User login
Proinflammatory diet may increase colorectal cancer risk
by increasing inflammation, new research suggests.
In the Jan. 18 online edition of JAMA Oncology, Fred K. Tabung, PhD, from the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, and coauthors presented analysis of data from 46,804 men in the Health Professionals Follow-up Study and 74,246 women in the Nurses’ Health Study, examining the relationship between proinflammatory diets and colorectal cancer.
The effect was more pronounced in men with highly inflammatory diets, who had a 44% higher risk of colorectal cancer, compared with those whose diets had low inflammatory potential (95% CI, 1.19-1.74; P less than .001). Women in the highest quintile had a 22% higher risk of colorectal cancer, compared with those in lowest quintile (95% CI, 1.02-1.45; P = .007). These increases in risk were seen even after adjusting for potential confounders such as age, family history of cancer, physical activity, smoking, NSAID use, and menopause status (JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844).
Overall, there were 2,699 cases of incident colorectal cancer over 2,571,831 person-years of follow-up. Researchers noted 38 more incidents of colorectal cancer per 100,000 person-years among men in the highest quintile, and 12 more among women in the highest quintile, compared with men and women in the lowest quintiles.
The score used in the study looks at intake of foods such as processed, red, and organ meat; fish, vegetables other than green leafy or dark yellow vegetables, refined grains, high-energy and low-energy drinks, and tomatoes, which are all positively related to concentrations of the inflammatory markers. Foods linked to low concentrations of inflammatory markers include beer, wine, tea, coffee, dark yellow vegetables, green leafy vegetables, snacks, fruit juice, and pizza.
The association between proinflammatory diet and colorectal cancer risk was found in all anatomic locations, including the proximal and distal colon. The only exception was for rectal cancer in women, where dietary inflammatory potential did not significantly change cancer risk.
“It is not entirely clear why associations for rectal cancer were stronger in men than in women but are unlikely to be due to chance given the large significant heterogeneity by sex,” wrote Dr. Tabung and coauthors. They suggested the difference in risk pattern for rectal cancer may relate to broader differences in risk patterns between sexes.
“For example, higher body weight strongly predisposes men to higher risk of proximal colon cancer, distal colon cancer, and rectal cancer, and predisposes women to mainly higher risk of distal colon cancer but not rectal cancer.”
This was also evident when the authors looked at subgroup effects based on body mass index and alcohol intake. Men who were overweight or obese and who were on the most proinflammatory diet had a significant, 48% higher risk of colorectal cancer, compared with the lowest quintile. The same effect was not seen in overweight or obese women.
When alcohol intake was examined, researchers saw a significant 62% higher risk of colorectal cancer among men who were in the highest quintile for dietary inflammatory potential, but who did not drink alcohol, compared with teetotalers in the lowest quintile. In women, there was a 33% higher risk in the highest quintile, compared with the lowest in those who did not drink.
A similar trend was evident in individuals who drank up to one alcoholic drink a day, but it was only borderline significant, and there was no significant effect seen in individuals who drank more than one alcoholic drink a day, despite the fact that a high intake of alcohol has been associated with cancer risk in both men and women.
“It is possible that the adverse effects of alcohol intake through other mechanisms may be more dominant than those of its effect on the EDIP and may partially explain the stronger associations among men and women not consuming alcohol than among alcohol consumers.”
The Health Professionals Follow-up Study and Nurses’ Health Study are supported by the National Institutes of Health. Three authors were also supported by grants from the National Institutes of Health, and one also received support from the Friends of the Dana-Farber Cancer Institute and the Dana-Farber Harvard Cancer Center. No conflicts of interest were declared.
SOURCE: Tabung FK et al. JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844
by increasing inflammation, new research suggests.
In the Jan. 18 online edition of JAMA Oncology, Fred K. Tabung, PhD, from the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, and coauthors presented analysis of data from 46,804 men in the Health Professionals Follow-up Study and 74,246 women in the Nurses’ Health Study, examining the relationship between proinflammatory diets and colorectal cancer.
The effect was more pronounced in men with highly inflammatory diets, who had a 44% higher risk of colorectal cancer, compared with those whose diets had low inflammatory potential (95% CI, 1.19-1.74; P less than .001). Women in the highest quintile had a 22% higher risk of colorectal cancer, compared with those in lowest quintile (95% CI, 1.02-1.45; P = .007). These increases in risk were seen even after adjusting for potential confounders such as age, family history of cancer, physical activity, smoking, NSAID use, and menopause status (JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844).
Overall, there were 2,699 cases of incident colorectal cancer over 2,571,831 person-years of follow-up. Researchers noted 38 more incidents of colorectal cancer per 100,000 person-years among men in the highest quintile, and 12 more among women in the highest quintile, compared with men and women in the lowest quintiles.
The score used in the study looks at intake of foods such as processed, red, and organ meat; fish, vegetables other than green leafy or dark yellow vegetables, refined grains, high-energy and low-energy drinks, and tomatoes, which are all positively related to concentrations of the inflammatory markers. Foods linked to low concentrations of inflammatory markers include beer, wine, tea, coffee, dark yellow vegetables, green leafy vegetables, snacks, fruit juice, and pizza.
The association between proinflammatory diet and colorectal cancer risk was found in all anatomic locations, including the proximal and distal colon. The only exception was for rectal cancer in women, where dietary inflammatory potential did not significantly change cancer risk.
“It is not entirely clear why associations for rectal cancer were stronger in men than in women but are unlikely to be due to chance given the large significant heterogeneity by sex,” wrote Dr. Tabung and coauthors. They suggested the difference in risk pattern for rectal cancer may relate to broader differences in risk patterns between sexes.
“For example, higher body weight strongly predisposes men to higher risk of proximal colon cancer, distal colon cancer, and rectal cancer, and predisposes women to mainly higher risk of distal colon cancer but not rectal cancer.”
This was also evident when the authors looked at subgroup effects based on body mass index and alcohol intake. Men who were overweight or obese and who were on the most proinflammatory diet had a significant, 48% higher risk of colorectal cancer, compared with the lowest quintile. The same effect was not seen in overweight or obese women.
When alcohol intake was examined, researchers saw a significant 62% higher risk of colorectal cancer among men who were in the highest quintile for dietary inflammatory potential, but who did not drink alcohol, compared with teetotalers in the lowest quintile. In women, there was a 33% higher risk in the highest quintile, compared with the lowest in those who did not drink.
A similar trend was evident in individuals who drank up to one alcoholic drink a day, but it was only borderline significant, and there was no significant effect seen in individuals who drank more than one alcoholic drink a day, despite the fact that a high intake of alcohol has been associated with cancer risk in both men and women.
“It is possible that the adverse effects of alcohol intake through other mechanisms may be more dominant than those of its effect on the EDIP and may partially explain the stronger associations among men and women not consuming alcohol than among alcohol consumers.”
The Health Professionals Follow-up Study and Nurses’ Health Study are supported by the National Institutes of Health. Three authors were also supported by grants from the National Institutes of Health, and one also received support from the Friends of the Dana-Farber Cancer Institute and the Dana-Farber Harvard Cancer Center. No conflicts of interest were declared.
SOURCE: Tabung FK et al. JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844
by increasing inflammation, new research suggests.
In the Jan. 18 online edition of JAMA Oncology, Fred K. Tabung, PhD, from the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, and coauthors presented analysis of data from 46,804 men in the Health Professionals Follow-up Study and 74,246 women in the Nurses’ Health Study, examining the relationship between proinflammatory diets and colorectal cancer.
The effect was more pronounced in men with highly inflammatory diets, who had a 44% higher risk of colorectal cancer, compared with those whose diets had low inflammatory potential (95% CI, 1.19-1.74; P less than .001). Women in the highest quintile had a 22% higher risk of colorectal cancer, compared with those in lowest quintile (95% CI, 1.02-1.45; P = .007). These increases in risk were seen even after adjusting for potential confounders such as age, family history of cancer, physical activity, smoking, NSAID use, and menopause status (JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844).
Overall, there were 2,699 cases of incident colorectal cancer over 2,571,831 person-years of follow-up. Researchers noted 38 more incidents of colorectal cancer per 100,000 person-years among men in the highest quintile, and 12 more among women in the highest quintile, compared with men and women in the lowest quintiles.
The score used in the study looks at intake of foods such as processed, red, and organ meat; fish, vegetables other than green leafy or dark yellow vegetables, refined grains, high-energy and low-energy drinks, and tomatoes, which are all positively related to concentrations of the inflammatory markers. Foods linked to low concentrations of inflammatory markers include beer, wine, tea, coffee, dark yellow vegetables, green leafy vegetables, snacks, fruit juice, and pizza.
The association between proinflammatory diet and colorectal cancer risk was found in all anatomic locations, including the proximal and distal colon. The only exception was for rectal cancer in women, where dietary inflammatory potential did not significantly change cancer risk.
“It is not entirely clear why associations for rectal cancer were stronger in men than in women but are unlikely to be due to chance given the large significant heterogeneity by sex,” wrote Dr. Tabung and coauthors. They suggested the difference in risk pattern for rectal cancer may relate to broader differences in risk patterns between sexes.
“For example, higher body weight strongly predisposes men to higher risk of proximal colon cancer, distal colon cancer, and rectal cancer, and predisposes women to mainly higher risk of distal colon cancer but not rectal cancer.”
This was also evident when the authors looked at subgroup effects based on body mass index and alcohol intake. Men who were overweight or obese and who were on the most proinflammatory diet had a significant, 48% higher risk of colorectal cancer, compared with the lowest quintile. The same effect was not seen in overweight or obese women.
When alcohol intake was examined, researchers saw a significant 62% higher risk of colorectal cancer among men who were in the highest quintile for dietary inflammatory potential, but who did not drink alcohol, compared with teetotalers in the lowest quintile. In women, there was a 33% higher risk in the highest quintile, compared with the lowest in those who did not drink.
A similar trend was evident in individuals who drank up to one alcoholic drink a day, but it was only borderline significant, and there was no significant effect seen in individuals who drank more than one alcoholic drink a day, despite the fact that a high intake of alcohol has been associated with cancer risk in both men and women.
“It is possible that the adverse effects of alcohol intake through other mechanisms may be more dominant than those of its effect on the EDIP and may partially explain the stronger associations among men and women not consuming alcohol than among alcohol consumers.”
The Health Professionals Follow-up Study and Nurses’ Health Study are supported by the National Institutes of Health. Three authors were also supported by grants from the National Institutes of Health, and one also received support from the Friends of the Dana-Farber Cancer Institute and the Dana-Farber Harvard Cancer Center. No conflicts of interest were declared.
SOURCE: Tabung FK et al. JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844
FROM JAMA ONCOLOGY
Key clinical point: A diet high in foods such as processed meats, refined grains, and soda may increase the risk of colorectal cancer by promoting inflammation.
Major finding: People in the highest quintile of dietary inflammatory score had a 32% higher risk of developing colorectal cancer, compared with those in the lowest quintile.
Data source: Analysis of data from 46,804 men in the Health Professionals Follow-up Study, and 74,246 women in the Nurses’ Health Study.
Disclosures: The Health Professionals Follow-up Study and Nurses’ Health Study are supported by the National Institutes of Health. Three authors were supported by grants from the National Institutes of Health, and one also received support from the Friends of the Dana-Farber Cancer Institute, and the Dana-Farber Harvard Cancer Center. No conflicts of interest were declared.
Source: JAMA Oncology. 2018 Jan 18. doi: 10.1001/jamaoncol.2017.4844
Atopic march largely attributed to genetic factors
according to a systematic review.
PhD candidate Sabria Khan and her colleagues at the Centre for Epidemiology and Biostatistics at the University of Melbourne said that the atopic march concept “asserts that allergic diseases start in early life with eczema, progress through food allergy, and culminate with hay fever and asthma.”
This systematic review of ten twin and sibling studies looked at known, measured environmental and genetic influences on the associations between the atopic phenotypes of the atopic march.
The studies of asthma and hay fever suggested that the prevalence of having both conditions was high (32%) and that they were more likely to occur together in monozygotic twins than they were in dizygotic twins. Similarly, other studies found a high phenotypic overlap between eczema and asthma and between eczema and hay fever, which was more pronounced in monozygotic twins than in dizygotic twins.
“Asthma is linked to hay fever and eczema through intermediate phenotypes like clinical measures of lung function, physiological measures of airway responsiveness and the biomarker exhaled nitric oxide, all of which are influenced by hereditary factors,” the authors said.
Overall, they concluded that genetic factors account for 75% of eczema cases, 70%-91% of asthma cases, and 72%-84% of hay fever cases, making them all highly heritable diseases.
“Our study found that the contribution of shared environmental factors to the proportion of correlation are very low (from 4% to 18%) and does not explain the familial patterns seen for asthma and hay fever,” they reported. “This finding contradicts various analyses where smoking behavior, indoor-outdoor pollution, and house dust mites were found to be significant risk factor for asthma and hay fever that are shared by siblings.”
The authors commented that preventing the onset of the atopic march, or arresting its development, could have significant public health implications. They suggested that interventions such as oral antihistamines could be introduced either before a child gets eczema or before a child with eczema goes on to develop asthma or hay fever. “Two randomized controlled trials showed moisturizing the skin can prevent mild to moderate eczema, and long-term studies are needed to see whether such intervention will prevent development of asthma and hay fever,” they said.
No conflicts of interest were declared.
SOURCE: Khan SJ et al. Allergy. 2018 Jan;73(1):17-28.
according to a systematic review.
PhD candidate Sabria Khan and her colleagues at the Centre for Epidemiology and Biostatistics at the University of Melbourne said that the atopic march concept “asserts that allergic diseases start in early life with eczema, progress through food allergy, and culminate with hay fever and asthma.”
This systematic review of ten twin and sibling studies looked at known, measured environmental and genetic influences on the associations between the atopic phenotypes of the atopic march.
The studies of asthma and hay fever suggested that the prevalence of having both conditions was high (32%) and that they were more likely to occur together in monozygotic twins than they were in dizygotic twins. Similarly, other studies found a high phenotypic overlap between eczema and asthma and between eczema and hay fever, which was more pronounced in monozygotic twins than in dizygotic twins.
“Asthma is linked to hay fever and eczema through intermediate phenotypes like clinical measures of lung function, physiological measures of airway responsiveness and the biomarker exhaled nitric oxide, all of which are influenced by hereditary factors,” the authors said.
Overall, they concluded that genetic factors account for 75% of eczema cases, 70%-91% of asthma cases, and 72%-84% of hay fever cases, making them all highly heritable diseases.
“Our study found that the contribution of shared environmental factors to the proportion of correlation are very low (from 4% to 18%) and does not explain the familial patterns seen for asthma and hay fever,” they reported. “This finding contradicts various analyses where smoking behavior, indoor-outdoor pollution, and house dust mites were found to be significant risk factor for asthma and hay fever that are shared by siblings.”
The authors commented that preventing the onset of the atopic march, or arresting its development, could have significant public health implications. They suggested that interventions such as oral antihistamines could be introduced either before a child gets eczema or before a child with eczema goes on to develop asthma or hay fever. “Two randomized controlled trials showed moisturizing the skin can prevent mild to moderate eczema, and long-term studies are needed to see whether such intervention will prevent development of asthma and hay fever,” they said.
No conflicts of interest were declared.
SOURCE: Khan SJ et al. Allergy. 2018 Jan;73(1):17-28.
according to a systematic review.
PhD candidate Sabria Khan and her colleagues at the Centre for Epidemiology and Biostatistics at the University of Melbourne said that the atopic march concept “asserts that allergic diseases start in early life with eczema, progress through food allergy, and culminate with hay fever and asthma.”
This systematic review of ten twin and sibling studies looked at known, measured environmental and genetic influences on the associations between the atopic phenotypes of the atopic march.
The studies of asthma and hay fever suggested that the prevalence of having both conditions was high (32%) and that they were more likely to occur together in monozygotic twins than they were in dizygotic twins. Similarly, other studies found a high phenotypic overlap between eczema and asthma and between eczema and hay fever, which was more pronounced in monozygotic twins than in dizygotic twins.
“Asthma is linked to hay fever and eczema through intermediate phenotypes like clinical measures of lung function, physiological measures of airway responsiveness and the biomarker exhaled nitric oxide, all of which are influenced by hereditary factors,” the authors said.
Overall, they concluded that genetic factors account for 75% of eczema cases, 70%-91% of asthma cases, and 72%-84% of hay fever cases, making them all highly heritable diseases.
“Our study found that the contribution of shared environmental factors to the proportion of correlation are very low (from 4% to 18%) and does not explain the familial patterns seen for asthma and hay fever,” they reported. “This finding contradicts various analyses where smoking behavior, indoor-outdoor pollution, and house dust mites were found to be significant risk factor for asthma and hay fever that are shared by siblings.”
The authors commented that preventing the onset of the atopic march, or arresting its development, could have significant public health implications. They suggested that interventions such as oral antihistamines could be introduced either before a child gets eczema or before a child with eczema goes on to develop asthma or hay fever. “Two randomized controlled trials showed moisturizing the skin can prevent mild to moderate eczema, and long-term studies are needed to see whether such intervention will prevent development of asthma and hay fever,” they said.
No conflicts of interest were declared.
SOURCE: Khan SJ et al. Allergy. 2018 Jan;73(1):17-28.
FROM ALLERGY
Key clinical point: Twin and sibling studies suggest that genetics play a far more significant role than environmental factors in the progression of atopic disease in childhood known as the “atopic march.”
Major finding: Genetic factors account for 75% of eczema, 70%-91% of asthma, and 72%-84% of hay fever.
Data source: Systematic review of ten twin and sibling studies.
Disclosures: No conflicts of interest were declared.
Source: Khan SJ et al. Allergy. 2018 Jan;73(1):17-28.
Breastfeeding lowers later diabetes risk in women
Breastfeeding may reduce a woman’s risk of developing diabetes, with a prospective cohort study showing a strong, inverse association between lactation duration and risk of diabetes.
In a report published in the Jan. 16 online edition of JAMA Internal Medicine, researchers analyzed data from the Coronary Artery Risk Development Study in Young Adults (CARDIA), which followed 1,238 women aged 18-30 for 30 years, with multiple assessments of glucose tolerance over the course of the study.
Women who breastfed for 6-12 months had a 48% reduction in the risk of diabetes (95% CI, 0.31-0.87), and those who breastfed for up to 6 months had a 25% lower risk (95% CI, 0.51-1.09), with the trend being significant.
In women with a history of gestational diabetes, those who did not breastfeed at all had a 2.08% higher excess risk of incident diabetes per year, compared with women who breastfed for at least 12 months. The increase in excess risk for the same comparison in women without a history of gestational diabetes was 0.48% per year.
Erica P. Gunderson, PhD, of Kaiser Permanente Northern California, Oakland, and her coauthors noted that previous meta-analyses of the effect of lactation on diabetes incidence or prevalence pointed to protective summary estimates of 9%-11% per year of lactation.
“Lactating women have lower circulating glucose in both fasting and post absorptive states, as well as lower insulin secretion, despite increased glucose production rates,” the authors wrote. “About 50 g of glucose per 24 hours is diverted into the mammary gland for milk synthesis via non–insulin mediated pathways.”
Studies in mice have also suggested that lactating animals have greater pancreatic beta-cell proliferation.
While the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists recommend breastfeeding for 1 year, only 55% of women in the United States are still breastfeeding at 6 months and 33% are still breastfeeding at 1 year after birth. Black women are also less likely to breastfeed, regardless of socioeconomic status or body size.
“Lactation is a natural biological process with the enormous potential to provide long-term benefits to maternal health, but has been underappreciated as a potential key strategy for early primary prevention of metabolic diseases in women across the childbearing years and beyond.”
The study and analyses were supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and two authors declared funding from pharmaceutical companies.
SOURCE: JAMA Intern Med. 2016 Jan 16. doi: 10.1001/jamainternmed.2017.7978.
Breastfeeding may reduce a woman’s risk of developing diabetes, with a prospective cohort study showing a strong, inverse association between lactation duration and risk of diabetes.
In a report published in the Jan. 16 online edition of JAMA Internal Medicine, researchers analyzed data from the Coronary Artery Risk Development Study in Young Adults (CARDIA), which followed 1,238 women aged 18-30 for 30 years, with multiple assessments of glucose tolerance over the course of the study.
Women who breastfed for 6-12 months had a 48% reduction in the risk of diabetes (95% CI, 0.31-0.87), and those who breastfed for up to 6 months had a 25% lower risk (95% CI, 0.51-1.09), with the trend being significant.
In women with a history of gestational diabetes, those who did not breastfeed at all had a 2.08% higher excess risk of incident diabetes per year, compared with women who breastfed for at least 12 months. The increase in excess risk for the same comparison in women without a history of gestational diabetes was 0.48% per year.
Erica P. Gunderson, PhD, of Kaiser Permanente Northern California, Oakland, and her coauthors noted that previous meta-analyses of the effect of lactation on diabetes incidence or prevalence pointed to protective summary estimates of 9%-11% per year of lactation.
“Lactating women have lower circulating glucose in both fasting and post absorptive states, as well as lower insulin secretion, despite increased glucose production rates,” the authors wrote. “About 50 g of glucose per 24 hours is diverted into the mammary gland for milk synthesis via non–insulin mediated pathways.”
Studies in mice have also suggested that lactating animals have greater pancreatic beta-cell proliferation.
While the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists recommend breastfeeding for 1 year, only 55% of women in the United States are still breastfeeding at 6 months and 33% are still breastfeeding at 1 year after birth. Black women are also less likely to breastfeed, regardless of socioeconomic status or body size.
“Lactation is a natural biological process with the enormous potential to provide long-term benefits to maternal health, but has been underappreciated as a potential key strategy for early primary prevention of metabolic diseases in women across the childbearing years and beyond.”
The study and analyses were supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and two authors declared funding from pharmaceutical companies.
SOURCE: JAMA Intern Med. 2016 Jan 16. doi: 10.1001/jamainternmed.2017.7978.
Breastfeeding may reduce a woman’s risk of developing diabetes, with a prospective cohort study showing a strong, inverse association between lactation duration and risk of diabetes.
In a report published in the Jan. 16 online edition of JAMA Internal Medicine, researchers analyzed data from the Coronary Artery Risk Development Study in Young Adults (CARDIA), which followed 1,238 women aged 18-30 for 30 years, with multiple assessments of glucose tolerance over the course of the study.
Women who breastfed for 6-12 months had a 48% reduction in the risk of diabetes (95% CI, 0.31-0.87), and those who breastfed for up to 6 months had a 25% lower risk (95% CI, 0.51-1.09), with the trend being significant.
In women with a history of gestational diabetes, those who did not breastfeed at all had a 2.08% higher excess risk of incident diabetes per year, compared with women who breastfed for at least 12 months. The increase in excess risk for the same comparison in women without a history of gestational diabetes was 0.48% per year.
Erica P. Gunderson, PhD, of Kaiser Permanente Northern California, Oakland, and her coauthors noted that previous meta-analyses of the effect of lactation on diabetes incidence or prevalence pointed to protective summary estimates of 9%-11% per year of lactation.
“Lactating women have lower circulating glucose in both fasting and post absorptive states, as well as lower insulin secretion, despite increased glucose production rates,” the authors wrote. “About 50 g of glucose per 24 hours is diverted into the mammary gland for milk synthesis via non–insulin mediated pathways.”
Studies in mice have also suggested that lactating animals have greater pancreatic beta-cell proliferation.
While the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists recommend breastfeeding for 1 year, only 55% of women in the United States are still breastfeeding at 6 months and 33% are still breastfeeding at 1 year after birth. Black women are also less likely to breastfeed, regardless of socioeconomic status or body size.
“Lactation is a natural biological process with the enormous potential to provide long-term benefits to maternal health, but has been underappreciated as a potential key strategy for early primary prevention of metabolic diseases in women across the childbearing years and beyond.”
The study and analyses were supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and two authors declared funding from pharmaceutical companies.
SOURCE: JAMA Intern Med. 2016 Jan 16. doi: 10.1001/jamainternmed.2017.7978.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Duration of breastfeeding is inversely associated with risk of developing diabetes.
Major finding: Women who breastfeed for 12 months or more have a 47% lower risk of developing diabetes compared with women who do not breastfeed at all.
Data source: Analysis of data from the CARDIA population-based prospective cohort study in 1,238 women.
Disclosures: The study and analyses were supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and two authors declared funding from pharmaceutical companies.
Source: JAMA Intern Med. 2016 Jan 16. doi: 10.1001/jamainternmed.2017.7978.
Hormone therapy may reduce depressive symptoms in early menopause
Hormone therapy may reduce the likelihood of depressive symptoms during early menopause, according to data from a placebo-controlled study published online Jan. 10 in JAMA Psychiatry.
Researchers randomized 172 medically healthy women who were perimenopausal or early postmenopausal to either patches of 0.1 mg of 17 beta-estradiol or placebo patches for 12 months, as well as oral micronized progesterone or placebo every 2-3 months.
Women in the placebo arm were more than twice as likely to score at least 16 on the Center for Epidemiologic Studies–Depression Scale (CES-D) at least once during the study, compared with women in the hormone therapy arm (32.3% vs. 17.3%; odds ratio, 2.5; 95% confidence interval, 1.1-5.7; P = .03). They also scored at least 16 on the scale during more visits than did women in the intervention arm (P = .002).
Women who received placebo had a significantly higher mean CES-D score over the course of the 12-month study, compared with those in the intervention arm. At 6 months, mean unadjusted CES-D scores were 5.6 in the placebo group and 4.2 in the intervention group. At 12 months, the scores were 5.7 and 4 respectively.
“Importantly, these results were significant despite statistically adjusting for change in vasomotor symptom bother, suggesting that TE+IMP [transdermal estradiol + intermittent micronized progesterone] has direct prophylactic mood benefits that are independent from its beneficial effects on menopausal symptoms, as previously observed in women with major depressive disorder treated with TE+IMP,” wrote Jennifer L. Gordon, PhD, of the psychology department at the University of Regina, Saskatchewan, and her associates.
The effect of hormone therapy on depression scores changed significantly depending on the women’s stage of menopause. Women in the early menopause transition experienced significant mood benefits, but women in the late menopause transition and those who were postmenopausal did not.
Treatment effects also were influenced by stress, as women who reported experiencing more stressful life events in the 6 months before enrolling in the study showed greater mood benefits than did those with fewer stressful life events.
However, baseline estradiol levels, baseline vasomotor symptoms, history of depression, and history of abuse did not significantly change the effects of treatment.
, compared with the placebo group, and one woman in the treatment group experienced acute deep vein thrombosis that led her to stop treatment and receive medical care. Two cases of major depressive disorder were noted in the placebo group.
Dr. Gordon and her associates cited several limitations. For example, they said, the active and placebo patches used in the study were not identical. “However, our findings remain significant when adjusting for participants’ beliefs about their treatment condition,” they wrote.
The study was supported by the National Institutes of Health, and one author was supported by a fellowship of the Fonds de la Recherche du Québec–Santé. No conflicts of interest were declared.
SOURCE: Gordon JL et al. JAMA Psychiatry. 2018 Jan 10. doi: 1010/jamapsychiatry.2018.3998.
Hormone therapy may reduce the likelihood of depressive symptoms during early menopause, according to data from a placebo-controlled study published online Jan. 10 in JAMA Psychiatry.
Researchers randomized 172 medically healthy women who were perimenopausal or early postmenopausal to either patches of 0.1 mg of 17 beta-estradiol or placebo patches for 12 months, as well as oral micronized progesterone or placebo every 2-3 months.
Women in the placebo arm were more than twice as likely to score at least 16 on the Center for Epidemiologic Studies–Depression Scale (CES-D) at least once during the study, compared with women in the hormone therapy arm (32.3% vs. 17.3%; odds ratio, 2.5; 95% confidence interval, 1.1-5.7; P = .03). They also scored at least 16 on the scale during more visits than did women in the intervention arm (P = .002).
Women who received placebo had a significantly higher mean CES-D score over the course of the 12-month study, compared with those in the intervention arm. At 6 months, mean unadjusted CES-D scores were 5.6 in the placebo group and 4.2 in the intervention group. At 12 months, the scores were 5.7 and 4 respectively.
“Importantly, these results were significant despite statistically adjusting for change in vasomotor symptom bother, suggesting that TE+IMP [transdermal estradiol + intermittent micronized progesterone] has direct prophylactic mood benefits that are independent from its beneficial effects on menopausal symptoms, as previously observed in women with major depressive disorder treated with TE+IMP,” wrote Jennifer L. Gordon, PhD, of the psychology department at the University of Regina, Saskatchewan, and her associates.
The effect of hormone therapy on depression scores changed significantly depending on the women’s stage of menopause. Women in the early menopause transition experienced significant mood benefits, but women in the late menopause transition and those who were postmenopausal did not.
Treatment effects also were influenced by stress, as women who reported experiencing more stressful life events in the 6 months before enrolling in the study showed greater mood benefits than did those with fewer stressful life events.
However, baseline estradiol levels, baseline vasomotor symptoms, history of depression, and history of abuse did not significantly change the effects of treatment.
, compared with the placebo group, and one woman in the treatment group experienced acute deep vein thrombosis that led her to stop treatment and receive medical care. Two cases of major depressive disorder were noted in the placebo group.
Dr. Gordon and her associates cited several limitations. For example, they said, the active and placebo patches used in the study were not identical. “However, our findings remain significant when adjusting for participants’ beliefs about their treatment condition,” they wrote.
The study was supported by the National Institutes of Health, and one author was supported by a fellowship of the Fonds de la Recherche du Québec–Santé. No conflicts of interest were declared.
SOURCE: Gordon JL et al. JAMA Psychiatry. 2018 Jan 10. doi: 1010/jamapsychiatry.2018.3998.
Hormone therapy may reduce the likelihood of depressive symptoms during early menopause, according to data from a placebo-controlled study published online Jan. 10 in JAMA Psychiatry.
Researchers randomized 172 medically healthy women who were perimenopausal or early postmenopausal to either patches of 0.1 mg of 17 beta-estradiol or placebo patches for 12 months, as well as oral micronized progesterone or placebo every 2-3 months.
Women in the placebo arm were more than twice as likely to score at least 16 on the Center for Epidemiologic Studies–Depression Scale (CES-D) at least once during the study, compared with women in the hormone therapy arm (32.3% vs. 17.3%; odds ratio, 2.5; 95% confidence interval, 1.1-5.7; P = .03). They also scored at least 16 on the scale during more visits than did women in the intervention arm (P = .002).
Women who received placebo had a significantly higher mean CES-D score over the course of the 12-month study, compared with those in the intervention arm. At 6 months, mean unadjusted CES-D scores were 5.6 in the placebo group and 4.2 in the intervention group. At 12 months, the scores were 5.7 and 4 respectively.
“Importantly, these results were significant despite statistically adjusting for change in vasomotor symptom bother, suggesting that TE+IMP [transdermal estradiol + intermittent micronized progesterone] has direct prophylactic mood benefits that are independent from its beneficial effects on menopausal symptoms, as previously observed in women with major depressive disorder treated with TE+IMP,” wrote Jennifer L. Gordon, PhD, of the psychology department at the University of Regina, Saskatchewan, and her associates.
The effect of hormone therapy on depression scores changed significantly depending on the women’s stage of menopause. Women in the early menopause transition experienced significant mood benefits, but women in the late menopause transition and those who were postmenopausal did not.
Treatment effects also were influenced by stress, as women who reported experiencing more stressful life events in the 6 months before enrolling in the study showed greater mood benefits than did those with fewer stressful life events.
However, baseline estradiol levels, baseline vasomotor symptoms, history of depression, and history of abuse did not significantly change the effects of treatment.
, compared with the placebo group, and one woman in the treatment group experienced acute deep vein thrombosis that led her to stop treatment and receive medical care. Two cases of major depressive disorder were noted in the placebo group.
Dr. Gordon and her associates cited several limitations. For example, they said, the active and placebo patches used in the study were not identical. “However, our findings remain significant when adjusting for participants’ beliefs about their treatment condition,” they wrote.
The study was supported by the National Institutes of Health, and one author was supported by a fellowship of the Fonds de la Recherche du Québec–Santé. No conflicts of interest were declared.
SOURCE: Gordon JL et al. JAMA Psychiatry. 2018 Jan 10. doi: 1010/jamapsychiatry.2018.3998.
FROM JAMA PSYCHIATRY
Key clinical point: Transdermal hormone therapy may reduce the likelihood of depressive symptoms in early menopause.
Major finding: Women who did not receive hormone therapy had more than a twofold higher likelihood of high depression scores, compared with women who were treated with transdermal estradiol.
Data source: Placebo-controlled, double-blind, randomized trial of 172 perimenopausal women.
Disclosures: The study was supported by the National Institutes of Health, and one author was supported by a fellowship of the Fonds de la Recherche du Québec–Santé. No conflicts of interest were declared.
Source: Gordon JL et al. JAMA Psychiatry. 2018 Jan 10. doi: 1010/jamapsychiatry.2018.3998.
views
The authors of this study are to be commended for conducting this randomized controlled trial to investigate the efficacy of a biologically rational strategy to prevent depressive symptoms in menopausal women, Hadine Joffe, MD, and Martha Hickey, MD, wrote in an accompanying editorial (JAMA Psychiatry. 2018 Jan 10. doi: 10.1001/jamapsychiatry.2017.3945).
The mechanisms of depression in menopause are not well understood, but increased changes in estradiol concentrations, nocturnal hot flashes, and sleep disturbance have been linked to the emergence of depressive symptoms. However, this study did not report accurate measures of hot flashes and sleep disturbance, which is important because of the central role of those symptoms in menopause associated mood disturbance.
Using hormone therapy in an effort to prevent depressive symptoms throughout perimenopause must be balanced with the adverse effects associated with extended exposure to hormone therapy, such as breast cancer. The results of this trial illustrate a potential role of hormone therapy in mood regulation, but they do not support changes in clinical guidance.
Dr. Joffe is affiliated with the Connors Center for Women’s Health and Gender Biology at Brigham and Women’s Hospital, the department of psychiatry at the hospital, and Harvard Medical School, all in Boston. Dr. Hickey is affiliated with the department of obstetrics and gynaecology at The University of Melbourne. Dr. Joffe was supported by the National Institute on Aging, and she declared research funding and consultancies from private industry, including Merck and SAGE. Dr. Hickey was supported by the Australian National Health and Medical Research Council and had no conflicts of interest to declare.
Self-administered subcutaneous Depo-Provera ‘feasible and acceptable’
Self-administration of subcutaneous depot medroxyprogesterone acetate contraceptive is a “feasible and acceptable” alternative to clinic-based administration, according to a study published online Dec. 12 in Contraception.
Researchers randomized 401 women aged 15-44 years – who had requested depot medroxyprogesterone acetate – to four injections over the year, administered by clinic staff at one of three Planned Parenthood health centers or self-administered at home.
The self-administered group performed the first injection at the clinic under supervision, then received instructions and three doses for home use.
At the 12-month follow-up, the rates of continuous use of the contraceptive – defined as reporting two additional doses on the 6-month survey and at least one additional dose on the 12-month survey – were 69% in the self-administration group and 54% in the clinic group (P = .005).
A significantly greater proportion of women in the self-administration group reported receiving at least four shots over the study period, compared with the clinic administration group (78% vs. 64%; P = .008). The authors also noted that 64% of the self-administration group reported receiving five doses – an additional poststudy shot – compared with 52% of the clinic group.
The self-administered injection was well received by women in that group, with 97% saying it was very or somewhat easy to administer, and 87% saying they would recommend self-injection to a friend.
Around half of the participants in the clinic group said they would be interested in self-administration in the future, but both groups reported similar levels of satisfaction with the injectable contraceptive overall.
“A survey study found that women who had difficulty obtaining or refilling a prescription in the past year were almost twice as likely to be interested in self-administration compared to those without such difficulty, suggesting that self-administration has potential to reduce barriers and disparities in access to effective contraception,” wrote Julia E. Kohn, PhD, MPA, and her colleagues at Planned Parenthood. “It is important to note, however, that potential barriers related to insurance coverage and reimbursement will need to be identified and addressed for self-administration to be successfully put into practice.”
There were three pregnancies. They were in the clinic group in women who had discontinued the contraceptive, and one was a planned pregnancy. There were two reports of pain and two reports of bruising at the injection site, but no serious adverse events were reported.
While the study relied on self-report of successful injections, the authors pointed to a recent study in which all women who reported self-injections had therapeutic levels of depot medroxyprogesterone acetate confirmed by blood testing.
Tara Health Foundation supported the study. Pfizer provided the study drug through an investigator-initiated research grant. No disclosures were made.
SOURCE: Kohn JE et al. Contraception. 2017 Dec 7. doi: 10.1016/j.contraception.2017.11.009
Self-administration of subcutaneous depot medroxyprogesterone acetate contraceptive is a “feasible and acceptable” alternative to clinic-based administration, according to a study published online Dec. 12 in Contraception.
Researchers randomized 401 women aged 15-44 years – who had requested depot medroxyprogesterone acetate – to four injections over the year, administered by clinic staff at one of three Planned Parenthood health centers or self-administered at home.
The self-administered group performed the first injection at the clinic under supervision, then received instructions and three doses for home use.
At the 12-month follow-up, the rates of continuous use of the contraceptive – defined as reporting two additional doses on the 6-month survey and at least one additional dose on the 12-month survey – were 69% in the self-administration group and 54% in the clinic group (P = .005).
A significantly greater proportion of women in the self-administration group reported receiving at least four shots over the study period, compared with the clinic administration group (78% vs. 64%; P = .008). The authors also noted that 64% of the self-administration group reported receiving five doses – an additional poststudy shot – compared with 52% of the clinic group.
The self-administered injection was well received by women in that group, with 97% saying it was very or somewhat easy to administer, and 87% saying they would recommend self-injection to a friend.
Around half of the participants in the clinic group said they would be interested in self-administration in the future, but both groups reported similar levels of satisfaction with the injectable contraceptive overall.
“A survey study found that women who had difficulty obtaining or refilling a prescription in the past year were almost twice as likely to be interested in self-administration compared to those without such difficulty, suggesting that self-administration has potential to reduce barriers and disparities in access to effective contraception,” wrote Julia E. Kohn, PhD, MPA, and her colleagues at Planned Parenthood. “It is important to note, however, that potential barriers related to insurance coverage and reimbursement will need to be identified and addressed for self-administration to be successfully put into practice.”
There were three pregnancies. They were in the clinic group in women who had discontinued the contraceptive, and one was a planned pregnancy. There were two reports of pain and two reports of bruising at the injection site, but no serious adverse events were reported.
While the study relied on self-report of successful injections, the authors pointed to a recent study in which all women who reported self-injections had therapeutic levels of depot medroxyprogesterone acetate confirmed by blood testing.
Tara Health Foundation supported the study. Pfizer provided the study drug through an investigator-initiated research grant. No disclosures were made.
SOURCE: Kohn JE et al. Contraception. 2017 Dec 7. doi: 10.1016/j.contraception.2017.11.009
Self-administration of subcutaneous depot medroxyprogesterone acetate contraceptive is a “feasible and acceptable” alternative to clinic-based administration, according to a study published online Dec. 12 in Contraception.
Researchers randomized 401 women aged 15-44 years – who had requested depot medroxyprogesterone acetate – to four injections over the year, administered by clinic staff at one of three Planned Parenthood health centers or self-administered at home.
The self-administered group performed the first injection at the clinic under supervision, then received instructions and three doses for home use.
At the 12-month follow-up, the rates of continuous use of the contraceptive – defined as reporting two additional doses on the 6-month survey and at least one additional dose on the 12-month survey – were 69% in the self-administration group and 54% in the clinic group (P = .005).
A significantly greater proportion of women in the self-administration group reported receiving at least four shots over the study period, compared with the clinic administration group (78% vs. 64%; P = .008). The authors also noted that 64% of the self-administration group reported receiving five doses – an additional poststudy shot – compared with 52% of the clinic group.
The self-administered injection was well received by women in that group, with 97% saying it was very or somewhat easy to administer, and 87% saying they would recommend self-injection to a friend.
Around half of the participants in the clinic group said they would be interested in self-administration in the future, but both groups reported similar levels of satisfaction with the injectable contraceptive overall.
“A survey study found that women who had difficulty obtaining or refilling a prescription in the past year were almost twice as likely to be interested in self-administration compared to those without such difficulty, suggesting that self-administration has potential to reduce barriers and disparities in access to effective contraception,” wrote Julia E. Kohn, PhD, MPA, and her colleagues at Planned Parenthood. “It is important to note, however, that potential barriers related to insurance coverage and reimbursement will need to be identified and addressed for self-administration to be successfully put into practice.”
There were three pregnancies. They were in the clinic group in women who had discontinued the contraceptive, and one was a planned pregnancy. There were two reports of pain and two reports of bruising at the injection site, but no serious adverse events were reported.
While the study relied on self-report of successful injections, the authors pointed to a recent study in which all women who reported self-injections had therapeutic levels of depot medroxyprogesterone acetate confirmed by blood testing.
Tara Health Foundation supported the study. Pfizer provided the study drug through an investigator-initiated research grant. No disclosures were made.
SOURCE: Kohn JE et al. Contraception. 2017 Dec 7. doi: 10.1016/j.contraception.2017.11.009
FROM CONTRACEPTION
Key clinical point: Self-administration of subcutaneous depot medroxyprogesterone acetate contraceptive is a “feasible and acceptable” alternative to administration in a clinic.
Major finding: The rates of continuous use of subcutaneous depot medroxyprogesterone acetate were 69% in the self-administration group and 54% in the clinic group.
Data source: Randomized controlled trial of 401 women.
Disclosures: Tara Health Foundation supported the study. Pfizer provided the study drug through an investigator-initiated research grant. No disclosures were made.
Source: Kohn JE et al. Contraception. 2017 Dec 7. doi: 10.1016/j.contraception.2017.11.009.
New HIV vaccine trial launched in Africa
The National Institutes of Health has partnered with Janssen Vaccines & Prevention B.V. to conduct the phase 2b proof-of-concept “Imbokodo” study of a quadrivalent HIV vaccine regimen using “mosaic” immunogens, which means the vaccine components are designed to trigger an immune response against a variety of HIV strains.
Preclinical studies in monkeys suggest this approach can protect against HIV infection, and two early-stage clinical trials in humans showed the vaccine was well tolerated and generated immune responses against HIV in healthy individuals.
The four doses of the vaccine will be spread out over a year, and the final two doses will be given together with doses of an HIV protein, clade C gp140, and an aluminum phosphate adjuvant to boost immune responses. The study has already begun to immunize some of the 2,600 HIV-negative sub-Saharan African women to be recruited for the study.
The study is sponsored by Janssen Vaccines & Prevention B.V. and cofunded by the Bill & Melinda Gates Foundation and the NIH.
SOURCE: National Institute of Allergy and Infectious Diseases News Releases Nov. 30, 2017.
The National Institutes of Health has partnered with Janssen Vaccines & Prevention B.V. to conduct the phase 2b proof-of-concept “Imbokodo” study of a quadrivalent HIV vaccine regimen using “mosaic” immunogens, which means the vaccine components are designed to trigger an immune response against a variety of HIV strains.
Preclinical studies in monkeys suggest this approach can protect against HIV infection, and two early-stage clinical trials in humans showed the vaccine was well tolerated and generated immune responses against HIV in healthy individuals.
The four doses of the vaccine will be spread out over a year, and the final two doses will be given together with doses of an HIV protein, clade C gp140, and an aluminum phosphate adjuvant to boost immune responses. The study has already begun to immunize some of the 2,600 HIV-negative sub-Saharan African women to be recruited for the study.
The study is sponsored by Janssen Vaccines & Prevention B.V. and cofunded by the Bill & Melinda Gates Foundation and the NIH.
SOURCE: National Institute of Allergy and Infectious Diseases News Releases Nov. 30, 2017.
The National Institutes of Health has partnered with Janssen Vaccines & Prevention B.V. to conduct the phase 2b proof-of-concept “Imbokodo” study of a quadrivalent HIV vaccine regimen using “mosaic” immunogens, which means the vaccine components are designed to trigger an immune response against a variety of HIV strains.
Preclinical studies in monkeys suggest this approach can protect against HIV infection, and two early-stage clinical trials in humans showed the vaccine was well tolerated and generated immune responses against HIV in healthy individuals.
The four doses of the vaccine will be spread out over a year, and the final two doses will be given together with doses of an HIV protein, clade C gp140, and an aluminum phosphate adjuvant to boost immune responses. The study has already begun to immunize some of the 2,600 HIV-negative sub-Saharan African women to be recruited for the study.
The study is sponsored by Janssen Vaccines & Prevention B.V. and cofunded by the Bill & Melinda Gates Foundation and the NIH.
SOURCE: National Institute of Allergy and Infectious Diseases News Releases Nov. 30, 2017.
Long-acting injectable PrEP trial launched in Africa
The first is a phase 3 trial comparing an injectable version of the investigational integrase inhibitor cabotegravir with pre-exposure prophylaxis (PrEP) using daily oral Truvada (emtricitabine). Researchers aim to recruit 3,200 sexually active women from across Southern and Eastern Africa, and follow them for an average of 2.6 years.
“Taking a daily pill can be challenging for some people. For some women, a long-acting injectable form of protection may be an easier, more desirable and discreet alternative,” he said.
Women currently account for 58% of new HIV infections in adults in Southern and Eastern Africa, but preventive tools can be difficult to negotiate with a new partner.
A similar study is already underway in men and transgender women who have sex with men. The study is cofunded by ViiV Healthcare and the Bill & Melinda Gates Foundation, and ViiV Healthcare and Gilead Sciences are providing the study medications.
SOURCE: National Institute of Allergy and Infectious Diseases News Releases Nov. 30, 2017.
The first is a phase 3 trial comparing an injectable version of the investigational integrase inhibitor cabotegravir with pre-exposure prophylaxis (PrEP) using daily oral Truvada (emtricitabine). Researchers aim to recruit 3,200 sexually active women from across Southern and Eastern Africa, and follow them for an average of 2.6 years.
“Taking a daily pill can be challenging for some people. For some women, a long-acting injectable form of protection may be an easier, more desirable and discreet alternative,” he said.
Women currently account for 58% of new HIV infections in adults in Southern and Eastern Africa, but preventive tools can be difficult to negotiate with a new partner.
A similar study is already underway in men and transgender women who have sex with men. The study is cofunded by ViiV Healthcare and the Bill & Melinda Gates Foundation, and ViiV Healthcare and Gilead Sciences are providing the study medications.
SOURCE: National Institute of Allergy and Infectious Diseases News Releases Nov. 30, 2017.
The first is a phase 3 trial comparing an injectable version of the investigational integrase inhibitor cabotegravir with pre-exposure prophylaxis (PrEP) using daily oral Truvada (emtricitabine). Researchers aim to recruit 3,200 sexually active women from across Southern and Eastern Africa, and follow them for an average of 2.6 years.
“Taking a daily pill can be challenging for some people. For some women, a long-acting injectable form of protection may be an easier, more desirable and discreet alternative,” he said.
Women currently account for 58% of new HIV infections in adults in Southern and Eastern Africa, but preventive tools can be difficult to negotiate with a new partner.
A similar study is already underway in men and transgender women who have sex with men. The study is cofunded by ViiV Healthcare and the Bill & Melinda Gates Foundation, and ViiV Healthcare and Gilead Sciences are providing the study medications.
SOURCE: National Institute of Allergy and Infectious Diseases News Releases Nov. 30, 2017.
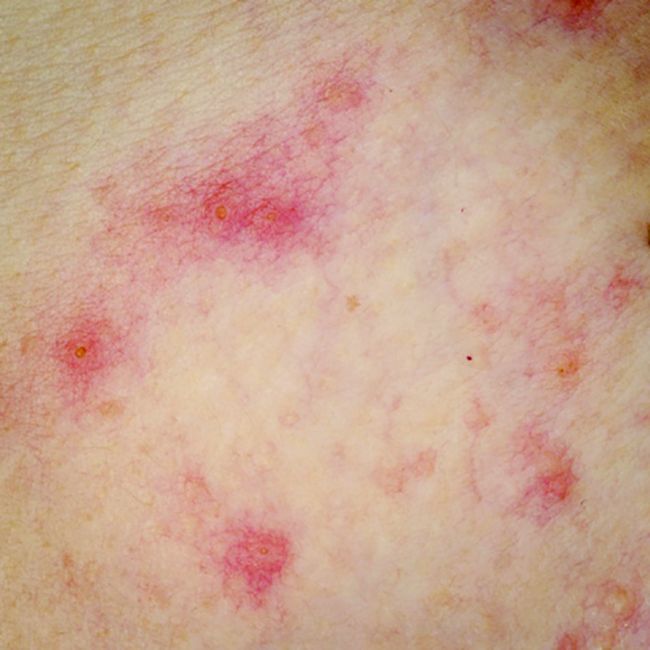
Obesity an independent risk factor for rosacea in cohort study of U.S. women
Obesity was associated with a significantly higher risk of rosacea, compared with a healthy weight, particularly when the weight was gained after age 18 years, according to an analysis of data from the Nurses Health Study II.
Investigators evaluated data on 89,886 women in the U.S. study from 1991 through 2005 – 5,249 diagnosed with rosacea – over 14 years to determine what, if any, relationship existed between body mass index (BMI) and rosacea risk.
As BMI increased, the risk of rosacea increased: Compared with participants with a BMI of 21.0 to 22.9 kg/m2 (considered a healthy weight), the risk of rosacea for those with a BMI of 25.0-29.9 (overweight) was 11% greater (95% confidence interval, 1.02-1.21), and among those with a BMI of 30.0-34.9, was 21% greater (95% CI, 1.09-1.34). The risk was 48% greater among those with a BMI of 35 or above (95% CI, 1.33-1.64). (A BMI over 30 is considered obese). The results were published in the December issue of the Journal of the American Academy of Dermatology.
The association between weight and rosacea risk was only significant after age 18 years, when the risk of rosacea increased 4% for every 10 pounds of weight gain, even after adjusting for BMI.
The investigators also found a significant relationship between waist circumference and rosacea risk that was independent of BMI, with the highest quintile of waist circumference associated with a 32% greater risk of rosacea, compared with the lowest quintile. Similarly, the highest quintile of hip circumference was associated with a 38% higher risk of rosacea, also independent of BMI. However there was no association between waist-to-hip ratio and rosacea risk.
While there were associations between the risk of rosacea and smoking, alcohol intake, and physical activity, the relationship between BMI and rosacea risk was not modified by these other risk factors.
Suyun Li, PhD, of the Guangzhou (China) Medical University School of Public Health and coauthors from the department of dermatology, Brown University, Providence, R.I.; and Brigham and Women’s Hospital, Boston, wrote that previous epidemiologic studies examining the interaction between obesity and rosacea have shown inconsistent results. Longitudinal studies on the issue had focused only on BMI and ignored other measures of central obesity.
“To our knowledge, this is the first cohort study on the association between obesity and risk for incident rosacea,” they wrote. “The study contributes to the understanding of rosacea etiology and informs clinical practice related to rosacea prevention and patient care.”
They suggested a number of different mechanisms that might explain how obesity increases the risk of rosacea, including the chronic, low-grade inflammatory state associated with obesity. “Adiposity can augment proinflammatory cytokine expression, such as interleukin 6 and tumor necrosis factor–alpha, both relevant to rosacea pathophysiology,” they noted. “Vascular changes associated with obesity might be another mechanism, considering obesity can lead to abnormalities of vascular function and structure, which might lead to the vasodilatation in rosacea.”
In the NHS II study, data are collected every 2 years, which the authors said ensured they had the most up-to-date information. While rosacea diagnoses relied on self-report, the authors said the study participants – nurses – were likely to have a high validity of self-reporting of rosacea. They acknowledged, however, that the lack of information on rosacea subtypes was a limitation of the study.
The study was supported by the department of dermatology, Brown University and a Nurses’ Health Study II grant. Dr. Li was supported by a research grant from the National Rosacea Society and the Dermatology Foundation. Another author declared having served as an investigator and receiving research funds from Sanofi and Regeneron; serving as a consultant for Sanofi and RTI Health Solutions, and having received honoraria from Astellas Canada, Prime, and Spire Learning. The remaining three authors had no disclosures.
SOURCE: Li S et al. J Am Acad Dermatol. 2017 Dec;77(6):1083-7.E5.
Obesity was associated with a significantly higher risk of rosacea, compared with a healthy weight, particularly when the weight was gained after age 18 years, according to an analysis of data from the Nurses Health Study II.
Investigators evaluated data on 89,886 women in the U.S. study from 1991 through 2005 – 5,249 diagnosed with rosacea – over 14 years to determine what, if any, relationship existed between body mass index (BMI) and rosacea risk.
As BMI increased, the risk of rosacea increased: Compared with participants with a BMI of 21.0 to 22.9 kg/m2 (considered a healthy weight), the risk of rosacea for those with a BMI of 25.0-29.9 (overweight) was 11% greater (95% confidence interval, 1.02-1.21), and among those with a BMI of 30.0-34.9, was 21% greater (95% CI, 1.09-1.34). The risk was 48% greater among those with a BMI of 35 or above (95% CI, 1.33-1.64). (A BMI over 30 is considered obese). The results were published in the December issue of the Journal of the American Academy of Dermatology.
The association between weight and rosacea risk was only significant after age 18 years, when the risk of rosacea increased 4% for every 10 pounds of weight gain, even after adjusting for BMI.
The investigators also found a significant relationship between waist circumference and rosacea risk that was independent of BMI, with the highest quintile of waist circumference associated with a 32% greater risk of rosacea, compared with the lowest quintile. Similarly, the highest quintile of hip circumference was associated with a 38% higher risk of rosacea, also independent of BMI. However there was no association between waist-to-hip ratio and rosacea risk.
While there were associations between the risk of rosacea and smoking, alcohol intake, and physical activity, the relationship between BMI and rosacea risk was not modified by these other risk factors.
Suyun Li, PhD, of the Guangzhou (China) Medical University School of Public Health and coauthors from the department of dermatology, Brown University, Providence, R.I.; and Brigham and Women’s Hospital, Boston, wrote that previous epidemiologic studies examining the interaction between obesity and rosacea have shown inconsistent results. Longitudinal studies on the issue had focused only on BMI and ignored other measures of central obesity.
“To our knowledge, this is the first cohort study on the association between obesity and risk for incident rosacea,” they wrote. “The study contributes to the understanding of rosacea etiology and informs clinical practice related to rosacea prevention and patient care.”
They suggested a number of different mechanisms that might explain how obesity increases the risk of rosacea, including the chronic, low-grade inflammatory state associated with obesity. “Adiposity can augment proinflammatory cytokine expression, such as interleukin 6 and tumor necrosis factor–alpha, both relevant to rosacea pathophysiology,” they noted. “Vascular changes associated with obesity might be another mechanism, considering obesity can lead to abnormalities of vascular function and structure, which might lead to the vasodilatation in rosacea.”
In the NHS II study, data are collected every 2 years, which the authors said ensured they had the most up-to-date information. While rosacea diagnoses relied on self-report, the authors said the study participants – nurses – were likely to have a high validity of self-reporting of rosacea. They acknowledged, however, that the lack of information on rosacea subtypes was a limitation of the study.
The study was supported by the department of dermatology, Brown University and a Nurses’ Health Study II grant. Dr. Li was supported by a research grant from the National Rosacea Society and the Dermatology Foundation. Another author declared having served as an investigator and receiving research funds from Sanofi and Regeneron; serving as a consultant for Sanofi and RTI Health Solutions, and having received honoraria from Astellas Canada, Prime, and Spire Learning. The remaining three authors had no disclosures.
SOURCE: Li S et al. J Am Acad Dermatol. 2017 Dec;77(6):1083-7.E5.
Obesity was associated with a significantly higher risk of rosacea, compared with a healthy weight, particularly when the weight was gained after age 18 years, according to an analysis of data from the Nurses Health Study II.
Investigators evaluated data on 89,886 women in the U.S. study from 1991 through 2005 – 5,249 diagnosed with rosacea – over 14 years to determine what, if any, relationship existed between body mass index (BMI) and rosacea risk.
As BMI increased, the risk of rosacea increased: Compared with participants with a BMI of 21.0 to 22.9 kg/m2 (considered a healthy weight), the risk of rosacea for those with a BMI of 25.0-29.9 (overweight) was 11% greater (95% confidence interval, 1.02-1.21), and among those with a BMI of 30.0-34.9, was 21% greater (95% CI, 1.09-1.34). The risk was 48% greater among those with a BMI of 35 or above (95% CI, 1.33-1.64). (A BMI over 30 is considered obese). The results were published in the December issue of the Journal of the American Academy of Dermatology.
The association between weight and rosacea risk was only significant after age 18 years, when the risk of rosacea increased 4% for every 10 pounds of weight gain, even after adjusting for BMI.
The investigators also found a significant relationship between waist circumference and rosacea risk that was independent of BMI, with the highest quintile of waist circumference associated with a 32% greater risk of rosacea, compared with the lowest quintile. Similarly, the highest quintile of hip circumference was associated with a 38% higher risk of rosacea, also independent of BMI. However there was no association between waist-to-hip ratio and rosacea risk.
While there were associations between the risk of rosacea and smoking, alcohol intake, and physical activity, the relationship between BMI and rosacea risk was not modified by these other risk factors.
Suyun Li, PhD, of the Guangzhou (China) Medical University School of Public Health and coauthors from the department of dermatology, Brown University, Providence, R.I.; and Brigham and Women’s Hospital, Boston, wrote that previous epidemiologic studies examining the interaction between obesity and rosacea have shown inconsistent results. Longitudinal studies on the issue had focused only on BMI and ignored other measures of central obesity.
“To our knowledge, this is the first cohort study on the association between obesity and risk for incident rosacea,” they wrote. “The study contributes to the understanding of rosacea etiology and informs clinical practice related to rosacea prevention and patient care.”
They suggested a number of different mechanisms that might explain how obesity increases the risk of rosacea, including the chronic, low-grade inflammatory state associated with obesity. “Adiposity can augment proinflammatory cytokine expression, such as interleukin 6 and tumor necrosis factor–alpha, both relevant to rosacea pathophysiology,” they noted. “Vascular changes associated with obesity might be another mechanism, considering obesity can lead to abnormalities of vascular function and structure, which might lead to the vasodilatation in rosacea.”
In the NHS II study, data are collected every 2 years, which the authors said ensured they had the most up-to-date information. While rosacea diagnoses relied on self-report, the authors said the study participants – nurses – were likely to have a high validity of self-reporting of rosacea. They acknowledged, however, that the lack of information on rosacea subtypes was a limitation of the study.
The study was supported by the department of dermatology, Brown University and a Nurses’ Health Study II grant. Dr. Li was supported by a research grant from the National Rosacea Society and the Dermatology Foundation. Another author declared having served as an investigator and receiving research funds from Sanofi and Regeneron; serving as a consultant for Sanofi and RTI Health Solutions, and having received honoraria from Astellas Canada, Prime, and Spire Learning. The remaining three authors had no disclosures.
SOURCE: Li S et al. J Am Acad Dermatol. 2017 Dec;77(6):1083-7.E5.
FROM JAAD
Key clinical point: independent of other risk factors such as smoking and alcohol intake.
Major finding: A BMI greater than 35 is associated with a 48% higher risk of rosacea, compared with a BMI of 21.
Data source: Information on rosacea diagnoses among 89,886 Nurses’ Health Study II participants.
Disclosures: The study was supported by the Warren Alpert Medical School of Brown University and a Nurses’ Health Study II grant. One author was supported by a research grant from the National Rosacea Society and the Dermatology Foundation; another author declared research funding, consultancies, and honoraria from the pharmaceutical industry.
Source: Li S et al. J Am Acad Dermatol. 2017 Dec;77(6):1083-7.E5.
Mammography screening’s benefits for breast cancer mortality questioned
Twenty-four years’ worth of data from the Netherlands’ mammography screening program suggest that it has achieved only a marginal impact on breast cancer mortality, according to a paper published online Dec. 5 in the British Medical Journal.
Researchers used data on the stage-specific incidence of breast cancer in the Netherlands during 1989-2012 and estimated the mortality effect of a nationwide, population-based mammography breast cancer screening program, which was introduced in 1988 and targeted women aged 50-75 years.
While there were considerable increases in the incidence of in situ tumors and stage 1 cancers during 1989-2012, the incidence of stages 2-4 cancers was relatively stable over this period. In women aged more than 50 years, who would have been eligible to participate in the screening program, the incidence of stages 2-4 cancers decreased by a nonsignificant 0.16%; from 168/100,000 women in 1989 to 166/100,000 in 2012.
Even when researchers limited their analysis to the period from 1995 to 2012, when the screening program was fully operational and participation rates were around 80%, the incidence of stages 2-4 cancers remained steady. It was also stable in women younger than 50 years, who would not have been eligible for screening.
To estimate the effects of mammography screening on mortality, researchers assumed a scenario without efficient treatment of breast cancer, in which the mortality increase of 0.09% per year seen from 1967 to 1995 would persist until 2012. Under this scenario, they calculated that screening would have at least prevented the predicted 2% increase in breast cancer mortality over that period. Combined with the 0.16% nonsignificant reduction in stages 2-4 cancers over the 23 years of screening, this equated to a total mortality decrease of around 3%.
This reduction paled in comparison to the contributions made by improved treatment and patient management, which the authors suggested would be associated with a 28% reduction in mortality.
“The data on advanced breast cancer in the Netherlands indicate that the Dutch national mammography screening programme would have had little influence on the decreases in breast cancer mortality observed over the past 24 years,” wrote Philippe Autier, MD, and his colleagues from the International Prevention Research Institute in Lyon, France. “This conclusion accords with the mounting evidence that randomised trials have overestimated the ability of mammography screening to reduce the risk of deaths from breast cancer in the entire life period after first exposure to mammography screening.”
However, mammography screening also was associated with a sixfold increase in the incidence of in situ cancers among women aged 50-74 years, over the 23-year study period.
The incidence of stage 1 cancers tripled in woman aged 50-69 years, and increased 3.5-fold in those aged 70-74 years. In comparison, over the same period the rates of stage 1 cancers in women younger than 50 years or older than 75 years increased 1.3-fold.
This amounted to a 50% increase in in situ and stage 1 cancers diagnosed among women who were invited to screening, compared with those younger than 50 years. Even in a best-case scenario, the advent of digital mammography would mean 10,038 overdiagnosed cancers for 640 breast cancer deaths prevented by screening.
“Thus for 1 woman who would not die from breast cancer because of screening, about 16 women would be overdiagnosed with an in situ or a stage 1 cancer,” the authors wrote. “Hence, the advent of digital technologies has probably worsened the overdiagnosis problem without clear evidence for improvements in the ability of screening to curb the risk of breast cancer death.”
The study was partly supported by the International Prevention Research Institute. No conflicts of interest were declared.
SOURCE: Autier P et al. BMJ. 2017 Dec 5;359:j5224.
Twenty-four years’ worth of data from the Netherlands’ mammography screening program suggest that it has achieved only a marginal impact on breast cancer mortality, according to a paper published online Dec. 5 in the British Medical Journal.
Researchers used data on the stage-specific incidence of breast cancer in the Netherlands during 1989-2012 and estimated the mortality effect of a nationwide, population-based mammography breast cancer screening program, which was introduced in 1988 and targeted women aged 50-75 years.
While there were considerable increases in the incidence of in situ tumors and stage 1 cancers during 1989-2012, the incidence of stages 2-4 cancers was relatively stable over this period. In women aged more than 50 years, who would have been eligible to participate in the screening program, the incidence of stages 2-4 cancers decreased by a nonsignificant 0.16%; from 168/100,000 women in 1989 to 166/100,000 in 2012.
Even when researchers limited their analysis to the period from 1995 to 2012, when the screening program was fully operational and participation rates were around 80%, the incidence of stages 2-4 cancers remained steady. It was also stable in women younger than 50 years, who would not have been eligible for screening.
To estimate the effects of mammography screening on mortality, researchers assumed a scenario without efficient treatment of breast cancer, in which the mortality increase of 0.09% per year seen from 1967 to 1995 would persist until 2012. Under this scenario, they calculated that screening would have at least prevented the predicted 2% increase in breast cancer mortality over that period. Combined with the 0.16% nonsignificant reduction in stages 2-4 cancers over the 23 years of screening, this equated to a total mortality decrease of around 3%.
This reduction paled in comparison to the contributions made by improved treatment and patient management, which the authors suggested would be associated with a 28% reduction in mortality.
“The data on advanced breast cancer in the Netherlands indicate that the Dutch national mammography screening programme would have had little influence on the decreases in breast cancer mortality observed over the past 24 years,” wrote Philippe Autier, MD, and his colleagues from the International Prevention Research Institute in Lyon, France. “This conclusion accords with the mounting evidence that randomised trials have overestimated the ability of mammography screening to reduce the risk of deaths from breast cancer in the entire life period after first exposure to mammography screening.”
However, mammography screening also was associated with a sixfold increase in the incidence of in situ cancers among women aged 50-74 years, over the 23-year study period.
The incidence of stage 1 cancers tripled in woman aged 50-69 years, and increased 3.5-fold in those aged 70-74 years. In comparison, over the same period the rates of stage 1 cancers in women younger than 50 years or older than 75 years increased 1.3-fold.
This amounted to a 50% increase in in situ and stage 1 cancers diagnosed among women who were invited to screening, compared with those younger than 50 years. Even in a best-case scenario, the advent of digital mammography would mean 10,038 overdiagnosed cancers for 640 breast cancer deaths prevented by screening.
“Thus for 1 woman who would not die from breast cancer because of screening, about 16 women would be overdiagnosed with an in situ or a stage 1 cancer,” the authors wrote. “Hence, the advent of digital technologies has probably worsened the overdiagnosis problem without clear evidence for improvements in the ability of screening to curb the risk of breast cancer death.”
The study was partly supported by the International Prevention Research Institute. No conflicts of interest were declared.
SOURCE: Autier P et al. BMJ. 2017 Dec 5;359:j5224.
Twenty-four years’ worth of data from the Netherlands’ mammography screening program suggest that it has achieved only a marginal impact on breast cancer mortality, according to a paper published online Dec. 5 in the British Medical Journal.
Researchers used data on the stage-specific incidence of breast cancer in the Netherlands during 1989-2012 and estimated the mortality effect of a nationwide, population-based mammography breast cancer screening program, which was introduced in 1988 and targeted women aged 50-75 years.
While there were considerable increases in the incidence of in situ tumors and stage 1 cancers during 1989-2012, the incidence of stages 2-4 cancers was relatively stable over this period. In women aged more than 50 years, who would have been eligible to participate in the screening program, the incidence of stages 2-4 cancers decreased by a nonsignificant 0.16%; from 168/100,000 women in 1989 to 166/100,000 in 2012.
Even when researchers limited their analysis to the period from 1995 to 2012, when the screening program was fully operational and participation rates were around 80%, the incidence of stages 2-4 cancers remained steady. It was also stable in women younger than 50 years, who would not have been eligible for screening.
To estimate the effects of mammography screening on mortality, researchers assumed a scenario without efficient treatment of breast cancer, in which the mortality increase of 0.09% per year seen from 1967 to 1995 would persist until 2012. Under this scenario, they calculated that screening would have at least prevented the predicted 2% increase in breast cancer mortality over that period. Combined with the 0.16% nonsignificant reduction in stages 2-4 cancers over the 23 years of screening, this equated to a total mortality decrease of around 3%.
This reduction paled in comparison to the contributions made by improved treatment and patient management, which the authors suggested would be associated with a 28% reduction in mortality.
“The data on advanced breast cancer in the Netherlands indicate that the Dutch national mammography screening programme would have had little influence on the decreases in breast cancer mortality observed over the past 24 years,” wrote Philippe Autier, MD, and his colleagues from the International Prevention Research Institute in Lyon, France. “This conclusion accords with the mounting evidence that randomised trials have overestimated the ability of mammography screening to reduce the risk of deaths from breast cancer in the entire life period after first exposure to mammography screening.”
However, mammography screening also was associated with a sixfold increase in the incidence of in situ cancers among women aged 50-74 years, over the 23-year study period.
The incidence of stage 1 cancers tripled in woman aged 50-69 years, and increased 3.5-fold in those aged 70-74 years. In comparison, over the same period the rates of stage 1 cancers in women younger than 50 years or older than 75 years increased 1.3-fold.
This amounted to a 50% increase in in situ and stage 1 cancers diagnosed among women who were invited to screening, compared with those younger than 50 years. Even in a best-case scenario, the advent of digital mammography would mean 10,038 overdiagnosed cancers for 640 breast cancer deaths prevented by screening.
“Thus for 1 woman who would not die from breast cancer because of screening, about 16 women would be overdiagnosed with an in situ or a stage 1 cancer,” the authors wrote. “Hence, the advent of digital technologies has probably worsened the overdiagnosis problem without clear evidence for improvements in the ability of screening to curb the risk of breast cancer death.”
The study was partly supported by the International Prevention Research Institute. No conflicts of interest were declared.
SOURCE: Autier P et al. BMJ. 2017 Dec 5;359:j5224.
FROM BMJ
Key clinical point: Data from the Netherlands’ mammography screening program suggests it has only achieved a small, nonsignificant reduction in breast cancer mortality at a cost of a significant increase in overdiagnosis of stage 1 and in situ breast cancers.
Major finding: The incidence of stages 2-4 cancers decreased only by a nonsignificant 0.16% over the 23 years of mammography screening in the Netherlands.
Data source: Population-based study using 23 years worth of data from the Netherlands.
Disclosures: The study was partly supported by the International Prevention Research Institute. No conflicts of interest were declared.
Source: Autier P et al. BMJ. 2017 Dec 5;359:j5224.
Fecal microbiota transplants by oral capsule noninferior to colonoscopy
Fecal microbiota transplantation (FMT) using oral capsules as the delivery method has been shown to be noninferior to delivery using colonoscopy for the treatment of Clostridium difficile infection, but with a significantly lower price tag.
In an unblended noninferiority trial, published in the Nov. 28 issue of JAMA, 116 adults with at least three documented episodes of C. difficile infection were randomized to either 360 mL of fecal slurry delivered to the cecum via colonoscopy or to 40 capsules of processed fecal microbiota swallowed under direct observation.
Dina Kao, MD, of the department of medicine at the University of Alberta, Edmonton, and coauthors commented that the response rate with the capsules was higher than that seen in other studies of fecal microbiota capsules, which they suggested may partly be due to the larger amount of donor stool used in the study: 80-100 g, compared with 17 g and 25 g used in other studies.
“The higher efficacy observed in this study suggests a dose-dependent response to FMT, and a benefit of bowel lavage prior to FMT, because residual vancomycin was detected up to 8 days despite its discontinuation,” they wrote.
Both treatment modalities achieved similar quality of life improvements. Both groups reported major improvements in domains including physical and emotional health, physical and social functioning, and general health, with no significant differences between the two arms of the study.
The cost per treatment in the colonoscopy group was $874 per patient, compared with $308 per patient in the capsule group.
“Although colonoscopy delivery is more invasive, resource intensive, costly, and inconvenient for patients, it has the advantage of identifying alternative diagnoses,” the authors wrote. “Conversely, when FMT is given by oral capsules, it can be administered in an office setting, which could substantially reduce cost and wait time.”
Both groups also showed significantly improved gut microbiota diversity, which approached that of the donor just 1 week after administration of the treatment.
While 30% of patients characterized FMTs as “unpleasant, gross, or disgusting,” 79% of participants said the unpleasantness was the same or less than anticipated, and 97% said they would undergo the same treatment by the same delivery method again if needed.
However, significantly more patients in the capsule group described their experience as “not at all unpleasant,” compared with the colonoscopy group (66% vs. 44%; 95% CI, 3%-40%; P = .01).
There were no colonic perforations seen in the colonoscopy group, and no infectious complications relating to the treatment in either group. One patient in each group died of underlying cardiopulmonary illness that was unrelated to the treatment, and the rate of minor adverse events was 5.4% in the capsule group and 12.5% in the colonoscopy group.
The authors acknowledged that the lack of a placebo group in the study meant they were not able to measure the size of the effect of fecal microbiota transplantation by either route. One earlier trial had also shown a placebo response rate of 45%.
The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
The AGA Center for Gut Microbiome Research and Education serves as a virtual “home” for AGA activities related to the gut microbiome, including the AGA FMT National Registry, which will assess short- and long-term patient outcomes associated with FMT. Learn more at http://www.gastro.org/about/initiatives/aga-center-for-gut-microbiome-research-education.
Clostridium difficile infection costs the U.S. health care system an estimated $1.5 billion each year, with 450,000 cases reported annually, 20% of which involve a recurrence of the infection. Fecal microbiota transplantation is increasingly being used as a treatment, but more widespread adoption is limited partly by the logistical difficulties of delivery.
This study offers encouraging data on delivery of fecal microbiota transplants via capsule, which may reduce barriers to adoption of this treatment; however, there are still some questions to be answered about the treatment’s efficacy, such as the timing of delivery and the relative importance of stool components.
There are also other approaches that should be considered in future research on C. difficile infection, including the use of vancomycin tapers with and without “chasers” of fidaxomicin/rifaximin, the use of defined microbial communities, and the use of sterile, fecal-derived products, which may even supplant standard fecal microbial transplants in the future.
Krishna Rao, MD, Vincent B. Young, MD, PhD, and Preeti N. Malani, MD, are with the division of infectious diseases in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are taken from an accompanying editorial (JAMA. 2017 Nov 28;381:1979-80. doi: 10.1001/jama.2017.17969). Dr. Young reported consulting fees from Vedanta, Merck, and Finch Therapeutics and grants from MedImmune. No other disclosures were reported.
Clostridium difficile infection costs the U.S. health care system an estimated $1.5 billion each year, with 450,000 cases reported annually, 20% of which involve a recurrence of the infection. Fecal microbiota transplantation is increasingly being used as a treatment, but more widespread adoption is limited partly by the logistical difficulties of delivery.
This study offers encouraging data on delivery of fecal microbiota transplants via capsule, which may reduce barriers to adoption of this treatment; however, there are still some questions to be answered about the treatment’s efficacy, such as the timing of delivery and the relative importance of stool components.
There are also other approaches that should be considered in future research on C. difficile infection, including the use of vancomycin tapers with and without “chasers” of fidaxomicin/rifaximin, the use of defined microbial communities, and the use of sterile, fecal-derived products, which may even supplant standard fecal microbial transplants in the future.
Krishna Rao, MD, Vincent B. Young, MD, PhD, and Preeti N. Malani, MD, are with the division of infectious diseases in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are taken from an accompanying editorial (JAMA. 2017 Nov 28;381:1979-80. doi: 10.1001/jama.2017.17969). Dr. Young reported consulting fees from Vedanta, Merck, and Finch Therapeutics and grants from MedImmune. No other disclosures were reported.
Clostridium difficile infection costs the U.S. health care system an estimated $1.5 billion each year, with 450,000 cases reported annually, 20% of which involve a recurrence of the infection. Fecal microbiota transplantation is increasingly being used as a treatment, but more widespread adoption is limited partly by the logistical difficulties of delivery.
This study offers encouraging data on delivery of fecal microbiota transplants via capsule, which may reduce barriers to adoption of this treatment; however, there are still some questions to be answered about the treatment’s efficacy, such as the timing of delivery and the relative importance of stool components.
There are also other approaches that should be considered in future research on C. difficile infection, including the use of vancomycin tapers with and without “chasers” of fidaxomicin/rifaximin, the use of defined microbial communities, and the use of sterile, fecal-derived products, which may even supplant standard fecal microbial transplants in the future.
Krishna Rao, MD, Vincent B. Young, MD, PhD, and Preeti N. Malani, MD, are with the division of infectious diseases in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are taken from an accompanying editorial (JAMA. 2017 Nov 28;381:1979-80. doi: 10.1001/jama.2017.17969). Dr. Young reported consulting fees from Vedanta, Merck, and Finch Therapeutics and grants from MedImmune. No other disclosures were reported.
Fecal microbiota transplantation (FMT) using oral capsules as the delivery method has been shown to be noninferior to delivery using colonoscopy for the treatment of Clostridium difficile infection, but with a significantly lower price tag.
In an unblended noninferiority trial, published in the Nov. 28 issue of JAMA, 116 adults with at least three documented episodes of C. difficile infection were randomized to either 360 mL of fecal slurry delivered to the cecum via colonoscopy or to 40 capsules of processed fecal microbiota swallowed under direct observation.
Dina Kao, MD, of the department of medicine at the University of Alberta, Edmonton, and coauthors commented that the response rate with the capsules was higher than that seen in other studies of fecal microbiota capsules, which they suggested may partly be due to the larger amount of donor stool used in the study: 80-100 g, compared with 17 g and 25 g used in other studies.
“The higher efficacy observed in this study suggests a dose-dependent response to FMT, and a benefit of bowel lavage prior to FMT, because residual vancomycin was detected up to 8 days despite its discontinuation,” they wrote.
Both treatment modalities achieved similar quality of life improvements. Both groups reported major improvements in domains including physical and emotional health, physical and social functioning, and general health, with no significant differences between the two arms of the study.
The cost per treatment in the colonoscopy group was $874 per patient, compared with $308 per patient in the capsule group.
“Although colonoscopy delivery is more invasive, resource intensive, costly, and inconvenient for patients, it has the advantage of identifying alternative diagnoses,” the authors wrote. “Conversely, when FMT is given by oral capsules, it can be administered in an office setting, which could substantially reduce cost and wait time.”
Both groups also showed significantly improved gut microbiota diversity, which approached that of the donor just 1 week after administration of the treatment.
While 30% of patients characterized FMTs as “unpleasant, gross, or disgusting,” 79% of participants said the unpleasantness was the same or less than anticipated, and 97% said they would undergo the same treatment by the same delivery method again if needed.
However, significantly more patients in the capsule group described their experience as “not at all unpleasant,” compared with the colonoscopy group (66% vs. 44%; 95% CI, 3%-40%; P = .01).
There were no colonic perforations seen in the colonoscopy group, and no infectious complications relating to the treatment in either group. One patient in each group died of underlying cardiopulmonary illness that was unrelated to the treatment, and the rate of minor adverse events was 5.4% in the capsule group and 12.5% in the colonoscopy group.
The authors acknowledged that the lack of a placebo group in the study meant they were not able to measure the size of the effect of fecal microbiota transplantation by either route. One earlier trial had also shown a placebo response rate of 45%.
The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
The AGA Center for Gut Microbiome Research and Education serves as a virtual “home” for AGA activities related to the gut microbiome, including the AGA FMT National Registry, which will assess short- and long-term patient outcomes associated with FMT. Learn more at http://www.gastro.org/about/initiatives/aga-center-for-gut-microbiome-research-education.
Fecal microbiota transplantation (FMT) using oral capsules as the delivery method has been shown to be noninferior to delivery using colonoscopy for the treatment of Clostridium difficile infection, but with a significantly lower price tag.
In an unblended noninferiority trial, published in the Nov. 28 issue of JAMA, 116 adults with at least three documented episodes of C. difficile infection were randomized to either 360 mL of fecal slurry delivered to the cecum via colonoscopy or to 40 capsules of processed fecal microbiota swallowed under direct observation.
Dina Kao, MD, of the department of medicine at the University of Alberta, Edmonton, and coauthors commented that the response rate with the capsules was higher than that seen in other studies of fecal microbiota capsules, which they suggested may partly be due to the larger amount of donor stool used in the study: 80-100 g, compared with 17 g and 25 g used in other studies.
“The higher efficacy observed in this study suggests a dose-dependent response to FMT, and a benefit of bowel lavage prior to FMT, because residual vancomycin was detected up to 8 days despite its discontinuation,” they wrote.
Both treatment modalities achieved similar quality of life improvements. Both groups reported major improvements in domains including physical and emotional health, physical and social functioning, and general health, with no significant differences between the two arms of the study.
The cost per treatment in the colonoscopy group was $874 per patient, compared with $308 per patient in the capsule group.
“Although colonoscopy delivery is more invasive, resource intensive, costly, and inconvenient for patients, it has the advantage of identifying alternative diagnoses,” the authors wrote. “Conversely, when FMT is given by oral capsules, it can be administered in an office setting, which could substantially reduce cost and wait time.”
Both groups also showed significantly improved gut microbiota diversity, which approached that of the donor just 1 week after administration of the treatment.
While 30% of patients characterized FMTs as “unpleasant, gross, or disgusting,” 79% of participants said the unpleasantness was the same or less than anticipated, and 97% said they would undergo the same treatment by the same delivery method again if needed.
However, significantly more patients in the capsule group described their experience as “not at all unpleasant,” compared with the colonoscopy group (66% vs. 44%; 95% CI, 3%-40%; P = .01).
There were no colonic perforations seen in the colonoscopy group, and no infectious complications relating to the treatment in either group. One patient in each group died of underlying cardiopulmonary illness that was unrelated to the treatment, and the rate of minor adverse events was 5.4% in the capsule group and 12.5% in the colonoscopy group.
The authors acknowledged that the lack of a placebo group in the study meant they were not able to measure the size of the effect of fecal microbiota transplantation by either route. One earlier trial had also shown a placebo response rate of 45%.
The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
The AGA Center for Gut Microbiome Research and Education serves as a virtual “home” for AGA activities related to the gut microbiome, including the AGA FMT National Registry, which will assess short- and long-term patient outcomes associated with FMT. Learn more at http://www.gastro.org/about/initiatives/aga-center-for-gut-microbiome-research-education.
FROM JAMA
Key clinical point: Delivering fecal microbiota transplants using oral capsules is noninferior to delivery via colonoscopy in the treatment of Clostridium difficile infection.
Major finding: The rates of resolution of recurrent C. difficile infection with fecal microbiota transplants are similar for delivery via oral capsule or via colonoscopy.
Data source: A randomized, unblended noninferiority trial in 116 adults with recurrent C. difficile infection.
Disclosures: The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.