User login
Psychiatrist’s killer gets life in prison
A patient has been sentenced to life in prison 4 years after brutally murdering his psychiatrist.
According to news reports, Umar Dutt, then age 21, went to the office of psychiatrist Achutha Reddy, MD, in Wichita, Kan., on Sept. 19, 2017, aiming to hold the doctor hostage. Dr. Reddy’s office manager reportedly heard noise coming from the closed office and after entering, found Mr. Dutt assaulting the 57-year-old Dr. Reddy.
She intervened, and Dr. Reddy fled the building, but Mr. Dutt followed him and ultimately stabbed the physician more than 160 times. Mr. Dutt than ran over Dr. Reddy’s body.
The patient was arrested that day elsewhere and initially entered a “not guilty” plea in Sedgwick County District Court in 2019. Mr. Dutt was held in the county jail on a $1 million bond.
In September 2021, he changed his plea to guilty. He was sentenced on Nov. 9.
He received credit for time served of 4 years. The prosecutors and defense attorneys and the judge recommended that Mr. Dutt serve his sentence at Larned Correctional Mental Health Facility because of a history of mental illness.
KWCH reports that the Kansas Department of Corrections will ultimately decide where Mr. Dutt will be incarcerated.
Dr. Reddy left behind a wife and three children.
At Mr. Dutt’s sentencing hearing, Dr. Reddy’s widow, Beena Reddy, MD, a Wichita-based anesthesiologist, reportedly told the court: “My children and I have been devastated by Achutha’s death. Our stability, our security, our peace of mind, has been destroyed by the premeditated, evil actions of Umar Dutt.”
A version of this article first appeared on Medscape.com.
A patient has been sentenced to life in prison 4 years after brutally murdering his psychiatrist.
According to news reports, Umar Dutt, then age 21, went to the office of psychiatrist Achutha Reddy, MD, in Wichita, Kan., on Sept. 19, 2017, aiming to hold the doctor hostage. Dr. Reddy’s office manager reportedly heard noise coming from the closed office and after entering, found Mr. Dutt assaulting the 57-year-old Dr. Reddy.
She intervened, and Dr. Reddy fled the building, but Mr. Dutt followed him and ultimately stabbed the physician more than 160 times. Mr. Dutt than ran over Dr. Reddy’s body.
The patient was arrested that day elsewhere and initially entered a “not guilty” plea in Sedgwick County District Court in 2019. Mr. Dutt was held in the county jail on a $1 million bond.
In September 2021, he changed his plea to guilty. He was sentenced on Nov. 9.
He received credit for time served of 4 years. The prosecutors and defense attorneys and the judge recommended that Mr. Dutt serve his sentence at Larned Correctional Mental Health Facility because of a history of mental illness.
KWCH reports that the Kansas Department of Corrections will ultimately decide where Mr. Dutt will be incarcerated.
Dr. Reddy left behind a wife and three children.
At Mr. Dutt’s sentencing hearing, Dr. Reddy’s widow, Beena Reddy, MD, a Wichita-based anesthesiologist, reportedly told the court: “My children and I have been devastated by Achutha’s death. Our stability, our security, our peace of mind, has been destroyed by the premeditated, evil actions of Umar Dutt.”
A version of this article first appeared on Medscape.com.
A patient has been sentenced to life in prison 4 years after brutally murdering his psychiatrist.
According to news reports, Umar Dutt, then age 21, went to the office of psychiatrist Achutha Reddy, MD, in Wichita, Kan., on Sept. 19, 2017, aiming to hold the doctor hostage. Dr. Reddy’s office manager reportedly heard noise coming from the closed office and after entering, found Mr. Dutt assaulting the 57-year-old Dr. Reddy.
She intervened, and Dr. Reddy fled the building, but Mr. Dutt followed him and ultimately stabbed the physician more than 160 times. Mr. Dutt than ran over Dr. Reddy’s body.
The patient was arrested that day elsewhere and initially entered a “not guilty” plea in Sedgwick County District Court in 2019. Mr. Dutt was held in the county jail on a $1 million bond.
In September 2021, he changed his plea to guilty. He was sentenced on Nov. 9.
He received credit for time served of 4 years. The prosecutors and defense attorneys and the judge recommended that Mr. Dutt serve his sentence at Larned Correctional Mental Health Facility because of a history of mental illness.
KWCH reports that the Kansas Department of Corrections will ultimately decide where Mr. Dutt will be incarcerated.
Dr. Reddy left behind a wife and three children.
At Mr. Dutt’s sentencing hearing, Dr. Reddy’s widow, Beena Reddy, MD, a Wichita-based anesthesiologist, reportedly told the court: “My children and I have been devastated by Achutha’s death. Our stability, our security, our peace of mind, has been destroyed by the premeditated, evil actions of Umar Dutt.”
A version of this article first appeared on Medscape.com.
CDC: Thirty percent of hospital workers in U.S. still unvaccinated
, according to a new survey by the Centers for Disease Control and Prevention.
The snapshot in time – Jan. 20, 2021 to Sept. 15, 2021 – is based on voluntary weekly reports from hospitals. Only about 48% of the 5,085 hospitals in the U.S. Health and Human Services department’s Unified Hospital Data Surveillance System reported data on vaccination coverage during the period, and, after validation checks, the study included reports from 2,086 facilities, or just 41% of all hospitals, covering 3.35 million workers.
Overall, the number who were fully vaccinated rose from 36.1% in Jan. 2021 to 60.2% in April 2021, and then crept slowly up to 70% by Sept. 15, the CDC researchers reported in the American Journal of Infection Control.
The slowdown among hospital workers seems to mirror the same decline as in the general population.
Arjun Srinivasan, MD, associate director for health care–associated infection prevention programs at the CDC, said the decline in part may be the result of misinformation.
Health care personnel “are not fully immune from vaccine misinformation,” he said, adding that such misinformation “is contributing to decreased vaccine uptake among non–health care personnel.”
“The take-home message is that there is a lot of work to do in health care settings in order to get all of our health care personnel vaccinated,” Dr. Srinivasan told this news organization. “We need them to be vaccinated to protect themselves. It is also really important that we as health care personnel get vaccinated to protect our patients.”
Vaccine mandates
The analysis shows that workers were more likely to be vaccinated if they worked at a children’s hospital (77%), lived in metropolitan counties (71%), or worked in a hospital with lower cumulative admissions of COVID-19 patients, or lower cumulative COVID-19 cases.
The odds of being fully vaccinated were lower if the surrounding community had lower vaccination coverage. Workers in non-metropolitan counties (63.3%) and in rural counties (65.1%) were also less likely to be fully vaccinated, as well as those who were in critical access hospitals (64%) or long-term acute care hospitals (68.8%).
Surveys have shown that health care personnel who are vaccine-hesitant cited concerns they had about vaccine efficacy, adverse effects, the speed of vaccine development, and lack of full Food and Drug Administration approval, the study authors noted. In addition, many reported low trust in the government.
A Medscape survey this past April found that 25% of health care workers said they did not plan to be fully vaccinated. Some 40% of the 9,349 workers who responded said that employers should never require a COVID-19 vaccine for clinicians.
But the Centers for Medicare & Medicaid Services is attempting to require all health care facilities that receive Medicare or Medicaid payment to vaccinate workers. All eligible staff must receive the first dose of a two-dose COVID-19 vaccine or a one-dose vaccine by Dec. 6, and a second dose by Jan. 4, 2022. The policy allows exemptions based on recognized medical conditions or religious beliefs.
Some hospitals and health systems and various states and cities have already begun implementing vaccine mandates. Northwell Health in New York, for instance, lost 1,400 workers (evenly split between clinical and nonclinical staff), or 2% of its 77,000 employees, as a result of the state’s mandate.
Northwell’s workforce is now considered 100% vaccinated, a hospital spokesman said in an interview. In addition, “we have allowed for team members who changed their minds and presented proof of vaccination to return,” said the spokesman, adding that “a couple of hundred employees have done just that.”
Ten states sued the Biden administration recently, aiming to stop the health care worker vaccine mandate. Other challenges to vaccine mandates have generally been unsuccessful. The U.S. Supreme Court, for example, in October declined to hear a challenge to Maine’s mandate for health care workers, even though it did not allow religious exemptions, according to the Washington Post.
“The courts seem to agree that health care personnel are different, and could be subject to these mandates,” said Dr. Srinivasan.
A version of this article first appeared on Medscape.com.
, according to a new survey by the Centers for Disease Control and Prevention.
The snapshot in time – Jan. 20, 2021 to Sept. 15, 2021 – is based on voluntary weekly reports from hospitals. Only about 48% of the 5,085 hospitals in the U.S. Health and Human Services department’s Unified Hospital Data Surveillance System reported data on vaccination coverage during the period, and, after validation checks, the study included reports from 2,086 facilities, or just 41% of all hospitals, covering 3.35 million workers.
Overall, the number who were fully vaccinated rose from 36.1% in Jan. 2021 to 60.2% in April 2021, and then crept slowly up to 70% by Sept. 15, the CDC researchers reported in the American Journal of Infection Control.
The slowdown among hospital workers seems to mirror the same decline as in the general population.
Arjun Srinivasan, MD, associate director for health care–associated infection prevention programs at the CDC, said the decline in part may be the result of misinformation.
Health care personnel “are not fully immune from vaccine misinformation,” he said, adding that such misinformation “is contributing to decreased vaccine uptake among non–health care personnel.”
“The take-home message is that there is a lot of work to do in health care settings in order to get all of our health care personnel vaccinated,” Dr. Srinivasan told this news organization. “We need them to be vaccinated to protect themselves. It is also really important that we as health care personnel get vaccinated to protect our patients.”
Vaccine mandates
The analysis shows that workers were more likely to be vaccinated if they worked at a children’s hospital (77%), lived in metropolitan counties (71%), or worked in a hospital with lower cumulative admissions of COVID-19 patients, or lower cumulative COVID-19 cases.
The odds of being fully vaccinated were lower if the surrounding community had lower vaccination coverage. Workers in non-metropolitan counties (63.3%) and in rural counties (65.1%) were also less likely to be fully vaccinated, as well as those who were in critical access hospitals (64%) or long-term acute care hospitals (68.8%).
Surveys have shown that health care personnel who are vaccine-hesitant cited concerns they had about vaccine efficacy, adverse effects, the speed of vaccine development, and lack of full Food and Drug Administration approval, the study authors noted. In addition, many reported low trust in the government.
A Medscape survey this past April found that 25% of health care workers said they did not plan to be fully vaccinated. Some 40% of the 9,349 workers who responded said that employers should never require a COVID-19 vaccine for clinicians.
But the Centers for Medicare & Medicaid Services is attempting to require all health care facilities that receive Medicare or Medicaid payment to vaccinate workers. All eligible staff must receive the first dose of a two-dose COVID-19 vaccine or a one-dose vaccine by Dec. 6, and a second dose by Jan. 4, 2022. The policy allows exemptions based on recognized medical conditions or religious beliefs.
Some hospitals and health systems and various states and cities have already begun implementing vaccine mandates. Northwell Health in New York, for instance, lost 1,400 workers (evenly split between clinical and nonclinical staff), or 2% of its 77,000 employees, as a result of the state’s mandate.
Northwell’s workforce is now considered 100% vaccinated, a hospital spokesman said in an interview. In addition, “we have allowed for team members who changed their minds and presented proof of vaccination to return,” said the spokesman, adding that “a couple of hundred employees have done just that.”
Ten states sued the Biden administration recently, aiming to stop the health care worker vaccine mandate. Other challenges to vaccine mandates have generally been unsuccessful. The U.S. Supreme Court, for example, in October declined to hear a challenge to Maine’s mandate for health care workers, even though it did not allow religious exemptions, according to the Washington Post.
“The courts seem to agree that health care personnel are different, and could be subject to these mandates,” said Dr. Srinivasan.
A version of this article first appeared on Medscape.com.
, according to a new survey by the Centers for Disease Control and Prevention.
The snapshot in time – Jan. 20, 2021 to Sept. 15, 2021 – is based on voluntary weekly reports from hospitals. Only about 48% of the 5,085 hospitals in the U.S. Health and Human Services department’s Unified Hospital Data Surveillance System reported data on vaccination coverage during the period, and, after validation checks, the study included reports from 2,086 facilities, or just 41% of all hospitals, covering 3.35 million workers.
Overall, the number who were fully vaccinated rose from 36.1% in Jan. 2021 to 60.2% in April 2021, and then crept slowly up to 70% by Sept. 15, the CDC researchers reported in the American Journal of Infection Control.
The slowdown among hospital workers seems to mirror the same decline as in the general population.
Arjun Srinivasan, MD, associate director for health care–associated infection prevention programs at the CDC, said the decline in part may be the result of misinformation.
Health care personnel “are not fully immune from vaccine misinformation,” he said, adding that such misinformation “is contributing to decreased vaccine uptake among non–health care personnel.”
“The take-home message is that there is a lot of work to do in health care settings in order to get all of our health care personnel vaccinated,” Dr. Srinivasan told this news organization. “We need them to be vaccinated to protect themselves. It is also really important that we as health care personnel get vaccinated to protect our patients.”
Vaccine mandates
The analysis shows that workers were more likely to be vaccinated if they worked at a children’s hospital (77%), lived in metropolitan counties (71%), or worked in a hospital with lower cumulative admissions of COVID-19 patients, or lower cumulative COVID-19 cases.
The odds of being fully vaccinated were lower if the surrounding community had lower vaccination coverage. Workers in non-metropolitan counties (63.3%) and in rural counties (65.1%) were also less likely to be fully vaccinated, as well as those who were in critical access hospitals (64%) or long-term acute care hospitals (68.8%).
Surveys have shown that health care personnel who are vaccine-hesitant cited concerns they had about vaccine efficacy, adverse effects, the speed of vaccine development, and lack of full Food and Drug Administration approval, the study authors noted. In addition, many reported low trust in the government.
A Medscape survey this past April found that 25% of health care workers said they did not plan to be fully vaccinated. Some 40% of the 9,349 workers who responded said that employers should never require a COVID-19 vaccine for clinicians.
But the Centers for Medicare & Medicaid Services is attempting to require all health care facilities that receive Medicare or Medicaid payment to vaccinate workers. All eligible staff must receive the first dose of a two-dose COVID-19 vaccine or a one-dose vaccine by Dec. 6, and a second dose by Jan. 4, 2022. The policy allows exemptions based on recognized medical conditions or religious beliefs.
Some hospitals and health systems and various states and cities have already begun implementing vaccine mandates. Northwell Health in New York, for instance, lost 1,400 workers (evenly split between clinical and nonclinical staff), or 2% of its 77,000 employees, as a result of the state’s mandate.
Northwell’s workforce is now considered 100% vaccinated, a hospital spokesman said in an interview. In addition, “we have allowed for team members who changed their minds and presented proof of vaccination to return,” said the spokesman, adding that “a couple of hundred employees have done just that.”
Ten states sued the Biden administration recently, aiming to stop the health care worker vaccine mandate. Other challenges to vaccine mandates have generally been unsuccessful. The U.S. Supreme Court, for example, in October declined to hear a challenge to Maine’s mandate for health care workers, even though it did not allow religious exemptions, according to the Washington Post.
“The courts seem to agree that health care personnel are different, and could be subject to these mandates,” said Dr. Srinivasan.
A version of this article first appeared on Medscape.com.
U.S. overdose deaths hit an all-time high
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
a 28.5% increase from the previous year.
Deaths in some states rose even more precipitously. Vermont saw an almost 70% increase, and drug overdose deaths in West Virginia increased by 62%. Many states, including Alabama, California, Kansas, Kentucky, Louisiana, Tennessee, and Washington, had a 45%-50% rise in overdose deaths.
The data released by the CDC was provisional, as there is generally a lag between a reported overdose and confirmation of the death to the National Vital Statistics System. The agency uses statistical models that render the counts almost 100% accurate, the CDC says.
The vast majority (73,757) of overdose deaths involved opioids – with most of those (62,338) involving synthetic opioids such as fentanyl. Federal officials said that one American died every 5 minutes from an overdose, or 265 a day.
“We have to acknowledge what this is – it is a crisis,” Department of Health & Human Services Secretary Xavier Becerra told reporters on a call.
“As much as the numbers speak so vividly, they don’t tell the whole story. We see it in the faces of grieving families and all those overworked caregivers. You hear it every time you get that panicked 911 phone call, you read it in obituaries of sons and daughters who left us way too soon,” Mr. Becerra said.
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said that “this is unacceptable, and it requires an unprecedented response.”
Dr. Gupta, who noted that he has a waiver to treat substance use disorder patients with buprenorphine, said he’s seen “first-hand the heartbreak of the overdose epidemic,” adding that, with 23 years in practice, “I’ve learned that an overdose is a cry for help and for far too many people that cry goes unanswered.”
Both Mr. Becerra and Dr. Gupta called on Congress to pass President Joe Biden’s fiscal 2022 budget request, noting that it calls for $41 billion – a $669 million increase from fiscal year 2021 – to go to agencies working on drug interdiction and substance use prevention, treatment, and recovery support.
Dr. Gupta also announced that the administration was releasing a model law that could be used by state legislatures to help standardize policies on making the overdose antidote naloxone more accessible. Currently, such policies are a patchwork across the nation.
In addition, the federal government is newly supporting harm reduction, Mr. Becerra said. This means federal money can be used by clinics and outreach programs to buy fentanyl test strips, which they can then distribute to drug users.
“It’s important for Americans to have the ability to make sure that they can test for fentanyl in the substance,” Dr. Gupta said.
Fake pills, fentanyl a huge issue
Federal officials said that both fentanyl and methamphetamine are contributing to rising numbers of fatalities.
“Drug cartels in Mexico are mass-producing fentanyl and methamphetamine largely sourced from chemicals in China and they are distributing these substances throughout the United States,” Anne Milgram, administrator of the Drug Enforcement Administration, said on the call.
Ms. Milgram said the agency had seized 12,000 pounds of fentanyl in 2021, enough to provide every American with a lethal dose. Fentanyl is also mixed in with cocaine, heroin, methamphetamine, and marijuana – often in counterfeit pills, Ms. Milgram said.
The DEA and other law enforcement agencies have seized more than 14 million such pills in 2021. “These types of pills are easily accessible today on social media and e-commerce platforms, Ms. Milgram said.
“Drug dealers are now in our homes,” she said. “Wherever there is a smart phone or a computer, a dealer is one click away,” Ms. Milgram said.
National Institute on Drug Abuse Director Nora D. Volkow, MD, said that dealers will continue to push both fentanyl and methamphetamine because they are among the most addictive substances. They also are more profitable because they don’t require cultivation and harvesting, she said on the call.
Dr. Volkow also noted that naloxone is not as effective in reversing fentanyl overdoses because fentanyl is more potent than heroin and other opioids, and “it gets into the brain extremely rapidly.”
Ongoing research is aimed at developing a faster delivery mechanism and a longer-lasting formulation to counter overdoses, Dr. Volkow said.
A version of this article first appeared on Medscape.com.
Majority of justices seem receptive to bid to stop Texas abortion law
During over 3 hours of oral arguments on Nov. 1,
They seemed less certain about whether the federal government — which is also challenging the law — was within its rights to sue Texas.
Senate Bill 8, which went into effect September 1, allows any private citizen to file suit anywhere in the state against anyone who performs, induces, or “aids or abets” an abortion. If successful in court, the plaintiff is entitled to at least $10,000 and does not have to pay attorneys’ fees; rather, defendants are required to pay all legal costs.
In September, most justices denied an emergency request to stop the law but agreed to quickly hear the challenges in person.
At the Nov. 1 hearing, it appeared that a few justices who had let the law stand — notably conservatives Amy Coney Barrett and Brett Kavanaugh — were now agreeing that its challengers, in particular the abortion provider Whole Woman’s Health, might have a legal basis to move forward.
“I think it’s pretty likely the Court is going to do something that allows ‘someone’s’ suit against SB 8 to go ahead,” tweeted Raffi Melkonian, a Houston attorney, after the hearing. “I don’t know when they’re going to do that.”
The Supreme Court usually issues its opinions months after arguments. Since these two challenges — Whole Woman’s Health v. Jackson and US v. Texas — were heard on a faster schedule, there’s speculation that a decision could also come quickly.
“The court clearly is in a hurry,” wrote Florida State University law professor Mary Ziegler before the hearing in a post on court-tracking site SCOTUSblog. She said the court seems to be taking the abortion issue as seriously as most Americans, and that the justices could rule before it hears oral arguments on December 1 in a Mississippi case directly challenging Roe v. Wade.
In addition, data shows abortions have been severely curtailed in Texas since the law took effect — by as much as 50% according to researchers at the University of Texas at Austin. They reported that 2,164 abortions were provided in September 2021, compared with 4,313 in September 2020.
“The actual provisions in this law have prevented every woman in Texas from exercising a constitutional right as declared by this court,” said Justice Elena Kagan, clarifying that it was every woman who had not made a decision by 6 weeks.
“Usually, in these chilling effect cases, we’re kind of guessing,” she said. “Here, we’re not guessing. We know exactly what has happened as a result of this law. It has chilled everybody on the ground.”
Judge Edward Stone II, an attorney with the Texas Attorney General’s Office who argued for the state, denied Justice Kagan’s assertion.
Nineteen medical organizations, including the American Medical Association, American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, and the American College of Physicians, filed a friend of the court brief supporting both challenges, saying the Texas law allows legislators to interfere with the patient–doctor relationship and that it limits treatment options.
Texas argued that the only way to challenge the law at the federal level would be to be sued first.
Marc A. Hearron, a lawyer with the Center for Reproductive Rights who argued for Whole Woman’s Health, said that was untenable.
“What my friends on the other side are saying is that clinics should just violate the law,” and “subject themselves to the risk that they will be forced to close their doors,” said Mr. Hearron.
But even if providers decide to violate the law, “they may not find physicians, nurses, ultrasound technicians, staff members willing to work behind the desk, because this law targets all of them,” he said.
Plus, clinics run the risk of becoming permanent defendants because the law does not prohibit multiple suits, he said.
Whole Woman’s Health asked the justices to stop the law by preventing the state’s clerks from filing cases.
Federal standing not as clear
The U.S. Department of Justice sued Texas on September 9, saying the law negated the constitutional right to an abortion.
“The Act is clearly unconstitutional under longstanding Supreme Court precedent,” Attorney General Merrick Garland said at the time.
At the court, U.S. Solicitor General Elizabeth B. Prelogar called it a “brazen attack” on the supremacy of federal law and said it would open the door to other states mounting similar challenges.
Justice Kagan seemed to agree.
“The entire point of this law, its purpose, and its effect is to find the chink in the armor of Ex parte Young,” a 1908 law that “set out a basic principle of how our government is supposed to work and how people can seek review of unconstitutional state laws,” she said, decrying that “after all these many years, some geniuses came up with a way to evade the commands of that decision.”
Judge Stone waved off the concerns. “Nothing in this law even pretends that Texas courts could evade that because it can’t,” he said.
“Essentially, we would be inviting states, all 50 of them, with respect to their unpreferred constitutional rights, to try to nullify the law — that this Court has laid down as to the content of those rights,” said Justice Kagan.
Justice Kavanaugh also seemed concerned about that possibility.
“It could be free speech rights. It could be free exercise of religion rights. It could be Second Amendment rights if this position is accepted here,” he said, citing a brief submitted by the Firearms Policy Coalition that supported the Whole Woman’s Health challenge.
Justice Neil Gorsuch seemed dubious that the Texas law would undercut anybody’s right to challenge.
“Often constitutional rights, of course, can only be enforced in a defensive posture, when an individual is faced either with potential liability, punitive damages, but also, of course, civil fines — fines and even criminal sanction, including prison time,” he said.
Judge Stone argued that the U.S. government is “not a proper plaintiff” and did not have the right to sue Texas or any of its officials because none were involved in enforcing the law. If the federal government didn’t like the law, it should ask Congress to fix it, said Judge Stone.
After the hearing, Texas Attorney General Ken Paxton reiterated that position.
“The Biden Administration does not have the power to sue a state, such as Texas, just because it disagrees with a state law that protects the unborn,” he said in a statement.
A ruling on the challenges will not put an end to the litigation over SB 8.
“Even if the Supreme Court does rule that the abortion provider plaintiffs are allowed to sue, it is likely that there will still need to be more litigation in a federal trial court before SB 8 is actually determined to be unconstitutional and is blocked by a court order,” wrote Ian Millhiser, a Supreme Court scholar, after the hearing.
A federal judge in Austin did approve the Department of Justice’s request for a temporary halt to the law in October, but days later, the Fifth Circuit Court of Appeals ruled it could go back into effect while the legal questions were being pondered in the courts.
A version of this article first appeared on Medscape.com.
During over 3 hours of oral arguments on Nov. 1,
They seemed less certain about whether the federal government — which is also challenging the law — was within its rights to sue Texas.
Senate Bill 8, which went into effect September 1, allows any private citizen to file suit anywhere in the state against anyone who performs, induces, or “aids or abets” an abortion. If successful in court, the plaintiff is entitled to at least $10,000 and does not have to pay attorneys’ fees; rather, defendants are required to pay all legal costs.
In September, most justices denied an emergency request to stop the law but agreed to quickly hear the challenges in person.
At the Nov. 1 hearing, it appeared that a few justices who had let the law stand — notably conservatives Amy Coney Barrett and Brett Kavanaugh — were now agreeing that its challengers, in particular the abortion provider Whole Woman’s Health, might have a legal basis to move forward.
“I think it’s pretty likely the Court is going to do something that allows ‘someone’s’ suit against SB 8 to go ahead,” tweeted Raffi Melkonian, a Houston attorney, after the hearing. “I don’t know when they’re going to do that.”
The Supreme Court usually issues its opinions months after arguments. Since these two challenges — Whole Woman’s Health v. Jackson and US v. Texas — were heard on a faster schedule, there’s speculation that a decision could also come quickly.
“The court clearly is in a hurry,” wrote Florida State University law professor Mary Ziegler before the hearing in a post on court-tracking site SCOTUSblog. She said the court seems to be taking the abortion issue as seriously as most Americans, and that the justices could rule before it hears oral arguments on December 1 in a Mississippi case directly challenging Roe v. Wade.
In addition, data shows abortions have been severely curtailed in Texas since the law took effect — by as much as 50% according to researchers at the University of Texas at Austin. They reported that 2,164 abortions were provided in September 2021, compared with 4,313 in September 2020.
“The actual provisions in this law have prevented every woman in Texas from exercising a constitutional right as declared by this court,” said Justice Elena Kagan, clarifying that it was every woman who had not made a decision by 6 weeks.
“Usually, in these chilling effect cases, we’re kind of guessing,” she said. “Here, we’re not guessing. We know exactly what has happened as a result of this law. It has chilled everybody on the ground.”
Judge Edward Stone II, an attorney with the Texas Attorney General’s Office who argued for the state, denied Justice Kagan’s assertion.
Nineteen medical organizations, including the American Medical Association, American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, and the American College of Physicians, filed a friend of the court brief supporting both challenges, saying the Texas law allows legislators to interfere with the patient–doctor relationship and that it limits treatment options.
Texas argued that the only way to challenge the law at the federal level would be to be sued first.
Marc A. Hearron, a lawyer with the Center for Reproductive Rights who argued for Whole Woman’s Health, said that was untenable.
“What my friends on the other side are saying is that clinics should just violate the law,” and “subject themselves to the risk that they will be forced to close their doors,” said Mr. Hearron.
But even if providers decide to violate the law, “they may not find physicians, nurses, ultrasound technicians, staff members willing to work behind the desk, because this law targets all of them,” he said.
Plus, clinics run the risk of becoming permanent defendants because the law does not prohibit multiple suits, he said.
Whole Woman’s Health asked the justices to stop the law by preventing the state’s clerks from filing cases.
Federal standing not as clear
The U.S. Department of Justice sued Texas on September 9, saying the law negated the constitutional right to an abortion.
“The Act is clearly unconstitutional under longstanding Supreme Court precedent,” Attorney General Merrick Garland said at the time.
At the court, U.S. Solicitor General Elizabeth B. Prelogar called it a “brazen attack” on the supremacy of federal law and said it would open the door to other states mounting similar challenges.
Justice Kagan seemed to agree.
“The entire point of this law, its purpose, and its effect is to find the chink in the armor of Ex parte Young,” a 1908 law that “set out a basic principle of how our government is supposed to work and how people can seek review of unconstitutional state laws,” she said, decrying that “after all these many years, some geniuses came up with a way to evade the commands of that decision.”
Judge Stone waved off the concerns. “Nothing in this law even pretends that Texas courts could evade that because it can’t,” he said.
“Essentially, we would be inviting states, all 50 of them, with respect to their unpreferred constitutional rights, to try to nullify the law — that this Court has laid down as to the content of those rights,” said Justice Kagan.
Justice Kavanaugh also seemed concerned about that possibility.
“It could be free speech rights. It could be free exercise of religion rights. It could be Second Amendment rights if this position is accepted here,” he said, citing a brief submitted by the Firearms Policy Coalition that supported the Whole Woman’s Health challenge.
Justice Neil Gorsuch seemed dubious that the Texas law would undercut anybody’s right to challenge.
“Often constitutional rights, of course, can only be enforced in a defensive posture, when an individual is faced either with potential liability, punitive damages, but also, of course, civil fines — fines and even criminal sanction, including prison time,” he said.
Judge Stone argued that the U.S. government is “not a proper plaintiff” and did not have the right to sue Texas or any of its officials because none were involved in enforcing the law. If the federal government didn’t like the law, it should ask Congress to fix it, said Judge Stone.
After the hearing, Texas Attorney General Ken Paxton reiterated that position.
“The Biden Administration does not have the power to sue a state, such as Texas, just because it disagrees with a state law that protects the unborn,” he said in a statement.
A ruling on the challenges will not put an end to the litigation over SB 8.
“Even if the Supreme Court does rule that the abortion provider plaintiffs are allowed to sue, it is likely that there will still need to be more litigation in a federal trial court before SB 8 is actually determined to be unconstitutional and is blocked by a court order,” wrote Ian Millhiser, a Supreme Court scholar, after the hearing.
A federal judge in Austin did approve the Department of Justice’s request for a temporary halt to the law in October, but days later, the Fifth Circuit Court of Appeals ruled it could go back into effect while the legal questions were being pondered in the courts.
A version of this article first appeared on Medscape.com.
During over 3 hours of oral arguments on Nov. 1,
They seemed less certain about whether the federal government — which is also challenging the law — was within its rights to sue Texas.
Senate Bill 8, which went into effect September 1, allows any private citizen to file suit anywhere in the state against anyone who performs, induces, or “aids or abets” an abortion. If successful in court, the plaintiff is entitled to at least $10,000 and does not have to pay attorneys’ fees; rather, defendants are required to pay all legal costs.
In September, most justices denied an emergency request to stop the law but agreed to quickly hear the challenges in person.
At the Nov. 1 hearing, it appeared that a few justices who had let the law stand — notably conservatives Amy Coney Barrett and Brett Kavanaugh — were now agreeing that its challengers, in particular the abortion provider Whole Woman’s Health, might have a legal basis to move forward.
“I think it’s pretty likely the Court is going to do something that allows ‘someone’s’ suit against SB 8 to go ahead,” tweeted Raffi Melkonian, a Houston attorney, after the hearing. “I don’t know when they’re going to do that.”
The Supreme Court usually issues its opinions months after arguments. Since these two challenges — Whole Woman’s Health v. Jackson and US v. Texas — were heard on a faster schedule, there’s speculation that a decision could also come quickly.
“The court clearly is in a hurry,” wrote Florida State University law professor Mary Ziegler before the hearing in a post on court-tracking site SCOTUSblog. She said the court seems to be taking the abortion issue as seriously as most Americans, and that the justices could rule before it hears oral arguments on December 1 in a Mississippi case directly challenging Roe v. Wade.
In addition, data shows abortions have been severely curtailed in Texas since the law took effect — by as much as 50% according to researchers at the University of Texas at Austin. They reported that 2,164 abortions were provided in September 2021, compared with 4,313 in September 2020.
“The actual provisions in this law have prevented every woman in Texas from exercising a constitutional right as declared by this court,” said Justice Elena Kagan, clarifying that it was every woman who had not made a decision by 6 weeks.
“Usually, in these chilling effect cases, we’re kind of guessing,” she said. “Here, we’re not guessing. We know exactly what has happened as a result of this law. It has chilled everybody on the ground.”
Judge Edward Stone II, an attorney with the Texas Attorney General’s Office who argued for the state, denied Justice Kagan’s assertion.
Nineteen medical organizations, including the American Medical Association, American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, and the American College of Physicians, filed a friend of the court brief supporting both challenges, saying the Texas law allows legislators to interfere with the patient–doctor relationship and that it limits treatment options.
Texas argued that the only way to challenge the law at the federal level would be to be sued first.
Marc A. Hearron, a lawyer with the Center for Reproductive Rights who argued for Whole Woman’s Health, said that was untenable.
“What my friends on the other side are saying is that clinics should just violate the law,” and “subject themselves to the risk that they will be forced to close their doors,” said Mr. Hearron.
But even if providers decide to violate the law, “they may not find physicians, nurses, ultrasound technicians, staff members willing to work behind the desk, because this law targets all of them,” he said.
Plus, clinics run the risk of becoming permanent defendants because the law does not prohibit multiple suits, he said.
Whole Woman’s Health asked the justices to stop the law by preventing the state’s clerks from filing cases.
Federal standing not as clear
The U.S. Department of Justice sued Texas on September 9, saying the law negated the constitutional right to an abortion.
“The Act is clearly unconstitutional under longstanding Supreme Court precedent,” Attorney General Merrick Garland said at the time.
At the court, U.S. Solicitor General Elizabeth B. Prelogar called it a “brazen attack” on the supremacy of federal law and said it would open the door to other states mounting similar challenges.
Justice Kagan seemed to agree.
“The entire point of this law, its purpose, and its effect is to find the chink in the armor of Ex parte Young,” a 1908 law that “set out a basic principle of how our government is supposed to work and how people can seek review of unconstitutional state laws,” she said, decrying that “after all these many years, some geniuses came up with a way to evade the commands of that decision.”
Judge Stone waved off the concerns. “Nothing in this law even pretends that Texas courts could evade that because it can’t,” he said.
“Essentially, we would be inviting states, all 50 of them, with respect to their unpreferred constitutional rights, to try to nullify the law — that this Court has laid down as to the content of those rights,” said Justice Kagan.
Justice Kavanaugh also seemed concerned about that possibility.
“It could be free speech rights. It could be free exercise of religion rights. It could be Second Amendment rights if this position is accepted here,” he said, citing a brief submitted by the Firearms Policy Coalition that supported the Whole Woman’s Health challenge.
Justice Neil Gorsuch seemed dubious that the Texas law would undercut anybody’s right to challenge.
“Often constitutional rights, of course, can only be enforced in a defensive posture, when an individual is faced either with potential liability, punitive damages, but also, of course, civil fines — fines and even criminal sanction, including prison time,” he said.
Judge Stone argued that the U.S. government is “not a proper plaintiff” and did not have the right to sue Texas or any of its officials because none were involved in enforcing the law. If the federal government didn’t like the law, it should ask Congress to fix it, said Judge Stone.
After the hearing, Texas Attorney General Ken Paxton reiterated that position.
“The Biden Administration does not have the power to sue a state, such as Texas, just because it disagrees with a state law that protects the unborn,” he said in a statement.
A ruling on the challenges will not put an end to the litigation over SB 8.
“Even if the Supreme Court does rule that the abortion provider plaintiffs are allowed to sue, it is likely that there will still need to be more litigation in a federal trial court before SB 8 is actually determined to be unconstitutional and is blocked by a court order,” wrote Ian Millhiser, a Supreme Court scholar, after the hearing.
A federal judge in Austin did approve the Department of Justice’s request for a temporary halt to the law in October, but days later, the Fifth Circuit Court of Appeals ruled it could go back into effect while the legal questions were being pondered in the courts.
A version of this article first appeared on Medscape.com.
Which specialties get the biggest markups over Medicare rates?
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .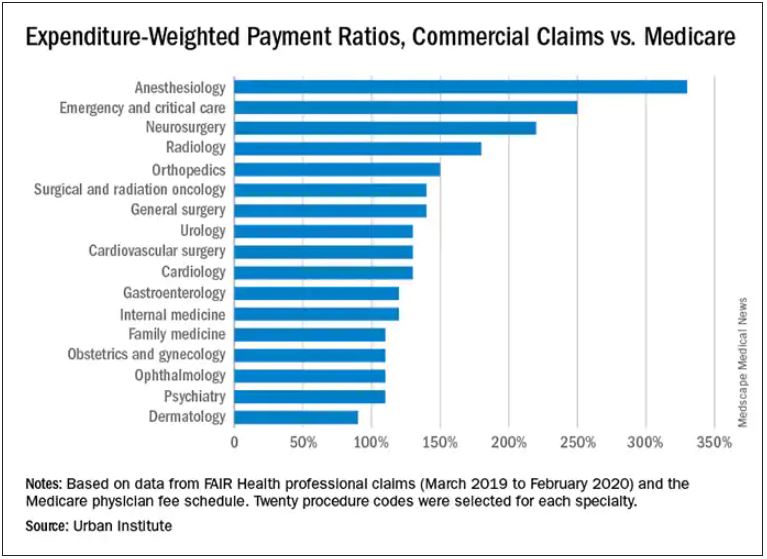
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
HHS okays first U.S. pilot to mandate coverage of gender-affirming care
The approval means transgender-related care must be included as part of the essential benefits offered on the state’s Affordable Care Act marketplace, which includes private individual and small group insurance plans. The coverage will start Jan. 1, 2023. Colorado is the first state in the United States to require such coverage.
The HHS notes that gender-affirming treatments to be covered include eye and lid modifications, face tightening, facial bone remodeling for facial feminization, breast/chest construction and reductions, and laser hair removal.
“I am proud to stand with Colorado to remove barriers that have historically made it difficult for transgender people to access health coverage and medical care,” said HHS Secretary Xavier Becerra in a statement.
“Colorado’s expansion of their essential health benefits to include gender-affirming surgery and other treatments is a model for other states to follow, and we invite other states to follow suit,” said Centers for Medicare & Medicaid Services Administrator Chiquita Brooks-LaSure in the statement.
Medicaid already covers comprehensive transgender care in Colorado.
The LGBTQ+ advocacy group One Colorado estimated that, thanks to the Affordable Care Act, only 5% of the state’s LGBTQ+ community was uninsured in 2019, compared to 10% in 2011.
However, 34% of transgender respondents to a One Colorado poll in 2018 said they had been denied coverage for an LGBTQ-specific medical service, such as gender-affirming care. Sixty-two percent said that a lack of insurance or limited insurance was a barrier to care; 84% said another barrier was the lack of adequately trained mental and behavioral health professionals.
Mental health also covered
The Colorado plan requires individual and small group plans to cover an annual 45- to 60-minute mental health wellness exam with a qualified mental health care practitioner. The visit can include behavioral health screening, education and consultation about healthy lifestyle changes, referrals to mental health treatment, and discussion of potential medication options.
The plans also must cover an additional 15 medications as alternatives to opioids and up to six acupuncture visits annually.
“This plan expands access to mental health services for Coloradans while helping those fighting substance abuse to overcome their addiction,” said Governor Jared Polis in a statement.
“This improves care for Coloradans and ensures that even more Coloradans have access to help when they need it,” he said.
A version of this article first appeared on Medscape.com.
The approval means transgender-related care must be included as part of the essential benefits offered on the state’s Affordable Care Act marketplace, which includes private individual and small group insurance plans. The coverage will start Jan. 1, 2023. Colorado is the first state in the United States to require such coverage.
The HHS notes that gender-affirming treatments to be covered include eye and lid modifications, face tightening, facial bone remodeling for facial feminization, breast/chest construction and reductions, and laser hair removal.
“I am proud to stand with Colorado to remove barriers that have historically made it difficult for transgender people to access health coverage and medical care,” said HHS Secretary Xavier Becerra in a statement.
“Colorado’s expansion of their essential health benefits to include gender-affirming surgery and other treatments is a model for other states to follow, and we invite other states to follow suit,” said Centers for Medicare & Medicaid Services Administrator Chiquita Brooks-LaSure in the statement.
Medicaid already covers comprehensive transgender care in Colorado.
The LGBTQ+ advocacy group One Colorado estimated that, thanks to the Affordable Care Act, only 5% of the state’s LGBTQ+ community was uninsured in 2019, compared to 10% in 2011.
However, 34% of transgender respondents to a One Colorado poll in 2018 said they had been denied coverage for an LGBTQ-specific medical service, such as gender-affirming care. Sixty-two percent said that a lack of insurance or limited insurance was a barrier to care; 84% said another barrier was the lack of adequately trained mental and behavioral health professionals.
Mental health also covered
The Colorado plan requires individual and small group plans to cover an annual 45- to 60-minute mental health wellness exam with a qualified mental health care practitioner. The visit can include behavioral health screening, education and consultation about healthy lifestyle changes, referrals to mental health treatment, and discussion of potential medication options.
The plans also must cover an additional 15 medications as alternatives to opioids and up to six acupuncture visits annually.
“This plan expands access to mental health services for Coloradans while helping those fighting substance abuse to overcome their addiction,” said Governor Jared Polis in a statement.
“This improves care for Coloradans and ensures that even more Coloradans have access to help when they need it,” he said.
A version of this article first appeared on Medscape.com.
The approval means transgender-related care must be included as part of the essential benefits offered on the state’s Affordable Care Act marketplace, which includes private individual and small group insurance plans. The coverage will start Jan. 1, 2023. Colorado is the first state in the United States to require such coverage.
The HHS notes that gender-affirming treatments to be covered include eye and lid modifications, face tightening, facial bone remodeling for facial feminization, breast/chest construction and reductions, and laser hair removal.
“I am proud to stand with Colorado to remove barriers that have historically made it difficult for transgender people to access health coverage and medical care,” said HHS Secretary Xavier Becerra in a statement.
“Colorado’s expansion of their essential health benefits to include gender-affirming surgery and other treatments is a model for other states to follow, and we invite other states to follow suit,” said Centers for Medicare & Medicaid Services Administrator Chiquita Brooks-LaSure in the statement.
Medicaid already covers comprehensive transgender care in Colorado.
The LGBTQ+ advocacy group One Colorado estimated that, thanks to the Affordable Care Act, only 5% of the state’s LGBTQ+ community was uninsured in 2019, compared to 10% in 2011.
However, 34% of transgender respondents to a One Colorado poll in 2018 said they had been denied coverage for an LGBTQ-specific medical service, such as gender-affirming care. Sixty-two percent said that a lack of insurance or limited insurance was a barrier to care; 84% said another barrier was the lack of adequately trained mental and behavioral health professionals.
Mental health also covered
The Colorado plan requires individual and small group plans to cover an annual 45- to 60-minute mental health wellness exam with a qualified mental health care practitioner. The visit can include behavioral health screening, education and consultation about healthy lifestyle changes, referrals to mental health treatment, and discussion of potential medication options.
The plans also must cover an additional 15 medications as alternatives to opioids and up to six acupuncture visits annually.
“This plan expands access to mental health services for Coloradans while helping those fighting substance abuse to overcome their addiction,” said Governor Jared Polis in a statement.
“This improves care for Coloradans and ensures that even more Coloradans have access to help when they need it,” he said.
A version of this article first appeared on Medscape.com.
Alleged on-the-job violence, racism, prompts psych workers to head to D.C.
A dozen workers from a psychiatric hospital near Seattle flew to Washington, D.C. to picket the National Association for Behavioral Healthcare’s annual meeting in an effort to get their employer to meet demands for a safer work environment, better staffing, and the hiring of security professionals.
They are also demanding that their employer, Cascade Behavioral Health Hospital, a private psychiatric facility owned by Acadia Healthcare and located in Tukwila, Washington, address what they call “racist harassment” by managers who have allegedly told many workers, who are primarily people of color, that they are going to be “filtered out,” Alazar Yirgu, a mental health technician at the facility, told this news organization.
The workers have been conducting a “safety strike” to protest working conditions at Cascade since early August. The protest in Tukwila began after a dozen or more workers were hurt in an August 1 incident during which they had attempted to restrain a violent patient.
“We’ve been out there for 2 months, and we will continue until our voice is heard,” said Mr. Yirgu, who was hospitalized as a result of the August patient outburst that he said has left him unable to work since the incident.
On Oct. 7, Mr. Yirgu and coworkers brought the protest to Washington, D.C., in a continued effort to voice their need for adequate personal protective equipment, increased staffing, and the hiring of security personnel.
“Any health care professional should not be fearful to do their job, because once they are in that state of mind, once they are fearful for themselves, then they are not doing their jobs; they are preoccupied with their fears,” said Mr. Yirgu, who has worked as a technician for 6 years.
Unsafe patient load
The workers reacted quickly after the August 1 patient outburst because there have been multiple previous incidents, Mr. Yirgu said.
In a 2019 news story by the Seattle Times, the newspaper reported there had been 65 assaults on patients or staff at Cascade from 2016 to 2018, resulting in concussions and broken bones in some instances.
Mr. Yirgu said that more recently, a patient broke a second story window, jumped to the ground, and ran off.
At the facility, workers are often assigned to as many as a dozen or more patients, he said, noting that at other psychiatric institutions, he’s cared for a maximum of five patients at once.
The Tukwila police have pushed back against the workers’ description of the incident in which Mr. Yirgu was injured, and Cascade Behavioral Health has aggressively defended its facility.
According to Mr. Yirgu, the expletive-spewing patient was clearly a danger to himself and others – especially after he stole a key card that would give him access to the entire facility, including the kitchen where knives were stored.
When more than a dozen staff answered the unit’s “Code Gray,” they were unable to subdue or restrain him. Mr. Yirgu ended up on the floor underneath the patient after the patient had jumped off a table.
As the incident unfolded, several workers called the police, who initially refused to go to the facility, saying that a new law prevented them from assisting with the restraint if there was no assault.
The Tukwila Police Department report shows that officers finally did go to the facility and determined that “a crime had not been committed based on the information presented to them, that there was no imminent threat of bodily harm, and that there was no legal grounds or authority for them to assist medical staff with physically restraining a patient.”
Cascade pushes back
A Service Employees International Union (SEIU) report shows about 70 workers refused to come in to work after the incident and began picketing outside the facility.
Cascade called it an illegal strike because the protesters had not given 10-days’ notice, as required by federal law, and moved to terminate those who participated. The local SEIU chapter, 1199NW, suggested the workers call their walkout a “safety strike,” because it was organized primarily to protest working conditions.
Meanwhile Cascade, which has erected a large fence so that no one in the facility can see the protesters, has said the strike is primarily about ongoing contract negotiations with the facility’s nurses and its union.
“The Union has been trying to apply unfair – and in some cases we believe unlawful – external pressures to this process, including picketing, work stoppages, smear campaigns, and false accusations,” Cascade CEO Christopher West wrote on the company’s website in mid-August.
He said the facility had “ample personal protective equipment” and that the “well-being and safety of our patients and staff always have been and will be our key priorities.”
In response to a request for comment, Cascade said in an emailed statement that physical confrontations had decreased by almost 50% and elopements (unauthorized leaving of the facility) by 80% from 2018 to 2021.
Cascade spokesperson Gretchen Hommrich said in the statement that the workers it has terminated “were let go for cause in violation to their employment agreement” and said the company still aimed to negotiate a new agreement with the union.
The “efforts outside of the bargaining process serve no productive purpose and have only brought harm to the residents they claim to serve,” said Ms. Hommrich.
‘Safety is the sole purpose’
Mr. Yirgu said it was outrageous to suggest workers were picketing over contract negotiations. “Safety is the sole purpose of this strike,” he said.
He noted that his patient care goal is to have a lot of one-on-one time with his patients, helping them navigate back to the outside world. The facility is supposed to be a safe place, Mr. Yirgu added. Violence inside the facility traumatizes the patients and may worsen their condition and delay their progress, he said.
“If I can’t keep them safe, there’s no way I’m going to be able to see them eye-to-eye when I told them I’d keep them safe and then they’re not anymore,” said Mr. Yirgu.
So far, 22 workers have been “terminated,” meaning they received a termination notice, have been taken off the work schedule by the employer, or otherwise been informed that the employer has deemed them to be separated, the SEIU reports. The organization has filed unfair labor practice (ULPs) for all 22.
A version of this article first appeared on Medscape.com.
A dozen workers from a psychiatric hospital near Seattle flew to Washington, D.C. to picket the National Association for Behavioral Healthcare’s annual meeting in an effort to get their employer to meet demands for a safer work environment, better staffing, and the hiring of security professionals.
They are also demanding that their employer, Cascade Behavioral Health Hospital, a private psychiatric facility owned by Acadia Healthcare and located in Tukwila, Washington, address what they call “racist harassment” by managers who have allegedly told many workers, who are primarily people of color, that they are going to be “filtered out,” Alazar Yirgu, a mental health technician at the facility, told this news organization.
The workers have been conducting a “safety strike” to protest working conditions at Cascade since early August. The protest in Tukwila began after a dozen or more workers were hurt in an August 1 incident during which they had attempted to restrain a violent patient.
“We’ve been out there for 2 months, and we will continue until our voice is heard,” said Mr. Yirgu, who was hospitalized as a result of the August patient outburst that he said has left him unable to work since the incident.
On Oct. 7, Mr. Yirgu and coworkers brought the protest to Washington, D.C., in a continued effort to voice their need for adequate personal protective equipment, increased staffing, and the hiring of security personnel.
“Any health care professional should not be fearful to do their job, because once they are in that state of mind, once they are fearful for themselves, then they are not doing their jobs; they are preoccupied with their fears,” said Mr. Yirgu, who has worked as a technician for 6 years.
Unsafe patient load
The workers reacted quickly after the August 1 patient outburst because there have been multiple previous incidents, Mr. Yirgu said.
In a 2019 news story by the Seattle Times, the newspaper reported there had been 65 assaults on patients or staff at Cascade from 2016 to 2018, resulting in concussions and broken bones in some instances.
Mr. Yirgu said that more recently, a patient broke a second story window, jumped to the ground, and ran off.
At the facility, workers are often assigned to as many as a dozen or more patients, he said, noting that at other psychiatric institutions, he’s cared for a maximum of five patients at once.
The Tukwila police have pushed back against the workers’ description of the incident in which Mr. Yirgu was injured, and Cascade Behavioral Health has aggressively defended its facility.
According to Mr. Yirgu, the expletive-spewing patient was clearly a danger to himself and others – especially after he stole a key card that would give him access to the entire facility, including the kitchen where knives were stored.
When more than a dozen staff answered the unit’s “Code Gray,” they were unable to subdue or restrain him. Mr. Yirgu ended up on the floor underneath the patient after the patient had jumped off a table.
As the incident unfolded, several workers called the police, who initially refused to go to the facility, saying that a new law prevented them from assisting with the restraint if there was no assault.
The Tukwila Police Department report shows that officers finally did go to the facility and determined that “a crime had not been committed based on the information presented to them, that there was no imminent threat of bodily harm, and that there was no legal grounds or authority for them to assist medical staff with physically restraining a patient.”
Cascade pushes back
A Service Employees International Union (SEIU) report shows about 70 workers refused to come in to work after the incident and began picketing outside the facility.
Cascade called it an illegal strike because the protesters had not given 10-days’ notice, as required by federal law, and moved to terminate those who participated. The local SEIU chapter, 1199NW, suggested the workers call their walkout a “safety strike,” because it was organized primarily to protest working conditions.
Meanwhile Cascade, which has erected a large fence so that no one in the facility can see the protesters, has said the strike is primarily about ongoing contract negotiations with the facility’s nurses and its union.
“The Union has been trying to apply unfair – and in some cases we believe unlawful – external pressures to this process, including picketing, work stoppages, smear campaigns, and false accusations,” Cascade CEO Christopher West wrote on the company’s website in mid-August.
He said the facility had “ample personal protective equipment” and that the “well-being and safety of our patients and staff always have been and will be our key priorities.”
In response to a request for comment, Cascade said in an emailed statement that physical confrontations had decreased by almost 50% and elopements (unauthorized leaving of the facility) by 80% from 2018 to 2021.
Cascade spokesperson Gretchen Hommrich said in the statement that the workers it has terminated “were let go for cause in violation to their employment agreement” and said the company still aimed to negotiate a new agreement with the union.
The “efforts outside of the bargaining process serve no productive purpose and have only brought harm to the residents they claim to serve,” said Ms. Hommrich.
‘Safety is the sole purpose’
Mr. Yirgu said it was outrageous to suggest workers were picketing over contract negotiations. “Safety is the sole purpose of this strike,” he said.
He noted that his patient care goal is to have a lot of one-on-one time with his patients, helping them navigate back to the outside world. The facility is supposed to be a safe place, Mr. Yirgu added. Violence inside the facility traumatizes the patients and may worsen their condition and delay their progress, he said.
“If I can’t keep them safe, there’s no way I’m going to be able to see them eye-to-eye when I told them I’d keep them safe and then they’re not anymore,” said Mr. Yirgu.
So far, 22 workers have been “terminated,” meaning they received a termination notice, have been taken off the work schedule by the employer, or otherwise been informed that the employer has deemed them to be separated, the SEIU reports. The organization has filed unfair labor practice (ULPs) for all 22.
A version of this article first appeared on Medscape.com.
A dozen workers from a psychiatric hospital near Seattle flew to Washington, D.C. to picket the National Association for Behavioral Healthcare’s annual meeting in an effort to get their employer to meet demands for a safer work environment, better staffing, and the hiring of security professionals.
They are also demanding that their employer, Cascade Behavioral Health Hospital, a private psychiatric facility owned by Acadia Healthcare and located in Tukwila, Washington, address what they call “racist harassment” by managers who have allegedly told many workers, who are primarily people of color, that they are going to be “filtered out,” Alazar Yirgu, a mental health technician at the facility, told this news organization.
The workers have been conducting a “safety strike” to protest working conditions at Cascade since early August. The protest in Tukwila began after a dozen or more workers were hurt in an August 1 incident during which they had attempted to restrain a violent patient.
“We’ve been out there for 2 months, and we will continue until our voice is heard,” said Mr. Yirgu, who was hospitalized as a result of the August patient outburst that he said has left him unable to work since the incident.
On Oct. 7, Mr. Yirgu and coworkers brought the protest to Washington, D.C., in a continued effort to voice their need for adequate personal protective equipment, increased staffing, and the hiring of security personnel.
“Any health care professional should not be fearful to do their job, because once they are in that state of mind, once they are fearful for themselves, then they are not doing their jobs; they are preoccupied with their fears,” said Mr. Yirgu, who has worked as a technician for 6 years.
Unsafe patient load
The workers reacted quickly after the August 1 patient outburst because there have been multiple previous incidents, Mr. Yirgu said.
In a 2019 news story by the Seattle Times, the newspaper reported there had been 65 assaults on patients or staff at Cascade from 2016 to 2018, resulting in concussions and broken bones in some instances.
Mr. Yirgu said that more recently, a patient broke a second story window, jumped to the ground, and ran off.
At the facility, workers are often assigned to as many as a dozen or more patients, he said, noting that at other psychiatric institutions, he’s cared for a maximum of five patients at once.
The Tukwila police have pushed back against the workers’ description of the incident in which Mr. Yirgu was injured, and Cascade Behavioral Health has aggressively defended its facility.
According to Mr. Yirgu, the expletive-spewing patient was clearly a danger to himself and others – especially after he stole a key card that would give him access to the entire facility, including the kitchen where knives were stored.
When more than a dozen staff answered the unit’s “Code Gray,” they were unable to subdue or restrain him. Mr. Yirgu ended up on the floor underneath the patient after the patient had jumped off a table.
As the incident unfolded, several workers called the police, who initially refused to go to the facility, saying that a new law prevented them from assisting with the restraint if there was no assault.
The Tukwila Police Department report shows that officers finally did go to the facility and determined that “a crime had not been committed based on the information presented to them, that there was no imminent threat of bodily harm, and that there was no legal grounds or authority for them to assist medical staff with physically restraining a patient.”
Cascade pushes back
A Service Employees International Union (SEIU) report shows about 70 workers refused to come in to work after the incident and began picketing outside the facility.
Cascade called it an illegal strike because the protesters had not given 10-days’ notice, as required by federal law, and moved to terminate those who participated. The local SEIU chapter, 1199NW, suggested the workers call their walkout a “safety strike,” because it was organized primarily to protest working conditions.
Meanwhile Cascade, which has erected a large fence so that no one in the facility can see the protesters, has said the strike is primarily about ongoing contract negotiations with the facility’s nurses and its union.
“The Union has been trying to apply unfair – and in some cases we believe unlawful – external pressures to this process, including picketing, work stoppages, smear campaigns, and false accusations,” Cascade CEO Christopher West wrote on the company’s website in mid-August.
He said the facility had “ample personal protective equipment” and that the “well-being and safety of our patients and staff always have been and will be our key priorities.”
In response to a request for comment, Cascade said in an emailed statement that physical confrontations had decreased by almost 50% and elopements (unauthorized leaving of the facility) by 80% from 2018 to 2021.
Cascade spokesperson Gretchen Hommrich said in the statement that the workers it has terminated “were let go for cause in violation to their employment agreement” and said the company still aimed to negotiate a new agreement with the union.
The “efforts outside of the bargaining process serve no productive purpose and have only brought harm to the residents they claim to serve,” said Ms. Hommrich.
‘Safety is the sole purpose’
Mr. Yirgu said it was outrageous to suggest workers were picketing over contract negotiations. “Safety is the sole purpose of this strike,” he said.
He noted that his patient care goal is to have a lot of one-on-one time with his patients, helping them navigate back to the outside world. The facility is supposed to be a safe place, Mr. Yirgu added. Violence inside the facility traumatizes the patients and may worsen their condition and delay their progress, he said.
“If I can’t keep them safe, there’s no way I’m going to be able to see them eye-to-eye when I told them I’d keep them safe and then they’re not anymore,” said Mr. Yirgu.
So far, 22 workers have been “terminated,” meaning they received a termination notice, have been taken off the work schedule by the employer, or otherwise been informed that the employer has deemed them to be separated, the SEIU reports. The organization has filed unfair labor practice (ULPs) for all 22.
A version of this article first appeared on Medscape.com.
New finasteride lawsuit brings renewed attention to psychiatric, ED adverse event reports
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
Baylor gets restraining order against COVID-19 vaccine–skeptic doc
in which he agreed to stop mentioning his prior leadership and academic appointments.
Baylor was the first institution to cut ties with Dr. McCullough, who has promoted the use of therapies seen as unproven for the treatment of COVID-19 and has questioned the effectiveness of COVID-19 vaccines. Since the Baylor suit, the Texas A&M College of Medicine, and the Texas Christian University (TCU) and University of North Texas Health Science Center (UNTHSC) School of Medicine have both removed Dr. McCullough from their faculties.
Granted by the 191st District Court in Dallas County, Tex., the Baylor restraining order – which is in effect at least until a hearing on the case on September 30 – was sought as part of Baylor Scott & White’s breach of contract suit against McCullough, who had previously been known as a well-respected expert in cardiorenal issues. The suit is seeking $1 million in damages, as well as attorneys’ fees.
The suit seeks to “enforce the terms” of the confidential employment separation agreement signed by Dr. McCullough in February and prevent Dr. McCullough from continuing “improper use of titles and claimed affiliations that have already confused the media, the medical community and the public,” it reads.
“This ongoing confusion regarding [Dr.] McCullough’s affiliations, and whether Plaintiffs support his opinions, is exactly what Plaintiffs bargained to avoid in the Separation Agreement,” and is likely to cause “irreparable reputational and business harm that is incapable of remedy by money damages alone,” the suit states.
One of Dr. McCullough’s attorneys, Clinton Mikel, maintains that all the times the physician was identified in the “thousands of hours of media interviews and countless publications since his departure from Baylor” were “said/printed by a third party with no encouragement from Dr. McCullough,” and that the doctor “does not and cannot control third parties.”
Mr. Mikel said in a statement emailed to this news organization by Dr. McCullough that the suit is “a politically motivated attempt to silence Dr. McCullough,” because it was filed on the same day the organization mandated COVID-19 vaccination for employees.
Dr. McCullough “intends to vigorously defend against Baylor’s unfounded lawsuit,” will seek to dissolve the restraining order, and recover “all payments due him from Baylor under the terms of the settlement agreement,” wrote Mr. Mikel.
The cardiologist’s legal team filed a motion to dismiss the suit on Aug. 9, essentially arguing that Baylor Scott & White’s action restricted Dr. McCullough’s right to free speech under the Texas Citizen’s Participation Act.
COVID-19 vaccines = bioterrorism?
Dr. McCullough accumulated a following in 2020 by promoting early at-home multidrug treatment of COVID-19 in interviews with conservative websites and at a U.S. Senate hearing in November.
Although Dr. McCullough does not appear to have any personal social media accounts, his broadcast and podcast interviews are tweeted by thousands daily around the world and featured on Facebook pages like “Pandemic Debate.”
Some Facebook posts with Dr. McCullough’s pronouncements have been labeled as misinformation or removed. Some of his videos remain on YouTube, where they are posted by the Association of American Physicians and Surgeons, a group that believes Dr. McCullough is “under fierce attack for speaking out about COVID-19 early treatment and vaccine safety.”
Dr. McCullough’s March 2021 testimony to the Texas Senate’s Health and Human Services Committee – in which he claimed that COVID-19 patients are being denied what he called proven treatments like hydroxychloroquine – has been viewed more than 3.7 million times on YouTube. The appearance has also been tweeted repeatedly.
Most of Dr. McCullough’s interviews and presentations are aggregated on Rumble, an alternative to YouTube.
In interviews, Dr. McCullough promotes the use of zinc, hydroxychloroquine, azithromycin, doxycycline, favipiravir, prednisone, and ivermectin as COVID-19 treatments – based on an outpatient treatment algorithm published in August 2020 in the American Journal of Medicine. The cardiologist was the lead author of that paper, which proposed treating people with COVID-like symptoms whether or not they had confirmed infection.
Dr. McCullough and colleagues published a follow-up paper that added colchicine to the mix in Reviews in Cardiovascular Medicine. Dr. McCullough is editor-in-chief of the journal, but this was not noted in the disclosures.
Similarly, Dr. McCullough has not disclosed in his COVID-19 publications or any interviews that he has received consulting fees from a host of pharmaceutical manufacturers that produce COVID-19 drugs and vaccines, including AstraZeneca, Eli Lilly, and Regeneron Pharmaceuticals. According to the Centers for Medicare & Medicaid Services’ Open Payments database, Dr. McCullough was paid about $300,000 annually by drug companies from 2014 to 2019, mostly for consulting on cardiovascular and diabetes medications. His payments dropped to $169,406.06 in 2020.
Dr. McCullough appeared on “The Ingraham Angle” on Fox News in December 2020, claiming that sequential, early treatment with “anti-infectives, corticosteroids, and then antithrombotics” could “reduce [COVID-19] hospitalizations by 85% and cut mortality in half.”
He repeated the claim on the Ingraham show in July and agreed with host Laura Ingraham that the vast majority of healthy people would do fine if they got COVID. He also made the claim that 84% of the COVID-19 cases in Israel were in people who had been vaccinated. “So it’s clear, we can’t vaccinate our way out of this,” he said. An Associated Press “fact check” report has pushed back on similar assertions about vaccine data from Israel.
In a separate interview posted in June, Dr. McCullough called the pandemic the first phase of a bioterrorism event, which was “all about keeping the population in fear and in isolation and preparing them to accept the vaccine, which appears to be phase two of a bioterrorism operation.”
In addition, he said, “good doctors are doing unthinkable things like injecting biologically active messenger RNA that produces this pathogenic spike protein into pregnant women.”
According to the Centers for Disease Control and Prevention, the vaccines teach the body to produce the spike protein, which then triggers an immune response that creates antibodies that will attack the virus.
A PolitiFact review debunks the notion that the mRNA vaccines are toxic, cytotoxic, or introduce live, active virus proteins into the body.
FactCheck.org also disputed Dr. McCullough’s claim in a July 13 Ingraham Angle appearance that the mRNA vaccines are ineffective against the Delta variant.
In the FactCheck article, Frederic Bushman, codirector of the University of Pennsylvania’s Center for Research on Coronaviruses and Other Emerging Pathogens, said that people were much better off being vaccinated than not,” adding, “the Delta variant may reduce the effectiveness [of the vaccines] a little, but still, they’re so effective that you get a lot of benefit.”
“The vaccines are failing,” Dr. McCullough asserted in an Aug. 3 video interview posted on Odysee. “As we sit here today, we have 11,000 Americans that the CDC has certified have died after the vaccine,” he said, citing two analyses – one by Jessica Rose, PhD, and another by British researchers.
Similar figures reportedly based on cases reported to the Food and Drug Administration’s Vaccine Adverse Events Reporting System (VAERS) were forwarded to this news organization by Dr. McCullough.
The CDC website notes that the agency has received reports of 7,653 deaths in people who received a vaccine as of Sept. 13 (0.0020% of vaccine doses given since Dec. 14, 2020), but it cautions that those deaths do not mean the vaccine was the cause.
Dr. McCullough repeatedly claimed in the Aug. 3 interview that the government has not been transparent on vaccine safety. Since June 2020, the CDC’s Advisory Committee on Immunization Practices has held 16 public meetings on the COVID-19 vaccines.
To date, the agency has advised clinicians to monitor for rare side effects including Guillain-Barré syndrome and thrombosis with thrombocytopenia syndrome after the Johnson & Johnson vaccine and myocarditis after mRNA (Pfizer-BioNTech and Moderna) vaccines.
Med schools distance themselves
According to the Baylor Scott & White suit, Dr. McCullough agreed on Feb. 24 in a confidential separation agreement that he would no longer use his academic or leadership titles nor hold himself out to be affiliated with Baylor University Medical Center, Baylor Heart and Vascular Institute, the Baylor Research Institute, or any other related institutions.
However, as of August, according to a Baylor spokesperson, McCullough continued to have privileges at Baylor University Medical Center and Baylor Scott & White Heart and Vascular Hospital, Dallas.
The lawsuit points to three interviews posted in June and July where Dr. McCullough is identified as a “vice chief of medicine” or a “vice chief of internal medicine,” both at Baylor University. It also cites a profile at the Cardiometabolic Health Congress website – which this news organization had also viewed – that was still active in late July with a similar title. The profile was later scrubbed from the site.
Social media posts and other media continue to refer to Dr. McCullough’s Baylor credentials. An episode of the Faith and Freedom podcast posted on Aug. 2 identified McCullough as a “professor of medicine at Baylor University Medical Center.”
As of Sept. 16, Dr. McCullough’s bio page at his current practice, Heart Place, lists him as a professor of medicine at Texas A&M College of Medicine. A spokesperson for Texas A&M told this news organization that McCullough is no longer affiliated with the school.
Dr. McCullough acknowledged in the Aug. 3 interview that his Texas A&M title had been “stripped away” at “around the same time this lawsuit was filed.”
He was still a professor of medicine at the TCU and UNTHSC School of Medicine in Fort Worth, but a school spokesperson notified this news organization on Aug. 19 that Dr. McCullough was no longer with the school.
Dr. McCullough has portrayed himself as both a victim and a truth-teller, a “concerned physician” warning the world about the dangers of COVID-19 vaccines. The Baylor Scott & White lawsuit “is really a strong-armed tactic,” he said in the Aug. 3 interview. “I’m just a little guy, so I have to hire my legal teams, and in a sense be drained dry on legal fees,” he said.
But Dr. McCullough apparently has a plan for helping to defray his legal costs. In the Aug. 3 interview, he said a foundation he helped start, Truth for Health, has a “donation side to it,” adding “some of that may be used for legal expense.”
Cheryl Jones, an attorney with PK Law in Towson, Md., said that might draw interest from the Internal Revenue Service. “I would expect IRS scrutiny if contributions to the Medical Censorship Defense Fund are used to defend Dr McCullough in his personal breach of contract lawsuit,” she told this news organization.
The IRS generally recognizes defending “human and civil rights secured by law” as a legitimate charitable purpose for a legal defense fund, she said, adding that such a fund “must serve only public, rather than private, interests.”
Misinformation from a physician more damaging?
Some in the medical field have refuted Dr. McCullough’s pronouncements on how to treat COVID-19, including two infectious disease specialists with Monash University, Melbourne, who responded to the cardiologist’s original paper in the American Journal of Medicine.
Tony Korman, MBBS, a professor at the Centre for Inflammatory Diseases at Monash, told this news organization, “we had concerns that reputable medical journals would accept and publish papers proposing treatment of COVID-19 which was not supported by evidence.”
The website Healthfeedback.org has also challenged McCullough’s and his supporters’ claims, including that the American Journal of Medicine endorsed the use of hydroxychloroquine and that the COVID-19 vaccines have caused thousands of deaths.
David Broniatowski, PhD, associate director for the Institute for Data, Democracy and Politics at George Washington University, Washington, said in an interview that Dr. McCullough’s casting himself as a “rebel doctor” is a well-known trope in the vaccine misinformation universe.
Although he was not familiar with Dr. McCullough, Dr. Broniatowski said the cardiologist’s claims are not unique – they’ve been circulating among antivaccine and conspiracy-oriented groups for months.
For instance, Dr. McCullough has claimed in interviews that a whistleblower within the CDC knows of 50,000 vaccine-related deaths. Using data from the supposed whistleblower, the group America’s Frontline Doctors sued the federal government in July to stop the administration of COVID-19 vaccines to those under 18, people who have already had COVID, and individuals who the group said have not been adequately informed about the risks.
The idea of a whistleblower inside the CDC is recycled from antivaccine claims from decades ago, Dr. Broniatowski said.
But, he added, “somebody who speaks with the credibility of a major institution will be more likely to be listened to by some people.” That vulnerable group is “being taken advantage of by a relatively small number of disinformation purveyors, who, in some cases, profit from that disinformation,” said Dr. Broniatowski.
“We rely on our doctors because we trust them,” he said. “And we trust them because we believe that as physicians, their value system places the patient’s best interests first. That’s why it’s so much of a disappointment when you have a physician that appears to be exercising this sort of bad judgment.”
Paul Offit, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, also said that he was not familiar with Dr. McCullough. But apprised of his claims, Dr. Offit told this news organization, “Peter McCullough is a friend of the virus.”
“The kind of information he promotes allows the virus to continue to spread, continue to do an enormous amount of harm, and continue to mutate and create variants that have become more contagious and more resistant to vaccine-induced immunity,” said Dr. Offit, the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia.
Dr. Offit added that the war should be against SARS-CoV-2, but “because this virus has so many supporters, the war in essence becomes a war against ourselves, which is much harder.”
Dr. McCullough maintains he is doing a service to his patients. “I’m just giving and trying to help America understand the pandemic,” he told Ms. Ingraham on Fox News on July 29.
But he acknowledged concern about the Federation of State Medical Board’s announcement that physicians who spread COVID-19 vaccine misinformation risk suspension or revocation of their license.
“I have to tell you I’m worried – that no matter what I do and how careful I am to cite the scientific studies, I’m still gonna be hunted down for quote, misinformation,” he said in the Aug. 3 interview.
A version of this article first appeared on Medscape.com.
in which he agreed to stop mentioning his prior leadership and academic appointments.
Baylor was the first institution to cut ties with Dr. McCullough, who has promoted the use of therapies seen as unproven for the treatment of COVID-19 and has questioned the effectiveness of COVID-19 vaccines. Since the Baylor suit, the Texas A&M College of Medicine, and the Texas Christian University (TCU) and University of North Texas Health Science Center (UNTHSC) School of Medicine have both removed Dr. McCullough from their faculties.
Granted by the 191st District Court in Dallas County, Tex., the Baylor restraining order – which is in effect at least until a hearing on the case on September 30 – was sought as part of Baylor Scott & White’s breach of contract suit against McCullough, who had previously been known as a well-respected expert in cardiorenal issues. The suit is seeking $1 million in damages, as well as attorneys’ fees.
The suit seeks to “enforce the terms” of the confidential employment separation agreement signed by Dr. McCullough in February and prevent Dr. McCullough from continuing “improper use of titles and claimed affiliations that have already confused the media, the medical community and the public,” it reads.
“This ongoing confusion regarding [Dr.] McCullough’s affiliations, and whether Plaintiffs support his opinions, is exactly what Plaintiffs bargained to avoid in the Separation Agreement,” and is likely to cause “irreparable reputational and business harm that is incapable of remedy by money damages alone,” the suit states.
One of Dr. McCullough’s attorneys, Clinton Mikel, maintains that all the times the physician was identified in the “thousands of hours of media interviews and countless publications since his departure from Baylor” were “said/printed by a third party with no encouragement from Dr. McCullough,” and that the doctor “does not and cannot control third parties.”
Mr. Mikel said in a statement emailed to this news organization by Dr. McCullough that the suit is “a politically motivated attempt to silence Dr. McCullough,” because it was filed on the same day the organization mandated COVID-19 vaccination for employees.
Dr. McCullough “intends to vigorously defend against Baylor’s unfounded lawsuit,” will seek to dissolve the restraining order, and recover “all payments due him from Baylor under the terms of the settlement agreement,” wrote Mr. Mikel.
The cardiologist’s legal team filed a motion to dismiss the suit on Aug. 9, essentially arguing that Baylor Scott & White’s action restricted Dr. McCullough’s right to free speech under the Texas Citizen’s Participation Act.
COVID-19 vaccines = bioterrorism?
Dr. McCullough accumulated a following in 2020 by promoting early at-home multidrug treatment of COVID-19 in interviews with conservative websites and at a U.S. Senate hearing in November.
Although Dr. McCullough does not appear to have any personal social media accounts, his broadcast and podcast interviews are tweeted by thousands daily around the world and featured on Facebook pages like “Pandemic Debate.”
Some Facebook posts with Dr. McCullough’s pronouncements have been labeled as misinformation or removed. Some of his videos remain on YouTube, where they are posted by the Association of American Physicians and Surgeons, a group that believes Dr. McCullough is “under fierce attack for speaking out about COVID-19 early treatment and vaccine safety.”
Dr. McCullough’s March 2021 testimony to the Texas Senate’s Health and Human Services Committee – in which he claimed that COVID-19 patients are being denied what he called proven treatments like hydroxychloroquine – has been viewed more than 3.7 million times on YouTube. The appearance has also been tweeted repeatedly.
Most of Dr. McCullough’s interviews and presentations are aggregated on Rumble, an alternative to YouTube.
In interviews, Dr. McCullough promotes the use of zinc, hydroxychloroquine, azithromycin, doxycycline, favipiravir, prednisone, and ivermectin as COVID-19 treatments – based on an outpatient treatment algorithm published in August 2020 in the American Journal of Medicine. The cardiologist was the lead author of that paper, which proposed treating people with COVID-like symptoms whether or not they had confirmed infection.
Dr. McCullough and colleagues published a follow-up paper that added colchicine to the mix in Reviews in Cardiovascular Medicine. Dr. McCullough is editor-in-chief of the journal, but this was not noted in the disclosures.
Similarly, Dr. McCullough has not disclosed in his COVID-19 publications or any interviews that he has received consulting fees from a host of pharmaceutical manufacturers that produce COVID-19 drugs and vaccines, including AstraZeneca, Eli Lilly, and Regeneron Pharmaceuticals. According to the Centers for Medicare & Medicaid Services’ Open Payments database, Dr. McCullough was paid about $300,000 annually by drug companies from 2014 to 2019, mostly for consulting on cardiovascular and diabetes medications. His payments dropped to $169,406.06 in 2020.
Dr. McCullough appeared on “The Ingraham Angle” on Fox News in December 2020, claiming that sequential, early treatment with “anti-infectives, corticosteroids, and then antithrombotics” could “reduce [COVID-19] hospitalizations by 85% and cut mortality in half.”
He repeated the claim on the Ingraham show in July and agreed with host Laura Ingraham that the vast majority of healthy people would do fine if they got COVID. He also made the claim that 84% of the COVID-19 cases in Israel were in people who had been vaccinated. “So it’s clear, we can’t vaccinate our way out of this,” he said. An Associated Press “fact check” report has pushed back on similar assertions about vaccine data from Israel.
In a separate interview posted in June, Dr. McCullough called the pandemic the first phase of a bioterrorism event, which was “all about keeping the population in fear and in isolation and preparing them to accept the vaccine, which appears to be phase two of a bioterrorism operation.”
In addition, he said, “good doctors are doing unthinkable things like injecting biologically active messenger RNA that produces this pathogenic spike protein into pregnant women.”
According to the Centers for Disease Control and Prevention, the vaccines teach the body to produce the spike protein, which then triggers an immune response that creates antibodies that will attack the virus.
A PolitiFact review debunks the notion that the mRNA vaccines are toxic, cytotoxic, or introduce live, active virus proteins into the body.
FactCheck.org also disputed Dr. McCullough’s claim in a July 13 Ingraham Angle appearance that the mRNA vaccines are ineffective against the Delta variant.
In the FactCheck article, Frederic Bushman, codirector of the University of Pennsylvania’s Center for Research on Coronaviruses and Other Emerging Pathogens, said that people were much better off being vaccinated than not,” adding, “the Delta variant may reduce the effectiveness [of the vaccines] a little, but still, they’re so effective that you get a lot of benefit.”
“The vaccines are failing,” Dr. McCullough asserted in an Aug. 3 video interview posted on Odysee. “As we sit here today, we have 11,000 Americans that the CDC has certified have died after the vaccine,” he said, citing two analyses – one by Jessica Rose, PhD, and another by British researchers.
Similar figures reportedly based on cases reported to the Food and Drug Administration’s Vaccine Adverse Events Reporting System (VAERS) were forwarded to this news organization by Dr. McCullough.
The CDC website notes that the agency has received reports of 7,653 deaths in people who received a vaccine as of Sept. 13 (0.0020% of vaccine doses given since Dec. 14, 2020), but it cautions that those deaths do not mean the vaccine was the cause.
Dr. McCullough repeatedly claimed in the Aug. 3 interview that the government has not been transparent on vaccine safety. Since June 2020, the CDC’s Advisory Committee on Immunization Practices has held 16 public meetings on the COVID-19 vaccines.
To date, the agency has advised clinicians to monitor for rare side effects including Guillain-Barré syndrome and thrombosis with thrombocytopenia syndrome after the Johnson & Johnson vaccine and myocarditis after mRNA (Pfizer-BioNTech and Moderna) vaccines.
Med schools distance themselves
According to the Baylor Scott & White suit, Dr. McCullough agreed on Feb. 24 in a confidential separation agreement that he would no longer use his academic or leadership titles nor hold himself out to be affiliated with Baylor University Medical Center, Baylor Heart and Vascular Institute, the Baylor Research Institute, or any other related institutions.
However, as of August, according to a Baylor spokesperson, McCullough continued to have privileges at Baylor University Medical Center and Baylor Scott & White Heart and Vascular Hospital, Dallas.
The lawsuit points to three interviews posted in June and July where Dr. McCullough is identified as a “vice chief of medicine” or a “vice chief of internal medicine,” both at Baylor University. It also cites a profile at the Cardiometabolic Health Congress website – which this news organization had also viewed – that was still active in late July with a similar title. The profile was later scrubbed from the site.
Social media posts and other media continue to refer to Dr. McCullough’s Baylor credentials. An episode of the Faith and Freedom podcast posted on Aug. 2 identified McCullough as a “professor of medicine at Baylor University Medical Center.”
As of Sept. 16, Dr. McCullough’s bio page at his current practice, Heart Place, lists him as a professor of medicine at Texas A&M College of Medicine. A spokesperson for Texas A&M told this news organization that McCullough is no longer affiliated with the school.
Dr. McCullough acknowledged in the Aug. 3 interview that his Texas A&M title had been “stripped away” at “around the same time this lawsuit was filed.”
He was still a professor of medicine at the TCU and UNTHSC School of Medicine in Fort Worth, but a school spokesperson notified this news organization on Aug. 19 that Dr. McCullough was no longer with the school.
Dr. McCullough has portrayed himself as both a victim and a truth-teller, a “concerned physician” warning the world about the dangers of COVID-19 vaccines. The Baylor Scott & White lawsuit “is really a strong-armed tactic,” he said in the Aug. 3 interview. “I’m just a little guy, so I have to hire my legal teams, and in a sense be drained dry on legal fees,” he said.
But Dr. McCullough apparently has a plan for helping to defray his legal costs. In the Aug. 3 interview, he said a foundation he helped start, Truth for Health, has a “donation side to it,” adding “some of that may be used for legal expense.”
Cheryl Jones, an attorney with PK Law in Towson, Md., said that might draw interest from the Internal Revenue Service. “I would expect IRS scrutiny if contributions to the Medical Censorship Defense Fund are used to defend Dr McCullough in his personal breach of contract lawsuit,” she told this news organization.
The IRS generally recognizes defending “human and civil rights secured by law” as a legitimate charitable purpose for a legal defense fund, she said, adding that such a fund “must serve only public, rather than private, interests.”
Misinformation from a physician more damaging?
Some in the medical field have refuted Dr. McCullough’s pronouncements on how to treat COVID-19, including two infectious disease specialists with Monash University, Melbourne, who responded to the cardiologist’s original paper in the American Journal of Medicine.
Tony Korman, MBBS, a professor at the Centre for Inflammatory Diseases at Monash, told this news organization, “we had concerns that reputable medical journals would accept and publish papers proposing treatment of COVID-19 which was not supported by evidence.”
The website Healthfeedback.org has also challenged McCullough’s and his supporters’ claims, including that the American Journal of Medicine endorsed the use of hydroxychloroquine and that the COVID-19 vaccines have caused thousands of deaths.
David Broniatowski, PhD, associate director for the Institute for Data, Democracy and Politics at George Washington University, Washington, said in an interview that Dr. McCullough’s casting himself as a “rebel doctor” is a well-known trope in the vaccine misinformation universe.
Although he was not familiar with Dr. McCullough, Dr. Broniatowski said the cardiologist’s claims are not unique – they’ve been circulating among antivaccine and conspiracy-oriented groups for months.
For instance, Dr. McCullough has claimed in interviews that a whistleblower within the CDC knows of 50,000 vaccine-related deaths. Using data from the supposed whistleblower, the group America’s Frontline Doctors sued the federal government in July to stop the administration of COVID-19 vaccines to those under 18, people who have already had COVID, and individuals who the group said have not been adequately informed about the risks.
The idea of a whistleblower inside the CDC is recycled from antivaccine claims from decades ago, Dr. Broniatowski said.
But, he added, “somebody who speaks with the credibility of a major institution will be more likely to be listened to by some people.” That vulnerable group is “being taken advantage of by a relatively small number of disinformation purveyors, who, in some cases, profit from that disinformation,” said Dr. Broniatowski.
“We rely on our doctors because we trust them,” he said. “And we trust them because we believe that as physicians, their value system places the patient’s best interests first. That’s why it’s so much of a disappointment when you have a physician that appears to be exercising this sort of bad judgment.”
Paul Offit, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, also said that he was not familiar with Dr. McCullough. But apprised of his claims, Dr. Offit told this news organization, “Peter McCullough is a friend of the virus.”
“The kind of information he promotes allows the virus to continue to spread, continue to do an enormous amount of harm, and continue to mutate and create variants that have become more contagious and more resistant to vaccine-induced immunity,” said Dr. Offit, the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia.
Dr. Offit added that the war should be against SARS-CoV-2, but “because this virus has so many supporters, the war in essence becomes a war against ourselves, which is much harder.”
Dr. McCullough maintains he is doing a service to his patients. “I’m just giving and trying to help America understand the pandemic,” he told Ms. Ingraham on Fox News on July 29.
But he acknowledged concern about the Federation of State Medical Board’s announcement that physicians who spread COVID-19 vaccine misinformation risk suspension or revocation of their license.
“I have to tell you I’m worried – that no matter what I do and how careful I am to cite the scientific studies, I’m still gonna be hunted down for quote, misinformation,” he said in the Aug. 3 interview.
A version of this article first appeared on Medscape.com.
in which he agreed to stop mentioning his prior leadership and academic appointments.
Baylor was the first institution to cut ties with Dr. McCullough, who has promoted the use of therapies seen as unproven for the treatment of COVID-19 and has questioned the effectiveness of COVID-19 vaccines. Since the Baylor suit, the Texas A&M College of Medicine, and the Texas Christian University (TCU) and University of North Texas Health Science Center (UNTHSC) School of Medicine have both removed Dr. McCullough from their faculties.
Granted by the 191st District Court in Dallas County, Tex., the Baylor restraining order – which is in effect at least until a hearing on the case on September 30 – was sought as part of Baylor Scott & White’s breach of contract suit against McCullough, who had previously been known as a well-respected expert in cardiorenal issues. The suit is seeking $1 million in damages, as well as attorneys’ fees.
The suit seeks to “enforce the terms” of the confidential employment separation agreement signed by Dr. McCullough in February and prevent Dr. McCullough from continuing “improper use of titles and claimed affiliations that have already confused the media, the medical community and the public,” it reads.
“This ongoing confusion regarding [Dr.] McCullough’s affiliations, and whether Plaintiffs support his opinions, is exactly what Plaintiffs bargained to avoid in the Separation Agreement,” and is likely to cause “irreparable reputational and business harm that is incapable of remedy by money damages alone,” the suit states.
One of Dr. McCullough’s attorneys, Clinton Mikel, maintains that all the times the physician was identified in the “thousands of hours of media interviews and countless publications since his departure from Baylor” were “said/printed by a third party with no encouragement from Dr. McCullough,” and that the doctor “does not and cannot control third parties.”
Mr. Mikel said in a statement emailed to this news organization by Dr. McCullough that the suit is “a politically motivated attempt to silence Dr. McCullough,” because it was filed on the same day the organization mandated COVID-19 vaccination for employees.
Dr. McCullough “intends to vigorously defend against Baylor’s unfounded lawsuit,” will seek to dissolve the restraining order, and recover “all payments due him from Baylor under the terms of the settlement agreement,” wrote Mr. Mikel.
The cardiologist’s legal team filed a motion to dismiss the suit on Aug. 9, essentially arguing that Baylor Scott & White’s action restricted Dr. McCullough’s right to free speech under the Texas Citizen’s Participation Act.
COVID-19 vaccines = bioterrorism?
Dr. McCullough accumulated a following in 2020 by promoting early at-home multidrug treatment of COVID-19 in interviews with conservative websites and at a U.S. Senate hearing in November.
Although Dr. McCullough does not appear to have any personal social media accounts, his broadcast and podcast interviews are tweeted by thousands daily around the world and featured on Facebook pages like “Pandemic Debate.”
Some Facebook posts with Dr. McCullough’s pronouncements have been labeled as misinformation or removed. Some of his videos remain on YouTube, where they are posted by the Association of American Physicians and Surgeons, a group that believes Dr. McCullough is “under fierce attack for speaking out about COVID-19 early treatment and vaccine safety.”
Dr. McCullough’s March 2021 testimony to the Texas Senate’s Health and Human Services Committee – in which he claimed that COVID-19 patients are being denied what he called proven treatments like hydroxychloroquine – has been viewed more than 3.7 million times on YouTube. The appearance has also been tweeted repeatedly.
Most of Dr. McCullough’s interviews and presentations are aggregated on Rumble, an alternative to YouTube.
In interviews, Dr. McCullough promotes the use of zinc, hydroxychloroquine, azithromycin, doxycycline, favipiravir, prednisone, and ivermectin as COVID-19 treatments – based on an outpatient treatment algorithm published in August 2020 in the American Journal of Medicine. The cardiologist was the lead author of that paper, which proposed treating people with COVID-like symptoms whether or not they had confirmed infection.
Dr. McCullough and colleagues published a follow-up paper that added colchicine to the mix in Reviews in Cardiovascular Medicine. Dr. McCullough is editor-in-chief of the journal, but this was not noted in the disclosures.
Similarly, Dr. McCullough has not disclosed in his COVID-19 publications or any interviews that he has received consulting fees from a host of pharmaceutical manufacturers that produce COVID-19 drugs and vaccines, including AstraZeneca, Eli Lilly, and Regeneron Pharmaceuticals. According to the Centers for Medicare & Medicaid Services’ Open Payments database, Dr. McCullough was paid about $300,000 annually by drug companies from 2014 to 2019, mostly for consulting on cardiovascular and diabetes medications. His payments dropped to $169,406.06 in 2020.
Dr. McCullough appeared on “The Ingraham Angle” on Fox News in December 2020, claiming that sequential, early treatment with “anti-infectives, corticosteroids, and then antithrombotics” could “reduce [COVID-19] hospitalizations by 85% and cut mortality in half.”
He repeated the claim on the Ingraham show in July and agreed with host Laura Ingraham that the vast majority of healthy people would do fine if they got COVID. He also made the claim that 84% of the COVID-19 cases in Israel were in people who had been vaccinated. “So it’s clear, we can’t vaccinate our way out of this,” he said. An Associated Press “fact check” report has pushed back on similar assertions about vaccine data from Israel.
In a separate interview posted in June, Dr. McCullough called the pandemic the first phase of a bioterrorism event, which was “all about keeping the population in fear and in isolation and preparing them to accept the vaccine, which appears to be phase two of a bioterrorism operation.”
In addition, he said, “good doctors are doing unthinkable things like injecting biologically active messenger RNA that produces this pathogenic spike protein into pregnant women.”
According to the Centers for Disease Control and Prevention, the vaccines teach the body to produce the spike protein, which then triggers an immune response that creates antibodies that will attack the virus.
A PolitiFact review debunks the notion that the mRNA vaccines are toxic, cytotoxic, or introduce live, active virus proteins into the body.
FactCheck.org also disputed Dr. McCullough’s claim in a July 13 Ingraham Angle appearance that the mRNA vaccines are ineffective against the Delta variant.
In the FactCheck article, Frederic Bushman, codirector of the University of Pennsylvania’s Center for Research on Coronaviruses and Other Emerging Pathogens, said that people were much better off being vaccinated than not,” adding, “the Delta variant may reduce the effectiveness [of the vaccines] a little, but still, they’re so effective that you get a lot of benefit.”
“The vaccines are failing,” Dr. McCullough asserted in an Aug. 3 video interview posted on Odysee. “As we sit here today, we have 11,000 Americans that the CDC has certified have died after the vaccine,” he said, citing two analyses – one by Jessica Rose, PhD, and another by British researchers.
Similar figures reportedly based on cases reported to the Food and Drug Administration’s Vaccine Adverse Events Reporting System (VAERS) were forwarded to this news organization by Dr. McCullough.
The CDC website notes that the agency has received reports of 7,653 deaths in people who received a vaccine as of Sept. 13 (0.0020% of vaccine doses given since Dec. 14, 2020), but it cautions that those deaths do not mean the vaccine was the cause.
Dr. McCullough repeatedly claimed in the Aug. 3 interview that the government has not been transparent on vaccine safety. Since June 2020, the CDC’s Advisory Committee on Immunization Practices has held 16 public meetings on the COVID-19 vaccines.
To date, the agency has advised clinicians to monitor for rare side effects including Guillain-Barré syndrome and thrombosis with thrombocytopenia syndrome after the Johnson & Johnson vaccine and myocarditis after mRNA (Pfizer-BioNTech and Moderna) vaccines.
Med schools distance themselves
According to the Baylor Scott & White suit, Dr. McCullough agreed on Feb. 24 in a confidential separation agreement that he would no longer use his academic or leadership titles nor hold himself out to be affiliated with Baylor University Medical Center, Baylor Heart and Vascular Institute, the Baylor Research Institute, or any other related institutions.
However, as of August, according to a Baylor spokesperson, McCullough continued to have privileges at Baylor University Medical Center and Baylor Scott & White Heart and Vascular Hospital, Dallas.
The lawsuit points to three interviews posted in June and July where Dr. McCullough is identified as a “vice chief of medicine” or a “vice chief of internal medicine,” both at Baylor University. It also cites a profile at the Cardiometabolic Health Congress website – which this news organization had also viewed – that was still active in late July with a similar title. The profile was later scrubbed from the site.
Social media posts and other media continue to refer to Dr. McCullough’s Baylor credentials. An episode of the Faith and Freedom podcast posted on Aug. 2 identified McCullough as a “professor of medicine at Baylor University Medical Center.”
As of Sept. 16, Dr. McCullough’s bio page at his current practice, Heart Place, lists him as a professor of medicine at Texas A&M College of Medicine. A spokesperson for Texas A&M told this news organization that McCullough is no longer affiliated with the school.
Dr. McCullough acknowledged in the Aug. 3 interview that his Texas A&M title had been “stripped away” at “around the same time this lawsuit was filed.”
He was still a professor of medicine at the TCU and UNTHSC School of Medicine in Fort Worth, but a school spokesperson notified this news organization on Aug. 19 that Dr. McCullough was no longer with the school.
Dr. McCullough has portrayed himself as both a victim and a truth-teller, a “concerned physician” warning the world about the dangers of COVID-19 vaccines. The Baylor Scott & White lawsuit “is really a strong-armed tactic,” he said in the Aug. 3 interview. “I’m just a little guy, so I have to hire my legal teams, and in a sense be drained dry on legal fees,” he said.
But Dr. McCullough apparently has a plan for helping to defray his legal costs. In the Aug. 3 interview, he said a foundation he helped start, Truth for Health, has a “donation side to it,” adding “some of that may be used for legal expense.”
Cheryl Jones, an attorney with PK Law in Towson, Md., said that might draw interest from the Internal Revenue Service. “I would expect IRS scrutiny if contributions to the Medical Censorship Defense Fund are used to defend Dr McCullough in his personal breach of contract lawsuit,” she told this news organization.
The IRS generally recognizes defending “human and civil rights secured by law” as a legitimate charitable purpose for a legal defense fund, she said, adding that such a fund “must serve only public, rather than private, interests.”
Misinformation from a physician more damaging?
Some in the medical field have refuted Dr. McCullough’s pronouncements on how to treat COVID-19, including two infectious disease specialists with Monash University, Melbourne, who responded to the cardiologist’s original paper in the American Journal of Medicine.
Tony Korman, MBBS, a professor at the Centre for Inflammatory Diseases at Monash, told this news organization, “we had concerns that reputable medical journals would accept and publish papers proposing treatment of COVID-19 which was not supported by evidence.”
The website Healthfeedback.org has also challenged McCullough’s and his supporters’ claims, including that the American Journal of Medicine endorsed the use of hydroxychloroquine and that the COVID-19 vaccines have caused thousands of deaths.
David Broniatowski, PhD, associate director for the Institute for Data, Democracy and Politics at George Washington University, Washington, said in an interview that Dr. McCullough’s casting himself as a “rebel doctor” is a well-known trope in the vaccine misinformation universe.
Although he was not familiar with Dr. McCullough, Dr. Broniatowski said the cardiologist’s claims are not unique – they’ve been circulating among antivaccine and conspiracy-oriented groups for months.
For instance, Dr. McCullough has claimed in interviews that a whistleblower within the CDC knows of 50,000 vaccine-related deaths. Using data from the supposed whistleblower, the group America’s Frontline Doctors sued the federal government in July to stop the administration of COVID-19 vaccines to those under 18, people who have already had COVID, and individuals who the group said have not been adequately informed about the risks.
The idea of a whistleblower inside the CDC is recycled from antivaccine claims from decades ago, Dr. Broniatowski said.
But, he added, “somebody who speaks with the credibility of a major institution will be more likely to be listened to by some people.” That vulnerable group is “being taken advantage of by a relatively small number of disinformation purveyors, who, in some cases, profit from that disinformation,” said Dr. Broniatowski.
“We rely on our doctors because we trust them,” he said. “And we trust them because we believe that as physicians, their value system places the patient’s best interests first. That’s why it’s so much of a disappointment when you have a physician that appears to be exercising this sort of bad judgment.”
Paul Offit, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, also said that he was not familiar with Dr. McCullough. But apprised of his claims, Dr. Offit told this news organization, “Peter McCullough is a friend of the virus.”
“The kind of information he promotes allows the virus to continue to spread, continue to do an enormous amount of harm, and continue to mutate and create variants that have become more contagious and more resistant to vaccine-induced immunity,” said Dr. Offit, the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia.
Dr. Offit added that the war should be against SARS-CoV-2, but “because this virus has so many supporters, the war in essence becomes a war against ourselves, which is much harder.”
Dr. McCullough maintains he is doing a service to his patients. “I’m just giving and trying to help America understand the pandemic,” he told Ms. Ingraham on Fox News on July 29.
But he acknowledged concern about the Federation of State Medical Board’s announcement that physicians who spread COVID-19 vaccine misinformation risk suspension or revocation of their license.
“I have to tell you I’m worried – that no matter what I do and how careful I am to cite the scientific studies, I’m still gonna be hunted down for quote, misinformation,” he said in the Aug. 3 interview.
A version of this article first appeared on Medscape.com.
COVID vaccine preprint study prompts Twitter outrage
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.




