User login
A 16-Year-Old Hispanic Male with a History of Hyperlipidemia Reports a Pruritic Rash on His Neck and Chest
Discussion
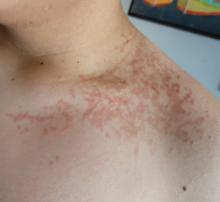
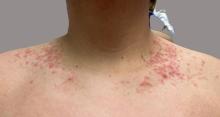
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Case Report
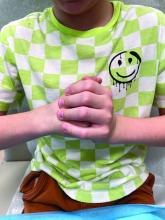
A 16-year-old Hispanic male with a history of hyperlipidemia presents to his pediatrician's office for a routine well-child check-up. He reports a pruritic rash on his neck and chest that has been present for the past 1.5 weeks. The rash is itchy, and although a cream from Mexico initially helped, it has not been effective recently. The patient mentions that he has increased his gym workouts and has been training for basketball. He has a history of obesity but has lost almost 100 pounds in the last 6 months. Most recently, he has stopped consuming carbohydrates and has been fasting in the mornings.
There is no history of eczema or psoriasis, either in the patient or his family.
Physical Examination
The patient weighs 147 pounds, a significant decrease from his previous weight of 270 pounds 6 months ago. Other vital signs are within normal limits.
On physical examination, the patient presents with net-like, pink, scaly plaques on his neck, with no other rashes on the body (see Pictures 1 and 2).
An 81-Year-Old White Woman Presented With a 2-Week History of a Painful Lesion on Her Left Calf
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
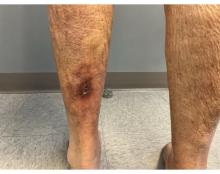
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
An 81-year-old White woman with a medical history significant for end stage renal disease (ESRD) on dialysis, diabetes, and a cerebrovascular accident presented with a 2-week history of a very painful lesion on her left calf. Upon physical exam, she was also noted to have tender subcutaneous nodules on her left anterolateral thigh that had been present for several weeks.
What's your diagnosis?
A 58-year-old White male presented with lesions on his index and middle finger for 3 months
Syphilis
Two biopsies by punch technique were performed; one for pathology and one for tissue culture (fungal and atypical mycobacteria). Tissue cultures showed no growth at 4 and 6 weeks, respectively. The lesions were swabbed for bacterial and viral cultures. Bacterial culture was positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and group C Streptococcus. Viral culture for herpes simplex virus (HSV) and varicella zoster virus (VZV) was negative. Histopathology confirmed the diagnosis of syphilis. Immunoperoxidase stain was positive for Treponema pallidum, and negative for HSV-1, HSV-2, and VZV. Special stains for PAS, GMS, Fite, and AFB were negative for organisms.
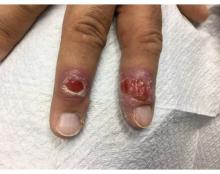
Syphilis, also known as Lues disease, is a contagious, sexually acquired disease caused by the spirochete T pallidum. The skin and mucous membranes are primarily infected. There are primary, secondary, and tertiary stages. In the primary or initial stage of syphilis, a chancre appears, usually 3-4 weeks after infection. The chancre is a painless papule or erosion that progresses to a firm ulceration. Lymphadenopathy may be present. Less often, multiple chancres may be present. Primary chancre on the finger has been reported in the literature, although it is far less common to have extragenital primary syphilis. The incidence ranges from 2% to 10%. Other extragenital areas that can be affected include lips, intraoral lesions, and the anus. Atypical chancres can be formed when other microbial agents are also present. Generally, an untreated chancre will heal spontaneously within a few months.
The patient referred to the department of health for treatment with penicillin G and further workup of sexually transmitted diseases. He was also seen by infectious disease for treatment of the superimposed bacterial infections and treated with an antibiotic regimen.
The case and photo were submitted by Dr. Bilu Martin.
Dr Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Ramoni S et al. Sex Transm Dis. 2010 Jul;37(7):468. doi: 10.1097/OLQ.0b013e3181e2cfac.
Starzycki Z. Br J Vener Dis. 1983 Jun;59(3):169-71. doi: 10.1136/sti.59.3.169.
Syphilis
Two biopsies by punch technique were performed; one for pathology and one for tissue culture (fungal and atypical mycobacteria). Tissue cultures showed no growth at 4 and 6 weeks, respectively. The lesions were swabbed for bacterial and viral cultures. Bacterial culture was positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and group C Streptococcus. Viral culture for herpes simplex virus (HSV) and varicella zoster virus (VZV) was negative. Histopathology confirmed the diagnosis of syphilis. Immunoperoxidase stain was positive for Treponema pallidum, and negative for HSV-1, HSV-2, and VZV. Special stains for PAS, GMS, Fite, and AFB were negative for organisms.
Syphilis, also known as Lues disease, is a contagious, sexually acquired disease caused by the spirochete T pallidum. The skin and mucous membranes are primarily infected. There are primary, secondary, and tertiary stages. In the primary or initial stage of syphilis, a chancre appears, usually 3-4 weeks after infection. The chancre is a painless papule or erosion that progresses to a firm ulceration. Lymphadenopathy may be present. Less often, multiple chancres may be present. Primary chancre on the finger has been reported in the literature, although it is far less common to have extragenital primary syphilis. The incidence ranges from 2% to 10%. Other extragenital areas that can be affected include lips, intraoral lesions, and the anus. Atypical chancres can be formed when other microbial agents are also present. Generally, an untreated chancre will heal spontaneously within a few months.
The patient referred to the department of health for treatment with penicillin G and further workup of sexually transmitted diseases. He was also seen by infectious disease for treatment of the superimposed bacterial infections and treated with an antibiotic regimen.
The case and photo were submitted by Dr. Bilu Martin.
Dr Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Ramoni S et al. Sex Transm Dis. 2010 Jul;37(7):468. doi: 10.1097/OLQ.0b013e3181e2cfac.
Starzycki Z. Br J Vener Dis. 1983 Jun;59(3):169-71. doi: 10.1136/sti.59.3.169.
Syphilis
Two biopsies by punch technique were performed; one for pathology and one for tissue culture (fungal and atypical mycobacteria). Tissue cultures showed no growth at 4 and 6 weeks, respectively. The lesions were swabbed for bacterial and viral cultures. Bacterial culture was positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and group C Streptococcus. Viral culture for herpes simplex virus (HSV) and varicella zoster virus (VZV) was negative. Histopathology confirmed the diagnosis of syphilis. Immunoperoxidase stain was positive for Treponema pallidum, and negative for HSV-1, HSV-2, and VZV. Special stains for PAS, GMS, Fite, and AFB were negative for organisms.
Syphilis, also known as Lues disease, is a contagious, sexually acquired disease caused by the spirochete T pallidum. The skin and mucous membranes are primarily infected. There are primary, secondary, and tertiary stages. In the primary or initial stage of syphilis, a chancre appears, usually 3-4 weeks after infection. The chancre is a painless papule or erosion that progresses to a firm ulceration. Lymphadenopathy may be present. Less often, multiple chancres may be present. Primary chancre on the finger has been reported in the literature, although it is far less common to have extragenital primary syphilis. The incidence ranges from 2% to 10%. Other extragenital areas that can be affected include lips, intraoral lesions, and the anus. Atypical chancres can be formed when other microbial agents are also present. Generally, an untreated chancre will heal spontaneously within a few months.
The patient referred to the department of health for treatment with penicillin G and further workup of sexually transmitted diseases. He was also seen by infectious disease for treatment of the superimposed bacterial infections and treated with an antibiotic regimen.
The case and photo were submitted by Dr. Bilu Martin.
Dr Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Ramoni S et al. Sex Transm Dis. 2010 Jul;37(7):468. doi: 10.1097/OLQ.0b013e3181e2cfac.
Starzycki Z. Br J Vener Dis. 1983 Jun;59(3):169-71. doi: 10.1136/sti.59.3.169.
A 58-year-old White male with no significant past medical history presented with lesions on his right index and middle fingers, which had been present for 3 months. The lesions were painless. The patient has a history of hand dermatitis. Upon questioning, the patient said he had not fished or cleaned fish tanks. He did garden occasionally (no roses). He has been using Neosporin on the lesions. He denied any fever or systemic symptoms and had no lymphadenopathy.
What's your diagnosis?
An 8-Year-Old Male With Asymptomatic Brown Rough Plaques on the Dorsum of the Right Hand and Fingers, Accompanied by Widening of the Knuckles
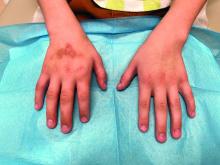
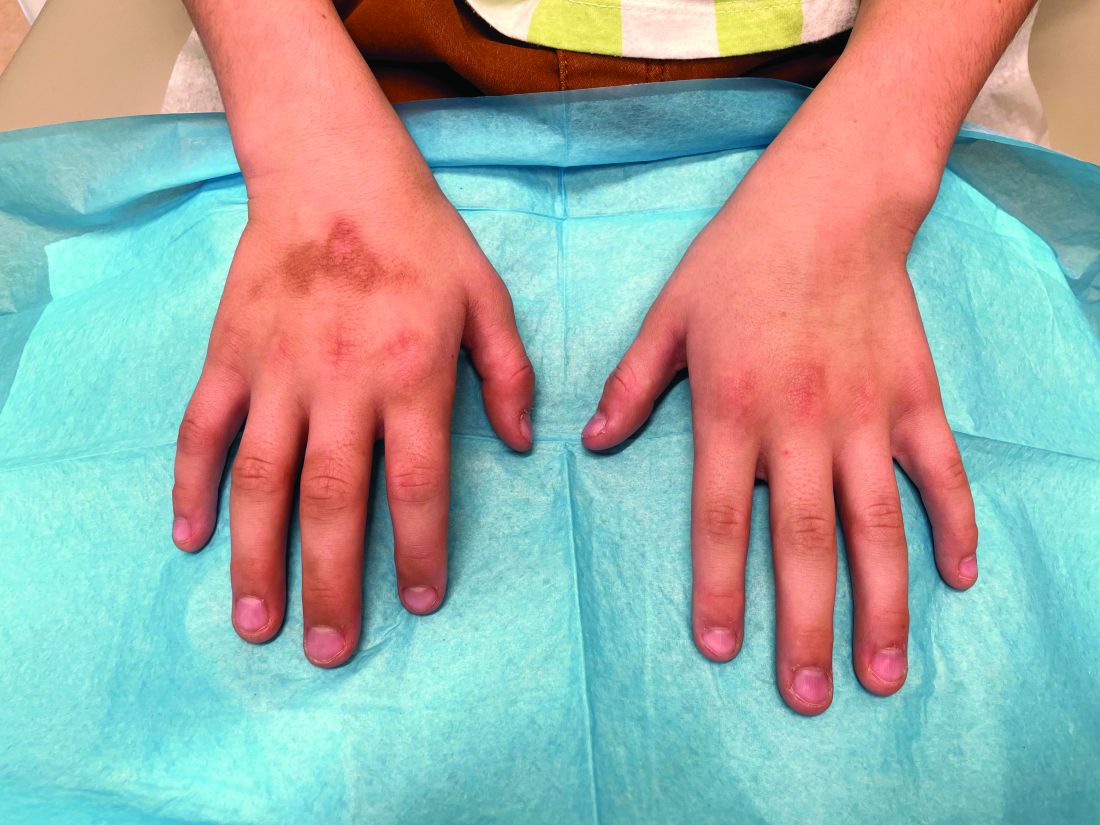
During examination, the patient was observed repetitively cracking his knuckles, making a fist with the right hand, placing the left hand on top, and rubbing the hand, a behavior he routinely did multiple times daily. The observed pattern of finger involvement on the dorsum of the right hand corresponded to areas subjected to significant pressure during the described activity. Consequently, a diagnosis of lichen simplex chronicus (LSC) secondary to mechanical rubbing, along with associated pachydermodactyly on the fingers of the right hand, was established.
Lichen simplex chronicus and pachydermodactyly are both attributed to microtrauma inflicted upon the skin. Lichen simplex chronicus often constitutes a diagnosis of exclusion and is characterized by repetitive trauma-induced keratinocyte proliferation and melanocyte activation, resulting in hyperpigmentation and skin thickening. Although typically observed in women between the fourth and fifth decades of life, LSC is rarely reported in children. In adults, LSC-related rubbing or scratching frequently arises from chronic pruritic dermatitis such as eczema or psoriasis, neurodermatitis from dysesthesia, or habitual movements, as exhibited by this young patient. While generally benign, LSC may become infected. In rare instances, malignant transformation may occur.
The association with pachydermodactyly implicates microtrauma, necessitating careful observation and questioning to elucidate the cause, as demonstrated in this case. Lesions are typically hyperpigmented, though cases of associated hypopigmentation or depigmentation have been documented. Affected areas typically fall within the patient’s hand and finger reach, with lesion improvement over several months achievable through trigger avoidance.
Pachydermodactyly, a rare but benign fibromatosis around the proximal interphalangeal joints, is often misdiagnosed as juvenile idiopathic arthritis, potentially leading to unnecessary treatments and patient anxiety. Microtrauma history due to digit manipulation is prevalent among affected individuals, with most also exhibiting neuropsychiatric disorders. Histological examination of pachydermodactyly reveals hypergranulosis and dermal thickening, accompanied by increased fibroblasts and collagen types I, III, and V, differing from the epidermal changes seen in LSC.
The differential diagnosis also included phytophotodermatitis, a phototoxic dermatologic reaction following exposure to ultraviolet light subsequent to contact with furocoumarin-containing plant chemicals. However, the persistence of the patient’s lesions for over a year precluded this diagnosis. Secondary hyperpigmentation was also contemplated but excluded due to the absence of preceding inflammatory dermatitis.
Treatment of LSC primarily involves identifying and addressing any underlying conditions, repairing the skin barrier, reducing inflammation, and modifying behaviors contributing to chronic microtrauma, as observed in this patient. Topical corticosteroids may aid in decreasing epidermal thickening and discoloration, though lesion resolution necessitates behavior cessation.
It’s important to identify these types of skin changes in children to avoid unnecessary medical treatments for these benign conditions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Seier JA, Dissemond J. Lichen Simplex Chronicus Due to Mechanical Irritation. Dtsch Arztebl Int. 2022 Nov 18;119(46):802. doi: 10.3238/arztebl.m2022.0213.
Small S et al. A 12-Year-Old Boy Presenting With Unilateral Proximal Interphalangeal Joint Swelling. BMJ Case Rep. 2011 Apr 13:2011:bcr0120113719. doi: 10.1136/bcr.01.2011.3719.
Voicu C et al Lichen Simplex Chronicus as an Essential Part of the Dermatologic Masquerade. Open Access Maced J Med Sci. 2017 Jul 24;5(4):556-557. doi: 10.3889/oamjms.2017.133.
During examination, the patient was observed repetitively cracking his knuckles, making a fist with the right hand, placing the left hand on top, and rubbing the hand, a behavior he routinely did multiple times daily. The observed pattern of finger involvement on the dorsum of the right hand corresponded to areas subjected to significant pressure during the described activity. Consequently, a diagnosis of lichen simplex chronicus (LSC) secondary to mechanical rubbing, along with associated pachydermodactyly on the fingers of the right hand, was established.
Lichen simplex chronicus and pachydermodactyly are both attributed to microtrauma inflicted upon the skin. Lichen simplex chronicus often constitutes a diagnosis of exclusion and is characterized by repetitive trauma-induced keratinocyte proliferation and melanocyte activation, resulting in hyperpigmentation and skin thickening. Although typically observed in women between the fourth and fifth decades of life, LSC is rarely reported in children. In adults, LSC-related rubbing or scratching frequently arises from chronic pruritic dermatitis such as eczema or psoriasis, neurodermatitis from dysesthesia, or habitual movements, as exhibited by this young patient. While generally benign, LSC may become infected. In rare instances, malignant transformation may occur.
The association with pachydermodactyly implicates microtrauma, necessitating careful observation and questioning to elucidate the cause, as demonstrated in this case. Lesions are typically hyperpigmented, though cases of associated hypopigmentation or depigmentation have been documented. Affected areas typically fall within the patient’s hand and finger reach, with lesion improvement over several months achievable through trigger avoidance.
Pachydermodactyly, a rare but benign fibromatosis around the proximal interphalangeal joints, is often misdiagnosed as juvenile idiopathic arthritis, potentially leading to unnecessary treatments and patient anxiety. Microtrauma history due to digit manipulation is prevalent among affected individuals, with most also exhibiting neuropsychiatric disorders. Histological examination of pachydermodactyly reveals hypergranulosis and dermal thickening, accompanied by increased fibroblasts and collagen types I, III, and V, differing from the epidermal changes seen in LSC.
The differential diagnosis also included phytophotodermatitis, a phototoxic dermatologic reaction following exposure to ultraviolet light subsequent to contact with furocoumarin-containing plant chemicals. However, the persistence of the patient’s lesions for over a year precluded this diagnosis. Secondary hyperpigmentation was also contemplated but excluded due to the absence of preceding inflammatory dermatitis.
Treatment of LSC primarily involves identifying and addressing any underlying conditions, repairing the skin barrier, reducing inflammation, and modifying behaviors contributing to chronic microtrauma, as observed in this patient. Topical corticosteroids may aid in decreasing epidermal thickening and discoloration, though lesion resolution necessitates behavior cessation.
It’s important to identify these types of skin changes in children to avoid unnecessary medical treatments for these benign conditions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Seier JA, Dissemond J. Lichen Simplex Chronicus Due to Mechanical Irritation. Dtsch Arztebl Int. 2022 Nov 18;119(46):802. doi: 10.3238/arztebl.m2022.0213.
Small S et al. A 12-Year-Old Boy Presenting With Unilateral Proximal Interphalangeal Joint Swelling. BMJ Case Rep. 2011 Apr 13:2011:bcr0120113719. doi: 10.1136/bcr.01.2011.3719.
Voicu C et al Lichen Simplex Chronicus as an Essential Part of the Dermatologic Masquerade. Open Access Maced J Med Sci. 2017 Jul 24;5(4):556-557. doi: 10.3889/oamjms.2017.133.
During examination, the patient was observed repetitively cracking his knuckles, making a fist with the right hand, placing the left hand on top, and rubbing the hand, a behavior he routinely did multiple times daily. The observed pattern of finger involvement on the dorsum of the right hand corresponded to areas subjected to significant pressure during the described activity. Consequently, a diagnosis of lichen simplex chronicus (LSC) secondary to mechanical rubbing, along with associated pachydermodactyly on the fingers of the right hand, was established.
Lichen simplex chronicus and pachydermodactyly are both attributed to microtrauma inflicted upon the skin. Lichen simplex chronicus often constitutes a diagnosis of exclusion and is characterized by repetitive trauma-induced keratinocyte proliferation and melanocyte activation, resulting in hyperpigmentation and skin thickening. Although typically observed in women between the fourth and fifth decades of life, LSC is rarely reported in children. In adults, LSC-related rubbing or scratching frequently arises from chronic pruritic dermatitis such as eczema or psoriasis, neurodermatitis from dysesthesia, or habitual movements, as exhibited by this young patient. While generally benign, LSC may become infected. In rare instances, malignant transformation may occur.
The association with pachydermodactyly implicates microtrauma, necessitating careful observation and questioning to elucidate the cause, as demonstrated in this case. Lesions are typically hyperpigmented, though cases of associated hypopigmentation or depigmentation have been documented. Affected areas typically fall within the patient’s hand and finger reach, with lesion improvement over several months achievable through trigger avoidance.
Pachydermodactyly, a rare but benign fibromatosis around the proximal interphalangeal joints, is often misdiagnosed as juvenile idiopathic arthritis, potentially leading to unnecessary treatments and patient anxiety. Microtrauma history due to digit manipulation is prevalent among affected individuals, with most also exhibiting neuropsychiatric disorders. Histological examination of pachydermodactyly reveals hypergranulosis and dermal thickening, accompanied by increased fibroblasts and collagen types I, III, and V, differing from the epidermal changes seen in LSC.
The differential diagnosis also included phytophotodermatitis, a phototoxic dermatologic reaction following exposure to ultraviolet light subsequent to contact with furocoumarin-containing plant chemicals. However, the persistence of the patient’s lesions for over a year precluded this diagnosis. Secondary hyperpigmentation was also contemplated but excluded due to the absence of preceding inflammatory dermatitis.
Treatment of LSC primarily involves identifying and addressing any underlying conditions, repairing the skin barrier, reducing inflammation, and modifying behaviors contributing to chronic microtrauma, as observed in this patient. Topical corticosteroids may aid in decreasing epidermal thickening and discoloration, though lesion resolution necessitates behavior cessation.
It’s important to identify these types of skin changes in children to avoid unnecessary medical treatments for these benign conditions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Seier JA, Dissemond J. Lichen Simplex Chronicus Due to Mechanical Irritation. Dtsch Arztebl Int. 2022 Nov 18;119(46):802. doi: 10.3238/arztebl.m2022.0213.
Small S et al. A 12-Year-Old Boy Presenting With Unilateral Proximal Interphalangeal Joint Swelling. BMJ Case Rep. 2011 Apr 13:2011:bcr0120113719. doi: 10.1136/bcr.01.2011.3719.
Voicu C et al Lichen Simplex Chronicus as an Essential Part of the Dermatologic Masquerade. Open Access Maced J Med Sci. 2017 Jul 24;5(4):556-557. doi: 10.3889/oamjms.2017.133.
The patient was otherwise healthy, with no current medication intake, and he engaged in baseball and soccer activities. Upon physical examination, a hyperpigmented lichenified irregular plaque was observed on the dorsum of the right hand, along with irregular hyperpigmented macules and plaques on the fingers. Fusiform widening of the interphalangeal joints on the second, third, and fourth fingers of the right hand was noted, without associated pain, edema, or erythema.
Do you agree with recent authors that patient satisfaction questionnaires should be modified to account for inherent societal biases, such as gender inequality and racism, to improve patient feedback?
[polldaddy:12782738]
[polldaddy:12782738]
[polldaddy:12782738]
When having discussions with your patients about recommended cancer screenings, have you been asked to answer questions related to liquid biopsy technology?
[polldaddy:11991465]
[polldaddy:11991465]
[polldaddy:11991465]
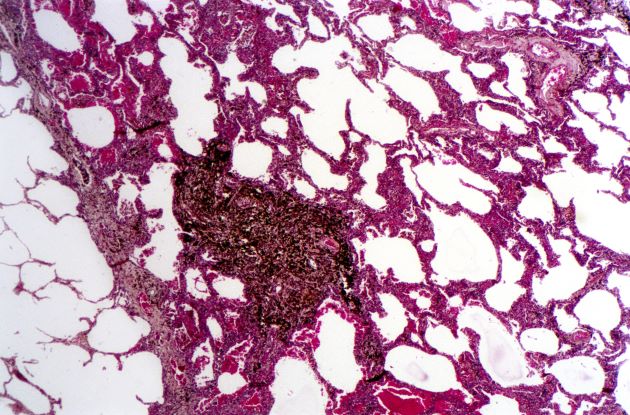
Woman presents with cough and bronchorrhea
Bronchioalveolar cell carcinoma (BAC) is a variant of non–small cell lung cancer (NSCLC) that, in recent years, has received a new identity in some of the literature. Adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) are relatively new entities that in some published literature have replaced the term BAC. The National Comprehensive Cancer Network recognizes these terms. AIS is defined as a localized adenocarcinoma of < 3 cm that exhibits a lepidic growth pattern, with neoplastic cells along the alveolar structures but without stromal, vascular, or pleural invasion. MIA refers to small, solitary adenocarcinomas < 3 cm with either pure lepidic growth or predominant lepidic growth with ≤ 5 mm of stromal invasion. BAC has unique epidemiologic, pathologic, and clinical features compared with other NSCLC subtypes. For example, although it is smoking-related, the relationship of BAC to smoking is less strong than with other types of NSCLC. About a third of patients with BAC are never-smokers.
There are also some unique radiographic features — its presentation may be confused with pneumonia or other inflammatory conditions in the lung, and only after a patient does not improve after a course of antibiotics should a diagnosis of BAC be considered. Unlike other types of lung cancer where chemotherapy may be the first plan of attack, surgery is often the first choice for treating BAC, particularly when there is no mediastinal node involvement (10%-25% of cases) or distal metastases (5% of cases). BAC usually harbors EGFR mutation. It is responsive to new targeted therapies for lung cancer, particularly osimertinib, afatinib, erlotinib, and gefitinib. Thus, people with BAC are good candidates for genetic testing.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Bronchioalveolar cell carcinoma (BAC) is a variant of non–small cell lung cancer (NSCLC) that, in recent years, has received a new identity in some of the literature. Adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) are relatively new entities that in some published literature have replaced the term BAC. The National Comprehensive Cancer Network recognizes these terms. AIS is defined as a localized adenocarcinoma of < 3 cm that exhibits a lepidic growth pattern, with neoplastic cells along the alveolar structures but without stromal, vascular, or pleural invasion. MIA refers to small, solitary adenocarcinomas < 3 cm with either pure lepidic growth or predominant lepidic growth with ≤ 5 mm of stromal invasion. BAC has unique epidemiologic, pathologic, and clinical features compared with other NSCLC subtypes. For example, although it is smoking-related, the relationship of BAC to smoking is less strong than with other types of NSCLC. About a third of patients with BAC are never-smokers.
There are also some unique radiographic features — its presentation may be confused with pneumonia or other inflammatory conditions in the lung, and only after a patient does not improve after a course of antibiotics should a diagnosis of BAC be considered. Unlike other types of lung cancer where chemotherapy may be the first plan of attack, surgery is often the first choice for treating BAC, particularly when there is no mediastinal node involvement (10%-25% of cases) or distal metastases (5% of cases). BAC usually harbors EGFR mutation. It is responsive to new targeted therapies for lung cancer, particularly osimertinib, afatinib, erlotinib, and gefitinib. Thus, people with BAC are good candidates for genetic testing.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Bronchioalveolar cell carcinoma (BAC) is a variant of non–small cell lung cancer (NSCLC) that, in recent years, has received a new identity in some of the literature. Adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) are relatively new entities that in some published literature have replaced the term BAC. The National Comprehensive Cancer Network recognizes these terms. AIS is defined as a localized adenocarcinoma of < 3 cm that exhibits a lepidic growth pattern, with neoplastic cells along the alveolar structures but without stromal, vascular, or pleural invasion. MIA refers to small, solitary adenocarcinomas < 3 cm with either pure lepidic growth or predominant lepidic growth with ≤ 5 mm of stromal invasion. BAC has unique epidemiologic, pathologic, and clinical features compared with other NSCLC subtypes. For example, although it is smoking-related, the relationship of BAC to smoking is less strong than with other types of NSCLC. About a third of patients with BAC are never-smokers.
There are also some unique radiographic features — its presentation may be confused with pneumonia or other inflammatory conditions in the lung, and only after a patient does not improve after a course of antibiotics should a diagnosis of BAC be considered. Unlike other types of lung cancer where chemotherapy may be the first plan of attack, surgery is often the first choice for treating BAC, particularly when there is no mediastinal node involvement (10%-25% of cases) or distal metastases (5% of cases). BAC usually harbors EGFR mutation. It is responsive to new targeted therapies for lung cancer, particularly osimertinib, afatinib, erlotinib, and gefitinib. Thus, people with BAC are good candidates for genetic testing.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
A 50-year-old woman, a never-smoker, presented with complaints of intermittent cough and shortness of breath for 3 months, associated with bronchorrhea (copious watery sputum production). She had lost 15 pounds in the past 2 months and had dyspnea on exertion for 1 month. Her pulse rate was 88/min, respiratory rate 18/min, and oxygen saturation 96% on room air. A chest x-ray (posteroanterior view) showed dense opacity in the right lower zone. Contrast-enhanced CT of the thorax showed diffuse ground-glass opacities around nodules and consolidation involving the apical and basal segments of the right lower lobe. Despite several courses of antimicrobials, bronchodilators, and IV corticosteroid therapy, the patient's condition worsened.
Question 2
Q2. Correct answer: A - No monitoring of PPI side effects.
Rationale
There are several putative risks associated with long-term PPI use: chronic kidney disease, dementia, vitamin and mineral deficiencies, and others. However, the overall quality of evidence to support these conclusions is low or very low, and the majority of the findings have low effect sizes that may be attributed to confounding. An American Gastroenterological Association clinical practice update recommended against routine monitoring for patients receiving long-term PPI treatment. However, data show that more than one-third of gastroenterologists still check for PPI side effects at least annually in their patients.
References
Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-Term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-15. doi: 10.1053/j.gastro.2017.01.031.
Leiman DA, Ravi K, Freedberg DE, Gyawali CP. Proton Pump Inhibitor Prescribing and Monitoring Patterns Among Gastroenterology Practitioners (published online ahead of print, 2021 Oct 4). J Clin Gastroenterol. 2021;10.1097/MCG.0000000000001623. doi: 10.1097/MCG.0000000000001623.
Q2. Correct answer: A - No monitoring of PPI side effects.
Rationale
There are several putative risks associated with long-term PPI use: chronic kidney disease, dementia, vitamin and mineral deficiencies, and others. However, the overall quality of evidence to support these conclusions is low or very low, and the majority of the findings have low effect sizes that may be attributed to confounding. An American Gastroenterological Association clinical practice update recommended against routine monitoring for patients receiving long-term PPI treatment. However, data show that more than one-third of gastroenterologists still check for PPI side effects at least annually in their patients.
References
Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-Term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-15. doi: 10.1053/j.gastro.2017.01.031.
Leiman DA, Ravi K, Freedberg DE, Gyawali CP. Proton Pump Inhibitor Prescribing and Monitoring Patterns Among Gastroenterology Practitioners (published online ahead of print, 2021 Oct 4). J Clin Gastroenterol. 2021;10.1097/MCG.0000000000001623. doi: 10.1097/MCG.0000000000001623.
Q2. Correct answer: A - No monitoring of PPI side effects.
Rationale
There are several putative risks associated with long-term PPI use: chronic kidney disease, dementia, vitamin and mineral deficiencies, and others. However, the overall quality of evidence to support these conclusions is low or very low, and the majority of the findings have low effect sizes that may be attributed to confounding. An American Gastroenterological Association clinical practice update recommended against routine monitoring for patients receiving long-term PPI treatment. However, data show that more than one-third of gastroenterologists still check for PPI side effects at least annually in their patients.
References
Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-Term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-15. doi: 10.1053/j.gastro.2017.01.031.
Leiman DA, Ravi K, Freedberg DE, Gyawali CP. Proton Pump Inhibitor Prescribing and Monitoring Patterns Among Gastroenterology Practitioners (published online ahead of print, 2021 Oct 4). J Clin Gastroenterol. 2021;10.1097/MCG.0000000000001623. doi: 10.1097/MCG.0000000000001623.
Q2. A 76-year-old man with atrial fibrillation treated with long-term anticoagulation with warfarin and coronary artery disease treated with aspirin was recently admitted with melena. Upper endoscopy revealed a duodenal ulcer with visible vessel. Endoscopic therapy was performed, and he was started on twice-daily proton-pump inhibitors (PPIs).
Question 1
Q1. Correct answer: B - Adding calcium carbonate (antacid) to her current regimen
Rationale
Compared with proton pump inhibitors (PPIs), vonoprazan is a potassium-competitive acid blocker (PCAB), which inhibits acid secretion by competitively blocking availability of potassium to hydrogen-potassium ATPase. Vonoprazan is rapidly absorbed independent of eating and is not affected by CYP2C19 polymorphisms. Several studies have compared PPIs with vonoprazan. Although vonoprazan is highly effective for treating LA Grade A and B esophagitis, so is lansoprazole, and healing rates at 8 weeks are 100% versus 99.2%, respectively. In contrast, vonoprazan healing of LA Grade C and D esophagitis at 8 weeks is 98.7% compared with 87.5% for lansoprazole.
Sleeping on pillows is not a reliable way to reduce reflux, as patients often move during sleep and lose any benefit from being propped on them. Antacids would not provide superior acid inhibition, compared with vonoprazan, and avoiding spicy foods would not address the underlying permissive reflux barrier that exists (hiatal hernia).
Reference
Graham DY and Dore MP. Update on the Use of Vonoprazan: A Competitive Acid Blocker. Gastroenterology. 2018;154(3):462-6. doi: 10.1053/j.gastro.2018.01.018.
Q1. Correct answer: B - Adding calcium carbonate (antacid) to her current regimen
Rationale
Compared with proton pump inhibitors (PPIs), vonoprazan is a potassium-competitive acid blocker (PCAB), which inhibits acid secretion by competitively blocking availability of potassium to hydrogen-potassium ATPase. Vonoprazan is rapidly absorbed independent of eating and is not affected by CYP2C19 polymorphisms. Several studies have compared PPIs with vonoprazan. Although vonoprazan is highly effective for treating LA Grade A and B esophagitis, so is lansoprazole, and healing rates at 8 weeks are 100% versus 99.2%, respectively. In contrast, vonoprazan healing of LA Grade C and D esophagitis at 8 weeks is 98.7% compared with 87.5% for lansoprazole.
Sleeping on pillows is not a reliable way to reduce reflux, as patients often move during sleep and lose any benefit from being propped on them. Antacids would not provide superior acid inhibition, compared with vonoprazan, and avoiding spicy foods would not address the underlying permissive reflux barrier that exists (hiatal hernia).
Reference
Graham DY and Dore MP. Update on the Use of Vonoprazan: A Competitive Acid Blocker. Gastroenterology. 2018;154(3):462-6. doi: 10.1053/j.gastro.2018.01.018.
Q1. Correct answer: B - Adding calcium carbonate (antacid) to her current regimen
Rationale
Compared with proton pump inhibitors (PPIs), vonoprazan is a potassium-competitive acid blocker (PCAB), which inhibits acid secretion by competitively blocking availability of potassium to hydrogen-potassium ATPase. Vonoprazan is rapidly absorbed independent of eating and is not affected by CYP2C19 polymorphisms. Several studies have compared PPIs with vonoprazan. Although vonoprazan is highly effective for treating LA Grade A and B esophagitis, so is lansoprazole, and healing rates at 8 weeks are 100% versus 99.2%, respectively. In contrast, vonoprazan healing of LA Grade C and D esophagitis at 8 weeks is 98.7% compared with 87.5% for lansoprazole.
Sleeping on pillows is not a reliable way to reduce reflux, as patients often move during sleep and lose any benefit from being propped on them. Antacids would not provide superior acid inhibition, compared with vonoprazan, and avoiding spicy foods would not address the underlying permissive reflux barrier that exists (hiatal hernia).
Reference
Graham DY and Dore MP. Update on the Use of Vonoprazan: A Competitive Acid Blocker. Gastroenterology. 2018;154(3):462-6. doi: 10.1053/j.gastro.2018.01.018.
Q1. A 62-year-old woman with rheumatoid arthritis reports regurgitation, heartburn, and dysphagia. She undergoes upper endoscopy, which reveals a 3-cm hiatal hernia and Los Angeles (LA) Grade D esophagitis. Previously performed esophageal function tests revealed absent contractility and a total acid exposure time of 8.2%. Her thoracic surgeon is concerned about the postoperative risks of dysphagia with hernia repair; therefore, surgery is deferred. Although improved, she continues to have symptoms of heartburn with daily lansoprazole.
Quick Quiz Question 2
Q2. Correct answer: A. Enteric infection
Rationale
Despite the numerous side effects associated with long-term PPI use, the quality of evidence and risk of confounding from these studies limits the ability to ascribe sufficient cause and effect between PPI use and these outcomes. However, a recent large randomized controlled trial that evaluated the use of pantoprazole versus placebo demonstrated a statistically significant difference between the pantoprazole and placebo groups only in enteric infections (1.4% vs 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Despite a nearly double increased risk of Clostridioides difficile infection in the PPI group, compared with the placebo group, the number of events was low, and the difference did not reach statistical significance. In the context of these data, and more recent studies suggesting an increased risk of COVID-19 in patients who take PPIs, compared with those who do not, the risk of enteric infections is likely small but significantly increased among long-term PPI users.
References
- Freedberg DE et al. Gastroenterology. 2017;152(4):706-15. doi: 10.1053/j.gastro.2017.01.031.
- Moayyedi P et al. Gastroenterology. 2019;157(3):682-91.e2. doi: 10.1053/j.gastro.2019.05.056.
Q2. Correct answer: A. Enteric infection
Rationale
Despite the numerous side effects associated with long-term PPI use, the quality of evidence and risk of confounding from these studies limits the ability to ascribe sufficient cause and effect between PPI use and these outcomes. However, a recent large randomized controlled trial that evaluated the use of pantoprazole versus placebo demonstrated a statistically significant difference between the pantoprazole and placebo groups only in enteric infections (1.4% vs 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Despite a nearly double increased risk of Clostridioides difficile infection in the PPI group, compared with the placebo group, the number of events was low, and the difference did not reach statistical significance. In the context of these data, and more recent studies suggesting an increased risk of COVID-19 in patients who take PPIs, compared with those who do not, the risk of enteric infections is likely small but significantly increased among long-term PPI users.
References
- Freedberg DE et al. Gastroenterology. 2017;152(4):706-15. doi: 10.1053/j.gastro.2017.01.031.
- Moayyedi P et al. Gastroenterology. 2019;157(3):682-91.e2. doi: 10.1053/j.gastro.2019.05.056.
Q2. Correct answer: A. Enteric infection
Rationale
Despite the numerous side effects associated with long-term PPI use, the quality of evidence and risk of confounding from these studies limits the ability to ascribe sufficient cause and effect between PPI use and these outcomes. However, a recent large randomized controlled trial that evaluated the use of pantoprazole versus placebo demonstrated a statistically significant difference between the pantoprazole and placebo groups only in enteric infections (1.4% vs 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Despite a nearly double increased risk of Clostridioides difficile infection in the PPI group, compared with the placebo group, the number of events was low, and the difference did not reach statistical significance. In the context of these data, and more recent studies suggesting an increased risk of COVID-19 in patients who take PPIs, compared with those who do not, the risk of enteric infections is likely small but significantly increased among long-term PPI users.
References
- Freedberg DE et al. Gastroenterology. 2017;152(4):706-15. doi: 10.1053/j.gastro.2017.01.031.
- Moayyedi P et al. Gastroenterology. 2019;157(3):682-91.e2. doi: 10.1053/j.gastro.2019.05.056.
.