User login
Shorter colonoscopies tied to higher colorectal cancer rates
Risk of interval colorectal cancer (CRC) more than doubled when average withdrawal times during routine colonoscopy were less than 6 minutes, investigators reported in the October issue of Gastroenterology.
The results reinforce current guidelines that set a withdrawal time of at least 6 minutes as a quality indicator for screening colonoscopy, said Dr. Aasma Shaukat at the Minneapolis Veterans Affairs Health Care System and her associates. “Focusing quality-improvement efforts on withdrawal time [less] than 6 minutes would likely have the most impact,” although rates of interval CRC were “lowest and relatively constant” when withdrawal times were at least 8 minutes, the investigators said.
Short scope withdrawal times are known to decrease adenoma detection rates, but their impact on risk of interval CRC had not been examined before, the investigators said. Their retrospective, multicenter, community-based study included 76,810 colonoscopies performed by 51 endoscopists in and near Minneapolis and St. Paul between 2004 and 2009. Follow-up time was 5.5 years or until cancer diagnosis (Gastroenterology 2015 June 5 [doi: 10.1053/j.gastro.2015.06.001]) .
Patients of endoscopists whose withdrawal times averaged less than 6 minutes had a 2.3-fold higher rate of interval CRC, compared with patients whose physicians took at least 6 minutes to withdraw the scope (incidence rate ratio, 2.3; 95% confidence interval, 1.5-3.4; P less than .0001), even after they had accounted for age, sex, and quality of preparation for colonoscopy, the researchers reported. Every extra minute of withdrawal time also led to a 3.6% rise in the chances of detecting an adenoma (95% CI, 2.4%-4.8%; P less than .0001). Physicians whose adenoma detection rates averaged at least 25% had somewhat lower interval CRC rates among their patients than did those whose adenoma detection rates (ADRs) were less than 25%, although the difference was not significant. Adjusting for ADRs also did not alter the link between withdrawal time and risk of interval CRC, but restricting the analysis to 2007 and later weakened the association slightly (IRR, 2.0; 95% CI; 1.0-3.9; P = .04). Finally, patients who were aged 60-69 years had interval CRCs almost three times more often than did younger patients, and patients who were at least 70 years old had 6.2 times the frequency of interval CRCs, compared with the youngest age group, the investigators reported.The researchers designed the study around the 6-minute withdrawal time threshold because of current guidelines but found “no obvious cut point for defining poor quality for screening colonoscopies,” they said. Although the patient sample represented the community-based target population for screening programs, the study had some limitations, the investigators added. For example, they used 2007 withdrawal times for the years 2004-2006 because they lacked data for those years, and as a result, they could have overestimated the association between withdrawal time and risk of interval CRC, they said. They also would have missed any cancer cases diagnosed in other states and could not distinguish between cancers diagnosed because of symptoms and those that were first detected on screening colonoscopy, they noted.
A Veterans Affairs Career Development program and the Center for Chronic Disease Outcomes Research funded the study. The authors reported having no conflicts of interest.
In this well-planned study by Dr. Shaukat and her colleagues, the authors retrospectively examined the association between an endoscopist’s mean withdrawal time, adenoma detection rate (ADR), and interval cancer rate by using data from a large community gastroenterology practice and a state cancer registry. Prior studies have examined the association between mean withdrawal time and ADR, and ADR and interval cancer, but no study has examined the association between withdrawal time (a process measure of colonoscopy quality) and interval cancer (an outcome measure of colonoscopy quality). In this study, the authors found a statistically significant association between mean withdrawal time and ADR, and mean withdrawal time and interval cancer. Specifically, for mean withdrawal times less than 8 minutes, lower withdrawal times were associated with higher rates of interval cancer. Compared to physicians with mean withdrawal times greater than 6 minutes, those with withdrawal times less than 6 minutes were 2.3 times more likely to have a patient develop an interval cancer. The authors did not find a statistically significant association between ADR and interval cancers, which they attributed to confounding related to the increased risk of cancer in populations with higher adenoma prevalence and the increased likelihood of early cancer detection related to more frequent surveillance examinations. These data provide clear evidence of a link between a modifiable physician characteristic (mean withdrawal time), a readily measurable intermediate outcome measure (ADR), and an important clinical outcome (interval cancer). Such data should prove useful to those who seek not only to measure, but also to improve, the quality of colonoscopy.
Dr. Sameer D. Saini is assistant professor of internal medicine at the University of Michigan Health System, Ann Arbor. He has no conflicts of interest.
In this well-planned study by Dr. Shaukat and her colleagues, the authors retrospectively examined the association between an endoscopist’s mean withdrawal time, adenoma detection rate (ADR), and interval cancer rate by using data from a large community gastroenterology practice and a state cancer registry. Prior studies have examined the association between mean withdrawal time and ADR, and ADR and interval cancer, but no study has examined the association between withdrawal time (a process measure of colonoscopy quality) and interval cancer (an outcome measure of colonoscopy quality). In this study, the authors found a statistically significant association between mean withdrawal time and ADR, and mean withdrawal time and interval cancer. Specifically, for mean withdrawal times less than 8 minutes, lower withdrawal times were associated with higher rates of interval cancer. Compared to physicians with mean withdrawal times greater than 6 minutes, those with withdrawal times less than 6 minutes were 2.3 times more likely to have a patient develop an interval cancer. The authors did not find a statistically significant association between ADR and interval cancers, which they attributed to confounding related to the increased risk of cancer in populations with higher adenoma prevalence and the increased likelihood of early cancer detection related to more frequent surveillance examinations. These data provide clear evidence of a link between a modifiable physician characteristic (mean withdrawal time), a readily measurable intermediate outcome measure (ADR), and an important clinical outcome (interval cancer). Such data should prove useful to those who seek not only to measure, but also to improve, the quality of colonoscopy.
Dr. Sameer D. Saini is assistant professor of internal medicine at the University of Michigan Health System, Ann Arbor. He has no conflicts of interest.
In this well-planned study by Dr. Shaukat and her colleagues, the authors retrospectively examined the association between an endoscopist’s mean withdrawal time, adenoma detection rate (ADR), and interval cancer rate by using data from a large community gastroenterology practice and a state cancer registry. Prior studies have examined the association between mean withdrawal time and ADR, and ADR and interval cancer, but no study has examined the association between withdrawal time (a process measure of colonoscopy quality) and interval cancer (an outcome measure of colonoscopy quality). In this study, the authors found a statistically significant association between mean withdrawal time and ADR, and mean withdrawal time and interval cancer. Specifically, for mean withdrawal times less than 8 minutes, lower withdrawal times were associated with higher rates of interval cancer. Compared to physicians with mean withdrawal times greater than 6 minutes, those with withdrawal times less than 6 minutes were 2.3 times more likely to have a patient develop an interval cancer. The authors did not find a statistically significant association between ADR and interval cancers, which they attributed to confounding related to the increased risk of cancer in populations with higher adenoma prevalence and the increased likelihood of early cancer detection related to more frequent surveillance examinations. These data provide clear evidence of a link between a modifiable physician characteristic (mean withdrawal time), a readily measurable intermediate outcome measure (ADR), and an important clinical outcome (interval cancer). Such data should prove useful to those who seek not only to measure, but also to improve, the quality of colonoscopy.
Dr. Sameer D. Saini is assistant professor of internal medicine at the University of Michigan Health System, Ann Arbor. He has no conflicts of interest.
Risk of interval colorectal cancer (CRC) more than doubled when average withdrawal times during routine colonoscopy were less than 6 minutes, investigators reported in the October issue of Gastroenterology.
The results reinforce current guidelines that set a withdrawal time of at least 6 minutes as a quality indicator for screening colonoscopy, said Dr. Aasma Shaukat at the Minneapolis Veterans Affairs Health Care System and her associates. “Focusing quality-improvement efforts on withdrawal time [less] than 6 minutes would likely have the most impact,” although rates of interval CRC were “lowest and relatively constant” when withdrawal times were at least 8 minutes, the investigators said.
Short scope withdrawal times are known to decrease adenoma detection rates, but their impact on risk of interval CRC had not been examined before, the investigators said. Their retrospective, multicenter, community-based study included 76,810 colonoscopies performed by 51 endoscopists in and near Minneapolis and St. Paul between 2004 and 2009. Follow-up time was 5.5 years or until cancer diagnosis (Gastroenterology 2015 June 5 [doi: 10.1053/j.gastro.2015.06.001]) .
Patients of endoscopists whose withdrawal times averaged less than 6 minutes had a 2.3-fold higher rate of interval CRC, compared with patients whose physicians took at least 6 minutes to withdraw the scope (incidence rate ratio, 2.3; 95% confidence interval, 1.5-3.4; P less than .0001), even after they had accounted for age, sex, and quality of preparation for colonoscopy, the researchers reported. Every extra minute of withdrawal time also led to a 3.6% rise in the chances of detecting an adenoma (95% CI, 2.4%-4.8%; P less than .0001). Physicians whose adenoma detection rates averaged at least 25% had somewhat lower interval CRC rates among their patients than did those whose adenoma detection rates (ADRs) were less than 25%, although the difference was not significant. Adjusting for ADRs also did not alter the link between withdrawal time and risk of interval CRC, but restricting the analysis to 2007 and later weakened the association slightly (IRR, 2.0; 95% CI; 1.0-3.9; P = .04). Finally, patients who were aged 60-69 years had interval CRCs almost three times more often than did younger patients, and patients who were at least 70 years old had 6.2 times the frequency of interval CRCs, compared with the youngest age group, the investigators reported.The researchers designed the study around the 6-minute withdrawal time threshold because of current guidelines but found “no obvious cut point for defining poor quality for screening colonoscopies,” they said. Although the patient sample represented the community-based target population for screening programs, the study had some limitations, the investigators added. For example, they used 2007 withdrawal times for the years 2004-2006 because they lacked data for those years, and as a result, they could have overestimated the association between withdrawal time and risk of interval CRC, they said. They also would have missed any cancer cases diagnosed in other states and could not distinguish between cancers diagnosed because of symptoms and those that were first detected on screening colonoscopy, they noted.
A Veterans Affairs Career Development program and the Center for Chronic Disease Outcomes Research funded the study. The authors reported having no conflicts of interest.
Risk of interval colorectal cancer (CRC) more than doubled when average withdrawal times during routine colonoscopy were less than 6 minutes, investigators reported in the October issue of Gastroenterology.
The results reinforce current guidelines that set a withdrawal time of at least 6 minutes as a quality indicator for screening colonoscopy, said Dr. Aasma Shaukat at the Minneapolis Veterans Affairs Health Care System and her associates. “Focusing quality-improvement efforts on withdrawal time [less] than 6 minutes would likely have the most impact,” although rates of interval CRC were “lowest and relatively constant” when withdrawal times were at least 8 minutes, the investigators said.
Short scope withdrawal times are known to decrease adenoma detection rates, but their impact on risk of interval CRC had not been examined before, the investigators said. Their retrospective, multicenter, community-based study included 76,810 colonoscopies performed by 51 endoscopists in and near Minneapolis and St. Paul between 2004 and 2009. Follow-up time was 5.5 years or until cancer diagnosis (Gastroenterology 2015 June 5 [doi: 10.1053/j.gastro.2015.06.001]) .
Patients of endoscopists whose withdrawal times averaged less than 6 minutes had a 2.3-fold higher rate of interval CRC, compared with patients whose physicians took at least 6 minutes to withdraw the scope (incidence rate ratio, 2.3; 95% confidence interval, 1.5-3.4; P less than .0001), even after they had accounted for age, sex, and quality of preparation for colonoscopy, the researchers reported. Every extra minute of withdrawal time also led to a 3.6% rise in the chances of detecting an adenoma (95% CI, 2.4%-4.8%; P less than .0001). Physicians whose adenoma detection rates averaged at least 25% had somewhat lower interval CRC rates among their patients than did those whose adenoma detection rates (ADRs) were less than 25%, although the difference was not significant. Adjusting for ADRs also did not alter the link between withdrawal time and risk of interval CRC, but restricting the analysis to 2007 and later weakened the association slightly (IRR, 2.0; 95% CI; 1.0-3.9; P = .04). Finally, patients who were aged 60-69 years had interval CRCs almost three times more often than did younger patients, and patients who were at least 70 years old had 6.2 times the frequency of interval CRCs, compared with the youngest age group, the investigators reported.The researchers designed the study around the 6-minute withdrawal time threshold because of current guidelines but found “no obvious cut point for defining poor quality for screening colonoscopies,” they said. Although the patient sample represented the community-based target population for screening programs, the study had some limitations, the investigators added. For example, they used 2007 withdrawal times for the years 2004-2006 because they lacked data for those years, and as a result, they could have overestimated the association between withdrawal time and risk of interval CRC, they said. They also would have missed any cancer cases diagnosed in other states and could not distinguish between cancers diagnosed because of symptoms and those that were first detected on screening colonoscopy, they noted.
A Veterans Affairs Career Development program and the Center for Chronic Disease Outcomes Research funded the study. The authors reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Colonoscope withdrawal times less than 6 minutes are associated with significantly higher rates of interval colorectal cancer.
Major finding: Rates of interval CRC were 2.3 times higher when physicians’ average withdrawal times were less than 6 minutes vs. greater than 6 minutes (P less than .001).
Data source: Retrospective study of 76,810 colonoscopies performed by 51 gastroenterologists.
Disclosures: A Veterans Affairs Career Development program and the Center for Chronic Disease Outcomes Research funded the study. The authors reported having no conflicts of interest.
High death rates for IBD patients who underwent emergency resections
Patients with inflammatory bowel disease (IBD) were about five to eight times more likely to die after emergency intestinal resection as opposed to elective surgery, a large meta-analysis found.
Overall mortality rates after emergency intestinal resection were 5.3% for patients with ulcerative colitis (UC) and 3.6% for patients with Crohn’s disease (CD), said Dr. Sunny Singh and his associates at the University of Calgary in Alberta, Canada. In contrast, only 0.6%-0.7% of patients died after elective resection, the researchers reported in the October issue of Gastroenterology (2015 Jun 5. doi: 10.1053/j.gastro.2015.06.001).
Source: American Gastroenterological AssociationClinicians should optimize medical management to avoid emergency resection, seek ways to reduce associated mortality, and use the data when counseling patients and weighing medical and surgical management options, they added.
Intestinal resection is less common among patients with IBD than in decades past, but almost half of CD patients undergo the surgery within 10 years of diagnosis, as do 16% of UC patients, according to another meta-analysis (Gastroenterology 2013;145:996-1006). Past studies have reported divergent rates of death after these surgeries, the researchers noted. To better understand mortality rates and relevant risk factors, they reviewed 18 original research articles and three abstracts published between 1990 and 2015, all of which were indexed in Medline, EMBASE, or PubMed. The studies included 67,057 UC patients and 75,971 CD patients from 15 countries.
Rates of mortality after elective resection were significantly lower than after emergency resection, whether patients had CD (elective, 0.6%; 95% confidence interval, 0.2%-1.7%; emergency, 3.6%; 1.8%-6.9%) or UC (elective, 0.7%; 0.6%-0.9%; emergency, 5.3%; 3.8%-7.3%), the researchers found. Death rates did not significantly differ based on disease type. Postoperative mortality dropped significantly after the 1990s among CD patients only, perhaps because emergency surgery has become less common in Calgary since 1997, the researchers said. However, they were unable to compare changes in death rates over time by surgery type, they said.
Several factors could explain the high fatality rates after emergency intestinal resection, the researchers said. Patients tended to have worse disease activity and higher rates of intestinal obstruction, intra-abdominal abscess, toxic megacolon, preoperative clostridial diarrhea, venous thromboembolism, malnourishment, or prolonged treatment with intravenous corticosteroids, they said. General surgeons are more likely to perform emergency resections than elective cases, which are typically handled by more experienced colorectal surgeons, they added. Emergency resections also are less likely to be performed laparoscopically than are elective resections, they noted. “The low risk of death associated with elective intestinal resections for CD and UC could be used as a quality assurance benchmark to compare outcomes between hospitals and surgeons,” they added.
The research was funded by the Canadian Institute of Health Research, Alberta-Innovates Health-Solutions, the Alberta IBD Consortium. Dr. Singh reported no conflicts of interest. Senior author Dr. Gilaad Kaplan and four coauthors disclosed speaker, advisory board, and funding relationships with a number of pharmaceutical companies.
Patients with inflammatory bowel disease (IBD) were about five to eight times more likely to die after emergency intestinal resection as opposed to elective surgery, a large meta-analysis found.
Overall mortality rates after emergency intestinal resection were 5.3% for patients with ulcerative colitis (UC) and 3.6% for patients with Crohn’s disease (CD), said Dr. Sunny Singh and his associates at the University of Calgary in Alberta, Canada. In contrast, only 0.6%-0.7% of patients died after elective resection, the researchers reported in the October issue of Gastroenterology (2015 Jun 5. doi: 10.1053/j.gastro.2015.06.001).
Source: American Gastroenterological AssociationClinicians should optimize medical management to avoid emergency resection, seek ways to reduce associated mortality, and use the data when counseling patients and weighing medical and surgical management options, they added.
Intestinal resection is less common among patients with IBD than in decades past, but almost half of CD patients undergo the surgery within 10 years of diagnosis, as do 16% of UC patients, according to another meta-analysis (Gastroenterology 2013;145:996-1006). Past studies have reported divergent rates of death after these surgeries, the researchers noted. To better understand mortality rates and relevant risk factors, they reviewed 18 original research articles and three abstracts published between 1990 and 2015, all of which were indexed in Medline, EMBASE, or PubMed. The studies included 67,057 UC patients and 75,971 CD patients from 15 countries.
Rates of mortality after elective resection were significantly lower than after emergency resection, whether patients had CD (elective, 0.6%; 95% confidence interval, 0.2%-1.7%; emergency, 3.6%; 1.8%-6.9%) or UC (elective, 0.7%; 0.6%-0.9%; emergency, 5.3%; 3.8%-7.3%), the researchers found. Death rates did not significantly differ based on disease type. Postoperative mortality dropped significantly after the 1990s among CD patients only, perhaps because emergency surgery has become less common in Calgary since 1997, the researchers said. However, they were unable to compare changes in death rates over time by surgery type, they said.
Several factors could explain the high fatality rates after emergency intestinal resection, the researchers said. Patients tended to have worse disease activity and higher rates of intestinal obstruction, intra-abdominal abscess, toxic megacolon, preoperative clostridial diarrhea, venous thromboembolism, malnourishment, or prolonged treatment with intravenous corticosteroids, they said. General surgeons are more likely to perform emergency resections than elective cases, which are typically handled by more experienced colorectal surgeons, they added. Emergency resections also are less likely to be performed laparoscopically than are elective resections, they noted. “The low risk of death associated with elective intestinal resections for CD and UC could be used as a quality assurance benchmark to compare outcomes between hospitals and surgeons,” they added.
The research was funded by the Canadian Institute of Health Research, Alberta-Innovates Health-Solutions, the Alberta IBD Consortium. Dr. Singh reported no conflicts of interest. Senior author Dr. Gilaad Kaplan and four coauthors disclosed speaker, advisory board, and funding relationships with a number of pharmaceutical companies.
Patients with inflammatory bowel disease (IBD) were about five to eight times more likely to die after emergency intestinal resection as opposed to elective surgery, a large meta-analysis found.
Overall mortality rates after emergency intestinal resection were 5.3% for patients with ulcerative colitis (UC) and 3.6% for patients with Crohn’s disease (CD), said Dr. Sunny Singh and his associates at the University of Calgary in Alberta, Canada. In contrast, only 0.6%-0.7% of patients died after elective resection, the researchers reported in the October issue of Gastroenterology (2015 Jun 5. doi: 10.1053/j.gastro.2015.06.001).
Source: American Gastroenterological AssociationClinicians should optimize medical management to avoid emergency resection, seek ways to reduce associated mortality, and use the data when counseling patients and weighing medical and surgical management options, they added.
Intestinal resection is less common among patients with IBD than in decades past, but almost half of CD patients undergo the surgery within 10 years of diagnosis, as do 16% of UC patients, according to another meta-analysis (Gastroenterology 2013;145:996-1006). Past studies have reported divergent rates of death after these surgeries, the researchers noted. To better understand mortality rates and relevant risk factors, they reviewed 18 original research articles and three abstracts published between 1990 and 2015, all of which were indexed in Medline, EMBASE, or PubMed. The studies included 67,057 UC patients and 75,971 CD patients from 15 countries.
Rates of mortality after elective resection were significantly lower than after emergency resection, whether patients had CD (elective, 0.6%; 95% confidence interval, 0.2%-1.7%; emergency, 3.6%; 1.8%-6.9%) or UC (elective, 0.7%; 0.6%-0.9%; emergency, 5.3%; 3.8%-7.3%), the researchers found. Death rates did not significantly differ based on disease type. Postoperative mortality dropped significantly after the 1990s among CD patients only, perhaps because emergency surgery has become less common in Calgary since 1997, the researchers said. However, they were unable to compare changes in death rates over time by surgery type, they said.
Several factors could explain the high fatality rates after emergency intestinal resection, the researchers said. Patients tended to have worse disease activity and higher rates of intestinal obstruction, intra-abdominal abscess, toxic megacolon, preoperative clostridial diarrhea, venous thromboembolism, malnourishment, or prolonged treatment with intravenous corticosteroids, they said. General surgeons are more likely to perform emergency resections than elective cases, which are typically handled by more experienced colorectal surgeons, they added. Emergency resections also are less likely to be performed laparoscopically than are elective resections, they noted. “The low risk of death associated with elective intestinal resections for CD and UC could be used as a quality assurance benchmark to compare outcomes between hospitals and surgeons,” they added.
The research was funded by the Canadian Institute of Health Research, Alberta-Innovates Health-Solutions, the Alberta IBD Consortium. Dr. Singh reported no conflicts of interest. Senior author Dr. Gilaad Kaplan and four coauthors disclosed speaker, advisory board, and funding relationships with a number of pharmaceutical companies.
FROM GASTROENTEROLOGY
Key clinical point: Patients with IBD were about five to eight times more likely to die after emergency intestinal resection as opposed to elective surgery.
Major finding: Overall mortality rates after emergency intestinal resection were 5.3% for patients with ulcerative colitis and 3.6% for Crohn’s disease; mortality rates after elective surgery were 0.7% and 0.6%, respectively.
Data source: Meta-analysis of 18 original research studies and three abstracts published between 1990 and 2015.
Disclosures: The research was funded by the Canadian Institute of Health Research, Alberta-Innovates Health-Solutions, the Alberta IBD Consortium. Dr. Singh reported no conflicts of interest. Senior author Dr. Gilaad Kaplan and four coauthors disclosed speaker, advisory board, and funding relationships with a number of pharmaceutical companies.
Brodalumab psoriasis results published, development plans continue
Brodalumab met all primary endpoints against its first-in-class rival, ustekinumab, and against placebo in two phase III trials of more than 3,700 patients with moderate to severe plaque psoriasis, investigators reported in the New England Journal of Medicine.
The results of the two studies, AMAGINE-2 and AMAGINE-3, were published on Sept. 30 (N Eng J Med. 2015;373:1318-28).
“The data for brodalumab are the best data we’ve seen. It literally is twice as effective at achieving PASI [Psoriasis Area Severity Index] 100 as ustekinumab, which is a spectacular drug,” lead investigator Dr. Mark Lebwohl said in an interview. “More than two-thirds of patients achieved PASI 90. We’ve never seen data like this before,” said Dr. Lebwohl, professor and chairman of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
Dermatologists and patients have closely followed brodalumab, an investigational interleukin-17 receptor A inhibitor, which, in March 2015, was reported to have doubled the PASI 100 response rate of ustekinumab in the AMAGINE-2 trial. But 2 months later, Amgen pulled funding and ended its partnership with AstraZeneca on the biologic in the wake of suicides of two patients who had recently completed brodalumab treatment. At the time, Amgen stated that it was concerned that brodalumab would receive restrictive labeling related to suicidal ideation and behavior. An Amgen spokeswoman on Sept. 29 declined to elaborate.
On Sept. 1, however, Valeant Pharmaceuticals announced that it was partnering with AstraZeneca to continue with plans to develop and commercialize brodalumab and that regulatory submissions in the United States and the European Union were planned for the 4th quarter of 2015.
In the interview, Dr. Lebwohl said he did not know of a mechanism by which blocking the IL-17 receptor might increase the risk of depression or suicide. “But psoriasis is certainly associated with depression,” he added. “Two out of more than 3,700 patients committed suicide over more than a year, and that could certainly be attributable to the depression associated with the underlying skin disease.”
AMAGINE-2 and AMAGINE-3 were replicate phase III, double-blind, randomized, controlled trials of 3,712 patients with moderate to severe plaque psoriasis. In the two studies, week 12 PASI 75 rates were about 85% with 210 mg of brodalumab, about 68% for 140 mg of brodalumab, and 6%-8% for placebo (P less than .001). Moreover, week 12 PASI 100 response rates with 210 mg of brodalumab were 37% and 44%, compared with 19% and 22% for ustekinumab (P less than .001), the investigators reported.
Median time to PASI 75 on 210 mg of brodalumab was 4 weeks – about twice as fast as for ustekinumab. Rates of static Physician’s Global Assessment (sPGA) scores of 0 or 1 (clear or almost clear skin) also were significantly higher for brodalumab, compared with placebo (P less than .001).
Mild to moderate candidiasis was more common during induction of brodalumab, compared with ustekinumab or placebo, underscoring the role of interleukin-17A in microbial surveillance, Dr. Lebwohl and his associates pointed out. They estimated that 1-1.3 serious infections occurred for every 100 patient-years of exposure to brodalumab, through 52 weeks.
The investigators reported but did not comment on the suicides, stating only that the study populations were large enough to assess common adverse events but “may have been inadequate for the detection of rare adverse events, which would require longer follow-up of large numbers of patients to provide a full understanding of the safety profile of brodalumab.”
Ustekinumab is marketed as Stelara.
Amgen funded the AMAGINE-2 and AMAGINE-3 studies. Dr. Lebwohl reported having received grant support from Amgen, AbbVie, Janssen Biotech, UCB Pharma, Pfizer, Celgene, Eli Lilly, and Novartis outside the submitted work. Twenty-four coauthors reported grant support or personal fees from Amgen, Abbvie, Merck, Janssen, and several other pharmaceutical companies. The other investigators declared no competing interests.
Brodalumab met all primary endpoints against its first-in-class rival, ustekinumab, and against placebo in two phase III trials of more than 3,700 patients with moderate to severe plaque psoriasis, investigators reported in the New England Journal of Medicine.
The results of the two studies, AMAGINE-2 and AMAGINE-3, were published on Sept. 30 (N Eng J Med. 2015;373:1318-28).
“The data for brodalumab are the best data we’ve seen. It literally is twice as effective at achieving PASI [Psoriasis Area Severity Index] 100 as ustekinumab, which is a spectacular drug,” lead investigator Dr. Mark Lebwohl said in an interview. “More than two-thirds of patients achieved PASI 90. We’ve never seen data like this before,” said Dr. Lebwohl, professor and chairman of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
Dermatologists and patients have closely followed brodalumab, an investigational interleukin-17 receptor A inhibitor, which, in March 2015, was reported to have doubled the PASI 100 response rate of ustekinumab in the AMAGINE-2 trial. But 2 months later, Amgen pulled funding and ended its partnership with AstraZeneca on the biologic in the wake of suicides of two patients who had recently completed brodalumab treatment. At the time, Amgen stated that it was concerned that brodalumab would receive restrictive labeling related to suicidal ideation and behavior. An Amgen spokeswoman on Sept. 29 declined to elaborate.
On Sept. 1, however, Valeant Pharmaceuticals announced that it was partnering with AstraZeneca to continue with plans to develop and commercialize brodalumab and that regulatory submissions in the United States and the European Union were planned for the 4th quarter of 2015.
In the interview, Dr. Lebwohl said he did not know of a mechanism by which blocking the IL-17 receptor might increase the risk of depression or suicide. “But psoriasis is certainly associated with depression,” he added. “Two out of more than 3,700 patients committed suicide over more than a year, and that could certainly be attributable to the depression associated with the underlying skin disease.”
AMAGINE-2 and AMAGINE-3 were replicate phase III, double-blind, randomized, controlled trials of 3,712 patients with moderate to severe plaque psoriasis. In the two studies, week 12 PASI 75 rates were about 85% with 210 mg of brodalumab, about 68% for 140 mg of brodalumab, and 6%-8% for placebo (P less than .001). Moreover, week 12 PASI 100 response rates with 210 mg of brodalumab were 37% and 44%, compared with 19% and 22% for ustekinumab (P less than .001), the investigators reported.
Median time to PASI 75 on 210 mg of brodalumab was 4 weeks – about twice as fast as for ustekinumab. Rates of static Physician’s Global Assessment (sPGA) scores of 0 or 1 (clear or almost clear skin) also were significantly higher for brodalumab, compared with placebo (P less than .001).
Mild to moderate candidiasis was more common during induction of brodalumab, compared with ustekinumab or placebo, underscoring the role of interleukin-17A in microbial surveillance, Dr. Lebwohl and his associates pointed out. They estimated that 1-1.3 serious infections occurred for every 100 patient-years of exposure to brodalumab, through 52 weeks.
The investigators reported but did not comment on the suicides, stating only that the study populations were large enough to assess common adverse events but “may have been inadequate for the detection of rare adverse events, which would require longer follow-up of large numbers of patients to provide a full understanding of the safety profile of brodalumab.”
Ustekinumab is marketed as Stelara.
Amgen funded the AMAGINE-2 and AMAGINE-3 studies. Dr. Lebwohl reported having received grant support from Amgen, AbbVie, Janssen Biotech, UCB Pharma, Pfizer, Celgene, Eli Lilly, and Novartis outside the submitted work. Twenty-four coauthors reported grant support or personal fees from Amgen, Abbvie, Merck, Janssen, and several other pharmaceutical companies. The other investigators declared no competing interests.
Brodalumab met all primary endpoints against its first-in-class rival, ustekinumab, and against placebo in two phase III trials of more than 3,700 patients with moderate to severe plaque psoriasis, investigators reported in the New England Journal of Medicine.
The results of the two studies, AMAGINE-2 and AMAGINE-3, were published on Sept. 30 (N Eng J Med. 2015;373:1318-28).
“The data for brodalumab are the best data we’ve seen. It literally is twice as effective at achieving PASI [Psoriasis Area Severity Index] 100 as ustekinumab, which is a spectacular drug,” lead investigator Dr. Mark Lebwohl said in an interview. “More than two-thirds of patients achieved PASI 90. We’ve never seen data like this before,” said Dr. Lebwohl, professor and chairman of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
Dermatologists and patients have closely followed brodalumab, an investigational interleukin-17 receptor A inhibitor, which, in March 2015, was reported to have doubled the PASI 100 response rate of ustekinumab in the AMAGINE-2 trial. But 2 months later, Amgen pulled funding and ended its partnership with AstraZeneca on the biologic in the wake of suicides of two patients who had recently completed brodalumab treatment. At the time, Amgen stated that it was concerned that brodalumab would receive restrictive labeling related to suicidal ideation and behavior. An Amgen spokeswoman on Sept. 29 declined to elaborate.
On Sept. 1, however, Valeant Pharmaceuticals announced that it was partnering with AstraZeneca to continue with plans to develop and commercialize brodalumab and that regulatory submissions in the United States and the European Union were planned for the 4th quarter of 2015.
In the interview, Dr. Lebwohl said he did not know of a mechanism by which blocking the IL-17 receptor might increase the risk of depression or suicide. “But psoriasis is certainly associated with depression,” he added. “Two out of more than 3,700 patients committed suicide over more than a year, and that could certainly be attributable to the depression associated with the underlying skin disease.”
AMAGINE-2 and AMAGINE-3 were replicate phase III, double-blind, randomized, controlled trials of 3,712 patients with moderate to severe plaque psoriasis. In the two studies, week 12 PASI 75 rates were about 85% with 210 mg of brodalumab, about 68% for 140 mg of brodalumab, and 6%-8% for placebo (P less than .001). Moreover, week 12 PASI 100 response rates with 210 mg of brodalumab were 37% and 44%, compared with 19% and 22% for ustekinumab (P less than .001), the investigators reported.
Median time to PASI 75 on 210 mg of brodalumab was 4 weeks – about twice as fast as for ustekinumab. Rates of static Physician’s Global Assessment (sPGA) scores of 0 or 1 (clear or almost clear skin) also were significantly higher for brodalumab, compared with placebo (P less than .001).
Mild to moderate candidiasis was more common during induction of brodalumab, compared with ustekinumab or placebo, underscoring the role of interleukin-17A in microbial surveillance, Dr. Lebwohl and his associates pointed out. They estimated that 1-1.3 serious infections occurred for every 100 patient-years of exposure to brodalumab, through 52 weeks.
The investigators reported but did not comment on the suicides, stating only that the study populations were large enough to assess common adverse events but “may have been inadequate for the detection of rare adverse events, which would require longer follow-up of large numbers of patients to provide a full understanding of the safety profile of brodalumab.”
Ustekinumab is marketed as Stelara.
Amgen funded the AMAGINE-2 and AMAGINE-3 studies. Dr. Lebwohl reported having received grant support from Amgen, AbbVie, Janssen Biotech, UCB Pharma, Pfizer, Celgene, Eli Lilly, and Novartis outside the submitted work. Twenty-four coauthors reported grant support or personal fees from Amgen, Abbvie, Merck, Janssen, and several other pharmaceutical companies. The other investigators declared no competing interests.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:Brodalumab met all primary endpoints against ustekinumab and placebo in patients with moderate to severe plaque psoriasis.
Major finding: Week 12 PASI 100 response rates were 37% and 44% with 210 mg biweekly brodalumab, compared with 19% and 22% for ustekinumab (P less than .001).
Data source: AMAGINE-2 and AMAGINE-3 were replicate phase III double-blind, randomized, placebo- and active-comparator controlled trials of 3,712 patients with moderate to severe plaque psoriasis.
Disclosures: Amgen funded the studies. Dr. Lebwohl reported having received grant support from Amgen, AbbVie, Janssen Biotech, UCB Pharma, Pfizer, Celgene, Eli Lilly, and Novartis outside the submitted work. Twenty-four coauthors reported financial relationships outside the submitted work with Abbvie, Merck, Janssen, and several other pharmaceutical companies. The other investigators declared no competing interests.
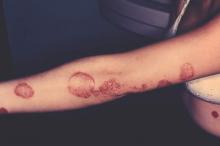
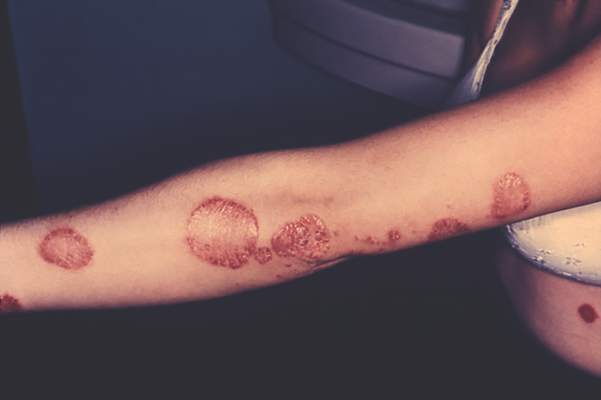
Large Study Identifies Psoriasis as an Independent Risk Factor for Major Depression
Self-reported psoriasis more than doubled the odds of major depression, even after accounting for demographic factors and comorbidities, reported the authors of a study that used data from the National Health and Nutrition Examination Survey.
In addition, depressive symptoms adversely affected the work and social lives of psoriasis patients in this first-in-kind study, said Dr. Brandon Cohen and his associates of the department of dermatology, New York University. “The risk of major depression was not associated with the self-reported degree of psoriasis. Therefore, our study supports that all patients with psoriasis, regardless of severity, are at risk for depressive symptoms and may benefit from depression screening,” they wrote. The study was published online on Sept. 30 in JAMA Dermatology (2015 Sep 30. doi: 10.1001/jamadermatol.2015.3605).
Psoriasis is known to increase the risk of major depression, as well as anxiety disorders, substance abuse, and suicidal ideation. To better understand whether disease severity or comorbidities affect the psoriasis-depression link, the researchers analyzed self-reported health data from 12,382 individuals who had responded to the National Health and Nutrition Examination Survey between 2009 and 2012. The investigators defined major depression as a score of at least 10 on the 27-point Patient Health Questionnaire-9 (PHQ-9). Psoriasis was self-reported, and some psoriasis patients were also asked to rate the severity of their skin disease.
After adjusting for factors such as age, sex, race, and history of myocardial infarction, stroke, and diabetes, the 351 individuals with psoriasis had more than twice the odds of major depression, compared with respondents without psoriasis (odds ratio, 2.09; 95% confidence interval, 1.41-3.11; P less than .001). Furthermore, the average PHQ-9 score among psoriasis patients was significantly higher than that among other respondents (4.54 vs. 3.22; P less than .001).
Neither severity of skin disease nor history of MI or stroke affected the relationship between psoriasis and depression. “The psychiatric burden of psoriasis may be more closely tied to patients’ perception of the social response to their appearance than objective disease severity,” the investigators said. They noted that as far as they know, this is the first study to use a nationally representative sample of patients to evaluate the association between psoriasis and major depression.
The investigators reported no funding sources and declared no competing interests.
Self-reported psoriasis more than doubled the odds of major depression, even after accounting for demographic factors and comorbidities, reported the authors of a study that used data from the National Health and Nutrition Examination Survey.
In addition, depressive symptoms adversely affected the work and social lives of psoriasis patients in this first-in-kind study, said Dr. Brandon Cohen and his associates of the department of dermatology, New York University. “The risk of major depression was not associated with the self-reported degree of psoriasis. Therefore, our study supports that all patients with psoriasis, regardless of severity, are at risk for depressive symptoms and may benefit from depression screening,” they wrote. The study was published online on Sept. 30 in JAMA Dermatology (2015 Sep 30. doi: 10.1001/jamadermatol.2015.3605).
Psoriasis is known to increase the risk of major depression, as well as anxiety disorders, substance abuse, and suicidal ideation. To better understand whether disease severity or comorbidities affect the psoriasis-depression link, the researchers analyzed self-reported health data from 12,382 individuals who had responded to the National Health and Nutrition Examination Survey between 2009 and 2012. The investigators defined major depression as a score of at least 10 on the 27-point Patient Health Questionnaire-9 (PHQ-9). Psoriasis was self-reported, and some psoriasis patients were also asked to rate the severity of their skin disease.
After adjusting for factors such as age, sex, race, and history of myocardial infarction, stroke, and diabetes, the 351 individuals with psoriasis had more than twice the odds of major depression, compared with respondents without psoriasis (odds ratio, 2.09; 95% confidence interval, 1.41-3.11; P less than .001). Furthermore, the average PHQ-9 score among psoriasis patients was significantly higher than that among other respondents (4.54 vs. 3.22; P less than .001).
Neither severity of skin disease nor history of MI or stroke affected the relationship between psoriasis and depression. “The psychiatric burden of psoriasis may be more closely tied to patients’ perception of the social response to their appearance than objective disease severity,” the investigators said. They noted that as far as they know, this is the first study to use a nationally representative sample of patients to evaluate the association between psoriasis and major depression.
The investigators reported no funding sources and declared no competing interests.
Self-reported psoriasis more than doubled the odds of major depression, even after accounting for demographic factors and comorbidities, reported the authors of a study that used data from the National Health and Nutrition Examination Survey.
In addition, depressive symptoms adversely affected the work and social lives of psoriasis patients in this first-in-kind study, said Dr. Brandon Cohen and his associates of the department of dermatology, New York University. “The risk of major depression was not associated with the self-reported degree of psoriasis. Therefore, our study supports that all patients with psoriasis, regardless of severity, are at risk for depressive symptoms and may benefit from depression screening,” they wrote. The study was published online on Sept. 30 in JAMA Dermatology (2015 Sep 30. doi: 10.1001/jamadermatol.2015.3605).
Psoriasis is known to increase the risk of major depression, as well as anxiety disorders, substance abuse, and suicidal ideation. To better understand whether disease severity or comorbidities affect the psoriasis-depression link, the researchers analyzed self-reported health data from 12,382 individuals who had responded to the National Health and Nutrition Examination Survey between 2009 and 2012. The investigators defined major depression as a score of at least 10 on the 27-point Patient Health Questionnaire-9 (PHQ-9). Psoriasis was self-reported, and some psoriasis patients were also asked to rate the severity of their skin disease.
After adjusting for factors such as age, sex, race, and history of myocardial infarction, stroke, and diabetes, the 351 individuals with psoriasis had more than twice the odds of major depression, compared with respondents without psoriasis (odds ratio, 2.09; 95% confidence interval, 1.41-3.11; P less than .001). Furthermore, the average PHQ-9 score among psoriasis patients was significantly higher than that among other respondents (4.54 vs. 3.22; P less than .001).
Neither severity of skin disease nor history of MI or stroke affected the relationship between psoriasis and depression. “The psychiatric burden of psoriasis may be more closely tied to patients’ perception of the social response to their appearance than objective disease severity,” the investigators said. They noted that as far as they know, this is the first study to use a nationally representative sample of patients to evaluate the association between psoriasis and major depression.
The investigators reported no funding sources and declared no competing interests.
FROM JAMA DERMATOLOGY
Large study identifies psoriasis as an independent risk factor for major depression
Self-reported psoriasis more than doubled the odds of major depression, even after accounting for demographic factors and comorbidities, reported the authors of a study that used data from the National Health and Nutrition Examination Survey.
In addition, depressive symptoms adversely affected the work and social lives of psoriasis patients in this first-in-kind study, said Dr. Brandon Cohen and his associates of the department of dermatology, New York University. “The risk of major depression was not associated with the self-reported degree of psoriasis. Therefore, our study supports that all patients with psoriasis, regardless of severity, are at risk for depressive symptoms and may benefit from depression screening,” they wrote. The study was published online on Sept. 30 in JAMA Dermatology (2015 Sep 30. doi: 10.1001/jamadermatol.2015.3605).
Psoriasis is known to increase the risk of major depression, as well as anxiety disorders, substance abuse, and suicidal ideation. To better understand whether disease severity or comorbidities affect the psoriasis-depression link, the researchers analyzed self-reported health data from 12,382 individuals who had responded to the National Health and Nutrition Examination Survey between 2009 and 2012. The investigators defined major depression as a score of at least 10 on the 27-point Patient Health Questionnaire-9 (PHQ-9). Psoriasis was self-reported, and some psoriasis patients were also asked to rate the severity of their skin disease.
After adjusting for factors such as age, sex, race, and history of myocardial infarction, stroke, and diabetes, the 351 individuals with psoriasis had more than twice the odds of major depression, compared with respondents without psoriasis (odds ratio, 2.09; 95% confidence interval, 1.41-3.11; P less than .001). Furthermore, the average PHQ-9 score among psoriasis patients was significantly higher than that among other respondents (4.54 vs. 3.22; P less than .001).
Neither severity of skin disease nor history of MI or stroke affected the relationship between psoriasis and depression. “The psychiatric burden of psoriasis may be more closely tied to patients’ perception of the social response to their appearance than objective disease severity,” the investigators said. They noted that as far as they know, this is the first study to use a nationally representative sample of patients to evaluate the association between psoriasis and major depression.
The investigators reported no funding sources and declared no competing interests.
Self-reported psoriasis more than doubled the odds of major depression, even after accounting for demographic factors and comorbidities, reported the authors of a study that used data from the National Health and Nutrition Examination Survey.
In addition, depressive symptoms adversely affected the work and social lives of psoriasis patients in this first-in-kind study, said Dr. Brandon Cohen and his associates of the department of dermatology, New York University. “The risk of major depression was not associated with the self-reported degree of psoriasis. Therefore, our study supports that all patients with psoriasis, regardless of severity, are at risk for depressive symptoms and may benefit from depression screening,” they wrote. The study was published online on Sept. 30 in JAMA Dermatology (2015 Sep 30. doi: 10.1001/jamadermatol.2015.3605).
Psoriasis is known to increase the risk of major depression, as well as anxiety disorders, substance abuse, and suicidal ideation. To better understand whether disease severity or comorbidities affect the psoriasis-depression link, the researchers analyzed self-reported health data from 12,382 individuals who had responded to the National Health and Nutrition Examination Survey between 2009 and 2012. The investigators defined major depression as a score of at least 10 on the 27-point Patient Health Questionnaire-9 (PHQ-9). Psoriasis was self-reported, and some psoriasis patients were also asked to rate the severity of their skin disease.
After adjusting for factors such as age, sex, race, and history of myocardial infarction, stroke, and diabetes, the 351 individuals with psoriasis had more than twice the odds of major depression, compared with respondents without psoriasis (odds ratio, 2.09; 95% confidence interval, 1.41-3.11; P less than .001). Furthermore, the average PHQ-9 score among psoriasis patients was significantly higher than that among other respondents (4.54 vs. 3.22; P less than .001).
Neither severity of skin disease nor history of MI or stroke affected the relationship between psoriasis and depression. “The psychiatric burden of psoriasis may be more closely tied to patients’ perception of the social response to their appearance than objective disease severity,” the investigators said. They noted that as far as they know, this is the first study to use a nationally representative sample of patients to evaluate the association between psoriasis and major depression.
The investigators reported no funding sources and declared no competing interests.
Self-reported psoriasis more than doubled the odds of major depression, even after accounting for demographic factors and comorbidities, reported the authors of a study that used data from the National Health and Nutrition Examination Survey.
In addition, depressive symptoms adversely affected the work and social lives of psoriasis patients in this first-in-kind study, said Dr. Brandon Cohen and his associates of the department of dermatology, New York University. “The risk of major depression was not associated with the self-reported degree of psoriasis. Therefore, our study supports that all patients with psoriasis, regardless of severity, are at risk for depressive symptoms and may benefit from depression screening,” they wrote. The study was published online on Sept. 30 in JAMA Dermatology (2015 Sep 30. doi: 10.1001/jamadermatol.2015.3605).
Psoriasis is known to increase the risk of major depression, as well as anxiety disorders, substance abuse, and suicidal ideation. To better understand whether disease severity or comorbidities affect the psoriasis-depression link, the researchers analyzed self-reported health data from 12,382 individuals who had responded to the National Health and Nutrition Examination Survey between 2009 and 2012. The investigators defined major depression as a score of at least 10 on the 27-point Patient Health Questionnaire-9 (PHQ-9). Psoriasis was self-reported, and some psoriasis patients were also asked to rate the severity of their skin disease.
After adjusting for factors such as age, sex, race, and history of myocardial infarction, stroke, and diabetes, the 351 individuals with psoriasis had more than twice the odds of major depression, compared with respondents without psoriasis (odds ratio, 2.09; 95% confidence interval, 1.41-3.11; P less than .001). Furthermore, the average PHQ-9 score among psoriasis patients was significantly higher than that among other respondents (4.54 vs. 3.22; P less than .001).
Neither severity of skin disease nor history of MI or stroke affected the relationship between psoriasis and depression. “The psychiatric burden of psoriasis may be more closely tied to patients’ perception of the social response to their appearance than objective disease severity,” the investigators said. They noted that as far as they know, this is the first study to use a nationally representative sample of patients to evaluate the association between psoriasis and major depression.
The investigators reported no funding sources and declared no competing interests.
FROM JAMA DERMATOLOGY
Key clinical point:Psoriasis was associated with a significantly increased risk of major depression in a large population-based U.S. study.
Major finding: Self-reported psoriasis more than doubled the odds of meeting criteria for major depression (OR, 2.09; P less than .001).
Data source: The cross-sectional study evaluated data from 12,382 National Health and Nutrition Examination Survey participants, who responded between 2009 and 2012.
Disclosures: The authors reported no funding sources and declared no competing interests.
Cardiac biomarkers predict cancer mortality
High circulating levels of six cardiovascular biomarkers predicted cancer mortality even before the start of treatment and regardless of tumor type or stage, according to a study published online Sept. 28 in Heart.
Cancer patients had high levels of these biomarkers even though they had no clinical signs of heart disease or concurrent infections, wrote Noemi Pavo of Medical University of Vienna. The findings suggest that these patients could benefit from enhanced heart failure therapies that go beyond the current focus on preventing cardiotoxic side effects of chemotherapy and radiotherapy, noted Dr. Pavo and her associates.
Cardiovascular hormone and peptide levels can rise during cancer treatment, but whether cancer itself affects these biomarkers has been unclear, the investigators said. Therefore, they prospectively measured levels of five cardiovascular hormones – NT-proBNP, MR-proANP, MR-proADM, CT-pro-ET, and copeptin – in addition to high-sensitive troponin, the proinflammatory markers interleukin 6 and C-reactive protein, and cytokines serum amyloid A, haptoglobin, and fibronectin, in 555 patients with newly diagnosed, as-yet-untreated cancer (Heart 2015 Sep 28. doi:10.1136/heartjnl-2015-307848).
A total of 186 patients (34%) died after an average of 25 months of follow-up, the researchers reported. Levels of all cardiovascular hormones and hsTnT increased with tumor stage progression. After the researchers controlled for age, tumor type, tumor stage, glomerular filtration rate, and cardiac status, rising levels of all five cardiac hormones and hsTnT independently predicted mortality, with adjusted hazard ratios ranging from 1.21 for CT-proET-1 and hsTnT to 1.54 for the natural logarithm of NT-proBNP, and P values ranging from .014 to less than.001.
“All of these markers are strongly related to mortality, implying a direct association with disease progression,” the researchers said. “While our endpoint [was] all-cause mortality, precise information about the percentage of cardiovascular-related death would certainly be of important clinical interest. Since post hoc interpretations of certifications of death are not reliable, the development of a cardiac disease during cancer progression should be documented in longitudinal studies in the future.”
An unrestricted grant from Thermo Fisher funded the study. The researchers declared no competing interests.
This study opens the potential for new management strategies integrating cardiology and oncology. Treating overt and perhaps subclinical cardiac dysfunction may improve outcomes in patients with cancer, and may possibly improve progression-free cancer survival based on optimizing cancer treatment and preventing interruptions. These biomarkers could serve as a surveillance strategy for both cardiologists and oncologists. It would be equally tantalizing to know whether treating the cancer effectively improves cardiac outcomes, but this will be more challenging to unravel if the cancer treatment imparts potential cardiotoxicity.
The key next stage will be to reproduce these findings in a prospectively designed multicenter study with larger numbers to validate the conclusion, collect the mode of death, and also to consider whether serial biomarker assessment adds further predictive power. Pavo et al. should be congratulated on bringing to our attention the widening complexity of biomarker biology and the potential to identify single biomarkers with the unique properties to predict both cardiovascular and oncology outcomes.
Dr. Alexander Lyon is at Imperial College and Royal Brompton Hospital in London. He reported receiving research funding and having consulting and advisory relationships with Pfizer, Onyx Pharmaceuticals, Ferring Pharmaceuticals, and Clinigen Group. These remarks are from his editorial (Heart 2015 Sep. 28. doi:10.1136/heartjnl-2015-308208).
This study opens the potential for new management strategies integrating cardiology and oncology. Treating overt and perhaps subclinical cardiac dysfunction may improve outcomes in patients with cancer, and may possibly improve progression-free cancer survival based on optimizing cancer treatment and preventing interruptions. These biomarkers could serve as a surveillance strategy for both cardiologists and oncologists. It would be equally tantalizing to know whether treating the cancer effectively improves cardiac outcomes, but this will be more challenging to unravel if the cancer treatment imparts potential cardiotoxicity.
The key next stage will be to reproduce these findings in a prospectively designed multicenter study with larger numbers to validate the conclusion, collect the mode of death, and also to consider whether serial biomarker assessment adds further predictive power. Pavo et al. should be congratulated on bringing to our attention the widening complexity of biomarker biology and the potential to identify single biomarkers with the unique properties to predict both cardiovascular and oncology outcomes.
Dr. Alexander Lyon is at Imperial College and Royal Brompton Hospital in London. He reported receiving research funding and having consulting and advisory relationships with Pfizer, Onyx Pharmaceuticals, Ferring Pharmaceuticals, and Clinigen Group. These remarks are from his editorial (Heart 2015 Sep. 28. doi:10.1136/heartjnl-2015-308208).
This study opens the potential for new management strategies integrating cardiology and oncology. Treating overt and perhaps subclinical cardiac dysfunction may improve outcomes in patients with cancer, and may possibly improve progression-free cancer survival based on optimizing cancer treatment and preventing interruptions. These biomarkers could serve as a surveillance strategy for both cardiologists and oncologists. It would be equally tantalizing to know whether treating the cancer effectively improves cardiac outcomes, but this will be more challenging to unravel if the cancer treatment imparts potential cardiotoxicity.
The key next stage will be to reproduce these findings in a prospectively designed multicenter study with larger numbers to validate the conclusion, collect the mode of death, and also to consider whether serial biomarker assessment adds further predictive power. Pavo et al. should be congratulated on bringing to our attention the widening complexity of biomarker biology and the potential to identify single biomarkers with the unique properties to predict both cardiovascular and oncology outcomes.
Dr. Alexander Lyon is at Imperial College and Royal Brompton Hospital in London. He reported receiving research funding and having consulting and advisory relationships with Pfizer, Onyx Pharmaceuticals, Ferring Pharmaceuticals, and Clinigen Group. These remarks are from his editorial (Heart 2015 Sep. 28. doi:10.1136/heartjnl-2015-308208).
High circulating levels of six cardiovascular biomarkers predicted cancer mortality even before the start of treatment and regardless of tumor type or stage, according to a study published online Sept. 28 in Heart.
Cancer patients had high levels of these biomarkers even though they had no clinical signs of heart disease or concurrent infections, wrote Noemi Pavo of Medical University of Vienna. The findings suggest that these patients could benefit from enhanced heart failure therapies that go beyond the current focus on preventing cardiotoxic side effects of chemotherapy and radiotherapy, noted Dr. Pavo and her associates.
Cardiovascular hormone and peptide levels can rise during cancer treatment, but whether cancer itself affects these biomarkers has been unclear, the investigators said. Therefore, they prospectively measured levels of five cardiovascular hormones – NT-proBNP, MR-proANP, MR-proADM, CT-pro-ET, and copeptin – in addition to high-sensitive troponin, the proinflammatory markers interleukin 6 and C-reactive protein, and cytokines serum amyloid A, haptoglobin, and fibronectin, in 555 patients with newly diagnosed, as-yet-untreated cancer (Heart 2015 Sep 28. doi:10.1136/heartjnl-2015-307848).
A total of 186 patients (34%) died after an average of 25 months of follow-up, the researchers reported. Levels of all cardiovascular hormones and hsTnT increased with tumor stage progression. After the researchers controlled for age, tumor type, tumor stage, glomerular filtration rate, and cardiac status, rising levels of all five cardiac hormones and hsTnT independently predicted mortality, with adjusted hazard ratios ranging from 1.21 for CT-proET-1 and hsTnT to 1.54 for the natural logarithm of NT-proBNP, and P values ranging from .014 to less than.001.
“All of these markers are strongly related to mortality, implying a direct association with disease progression,” the researchers said. “While our endpoint [was] all-cause mortality, precise information about the percentage of cardiovascular-related death would certainly be of important clinical interest. Since post hoc interpretations of certifications of death are not reliable, the development of a cardiac disease during cancer progression should be documented in longitudinal studies in the future.”
An unrestricted grant from Thermo Fisher funded the study. The researchers declared no competing interests.
High circulating levels of six cardiovascular biomarkers predicted cancer mortality even before the start of treatment and regardless of tumor type or stage, according to a study published online Sept. 28 in Heart.
Cancer patients had high levels of these biomarkers even though they had no clinical signs of heart disease or concurrent infections, wrote Noemi Pavo of Medical University of Vienna. The findings suggest that these patients could benefit from enhanced heart failure therapies that go beyond the current focus on preventing cardiotoxic side effects of chemotherapy and radiotherapy, noted Dr. Pavo and her associates.
Cardiovascular hormone and peptide levels can rise during cancer treatment, but whether cancer itself affects these biomarkers has been unclear, the investigators said. Therefore, they prospectively measured levels of five cardiovascular hormones – NT-proBNP, MR-proANP, MR-proADM, CT-pro-ET, and copeptin – in addition to high-sensitive troponin, the proinflammatory markers interleukin 6 and C-reactive protein, and cytokines serum amyloid A, haptoglobin, and fibronectin, in 555 patients with newly diagnosed, as-yet-untreated cancer (Heart 2015 Sep 28. doi:10.1136/heartjnl-2015-307848).
A total of 186 patients (34%) died after an average of 25 months of follow-up, the researchers reported. Levels of all cardiovascular hormones and hsTnT increased with tumor stage progression. After the researchers controlled for age, tumor type, tumor stage, glomerular filtration rate, and cardiac status, rising levels of all five cardiac hormones and hsTnT independently predicted mortality, with adjusted hazard ratios ranging from 1.21 for CT-proET-1 and hsTnT to 1.54 for the natural logarithm of NT-proBNP, and P values ranging from .014 to less than.001.
“All of these markers are strongly related to mortality, implying a direct association with disease progression,” the researchers said. “While our endpoint [was] all-cause mortality, precise information about the percentage of cardiovascular-related death would certainly be of important clinical interest. Since post hoc interpretations of certifications of death are not reliable, the development of a cardiac disease during cancer progression should be documented in longitudinal studies in the future.”
An unrestricted grant from Thermo Fisher funded the study. The researchers declared no competing interests.
FROM HEART
Key clinical point: High levels of cardiac hormones and peptides predicted mortality in patients with first-time, as-yet-untreated cancer.
Major finding: Rising levels of all biomarkers significantly predicted mortality, with adjusted hazard ratios ranging from 1.21 to 1.54.
Data source: Prospective observational study of 555 cancer patients.
Disclosures: An unrestricted grant from Thermo Fisher funded the study. The researchers declared no competing interests.
Metformin associated with small height gain in children
Children who received at least 274 mg of metformin for any reason grew about 1 cm more than controls, authors of a meta-analysis reported online in JAMA Pediatrics.
The relative increase “may appear small, [but] is likely underestimated, given that many studies were of short duration and included older adolescents, potentially after epiphyseal growth plate closure,” wrote Nicholas Kuzik of the University of Alberta in Edmonton, Canada, and his associates. “It is possible that longer treatment periods or treatments concentrated at times of greater growth may lead to even greater height changes,” the researchers added.
Metformin has been increasingly used off label for children and adolescents who have impaired glucose tolerance, nonalcoholic fatty liver disease, obesity, or polycystic ovary syndrome, the investigators noted. Their meta-analysis included 10 randomized controlled trials of 562 such patients who were 8 to almost 16 years old. A total of 59% of children were female and body mass index ranged from 18.4 to 41 (JAMA Pediatr. 2015 Sept. 28. doi: 10.1001/jamapediatrics.2015.2186).
Height gains did not statistically differ between groups when the researchers analyzed all 10 studies together. The 1-cm greater average increase with metformin, compared with controls (95% confidence interval, 0.0 to 2.0 cm) applied only to the five studies that used the highest cumulative doses of at least 274 mg. Metformin conferred no relative gains in height for the five trials that used lower cumulative doses of 91-186 mg. The lower-dose studies also involved 3-6 months of treatment, compared with 6-48 months for the high-dose trials, said Mr. Kuzik and his associates.
Metformin affects several factors that mediate growth, including sex hormones, insulin or insulin-like growth factor 1, and adenosine monophosphate–activated protein kinase, the investigators noted. They recommended longer-term studies of younger children.
The investigators declared no funding sources or competing interests.
Children who received at least 274 mg of metformin for any reason grew about 1 cm more than controls, authors of a meta-analysis reported online in JAMA Pediatrics.
The relative increase “may appear small, [but] is likely underestimated, given that many studies were of short duration and included older adolescents, potentially after epiphyseal growth plate closure,” wrote Nicholas Kuzik of the University of Alberta in Edmonton, Canada, and his associates. “It is possible that longer treatment periods or treatments concentrated at times of greater growth may lead to even greater height changes,” the researchers added.
Metformin has been increasingly used off label for children and adolescents who have impaired glucose tolerance, nonalcoholic fatty liver disease, obesity, or polycystic ovary syndrome, the investigators noted. Their meta-analysis included 10 randomized controlled trials of 562 such patients who were 8 to almost 16 years old. A total of 59% of children were female and body mass index ranged from 18.4 to 41 (JAMA Pediatr. 2015 Sept. 28. doi: 10.1001/jamapediatrics.2015.2186).
Height gains did not statistically differ between groups when the researchers analyzed all 10 studies together. The 1-cm greater average increase with metformin, compared with controls (95% confidence interval, 0.0 to 2.0 cm) applied only to the five studies that used the highest cumulative doses of at least 274 mg. Metformin conferred no relative gains in height for the five trials that used lower cumulative doses of 91-186 mg. The lower-dose studies also involved 3-6 months of treatment, compared with 6-48 months for the high-dose trials, said Mr. Kuzik and his associates.
Metformin affects several factors that mediate growth, including sex hormones, insulin or insulin-like growth factor 1, and adenosine monophosphate–activated protein kinase, the investigators noted. They recommended longer-term studies of younger children.
The investigators declared no funding sources or competing interests.
Children who received at least 274 mg of metformin for any reason grew about 1 cm more than controls, authors of a meta-analysis reported online in JAMA Pediatrics.
The relative increase “may appear small, [but] is likely underestimated, given that many studies were of short duration and included older adolescents, potentially after epiphyseal growth plate closure,” wrote Nicholas Kuzik of the University of Alberta in Edmonton, Canada, and his associates. “It is possible that longer treatment periods or treatments concentrated at times of greater growth may lead to even greater height changes,” the researchers added.
Metformin has been increasingly used off label for children and adolescents who have impaired glucose tolerance, nonalcoholic fatty liver disease, obesity, or polycystic ovary syndrome, the investigators noted. Their meta-analysis included 10 randomized controlled trials of 562 such patients who were 8 to almost 16 years old. A total of 59% of children were female and body mass index ranged from 18.4 to 41 (JAMA Pediatr. 2015 Sept. 28. doi: 10.1001/jamapediatrics.2015.2186).
Height gains did not statistically differ between groups when the researchers analyzed all 10 studies together. The 1-cm greater average increase with metformin, compared with controls (95% confidence interval, 0.0 to 2.0 cm) applied only to the five studies that used the highest cumulative doses of at least 274 mg. Metformin conferred no relative gains in height for the five trials that used lower cumulative doses of 91-186 mg. The lower-dose studies also involved 3-6 months of treatment, compared with 6-48 months for the high-dose trials, said Mr. Kuzik and his associates.
Metformin affects several factors that mediate growth, including sex hormones, insulin or insulin-like growth factor 1, and adenosine monophosphate–activated protein kinase, the investigators noted. They recommended longer-term studies of younger children.
The investigators declared no funding sources or competing interests.
FROM JAMA PEDIATRICS
Key clinical point: Metformin was associated with a small height increase among children in a meta-analysis.
Major finding: Cumulative doses of at least 274 mg were associated with a 1-cm height increase.
Data source: A meta-analysis of 10 randomized controlled trials.
Disclosures: The researchers declared no funding sources or conflicts of interest.
CBT improves depression but not self-care in heart failure patients
Cognitive behavioral therapy significantly improved major depression but did not improve self-care by heart failure patients, investigators reported online in JAMA Internal Medicine.
“The results suggest that CBT is superior to usual care for depression in patients with heart failure,” said Dr. Kenneth Freedland and his associates at Washington University in St. Louis. They called the findings “especially encouraging” in light of recent negative results from the SADHART-CHF and MOOD-HF trials of selective serotonin reuptake inhibitors in this population.Patients in heart failure often have major depression, which increases their chances of poor self-care, hospitalization, and mortality, the researchers noted. Their single-blind, randomized trial included 158 patients who were in New York Heart Association class I, II, or III heart failure and met criteria for major depression. Patients in the intervention group received standard medical care, plus up to 6 months of CBT designed for cardiac patients.Patients received CBT weekly, then biweekly, and then monthly as they reached their treatment goals, but they also received telephone follow-up to help prevent relapse. The control group received standard medical care plus consultation with a cardiac nurse, written materials on heart failure self-care, and three follow-up phone calls with the nurse (JAMA Intern Med. 2015 Sept. 28. doi:10.1001/jamainternmed.2015.5220). At 6 months, the CBT group scored significantly lower on the BDI-II than did controls (mean score, 12.8 [standard deviation, 10.6] vs. 17.3 [10.7]; P = .008), the researchers said. Remission rates with CBT were 46% based on the BDI-II and 51% based on the Hamilton Depression Scale, both of which significantly exceeded remission rates of 19-20% for controls. The CBT group also improved significantly more than did controls on standard measures for anxiety, heart failure-related quality of life, mental health–related quality of life, fatigue, and social functioning, but not on measures of physical functioning, the researchers reported.
The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no competing interests.
When depression occurs in patients with heart failure, which is often, the illness burden and management complexity increase multifold. Freedland et al. tested the hypothesis that the effective treatment of comorbid depression with cognitive behavioral therapy (CBT) would also lead to improvements in heart failure self-care and physical functioning and found that it did not. The good news is that CBT did significantly improve emotional health and overall quality of life, and the improvement in depressive symptoms associated with CBT was larger than observed in pharmacotherapy trials for depression in patients with heart disease. This supports evidence for a shift in practice away from so much pharmacotherapy and more use of psychotherapy to achieve better mental health and overall quality of life outcomes in patients with heart failure. In reframing how we think about the management of depression in patients with heart failure, we should be talking more and prescribing less.
Dr. Patrick G. O’Malley is deputy editor of JAMA Internal Medicine. He declared no competing interests. These comments were taken from his accompanying editorial (JAMA Intern Med. 2015 Sept. 28).
When depression occurs in patients with heart failure, which is often, the illness burden and management complexity increase multifold. Freedland et al. tested the hypothesis that the effective treatment of comorbid depression with cognitive behavioral therapy (CBT) would also lead to improvements in heart failure self-care and physical functioning and found that it did not. The good news is that CBT did significantly improve emotional health and overall quality of life, and the improvement in depressive symptoms associated with CBT was larger than observed in pharmacotherapy trials for depression in patients with heart disease. This supports evidence for a shift in practice away from so much pharmacotherapy and more use of psychotherapy to achieve better mental health and overall quality of life outcomes in patients with heart failure. In reframing how we think about the management of depression in patients with heart failure, we should be talking more and prescribing less.
Dr. Patrick G. O’Malley is deputy editor of JAMA Internal Medicine. He declared no competing interests. These comments were taken from his accompanying editorial (JAMA Intern Med. 2015 Sept. 28).
When depression occurs in patients with heart failure, which is often, the illness burden and management complexity increase multifold. Freedland et al. tested the hypothesis that the effective treatment of comorbid depression with cognitive behavioral therapy (CBT) would also lead to improvements in heart failure self-care and physical functioning and found that it did not. The good news is that CBT did significantly improve emotional health and overall quality of life, and the improvement in depressive symptoms associated with CBT was larger than observed in pharmacotherapy trials for depression in patients with heart disease. This supports evidence for a shift in practice away from so much pharmacotherapy and more use of psychotherapy to achieve better mental health and overall quality of life outcomes in patients with heart failure. In reframing how we think about the management of depression in patients with heart failure, we should be talking more and prescribing less.
Dr. Patrick G. O’Malley is deputy editor of JAMA Internal Medicine. He declared no competing interests. These comments were taken from his accompanying editorial (JAMA Intern Med. 2015 Sept. 28).
Cognitive behavioral therapy significantly improved major depression but did not improve self-care by heart failure patients, investigators reported online in JAMA Internal Medicine.
“The results suggest that CBT is superior to usual care for depression in patients with heart failure,” said Dr. Kenneth Freedland and his associates at Washington University in St. Louis. They called the findings “especially encouraging” in light of recent negative results from the SADHART-CHF and MOOD-HF trials of selective serotonin reuptake inhibitors in this population.Patients in heart failure often have major depression, which increases their chances of poor self-care, hospitalization, and mortality, the researchers noted. Their single-blind, randomized trial included 158 patients who were in New York Heart Association class I, II, or III heart failure and met criteria for major depression. Patients in the intervention group received standard medical care, plus up to 6 months of CBT designed for cardiac patients.Patients received CBT weekly, then biweekly, and then monthly as they reached their treatment goals, but they also received telephone follow-up to help prevent relapse. The control group received standard medical care plus consultation with a cardiac nurse, written materials on heart failure self-care, and three follow-up phone calls with the nurse (JAMA Intern Med. 2015 Sept. 28. doi:10.1001/jamainternmed.2015.5220). At 6 months, the CBT group scored significantly lower on the BDI-II than did controls (mean score, 12.8 [standard deviation, 10.6] vs. 17.3 [10.7]; P = .008), the researchers said. Remission rates with CBT were 46% based on the BDI-II and 51% based on the Hamilton Depression Scale, both of which significantly exceeded remission rates of 19-20% for controls. The CBT group also improved significantly more than did controls on standard measures for anxiety, heart failure-related quality of life, mental health–related quality of life, fatigue, and social functioning, but not on measures of physical functioning, the researchers reported.
The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no competing interests.
Cognitive behavioral therapy significantly improved major depression but did not improve self-care by heart failure patients, investigators reported online in JAMA Internal Medicine.
“The results suggest that CBT is superior to usual care for depression in patients with heart failure,” said Dr. Kenneth Freedland and his associates at Washington University in St. Louis. They called the findings “especially encouraging” in light of recent negative results from the SADHART-CHF and MOOD-HF trials of selective serotonin reuptake inhibitors in this population.Patients in heart failure often have major depression, which increases their chances of poor self-care, hospitalization, and mortality, the researchers noted. Their single-blind, randomized trial included 158 patients who were in New York Heart Association class I, II, or III heart failure and met criteria for major depression. Patients in the intervention group received standard medical care, plus up to 6 months of CBT designed for cardiac patients.Patients received CBT weekly, then biweekly, and then monthly as they reached their treatment goals, but they also received telephone follow-up to help prevent relapse. The control group received standard medical care plus consultation with a cardiac nurse, written materials on heart failure self-care, and three follow-up phone calls with the nurse (JAMA Intern Med. 2015 Sept. 28. doi:10.1001/jamainternmed.2015.5220). At 6 months, the CBT group scored significantly lower on the BDI-II than did controls (mean score, 12.8 [standard deviation, 10.6] vs. 17.3 [10.7]; P = .008), the researchers said. Remission rates with CBT were 46% based on the BDI-II and 51% based on the Hamilton Depression Scale, both of which significantly exceeded remission rates of 19-20% for controls. The CBT group also improved significantly more than did controls on standard measures for anxiety, heart failure-related quality of life, mental health–related quality of life, fatigue, and social functioning, but not on measures of physical functioning, the researchers reported.
The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no competing interests.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Cognitive behavior therapy significantly improved major depression but not self-care among patients with heart failure.
Major finding: At 6 months, mean BDI-II scores were 12.8 for CBT vs. 17.3 for enhanced usual care (P = .008).
Data source: Single-blind, randomized trial of 158 patients.
Disclosures: The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no conflicts of interest.
AACE Releases New Position Statement on Testosterone Replacement Therapy in Men
Rigorous studies have not linked testosterone replacement therapy to heart attack or stroke, and the decision to prescribe testosterone replacement therapy should be based on a full diagnostic work-up – not the underlying cause of hypogonadism, according to a new position statement from the American Association of Clinical Endocrinologists.
The statement challenges several aspects of a recent Food and Drug Administration safety announcement warning about “possible” increased risks of heart attack and stroke with testosterone replacement therapy (TRT) and approving its use only for testicular, pituitary, or brain disorders that cause low testosterone, not for age-associated hypogonadism.
In fact, AACE rejoined, randomized controlled trials have lacked the power to assess whether TRT increases the chances of cardiovascular events or death. Data linking TRT to cardiovascular problems come from a few retrospective studies, the “major flaws” of which limit their ability to assess risk. “Large-scale prospective randomized controlled trials on testosterone therapy, focusing on cardiovascular benefits and risks, are clearly needed. As with therapeutics in general, common sense, experience, and an individualized approach are recommended” (Endocr Pract. 2015;21:1066-73).
The benefits and risks of TRT in age-associated hypogonadism remain uncertain, according to both the FDA and AACE. Until better studies are available, AACE recommends that clinicians consider TRT for men with signs and symptoms that are consistent with hypogonadism, regardless of cause, and who have at least two “unequivocally low” testosterone levels in samples drawn before 10 a.m.
Clinicians also should educate patients about the possible cardiovascular risks of TRT, should be “extra cautious” when considering TRT for symptomatic elderly men with low testosterone levels, and should avoid TRT entirely in frail elderly men “until better outcome data are available,” AACE also recommended. Furthermore, clinicians should avoid TRT for patients with uncontrolled or poorly controlled heart failure, a history of heart attack or cerebrovascular accident within the past 6 months, an individual or family history of a procoagulant state, or an individual history of thromboembolism, AACE stated.
Although TRT can improve some cardiovascular risk factors by promoting muscle gain and fat loss, decreasing insulin resistance, and potentially reversing metabolic syndrome, it remains unclear whether low testosterone is a marker of cardiovascular illness or a causal factor, AACE noted. Replacement therapy is most likely to benefit men with very low testosterone levels, not those whose levels are just below normal, according to AACE.
The American Urological Association has echoed several recommendations from AACE, emphasizing in its own statement that “testosterone therapy in the absence of hypogonadism is inappropriate” and calling for more federal and industry funding for studies of the indications, benefits, and risks of approved treatments for hypogonadism as well as studies of new potential therapies. “Current evidence does not provide any definitive answers regarding the risks of testosterone therapy on prostate cancer and cardiovascular disease, and patients should be so informed,” noted the statement from the AUA, which was last updated in August 2015.
The AACE Reproductive Endocrinology Scientific Committee listed the following disclosures: first author Dr. Neil Goodman reported serving on the AbbVie speaker bureau for AndroGel and senior author Dr. Glenn Cunningham reported receiving research support from Abbvie, having served on advisory panels for Abbvie, Apricus, Clarus Therapeutics, Endo Pharma, and Lilly and having consulted for Clarus Therapeutics, Endo Pharma, Ferring, Purdue Pharma, and Repros Therapeutics. Two other coauthors declared financial relationships with Abbvie, GlaxoSmithKline, Merck, Sanofi-Aventies, and a number of other pharmaceutical companies. The other two coauthors declared no competing interests.
Rigorous studies have not linked testosterone replacement therapy to heart attack or stroke, and the decision to prescribe testosterone replacement therapy should be based on a full diagnostic work-up – not the underlying cause of hypogonadism, according to a new position statement from the American Association of Clinical Endocrinologists.
The statement challenges several aspects of a recent Food and Drug Administration safety announcement warning about “possible” increased risks of heart attack and stroke with testosterone replacement therapy (TRT) and approving its use only for testicular, pituitary, or brain disorders that cause low testosterone, not for age-associated hypogonadism.
In fact, AACE rejoined, randomized controlled trials have lacked the power to assess whether TRT increases the chances of cardiovascular events or death. Data linking TRT to cardiovascular problems come from a few retrospective studies, the “major flaws” of which limit their ability to assess risk. “Large-scale prospective randomized controlled trials on testosterone therapy, focusing on cardiovascular benefits and risks, are clearly needed. As with therapeutics in general, common sense, experience, and an individualized approach are recommended” (Endocr Pract. 2015;21:1066-73).
The benefits and risks of TRT in age-associated hypogonadism remain uncertain, according to both the FDA and AACE. Until better studies are available, AACE recommends that clinicians consider TRT for men with signs and symptoms that are consistent with hypogonadism, regardless of cause, and who have at least two “unequivocally low” testosterone levels in samples drawn before 10 a.m.
Clinicians also should educate patients about the possible cardiovascular risks of TRT, should be “extra cautious” when considering TRT for symptomatic elderly men with low testosterone levels, and should avoid TRT entirely in frail elderly men “until better outcome data are available,” AACE also recommended. Furthermore, clinicians should avoid TRT for patients with uncontrolled or poorly controlled heart failure, a history of heart attack or cerebrovascular accident within the past 6 months, an individual or family history of a procoagulant state, or an individual history of thromboembolism, AACE stated.
Although TRT can improve some cardiovascular risk factors by promoting muscle gain and fat loss, decreasing insulin resistance, and potentially reversing metabolic syndrome, it remains unclear whether low testosterone is a marker of cardiovascular illness or a causal factor, AACE noted. Replacement therapy is most likely to benefit men with very low testosterone levels, not those whose levels are just below normal, according to AACE.
The American Urological Association has echoed several recommendations from AACE, emphasizing in its own statement that “testosterone therapy in the absence of hypogonadism is inappropriate” and calling for more federal and industry funding for studies of the indications, benefits, and risks of approved treatments for hypogonadism as well as studies of new potential therapies. “Current evidence does not provide any definitive answers regarding the risks of testosterone therapy on prostate cancer and cardiovascular disease, and patients should be so informed,” noted the statement from the AUA, which was last updated in August 2015.
The AACE Reproductive Endocrinology Scientific Committee listed the following disclosures: first author Dr. Neil Goodman reported serving on the AbbVie speaker bureau for AndroGel and senior author Dr. Glenn Cunningham reported receiving research support from Abbvie, having served on advisory panels for Abbvie, Apricus, Clarus Therapeutics, Endo Pharma, and Lilly and having consulted for Clarus Therapeutics, Endo Pharma, Ferring, Purdue Pharma, and Repros Therapeutics. Two other coauthors declared financial relationships with Abbvie, GlaxoSmithKline, Merck, Sanofi-Aventies, and a number of other pharmaceutical companies. The other two coauthors declared no competing interests.
Rigorous studies have not linked testosterone replacement therapy to heart attack or stroke, and the decision to prescribe testosterone replacement therapy should be based on a full diagnostic work-up – not the underlying cause of hypogonadism, according to a new position statement from the American Association of Clinical Endocrinologists.
The statement challenges several aspects of a recent Food and Drug Administration safety announcement warning about “possible” increased risks of heart attack and stroke with testosterone replacement therapy (TRT) and approving its use only for testicular, pituitary, or brain disorders that cause low testosterone, not for age-associated hypogonadism.
In fact, AACE rejoined, randomized controlled trials have lacked the power to assess whether TRT increases the chances of cardiovascular events or death. Data linking TRT to cardiovascular problems come from a few retrospective studies, the “major flaws” of which limit their ability to assess risk. “Large-scale prospective randomized controlled trials on testosterone therapy, focusing on cardiovascular benefits and risks, are clearly needed. As with therapeutics in general, common sense, experience, and an individualized approach are recommended” (Endocr Pract. 2015;21:1066-73).
The benefits and risks of TRT in age-associated hypogonadism remain uncertain, according to both the FDA and AACE. Until better studies are available, AACE recommends that clinicians consider TRT for men with signs and symptoms that are consistent with hypogonadism, regardless of cause, and who have at least two “unequivocally low” testosterone levels in samples drawn before 10 a.m.
Clinicians also should educate patients about the possible cardiovascular risks of TRT, should be “extra cautious” when considering TRT for symptomatic elderly men with low testosterone levels, and should avoid TRT entirely in frail elderly men “until better outcome data are available,” AACE also recommended. Furthermore, clinicians should avoid TRT for patients with uncontrolled or poorly controlled heart failure, a history of heart attack or cerebrovascular accident within the past 6 months, an individual or family history of a procoagulant state, or an individual history of thromboembolism, AACE stated.
Although TRT can improve some cardiovascular risk factors by promoting muscle gain and fat loss, decreasing insulin resistance, and potentially reversing metabolic syndrome, it remains unclear whether low testosterone is a marker of cardiovascular illness or a causal factor, AACE noted. Replacement therapy is most likely to benefit men with very low testosterone levels, not those whose levels are just below normal, according to AACE.
The American Urological Association has echoed several recommendations from AACE, emphasizing in its own statement that “testosterone therapy in the absence of hypogonadism is inappropriate” and calling for more federal and industry funding for studies of the indications, benefits, and risks of approved treatments for hypogonadism as well as studies of new potential therapies. “Current evidence does not provide any definitive answers regarding the risks of testosterone therapy on prostate cancer and cardiovascular disease, and patients should be so informed,” noted the statement from the AUA, which was last updated in August 2015.
The AACE Reproductive Endocrinology Scientific Committee listed the following disclosures: first author Dr. Neil Goodman reported serving on the AbbVie speaker bureau for AndroGel and senior author Dr. Glenn Cunningham reported receiving research support from Abbvie, having served on advisory panels for Abbvie, Apricus, Clarus Therapeutics, Endo Pharma, and Lilly and having consulted for Clarus Therapeutics, Endo Pharma, Ferring, Purdue Pharma, and Repros Therapeutics. Two other coauthors declared financial relationships with Abbvie, GlaxoSmithKline, Merck, Sanofi-Aventies, and a number of other pharmaceutical companies. The other two coauthors declared no competing interests.
FROM ENDOCRINE PRACTICE
AACE releases new position statement on testosterone replacement therapy in men
Rigorous studies have not linked testosterone replacement therapy to heart attack or stroke, and the decision to prescribe testosterone replacement therapy should be based on a full diagnostic work-up – not the underlying cause of hypogonadism, according to a new position statement from the American Association of Clinical Endocrinologists.
The statement challenges several aspects of a recent Food and Drug Administration safety announcement warning about “possible” increased risks of heart attack and stroke with testosterone replacement therapy (TRT) and approving its use only for testicular, pituitary, or brain disorders that cause low testosterone, not for age-associated hypogonadism.
In fact, AACE rejoined, randomized controlled trials have lacked the power to assess whether TRT increases the chances of cardiovascular events or death. Data linking TRT to cardiovascular problems come from a few retrospective studies, the “major flaws” of which limit their ability to assess risk. “Large-scale prospective randomized controlled trials on testosterone therapy, focusing on cardiovascular benefits and risks, are clearly needed. As with therapeutics in general, common sense, experience, and an individualized approach are recommended” (Endocr Pract. 2015;21:1066-73).
The benefits and risks of TRT in age-associated hypogonadism remain uncertain, according to both the FDA and AACE. Until better studies are available, AACE recommends that clinicians consider TRT for men with signs and symptoms that are consistent with hypogonadism, regardless of cause, and who have at least two “unequivocally low” testosterone levels in samples drawn before 10 a.m.
Clinicians also should educate patients about the possible cardiovascular risks of TRT, should be “extra cautious” when considering TRT for symptomatic elderly men with low testosterone levels, and should avoid TRT entirely in frail elderly men “until better outcome data are available,” AACE also recommended. Furthermore, clinicians should avoid TRT for patients with uncontrolled or poorly controlled heart failure, a history of heart attack or cerebrovascular accident within the past 6 months, an individual or family history of a procoagulant state, or an individual history of thromboembolism, AACE stated.
Although TRT can improve some cardiovascular risk factors by promoting muscle gain and fat loss, decreasing insulin resistance, and potentially reversing metabolic syndrome, it remains unclear whether low testosterone is a marker of cardiovascular illness or a causal factor, AACE noted. Replacement therapy is most likely to benefit men with very low testosterone levels, not those whose levels are just below normal, according to AACE.
The American Urological Association has echoed several recommendations from AACE, emphasizing in its own statement that “testosterone therapy in the absence of hypogonadism is inappropriate” and calling for more federal and industry funding for studies of the indications, benefits, and risks of approved treatments for hypogonadism as well as studies of new potential therapies. “Current evidence does not provide any definitive answers regarding the risks of testosterone therapy on prostate cancer and cardiovascular disease, and patients should be so informed,” noted the statement from the AUA, which was last updated in August 2015.
The AACE Reproductive Endocrinology Scientific Committee listed the following disclosures: first author Dr. Neil Goodman reported serving on the AbbVie speaker bureau for AndroGel and senior author Dr. Glenn Cunningham reported receiving research support from Abbvie, having served on advisory panels for Abbvie, Apricus, Clarus Therapeutics, Endo Pharma, and Lilly and having consulted for Clarus Therapeutics, Endo Pharma, Ferring, Purdue Pharma, and Repros Therapeutics. Two other coauthors declared financial relationships with Abbvie, GlaxoSmithKline, Merck, Sanofi-Aventies, and a number of other pharmaceutical companies. The other two coauthors declared no competing interests.
Rigorous studies have not linked testosterone replacement therapy to heart attack or stroke, and the decision to prescribe testosterone replacement therapy should be based on a full diagnostic work-up – not the underlying cause of hypogonadism, according to a new position statement from the American Association of Clinical Endocrinologists.
The statement challenges several aspects of a recent Food and Drug Administration safety announcement warning about “possible” increased risks of heart attack and stroke with testosterone replacement therapy (TRT) and approving its use only for testicular, pituitary, or brain disorders that cause low testosterone, not for age-associated hypogonadism.
In fact, AACE rejoined, randomized controlled trials have lacked the power to assess whether TRT increases the chances of cardiovascular events or death. Data linking TRT to cardiovascular problems come from a few retrospective studies, the “major flaws” of which limit their ability to assess risk. “Large-scale prospective randomized controlled trials on testosterone therapy, focusing on cardiovascular benefits and risks, are clearly needed. As with therapeutics in general, common sense, experience, and an individualized approach are recommended” (Endocr Pract. 2015;21:1066-73).
The benefits and risks of TRT in age-associated hypogonadism remain uncertain, according to both the FDA and AACE. Until better studies are available, AACE recommends that clinicians consider TRT for men with signs and symptoms that are consistent with hypogonadism, regardless of cause, and who have at least two “unequivocally low” testosterone levels in samples drawn before 10 a.m.
Clinicians also should educate patients about the possible cardiovascular risks of TRT, should be “extra cautious” when considering TRT for symptomatic elderly men with low testosterone levels, and should avoid TRT entirely in frail elderly men “until better outcome data are available,” AACE also recommended. Furthermore, clinicians should avoid TRT for patients with uncontrolled or poorly controlled heart failure, a history of heart attack or cerebrovascular accident within the past 6 months, an individual or family history of a procoagulant state, or an individual history of thromboembolism, AACE stated.
Although TRT can improve some cardiovascular risk factors by promoting muscle gain and fat loss, decreasing insulin resistance, and potentially reversing metabolic syndrome, it remains unclear whether low testosterone is a marker of cardiovascular illness or a causal factor, AACE noted. Replacement therapy is most likely to benefit men with very low testosterone levels, not those whose levels are just below normal, according to AACE.
The American Urological Association has echoed several recommendations from AACE, emphasizing in its own statement that “testosterone therapy in the absence of hypogonadism is inappropriate” and calling for more federal and industry funding for studies of the indications, benefits, and risks of approved treatments for hypogonadism as well as studies of new potential therapies. “Current evidence does not provide any definitive answers regarding the risks of testosterone therapy on prostate cancer and cardiovascular disease, and patients should be so informed,” noted the statement from the AUA, which was last updated in August 2015.
The AACE Reproductive Endocrinology Scientific Committee listed the following disclosures: first author Dr. Neil Goodman reported serving on the AbbVie speaker bureau for AndroGel and senior author Dr. Glenn Cunningham reported receiving research support from Abbvie, having served on advisory panels for Abbvie, Apricus, Clarus Therapeutics, Endo Pharma, and Lilly and having consulted for Clarus Therapeutics, Endo Pharma, Ferring, Purdue Pharma, and Repros Therapeutics. Two other coauthors declared financial relationships with Abbvie, GlaxoSmithKline, Merck, Sanofi-Aventies, and a number of other pharmaceutical companies. The other two coauthors declared no competing interests.
Rigorous studies have not linked testosterone replacement therapy to heart attack or stroke, and the decision to prescribe testosterone replacement therapy should be based on a full diagnostic work-up – not the underlying cause of hypogonadism, according to a new position statement from the American Association of Clinical Endocrinologists.
The statement challenges several aspects of a recent Food and Drug Administration safety announcement warning about “possible” increased risks of heart attack and stroke with testosterone replacement therapy (TRT) and approving its use only for testicular, pituitary, or brain disorders that cause low testosterone, not for age-associated hypogonadism.
In fact, AACE rejoined, randomized controlled trials have lacked the power to assess whether TRT increases the chances of cardiovascular events or death. Data linking TRT to cardiovascular problems come from a few retrospective studies, the “major flaws” of which limit their ability to assess risk. “Large-scale prospective randomized controlled trials on testosterone therapy, focusing on cardiovascular benefits and risks, are clearly needed. As with therapeutics in general, common sense, experience, and an individualized approach are recommended” (Endocr Pract. 2015;21:1066-73).
The benefits and risks of TRT in age-associated hypogonadism remain uncertain, according to both the FDA and AACE. Until better studies are available, AACE recommends that clinicians consider TRT for men with signs and symptoms that are consistent with hypogonadism, regardless of cause, and who have at least two “unequivocally low” testosterone levels in samples drawn before 10 a.m.
Clinicians also should educate patients about the possible cardiovascular risks of TRT, should be “extra cautious” when considering TRT for symptomatic elderly men with low testosterone levels, and should avoid TRT entirely in frail elderly men “until better outcome data are available,” AACE also recommended. Furthermore, clinicians should avoid TRT for patients with uncontrolled or poorly controlled heart failure, a history of heart attack or cerebrovascular accident within the past 6 months, an individual or family history of a procoagulant state, or an individual history of thromboembolism, AACE stated.
Although TRT can improve some cardiovascular risk factors by promoting muscle gain and fat loss, decreasing insulin resistance, and potentially reversing metabolic syndrome, it remains unclear whether low testosterone is a marker of cardiovascular illness or a causal factor, AACE noted. Replacement therapy is most likely to benefit men with very low testosterone levels, not those whose levels are just below normal, according to AACE.
The American Urological Association has echoed several recommendations from AACE, emphasizing in its own statement that “testosterone therapy in the absence of hypogonadism is inappropriate” and calling for more federal and industry funding for studies of the indications, benefits, and risks of approved treatments for hypogonadism as well as studies of new potential therapies. “Current evidence does not provide any definitive answers regarding the risks of testosterone therapy on prostate cancer and cardiovascular disease, and patients should be so informed,” noted the statement from the AUA, which was last updated in August 2015.
The AACE Reproductive Endocrinology Scientific Committee listed the following disclosures: first author Dr. Neil Goodman reported serving on the AbbVie speaker bureau for AndroGel and senior author Dr. Glenn Cunningham reported receiving research support from Abbvie, having served on advisory panels for Abbvie, Apricus, Clarus Therapeutics, Endo Pharma, and Lilly and having consulted for Clarus Therapeutics, Endo Pharma, Ferring, Purdue Pharma, and Repros Therapeutics. Two other coauthors declared financial relationships with Abbvie, GlaxoSmithKline, Merck, Sanofi-Aventies, and a number of other pharmaceutical companies. The other two coauthors declared no competing interests.
FROM ENDOCRINE PRACTICE