User login
VIDEO: Start probiotics within 2 days of antibiotics to prevent CDI
Starting probiotics within 2 days of the first antibiotic dose could cut the risk of Clostridium difficile infection among hospitalized adults by more than 50%, according to the results of a systemic review and metaregression analysis.
The protective effect waned when patients delayed starting probiotics, reported Nicole T. Shen, MD, of Cornell University, New York, and her associates. The study appears in Gastroenterology (doi: 10.1053/j.gastro.2017.02.003). “Given the magnitude of benefit and the low cost of probiotics, the decision is likely to be highly cost effective,” they added.
Systematic reviews support the use of probiotics for preventing Clostridium difficile infection (CDI), but guidelines do not reflect these findings. To help guide clinical practice, the reviewers searched MEDLINE, EMBASE, the International Journal of Probiotics and Prebiotics, and the Cochrane Library databases for randomized controlled trials of probiotics and CDI among hospitalized adults taking antibiotics. This search yielded 19 published studies of 6,261 patients. Two reviewers separately extracted data from these studies and examined quality of evidence and risk of bias.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
A total of 54 patients in the probiotic cohort (1.6%) developed CDI, compared with 115 controls (3.9%), a statistically significant difference (P less than .001). In regression analysis, the probiotic group was about 58% less likely to develop CDI than controls (hazard ratio, 0.42; 95% confidence interval, 0.30-0.57; P less than .001). Importantly, probiotics were significantly effective against CDI only when started within 2 days of antibiotic initiation (relative risk, 0.32; 95% CI, 0.22-0.48), not when started within 3-7 days (RR, 0.70, 95% CI, 0.40-1.23). The difference between these estimated risk ratios was statistically significant (P = .02).
In 18 of the 19 studies, patients received probiotics within 3 days of starting antibiotics, while patients in the remaining study could start probiotics any time within 7 days of antibiotic initiation. “Not only was [this] study unusual with respect to probiotic timing, it was also much larger than all other studies, and its results were statistically insignificant,” the reviewers wrote. Metaregression analyses of all studies and of all but the outlier study linked delaying probiotics with a decrease in efficacy against CDI, with P values of .04 and .09, respectively. Those findings “suggest that the decrement in efficacy with delay in starting probiotics is not sensitive to inclusion of a single large ‘outlier’ study,” the reviewers emphasized. “In fact, inclusion only dampens the magnitude of the decrement in efficacy, although it is still clinically important and statistically significant.”
The trials included 12 probiotic formulas containing Lactobacillus, Saccharomyces, Bifidobacterium, and Streptococcus, either alone or in combination. Probiotics were not associated with adverse effects in the trials. Quality of evidence was generally high, but seven trials had missing data on the primary outcome. Furthermore, two studies lacked a placebo group, and lead authors of two studies disclosed ties to the probiotic manufacturers that provided funding.
One reviewer received fellowship support from the Louis and Rachel Rudin Foundation. None had conflicts of interest.
Starting probiotics within 2 days of the first antibiotic dose could cut the risk of Clostridium difficile infection among hospitalized adults by more than 50%, according to the results of a systemic review and metaregression analysis.
The protective effect waned when patients delayed starting probiotics, reported Nicole T. Shen, MD, of Cornell University, New York, and her associates. The study appears in Gastroenterology (doi: 10.1053/j.gastro.2017.02.003). “Given the magnitude of benefit and the low cost of probiotics, the decision is likely to be highly cost effective,” they added.
Systematic reviews support the use of probiotics for preventing Clostridium difficile infection (CDI), but guidelines do not reflect these findings. To help guide clinical practice, the reviewers searched MEDLINE, EMBASE, the International Journal of Probiotics and Prebiotics, and the Cochrane Library databases for randomized controlled trials of probiotics and CDI among hospitalized adults taking antibiotics. This search yielded 19 published studies of 6,261 patients. Two reviewers separately extracted data from these studies and examined quality of evidence and risk of bias.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
A total of 54 patients in the probiotic cohort (1.6%) developed CDI, compared with 115 controls (3.9%), a statistically significant difference (P less than .001). In regression analysis, the probiotic group was about 58% less likely to develop CDI than controls (hazard ratio, 0.42; 95% confidence interval, 0.30-0.57; P less than .001). Importantly, probiotics were significantly effective against CDI only when started within 2 days of antibiotic initiation (relative risk, 0.32; 95% CI, 0.22-0.48), not when started within 3-7 days (RR, 0.70, 95% CI, 0.40-1.23). The difference between these estimated risk ratios was statistically significant (P = .02).
In 18 of the 19 studies, patients received probiotics within 3 days of starting antibiotics, while patients in the remaining study could start probiotics any time within 7 days of antibiotic initiation. “Not only was [this] study unusual with respect to probiotic timing, it was also much larger than all other studies, and its results were statistically insignificant,” the reviewers wrote. Metaregression analyses of all studies and of all but the outlier study linked delaying probiotics with a decrease in efficacy against CDI, with P values of .04 and .09, respectively. Those findings “suggest that the decrement in efficacy with delay in starting probiotics is not sensitive to inclusion of a single large ‘outlier’ study,” the reviewers emphasized. “In fact, inclusion only dampens the magnitude of the decrement in efficacy, although it is still clinically important and statistically significant.”
The trials included 12 probiotic formulas containing Lactobacillus, Saccharomyces, Bifidobacterium, and Streptococcus, either alone or in combination. Probiotics were not associated with adverse effects in the trials. Quality of evidence was generally high, but seven trials had missing data on the primary outcome. Furthermore, two studies lacked a placebo group, and lead authors of two studies disclosed ties to the probiotic manufacturers that provided funding.
One reviewer received fellowship support from the Louis and Rachel Rudin Foundation. None had conflicts of interest.
Starting probiotics within 2 days of the first antibiotic dose could cut the risk of Clostridium difficile infection among hospitalized adults by more than 50%, according to the results of a systemic review and metaregression analysis.
The protective effect waned when patients delayed starting probiotics, reported Nicole T. Shen, MD, of Cornell University, New York, and her associates. The study appears in Gastroenterology (doi: 10.1053/j.gastro.2017.02.003). “Given the magnitude of benefit and the low cost of probiotics, the decision is likely to be highly cost effective,” they added.
Systematic reviews support the use of probiotics for preventing Clostridium difficile infection (CDI), but guidelines do not reflect these findings. To help guide clinical practice, the reviewers searched MEDLINE, EMBASE, the International Journal of Probiotics and Prebiotics, and the Cochrane Library databases for randomized controlled trials of probiotics and CDI among hospitalized adults taking antibiotics. This search yielded 19 published studies of 6,261 patients. Two reviewers separately extracted data from these studies and examined quality of evidence and risk of bias.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
A total of 54 patients in the probiotic cohort (1.6%) developed CDI, compared with 115 controls (3.9%), a statistically significant difference (P less than .001). In regression analysis, the probiotic group was about 58% less likely to develop CDI than controls (hazard ratio, 0.42; 95% confidence interval, 0.30-0.57; P less than .001). Importantly, probiotics were significantly effective against CDI only when started within 2 days of antibiotic initiation (relative risk, 0.32; 95% CI, 0.22-0.48), not when started within 3-7 days (RR, 0.70, 95% CI, 0.40-1.23). The difference between these estimated risk ratios was statistically significant (P = .02).
In 18 of the 19 studies, patients received probiotics within 3 days of starting antibiotics, while patients in the remaining study could start probiotics any time within 7 days of antibiotic initiation. “Not only was [this] study unusual with respect to probiotic timing, it was also much larger than all other studies, and its results were statistically insignificant,” the reviewers wrote. Metaregression analyses of all studies and of all but the outlier study linked delaying probiotics with a decrease in efficacy against CDI, with P values of .04 and .09, respectively. Those findings “suggest that the decrement in efficacy with delay in starting probiotics is not sensitive to inclusion of a single large ‘outlier’ study,” the reviewers emphasized. “In fact, inclusion only dampens the magnitude of the decrement in efficacy, although it is still clinically important and statistically significant.”
The trials included 12 probiotic formulas containing Lactobacillus, Saccharomyces, Bifidobacterium, and Streptococcus, either alone or in combination. Probiotics were not associated with adverse effects in the trials. Quality of evidence was generally high, but seven trials had missing data on the primary outcome. Furthermore, two studies lacked a placebo group, and lead authors of two studies disclosed ties to the probiotic manufacturers that provided funding.
One reviewer received fellowship support from the Louis and Rachel Rudin Foundation. None had conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Starting probiotics within 2 days of antibiotics was associated with a significantly reduced risk of Clostridium difficile infection among hospitalized patients.
Major finding: Probiotics were significantly effective against CDI only when started within 2 days of antibiotic initiation (relative risk, 0.32; 95% CI, 0.22-0.48), not when started within 3-7 days (RR, 0.70; 95% CI, 0.40-1.23).
Data source: A systematic review and metaregression analysis of 19 studies of 6,261 patients.
Disclosures: One reviewer received fellowship support from the Louis and Rachel Rudin Foundation. None had conflicts of interest.
Distance from transplant center predicted mortality in chronic liver disease
Living more than 150 miles from a liver transplant center was associated with a higher risk of mortality among patients with chronic liver failure, regardless of etiology, transplantation status, or whether patients had decompensated cirrhosis or hepatocellular carcinoma, according to a first-in-kind, population-based study reported in the June issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.02.023).
The findings underscore the need for accessible, specialized liver care irrespective of whether patients with chronic liver failure (CLF) are destined for transplantation, David S. Goldberg, MD, of the University of Pennsylvania, Philadelphia, wrote with his associates. The associations “do not provide cause and effect,” but underscore the need to consider “the broader impact of transplant-related policies that could decrease transplant volumes and threaten closures of smaller liver transplant centers that serve geographically isolated populations in the Southeast and Midwest,” they added.
A total of 879 (5.2%) patients lived more than 150 miles from the nearest liver transplant center, the analysis showed. Even after controlling for etiology of liver disease, this subgroup was at significantly greater risk of mortality (hazard ratio, 1.2; 95% confidence interval, 1.1-1.3; P less than .001) and of dying without undergoing transplantation (HR, 1.2; 95% CI, 1.1-1.3; P = .003) than were patients who were less geographically isolated. Distance from a transplant center also predicted overall and transplant-free mortality when modeled as a continuous variable, with hazard ratios of 1.02 (P = .02) and 1.03 (P = .04), respectively. “Although patients living more than 150 miles from a liver transplant center had fewer outpatient gastroenterologist visits, this covariate did not affect the final models,” the investigators reported. Rural locality did not predict mortality after controlling for distance from a transplant center, and neither did living in a low-income zip code, they added.
Data from the Centers for Disease Control and Prevention indicate that age-adjusted rates of death from liver disease are lowest in New York, where the entire population lives within 150 miles of a liver transplant center, the researchers noted. “By contrast, New Mexico and Wyoming have the highest age-adjusted death rates, and more than 95% of those states’ populations live more than 150 miles from a [transplant] center,” they emphasized. “The management of most patients with CLF is not centered on transplantation, but rather the spectrum of care for decompensated cirrhosis and hepatocellular carcinoma. Thus, maintaining access to specialized liver care is important for patients with CLF.”
Dr. Goldberg received support from the National Institutes of Health. The investigators had no conflicts.
Living more than 150 miles from a liver transplant center was associated with a higher risk of mortality among patients with chronic liver failure, regardless of etiology, transplantation status, or whether patients had decompensated cirrhosis or hepatocellular carcinoma, according to a first-in-kind, population-based study reported in the June issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.02.023).
The findings underscore the need for accessible, specialized liver care irrespective of whether patients with chronic liver failure (CLF) are destined for transplantation, David S. Goldberg, MD, of the University of Pennsylvania, Philadelphia, wrote with his associates. The associations “do not provide cause and effect,” but underscore the need to consider “the broader impact of transplant-related policies that could decrease transplant volumes and threaten closures of smaller liver transplant centers that serve geographically isolated populations in the Southeast and Midwest,” they added.
A total of 879 (5.2%) patients lived more than 150 miles from the nearest liver transplant center, the analysis showed. Even after controlling for etiology of liver disease, this subgroup was at significantly greater risk of mortality (hazard ratio, 1.2; 95% confidence interval, 1.1-1.3; P less than .001) and of dying without undergoing transplantation (HR, 1.2; 95% CI, 1.1-1.3; P = .003) than were patients who were less geographically isolated. Distance from a transplant center also predicted overall and transplant-free mortality when modeled as a continuous variable, with hazard ratios of 1.02 (P = .02) and 1.03 (P = .04), respectively. “Although patients living more than 150 miles from a liver transplant center had fewer outpatient gastroenterologist visits, this covariate did not affect the final models,” the investigators reported. Rural locality did not predict mortality after controlling for distance from a transplant center, and neither did living in a low-income zip code, they added.
Data from the Centers for Disease Control and Prevention indicate that age-adjusted rates of death from liver disease are lowest in New York, where the entire population lives within 150 miles of a liver transplant center, the researchers noted. “By contrast, New Mexico and Wyoming have the highest age-adjusted death rates, and more than 95% of those states’ populations live more than 150 miles from a [transplant] center,” they emphasized. “The management of most patients with CLF is not centered on transplantation, but rather the spectrum of care for decompensated cirrhosis and hepatocellular carcinoma. Thus, maintaining access to specialized liver care is important for patients with CLF.”
Dr. Goldberg received support from the National Institutes of Health. The investigators had no conflicts.
Living more than 150 miles from a liver transplant center was associated with a higher risk of mortality among patients with chronic liver failure, regardless of etiology, transplantation status, or whether patients had decompensated cirrhosis or hepatocellular carcinoma, according to a first-in-kind, population-based study reported in the June issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.02.023).
The findings underscore the need for accessible, specialized liver care irrespective of whether patients with chronic liver failure (CLF) are destined for transplantation, David S. Goldberg, MD, of the University of Pennsylvania, Philadelphia, wrote with his associates. The associations “do not provide cause and effect,” but underscore the need to consider “the broader impact of transplant-related policies that could decrease transplant volumes and threaten closures of smaller liver transplant centers that serve geographically isolated populations in the Southeast and Midwest,” they added.
A total of 879 (5.2%) patients lived more than 150 miles from the nearest liver transplant center, the analysis showed. Even after controlling for etiology of liver disease, this subgroup was at significantly greater risk of mortality (hazard ratio, 1.2; 95% confidence interval, 1.1-1.3; P less than .001) and of dying without undergoing transplantation (HR, 1.2; 95% CI, 1.1-1.3; P = .003) than were patients who were less geographically isolated. Distance from a transplant center also predicted overall and transplant-free mortality when modeled as a continuous variable, with hazard ratios of 1.02 (P = .02) and 1.03 (P = .04), respectively. “Although patients living more than 150 miles from a liver transplant center had fewer outpatient gastroenterologist visits, this covariate did not affect the final models,” the investigators reported. Rural locality did not predict mortality after controlling for distance from a transplant center, and neither did living in a low-income zip code, they added.
Data from the Centers for Disease Control and Prevention indicate that age-adjusted rates of death from liver disease are lowest in New York, where the entire population lives within 150 miles of a liver transplant center, the researchers noted. “By contrast, New Mexico and Wyoming have the highest age-adjusted death rates, and more than 95% of those states’ populations live more than 150 miles from a [transplant] center,” they emphasized. “The management of most patients with CLF is not centered on transplantation, but rather the spectrum of care for decompensated cirrhosis and hepatocellular carcinoma. Thus, maintaining access to specialized liver care is important for patients with CLF.”
Dr. Goldberg received support from the National Institutes of Health. The investigators had no conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Geographic isolation from a liver transplant center independently predicted mortality among patients with chronic liver failure.
Major finding: In adjusted analyses, patients who lived more than 150 miles from a liver transplant center were at significantly greater risk of mortality (HR, 1.2; 95% CI, 1.1-1.3; P less than .001) and of dying without undergoing transplantation (HR, 1.2; 95% CI, 1.1-1.3; P = .003) than were patients who were less geographically isolated.
Data source: A retrospective cohort study of 16,824 patients with chronic liver failure who were included in the Healthcare Integrated Research Database between 2006 and 2014.
Disclosures: Dr. Goldberg received support from the National Institutes of Health. The investigators had no conflicts.
Persistently nondysplastic Barrett’s esophagus did not protect against progression
Patients with at least five biopsies showing nondysplastic Barrett’s esophagus were statistically as likely to progress to high-grade dysplasia or esophageal adenocarcinoma as patients with a single such biopsy, according to a multicenter prospective registry study reported in the June issue of Clinical Gastroenterology and Hepatology (doi: org/10.1016/j.cgh.2017.02.019).
The findings, which contradict those from another recent multicenter cohort study (Gastroenterology. 2013;145[3]:548-53), highlight the need for more studies before lengthening the time between surveillance biopsies in patients with nondysplastic Barrett’s esophagus, Rajesh Krishnamoorthi, MD, of Mayo Clinic in Rochester, Minn., wrote with his associates.
Barrett’s esophagus is the strongest predictor of esophageal adenocarcinoma, but studies have reported mixed results as to whether the risk of this cancer increases over time or wanes with consecutive biopsies that indicate nondysplasia, the researchers noted. Therefore, they studied the prospective, multicenter Mayo Clinic Esophageal Adenocarcinoma and Barrett’s Esophagus registry, excluding patients who progressed to adenocarcinoma within 12 months, had missing data, or had no follow-up biopsies. This approach left 480 subjects for analysis. Patients averaged 63 years of age, 78% were male, the mean length of Barrett’s esophagus was 5.7 cm, and the average time between biopsies was 1.8 years, with a standard deviation of 1.3 years.
A total of 16 patients progressed to high-grade dysplasia or esophageal adenocarcinoma over 1,832 patient-years of follow-up, for an overall annual risk of progression of 0.87%. Two patients progressed to esophageal adenocarcinoma (annual risk, 0.11%; 95% confidence interval, 0.03% to 0.44%), while 14 patients progressed to high-grade dysplasia (annual risk, 0.76%; 95% CI, 0.45% to 1.29%). Eight patients progressed to one of these two outcomes after a single nondysplastic biopsy, three progressed after two such biopsies, three progressed after three such biopsies, none progressed after four such biopsies, and two progressed after five such biopsies. Statistically, patients with at least five consecutive nondysplastic biopsies were no less likely to progress than were patients with only one nondysplastic biopsy (hazard ratio, 0.48; 95% CI, 0.07 to 1.92; P = .32). Hazard ratios for the other groups ranged between 0.0 and 0.85, with no significant difference in estimated risk between groups (P = .68) after controlling for age, sex, and length of Barrett’s esophagus.
The previous multicenter cohort study linked persistently nondysplastic Barrett’s esophagus with a lower rate of progression to esophageal adenocarcinoma, and, based on those findings, the authors suggested lengthening intervals between biopsy surveillance or even stopping surveillance, Dr. Krishnamoorthi and his associates noted. However, that study did not have mutually exclusive groups. “Additional data are required before increasing the interval between surveillance endoscopies based on persistence of nondysplastic Barrett’s esophagus,” they concluded.
The study lacked misclassification bias given long-segment Barrett’s esophagus, and specialized gastrointestinal pathologists interpreted all histology specimens, the researchers noted. “The small number of progressors is a potential limitation, reducing power to assess associations,” they added.
The investigators did not report funding sources. They reported having no conflicts of interest.
Current practice guidelines recommend endoscopic surveillance in Barrett’s esophagus (BE) patients to detect esophageal adenocarcinoma (EAC) at an early and potentially curable stage.
As currently practiced, endoscopic surveillance of BE has numerous limitations and provides the impetus for improved risk-stratification and, ultimately, the effectiveness of current surveillance strategies. Persistence of nondysplastic BE (NDBE) has previously been shown to be an indicator of lower risk of progression to high-grade dysplasia (HGD)/EAC. However, outcomes studies on this topic have reported conflicting results.
Where do we stand with regard to persistence of NDBE and its impact on surveillance intervals? Future large cohort studies are required that address all potential confounders and include a large number of patients with progression to HGD/EAC (a challenge given the rarity of this outcome). At the present time, based on the available data, surveillance intervals cannot be lengthened in patients with persistent NDBE. Future studies also need to focus on the development and validation of prediction models that incorporate clinical, endoscopic, and histologic factors in risk stratification. Until then, meticulous examination techniques, cognitive knowledge and training, use of standardized grading systems, and use of high-definition white light endoscopy are critical in improving effectiveness of surveillance programs in BE patients.
Sachin Wani, MD, is associate professor of medicine and Medical codirector of the Esophageal and Gastric Center of Excellence, division of gastroenterology and hepatology, University of Colorado at Denver, Aurora. He is supported by the University of Colorado Department of Medicine Outstanding Early Scholars Program and is a consultant for Medtronic and Boston Scientific.
Current practice guidelines recommend endoscopic surveillance in Barrett’s esophagus (BE) patients to detect esophageal adenocarcinoma (EAC) at an early and potentially curable stage.
As currently practiced, endoscopic surveillance of BE has numerous limitations and provides the impetus for improved risk-stratification and, ultimately, the effectiveness of current surveillance strategies. Persistence of nondysplastic BE (NDBE) has previously been shown to be an indicator of lower risk of progression to high-grade dysplasia (HGD)/EAC. However, outcomes studies on this topic have reported conflicting results.
Where do we stand with regard to persistence of NDBE and its impact on surveillance intervals? Future large cohort studies are required that address all potential confounders and include a large number of patients with progression to HGD/EAC (a challenge given the rarity of this outcome). At the present time, based on the available data, surveillance intervals cannot be lengthened in patients with persistent NDBE. Future studies also need to focus on the development and validation of prediction models that incorporate clinical, endoscopic, and histologic factors in risk stratification. Until then, meticulous examination techniques, cognitive knowledge and training, use of standardized grading systems, and use of high-definition white light endoscopy are critical in improving effectiveness of surveillance programs in BE patients.
Sachin Wani, MD, is associate professor of medicine and Medical codirector of the Esophageal and Gastric Center of Excellence, division of gastroenterology and hepatology, University of Colorado at Denver, Aurora. He is supported by the University of Colorado Department of Medicine Outstanding Early Scholars Program and is a consultant for Medtronic and Boston Scientific.
Current practice guidelines recommend endoscopic surveillance in Barrett’s esophagus (BE) patients to detect esophageal adenocarcinoma (EAC) at an early and potentially curable stage.
As currently practiced, endoscopic surveillance of BE has numerous limitations and provides the impetus for improved risk-stratification and, ultimately, the effectiveness of current surveillance strategies. Persistence of nondysplastic BE (NDBE) has previously been shown to be an indicator of lower risk of progression to high-grade dysplasia (HGD)/EAC. However, outcomes studies on this topic have reported conflicting results.
Where do we stand with regard to persistence of NDBE and its impact on surveillance intervals? Future large cohort studies are required that address all potential confounders and include a large number of patients with progression to HGD/EAC (a challenge given the rarity of this outcome). At the present time, based on the available data, surveillance intervals cannot be lengthened in patients with persistent NDBE. Future studies also need to focus on the development and validation of prediction models that incorporate clinical, endoscopic, and histologic factors in risk stratification. Until then, meticulous examination techniques, cognitive knowledge and training, use of standardized grading systems, and use of high-definition white light endoscopy are critical in improving effectiveness of surveillance programs in BE patients.
Sachin Wani, MD, is associate professor of medicine and Medical codirector of the Esophageal and Gastric Center of Excellence, division of gastroenterology and hepatology, University of Colorado at Denver, Aurora. He is supported by the University of Colorado Department of Medicine Outstanding Early Scholars Program and is a consultant for Medtronic and Boston Scientific.
Patients with at least five biopsies showing nondysplastic Barrett’s esophagus were statistically as likely to progress to high-grade dysplasia or esophageal adenocarcinoma as patients with a single such biopsy, according to a multicenter prospective registry study reported in the June issue of Clinical Gastroenterology and Hepatology (doi: org/10.1016/j.cgh.2017.02.019).
The findings, which contradict those from another recent multicenter cohort study (Gastroenterology. 2013;145[3]:548-53), highlight the need for more studies before lengthening the time between surveillance biopsies in patients with nondysplastic Barrett’s esophagus, Rajesh Krishnamoorthi, MD, of Mayo Clinic in Rochester, Minn., wrote with his associates.
Barrett’s esophagus is the strongest predictor of esophageal adenocarcinoma, but studies have reported mixed results as to whether the risk of this cancer increases over time or wanes with consecutive biopsies that indicate nondysplasia, the researchers noted. Therefore, they studied the prospective, multicenter Mayo Clinic Esophageal Adenocarcinoma and Barrett’s Esophagus registry, excluding patients who progressed to adenocarcinoma within 12 months, had missing data, or had no follow-up biopsies. This approach left 480 subjects for analysis. Patients averaged 63 years of age, 78% were male, the mean length of Barrett’s esophagus was 5.7 cm, and the average time between biopsies was 1.8 years, with a standard deviation of 1.3 years.
A total of 16 patients progressed to high-grade dysplasia or esophageal adenocarcinoma over 1,832 patient-years of follow-up, for an overall annual risk of progression of 0.87%. Two patients progressed to esophageal adenocarcinoma (annual risk, 0.11%; 95% confidence interval, 0.03% to 0.44%), while 14 patients progressed to high-grade dysplasia (annual risk, 0.76%; 95% CI, 0.45% to 1.29%). Eight patients progressed to one of these two outcomes after a single nondysplastic biopsy, three progressed after two such biopsies, three progressed after three such biopsies, none progressed after four such biopsies, and two progressed after five such biopsies. Statistically, patients with at least five consecutive nondysplastic biopsies were no less likely to progress than were patients with only one nondysplastic biopsy (hazard ratio, 0.48; 95% CI, 0.07 to 1.92; P = .32). Hazard ratios for the other groups ranged between 0.0 and 0.85, with no significant difference in estimated risk between groups (P = .68) after controlling for age, sex, and length of Barrett’s esophagus.
The previous multicenter cohort study linked persistently nondysplastic Barrett’s esophagus with a lower rate of progression to esophageal adenocarcinoma, and, based on those findings, the authors suggested lengthening intervals between biopsy surveillance or even stopping surveillance, Dr. Krishnamoorthi and his associates noted. However, that study did not have mutually exclusive groups. “Additional data are required before increasing the interval between surveillance endoscopies based on persistence of nondysplastic Barrett’s esophagus,” they concluded.
The study lacked misclassification bias given long-segment Barrett’s esophagus, and specialized gastrointestinal pathologists interpreted all histology specimens, the researchers noted. “The small number of progressors is a potential limitation, reducing power to assess associations,” they added.
The investigators did not report funding sources. They reported having no conflicts of interest.
Patients with at least five biopsies showing nondysplastic Barrett’s esophagus were statistically as likely to progress to high-grade dysplasia or esophageal adenocarcinoma as patients with a single such biopsy, according to a multicenter prospective registry study reported in the June issue of Clinical Gastroenterology and Hepatology (doi: org/10.1016/j.cgh.2017.02.019).
The findings, which contradict those from another recent multicenter cohort study (Gastroenterology. 2013;145[3]:548-53), highlight the need for more studies before lengthening the time between surveillance biopsies in patients with nondysplastic Barrett’s esophagus, Rajesh Krishnamoorthi, MD, of Mayo Clinic in Rochester, Minn., wrote with his associates.
Barrett’s esophagus is the strongest predictor of esophageal adenocarcinoma, but studies have reported mixed results as to whether the risk of this cancer increases over time or wanes with consecutive biopsies that indicate nondysplasia, the researchers noted. Therefore, they studied the prospective, multicenter Mayo Clinic Esophageal Adenocarcinoma and Barrett’s Esophagus registry, excluding patients who progressed to adenocarcinoma within 12 months, had missing data, or had no follow-up biopsies. This approach left 480 subjects for analysis. Patients averaged 63 years of age, 78% were male, the mean length of Barrett’s esophagus was 5.7 cm, and the average time between biopsies was 1.8 years, with a standard deviation of 1.3 years.
A total of 16 patients progressed to high-grade dysplasia or esophageal adenocarcinoma over 1,832 patient-years of follow-up, for an overall annual risk of progression of 0.87%. Two patients progressed to esophageal adenocarcinoma (annual risk, 0.11%; 95% confidence interval, 0.03% to 0.44%), while 14 patients progressed to high-grade dysplasia (annual risk, 0.76%; 95% CI, 0.45% to 1.29%). Eight patients progressed to one of these two outcomes after a single nondysplastic biopsy, three progressed after two such biopsies, three progressed after three such biopsies, none progressed after four such biopsies, and two progressed after five such biopsies. Statistically, patients with at least five consecutive nondysplastic biopsies were no less likely to progress than were patients with only one nondysplastic biopsy (hazard ratio, 0.48; 95% CI, 0.07 to 1.92; P = .32). Hazard ratios for the other groups ranged between 0.0 and 0.85, with no significant difference in estimated risk between groups (P = .68) after controlling for age, sex, and length of Barrett’s esophagus.
The previous multicenter cohort study linked persistently nondysplastic Barrett’s esophagus with a lower rate of progression to esophageal adenocarcinoma, and, based on those findings, the authors suggested lengthening intervals between biopsy surveillance or even stopping surveillance, Dr. Krishnamoorthi and his associates noted. However, that study did not have mutually exclusive groups. “Additional data are required before increasing the interval between surveillance endoscopies based on persistence of nondysplastic Barrett’s esophagus,” they concluded.
The study lacked misclassification bias given long-segment Barrett’s esophagus, and specialized gastrointestinal pathologists interpreted all histology specimens, the researchers noted. “The small number of progressors is a potential limitation, reducing power to assess associations,” they added.
The investigators did not report funding sources. They reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Patients with multiple consecutive biopsies showing nondysplastic Barrett’s esophagus were statistically as likely to progress to esophageal adenocarcinoma or high-grade dysplasia as those with a single nondysplastic biopsy.
Major finding: Hazard ratios for progression ranged between 0.00 and 0.85, with no significant difference in estimated risk among groups stratified by number of consecutive nondysplastic biopsies (P = .68), after controlling for age, sex, and length of Barrett’s esophagus.
Data source: A prospective multicenter registry of 480 patients with nondysplastic Barrett’s esophagus and multiple surveillance biopsies.
Disclosures: The investigators did not report funding sources. They reported having no conflicts of interest.
Study confirms new mutation, possible therapeutic target in epidermolysis bullosa
PORTLAND, ORE. – New research has uncovered “a new kid on the block” of genes underlying epidermolysis bullosa simplex (EBS), which may someday pave the way for new therapies, John McGrath, MD, said at the annual meeting of the Society for Investigative Dermatology.
The gene encodes kelch-like family member 24 (KLHL24), the substrate receptor of cullin 3-RBX1-KLHL24 complex, explained Dr. McGrath, professor of molecular dermatology, St. John’s Institute of Dermatology, King’s College London, where he studies heritable skin diseases. Keratin 14 is the substrate of this complex. In EBS, KLHL24 mutations affect methionine initiation codons, resulting in the loss of the first 28 amino acids of KLHL24 protein. As a result, the protein becomes overly stable, which leads to excessive ubiquitination and breakdown of keratin 14.
KLHL24 is the 19th gene to be implicated in EBS. News of the finding broke in late 2016, when two publications identified KLHL24 mutations in 19 patients with EBS from 10 different families. The first study, conducted in China, identified start-codon mutations in the KLHL24 gene in five patients, confirmed keratin 14 as the substrate of the protein, and established that KLHL24 mutations induce disproportionate ubiquitination and fragmentation of keratin 14. The researchers confirmed these results by using a knock-in mouse model (Nat Genet. 2016 Dec;48 [12]:1508-16).
Meanwhile, European researchers identified KLHL24 mutations in families from Israel, Germany, Switzerland, Finland, Qatar, and Italy, and confirmed that these mutations affect the equilibrium between intermediate keratin filaments and keratin breakdown that is necessary for skin integrity (Am J Hum Genet. 2016 Dec 1;99[6]:1395-1404).
EBS affects more than 400,000 individuals worldwide, and is usually linked to heterozygous missense mutations in genes encoding keratins 5 and 14, Dr. McGrath noted. “However, EBS is clinically and genetically heterogeneous,” he added. “About 20% of cases have no mutation in known genes.” Pursuing an accurate diagnosis of EBS subtype is important for genetic counseling and clinical care, he emphasized.
Therefore, to help confirm the findings of the studies in China and Europe, Dr. McGrath and his associates performed Sanger sequencing, skin pathology, clinical phenotyping, and electron microscopy of samples from undiagnosed cases of EB simplex that were banked at the U.K.’s National Diagnostic EB Laboratory. Among 183 cases (about 20% of all EBS referrals to the laboratory) that had not been linked to other mutations, 7 from six families had heterozygous KLHL24 mutations.
Together, the three studies name five KLHL24 mutations from 26 individuals, 16 families, and eight countries, Dr. McGrath said. “All these mutations target the initiation codon of methionine,” he added. As has previously been reported in EBS, immunostaining for keratin 14 is positive, outlining areas of cleavage within the basal cell layer of the skin. Semi-thin sections reveal pallor in the basal layer of keratinocytes, and electron microscopy of this layer shows blister formation.
“In nonlesional basal keratinocytes, there are few keratin filaments and mitochondria are prominent,” Dr. McGrath explained. “Higher magnification confirms the lack of identifiable keratin filaments, and reveals prominent microtubules.” Lesional skin shows not only the paucity of keratin filaments, but clear evidence of cytolysis, with numerous autophagosomes, autolysosomes, and disruption of organelles in the cellular cytoplasm. “The keratin has been chopped up, digested, and is being organically recycled. That’s essentially what’s going on here,” he said.
These studies show how clinician scientists can help advance the diagnosis and treatment of skin diseases by linking clinical and molecular pathology, Dr. McGrath said. But just as importantly, they might reveal a new approach to treating EBS. “Instead of gene editing or protein therapy, we have a new mechanism that may be ripe for proteasomal inhibitors or other targets of the ubiquitination pathway,” he added. “It’s an intriguing way forward when thinking about drugs and small molecules for EB therapy.”
The National Institute for Health Research and the NHS Foundation Trust funded the study. Dr. McGrath had no relevant financial disclosures.
PORTLAND, ORE. – New research has uncovered “a new kid on the block” of genes underlying epidermolysis bullosa simplex (EBS), which may someday pave the way for new therapies, John McGrath, MD, said at the annual meeting of the Society for Investigative Dermatology.
The gene encodes kelch-like family member 24 (KLHL24), the substrate receptor of cullin 3-RBX1-KLHL24 complex, explained Dr. McGrath, professor of molecular dermatology, St. John’s Institute of Dermatology, King’s College London, where he studies heritable skin diseases. Keratin 14 is the substrate of this complex. In EBS, KLHL24 mutations affect methionine initiation codons, resulting in the loss of the first 28 amino acids of KLHL24 protein. As a result, the protein becomes overly stable, which leads to excessive ubiquitination and breakdown of keratin 14.
KLHL24 is the 19th gene to be implicated in EBS. News of the finding broke in late 2016, when two publications identified KLHL24 mutations in 19 patients with EBS from 10 different families. The first study, conducted in China, identified start-codon mutations in the KLHL24 gene in five patients, confirmed keratin 14 as the substrate of the protein, and established that KLHL24 mutations induce disproportionate ubiquitination and fragmentation of keratin 14. The researchers confirmed these results by using a knock-in mouse model (Nat Genet. 2016 Dec;48 [12]:1508-16).
Meanwhile, European researchers identified KLHL24 mutations in families from Israel, Germany, Switzerland, Finland, Qatar, and Italy, and confirmed that these mutations affect the equilibrium between intermediate keratin filaments and keratin breakdown that is necessary for skin integrity (Am J Hum Genet. 2016 Dec 1;99[6]:1395-1404).
EBS affects more than 400,000 individuals worldwide, and is usually linked to heterozygous missense mutations in genes encoding keratins 5 and 14, Dr. McGrath noted. “However, EBS is clinically and genetically heterogeneous,” he added. “About 20% of cases have no mutation in known genes.” Pursuing an accurate diagnosis of EBS subtype is important for genetic counseling and clinical care, he emphasized.
Therefore, to help confirm the findings of the studies in China and Europe, Dr. McGrath and his associates performed Sanger sequencing, skin pathology, clinical phenotyping, and electron microscopy of samples from undiagnosed cases of EB simplex that were banked at the U.K.’s National Diagnostic EB Laboratory. Among 183 cases (about 20% of all EBS referrals to the laboratory) that had not been linked to other mutations, 7 from six families had heterozygous KLHL24 mutations.
Together, the three studies name five KLHL24 mutations from 26 individuals, 16 families, and eight countries, Dr. McGrath said. “All these mutations target the initiation codon of methionine,” he added. As has previously been reported in EBS, immunostaining for keratin 14 is positive, outlining areas of cleavage within the basal cell layer of the skin. Semi-thin sections reveal pallor in the basal layer of keratinocytes, and electron microscopy of this layer shows blister formation.
“In nonlesional basal keratinocytes, there are few keratin filaments and mitochondria are prominent,” Dr. McGrath explained. “Higher magnification confirms the lack of identifiable keratin filaments, and reveals prominent microtubules.” Lesional skin shows not only the paucity of keratin filaments, but clear evidence of cytolysis, with numerous autophagosomes, autolysosomes, and disruption of organelles in the cellular cytoplasm. “The keratin has been chopped up, digested, and is being organically recycled. That’s essentially what’s going on here,” he said.
These studies show how clinician scientists can help advance the diagnosis and treatment of skin diseases by linking clinical and molecular pathology, Dr. McGrath said. But just as importantly, they might reveal a new approach to treating EBS. “Instead of gene editing or protein therapy, we have a new mechanism that may be ripe for proteasomal inhibitors or other targets of the ubiquitination pathway,” he added. “It’s an intriguing way forward when thinking about drugs and small molecules for EB therapy.”
The National Institute for Health Research and the NHS Foundation Trust funded the study. Dr. McGrath had no relevant financial disclosures.
PORTLAND, ORE. – New research has uncovered “a new kid on the block” of genes underlying epidermolysis bullosa simplex (EBS), which may someday pave the way for new therapies, John McGrath, MD, said at the annual meeting of the Society for Investigative Dermatology.
The gene encodes kelch-like family member 24 (KLHL24), the substrate receptor of cullin 3-RBX1-KLHL24 complex, explained Dr. McGrath, professor of molecular dermatology, St. John’s Institute of Dermatology, King’s College London, where he studies heritable skin diseases. Keratin 14 is the substrate of this complex. In EBS, KLHL24 mutations affect methionine initiation codons, resulting in the loss of the first 28 amino acids of KLHL24 protein. As a result, the protein becomes overly stable, which leads to excessive ubiquitination and breakdown of keratin 14.
KLHL24 is the 19th gene to be implicated in EBS. News of the finding broke in late 2016, when two publications identified KLHL24 mutations in 19 patients with EBS from 10 different families. The first study, conducted in China, identified start-codon mutations in the KLHL24 gene in five patients, confirmed keratin 14 as the substrate of the protein, and established that KLHL24 mutations induce disproportionate ubiquitination and fragmentation of keratin 14. The researchers confirmed these results by using a knock-in mouse model (Nat Genet. 2016 Dec;48 [12]:1508-16).
Meanwhile, European researchers identified KLHL24 mutations in families from Israel, Germany, Switzerland, Finland, Qatar, and Italy, and confirmed that these mutations affect the equilibrium between intermediate keratin filaments and keratin breakdown that is necessary for skin integrity (Am J Hum Genet. 2016 Dec 1;99[6]:1395-1404).
EBS affects more than 400,000 individuals worldwide, and is usually linked to heterozygous missense mutations in genes encoding keratins 5 and 14, Dr. McGrath noted. “However, EBS is clinically and genetically heterogeneous,” he added. “About 20% of cases have no mutation in known genes.” Pursuing an accurate diagnosis of EBS subtype is important for genetic counseling and clinical care, he emphasized.
Therefore, to help confirm the findings of the studies in China and Europe, Dr. McGrath and his associates performed Sanger sequencing, skin pathology, clinical phenotyping, and electron microscopy of samples from undiagnosed cases of EB simplex that were banked at the U.K.’s National Diagnostic EB Laboratory. Among 183 cases (about 20% of all EBS referrals to the laboratory) that had not been linked to other mutations, 7 from six families had heterozygous KLHL24 mutations.
Together, the three studies name five KLHL24 mutations from 26 individuals, 16 families, and eight countries, Dr. McGrath said. “All these mutations target the initiation codon of methionine,” he added. As has previously been reported in EBS, immunostaining for keratin 14 is positive, outlining areas of cleavage within the basal cell layer of the skin. Semi-thin sections reveal pallor in the basal layer of keratinocytes, and electron microscopy of this layer shows blister formation.
“In nonlesional basal keratinocytes, there are few keratin filaments and mitochondria are prominent,” Dr. McGrath explained. “Higher magnification confirms the lack of identifiable keratin filaments, and reveals prominent microtubules.” Lesional skin shows not only the paucity of keratin filaments, but clear evidence of cytolysis, with numerous autophagosomes, autolysosomes, and disruption of organelles in the cellular cytoplasm. “The keratin has been chopped up, digested, and is being organically recycled. That’s essentially what’s going on here,” he said.
These studies show how clinician scientists can help advance the diagnosis and treatment of skin diseases by linking clinical and molecular pathology, Dr. McGrath said. But just as importantly, they might reveal a new approach to treating EBS. “Instead of gene editing or protein therapy, we have a new mechanism that may be ripe for proteasomal inhibitors or other targets of the ubiquitination pathway,” he added. “It’s an intriguing way forward when thinking about drugs and small molecules for EB therapy.”
The National Institute for Health Research and the NHS Foundation Trust funded the study. Dr. McGrath had no relevant financial disclosures.
AT SID 2017
Key clinical point: Studies that have identified genes related to epidermolysis bullosa may lead to new treatment approaches for patients with epidermolysis bullosa simplex (EBS).
Major finding: Of 183 patients with EBS, not linked to other mutations, testing determined that 7 had heterozygous KLHL24 mutations, recently identified in patients with EB.
Data source: Samples from the 183 patients were banked at the United Kingdom’s National Diagnostic EB laboratory.
Disclosures: The National Institute for Health Research and the NHS Foundation Trust funded the study. Dr. McGrath had no relevant financial disclosures.
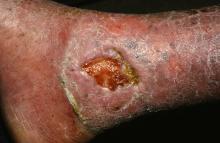
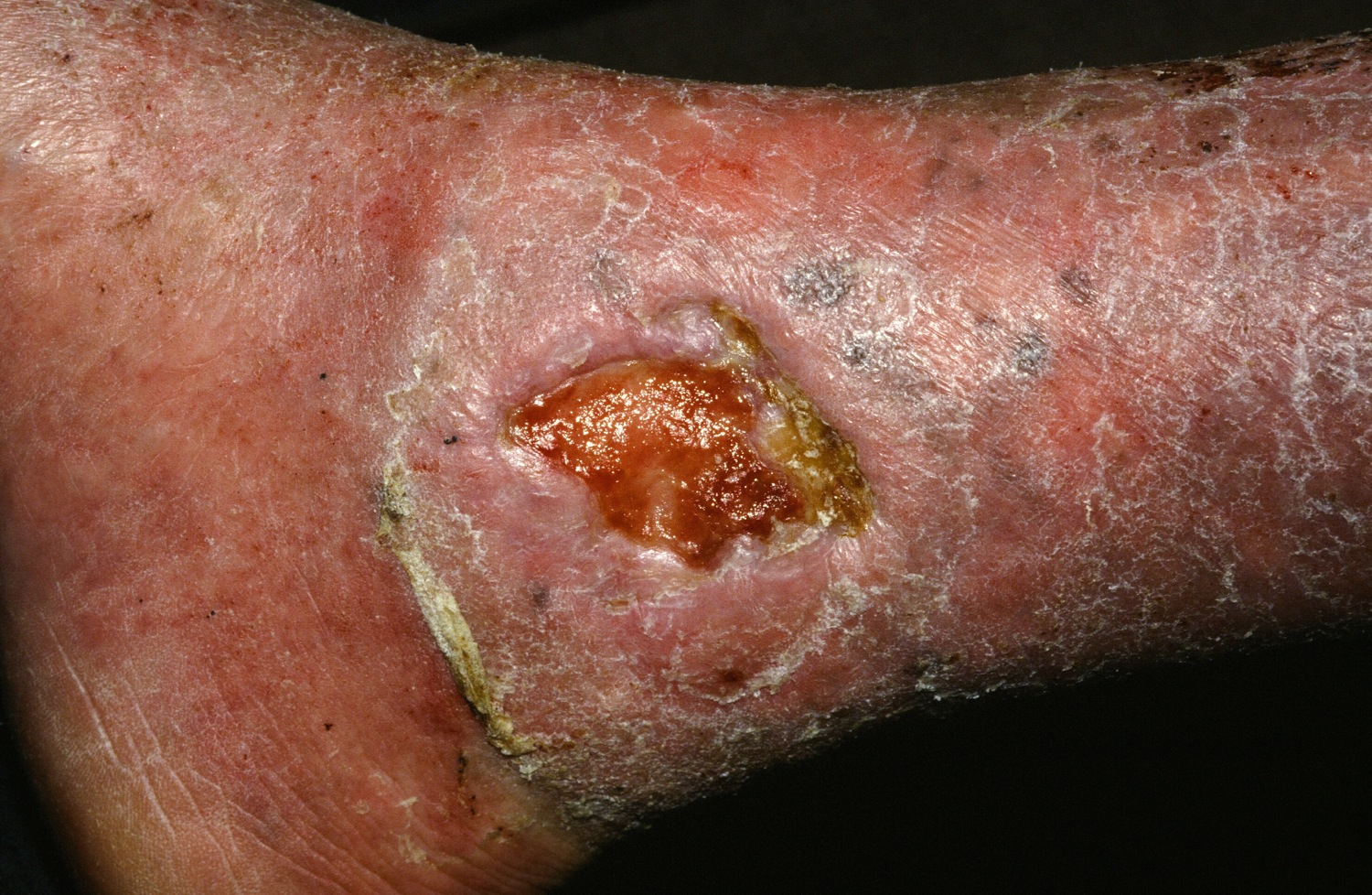
Stem cell therapy significantly improves ulcer healing
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
At SID 2017
Key clinical point: Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy.
Major finding: Neither control group achieved meaningful wound closure by week 24, while stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week (P less than .0005 for difference in healing rates among groups).
Data source: A randomized, controlled, double-blind pilot trial of 11 patients.
Disclosures: The National Institutes of Health supported the study. Dr. Grada had no conflicts of interest.
Mole count predicted melanoma death, especially among men
PORTLAND, ORE. – Among white men, the presence of at least one cutaneous nevus measuring 3 mm or more significantly predicted death from melanoma, in an adjusted analysis of a large prospective cohort study.
Mole count also predicted melanoma death among white women, but the association reached statistical significance only when women had at least three cutaneous nevi measuring 3 mm or more, Eunyoung Cho, ScD, said at the annual meeting of the Society for Investigative Dermatology. The reasons why these associations varied by sex is unclear, although previous studies have documented higher rates of melanoma death among men than among women, and even male physicians tend to seek health care less frequently than their female counterparts, Dr. Cho said in her poster presentation.
In the Nurses’ Health Study, white women with at least three moles measuring at least 3 mm in diameter were at significantly increased risk of dying of melanoma, compared with those with no moles that size (hazard ratio, 2.5; 95% confidence interval, 1.5-4.1), even after the investigators controlled for many other potential confounders, including sunburn history, skin reaction to sun during childhood, tanning ability, family history of melanoma, personal history of nonmelanoma skin cancer, age, activity level, smoking, body mass index, alcohol intake, and hair color. Women with one or two moles also showed a trend toward increased risk of melanoma death (HR, 1.4), but the 95% confidence interval for the hazard ratio did not reach statistical significance (0.9-2.3).
The investigators estimated that among white women, each additional mole measuring 3 mm or more conferred about a 12% increase in the melanoma death rate, even after confounders were controlled for.
In the Health Professionals Follow-Up Study, men with one or two moles of at least 3 mm had about twice the melanoma death rate as men without moles of this size (HR, 2.0; 95% CI, 1.3-3.3), even after investigators controlled for potential confounders. The risk of melanoma death was even greater among men with at least three moles (HR, 4.0; 95% CI, 2.5-6.2), and the difference in rates was statistically significant (P less than .0001). After confounders were accounted for, each additional mole measuring at least 3 mm conferred a 20% increase in the rate of melanoma death.
A different picture emerged after narrowing the adjusted analyses to include only people diagnosed with melanoma: In this group, mole count did not predict melanoma death among women, but continued to do so among men with melanoma who had at least three moles at baseline (HR, 1.8; 95% CI, 1.1-3.0), Dr. Cho reported. Among men, higher mole count also predicted melanoma of at least 1-mm Breslow thickness, an important prognostic factor, she added. Hazard ratios for these “thicker melanomas” were 1.9 (95% CI, 1.1-3.3) among men with one or two moles, and 2.5 (95% CI, 1.5-4.4) among men with three or more moles. Among women with melanoma, mole count did not predict Breslow thickness.
The extent to which sex affected trends in this analysis highlights the need for more studies of sex and other phenotypic risk factors for melanoma death, Dr. Cho concluded. She presented on behalf of lead author Wen-Qing Li, PhD, also of Brown University.
The National Institutes of Health and the Dermatology Foundation provided funding. Dr. Cho and Dr. Li had no relevant financial disclosures.
PORTLAND, ORE. – Among white men, the presence of at least one cutaneous nevus measuring 3 mm or more significantly predicted death from melanoma, in an adjusted analysis of a large prospective cohort study.
Mole count also predicted melanoma death among white women, but the association reached statistical significance only when women had at least three cutaneous nevi measuring 3 mm or more, Eunyoung Cho, ScD, said at the annual meeting of the Society for Investigative Dermatology. The reasons why these associations varied by sex is unclear, although previous studies have documented higher rates of melanoma death among men than among women, and even male physicians tend to seek health care less frequently than their female counterparts, Dr. Cho said in her poster presentation.
In the Nurses’ Health Study, white women with at least three moles measuring at least 3 mm in diameter were at significantly increased risk of dying of melanoma, compared with those with no moles that size (hazard ratio, 2.5; 95% confidence interval, 1.5-4.1), even after the investigators controlled for many other potential confounders, including sunburn history, skin reaction to sun during childhood, tanning ability, family history of melanoma, personal history of nonmelanoma skin cancer, age, activity level, smoking, body mass index, alcohol intake, and hair color. Women with one or two moles also showed a trend toward increased risk of melanoma death (HR, 1.4), but the 95% confidence interval for the hazard ratio did not reach statistical significance (0.9-2.3).
The investigators estimated that among white women, each additional mole measuring 3 mm or more conferred about a 12% increase in the melanoma death rate, even after confounders were controlled for.
In the Health Professionals Follow-Up Study, men with one or two moles of at least 3 mm had about twice the melanoma death rate as men without moles of this size (HR, 2.0; 95% CI, 1.3-3.3), even after investigators controlled for potential confounders. The risk of melanoma death was even greater among men with at least three moles (HR, 4.0; 95% CI, 2.5-6.2), and the difference in rates was statistically significant (P less than .0001). After confounders were accounted for, each additional mole measuring at least 3 mm conferred a 20% increase in the rate of melanoma death.
A different picture emerged after narrowing the adjusted analyses to include only people diagnosed with melanoma: In this group, mole count did not predict melanoma death among women, but continued to do so among men with melanoma who had at least three moles at baseline (HR, 1.8; 95% CI, 1.1-3.0), Dr. Cho reported. Among men, higher mole count also predicted melanoma of at least 1-mm Breslow thickness, an important prognostic factor, she added. Hazard ratios for these “thicker melanomas” were 1.9 (95% CI, 1.1-3.3) among men with one or two moles, and 2.5 (95% CI, 1.5-4.4) among men with three or more moles. Among women with melanoma, mole count did not predict Breslow thickness.
The extent to which sex affected trends in this analysis highlights the need for more studies of sex and other phenotypic risk factors for melanoma death, Dr. Cho concluded. She presented on behalf of lead author Wen-Qing Li, PhD, also of Brown University.
The National Institutes of Health and the Dermatology Foundation provided funding. Dr. Cho and Dr. Li had no relevant financial disclosures.
PORTLAND, ORE. – Among white men, the presence of at least one cutaneous nevus measuring 3 mm or more significantly predicted death from melanoma, in an adjusted analysis of a large prospective cohort study.
Mole count also predicted melanoma death among white women, but the association reached statistical significance only when women had at least three cutaneous nevi measuring 3 mm or more, Eunyoung Cho, ScD, said at the annual meeting of the Society for Investigative Dermatology. The reasons why these associations varied by sex is unclear, although previous studies have documented higher rates of melanoma death among men than among women, and even male physicians tend to seek health care less frequently than their female counterparts, Dr. Cho said in her poster presentation.
In the Nurses’ Health Study, white women with at least three moles measuring at least 3 mm in diameter were at significantly increased risk of dying of melanoma, compared with those with no moles that size (hazard ratio, 2.5; 95% confidence interval, 1.5-4.1), even after the investigators controlled for many other potential confounders, including sunburn history, skin reaction to sun during childhood, tanning ability, family history of melanoma, personal history of nonmelanoma skin cancer, age, activity level, smoking, body mass index, alcohol intake, and hair color. Women with one or two moles also showed a trend toward increased risk of melanoma death (HR, 1.4), but the 95% confidence interval for the hazard ratio did not reach statistical significance (0.9-2.3).
The investigators estimated that among white women, each additional mole measuring 3 mm or more conferred about a 12% increase in the melanoma death rate, even after confounders were controlled for.
In the Health Professionals Follow-Up Study, men with one or two moles of at least 3 mm had about twice the melanoma death rate as men without moles of this size (HR, 2.0; 95% CI, 1.3-3.3), even after investigators controlled for potential confounders. The risk of melanoma death was even greater among men with at least three moles (HR, 4.0; 95% CI, 2.5-6.2), and the difference in rates was statistically significant (P less than .0001). After confounders were accounted for, each additional mole measuring at least 3 mm conferred a 20% increase in the rate of melanoma death.
A different picture emerged after narrowing the adjusted analyses to include only people diagnosed with melanoma: In this group, mole count did not predict melanoma death among women, but continued to do so among men with melanoma who had at least three moles at baseline (HR, 1.8; 95% CI, 1.1-3.0), Dr. Cho reported. Among men, higher mole count also predicted melanoma of at least 1-mm Breslow thickness, an important prognostic factor, she added. Hazard ratios for these “thicker melanomas” were 1.9 (95% CI, 1.1-3.3) among men with one or two moles, and 2.5 (95% CI, 1.5-4.4) among men with three or more moles. Among women with melanoma, mole count did not predict Breslow thickness.
The extent to which sex affected trends in this analysis highlights the need for more studies of sex and other phenotypic risk factors for melanoma death, Dr. Cho concluded. She presented on behalf of lead author Wen-Qing Li, PhD, also of Brown University.
The National Institutes of Health and the Dermatology Foundation provided funding. Dr. Cho and Dr. Li had no relevant financial disclosures.
AT SID 2017
Key clinical point: Mole count was an independent risk factor for melanoma death among men and, to a lesser extent, among women.
Major finding: Adjusted hazard ratios were 2.0 among white men with one or two moles at least 3 mm in diameter and 4.0 among those with at least three moles, but among white women, the association was not significant unless they had at least three moles (HR, 2.5).
Data source: Adjusted analyses of 77,288 white women from the Nurses’ Health Study and 32,455 white men from the Health Professionals Follow-Up Study for 1986 through 2012.
Disclosures: The National Institutes of Health and the Dermatology Foundation provided funding for the study. Dr. Cho and Dr. Li had no relevant financial disclosures.
Teletriage cut dermatology wait times ninefold for patients at a free clinic
PORTLAND – For uninsured patients with limited health care access, a teledermatology triage protocol cut average appointment wait times by ninefold, and usually provided adequate dermatologic care without the need for in-person follow-up, Peter B. Chansky reported at the annual meeting of the Society for Investigative Dermatology.
“In our study, teledermatology was sufficient to triage 70% of cases, which significantly reduced time to evaluation, increased the availability of in-person appointments, and provided a new chance for volunteer dermatologists to serve disadvantaged populations that do not have access to specialty providers,” Mr. Chansky, a medical student at the University of Pennsylvania, Philadelphia, said during an oral presentation of his poster.
Puentes de Salud is a nonprofit, multidisciplinary health care clinic that serves uninsured Latino immigrants in southern Philadelphia, explained Mr. Chansky, who conducted the study under the mentorship of Jules B. Lipoff, MD, of the department of dermatology, at the University of Pennsylvania. Volunteer dermatologists hold a clinic at Puentes de Salud once per month, but patients’ need substantially outpaces supply, which has fueled long wait times and delays in care.
To test an alternative, the volunteer dermatologists created a “teletriage” system for primary care providers to turn to first, before attempting to schedule in-person dermatology appointments at Puentes de Salud. The results were striking: Teledermatology cut average wait times by a factor of 9.3, and patients who typically had gone months with unevaluated skin lesions waited an average of 1.4 days (standard deviation, 3.1 days) for a teledermatology consult, instead of 13.4 days (SD, 1.9 days) for an in-person appointment (P less than .0001).
Just as notably, teledermatologists changed or expanded on 70% of primary care providers’ diagnoses and altered their treatment plans 95% of the time. “Teledermatology also reclaimed 18% of monthly in-person clinic appointments for patients who needed face-to-face consultation,” Mr. Chansky said. “Access to dermatologic care is especially limited among uninsured patients, and using teledermatology to triage patients in a volunteer free clinic has never been evaluated,” he noted.
The analysis included 60 teletriage referrals from nurses and physicians over 2.5 years. Patients were usually male, averaged 32 years in age, and reported an average symptom duration of 15 months. Most lesions had not previously been treated. Cases were usually inflammatory in nature (45%), while 18% were neoplastic, 17% were infectious, and 8% were pigmented lesions. Lesions were usually located on visible areas of skin, including the face, hands, and arms.
This protocol relied on volunteer dermatologists, but teletriage repeatedly has been shown to provide effective dermatologic care in a variety of health care settings, Mr. Chansky noted. “Teledermatology is an accurate, cost-effective, and efficient tool for improving access to dermatologic care,” he added.
Mr. Chansky did not acknowledge external funding sources and had no conflicts of interest.
PORTLAND – For uninsured patients with limited health care access, a teledermatology triage protocol cut average appointment wait times by ninefold, and usually provided adequate dermatologic care without the need for in-person follow-up, Peter B. Chansky reported at the annual meeting of the Society for Investigative Dermatology.
“In our study, teledermatology was sufficient to triage 70% of cases, which significantly reduced time to evaluation, increased the availability of in-person appointments, and provided a new chance for volunteer dermatologists to serve disadvantaged populations that do not have access to specialty providers,” Mr. Chansky, a medical student at the University of Pennsylvania, Philadelphia, said during an oral presentation of his poster.
Puentes de Salud is a nonprofit, multidisciplinary health care clinic that serves uninsured Latino immigrants in southern Philadelphia, explained Mr. Chansky, who conducted the study under the mentorship of Jules B. Lipoff, MD, of the department of dermatology, at the University of Pennsylvania. Volunteer dermatologists hold a clinic at Puentes de Salud once per month, but patients’ need substantially outpaces supply, which has fueled long wait times and delays in care.
To test an alternative, the volunteer dermatologists created a “teletriage” system for primary care providers to turn to first, before attempting to schedule in-person dermatology appointments at Puentes de Salud. The results were striking: Teledermatology cut average wait times by a factor of 9.3, and patients who typically had gone months with unevaluated skin lesions waited an average of 1.4 days (standard deviation, 3.1 days) for a teledermatology consult, instead of 13.4 days (SD, 1.9 days) for an in-person appointment (P less than .0001).
Just as notably, teledermatologists changed or expanded on 70% of primary care providers’ diagnoses and altered their treatment plans 95% of the time. “Teledermatology also reclaimed 18% of monthly in-person clinic appointments for patients who needed face-to-face consultation,” Mr. Chansky said. “Access to dermatologic care is especially limited among uninsured patients, and using teledermatology to triage patients in a volunteer free clinic has never been evaluated,” he noted.
The analysis included 60 teletriage referrals from nurses and physicians over 2.5 years. Patients were usually male, averaged 32 years in age, and reported an average symptom duration of 15 months. Most lesions had not previously been treated. Cases were usually inflammatory in nature (45%), while 18% were neoplastic, 17% were infectious, and 8% were pigmented lesions. Lesions were usually located on visible areas of skin, including the face, hands, and arms.
This protocol relied on volunteer dermatologists, but teletriage repeatedly has been shown to provide effective dermatologic care in a variety of health care settings, Mr. Chansky noted. “Teledermatology is an accurate, cost-effective, and efficient tool for improving access to dermatologic care,” he added.
Mr. Chansky did not acknowledge external funding sources and had no conflicts of interest.
PORTLAND – For uninsured patients with limited health care access, a teledermatology triage protocol cut average appointment wait times by ninefold, and usually provided adequate dermatologic care without the need for in-person follow-up, Peter B. Chansky reported at the annual meeting of the Society for Investigative Dermatology.
“In our study, teledermatology was sufficient to triage 70% of cases, which significantly reduced time to evaluation, increased the availability of in-person appointments, and provided a new chance for volunteer dermatologists to serve disadvantaged populations that do not have access to specialty providers,” Mr. Chansky, a medical student at the University of Pennsylvania, Philadelphia, said during an oral presentation of his poster.
Puentes de Salud is a nonprofit, multidisciplinary health care clinic that serves uninsured Latino immigrants in southern Philadelphia, explained Mr. Chansky, who conducted the study under the mentorship of Jules B. Lipoff, MD, of the department of dermatology, at the University of Pennsylvania. Volunteer dermatologists hold a clinic at Puentes de Salud once per month, but patients’ need substantially outpaces supply, which has fueled long wait times and delays in care.
To test an alternative, the volunteer dermatologists created a “teletriage” system for primary care providers to turn to first, before attempting to schedule in-person dermatology appointments at Puentes de Salud. The results were striking: Teledermatology cut average wait times by a factor of 9.3, and patients who typically had gone months with unevaluated skin lesions waited an average of 1.4 days (standard deviation, 3.1 days) for a teledermatology consult, instead of 13.4 days (SD, 1.9 days) for an in-person appointment (P less than .0001).
Just as notably, teledermatologists changed or expanded on 70% of primary care providers’ diagnoses and altered their treatment plans 95% of the time. “Teledermatology also reclaimed 18% of monthly in-person clinic appointments for patients who needed face-to-face consultation,” Mr. Chansky said. “Access to dermatologic care is especially limited among uninsured patients, and using teledermatology to triage patients in a volunteer free clinic has never been evaluated,” he noted.
The analysis included 60 teletriage referrals from nurses and physicians over 2.5 years. Patients were usually male, averaged 32 years in age, and reported an average symptom duration of 15 months. Most lesions had not previously been treated. Cases were usually inflammatory in nature (45%), while 18% were neoplastic, 17% were infectious, and 8% were pigmented lesions. Lesions were usually located on visible areas of skin, including the face, hands, and arms.
This protocol relied on volunteer dermatologists, but teletriage repeatedly has been shown to provide effective dermatologic care in a variety of health care settings, Mr. Chansky noted. “Teledermatology is an accurate, cost-effective, and efficient tool for improving access to dermatologic care,” he added.
Mr. Chansky did not acknowledge external funding sources and had no conflicts of interest.
AT SID 2017
Key clinical point: For uninsured patients with limited health care access, teledermatology triage protocol can significantly cut appointment wait times and usually obviates the need for in-person follow-up.
Major finding: Teledermatology triage cut average appointment wait times by a factor of 9.3, and 70% of patients did not need additional in-person care.
Data source: An analysis of 60 referrals to teletriage over 2.5 years, among patients seen at a free clinic in Philadelphia.
Disclosures: Mr. Chansky did not acknowledge external funding sources, and had no conflicts of interest.
Modern estrogen ‘microdoses’ in contraceptives did not increase risk of melanoma
PORTLAND, ORE. – Long-term exposure to commonly used estrogen-based contraceptives was not associated with malignant melanoma in a single-center retrospective study of more than 77,000 women.
This null result belied decades-old studies performed when contraceptives contained much higher doses of estrogen, Kelly A. Mueller of Northwestern University, Chicago, said in a poster presented at the annual meeting of the Society for Investigative Dermatology. Current microdosing of ethinyl estradiol (EE) “is not associated with subsequent diagnosis of melanoma in our population, and is not inconsistent with the full prescribing information for commonly prescribed contraceptives containing EE,” she said. The study also shows how large retrospective analyses of long-term follow-up data can inform pharmacovigilance for rare, serious medical events.
To help clarify whether current microdosing (10-40 mcg/day) of EE can increase melanoma risk, the researchers compared 2,425 women prescribed oral, vaginal ring, or skin patch EE contraceptives for at least 12 months with 74,868 unexposed women. For both groups, initial clinical encounters occurred between 2001 and 2011, women were followed for at least 5 years, and none had a baseline history of melanoma or exogenous estrogen exposure. The data source was the Northwestern Medicine Enterprise Data Warehouse, which integrates electronic medical records from more than 4 million patients in the urban Midwest.
When first seen, patients tended to be in their late 20s and ranged in age between 18 and 40 years. Excluding cutaneous malignant melanomas diagnosed within 12 months of initial contraceptive prescription left three cases in the exposed group and 194 cases in the unexposed group, which translated to statistically similar rates of melanoma (0.1% and 0.3%, respectively; P = 0.3). The three cases in the exposed group were diagnosed between 37 and 92 months after initial prescription of EE contraceptives, but “the limited sample size for the outcome of interest did not allow for further analyses,” she reported. Nevertheless, the findings suggest no link between long-term microdosing of EE exposure and cutaneous melanoma, Ms. Mueller added.
The National Institutes of Health helps support the Northwestern Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
PORTLAND, ORE. – Long-term exposure to commonly used estrogen-based contraceptives was not associated with malignant melanoma in a single-center retrospective study of more than 77,000 women.
This null result belied decades-old studies performed when contraceptives contained much higher doses of estrogen, Kelly A. Mueller of Northwestern University, Chicago, said in a poster presented at the annual meeting of the Society for Investigative Dermatology. Current microdosing of ethinyl estradiol (EE) “is not associated with subsequent diagnosis of melanoma in our population, and is not inconsistent with the full prescribing information for commonly prescribed contraceptives containing EE,” she said. The study also shows how large retrospective analyses of long-term follow-up data can inform pharmacovigilance for rare, serious medical events.
To help clarify whether current microdosing (10-40 mcg/day) of EE can increase melanoma risk, the researchers compared 2,425 women prescribed oral, vaginal ring, or skin patch EE contraceptives for at least 12 months with 74,868 unexposed women. For both groups, initial clinical encounters occurred between 2001 and 2011, women were followed for at least 5 years, and none had a baseline history of melanoma or exogenous estrogen exposure. The data source was the Northwestern Medicine Enterprise Data Warehouse, which integrates electronic medical records from more than 4 million patients in the urban Midwest.
When first seen, patients tended to be in their late 20s and ranged in age between 18 and 40 years. Excluding cutaneous malignant melanomas diagnosed within 12 months of initial contraceptive prescription left three cases in the exposed group and 194 cases in the unexposed group, which translated to statistically similar rates of melanoma (0.1% and 0.3%, respectively; P = 0.3). The three cases in the exposed group were diagnosed between 37 and 92 months after initial prescription of EE contraceptives, but “the limited sample size for the outcome of interest did not allow for further analyses,” she reported. Nevertheless, the findings suggest no link between long-term microdosing of EE exposure and cutaneous melanoma, Ms. Mueller added.
The National Institutes of Health helps support the Northwestern Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
PORTLAND, ORE. – Long-term exposure to commonly used estrogen-based contraceptives was not associated with malignant melanoma in a single-center retrospective study of more than 77,000 women.
This null result belied decades-old studies performed when contraceptives contained much higher doses of estrogen, Kelly A. Mueller of Northwestern University, Chicago, said in a poster presented at the annual meeting of the Society for Investigative Dermatology. Current microdosing of ethinyl estradiol (EE) “is not associated with subsequent diagnosis of melanoma in our population, and is not inconsistent with the full prescribing information for commonly prescribed contraceptives containing EE,” she said. The study also shows how large retrospective analyses of long-term follow-up data can inform pharmacovigilance for rare, serious medical events.
To help clarify whether current microdosing (10-40 mcg/day) of EE can increase melanoma risk, the researchers compared 2,425 women prescribed oral, vaginal ring, or skin patch EE contraceptives for at least 12 months with 74,868 unexposed women. For both groups, initial clinical encounters occurred between 2001 and 2011, women were followed for at least 5 years, and none had a baseline history of melanoma or exogenous estrogen exposure. The data source was the Northwestern Medicine Enterprise Data Warehouse, which integrates electronic medical records from more than 4 million patients in the urban Midwest.
When first seen, patients tended to be in their late 20s and ranged in age between 18 and 40 years. Excluding cutaneous malignant melanomas diagnosed within 12 months of initial contraceptive prescription left three cases in the exposed group and 194 cases in the unexposed group, which translated to statistically similar rates of melanoma (0.1% and 0.3%, respectively; P = 0.3). The three cases in the exposed group were diagnosed between 37 and 92 months after initial prescription of EE contraceptives, but “the limited sample size for the outcome of interest did not allow for further analyses,” she reported. Nevertheless, the findings suggest no link between long-term microdosing of EE exposure and cutaneous melanoma, Ms. Mueller added.
The National Institutes of Health helps support the Northwestern Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
AT SID 2017
Key clinical point: Long-term exposure to modern “microdoses” of ethinyl estradiol in contraceptives was not associated with malignant melanoma.
Major finding: Rates were 0.1% in the exposed group and 0.3% in the unexposed group (P = .3).
Data source: A retrospective cohort study of 77,293 women.
Disclosures: The National Institutes of Health helps support the Northwestern Medicine Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
Systems modeling advances precision medicine in alopecia
PORTLAND – Alopecia areata can resist treatment stubbornly, but dermatologists might soon have better tools to predict response to therapy.
Personalized gene sequencing is key to this type of precision medicine, but conventional sequencing can be “extremely cumbersome and clinically impractical,” James C. Chen, PhD, said at the annual meeting of the Society for Investigative Dermatology.
During alopecia trials at Columbia, researchers routinely perform RNA sequencing of scalp biopsies to analyze therapeutic response on a molecular level. Using these RNAseq data from patients with untreated alopecia areata and gene regulatory network analysis data from the Algorithm for the Reconstruction of Accurate Cellular Networks, Dr. Chen and his associates modeled the molecular mechanisms of action of the pan–Janus kinase inhibitor tofacitinib, the JAK1/JAK2 inhibitor ruxolitinib, the CTLA4 inhibitor abatacept, and intralesional triamcinolone acetonide (IL-TAC). Heat maps of molecular responses to treatment showed distinct mechanisms of action between IL-TAC and abatacept, Dr. Chen said.
Furthermore, these therapies showed distinct and much less robust molecular effects than either ruxolitinib or tofacitinib. A Venn diagram of the biosignatures and molecular mechanisms of action of all four therapies showed little overlap. In fact, the probability of so little overlap between tofacitinib and IL-TAC occurring by chance was 0.023. The lack of overlap between the two JAK inhibitors was even more pronounced (P = 2.21 x 10–11).
Only 5-10 transcription factors are needed to capture these molecular mechanisms of action, which could greatly streamline precision dermatology in the future, according to Dr. Chen. “Systems biology offers a foundation for developing precision medicine strategies and selecting treatments for patients based on their individual molecular pathology,” he concluded. “Even when patients with alopecia areata have the same clinical phenotype, the molecular pathways they take to get there are not necessarily the same. We need to define those paths to maximize our chances of matching drugs to patients.”
Dr. Chen acknowledged support from the National Institutes of Health, epiCURE, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. He had no relevant financial conflicts of interest.
PORTLAND – Alopecia areata can resist treatment stubbornly, but dermatologists might soon have better tools to predict response to therapy.
Personalized gene sequencing is key to this type of precision medicine, but conventional sequencing can be “extremely cumbersome and clinically impractical,” James C. Chen, PhD, said at the annual meeting of the Society for Investigative Dermatology.
During alopecia trials at Columbia, researchers routinely perform RNA sequencing of scalp biopsies to analyze therapeutic response on a molecular level. Using these RNAseq data from patients with untreated alopecia areata and gene regulatory network analysis data from the Algorithm for the Reconstruction of Accurate Cellular Networks, Dr. Chen and his associates modeled the molecular mechanisms of action of the pan–Janus kinase inhibitor tofacitinib, the JAK1/JAK2 inhibitor ruxolitinib, the CTLA4 inhibitor abatacept, and intralesional triamcinolone acetonide (IL-TAC). Heat maps of molecular responses to treatment showed distinct mechanisms of action between IL-TAC and abatacept, Dr. Chen said.
Furthermore, these therapies showed distinct and much less robust molecular effects than either ruxolitinib or tofacitinib. A Venn diagram of the biosignatures and molecular mechanisms of action of all four therapies showed little overlap. In fact, the probability of so little overlap between tofacitinib and IL-TAC occurring by chance was 0.023. The lack of overlap between the two JAK inhibitors was even more pronounced (P = 2.21 x 10–11).
Only 5-10 transcription factors are needed to capture these molecular mechanisms of action, which could greatly streamline precision dermatology in the future, according to Dr. Chen. “Systems biology offers a foundation for developing precision medicine strategies and selecting treatments for patients based on their individual molecular pathology,” he concluded. “Even when patients with alopecia areata have the same clinical phenotype, the molecular pathways they take to get there are not necessarily the same. We need to define those paths to maximize our chances of matching drugs to patients.”
Dr. Chen acknowledged support from the National Institutes of Health, epiCURE, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. He had no relevant financial conflicts of interest.
PORTLAND – Alopecia areata can resist treatment stubbornly, but dermatologists might soon have better tools to predict response to therapy.
Personalized gene sequencing is key to this type of precision medicine, but conventional sequencing can be “extremely cumbersome and clinically impractical,” James C. Chen, PhD, said at the annual meeting of the Society for Investigative Dermatology.
During alopecia trials at Columbia, researchers routinely perform RNA sequencing of scalp biopsies to analyze therapeutic response on a molecular level. Using these RNAseq data from patients with untreated alopecia areata and gene regulatory network analysis data from the Algorithm for the Reconstruction of Accurate Cellular Networks, Dr. Chen and his associates modeled the molecular mechanisms of action of the pan–Janus kinase inhibitor tofacitinib, the JAK1/JAK2 inhibitor ruxolitinib, the CTLA4 inhibitor abatacept, and intralesional triamcinolone acetonide (IL-TAC). Heat maps of molecular responses to treatment showed distinct mechanisms of action between IL-TAC and abatacept, Dr. Chen said.
Furthermore, these therapies showed distinct and much less robust molecular effects than either ruxolitinib or tofacitinib. A Venn diagram of the biosignatures and molecular mechanisms of action of all four therapies showed little overlap. In fact, the probability of so little overlap between tofacitinib and IL-TAC occurring by chance was 0.023. The lack of overlap between the two JAK inhibitors was even more pronounced (P = 2.21 x 10–11).
Only 5-10 transcription factors are needed to capture these molecular mechanisms of action, which could greatly streamline precision dermatology in the future, according to Dr. Chen. “Systems biology offers a foundation for developing precision medicine strategies and selecting treatments for patients based on their individual molecular pathology,” he concluded. “Even when patients with alopecia areata have the same clinical phenotype, the molecular pathways they take to get there are not necessarily the same. We need to define those paths to maximize our chances of matching drugs to patients.”
Dr. Chen acknowledged support from the National Institutes of Health, epiCURE, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. He had no relevant financial conflicts of interest.
EXPERT ANALYSIS FROM SID 2017
Topical JAK inhibitor showed promise in facial vitiligo
PORTLAND – Twice-daily topical therapy with the Janus kinase (JAK) inhibitor ruxolitinib led to significant improvements in facial vitiligo in a small, uncontrolled, open-label, proof-of-concept study.
Four patients with significant baseline facial involvement improved by an average of 76% on the facial Vitiligo Area Scoring Index, or VASI (95% confidence interval, 53%-99%, P = .001), Brooke Rothstein reported at the annual meeting of the Society for Investigative Dermatology. The results suggest that topical JAK inhibition might help treat facial vitiligo, while potentially sparing patients from the side effects of oral therapy, said Ms. Rothstein, a medical student at Tufts University, Boston, who conducted the study under the mentorship of David Rosmarin, MD, of the department of dermatology at Tufts.
The study included 11 patients with vitiligo affecting at least 1% of body surface area. In all, 54% were male and the average age was 52 years. Patients applied ruxolitinib 1.5% phosphate cream to affected areas twice daily for 20 weeks. The primary outcome was percent improvement in VASI from baseline, Ms. Rothstein said.
By week 20, eight (73%) patients responded to treatment. Overall VASI scores improved by 23% (95% CI, 4%-43%; P = .02) when considering all patients and affected body regions. Three of eight patients responded on the body, and one of these eight patients also improved on acral surfaces, but these improvements were modest – less than 10%, compared with baseline, which was statistically insignificant.
Adverse events were generally mild and included erythema, hyperpigmentation, and transient acne, Ms. Rothstein reported. Despite the small sample size and open-label design of this study, the findings support further studies of topical JAK inhibition in vitiligo and add to mounting evidence that targeting interferon-gamma and its associated chemokines might stimulate repigmentation of skin in affected patients, she concluded.
This study also was published online in the Journal of the American Academy of Dermatology (J Am Acad Dermatol. 2017 Apr 5. doi: 10.1016/j.jaad.2017.02.049). The work was partially supported by Incyte, manufacturer of ruxolitinib (Jakafi), which supplied the study drug and reviewed the manuscript, but did not have final approval or control over the decision to submit for publication. An Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship also helped support the work. Ms. Rothstein and her coinvestigators reported having no financial conflicts of interest.
Ruxolitinib, in a tablet formulation, is approved by the Food and Drug Administration for treating myelofibrosis and polycythemia vera.
PORTLAND – Twice-daily topical therapy with the Janus kinase (JAK) inhibitor ruxolitinib led to significant improvements in facial vitiligo in a small, uncontrolled, open-label, proof-of-concept study.
Four patients with significant baseline facial involvement improved by an average of 76% on the facial Vitiligo Area Scoring Index, or VASI (95% confidence interval, 53%-99%, P = .001), Brooke Rothstein reported at the annual meeting of the Society for Investigative Dermatology. The results suggest that topical JAK inhibition might help treat facial vitiligo, while potentially sparing patients from the side effects of oral therapy, said Ms. Rothstein, a medical student at Tufts University, Boston, who conducted the study under the mentorship of David Rosmarin, MD, of the department of dermatology at Tufts.
The study included 11 patients with vitiligo affecting at least 1% of body surface area. In all, 54% were male and the average age was 52 years. Patients applied ruxolitinib 1.5% phosphate cream to affected areas twice daily for 20 weeks. The primary outcome was percent improvement in VASI from baseline, Ms. Rothstein said.
By week 20, eight (73%) patients responded to treatment. Overall VASI scores improved by 23% (95% CI, 4%-43%; P = .02) when considering all patients and affected body regions. Three of eight patients responded on the body, and one of these eight patients also improved on acral surfaces, but these improvements were modest – less than 10%, compared with baseline, which was statistically insignificant.
Adverse events were generally mild and included erythema, hyperpigmentation, and transient acne, Ms. Rothstein reported. Despite the small sample size and open-label design of this study, the findings support further studies of topical JAK inhibition in vitiligo and add to mounting evidence that targeting interferon-gamma and its associated chemokines might stimulate repigmentation of skin in affected patients, she concluded.
This study also was published online in the Journal of the American Academy of Dermatology (J Am Acad Dermatol. 2017 Apr 5. doi: 10.1016/j.jaad.2017.02.049). The work was partially supported by Incyte, manufacturer of ruxolitinib (Jakafi), which supplied the study drug and reviewed the manuscript, but did not have final approval or control over the decision to submit for publication. An Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship also helped support the work. Ms. Rothstein and her coinvestigators reported having no financial conflicts of interest.
Ruxolitinib, in a tablet formulation, is approved by the Food and Drug Administration for treating myelofibrosis and polycythemia vera.
PORTLAND – Twice-daily topical therapy with the Janus kinase (JAK) inhibitor ruxolitinib led to significant improvements in facial vitiligo in a small, uncontrolled, open-label, proof-of-concept study.
Four patients with significant baseline facial involvement improved by an average of 76% on the facial Vitiligo Area Scoring Index, or VASI (95% confidence interval, 53%-99%, P = .001), Brooke Rothstein reported at the annual meeting of the Society for Investigative Dermatology. The results suggest that topical JAK inhibition might help treat facial vitiligo, while potentially sparing patients from the side effects of oral therapy, said Ms. Rothstein, a medical student at Tufts University, Boston, who conducted the study under the mentorship of David Rosmarin, MD, of the department of dermatology at Tufts.
The study included 11 patients with vitiligo affecting at least 1% of body surface area. In all, 54% were male and the average age was 52 years. Patients applied ruxolitinib 1.5% phosphate cream to affected areas twice daily for 20 weeks. The primary outcome was percent improvement in VASI from baseline, Ms. Rothstein said.
By week 20, eight (73%) patients responded to treatment. Overall VASI scores improved by 23% (95% CI, 4%-43%; P = .02) when considering all patients and affected body regions. Three of eight patients responded on the body, and one of these eight patients also improved on acral surfaces, but these improvements were modest – less than 10%, compared with baseline, which was statistically insignificant.
Adverse events were generally mild and included erythema, hyperpigmentation, and transient acne, Ms. Rothstein reported. Despite the small sample size and open-label design of this study, the findings support further studies of topical JAK inhibition in vitiligo and add to mounting evidence that targeting interferon-gamma and its associated chemokines might stimulate repigmentation of skin in affected patients, she concluded.
This study also was published online in the Journal of the American Academy of Dermatology (J Am Acad Dermatol. 2017 Apr 5. doi: 10.1016/j.jaad.2017.02.049). The work was partially supported by Incyte, manufacturer of ruxolitinib (Jakafi), which supplied the study drug and reviewed the manuscript, but did not have final approval or control over the decision to submit for publication. An Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship also helped support the work. Ms. Rothstein and her coinvestigators reported having no financial conflicts of interest.
Ruxolitinib, in a tablet formulation, is approved by the Food and Drug Administration for treating myelofibrosis and polycythemia vera.
AT SID 2017
Key clinical point:
Major finding: Four patients with significant facial vitiligo improved by 76% on the facial Vitiligo Area Scoring Index, from baseline (P = .001).
Data source: An uncontrolled, open-label pilot study of 11 patients with vitiligo affecting more than 1% of body surface area.
Disclosures: The work was partially supported by Incyte, manufacturer of ruxolitinib, which supplied the study drug and reviewed the manuscript, but did not have final approval or control over the decision to submit for publication. An Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship also helped support the work. Ms. Rothstein and her coinvestigators reported having no financial conflicts of interest.