User login
Minilaparoscopy is a relevant surgical technique
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
Full disclosure
I have nothing to disclose.
That is the first line on my second slide in just about every talk I give. I have no financial conflicts of interest. I no longer accept meals from pharmaceutical companies, I no longer conduct pharmaceutical company sponsored research, and I no longer give talks that include honoraria from pharmaceutical companies. I turn down payments from pharmaceutical companies when I participate in drug-monitoring safety boards and advisory committees. I do not have a financial conflict of interest.
Or do I?
In preparing to write this essay, I searched the Open Payments website (www.cms.gov/OpenPayments/index.html) for my name. Open Payments is the product of the Physician Payments Sunshine Act passed in 2010 as part of the Affordable Care Act. The website went live in September 2014 with the intention of making public all payments made to physicians from device and drug makers. I was happy to confirm that I have received no “General Payments,” which are payments for meals, travel, honoraria, consulting, and the like. However, I was surprised to learn that I did receive “Associated Research” payments. According to the website, an Associated Research payment is “funding for a research project or study where the physician is named as a principal investigator.”
I still have a few trials open under my name, but none have accrued a patient in more than 7 years. Nonetheless, I am on record, and publicly so, for accepting an Associated payment for research to the tune of $1,308,360.06.
Upon learning this, my thoughts turned to the New York Times. The Times recently published an expose in cooperation with ProPublica. In it, a prominent cancer researcher at Memorial Sloan Kettering was accused of repeatedly failing to disclose his substantial financial conflicts of interest. The payments creating the conflict were listed on the Open Payments website. Since financial disclosure is almost always required for a manuscript listed in PubMed, a simple comparison of two public websites provided the journalists with nearly all the information they needed to conclude malfeasance in disclosure.
In response, the accused admitted the failure to disclose, but attributed it to an unintentional error. In the frenzy that followed, a man of towering stature, a paragon of cancer research, submitted his resignation. The sequence of events was tragic. Had the payments been for research instead of services rendered would the consequences have been the same?
Most of us believe corporate payments for research are less likely to influence our prescribing and consulting habits than are general payments for entertainment and speaking engagements. I remember receiving my first research grant from the now defunct pharma company Immunex. It was for $10,000 – a paltry sum – but enough for me to set up a clinical trial using Immunex’s drugs. I was flattered, indebted, and conflicted from that point forward. Funded research propels our careers forward. Thinking research payments bias our decision making less than direct payments is naive. Money corrupts, and that is why research dollars need to be disclosed whenever we discuss research at the podium or in print.
With appropriate indignation, I explored the Open Payments website to learn more of my hitherto unknown payment. It was attached to a multicenter, randomized clinical trial for which I served as local principal investigator. The payment was made in January 2017 and our research team cannot verify such a payment was ever received. According to the website, the payment was not disputed. I sought to dispute it.
Our friends at the Centers for Medicare and Medicaid Services do not make filing a dispute easy. I first had to register with my home address and create a new password that, of course, needs to be changed every 60 days. I duly registered and logged into the website as instructed. I followed instructions and filled in data fields for about an additional 10 pages before being informed that I needed to logout, then log back in, to access the Open Payments application. When I did that, I was greeted with instructions to register in the Open Payments system. I then realized that all I had done to that point was register with the CMS.gov portal, not Open Payments. In for a dime, in for a dollar, I registered with Open Payments.
I almost gave up when they asked me to provide a Physician Taxonomy Code. It took me a long time to find it. For those interested, the code for Hematology is 207RH0000X. With that code entered in the right box, I was only two pages away from being registered and ready to open the dispute. Failure hit me like a lake effect snow storm. Despite my diligence, I was not “vetted” and could not file a dispute. I must have done something wrong and cannot seem to investigate the payment further, but I’m sure the New York Times could.
Now, I don’t know if I have anything to disclose or not. I do know that I have to investigate my payment the best I can, that I have to disclose it if it is real, and that I have to check Open Payments every so often to make sure I am not surprised by an investigative journalist’s report in the future. Add these to the pantheon of onerous requirements for a successful academic career.
Many wear their entanglements as a badge of honor on slides highlighting a long list of conflicts. One speaker joked that she had so many conflicts that she had no conflicts. Clearly, much like alarm fatigue, the constant display of financial conflict of interest disclosures rarely raises red flags in an audience of peers. To an audience of interested lay persons, though, those conflicts may be very important and relevant.
It is our duty to accurately account for and report them no matter the difficulty in doing so. Failure to do so can carry tragic consequences.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
I have nothing to disclose.
That is the first line on my second slide in just about every talk I give. I have no financial conflicts of interest. I no longer accept meals from pharmaceutical companies, I no longer conduct pharmaceutical company sponsored research, and I no longer give talks that include honoraria from pharmaceutical companies. I turn down payments from pharmaceutical companies when I participate in drug-monitoring safety boards and advisory committees. I do not have a financial conflict of interest.
Or do I?
In preparing to write this essay, I searched the Open Payments website (www.cms.gov/OpenPayments/index.html) for my name. Open Payments is the product of the Physician Payments Sunshine Act passed in 2010 as part of the Affordable Care Act. The website went live in September 2014 with the intention of making public all payments made to physicians from device and drug makers. I was happy to confirm that I have received no “General Payments,” which are payments for meals, travel, honoraria, consulting, and the like. However, I was surprised to learn that I did receive “Associated Research” payments. According to the website, an Associated Research payment is “funding for a research project or study where the physician is named as a principal investigator.”
I still have a few trials open under my name, but none have accrued a patient in more than 7 years. Nonetheless, I am on record, and publicly so, for accepting an Associated payment for research to the tune of $1,308,360.06.
Upon learning this, my thoughts turned to the New York Times. The Times recently published an expose in cooperation with ProPublica. In it, a prominent cancer researcher at Memorial Sloan Kettering was accused of repeatedly failing to disclose his substantial financial conflicts of interest. The payments creating the conflict were listed on the Open Payments website. Since financial disclosure is almost always required for a manuscript listed in PubMed, a simple comparison of two public websites provided the journalists with nearly all the information they needed to conclude malfeasance in disclosure.
In response, the accused admitted the failure to disclose, but attributed it to an unintentional error. In the frenzy that followed, a man of towering stature, a paragon of cancer research, submitted his resignation. The sequence of events was tragic. Had the payments been for research instead of services rendered would the consequences have been the same?
Most of us believe corporate payments for research are less likely to influence our prescribing and consulting habits than are general payments for entertainment and speaking engagements. I remember receiving my first research grant from the now defunct pharma company Immunex. It was for $10,000 – a paltry sum – but enough for me to set up a clinical trial using Immunex’s drugs. I was flattered, indebted, and conflicted from that point forward. Funded research propels our careers forward. Thinking research payments bias our decision making less than direct payments is naive. Money corrupts, and that is why research dollars need to be disclosed whenever we discuss research at the podium or in print.
With appropriate indignation, I explored the Open Payments website to learn more of my hitherto unknown payment. It was attached to a multicenter, randomized clinical trial for which I served as local principal investigator. The payment was made in January 2017 and our research team cannot verify such a payment was ever received. According to the website, the payment was not disputed. I sought to dispute it.
Our friends at the Centers for Medicare and Medicaid Services do not make filing a dispute easy. I first had to register with my home address and create a new password that, of course, needs to be changed every 60 days. I duly registered and logged into the website as instructed. I followed instructions and filled in data fields for about an additional 10 pages before being informed that I needed to logout, then log back in, to access the Open Payments application. When I did that, I was greeted with instructions to register in the Open Payments system. I then realized that all I had done to that point was register with the CMS.gov portal, not Open Payments. In for a dime, in for a dollar, I registered with Open Payments.
I almost gave up when they asked me to provide a Physician Taxonomy Code. It took me a long time to find it. For those interested, the code for Hematology is 207RH0000X. With that code entered in the right box, I was only two pages away from being registered and ready to open the dispute. Failure hit me like a lake effect snow storm. Despite my diligence, I was not “vetted” and could not file a dispute. I must have done something wrong and cannot seem to investigate the payment further, but I’m sure the New York Times could.
Now, I don’t know if I have anything to disclose or not. I do know that I have to investigate my payment the best I can, that I have to disclose it if it is real, and that I have to check Open Payments every so often to make sure I am not surprised by an investigative journalist’s report in the future. Add these to the pantheon of onerous requirements for a successful academic career.
Many wear their entanglements as a badge of honor on slides highlighting a long list of conflicts. One speaker joked that she had so many conflicts that she had no conflicts. Clearly, much like alarm fatigue, the constant display of financial conflict of interest disclosures rarely raises red flags in an audience of peers. To an audience of interested lay persons, though, those conflicts may be very important and relevant.
It is our duty to accurately account for and report them no matter the difficulty in doing so. Failure to do so can carry tragic consequences.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
I have nothing to disclose.
That is the first line on my second slide in just about every talk I give. I have no financial conflicts of interest. I no longer accept meals from pharmaceutical companies, I no longer conduct pharmaceutical company sponsored research, and I no longer give talks that include honoraria from pharmaceutical companies. I turn down payments from pharmaceutical companies when I participate in drug-monitoring safety boards and advisory committees. I do not have a financial conflict of interest.
Or do I?
In preparing to write this essay, I searched the Open Payments website (www.cms.gov/OpenPayments/index.html) for my name. Open Payments is the product of the Physician Payments Sunshine Act passed in 2010 as part of the Affordable Care Act. The website went live in September 2014 with the intention of making public all payments made to physicians from device and drug makers. I was happy to confirm that I have received no “General Payments,” which are payments for meals, travel, honoraria, consulting, and the like. However, I was surprised to learn that I did receive “Associated Research” payments. According to the website, an Associated Research payment is “funding for a research project or study where the physician is named as a principal investigator.”
I still have a few trials open under my name, but none have accrued a patient in more than 7 years. Nonetheless, I am on record, and publicly so, for accepting an Associated payment for research to the tune of $1,308,360.06.
Upon learning this, my thoughts turned to the New York Times. The Times recently published an expose in cooperation with ProPublica. In it, a prominent cancer researcher at Memorial Sloan Kettering was accused of repeatedly failing to disclose his substantial financial conflicts of interest. The payments creating the conflict were listed on the Open Payments website. Since financial disclosure is almost always required for a manuscript listed in PubMed, a simple comparison of two public websites provided the journalists with nearly all the information they needed to conclude malfeasance in disclosure.
In response, the accused admitted the failure to disclose, but attributed it to an unintentional error. In the frenzy that followed, a man of towering stature, a paragon of cancer research, submitted his resignation. The sequence of events was tragic. Had the payments been for research instead of services rendered would the consequences have been the same?
Most of us believe corporate payments for research are less likely to influence our prescribing and consulting habits than are general payments for entertainment and speaking engagements. I remember receiving my first research grant from the now defunct pharma company Immunex. It was for $10,000 – a paltry sum – but enough for me to set up a clinical trial using Immunex’s drugs. I was flattered, indebted, and conflicted from that point forward. Funded research propels our careers forward. Thinking research payments bias our decision making less than direct payments is naive. Money corrupts, and that is why research dollars need to be disclosed whenever we discuss research at the podium or in print.
With appropriate indignation, I explored the Open Payments website to learn more of my hitherto unknown payment. It was attached to a multicenter, randomized clinical trial for which I served as local principal investigator. The payment was made in January 2017 and our research team cannot verify such a payment was ever received. According to the website, the payment was not disputed. I sought to dispute it.
Our friends at the Centers for Medicare and Medicaid Services do not make filing a dispute easy. I first had to register with my home address and create a new password that, of course, needs to be changed every 60 days. I duly registered and logged into the website as instructed. I followed instructions and filled in data fields for about an additional 10 pages before being informed that I needed to logout, then log back in, to access the Open Payments application. When I did that, I was greeted with instructions to register in the Open Payments system. I then realized that all I had done to that point was register with the CMS.gov portal, not Open Payments. In for a dime, in for a dollar, I registered with Open Payments.
I almost gave up when they asked me to provide a Physician Taxonomy Code. It took me a long time to find it. For those interested, the code for Hematology is 207RH0000X. With that code entered in the right box, I was only two pages away from being registered and ready to open the dispute. Failure hit me like a lake effect snow storm. Despite my diligence, I was not “vetted” and could not file a dispute. I must have done something wrong and cannot seem to investigate the payment further, but I’m sure the New York Times could.
Now, I don’t know if I have anything to disclose or not. I do know that I have to investigate my payment the best I can, that I have to disclose it if it is real, and that I have to check Open Payments every so often to make sure I am not surprised by an investigative journalist’s report in the future. Add these to the pantheon of onerous requirements for a successful academic career.
Many wear their entanglements as a badge of honor on slides highlighting a long list of conflicts. One speaker joked that she had so many conflicts that she had no conflicts. Clearly, much like alarm fatigue, the constant display of financial conflict of interest disclosures rarely raises red flags in an audience of peers. To an audience of interested lay persons, though, those conflicts may be very important and relevant.
It is our duty to accurately account for and report them no matter the difficulty in doing so. Failure to do so can carry tragic consequences.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
White coats and provider attire: Does it matter to patients?
What is appropriate “ward garb”?
The question of appropriate ward garb is a problem for the ages. Compared with photo stills and films from the 1960s, the doctors of today appear like vagabonds. No ties, no lab coats, and scrub tops have become the norm for a number (a majority?) of hospital-based docs – and even more so on the surgical wards and in the ER.
Past studies have addressed patient preferences for provider dress, but none like the results of a recent survey.
From the University of Michigan, Ann Arbor, comes a physician attire survey of a convenience sample of 4,000 patients at 10 U.S. academic medical centers. It included both inpatients and outpatients, and used the design of many previous studies, showing patients the same doctor dressed seven different ways. After viewing the photographs, the patients received surveys as to their preference of physician based on attire, as well as being asked to rate the physician in the areas of knowledge, trust, care, approachability, and comfort.
You can see the domains: casual, scrubs, and formal, each with and without a lab coat. The seventh category is business attire (future C-suite wannabes – you know who you are).
Over half of the participants indicated that how a physician dresses was important to them, with more than one in three stating that this influenced how happy they were with care received. Overall, respondents indicated that formal attire with white coats was the most preferred form of physician dress.
I found the discussion in the study worthwhile, along with the strengths and weaknesses of the author’s outline. They went to great lengths to design a nonbiased questionnaire and used a consistent approach to shooting their photos. They also discussed lab coats, long sleeves, and hygiene.
But what to draw from the findings? Does patient satisfaction matter or just clinical outcomes? Is patient happiness a means to an end or an end unto itself? Can I even get you exercised about a score of 6 versus 8 (a 25% difference)? For instance, imagine the worst-dressed doc – say shorts and flip-flops. Is that a 5.8 or a 2.3? The anchor matters, and it helps to put the ratings in context.
Read the full post at hospitalleader.org.
Dr. Flansbaum works for Geisinger Health System in Danville, Pa., in both the divisions of hospital medicine and population health. He is a founding member of the Society of Hospital Medicine and served as a board member and officer.
Also in The Hospital Leader
•Hospitalists Can Improve Patient Trust…in Their Colleagues by Chris Moriates, MD, SFHM
•Treatment of Type II MIs by Brad Flansbaum, MD, MPH, MHM
•The $64,000 Question: How Can Hospitalists Improve Their HCAHPS Scores? by Leslie Flores, MHA, SFHM
What is appropriate “ward garb”?
What is appropriate “ward garb”?
The question of appropriate ward garb is a problem for the ages. Compared with photo stills and films from the 1960s, the doctors of today appear like vagabonds. No ties, no lab coats, and scrub tops have become the norm for a number (a majority?) of hospital-based docs – and even more so on the surgical wards and in the ER.
Past studies have addressed patient preferences for provider dress, but none like the results of a recent survey.
From the University of Michigan, Ann Arbor, comes a physician attire survey of a convenience sample of 4,000 patients at 10 U.S. academic medical centers. It included both inpatients and outpatients, and used the design of many previous studies, showing patients the same doctor dressed seven different ways. After viewing the photographs, the patients received surveys as to their preference of physician based on attire, as well as being asked to rate the physician in the areas of knowledge, trust, care, approachability, and comfort.
You can see the domains: casual, scrubs, and formal, each with and without a lab coat. The seventh category is business attire (future C-suite wannabes – you know who you are).
Over half of the participants indicated that how a physician dresses was important to them, with more than one in three stating that this influenced how happy they were with care received. Overall, respondents indicated that formal attire with white coats was the most preferred form of physician dress.
I found the discussion in the study worthwhile, along with the strengths and weaknesses of the author’s outline. They went to great lengths to design a nonbiased questionnaire and used a consistent approach to shooting their photos. They also discussed lab coats, long sleeves, and hygiene.
But what to draw from the findings? Does patient satisfaction matter or just clinical outcomes? Is patient happiness a means to an end or an end unto itself? Can I even get you exercised about a score of 6 versus 8 (a 25% difference)? For instance, imagine the worst-dressed doc – say shorts and flip-flops. Is that a 5.8 or a 2.3? The anchor matters, and it helps to put the ratings in context.
Read the full post at hospitalleader.org.
Dr. Flansbaum works for Geisinger Health System in Danville, Pa., in both the divisions of hospital medicine and population health. He is a founding member of the Society of Hospital Medicine and served as a board member and officer.
Also in The Hospital Leader
•Hospitalists Can Improve Patient Trust…in Their Colleagues by Chris Moriates, MD, SFHM
•Treatment of Type II MIs by Brad Flansbaum, MD, MPH, MHM
•The $64,000 Question: How Can Hospitalists Improve Their HCAHPS Scores? by Leslie Flores, MHA, SFHM
The question of appropriate ward garb is a problem for the ages. Compared with photo stills and films from the 1960s, the doctors of today appear like vagabonds. No ties, no lab coats, and scrub tops have become the norm for a number (a majority?) of hospital-based docs – and even more so on the surgical wards and in the ER.
Past studies have addressed patient preferences for provider dress, but none like the results of a recent survey.
From the University of Michigan, Ann Arbor, comes a physician attire survey of a convenience sample of 4,000 patients at 10 U.S. academic medical centers. It included both inpatients and outpatients, and used the design of many previous studies, showing patients the same doctor dressed seven different ways. After viewing the photographs, the patients received surveys as to their preference of physician based on attire, as well as being asked to rate the physician in the areas of knowledge, trust, care, approachability, and comfort.
You can see the domains: casual, scrubs, and formal, each with and without a lab coat. The seventh category is business attire (future C-suite wannabes – you know who you are).
Over half of the participants indicated that how a physician dresses was important to them, with more than one in three stating that this influenced how happy they were with care received. Overall, respondents indicated that formal attire with white coats was the most preferred form of physician dress.
I found the discussion in the study worthwhile, along with the strengths and weaknesses of the author’s outline. They went to great lengths to design a nonbiased questionnaire and used a consistent approach to shooting their photos. They also discussed lab coats, long sleeves, and hygiene.
But what to draw from the findings? Does patient satisfaction matter or just clinical outcomes? Is patient happiness a means to an end or an end unto itself? Can I even get you exercised about a score of 6 versus 8 (a 25% difference)? For instance, imagine the worst-dressed doc – say shorts and flip-flops. Is that a 5.8 or a 2.3? The anchor matters, and it helps to put the ratings in context.
Read the full post at hospitalleader.org.
Dr. Flansbaum works for Geisinger Health System in Danville, Pa., in both the divisions of hospital medicine and population health. He is a founding member of the Society of Hospital Medicine and served as a board member and officer.
Also in The Hospital Leader
•Hospitalists Can Improve Patient Trust…in Their Colleagues by Chris Moriates, MD, SFHM
•Treatment of Type II MIs by Brad Flansbaum, MD, MPH, MHM
•The $64,000 Question: How Can Hospitalists Improve Their HCAHPS Scores? by Leslie Flores, MHA, SFHM
Inpatient vs. outpatient addiction treatment: Which is best?
In the course of my general psychiatry practice, there are times when I am unable to manage a patient’s substance abuse issues, and I have referred patients to a higher level of care – often to an intensive outpatient program (IOP) that meets for 3 hours a day, or to an inpatient rehabilitation, usually for 28 days. I’m not always sure who can be managed in which setting, and I usually honor the patient’s wishes. If the patient is motivated, has a support system in place, and is concerned that his job will be in jeopardy if he takes time off work, then I refer to Kolmac Outpatient Recovery Centers, a local outpatient treatment center that gives patients the option of attending in the mornings or evenings and allows most people to continue working. If I think I may have only a single shot at getting a patient engaged in care, and the patient is willing to go to an inpatient setting, I refer to a residential treatment facility. It has occurred to me that this is not a very scientific way of making a treatment decision.
George Kolodner, MD, is the chief clinical officer of Kolmac. He has been a member of the American Society of Addiction Medicine’s (ASAM) treatment criteria committee. When I spoke with Dr. Kolodner, he noted: “Discussions between third-party payers and treatment programs about what is the appropriate level of care for a particular individual have been adversarial. ASAM has spent many years developing the ASAM Criteria, a document that attempts to mediate these disagreements by developing objective criteria for where people ought to be treated. Because it is so comprehensive and the variables are so many, it can be difficult to use. A computerized version, called ‘Continuum,’ has been developed to make the criteria more user-friendly.”
“My 45-year experience,” Dr. Kolodner continued, “is that detoxification and rehabilitation can usually be done successfully on an outpatient basis if an appropriate facility is available and the patient has both a supportive living environment and can get to the treatment. Hospitalization and residential rehabilitation is an essential level of care when those conditions do not exist or when outpatient treatment proves to be insufficient.”
One problem with comparing the success of IOPs to inpatient programs is that these settings differ widely in which services they offer to patients.
“There’s no standardization,” Dr. Kolodner said. “The services may be watered down, they may not have a medical staff or a psychiatrist, and people get sucked into inappropriate treatments. When it comes to both IOPs and inpatient facilities, there is no uniformity, and right now it’s caveat emptor.”
Marc Fishman, MD, is medical director of Maryland Treatment Centers/Mountain Manor Treatment Center, a coeditor of the ASAM Criteria, and, with Dr. Kolodner, a member of ASAM’s treatment criteria committee. He, too, talked about the absence of standardization across treatment settings.
“Bed-based and non–bed-based care exist in many flavors and subflavors. You have to remember,” Dr. Fishman said, “this is a marathon, not a sprint, and one of the most important goals of bed-based care is that it serves as a stepping stone for outpatient treatment.”
Dr. Fishman talked about a list of criteria he uses to decide whether someone can be treated as an outpatient. “First, someone has to be able to access outpatient treatment; it may not be available. Can they get back and forth? How chaotic are their lives? Is there support at home, or is it a toxic environment in which others are using? Are they likely to keep using and drop out? What is the patient’s level of motivation? If a person is very ambivalent, you may need a high-intensity motivational milieu. Are their psychiatric symptoms severe enough to require 24-hour monitoring and supervision? Most detoxification we can do on an outpatient basis, but some complex multisubstance withdrawal may need more monitoring.
“Also, we have an increasing armamentarium of medications to promote abstinence, and sometimes it makes sense to start them in higher-level treatment settings; for example long-acting injectable naltrexone (Vivitrol) needs a 10-day postdetox opioid-free washout before it can be started.”
Dr. Fishman was careful to note that imminent danger is usually not a reason to use an inpatient rehab setting. “When you’re talking about safety issues, then people usually need a hospital. Most rehabs are not equipped to deal with dangerous patients.”
In choosing from the different treatment options, the first question should be to ask which forms of treatment are available with high-quality care. Can the patient access an outpatient center, will he be able to get to treatment, and will he be able to remain sober between visits? Will he be offered a full range of treatment options in that setting? Can substance withdrawal be managed safely? If the patient fails at outpatient care, will he be willing to consider inpatient treatment as a next step? What is the risk associated with relapse in a setting that allows for access to substances between sessions? Is the patient someone who is at high risk for a fatal overdose, or at high risk for endangering others, for example, someone who has been revived from overdoses or has driven while inebriated? Would this patient benefit from more intensive psychotherapeutic care? And the question that always haunts me: If there is a bad outcome, will I regret that I did not recommend more?”
Often, I’m left with the idea that it would be nice if we were all given crystal balls at the end of training. In hindsight, if a patient does well, the treatment that was offered was enough, and perhaps even too much in terms of cost. If a patient does not do well, we may be left to ask if he would have been better off if we had recommended a higher level of care, assuming that care could be financed and accessed, and that the patient complied with the treatment recommendations.
Both experts agree that treatment is often effective, and the news here is good. But treatment only works if a patient actually follows through on it, so the best treatment is often the one the patient is willing to accept.
Dr. Miller is the coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016).
In the course of my general psychiatry practice, there are times when I am unable to manage a patient’s substance abuse issues, and I have referred patients to a higher level of care – often to an intensive outpatient program (IOP) that meets for 3 hours a day, or to an inpatient rehabilitation, usually for 28 days. I’m not always sure who can be managed in which setting, and I usually honor the patient’s wishes. If the patient is motivated, has a support system in place, and is concerned that his job will be in jeopardy if he takes time off work, then I refer to Kolmac Outpatient Recovery Centers, a local outpatient treatment center that gives patients the option of attending in the mornings or evenings and allows most people to continue working. If I think I may have only a single shot at getting a patient engaged in care, and the patient is willing to go to an inpatient setting, I refer to a residential treatment facility. It has occurred to me that this is not a very scientific way of making a treatment decision.
George Kolodner, MD, is the chief clinical officer of Kolmac. He has been a member of the American Society of Addiction Medicine’s (ASAM) treatment criteria committee. When I spoke with Dr. Kolodner, he noted: “Discussions between third-party payers and treatment programs about what is the appropriate level of care for a particular individual have been adversarial. ASAM has spent many years developing the ASAM Criteria, a document that attempts to mediate these disagreements by developing objective criteria for where people ought to be treated. Because it is so comprehensive and the variables are so many, it can be difficult to use. A computerized version, called ‘Continuum,’ has been developed to make the criteria more user-friendly.”
“My 45-year experience,” Dr. Kolodner continued, “is that detoxification and rehabilitation can usually be done successfully on an outpatient basis if an appropriate facility is available and the patient has both a supportive living environment and can get to the treatment. Hospitalization and residential rehabilitation is an essential level of care when those conditions do not exist or when outpatient treatment proves to be insufficient.”
One problem with comparing the success of IOPs to inpatient programs is that these settings differ widely in which services they offer to patients.
“There’s no standardization,” Dr. Kolodner said. “The services may be watered down, they may not have a medical staff or a psychiatrist, and people get sucked into inappropriate treatments. When it comes to both IOPs and inpatient facilities, there is no uniformity, and right now it’s caveat emptor.”
Marc Fishman, MD, is medical director of Maryland Treatment Centers/Mountain Manor Treatment Center, a coeditor of the ASAM Criteria, and, with Dr. Kolodner, a member of ASAM’s treatment criteria committee. He, too, talked about the absence of standardization across treatment settings.
“Bed-based and non–bed-based care exist in many flavors and subflavors. You have to remember,” Dr. Fishman said, “this is a marathon, not a sprint, and one of the most important goals of bed-based care is that it serves as a stepping stone for outpatient treatment.”
Dr. Fishman talked about a list of criteria he uses to decide whether someone can be treated as an outpatient. “First, someone has to be able to access outpatient treatment; it may not be available. Can they get back and forth? How chaotic are their lives? Is there support at home, or is it a toxic environment in which others are using? Are they likely to keep using and drop out? What is the patient’s level of motivation? If a person is very ambivalent, you may need a high-intensity motivational milieu. Are their psychiatric symptoms severe enough to require 24-hour monitoring and supervision? Most detoxification we can do on an outpatient basis, but some complex multisubstance withdrawal may need more monitoring.
“Also, we have an increasing armamentarium of medications to promote abstinence, and sometimes it makes sense to start them in higher-level treatment settings; for example long-acting injectable naltrexone (Vivitrol) needs a 10-day postdetox opioid-free washout before it can be started.”
Dr. Fishman was careful to note that imminent danger is usually not a reason to use an inpatient rehab setting. “When you’re talking about safety issues, then people usually need a hospital. Most rehabs are not equipped to deal with dangerous patients.”
In choosing from the different treatment options, the first question should be to ask which forms of treatment are available with high-quality care. Can the patient access an outpatient center, will he be able to get to treatment, and will he be able to remain sober between visits? Will he be offered a full range of treatment options in that setting? Can substance withdrawal be managed safely? If the patient fails at outpatient care, will he be willing to consider inpatient treatment as a next step? What is the risk associated with relapse in a setting that allows for access to substances between sessions? Is the patient someone who is at high risk for a fatal overdose, or at high risk for endangering others, for example, someone who has been revived from overdoses or has driven while inebriated? Would this patient benefit from more intensive psychotherapeutic care? And the question that always haunts me: If there is a bad outcome, will I regret that I did not recommend more?”
Often, I’m left with the idea that it would be nice if we were all given crystal balls at the end of training. In hindsight, if a patient does well, the treatment that was offered was enough, and perhaps even too much in terms of cost. If a patient does not do well, we may be left to ask if he would have been better off if we had recommended a higher level of care, assuming that care could be financed and accessed, and that the patient complied with the treatment recommendations.
Both experts agree that treatment is often effective, and the news here is good. But treatment only works if a patient actually follows through on it, so the best treatment is often the one the patient is willing to accept.
Dr. Miller is the coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016).
In the course of my general psychiatry practice, there are times when I am unable to manage a patient’s substance abuse issues, and I have referred patients to a higher level of care – often to an intensive outpatient program (IOP) that meets for 3 hours a day, or to an inpatient rehabilitation, usually for 28 days. I’m not always sure who can be managed in which setting, and I usually honor the patient’s wishes. If the patient is motivated, has a support system in place, and is concerned that his job will be in jeopardy if he takes time off work, then I refer to Kolmac Outpatient Recovery Centers, a local outpatient treatment center that gives patients the option of attending in the mornings or evenings and allows most people to continue working. If I think I may have only a single shot at getting a patient engaged in care, and the patient is willing to go to an inpatient setting, I refer to a residential treatment facility. It has occurred to me that this is not a very scientific way of making a treatment decision.
George Kolodner, MD, is the chief clinical officer of Kolmac. He has been a member of the American Society of Addiction Medicine’s (ASAM) treatment criteria committee. When I spoke with Dr. Kolodner, he noted: “Discussions between third-party payers and treatment programs about what is the appropriate level of care for a particular individual have been adversarial. ASAM has spent many years developing the ASAM Criteria, a document that attempts to mediate these disagreements by developing objective criteria for where people ought to be treated. Because it is so comprehensive and the variables are so many, it can be difficult to use. A computerized version, called ‘Continuum,’ has been developed to make the criteria more user-friendly.”
“My 45-year experience,” Dr. Kolodner continued, “is that detoxification and rehabilitation can usually be done successfully on an outpatient basis if an appropriate facility is available and the patient has both a supportive living environment and can get to the treatment. Hospitalization and residential rehabilitation is an essential level of care when those conditions do not exist or when outpatient treatment proves to be insufficient.”
One problem with comparing the success of IOPs to inpatient programs is that these settings differ widely in which services they offer to patients.
“There’s no standardization,” Dr. Kolodner said. “The services may be watered down, they may not have a medical staff or a psychiatrist, and people get sucked into inappropriate treatments. When it comes to both IOPs and inpatient facilities, there is no uniformity, and right now it’s caveat emptor.”
Marc Fishman, MD, is medical director of Maryland Treatment Centers/Mountain Manor Treatment Center, a coeditor of the ASAM Criteria, and, with Dr. Kolodner, a member of ASAM’s treatment criteria committee. He, too, talked about the absence of standardization across treatment settings.
“Bed-based and non–bed-based care exist in many flavors and subflavors. You have to remember,” Dr. Fishman said, “this is a marathon, not a sprint, and one of the most important goals of bed-based care is that it serves as a stepping stone for outpatient treatment.”
Dr. Fishman talked about a list of criteria he uses to decide whether someone can be treated as an outpatient. “First, someone has to be able to access outpatient treatment; it may not be available. Can they get back and forth? How chaotic are their lives? Is there support at home, or is it a toxic environment in which others are using? Are they likely to keep using and drop out? What is the patient’s level of motivation? If a person is very ambivalent, you may need a high-intensity motivational milieu. Are their psychiatric symptoms severe enough to require 24-hour monitoring and supervision? Most detoxification we can do on an outpatient basis, but some complex multisubstance withdrawal may need more monitoring.
“Also, we have an increasing armamentarium of medications to promote abstinence, and sometimes it makes sense to start them in higher-level treatment settings; for example long-acting injectable naltrexone (Vivitrol) needs a 10-day postdetox opioid-free washout before it can be started.”
Dr. Fishman was careful to note that imminent danger is usually not a reason to use an inpatient rehab setting. “When you’re talking about safety issues, then people usually need a hospital. Most rehabs are not equipped to deal with dangerous patients.”
In choosing from the different treatment options, the first question should be to ask which forms of treatment are available with high-quality care. Can the patient access an outpatient center, will he be able to get to treatment, and will he be able to remain sober between visits? Will he be offered a full range of treatment options in that setting? Can substance withdrawal be managed safely? If the patient fails at outpatient care, will he be willing to consider inpatient treatment as a next step? What is the risk associated with relapse in a setting that allows for access to substances between sessions? Is the patient someone who is at high risk for a fatal overdose, or at high risk for endangering others, for example, someone who has been revived from overdoses or has driven while inebriated? Would this patient benefit from more intensive psychotherapeutic care? And the question that always haunts me: If there is a bad outcome, will I regret that I did not recommend more?”
Often, I’m left with the idea that it would be nice if we were all given crystal balls at the end of training. In hindsight, if a patient does well, the treatment that was offered was enough, and perhaps even too much in terms of cost. If a patient does not do well, we may be left to ask if he would have been better off if we had recommended a higher level of care, assuming that care could be financed and accessed, and that the patient complied with the treatment recommendations.
Both experts agree that treatment is often effective, and the news here is good. But treatment only works if a patient actually follows through on it, so the best treatment is often the one the patient is willing to accept.
Dr. Miller is the coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016).
Slowing down
This past Labor Day weekend, I did something radical. I slowed down. Way down. My wife slowed down with me, which helped. We spent the weekend close to home walking, talking, reading, contemplating, planning, assessing, doing puzzles and crosswords, and imbibing a craft beer or two, slowly, of course. Why? Because of Adam Grant, PhD, the organizational psychologist at the University of Pennsylvania’s Wharton School of Business, Philadelphia. I had recently reread his 2016 book I’m a big fan; he’s one of those professors who makes you fervently wish you were a student again, someone who will provoke you and challenge your way of thinking.
Dr. Grant’s basic premise, which he has proved through research, is that procrastination boosts productivity. Here’s how: Let’s say you’re facing a challenge or difficult task. He says to start working on it immediately, then take some time away for reflection. This “quick to start and slow to finish” method allows your brain to continually percolate on the problem. An incomplete task stays partially active in your brain. When you come back to it you often see it with fresh eyes. You will experience your highest productivity when you are toggling between these two modes.
This makes sense, and Dr. Grant cites numerous examples from Leonardo da Vinci to the founders of Warby-Parker, as examples of success. But how can it benefit physicians? Many of us are “precrastinators,” people who tend to complete or at least begin tasks as soon as possible, even when it’s unnecessary or not urgent. Unlike some jobs in which it’s easier to take a break from a project and return to it with more creative solutions, we often are racing against a clock to see more patients, read more slides, answer more emails, and make more phone calls. We are perpetually frenetic, which is not conducive to original thinking.
If this sounds like you, then you are likely to benefit from deliberate procrastination. Here are a few ways to slow down:
- Put it on your calendar. Yes, I see the irony, but it works. Start by scheduling one hour a week where you are to accomplish nothing. You can fill this time with whatever your mind wants to do at that moment.
- When faced with a diagnostic dilemma or treatment failure, resist the urge to solve that problem in that moment. Save that note for later, tell the patient you will call him back or bring him back for a visit later. Even if you’re not actively working on it, it will incubate somewhere in your brain, allowing more divergent thought processes to take over. It’s a little like trying to solve a crossword that seems impossible in the moment and then answers suddenly appear without effort.
- Take up a hobby: Play the guitar, learn to make pasta, climb a big rock. When you are fully engaged in such pursuits it requires complete mental focus. When you revisit the difficult problem you’re working on, you will likely see it from different perspectives.
- Meditate: Meditation requires our brains and bodies to slow down. It can help reduce self-doubt and criticism which stifle problem solving.
- Watch Slow TV. Slow TV is a Scandinavian phenomenon where you sit and watch meditative video such as a 7-hour train cam from Bergen, Norway, to Oslo. There’s no dialogue, no plot, no commercials. It’s just 7 hours of track and train and is weirdly comforting.
If you want to learn more, then when you get a chance, Google “slow living” and explore. Of course, some of you precrastinators probably have already started before finishing this column.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
This past Labor Day weekend, I did something radical. I slowed down. Way down. My wife slowed down with me, which helped. We spent the weekend close to home walking, talking, reading, contemplating, planning, assessing, doing puzzles and crosswords, and imbibing a craft beer or two, slowly, of course. Why? Because of Adam Grant, PhD, the organizational psychologist at the University of Pennsylvania’s Wharton School of Business, Philadelphia. I had recently reread his 2016 book I’m a big fan; he’s one of those professors who makes you fervently wish you were a student again, someone who will provoke you and challenge your way of thinking.
Dr. Grant’s basic premise, which he has proved through research, is that procrastination boosts productivity. Here’s how: Let’s say you’re facing a challenge or difficult task. He says to start working on it immediately, then take some time away for reflection. This “quick to start and slow to finish” method allows your brain to continually percolate on the problem. An incomplete task stays partially active in your brain. When you come back to it you often see it with fresh eyes. You will experience your highest productivity when you are toggling between these two modes.
This makes sense, and Dr. Grant cites numerous examples from Leonardo da Vinci to the founders of Warby-Parker, as examples of success. But how can it benefit physicians? Many of us are “precrastinators,” people who tend to complete or at least begin tasks as soon as possible, even when it’s unnecessary or not urgent. Unlike some jobs in which it’s easier to take a break from a project and return to it with more creative solutions, we often are racing against a clock to see more patients, read more slides, answer more emails, and make more phone calls. We are perpetually frenetic, which is not conducive to original thinking.
If this sounds like you, then you are likely to benefit from deliberate procrastination. Here are a few ways to slow down:
- Put it on your calendar. Yes, I see the irony, but it works. Start by scheduling one hour a week where you are to accomplish nothing. You can fill this time with whatever your mind wants to do at that moment.
- When faced with a diagnostic dilemma or treatment failure, resist the urge to solve that problem in that moment. Save that note for later, tell the patient you will call him back or bring him back for a visit later. Even if you’re not actively working on it, it will incubate somewhere in your brain, allowing more divergent thought processes to take over. It’s a little like trying to solve a crossword that seems impossible in the moment and then answers suddenly appear without effort.
- Take up a hobby: Play the guitar, learn to make pasta, climb a big rock. When you are fully engaged in such pursuits it requires complete mental focus. When you revisit the difficult problem you’re working on, you will likely see it from different perspectives.
- Meditate: Meditation requires our brains and bodies to slow down. It can help reduce self-doubt and criticism which stifle problem solving.
- Watch Slow TV. Slow TV is a Scandinavian phenomenon where you sit and watch meditative video such as a 7-hour train cam from Bergen, Norway, to Oslo. There’s no dialogue, no plot, no commercials. It’s just 7 hours of track and train and is weirdly comforting.
If you want to learn more, then when you get a chance, Google “slow living” and explore. Of course, some of you precrastinators probably have already started before finishing this column.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
This past Labor Day weekend, I did something radical. I slowed down. Way down. My wife slowed down with me, which helped. We spent the weekend close to home walking, talking, reading, contemplating, planning, assessing, doing puzzles and crosswords, and imbibing a craft beer or two, slowly, of course. Why? Because of Adam Grant, PhD, the organizational psychologist at the University of Pennsylvania’s Wharton School of Business, Philadelphia. I had recently reread his 2016 book I’m a big fan; he’s one of those professors who makes you fervently wish you were a student again, someone who will provoke you and challenge your way of thinking.
Dr. Grant’s basic premise, which he has proved through research, is that procrastination boosts productivity. Here’s how: Let’s say you’re facing a challenge or difficult task. He says to start working on it immediately, then take some time away for reflection. This “quick to start and slow to finish” method allows your brain to continually percolate on the problem. An incomplete task stays partially active in your brain. When you come back to it you often see it with fresh eyes. You will experience your highest productivity when you are toggling between these two modes.
This makes sense, and Dr. Grant cites numerous examples from Leonardo da Vinci to the founders of Warby-Parker, as examples of success. But how can it benefit physicians? Many of us are “precrastinators,” people who tend to complete or at least begin tasks as soon as possible, even when it’s unnecessary or not urgent. Unlike some jobs in which it’s easier to take a break from a project and return to it with more creative solutions, we often are racing against a clock to see more patients, read more slides, answer more emails, and make more phone calls. We are perpetually frenetic, which is not conducive to original thinking.
If this sounds like you, then you are likely to benefit from deliberate procrastination. Here are a few ways to slow down:
- Put it on your calendar. Yes, I see the irony, but it works. Start by scheduling one hour a week where you are to accomplish nothing. You can fill this time with whatever your mind wants to do at that moment.
- When faced with a diagnostic dilemma or treatment failure, resist the urge to solve that problem in that moment. Save that note for later, tell the patient you will call him back or bring him back for a visit later. Even if you’re not actively working on it, it will incubate somewhere in your brain, allowing more divergent thought processes to take over. It’s a little like trying to solve a crossword that seems impossible in the moment and then answers suddenly appear without effort.
- Take up a hobby: Play the guitar, learn to make pasta, climb a big rock. When you are fully engaged in such pursuits it requires complete mental focus. When you revisit the difficult problem you’re working on, you will likely see it from different perspectives.
- Meditate: Meditation requires our brains and bodies to slow down. It can help reduce self-doubt and criticism which stifle problem solving.
- Watch Slow TV. Slow TV is a Scandinavian phenomenon where you sit and watch meditative video such as a 7-hour train cam from Bergen, Norway, to Oslo. There’s no dialogue, no plot, no commercials. It’s just 7 hours of track and train and is weirdly comforting.
If you want to learn more, then when you get a chance, Google “slow living” and explore. Of course, some of you precrastinators probably have already started before finishing this column.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
October 2018
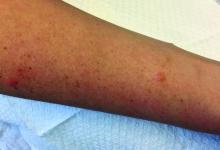
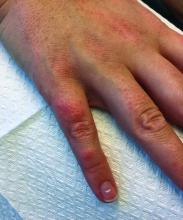
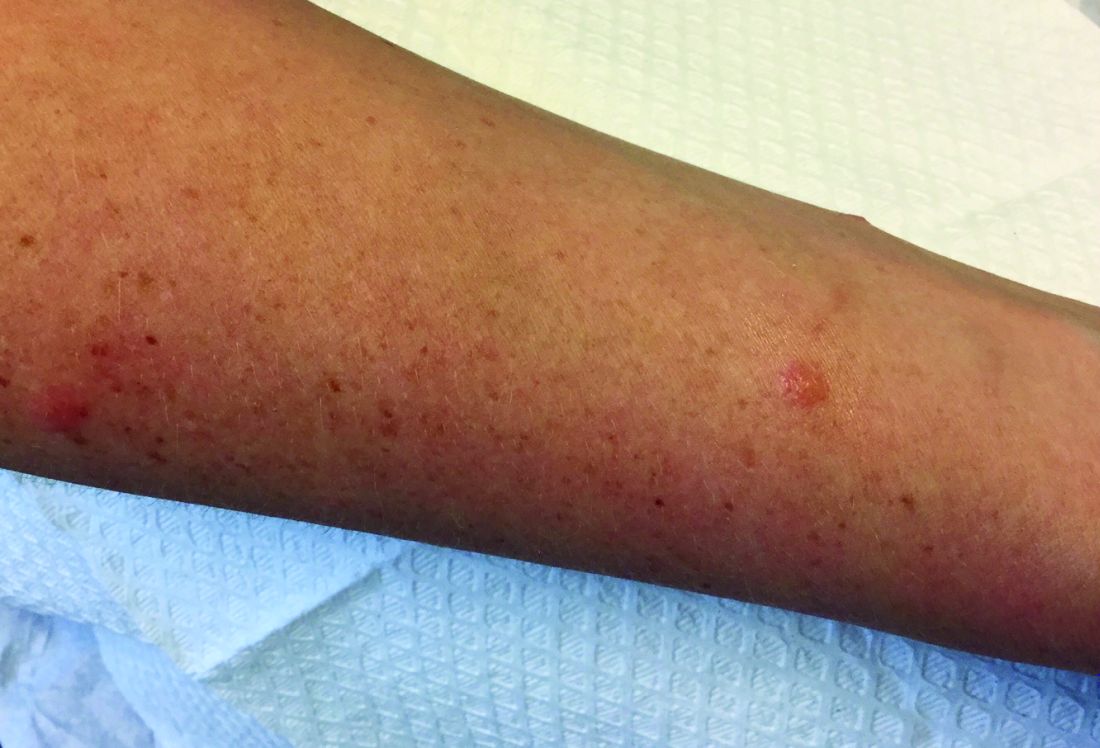
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
A 30-year-old female presented with 2 days of intensely pruritic erythematous papules and vesicles on her bilateral arms and hands. The lesions began appearing 1 day after a camping trip. Her neck, chest, and upper back were clear.
Don’t forget about OSHA
With the bewildering array of new bureaucracies that private practices are now forced to contend with, it is easy to forget about the older ones – especially the Occupational Health and Safety Administration (OSHA).
with all the applicable regulations. Even if you hold regular safety meetings (which all too often is not the case), the occasional comprehensive review is always a good idea, and could save you a bundle in fines.
For starters, do you have an official OSHA poster, enumerating employee rights and explaining how to file complaints? Every office must have one posted in plain site, and it is the first thing an OSHA inspector will look for. You can download one from OSHA’s Web site or order it at no charge by calling 800-321-OSHA.
Next, how old is your written exposure control plan for blood-borne pathogens? It should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, and your implementation of universal precautions – and it is supposed to be updated annually, to reflect changes in technology.
You need not adopt every new safety device as it comes on the market, but you should document which ones you are using – and which you pass up – and why. For example, you and your employees may decide not to purchase a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it’s worth; but you should document how you arrived at your decision and why you feel that your current protocol is as good or better.
Review your list of hazardous substances, which all employees have a right to know about. Keep in mind that OSHA’s list includes alcohol, hydrogen peroxide, acetone, and other substances that you might not consider particularly dangerous, but are nevertheless classified as “hazardous.” (My favorite in that category is liquid nitrogen; it’s hard to envision anything less hazardous, since it evaporates instantly if spilled, and cannot injure skin, or anything else, without purposeful, sustained exposure – and is great, incidentally, for extinguishing small fires.) For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
Check out your building’s exits. Everyone must be able to evacuate your office quickly in case of fire or other emergencies. At a minimum, you (or the owner of the building) are expected to establish exit routes to accommodate all employees and to post easily visible evacuation diagrams.
Examine all electrical devices and their power sources. All electrically powered equipment – medical, clerical, or anything else in the office – must operate safely. Pay particular attention to the way wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning. And beware the common situation of too many gadgets running off a single circuit.
You must provide all at-risk employees with hepatitis B vaccine at no cost to them. You also must provide and pay for appropriate medical treatment and follow-up after any exposure to a dangerous pathogen.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Federal OSHA regulations do not require medical and dental offices to keep an injury and illness log, as other businesses must; but your state may have a requirement that supersedes the federal law. Check with your state, or with your local OSHA office, regarding any such requirements.
It is a mistake to take OSHA regulations lightly; failure to comply with them can result in stiff penalties running into many thousands of dollars.
How can you be certain you are complying with all the rules? The easiest and cheapest way is to call your local OSHA office and request an inspection. Why would you do that? Because OSHA issues no citations during voluntary inspections, as long as you agree to remedy any violations they find.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
With the bewildering array of new bureaucracies that private practices are now forced to contend with, it is easy to forget about the older ones – especially the Occupational Health and Safety Administration (OSHA).
with all the applicable regulations. Even if you hold regular safety meetings (which all too often is not the case), the occasional comprehensive review is always a good idea, and could save you a bundle in fines.
For starters, do you have an official OSHA poster, enumerating employee rights and explaining how to file complaints? Every office must have one posted in plain site, and it is the first thing an OSHA inspector will look for. You can download one from OSHA’s Web site or order it at no charge by calling 800-321-OSHA.
Next, how old is your written exposure control plan for blood-borne pathogens? It should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, and your implementation of universal precautions – and it is supposed to be updated annually, to reflect changes in technology.
You need not adopt every new safety device as it comes on the market, but you should document which ones you are using – and which you pass up – and why. For example, you and your employees may decide not to purchase a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it’s worth; but you should document how you arrived at your decision and why you feel that your current protocol is as good or better.
Review your list of hazardous substances, which all employees have a right to know about. Keep in mind that OSHA’s list includes alcohol, hydrogen peroxide, acetone, and other substances that you might not consider particularly dangerous, but are nevertheless classified as “hazardous.” (My favorite in that category is liquid nitrogen; it’s hard to envision anything less hazardous, since it evaporates instantly if spilled, and cannot injure skin, or anything else, without purposeful, sustained exposure – and is great, incidentally, for extinguishing small fires.) For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
Check out your building’s exits. Everyone must be able to evacuate your office quickly in case of fire or other emergencies. At a minimum, you (or the owner of the building) are expected to establish exit routes to accommodate all employees and to post easily visible evacuation diagrams.
Examine all electrical devices and their power sources. All electrically powered equipment – medical, clerical, or anything else in the office – must operate safely. Pay particular attention to the way wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning. And beware the common situation of too many gadgets running off a single circuit.
You must provide all at-risk employees with hepatitis B vaccine at no cost to them. You also must provide and pay for appropriate medical treatment and follow-up after any exposure to a dangerous pathogen.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Federal OSHA regulations do not require medical and dental offices to keep an injury and illness log, as other businesses must; but your state may have a requirement that supersedes the federal law. Check with your state, or with your local OSHA office, regarding any such requirements.
It is a mistake to take OSHA regulations lightly; failure to comply with them can result in stiff penalties running into many thousands of dollars.
How can you be certain you are complying with all the rules? The easiest and cheapest way is to call your local OSHA office and request an inspection. Why would you do that? Because OSHA issues no citations during voluntary inspections, as long as you agree to remedy any violations they find.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
With the bewildering array of new bureaucracies that private practices are now forced to contend with, it is easy to forget about the older ones – especially the Occupational Health and Safety Administration (OSHA).
with all the applicable regulations. Even if you hold regular safety meetings (which all too often is not the case), the occasional comprehensive review is always a good idea, and could save you a bundle in fines.
For starters, do you have an official OSHA poster, enumerating employee rights and explaining how to file complaints? Every office must have one posted in plain site, and it is the first thing an OSHA inspector will look for. You can download one from OSHA’s Web site or order it at no charge by calling 800-321-OSHA.
Next, how old is your written exposure control plan for blood-borne pathogens? It should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, and your implementation of universal precautions – and it is supposed to be updated annually, to reflect changes in technology.
You need not adopt every new safety device as it comes on the market, but you should document which ones you are using – and which you pass up – and why. For example, you and your employees may decide not to purchase a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it’s worth; but you should document how you arrived at your decision and why you feel that your current protocol is as good or better.
Review your list of hazardous substances, which all employees have a right to know about. Keep in mind that OSHA’s list includes alcohol, hydrogen peroxide, acetone, and other substances that you might not consider particularly dangerous, but are nevertheless classified as “hazardous.” (My favorite in that category is liquid nitrogen; it’s hard to envision anything less hazardous, since it evaporates instantly if spilled, and cannot injure skin, or anything else, without purposeful, sustained exposure – and is great, incidentally, for extinguishing small fires.) For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
Check out your building’s exits. Everyone must be able to evacuate your office quickly in case of fire or other emergencies. At a minimum, you (or the owner of the building) are expected to establish exit routes to accommodate all employees and to post easily visible evacuation diagrams.
Examine all electrical devices and their power sources. All electrically powered equipment – medical, clerical, or anything else in the office – must operate safely. Pay particular attention to the way wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning. And beware the common situation of too many gadgets running off a single circuit.
You must provide all at-risk employees with hepatitis B vaccine at no cost to them. You also must provide and pay for appropriate medical treatment and follow-up after any exposure to a dangerous pathogen.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Federal OSHA regulations do not require medical and dental offices to keep an injury and illness log, as other businesses must; but your state may have a requirement that supersedes the federal law. Check with your state, or with your local OSHA office, regarding any such requirements.
It is a mistake to take OSHA regulations lightly; failure to comply with them can result in stiff penalties running into many thousands of dollars.
How can you be certain you are complying with all the rules? The easiest and cheapest way is to call your local OSHA office and request an inspection. Why would you do that? Because OSHA issues no citations during voluntary inspections, as long as you agree to remedy any violations they find.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
No-shows: Trying to predict and reduce the unpredictable
Why do patients no-show?
The reasons are, obviously, widely variable among patients and circumstances. Some are more understandable than others, but all of them add up to an empty chair across the desk and loss of income for that time slot.
A recent study in Neurology: Clinical Practice looked into this question. Interestingly, it found that people with certain chronic diseases, such as medication-overuse headaches, chronic daily headaches, and seizures, were among those with the highest no-show rates.
These are all conditions that require medication fine tuning, but this can be difficult without the patient coming in. There’s only so much that can be done on the phone, and in this business a direct face-to-face conversation is often needed.
On the opposite side, they noted that people with degenerative disorders that have more limited treatments, such as Alzheimer’s and Parkinson’s diseases, had the highest rate of making it to the appointment, though this may be due more to caretakers than the patients themselves.
Financial issues come into play. Younger patients with chronic diseases may have more difficulty taking time off work, or may just simply not have the money for a copay. They could also be too depressed from their situation to come in. Granted, it would be nice if they’d call to let us know they weren’t coming (at my office we don’t ask questions), but many don’t bother.
All of us are affected by this problem. Seeing patients is what drives the economics of every medical practice. An empty exam room is a financial hit, and it denies another patient who needs help a chance to be seen.
Fifteen years ago, my billing company ran some numbers and found that patients on one specific insurance plan had two to three times the rate of no-shows of any of my other contracts. With a number like that, I couldn’t see a reason to stay with them, and I dropped that plan. I felt bad for the reliable patients affected, but the hard truth is that if I can’t keep my practice open, I can’t help anyone. Why this plan had so many no-shows could be from a number of factors, but the end result was the same. Regardless of the reason, it was having a negative impact on my bottom line.
We try all kinds of different ways to remind people of their appointments. My secretary makes reminder calls. Other offices send texts or emails, or have a robocall system. These can only help to a certain degree. At some point, this becomes the “you can lead a horse to water ...” adage.
There’s no real easy answer, either. At my office, we don’t overbook. It seems to be an unwritten rule that every time we gamble that someone won’t come in and then put someone else in the slot, they both show up.
Research like this is interesting, and maybe helpful at making a predictive model about no-shows. But I’m not convinced it will eventually have everyday use in a real-world practice.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Why do patients no-show?
The reasons are, obviously, widely variable among patients and circumstances. Some are more understandable than others, but all of them add up to an empty chair across the desk and loss of income for that time slot.
A recent study in Neurology: Clinical Practice looked into this question. Interestingly, it found that people with certain chronic diseases, such as medication-overuse headaches, chronic daily headaches, and seizures, were among those with the highest no-show rates.
These are all conditions that require medication fine tuning, but this can be difficult without the patient coming in. There’s only so much that can be done on the phone, and in this business a direct face-to-face conversation is often needed.
On the opposite side, they noted that people with degenerative disorders that have more limited treatments, such as Alzheimer’s and Parkinson’s diseases, had the highest rate of making it to the appointment, though this may be due more to caretakers than the patients themselves.
Financial issues come into play. Younger patients with chronic diseases may have more difficulty taking time off work, or may just simply not have the money for a copay. They could also be too depressed from their situation to come in. Granted, it would be nice if they’d call to let us know they weren’t coming (at my office we don’t ask questions), but many don’t bother.
All of us are affected by this problem. Seeing patients is what drives the economics of every medical practice. An empty exam room is a financial hit, and it denies another patient who needs help a chance to be seen.
Fifteen years ago, my billing company ran some numbers and found that patients on one specific insurance plan had two to three times the rate of no-shows of any of my other contracts. With a number like that, I couldn’t see a reason to stay with them, and I dropped that plan. I felt bad for the reliable patients affected, but the hard truth is that if I can’t keep my practice open, I can’t help anyone. Why this plan had so many no-shows could be from a number of factors, but the end result was the same. Regardless of the reason, it was having a negative impact on my bottom line.
We try all kinds of different ways to remind people of their appointments. My secretary makes reminder calls. Other offices send texts or emails, or have a robocall system. These can only help to a certain degree. At some point, this becomes the “you can lead a horse to water ...” adage.
There’s no real easy answer, either. At my office, we don’t overbook. It seems to be an unwritten rule that every time we gamble that someone won’t come in and then put someone else in the slot, they both show up.
Research like this is interesting, and maybe helpful at making a predictive model about no-shows. But I’m not convinced it will eventually have everyday use in a real-world practice.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Why do patients no-show?
The reasons are, obviously, widely variable among patients and circumstances. Some are more understandable than others, but all of them add up to an empty chair across the desk and loss of income for that time slot.
A recent study in Neurology: Clinical Practice looked into this question. Interestingly, it found that people with certain chronic diseases, such as medication-overuse headaches, chronic daily headaches, and seizures, were among those with the highest no-show rates.
These are all conditions that require medication fine tuning, but this can be difficult without the patient coming in. There’s only so much that can be done on the phone, and in this business a direct face-to-face conversation is often needed.
On the opposite side, they noted that people with degenerative disorders that have more limited treatments, such as Alzheimer’s and Parkinson’s diseases, had the highest rate of making it to the appointment, though this may be due more to caretakers than the patients themselves.
Financial issues come into play. Younger patients with chronic diseases may have more difficulty taking time off work, or may just simply not have the money for a copay. They could also be too depressed from their situation to come in. Granted, it would be nice if they’d call to let us know they weren’t coming (at my office we don’t ask questions), but many don’t bother.
All of us are affected by this problem. Seeing patients is what drives the economics of every medical practice. An empty exam room is a financial hit, and it denies another patient who needs help a chance to be seen.
Fifteen years ago, my billing company ran some numbers and found that patients on one specific insurance plan had two to three times the rate of no-shows of any of my other contracts. With a number like that, I couldn’t see a reason to stay with them, and I dropped that plan. I felt bad for the reliable patients affected, but the hard truth is that if I can’t keep my practice open, I can’t help anyone. Why this plan had so many no-shows could be from a number of factors, but the end result was the same. Regardless of the reason, it was having a negative impact on my bottom line.
We try all kinds of different ways to remind people of their appointments. My secretary makes reminder calls. Other offices send texts or emails, or have a robocall system. These can only help to a certain degree. At some point, this becomes the “you can lead a horse to water ...” adage.
There’s no real easy answer, either. At my office, we don’t overbook. It seems to be an unwritten rule that every time we gamble that someone won’t come in and then put someone else in the slot, they both show up.
Research like this is interesting, and maybe helpful at making a predictive model about no-shows. But I’m not convinced it will eventually have everyday use in a real-world practice.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Professional psychology
, the ability to figure out how to get people to accept what you’re trying to do for them.
Expertise and psychology: Every profession needs both, including our own. Over my years in practice, I’ve met people in many walks of life who develop the same combination. Here are some favorites:
1. Wedding planners
Venues, décor, dresses, floral arrangements, caterers, bands. Wedding planners must know about all of these. And that’s just the start.
Weddings make everyone a bit crazy, or more than a bit. There are parents trying not to let go, children trying to pull away (a Destination Wedding in Patagonia – perfect – none of the family can come!), cultural and taste gaps between the sides (tipplers from Tinseltown and teetotalers from Tupelo), culling the guest list (see Patagonia). Every wedding planner I’ve met could write a book, but won’t. Legal fees would be too high.
Given all this turmoil, some wedding planners might advise elopement and put themselves out of business. No fear of that happening.
2. Event planners
See Wedding Planners, only add: arbitrary and capricious bosses, incompetent implementers, acts of God, acts of man, and everything that goes wrong when there are too many moving parts. One close friend who organizes professional conventions says that every year one attendee posts this complaint on the message board: “Why is there no Diet Mountain Dew?!!!”
3. Dressmakers
Again, see Wedding Planners. Knowing how to design, create, and fit a dress demands a set of skills that earns my admiration and respect. Knowing how to deal with the people who are going to wear the dresses deserves not respect but awe. Even if I knew how to sew, I wouldn’t last a week in this business.
4. Financial planners
Every financial planner I meet describes what they do as “mostly psychology.” Of course, they need to recommend investments that suit the age, life status, and plans of their clients. That’s the easy part.
“When the market is dropping,” says Phil, “people call to scream that they’re losing their shirt. When the market is going up, they call to scream that they’re not making out as well as their friends claim they are.
“Either way, I just hold the phone far enough away from my ear to save my hearing until they’re done venting. Then I try to calm them down and assure them that investing is a long game, and over time they’ll do better staying the course we agreed on than jumping around with every market swing, up or down.”
“Do they listen?”
“Most of them. Eventually.”
4. Speakers’ booking agents
Matching clients with speakers can bring curious challenges. Celebrity speakers in particular may have unique requirements that the agents who book them must figure out and comply with. Or else.
For instance, one young man I met had to book a distinguished jurist. He needed to be picked up in a limo. The limo had to be a Bentley. And the Bentley had to be gray.
Well, excuse me.
5. Waiters
I think the favored term these days is “Servers.” Servers serve strangers. Some strangers are pleasant and courteous. Not all. Waiters mean to please, but to do that they have to put up with a lot. Always with a smile, of course.
Customers ask silly questions. (“What’s good here?”) Some don’t find the menu detailed enough. (“Can I have half of this and some of that, with the sauce on the side?”) They may find the food too hot. Or too cold. Or too spicy. Or too bland. After all that, they may tip a little. Or not.
But the server still has to ask, “Is everything satisfactory?”
6. Psychologists
Never mind.
7. Parking meter readers
Just kidding. Meter readers write parking tickets.
They know perfectly well that everyone knows exactly what they do. And they don’t care.
8. People who field complaints at call centers
Requirement: Patience, savvy, Xanax.
9. Dermatologists
You bet, in spades. I wrote a whole book on the subject. You can read it if you want to. If you disagree, don’t tell me.
I don’t need readers to disagree with me. I already have patients.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected] .
, the ability to figure out how to get people to accept what you’re trying to do for them.
Expertise and psychology: Every profession needs both, including our own. Over my years in practice, I’ve met people in many walks of life who develop the same combination. Here are some favorites:
1. Wedding planners
Venues, décor, dresses, floral arrangements, caterers, bands. Wedding planners must know about all of these. And that’s just the start.
Weddings make everyone a bit crazy, or more than a bit. There are parents trying not to let go, children trying to pull away (a Destination Wedding in Patagonia – perfect – none of the family can come!), cultural and taste gaps between the sides (tipplers from Tinseltown and teetotalers from Tupelo), culling the guest list (see Patagonia). Every wedding planner I’ve met could write a book, but won’t. Legal fees would be too high.
Given all this turmoil, some wedding planners might advise elopement and put themselves out of business. No fear of that happening.
2. Event planners
See Wedding Planners, only add: arbitrary and capricious bosses, incompetent implementers, acts of God, acts of man, and everything that goes wrong when there are too many moving parts. One close friend who organizes professional conventions says that every year one attendee posts this complaint on the message board: “Why is there no Diet Mountain Dew?!!!”
3. Dressmakers
Again, see Wedding Planners. Knowing how to design, create, and fit a dress demands a set of skills that earns my admiration and respect. Knowing how to deal with the people who are going to wear the dresses deserves not respect but awe. Even if I knew how to sew, I wouldn’t last a week in this business.
4. Financial planners
Every financial planner I meet describes what they do as “mostly psychology.” Of course, they need to recommend investments that suit the age, life status, and plans of their clients. That’s the easy part.
“When the market is dropping,” says Phil, “people call to scream that they’re losing their shirt. When the market is going up, they call to scream that they’re not making out as well as their friends claim they are.
“Either way, I just hold the phone far enough away from my ear to save my hearing until they’re done venting. Then I try to calm them down and assure them that investing is a long game, and over time they’ll do better staying the course we agreed on than jumping around with every market swing, up or down.”
“Do they listen?”
“Most of them. Eventually.”
4. Speakers’ booking agents
Matching clients with speakers can bring curious challenges. Celebrity speakers in particular may have unique requirements that the agents who book them must figure out and comply with. Or else.
For instance, one young man I met had to book a distinguished jurist. He needed to be picked up in a limo. The limo had to be a Bentley. And the Bentley had to be gray.
Well, excuse me.
5. Waiters
I think the favored term these days is “Servers.” Servers serve strangers. Some strangers are pleasant and courteous. Not all. Waiters mean to please, but to do that they have to put up with a lot. Always with a smile, of course.
Customers ask silly questions. (“What’s good here?”) Some don’t find the menu detailed enough. (“Can I have half of this and some of that, with the sauce on the side?”) They may find the food too hot. Or too cold. Or too spicy. Or too bland. After all that, they may tip a little. Or not.
But the server still has to ask, “Is everything satisfactory?”
6. Psychologists
Never mind.
7. Parking meter readers
Just kidding. Meter readers write parking tickets.
They know perfectly well that everyone knows exactly what they do. And they don’t care.
8. People who field complaints at call centers
Requirement: Patience, savvy, Xanax.
9. Dermatologists
You bet, in spades. I wrote a whole book on the subject. You can read it if you want to. If you disagree, don’t tell me.
I don’t need readers to disagree with me. I already have patients.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected] .
, the ability to figure out how to get people to accept what you’re trying to do for them.
Expertise and psychology: Every profession needs both, including our own. Over my years in practice, I’ve met people in many walks of life who develop the same combination. Here are some favorites:
1. Wedding planners
Venues, décor, dresses, floral arrangements, caterers, bands. Wedding planners must know about all of these. And that’s just the start.
Weddings make everyone a bit crazy, or more than a bit. There are parents trying not to let go, children trying to pull away (a Destination Wedding in Patagonia – perfect – none of the family can come!), cultural and taste gaps between the sides (tipplers from Tinseltown and teetotalers from Tupelo), culling the guest list (see Patagonia). Every wedding planner I’ve met could write a book, but won’t. Legal fees would be too high.
Given all this turmoil, some wedding planners might advise elopement and put themselves out of business. No fear of that happening.
2. Event planners
See Wedding Planners, only add: arbitrary and capricious bosses, incompetent implementers, acts of God, acts of man, and everything that goes wrong when there are too many moving parts. One close friend who organizes professional conventions says that every year one attendee posts this complaint on the message board: “Why is there no Diet Mountain Dew?!!!”
3. Dressmakers
Again, see Wedding Planners. Knowing how to design, create, and fit a dress demands a set of skills that earns my admiration and respect. Knowing how to deal with the people who are going to wear the dresses deserves not respect but awe. Even if I knew how to sew, I wouldn’t last a week in this business.
4. Financial planners
Every financial planner I meet describes what they do as “mostly psychology.” Of course, they need to recommend investments that suit the age, life status, and plans of their clients. That’s the easy part.
“When the market is dropping,” says Phil, “people call to scream that they’re losing their shirt. When the market is going up, they call to scream that they’re not making out as well as their friends claim they are.
“Either way, I just hold the phone far enough away from my ear to save my hearing until they’re done venting. Then I try to calm them down and assure them that investing is a long game, and over time they’ll do better staying the course we agreed on than jumping around with every market swing, up or down.”
“Do they listen?”
“Most of them. Eventually.”
4. Speakers’ booking agents
Matching clients with speakers can bring curious challenges. Celebrity speakers in particular may have unique requirements that the agents who book them must figure out and comply with. Or else.
For instance, one young man I met had to book a distinguished jurist. He needed to be picked up in a limo. The limo had to be a Bentley. And the Bentley had to be gray.
Well, excuse me.
5. Waiters
I think the favored term these days is “Servers.” Servers serve strangers. Some strangers are pleasant and courteous. Not all. Waiters mean to please, but to do that they have to put up with a lot. Always with a smile, of course.
Customers ask silly questions. (“What’s good here?”) Some don’t find the menu detailed enough. (“Can I have half of this and some of that, with the sauce on the side?”) They may find the food too hot. Or too cold. Or too spicy. Or too bland. After all that, they may tip a little. Or not.
But the server still has to ask, “Is everything satisfactory?”
6. Psychologists
Never mind.
7. Parking meter readers
Just kidding. Meter readers write parking tickets.
They know perfectly well that everyone knows exactly what they do. And they don’t care.
8. People who field complaints at call centers
Requirement: Patience, savvy, Xanax.
9. Dermatologists
You bet, in spades. I wrote a whole book on the subject. You can read it if you want to. If you disagree, don’t tell me.
I don’t need readers to disagree with me. I already have patients.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected] .
Patient-reported outcomes in esophageal diseases
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.