User login
Will interchangeable insulin be more affordable in the U.S.?
When the Food and Drug Administration approved Semglee, the first interchangeable biosimilar insulin, the agency pitched it as having the potential to be less costly than insulins currently on the market, but lack of transparency in pharmaceutical pricing has left analysts and advocates guessing whether it will indeed be a source of relief.
Semglee (Mylan Pharmaceuticals), first approved as a biosimilar in June 2020, costs about $100 a vial.
But receiving the “interchangeable designation” in July 2021, the first for any insulin, now allows Semglee to be substituted for the branded Lantus (insulin glargine, Sanofi) at the pharmacy without the need for a separate prescription, the same way as generic medicines.
A spokesperson for Viatris – Mylan’s parent company told this news organization that the interchangeable, with its new labeling, will be “introduced before the end of the year,” but it would not give any more details.
“Additional information, including pricing information, for interchangeable biosimilar Semglee will be provided at the time of product launch,” said the spokesperson.
Even at $100 a vial, it is not cheap
Ian Devaney, a spokesman for the advocacy group T1 International, said the organization is optimistic, given that “another player has been able to enter into a space that has for so long been dominated by Eli Lilly, Novo Nordisk, and Sanofi.” Increased competition “will help drive down the overall costs of insulin,” Mr. Devaney said in an interview. But, he added, for many people, especially in low-income countries, Semglee’s launch will have little to no impact on price.
Even at $100 a vial in the United States, “this is not an insignificant amount of money and presents a very difficult financial challenge for those dependent on insulin to survive,” he said.
A current Semglee user agreed, sharing her story with this news organization via T1 International. “My son uses three to five vials of long-acting insulin per month, and I use one to three vials per month,” said the woman, who prefers to remain anonymous. “If we were to lose Medicaid, we would still be paying up to $800 out of pocket monthly to survive, and that’s not even counting fast-acting insulin or other supplies. While $100 a vial may be cheaper, these costs are still outrageous.”
The woman also noted that, while new competitors are welcome, they also have been disruptive. After her doctor switched her to Semglee, she was notified that it was on back order. “It took a week to get it filled, and when it finally came in, it was in short supply,” she said, noting that she and her son received one Semglee pen each, “well short of the three and five each we were expecting.”
U.S. pricing is all ‘smoke and mirrors’
Sara W. Koblitz, a food and drug law attorney with Hyman Phelps in Washington, D.C., noted in a blog post that interchangeable Semglee will likely be awarded a year of marketing exclusivity, which will block other interchangeable competitors from entering the market during that time.
With no competition, “Mylan can price Semglee only slightly less than Lantus and still take market share, only marginally reducing costs to consumers,” she wrote.
Jing Luo, MD, MPH, an assistant professor of medicine at the University of Pittsburgh, who has studied insulin access and costs, said that having just one interchangeable on the market might not be enough to drive insulin costs down.
And, he told this news organization, “there’s even a possibility that Semglee prices will go up, but hopefully that will not be the case.”
Manufacturers like Mylan can also offer confidential discounts and rebates to pharmacy benefit managers (PBMs), health plans, and health plan sponsors (usually large companies that are self-insured) that make it difficult to assess the true cost, said David Steinberg, PharmD, director of pharmacy insights at Scripta Insights. The Wellesley, Mass.–based company advises self-insured employers on how to optimize pharmacy benefits.
When it comes to pricing, “it’s a lot of smoke and mirrors,” Dr. Steinberg said in an interview.
Dr. Steinberg also noted that some PBMs might choose to continue contracts with Sanofi that offer rebates for Lantus, leaving Semglee in a less-preferred position on a formulary, which could increase how much the patient pays at the pharmacy counter.
Medicare and Medicaid, however, can put Semglee in the top-tier preferred formulary position. Most Medicaid plans cover Semglee, but it appears that Medicare has not added coverage yet.
Does current pricing predict the future?
The currently marketed Semglee has an average wholesale price (AWP) that is one third of Lantus’, and about half of what is published for Basaglar (insulin glargine, Eli Lilly), a “follow-on biologic” approved in 2015 that is similar to Lantus, Dr. Steinberg said.
The AWP is often cited by analysts when talking about costs. The AWP of the current Semglee 10-mL vial is $118.38; the Lantus 10-mL vial is $340.27, said Steinberg.
Five prefilled Semglee pens (each 3 mL) are $177.58; for Lantus, the AWP for five 3-mL pens is $510.37.
Dr. Luo said he has seen a box of Semglee pens retail between $177 and $195, compared with about $500 retail for the Lantus pens.
Currently, people with commercial insurance can get Semglee for $0-75 a month, for up to a year, using the company’s savings program.
Steinberg said it’s possible that Mylan could increase the list price for the interchangeable Semglee, but that move could backfire. “I think their goal initially is to get market share,” he said.
After Basaglar came on the market – in late 2016 – the price of Lantus came down significantly over the next few years, according to a 2019 study by Dr. Luo’s colleagues at the University of Pittsburgh.
But Basaglar has not hung on to market share, according to Scott Strumello, a person with autoimmune type 1 diabetes who tweets and blogs about insulin and other issues.
In early August, Mr. Strumello tweeted some Lilly data that showed U.S. sales of Basaglar declined 42% in the first two quarters of 2021, compared with the same period in 2020.
Dr. Steinberg noted that the decline may have to do with rebates being given to PBMs by competitors Sanofi and Novo Nordisk. Sanofi “is very aggressive when it comes to pricing with their PBM partners,” he said.
While Mr. Devaney said people with diabetes are hopeful that Semglee can break the big three manufacturers’ monopoly, he added: “We don’t see Semglee as something that is solving the root cause of the insulin price crisis, which is high list prices and pharmaceutical industry greed.”
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration approved Semglee, the first interchangeable biosimilar insulin, the agency pitched it as having the potential to be less costly than insulins currently on the market, but lack of transparency in pharmaceutical pricing has left analysts and advocates guessing whether it will indeed be a source of relief.
Semglee (Mylan Pharmaceuticals), first approved as a biosimilar in June 2020, costs about $100 a vial.
But receiving the “interchangeable designation” in July 2021, the first for any insulin, now allows Semglee to be substituted for the branded Lantus (insulin glargine, Sanofi) at the pharmacy without the need for a separate prescription, the same way as generic medicines.
A spokesperson for Viatris – Mylan’s parent company told this news organization that the interchangeable, with its new labeling, will be “introduced before the end of the year,” but it would not give any more details.
“Additional information, including pricing information, for interchangeable biosimilar Semglee will be provided at the time of product launch,” said the spokesperson.
Even at $100 a vial, it is not cheap
Ian Devaney, a spokesman for the advocacy group T1 International, said the organization is optimistic, given that “another player has been able to enter into a space that has for so long been dominated by Eli Lilly, Novo Nordisk, and Sanofi.” Increased competition “will help drive down the overall costs of insulin,” Mr. Devaney said in an interview. But, he added, for many people, especially in low-income countries, Semglee’s launch will have little to no impact on price.
Even at $100 a vial in the United States, “this is not an insignificant amount of money and presents a very difficult financial challenge for those dependent on insulin to survive,” he said.
A current Semglee user agreed, sharing her story with this news organization via T1 International. “My son uses three to five vials of long-acting insulin per month, and I use one to three vials per month,” said the woman, who prefers to remain anonymous. “If we were to lose Medicaid, we would still be paying up to $800 out of pocket monthly to survive, and that’s not even counting fast-acting insulin or other supplies. While $100 a vial may be cheaper, these costs are still outrageous.”
The woman also noted that, while new competitors are welcome, they also have been disruptive. After her doctor switched her to Semglee, she was notified that it was on back order. “It took a week to get it filled, and when it finally came in, it was in short supply,” she said, noting that she and her son received one Semglee pen each, “well short of the three and five each we were expecting.”
U.S. pricing is all ‘smoke and mirrors’
Sara W. Koblitz, a food and drug law attorney with Hyman Phelps in Washington, D.C., noted in a blog post that interchangeable Semglee will likely be awarded a year of marketing exclusivity, which will block other interchangeable competitors from entering the market during that time.
With no competition, “Mylan can price Semglee only slightly less than Lantus and still take market share, only marginally reducing costs to consumers,” she wrote.
Jing Luo, MD, MPH, an assistant professor of medicine at the University of Pittsburgh, who has studied insulin access and costs, said that having just one interchangeable on the market might not be enough to drive insulin costs down.
And, he told this news organization, “there’s even a possibility that Semglee prices will go up, but hopefully that will not be the case.”
Manufacturers like Mylan can also offer confidential discounts and rebates to pharmacy benefit managers (PBMs), health plans, and health plan sponsors (usually large companies that are self-insured) that make it difficult to assess the true cost, said David Steinberg, PharmD, director of pharmacy insights at Scripta Insights. The Wellesley, Mass.–based company advises self-insured employers on how to optimize pharmacy benefits.
When it comes to pricing, “it’s a lot of smoke and mirrors,” Dr. Steinberg said in an interview.
Dr. Steinberg also noted that some PBMs might choose to continue contracts with Sanofi that offer rebates for Lantus, leaving Semglee in a less-preferred position on a formulary, which could increase how much the patient pays at the pharmacy counter.
Medicare and Medicaid, however, can put Semglee in the top-tier preferred formulary position. Most Medicaid plans cover Semglee, but it appears that Medicare has not added coverage yet.
Does current pricing predict the future?
The currently marketed Semglee has an average wholesale price (AWP) that is one third of Lantus’, and about half of what is published for Basaglar (insulin glargine, Eli Lilly), a “follow-on biologic” approved in 2015 that is similar to Lantus, Dr. Steinberg said.
The AWP is often cited by analysts when talking about costs. The AWP of the current Semglee 10-mL vial is $118.38; the Lantus 10-mL vial is $340.27, said Steinberg.
Five prefilled Semglee pens (each 3 mL) are $177.58; for Lantus, the AWP for five 3-mL pens is $510.37.
Dr. Luo said he has seen a box of Semglee pens retail between $177 and $195, compared with about $500 retail for the Lantus pens.
Currently, people with commercial insurance can get Semglee for $0-75 a month, for up to a year, using the company’s savings program.
Steinberg said it’s possible that Mylan could increase the list price for the interchangeable Semglee, but that move could backfire. “I think their goal initially is to get market share,” he said.
After Basaglar came on the market – in late 2016 – the price of Lantus came down significantly over the next few years, according to a 2019 study by Dr. Luo’s colleagues at the University of Pittsburgh.
But Basaglar has not hung on to market share, according to Scott Strumello, a person with autoimmune type 1 diabetes who tweets and blogs about insulin and other issues.
In early August, Mr. Strumello tweeted some Lilly data that showed U.S. sales of Basaglar declined 42% in the first two quarters of 2021, compared with the same period in 2020.
Dr. Steinberg noted that the decline may have to do with rebates being given to PBMs by competitors Sanofi and Novo Nordisk. Sanofi “is very aggressive when it comes to pricing with their PBM partners,” he said.
While Mr. Devaney said people with diabetes are hopeful that Semglee can break the big three manufacturers’ monopoly, he added: “We don’t see Semglee as something that is solving the root cause of the insulin price crisis, which is high list prices and pharmaceutical industry greed.”
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration approved Semglee, the first interchangeable biosimilar insulin, the agency pitched it as having the potential to be less costly than insulins currently on the market, but lack of transparency in pharmaceutical pricing has left analysts and advocates guessing whether it will indeed be a source of relief.
Semglee (Mylan Pharmaceuticals), first approved as a biosimilar in June 2020, costs about $100 a vial.
But receiving the “interchangeable designation” in July 2021, the first for any insulin, now allows Semglee to be substituted for the branded Lantus (insulin glargine, Sanofi) at the pharmacy without the need for a separate prescription, the same way as generic medicines.
A spokesperson for Viatris – Mylan’s parent company told this news organization that the interchangeable, with its new labeling, will be “introduced before the end of the year,” but it would not give any more details.
“Additional information, including pricing information, for interchangeable biosimilar Semglee will be provided at the time of product launch,” said the spokesperson.
Even at $100 a vial, it is not cheap
Ian Devaney, a spokesman for the advocacy group T1 International, said the organization is optimistic, given that “another player has been able to enter into a space that has for so long been dominated by Eli Lilly, Novo Nordisk, and Sanofi.” Increased competition “will help drive down the overall costs of insulin,” Mr. Devaney said in an interview. But, he added, for many people, especially in low-income countries, Semglee’s launch will have little to no impact on price.
Even at $100 a vial in the United States, “this is not an insignificant amount of money and presents a very difficult financial challenge for those dependent on insulin to survive,” he said.
A current Semglee user agreed, sharing her story with this news organization via T1 International. “My son uses three to five vials of long-acting insulin per month, and I use one to three vials per month,” said the woman, who prefers to remain anonymous. “If we were to lose Medicaid, we would still be paying up to $800 out of pocket monthly to survive, and that’s not even counting fast-acting insulin or other supplies. While $100 a vial may be cheaper, these costs are still outrageous.”
The woman also noted that, while new competitors are welcome, they also have been disruptive. After her doctor switched her to Semglee, she was notified that it was on back order. “It took a week to get it filled, and when it finally came in, it was in short supply,” she said, noting that she and her son received one Semglee pen each, “well short of the three and five each we were expecting.”
U.S. pricing is all ‘smoke and mirrors’
Sara W. Koblitz, a food and drug law attorney with Hyman Phelps in Washington, D.C., noted in a blog post that interchangeable Semglee will likely be awarded a year of marketing exclusivity, which will block other interchangeable competitors from entering the market during that time.
With no competition, “Mylan can price Semglee only slightly less than Lantus and still take market share, only marginally reducing costs to consumers,” she wrote.
Jing Luo, MD, MPH, an assistant professor of medicine at the University of Pittsburgh, who has studied insulin access and costs, said that having just one interchangeable on the market might not be enough to drive insulin costs down.
And, he told this news organization, “there’s even a possibility that Semglee prices will go up, but hopefully that will not be the case.”
Manufacturers like Mylan can also offer confidential discounts and rebates to pharmacy benefit managers (PBMs), health plans, and health plan sponsors (usually large companies that are self-insured) that make it difficult to assess the true cost, said David Steinberg, PharmD, director of pharmacy insights at Scripta Insights. The Wellesley, Mass.–based company advises self-insured employers on how to optimize pharmacy benefits.
When it comes to pricing, “it’s a lot of smoke and mirrors,” Dr. Steinberg said in an interview.
Dr. Steinberg also noted that some PBMs might choose to continue contracts with Sanofi that offer rebates for Lantus, leaving Semglee in a less-preferred position on a formulary, which could increase how much the patient pays at the pharmacy counter.
Medicare and Medicaid, however, can put Semglee in the top-tier preferred formulary position. Most Medicaid plans cover Semglee, but it appears that Medicare has not added coverage yet.
Does current pricing predict the future?
The currently marketed Semglee has an average wholesale price (AWP) that is one third of Lantus’, and about half of what is published for Basaglar (insulin glargine, Eli Lilly), a “follow-on biologic” approved in 2015 that is similar to Lantus, Dr. Steinberg said.
The AWP is often cited by analysts when talking about costs. The AWP of the current Semglee 10-mL vial is $118.38; the Lantus 10-mL vial is $340.27, said Steinberg.
Five prefilled Semglee pens (each 3 mL) are $177.58; for Lantus, the AWP for five 3-mL pens is $510.37.
Dr. Luo said he has seen a box of Semglee pens retail between $177 and $195, compared with about $500 retail for the Lantus pens.
Currently, people with commercial insurance can get Semglee for $0-75 a month, for up to a year, using the company’s savings program.
Steinberg said it’s possible that Mylan could increase the list price for the interchangeable Semglee, but that move could backfire. “I think their goal initially is to get market share,” he said.
After Basaglar came on the market – in late 2016 – the price of Lantus came down significantly over the next few years, according to a 2019 study by Dr. Luo’s colleagues at the University of Pittsburgh.
But Basaglar has not hung on to market share, according to Scott Strumello, a person with autoimmune type 1 diabetes who tweets and blogs about insulin and other issues.
In early August, Mr. Strumello tweeted some Lilly data that showed U.S. sales of Basaglar declined 42% in the first two quarters of 2021, compared with the same period in 2020.
Dr. Steinberg noted that the decline may have to do with rebates being given to PBMs by competitors Sanofi and Novo Nordisk. Sanofi “is very aggressive when it comes to pricing with their PBM partners,” he said.
While Mr. Devaney said people with diabetes are hopeful that Semglee can break the big three manufacturers’ monopoly, he added: “We don’t see Semglee as something that is solving the root cause of the insulin price crisis, which is high list prices and pharmaceutical industry greed.”
A version of this article first appeared on Medscape.com.
United Healthcare settles claims it violated mental health parity
The nation’s largest health care insurer has agreed to pay $16 million in restitution and penalties to consumers, the federal government, and the state of New York to settle complaints that it illegally limited outpatient psychotherapy.
The settlement resolves a joint U.S. Department of Labor and New York Attorney General investigation and lawsuit that found that United Healthcare and United Behavioral Health (UBH) had, since at least 2013, violated the federal Mental Health Parity and Addiction Equity Act of 2008.
United Healthcare and UBH are fighting similar claims in Wit v United Behavioral Health, which is currently on appeal at the U.S. Court of Appeals for the Ninth Circuit. If that complaint is decided against UBH, the insurer might have to reprocess more than 67,000 claims.
The joint New York–federal investigation determined that United Healthcare reduced reimbursement rates for out-of-network mental health services and denied payment for many services using its Algorithms for Effective Reporting and Treatment (ALERT) utilization review program.
All outpatient psychotherapy was subjected to review; by comparison, only a handful of medical and surgical services were. That violated parity laws, the New York Attorney General’s office said in a statement.
“United’s denial of these vital services was both unlawful and dangerous,” New York Attorney General Letitia James said in the statement.
“Protecting access to mental health and substance disorder treatment is a priority for the Department of Labor and something I believe in strongly as a person in long-term recovery,” U.S. Secretary of Labor Marty Walsh said in another statement.
Mr. Walsh added that the settlement “provides compensation for many people who were denied full benefits and equitable treatment.”
‘Investigations change behavior’
Commenting for this news organization, Joe Parks, MD, medical director of the National Council for Mental Wellbeing, applauded the settlement.
“These kinds of investigations change behavior, and people get better coverage,” Dr. Parks said.
He also applauded New York, saying that it “has been one of the more assertive states in expecting companies to comply with parity requirements. They should be emulated by other states.”
Dr. Parks noted that a 2019 U.S. Government Accountability Office report showed that only 20 states routinely conducted parity compliance reviews.
A spokesperson for the American Psychiatric Association said it was notable that the Department of Labor focused on three issues that it had not publicly investigated before.
These were payment parity, the use of algorithms in mental health to identify practitioners and patients who use more health care than expected of the “average” person, and requiring disclosure of how the insurer set rates, guidelines, and medical necessity standards.
The American Psychological Association’s chief of psychological practice, Jared L. Skillings, PhD, said the group was gratified that the Department of Labor and New York took complaints seriously and that it “is encouraged that United Behavioral Health and United Healthcare have agreed to change their unfair procedures regarding reimbursement and denial of psychological care.”
The association “will monitor implementation of the settlement with high hopes for fair treatment for patients with mental health needs,” Dr. Skillings said in an interview.
Across-the-board cuts for therapy
The investigation found that United Healthcare reduced the allowable reimbursement amount for all nonphysicians across the board for psychotherapy – by 25% for PhD-level psychologists and by 35% for all MA-level therapists.
The company’s ALERT system used arbitrary thresholds to trigger utilization review, which “often led to denials of coverage when providers could not justify continued treatment after 20 sessions,” said the New York attorney general.
Beneficiaries had to figure out how to pay for continuing services or abruptly end necessary treatment.
United Healthcare agreed to stop reducing reimbursement and to not employ a similar policy in New York for at least 2 years. It will stop using ALERT and stop issuing denials for psychotherapy through at least 2023.
In addition, “We are committed to ensuring all our members have access to care and to reimbursing providers consistent with the terms of the member’s health plan and state and federal rules,” United Healthcare said.
Dr. Parks noted that United Healthcare had stopped using ALERT long before the settlement and was instead employing Level of Care Utilization System for Psychiatric and Addiction Services (LOCUS) and American Society of Addiction Medicine criteria, which he called “a good step forward.”
‘Meaningful’ payout
United Healthcare will pay $14.3 million in restitution to consumers, including $9 million to 20,000 New Yorkers who received denials of or reductions in reimbursement. The company will also pay more than $2 million in penalties, with $1.3 million going to New York.
The insurer has annual revenues of some $250 billion.
“It’s not a large amount of money in United’s overall business operation,” said Dr. Parks.
However, “It certainly could be a meaningful amount of money for the individuals that didn’t get coverage that paid out of pocket,” he added.
A version of this article first appeared on Medscape.com.
The nation’s largest health care insurer has agreed to pay $16 million in restitution and penalties to consumers, the federal government, and the state of New York to settle complaints that it illegally limited outpatient psychotherapy.
The settlement resolves a joint U.S. Department of Labor and New York Attorney General investigation and lawsuit that found that United Healthcare and United Behavioral Health (UBH) had, since at least 2013, violated the federal Mental Health Parity and Addiction Equity Act of 2008.
United Healthcare and UBH are fighting similar claims in Wit v United Behavioral Health, which is currently on appeal at the U.S. Court of Appeals for the Ninth Circuit. If that complaint is decided against UBH, the insurer might have to reprocess more than 67,000 claims.
The joint New York–federal investigation determined that United Healthcare reduced reimbursement rates for out-of-network mental health services and denied payment for many services using its Algorithms for Effective Reporting and Treatment (ALERT) utilization review program.
All outpatient psychotherapy was subjected to review; by comparison, only a handful of medical and surgical services were. That violated parity laws, the New York Attorney General’s office said in a statement.
“United’s denial of these vital services was both unlawful and dangerous,” New York Attorney General Letitia James said in the statement.
“Protecting access to mental health and substance disorder treatment is a priority for the Department of Labor and something I believe in strongly as a person in long-term recovery,” U.S. Secretary of Labor Marty Walsh said in another statement.
Mr. Walsh added that the settlement “provides compensation for many people who were denied full benefits and equitable treatment.”
‘Investigations change behavior’
Commenting for this news organization, Joe Parks, MD, medical director of the National Council for Mental Wellbeing, applauded the settlement.
“These kinds of investigations change behavior, and people get better coverage,” Dr. Parks said.
He also applauded New York, saying that it “has been one of the more assertive states in expecting companies to comply with parity requirements. They should be emulated by other states.”
Dr. Parks noted that a 2019 U.S. Government Accountability Office report showed that only 20 states routinely conducted parity compliance reviews.
A spokesperson for the American Psychiatric Association said it was notable that the Department of Labor focused on three issues that it had not publicly investigated before.
These were payment parity, the use of algorithms in mental health to identify practitioners and patients who use more health care than expected of the “average” person, and requiring disclosure of how the insurer set rates, guidelines, and medical necessity standards.
The American Psychological Association’s chief of psychological practice, Jared L. Skillings, PhD, said the group was gratified that the Department of Labor and New York took complaints seriously and that it “is encouraged that United Behavioral Health and United Healthcare have agreed to change their unfair procedures regarding reimbursement and denial of psychological care.”
The association “will monitor implementation of the settlement with high hopes for fair treatment for patients with mental health needs,” Dr. Skillings said in an interview.
Across-the-board cuts for therapy
The investigation found that United Healthcare reduced the allowable reimbursement amount for all nonphysicians across the board for psychotherapy – by 25% for PhD-level psychologists and by 35% for all MA-level therapists.
The company’s ALERT system used arbitrary thresholds to trigger utilization review, which “often led to denials of coverage when providers could not justify continued treatment after 20 sessions,” said the New York attorney general.
Beneficiaries had to figure out how to pay for continuing services or abruptly end necessary treatment.
United Healthcare agreed to stop reducing reimbursement and to not employ a similar policy in New York for at least 2 years. It will stop using ALERT and stop issuing denials for psychotherapy through at least 2023.
In addition, “We are committed to ensuring all our members have access to care and to reimbursing providers consistent with the terms of the member’s health plan and state and federal rules,” United Healthcare said.
Dr. Parks noted that United Healthcare had stopped using ALERT long before the settlement and was instead employing Level of Care Utilization System for Psychiatric and Addiction Services (LOCUS) and American Society of Addiction Medicine criteria, which he called “a good step forward.”
‘Meaningful’ payout
United Healthcare will pay $14.3 million in restitution to consumers, including $9 million to 20,000 New Yorkers who received denials of or reductions in reimbursement. The company will also pay more than $2 million in penalties, with $1.3 million going to New York.
The insurer has annual revenues of some $250 billion.
“It’s not a large amount of money in United’s overall business operation,” said Dr. Parks.
However, “It certainly could be a meaningful amount of money for the individuals that didn’t get coverage that paid out of pocket,” he added.
A version of this article first appeared on Medscape.com.
The nation’s largest health care insurer has agreed to pay $16 million in restitution and penalties to consumers, the federal government, and the state of New York to settle complaints that it illegally limited outpatient psychotherapy.
The settlement resolves a joint U.S. Department of Labor and New York Attorney General investigation and lawsuit that found that United Healthcare and United Behavioral Health (UBH) had, since at least 2013, violated the federal Mental Health Parity and Addiction Equity Act of 2008.
United Healthcare and UBH are fighting similar claims in Wit v United Behavioral Health, which is currently on appeal at the U.S. Court of Appeals for the Ninth Circuit. If that complaint is decided against UBH, the insurer might have to reprocess more than 67,000 claims.
The joint New York–federal investigation determined that United Healthcare reduced reimbursement rates for out-of-network mental health services and denied payment for many services using its Algorithms for Effective Reporting and Treatment (ALERT) utilization review program.
All outpatient psychotherapy was subjected to review; by comparison, only a handful of medical and surgical services were. That violated parity laws, the New York Attorney General’s office said in a statement.
“United’s denial of these vital services was both unlawful and dangerous,” New York Attorney General Letitia James said in the statement.
“Protecting access to mental health and substance disorder treatment is a priority for the Department of Labor and something I believe in strongly as a person in long-term recovery,” U.S. Secretary of Labor Marty Walsh said in another statement.
Mr. Walsh added that the settlement “provides compensation for many people who were denied full benefits and equitable treatment.”
‘Investigations change behavior’
Commenting for this news organization, Joe Parks, MD, medical director of the National Council for Mental Wellbeing, applauded the settlement.
“These kinds of investigations change behavior, and people get better coverage,” Dr. Parks said.
He also applauded New York, saying that it “has been one of the more assertive states in expecting companies to comply with parity requirements. They should be emulated by other states.”
Dr. Parks noted that a 2019 U.S. Government Accountability Office report showed that only 20 states routinely conducted parity compliance reviews.
A spokesperson for the American Psychiatric Association said it was notable that the Department of Labor focused on three issues that it had not publicly investigated before.
These were payment parity, the use of algorithms in mental health to identify practitioners and patients who use more health care than expected of the “average” person, and requiring disclosure of how the insurer set rates, guidelines, and medical necessity standards.
The American Psychological Association’s chief of psychological practice, Jared L. Skillings, PhD, said the group was gratified that the Department of Labor and New York took complaints seriously and that it “is encouraged that United Behavioral Health and United Healthcare have agreed to change their unfair procedures regarding reimbursement and denial of psychological care.”
The association “will monitor implementation of the settlement with high hopes for fair treatment for patients with mental health needs,” Dr. Skillings said in an interview.
Across-the-board cuts for therapy
The investigation found that United Healthcare reduced the allowable reimbursement amount for all nonphysicians across the board for psychotherapy – by 25% for PhD-level psychologists and by 35% for all MA-level therapists.
The company’s ALERT system used arbitrary thresholds to trigger utilization review, which “often led to denials of coverage when providers could not justify continued treatment after 20 sessions,” said the New York attorney general.
Beneficiaries had to figure out how to pay for continuing services or abruptly end necessary treatment.
United Healthcare agreed to stop reducing reimbursement and to not employ a similar policy in New York for at least 2 years. It will stop using ALERT and stop issuing denials for psychotherapy through at least 2023.
In addition, “We are committed to ensuring all our members have access to care and to reimbursing providers consistent with the terms of the member’s health plan and state and federal rules,” United Healthcare said.
Dr. Parks noted that United Healthcare had stopped using ALERT long before the settlement and was instead employing Level of Care Utilization System for Psychiatric and Addiction Services (LOCUS) and American Society of Addiction Medicine criteria, which he called “a good step forward.”
‘Meaningful’ payout
United Healthcare will pay $14.3 million in restitution to consumers, including $9 million to 20,000 New Yorkers who received denials of or reductions in reimbursement. The company will also pay more than $2 million in penalties, with $1.3 million going to New York.
The insurer has annual revenues of some $250 billion.
“It’s not a large amount of money in United’s overall business operation,” said Dr. Parks.
However, “It certainly could be a meaningful amount of money for the individuals that didn’t get coverage that paid out of pocket,” he added.
A version of this article first appeared on Medscape.com.
‘Deeper dive’ into opioid overdose deaths during COVID pandemic
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Pups for veterans with PTSD: Biden signs PAWS act into law
Service members with posttraumatic stress disorder and other mental health conditions may eventually have expanded access to service dogs through legislation recently signed into law by President Joseph R. Biden.
The Puppies Assisting Wounded Servicemembers (PAWS) for Veterans Therapy Act (HR 1448) orders the Department of Veterans Affairs to begin a pilot program that over the course of 5 years will examine the utility and effectiveness of service dogs for improving the mental health of military veterans.
The legislation does not set a specific start date for the pilot program, but Rory Diamond, CEO of K9s for Warriors, a nonprofit organization based in Ponte Vedra, Fla., noted that K9s for Warriors and other organizations will be pushing the VA to start in 2022.
“We commend the White House for supporting this bill as a critical step in combating veteran suicide, and we’re confident in the path ahead for service dogs ultimately becoming a covered VA benefit to veterans with PTSD,” Mr. Diamond said in a statement provided to this news organization.
“For servicemembers relying on task-trained service dogs for PTSD, the HR 1448 is a giant leap towards supporting veterans and their service dogs in an equitable way,” Canine Companions, a national nonprofit organization that trains and provides service dogs, said in its own statement.
“It might mean the difference between having a veteran who won’t be here tomorrow and having one that will,” the group added.
Invisible wounds of war
In another statement, Bill McCabe, legislative affairs director at the Enlisted Association, said that “now, more than ever, veterans suffering from invisible wounds of war need access to trained service dogs, which have been scientifically proven to help alleviate symptoms of posttraumatic stress,” as well as traumatic brain injuries (TBIs) and military sexual trauma.
“We thank President Biden for recognizing veterans need every possible option when seeking mental health treatments, and look forward to working with the Department of Veterans Affairs to implement this important program,” Mr. McCabe said.
A recent VA report showed that in 2014, 40% of veterans had mental health conditions such as PTSD and substance use. An average of 20 veterans per day died by suicide that year.
Veterans with problems regarding mobility, hearing, and sight, as well as some mental health problems, have been eligible to have costs of veterinary care for service dogs paid by the VA, although the VA has not paid for the training of the animals.
The PAWS Act, which was bipartisan legislation introduced by U.S. Senators Thom Tillis (R-N.C.), Kyrsten Sinema (D-Ariz.), Kevin Cramer (R-N.D.), and Dianne Feinstein (D-Calif.), aims to expand eligibility to those with any mental health problems.
For at least a decade, various service dog and veterans’ organizations have pushed to have the VA expand the service dog benefit. This new law is a “first step,” said Mr. Diamond. “We had to kick open the door,” he said, adding that “the VA has essentially said no for almost 15 years.”
Mr. Diamond noted that there is “overwhelming” evidence showing that service dogs improve quality of life and reduce distress for veterans with PTSD and other diagnoses.
‘No excuse’
Results from a VA study showed that suicidal ideation was reduced in veterans who were paired with service dogs, compared with veterans paired with emotional support dogs. The study, which was made public in March, found no reduction in overall disability, according to a report by Military.com.
K9s for Warriors cites numerous other studies, published in peer-reviewed journals, that have shown that service dogs reduce PTSD symptoms, especially hypervigilance.
“There really is no excuse not to have the VA engaged in helping veterans suffering from posttraumatic stress who are extremely high risk of suicide to get a lifesaving service dog,” Mr. Diamond said.
His organization has paired 700 veterans suffering from TBI, PTSD, or military sexual trauma with a service dog. The organization provides a 3-week training program for the veteran and his or her dog.
Although about 200 of the graduates have been eligible to receive coverage from the VA for veterinary care for the dogs, it requires a lot of paperwork, and the criteria for who can be certified to receive that benefit are somewhat vague, Mr. Diamond noted.
Under current policy, the dog and veteran must have successfully completed a training program offered by an organization accredited by Assistance Dogs International or the International Guide Dog Federation.
The new pilot program will enable eligible veterans to receive dog training instruction from accredited nonprofit service dog training organizations, and it will give them the opportunity to adopt a dog that they actively assisted in training.
A version of this article first appeared on Medscape.com.
Service members with posttraumatic stress disorder and other mental health conditions may eventually have expanded access to service dogs through legislation recently signed into law by President Joseph R. Biden.
The Puppies Assisting Wounded Servicemembers (PAWS) for Veterans Therapy Act (HR 1448) orders the Department of Veterans Affairs to begin a pilot program that over the course of 5 years will examine the utility and effectiveness of service dogs for improving the mental health of military veterans.
The legislation does not set a specific start date for the pilot program, but Rory Diamond, CEO of K9s for Warriors, a nonprofit organization based in Ponte Vedra, Fla., noted that K9s for Warriors and other organizations will be pushing the VA to start in 2022.
“We commend the White House for supporting this bill as a critical step in combating veteran suicide, and we’re confident in the path ahead for service dogs ultimately becoming a covered VA benefit to veterans with PTSD,” Mr. Diamond said in a statement provided to this news organization.
“For servicemembers relying on task-trained service dogs for PTSD, the HR 1448 is a giant leap towards supporting veterans and their service dogs in an equitable way,” Canine Companions, a national nonprofit organization that trains and provides service dogs, said in its own statement.
“It might mean the difference between having a veteran who won’t be here tomorrow and having one that will,” the group added.
Invisible wounds of war
In another statement, Bill McCabe, legislative affairs director at the Enlisted Association, said that “now, more than ever, veterans suffering from invisible wounds of war need access to trained service dogs, which have been scientifically proven to help alleviate symptoms of posttraumatic stress,” as well as traumatic brain injuries (TBIs) and military sexual trauma.
“We thank President Biden for recognizing veterans need every possible option when seeking mental health treatments, and look forward to working with the Department of Veterans Affairs to implement this important program,” Mr. McCabe said.
A recent VA report showed that in 2014, 40% of veterans had mental health conditions such as PTSD and substance use. An average of 20 veterans per day died by suicide that year.
Veterans with problems regarding mobility, hearing, and sight, as well as some mental health problems, have been eligible to have costs of veterinary care for service dogs paid by the VA, although the VA has not paid for the training of the animals.
The PAWS Act, which was bipartisan legislation introduced by U.S. Senators Thom Tillis (R-N.C.), Kyrsten Sinema (D-Ariz.), Kevin Cramer (R-N.D.), and Dianne Feinstein (D-Calif.), aims to expand eligibility to those with any mental health problems.
For at least a decade, various service dog and veterans’ organizations have pushed to have the VA expand the service dog benefit. This new law is a “first step,” said Mr. Diamond. “We had to kick open the door,” he said, adding that “the VA has essentially said no for almost 15 years.”
Mr. Diamond noted that there is “overwhelming” evidence showing that service dogs improve quality of life and reduce distress for veterans with PTSD and other diagnoses.
‘No excuse’
Results from a VA study showed that suicidal ideation was reduced in veterans who were paired with service dogs, compared with veterans paired with emotional support dogs. The study, which was made public in March, found no reduction in overall disability, according to a report by Military.com.
K9s for Warriors cites numerous other studies, published in peer-reviewed journals, that have shown that service dogs reduce PTSD symptoms, especially hypervigilance.
“There really is no excuse not to have the VA engaged in helping veterans suffering from posttraumatic stress who are extremely high risk of suicide to get a lifesaving service dog,” Mr. Diamond said.
His organization has paired 700 veterans suffering from TBI, PTSD, or military sexual trauma with a service dog. The organization provides a 3-week training program for the veteran and his or her dog.
Although about 200 of the graduates have been eligible to receive coverage from the VA for veterinary care for the dogs, it requires a lot of paperwork, and the criteria for who can be certified to receive that benefit are somewhat vague, Mr. Diamond noted.
Under current policy, the dog and veteran must have successfully completed a training program offered by an organization accredited by Assistance Dogs International or the International Guide Dog Federation.
The new pilot program will enable eligible veterans to receive dog training instruction from accredited nonprofit service dog training organizations, and it will give them the opportunity to adopt a dog that they actively assisted in training.
A version of this article first appeared on Medscape.com.
Service members with posttraumatic stress disorder and other mental health conditions may eventually have expanded access to service dogs through legislation recently signed into law by President Joseph R. Biden.
The Puppies Assisting Wounded Servicemembers (PAWS) for Veterans Therapy Act (HR 1448) orders the Department of Veterans Affairs to begin a pilot program that over the course of 5 years will examine the utility and effectiveness of service dogs for improving the mental health of military veterans.
The legislation does not set a specific start date for the pilot program, but Rory Diamond, CEO of K9s for Warriors, a nonprofit organization based in Ponte Vedra, Fla., noted that K9s for Warriors and other organizations will be pushing the VA to start in 2022.
“We commend the White House for supporting this bill as a critical step in combating veteran suicide, and we’re confident in the path ahead for service dogs ultimately becoming a covered VA benefit to veterans with PTSD,” Mr. Diamond said in a statement provided to this news organization.
“For servicemembers relying on task-trained service dogs for PTSD, the HR 1448 is a giant leap towards supporting veterans and their service dogs in an equitable way,” Canine Companions, a national nonprofit organization that trains and provides service dogs, said in its own statement.
“It might mean the difference between having a veteran who won’t be here tomorrow and having one that will,” the group added.
Invisible wounds of war
In another statement, Bill McCabe, legislative affairs director at the Enlisted Association, said that “now, more than ever, veterans suffering from invisible wounds of war need access to trained service dogs, which have been scientifically proven to help alleviate symptoms of posttraumatic stress,” as well as traumatic brain injuries (TBIs) and military sexual trauma.
“We thank President Biden for recognizing veterans need every possible option when seeking mental health treatments, and look forward to working with the Department of Veterans Affairs to implement this important program,” Mr. McCabe said.
A recent VA report showed that in 2014, 40% of veterans had mental health conditions such as PTSD and substance use. An average of 20 veterans per day died by suicide that year.
Veterans with problems regarding mobility, hearing, and sight, as well as some mental health problems, have been eligible to have costs of veterinary care for service dogs paid by the VA, although the VA has not paid for the training of the animals.
The PAWS Act, which was bipartisan legislation introduced by U.S. Senators Thom Tillis (R-N.C.), Kyrsten Sinema (D-Ariz.), Kevin Cramer (R-N.D.), and Dianne Feinstein (D-Calif.), aims to expand eligibility to those with any mental health problems.
For at least a decade, various service dog and veterans’ organizations have pushed to have the VA expand the service dog benefit. This new law is a “first step,” said Mr. Diamond. “We had to kick open the door,” he said, adding that “the VA has essentially said no for almost 15 years.”
Mr. Diamond noted that there is “overwhelming” evidence showing that service dogs improve quality of life and reduce distress for veterans with PTSD and other diagnoses.
‘No excuse’
Results from a VA study showed that suicidal ideation was reduced in veterans who were paired with service dogs, compared with veterans paired with emotional support dogs. The study, which was made public in March, found no reduction in overall disability, according to a report by Military.com.
K9s for Warriors cites numerous other studies, published in peer-reviewed journals, that have shown that service dogs reduce PTSD symptoms, especially hypervigilance.
“There really is no excuse not to have the VA engaged in helping veterans suffering from posttraumatic stress who are extremely high risk of suicide to get a lifesaving service dog,” Mr. Diamond said.
His organization has paired 700 veterans suffering from TBI, PTSD, or military sexual trauma with a service dog. The organization provides a 3-week training program for the veteran and his or her dog.
Although about 200 of the graduates have been eligible to receive coverage from the VA for veterinary care for the dogs, it requires a lot of paperwork, and the criteria for who can be certified to receive that benefit are somewhat vague, Mr. Diamond noted.
Under current policy, the dog and veteran must have successfully completed a training program offered by an organization accredited by Assistance Dogs International or the International Guide Dog Federation.
The new pilot program will enable eligible veterans to receive dog training instruction from accredited nonprofit service dog training organizations, and it will give them the opportunity to adopt a dog that they actively assisted in training.
A version of this article first appeared on Medscape.com.
‘Munchausen by Internet’ crises a warning for all HCPs
A new study documents a handful of cases of women with Munchausen syndrome by Internet who targeted doulas in the United Kingdom during the COVID-19 lockdown.
The five cases were investigated by Kathryn Newns, MSc, DClinPsy, a clinical psychologist in Cambridge, England, who said the cases were brought to her attention by a doula she herself had used for the birth of her own child a decade earlier.
Dr. Newns said she believes these are not isolated cases – either geographically or in terms of the specialty involved.
“I don’t think it is likely that this is only happening in the United Kingdom. And I’m sure it’s not just happening in the doula world,” Dr. Newns told this news organization.
Coinvestigator Marc Feldman, MD, a clinical professor of psychiatry at the University of Alabama, Tuscaloosa, coined the term “Munchausen by Internet” in a 2000 article. The expression refers to use of electronic media to perpetrate hoaxes that reward posers with sympathy, control, or emotional gratification. The hoaxers do not seek financial gain.
“The ease of carrying out Munchausen behaviors makes me think that it must be much more common than it ever was,” Dr. Feldman said in an interview.
He noted that the new DSM-5 will eliminate the terms “Munchausen” and “Munchausen by Internet” and will clarify that “factitious disorder” can be partly or wholly carried out online.
The study was published in the May issue of the Annals of Clinical Psychiatry.
A warning for others
In the past, those with factitious disorder had to go to medical libraries to study up on the ailment they wanted to feign. They would then present to an emergency department or a doctor’s office and act convincingly, Dr. Feldman said.
“Now all you have to do is go to Wikipedia and you can become an expert on a medical ailment within a few minutes,” he added.
In the five cases described in the study, the hoaxers created rich stories, especially in cases 1 and 2. In those cases, the perpetrator turned out to be the same person. Subterfuge “obviously made it much harder to know she wasn’t who she purported to be,” said Dr. Newns.
Dr. Feldman noted that in Munchausen by Internet, there may be some element of truth within the stories.
For health care professionals, “it takes a considerable leap to assume that somebody who’s talking about some dreaded ailment is in fact exaggerating or outright lying,” he said.
In the five cases described in the study, persons contacted doulas, then related traumatic stories and described dramatic, immediate needs. All of the doulas were working remotely because of the COVID-19 pandemic. This likely made it easier for the perpetrators to pull off the hoaxes. The health care professionals agreed to share their experiences in the hopes of warning others.
Elaborate scenarios
The first two cases were ultimately determined to involve one person who had created elaborate scenarios.
In case 1, the hoaxer, who called herself “Jessica,” texted the doula “Charlotte” when she was allegedly 39 weeks’ pregnant. She said she was unable to go to the hospital because of the COVID-19 risks to her husband, who had cystic fibrosis and had recently undergone a heart and lung transplant.
The husband “Jordan” took over communications, using the same WhatsApp number as Jessica, as Jessica went into labor.
Ostensibly, a midwife team had come to Jessica’s and Jordan’s house. When the doula was on the phone with Jordan, she heard Jessica crying, grunting, and screaming, and then, at 2:00 a.m., she heard the sound of a baby crying. A photo of the baby was texted to Charlotte.
Soon, there were many problems. Jessica allegedly had a postpartum hemorrhage, and mother and baby were taken to separate hospitals. The baby was then diagnosed with congenital heart disease.
Over the next week, “midwives” started texting back and forth with Charlotte. The doula began to have doubts and asked a midwife to share a visual communication.
After receiving no response, Charlotte used a video call, got Jessica on screen, and told her she thought there was no baby. Jessica said the baby was real and showed a “growth chart” as proof of the 5-day-old baby’s existence. The birth and baby noises were later determined to be recordings.
Child deaths
After sharing information among themselves on a private Facebook group, the doulas determined that the person in case 2, “Dakota,” was the same woman who was involved in case 1.
In case 2, a doula had spent 2 years supporting Dakota through the deaths of a parent and her baby, who had a congenital defect. A baby-loss charity had also worked with Dakota but could not confirm the baby’s existence.
Dakota had gone so far as to make a video for the doula that showed a hospital room. In a voice-over, Dakota thanks everyone for the support she received as the baby died.
In case 3, “Hannah” texted a doula seeking emotional but not birth support. The doula, Nikki Barrow, has recounted the case on her own blog.
Hannah became desperate when she went into labor. Ms. Barrow remained close via texts, phone, and video calls, even as the baby supposedly died after 3 days. The doula lit a candle for the baby and cried with Hannah.
Ms. Barrow was eventually able to break away from Hannah, saying she was not a bereavement specialist. However, days later, Hannah tracked her down and claimed she had an infection in her heart and did not have much time to live. At that point, Ms. Barrow stopped all contact.
She determined from other doulas that Hannah had been hoaxing doulas for 4 or 5 years. Some had offered to get her help, but she refused and ended all contact.
Multiple COVID crises?
In case 4, a woman sought support on a doula-centered Facebook page and said her partner “Jack” would be in touch. Jack sent the doula hundreds of emails, texts, and WhatsApp messages and then said he was hospitalized with COVID. The woman, “Hayley,” was also soon diagnosed with COVID.
Hayley refused video contact and did not share photos. Drama continued to unfold. She reported that her baby was breach, that she had a second uterus with a second pregnancy simultaneously, and that the baby had COVID.
Hayley also claimed that her partner had come to the hospital, had raped her, and had brandished a gun. When the doula called the police, they did not find Hayley at the hospital or elsewhere.
In case 5, a “grandmother” contacted “Lisa” to find a doula for her daughter-in-law, “Anna.” Hours later, Anna was giving birth, and the baby had to be taken to the hospital because of cardiac and breathing problems. The doula heard nothing more after a few weeks.
However, at least three other doulas said they had supported the same “family.”
Online training program
In all cases, the doulas were not paid for their time. Reports to the police prompted no action because no money had changed hands. Some doulas said they felt bereaved, angry, or “silly” that they had been hoodwinked. All noted how difficult it was to disengage from clients who seemed to be in peril.
Ms. Barrow decided to create an online training program in which doulas are advised on how to stay safe while working online.
DoulaMatch, which matches birth support specialists with women in the United States and Canada, offers tips to help protect doulas from hoaxes.
Kim James, BDT(DONA), ICCE, LCCE, CLE, the owner and operator of DoulaMatch, said the organization is aware of “scammers who waste everyone’s time and have found doulas to be the latest easy targets.”
However, she noted, “I’ve only very occasionally and anecdotally heard about people fabricating a pregnancy for emotional gratification.”
In his 2000 article, Dr. Feldman offers clues to help detect hoaxers. He advises clinicians to be wary of the following:
- Cases in which the length, frequency, and duration of posts are incongruous with the severity of the illness the person is claiming to have; for example, someone who claims to be in submitting detailed posts.
- Near-fatal exacerbations of illness alternating with miraculous recoveries.
- Personal claims that are fantastic, are contradicted by later posts, or are disproved.
- Continual dramatic events occurring in the person’s life, especially when others in a group become the focus of attention.
- Others ostensibly posting on behalf of the individual who have identical patterns of writing, such as making grammatical errors, misspellings, and using stylistic idiosyncrasies.
A version of this article first appeared on Medscape.com.
A new study documents a handful of cases of women with Munchausen syndrome by Internet who targeted doulas in the United Kingdom during the COVID-19 lockdown.
The five cases were investigated by Kathryn Newns, MSc, DClinPsy, a clinical psychologist in Cambridge, England, who said the cases were brought to her attention by a doula she herself had used for the birth of her own child a decade earlier.
Dr. Newns said she believes these are not isolated cases – either geographically or in terms of the specialty involved.
“I don’t think it is likely that this is only happening in the United Kingdom. And I’m sure it’s not just happening in the doula world,” Dr. Newns told this news organization.
Coinvestigator Marc Feldman, MD, a clinical professor of psychiatry at the University of Alabama, Tuscaloosa, coined the term “Munchausen by Internet” in a 2000 article. The expression refers to use of electronic media to perpetrate hoaxes that reward posers with sympathy, control, or emotional gratification. The hoaxers do not seek financial gain.
“The ease of carrying out Munchausen behaviors makes me think that it must be much more common than it ever was,” Dr. Feldman said in an interview.
He noted that the new DSM-5 will eliminate the terms “Munchausen” and “Munchausen by Internet” and will clarify that “factitious disorder” can be partly or wholly carried out online.
The study was published in the May issue of the Annals of Clinical Psychiatry.
A warning for others
In the past, those with factitious disorder had to go to medical libraries to study up on the ailment they wanted to feign. They would then present to an emergency department or a doctor’s office and act convincingly, Dr. Feldman said.
“Now all you have to do is go to Wikipedia and you can become an expert on a medical ailment within a few minutes,” he added.
In the five cases described in the study, the hoaxers created rich stories, especially in cases 1 and 2. In those cases, the perpetrator turned out to be the same person. Subterfuge “obviously made it much harder to know she wasn’t who she purported to be,” said Dr. Newns.
Dr. Feldman noted that in Munchausen by Internet, there may be some element of truth within the stories.
For health care professionals, “it takes a considerable leap to assume that somebody who’s talking about some dreaded ailment is in fact exaggerating or outright lying,” he said.
In the five cases described in the study, persons contacted doulas, then related traumatic stories and described dramatic, immediate needs. All of the doulas were working remotely because of the COVID-19 pandemic. This likely made it easier for the perpetrators to pull off the hoaxes. The health care professionals agreed to share their experiences in the hopes of warning others.
Elaborate scenarios
The first two cases were ultimately determined to involve one person who had created elaborate scenarios.
In case 1, the hoaxer, who called herself “Jessica,” texted the doula “Charlotte” when she was allegedly 39 weeks’ pregnant. She said she was unable to go to the hospital because of the COVID-19 risks to her husband, who had cystic fibrosis and had recently undergone a heart and lung transplant.
The husband “Jordan” took over communications, using the same WhatsApp number as Jessica, as Jessica went into labor.
Ostensibly, a midwife team had come to Jessica’s and Jordan’s house. When the doula was on the phone with Jordan, she heard Jessica crying, grunting, and screaming, and then, at 2:00 a.m., she heard the sound of a baby crying. A photo of the baby was texted to Charlotte.
Soon, there were many problems. Jessica allegedly had a postpartum hemorrhage, and mother and baby were taken to separate hospitals. The baby was then diagnosed with congenital heart disease.
Over the next week, “midwives” started texting back and forth with Charlotte. The doula began to have doubts and asked a midwife to share a visual communication.
After receiving no response, Charlotte used a video call, got Jessica on screen, and told her she thought there was no baby. Jessica said the baby was real and showed a “growth chart” as proof of the 5-day-old baby’s existence. The birth and baby noises were later determined to be recordings.
Child deaths
After sharing information among themselves on a private Facebook group, the doulas determined that the person in case 2, “Dakota,” was the same woman who was involved in case 1.
In case 2, a doula had spent 2 years supporting Dakota through the deaths of a parent and her baby, who had a congenital defect. A baby-loss charity had also worked with Dakota but could not confirm the baby’s existence.
Dakota had gone so far as to make a video for the doula that showed a hospital room. In a voice-over, Dakota thanks everyone for the support she received as the baby died.
In case 3, “Hannah” texted a doula seeking emotional but not birth support. The doula, Nikki Barrow, has recounted the case on her own blog.
Hannah became desperate when she went into labor. Ms. Barrow remained close via texts, phone, and video calls, even as the baby supposedly died after 3 days. The doula lit a candle for the baby and cried with Hannah.
Ms. Barrow was eventually able to break away from Hannah, saying she was not a bereavement specialist. However, days later, Hannah tracked her down and claimed she had an infection in her heart and did not have much time to live. At that point, Ms. Barrow stopped all contact.
She determined from other doulas that Hannah had been hoaxing doulas for 4 or 5 years. Some had offered to get her help, but she refused and ended all contact.
Multiple COVID crises?
In case 4, a woman sought support on a doula-centered Facebook page and said her partner “Jack” would be in touch. Jack sent the doula hundreds of emails, texts, and WhatsApp messages and then said he was hospitalized with COVID. The woman, “Hayley,” was also soon diagnosed with COVID.
Hayley refused video contact and did not share photos. Drama continued to unfold. She reported that her baby was breach, that she had a second uterus with a second pregnancy simultaneously, and that the baby had COVID.
Hayley also claimed that her partner had come to the hospital, had raped her, and had brandished a gun. When the doula called the police, they did not find Hayley at the hospital or elsewhere.
In case 5, a “grandmother” contacted “Lisa” to find a doula for her daughter-in-law, “Anna.” Hours later, Anna was giving birth, and the baby had to be taken to the hospital because of cardiac and breathing problems. The doula heard nothing more after a few weeks.
However, at least three other doulas said they had supported the same “family.”
Online training program
In all cases, the doulas were not paid for their time. Reports to the police prompted no action because no money had changed hands. Some doulas said they felt bereaved, angry, or “silly” that they had been hoodwinked. All noted how difficult it was to disengage from clients who seemed to be in peril.
Ms. Barrow decided to create an online training program in which doulas are advised on how to stay safe while working online.
DoulaMatch, which matches birth support specialists with women in the United States and Canada, offers tips to help protect doulas from hoaxes.
Kim James, BDT(DONA), ICCE, LCCE, CLE, the owner and operator of DoulaMatch, said the organization is aware of “scammers who waste everyone’s time and have found doulas to be the latest easy targets.”
However, she noted, “I’ve only very occasionally and anecdotally heard about people fabricating a pregnancy for emotional gratification.”
In his 2000 article, Dr. Feldman offers clues to help detect hoaxers. He advises clinicians to be wary of the following:
- Cases in which the length, frequency, and duration of posts are incongruous with the severity of the illness the person is claiming to have; for example, someone who claims to be in submitting detailed posts.
- Near-fatal exacerbations of illness alternating with miraculous recoveries.
- Personal claims that are fantastic, are contradicted by later posts, or are disproved.
- Continual dramatic events occurring in the person’s life, especially when others in a group become the focus of attention.
- Others ostensibly posting on behalf of the individual who have identical patterns of writing, such as making grammatical errors, misspellings, and using stylistic idiosyncrasies.
A version of this article first appeared on Medscape.com.
A new study documents a handful of cases of women with Munchausen syndrome by Internet who targeted doulas in the United Kingdom during the COVID-19 lockdown.
The five cases were investigated by Kathryn Newns, MSc, DClinPsy, a clinical psychologist in Cambridge, England, who said the cases were brought to her attention by a doula she herself had used for the birth of her own child a decade earlier.
Dr. Newns said she believes these are not isolated cases – either geographically or in terms of the specialty involved.
“I don’t think it is likely that this is only happening in the United Kingdom. And I’m sure it’s not just happening in the doula world,” Dr. Newns told this news organization.
Coinvestigator Marc Feldman, MD, a clinical professor of psychiatry at the University of Alabama, Tuscaloosa, coined the term “Munchausen by Internet” in a 2000 article. The expression refers to use of electronic media to perpetrate hoaxes that reward posers with sympathy, control, or emotional gratification. The hoaxers do not seek financial gain.
“The ease of carrying out Munchausen behaviors makes me think that it must be much more common than it ever was,” Dr. Feldman said in an interview.
He noted that the new DSM-5 will eliminate the terms “Munchausen” and “Munchausen by Internet” and will clarify that “factitious disorder” can be partly or wholly carried out online.
The study was published in the May issue of the Annals of Clinical Psychiatry.
A warning for others
In the past, those with factitious disorder had to go to medical libraries to study up on the ailment they wanted to feign. They would then present to an emergency department or a doctor’s office and act convincingly, Dr. Feldman said.
“Now all you have to do is go to Wikipedia and you can become an expert on a medical ailment within a few minutes,” he added.
In the five cases described in the study, the hoaxers created rich stories, especially in cases 1 and 2. In those cases, the perpetrator turned out to be the same person. Subterfuge “obviously made it much harder to know she wasn’t who she purported to be,” said Dr. Newns.
Dr. Feldman noted that in Munchausen by Internet, there may be some element of truth within the stories.
For health care professionals, “it takes a considerable leap to assume that somebody who’s talking about some dreaded ailment is in fact exaggerating or outright lying,” he said.
In the five cases described in the study, persons contacted doulas, then related traumatic stories and described dramatic, immediate needs. All of the doulas were working remotely because of the COVID-19 pandemic. This likely made it easier for the perpetrators to pull off the hoaxes. The health care professionals agreed to share their experiences in the hopes of warning others.
Elaborate scenarios
The first two cases were ultimately determined to involve one person who had created elaborate scenarios.
In case 1, the hoaxer, who called herself “Jessica,” texted the doula “Charlotte” when she was allegedly 39 weeks’ pregnant. She said she was unable to go to the hospital because of the COVID-19 risks to her husband, who had cystic fibrosis and had recently undergone a heart and lung transplant.
The husband “Jordan” took over communications, using the same WhatsApp number as Jessica, as Jessica went into labor.
Ostensibly, a midwife team had come to Jessica’s and Jordan’s house. When the doula was on the phone with Jordan, she heard Jessica crying, grunting, and screaming, and then, at 2:00 a.m., she heard the sound of a baby crying. A photo of the baby was texted to Charlotte.
Soon, there were many problems. Jessica allegedly had a postpartum hemorrhage, and mother and baby were taken to separate hospitals. The baby was then diagnosed with congenital heart disease.
Over the next week, “midwives” started texting back and forth with Charlotte. The doula began to have doubts and asked a midwife to share a visual communication.
After receiving no response, Charlotte used a video call, got Jessica on screen, and told her she thought there was no baby. Jessica said the baby was real and showed a “growth chart” as proof of the 5-day-old baby’s existence. The birth and baby noises were later determined to be recordings.
Child deaths
After sharing information among themselves on a private Facebook group, the doulas determined that the person in case 2, “Dakota,” was the same woman who was involved in case 1.
In case 2, a doula had spent 2 years supporting Dakota through the deaths of a parent and her baby, who had a congenital defect. A baby-loss charity had also worked with Dakota but could not confirm the baby’s existence.
Dakota had gone so far as to make a video for the doula that showed a hospital room. In a voice-over, Dakota thanks everyone for the support she received as the baby died.
In case 3, “Hannah” texted a doula seeking emotional but not birth support. The doula, Nikki Barrow, has recounted the case on her own blog.
Hannah became desperate when she went into labor. Ms. Barrow remained close via texts, phone, and video calls, even as the baby supposedly died after 3 days. The doula lit a candle for the baby and cried with Hannah.
Ms. Barrow was eventually able to break away from Hannah, saying she was not a bereavement specialist. However, days later, Hannah tracked her down and claimed she had an infection in her heart and did not have much time to live. At that point, Ms. Barrow stopped all contact.
She determined from other doulas that Hannah had been hoaxing doulas for 4 or 5 years. Some had offered to get her help, but she refused and ended all contact.
Multiple COVID crises?
In case 4, a woman sought support on a doula-centered Facebook page and said her partner “Jack” would be in touch. Jack sent the doula hundreds of emails, texts, and WhatsApp messages and then said he was hospitalized with COVID. The woman, “Hayley,” was also soon diagnosed with COVID.
Hayley refused video contact and did not share photos. Drama continued to unfold. She reported that her baby was breach, that she had a second uterus with a second pregnancy simultaneously, and that the baby had COVID.
Hayley also claimed that her partner had come to the hospital, had raped her, and had brandished a gun. When the doula called the police, they did not find Hayley at the hospital or elsewhere.
In case 5, a “grandmother” contacted “Lisa” to find a doula for her daughter-in-law, “Anna.” Hours later, Anna was giving birth, and the baby had to be taken to the hospital because of cardiac and breathing problems. The doula heard nothing more after a few weeks.
However, at least three other doulas said they had supported the same “family.”
Online training program
In all cases, the doulas were not paid for their time. Reports to the police prompted no action because no money had changed hands. Some doulas said they felt bereaved, angry, or “silly” that they had been hoodwinked. All noted how difficult it was to disengage from clients who seemed to be in peril.
Ms. Barrow decided to create an online training program in which doulas are advised on how to stay safe while working online.
DoulaMatch, which matches birth support specialists with women in the United States and Canada, offers tips to help protect doulas from hoaxes.
Kim James, BDT(DONA), ICCE, LCCE, CLE, the owner and operator of DoulaMatch, said the organization is aware of “scammers who waste everyone’s time and have found doulas to be the latest easy targets.”
However, she noted, “I’ve only very occasionally and anecdotally heard about people fabricating a pregnancy for emotional gratification.”
In his 2000 article, Dr. Feldman offers clues to help detect hoaxers. He advises clinicians to be wary of the following:
- Cases in which the length, frequency, and duration of posts are incongruous with the severity of the illness the person is claiming to have; for example, someone who claims to be in submitting detailed posts.
- Near-fatal exacerbations of illness alternating with miraculous recoveries.
- Personal claims that are fantastic, are contradicted by later posts, or are disproved.
- Continual dramatic events occurring in the person’s life, especially when others in a group become the focus of attention.
- Others ostensibly posting on behalf of the individual who have identical patterns of writing, such as making grammatical errors, misspellings, and using stylistic idiosyncrasies.
A version of this article first appeared on Medscape.com.
Are you at legal risk for speaking at conferences?
When Jerry Gardner, MD, and a junior colleague received the acceptance notification for their abstract to be presented at Digestive Diseases Week® (DDW) 2021, a clause in the mandatory participation agreement gave Dr. Gardner pause. It required his colleague, as the submitting author, to completely accept any and all legal responsibility for any claims that might arise out of their presentation.
The clause was a red flag to Dr. Gardner, president of Science for Organizations, a Mill Valley, Calif.–based consulting firm. The gastroenterologist and former head of the digestive diseases branch at the National Institute of Diabetes and Digestive and Kidney Diseases – who has made hundreds of presentations and had participated in DDW for 40 years – had never encountered such a broad indemnity clause.
This news organization investigated just how risky it is to make a presentation at a conference – more than a dozen professional societies were contacted. Although DDW declined to discuss its agreement, Houston health care attorney Rachel V. Rose said that Dr. Gardner was smart to be cautious. “I would not sign that agreement. I have never seen anything that broad and all encompassing,” she said.
The DDW requirement “means that participants must put themselves at great potential financial risk in order to present their work,” Dr. Gardner said. He added that he and his colleague would not have submitted an abstract had they known about the indemnification clause up front.
Dr. Gardner advised his colleague not to sign the DDW agreement. She did not, and both missed the meeting.
Speakers ‘have to be careful’
Dr. Gardner may be an exception. How many doctors are willing to forgo a presentation because of a concern about something in an agreement?
John Mandrola, MD, said he operates under the assumption that if he does not sign the agreement, then he won’t be able to give his presentation. He admits that he generally just signs them and is careful with his presentations. “I’ve never really paid much attention to them,” said Dr. Mandrola, a cardiac electrophysiologist in Louisville, Ky., and chief cardiology correspondent for Medscape.
Not everyone takes that approach. “I do think that people read them, but they also take them with a grain of salt,” said E. Magnus Ohman, MBBS, professor of medicine at Duke University, Durham, N.C. He said he’s pragmatic and regards the agreements as a necessary evil in a litigious nation. Speakers “have to be careful, obviously,” Dr. Ohman said in an interview.
Some argue that the requirements are not only fair but also understandable. David Johnson, MD, a former president of the American College of Gastroenterology, said he has never had questions about agreements for meetings he has been involved with. “To me, this is not anything other than standard operating procedure,” he said.
Presenters participate by invitation, noted Dr. Johnson, a professor of medicine and chief of gastroenterology at the Eastern Virginia Medical School, Norfolk, who is a contributor to this news organization. “If they stand up and do something egregious, I would concur that the society should not be liable,” he said.
Big asks, big secrecy
Even for those who generally agree with Dr. Johnson’s position, it may be hard to completely understand what’s at stake without an attorney.
Although many declined to discuss their policies, a handful of professional societies provided their agreements for review. In general, the agreements appear to offer broad protection and rights to the organizers and large liability exposure for the participants. Participants are charged with a wide range of responsibilities, such as ensuring against copyright violations and intellectual property infringement, and that they also agree to unlimited use of their presentations and their name and likeness.
The American Academy of Neurology, which held its meeting virtually in 2021, required participants to indemnify the organization against all “losses, expenses, damages, or liabilities,” including “reasonable attorneys’ fees.” Federal employees, however, could opt out of indemnification.
The American Society of Clinical Oncology said that it does not usually require indemnification from its meeting participants. However, a spokesperson noted that ASCO did require participants at its 2021 virtual meeting to abide by the terms of use for content posted to the ASCO website. Those terms specify that users agree to indemnify ASCO from damages related to posts.
The American Psychiatric Association said it does not require any indemnification but did not make its agreement available. The American Academy of Pediatrics also said it did not require indemnification but would not share its agreement.
An American Diabetes Association spokesperson said that “every association is different in what they ask or require from speakers,” but would not share its requirements.
The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the Endocrine Society all declined to participate.
The organizations that withheld agreements “probably don’t want anybody picking apart their documents,” said Kyle Claussen, CEO of the Resolve Physician Agency, which reviews employment contracts and other contracts for physicians. “The more fair a document, the more likely they would be willing to disclose that, because they have nothing to hide,” he said.
‘It’s all on you’
Requiring indemnification for any and all aspects of a presentation appears to be increasingly common, said the attorneys interviewed for this article. As organizations repackage meeting presentations for sale, they put the content further out into the world and for a longer period, which increases liability exposure.
“If I’m the attorney for DDW, I certainly think I’d want to have this in place,” said Mr. Claussen.
“It’s good business sense for them because it reduces their risk,” said Courtney H. A. Thompson, an attorney with Fredrikson & Byron in Minneapolis, who advises regional and national corporations and ad agencies on advertising, marketing, and trademark law. She also works with clients who speak at meetings and who thus encounter meeting agreements.
Ms. Thompson said indemnity clauses have become fairly common over the past decade, especially as more companies and organizations have sought to protect trademarks, copyrights, and intellectual property and to minimize litigation costs.
A conference organizer “doesn’t want a third party to come after them for intellectual property, privacy, or publicity right infringement based on the participation of the customer or, in this case, the speaker,” said Ms. Thompson.
The agreements also reflect America’s litigation-prone culture.
Dean Fanelli, a patent attorney in the Washington, D.C., office of Cooley LLP, said the agreements he’s been asked to sign as a speaker increasingly seem “overly lawyerly.”
Two decades ago, a speaker might have been asked to sign a paragraph or a one-page form. Now “they often look more like formalized legal agreements,” Mr. Fanelli told this news organization.
The DDW agreement, for instance, ran four pages and contained 21 detailed clauses.
The increasingly complicated agreements “are a little over the top,” said Mr. Fanelli. But as an attorney who works with clients in the pharmaceutical industry, he said he understands that meeting organizers want to protect their rights.
DDW’s main indemnification clause requires the participant to indemnify DDW and its agents, directors, and employees “against any and all claims, demands, causes of action, losses, damages, liabilities, costs, and expenses,” including attorneys’ fees “arising out of a claim, action or proceeding” based on a breach or “alleged breach” by the participant.
“You’re releasing this information to them and then you’re also giving them blanket indemnity back, saying if there’s any type of intellectual property violation on your end – if you’ve included any type of work that’s protected, if this causes any problems – it’s all on you,” said Mr. Claussen.
Other potential pitfalls
Aside from indemnification, participation agreements can contain other potentially worrisome clauses, including onerous terms for cancellation and reuse of content without remuneration.
DDW requires royalty-free licensing of a speaker’s content; the organization can reproduce it in perpetuity without royalties. Many organizations have such a clause in their agreements, including the AAN and the American College of Cardiology.
ASCO’s general authorization form for meeting participants requires that they assign to ASCO rights to their content “perpetually, irrevocably, worldwide and royalty free.” Participants can contact the organization if they seek to opt out, but it’s not clear whether ASCO grants such requests.
Participants in the upcoming American Heart Association annual meeting can deny permission to record their presentation. But if they allow recording and do not agree to assign all rights and copyright ownership to the AHA, the work will be excluded from publication in the meeting program, e-posters, and the meeting supplement in Circulation.
Mr. Claussen said granting royalty-free rights presents a conundrum. Having content reproduced in various formats “might be better for your personal brand,” but it’s not likely to result in any direct compensation and could increase liability exposure, he said.
How presenters must prepare
Mr. Claussen and Ms. Rose said speakers should be vigilant about their own rights and responsibilities, including ensuring that they do not violate copyrights or infringe on intellectual property rights.
“I would recommend that folks be meticulous about what is in their slide deck and materials,” said Ms. Thompson. He said that presenters should be sure they have the right to share material. Technologies crawl the internet seeking out infringement, which often leads to cease and desist letters from attorneys, she said.
It’s better to head off such a letter, Ms. Thompson said. “You need to defend it whether or not it’s a viable claim,” and that can be costly, she said.
Both Ms. Thompson and Mr. Fanelli also warn about disclosing anything that might be considered a trade secret. Many agreements prohibit presenters from engaging in commercial promotion, but if a talk includes information about a drug or device, the manufacturer will want to review the presentation before it’s made public, said Mr. Fanelli.
Many organizations prohibit attendees from photographing, recording, or tweeting at meetings and often require speakers to warn the audience about doing so. DDW goes further by holding presenters liable if someone violates the rule.
“That’s a huge problem,” said Dr. Mandrola. He noted that although it might be easy to police journalists attending a meeting, “it seems hard to enforce that rule amongst just regular attendees.”
Accept or negotiate?
Individuals who submit work to an organization might feel they must sign an agreement as is, especially if they are looking to advance their career or expand knowledge by presenting work at a meeting. But some attorneys said it might be possible to negotiate with meeting organizers.
“My personal opinion is that it never hurts to ask,” said Ms. Thompson. If she were speaking at a legal conference, she would mark up a contract and “see what happens.” The more times pushback is accepted – say, if it works with three out of five speaking engagements – the more it reduces overall liability exposure.
Mr. Fanelli, however, said that although he always reads over an agreement, he typically signs without negotiating. “I don’t usually worry about it because I’m just trying to talk at a particular seminar,” he said.
Prospective presenters “have to weigh that balance – do you want to talk at a seminar, or are you concerned about the legal issues?” said Mr. Fanelli.
If in doubt, talk with a lawyer.
“If you ever have a question on whether or not you should consult an attorney, the answer is always yes,” said Mr. Claussen. It would be “an ounce of prevention,” especially if it’s just a short agreement, he said.
Dr. Ohman, however, said that he believed “it would be fairly costly” and potentially unwieldy. “You can’t litigate everything in life,” he added.
As for Dr. Gardner, he said he would not be as likely to attend DDW in the future if he has to agree to cover any and all liability. “I can’t conceive of ever agreeing to personally indemnify DDW in order to make a presentation at the annual meeting,” he said.
A version of this article first appeared on Medscape.com.
When Jerry Gardner, MD, and a junior colleague received the acceptance notification for their abstract to be presented at Digestive Diseases Week® (DDW) 2021, a clause in the mandatory participation agreement gave Dr. Gardner pause. It required his colleague, as the submitting author, to completely accept any and all legal responsibility for any claims that might arise out of their presentation.
The clause was a red flag to Dr. Gardner, president of Science for Organizations, a Mill Valley, Calif.–based consulting firm. The gastroenterologist and former head of the digestive diseases branch at the National Institute of Diabetes and Digestive and Kidney Diseases – who has made hundreds of presentations and had participated in DDW for 40 years – had never encountered such a broad indemnity clause.
This news organization investigated just how risky it is to make a presentation at a conference – more than a dozen professional societies were contacted. Although DDW declined to discuss its agreement, Houston health care attorney Rachel V. Rose said that Dr. Gardner was smart to be cautious. “I would not sign that agreement. I have never seen anything that broad and all encompassing,” she said.
The DDW requirement “means that participants must put themselves at great potential financial risk in order to present their work,” Dr. Gardner said. He added that he and his colleague would not have submitted an abstract had they known about the indemnification clause up front.
Dr. Gardner advised his colleague not to sign the DDW agreement. She did not, and both missed the meeting.
Speakers ‘have to be careful’
Dr. Gardner may be an exception. How many doctors are willing to forgo a presentation because of a concern about something in an agreement?
John Mandrola, MD, said he operates under the assumption that if he does not sign the agreement, then he won’t be able to give his presentation. He admits that he generally just signs them and is careful with his presentations. “I’ve never really paid much attention to them,” said Dr. Mandrola, a cardiac electrophysiologist in Louisville, Ky., and chief cardiology correspondent for Medscape.
Not everyone takes that approach. “I do think that people read them, but they also take them with a grain of salt,” said E. Magnus Ohman, MBBS, professor of medicine at Duke University, Durham, N.C. He said he’s pragmatic and regards the agreements as a necessary evil in a litigious nation. Speakers “have to be careful, obviously,” Dr. Ohman said in an interview.
Some argue that the requirements are not only fair but also understandable. David Johnson, MD, a former president of the American College of Gastroenterology, said he has never had questions about agreements for meetings he has been involved with. “To me, this is not anything other than standard operating procedure,” he said.
Presenters participate by invitation, noted Dr. Johnson, a professor of medicine and chief of gastroenterology at the Eastern Virginia Medical School, Norfolk, who is a contributor to this news organization. “If they stand up and do something egregious, I would concur that the society should not be liable,” he said.
Big asks, big secrecy
Even for those who generally agree with Dr. Johnson’s position, it may be hard to completely understand what’s at stake without an attorney.
Although many declined to discuss their policies, a handful of professional societies provided their agreements for review. In general, the agreements appear to offer broad protection and rights to the organizers and large liability exposure for the participants. Participants are charged with a wide range of responsibilities, such as ensuring against copyright violations and intellectual property infringement, and that they also agree to unlimited use of their presentations and their name and likeness.
The American Academy of Neurology, which held its meeting virtually in 2021, required participants to indemnify the organization against all “losses, expenses, damages, or liabilities,” including “reasonable attorneys’ fees.” Federal employees, however, could opt out of indemnification.
The American Society of Clinical Oncology said that it does not usually require indemnification from its meeting participants. However, a spokesperson noted that ASCO did require participants at its 2021 virtual meeting to abide by the terms of use for content posted to the ASCO website. Those terms specify that users agree to indemnify ASCO from damages related to posts.
The American Psychiatric Association said it does not require any indemnification but did not make its agreement available. The American Academy of Pediatrics also said it did not require indemnification but would not share its agreement.
An American Diabetes Association spokesperson said that “every association is different in what they ask or require from speakers,” but would not share its requirements.
The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the Endocrine Society all declined to participate.
The organizations that withheld agreements “probably don’t want anybody picking apart their documents,” said Kyle Claussen, CEO of the Resolve Physician Agency, which reviews employment contracts and other contracts for physicians. “The more fair a document, the more likely they would be willing to disclose that, because they have nothing to hide,” he said.
‘It’s all on you’
Requiring indemnification for any and all aspects of a presentation appears to be increasingly common, said the attorneys interviewed for this article. As organizations repackage meeting presentations for sale, they put the content further out into the world and for a longer period, which increases liability exposure.
“If I’m the attorney for DDW, I certainly think I’d want to have this in place,” said Mr. Claussen.
“It’s good business sense for them because it reduces their risk,” said Courtney H. A. Thompson, an attorney with Fredrikson & Byron in Minneapolis, who advises regional and national corporations and ad agencies on advertising, marketing, and trademark law. She also works with clients who speak at meetings and who thus encounter meeting agreements.
Ms. Thompson said indemnity clauses have become fairly common over the past decade, especially as more companies and organizations have sought to protect trademarks, copyrights, and intellectual property and to minimize litigation costs.
A conference organizer “doesn’t want a third party to come after them for intellectual property, privacy, or publicity right infringement based on the participation of the customer or, in this case, the speaker,” said Ms. Thompson.
The agreements also reflect America’s litigation-prone culture.
Dean Fanelli, a patent attorney in the Washington, D.C., office of Cooley LLP, said the agreements he’s been asked to sign as a speaker increasingly seem “overly lawyerly.”
Two decades ago, a speaker might have been asked to sign a paragraph or a one-page form. Now “they often look more like formalized legal agreements,” Mr. Fanelli told this news organization.
The DDW agreement, for instance, ran four pages and contained 21 detailed clauses.
The increasingly complicated agreements “are a little over the top,” said Mr. Fanelli. But as an attorney who works with clients in the pharmaceutical industry, he said he understands that meeting organizers want to protect their rights.
DDW’s main indemnification clause requires the participant to indemnify DDW and its agents, directors, and employees “against any and all claims, demands, causes of action, losses, damages, liabilities, costs, and expenses,” including attorneys’ fees “arising out of a claim, action or proceeding” based on a breach or “alleged breach” by the participant.
“You’re releasing this information to them and then you’re also giving them blanket indemnity back, saying if there’s any type of intellectual property violation on your end – if you’ve included any type of work that’s protected, if this causes any problems – it’s all on you,” said Mr. Claussen.
Other potential pitfalls
Aside from indemnification, participation agreements can contain other potentially worrisome clauses, including onerous terms for cancellation and reuse of content without remuneration.
DDW requires royalty-free licensing of a speaker’s content; the organization can reproduce it in perpetuity without royalties. Many organizations have such a clause in their agreements, including the AAN and the American College of Cardiology.
ASCO’s general authorization form for meeting participants requires that they assign to ASCO rights to their content “perpetually, irrevocably, worldwide and royalty free.” Participants can contact the organization if they seek to opt out, but it’s not clear whether ASCO grants such requests.
Participants in the upcoming American Heart Association annual meeting can deny permission to record their presentation. But if they allow recording and do not agree to assign all rights and copyright ownership to the AHA, the work will be excluded from publication in the meeting program, e-posters, and the meeting supplement in Circulation.
Mr. Claussen said granting royalty-free rights presents a conundrum. Having content reproduced in various formats “might be better for your personal brand,” but it’s not likely to result in any direct compensation and could increase liability exposure, he said.
How presenters must prepare
Mr. Claussen and Ms. Rose said speakers should be vigilant about their own rights and responsibilities, including ensuring that they do not violate copyrights or infringe on intellectual property rights.
“I would recommend that folks be meticulous about what is in their slide deck and materials,” said Ms. Thompson. He said that presenters should be sure they have the right to share material. Technologies crawl the internet seeking out infringement, which often leads to cease and desist letters from attorneys, she said.
It’s better to head off such a letter, Ms. Thompson said. “You need to defend it whether or not it’s a viable claim,” and that can be costly, she said.
Both Ms. Thompson and Mr. Fanelli also warn about disclosing anything that might be considered a trade secret. Many agreements prohibit presenters from engaging in commercial promotion, but if a talk includes information about a drug or device, the manufacturer will want to review the presentation before it’s made public, said Mr. Fanelli.
Many organizations prohibit attendees from photographing, recording, or tweeting at meetings and often require speakers to warn the audience about doing so. DDW goes further by holding presenters liable if someone violates the rule.
“That’s a huge problem,” said Dr. Mandrola. He noted that although it might be easy to police journalists attending a meeting, “it seems hard to enforce that rule amongst just regular attendees.”
Accept or negotiate?
Individuals who submit work to an organization might feel they must sign an agreement as is, especially if they are looking to advance their career or expand knowledge by presenting work at a meeting. But some attorneys said it might be possible to negotiate with meeting organizers.
“My personal opinion is that it never hurts to ask,” said Ms. Thompson. If she were speaking at a legal conference, she would mark up a contract and “see what happens.” The more times pushback is accepted – say, if it works with three out of five speaking engagements – the more it reduces overall liability exposure.
Mr. Fanelli, however, said that although he always reads over an agreement, he typically signs without negotiating. “I don’t usually worry about it because I’m just trying to talk at a particular seminar,” he said.
Prospective presenters “have to weigh that balance – do you want to talk at a seminar, or are you concerned about the legal issues?” said Mr. Fanelli.
If in doubt, talk with a lawyer.
“If you ever have a question on whether or not you should consult an attorney, the answer is always yes,” said Mr. Claussen. It would be “an ounce of prevention,” especially if it’s just a short agreement, he said.
Dr. Ohman, however, said that he believed “it would be fairly costly” and potentially unwieldy. “You can’t litigate everything in life,” he added.
As for Dr. Gardner, he said he would not be as likely to attend DDW in the future if he has to agree to cover any and all liability. “I can’t conceive of ever agreeing to personally indemnify DDW in order to make a presentation at the annual meeting,” he said.
A version of this article first appeared on Medscape.com.
When Jerry Gardner, MD, and a junior colleague received the acceptance notification for their abstract to be presented at Digestive Diseases Week® (DDW) 2021, a clause in the mandatory participation agreement gave Dr. Gardner pause. It required his colleague, as the submitting author, to completely accept any and all legal responsibility for any claims that might arise out of their presentation.
The clause was a red flag to Dr. Gardner, president of Science for Organizations, a Mill Valley, Calif.–based consulting firm. The gastroenterologist and former head of the digestive diseases branch at the National Institute of Diabetes and Digestive and Kidney Diseases – who has made hundreds of presentations and had participated in DDW for 40 years – had never encountered such a broad indemnity clause.
This news organization investigated just how risky it is to make a presentation at a conference – more than a dozen professional societies were contacted. Although DDW declined to discuss its agreement, Houston health care attorney Rachel V. Rose said that Dr. Gardner was smart to be cautious. “I would not sign that agreement. I have never seen anything that broad and all encompassing,” she said.
The DDW requirement “means that participants must put themselves at great potential financial risk in order to present their work,” Dr. Gardner said. He added that he and his colleague would not have submitted an abstract had they known about the indemnification clause up front.
Dr. Gardner advised his colleague not to sign the DDW agreement. She did not, and both missed the meeting.
Speakers ‘have to be careful’
Dr. Gardner may be an exception. How many doctors are willing to forgo a presentation because of a concern about something in an agreement?
John Mandrola, MD, said he operates under the assumption that if he does not sign the agreement, then he won’t be able to give his presentation. He admits that he generally just signs them and is careful with his presentations. “I’ve never really paid much attention to them,” said Dr. Mandrola, a cardiac electrophysiologist in Louisville, Ky., and chief cardiology correspondent for Medscape.
Not everyone takes that approach. “I do think that people read them, but they also take them with a grain of salt,” said E. Magnus Ohman, MBBS, professor of medicine at Duke University, Durham, N.C. He said he’s pragmatic and regards the agreements as a necessary evil in a litigious nation. Speakers “have to be careful, obviously,” Dr. Ohman said in an interview.
Some argue that the requirements are not only fair but also understandable. David Johnson, MD, a former president of the American College of Gastroenterology, said he has never had questions about agreements for meetings he has been involved with. “To me, this is not anything other than standard operating procedure,” he said.
Presenters participate by invitation, noted Dr. Johnson, a professor of medicine and chief of gastroenterology at the Eastern Virginia Medical School, Norfolk, who is a contributor to this news organization. “If they stand up and do something egregious, I would concur that the society should not be liable,” he said.
Big asks, big secrecy
Even for those who generally agree with Dr. Johnson’s position, it may be hard to completely understand what’s at stake without an attorney.
Although many declined to discuss their policies, a handful of professional societies provided their agreements for review. In general, the agreements appear to offer broad protection and rights to the organizers and large liability exposure for the participants. Participants are charged with a wide range of responsibilities, such as ensuring against copyright violations and intellectual property infringement, and that they also agree to unlimited use of their presentations and their name and likeness.
The American Academy of Neurology, which held its meeting virtually in 2021, required participants to indemnify the organization against all “losses, expenses, damages, or liabilities,” including “reasonable attorneys’ fees.” Federal employees, however, could opt out of indemnification.
The American Society of Clinical Oncology said that it does not usually require indemnification from its meeting participants. However, a spokesperson noted that ASCO did require participants at its 2021 virtual meeting to abide by the terms of use for content posted to the ASCO website. Those terms specify that users agree to indemnify ASCO from damages related to posts.
The American Psychiatric Association said it does not require any indemnification but did not make its agreement available. The American Academy of Pediatrics also said it did not require indemnification but would not share its agreement.
An American Diabetes Association spokesperson said that “every association is different in what they ask or require from speakers,” but would not share its requirements.
The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the Endocrine Society all declined to participate.
The organizations that withheld agreements “probably don’t want anybody picking apart their documents,” said Kyle Claussen, CEO of the Resolve Physician Agency, which reviews employment contracts and other contracts for physicians. “The more fair a document, the more likely they would be willing to disclose that, because they have nothing to hide,” he said.
‘It’s all on you’
Requiring indemnification for any and all aspects of a presentation appears to be increasingly common, said the attorneys interviewed for this article. As organizations repackage meeting presentations for sale, they put the content further out into the world and for a longer period, which increases liability exposure.
“If I’m the attorney for DDW, I certainly think I’d want to have this in place,” said Mr. Claussen.
“It’s good business sense for them because it reduces their risk,” said Courtney H. A. Thompson, an attorney with Fredrikson & Byron in Minneapolis, who advises regional and national corporations and ad agencies on advertising, marketing, and trademark law. She also works with clients who speak at meetings and who thus encounter meeting agreements.
Ms. Thompson said indemnity clauses have become fairly common over the past decade, especially as more companies and organizations have sought to protect trademarks, copyrights, and intellectual property and to minimize litigation costs.
A conference organizer “doesn’t want a third party to come after them for intellectual property, privacy, or publicity right infringement based on the participation of the customer or, in this case, the speaker,” said Ms. Thompson.
The agreements also reflect America’s litigation-prone culture.
Dean Fanelli, a patent attorney in the Washington, D.C., office of Cooley LLP, said the agreements he’s been asked to sign as a speaker increasingly seem “overly lawyerly.”
Two decades ago, a speaker might have been asked to sign a paragraph or a one-page form. Now “they often look more like formalized legal agreements,” Mr. Fanelli told this news organization.
The DDW agreement, for instance, ran four pages and contained 21 detailed clauses.
The increasingly complicated agreements “are a little over the top,” said Mr. Fanelli. But as an attorney who works with clients in the pharmaceutical industry, he said he understands that meeting organizers want to protect their rights.
DDW’s main indemnification clause requires the participant to indemnify DDW and its agents, directors, and employees “against any and all claims, demands, causes of action, losses, damages, liabilities, costs, and expenses,” including attorneys’ fees “arising out of a claim, action or proceeding” based on a breach or “alleged breach” by the participant.
“You’re releasing this information to them and then you’re also giving them blanket indemnity back, saying if there’s any type of intellectual property violation on your end – if you’ve included any type of work that’s protected, if this causes any problems – it’s all on you,” said Mr. Claussen.
Other potential pitfalls
Aside from indemnification, participation agreements can contain other potentially worrisome clauses, including onerous terms for cancellation and reuse of content without remuneration.
DDW requires royalty-free licensing of a speaker’s content; the organization can reproduce it in perpetuity without royalties. Many organizations have such a clause in their agreements, including the AAN and the American College of Cardiology.
ASCO’s general authorization form for meeting participants requires that they assign to ASCO rights to their content “perpetually, irrevocably, worldwide and royalty free.” Participants can contact the organization if they seek to opt out, but it’s not clear whether ASCO grants such requests.
Participants in the upcoming American Heart Association annual meeting can deny permission to record their presentation. But if they allow recording and do not agree to assign all rights and copyright ownership to the AHA, the work will be excluded from publication in the meeting program, e-posters, and the meeting supplement in Circulation.
Mr. Claussen said granting royalty-free rights presents a conundrum. Having content reproduced in various formats “might be better for your personal brand,” but it’s not likely to result in any direct compensation and could increase liability exposure, he said.
How presenters must prepare
Mr. Claussen and Ms. Rose said speakers should be vigilant about their own rights and responsibilities, including ensuring that they do not violate copyrights or infringe on intellectual property rights.
“I would recommend that folks be meticulous about what is in their slide deck and materials,” said Ms. Thompson. He said that presenters should be sure they have the right to share material. Technologies crawl the internet seeking out infringement, which often leads to cease and desist letters from attorneys, she said.
It’s better to head off such a letter, Ms. Thompson said. “You need to defend it whether or not it’s a viable claim,” and that can be costly, she said.
Both Ms. Thompson and Mr. Fanelli also warn about disclosing anything that might be considered a trade secret. Many agreements prohibit presenters from engaging in commercial promotion, but if a talk includes information about a drug or device, the manufacturer will want to review the presentation before it’s made public, said Mr. Fanelli.
Many organizations prohibit attendees from photographing, recording, or tweeting at meetings and often require speakers to warn the audience about doing so. DDW goes further by holding presenters liable if someone violates the rule.
“That’s a huge problem,” said Dr. Mandrola. He noted that although it might be easy to police journalists attending a meeting, “it seems hard to enforce that rule amongst just regular attendees.”
Accept or negotiate?
Individuals who submit work to an organization might feel they must sign an agreement as is, especially if they are looking to advance their career or expand knowledge by presenting work at a meeting. But some attorneys said it might be possible to negotiate with meeting organizers.
“My personal opinion is that it never hurts to ask,” said Ms. Thompson. If she were speaking at a legal conference, she would mark up a contract and “see what happens.” The more times pushback is accepted – say, if it works with three out of five speaking engagements – the more it reduces overall liability exposure.
Mr. Fanelli, however, said that although he always reads over an agreement, he typically signs without negotiating. “I don’t usually worry about it because I’m just trying to talk at a particular seminar,” he said.
Prospective presenters “have to weigh that balance – do you want to talk at a seminar, or are you concerned about the legal issues?” said Mr. Fanelli.
If in doubt, talk with a lawyer.
“If you ever have a question on whether or not you should consult an attorney, the answer is always yes,” said Mr. Claussen. It would be “an ounce of prevention,” especially if it’s just a short agreement, he said.
Dr. Ohman, however, said that he believed “it would be fairly costly” and potentially unwieldy. “You can’t litigate everything in life,” he added.
As for Dr. Gardner, he said he would not be as likely to attend DDW in the future if he has to agree to cover any and all liability. “I can’t conceive of ever agreeing to personally indemnify DDW in order to make a presentation at the annual meeting,” he said.
A version of this article first appeared on Medscape.com.
Watchdog group demands removal of FDA leaders after aducanumab approval
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
Physician convicted in buprenorphine scheme faces up to 20 years in prison
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
Study finds little impact of private equity on dermatology practices
A new
The authors reported that – with an average of five quarters postacquisition – there was no statistically significant differential between investor-owned and non–investor-owned practices “in total spending, overall use of dermatology procedures per patient, or specific high-volume and profitable procedures.”
Essentially, the study findings were equivocal, reported Robert Tyler Braun, PhD and his colleagues at Weill Cornell Medicine, New York. “The results provide mixed support for both proponents and opponents of private equity acquisitions,” they wrote in the study, which was published in Health Affairs.
But two dermatologists not involved with the study said the analysis has significant limitations, including a lack of pathology data, a lack of Medicare data, and a lack of insight into how advanced practice clinicians, such as nurse practitioners and physician assistants, were used by the private equity (PE)–owned practices. The study was not able to track “incident to billing.”
Leaving out Medicare data is a “huge oversight,” Joseph K. Francis, MD, a Mohs surgeon at the University of Florida, Gainesville, said in an interview. “The study is fundamentally flawed.”
“With all of these limitations, it’s difficult to draw meaningful conclusions,” agreed Clifford Perlis, MD, Mbe, of Keystone Dermatology in King of Prussia, Pa.
Both Dr. Francis and Dr. Perlis also questioned the influence of one of the study’s primary sponsors, the Physicians Foundation, formed out of the settlement of a class action lawsuit against third-party payers.
In addition, Dr. Francis and Dr. Perlis said they thought the study did not follow the PE-owned practices for a long enough period of time after acquisition to detect any differences, and that the dataset – looking at practice acquisitions from 2012 to 2017 – was too old to paint a reliable picture of the current state of PE-owned practices. Acquisitions have accelerated since 2017.
In March 2021, Harvard researchers reported in JAMA Health Forum that PE purchases in health care peaked in the first quarter of 2018 and surged to almost as high a level in the fourth quarter of 2020, with 153 deals announced in the second half of the year. Of the 153 acquisitions, 98, or 64%, were for physician practices or other health care services.
Dr. Braun said his study focused on 2012-2017 because it was an available data set. And, he defended the snapshot, saying that he and his colleagues had as much as 4 years of postacquisition data for the practices that were purchased in 2013. He acknowledged there were less data on practices purchased from 2014 to 2016.
“It is possible that our results would change with a longer postacquisition period,” Dr. Braun said in an interview. But, he said there was no way to predict whether that change “would look better or worse for private equity.”
Modest price increases
The authors analyzed data from the Health Care Cost Institute, which aggregates claims for some 50 million individuals insured by Aetna, Humana, and United Healthcare. The data do not include Medicare claims.
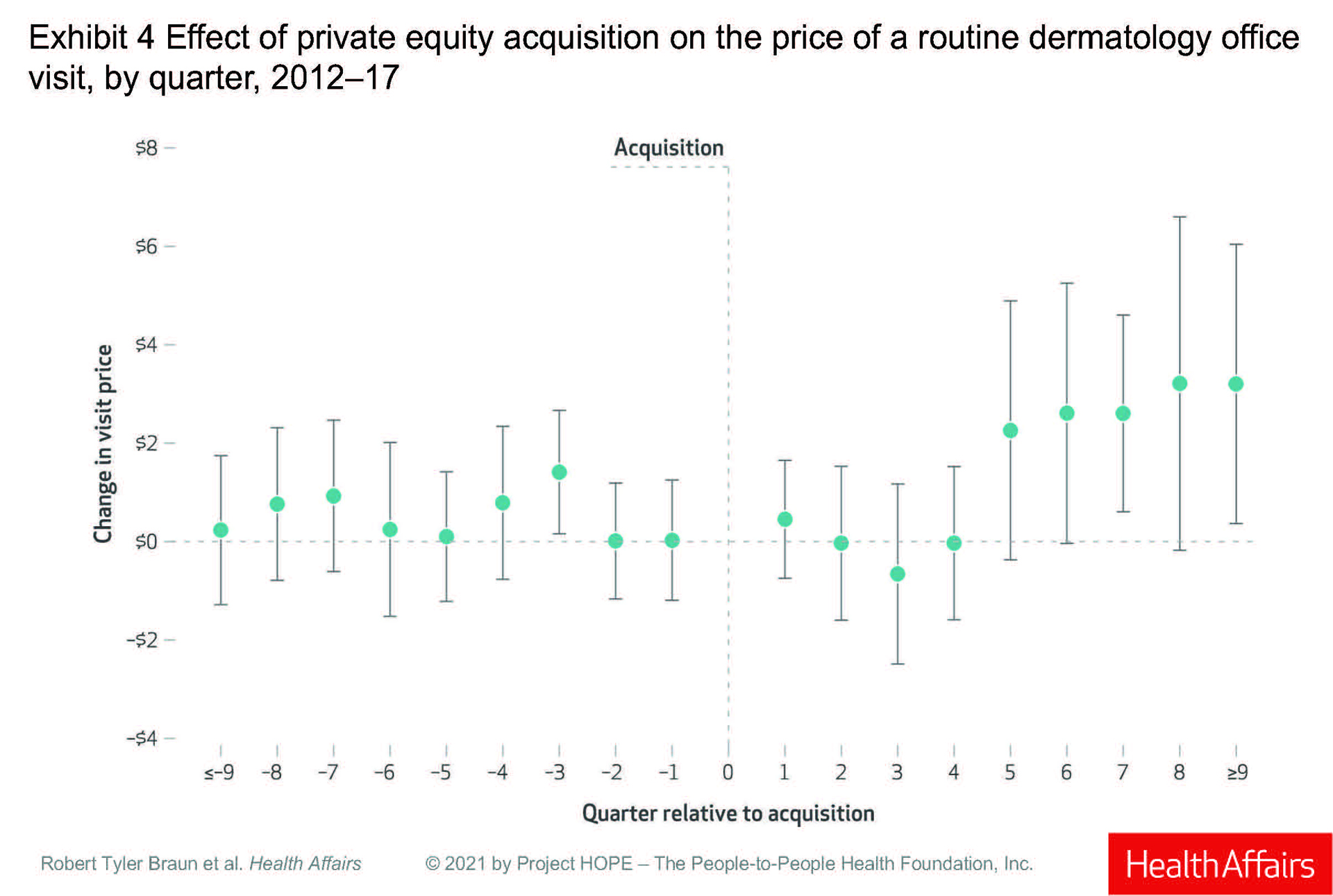
They examined dermatologists in practices bought between 2013 and 2016 and compared them to dermatologists who were in practices not owned by private equity. Each dermatologist had to have at least 2 years of data, and the authors compared preacquisition with postacquisition data for those in PE-owned practices.
They identified 64 practices – with 246 dermatologists – bought by private investors. Preacquisition, PE practices were larger than non-PE practices, with 4.2 clinicians (including advanced practitioners) per practice, compared with 1.7 in non–investor-owned practices.
The authors looked at volume and prices for routine office visits (CPT code 99213), biopsies (11101), excisions (11602), destruction of first lesion (cryotherapy; 17000), and Mohs micrographic surgery (17311).
Prices for a routine office visit rose nominally in the first quarter after acquisition (under $1), stayed at 0 in the second quarter, decreased in the third quarter, and was 0 again in the fourth quarter. It was not until the fifth quarter post acquisition that prices rose, increasing by 3% ($2.26), and then rising 5% ($3.20) in the ninth quarter.
Dr. Braun said the price increases make sense because practices get “rolled up” into larger platforms, theoretically giving them more negotiating leverage with insurers. And he said the paper’s results “are consistent with physician practice consolidation research – mainly hospitals acquiring practices – that prices increase after acquisition.” He acknowledged that the dermatology paper found “more modest effects,” than other studies.
Dr. Francis, however, said the increases are basically “pocket change,” and that they reflect a failed promise from private investors that clinicians in PE-owned practices will be paid more. The small differences in pay may also mean that insurers are likely not acquiescing to the theoretical leverage of larger dermatology entities.
PE-owned dermatologists saw about 5% more patients per quarter initially, rising to 17% more per quarter by eight quarters after purchase, according to the study.
The study reported a significant increase in Mohs surgery and cryotherapy in the first quarter post purchase, and a significant increase in biopsies after eight quarters. But Dr. Braun and colleagues concluded that total spending per patient did not change significantly after acquisition. “That says that maybe physicians aren’t changing their behaviors that much,” Dr. Braun said in an interview.
Dr. Perlis disagreed, noting that practices rarely change quickly. “Anecdotally, most groups that are taken over, nothing changes initially,” while the new owners are getting a feel for the practice.
He also said the paper erred in not addressing quality of and access to care. “Quality and patient satisfaction and access are also other important factors that need to be examined.”
Not benign players
Both Dr. Perlis and Dr. Francis said the study may have been improved by having a dermatologist as a coauthor. Dr. Braun countered that he and his colleagues consulted several dermatologists during the course of the study, and that they also conducted 30 interviews with proponents and opponents of PE ownership.
The authors warned of what they viewed as some disturbing trends in PE-owned practices, including what Dr. Braun called “stealth” consolidation – investors making small purchases that fall outside of federal regulation, and then amassing them into large entities.
He also commented that it was “alarming” that PE-owned practices were hiring a larger number of advanced practitioners. The authors also expressed concern about leveraged buyouts, in which investors require a practice to carry high debt loads that can eventually drive it into bankruptcy.
“These are not benign players,” said Dr. Francis. He noted that it took “an act of Congress to stop surprise billing,” a tactic employed by PE-owned health care entities. “Policy makers should be looking at what’s best for patients, especially Medicare and vulnerable patients.”
Dr. Perlis also has qualms about PE-owned practices. “The money to support returns to investors has to come from somewhere and that creates an inherent tension between patient care and optimizing revenue for investors,” he said. “It’s a pretty head-on conflict.”
A new
The authors reported that – with an average of five quarters postacquisition – there was no statistically significant differential between investor-owned and non–investor-owned practices “in total spending, overall use of dermatology procedures per patient, or specific high-volume and profitable procedures.”
Essentially, the study findings were equivocal, reported Robert Tyler Braun, PhD and his colleagues at Weill Cornell Medicine, New York. “The results provide mixed support for both proponents and opponents of private equity acquisitions,” they wrote in the study, which was published in Health Affairs.
But two dermatologists not involved with the study said the analysis has significant limitations, including a lack of pathology data, a lack of Medicare data, and a lack of insight into how advanced practice clinicians, such as nurse practitioners and physician assistants, were used by the private equity (PE)–owned practices. The study was not able to track “incident to billing.”
Leaving out Medicare data is a “huge oversight,” Joseph K. Francis, MD, a Mohs surgeon at the University of Florida, Gainesville, said in an interview. “The study is fundamentally flawed.”
“With all of these limitations, it’s difficult to draw meaningful conclusions,” agreed Clifford Perlis, MD, Mbe, of Keystone Dermatology in King of Prussia, Pa.
Both Dr. Francis and Dr. Perlis also questioned the influence of one of the study’s primary sponsors, the Physicians Foundation, formed out of the settlement of a class action lawsuit against third-party payers.
In addition, Dr. Francis and Dr. Perlis said they thought the study did not follow the PE-owned practices for a long enough period of time after acquisition to detect any differences, and that the dataset – looking at practice acquisitions from 2012 to 2017 – was too old to paint a reliable picture of the current state of PE-owned practices. Acquisitions have accelerated since 2017.
In March 2021, Harvard researchers reported in JAMA Health Forum that PE purchases in health care peaked in the first quarter of 2018 and surged to almost as high a level in the fourth quarter of 2020, with 153 deals announced in the second half of the year. Of the 153 acquisitions, 98, or 64%, were for physician practices or other health care services.
Dr. Braun said his study focused on 2012-2017 because it was an available data set. And, he defended the snapshot, saying that he and his colleagues had as much as 4 years of postacquisition data for the practices that were purchased in 2013. He acknowledged there were less data on practices purchased from 2014 to 2016.
“It is possible that our results would change with a longer postacquisition period,” Dr. Braun said in an interview. But, he said there was no way to predict whether that change “would look better or worse for private equity.”
Modest price increases
The authors analyzed data from the Health Care Cost Institute, which aggregates claims for some 50 million individuals insured by Aetna, Humana, and United Healthcare. The data do not include Medicare claims.

They examined dermatologists in practices bought between 2013 and 2016 and compared them to dermatologists who were in practices not owned by private equity. Each dermatologist had to have at least 2 years of data, and the authors compared preacquisition with postacquisition data for those in PE-owned practices.
They identified 64 practices – with 246 dermatologists – bought by private investors. Preacquisition, PE practices were larger than non-PE practices, with 4.2 clinicians (including advanced practitioners) per practice, compared with 1.7 in non–investor-owned practices.
The authors looked at volume and prices for routine office visits (CPT code 99213), biopsies (11101), excisions (11602), destruction of first lesion (cryotherapy; 17000), and Mohs micrographic surgery (17311).
Prices for a routine office visit rose nominally in the first quarter after acquisition (under $1), stayed at 0 in the second quarter, decreased in the third quarter, and was 0 again in the fourth quarter. It was not until the fifth quarter post acquisition that prices rose, increasing by 3% ($2.26), and then rising 5% ($3.20) in the ninth quarter.
Dr. Braun said the price increases make sense because practices get “rolled up” into larger platforms, theoretically giving them more negotiating leverage with insurers. And he said the paper’s results “are consistent with physician practice consolidation research – mainly hospitals acquiring practices – that prices increase after acquisition.” He acknowledged that the dermatology paper found “more modest effects,” than other studies.
Dr. Francis, however, said the increases are basically “pocket change,” and that they reflect a failed promise from private investors that clinicians in PE-owned practices will be paid more. The small differences in pay may also mean that insurers are likely not acquiescing to the theoretical leverage of larger dermatology entities.
PE-owned dermatologists saw about 5% more patients per quarter initially, rising to 17% more per quarter by eight quarters after purchase, according to the study.
The study reported a significant increase in Mohs surgery and cryotherapy in the first quarter post purchase, and a significant increase in biopsies after eight quarters. But Dr. Braun and colleagues concluded that total spending per patient did not change significantly after acquisition. “That says that maybe physicians aren’t changing their behaviors that much,” Dr. Braun said in an interview.
Dr. Perlis disagreed, noting that practices rarely change quickly. “Anecdotally, most groups that are taken over, nothing changes initially,” while the new owners are getting a feel for the practice.
He also said the paper erred in not addressing quality of and access to care. “Quality and patient satisfaction and access are also other important factors that need to be examined.”
Not benign players
Both Dr. Perlis and Dr. Francis said the study may have been improved by having a dermatologist as a coauthor. Dr. Braun countered that he and his colleagues consulted several dermatologists during the course of the study, and that they also conducted 30 interviews with proponents and opponents of PE ownership.
The authors warned of what they viewed as some disturbing trends in PE-owned practices, including what Dr. Braun called “stealth” consolidation – investors making small purchases that fall outside of federal regulation, and then amassing them into large entities.
He also commented that it was “alarming” that PE-owned practices were hiring a larger number of advanced practitioners. The authors also expressed concern about leveraged buyouts, in which investors require a practice to carry high debt loads that can eventually drive it into bankruptcy.
“These are not benign players,” said Dr. Francis. He noted that it took “an act of Congress to stop surprise billing,” a tactic employed by PE-owned health care entities. “Policy makers should be looking at what’s best for patients, especially Medicare and vulnerable patients.”
Dr. Perlis also has qualms about PE-owned practices. “The money to support returns to investors has to come from somewhere and that creates an inherent tension between patient care and optimizing revenue for investors,” he said. “It’s a pretty head-on conflict.”
A new
The authors reported that – with an average of five quarters postacquisition – there was no statistically significant differential between investor-owned and non–investor-owned practices “in total spending, overall use of dermatology procedures per patient, or specific high-volume and profitable procedures.”
Essentially, the study findings were equivocal, reported Robert Tyler Braun, PhD and his colleagues at Weill Cornell Medicine, New York. “The results provide mixed support for both proponents and opponents of private equity acquisitions,” they wrote in the study, which was published in Health Affairs.
But two dermatologists not involved with the study said the analysis has significant limitations, including a lack of pathology data, a lack of Medicare data, and a lack of insight into how advanced practice clinicians, such as nurse practitioners and physician assistants, were used by the private equity (PE)–owned practices. The study was not able to track “incident to billing.”
Leaving out Medicare data is a “huge oversight,” Joseph K. Francis, MD, a Mohs surgeon at the University of Florida, Gainesville, said in an interview. “The study is fundamentally flawed.”
“With all of these limitations, it’s difficult to draw meaningful conclusions,” agreed Clifford Perlis, MD, Mbe, of Keystone Dermatology in King of Prussia, Pa.
Both Dr. Francis and Dr. Perlis also questioned the influence of one of the study’s primary sponsors, the Physicians Foundation, formed out of the settlement of a class action lawsuit against third-party payers.
In addition, Dr. Francis and Dr. Perlis said they thought the study did not follow the PE-owned practices for a long enough period of time after acquisition to detect any differences, and that the dataset – looking at practice acquisitions from 2012 to 2017 – was too old to paint a reliable picture of the current state of PE-owned practices. Acquisitions have accelerated since 2017.
In March 2021, Harvard researchers reported in JAMA Health Forum that PE purchases in health care peaked in the first quarter of 2018 and surged to almost as high a level in the fourth quarter of 2020, with 153 deals announced in the second half of the year. Of the 153 acquisitions, 98, or 64%, were for physician practices or other health care services.
Dr. Braun said his study focused on 2012-2017 because it was an available data set. And, he defended the snapshot, saying that he and his colleagues had as much as 4 years of postacquisition data for the practices that were purchased in 2013. He acknowledged there were less data on practices purchased from 2014 to 2016.
“It is possible that our results would change with a longer postacquisition period,” Dr. Braun said in an interview. But, he said there was no way to predict whether that change “would look better or worse for private equity.”
Modest price increases
The authors analyzed data from the Health Care Cost Institute, which aggregates claims for some 50 million individuals insured by Aetna, Humana, and United Healthcare. The data do not include Medicare claims.

They examined dermatologists in practices bought between 2013 and 2016 and compared them to dermatologists who were in practices not owned by private equity. Each dermatologist had to have at least 2 years of data, and the authors compared preacquisition with postacquisition data for those in PE-owned practices.
They identified 64 practices – with 246 dermatologists – bought by private investors. Preacquisition, PE practices were larger than non-PE practices, with 4.2 clinicians (including advanced practitioners) per practice, compared with 1.7 in non–investor-owned practices.
The authors looked at volume and prices for routine office visits (CPT code 99213), biopsies (11101), excisions (11602), destruction of first lesion (cryotherapy; 17000), and Mohs micrographic surgery (17311).
Prices for a routine office visit rose nominally in the first quarter after acquisition (under $1), stayed at 0 in the second quarter, decreased in the third quarter, and was 0 again in the fourth quarter. It was not until the fifth quarter post acquisition that prices rose, increasing by 3% ($2.26), and then rising 5% ($3.20) in the ninth quarter.
Dr. Braun said the price increases make sense because practices get “rolled up” into larger platforms, theoretically giving them more negotiating leverage with insurers. And he said the paper’s results “are consistent with physician practice consolidation research – mainly hospitals acquiring practices – that prices increase after acquisition.” He acknowledged that the dermatology paper found “more modest effects,” than other studies.
Dr. Francis, however, said the increases are basically “pocket change,” and that they reflect a failed promise from private investors that clinicians in PE-owned practices will be paid more. The small differences in pay may also mean that insurers are likely not acquiescing to the theoretical leverage of larger dermatology entities.
PE-owned dermatologists saw about 5% more patients per quarter initially, rising to 17% more per quarter by eight quarters after purchase, according to the study.
The study reported a significant increase in Mohs surgery and cryotherapy in the first quarter post purchase, and a significant increase in biopsies after eight quarters. But Dr. Braun and colleagues concluded that total spending per patient did not change significantly after acquisition. “That says that maybe physicians aren’t changing their behaviors that much,” Dr. Braun said in an interview.
Dr. Perlis disagreed, noting that practices rarely change quickly. “Anecdotally, most groups that are taken over, nothing changes initially,” while the new owners are getting a feel for the practice.
He also said the paper erred in not addressing quality of and access to care. “Quality and patient satisfaction and access are also other important factors that need to be examined.”
Not benign players
Both Dr. Perlis and Dr. Francis said the study may have been improved by having a dermatologist as a coauthor. Dr. Braun countered that he and his colleagues consulted several dermatologists during the course of the study, and that they also conducted 30 interviews with proponents and opponents of PE ownership.
The authors warned of what they viewed as some disturbing trends in PE-owned practices, including what Dr. Braun called “stealth” consolidation – investors making small purchases that fall outside of federal regulation, and then amassing them into large entities.
He also commented that it was “alarming” that PE-owned practices were hiring a larger number of advanced practitioners. The authors also expressed concern about leveraged buyouts, in which investors require a practice to carry high debt loads that can eventually drive it into bankruptcy.
“These are not benign players,” said Dr. Francis. He noted that it took “an act of Congress to stop surprise billing,” a tactic employed by PE-owned health care entities. “Policy makers should be looking at what’s best for patients, especially Medicare and vulnerable patients.”
Dr. Perlis also has qualms about PE-owned practices. “The money to support returns to investors has to come from somewhere and that creates an inherent tension between patient care and optimizing revenue for investors,” he said. “It’s a pretty head-on conflict.”
FROM HEALTH AFFAIRS
APA, AMA, others move to stop insurer from overturning mental health claims ruling
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.


















