antibody. If you eat a food, it is a normal response to produce IgG to it, but these companies will test all these things and when it comes back elevated, they say ‘Aha! This is your food sensitivity and this is why you’re not sleeping well at night.’ ” To illustrate the harm that can come from food allergy tests he discussed a 6-year-old girl who presented to his clinic several years ago with typical symptoms of allergic rhinitis. The parent reported a history of sneezing around dogs, itchy, watery eyes in the spring, recurrent cough, and frequent upper respiratory infections.
The referring physician had ordered an allergy panel, which flagged a long list of foods that the girl was supposedly allergic to, including banana, egg white, cod, and peanut. “This family was told to take all of these foods out of her diet,” Dr. Stukus said. “Interestingly, she had been seen by this physician for evaluation of environmental allergies, but the only ones included in the test were cat, cockroach, dog, and dust mite. They didn’t even include the spring pollen allergies. You want to avoid tests like this.”
Food sensitization is not the same as food allergy, he continued, noting that about 30% of all children will have detectable IgE toward peanuts, milk, egg, and shrimp, but that only about 5% are truly allergic to those foods.
“If we go by IgE testing alone, we’re going to overdiagnose the vast majority of people with food allergies that they don’t actually have,” he said. “Food allergy is diagnosed by the history and then confirmed by testing. With IgE-mediated food allergies we know that milk, egg, wheat, soy, finned fish, shellfish, and peanuts account for more than 90% of all food allergy reactions. Can any food potentially cause a food allergy? Yes, potentially, but we know that most fruits and vegetables and grains are very unlikely to cause an allergy.”
IgE-mediated food allergies are objective, immediate onset, and reproducible with every exposure to the offending food, no matter what form. Typical symptoms include hives, swelling, vomiting, runny nose/congestion, wheezing, hypotension, and anaphylaxis.
“We can also accurately identify infants that are more at risk to develop food allergies,” Dr. Stukus said. Infants with refractory atopic dermatitis often progress from eczema to food allergies to allergic rhinitis and asthma, the so-called “allergic march.” “Family history does have a role as well, but it’s not as significant,” he said. As for diagnostic tools, skin prick testing detects the presence of specific IgE bound to cutaneous mast cells and has a high negative predictive value and a low positive predictive value (around 50%).
With serum-specific IgE testing, levels of IgE for food and/or inhalant allergen can be obtained conveniently through routine venipuncture. Results are reported in ranges from 0.1 kU/L to 100 kU/L, and some are reported as arbitrary classes in levels of severity from 1 to 5.
“I highly discourage anybody from paying attention to arbitrary classes [on these reports],” Dr. Stukus said. “Those are meaningless. The absolute value is all that matters.”
He added that both skin and blood testing have high rates of false positive results. “We really need to use the history to help guide what tests we do; they were never designed to be used as screening tests, yet they’re used as screening tests on a regular basis,” he said. “There is also no indication to do shotgun testing. The reason why is because we see lots of cross reactivity on testing. If we have someone with peanut allergy and we start doing specific IgE testing for all legumes, more often than not we’re going to find detectable IgE, but it’s much less likely that they actually have clinical reactivity to foods like soy and beans.”
Dr. Stukus advises clinicians to consider certain questions before they order an allergen panel, the first being: Do I have the knowledge and experience to properly interpret the results?
“If you don’t know how to interpret the test, you probably shouldn’t order it in the first place,” he said. “If you do have the knowledge to interpret the results, will the results help to determine the diagnosis or change management? If not, why are you testing just to test? There is zero clinical indication to order a food allergy panel.” Dr. Stukus recommended a review of unproven tests for adverse reactions to foods published in 2018 in The Journal of Allergy and Clinical Immunology.
According to Dr. Stukus, potential harms from unproven food allergy tests include cost, unnecessary dietary avoidance, and a delay in diagnosis for the underlying condition. During the COVID-19 pandemic, he observed an increase in the number of patients with orthorexia, which he described as an eating disorder characterized by having an unsafe obsession with healthy food that becomes deeply rooted in the individual’s way of thinking to the point that it interferes with daily life.
“If you take someone who has anxiety at baseline, and then you give them a list of foods that they allegedly can’t eat, that’s going to cause worse anxiety,” he added. “We’re seeing that from the results of these tests.”
Dr. Stukus disclosed that he is a consultant for Before Brands, Kaleo, and Novartis. He is also associate editor of the Annals of Allergy, Asthma and Immunology.
INDIANAPOLIS – If there’s one truth that David R. Stukus, MD, has come to realize from his 2 years as director of a food allergy treatment center, it’s that
“When they’re given a diagnosis of food allergy, many families do not receive proper education to help them understand the risk as well as self-management and prognosis,” he said at the annual meeting of the Society for Pediatric Dermatology. “They are left to fend for themselves, which leads to increased anxiety. If they don’t understand what it means to manage their child’s food allergy, they’re going to think that they’re a ticking time bomb,” said Dr. Stukus, director of the Food Allergy Treatment Center and professor of pediatrics in the division of allergy and immunology at Nationwide Children’s Hospital in Columbus, Ohio.
During his presentation, he toured clinicians through best practices to diagnose and treat food allergies and shared cautionary tales of unsupported claims, unnecessary testing, and potential harm to misdiagnosed patients.
While food allergies can be serious and life-threatening, they are also manageable, he continued. It doesn’t mean that children with food allergies can’t go to school, attend baseball games, or participate in activities that any other child would. “Telling someone to adopt a restricted diet is not a benign recommendation,” he said. “That can cause real harm.”
Dr. Stukus defined food allergy as an immunologic response to an allergen that results in reproducible symptoms with every exposure. “Most commonly we’re going to see IgE-mediated food allergies, which often occur within minutes of eating certain foods,” he said.
Food intolerance, on the other hand, is a nonimmunologic response to a food that causes gastrointestinal symptoms with exposure. “This can come and go over time,” he said. “The most common example is lactose intolerance.”
Then there’s food sensitivity, which Dr. Stukus said is not a medical term but a marketing term often applied to a variety of symptoms without evidence to support its use.
“On the Internet you will find many companies marketing food sensitivity tests,” he said. “Gluten-free foods are now a billion-dollar industry. There are no validated tests to diagnose food sensitivity. All the blood tests measure IgG, which is memory antibody. If you eat a food, it is a normal response to produce IgG to it, but these companies will test all these things and when it comes back elevated, they say ‘Aha! This is your food sensitivity and this is why you’re not sleeping well at night.’ ” To illustrate the harm that can come from food allergy tests he discussed a 6-year-old girl who presented to his clinic several years ago with typical symptoms of allergic rhinitis. The parent reported a history of sneezing around dogs, itchy, watery eyes in the spring, recurrent cough, and frequent upper respiratory infections.
The referring physician had ordered an allergy panel, which flagged a long list of foods that the girl was supposedly allergic to, including banana, egg white, cod, and peanut. “This family was told to take all of these foods out of her diet,” Dr. Stukus said. “Interestingly, she had been seen by this physician for evaluation of environmental allergies, but the only ones included in the test were cat, cockroach, dog, and dust mite. They didn’t even include the spring pollen allergies. You want to avoid tests like this.”
Food sensitization is not the same as food allergy, he continued, noting that about 30% of all children will have detectable IgE toward peanuts, milk, egg, and shrimp, but that only about 5% are truly allergic to those foods.
“If we go by IgE testing alone, we’re going to overdiagnose the vast majority of people with food allergies that they don’t actually have,” he said. “Food allergy is diagnosed by the history and then confirmed by testing. With IgE-mediated food allergies we know that milk, egg, wheat, soy, finned fish, shellfish, and peanuts account for more than 90% of all food allergy reactions. Can any food potentially cause a food allergy? Yes, potentially, but we know that most fruits and vegetables and grains are very unlikely to cause an allergy.”
IgE-mediated food allergies are objective, immediate onset, and reproducible with every exposure to the offending food, no matter what form. Typical symptoms include hives, swelling, vomiting, runny nose/congestion, wheezing, hypotension, and anaphylaxis.
“We can also accurately identify infants that are more at risk to develop food allergies,” Dr. Stukus said. Infants with refractory atopic dermatitis often progress from eczema to food allergies to allergic rhinitis and asthma, the so-called “allergic march.” “Family history does have a role as well, but it’s not as significant,” he said. As for diagnostic tools, skin prick testing detects the presence of specific IgE bound to cutaneous mast cells and has a high negative predictive value and a low positive predictive value (around 50%).
With serum-specific IgE testing, levels of IgE for food and/or inhalant allergen can be obtained conveniently through routine venipuncture. Results are reported in ranges from 0.1 kU/L to 100 kU/L, and some are reported as arbitrary classes in levels of severity from 1 to 5.
“I highly discourage anybody from paying attention to arbitrary classes [on these reports],” Dr. Stukus said. “Those are meaningless. The absolute value is all that matters.”
He added that both skin and blood testing have high rates of false positive results. “We really need to use the history to help guide what tests we do; they were never designed to be used as screening tests, yet they’re used as screening tests on a regular basis,” he said. “There is also no indication to do shotgun testing. The reason why is because we see lots of cross reactivity on testing. If we have someone with peanut allergy and we start doing specific IgE testing for all legumes, more often than not we’re going to find detectable IgE, but it’s much less likely that they actually have clinical reactivity to foods like soy and beans.”
Dr. Stukus advises clinicians to consider certain questions before they order an allergen panel, the first being: Do I have the knowledge and experience to properly interpret the results?
“If you don’t know how to interpret the test, you probably shouldn’t order it in the first place,” he said. “If you do have the knowledge to interpret the results, will the results help to determine the diagnosis or change management? If not, why are you testing just to test? There is zero clinical indication to order a food allergy panel.” Dr. Stukus recommended a review of unproven tests for adverse reactions to foods published in 2018 in The Journal of Allergy and Clinical Immunology.
According to Dr. Stukus, potential harms from unproven food allergy tests include cost, unnecessary dietary avoidance, and a delay in diagnosis for the underlying condition. During the COVID-19 pandemic, he observed an increase in the number of patients with orthorexia, which he described as an eating disorder characterized by having an unsafe obsession with healthy food that becomes deeply rooted in the individual’s way of thinking to the point that it interferes with daily life.
“If you take someone who has anxiety at baseline, and then you give them a list of foods that they allegedly can’t eat, that’s going to cause worse anxiety,” he added. “We’re seeing that from the results of these tests.”
Dr. Stukus disclosed that he is a consultant for Before Brands, Kaleo, and Novartis. He is also associate editor of the Annals of Allergy, Asthma and Immunology.
AT SPD 2022
What are your treatment options when isotretinoin fails?
INDIANAPOLIS – – which is known to increase the drug’s bioavailability, advises James R. Treat, MD, a pediatric dermatologist at Children’s Hospital of Philadelphia.
“We see lots of teenagers who are on a restrictive diet,” which is “certainly one reason they could be failing isotretinoin,” Dr. Treat said at the annual meeting of the Society for Pediatric Dermatology.
Often, patients say that they have been referred to him because they had no response to 20 mg or 30 mg per day of isotretinoin. But after a dose escalation to 60 mg per day, their acne worsened.
If the patient’s acne is worsening with a cystic flare, “tripling the dose of isotretinoin is not something that you should do,” Dr. Treat said. “You should lower the dose and consider adding steroids.” For evidence-based recommendations on managing acne fulminans, he recommended an article published in the Journal of the American Academy of Dermatology in 2017.
Skin picking is another common reason for failure of isotretinoin, as well as with other acne therapies. These patients may have associated anxiety, which “might be a contraindication or at least something to consider before you put them on isotretinoin,” he noted.
In his experience, off-label use of N-acetylcysteine, an antioxidant and cysteine prodrug, has been “extremely effective” for patients with excoriation disorder. In a randomized trial of adults 18-60 years of age, 47% patients who took 1,200-3,000 mg per day doses of N-acetylcysteine for 12 weeks reported that their skin picking was much or very much improved, compared to 19% of those who took placebo (P = .03). The authors wrote that N-acetylcysteine “increases extracellular levels of glutamate in the nucleus accumbens,” and that these results support the hypothesis that “pharmacologic manipulation of the glutamate system may target core symptoms of compulsive behaviors.”
The tumor necrosis factor (TNF)-alpha blocker adalimumab is a reasonable option for patients with severe cystic inflammatory acne who fail isotretinoin, Dr. Treat said. In one published case, clinicians administered adalimumab 40 mg every other week for a 16-year-old male patient who received isotretinoin for moderate acne vulgaris, which caused sudden development of acne fulminans and incapacitating acute sacroiliitis with bilateral hip arthritis. Inflammatory lesions started to clear in 1 month and comedones improved by 3 months of treatment. Adalimumab was discontinued after 1 year and the patient remained clear.
“There are now multiple reports as well as some case series showing TNF-alpha agents causing clearance of acne,” said Dr. Treat, who directs the hospital’s pediatric dermatology fellowship program. A literature review of adalimumab, etanercept, and infliximab for treatment-resistant acne found that all agents had similar efficacy after 3-6 months of therapy. “We see this in our GI population, where TNF-alpha agents are helping their acne also,” he said. “We just have to augment it with some topical medications.”
Certain medications can drive the development of acne, including phenytoin, phenobarbital, lithium, MEK inhibitors, EGFR inhibitors, systemic steroids, and unopposed progesterone contraceptives. Some genetic conditions also predispose patients to acne, including mutations in the NCSTN gene and trisomy 13.
Dr. Treat discussed one of his patients with severe acne who had trisomy 13. The patient failed 12 months of doxycycline and amoxicillin in combination with a topical retinoid. He also failed low- and high-dose isotretinoin in combination with prednisone, as well as oral dapsone at a dose of 1 mg/kg per day for 3 months. He was started on adalimumab, but that was stopped after he flared. The patient is now maintained on ustekinumab monthly at a dose of 45 mg.
“I’ve only had a few patients where isotretinoin truly has failed,” Dr. Treat said. He described one patient with severe acne who had a hidradenitis-like appearance in his axilla and groin. “I treated with isotretinoin very gingerly in the beginning, [but] he flared significantly. I had given him concomitant steroids from the very beginning and transitioned to multiple different therapies – all of which failed.”
Next, Dr. Treat tried a course of systemic dapsone, and the patient responded nicely. “As an anti-inflammatory agent, dapsone is very reasonable” to consider, he said. “It’s something to add to your armamentarium.”
Dr. Treat disclosed that he is a consultant for Palvella and Regeneron. He has ownership interests in Matinas Biopharma Holdings, Axsome, Sorrento, and Amarin.
INDIANAPOLIS – – which is known to increase the drug’s bioavailability, advises James R. Treat, MD, a pediatric dermatologist at Children’s Hospital of Philadelphia.
“We see lots of teenagers who are on a restrictive diet,” which is “certainly one reason they could be failing isotretinoin,” Dr. Treat said at the annual meeting of the Society for Pediatric Dermatology.
Often, patients say that they have been referred to him because they had no response to 20 mg or 30 mg per day of isotretinoin. But after a dose escalation to 60 mg per day, their acne worsened.
If the patient’s acne is worsening with a cystic flare, “tripling the dose of isotretinoin is not something that you should do,” Dr. Treat said. “You should lower the dose and consider adding steroids.” For evidence-based recommendations on managing acne fulminans, he recommended an article published in the Journal of the American Academy of Dermatology in 2017.
Skin picking is another common reason for failure of isotretinoin, as well as with other acne therapies. These patients may have associated anxiety, which “might be a contraindication or at least something to consider before you put them on isotretinoin,” he noted.
In his experience, off-label use of N-acetylcysteine, an antioxidant and cysteine prodrug, has been “extremely effective” for patients with excoriation disorder. In a randomized trial of adults 18-60 years of age, 47% patients who took 1,200-3,000 mg per day doses of N-acetylcysteine for 12 weeks reported that their skin picking was much or very much improved, compared to 19% of those who took placebo (P = .03). The authors wrote that N-acetylcysteine “increases extracellular levels of glutamate in the nucleus accumbens,” and that these results support the hypothesis that “pharmacologic manipulation of the glutamate system may target core symptoms of compulsive behaviors.”
The tumor necrosis factor (TNF)-alpha blocker adalimumab is a reasonable option for patients with severe cystic inflammatory acne who fail isotretinoin, Dr. Treat said. In one published case, clinicians administered adalimumab 40 mg every other week for a 16-year-old male patient who received isotretinoin for moderate acne vulgaris, which caused sudden development of acne fulminans and incapacitating acute sacroiliitis with bilateral hip arthritis. Inflammatory lesions started to clear in 1 month and comedones improved by 3 months of treatment. Adalimumab was discontinued after 1 year and the patient remained clear.
“There are now multiple reports as well as some case series showing TNF-alpha agents causing clearance of acne,” said Dr. Treat, who directs the hospital’s pediatric dermatology fellowship program. A literature review of adalimumab, etanercept, and infliximab for treatment-resistant acne found that all agents had similar efficacy after 3-6 months of therapy. “We see this in our GI population, where TNF-alpha agents are helping their acne also,” he said. “We just have to augment it with some topical medications.”
Certain medications can drive the development of acne, including phenytoin, phenobarbital, lithium, MEK inhibitors, EGFR inhibitors, systemic steroids, and unopposed progesterone contraceptives. Some genetic conditions also predispose patients to acne, including mutations in the NCSTN gene and trisomy 13.
Dr. Treat discussed one of his patients with severe acne who had trisomy 13. The patient failed 12 months of doxycycline and amoxicillin in combination with a topical retinoid. He also failed low- and high-dose isotretinoin in combination with prednisone, as well as oral dapsone at a dose of 1 mg/kg per day for 3 months. He was started on adalimumab, but that was stopped after he flared. The patient is now maintained on ustekinumab monthly at a dose of 45 mg.
“I’ve only had a few patients where isotretinoin truly has failed,” Dr. Treat said. He described one patient with severe acne who had a hidradenitis-like appearance in his axilla and groin. “I treated with isotretinoin very gingerly in the beginning, [but] he flared significantly. I had given him concomitant steroids from the very beginning and transitioned to multiple different therapies – all of which failed.”
Next, Dr. Treat tried a course of systemic dapsone, and the patient responded nicely. “As an anti-inflammatory agent, dapsone is very reasonable” to consider, he said. “It’s something to add to your armamentarium.”
Dr. Treat disclosed that he is a consultant for Palvella and Regeneron. He has ownership interests in Matinas Biopharma Holdings, Axsome, Sorrento, and Amarin.
INDIANAPOLIS – – which is known to increase the drug’s bioavailability, advises James R. Treat, MD, a pediatric dermatologist at Children’s Hospital of Philadelphia.
“We see lots of teenagers who are on a restrictive diet,” which is “certainly one reason they could be failing isotretinoin,” Dr. Treat said at the annual meeting of the Society for Pediatric Dermatology.
Often, patients say that they have been referred to him because they had no response to 20 mg or 30 mg per day of isotretinoin. But after a dose escalation to 60 mg per day, their acne worsened.
If the patient’s acne is worsening with a cystic flare, “tripling the dose of isotretinoin is not something that you should do,” Dr. Treat said. “You should lower the dose and consider adding steroids.” For evidence-based recommendations on managing acne fulminans, he recommended an article published in the Journal of the American Academy of Dermatology in 2017.
Skin picking is another common reason for failure of isotretinoin, as well as with other acne therapies. These patients may have associated anxiety, which “might be a contraindication or at least something to consider before you put them on isotretinoin,” he noted.
In his experience, off-label use of N-acetylcysteine, an antioxidant and cysteine prodrug, has been “extremely effective” for patients with excoriation disorder. In a randomized trial of adults 18-60 years of age, 47% patients who took 1,200-3,000 mg per day doses of N-acetylcysteine for 12 weeks reported that their skin picking was much or very much improved, compared to 19% of those who took placebo (P = .03). The authors wrote that N-acetylcysteine “increases extracellular levels of glutamate in the nucleus accumbens,” and that these results support the hypothesis that “pharmacologic manipulation of the glutamate system may target core symptoms of compulsive behaviors.”
The tumor necrosis factor (TNF)-alpha blocker adalimumab is a reasonable option for patients with severe cystic inflammatory acne who fail isotretinoin, Dr. Treat said. In one published case, clinicians administered adalimumab 40 mg every other week for a 16-year-old male patient who received isotretinoin for moderate acne vulgaris, which caused sudden development of acne fulminans and incapacitating acute sacroiliitis with bilateral hip arthritis. Inflammatory lesions started to clear in 1 month and comedones improved by 3 months of treatment. Adalimumab was discontinued after 1 year and the patient remained clear.
“There are now multiple reports as well as some case series showing TNF-alpha agents causing clearance of acne,” said Dr. Treat, who directs the hospital’s pediatric dermatology fellowship program. A literature review of adalimumab, etanercept, and infliximab for treatment-resistant acne found that all agents had similar efficacy after 3-6 months of therapy. “We see this in our GI population, where TNF-alpha agents are helping their acne also,” he said. “We just have to augment it with some topical medications.”
Certain medications can drive the development of acne, including phenytoin, phenobarbital, lithium, MEK inhibitors, EGFR inhibitors, systemic steroids, and unopposed progesterone contraceptives. Some genetic conditions also predispose patients to acne, including mutations in the NCSTN gene and trisomy 13.
Dr. Treat discussed one of his patients with severe acne who had trisomy 13. The patient failed 12 months of doxycycline and amoxicillin in combination with a topical retinoid. He also failed low- and high-dose isotretinoin in combination with prednisone, as well as oral dapsone at a dose of 1 mg/kg per day for 3 months. He was started on adalimumab, but that was stopped after he flared. The patient is now maintained on ustekinumab monthly at a dose of 45 mg.
“I’ve only had a few patients where isotretinoin truly has failed,” Dr. Treat said. He described one patient with severe acne who had a hidradenitis-like appearance in his axilla and groin. “I treated with isotretinoin very gingerly in the beginning, [but] he flared significantly. I had given him concomitant steroids from the very beginning and transitioned to multiple different therapies – all of which failed.”
Next, Dr. Treat tried a course of systemic dapsone, and the patient responded nicely. “As an anti-inflammatory agent, dapsone is very reasonable” to consider, he said. “It’s something to add to your armamentarium.”
Dr. Treat disclosed that he is a consultant for Palvella and Regeneron. He has ownership interests in Matinas Biopharma Holdings, Axsome, Sorrento, and Amarin.
AT SPD 2022
Topical gene therapy for dystrophic epidermolysis bullosa shows promise
INDIANAPOLIS – An investigational compared with placebo, according to results from a small phase 3 study.
DEB is a serious, ultra-rare genetic blistering disease caused by mutations in the COL7A1 gene, encoding for type VII collagen and leading to skin fragility and wounds. No approved therapies are currently available. In the study, treatment was generally well tolerated.
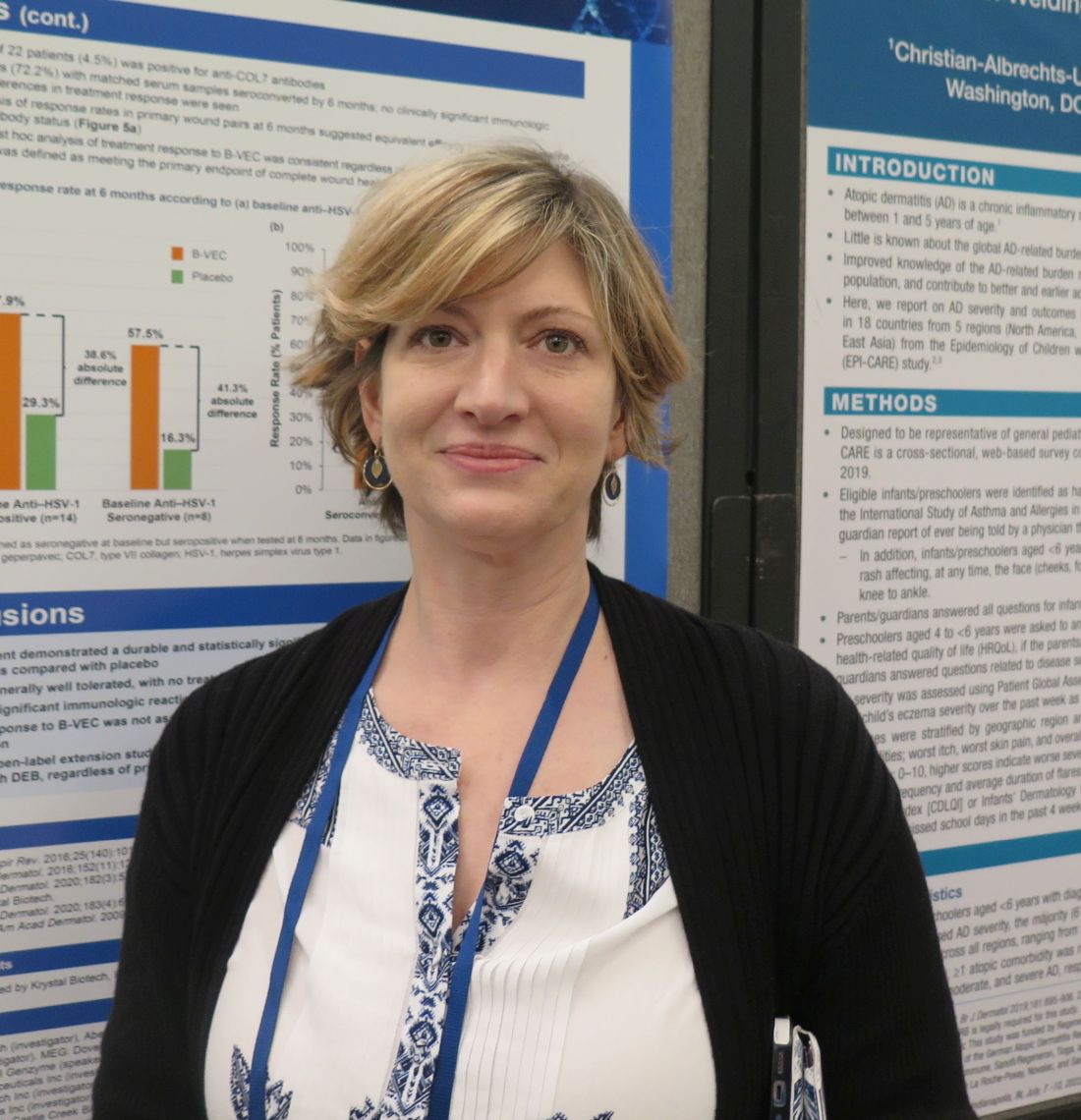
“B-VEC is the first treatment that has not only been shown to be effective, but the first to directly target the defect through topical application,” the study’s principal investigator, Shireen V. Guide, MD, said in an interview during a poster session at the annual meeting of the Society for Pediatric Dermatology. “It delivers type VII collagen gene therapy to these patients, which allows healing in areas that they may have had open since birth. It’s been life-changing for them.”
B-VEC is a herpes simplex virus (HSV-1)-based topical, redosable gene therapy being developed by Krystal Biotech that is designed to restore functional COL7 protein by delivering the COL7A1 gene. For the phase 3, multicenter, double-blind, placebo-controlled study known GEM-3, Dr. Guide, who practices dermatology in Rancho Santa Margarita, Calif., and her colleagues, including Peter Marinkovich, MD, from Stanford (Calif.) University, and Mercedes Gonzalez, MD, from the University of Miami, enrolled 31 patients aged 6 months and older with genetically confirmed DEB. Each patient had one wound treated randomized 1:1 to treatment with B-VEC once a week or placebo for 6 months. The mean age of the 31 study participants was 17 years, 65% were male, 65% were White, and 19% were Asian.
The primary endpoint was complete wound healing (defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage) at 6 months. Additional endpoints included complete wound healing at 3 months and change in pain associated with wound dressing changes.
At 3 months, 70% of wounds treated with B-VEC met the endpoint of complete wound healing, compared with 20% of wounds treated with placebo (P < .005). At 6 months, 67% of wounds treated with B-VEC met the endpoint of complete wound healing compared with 22% of those treated with placebo (P < .005).
Of the total wounds that closed at 3 months, 67% of wounds treated with B-VEC were also closed at 6 months, compared with 33% of those treated with placebo (P = .02). In other findings, a trend toward decreased pain was observed in wounds treated with B-VEC vs. those treated with placebo.
B-VEC was well tolerated with no treatment-related serious adverse events or discontinuations. Three patients experienced a total of five serious adverse events during the study: anemia (two events), and cellulitis, diarrhea, and positive blood culture (one event each). None were considered related to the study drug.
Dr. Guide, who is on staff at Children’s Health of Orange County, Orange, Calif., characterized B-VEC as “very novel because it’s very practical.”
To date, all treatments for DEB “have been extremely labor intensive, including skin grafting and hospitalizations. It’s a topical application that can be done in the office and potentially applied at home in the future. It’s also durable. Not only are the [treated] areas closing, but they are staying closed.”
Kalyani S. Marathe, MD, MPH, director of the dermatology division at Cincinnati Children’s Hospital, who was asked to comment on the study, said that topical application of B-VEC “allows the side effect profile to be very favorable. The results are remarkable in the amount of wound healing and reduction in pain.”
The tolerability of this medication “is crucial,” she added. “EB patients have a lot of pain from their wounds and so any treatment needs to be as painless as possible for it to be usable. I’m very excited about the next phase of studies for this medication and hopeful that it heralds new treatments for our EB patients.”
In June 2022, the manufacturer announced that it had submitted a biologics license application to the Food and Drug Administration for approval of B-VEC for the treatment of DEB, and that it anticipates submitting an application for marketing authorization with the European Medical Agency (EMA) in the second half of 2022.
Dr. Guide disclosed that she has served as an investigator for Krystal Biotech, Innovaderm Research, Arcutis, Premier Research, Paidion, and Castle Biosciences. Dr. Marathe disclosed that she has served as an adviser for Verrica, and that Cincinnati Children’s Hospital is a site for the next phase studies for B-VEC.
*This story was updated on July 25.
INDIANAPOLIS – An investigational compared with placebo, according to results from a small phase 3 study.
DEB is a serious, ultra-rare genetic blistering disease caused by mutations in the COL7A1 gene, encoding for type VII collagen and leading to skin fragility and wounds. No approved therapies are currently available. In the study, treatment was generally well tolerated.
“B-VEC is the first treatment that has not only been shown to be effective, but the first to directly target the defect through topical application,” the study’s principal investigator, Shireen V. Guide, MD, said in an interview during a poster session at the annual meeting of the Society for Pediatric Dermatology. “It delivers type VII collagen gene therapy to these patients, which allows healing in areas that they may have had open since birth. It’s been life-changing for them.”
B-VEC is a herpes simplex virus (HSV-1)-based topical, redosable gene therapy being developed by Krystal Biotech that is designed to restore functional COL7 protein by delivering the COL7A1 gene. For the phase 3, multicenter, double-blind, placebo-controlled study known GEM-3, Dr. Guide, who practices dermatology in Rancho Santa Margarita, Calif., and her colleagues, including Peter Marinkovich, MD, from Stanford (Calif.) University, and Mercedes Gonzalez, MD, from the University of Miami, enrolled 31 patients aged 6 months and older with genetically confirmed DEB. Each patient had one wound treated randomized 1:1 to treatment with B-VEC once a week or placebo for 6 months. The mean age of the 31 study participants was 17 years, 65% were male, 65% were White, and 19% were Asian.
The primary endpoint was complete wound healing (defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage) at 6 months. Additional endpoints included complete wound healing at 3 months and change in pain associated with wound dressing changes.
At 3 months, 70% of wounds treated with B-VEC met the endpoint of complete wound healing, compared with 20% of wounds treated with placebo (P < .005). At 6 months, 67% of wounds treated with B-VEC met the endpoint of complete wound healing compared with 22% of those treated with placebo (P < .005).
Of the total wounds that closed at 3 months, 67% of wounds treated with B-VEC were also closed at 6 months, compared with 33% of those treated with placebo (P = .02). In other findings, a trend toward decreased pain was observed in wounds treated with B-VEC vs. those treated with placebo.
B-VEC was well tolerated with no treatment-related serious adverse events or discontinuations. Three patients experienced a total of five serious adverse events during the study: anemia (two events), and cellulitis, diarrhea, and positive blood culture (one event each). None were considered related to the study drug.
Dr. Guide, who is on staff at Children’s Health of Orange County, Orange, Calif., characterized B-VEC as “very novel because it’s very practical.”
To date, all treatments for DEB “have been extremely labor intensive, including skin grafting and hospitalizations. It’s a topical application that can be done in the office and potentially applied at home in the future. It’s also durable. Not only are the [treated] areas closing, but they are staying closed.”
Kalyani S. Marathe, MD, MPH, director of the dermatology division at Cincinnati Children’s Hospital, who was asked to comment on the study, said that topical application of B-VEC “allows the side effect profile to be very favorable. The results are remarkable in the amount of wound healing and reduction in pain.”
The tolerability of this medication “is crucial,” she added. “EB patients have a lot of pain from their wounds and so any treatment needs to be as painless as possible for it to be usable. I’m very excited about the next phase of studies for this medication and hopeful that it heralds new treatments for our EB patients.”
In June 2022, the manufacturer announced that it had submitted a biologics license application to the Food and Drug Administration for approval of B-VEC for the treatment of DEB, and that it anticipates submitting an application for marketing authorization with the European Medical Agency (EMA) in the second half of 2022.
Dr. Guide disclosed that she has served as an investigator for Krystal Biotech, Innovaderm Research, Arcutis, Premier Research, Paidion, and Castle Biosciences. Dr. Marathe disclosed that she has served as an adviser for Verrica, and that Cincinnati Children’s Hospital is a site for the next phase studies for B-VEC.
*This story was updated on July 25.
INDIANAPOLIS – An investigational compared with placebo, according to results from a small phase 3 study.
DEB is a serious, ultra-rare genetic blistering disease caused by mutations in the COL7A1 gene, encoding for type VII collagen and leading to skin fragility and wounds. No approved therapies are currently available. In the study, treatment was generally well tolerated.
“B-VEC is the first treatment that has not only been shown to be effective, but the first to directly target the defect through topical application,” the study’s principal investigator, Shireen V. Guide, MD, said in an interview during a poster session at the annual meeting of the Society for Pediatric Dermatology. “It delivers type VII collagen gene therapy to these patients, which allows healing in areas that they may have had open since birth. It’s been life-changing for them.”
B-VEC is a herpes simplex virus (HSV-1)-based topical, redosable gene therapy being developed by Krystal Biotech that is designed to restore functional COL7 protein by delivering the COL7A1 gene. For the phase 3, multicenter, double-blind, placebo-controlled study known GEM-3, Dr. Guide, who practices dermatology in Rancho Santa Margarita, Calif., and her colleagues, including Peter Marinkovich, MD, from Stanford (Calif.) University, and Mercedes Gonzalez, MD, from the University of Miami, enrolled 31 patients aged 6 months and older with genetically confirmed DEB. Each patient had one wound treated randomized 1:1 to treatment with B-VEC once a week or placebo for 6 months. The mean age of the 31 study participants was 17 years, 65% were male, 65% were White, and 19% were Asian.
The primary endpoint was complete wound healing (defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage) at 6 months. Additional endpoints included complete wound healing at 3 months and change in pain associated with wound dressing changes.
At 3 months, 70% of wounds treated with B-VEC met the endpoint of complete wound healing, compared with 20% of wounds treated with placebo (P < .005). At 6 months, 67% of wounds treated with B-VEC met the endpoint of complete wound healing compared with 22% of those treated with placebo (P < .005).
Of the total wounds that closed at 3 months, 67% of wounds treated with B-VEC were also closed at 6 months, compared with 33% of those treated with placebo (P = .02). In other findings, a trend toward decreased pain was observed in wounds treated with B-VEC vs. those treated with placebo.
B-VEC was well tolerated with no treatment-related serious adverse events or discontinuations. Three patients experienced a total of five serious adverse events during the study: anemia (two events), and cellulitis, diarrhea, and positive blood culture (one event each). None were considered related to the study drug.
Dr. Guide, who is on staff at Children’s Health of Orange County, Orange, Calif., characterized B-VEC as “very novel because it’s very practical.”
To date, all treatments for DEB “have been extremely labor intensive, including skin grafting and hospitalizations. It’s a topical application that can be done in the office and potentially applied at home in the future. It’s also durable. Not only are the [treated] areas closing, but they are staying closed.”
Kalyani S. Marathe, MD, MPH, director of the dermatology division at Cincinnati Children’s Hospital, who was asked to comment on the study, said that topical application of B-VEC “allows the side effect profile to be very favorable. The results are remarkable in the amount of wound healing and reduction in pain.”
The tolerability of this medication “is crucial,” she added. “EB patients have a lot of pain from their wounds and so any treatment needs to be as painless as possible for it to be usable. I’m very excited about the next phase of studies for this medication and hopeful that it heralds new treatments for our EB patients.”
In June 2022, the manufacturer announced that it had submitted a biologics license application to the Food and Drug Administration for approval of B-VEC for the treatment of DEB, and that it anticipates submitting an application for marketing authorization with the European Medical Agency (EMA) in the second half of 2022.
Dr. Guide disclosed that she has served as an investigator for Krystal Biotech, Innovaderm Research, Arcutis, Premier Research, Paidion, and Castle Biosciences. Dr. Marathe disclosed that she has served as an adviser for Verrica, and that Cincinnati Children’s Hospital is a site for the next phase studies for B-VEC.
*This story was updated on July 25.
AT SPD 2022
Clinical characteristics of recurrent RIME elucidated in chart review
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
Think of pediatric morphea as a systemic, chronic disease, expert advises
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
AT SPD 2022
Study eyes characteristics of pediatric patients with hidradenitis suppurativa
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.

“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.

“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.

“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
AT SPD 2022
What influences a trainee’s decision to choose pediatric dermatology as a career?
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
AT SPD 2022
Eczema causes substantial burden for many infants and preschoolers
INDIANAPOLIS – . Those are key findings from a large international web-based survey that was presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
“Improved knowledge of the AD-related burden may help reinforce the medical need in the pediatric population and contribute to better and earlier adequate management of the disease,” authors led by Stephan Weidinger , MD, PhD, vice head of the department of dermatology at University Hospital Schleswig-Holstein, Kiel, Germany, wrote in the abstract.
For the study, Dr. Weidinger and colleagues evaluated 1,486 infants and preschoolers with AD aged 6 months to under 6 years, who participated in the Epidemiology of Children with Atopic Dermatitis Reporting on their Experience (EPI-CARE), an international, cross-sectional, web-based survey of children and adolescents. The study population resided in 18 countries from five regions of the world, including North America, Latin America, Europe, Middle East/Eurasia, and East Asia. Parents or guardians answered all questions for infants/preschoolers younger than 4 years of age, while preschoolers aged 4 to younger than 6 years were asked to answer questions related to the impact of AD on their health-related quality of life.
AD severity was assessed using Patient Global Assessment (PtGA), where parents or guardians described their child’s eczema severity over the last week as mild, moderate, or severe. The researchers stratified outcomes by geographic region and AD severity, which included the following atopic comorbidities: worst itch, worst skin pain, and overall sleep disturbance in the past 24 hours as measured by the 0-10 numeric rating scale, where higher scores indicate worse severity; eczema-related hospitalization in the past 12 months; and frequency and average duration of flares over the past month.
The mean age of the study participants was 3 years and 61.6% had mild disease. The most common atopic comorbidities were hay fever, asthma, and seasonal allergies, and the incidence of atopic comorbidities increased with increasing AD severity. One or more atopic comorbidities was reported in 88.3% of patients with mild AD, compared with 92.1% of those with moderate disease and 95.8% of those with severe disease. In addition, infants and preschoolers with moderate or severe AD had worse itch, skin pain, and sleep disturbances over the past 24 hours, compared with those who had mild AD.
More than half of infants and preschoolers with severe AD (54.1%) were reported to have been hospitalized in the past 12 months (this ranged from 30.2% to 71.3% across regions), as did 35% of patients with moderate AD and 32.1% of those with mild AD. In addition, 50.6% of infants and preschoolers with severe AD had more than two flares in the past month, compared with 18.1% of those with moderate AD and 6.3% of those with mild disease.
In other findings, 50.7% of infants and preschoolers with severe AD had flares than lasted an average of 2 or more weeks, compared with 20.8% of those with moderate disease and 10% of those with mild disease. Also, 78.3% of preschoolers aged 4 to less than 6 years had missed one or more days of school in the previous 4 weeks: a mean of 5.1 days among those with mild AD, a mean of 7.3 days among those with moderate AD, and a mean of 12.1 days among those with severe disease.
Raj J. Chovatiya MD, PhD, of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that infants and preschoolers remain an understudied group despite the high prevalence of AD in this age range. “The results of this study demonstrate a substantial burden of disease in this population, particularly among those with more severe disease,” said Dr. Chovatiya, who also directs the university’s Center for Eczema and Itch. “This includes longer and more frequent AD flares as well as high rates of inpatient hospitalization. These findings suggest that additional research is needed to better characterize disease burden and optimize outcomes for young children with AD.”
The study was funded by Regeneron Pharmaceuticals and Sanofi. Dr. Weidinger and other coauthors reported having received institutional research grants and consulting fees from many pharmaceutical companies that manufacture drugs used for the treatment of psoriasis and eczema.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, L’Oréal, the National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB.
INDIANAPOLIS – . Those are key findings from a large international web-based survey that was presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
“Improved knowledge of the AD-related burden may help reinforce the medical need in the pediatric population and contribute to better and earlier adequate management of the disease,” authors led by Stephan Weidinger , MD, PhD, vice head of the department of dermatology at University Hospital Schleswig-Holstein, Kiel, Germany, wrote in the abstract.
For the study, Dr. Weidinger and colleagues evaluated 1,486 infants and preschoolers with AD aged 6 months to under 6 years, who participated in the Epidemiology of Children with Atopic Dermatitis Reporting on their Experience (EPI-CARE), an international, cross-sectional, web-based survey of children and adolescents. The study population resided in 18 countries from five regions of the world, including North America, Latin America, Europe, Middle East/Eurasia, and East Asia. Parents or guardians answered all questions for infants/preschoolers younger than 4 years of age, while preschoolers aged 4 to younger than 6 years were asked to answer questions related to the impact of AD on their health-related quality of life.
AD severity was assessed using Patient Global Assessment (PtGA), where parents or guardians described their child’s eczema severity over the last week as mild, moderate, or severe. The researchers stratified outcomes by geographic region and AD severity, which included the following atopic comorbidities: worst itch, worst skin pain, and overall sleep disturbance in the past 24 hours as measured by the 0-10 numeric rating scale, where higher scores indicate worse severity; eczema-related hospitalization in the past 12 months; and frequency and average duration of flares over the past month.
The mean age of the study participants was 3 years and 61.6% had mild disease. The most common atopic comorbidities were hay fever, asthma, and seasonal allergies, and the incidence of atopic comorbidities increased with increasing AD severity. One or more atopic comorbidities was reported in 88.3% of patients with mild AD, compared with 92.1% of those with moderate disease and 95.8% of those with severe disease. In addition, infants and preschoolers with moderate or severe AD had worse itch, skin pain, and sleep disturbances over the past 24 hours, compared with those who had mild AD.
More than half of infants and preschoolers with severe AD (54.1%) were reported to have been hospitalized in the past 12 months (this ranged from 30.2% to 71.3% across regions), as did 35% of patients with moderate AD and 32.1% of those with mild AD. In addition, 50.6% of infants and preschoolers with severe AD had more than two flares in the past month, compared with 18.1% of those with moderate AD and 6.3% of those with mild disease.
In other findings, 50.7% of infants and preschoolers with severe AD had flares than lasted an average of 2 or more weeks, compared with 20.8% of those with moderate disease and 10% of those with mild disease. Also, 78.3% of preschoolers aged 4 to less than 6 years had missed one or more days of school in the previous 4 weeks: a mean of 5.1 days among those with mild AD, a mean of 7.3 days among those with moderate AD, and a mean of 12.1 days among those with severe disease.
Raj J. Chovatiya MD, PhD, of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that infants and preschoolers remain an understudied group despite the high prevalence of AD in this age range. “The results of this study demonstrate a substantial burden of disease in this population, particularly among those with more severe disease,” said Dr. Chovatiya, who also directs the university’s Center for Eczema and Itch. “This includes longer and more frequent AD flares as well as high rates of inpatient hospitalization. These findings suggest that additional research is needed to better characterize disease burden and optimize outcomes for young children with AD.”
The study was funded by Regeneron Pharmaceuticals and Sanofi. Dr. Weidinger and other coauthors reported having received institutional research grants and consulting fees from many pharmaceutical companies that manufacture drugs used for the treatment of psoriasis and eczema.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, L’Oréal, the National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB.
INDIANAPOLIS – . Those are key findings from a large international web-based survey that was presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
“Improved knowledge of the AD-related burden may help reinforce the medical need in the pediatric population and contribute to better and earlier adequate management of the disease,” authors led by Stephan Weidinger , MD, PhD, vice head of the department of dermatology at University Hospital Schleswig-Holstein, Kiel, Germany, wrote in the abstract.
For the study, Dr. Weidinger and colleagues evaluated 1,486 infants and preschoolers with AD aged 6 months to under 6 years, who participated in the Epidemiology of Children with Atopic Dermatitis Reporting on their Experience (EPI-CARE), an international, cross-sectional, web-based survey of children and adolescents. The study population resided in 18 countries from five regions of the world, including North America, Latin America, Europe, Middle East/Eurasia, and East Asia. Parents or guardians answered all questions for infants/preschoolers younger than 4 years of age, while preschoolers aged 4 to younger than 6 years were asked to answer questions related to the impact of AD on their health-related quality of life.
AD severity was assessed using Patient Global Assessment (PtGA), where parents or guardians described their child’s eczema severity over the last week as mild, moderate, or severe. The researchers stratified outcomes by geographic region and AD severity, which included the following atopic comorbidities: worst itch, worst skin pain, and overall sleep disturbance in the past 24 hours as measured by the 0-10 numeric rating scale, where higher scores indicate worse severity; eczema-related hospitalization in the past 12 months; and frequency and average duration of flares over the past month.
The mean age of the study participants was 3 years and 61.6% had mild disease. The most common atopic comorbidities were hay fever, asthma, and seasonal allergies, and the incidence of atopic comorbidities increased with increasing AD severity. One or more atopic comorbidities was reported in 88.3% of patients with mild AD, compared with 92.1% of those with moderate disease and 95.8% of those with severe disease. In addition, infants and preschoolers with moderate or severe AD had worse itch, skin pain, and sleep disturbances over the past 24 hours, compared with those who had mild AD.
More than half of infants and preschoolers with severe AD (54.1%) were reported to have been hospitalized in the past 12 months (this ranged from 30.2% to 71.3% across regions), as did 35% of patients with moderate AD and 32.1% of those with mild AD. In addition, 50.6% of infants and preschoolers with severe AD had more than two flares in the past month, compared with 18.1% of those with moderate AD and 6.3% of those with mild disease.
In other findings, 50.7% of infants and preschoolers with severe AD had flares than lasted an average of 2 or more weeks, compared with 20.8% of those with moderate disease and 10% of those with mild disease. Also, 78.3% of preschoolers aged 4 to less than 6 years had missed one or more days of school in the previous 4 weeks: a mean of 5.1 days among those with mild AD, a mean of 7.3 days among those with moderate AD, and a mean of 12.1 days among those with severe disease.
Raj J. Chovatiya MD, PhD, of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that infants and preschoolers remain an understudied group despite the high prevalence of AD in this age range. “The results of this study demonstrate a substantial burden of disease in this population, particularly among those with more severe disease,” said Dr. Chovatiya, who also directs the university’s Center for Eczema and Itch. “This includes longer and more frequent AD flares as well as high rates of inpatient hospitalization. These findings suggest that additional research is needed to better characterize disease burden and optimize outcomes for young children with AD.”
The study was funded by Regeneron Pharmaceuticals and Sanofi. Dr. Weidinger and other coauthors reported having received institutional research grants and consulting fees from many pharmaceutical companies that manufacture drugs used for the treatment of psoriasis and eczema.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, L’Oréal, the National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB.
AT SPD 2022
Eczema severity, time spent on management strongly associated with overall disease burden
. However, AD severity and spending 11 hours or more per week managing the condition did correlate with higher overall disease burden.
“Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain,” Aaron M. Drucker, MD, of the division of dermatology at the University of Toronto, and colleagues wrote in the study published online in JAMA Dermatology. “Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact.”
Dr. Drucker and colleagues collected data from an externally led patient-focused drug development survey on AD, a 32-item questionnaire that was administered electronically between Aug. 1, 2019, and Oct. 11, 2019. Respondents were asked to rate the overall impact of their AD in the past months and the specific elements of disease burden on a 1-5 scale, with 1 meaning no impact, and 5 meaning a significant impact. They were also asked to rate current mood changes and mood changes at the worst point of AD on a 4-point scale that ranged from “not present” to “severe.” The researchers used multivariable ordinal regression to examine associations between demographic and clinical variables and patient-reported overall AD impact scores.
Survey results
Of the 1,065 respondents, 33% were aged 18-34 years, 50% were aged 35-50 years, 17% were aged 65 years or older, and 83% were female. Nearly half (45%) reported having moderate AD, while 28% had severe AD. When asked about the overall disease burden of AD symptoms in the past month, 30% reported a significant impact on life, 28% reported a moderate impact score, 21% reported a high impact score, 18% reported a low impact score, and 3% of respondents reported no impact.
In the multivariable proportional odds analysis, moderate AD (odds ratio [OR], 4.13) and severe AD (OR, 13.63) were both associated with greater disease burden compared with mild AD. Also, spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (an OR of 2.67 for 11-20 hours per week spent managing AD and OR of 5.34 for 21 or more hours per week spent managing AD).
Correlations between specific impact domains such as sleep, cognitive thinking, and physical activity and overall AD impact scores ranged from weak to moderate, and no individual aspect of disease burden correlated strongly with overall impact scores. The researchers observed similar results after they stratified the analysis by age, current severity, and time spent managing AD.
In other findings, 40% of study participants reported mild changes in mood related to their AD, 30% reported moderate changes, 9% reported severe changes, while the remainder reported no changes in mood. The variable most strongly associated with current mood changes was having severe AD at the time of the survey (OR 5.29).
Understanding of disease burden ‘limited’
“Atopic dermatitis is associated with an immense clinical burden,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “However, our understanding of disease burden from the patient perspective is limited,” he added.
“Interestingly, no single specific element of disease burden was strongly correlated with overall burden, further supporting the complex, multidimensional nature” of the impact of AD, he said, noting that the study “highlights the need for clinicians to look beyond the skin when it comes to AD and underscores the need for additional research to better understand the patient and caregiver perspective.”
Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was also asked to comment on the study, noted that aside from the well discussed impact and burden of itch and its impact on sleep loss, much remains to be learned about the full impact of AD, particularly among adults.
“For example, it is commonly accepted and expected that patients with more severe AD likely experience higher disease burden, but are there other factors that can influence this risk?” she asked. “Can we explain the high impact of AD disease aside from the level of disease severity, particularly among adults with AD?”
The study, she added, “is important because it provides additional insights into those possible factors, including ‘time spent managing their disease’ and ‘associated depression.’ In particular, understanding the association between ‘time spent managing their disease’ and higher disease burden is critical because, in my opinion, it emphasizes the need to develop better strategies for improving the care of patients with AD including the development of more efficacious and safer treatment strategies.”
Dr. Drucker and colleagues acknowledged certain limitations of the analysis, including its cross-sectional design, the potential for selection bias, and the fact that it did not use the patient-oriented outcome measure or the dermatology life quality index. “Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed,” they concluded.
The work was supported in part by a grant from the National Eczema Association (NEA). Dr. Drucker reported that he receives compensation from the British Journal of Dermatology (as reviewer and section editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer). Coauthors representing the NEA and other patient organizations including the Allergy & Asthma Network, Asthma and Allergy Foundation of America, Global Parents for Eczema Research, and International Topical Steroid Awareness Network received organizational grants (Pfizer) and sponsorship funding for these analyses from AbbVie, Eli Lilly, Incyte, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, EPI Health, Incyte, L’Oréal, the NEA, Pfizer, Regeneron, Sanofi, and UCB.
Dr. Chiesa Fuxench disclosed that she has received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, and Vanda for work related to AD She has served as consultant for the Asthma and Allergy Foundation of America, NEA, AbbVie, Incyte Corporation, and Pfizer; and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer.
. However, AD severity and spending 11 hours or more per week managing the condition did correlate with higher overall disease burden.
“Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain,” Aaron M. Drucker, MD, of the division of dermatology at the University of Toronto, and colleagues wrote in the study published online in JAMA Dermatology. “Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact.”
Dr. Drucker and colleagues collected data from an externally led patient-focused drug development survey on AD, a 32-item questionnaire that was administered electronically between Aug. 1, 2019, and Oct. 11, 2019. Respondents were asked to rate the overall impact of their AD in the past months and the specific elements of disease burden on a 1-5 scale, with 1 meaning no impact, and 5 meaning a significant impact. They were also asked to rate current mood changes and mood changes at the worst point of AD on a 4-point scale that ranged from “not present” to “severe.” The researchers used multivariable ordinal regression to examine associations between demographic and clinical variables and patient-reported overall AD impact scores.
Survey results
Of the 1,065 respondents, 33% were aged 18-34 years, 50% were aged 35-50 years, 17% were aged 65 years or older, and 83% were female. Nearly half (45%) reported having moderate AD, while 28% had severe AD. When asked about the overall disease burden of AD symptoms in the past month, 30% reported a significant impact on life, 28% reported a moderate impact score, 21% reported a high impact score, 18% reported a low impact score, and 3% of respondents reported no impact.
In the multivariable proportional odds analysis, moderate AD (odds ratio [OR], 4.13) and severe AD (OR, 13.63) were both associated with greater disease burden compared with mild AD. Also, spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (an OR of 2.67 for 11-20 hours per week spent managing AD and OR of 5.34 for 21 or more hours per week spent managing AD).
Correlations between specific impact domains such as sleep, cognitive thinking, and physical activity and overall AD impact scores ranged from weak to moderate, and no individual aspect of disease burden correlated strongly with overall impact scores. The researchers observed similar results after they stratified the analysis by age, current severity, and time spent managing AD.
In other findings, 40% of study participants reported mild changes in mood related to their AD, 30% reported moderate changes, 9% reported severe changes, while the remainder reported no changes in mood. The variable most strongly associated with current mood changes was having severe AD at the time of the survey (OR 5.29).
Understanding of disease burden ‘limited’
“Atopic dermatitis is associated with an immense clinical burden,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “However, our understanding of disease burden from the patient perspective is limited,” he added.
“Interestingly, no single specific element of disease burden was strongly correlated with overall burden, further supporting the complex, multidimensional nature” of the impact of AD, he said, noting that the study “highlights the need for clinicians to look beyond the skin when it comes to AD and underscores the need for additional research to better understand the patient and caregiver perspective.”
Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was also asked to comment on the study, noted that aside from the well discussed impact and burden of itch and its impact on sleep loss, much remains to be learned about the full impact of AD, particularly among adults.
“For example, it is commonly accepted and expected that patients with more severe AD likely experience higher disease burden, but are there other factors that can influence this risk?” she asked. “Can we explain the high impact of AD disease aside from the level of disease severity, particularly among adults with AD?”
The study, she added, “is important because it provides additional insights into those possible factors, including ‘time spent managing their disease’ and ‘associated depression.’ In particular, understanding the association between ‘time spent managing their disease’ and higher disease burden is critical because, in my opinion, it emphasizes the need to develop better strategies for improving the care of patients with AD including the development of more efficacious and safer treatment strategies.”
Dr. Drucker and colleagues acknowledged certain limitations of the analysis, including its cross-sectional design, the potential for selection bias, and the fact that it did not use the patient-oriented outcome measure or the dermatology life quality index. “Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed,” they concluded.
The work was supported in part by a grant from the National Eczema Association (NEA). Dr. Drucker reported that he receives compensation from the British Journal of Dermatology (as reviewer and section editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer). Coauthors representing the NEA and other patient organizations including the Allergy & Asthma Network, Asthma and Allergy Foundation of America, Global Parents for Eczema Research, and International Topical Steroid Awareness Network received organizational grants (Pfizer) and sponsorship funding for these analyses from AbbVie, Eli Lilly, Incyte, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, EPI Health, Incyte, L’Oréal, the NEA, Pfizer, Regeneron, Sanofi, and UCB.
Dr. Chiesa Fuxench disclosed that she has received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, and Vanda for work related to AD She has served as consultant for the Asthma and Allergy Foundation of America, NEA, AbbVie, Incyte Corporation, and Pfizer; and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer.
. However, AD severity and spending 11 hours or more per week managing the condition did correlate with higher overall disease burden.
“Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain,” Aaron M. Drucker, MD, of the division of dermatology at the University of Toronto, and colleagues wrote in the study published online in JAMA Dermatology. “Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact.”
Dr. Drucker and colleagues collected data from an externally led patient-focused drug development survey on AD, a 32-item questionnaire that was administered electronically between Aug. 1, 2019, and Oct. 11, 2019. Respondents were asked to rate the overall impact of their AD in the past months and the specific elements of disease burden on a 1-5 scale, with 1 meaning no impact, and 5 meaning a significant impact. They were also asked to rate current mood changes and mood changes at the worst point of AD on a 4-point scale that ranged from “not present” to “severe.” The researchers used multivariable ordinal regression to examine associations between demographic and clinical variables and patient-reported overall AD impact scores.
Survey results
Of the 1,065 respondents, 33% were aged 18-34 years, 50% were aged 35-50 years, 17% were aged 65 years or older, and 83% were female. Nearly half (45%) reported having moderate AD, while 28% had severe AD. When asked about the overall disease burden of AD symptoms in the past month, 30% reported a significant impact on life, 28% reported a moderate impact score, 21% reported a high impact score, 18% reported a low impact score, and 3% of respondents reported no impact.
In the multivariable proportional odds analysis, moderate AD (odds ratio [OR], 4.13) and severe AD (OR, 13.63) were both associated with greater disease burden compared with mild AD. Also, spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (an OR of 2.67 for 11-20 hours per week spent managing AD and OR of 5.34 for 21 or more hours per week spent managing AD).
Correlations between specific impact domains such as sleep, cognitive thinking, and physical activity and overall AD impact scores ranged from weak to moderate, and no individual aspect of disease burden correlated strongly with overall impact scores. The researchers observed similar results after they stratified the analysis by age, current severity, and time spent managing AD.
In other findings, 40% of study participants reported mild changes in mood related to their AD, 30% reported moderate changes, 9% reported severe changes, while the remainder reported no changes in mood. The variable most strongly associated with current mood changes was having severe AD at the time of the survey (OR 5.29).
Understanding of disease burden ‘limited’
“Atopic dermatitis is associated with an immense clinical burden,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “However, our understanding of disease burden from the patient perspective is limited,” he added.
“Interestingly, no single specific element of disease burden was strongly correlated with overall burden, further supporting the complex, multidimensional nature” of the impact of AD, he said, noting that the study “highlights the need for clinicians to look beyond the skin when it comes to AD and underscores the need for additional research to better understand the patient and caregiver perspective.”
Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was also asked to comment on the study, noted that aside from the well discussed impact and burden of itch and its impact on sleep loss, much remains to be learned about the full impact of AD, particularly among adults.
“For example, it is commonly accepted and expected that patients with more severe AD likely experience higher disease burden, but are there other factors that can influence this risk?” she asked. “Can we explain the high impact of AD disease aside from the level of disease severity, particularly among adults with AD?”
The study, she added, “is important because it provides additional insights into those possible factors, including ‘time spent managing their disease’ and ‘associated depression.’ In particular, understanding the association between ‘time spent managing their disease’ and higher disease burden is critical because, in my opinion, it emphasizes the need to develop better strategies for improving the care of patients with AD including the development of more efficacious and safer treatment strategies.”
Dr. Drucker and colleagues acknowledged certain limitations of the analysis, including its cross-sectional design, the potential for selection bias, and the fact that it did not use the patient-oriented outcome measure or the dermatology life quality index. “Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed,” they concluded.
The work was supported in part by a grant from the National Eczema Association (NEA). Dr. Drucker reported that he receives compensation from the British Journal of Dermatology (as reviewer and section editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer). Coauthors representing the NEA and other patient organizations including the Allergy & Asthma Network, Asthma and Allergy Foundation of America, Global Parents for Eczema Research, and International Topical Steroid Awareness Network received organizational grants (Pfizer) and sponsorship funding for these analyses from AbbVie, Eli Lilly, Incyte, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, EPI Health, Incyte, L’Oréal, the NEA, Pfizer, Regeneron, Sanofi, and UCB.
Dr. Chiesa Fuxench disclosed that she has received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, and Vanda for work related to AD She has served as consultant for the Asthma and Allergy Foundation of America, NEA, AbbVie, Incyte Corporation, and Pfizer; and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer.
FROM JAMA DERMATOLOGY