User login
Is respiratory compromise the new “sepsis”?
Hospitalists can play a key role in prevention
Clinicians and even the general public are aware of the dangers of sepsis, the life-threatening illness caused by a body’s response to an infection. Irrespective of one’s perception of pharmaceutical marketing materials or the evidence-based medicine used, awareness about sepsis has led to earlier diagnosis and interventions that have likely saved countless patients’ lives.
Moreover, hospitalists have played a key role in sepsis prevention. In their research, “Improving Survival from Sepsis in Noncritical Units: Role of Hospitalists and Sepsis Team in Early Detection and Initial Treatment of Septic Patients,” Adriana Ducci, MD, and her colleagues showed that a hospitalist-managed sepsis protocol improved sepsis case notifications and patient outcomes.
Although sepsis and respiratory compromise are clearly very different conditions, I believe that greater awareness about respiratory compromise will lead to earlier diagnosis and interventions, which will theoretically improve patient outcomes. Moreover, as with the sepsis awareness campaign, hospitalists can play a key role in recognizing respiratory compromise and in the implementation of appropriate interventions.
As defined by the Respiratory Compromise Institute, “respiratory compromise” is defined as a state in which there is a high likelihood of decompensation into respiratory failure and/or death, but, in which specific interventions – be it therapeutic and/or monitoring – might prevent or mitigate this decompensation.
A significant segment of patients who may be at risk for respiratory compromise are those receiving opioids. The cost of opioid-related adverse events, in terms of both human life and hospital expenses, remains at the forefront of the public eye. It has been estimated that yearly costs in the United States associated with opioid-related postoperative respiratory failure were estimated at $2 billion.
Thomas W. Frederickson MD, FACP, SFHM, MBA, the lead author of the Society of Hospital Medicine guide for Reducing Adverse Drug Events Related to Opioids (RADEO), emphasized in a podcast with the Physician-Patient Alliance for Health & Safety the need to identify patient conditions that pose a greater risk of respiratory compromise.
In particular, Dr. Frederickson pointed out the need to screen for obstructive sleep apnea (OSA): “Patients with obstructive sleep apnea are dependent upon their arousal mechanism in order to avoid respiratory depression and eventual respiratory failure. When these patients receive opioid medication, it decreases this ability for arousal. That puts them at risk for a sudden spiral that includes respiratory insufficiency and respiratory arrest. This can happen very quickly and part of the risk is that the traditional monitoring for sedation that we use in the hospital – that is on a periodic basis and depends upon nursing interventions and questioning – really becomes much less effective in this patient population that can have a respiratory arrest, because of failure to arouse, very quickly. So, a monitoring regimen that takes place every 60 minutes is likely to be ineffective.”
Patient conditions such as OSA should be considered, along with other comorbidities. As the RADEO Guide states: “Before starting opioid therapy, either in surgical or non-surgical settings, it is important to identify any real or potential risks of respiratory depression or other opioid-related adverse effects. Patient comorbidities such as OSA, neurologic disorders, organ impairment, substance abuse history, and other medication use are important aspects to consider.”
Although we have clearly recognized a significant increase in respiratory complications associated with opioid administration, there are other areas, which are non–opioid related, that can create respiratory compromise. We view many patients with stable or underlying respiratory conditions, whether it be COPD, sleep apnea, or preexisting pathophysiology, where either due to sedative agents, or an acute illness – like pneumonia – they can go from a stable condition to respiratory compromise and become at risk for respiratory failure.
A classic example of that in my world of anesthesia has been the well-recognized area of non–operating room anesthesia – in particular, in endoscopy suites where numerous endoscopy procedures are performed under the administration of propofol or other anxiolytic-like drugs. There has been a well-recognized incidence of sentinel events related to oxygenation and ventilation, including death.
Many clinicians see sedation as a benign introduction of relatively limited-effect drugs, which isn’t always true. So, therefore, it is essential that clinicians understand three things:
1. The drugs we employ as sedative agents can have variable effects on individuals depending on their tolerance and their underlying medical condition.
2. The dosages and particular combination of drugs employed may cause an adverse event – for example, the combination of opioids and benzodiazepines.
3. There are factors that can distract from the clinical assessment of routine vital signs, such as respiratory rate, heart rate, and blood pressure. For example, when pulse oximetry is administered with oxygen therapy, there can often be a delay in the recognition of hypoventilation. Consequently, that’s why more and more clinicians are beginning to utilize capnography, or CO2 monitoring, in the expired gas to earlier detect depressed respiratory rate and/or apnea, as well as signs of hypoventilation or inadequate ventilation.
There clearly are obstacles to continuous patient monitoring, such as the associated cost, familiarity with the utilization, the benefit, as well as the limitations of specific monitors in different clinical situations, which mandates an educational process to employ these. However, currently, patient monitoring provides the best early indicator of a patient’s deterioration and the possibility of respiratory compromise.
In my field, we have become very comfortable with capnography and patient monitoring, because for decades it’s been a standard of care for monitoring in the operating room. The role for utilization of capnography for patients who are receiving an opioid or sedative agent outside of the operating room needs to be further assessed. However, technology is not a silver bullet and should be used as an adjunct to clinical judgment in at-risk populations.
Simple recognition and greater awareness of respiratory compromise, just as with sepsis awareness campaigns, will mean more patients are diagnosed earlier, more appropriate interventions are made, and hopefully more adverse events and patient deaths are averted.
Dr. Vender is the emeritus Harris Family Foundation chairman of the department of anesthesiology at NorthShore University Health System in Evanston, Ill. He is clinical professor at the University of Chicago Pritzker School of Medicine and chairman, Clinical Advisory Committee, Respiratory Compromise Institute. Dr. Vender has consulted with Medtronic.
Hospitalists can play a key role in prevention
Hospitalists can play a key role in prevention
Clinicians and even the general public are aware of the dangers of sepsis, the life-threatening illness caused by a body’s response to an infection. Irrespective of one’s perception of pharmaceutical marketing materials or the evidence-based medicine used, awareness about sepsis has led to earlier diagnosis and interventions that have likely saved countless patients’ lives.
Moreover, hospitalists have played a key role in sepsis prevention. In their research, “Improving Survival from Sepsis in Noncritical Units: Role of Hospitalists and Sepsis Team in Early Detection and Initial Treatment of Septic Patients,” Adriana Ducci, MD, and her colleagues showed that a hospitalist-managed sepsis protocol improved sepsis case notifications and patient outcomes.
Although sepsis and respiratory compromise are clearly very different conditions, I believe that greater awareness about respiratory compromise will lead to earlier diagnosis and interventions, which will theoretically improve patient outcomes. Moreover, as with the sepsis awareness campaign, hospitalists can play a key role in recognizing respiratory compromise and in the implementation of appropriate interventions.
As defined by the Respiratory Compromise Institute, “respiratory compromise” is defined as a state in which there is a high likelihood of decompensation into respiratory failure and/or death, but, in which specific interventions – be it therapeutic and/or monitoring – might prevent or mitigate this decompensation.
A significant segment of patients who may be at risk for respiratory compromise are those receiving opioids. The cost of opioid-related adverse events, in terms of both human life and hospital expenses, remains at the forefront of the public eye. It has been estimated that yearly costs in the United States associated with opioid-related postoperative respiratory failure were estimated at $2 billion.
Thomas W. Frederickson MD, FACP, SFHM, MBA, the lead author of the Society of Hospital Medicine guide for Reducing Adverse Drug Events Related to Opioids (RADEO), emphasized in a podcast with the Physician-Patient Alliance for Health & Safety the need to identify patient conditions that pose a greater risk of respiratory compromise.
In particular, Dr. Frederickson pointed out the need to screen for obstructive sleep apnea (OSA): “Patients with obstructive sleep apnea are dependent upon their arousal mechanism in order to avoid respiratory depression and eventual respiratory failure. When these patients receive opioid medication, it decreases this ability for arousal. That puts them at risk for a sudden spiral that includes respiratory insufficiency and respiratory arrest. This can happen very quickly and part of the risk is that the traditional monitoring for sedation that we use in the hospital – that is on a periodic basis and depends upon nursing interventions and questioning – really becomes much less effective in this patient population that can have a respiratory arrest, because of failure to arouse, very quickly. So, a monitoring regimen that takes place every 60 minutes is likely to be ineffective.”
Patient conditions such as OSA should be considered, along with other comorbidities. As the RADEO Guide states: “Before starting opioid therapy, either in surgical or non-surgical settings, it is important to identify any real or potential risks of respiratory depression or other opioid-related adverse effects. Patient comorbidities such as OSA, neurologic disorders, organ impairment, substance abuse history, and other medication use are important aspects to consider.”
Although we have clearly recognized a significant increase in respiratory complications associated with opioid administration, there are other areas, which are non–opioid related, that can create respiratory compromise. We view many patients with stable or underlying respiratory conditions, whether it be COPD, sleep apnea, or preexisting pathophysiology, where either due to sedative agents, or an acute illness – like pneumonia – they can go from a stable condition to respiratory compromise and become at risk for respiratory failure.
A classic example of that in my world of anesthesia has been the well-recognized area of non–operating room anesthesia – in particular, in endoscopy suites where numerous endoscopy procedures are performed under the administration of propofol or other anxiolytic-like drugs. There has been a well-recognized incidence of sentinel events related to oxygenation and ventilation, including death.
Many clinicians see sedation as a benign introduction of relatively limited-effect drugs, which isn’t always true. So, therefore, it is essential that clinicians understand three things:
1. The drugs we employ as sedative agents can have variable effects on individuals depending on their tolerance and their underlying medical condition.
2. The dosages and particular combination of drugs employed may cause an adverse event – for example, the combination of opioids and benzodiazepines.
3. There are factors that can distract from the clinical assessment of routine vital signs, such as respiratory rate, heart rate, and blood pressure. For example, when pulse oximetry is administered with oxygen therapy, there can often be a delay in the recognition of hypoventilation. Consequently, that’s why more and more clinicians are beginning to utilize capnography, or CO2 monitoring, in the expired gas to earlier detect depressed respiratory rate and/or apnea, as well as signs of hypoventilation or inadequate ventilation.
There clearly are obstacles to continuous patient monitoring, such as the associated cost, familiarity with the utilization, the benefit, as well as the limitations of specific monitors in different clinical situations, which mandates an educational process to employ these. However, currently, patient monitoring provides the best early indicator of a patient’s deterioration and the possibility of respiratory compromise.
In my field, we have become very comfortable with capnography and patient monitoring, because for decades it’s been a standard of care for monitoring in the operating room. The role for utilization of capnography for patients who are receiving an opioid or sedative agent outside of the operating room needs to be further assessed. However, technology is not a silver bullet and should be used as an adjunct to clinical judgment in at-risk populations.
Simple recognition and greater awareness of respiratory compromise, just as with sepsis awareness campaigns, will mean more patients are diagnosed earlier, more appropriate interventions are made, and hopefully more adverse events and patient deaths are averted.
Dr. Vender is the emeritus Harris Family Foundation chairman of the department of anesthesiology at NorthShore University Health System in Evanston, Ill. He is clinical professor at the University of Chicago Pritzker School of Medicine and chairman, Clinical Advisory Committee, Respiratory Compromise Institute. Dr. Vender has consulted with Medtronic.
Clinicians and even the general public are aware of the dangers of sepsis, the life-threatening illness caused by a body’s response to an infection. Irrespective of one’s perception of pharmaceutical marketing materials or the evidence-based medicine used, awareness about sepsis has led to earlier diagnosis and interventions that have likely saved countless patients’ lives.
Moreover, hospitalists have played a key role in sepsis prevention. In their research, “Improving Survival from Sepsis in Noncritical Units: Role of Hospitalists and Sepsis Team in Early Detection and Initial Treatment of Septic Patients,” Adriana Ducci, MD, and her colleagues showed that a hospitalist-managed sepsis protocol improved sepsis case notifications and patient outcomes.
Although sepsis and respiratory compromise are clearly very different conditions, I believe that greater awareness about respiratory compromise will lead to earlier diagnosis and interventions, which will theoretically improve patient outcomes. Moreover, as with the sepsis awareness campaign, hospitalists can play a key role in recognizing respiratory compromise and in the implementation of appropriate interventions.
As defined by the Respiratory Compromise Institute, “respiratory compromise” is defined as a state in which there is a high likelihood of decompensation into respiratory failure and/or death, but, in which specific interventions – be it therapeutic and/or monitoring – might prevent or mitigate this decompensation.
A significant segment of patients who may be at risk for respiratory compromise are those receiving opioids. The cost of opioid-related adverse events, in terms of both human life and hospital expenses, remains at the forefront of the public eye. It has been estimated that yearly costs in the United States associated with opioid-related postoperative respiratory failure were estimated at $2 billion.
Thomas W. Frederickson MD, FACP, SFHM, MBA, the lead author of the Society of Hospital Medicine guide for Reducing Adverse Drug Events Related to Opioids (RADEO), emphasized in a podcast with the Physician-Patient Alliance for Health & Safety the need to identify patient conditions that pose a greater risk of respiratory compromise.
In particular, Dr. Frederickson pointed out the need to screen for obstructive sleep apnea (OSA): “Patients with obstructive sleep apnea are dependent upon their arousal mechanism in order to avoid respiratory depression and eventual respiratory failure. When these patients receive opioid medication, it decreases this ability for arousal. That puts them at risk for a sudden spiral that includes respiratory insufficiency and respiratory arrest. This can happen very quickly and part of the risk is that the traditional monitoring for sedation that we use in the hospital – that is on a periodic basis and depends upon nursing interventions and questioning – really becomes much less effective in this patient population that can have a respiratory arrest, because of failure to arouse, very quickly. So, a monitoring regimen that takes place every 60 minutes is likely to be ineffective.”
Patient conditions such as OSA should be considered, along with other comorbidities. As the RADEO Guide states: “Before starting opioid therapy, either in surgical or non-surgical settings, it is important to identify any real or potential risks of respiratory depression or other opioid-related adverse effects. Patient comorbidities such as OSA, neurologic disorders, organ impairment, substance abuse history, and other medication use are important aspects to consider.”
Although we have clearly recognized a significant increase in respiratory complications associated with opioid administration, there are other areas, which are non–opioid related, that can create respiratory compromise. We view many patients with stable or underlying respiratory conditions, whether it be COPD, sleep apnea, or preexisting pathophysiology, where either due to sedative agents, or an acute illness – like pneumonia – they can go from a stable condition to respiratory compromise and become at risk for respiratory failure.
A classic example of that in my world of anesthesia has been the well-recognized area of non–operating room anesthesia – in particular, in endoscopy suites where numerous endoscopy procedures are performed under the administration of propofol or other anxiolytic-like drugs. There has been a well-recognized incidence of sentinel events related to oxygenation and ventilation, including death.
Many clinicians see sedation as a benign introduction of relatively limited-effect drugs, which isn’t always true. So, therefore, it is essential that clinicians understand three things:
1. The drugs we employ as sedative agents can have variable effects on individuals depending on their tolerance and their underlying medical condition.
2. The dosages and particular combination of drugs employed may cause an adverse event – for example, the combination of opioids and benzodiazepines.
3. There are factors that can distract from the clinical assessment of routine vital signs, such as respiratory rate, heart rate, and blood pressure. For example, when pulse oximetry is administered with oxygen therapy, there can often be a delay in the recognition of hypoventilation. Consequently, that’s why more and more clinicians are beginning to utilize capnography, or CO2 monitoring, in the expired gas to earlier detect depressed respiratory rate and/or apnea, as well as signs of hypoventilation or inadequate ventilation.
There clearly are obstacles to continuous patient monitoring, such as the associated cost, familiarity with the utilization, the benefit, as well as the limitations of specific monitors in different clinical situations, which mandates an educational process to employ these. However, currently, patient monitoring provides the best early indicator of a patient’s deterioration and the possibility of respiratory compromise.
In my field, we have become very comfortable with capnography and patient monitoring, because for decades it’s been a standard of care for monitoring in the operating room. The role for utilization of capnography for patients who are receiving an opioid or sedative agent outside of the operating room needs to be further assessed. However, technology is not a silver bullet and should be used as an adjunct to clinical judgment in at-risk populations.
Simple recognition and greater awareness of respiratory compromise, just as with sepsis awareness campaigns, will mean more patients are diagnosed earlier, more appropriate interventions are made, and hopefully more adverse events and patient deaths are averted.
Dr. Vender is the emeritus Harris Family Foundation chairman of the department of anesthesiology at NorthShore University Health System in Evanston, Ill. He is clinical professor at the University of Chicago Pritzker School of Medicine and chairman, Clinical Advisory Committee, Respiratory Compromise Institute. Dr. Vender has consulted with Medtronic.
Understanding hypertensive disorders in pregnancy
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Netflix addiction, COPD blues, waistline-busting memes
The hottest new drug? Netflix
We all knew this was coming eventually. The first case of “Netflix addiction” has emerged, as a 26-year-old man in India has reportedly sought help at an addiction treatment center in Bangalore. Symptoms included 7-10 hours of TV watching per day and increasing isolation from others. Honestly, sounds like an ideal weekend. A clinical psychology professor at the treatment center warns that this instance is very similar to cases where patients are addicted to video games or social media, wherein the virtual world takes precedence over the real one. No reports yet about exactly what he was bingeing on, but our money’s on “The Great British Baking Show.” And can you blame him? The things they make on that show! This is the first case of Netflix addiction but undoubtedly will not be the last. It’s just so easy to watch 19 episodes of “Law & Order” in a row! I’m not an addict, I’m just a dedicated fan. Please don’t take my computer away from me.
Losin’ the COPD blues
The pursuit of improved therapies for chronic obstructive pulmonary disease has produced a wealth of treatment options. But one new approach sounds better than them all. In a pilot study, an inexpensive, handheld device improved breathing control and self-confidence in people with COPD. And it boosted their quality of life. In fact, 3 months of use for only about a half hour a day most days of the week improved several pulmonary outcome measures, including maximal inspiratory pressure, maximal expiratory pressure, and distance on the 6-minute walk test. The 14 patients, all ex-smokers, even posted significant improvements in performance of “Happy Birthday,” “You Are My Sunshine,” and the respiratorily challenging Johnny Cash’s “Ring of Fire.” Technically called a “free reed wind instrument,” the pulmonary rehab device is also known as a “harmonica.” Can a mouth organ really counter emphysema? Well, neither of the two hard-blowing blues harmonica legends named Sonny Boy Williamson succumbed to COPD.
This is why you’re fat
Apparently, the real reason for rising levels of obesity is not sugar or lack of exercise – it’s memes. Researchers from England’s Loughborough University sent a memo to Parliament displaying evidence that Internet memes are contributing to unhealthy eating habits and sending damaging messages to today’s Internet-loving youth. Researchers blamed such memes as a picture of an obese child with the words “Free food? Count me in!” and a series of toned bodies next to a body made of hot dogs and pizza captioned “me.” Clearly, researchers have never experienced the pure joy of eating too much pizza and truly feeling like you are one with the pie. The report didn’t mention whether more-fit countries meme less or just work out more. We’re inclined to believe a healthy lifestyle includes eating in moderation, staying fit, and meme-ing to your heart’s content.
Mad for vittles
You may not think you need to warn patients about eating rodent brains, but a Rochester, N.Y., man landed in a local hospital after his cognitive abilities took a plunge and his grip on everyday reality had loosened considerably. He’d also misplaced the ability to ambulate on his own. An MRI of his brain revealed a strange, tragic condition: The images bore a striking similarity to the brains of victims suffering from variant Creutzfeldt-Jakob disease, the prion-fueled fatal brain condition known more colloquially as “mad cow disease.” Yet most of the few hundred cases ever encountered were the result of eating bad beef in the United Kingdom more than a quarter century ago. How did this Empire State citizen succumb to a notorious English affliction? The culprits: squirrels. Seems the victim was an avid hunter who’d enjoyed his share of bushy-tailed acorn eaters, and who’d on occasion ensured that no part of his twitchy prey had gone to culinary waste. Including their brains.
The hottest new drug? Netflix
We all knew this was coming eventually. The first case of “Netflix addiction” has emerged, as a 26-year-old man in India has reportedly sought help at an addiction treatment center in Bangalore. Symptoms included 7-10 hours of TV watching per day and increasing isolation from others. Honestly, sounds like an ideal weekend. A clinical psychology professor at the treatment center warns that this instance is very similar to cases where patients are addicted to video games or social media, wherein the virtual world takes precedence over the real one. No reports yet about exactly what he was bingeing on, but our money’s on “The Great British Baking Show.” And can you blame him? The things they make on that show! This is the first case of Netflix addiction but undoubtedly will not be the last. It’s just so easy to watch 19 episodes of “Law & Order” in a row! I’m not an addict, I’m just a dedicated fan. Please don’t take my computer away from me.
Losin’ the COPD blues
The pursuit of improved therapies for chronic obstructive pulmonary disease has produced a wealth of treatment options. But one new approach sounds better than them all. In a pilot study, an inexpensive, handheld device improved breathing control and self-confidence in people with COPD. And it boosted their quality of life. In fact, 3 months of use for only about a half hour a day most days of the week improved several pulmonary outcome measures, including maximal inspiratory pressure, maximal expiratory pressure, and distance on the 6-minute walk test. The 14 patients, all ex-smokers, even posted significant improvements in performance of “Happy Birthday,” “You Are My Sunshine,” and the respiratorily challenging Johnny Cash’s “Ring of Fire.” Technically called a “free reed wind instrument,” the pulmonary rehab device is also known as a “harmonica.” Can a mouth organ really counter emphysema? Well, neither of the two hard-blowing blues harmonica legends named Sonny Boy Williamson succumbed to COPD.
This is why you’re fat
Apparently, the real reason for rising levels of obesity is not sugar or lack of exercise – it’s memes. Researchers from England’s Loughborough University sent a memo to Parliament displaying evidence that Internet memes are contributing to unhealthy eating habits and sending damaging messages to today’s Internet-loving youth. Researchers blamed such memes as a picture of an obese child with the words “Free food? Count me in!” and a series of toned bodies next to a body made of hot dogs and pizza captioned “me.” Clearly, researchers have never experienced the pure joy of eating too much pizza and truly feeling like you are one with the pie. The report didn’t mention whether more-fit countries meme less or just work out more. We’re inclined to believe a healthy lifestyle includes eating in moderation, staying fit, and meme-ing to your heart’s content.
Mad for vittles
You may not think you need to warn patients about eating rodent brains, but a Rochester, N.Y., man landed in a local hospital after his cognitive abilities took a plunge and his grip on everyday reality had loosened considerably. He’d also misplaced the ability to ambulate on his own. An MRI of his brain revealed a strange, tragic condition: The images bore a striking similarity to the brains of victims suffering from variant Creutzfeldt-Jakob disease, the prion-fueled fatal brain condition known more colloquially as “mad cow disease.” Yet most of the few hundred cases ever encountered were the result of eating bad beef in the United Kingdom more than a quarter century ago. How did this Empire State citizen succumb to a notorious English affliction? The culprits: squirrels. Seems the victim was an avid hunter who’d enjoyed his share of bushy-tailed acorn eaters, and who’d on occasion ensured that no part of his twitchy prey had gone to culinary waste. Including their brains.
The hottest new drug? Netflix
We all knew this was coming eventually. The first case of “Netflix addiction” has emerged, as a 26-year-old man in India has reportedly sought help at an addiction treatment center in Bangalore. Symptoms included 7-10 hours of TV watching per day and increasing isolation from others. Honestly, sounds like an ideal weekend. A clinical psychology professor at the treatment center warns that this instance is very similar to cases where patients are addicted to video games or social media, wherein the virtual world takes precedence over the real one. No reports yet about exactly what he was bingeing on, but our money’s on “The Great British Baking Show.” And can you blame him? The things they make on that show! This is the first case of Netflix addiction but undoubtedly will not be the last. It’s just so easy to watch 19 episodes of “Law & Order” in a row! I’m not an addict, I’m just a dedicated fan. Please don’t take my computer away from me.
Losin’ the COPD blues
The pursuit of improved therapies for chronic obstructive pulmonary disease has produced a wealth of treatment options. But one new approach sounds better than them all. In a pilot study, an inexpensive, handheld device improved breathing control and self-confidence in people with COPD. And it boosted their quality of life. In fact, 3 months of use for only about a half hour a day most days of the week improved several pulmonary outcome measures, including maximal inspiratory pressure, maximal expiratory pressure, and distance on the 6-minute walk test. The 14 patients, all ex-smokers, even posted significant improvements in performance of “Happy Birthday,” “You Are My Sunshine,” and the respiratorily challenging Johnny Cash’s “Ring of Fire.” Technically called a “free reed wind instrument,” the pulmonary rehab device is also known as a “harmonica.” Can a mouth organ really counter emphysema? Well, neither of the two hard-blowing blues harmonica legends named Sonny Boy Williamson succumbed to COPD.
This is why you’re fat
Apparently, the real reason for rising levels of obesity is not sugar or lack of exercise – it’s memes. Researchers from England’s Loughborough University sent a memo to Parliament displaying evidence that Internet memes are contributing to unhealthy eating habits and sending damaging messages to today’s Internet-loving youth. Researchers blamed such memes as a picture of an obese child with the words “Free food? Count me in!” and a series of toned bodies next to a body made of hot dogs and pizza captioned “me.” Clearly, researchers have never experienced the pure joy of eating too much pizza and truly feeling like you are one with the pie. The report didn’t mention whether more-fit countries meme less or just work out more. We’re inclined to believe a healthy lifestyle includes eating in moderation, staying fit, and meme-ing to your heart’s content.
Mad for vittles
You may not think you need to warn patients about eating rodent brains, but a Rochester, N.Y., man landed in a local hospital after his cognitive abilities took a plunge and his grip on everyday reality had loosened considerably. He’d also misplaced the ability to ambulate on his own. An MRI of his brain revealed a strange, tragic condition: The images bore a striking similarity to the brains of victims suffering from variant Creutzfeldt-Jakob disease, the prion-fueled fatal brain condition known more colloquially as “mad cow disease.” Yet most of the few hundred cases ever encountered were the result of eating bad beef in the United Kingdom more than a quarter century ago. How did this Empire State citizen succumb to a notorious English affliction? The culprits: squirrels. Seems the victim was an avid hunter who’d enjoyed his share of bushy-tailed acorn eaters, and who’d on occasion ensured that no part of his twitchy prey had gone to culinary waste. Including their brains.
Private practice gastroenterology models: Weighing the options
Editor’s note: It is my pleasure to introduce this new quarterly column in The New Gastroenterologist that will be dedicated to addressing important topics for early-career GIs who are either considering a career or starting a career as an independent GI physician in practice. This column is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA), a national advocacy organization of more than 1,800 gastroenterologists in 79 member practices, which is focused exclusively on policies that promote and protect the high-quality, cost-efficient care provided to patients in the independent GI-practice setting.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Educating and training for your life’s work has likely occupied much of the past 10 years including medical school, residency, and fellowship. When it comes to deciding the next steps, the options can seem daunting.
As a long-standing private practice gastroenterologist, I think it is important for early-career GIs to understand the different private practice options and the new challenges and opportunities that private practitioners are experiencing.
According to recent data, there are approximately 12,500 clinical gastroenterologists divided among private gastroenterology practice models, hospital-based employees, and large multispecialty groups. There are nearly 6,000 private practice gastroenterologists.
There has been ongoing dialogue among all health care system stakeholders and the public regarding health care delivery, access, and financing. For the past several years, private practice advocacy groups, including DHPA, have been urging the elimination of the Medicare “site of service” differential between hospital and nonhospital settings, which typically results in substantially higher costs for hospital-based services.
In the 2015 Balanced Budget Act, Congress mandated that patient services provided in off-campus locations acquired by hospitals after Nov.1, 2015, be paid at the nonhospital rate. The 2019 Medicare Outpatient Prospective Payment System/Ambulatory Surgical Center proposed rule takes additional steps in achieving site neutrality and will likely have the effect of tamping down hospitals’ incentives to acquire independent physician practices.
This is a positive step forward in preserving the cost-efficient, high-quality care provided in the independent GI medical setting. And, with the growing health needs of an aging population and an aging GI physician workforce (nearly half of gastroenterologists are older than 55 years old), there is sure to be an ongoing high demand for providers in our specialty.
Selecting the career path that best fits your goals, ambitions, and lifestyle can be challenging. But, our professional training has taught us that the best method for arriving at the correct course of action is to first understand the questions and then seek the answers – let’s get to it.
Private practice models: What are the options?
A lot has changed since I completed my fellowship training in 1978. But the most dramatic changes have happened in the past decade, including the trend of smaller practices consolidating into larger groups.
Traditionally, physicians and patients have favored individual and very-small-group practices. Patients view small-group practices as highly personalized. They come to appreciate knowing all the physicians and staff in a practice. These long-standing “family type” relationships among patients and providers that often develop in this clinical care setting engenders in both the patient and provider a high level of satisfaction with the type and experience of care provided.
New physicians who are part of a small group practice often have the opportunity to take an earlier and more active role within the leadership of the practice. Small groups typically look to new physicians to function as “innovators” who can introduce into the practice those cutting-edge treatments and procedures learned during fellowship.
In the past decade, however, the trend toward the disappearance of solo and very-small-group practices has accelerated. Today, very small groups face several challenges. Providing all the “necessities” that are now part of today’s medical practice can be daunting. Small-sized practices are less likely to integrate ancillary services (e.g., lab and pathology services, in-office infusions, dietary and weight loss management) that are more typically seen in larger practices. Patients may find this fragmentation of care burdensome when they have to go to several providers for treatment.
The difficulties of implementing and maintaining information technology, EHRs, and patient-engagement tools are often inversely related to group size. In addition, the ability and effectiveness of a group to negotiate with hospital systems and insurance companies can be easier for larger practices, although other local factors will also come into play.
Beyond the administrative aspects of running a small group practice, your views about work-life balance should also be an important consideration when choosing a career path. Understandably, issues of call coverage and time off can be more restrictive in small groups.
Is bigger better?
The consolidation seen in hospital systems and multispecialty groups has found its way into single specialty practices. Many urban areas now have GI group practices of 10 or more physicians. There are now approximately 15 groups with 40 or more gastroenterologists, including a few GI practices with 100 or more physicians.
Increasing the size of a practice has obvious potential advantages, including less burdensome on-call requirements and a lower per-physician cost of maintaining and operating the practice. Larger groups often have dedicated software development and IT support staff. Patients are engaged and can connect with their providers through all manner of social media.
Large practice size also can make it possible to enable physicians who may choose to focus on single areas of gastroenterology. This means that a physician who wants to subspecialize in areas such as inflammatory bowel disease, hepatology, woman’s health, and advanced therapeutic endoscopy, would have the requisite large patient base, through internal practice referral, to support subspecialization. Larger groups can also integrate ancillary services into their practice such as pathology, infusion therapy, and nonhospital-based endoscopy services.
However, there can be disadvantages to choosing a larger practice. As in other larger institutions, physicians practicing in larger-sized groups may feel somewhat removed from practice management decisions. It may take several years to become a partner in a large practice – if you are more interested in the opportunity to be involved in practice decisions, a smaller group may be right for you.
New trends in practice groups
Physicians are continuously looking for ways to practice effectively and efficiently while expanding the range of services offered (think obesity management). Independent practice physicians are finding it increasingly difficult to grow and manage successful organizations while they care for their patients. Larger practices now typically include areas such as nursing, information technology, human resources, billing, and practice administration. I trained to treat patients, not run a business – there was much I’ve learned along the way. Many schools now offer joint MD/MBA programs. This may help blend the clinical, operational, and business components of practice.
In a newly developing trend, practice groups are exploring strategic partnerships with private equity/venture capital, practice management companies, national ambulatory surgery center companies, and even managed care insurance companies. This creates the opportunity to forge partnerships with these various health care–focused groups, and results in investment in GI practices seeking experienced business leadership and management while remaining independent of a health system. Already well established in dermatology, ophthalmology, and anesthesia, this phenomenon is now beginning in gastroenterology.
There are many things to consider when choosing a career path. Independent practice in gastroenterology continues as a vitally important component of care delivery, and it’s my hope that the new generation of gastroenterologists finds their journey as rewarding and personally satisfying as mine has been.
Fred B. Rosenberg, MD, is a board-certified gastroenterologist and the medical director of the North Shore Endoscopy Center in Lake Bluff, Ill., the founding president of Illinois Gastroenterology Group, and immediate past president of DHPA.
Editor’s note: It is my pleasure to introduce this new quarterly column in The New Gastroenterologist that will be dedicated to addressing important topics for early-career GIs who are either considering a career or starting a career as an independent GI physician in practice. This column is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA), a national advocacy organization of more than 1,800 gastroenterologists in 79 member practices, which is focused exclusively on policies that promote and protect the high-quality, cost-efficient care provided to patients in the independent GI-practice setting.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Educating and training for your life’s work has likely occupied much of the past 10 years including medical school, residency, and fellowship. When it comes to deciding the next steps, the options can seem daunting.
As a long-standing private practice gastroenterologist, I think it is important for early-career GIs to understand the different private practice options and the new challenges and opportunities that private practitioners are experiencing.
According to recent data, there are approximately 12,500 clinical gastroenterologists divided among private gastroenterology practice models, hospital-based employees, and large multispecialty groups. There are nearly 6,000 private practice gastroenterologists.
There has been ongoing dialogue among all health care system stakeholders and the public regarding health care delivery, access, and financing. For the past several years, private practice advocacy groups, including DHPA, have been urging the elimination of the Medicare “site of service” differential between hospital and nonhospital settings, which typically results in substantially higher costs for hospital-based services.
In the 2015 Balanced Budget Act, Congress mandated that patient services provided in off-campus locations acquired by hospitals after Nov.1, 2015, be paid at the nonhospital rate. The 2019 Medicare Outpatient Prospective Payment System/Ambulatory Surgical Center proposed rule takes additional steps in achieving site neutrality and will likely have the effect of tamping down hospitals’ incentives to acquire independent physician practices.
This is a positive step forward in preserving the cost-efficient, high-quality care provided in the independent GI medical setting. And, with the growing health needs of an aging population and an aging GI physician workforce (nearly half of gastroenterologists are older than 55 years old), there is sure to be an ongoing high demand for providers in our specialty.
Selecting the career path that best fits your goals, ambitions, and lifestyle can be challenging. But, our professional training has taught us that the best method for arriving at the correct course of action is to first understand the questions and then seek the answers – let’s get to it.
Private practice models: What are the options?
A lot has changed since I completed my fellowship training in 1978. But the most dramatic changes have happened in the past decade, including the trend of smaller practices consolidating into larger groups.
Traditionally, physicians and patients have favored individual and very-small-group practices. Patients view small-group practices as highly personalized. They come to appreciate knowing all the physicians and staff in a practice. These long-standing “family type” relationships among patients and providers that often develop in this clinical care setting engenders in both the patient and provider a high level of satisfaction with the type and experience of care provided.
New physicians who are part of a small group practice often have the opportunity to take an earlier and more active role within the leadership of the practice. Small groups typically look to new physicians to function as “innovators” who can introduce into the practice those cutting-edge treatments and procedures learned during fellowship.
In the past decade, however, the trend toward the disappearance of solo and very-small-group practices has accelerated. Today, very small groups face several challenges. Providing all the “necessities” that are now part of today’s medical practice can be daunting. Small-sized practices are less likely to integrate ancillary services (e.g., lab and pathology services, in-office infusions, dietary and weight loss management) that are more typically seen in larger practices. Patients may find this fragmentation of care burdensome when they have to go to several providers for treatment.
The difficulties of implementing and maintaining information technology, EHRs, and patient-engagement tools are often inversely related to group size. In addition, the ability and effectiveness of a group to negotiate with hospital systems and insurance companies can be easier for larger practices, although other local factors will also come into play.
Beyond the administrative aspects of running a small group practice, your views about work-life balance should also be an important consideration when choosing a career path. Understandably, issues of call coverage and time off can be more restrictive in small groups.
Is bigger better?
The consolidation seen in hospital systems and multispecialty groups has found its way into single specialty practices. Many urban areas now have GI group practices of 10 or more physicians. There are now approximately 15 groups with 40 or more gastroenterologists, including a few GI practices with 100 or more physicians.
Increasing the size of a practice has obvious potential advantages, including less burdensome on-call requirements and a lower per-physician cost of maintaining and operating the practice. Larger groups often have dedicated software development and IT support staff. Patients are engaged and can connect with their providers through all manner of social media.
Large practice size also can make it possible to enable physicians who may choose to focus on single areas of gastroenterology. This means that a physician who wants to subspecialize in areas such as inflammatory bowel disease, hepatology, woman’s health, and advanced therapeutic endoscopy, would have the requisite large patient base, through internal practice referral, to support subspecialization. Larger groups can also integrate ancillary services into their practice such as pathology, infusion therapy, and nonhospital-based endoscopy services.
However, there can be disadvantages to choosing a larger practice. As in other larger institutions, physicians practicing in larger-sized groups may feel somewhat removed from practice management decisions. It may take several years to become a partner in a large practice – if you are more interested in the opportunity to be involved in practice decisions, a smaller group may be right for you.
New trends in practice groups
Physicians are continuously looking for ways to practice effectively and efficiently while expanding the range of services offered (think obesity management). Independent practice physicians are finding it increasingly difficult to grow and manage successful organizations while they care for their patients. Larger practices now typically include areas such as nursing, information technology, human resources, billing, and practice administration. I trained to treat patients, not run a business – there was much I’ve learned along the way. Many schools now offer joint MD/MBA programs. This may help blend the clinical, operational, and business components of practice.
In a newly developing trend, practice groups are exploring strategic partnerships with private equity/venture capital, practice management companies, national ambulatory surgery center companies, and even managed care insurance companies. This creates the opportunity to forge partnerships with these various health care–focused groups, and results in investment in GI practices seeking experienced business leadership and management while remaining independent of a health system. Already well established in dermatology, ophthalmology, and anesthesia, this phenomenon is now beginning in gastroenterology.
There are many things to consider when choosing a career path. Independent practice in gastroenterology continues as a vitally important component of care delivery, and it’s my hope that the new generation of gastroenterologists finds their journey as rewarding and personally satisfying as mine has been.
Fred B. Rosenberg, MD, is a board-certified gastroenterologist and the medical director of the North Shore Endoscopy Center in Lake Bluff, Ill., the founding president of Illinois Gastroenterology Group, and immediate past president of DHPA.
Editor’s note: It is my pleasure to introduce this new quarterly column in The New Gastroenterologist that will be dedicated to addressing important topics for early-career GIs who are either considering a career or starting a career as an independent GI physician in practice. This column is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA), a national advocacy organization of more than 1,800 gastroenterologists in 79 member practices, which is focused exclusively on policies that promote and protect the high-quality, cost-efficient care provided to patients in the independent GI-practice setting.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Educating and training for your life’s work has likely occupied much of the past 10 years including medical school, residency, and fellowship. When it comes to deciding the next steps, the options can seem daunting.
As a long-standing private practice gastroenterologist, I think it is important for early-career GIs to understand the different private practice options and the new challenges and opportunities that private practitioners are experiencing.
According to recent data, there are approximately 12,500 clinical gastroenterologists divided among private gastroenterology practice models, hospital-based employees, and large multispecialty groups. There are nearly 6,000 private practice gastroenterologists.
There has been ongoing dialogue among all health care system stakeholders and the public regarding health care delivery, access, and financing. For the past several years, private practice advocacy groups, including DHPA, have been urging the elimination of the Medicare “site of service” differential between hospital and nonhospital settings, which typically results in substantially higher costs for hospital-based services.
In the 2015 Balanced Budget Act, Congress mandated that patient services provided in off-campus locations acquired by hospitals after Nov.1, 2015, be paid at the nonhospital rate. The 2019 Medicare Outpatient Prospective Payment System/Ambulatory Surgical Center proposed rule takes additional steps in achieving site neutrality and will likely have the effect of tamping down hospitals’ incentives to acquire independent physician practices.
This is a positive step forward in preserving the cost-efficient, high-quality care provided in the independent GI medical setting. And, with the growing health needs of an aging population and an aging GI physician workforce (nearly half of gastroenterologists are older than 55 years old), there is sure to be an ongoing high demand for providers in our specialty.
Selecting the career path that best fits your goals, ambitions, and lifestyle can be challenging. But, our professional training has taught us that the best method for arriving at the correct course of action is to first understand the questions and then seek the answers – let’s get to it.
Private practice models: What are the options?
A lot has changed since I completed my fellowship training in 1978. But the most dramatic changes have happened in the past decade, including the trend of smaller practices consolidating into larger groups.
Traditionally, physicians and patients have favored individual and very-small-group practices. Patients view small-group practices as highly personalized. They come to appreciate knowing all the physicians and staff in a practice. These long-standing “family type” relationships among patients and providers that often develop in this clinical care setting engenders in both the patient and provider a high level of satisfaction with the type and experience of care provided.
New physicians who are part of a small group practice often have the opportunity to take an earlier and more active role within the leadership of the practice. Small groups typically look to new physicians to function as “innovators” who can introduce into the practice those cutting-edge treatments and procedures learned during fellowship.
In the past decade, however, the trend toward the disappearance of solo and very-small-group practices has accelerated. Today, very small groups face several challenges. Providing all the “necessities” that are now part of today’s medical practice can be daunting. Small-sized practices are less likely to integrate ancillary services (e.g., lab and pathology services, in-office infusions, dietary and weight loss management) that are more typically seen in larger practices. Patients may find this fragmentation of care burdensome when they have to go to several providers for treatment.
The difficulties of implementing and maintaining information technology, EHRs, and patient-engagement tools are often inversely related to group size. In addition, the ability and effectiveness of a group to negotiate with hospital systems and insurance companies can be easier for larger practices, although other local factors will also come into play.
Beyond the administrative aspects of running a small group practice, your views about work-life balance should also be an important consideration when choosing a career path. Understandably, issues of call coverage and time off can be more restrictive in small groups.
Is bigger better?
The consolidation seen in hospital systems and multispecialty groups has found its way into single specialty practices. Many urban areas now have GI group practices of 10 or more physicians. There are now approximately 15 groups with 40 or more gastroenterologists, including a few GI practices with 100 or more physicians.
Increasing the size of a practice has obvious potential advantages, including less burdensome on-call requirements and a lower per-physician cost of maintaining and operating the practice. Larger groups often have dedicated software development and IT support staff. Patients are engaged and can connect with their providers through all manner of social media.
Large practice size also can make it possible to enable physicians who may choose to focus on single areas of gastroenterology. This means that a physician who wants to subspecialize in areas such as inflammatory bowel disease, hepatology, woman’s health, and advanced therapeutic endoscopy, would have the requisite large patient base, through internal practice referral, to support subspecialization. Larger groups can also integrate ancillary services into their practice such as pathology, infusion therapy, and nonhospital-based endoscopy services.
However, there can be disadvantages to choosing a larger practice. As in other larger institutions, physicians practicing in larger-sized groups may feel somewhat removed from practice management decisions. It may take several years to become a partner in a large practice – if you are more interested in the opportunity to be involved in practice decisions, a smaller group may be right for you.
New trends in practice groups
Physicians are continuously looking for ways to practice effectively and efficiently while expanding the range of services offered (think obesity management). Independent practice physicians are finding it increasingly difficult to grow and manage successful organizations while they care for their patients. Larger practices now typically include areas such as nursing, information technology, human resources, billing, and practice administration. I trained to treat patients, not run a business – there was much I’ve learned along the way. Many schools now offer joint MD/MBA programs. This may help blend the clinical, operational, and business components of practice.
In a newly developing trend, practice groups are exploring strategic partnerships with private equity/venture capital, practice management companies, national ambulatory surgery center companies, and even managed care insurance companies. This creates the opportunity to forge partnerships with these various health care–focused groups, and results in investment in GI practices seeking experienced business leadership and management while remaining independent of a health system. Already well established in dermatology, ophthalmology, and anesthesia, this phenomenon is now beginning in gastroenterology.
There are many things to consider when choosing a career path. Independent practice in gastroenterology continues as a vitally important component of care delivery, and it’s my hope that the new generation of gastroenterologists finds their journey as rewarding and personally satisfying as mine has been.
Fred B. Rosenberg, MD, is a board-certified gastroenterologist and the medical director of the North Shore Endoscopy Center in Lake Bluff, Ill., the founding president of Illinois Gastroenterology Group, and immediate past president of DHPA.
Chinese American man with high risk of psychosis
Editors’ Note: Curbside Consult is an occasional column aimed at helping psychiatrists think through family and cultural considerations when treating patients. It examines case vignettes and is written by two Group for the Advancement of Psychiatry (GAP) committees – the Committee on Family Psychiatry and the Committee on Cultural Psychiatry. The contributors have revised selected patient details to shield the identities of the patients/cases and to comply with HIPAA requirements.
Case vignette
Bill is a 25-year-old man of Chinese descent who sought psychiatric evaluation of his psychosis risk. His parents emigrated from China to Canada more than 30 years ago; Bill was born in Canada, and moved to the United States with his parents and two siblings at age 7.
His family has a strong history of mental illness. His older sister was diagnosed with schizophrenia; she frequently got into verbal altercations with her parents. When Bill was 15, she walked out of the house after a fight with the family and never returned. Bill’s family thinks his father has had paranoid delusions. In the past, he attempted to call the police multiple times because he suspected the neighbors had planted a listening device in his front yard. The family stopped him from making the actual calls. However, the family never brought him to psychiatric evaluation because of perceived stigma and social discrimination in their community. He also was emotionally and physically abusive to Bill during his childhood by calling him names and hitting him with a belt. As an adult, Bill still has posttraumatic stress disorder symptoms including flashbacks, nightmares, and avoidance when thinking about his father.
Bill identifies himself as Chinese American and speaks English only. He often perceives himself as a newcomer to U.S. society, making comments such as: “I guess I should live my life like this to fulfill the American dream.” Bill’s parents placed a strong emphasis on academic success, often at the expense of their children’s social interaction and playtime activities. Bill describes himself as a “loner” with few friends. He maintained high academic achievement during high school and was accepted by a prestigious college. Although he was interested in music composition, Bill was “forced” by his parents to major in courses deemed good preparation for law school.
During college, he suffered severe depression with insomnia, low energy, hopelessness, anhedonia, social withdrawal, poor appetite with weight loss, and ruminative thoughts but without delusional thoughts or perceptual disturbances. He had one near-lethal suicide attempt, during which he impulsively took the contents of an entire bottle of Tylenol in the context of family conflicts, resulting in psychiatric hospitalization. Bill recalled with animosity the inpatient psychiatrists who put him on psychotropic medications during a 3-day hospitalization. He was not adherent to the medication and did not follow up with postdischarge outpatient care. He did not remember the medication trial he had during the hospitalization, nor did he give consent to obtain medical records from there. Bill withdrew from college in sophomore year, because of his declining academic performance secondary to his mental illness. He currently works at a gas station.
Over the last year, Bill’s interpersonal communication has become disorganized in both work and social settings, and he has developed thought blocking, causing him substantial distress. He intermittently hears voices of strangers in the background; these have gradually become more frequent, now occurring 3-4 times a week. Bill also is more depressed, with frequent crying episodes and worsening social isolation. He often thinks that life is not worth living, but he has no active suicidal plans or intent.
Bill’s supervisor and coworkers strongly suggested that he seek medical evaluation. As an outpatient, Bill started weekly cognitive-behavioral therapy (CBT) and biweekly medication sessions for early psychosis symptoms, receiving low-dose risperidone (1 mg b.i.d.) and fluoxetine (20 mg daily). Despite initial improvement, he was very skeptical about continuing the medications because of concern that they will cause a “change in his identity” by altering his body chemistry. His parents have been reluctant to join family meetings, because they were ambivalent about Bill’s ongoing psychiatric treatment.
Treatment team’s impressions
Clinical high risk (CHR) syndrome refers to the prodromal phase before a full psychotic disorder. As one of the three subcategories of CHR, genetic risk and deterioration (GRD) prodromal syndrome is defined by having a genetic risk for psychosis (first-degree relative with a psychotic disorder or meeting criteria for schizotypal personality disorder) and a recent decline in daily functioning equivalent to a 30% drop in Global Assessment of Functioning rating.1 Due to Bill’s family history of psychosis, new difficulties in self-care, psychotic-spectrum symptoms, and declining social/executive function, he meets criteria for GRD prodromal syndrome. In addition, major depressive disorder should be considered on his differential diagnosis.
Bill has not received a diagnosis of acute psychosis, and instead is judged to be in the CHR spectrum for psychosis, because of his level of insight that the occasional perceptual disturbance and abnormal thought content are in his own mind. Since 26% of individuals with CHR in mainland China2 and 35% in the general U.S. population develop fully psychotic symptoms within 2-3 years, Bill’s current presentation warrants secondary preventive care (early intervention) to promote improved clinical outcomes. Given the high rates of comorbid depressive and anxiety disorders among individuals with CHR, Bill’s mood symptoms and passive suicidality also require psychiatric intervention. The treatment team raised three questions, which are answered below.
1. How can we understand Bill’s and his family’s resistance to mental health treatment?
Chinese Americans tend to access mental health services at a lower rate than that of the general U.S. population.3 They also tend to exhibit elevated discontinuation from mental health care, compared with non-Latino whites.4 Since first- and second-generation Chinese Americans have similar use rates, it is likely the barriers to care are not immigration specific but also related to factors that endure across generations, including culture-related aspects. These include cultural concepts of illness and how to interpret prodromal symptoms such as Bill’s, stigma and interpersonal shame regarding mental illness and psychiatric treatment, and value orientations such as self-reliance, avoidance of direct expressions of interpersonal conflict, and family privacy.
For example, Chinese Americans tend to emphasize the physical-symptom components of psychiatric problems, partly because of mind-body holism – which combines physical and psychological symptoms into cultural syndromes and idioms of distress – and partly because of concerns about stigma and shame regarding mental health symptoms. Hence, they are more likely to seek help from primary care clinicians for psychological distress before any mental health provider. Many Chinese Americans interpret mild to moderate psychological distress as “mental weakness” or “excessive thinking” (xiang tai duo), which does not require clinical evaluation. In this view, only alarmingly bizarre or disruptive behaviors warrant formal psychiatric treatment.
Some Chinese parents perceive psychosocial stressors, including hardships, interpersonal conflicts, and academic burdens, as understandable triggers for symptoms that clinicians would classify as schizophrenia or attenuated psychosis syndrome.5 Those views of illness can be associated in the Chinese American community with delayed diagnosis and intervention for emerging psychiatric disorders.
Social stigma regarding mental illness is pervasive in many Chinese American communities. Stigma hinders service use, to avoid shame and save the family’s “face.” Although many Chinese Americans acknowledge the efficacy of biomedical treatments for mental illness, they also remain concerned about enduring shame in their communities if they access those services.6 In line with collectivistic values traditionally held by many Chinese Americans, individuals tend to turn to relatives for help first and keep mental illness confidential within the family group to avoid disgracing the family name.7 Hence, social stigma in the Chinese community can be a key barrier to early detection and early intervention for youth at high risk of psychosis.
Culturally influenced cognitions, emotions, and values also might contribute to underuse of formal mental health services in this population. Many Chinese Americans perceive the roots of mental illness in a lack of willpower and the unwise indulgence of morbid thoughts. In these communities, direct communication of strong emotions can be discouraged – in the name of maintaining harmony, collective interests, and tolerance.8 Hence, many Chinese Americans find Western models of psychotherapy that focus on intrapsychic conflicts and/or intense interpersonal emotions incongruous with their treatment expectations. Psychiatric interview processes that explore gloomy or disturbing thoughts can be perceived as disquieting and antithetical to the goals of treatment. In addition, some individuals rooted in collectivist communities would rather keep personal psychological problems private within the family than seek counseling from an expert who is an out-group stranger.
2. How does Bill’s cultural and social context affect his prognosis?
Individuals with psychosis and their close relatives are generally vulnerable to stigma and discrimination. Mental health stigma has a substantial effect on the lives of patients with psychosis and their family members. The magnitude of the perceived stigma tends to be greater if the patient has more severe positive symptoms, is more educated, or resides in a highly urbanized area.
Acutely ill patients usually face more negative community responses than do milder cases, since their close relatives are blamed for failing to uphold the moral and legal responsibility of ensuring that the patients control their behavior. The effect of stigma in Chinese society also is greater among male patients with early-onset illness, because of the expectation that men marry and become the family breadwinner to attain higher social status. Hence, young males who are unable to achieve these socially determined adult milestones can be considered socially inferior, and suffer more community discrimination and exclusion, which are risk factors for clinical deterioration and functional impairment.
Social stigma can intensify relationship conflicts within the family and magnify expressed emotion (EE), which is defined as caregivers’ attitude toward a person with mental illness as reflected by their comments and interaction patterns. “High EE” comprises three behavioral patterns: criticism, hostility, and emotional overinvolvement. High EE is associated with psychiatric symptom relapse among individuals with schizophrenia and other disorders.9
Currently, most of the literature on EE is limited to white samples in Western countries. Some researchers have studied the relationship between the EE index of emotional overinvolvement and schizophrenia relapse among Hispanic populations.10 However, there are limited data on cultural congruence of EE research in Asian populations. Therefore, clinicians should carefully evaluate the contribution of high EE to Bill’s family’s situation during his course of treatment.
Higher education often is associated with greater levels of EE and can result in anxious and fearful responses to the person’s illness.11 This may be attributable to more negative reactions to actual or feared stigma and discrimination, possibly because relatives feel they have more to lose regarding the family’s social status, especially in densely populated urban areas where it might be harder to keep the patient’s mental illness as a “family secret.”
On the other hand, certain explanatory models of psychosis can modulate Chinese community members’ perceptions and allow ill individuals to remain socially integrated. Cultural idioms such as “excessive thinking” (xiang tai duo), “taking things too hard” (xiang bu kai), and “narrow-mindedness” (xiao xin yan) promote socially accommodating behaviors that facilitate acceptance of mildly to moderately ill individuals as full-status community members.12
Another important contributor to psychosis risk is Bill’s acculturative stress about his cultural identity. Linguistic challenges, limited social support, perceived discrimination, and an acculturation gap between parents and children are major sources of acculturative stress among Chinese American college students.13 Greater acculturative stress elevates the risk of mental illness and symptom deterioration. However, highly acculturated Chinese Americans with above-average bicultural self-efficacy tend to express more positive attitudes toward mental health services.
3. Are there culturally appropriate interventions that can help Bill and his family?
A major predictor among Chinese Americans of the intent to use services is the perceived credibility of the treatment and the provider. Ethnic-specific services staffed by bicultural/bilingual mental health clinicians delivering culturally responsive interventions are increasingly available in many metropolitan areas with major Asian communities. These programs have shown clinical efficacy in encouraging service use and promoting treatment persistence. Bill and his family may benefit from referral to ethnic-specific services, where they can obtain culturally sensitive psychoeducation about his mental illness and treatment plan.
Other services that might be useful for Bill and his family include family psychoeducation programs and supportive groups specifically designed for Chinese American families; these can improve the entire family’s psychosocial health, promote medication adherence, and reduce the risk of symptom relapse through family-centered intervention models.14 Connecting with local National Alliance on Mental Illness (NAMI) programs might help Bill’s parents obtain social support from Chinese American families with similar caregiver experiences.
However, services that are not designed specifically for Chinese-origin patients also can provide excellent care for these patients, and be perceived as credible and effective. A thorough cultural assessment is necessary, as well as inclusion of the information obtained in the treatment plan. As described in the DSM-5 Outline for Cultural Formulation and operationalized in the Cultural Formulation Interview, clinicians should assess possible cultural differences among Bill, his family, his community, and his clinicians regarding their cultural concepts of distress and illness and expectations of care in order to formulate a treatment plan acceptable to patient and family. Practical cultural barriers should be addressed, such as Bill’s parents’ limited English proficiency, in which case a bilingual clinician or trained interpreter should be included in the treatment team.
With Bill’s consent, the treatment team also should consider reaching out to his parents, especially his mother, to understand and empathize with their cultural concepts of distress and illness as well as expectations for care. In addition to providing psychoeducation, the clinicians should validate the parents’ experience of shame, fear, and worry about their son. Bill’s brother, for example, might be a useful bridge in communicating with the parents given his higher acculturation and potentially greater acceptance of psychiatric care. He might help alleviate the tension between Bill and his parents and encourage them to seek family-based help.
Take-home points
- Clinical training programs should offer cultural competency training about underserved populations, including communities of color.
- Certain key concepts, such as traditional idioms of distress and explanatory models, social stigma, and acculturative stress, should be included in these trainings and evaluated in a comprehensive psychosocial assessment.
- High expressed emotion among family caregivers is associated with higher rates of psychiatric symptom relapse, whereas families with above-average bicultural self-efficacy have more positive attitudes toward mental health services.
- Clinicians should incorporate culturally appropriate educational materials (for example, CHR warning signs) and interventions to engage underserved patients and their families in mental health treatment.
Contributors
Emily Wu, MD – Harvard Medical School, Boston
Francis Lu, MD – University of California, Davis
John Sargent, MD – Tufts Medical Center, Boston
Roberto Lewis-Fernández, MD – Columbia College of Physicians & Surgeons, New York
If you would like to a submit case in which your understanding and treatment are affected by challenging cultural and family values, send it to [email protected]. We will then write back with our best answers about how one might proceed in such a case. Your case and our response will then be published at mdedge.com/psychiatry. This column is meant to be educational and does not constitute medical advice. The opinions expressed are those of the contributors and do not represent those of the organizations they are employed by or affiliated with or the Group for the Advancement of Psychiatry.
References
1. J Nerv Ment Dis. 2013 Jun;20(6);484-9.
2. Schizophr Res. 2014 Feb;152(2-3):391-9.
3. Perspect Psychiatr Care. 2013;49(4):288-92.
4. Ment Health Serv Res. 2001 Dec;3(4):201-14.
5. Br J Psychiatry. 2000 Jul;177;20-5.
6. Cultr Divers Ethnic Minor Psychol. 2008 Jan;14(1):10-8.
7. Couns Psychol. 2003 May1;31:343-61.
8. Emotion. 2002 Dec;2(4):341-60.
9. Am J Psychiatry. 1986 Nov;143(11):1361-73.
10. J Nerv Ment Dis. 2013 Oct;201(10):833-40.
11. Schizophr Bull. 1981;7(1):43-4.
12. Schizophr Bull. 2010 Jul;36(4):836-45.
13. Am J Orthopsychiatry. 2011 Oct;81(4):489-97.
14. Patient Educ Couns. 2009 Apr;75(1):67-76.
Editors’ Note: Curbside Consult is an occasional column aimed at helping psychiatrists think through family and cultural considerations when treating patients. It examines case vignettes and is written by two Group for the Advancement of Psychiatry (GAP) committees – the Committee on Family Psychiatry and the Committee on Cultural Psychiatry. The contributors have revised selected patient details to shield the identities of the patients/cases and to comply with HIPAA requirements.
Case vignette
Bill is a 25-year-old man of Chinese descent who sought psychiatric evaluation of his psychosis risk. His parents emigrated from China to Canada more than 30 years ago; Bill was born in Canada, and moved to the United States with his parents and two siblings at age 7.
His family has a strong history of mental illness. His older sister was diagnosed with schizophrenia; she frequently got into verbal altercations with her parents. When Bill was 15, she walked out of the house after a fight with the family and never returned. Bill’s family thinks his father has had paranoid delusions. In the past, he attempted to call the police multiple times because he suspected the neighbors had planted a listening device in his front yard. The family stopped him from making the actual calls. However, the family never brought him to psychiatric evaluation because of perceived stigma and social discrimination in their community. He also was emotionally and physically abusive to Bill during his childhood by calling him names and hitting him with a belt. As an adult, Bill still has posttraumatic stress disorder symptoms including flashbacks, nightmares, and avoidance when thinking about his father.
Bill identifies himself as Chinese American and speaks English only. He often perceives himself as a newcomer to U.S. society, making comments such as: “I guess I should live my life like this to fulfill the American dream.” Bill’s parents placed a strong emphasis on academic success, often at the expense of their children’s social interaction and playtime activities. Bill describes himself as a “loner” with few friends. He maintained high academic achievement during high school and was accepted by a prestigious college. Although he was interested in music composition, Bill was “forced” by his parents to major in courses deemed good preparation for law school.
During college, he suffered severe depression with insomnia, low energy, hopelessness, anhedonia, social withdrawal, poor appetite with weight loss, and ruminative thoughts but without delusional thoughts or perceptual disturbances. He had one near-lethal suicide attempt, during which he impulsively took the contents of an entire bottle of Tylenol in the context of family conflicts, resulting in psychiatric hospitalization. Bill recalled with animosity the inpatient psychiatrists who put him on psychotropic medications during a 3-day hospitalization. He was not adherent to the medication and did not follow up with postdischarge outpatient care. He did not remember the medication trial he had during the hospitalization, nor did he give consent to obtain medical records from there. Bill withdrew from college in sophomore year, because of his declining academic performance secondary to his mental illness. He currently works at a gas station.
Over the last year, Bill’s interpersonal communication has become disorganized in both work and social settings, and he has developed thought blocking, causing him substantial distress. He intermittently hears voices of strangers in the background; these have gradually become more frequent, now occurring 3-4 times a week. Bill also is more depressed, with frequent crying episodes and worsening social isolation. He often thinks that life is not worth living, but he has no active suicidal plans or intent.
Bill’s supervisor and coworkers strongly suggested that he seek medical evaluation. As an outpatient, Bill started weekly cognitive-behavioral therapy (CBT) and biweekly medication sessions for early psychosis symptoms, receiving low-dose risperidone (1 mg b.i.d.) and fluoxetine (20 mg daily). Despite initial improvement, he was very skeptical about continuing the medications because of concern that they will cause a “change in his identity” by altering his body chemistry. His parents have been reluctant to join family meetings, because they were ambivalent about Bill’s ongoing psychiatric treatment.
Treatment team’s impressions
Clinical high risk (CHR) syndrome refers to the prodromal phase before a full psychotic disorder. As one of the three subcategories of CHR, genetic risk and deterioration (GRD) prodromal syndrome is defined by having a genetic risk for psychosis (first-degree relative with a psychotic disorder or meeting criteria for schizotypal personality disorder) and a recent decline in daily functioning equivalent to a 30% drop in Global Assessment of Functioning rating.1 Due to Bill’s family history of psychosis, new difficulties in self-care, psychotic-spectrum symptoms, and declining social/executive function, he meets criteria for GRD prodromal syndrome. In addition, major depressive disorder should be considered on his differential diagnosis.
Bill has not received a diagnosis of acute psychosis, and instead is judged to be in the CHR spectrum for psychosis, because of his level of insight that the occasional perceptual disturbance and abnormal thought content are in his own mind. Since 26% of individuals with CHR in mainland China2 and 35% in the general U.S. population develop fully psychotic symptoms within 2-3 years, Bill’s current presentation warrants secondary preventive care (early intervention) to promote improved clinical outcomes. Given the high rates of comorbid depressive and anxiety disorders among individuals with CHR, Bill’s mood symptoms and passive suicidality also require psychiatric intervention. The treatment team raised three questions, which are answered below.
1. How can we understand Bill’s and his family’s resistance to mental health treatment?
Chinese Americans tend to access mental health services at a lower rate than that of the general U.S. population.3 They also tend to exhibit elevated discontinuation from mental health care, compared with non-Latino whites.4 Since first- and second-generation Chinese Americans have similar use rates, it is likely the barriers to care are not immigration specific but also related to factors that endure across generations, including culture-related aspects. These include cultural concepts of illness and how to interpret prodromal symptoms such as Bill’s, stigma and interpersonal shame regarding mental illness and psychiatric treatment, and value orientations such as self-reliance, avoidance of direct expressions of interpersonal conflict, and family privacy.
For example, Chinese Americans tend to emphasize the physical-symptom components of psychiatric problems, partly because of mind-body holism – which combines physical and psychological symptoms into cultural syndromes and idioms of distress – and partly because of concerns about stigma and shame regarding mental health symptoms. Hence, they are more likely to seek help from primary care clinicians for psychological distress before any mental health provider. Many Chinese Americans interpret mild to moderate psychological distress as “mental weakness” or “excessive thinking” (xiang tai duo), which does not require clinical evaluation. In this view, only alarmingly bizarre or disruptive behaviors warrant formal psychiatric treatment.
Some Chinese parents perceive psychosocial stressors, including hardships, interpersonal conflicts, and academic burdens, as understandable triggers for symptoms that clinicians would classify as schizophrenia or attenuated psychosis syndrome.5 Those views of illness can be associated in the Chinese American community with delayed diagnosis and intervention for emerging psychiatric disorders.
Social stigma regarding mental illness is pervasive in many Chinese American communities. Stigma hinders service use, to avoid shame and save the family’s “face.” Although many Chinese Americans acknowledge the efficacy of biomedical treatments for mental illness, they also remain concerned about enduring shame in their communities if they access those services.6 In line with collectivistic values traditionally held by many Chinese Americans, individuals tend to turn to relatives for help first and keep mental illness confidential within the family group to avoid disgracing the family name.7 Hence, social stigma in the Chinese community can be a key barrier to early detection and early intervention for youth at high risk of psychosis.
Culturally influenced cognitions, emotions, and values also might contribute to underuse of formal mental health services in this population. Many Chinese Americans perceive the roots of mental illness in a lack of willpower and the unwise indulgence of morbid thoughts. In these communities, direct communication of strong emotions can be discouraged – in the name of maintaining harmony, collective interests, and tolerance.8 Hence, many Chinese Americans find Western models of psychotherapy that focus on intrapsychic conflicts and/or intense interpersonal emotions incongruous with their treatment expectations. Psychiatric interview processes that explore gloomy or disturbing thoughts can be perceived as disquieting and antithetical to the goals of treatment. In addition, some individuals rooted in collectivist communities would rather keep personal psychological problems private within the family than seek counseling from an expert who is an out-group stranger.
2. How does Bill’s cultural and social context affect his prognosis?
Individuals with psychosis and their close relatives are generally vulnerable to stigma and discrimination. Mental health stigma has a substantial effect on the lives of patients with psychosis and their family members. The magnitude of the perceived stigma tends to be greater if the patient has more severe positive symptoms, is more educated, or resides in a highly urbanized area.
Acutely ill patients usually face more negative community responses than do milder cases, since their close relatives are blamed for failing to uphold the moral and legal responsibility of ensuring that the patients control their behavior. The effect of stigma in Chinese society also is greater among male patients with early-onset illness, because of the expectation that men marry and become the family breadwinner to attain higher social status. Hence, young males who are unable to achieve these socially determined adult milestones can be considered socially inferior, and suffer more community discrimination and exclusion, which are risk factors for clinical deterioration and functional impairment.
Social stigma can intensify relationship conflicts within the family and magnify expressed emotion (EE), which is defined as caregivers’ attitude toward a person with mental illness as reflected by their comments and interaction patterns. “High EE” comprises three behavioral patterns: criticism, hostility, and emotional overinvolvement. High EE is associated with psychiatric symptom relapse among individuals with schizophrenia and other disorders.9
Currently, most of the literature on EE is limited to white samples in Western countries. Some researchers have studied the relationship between the EE index of emotional overinvolvement and schizophrenia relapse among Hispanic populations.10 However, there are limited data on cultural congruence of EE research in Asian populations. Therefore, clinicians should carefully evaluate the contribution of high EE to Bill’s family’s situation during his course of treatment.
Higher education often is associated with greater levels of EE and can result in anxious and fearful responses to the person’s illness.11 This may be attributable to more negative reactions to actual or feared stigma and discrimination, possibly because relatives feel they have more to lose regarding the family’s social status, especially in densely populated urban areas where it might be harder to keep the patient’s mental illness as a “family secret.”
On the other hand, certain explanatory models of psychosis can modulate Chinese community members’ perceptions and allow ill individuals to remain socially integrated. Cultural idioms such as “excessive thinking” (xiang tai duo), “taking things too hard” (xiang bu kai), and “narrow-mindedness” (xiao xin yan) promote socially accommodating behaviors that facilitate acceptance of mildly to moderately ill individuals as full-status community members.12
Another important contributor to psychosis risk is Bill’s acculturative stress about his cultural identity. Linguistic challenges, limited social support, perceived discrimination, and an acculturation gap between parents and children are major sources of acculturative stress among Chinese American college students.13 Greater acculturative stress elevates the risk of mental illness and symptom deterioration. However, highly acculturated Chinese Americans with above-average bicultural self-efficacy tend to express more positive attitudes toward mental health services.
3. Are there culturally appropriate interventions that can help Bill and his family?
A major predictor among Chinese Americans of the intent to use services is the perceived credibility of the treatment and the provider. Ethnic-specific services staffed by bicultural/bilingual mental health clinicians delivering culturally responsive interventions are increasingly available in many metropolitan areas with major Asian communities. These programs have shown clinical efficacy in encouraging service use and promoting treatment persistence. Bill and his family may benefit from referral to ethnic-specific services, where they can obtain culturally sensitive psychoeducation about his mental illness and treatment plan.
Other services that might be useful for Bill and his family include family psychoeducation programs and supportive groups specifically designed for Chinese American families; these can improve the entire family’s psychosocial health, promote medication adherence, and reduce the risk of symptom relapse through family-centered intervention models.14 Connecting with local National Alliance on Mental Illness (NAMI) programs might help Bill’s parents obtain social support from Chinese American families with similar caregiver experiences.
However, services that are not designed specifically for Chinese-origin patients also can provide excellent care for these patients, and be perceived as credible and effective. A thorough cultural assessment is necessary, as well as inclusion of the information obtained in the treatment plan. As described in the DSM-5 Outline for Cultural Formulation and operationalized in the Cultural Formulation Interview, clinicians should assess possible cultural differences among Bill, his family, his community, and his clinicians regarding their cultural concepts of distress and illness and expectations of care in order to formulate a treatment plan acceptable to patient and family. Practical cultural barriers should be addressed, such as Bill’s parents’ limited English proficiency, in which case a bilingual clinician or trained interpreter should be included in the treatment team.
With Bill’s consent, the treatment team also should consider reaching out to his parents, especially his mother, to understand and empathize with their cultural concepts of distress and illness as well as expectations for care. In addition to providing psychoeducation, the clinicians should validate the parents’ experience of shame, fear, and worry about their son. Bill’s brother, for example, might be a useful bridge in communicating with the parents given his higher acculturation and potentially greater acceptance of psychiatric care. He might help alleviate the tension between Bill and his parents and encourage them to seek family-based help.
Take-home points
- Clinical training programs should offer cultural competency training about underserved populations, including communities of color.
- Certain key concepts, such as traditional idioms of distress and explanatory models, social stigma, and acculturative stress, should be included in these trainings and evaluated in a comprehensive psychosocial assessment.
- High expressed emotion among family caregivers is associated with higher rates of psychiatric symptom relapse, whereas families with above-average bicultural self-efficacy have more positive attitudes toward mental health services.
- Clinicians should incorporate culturally appropriate educational materials (for example, CHR warning signs) and interventions to engage underserved patients and their families in mental health treatment.
Contributors
Emily Wu, MD – Harvard Medical School, Boston
Francis Lu, MD – University of California, Davis
John Sargent, MD – Tufts Medical Center, Boston
Roberto Lewis-Fernández, MD – Columbia College of Physicians & Surgeons, New York
If you would like to a submit case in which your understanding and treatment are affected by challenging cultural and family values, send it to [email protected]. We will then write back with our best answers about how one might proceed in such a case. Your case and our response will then be published at mdedge.com/psychiatry. This column is meant to be educational and does not constitute medical advice. The opinions expressed are those of the contributors and do not represent those of the organizations they are employed by or affiliated with or the Group for the Advancement of Psychiatry.
References
1. J Nerv Ment Dis. 2013 Jun;20(6);484-9.
2. Schizophr Res. 2014 Feb;152(2-3):391-9.
3. Perspect Psychiatr Care. 2013;49(4):288-92.
4. Ment Health Serv Res. 2001 Dec;3(4):201-14.
5. Br J Psychiatry. 2000 Jul;177;20-5.
6. Cultr Divers Ethnic Minor Psychol. 2008 Jan;14(1):10-8.
7. Couns Psychol. 2003 May1;31:343-61.
8. Emotion. 2002 Dec;2(4):341-60.
9. Am J Psychiatry. 1986 Nov;143(11):1361-73.
10. J Nerv Ment Dis. 2013 Oct;201(10):833-40.
11. Schizophr Bull. 1981;7(1):43-4.
12. Schizophr Bull. 2010 Jul;36(4):836-45.
13. Am J Orthopsychiatry. 2011 Oct;81(4):489-97.
14. Patient Educ Couns. 2009 Apr;75(1):67-76.
Editors’ Note: Curbside Consult is an occasional column aimed at helping psychiatrists think through family and cultural considerations when treating patients. It examines case vignettes and is written by two Group for the Advancement of Psychiatry (GAP) committees – the Committee on Family Psychiatry and the Committee on Cultural Psychiatry. The contributors have revised selected patient details to shield the identities of the patients/cases and to comply with HIPAA requirements.
Case vignette
Bill is a 25-year-old man of Chinese descent who sought psychiatric evaluation of his psychosis risk. His parents emigrated from China to Canada more than 30 years ago; Bill was born in Canada, and moved to the United States with his parents and two siblings at age 7.
His family has a strong history of mental illness. His older sister was diagnosed with schizophrenia; she frequently got into verbal altercations with her parents. When Bill was 15, she walked out of the house after a fight with the family and never returned. Bill’s family thinks his father has had paranoid delusions. In the past, he attempted to call the police multiple times because he suspected the neighbors had planted a listening device in his front yard. The family stopped him from making the actual calls. However, the family never brought him to psychiatric evaluation because of perceived stigma and social discrimination in their community. He also was emotionally and physically abusive to Bill during his childhood by calling him names and hitting him with a belt. As an adult, Bill still has posttraumatic stress disorder symptoms including flashbacks, nightmares, and avoidance when thinking about his father.
Bill identifies himself as Chinese American and speaks English only. He often perceives himself as a newcomer to U.S. society, making comments such as: “I guess I should live my life like this to fulfill the American dream.” Bill’s parents placed a strong emphasis on academic success, often at the expense of their children’s social interaction and playtime activities. Bill describes himself as a “loner” with few friends. He maintained high academic achievement during high school and was accepted by a prestigious college. Although he was interested in music composition, Bill was “forced” by his parents to major in courses deemed good preparation for law school.
During college, he suffered severe depression with insomnia, low energy, hopelessness, anhedonia, social withdrawal, poor appetite with weight loss, and ruminative thoughts but without delusional thoughts or perceptual disturbances. He had one near-lethal suicide attempt, during which he impulsively took the contents of an entire bottle of Tylenol in the context of family conflicts, resulting in psychiatric hospitalization. Bill recalled with animosity the inpatient psychiatrists who put him on psychotropic medications during a 3-day hospitalization. He was not adherent to the medication and did not follow up with postdischarge outpatient care. He did not remember the medication trial he had during the hospitalization, nor did he give consent to obtain medical records from there. Bill withdrew from college in sophomore year, because of his declining academic performance secondary to his mental illness. He currently works at a gas station.
Over the last year, Bill’s interpersonal communication has become disorganized in both work and social settings, and he has developed thought blocking, causing him substantial distress. He intermittently hears voices of strangers in the background; these have gradually become more frequent, now occurring 3-4 times a week. Bill also is more depressed, with frequent crying episodes and worsening social isolation. He often thinks that life is not worth living, but he has no active suicidal plans or intent.
Bill’s supervisor and coworkers strongly suggested that he seek medical evaluation. As an outpatient, Bill started weekly cognitive-behavioral therapy (CBT) and biweekly medication sessions for early psychosis symptoms, receiving low-dose risperidone (1 mg b.i.d.) and fluoxetine (20 mg daily). Despite initial improvement, he was very skeptical about continuing the medications because of concern that they will cause a “change in his identity” by altering his body chemistry. His parents have been reluctant to join family meetings, because they were ambivalent about Bill’s ongoing psychiatric treatment.
Treatment team’s impressions
Clinical high risk (CHR) syndrome refers to the prodromal phase before a full psychotic disorder. As one of the three subcategories of CHR, genetic risk and deterioration (GRD) prodromal syndrome is defined by having a genetic risk for psychosis (first-degree relative with a psychotic disorder or meeting criteria for schizotypal personality disorder) and a recent decline in daily functioning equivalent to a 30% drop in Global Assessment of Functioning rating.1 Due to Bill’s family history of psychosis, new difficulties in self-care, psychotic-spectrum symptoms, and declining social/executive function, he meets criteria for GRD prodromal syndrome. In addition, major depressive disorder should be considered on his differential diagnosis.
Bill has not received a diagnosis of acute psychosis, and instead is judged to be in the CHR spectrum for psychosis, because of his level of insight that the occasional perceptual disturbance and abnormal thought content are in his own mind. Since 26% of individuals with CHR in mainland China2 and 35% in the general U.S. population develop fully psychotic symptoms within 2-3 years, Bill’s current presentation warrants secondary preventive care (early intervention) to promote improved clinical outcomes. Given the high rates of comorbid depressive and anxiety disorders among individuals with CHR, Bill’s mood symptoms and passive suicidality also require psychiatric intervention. The treatment team raised three questions, which are answered below.
1. How can we understand Bill’s and his family’s resistance to mental health treatment?
Chinese Americans tend to access mental health services at a lower rate than that of the general U.S. population.3 They also tend to exhibit elevated discontinuation from mental health care, compared with non-Latino whites.4 Since first- and second-generation Chinese Americans have similar use rates, it is likely the barriers to care are not immigration specific but also related to factors that endure across generations, including culture-related aspects. These include cultural concepts of illness and how to interpret prodromal symptoms such as Bill’s, stigma and interpersonal shame regarding mental illness and psychiatric treatment, and value orientations such as self-reliance, avoidance of direct expressions of interpersonal conflict, and family privacy.
For example, Chinese Americans tend to emphasize the physical-symptom components of psychiatric problems, partly because of mind-body holism – which combines physical and psychological symptoms into cultural syndromes and idioms of distress – and partly because of concerns about stigma and shame regarding mental health symptoms. Hence, they are more likely to seek help from primary care clinicians for psychological distress before any mental health provider. Many Chinese Americans interpret mild to moderate psychological distress as “mental weakness” or “excessive thinking” (xiang tai duo), which does not require clinical evaluation. In this view, only alarmingly bizarre or disruptive behaviors warrant formal psychiatric treatment.
Some Chinese parents perceive psychosocial stressors, including hardships, interpersonal conflicts, and academic burdens, as understandable triggers for symptoms that clinicians would classify as schizophrenia or attenuated psychosis syndrome.5 Those views of illness can be associated in the Chinese American community with delayed diagnosis and intervention for emerging psychiatric disorders.
Social stigma regarding mental illness is pervasive in many Chinese American communities. Stigma hinders service use, to avoid shame and save the family’s “face.” Although many Chinese Americans acknowledge the efficacy of biomedical treatments for mental illness, they also remain concerned about enduring shame in their communities if they access those services.6 In line with collectivistic values traditionally held by many Chinese Americans, individuals tend to turn to relatives for help first and keep mental illness confidential within the family group to avoid disgracing the family name.7 Hence, social stigma in the Chinese community can be a key barrier to early detection and early intervention for youth at high risk of psychosis.
Culturally influenced cognitions, emotions, and values also might contribute to underuse of formal mental health services in this population. Many Chinese Americans perceive the roots of mental illness in a lack of willpower and the unwise indulgence of morbid thoughts. In these communities, direct communication of strong emotions can be discouraged – in the name of maintaining harmony, collective interests, and tolerance.8 Hence, many Chinese Americans find Western models of psychotherapy that focus on intrapsychic conflicts and/or intense interpersonal emotions incongruous with their treatment expectations. Psychiatric interview processes that explore gloomy or disturbing thoughts can be perceived as disquieting and antithetical to the goals of treatment. In addition, some individuals rooted in collectivist communities would rather keep personal psychological problems private within the family than seek counseling from an expert who is an out-group stranger.
2. How does Bill’s cultural and social context affect his prognosis?
Individuals with psychosis and their close relatives are generally vulnerable to stigma and discrimination. Mental health stigma has a substantial effect on the lives of patients with psychosis and their family members. The magnitude of the perceived stigma tends to be greater if the patient has more severe positive symptoms, is more educated, or resides in a highly urbanized area.
Acutely ill patients usually face more negative community responses than do milder cases, since their close relatives are blamed for failing to uphold the moral and legal responsibility of ensuring that the patients control their behavior. The effect of stigma in Chinese society also is greater among male patients with early-onset illness, because of the expectation that men marry and become the family breadwinner to attain higher social status. Hence, young males who are unable to achieve these socially determined adult milestones can be considered socially inferior, and suffer more community discrimination and exclusion, which are risk factors for clinical deterioration and functional impairment.
Social stigma can intensify relationship conflicts within the family and magnify expressed emotion (EE), which is defined as caregivers’ attitude toward a person with mental illness as reflected by their comments and interaction patterns. “High EE” comprises three behavioral patterns: criticism, hostility, and emotional overinvolvement. High EE is associated with psychiatric symptom relapse among individuals with schizophrenia and other disorders.9
Currently, most of the literature on EE is limited to white samples in Western countries. Some researchers have studied the relationship between the EE index of emotional overinvolvement and schizophrenia relapse among Hispanic populations.10 However, there are limited data on cultural congruence of EE research in Asian populations. Therefore, clinicians should carefully evaluate the contribution of high EE to Bill’s family’s situation during his course of treatment.
Higher education often is associated with greater levels of EE and can result in anxious and fearful responses to the person’s illness.11 This may be attributable to more negative reactions to actual or feared stigma and discrimination, possibly because relatives feel they have more to lose regarding the family’s social status, especially in densely populated urban areas where it might be harder to keep the patient’s mental illness as a “family secret.”
On the other hand, certain explanatory models of psychosis can modulate Chinese community members’ perceptions and allow ill individuals to remain socially integrated. Cultural idioms such as “excessive thinking” (xiang tai duo), “taking things too hard” (xiang bu kai), and “narrow-mindedness” (xiao xin yan) promote socially accommodating behaviors that facilitate acceptance of mildly to moderately ill individuals as full-status community members.12
Another important contributor to psychosis risk is Bill’s acculturative stress about his cultural identity. Linguistic challenges, limited social support, perceived discrimination, and an acculturation gap between parents and children are major sources of acculturative stress among Chinese American college students.13 Greater acculturative stress elevates the risk of mental illness and symptom deterioration. However, highly acculturated Chinese Americans with above-average bicultural self-efficacy tend to express more positive attitudes toward mental health services.
3. Are there culturally appropriate interventions that can help Bill and his family?
A major predictor among Chinese Americans of the intent to use services is the perceived credibility of the treatment and the provider. Ethnic-specific services staffed by bicultural/bilingual mental health clinicians delivering culturally responsive interventions are increasingly available in many metropolitan areas with major Asian communities. These programs have shown clinical efficacy in encouraging service use and promoting treatment persistence. Bill and his family may benefit from referral to ethnic-specific services, where they can obtain culturally sensitive psychoeducation about his mental illness and treatment plan.
Other services that might be useful for Bill and his family include family psychoeducation programs and supportive groups specifically designed for Chinese American families; these can improve the entire family’s psychosocial health, promote medication adherence, and reduce the risk of symptom relapse through family-centered intervention models.14 Connecting with local National Alliance on Mental Illness (NAMI) programs might help Bill’s parents obtain social support from Chinese American families with similar caregiver experiences.
However, services that are not designed specifically for Chinese-origin patients also can provide excellent care for these patients, and be perceived as credible and effective. A thorough cultural assessment is necessary, as well as inclusion of the information obtained in the treatment plan. As described in the DSM-5 Outline for Cultural Formulation and operationalized in the Cultural Formulation Interview, clinicians should assess possible cultural differences among Bill, his family, his community, and his clinicians regarding their cultural concepts of distress and illness and expectations of care in order to formulate a treatment plan acceptable to patient and family. Practical cultural barriers should be addressed, such as Bill’s parents’ limited English proficiency, in which case a bilingual clinician or trained interpreter should be included in the treatment team.
With Bill’s consent, the treatment team also should consider reaching out to his parents, especially his mother, to understand and empathize with their cultural concepts of distress and illness as well as expectations for care. In addition to providing psychoeducation, the clinicians should validate the parents’ experience of shame, fear, and worry about their son. Bill’s brother, for example, might be a useful bridge in communicating with the parents given his higher acculturation and potentially greater acceptance of psychiatric care. He might help alleviate the tension between Bill and his parents and encourage them to seek family-based help.
Take-home points
- Clinical training programs should offer cultural competency training about underserved populations, including communities of color.
- Certain key concepts, such as traditional idioms of distress and explanatory models, social stigma, and acculturative stress, should be included in these trainings and evaluated in a comprehensive psychosocial assessment.
- High expressed emotion among family caregivers is associated with higher rates of psychiatric symptom relapse, whereas families with above-average bicultural self-efficacy have more positive attitudes toward mental health services.
- Clinicians should incorporate culturally appropriate educational materials (for example, CHR warning signs) and interventions to engage underserved patients and their families in mental health treatment.
Contributors
Emily Wu, MD – Harvard Medical School, Boston
Francis Lu, MD – University of California, Davis
John Sargent, MD – Tufts Medical Center, Boston
Roberto Lewis-Fernández, MD – Columbia College of Physicians & Surgeons, New York
If you would like to a submit case in which your understanding and treatment are affected by challenging cultural and family values, send it to [email protected]. We will then write back with our best answers about how one might proceed in such a case. Your case and our response will then be published at mdedge.com/psychiatry. This column is meant to be educational and does not constitute medical advice. The opinions expressed are those of the contributors and do not represent those of the organizations they are employed by or affiliated with or the Group for the Advancement of Psychiatry.
References
1. J Nerv Ment Dis. 2013 Jun;20(6);484-9.
2. Schizophr Res. 2014 Feb;152(2-3):391-9.
3. Perspect Psychiatr Care. 2013;49(4):288-92.
4. Ment Health Serv Res. 2001 Dec;3(4):201-14.
5. Br J Psychiatry. 2000 Jul;177;20-5.
6. Cultr Divers Ethnic Minor Psychol. 2008 Jan;14(1):10-8.
7. Couns Psychol. 2003 May1;31:343-61.
8. Emotion. 2002 Dec;2(4):341-60.
9. Am J Psychiatry. 1986 Nov;143(11):1361-73.
10. J Nerv Ment Dis. 2013 Oct;201(10):833-40.
11. Schizophr Bull. 1981;7(1):43-4.
12. Schizophr Bull. 2010 Jul;36(4):836-45.
13. Am J Orthopsychiatry. 2011 Oct;81(4):489-97.
14. Patient Educ Couns. 2009 Apr;75(1):67-76.
Severe chronic malnutrition: What it is and how to diagnose it
My wife and I have traveled a number of times as far east as Kyrgyzstan and as far south as Paraguay to participate in short 1- to 2-week medical clinics. When I participated in a week-long medical clinic in Haiti in early 2017, the CEO of the hosting U.S. organization asked, “I wonder if we are doing any good here?” His organization had been to Onaville, Haiti for the last 4-5 years.
So my wife Stacy, a retired licensed practical nurse, and I, a general pediatrician with an interest in severe acute malnutrition, went on a 3-month medical sabbatical to Onaville. We were self-funded, with the exception of our home church in Senoia, Ga., paying the cost of our lodging during that time.
Prior to the 2010 Haiti earthquake, the government planned Onaville to be a retirement community area, with a population of only about 1,500, I was told. After the devastating temblor, it became one of several areas where the government sent people displaced from Port-au-Prince. The population today is possibly 250,000 or more.
The poverty in this area has “newer” flavor than areas such as Cité Soleil, which has been there for decades. What we found in Onaville – and probably all of Haiti – is an appalling lack of understanding and appreciation about the nature of malnutrition.
Methods and materials for study
The 1981 World Health Organization’s last printed monograph about severe acute nutrition remains essentially today’s cookbook recipe for treatment. Little seems to have changed since then in the literature I’ve reviewed. It didn’t take long after we started seeing the children in Onaville to shift that interest to something much more serious and widespread.
I wanted to start with basic health assessments in the Onaville children around 5 years and under. These children rarely see a physician, and only about half or so get any vaccine. Most parents do not have any immunization records in their possession to even review.
We decided to measure head size, mid-upper arm circumference, height, weight, and hemoglobin levels. Date of birth was recorded, if known or could at best be closely estimated. Vaccination was recorded as a yes or no response. All children also were examined for evidence of things like swelling, marasmic appearance (wasting, loss of body fat and muscle), yellowed hair, eye findings of vitamin A deficiency, etc. I wanted to get some impression about the health of these children in the same way that most mobile medical clinics do in Haiti.
Being a database programmer since I bought my first computer in 1985, and having written and deployed my office’s current EMR system in 2000, I decided before ever arriving in Haiti to write the software needed for this task. Unlike regular office EMRs, there were some special considerations.
Growth charts needed not only to be generated for individuals, but in aggregate. Hemoglobins levels, too, needed charting. While in the United States, I use Centers for Disease Control and Prevention growth chart data, but for Haiti I used WHO growth data. I was able to procure hemoglobin charting data as well. Aggregate data turned out to be key to our conclusions.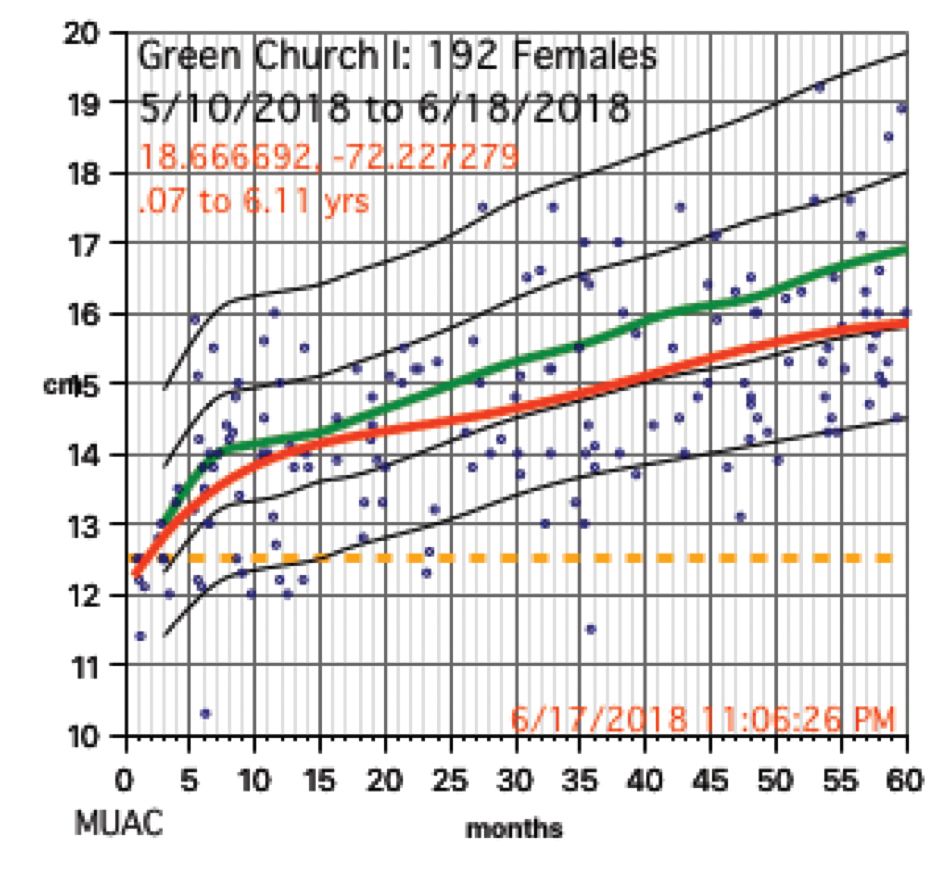
We used a regular consumer quality digital bathroom scale for weights. A sewing tape attached with duct tape to a wall or pillar was used to measure height. Standard head circumference tapes were use to measure heads and arms.
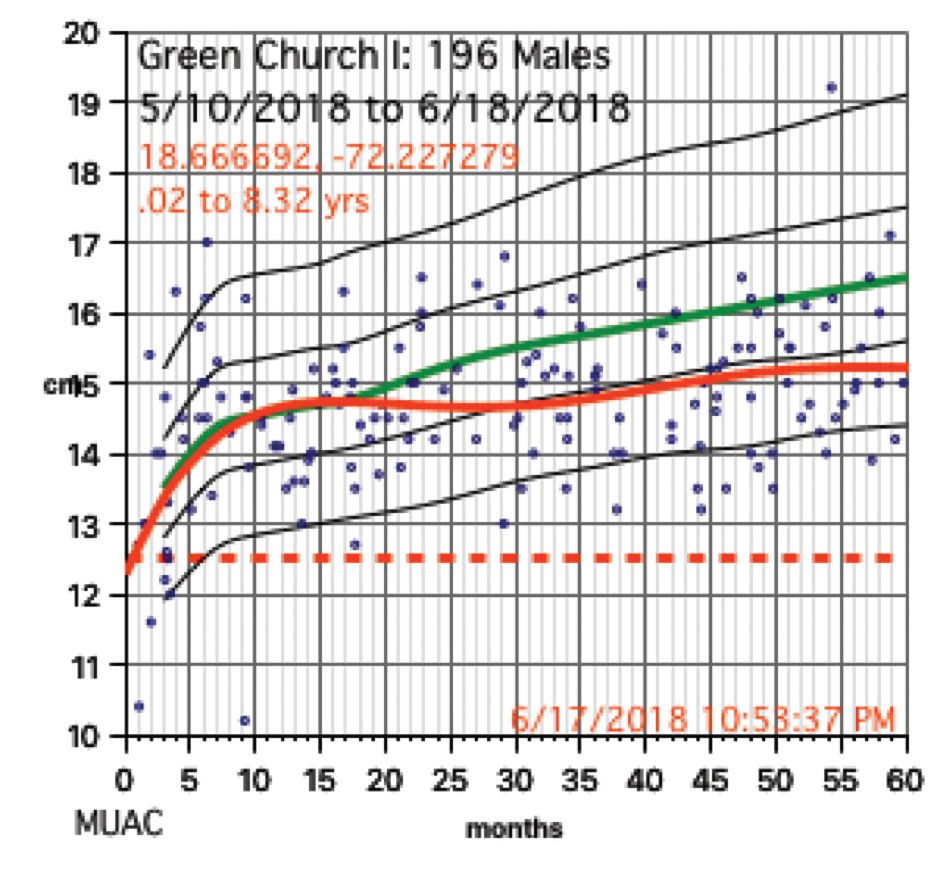
The hemoglobin was measured with a HemoCue Hb 201+ instrument. Size, ruggedness, and cost dictated all our choices because, except for food, we had to carry everything with us. The cost of a new HemoCue was under $400 and each microcuvette test was about $1.50.
Severe anemia
In total, we saw about 386 children, mostly 5 years and under, in Onaville. Toward the end of the 3 months, we were seeing some of those back as follow-ups. One of the first hemoglobins was 4.9 g/dL, with a 5.4 g/dL on repeat. This stunned us. In the first few days, we were seeing what we saw consistently throughout the course of 3 months.
About 19% of these children had hemoglobins from below 9.0 g/dL to below 6 g/dL. More importantly, there was little on physical exam that would trigger one to do a hemoglobin. Low hemoglobins were not associated with yellow-orange hair. No cases of the swelling of kwashiorkor or pencil-like frames of marasmus were seen. 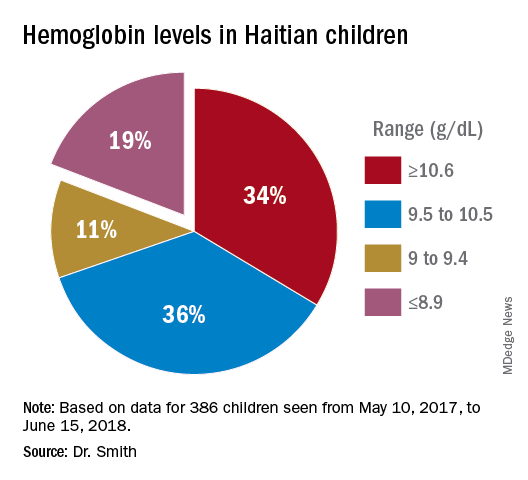
Severe chronic malnutrition
The scatter charts are very telling and the hemoglobin graphs are explosive. What is demonstrated is that this recent population is slowly starving to death. How can the hemoglobins be so very low in comparison to the only slightly lowered mean averages (the solid red line)?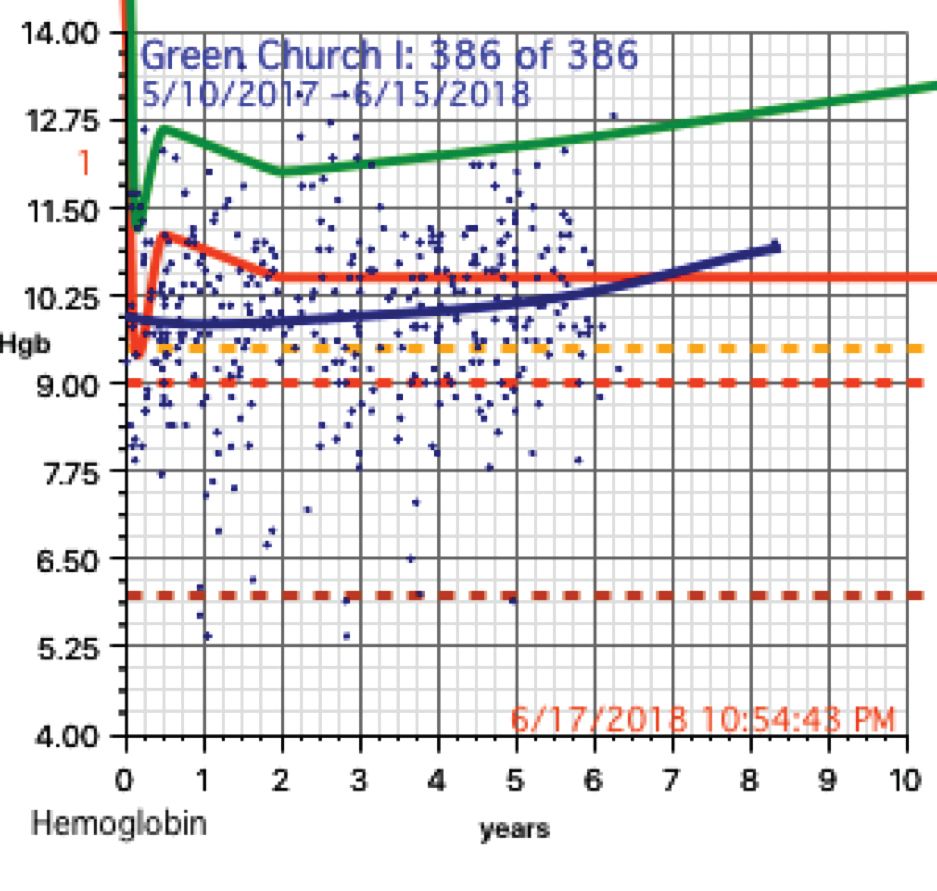
In over 3 decades of pediatric medicine, I rarely have seen children in the United States with hemoglobins below 9.5 g/dL. Often they have other illnesses that clearly point to the cause. Could the 19% of children with severely lowered hemoglobins (below 9.0 g/dL) be caused by sickle cell disease or something else in these Haitian children?
A search for articles where sickle cell was studied revealed a study done at St. Damien Pediatrics Hospital in Port-au-Prince (Blood. 2012;120:4235). The overall incidence of sickle cell disease was this: “Of the 2,258 samples tested, 247 had HbS, fifty-seven had HbC, ten had HbSS, and three had HbSC.” Only 0.57% of these children had sickle or sickle-C disease where one could expect hemoglobins to be as low as in the children of Onaville. Applying that percentage to the 386 children we saw would account for about only 2 children who might have sickling anemia. Yet we had 73 children in our study with severely lowered hemoglobins below 9.0 g/dL. If you estimate that half of the 250,000 people in Onaville are children, that extrapolates to over 47,000 with severe anemia! I think that a study larger than ours needs to be done to better assess that, however.
My best thought is that these children who have little external evidence of abnormality and mildly lowered growth data represent a type of malnutrition that has not been defined, much less addressed. I call this severe chronic malnutrition. The very low hemoglobins indicate to me that this is not simply a lack of iron – although certainly that is a factor – but rather that these children are in a state of chronic protein deprivation. They represent a large pool of children who exist between those with normal nutritional states and those with the kwashiorkor or marasmus of severe acute malnutrition.
A search of the 69,823 ICD-10 codes in my database for “malnutrition” only turns up the ill-defined terms, “Unspecified severe protein-calorie malnutrition,” “Moderate, and Mild protein-calorie malnutrition,” “Unspecified protein-calorie malnutrition,” and “Sequelae of protein-calorie malnutrition.” Whatever each of those means is purely subjective in my opinion.
Without a clear understanding or definition of what is severe chronic malnutrition, we are like the Titanic trying to avoid icebergs on a moonless night. I think we must define severe chronic malnutrition before we really can understand the pathophysiology and treatment of severe acute malnutrition.
The WHO published its last printed monograph, “The treatment and management of severe acute protein-energy malnutrition,” in 1981. This publication is essentially a cookbook approach for what to do, with no clear presentation of the chemical processes and medicine involved. The primary focus for the WHO is mid-upper arm circumference and weight for height. Reading this document might lead one to believe that all malnutrition is acutely severe. It is most certainly not.
Conclusion
The answer to why some children show the swelling of kwashiorkor and some show marasmus probably will not be found in the study of severe acute malnutrition or refeeding syndrome alone. We must go far beyond the WHO’s cookbook recipe.
I think we must start with the study, definition, and treatment of severe chronic malnutrition.
While in Haiti, we shared these data with three organization that are working to provide nutrition in a starving nation. Together, the Baptist Haiti Mission, Mission of Hope Haiti, and Trinity Hope may well be supplying 175,000 meals a day through school lunches and other avenues throughout the country. Their response was telling. Those at Baptist Haiti Mission, an organization with a presence of almost 80 years there, told us that this information was a “big deal.”
The issue for them is the answer to the question, “How can we tell if we are doing any good in our feeding programs?” A lot of money is being thrown into nutrition without tangible ways to assess impact. Clearly parameters such as mid-upper arm circumference and weight for height that WHO advocates is not adequate, as our plots revealed.
We think that a simple, cheap, hemoglobin finger stick can tell us who is falling through the cracks into severe chronic malnutrition and those at risk for severe acute malnutrition. I am an advocate for instituting hemoglobin surveillance as part of all feeding programs. Then we can come up with the cheapest and most effective in-country mechanisms to treat these children.
Indeed that is our next step in working in Haiti.
Dr. Smith is a board certified pediatrician working in McDonough, Ga., with an interest in malnutrition among the children of Haiti. Email him at [email protected].
My wife and I have traveled a number of times as far east as Kyrgyzstan and as far south as Paraguay to participate in short 1- to 2-week medical clinics. When I participated in a week-long medical clinic in Haiti in early 2017, the CEO of the hosting U.S. organization asked, “I wonder if we are doing any good here?” His organization had been to Onaville, Haiti for the last 4-5 years.
So my wife Stacy, a retired licensed practical nurse, and I, a general pediatrician with an interest in severe acute malnutrition, went on a 3-month medical sabbatical to Onaville. We were self-funded, with the exception of our home church in Senoia, Ga., paying the cost of our lodging during that time.
Prior to the 2010 Haiti earthquake, the government planned Onaville to be a retirement community area, with a population of only about 1,500, I was told. After the devastating temblor, it became one of several areas where the government sent people displaced from Port-au-Prince. The population today is possibly 250,000 or more.
The poverty in this area has “newer” flavor than areas such as Cité Soleil, which has been there for decades. What we found in Onaville – and probably all of Haiti – is an appalling lack of understanding and appreciation about the nature of malnutrition.
Methods and materials for study
The 1981 World Health Organization’s last printed monograph about severe acute nutrition remains essentially today’s cookbook recipe for treatment. Little seems to have changed since then in the literature I’ve reviewed. It didn’t take long after we started seeing the children in Onaville to shift that interest to something much more serious and widespread.
I wanted to start with basic health assessments in the Onaville children around 5 years and under. These children rarely see a physician, and only about half or so get any vaccine. Most parents do not have any immunization records in their possession to even review.
We decided to measure head size, mid-upper arm circumference, height, weight, and hemoglobin levels. Date of birth was recorded, if known or could at best be closely estimated. Vaccination was recorded as a yes or no response. All children also were examined for evidence of things like swelling, marasmic appearance (wasting, loss of body fat and muscle), yellowed hair, eye findings of vitamin A deficiency, etc. I wanted to get some impression about the health of these children in the same way that most mobile medical clinics do in Haiti.
Being a database programmer since I bought my first computer in 1985, and having written and deployed my office’s current EMR system in 2000, I decided before ever arriving in Haiti to write the software needed for this task. Unlike regular office EMRs, there were some special considerations.
Growth charts needed not only to be generated for individuals, but in aggregate. Hemoglobins levels, too, needed charting. While in the United States, I use Centers for Disease Control and Prevention growth chart data, but for Haiti I used WHO growth data. I was able to procure hemoglobin charting data as well. Aggregate data turned out to be key to our conclusions.
We used a regular consumer quality digital bathroom scale for weights. A sewing tape attached with duct tape to a wall or pillar was used to measure height. Standard head circumference tapes were use to measure heads and arms.

The hemoglobin was measured with a HemoCue Hb 201+ instrument. Size, ruggedness, and cost dictated all our choices because, except for food, we had to carry everything with us. The cost of a new HemoCue was under $400 and each microcuvette test was about $1.50.
Severe anemia
In total, we saw about 386 children, mostly 5 years and under, in Onaville. Toward the end of the 3 months, we were seeing some of those back as follow-ups. One of the first hemoglobins was 4.9 g/dL, with a 5.4 g/dL on repeat. This stunned us. In the first few days, we were seeing what we saw consistently throughout the course of 3 months.
About 19% of these children had hemoglobins from below 9.0 g/dL to below 6 g/dL. More importantly, there was little on physical exam that would trigger one to do a hemoglobin. Low hemoglobins were not associated with yellow-orange hair. No cases of the swelling of kwashiorkor or pencil-like frames of marasmus were seen. 
Severe chronic malnutrition
The scatter charts are very telling and the hemoglobin graphs are explosive. What is demonstrated is that this recent population is slowly starving to death. How can the hemoglobins be so very low in comparison to the only slightly lowered mean averages (the solid red line)?
In over 3 decades of pediatric medicine, I rarely have seen children in the United States with hemoglobins below 9.5 g/dL. Often they have other illnesses that clearly point to the cause. Could the 19% of children with severely lowered hemoglobins (below 9.0 g/dL) be caused by sickle cell disease or something else in these Haitian children?
A search for articles where sickle cell was studied revealed a study done at St. Damien Pediatrics Hospital in Port-au-Prince (Blood. 2012;120:4235). The overall incidence of sickle cell disease was this: “Of the 2,258 samples tested, 247 had HbS, fifty-seven had HbC, ten had HbSS, and three had HbSC.” Only 0.57% of these children had sickle or sickle-C disease where one could expect hemoglobins to be as low as in the children of Onaville. Applying that percentage to the 386 children we saw would account for about only 2 children who might have sickling anemia. Yet we had 73 children in our study with severely lowered hemoglobins below 9.0 g/dL. If you estimate that half of the 250,000 people in Onaville are children, that extrapolates to over 47,000 with severe anemia! I think that a study larger than ours needs to be done to better assess that, however.
My best thought is that these children who have little external evidence of abnormality and mildly lowered growth data represent a type of malnutrition that has not been defined, much less addressed. I call this severe chronic malnutrition. The very low hemoglobins indicate to me that this is not simply a lack of iron – although certainly that is a factor – but rather that these children are in a state of chronic protein deprivation. They represent a large pool of children who exist between those with normal nutritional states and those with the kwashiorkor or marasmus of severe acute malnutrition.
A search of the 69,823 ICD-10 codes in my database for “malnutrition” only turns up the ill-defined terms, “Unspecified severe protein-calorie malnutrition,” “Moderate, and Mild protein-calorie malnutrition,” “Unspecified protein-calorie malnutrition,” and “Sequelae of protein-calorie malnutrition.” Whatever each of those means is purely subjective in my opinion.
Without a clear understanding or definition of what is severe chronic malnutrition, we are like the Titanic trying to avoid icebergs on a moonless night. I think we must define severe chronic malnutrition before we really can understand the pathophysiology and treatment of severe acute malnutrition.
The WHO published its last printed monograph, “The treatment and management of severe acute protein-energy malnutrition,” in 1981. This publication is essentially a cookbook approach for what to do, with no clear presentation of the chemical processes and medicine involved. The primary focus for the WHO is mid-upper arm circumference and weight for height. Reading this document might lead one to believe that all malnutrition is acutely severe. It is most certainly not.
Conclusion
The answer to why some children show the swelling of kwashiorkor and some show marasmus probably will not be found in the study of severe acute malnutrition or refeeding syndrome alone. We must go far beyond the WHO’s cookbook recipe.
I think we must start with the study, definition, and treatment of severe chronic malnutrition.
While in Haiti, we shared these data with three organization that are working to provide nutrition in a starving nation. Together, the Baptist Haiti Mission, Mission of Hope Haiti, and Trinity Hope may well be supplying 175,000 meals a day through school lunches and other avenues throughout the country. Their response was telling. Those at Baptist Haiti Mission, an organization with a presence of almost 80 years there, told us that this information was a “big deal.”
The issue for them is the answer to the question, “How can we tell if we are doing any good in our feeding programs?” A lot of money is being thrown into nutrition without tangible ways to assess impact. Clearly parameters such as mid-upper arm circumference and weight for height that WHO advocates is not adequate, as our plots revealed.
We think that a simple, cheap, hemoglobin finger stick can tell us who is falling through the cracks into severe chronic malnutrition and those at risk for severe acute malnutrition. I am an advocate for instituting hemoglobin surveillance as part of all feeding programs. Then we can come up with the cheapest and most effective in-country mechanisms to treat these children.
Indeed that is our next step in working in Haiti.
Dr. Smith is a board certified pediatrician working in McDonough, Ga., with an interest in malnutrition among the children of Haiti. Email him at [email protected].
My wife and I have traveled a number of times as far east as Kyrgyzstan and as far south as Paraguay to participate in short 1- to 2-week medical clinics. When I participated in a week-long medical clinic in Haiti in early 2017, the CEO of the hosting U.S. organization asked, “I wonder if we are doing any good here?” His organization had been to Onaville, Haiti for the last 4-5 years.
So my wife Stacy, a retired licensed practical nurse, and I, a general pediatrician with an interest in severe acute malnutrition, went on a 3-month medical sabbatical to Onaville. We were self-funded, with the exception of our home church in Senoia, Ga., paying the cost of our lodging during that time.
Prior to the 2010 Haiti earthquake, the government planned Onaville to be a retirement community area, with a population of only about 1,500, I was told. After the devastating temblor, it became one of several areas where the government sent people displaced from Port-au-Prince. The population today is possibly 250,000 or more.
The poverty in this area has “newer” flavor than areas such as Cité Soleil, which has been there for decades. What we found in Onaville – and probably all of Haiti – is an appalling lack of understanding and appreciation about the nature of malnutrition.
Methods and materials for study
The 1981 World Health Organization’s last printed monograph about severe acute nutrition remains essentially today’s cookbook recipe for treatment. Little seems to have changed since then in the literature I’ve reviewed. It didn’t take long after we started seeing the children in Onaville to shift that interest to something much more serious and widespread.
I wanted to start with basic health assessments in the Onaville children around 5 years and under. These children rarely see a physician, and only about half or so get any vaccine. Most parents do not have any immunization records in their possession to even review.
We decided to measure head size, mid-upper arm circumference, height, weight, and hemoglobin levels. Date of birth was recorded, if known or could at best be closely estimated. Vaccination was recorded as a yes or no response. All children also were examined for evidence of things like swelling, marasmic appearance (wasting, loss of body fat and muscle), yellowed hair, eye findings of vitamin A deficiency, etc. I wanted to get some impression about the health of these children in the same way that most mobile medical clinics do in Haiti.
Being a database programmer since I bought my first computer in 1985, and having written and deployed my office’s current EMR system in 2000, I decided before ever arriving in Haiti to write the software needed for this task. Unlike regular office EMRs, there were some special considerations.
Growth charts needed not only to be generated for individuals, but in aggregate. Hemoglobins levels, too, needed charting. While in the United States, I use Centers for Disease Control and Prevention growth chart data, but for Haiti I used WHO growth data. I was able to procure hemoglobin charting data as well. Aggregate data turned out to be key to our conclusions.
We used a regular consumer quality digital bathroom scale for weights. A sewing tape attached with duct tape to a wall or pillar was used to measure height. Standard head circumference tapes were use to measure heads and arms.

The hemoglobin was measured with a HemoCue Hb 201+ instrument. Size, ruggedness, and cost dictated all our choices because, except for food, we had to carry everything with us. The cost of a new HemoCue was under $400 and each microcuvette test was about $1.50.
Severe anemia
In total, we saw about 386 children, mostly 5 years and under, in Onaville. Toward the end of the 3 months, we were seeing some of those back as follow-ups. One of the first hemoglobins was 4.9 g/dL, with a 5.4 g/dL on repeat. This stunned us. In the first few days, we were seeing what we saw consistently throughout the course of 3 months.
About 19% of these children had hemoglobins from below 9.0 g/dL to below 6 g/dL. More importantly, there was little on physical exam that would trigger one to do a hemoglobin. Low hemoglobins were not associated with yellow-orange hair. No cases of the swelling of kwashiorkor or pencil-like frames of marasmus were seen. 
Severe chronic malnutrition
The scatter charts are very telling and the hemoglobin graphs are explosive. What is demonstrated is that this recent population is slowly starving to death. How can the hemoglobins be so very low in comparison to the only slightly lowered mean averages (the solid red line)?
In over 3 decades of pediatric medicine, I rarely have seen children in the United States with hemoglobins below 9.5 g/dL. Often they have other illnesses that clearly point to the cause. Could the 19% of children with severely lowered hemoglobins (below 9.0 g/dL) be caused by sickle cell disease or something else in these Haitian children?
A search for articles where sickle cell was studied revealed a study done at St. Damien Pediatrics Hospital in Port-au-Prince (Blood. 2012;120:4235). The overall incidence of sickle cell disease was this: “Of the 2,258 samples tested, 247 had HbS, fifty-seven had HbC, ten had HbSS, and three had HbSC.” Only 0.57% of these children had sickle or sickle-C disease where one could expect hemoglobins to be as low as in the children of Onaville. Applying that percentage to the 386 children we saw would account for about only 2 children who might have sickling anemia. Yet we had 73 children in our study with severely lowered hemoglobins below 9.0 g/dL. If you estimate that half of the 250,000 people in Onaville are children, that extrapolates to over 47,000 with severe anemia! I think that a study larger than ours needs to be done to better assess that, however.
My best thought is that these children who have little external evidence of abnormality and mildly lowered growth data represent a type of malnutrition that has not been defined, much less addressed. I call this severe chronic malnutrition. The very low hemoglobins indicate to me that this is not simply a lack of iron – although certainly that is a factor – but rather that these children are in a state of chronic protein deprivation. They represent a large pool of children who exist between those with normal nutritional states and those with the kwashiorkor or marasmus of severe acute malnutrition.
A search of the 69,823 ICD-10 codes in my database for “malnutrition” only turns up the ill-defined terms, “Unspecified severe protein-calorie malnutrition,” “Moderate, and Mild protein-calorie malnutrition,” “Unspecified protein-calorie malnutrition,” and “Sequelae of protein-calorie malnutrition.” Whatever each of those means is purely subjective in my opinion.
Without a clear understanding or definition of what is severe chronic malnutrition, we are like the Titanic trying to avoid icebergs on a moonless night. I think we must define severe chronic malnutrition before we really can understand the pathophysiology and treatment of severe acute malnutrition.
The WHO published its last printed monograph, “The treatment and management of severe acute protein-energy malnutrition,” in 1981. This publication is essentially a cookbook approach for what to do, with no clear presentation of the chemical processes and medicine involved. The primary focus for the WHO is mid-upper arm circumference and weight for height. Reading this document might lead one to believe that all malnutrition is acutely severe. It is most certainly not.
Conclusion
The answer to why some children show the swelling of kwashiorkor and some show marasmus probably will not be found in the study of severe acute malnutrition or refeeding syndrome alone. We must go far beyond the WHO’s cookbook recipe.
I think we must start with the study, definition, and treatment of severe chronic malnutrition.
While in Haiti, we shared these data with three organization that are working to provide nutrition in a starving nation. Together, the Baptist Haiti Mission, Mission of Hope Haiti, and Trinity Hope may well be supplying 175,000 meals a day through school lunches and other avenues throughout the country. Their response was telling. Those at Baptist Haiti Mission, an organization with a presence of almost 80 years there, told us that this information was a “big deal.”
The issue for them is the answer to the question, “How can we tell if we are doing any good in our feeding programs?” A lot of money is being thrown into nutrition without tangible ways to assess impact. Clearly parameters such as mid-upper arm circumference and weight for height that WHO advocates is not adequate, as our plots revealed.
We think that a simple, cheap, hemoglobin finger stick can tell us who is falling through the cracks into severe chronic malnutrition and those at risk for severe acute malnutrition. I am an advocate for instituting hemoglobin surveillance as part of all feeding programs. Then we can come up with the cheapest and most effective in-country mechanisms to treat these children.
Indeed that is our next step in working in Haiti.
Dr. Smith is a board certified pediatrician working in McDonough, Ga., with an interest in malnutrition among the children of Haiti. Email him at [email protected].
November 2018
Desmoplastic trichilemmoma
that presents as a solitary, skin colored lesion on the midface. Lesions may appear smooth or verrucous. Lesions may occur alongside trichoepitheliomas. They may also occur on genital skin and resemble condyloma acuminata.
Histopathology reveals downward lobular growth of the epidermis. Keratinocytes are clear secondary to periodic acid-Schiff (PAS)–positive glycogen in the cells. In desmoplastic trichilemmoma, small clusters of cells are arranged in an infiltrative pattern that resembles invasive carcinoma. Often, the desmoplastic areas are surrounded by benign-appearing trichilemmomas, which helps to make the diagnosis. Desmoplastic trichilemmomas can also occur within nevus sebaceous. As trichilemmoma is a benign growth; no treatment is needed. However, if further removal is desired, electrodesiccation, cryotherapy, shave removal, or excision are treatment options. Rarely seen, the malignant counterpart to trichilemmomas is a trichilemmal carcinoma, which requires surgical excision or Mohs.
The appearance of multiple trichilemmomas is a marker for Cowden syndrome. Cowden syndrome is a rare autosomal dominant disorder in which there is a mutation in a tumor-suppressor gene called PTEN. Patients may have oral mucosal papillomas, sclerotic fibromas, acral keratotic papules, and are at risk for the development of adenocarcinoma of the breast, gastrointestinal tract, and thyroid.
Trichoepithelioma is a benign neoplasm derived from follicular germ cells that presents as a skin-colored papule on the midface, especially the nose. Multiple trichoepitheliomas are a marker for Brooke-Spiegler syndrome. A desmoplastic trichoepithelioma is a variant that has stromal sclerosis on pathology. It is a benign lesion, although may be difficult to differentiate from sclerosing basal cell or microcystic adnexal carcinoma.
Angiofibroma, or fibrous papule, is a commonly seen, benign, skin-colored papule also often occurring on the nose. They can be treated for cosmetic purposes. Multiple lesions are associated with tuberous sclerosis.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Desmoplastic trichilemmoma
that presents as a solitary, skin colored lesion on the midface. Lesions may appear smooth or verrucous. Lesions may occur alongside trichoepitheliomas. They may also occur on genital skin and resemble condyloma acuminata.
Histopathology reveals downward lobular growth of the epidermis. Keratinocytes are clear secondary to periodic acid-Schiff (PAS)–positive glycogen in the cells. In desmoplastic trichilemmoma, small clusters of cells are arranged in an infiltrative pattern that resembles invasive carcinoma. Often, the desmoplastic areas are surrounded by benign-appearing trichilemmomas, which helps to make the diagnosis. Desmoplastic trichilemmomas can also occur within nevus sebaceous. As trichilemmoma is a benign growth; no treatment is needed. However, if further removal is desired, electrodesiccation, cryotherapy, shave removal, or excision are treatment options. Rarely seen, the malignant counterpart to trichilemmomas is a trichilemmal carcinoma, which requires surgical excision or Mohs.
The appearance of multiple trichilemmomas is a marker for Cowden syndrome. Cowden syndrome is a rare autosomal dominant disorder in which there is a mutation in a tumor-suppressor gene called PTEN. Patients may have oral mucosal papillomas, sclerotic fibromas, acral keratotic papules, and are at risk for the development of adenocarcinoma of the breast, gastrointestinal tract, and thyroid.
Trichoepithelioma is a benign neoplasm derived from follicular germ cells that presents as a skin-colored papule on the midface, especially the nose. Multiple trichoepitheliomas are a marker for Brooke-Spiegler syndrome. A desmoplastic trichoepithelioma is a variant that has stromal sclerosis on pathology. It is a benign lesion, although may be difficult to differentiate from sclerosing basal cell or microcystic adnexal carcinoma.
Angiofibroma, or fibrous papule, is a commonly seen, benign, skin-colored papule also often occurring on the nose. They can be treated for cosmetic purposes. Multiple lesions are associated with tuberous sclerosis.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Desmoplastic trichilemmoma
that presents as a solitary, skin colored lesion on the midface. Lesions may appear smooth or verrucous. Lesions may occur alongside trichoepitheliomas. They may also occur on genital skin and resemble condyloma acuminata.
Histopathology reveals downward lobular growth of the epidermis. Keratinocytes are clear secondary to periodic acid-Schiff (PAS)–positive glycogen in the cells. In desmoplastic trichilemmoma, small clusters of cells are arranged in an infiltrative pattern that resembles invasive carcinoma. Often, the desmoplastic areas are surrounded by benign-appearing trichilemmomas, which helps to make the diagnosis. Desmoplastic trichilemmomas can also occur within nevus sebaceous. As trichilemmoma is a benign growth; no treatment is needed. However, if further removal is desired, electrodesiccation, cryotherapy, shave removal, or excision are treatment options. Rarely seen, the malignant counterpart to trichilemmomas is a trichilemmal carcinoma, which requires surgical excision or Mohs.
The appearance of multiple trichilemmomas is a marker for Cowden syndrome. Cowden syndrome is a rare autosomal dominant disorder in which there is a mutation in a tumor-suppressor gene called PTEN. Patients may have oral mucosal papillomas, sclerotic fibromas, acral keratotic papules, and are at risk for the development of adenocarcinoma of the breast, gastrointestinal tract, and thyroid.
Trichoepithelioma is a benign neoplasm derived from follicular germ cells that presents as a skin-colored papule on the midface, especially the nose. Multiple trichoepitheliomas are a marker for Brooke-Spiegler syndrome. A desmoplastic trichoepithelioma is a variant that has stromal sclerosis on pathology. It is a benign lesion, although may be difficult to differentiate from sclerosing basal cell or microcystic adnexal carcinoma.
Angiofibroma, or fibrous papule, is a commonly seen, benign, skin-colored papule also often occurring on the nose. They can be treated for cosmetic purposes. Multiple lesions are associated with tuberous sclerosis.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Overcoming social media’s false narratives; using fitness to fight addictions
For most people, life is a roller coaster of satisfaction and challenge. And in the midst of days filled with the latter, the social media chronicles of someone’s seemingly perfect life can set the teeth on edge. But should seeing those adventures from afar generate feelings of envy and self-loathing?
No, argues a piece written in The Guardian. Social media has created a world in which everyone seems ecstatic – apart from us.
Is there any way for people to curb their resentment? Yes, said Ethan Kross, PhD, professor of psychology at the University of Michigan, Ann Arbor, who studies Facebook’s impact on well-being. Interviewed for The Guardian article, Dr. Kross remarks that “envy is being taken to an extreme. We are constantly bombarded by ‘photoshopped lives’ and that exerts a toll on us the likes of which we have never experienced in the history of our species. And it is not particularly pleasant.”
Negotiating the era of envy requires a conscious effort to not compare one’s life with those of others, especially since their social presence may choose to gloss over their real-life troubles. Heavy lifting to boost personal self-esteem can be beneficial.
But these steps are far easier in theory than in practice. “ – we know that these images and narratives that are presented aren’t real, we can talk about it and rationalize it – but on an emotional level, it’s still pushing buttons,” clinical psychologist Rachel Andrew, ClinPsyD, said in the article. “If those images or narratives tap into what we aspire to, but what we don’t have, then it becomes very powerful.”
Gym seeks to help people stay in recovery
The world for those who are trying to rid themselves of substance use/addiction can be a fragile place. Having support can be the difference between a new clear-headed life and the slide back to darkness. For people with addictions in several U.S. cities, community gyms that operate under the moniker “The Phoenix” can be help.
The Phoenix was started by Scott Strode as a way to help people generate some sweat to stay sober. He has been sober for 21 years. There are no initiation fees to join and no monthly dues; funding comes from donations and grants. The absence of a financial burden comes with the requirements of 48 hours of sobriety, and the desire for that to continue.
The 14 Phoenix gyms in the United States have helped an estimated 26,000 people with their recovery.
“The hardest part about coming to Phoenix is opening the front door. But we’ve removed all those other barriers to access. Because it’s free, it doesn’t matter what insurance you have or how much money is in the bank account or what your addiction story is,” Mr. Strode said in an interview with “CBS This Morning: Saturday.”
Dana Smith has been sober for 9 years. Her introduction to The Phoenix was in prison, serving a sentence for a fatal traffic accident she caused while driving drunk. When asked by the interviewer how she lives with the reality that she took a life, Ms. Smith replies: “That’s another reason it was so important for me to come to Phoenix. I knew that I needed to be in a place where I felt comfortable talking about it. And I felt open and able to share ... I can help others ... and I have to listen the way that people listened for me and the way that people helped me to heal.”
Mr. Strode still burns with passion about the importance of The Phoenix. “For me, getting out of my addiction was like getting out of a burning building. And I just don’t feel like I can walk away if I know people are still in there,” he said.
Success vs. happiness: An illusion?
Harvard University academic Todd Rose, EdD, has taken on the idea that we can be happy or successful, but not both. In the book, “Dark Horse: Achieving Success Through the Pursuit of Fulfillment,” Dr. Rose and his coauthor Ogi Ogas, PhD, posit that striving for personal fulfillment can generate career success, and that this success does not come at the expense of happiness.
“For most of us, when we think about success, it’s pretty narrow, and we end up thinking about things like wealth, status, power. And we sort of think that you have to choose between that and being happy – and dark horses show us that you actually don’t have to choose,” Dr. Rose said in an interview on “CBS This Morning.”
There was a time when Dr. Rose was a young father on welfare with a bleak outlook. Following his father’s advice to find his motivation and pursue it changed his life.
“Think about the things that you enjoy doing and ask yourself why. ... The more you think about those things, the more you know what really moves you. And if you ask yourself that question often enough, it will reveal your broader motives and that will put you on a path to fulfillment,” Dr. Rose said.
The same advice goes for parents trying to counsel their children about career choices. “But if you think about us as parents, we actually don’t ask our kids (what motivates them) very often. We spend a lot of time telling them what should matter and very little time helping them figure it out for themselves,” Dr. Rose said. “They need to figure out what really matters to them and what motivates them, and we can help them by asking.”
Healthy elders break stereotypes
Medical care is focused on helping patients get better. Another aspect of medical care – keeping healthy people healthy – is not always high on the learning agenda. But at more than 20 medical schools in the United States, second-year students are getting another perspective on health care from healthy seniors.
Eighty two-year-old Elizabeth Shepherd is a participant in the program being offered to medical students at Cornell University in New York City. Ms. Shepherd acquaints the students with her everyday life, which includes the occasional fall, dealing with macular degeneration, and the desire for more sexual activity. “It’s important that they don’t think life stops as you get older,” Ms. Shepherd said in an interview with The New York Times. “So I decided I would be frank with them.”
The program can help re-jig the sometimes distorted view that med students have of older adults. “Unfortunately, most education takes place within the hospital,” said Ronald D. Adelman, MD, who developed the program at Cornell. “If you’re only seeing the hospitalized elderly, you’re seeing the debilitated, the physically deteriorating, the demented. It’s easy to pick up ageist stereotypes.”
A powerful take-home message for the students is that all people are worth treating, regardless of age.
Family separations worse than thought
The trauma of the separation of children from family members seeking to enter the United States from Mexico and countries farther south is undeniable. Now, as reported in Mother Jones, Amnesty International indicates far more families than officially tallied have been separated.
“The Trump administration is waging a deliberate campaign of widespread human rights violations in order to punish and deter people seeking safety at the U.S.-Mexico border,” Erika Guevara-Rosas, the Americas director at Amnesty International, said in a statement.
The American Psychiatric Association has called for an end to the policy on mental health grounds. “Children depend on their parents for safety and support. Any forced separation is highly stressful for children and can cause lifelong trauma, as well as an increased risk of other mental illnesses, such as depression, anxiety, and posttraumatic stress disorder. The evidence is clear that this level of trauma also results in serious medical and health consequences for these children and their caregivers,” according to the APA statement.
Compounding the trauma, if a child’s parents are deported while the child is in detention, the child could be put up for adoption without notification of the parents. Reports of abuse of children at some detention centers have heightened criticism of the policy.
For most people, life is a roller coaster of satisfaction and challenge. And in the midst of days filled with the latter, the social media chronicles of someone’s seemingly perfect life can set the teeth on edge. But should seeing those adventures from afar generate feelings of envy and self-loathing?
No, argues a piece written in The Guardian. Social media has created a world in which everyone seems ecstatic – apart from us.
Is there any way for people to curb their resentment? Yes, said Ethan Kross, PhD, professor of psychology at the University of Michigan, Ann Arbor, who studies Facebook’s impact on well-being. Interviewed for The Guardian article, Dr. Kross remarks that “envy is being taken to an extreme. We are constantly bombarded by ‘photoshopped lives’ and that exerts a toll on us the likes of which we have never experienced in the history of our species. And it is not particularly pleasant.”
Negotiating the era of envy requires a conscious effort to not compare one’s life with those of others, especially since their social presence may choose to gloss over their real-life troubles. Heavy lifting to boost personal self-esteem can be beneficial.
But these steps are far easier in theory than in practice. “ – we know that these images and narratives that are presented aren’t real, we can talk about it and rationalize it – but on an emotional level, it’s still pushing buttons,” clinical psychologist Rachel Andrew, ClinPsyD, said in the article. “If those images or narratives tap into what we aspire to, but what we don’t have, then it becomes very powerful.”
Gym seeks to help people stay in recovery
The world for those who are trying to rid themselves of substance use/addiction can be a fragile place. Having support can be the difference between a new clear-headed life and the slide back to darkness. For people with addictions in several U.S. cities, community gyms that operate under the moniker “The Phoenix” can be help.
The Phoenix was started by Scott Strode as a way to help people generate some sweat to stay sober. He has been sober for 21 years. There are no initiation fees to join and no monthly dues; funding comes from donations and grants. The absence of a financial burden comes with the requirements of 48 hours of sobriety, and the desire for that to continue.
The 14 Phoenix gyms in the United States have helped an estimated 26,000 people with their recovery.
“The hardest part about coming to Phoenix is opening the front door. But we’ve removed all those other barriers to access. Because it’s free, it doesn’t matter what insurance you have or how much money is in the bank account or what your addiction story is,” Mr. Strode said in an interview with “CBS This Morning: Saturday.”
Dana Smith has been sober for 9 years. Her introduction to The Phoenix was in prison, serving a sentence for a fatal traffic accident she caused while driving drunk. When asked by the interviewer how she lives with the reality that she took a life, Ms. Smith replies: “That’s another reason it was so important for me to come to Phoenix. I knew that I needed to be in a place where I felt comfortable talking about it. And I felt open and able to share ... I can help others ... and I have to listen the way that people listened for me and the way that people helped me to heal.”
Mr. Strode still burns with passion about the importance of The Phoenix. “For me, getting out of my addiction was like getting out of a burning building. And I just don’t feel like I can walk away if I know people are still in there,” he said.
Success vs. happiness: An illusion?
Harvard University academic Todd Rose, EdD, has taken on the idea that we can be happy or successful, but not both. In the book, “Dark Horse: Achieving Success Through the Pursuit of Fulfillment,” Dr. Rose and his coauthor Ogi Ogas, PhD, posit that striving for personal fulfillment can generate career success, and that this success does not come at the expense of happiness.
“For most of us, when we think about success, it’s pretty narrow, and we end up thinking about things like wealth, status, power. And we sort of think that you have to choose between that and being happy – and dark horses show us that you actually don’t have to choose,” Dr. Rose said in an interview on “CBS This Morning.”
There was a time when Dr. Rose was a young father on welfare with a bleak outlook. Following his father’s advice to find his motivation and pursue it changed his life.
“Think about the things that you enjoy doing and ask yourself why. ... The more you think about those things, the more you know what really moves you. And if you ask yourself that question often enough, it will reveal your broader motives and that will put you on a path to fulfillment,” Dr. Rose said.
The same advice goes for parents trying to counsel their children about career choices. “But if you think about us as parents, we actually don’t ask our kids (what motivates them) very often. We spend a lot of time telling them what should matter and very little time helping them figure it out for themselves,” Dr. Rose said. “They need to figure out what really matters to them and what motivates them, and we can help them by asking.”
Healthy elders break stereotypes
Medical care is focused on helping patients get better. Another aspect of medical care – keeping healthy people healthy – is not always high on the learning agenda. But at more than 20 medical schools in the United States, second-year students are getting another perspective on health care from healthy seniors.
Eighty two-year-old Elizabeth Shepherd is a participant in the program being offered to medical students at Cornell University in New York City. Ms. Shepherd acquaints the students with her everyday life, which includes the occasional fall, dealing with macular degeneration, and the desire for more sexual activity. “It’s important that they don’t think life stops as you get older,” Ms. Shepherd said in an interview with The New York Times. “So I decided I would be frank with them.”
The program can help re-jig the sometimes distorted view that med students have of older adults. “Unfortunately, most education takes place within the hospital,” said Ronald D. Adelman, MD, who developed the program at Cornell. “If you’re only seeing the hospitalized elderly, you’re seeing the debilitated, the physically deteriorating, the demented. It’s easy to pick up ageist stereotypes.”
A powerful take-home message for the students is that all people are worth treating, regardless of age.
Family separations worse than thought
The trauma of the separation of children from family members seeking to enter the United States from Mexico and countries farther south is undeniable. Now, as reported in Mother Jones, Amnesty International indicates far more families than officially tallied have been separated.
“The Trump administration is waging a deliberate campaign of widespread human rights violations in order to punish and deter people seeking safety at the U.S.-Mexico border,” Erika Guevara-Rosas, the Americas director at Amnesty International, said in a statement.
The American Psychiatric Association has called for an end to the policy on mental health grounds. “Children depend on their parents for safety and support. Any forced separation is highly stressful for children and can cause lifelong trauma, as well as an increased risk of other mental illnesses, such as depression, anxiety, and posttraumatic stress disorder. The evidence is clear that this level of trauma also results in serious medical and health consequences for these children and their caregivers,” according to the APA statement.
Compounding the trauma, if a child’s parents are deported while the child is in detention, the child could be put up for adoption without notification of the parents. Reports of abuse of children at some detention centers have heightened criticism of the policy.
For most people, life is a roller coaster of satisfaction and challenge. And in the midst of days filled with the latter, the social media chronicles of someone’s seemingly perfect life can set the teeth on edge. But should seeing those adventures from afar generate feelings of envy and self-loathing?
No, argues a piece written in The Guardian. Social media has created a world in which everyone seems ecstatic – apart from us.
Is there any way for people to curb their resentment? Yes, said Ethan Kross, PhD, professor of psychology at the University of Michigan, Ann Arbor, who studies Facebook’s impact on well-being. Interviewed for The Guardian article, Dr. Kross remarks that “envy is being taken to an extreme. We are constantly bombarded by ‘photoshopped lives’ and that exerts a toll on us the likes of which we have never experienced in the history of our species. And it is not particularly pleasant.”
Negotiating the era of envy requires a conscious effort to not compare one’s life with those of others, especially since their social presence may choose to gloss over their real-life troubles. Heavy lifting to boost personal self-esteem can be beneficial.
But these steps are far easier in theory than in practice. “ – we know that these images and narratives that are presented aren’t real, we can talk about it and rationalize it – but on an emotional level, it’s still pushing buttons,” clinical psychologist Rachel Andrew, ClinPsyD, said in the article. “If those images or narratives tap into what we aspire to, but what we don’t have, then it becomes very powerful.”
Gym seeks to help people stay in recovery
The world for those who are trying to rid themselves of substance use/addiction can be a fragile place. Having support can be the difference between a new clear-headed life and the slide back to darkness. For people with addictions in several U.S. cities, community gyms that operate under the moniker “The Phoenix” can be help.
The Phoenix was started by Scott Strode as a way to help people generate some sweat to stay sober. He has been sober for 21 years. There are no initiation fees to join and no monthly dues; funding comes from donations and grants. The absence of a financial burden comes with the requirements of 48 hours of sobriety, and the desire for that to continue.
The 14 Phoenix gyms in the United States have helped an estimated 26,000 people with their recovery.
“The hardest part about coming to Phoenix is opening the front door. But we’ve removed all those other barriers to access. Because it’s free, it doesn’t matter what insurance you have or how much money is in the bank account or what your addiction story is,” Mr. Strode said in an interview with “CBS This Morning: Saturday.”
Dana Smith has been sober for 9 years. Her introduction to The Phoenix was in prison, serving a sentence for a fatal traffic accident she caused while driving drunk. When asked by the interviewer how she lives with the reality that she took a life, Ms. Smith replies: “That’s another reason it was so important for me to come to Phoenix. I knew that I needed to be in a place where I felt comfortable talking about it. And I felt open and able to share ... I can help others ... and I have to listen the way that people listened for me and the way that people helped me to heal.”
Mr. Strode still burns with passion about the importance of The Phoenix. “For me, getting out of my addiction was like getting out of a burning building. And I just don’t feel like I can walk away if I know people are still in there,” he said.
Success vs. happiness: An illusion?
Harvard University academic Todd Rose, EdD, has taken on the idea that we can be happy or successful, but not both. In the book, “Dark Horse: Achieving Success Through the Pursuit of Fulfillment,” Dr. Rose and his coauthor Ogi Ogas, PhD, posit that striving for personal fulfillment can generate career success, and that this success does not come at the expense of happiness.
“For most of us, when we think about success, it’s pretty narrow, and we end up thinking about things like wealth, status, power. And we sort of think that you have to choose between that and being happy – and dark horses show us that you actually don’t have to choose,” Dr. Rose said in an interview on “CBS This Morning.”
There was a time when Dr. Rose was a young father on welfare with a bleak outlook. Following his father’s advice to find his motivation and pursue it changed his life.
“Think about the things that you enjoy doing and ask yourself why. ... The more you think about those things, the more you know what really moves you. And if you ask yourself that question often enough, it will reveal your broader motives and that will put you on a path to fulfillment,” Dr. Rose said.
The same advice goes for parents trying to counsel their children about career choices. “But if you think about us as parents, we actually don’t ask our kids (what motivates them) very often. We spend a lot of time telling them what should matter and very little time helping them figure it out for themselves,” Dr. Rose said. “They need to figure out what really matters to them and what motivates them, and we can help them by asking.”
Healthy elders break stereotypes
Medical care is focused on helping patients get better. Another aspect of medical care – keeping healthy people healthy – is not always high on the learning agenda. But at more than 20 medical schools in the United States, second-year students are getting another perspective on health care from healthy seniors.
Eighty two-year-old Elizabeth Shepherd is a participant in the program being offered to medical students at Cornell University in New York City. Ms. Shepherd acquaints the students with her everyday life, which includes the occasional fall, dealing with macular degeneration, and the desire for more sexual activity. “It’s important that they don’t think life stops as you get older,” Ms. Shepherd said in an interview with The New York Times. “So I decided I would be frank with them.”
The program can help re-jig the sometimes distorted view that med students have of older adults. “Unfortunately, most education takes place within the hospital,” said Ronald D. Adelman, MD, who developed the program at Cornell. “If you’re only seeing the hospitalized elderly, you’re seeing the debilitated, the physically deteriorating, the demented. It’s easy to pick up ageist stereotypes.”
A powerful take-home message for the students is that all people are worth treating, regardless of age.
Family separations worse than thought
The trauma of the separation of children from family members seeking to enter the United States from Mexico and countries farther south is undeniable. Now, as reported in Mother Jones, Amnesty International indicates far more families than officially tallied have been separated.
“The Trump administration is waging a deliberate campaign of widespread human rights violations in order to punish and deter people seeking safety at the U.S.-Mexico border,” Erika Guevara-Rosas, the Americas director at Amnesty International, said in a statement.
The American Psychiatric Association has called for an end to the policy on mental health grounds. “Children depend on their parents for safety and support. Any forced separation is highly stressful for children and can cause lifelong trauma, as well as an increased risk of other mental illnesses, such as depression, anxiety, and posttraumatic stress disorder. The evidence is clear that this level of trauma also results in serious medical and health consequences for these children and their caregivers,” according to the APA statement.
Compounding the trauma, if a child’s parents are deported while the child is in detention, the child could be put up for adoption without notification of the parents. Reports of abuse of children at some detention centers have heightened criticism of the policy.
Book Review: DuPont’s approach to addiction is tough, yet compassionate
What do Queen Silvia of Sweden, Pope Francis, and the first director of the National Institute on Drug Abuse (NIDA) have in common? They all share a deep and sobering commitment to fighting the global disease of addiction.
Robert L. DuPont, MD, has written a beautiful and surprisingly spiritual guide into the American addiction epidemic. He is the author of “The Selfish Brain: Learning From Addiction” (Center City, Minn.: Hazelden, 2000) and with his newest publication, “Chemical Slavery: Understanding Addiction and Stopping the Drug Epidemic” (Institute for Behavior and Health, 2018), he writes a clear-eyed tome detailing the history of drug and alcohol use within the United States and the current state of America’s drug epidemic.
Dr. DuPont is well known within the American addiction community as NIDA’s first director and as the second drug czar, under two presidents, Richard M. Nixon and Gerald R. Ford. His breadth of experience, spanning 50-plus years, dates from his early career with the District of Columbia Department of Corrections, into his work in public policy on drugs and alcohol. This experience infuses his book with the hard science of addiction and the common-sense compassion required to shepherd people into recovery. One of us has worked with and been influenced by him since the 1970s. The other, also an addiction psychiatrist, finished this book both invigorated and compelled to say thank you to Dr. DuPont and his life’s work! He remains relevant, insightful, and always optimistic about the future of addiction treatment.
Harm reduction explored
The book begins with a cogent history of drug and alcohol use in America. Dr. DuPont details this as well as the public policies that have evolved to address them. He weaves into our national history reasoning behind why, as a “mass consumer” culture, we are more prone to addiction than ever before. The loss of cultural and societal pressures has a role to play in the rise along with genetics and environmental stress. The adolescent brain is prominently discussed throughout this first section as a highly vulnerable organ that can lead to lifelong addiction if primed early by addictive chemicals.
Dr. DuPont addresses the biology of addiction, delving into both the biological mechanisms within the brain, making those details accessible and understandable – to a practicing physician as well as a family member or patient struggling with addiction.
He also addresses harm reduction, a fairly new concept within the field. Harm reduction has taken on more prominence with localities across the country providing people with addictions with safe places and clean needles to continue their substance use without risks of serious or life-threatening diseases and crime. He challenges the idea that harm reduction is active recovery from substance addiction. Instead, he opines that harm reduction must be tethered to and must lead to real recovery work or it risks becoming an organizational enabling of the addict’s behavior.
Dr. DuPont pulls no punches with his language. He uses words such as “fatal,” “addict,” and “alcoholic.” He addresses the concerns by some in the field that those kinds of words are harsh, derogatory, and prejudicial by calling out addiction as a disease hallmarked primarily by loss of control and by dishonesty. To shun those words is to perpetuate the disease, delaying life-saving treatment.
Compassion is a theme throughout. He says we must stigmatize the addiction but not the addict. He advocates for real consequences to addictive behaviors as a key to getting addicted physicians and others with this disease into recovery. Treatment works, but we also have studied specific approaches that work and why.1 His writing conveys a genuine empathy for his patients and argues that treatment is delayed when serious and negative consequences for patients are removed.
Focus on prevention
A clear passion for prevention is evident within his chapters on youth addiction. For adolescents, Dr. DuPont presents a “One Choice approach,” which requires complete abstinence from alcohol, tobacco, and marijuana. He also presents science showing that patients younger than age 21 will have a greater risk of developing lifelong and debilitating addictions if they use these chemicals prior to this age. His emphasis for the One Choice approach carries ramifications throughout the primary care, pediatric, and family practice communities.
Dr. DuPont is nothing if not an optimist. Though he clearly defines addiction as an often-fatal disease, he remains positive about the future of addiction treatment, both with changes in public policy and with advancements in the medicine of addiction. He makes a compelling argument regarding Sweden’s approach to the drug problem, citing Queen Silvia’s lifelong commitment to prevent, control, and treat drug and alcohol addiction. Sweden’s model is, indeed, intriguing, and that country’s outcomes present a strong argument for the marriage of the criminal justice system with medical intervention – an approach that the United States has adopted only in a patchwork fashion.
The book describes controversies throughout, such as Dr. DuPont’s furtherance of our work on how best to treat dual disorders.2 He is not impressed with the self-medication hypothesis as well as the dive into the U.S. national medical marijuana experiment. We had looked at college students having new-onset memory or attention-deficit/hyperactivity problems, only to find that it was likely psychostimulant seeking to reverse marijuana effects.3 Physicians, families, and patients would be well served to review his arguments on the clear definition of “medicine” with regard to marijuana and the risks taken when we medicalize a known addictive chemical. He tackles the push for legalization as well, and the risks of increased societal acceptance and commercialization power that comes with this. He has led the field in thinking about the role of early drug exposure, brain training, and hijacking in the addictive process. This gateway hypothesis can occur whether the first teen drugs are cannabis or tobacco4 or alcohol.
Dr. DuPont finishes his work with a detailed biography, in which he addresses his family history of addiction, and his own overuse of alcohol in his late teens and early 20s as well as a confession of onetime use of marijuana during medical school. He always has led the field in trying to explain the disease of addiction, intervention, treatment, and recovery. But this self-reflection and brutal honesty is refreshing and in step with themes in his book, which promote the idea of recovery as an embrace of honesty, in mind, body, and spirit.
Dr. Jorandby trained in addiction psychiatry at Yale University, New Haven, Conn., and works as an addiction psychiatrist with Amen Clinics in Washington. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis.
References
1. J Subst Abuse Treat. 2009 Mar;36(2):159-71.
2. J Addict Dis. 2007;26 Suppl 1:13-23.
3. Am J Psychiatry. 2007 Jun;164(6):973.
4. J Addict Dis. 2003;22(3):51-62.
What do Queen Silvia of Sweden, Pope Francis, and the first director of the National Institute on Drug Abuse (NIDA) have in common? They all share a deep and sobering commitment to fighting the global disease of addiction.
Robert L. DuPont, MD, has written a beautiful and surprisingly spiritual guide into the American addiction epidemic. He is the author of “The Selfish Brain: Learning From Addiction” (Center City, Minn.: Hazelden, 2000) and with his newest publication, “Chemical Slavery: Understanding Addiction and Stopping the Drug Epidemic” (Institute for Behavior and Health, 2018), he writes a clear-eyed tome detailing the history of drug and alcohol use within the United States and the current state of America’s drug epidemic.
Dr. DuPont is well known within the American addiction community as NIDA’s first director and as the second drug czar, under two presidents, Richard M. Nixon and Gerald R. Ford. His breadth of experience, spanning 50-plus years, dates from his early career with the District of Columbia Department of Corrections, into his work in public policy on drugs and alcohol. This experience infuses his book with the hard science of addiction and the common-sense compassion required to shepherd people into recovery. One of us has worked with and been influenced by him since the 1970s. The other, also an addiction psychiatrist, finished this book both invigorated and compelled to say thank you to Dr. DuPont and his life’s work! He remains relevant, insightful, and always optimistic about the future of addiction treatment.
Harm reduction explored
The book begins with a cogent history of drug and alcohol use in America. Dr. DuPont details this as well as the public policies that have evolved to address them. He weaves into our national history reasoning behind why, as a “mass consumer” culture, we are more prone to addiction than ever before. The loss of cultural and societal pressures has a role to play in the rise along with genetics and environmental stress. The adolescent brain is prominently discussed throughout this first section as a highly vulnerable organ that can lead to lifelong addiction if primed early by addictive chemicals.
Dr. DuPont addresses the biology of addiction, delving into both the biological mechanisms within the brain, making those details accessible and understandable – to a practicing physician as well as a family member or patient struggling with addiction.
He also addresses harm reduction, a fairly new concept within the field. Harm reduction has taken on more prominence with localities across the country providing people with addictions with safe places and clean needles to continue their substance use without risks of serious or life-threatening diseases and crime. He challenges the idea that harm reduction is active recovery from substance addiction. Instead, he opines that harm reduction must be tethered to and must lead to real recovery work or it risks becoming an organizational enabling of the addict’s behavior.
Dr. DuPont pulls no punches with his language. He uses words such as “fatal,” “addict,” and “alcoholic.” He addresses the concerns by some in the field that those kinds of words are harsh, derogatory, and prejudicial by calling out addiction as a disease hallmarked primarily by loss of control and by dishonesty. To shun those words is to perpetuate the disease, delaying life-saving treatment.
Compassion is a theme throughout. He says we must stigmatize the addiction but not the addict. He advocates for real consequences to addictive behaviors as a key to getting addicted physicians and others with this disease into recovery. Treatment works, but we also have studied specific approaches that work and why.1 His writing conveys a genuine empathy for his patients and argues that treatment is delayed when serious and negative consequences for patients are removed.
Focus on prevention
A clear passion for prevention is evident within his chapters on youth addiction. For adolescents, Dr. DuPont presents a “One Choice approach,” which requires complete abstinence from alcohol, tobacco, and marijuana. He also presents science showing that patients younger than age 21 will have a greater risk of developing lifelong and debilitating addictions if they use these chemicals prior to this age. His emphasis for the One Choice approach carries ramifications throughout the primary care, pediatric, and family practice communities.
Dr. DuPont is nothing if not an optimist. Though he clearly defines addiction as an often-fatal disease, he remains positive about the future of addiction treatment, both with changes in public policy and with advancements in the medicine of addiction. He makes a compelling argument regarding Sweden’s approach to the drug problem, citing Queen Silvia’s lifelong commitment to prevent, control, and treat drug and alcohol addiction. Sweden’s model is, indeed, intriguing, and that country’s outcomes present a strong argument for the marriage of the criminal justice system with medical intervention – an approach that the United States has adopted only in a patchwork fashion.
The book describes controversies throughout, such as Dr. DuPont’s furtherance of our work on how best to treat dual disorders.2 He is not impressed with the self-medication hypothesis as well as the dive into the U.S. national medical marijuana experiment. We had looked at college students having new-onset memory or attention-deficit/hyperactivity problems, only to find that it was likely psychostimulant seeking to reverse marijuana effects.3 Physicians, families, and patients would be well served to review his arguments on the clear definition of “medicine” with regard to marijuana and the risks taken when we medicalize a known addictive chemical. He tackles the push for legalization as well, and the risks of increased societal acceptance and commercialization power that comes with this. He has led the field in thinking about the role of early drug exposure, brain training, and hijacking in the addictive process. This gateway hypothesis can occur whether the first teen drugs are cannabis or tobacco4 or alcohol.
Dr. DuPont finishes his work with a detailed biography, in which he addresses his family history of addiction, and his own overuse of alcohol in his late teens and early 20s as well as a confession of onetime use of marijuana during medical school. He always has led the field in trying to explain the disease of addiction, intervention, treatment, and recovery. But this self-reflection and brutal honesty is refreshing and in step with themes in his book, which promote the idea of recovery as an embrace of honesty, in mind, body, and spirit.
Dr. Jorandby trained in addiction psychiatry at Yale University, New Haven, Conn., and works as an addiction psychiatrist with Amen Clinics in Washington. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis.
References
1. J Subst Abuse Treat. 2009 Mar;36(2):159-71.
2. J Addict Dis. 2007;26 Suppl 1:13-23.
3. Am J Psychiatry. 2007 Jun;164(6):973.
4. J Addict Dis. 2003;22(3):51-62.
What do Queen Silvia of Sweden, Pope Francis, and the first director of the National Institute on Drug Abuse (NIDA) have in common? They all share a deep and sobering commitment to fighting the global disease of addiction.
Robert L. DuPont, MD, has written a beautiful and surprisingly spiritual guide into the American addiction epidemic. He is the author of “The Selfish Brain: Learning From Addiction” (Center City, Minn.: Hazelden, 2000) and with his newest publication, “Chemical Slavery: Understanding Addiction and Stopping the Drug Epidemic” (Institute for Behavior and Health, 2018), he writes a clear-eyed tome detailing the history of drug and alcohol use within the United States and the current state of America’s drug epidemic.
Dr. DuPont is well known within the American addiction community as NIDA’s first director and as the second drug czar, under two presidents, Richard M. Nixon and Gerald R. Ford. His breadth of experience, spanning 50-plus years, dates from his early career with the District of Columbia Department of Corrections, into his work in public policy on drugs and alcohol. This experience infuses his book with the hard science of addiction and the common-sense compassion required to shepherd people into recovery. One of us has worked with and been influenced by him since the 1970s. The other, also an addiction psychiatrist, finished this book both invigorated and compelled to say thank you to Dr. DuPont and his life’s work! He remains relevant, insightful, and always optimistic about the future of addiction treatment.
Harm reduction explored
The book begins with a cogent history of drug and alcohol use in America. Dr. DuPont details this as well as the public policies that have evolved to address them. He weaves into our national history reasoning behind why, as a “mass consumer” culture, we are more prone to addiction than ever before. The loss of cultural and societal pressures has a role to play in the rise along with genetics and environmental stress. The adolescent brain is prominently discussed throughout this first section as a highly vulnerable organ that can lead to lifelong addiction if primed early by addictive chemicals.
Dr. DuPont addresses the biology of addiction, delving into both the biological mechanisms within the brain, making those details accessible and understandable – to a practicing physician as well as a family member or patient struggling with addiction.
He also addresses harm reduction, a fairly new concept within the field. Harm reduction has taken on more prominence with localities across the country providing people with addictions with safe places and clean needles to continue their substance use without risks of serious or life-threatening diseases and crime. He challenges the idea that harm reduction is active recovery from substance addiction. Instead, he opines that harm reduction must be tethered to and must lead to real recovery work or it risks becoming an organizational enabling of the addict’s behavior.
Dr. DuPont pulls no punches with his language. He uses words such as “fatal,” “addict,” and “alcoholic.” He addresses the concerns by some in the field that those kinds of words are harsh, derogatory, and prejudicial by calling out addiction as a disease hallmarked primarily by loss of control and by dishonesty. To shun those words is to perpetuate the disease, delaying life-saving treatment.
Compassion is a theme throughout. He says we must stigmatize the addiction but not the addict. He advocates for real consequences to addictive behaviors as a key to getting addicted physicians and others with this disease into recovery. Treatment works, but we also have studied specific approaches that work and why.1 His writing conveys a genuine empathy for his patients and argues that treatment is delayed when serious and negative consequences for patients are removed.
Focus on prevention
A clear passion for prevention is evident within his chapters on youth addiction. For adolescents, Dr. DuPont presents a “One Choice approach,” which requires complete abstinence from alcohol, tobacco, and marijuana. He also presents science showing that patients younger than age 21 will have a greater risk of developing lifelong and debilitating addictions if they use these chemicals prior to this age. His emphasis for the One Choice approach carries ramifications throughout the primary care, pediatric, and family practice communities.
Dr. DuPont is nothing if not an optimist. Though he clearly defines addiction as an often-fatal disease, he remains positive about the future of addiction treatment, both with changes in public policy and with advancements in the medicine of addiction. He makes a compelling argument regarding Sweden’s approach to the drug problem, citing Queen Silvia’s lifelong commitment to prevent, control, and treat drug and alcohol addiction. Sweden’s model is, indeed, intriguing, and that country’s outcomes present a strong argument for the marriage of the criminal justice system with medical intervention – an approach that the United States has adopted only in a patchwork fashion.
The book describes controversies throughout, such as Dr. DuPont’s furtherance of our work on how best to treat dual disorders.2 He is not impressed with the self-medication hypothesis as well as the dive into the U.S. national medical marijuana experiment. We had looked at college students having new-onset memory or attention-deficit/hyperactivity problems, only to find that it was likely psychostimulant seeking to reverse marijuana effects.3 Physicians, families, and patients would be well served to review his arguments on the clear definition of “medicine” with regard to marijuana and the risks taken when we medicalize a known addictive chemical. He tackles the push for legalization as well, and the risks of increased societal acceptance and commercialization power that comes with this. He has led the field in thinking about the role of early drug exposure, brain training, and hijacking in the addictive process. This gateway hypothesis can occur whether the first teen drugs are cannabis or tobacco4 or alcohol.
Dr. DuPont finishes his work with a detailed biography, in which he addresses his family history of addiction, and his own overuse of alcohol in his late teens and early 20s as well as a confession of onetime use of marijuana during medical school. He always has led the field in trying to explain the disease of addiction, intervention, treatment, and recovery. But this self-reflection and brutal honesty is refreshing and in step with themes in his book, which promote the idea of recovery as an embrace of honesty, in mind, body, and spirit.
Dr. Jorandby trained in addiction psychiatry at Yale University, New Haven, Conn., and works as an addiction psychiatrist with Amen Clinics in Washington. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis.
References
1. J Subst Abuse Treat. 2009 Mar;36(2):159-71.
2. J Addict Dis. 2007;26 Suppl 1:13-23.
3. Am J Psychiatry. 2007 Jun;164(6):973.
4. J Addict Dis. 2003;22(3):51-62.
How much more proof do you need?
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.