User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
High percentage of nursing home residents found to harbor MDROs
SAN DIEGO – Nearly half of nursing home residents harbored multi-drug resistant organisms on their skin, results from a large multi-center surveillance study showed.
“Residents in skilled nursing homes are the most vulnerable patients in the health care system,” lead study author James A. McKinnell, MD, said in an interview in advance of an annual scientific meeting on infectious diseases. “Many residents depend on help from health care workers for routine needs like eating or bathing. Skilled nursing facilities have an obligation to optimize the personal hygiene and environmental cleanliness in skilled nursing facilities.”

The researchers obtained 2,797 body swabs from 1,400 residents in all. Swabs were processed for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), extended spectrum beta-lactamase producers (ESBLs), and carbapenem-resistant Enterobacteriaceae (CRE). “MRSA is a relatively well known bacteria, but CRE is the new pathogen that the CDC has defined as an urgent public health threat,” Dr. McKinnell said.
The researchers also conducted environmental surveillance of commonly touched items in skilled nursing facilities. The five surfaces tested in resident rooms were the bedside table, TV remote, door knobs, light switch, and bathrooms. The five surfaces tested in common areas were the nursing station counter, tables, chairs, hallway hand rails, and drinking fountains.
Overall, 49% of residents harbored MDROs. MRSA was found in 37% of residents, followed by ESBL in 16%, VRE in 7%, and CRE in 1%. Resident MDRO status was known for 11% of MRSA carriers, compared with 18% of ESBL, 4% of VRE, and none of the CRE carriers. Rates of colonization did not differ whether residents had long stays at the facility or postacute stays (49% vs. 48%, respectively), but bed-bound residents were more likely to be MDRO colonized, compared with ambulatory residents (59% vs. 46%; P less than .001). In the analysis of environmental swabs, 93% of common areas and 74% of resident rooms had an MDRO-positive object, with an average of 2.5 and 1.9 objects, respectively, found to be contaminated.
“The fact that about half of patients were carrying a bacteria that could cause infection on their skin was very high,” said Dr. McKinnell, who is a member of the Infectious Disease Clinical Outcome Research Unit at the Los Angeles Biomedical Research Institute at Harbor-UCLA. “Studies conducted in other care settings, we would typically see less than a quarter of patients carry these types of bacteria. I was also surprised to see that 1% of patients were carrying the CRE bacteria on their skin.”
He acknowledged certain limitations of the study, including the fact that the data were taken from a select group of nursing homes that are participating in an interventional study to improve personal hygiene for skilled nursing facility residents. “They may not be representative of all skilled nursing facilities,” he said.
The Agency for Healthcare Research and Quality funded the study. Dr. McKinnell disclosed that he is conducting studies in health care facilities with products supplied from 3M, Clorox, Sage, and Xttrium Laboratories. Many of his coauthors disclosed numerous financial ties to the pharmaceutical industry. The event was the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
SAN DIEGO – Nearly half of nursing home residents harbored multi-drug resistant organisms on their skin, results from a large multi-center surveillance study showed.
“Residents in skilled nursing homes are the most vulnerable patients in the health care system,” lead study author James A. McKinnell, MD, said in an interview in advance of an annual scientific meeting on infectious diseases. “Many residents depend on help from health care workers for routine needs like eating or bathing. Skilled nursing facilities have an obligation to optimize the personal hygiene and environmental cleanliness in skilled nursing facilities.”

The researchers obtained 2,797 body swabs from 1,400 residents in all. Swabs were processed for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), extended spectrum beta-lactamase producers (ESBLs), and carbapenem-resistant Enterobacteriaceae (CRE). “MRSA is a relatively well known bacteria, but CRE is the new pathogen that the CDC has defined as an urgent public health threat,” Dr. McKinnell said.
The researchers also conducted environmental surveillance of commonly touched items in skilled nursing facilities. The five surfaces tested in resident rooms were the bedside table, TV remote, door knobs, light switch, and bathrooms. The five surfaces tested in common areas were the nursing station counter, tables, chairs, hallway hand rails, and drinking fountains.
Overall, 49% of residents harbored MDROs. MRSA was found in 37% of residents, followed by ESBL in 16%, VRE in 7%, and CRE in 1%. Resident MDRO status was known for 11% of MRSA carriers, compared with 18% of ESBL, 4% of VRE, and none of the CRE carriers. Rates of colonization did not differ whether residents had long stays at the facility or postacute stays (49% vs. 48%, respectively), but bed-bound residents were more likely to be MDRO colonized, compared with ambulatory residents (59% vs. 46%; P less than .001). In the analysis of environmental swabs, 93% of common areas and 74% of resident rooms had an MDRO-positive object, with an average of 2.5 and 1.9 objects, respectively, found to be contaminated.
“The fact that about half of patients were carrying a bacteria that could cause infection on their skin was very high,” said Dr. McKinnell, who is a member of the Infectious Disease Clinical Outcome Research Unit at the Los Angeles Biomedical Research Institute at Harbor-UCLA. “Studies conducted in other care settings, we would typically see less than a quarter of patients carry these types of bacteria. I was also surprised to see that 1% of patients were carrying the CRE bacteria on their skin.”
He acknowledged certain limitations of the study, including the fact that the data were taken from a select group of nursing homes that are participating in an interventional study to improve personal hygiene for skilled nursing facility residents. “They may not be representative of all skilled nursing facilities,” he said.
The Agency for Healthcare Research and Quality funded the study. Dr. McKinnell disclosed that he is conducting studies in health care facilities with products supplied from 3M, Clorox, Sage, and Xttrium Laboratories. Many of his coauthors disclosed numerous financial ties to the pharmaceutical industry. The event was the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
SAN DIEGO – Nearly half of nursing home residents harbored multi-drug resistant organisms on their skin, results from a large multi-center surveillance study showed.
“Residents in skilled nursing homes are the most vulnerable patients in the health care system,” lead study author James A. McKinnell, MD, said in an interview in advance of an annual scientific meeting on infectious diseases. “Many residents depend on help from health care workers for routine needs like eating or bathing. Skilled nursing facilities have an obligation to optimize the personal hygiene and environmental cleanliness in skilled nursing facilities.”

The researchers obtained 2,797 body swabs from 1,400 residents in all. Swabs were processed for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), extended spectrum beta-lactamase producers (ESBLs), and carbapenem-resistant Enterobacteriaceae (CRE). “MRSA is a relatively well known bacteria, but CRE is the new pathogen that the CDC has defined as an urgent public health threat,” Dr. McKinnell said.
The researchers also conducted environmental surveillance of commonly touched items in skilled nursing facilities. The five surfaces tested in resident rooms were the bedside table, TV remote, door knobs, light switch, and bathrooms. The five surfaces tested in common areas were the nursing station counter, tables, chairs, hallway hand rails, and drinking fountains.
Overall, 49% of residents harbored MDROs. MRSA was found in 37% of residents, followed by ESBL in 16%, VRE in 7%, and CRE in 1%. Resident MDRO status was known for 11% of MRSA carriers, compared with 18% of ESBL, 4% of VRE, and none of the CRE carriers. Rates of colonization did not differ whether residents had long stays at the facility or postacute stays (49% vs. 48%, respectively), but bed-bound residents were more likely to be MDRO colonized, compared with ambulatory residents (59% vs. 46%; P less than .001). In the analysis of environmental swabs, 93% of common areas and 74% of resident rooms had an MDRO-positive object, with an average of 2.5 and 1.9 objects, respectively, found to be contaminated.
“The fact that about half of patients were carrying a bacteria that could cause infection on their skin was very high,” said Dr. McKinnell, who is a member of the Infectious Disease Clinical Outcome Research Unit at the Los Angeles Biomedical Research Institute at Harbor-UCLA. “Studies conducted in other care settings, we would typically see less than a quarter of patients carry these types of bacteria. I was also surprised to see that 1% of patients were carrying the CRE bacteria on their skin.”
He acknowledged certain limitations of the study, including the fact that the data were taken from a select group of nursing homes that are participating in an interventional study to improve personal hygiene for skilled nursing facility residents. “They may not be representative of all skilled nursing facilities,” he said.
The Agency for Healthcare Research and Quality funded the study. Dr. McKinnell disclosed that he is conducting studies in health care facilities with products supplied from 3M, Clorox, Sage, and Xttrium Laboratories. Many of his coauthors disclosed numerous financial ties to the pharmaceutical industry. The event was the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
AT ID WEEK 2017
Key clinical point: About one of every two nursing home residents is colonized with multidrug resistant organisms.
Major finding:
Study details: A surveillance study of 1,400 residents at 28 skilled nursing facilities in Southern California.
Disclosures: The Agency for Healthcare Research and Quality funded the study. Dr. McKinnell disclosed that he is conducting studies in health care facilities with products supplied from 3M, Clorox, Sage, and Xttrium Laboratories. Many of his coauthors disclosed numerous financial ties to the pharmaceutical industry.
Is the patient-centered medical home on life support?
The way Edward J. Bujold, MD, sees it, the patient-centered medical home (PCMH) as a model of care has become unsustainable in the current health care landscape.
In an opinion piece published online in JAMA Internal Medicine, Dr. Bujold describes how he reinvested Medicare incentive payments to build a solo family medicine practice into a patient-centered medical home with a staff of 14 full-time and part-time employees, including an embedded psychologist, pharmacist, physical therapist, and dietitian.
“Although only about one-quarter of the patients cared for at our practice are Medicare beneficiaries, other insurers and those they insure have benefited immensely,” wrote Dr. Bujold, owner of Granite Falls (N.C.) Family Medical Care Center (JAMA Internal Med. 2017 Sept 25. doi: 10.1001/jamainternmed.2017.4651).
The bonuses from the Center for Medicare & Medicaid Services’ Meaningful Use and Physician Quality Reporting System “enabled us to develop and support the financial structure required to create the PCMH,” he noted. “For the most part, this financial support ended in December 2015. Our financial losses in 2016 are enough to jeopardize the foundation of our PCMH.”
When Congress voted to end the Medicare Sustainable Growth Rate formula, the Primary Care Incentive Payment Program authorized under the Affordable Care Act also ended.
“During 2017, a value-based payment system to replace and hopefully increase our reimbursement is to be phased in,” Dr. Bujold wrote. “The problem for practices like ours is the Medicare Access and Children’s Health Insurance Program Reauthorization Act of 2015/Merit-Based Incentive Payment System (MACRA/MIPS) does not take effect until 2019, and there is no guarantee we will receive any more money than we receive now. We may receive less.”
Citing studies from Oregon and from the Patient-Centered Primary Care Collaborative, Dr. Bujold said that for every dollar invested in primary care, the overarching health system saves about $13.
“The 80% reduction in hospital admissions for our practice is a testimony to these savings,” he stated. “The irony for me is that, 5 years ago, I generated a substantial amount of my income from the hospital portion of my practice, because I was seeing an average of five patients per day in the hospital.”
As a result of transitioning his practice to a PCMH, however, he now sees “one or two and sometimes no hospital patients per day.” At the same time, his office’s overhead cost is 65% and increasing – up from 50% when he opened his practice 31 years ago.
Dr. Bujold noted that about 60% of primary care physicians are employed by hospital organizations, which are able to charge a facility fee “above and beyond what the independent primary care physician charges in his or her office. This increases the cost of care in general by 20%-30% and the patient out-of-pocket costs by a factor of 2 or 3.”
In his view, “we have large hospital systems, the pharmaceutical industry, large insurance companies, and other moneyed players and their lobbyists with vested financial interests. The view from my practice is that these industries seem to hold all of the cards, and it is very difficult to move forward. I am more pessimistic than I was 5 years ago.”
Despite his current misgivings, Dr. Bujold ended his opinion piece by stating that the best way to “heal the health care system in the United States” is one directed by primary care and rooted in high-functioning PCMHs.
“The trajectory must be changed,” Dr. Bujold wrote. “In many areas, payers are not supporting PCMH transformation, and states are not engaging practices or working with Medicare to support transformation. Investing in high-functioning PCMH practices is the right thing to do.”
Dr. Bujold disclosed that he is employed by KPN Health as chief physician strategist and is an advisory board member of the Patient-Centered Primary Care Collaborative.
Financial support for primary care varies around the country and can also vary by payer within an individual region. Dr. Bujold describes very limited commercial-payer support for the care his practice delivers. In contrast, Rhode Island required that all payers increase the percent of total medical dollars for fully insured members paid to primary care by 1% annually from 2010 to 2014 and then sustain that increased percentage. This requirement resulted in substantial support for sustained practice changes in care delivery.
Starting in 2011, the Centers for Medicare & Medicaid Services (CMS) began programs to test new changes in primary care delivery and payment. Comprehensive Primary Care Plus, the largest such Medicare model to date, is being tested in 14 regions of the country, as well as 4 more regions starting in 2018, and is evaluated independently by region; the 5-year program is designed to strengthen primary care through multipayer-payment reform and care-delivery transformation.
The model includes two primary care practice tracks with incrementally advanced care-delivery requirements and payment options. Practices in both tracks work to improve patient access and continuity, provide care management to the patients at highest risk, engage patients and caregivers, focus on planned care and population health, and increase the comprehensiveness and coordination of care.
Primary care – and the patient-centered medical home – can be saved, but there is considerable work to be done. In our view, the delivery of comprehensive primary care requires population-based payments aligned across payers to ensure adequate support for care-delivery changes for all the patients in the practice. Policies and payment across the health care system should be aligned to support a robust primary care infrastructure that delivers on its promise of higher health care quality at lower costs.
Patients should experience the benefits of comprehensive primary care. They should expect their insurer to pay for it and their primary care practice to deliver it.
Financial support for primary care varies around the country and can also vary by payer within an individual region. Dr. Bujold describes very limited commercial-payer support for the care his practice delivers. In contrast, Rhode Island required that all payers increase the percent of total medical dollars for fully insured members paid to primary care by 1% annually from 2010 to 2014 and then sustain that increased percentage. This requirement resulted in substantial support for sustained practice changes in care delivery.
Starting in 2011, the Centers for Medicare & Medicaid Services (CMS) began programs to test new changes in primary care delivery and payment. Comprehensive Primary Care Plus, the largest such Medicare model to date, is being tested in 14 regions of the country, as well as 4 more regions starting in 2018, and is evaluated independently by region; the 5-year program is designed to strengthen primary care through multipayer-payment reform and care-delivery transformation.
The model includes two primary care practice tracks with incrementally advanced care-delivery requirements and payment options. Practices in both tracks work to improve patient access and continuity, provide care management to the patients at highest risk, engage patients and caregivers, focus on planned care and population health, and increase the comprehensiveness and coordination of care.
Primary care – and the patient-centered medical home – can be saved, but there is considerable work to be done. In our view, the delivery of comprehensive primary care requires population-based payments aligned across payers to ensure adequate support for care-delivery changes for all the patients in the practice. Policies and payment across the health care system should be aligned to support a robust primary care infrastructure that delivers on its promise of higher health care quality at lower costs.
Patients should experience the benefits of comprehensive primary care. They should expect their insurer to pay for it and their primary care practice to deliver it.
Financial support for primary care varies around the country and can also vary by payer within an individual region. Dr. Bujold describes very limited commercial-payer support for the care his practice delivers. In contrast, Rhode Island required that all payers increase the percent of total medical dollars for fully insured members paid to primary care by 1% annually from 2010 to 2014 and then sustain that increased percentage. This requirement resulted in substantial support for sustained practice changes in care delivery.
Starting in 2011, the Centers for Medicare & Medicaid Services (CMS) began programs to test new changes in primary care delivery and payment. Comprehensive Primary Care Plus, the largest such Medicare model to date, is being tested in 14 regions of the country, as well as 4 more regions starting in 2018, and is evaluated independently by region; the 5-year program is designed to strengthen primary care through multipayer-payment reform and care-delivery transformation.
The model includes two primary care practice tracks with incrementally advanced care-delivery requirements and payment options. Practices in both tracks work to improve patient access and continuity, provide care management to the patients at highest risk, engage patients and caregivers, focus on planned care and population health, and increase the comprehensiveness and coordination of care.
Primary care – and the patient-centered medical home – can be saved, but there is considerable work to be done. In our view, the delivery of comprehensive primary care requires population-based payments aligned across payers to ensure adequate support for care-delivery changes for all the patients in the practice. Policies and payment across the health care system should be aligned to support a robust primary care infrastructure that delivers on its promise of higher health care quality at lower costs.
Patients should experience the benefits of comprehensive primary care. They should expect their insurer to pay for it and their primary care practice to deliver it.
The way Edward J. Bujold, MD, sees it, the patient-centered medical home (PCMH) as a model of care has become unsustainable in the current health care landscape.
In an opinion piece published online in JAMA Internal Medicine, Dr. Bujold describes how he reinvested Medicare incentive payments to build a solo family medicine practice into a patient-centered medical home with a staff of 14 full-time and part-time employees, including an embedded psychologist, pharmacist, physical therapist, and dietitian.
“Although only about one-quarter of the patients cared for at our practice are Medicare beneficiaries, other insurers and those they insure have benefited immensely,” wrote Dr. Bujold, owner of Granite Falls (N.C.) Family Medical Care Center (JAMA Internal Med. 2017 Sept 25. doi: 10.1001/jamainternmed.2017.4651).
The bonuses from the Center for Medicare & Medicaid Services’ Meaningful Use and Physician Quality Reporting System “enabled us to develop and support the financial structure required to create the PCMH,” he noted. “For the most part, this financial support ended in December 2015. Our financial losses in 2016 are enough to jeopardize the foundation of our PCMH.”
When Congress voted to end the Medicare Sustainable Growth Rate formula, the Primary Care Incentive Payment Program authorized under the Affordable Care Act also ended.
“During 2017, a value-based payment system to replace and hopefully increase our reimbursement is to be phased in,” Dr. Bujold wrote. “The problem for practices like ours is the Medicare Access and Children’s Health Insurance Program Reauthorization Act of 2015/Merit-Based Incentive Payment System (MACRA/MIPS) does not take effect until 2019, and there is no guarantee we will receive any more money than we receive now. We may receive less.”
Citing studies from Oregon and from the Patient-Centered Primary Care Collaborative, Dr. Bujold said that for every dollar invested in primary care, the overarching health system saves about $13.
“The 80% reduction in hospital admissions for our practice is a testimony to these savings,” he stated. “The irony for me is that, 5 years ago, I generated a substantial amount of my income from the hospital portion of my practice, because I was seeing an average of five patients per day in the hospital.”
As a result of transitioning his practice to a PCMH, however, he now sees “one or two and sometimes no hospital patients per day.” At the same time, his office’s overhead cost is 65% and increasing – up from 50% when he opened his practice 31 years ago.
Dr. Bujold noted that about 60% of primary care physicians are employed by hospital organizations, which are able to charge a facility fee “above and beyond what the independent primary care physician charges in his or her office. This increases the cost of care in general by 20%-30% and the patient out-of-pocket costs by a factor of 2 or 3.”
In his view, “we have large hospital systems, the pharmaceutical industry, large insurance companies, and other moneyed players and their lobbyists with vested financial interests. The view from my practice is that these industries seem to hold all of the cards, and it is very difficult to move forward. I am more pessimistic than I was 5 years ago.”
Despite his current misgivings, Dr. Bujold ended his opinion piece by stating that the best way to “heal the health care system in the United States” is one directed by primary care and rooted in high-functioning PCMHs.
“The trajectory must be changed,” Dr. Bujold wrote. “In many areas, payers are not supporting PCMH transformation, and states are not engaging practices or working with Medicare to support transformation. Investing in high-functioning PCMH practices is the right thing to do.”
Dr. Bujold disclosed that he is employed by KPN Health as chief physician strategist and is an advisory board member of the Patient-Centered Primary Care Collaborative.
The way Edward J. Bujold, MD, sees it, the patient-centered medical home (PCMH) as a model of care has become unsustainable in the current health care landscape.
In an opinion piece published online in JAMA Internal Medicine, Dr. Bujold describes how he reinvested Medicare incentive payments to build a solo family medicine practice into a patient-centered medical home with a staff of 14 full-time and part-time employees, including an embedded psychologist, pharmacist, physical therapist, and dietitian.
“Although only about one-quarter of the patients cared for at our practice are Medicare beneficiaries, other insurers and those they insure have benefited immensely,” wrote Dr. Bujold, owner of Granite Falls (N.C.) Family Medical Care Center (JAMA Internal Med. 2017 Sept 25. doi: 10.1001/jamainternmed.2017.4651).
The bonuses from the Center for Medicare & Medicaid Services’ Meaningful Use and Physician Quality Reporting System “enabled us to develop and support the financial structure required to create the PCMH,” he noted. “For the most part, this financial support ended in December 2015. Our financial losses in 2016 are enough to jeopardize the foundation of our PCMH.”
When Congress voted to end the Medicare Sustainable Growth Rate formula, the Primary Care Incentive Payment Program authorized under the Affordable Care Act also ended.
“During 2017, a value-based payment system to replace and hopefully increase our reimbursement is to be phased in,” Dr. Bujold wrote. “The problem for practices like ours is the Medicare Access and Children’s Health Insurance Program Reauthorization Act of 2015/Merit-Based Incentive Payment System (MACRA/MIPS) does not take effect until 2019, and there is no guarantee we will receive any more money than we receive now. We may receive less.”
Citing studies from Oregon and from the Patient-Centered Primary Care Collaborative, Dr. Bujold said that for every dollar invested in primary care, the overarching health system saves about $13.
“The 80% reduction in hospital admissions for our practice is a testimony to these savings,” he stated. “The irony for me is that, 5 years ago, I generated a substantial amount of my income from the hospital portion of my practice, because I was seeing an average of five patients per day in the hospital.”
As a result of transitioning his practice to a PCMH, however, he now sees “one or two and sometimes no hospital patients per day.” At the same time, his office’s overhead cost is 65% and increasing – up from 50% when he opened his practice 31 years ago.
Dr. Bujold noted that about 60% of primary care physicians are employed by hospital organizations, which are able to charge a facility fee “above and beyond what the independent primary care physician charges in his or her office. This increases the cost of care in general by 20%-30% and the patient out-of-pocket costs by a factor of 2 or 3.”
In his view, “we have large hospital systems, the pharmaceutical industry, large insurance companies, and other moneyed players and their lobbyists with vested financial interests. The view from my practice is that these industries seem to hold all of the cards, and it is very difficult to move forward. I am more pessimistic than I was 5 years ago.”
Despite his current misgivings, Dr. Bujold ended his opinion piece by stating that the best way to “heal the health care system in the United States” is one directed by primary care and rooted in high-functioning PCMHs.
“The trajectory must be changed,” Dr. Bujold wrote. “In many areas, payers are not supporting PCMH transformation, and states are not engaging practices or working with Medicare to support transformation. Investing in high-functioning PCMH practices is the right thing to do.”
Dr. Bujold disclosed that he is employed by KPN Health as chief physician strategist and is an advisory board member of the Patient-Centered Primary Care Collaborative.
FROM JAMA INTERNAL MEDICINE
A spike in syphilis puts prenatal care in focus
Fifteen years ago, reported cases of syphilis in the United States were so infrequent that public health officials thought it might join the ranks of malaria, polio, and smallpox as an eradicated disease. That turned out to be wishful thinking.
According to data from the Centers for Disease Control and Prevention, between 2012 and 2015, the overall rates of syphilis in the United States increased by 48%, while the rates of primary and secondary infection among women spiked by 56%. That was a compelling enough rise, but fresh data from the agency indicate that the overall rates of syphilis increased by 17.6% between 2015 and 2016, and by 74% between 2012 and 2016. 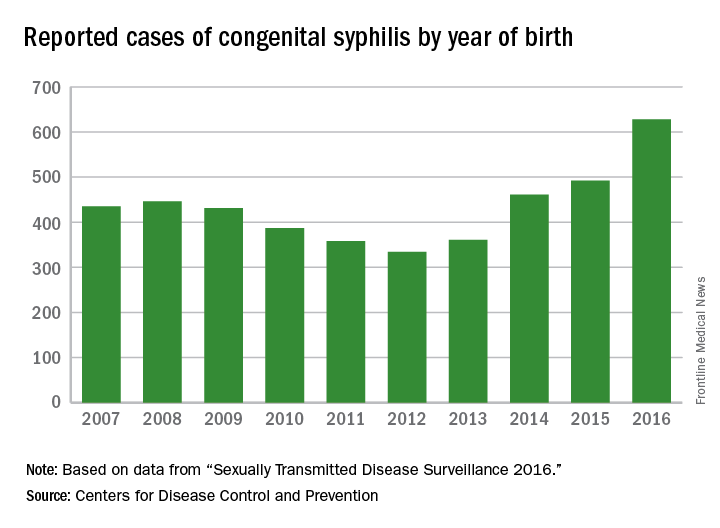
These trends prompted the CDC to launch a “call to action” educational campaign in an effort to curb the rising syphilis rates. The United States Preventive Services Task Force also is taking action. It recently posted a research plan on screening pregnant women for syphilis that will form the basis of a forthcoming evidence review and, potentially, new recommendations.

observed in all regions of the United States during the same time period, said Dr. Kidd, who coauthored a 2015 Morbidity and Mortality Weekly Report on the topic (MMWR. 2015 Nov 13;64[44]:1241-5). That analysis found that during 2012-2014, the number of reported CS cases in the United States increased from 334 to 458, which represents a rate increase from 8.4 to 11.6 cases per 100,000 live births. This contrasted with earlier data, which found that the overall rate of reported CS had decreased from 10.5 to 8.4 cases per 100,000 live births during 2008-2012.
In 2016, there were 628 reported cases of CS, including 41 syphilitic stillbirths, according to the CDC.
“Congenital syphilis rates tend to track female syphilis rates; so as female rates go up, we know we’re going to see a rise in congenital syphilis rates,” Dr. Kidd said. “One way to prevent syphilis is to prevent female syphilis altogether. Another way is to prevent the transmission from mother to infant when you have a pregnant woman with syphilis.”
Lack of prenatal care
CDC guidelines recommend that all pregnant women undergo routine serologic screening for syphilis during their first prenatal visit. Additional testing at 28 weeks’ gestation and again at delivery is warranted for women who are at increased risk or live in communities with increased prevalence of syphilis infection. That approach may seem sensible, but such prevention measures are ineffective when mothers don’t receive any prenatal care or receive it late, which happens in about half of all CS cases, Dr. Kidd said.
Inconsistent, inadequate, or a total absence of prenatal care is “probably the biggest risk factor for vertical transmission, especially among high-risk populations, where there is an increased background prevalence of syphilis in childbearing women,” said Robert Maupin, MD, professor of clinical obstetrics and gynecology in the section of maternal-fetal medicine at Louisiana State University Health Sciences Center, New Orleans.
To complicate matters, women who receive no or inconsistent prenatal care face an increased risk for preterm birth, Dr. Maupin noted. So while a clinician might follow CDC recommendations that pregnant women with confirmed or suspected syphilis complete a course of long-acting penicillin G for at least 30 days or longer before the child is born, “the timing of being able to implement effective prevention and treatment prior to that 30-day window can sometimes be compromised by the fact that she ends up delivering prematurely,” he said. “If someone’s not adequately linked to consistent prenatal care, she may not complete that full course of prevention. Additionally, patterns of care are often fragmented, meaning that patients may go to one clinic or one provider, may not return, and may end up switching to a different clinic. That translates into a potential lag in implementing treatment or making a diagnosis in the first place, and that may be disruptive in the context of our attempted prevention measures.”
Precise reasons why some pregnant women in the United States receive no or inadequate prenatal care remain unclear.
“Anecdotally, in the West, I hear that women with drug abuse histories or drug abuse issues [are vulnerable], or they may be homeless or have mental health issues,” Dr. Kidd said. “In other areas of the country, people feel that it’s more of an insurance or access to care issue, but we don’t have data on that here at the CDC.”
Repeat screening
In 2015, a large analysis of women who were commercially-insured or Medicaid-insured found that more than 95% who received prenatal care were screened for syphilis at least once during pregnancy (Obstet Gynecol. 2015;125[5]:1211-6). However, CDC data of CS cases shows that about 15% of their mothers are infected during pregnancy, which would occur after that first screening test.
“That’s where the repeat screening early in the third trimester and at delivery becomes the real issue,” Dr. Kidd said. “For high-risk women, including those who live in the high morbidity areas, they should be screened again later in pregnancy. Many ob.gyns. may not be aware of that recommendation, or may not be aware they’re in an area that does have a high syphilis morbidity, and that the pregnant women who are seeing them may be at increased risk of syphilis.”
Dr. Maupin, who is associate dean of diversity and community engagement at LSU Health Sciences Center, advised clinicians to view CS with the same sense of urgency that existed in previous years with perinatal HIV transmission.
“In the last decade and a half we’ve seen a substantial decline in perinatal HIV transmission because of intensive efforts on the public health side in terms of both screening and use of treatment,” he said. “If we look at this with a similar level of contemporary urgency, it will bear similar fruit over time. Additionally, from a maternal-fetal medicine standpoint, the more effectively we treat and/or control diseases and comorbidities prior to pregnancy, the less likely those things will have an adverse impact on the health and well-being of the newborn.”
Steps you can take to curb CS
In its “call to action” on syphilis, the Centers for Disease Control and Prevention cited several practical ways that clinicians can combat the spread of congenital syphilis (CS).
1. Complete a sexual history for your patients. The CDC recommends following this with STD counseling for those at risk and contraception counseling for women at risk of unintended pregnancy.
2. Test all pregnant women for syphilis. This should be done at the first prenatal visit, with repeat screening for pregnant women at high risk and in areas of high prevalence at the beginning of the third trimester and again at delivery.
3. Treat women infected with syphilis immediately. If a woman has syphilis or suspected syphilis, she should be treated with long-acting penicillin G, especially if she is pregnant. CDC also calls for testing and treating the infected woman’s sex partner(s) to avoid reinfection.
4. Confirm syphilis testing at delivery. Before discharging the mother or infant from the hospital, check that the mother has been tested for syphilis at least once during pregnancy or at delivery. All women who deliver a stillborn infant should be tested for syphilis.
5. Report CS cases to the local or state health department within 24 hours.
Fifteen years ago, reported cases of syphilis in the United States were so infrequent that public health officials thought it might join the ranks of malaria, polio, and smallpox as an eradicated disease. That turned out to be wishful thinking.
According to data from the Centers for Disease Control and Prevention, between 2012 and 2015, the overall rates of syphilis in the United States increased by 48%, while the rates of primary and secondary infection among women spiked by 56%. That was a compelling enough rise, but fresh data from the agency indicate that the overall rates of syphilis increased by 17.6% between 2015 and 2016, and by 74% between 2012 and 2016. 
These trends prompted the CDC to launch a “call to action” educational campaign in an effort to curb the rising syphilis rates. The United States Preventive Services Task Force also is taking action. It recently posted a research plan on screening pregnant women for syphilis that will form the basis of a forthcoming evidence review and, potentially, new recommendations.

observed in all regions of the United States during the same time period, said Dr. Kidd, who coauthored a 2015 Morbidity and Mortality Weekly Report on the topic (MMWR. 2015 Nov 13;64[44]:1241-5). That analysis found that during 2012-2014, the number of reported CS cases in the United States increased from 334 to 458, which represents a rate increase from 8.4 to 11.6 cases per 100,000 live births. This contrasted with earlier data, which found that the overall rate of reported CS had decreased from 10.5 to 8.4 cases per 100,000 live births during 2008-2012.
In 2016, there were 628 reported cases of CS, including 41 syphilitic stillbirths, according to the CDC.
“Congenital syphilis rates tend to track female syphilis rates; so as female rates go up, we know we’re going to see a rise in congenital syphilis rates,” Dr. Kidd said. “One way to prevent syphilis is to prevent female syphilis altogether. Another way is to prevent the transmission from mother to infant when you have a pregnant woman with syphilis.”
Lack of prenatal care
CDC guidelines recommend that all pregnant women undergo routine serologic screening for syphilis during their first prenatal visit. Additional testing at 28 weeks’ gestation and again at delivery is warranted for women who are at increased risk or live in communities with increased prevalence of syphilis infection. That approach may seem sensible, but such prevention measures are ineffective when mothers don’t receive any prenatal care or receive it late, which happens in about half of all CS cases, Dr. Kidd said.
Inconsistent, inadequate, or a total absence of prenatal care is “probably the biggest risk factor for vertical transmission, especially among high-risk populations, where there is an increased background prevalence of syphilis in childbearing women,” said Robert Maupin, MD, professor of clinical obstetrics and gynecology in the section of maternal-fetal medicine at Louisiana State University Health Sciences Center, New Orleans.
To complicate matters, women who receive no or inconsistent prenatal care face an increased risk for preterm birth, Dr. Maupin noted. So while a clinician might follow CDC recommendations that pregnant women with confirmed or suspected syphilis complete a course of long-acting penicillin G for at least 30 days or longer before the child is born, “the timing of being able to implement effective prevention and treatment prior to that 30-day window can sometimes be compromised by the fact that she ends up delivering prematurely,” he said. “If someone’s not adequately linked to consistent prenatal care, she may not complete that full course of prevention. Additionally, patterns of care are often fragmented, meaning that patients may go to one clinic or one provider, may not return, and may end up switching to a different clinic. That translates into a potential lag in implementing treatment or making a diagnosis in the first place, and that may be disruptive in the context of our attempted prevention measures.”
Precise reasons why some pregnant women in the United States receive no or inadequate prenatal care remain unclear.
“Anecdotally, in the West, I hear that women with drug abuse histories or drug abuse issues [are vulnerable], or they may be homeless or have mental health issues,” Dr. Kidd said. “In other areas of the country, people feel that it’s more of an insurance or access to care issue, but we don’t have data on that here at the CDC.”
Repeat screening
In 2015, a large analysis of women who were commercially-insured or Medicaid-insured found that more than 95% who received prenatal care were screened for syphilis at least once during pregnancy (Obstet Gynecol. 2015;125[5]:1211-6). However, CDC data of CS cases shows that about 15% of their mothers are infected during pregnancy, which would occur after that first screening test.
“That’s where the repeat screening early in the third trimester and at delivery becomes the real issue,” Dr. Kidd said. “For high-risk women, including those who live in the high morbidity areas, they should be screened again later in pregnancy. Many ob.gyns. may not be aware of that recommendation, or may not be aware they’re in an area that does have a high syphilis morbidity, and that the pregnant women who are seeing them may be at increased risk of syphilis.”
Dr. Maupin, who is associate dean of diversity and community engagement at LSU Health Sciences Center, advised clinicians to view CS with the same sense of urgency that existed in previous years with perinatal HIV transmission.
“In the last decade and a half we’ve seen a substantial decline in perinatal HIV transmission because of intensive efforts on the public health side in terms of both screening and use of treatment,” he said. “If we look at this with a similar level of contemporary urgency, it will bear similar fruit over time. Additionally, from a maternal-fetal medicine standpoint, the more effectively we treat and/or control diseases and comorbidities prior to pregnancy, the less likely those things will have an adverse impact on the health and well-being of the newborn.”
Steps you can take to curb CS
In its “call to action” on syphilis, the Centers for Disease Control and Prevention cited several practical ways that clinicians can combat the spread of congenital syphilis (CS).
1. Complete a sexual history for your patients. The CDC recommends following this with STD counseling for those at risk and contraception counseling for women at risk of unintended pregnancy.
2. Test all pregnant women for syphilis. This should be done at the first prenatal visit, with repeat screening for pregnant women at high risk and in areas of high prevalence at the beginning of the third trimester and again at delivery.
3. Treat women infected with syphilis immediately. If a woman has syphilis or suspected syphilis, she should be treated with long-acting penicillin G, especially if she is pregnant. CDC also calls for testing and treating the infected woman’s sex partner(s) to avoid reinfection.
4. Confirm syphilis testing at delivery. Before discharging the mother or infant from the hospital, check that the mother has been tested for syphilis at least once during pregnancy or at delivery. All women who deliver a stillborn infant should be tested for syphilis.
5. Report CS cases to the local or state health department within 24 hours.
Fifteen years ago, reported cases of syphilis in the United States were so infrequent that public health officials thought it might join the ranks of malaria, polio, and smallpox as an eradicated disease. That turned out to be wishful thinking.
According to data from the Centers for Disease Control and Prevention, between 2012 and 2015, the overall rates of syphilis in the United States increased by 48%, while the rates of primary and secondary infection among women spiked by 56%. That was a compelling enough rise, but fresh data from the agency indicate that the overall rates of syphilis increased by 17.6% between 2015 and 2016, and by 74% between 2012 and 2016. 
These trends prompted the CDC to launch a “call to action” educational campaign in an effort to curb the rising syphilis rates. The United States Preventive Services Task Force also is taking action. It recently posted a research plan on screening pregnant women for syphilis that will form the basis of a forthcoming evidence review and, potentially, new recommendations.

observed in all regions of the United States during the same time period, said Dr. Kidd, who coauthored a 2015 Morbidity and Mortality Weekly Report on the topic (MMWR. 2015 Nov 13;64[44]:1241-5). That analysis found that during 2012-2014, the number of reported CS cases in the United States increased from 334 to 458, which represents a rate increase from 8.4 to 11.6 cases per 100,000 live births. This contrasted with earlier data, which found that the overall rate of reported CS had decreased from 10.5 to 8.4 cases per 100,000 live births during 2008-2012.
In 2016, there were 628 reported cases of CS, including 41 syphilitic stillbirths, according to the CDC.
“Congenital syphilis rates tend to track female syphilis rates; so as female rates go up, we know we’re going to see a rise in congenital syphilis rates,” Dr. Kidd said. “One way to prevent syphilis is to prevent female syphilis altogether. Another way is to prevent the transmission from mother to infant when you have a pregnant woman with syphilis.”
Lack of prenatal care
CDC guidelines recommend that all pregnant women undergo routine serologic screening for syphilis during their first prenatal visit. Additional testing at 28 weeks’ gestation and again at delivery is warranted for women who are at increased risk or live in communities with increased prevalence of syphilis infection. That approach may seem sensible, but such prevention measures are ineffective when mothers don’t receive any prenatal care or receive it late, which happens in about half of all CS cases, Dr. Kidd said.
Inconsistent, inadequate, or a total absence of prenatal care is “probably the biggest risk factor for vertical transmission, especially among high-risk populations, where there is an increased background prevalence of syphilis in childbearing women,” said Robert Maupin, MD, professor of clinical obstetrics and gynecology in the section of maternal-fetal medicine at Louisiana State University Health Sciences Center, New Orleans.
To complicate matters, women who receive no or inconsistent prenatal care face an increased risk for preterm birth, Dr. Maupin noted. So while a clinician might follow CDC recommendations that pregnant women with confirmed or suspected syphilis complete a course of long-acting penicillin G for at least 30 days or longer before the child is born, “the timing of being able to implement effective prevention and treatment prior to that 30-day window can sometimes be compromised by the fact that she ends up delivering prematurely,” he said. “If someone’s not adequately linked to consistent prenatal care, she may not complete that full course of prevention. Additionally, patterns of care are often fragmented, meaning that patients may go to one clinic or one provider, may not return, and may end up switching to a different clinic. That translates into a potential lag in implementing treatment or making a diagnosis in the first place, and that may be disruptive in the context of our attempted prevention measures.”
Precise reasons why some pregnant women in the United States receive no or inadequate prenatal care remain unclear.
“Anecdotally, in the West, I hear that women with drug abuse histories or drug abuse issues [are vulnerable], or they may be homeless or have mental health issues,” Dr. Kidd said. “In other areas of the country, people feel that it’s more of an insurance or access to care issue, but we don’t have data on that here at the CDC.”
Repeat screening
In 2015, a large analysis of women who were commercially-insured or Medicaid-insured found that more than 95% who received prenatal care were screened for syphilis at least once during pregnancy (Obstet Gynecol. 2015;125[5]:1211-6). However, CDC data of CS cases shows that about 15% of their mothers are infected during pregnancy, which would occur after that first screening test.
“That’s where the repeat screening early in the third trimester and at delivery becomes the real issue,” Dr. Kidd said. “For high-risk women, including those who live in the high morbidity areas, they should be screened again later in pregnancy. Many ob.gyns. may not be aware of that recommendation, or may not be aware they’re in an area that does have a high syphilis morbidity, and that the pregnant women who are seeing them may be at increased risk of syphilis.”
Dr. Maupin, who is associate dean of diversity and community engagement at LSU Health Sciences Center, advised clinicians to view CS with the same sense of urgency that existed in previous years with perinatal HIV transmission.
“In the last decade and a half we’ve seen a substantial decline in perinatal HIV transmission because of intensive efforts on the public health side in terms of both screening and use of treatment,” he said. “If we look at this with a similar level of contemporary urgency, it will bear similar fruit over time. Additionally, from a maternal-fetal medicine standpoint, the more effectively we treat and/or control diseases and comorbidities prior to pregnancy, the less likely those things will have an adverse impact on the health and well-being of the newborn.”
Steps you can take to curb CS
In its “call to action” on syphilis, the Centers for Disease Control and Prevention cited several practical ways that clinicians can combat the spread of congenital syphilis (CS).
1. Complete a sexual history for your patients. The CDC recommends following this with STD counseling for those at risk and contraception counseling for women at risk of unintended pregnancy.
2. Test all pregnant women for syphilis. This should be done at the first prenatal visit, with repeat screening for pregnant women at high risk and in areas of high prevalence at the beginning of the third trimester and again at delivery.
3. Treat women infected with syphilis immediately. If a woman has syphilis or suspected syphilis, she should be treated with long-acting penicillin G, especially if she is pregnant. CDC also calls for testing and treating the infected woman’s sex partner(s) to avoid reinfection.
4. Confirm syphilis testing at delivery. Before discharging the mother or infant from the hospital, check that the mother has been tested for syphilis at least once during pregnancy or at delivery. All women who deliver a stillborn infant should be tested for syphilis.
5. Report CS cases to the local or state health department within 24 hours.
Immunogenicity concerns for biosimilars so far don’t go beyond originator biologics
A key factor that impacts the efficacy and the toxicity of biologics used for rheumatic diseases is their immunogenicity, and this factor doesn’t appear to be any different for biosimilars in studies conducted so far.
In a meta-analysis of 63 studies of tumor necrosis factor (TNF) inhibitors, investigators including Daniel E. Furst, MD, professor of rheumatology at the University of Washington, Seattle, who also is affiliated with the University of California, Los Angeles, and the University of Florence, Italy, found that antidrug antibodies developed in 17% of patients (BioDrugs 2015;29:241-58).
“That doesn’t sound too bad, but does it differ by medication?” asked Dr. Furst, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “For infliximab, 30% of the time, there are antidrug antibodies. So if that has a clinical effect, that’s a big deal. For certolizumab ... it’s only 6%, so there is a huge difference within the TNF inhibitors.” At the same time, antidrug antibodies developed in patients on adalimumab 23% of the time, followed by certolizumab (6%), golimumab (4%), and etanercept (2%).
One approach to circumventing the impact of antidrug antibodies on clinical response is by using immunosuppression, which in the meta-analysis had a 70% probability for decreasing antidrug antibodies. But this approach comes with a hitch, Dr. Furst said. The effect of immunosuppression on antidrug antibody positivity differs by disease. Immunosuppression had a 78% probability for decreasing antidrug antibody positivity in RA, 63% probability in inflammatory bowel disease, and 32% probability in ankylosing spondylitis. “That’s a wild mix of immunogenicity and the possibility that it’s going to affect the underlying response,” he said. The best studies in this area are of infliximab and adalimumab, which showed that antidrug antibodies in patients on infliximab reduced the probability of a clinical response by 54% and for adalimumab by 65%.
The same meta-analysis found that there were more antibodies to adalimumab than to certolizumab, golimumab, or etanercept, and also more antibodies to infliximab than to certolizumab, golimumab, or etanercept, but no difference between adalimumab and infliximab. “You’d think that maybe there is a difference between adalimumab and infliximab, but that’s not true,” Dr. Furst said. The apparent effect on the percentage of antidrug antibodies depends on what type of assay is used. For example, radioimmunoassay measures about 11% more antidrug antibodies, compared with enzyme-linked immunosorbent assay. Disease duration also matters. Each year of disease duration increases the antidrug antibodies by 1%.
What about the non-TNF inhibitors? A meta-analysis of five core trials of tocilizumab for RA found that the antidrug antibodies ranged from 1.7% at baseline to 2.3% during the respective trials (Clin Ther. 2010;32:1597-1609). “Anywhere along the way, the percentage of antidrug antibodies was about 1.5%,” said Dr. Furst, who was not involved with the study. “So as opposed to the TNF inhibitors, when you look at tocilizumab, this whole immunity question is probably a non-issue. The same is true for abatacept. With rituximab, the question is a little bit different. In one randomized, controlled trial, immunogenicity was 2.9%, while in the next it was 7.9%. In an open-label trial, it was 11.5%. So I think for rituximab we have to assume that you have some potentially important antidrug antibodies that might affect response.”
Studies have shown that antidrug antibodies do affect the pharmacokinetics of a biologic (and thereby clinical response) by producing lower trough levels of the biologic through an increase in its clearance. However, studies of infliximab and its biosimilars have not yielded any significant differences in clinical responses rates despite small differences in rates of antidrug antibodies or in rates of treatment-emergent adverse events, he said.
There has also been no difference in disease worsening in the only reported double-blind, randomized, switching study for infliximab and an infliximab biosimilar, the NOR-SWITCH study. However, the small numbers of patients in the study with certain diseases for which infliximab is indicated do not allow for conclusions to be drawn for specific diseases, Dr. Furst said.
Dr. Furst disclosed that he has received grant/research support from AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, and Roche/Genentech. He is also a consultant for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Cytori, Novartis, Pfizer, and Roche/Genentech. Global Academy for Medical Education and this news organization are owned by the same parent company.
A key factor that impacts the efficacy and the toxicity of biologics used for rheumatic diseases is their immunogenicity, and this factor doesn’t appear to be any different for biosimilars in studies conducted so far.
In a meta-analysis of 63 studies of tumor necrosis factor (TNF) inhibitors, investigators including Daniel E. Furst, MD, professor of rheumatology at the University of Washington, Seattle, who also is affiliated with the University of California, Los Angeles, and the University of Florence, Italy, found that antidrug antibodies developed in 17% of patients (BioDrugs 2015;29:241-58).
“That doesn’t sound too bad, but does it differ by medication?” asked Dr. Furst, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “For infliximab, 30% of the time, there are antidrug antibodies. So if that has a clinical effect, that’s a big deal. For certolizumab ... it’s only 6%, so there is a huge difference within the TNF inhibitors.” At the same time, antidrug antibodies developed in patients on adalimumab 23% of the time, followed by certolizumab (6%), golimumab (4%), and etanercept (2%).
One approach to circumventing the impact of antidrug antibodies on clinical response is by using immunosuppression, which in the meta-analysis had a 70% probability for decreasing antidrug antibodies. But this approach comes with a hitch, Dr. Furst said. The effect of immunosuppression on antidrug antibody positivity differs by disease. Immunosuppression had a 78% probability for decreasing antidrug antibody positivity in RA, 63% probability in inflammatory bowel disease, and 32% probability in ankylosing spondylitis. “That’s a wild mix of immunogenicity and the possibility that it’s going to affect the underlying response,” he said. The best studies in this area are of infliximab and adalimumab, which showed that antidrug antibodies in patients on infliximab reduced the probability of a clinical response by 54% and for adalimumab by 65%.
The same meta-analysis found that there were more antibodies to adalimumab than to certolizumab, golimumab, or etanercept, and also more antibodies to infliximab than to certolizumab, golimumab, or etanercept, but no difference between adalimumab and infliximab. “You’d think that maybe there is a difference between adalimumab and infliximab, but that’s not true,” Dr. Furst said. The apparent effect on the percentage of antidrug antibodies depends on what type of assay is used. For example, radioimmunoassay measures about 11% more antidrug antibodies, compared with enzyme-linked immunosorbent assay. Disease duration also matters. Each year of disease duration increases the antidrug antibodies by 1%.
What about the non-TNF inhibitors? A meta-analysis of five core trials of tocilizumab for RA found that the antidrug antibodies ranged from 1.7% at baseline to 2.3% during the respective trials (Clin Ther. 2010;32:1597-1609). “Anywhere along the way, the percentage of antidrug antibodies was about 1.5%,” said Dr. Furst, who was not involved with the study. “So as opposed to the TNF inhibitors, when you look at tocilizumab, this whole immunity question is probably a non-issue. The same is true for abatacept. With rituximab, the question is a little bit different. In one randomized, controlled trial, immunogenicity was 2.9%, while in the next it was 7.9%. In an open-label trial, it was 11.5%. So I think for rituximab we have to assume that you have some potentially important antidrug antibodies that might affect response.”
Studies have shown that antidrug antibodies do affect the pharmacokinetics of a biologic (and thereby clinical response) by producing lower trough levels of the biologic through an increase in its clearance. However, studies of infliximab and its biosimilars have not yielded any significant differences in clinical responses rates despite small differences in rates of antidrug antibodies or in rates of treatment-emergent adverse events, he said.
There has also been no difference in disease worsening in the only reported double-blind, randomized, switching study for infliximab and an infliximab biosimilar, the NOR-SWITCH study. However, the small numbers of patients in the study with certain diseases for which infliximab is indicated do not allow for conclusions to be drawn for specific diseases, Dr. Furst said.
Dr. Furst disclosed that he has received grant/research support from AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, and Roche/Genentech. He is also a consultant for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Cytori, Novartis, Pfizer, and Roche/Genentech. Global Academy for Medical Education and this news organization are owned by the same parent company.
A key factor that impacts the efficacy and the toxicity of biologics used for rheumatic diseases is their immunogenicity, and this factor doesn’t appear to be any different for biosimilars in studies conducted so far.
In a meta-analysis of 63 studies of tumor necrosis factor (TNF) inhibitors, investigators including Daniel E. Furst, MD, professor of rheumatology at the University of Washington, Seattle, who also is affiliated with the University of California, Los Angeles, and the University of Florence, Italy, found that antidrug antibodies developed in 17% of patients (BioDrugs 2015;29:241-58).
“That doesn’t sound too bad, but does it differ by medication?” asked Dr. Furst, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “For infliximab, 30% of the time, there are antidrug antibodies. So if that has a clinical effect, that’s a big deal. For certolizumab ... it’s only 6%, so there is a huge difference within the TNF inhibitors.” At the same time, antidrug antibodies developed in patients on adalimumab 23% of the time, followed by certolizumab (6%), golimumab (4%), and etanercept (2%).
One approach to circumventing the impact of antidrug antibodies on clinical response is by using immunosuppression, which in the meta-analysis had a 70% probability for decreasing antidrug antibodies. But this approach comes with a hitch, Dr. Furst said. The effect of immunosuppression on antidrug antibody positivity differs by disease. Immunosuppression had a 78% probability for decreasing antidrug antibody positivity in RA, 63% probability in inflammatory bowel disease, and 32% probability in ankylosing spondylitis. “That’s a wild mix of immunogenicity and the possibility that it’s going to affect the underlying response,” he said. The best studies in this area are of infliximab and adalimumab, which showed that antidrug antibodies in patients on infliximab reduced the probability of a clinical response by 54% and for adalimumab by 65%.
The same meta-analysis found that there were more antibodies to adalimumab than to certolizumab, golimumab, or etanercept, and also more antibodies to infliximab than to certolizumab, golimumab, or etanercept, but no difference between adalimumab and infliximab. “You’d think that maybe there is a difference between adalimumab and infliximab, but that’s not true,” Dr. Furst said. The apparent effect on the percentage of antidrug antibodies depends on what type of assay is used. For example, radioimmunoassay measures about 11% more antidrug antibodies, compared with enzyme-linked immunosorbent assay. Disease duration also matters. Each year of disease duration increases the antidrug antibodies by 1%.
What about the non-TNF inhibitors? A meta-analysis of five core trials of tocilizumab for RA found that the antidrug antibodies ranged from 1.7% at baseline to 2.3% during the respective trials (Clin Ther. 2010;32:1597-1609). “Anywhere along the way, the percentage of antidrug antibodies was about 1.5%,” said Dr. Furst, who was not involved with the study. “So as opposed to the TNF inhibitors, when you look at tocilizumab, this whole immunity question is probably a non-issue. The same is true for abatacept. With rituximab, the question is a little bit different. In one randomized, controlled trial, immunogenicity was 2.9%, while in the next it was 7.9%. In an open-label trial, it was 11.5%. So I think for rituximab we have to assume that you have some potentially important antidrug antibodies that might affect response.”
Studies have shown that antidrug antibodies do affect the pharmacokinetics of a biologic (and thereby clinical response) by producing lower trough levels of the biologic through an increase in its clearance. However, studies of infliximab and its biosimilars have not yielded any significant differences in clinical responses rates despite small differences in rates of antidrug antibodies or in rates of treatment-emergent adverse events, he said.
There has also been no difference in disease worsening in the only reported double-blind, randomized, switching study for infliximab and an infliximab biosimilar, the NOR-SWITCH study. However, the small numbers of patients in the study with certain diseases for which infliximab is indicated do not allow for conclusions to be drawn for specific diseases, Dr. Furst said.
Dr. Furst disclosed that he has received grant/research support from AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, and Roche/Genentech. He is also a consultant for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Cytori, Novartis, Pfizer, and Roche/Genentech. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Early ankylosing spondylitis treatment stops transition from inflammatory to bone-forming fatty lesions
Structural damage in axial and peripheral ankylosing spondylitis is the result of a combination of destruction and new bone formation, which can be halted if treated early and for long enough, according to Dirk Elewaut, MD, PhD.
“There is a longstanding paradox in the field of ankylosing spondylitis, as to whether there is a strict relationship between the inflammation that leads to the signs and symptoms in patients, and the structural progression,” said Dr. Elewaut, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “ ”
One key reason why the early TNF inhibition trials showed no effect of treatment on structural progression stemmed from inadequate study design. “The original studies were underpowered, and the patients used NSAIDs,” he said. “We also know that the anti-inflammatory effect [of TNF inhibition] is not 100%. So you have a clear drop in inflammation with biologics, but it’s not completely stopped. This led to a lot of speculation as to whether inflammation and ankyloses are coupled or not.”
More recently, researchers have honed in on the transition from acute inflammatory lesions visible on MRI by bone marrow edema to so-called fatty lesions, which are also visible on MRI. They have observed that fatty lesions are associated with new bone formation. “Lesions containing more fat are thought to be more difficult to modulate by biological therapy,” Dr. Elewaut said. “Once you have a fatty lesion, you have some kind of metabolic disease of the bone, and it’s a one-way road to the development of new syndesmophytes. So in other words, it is essential to assess the relationship between inflammation and new bone formation with both the STIR [short tau inversion recovery images] and T1-weighted MRI.”
A working hypothesis among AS researchers is that the effect of anti-TNF therapy on radiologic progression depends on the relative number of acute and mature inflammatory lesions that individual patients have. “Early diagnosis is a prerequisite for advances in disease modification,” he said. At least three “windows of opportunity” for disease modification exist, Dr. Elewaut continued. One is that the link between inflammation as measured by clinical parameters and new bone formation will be more evident in early AS. The second is that early treatment of patients with axial AS and spinal inflammation with anti-TNF agents will prevent new bone formation. The third is that reduction of new bone formation in established AS will be observed only with long-term anti-TNF therapy after mature lesions have resolved/repaired, and no new inflammatory lesions are being formed. One study found that patients with a delay of more than 10 years starting anti-TNF therapy were more likely to progress, compared with patients who received therapy earlier (odds ratio, 2.4; P = .03; see Arthritis Rheum. 2013;65:645-54). “The message here is quite clear,” Dr. Elewaut said. “The earlier we treat intensively, the better impact you have on structural outcomes.”
In a trial known as CRESPA (Clinical Remission in peripheral SPondyloArthritis), researchers including Dr. Elewaut evaluated the efficacy and safety of golimumab (Simponi) to induce clinical remission in patients with early, active peripheral AS (Ann Rheum Dis. 2017;76:1389-95). In all, 60 patients were randomized to golimumab or placebo for 24 weeks. At week 24, a significantly higher percentage of patients receiving golimumab achieved clinical remission, compared with placebo (75% vs. 20%, respectively; P less than .001). At week 12, similar results were observed (70% vs. 15%; P less than .001).
“These were very striking results in this early disease population,” Dr. Elewaut said. In a follow-up analysis, the researchers withdrew therapy in patients who reached clinical remission, to see what would happen. “At 1.5 years of follow-up, 57% of patients are in drug-free remission,” Dr. Elewaut said. “This is interesting and suggests that at least in a fraction of those patients, you can actually achieve a drug-free remission.” Predictors for relapse included pre-existing psoriasis and having more than five swollen joints.
Dr. Elewaut disclosed that he is a member of the speakers bureau for AbbVie, Novartis, Pfizer, and UCB. Global Academy for Medical Education and this news organization are owned by the same parent company.
Structural damage in axial and peripheral ankylosing spondylitis is the result of a combination of destruction and new bone formation, which can be halted if treated early and for long enough, according to Dirk Elewaut, MD, PhD.
“There is a longstanding paradox in the field of ankylosing spondylitis, as to whether there is a strict relationship between the inflammation that leads to the signs and symptoms in patients, and the structural progression,” said Dr. Elewaut, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “ ”
One key reason why the early TNF inhibition trials showed no effect of treatment on structural progression stemmed from inadequate study design. “The original studies were underpowered, and the patients used NSAIDs,” he said. “We also know that the anti-inflammatory effect [of TNF inhibition] is not 100%. So you have a clear drop in inflammation with biologics, but it’s not completely stopped. This led to a lot of speculation as to whether inflammation and ankyloses are coupled or not.”
More recently, researchers have honed in on the transition from acute inflammatory lesions visible on MRI by bone marrow edema to so-called fatty lesions, which are also visible on MRI. They have observed that fatty lesions are associated with new bone formation. “Lesions containing more fat are thought to be more difficult to modulate by biological therapy,” Dr. Elewaut said. “Once you have a fatty lesion, you have some kind of metabolic disease of the bone, and it’s a one-way road to the development of new syndesmophytes. So in other words, it is essential to assess the relationship between inflammation and new bone formation with both the STIR [short tau inversion recovery images] and T1-weighted MRI.”
A working hypothesis among AS researchers is that the effect of anti-TNF therapy on radiologic progression depends on the relative number of acute and mature inflammatory lesions that individual patients have. “Early diagnosis is a prerequisite for advances in disease modification,” he said. At least three “windows of opportunity” for disease modification exist, Dr. Elewaut continued. One is that the link between inflammation as measured by clinical parameters and new bone formation will be more evident in early AS. The second is that early treatment of patients with axial AS and spinal inflammation with anti-TNF agents will prevent new bone formation. The third is that reduction of new bone formation in established AS will be observed only with long-term anti-TNF therapy after mature lesions have resolved/repaired, and no new inflammatory lesions are being formed. One study found that patients with a delay of more than 10 years starting anti-TNF therapy were more likely to progress, compared with patients who received therapy earlier (odds ratio, 2.4; P = .03; see Arthritis Rheum. 2013;65:645-54). “The message here is quite clear,” Dr. Elewaut said. “The earlier we treat intensively, the better impact you have on structural outcomes.”
In a trial known as CRESPA (Clinical Remission in peripheral SPondyloArthritis), researchers including Dr. Elewaut evaluated the efficacy and safety of golimumab (Simponi) to induce clinical remission in patients with early, active peripheral AS (Ann Rheum Dis. 2017;76:1389-95). In all, 60 patients were randomized to golimumab or placebo for 24 weeks. At week 24, a significantly higher percentage of patients receiving golimumab achieved clinical remission, compared with placebo (75% vs. 20%, respectively; P less than .001). At week 12, similar results were observed (70% vs. 15%; P less than .001).
“These were very striking results in this early disease population,” Dr. Elewaut said. In a follow-up analysis, the researchers withdrew therapy in patients who reached clinical remission, to see what would happen. “At 1.5 years of follow-up, 57% of patients are in drug-free remission,” Dr. Elewaut said. “This is interesting and suggests that at least in a fraction of those patients, you can actually achieve a drug-free remission.” Predictors for relapse included pre-existing psoriasis and having more than five swollen joints.
Dr. Elewaut disclosed that he is a member of the speakers bureau for AbbVie, Novartis, Pfizer, and UCB. Global Academy for Medical Education and this news organization are owned by the same parent company.
Structural damage in axial and peripheral ankylosing spondylitis is the result of a combination of destruction and new bone formation, which can be halted if treated early and for long enough, according to Dirk Elewaut, MD, PhD.
“There is a longstanding paradox in the field of ankylosing spondylitis, as to whether there is a strict relationship between the inflammation that leads to the signs and symptoms in patients, and the structural progression,” said Dr. Elewaut, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “ ”
One key reason why the early TNF inhibition trials showed no effect of treatment on structural progression stemmed from inadequate study design. “The original studies were underpowered, and the patients used NSAIDs,” he said. “We also know that the anti-inflammatory effect [of TNF inhibition] is not 100%. So you have a clear drop in inflammation with biologics, but it’s not completely stopped. This led to a lot of speculation as to whether inflammation and ankyloses are coupled or not.”
More recently, researchers have honed in on the transition from acute inflammatory lesions visible on MRI by bone marrow edema to so-called fatty lesions, which are also visible on MRI. They have observed that fatty lesions are associated with new bone formation. “Lesions containing more fat are thought to be more difficult to modulate by biological therapy,” Dr. Elewaut said. “Once you have a fatty lesion, you have some kind of metabolic disease of the bone, and it’s a one-way road to the development of new syndesmophytes. So in other words, it is essential to assess the relationship between inflammation and new bone formation with both the STIR [short tau inversion recovery images] and T1-weighted MRI.”
A working hypothesis among AS researchers is that the effect of anti-TNF therapy on radiologic progression depends on the relative number of acute and mature inflammatory lesions that individual patients have. “Early diagnosis is a prerequisite for advances in disease modification,” he said. At least three “windows of opportunity” for disease modification exist, Dr. Elewaut continued. One is that the link between inflammation as measured by clinical parameters and new bone formation will be more evident in early AS. The second is that early treatment of patients with axial AS and spinal inflammation with anti-TNF agents will prevent new bone formation. The third is that reduction of new bone formation in established AS will be observed only with long-term anti-TNF therapy after mature lesions have resolved/repaired, and no new inflammatory lesions are being formed. One study found that patients with a delay of more than 10 years starting anti-TNF therapy were more likely to progress, compared with patients who received therapy earlier (odds ratio, 2.4; P = .03; see Arthritis Rheum. 2013;65:645-54). “The message here is quite clear,” Dr. Elewaut said. “The earlier we treat intensively, the better impact you have on structural outcomes.”
In a trial known as CRESPA (Clinical Remission in peripheral SPondyloArthritis), researchers including Dr. Elewaut evaluated the efficacy and safety of golimumab (Simponi) to induce clinical remission in patients with early, active peripheral AS (Ann Rheum Dis. 2017;76:1389-95). In all, 60 patients were randomized to golimumab or placebo for 24 weeks. At week 24, a significantly higher percentage of patients receiving golimumab achieved clinical remission, compared with placebo (75% vs. 20%, respectively; P less than .001). At week 12, similar results were observed (70% vs. 15%; P less than .001).
“These were very striking results in this early disease population,” Dr. Elewaut said. In a follow-up analysis, the researchers withdrew therapy in patients who reached clinical remission, to see what would happen. “At 1.5 years of follow-up, 57% of patients are in drug-free remission,” Dr. Elewaut said. “This is interesting and suggests that at least in a fraction of those patients, you can actually achieve a drug-free remission.” Predictors for relapse included pre-existing psoriasis and having more than five swollen joints.
Dr. Elewaut disclosed that he is a member of the speakers bureau for AbbVie, Novartis, Pfizer, and UCB. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Picosecond lasers emerging as a go-to for tattoo removal
SAN DIEGO – When counseling patients about laser tattoo removal, resist the temptation to promise clearance in a certain number of treatments.
“You will regret it,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium. “If you say, ‘This looks like this is going to take 6-8 treatments, this looks very simple to me,’ you’ll find that you’ll have someone who requires 15-18 treatments. Further, partial clearing may be cosmetically inferior than nontreatment.”
“These target the microscopic tattoo particles located inside dermal phagocytic cells and scattered extracellularly throughout the dermis,” Dr. Avram explained. The Q-switched laser heats particles to more than 1,000º C within nanoseconds, or billionths of a second. “It produces extreme heat, cavitation, and cell rupture,” he said. “The clinical endpoint is immediate epidermal whitening of tattooed skin.” The process causes transdermal elimination; some of it flows into the lymphatic system, while the rest undergoes rephagocytosis by dermal scavenger cells.
Picosecond lasers are even faster than their Q-switched counterparts, delivering high energies in trillionths of a second. “A picosecond is to a second as 1 second is to 37,000 years,” Dr. Avram said. Commercially available picosecond (ps) lasers include devices with wavelengths of 532 nm, 755 nm, and 1,064 nm that deliver energy in a range of 300-750 ps. The Nd:YAG lasers work best for red and black ink, while Alexandrite lasers work best for green and blue ink.
In Dr. Avram’s experience, ps lasers are generally more effective for tattoo removal, compared with nanosecond lasers. “There’s some nonselective targeting of other pigments, and they’re particularly effective for faded tattoos,” he said. “Combining nanosecond and picosecond devices provides enhanced results, but picosecond lasers are more expensive.”
The clinical endpoint for ps lasers is the same as for nanosecond lasers: epidermal whitening. He said he schedules about 8 weeks between treatments. “If you don’t inform patients of the expectations, they’re going to be very disappointed with you,” Dr. Avram said. “You need to tell them that it’s going to take a lot of treatments and that it may not clear completely. You may be working with them for a year or 2.”
The checklist prior to the first treatment with any laser involves assessing the type of tattoo (amateur or professional), the color of the tattoo, patient skin type, and the duration of the tattoo. “You also want to palpate for an existing scar,” he said. “A lot of times, patients don’t recognize they have a scar on the treatment site. You don’t want to own a complication that has nothing to do with your treatments. Photographing the scar is also important.”
Hyperpigmentation or hypopigmentation is a greater concern in darker skin types or tanned individuals, compared with fairer-skinned patients. “The 1,064-nm Q-switched Nd:YAG laser is the least likely to affect skin pigment,” said Dr. Avram, who is codirector of the Massachusetts General Hospital/Wellman Laser and Cosmetic Fellowship. “It’s safest for Fitzpatrick skin types IV-VI but it’s not very effective for green, blue, and red tattoo ink colors. Some degree of dyspigmentation occurs in most patients regardless of skin type. Much of this is temporary and improves with time, but it may take months to years.”
Professional tattoos are the most difficult to treat because they often feature dense and deeply placed tattoo ink and require 6-20 or more treatments to improve, he said. On the other hand, amateur tattoos, traumatic tattoos, and radiation tattoos improve more rapidly and generally require fewer treatment to yield improvement.
“Color is key,” Dr. Avram said. “If you have different colors in one tattoo, it is going to be more difficult to clear.” Black and dark-blue tattoos respond best to laser, while light blue and green also respond well. Red responds well, but purple can be challenging. “Yellow and orange do not respond well, but they respond partially,” he said.
Researchers who conducted a large cohort trial of variables influencing the outcome of tattoos treated by Q-switched lasers found that 47% of tattoos were cleared after 10 treatment sessions, while 75% were cleared after 15 sessions (Arch Dermatol. 2012;148[12]:1364-9). Predictors of poor response included smoking, the presence of colors other than black and red, tattoo size larger than 30 cm2, location on the feet or legs, duration greater than 36 months, high color density, and treatment intervals of 8 weeks or less.
Dr. Avram cautioned against taking a “cookbook” approach to treating tattoos and underscored the importance of decreasing the fluence if tissue “splatter” occurs, as this may produce scarring. “The treating clinician should follow the treatment endpoint, not the laser fluences,” he said. “Do not use IPL [intense pulsed light therapy] for tattoos; that’s inappropriate and you may end up scarring your patient.”
Common adverse effects include erythema, blistering, hyper- and hypopigmentation, and scarring. Less common adverse effects include an allergic reaction, darkening of the cosmetic tattoo, an immune reaction, and chrysiasis, a dark-blue pigmentation caused by Q-switched laser treatment in patients with a history of gold-salt ingestion. “Any history of gold ingestion will produce this finding, even if they ingested 40 years ago,” he said. “This is very difficult to correct.”
The optimal interval between treatments continues to be explored. For example, the R20 method consists of four treatments separated by 20 minutes. The initial study found that this approach led to better outcomes, compared with conventional, single-pass laser treatment (J Am Acad Dermatol. 2012;66[2]:271-7). A companion technology that is playing a role in such repeat treatments is a Food and Drug Administration–approved transparent silicone patch infused with perfluorodecalin that helps reduce scattering and improves efficacy.
“It also allows for performing consecutive repeat laser treatments at the same visit,” Dr. Avram said. In one study, 11 of 17 patients had more rapid clearance on the side treated with the perfluorodecalin patch, compared with the side that was treated without the patch (Laser Surg Med. 2015;47[8]:613-8).
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
[email protected]
SAN DIEGO – When counseling patients about laser tattoo removal, resist the temptation to promise clearance in a certain number of treatments.
“You will regret it,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium. “If you say, ‘This looks like this is going to take 6-8 treatments, this looks very simple to me,’ you’ll find that you’ll have someone who requires 15-18 treatments. Further, partial clearing may be cosmetically inferior than nontreatment.”
“These target the microscopic tattoo particles located inside dermal phagocytic cells and scattered extracellularly throughout the dermis,” Dr. Avram explained. The Q-switched laser heats particles to more than 1,000º C within nanoseconds, or billionths of a second. “It produces extreme heat, cavitation, and cell rupture,” he said. “The clinical endpoint is immediate epidermal whitening of tattooed skin.” The process causes transdermal elimination; some of it flows into the lymphatic system, while the rest undergoes rephagocytosis by dermal scavenger cells.
Picosecond lasers are even faster than their Q-switched counterparts, delivering high energies in trillionths of a second. “A picosecond is to a second as 1 second is to 37,000 years,” Dr. Avram said. Commercially available picosecond (ps) lasers include devices with wavelengths of 532 nm, 755 nm, and 1,064 nm that deliver energy in a range of 300-750 ps. The Nd:YAG lasers work best for red and black ink, while Alexandrite lasers work best for green and blue ink.
In Dr. Avram’s experience, ps lasers are generally more effective for tattoo removal, compared with nanosecond lasers. “There’s some nonselective targeting of other pigments, and they’re particularly effective for faded tattoos,” he said. “Combining nanosecond and picosecond devices provides enhanced results, but picosecond lasers are more expensive.”
The clinical endpoint for ps lasers is the same as for nanosecond lasers: epidermal whitening. He said he schedules about 8 weeks between treatments. “If you don’t inform patients of the expectations, they’re going to be very disappointed with you,” Dr. Avram said. “You need to tell them that it’s going to take a lot of treatments and that it may not clear completely. You may be working with them for a year or 2.”
The checklist prior to the first treatment with any laser involves assessing the type of tattoo (amateur or professional), the color of the tattoo, patient skin type, and the duration of the tattoo. “You also want to palpate for an existing scar,” he said. “A lot of times, patients don’t recognize they have a scar on the treatment site. You don’t want to own a complication that has nothing to do with your treatments. Photographing the scar is also important.”
Hyperpigmentation or hypopigmentation is a greater concern in darker skin types or tanned individuals, compared with fairer-skinned patients. “The 1,064-nm Q-switched Nd:YAG laser is the least likely to affect skin pigment,” said Dr. Avram, who is codirector of the Massachusetts General Hospital/Wellman Laser and Cosmetic Fellowship. “It’s safest for Fitzpatrick skin types IV-VI but it’s not very effective for green, blue, and red tattoo ink colors. Some degree of dyspigmentation occurs in most patients regardless of skin type. Much of this is temporary and improves with time, but it may take months to years.”
Professional tattoos are the most difficult to treat because they often feature dense and deeply placed tattoo ink and require 6-20 or more treatments to improve, he said. On the other hand, amateur tattoos, traumatic tattoos, and radiation tattoos improve more rapidly and generally require fewer treatment to yield improvement.
“Color is key,” Dr. Avram said. “If you have different colors in one tattoo, it is going to be more difficult to clear.” Black and dark-blue tattoos respond best to laser, while light blue and green also respond well. Red responds well, but purple can be challenging. “Yellow and orange do not respond well, but they respond partially,” he said.
Researchers who conducted a large cohort trial of variables influencing the outcome of tattoos treated by Q-switched lasers found that 47% of tattoos were cleared after 10 treatment sessions, while 75% were cleared after 15 sessions (Arch Dermatol. 2012;148[12]:1364-9). Predictors of poor response included smoking, the presence of colors other than black and red, tattoo size larger than 30 cm2, location on the feet or legs, duration greater than 36 months, high color density, and treatment intervals of 8 weeks or less.
Dr. Avram cautioned against taking a “cookbook” approach to treating tattoos and underscored the importance of decreasing the fluence if tissue “splatter” occurs, as this may produce scarring. “The treating clinician should follow the treatment endpoint, not the laser fluences,” he said. “Do not use IPL [intense pulsed light therapy] for tattoos; that’s inappropriate and you may end up scarring your patient.”
Common adverse effects include erythema, blistering, hyper- and hypopigmentation, and scarring. Less common adverse effects include an allergic reaction, darkening of the cosmetic tattoo, an immune reaction, and chrysiasis, a dark-blue pigmentation caused by Q-switched laser treatment in patients with a history of gold-salt ingestion. “Any history of gold ingestion will produce this finding, even if they ingested 40 years ago,” he said. “This is very difficult to correct.”
The optimal interval between treatments continues to be explored. For example, the R20 method consists of four treatments separated by 20 minutes. The initial study found that this approach led to better outcomes, compared with conventional, single-pass laser treatment (J Am Acad Dermatol. 2012;66[2]:271-7). A companion technology that is playing a role in such repeat treatments is a Food and Drug Administration–approved transparent silicone patch infused with perfluorodecalin that helps reduce scattering and improves efficacy.
“It also allows for performing consecutive repeat laser treatments at the same visit,” Dr. Avram said. In one study, 11 of 17 patients had more rapid clearance on the side treated with the perfluorodecalin patch, compared with the side that was treated without the patch (Laser Surg Med. 2015;47[8]:613-8).
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
[email protected]
SAN DIEGO – When counseling patients about laser tattoo removal, resist the temptation to promise clearance in a certain number of treatments.
“You will regret it,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium. “If you say, ‘This looks like this is going to take 6-8 treatments, this looks very simple to me,’ you’ll find that you’ll have someone who requires 15-18 treatments. Further, partial clearing may be cosmetically inferior than nontreatment.”
“These target the microscopic tattoo particles located inside dermal phagocytic cells and scattered extracellularly throughout the dermis,” Dr. Avram explained. The Q-switched laser heats particles to more than 1,000º C within nanoseconds, or billionths of a second. “It produces extreme heat, cavitation, and cell rupture,” he said. “The clinical endpoint is immediate epidermal whitening of tattooed skin.” The process causes transdermal elimination; some of it flows into the lymphatic system, while the rest undergoes rephagocytosis by dermal scavenger cells.
Picosecond lasers are even faster than their Q-switched counterparts, delivering high energies in trillionths of a second. “A picosecond is to a second as 1 second is to 37,000 years,” Dr. Avram said. Commercially available picosecond (ps) lasers include devices with wavelengths of 532 nm, 755 nm, and 1,064 nm that deliver energy in a range of 300-750 ps. The Nd:YAG lasers work best for red and black ink, while Alexandrite lasers work best for green and blue ink.
In Dr. Avram’s experience, ps lasers are generally more effective for tattoo removal, compared with nanosecond lasers. “There’s some nonselective targeting of other pigments, and they’re particularly effective for faded tattoos,” he said. “Combining nanosecond and picosecond devices provides enhanced results, but picosecond lasers are more expensive.”
The clinical endpoint for ps lasers is the same as for nanosecond lasers: epidermal whitening. He said he schedules about 8 weeks between treatments. “If you don’t inform patients of the expectations, they’re going to be very disappointed with you,” Dr. Avram said. “You need to tell them that it’s going to take a lot of treatments and that it may not clear completely. You may be working with them for a year or 2.”
The checklist prior to the first treatment with any laser involves assessing the type of tattoo (amateur or professional), the color of the tattoo, patient skin type, and the duration of the tattoo. “You also want to palpate for an existing scar,” he said. “A lot of times, patients don’t recognize they have a scar on the treatment site. You don’t want to own a complication that has nothing to do with your treatments. Photographing the scar is also important.”
Hyperpigmentation or hypopigmentation is a greater concern in darker skin types or tanned individuals, compared with fairer-skinned patients. “The 1,064-nm Q-switched Nd:YAG laser is the least likely to affect skin pigment,” said Dr. Avram, who is codirector of the Massachusetts General Hospital/Wellman Laser and Cosmetic Fellowship. “It’s safest for Fitzpatrick skin types IV-VI but it’s not very effective for green, blue, and red tattoo ink colors. Some degree of dyspigmentation occurs in most patients regardless of skin type. Much of this is temporary and improves with time, but it may take months to years.”
Professional tattoos are the most difficult to treat because they often feature dense and deeply placed tattoo ink and require 6-20 or more treatments to improve, he said. On the other hand, amateur tattoos, traumatic tattoos, and radiation tattoos improve more rapidly and generally require fewer treatment to yield improvement.
“Color is key,” Dr. Avram said. “If you have different colors in one tattoo, it is going to be more difficult to clear.” Black and dark-blue tattoos respond best to laser, while light blue and green also respond well. Red responds well, but purple can be challenging. “Yellow and orange do not respond well, but they respond partially,” he said.
Researchers who conducted a large cohort trial of variables influencing the outcome of tattoos treated by Q-switched lasers found that 47% of tattoos were cleared after 10 treatment sessions, while 75% were cleared after 15 sessions (Arch Dermatol. 2012;148[12]:1364-9). Predictors of poor response included smoking, the presence of colors other than black and red, tattoo size larger than 30 cm2, location on the feet or legs, duration greater than 36 months, high color density, and treatment intervals of 8 weeks or less.
Dr. Avram cautioned against taking a “cookbook” approach to treating tattoos and underscored the importance of decreasing the fluence if tissue “splatter” occurs, as this may produce scarring. “The treating clinician should follow the treatment endpoint, not the laser fluences,” he said. “Do not use IPL [intense pulsed light therapy] for tattoos; that’s inappropriate and you may end up scarring your patient.”
Common adverse effects include erythema, blistering, hyper- and hypopigmentation, and scarring. Less common adverse effects include an allergic reaction, darkening of the cosmetic tattoo, an immune reaction, and chrysiasis, a dark-blue pigmentation caused by Q-switched laser treatment in patients with a history of gold-salt ingestion. “Any history of gold ingestion will produce this finding, even if they ingested 40 years ago,” he said. “This is very difficult to correct.”
The optimal interval between treatments continues to be explored. For example, the R20 method consists of four treatments separated by 20 minutes. The initial study found that this approach led to better outcomes, compared with conventional, single-pass laser treatment (J Am Acad Dermatol. 2012;66[2]:271-7). A companion technology that is playing a role in such repeat treatments is a Food and Drug Administration–approved transparent silicone patch infused with perfluorodecalin that helps reduce scattering and improves efficacy.
“It also allows for performing consecutive repeat laser treatments at the same visit,” Dr. Avram said. In one study, 11 of 17 patients had more rapid clearance on the side treated with the perfluorodecalin patch, compared with the side that was treated without the patch (Laser Surg Med. 2015;47[8]:613-8).
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
[email protected]
AT MOAS 2017
Platelet-rich plasma treatment for hair loss continues to be refined
SAN DIEGO – There is currently no standard protocol for injecting autologous platelet-rich plasma to stimulate hair growth, but the technique appears to be about 50% effective, according to Marc R. Avram, MD.
“I tell patients that this is not FDA [Food and Drug Administration] approved, but we think it to be safe,” said Dr. Avram, clinical professor of dermatology at the Cornell University, New York, said at the annual Masters of Aesthetics Symposium. “We don’t know how well it’s going to work. There are a lot of published data on it, but none of [them are] randomized or controlled long-term.”
In Dr. Avram’s experience, he has found that PRP is a good option for patients with difficult hair loss, such as those who had extensive hair loss after chemotherapy but the hair never grew back in the same fashion, or patients who have failed treatment with finasteride and minoxidil.
Currently, there is no standard protocol for using PRP to stimulate hair growth, but the approach Dr. Avram follows is modeled on his experience of injecting thousands of patients with triamcinolone acetonide (Kenalog) for hair loss every 4-6 weeks. After drawing 20 ccs-30 ccs of blood from the patient, the vial is placed in a centrifuge for 10 minutes, a process that separates PRP from red blood cells. Next, the clinician injects PRP into the deep dermis/superficial subcutaneous tissue of the desired treatment area. An average of 4 ccs-8 ccs is injected during each session.
After three monthly treatments, patients follow up at 3 and 6 months after the last treatment to evaluate efficacy. “All patients are told if there is regrowth or thickening of terminal hair, maintenance treatments will be needed every 6-9 months,” he said.
Published clinical trials of PRP include a follow-up period of 3-12 months and most demonstrate an efficacy in the range of 50%-70%. “It seems to be more effective for earlier stages of hair loss, and there are no known side effects to date,” said Dr. Avram, who has authored five textbooks on hair and cosmetic dermatology. “I had one patient call up to say he thought he had an increase in hair loss 2-3 weeks after treatment, but that’s one patient in a couple hundred. This may be similar to the effect minoxidil has on some patients. I’ve had no other issues with side effects.”
In his opinion, future challenges in the use of PRP for restoring hair loss include better defining optimal candidates for the procedure and establishing a better treatment protocol. “How often should maintenance be done?” he asked. “Is this going to be helpful for alopecia areata and scarring alopecia? Also, we need to determine if finasteride, minoxidil, low-level light laser therapy, or any other medications can enhance PRP efficacy in combination. What’s the optimal combination for patients? We don’t know yet. But I think in the future we will.”
Dr. Avram disclosed that he is a consultant for Restoration Robotics.
SAN DIEGO – There is currently no standard protocol for injecting autologous platelet-rich plasma to stimulate hair growth, but the technique appears to be about 50% effective, according to Marc R. Avram, MD.
“I tell patients that this is not FDA [Food and Drug Administration] approved, but we think it to be safe,” said Dr. Avram, clinical professor of dermatology at the Cornell University, New York, said at the annual Masters of Aesthetics Symposium. “We don’t know how well it’s going to work. There are a lot of published data on it, but none of [them are] randomized or controlled long-term.”
In Dr. Avram’s experience, he has found that PRP is a good option for patients with difficult hair loss, such as those who had extensive hair loss after chemotherapy but the hair never grew back in the same fashion, or patients who have failed treatment with finasteride and minoxidil.
Currently, there is no standard protocol for using PRP to stimulate hair growth, but the approach Dr. Avram follows is modeled on his experience of injecting thousands of patients with triamcinolone acetonide (Kenalog) for hair loss every 4-6 weeks. After drawing 20 ccs-30 ccs of blood from the patient, the vial is placed in a centrifuge for 10 minutes, a process that separates PRP from red blood cells. Next, the clinician injects PRP into the deep dermis/superficial subcutaneous tissue of the desired treatment area. An average of 4 ccs-8 ccs is injected during each session.
After three monthly treatments, patients follow up at 3 and 6 months after the last treatment to evaluate efficacy. “All patients are told if there is regrowth or thickening of terminal hair, maintenance treatments will be needed every 6-9 months,” he said.
Published clinical trials of PRP include a follow-up period of 3-12 months and most demonstrate an efficacy in the range of 50%-70%. “It seems to be more effective for earlier stages of hair loss, and there are no known side effects to date,” said Dr. Avram, who has authored five textbooks on hair and cosmetic dermatology. “I had one patient call up to say he thought he had an increase in hair loss 2-3 weeks after treatment, but that’s one patient in a couple hundred. This may be similar to the effect minoxidil has on some patients. I’ve had no other issues with side effects.”
In his opinion, future challenges in the use of PRP for restoring hair loss include better defining optimal candidates for the procedure and establishing a better treatment protocol. “How often should maintenance be done?” he asked. “Is this going to be helpful for alopecia areata and scarring alopecia? Also, we need to determine if finasteride, minoxidil, low-level light laser therapy, or any other medications can enhance PRP efficacy in combination. What’s the optimal combination for patients? We don’t know yet. But I think in the future we will.”
Dr. Avram disclosed that he is a consultant for Restoration Robotics.
SAN DIEGO – There is currently no standard protocol for injecting autologous platelet-rich plasma to stimulate hair growth, but the technique appears to be about 50% effective, according to Marc R. Avram, MD.
“I tell patients that this is not FDA [Food and Drug Administration] approved, but we think it to be safe,” said Dr. Avram, clinical professor of dermatology at the Cornell University, New York, said at the annual Masters of Aesthetics Symposium. “We don’t know how well it’s going to work. There are a lot of published data on it, but none of [them are] randomized or controlled long-term.”
In Dr. Avram’s experience, he has found that PRP is a good option for patients with difficult hair loss, such as those who had extensive hair loss after chemotherapy but the hair never grew back in the same fashion, or patients who have failed treatment with finasteride and minoxidil.
Currently, there is no standard protocol for using PRP to stimulate hair growth, but the approach Dr. Avram follows is modeled on his experience of injecting thousands of patients with triamcinolone acetonide (Kenalog) for hair loss every 4-6 weeks. After drawing 20 ccs-30 ccs of blood from the patient, the vial is placed in a centrifuge for 10 minutes, a process that separates PRP from red blood cells. Next, the clinician injects PRP into the deep dermis/superficial subcutaneous tissue of the desired treatment area. An average of 4 ccs-8 ccs is injected during each session.
After three monthly treatments, patients follow up at 3 and 6 months after the last treatment to evaluate efficacy. “All patients are told if there is regrowth or thickening of terminal hair, maintenance treatments will be needed every 6-9 months,” he said.
Published clinical trials of PRP include a follow-up period of 3-12 months and most demonstrate an efficacy in the range of 50%-70%. “It seems to be more effective for earlier stages of hair loss, and there are no known side effects to date,” said Dr. Avram, who has authored five textbooks on hair and cosmetic dermatology. “I had one patient call up to say he thought he had an increase in hair loss 2-3 weeks after treatment, but that’s one patient in a couple hundred. This may be similar to the effect minoxidil has on some patients. I’ve had no other issues with side effects.”
In his opinion, future challenges in the use of PRP for restoring hair loss include better defining optimal candidates for the procedure and establishing a better treatment protocol. “How often should maintenance be done?” he asked. “Is this going to be helpful for alopecia areata and scarring alopecia? Also, we need to determine if finasteride, minoxidil, low-level light laser therapy, or any other medications can enhance PRP efficacy in combination. What’s the optimal combination for patients? We don’t know yet. But I think in the future we will.”
Dr. Avram disclosed that he is a consultant for Restoration Robotics.
AT MOAS 2017
Endocrine Society updates treatment guidelines for transgender persons
Previous clinical recommendations on caring for transgender individuals have advised that hormone treatment begin no earlier than age 16 years, but
“Sixteen is the typical age cutoff in many areas of the world for some decision-making capacity from a legal perspective, but when you think about hormones and puberty, 16 is pretty late,” Joshua D. Safer, MD, one of the task force members who authored the guideline, said in an interview. “If we’re going to use biology for guidance, then hormone interventions for transgender kids should begin occurring earlier, when puberty really happens, like around age 12, 13, or 14. However, we’re in a situation where we lack a test. We can’t diagnose anybody as transgender with excellent confidence, outside of talking to those kids. When we start talking about hormone therapies, we talk about some things that will be irreversible. That’s a fraught place to go, but we recognize that people are going to treat kids under 16 in many instances.”
Over several years, Dr. Safer and nine other task force members, chaired by Wylie Hembree, MD, of the College of Physicians and Surgeons at Columbia University, New York, worked to establish a framework for the appropriate treatment of transgender individuals. The efforts of the task force were framed around a durable biological underpinning to gender identity. “That’s state of the art right now,” said Dr. Safer, who is the medical director of the Center for Transgender Medicine and Surgery at Boston University Medical Center. “People think there’s debate about whether there’s a substantial biological component. I think that the data are pretty strong, so I don’t think there’s a lot of debate about that in the scientific world. The debate is more about what that biology might be. That’s all over the map.”
That notion of a biological basis for gender identity contributed to a second major change from the Endocrine Society’s 2009 guideline, which recommended that the diagnosis of gender identity disorder be made by a mental health professional. The current guideline states that for the care of peripubertal youths and older adolescents, “we recommend that an expert multidisciplinary team comprised of medical professionals and mental health professionals manage this treatment. The treating physician must confirm the criteria for treatment used by the referring mental health practitioner and collaborate with them in decisions about gender-affirming surgery in older adolescents.” Meanwhile, for adult gender-dysphoric/gender-incongruent persons, “the treating clinicians (collectively) should have expertise in transgender-specific diagnostic criteria, mental health, primary care, hormone treatment, and surgery, as needed by the patient.” Dr. Safer described this new approach as “a major change in terms of trying to gain access to care by liberalizing the variety of those in the medical community who can be associated with the diagnosis, at least on the adult side.”
A number of associations cosponsored the guideline, including the American Association of Clinical Endocrinologists, American Society of Andrology, European Society for Paediatric Endocrinology, European Society of Endocrinology, Pediatric Endocrine Society, and World Professional Association for Transgender Health. Other key recommendations from the guideline include:
- All individuals seeking gender-affirming medical treatment should receive information and counsel on options for fertility preservation prior to initiating puberty suppression in adolescents and prior to treating with hormonal therapy of the affirmed gender in both adolescents and adults.
- Removal of gonads may be considered when high doses of sex steroids are required to suppress the body’s secretion of hormones and/or to reduce steroid levels in advanced age.
- During sex-steroid treatment, clinicians should monitor, in both transgender males (female to male) and transgender females (male to female), prolactin, metabolic disorders, and bone loss, as well as cancer risks in individuals who have not undergone surgical treatment.
Concurrent with the release of the new guideline, the Endocrine Society issued a position statement that calls on federal and private insurers to cover medical interventions for transgender individuals as prescribed by a physician. “I live in Massachusetts, where our insurance commissioner deemed insurance coverage obligatory for transgender individuals as of 2015,” said Dr. Safer, who is also director of the endocrinology fellowship training program at Boston University. “I’ve spoken to the medical directors of our large insurers, like Blue Cross/Blue Shield. What’s notable is that there has been no push back [on coverage for transgender individuals] from the insurance companies. These services are not expensive: the primary care, the mental health care, and the hormones. Many of the patients are not opting for surgeries. The theme of their concern was to get their [health insurance] policies right as quickly as possible so that they could stop wasting time talking about it, and they could focus their energy on other, more expensive health care concerns.”
In the guideline, Dr. Safer and the other task force members called for more rigorous evaluations of the effectiveness and safety of endocrine and surgical protocols in the future. “Specifically, endocrine treatment protocols for GD/gender incongruence should include the careful assessment of the following: (1) the effects of prolonged delay of puberty in adolescents on bone health, gonadal function, and the brain (including effects on cognitive, emotional, social, and sexual development); (2) the effects of treatment in adults on sex hormone levels; (3) the requirement for and the effects of progestins and other agents used to suppress endogenous sex steroids during treatment; and (4) the risks and benefits of gender-affirming hormone treatment in older transgender people.”
Dr. Safer reported having no financial disclosures.
Previous clinical recommendations on caring for transgender individuals have advised that hormone treatment begin no earlier than age 16 years, but
“Sixteen is the typical age cutoff in many areas of the world for some decision-making capacity from a legal perspective, but when you think about hormones and puberty, 16 is pretty late,” Joshua D. Safer, MD, one of the task force members who authored the guideline, said in an interview. “If we’re going to use biology for guidance, then hormone interventions for transgender kids should begin occurring earlier, when puberty really happens, like around age 12, 13, or 14. However, we’re in a situation where we lack a test. We can’t diagnose anybody as transgender with excellent confidence, outside of talking to those kids. When we start talking about hormone therapies, we talk about some things that will be irreversible. That’s a fraught place to go, but we recognize that people are going to treat kids under 16 in many instances.”
Over several years, Dr. Safer and nine other task force members, chaired by Wylie Hembree, MD, of the College of Physicians and Surgeons at Columbia University, New York, worked to establish a framework for the appropriate treatment of transgender individuals. The efforts of the task force were framed around a durable biological underpinning to gender identity. “That’s state of the art right now,” said Dr. Safer, who is the medical director of the Center for Transgender Medicine and Surgery at Boston University Medical Center. “People think there’s debate about whether there’s a substantial biological component. I think that the data are pretty strong, so I don’t think there’s a lot of debate about that in the scientific world. The debate is more about what that biology might be. That’s all over the map.”
That notion of a biological basis for gender identity contributed to a second major change from the Endocrine Society’s 2009 guideline, which recommended that the diagnosis of gender identity disorder be made by a mental health professional. The current guideline states that for the care of peripubertal youths and older adolescents, “we recommend that an expert multidisciplinary team comprised of medical professionals and mental health professionals manage this treatment. The treating physician must confirm the criteria for treatment used by the referring mental health practitioner and collaborate with them in decisions about gender-affirming surgery in older adolescents.” Meanwhile, for adult gender-dysphoric/gender-incongruent persons, “the treating clinicians (collectively) should have expertise in transgender-specific diagnostic criteria, mental health, primary care, hormone treatment, and surgery, as needed by the patient.” Dr. Safer described this new approach as “a major change in terms of trying to gain access to care by liberalizing the variety of those in the medical community who can be associated with the diagnosis, at least on the adult side.”
A number of associations cosponsored the guideline, including the American Association of Clinical Endocrinologists, American Society of Andrology, European Society for Paediatric Endocrinology, European Society of Endocrinology, Pediatric Endocrine Society, and World Professional Association for Transgender Health. Other key recommendations from the guideline include:
- All individuals seeking gender-affirming medical treatment should receive information and counsel on options for fertility preservation prior to initiating puberty suppression in adolescents and prior to treating with hormonal therapy of the affirmed gender in both adolescents and adults.
- Removal of gonads may be considered when high doses of sex steroids are required to suppress the body’s secretion of hormones and/or to reduce steroid levels in advanced age.
- During sex-steroid treatment, clinicians should monitor, in both transgender males (female to male) and transgender females (male to female), prolactin, metabolic disorders, and bone loss, as well as cancer risks in individuals who have not undergone surgical treatment.
Concurrent with the release of the new guideline, the Endocrine Society issued a position statement that calls on federal and private insurers to cover medical interventions for transgender individuals as prescribed by a physician. “I live in Massachusetts, where our insurance commissioner deemed insurance coverage obligatory for transgender individuals as of 2015,” said Dr. Safer, who is also director of the endocrinology fellowship training program at Boston University. “I’ve spoken to the medical directors of our large insurers, like Blue Cross/Blue Shield. What’s notable is that there has been no push back [on coverage for transgender individuals] from the insurance companies. These services are not expensive: the primary care, the mental health care, and the hormones. Many of the patients are not opting for surgeries. The theme of their concern was to get their [health insurance] policies right as quickly as possible so that they could stop wasting time talking about it, and they could focus their energy on other, more expensive health care concerns.”
In the guideline, Dr. Safer and the other task force members called for more rigorous evaluations of the effectiveness and safety of endocrine and surgical protocols in the future. “Specifically, endocrine treatment protocols for GD/gender incongruence should include the careful assessment of the following: (1) the effects of prolonged delay of puberty in adolescents on bone health, gonadal function, and the brain (including effects on cognitive, emotional, social, and sexual development); (2) the effects of treatment in adults on sex hormone levels; (3) the requirement for and the effects of progestins and other agents used to suppress endogenous sex steroids during treatment; and (4) the risks and benefits of gender-affirming hormone treatment in older transgender people.”
Dr. Safer reported having no financial disclosures.
Previous clinical recommendations on caring for transgender individuals have advised that hormone treatment begin no earlier than age 16 years, but
“Sixteen is the typical age cutoff in many areas of the world for some decision-making capacity from a legal perspective, but when you think about hormones and puberty, 16 is pretty late,” Joshua D. Safer, MD, one of the task force members who authored the guideline, said in an interview. “If we’re going to use biology for guidance, then hormone interventions for transgender kids should begin occurring earlier, when puberty really happens, like around age 12, 13, or 14. However, we’re in a situation where we lack a test. We can’t diagnose anybody as transgender with excellent confidence, outside of talking to those kids. When we start talking about hormone therapies, we talk about some things that will be irreversible. That’s a fraught place to go, but we recognize that people are going to treat kids under 16 in many instances.”
Over several years, Dr. Safer and nine other task force members, chaired by Wylie Hembree, MD, of the College of Physicians and Surgeons at Columbia University, New York, worked to establish a framework for the appropriate treatment of transgender individuals. The efforts of the task force were framed around a durable biological underpinning to gender identity. “That’s state of the art right now,” said Dr. Safer, who is the medical director of the Center for Transgender Medicine and Surgery at Boston University Medical Center. “People think there’s debate about whether there’s a substantial biological component. I think that the data are pretty strong, so I don’t think there’s a lot of debate about that in the scientific world. The debate is more about what that biology might be. That’s all over the map.”
That notion of a biological basis for gender identity contributed to a second major change from the Endocrine Society’s 2009 guideline, which recommended that the diagnosis of gender identity disorder be made by a mental health professional. The current guideline states that for the care of peripubertal youths and older adolescents, “we recommend that an expert multidisciplinary team comprised of medical professionals and mental health professionals manage this treatment. The treating physician must confirm the criteria for treatment used by the referring mental health practitioner and collaborate with them in decisions about gender-affirming surgery in older adolescents.” Meanwhile, for adult gender-dysphoric/gender-incongruent persons, “the treating clinicians (collectively) should have expertise in transgender-specific diagnostic criteria, mental health, primary care, hormone treatment, and surgery, as needed by the patient.” Dr. Safer described this new approach as “a major change in terms of trying to gain access to care by liberalizing the variety of those in the medical community who can be associated with the diagnosis, at least on the adult side.”
A number of associations cosponsored the guideline, including the American Association of Clinical Endocrinologists, American Society of Andrology, European Society for Paediatric Endocrinology, European Society of Endocrinology, Pediatric Endocrine Society, and World Professional Association for Transgender Health. Other key recommendations from the guideline include:
- All individuals seeking gender-affirming medical treatment should receive information and counsel on options for fertility preservation prior to initiating puberty suppression in adolescents and prior to treating with hormonal therapy of the affirmed gender in both adolescents and adults.
- Removal of gonads may be considered when high doses of sex steroids are required to suppress the body’s secretion of hormones and/or to reduce steroid levels in advanced age.
- During sex-steroid treatment, clinicians should monitor, in both transgender males (female to male) and transgender females (male to female), prolactin, metabolic disorders, and bone loss, as well as cancer risks in individuals who have not undergone surgical treatment.
Concurrent with the release of the new guideline, the Endocrine Society issued a position statement that calls on federal and private insurers to cover medical interventions for transgender individuals as prescribed by a physician. “I live in Massachusetts, where our insurance commissioner deemed insurance coverage obligatory for transgender individuals as of 2015,” said Dr. Safer, who is also director of the endocrinology fellowship training program at Boston University. “I’ve spoken to the medical directors of our large insurers, like Blue Cross/Blue Shield. What’s notable is that there has been no push back [on coverage for transgender individuals] from the insurance companies. These services are not expensive: the primary care, the mental health care, and the hormones. Many of the patients are not opting for surgeries. The theme of their concern was to get their [health insurance] policies right as quickly as possible so that they could stop wasting time talking about it, and they could focus their energy on other, more expensive health care concerns.”
In the guideline, Dr. Safer and the other task force members called for more rigorous evaluations of the effectiveness and safety of endocrine and surgical protocols in the future. “Specifically, endocrine treatment protocols for GD/gender incongruence should include the careful assessment of the following: (1) the effects of prolonged delay of puberty in adolescents on bone health, gonadal function, and the brain (including effects on cognitive, emotional, social, and sexual development); (2) the effects of treatment in adults on sex hormone levels; (3) the requirement for and the effects of progestins and other agents used to suppress endogenous sex steroids during treatment; and (4) the risks and benefits of gender-affirming hormone treatment in older transgender people.”
Dr. Safer reported having no financial disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Riociguat may benefit subset of PAH patients
Switching to riociguat may be an effective strategy for pulmonary arterial hypertension (PAH) patients who respond inadequately to phosphodiesterase-5 inhibitors, results from a small open-label study demonstrated.
“This study represents an important step towards determining if this new treatment strategy is an effective approach to the management of patients with PAH, although additional data from larger, randomised, controlled studies are needed to further establish the safety and efficacy of this approach,” researchers led by Marius M. Hoeper, MD, wrote in a study published online Sept. 9, 2017, in the European Respiratory Journal.
Current clinical data indicate that many patients with PAH who receive phosphodiesterase-5 inhibitors do not reach treatment goals. “For example, in the AMBITION study, 73% of patients with PAH receiving tadalafil monotherapy and 61% of those receiving tadalafil in combination with ambrisentan did not achieve a satisfactory clinical response at week 24 of the study (N Engl J Med. 2015;373:834-44),” Dr. Hoeper of the Clinic for Respiratory Medicine at Hannover Medical School Germany and his associates wrote. “Furthermore, in the SERAPHIN study, event-free survival of patients receiving [phosphodiesterase-5 inhibitors] monotherapy was approximately 50% at 3 years (N Engl J Med. 2013;369:809-18).”
For the current trial, known as RESPITE, investigators from nine countries in Europe and North America enrolled 61 PAH patients in a 24-week, open-label uncontrolled analysis to investigate the safety, feasibility, and benefit of switching them from phosphodiesterase-5 inhibitors to riociguat. The patients underwent 1-3 days free of phosphodiesterase-5 inhibitors before receiving riociguat in a maximum dose of up to 2.5 mg t.i.d. Most patients (74%) were female, and 92% were Caucasian. In all, 51 patients (84%) completed all 24 weeks of treatment, while the remaining 10 discontinued treatment, 4 of whom due to adverse events.
Additionally, 54% of the patients studied experienced an improvement in their the World Health Organization Functional Class. However, 32 patients (52%) experienced study drug–related adverse events and 10 (16%) experienced serious adverse events, two of which were related to the drug being studied. Six patients (10%) experienced clinical worsening, including death in two, though the deaths were deemed to be unrelated to the drug being studied.
“Although not mechanistically studied, the findings of RESPITE support the hypothesis that a defective [nitric oxide–soluble guanylate cyclase–cyclic guanosine monophosphate] pathway might explain why some patients have no sufficient or sustained response to [phosphodiesterase-5 inhibitors] therapy,” the researchers noted. “In such patients, direct stimulation of [soluble guanylate cyclase] may be more effective than inhibition of [phosphodiesterase-5], but this hypothesis is still unproven.”
They acknowledged certain limitations of the study, including its prospective design and the relatively homogenous patient population. “Other limitations include the lack of a long-term continuation phase, and the absence of mechanistic data allowing identification of patients likely to respond or not respond to switching,” they wrote. “Two deaths were observed in this study, which might raise concerns, although neither of the deaths (one due to pneumonia and one due to subdural haematoma) was considered by the investigators to be study drug-related or due to worsening PAH. Given the lack of a control group and the rate of study withdrawals and clinical worsening events, further evaluation to clarify the safety of switching is required.”
The study was funded by Bayer AG, Berlin. Dr. Hoeper and his coauthors disclosed having financial ties to numerous pharmaceutical companies, including Bayer, which makes riociguat.
PRIMARY SOURCE: Eur Respir J. 2017 Sep 9. doi: 10.1183/13993003.02425-2016.
Switching to riociguat may be an effective strategy for pulmonary arterial hypertension (PAH) patients who respond inadequately to phosphodiesterase-5 inhibitors, results from a small open-label study demonstrated.
“This study represents an important step towards determining if this new treatment strategy is an effective approach to the management of patients with PAH, although additional data from larger, randomised, controlled studies are needed to further establish the safety and efficacy of this approach,” researchers led by Marius M. Hoeper, MD, wrote in a study published online Sept. 9, 2017, in the European Respiratory Journal.
Current clinical data indicate that many patients with PAH who receive phosphodiesterase-5 inhibitors do not reach treatment goals. “For example, in the AMBITION study, 73% of patients with PAH receiving tadalafil monotherapy and 61% of those receiving tadalafil in combination with ambrisentan did not achieve a satisfactory clinical response at week 24 of the study (N Engl J Med. 2015;373:834-44),” Dr. Hoeper of the Clinic for Respiratory Medicine at Hannover Medical School Germany and his associates wrote. “Furthermore, in the SERAPHIN study, event-free survival of patients receiving [phosphodiesterase-5 inhibitors] monotherapy was approximately 50% at 3 years (N Engl J Med. 2013;369:809-18).”
For the current trial, known as RESPITE, investigators from nine countries in Europe and North America enrolled 61 PAH patients in a 24-week, open-label uncontrolled analysis to investigate the safety, feasibility, and benefit of switching them from phosphodiesterase-5 inhibitors to riociguat. The patients underwent 1-3 days free of phosphodiesterase-5 inhibitors before receiving riociguat in a maximum dose of up to 2.5 mg t.i.d. Most patients (74%) were female, and 92% were Caucasian. In all, 51 patients (84%) completed all 24 weeks of treatment, while the remaining 10 discontinued treatment, 4 of whom due to adverse events.
Additionally, 54% of the patients studied experienced an improvement in their the World Health Organization Functional Class. However, 32 patients (52%) experienced study drug–related adverse events and 10 (16%) experienced serious adverse events, two of which were related to the drug being studied. Six patients (10%) experienced clinical worsening, including death in two, though the deaths were deemed to be unrelated to the drug being studied.
“Although not mechanistically studied, the findings of RESPITE support the hypothesis that a defective [nitric oxide–soluble guanylate cyclase–cyclic guanosine monophosphate] pathway might explain why some patients have no sufficient or sustained response to [phosphodiesterase-5 inhibitors] therapy,” the researchers noted. “In such patients, direct stimulation of [soluble guanylate cyclase] may be more effective than inhibition of [phosphodiesterase-5], but this hypothesis is still unproven.”
They acknowledged certain limitations of the study, including its prospective design and the relatively homogenous patient population. “Other limitations include the lack of a long-term continuation phase, and the absence of mechanistic data allowing identification of patients likely to respond or not respond to switching,” they wrote. “Two deaths were observed in this study, which might raise concerns, although neither of the deaths (one due to pneumonia and one due to subdural haematoma) was considered by the investigators to be study drug-related or due to worsening PAH. Given the lack of a control group and the rate of study withdrawals and clinical worsening events, further evaluation to clarify the safety of switching is required.”
The study was funded by Bayer AG, Berlin. Dr. Hoeper and his coauthors disclosed having financial ties to numerous pharmaceutical companies, including Bayer, which makes riociguat.
PRIMARY SOURCE: Eur Respir J. 2017 Sep 9. doi: 10.1183/13993003.02425-2016.
Switching to riociguat may be an effective strategy for pulmonary arterial hypertension (PAH) patients who respond inadequately to phosphodiesterase-5 inhibitors, results from a small open-label study demonstrated.
“This study represents an important step towards determining if this new treatment strategy is an effective approach to the management of patients with PAH, although additional data from larger, randomised, controlled studies are needed to further establish the safety and efficacy of this approach,” researchers led by Marius M. Hoeper, MD, wrote in a study published online Sept. 9, 2017, in the European Respiratory Journal.
Current clinical data indicate that many patients with PAH who receive phosphodiesterase-5 inhibitors do not reach treatment goals. “For example, in the AMBITION study, 73% of patients with PAH receiving tadalafil monotherapy and 61% of those receiving tadalafil in combination with ambrisentan did not achieve a satisfactory clinical response at week 24 of the study (N Engl J Med. 2015;373:834-44),” Dr. Hoeper of the Clinic for Respiratory Medicine at Hannover Medical School Germany and his associates wrote. “Furthermore, in the SERAPHIN study, event-free survival of patients receiving [phosphodiesterase-5 inhibitors] monotherapy was approximately 50% at 3 years (N Engl J Med. 2013;369:809-18).”
For the current trial, known as RESPITE, investigators from nine countries in Europe and North America enrolled 61 PAH patients in a 24-week, open-label uncontrolled analysis to investigate the safety, feasibility, and benefit of switching them from phosphodiesterase-5 inhibitors to riociguat. The patients underwent 1-3 days free of phosphodiesterase-5 inhibitors before receiving riociguat in a maximum dose of up to 2.5 mg t.i.d. Most patients (74%) were female, and 92% were Caucasian. In all, 51 patients (84%) completed all 24 weeks of treatment, while the remaining 10 discontinued treatment, 4 of whom due to adverse events.
Additionally, 54% of the patients studied experienced an improvement in their the World Health Organization Functional Class. However, 32 patients (52%) experienced study drug–related adverse events and 10 (16%) experienced serious adverse events, two of which were related to the drug being studied. Six patients (10%) experienced clinical worsening, including death in two, though the deaths were deemed to be unrelated to the drug being studied.
“Although not mechanistically studied, the findings of RESPITE support the hypothesis that a defective [nitric oxide–soluble guanylate cyclase–cyclic guanosine monophosphate] pathway might explain why some patients have no sufficient or sustained response to [phosphodiesterase-5 inhibitors] therapy,” the researchers noted. “In such patients, direct stimulation of [soluble guanylate cyclase] may be more effective than inhibition of [phosphodiesterase-5], but this hypothesis is still unproven.”
They acknowledged certain limitations of the study, including its prospective design and the relatively homogenous patient population. “Other limitations include the lack of a long-term continuation phase, and the absence of mechanistic data allowing identification of patients likely to respond or not respond to switching,” they wrote. “Two deaths were observed in this study, which might raise concerns, although neither of the deaths (one due to pneumonia and one due to subdural haematoma) was considered by the investigators to be study drug-related or due to worsening PAH. Given the lack of a control group and the rate of study withdrawals and clinical worsening events, further evaluation to clarify the safety of switching is required.”
The study was funded by Bayer AG, Berlin. Dr. Hoeper and his coauthors disclosed having financial ties to numerous pharmaceutical companies, including Bayer, which makes riociguat.
PRIMARY SOURCE: Eur Respir J. 2017 Sep 9. doi: 10.1183/13993003.02425-2016.
FROM THE EUROPEAN RESPIRATORY JOURNAL
Key clinical point: Selected patients with pulmonary artery hypertension (PAH) may benefit from switching from phosphodiesterase-5 inhibitors to riociguat.
Major finding: Among patients who completed all 24 weeks of treatment with riociguat, their mean 6-minute walking distance had increased by a mean of 31 meters, and their N-terminal pro b-type natriuretic peptide level decreased by a mean of 347 pg/mL.
Study details: A multicenter, open-label trial of 61 patients with PAH.
Disclosures: The study was funded by Bayer AG, Berlin. Dr. Hoeper and his coauthors disclosed having financial ties to numerous pharmaceutical companies, including Bayer, which makes riociguat.
Source: Marius M. Hoeper, et al. RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphdiesterase-5 inhibitors. Eur Resp J. 2017 Sep 09. doi: 10.1183/13993003.02425-2016.
No increased overall cardiovascular risk seen with exenatide use
“We did not observe any specific safety issues during our trial; there was no adverse signal with respect to heart failure, despite the higher mean heart rate in the exenatide group than in the placebo group, and events of acute pancreatitis and pancreatic cancer were rare, with similar rates in the two groups,” according to researchers led by Rury R. Holman, F.Med.Sci. Their study was published online Sept. 14 in the New England Journal of Medicine, and was presented at the annual meeting of the European Association for the Study of Diabetes.
For the trial, which was funded by Amylin Pharmaceuticals and known as the Exenatide Study of Cardiovascular Event Lowering (EXSCEL), the researchers randomly assigned 14,752 patients with type 2 diabetes at 687 sites in 35 countries to receive subcutaneous injections of extended-released exenatide at a dose of 2 mg, or matching placebo once per week, from June 18, 2010, through Sept. 16, 2015. The patients were followed for a median of 3.2 years and the main outcome of interest was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or stroke.
In the meantime, the intention-to-treat analysis revealed that exenatide was noninferior to placebo with respect to safety (P less than .001) but was not superior to placebo with respect to safety (P = .06). The researchers observed no significant differences between the two groups in the rates of death from cardiovascular causes, fatal or nonfatal MI, or stroke, hospitalization for heart failure or for acute coronary syndrome, or in the incidence of cute pancreatitis, pancreatic cancer, medullary thyroid carcinoma, and serious adverse events.
“The pragmatic design of the trial included integration with usual care and wide-ranging eligibility criteria,” the researchers wrote. “For example, patients with any degree of cardiovascular risk who were at least 18 years of age (with no upper age limit) were eligible. To further augment the potential generalizability of any findings, we evaluated the cardiovascular effect of once-weekly extended-release exenatide in the usual-care setting by maintaining the focus of management of diabetes and cardiovascular risk with the usual-care provider.”
The researchers acknowledged certain limitations of the study, including the rate of premature discontinuation of the trial regimen, “which was driven by patient decision. We speculate that probable factors for discontinuation were the complexity of the first-generation injection device that was used and the fact that our trial had no run-in period.”
The trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trial Unit, in collaboration with Amylin Pharmaceuticals. Dr. Holman and his coauthors reported having numerous financial ties to the pharmaceutical industry.
PRIMARY SOURCE: N Engl J Med 2017 Sept. 14. doi: 10.1056/NEJMoa1612917
“We did not observe any specific safety issues during our trial; there was no adverse signal with respect to heart failure, despite the higher mean heart rate in the exenatide group than in the placebo group, and events of acute pancreatitis and pancreatic cancer were rare, with similar rates in the two groups,” according to researchers led by Rury R. Holman, F.Med.Sci. Their study was published online Sept. 14 in the New England Journal of Medicine, and was presented at the annual meeting of the European Association for the Study of Diabetes.
For the trial, which was funded by Amylin Pharmaceuticals and known as the Exenatide Study of Cardiovascular Event Lowering (EXSCEL), the researchers randomly assigned 14,752 patients with type 2 diabetes at 687 sites in 35 countries to receive subcutaneous injections of extended-released exenatide at a dose of 2 mg, or matching placebo once per week, from June 18, 2010, through Sept. 16, 2015. The patients were followed for a median of 3.2 years and the main outcome of interest was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or stroke.
In the meantime, the intention-to-treat analysis revealed that exenatide was noninferior to placebo with respect to safety (P less than .001) but was not superior to placebo with respect to safety (P = .06). The researchers observed no significant differences between the two groups in the rates of death from cardiovascular causes, fatal or nonfatal MI, or stroke, hospitalization for heart failure or for acute coronary syndrome, or in the incidence of cute pancreatitis, pancreatic cancer, medullary thyroid carcinoma, and serious adverse events.
“The pragmatic design of the trial included integration with usual care and wide-ranging eligibility criteria,” the researchers wrote. “For example, patients with any degree of cardiovascular risk who were at least 18 years of age (with no upper age limit) were eligible. To further augment the potential generalizability of any findings, we evaluated the cardiovascular effect of once-weekly extended-release exenatide in the usual-care setting by maintaining the focus of management of diabetes and cardiovascular risk with the usual-care provider.”
The researchers acknowledged certain limitations of the study, including the rate of premature discontinuation of the trial regimen, “which was driven by patient decision. We speculate that probable factors for discontinuation were the complexity of the first-generation injection device that was used and the fact that our trial had no run-in period.”
The trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trial Unit, in collaboration with Amylin Pharmaceuticals. Dr. Holman and his coauthors reported having numerous financial ties to the pharmaceutical industry.
PRIMARY SOURCE: N Engl J Med 2017 Sept. 14. doi: 10.1056/NEJMoa1612917
“We did not observe any specific safety issues during our trial; there was no adverse signal with respect to heart failure, despite the higher mean heart rate in the exenatide group than in the placebo group, and events of acute pancreatitis and pancreatic cancer were rare, with similar rates in the two groups,” according to researchers led by Rury R. Holman, F.Med.Sci. Their study was published online Sept. 14 in the New England Journal of Medicine, and was presented at the annual meeting of the European Association for the Study of Diabetes.
For the trial, which was funded by Amylin Pharmaceuticals and known as the Exenatide Study of Cardiovascular Event Lowering (EXSCEL), the researchers randomly assigned 14,752 patients with type 2 diabetes at 687 sites in 35 countries to receive subcutaneous injections of extended-released exenatide at a dose of 2 mg, or matching placebo once per week, from June 18, 2010, through Sept. 16, 2015. The patients were followed for a median of 3.2 years and the main outcome of interest was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or stroke.
In the meantime, the intention-to-treat analysis revealed that exenatide was noninferior to placebo with respect to safety (P less than .001) but was not superior to placebo with respect to safety (P = .06). The researchers observed no significant differences between the two groups in the rates of death from cardiovascular causes, fatal or nonfatal MI, or stroke, hospitalization for heart failure or for acute coronary syndrome, or in the incidence of cute pancreatitis, pancreatic cancer, medullary thyroid carcinoma, and serious adverse events.
“The pragmatic design of the trial included integration with usual care and wide-ranging eligibility criteria,” the researchers wrote. “For example, patients with any degree of cardiovascular risk who were at least 18 years of age (with no upper age limit) were eligible. To further augment the potential generalizability of any findings, we evaluated the cardiovascular effect of once-weekly extended-release exenatide in the usual-care setting by maintaining the focus of management of diabetes and cardiovascular risk with the usual-care provider.”
The researchers acknowledged certain limitations of the study, including the rate of premature discontinuation of the trial regimen, “which was driven by patient decision. We speculate that probable factors for discontinuation were the complexity of the first-generation injection device that was used and the fact that our trial had no run-in period.”
The trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trial Unit, in collaboration with Amylin Pharmaceuticals. Dr. Holman and his coauthors reported having numerous financial ties to the pharmaceutical industry.
PRIMARY SOURCE: N Engl J Med 2017 Sept. 14. doi: 10.1056/NEJMoa1612917
FROM EASD 2017
Key clinical point: Exenatide was noninferior to placebo with respect to cardiovascular safety but was not superior with respect to efficacy.
Major finding: Among patients with type 2 diabetes with and without previous cardiovascular disease, once-weekly administration of exenatide does not appear to cause an increase in their overall cardiovascular risk.
Study details: A randomized, placebo-controlled trial of 14,752 diabetic patients with or without previous cardiovascular disease.
Disclosures: The trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trial Unit, in collaboration with Amylin Pharmaceuticals. Dr. Holman and his coauthors reported having numerous financial ties to the pharmaceutical industry.
Source: Rury R. Holman, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017 Sept 14. doi: 10.1056/NEJMoa1612917.












