User login
Consider measles vaccine booster in HIV-positive patients
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
REPORTING FROM ESPID 2019
Breastfeeding protects against intussusception
LJUBLJANA, SLOVENIA – in a German case-control study.
Two other potent risk factors for intussusception in children less than 1 year old were identified: a family history of intussusception, and an episode of gastroenteritis, Doris F. Oberle, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Oberle, of the Paul Ehrlich Institute in Langen, Germany, presented a retrospective study of 116 meticulously validated cases of intussusception in infancy treated at 19 German pediatric centers during 2010-2014 and 272 controls matched by birth month, sex, and location. A standardized interview was conducted with the parents of all study participants.
Rotavirus vaccine was added to the German national vaccination schedule in 2013. In a multivariate logistic regression analysis, the risk of intussusception was increased by 5.4-fold following the first dose of the vaccine, compared with nonrecipients. However, subsequent doses of rotavirus vaccine were not associated with any excess risk.
In addition, a family history of intussusception was linked to a 4.2-fold increased risk, while an episode of gastroenteritis during the first year of life was associated with a 4.7-fold elevated risk.
In a novel finding, breastfeeding was independently associated with a 44% reduction in the risk of intussusception, compared with that of bottle-fed babies.
The most common presenting signs and symptoms of intussusception were vomiting, abdominal pain, hematochezia, pallor, and reduced appetite, each present in at least half of affected infants.
Dr. Oberle reported having no financial conflicts regarding her study, supported by the Paul Ehrlich Institute.
LJUBLJANA, SLOVENIA – in a German case-control study.
Two other potent risk factors for intussusception in children less than 1 year old were identified: a family history of intussusception, and an episode of gastroenteritis, Doris F. Oberle, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Oberle, of the Paul Ehrlich Institute in Langen, Germany, presented a retrospective study of 116 meticulously validated cases of intussusception in infancy treated at 19 German pediatric centers during 2010-2014 and 272 controls matched by birth month, sex, and location. A standardized interview was conducted with the parents of all study participants.
Rotavirus vaccine was added to the German national vaccination schedule in 2013. In a multivariate logistic regression analysis, the risk of intussusception was increased by 5.4-fold following the first dose of the vaccine, compared with nonrecipients. However, subsequent doses of rotavirus vaccine were not associated with any excess risk.
In addition, a family history of intussusception was linked to a 4.2-fold increased risk, while an episode of gastroenteritis during the first year of life was associated with a 4.7-fold elevated risk.
In a novel finding, breastfeeding was independently associated with a 44% reduction in the risk of intussusception, compared with that of bottle-fed babies.
The most common presenting signs and symptoms of intussusception were vomiting, abdominal pain, hematochezia, pallor, and reduced appetite, each present in at least half of affected infants.
Dr. Oberle reported having no financial conflicts regarding her study, supported by the Paul Ehrlich Institute.
LJUBLJANA, SLOVENIA – in a German case-control study.
Two other potent risk factors for intussusception in children less than 1 year old were identified: a family history of intussusception, and an episode of gastroenteritis, Doris F. Oberle, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Oberle, of the Paul Ehrlich Institute in Langen, Germany, presented a retrospective study of 116 meticulously validated cases of intussusception in infancy treated at 19 German pediatric centers during 2010-2014 and 272 controls matched by birth month, sex, and location. A standardized interview was conducted with the parents of all study participants.
Rotavirus vaccine was added to the German national vaccination schedule in 2013. In a multivariate logistic regression analysis, the risk of intussusception was increased by 5.4-fold following the first dose of the vaccine, compared with nonrecipients. However, subsequent doses of rotavirus vaccine were not associated with any excess risk.
In addition, a family history of intussusception was linked to a 4.2-fold increased risk, while an episode of gastroenteritis during the first year of life was associated with a 4.7-fold elevated risk.
In a novel finding, breastfeeding was independently associated with a 44% reduction in the risk of intussusception, compared with that of bottle-fed babies.
The most common presenting signs and symptoms of intussusception were vomiting, abdominal pain, hematochezia, pallor, and reduced appetite, each present in at least half of affected infants.
Dr. Oberle reported having no financial conflicts regarding her study, supported by the Paul Ehrlich Institute.
REPORTING FROM ESPID 2019
Expanded indication being considered for meningococcal group B vaccine
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
EXPERT ANALYSIS FROM ESPID 2019
Five rules for evaluating melanonychia
WAIKOLOA, HAWAII – Many dermatologists find melanonychia to be intimidating. The clinical features are ambiguous, and the prospect of doing a painful nail apparatus biopsy can be daunting for the inexperienced. As a result, the biopsy gets delayed and melanoma of the nail is often initially a missed diagnosis, not uncommonly for years, with devastating consequences.
Here are five at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Rule #1: Always look beyond the nail
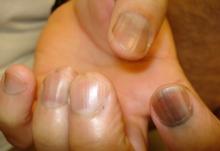
When a light-skinned person presents with more than one nail with pigmentation, the likelihood that one of them is melanoma is much less than if there is only one nail with melanonychia, according to Dr. Jellinek, a dermatologist in private practice in East Greenwich, R.I.
Also, be sure to look at the skin and mucosa. Consider the medications the patients may be taking: For example, cyclophosphamide (Cytoxan) is notorious for causing nail changes as a side effect. A past medical history of lichen planus, carpal tunnel syndrome, Addison disease, or other conditions may explain the melanonychia.
Laugier-Hunziker syndrome is a condition worth getting to know. It’s an acquired disorder characterized longitudinal melanonychia and other pigmentary changes, which may include diffuse hyperpigmentation of the orolabial mucosa, ocular pigment, and/or pigmented palmoplantar lesions. It’s said to be rare, but Dr. Jellinek disagrees.
“Learn this one if you don’t know it. I see a case about every 2 weeks. It’s not heritable and not associated with any other medical condition,” he said.
Rule #2: Your dermatoscope is great for nails
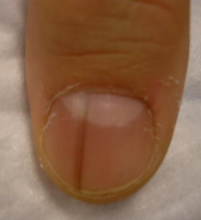
What Dr. Jellinek considers to be among the all-time best papers on the value of dermoscopy for nail pigmentation was authored by French investigators. They analyzed 148 consecutive cases of longitudinal melanonychia and concluded that the dermoscopic combination of a brown background coupled with irregular longitudinal lines in terms of color, spacing, diameter, and/or lack of parallelism strongly suggests melanoma. A micro-Hutchinson’s sign, while a rare finding, occurred only in melanoma, where it represented periungual spread of a radial growth phase malignancy (Arch Dermatol. 2002 Oct;138[10]:1327-33).
“I think nail dermoscopy is most helpful for subungual hemorrhage. I average one referral per week for hemorrhage under the nail. On dermoscopy it’s as if someone took paint and threw it at the nail. Purple to brown blood spots, with no background color. This should be a doorway diagnosis of hemorrhage,” Dr. Jellinek said.
Rule #3: Know when you don’t know
“This is really the key for me,” the dermatologist commented. “There are automatic cases for biopsy, and more commonly routine cases for reassurance. But the gray zone, when you know you don’t know, is the key decision making moment.”
When something just doesn’t feel right, there’s absolutely nothing wrong with getting a second opinion, he stressed.
“It’s worthwhile getting to know people whose opinions you trust. There’s a saying I like to teach our fellows: ‘Never worry alone.’ So if you’re worried about someone, listen to that inner voice. There’s no shame in getting a second opinion. It’s great! Patients are never upset, either. They feel really well taken care of,” he said.
Rule #4: Don’t wimp out when a biopsy is warranted
Many dermatologists hem and haw about doing a biopsy for a concerning lesion on the nail, when they wouldn’t hesitate to biopsy a similarly suspicious lesion on the face.
But it’s essential to biopsy the right area, he added. For longitudinal melanonychia, that’s the matrix. The nail plate is the wrong place; a biopsy obtained there will result in an inappropriate benign diagnosis.
“The starter set is to do a punch biopsy. This is your gateway drug to the world of nail surgery. Lots of dermatologists are intimidated by nail surgery, but if you can do any minor surgery, you can do a punch of the matrix. All it takes is a little practice. And if all you can do is punch biopsies, you’re good for your career. If you can do that, you’re golden. There are people who’ve just done punch biopsies for their whole career and they don’t miss melanomas,” he said.
Step one is to undermine the proximal nail fold using a pediatric elevator, which costs only about $30. “If you’re going to do a lot of nail surgery, they’re really helpful,” he said.
There’s no need at all to evulse the nail. Just make oblique incisions in the proximal nail fold in order to reflect it and look at the matrix. A 3-mm punch is standard, directed right over the origin of the pigment. Resist the temptation to force or squeeze the specimen in order to extract it. Instead, use really fine-tipped scissors to nibble at the base of the specimen, then gently pull it out, making an effort to keep the nail plate attached to the digit and avoid getting it stuck up in the punch.
Rule #5: Have dermatopathologists extensively experienced with nail pathology on your Rolodex
The histopathologic findings present in early subungual melanoma in situ are often too subtle for general dermatopathologists to appreciate, in Dr. Jellinek’s experience. He cited other investigators’ study of 18 cases of subungual melanoma in situ, all marked by longitudinal melanonychia. Only half showed the classic giveaway on the original nail matrix biopsy, consisting of a significantly increased number of atypical melanocytes with marked nuclear atypia. Blatant pagetoid spread was infrequent. However, all 18 cases displayed a novel, more subtle, and previously undescribed finding: haphazard and uneven distribution of atypical solitary melanocytes with variably sized and shaped hyperchromatic nuclei (J Cutan Pathol. 2016 Jan;43[1]:41-52).
Dr. Jellinek reported having no financial conflicts regarding his presentation. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Many dermatologists find melanonychia to be intimidating. The clinical features are ambiguous, and the prospect of doing a painful nail apparatus biopsy can be daunting for the inexperienced. As a result, the biopsy gets delayed and melanoma of the nail is often initially a missed diagnosis, not uncommonly for years, with devastating consequences.
Here are five at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Rule #1: Always look beyond the nail
When a light-skinned person presents with more than one nail with pigmentation, the likelihood that one of them is melanoma is much less than if there is only one nail with melanonychia, according to Dr. Jellinek, a dermatologist in private practice in East Greenwich, R.I.
Also, be sure to look at the skin and mucosa. Consider the medications the patients may be taking: For example, cyclophosphamide (Cytoxan) is notorious for causing nail changes as a side effect. A past medical history of lichen planus, carpal tunnel syndrome, Addison disease, or other conditions may explain the melanonychia.
Laugier-Hunziker syndrome is a condition worth getting to know. It’s an acquired disorder characterized longitudinal melanonychia and other pigmentary changes, which may include diffuse hyperpigmentation of the orolabial mucosa, ocular pigment, and/or pigmented palmoplantar lesions. It’s said to be rare, but Dr. Jellinek disagrees.
“Learn this one if you don’t know it. I see a case about every 2 weeks. It’s not heritable and not associated with any other medical condition,” he said.
Rule #2: Your dermatoscope is great for nails
What Dr. Jellinek considers to be among the all-time best papers on the value of dermoscopy for nail pigmentation was authored by French investigators. They analyzed 148 consecutive cases of longitudinal melanonychia and concluded that the dermoscopic combination of a brown background coupled with irregular longitudinal lines in terms of color, spacing, diameter, and/or lack of parallelism strongly suggests melanoma. A micro-Hutchinson’s sign, while a rare finding, occurred only in melanoma, where it represented periungual spread of a radial growth phase malignancy (Arch Dermatol. 2002 Oct;138[10]:1327-33).
“I think nail dermoscopy is most helpful for subungual hemorrhage. I average one referral per week for hemorrhage under the nail. On dermoscopy it’s as if someone took paint and threw it at the nail. Purple to brown blood spots, with no background color. This should be a doorway diagnosis of hemorrhage,” Dr. Jellinek said.
Rule #3: Know when you don’t know
“This is really the key for me,” the dermatologist commented. “There are automatic cases for biopsy, and more commonly routine cases for reassurance. But the gray zone, when you know you don’t know, is the key decision making moment.”
When something just doesn’t feel right, there’s absolutely nothing wrong with getting a second opinion, he stressed.
“It’s worthwhile getting to know people whose opinions you trust. There’s a saying I like to teach our fellows: ‘Never worry alone.’ So if you’re worried about someone, listen to that inner voice. There’s no shame in getting a second opinion. It’s great! Patients are never upset, either. They feel really well taken care of,” he said.
Rule #4: Don’t wimp out when a biopsy is warranted
Many dermatologists hem and haw about doing a biopsy for a concerning lesion on the nail, when they wouldn’t hesitate to biopsy a similarly suspicious lesion on the face.
But it’s essential to biopsy the right area, he added. For longitudinal melanonychia, that’s the matrix. The nail plate is the wrong place; a biopsy obtained there will result in an inappropriate benign diagnosis.
“The starter set is to do a punch biopsy. This is your gateway drug to the world of nail surgery. Lots of dermatologists are intimidated by nail surgery, but if you can do any minor surgery, you can do a punch of the matrix. All it takes is a little practice. And if all you can do is punch biopsies, you’re good for your career. If you can do that, you’re golden. There are people who’ve just done punch biopsies for their whole career and they don’t miss melanomas,” he said.
Step one is to undermine the proximal nail fold using a pediatric elevator, which costs only about $30. “If you’re going to do a lot of nail surgery, they’re really helpful,” he said.
There’s no need at all to evulse the nail. Just make oblique incisions in the proximal nail fold in order to reflect it and look at the matrix. A 3-mm punch is standard, directed right over the origin of the pigment. Resist the temptation to force or squeeze the specimen in order to extract it. Instead, use really fine-tipped scissors to nibble at the base of the specimen, then gently pull it out, making an effort to keep the nail plate attached to the digit and avoid getting it stuck up in the punch.
Rule #5: Have dermatopathologists extensively experienced with nail pathology on your Rolodex
The histopathologic findings present in early subungual melanoma in situ are often too subtle for general dermatopathologists to appreciate, in Dr. Jellinek’s experience. He cited other investigators’ study of 18 cases of subungual melanoma in situ, all marked by longitudinal melanonychia. Only half showed the classic giveaway on the original nail matrix biopsy, consisting of a significantly increased number of atypical melanocytes with marked nuclear atypia. Blatant pagetoid spread was infrequent. However, all 18 cases displayed a novel, more subtle, and previously undescribed finding: haphazard and uneven distribution of atypical solitary melanocytes with variably sized and shaped hyperchromatic nuclei (J Cutan Pathol. 2016 Jan;43[1]:41-52).
Dr. Jellinek reported having no financial conflicts regarding his presentation. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Many dermatologists find melanonychia to be intimidating. The clinical features are ambiguous, and the prospect of doing a painful nail apparatus biopsy can be daunting for the inexperienced. As a result, the biopsy gets delayed and melanoma of the nail is often initially a missed diagnosis, not uncommonly for years, with devastating consequences.
Here are five at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Rule #1: Always look beyond the nail
When a light-skinned person presents with more than one nail with pigmentation, the likelihood that one of them is melanoma is much less than if there is only one nail with melanonychia, according to Dr. Jellinek, a dermatologist in private practice in East Greenwich, R.I.
Also, be sure to look at the skin and mucosa. Consider the medications the patients may be taking: For example, cyclophosphamide (Cytoxan) is notorious for causing nail changes as a side effect. A past medical history of lichen planus, carpal tunnel syndrome, Addison disease, or other conditions may explain the melanonychia.
Laugier-Hunziker syndrome is a condition worth getting to know. It’s an acquired disorder characterized longitudinal melanonychia and other pigmentary changes, which may include diffuse hyperpigmentation of the orolabial mucosa, ocular pigment, and/or pigmented palmoplantar lesions. It’s said to be rare, but Dr. Jellinek disagrees.
“Learn this one if you don’t know it. I see a case about every 2 weeks. It’s not heritable and not associated with any other medical condition,” he said.
Rule #2: Your dermatoscope is great for nails
What Dr. Jellinek considers to be among the all-time best papers on the value of dermoscopy for nail pigmentation was authored by French investigators. They analyzed 148 consecutive cases of longitudinal melanonychia and concluded that the dermoscopic combination of a brown background coupled with irregular longitudinal lines in terms of color, spacing, diameter, and/or lack of parallelism strongly suggests melanoma. A micro-Hutchinson’s sign, while a rare finding, occurred only in melanoma, where it represented periungual spread of a radial growth phase malignancy (Arch Dermatol. 2002 Oct;138[10]:1327-33).
“I think nail dermoscopy is most helpful for subungual hemorrhage. I average one referral per week for hemorrhage under the nail. On dermoscopy it’s as if someone took paint and threw it at the nail. Purple to brown blood spots, with no background color. This should be a doorway diagnosis of hemorrhage,” Dr. Jellinek said.
Rule #3: Know when you don’t know
“This is really the key for me,” the dermatologist commented. “There are automatic cases for biopsy, and more commonly routine cases for reassurance. But the gray zone, when you know you don’t know, is the key decision making moment.”
When something just doesn’t feel right, there’s absolutely nothing wrong with getting a second opinion, he stressed.
“It’s worthwhile getting to know people whose opinions you trust. There’s a saying I like to teach our fellows: ‘Never worry alone.’ So if you’re worried about someone, listen to that inner voice. There’s no shame in getting a second opinion. It’s great! Patients are never upset, either. They feel really well taken care of,” he said.
Rule #4: Don’t wimp out when a biopsy is warranted
Many dermatologists hem and haw about doing a biopsy for a concerning lesion on the nail, when they wouldn’t hesitate to biopsy a similarly suspicious lesion on the face.
But it’s essential to biopsy the right area, he added. For longitudinal melanonychia, that’s the matrix. The nail plate is the wrong place; a biopsy obtained there will result in an inappropriate benign diagnosis.
“The starter set is to do a punch biopsy. This is your gateway drug to the world of nail surgery. Lots of dermatologists are intimidated by nail surgery, but if you can do any minor surgery, you can do a punch of the matrix. All it takes is a little practice. And if all you can do is punch biopsies, you’re good for your career. If you can do that, you’re golden. There are people who’ve just done punch biopsies for their whole career and they don’t miss melanomas,” he said.
Step one is to undermine the proximal nail fold using a pediatric elevator, which costs only about $30. “If you’re going to do a lot of nail surgery, they’re really helpful,” he said.
There’s no need at all to evulse the nail. Just make oblique incisions in the proximal nail fold in order to reflect it and look at the matrix. A 3-mm punch is standard, directed right over the origin of the pigment. Resist the temptation to force or squeeze the specimen in order to extract it. Instead, use really fine-tipped scissors to nibble at the base of the specimen, then gently pull it out, making an effort to keep the nail plate attached to the digit and avoid getting it stuck up in the punch.
Rule #5: Have dermatopathologists extensively experienced with nail pathology on your Rolodex
The histopathologic findings present in early subungual melanoma in situ are often too subtle for general dermatopathologists to appreciate, in Dr. Jellinek’s experience. He cited other investigators’ study of 18 cases of subungual melanoma in situ, all marked by longitudinal melanonychia. Only half showed the classic giveaway on the original nail matrix biopsy, consisting of a significantly increased number of atypical melanocytes with marked nuclear atypia. Blatant pagetoid spread was infrequent. However, all 18 cases displayed a novel, more subtle, and previously undescribed finding: haphazard and uneven distribution of atypical solitary melanocytes with variably sized and shaped hyperchromatic nuclei (J Cutan Pathol. 2016 Jan;43[1]:41-52).
Dr. Jellinek reported having no financial conflicts regarding his presentation. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Methotrexate significantly reduced knee OA pain
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.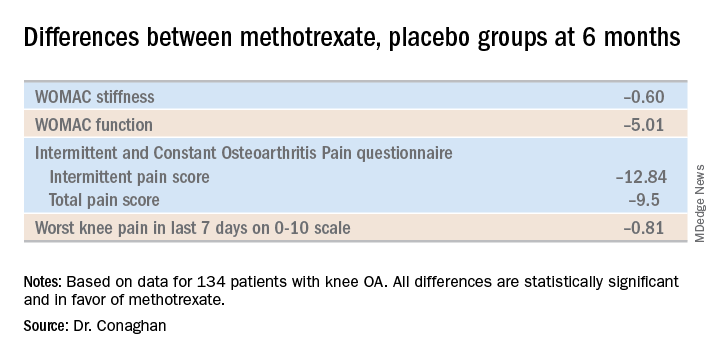
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
REPORTING FROM OARSI 2019
Obesity doesn’t hamper flu vaccine response in pregnancy
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
REPORTING FROM ESPID 2019
Key clinical point: High BMI doesn’t impair influenza vaccine responses in pregnant women.
Major finding: Protective antibody levels against all three vaccine antigens were documented 1 month post vaccination in 92% of the obese and 74% of the nonobese mothers.
Study details: This was a prospective observational study of 90 women vaccinated against influenza during pregnancy, 24 of whom were obese.
Disclosures: The study was supported by the University of Adelaide Women’s and Children’s Hospital Research Foundation.
Some Brits snuff out TORCH screen to raise awareness of congenital syphilis
LJUBLJANA, SLOVENIA – Pediatricians in the south of England are so concerned about the recent national increase in the diagnosis of syphilis in adults and its ramifications for neonates that they’ve ditched the traditional TORCH newborn screen because the acronym doesn’t specifically remind clinicians to think about congenital syphilis, Mildred A. Iro, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“ explained Dr. Iro of the University of Southampton (England).
She highlighted salient features of three recent cases of congenital syphilis managed at Southampton Children’s Hospital.
“The key message that we’d like to share is that we just need to be more aware about congenital syphilis. Retest mothers if their risk factor status changes, and test suspected infants and children,” Dr. Iro said.
As a practical matter, however, even though current guidelines recommend retesting mothers whose risk factor status becomes heightened following an initial negative syphilis serology result early in pregnancy, clinicians often are unaware that a mother’s risk status has changed. And retesting all mothers during pregnancy isn’t attractive from a cost-benefit standpoint. This makes scrupulous screening of newborns all the more important. And yet TORCH, which stands for Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes infections, isn’t an acronym that promotes awareness of congenital syphilis, a disease which occupies an obscure position in TORCH under the “O” for “Other” heading. That’s why the term “congenital infection screen” has become the new norm in the south of England, she explained.
However, one pediatrician who didn’t consider congenital infection screen to be an improvement in terminology over TORCH had an alternative suggestion, which struck a favorable chord with his fellow audience members: Simply change the acronym to TORCHS, with the S standing for syphilis.
Dr. Iro noted that two of the three affected children were diagnosed at age 7-8 weeks. The third wasn’t diagnosed until age 15 months, when the mother tested positive for syphilis in a subsequent pregnancy. As is typical of the disease known as “the great masquerader,” while all three of the affected children were unwell early in infancy, they presented with a wide range of symptoms. Among the more prominent features were prolonged irritability, respiratory distress, odd rashes, anemia, hepatomegaly, and tachypnea. One infant had reduced movement and pain in one arm.
All three children underwent extensive testing. None had neurosyphilis. All achieved good outcomes on standard guideline-directed therapy.
As for the mothers, they were aged 19, 21, and 23 years when diagnosed with syphilis. All were Caucasian, and antenatal blood testing was negative in all three. None were retested during pregnancy, even though two of them had a male partner or former partner who was positive for syphilis, and the partner of the third disclosed to her that he had sex with men.
At diagnosis, all three women had a strongly positive Treponema pallidum particle agglutination assay, a high rapid plasma reagin, and a positive syphilis IgM assay.
Dr. Iro reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – Pediatricians in the south of England are so concerned about the recent national increase in the diagnosis of syphilis in adults and its ramifications for neonates that they’ve ditched the traditional TORCH newborn screen because the acronym doesn’t specifically remind clinicians to think about congenital syphilis, Mildred A. Iro, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“ explained Dr. Iro of the University of Southampton (England).
She highlighted salient features of three recent cases of congenital syphilis managed at Southampton Children’s Hospital.
“The key message that we’d like to share is that we just need to be more aware about congenital syphilis. Retest mothers if their risk factor status changes, and test suspected infants and children,” Dr. Iro said.
As a practical matter, however, even though current guidelines recommend retesting mothers whose risk factor status becomes heightened following an initial negative syphilis serology result early in pregnancy, clinicians often are unaware that a mother’s risk status has changed. And retesting all mothers during pregnancy isn’t attractive from a cost-benefit standpoint. This makes scrupulous screening of newborns all the more important. And yet TORCH, which stands for Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes infections, isn’t an acronym that promotes awareness of congenital syphilis, a disease which occupies an obscure position in TORCH under the “O” for “Other” heading. That’s why the term “congenital infection screen” has become the new norm in the south of England, she explained.
However, one pediatrician who didn’t consider congenital infection screen to be an improvement in terminology over TORCH had an alternative suggestion, which struck a favorable chord with his fellow audience members: Simply change the acronym to TORCHS, with the S standing for syphilis.
Dr. Iro noted that two of the three affected children were diagnosed at age 7-8 weeks. The third wasn’t diagnosed until age 15 months, when the mother tested positive for syphilis in a subsequent pregnancy. As is typical of the disease known as “the great masquerader,” while all three of the affected children were unwell early in infancy, they presented with a wide range of symptoms. Among the more prominent features were prolonged irritability, respiratory distress, odd rashes, anemia, hepatomegaly, and tachypnea. One infant had reduced movement and pain in one arm.
All three children underwent extensive testing. None had neurosyphilis. All achieved good outcomes on standard guideline-directed therapy.
As for the mothers, they were aged 19, 21, and 23 years when diagnosed with syphilis. All were Caucasian, and antenatal blood testing was negative in all three. None were retested during pregnancy, even though two of them had a male partner or former partner who was positive for syphilis, and the partner of the third disclosed to her that he had sex with men.
At diagnosis, all three women had a strongly positive Treponema pallidum particle agglutination assay, a high rapid plasma reagin, and a positive syphilis IgM assay.
Dr. Iro reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – Pediatricians in the south of England are so concerned about the recent national increase in the diagnosis of syphilis in adults and its ramifications for neonates that they’ve ditched the traditional TORCH newborn screen because the acronym doesn’t specifically remind clinicians to think about congenital syphilis, Mildred A. Iro, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“ explained Dr. Iro of the University of Southampton (England).
She highlighted salient features of three recent cases of congenital syphilis managed at Southampton Children’s Hospital.
“The key message that we’d like to share is that we just need to be more aware about congenital syphilis. Retest mothers if their risk factor status changes, and test suspected infants and children,” Dr. Iro said.
As a practical matter, however, even though current guidelines recommend retesting mothers whose risk factor status becomes heightened following an initial negative syphilis serology result early in pregnancy, clinicians often are unaware that a mother’s risk status has changed. And retesting all mothers during pregnancy isn’t attractive from a cost-benefit standpoint. This makes scrupulous screening of newborns all the more important. And yet TORCH, which stands for Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes infections, isn’t an acronym that promotes awareness of congenital syphilis, a disease which occupies an obscure position in TORCH under the “O” for “Other” heading. That’s why the term “congenital infection screen” has become the new norm in the south of England, she explained.
However, one pediatrician who didn’t consider congenital infection screen to be an improvement in terminology over TORCH had an alternative suggestion, which struck a favorable chord with his fellow audience members: Simply change the acronym to TORCHS, with the S standing for syphilis.
Dr. Iro noted that two of the three affected children were diagnosed at age 7-8 weeks. The third wasn’t diagnosed until age 15 months, when the mother tested positive for syphilis in a subsequent pregnancy. As is typical of the disease known as “the great masquerader,” while all three of the affected children were unwell early in infancy, they presented with a wide range of symptoms. Among the more prominent features were prolonged irritability, respiratory distress, odd rashes, anemia, hepatomegaly, and tachypnea. One infant had reduced movement and pain in one arm.
All three children underwent extensive testing. None had neurosyphilis. All achieved good outcomes on standard guideline-directed therapy.
As for the mothers, they were aged 19, 21, and 23 years when diagnosed with syphilis. All were Caucasian, and antenatal blood testing was negative in all three. None were retested during pregnancy, even though two of them had a male partner or former partner who was positive for syphilis, and the partner of the third disclosed to her that he had sex with men.
At diagnosis, all three women had a strongly positive Treponema pallidum particle agglutination assay, a high rapid plasma reagin, and a positive syphilis IgM assay.
Dr. Iro reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2019
C-section linked to serious infection in preschoolers
LJUBLJANA, SLOVENIA – Delivery by C-section – especially when elective – carries a significantly higher hospitalization risk for severe infection in the first 5 years of life than vaginal delivery in a study of nearly 7.3 million singleton deliveries in four asset-rich countries, David Burgner, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This is something that obstetricians might need to consider when discussing with the family the pros and cons for an elective C-section, particularly one that isn’t otherwise indicated for the baby or the mother,” said Dr. Burgner of the Murdoch Children’s Research Institute in Melbourne.
He presented an observational study of 7.29 million singleton births in Denmark, Great Britain, Scotland, and two Australian states during 1996-2015. C-section rates ranged from a low of 17.5% in Denmark to 29.4% in Western Australia, all of which are greater than the 10%-15% rate endorsed by the World Health Organization. Elective C-section rates varied by country from 39% to 57%. Of note, pediatric hospital care in all four countries is free, so economic considerations didn’t drive admission.
The impetus for this international collaboration was to gain new insight into the differential susceptibility to childhood infection, he explained.
“We know from our clinical practice that pretty much all of the children are exposed to pretty much all potentially serious pathogens during early life. And yet it’s only a minority that develop severe infection. It’s an extremely interesting scientific question and an extremely important clinical question as to what’s driving that differential susceptibility,” according to the pediatric infectious disease specialist.
There are a number of established risk factors for infection-related hospitalization in children, including parental smoking, maternal antibiotic exposure during pregnancy, and growth measurements at birth. Dr. Burgner and coinvestigators hypothesized that another important risk factor is the nature of the microbiome transmitted from mother to baby during delivery. This postnatal microbiome varies depending upon mode of delivery: Vaginal delivery transmits the maternal enteric microbiome, which they reasoned might be through direct immunomodulation that sets up protective immune responses early in life, especially against respiratory and gastrointestinal tract infections. In contrast, delivery by C-section causes the baby to pick up the maternal skin and hospital environment microbiomes, but not the maternal enteric microbiome.
Thus, the investigators hypothesized that C-section poses a greater risk of infection-related hospitalization during the first 5 years of life than does vaginal delivery, and that elective C-section poses a higher risk than does emergency C-section because it is more likely to involve rupture of membranes.
The center-specific rates of C-section and infection-related pediatric infection, when combined into a meta-analysis, bore out the study hypothesis. Emergency C-section was associated with a 9% greater risk of infection-related hospitalization through 5 years of age than was vaginal delivery, while elective C-section was associated with a 13% increased risk, both of which were statistically significant and clinically important.
“We were quite taken with these results. We think they provide evidence that C-section is consistently associated with infection-related hospitalization. It’s an association study that can’t prove causality, but the results implicate the postnatal microbiome as the most plausible explanation in terms of what’s driving this association,” according to Dr. Burgner.
The association between C-section and infection-related hospitalization was persistent throughout the preschool years. For example, the increased risk associated with elective C-section was 16% during age 0-3 months, 20% during months 4-6, 14% in months 7-12, 13% during ages 1-2 years, and 11% among 2- to 5-year-olds, he continued.
The increased risk of severe preschool infection was highest for upper and lower respiratory tract and gastrointestinal infections, which involve the organ systems most likely to experience direct inoculation of the maternal microbiome, he noted.
Because the investigators recognized that the study results were potentially vulnerable to confounding by indication – that is, that the reason for doing a C-section might itself confer increased risk of subsequent preschool infection-related hospitalization – they repeated their analysis in a predefined low-risk subpopulation. The results closely mirrored those in the overall study population: an 8% increased risk in the emergency C-section group and a 14% increased risk with elective C-section.
Results of this large multinational study should provide further support for ongoing research aimed at supporting the infant microbiome after delivery by C-section via vaginal microbial transfer and other methods, he observed.
Dr. Burgner reported having no financial conflicts regarding the study, which was cosponsored by the National Health and Medical Research Council of Australia, the Danish Council for Independent Research, and nonprofit foundations.
LJUBLJANA, SLOVENIA – Delivery by C-section – especially when elective – carries a significantly higher hospitalization risk for severe infection in the first 5 years of life than vaginal delivery in a study of nearly 7.3 million singleton deliveries in four asset-rich countries, David Burgner, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This is something that obstetricians might need to consider when discussing with the family the pros and cons for an elective C-section, particularly one that isn’t otherwise indicated for the baby or the mother,” said Dr. Burgner of the Murdoch Children’s Research Institute in Melbourne.
He presented an observational study of 7.29 million singleton births in Denmark, Great Britain, Scotland, and two Australian states during 1996-2015. C-section rates ranged from a low of 17.5% in Denmark to 29.4% in Western Australia, all of which are greater than the 10%-15% rate endorsed by the World Health Organization. Elective C-section rates varied by country from 39% to 57%. Of note, pediatric hospital care in all four countries is free, so economic considerations didn’t drive admission.
The impetus for this international collaboration was to gain new insight into the differential susceptibility to childhood infection, he explained.
“We know from our clinical practice that pretty much all of the children are exposed to pretty much all potentially serious pathogens during early life. And yet it’s only a minority that develop severe infection. It’s an extremely interesting scientific question and an extremely important clinical question as to what’s driving that differential susceptibility,” according to the pediatric infectious disease specialist.
There are a number of established risk factors for infection-related hospitalization in children, including parental smoking, maternal antibiotic exposure during pregnancy, and growth measurements at birth. Dr. Burgner and coinvestigators hypothesized that another important risk factor is the nature of the microbiome transmitted from mother to baby during delivery. This postnatal microbiome varies depending upon mode of delivery: Vaginal delivery transmits the maternal enteric microbiome, which they reasoned might be through direct immunomodulation that sets up protective immune responses early in life, especially against respiratory and gastrointestinal tract infections. In contrast, delivery by C-section causes the baby to pick up the maternal skin and hospital environment microbiomes, but not the maternal enteric microbiome.
Thus, the investigators hypothesized that C-section poses a greater risk of infection-related hospitalization during the first 5 years of life than does vaginal delivery, and that elective C-section poses a higher risk than does emergency C-section because it is more likely to involve rupture of membranes.
The center-specific rates of C-section and infection-related pediatric infection, when combined into a meta-analysis, bore out the study hypothesis. Emergency C-section was associated with a 9% greater risk of infection-related hospitalization through 5 years of age than was vaginal delivery, while elective C-section was associated with a 13% increased risk, both of which were statistically significant and clinically important.
“We were quite taken with these results. We think they provide evidence that C-section is consistently associated with infection-related hospitalization. It’s an association study that can’t prove causality, but the results implicate the postnatal microbiome as the most plausible explanation in terms of what’s driving this association,” according to Dr. Burgner.
The association between C-section and infection-related hospitalization was persistent throughout the preschool years. For example, the increased risk associated with elective C-section was 16% during age 0-3 months, 20% during months 4-6, 14% in months 7-12, 13% during ages 1-2 years, and 11% among 2- to 5-year-olds, he continued.
The increased risk of severe preschool infection was highest for upper and lower respiratory tract and gastrointestinal infections, which involve the organ systems most likely to experience direct inoculation of the maternal microbiome, he noted.
Because the investigators recognized that the study results were potentially vulnerable to confounding by indication – that is, that the reason for doing a C-section might itself confer increased risk of subsequent preschool infection-related hospitalization – they repeated their analysis in a predefined low-risk subpopulation. The results closely mirrored those in the overall study population: an 8% increased risk in the emergency C-section group and a 14% increased risk with elective C-section.
Results of this large multinational study should provide further support for ongoing research aimed at supporting the infant microbiome after delivery by C-section via vaginal microbial transfer and other methods, he observed.
Dr. Burgner reported having no financial conflicts regarding the study, which was cosponsored by the National Health and Medical Research Council of Australia, the Danish Council for Independent Research, and nonprofit foundations.
LJUBLJANA, SLOVENIA – Delivery by C-section – especially when elective – carries a significantly higher hospitalization risk for severe infection in the first 5 years of life than vaginal delivery in a study of nearly 7.3 million singleton deliveries in four asset-rich countries, David Burgner, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This is something that obstetricians might need to consider when discussing with the family the pros and cons for an elective C-section, particularly one that isn’t otherwise indicated for the baby or the mother,” said Dr. Burgner of the Murdoch Children’s Research Institute in Melbourne.
He presented an observational study of 7.29 million singleton births in Denmark, Great Britain, Scotland, and two Australian states during 1996-2015. C-section rates ranged from a low of 17.5% in Denmark to 29.4% in Western Australia, all of which are greater than the 10%-15% rate endorsed by the World Health Organization. Elective C-section rates varied by country from 39% to 57%. Of note, pediatric hospital care in all four countries is free, so economic considerations didn’t drive admission.
The impetus for this international collaboration was to gain new insight into the differential susceptibility to childhood infection, he explained.
“We know from our clinical practice that pretty much all of the children are exposed to pretty much all potentially serious pathogens during early life. And yet it’s only a minority that develop severe infection. It’s an extremely interesting scientific question and an extremely important clinical question as to what’s driving that differential susceptibility,” according to the pediatric infectious disease specialist.
There are a number of established risk factors for infection-related hospitalization in children, including parental smoking, maternal antibiotic exposure during pregnancy, and growth measurements at birth. Dr. Burgner and coinvestigators hypothesized that another important risk factor is the nature of the microbiome transmitted from mother to baby during delivery. This postnatal microbiome varies depending upon mode of delivery: Vaginal delivery transmits the maternal enteric microbiome, which they reasoned might be through direct immunomodulation that sets up protective immune responses early in life, especially against respiratory and gastrointestinal tract infections. In contrast, delivery by C-section causes the baby to pick up the maternal skin and hospital environment microbiomes, but not the maternal enteric microbiome.
Thus, the investigators hypothesized that C-section poses a greater risk of infection-related hospitalization during the first 5 years of life than does vaginal delivery, and that elective C-section poses a higher risk than does emergency C-section because it is more likely to involve rupture of membranes.
The center-specific rates of C-section and infection-related pediatric infection, when combined into a meta-analysis, bore out the study hypothesis. Emergency C-section was associated with a 9% greater risk of infection-related hospitalization through 5 years of age than was vaginal delivery, while elective C-section was associated with a 13% increased risk, both of which were statistically significant and clinically important.
“We were quite taken with these results. We think they provide evidence that C-section is consistently associated with infection-related hospitalization. It’s an association study that can’t prove causality, but the results implicate the postnatal microbiome as the most plausible explanation in terms of what’s driving this association,” according to Dr. Burgner.
The association between C-section and infection-related hospitalization was persistent throughout the preschool years. For example, the increased risk associated with elective C-section was 16% during age 0-3 months, 20% during months 4-6, 14% in months 7-12, 13% during ages 1-2 years, and 11% among 2- to 5-year-olds, he continued.
The increased risk of severe preschool infection was highest for upper and lower respiratory tract and gastrointestinal infections, which involve the organ systems most likely to experience direct inoculation of the maternal microbiome, he noted.
Because the investigators recognized that the study results were potentially vulnerable to confounding by indication – that is, that the reason for doing a C-section might itself confer increased risk of subsequent preschool infection-related hospitalization – they repeated their analysis in a predefined low-risk subpopulation. The results closely mirrored those in the overall study population: an 8% increased risk in the emergency C-section group and a 14% increased risk with elective C-section.
Results of this large multinational study should provide further support for ongoing research aimed at supporting the infant microbiome after delivery by C-section via vaginal microbial transfer and other methods, he observed.
Dr. Burgner reported having no financial conflicts regarding the study, which was cosponsored by the National Health and Medical Research Council of Australia, the Danish Council for Independent Research, and nonprofit foundations.
REPORTING FROM ESPID 2019
HPV vaccine: Is one dose enough?
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2019
Building better flu vaccines is daunting
LJUBLJANA, SLOVENIA – Don’t hold your breath waiting for a substantially better, more reliably effective influenza vaccine.
That was a key cautionary message provided by vaccine expert Edward A. Belongia, MD, at the annual meeting of the European Society for Paediatric Infectious Diseases.
The effectiveness of seasonal influenza vaccine varies from 10% to 60% year by year, leaving enormous room for improvement. But many obstacles exist to developing a more consistent and reliably effective version of the seasonal influenza vaccine. And the lofty goal of creating a universal vaccine is even more ambitious, although the National Institute of Allergy and Infectious Diseases has declared it to be a top priority and mapped out a strategic plan for getting there (J Infect Dis. 2018 Jul 2;218[3]:347-54).
“Ultimately the Holy Grail is a universal flu vaccine that would provide pan-A and pan-B protection that would last for more than 1 year, with protection against avian and pandemic viruses, and would work for both children and adults. We are nowhere near that. Every 5 years someone says we’re 5 years away, and then 5 years go by and we’re still 5 years away. So I’m not making any predictions on that,” said Dr. Belongia, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wisc.) Clinic Research Institute, which is part of the U.S. Influenza Vaccine Effectiveness Network.
One of the big problems in creating a more effective flu vaccine, particularly for children, is the H3N2 virus subtype. Dr. Belongia was first author of a systematic review and meta-analysis of studies of more than a dozen recent flu seasons showing that although vaccine effectiveness against H3N2 varied widely from year to year, it was consistently lower than against influenza type B and H1N1 (Lancet Infect Dis. 2016 Aug;16[8]:942-51).
And that’s especially true in children and adolescents. Notably, in the 2014-2015 U.S. flu season, vaccine effectiveness against H3N2 in children aged 6 months to 8 years was low at 23%, but shockingly lower at a mere 7% in the 9- to 17-year-olds. Whereas in the 2017-2018 season, vaccine effectiveness against H3N2 in the 9- to 17-year-olds jumped to 46% while remaining low but consistent at 22% in the younger children.
“We see a very different age pattern here for the older children compared to the younger children, and quite frankly we don’t really understand what’s doing this,” said Dr. Belongia.
What is well understood, however, is that the problematic performance of influenza vaccines when it comes to protecting against H3N2 is a complicated matter stemming from three sources: the virus itself; the current egg-based vaccine manufacturing methodology, which is now 7 decades old; and host factors.
That troublesome H3N2 virus
Antigenic evolution of the H3N2 virus occurs at a 5- to 6-fold higher rate than for influenza B virus and roughly 17-fold faster than for H1N1. That high mutation rate makes for a moving target that’s a real problem when trying to keep a vaccine current. Also, the globular head of the virus is prone to glycosylation, which enables the virus to evade immune detection.
Vaccine-related factors
It’s likely that the availability of the flu vaccine for the upcoming 2019-2020 season is going to be delayed because of late selection of the strains for inclusion. The World Health Organization ordinarily selects strains for vaccines for the Northern Hemisphere in February, giving vaccine manufacturers 6-8 months to produce their vaccines and ship them in time for administration from September through November. This year, however, the WHO delayed selection of the H3N2 component until March because of the high level of antigenic and genetic diversity of circulating strains.
“This hasn’t happened since 2003 – it’s a very rare occurrence – but it does increase the potential that there’s going to be a delay in the availability of the vaccine in the fall,” he explained.
Eventually, the WHO selected a new clade 3C.3a virus called A/Kansas/14/2017 for the 2019-2020 vaccine. It should cover the circulating strains of H3N2 “reasonably well,” according to the physician.
Another issue: H3N2 has become adapted to the mammalian environment, so growing the virus in eggs introduces strong selection pressure for mutations leading to reduced vaccine effectiveness. Yet only two flu vaccines licensed in the United States are manufactured without eggs: Flucelvax, marketed by Seqirus for patients aged 4 years and up, and Sanofi’s Flublok, which is licensed for individuals who are 18 years of age or older. Studies are underway looking at the relative effectiveness of egg-based versus cell culture-manufactured flu vaccines in real-world settings.
Host factors
Hemagglutinin imprinting, sometimes referred to as “original antigenic sin,” is a decades-old concept whereby early childhood exposure to influenza viruses shapes future vaccine response.
“It suggests there could be some birth cohort effects in vaccine responsiveness, depending on what was circulating in the first 2-3 years after birth. It would complicate vaccine strategy quite a bit if you had to have different strategies for different birth cohorts,” Dr. Belongia observed.
Another host factor issue is the controversial topic of negative interference stemming from repeated vaccinations. It’s unclear how important this is in the real world, because studies have been inconsistent. Reassuringly, Dr. Belongia and coworkers found no association between prior-season influenza vaccination and diminished vaccine effectiveness in 3,369 U.S. children aged 2-17 years studied during the 2013-14 through 2015-16 flu seasons (JAMA Netw Open. 2018 Oct 5;1[6]:e183742. doi: 10.1001/jamanetworkopen.2018.3742).
“We found no suggestion at all of a problem with being vaccinated two seasons in a row,” according to Dr. Belongia.
How to build a better influenza vaccine for children
“I would say that even before we get to a universal vaccine, the next generation of flu vaccines that are more effective are not going to be manufactured using eggs, although we’re not real close to that. But I think that’s eventually where we’re going,” he said.
“I think it’s going to take a systems biology approach in order to really understand the adaptive immune response to infection and vaccination in early life. That means a much more detailed understanding of what is underlying the imprinting mechanisms and what is the adaptive response to repeated vaccination and infection. I think this is going to take prospective infant cohort studies; the National Institutes of Health is funding some that will begin within the next year,” Dr. Belongia added.
Many investigational approaches to improving influenza virus subtype-level protection are being explored. These include novel adjuvants, nanoparticle vaccines, computationally optimized broadly reactive antigens, and standardization of neuraminidase content.
And as for the much-desired universal flu vaccine?
“I will say that if a universal vaccine is going to work it’s probably going to work first in children. They have a much shorter immune history and their antibody landscape is a lot smaller, so you have a much better opportunity, I think, to generate a broad response to a universal vaccine compared to adults, who have much more complex immune landscapes,” he said.
Dr. Belongia reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – Don’t hold your breath waiting for a substantially better, more reliably effective influenza vaccine.
That was a key cautionary message provided by vaccine expert Edward A. Belongia, MD, at the annual meeting of the European Society for Paediatric Infectious Diseases.
The effectiveness of seasonal influenza vaccine varies from 10% to 60% year by year, leaving enormous room for improvement. But many obstacles exist to developing a more consistent and reliably effective version of the seasonal influenza vaccine. And the lofty goal of creating a universal vaccine is even more ambitious, although the National Institute of Allergy and Infectious Diseases has declared it to be a top priority and mapped out a strategic plan for getting there (J Infect Dis. 2018 Jul 2;218[3]:347-54).
“Ultimately the Holy Grail is a universal flu vaccine that would provide pan-A and pan-B protection that would last for more than 1 year, with protection against avian and pandemic viruses, and would work for both children and adults. We are nowhere near that. Every 5 years someone says we’re 5 years away, and then 5 years go by and we’re still 5 years away. So I’m not making any predictions on that,” said Dr. Belongia, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wisc.) Clinic Research Institute, which is part of the U.S. Influenza Vaccine Effectiveness Network.
One of the big problems in creating a more effective flu vaccine, particularly for children, is the H3N2 virus subtype. Dr. Belongia was first author of a systematic review and meta-analysis of studies of more than a dozen recent flu seasons showing that although vaccine effectiveness against H3N2 varied widely from year to year, it was consistently lower than against influenza type B and H1N1 (Lancet Infect Dis. 2016 Aug;16[8]:942-51).
And that’s especially true in children and adolescents. Notably, in the 2014-2015 U.S. flu season, vaccine effectiveness against H3N2 in children aged 6 months to 8 years was low at 23%, but shockingly lower at a mere 7% in the 9- to 17-year-olds. Whereas in the 2017-2018 season, vaccine effectiveness against H3N2 in the 9- to 17-year-olds jumped to 46% while remaining low but consistent at 22% in the younger children.
“We see a very different age pattern here for the older children compared to the younger children, and quite frankly we don’t really understand what’s doing this,” said Dr. Belongia.
What is well understood, however, is that the problematic performance of influenza vaccines when it comes to protecting against H3N2 is a complicated matter stemming from three sources: the virus itself; the current egg-based vaccine manufacturing methodology, which is now 7 decades old; and host factors.
That troublesome H3N2 virus
Antigenic evolution of the H3N2 virus occurs at a 5- to 6-fold higher rate than for influenza B virus and roughly 17-fold faster than for H1N1. That high mutation rate makes for a moving target that’s a real problem when trying to keep a vaccine current. Also, the globular head of the virus is prone to glycosylation, which enables the virus to evade immune detection.
Vaccine-related factors
It’s likely that the availability of the flu vaccine for the upcoming 2019-2020 season is going to be delayed because of late selection of the strains for inclusion. The World Health Organization ordinarily selects strains for vaccines for the Northern Hemisphere in February, giving vaccine manufacturers 6-8 months to produce their vaccines and ship them in time for administration from September through November. This year, however, the WHO delayed selection of the H3N2 component until March because of the high level of antigenic and genetic diversity of circulating strains.
“This hasn’t happened since 2003 – it’s a very rare occurrence – but it does increase the potential that there’s going to be a delay in the availability of the vaccine in the fall,” he explained.
Eventually, the WHO selected a new clade 3C.3a virus called A/Kansas/14/2017 for the 2019-2020 vaccine. It should cover the circulating strains of H3N2 “reasonably well,” according to the physician.
Another issue: H3N2 has become adapted to the mammalian environment, so growing the virus in eggs introduces strong selection pressure for mutations leading to reduced vaccine effectiveness. Yet only two flu vaccines licensed in the United States are manufactured without eggs: Flucelvax, marketed by Seqirus for patients aged 4 years and up, and Sanofi’s Flublok, which is licensed for individuals who are 18 years of age or older. Studies are underway looking at the relative effectiveness of egg-based versus cell culture-manufactured flu vaccines in real-world settings.
Host factors
Hemagglutinin imprinting, sometimes referred to as “original antigenic sin,” is a decades-old concept whereby early childhood exposure to influenza viruses shapes future vaccine response.
“It suggests there could be some birth cohort effects in vaccine responsiveness, depending on what was circulating in the first 2-3 years after birth. It would complicate vaccine strategy quite a bit if you had to have different strategies for different birth cohorts,” Dr. Belongia observed.
Another host factor issue is the controversial topic of negative interference stemming from repeated vaccinations. It’s unclear how important this is in the real world, because studies have been inconsistent. Reassuringly, Dr. Belongia and coworkers found no association between prior-season influenza vaccination and diminished vaccine effectiveness in 3,369 U.S. children aged 2-17 years studied during the 2013-14 through 2015-16 flu seasons (JAMA Netw Open. 2018 Oct 5;1[6]:e183742. doi: 10.1001/jamanetworkopen.2018.3742).
“We found no suggestion at all of a problem with being vaccinated two seasons in a row,” according to Dr. Belongia.
How to build a better influenza vaccine for children
“I would say that even before we get to a universal vaccine, the next generation of flu vaccines that are more effective are not going to be manufactured using eggs, although we’re not real close to that. But I think that’s eventually where we’re going,” he said.
“I think it’s going to take a systems biology approach in order to really understand the adaptive immune response to infection and vaccination in early life. That means a much more detailed understanding of what is underlying the imprinting mechanisms and what is the adaptive response to repeated vaccination and infection. I think this is going to take prospective infant cohort studies; the National Institutes of Health is funding some that will begin within the next year,” Dr. Belongia added.
Many investigational approaches to improving influenza virus subtype-level protection are being explored. These include novel adjuvants, nanoparticle vaccines, computationally optimized broadly reactive antigens, and standardization of neuraminidase content.
And as for the much-desired universal flu vaccine?
“I will say that if a universal vaccine is going to work it’s probably going to work first in children. They have a much shorter immune history and their antibody landscape is a lot smaller, so you have a much better opportunity, I think, to generate a broad response to a universal vaccine compared to adults, who have much more complex immune landscapes,” he said.
Dr. Belongia reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – Don’t hold your breath waiting for a substantially better, more reliably effective influenza vaccine.
That was a key cautionary message provided by vaccine expert Edward A. Belongia, MD, at the annual meeting of the European Society for Paediatric Infectious Diseases.
The effectiveness of seasonal influenza vaccine varies from 10% to 60% year by year, leaving enormous room for improvement. But many obstacles exist to developing a more consistent and reliably effective version of the seasonal influenza vaccine. And the lofty goal of creating a universal vaccine is even more ambitious, although the National Institute of Allergy and Infectious Diseases has declared it to be a top priority and mapped out a strategic plan for getting there (J Infect Dis. 2018 Jul 2;218[3]:347-54).
“Ultimately the Holy Grail is a universal flu vaccine that would provide pan-A and pan-B protection that would last for more than 1 year, with protection against avian and pandemic viruses, and would work for both children and adults. We are nowhere near that. Every 5 years someone says we’re 5 years away, and then 5 years go by and we’re still 5 years away. So I’m not making any predictions on that,” said Dr. Belongia, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wisc.) Clinic Research Institute, which is part of the U.S. Influenza Vaccine Effectiveness Network.
One of the big problems in creating a more effective flu vaccine, particularly for children, is the H3N2 virus subtype. Dr. Belongia was first author of a systematic review and meta-analysis of studies of more than a dozen recent flu seasons showing that although vaccine effectiveness against H3N2 varied widely from year to year, it was consistently lower than against influenza type B and H1N1 (Lancet Infect Dis. 2016 Aug;16[8]:942-51).
And that’s especially true in children and adolescents. Notably, in the 2014-2015 U.S. flu season, vaccine effectiveness against H3N2 in children aged 6 months to 8 years was low at 23%, but shockingly lower at a mere 7% in the 9- to 17-year-olds. Whereas in the 2017-2018 season, vaccine effectiveness against H3N2 in the 9- to 17-year-olds jumped to 46% while remaining low but consistent at 22% in the younger children.
“We see a very different age pattern here for the older children compared to the younger children, and quite frankly we don’t really understand what’s doing this,” said Dr. Belongia.
What is well understood, however, is that the problematic performance of influenza vaccines when it comes to protecting against H3N2 is a complicated matter stemming from three sources: the virus itself; the current egg-based vaccine manufacturing methodology, which is now 7 decades old; and host factors.
That troublesome H3N2 virus
Antigenic evolution of the H3N2 virus occurs at a 5- to 6-fold higher rate than for influenza B virus and roughly 17-fold faster than for H1N1. That high mutation rate makes for a moving target that’s a real problem when trying to keep a vaccine current. Also, the globular head of the virus is prone to glycosylation, which enables the virus to evade immune detection.
Vaccine-related factors
It’s likely that the availability of the flu vaccine for the upcoming 2019-2020 season is going to be delayed because of late selection of the strains for inclusion. The World Health Organization ordinarily selects strains for vaccines for the Northern Hemisphere in February, giving vaccine manufacturers 6-8 months to produce their vaccines and ship them in time for administration from September through November. This year, however, the WHO delayed selection of the H3N2 component until March because of the high level of antigenic and genetic diversity of circulating strains.
“This hasn’t happened since 2003 – it’s a very rare occurrence – but it does increase the potential that there’s going to be a delay in the availability of the vaccine in the fall,” he explained.
Eventually, the WHO selected a new clade 3C.3a virus called A/Kansas/14/2017 for the 2019-2020 vaccine. It should cover the circulating strains of H3N2 “reasonably well,” according to the physician.
Another issue: H3N2 has become adapted to the mammalian environment, so growing the virus in eggs introduces strong selection pressure for mutations leading to reduced vaccine effectiveness. Yet only two flu vaccines licensed in the United States are manufactured without eggs: Flucelvax, marketed by Seqirus for patients aged 4 years and up, and Sanofi’s Flublok, which is licensed for individuals who are 18 years of age or older. Studies are underway looking at the relative effectiveness of egg-based versus cell culture-manufactured flu vaccines in real-world settings.
Host factors
Hemagglutinin imprinting, sometimes referred to as “original antigenic sin,” is a decades-old concept whereby early childhood exposure to influenza viruses shapes future vaccine response.
“It suggests there could be some birth cohort effects in vaccine responsiveness, depending on what was circulating in the first 2-3 years after birth. It would complicate vaccine strategy quite a bit if you had to have different strategies for different birth cohorts,” Dr. Belongia observed.
Another host factor issue is the controversial topic of negative interference stemming from repeated vaccinations. It’s unclear how important this is in the real world, because studies have been inconsistent. Reassuringly, Dr. Belongia and coworkers found no association between prior-season influenza vaccination and diminished vaccine effectiveness in 3,369 U.S. children aged 2-17 years studied during the 2013-14 through 2015-16 flu seasons (JAMA Netw Open. 2018 Oct 5;1[6]:e183742. doi: 10.1001/jamanetworkopen.2018.3742).
“We found no suggestion at all of a problem with being vaccinated two seasons in a row,” according to Dr. Belongia.
How to build a better influenza vaccine for children
“I would say that even before we get to a universal vaccine, the next generation of flu vaccines that are more effective are not going to be manufactured using eggs, although we’re not real close to that. But I think that’s eventually where we’re going,” he said.
“I think it’s going to take a systems biology approach in order to really understand the adaptive immune response to infection and vaccination in early life. That means a much more detailed understanding of what is underlying the imprinting mechanisms and what is the adaptive response to repeated vaccination and infection. I think this is going to take prospective infant cohort studies; the National Institutes of Health is funding some that will begin within the next year,” Dr. Belongia added.
Many investigational approaches to improving influenza virus subtype-level protection are being explored. These include novel adjuvants, nanoparticle vaccines, computationally optimized broadly reactive antigens, and standardization of neuraminidase content.
And as for the much-desired universal flu vaccine?
“I will say that if a universal vaccine is going to work it’s probably going to work first in children. They have a much shorter immune history and their antibody landscape is a lot smaller, so you have a much better opportunity, I think, to generate a broad response to a universal vaccine compared to adults, who have much more complex immune landscapes,” he said.
Dr. Belongia reported having no financial conflicts regarding his presentation.
REPORTING FROM ESPID 2019