User login
Christopher Palmer has been an associate editor at MDedge News since 2017. When he's not tidying grammar, he writes short pieces about breaking FDA announcements and approvals, as well as journal articles. He proudly holds a BA in English and philosophy. Follow him on Twitter @cmacmpalm.
MS medication withdrawn because of safety concerns
Citing concerns about safety, Biogen and AbbVie announced March 2 that they will be withdrawing daclizumab (Zinbryta) from worldwide markets. Daclizumab has known risks, so it was usually prescribed only for people with relapsing multiple sclerosis who had tried two or more other medications that hadn’t worked well enough.
Reports of inflammatory encephalitis and meningoencephalitis led the European Medicines Agency to initiate an Article 20 referral procedure. In such referrals, a medicine or class of medicines are scientifically assessed because of concerns over safety or quality.
However, Biogen and AbbVie concluded that, because of the complex nature of these reports and how few patients were taking daclizumab, it would be difficult to characterize the nature of the medication’s harms and benefits, so the companies instead have decided to withdraw the medication from the market.
On March 14, the Food and Drug Administration announced that it is conducting a review of similar adverse event reports it has received.
The drug will continue to be available to patients until April 30, 2018. Patients taking daclizumab should not stop taking the drug without talking to their doctor and should contact their doctor if they have any new or unexplained symptoms, the FDA said. More information can be found in the press release.
**Story updated 3/14/2018.
Citing concerns about safety, Biogen and AbbVie announced March 2 that they will be withdrawing daclizumab (Zinbryta) from worldwide markets. Daclizumab has known risks, so it was usually prescribed only for people with relapsing multiple sclerosis who had tried two or more other medications that hadn’t worked well enough.
Reports of inflammatory encephalitis and meningoencephalitis led the European Medicines Agency to initiate an Article 20 referral procedure. In such referrals, a medicine or class of medicines are scientifically assessed because of concerns over safety or quality.
However, Biogen and AbbVie concluded that, because of the complex nature of these reports and how few patients were taking daclizumab, it would be difficult to characterize the nature of the medication’s harms and benefits, so the companies instead have decided to withdraw the medication from the market.
On March 14, the Food and Drug Administration announced that it is conducting a review of similar adverse event reports it has received.
The drug will continue to be available to patients until April 30, 2018. Patients taking daclizumab should not stop taking the drug without talking to their doctor and should contact their doctor if they have any new or unexplained symptoms, the FDA said. More information can be found in the press release.
**Story updated 3/14/2018.
Citing concerns about safety, Biogen and AbbVie announced March 2 that they will be withdrawing daclizumab (Zinbryta) from worldwide markets. Daclizumab has known risks, so it was usually prescribed only for people with relapsing multiple sclerosis who had tried two or more other medications that hadn’t worked well enough.
Reports of inflammatory encephalitis and meningoencephalitis led the European Medicines Agency to initiate an Article 20 referral procedure. In such referrals, a medicine or class of medicines are scientifically assessed because of concerns over safety or quality.
However, Biogen and AbbVie concluded that, because of the complex nature of these reports and how few patients were taking daclizumab, it would be difficult to characterize the nature of the medication’s harms and benefits, so the companies instead have decided to withdraw the medication from the market.
On March 14, the Food and Drug Administration announced that it is conducting a review of similar adverse event reports it has received.
The drug will continue to be available to patients until April 30, 2018. Patients taking daclizumab should not stop taking the drug without talking to their doctor and should contact their doctor if they have any new or unexplained symptoms, the FDA said. More information can be found in the press release.
**Story updated 3/14/2018.
FDA alerts doctors to stop administering compounded products
The Food and Drug Administration today alerted physicians to stop administering or providing drugs manufactured by Cantrell Drug, including opioid products and other drugs intended for sterile injection, because the company has failed to ensure quality and sterility in its compounding operations, which could seriously compromise patient safety.
Health care providers should check medical supplies and separate anything produced by Cantrell Drug, which can be identified by finding the company’s name on the label. The FDA also recommended that physicians seek alternative arrangements for medicines and that any patients who have any products made by Cantrell contact their physicians.
So far, the FDA is not aware of any illness or injuries caused by potentially contaminated products from Cantrell, but any that do arise should be reported to the FDA’s MedWatch program.
Read more about this alert in the FDA website.
The Food and Drug Administration today alerted physicians to stop administering or providing drugs manufactured by Cantrell Drug, including opioid products and other drugs intended for sterile injection, because the company has failed to ensure quality and sterility in its compounding operations, which could seriously compromise patient safety.
Health care providers should check medical supplies and separate anything produced by Cantrell Drug, which can be identified by finding the company’s name on the label. The FDA also recommended that physicians seek alternative arrangements for medicines and that any patients who have any products made by Cantrell contact their physicians.
So far, the FDA is not aware of any illness or injuries caused by potentially contaminated products from Cantrell, but any that do arise should be reported to the FDA’s MedWatch program.
Read more about this alert in the FDA website.
The Food and Drug Administration today alerted physicians to stop administering or providing drugs manufactured by Cantrell Drug, including opioid products and other drugs intended for sterile injection, because the company has failed to ensure quality and sterility in its compounding operations, which could seriously compromise patient safety.
Health care providers should check medical supplies and separate anything produced by Cantrell Drug, which can be identified by finding the company’s name on the label. The FDA also recommended that physicians seek alternative arrangements for medicines and that any patients who have any products made by Cantrell contact their physicians.
So far, the FDA is not aware of any illness or injuries caused by potentially contaminated products from Cantrell, but any that do arise should be reported to the FDA’s MedWatch program.
Read more about this alert in the FDA website.
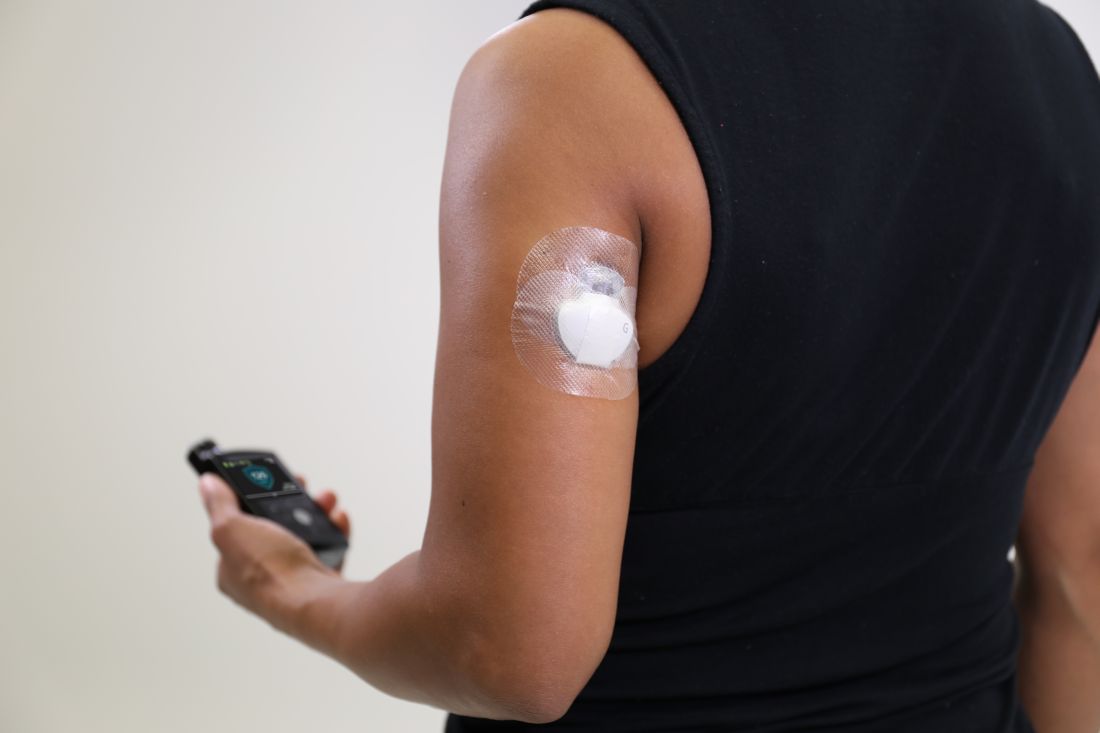
Arm-placement indication for CGM approved by FDA
The Food and Drug Administration has approved a new indication for the Guardian Sensor 3 continuous glucose monitor (CGM), allowing patients to position it on their arms, according to an announcement by Medtronic, the sensor’s manufacturer.
Although previously only the abdomen had FDA approval as a CGM site, findings from at least one study have shown that most patients prefer to position their sensors elsewhere. This new indication gives these patients more flexibility in where they can place their CGMs. The site of CGM placement did not affect the failure rate or noncompliance.
The sensor works in tandem with an automated insulin delivery system known as the MiniMed 670G system, also manufactured by Medtronic. This system is approved for people with type 1 diabetes aged 14 years and older and adjusts basal insulin levels every 5 minutes – “based on real-time data delivered by the Guardian Sensor 3,” according to Medtronic.
Read more in Medtronic’s press release.
Correction, 3/21/18: A previous version of this article contained a photo of a device that was not the Guardian 3 Sensor.
The Food and Drug Administration has approved a new indication for the Guardian Sensor 3 continuous glucose monitor (CGM), allowing patients to position it on their arms, according to an announcement by Medtronic, the sensor’s manufacturer.
Although previously only the abdomen had FDA approval as a CGM site, findings from at least one study have shown that most patients prefer to position their sensors elsewhere. This new indication gives these patients more flexibility in where they can place their CGMs. The site of CGM placement did not affect the failure rate or noncompliance.
The sensor works in tandem with an automated insulin delivery system known as the MiniMed 670G system, also manufactured by Medtronic. This system is approved for people with type 1 diabetes aged 14 years and older and adjusts basal insulin levels every 5 minutes – “based on real-time data delivered by the Guardian Sensor 3,” according to Medtronic.
Read more in Medtronic’s press release.
Correction, 3/21/18: A previous version of this article contained a photo of a device that was not the Guardian 3 Sensor.
The Food and Drug Administration has approved a new indication for the Guardian Sensor 3 continuous glucose monitor (CGM), allowing patients to position it on their arms, according to an announcement by Medtronic, the sensor’s manufacturer.
Although previously only the abdomen had FDA approval as a CGM site, findings from at least one study have shown that most patients prefer to position their sensors elsewhere. This new indication gives these patients more flexibility in where they can place their CGMs. The site of CGM placement did not affect the failure rate or noncompliance.
The sensor works in tandem with an automated insulin delivery system known as the MiniMed 670G system, also manufactured by Medtronic. This system is approved for people with type 1 diabetes aged 14 years and older and adjusts basal insulin levels every 5 minutes – “based on real-time data delivered by the Guardian Sensor 3,” according to Medtronic.
Read more in Medtronic’s press release.
Correction, 3/21/18: A previous version of this article contained a photo of a device that was not the Guardian 3 Sensor.
Insomnia, major depressive episode linked in U.S. soldiers
Insomnia is prevalent among U.S. soldiers, and the highest prevalence rate is among those with current major depressive episode, according to a cross-sectional analysis.
“Psychiatric disorders moderated the relationship between insomnia and memory/concentration problems, suggesting the psychiatric disorders contribute unique variance to cognitive problems,” wrote Janeese A. Brownlow, PhD, of the University of Pennsylvania, Philadelphia, and her associates. “Results highlight the importance of considering both insomnia and psychiatric disorders in the diagnosis and treatment of cognitive deficits in military soldiers.”
The researchers used the All Army Study of the Army Study to Assess Risk and Resilience in Servicemembers as their data source. They used the Composite International Diagnostic Interview (CIDI) and the Posttraumatic Stress Disorder Checklist to assess psychiatric disorders; the CIDI also helped assess cognitive problems. One of the strengths of this study was its large sample size: It had an unweighted sample of 21,449 soldiers.
. The prevalence was 82.6% among soldiers with generalized anxiety disorder and 69.7% among those with PTSD. The likelihood of having insomnia grew with the number of comorbid psychiatric disorders.
One of the limitations of the study was that many of the measures were self-reported; for example, the psychiatric diagnoses and the determinations regarding insomnia were based on surveys and questionnaires rather than clinical interviews and assessments. Furthermore, the absence of a comprehensive neurocognitive battery might have limited the study’s ability to assess cognitive problems. Nevertheless, the researchers wrote, “addressing insomnia may increase resiliency and the ability to perform and cope with the complexities of active duty.”
Read more about the study in the Journal of Affective Disorders.
Insomnia is prevalent among U.S. soldiers, and the highest prevalence rate is among those with current major depressive episode, according to a cross-sectional analysis.
“Psychiatric disorders moderated the relationship between insomnia and memory/concentration problems, suggesting the psychiatric disorders contribute unique variance to cognitive problems,” wrote Janeese A. Brownlow, PhD, of the University of Pennsylvania, Philadelphia, and her associates. “Results highlight the importance of considering both insomnia and psychiatric disorders in the diagnosis and treatment of cognitive deficits in military soldiers.”
The researchers used the All Army Study of the Army Study to Assess Risk and Resilience in Servicemembers as their data source. They used the Composite International Diagnostic Interview (CIDI) and the Posttraumatic Stress Disorder Checklist to assess psychiatric disorders; the CIDI also helped assess cognitive problems. One of the strengths of this study was its large sample size: It had an unweighted sample of 21,449 soldiers.
. The prevalence was 82.6% among soldiers with generalized anxiety disorder and 69.7% among those with PTSD. The likelihood of having insomnia grew with the number of comorbid psychiatric disorders.
One of the limitations of the study was that many of the measures were self-reported; for example, the psychiatric diagnoses and the determinations regarding insomnia were based on surveys and questionnaires rather than clinical interviews and assessments. Furthermore, the absence of a comprehensive neurocognitive battery might have limited the study’s ability to assess cognitive problems. Nevertheless, the researchers wrote, “addressing insomnia may increase resiliency and the ability to perform and cope with the complexities of active duty.”
Read more about the study in the Journal of Affective Disorders.
Insomnia is prevalent among U.S. soldiers, and the highest prevalence rate is among those with current major depressive episode, according to a cross-sectional analysis.
“Psychiatric disorders moderated the relationship between insomnia and memory/concentration problems, suggesting the psychiatric disorders contribute unique variance to cognitive problems,” wrote Janeese A. Brownlow, PhD, of the University of Pennsylvania, Philadelphia, and her associates. “Results highlight the importance of considering both insomnia and psychiatric disorders in the diagnosis and treatment of cognitive deficits in military soldiers.”
The researchers used the All Army Study of the Army Study to Assess Risk and Resilience in Servicemembers as their data source. They used the Composite International Diagnostic Interview (CIDI) and the Posttraumatic Stress Disorder Checklist to assess psychiatric disorders; the CIDI also helped assess cognitive problems. One of the strengths of this study was its large sample size: It had an unweighted sample of 21,449 soldiers.
. The prevalence was 82.6% among soldiers with generalized anxiety disorder and 69.7% among those with PTSD. The likelihood of having insomnia grew with the number of comorbid psychiatric disorders.
One of the limitations of the study was that many of the measures were self-reported; for example, the psychiatric diagnoses and the determinations regarding insomnia were based on surveys and questionnaires rather than clinical interviews and assessments. Furthermore, the absence of a comprehensive neurocognitive battery might have limited the study’s ability to assess cognitive problems. Nevertheless, the researchers wrote, “addressing insomnia may increase resiliency and the ability to perform and cope with the complexities of active duty.”
Read more about the study in the Journal of Affective Disorders.
FROM JOURNAL OF AFFECTIVE DISORDERS
Poor sleep, suicide risk linked in college students
Suicidal behaviors are associated with poor sleep in college students, even after controlling for depression, a cross-sectional analysis shows. Specifically, students were 6.54 times as likely to be classified with suicide risk if they were depressed and 2.70 times as likely if they had poor quality of sleep.
“Our findings add to a growing body of literature pointing to sleep as an important component to include in screening and intervention efforts to prevent suicidal ideation and attempts on college campuses,” wrote Stephen P. Becker, PhD, a pediatric psychologist affiliated with Cincinnati Children’s Hospital Medical Center, and his colleagues.
Of the 1,700 college students included in this study (J Psychiatr Res. 2018 Jan 12:99:122-8), 82.7% of those classified with suicide risk had poor sleep quality, but only 31.3% of those with poor sleep quality were classified with suicide risk.
Most previous studies had looked only at insomnia or bad dreams rather than other aspects of poor sleep quality, and because this study used the Pittsburgh Sleep Quality Index, it was able to evaluate additional well-validated components of sleep. so that screening and intervention based on sleep quality can be more effective. This is important, because suicide is one of the leading causes of death among young adults.
The researchers declared no conflicts of interest.
Read more about this study in the Journal of Psychiatric Research.
Suicidal behaviors are associated with poor sleep in college students, even after controlling for depression, a cross-sectional analysis shows. Specifically, students were 6.54 times as likely to be classified with suicide risk if they were depressed and 2.70 times as likely if they had poor quality of sleep.
“Our findings add to a growing body of literature pointing to sleep as an important component to include in screening and intervention efforts to prevent suicidal ideation and attempts on college campuses,” wrote Stephen P. Becker, PhD, a pediatric psychologist affiliated with Cincinnati Children’s Hospital Medical Center, and his colleagues.
Of the 1,700 college students included in this study (J Psychiatr Res. 2018 Jan 12:99:122-8), 82.7% of those classified with suicide risk had poor sleep quality, but only 31.3% of those with poor sleep quality were classified with suicide risk.
Most previous studies had looked only at insomnia or bad dreams rather than other aspects of poor sleep quality, and because this study used the Pittsburgh Sleep Quality Index, it was able to evaluate additional well-validated components of sleep. so that screening and intervention based on sleep quality can be more effective. This is important, because suicide is one of the leading causes of death among young adults.
The researchers declared no conflicts of interest.
Read more about this study in the Journal of Psychiatric Research.
Suicidal behaviors are associated with poor sleep in college students, even after controlling for depression, a cross-sectional analysis shows. Specifically, students were 6.54 times as likely to be classified with suicide risk if they were depressed and 2.70 times as likely if they had poor quality of sleep.
“Our findings add to a growing body of literature pointing to sleep as an important component to include in screening and intervention efforts to prevent suicidal ideation and attempts on college campuses,” wrote Stephen P. Becker, PhD, a pediatric psychologist affiliated with Cincinnati Children’s Hospital Medical Center, and his colleagues.
Of the 1,700 college students included in this study (J Psychiatr Res. 2018 Jan 12:99:122-8), 82.7% of those classified with suicide risk had poor sleep quality, but only 31.3% of those with poor sleep quality were classified with suicide risk.
Most previous studies had looked only at insomnia or bad dreams rather than other aspects of poor sleep quality, and because this study used the Pittsburgh Sleep Quality Index, it was able to evaluate additional well-validated components of sleep. so that screening and intervention based on sleep quality can be more effective. This is important, because suicide is one of the leading causes of death among young adults.
The researchers declared no conflicts of interest.
Read more about this study in the Journal of Psychiatric Research.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Delayed treatment for psychosis can have ‘deleterious’ effects
The longer that patients with schizophrenia go without treatment for a psychotic episode, the more their hippocampus atrophies, suggests a study published Feb. 21 in JAMA Psychiatry.
To conduct the study, researchers studied 71 patients between March 5, 2013, and Oct. 8, 2014, who were experiencing their first psychotic episode and were antipsychotic naive. The research team matched those patients with 73 healthy controls, treated the patients with antipsychotics, and performed MRIs on members of both groups at baseline and 8 weeks later. The primary outcome measure was change in left and right hippocampal volume integrity, which is inversely associated with hippocampal atrophy.
Results showed that many patients with psychosis had lower median hippocampal volume integrity. Furthermore, those . The duration of untreated psychosis was correlated with both decreases, but this association was only significant with left hippocampal volume integrity.
This relationship is “consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure,” wrote Donald C. Goff, MD, of the psychiatry department at New York University, and his associates. “Larger longitudinal studies of longer duration are needed to examine the association between [duration of untreated psychosis], hippocampal volume, and clinical outcomes.”
Dr. Goff reported receiving research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Avanir Pharmaceuticals. Another author reported receiving support from numerous entities and honoraria for serving on an advisory board for Allergan. No other disclosures were reported.
Read more at JAMA Psychiatry.
The longer that patients with schizophrenia go without treatment for a psychotic episode, the more their hippocampus atrophies, suggests a study published Feb. 21 in JAMA Psychiatry.
To conduct the study, researchers studied 71 patients between March 5, 2013, and Oct. 8, 2014, who were experiencing their first psychotic episode and were antipsychotic naive. The research team matched those patients with 73 healthy controls, treated the patients with antipsychotics, and performed MRIs on members of both groups at baseline and 8 weeks later. The primary outcome measure was change in left and right hippocampal volume integrity, which is inversely associated with hippocampal atrophy.
Results showed that many patients with psychosis had lower median hippocampal volume integrity. Furthermore, those . The duration of untreated psychosis was correlated with both decreases, but this association was only significant with left hippocampal volume integrity.
This relationship is “consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure,” wrote Donald C. Goff, MD, of the psychiatry department at New York University, and his associates. “Larger longitudinal studies of longer duration are needed to examine the association between [duration of untreated psychosis], hippocampal volume, and clinical outcomes.”
Dr. Goff reported receiving research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Avanir Pharmaceuticals. Another author reported receiving support from numerous entities and honoraria for serving on an advisory board for Allergan. No other disclosures were reported.
Read more at JAMA Psychiatry.
The longer that patients with schizophrenia go without treatment for a psychotic episode, the more their hippocampus atrophies, suggests a study published Feb. 21 in JAMA Psychiatry.
To conduct the study, researchers studied 71 patients between March 5, 2013, and Oct. 8, 2014, who were experiencing their first psychotic episode and were antipsychotic naive. The research team matched those patients with 73 healthy controls, treated the patients with antipsychotics, and performed MRIs on members of both groups at baseline and 8 weeks later. The primary outcome measure was change in left and right hippocampal volume integrity, which is inversely associated with hippocampal atrophy.
Results showed that many patients with psychosis had lower median hippocampal volume integrity. Furthermore, those . The duration of untreated psychosis was correlated with both decreases, but this association was only significant with left hippocampal volume integrity.
This relationship is “consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure,” wrote Donald C. Goff, MD, of the psychiatry department at New York University, and his associates. “Larger longitudinal studies of longer duration are needed to examine the association between [duration of untreated psychosis], hippocampal volume, and clinical outcomes.”
Dr. Goff reported receiving research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Avanir Pharmaceuticals. Another author reported receiving support from numerous entities and honoraria for serving on an advisory board for Allergan. No other disclosures were reported.
Read more at JAMA Psychiatry.
FROM JAMA PSYCHIATRY
Blood test approved for patients with concussions
The Food and Drug Administration approved a new blood test on Feb. 14 – the Banyan Brain Trauma Indicator – for assessing patients with mild traumatic brain injuries, also known as concussions.
Most traumatic brain injuries are classified as “mild,” and the majority of patients have negative CT scans, according to an FDA announcement. Within a matter of hours, this test can help predict which patients will have negative scans by measuring certain proteins released by brain tissue, thereby potentially eliminating unnecessary imaging – and the costs and radiation exposure that would go along with it.
Read more in the FDA’s press release.
The Food and Drug Administration approved a new blood test on Feb. 14 – the Banyan Brain Trauma Indicator – for assessing patients with mild traumatic brain injuries, also known as concussions.
Most traumatic brain injuries are classified as “mild,” and the majority of patients have negative CT scans, according to an FDA announcement. Within a matter of hours, this test can help predict which patients will have negative scans by measuring certain proteins released by brain tissue, thereby potentially eliminating unnecessary imaging – and the costs and radiation exposure that would go along with it.
Read more in the FDA’s press release.
The Food and Drug Administration approved a new blood test on Feb. 14 – the Banyan Brain Trauma Indicator – for assessing patients with mild traumatic brain injuries, also known as concussions.
Most traumatic brain injuries are classified as “mild,” and the majority of patients have negative CT scans, according to an FDA announcement. Within a matter of hours, this test can help predict which patients will have negative scans by measuring certain proteins released by brain tissue, thereby potentially eliminating unnecessary imaging – and the costs and radiation exposure that would go along with it.
Read more in the FDA’s press release.
Exploding e-cigs can cause grievous injuries
With the increasing use of e-cigarettes among adolescents, explosions involving these devices are a growing concern because they can cause serious injuries.
One such incident, described by Elizabeth Ackley, MD, of the University of Colorado at Denver, Aurora, and her coauthors, involved a 17-year-old youth whose electronic nicotine-delivery systems (ENDS) exploded as he was about to take a puff. He presented with a burned left thumb with sensory loss, reduced motor control, and heavy bleeding. He underwent debridement, multiple antibiotic courses, and six operative procedures that ultimately led to removal of the lateral aspect of his thumb.
Dr. Ackley and her associates emphasized. This can occur with overheating, water exposure, excessive charging, improper charging with incompatible devices, contact with metallic objects such as keys or coins, or damage of the battery.
Mineral oil should be used for initial wound irrigation, the authors advise, and “surgical debridement is the definitive treatment for injuries and should remove any remaining alkaline material from tissues.” Delay use of water-based irrigation until after surgical debridement, and one report suggests probing the wound with litmus paper to ensure the pH is no longer alkaline prior to using water irrigation.
Although there are other talking points pediatricians may use when counseling teens about use of e-cigs and other tobacco products, the authors suggested that “the potential for major and disfiguring injury from ENDS explosions may be a more compelling talking point with teens instead of long-term or other nebulous adverse effects of ENDS and tobacco products.”
Read more about the subject at J Pediatr. 2018. doi: 10.1016/j.jpeds.2017.12.032.
With the increasing use of e-cigarettes among adolescents, explosions involving these devices are a growing concern because they can cause serious injuries.
One such incident, described by Elizabeth Ackley, MD, of the University of Colorado at Denver, Aurora, and her coauthors, involved a 17-year-old youth whose electronic nicotine-delivery systems (ENDS) exploded as he was about to take a puff. He presented with a burned left thumb with sensory loss, reduced motor control, and heavy bleeding. He underwent debridement, multiple antibiotic courses, and six operative procedures that ultimately led to removal of the lateral aspect of his thumb.
Dr. Ackley and her associates emphasized. This can occur with overheating, water exposure, excessive charging, improper charging with incompatible devices, contact with metallic objects such as keys or coins, or damage of the battery.
Mineral oil should be used for initial wound irrigation, the authors advise, and “surgical debridement is the definitive treatment for injuries and should remove any remaining alkaline material from tissues.” Delay use of water-based irrigation until after surgical debridement, and one report suggests probing the wound with litmus paper to ensure the pH is no longer alkaline prior to using water irrigation.
Although there are other talking points pediatricians may use when counseling teens about use of e-cigs and other tobacco products, the authors suggested that “the potential for major and disfiguring injury from ENDS explosions may be a more compelling talking point with teens instead of long-term or other nebulous adverse effects of ENDS and tobacco products.”
Read more about the subject at J Pediatr. 2018. doi: 10.1016/j.jpeds.2017.12.032.
With the increasing use of e-cigarettes among adolescents, explosions involving these devices are a growing concern because they can cause serious injuries.
One such incident, described by Elizabeth Ackley, MD, of the University of Colorado at Denver, Aurora, and her coauthors, involved a 17-year-old youth whose electronic nicotine-delivery systems (ENDS) exploded as he was about to take a puff. He presented with a burned left thumb with sensory loss, reduced motor control, and heavy bleeding. He underwent debridement, multiple antibiotic courses, and six operative procedures that ultimately led to removal of the lateral aspect of his thumb.
Dr. Ackley and her associates emphasized. This can occur with overheating, water exposure, excessive charging, improper charging with incompatible devices, contact with metallic objects such as keys or coins, or damage of the battery.
Mineral oil should be used for initial wound irrigation, the authors advise, and “surgical debridement is the definitive treatment for injuries and should remove any remaining alkaline material from tissues.” Delay use of water-based irrigation until after surgical debridement, and one report suggests probing the wound with litmus paper to ensure the pH is no longer alkaline prior to using water irrigation.
Although there are other talking points pediatricians may use when counseling teens about use of e-cigs and other tobacco products, the authors suggested that “the potential for major and disfiguring injury from ENDS explosions may be a more compelling talking point with teens instead of long-term or other nebulous adverse effects of ENDS and tobacco products.”
Read more about the subject at J Pediatr. 2018. doi: 10.1016/j.jpeds.2017.12.032.
Pulmonary Fibrosis Foundation offers trial-finding app
The Pulmonary Fibrosis Foundation (PFF) has begun offering a tool to help patients navigate through more than 100 clinical trials aimed at advancing the treatment of pulmonary fibrosis, according to a statement from the Foundation.
The platform includes trials for patients with idiopathic pulmonary fibrosis.
“Before this, it was pretty impossible to search for clinical trials,” noted Bill Burke, a PF patient and support group leader from Williamsburg, Va., in the statement.
The tool, which is available both on the PFF website and as a free app, draws on information from ClinicalTrials.gov.
The Foundation intends for the new tool to give patients “a voice in their care process,” according to the statement.
“We want to empower patients to actively participate in identifying clinical trials,” said Harold R. Collard, MD, senior medical adviser of research and development for the Pulmonary Fibrosis Foundation.
More information about the trial finder is available on the PFF’s website.
The Pulmonary Fibrosis Foundation (PFF) has begun offering a tool to help patients navigate through more than 100 clinical trials aimed at advancing the treatment of pulmonary fibrosis, according to a statement from the Foundation.
The platform includes trials for patients with idiopathic pulmonary fibrosis.
“Before this, it was pretty impossible to search for clinical trials,” noted Bill Burke, a PF patient and support group leader from Williamsburg, Va., in the statement.
The tool, which is available both on the PFF website and as a free app, draws on information from ClinicalTrials.gov.
The Foundation intends for the new tool to give patients “a voice in their care process,” according to the statement.
“We want to empower patients to actively participate in identifying clinical trials,” said Harold R. Collard, MD, senior medical adviser of research and development for the Pulmonary Fibrosis Foundation.
More information about the trial finder is available on the PFF’s website.
The Pulmonary Fibrosis Foundation (PFF) has begun offering a tool to help patients navigate through more than 100 clinical trials aimed at advancing the treatment of pulmonary fibrosis, according to a statement from the Foundation.
The platform includes trials for patients with idiopathic pulmonary fibrosis.
“Before this, it was pretty impossible to search for clinical trials,” noted Bill Burke, a PF patient and support group leader from Williamsburg, Va., in the statement.
The tool, which is available both on the PFF website and as a free app, draws on information from ClinicalTrials.gov.
The Foundation intends for the new tool to give patients “a voice in their care process,” according to the statement.
“We want to empower patients to actively participate in identifying clinical trials,” said Harold R. Collard, MD, senior medical adviser of research and development for the Pulmonary Fibrosis Foundation.
More information about the trial finder is available on the PFF’s website.
Drug combo indicated for bacterial pneumonia
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.