User login
HCV patients had distinct mucosal microbiome
SAN DIEGO – Patients with hepatitis C virus (HCV) infections had distinct duodenal mucosal microbiomes and greater intestinal permeability, compared with healthy controls and patients with other chronic liver diseases, Dr. Ashok Raj reported.
The findings might one day lead to therapies that aim to restore or normalize the microbiomes of patients with HCV, Dr. Raj said in an interview at the annual Digestive Disease Week.
Chronic liver disease (CLD) has been linked to dysbiosis, or abnormal shifts of the microbiome. But most studies have focused on fecal specimens, and “recent evidence suggests that the mucosal microbiota differ from fecal microbiota,” said Dr. Raj, a gastroenterologist and hepatologist at Princess Alexandra Hospital in Brisbane, Australia, and a PhD candidate at the University of Queensland at Brisbane.
“The small-intestinal mucosal microbiota are of particular interest to us,” Dr. Raj explained. “Anatomically, all the blood from this region of the gut drains into the portal vein and flows directly to the liver. Because of small-intestinal permeability, either bacteria or their products could travel to the liver and contribute to disease. But very little is known about this microbiota in CLD.”
Therefore, Dr. Raj and his associates sequenced bacterial DNA from mucosal biopsies of the second part of the duodenum from 38 prospectively recruited endoscopy patients with CLD and 13 healthy controls. The researchers also evaluated dietary habits, intestinal permeability, hepatic stiffness based on transient elastography, and the presence of metabolic syndrome, as measured by the International Diabetes Federation/American Heart Association/National Heart, Lung, and Blood Institute 2009 Consensus criteria. The CLD group included 28 men and 10 women aged 36-82 years, including 16 patients with HCV, 10 patients with nonalcoholic fatty liver disease, 7 patients with fatty liver disease, 3 patients with autoimmune hepatitis, and 2 patients with hepatitis B virus infection. The controls were between 24 and 73 years old, and 70% were women.
Sequencing of bacteria DNA revealed significant differences between patients and controls, particularly among patients with HCV, Dr. Raj said. The HCV patients not only had significantly less microbial diversity (P less than .02), but the overall changes in their microbiota were significant enough for them to cluster separately from controls and from patients with other types of CLD (P less than .01 for both comparisons). Furthermore, HCV patients had significantly greater small-intestinal permeability (mean ± SD log lactulose to rhamnose ratio, 1.57 ± 0.27) than controls (1.21 ± 0.25; P less than .01) or patients with other CLDs (1.24 ± 0.39; P = .01).
“Additionally, for the HCV patients, dietary fat intake showed a moderately strong positive correlation with intestinal permeability,” Dr. Raj said (r = 0.58; P = .03). “These findings are in keeping with animal models, which have shown that dietary fat can change the microbiota and also increase intestinal permeability.” However, the multivariate analysis found no links between microbial characteristics and hepatic stiffness or metabolic syndrome – perhaps because most patients were “at the cirrhotic end of the spectrum, reflecting their indication for endoscopy,” or because “these relationships are subtler and require larger sample numbers,” he said.
“Patients with HCV may have a unique small-intestinal microbiome,” Dr. Raj concluded. “These patients had higher intestinal permeability, and it is possible that the microbiota have a part to play in that.” Exactly how microbiota and gut permeability contribute to disease remains unclear, but pathology in the small intestine could help explain some features of the HCV trajectory, such as extrahepatic manifestations or variations in disease progression, he added. “Future studies may lead to targeting the small-intestinal gut microbiome to modulate and even treat HCV.”
The study was funded by a postgraduate award from the government of Australia and by the Princess Alexandra Hospital Research Foundation. Dr. Raj had no disclosures.
SAN DIEGO – Patients with hepatitis C virus (HCV) infections had distinct duodenal mucosal microbiomes and greater intestinal permeability, compared with healthy controls and patients with other chronic liver diseases, Dr. Ashok Raj reported.
The findings might one day lead to therapies that aim to restore or normalize the microbiomes of patients with HCV, Dr. Raj said in an interview at the annual Digestive Disease Week.
Chronic liver disease (CLD) has been linked to dysbiosis, or abnormal shifts of the microbiome. But most studies have focused on fecal specimens, and “recent evidence suggests that the mucosal microbiota differ from fecal microbiota,” said Dr. Raj, a gastroenterologist and hepatologist at Princess Alexandra Hospital in Brisbane, Australia, and a PhD candidate at the University of Queensland at Brisbane.
“The small-intestinal mucosal microbiota are of particular interest to us,” Dr. Raj explained. “Anatomically, all the blood from this region of the gut drains into the portal vein and flows directly to the liver. Because of small-intestinal permeability, either bacteria or their products could travel to the liver and contribute to disease. But very little is known about this microbiota in CLD.”
Therefore, Dr. Raj and his associates sequenced bacterial DNA from mucosal biopsies of the second part of the duodenum from 38 prospectively recruited endoscopy patients with CLD and 13 healthy controls. The researchers also evaluated dietary habits, intestinal permeability, hepatic stiffness based on transient elastography, and the presence of metabolic syndrome, as measured by the International Diabetes Federation/American Heart Association/National Heart, Lung, and Blood Institute 2009 Consensus criteria. The CLD group included 28 men and 10 women aged 36-82 years, including 16 patients with HCV, 10 patients with nonalcoholic fatty liver disease, 7 patients with fatty liver disease, 3 patients with autoimmune hepatitis, and 2 patients with hepatitis B virus infection. The controls were between 24 and 73 years old, and 70% were women.
Sequencing of bacteria DNA revealed significant differences between patients and controls, particularly among patients with HCV, Dr. Raj said. The HCV patients not only had significantly less microbial diversity (P less than .02), but the overall changes in their microbiota were significant enough for them to cluster separately from controls and from patients with other types of CLD (P less than .01 for both comparisons). Furthermore, HCV patients had significantly greater small-intestinal permeability (mean ± SD log lactulose to rhamnose ratio, 1.57 ± 0.27) than controls (1.21 ± 0.25; P less than .01) or patients with other CLDs (1.24 ± 0.39; P = .01).
“Additionally, for the HCV patients, dietary fat intake showed a moderately strong positive correlation with intestinal permeability,” Dr. Raj said (r = 0.58; P = .03). “These findings are in keeping with animal models, which have shown that dietary fat can change the microbiota and also increase intestinal permeability.” However, the multivariate analysis found no links between microbial characteristics and hepatic stiffness or metabolic syndrome – perhaps because most patients were “at the cirrhotic end of the spectrum, reflecting their indication for endoscopy,” or because “these relationships are subtler and require larger sample numbers,” he said.
“Patients with HCV may have a unique small-intestinal microbiome,” Dr. Raj concluded. “These patients had higher intestinal permeability, and it is possible that the microbiota have a part to play in that.” Exactly how microbiota and gut permeability contribute to disease remains unclear, but pathology in the small intestine could help explain some features of the HCV trajectory, such as extrahepatic manifestations or variations in disease progression, he added. “Future studies may lead to targeting the small-intestinal gut microbiome to modulate and even treat HCV.”
The study was funded by a postgraduate award from the government of Australia and by the Princess Alexandra Hospital Research Foundation. Dr. Raj had no disclosures.
SAN DIEGO – Patients with hepatitis C virus (HCV) infections had distinct duodenal mucosal microbiomes and greater intestinal permeability, compared with healthy controls and patients with other chronic liver diseases, Dr. Ashok Raj reported.
The findings might one day lead to therapies that aim to restore or normalize the microbiomes of patients with HCV, Dr. Raj said in an interview at the annual Digestive Disease Week.
Chronic liver disease (CLD) has been linked to dysbiosis, or abnormal shifts of the microbiome. But most studies have focused on fecal specimens, and “recent evidence suggests that the mucosal microbiota differ from fecal microbiota,” said Dr. Raj, a gastroenterologist and hepatologist at Princess Alexandra Hospital in Brisbane, Australia, and a PhD candidate at the University of Queensland at Brisbane.
“The small-intestinal mucosal microbiota are of particular interest to us,” Dr. Raj explained. “Anatomically, all the blood from this region of the gut drains into the portal vein and flows directly to the liver. Because of small-intestinal permeability, either bacteria or their products could travel to the liver and contribute to disease. But very little is known about this microbiota in CLD.”
Therefore, Dr. Raj and his associates sequenced bacterial DNA from mucosal biopsies of the second part of the duodenum from 38 prospectively recruited endoscopy patients with CLD and 13 healthy controls. The researchers also evaluated dietary habits, intestinal permeability, hepatic stiffness based on transient elastography, and the presence of metabolic syndrome, as measured by the International Diabetes Federation/American Heart Association/National Heart, Lung, and Blood Institute 2009 Consensus criteria. The CLD group included 28 men and 10 women aged 36-82 years, including 16 patients with HCV, 10 patients with nonalcoholic fatty liver disease, 7 patients with fatty liver disease, 3 patients with autoimmune hepatitis, and 2 patients with hepatitis B virus infection. The controls were between 24 and 73 years old, and 70% were women.
Sequencing of bacteria DNA revealed significant differences between patients and controls, particularly among patients with HCV, Dr. Raj said. The HCV patients not only had significantly less microbial diversity (P less than .02), but the overall changes in their microbiota were significant enough for them to cluster separately from controls and from patients with other types of CLD (P less than .01 for both comparisons). Furthermore, HCV patients had significantly greater small-intestinal permeability (mean ± SD log lactulose to rhamnose ratio, 1.57 ± 0.27) than controls (1.21 ± 0.25; P less than .01) or patients with other CLDs (1.24 ± 0.39; P = .01).
“Additionally, for the HCV patients, dietary fat intake showed a moderately strong positive correlation with intestinal permeability,” Dr. Raj said (r = 0.58; P = .03). “These findings are in keeping with animal models, which have shown that dietary fat can change the microbiota and also increase intestinal permeability.” However, the multivariate analysis found no links between microbial characteristics and hepatic stiffness or metabolic syndrome – perhaps because most patients were “at the cirrhotic end of the spectrum, reflecting their indication for endoscopy,” or because “these relationships are subtler and require larger sample numbers,” he said.
“Patients with HCV may have a unique small-intestinal microbiome,” Dr. Raj concluded. “These patients had higher intestinal permeability, and it is possible that the microbiota have a part to play in that.” Exactly how microbiota and gut permeability contribute to disease remains unclear, but pathology in the small intestine could help explain some features of the HCV trajectory, such as extrahepatic manifestations or variations in disease progression, he added. “Future studies may lead to targeting the small-intestinal gut microbiome to modulate and even treat HCV.”
The study was funded by a postgraduate award from the government of Australia and by the Princess Alexandra Hospital Research Foundation. Dr. Raj had no disclosures.
AT DDW® 2016
Key clinical point: Patients with hepatitis C virus infection had unique mucosal microbiomes, compared with controls or patients with other chronic liver diseases.
Major finding: The HCV patients not only had significantly less microbial diversity (P less than .02), but the overall changes in their microbiota were significant enough for them to cluster separately from controls and from patients with other types of chronic liver disease (P less than .01 for both comparisons).
Data source: Bacterial DNA sequencing of duodenal mucosal biopsies from 38 patients with chronic liver diseases and 10 controls.
Disclosures: The study was funded by a postgraduate award from the government of Australia and by the Princess Alexandra Hospital Research Foundation. Dr. Raj had no disclosures.
Empagliflozin slows renal disease progression in type 2 diabetes
Empagliflozin was associated with a significant 39% decrease in risk of new or worsening nephropathy, compared with placebo among adults with type 2 diabetes at high risk for cardiovascular events, based on a secondary analysis of the phase III, randomized, double-blind EMPA-REG OUTCOME trial.
“Patients in the empagliflozin group also had a significantly lower risk of progression to macroalbuminuria or clinically relevant renal outcomes, such as a doubling of the serum creatinine level and initiation of renal-replacement therapy, than did those in the placebo group,” Dr. Christoph Wanner of Würzburg (Germany) University Clinic, and his associates reported in the June 14 New England Journal of Medicine.
Empagliflozin(Jardiance) is a selective sodium–glucose cotransporter-2 inhibitor that was approved in the United States in 2014 to improve glycemic control among patients with type 2 diabetes mellitus. In the initial analysis of the EMPA-REG OUTCOME trial, empagliflozin was associated with a significantly lower rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke, as compared with placebo among patients with type 2 diabetes at high cardiovascular risk (N Engl J Med. 2015 Nov 26 doi: 10.1056/NEJMoa1504720).
For the current secondary analysis, Dr. Wanner and his associates focused on renal microvascular outcomes, including new or worsening nephropathy (macroalbuminuria, defined as greater than 300 mg of urinary albumin/gram of creatinine), a doubling of the serum creatinine level accompanied by an epidermal growth factor receptor (eGFR) of less than or equal to 45 mL/minute per 1.73 m2of body surface area, a new need for renal-replacement therapy, and death from renal disease. A total of 7,020 patients with type 2 diabetes and an eGFR of at least 30 mL/minute per 1.73 m2 of body-surface area received either 10 mg or 25 mg of empagliflozin or placebo once daily, plus standard diabetes care (N Engl J Med. 2016 Jun 14. doi: 10.1056/NEJMoa1515920).
New or worsening nephropathy occurred among 525 (12.7%) patients who received empagliflozin, compared with 388 (18.8%) patients who received placebo, for a statistically significant 39% decrease in relative risk of this outcome (hazard ratio, 0.61; P less than .001). This benefit persisted at both doses of empagliflozin, among patients with and without baseline chronic kidney disease (eGFR greater than or equal to 60 mL/min/1.73m2), and across other subgroups stratified by sex, race, body mass index, number of cardiovascular risk factors, and diabetes history and treatment.
A total of 70 patients (1.5%) in the empagliflozin group had a doubling of serum creatinine, vs. 60 patients (2.6%) in the placebo group, for a significant relative risk reduction of 44% (hazard ratio, 0.56; P less than .001). In addition, the proportion of patients starting renal-replacement therapy was twice as high in the placebo group than in the empagliflozin group (0.6% and 0.3%, respectively; HR, 0.45; P less .01).
The groups did not significantly differ in the rate of incident albuminuria, the researchers said. “There were three deaths from renal disease in the empagliflozin group (0.1%) and none in the placebo group,” they added.
The study uncovered no safety signals related to hypoglycemia, diabetic ketoacidosis, thromboembolic events, bone fractures, or volume depletion, the investigators said. Rates of overall adverse events, serious adverse events, and adverse events leading to treatment discontinuation were similar across groups. Rates of complicated urinary tract infections did not significantly vary according to treatment or based on the presence or absence of chronic kidney disease (CKD).Although empagliflozin was associated with a doubling in the rate of urosepsis, these events were rare, affecting only 0.3% and 0.7% of patients with baseline CKD and 0.1% and 0.2% of patients without baseline CKD.
The study was funded by the Boehringer Ingelheim and Eli Lilly and the Diabetes Alliance. Dr. Wanner disclosed grant support from the European Foundation for the Study of Diabetes and personal fees from Boehringer Ingelheim, Janssen, and Novo Nordisk.
[In the EMPA-REG OUTCOME trial,] empagliflozin was associated with a slower progression of kidney disease and lower rates of clinically relevant renal events than was placebo when added to standard of care in patients at high cardiovascular risk. [In the LEADER trial,] the rate of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke among patients with type 2 diabetes mellitus was lower with liraglutide than placebo.
Why do the EMPA-REG OUTCOME and LEADER trials show cardiovascular and microvascular benefit, whereas other trials have come close yet have not shown similar results? Although there may have been differences among the participants that account for the positive results, such differences alone do not fully explain the [findings]. We are left with differences that appear encouraging, yet are not a “home run” with regard to the management of diabetes. In the coming years, controlled and comparative effectiveness trials that uniformly combine newer agents with older agents may help to delineate an event more effective treatment plan for the millions of people whose lives are affected by type 2 diabetes.
Dr. Julie R. Ingelfinger is employed by the New England Journal of Medicine as deputy editor. Dr. Clifford J. Rosen is at the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough, and is an associate editor for the New England Journal of Medicine. These comments are from their accompanying editorial (N Engl J Med. 2016 Jun 14. doi: 10.1056/NEJMe1607413).
[In the EMPA-REG OUTCOME trial,] empagliflozin was associated with a slower progression of kidney disease and lower rates of clinically relevant renal events than was placebo when added to standard of care in patients at high cardiovascular risk. [In the LEADER trial,] the rate of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke among patients with type 2 diabetes mellitus was lower with liraglutide than placebo.
Why do the EMPA-REG OUTCOME and LEADER trials show cardiovascular and microvascular benefit, whereas other trials have come close yet have not shown similar results? Although there may have been differences among the participants that account for the positive results, such differences alone do not fully explain the [findings]. We are left with differences that appear encouraging, yet are not a “home run” with regard to the management of diabetes. In the coming years, controlled and comparative effectiveness trials that uniformly combine newer agents with older agents may help to delineate an event more effective treatment plan for the millions of people whose lives are affected by type 2 diabetes.
Dr. Julie R. Ingelfinger is employed by the New England Journal of Medicine as deputy editor. Dr. Clifford J. Rosen is at the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough, and is an associate editor for the New England Journal of Medicine. These comments are from their accompanying editorial (N Engl J Med. 2016 Jun 14. doi: 10.1056/NEJMe1607413).
[In the EMPA-REG OUTCOME trial,] empagliflozin was associated with a slower progression of kidney disease and lower rates of clinically relevant renal events than was placebo when added to standard of care in patients at high cardiovascular risk. [In the LEADER trial,] the rate of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke among patients with type 2 diabetes mellitus was lower with liraglutide than placebo.
Why do the EMPA-REG OUTCOME and LEADER trials show cardiovascular and microvascular benefit, whereas other trials have come close yet have not shown similar results? Although there may have been differences among the participants that account for the positive results, such differences alone do not fully explain the [findings]. We are left with differences that appear encouraging, yet are not a “home run” with regard to the management of diabetes. In the coming years, controlled and comparative effectiveness trials that uniformly combine newer agents with older agents may help to delineate an event more effective treatment plan for the millions of people whose lives are affected by type 2 diabetes.
Dr. Julie R. Ingelfinger is employed by the New England Journal of Medicine as deputy editor. Dr. Clifford J. Rosen is at the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough, and is an associate editor for the New England Journal of Medicine. These comments are from their accompanying editorial (N Engl J Med. 2016 Jun 14. doi: 10.1056/NEJMe1607413).
Empagliflozin was associated with a significant 39% decrease in risk of new or worsening nephropathy, compared with placebo among adults with type 2 diabetes at high risk for cardiovascular events, based on a secondary analysis of the phase III, randomized, double-blind EMPA-REG OUTCOME trial.
“Patients in the empagliflozin group also had a significantly lower risk of progression to macroalbuminuria or clinically relevant renal outcomes, such as a doubling of the serum creatinine level and initiation of renal-replacement therapy, than did those in the placebo group,” Dr. Christoph Wanner of Würzburg (Germany) University Clinic, and his associates reported in the June 14 New England Journal of Medicine.
Empagliflozin(Jardiance) is a selective sodium–glucose cotransporter-2 inhibitor that was approved in the United States in 2014 to improve glycemic control among patients with type 2 diabetes mellitus. In the initial analysis of the EMPA-REG OUTCOME trial, empagliflozin was associated with a significantly lower rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke, as compared with placebo among patients with type 2 diabetes at high cardiovascular risk (N Engl J Med. 2015 Nov 26 doi: 10.1056/NEJMoa1504720).
For the current secondary analysis, Dr. Wanner and his associates focused on renal microvascular outcomes, including new or worsening nephropathy (macroalbuminuria, defined as greater than 300 mg of urinary albumin/gram of creatinine), a doubling of the serum creatinine level accompanied by an epidermal growth factor receptor (eGFR) of less than or equal to 45 mL/minute per 1.73 m2of body surface area, a new need for renal-replacement therapy, and death from renal disease. A total of 7,020 patients with type 2 diabetes and an eGFR of at least 30 mL/minute per 1.73 m2 of body-surface area received either 10 mg or 25 mg of empagliflozin or placebo once daily, plus standard diabetes care (N Engl J Med. 2016 Jun 14. doi: 10.1056/NEJMoa1515920).
New or worsening nephropathy occurred among 525 (12.7%) patients who received empagliflozin, compared with 388 (18.8%) patients who received placebo, for a statistically significant 39% decrease in relative risk of this outcome (hazard ratio, 0.61; P less than .001). This benefit persisted at both doses of empagliflozin, among patients with and without baseline chronic kidney disease (eGFR greater than or equal to 60 mL/min/1.73m2), and across other subgroups stratified by sex, race, body mass index, number of cardiovascular risk factors, and diabetes history and treatment.
A total of 70 patients (1.5%) in the empagliflozin group had a doubling of serum creatinine, vs. 60 patients (2.6%) in the placebo group, for a significant relative risk reduction of 44% (hazard ratio, 0.56; P less than .001). In addition, the proportion of patients starting renal-replacement therapy was twice as high in the placebo group than in the empagliflozin group (0.6% and 0.3%, respectively; HR, 0.45; P less .01).
The groups did not significantly differ in the rate of incident albuminuria, the researchers said. “There were three deaths from renal disease in the empagliflozin group (0.1%) and none in the placebo group,” they added.
The study uncovered no safety signals related to hypoglycemia, diabetic ketoacidosis, thromboembolic events, bone fractures, or volume depletion, the investigators said. Rates of overall adverse events, serious adverse events, and adverse events leading to treatment discontinuation were similar across groups. Rates of complicated urinary tract infections did not significantly vary according to treatment or based on the presence or absence of chronic kidney disease (CKD).Although empagliflozin was associated with a doubling in the rate of urosepsis, these events were rare, affecting only 0.3% and 0.7% of patients with baseline CKD and 0.1% and 0.2% of patients without baseline CKD.
The study was funded by the Boehringer Ingelheim and Eli Lilly and the Diabetes Alliance. Dr. Wanner disclosed grant support from the European Foundation for the Study of Diabetes and personal fees from Boehringer Ingelheim, Janssen, and Novo Nordisk.
Empagliflozin was associated with a significant 39% decrease in risk of new or worsening nephropathy, compared with placebo among adults with type 2 diabetes at high risk for cardiovascular events, based on a secondary analysis of the phase III, randomized, double-blind EMPA-REG OUTCOME trial.
“Patients in the empagliflozin group also had a significantly lower risk of progression to macroalbuminuria or clinically relevant renal outcomes, such as a doubling of the serum creatinine level and initiation of renal-replacement therapy, than did those in the placebo group,” Dr. Christoph Wanner of Würzburg (Germany) University Clinic, and his associates reported in the June 14 New England Journal of Medicine.
Empagliflozin(Jardiance) is a selective sodium–glucose cotransporter-2 inhibitor that was approved in the United States in 2014 to improve glycemic control among patients with type 2 diabetes mellitus. In the initial analysis of the EMPA-REG OUTCOME trial, empagliflozin was associated with a significantly lower rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke, as compared with placebo among patients with type 2 diabetes at high cardiovascular risk (N Engl J Med. 2015 Nov 26 doi: 10.1056/NEJMoa1504720).
For the current secondary analysis, Dr. Wanner and his associates focused on renal microvascular outcomes, including new or worsening nephropathy (macroalbuminuria, defined as greater than 300 mg of urinary albumin/gram of creatinine), a doubling of the serum creatinine level accompanied by an epidermal growth factor receptor (eGFR) of less than or equal to 45 mL/minute per 1.73 m2of body surface area, a new need for renal-replacement therapy, and death from renal disease. A total of 7,020 patients with type 2 diabetes and an eGFR of at least 30 mL/minute per 1.73 m2 of body-surface area received either 10 mg or 25 mg of empagliflozin or placebo once daily, plus standard diabetes care (N Engl J Med. 2016 Jun 14. doi: 10.1056/NEJMoa1515920).
New or worsening nephropathy occurred among 525 (12.7%) patients who received empagliflozin, compared with 388 (18.8%) patients who received placebo, for a statistically significant 39% decrease in relative risk of this outcome (hazard ratio, 0.61; P less than .001). This benefit persisted at both doses of empagliflozin, among patients with and without baseline chronic kidney disease (eGFR greater than or equal to 60 mL/min/1.73m2), and across other subgroups stratified by sex, race, body mass index, number of cardiovascular risk factors, and diabetes history and treatment.
A total of 70 patients (1.5%) in the empagliflozin group had a doubling of serum creatinine, vs. 60 patients (2.6%) in the placebo group, for a significant relative risk reduction of 44% (hazard ratio, 0.56; P less than .001). In addition, the proportion of patients starting renal-replacement therapy was twice as high in the placebo group than in the empagliflozin group (0.6% and 0.3%, respectively; HR, 0.45; P less .01).
The groups did not significantly differ in the rate of incident albuminuria, the researchers said. “There were three deaths from renal disease in the empagliflozin group (0.1%) and none in the placebo group,” they added.
The study uncovered no safety signals related to hypoglycemia, diabetic ketoacidosis, thromboembolic events, bone fractures, or volume depletion, the investigators said. Rates of overall adverse events, serious adverse events, and adverse events leading to treatment discontinuation were similar across groups. Rates of complicated urinary tract infections did not significantly vary according to treatment or based on the presence or absence of chronic kidney disease (CKD).Although empagliflozin was associated with a doubling in the rate of urosepsis, these events were rare, affecting only 0.3% and 0.7% of patients with baseline CKD and 0.1% and 0.2% of patients without baseline CKD.
The study was funded by the Boehringer Ingelheim and Eli Lilly and the Diabetes Alliance. Dr. Wanner disclosed grant support from the European Foundation for the Study of Diabetes and personal fees from Boehringer Ingelheim, Janssen, and Novo Nordisk.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Compared with placebo, empagliflozin was associated with better renal outcomes among patients with type 2 diabetes and high cardiovascular risk.
Major finding: New or worsening nephropathy affected 525 (12.7%) empagliflozin patients, vs. 388 (18.8%) placebo patients (HR, 0.61; P less than .001).
Data source: A secondary analysis of data for 4,124 patients from the international, phase III, double-blind EMPA-REG OUTCOMES trial.
Disclosures: The study was funded by the Boehringer Ingelheim and Eli Lilly and the Diabetes Alliance. Dr. Wanner disclosed grant support from the European Foundation for the Study of Diabetes and personal fees from Boehringer Ingelheim, Janssen, and Novo Nordisk.
Skin patch testing pinpoints dietary triggers of IBS
SAN DIEGO – About 90% of patients reported improvement in symptoms of irritable bowel syndrome after avoiding type 4 food allergens identified by skin patch testing, according to an uncontrolled study.
Furthermore, 69% of patients reported at least moderate improvement after eliminating foods to which they reacted, said Dr. Michael Stierstorfer, a dermatologist at East Penn Dermatology in North Wales, Pa., who partnered with gastroenterologists at Temple University to conduct the study. “This raises questions about a possible overlap between IBS and allergic contact enteritis,” the researchers stated in a poster presented at the annual Digestive Disease Week.
Irritable bowel syndrome is often treatment refractory and tends to elude conventional diagnostics. That was the case for Dr. Stierstorfer, who several years ago developed symptoms of IBS with constipation (IBS-C) that eventually affected him about half the time, he said in an interview. A hydrogen breath test, upper endoscopy, colonoscopy, abdominal/pelvic CT, and tests for gluten-sensitive enteropathy and parasites revealed no abnormalities except decreased small intestinal motility, he said.
But after “flaring badly” twice when he ate Indian food, he began to suspect a cause. “I stopped eating garlic and within a day, I was absolutely fine,” Dr. Stierstorfer said. “The symptoms recurred only if I accidentally ate garlic again.”
Studies had refuted links between IBS and type 1 hypersensitivity but had not explored the role of type 4 (delayed) hypersensitivity in the disorder, Dr. Stierstorfer discovered. “Dermatologists do patch testing all the time for patients with refractory eczema to search for type 4 allergic contact factors that might be causing their rash,” he said. “I performed a patch test of garlic on myself to look for a type 4 allergy, and it was strongly positive. I thought I probably wasn’t the only person walking around with symptoms that mimicked IBS but were really from a type 4 food allergy.”
He tested that idea by skin patch testing 50 patients with IBS symptoms whom he recruited through his dermatology practice. In all, 30 (60%) patients reacted to at least one food allergen, of whom 14 (46%) reported symptomatic improvement after eliminating the suspected triggers from their diets. The findings appeared in the March 2013 Journal of the American Academy of Dermatology (68:377-84).
Next, Dr. Stierstorfer partnered with Dr. Grace Shin, a 3rd-year gastroenterology fellow at Temple University, Philadelphia, and her colleagues. Together, they tested 57 patients with physician-diagnosed IBS with diarrhea (about 43% of patients), IBS with constipation (16%), mixed IBS (30%), or unsubtyped IBS (11%). Patients averaged 41 years of age (standard deviation, 15 years) and 77% were female. Each patient had between 118 and 122 individual allergen patches placed on his or her back. Two days later, the patches were removed and the skin evaluated for macular erythema consistent with a type 4 hypersensitivity reaction. The patients were checked again a day or 2 later to catch any highly delayed reactions.
In all, 56 patients (98%) showed evidence of at least one hypersensitivity, and most reacted to between two and three allergens, Dr. Stierstorfer said. The most commonly identified triggers were cinnamon bark (35 patients; 61%) and sodium bisulfite (26 patients; 46%). At baseline, patients rated their abdominal pain or discomfort at an average of 6.7 on a 10-point severity scale (SD, 2.3 points). After 2-4 weeks of avoiding allergens to which they developed macular edema, they reported a mean 4.4-point improvement in their abdominal symptoms (SD, 2.7 points; P less than .001).
The patients also reported an average 5.8-point improvement on a 10-point scale of global IBS symptom severity (SD, 3.2 points; P less than .001). Overall, 91% of patients reported at least partial relief of abdominal symptoms, while 89% of patients reported at least partial relief of global symptoms, the investigators reported.
Based on these results, “food-related type 4 hypersensitivity reactions may contribute to the pathogenesis of IBS and IBS-like symptoms,” Dr. Shin said in an interview. “The idea of allergic contact enteritis intrigued me, because it made me think that some patients diagnosed with IBS, especially IBS with diarrhea, might benefit from allergy testing when the standard approaches don’t work.”
Another dietary intervention for IBS, the low-FODMAP diet, can help relieve symptoms, “but it’s a hard diet to follow,” Dr. Shin added. “Being able to focus on eliminating one or two things would be easier than eliminating multiple classes of foods that are so common to an American diet.”
Next, the team is planning a controlled trial of the skin patch test. “There is still more validation work to do,” said Dr. Stierstorfer. “But I think this shows that looking at something from a unique perspective – in this case, a dermatologic perspective for a GI problem – can result in a new approach, and potentially an advance in medicine.”
Dr. Shin had no disclosures. Dr. Stierstorfer disclosed financial ties to IBS Centers for Advanced Food Allergy Testing.
SAN DIEGO – About 90% of patients reported improvement in symptoms of irritable bowel syndrome after avoiding type 4 food allergens identified by skin patch testing, according to an uncontrolled study.
Furthermore, 69% of patients reported at least moderate improvement after eliminating foods to which they reacted, said Dr. Michael Stierstorfer, a dermatologist at East Penn Dermatology in North Wales, Pa., who partnered with gastroenterologists at Temple University to conduct the study. “This raises questions about a possible overlap between IBS and allergic contact enteritis,” the researchers stated in a poster presented at the annual Digestive Disease Week.
Irritable bowel syndrome is often treatment refractory and tends to elude conventional diagnostics. That was the case for Dr. Stierstorfer, who several years ago developed symptoms of IBS with constipation (IBS-C) that eventually affected him about half the time, he said in an interview. A hydrogen breath test, upper endoscopy, colonoscopy, abdominal/pelvic CT, and tests for gluten-sensitive enteropathy and parasites revealed no abnormalities except decreased small intestinal motility, he said.
But after “flaring badly” twice when he ate Indian food, he began to suspect a cause. “I stopped eating garlic and within a day, I was absolutely fine,” Dr. Stierstorfer said. “The symptoms recurred only if I accidentally ate garlic again.”
Studies had refuted links between IBS and type 1 hypersensitivity but had not explored the role of type 4 (delayed) hypersensitivity in the disorder, Dr. Stierstorfer discovered. “Dermatologists do patch testing all the time for patients with refractory eczema to search for type 4 allergic contact factors that might be causing their rash,” he said. “I performed a patch test of garlic on myself to look for a type 4 allergy, and it was strongly positive. I thought I probably wasn’t the only person walking around with symptoms that mimicked IBS but were really from a type 4 food allergy.”
He tested that idea by skin patch testing 50 patients with IBS symptoms whom he recruited through his dermatology practice. In all, 30 (60%) patients reacted to at least one food allergen, of whom 14 (46%) reported symptomatic improvement after eliminating the suspected triggers from their diets. The findings appeared in the March 2013 Journal of the American Academy of Dermatology (68:377-84).
Next, Dr. Stierstorfer partnered with Dr. Grace Shin, a 3rd-year gastroenterology fellow at Temple University, Philadelphia, and her colleagues. Together, they tested 57 patients with physician-diagnosed IBS with diarrhea (about 43% of patients), IBS with constipation (16%), mixed IBS (30%), or unsubtyped IBS (11%). Patients averaged 41 years of age (standard deviation, 15 years) and 77% were female. Each patient had between 118 and 122 individual allergen patches placed on his or her back. Two days later, the patches were removed and the skin evaluated for macular erythema consistent with a type 4 hypersensitivity reaction. The patients were checked again a day or 2 later to catch any highly delayed reactions.
In all, 56 patients (98%) showed evidence of at least one hypersensitivity, and most reacted to between two and three allergens, Dr. Stierstorfer said. The most commonly identified triggers were cinnamon bark (35 patients; 61%) and sodium bisulfite (26 patients; 46%). At baseline, patients rated their abdominal pain or discomfort at an average of 6.7 on a 10-point severity scale (SD, 2.3 points). After 2-4 weeks of avoiding allergens to which they developed macular edema, they reported a mean 4.4-point improvement in their abdominal symptoms (SD, 2.7 points; P less than .001).
The patients also reported an average 5.8-point improvement on a 10-point scale of global IBS symptom severity (SD, 3.2 points; P less than .001). Overall, 91% of patients reported at least partial relief of abdominal symptoms, while 89% of patients reported at least partial relief of global symptoms, the investigators reported.
Based on these results, “food-related type 4 hypersensitivity reactions may contribute to the pathogenesis of IBS and IBS-like symptoms,” Dr. Shin said in an interview. “The idea of allergic contact enteritis intrigued me, because it made me think that some patients diagnosed with IBS, especially IBS with diarrhea, might benefit from allergy testing when the standard approaches don’t work.”
Another dietary intervention for IBS, the low-FODMAP diet, can help relieve symptoms, “but it’s a hard diet to follow,” Dr. Shin added. “Being able to focus on eliminating one or two things would be easier than eliminating multiple classes of foods that are so common to an American diet.”
Next, the team is planning a controlled trial of the skin patch test. “There is still more validation work to do,” said Dr. Stierstorfer. “But I think this shows that looking at something from a unique perspective – in this case, a dermatologic perspective for a GI problem – can result in a new approach, and potentially an advance in medicine.”
Dr. Shin had no disclosures. Dr. Stierstorfer disclosed financial ties to IBS Centers for Advanced Food Allergy Testing.
SAN DIEGO – About 90% of patients reported improvement in symptoms of irritable bowel syndrome after avoiding type 4 food allergens identified by skin patch testing, according to an uncontrolled study.
Furthermore, 69% of patients reported at least moderate improvement after eliminating foods to which they reacted, said Dr. Michael Stierstorfer, a dermatologist at East Penn Dermatology in North Wales, Pa., who partnered with gastroenterologists at Temple University to conduct the study. “This raises questions about a possible overlap between IBS and allergic contact enteritis,” the researchers stated in a poster presented at the annual Digestive Disease Week.
Irritable bowel syndrome is often treatment refractory and tends to elude conventional diagnostics. That was the case for Dr. Stierstorfer, who several years ago developed symptoms of IBS with constipation (IBS-C) that eventually affected him about half the time, he said in an interview. A hydrogen breath test, upper endoscopy, colonoscopy, abdominal/pelvic CT, and tests for gluten-sensitive enteropathy and parasites revealed no abnormalities except decreased small intestinal motility, he said.
But after “flaring badly” twice when he ate Indian food, he began to suspect a cause. “I stopped eating garlic and within a day, I was absolutely fine,” Dr. Stierstorfer said. “The symptoms recurred only if I accidentally ate garlic again.”
Studies had refuted links between IBS and type 1 hypersensitivity but had not explored the role of type 4 (delayed) hypersensitivity in the disorder, Dr. Stierstorfer discovered. “Dermatologists do patch testing all the time for patients with refractory eczema to search for type 4 allergic contact factors that might be causing their rash,” he said. “I performed a patch test of garlic on myself to look for a type 4 allergy, and it was strongly positive. I thought I probably wasn’t the only person walking around with symptoms that mimicked IBS but were really from a type 4 food allergy.”
He tested that idea by skin patch testing 50 patients with IBS symptoms whom he recruited through his dermatology practice. In all, 30 (60%) patients reacted to at least one food allergen, of whom 14 (46%) reported symptomatic improvement after eliminating the suspected triggers from their diets. The findings appeared in the March 2013 Journal of the American Academy of Dermatology (68:377-84).
Next, Dr. Stierstorfer partnered with Dr. Grace Shin, a 3rd-year gastroenterology fellow at Temple University, Philadelphia, and her colleagues. Together, they tested 57 patients with physician-diagnosed IBS with diarrhea (about 43% of patients), IBS with constipation (16%), mixed IBS (30%), or unsubtyped IBS (11%). Patients averaged 41 years of age (standard deviation, 15 years) and 77% were female. Each patient had between 118 and 122 individual allergen patches placed on his or her back. Two days later, the patches were removed and the skin evaluated for macular erythema consistent with a type 4 hypersensitivity reaction. The patients were checked again a day or 2 later to catch any highly delayed reactions.
In all, 56 patients (98%) showed evidence of at least one hypersensitivity, and most reacted to between two and three allergens, Dr. Stierstorfer said. The most commonly identified triggers were cinnamon bark (35 patients; 61%) and sodium bisulfite (26 patients; 46%). At baseline, patients rated their abdominal pain or discomfort at an average of 6.7 on a 10-point severity scale (SD, 2.3 points). After 2-4 weeks of avoiding allergens to which they developed macular edema, they reported a mean 4.4-point improvement in their abdominal symptoms (SD, 2.7 points; P less than .001).
The patients also reported an average 5.8-point improvement on a 10-point scale of global IBS symptom severity (SD, 3.2 points; P less than .001). Overall, 91% of patients reported at least partial relief of abdominal symptoms, while 89% of patients reported at least partial relief of global symptoms, the investigators reported.
Based on these results, “food-related type 4 hypersensitivity reactions may contribute to the pathogenesis of IBS and IBS-like symptoms,” Dr. Shin said in an interview. “The idea of allergic contact enteritis intrigued me, because it made me think that some patients diagnosed with IBS, especially IBS with diarrhea, might benefit from allergy testing when the standard approaches don’t work.”
Another dietary intervention for IBS, the low-FODMAP diet, can help relieve symptoms, “but it’s a hard diet to follow,” Dr. Shin added. “Being able to focus on eliminating one or two things would be easier than eliminating multiple classes of foods that are so common to an American diet.”
Next, the team is planning a controlled trial of the skin patch test. “There is still more validation work to do,” said Dr. Stierstorfer. “But I think this shows that looking at something from a unique perspective – in this case, a dermatologic perspective for a GI problem – can result in a new approach, and potentially an advance in medicine.”
Dr. Shin had no disclosures. Dr. Stierstorfer disclosed financial ties to IBS Centers for Advanced Food Allergy Testing.
AT DDW® 2016
Key clinical point: Avoiding food allergens identified by skin patch testing significantly improved self-reported symptoms of irritable bowel syndrome.
Major finding: In all, 69% of patients reported at least moderate improvement after eliminating foods to which they reacted.
Data source: A single-arm proof-of-concept study of 57 patients with physician-diagnosed IBS.
Disclosures: Dr. Shin had no disclosures. Dr. Stierstorfer disclosed financial ties to IBS Centers for Advanced Food Allergy Testing.
Novel flow cytometry identified T-cell signatures of vedolizumab responders
SAN DIEGO – A novel test distinguished patients with inflammatory bowel disease (IBD) who responded to vedolizumab from nonresponders, investigators reported at the annual Digestive Disease Week.
“If we can optimize and simplify this prescreening flow cytometry panel, it would allow nonresponding patients to avoid unnecessary side effects of treatment,” said lead author Sophia Diaz, a researcher at the University of Miami Health System.
Exactly how patients develop IBD remains unclear, but its pathogenesis involves chronic T-cell mediated inflammation and subsequent tissue damage, Ms. Diaz noted. Vedolizumab (Entyvio) is a monoclonal antibody – specifically, an alpha4-beta7 integrin blocker – that stops T cells from homing to lymphoid tissue in the gut, and is approved for treating IBD in the United States and Europe. The biologic offers specificity and efficacy across a range of patient types, and a good safety profile, but less than half of patients responded to it in the pivotal GEMINI trials, Ms. Diaz noted. “We wanted to find a biomarker that tracked with vedolizumab to enable physicians to prescribe it on a more informed basis, compared with other drugs,” she said.
The researchers therefore used flow cytometry to seek T-cell signatures that reliably discriminated between vedolizumab responders and nonresponders. They isolated peripheral leukocytes and lamina propria T cells from 14 active IBD patients before and about 16 weeks after starting vedolizumab and going to a maintenance dose. Next, the investigators used flow cytometry to probe the T cells for a number of cell surface antigens. They also tested T cells for chemokine receptors that are involved in gut homing and activation. They sorted the results based on response to vedolizumab maintenance therapy, defined as a 30% decrease in partial Mayo scores (for ulcerative colitis) or Harvey-Bradshaw index scores (for Crohn’s disease).
The study revealed several direct and inverse correlates of vedolizumab response, Ms. Diaz said. For example, compared with nonresponders, responders had a higher proportion of alpha4-beta7+MDR1+RO+ effector T cells, and lower percentages of effector T cells that were MDR1+RO-CD8a+ or CCR9+RO-CD8a+. Notably, the percentage of MDR1+RO-CD8a+ effector T cells was significantly higher among nonresponders than responders, both before (P = .048) and after (P = .005) treatment. “I thought that was interesting, because the difference is already significant at this small sample size,” Ms. Diaz said. “That’s a very powerful thing, because it indicates there is something within these patients that is stable that is telling us about their response.”
Taken together, the results suggest that the percentage of beta7+MDR1+ T cells directly predicts vedolizumab response, while MDR1+CD8a+ T cells and CCR9+CD8a+ T cells inversely correlate with response, Ms. Diaz said. The researchers plan to validate the panel in more patients, including multicenter cohorts of patients, both with ulcerative colitis and Crohn’s disease, she added.
The study was partially funded by Takeda. Ms. Diaz had no disclosures.
SAN DIEGO – A novel test distinguished patients with inflammatory bowel disease (IBD) who responded to vedolizumab from nonresponders, investigators reported at the annual Digestive Disease Week.
“If we can optimize and simplify this prescreening flow cytometry panel, it would allow nonresponding patients to avoid unnecessary side effects of treatment,” said lead author Sophia Diaz, a researcher at the University of Miami Health System.
Exactly how patients develop IBD remains unclear, but its pathogenesis involves chronic T-cell mediated inflammation and subsequent tissue damage, Ms. Diaz noted. Vedolizumab (Entyvio) is a monoclonal antibody – specifically, an alpha4-beta7 integrin blocker – that stops T cells from homing to lymphoid tissue in the gut, and is approved for treating IBD in the United States and Europe. The biologic offers specificity and efficacy across a range of patient types, and a good safety profile, but less than half of patients responded to it in the pivotal GEMINI trials, Ms. Diaz noted. “We wanted to find a biomarker that tracked with vedolizumab to enable physicians to prescribe it on a more informed basis, compared with other drugs,” she said.
The researchers therefore used flow cytometry to seek T-cell signatures that reliably discriminated between vedolizumab responders and nonresponders. They isolated peripheral leukocytes and lamina propria T cells from 14 active IBD patients before and about 16 weeks after starting vedolizumab and going to a maintenance dose. Next, the investigators used flow cytometry to probe the T cells for a number of cell surface antigens. They also tested T cells for chemokine receptors that are involved in gut homing and activation. They sorted the results based on response to vedolizumab maintenance therapy, defined as a 30% decrease in partial Mayo scores (for ulcerative colitis) or Harvey-Bradshaw index scores (for Crohn’s disease).
The study revealed several direct and inverse correlates of vedolizumab response, Ms. Diaz said. For example, compared with nonresponders, responders had a higher proportion of alpha4-beta7+MDR1+RO+ effector T cells, and lower percentages of effector T cells that were MDR1+RO-CD8a+ or CCR9+RO-CD8a+. Notably, the percentage of MDR1+RO-CD8a+ effector T cells was significantly higher among nonresponders than responders, both before (P = .048) and after (P = .005) treatment. “I thought that was interesting, because the difference is already significant at this small sample size,” Ms. Diaz said. “That’s a very powerful thing, because it indicates there is something within these patients that is stable that is telling us about their response.”
Taken together, the results suggest that the percentage of beta7+MDR1+ T cells directly predicts vedolizumab response, while MDR1+CD8a+ T cells and CCR9+CD8a+ T cells inversely correlate with response, Ms. Diaz said. The researchers plan to validate the panel in more patients, including multicenter cohorts of patients, both with ulcerative colitis and Crohn’s disease, she added.
The study was partially funded by Takeda. Ms. Diaz had no disclosures.
SAN DIEGO – A novel test distinguished patients with inflammatory bowel disease (IBD) who responded to vedolizumab from nonresponders, investigators reported at the annual Digestive Disease Week.
“If we can optimize and simplify this prescreening flow cytometry panel, it would allow nonresponding patients to avoid unnecessary side effects of treatment,” said lead author Sophia Diaz, a researcher at the University of Miami Health System.
Exactly how patients develop IBD remains unclear, but its pathogenesis involves chronic T-cell mediated inflammation and subsequent tissue damage, Ms. Diaz noted. Vedolizumab (Entyvio) is a monoclonal antibody – specifically, an alpha4-beta7 integrin blocker – that stops T cells from homing to lymphoid tissue in the gut, and is approved for treating IBD in the United States and Europe. The biologic offers specificity and efficacy across a range of patient types, and a good safety profile, but less than half of patients responded to it in the pivotal GEMINI trials, Ms. Diaz noted. “We wanted to find a biomarker that tracked with vedolizumab to enable physicians to prescribe it on a more informed basis, compared with other drugs,” she said.
The researchers therefore used flow cytometry to seek T-cell signatures that reliably discriminated between vedolizumab responders and nonresponders. They isolated peripheral leukocytes and lamina propria T cells from 14 active IBD patients before and about 16 weeks after starting vedolizumab and going to a maintenance dose. Next, the investigators used flow cytometry to probe the T cells for a number of cell surface antigens. They also tested T cells for chemokine receptors that are involved in gut homing and activation. They sorted the results based on response to vedolizumab maintenance therapy, defined as a 30% decrease in partial Mayo scores (for ulcerative colitis) or Harvey-Bradshaw index scores (for Crohn’s disease).
The study revealed several direct and inverse correlates of vedolizumab response, Ms. Diaz said. For example, compared with nonresponders, responders had a higher proportion of alpha4-beta7+MDR1+RO+ effector T cells, and lower percentages of effector T cells that were MDR1+RO-CD8a+ or CCR9+RO-CD8a+. Notably, the percentage of MDR1+RO-CD8a+ effector T cells was significantly higher among nonresponders than responders, both before (P = .048) and after (P = .005) treatment. “I thought that was interesting, because the difference is already significant at this small sample size,” Ms. Diaz said. “That’s a very powerful thing, because it indicates there is something within these patients that is stable that is telling us about their response.”
Taken together, the results suggest that the percentage of beta7+MDR1+ T cells directly predicts vedolizumab response, while MDR1+CD8a+ T cells and CCR9+CD8a+ T cells inversely correlate with response, Ms. Diaz said. The researchers plan to validate the panel in more patients, including multicenter cohorts of patients, both with ulcerative colitis and Crohn’s disease, she added.
The study was partially funded by Takeda. Ms. Diaz had no disclosures.
AT DDW® 2016
Key clinical point: A novel flow cytometry panel shows promise for determining if patients with inflammatory bowel disease will respond to vedolizumab.
Major finding: The percentage of MDR1+RO-CD8a+ effector T cells was significantly higher among nonresponders than responders, both before (P = .048) and after (P = .005) treatment.
Data source: Flow cytometry of 14 patients with active ulcerative colitis or Crohn’s disease.
Disclosures: The study was partially funded by Takeda. Ms. Diaz had no disclosures.
Tips for collaborations among GI investigators, industry, FDA
SAN DIEGO – Tensions among academic investigators, industry sponsors, and the Food and Drug Administration can hinder new drug approvals and slow or block communication of important results, experts said at the annual Digestive Disease Week.
Relationships between investigators and industry have become especially strained, according to Dr. M. Scott Harris, cofounder of Lyric Pharmaceuticals in San Francisco. “I can tell you as a former investigator, and someone who speaks to investigators all the time, they feel disenfranchised,” he said.
Several steps can help. Industry should focus on empowering investigators, “not study sites,” said Dr. Harris. “Have protocol development meetings, not investigator meetings. Have open dialogue so that investigators can share in the excitement of the study, the results, and the science.”
Intellectual property is a particularly hot topic, acknowledged Dr. Harris, who started out in academic gastroenterology before making the jump to working for pharmaceutical and biotechnology companies. “Intellectual property is the lifeblood of a company – the only thing that generates the likelihood of a return on investment,” he emphasized. “Please do not push back if some information cannot be shared with you.” But within those constraints, industry should “force itself to be as patient as possible,” he said. “A balance has to be struck between the need for IP [intellectual property] and the need to share knowledge.”
Sharing knowledge also means that industry sponsors need to commit to a clear publication strategy, said Dr. Harris. “Investigators want the results of the studies to be communicated, including reasons for failure. We have failed at this as an industry, and this is not acceptable.”
But academic investigators need to make some changes, too. Successfully joining a trial means engaging actively with the sponsor and protocol, Dr. Harris emphasized. “Don’t tell your staff you’re too busy to talk to me when I call. Focus on ethical study conduct, good clinical practice, training, data quality, and meeting timelines.”
Dr. Gary Lichtenstein agreed. A gastroenterologist at the University of Pennsylvania, Philadelphia, with more than 30 years of experience in clinical trials, he knows that successful academic study sites have “efficient and businesslike operations,” a proven internal audit system, and solid, reasonable budgeting for staff time, overhead, equipment, and storage. In particular, academic investigators should double-check training requirements, the qualifications of the study coordinator, and who will handle regulatory, legal, and budgeting concerns, he said.
Vetting a potential industry sponsor is just as important. Ask “if they have the staff, time, equipment, and space to do the study,” Dr. Lichtenstein stressed. “Communicate expectations in writing back and forth to avoid misunderstandings. An indemnification clause is also very important to hold the consultant harmless from and against any claim, loss, or damage whatsoever.”
The FDA, for its part, needs to respond faster to meeting requests and offer clearer guidance about appropriate study designs and outcome measures, both experts emphasized. The median time for FDA to approve a drug application is about 180 days, while approving new gastroenterology agents takes nearly twice as long, according to Dr. Lichtenstein. “There is clearly a discrepancy, and it would be nice if we moved the bar closer. As an investigator, I need to know which trials are acceptable in design, and what endpoints are clearly defined, with examples,” he said. “If I have a question, I need a point of contact to call to get an answer in rapid time, instead of having to wait for months, and I need to know which biomarkers are appropriate to use.”
Dr. Harris agreed. “There should be tension between FDA and industry – that is part of the process,” he said. “But open communication and rapid response to meeting requests are crucial.”
Timeliness and transparency are especially important as FDA transitions “from being a classic regulator to a proactive partner in drug development,” Dr. Harris said. “What industry needs and expects from FDA is greater certainty on the path. Companies may or may not like a particular FDA guidance document, but they greatly appreciate the clarity that guidance documents provide.”
Dr. Lichtenstein disclosed ties to AbbVie, Hospira, Pfizer, and numerous other pharmaceutical companies. Dr. Harris is employed by Lyric Pharmaceuticals and disclosed relationships with several other biopharmaceutical companies.
SAN DIEGO – Tensions among academic investigators, industry sponsors, and the Food and Drug Administration can hinder new drug approvals and slow or block communication of important results, experts said at the annual Digestive Disease Week.
Relationships between investigators and industry have become especially strained, according to Dr. M. Scott Harris, cofounder of Lyric Pharmaceuticals in San Francisco. “I can tell you as a former investigator, and someone who speaks to investigators all the time, they feel disenfranchised,” he said.
Several steps can help. Industry should focus on empowering investigators, “not study sites,” said Dr. Harris. “Have protocol development meetings, not investigator meetings. Have open dialogue so that investigators can share in the excitement of the study, the results, and the science.”
Intellectual property is a particularly hot topic, acknowledged Dr. Harris, who started out in academic gastroenterology before making the jump to working for pharmaceutical and biotechnology companies. “Intellectual property is the lifeblood of a company – the only thing that generates the likelihood of a return on investment,” he emphasized. “Please do not push back if some information cannot be shared with you.” But within those constraints, industry should “force itself to be as patient as possible,” he said. “A balance has to be struck between the need for IP [intellectual property] and the need to share knowledge.”
Sharing knowledge also means that industry sponsors need to commit to a clear publication strategy, said Dr. Harris. “Investigators want the results of the studies to be communicated, including reasons for failure. We have failed at this as an industry, and this is not acceptable.”
But academic investigators need to make some changes, too. Successfully joining a trial means engaging actively with the sponsor and protocol, Dr. Harris emphasized. “Don’t tell your staff you’re too busy to talk to me when I call. Focus on ethical study conduct, good clinical practice, training, data quality, and meeting timelines.”
Dr. Gary Lichtenstein agreed. A gastroenterologist at the University of Pennsylvania, Philadelphia, with more than 30 years of experience in clinical trials, he knows that successful academic study sites have “efficient and businesslike operations,” a proven internal audit system, and solid, reasonable budgeting for staff time, overhead, equipment, and storage. In particular, academic investigators should double-check training requirements, the qualifications of the study coordinator, and who will handle regulatory, legal, and budgeting concerns, he said.
Vetting a potential industry sponsor is just as important. Ask “if they have the staff, time, equipment, and space to do the study,” Dr. Lichtenstein stressed. “Communicate expectations in writing back and forth to avoid misunderstandings. An indemnification clause is also very important to hold the consultant harmless from and against any claim, loss, or damage whatsoever.”
The FDA, for its part, needs to respond faster to meeting requests and offer clearer guidance about appropriate study designs and outcome measures, both experts emphasized. The median time for FDA to approve a drug application is about 180 days, while approving new gastroenterology agents takes nearly twice as long, according to Dr. Lichtenstein. “There is clearly a discrepancy, and it would be nice if we moved the bar closer. As an investigator, I need to know which trials are acceptable in design, and what endpoints are clearly defined, with examples,” he said. “If I have a question, I need a point of contact to call to get an answer in rapid time, instead of having to wait for months, and I need to know which biomarkers are appropriate to use.”
Dr. Harris agreed. “There should be tension between FDA and industry – that is part of the process,” he said. “But open communication and rapid response to meeting requests are crucial.”
Timeliness and transparency are especially important as FDA transitions “from being a classic regulator to a proactive partner in drug development,” Dr. Harris said. “What industry needs and expects from FDA is greater certainty on the path. Companies may or may not like a particular FDA guidance document, but they greatly appreciate the clarity that guidance documents provide.”
Dr. Lichtenstein disclosed ties to AbbVie, Hospira, Pfizer, and numerous other pharmaceutical companies. Dr. Harris is employed by Lyric Pharmaceuticals and disclosed relationships with several other biopharmaceutical companies.
SAN DIEGO – Tensions among academic investigators, industry sponsors, and the Food and Drug Administration can hinder new drug approvals and slow or block communication of important results, experts said at the annual Digestive Disease Week.
Relationships between investigators and industry have become especially strained, according to Dr. M. Scott Harris, cofounder of Lyric Pharmaceuticals in San Francisco. “I can tell you as a former investigator, and someone who speaks to investigators all the time, they feel disenfranchised,” he said.
Several steps can help. Industry should focus on empowering investigators, “not study sites,” said Dr. Harris. “Have protocol development meetings, not investigator meetings. Have open dialogue so that investigators can share in the excitement of the study, the results, and the science.”
Intellectual property is a particularly hot topic, acknowledged Dr. Harris, who started out in academic gastroenterology before making the jump to working for pharmaceutical and biotechnology companies. “Intellectual property is the lifeblood of a company – the only thing that generates the likelihood of a return on investment,” he emphasized. “Please do not push back if some information cannot be shared with you.” But within those constraints, industry should “force itself to be as patient as possible,” he said. “A balance has to be struck between the need for IP [intellectual property] and the need to share knowledge.”
Sharing knowledge also means that industry sponsors need to commit to a clear publication strategy, said Dr. Harris. “Investigators want the results of the studies to be communicated, including reasons for failure. We have failed at this as an industry, and this is not acceptable.”
But academic investigators need to make some changes, too. Successfully joining a trial means engaging actively with the sponsor and protocol, Dr. Harris emphasized. “Don’t tell your staff you’re too busy to talk to me when I call. Focus on ethical study conduct, good clinical practice, training, data quality, and meeting timelines.”
Dr. Gary Lichtenstein agreed. A gastroenterologist at the University of Pennsylvania, Philadelphia, with more than 30 years of experience in clinical trials, he knows that successful academic study sites have “efficient and businesslike operations,” a proven internal audit system, and solid, reasonable budgeting for staff time, overhead, equipment, and storage. In particular, academic investigators should double-check training requirements, the qualifications of the study coordinator, and who will handle regulatory, legal, and budgeting concerns, he said.
Vetting a potential industry sponsor is just as important. Ask “if they have the staff, time, equipment, and space to do the study,” Dr. Lichtenstein stressed. “Communicate expectations in writing back and forth to avoid misunderstandings. An indemnification clause is also very important to hold the consultant harmless from and against any claim, loss, or damage whatsoever.”
The FDA, for its part, needs to respond faster to meeting requests and offer clearer guidance about appropriate study designs and outcome measures, both experts emphasized. The median time for FDA to approve a drug application is about 180 days, while approving new gastroenterology agents takes nearly twice as long, according to Dr. Lichtenstein. “There is clearly a discrepancy, and it would be nice if we moved the bar closer. As an investigator, I need to know which trials are acceptable in design, and what endpoints are clearly defined, with examples,” he said. “If I have a question, I need a point of contact to call to get an answer in rapid time, instead of having to wait for months, and I need to know which biomarkers are appropriate to use.”
Dr. Harris agreed. “There should be tension between FDA and industry – that is part of the process,” he said. “But open communication and rapid response to meeting requests are crucial.”
Timeliness and transparency are especially important as FDA transitions “from being a classic regulator to a proactive partner in drug development,” Dr. Harris said. “What industry needs and expects from FDA is greater certainty on the path. Companies may or may not like a particular FDA guidance document, but they greatly appreciate the clarity that guidance documents provide.”
Dr. Lichtenstein disclosed ties to AbbVie, Hospira, Pfizer, and numerous other pharmaceutical companies. Dr. Harris is employed by Lyric Pharmaceuticals and disclosed relationships with several other biopharmaceutical companies.
AT DDW® 2016
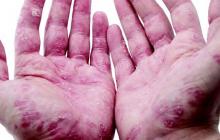
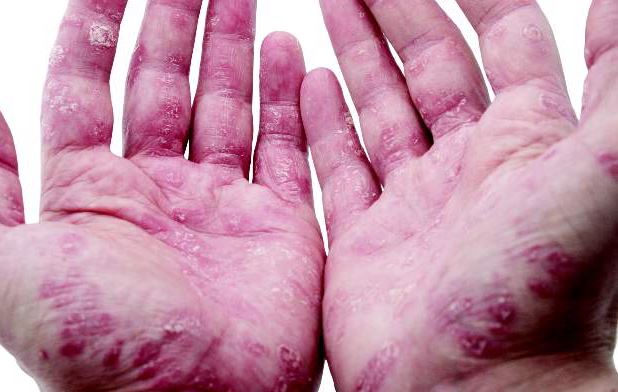
Study: TNF inhibitors improve extraintestinal IBD manifestations
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
AT DDW® 2016
Key clinical point: Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease among more than half of affected patients.
Major finding: About 55% of patients who received infliximab, adalimumab, or certolizumab had a clinical response.
Data source: A study of 1,249 patients with IBD from a national cohort.
Disclosures: A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
Follicular lymphoma with histologic transformation may merit ASCT
Among patients with high tumor burden follicular lymphoma (FL) that responded to rituximab chemotherapy but then underwent histologic transformation, median overall survival was not reached when patients received autologous stem cell transplantation (ASCT), but was only 1.7 years otherwise, based on results of an ancillary study of a clinical trial.
In contrast, ASCT did not affect overall survival when patients progressed to untransformed FL, said Dr. Clémentine Sarkozy of Centre Hospitalier Lyon-Sud in Pierre Bénite, France, and her associates. Fully 58% of histologic transformations occurred in the first year of follow-up, highlighting “the necessity for biopsy at the first recurrence of FL,” they wrote online June 13 in the Journal of Clinical Oncology.
Histologic transformation in FL signifies progression to aggressive lymphoma. Studies of histologic transformation and subsequent overall survival in the rituximab era have been retrospective, with variable patient populations and initial management regimens, according to the investigators. Therefore, they followed 1,018 patients from the multicenter, randomized, phase III PRIMA (Primary Rituximab and Maintenance) trial, which evaluated maintenance rituximab therapy among patients with symptomatic FL who had responded to induction chemotherapy plus rituximab (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.65.7163).
A total of 463 patients (45.5%) experienced disease recurrence or progression, and 194 (42%) were biopsied over a median follow-up time of 6 years. A total of 40 (20.6%) biopsies showed histologic transformation, while 154 (79.4%) had untransformed FL. Median time to recurrence was 9.6 months for patients with histologic transformation and 22.8 months for patients with untransformed FL (P = .02). Median overall survival with histologic transformation was worse than with untransformed FL (3.8 years vs. 6.4 years; hazard ratio, 3.9; 95% confidence interval, 2.2-6.9; P = .001). Furthermore, among patients who progressed within 12 months, median overall survival with histologic transformation was 2 years, compared with 6.4 years for patients with untransformed FL (P = .007).
After salvage therapy, 17 (42%) patients with histologic transformation underwent consolidation with high-dose chemotherapy and ASCT. Median overall survival for these patients was not reached, versus 1.7 years when they did not undergo ASCT. In contrast, ASCT did not improve overall survival among patients with untransformed FL. Results were similar after excluding patients with early progression and patients who were older than 65 years, the investigators reported.
Risk factors for histologic transformation in the univariate analysis included performance status, anemia, high lactate dehydrogenase level, “B” symptoms, histologic grade 3a, and high Follicular Lymphoma International Prognostic Index scores at diagnosis. However, only Eastern Cooperative Oncology Group performance status of 2 to 4 (HR, 5.6; 95% CI, 1.7-17.7), and anemia (HR, 3.7; 95% CI, 1.4-9.7) remained significant in the multivariate analysis. Neither the choice of induction regimen nor the quality of response seemed to affect the likelihood of histologic transformation, and rituximab maintenance therapy did not seem to alter response to salvage treatment or survival after histologic transformation. By necessity, the study excluded patients who did not respond to initial immunochemotherapy, which could have limited the generalizability of the findings, the investigators noted.
The study was funded by Sandoz and Takeda Pharmaceuticals. Dr. Sarkozy disclosed research funding from Sandoz and Takeda Pharmaceuticals and honoraria from Gilead Sciences. Twelve coinvestigators also disclosed ties to Takeda and a number of other pharmaceutical companies. The other seven coinvestigators had no disclosures.
In just 3 years, prospective observational studies and [this] clever ancillary analysis of a prospective clinical trial have better informed the lymphoma community about the expected incidence and timing of transformation in patients with follicular lymphoma after being treated with modern management strategies. But we are still limited by clumsy predictive tools for identifying patients at highest risk. Deeper understanding of biologic and genetic factors of FL subclonal populations as well as the tumor microenvironment will allow for more precise identification of patients truly at risk and potentially will provide actionable targets for abrogating that risk. Future [studies of] transformed lymphoma will hopefully replace variables such as anthracyclines, the Follicular Lymphoma International Prognostic Index, lactate dehydrogenase, and ASCT with promising new variables such as IRF-4, miR-31, bcl-2, pleuripotency, and nuclear factor kappa B pathway genes or new therapies that target these variables. Future analyses should not simply prognosticate who is at risk for transformation, but should predict a specific intervention to either prevent or treat such an event.
Dr. Brian K. Link is at the University of Iowa, Iowa City. He reported ties to AbbVie, Gilead Sciences, Genentech, Sandoz, Pharmacyclics, Millennium Pharmaceuticals, Genentech, Kite Pharma, Seattle Genetics, and Dynavax Technologies. These comments are from his editorial accompanying the report (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.67.4234).
In just 3 years, prospective observational studies and [this] clever ancillary analysis of a prospective clinical trial have better informed the lymphoma community about the expected incidence and timing of transformation in patients with follicular lymphoma after being treated with modern management strategies. But we are still limited by clumsy predictive tools for identifying patients at highest risk. Deeper understanding of biologic and genetic factors of FL subclonal populations as well as the tumor microenvironment will allow for more precise identification of patients truly at risk and potentially will provide actionable targets for abrogating that risk. Future [studies of] transformed lymphoma will hopefully replace variables such as anthracyclines, the Follicular Lymphoma International Prognostic Index, lactate dehydrogenase, and ASCT with promising new variables such as IRF-4, miR-31, bcl-2, pleuripotency, and nuclear factor kappa B pathway genes or new therapies that target these variables. Future analyses should not simply prognosticate who is at risk for transformation, but should predict a specific intervention to either prevent or treat such an event.
Dr. Brian K. Link is at the University of Iowa, Iowa City. He reported ties to AbbVie, Gilead Sciences, Genentech, Sandoz, Pharmacyclics, Millennium Pharmaceuticals, Genentech, Kite Pharma, Seattle Genetics, and Dynavax Technologies. These comments are from his editorial accompanying the report (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.67.4234).
In just 3 years, prospective observational studies and [this] clever ancillary analysis of a prospective clinical trial have better informed the lymphoma community about the expected incidence and timing of transformation in patients with follicular lymphoma after being treated with modern management strategies. But we are still limited by clumsy predictive tools for identifying patients at highest risk. Deeper understanding of biologic and genetic factors of FL subclonal populations as well as the tumor microenvironment will allow for more precise identification of patients truly at risk and potentially will provide actionable targets for abrogating that risk. Future [studies of] transformed lymphoma will hopefully replace variables such as anthracyclines, the Follicular Lymphoma International Prognostic Index, lactate dehydrogenase, and ASCT with promising new variables such as IRF-4, miR-31, bcl-2, pleuripotency, and nuclear factor kappa B pathway genes or new therapies that target these variables. Future analyses should not simply prognosticate who is at risk for transformation, but should predict a specific intervention to either prevent or treat such an event.
Dr. Brian K. Link is at the University of Iowa, Iowa City. He reported ties to AbbVie, Gilead Sciences, Genentech, Sandoz, Pharmacyclics, Millennium Pharmaceuticals, Genentech, Kite Pharma, Seattle Genetics, and Dynavax Technologies. These comments are from his editorial accompanying the report (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.67.4234).
Among patients with high tumor burden follicular lymphoma (FL) that responded to rituximab chemotherapy but then underwent histologic transformation, median overall survival was not reached when patients received autologous stem cell transplantation (ASCT), but was only 1.7 years otherwise, based on results of an ancillary study of a clinical trial.
In contrast, ASCT did not affect overall survival when patients progressed to untransformed FL, said Dr. Clémentine Sarkozy of Centre Hospitalier Lyon-Sud in Pierre Bénite, France, and her associates. Fully 58% of histologic transformations occurred in the first year of follow-up, highlighting “the necessity for biopsy at the first recurrence of FL,” they wrote online June 13 in the Journal of Clinical Oncology.
Histologic transformation in FL signifies progression to aggressive lymphoma. Studies of histologic transformation and subsequent overall survival in the rituximab era have been retrospective, with variable patient populations and initial management regimens, according to the investigators. Therefore, they followed 1,018 patients from the multicenter, randomized, phase III PRIMA (Primary Rituximab and Maintenance) trial, which evaluated maintenance rituximab therapy among patients with symptomatic FL who had responded to induction chemotherapy plus rituximab (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.65.7163).
A total of 463 patients (45.5%) experienced disease recurrence or progression, and 194 (42%) were biopsied over a median follow-up time of 6 years. A total of 40 (20.6%) biopsies showed histologic transformation, while 154 (79.4%) had untransformed FL. Median time to recurrence was 9.6 months for patients with histologic transformation and 22.8 months for patients with untransformed FL (P = .02). Median overall survival with histologic transformation was worse than with untransformed FL (3.8 years vs. 6.4 years; hazard ratio, 3.9; 95% confidence interval, 2.2-6.9; P = .001). Furthermore, among patients who progressed within 12 months, median overall survival with histologic transformation was 2 years, compared with 6.4 years for patients with untransformed FL (P = .007).
After salvage therapy, 17 (42%) patients with histologic transformation underwent consolidation with high-dose chemotherapy and ASCT. Median overall survival for these patients was not reached, versus 1.7 years when they did not undergo ASCT. In contrast, ASCT did not improve overall survival among patients with untransformed FL. Results were similar after excluding patients with early progression and patients who were older than 65 years, the investigators reported.
Risk factors for histologic transformation in the univariate analysis included performance status, anemia, high lactate dehydrogenase level, “B” symptoms, histologic grade 3a, and high Follicular Lymphoma International Prognostic Index scores at diagnosis. However, only Eastern Cooperative Oncology Group performance status of 2 to 4 (HR, 5.6; 95% CI, 1.7-17.7), and anemia (HR, 3.7; 95% CI, 1.4-9.7) remained significant in the multivariate analysis. Neither the choice of induction regimen nor the quality of response seemed to affect the likelihood of histologic transformation, and rituximab maintenance therapy did not seem to alter response to salvage treatment or survival after histologic transformation. By necessity, the study excluded patients who did not respond to initial immunochemotherapy, which could have limited the generalizability of the findings, the investigators noted.
The study was funded by Sandoz and Takeda Pharmaceuticals. Dr. Sarkozy disclosed research funding from Sandoz and Takeda Pharmaceuticals and honoraria from Gilead Sciences. Twelve coinvestigators also disclosed ties to Takeda and a number of other pharmaceutical companies. The other seven coinvestigators had no disclosures.
Among patients with high tumor burden follicular lymphoma (FL) that responded to rituximab chemotherapy but then underwent histologic transformation, median overall survival was not reached when patients received autologous stem cell transplantation (ASCT), but was only 1.7 years otherwise, based on results of an ancillary study of a clinical trial.
In contrast, ASCT did not affect overall survival when patients progressed to untransformed FL, said Dr. Clémentine Sarkozy of Centre Hospitalier Lyon-Sud in Pierre Bénite, France, and her associates. Fully 58% of histologic transformations occurred in the first year of follow-up, highlighting “the necessity for biopsy at the first recurrence of FL,” they wrote online June 13 in the Journal of Clinical Oncology.
Histologic transformation in FL signifies progression to aggressive lymphoma. Studies of histologic transformation and subsequent overall survival in the rituximab era have been retrospective, with variable patient populations and initial management regimens, according to the investigators. Therefore, they followed 1,018 patients from the multicenter, randomized, phase III PRIMA (Primary Rituximab and Maintenance) trial, which evaluated maintenance rituximab therapy among patients with symptomatic FL who had responded to induction chemotherapy plus rituximab (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.65.7163).
A total of 463 patients (45.5%) experienced disease recurrence or progression, and 194 (42%) were biopsied over a median follow-up time of 6 years. A total of 40 (20.6%) biopsies showed histologic transformation, while 154 (79.4%) had untransformed FL. Median time to recurrence was 9.6 months for patients with histologic transformation and 22.8 months for patients with untransformed FL (P = .02). Median overall survival with histologic transformation was worse than with untransformed FL (3.8 years vs. 6.4 years; hazard ratio, 3.9; 95% confidence interval, 2.2-6.9; P = .001). Furthermore, among patients who progressed within 12 months, median overall survival with histologic transformation was 2 years, compared with 6.4 years for patients with untransformed FL (P = .007).
After salvage therapy, 17 (42%) patients with histologic transformation underwent consolidation with high-dose chemotherapy and ASCT. Median overall survival for these patients was not reached, versus 1.7 years when they did not undergo ASCT. In contrast, ASCT did not improve overall survival among patients with untransformed FL. Results were similar after excluding patients with early progression and patients who were older than 65 years, the investigators reported.
Risk factors for histologic transformation in the univariate analysis included performance status, anemia, high lactate dehydrogenase level, “B” symptoms, histologic grade 3a, and high Follicular Lymphoma International Prognostic Index scores at diagnosis. However, only Eastern Cooperative Oncology Group performance status of 2 to 4 (HR, 5.6; 95% CI, 1.7-17.7), and anemia (HR, 3.7; 95% CI, 1.4-9.7) remained significant in the multivariate analysis. Neither the choice of induction regimen nor the quality of response seemed to affect the likelihood of histologic transformation, and rituximab maintenance therapy did not seem to alter response to salvage treatment or survival after histologic transformation. By necessity, the study excluded patients who did not respond to initial immunochemotherapy, which could have limited the generalizability of the findings, the investigators noted.
The study was funded by Sandoz and Takeda Pharmaceuticals. Dr. Sarkozy disclosed research funding from Sandoz and Takeda Pharmaceuticals and honoraria from Gilead Sciences. Twelve coinvestigators also disclosed ties to Takeda and a number of other pharmaceutical companies. The other seven coinvestigators had no disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Histologic transformation of follicular lymphoma tends to occur early and may merit intensive salvage with autologous stem cell transplantation.
Major finding: Median overall survival was not reached among patients who received ASCT and was 1.7 years in those who didn’t have ASCT.
Data source: A study of 1,018 patients from the multicenter, randomized, phase III PRIMA (Primary Rituximab and Maintenance) trial.
Disclosures: The study was funded by Sandoz and Takeda Pharmaceuticals. Dr. Sarkozy disclosed research funding from Sandoz and Takeda Pharmaceuticals and honoraria from Gilead Sciences. Twelve coinvestigators also disclosed ties to Takeda and a number of other pharmaceutical companies. The other seven coinvestigators had no disclosures.
Tyrosine kinase inhibitors may boost cardiac risk in chronic myeloid leukemia
Patients with chronic myeloid leukemia (CML) who received tyrosine kinase inhibitors (TKIs) had 1.7 times the rate of arterial or venous vascular events of population-based controls in a large retrospective cohort study.
In addition, second-generation TKIs were associated with higher rates of myocardial infarction than was first-generation imatinib, Dr. Torsten Dahlén of Karolinska University Hospital Solna, Stockholm, and his associates reported. Although absolute numbers of cardiovascular events were low, physicians “should be aware of these risk factors when initiating TKI therapy in patients with CML,” the authors wrote in a study published online June 13 in the Annals of Internal Medicine .
Tyrosine kinase inhibitors have “revolutionized” the prognosis of CML and are generally well tolerated, the researchers noted. But case reports and follow-up studies of clinical trial participants have raised concerns about cardiovascular toxicities with second-generation TKIs, such as nilotinib, they added.
To further study the issue, the investigators compared 896 patients in Sweden who were diagnosed with CML between 2002 and 2012 with 4,438 age- and sex-matched controls from the national population register. By crosschecking both groups against a national patient database, the investigators calculated rates of venous thrombosis, pulmonary embolism, myocardial infarction, cerebrovascular ischemia, and other arterial thromboses (Ann. Intern. Med. 2016 Jun 13. doi: 10.7326/M15-2306).
A total of 846 CML patients (94%) received a TKI during a median of 4.2 years of follow-up, the investigators reported. First-line therapy usually consisted of imatinib (89%), followed by nilotinib (9%) and dasatinib (1%).
The TKI cohort had 78 arterial and venous events during 3,969 person-years of follow-up, compared with 250 events during 21,917 person-years of follow-up for controls, for a statistically significant incidence rate ratio (IRR) of 1.7 (95% confidence interval, 1.3-2.2). Individual IRRs for arterial and venous events also reached statistical significance at 1.5 (95% CI, 1.1-2.1) and 2.0 (95% CI, 1.2-3.3), respectively. Deep venous thrombosis and myocardial infarction accounted for most of the excess risk, with IRRs of 2.2 (95% CI, 1.1-4.4) and 1.9 (95% CI, 1.3-2.7), respectively.
When investigators looked only at the TKI cohort, they found that the rates of arterial thromboembolic events were highest for nilotinib (29 events per 1,000 person-years), followed by dasatinib (19 events per 1,000 person-years) and imatinib (13 events per 1,000 person-years). Nilotinib also was associated with a substantially higher rate of all arterial and venous events (42/1,000 person-years) than dasatinib (20/1,000 person-years) and imatinib (16/1,000 person-years).
Furthermore, nilotinib and dasatinib were associated with higher rates of myocardial infarctions (29 and 19 per 1,000 person-years, respectively) and cerebrovascular ischemic events (11 and 4 events per 1,000 person-years, respectively) than was imatinib (8 events per 1,000 person-years and 4 events per 1,000 person-years, respectively). However, the absolute numbers of events were too small to allow for statistical comparisons, the researchers said.
“The observed increase in thrombotic events may be related to CML itself, the treatment administered, or both,” they noted, but “the prevalence of myocardial infarction in patients with CML before diagnosis was similar to that of the control population, [which] might indicate a treatment-related association.”
Among the 31 patients on TKIs who had a myocardial infarction, 26 (84%) had been previously diagnosed with at least one risk factor for cardiovascular disease, including diabetes (19%), atrial fibrillation (26%), angina pectoris (39%), hypertension (55%), and hyperlipidemia (23%).
Most patients who received nilotinib or dasatinib had previously received imatinib, meaning that they could have had more advanced disease that increased their risk of adverse events, according to the researchers.
“The small number of events also leads us to exercise caution in drawing any strong conclusions,” they added. “Future data from the Swedish CML register will provide more robust evidence regarding the risks of individual drugs as exposure time increases.”
The researchers received no funding for the work. Dr. Dahlén disclosed grant support from Merck outside the submitted work. Two coinvestigators disclosed ties to Ariad, Bristol-Myers, Novartis, and Squibb.
Patients with chronic myeloid leukemia (CML) who received tyrosine kinase inhibitors (TKIs) had 1.7 times the rate of arterial or venous vascular events of population-based controls in a large retrospective cohort study.
In addition, second-generation TKIs were associated with higher rates of myocardial infarction than was first-generation imatinib, Dr. Torsten Dahlén of Karolinska University Hospital Solna, Stockholm, and his associates reported. Although absolute numbers of cardiovascular events were low, physicians “should be aware of these risk factors when initiating TKI therapy in patients with CML,” the authors wrote in a study published online June 13 in the Annals of Internal Medicine .
Tyrosine kinase inhibitors have “revolutionized” the prognosis of CML and are generally well tolerated, the researchers noted. But case reports and follow-up studies of clinical trial participants have raised concerns about cardiovascular toxicities with second-generation TKIs, such as nilotinib, they added.
To further study the issue, the investigators compared 896 patients in Sweden who were diagnosed with CML between 2002 and 2012 with 4,438 age- and sex-matched controls from the national population register. By crosschecking both groups against a national patient database, the investigators calculated rates of venous thrombosis, pulmonary embolism, myocardial infarction, cerebrovascular ischemia, and other arterial thromboses (Ann. Intern. Med. 2016 Jun 13. doi: 10.7326/M15-2306).
A total of 846 CML patients (94%) received a TKI during a median of 4.2 years of follow-up, the investigators reported. First-line therapy usually consisted of imatinib (89%), followed by nilotinib (9%) and dasatinib (1%).
The TKI cohort had 78 arterial and venous events during 3,969 person-years of follow-up, compared with 250 events during 21,917 person-years of follow-up for controls, for a statistically significant incidence rate ratio (IRR) of 1.7 (95% confidence interval, 1.3-2.2). Individual IRRs for arterial and venous events also reached statistical significance at 1.5 (95% CI, 1.1-2.1) and 2.0 (95% CI, 1.2-3.3), respectively. Deep venous thrombosis and myocardial infarction accounted for most of the excess risk, with IRRs of 2.2 (95% CI, 1.1-4.4) and 1.9 (95% CI, 1.3-2.7), respectively.
When investigators looked only at the TKI cohort, they found that the rates of arterial thromboembolic events were highest for nilotinib (29 events per 1,000 person-years), followed by dasatinib (19 events per 1,000 person-years) and imatinib (13 events per 1,000 person-years). Nilotinib also was associated with a substantially higher rate of all arterial and venous events (42/1,000 person-years) than dasatinib (20/1,000 person-years) and imatinib (16/1,000 person-years).
Furthermore, nilotinib and dasatinib were associated with higher rates of myocardial infarctions (29 and 19 per 1,000 person-years, respectively) and cerebrovascular ischemic events (11 and 4 events per 1,000 person-years, respectively) than was imatinib (8 events per 1,000 person-years and 4 events per 1,000 person-years, respectively). However, the absolute numbers of events were too small to allow for statistical comparisons, the researchers said.
“The observed increase in thrombotic events may be related to CML itself, the treatment administered, or both,” they noted, but “the prevalence of myocardial infarction in patients with CML before diagnosis was similar to that of the control population, [which] might indicate a treatment-related association.”
Among the 31 patients on TKIs who had a myocardial infarction, 26 (84%) had been previously diagnosed with at least one risk factor for cardiovascular disease, including diabetes (19%), atrial fibrillation (26%), angina pectoris (39%), hypertension (55%), and hyperlipidemia (23%).
Most patients who received nilotinib or dasatinib had previously received imatinib, meaning that they could have had more advanced disease that increased their risk of adverse events, according to the researchers.
“The small number of events also leads us to exercise caution in drawing any strong conclusions,” they added. “Future data from the Swedish CML register will provide more robust evidence regarding the risks of individual drugs as exposure time increases.”
The researchers received no funding for the work. Dr. Dahlén disclosed grant support from Merck outside the submitted work. Two coinvestigators disclosed ties to Ariad, Bristol-Myers, Novartis, and Squibb.
Patients with chronic myeloid leukemia (CML) who received tyrosine kinase inhibitors (TKIs) had 1.7 times the rate of arterial or venous vascular events of population-based controls in a large retrospective cohort study.
In addition, second-generation TKIs were associated with higher rates of myocardial infarction than was first-generation imatinib, Dr. Torsten Dahlén of Karolinska University Hospital Solna, Stockholm, and his associates reported. Although absolute numbers of cardiovascular events were low, physicians “should be aware of these risk factors when initiating TKI therapy in patients with CML,” the authors wrote in a study published online June 13 in the Annals of Internal Medicine .
Tyrosine kinase inhibitors have “revolutionized” the prognosis of CML and are generally well tolerated, the researchers noted. But case reports and follow-up studies of clinical trial participants have raised concerns about cardiovascular toxicities with second-generation TKIs, such as nilotinib, they added.
To further study the issue, the investigators compared 896 patients in Sweden who were diagnosed with CML between 2002 and 2012 with 4,438 age- and sex-matched controls from the national population register. By crosschecking both groups against a national patient database, the investigators calculated rates of venous thrombosis, pulmonary embolism, myocardial infarction, cerebrovascular ischemia, and other arterial thromboses (Ann. Intern. Med. 2016 Jun 13. doi: 10.7326/M15-2306).
A total of 846 CML patients (94%) received a TKI during a median of 4.2 years of follow-up, the investigators reported. First-line therapy usually consisted of imatinib (89%), followed by nilotinib (9%) and dasatinib (1%).
The TKI cohort had 78 arterial and venous events during 3,969 person-years of follow-up, compared with 250 events during 21,917 person-years of follow-up for controls, for a statistically significant incidence rate ratio (IRR) of 1.7 (95% confidence interval, 1.3-2.2). Individual IRRs for arterial and venous events also reached statistical significance at 1.5 (95% CI, 1.1-2.1) and 2.0 (95% CI, 1.2-3.3), respectively. Deep venous thrombosis and myocardial infarction accounted for most of the excess risk, with IRRs of 2.2 (95% CI, 1.1-4.4) and 1.9 (95% CI, 1.3-2.7), respectively.
When investigators looked only at the TKI cohort, they found that the rates of arterial thromboembolic events were highest for nilotinib (29 events per 1,000 person-years), followed by dasatinib (19 events per 1,000 person-years) and imatinib (13 events per 1,000 person-years). Nilotinib also was associated with a substantially higher rate of all arterial and venous events (42/1,000 person-years) than dasatinib (20/1,000 person-years) and imatinib (16/1,000 person-years).
Furthermore, nilotinib and dasatinib were associated with higher rates of myocardial infarctions (29 and 19 per 1,000 person-years, respectively) and cerebrovascular ischemic events (11 and 4 events per 1,000 person-years, respectively) than was imatinib (8 events per 1,000 person-years and 4 events per 1,000 person-years, respectively). However, the absolute numbers of events were too small to allow for statistical comparisons, the researchers said.
“The observed increase in thrombotic events may be related to CML itself, the treatment administered, or both,” they noted, but “the prevalence of myocardial infarction in patients with CML before diagnosis was similar to that of the control population, [which] might indicate a treatment-related association.”
Among the 31 patients on TKIs who had a myocardial infarction, 26 (84%) had been previously diagnosed with at least one risk factor for cardiovascular disease, including diabetes (19%), atrial fibrillation (26%), angina pectoris (39%), hypertension (55%), and hyperlipidemia (23%).
Most patients who received nilotinib or dasatinib had previously received imatinib, meaning that they could have had more advanced disease that increased their risk of adverse events, according to the researchers.
“The small number of events also leads us to exercise caution in drawing any strong conclusions,” they added. “Future data from the Swedish CML register will provide more robust evidence regarding the risks of individual drugs as exposure time increases.”
The researchers received no funding for the work. Dr. Dahlén disclosed grant support from Merck outside the submitted work. Two coinvestigators disclosed ties to Ariad, Bristol-Myers, Novartis, and Squibb.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Tyrosine kinase inhibitors were associated with a significantly increased risk of cardiovascular events among chronic myeloid leukemia patients.
Major finding: These patients had 1.7 times the rate of arterial or venous events, compared with the general population (95% confidence interval, 1.3-2.2).
Data source: A retrospective, registry-based cohort study of 896 patients with CML and 4,438 population-based controls matched by age and sex.
Disclosures: The researchers received no funding for the work. Dr. Dahlén disclosed grant support from Merck outside the submitted work. Two coinvestigators disclosed ties to Ariad, Bristol-Myers, Novartis, and Squibb.
Adding calcipotriene to 5-FU dramatically reduced AKs
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: A combination of 5% 5-fluorouracil cream and 0.005% calcipotriene was significantly more effective at removing actinic keratoses at different anatomic sites as 5-FU monotherapy.
Major finding: At week 8, the combination group had an average 86% reduction in the number of facial AKs, compared with 26% with 5-FU monotherapy.
Data source: A randomized, double-blind, controlled study of 131 patients with at least four AKs on the face, scalp, and/or upper arms.
Disclosures: Dr. Demehri had no disclosures.
Keys to alopecia areata might lie in gut microbiome
SCOTTSDALE, ARIZ. – Wiping out the gut microbiome with antibiotics prevented alopecia areata in a study of mice, providing evidence that the gut microbiome may play a role in alopecia, Dr. James Chen reported at the annual meeting of the Society for Investigative Dermatology.
The finding shows that the bacterial culprits in alopecia “reside in the gut microbiome, and not in the skin,” said Dr. Chen, a postdoctoral research fellow in medical genetics at Columbia University, New York.
Alopecia areata is mediated by autoreactive NKG2D+ CD8+ T cells. Aberrations in the human microbiome underlie several other autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and type I diabetes, Dr. Chen noted. “The gut microbiome also has been linked to skin conditions, such as acne, psoriasis, and atopic dermatitis,” he added. “So we asked, if we deplete this microbiome with an antibiotic cocktail, do we see an effect on alopecia?”
To find out, he and his coinvestigators grafted skin from C3H/Hej mice, which spontaneously develop alopecia, onto healthy younger mice, causing them to develop alopecia 6-10 weeks later. “Strikingly, we found that treating unaffected mice with an oral antibiotic cocktail prior to grafting completely prevented the development of alopecia areata, and this remained true through 15 weeks,” he said. “This is the first evidence that the gut microbiome could be implicated in alopecia, based on the absence of the phenotype that we see in treated mice.”
The researchers also evaluated whether the skin microbiomes of antibiotic-treated and control mice differed, and determined that the skin samples resembled each other in terms of overall bacterial load and bacterial taxonomic clustering patterns. That suggests that the skin microbiome is not involved in alopecia areata, Dr. Chen said.
Finally, the investigators transferred NKG2D+ CD8+ T cells from the cutaneous lymph nodes of alopecic mice to normal mice that had been pretreated with antibiotics. The treated mice had little infiltration of these T cells into the skin, and lower overall T-cell levels than control mice, Dr. Chen reported.
The investigators are now testing combinations of antibiotics and fecal transplants to pinpoint which gut bacteria make mice susceptible to hair loss. Doing so “will have significant implications on both our understanding of alopecia areata susceptibility, as well as actionable therapeutic targets for treatment” in humans, Dr. Chen said.
The study was funded by the National Institutes of Health, the Medical Research Council, the Dermatology Foundation, Locks of Love Foundation, and NYSTEM (New York State Stem Cell Science). Dr. Chen had no financial disclosures.
SCOTTSDALE, ARIZ. – Wiping out the gut microbiome with antibiotics prevented alopecia areata in a study of mice, providing evidence that the gut microbiome may play a role in alopecia, Dr. James Chen reported at the annual meeting of the Society for Investigative Dermatology.
The finding shows that the bacterial culprits in alopecia “reside in the gut microbiome, and not in the skin,” said Dr. Chen, a postdoctoral research fellow in medical genetics at Columbia University, New York.
Alopecia areata is mediated by autoreactive NKG2D+ CD8+ T cells. Aberrations in the human microbiome underlie several other autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and type I diabetes, Dr. Chen noted. “The gut microbiome also has been linked to skin conditions, such as acne, psoriasis, and atopic dermatitis,” he added. “So we asked, if we deplete this microbiome with an antibiotic cocktail, do we see an effect on alopecia?”
To find out, he and his coinvestigators grafted skin from C3H/Hej mice, which spontaneously develop alopecia, onto healthy younger mice, causing them to develop alopecia 6-10 weeks later. “Strikingly, we found that treating unaffected mice with an oral antibiotic cocktail prior to grafting completely prevented the development of alopecia areata, and this remained true through 15 weeks,” he said. “This is the first evidence that the gut microbiome could be implicated in alopecia, based on the absence of the phenotype that we see in treated mice.”
The researchers also evaluated whether the skin microbiomes of antibiotic-treated and control mice differed, and determined that the skin samples resembled each other in terms of overall bacterial load and bacterial taxonomic clustering patterns. That suggests that the skin microbiome is not involved in alopecia areata, Dr. Chen said.
Finally, the investigators transferred NKG2D+ CD8+ T cells from the cutaneous lymph nodes of alopecic mice to normal mice that had been pretreated with antibiotics. The treated mice had little infiltration of these T cells into the skin, and lower overall T-cell levels than control mice, Dr. Chen reported.
The investigators are now testing combinations of antibiotics and fecal transplants to pinpoint which gut bacteria make mice susceptible to hair loss. Doing so “will have significant implications on both our understanding of alopecia areata susceptibility, as well as actionable therapeutic targets for treatment” in humans, Dr. Chen said.
The study was funded by the National Institutes of Health, the Medical Research Council, the Dermatology Foundation, Locks of Love Foundation, and NYSTEM (New York State Stem Cell Science). Dr. Chen had no financial disclosures.
SCOTTSDALE, ARIZ. – Wiping out the gut microbiome with antibiotics prevented alopecia areata in a study of mice, providing evidence that the gut microbiome may play a role in alopecia, Dr. James Chen reported at the annual meeting of the Society for Investigative Dermatology.
The finding shows that the bacterial culprits in alopecia “reside in the gut microbiome, and not in the skin,” said Dr. Chen, a postdoctoral research fellow in medical genetics at Columbia University, New York.
Alopecia areata is mediated by autoreactive NKG2D+ CD8+ T cells. Aberrations in the human microbiome underlie several other autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and type I diabetes, Dr. Chen noted. “The gut microbiome also has been linked to skin conditions, such as acne, psoriasis, and atopic dermatitis,” he added. “So we asked, if we deplete this microbiome with an antibiotic cocktail, do we see an effect on alopecia?”
To find out, he and his coinvestigators grafted skin from C3H/Hej mice, which spontaneously develop alopecia, onto healthy younger mice, causing them to develop alopecia 6-10 weeks later. “Strikingly, we found that treating unaffected mice with an oral antibiotic cocktail prior to grafting completely prevented the development of alopecia areata, and this remained true through 15 weeks,” he said. “This is the first evidence that the gut microbiome could be implicated in alopecia, based on the absence of the phenotype that we see in treated mice.”
The researchers also evaluated whether the skin microbiomes of antibiotic-treated and control mice differed, and determined that the skin samples resembled each other in terms of overall bacterial load and bacterial taxonomic clustering patterns. That suggests that the skin microbiome is not involved in alopecia areata, Dr. Chen said.
Finally, the investigators transferred NKG2D+ CD8+ T cells from the cutaneous lymph nodes of alopecic mice to normal mice that had been pretreated with antibiotics. The treated mice had little infiltration of these T cells into the skin, and lower overall T-cell levels than control mice, Dr. Chen reported.
The investigators are now testing combinations of antibiotics and fecal transplants to pinpoint which gut bacteria make mice susceptible to hair loss. Doing so “will have significant implications on both our understanding of alopecia areata susceptibility, as well as actionable therapeutic targets for treatment” in humans, Dr. Chen said.
The study was funded by the National Institutes of Health, the Medical Research Council, the Dermatology Foundation, Locks of Love Foundation, and NYSTEM (New York State Stem Cell Science). Dr. Chen had no financial disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Using antibiotics to eliminate the gut microbiome in mice prevented them from developing alopecia.
Major finding: The mice also had lower levels of cytotoxic T-cell infiltration into the skin, compared with alopecic controls.
Data source: A study of C3H/Hej (alopecic) mice and healthy young mice that received skin grafts from the alopecic phenotype.
Disclosures: The study was funded by the National Institutes of Health, the Medical Research Council, the Dermatology Foundation, Locks of Love Foundation, and NYSTEM (New York State Stem Cell Science). Dr. Chen had no financial disclosures.