User login
‘The birth of a mother is a complex process’
Softening the blow to women and families of severe perinatal, postpartum psychiatric disorders
Editor’s Note: Alison M. Heru, MD, the Families in Psychiatry columnist, invited Dr. Reinstein to address this topic.
“But this was not what I expected!” That’s a statement I have heard from countless new mothers.
Women often envision pregnancy and the postpartum period as a time of pure joy. The glow of an expectant woman and the excitement of the arrival of a new baby masks the reality that many women struggle emotionally when transitioning to motherhood. Like the birth of a child, the birth of a mother is a complex process. Upholding the myth that all women seamlessly transform into mothers can have devastating effects and hinder access to mental health care.
As a psychiatrist working on a women’s inpatient unit with a perinatal program, I treat women at times of crisis. What may have begun as mild anxiety or depression sometimes quickly spirals into severe psychiatric illness. The sheer force of these severe perinatal and postpartum psychiatric disorders often leaves women and families shocked and confused, wondering what happened to their crumbled dreams of early motherhood.
What must general psychiatrists know about perinatal and postpartum psychiatric disorders? Why is maternal mental health so important? What are the barriers to treatment for these women? How can general psychiatrists best support and treat these new mothers and their families?
What data show
Maternal depression is now known to be one of the most common complications of pregnancy. Studies have suggested that about 11% of women experience depression during pregnancy1 and approximately 17% of women are depressed in the postpartum period.2 Perinatal generalized anxiety disorder has been shown to have a prevalence of 8.5%-10.5% during pregnancy with a wider variance post partum.3 Approximately 3% of women in the general community develop PTSD symptoms following childbirth.4 Research suggests that about 2% of women develop obsessive-compulsive disorder symptoms in the postpartum period.5 Postpartum psychosis, a rare but potentially devastating illness, occurs after 0.1%-0.2% of births.6
Importance of maternal mental health
There is a growing body of literature supporting both obstetric and pediatric adverse outcomes related to untreated psychiatric illness. Untreated maternal depression has been associated with obstetric complications, such as preterm delivery, preeclampsia, low birth weight, as well as the child’s developing cognitive function.7 Anxiety during pregnancy has been associated with both a shorter gestational period and adverse implications for fetal neurodevelopment.
These adverse effects were found to be even more potent in “pregnancy anxiety,” or anxiety specifically focused on the pregnancy, the birth experience, and the transition to motherhood.8 The psychotic symptoms occurring during postpartum psychosis can jeopardize the lives of both a woman and her child and carries a 4% risk of infanticide.9 Although there are limited data about the long-term effects of postpartum obsessive-compulsive disorders and PTSD, it is reasonable to assume that they might carry negative long-term implications for the mother and possibly her child.
Barriers to treatment
Despite the significant rates of mental illness, pregnant and new mothers often face barriers to receiving treatment. Many psychiatrists are hesitant to prescribe psychiatric medication to pregnant women because of concerns about teratogenic potential of psychiatric medications; similar concerns exist for newborn babies when prescribing medications to lactating mothers. In addition, the field of reproductive psychiatry is evolving at a rapid pace, making it difficult for busy psychiatrists to keep up with the ever-growing literature.
Also, it is hard to imagine a population that has more barriers to attending outpatient appointments. For many new mothers, the exhaustion and all-consuming work involved with taking care of a newborn are insurmountable barriers to obtaining mental health care. In addition, despite the awareness that new mothers often are more emotional, families can be slow to recognize the developing severity of a psychiatric illness during the peripartum and postpartum periods.
Supporting and treating new mothers
As general psychiatrists, there are several ways to directly help these women.
1. Expect the expected. Even in women with no prior psychiatric history, a significant percentage of expectant and postpartum women will develop acute psychiatric symptoms. Learn about the different presentations and treatments of perinatal and postpartum psychiatric disorders. For example, a woman might have thoughts of harming her baby in both postpartum psychosis and obsessive-compulsive disorder. However, the acuity and treatment of these two conditions drastically differ.
2. Learn more about psychiatric medications. Several apps and websites are available to psychiatrists to learn about the safety profile of psychiatric medications, such as Reprotox.org, mothertobaby.org, lactmed, and womensmentalhealth.org. Many medications are considered to be relatively safe during pregnancy and breastfeeding. It is important for psychiatrists to appreciate the risks when choosing not to prescribe to pregnant and postpartum women. Sometimes a known risk of a specific medication may be preferable to the unknown risk of leaving a woman susceptible to a severe psychiatric decompensation.
3. Involve all members of the family. A mother’s mental health has significant implications for the entire family. Psychoeducation for the family as well as frequent family sessions are key tools when treating this population. In addition, prescribing to pregnant women provides the opportunity for a psychiatrist to refine skills in joint decision making; it is crucial to involve both a patient and her spouse when discussing psychiatric medications.
4. Provide ready access and collaborate care. It is important to understand the potential rapid onset of psychiatric symptoms during the pregnancy and postpartum period. Psychiatrists should be prepared to collaborate care with other specialties. It is important to establish relationships with community psychotherapists specializing in maternal mental health, pediatricians, as well as obstetricians.
5. Learn when to seek a higher level of care. Although many women with perinatal and postpartum psychiatric symptoms can be managed as outpatients, women at times need a higher level of care. Similar to general psychiatry, women who are acutely suicidal or homicidal or have a sudden onset of psychotic and manic symptoms all should be evaluated immediately for inpatient hospitalization. Women with less severe symptoms but who require a higher level of care than typically offered in standard outpatient treatment should be candidates for partial hospitalization programs.
General intensive programs usually can accommodate these women, but it is ideal to refer this population to perinatal intensive programs. Postpartum Support International (postpartum.net) lists the nationwide inpatient and partial perinatal programs as well as regional and local services. An example of inpatient perinatal care is the women’s unit at Zucker Hillside Hospital (Northwell Health System, Glen Oaks, N.Y.), which houses an inpatient perinatal program. As a psychiatrist on the unit, I treat acute symptoms such as depression, anxiety, psychosis, mania, and catatonia that occur during the perinatal and postpartum periods. Given the severity of symptoms, I use a wide range of psychiatric medications with the possibility of electroconvulsive therapy when indicated. Psychotherapy staff on the unit offer specialized perinatal, mothers, and dialectical behavioral therapy groups. Breast pumps are available for women who wish to breastfeed. Accommodations are made for babies and children to visit their mother when clinically appropriate. Once discharged, women often are referred to Zucker Hillside’s own perinatal outpatient clinic for continued treatment. Similar models exist in select inpatient units as well as an increasing number of partial programs across the United States.
Conclusion
Psychiatric care for pregnant and new mothers can be challenging, but it is also immensely rewarding. Restoring a mother’s mental health usually leads to increased emotional stability for her entire family. Given the prevalence of maternal mental health disorders, psychiatrists in nearly every setting will encounter this population of women. With dedicated time devoted to reviewing the literature and learning about local resources, psychiatrists can feel comfortable treating women throughout the childbearing experience.
References
1. J Affect Disord. 2017 Sep;219:86-92.
2. J Psychiatr Res. 2018 Sep;104:235-48.
3. J Womens Health. (Larchmt). 2015 Sep;24(9):762-70.
4. Clin Psychol Rev. 2014 Jul;34(5):389-401.
5. Compr Psychiatry. 2009 Nov-Dec;50(6):503-9.
6. Int Rev Psychiatry. 2003 Aug;15(3):231-42.
7. Clin Obstet Gynecol. 2018 Sep;61(3):533-43.
8. Curr Opinion Psychiatry. 2012 Mar;25(2):141-8.
9. Am J Psychiatry. 2009 Apr;166(4):405-8.
Dr. Reinstein is a psychiatry attending at Zucker Hillside Hospital. Her clinical interests include reproductive psychiatry and family therapy, with a specific focus on maternal mental health. Dr. Reinstein completed her adult psychiatry residency training at Montefiore Hospital/Albert Einstein College of Medicine, New York, after graduating from the Albert Einstein College of Medicine and Yeshiva University, New York, with a BA in biology. She is one of the recipients of the 4th Annual Resident Recognition Award for Excellence in Family Oriented Care.
Softening the blow to women and families of severe perinatal, postpartum psychiatric disorders
Softening the blow to women and families of severe perinatal, postpartum psychiatric disorders
Editor’s Note: Alison M. Heru, MD, the Families in Psychiatry columnist, invited Dr. Reinstein to address this topic.
“But this was not what I expected!” That’s a statement I have heard from countless new mothers.
Women often envision pregnancy and the postpartum period as a time of pure joy. The glow of an expectant woman and the excitement of the arrival of a new baby masks the reality that many women struggle emotionally when transitioning to motherhood. Like the birth of a child, the birth of a mother is a complex process. Upholding the myth that all women seamlessly transform into mothers can have devastating effects and hinder access to mental health care.
As a psychiatrist working on a women’s inpatient unit with a perinatal program, I treat women at times of crisis. What may have begun as mild anxiety or depression sometimes quickly spirals into severe psychiatric illness. The sheer force of these severe perinatal and postpartum psychiatric disorders often leaves women and families shocked and confused, wondering what happened to their crumbled dreams of early motherhood.
What must general psychiatrists know about perinatal and postpartum psychiatric disorders? Why is maternal mental health so important? What are the barriers to treatment for these women? How can general psychiatrists best support and treat these new mothers and their families?
What data show
Maternal depression is now known to be one of the most common complications of pregnancy. Studies have suggested that about 11% of women experience depression during pregnancy1 and approximately 17% of women are depressed in the postpartum period.2 Perinatal generalized anxiety disorder has been shown to have a prevalence of 8.5%-10.5% during pregnancy with a wider variance post partum.3 Approximately 3% of women in the general community develop PTSD symptoms following childbirth.4 Research suggests that about 2% of women develop obsessive-compulsive disorder symptoms in the postpartum period.5 Postpartum psychosis, a rare but potentially devastating illness, occurs after 0.1%-0.2% of births.6
Importance of maternal mental health
There is a growing body of literature supporting both obstetric and pediatric adverse outcomes related to untreated psychiatric illness. Untreated maternal depression has been associated with obstetric complications, such as preterm delivery, preeclampsia, low birth weight, as well as the child’s developing cognitive function.7 Anxiety during pregnancy has been associated with both a shorter gestational period and adverse implications for fetal neurodevelopment.
These adverse effects were found to be even more potent in “pregnancy anxiety,” or anxiety specifically focused on the pregnancy, the birth experience, and the transition to motherhood.8 The psychotic symptoms occurring during postpartum psychosis can jeopardize the lives of both a woman and her child and carries a 4% risk of infanticide.9 Although there are limited data about the long-term effects of postpartum obsessive-compulsive disorders and PTSD, it is reasonable to assume that they might carry negative long-term implications for the mother and possibly her child.
Barriers to treatment
Despite the significant rates of mental illness, pregnant and new mothers often face barriers to receiving treatment. Many psychiatrists are hesitant to prescribe psychiatric medication to pregnant women because of concerns about teratogenic potential of psychiatric medications; similar concerns exist for newborn babies when prescribing medications to lactating mothers. In addition, the field of reproductive psychiatry is evolving at a rapid pace, making it difficult for busy psychiatrists to keep up with the ever-growing literature.
Also, it is hard to imagine a population that has more barriers to attending outpatient appointments. For many new mothers, the exhaustion and all-consuming work involved with taking care of a newborn are insurmountable barriers to obtaining mental health care. In addition, despite the awareness that new mothers often are more emotional, families can be slow to recognize the developing severity of a psychiatric illness during the peripartum and postpartum periods.
Supporting and treating new mothers
As general psychiatrists, there are several ways to directly help these women.
1. Expect the expected. Even in women with no prior psychiatric history, a significant percentage of expectant and postpartum women will develop acute psychiatric symptoms. Learn about the different presentations and treatments of perinatal and postpartum psychiatric disorders. For example, a woman might have thoughts of harming her baby in both postpartum psychosis and obsessive-compulsive disorder. However, the acuity and treatment of these two conditions drastically differ.
2. Learn more about psychiatric medications. Several apps and websites are available to psychiatrists to learn about the safety profile of psychiatric medications, such as Reprotox.org, mothertobaby.org, lactmed, and womensmentalhealth.org. Many medications are considered to be relatively safe during pregnancy and breastfeeding. It is important for psychiatrists to appreciate the risks when choosing not to prescribe to pregnant and postpartum women. Sometimes a known risk of a specific medication may be preferable to the unknown risk of leaving a woman susceptible to a severe psychiatric decompensation.
3. Involve all members of the family. A mother’s mental health has significant implications for the entire family. Psychoeducation for the family as well as frequent family sessions are key tools when treating this population. In addition, prescribing to pregnant women provides the opportunity for a psychiatrist to refine skills in joint decision making; it is crucial to involve both a patient and her spouse when discussing psychiatric medications.
4. Provide ready access and collaborate care. It is important to understand the potential rapid onset of psychiatric symptoms during the pregnancy and postpartum period. Psychiatrists should be prepared to collaborate care with other specialties. It is important to establish relationships with community psychotherapists specializing in maternal mental health, pediatricians, as well as obstetricians.
5. Learn when to seek a higher level of care. Although many women with perinatal and postpartum psychiatric symptoms can be managed as outpatients, women at times need a higher level of care. Similar to general psychiatry, women who are acutely suicidal or homicidal or have a sudden onset of psychotic and manic symptoms all should be evaluated immediately for inpatient hospitalization. Women with less severe symptoms but who require a higher level of care than typically offered in standard outpatient treatment should be candidates for partial hospitalization programs.
General intensive programs usually can accommodate these women, but it is ideal to refer this population to perinatal intensive programs. Postpartum Support International (postpartum.net) lists the nationwide inpatient and partial perinatal programs as well as regional and local services. An example of inpatient perinatal care is the women’s unit at Zucker Hillside Hospital (Northwell Health System, Glen Oaks, N.Y.), which houses an inpatient perinatal program. As a psychiatrist on the unit, I treat acute symptoms such as depression, anxiety, psychosis, mania, and catatonia that occur during the perinatal and postpartum periods. Given the severity of symptoms, I use a wide range of psychiatric medications with the possibility of electroconvulsive therapy when indicated. Psychotherapy staff on the unit offer specialized perinatal, mothers, and dialectical behavioral therapy groups. Breast pumps are available for women who wish to breastfeed. Accommodations are made for babies and children to visit their mother when clinically appropriate. Once discharged, women often are referred to Zucker Hillside’s own perinatal outpatient clinic for continued treatment. Similar models exist in select inpatient units as well as an increasing number of partial programs across the United States.
Conclusion
Psychiatric care for pregnant and new mothers can be challenging, but it is also immensely rewarding. Restoring a mother’s mental health usually leads to increased emotional stability for her entire family. Given the prevalence of maternal mental health disorders, psychiatrists in nearly every setting will encounter this population of women. With dedicated time devoted to reviewing the literature and learning about local resources, psychiatrists can feel comfortable treating women throughout the childbearing experience.
References
1. J Affect Disord. 2017 Sep;219:86-92.
2. J Psychiatr Res. 2018 Sep;104:235-48.
3. J Womens Health. (Larchmt). 2015 Sep;24(9):762-70.
4. Clin Psychol Rev. 2014 Jul;34(5):389-401.
5. Compr Psychiatry. 2009 Nov-Dec;50(6):503-9.
6. Int Rev Psychiatry. 2003 Aug;15(3):231-42.
7. Clin Obstet Gynecol. 2018 Sep;61(3):533-43.
8. Curr Opinion Psychiatry. 2012 Mar;25(2):141-8.
9. Am J Psychiatry. 2009 Apr;166(4):405-8.
Dr. Reinstein is a psychiatry attending at Zucker Hillside Hospital. Her clinical interests include reproductive psychiatry and family therapy, with a specific focus on maternal mental health. Dr. Reinstein completed her adult psychiatry residency training at Montefiore Hospital/Albert Einstein College of Medicine, New York, after graduating from the Albert Einstein College of Medicine and Yeshiva University, New York, with a BA in biology. She is one of the recipients of the 4th Annual Resident Recognition Award for Excellence in Family Oriented Care.
Editor’s Note: Alison M. Heru, MD, the Families in Psychiatry columnist, invited Dr. Reinstein to address this topic.
“But this was not what I expected!” That’s a statement I have heard from countless new mothers.
Women often envision pregnancy and the postpartum period as a time of pure joy. The glow of an expectant woman and the excitement of the arrival of a new baby masks the reality that many women struggle emotionally when transitioning to motherhood. Like the birth of a child, the birth of a mother is a complex process. Upholding the myth that all women seamlessly transform into mothers can have devastating effects and hinder access to mental health care.
As a psychiatrist working on a women’s inpatient unit with a perinatal program, I treat women at times of crisis. What may have begun as mild anxiety or depression sometimes quickly spirals into severe psychiatric illness. The sheer force of these severe perinatal and postpartum psychiatric disorders often leaves women and families shocked and confused, wondering what happened to their crumbled dreams of early motherhood.
What must general psychiatrists know about perinatal and postpartum psychiatric disorders? Why is maternal mental health so important? What are the barriers to treatment for these women? How can general psychiatrists best support and treat these new mothers and their families?
What data show
Maternal depression is now known to be one of the most common complications of pregnancy. Studies have suggested that about 11% of women experience depression during pregnancy1 and approximately 17% of women are depressed in the postpartum period.2 Perinatal generalized anxiety disorder has been shown to have a prevalence of 8.5%-10.5% during pregnancy with a wider variance post partum.3 Approximately 3% of women in the general community develop PTSD symptoms following childbirth.4 Research suggests that about 2% of women develop obsessive-compulsive disorder symptoms in the postpartum period.5 Postpartum psychosis, a rare but potentially devastating illness, occurs after 0.1%-0.2% of births.6
Importance of maternal mental health
There is a growing body of literature supporting both obstetric and pediatric adverse outcomes related to untreated psychiatric illness. Untreated maternal depression has been associated with obstetric complications, such as preterm delivery, preeclampsia, low birth weight, as well as the child’s developing cognitive function.7 Anxiety during pregnancy has been associated with both a shorter gestational period and adverse implications for fetal neurodevelopment.
These adverse effects were found to be even more potent in “pregnancy anxiety,” or anxiety specifically focused on the pregnancy, the birth experience, and the transition to motherhood.8 The psychotic symptoms occurring during postpartum psychosis can jeopardize the lives of both a woman and her child and carries a 4% risk of infanticide.9 Although there are limited data about the long-term effects of postpartum obsessive-compulsive disorders and PTSD, it is reasonable to assume that they might carry negative long-term implications for the mother and possibly her child.
Barriers to treatment
Despite the significant rates of mental illness, pregnant and new mothers often face barriers to receiving treatment. Many psychiatrists are hesitant to prescribe psychiatric medication to pregnant women because of concerns about teratogenic potential of psychiatric medications; similar concerns exist for newborn babies when prescribing medications to lactating mothers. In addition, the field of reproductive psychiatry is evolving at a rapid pace, making it difficult for busy psychiatrists to keep up with the ever-growing literature.
Also, it is hard to imagine a population that has more barriers to attending outpatient appointments. For many new mothers, the exhaustion and all-consuming work involved with taking care of a newborn are insurmountable barriers to obtaining mental health care. In addition, despite the awareness that new mothers often are more emotional, families can be slow to recognize the developing severity of a psychiatric illness during the peripartum and postpartum periods.
Supporting and treating new mothers
As general psychiatrists, there are several ways to directly help these women.
1. Expect the expected. Even in women with no prior psychiatric history, a significant percentage of expectant and postpartum women will develop acute psychiatric symptoms. Learn about the different presentations and treatments of perinatal and postpartum psychiatric disorders. For example, a woman might have thoughts of harming her baby in both postpartum psychosis and obsessive-compulsive disorder. However, the acuity and treatment of these two conditions drastically differ.
2. Learn more about psychiatric medications. Several apps and websites are available to psychiatrists to learn about the safety profile of psychiatric medications, such as Reprotox.org, mothertobaby.org, lactmed, and womensmentalhealth.org. Many medications are considered to be relatively safe during pregnancy and breastfeeding. It is important for psychiatrists to appreciate the risks when choosing not to prescribe to pregnant and postpartum women. Sometimes a known risk of a specific medication may be preferable to the unknown risk of leaving a woman susceptible to a severe psychiatric decompensation.
3. Involve all members of the family. A mother’s mental health has significant implications for the entire family. Psychoeducation for the family as well as frequent family sessions are key tools when treating this population. In addition, prescribing to pregnant women provides the opportunity for a psychiatrist to refine skills in joint decision making; it is crucial to involve both a patient and her spouse when discussing psychiatric medications.
4. Provide ready access and collaborate care. It is important to understand the potential rapid onset of psychiatric symptoms during the pregnancy and postpartum period. Psychiatrists should be prepared to collaborate care with other specialties. It is important to establish relationships with community psychotherapists specializing in maternal mental health, pediatricians, as well as obstetricians.
5. Learn when to seek a higher level of care. Although many women with perinatal and postpartum psychiatric symptoms can be managed as outpatients, women at times need a higher level of care. Similar to general psychiatry, women who are acutely suicidal or homicidal or have a sudden onset of psychotic and manic symptoms all should be evaluated immediately for inpatient hospitalization. Women with less severe symptoms but who require a higher level of care than typically offered in standard outpatient treatment should be candidates for partial hospitalization programs.
General intensive programs usually can accommodate these women, but it is ideal to refer this population to perinatal intensive programs. Postpartum Support International (postpartum.net) lists the nationwide inpatient and partial perinatal programs as well as regional and local services. An example of inpatient perinatal care is the women’s unit at Zucker Hillside Hospital (Northwell Health System, Glen Oaks, N.Y.), which houses an inpatient perinatal program. As a psychiatrist on the unit, I treat acute symptoms such as depression, anxiety, psychosis, mania, and catatonia that occur during the perinatal and postpartum periods. Given the severity of symptoms, I use a wide range of psychiatric medications with the possibility of electroconvulsive therapy when indicated. Psychotherapy staff on the unit offer specialized perinatal, mothers, and dialectical behavioral therapy groups. Breast pumps are available for women who wish to breastfeed. Accommodations are made for babies and children to visit their mother when clinically appropriate. Once discharged, women often are referred to Zucker Hillside’s own perinatal outpatient clinic for continued treatment. Similar models exist in select inpatient units as well as an increasing number of partial programs across the United States.
Conclusion
Psychiatric care for pregnant and new mothers can be challenging, but it is also immensely rewarding. Restoring a mother’s mental health usually leads to increased emotional stability for her entire family. Given the prevalence of maternal mental health disorders, psychiatrists in nearly every setting will encounter this population of women. With dedicated time devoted to reviewing the literature and learning about local resources, psychiatrists can feel comfortable treating women throughout the childbearing experience.
References
1. J Affect Disord. 2017 Sep;219:86-92.
2. J Psychiatr Res. 2018 Sep;104:235-48.
3. J Womens Health. (Larchmt). 2015 Sep;24(9):762-70.
4. Clin Psychol Rev. 2014 Jul;34(5):389-401.
5. Compr Psychiatry. 2009 Nov-Dec;50(6):503-9.
6. Int Rev Psychiatry. 2003 Aug;15(3):231-42.
7. Clin Obstet Gynecol. 2018 Sep;61(3):533-43.
8. Curr Opinion Psychiatry. 2012 Mar;25(2):141-8.
9. Am J Psychiatry. 2009 Apr;166(4):405-8.
Dr. Reinstein is a psychiatry attending at Zucker Hillside Hospital. Her clinical interests include reproductive psychiatry and family therapy, with a specific focus on maternal mental health. Dr. Reinstein completed her adult psychiatry residency training at Montefiore Hospital/Albert Einstein College of Medicine, New York, after graduating from the Albert Einstein College of Medicine and Yeshiva University, New York, with a BA in biology. She is one of the recipients of the 4th Annual Resident Recognition Award for Excellence in Family Oriented Care.
Firing patients
After last month’s
One might assume that, just as patients are free to choose or reject their doctors, physicians have an equal right to reject their patients; and to a certain extent, that’s true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, sex, sexual orientation, and so on. However, our ethical obligations to “do no harm” and to place our patients’ welfare above our own self-interests dictate that dismissing a patient should be the absolute last resort, after all other options have been exhausted.
First, to avoid charges of arbitrary termination, you should draw up a specific list of situations that could merit a dismissal from your office, and add it to your office manual. Every list will probably differ in some respects, but for the sake of example, here is mine:
- Threats or violence toward physicians or staff.
- Inappropriate sexual advances toward physicians or staff.
- Providing false or misleading medical history.
- Repeated rude or disruptive behavior.
- Demands for unapproved, unindicated, or inappropriate treatments or medications (particularly controlled substances).
- Refusal to adhere to agreed-upon treatment plans.
- Repeated failure to keep scheduled appointments.
- Repeated failure to pay medical bills.
As with pretty much everything in a private practice, accurate and written documentation of dismissible behavior is essential. Record all incidents and assemble as much material evidence as possible from all available sources.
In most cases (except the first two infractions on our list, for which we have zero tolerance), we make every effort to resolve the problem amicably. We communicate with the patients in question, explain our concerns, and discuss options for resolution. I also may send a letter, repeating my concerns and proposed solutions, as further documentation of our efforts to achieve an amicable resolution. All verbal and written warnings are, of course, documented as well. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating these could lead to intervention by your state licensing board. There also is the risk of civil litigation, which typically is not covered by malpractice policies and may not be covered by your general liability policy either.
Patients who feel that termination was unjustified also may respond with negative reviews on social media, which I’ve discussed in recent columns, and will again, soon.
If something untrue is posted about you on a doctor-rating site, take action. Reputable sites have their own reputations to protect and can usually be persuaded to remove anything that is demonstrably false, although you may need a lawyer’s letter to get their attention. Try to get the error removed entirely or corrected within the original posting. An erratum on some distant page of the website is likely to be ignored, and will leave the false information online, intact.
Unfair comments are unlikely to be removed unless they are blatantly libelous; but many sites allow you to post a response, giving your side of the story. (More on that in the near future.) Also, there is nothing wrong with encouraging happy patients to write favorable reviews on those same sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
After last month’s
One might assume that, just as patients are free to choose or reject their doctors, physicians have an equal right to reject their patients; and to a certain extent, that’s true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, sex, sexual orientation, and so on. However, our ethical obligations to “do no harm” and to place our patients’ welfare above our own self-interests dictate that dismissing a patient should be the absolute last resort, after all other options have been exhausted.
First, to avoid charges of arbitrary termination, you should draw up a specific list of situations that could merit a dismissal from your office, and add it to your office manual. Every list will probably differ in some respects, but for the sake of example, here is mine:
- Threats or violence toward physicians or staff.
- Inappropriate sexual advances toward physicians or staff.
- Providing false or misleading medical history.
- Repeated rude or disruptive behavior.
- Demands for unapproved, unindicated, or inappropriate treatments or medications (particularly controlled substances).
- Refusal to adhere to agreed-upon treatment plans.
- Repeated failure to keep scheduled appointments.
- Repeated failure to pay medical bills.
As with pretty much everything in a private practice, accurate and written documentation of dismissible behavior is essential. Record all incidents and assemble as much material evidence as possible from all available sources.
In most cases (except the first two infractions on our list, for which we have zero tolerance), we make every effort to resolve the problem amicably. We communicate with the patients in question, explain our concerns, and discuss options for resolution. I also may send a letter, repeating my concerns and proposed solutions, as further documentation of our efforts to achieve an amicable resolution. All verbal and written warnings are, of course, documented as well. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating these could lead to intervention by your state licensing board. There also is the risk of civil litigation, which typically is not covered by malpractice policies and may not be covered by your general liability policy either.
Patients who feel that termination was unjustified also may respond with negative reviews on social media, which I’ve discussed in recent columns, and will again, soon.
If something untrue is posted about you on a doctor-rating site, take action. Reputable sites have their own reputations to protect and can usually be persuaded to remove anything that is demonstrably false, although you may need a lawyer’s letter to get their attention. Try to get the error removed entirely or corrected within the original posting. An erratum on some distant page of the website is likely to be ignored, and will leave the false information online, intact.
Unfair comments are unlikely to be removed unless they are blatantly libelous; but many sites allow you to post a response, giving your side of the story. (More on that in the near future.) Also, there is nothing wrong with encouraging happy patients to write favorable reviews on those same sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
After last month’s
One might assume that, just as patients are free to choose or reject their doctors, physicians have an equal right to reject their patients; and to a certain extent, that’s true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, sex, sexual orientation, and so on. However, our ethical obligations to “do no harm” and to place our patients’ welfare above our own self-interests dictate that dismissing a patient should be the absolute last resort, after all other options have been exhausted.
First, to avoid charges of arbitrary termination, you should draw up a specific list of situations that could merit a dismissal from your office, and add it to your office manual. Every list will probably differ in some respects, but for the sake of example, here is mine:
- Threats or violence toward physicians or staff.
- Inappropriate sexual advances toward physicians or staff.
- Providing false or misleading medical history.
- Repeated rude or disruptive behavior.
- Demands for unapproved, unindicated, or inappropriate treatments or medications (particularly controlled substances).
- Refusal to adhere to agreed-upon treatment plans.
- Repeated failure to keep scheduled appointments.
- Repeated failure to pay medical bills.
As with pretty much everything in a private practice, accurate and written documentation of dismissible behavior is essential. Record all incidents and assemble as much material evidence as possible from all available sources.
In most cases (except the first two infractions on our list, for which we have zero tolerance), we make every effort to resolve the problem amicably. We communicate with the patients in question, explain our concerns, and discuss options for resolution. I also may send a letter, repeating my concerns and proposed solutions, as further documentation of our efforts to achieve an amicable resolution. All verbal and written warnings are, of course, documented as well. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating these could lead to intervention by your state licensing board. There also is the risk of civil litigation, which typically is not covered by malpractice policies and may not be covered by your general liability policy either.
Patients who feel that termination was unjustified also may respond with negative reviews on social media, which I’ve discussed in recent columns, and will again, soon.
If something untrue is posted about you on a doctor-rating site, take action. Reputable sites have their own reputations to protect and can usually be persuaded to remove anything that is demonstrably false, although you may need a lawyer’s letter to get their attention. Try to get the error removed entirely or corrected within the original posting. An erratum on some distant page of the website is likely to be ignored, and will leave the false information online, intact.
Unfair comments are unlikely to be removed unless they are blatantly libelous; but many sites allow you to post a response, giving your side of the story. (More on that in the near future.) Also, there is nothing wrong with encouraging happy patients to write favorable reviews on those same sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
An update on treatment of depression
Paul is 13-year-old male in seventh grade with a history of inattentive ADHD and a positive family history of depression and anxiety in his mother. He always has had a few friends, but recently they have not wanted to hang out with him; he feels like people are ignoring him. For the past 2 months, Paul’s mood has gotten very low. He feels sad and also bored because he is not enjoying anything anymore. He feels as though he is “a loser,” and as though nothing will ever get better. His grades have dropped. He has thoughts of wishing he were dead, although he has no specific plan and says he wouldn’t do it because he doesn’t want to hurt his parents. He is looking at his phone at night and gets to bed late, then doesn’t want to get up in the morning. He sleeps until noon on weekends. Appetite is increased. He doesn’t have energy to do things on the weekends.
Discussion
Paul clearly meets diagnostic criteria for depression. He feels sad and has lost pleasure in activities he used to enjoy. He has negative, hopeless thoughts, and vague thoughts of death although no specific plans. He has vegetative signs of depression with increased appetite and sleep; he likely has worse concentration than usual, given that his grades have dropped. Energy is low.
Meta-analyses have demonstrated the efficacy of SSRIs (fluoxetine, sertraline, citalopram, escitalopram) as well as venlafaxine, mirtazapine, and nefazodone with small to very small effect sizes.1 A large placebo effect is seen in many of these studies, correlating with the number of study sites – a feature of many industry-sponsored studies.
John Walkup, MD, a leading researcher on both medication and psychotherapeutic interventions in children’s mood disorders, has pointed out that the quality of industry-sponsored studies (vs. National Institute of Mental Health–sponsored studies) is likely lower, with more pressure to get in large numbers of patients in a short period of time, less trained investigators leading to less clear-cut diagnoses, and other sources of bias.2 This raises the question of whether we should weight NIMH-sponsored studies more heavily in meta-analyses.
A second factor to consider is the risk of harm, and a significant issue here is the question of whether suicidal ideation is increased among those patients taking SSRIs. Meta-analyses from the late 2000s, which balanced the number needed to treat vs. the number needed to harm, judged that for children under age 13 years, fluoxetine was the only antidepressant that was worth the cost-benefit ratio. However, in the past several years there has been a major improvement in the assessment of suicidal ideation in the form of the Columbia Suicide Severity Rating Scale, a standardized method of assessing the presence and significance of suicidal thoughts and behaviors. Studies that have used this assessment have found no significant increase in suicidal ideation with SSRIs vs. placebo.
The takeaway here is that the SSRIs can work, with fluoxetine, sertraline, and escitalopram leading the evidence, and that with refinements of the assessment they do not appear to increase the risk of suicidal ideation.3 Of course, it remains important to discuss this issue with families.
Psychotherapy is the other major treatment for depression. Cognitive behavioral therapy (CBT) and Interpersonal therapy (IPT) for adolescents show effectiveness in teens.4 Recent meta-analyses have gotten stronger through the use of stringent quality criteria and the inclusion of negative studies; these therapies continue to be considered well established. It is worthwhile to talk to therapists in your community to understand what type of treatment they offer. If you are hiring therapists to be embedded in your practice, look for people who have been trained in CBT or IPT. It is particularly helpful to know whether therapists have seen patients using CBT or IPT while getting supervision in these modalities.
CBT and IPT are different. CBT puts an emphasis on the thought-feeling-behavior triangle while IPT focuses more on relationships. Someone who has tried one and has not benefited nevertheless may benefit from the other.
Working with your patients to choose what type of psychotherapy modality for depression they would like is particularly effective.
Finally, be aware of how the environment may be affecting your patient. School issues related to peers, learning style or disabilities, and organization have a major effect on teens. In this case, Paul is looking at his phone nightly, which may be affecting both his sleep and self-esteem. Family issues continue to play a key role.
Paul was referred for CBT therapy, which was moderately helpful. After a few months, sertraline was added with further improvement. A key element in fully resolving Paul’s depression was his becoming involved in the drama club, which gave him the chance to meet a group of peers who shared his interests.
Dr. Hall is assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. JAMA. 2007 Apr 18;297(15):1683-96.
2. Am J Psychiatry. 2017 May 1;174(5):430-7.
3. J Child Adolesc Psychopharmacol. 2018. doi: 10.1089/cap.2017.0174.
4. J Clin Child Adolesc Psychol. 2017 Jan-Feb;46(1):11-43.
Paul is 13-year-old male in seventh grade with a history of inattentive ADHD and a positive family history of depression and anxiety in his mother. He always has had a few friends, but recently they have not wanted to hang out with him; he feels like people are ignoring him. For the past 2 months, Paul’s mood has gotten very low. He feels sad and also bored because he is not enjoying anything anymore. He feels as though he is “a loser,” and as though nothing will ever get better. His grades have dropped. He has thoughts of wishing he were dead, although he has no specific plan and says he wouldn’t do it because he doesn’t want to hurt his parents. He is looking at his phone at night and gets to bed late, then doesn’t want to get up in the morning. He sleeps until noon on weekends. Appetite is increased. He doesn’t have energy to do things on the weekends.
Discussion
Paul clearly meets diagnostic criteria for depression. He feels sad and has lost pleasure in activities he used to enjoy. He has negative, hopeless thoughts, and vague thoughts of death although no specific plans. He has vegetative signs of depression with increased appetite and sleep; he likely has worse concentration than usual, given that his grades have dropped. Energy is low.
Meta-analyses have demonstrated the efficacy of SSRIs (fluoxetine, sertraline, citalopram, escitalopram) as well as venlafaxine, mirtazapine, and nefazodone with small to very small effect sizes.1 A large placebo effect is seen in many of these studies, correlating with the number of study sites – a feature of many industry-sponsored studies.
John Walkup, MD, a leading researcher on both medication and psychotherapeutic interventions in children’s mood disorders, has pointed out that the quality of industry-sponsored studies (vs. National Institute of Mental Health–sponsored studies) is likely lower, with more pressure to get in large numbers of patients in a short period of time, less trained investigators leading to less clear-cut diagnoses, and other sources of bias.2 This raises the question of whether we should weight NIMH-sponsored studies more heavily in meta-analyses.
A second factor to consider is the risk of harm, and a significant issue here is the question of whether suicidal ideation is increased among those patients taking SSRIs. Meta-analyses from the late 2000s, which balanced the number needed to treat vs. the number needed to harm, judged that for children under age 13 years, fluoxetine was the only antidepressant that was worth the cost-benefit ratio. However, in the past several years there has been a major improvement in the assessment of suicidal ideation in the form of the Columbia Suicide Severity Rating Scale, a standardized method of assessing the presence and significance of suicidal thoughts and behaviors. Studies that have used this assessment have found no significant increase in suicidal ideation with SSRIs vs. placebo.
The takeaway here is that the SSRIs can work, with fluoxetine, sertraline, and escitalopram leading the evidence, and that with refinements of the assessment they do not appear to increase the risk of suicidal ideation.3 Of course, it remains important to discuss this issue with families.
Psychotherapy is the other major treatment for depression. Cognitive behavioral therapy (CBT) and Interpersonal therapy (IPT) for adolescents show effectiveness in teens.4 Recent meta-analyses have gotten stronger through the use of stringent quality criteria and the inclusion of negative studies; these therapies continue to be considered well established. It is worthwhile to talk to therapists in your community to understand what type of treatment they offer. If you are hiring therapists to be embedded in your practice, look for people who have been trained in CBT or IPT. It is particularly helpful to know whether therapists have seen patients using CBT or IPT while getting supervision in these modalities.
CBT and IPT are different. CBT puts an emphasis on the thought-feeling-behavior triangle while IPT focuses more on relationships. Someone who has tried one and has not benefited nevertheless may benefit from the other.
Working with your patients to choose what type of psychotherapy modality for depression they would like is particularly effective.
Finally, be aware of how the environment may be affecting your patient. School issues related to peers, learning style or disabilities, and organization have a major effect on teens. In this case, Paul is looking at his phone nightly, which may be affecting both his sleep and self-esteem. Family issues continue to play a key role.
Paul was referred for CBT therapy, which was moderately helpful. After a few months, sertraline was added with further improvement. A key element in fully resolving Paul’s depression was his becoming involved in the drama club, which gave him the chance to meet a group of peers who shared his interests.
Dr. Hall is assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. JAMA. 2007 Apr 18;297(15):1683-96.
2. Am J Psychiatry. 2017 May 1;174(5):430-7.
3. J Child Adolesc Psychopharmacol. 2018. doi: 10.1089/cap.2017.0174.
4. J Clin Child Adolesc Psychol. 2017 Jan-Feb;46(1):11-43.
Paul is 13-year-old male in seventh grade with a history of inattentive ADHD and a positive family history of depression and anxiety in his mother. He always has had a few friends, but recently they have not wanted to hang out with him; he feels like people are ignoring him. For the past 2 months, Paul’s mood has gotten very low. He feels sad and also bored because he is not enjoying anything anymore. He feels as though he is “a loser,” and as though nothing will ever get better. His grades have dropped. He has thoughts of wishing he were dead, although he has no specific plan and says he wouldn’t do it because he doesn’t want to hurt his parents. He is looking at his phone at night and gets to bed late, then doesn’t want to get up in the morning. He sleeps until noon on weekends. Appetite is increased. He doesn’t have energy to do things on the weekends.
Discussion
Paul clearly meets diagnostic criteria for depression. He feels sad and has lost pleasure in activities he used to enjoy. He has negative, hopeless thoughts, and vague thoughts of death although no specific plans. He has vegetative signs of depression with increased appetite and sleep; he likely has worse concentration than usual, given that his grades have dropped. Energy is low.
Meta-analyses have demonstrated the efficacy of SSRIs (fluoxetine, sertraline, citalopram, escitalopram) as well as venlafaxine, mirtazapine, and nefazodone with small to very small effect sizes.1 A large placebo effect is seen in many of these studies, correlating with the number of study sites – a feature of many industry-sponsored studies.
John Walkup, MD, a leading researcher on both medication and psychotherapeutic interventions in children’s mood disorders, has pointed out that the quality of industry-sponsored studies (vs. National Institute of Mental Health–sponsored studies) is likely lower, with more pressure to get in large numbers of patients in a short period of time, less trained investigators leading to less clear-cut diagnoses, and other sources of bias.2 This raises the question of whether we should weight NIMH-sponsored studies more heavily in meta-analyses.
A second factor to consider is the risk of harm, and a significant issue here is the question of whether suicidal ideation is increased among those patients taking SSRIs. Meta-analyses from the late 2000s, which balanced the number needed to treat vs. the number needed to harm, judged that for children under age 13 years, fluoxetine was the only antidepressant that was worth the cost-benefit ratio. However, in the past several years there has been a major improvement in the assessment of suicidal ideation in the form of the Columbia Suicide Severity Rating Scale, a standardized method of assessing the presence and significance of suicidal thoughts and behaviors. Studies that have used this assessment have found no significant increase in suicidal ideation with SSRIs vs. placebo.
The takeaway here is that the SSRIs can work, with fluoxetine, sertraline, and escitalopram leading the evidence, and that with refinements of the assessment they do not appear to increase the risk of suicidal ideation.3 Of course, it remains important to discuss this issue with families.
Psychotherapy is the other major treatment for depression. Cognitive behavioral therapy (CBT) and Interpersonal therapy (IPT) for adolescents show effectiveness in teens.4 Recent meta-analyses have gotten stronger through the use of stringent quality criteria and the inclusion of negative studies; these therapies continue to be considered well established. It is worthwhile to talk to therapists in your community to understand what type of treatment they offer. If you are hiring therapists to be embedded in your practice, look for people who have been trained in CBT or IPT. It is particularly helpful to know whether therapists have seen patients using CBT or IPT while getting supervision in these modalities.
CBT and IPT are different. CBT puts an emphasis on the thought-feeling-behavior triangle while IPT focuses more on relationships. Someone who has tried one and has not benefited nevertheless may benefit from the other.
Working with your patients to choose what type of psychotherapy modality for depression they would like is particularly effective.
Finally, be aware of how the environment may be affecting your patient. School issues related to peers, learning style or disabilities, and organization have a major effect on teens. In this case, Paul is looking at his phone nightly, which may be affecting both his sleep and self-esteem. Family issues continue to play a key role.
Paul was referred for CBT therapy, which was moderately helpful. After a few months, sertraline was added with further improvement. A key element in fully resolving Paul’s depression was his becoming involved in the drama club, which gave him the chance to meet a group of peers who shared his interests.
Dr. Hall is assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. JAMA. 2007 Apr 18;297(15):1683-96.
2. Am J Psychiatry. 2017 May 1;174(5):430-7.
3. J Child Adolesc Psychopharmacol. 2018. doi: 10.1089/cap.2017.0174.
4. J Clin Child Adolesc Psychol. 2017 Jan-Feb;46(1):11-43.
Prior authorization revisited: An update from the APA
You may have noticed that one of topics I like to write about in this column is the assault on the practice of medicine: the obligations that steal our time from patients without either the value or outcomes our patients see. It is these extraneous demands on our time and on our psyches that dehumanize medical practice as experienced by our patients and contribute to physician burnout. High on my list is the requirement for prior authorization for medications.
In 2015, I wrote a column, “Prior Authorization for Medications: Who oversees placement of the hoops?” The article followed my unsuccessful 6-week-long endeavor to get modafinil authorized for a patient. My efforts included interactions with the insurance company’s chief medical officer, the insurance commissioners in three states, my U.S. senator, and finally, the American Psychiatric Association’s attorney, Colleen Coyle, who has been working on this issue along with the American Medical Association and other medical specialty organizations for years now. The irony of the article is that, after it came out, a reader informed me that modafinil could be obtained from Costco for $34 for 30 pills, while every pharmacy I had checked was selling the generic medication for nearly $1,000 for the same number of pills!
One might make the case that prior authorization saves money by rationing the most expensive medications. And I might counter that physicians should be willing to try less expensive medications first – if we only knew the price of the medications we are prescribing. There is, however, no clear logic to the tremendous variability in price across pharmacies, which can amount to hundreds of dollars a month in the cost to an uninsured patient, and none of which reflects what might be a negotiated cost for those using health insurance. The newest medications are expensive from any retailer, but when it comes to older generics, it’s a crapshoot.
Years have passed since my prior authorization fiasco. Today, I keep a GoodRx app on my phone and often reference it when prescribing medications that might be expensive at one pharmacy but not at another. The app tells me that I can now buy 30 pills of the modafinil for $40.34 at grocery store pharmacy that is 2.7 miles from my office or for $305.50 at Walgreens, 3.6 miles away. A quick call to Costco shows that the price there has gone up to $40.89. For the prescriptions that I have written since that article, I have told patients that their insurance providers may not authorize the medication and if that is the case, they should price shop, as I don’t have the tenacity to go through the prior authorization process I endured in 2015.
So what progress has the APA made over the past 4 years? I wrote back to the APA’s attorney, Ms. Coyle, to ask for an update. Ms. Coyle was kind enough to respond in some detail. , and there is one less obstacle to obtaining care for a growing number of patients during our overdose epidemic.
Other news, however, is not all good. Medicare plans to increase the use of prior authorization.
Ms. Coyle provided data that confirmed what most physicians suspected. “The AMA did a survey1 and found 92% of physicians report that ‘prior authorizations programs have a negative impact on patient clinical outcomes.’ The AMA study revealed that ‘every week a medical practice completes an average of 29.1 prior authorization requirements per physician, which takes an average of 14.6 hours to process – the equivalent of nearly two business days.’ ”
She recounted the efforts the APA has made on behalf of psychiatrists. “We are working with the AMA and other physician groups to address the issue and recommended [Health and Human Services] address the burden of prior authorization as part of its Patients over Paperwork Initiative.2 Through our website and the helpline, we collected stories from our members about the burden it causes and the negative impact it has on patient care. We’ve shared the stories with the American Medical Association to use in joint advocacy efforts with the administration and private insurers.”
Finally, the APA has written a letter to the Centers for Medicare & Medicaid Services in opposition to a proposal for increased utilization review of Medicare Part D protected classes medications, which include antipsychotics and antidepressants. “We strongly oppose the proposal,” Ms. Coyle noted, “and asked our district branches to also submit letters in opposition.”
What can individual psychiatrists do to advocate? I noted that many of my efforts were futile. My U.S. senator responded with a letter that said my problem was not in his domain.
“As for the best way to approach the issue in the state, we recommend working with the state medical society to push for legislation. The AMA has draft legislation that may be used. It would also be helpful to collect the stories from the members about their challenges with prior authorization and impact on patient care/outcomes to share with state legislatures. It may also be beneficial to share these concerns with the state insurance commissioner and Department of Health and Human Services,” Ms. Coyle wrote. She noted it would be helpful for members to work directly with the district branches of the APA.
I am still left with some sense of futility. In 2014, Danielle Ofri, MD, PhD, a physician and writer, wrote an op-ed3 for the New York Times, “Adventures in ‘Prior Authorization,’ ” where she detailed the egregious practice. In the past 5 years, not much has changed, and insurers have not taken to the idea that preserving physician time for clinical care and face-to-face interactions with patients is the priority that we all might like it to be.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
References
1. AMA Prior Authorization Physician Survey, 2017.
2. CMS Patients Over Paperwork Initiative, CMS.gov.
3. New York Times, Aug. 3, 2014.
You may have noticed that one of topics I like to write about in this column is the assault on the practice of medicine: the obligations that steal our time from patients without either the value or outcomes our patients see. It is these extraneous demands on our time and on our psyches that dehumanize medical practice as experienced by our patients and contribute to physician burnout. High on my list is the requirement for prior authorization for medications.
In 2015, I wrote a column, “Prior Authorization for Medications: Who oversees placement of the hoops?” The article followed my unsuccessful 6-week-long endeavor to get modafinil authorized for a patient. My efforts included interactions with the insurance company’s chief medical officer, the insurance commissioners in three states, my U.S. senator, and finally, the American Psychiatric Association’s attorney, Colleen Coyle, who has been working on this issue along with the American Medical Association and other medical specialty organizations for years now. The irony of the article is that, after it came out, a reader informed me that modafinil could be obtained from Costco for $34 for 30 pills, while every pharmacy I had checked was selling the generic medication for nearly $1,000 for the same number of pills!
One might make the case that prior authorization saves money by rationing the most expensive medications. And I might counter that physicians should be willing to try less expensive medications first – if we only knew the price of the medications we are prescribing. There is, however, no clear logic to the tremendous variability in price across pharmacies, which can amount to hundreds of dollars a month in the cost to an uninsured patient, and none of which reflects what might be a negotiated cost for those using health insurance. The newest medications are expensive from any retailer, but when it comes to older generics, it’s a crapshoot.
Years have passed since my prior authorization fiasco. Today, I keep a GoodRx app on my phone and often reference it when prescribing medications that might be expensive at one pharmacy but not at another. The app tells me that I can now buy 30 pills of the modafinil for $40.34 at grocery store pharmacy that is 2.7 miles from my office or for $305.50 at Walgreens, 3.6 miles away. A quick call to Costco shows that the price there has gone up to $40.89. For the prescriptions that I have written since that article, I have told patients that their insurance providers may not authorize the medication and if that is the case, they should price shop, as I don’t have the tenacity to go through the prior authorization process I endured in 2015.
So what progress has the APA made over the past 4 years? I wrote back to the APA’s attorney, Ms. Coyle, to ask for an update. Ms. Coyle was kind enough to respond in some detail. , and there is one less obstacle to obtaining care for a growing number of patients during our overdose epidemic.
Other news, however, is not all good. Medicare plans to increase the use of prior authorization.
Ms. Coyle provided data that confirmed what most physicians suspected. “The AMA did a survey1 and found 92% of physicians report that ‘prior authorizations programs have a negative impact on patient clinical outcomes.’ The AMA study revealed that ‘every week a medical practice completes an average of 29.1 prior authorization requirements per physician, which takes an average of 14.6 hours to process – the equivalent of nearly two business days.’ ”
She recounted the efforts the APA has made on behalf of psychiatrists. “We are working with the AMA and other physician groups to address the issue and recommended [Health and Human Services] address the burden of prior authorization as part of its Patients over Paperwork Initiative.2 Through our website and the helpline, we collected stories from our members about the burden it causes and the negative impact it has on patient care. We’ve shared the stories with the American Medical Association to use in joint advocacy efforts with the administration and private insurers.”
Finally, the APA has written a letter to the Centers for Medicare & Medicaid Services in opposition to a proposal for increased utilization review of Medicare Part D protected classes medications, which include antipsychotics and antidepressants. “We strongly oppose the proposal,” Ms. Coyle noted, “and asked our district branches to also submit letters in opposition.”
What can individual psychiatrists do to advocate? I noted that many of my efforts were futile. My U.S. senator responded with a letter that said my problem was not in his domain.
“As for the best way to approach the issue in the state, we recommend working with the state medical society to push for legislation. The AMA has draft legislation that may be used. It would also be helpful to collect the stories from the members about their challenges with prior authorization and impact on patient care/outcomes to share with state legislatures. It may also be beneficial to share these concerns with the state insurance commissioner and Department of Health and Human Services,” Ms. Coyle wrote. She noted it would be helpful for members to work directly with the district branches of the APA.
I am still left with some sense of futility. In 2014, Danielle Ofri, MD, PhD, a physician and writer, wrote an op-ed3 for the New York Times, “Adventures in ‘Prior Authorization,’ ” where she detailed the egregious practice. In the past 5 years, not much has changed, and insurers have not taken to the idea that preserving physician time for clinical care and face-to-face interactions with patients is the priority that we all might like it to be.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
References
1. AMA Prior Authorization Physician Survey, 2017.
2. CMS Patients Over Paperwork Initiative, CMS.gov.
3. New York Times, Aug. 3, 2014.
You may have noticed that one of topics I like to write about in this column is the assault on the practice of medicine: the obligations that steal our time from patients without either the value or outcomes our patients see. It is these extraneous demands on our time and on our psyches that dehumanize medical practice as experienced by our patients and contribute to physician burnout. High on my list is the requirement for prior authorization for medications.
In 2015, I wrote a column, “Prior Authorization for Medications: Who oversees placement of the hoops?” The article followed my unsuccessful 6-week-long endeavor to get modafinil authorized for a patient. My efforts included interactions with the insurance company’s chief medical officer, the insurance commissioners in three states, my U.S. senator, and finally, the American Psychiatric Association’s attorney, Colleen Coyle, who has been working on this issue along with the American Medical Association and other medical specialty organizations for years now. The irony of the article is that, after it came out, a reader informed me that modafinil could be obtained from Costco for $34 for 30 pills, while every pharmacy I had checked was selling the generic medication for nearly $1,000 for the same number of pills!
One might make the case that prior authorization saves money by rationing the most expensive medications. And I might counter that physicians should be willing to try less expensive medications first – if we only knew the price of the medications we are prescribing. There is, however, no clear logic to the tremendous variability in price across pharmacies, which can amount to hundreds of dollars a month in the cost to an uninsured patient, and none of which reflects what might be a negotiated cost for those using health insurance. The newest medications are expensive from any retailer, but when it comes to older generics, it’s a crapshoot.
Years have passed since my prior authorization fiasco. Today, I keep a GoodRx app on my phone and often reference it when prescribing medications that might be expensive at one pharmacy but not at another. The app tells me that I can now buy 30 pills of the modafinil for $40.34 at grocery store pharmacy that is 2.7 miles from my office or for $305.50 at Walgreens, 3.6 miles away. A quick call to Costco shows that the price there has gone up to $40.89. For the prescriptions that I have written since that article, I have told patients that their insurance providers may not authorize the medication and if that is the case, they should price shop, as I don’t have the tenacity to go through the prior authorization process I endured in 2015.
So what progress has the APA made over the past 4 years? I wrote back to the APA’s attorney, Ms. Coyle, to ask for an update. Ms. Coyle was kind enough to respond in some detail. , and there is one less obstacle to obtaining care for a growing number of patients during our overdose epidemic.
Other news, however, is not all good. Medicare plans to increase the use of prior authorization.
Ms. Coyle provided data that confirmed what most physicians suspected. “The AMA did a survey1 and found 92% of physicians report that ‘prior authorizations programs have a negative impact on patient clinical outcomes.’ The AMA study revealed that ‘every week a medical practice completes an average of 29.1 prior authorization requirements per physician, which takes an average of 14.6 hours to process – the equivalent of nearly two business days.’ ”
She recounted the efforts the APA has made on behalf of psychiatrists. “We are working with the AMA and other physician groups to address the issue and recommended [Health and Human Services] address the burden of prior authorization as part of its Patients over Paperwork Initiative.2 Through our website and the helpline, we collected stories from our members about the burden it causes and the negative impact it has on patient care. We’ve shared the stories with the American Medical Association to use in joint advocacy efforts with the administration and private insurers.”
Finally, the APA has written a letter to the Centers for Medicare & Medicaid Services in opposition to a proposal for increased utilization review of Medicare Part D protected classes medications, which include antipsychotics and antidepressants. “We strongly oppose the proposal,” Ms. Coyle noted, “and asked our district branches to also submit letters in opposition.”
What can individual psychiatrists do to advocate? I noted that many of my efforts were futile. My U.S. senator responded with a letter that said my problem was not in his domain.
“As for the best way to approach the issue in the state, we recommend working with the state medical society to push for legislation. The AMA has draft legislation that may be used. It would also be helpful to collect the stories from the members about their challenges with prior authorization and impact on patient care/outcomes to share with state legislatures. It may also be beneficial to share these concerns with the state insurance commissioner and Department of Health and Human Services,” Ms. Coyle wrote. She noted it would be helpful for members to work directly with the district branches of the APA.
I am still left with some sense of futility. In 2014, Danielle Ofri, MD, PhD, a physician and writer, wrote an op-ed3 for the New York Times, “Adventures in ‘Prior Authorization,’ ” where she detailed the egregious practice. In the past 5 years, not much has changed, and insurers have not taken to the idea that preserving physician time for clinical care and face-to-face interactions with patients is the priority that we all might like it to be.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
References
1. AMA Prior Authorization Physician Survey, 2017.
2. CMS Patients Over Paperwork Initiative, CMS.gov.
3. New York Times, Aug. 3, 2014.
What’s driving burnout?
Working fewer hours but still struggling
According to a new survey report released by The Physicians Foundation, 80% of physicians across all specialties report being at full capacity or overextended, and 78% reported sometimes, often, or always experiencing feelings of burnout.
Sixty-two percent of U.S. doctors are pessimistic about the future of medicine, and 49% wouldn’t recommend medicine as a career to their children. This paints a pretty grim picture of medical practice in the United States in 2018.
The survey is conducted every other year by The Physicians Foundation with the assistance of Merritt Hawkins, and I wrote a blog post about the 2016 survey results, which showed alarming levels of disengagement and burnout. So, I thought it would be worthwhile looking over the 2018 report to see if anything has improved.
It appears that things haven’t changed much for doctors since 2016 regarding their attitudes toward their work. The biggest take-away from this year’s survey is that doctors overall are working fewer hours and seeing fewer patients but still struggling with morale and burnout. One important trend that was highlighted is the move toward employment by hospitals or integrated delivery systems; only 31% of physicians are independent practice owners or partners, vs. 49% in the first such survey conducted in 2012. Interestingly, employed doctors tend to work longer hours but see fewer patients compared with their practice-owner colleagues.
The 39-question survey is sent out via e-mail to more than 700,000 physicians (everyone the AMA or Merritt Hawkins has in their databases), and this year 8,774 physicians responded; the statistics geniuses at the University of Tennessee say the survey results have a margin of error of +/– 1.057%. Interestingly, that’s less than half of the 17,236 physicians who responded to the survey in 2016, and I wonder if the reduction in response rate itself indicates an increased level of disengagement among doctors.
Doctors expressed similar frustrations with specific aspects of their work this year, compared with 2016. The single biggest frustration cited by doctors was EHRs (39% this year vs. 27% in 2016), followed by regulatory/insurance requirements (down to 38% from 58% in 2016) and loss of clinical autonomy (37% vs. 32% in 2016). Survey respondents reported working an average of 51.4 hours per week, of which 11.4 hours (22%) are spent on nonclinical (paperwork) duties.
Read the full post at hospitalleader.org.
Also on The Hospital Leader
- I don’t want someone like you caring for me by Gopi Astik, MD
- CMS added care transition codes a few years back. How’s that goin’? by Brad Flansbaum, DO, MPH, MHM
- Sleepless in the hospital no more: Lessons learned during the SIESTA Study by Vineet Arora, MD, MAPP, MHM
- Are you committing malpractice by not treating opioid use disorder in the hospital? by Chris Moriates, MD
Working fewer hours but still struggling
Working fewer hours but still struggling
According to a new survey report released by The Physicians Foundation, 80% of physicians across all specialties report being at full capacity or overextended, and 78% reported sometimes, often, or always experiencing feelings of burnout.
Sixty-two percent of U.S. doctors are pessimistic about the future of medicine, and 49% wouldn’t recommend medicine as a career to their children. This paints a pretty grim picture of medical practice in the United States in 2018.
The survey is conducted every other year by The Physicians Foundation with the assistance of Merritt Hawkins, and I wrote a blog post about the 2016 survey results, which showed alarming levels of disengagement and burnout. So, I thought it would be worthwhile looking over the 2018 report to see if anything has improved.
It appears that things haven’t changed much for doctors since 2016 regarding their attitudes toward their work. The biggest take-away from this year’s survey is that doctors overall are working fewer hours and seeing fewer patients but still struggling with morale and burnout. One important trend that was highlighted is the move toward employment by hospitals or integrated delivery systems; only 31% of physicians are independent practice owners or partners, vs. 49% in the first such survey conducted in 2012. Interestingly, employed doctors tend to work longer hours but see fewer patients compared with their practice-owner colleagues.
The 39-question survey is sent out via e-mail to more than 700,000 physicians (everyone the AMA or Merritt Hawkins has in their databases), and this year 8,774 physicians responded; the statistics geniuses at the University of Tennessee say the survey results have a margin of error of +/– 1.057%. Interestingly, that’s less than half of the 17,236 physicians who responded to the survey in 2016, and I wonder if the reduction in response rate itself indicates an increased level of disengagement among doctors.
Doctors expressed similar frustrations with specific aspects of their work this year, compared with 2016. The single biggest frustration cited by doctors was EHRs (39% this year vs. 27% in 2016), followed by regulatory/insurance requirements (down to 38% from 58% in 2016) and loss of clinical autonomy (37% vs. 32% in 2016). Survey respondents reported working an average of 51.4 hours per week, of which 11.4 hours (22%) are spent on nonclinical (paperwork) duties.
Read the full post at hospitalleader.org.
Also on The Hospital Leader
- I don’t want someone like you caring for me by Gopi Astik, MD
- CMS added care transition codes a few years back. How’s that goin’? by Brad Flansbaum, DO, MPH, MHM
- Sleepless in the hospital no more: Lessons learned during the SIESTA Study by Vineet Arora, MD, MAPP, MHM
- Are you committing malpractice by not treating opioid use disorder in the hospital? by Chris Moriates, MD
According to a new survey report released by The Physicians Foundation, 80% of physicians across all specialties report being at full capacity or overextended, and 78% reported sometimes, often, or always experiencing feelings of burnout.
Sixty-two percent of U.S. doctors are pessimistic about the future of medicine, and 49% wouldn’t recommend medicine as a career to their children. This paints a pretty grim picture of medical practice in the United States in 2018.
The survey is conducted every other year by The Physicians Foundation with the assistance of Merritt Hawkins, and I wrote a blog post about the 2016 survey results, which showed alarming levels of disengagement and burnout. So, I thought it would be worthwhile looking over the 2018 report to see if anything has improved.
It appears that things haven’t changed much for doctors since 2016 regarding their attitudes toward their work. The biggest take-away from this year’s survey is that doctors overall are working fewer hours and seeing fewer patients but still struggling with morale and burnout. One important trend that was highlighted is the move toward employment by hospitals or integrated delivery systems; only 31% of physicians are independent practice owners or partners, vs. 49% in the first such survey conducted in 2012. Interestingly, employed doctors tend to work longer hours but see fewer patients compared with their practice-owner colleagues.
The 39-question survey is sent out via e-mail to more than 700,000 physicians (everyone the AMA or Merritt Hawkins has in their databases), and this year 8,774 physicians responded; the statistics geniuses at the University of Tennessee say the survey results have a margin of error of +/– 1.057%. Interestingly, that’s less than half of the 17,236 physicians who responded to the survey in 2016, and I wonder if the reduction in response rate itself indicates an increased level of disengagement among doctors.
Doctors expressed similar frustrations with specific aspects of their work this year, compared with 2016. The single biggest frustration cited by doctors was EHRs (39% this year vs. 27% in 2016), followed by regulatory/insurance requirements (down to 38% from 58% in 2016) and loss of clinical autonomy (37% vs. 32% in 2016). Survey respondents reported working an average of 51.4 hours per week, of which 11.4 hours (22%) are spent on nonclinical (paperwork) duties.
Read the full post at hospitalleader.org.
Also on The Hospital Leader
- I don’t want someone like you caring for me by Gopi Astik, MD
- CMS added care transition codes a few years back. How’s that goin’? by Brad Flansbaum, DO, MPH, MHM
- Sleepless in the hospital no more: Lessons learned during the SIESTA Study by Vineet Arora, MD, MAPP, MHM
- Are you committing malpractice by not treating opioid use disorder in the hospital? by Chris Moriates, MD
Patient, heal thyself!
Octavio has prostate cancer. His prostate growth is large but localized.
“What do your doctors suggest?” I asked him.
“They sent me to two specialists at the medical center,” he said. “One does robotic surgery, the other does radiation. Each one told me why they recommend their technique.”
“How will you decide?”
“I’ll do some reading,” he said.
“What about the doctor who sent you to them?”
“He hasn’t discussed the choice with me, just sent me to get opinions. I have to make up own mind.”
Out of training for some time, I gather from students and family medical interactions that patient autonomy is now a reigning principle. Here is one definition:
. Patient autonomy does allow for health care providers to educate the patient but does not allow the health care provider to make the decision for the patient.
This sounds sensible, even admirable: no more paternalistic physicians talking down to patients and ordering them around. Yet a closer look shows a contradiction:
1. The second sentence says that patient autonomy “does not allow the health care provider to make the decision for the patient.”
2. But the first one says that patients should decide, “without their health care provider trying to influence the decision.”
Is “trying to influence” the same as making the decision for the patient?
Some would argue that it is: The power discrepancy between the parties makes a doctor’s attempt to influence amount to coercion.
Do you agree, esteemed colleagues, those of you who, like me, treat patients all day? If the choice is between freezing an actinic keratosis, burning it, or using topical chemotherapy, do you just lay all three options out there and ask the patient to pick one? What if your patient works in public and doesn’t have 2 weeks to wait while the reaction to topical 5-fluorouracil that makes his skin look like raw lobster subsides? Can you point that out? Or would that be “trying to influence” and thus not allowed?
You and I can think of many other examples, about medical choices large and small, where we could pose similar questions. This is not abstract philosophy; it is what we do all day.
Look up robot-assisted surgery and radiation for prostate cancer. You will find proponents of both, each making claims concerning survival, recurrence, discomfort, complications. Which is more important – a 15% greater chance of living 2 years longer or a 22% lower risk of incontinence? Will reading such statistics make your choice easier? What if other studies show different numbers?
Octavio chose surgery. I asked him how he decided.
“I talked with an internist I know socially,” he said. “He shared his experience with patients he’s referred for my problem and advised surgery as the better choice. I also saw a story online about a lawyer who chose one method, then 5 years later had to do the other.”
Octavio is sophisticated and well read. He lives near Boston, the self-described hub of medical expertise and academic excellence. Yet he makes up his mind the way everybody does: by asking a trusted adviser, by hearing an arresting anecdote. It’s not science. It’s how people think.
You don’t have to be a behavioral psychologist to know how hard it is for patients, especially frightened ones, to interpret statistical variances or compare disparate categories. Which is better – shorter life with less pain or longer life with more? How much less? How much more? There are ways to address such questions, but having an expert, trusted, and sympathetic adviser is a pretty good way to start. Only an abstract ethicist with no practical exposure to (or sympathy with) actual existing patients and their actual existing providers could possibly think otherwise.
“Let’s freeze those actinics off,” I suggest to a patient. “That won’t scar, you won’t need a dozen shots of lidocaine, and you won’t have to hide for 3 weeks.”
Did I influence her health care decision? Sure. Guilty as charged, with no apologies. When I am a patient, I want nothing less for myself: sympathetic, experienced guidance, shared by someone who knows me and appears to care one way or the other how I do.
Lord preserve us, doctors and patients both, from dogmatists who would demand otherwise.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Octavio has prostate cancer. His prostate growth is large but localized.
“What do your doctors suggest?” I asked him.
“They sent me to two specialists at the medical center,” he said. “One does robotic surgery, the other does radiation. Each one told me why they recommend their technique.”
“How will you decide?”
“I’ll do some reading,” he said.
“What about the doctor who sent you to them?”
“He hasn’t discussed the choice with me, just sent me to get opinions. I have to make up own mind.”
Out of training for some time, I gather from students and family medical interactions that patient autonomy is now a reigning principle. Here is one definition:
. Patient autonomy does allow for health care providers to educate the patient but does not allow the health care provider to make the decision for the patient.
This sounds sensible, even admirable: no more paternalistic physicians talking down to patients and ordering them around. Yet a closer look shows a contradiction:
1. The second sentence says that patient autonomy “does not allow the health care provider to make the decision for the patient.”
2. But the first one says that patients should decide, “without their health care provider trying to influence the decision.”
Is “trying to influence” the same as making the decision for the patient?
Some would argue that it is: The power discrepancy between the parties makes a doctor’s attempt to influence amount to coercion.
Do you agree, esteemed colleagues, those of you who, like me, treat patients all day? If the choice is between freezing an actinic keratosis, burning it, or using topical chemotherapy, do you just lay all three options out there and ask the patient to pick one? What if your patient works in public and doesn’t have 2 weeks to wait while the reaction to topical 5-fluorouracil that makes his skin look like raw lobster subsides? Can you point that out? Or would that be “trying to influence” and thus not allowed?
You and I can think of many other examples, about medical choices large and small, where we could pose similar questions. This is not abstract philosophy; it is what we do all day.
Look up robot-assisted surgery and radiation for prostate cancer. You will find proponents of both, each making claims concerning survival, recurrence, discomfort, complications. Which is more important – a 15% greater chance of living 2 years longer or a 22% lower risk of incontinence? Will reading such statistics make your choice easier? What if other studies show different numbers?
Octavio chose surgery. I asked him how he decided.
“I talked with an internist I know socially,” he said. “He shared his experience with patients he’s referred for my problem and advised surgery as the better choice. I also saw a story online about a lawyer who chose one method, then 5 years later had to do the other.”
Octavio is sophisticated and well read. He lives near Boston, the self-described hub of medical expertise and academic excellence. Yet he makes up his mind the way everybody does: by asking a trusted adviser, by hearing an arresting anecdote. It’s not science. It’s how people think.
You don’t have to be a behavioral psychologist to know how hard it is for patients, especially frightened ones, to interpret statistical variances or compare disparate categories. Which is better – shorter life with less pain or longer life with more? How much less? How much more? There are ways to address such questions, but having an expert, trusted, and sympathetic adviser is a pretty good way to start. Only an abstract ethicist with no practical exposure to (or sympathy with) actual existing patients and their actual existing providers could possibly think otherwise.
“Let’s freeze those actinics off,” I suggest to a patient. “That won’t scar, you won’t need a dozen shots of lidocaine, and you won’t have to hide for 3 weeks.”
Did I influence her health care decision? Sure. Guilty as charged, with no apologies. When I am a patient, I want nothing less for myself: sympathetic, experienced guidance, shared by someone who knows me and appears to care one way or the other how I do.
Lord preserve us, doctors and patients both, from dogmatists who would demand otherwise.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Octavio has prostate cancer. His prostate growth is large but localized.
“What do your doctors suggest?” I asked him.
“They sent me to two specialists at the medical center,” he said. “One does robotic surgery, the other does radiation. Each one told me why they recommend their technique.”
“How will you decide?”
“I’ll do some reading,” he said.
“What about the doctor who sent you to them?”
“He hasn’t discussed the choice with me, just sent me to get opinions. I have to make up own mind.”
Out of training for some time, I gather from students and family medical interactions that patient autonomy is now a reigning principle. Here is one definition:
. Patient autonomy does allow for health care providers to educate the patient but does not allow the health care provider to make the decision for the patient.
This sounds sensible, even admirable: no more paternalistic physicians talking down to patients and ordering them around. Yet a closer look shows a contradiction:
1. The second sentence says that patient autonomy “does not allow the health care provider to make the decision for the patient.”
2. But the first one says that patients should decide, “without their health care provider trying to influence the decision.”
Is “trying to influence” the same as making the decision for the patient?
Some would argue that it is: The power discrepancy between the parties makes a doctor’s attempt to influence amount to coercion.
Do you agree, esteemed colleagues, those of you who, like me, treat patients all day? If the choice is between freezing an actinic keratosis, burning it, or using topical chemotherapy, do you just lay all three options out there and ask the patient to pick one? What if your patient works in public and doesn’t have 2 weeks to wait while the reaction to topical 5-fluorouracil that makes his skin look like raw lobster subsides? Can you point that out? Or would that be “trying to influence” and thus not allowed?
You and I can think of many other examples, about medical choices large and small, where we could pose similar questions. This is not abstract philosophy; it is what we do all day.
Look up robot-assisted surgery and radiation for prostate cancer. You will find proponents of both, each making claims concerning survival, recurrence, discomfort, complications. Which is more important – a 15% greater chance of living 2 years longer or a 22% lower risk of incontinence? Will reading such statistics make your choice easier? What if other studies show different numbers?
Octavio chose surgery. I asked him how he decided.
“I talked with an internist I know socially,” he said. “He shared his experience with patients he’s referred for my problem and advised surgery as the better choice. I also saw a story online about a lawyer who chose one method, then 5 years later had to do the other.”
Octavio is sophisticated and well read. He lives near Boston, the self-described hub of medical expertise and academic excellence. Yet he makes up his mind the way everybody does: by asking a trusted adviser, by hearing an arresting anecdote. It’s not science. It’s how people think.
You don’t have to be a behavioral psychologist to know how hard it is for patients, especially frightened ones, to interpret statistical variances or compare disparate categories. Which is better – shorter life with less pain or longer life with more? How much less? How much more? There are ways to address such questions, but having an expert, trusted, and sympathetic adviser is a pretty good way to start. Only an abstract ethicist with no practical exposure to (or sympathy with) actual existing patients and their actual existing providers could possibly think otherwise.
“Let’s freeze those actinics off,” I suggest to a patient. “That won’t scar, you won’t need a dozen shots of lidocaine, and you won’t have to hide for 3 weeks.”
Did I influence her health care decision? Sure. Guilty as charged, with no apologies. When I am a patient, I want nothing less for myself: sympathetic, experienced guidance, shared by someone who knows me and appears to care one way or the other how I do.
Lord preserve us, doctors and patients both, from dogmatists who would demand otherwise.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Boosting Alzheimer’s trial participation via Medicare Advantage ‘memory fitness programs’
Clinical trials represent future hope for patients seeking better care, and there is no disease more in need of better care than Alzheimer’s disease. While death rates among most cancers, as well as heart disease, HIV-related illness, and other categories, have declined in the past decade, there has been no progress for Alzheimer’s disease. Better health and wellness overall may be having a beneficial effect that has produced a reduction in age-adjusted dementia rates, but with the aging of the population there are a greater absolute number of dementia cases than ever before, and that number is expected to continue rising. Finding a disease-modifying therapy seems to be the best hope for changing this dim outlook. Clinical trials intend to do just that but are hampered by patient enrollment rates that remain low. Far fewer eligible patients enroll than are needed, causing studies to take longer to complete, driving up their costs and essentially slowing progress. There is a need to increase patient enrollment, and there has been a variety of efforts intended to address this, not the least of which has been an explosion of media coverage of Alzheimer’s disease.
The Global Alzheimer’s Platform (GAP) Foundation, a nonprofit, self-described patient-centric entity dedicated to reducing the time and cost of Alzheimer’s disease clinical trials, recently announced an initiative to increase participation in Alzheimer’s clinical trials by supporting and collaborating with “memory fitness programs” through select Medicare Advantage plans. At worst, this seems a harmless way to increase attention and hopefully interest in clinical trial participation. At best, this may be a cost-effective way to increase enrollment and even improve dementia care. Dementia is notoriously underdiagnosed, especially by overworked, busy primary care providers who simply lack the time to perform the time-consuming testing that is typically required to diagnose and follow such patients.
There are some caveats to consider. First, memory fitness programs are of dubious benefit. They generally fit the description of being harmless, but there is little compelling evidence that they preserve or improve memory.
Second, enrollment in a clinical trial, for a patient, is not always a winning proposition. To date, there has been little success and in the absence of benefit, any downside – even if simply an inconvenience – is a net negative. Recently at the 2018 Clinical Trials on Alzheimer’s Disease meeting, Merck reported that patients with mild cognitive impairment receiving active treatment in the BACE1 inhibitor verubecestat trial actually declined at a more rapid rate than did those on placebo. While the absolute difference was small, and one could argue whether it was clinically significant or simply a random occurrence, it was a reminder that intervention with an experimental agent is not necessarily benign.
Third, Medicare Advantage plans, while popular in some circles, are not considered advantageous to providers so that the proliferation of inadequate reimbursement will potentially fuel the accelerating number of providers who opt out of insurance plans altogether. This is not necessarily an issue for the GAP Foundation specifically but is nonetheless an issue for anything that promotes MA plans).
Finally, it remains important to help patients and families maintain a positive outlook, especially when we have nothing better to offer. Alzheimer’s disease is not a death sentence for every patient affected. While many have difficult and heartbreaking courses, some have slowly progressive courses with relatively little impairment for an extended period of time. There are also the dementia-phobic, cognitively unimpaired individuals (or who simply have normal age-associated cognitive changes) in whom the continued drumbeat of dementia awareness and memory testing raises their paranoia ever higher. We treat deficits (or try to), but we have to live based on our preserved skills. The challenge clinicians must face with patients and families is how to maximize function while compensating for deficits and making sure that patients and families maintain their hope.
Dr. Caselli is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Clinical trials represent future hope for patients seeking better care, and there is no disease more in need of better care than Alzheimer’s disease. While death rates among most cancers, as well as heart disease, HIV-related illness, and other categories, have declined in the past decade, there has been no progress for Alzheimer’s disease. Better health and wellness overall may be having a beneficial effect that has produced a reduction in age-adjusted dementia rates, but with the aging of the population there are a greater absolute number of dementia cases than ever before, and that number is expected to continue rising. Finding a disease-modifying therapy seems to be the best hope for changing this dim outlook. Clinical trials intend to do just that but are hampered by patient enrollment rates that remain low. Far fewer eligible patients enroll than are needed, causing studies to take longer to complete, driving up their costs and essentially slowing progress. There is a need to increase patient enrollment, and there has been a variety of efforts intended to address this, not the least of which has been an explosion of media coverage of Alzheimer’s disease.
The Global Alzheimer’s Platform (GAP) Foundation, a nonprofit, self-described patient-centric entity dedicated to reducing the time and cost of Alzheimer’s disease clinical trials, recently announced an initiative to increase participation in Alzheimer’s clinical trials by supporting and collaborating with “memory fitness programs” through select Medicare Advantage plans. At worst, this seems a harmless way to increase attention and hopefully interest in clinical trial participation. At best, this may be a cost-effective way to increase enrollment and even improve dementia care. Dementia is notoriously underdiagnosed, especially by overworked, busy primary care providers who simply lack the time to perform the time-consuming testing that is typically required to diagnose and follow such patients.
There are some caveats to consider. First, memory fitness programs are of dubious benefit. They generally fit the description of being harmless, but there is little compelling evidence that they preserve or improve memory.
Second, enrollment in a clinical trial, for a patient, is not always a winning proposition. To date, there has been little success and in the absence of benefit, any downside – even if simply an inconvenience – is a net negative. Recently at the 2018 Clinical Trials on Alzheimer’s Disease meeting, Merck reported that patients with mild cognitive impairment receiving active treatment in the BACE1 inhibitor verubecestat trial actually declined at a more rapid rate than did those on placebo. While the absolute difference was small, and one could argue whether it was clinically significant or simply a random occurrence, it was a reminder that intervention with an experimental agent is not necessarily benign.
Third, Medicare Advantage plans, while popular in some circles, are not considered advantageous to providers so that the proliferation of inadequate reimbursement will potentially fuel the accelerating number of providers who opt out of insurance plans altogether. This is not necessarily an issue for the GAP Foundation specifically but is nonetheless an issue for anything that promotes MA plans).
Finally, it remains important to help patients and families maintain a positive outlook, especially when we have nothing better to offer. Alzheimer’s disease is not a death sentence for every patient affected. While many have difficult and heartbreaking courses, some have slowly progressive courses with relatively little impairment for an extended period of time. There are also the dementia-phobic, cognitively unimpaired individuals (or who simply have normal age-associated cognitive changes) in whom the continued drumbeat of dementia awareness and memory testing raises their paranoia ever higher. We treat deficits (or try to), but we have to live based on our preserved skills. The challenge clinicians must face with patients and families is how to maximize function while compensating for deficits and making sure that patients and families maintain their hope.
Dr. Caselli is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Clinical trials represent future hope for patients seeking better care, and there is no disease more in need of better care than Alzheimer’s disease. While death rates among most cancers, as well as heart disease, HIV-related illness, and other categories, have declined in the past decade, there has been no progress for Alzheimer’s disease. Better health and wellness overall may be having a beneficial effect that has produced a reduction in age-adjusted dementia rates, but with the aging of the population there are a greater absolute number of dementia cases than ever before, and that number is expected to continue rising. Finding a disease-modifying therapy seems to be the best hope for changing this dim outlook. Clinical trials intend to do just that but are hampered by patient enrollment rates that remain low. Far fewer eligible patients enroll than are needed, causing studies to take longer to complete, driving up their costs and essentially slowing progress. There is a need to increase patient enrollment, and there has been a variety of efforts intended to address this, not the least of which has been an explosion of media coverage of Alzheimer’s disease.
The Global Alzheimer’s Platform (GAP) Foundation, a nonprofit, self-described patient-centric entity dedicated to reducing the time and cost of Alzheimer’s disease clinical trials, recently announced an initiative to increase participation in Alzheimer’s clinical trials by supporting and collaborating with “memory fitness programs” through select Medicare Advantage plans. At worst, this seems a harmless way to increase attention and hopefully interest in clinical trial participation. At best, this may be a cost-effective way to increase enrollment and even improve dementia care. Dementia is notoriously underdiagnosed, especially by overworked, busy primary care providers who simply lack the time to perform the time-consuming testing that is typically required to diagnose and follow such patients.
There are some caveats to consider. First, memory fitness programs are of dubious benefit. They generally fit the description of being harmless, but there is little compelling evidence that they preserve or improve memory.
Second, enrollment in a clinical trial, for a patient, is not always a winning proposition. To date, there has been little success and in the absence of benefit, any downside – even if simply an inconvenience – is a net negative. Recently at the 2018 Clinical Trials on Alzheimer’s Disease meeting, Merck reported that patients with mild cognitive impairment receiving active treatment in the BACE1 inhibitor verubecestat trial actually declined at a more rapid rate than did those on placebo. While the absolute difference was small, and one could argue whether it was clinically significant or simply a random occurrence, it was a reminder that intervention with an experimental agent is not necessarily benign.
Third, Medicare Advantage plans, while popular in some circles, are not considered advantageous to providers so that the proliferation of inadequate reimbursement will potentially fuel the accelerating number of providers who opt out of insurance plans altogether. This is not necessarily an issue for the GAP Foundation specifically but is nonetheless an issue for anything that promotes MA plans).
Finally, it remains important to help patients and families maintain a positive outlook, especially when we have nothing better to offer. Alzheimer’s disease is not a death sentence for every patient affected. While many have difficult and heartbreaking courses, some have slowly progressive courses with relatively little impairment for an extended period of time. There are also the dementia-phobic, cognitively unimpaired individuals (or who simply have normal age-associated cognitive changes) in whom the continued drumbeat of dementia awareness and memory testing raises their paranoia ever higher. We treat deficits (or try to), but we have to live based on our preserved skills. The challenge clinicians must face with patients and families is how to maximize function while compensating for deficits and making sure that patients and families maintain their hope.
Dr. Caselli is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Is intra-articular platelet-rich plasma injection an effective treatment for knee OA?
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).
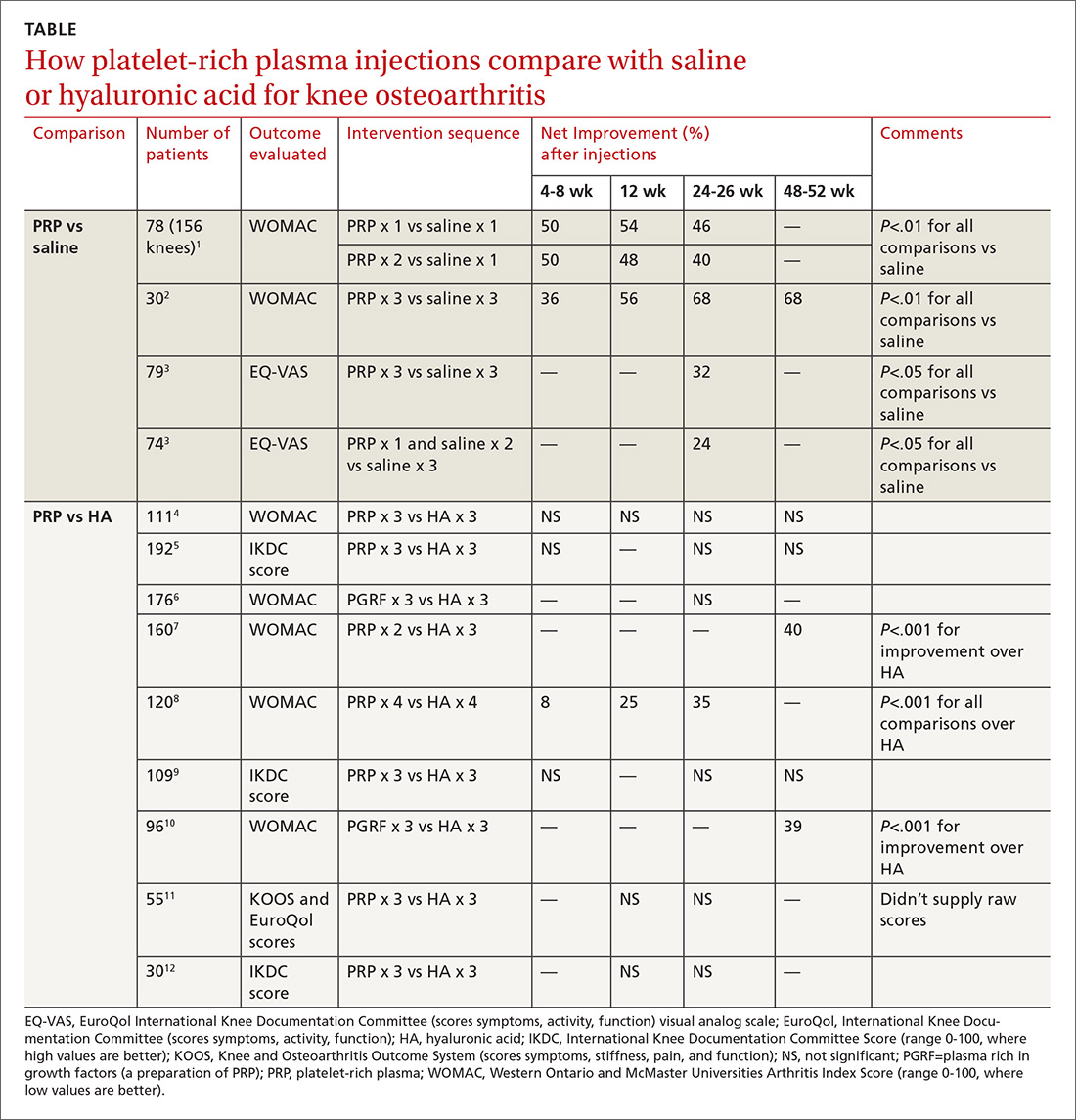
The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).

The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).

The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
EVIDENCE-BASED ANSWER:
Probably not, based on the balance of evidence. While low-quality evidence may suggest potential benefit, the balance of evidence suggests it is no better than placebo.
Compared with saline placebo, platelet-rich plasma (PRP) injections may improve standardized scores for knee osteoarthritis (OA) pain, function, and stiffness by 24% to 70% for periods of 6 to 52 weeks in patients with early to moderate OA (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with methodologic flaws).
Compared with hyaluronic acid (HA), PRP probably improves scores by a similar amount for periods of 8 to 52 weeks (SOR: B, multiple RCTs with conflicting results favoring no difference). However, since HA alone likely doesn’t improve scores more than placebo (SOR: B, RCTs of moderate quality), if both HA and PRP are about the same, then both are not better than placebo.
Vaginal approach is the most cost-effective route
Vaginal approach is the most cost-effective route
I applaud Drs. Kotha and Sanfilippo for addressing the “elephant in the room.” At the hospitals where I work, surgeons pay little or no attention to the cost of the disposables and operating room time used for their laparoscopic procedures. I believe the authors were remiss, though, to not at least mention the most minimally invasive approach to hysterectomy—the vaginal approach—which is by far the most cost-effective and safest route.
Thomas Powers, MD
Arcadia, California
Drs. Kotha and Sanfilippo respond
We appreciate Dr. Powers’ comments regarding our article on cost-conscious choices for minimally invasive gynecologic surgery. Indeed, he provides an important point. As the article’s overall focus, however, was on minimally invasive gynecologic surgery, we did not include a comparison with either abdominal or vaginal hysterectomy. Of interest, Warren and colleagues conducted a retrospective analysis of claims data in which expenditures for minimally invasive procedures (MIP) were compared with those of vaginal hysterectomy.1 For 15,404 patients who underwent surgery, costs were compared between MIP and abdominal as well as vaginal hysterectomy. Costs were as follows:
- abdominal hysterectomy, $12,086
- MIP, $10,868
- vaginal hysterectomy, $9,544.
Vaginal hysterectomy cost was statistically significantly less (P<.05) compared with other methods. The authors concluded that the laparoscopic approach should be considered when the option of an abdominal versus laparoscopic procedure is entertained. For the gynecologic surgeon considering a laparoscopic approach, the information we provided in our article merits strong consideration.
- Warren L, Ladapo JA, Borah BJ, et al. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581-588.
Vaginal approach is the most cost-effective route
I applaud Drs. Kotha and Sanfilippo for addressing the “elephant in the room.” At the hospitals where I work, surgeons pay little or no attention to the cost of the disposables and operating room time used for their laparoscopic procedures. I believe the authors were remiss, though, to not at least mention the most minimally invasive approach to hysterectomy—the vaginal approach—which is by far the most cost-effective and safest route.
Thomas Powers, MD
Arcadia, California
Drs. Kotha and Sanfilippo respond
We appreciate Dr. Powers’ comments regarding our article on cost-conscious choices for minimally invasive gynecologic surgery. Indeed, he provides an important point. As the article’s overall focus, however, was on minimally invasive gynecologic surgery, we did not include a comparison with either abdominal or vaginal hysterectomy. Of interest, Warren and colleagues conducted a retrospective analysis of claims data in which expenditures for minimally invasive procedures (MIP) were compared with those of vaginal hysterectomy.1 For 15,404 patients who underwent surgery, costs were compared between MIP and abdominal as well as vaginal hysterectomy. Costs were as follows:
- abdominal hysterectomy, $12,086
- MIP, $10,868
- vaginal hysterectomy, $9,544.
Vaginal hysterectomy cost was statistically significantly less (P<.05) compared with other methods. The authors concluded that the laparoscopic approach should be considered when the option of an abdominal versus laparoscopic procedure is entertained. For the gynecologic surgeon considering a laparoscopic approach, the information we provided in our article merits strong consideration.
Vaginal approach is the most cost-effective route
I applaud Drs. Kotha and Sanfilippo for addressing the “elephant in the room.” At the hospitals where I work, surgeons pay little or no attention to the cost of the disposables and operating room time used for their laparoscopic procedures. I believe the authors were remiss, though, to not at least mention the most minimally invasive approach to hysterectomy—the vaginal approach—which is by far the most cost-effective and safest route.
Thomas Powers, MD
Arcadia, California
Drs. Kotha and Sanfilippo respond
We appreciate Dr. Powers’ comments regarding our article on cost-conscious choices for minimally invasive gynecologic surgery. Indeed, he provides an important point. As the article’s overall focus, however, was on minimally invasive gynecologic surgery, we did not include a comparison with either abdominal or vaginal hysterectomy. Of interest, Warren and colleagues conducted a retrospective analysis of claims data in which expenditures for minimally invasive procedures (MIP) were compared with those of vaginal hysterectomy.1 For 15,404 patients who underwent surgery, costs were compared between MIP and abdominal as well as vaginal hysterectomy. Costs were as follows:
- abdominal hysterectomy, $12,086
- MIP, $10,868
- vaginal hysterectomy, $9,544.
Vaginal hysterectomy cost was statistically significantly less (P<.05) compared with other methods. The authors concluded that the laparoscopic approach should be considered when the option of an abdominal versus laparoscopic procedure is entertained. For the gynecologic surgeon considering a laparoscopic approach, the information we provided in our article merits strong consideration.
- Warren L, Ladapo JA, Borah BJ, et al. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581-588.
- Warren L, Ladapo JA, Borah BJ, et al. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581-588.
IV ketamine, intranasal esketamine likely to ‘happily coexist’
Dr. Steven Levine offers perspective on how the FDA approval will affect patients, practice
Q: Why is this a “banner day” for psychiatry?
A: This is truly the first new option for depression in 60 years. The selective serotonin reuptake inhibitors (SSRIs) developed in the mid-’80s were not truly new, not much different from the monoamine oxidase inhibitors (MAOI) and tricyclic antidepressants. In fact, they work much like watered-down MAOIs. Esketamine works by a truly novel mechanism.
Even though it constitutes a relatively new treatment, ketamine is a very old medicine, and we probably know more about the pharmacology and mechanisms in depression than for the SSRIs.
The idea of SSRIs working by increasing levels of neurotransmitters like serotonin has never held water. We never really believed that, but for people who respond to them – and many are helped – what is really happening weeks to months down the line is that these drugs increase the plasticity of the brain. Depression, like other mental health conditions, disrupts connections between important brain regions, reducing the number, function, and quality of the connections, and we believe SSRIs improve these.
Ketamine does these same things by a different route and much, much more quickly.
Q: What is esketamine’s method of action, and how long will a dose last?
A: Ketamine and esketamine bind to and block glutamate N-methyl-D-aspartate (NMDA) receptors. This leads to the release of several chemical messengers, the result of which increases the production of neurotrophic factors, in particular brain-derived neurotrophic factor (BDNF), that play a key role in healing damaged connections in the brain. A single dose of esketamine would only be expected to relieve depression symptoms for days, up to a week or 2. Multiple doses over the first few weeks can extend the durability of response to several weeks and sometimes months.
It is not true for every patient, but some do have improvement within 2-4 hours that correlates with physiologic changes. Others can be later responders and require up to 6 exposures.
Q: The FDA approval requires those who administer the drug to complete special training and meet licensure requirements. Is this realistic for small practices?
A: Initially, not every psychiatrist will be able to offer esketamine, and I think it might be beyond the reach of small practices, and that’s probably okay. Enough people and enough centers will be able to offer it to meet the initial demand.
Q: Is esketamine “better” than ketamine infusions? With the approved drug available, will ketamine infusion clinics still have a place?
A: There are major pros with this. The FDA approval takes out of the gray area of off-label administration. It will most likely be covered by insurance now – a huge advantage that will put this in the reach of so many patients who haven’t been able to access this treatment.
I think that, because there are advantages and disadvantages for both IV ketamine and nasal esketamine, they will happily coexist for years to come. However, because nasal esketamine will likely be restricted to prescription by psychiatrists, it may have more impact on non-psychiatrist-led practices. In this way,
Dr. Steven Levine is the founder of Actify Neurotherapies, which operates nine clinics providing ketamine treatment for depression.
Dr. Steven Levine offers perspective on how the FDA approval will affect patients, practice
Dr. Steven Levine offers perspective on how the FDA approval will affect patients, practice
Q: Why is this a “banner day” for psychiatry?
A: This is truly the first new option for depression in 60 years. The selective serotonin reuptake inhibitors (SSRIs) developed in the mid-’80s were not truly new, not much different from the monoamine oxidase inhibitors (MAOI) and tricyclic antidepressants. In fact, they work much like watered-down MAOIs. Esketamine works by a truly novel mechanism.
Even though it constitutes a relatively new treatment, ketamine is a very old medicine, and we probably know more about the pharmacology and mechanisms in depression than for the SSRIs.
The idea of SSRIs working by increasing levels of neurotransmitters like serotonin has never held water. We never really believed that, but for people who respond to them – and many are helped – what is really happening weeks to months down the line is that these drugs increase the plasticity of the brain. Depression, like other mental health conditions, disrupts connections between important brain regions, reducing the number, function, and quality of the connections, and we believe SSRIs improve these.
Ketamine does these same things by a different route and much, much more quickly.
Q: What is esketamine’s method of action, and how long will a dose last?
A: Ketamine and esketamine bind to and block glutamate N-methyl-D-aspartate (NMDA) receptors. This leads to the release of several chemical messengers, the result of which increases the production of neurotrophic factors, in particular brain-derived neurotrophic factor (BDNF), that play a key role in healing damaged connections in the brain. A single dose of esketamine would only be expected to relieve depression symptoms for days, up to a week or 2. Multiple doses over the first few weeks can extend the durability of response to several weeks and sometimes months.
It is not true for every patient, but some do have improvement within 2-4 hours that correlates with physiologic changes. Others can be later responders and require up to 6 exposures.
Q: The FDA approval requires those who administer the drug to complete special training and meet licensure requirements. Is this realistic for small practices?
A: Initially, not every psychiatrist will be able to offer esketamine, and I think it might be beyond the reach of small practices, and that’s probably okay. Enough people and enough centers will be able to offer it to meet the initial demand.
Q: Is esketamine “better” than ketamine infusions? With the approved drug available, will ketamine infusion clinics still have a place?
A: There are major pros with this. The FDA approval takes out of the gray area of off-label administration. It will most likely be covered by insurance now – a huge advantage that will put this in the reach of so many patients who haven’t been able to access this treatment.
I think that, because there are advantages and disadvantages for both IV ketamine and nasal esketamine, they will happily coexist for years to come. However, because nasal esketamine will likely be restricted to prescription by psychiatrists, it may have more impact on non-psychiatrist-led practices. In this way,
Dr. Steven Levine is the founder of Actify Neurotherapies, which operates nine clinics providing ketamine treatment for depression.
Q: Why is this a “banner day” for psychiatry?
A: This is truly the first new option for depression in 60 years. The selective serotonin reuptake inhibitors (SSRIs) developed in the mid-’80s were not truly new, not much different from the monoamine oxidase inhibitors (MAOI) and tricyclic antidepressants. In fact, they work much like watered-down MAOIs. Esketamine works by a truly novel mechanism.
Even though it constitutes a relatively new treatment, ketamine is a very old medicine, and we probably know more about the pharmacology and mechanisms in depression than for the SSRIs.
The idea of SSRIs working by increasing levels of neurotransmitters like serotonin has never held water. We never really believed that, but for people who respond to them – and many are helped – what is really happening weeks to months down the line is that these drugs increase the plasticity of the brain. Depression, like other mental health conditions, disrupts connections between important brain regions, reducing the number, function, and quality of the connections, and we believe SSRIs improve these.
Ketamine does these same things by a different route and much, much more quickly.
Q: What is esketamine’s method of action, and how long will a dose last?
A: Ketamine and esketamine bind to and block glutamate N-methyl-D-aspartate (NMDA) receptors. This leads to the release of several chemical messengers, the result of which increases the production of neurotrophic factors, in particular brain-derived neurotrophic factor (BDNF), that play a key role in healing damaged connections in the brain. A single dose of esketamine would only be expected to relieve depression symptoms for days, up to a week or 2. Multiple doses over the first few weeks can extend the durability of response to several weeks and sometimes months.
It is not true for every patient, but some do have improvement within 2-4 hours that correlates with physiologic changes. Others can be later responders and require up to 6 exposures.
Q: The FDA approval requires those who administer the drug to complete special training and meet licensure requirements. Is this realistic for small practices?
A: Initially, not every psychiatrist will be able to offer esketamine, and I think it might be beyond the reach of small practices, and that’s probably okay. Enough people and enough centers will be able to offer it to meet the initial demand.
Q: Is esketamine “better” than ketamine infusions? With the approved drug available, will ketamine infusion clinics still have a place?
A: There are major pros with this. The FDA approval takes out of the gray area of off-label administration. It will most likely be covered by insurance now – a huge advantage that will put this in the reach of so many patients who haven’t been able to access this treatment.
I think that, because there are advantages and disadvantages for both IV ketamine and nasal esketamine, they will happily coexist for years to come. However, because nasal esketamine will likely be restricted to prescription by psychiatrists, it may have more impact on non-psychiatrist-led practices. In this way,
Dr. Steven Levine is the founder of Actify Neurotherapies, which operates nine clinics providing ketamine treatment for depression.