User login
Poor physical fitness upped diabetes risk regardless of weight
Young, out-of-shape men were about three times more likely than physically fit men to develop type 2 diabetes later in life, even if their body weight was normal, reported the authors of a large registry study.
“These findings suggest that interventions to improve aerobic and muscle fitness levels early in life could help reduce risk for type 2 diabetes mellitus in adulthood,” Dr. Casey Crump, at the Icahn School of Medicine at Mount Sinai, New York, and his associates wrote in a study published online March 7 in Annals of Internal Medicine.
Future longitudinal studies of physical fitness could help identify “windows of susceptibility” and the best preventive measures, the researchers added.
A sedentary lifestyle is known to increase the risk of type 2 diabetes, but less is known about how physical fitness affects risk.
To explore the question, the researchers identified 1,534,425 men without baseline diabetes who underwent military conscription physical examinations between 1969 and 1997. They tracked the men until up to 62 years of age by analyzing both the Swedish Hospital Registry and the Swedish Outpatient Registry (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M15-2002).
In all, 34,008 men developed type 2 diabetes over 39.4 million years of follow-up, the investigators said. Both low cardiorespiratory fitness and low muscle strength independently increased the risk for type 2 diabetes, regardless of whether the men had a high or normal body weight.
Moreover, the combination of low cardiorespiratory fitness and poor muscular fitness increased type 2 diabetes risk threefold (adjusted hazard ratio, 3.07; 95% confidence interval, 2.88 to 3.27; P less than .001), with a positive additive interaction (P less than .001).
Accounting for smoking lowered the associations between poor baseline fitness and type 2 diabetes by about 9%; but they remained significant (P less than .001), suggesting that unmeasured confounding “had little influence on our main findings,” the investigators said. If the associations are causal, then aerobic conditioning programs targeting men with low muscle strength might have the greatest public health impact, they added.
The U.S. National Institutes of Health, the Swedish Research Council, and Region Skåne/Lund University funded the study. The researchers had no disclosures.
Given the global increase in type 2 diabetes prevalence, there is a pressing need for intervention strategies that reduce the progression of the disease as well as the risk for related complications and premature death. The study by Dr. Crump and his colleagues fills an important research gap by demonstrating a strong inverse association between physical fitness at a young age and long-term risk for type 2 diabetes, independent of weight status. The enhancement of cardiorespiratory and muscular fitness through habitual physical activity in all persons should be recommended as a frontline therapy to address the public health burden of type 2 diabetes.
Peter T. Katzmarzyk, Ph.D., is at Pennington Biomedical Research Center in Baton Rouge, La. He had no disclosures. These comments were taken from his accompanying editorial (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M16-0269).
Given the global increase in type 2 diabetes prevalence, there is a pressing need for intervention strategies that reduce the progression of the disease as well as the risk for related complications and premature death. The study by Dr. Crump and his colleagues fills an important research gap by demonstrating a strong inverse association between physical fitness at a young age and long-term risk for type 2 diabetes, independent of weight status. The enhancement of cardiorespiratory and muscular fitness through habitual physical activity in all persons should be recommended as a frontline therapy to address the public health burden of type 2 diabetes.
Peter T. Katzmarzyk, Ph.D., is at Pennington Biomedical Research Center in Baton Rouge, La. He had no disclosures. These comments were taken from his accompanying editorial (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M16-0269).
Given the global increase in type 2 diabetes prevalence, there is a pressing need for intervention strategies that reduce the progression of the disease as well as the risk for related complications and premature death. The study by Dr. Crump and his colleagues fills an important research gap by demonstrating a strong inverse association between physical fitness at a young age and long-term risk for type 2 diabetes, independent of weight status. The enhancement of cardiorespiratory and muscular fitness through habitual physical activity in all persons should be recommended as a frontline therapy to address the public health burden of type 2 diabetes.
Peter T. Katzmarzyk, Ph.D., is at Pennington Biomedical Research Center in Baton Rouge, La. He had no disclosures. These comments were taken from his accompanying editorial (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M16-0269).
Young, out-of-shape men were about three times more likely than physically fit men to develop type 2 diabetes later in life, even if their body weight was normal, reported the authors of a large registry study.
“These findings suggest that interventions to improve aerobic and muscle fitness levels early in life could help reduce risk for type 2 diabetes mellitus in adulthood,” Dr. Casey Crump, at the Icahn School of Medicine at Mount Sinai, New York, and his associates wrote in a study published online March 7 in Annals of Internal Medicine.
Future longitudinal studies of physical fitness could help identify “windows of susceptibility” and the best preventive measures, the researchers added.
A sedentary lifestyle is known to increase the risk of type 2 diabetes, but less is known about how physical fitness affects risk.
To explore the question, the researchers identified 1,534,425 men without baseline diabetes who underwent military conscription physical examinations between 1969 and 1997. They tracked the men until up to 62 years of age by analyzing both the Swedish Hospital Registry and the Swedish Outpatient Registry (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M15-2002).
In all, 34,008 men developed type 2 diabetes over 39.4 million years of follow-up, the investigators said. Both low cardiorespiratory fitness and low muscle strength independently increased the risk for type 2 diabetes, regardless of whether the men had a high or normal body weight.
Moreover, the combination of low cardiorespiratory fitness and poor muscular fitness increased type 2 diabetes risk threefold (adjusted hazard ratio, 3.07; 95% confidence interval, 2.88 to 3.27; P less than .001), with a positive additive interaction (P less than .001).
Accounting for smoking lowered the associations between poor baseline fitness and type 2 diabetes by about 9%; but they remained significant (P less than .001), suggesting that unmeasured confounding “had little influence on our main findings,” the investigators said. If the associations are causal, then aerobic conditioning programs targeting men with low muscle strength might have the greatest public health impact, they added.
The U.S. National Institutes of Health, the Swedish Research Council, and Region Skåne/Lund University funded the study. The researchers had no disclosures.
Young, out-of-shape men were about three times more likely than physically fit men to develop type 2 diabetes later in life, even if their body weight was normal, reported the authors of a large registry study.
“These findings suggest that interventions to improve aerobic and muscle fitness levels early in life could help reduce risk for type 2 diabetes mellitus in adulthood,” Dr. Casey Crump, at the Icahn School of Medicine at Mount Sinai, New York, and his associates wrote in a study published online March 7 in Annals of Internal Medicine.
Future longitudinal studies of physical fitness could help identify “windows of susceptibility” and the best preventive measures, the researchers added.
A sedentary lifestyle is known to increase the risk of type 2 diabetes, but less is known about how physical fitness affects risk.
To explore the question, the researchers identified 1,534,425 men without baseline diabetes who underwent military conscription physical examinations between 1969 and 1997. They tracked the men until up to 62 years of age by analyzing both the Swedish Hospital Registry and the Swedish Outpatient Registry (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M15-2002).
In all, 34,008 men developed type 2 diabetes over 39.4 million years of follow-up, the investigators said. Both low cardiorespiratory fitness and low muscle strength independently increased the risk for type 2 diabetes, regardless of whether the men had a high or normal body weight.
Moreover, the combination of low cardiorespiratory fitness and poor muscular fitness increased type 2 diabetes risk threefold (adjusted hazard ratio, 3.07; 95% confidence interval, 2.88 to 3.27; P less than .001), with a positive additive interaction (P less than .001).
Accounting for smoking lowered the associations between poor baseline fitness and type 2 diabetes by about 9%; but they remained significant (P less than .001), suggesting that unmeasured confounding “had little influence on our main findings,” the investigators said. If the associations are causal, then aerobic conditioning programs targeting men with low muscle strength might have the greatest public health impact, they added.
The U.S. National Institutes of Health, the Swedish Research Council, and Region Skåne/Lund University funded the study. The researchers had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: In young men, both low cardiorespiratory fitness and low muscle strength independently increased the risk for type 2 diabetes mellitus, regardless of body weight.
Major finding: Low cardiorespiratory fitness combined with poor muscular fitness increased T2DM risk threefold (adjusted hazard ratio, 3.07; P less than .001).
Data source: A registry study of 1,534,425 Swedish men without baseline diabetes mellitus who underwent military conscription physical examinations between 1969 and 1997.
Disclosures: The U.S. National Institutes of Health, the Swedish Research Council, and Region Skåne/Lund University funded the study. The researchers had no disclosures.
Brodalumab met primary endpoints, deaths called ‘unrelated’
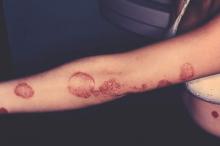
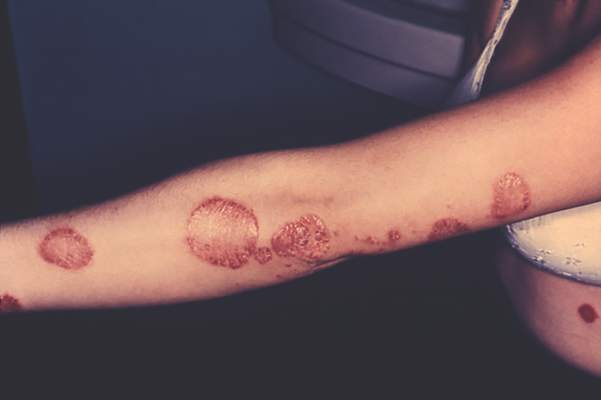
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
Key clinical point: The interleukin-17 inhibitor brodalumab met its coprimary endpoints in a phase III trial of patients with moderate to severe plaque psoriasis.
Major finding: At week 12, 83% of patients on 210 mg brodalumab achieved PASI 75, as did 60% of 140-mg patients and 3% of the placebo group. The coprimary endpoint, a static Physician Global Assessment score of 0/1, was met for 76% of 210-mg patients, 54% of 140-mg patients, and 1% of placebo patients. All four deaths were considered unrelated to treatment.
Data source: AMAGINE-1, a phase III, multicenter, randomized, double-blind study of brodalumab (210 or 140 mg) or placebo in 661 patients.
Disclosures: Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
High Gluten Consumption Early in Life Upped Risk for Celiac Disease
Children who were genetically susceptible to celiac disease and consumed high amounts of gluten at 12 months of age were at least twice as likely to develop the autoimmune disorder as genetically predisposed children who consumed less gluten, researchers reported in the March issue of Clinical Gastroenterology and Hepatology.
The association was similar among children who carried any of the major human leukocyte antigen (HLA) risk genotypes for celiac disease, said Dr. Carin Aronsson at Lund University in Sweden and her associates. “Because these HLA risk genotypes are widely distributed in the general population, these findings may have consequence for future infant feeding recommendations,” they said. They recommended repeating the study in other countries to confirm the link.
In order to develop celiac disease, patients must consume gluten and carry at least one of the relevant DR3-DQ2 and DR4-DQ8 HLA risk haplotypes. But because gluten is widely consumed in products containing wheat, rye, and barley, and because about half of whites have at least one of the two haplotypes, gluten intolerance probably depends on other environmental factors, the researchers said. To further study these factors, they compared 3-day food diaries collected at ages 9, 12, 18, and 24 months for 146 children with positive tissue transglutaminase autoantibody (tTGA) assays and biopsy-confirmed celiac disease (cases) and 436 tTGA-negative children (controls). Cases and controls were matched by age, sex, and HLA genotype (Clin Gastroenterol Hepatol. 2015 Oct 7. doi: 10.1016/j.cgh.2015.09.030).
The food diaries revealed higher gluten intake among cases, compared with controls, beginning at the age of 12 months, said the researchers. Notably, cases consumed a median of 4.9 g of gluten a day before tTGA seroconversion, 1 g more than the median amount for controls of the same age (odds ratio, 1.3; 95% confidence interval, 1.1-1.5; P = .0002). Furthermore, significantly more cases than controls consumed the highest tertile of gluten, more than 5 g per day, before seroconversion (OR, 2.7; 95% CI, 1.7-4.1; P less than .0001). These associations were similar among children of all haplotype profiles and trended in the same direction among children with and without first-degree relatives with celiac disease.
Cases and controls resembled each other in terms of breastfeeding duration, age at first introduction to gluten, and total daily caloric intake, the investigators noted. “The prospective design of this birth cohort study enabled us to obtain the diet information before seroconversion of tTGA as a marker of celiac disease,” they said. “This eliminated the risk of reporting biases or a change in feeding habits because of the knowledge of serology results or disease status.” But they did not analyze the number of daily servings of foods that contained gluten. “We cannot exclude the possibility that the number of portions given frequently during the course of the day may have different effects on disease risk,” they said.
The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
Long-suffering Swedish children probably have the highest rate of celiac disease in the world. This rate has dramatically increased. Why and why not? Previous studies have shown that it is not breastfeeding. It is not age or timing of introduction of gluten. It is not likely to be infections. This study shows that it is the amount of gluten that drives children with the highest genetic risk for celiac disease to develop the disease early in life. This conversion is preceded by a high intake of gluten. While these results alone should not determine general infant feeding practices, it suggests that if you are a Swedish child who carries these high-risk genes, high quantities of gluten early in life are not for you.
This study also raises the question of the effect high-dose gluten in adults at risk. Previously, studies have shown that the prevalence of celiac disease in adults in Sweden is not much different from the pediatric population. This study needs to be expanded to other Western populations where the rate of celiac disease is not so high. While nutritional engineering on a grand scale should not be undertaken lightly given the possibility of unexpected consequences, it behooves at least the Swedish population to perhaps reexamine their cultural practices of incorporating high gluten-containing cereals early in the lives of children, most especially those at particular risk for celiac disease.
Dr. Joseph A. Murray, AGAF, is professor of medicine, consultant, division of gastroenterology and hepatology, and department of immunology, and director of the Celiac Disease Program at the Mayo Clinic, Rochester, Minn.
Long-suffering Swedish children probably have the highest rate of celiac disease in the world. This rate has dramatically increased. Why and why not? Previous studies have shown that it is not breastfeeding. It is not age or timing of introduction of gluten. It is not likely to be infections. This study shows that it is the amount of gluten that drives children with the highest genetic risk for celiac disease to develop the disease early in life. This conversion is preceded by a high intake of gluten. While these results alone should not determine general infant feeding practices, it suggests that if you are a Swedish child who carries these high-risk genes, high quantities of gluten early in life are not for you.
This study also raises the question of the effect high-dose gluten in adults at risk. Previously, studies have shown that the prevalence of celiac disease in adults in Sweden is not much different from the pediatric population. This study needs to be expanded to other Western populations where the rate of celiac disease is not so high. While nutritional engineering on a grand scale should not be undertaken lightly given the possibility of unexpected consequences, it behooves at least the Swedish population to perhaps reexamine their cultural practices of incorporating high gluten-containing cereals early in the lives of children, most especially those at particular risk for celiac disease.
Dr. Joseph A. Murray, AGAF, is professor of medicine, consultant, division of gastroenterology and hepatology, and department of immunology, and director of the Celiac Disease Program at the Mayo Clinic, Rochester, Minn.
Long-suffering Swedish children probably have the highest rate of celiac disease in the world. This rate has dramatically increased. Why and why not? Previous studies have shown that it is not breastfeeding. It is not age or timing of introduction of gluten. It is not likely to be infections. This study shows that it is the amount of gluten that drives children with the highest genetic risk for celiac disease to develop the disease early in life. This conversion is preceded by a high intake of gluten. While these results alone should not determine general infant feeding practices, it suggests that if you are a Swedish child who carries these high-risk genes, high quantities of gluten early in life are not for you.
This study also raises the question of the effect high-dose gluten in adults at risk. Previously, studies have shown that the prevalence of celiac disease in adults in Sweden is not much different from the pediatric population. This study needs to be expanded to other Western populations where the rate of celiac disease is not so high. While nutritional engineering on a grand scale should not be undertaken lightly given the possibility of unexpected consequences, it behooves at least the Swedish population to perhaps reexamine their cultural practices of incorporating high gluten-containing cereals early in the lives of children, most especially those at particular risk for celiac disease.
Dr. Joseph A. Murray, AGAF, is professor of medicine, consultant, division of gastroenterology and hepatology, and department of immunology, and director of the Celiac Disease Program at the Mayo Clinic, Rochester, Minn.
Children who were genetically susceptible to celiac disease and consumed high amounts of gluten at 12 months of age were at least twice as likely to develop the autoimmune disorder as genetically predisposed children who consumed less gluten, researchers reported in the March issue of Clinical Gastroenterology and Hepatology.
The association was similar among children who carried any of the major human leukocyte antigen (HLA) risk genotypes for celiac disease, said Dr. Carin Aronsson at Lund University in Sweden and her associates. “Because these HLA risk genotypes are widely distributed in the general population, these findings may have consequence for future infant feeding recommendations,” they said. They recommended repeating the study in other countries to confirm the link.
In order to develop celiac disease, patients must consume gluten and carry at least one of the relevant DR3-DQ2 and DR4-DQ8 HLA risk haplotypes. But because gluten is widely consumed in products containing wheat, rye, and barley, and because about half of whites have at least one of the two haplotypes, gluten intolerance probably depends on other environmental factors, the researchers said. To further study these factors, they compared 3-day food diaries collected at ages 9, 12, 18, and 24 months for 146 children with positive tissue transglutaminase autoantibody (tTGA) assays and biopsy-confirmed celiac disease (cases) and 436 tTGA-negative children (controls). Cases and controls were matched by age, sex, and HLA genotype (Clin Gastroenterol Hepatol. 2015 Oct 7. doi: 10.1016/j.cgh.2015.09.030).
The food diaries revealed higher gluten intake among cases, compared with controls, beginning at the age of 12 months, said the researchers. Notably, cases consumed a median of 4.9 g of gluten a day before tTGA seroconversion, 1 g more than the median amount for controls of the same age (odds ratio, 1.3; 95% confidence interval, 1.1-1.5; P = .0002). Furthermore, significantly more cases than controls consumed the highest tertile of gluten, more than 5 g per day, before seroconversion (OR, 2.7; 95% CI, 1.7-4.1; P less than .0001). These associations were similar among children of all haplotype profiles and trended in the same direction among children with and without first-degree relatives with celiac disease.
Cases and controls resembled each other in terms of breastfeeding duration, age at first introduction to gluten, and total daily caloric intake, the investigators noted. “The prospective design of this birth cohort study enabled us to obtain the diet information before seroconversion of tTGA as a marker of celiac disease,” they said. “This eliminated the risk of reporting biases or a change in feeding habits because of the knowledge of serology results or disease status.” But they did not analyze the number of daily servings of foods that contained gluten. “We cannot exclude the possibility that the number of portions given frequently during the course of the day may have different effects on disease risk,” they said.
The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
Children who were genetically susceptible to celiac disease and consumed high amounts of gluten at 12 months of age were at least twice as likely to develop the autoimmune disorder as genetically predisposed children who consumed less gluten, researchers reported in the March issue of Clinical Gastroenterology and Hepatology.
The association was similar among children who carried any of the major human leukocyte antigen (HLA) risk genotypes for celiac disease, said Dr. Carin Aronsson at Lund University in Sweden and her associates. “Because these HLA risk genotypes are widely distributed in the general population, these findings may have consequence for future infant feeding recommendations,” they said. They recommended repeating the study in other countries to confirm the link.
In order to develop celiac disease, patients must consume gluten and carry at least one of the relevant DR3-DQ2 and DR4-DQ8 HLA risk haplotypes. But because gluten is widely consumed in products containing wheat, rye, and barley, and because about half of whites have at least one of the two haplotypes, gluten intolerance probably depends on other environmental factors, the researchers said. To further study these factors, they compared 3-day food diaries collected at ages 9, 12, 18, and 24 months for 146 children with positive tissue transglutaminase autoantibody (tTGA) assays and biopsy-confirmed celiac disease (cases) and 436 tTGA-negative children (controls). Cases and controls were matched by age, sex, and HLA genotype (Clin Gastroenterol Hepatol. 2015 Oct 7. doi: 10.1016/j.cgh.2015.09.030).
The food diaries revealed higher gluten intake among cases, compared with controls, beginning at the age of 12 months, said the researchers. Notably, cases consumed a median of 4.9 g of gluten a day before tTGA seroconversion, 1 g more than the median amount for controls of the same age (odds ratio, 1.3; 95% confidence interval, 1.1-1.5; P = .0002). Furthermore, significantly more cases than controls consumed the highest tertile of gluten, more than 5 g per day, before seroconversion (OR, 2.7; 95% CI, 1.7-4.1; P less than .0001). These associations were similar among children of all haplotype profiles and trended in the same direction among children with and without first-degree relatives with celiac disease.
Cases and controls resembled each other in terms of breastfeeding duration, age at first introduction to gluten, and total daily caloric intake, the investigators noted. “The prospective design of this birth cohort study enabled us to obtain the diet information before seroconversion of tTGA as a marker of celiac disease,” they said. “This eliminated the risk of reporting biases or a change in feeding habits because of the knowledge of serology results or disease status.” But they did not analyze the number of daily servings of foods that contained gluten. “We cannot exclude the possibility that the number of portions given frequently during the course of the day may have different effects on disease risk,” they said.
The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
High gluten consumption early in life upped risk of celiac disease
Children who were genetically susceptible to celiac disease and consumed high amounts of gluten at 12 months of age were at least twice as likely to develop the autoimmune disorder as genetically predisposed children who consumed less gluten, researchers reported in the March issue of Clinical Gastroenterology and Hepatology.
The association was similar among children who carried any of the major human leukocyte antigen (HLA) risk genotypes for celiac disease, said Dr. Carin Aronsson at Lund University in Sweden and her associates. “Because these HLA risk genotypes are widely distributed in the general population, these findings may have consequence for future infant feeding recommendations,” they said. They recommended repeating the study in other countries to confirm the link.
In order to develop celiac disease, patients must consume gluten and carry at least one of the relevant DR3-DQ2 and DR4-DQ8 HLA risk haplotypes. But because gluten is widely consumed in products containing wheat, rye, and barley, and because about half of whites have at least one of the two haplotypes, gluten intolerance probably depends on other environmental factors, the researchers said. To further study these factors, they compared 3-day food diaries collected at ages 9, 12, 18, and 24 months for 146 children with positive tissue transglutaminase autoantibody (tTGA) assays and biopsy-confirmed celiac disease (cases) and 436 tTGA-negative children (controls). Cases and controls were matched by age, sex, and HLA genotype (Clin Gastroenterol Hepatol. 2015 Oct 7. doi: 10.1016/j.cgh.2015.09.030).
The food diaries revealed higher gluten intake among cases, compared with controls, beginning at the age of 12 months, said the researchers. Notably, cases consumed a median of 4.9 g of gluten a day before tTGA seroconversion, 1 g more than the median amount for controls of the same age (odds ratio, 1.3; 95% confidence interval, 1.1-1.5; P = .0002). Furthermore, significantly more cases than controls consumed the highest tertile of gluten, more than 5 g per day, before seroconversion (OR, 2.7; 95% CI, 1.7-4.1; P less than .0001). These associations were similar among children of all haplotype profiles and trended in the same direction among children with and without first-degree relatives with celiac disease.
Cases and controls resembled each other in terms of breastfeeding duration, age at first introduction to gluten, and total daily caloric intake, the investigators noted. “The prospective design of this birth cohort study enabled us to obtain the diet information before seroconversion of tTGA as a marker of celiac disease,” they said. “This eliminated the risk of reporting biases or a change in feeding habits because of the knowledge of serology results or disease status.” But they did not analyze the number of daily servings of foods that contained gluten. “We cannot exclude the possibility that the number of portions given frequently during the course of the day may have different effects on disease risk,” they said.
The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
Long-suffering Swedish children probably have the highest rate of celiac disease in the world. This rate has dramatically increased. Why and why not? Previous studies have shown that it is not breastfeeding. It is not age or timing of introduction of gluten. It is not likely to be infections. This study shows that it is the amount of gluten that drives children with the highest genetic risk for celiac disease to develop the disease early in life. This conversion is preceded by a high intake of gluten. While these results alone should not determine general infant feeding practices, it suggests that if you are a Swedish child who carries these high-risk genes, high quantities of gluten early in life are not for you.
This study also raises the question of the effect high-dose gluten in adults at risk. Previously, studies have shown that the prevalence of celiac disease in adults in Sweden is not much different from the pediatric population. This study needs to be expanded to other Western populations where the rate of celiac disease is not so high. While nutritional engineering on a grand scale should not be undertaken lightly given the possibility of unexpected consequences, it behooves at least the Swedish population to perhaps reexamine their cultural practices of incorporating high gluten-containing cereals early in the lives of children, most especially those at particular risk for celiac disease.
Dr. Joseph A. Murray, AGAF, is professor of medicine, consultant, division of gastroenterology and hepatology, and department of immunology, and director of the Celiac Disease Program at the Mayo Clinic, Rochester, Minn.
Long-suffering Swedish children probably have the highest rate of celiac disease in the world. This rate has dramatically increased. Why and why not? Previous studies have shown that it is not breastfeeding. It is not age or timing of introduction of gluten. It is not likely to be infections. This study shows that it is the amount of gluten that drives children with the highest genetic risk for celiac disease to develop the disease early in life. This conversion is preceded by a high intake of gluten. While these results alone should not determine general infant feeding practices, it suggests that if you are a Swedish child who carries these high-risk genes, high quantities of gluten early in life are not for you.
This study also raises the question of the effect high-dose gluten in adults at risk. Previously, studies have shown that the prevalence of celiac disease in adults in Sweden is not much different from the pediatric population. This study needs to be expanded to other Western populations where the rate of celiac disease is not so high. While nutritional engineering on a grand scale should not be undertaken lightly given the possibility of unexpected consequences, it behooves at least the Swedish population to perhaps reexamine their cultural practices of incorporating high gluten-containing cereals early in the lives of children, most especially those at particular risk for celiac disease.
Dr. Joseph A. Murray, AGAF, is professor of medicine, consultant, division of gastroenterology and hepatology, and department of immunology, and director of the Celiac Disease Program at the Mayo Clinic, Rochester, Minn.
Long-suffering Swedish children probably have the highest rate of celiac disease in the world. This rate has dramatically increased. Why and why not? Previous studies have shown that it is not breastfeeding. It is not age or timing of introduction of gluten. It is not likely to be infections. This study shows that it is the amount of gluten that drives children with the highest genetic risk for celiac disease to develop the disease early in life. This conversion is preceded by a high intake of gluten. While these results alone should not determine general infant feeding practices, it suggests that if you are a Swedish child who carries these high-risk genes, high quantities of gluten early in life are not for you.
This study also raises the question of the effect high-dose gluten in adults at risk. Previously, studies have shown that the prevalence of celiac disease in adults in Sweden is not much different from the pediatric population. This study needs to be expanded to other Western populations where the rate of celiac disease is not so high. While nutritional engineering on a grand scale should not be undertaken lightly given the possibility of unexpected consequences, it behooves at least the Swedish population to perhaps reexamine their cultural practices of incorporating high gluten-containing cereals early in the lives of children, most especially those at particular risk for celiac disease.
Dr. Joseph A. Murray, AGAF, is professor of medicine, consultant, division of gastroenterology and hepatology, and department of immunology, and director of the Celiac Disease Program at the Mayo Clinic, Rochester, Minn.
Children who were genetically susceptible to celiac disease and consumed high amounts of gluten at 12 months of age were at least twice as likely to develop the autoimmune disorder as genetically predisposed children who consumed less gluten, researchers reported in the March issue of Clinical Gastroenterology and Hepatology.
The association was similar among children who carried any of the major human leukocyte antigen (HLA) risk genotypes for celiac disease, said Dr. Carin Aronsson at Lund University in Sweden and her associates. “Because these HLA risk genotypes are widely distributed in the general population, these findings may have consequence for future infant feeding recommendations,” they said. They recommended repeating the study in other countries to confirm the link.
In order to develop celiac disease, patients must consume gluten and carry at least one of the relevant DR3-DQ2 and DR4-DQ8 HLA risk haplotypes. But because gluten is widely consumed in products containing wheat, rye, and barley, and because about half of whites have at least one of the two haplotypes, gluten intolerance probably depends on other environmental factors, the researchers said. To further study these factors, they compared 3-day food diaries collected at ages 9, 12, 18, and 24 months for 146 children with positive tissue transglutaminase autoantibody (tTGA) assays and biopsy-confirmed celiac disease (cases) and 436 tTGA-negative children (controls). Cases and controls were matched by age, sex, and HLA genotype (Clin Gastroenterol Hepatol. 2015 Oct 7. doi: 10.1016/j.cgh.2015.09.030).
The food diaries revealed higher gluten intake among cases, compared with controls, beginning at the age of 12 months, said the researchers. Notably, cases consumed a median of 4.9 g of gluten a day before tTGA seroconversion, 1 g more than the median amount for controls of the same age (odds ratio, 1.3; 95% confidence interval, 1.1-1.5; P = .0002). Furthermore, significantly more cases than controls consumed the highest tertile of gluten, more than 5 g per day, before seroconversion (OR, 2.7; 95% CI, 1.7-4.1; P less than .0001). These associations were similar among children of all haplotype profiles and trended in the same direction among children with and without first-degree relatives with celiac disease.
Cases and controls resembled each other in terms of breastfeeding duration, age at first introduction to gluten, and total daily caloric intake, the investigators noted. “The prospective design of this birth cohort study enabled us to obtain the diet information before seroconversion of tTGA as a marker of celiac disease,” they said. “This eliminated the risk of reporting biases or a change in feeding habits because of the knowledge of serology results or disease status.” But they did not analyze the number of daily servings of foods that contained gluten. “We cannot exclude the possibility that the number of portions given frequently during the course of the day may have different effects on disease risk,” they said.
The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
Children who were genetically susceptible to celiac disease and consumed high amounts of gluten at 12 months of age were at least twice as likely to develop the autoimmune disorder as genetically predisposed children who consumed less gluten, researchers reported in the March issue of Clinical Gastroenterology and Hepatology.
The association was similar among children who carried any of the major human leukocyte antigen (HLA) risk genotypes for celiac disease, said Dr. Carin Aronsson at Lund University in Sweden and her associates. “Because these HLA risk genotypes are widely distributed in the general population, these findings may have consequence for future infant feeding recommendations,” they said. They recommended repeating the study in other countries to confirm the link.
In order to develop celiac disease, patients must consume gluten and carry at least one of the relevant DR3-DQ2 and DR4-DQ8 HLA risk haplotypes. But because gluten is widely consumed in products containing wheat, rye, and barley, and because about half of whites have at least one of the two haplotypes, gluten intolerance probably depends on other environmental factors, the researchers said. To further study these factors, they compared 3-day food diaries collected at ages 9, 12, 18, and 24 months for 146 children with positive tissue transglutaminase autoantibody (tTGA) assays and biopsy-confirmed celiac disease (cases) and 436 tTGA-negative children (controls). Cases and controls were matched by age, sex, and HLA genotype (Clin Gastroenterol Hepatol. 2015 Oct 7. doi: 10.1016/j.cgh.2015.09.030).
The food diaries revealed higher gluten intake among cases, compared with controls, beginning at the age of 12 months, said the researchers. Notably, cases consumed a median of 4.9 g of gluten a day before tTGA seroconversion, 1 g more than the median amount for controls of the same age (odds ratio, 1.3; 95% confidence interval, 1.1-1.5; P = .0002). Furthermore, significantly more cases than controls consumed the highest tertile of gluten, more than 5 g per day, before seroconversion (OR, 2.7; 95% CI, 1.7-4.1; P less than .0001). These associations were similar among children of all haplotype profiles and trended in the same direction among children with and without first-degree relatives with celiac disease.
Cases and controls resembled each other in terms of breastfeeding duration, age at first introduction to gluten, and total daily caloric intake, the investigators noted. “The prospective design of this birth cohort study enabled us to obtain the diet information before seroconversion of tTGA as a marker of celiac disease,” they said. “This eliminated the risk of reporting biases or a change in feeding habits because of the knowledge of serology results or disease status.” But they did not analyze the number of daily servings of foods that contained gluten. “We cannot exclude the possibility that the number of portions given frequently during the course of the day may have different effects on disease risk,” they said.
The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: High levels of gluten consumption in early life significantly increased the risk of celiac disease.
Major finding: The odds of celiac disease were more than twice as high among children who consumed more than 5 g of gluten a day, compared with those who consumed less gluten (OR, 2.65; P less than .0001).
Data source: A 1 to 3 matched nested case-control study of 146 children with biopsy-confirmed celiac disease (cases) and 436 tissue transglutaminase (tTGA)-negative controls.
Disclosures: The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Bedside asthma medication delivery tied to lower ED readmissions
A bedside medication delivery service increased the percentage of asthma patients discharged with medications in hand from 0% to 75%, which helped prevent emergency department readmissions within the next month, according to an exploratory, retrospective analysis.
“To our knowledge this report is the first to detail specific strategies to reliably discharge patients with meds in hand,” said Dr. Jonathan Hatoun of Boston University Medical Center and his associates. “Although this study was not designed to detect adherence, families may feel more comfortable administering medications after receiving teaching from and having questions answered by their inpatient nurse, using the same medications and equipment they will use at home.”
Before the intervention, the hospital previously had routinely discharged asthma patients without medications in hand, and in 2011, a survey showed that 37% never filled their prescriptions. Concerned that patients were “unnecessarily suffering,” Dr. Hatoun and his associates assembled a multidisciplinary team that worked for 2 years to improve this outcome measure. They initially asked residents to write prescriptions at least a day before discharge, but they were concerned that treatment plans could change. Next, they asked families to pick up medications at the hospital pharmacy, but parents were reluctant to leave their sick child’s bedside. Therefore, the researchers designed an in-room service in which pharmacists delivered the medications to the child’s room when a parent was present (Pediatrics 2016 Feb 24. doi: 10.1542/peds.2015-0461). “Copayments were collected in the room, either in cash or with a mobile credit payment system purchased by the pharmacy,” the researchers explained. “Unlike traditional pharmacy pickup, the delivery service allows the patient, parent, nurse, and pharmacist to be together in the patient’s hospital room for teaching with the actual medications available for demonstration.”
The delivery service not only met the project goal to increase the “meds in hand” rate from 0% to 75%, but an analysis of patients with complete insurance claims showed that patients discharged with medications in hand were significantly less likely to return to the emergency department within 30 days of discharge, for any reason, compared with patients who received usual care (odds ratio, 0.22; 95% confidence interval, 0.05-0.99).
“Although more evidence on the impact of being discharged in possession of discharge medications is needed, a service that provides admitted patients with their outpatient medications before they leave the hospital has many potential benefits,” the investigators noted. “Additional areas of exploration could include how the Meds-in-Hand service affects the patient experience, hospital finances, and clinical outcomes for other medical conditions.”
The authors had no external funding sources or disclosures.
A bedside medication delivery service increased the percentage of asthma patients discharged with medications in hand from 0% to 75%, which helped prevent emergency department readmissions within the next month, according to an exploratory, retrospective analysis.
“To our knowledge this report is the first to detail specific strategies to reliably discharge patients with meds in hand,” said Dr. Jonathan Hatoun of Boston University Medical Center and his associates. “Although this study was not designed to detect adherence, families may feel more comfortable administering medications after receiving teaching from and having questions answered by their inpatient nurse, using the same medications and equipment they will use at home.”
Before the intervention, the hospital previously had routinely discharged asthma patients without medications in hand, and in 2011, a survey showed that 37% never filled their prescriptions. Concerned that patients were “unnecessarily suffering,” Dr. Hatoun and his associates assembled a multidisciplinary team that worked for 2 years to improve this outcome measure. They initially asked residents to write prescriptions at least a day before discharge, but they were concerned that treatment plans could change. Next, they asked families to pick up medications at the hospital pharmacy, but parents were reluctant to leave their sick child’s bedside. Therefore, the researchers designed an in-room service in which pharmacists delivered the medications to the child’s room when a parent was present (Pediatrics 2016 Feb 24. doi: 10.1542/peds.2015-0461). “Copayments were collected in the room, either in cash or with a mobile credit payment system purchased by the pharmacy,” the researchers explained. “Unlike traditional pharmacy pickup, the delivery service allows the patient, parent, nurse, and pharmacist to be together in the patient’s hospital room for teaching with the actual medications available for demonstration.”
The delivery service not only met the project goal to increase the “meds in hand” rate from 0% to 75%, but an analysis of patients with complete insurance claims showed that patients discharged with medications in hand were significantly less likely to return to the emergency department within 30 days of discharge, for any reason, compared with patients who received usual care (odds ratio, 0.22; 95% confidence interval, 0.05-0.99).
“Although more evidence on the impact of being discharged in possession of discharge medications is needed, a service that provides admitted patients with their outpatient medications before they leave the hospital has many potential benefits,” the investigators noted. “Additional areas of exploration could include how the Meds-in-Hand service affects the patient experience, hospital finances, and clinical outcomes for other medical conditions.”
The authors had no external funding sources or disclosures.
A bedside medication delivery service increased the percentage of asthma patients discharged with medications in hand from 0% to 75%, which helped prevent emergency department readmissions within the next month, according to an exploratory, retrospective analysis.
“To our knowledge this report is the first to detail specific strategies to reliably discharge patients with meds in hand,” said Dr. Jonathan Hatoun of Boston University Medical Center and his associates. “Although this study was not designed to detect adherence, families may feel more comfortable administering medications after receiving teaching from and having questions answered by their inpatient nurse, using the same medications and equipment they will use at home.”
Before the intervention, the hospital previously had routinely discharged asthma patients without medications in hand, and in 2011, a survey showed that 37% never filled their prescriptions. Concerned that patients were “unnecessarily suffering,” Dr. Hatoun and his associates assembled a multidisciplinary team that worked for 2 years to improve this outcome measure. They initially asked residents to write prescriptions at least a day before discharge, but they were concerned that treatment plans could change. Next, they asked families to pick up medications at the hospital pharmacy, but parents were reluctant to leave their sick child’s bedside. Therefore, the researchers designed an in-room service in which pharmacists delivered the medications to the child’s room when a parent was present (Pediatrics 2016 Feb 24. doi: 10.1542/peds.2015-0461). “Copayments were collected in the room, either in cash or with a mobile credit payment system purchased by the pharmacy,” the researchers explained. “Unlike traditional pharmacy pickup, the delivery service allows the patient, parent, nurse, and pharmacist to be together in the patient’s hospital room for teaching with the actual medications available for demonstration.”
The delivery service not only met the project goal to increase the “meds in hand” rate from 0% to 75%, but an analysis of patients with complete insurance claims showed that patients discharged with medications in hand were significantly less likely to return to the emergency department within 30 days of discharge, for any reason, compared with patients who received usual care (odds ratio, 0.22; 95% confidence interval, 0.05-0.99).
“Although more evidence on the impact of being discharged in possession of discharge medications is needed, a service that provides admitted patients with their outpatient medications before they leave the hospital has many potential benefits,” the investigators noted. “Additional areas of exploration could include how the Meds-in-Hand service affects the patient experience, hospital finances, and clinical outcomes for other medical conditions.”
The authors had no external funding sources or disclosures.
FROM PEDIATRICS
Key clinical point: A bedside medication delivery service ensured that most children hospitalized with asthma left with medications in hand, helping prevent 30-day readmissions.
Major finding: The rate of discharge with medications in hand rose from 0% to 75%. Discharge with medications in hand was associated with significantly decreased odds of 30-day all-cause emergency department readmission, compared with usual care (odds ratio, 0.22; 95% confidence interval, 0.05-0.99).
Data source: A single-center exploratory retrospective study.
Disclosures: The researchers had no external funding sources and no disclosures.
Mirtazapine improves functional dyspepsia in small study
The antidepressant mirtazapine improved weight loss, early satiation, nausea, and other signs and symptoms in patients with functional dyspepsia, said the authors of a placebo-controlled pilot study published in the March issue of Clinical Gastroenterology and Hepatology.
The findings suggest that mirtazapine “has the potential to become the treatment of choice for functional dyspepsia in patients with weight loss, and evaluation in larger multicenter studies is warranted,” said Dr. Jan Tack and his associates at the University of Leuven, Belgium.
Functional dyspepsia, one of the most prevalent gastrointestinal disorders, is characterized by early satiation, postprandial fullness, and epigastric pain and burning in the absence of underlying systemic or metabolic disease. Up to 40% of affected patients lose weight, an “alarm symptom” that until now has lacked effective treatment, the researchers said.
Mirtazapine, an antagonist of the H1, alpha2, 5-hydroxytryptamine (5-HT)2c, and 5-HT3 receptors, often causes weight gain when used to treat depression. Therefore, the investigators designed a double-blind single-center pilot trial of 34 patients with functional dyspepsia who had lost more than 10% of their original body weight. After a 2-week run-in period, half the patients were randomized to 15 mg of mirtazapine every evening and the other half to placebo (Clin Gastroenterol Hepatol. 2016 Jan 9. doi: 10.1016/j.cgh.2015.09.043).
The average weight of placebo patients remained almost unchanged throughout the trial, while patients on mirtazapine gained an average of 2.5 + 0.6 kg by week 4 (P = .003 for between-group comparison) and 3.9 + 0.7 kg, or 6.4% of their original body weight, by week 8 (P less than .0001). Mean scores on a validated dyspepsia symptom severity (DSS) questionnaire improved significantly between baseline and weeks 4 (P = .003) and 8 (P = .017) for mirtazapine but not placebo. Directly comparing the two groups in terms of the DSS revealed a large effect size that trended toward significance (P = .06) at week 4 but not at week 8 (P = .55). However, mirtazapine significantly outperformed placebo in measures of early satiety, quality of life, gastrointestinal-specific anxiety, and nutrient tolerance, “mostly with large effect sizes,” the investigators said.
Mirtazapine did not affect epigastric pain or gastric emptying, and had little effect on postprandial fullness. Moreover, 2 of 17 patients in the mirtazapine group dropped out of the study because of unacceptable levels of drowsiness, which is a common side effect of the medication.
Many patients with functional dyspepsia respond inadequately to first-line treatment with acid-suppressive or prokinetic drugs, the investigators noted. While tegaserod, buspirone, and acotiamide can improve gastric accommodation, it is unknown if they promote weight gain. The results for mirtazapine are promising, but the pilot trial included only tertiary care patients, and the small sample size precluded separate analyses of patients with postprandial distress syndrome as opposed to epigastric pain syndrome, the researchers said.
The study was funded by Leuven University, the FWO, and the KU Leuven Special Research Fund. Mirtazapine and placebo were supplied by MSD Belgium. The investigators had no disclosures.
The antidepressant mirtazapine improved weight loss, early satiation, nausea, and other signs and symptoms in patients with functional dyspepsia, said the authors of a placebo-controlled pilot study published in the March issue of Clinical Gastroenterology and Hepatology.
The findings suggest that mirtazapine “has the potential to become the treatment of choice for functional dyspepsia in patients with weight loss, and evaluation in larger multicenter studies is warranted,” said Dr. Jan Tack and his associates at the University of Leuven, Belgium.
Functional dyspepsia, one of the most prevalent gastrointestinal disorders, is characterized by early satiation, postprandial fullness, and epigastric pain and burning in the absence of underlying systemic or metabolic disease. Up to 40% of affected patients lose weight, an “alarm symptom” that until now has lacked effective treatment, the researchers said.
Mirtazapine, an antagonist of the H1, alpha2, 5-hydroxytryptamine (5-HT)2c, and 5-HT3 receptors, often causes weight gain when used to treat depression. Therefore, the investigators designed a double-blind single-center pilot trial of 34 patients with functional dyspepsia who had lost more than 10% of their original body weight. After a 2-week run-in period, half the patients were randomized to 15 mg of mirtazapine every evening and the other half to placebo (Clin Gastroenterol Hepatol. 2016 Jan 9. doi: 10.1016/j.cgh.2015.09.043).
The average weight of placebo patients remained almost unchanged throughout the trial, while patients on mirtazapine gained an average of 2.5 + 0.6 kg by week 4 (P = .003 for between-group comparison) and 3.9 + 0.7 kg, or 6.4% of their original body weight, by week 8 (P less than .0001). Mean scores on a validated dyspepsia symptom severity (DSS) questionnaire improved significantly between baseline and weeks 4 (P = .003) and 8 (P = .017) for mirtazapine but not placebo. Directly comparing the two groups in terms of the DSS revealed a large effect size that trended toward significance (P = .06) at week 4 but not at week 8 (P = .55). However, mirtazapine significantly outperformed placebo in measures of early satiety, quality of life, gastrointestinal-specific anxiety, and nutrient tolerance, “mostly with large effect sizes,” the investigators said.
Mirtazapine did not affect epigastric pain or gastric emptying, and had little effect on postprandial fullness. Moreover, 2 of 17 patients in the mirtazapine group dropped out of the study because of unacceptable levels of drowsiness, which is a common side effect of the medication.
Many patients with functional dyspepsia respond inadequately to first-line treatment with acid-suppressive or prokinetic drugs, the investigators noted. While tegaserod, buspirone, and acotiamide can improve gastric accommodation, it is unknown if they promote weight gain. The results for mirtazapine are promising, but the pilot trial included only tertiary care patients, and the small sample size precluded separate analyses of patients with postprandial distress syndrome as opposed to epigastric pain syndrome, the researchers said.
The study was funded by Leuven University, the FWO, and the KU Leuven Special Research Fund. Mirtazapine and placebo were supplied by MSD Belgium. The investigators had no disclosures.
The antidepressant mirtazapine improved weight loss, early satiation, nausea, and other signs and symptoms in patients with functional dyspepsia, said the authors of a placebo-controlled pilot study published in the March issue of Clinical Gastroenterology and Hepatology.
The findings suggest that mirtazapine “has the potential to become the treatment of choice for functional dyspepsia in patients with weight loss, and evaluation in larger multicenter studies is warranted,” said Dr. Jan Tack and his associates at the University of Leuven, Belgium.
Functional dyspepsia, one of the most prevalent gastrointestinal disorders, is characterized by early satiation, postprandial fullness, and epigastric pain and burning in the absence of underlying systemic or metabolic disease. Up to 40% of affected patients lose weight, an “alarm symptom” that until now has lacked effective treatment, the researchers said.
Mirtazapine, an antagonist of the H1, alpha2, 5-hydroxytryptamine (5-HT)2c, and 5-HT3 receptors, often causes weight gain when used to treat depression. Therefore, the investigators designed a double-blind single-center pilot trial of 34 patients with functional dyspepsia who had lost more than 10% of their original body weight. After a 2-week run-in period, half the patients were randomized to 15 mg of mirtazapine every evening and the other half to placebo (Clin Gastroenterol Hepatol. 2016 Jan 9. doi: 10.1016/j.cgh.2015.09.043).
The average weight of placebo patients remained almost unchanged throughout the trial, while patients on mirtazapine gained an average of 2.5 + 0.6 kg by week 4 (P = .003 for between-group comparison) and 3.9 + 0.7 kg, or 6.4% of their original body weight, by week 8 (P less than .0001). Mean scores on a validated dyspepsia symptom severity (DSS) questionnaire improved significantly between baseline and weeks 4 (P = .003) and 8 (P = .017) for mirtazapine but not placebo. Directly comparing the two groups in terms of the DSS revealed a large effect size that trended toward significance (P = .06) at week 4 but not at week 8 (P = .55). However, mirtazapine significantly outperformed placebo in measures of early satiety, quality of life, gastrointestinal-specific anxiety, and nutrient tolerance, “mostly with large effect sizes,” the investigators said.
Mirtazapine did not affect epigastric pain or gastric emptying, and had little effect on postprandial fullness. Moreover, 2 of 17 patients in the mirtazapine group dropped out of the study because of unacceptable levels of drowsiness, which is a common side effect of the medication.
Many patients with functional dyspepsia respond inadequately to first-line treatment with acid-suppressive or prokinetic drugs, the investigators noted. While tegaserod, buspirone, and acotiamide can improve gastric accommodation, it is unknown if they promote weight gain. The results for mirtazapine are promising, but the pilot trial included only tertiary care patients, and the small sample size precluded separate analyses of patients with postprandial distress syndrome as opposed to epigastric pain syndrome, the researchers said.
The study was funded by Leuven University, the FWO, and the KU Leuven Special Research Fund. Mirtazapine and placebo were supplied by MSD Belgium. The investigators had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Mirtazapine treatment led to weight gain and a number of other improvements among patients with functional dyspepsia and weight loss.
Major finding: Patients regained an average of 6.5% of their original body weight on mirtazapine, and did not regain weight on placebo.
Data source: A single-center randomized double-blind study of 34 patients with functional dyspepsia.
Disclosures: Leuven University, the FWO, and the KU Leuven Special Research Fund helped fund the study. Mirtazapine and placebo were supplied by MSD Belgium. The investigators had no disclosures.
Study backed familial component of advanced adenoma risk
Siblings of patients with advanced adenoma had sixfold higher odds of having the tumors themselves, as compared with controls, said the authors of a blinded cross-sectional study reported in the March issue of Gastroenterology.
The results reinforce the need for early screening of individuals whose siblings have advanced adenoma, said Dr. Siew Ng at the Chinese University of Hong Kong and her associates. The risk of advanced adenoma was even higher when affected probands were younger than average or had multiple adenomas, the researchers added.
Most studies that have purported to study the familial risk of adenoma actually studied the risk of adenoma in persons whose first-degree relatives have colorectal cancer, according to Dr. Ng and her associates. Their study included 200 asymptomatic (“exposed”) siblings of individuals with advanced adenomas as diagnosed on colonoscopy, and 400 controls whose siblings had no family history of colorectal cancer or colonoscopic evidence of neoplasia. The researchers defined advanced adenomas as those measuring at least 10 mm or that had high-grade dysplasia or villous or tubulovillous characteristics. “We focused on advanced lesions, as they have the greatest malignant potential, and removing these lesions can reduce colorectal cancer incidence and mortality,” they said (Gastroenterology. 2015 Nov 14. doi: 10.1053/j.gastro.2015.11.003).
Exposed siblings were consistently more likely to have adenomas themselves, compared with the control group, said the investigators. For example, the prevalence of any advanced adenoma was 11.5% among exposed siblings compared with only 2.5% among controls (matched odds ratio, 6.05; 95% confidence interval, 2.7-13.4; P less than .001). Similarly, the prevalence of adenomas measuring at least 10 mm was 10.5% among exposed individuals and 1.8% among controls (mOR, 8.6; 95% CI, 3.4-21.4; P less than .001). The prevalence of villous adenomas was 5.5% among exposed individuals and 1.3% among controls (mOR, 6.3; 95% CI, 2.0-19.5; P = .001) and the prevalence of all colorectal adenomas was 39% among exposed individuals and 19% among controls (mOR, 3.3; 95% CI, 2.2-5.0; P less than .001). Finally, two cases of colorectal cancer were detected among the exposed siblings, while no such cases were detected among the controls.
The exposed siblings and controls resembled each other in terms of aspirin use, smoking, body mass index, and metabolic diseases, the researchers said. However, the probands with adenoma were identified from a consecutive group of patients, while control siblings were enrolled through a screening program, they said. Therefore, the groups might have differed in terms of unmeasured environmental risk factors for cancer, such as physical activity and dietary habits. They also noted the difficulties in obtaining accurate family histories of colonic neoplasia, especially distinguishing adenoma from advanced adenoma. Finally, Hong Kong is ethnically homogenous, and the data might not be generalizable to other populations, although Asia and Western countries do tend to have comparable rates of advanced adenoma in average-risk individuals and in families with histories of colorectal neoplasias.
The Research Grants Council of the Hong Kong Special Administrative Region funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
Current guidelines recommend early screening and shorter surveillance intervals in individuals with a first-degree relative (FDR) with colorectal cancer (CRC) (Gastroenterology. 2008;134:1570-950). Existing literature is limited by either lack of an appropriate comparison group or inability to assess adenoma risk in subjects who have an FDR with adenomas.

|
| Dr. Harini S. Naidu |
To date, this is the first prospective study to demonstrate increased prevalence of advanced adenomas in siblings of probands with advanced adenomas detected during colonoscopy. The authors should be congratulated on completing an organized, well-powered study using colonoscopy and histopathology and were careful to limit familial clustering by randomly selecting only one sibling from each family. Although this study has important findings, there are a few points worthy of consideration.
First, it would be helpful to understand whether the siblings shared both parents, one parent, or were adopted, as this would affect the genetic implications of the findings.
Second, the analysis did not stratify probands and siblings based on whether the colonoscopy included in the study was the first or second screening, or surveillance colonoscopy. The risk of advanced adenomas is expected to be different in someone with numerous normal colonoscopies, compared with someone undergoing their initial screening colonoscopy, and this point deserves clarification.

|
| Dr. Audrey H. Calderwood |
Third, it would be helpful to know how many siblings in each group were excluded due to previous adenomas, which bias results towards the null. For example, exclusion of high-risk individuals with previous adenomas in the control group may make the prevalence of adenoma detection appear lower if only lower-risk individuals are included.
Lastly, this study was performed in a uniform Asian patient population, and may not be generalizable to other populations. Validation in a more ethnically heterogeneous setting is warranted. Overall, this is a solid, clinically relevant study that can help inform the impact of family history of advanced adenomas on CRC screening recommendations.
In addition, the study’s findings corroborate the American College of Gastroenterology’s recommendations for earlier CRC screening at shorter surveillance intervals in patients who have FDRs with advanced adenomas detected at age less than 60, or two FDRs diagnosed with advanced adenomas at any age (Am J Gastroenterol. 2009;104:739–50).
Dr. Harini S. Naidu and Dr. Audrey H. Calderwood are in the section of gastroenterology, Boston University. The authors have no conflicts of interest to declare.
Current guidelines recommend early screening and shorter surveillance intervals in individuals with a first-degree relative (FDR) with colorectal cancer (CRC) (Gastroenterology. 2008;134:1570-950). Existing literature is limited by either lack of an appropriate comparison group or inability to assess adenoma risk in subjects who have an FDR with adenomas.

|
| Dr. Harini S. Naidu |
To date, this is the first prospective study to demonstrate increased prevalence of advanced adenomas in siblings of probands with advanced adenomas detected during colonoscopy. The authors should be congratulated on completing an organized, well-powered study using colonoscopy and histopathology and were careful to limit familial clustering by randomly selecting only one sibling from each family. Although this study has important findings, there are a few points worthy of consideration.
First, it would be helpful to understand whether the siblings shared both parents, one parent, or were adopted, as this would affect the genetic implications of the findings.
Second, the analysis did not stratify probands and siblings based on whether the colonoscopy included in the study was the first or second screening, or surveillance colonoscopy. The risk of advanced adenomas is expected to be different in someone with numerous normal colonoscopies, compared with someone undergoing their initial screening colonoscopy, and this point deserves clarification.

|
| Dr. Audrey H. Calderwood |
Third, it would be helpful to know how many siblings in each group were excluded due to previous adenomas, which bias results towards the null. For example, exclusion of high-risk individuals with previous adenomas in the control group may make the prevalence of adenoma detection appear lower if only lower-risk individuals are included.
Lastly, this study was performed in a uniform Asian patient population, and may not be generalizable to other populations. Validation in a more ethnically heterogeneous setting is warranted. Overall, this is a solid, clinically relevant study that can help inform the impact of family history of advanced adenomas on CRC screening recommendations.
In addition, the study’s findings corroborate the American College of Gastroenterology’s recommendations for earlier CRC screening at shorter surveillance intervals in patients who have FDRs with advanced adenomas detected at age less than 60, or two FDRs diagnosed with advanced adenomas at any age (Am J Gastroenterol. 2009;104:739–50).
Dr. Harini S. Naidu and Dr. Audrey H. Calderwood are in the section of gastroenterology, Boston University. The authors have no conflicts of interest to declare.
Current guidelines recommend early screening and shorter surveillance intervals in individuals with a first-degree relative (FDR) with colorectal cancer (CRC) (Gastroenterology. 2008;134:1570-950). Existing literature is limited by either lack of an appropriate comparison group or inability to assess adenoma risk in subjects who have an FDR with adenomas.

|
| Dr. Harini S. Naidu |
To date, this is the first prospective study to demonstrate increased prevalence of advanced adenomas in siblings of probands with advanced adenomas detected during colonoscopy. The authors should be congratulated on completing an organized, well-powered study using colonoscopy and histopathology and were careful to limit familial clustering by randomly selecting only one sibling from each family. Although this study has important findings, there are a few points worthy of consideration.
First, it would be helpful to understand whether the siblings shared both parents, one parent, or were adopted, as this would affect the genetic implications of the findings.
Second, the analysis did not stratify probands and siblings based on whether the colonoscopy included in the study was the first or second screening, or surveillance colonoscopy. The risk of advanced adenomas is expected to be different in someone with numerous normal colonoscopies, compared with someone undergoing their initial screening colonoscopy, and this point deserves clarification.

|
| Dr. Audrey H. Calderwood |
Third, it would be helpful to know how many siblings in each group were excluded due to previous adenomas, which bias results towards the null. For example, exclusion of high-risk individuals with previous adenomas in the control group may make the prevalence of adenoma detection appear lower if only lower-risk individuals are included.
Lastly, this study was performed in a uniform Asian patient population, and may not be generalizable to other populations. Validation in a more ethnically heterogeneous setting is warranted. Overall, this is a solid, clinically relevant study that can help inform the impact of family history of advanced adenomas on CRC screening recommendations.
In addition, the study’s findings corroborate the American College of Gastroenterology’s recommendations for earlier CRC screening at shorter surveillance intervals in patients who have FDRs with advanced adenomas detected at age less than 60, or two FDRs diagnosed with advanced adenomas at any age (Am J Gastroenterol. 2009;104:739–50).
Dr. Harini S. Naidu and Dr. Audrey H. Calderwood are in the section of gastroenterology, Boston University. The authors have no conflicts of interest to declare.
Siblings of patients with advanced adenoma had sixfold higher odds of having the tumors themselves, as compared with controls, said the authors of a blinded cross-sectional study reported in the March issue of Gastroenterology.
The results reinforce the need for early screening of individuals whose siblings have advanced adenoma, said Dr. Siew Ng at the Chinese University of Hong Kong and her associates. The risk of advanced adenoma was even higher when affected probands were younger than average or had multiple adenomas, the researchers added.
Most studies that have purported to study the familial risk of adenoma actually studied the risk of adenoma in persons whose first-degree relatives have colorectal cancer, according to Dr. Ng and her associates. Their study included 200 asymptomatic (“exposed”) siblings of individuals with advanced adenomas as diagnosed on colonoscopy, and 400 controls whose siblings had no family history of colorectal cancer or colonoscopic evidence of neoplasia. The researchers defined advanced adenomas as those measuring at least 10 mm or that had high-grade dysplasia or villous or tubulovillous characteristics. “We focused on advanced lesions, as they have the greatest malignant potential, and removing these lesions can reduce colorectal cancer incidence and mortality,” they said (Gastroenterology. 2015 Nov 14. doi: 10.1053/j.gastro.2015.11.003).
Exposed siblings were consistently more likely to have adenomas themselves, compared with the control group, said the investigators. For example, the prevalence of any advanced adenoma was 11.5% among exposed siblings compared with only 2.5% among controls (matched odds ratio, 6.05; 95% confidence interval, 2.7-13.4; P less than .001). Similarly, the prevalence of adenomas measuring at least 10 mm was 10.5% among exposed individuals and 1.8% among controls (mOR, 8.6; 95% CI, 3.4-21.4; P less than .001). The prevalence of villous adenomas was 5.5% among exposed individuals and 1.3% among controls (mOR, 6.3; 95% CI, 2.0-19.5; P = .001) and the prevalence of all colorectal adenomas was 39% among exposed individuals and 19% among controls (mOR, 3.3; 95% CI, 2.2-5.0; P less than .001). Finally, two cases of colorectal cancer were detected among the exposed siblings, while no such cases were detected among the controls.
The exposed siblings and controls resembled each other in terms of aspirin use, smoking, body mass index, and metabolic diseases, the researchers said. However, the probands with adenoma were identified from a consecutive group of patients, while control siblings were enrolled through a screening program, they said. Therefore, the groups might have differed in terms of unmeasured environmental risk factors for cancer, such as physical activity and dietary habits. They also noted the difficulties in obtaining accurate family histories of colonic neoplasia, especially distinguishing adenoma from advanced adenoma. Finally, Hong Kong is ethnically homogenous, and the data might not be generalizable to other populations, although Asia and Western countries do tend to have comparable rates of advanced adenoma in average-risk individuals and in families with histories of colorectal neoplasias.
The Research Grants Council of the Hong Kong Special Administrative Region funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
Siblings of patients with advanced adenoma had sixfold higher odds of having the tumors themselves, as compared with controls, said the authors of a blinded cross-sectional study reported in the March issue of Gastroenterology.
The results reinforce the need for early screening of individuals whose siblings have advanced adenoma, said Dr. Siew Ng at the Chinese University of Hong Kong and her associates. The risk of advanced adenoma was even higher when affected probands were younger than average or had multiple adenomas, the researchers added.
Most studies that have purported to study the familial risk of adenoma actually studied the risk of adenoma in persons whose first-degree relatives have colorectal cancer, according to Dr. Ng and her associates. Their study included 200 asymptomatic (“exposed”) siblings of individuals with advanced adenomas as diagnosed on colonoscopy, and 400 controls whose siblings had no family history of colorectal cancer or colonoscopic evidence of neoplasia. The researchers defined advanced adenomas as those measuring at least 10 mm or that had high-grade dysplasia or villous or tubulovillous characteristics. “We focused on advanced lesions, as they have the greatest malignant potential, and removing these lesions can reduce colorectal cancer incidence and mortality,” they said (Gastroenterology. 2015 Nov 14. doi: 10.1053/j.gastro.2015.11.003).
Exposed siblings were consistently more likely to have adenomas themselves, compared with the control group, said the investigators. For example, the prevalence of any advanced adenoma was 11.5% among exposed siblings compared with only 2.5% among controls (matched odds ratio, 6.05; 95% confidence interval, 2.7-13.4; P less than .001). Similarly, the prevalence of adenomas measuring at least 10 mm was 10.5% among exposed individuals and 1.8% among controls (mOR, 8.6; 95% CI, 3.4-21.4; P less than .001). The prevalence of villous adenomas was 5.5% among exposed individuals and 1.3% among controls (mOR, 6.3; 95% CI, 2.0-19.5; P = .001) and the prevalence of all colorectal adenomas was 39% among exposed individuals and 19% among controls (mOR, 3.3; 95% CI, 2.2-5.0; P less than .001). Finally, two cases of colorectal cancer were detected among the exposed siblings, while no such cases were detected among the controls.
The exposed siblings and controls resembled each other in terms of aspirin use, smoking, body mass index, and metabolic diseases, the researchers said. However, the probands with adenoma were identified from a consecutive group of patients, while control siblings were enrolled through a screening program, they said. Therefore, the groups might have differed in terms of unmeasured environmental risk factors for cancer, such as physical activity and dietary habits. They also noted the difficulties in obtaining accurate family histories of colonic neoplasia, especially distinguishing adenoma from advanced adenoma. Finally, Hong Kong is ethnically homogenous, and the data might not be generalizable to other populations, although Asia and Western countries do tend to have comparable rates of advanced adenoma in average-risk individuals and in families with histories of colorectal neoplasias.
The Research Grants Council of the Hong Kong Special Administrative Region funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
FROM GASTROENTEROLOGY
Key clinical point: Siblings of patients with advanced adenoma were substantially more likely to also have advanced adenomas as compared with controls.
Major finding: The odds of advanced adenomas among exposed siblings were six times greater than for controls (95% confidence interval, 2.7-13.4; P less than .001).
Data source: A cross-sectional study of 200 asymptomatic siblings of individuals with advanced adenomas and 400 controls whose siblings had no family history of colorectal cancer or colonoscopic evidence of neoplasia.
Disclosures: The Research Grants Council of the Hong Kong Special Administrative Region funded the study. The investigators had no disclosures.
MRI topped transient elastography for staging nonalcoholic fatty liver disease
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Two specialized MRI techniques surpassed transient elastography for staging fibrosis and steatosis in nonalcoholic fatty liver disease.
Major finding: The areas under the curve for magnetic resonance elastography and the proton density fat fraction measure were significantly greater than those for transient elastography and the TE-based controlled attenuation parameter (P is less than .001 for both comparisons).
Data source: A cross-sectional study of 142 patients with nonalcoholic fatty liver disease and 10 controls.
Disclosures: The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
Labeled peptide bound the claudin-1 target in colorectal cancer models
An optically labeled peptide rapidly and specifically bound the claudin-1 membrane protein, a “promising” target for early detection of human colonic adenomas, according to a study published online in the March issue of Cellular and Molecular Gastroenterology and Hepatology.
If the peptide holds up in human studies, it might one day be used to screen high-risk patients with multiple polyps, inflammatory bowel disease, Lynch syndrome, or a family history of CRC, said Dr. Emily Rabinsky and her associates at the University of Michigan in Ann Arbor. Peptides can be delivered topically to mucosa in high concentrations to maximize binding and image contrast while minimizing the risk of systemic absorption and toxicity, they added.
Adenomatous polyps express early molecular targets that are candidates for enhanced CRC surveillance. This research area is important because more than 35% of premalignant colonic lesions are flat and therefore difficult to visualize. As a result, up to 25% of adenomas are missed during typical white light colonoscopy. Furthermore, premalignant adenomas cannot be distinguished grossly from noncancerous hyperplastic polyps, the researchers noted (Cell Mol Gastroenterol Hepatol. 2016 [doi: 10.1016/j.jcmgh.2015.12.001]). Claudin-1 is an integral membrane protein that is known to be overexpressed in human colorectal and squamous cell neoplasias as well as in cancers of the human pancreas, thyroid, cervix, stomach, and nasopharynx. For the study, the investigators used gene expression data to confirm that claudin-1 is an early target in colonic adenomas, and then performed phage display against the extracellular loop of claudin-1 to identify the claudin-1-specific RTSPSSR peptide. After labeling the peptide with Cy5.5, they characterized its binding parameters and confirmed that it also specifically binds to human CRC cells. Next, they performed in vivo fluorescent colonic endoscopy of CPC;Apc mice, which spontaneously develop colonic adenomas. They also used immunofluorescence to confirm that RTSPSSR binds specifically to adenomas from the proximal human colon.
Claudin-1 expression was 2.5 times greater in human colonic adenomas compared with normal colonic mucosa, the researchers found. Furthermore, the RTSPSSR peptide specifically bound claudin-1 in knockdown and competition studies. Binding to CRC cells occurred in 1.2 minutes, with an “adequate” affinity of 42 nmol per liter, the researchers said. Moreover, the peptide specifically bound to both flat and polyploid murine colonic adenomas, with a significantly higher target-to-background ratio compared with normal in vivo images. In addition, immunofluorescence was significantly more intense for peptide that bound to adenomas and sessile serrated adenomas from the proximal human colon compared with normal tissue and hyperplastic polyps.
“Future development of this peptide will require in vivo clinical validation in human studies,” said the investigators. “Although we found promising results with this peptide alone, disease heterogeneity in a broad patient population may require use of additional targets using multiplexed imaging methods.” Nonetheless, this peptide can help detect multiple targets with a single topical application and at relatively low expression levels, they noted.
The study was partially funded by the National Institutes of Health and by Mary L. Petrovich. Dr. Rabinsky and two coinvestigators are co-inventors on a provisional patent on the claudin-1 peptide. The other researchers had no disclosures.
The application of fluorescent affinity probes described in this study is groundbreaking. In the context of advanced imaging techniques, including chromoendoscopy, narrowband imaging, high magnification, and confocal endomicroscopy, this study describes a specific molecular probe. That is a major advance in the area of personalized medicine.
While most would agree that detection of polypoid adenomas does not generally require advanced imaging technologies, the genetically engineered mouse model used in this study is useful for proof of concept. It is, however, important to note that lesions were not detected from a broad area; polyps were labeled during a 5-minute incubation with the fluorescent-tagged peptide and the area was then washed. While the fluorescent intensity of lesions relative to surrounding nondysplastic mucosae were impressively elevated in both polypoid and flat adenomas relative, it is important to note that there was significant overlap between normal mucosae, hyperplastic polyps, sessile serrated adenomas/polyps, and traditional adenomas. While the limited sensitivity and specificity make it unlikely that the probe used here, which targets a surface protein that is only modestly upregulated in dysplasia, will be of great value. However, the idea of specifically detecting lesions using affinity probes does have promise.
On the basis of this study, some might ask whether biopsy and histopathologic examination can be replaced by intravital affinity labeling. At this point, the answer must be no, as the sensitivity and specificity of labeling techniques are far below that of traditional histopathologic examination, even for straightforward lesions such as those studied here. Yet as a means to enhance the sensitivity of sampling when surveying large areas, such as Barrett’s esophagus or long-standing ulcerative colitis, the approaches described in this study point the way to a bright future.
Jerrold R. Turner, M.D., Ph.D., AGAF, is in the departments of pathology and medicine (GI), Brigham and Women’s Hospital, Harvard Medical School, Boston.
The application of fluorescent affinity probes described in this study is groundbreaking. In the context of advanced imaging techniques, including chromoendoscopy, narrowband imaging, high magnification, and confocal endomicroscopy, this study describes a specific molecular probe. That is a major advance in the area of personalized medicine.
While most would agree that detection of polypoid adenomas does not generally require advanced imaging technologies, the genetically engineered mouse model used in this study is useful for proof of concept. It is, however, important to note that lesions were not detected from a broad area; polyps were labeled during a 5-minute incubation with the fluorescent-tagged peptide and the area was then washed. While the fluorescent intensity of lesions relative to surrounding nondysplastic mucosae were impressively elevated in both polypoid and flat adenomas relative, it is important to note that there was significant overlap between normal mucosae, hyperplastic polyps, sessile serrated adenomas/polyps, and traditional adenomas. While the limited sensitivity and specificity make it unlikely that the probe used here, which targets a surface protein that is only modestly upregulated in dysplasia, will be of great value. However, the idea of specifically detecting lesions using affinity probes does have promise.
On the basis of this study, some might ask whether biopsy and histopathologic examination can be replaced by intravital affinity labeling. At this point, the answer must be no, as the sensitivity and specificity of labeling techniques are far below that of traditional histopathologic examination, even for straightforward lesions such as those studied here. Yet as a means to enhance the sensitivity of sampling when surveying large areas, such as Barrett’s esophagus or long-standing ulcerative colitis, the approaches described in this study point the way to a bright future.
Jerrold R. Turner, M.D., Ph.D., AGAF, is in the departments of pathology and medicine (GI), Brigham and Women’s Hospital, Harvard Medical School, Boston.
The application of fluorescent affinity probes described in this study is groundbreaking. In the context of advanced imaging techniques, including chromoendoscopy, narrowband imaging, high magnification, and confocal endomicroscopy, this study describes a specific molecular probe. That is a major advance in the area of personalized medicine.
While most would agree that detection of polypoid adenomas does not generally require advanced imaging technologies, the genetically engineered mouse model used in this study is useful for proof of concept. It is, however, important to note that lesions were not detected from a broad area; polyps were labeled during a 5-minute incubation with the fluorescent-tagged peptide and the area was then washed. While the fluorescent intensity of lesions relative to surrounding nondysplastic mucosae were impressively elevated in both polypoid and flat adenomas relative, it is important to note that there was significant overlap between normal mucosae, hyperplastic polyps, sessile serrated adenomas/polyps, and traditional adenomas. While the limited sensitivity and specificity make it unlikely that the probe used here, which targets a surface protein that is only modestly upregulated in dysplasia, will be of great value. However, the idea of specifically detecting lesions using affinity probes does have promise.
On the basis of this study, some might ask whether biopsy and histopathologic examination can be replaced by intravital affinity labeling. At this point, the answer must be no, as the sensitivity and specificity of labeling techniques are far below that of traditional histopathologic examination, even for straightforward lesions such as those studied here. Yet as a means to enhance the sensitivity of sampling when surveying large areas, such as Barrett’s esophagus or long-standing ulcerative colitis, the approaches described in this study point the way to a bright future.
Jerrold R. Turner, M.D., Ph.D., AGAF, is in the departments of pathology and medicine (GI), Brigham and Women’s Hospital, Harvard Medical School, Boston.
An optically labeled peptide rapidly and specifically bound the claudin-1 membrane protein, a “promising” target for early detection of human colonic adenomas, according to a study published online in the March issue of Cellular and Molecular Gastroenterology and Hepatology.
If the peptide holds up in human studies, it might one day be used to screen high-risk patients with multiple polyps, inflammatory bowel disease, Lynch syndrome, or a family history of CRC, said Dr. Emily Rabinsky and her associates at the University of Michigan in Ann Arbor. Peptides can be delivered topically to mucosa in high concentrations to maximize binding and image contrast while minimizing the risk of systemic absorption and toxicity, they added.
Adenomatous polyps express early molecular targets that are candidates for enhanced CRC surveillance. This research area is important because more than 35% of premalignant colonic lesions are flat and therefore difficult to visualize. As a result, up to 25% of adenomas are missed during typical white light colonoscopy. Furthermore, premalignant adenomas cannot be distinguished grossly from noncancerous hyperplastic polyps, the researchers noted (Cell Mol Gastroenterol Hepatol. 2016 [doi: 10.1016/j.jcmgh.2015.12.001]). Claudin-1 is an integral membrane protein that is known to be overexpressed in human colorectal and squamous cell neoplasias as well as in cancers of the human pancreas, thyroid, cervix, stomach, and nasopharynx. For the study, the investigators used gene expression data to confirm that claudin-1 is an early target in colonic adenomas, and then performed phage display against the extracellular loop of claudin-1 to identify the claudin-1-specific RTSPSSR peptide. After labeling the peptide with Cy5.5, they characterized its binding parameters and confirmed that it also specifically binds to human CRC cells. Next, they performed in vivo fluorescent colonic endoscopy of CPC;Apc mice, which spontaneously develop colonic adenomas. They also used immunofluorescence to confirm that RTSPSSR binds specifically to adenomas from the proximal human colon.
Claudin-1 expression was 2.5 times greater in human colonic adenomas compared with normal colonic mucosa, the researchers found. Furthermore, the RTSPSSR peptide specifically bound claudin-1 in knockdown and competition studies. Binding to CRC cells occurred in 1.2 minutes, with an “adequate” affinity of 42 nmol per liter, the researchers said. Moreover, the peptide specifically bound to both flat and polyploid murine colonic adenomas, with a significantly higher target-to-background ratio compared with normal in vivo images. In addition, immunofluorescence was significantly more intense for peptide that bound to adenomas and sessile serrated adenomas from the proximal human colon compared with normal tissue and hyperplastic polyps.
“Future development of this peptide will require in vivo clinical validation in human studies,” said the investigators. “Although we found promising results with this peptide alone, disease heterogeneity in a broad patient population may require use of additional targets using multiplexed imaging methods.” Nonetheless, this peptide can help detect multiple targets with a single topical application and at relatively low expression levels, they noted.
The study was partially funded by the National Institutes of Health and by Mary L. Petrovich. Dr. Rabinsky and two coinvestigators are co-inventors on a provisional patent on the claudin-1 peptide. The other researchers had no disclosures.
An optically labeled peptide rapidly and specifically bound the claudin-1 membrane protein, a “promising” target for early detection of human colonic adenomas, according to a study published online in the March issue of Cellular and Molecular Gastroenterology and Hepatology.
If the peptide holds up in human studies, it might one day be used to screen high-risk patients with multiple polyps, inflammatory bowel disease, Lynch syndrome, or a family history of CRC, said Dr. Emily Rabinsky and her associates at the University of Michigan in Ann Arbor. Peptides can be delivered topically to mucosa in high concentrations to maximize binding and image contrast while minimizing the risk of systemic absorption and toxicity, they added.
Adenomatous polyps express early molecular targets that are candidates for enhanced CRC surveillance. This research area is important because more than 35% of premalignant colonic lesions are flat and therefore difficult to visualize. As a result, up to 25% of adenomas are missed during typical white light colonoscopy. Furthermore, premalignant adenomas cannot be distinguished grossly from noncancerous hyperplastic polyps, the researchers noted (Cell Mol Gastroenterol Hepatol. 2016 [doi: 10.1016/j.jcmgh.2015.12.001]). Claudin-1 is an integral membrane protein that is known to be overexpressed in human colorectal and squamous cell neoplasias as well as in cancers of the human pancreas, thyroid, cervix, stomach, and nasopharynx. For the study, the investigators used gene expression data to confirm that claudin-1 is an early target in colonic adenomas, and then performed phage display against the extracellular loop of claudin-1 to identify the claudin-1-specific RTSPSSR peptide. After labeling the peptide with Cy5.5, they characterized its binding parameters and confirmed that it also specifically binds to human CRC cells. Next, they performed in vivo fluorescent colonic endoscopy of CPC;Apc mice, which spontaneously develop colonic adenomas. They also used immunofluorescence to confirm that RTSPSSR binds specifically to adenomas from the proximal human colon.
Claudin-1 expression was 2.5 times greater in human colonic adenomas compared with normal colonic mucosa, the researchers found. Furthermore, the RTSPSSR peptide specifically bound claudin-1 in knockdown and competition studies. Binding to CRC cells occurred in 1.2 minutes, with an “adequate” affinity of 42 nmol per liter, the researchers said. Moreover, the peptide specifically bound to both flat and polyploid murine colonic adenomas, with a significantly higher target-to-background ratio compared with normal in vivo images. In addition, immunofluorescence was significantly more intense for peptide that bound to adenomas and sessile serrated adenomas from the proximal human colon compared with normal tissue and hyperplastic polyps.
“Future development of this peptide will require in vivo clinical validation in human studies,” said the investigators. “Although we found promising results with this peptide alone, disease heterogeneity in a broad patient population may require use of additional targets using multiplexed imaging methods.” Nonetheless, this peptide can help detect multiple targets with a single topical application and at relatively low expression levels, they noted.
The study was partially funded by the National Institutes of Health and by Mary L. Petrovich. Dr. Rabinsky and two coinvestigators are co-inventors on a provisional patent on the claudin-1 peptide. The other researchers had no disclosures.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The claudin-1 protein is overexpressed in human colonic adenomas and was bound by the labeled fluorescence RTSPSSR peptide.
Major finding: The peptide bound to claudin-1 in colorectal cancer cells in 1.2 minutes, with an “adequate” affinity of 42 nmol per liter. Immunofluorescence revealed significantly greater binding intensity for human colonic adenomas and sessile serrated adenomas than normal tissue or hyperplastic polyps.
Data source: An analysis of gene expression data, phage display, endoscopy of CPC;Apc mice, and immunofluorescence of normal and cancerous human proximal colon tissue.
Disclosures: The study was partially funded by the National Institutes of Health and by Mary L. Petrovich. Dr. Rabinsky and two coinvestigators are coinventors on a provisional patent on the peptide. The other researchers had no disclosures.
Labeled peptide binds the claudin-1 target in CRC
An optically labeled peptide rapidly and specifically bound the claudin-1 membrane protein, a “promising” target for early detection of human colonic adenomas, in a study published in the March issue of Cellular and Molecular Gastroenterology and Hepatology.
If the peptide holds up in human studies, it could be used to screen high-risk patients with multiple polyps, inflammatory bowel disease, Lynch syndrome, or a family history of (CRC), said Dr. Emily Rabinsky and associates at the University of Michigan, Ann Arbor. Peptides can be delivered topically to mucosa in high concentrations to maximize binding and image contrast while minimizing the risk of systemic absorption and toxicity, they added.
Adenomatous polyps express early molecular targets that are candidates for enhanced CRC surveillance. More than 35% of premalignant colonic lesions are flat and therefore difficult to visualize, so up to 25% of adenomas are missed during typical white light colonoscopy. Premalignant adenomas cannot be distinguished grossly from noncancerous hyperplastic polyps (Cell Mol Gastroenterol Hepatol. 2016. doi: 10.1016/j.jcmgh.2015.12.001).
The researchers used gene expression data to confirm that claudin-1 is an early target in colonic adenomas, and performed phage display against the extracellular loop of claudin-1 to identify the claudin-1-specific RTSPSSR peptide. After labeling the peptide with Cy5.5, they characterized its binding parameters and confirmed that it specifically binds to human CRC cells. They did in vivo fluorescent colonic endoscopy of CPC;Apc mice, which spontaneously develop colonic adenomas. They used immunofluorescence to confirm that RTSPSSR binds specifically to adenomas from the proximal human colon.
Claudin-1 expression was 2.5 times greater in human colonic adenomas than normal colonic mucosa, and the RTSPSSR peptide specifically bound claudin-1 in knockdown and competition studies. Binding to CRC cells occurred in 1.2 minutes, with an “adequate” affinity of 42 nmol/L. The peptide specifically bound to both flat and polyploid murine colonic adenomas, with a significantly higher target-to-background ratio compared with normal in vivo images.
The National Institutes of Health and Mary L. Petrovich provided funding. Dr. Rabinsky and two coauthors are coinventors on a provisional patent on the claudin-1 peptide.
An optically labeled peptide rapidly and specifically bound the claudin-1 membrane protein, a “promising” target for early detection of human colonic adenomas, in a study published in the March issue of Cellular and Molecular Gastroenterology and Hepatology.
If the peptide holds up in human studies, it could be used to screen high-risk patients with multiple polyps, inflammatory bowel disease, Lynch syndrome, or a family history of (CRC), said Dr. Emily Rabinsky and associates at the University of Michigan, Ann Arbor. Peptides can be delivered topically to mucosa in high concentrations to maximize binding and image contrast while minimizing the risk of systemic absorption and toxicity, they added.
Adenomatous polyps express early molecular targets that are candidates for enhanced CRC surveillance. More than 35% of premalignant colonic lesions are flat and therefore difficult to visualize, so up to 25% of adenomas are missed during typical white light colonoscopy. Premalignant adenomas cannot be distinguished grossly from noncancerous hyperplastic polyps (Cell Mol Gastroenterol Hepatol. 2016. doi: 10.1016/j.jcmgh.2015.12.001).
The researchers used gene expression data to confirm that claudin-1 is an early target in colonic adenomas, and performed phage display against the extracellular loop of claudin-1 to identify the claudin-1-specific RTSPSSR peptide. After labeling the peptide with Cy5.5, they characterized its binding parameters and confirmed that it specifically binds to human CRC cells. They did in vivo fluorescent colonic endoscopy of CPC;Apc mice, which spontaneously develop colonic adenomas. They used immunofluorescence to confirm that RTSPSSR binds specifically to adenomas from the proximal human colon.
Claudin-1 expression was 2.5 times greater in human colonic adenomas than normal colonic mucosa, and the RTSPSSR peptide specifically bound claudin-1 in knockdown and competition studies. Binding to CRC cells occurred in 1.2 minutes, with an “adequate” affinity of 42 nmol/L. The peptide specifically bound to both flat and polyploid murine colonic adenomas, with a significantly higher target-to-background ratio compared with normal in vivo images.
The National Institutes of Health and Mary L. Petrovich provided funding. Dr. Rabinsky and two coauthors are coinventors on a provisional patent on the claudin-1 peptide.
An optically labeled peptide rapidly and specifically bound the claudin-1 membrane protein, a “promising” target for early detection of human colonic adenomas, in a study published in the March issue of Cellular and Molecular Gastroenterology and Hepatology.
If the peptide holds up in human studies, it could be used to screen high-risk patients with multiple polyps, inflammatory bowel disease, Lynch syndrome, or a family history of (CRC), said Dr. Emily Rabinsky and associates at the University of Michigan, Ann Arbor. Peptides can be delivered topically to mucosa in high concentrations to maximize binding and image contrast while minimizing the risk of systemic absorption and toxicity, they added.
Adenomatous polyps express early molecular targets that are candidates for enhanced CRC surveillance. More than 35% of premalignant colonic lesions are flat and therefore difficult to visualize, so up to 25% of adenomas are missed during typical white light colonoscopy. Premalignant adenomas cannot be distinguished grossly from noncancerous hyperplastic polyps (Cell Mol Gastroenterol Hepatol. 2016. doi: 10.1016/j.jcmgh.2015.12.001).
The researchers used gene expression data to confirm that claudin-1 is an early target in colonic adenomas, and performed phage display against the extracellular loop of claudin-1 to identify the claudin-1-specific RTSPSSR peptide. After labeling the peptide with Cy5.5, they characterized its binding parameters and confirmed that it specifically binds to human CRC cells. They did in vivo fluorescent colonic endoscopy of CPC;Apc mice, which spontaneously develop colonic adenomas. They used immunofluorescence to confirm that RTSPSSR binds specifically to adenomas from the proximal human colon.
Claudin-1 expression was 2.5 times greater in human colonic adenomas than normal colonic mucosa, and the RTSPSSR peptide specifically bound claudin-1 in knockdown and competition studies. Binding to CRC cells occurred in 1.2 minutes, with an “adequate” affinity of 42 nmol/L. The peptide specifically bound to both flat and polyploid murine colonic adenomas, with a significantly higher target-to-background ratio compared with normal in vivo images.
The National Institutes of Health and Mary L. Petrovich provided funding. Dr. Rabinsky and two coauthors are coinventors on a provisional patent on the claudin-1 peptide.