User login
Ustekinumab misses primary endpoint in atopic dermatitis
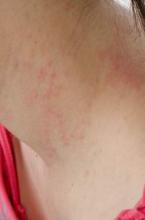
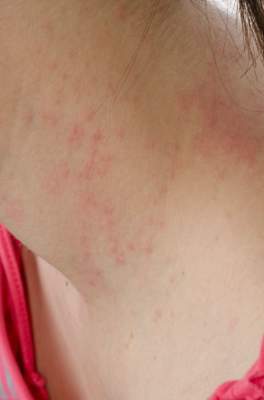
SCOTTSDALE, ARIZ. – Treatment with ustekinumab did not result in significant improvements in atopic dermatitis after 16 weeks, compared with placebo in a phase II study of adults with moderate to severe atopic dermatitis, Dr. Patrick Brunner reported at the annual meeting of the Society for Investigative Dermatology.
However, molecular studies revealed robust modulation of relevant transcriptomic genes after 1 month of ustekinumab, compared with placebo, said Dr. Brunner, who is with the Laboratory for Investigative Dermatology, Rockefeller University, New York. Since the crossover design of this trial and the use of topical corticosteroids by patients probably diluted the differences in clinical outcomes between the intervention and placebo groups, “another study with a different design and dosing is mandated,” he said in an oral presentation at the meeting.
Ustekinumab (Stelara) is a long-acting injectable human interleukin-12 and interleukin-23 antagonist that suppresses Th1, Th17, and Th22 activation. It was approved in 2009 in the United States for moderate to severe plaque psoriasis, and in 2013 for active psoriatic arthritis.
To investigate ustekinumab as therapy for atopic dermatitis, Dr. Brunner and his associates randomly assigned 33 adults aged 18-75 years with refractory atopic dermatitis and baseline scores on the Scoring Atopic Dermatitis (SCORAD) severity scale above 15 to subcutaneous injections of either placebo (17 patients) or ustekinumab (16) at weeks 0, 4, and 16. Ustekinumab was dosed the same way as in psoriasis: 45 mg per injection for patients at or under 100 kg, and 90 mg per injection for heavier patients. At week 16, all patients crossed over to the other treatment for another 16 weeks.
The groups were similar in terms of baseline demographics, intrinsic versus extrinsic IgE status, mean SCORAD, and average levels of IgE and eosinophils. To increase enrollment and retention, both groups were given triamcinolone acetonide 0.025%.
The proportion of SCORAD50 responders (patients with at least a 50% drop from the baseline SCORAD) was greater for ustekinumab than placebo at weeks 12, 16, and 20, but the differences never reached statistical significance, Dr. Brunner reported. At week 16, five (31%) ustekinumab-treated patients had achieved SCORAD50 (the primary endpoint), compared with three (18%) of those on placebo. The ustekinumab SCORAD50 response reached 50% by week 20, but by then patients had crossed over and the two groups began to resemble each other, he said.
“The lessons learned for designing atopic dermatitis trials are to avoid a crossover design with a long-lasting drug, and to keep in mind that even mild background topical steroids can tremendously confound detection of drug effects,” Dr. Brunner commented.
Studies of the atopic dermatitis molecular profile, or transcriptome, revealed similar gene expression levels for both trial arms at baseline, but substantially more gene modulation after 4 weeks of ustekinumab, compared with placebo, he reported. This molecular response involved the Th1, Th17, Th22, but also Th2-related atopic dermatitis genes, and in all cases the differences from placebo were statistically significant (P less than .05). Furthermore, gene modulation became more pronounced through the end of the trial and correlated with clinical response, he added.
The most common adverse effect associated with ustekinumab was respiratory infection, which affected two patients. There were no serious adverse effects reported and none of the patients stopped treatment because of adverse effects.
“Ustekinumab had clear clinical and molecular effects, but clinical outcomes might have been obscured by a profound placebo effect, most likely due to background topical glucocorticosteroids and possibly insufficient dosing for atopic dermatitis,” Dr. Brunner and his associates concluded in a report of their findings, which was published online after the meeting in Experimental Dermatology (Exp Dermatol. 2016 Jun 15. doi: 10.1111/exd.13112).
The study was supported by Janssen, the manufacturer of ustekinumab, and by the National Institutes of Health. Dr. Brunner had no disclosures.
SCOTTSDALE, ARIZ. – Treatment with ustekinumab did not result in significant improvements in atopic dermatitis after 16 weeks, compared with placebo in a phase II study of adults with moderate to severe atopic dermatitis, Dr. Patrick Brunner reported at the annual meeting of the Society for Investigative Dermatology.
However, molecular studies revealed robust modulation of relevant transcriptomic genes after 1 month of ustekinumab, compared with placebo, said Dr. Brunner, who is with the Laboratory for Investigative Dermatology, Rockefeller University, New York. Since the crossover design of this trial and the use of topical corticosteroids by patients probably diluted the differences in clinical outcomes between the intervention and placebo groups, “another study with a different design and dosing is mandated,” he said in an oral presentation at the meeting.
Ustekinumab (Stelara) is a long-acting injectable human interleukin-12 and interleukin-23 antagonist that suppresses Th1, Th17, and Th22 activation. It was approved in 2009 in the United States for moderate to severe plaque psoriasis, and in 2013 for active psoriatic arthritis.
To investigate ustekinumab as therapy for atopic dermatitis, Dr. Brunner and his associates randomly assigned 33 adults aged 18-75 years with refractory atopic dermatitis and baseline scores on the Scoring Atopic Dermatitis (SCORAD) severity scale above 15 to subcutaneous injections of either placebo (17 patients) or ustekinumab (16) at weeks 0, 4, and 16. Ustekinumab was dosed the same way as in psoriasis: 45 mg per injection for patients at or under 100 kg, and 90 mg per injection for heavier patients. At week 16, all patients crossed over to the other treatment for another 16 weeks.
The groups were similar in terms of baseline demographics, intrinsic versus extrinsic IgE status, mean SCORAD, and average levels of IgE and eosinophils. To increase enrollment and retention, both groups were given triamcinolone acetonide 0.025%.
The proportion of SCORAD50 responders (patients with at least a 50% drop from the baseline SCORAD) was greater for ustekinumab than placebo at weeks 12, 16, and 20, but the differences never reached statistical significance, Dr. Brunner reported. At week 16, five (31%) ustekinumab-treated patients had achieved SCORAD50 (the primary endpoint), compared with three (18%) of those on placebo. The ustekinumab SCORAD50 response reached 50% by week 20, but by then patients had crossed over and the two groups began to resemble each other, he said.
“The lessons learned for designing atopic dermatitis trials are to avoid a crossover design with a long-lasting drug, and to keep in mind that even mild background topical steroids can tremendously confound detection of drug effects,” Dr. Brunner commented.
Studies of the atopic dermatitis molecular profile, or transcriptome, revealed similar gene expression levels for both trial arms at baseline, but substantially more gene modulation after 4 weeks of ustekinumab, compared with placebo, he reported. This molecular response involved the Th1, Th17, Th22, but also Th2-related atopic dermatitis genes, and in all cases the differences from placebo were statistically significant (P less than .05). Furthermore, gene modulation became more pronounced through the end of the trial and correlated with clinical response, he added.
The most common adverse effect associated with ustekinumab was respiratory infection, which affected two patients. There were no serious adverse effects reported and none of the patients stopped treatment because of adverse effects.
“Ustekinumab had clear clinical and molecular effects, but clinical outcomes might have been obscured by a profound placebo effect, most likely due to background topical glucocorticosteroids and possibly insufficient dosing for atopic dermatitis,” Dr. Brunner and his associates concluded in a report of their findings, which was published online after the meeting in Experimental Dermatology (Exp Dermatol. 2016 Jun 15. doi: 10.1111/exd.13112).
The study was supported by Janssen, the manufacturer of ustekinumab, and by the National Institutes of Health. Dr. Brunner had no disclosures.
SCOTTSDALE, ARIZ. – Treatment with ustekinumab did not result in significant improvements in atopic dermatitis after 16 weeks, compared with placebo in a phase II study of adults with moderate to severe atopic dermatitis, Dr. Patrick Brunner reported at the annual meeting of the Society for Investigative Dermatology.
However, molecular studies revealed robust modulation of relevant transcriptomic genes after 1 month of ustekinumab, compared with placebo, said Dr. Brunner, who is with the Laboratory for Investigative Dermatology, Rockefeller University, New York. Since the crossover design of this trial and the use of topical corticosteroids by patients probably diluted the differences in clinical outcomes between the intervention and placebo groups, “another study with a different design and dosing is mandated,” he said in an oral presentation at the meeting.
Ustekinumab (Stelara) is a long-acting injectable human interleukin-12 and interleukin-23 antagonist that suppresses Th1, Th17, and Th22 activation. It was approved in 2009 in the United States for moderate to severe plaque psoriasis, and in 2013 for active psoriatic arthritis.
To investigate ustekinumab as therapy for atopic dermatitis, Dr. Brunner and his associates randomly assigned 33 adults aged 18-75 years with refractory atopic dermatitis and baseline scores on the Scoring Atopic Dermatitis (SCORAD) severity scale above 15 to subcutaneous injections of either placebo (17 patients) or ustekinumab (16) at weeks 0, 4, and 16. Ustekinumab was dosed the same way as in psoriasis: 45 mg per injection for patients at or under 100 kg, and 90 mg per injection for heavier patients. At week 16, all patients crossed over to the other treatment for another 16 weeks.
The groups were similar in terms of baseline demographics, intrinsic versus extrinsic IgE status, mean SCORAD, and average levels of IgE and eosinophils. To increase enrollment and retention, both groups were given triamcinolone acetonide 0.025%.
The proportion of SCORAD50 responders (patients with at least a 50% drop from the baseline SCORAD) was greater for ustekinumab than placebo at weeks 12, 16, and 20, but the differences never reached statistical significance, Dr. Brunner reported. At week 16, five (31%) ustekinumab-treated patients had achieved SCORAD50 (the primary endpoint), compared with three (18%) of those on placebo. The ustekinumab SCORAD50 response reached 50% by week 20, but by then patients had crossed over and the two groups began to resemble each other, he said.
“The lessons learned for designing atopic dermatitis trials are to avoid a crossover design with a long-lasting drug, and to keep in mind that even mild background topical steroids can tremendously confound detection of drug effects,” Dr. Brunner commented.
Studies of the atopic dermatitis molecular profile, or transcriptome, revealed similar gene expression levels for both trial arms at baseline, but substantially more gene modulation after 4 weeks of ustekinumab, compared with placebo, he reported. This molecular response involved the Th1, Th17, Th22, but also Th2-related atopic dermatitis genes, and in all cases the differences from placebo were statistically significant (P less than .05). Furthermore, gene modulation became more pronounced through the end of the trial and correlated with clinical response, he added.
The most common adverse effect associated with ustekinumab was respiratory infection, which affected two patients. There were no serious adverse effects reported and none of the patients stopped treatment because of adverse effects.
“Ustekinumab had clear clinical and molecular effects, but clinical outcomes might have been obscured by a profound placebo effect, most likely due to background topical glucocorticosteroids and possibly insufficient dosing for atopic dermatitis,” Dr. Brunner and his associates concluded in a report of their findings, which was published online after the meeting in Experimental Dermatology (Exp Dermatol. 2016 Jun 15. doi: 10.1111/exd.13112).
The study was supported by Janssen, the manufacturer of ustekinumab, and by the National Institutes of Health. Dr. Brunner had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Ustekinumab missed its primary endpoint in a phase II trial of adults with moderate to severe atopic dermatitis.
Major finding: At 16 weeks, five (31%) ustekinumab patients had achieved SCORAD50, compared with three (19%) placebo patients, a difference that was not statistically significant.
Data source: A randomized, double-blind study evaluated ustekinumab in 33 patients with moderate to severe atopic dermatitis.
Disclosures: The study was supported by Janssen, the maker of ustekinumab (Stelara), and by the National Institutes of Health. Dr. Brunner had no disclosures.
Midostaurin cut organ damage in systemic mastocytosis
Midostaurin completely resolved at least one type of organ damage for 45% of patients with advanced systemic mastocytosis, based on a multicenter, open-label, phase II, industry-sponsored trial.
“Response rates were similar regardless of the subtype of advanced systemic mastocytosis, KIT mutation status, or exposure to previous therapy,” reported Jason R. Gotlib, MD, of Stanford (Calif.) University, and his associates. Adverse effects led to dose reductions for 41% of patients, however, and caused 22% of patients to stop treatment, the researchers wrote online June 29 in the New England Journal of Medicine.
Systemic mastocytosis is related to a constitutively activated receptor tyrosine kinase encoded by the KIT D816V mutation. As neoplastic mast cells infiltrate and damage organs, patients develop cytopenias, hypoalbuminemia, osteolytic bone lesions, abnormal liver function, ascites, and weight loss. Mastocytosis lacks effective treatments, and patients with aggressive disease tend to live about 3.5 years, the researchers noted (N Engl J Med. 2016 Jun 29;374:2530-40).
Of 116 patients with advanced systemic mastocytosis, 27 lacked measurable signs of disease or had unrelated signs and symptoms. The remaining 89 patients included 16 with aggressive systemic disease, 57 with systemic disease and an associated hematologic neoplasm, and 16 with mast cell leukemia. Patients received 100 mg oral midostaurin twice daily in continuous 4-week cycles for a median of 11.4 months, with a median follow-up of 26 months.
In all, 53 (60%) patients experienced at least 50% improvement in one type of organ damage or improvement in more than one type of organ damage, said the researchers. These responders included 12 patients with aggressive systemic mastocytosis, 33 patients with systemic mastocytosis and a hematologic neoplasm, and eight patients with mast-cell leukemia. No one achieved complete remission, but after six treatment cycles, 45% of patients had complete resolution of at least one type of organ damage.
Patients typically experienced, at best, a nearly 60% drop in bone marrow mast cell burden and serum tryptase. The median duration of response was 24 months, median overall survival was 28.7 months, and median progression-free survival was 14.1 months. Mast cell leukemia and a history of treatment for mastocytosis were tied to shorter survival, while a 50% decrease in mast cell burden significantly improved survival (hazard ratio, 0.33; P = .01).
Grade 3 or 4 hematologic abnormalities included neutropenia (24% of patients), anemia (41%), and thrombocytopenia (29%). Marked myelosuppression was associated with baseline cytopenia and may have reflected either treatment-related effects or disease progression, the researchers said. The most common grade 3/4 nonhematologic adverse effects were fatigue (9% of patients) and diarrhea (8%).
Novartis Pharmaceuticals sponsored the study, and designed it and collected the data with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. The remaining four coinvestigators had no disclosures.
Midostaurin completely resolved at least one type of organ damage for 45% of patients with advanced systemic mastocytosis, based on a multicenter, open-label, phase II, industry-sponsored trial.
“Response rates were similar regardless of the subtype of advanced systemic mastocytosis, KIT mutation status, or exposure to previous therapy,” reported Jason R. Gotlib, MD, of Stanford (Calif.) University, and his associates. Adverse effects led to dose reductions for 41% of patients, however, and caused 22% of patients to stop treatment, the researchers wrote online June 29 in the New England Journal of Medicine.
Systemic mastocytosis is related to a constitutively activated receptor tyrosine kinase encoded by the KIT D816V mutation. As neoplastic mast cells infiltrate and damage organs, patients develop cytopenias, hypoalbuminemia, osteolytic bone lesions, abnormal liver function, ascites, and weight loss. Mastocytosis lacks effective treatments, and patients with aggressive disease tend to live about 3.5 years, the researchers noted (N Engl J Med. 2016 Jun 29;374:2530-40).
Of 116 patients with advanced systemic mastocytosis, 27 lacked measurable signs of disease or had unrelated signs and symptoms. The remaining 89 patients included 16 with aggressive systemic disease, 57 with systemic disease and an associated hematologic neoplasm, and 16 with mast cell leukemia. Patients received 100 mg oral midostaurin twice daily in continuous 4-week cycles for a median of 11.4 months, with a median follow-up of 26 months.
In all, 53 (60%) patients experienced at least 50% improvement in one type of organ damage or improvement in more than one type of organ damage, said the researchers. These responders included 12 patients with aggressive systemic mastocytosis, 33 patients with systemic mastocytosis and a hematologic neoplasm, and eight patients with mast-cell leukemia. No one achieved complete remission, but after six treatment cycles, 45% of patients had complete resolution of at least one type of organ damage.
Patients typically experienced, at best, a nearly 60% drop in bone marrow mast cell burden and serum tryptase. The median duration of response was 24 months, median overall survival was 28.7 months, and median progression-free survival was 14.1 months. Mast cell leukemia and a history of treatment for mastocytosis were tied to shorter survival, while a 50% decrease in mast cell burden significantly improved survival (hazard ratio, 0.33; P = .01).
Grade 3 or 4 hematologic abnormalities included neutropenia (24% of patients), anemia (41%), and thrombocytopenia (29%). Marked myelosuppression was associated with baseline cytopenia and may have reflected either treatment-related effects or disease progression, the researchers said. The most common grade 3/4 nonhematologic adverse effects were fatigue (9% of patients) and diarrhea (8%).
Novartis Pharmaceuticals sponsored the study, and designed it and collected the data with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. The remaining four coinvestigators had no disclosures.
Midostaurin completely resolved at least one type of organ damage for 45% of patients with advanced systemic mastocytosis, based on a multicenter, open-label, phase II, industry-sponsored trial.
“Response rates were similar regardless of the subtype of advanced systemic mastocytosis, KIT mutation status, or exposure to previous therapy,” reported Jason R. Gotlib, MD, of Stanford (Calif.) University, and his associates. Adverse effects led to dose reductions for 41% of patients, however, and caused 22% of patients to stop treatment, the researchers wrote online June 29 in the New England Journal of Medicine.
Systemic mastocytosis is related to a constitutively activated receptor tyrosine kinase encoded by the KIT D816V mutation. As neoplastic mast cells infiltrate and damage organs, patients develop cytopenias, hypoalbuminemia, osteolytic bone lesions, abnormal liver function, ascites, and weight loss. Mastocytosis lacks effective treatments, and patients with aggressive disease tend to live about 3.5 years, the researchers noted (N Engl J Med. 2016 Jun 29;374:2530-40).
Of 116 patients with advanced systemic mastocytosis, 27 lacked measurable signs of disease or had unrelated signs and symptoms. The remaining 89 patients included 16 with aggressive systemic disease, 57 with systemic disease and an associated hematologic neoplasm, and 16 with mast cell leukemia. Patients received 100 mg oral midostaurin twice daily in continuous 4-week cycles for a median of 11.4 months, with a median follow-up of 26 months.
In all, 53 (60%) patients experienced at least 50% improvement in one type of organ damage or improvement in more than one type of organ damage, said the researchers. These responders included 12 patients with aggressive systemic mastocytosis, 33 patients with systemic mastocytosis and a hematologic neoplasm, and eight patients with mast-cell leukemia. No one achieved complete remission, but after six treatment cycles, 45% of patients had complete resolution of at least one type of organ damage.
Patients typically experienced, at best, a nearly 60% drop in bone marrow mast cell burden and serum tryptase. The median duration of response was 24 months, median overall survival was 28.7 months, and median progression-free survival was 14.1 months. Mast cell leukemia and a history of treatment for mastocytosis were tied to shorter survival, while a 50% decrease in mast cell burden significantly improved survival (hazard ratio, 0.33; P = .01).
Grade 3 or 4 hematologic abnormalities included neutropenia (24% of patients), anemia (41%), and thrombocytopenia (29%). Marked myelosuppression was associated with baseline cytopenia and may have reflected either treatment-related effects or disease progression, the researchers said. The most common grade 3/4 nonhematologic adverse effects were fatigue (9% of patients) and diarrhea (8%).
Novartis Pharmaceuticals sponsored the study, and designed it and collected the data with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. The remaining four coinvestigators had no disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Midostaurin helped to resolve organ damage related to mastocytosis.
Major finding: In all, 45% of patients had complete resolution of at least one type of organ damage within six, 4-week treatment cycles.
Data source: An international, open-label, phase II study of 116 patients given 100 mg oral midostaurin twice daily.
Disclosures: Novartis Pharmaceuticals sponsored the study, designed the study, and collected the data together with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. Four coinvestigators had no disclosures.
Lenalidomide eliminated transfusion dependence for a minority of non-del(5q) MDS patients
Lenalidomide led to independence from red blood cell transfusions for 27% of patients with lower-risk non-del(5q) myelodysplastic syndromes who were refractory to or ineligible for erythropoiesis-stimulating agents, based on the results of a phase III placebo-controlled study.
Patients were most likely to reach this primary endpoint if their baseline erythropoietin (EPO) level was less than 100 mU/mL, Dr. Valeria Santini of the University of Florence (Italy) and her associates wrote online June 27 in the Journal of Clinical Oncology.
Erythropoiesis-stimulating agents (ESAs) are first-line therapy for anemia in lower-risk myelodysplastic syndrome (MDS) patients without a deletion 5q, but most patients stop responding over time. “Although azacitidine and decitabine are approved in the United States and other countries in this setting, no approved treatments exist in the European Union and other countries for ESA-refractory patients who are dependent on red blood cell transfusions,” the researchers wrote.
Their international double-blind study enrolled 239 adults with International Prognostic Scoring System lower- or intermediate-risk MDS who lacked the del(5q) mutation and needed at least 2 U of packed red blood cells every 28 days. Patients were randomly assigned 2:1 to lenalidomide (160 patients) or placebo (79 patients), given once daily for 28-day cycles. Patients whose creatinine clearance was 40-60 mL/min received 5 mg lenalidomide, while the rest received 10 mg. Randomization was stratified based on MDS treatment history, baseline transfusion requirements, and time from MDS diagnosis (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2015.66.0118). A total of 43 lenalidomide patients (27%) and 2 placebo patients (2.5%; P less than .001) did not require packed red blood cell transfusions for at least 8 weeks. Transfusion independence persisted through 24 weeks for 17% of lenalidomide patients and no placebo patients. Of 39 patients given 5-mg lenalidomide, 18% reached the primary endpoint (P = .006, compared with placebo). The median duration of response was nearly 31 weeks (95% confidence interval, 21-59 weeks). Prior use of ESAs was the only significant predictor of response in the multivariate analysis (odds ratio, 4.6; 95% confidence interval, 1.3-16.1; P = .01), although low baseline transfusion burden reached significance in the univariate model and borderline significance in the multivariate model (OR, 2.7; 95% CI, 0.96-7.6; P = .06).
The highest response rate (43%) occurred among the 40 patients who had previously received ESAs and had a baseline erythropoietin level under 100 mU/mL. The response rates for other patients previously treated with ESAs fell as baseline EPO level increased, and the response rate was only 9% among patients whose relatively high endogenous EPO level had made them ineligible for ESA treatment. “Patients with higher endogenous EPO levels may be less responsive due to marked intrinsic defects in erythroid signaling pathways, including signal transducer and activator of transcription extracellular regulated kinase 1/2, and lipid raft assembly, which are restored or stimulated by lenalidomide exposure,” the researchers wrote.
As in previous studies, grade 3-4 adverse events with lenalidomide usually were due to myelosuppression. Grade 3-4 neutropenia affected 99 lenalidomide patients (62%) and 10 placebo patients (13%). Grade 3-4 thrombocytopenia affected 57 lenalidomide patients (36%) and 3 placebo patients (4%). Lenalidomide was associated with a 3% rate of venous thrombosis but did not cause detectable pulmonary embolism. Lenalidomide was stopped in 32% of patients because of thrombocytopenia, neutropenia, or other adverse events, compared with 11% of placebo patients. In all, 2.5% of patients in each group died while on treatment.
Celgene makes lenalidomide and funded the study. Dr. Santini disclosed consulting or advisory roles and honoraria from Celgene, Janssen Pharmaceuticals, and Novartis. Seventeen coinvestigators also disclosed ties to Celgene.
Have goals of therapy been met in treating patients with lower-risk, non-del(5q) myelodysplastic syndromes with lenalidomide? For patients requiring a median of 3 U of packed red blood cells monthly, approximately one-quarter will respond to the drug for a median of little more than 7 months. In exchange, nearly three-quarters of patients will take a pill unnecessarily, with most experiencing cytopenias, for a median of more than 5 months. These data were submitted to the FDA in a supplementary new drug application for an expanded indication for lenalidomide in the non-del(5q) MDS population. The submission was subsequently withdrawn, however, when further analyses and data were requested to support the risk-benefit assessment.
For the majority of these patients, then, neither quality of life nor transfusion requirements improved, and lenalidomide use in patients with non-del(5q) MDS will continue to be relegated to the off-label realm. For a tantalizing few, including at least one patient whose response lasted for 5 years, the drug is extremely effective, and it is now our responsibility, as clinical and translational scientists, to determine how we can better identify those people, and make the long day’s journey into night even longer.
Dr. Mikkael A. Sekeres is at the Cleveland Clinic. He disclosed advisory or consulting ties to Celgene, the maker of lenalidomide. These comments are from his accompanying editorial (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2016.68.2492).
Have goals of therapy been met in treating patients with lower-risk, non-del(5q) myelodysplastic syndromes with lenalidomide? For patients requiring a median of 3 U of packed red blood cells monthly, approximately one-quarter will respond to the drug for a median of little more than 7 months. In exchange, nearly three-quarters of patients will take a pill unnecessarily, with most experiencing cytopenias, for a median of more than 5 months. These data were submitted to the FDA in a supplementary new drug application for an expanded indication for lenalidomide in the non-del(5q) MDS population. The submission was subsequently withdrawn, however, when further analyses and data were requested to support the risk-benefit assessment.
For the majority of these patients, then, neither quality of life nor transfusion requirements improved, and lenalidomide use in patients with non-del(5q) MDS will continue to be relegated to the off-label realm. For a tantalizing few, including at least one patient whose response lasted for 5 years, the drug is extremely effective, and it is now our responsibility, as clinical and translational scientists, to determine how we can better identify those people, and make the long day’s journey into night even longer.
Dr. Mikkael A. Sekeres is at the Cleveland Clinic. He disclosed advisory or consulting ties to Celgene, the maker of lenalidomide. These comments are from his accompanying editorial (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2016.68.2492).
Have goals of therapy been met in treating patients with lower-risk, non-del(5q) myelodysplastic syndromes with lenalidomide? For patients requiring a median of 3 U of packed red blood cells monthly, approximately one-quarter will respond to the drug for a median of little more than 7 months. In exchange, nearly three-quarters of patients will take a pill unnecessarily, with most experiencing cytopenias, for a median of more than 5 months. These data were submitted to the FDA in a supplementary new drug application for an expanded indication for lenalidomide in the non-del(5q) MDS population. The submission was subsequently withdrawn, however, when further analyses and data were requested to support the risk-benefit assessment.
For the majority of these patients, then, neither quality of life nor transfusion requirements improved, and lenalidomide use in patients with non-del(5q) MDS will continue to be relegated to the off-label realm. For a tantalizing few, including at least one patient whose response lasted for 5 years, the drug is extremely effective, and it is now our responsibility, as clinical and translational scientists, to determine how we can better identify those people, and make the long day’s journey into night even longer.
Dr. Mikkael A. Sekeres is at the Cleveland Clinic. He disclosed advisory or consulting ties to Celgene, the maker of lenalidomide. These comments are from his accompanying editorial (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2016.68.2492).
Lenalidomide led to independence from red blood cell transfusions for 27% of patients with lower-risk non-del(5q) myelodysplastic syndromes who were refractory to or ineligible for erythropoiesis-stimulating agents, based on the results of a phase III placebo-controlled study.
Patients were most likely to reach this primary endpoint if their baseline erythropoietin (EPO) level was less than 100 mU/mL, Dr. Valeria Santini of the University of Florence (Italy) and her associates wrote online June 27 in the Journal of Clinical Oncology.
Erythropoiesis-stimulating agents (ESAs) are first-line therapy for anemia in lower-risk myelodysplastic syndrome (MDS) patients without a deletion 5q, but most patients stop responding over time. “Although azacitidine and decitabine are approved in the United States and other countries in this setting, no approved treatments exist in the European Union and other countries for ESA-refractory patients who are dependent on red blood cell transfusions,” the researchers wrote.
Their international double-blind study enrolled 239 adults with International Prognostic Scoring System lower- or intermediate-risk MDS who lacked the del(5q) mutation and needed at least 2 U of packed red blood cells every 28 days. Patients were randomly assigned 2:1 to lenalidomide (160 patients) or placebo (79 patients), given once daily for 28-day cycles. Patients whose creatinine clearance was 40-60 mL/min received 5 mg lenalidomide, while the rest received 10 mg. Randomization was stratified based on MDS treatment history, baseline transfusion requirements, and time from MDS diagnosis (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2015.66.0118). A total of 43 lenalidomide patients (27%) and 2 placebo patients (2.5%; P less than .001) did not require packed red blood cell transfusions for at least 8 weeks. Transfusion independence persisted through 24 weeks for 17% of lenalidomide patients and no placebo patients. Of 39 patients given 5-mg lenalidomide, 18% reached the primary endpoint (P = .006, compared with placebo). The median duration of response was nearly 31 weeks (95% confidence interval, 21-59 weeks). Prior use of ESAs was the only significant predictor of response in the multivariate analysis (odds ratio, 4.6; 95% confidence interval, 1.3-16.1; P = .01), although low baseline transfusion burden reached significance in the univariate model and borderline significance in the multivariate model (OR, 2.7; 95% CI, 0.96-7.6; P = .06).
The highest response rate (43%) occurred among the 40 patients who had previously received ESAs and had a baseline erythropoietin level under 100 mU/mL. The response rates for other patients previously treated with ESAs fell as baseline EPO level increased, and the response rate was only 9% among patients whose relatively high endogenous EPO level had made them ineligible for ESA treatment. “Patients with higher endogenous EPO levels may be less responsive due to marked intrinsic defects in erythroid signaling pathways, including signal transducer and activator of transcription extracellular regulated kinase 1/2, and lipid raft assembly, which are restored or stimulated by lenalidomide exposure,” the researchers wrote.
As in previous studies, grade 3-4 adverse events with lenalidomide usually were due to myelosuppression. Grade 3-4 neutropenia affected 99 lenalidomide patients (62%) and 10 placebo patients (13%). Grade 3-4 thrombocytopenia affected 57 lenalidomide patients (36%) and 3 placebo patients (4%). Lenalidomide was associated with a 3% rate of venous thrombosis but did not cause detectable pulmonary embolism. Lenalidomide was stopped in 32% of patients because of thrombocytopenia, neutropenia, or other adverse events, compared with 11% of placebo patients. In all, 2.5% of patients in each group died while on treatment.
Celgene makes lenalidomide and funded the study. Dr. Santini disclosed consulting or advisory roles and honoraria from Celgene, Janssen Pharmaceuticals, and Novartis. Seventeen coinvestigators also disclosed ties to Celgene.
Lenalidomide led to independence from red blood cell transfusions for 27% of patients with lower-risk non-del(5q) myelodysplastic syndromes who were refractory to or ineligible for erythropoiesis-stimulating agents, based on the results of a phase III placebo-controlled study.
Patients were most likely to reach this primary endpoint if their baseline erythropoietin (EPO) level was less than 100 mU/mL, Dr. Valeria Santini of the University of Florence (Italy) and her associates wrote online June 27 in the Journal of Clinical Oncology.
Erythropoiesis-stimulating agents (ESAs) are first-line therapy for anemia in lower-risk myelodysplastic syndrome (MDS) patients without a deletion 5q, but most patients stop responding over time. “Although azacitidine and decitabine are approved in the United States and other countries in this setting, no approved treatments exist in the European Union and other countries for ESA-refractory patients who are dependent on red blood cell transfusions,” the researchers wrote.
Their international double-blind study enrolled 239 adults with International Prognostic Scoring System lower- or intermediate-risk MDS who lacked the del(5q) mutation and needed at least 2 U of packed red blood cells every 28 days. Patients were randomly assigned 2:1 to lenalidomide (160 patients) or placebo (79 patients), given once daily for 28-day cycles. Patients whose creatinine clearance was 40-60 mL/min received 5 mg lenalidomide, while the rest received 10 mg. Randomization was stratified based on MDS treatment history, baseline transfusion requirements, and time from MDS diagnosis (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2015.66.0118). A total of 43 lenalidomide patients (27%) and 2 placebo patients (2.5%; P less than .001) did not require packed red blood cell transfusions for at least 8 weeks. Transfusion independence persisted through 24 weeks for 17% of lenalidomide patients and no placebo patients. Of 39 patients given 5-mg lenalidomide, 18% reached the primary endpoint (P = .006, compared with placebo). The median duration of response was nearly 31 weeks (95% confidence interval, 21-59 weeks). Prior use of ESAs was the only significant predictor of response in the multivariate analysis (odds ratio, 4.6; 95% confidence interval, 1.3-16.1; P = .01), although low baseline transfusion burden reached significance in the univariate model and borderline significance in the multivariate model (OR, 2.7; 95% CI, 0.96-7.6; P = .06).
The highest response rate (43%) occurred among the 40 patients who had previously received ESAs and had a baseline erythropoietin level under 100 mU/mL. The response rates for other patients previously treated with ESAs fell as baseline EPO level increased, and the response rate was only 9% among patients whose relatively high endogenous EPO level had made them ineligible for ESA treatment. “Patients with higher endogenous EPO levels may be less responsive due to marked intrinsic defects in erythroid signaling pathways, including signal transducer and activator of transcription extracellular regulated kinase 1/2, and lipid raft assembly, which are restored or stimulated by lenalidomide exposure,” the researchers wrote.
As in previous studies, grade 3-4 adverse events with lenalidomide usually were due to myelosuppression. Grade 3-4 neutropenia affected 99 lenalidomide patients (62%) and 10 placebo patients (13%). Grade 3-4 thrombocytopenia affected 57 lenalidomide patients (36%) and 3 placebo patients (4%). Lenalidomide was associated with a 3% rate of venous thrombosis but did not cause detectable pulmonary embolism. Lenalidomide was stopped in 32% of patients because of thrombocytopenia, neutropenia, or other adverse events, compared with 11% of placebo patients. In all, 2.5% of patients in each group died while on treatment.
Celgene makes lenalidomide and funded the study. Dr. Santini disclosed consulting or advisory roles and honoraria from Celgene, Janssen Pharmaceuticals, and Novartis. Seventeen coinvestigators also disclosed ties to Celgene.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Lenalidomide usually does not free patients with lower-risk non-del(5q) myelodysplastic syndromes who are refractory to or ineligible for erythropoiesis-stimulating agents from needing packed red blood cell transfusions.
Major finding: A total of 27% of lenalidomide-treated and 2.5% of placebo-treated patients did not need transfusions for at least 8 weeks while on treatment (P less than .001).
Data source: An international randomized, double-blind phase III study of 239 adults with non-del(5q) MDS who were ineligible for or refractory to ESAs.
Disclosures: Celgene makes lenalidomide and funded the study. Dr. Santini disclosed consulting or advisory roles and honoraria from Celgene, Janssen Pharmaceuticals, and Novartis. Seventeen coinvestigators also disclosed ties to Celgene.
CABG tops PCI for nondiabetic patients with multivessel CAD
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Coronary artery bypass graft is superior to percutaneous coronary intervention for nondiabetic patients with multivessel coronary artery disease.
Major finding: All-cause mortality rates were 6% for CABG and 9.3% for PCI (HR, 0.65; P = .039).
Data source: A pooled analysis of 1,275 nondiabetic patients with two- or three-vessel CAD from the SYNTAX and BEST trials.
Disclosures: The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
Lisinopril tied to 19% drop in conduction system disease
Compared with chlorthalidone, the angiotensin-converting enzyme inhibitor lisinopril was associated with a 19% drop in new-onset conduction system disease among high-risk patients with hypertension, based on a secondary analysis of the randomized, double-blind Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Amlodipine besylate and pravastatin did not affect rates of conduction defects, added Dr. Thomas A. Dewland of Oregon Health and Science University, Portland, and his associates, who reported the findings online June 27 in JAMA Internal Medicine.
ALLHAT was a randomized, double-blind trial comparing chlorthalidone, amlodipine, lisinopril, or doxazosin mesylate in patients with hypertension and at least one other cardiac risk factor. Patients with elevated fasting low-density lipoprotein levels also were randomized to pravastatin sodium or usual care. Most (56%) patients were men, and the average age was about 67 years (standard deviation, 7.3 years). Twelve-lead electrocardiograms were obtained every 2 years, enabling the current analysis, the researchers said. In all, 21,004 patients without a baseline pacemaker or conduction system disease were followed for an average of 5 years (JAMA Intern Med. 2016 Jun 27. doi: 10.1001/jamainternmed.2016.250).
A total of 1,114 patients developed new-onset conduction system disease (3 events per 1,000 person-years), including 389 patients with left bundle branch block (4.5 events per 1,000 person-years), 570 patients with right bundle branch block (6.6 events per 1,000 person-years), and 155 patients with intraventricular conduction delay. The risk of conduction system disease was 19% lower with lisinopril than chlorthalidone (hazard ratio, 0.81; 95% confidence interval, 0.69-0.95; P = .01). Neither amlodipine nor pravastatin significantly affected the chances of conduction system disease, but patients who were older, white, or had diabetes or left ventricular hypertrophy were at significantly greater risk than the overall cohort.
“The antifibrotic effects of angiotensin-converting enzyme inhibitors may prevent or slow the development of conduction system disease, and future research is warranted to understand whether this treatment affects other conduction-related clinical outcomes,” including the need for pacemaker implantation, the researchers concluded.
The study was supported by the American Heart Association, the Joseph Drown Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by Pfizer. The investigators had no disclosures.
To our knowledge, this is the first report that incident conduction system disease may be prevented by drug therapy, in this case lisinopril. This important observation is consistent with established knowledge that angiotensin-converting enzyme inhibition has salutary effects on inflammation, hypertrophy, and fibrosis. We look forward to future studies to confirm that ECG conduction disease can indeed be prevented or progression slowed by relatively simple interventions, to define those individuals most likely to benefit, and to assess whether such preventive strategies can indeed ward off the need for invasive procedures such as pacemakers in selected patient subsets. This is indeed an exciting prospect!
Dr. Sanjiv M. Narayan, Dr. Tina Baykaner, and Dr. David J. Maron are at Falk Cardiovascular Research Center, Stanford University, Palo Alto, California. Dr. Narayan reported receiving research support from the National Institutes of Health; being coinventor of intellectual property owned by the University of California and licensed to Topera, in which he held equity; and receiving consulting fees from the American College of Cardiology and Uptodate.com and speaking fees from St. Jude Medical and Medtronic. The other authors had no disclosures. These comments are from their editorial (JAMA Intern Med. 2016 Jun 27. doi: 10.1001 /jamainternmed.2016.2502).
To our knowledge, this is the first report that incident conduction system disease may be prevented by drug therapy, in this case lisinopril. This important observation is consistent with established knowledge that angiotensin-converting enzyme inhibition has salutary effects on inflammation, hypertrophy, and fibrosis. We look forward to future studies to confirm that ECG conduction disease can indeed be prevented or progression slowed by relatively simple interventions, to define those individuals most likely to benefit, and to assess whether such preventive strategies can indeed ward off the need for invasive procedures such as pacemakers in selected patient subsets. This is indeed an exciting prospect!
Dr. Sanjiv M. Narayan, Dr. Tina Baykaner, and Dr. David J. Maron are at Falk Cardiovascular Research Center, Stanford University, Palo Alto, California. Dr. Narayan reported receiving research support from the National Institutes of Health; being coinventor of intellectual property owned by the University of California and licensed to Topera, in which he held equity; and receiving consulting fees from the American College of Cardiology and Uptodate.com and speaking fees from St. Jude Medical and Medtronic. The other authors had no disclosures. These comments are from their editorial (JAMA Intern Med. 2016 Jun 27. doi: 10.1001 /jamainternmed.2016.2502).
To our knowledge, this is the first report that incident conduction system disease may be prevented by drug therapy, in this case lisinopril. This important observation is consistent with established knowledge that angiotensin-converting enzyme inhibition has salutary effects on inflammation, hypertrophy, and fibrosis. We look forward to future studies to confirm that ECG conduction disease can indeed be prevented or progression slowed by relatively simple interventions, to define those individuals most likely to benefit, and to assess whether such preventive strategies can indeed ward off the need for invasive procedures such as pacemakers in selected patient subsets. This is indeed an exciting prospect!
Dr. Sanjiv M. Narayan, Dr. Tina Baykaner, and Dr. David J. Maron are at Falk Cardiovascular Research Center, Stanford University, Palo Alto, California. Dr. Narayan reported receiving research support from the National Institutes of Health; being coinventor of intellectual property owned by the University of California and licensed to Topera, in which he held equity; and receiving consulting fees from the American College of Cardiology and Uptodate.com and speaking fees from St. Jude Medical and Medtronic. The other authors had no disclosures. These comments are from their editorial (JAMA Intern Med. 2016 Jun 27. doi: 10.1001 /jamainternmed.2016.2502).
Compared with chlorthalidone, the angiotensin-converting enzyme inhibitor lisinopril was associated with a 19% drop in new-onset conduction system disease among high-risk patients with hypertension, based on a secondary analysis of the randomized, double-blind Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Amlodipine besylate and pravastatin did not affect rates of conduction defects, added Dr. Thomas A. Dewland of Oregon Health and Science University, Portland, and his associates, who reported the findings online June 27 in JAMA Internal Medicine.
ALLHAT was a randomized, double-blind trial comparing chlorthalidone, amlodipine, lisinopril, or doxazosin mesylate in patients with hypertension and at least one other cardiac risk factor. Patients with elevated fasting low-density lipoprotein levels also were randomized to pravastatin sodium or usual care. Most (56%) patients were men, and the average age was about 67 years (standard deviation, 7.3 years). Twelve-lead electrocardiograms were obtained every 2 years, enabling the current analysis, the researchers said. In all, 21,004 patients without a baseline pacemaker or conduction system disease were followed for an average of 5 years (JAMA Intern Med. 2016 Jun 27. doi: 10.1001/jamainternmed.2016.250).
A total of 1,114 patients developed new-onset conduction system disease (3 events per 1,000 person-years), including 389 patients with left bundle branch block (4.5 events per 1,000 person-years), 570 patients with right bundle branch block (6.6 events per 1,000 person-years), and 155 patients with intraventricular conduction delay. The risk of conduction system disease was 19% lower with lisinopril than chlorthalidone (hazard ratio, 0.81; 95% confidence interval, 0.69-0.95; P = .01). Neither amlodipine nor pravastatin significantly affected the chances of conduction system disease, but patients who were older, white, or had diabetes or left ventricular hypertrophy were at significantly greater risk than the overall cohort.
“The antifibrotic effects of angiotensin-converting enzyme inhibitors may prevent or slow the development of conduction system disease, and future research is warranted to understand whether this treatment affects other conduction-related clinical outcomes,” including the need for pacemaker implantation, the researchers concluded.
The study was supported by the American Heart Association, the Joseph Drown Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by Pfizer. The investigators had no disclosures.
Compared with chlorthalidone, the angiotensin-converting enzyme inhibitor lisinopril was associated with a 19% drop in new-onset conduction system disease among high-risk patients with hypertension, based on a secondary analysis of the randomized, double-blind Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Amlodipine besylate and pravastatin did not affect rates of conduction defects, added Dr. Thomas A. Dewland of Oregon Health and Science University, Portland, and his associates, who reported the findings online June 27 in JAMA Internal Medicine.
ALLHAT was a randomized, double-blind trial comparing chlorthalidone, amlodipine, lisinopril, or doxazosin mesylate in patients with hypertension and at least one other cardiac risk factor. Patients with elevated fasting low-density lipoprotein levels also were randomized to pravastatin sodium or usual care. Most (56%) patients were men, and the average age was about 67 years (standard deviation, 7.3 years). Twelve-lead electrocardiograms were obtained every 2 years, enabling the current analysis, the researchers said. In all, 21,004 patients without a baseline pacemaker or conduction system disease were followed for an average of 5 years (JAMA Intern Med. 2016 Jun 27. doi: 10.1001/jamainternmed.2016.250).
A total of 1,114 patients developed new-onset conduction system disease (3 events per 1,000 person-years), including 389 patients with left bundle branch block (4.5 events per 1,000 person-years), 570 patients with right bundle branch block (6.6 events per 1,000 person-years), and 155 patients with intraventricular conduction delay. The risk of conduction system disease was 19% lower with lisinopril than chlorthalidone (hazard ratio, 0.81; 95% confidence interval, 0.69-0.95; P = .01). Neither amlodipine nor pravastatin significantly affected the chances of conduction system disease, but patients who were older, white, or had diabetes or left ventricular hypertrophy were at significantly greater risk than the overall cohort.
“The antifibrotic effects of angiotensin-converting enzyme inhibitors may prevent or slow the development of conduction system disease, and future research is warranted to understand whether this treatment affects other conduction-related clinical outcomes,” including the need for pacemaker implantation, the researchers concluded.
The study was supported by the American Heart Association, the Joseph Drown Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by Pfizer. The investigators had no disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Compared with chlorthalidone, the angiotensin-converting enzyme inhibitor lisinopril was associated with a significant drop in new-onset conduction system disease among high-risk patients with hypertension.
Major finding: The risk of conduction system disease was 19% lower with lisinopril than chlorthalidone (hazard ratio, 0.81; 95% CI, 0.69 to 0.95; P = .01).
Data source: Serial 12-lead electrocardiograms of 21,004 patients with hypertension and at least one other cardiac risk factor, with an average of 5 years of follow-up.
Disclosures: The study was supported by the American Heart Association, the Joseph Drown Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by Pfizer, Inc. The investigators had no disclosures.
July 2016: Click for Credit
Here are 4 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Pregnancy Alters Pharmacodynamics of Anti-TNF Agents in Women With IBD
To take the posttest, go to: http://bit.ly/1VQFIHf
Expires May 24, 2017
VITALS
Key clinical point: Blood levels of infliximab rose during pregnancy, while adalimumab levels remained stable, even after researchers accounted for changes in albumin, body mass index, and C-reactive protein levels.
Major finding: Median infliximab concentrations rose from 8.5 mcg/mL in the first trimester to a peak of 21 mcg/mL during the middle of the third trimester (P = .04). Median adalimumab levels ranged between 8.6 and 12.2 mcg/mL during pregnancy.
Data source: A prospective study of 25 pregnant women with ulcerative colitis or Crohn's disease.
Disclosures: Dr. Seow disclosed ties with Janssen, AbbVie, Takeda, Shire, and Actavis.
2. Vascular Disease Linked to Sight Loss in Giant Cell Arteritis
To take the posttest, go to: http://bit.ly/1UqLuu5
Expires May 10, 2017
VITALS
Key clinical point: Patients with vascular disease who develop giant cell arteritis may require careful monitoring for sight loss.
Major finding: Overall, 42.9% of patients had some visual disturbance at first clinic review; 7.9% were blind at 6 months.
Data source: Analysis of 433 patients newly diagnosed with GCA participating in the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS).
Disclosures: The DCVAS study is supported by the American College of Rheumatology and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
3. Pediatric and Adolescent Mental Health
Part 1: Diagnoses, drug prescribing vary widely
To take the posttest, go to: http://bit.ly/24FHTxY
Expires April 1, 2017
VITALS
Key clinical point: A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications given in practices nationwide, a study has shown.
Major finding: Nationwide, 15% of pediatric patients received a mental health diagnosis, and 14% were prescribed psychotropic medications in primary care, regardless of colocated mental health services.
Data source: A retrospective study of electronic health records for 294,748 patients aged 4-18 years.
Disclosures: Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
Part 2: Disorders prevalent in young transgender women
To take the posttest, go to: http://bit.ly/24FCDdq
Expires March 21, 2017
VITALS
Key clinical point: Young transgender women have a high prevalence of psychiatric disorders that is two to four times higher than that in the general population.
Major finding: 41.5% of the study participants had at least one psychiatric disorder, such as major depressive disorder, suicidality, generalized anxiety, PTSD, and alcohol or substance dependence.
Data source: An observational cohort study involving 298 transgender women aged 16-29 years residing in Chicago and Boston.
Disclosures: This study was supported by the National Institute of Mental Health. Dr. Reisner and his associates reported having no relevant financial disclosures.
Here are 4 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Pregnancy Alters Pharmacodynamics of Anti-TNF Agents in Women With IBD
To take the posttest, go to: http://bit.ly/1VQFIHf
Expires May 24, 2017
VITALS
Key clinical point: Blood levels of infliximab rose during pregnancy, while adalimumab levels remained stable, even after researchers accounted for changes in albumin, body mass index, and C-reactive protein levels.
Major finding: Median infliximab concentrations rose from 8.5 mcg/mL in the first trimester to a peak of 21 mcg/mL during the middle of the third trimester (P = .04). Median adalimumab levels ranged between 8.6 and 12.2 mcg/mL during pregnancy.
Data source: A prospective study of 25 pregnant women with ulcerative colitis or Crohn's disease.
Disclosures: Dr. Seow disclosed ties with Janssen, AbbVie, Takeda, Shire, and Actavis.
2. Vascular Disease Linked to Sight Loss in Giant Cell Arteritis
To take the posttest, go to: http://bit.ly/1UqLuu5
Expires May 10, 2017
VITALS
Key clinical point: Patients with vascular disease who develop giant cell arteritis may require careful monitoring for sight loss.
Major finding: Overall, 42.9% of patients had some visual disturbance at first clinic review; 7.9% were blind at 6 months.
Data source: Analysis of 433 patients newly diagnosed with GCA participating in the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS).
Disclosures: The DCVAS study is supported by the American College of Rheumatology and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
3. Pediatric and Adolescent Mental Health
Part 1: Diagnoses, drug prescribing vary widely
To take the posttest, go to: http://bit.ly/24FHTxY
Expires April 1, 2017
VITALS
Key clinical point: A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications given in practices nationwide, a study has shown.
Major finding: Nationwide, 15% of pediatric patients received a mental health diagnosis, and 14% were prescribed psychotropic medications in primary care, regardless of colocated mental health services.
Data source: A retrospective study of electronic health records for 294,748 patients aged 4-18 years.
Disclosures: Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
Part 2: Disorders prevalent in young transgender women
To take the posttest, go to: http://bit.ly/24FCDdq
Expires March 21, 2017
VITALS
Key clinical point: Young transgender women have a high prevalence of psychiatric disorders that is two to four times higher than that in the general population.
Major finding: 41.5% of the study participants had at least one psychiatric disorder, such as major depressive disorder, suicidality, generalized anxiety, PTSD, and alcohol or substance dependence.
Data source: An observational cohort study involving 298 transgender women aged 16-29 years residing in Chicago and Boston.
Disclosures: This study was supported by the National Institute of Mental Health. Dr. Reisner and his associates reported having no relevant financial disclosures.
Here are 4 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Pregnancy Alters Pharmacodynamics of Anti-TNF Agents in Women With IBD
To take the posttest, go to: http://bit.ly/1VQFIHf
Expires May 24, 2017
VITALS
Key clinical point: Blood levels of infliximab rose during pregnancy, while adalimumab levels remained stable, even after researchers accounted for changes in albumin, body mass index, and C-reactive protein levels.
Major finding: Median infliximab concentrations rose from 8.5 mcg/mL in the first trimester to a peak of 21 mcg/mL during the middle of the third trimester (P = .04). Median adalimumab levels ranged between 8.6 and 12.2 mcg/mL during pregnancy.
Data source: A prospective study of 25 pregnant women with ulcerative colitis or Crohn's disease.
Disclosures: Dr. Seow disclosed ties with Janssen, AbbVie, Takeda, Shire, and Actavis.
2. Vascular Disease Linked to Sight Loss in Giant Cell Arteritis
To take the posttest, go to: http://bit.ly/1UqLuu5
Expires May 10, 2017
VITALS
Key clinical point: Patients with vascular disease who develop giant cell arteritis may require careful monitoring for sight loss.
Major finding: Overall, 42.9% of patients had some visual disturbance at first clinic review; 7.9% were blind at 6 months.
Data source: Analysis of 433 patients newly diagnosed with GCA participating in the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS).
Disclosures: The DCVAS study is supported by the American College of Rheumatology and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
3. Pediatric and Adolescent Mental Health
Part 1: Diagnoses, drug prescribing vary widely
To take the posttest, go to: http://bit.ly/24FHTxY
Expires April 1, 2017
VITALS
Key clinical point: A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications given in practices nationwide, a study has shown.
Major finding: Nationwide, 15% of pediatric patients received a mental health diagnosis, and 14% were prescribed psychotropic medications in primary care, regardless of colocated mental health services.
Data source: A retrospective study of electronic health records for 294,748 patients aged 4-18 years.
Disclosures: Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
Part 2: Disorders prevalent in young transgender women
To take the posttest, go to: http://bit.ly/24FCDdq
Expires March 21, 2017
VITALS
Key clinical point: Young transgender women have a high prevalence of psychiatric disorders that is two to four times higher than that in the general population.
Major finding: 41.5% of the study participants had at least one psychiatric disorder, such as major depressive disorder, suicidality, generalized anxiety, PTSD, and alcohol or substance dependence.
Data source: An observational cohort study involving 298 transgender women aged 16-29 years residing in Chicago and Boston.
Disclosures: This study was supported by the National Institute of Mental Health. Dr. Reisner and his associates reported having no relevant financial disclosures.
SEER data underscore mortality associated with thin melanomas
SCOTTSDALE, ARIZ. – Thin melanomas account for most melanoma deaths, even though T4 lesions have the worst prognosis, according to an analysis of melanoma data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
In 2015, a study from Queensland, Australia, reported that more patients died from T1 melanomas than did from T4 melanomas (J Invest Dermatol. 2015 Apr;135:1190-3). But a similar analysis in the United States was not available, so Dr. Shoshana Landow, a dermatologist at the Providence (R.I.) Veterans Affairs Medical Center,and her associates extracted SEER 13 data for invasive melanomas between 1992 through 2003. These registries covered Atlanta, Connecticut, Detroit, rural Georgia, Hawaii, Iowa, Los Angeles, New Mexico, San Francisco-Oakland, San Jose-Monterey, Seattle-Puget Sound, Utah, and the Alaska Native Tumor Registry, according to the SEER website.
Among 105,264 recorded melanomas during this period, 37,210 tumors were in situ, the investigators reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. They excluded those cases, as well as 4,789 cases that involved more than one primary melanoma, 8,637 that extended beyond the skin at diagnosis, 5,308 with no record of thickness, and one that was not known to be primary.
That left 49,319 invasive melanomas, of which 35,509 (72%) were T1 (0.01-1.00mm), 7,879 (16%) were T2 (1.01-2.00 mm), 3,948 (8%) were T3 (2.01-4.00 mm), and 1,983 (4%) were T4 (more than 4.00 mm). A total of 17% of the T1 lesions were 0.01-0.25 mm thick at diagnosis, while 42% were 0.26-0.50 mm thick, 25% were 0.51-0.75 mm thick, and 16% were 0.76-1.00 mm thick.
Ten years after diagnosis, 3,660 (7.4%) patients had died of melanoma, according to the study. These deaths included 1,072 T1 patients, 974 T2 patients, 985 T3 patients, and 629 T4 patients. But while T4 lesions accounted for the fewest number of deaths and made up only 4% of all invasive melanomas, the 10-year mortality rate for T4 lesions was nearly 32%, vs. 25% for T3 lesions, 12% for T2 lesions, and 3% for T1 lesions.
Most (42%) T1 lesions were 0.26-0.50 mm thick, 25% of T1 lesions were 0.51-0.75 mm thick, and the remaining T1 lesions were nearly evenly split between the thinnest (0.01-0.25 mm) and the thickest (0.76-1.00mm) categories, the researchers also reported. When considering only the T1 lesions, 10-year mortality rates were 3% for the 0.01-0.25 mm category, 1.9% for the 0.26-0.5 mm category, 3.7% for the 0.51-0.75 mm category, and 5.8% for the 0.76-1.00 mm category.
Thus, the thinnest invasive melanomas had a higher 10-year death rate than did the next-thinnest category, a finding that “demands explanation” and was not caused by ulceration, the researchers noted.
“The greatest total number of deaths was from T1 melanomas and the smallest number from T4, despite the fact that prognosis worsened as melanoma thickened from T1 to T4,” they concluded in their poster. “Our findings highlight the heavy death toll of thin melanomas.”
The authors did not specify funding sources, and had no disclosures.
SCOTTSDALE, ARIZ. – Thin melanomas account for most melanoma deaths, even though T4 lesions have the worst prognosis, according to an analysis of melanoma data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
In 2015, a study from Queensland, Australia, reported that more patients died from T1 melanomas than did from T4 melanomas (J Invest Dermatol. 2015 Apr;135:1190-3). But a similar analysis in the United States was not available, so Dr. Shoshana Landow, a dermatologist at the Providence (R.I.) Veterans Affairs Medical Center,and her associates extracted SEER 13 data for invasive melanomas between 1992 through 2003. These registries covered Atlanta, Connecticut, Detroit, rural Georgia, Hawaii, Iowa, Los Angeles, New Mexico, San Francisco-Oakland, San Jose-Monterey, Seattle-Puget Sound, Utah, and the Alaska Native Tumor Registry, according to the SEER website.
Among 105,264 recorded melanomas during this period, 37,210 tumors were in situ, the investigators reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. They excluded those cases, as well as 4,789 cases that involved more than one primary melanoma, 8,637 that extended beyond the skin at diagnosis, 5,308 with no record of thickness, and one that was not known to be primary.
That left 49,319 invasive melanomas, of which 35,509 (72%) were T1 (0.01-1.00mm), 7,879 (16%) were T2 (1.01-2.00 mm), 3,948 (8%) were T3 (2.01-4.00 mm), and 1,983 (4%) were T4 (more than 4.00 mm). A total of 17% of the T1 lesions were 0.01-0.25 mm thick at diagnosis, while 42% were 0.26-0.50 mm thick, 25% were 0.51-0.75 mm thick, and 16% were 0.76-1.00 mm thick.
Ten years after diagnosis, 3,660 (7.4%) patients had died of melanoma, according to the study. These deaths included 1,072 T1 patients, 974 T2 patients, 985 T3 patients, and 629 T4 patients. But while T4 lesions accounted for the fewest number of deaths and made up only 4% of all invasive melanomas, the 10-year mortality rate for T4 lesions was nearly 32%, vs. 25% for T3 lesions, 12% for T2 lesions, and 3% for T1 lesions.
Most (42%) T1 lesions were 0.26-0.50 mm thick, 25% of T1 lesions were 0.51-0.75 mm thick, and the remaining T1 lesions were nearly evenly split between the thinnest (0.01-0.25 mm) and the thickest (0.76-1.00mm) categories, the researchers also reported. When considering only the T1 lesions, 10-year mortality rates were 3% for the 0.01-0.25 mm category, 1.9% for the 0.26-0.5 mm category, 3.7% for the 0.51-0.75 mm category, and 5.8% for the 0.76-1.00 mm category.
Thus, the thinnest invasive melanomas had a higher 10-year death rate than did the next-thinnest category, a finding that “demands explanation” and was not caused by ulceration, the researchers noted.
“The greatest total number of deaths was from T1 melanomas and the smallest number from T4, despite the fact that prognosis worsened as melanoma thickened from T1 to T4,” they concluded in their poster. “Our findings highlight the heavy death toll of thin melanomas.”
The authors did not specify funding sources, and had no disclosures.
SCOTTSDALE, ARIZ. – Thin melanomas account for most melanoma deaths, even though T4 lesions have the worst prognosis, according to an analysis of melanoma data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
In 2015, a study from Queensland, Australia, reported that more patients died from T1 melanomas than did from T4 melanomas (J Invest Dermatol. 2015 Apr;135:1190-3). But a similar analysis in the United States was not available, so Dr. Shoshana Landow, a dermatologist at the Providence (R.I.) Veterans Affairs Medical Center,and her associates extracted SEER 13 data for invasive melanomas between 1992 through 2003. These registries covered Atlanta, Connecticut, Detroit, rural Georgia, Hawaii, Iowa, Los Angeles, New Mexico, San Francisco-Oakland, San Jose-Monterey, Seattle-Puget Sound, Utah, and the Alaska Native Tumor Registry, according to the SEER website.
Among 105,264 recorded melanomas during this period, 37,210 tumors were in situ, the investigators reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. They excluded those cases, as well as 4,789 cases that involved more than one primary melanoma, 8,637 that extended beyond the skin at diagnosis, 5,308 with no record of thickness, and one that was not known to be primary.
That left 49,319 invasive melanomas, of which 35,509 (72%) were T1 (0.01-1.00mm), 7,879 (16%) were T2 (1.01-2.00 mm), 3,948 (8%) were T3 (2.01-4.00 mm), and 1,983 (4%) were T4 (more than 4.00 mm). A total of 17% of the T1 lesions were 0.01-0.25 mm thick at diagnosis, while 42% were 0.26-0.50 mm thick, 25% were 0.51-0.75 mm thick, and 16% were 0.76-1.00 mm thick.
Ten years after diagnosis, 3,660 (7.4%) patients had died of melanoma, according to the study. These deaths included 1,072 T1 patients, 974 T2 patients, 985 T3 patients, and 629 T4 patients. But while T4 lesions accounted for the fewest number of deaths and made up only 4% of all invasive melanomas, the 10-year mortality rate for T4 lesions was nearly 32%, vs. 25% for T3 lesions, 12% for T2 lesions, and 3% for T1 lesions.
Most (42%) T1 lesions were 0.26-0.50 mm thick, 25% of T1 lesions were 0.51-0.75 mm thick, and the remaining T1 lesions were nearly evenly split between the thinnest (0.01-0.25 mm) and the thickest (0.76-1.00mm) categories, the researchers also reported. When considering only the T1 lesions, 10-year mortality rates were 3% for the 0.01-0.25 mm category, 1.9% for the 0.26-0.5 mm category, 3.7% for the 0.51-0.75 mm category, and 5.8% for the 0.76-1.00 mm category.
Thus, the thinnest invasive melanomas had a higher 10-year death rate than did the next-thinnest category, a finding that “demands explanation” and was not caused by ulceration, the researchers noted.
“The greatest total number of deaths was from T1 melanomas and the smallest number from T4, despite the fact that prognosis worsened as melanoma thickened from T1 to T4,” they concluded in their poster. “Our findings highlight the heavy death toll of thin melanomas.”
The authors did not specify funding sources, and had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Thin (T1) melanomas accounted for the greatest number of deaths from melanoma in a large study.
Major finding: Among 3,660 deaths from invasive melanoma, most (1,072) were in people with T1 lesions. While T4 lesions made up only 4% of invasive melanomas, their 10-year mortality rate was nearly 32%.
Data source: A study of 49,139 invasive melanomas recorded in the NCI’s SEER database.
Disclosures: The investigators did not specify funding sources and had no disclosures.
Study links mismatch repair defects to lower PFS in endometrial cancer
Endometrioid endometrial tumors with DNA mismatch repair (MMR) defects were associated with lower progression-free survival than were tumors that lacked these epigenetic defects, according to a large prospective study published online June 20 in the Journal of Clinical Oncology.
“The overall effect was less than would be expected given the strong association [between MMR defects and] higher grade (66% grade 2 or 3), higher stage (22% stage III or IV), and frequent lymphovascular space invasion (33%),” Dr. Scott McMeekin of the University of Oklahoma Health Sciences Center, Oklahoma City, and his associates wrote. Perhaps MMR-deficient tumors “are eliciting an antitumor immune response, as has been described for POLE ultramutated tumors,” they suggested.
Endometrioid endometrial tumors comprise about 80% of uterine cancers, and about 20%-40% of endometrioid endometrial cancer (EEC) tumors show loss of MMR, but “the relationship between MMR defects and outcomes in patients with endometrial cancer has not been fully established,” the researchers wrote.
Their study looked for links between MMR classes, clinicopathologic features, and outcomes, including response to adjuvant therapy. Understanding these links “will be critical to the design and implementation of trials for treating advanced-stage and recurrent EEC, including biologic therapies such as immune checkpoint blockade,” they wrote (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8722).
The study included 1,043 women with primary EEC prospectively recruited between 2003 and 2007 by the NRG/Gynecologic Oncology Group.
Tumor slides and clinical reports for a total of 1,024 EECs were centrally reviewed, and the tumors were tested for microsatellite instability (MSI), MLH1 methylation, and MMR protein expression. Each tumor was classified as being either MMR normal, having an epigenetic defect, having a probable mutation (that is, an MMR defect not attributable to MLH1 methylation), or as being MSI-low.
Progression-free survival was significantly lower among women whose tumors had epigenetic defects, compared with women whose tumors were MMR normal (hazard ratio, 1.37; 95% confidence interval, 1.00-1.86; P less than .05).
However, trends in disease-specific survival were similar, regardless of MMR type, the researchers wrote. Importantly, an exploratory analysis revealed a possible link between MMR status and response to adjuvant therapy. Specifically, women classified as having probable MMR mutations who received adjuvant therapy had substantially better progression-free survival, with a P-value that trended toward significant (HR, 0.24; 95% CI, 0.05-1.16; P = .07).
“No such effect was seen in the epigenetic defect group, and the differences in outcomes for patients with the two different classes of MMR defect are highly suggestive,” the researchers wrote. “Despite the large size of our study, our power to detect differences in survival is limited by the modest number of cases whose tumors were classified as probable mutation (99, 10% of cohort) and further by the fact that only 26 subjects received adjuvant therapy.”
Since many cancer centers regularly test tumor specimens for MMR defects and MLH1 methylation, “it should be possible to rapidly undertake retrospective studies to validate the findings we report here,” the researchers noted.
Dr. McMeekin reported having no financial disclosures. His coauthors reported ties to several companies, including Repros Therapeutics, Ethicon, Vitatex, Genentech, Janssen Oncology, Tesaro, AstraZeneca, and OvaGene Oncology.
Endometrioid endometrial tumors with DNA mismatch repair (MMR) defects were associated with lower progression-free survival than were tumors that lacked these epigenetic defects, according to a large prospective study published online June 20 in the Journal of Clinical Oncology.
“The overall effect was less than would be expected given the strong association [between MMR defects and] higher grade (66% grade 2 or 3), higher stage (22% stage III or IV), and frequent lymphovascular space invasion (33%),” Dr. Scott McMeekin of the University of Oklahoma Health Sciences Center, Oklahoma City, and his associates wrote. Perhaps MMR-deficient tumors “are eliciting an antitumor immune response, as has been described for POLE ultramutated tumors,” they suggested.
Endometrioid endometrial tumors comprise about 80% of uterine cancers, and about 20%-40% of endometrioid endometrial cancer (EEC) tumors show loss of MMR, but “the relationship between MMR defects and outcomes in patients with endometrial cancer has not been fully established,” the researchers wrote.
Their study looked for links between MMR classes, clinicopathologic features, and outcomes, including response to adjuvant therapy. Understanding these links “will be critical to the design and implementation of trials for treating advanced-stage and recurrent EEC, including biologic therapies such as immune checkpoint blockade,” they wrote (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8722).
The study included 1,043 women with primary EEC prospectively recruited between 2003 and 2007 by the NRG/Gynecologic Oncology Group.
Tumor slides and clinical reports for a total of 1,024 EECs were centrally reviewed, and the tumors were tested for microsatellite instability (MSI), MLH1 methylation, and MMR protein expression. Each tumor was classified as being either MMR normal, having an epigenetic defect, having a probable mutation (that is, an MMR defect not attributable to MLH1 methylation), or as being MSI-low.
Progression-free survival was significantly lower among women whose tumors had epigenetic defects, compared with women whose tumors were MMR normal (hazard ratio, 1.37; 95% confidence interval, 1.00-1.86; P less than .05).
However, trends in disease-specific survival were similar, regardless of MMR type, the researchers wrote. Importantly, an exploratory analysis revealed a possible link between MMR status and response to adjuvant therapy. Specifically, women classified as having probable MMR mutations who received adjuvant therapy had substantially better progression-free survival, with a P-value that trended toward significant (HR, 0.24; 95% CI, 0.05-1.16; P = .07).
“No such effect was seen in the epigenetic defect group, and the differences in outcomes for patients with the two different classes of MMR defect are highly suggestive,” the researchers wrote. “Despite the large size of our study, our power to detect differences in survival is limited by the modest number of cases whose tumors were classified as probable mutation (99, 10% of cohort) and further by the fact that only 26 subjects received adjuvant therapy.”
Since many cancer centers regularly test tumor specimens for MMR defects and MLH1 methylation, “it should be possible to rapidly undertake retrospective studies to validate the findings we report here,” the researchers noted.
Dr. McMeekin reported having no financial disclosures. His coauthors reported ties to several companies, including Repros Therapeutics, Ethicon, Vitatex, Genentech, Janssen Oncology, Tesaro, AstraZeneca, and OvaGene Oncology.
Endometrioid endometrial tumors with DNA mismatch repair (MMR) defects were associated with lower progression-free survival than were tumors that lacked these epigenetic defects, according to a large prospective study published online June 20 in the Journal of Clinical Oncology.
“The overall effect was less than would be expected given the strong association [between MMR defects and] higher grade (66% grade 2 or 3), higher stage (22% stage III or IV), and frequent lymphovascular space invasion (33%),” Dr. Scott McMeekin of the University of Oklahoma Health Sciences Center, Oklahoma City, and his associates wrote. Perhaps MMR-deficient tumors “are eliciting an antitumor immune response, as has been described for POLE ultramutated tumors,” they suggested.
Endometrioid endometrial tumors comprise about 80% of uterine cancers, and about 20%-40% of endometrioid endometrial cancer (EEC) tumors show loss of MMR, but “the relationship between MMR defects and outcomes in patients with endometrial cancer has not been fully established,” the researchers wrote.
Their study looked for links between MMR classes, clinicopathologic features, and outcomes, including response to adjuvant therapy. Understanding these links “will be critical to the design and implementation of trials for treating advanced-stage and recurrent EEC, including biologic therapies such as immune checkpoint blockade,” they wrote (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8722).
The study included 1,043 women with primary EEC prospectively recruited between 2003 and 2007 by the NRG/Gynecologic Oncology Group.
Tumor slides and clinical reports for a total of 1,024 EECs were centrally reviewed, and the tumors were tested for microsatellite instability (MSI), MLH1 methylation, and MMR protein expression. Each tumor was classified as being either MMR normal, having an epigenetic defect, having a probable mutation (that is, an MMR defect not attributable to MLH1 methylation), or as being MSI-low.
Progression-free survival was significantly lower among women whose tumors had epigenetic defects, compared with women whose tumors were MMR normal (hazard ratio, 1.37; 95% confidence interval, 1.00-1.86; P less than .05).
However, trends in disease-specific survival were similar, regardless of MMR type, the researchers wrote. Importantly, an exploratory analysis revealed a possible link between MMR status and response to adjuvant therapy. Specifically, women classified as having probable MMR mutations who received adjuvant therapy had substantially better progression-free survival, with a P-value that trended toward significant (HR, 0.24; 95% CI, 0.05-1.16; P = .07).
“No such effect was seen in the epigenetic defect group, and the differences in outcomes for patients with the two different classes of MMR defect are highly suggestive,” the researchers wrote. “Despite the large size of our study, our power to detect differences in survival is limited by the modest number of cases whose tumors were classified as probable mutation (99, 10% of cohort) and further by the fact that only 26 subjects received adjuvant therapy.”
Since many cancer centers regularly test tumor specimens for MMR defects and MLH1 methylation, “it should be possible to rapidly undertake retrospective studies to validate the findings we report here,” the researchers noted.
Dr. McMeekin reported having no financial disclosures. His coauthors reported ties to several companies, including Repros Therapeutics, Ethicon, Vitatex, Genentech, Janssen Oncology, Tesaro, AstraZeneca, and OvaGene Oncology.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: DNA mismatch repair defects have prognostic importance in endometrioid endometrial cancers.
Major finding: Progression-free survival was significantly lower among women whose tumors had epigenetic defects, compared with women whose tumors were MMR normal (HR, 1.37; 95% confidence interval, 1.00-1.86; P less than .05).
Data source: A prospective study of 1,024 endometrioid endometrial tumors, with central review of tumor slides and clinical data.
Disclosures: Dr. McMeekin reported having no financial disclosures. His coauthors reported ties to several companies, including Repros Therapeutics, Ethicon, Vitatex, Genentech, Janssen Oncology, Tesaro, AstraZeneca, and OvaGene Oncology.
Study confirms role of oral contraceptives in preventing ovarian cancer
Every 5-year increase in duration of oral contraceptive use was associated with about a 13% decrease in the risk of invasive ovarian cancers, and this protective effect persisted for all histologic subtypes except mucinous tumors, according to results of a meta-analysis.
“Oral contraceptives continue to be an important preventive factor for most types of ovarian cancer. Few other risk factors for ovarian cancer are modifiable, and those that are, such as smoking and obesity, did not show clear associations with serous carcinomas, the most common and fatal subtype,” Dr. Nicolas Wentzensen of the National Cancer Institute and his associates wrote June 20 in the Journal of Clinical Oncology.
Ovarian cancer is heterogeneous and its etiology poorly understood. Power limitations have prevented individual studies from parsing risk factors for distinct ovarian cancer histotypes, the researchers added. To solve this issue, they examined links between 14 different hormonal, reproductive, and lifestyle characteristics and histologic subtypes of tumors in the Ovarian Cancer Cohort Consortium.
Their analysis included 5,584 invasive ovarian cancers from more than 1.3 million women enrolled in 21 prospective studies (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8178).
Patients who reported having ever used oral contraceptives were about 16% less likely to develop any type of invasive epithelial ovarian cancer, compared with those who had never used oral contraceptives (relative risk, 0.84; 95% confidence interval, 0.79-0.89). Associations were similar for high-grade serous, endometrioid, and clear cell histotypes, although OC use did not appear to protect against mucinous tumors, the researchers wrote.
Some of the strongest associations were for endometrioid and clear cell carcinomas, they noted. For example, the risk of endometrioid carcinoma fell by about 22% for every birth (RR per birth, 0.78; 95% CI, 0.74-0.83), and the risk of clear cell carcinoma fell by about 32% per birth (RR, 0.68; 95% CI, 0.61-0.76).
“Likewise, age at menopause, endometriosis, and tubal ligation were associated only with clear cell and endometrioid tumors,” the researchers wrote.
Smoking was tied to a higher risk of mucinous tumors (RR per 20 pack-years, 1.20; 95% CI, 1.04-1.39) but was associated with a lower risk of clear cell tumors (RR, 0.68; 95% CI, 0.53-0.89).
“The substantial heterogeneity of individual risk factor associations across ovarian cancer subtypes [suggests] that subtypes are indeed different diseases, and underscores the importance of evaluating risk factors and biomarkers by ovarian cancer subtypes,” the researchers concluded. “Due to weaker associations observed for high-grade serous carcinomas, prediction of the clinically most important subtype may perform worse than for other types, which underscores the importance of finding better risk factors for serous carcinomas.”
The consortium plans to search for more tumor predictors by analyzing circulating biomarkers and genetic data.
Dr. Wentzensen reported having no financial disclosures. Other researchers reported financial relationships with GlaxoSmithKline and Cepheid.
Every 5-year increase in duration of oral contraceptive use was associated with about a 13% decrease in the risk of invasive ovarian cancers, and this protective effect persisted for all histologic subtypes except mucinous tumors, according to results of a meta-analysis.
“Oral contraceptives continue to be an important preventive factor for most types of ovarian cancer. Few other risk factors for ovarian cancer are modifiable, and those that are, such as smoking and obesity, did not show clear associations with serous carcinomas, the most common and fatal subtype,” Dr. Nicolas Wentzensen of the National Cancer Institute and his associates wrote June 20 in the Journal of Clinical Oncology.
Ovarian cancer is heterogeneous and its etiology poorly understood. Power limitations have prevented individual studies from parsing risk factors for distinct ovarian cancer histotypes, the researchers added. To solve this issue, they examined links between 14 different hormonal, reproductive, and lifestyle characteristics and histologic subtypes of tumors in the Ovarian Cancer Cohort Consortium.
Their analysis included 5,584 invasive ovarian cancers from more than 1.3 million women enrolled in 21 prospective studies (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8178).
Patients who reported having ever used oral contraceptives were about 16% less likely to develop any type of invasive epithelial ovarian cancer, compared with those who had never used oral contraceptives (relative risk, 0.84; 95% confidence interval, 0.79-0.89). Associations were similar for high-grade serous, endometrioid, and clear cell histotypes, although OC use did not appear to protect against mucinous tumors, the researchers wrote.
Some of the strongest associations were for endometrioid and clear cell carcinomas, they noted. For example, the risk of endometrioid carcinoma fell by about 22% for every birth (RR per birth, 0.78; 95% CI, 0.74-0.83), and the risk of clear cell carcinoma fell by about 32% per birth (RR, 0.68; 95% CI, 0.61-0.76).
“Likewise, age at menopause, endometriosis, and tubal ligation were associated only with clear cell and endometrioid tumors,” the researchers wrote.
Smoking was tied to a higher risk of mucinous tumors (RR per 20 pack-years, 1.20; 95% CI, 1.04-1.39) but was associated with a lower risk of clear cell tumors (RR, 0.68; 95% CI, 0.53-0.89).
“The substantial heterogeneity of individual risk factor associations across ovarian cancer subtypes [suggests] that subtypes are indeed different diseases, and underscores the importance of evaluating risk factors and biomarkers by ovarian cancer subtypes,” the researchers concluded. “Due to weaker associations observed for high-grade serous carcinomas, prediction of the clinically most important subtype may perform worse than for other types, which underscores the importance of finding better risk factors for serous carcinomas.”
The consortium plans to search for more tumor predictors by analyzing circulating biomarkers and genetic data.
Dr. Wentzensen reported having no financial disclosures. Other researchers reported financial relationships with GlaxoSmithKline and Cepheid.
Every 5-year increase in duration of oral contraceptive use was associated with about a 13% decrease in the risk of invasive ovarian cancers, and this protective effect persisted for all histologic subtypes except mucinous tumors, according to results of a meta-analysis.
“Oral contraceptives continue to be an important preventive factor for most types of ovarian cancer. Few other risk factors for ovarian cancer are modifiable, and those that are, such as smoking and obesity, did not show clear associations with serous carcinomas, the most common and fatal subtype,” Dr. Nicolas Wentzensen of the National Cancer Institute and his associates wrote June 20 in the Journal of Clinical Oncology.
Ovarian cancer is heterogeneous and its etiology poorly understood. Power limitations have prevented individual studies from parsing risk factors for distinct ovarian cancer histotypes, the researchers added. To solve this issue, they examined links between 14 different hormonal, reproductive, and lifestyle characteristics and histologic subtypes of tumors in the Ovarian Cancer Cohort Consortium.
Their analysis included 5,584 invasive ovarian cancers from more than 1.3 million women enrolled in 21 prospective studies (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8178).
Patients who reported having ever used oral contraceptives were about 16% less likely to develop any type of invasive epithelial ovarian cancer, compared with those who had never used oral contraceptives (relative risk, 0.84; 95% confidence interval, 0.79-0.89). Associations were similar for high-grade serous, endometrioid, and clear cell histotypes, although OC use did not appear to protect against mucinous tumors, the researchers wrote.
Some of the strongest associations were for endometrioid and clear cell carcinomas, they noted. For example, the risk of endometrioid carcinoma fell by about 22% for every birth (RR per birth, 0.78; 95% CI, 0.74-0.83), and the risk of clear cell carcinoma fell by about 32% per birth (RR, 0.68; 95% CI, 0.61-0.76).
“Likewise, age at menopause, endometriosis, and tubal ligation were associated only with clear cell and endometrioid tumors,” the researchers wrote.
Smoking was tied to a higher risk of mucinous tumors (RR per 20 pack-years, 1.20; 95% CI, 1.04-1.39) but was associated with a lower risk of clear cell tumors (RR, 0.68; 95% CI, 0.53-0.89).
“The substantial heterogeneity of individual risk factor associations across ovarian cancer subtypes [suggests] that subtypes are indeed different diseases, and underscores the importance of evaluating risk factors and biomarkers by ovarian cancer subtypes,” the researchers concluded. “Due to weaker associations observed for high-grade serous carcinomas, prediction of the clinically most important subtype may perform worse than for other types, which underscores the importance of finding better risk factors for serous carcinomas.”
The consortium plans to search for more tumor predictors by analyzing circulating biomarkers and genetic data.
Dr. Wentzensen reported having no financial disclosures. Other researchers reported financial relationships with GlaxoSmithKline and Cepheid.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Oral contraceptive (OC) use was inversely associated with the risk of several types of ovarian cancer.
Major finding: Use of OCs was associated with a 16% decrease in the risk of any type of ovarian cancer (relative risk, 0.84; 95% confidence interval, 0.79-0.89).
Data source: A meta-analysis of 21 prospective studies that included 5,584 invasive ovarian cancer cases from more than 1.3 million women.
Disclosures: Dr. Wentzensen reported having no financial disclosures. Other researchers reported financial relationships with GlaxoSmithKline and Cepheid.
Continuous Rd again beat MPT for transplant-ineligible multiple myeloma
Individuals with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation were 31% less likely to die or progress on continuous lenalidomide plus low-dose dexamethasone (Rd continuous) than were those on melphalan, prednisone, and thalidomide (MPT), according to an extended follow-up of patients from the FIRST trial.
In addition, Rd continuous was associated with a statistically significant 20% decrease in risk of death or disease progression, compared with MPT among patients older than 75 years, reported Dr. Cyrille Hulin of Bordeaux (France) Hospital University Center and associates. The finding “establishes continuous treatment with Rd until disease progression as a new standard of care for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, regardless of age. With proper monitoring and dose adjustment, Rd continuous is an effective and tolerable treatment option for even the most elderly patients,” they wrote online June 20 in the Journal of Clinical Oncology.
FIRST (Frontline Investigation of Revlimid Plus Dexamethasone Versus Standard Thalidomide) was an international, randomized, phase III open-label study of patients with untreated symptomatic multiple myeloma enrolled between 2008 and 2011. Patients were randomly assigned to Rd continuous, 72 weeks/18 cycles of Rd (Rd18), or MPT. The primary endpoint was progression-free survival (PFS). In the original analysis, Rd continuous led to a 28% lower risk of progression or death, compared with MPT, for a statistically significant hazard ratio (HR) of 0.72. The current study involved longer follow-up, with an updated data cutoff that was 3 years after the end of recruitment, the researchers said (J Clin Oncol. 2016 Jun 20. doi: 10.1200/JCO.2016.66.7295).
The cohort included 1,623 patients, of whom 567 (35%) were older than 75 years. The intention-to-treat populations included 535 Rd continuous patients, 541 Rd18 patients, and 547 MPT patients. Survivors were followed for a median of 45.5 months. As in the earlier analysis of FIRST data, the PFS was longer with Rd continuous than with MPT. For the overall intention-to-treat group, the median PFS was 26 months with Rd continuous, and 21.9 months with MPT (HR, 0.69; 95% confidence interval, 0.59-0.80). Among patients aged 75 years and younger, the median PFS was 28.1 months and 22.4 months, respectively (HR, 0.64; 95% CI, 0.53-0.77). Among patients over age 75 years, the median PFS was 20.3 months and 19.8 months, respectively (HR, 0.80, 95% CI, 0.62-1.03). In addition, the 4-year PFS “was more than doubled with Rd continuous versus MPT, regardless of age,” the investigators said. In contrast, Rd18 and MPT led to a similar median PFS, regardless of age.
As in the prior FIRST analysis, MPT was more often linked to grade 3 and 4 neutropenia (40% and 47% of older and younger patients, respectively, versus about 28% of Rd continuous patients), while Rd more often led to grade 3 and 4 infections (about 30% of Rd continuous patients, about 22% of Rd18 patients, and 16%-20% of MPT patients).
A total of 40% of younger Rd patients remained at their starting lenalidomide dose at 72 weeks, while only 16% of MPT patients stayed at their starting thalidomide dose. Similarly, 30% of older Rd continuous patients remained on their starting dose at 72 weeks, compared with 19% of older MPT patients.
Older age was associated with International Staging System stage III disease, renal impairment, and more comorbidities, but not with high-risk cytogenetics (that is, del[17p] and t[4;14]). “Although chronologic age is not necessarily an indicator of frailty, FIRST trial results did show greater PFS and OS [overall survival] benefits with Rd continuous versus MPT therapy, regardless of age,” the researchers commented.
The study was funded by Intergroupe Francophone du Myélome and Celgene. Dr. Hulin disclosed honoraria from Celgene, Amgen, Bristol-Myers Squibb, and Novartis.
Individuals with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation were 31% less likely to die or progress on continuous lenalidomide plus low-dose dexamethasone (Rd continuous) than were those on melphalan, prednisone, and thalidomide (MPT), according to an extended follow-up of patients from the FIRST trial.
In addition, Rd continuous was associated with a statistically significant 20% decrease in risk of death or disease progression, compared with MPT among patients older than 75 years, reported Dr. Cyrille Hulin of Bordeaux (France) Hospital University Center and associates. The finding “establishes continuous treatment with Rd until disease progression as a new standard of care for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, regardless of age. With proper monitoring and dose adjustment, Rd continuous is an effective and tolerable treatment option for even the most elderly patients,” they wrote online June 20 in the Journal of Clinical Oncology.
FIRST (Frontline Investigation of Revlimid Plus Dexamethasone Versus Standard Thalidomide) was an international, randomized, phase III open-label study of patients with untreated symptomatic multiple myeloma enrolled between 2008 and 2011. Patients were randomly assigned to Rd continuous, 72 weeks/18 cycles of Rd (Rd18), or MPT. The primary endpoint was progression-free survival (PFS). In the original analysis, Rd continuous led to a 28% lower risk of progression or death, compared with MPT, for a statistically significant hazard ratio (HR) of 0.72. The current study involved longer follow-up, with an updated data cutoff that was 3 years after the end of recruitment, the researchers said (J Clin Oncol. 2016 Jun 20. doi: 10.1200/JCO.2016.66.7295).
The cohort included 1,623 patients, of whom 567 (35%) were older than 75 years. The intention-to-treat populations included 535 Rd continuous patients, 541 Rd18 patients, and 547 MPT patients. Survivors were followed for a median of 45.5 months. As in the earlier analysis of FIRST data, the PFS was longer with Rd continuous than with MPT. For the overall intention-to-treat group, the median PFS was 26 months with Rd continuous, and 21.9 months with MPT (HR, 0.69; 95% confidence interval, 0.59-0.80). Among patients aged 75 years and younger, the median PFS was 28.1 months and 22.4 months, respectively (HR, 0.64; 95% CI, 0.53-0.77). Among patients over age 75 years, the median PFS was 20.3 months and 19.8 months, respectively (HR, 0.80, 95% CI, 0.62-1.03). In addition, the 4-year PFS “was more than doubled with Rd continuous versus MPT, regardless of age,” the investigators said. In contrast, Rd18 and MPT led to a similar median PFS, regardless of age.
As in the prior FIRST analysis, MPT was more often linked to grade 3 and 4 neutropenia (40% and 47% of older and younger patients, respectively, versus about 28% of Rd continuous patients), while Rd more often led to grade 3 and 4 infections (about 30% of Rd continuous patients, about 22% of Rd18 patients, and 16%-20% of MPT patients).
A total of 40% of younger Rd patients remained at their starting lenalidomide dose at 72 weeks, while only 16% of MPT patients stayed at their starting thalidomide dose. Similarly, 30% of older Rd continuous patients remained on their starting dose at 72 weeks, compared with 19% of older MPT patients.
Older age was associated with International Staging System stage III disease, renal impairment, and more comorbidities, but not with high-risk cytogenetics (that is, del[17p] and t[4;14]). “Although chronologic age is not necessarily an indicator of frailty, FIRST trial results did show greater PFS and OS [overall survival] benefits with Rd continuous versus MPT therapy, regardless of age,” the researchers commented.
The study was funded by Intergroupe Francophone du Myélome and Celgene. Dr. Hulin disclosed honoraria from Celgene, Amgen, Bristol-Myers Squibb, and Novartis.
Individuals with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation were 31% less likely to die or progress on continuous lenalidomide plus low-dose dexamethasone (Rd continuous) than were those on melphalan, prednisone, and thalidomide (MPT), according to an extended follow-up of patients from the FIRST trial.
In addition, Rd continuous was associated with a statistically significant 20% decrease in risk of death or disease progression, compared with MPT among patients older than 75 years, reported Dr. Cyrille Hulin of Bordeaux (France) Hospital University Center and associates. The finding “establishes continuous treatment with Rd until disease progression as a new standard of care for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, regardless of age. With proper monitoring and dose adjustment, Rd continuous is an effective and tolerable treatment option for even the most elderly patients,” they wrote online June 20 in the Journal of Clinical Oncology.
FIRST (Frontline Investigation of Revlimid Plus Dexamethasone Versus Standard Thalidomide) was an international, randomized, phase III open-label study of patients with untreated symptomatic multiple myeloma enrolled between 2008 and 2011. Patients were randomly assigned to Rd continuous, 72 weeks/18 cycles of Rd (Rd18), or MPT. The primary endpoint was progression-free survival (PFS). In the original analysis, Rd continuous led to a 28% lower risk of progression or death, compared with MPT, for a statistically significant hazard ratio (HR) of 0.72. The current study involved longer follow-up, with an updated data cutoff that was 3 years after the end of recruitment, the researchers said (J Clin Oncol. 2016 Jun 20. doi: 10.1200/JCO.2016.66.7295).
The cohort included 1,623 patients, of whom 567 (35%) were older than 75 years. The intention-to-treat populations included 535 Rd continuous patients, 541 Rd18 patients, and 547 MPT patients. Survivors were followed for a median of 45.5 months. As in the earlier analysis of FIRST data, the PFS was longer with Rd continuous than with MPT. For the overall intention-to-treat group, the median PFS was 26 months with Rd continuous, and 21.9 months with MPT (HR, 0.69; 95% confidence interval, 0.59-0.80). Among patients aged 75 years and younger, the median PFS was 28.1 months and 22.4 months, respectively (HR, 0.64; 95% CI, 0.53-0.77). Among patients over age 75 years, the median PFS was 20.3 months and 19.8 months, respectively (HR, 0.80, 95% CI, 0.62-1.03). In addition, the 4-year PFS “was more than doubled with Rd continuous versus MPT, regardless of age,” the investigators said. In contrast, Rd18 and MPT led to a similar median PFS, regardless of age.
As in the prior FIRST analysis, MPT was more often linked to grade 3 and 4 neutropenia (40% and 47% of older and younger patients, respectively, versus about 28% of Rd continuous patients), while Rd more often led to grade 3 and 4 infections (about 30% of Rd continuous patients, about 22% of Rd18 patients, and 16%-20% of MPT patients).
A total of 40% of younger Rd patients remained at their starting lenalidomide dose at 72 weeks, while only 16% of MPT patients stayed at their starting thalidomide dose. Similarly, 30% of older Rd continuous patients remained on their starting dose at 72 weeks, compared with 19% of older MPT patients.
Older age was associated with International Staging System stage III disease, renal impairment, and more comorbidities, but not with high-risk cytogenetics (that is, del[17p] and t[4;14]). “Although chronologic age is not necessarily an indicator of frailty, FIRST trial results did show greater PFS and OS [overall survival] benefits with Rd continuous versus MPT therapy, regardless of age,” the researchers commented.
The study was funded by Intergroupe Francophone du Myélome and Celgene. Dr. Hulin disclosed honoraria from Celgene, Amgen, Bristol-Myers Squibb, and Novartis.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: For patients with newly diagnosed multiple myeloma who were ineligible for transplant, including older patients, continuous lenalidomide plus low-dose dexamethasone (Rd continuous) was associated with significantly longer progression-free survival than was treatment with melphalan, prednisone, and thalidomide (MPT).
Major finding: Continuous Rd was associated with a 31% lower risk of death or progression in the overall intention-to-treat analysis, a 36% decrease among patients aged 75 years or younger, and a 20% decrease among patients older than 75 years.
Data source: An updated analysis of 1,623 patients from the FIRST trial.
Disclosures: The study was funded by Intergroupe Francophone du Myélome and Celgene. Dr. Hulin disclosed honoraria from Celgene, Amgen, Bristol-Myers Squibb, and Novartis.