User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Session highlights controversies in diverticulitis management
SAN DIEGO – During the annual clinical congress of the American College of Surgeons, a panel of experts discussed a wide range of evolving controversies in the management of complicated diverticulitis.
John Migaly, MD, kicked off the session by exploring the current evidence for laparoscopic lavage versus primary resection for Hinchey III diverticulitis. “Over the past two decades there has been a growing utilization of primary resection and reanastomoses with or without the use of ileostomy,” said Dr. Migaly, director of the general surgery residency program at Duke University, Durham, N.C. “And most recently over the last 5 or 10 years there’s been growing enthusiasm for laparoscopic lavage for Hinchey III diverticulitis. The enthusiasm for this procedure is based on [its accessibility] to all of us who operate on the colon in the acute setting.”
The DILALA trial makes the best case for laparoscopic lavage, “but it doesn’t make a very good one,” he commented. The trial was conducted in nine surgical departments in Sweden and Denmark (Ann Surg. 2016;263[1]:117-22). Hinchey III patients were randomized 1:1 to laparoscopic lavage or the Hartmann procedure. The primary outcome was reoperations within 12 months. An early analysis of the short-term outcomes in 83 patients found similar 30-day and 90-day mortality and morbidity. Its authors concluded that laparoscopic lavage had equivalent morbidity and mortality compared with radical resection, shorter operative time, shorter time in the recovery unit, a shorter hospital stay, but no difference in the rate of reoperation.
“It was found to be safe and feasible,” Dr. Migaly said. One year later, the researchers presented their 12-month outcomes and came to the same conclusions. Limitations of the data are that it was conducted in nine centers “but they enrolled only 83 patients, so it seems underpowered,” he said. “And there was no mention of the incidence of abdominal abscess requiring percutaneous drainage or episodes of diverticulitis. The data seem a little less granular than the LADIES trial.”
The SCANDIV trial is the largest study on the topic to date, a randomized clinical superiority trial conducted at 21 centers in Sweden and Norway (JAMA 2015;314[13]:1364-75). Of the 509 patients screened, 415 were eligible and 199 were enrolled: 101 to laparoscopic lavage, 98 to colon resection. The primary endpoint was severe postoperative complications within 90 days, defined as a Clavien-Dindo score of over 3. The researchers found no difference in major complications nor in 90-day mortality between the two groups. The rate of reoperation was significantly higher in the lavage group, compared with the resection group (20.3% vs. 5.7%, respectively). Four sigmoid cancers were missed in the lavage group and, while the length of operation was significantly shorter in the lavage group, there were no differences between the two groups in hospital length of stay or quality of life.
A meta-analysis of the three randomized controlled trials that Dr. Migaly reviewed concluded that laparoscopic lavage, compared with resection, for Hinchey III diverticulitis increased the rate of total reoperations, the rate of reoperation for infection, and the rate of subsequent percutaneous drainage (J Gastrointest Surg 2017;21[9]:1491-99). A larger, more recent meta-analysis of 589 patients, including the three randomized controlled trials that Dr. Migaly discussed, concluded that laparoscopic lavage patients, compared with resection patients, had three times the risk of persistent peritonitis, intra-abdominal abscess, and emergency reoperative surgery. “Therefore, a reasonable conclusion would be that data at this point does not support the use of laparoscopic lavage,” he said.
The initial procedure of choice for most patients is abscess drainage via CT-guided percutaneous drainage. “There are some patients who are not candidates for percutaneous drainage, [such as] if the abscess is not accessible to the radiologist, if the patient is anticoagulated, and if the patient requires emergent surgical intervention irrespective of the abscess,” said Dr. Maron, a colorectal surgeon who practices at Cleveland Clinic Florida, Weston. “There is also a question of cavity size. Most authors in the literature use a cut-off of 3-4 cm.”
The goal is complete drainage of the abscess, and sometimes multiple catheters will be required. One study found that predictors of successful abscess drainage included having a well-defined, unilocular abscess. The success rate fell to 63% for patients who presented with more complex abscesses, including those that were loculated, poorly defined, associated with a fistula, and contained feces or semisolid material (Dis Colon Rectum. 1997;40:1009-13).
The 2014 ASCRS Practice Parameters includes the recommendation that elective colectomy should typically be considered after the patient recovers from an episode of complicated diverticulitis, “but some of the data may be calling that into question,” Dr. Maron said. In one series of 18 patients with an abscess treated percutaneously, 11 refused surgery and 7 had significant comorbidity (Dis Colon Rectum. 2014;57:331-6). Three patients died of a pre-existing condition and 7 of the 15 surviving patients had recurrent diverticulitis. Three underwent surgery and four were treated medically. The authors found no association between long-term failure and abscess location or previous episodes of diverticulitis.
In a larger study, researchers identified 218 patients who were initially treated with intravenous antibiotics and percutaneous drain (Dis Colon Rectum. 2013;56:622-6). About 10% of the patients required an urgent operation, while most of the other patients underwent elective resection, but 15% of patients did not undergo a subsequent colectomy. “Most of these patients were medically unfit to undergo surgery,” he noted. Abscess location was more commonly paracolic than pelvic. The mean abscess size was 4.2 cm, and the drain was left in for a median of 20 days. The recurrence rate in this series was only 30%, but none of the recurrences required surgery. The authors found that abscesses greater than 5 cm in size were associated with a greater risk of recurrence (P = .003). They concluded that observation after percutaneous drainage is safe in selected patients.
Based on results from this and other more recent studies, Dr. Maron said that it remains unclear whether surgeons should wait and watch or operate after successful percutaneous drainage of diverticular abscess. The data are “not as robust as we’d like … most of these are retrospective studies,” he said. “There’s quite a bit of inherent selection bias, and there is no standardization with regard to length of time of percutaneous drain, rationale for nonoperative management versus elective colectomy. What we do know is that there are some patients who can be managed safely without surgery. Unfortunately, there is no good algorithm I can offer you: Perhaps larger abscesses can portend a higher recurrence. I don’t think we’ll have a good answer to this until we perform a prospective randomized trial. However, we may learn some data from patients managed by peritoneal lavage and drain placement.”
Next, consider how to perform the procedure: laparoscopic or open? “But again, you’re going to look at how stable your patient is, what your skill set is, what equipment is available, and if the patient has had previous abdominal surgeries,” she advised. “In the traditional Hartmann procedure, you resect the perforated segment. You try not to open any tissue planes that you don’t have to. You do just enough so you can bring up a colostomy; you close the rectum or you make a mucous fistula. The problem with this operation is that up to 80% of these patients have their colostomy closed. That is why there is all this controversy. If there is any question, this [procedure] is always the safest option; there’s no anastomosis.”
What about a performing resection and a colorectal anastomosis? This is usually done more commonly in the elective situation, “when things are perfect, when you have healthy tissue,” Dr. Hull said. “If you do it in the emergent situation you have to think to yourself, ‘Could my patient tolerate a leak?’ You won’t want to do this operation on a 72-year-old who’s on steroids for chronic pulmonary disease and has coronary artery disease, because if they had a leak, they’d probably die.”
The third procedural option is to resect bowel (usually sigmoid) with colorectal anastomosis and diversion (loop ileostomy). “This is always my preferred choice,” Dr. Hull said. “I always am thinking, ‘Why can’t I do this?’ The reason is, closure of ileostomy is much easier than a Hartmann reversal, and 90% of these patients get reversed.” In a multicenter trial conducted by Swedish researchers, 62 patients were randomized to Hartmann’s procedure versus primary anastomosis with diverting ileostomy for perforated left-sided diverticulitis (Ann Surg. 2012;256[5]:819-27). The mortality and complications were similar, but the number of patients who got their stoma reversed was significantly less in the Hartmann’s group, compared with the primary anastomosis group (57% vs. 90%, respectively), and the serious complications were much higher in the Hartmann’s group (20% vs. 0). She cited an article from the World Journal of Emergency Surgery as one of the most comprehensive reviews of the subject.

So what is a surgeon to do? For an anastomosis after resection, “consider patient factors: Are they stable?” Dr. Hull said. “What are their comorbid conditions? You have to think, ‘What is my preference? Am I comfortable putting this back together?’ But a primary anastomosis is feasible, even in the acute setting with or without diverting ileostomy. The laparoscopic approach is typically preferred, but if that’s not in your armamentarium, the open [approach] is just fine. You should always perform a leak test. You can also do on table colonic lavage if feasible, especially if you have a large stool burden.”
None of the speakers reported having financial disclosures.
SAN DIEGO – During the annual clinical congress of the American College of Surgeons, a panel of experts discussed a wide range of evolving controversies in the management of complicated diverticulitis.
John Migaly, MD, kicked off the session by exploring the current evidence for laparoscopic lavage versus primary resection for Hinchey III diverticulitis. “Over the past two decades there has been a growing utilization of primary resection and reanastomoses with or without the use of ileostomy,” said Dr. Migaly, director of the general surgery residency program at Duke University, Durham, N.C. “And most recently over the last 5 or 10 years there’s been growing enthusiasm for laparoscopic lavage for Hinchey III diverticulitis. The enthusiasm for this procedure is based on [its accessibility] to all of us who operate on the colon in the acute setting.”
The DILALA trial makes the best case for laparoscopic lavage, “but it doesn’t make a very good one,” he commented. The trial was conducted in nine surgical departments in Sweden and Denmark (Ann Surg. 2016;263[1]:117-22). Hinchey III patients were randomized 1:1 to laparoscopic lavage or the Hartmann procedure. The primary outcome was reoperations within 12 months. An early analysis of the short-term outcomes in 83 patients found similar 30-day and 90-day mortality and morbidity. Its authors concluded that laparoscopic lavage had equivalent morbidity and mortality compared with radical resection, shorter operative time, shorter time in the recovery unit, a shorter hospital stay, but no difference in the rate of reoperation.
“It was found to be safe and feasible,” Dr. Migaly said. One year later, the researchers presented their 12-month outcomes and came to the same conclusions. Limitations of the data are that it was conducted in nine centers “but they enrolled only 83 patients, so it seems underpowered,” he said. “And there was no mention of the incidence of abdominal abscess requiring percutaneous drainage or episodes of diverticulitis. The data seem a little less granular than the LADIES trial.”
The SCANDIV trial is the largest study on the topic to date, a randomized clinical superiority trial conducted at 21 centers in Sweden and Norway (JAMA 2015;314[13]:1364-75). Of the 509 patients screened, 415 were eligible and 199 were enrolled: 101 to laparoscopic lavage, 98 to colon resection. The primary endpoint was severe postoperative complications within 90 days, defined as a Clavien-Dindo score of over 3. The researchers found no difference in major complications nor in 90-day mortality between the two groups. The rate of reoperation was significantly higher in the lavage group, compared with the resection group (20.3% vs. 5.7%, respectively). Four sigmoid cancers were missed in the lavage group and, while the length of operation was significantly shorter in the lavage group, there were no differences between the two groups in hospital length of stay or quality of life.
A meta-analysis of the three randomized controlled trials that Dr. Migaly reviewed concluded that laparoscopic lavage, compared with resection, for Hinchey III diverticulitis increased the rate of total reoperations, the rate of reoperation for infection, and the rate of subsequent percutaneous drainage (J Gastrointest Surg 2017;21[9]:1491-99). A larger, more recent meta-analysis of 589 patients, including the three randomized controlled trials that Dr. Migaly discussed, concluded that laparoscopic lavage patients, compared with resection patients, had three times the risk of persistent peritonitis, intra-abdominal abscess, and emergency reoperative surgery. “Therefore, a reasonable conclusion would be that data at this point does not support the use of laparoscopic lavage,” he said.
The initial procedure of choice for most patients is abscess drainage via CT-guided percutaneous drainage. “There are some patients who are not candidates for percutaneous drainage, [such as] if the abscess is not accessible to the radiologist, if the patient is anticoagulated, and if the patient requires emergent surgical intervention irrespective of the abscess,” said Dr. Maron, a colorectal surgeon who practices at Cleveland Clinic Florida, Weston. “There is also a question of cavity size. Most authors in the literature use a cut-off of 3-4 cm.”
The goal is complete drainage of the abscess, and sometimes multiple catheters will be required. One study found that predictors of successful abscess drainage included having a well-defined, unilocular abscess. The success rate fell to 63% for patients who presented with more complex abscesses, including those that were loculated, poorly defined, associated with a fistula, and contained feces or semisolid material (Dis Colon Rectum. 1997;40:1009-13).
The 2014 ASCRS Practice Parameters includes the recommendation that elective colectomy should typically be considered after the patient recovers from an episode of complicated diverticulitis, “but some of the data may be calling that into question,” Dr. Maron said. In one series of 18 patients with an abscess treated percutaneously, 11 refused surgery and 7 had significant comorbidity (Dis Colon Rectum. 2014;57:331-6). Three patients died of a pre-existing condition and 7 of the 15 surviving patients had recurrent diverticulitis. Three underwent surgery and four were treated medically. The authors found no association between long-term failure and abscess location or previous episodes of diverticulitis.
In a larger study, researchers identified 218 patients who were initially treated with intravenous antibiotics and percutaneous drain (Dis Colon Rectum. 2013;56:622-6). About 10% of the patients required an urgent operation, while most of the other patients underwent elective resection, but 15% of patients did not undergo a subsequent colectomy. “Most of these patients were medically unfit to undergo surgery,” he noted. Abscess location was more commonly paracolic than pelvic. The mean abscess size was 4.2 cm, and the drain was left in for a median of 20 days. The recurrence rate in this series was only 30%, but none of the recurrences required surgery. The authors found that abscesses greater than 5 cm in size were associated with a greater risk of recurrence (P = .003). They concluded that observation after percutaneous drainage is safe in selected patients.
Based on results from this and other more recent studies, Dr. Maron said that it remains unclear whether surgeons should wait and watch or operate after successful percutaneous drainage of diverticular abscess. The data are “not as robust as we’d like … most of these are retrospective studies,” he said. “There’s quite a bit of inherent selection bias, and there is no standardization with regard to length of time of percutaneous drain, rationale for nonoperative management versus elective colectomy. What we do know is that there are some patients who can be managed safely without surgery. Unfortunately, there is no good algorithm I can offer you: Perhaps larger abscesses can portend a higher recurrence. I don’t think we’ll have a good answer to this until we perform a prospective randomized trial. However, we may learn some data from patients managed by peritoneal lavage and drain placement.”
Next, consider how to perform the procedure: laparoscopic or open? “But again, you’re going to look at how stable your patient is, what your skill set is, what equipment is available, and if the patient has had previous abdominal surgeries,” she advised. “In the traditional Hartmann procedure, you resect the perforated segment. You try not to open any tissue planes that you don’t have to. You do just enough so you can bring up a colostomy; you close the rectum or you make a mucous fistula. The problem with this operation is that up to 80% of these patients have their colostomy closed. That is why there is all this controversy. If there is any question, this [procedure] is always the safest option; there’s no anastomosis.”
What about a performing resection and a colorectal anastomosis? This is usually done more commonly in the elective situation, “when things are perfect, when you have healthy tissue,” Dr. Hull said. “If you do it in the emergent situation you have to think to yourself, ‘Could my patient tolerate a leak?’ You won’t want to do this operation on a 72-year-old who’s on steroids for chronic pulmonary disease and has coronary artery disease, because if they had a leak, they’d probably die.”
The third procedural option is to resect bowel (usually sigmoid) with colorectal anastomosis and diversion (loop ileostomy). “This is always my preferred choice,” Dr. Hull said. “I always am thinking, ‘Why can’t I do this?’ The reason is, closure of ileostomy is much easier than a Hartmann reversal, and 90% of these patients get reversed.” In a multicenter trial conducted by Swedish researchers, 62 patients were randomized to Hartmann’s procedure versus primary anastomosis with diverting ileostomy for perforated left-sided diverticulitis (Ann Surg. 2012;256[5]:819-27). The mortality and complications were similar, but the number of patients who got their stoma reversed was significantly less in the Hartmann’s group, compared with the primary anastomosis group (57% vs. 90%, respectively), and the serious complications were much higher in the Hartmann’s group (20% vs. 0). She cited an article from the World Journal of Emergency Surgery as one of the most comprehensive reviews of the subject.

So what is a surgeon to do? For an anastomosis after resection, “consider patient factors: Are they stable?” Dr. Hull said. “What are their comorbid conditions? You have to think, ‘What is my preference? Am I comfortable putting this back together?’ But a primary anastomosis is feasible, even in the acute setting with or without diverting ileostomy. The laparoscopic approach is typically preferred, but if that’s not in your armamentarium, the open [approach] is just fine. You should always perform a leak test. You can also do on table colonic lavage if feasible, especially if you have a large stool burden.”
None of the speakers reported having financial disclosures.
SAN DIEGO – During the annual clinical congress of the American College of Surgeons, a panel of experts discussed a wide range of evolving controversies in the management of complicated diverticulitis.
John Migaly, MD, kicked off the session by exploring the current evidence for laparoscopic lavage versus primary resection for Hinchey III diverticulitis. “Over the past two decades there has been a growing utilization of primary resection and reanastomoses with or without the use of ileostomy,” said Dr. Migaly, director of the general surgery residency program at Duke University, Durham, N.C. “And most recently over the last 5 or 10 years there’s been growing enthusiasm for laparoscopic lavage for Hinchey III diverticulitis. The enthusiasm for this procedure is based on [its accessibility] to all of us who operate on the colon in the acute setting.”
The DILALA trial makes the best case for laparoscopic lavage, “but it doesn’t make a very good one,” he commented. The trial was conducted in nine surgical departments in Sweden and Denmark (Ann Surg. 2016;263[1]:117-22). Hinchey III patients were randomized 1:1 to laparoscopic lavage or the Hartmann procedure. The primary outcome was reoperations within 12 months. An early analysis of the short-term outcomes in 83 patients found similar 30-day and 90-day mortality and morbidity. Its authors concluded that laparoscopic lavage had equivalent morbidity and mortality compared with radical resection, shorter operative time, shorter time in the recovery unit, a shorter hospital stay, but no difference in the rate of reoperation.
“It was found to be safe and feasible,” Dr. Migaly said. One year later, the researchers presented their 12-month outcomes and came to the same conclusions. Limitations of the data are that it was conducted in nine centers “but they enrolled only 83 patients, so it seems underpowered,” he said. “And there was no mention of the incidence of abdominal abscess requiring percutaneous drainage or episodes of diverticulitis. The data seem a little less granular than the LADIES trial.”
The SCANDIV trial is the largest study on the topic to date, a randomized clinical superiority trial conducted at 21 centers in Sweden and Norway (JAMA 2015;314[13]:1364-75). Of the 509 patients screened, 415 were eligible and 199 were enrolled: 101 to laparoscopic lavage, 98 to colon resection. The primary endpoint was severe postoperative complications within 90 days, defined as a Clavien-Dindo score of over 3. The researchers found no difference in major complications nor in 90-day mortality between the two groups. The rate of reoperation was significantly higher in the lavage group, compared with the resection group (20.3% vs. 5.7%, respectively). Four sigmoid cancers were missed in the lavage group and, while the length of operation was significantly shorter in the lavage group, there were no differences between the two groups in hospital length of stay or quality of life.
A meta-analysis of the three randomized controlled trials that Dr. Migaly reviewed concluded that laparoscopic lavage, compared with resection, for Hinchey III diverticulitis increased the rate of total reoperations, the rate of reoperation for infection, and the rate of subsequent percutaneous drainage (J Gastrointest Surg 2017;21[9]:1491-99). A larger, more recent meta-analysis of 589 patients, including the three randomized controlled trials that Dr. Migaly discussed, concluded that laparoscopic lavage patients, compared with resection patients, had three times the risk of persistent peritonitis, intra-abdominal abscess, and emergency reoperative surgery. “Therefore, a reasonable conclusion would be that data at this point does not support the use of laparoscopic lavage,” he said.
The initial procedure of choice for most patients is abscess drainage via CT-guided percutaneous drainage. “There are some patients who are not candidates for percutaneous drainage, [such as] if the abscess is not accessible to the radiologist, if the patient is anticoagulated, and if the patient requires emergent surgical intervention irrespective of the abscess,” said Dr. Maron, a colorectal surgeon who practices at Cleveland Clinic Florida, Weston. “There is also a question of cavity size. Most authors in the literature use a cut-off of 3-4 cm.”
The goal is complete drainage of the abscess, and sometimes multiple catheters will be required. One study found that predictors of successful abscess drainage included having a well-defined, unilocular abscess. The success rate fell to 63% for patients who presented with more complex abscesses, including those that were loculated, poorly defined, associated with a fistula, and contained feces or semisolid material (Dis Colon Rectum. 1997;40:1009-13).
The 2014 ASCRS Practice Parameters includes the recommendation that elective colectomy should typically be considered after the patient recovers from an episode of complicated diverticulitis, “but some of the data may be calling that into question,” Dr. Maron said. In one series of 18 patients with an abscess treated percutaneously, 11 refused surgery and 7 had significant comorbidity (Dis Colon Rectum. 2014;57:331-6). Three patients died of a pre-existing condition and 7 of the 15 surviving patients had recurrent diverticulitis. Three underwent surgery and four were treated medically. The authors found no association between long-term failure and abscess location or previous episodes of diverticulitis.
In a larger study, researchers identified 218 patients who were initially treated with intravenous antibiotics and percutaneous drain (Dis Colon Rectum. 2013;56:622-6). About 10% of the patients required an urgent operation, while most of the other patients underwent elective resection, but 15% of patients did not undergo a subsequent colectomy. “Most of these patients were medically unfit to undergo surgery,” he noted. Abscess location was more commonly paracolic than pelvic. The mean abscess size was 4.2 cm, and the drain was left in for a median of 20 days. The recurrence rate in this series was only 30%, but none of the recurrences required surgery. The authors found that abscesses greater than 5 cm in size were associated with a greater risk of recurrence (P = .003). They concluded that observation after percutaneous drainage is safe in selected patients.
Based on results from this and other more recent studies, Dr. Maron said that it remains unclear whether surgeons should wait and watch or operate after successful percutaneous drainage of diverticular abscess. The data are “not as robust as we’d like … most of these are retrospective studies,” he said. “There’s quite a bit of inherent selection bias, and there is no standardization with regard to length of time of percutaneous drain, rationale for nonoperative management versus elective colectomy. What we do know is that there are some patients who can be managed safely without surgery. Unfortunately, there is no good algorithm I can offer you: Perhaps larger abscesses can portend a higher recurrence. I don’t think we’ll have a good answer to this until we perform a prospective randomized trial. However, we may learn some data from patients managed by peritoneal lavage and drain placement.”
Next, consider how to perform the procedure: laparoscopic or open? “But again, you’re going to look at how stable your patient is, what your skill set is, what equipment is available, and if the patient has had previous abdominal surgeries,” she advised. “In the traditional Hartmann procedure, you resect the perforated segment. You try not to open any tissue planes that you don’t have to. You do just enough so you can bring up a colostomy; you close the rectum or you make a mucous fistula. The problem with this operation is that up to 80% of these patients have their colostomy closed. That is why there is all this controversy. If there is any question, this [procedure] is always the safest option; there’s no anastomosis.”
What about a performing resection and a colorectal anastomosis? This is usually done more commonly in the elective situation, “when things are perfect, when you have healthy tissue,” Dr. Hull said. “If you do it in the emergent situation you have to think to yourself, ‘Could my patient tolerate a leak?’ You won’t want to do this operation on a 72-year-old who’s on steroids for chronic pulmonary disease and has coronary artery disease, because if they had a leak, they’d probably die.”
The third procedural option is to resect bowel (usually sigmoid) with colorectal anastomosis and diversion (loop ileostomy). “This is always my preferred choice,” Dr. Hull said. “I always am thinking, ‘Why can’t I do this?’ The reason is, closure of ileostomy is much easier than a Hartmann reversal, and 90% of these patients get reversed.” In a multicenter trial conducted by Swedish researchers, 62 patients were randomized to Hartmann’s procedure versus primary anastomosis with diverting ileostomy for perforated left-sided diverticulitis (Ann Surg. 2012;256[5]:819-27). The mortality and complications were similar, but the number of patients who got their stoma reversed was significantly less in the Hartmann’s group, compared with the primary anastomosis group (57% vs. 90%, respectively), and the serious complications were much higher in the Hartmann’s group (20% vs. 0). She cited an article from the World Journal of Emergency Surgery as one of the most comprehensive reviews of the subject.

So what is a surgeon to do? For an anastomosis after resection, “consider patient factors: Are they stable?” Dr. Hull said. “What are their comorbid conditions? You have to think, ‘What is my preference? Am I comfortable putting this back together?’ But a primary anastomosis is feasible, even in the acute setting with or without diverting ileostomy. The laparoscopic approach is typically preferred, but if that’s not in your armamentarium, the open [approach] is just fine. You should always perform a leak test. You can also do on table colonic lavage if feasible, especially if you have a large stool burden.”
None of the speakers reported having financial disclosures.
AT THE ACS CLINICAL CONGRESS
Ribaxamase reduced new CDI by 71%
SAN DIEGO – , results from a phase 2b study showed.
At an annual scientific meeting on infectious diseases, lead investigator John F. Kokai-Kun, PhD, said that the finding represents a paradigm shift in the use of intravenous beta-lactam antibiotics to prevent opportunistic infections. “We currently treat Clostridium difficile infection (CDI) with antibiotics, which attack the vegetative cells,” said Dr. Kokai-Kun, vice president of nonclinical affairs for Rockville, Md.–based Synthetic Biologics, which is developing ribaxamase. “Since C. diff. is primarily a toxin-mediated disease, certain products seem to neutralize the toxin. There’s also been work with probiotics and prebiotics to try to strengthen and repair the dysbiotic colon. Fecal replacement therapy has been shown to be fairly effective for treatment of recurrent C. diff. infection. What if we could simply block the initial insult that leads to this cascade? That’s the damage caused to the gut microbiome by the antibiotic that’s excreted to the intestine.”
That’s where ribaxamase comes in, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Ribaxamase is an orally administered beta-lac-tamase designed to degrade penicillin and cephalosporins in the intestinal lumen. It’s formulated for release in the proximal small intestine and is expected to be given during or a short time after administration of IV beta-lactam antibiotics such as ceftriaxone. “This is expected to degrade the excess antibiotics that are excreted into the small intestine via the bile,” Dr. Kokai-Kun explained. “It’s designed to prevent disruption of the gut microbiome and thus protect from opportunistic GI infections like CDI.”
Early-stage clinical studies demonstrated that ribaxamase was well tolerated and that it is not systemically absorbed, while phase 2 studies showed that ribaxamase degrades ceftriaxone in the intestine to below the level of detection while not affecting the pharmacokinetics of ceftriaxone in the plasma.
For the current study, 412 patients were enrolled at 84 multinational clinical sites. These patients were admitted to the hospital for treatment of a lower respiratory tract infection and were randomized 1:1 to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo. Patients in both groups could also receive an oral macrolide at the discretion of the clinical investigator.
The researchers also obtained fecal samples at screening, 72 hours post antibiotic treatment, and at the end of a 4-week follow-up visit, to determine colonization by opportunistic pathogens and to examine changes in the gut microbiome.
Patients were monitored for 6 weeks for diarrhea and CDI. Diarrhea was defined as three or more loose or watery stools in a 24-hour period. “If that occurred, then we collected a sample, which was sent to the local lab to determine the presence of C. difficile toxins,” Dr. Kokai-Kun said.
The average age of study participants was 70 years, and about one-third in each arm received oral macrolides. The number of adverse events and serious adverse events were similar between active and placebo arms, and there was no trend associated with ribaxamase use. The lower respiratory tract infection cure rate to the ceftriaxone treatment was about 99% in both arms at 72 hours post treatment and at 2 weeks post treatment.
To analyze changes in the gut microbiome, the researchers conducted 16S rRNA sequencing of DNA extracted from fecal samples. In all, 652 samples were sequenced from 229 patients. Results from that analysis suggests that ribaxamase “appears to protect the gut microbiome from the onslaught of the ceftriaxone,” he said.
Ribaxamase reduced the incidence of new-onset CDI by 71%, compared with placebo (P = .045). “It apparently did this by protecting the integrity of the gut microbiome,” Dr. Kokai-Kun said. “There was also a significant reduction of new colonization by vancomycin-resistant enterococci at 72 hours and 4 weeks (P = .0001 and P = .0002, respectively) which is an opportunistic pathogen that is known to be able to inhabit gut microbiome when there is dysbiosis.”
The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
AGA Resource
Help your patients better understand C. difficile by using AGA’s patient education materials available here.
[email protected]
SAN DIEGO – , results from a phase 2b study showed.
At an annual scientific meeting on infectious diseases, lead investigator John F. Kokai-Kun, PhD, said that the finding represents a paradigm shift in the use of intravenous beta-lactam antibiotics to prevent opportunistic infections. “We currently treat Clostridium difficile infection (CDI) with antibiotics, which attack the vegetative cells,” said Dr. Kokai-Kun, vice president of nonclinical affairs for Rockville, Md.–based Synthetic Biologics, which is developing ribaxamase. “Since C. diff. is primarily a toxin-mediated disease, certain products seem to neutralize the toxin. There’s also been work with probiotics and prebiotics to try to strengthen and repair the dysbiotic colon. Fecal replacement therapy has been shown to be fairly effective for treatment of recurrent C. diff. infection. What if we could simply block the initial insult that leads to this cascade? That’s the damage caused to the gut microbiome by the antibiotic that’s excreted to the intestine.”
That’s where ribaxamase comes in, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Ribaxamase is an orally administered beta-lac-tamase designed to degrade penicillin and cephalosporins in the intestinal lumen. It’s formulated for release in the proximal small intestine and is expected to be given during or a short time after administration of IV beta-lactam antibiotics such as ceftriaxone. “This is expected to degrade the excess antibiotics that are excreted into the small intestine via the bile,” Dr. Kokai-Kun explained. “It’s designed to prevent disruption of the gut microbiome and thus protect from opportunistic GI infections like CDI.”
Early-stage clinical studies demonstrated that ribaxamase was well tolerated and that it is not systemically absorbed, while phase 2 studies showed that ribaxamase degrades ceftriaxone in the intestine to below the level of detection while not affecting the pharmacokinetics of ceftriaxone in the plasma.
For the current study, 412 patients were enrolled at 84 multinational clinical sites. These patients were admitted to the hospital for treatment of a lower respiratory tract infection and were randomized 1:1 to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo. Patients in both groups could also receive an oral macrolide at the discretion of the clinical investigator.
The researchers also obtained fecal samples at screening, 72 hours post antibiotic treatment, and at the end of a 4-week follow-up visit, to determine colonization by opportunistic pathogens and to examine changes in the gut microbiome.
Patients were monitored for 6 weeks for diarrhea and CDI. Diarrhea was defined as three or more loose or watery stools in a 24-hour period. “If that occurred, then we collected a sample, which was sent to the local lab to determine the presence of C. difficile toxins,” Dr. Kokai-Kun said.
The average age of study participants was 70 years, and about one-third in each arm received oral macrolides. The number of adverse events and serious adverse events were similar between active and placebo arms, and there was no trend associated with ribaxamase use. The lower respiratory tract infection cure rate to the ceftriaxone treatment was about 99% in both arms at 72 hours post treatment and at 2 weeks post treatment.
To analyze changes in the gut microbiome, the researchers conducted 16S rRNA sequencing of DNA extracted from fecal samples. In all, 652 samples were sequenced from 229 patients. Results from that analysis suggests that ribaxamase “appears to protect the gut microbiome from the onslaught of the ceftriaxone,” he said.
Ribaxamase reduced the incidence of new-onset CDI by 71%, compared with placebo (P = .045). “It apparently did this by protecting the integrity of the gut microbiome,” Dr. Kokai-Kun said. “There was also a significant reduction of new colonization by vancomycin-resistant enterococci at 72 hours and 4 weeks (P = .0001 and P = .0002, respectively) which is an opportunistic pathogen that is known to be able to inhabit gut microbiome when there is dysbiosis.”
The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
AGA Resource
Help your patients better understand C. difficile by using AGA’s patient education materials available here.
[email protected]
SAN DIEGO – , results from a phase 2b study showed.
At an annual scientific meeting on infectious diseases, lead investigator John F. Kokai-Kun, PhD, said that the finding represents a paradigm shift in the use of intravenous beta-lactam antibiotics to prevent opportunistic infections. “We currently treat Clostridium difficile infection (CDI) with antibiotics, which attack the vegetative cells,” said Dr. Kokai-Kun, vice president of nonclinical affairs for Rockville, Md.–based Synthetic Biologics, which is developing ribaxamase. “Since C. diff. is primarily a toxin-mediated disease, certain products seem to neutralize the toxin. There’s also been work with probiotics and prebiotics to try to strengthen and repair the dysbiotic colon. Fecal replacement therapy has been shown to be fairly effective for treatment of recurrent C. diff. infection. What if we could simply block the initial insult that leads to this cascade? That’s the damage caused to the gut microbiome by the antibiotic that’s excreted to the intestine.”
That’s where ribaxamase comes in, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Ribaxamase is an orally administered beta-lac-tamase designed to degrade penicillin and cephalosporins in the intestinal lumen. It’s formulated for release in the proximal small intestine and is expected to be given during or a short time after administration of IV beta-lactam antibiotics such as ceftriaxone. “This is expected to degrade the excess antibiotics that are excreted into the small intestine via the bile,” Dr. Kokai-Kun explained. “It’s designed to prevent disruption of the gut microbiome and thus protect from opportunistic GI infections like CDI.”
Early-stage clinical studies demonstrated that ribaxamase was well tolerated and that it is not systemically absorbed, while phase 2 studies showed that ribaxamase degrades ceftriaxone in the intestine to below the level of detection while not affecting the pharmacokinetics of ceftriaxone in the plasma.
For the current study, 412 patients were enrolled at 84 multinational clinical sites. These patients were admitted to the hospital for treatment of a lower respiratory tract infection and were randomized 1:1 to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo. Patients in both groups could also receive an oral macrolide at the discretion of the clinical investigator.
The researchers also obtained fecal samples at screening, 72 hours post antibiotic treatment, and at the end of a 4-week follow-up visit, to determine colonization by opportunistic pathogens and to examine changes in the gut microbiome.
Patients were monitored for 6 weeks for diarrhea and CDI. Diarrhea was defined as three or more loose or watery stools in a 24-hour period. “If that occurred, then we collected a sample, which was sent to the local lab to determine the presence of C. difficile toxins,” Dr. Kokai-Kun said.
The average age of study participants was 70 years, and about one-third in each arm received oral macrolides. The number of adverse events and serious adverse events were similar between active and placebo arms, and there was no trend associated with ribaxamase use. The lower respiratory tract infection cure rate to the ceftriaxone treatment was about 99% in both arms at 72 hours post treatment and at 2 weeks post treatment.
To analyze changes in the gut microbiome, the researchers conducted 16S rRNA sequencing of DNA extracted from fecal samples. In all, 652 samples were sequenced from 229 patients. Results from that analysis suggests that ribaxamase “appears to protect the gut microbiome from the onslaught of the ceftriaxone,” he said.
Ribaxamase reduced the incidence of new-onset CDI by 71%, compared with placebo (P = .045). “It apparently did this by protecting the integrity of the gut microbiome,” Dr. Kokai-Kun said. “There was also a significant reduction of new colonization by vancomycin-resistant enterococci at 72 hours and 4 weeks (P = .0001 and P = .0002, respectively) which is an opportunistic pathogen that is known to be able to inhabit gut microbiome when there is dysbiosis.”
The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
AGA Resource
Help your patients better understand C. difficile by using AGA’s patient education materials available here.
[email protected]
Cases of Legionnaires’ continue to rise in the United States
SAN DIEGO –
“Improved testing and surveillance are needed to improve understanding of disease and outbreak burden,” Laura A. Cooley, MD, said at an annual scientific meeting on infectious diseases. “There is more to learn about environmental sources of Legionella for cases not associated with known outbreaks and about the distribution of Legionella in the environment.”
A Gram-negative bacillus, Legionella is an intracellular parasite of free-living protozoa primarily found in freshwater. “It can live and grow in biofilm, and there are more than 60 species of the bacterium,” she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Cases are higher in the warmer months, and the rates are highest among the elderly, men, and those of black race. Currently, L. pneumophila accounts for about 90% of cases in the United States. “Once it’s transmitted, it has to hit a susceptible population to cause disease, generally older individuals and people with underlying conditions,” Dr. Cooley said.
A separate analysis evaluated Legionella cases reported among U.S. residents between 2005 and 2009 (MMWR. 2011;60[32]:1083-6). It found that only 4% were associated with outbreaks, and 96% were sporadic. “That doesn’t mean that [the cases] weren’t associated with the same kind of source, they just weren’t identified as an outbreak,” Dr. Cooley said. “It shows that there is a lot to learn about transmission of Legionella.”
Data from the National Notifiable Diseases Surveillance System indicate that rates of Legionella continue to rise nationwide, especially in the Midwest and Northeast. “Why? It’s possible that there are differences in testing preferences and reporting preferences in this region of the country,” Dr. Cooley noted. “Maybe people are more tuned in to the potential for outbreaks, but there are reasons why there could be differences in disease, like differences in infrastructure, climate, population density, and cooling tower density.” CDC data from 2015 indicate that most cases are not associated with a known exposure, and that the case fatality rate differs by exposure type: 12% for cases reporting health care exposure during the 10 days before symptom onset (25% for definite cases), 9% for cases reporting assisted or senior living exposure, 7% when no specific exposure is reported, and 4% for cases reporting travel exposure (MMWR. 2017;66[22]:584-9).
The U.S. case definition of Legionnaires’ disease consists of clinical or radiologic pneumonia plus confirmatory laboratory testing, either by urinary antigen test (UAT), lower respiratory culture, or appropriate serological testing. Polymerase chain reaction can be used as a presumptive test for a suspect case. “UAT is easy and it detects L. pneumophila serogroup 1 (Lp1), but it has some gaps,” Dr. Cooley said. “It isn’t completely sensitive for Lp1, and it doesn’t detect any other species or serogroups. That’s why we also recommend that a culture of respiratory secretions on selective media be performed at the same time. That being said, in the U.S., nearly all reported cases of Legionella are diagnosed by UAT only.”
A 2016 CDC MMWR and Vital Signs report found that almost all Legionella outbreaks could be prevented with effective water management, and the CDC has published a step-by-step guide to creating a water management program to reduce Legionella growth and spread in buildings. The 2017 MMWR Report found that definite health care–associated Legionnaires’ disease is deadly for one in four people who get it. The report also found that this issue is widespread; 76% of complete reporting jurisdictions reported at least one definite case of health care–associated Legionella disease in 2015. More recently, the Centers for Medicare & Medicaid Services issued a requirement to reduce risk in health care facility water systems to prevent cases and outbreaks. It applies to hospitals, skilled nursing facilities, and critical access hospitals.
Dr. Cooley reported having no financial disclosures.
SAN DIEGO –
“Improved testing and surveillance are needed to improve understanding of disease and outbreak burden,” Laura A. Cooley, MD, said at an annual scientific meeting on infectious diseases. “There is more to learn about environmental sources of Legionella for cases not associated with known outbreaks and about the distribution of Legionella in the environment.”
A Gram-negative bacillus, Legionella is an intracellular parasite of free-living protozoa primarily found in freshwater. “It can live and grow in biofilm, and there are more than 60 species of the bacterium,” she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Cases are higher in the warmer months, and the rates are highest among the elderly, men, and those of black race. Currently, L. pneumophila accounts for about 90% of cases in the United States. “Once it’s transmitted, it has to hit a susceptible population to cause disease, generally older individuals and people with underlying conditions,” Dr. Cooley said.
A separate analysis evaluated Legionella cases reported among U.S. residents between 2005 and 2009 (MMWR. 2011;60[32]:1083-6). It found that only 4% were associated with outbreaks, and 96% were sporadic. “That doesn’t mean that [the cases] weren’t associated with the same kind of source, they just weren’t identified as an outbreak,” Dr. Cooley said. “It shows that there is a lot to learn about transmission of Legionella.”
Data from the National Notifiable Diseases Surveillance System indicate that rates of Legionella continue to rise nationwide, especially in the Midwest and Northeast. “Why? It’s possible that there are differences in testing preferences and reporting preferences in this region of the country,” Dr. Cooley noted. “Maybe people are more tuned in to the potential for outbreaks, but there are reasons why there could be differences in disease, like differences in infrastructure, climate, population density, and cooling tower density.” CDC data from 2015 indicate that most cases are not associated with a known exposure, and that the case fatality rate differs by exposure type: 12% for cases reporting health care exposure during the 10 days before symptom onset (25% for definite cases), 9% for cases reporting assisted or senior living exposure, 7% when no specific exposure is reported, and 4% for cases reporting travel exposure (MMWR. 2017;66[22]:584-9).
The U.S. case definition of Legionnaires’ disease consists of clinical or radiologic pneumonia plus confirmatory laboratory testing, either by urinary antigen test (UAT), lower respiratory culture, or appropriate serological testing. Polymerase chain reaction can be used as a presumptive test for a suspect case. “UAT is easy and it detects L. pneumophila serogroup 1 (Lp1), but it has some gaps,” Dr. Cooley said. “It isn’t completely sensitive for Lp1, and it doesn’t detect any other species or serogroups. That’s why we also recommend that a culture of respiratory secretions on selective media be performed at the same time. That being said, in the U.S., nearly all reported cases of Legionella are diagnosed by UAT only.”
A 2016 CDC MMWR and Vital Signs report found that almost all Legionella outbreaks could be prevented with effective water management, and the CDC has published a step-by-step guide to creating a water management program to reduce Legionella growth and spread in buildings. The 2017 MMWR Report found that definite health care–associated Legionnaires’ disease is deadly for one in four people who get it. The report also found that this issue is widespread; 76% of complete reporting jurisdictions reported at least one definite case of health care–associated Legionella disease in 2015. More recently, the Centers for Medicare & Medicaid Services issued a requirement to reduce risk in health care facility water systems to prevent cases and outbreaks. It applies to hospitals, skilled nursing facilities, and critical access hospitals.
Dr. Cooley reported having no financial disclosures.
SAN DIEGO –
“Improved testing and surveillance are needed to improve understanding of disease and outbreak burden,” Laura A. Cooley, MD, said at an annual scientific meeting on infectious diseases. “There is more to learn about environmental sources of Legionella for cases not associated with known outbreaks and about the distribution of Legionella in the environment.”
A Gram-negative bacillus, Legionella is an intracellular parasite of free-living protozoa primarily found in freshwater. “It can live and grow in biofilm, and there are more than 60 species of the bacterium,” she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Cases are higher in the warmer months, and the rates are highest among the elderly, men, and those of black race. Currently, L. pneumophila accounts for about 90% of cases in the United States. “Once it’s transmitted, it has to hit a susceptible population to cause disease, generally older individuals and people with underlying conditions,” Dr. Cooley said.
A separate analysis evaluated Legionella cases reported among U.S. residents between 2005 and 2009 (MMWR. 2011;60[32]:1083-6). It found that only 4% were associated with outbreaks, and 96% were sporadic. “That doesn’t mean that [the cases] weren’t associated with the same kind of source, they just weren’t identified as an outbreak,” Dr. Cooley said. “It shows that there is a lot to learn about transmission of Legionella.”
Data from the National Notifiable Diseases Surveillance System indicate that rates of Legionella continue to rise nationwide, especially in the Midwest and Northeast. “Why? It’s possible that there are differences in testing preferences and reporting preferences in this region of the country,” Dr. Cooley noted. “Maybe people are more tuned in to the potential for outbreaks, but there are reasons why there could be differences in disease, like differences in infrastructure, climate, population density, and cooling tower density.” CDC data from 2015 indicate that most cases are not associated with a known exposure, and that the case fatality rate differs by exposure type: 12% for cases reporting health care exposure during the 10 days before symptom onset (25% for definite cases), 9% for cases reporting assisted or senior living exposure, 7% when no specific exposure is reported, and 4% for cases reporting travel exposure (MMWR. 2017;66[22]:584-9).
The U.S. case definition of Legionnaires’ disease consists of clinical or radiologic pneumonia plus confirmatory laboratory testing, either by urinary antigen test (UAT), lower respiratory culture, or appropriate serological testing. Polymerase chain reaction can be used as a presumptive test for a suspect case. “UAT is easy and it detects L. pneumophila serogroup 1 (Lp1), but it has some gaps,” Dr. Cooley said. “It isn’t completely sensitive for Lp1, and it doesn’t detect any other species or serogroups. That’s why we also recommend that a culture of respiratory secretions on selective media be performed at the same time. That being said, in the U.S., nearly all reported cases of Legionella are diagnosed by UAT only.”
A 2016 CDC MMWR and Vital Signs report found that almost all Legionella outbreaks could be prevented with effective water management, and the CDC has published a step-by-step guide to creating a water management program to reduce Legionella growth and spread in buildings. The 2017 MMWR Report found that definite health care–associated Legionnaires’ disease is deadly for one in four people who get it. The report also found that this issue is widespread; 76% of complete reporting jurisdictions reported at least one definite case of health care–associated Legionella disease in 2015. More recently, the Centers for Medicare & Medicaid Services issued a requirement to reduce risk in health care facility water systems to prevent cases and outbreaks. It applies to hospitals, skilled nursing facilities, and critical access hospitals.
Dr. Cooley reported having no financial disclosures.
REPORTING FROM ID WEEK 2017
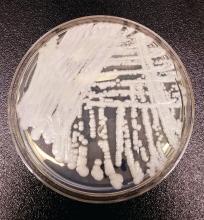
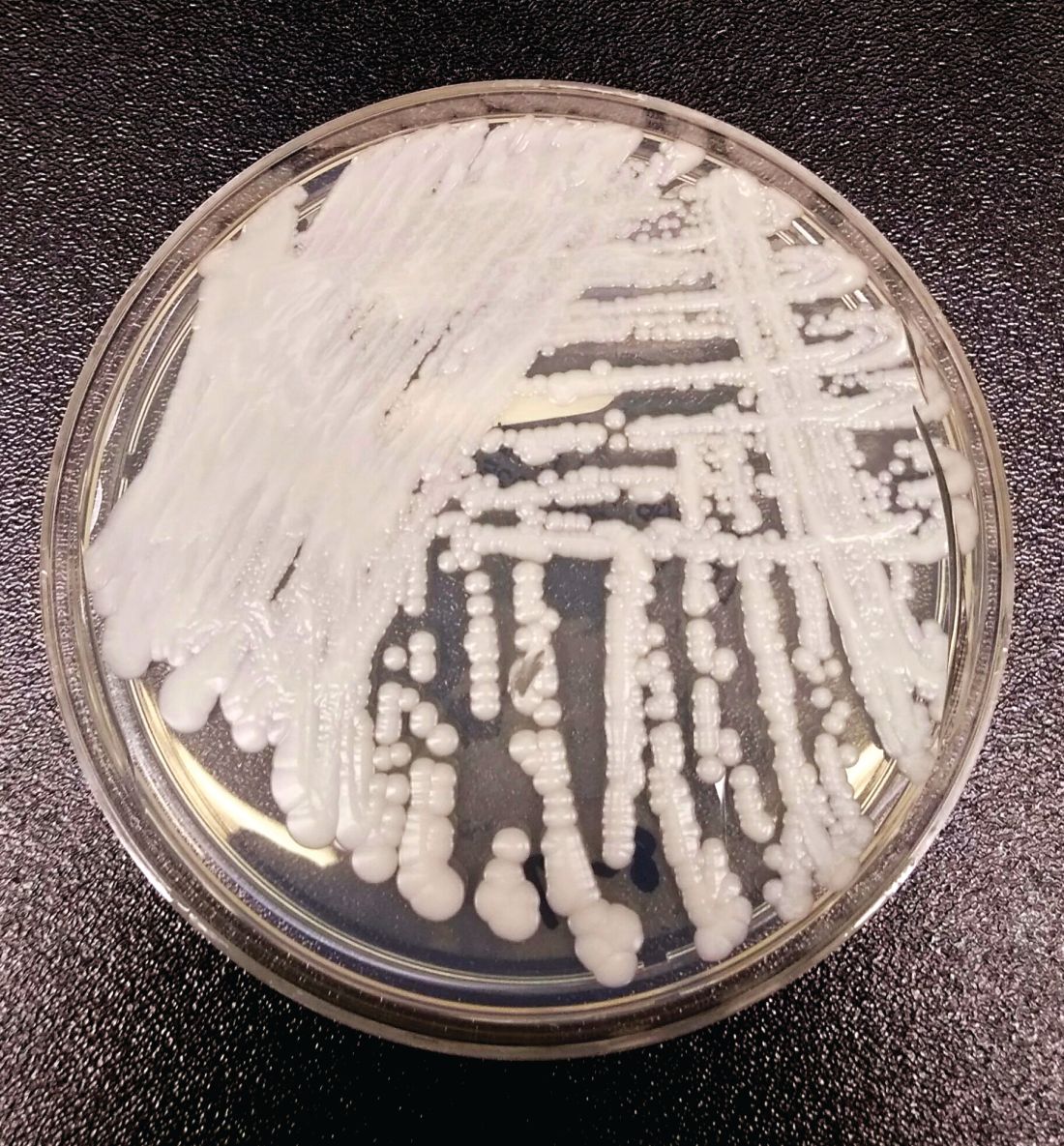
C. auris: ‘A yeast that acts like a bacteria’
SAN DIEGO – The rise of Candida auris as a superbug represents a paradigm shift, because, in the words of Dr. Tom M. Chiller, it’s a yeast that acts like a bacteria.
“Treatment resistance is now the norm,” Dr. Chiller, chief of the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta, said an annual scientific meeting on infectious diseases. “It thrives on skin, it contaminates patient rooms, and it spreads readily in health care settings.”
Since it was first described in Japan in 2009, C. auris has been identified in multiple countries in four continents, including the United States, prompting the CDC to issue a clinical alert to health care facilities in June of 2016. To date, more than 130 cases have been reported in 10 states, mostly in New York and New Jersey. At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, Dr. Chiller said that C. auris is a challenging superbug for four main reasons:
It’s not easily identified
Matrix assisted laser desorption ionization–time of flight (MALDI-TOF) or DNA sequencing are required to make the diagnosis. “It turns out that only about 25% of clinical labs have MALDI-TOF available, so we’re still lacking in our ability to identify it,” he said.
It’s easily transmitted
C. auris “is really happy in a hospital room,” Dr. Chiller said. “You can grow it from the floor, on the bottom of shoes, and on hand alcohol dispensers. It also likes the skin, and it also likes to grow in slightly higher temperatures. You find it readily in the axilla and groin. Those are the main locations we’re using for developing screening culture techniques.”
It’s difficult to treat
Treatment, if clinically indicated, includes an echinocandin such as micafungin, anidulafungin, and caspofungin at standard dosing. However, there have been cases of development of resistance to echinocandins while on therapy. “That bothers me,” Dr. Chiller said. “We don’t like to see that happen, and I am concerned. These bugs are really happy to be resistant, but based on the epidemiology, we remain convinced that it’s important to treat with an echinocandin.”
It can cause severe invasive disease and death
Global epidemiologic evaluation of the first 50 or so cases found that some patients were on antifungal treatment when C. auris was isolated. The mortality was greater than 60%, and there was a clustering in some hospitals. “Some hospitals reported that up to 40% of candidemia cases were from C. auris,” he said.
Among cases in the United States to date, the median age of affected patients is 70 years and patients’ 30-day mortality is about 30%. “They were quite ill, with multiple underlying conditions and indwelling devices,” Dr. Chiller said. They had “extensive health care exposure” with stays in acute care hospitals and nursing homes with ventilator units, and several recent cases with travel and health care exposures abroad, mainly to India, Pakistan, Venezuela, and South Africa.
Clinicians should report suspected cases to their local health department or to the CDC at [email protected].
“We also want them to implement and reinforce infection control measures,” Dr. Chiller advised. “Get the lab to review other potential Candida cases or Candida species you might have. Conduct contact tracing to identify other colonized patients, and consider point-prevalence surveys.”
He reported having no financial disclosures.
SAN DIEGO – The rise of Candida auris as a superbug represents a paradigm shift, because, in the words of Dr. Tom M. Chiller, it’s a yeast that acts like a bacteria.
“Treatment resistance is now the norm,” Dr. Chiller, chief of the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta, said an annual scientific meeting on infectious diseases. “It thrives on skin, it contaminates patient rooms, and it spreads readily in health care settings.”
Since it was first described in Japan in 2009, C. auris has been identified in multiple countries in four continents, including the United States, prompting the CDC to issue a clinical alert to health care facilities in June of 2016. To date, more than 130 cases have been reported in 10 states, mostly in New York and New Jersey. At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, Dr. Chiller said that C. auris is a challenging superbug for four main reasons:
It’s not easily identified
Matrix assisted laser desorption ionization–time of flight (MALDI-TOF) or DNA sequencing are required to make the diagnosis. “It turns out that only about 25% of clinical labs have MALDI-TOF available, so we’re still lacking in our ability to identify it,” he said.
It’s easily transmitted
C. auris “is really happy in a hospital room,” Dr. Chiller said. “You can grow it from the floor, on the bottom of shoes, and on hand alcohol dispensers. It also likes the skin, and it also likes to grow in slightly higher temperatures. You find it readily in the axilla and groin. Those are the main locations we’re using for developing screening culture techniques.”
It’s difficult to treat
Treatment, if clinically indicated, includes an echinocandin such as micafungin, anidulafungin, and caspofungin at standard dosing. However, there have been cases of development of resistance to echinocandins while on therapy. “That bothers me,” Dr. Chiller said. “We don’t like to see that happen, and I am concerned. These bugs are really happy to be resistant, but based on the epidemiology, we remain convinced that it’s important to treat with an echinocandin.”
It can cause severe invasive disease and death
Global epidemiologic evaluation of the first 50 or so cases found that some patients were on antifungal treatment when C. auris was isolated. The mortality was greater than 60%, and there was a clustering in some hospitals. “Some hospitals reported that up to 40% of candidemia cases were from C. auris,” he said.
Among cases in the United States to date, the median age of affected patients is 70 years and patients’ 30-day mortality is about 30%. “They were quite ill, with multiple underlying conditions and indwelling devices,” Dr. Chiller said. They had “extensive health care exposure” with stays in acute care hospitals and nursing homes with ventilator units, and several recent cases with travel and health care exposures abroad, mainly to India, Pakistan, Venezuela, and South Africa.
Clinicians should report suspected cases to their local health department or to the CDC at [email protected].
“We also want them to implement and reinforce infection control measures,” Dr. Chiller advised. “Get the lab to review other potential Candida cases or Candida species you might have. Conduct contact tracing to identify other colonized patients, and consider point-prevalence surveys.”
He reported having no financial disclosures.
SAN DIEGO – The rise of Candida auris as a superbug represents a paradigm shift, because, in the words of Dr. Tom M. Chiller, it’s a yeast that acts like a bacteria.
“Treatment resistance is now the norm,” Dr. Chiller, chief of the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta, said an annual scientific meeting on infectious diseases. “It thrives on skin, it contaminates patient rooms, and it spreads readily in health care settings.”
Since it was first described in Japan in 2009, C. auris has been identified in multiple countries in four continents, including the United States, prompting the CDC to issue a clinical alert to health care facilities in June of 2016. To date, more than 130 cases have been reported in 10 states, mostly in New York and New Jersey. At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, Dr. Chiller said that C. auris is a challenging superbug for four main reasons:
It’s not easily identified
Matrix assisted laser desorption ionization–time of flight (MALDI-TOF) or DNA sequencing are required to make the diagnosis. “It turns out that only about 25% of clinical labs have MALDI-TOF available, so we’re still lacking in our ability to identify it,” he said.
It’s easily transmitted
C. auris “is really happy in a hospital room,” Dr. Chiller said. “You can grow it from the floor, on the bottom of shoes, and on hand alcohol dispensers. It also likes the skin, and it also likes to grow in slightly higher temperatures. You find it readily in the axilla and groin. Those are the main locations we’re using for developing screening culture techniques.”
It’s difficult to treat
Treatment, if clinically indicated, includes an echinocandin such as micafungin, anidulafungin, and caspofungin at standard dosing. However, there have been cases of development of resistance to echinocandins while on therapy. “That bothers me,” Dr. Chiller said. “We don’t like to see that happen, and I am concerned. These bugs are really happy to be resistant, but based on the epidemiology, we remain convinced that it’s important to treat with an echinocandin.”
It can cause severe invasive disease and death
Global epidemiologic evaluation of the first 50 or so cases found that some patients were on antifungal treatment when C. auris was isolated. The mortality was greater than 60%, and there was a clustering in some hospitals. “Some hospitals reported that up to 40% of candidemia cases were from C. auris,” he said.
Among cases in the United States to date, the median age of affected patients is 70 years and patients’ 30-day mortality is about 30%. “They were quite ill, with multiple underlying conditions and indwelling devices,” Dr. Chiller said. They had “extensive health care exposure” with stays in acute care hospitals and nursing homes with ventilator units, and several recent cases with travel and health care exposures abroad, mainly to India, Pakistan, Venezuela, and South Africa.
Clinicians should report suspected cases to their local health department or to the CDC at [email protected].
“We also want them to implement and reinforce infection control measures,” Dr. Chiller advised. “Get the lab to review other potential Candida cases or Candida species you might have. Conduct contact tracing to identify other colonized patients, and consider point-prevalence surveys.”
He reported having no financial disclosures.
REPORTING FROM ID WEEK 2017
Delayed appropriate therapy affects outcomes in patients at risk for CRE infections
SAN DIEGO – Among patients with serious infections due to Enterobacteriaceae, delayed appropriate therapy has a stronger association with outcomes, relative to presence of carbapenem-resistant Enterobacteriaceae, according to an analysis of national hospital data.
“We need to reconsider how we approach patients with serious Gram-negative infections,” lead study author Thomas Lodise, PharmD, PhD, said at an annual scientific meeting on infectious diseases. “We kind of take this wait-and-see approach in infectious diseases; we wait a couple of days, then we get aggressive. You would never do this in oncology. I don’t know how many more studies we need to show that early therapy matters. We talk about antibiotic stewardship. One of the fundamental pillars of stewardship is getting it right the first time, and we fail to do this in the majority of patients with serious Gram-negative infections.”

At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, he noted that delayed appropriate therapy is associated with increased rates of clinical failure and mortality, longer lengths of stay, longer durations of antibiotic treatment, and greater in-hospital costs. “Similarly, patients with infections caused by carbapenem-resistant Enterobacteriaceae (CRE) have poorer outcomes, such as increased risk of mortality or of being discharged to a long-term care facility, compared with patients with infections caused by carbapenem-susceptible Enterobacteriaceae isolates,” said Dr. Lodise of the Albany (N.Y.) College of Pharmacy and Health Sciences. “Although CRE and delayed appropriate therapy have both been associated with worse outcomes, the impact of each of these factors on clinical and economic outcomes is not well understood.”
In an effort to assess the independent and combined impact of CRE and delayed appropriate therapy on clinical and economic outcomes among hospitalized U.S. patients with serious infections due to Enterobacteriaceae, Dr. Lodise and his associates drew from the Premier Hospital Database, which includes information for about 500 acute-care hospitals in the United States, including the 150 hospitals that provided admission records and microbiological data assessed in the current analysis.
The researchers evaluated adults hospitalized between July 2011 and September 2014. The index date was defined as the earliest culture positive for at least one Gram-negative bacteria of interest, and patients were stratified based on whether the pathogen was CRE or non-CRE. Appropriate therapy was defined as receipt of an antibiotic regimen with microbiological activity against all pathogens identified within the index culture on the index date or within the subsequent 2-day period. All subsequent receipt of such therapy was defined as delayed appropriate therapy.
In all, 50,069 patients with a mean age of 66 years were included in the study. Of these, 514 (1%) harbored infections caused by CRE, and 49,555 (99%) had infections caused by a pathogen other than CRE. Multivariate adjusted analysis revealed significant differences between the CRE group and the non-CRE group in duration of antibiotic therapy (a mean of 8.5 days vs. 7.5 days, respectively); length of stay (a mean of 8.4 days vs. 7.6 days), and in-hospital cost (a mean of $19,816 vs. a mean of $15,165; P less than .01 for all associations). In addition, CRE patients were less likely to be discharged home (odds ratio [OR], .3) and more likely to die in the hospital or be discharged to hospice (OR, 2.2).
When outcomes of patients infections due to Enterobacteriaceae species were stratified by timing of appropriate therapy (timely vs. delayed) and CRE status (CRE vs. non-CRE), without exception the burden of serious infections was least among patients with infections due to non-CRE who received timely appropriate therapy, and greatest among patients with infections due to CRE in whom appropriate therapy was delayed. A gradient effect was observed across strata, and weighted towards timing of receipt of initial therapy. For example, the mean LOS post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of 5 days) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of 8.8 days). Similarly, mean in-hospital costs post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of $9,875) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of $25,506).
“This study demonstrates the importance of early identification of patients at risk for delayed appropriate therapy, through the use of clinical criteria for risk stratification or rapid diagnostic tools,” Dr. Lodise concluded. “The findings also highlight the need to shift current treatment practices away from antibiotic escalation strategies that contribute to delayed appropriate therapy and toward early aggressive, appropriate therapy in patients at risk for CRE infection.”
Allergan funded the study. Dr. Lodise disclosed that he has received consulting fees or honoraria from Allergan. He has also been a consultant for Merck, Achaogen, Zavante, and The Medicines Company.
SAN DIEGO – Among patients with serious infections due to Enterobacteriaceae, delayed appropriate therapy has a stronger association with outcomes, relative to presence of carbapenem-resistant Enterobacteriaceae, according to an analysis of national hospital data.
“We need to reconsider how we approach patients with serious Gram-negative infections,” lead study author Thomas Lodise, PharmD, PhD, said at an annual scientific meeting on infectious diseases. “We kind of take this wait-and-see approach in infectious diseases; we wait a couple of days, then we get aggressive. You would never do this in oncology. I don’t know how many more studies we need to show that early therapy matters. We talk about antibiotic stewardship. One of the fundamental pillars of stewardship is getting it right the first time, and we fail to do this in the majority of patients with serious Gram-negative infections.”

At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, he noted that delayed appropriate therapy is associated with increased rates of clinical failure and mortality, longer lengths of stay, longer durations of antibiotic treatment, and greater in-hospital costs. “Similarly, patients with infections caused by carbapenem-resistant Enterobacteriaceae (CRE) have poorer outcomes, such as increased risk of mortality or of being discharged to a long-term care facility, compared with patients with infections caused by carbapenem-susceptible Enterobacteriaceae isolates,” said Dr. Lodise of the Albany (N.Y.) College of Pharmacy and Health Sciences. “Although CRE and delayed appropriate therapy have both been associated with worse outcomes, the impact of each of these factors on clinical and economic outcomes is not well understood.”
In an effort to assess the independent and combined impact of CRE and delayed appropriate therapy on clinical and economic outcomes among hospitalized U.S. patients with serious infections due to Enterobacteriaceae, Dr. Lodise and his associates drew from the Premier Hospital Database, which includes information for about 500 acute-care hospitals in the United States, including the 150 hospitals that provided admission records and microbiological data assessed in the current analysis.
The researchers evaluated adults hospitalized between July 2011 and September 2014. The index date was defined as the earliest culture positive for at least one Gram-negative bacteria of interest, and patients were stratified based on whether the pathogen was CRE or non-CRE. Appropriate therapy was defined as receipt of an antibiotic regimen with microbiological activity against all pathogens identified within the index culture on the index date or within the subsequent 2-day period. All subsequent receipt of such therapy was defined as delayed appropriate therapy.
In all, 50,069 patients with a mean age of 66 years were included in the study. Of these, 514 (1%) harbored infections caused by CRE, and 49,555 (99%) had infections caused by a pathogen other than CRE. Multivariate adjusted analysis revealed significant differences between the CRE group and the non-CRE group in duration of antibiotic therapy (a mean of 8.5 days vs. 7.5 days, respectively); length of stay (a mean of 8.4 days vs. 7.6 days), and in-hospital cost (a mean of $19,816 vs. a mean of $15,165; P less than .01 for all associations). In addition, CRE patients were less likely to be discharged home (odds ratio [OR], .3) and more likely to die in the hospital or be discharged to hospice (OR, 2.2).
When outcomes of patients infections due to Enterobacteriaceae species were stratified by timing of appropriate therapy (timely vs. delayed) and CRE status (CRE vs. non-CRE), without exception the burden of serious infections was least among patients with infections due to non-CRE who received timely appropriate therapy, and greatest among patients with infections due to CRE in whom appropriate therapy was delayed. A gradient effect was observed across strata, and weighted towards timing of receipt of initial therapy. For example, the mean LOS post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of 5 days) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of 8.8 days). Similarly, mean in-hospital costs post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of $9,875) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of $25,506).
“This study demonstrates the importance of early identification of patients at risk for delayed appropriate therapy, through the use of clinical criteria for risk stratification or rapid diagnostic tools,” Dr. Lodise concluded. “The findings also highlight the need to shift current treatment practices away from antibiotic escalation strategies that contribute to delayed appropriate therapy and toward early aggressive, appropriate therapy in patients at risk for CRE infection.”
Allergan funded the study. Dr. Lodise disclosed that he has received consulting fees or honoraria from Allergan. He has also been a consultant for Merck, Achaogen, Zavante, and The Medicines Company.
SAN DIEGO – Among patients with serious infections due to Enterobacteriaceae, delayed appropriate therapy has a stronger association with outcomes, relative to presence of carbapenem-resistant Enterobacteriaceae, according to an analysis of national hospital data.
“We need to reconsider how we approach patients with serious Gram-negative infections,” lead study author Thomas Lodise, PharmD, PhD, said at an annual scientific meeting on infectious diseases. “We kind of take this wait-and-see approach in infectious diseases; we wait a couple of days, then we get aggressive. You would never do this in oncology. I don’t know how many more studies we need to show that early therapy matters. We talk about antibiotic stewardship. One of the fundamental pillars of stewardship is getting it right the first time, and we fail to do this in the majority of patients with serious Gram-negative infections.”

At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, he noted that delayed appropriate therapy is associated with increased rates of clinical failure and mortality, longer lengths of stay, longer durations of antibiotic treatment, and greater in-hospital costs. “Similarly, patients with infections caused by carbapenem-resistant Enterobacteriaceae (CRE) have poorer outcomes, such as increased risk of mortality or of being discharged to a long-term care facility, compared with patients with infections caused by carbapenem-susceptible Enterobacteriaceae isolates,” said Dr. Lodise of the Albany (N.Y.) College of Pharmacy and Health Sciences. “Although CRE and delayed appropriate therapy have both been associated with worse outcomes, the impact of each of these factors on clinical and economic outcomes is not well understood.”
In an effort to assess the independent and combined impact of CRE and delayed appropriate therapy on clinical and economic outcomes among hospitalized U.S. patients with serious infections due to Enterobacteriaceae, Dr. Lodise and his associates drew from the Premier Hospital Database, which includes information for about 500 acute-care hospitals in the United States, including the 150 hospitals that provided admission records and microbiological data assessed in the current analysis.
The researchers evaluated adults hospitalized between July 2011 and September 2014. The index date was defined as the earliest culture positive for at least one Gram-negative bacteria of interest, and patients were stratified based on whether the pathogen was CRE or non-CRE. Appropriate therapy was defined as receipt of an antibiotic regimen with microbiological activity against all pathogens identified within the index culture on the index date or within the subsequent 2-day period. All subsequent receipt of such therapy was defined as delayed appropriate therapy.
In all, 50,069 patients with a mean age of 66 years were included in the study. Of these, 514 (1%) harbored infections caused by CRE, and 49,555 (99%) had infections caused by a pathogen other than CRE. Multivariate adjusted analysis revealed significant differences between the CRE group and the non-CRE group in duration of antibiotic therapy (a mean of 8.5 days vs. 7.5 days, respectively); length of stay (a mean of 8.4 days vs. 7.6 days), and in-hospital cost (a mean of $19,816 vs. a mean of $15,165; P less than .01 for all associations). In addition, CRE patients were less likely to be discharged home (odds ratio [OR], .3) and more likely to die in the hospital or be discharged to hospice (OR, 2.2).
When outcomes of patients infections due to Enterobacteriaceae species were stratified by timing of appropriate therapy (timely vs. delayed) and CRE status (CRE vs. non-CRE), without exception the burden of serious infections was least among patients with infections due to non-CRE who received timely appropriate therapy, and greatest among patients with infections due to CRE in whom appropriate therapy was delayed. A gradient effect was observed across strata, and weighted towards timing of receipt of initial therapy. For example, the mean LOS post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of 5 days) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of 8.8 days). Similarly, mean in-hospital costs post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of $9,875) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of $25,506).
“This study demonstrates the importance of early identification of patients at risk for delayed appropriate therapy, through the use of clinical criteria for risk stratification or rapid diagnostic tools,” Dr. Lodise concluded. “The findings also highlight the need to shift current treatment practices away from antibiotic escalation strategies that contribute to delayed appropriate therapy and toward early aggressive, appropriate therapy in patients at risk for CRE infection.”
Allergan funded the study. Dr. Lodise disclosed that he has received consulting fees or honoraria from Allergan. He has also been a consultant for Merck, Achaogen, Zavante, and The Medicines Company.
REPORTING FROM ID WEEK 2017
Key clinical point:
Major finding: The mean LOS post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of 5 days) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of 8.8 days).
Study details: An analysis of 50,069 adults hospitalized with serious infections due to Enterobacteriaceae between July 2011 and September 2014.
Disclosures: Allergan funded the study. Dr. Lodise disclosed that he has received consulting fees or honoraria from Allergan. He also has been a consultant for Merck, Achaogen, Zavante, and The Medicines Company.
VA study finds high MRSA infection risk among those colonized with the bacterium
SAN DIEGO – Patients colonized with MRSA are at high risk of MRSA infection both in the predischarge and postdischarge time periods, results from an 8-year Veterans Affairs study showed.
“MRSA colonization is recognized as being a strong predictor of subsequent infection,” Richard E. Nelson, PhD, said at an annual scientific meeting on infectious diseases. “What’s less understood is, are there differences in infection rates among patients who are colonized at different times? And, is there a difference between patients who import colonization with them to a hospital versus those who acquire it during a hospital stay? In addition, infection control efforts mainly focus on the predischarge time period. What about infections that develop post discharge?”
In an effort to investigate these questions, Dr. Nelson of the VA Salt Lake City Healthcare System, and his associates, evaluated more than 1.3 million acute care inpatient admissions to 125 VA hospitals nationwide from January 2008 through December 2015 who had surveillance tests performed for MRSA carriage.
The researchers restricted admissions to individuals with at least 365 days of VA activity prior to admission and categorized them into three groups: no colonization (defined as those who had no positive surveillance tests (n = 1,196,928); importation (defined as those who tested positive for MRSA colonization on admission (n = 95,833); and acquisition (defined as those who did not test positive for MRSA on admission but tested positive on a subsequent surveillance test during their admission (n = 15,146). Next, they captured MRSA infections in these individuals prior to discharge and at 30 and 90 days post discharge. Infections were defined as positive MRSA cultures taken from sterile sites, including blood, catheter site, or bone.
Overall, patients were in their mid-60s, and those who imported MRSA and those who acquired it were more likely to be male, less likely to be married, and more likely to not have health insurance. , which peaked in 2010 and declined through 2015,” said Dr. Nelson, who also holds a faculty position in University of Utah’s department of internal medicine, in the division of epidemiology. Specifically, the proportion of predischarge MRSA infections, compared with 30 days post discharge, were 40.4% vs. 59.6%, respectively, in the no colonization group; 63% vs. 37% in the importation group, and 80.8% vs. 19.2% in the acquisition group.
He also reported that the proportion of predischarge MRSA infections, compared with 90 days post discharge, were 20.5% vs. 79.5%, respectively, in the no colonization group; 47.3% vs. 52.7% in the importation group, and 70.5% vs. 29.5% in the acquisition group. The time from acquisition to infection was a mean of 8.7 days in the 30-day analysis and a mean of 22.4 days in the 90-day analysis.
Multivariate logistic regression revealed that the impact of colonization status on infection was highest in the acquisition group, compared with the importation group. Specifically, the odds ratio of developing a MRSA infection among the importation group was 29.22 in the predischarge period, OR 10.87 at post discharge 30 days, and OR 7.64 at post discharge 90 days (P less than .001 for all). Meanwhile, the OR among the acquisition group was 85.19 in the predischarge period, OR 13.01 at post discharge 30 days, and OR 8.26 at post discharge 90 days (P less than .001 for all).
Dr. Nelson acknowledged certain limitations of the study, including the fact that it only identified postdischarge infections that were detected in a VA facility. “This is likely an underestimate of postdischarge infections, because we’re missing the infection that occur in non-VA facilities,” he said at the event, which marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “Also, patients can be colonized in many different body locations, but the VA protocol is that the surveillance test be done in the nostrils. So we may have misclassified patients who were colonized in a different body location as being uncolonized, when in fact they were colonized.”
The study was funded by a grant from the VA. Dr. Nelson reported having no financial disclosures.
SAN DIEGO – Patients colonized with MRSA are at high risk of MRSA infection both in the predischarge and postdischarge time periods, results from an 8-year Veterans Affairs study showed.
“MRSA colonization is recognized as being a strong predictor of subsequent infection,” Richard E. Nelson, PhD, said at an annual scientific meeting on infectious diseases. “What’s less understood is, are there differences in infection rates among patients who are colonized at different times? And, is there a difference between patients who import colonization with them to a hospital versus those who acquire it during a hospital stay? In addition, infection control efforts mainly focus on the predischarge time period. What about infections that develop post discharge?”
In an effort to investigate these questions, Dr. Nelson of the VA Salt Lake City Healthcare System, and his associates, evaluated more than 1.3 million acute care inpatient admissions to 125 VA hospitals nationwide from January 2008 through December 2015 who had surveillance tests performed for MRSA carriage.
The researchers restricted admissions to individuals with at least 365 days of VA activity prior to admission and categorized them into three groups: no colonization (defined as those who had no positive surveillance tests (n = 1,196,928); importation (defined as those who tested positive for MRSA colonization on admission (n = 95,833); and acquisition (defined as those who did not test positive for MRSA on admission but tested positive on a subsequent surveillance test during their admission (n = 15,146). Next, they captured MRSA infections in these individuals prior to discharge and at 30 and 90 days post discharge. Infections were defined as positive MRSA cultures taken from sterile sites, including blood, catheter site, or bone.
Overall, patients were in their mid-60s, and those who imported MRSA and those who acquired it were more likely to be male, less likely to be married, and more likely to not have health insurance. , which peaked in 2010 and declined through 2015,” said Dr. Nelson, who also holds a faculty position in University of Utah’s department of internal medicine, in the division of epidemiology. Specifically, the proportion of predischarge MRSA infections, compared with 30 days post discharge, were 40.4% vs. 59.6%, respectively, in the no colonization group; 63% vs. 37% in the importation group, and 80.8% vs. 19.2% in the acquisition group.
He also reported that the proportion of predischarge MRSA infections, compared with 90 days post discharge, were 20.5% vs. 79.5%, respectively, in the no colonization group; 47.3% vs. 52.7% in the importation group, and 70.5% vs. 29.5% in the acquisition group. The time from acquisition to infection was a mean of 8.7 days in the 30-day analysis and a mean of 22.4 days in the 90-day analysis.
Multivariate logistic regression revealed that the impact of colonization status on infection was highest in the acquisition group, compared with the importation group. Specifically, the odds ratio of developing a MRSA infection among the importation group was 29.22 in the predischarge period, OR 10.87 at post discharge 30 days, and OR 7.64 at post discharge 90 days (P less than .001 for all). Meanwhile, the OR among the acquisition group was 85.19 in the predischarge period, OR 13.01 at post discharge 30 days, and OR 8.26 at post discharge 90 days (P less than .001 for all).
Dr. Nelson acknowledged certain limitations of the study, including the fact that it only identified postdischarge infections that were detected in a VA facility. “This is likely an underestimate of postdischarge infections, because we’re missing the infection that occur in non-VA facilities,” he said at the event, which marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “Also, patients can be colonized in many different body locations, but the VA protocol is that the surveillance test be done in the nostrils. So we may have misclassified patients who were colonized in a different body location as being uncolonized, when in fact they were colonized.”
The study was funded by a grant from the VA. Dr. Nelson reported having no financial disclosures.
SAN DIEGO – Patients colonized with MRSA are at high risk of MRSA infection both in the predischarge and postdischarge time periods, results from an 8-year Veterans Affairs study showed.
“MRSA colonization is recognized as being a strong predictor of subsequent infection,” Richard E. Nelson, PhD, said at an annual scientific meeting on infectious diseases. “What’s less understood is, are there differences in infection rates among patients who are colonized at different times? And, is there a difference between patients who import colonization with them to a hospital versus those who acquire it during a hospital stay? In addition, infection control efforts mainly focus on the predischarge time period. What about infections that develop post discharge?”
In an effort to investigate these questions, Dr. Nelson of the VA Salt Lake City Healthcare System, and his associates, evaluated more than 1.3 million acute care inpatient admissions to 125 VA hospitals nationwide from January 2008 through December 2015 who had surveillance tests performed for MRSA carriage.
The researchers restricted admissions to individuals with at least 365 days of VA activity prior to admission and categorized them into three groups: no colonization (defined as those who had no positive surveillance tests (n = 1,196,928); importation (defined as those who tested positive for MRSA colonization on admission (n = 95,833); and acquisition (defined as those who did not test positive for MRSA on admission but tested positive on a subsequent surveillance test during their admission (n = 15,146). Next, they captured MRSA infections in these individuals prior to discharge and at 30 and 90 days post discharge. Infections were defined as positive MRSA cultures taken from sterile sites, including blood, catheter site, or bone.
Overall, patients were in their mid-60s, and those who imported MRSA and those who acquired it were more likely to be male, less likely to be married, and more likely to not have health insurance. , which peaked in 2010 and declined through 2015,” said Dr. Nelson, who also holds a faculty position in University of Utah’s department of internal medicine, in the division of epidemiology. Specifically, the proportion of predischarge MRSA infections, compared with 30 days post discharge, were 40.4% vs. 59.6%, respectively, in the no colonization group; 63% vs. 37% in the importation group, and 80.8% vs. 19.2% in the acquisition group.
He also reported that the proportion of predischarge MRSA infections, compared with 90 days post discharge, were 20.5% vs. 79.5%, respectively, in the no colonization group; 47.3% vs. 52.7% in the importation group, and 70.5% vs. 29.5% in the acquisition group. The time from acquisition to infection was a mean of 8.7 days in the 30-day analysis and a mean of 22.4 days in the 90-day analysis.
Multivariate logistic regression revealed that the impact of colonization status on infection was highest in the acquisition group, compared with the importation group. Specifically, the odds ratio of developing a MRSA infection among the importation group was 29.22 in the predischarge period, OR 10.87 at post discharge 30 days, and OR 7.64 at post discharge 90 days (P less than .001 for all). Meanwhile, the OR among the acquisition group was 85.19 in the predischarge period, OR 13.01 at post discharge 30 days, and OR 8.26 at post discharge 90 days (P less than .001 for all).
Dr. Nelson acknowledged certain limitations of the study, including the fact that it only identified postdischarge infections that were detected in a VA facility. “This is likely an underestimate of postdischarge infections, because we’re missing the infection that occur in non-VA facilities,” he said at the event, which marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “Also, patients can be colonized in many different body locations, but the VA protocol is that the surveillance test be done in the nostrils. So we may have misclassified patients who were colonized in a different body location as being uncolonized, when in fact they were colonized.”
The study was funded by a grant from the VA. Dr. Nelson reported having no financial disclosures.
REPORTING FROM ID WEEK 2017
Key clinical point: About half of postdischarge MRSA infections were in patients who acquired the organism before discharge.
Major finding: The proportion of predischarge MRSA infections, compared with 30 days post discharge, were 40.4% vs. 59.6%, respectively, in the no colonization group; 63% vs. 37% in the importation group, and 80.8% vs. 19.2% in the acquisition group.
Study details: An analysis of more than 1.3 million acute care inpatient admissions to 125 VA hospitals nationwide from January 2008 through December 2015.
Disclosures: The study was funded by a grant from the VA. Dr. Nelson reported having no financial disclosures.
Ribaxamase reduced new CDI infection by 71%
SAN DIEGO – , results from a phase 2b study showed.
At an annual scientific meeting on infectious diseases, lead investigator John F. Kokai-Kun, PhD, said that the finding represents a paradigm shift in the use of intravenous beta-lactam antibiotics to prevent opportunistic infections. “We currently treat Clostridium difficile infection (CDI) with antibiotics, which attack the vegetative cells,” said Dr. Kokai-Kun, vice president of nonclinical affairs for Rockville, Md.–based Synthetic Biologics, which is developing ribaxamase. “Since C. diff. is primarily a toxin-mediated disease, certain products seem to neutralize the toxin. There’s also been work with probiotics and prebiotics to try to strengthen and repair the dysbiotic colon. Fecal replacement therapy has been shown to be fairly effective for treatment of recurrent C. diff. infection. What if we could simply block the initial insult that leads to this cascade? That’s the damage caused to the gut microbiome by the antibiotic that’s excreted to the intestine.”
That’s where ribaxamase comes in, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Ribaxamase is an orally administered beta-lactamase designed to degrade penicillin and cephalosporins in the intestinal lumen. It’s formulated for release in the proximal small intestine and is expected to be given during or a short time after administration of IV beta-lactam antibiotics such as ceftriaxone. “This is expected to degrade the excess antibiotics that are excreted into the small intestine via the bile,” Dr. Kokai-Kun explained. “It’s designed to prevent disruption of the gut microbiome and thus protect from opportunistic GI infections like CDI.” Early-stage clinical studies demonstrated that ribaxamase was well tolerated and that it is not systemically absorbed, while phase 2 studies showed that ribaxamase degrades ceftriaxone in the intestine to below the level of detection while not affecting the pharmacokinetics of ceftriaxone in the plasma.
For the current study, 412 patients were enrolled at 84 multinational clinical sites. These patients were admitted to the hospital for treatment of a lower respiratory tract infection and were randomized 1:1 to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo. Patients in both groups could also receive an oral macrolide at the discretion of the clinical investigator. The researchers also obtained fecal samples at screening, 72 hours post antibiotic treatment, and at the end of a 4-week follow-up visit, to determine colonization by opportunistic pathogens and to examine changes in the gut microbiome. Patients were monitored for 6 weeks for diarrhea and CDI. Diarrhea was defined as three or more loose or watery stools in a 24-hour period. “If that occurred, then we collected a sample, which was sent to the local lab to determine the presence of C. difficile toxins,” Dr. Kokai-Kun said.
The average age of study participants was 70 years, and about one-third in each arm received oral macrolides. The number of adverse events and serious adverse events were similar between active and placebo arms, and there was no trend associated with ribaxamase use. The lower respiratory tract infection cure rate to the ceftriaxone treatment was about 99% in both arms at 72 hours post treatment and at 2 weeks post treatment.
To analyze changes in the gut microbiome, the researchers conducted 16S rRNA sequencing of DNA extracted from fecal samples. In all, 652 samples were sequenced from 229 patients. Results from that analysis suggests that ribaxamase “appears to protect the gut microbiome from the onslaught of the ceftriaxone,” he said.
Ribaxamase reduced the incidence of new-onset CDI by 71%, compared with placebo (P = .045). “It apparently did this by protecting the integrity of the gut microbiome,” Dr. Kokai-Kun said. “There was also a significant reduction of new colonization by vancomycin-resistant enterococci at 72 hours and 4 weeks (P = .0001 and P = .0002, respectively) which is an opportunistic pathogen that is known to be able to inhabit gut microbiome when there is dysbiosis.”
The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
SAN DIEGO – , results from a phase 2b study showed.
At an annual scientific meeting on infectious diseases, lead investigator John F. Kokai-Kun, PhD, said that the finding represents a paradigm shift in the use of intravenous beta-lactam antibiotics to prevent opportunistic infections. “We currently treat Clostridium difficile infection (CDI) with antibiotics, which attack the vegetative cells,” said Dr. Kokai-Kun, vice president of nonclinical affairs for Rockville, Md.–based Synthetic Biologics, which is developing ribaxamase. “Since C. diff. is primarily a toxin-mediated disease, certain products seem to neutralize the toxin. There’s also been work with probiotics and prebiotics to try to strengthen and repair the dysbiotic colon. Fecal replacement therapy has been shown to be fairly effective for treatment of recurrent C. diff. infection. What if we could simply block the initial insult that leads to this cascade? That’s the damage caused to the gut microbiome by the antibiotic that’s excreted to the intestine.”
That’s where ribaxamase comes in, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Ribaxamase is an orally administered beta-lactamase designed to degrade penicillin and cephalosporins in the intestinal lumen. It’s formulated for release in the proximal small intestine and is expected to be given during or a short time after administration of IV beta-lactam antibiotics such as ceftriaxone. “This is expected to degrade the excess antibiotics that are excreted into the small intestine via the bile,” Dr. Kokai-Kun explained. “It’s designed to prevent disruption of the gut microbiome and thus protect from opportunistic GI infections like CDI.” Early-stage clinical studies demonstrated that ribaxamase was well tolerated and that it is not systemically absorbed, while phase 2 studies showed that ribaxamase degrades ceftriaxone in the intestine to below the level of detection while not affecting the pharmacokinetics of ceftriaxone in the plasma.
For the current study, 412 patients were enrolled at 84 multinational clinical sites. These patients were admitted to the hospital for treatment of a lower respiratory tract infection and were randomized 1:1 to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo. Patients in both groups could also receive an oral macrolide at the discretion of the clinical investigator. The researchers also obtained fecal samples at screening, 72 hours post antibiotic treatment, and at the end of a 4-week follow-up visit, to determine colonization by opportunistic pathogens and to examine changes in the gut microbiome. Patients were monitored for 6 weeks for diarrhea and CDI. Diarrhea was defined as three or more loose or watery stools in a 24-hour period. “If that occurred, then we collected a sample, which was sent to the local lab to determine the presence of C. difficile toxins,” Dr. Kokai-Kun said.
The average age of study participants was 70 years, and about one-third in each arm received oral macrolides. The number of adverse events and serious adverse events were similar between active and placebo arms, and there was no trend associated with ribaxamase use. The lower respiratory tract infection cure rate to the ceftriaxone treatment was about 99% in both arms at 72 hours post treatment and at 2 weeks post treatment.
To analyze changes in the gut microbiome, the researchers conducted 16S rRNA sequencing of DNA extracted from fecal samples. In all, 652 samples were sequenced from 229 patients. Results from that analysis suggests that ribaxamase “appears to protect the gut microbiome from the onslaught of the ceftriaxone,” he said.
Ribaxamase reduced the incidence of new-onset CDI by 71%, compared with placebo (P = .045). “It apparently did this by protecting the integrity of the gut microbiome,” Dr. Kokai-Kun said. “There was also a significant reduction of new colonization by vancomycin-resistant enterococci at 72 hours and 4 weeks (P = .0001 and P = .0002, respectively) which is an opportunistic pathogen that is known to be able to inhabit gut microbiome when there is dysbiosis.”
The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
SAN DIEGO – , results from a phase 2b study showed.
At an annual scientific meeting on infectious diseases, lead investigator John F. Kokai-Kun, PhD, said that the finding represents a paradigm shift in the use of intravenous beta-lactam antibiotics to prevent opportunistic infections. “We currently treat Clostridium difficile infection (CDI) with antibiotics, which attack the vegetative cells,” said Dr. Kokai-Kun, vice president of nonclinical affairs for Rockville, Md.–based Synthetic Biologics, which is developing ribaxamase. “Since C. diff. is primarily a toxin-mediated disease, certain products seem to neutralize the toxin. There’s also been work with probiotics and prebiotics to try to strengthen and repair the dysbiotic colon. Fecal replacement therapy has been shown to be fairly effective for treatment of recurrent C. diff. infection. What if we could simply block the initial insult that leads to this cascade? That’s the damage caused to the gut microbiome by the antibiotic that’s excreted to the intestine.”
That’s where ribaxamase comes in, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Ribaxamase is an orally administered beta-lactamase designed to degrade penicillin and cephalosporins in the intestinal lumen. It’s formulated for release in the proximal small intestine and is expected to be given during or a short time after administration of IV beta-lactam antibiotics such as ceftriaxone. “This is expected to degrade the excess antibiotics that are excreted into the small intestine via the bile,” Dr. Kokai-Kun explained. “It’s designed to prevent disruption of the gut microbiome and thus protect from opportunistic GI infections like CDI.” Early-stage clinical studies demonstrated that ribaxamase was well tolerated and that it is not systemically absorbed, while phase 2 studies showed that ribaxamase degrades ceftriaxone in the intestine to below the level of detection while not affecting the pharmacokinetics of ceftriaxone in the plasma.
For the current study, 412 patients were enrolled at 84 multinational clinical sites. These patients were admitted to the hospital for treatment of a lower respiratory tract infection and were randomized 1:1 to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo. Patients in both groups could also receive an oral macrolide at the discretion of the clinical investigator. The researchers also obtained fecal samples at screening, 72 hours post antibiotic treatment, and at the end of a 4-week follow-up visit, to determine colonization by opportunistic pathogens and to examine changes in the gut microbiome. Patients were monitored for 6 weeks for diarrhea and CDI. Diarrhea was defined as three or more loose or watery stools in a 24-hour period. “If that occurred, then we collected a sample, which was sent to the local lab to determine the presence of C. difficile toxins,” Dr. Kokai-Kun said.
The average age of study participants was 70 years, and about one-third in each arm received oral macrolides. The number of adverse events and serious adverse events were similar between active and placebo arms, and there was no trend associated with ribaxamase use. The lower respiratory tract infection cure rate to the ceftriaxone treatment was about 99% in both arms at 72 hours post treatment and at 2 weeks post treatment.
To analyze changes in the gut microbiome, the researchers conducted 16S rRNA sequencing of DNA extracted from fecal samples. In all, 652 samples were sequenced from 229 patients. Results from that analysis suggests that ribaxamase “appears to protect the gut microbiome from the onslaught of the ceftriaxone,” he said.
Ribaxamase reduced the incidence of new-onset CDI by 71%, compared with placebo (P = .045). “It apparently did this by protecting the integrity of the gut microbiome,” Dr. Kokai-Kun said. “There was also a significant reduction of new colonization by vancomycin-resistant enterococci at 72 hours and 4 weeks (P = .0001 and P = .0002, respectively) which is an opportunistic pathogen that is known to be able to inhabit gut microbiome when there is dysbiosis.”
The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
REPORTING FROM ID WEEK 2017
Key clinical point: Ribaxamase reduced new colonization with C. diff. and vancomycin-resistant enterococci.
Major finding: Ribaxamase reduced the incidence of new onset CDI by 71%, compared with placebo (P = 0.045).
Study details: A trial of 412 patients admitted to the hospital for treatment of a lower respiratory tract infection who were randomized to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo.
Disclosures: The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
Study IDs risk predictors of PrEP use in MSM
SAN DIEGO – Recent sexual risk behavior and partnership type may be important predictors of pre-exposure prophylaxis in men who have sex with men, results from a 48-week study suggest.
“We know from other studies including iPrEX, the Partners PrEP, and the Demo project that individuals who report higher risk behaviors are more likely to be adherent to pre-exposure prophylaxis (PrEP),” lead study author Jill Blumenthal, MD, said in an interview in advance of an annual scientific meeting on infectious diseases.
As part of a PrEP California Collaborative Treatment Group demonstration study of 398 HIV-negative at-risk men who have sex with men and transgender women, Dr. Blumenthal, of the Antiviral Research Center at the University of California, San Diego, and her associates estimated their HIV risk score at baseline and week 48. Their score was estimated as the probability of seroconversion over the next year based on number of condomless anal sex acts with HIV+/unknown partners in the last month and any sexually transmitted infection diagnosed at study visit. The researchers categorized HIV risk score as low (less than 0.12), moderate (0.12-0.59) and high (greater than 0.59) risk based on population seroconversion probabilities. They assigned partnership as no/single HIV- partner, single HIV+ partner, or multiple partners of any serostatus in the past 3 months. They estimated PrEP adherence by intracellular tenofovir-diphosphate (TFV-DP) levels as a continuous variable at week 48.
Of 313 study participants who completed week 48, the researchers observed no significant change in HIV risk category from baseline to week 48 (low: 44% to 42%; moderate: 27% to 24%; high: 28% to 34%; P=0.25). However, there was a significant change in partnership type, with the proportion of those with no or single HIV- partnerships increasing from 1% to 9% (P less than 0.001). Univariate analysis revealed that moderate and high risk groups had higher TFV-DP levels, compared with the low risk group at week 48 (P = 0.018). Participants with no/single HIV- partner had significantly lower TFV-DP levels, compared with those who had one HIV+ partner or multiple partners (P = 0.007). On multivariable linear regression, only low risk partnerships remained significant where no/single HIV- partnerships were associated with lower TFV-DP levels (P = 0.014).
“Although more individuals in our study reported having either no or a single HIV-negative partners by the end of the study, there was no decrease in risk behavior based on reported condomless anal sex acts and laboratory-confirmed STIs over time,” Dr. Blumenthal said. “However, those risk behaviors did not increase either, arguing against risk compensation. Individuals with higher HIV risk behaviors and in riskier partnerships (those with either a single HIV+ or multiple partners) had higher TFV-DP levels at week 48 suggesting a maintained, strong motivation for PrEP adherence.”
She acknowledged certain limitations of the study, including the fact that the risk behavior score used in the analysis has not been validated in prospective studies of HIV incidence. “In addition, participants in the study were not allowed to start and stop PrEP, so less risky individuals may have remained in the study in the event they wanted to restart PrEP,” she said. Dr. Blumenthal disclosed that she is a Gilead Educational Grant recipient and that the study drug was provided by Gilead.
SAN DIEGO – Recent sexual risk behavior and partnership type may be important predictors of pre-exposure prophylaxis in men who have sex with men, results from a 48-week study suggest.
“We know from other studies including iPrEX, the Partners PrEP, and the Demo project that individuals who report higher risk behaviors are more likely to be adherent to pre-exposure prophylaxis (PrEP),” lead study author Jill Blumenthal, MD, said in an interview in advance of an annual scientific meeting on infectious diseases.
As part of a PrEP California Collaborative Treatment Group demonstration study of 398 HIV-negative at-risk men who have sex with men and transgender women, Dr. Blumenthal, of the Antiviral Research Center at the University of California, San Diego, and her associates estimated their HIV risk score at baseline and week 48. Their score was estimated as the probability of seroconversion over the next year based on number of condomless anal sex acts with HIV+/unknown partners in the last month and any sexually transmitted infection diagnosed at study visit. The researchers categorized HIV risk score as low (less than 0.12), moderate (0.12-0.59) and high (greater than 0.59) risk based on population seroconversion probabilities. They assigned partnership as no/single HIV- partner, single HIV+ partner, or multiple partners of any serostatus in the past 3 months. They estimated PrEP adherence by intracellular tenofovir-diphosphate (TFV-DP) levels as a continuous variable at week 48.
Of 313 study participants who completed week 48, the researchers observed no significant change in HIV risk category from baseline to week 48 (low: 44% to 42%; moderate: 27% to 24%; high: 28% to 34%; P=0.25). However, there was a significant change in partnership type, with the proportion of those with no or single HIV- partnerships increasing from 1% to 9% (P less than 0.001). Univariate analysis revealed that moderate and high risk groups had higher TFV-DP levels, compared with the low risk group at week 48 (P = 0.018). Participants with no/single HIV- partner had significantly lower TFV-DP levels, compared with those who had one HIV+ partner or multiple partners (P = 0.007). On multivariable linear regression, only low risk partnerships remained significant where no/single HIV- partnerships were associated with lower TFV-DP levels (P = 0.014).
“Although more individuals in our study reported having either no or a single HIV-negative partners by the end of the study, there was no decrease in risk behavior based on reported condomless anal sex acts and laboratory-confirmed STIs over time,” Dr. Blumenthal said. “However, those risk behaviors did not increase either, arguing against risk compensation. Individuals with higher HIV risk behaviors and in riskier partnerships (those with either a single HIV+ or multiple partners) had higher TFV-DP levels at week 48 suggesting a maintained, strong motivation for PrEP adherence.”
She acknowledged certain limitations of the study, including the fact that the risk behavior score used in the analysis has not been validated in prospective studies of HIV incidence. “In addition, participants in the study were not allowed to start and stop PrEP, so less risky individuals may have remained in the study in the event they wanted to restart PrEP,” she said. Dr. Blumenthal disclosed that she is a Gilead Educational Grant recipient and that the study drug was provided by Gilead.
SAN DIEGO – Recent sexual risk behavior and partnership type may be important predictors of pre-exposure prophylaxis in men who have sex with men, results from a 48-week study suggest.
“We know from other studies including iPrEX, the Partners PrEP, and the Demo project that individuals who report higher risk behaviors are more likely to be adherent to pre-exposure prophylaxis (PrEP),” lead study author Jill Blumenthal, MD, said in an interview in advance of an annual scientific meeting on infectious diseases.
As part of a PrEP California Collaborative Treatment Group demonstration study of 398 HIV-negative at-risk men who have sex with men and transgender women, Dr. Blumenthal, of the Antiviral Research Center at the University of California, San Diego, and her associates estimated their HIV risk score at baseline and week 48. Their score was estimated as the probability of seroconversion over the next year based on number of condomless anal sex acts with HIV+/unknown partners in the last month and any sexually transmitted infection diagnosed at study visit. The researchers categorized HIV risk score as low (less than 0.12), moderate (0.12-0.59) and high (greater than 0.59) risk based on population seroconversion probabilities. They assigned partnership as no/single HIV- partner, single HIV+ partner, or multiple partners of any serostatus in the past 3 months. They estimated PrEP adherence by intracellular tenofovir-diphosphate (TFV-DP) levels as a continuous variable at week 48.
Of 313 study participants who completed week 48, the researchers observed no significant change in HIV risk category from baseline to week 48 (low: 44% to 42%; moderate: 27% to 24%; high: 28% to 34%; P=0.25). However, there was a significant change in partnership type, with the proportion of those with no or single HIV- partnerships increasing from 1% to 9% (P less than 0.001). Univariate analysis revealed that moderate and high risk groups had higher TFV-DP levels, compared with the low risk group at week 48 (P = 0.018). Participants with no/single HIV- partner had significantly lower TFV-DP levels, compared with those who had one HIV+ partner or multiple partners (P = 0.007). On multivariable linear regression, only low risk partnerships remained significant where no/single HIV- partnerships were associated with lower TFV-DP levels (P = 0.014).
“Although more individuals in our study reported having either no or a single HIV-negative partners by the end of the study, there was no decrease in risk behavior based on reported condomless anal sex acts and laboratory-confirmed STIs over time,” Dr. Blumenthal said. “However, those risk behaviors did not increase either, arguing against risk compensation. Individuals with higher HIV risk behaviors and in riskier partnerships (those with either a single HIV+ or multiple partners) had higher TFV-DP levels at week 48 suggesting a maintained, strong motivation for PrEP adherence.”
She acknowledged certain limitations of the study, including the fact that the risk behavior score used in the analysis has not been validated in prospective studies of HIV incidence. “In addition, participants in the study were not allowed to start and stop PrEP, so less risky individuals may have remained in the study in the event they wanted to restart PrEP,” she said. Dr. Blumenthal disclosed that she is a Gilead Educational Grant recipient and that the study drug was provided by Gilead.
ID WEEK 2017
Key clinical point: Recent HIV risk behavior and partnership type predict pre-exposure prophylaxis adherence in men who have sex with men.
Major finding: After 48 weeks, the proportion of those with no or single HIV- partnerships increased from 1% to 9% (P less than 0.001).
Study details: A demonstration study of 398 HIV-negative at-risk men who have sex with men and transgender women.
Disclosures: Dr. Blumenthal disclosed that she is a Gilead Educational Grant recipient and that the study drug was provided by Gilead.
Study eyes factors that may trigger breakthrough bacteremia
SAN DIEGO – , according to a detailed study of six isolates.
“Both patient and microbe factors can contribute to treatment failure, even involving pan-susceptible isolates,” Andrew Berti, PharmD, PhD, said in an interview prior to an annual scientific meeting on infectious diseases.

The researchers presented the case of a patient who experienced multiple episodes of breakthrough Staphylococcus aureus bacteremia over a period of 5 years in the setting of appropriately dosed antimicrobial suppressive therapy. They recovered six clinical bloodstream isolates from the patient during distinct episodes of methicillin-susceptible S. aureus (MSSA) bacteremia, and it was determined that a central line infection was the source for each episode. Isolates recovered were susceptible to the individual therapies received, which included oxacillin, daptomycin, and dalbavancin. The researchers used Illumina technology to collect bacterial whole genome sequence data. “Relatively little is known about bacterial evolution during human infection as these tend to be acute, rapidly resolved events,” Dr. Berti said. “This case was unique in that we could observe changes to a pathogen over a more than 6 years period as it adapted to survive host defenses and multiple antibiotic interventions.”
The researchers discovered that the first two isolates (ST256) and the last four isolates (ST5) “represent distinct populations and suggest that a distinct MSSA strain displaced the previous population between bacteremia episodes 2 and 3,” they wrote in their abstract. “Of note, all of these strains were able to survive and establish breakthrough bacteremias despite favorable susceptibility profiles to the agents used as suppressive therapy. Although the MICs remain low and in the susceptible range to oxacillin, daptomycin, and dalbavancin, these isolates progressively developed significant antimicrobial tolerance phenotypes, which coincided with mutations in walK (yycG), htrA2, ftsW, ebh, and ileS that may be advantageous to survival under antibiotic pressure.”
Dr. Berti acknowledged certain limitations of the analysis, including the inability to test for heteroresistance to some antibiotics, which may also contribute to treatment failure. “Specific therapeutic interventions that coincide with bacterial population changes are not necessarily causative of those changes,” he said. One of the study authors, Warren Rose, PharmD, disclosed having received research grants, speaker honoraria, and/or consulting fees from Theravance, Merck, The Medicines Company and Visante, Inc. All other authors reported having no financial disclosures.
SAN DIEGO – , according to a detailed study of six isolates.
“Both patient and microbe factors can contribute to treatment failure, even involving pan-susceptible isolates,” Andrew Berti, PharmD, PhD, said in an interview prior to an annual scientific meeting on infectious diseases.

The researchers presented the case of a patient who experienced multiple episodes of breakthrough Staphylococcus aureus bacteremia over a period of 5 years in the setting of appropriately dosed antimicrobial suppressive therapy. They recovered six clinical bloodstream isolates from the patient during distinct episodes of methicillin-susceptible S. aureus (MSSA) bacteremia, and it was determined that a central line infection was the source for each episode. Isolates recovered were susceptible to the individual therapies received, which included oxacillin, daptomycin, and dalbavancin. The researchers used Illumina technology to collect bacterial whole genome sequence data. “Relatively little is known about bacterial evolution during human infection as these tend to be acute, rapidly resolved events,” Dr. Berti said. “This case was unique in that we could observe changes to a pathogen over a more than 6 years period as it adapted to survive host defenses and multiple antibiotic interventions.”
The researchers discovered that the first two isolates (ST256) and the last four isolates (ST5) “represent distinct populations and suggest that a distinct MSSA strain displaced the previous population between bacteremia episodes 2 and 3,” they wrote in their abstract. “Of note, all of these strains were able to survive and establish breakthrough bacteremias despite favorable susceptibility profiles to the agents used as suppressive therapy. Although the MICs remain low and in the susceptible range to oxacillin, daptomycin, and dalbavancin, these isolates progressively developed significant antimicrobial tolerance phenotypes, which coincided with mutations in walK (yycG), htrA2, ftsW, ebh, and ileS that may be advantageous to survival under antibiotic pressure.”
Dr. Berti acknowledged certain limitations of the analysis, including the inability to test for heteroresistance to some antibiotics, which may also contribute to treatment failure. “Specific therapeutic interventions that coincide with bacterial population changes are not necessarily causative of those changes,” he said. One of the study authors, Warren Rose, PharmD, disclosed having received research grants, speaker honoraria, and/or consulting fees from Theravance, Merck, The Medicines Company and Visante, Inc. All other authors reported having no financial disclosures.
SAN DIEGO – , according to a detailed study of six isolates.
“Both patient and microbe factors can contribute to treatment failure, even involving pan-susceptible isolates,” Andrew Berti, PharmD, PhD, said in an interview prior to an annual scientific meeting on infectious diseases.

The researchers presented the case of a patient who experienced multiple episodes of breakthrough Staphylococcus aureus bacteremia over a period of 5 years in the setting of appropriately dosed antimicrobial suppressive therapy. They recovered six clinical bloodstream isolates from the patient during distinct episodes of methicillin-susceptible S. aureus (MSSA) bacteremia, and it was determined that a central line infection was the source for each episode. Isolates recovered were susceptible to the individual therapies received, which included oxacillin, daptomycin, and dalbavancin. The researchers used Illumina technology to collect bacterial whole genome sequence data. “Relatively little is known about bacterial evolution during human infection as these tend to be acute, rapidly resolved events,” Dr. Berti said. “This case was unique in that we could observe changes to a pathogen over a more than 6 years period as it adapted to survive host defenses and multiple antibiotic interventions.”
The researchers discovered that the first two isolates (ST256) and the last four isolates (ST5) “represent distinct populations and suggest that a distinct MSSA strain displaced the previous population between bacteremia episodes 2 and 3,” they wrote in their abstract. “Of note, all of these strains were able to survive and establish breakthrough bacteremias despite favorable susceptibility profiles to the agents used as suppressive therapy. Although the MICs remain low and in the susceptible range to oxacillin, daptomycin, and dalbavancin, these isolates progressively developed significant antimicrobial tolerance phenotypes, which coincided with mutations in walK (yycG), htrA2, ftsW, ebh, and ileS that may be advantageous to survival under antibiotic pressure.”
Dr. Berti acknowledged certain limitations of the analysis, including the inability to test for heteroresistance to some antibiotics, which may also contribute to treatment failure. “Specific therapeutic interventions that coincide with bacterial population changes are not necessarily causative of those changes,” he said. One of the study authors, Warren Rose, PharmD, disclosed having received research grants, speaker honoraria, and/or consulting fees from Theravance, Merck, The Medicines Company and Visante, Inc. All other authors reported having no financial disclosures.
REPORTING FROM ID WEEK 2017
Key clinical point: Both patient and microbe factors can contribute to antibitoic treatment failure.
Major finding: Two distinct Staphylococcus aureus lineages were islolated over a period of 5 years, and each was able to persist despite appropriate antibitoic interventions.
Study details: Clinical analysis of six bloodstream isolates from a singles patient over a 5-year period.
Disclosures: One of the study authors, Warren Rose, PharmD, disclosed having received research grants, speaker honoraria and/or consulting fees from Theravance, Merck, The Medicines Company and Visante. All other authors reported having no financial disclosures.
HIV antiretroviral resistance can affect more than 10% of pregnant women
SAN DIEGO – HIV antiretroviral resistance can affect more than 10% of pregnant women, even if they are previously treatment naive, results of a case-control study demonstrated.
“Furthermore, if there is an HIV-infected infant who received HIV prophylaxis with zidovudine and nevirapine, the infant may have developed resistance to the nonnucleoside reverse transcriptase inhibitors [NNRTIs] class of medications, and timely antiretroviral-resistant testing is an important step prior to choosing an appropriate regimen,” Nava Yeganeh, MD, said in an interview prior to an annual scientific meeting on infectious diseases.
In all, 140 infants were HIV infected, and 13 had drug-resistant mutations. Of the 606 women who had sufficient nucleic acid amplification for resistance testing, 63 (10.4%) had drug-resistant mutations against one or more classes of antiretrovirals. “These mothers may have been infected with a drug-resistant strain of HIV, which they then may have passed on to their infants,” Dr. Yeganeh said. “We also found that 3 of the 13 HIV-infected infants with drug-resistant mutations against NNRTIs were born to mothers who did not have a resistant strain of HIV. These three infants likely developed resistance because of the infant prophylaxis they received with nevirapine.”
Univariate and multivariate analyses revealed that drug-resistant mutation in mothers was not associated with increased risk of HIV mother-to-child transmission (adjusted odds ratio, 0.79). The only predictors of mother-to-child transmission were log HIV viral load (OR, 1.4) and infant prophylaxis arm with a two-drug regimen (OR, 1.6). In addition, the presence of drug-resistant mutations in mothers who transmitted was strongly associated with presence of drug-resistant mutations in infants (P less than .001).
A key limitation of the trial, Dr. Yeganeh said, was that it was completed in 2011. “Antiretroviral-resistant HIV may be even more common now that antiretrovirals are more available,” she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. She reported having no financial disclosures.
SAN DIEGO – HIV antiretroviral resistance can affect more than 10% of pregnant women, even if they are previously treatment naive, results of a case-control study demonstrated.
“Furthermore, if there is an HIV-infected infant who received HIV prophylaxis with zidovudine and nevirapine, the infant may have developed resistance to the nonnucleoside reverse transcriptase inhibitors [NNRTIs] class of medications, and timely antiretroviral-resistant testing is an important step prior to choosing an appropriate regimen,” Nava Yeganeh, MD, said in an interview prior to an annual scientific meeting on infectious diseases.
In all, 140 infants were HIV infected, and 13 had drug-resistant mutations. Of the 606 women who had sufficient nucleic acid amplification for resistance testing, 63 (10.4%) had drug-resistant mutations against one or more classes of antiretrovirals. “These mothers may have been infected with a drug-resistant strain of HIV, which they then may have passed on to their infants,” Dr. Yeganeh said. “We also found that 3 of the 13 HIV-infected infants with drug-resistant mutations against NNRTIs were born to mothers who did not have a resistant strain of HIV. These three infants likely developed resistance because of the infant prophylaxis they received with nevirapine.”
Univariate and multivariate analyses revealed that drug-resistant mutation in mothers was not associated with increased risk of HIV mother-to-child transmission (adjusted odds ratio, 0.79). The only predictors of mother-to-child transmission were log HIV viral load (OR, 1.4) and infant prophylaxis arm with a two-drug regimen (OR, 1.6). In addition, the presence of drug-resistant mutations in mothers who transmitted was strongly associated with presence of drug-resistant mutations in infants (P less than .001).
A key limitation of the trial, Dr. Yeganeh said, was that it was completed in 2011. “Antiretroviral-resistant HIV may be even more common now that antiretrovirals are more available,” she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. She reported having no financial disclosures.
SAN DIEGO – HIV antiretroviral resistance can affect more than 10% of pregnant women, even if they are previously treatment naive, results of a case-control study demonstrated.
“Furthermore, if there is an HIV-infected infant who received HIV prophylaxis with zidovudine and nevirapine, the infant may have developed resistance to the nonnucleoside reverse transcriptase inhibitors [NNRTIs] class of medications, and timely antiretroviral-resistant testing is an important step prior to choosing an appropriate regimen,” Nava Yeganeh, MD, said in an interview prior to an annual scientific meeting on infectious diseases.
In all, 140 infants were HIV infected, and 13 had drug-resistant mutations. Of the 606 women who had sufficient nucleic acid amplification for resistance testing, 63 (10.4%) had drug-resistant mutations against one or more classes of antiretrovirals. “These mothers may have been infected with a drug-resistant strain of HIV, which they then may have passed on to their infants,” Dr. Yeganeh said. “We also found that 3 of the 13 HIV-infected infants with drug-resistant mutations against NNRTIs were born to mothers who did not have a resistant strain of HIV. These three infants likely developed resistance because of the infant prophylaxis they received with nevirapine.”
Univariate and multivariate analyses revealed that drug-resistant mutation in mothers was not associated with increased risk of HIV mother-to-child transmission (adjusted odds ratio, 0.79). The only predictors of mother-to-child transmission were log HIV viral load (OR, 1.4) and infant prophylaxis arm with a two-drug regimen (OR, 1.6). In addition, the presence of drug-resistant mutations in mothers who transmitted was strongly associated with presence of drug-resistant mutations in infants (P less than .001).
A key limitation of the trial, Dr. Yeganeh said, was that it was completed in 2011. “Antiretroviral-resistant HIV may be even more common now that antiretrovirals are more available,” she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. She reported having no financial disclosures.
AT IDWEEK 2017
Key clinical point:
Major finding: Of 606 women who had sufficient nucleic acid amplification for resistance testing, 63 (10.4%) had drug-resistant mutations against one or more classes of antiretrovirals.
Study details: A case-control study of blood samples from 606 HIV-infected pregnant women and their infants.
Disclosures: Dr. Yeganeh reported having no financial disclosures.