User login
More cost compression coming
In mid-March, the President released his FY2020 budget proposal. Traditionally, the White House budget has little relation to the ultimate budget since Congress actually creates the final iteration (assuming the government can pass a budget at all). This budget cuts funding for the NIH, Medicare, Medicaid, and most agencies not related to defense, border security, or the TSA. No matter what the final version looks like, the federal deficit will balloon as a result of last year’s tax cuts that were combined with relentless increases in entitlement program spending. The message for health care leaders is clear: Since we are responsible for an enormous percentage of committed federal and state spending, we will be in the cross-hairs of cost compression.
As we enter the 2020 election cycle in earnest, politicians will argue about “Medicare for All” versus government overreach. We will wrestle with competing philosophies of States’ Rights versus Federalism. As physicians, we must advocate for a system of funds flow and regulatory power that we believe best serves our patients within a financially sustainable framework.
On to this month’s issue – there are two stories on early-age colon cancer. A page one story adds to our understanding of the molecular pathways involved (microsatellite instability) and tumor location. Another story points out that younger CRC patients often go undiagnosed or are misdiagnosed. The AGA has published important clinical guidance about pregnancy and IBD and switching from biologic medications to biosimilars. Finally, an enormously important study, published in The Lancet, confirmed that hepatitis C treatment with direct-acting antiviral medications reduces mortality and cancer risk – something we suspected but needed confirmed.
I hope to see everyone at DDW next month.
John I. Allen, MD, MBA, AGAF
Editor in Chief
In mid-March, the President released his FY2020 budget proposal. Traditionally, the White House budget has little relation to the ultimate budget since Congress actually creates the final iteration (assuming the government can pass a budget at all). This budget cuts funding for the NIH, Medicare, Medicaid, and most agencies not related to defense, border security, or the TSA. No matter what the final version looks like, the federal deficit will balloon as a result of last year’s tax cuts that were combined with relentless increases in entitlement program spending. The message for health care leaders is clear: Since we are responsible for an enormous percentage of committed federal and state spending, we will be in the cross-hairs of cost compression.
As we enter the 2020 election cycle in earnest, politicians will argue about “Medicare for All” versus government overreach. We will wrestle with competing philosophies of States’ Rights versus Federalism. As physicians, we must advocate for a system of funds flow and regulatory power that we believe best serves our patients within a financially sustainable framework.
On to this month’s issue – there are two stories on early-age colon cancer. A page one story adds to our understanding of the molecular pathways involved (microsatellite instability) and tumor location. Another story points out that younger CRC patients often go undiagnosed or are misdiagnosed. The AGA has published important clinical guidance about pregnancy and IBD and switching from biologic medications to biosimilars. Finally, an enormously important study, published in The Lancet, confirmed that hepatitis C treatment with direct-acting antiviral medications reduces mortality and cancer risk – something we suspected but needed confirmed.
I hope to see everyone at DDW next month.
John I. Allen, MD, MBA, AGAF
Editor in Chief
In mid-March, the President released his FY2020 budget proposal. Traditionally, the White House budget has little relation to the ultimate budget since Congress actually creates the final iteration (assuming the government can pass a budget at all). This budget cuts funding for the NIH, Medicare, Medicaid, and most agencies not related to defense, border security, or the TSA. No matter what the final version looks like, the federal deficit will balloon as a result of last year’s tax cuts that were combined with relentless increases in entitlement program spending. The message for health care leaders is clear: Since we are responsible for an enormous percentage of committed federal and state spending, we will be in the cross-hairs of cost compression.
As we enter the 2020 election cycle in earnest, politicians will argue about “Medicare for All” versus government overreach. We will wrestle with competing philosophies of States’ Rights versus Federalism. As physicians, we must advocate for a system of funds flow and regulatory power that we believe best serves our patients within a financially sustainable framework.
On to this month’s issue – there are two stories on early-age colon cancer. A page one story adds to our understanding of the molecular pathways involved (microsatellite instability) and tumor location. Another story points out that younger CRC patients often go undiagnosed or are misdiagnosed. The AGA has published important clinical guidance about pregnancy and IBD and switching from biologic medications to biosimilars. Finally, an enormously important study, published in The Lancet, confirmed that hepatitis C treatment with direct-acting antiviral medications reduces mortality and cancer risk – something we suspected but needed confirmed.
I hope to see everyone at DDW next month.
John I. Allen, MD, MBA, AGAF
Editor in Chief
A.I. and U 2
In a previous Letter from Maine I wrote about a study performed in China in which more than half a million patients were diagnosed by an artificial intelligence (A.I.) system that was able to extract and analyze information from their electronic medical records. The system was at least as accurate as physicians who had access to the same data (“A.I. Shows Promise Assisting Physicians,” by Cade Metz, The New York Times, Feb. 11, 2019). I ended my column with the hopeful assumption that despite incredible advances in A.I., the practice of medicine always would include a human element. However, I left unexplained exactly how physicians would fit into the post-A.I. revolution. In the weeks since I submitted that column, I have been searching for roles that might remain for physicians after A.I. has snatched their bread and butter of diagnosis and management.
I easily can envision a system in which the patient enters her chief complaint and current symptoms into her smartphone or tablet. Using its database of the patient’s past, family, and social history, the system generates a list of laboratory and imaging studies, some of which the patient may be able to submit directly from her handheld device. For example, the system may be able to use the patient’s phone to “examine” her. The A.I. system then generates a diagnosis.
If the diagnosed condition and management is simple and straightforward, such as a rash, the information could be communicated to the patient directly, with a short paragraph of explanation and list of persistent symptoms that would indicate that the condition was not improving as expected. A contact dermatitis comes to mind here.
However, suppose the A.I. system determines that the patient has a 90% chance of having stage IV pancreatic cancer, with a life expectancy of 6 months. Is this the kind of information you would like to learn about yourself by clicking “Your Diagnosis” box on your phone while you were having lunch with a friend? Obviously, a diagnosis of this severity should be communicated human to human, even though it was generated by a highly accurate computer system. And this communication would best be done in the form of a dialogue with someone who knows the patient and has some understanding of how she might understand and cope with the information. In the absence of a prior relationship, the dialogue should occur in real time and face to face at a minimum. I guess we have to acknowledge that FaceTime or Skype might be acceptable here.
Fortunately, stage IV cancers are rare, but there are a bazillion other conditions that, while not serious, require a nuanced explanation as part of a successful management plan that takes into account the patient’s level of anxiety and cognitive abilities. A boilerplate paragraph or two spit out by an A.I. system isn’t good health care. Although I know many physicians do rely on printed handouts for conditions they feel is a no-brainer.
The bottom line is that even when a machine may be better than we are at making some diagnoses, there always will be a role for a human to help other humans understand and cope with those diagnoses. At this point, physicians would appear be the obvious choice to fill that role. How we will get reimbursed for our communication skills is unclear.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In a previous Letter from Maine I wrote about a study performed in China in which more than half a million patients were diagnosed by an artificial intelligence (A.I.) system that was able to extract and analyze information from their electronic medical records. The system was at least as accurate as physicians who had access to the same data (“A.I. Shows Promise Assisting Physicians,” by Cade Metz, The New York Times, Feb. 11, 2019). I ended my column with the hopeful assumption that despite incredible advances in A.I., the practice of medicine always would include a human element. However, I left unexplained exactly how physicians would fit into the post-A.I. revolution. In the weeks since I submitted that column, I have been searching for roles that might remain for physicians after A.I. has snatched their bread and butter of diagnosis and management.
I easily can envision a system in which the patient enters her chief complaint and current symptoms into her smartphone or tablet. Using its database of the patient’s past, family, and social history, the system generates a list of laboratory and imaging studies, some of which the patient may be able to submit directly from her handheld device. For example, the system may be able to use the patient’s phone to “examine” her. The A.I. system then generates a diagnosis.
If the diagnosed condition and management is simple and straightforward, such as a rash, the information could be communicated to the patient directly, with a short paragraph of explanation and list of persistent symptoms that would indicate that the condition was not improving as expected. A contact dermatitis comes to mind here.
However, suppose the A.I. system determines that the patient has a 90% chance of having stage IV pancreatic cancer, with a life expectancy of 6 months. Is this the kind of information you would like to learn about yourself by clicking “Your Diagnosis” box on your phone while you were having lunch with a friend? Obviously, a diagnosis of this severity should be communicated human to human, even though it was generated by a highly accurate computer system. And this communication would best be done in the form of a dialogue with someone who knows the patient and has some understanding of how she might understand and cope with the information. In the absence of a prior relationship, the dialogue should occur in real time and face to face at a minimum. I guess we have to acknowledge that FaceTime or Skype might be acceptable here.
Fortunately, stage IV cancers are rare, but there are a bazillion other conditions that, while not serious, require a nuanced explanation as part of a successful management plan that takes into account the patient’s level of anxiety and cognitive abilities. A boilerplate paragraph or two spit out by an A.I. system isn’t good health care. Although I know many physicians do rely on printed handouts for conditions they feel is a no-brainer.
The bottom line is that even when a machine may be better than we are at making some diagnoses, there always will be a role for a human to help other humans understand and cope with those diagnoses. At this point, physicians would appear be the obvious choice to fill that role. How we will get reimbursed for our communication skills is unclear.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In a previous Letter from Maine I wrote about a study performed in China in which more than half a million patients were diagnosed by an artificial intelligence (A.I.) system that was able to extract and analyze information from their electronic medical records. The system was at least as accurate as physicians who had access to the same data (“A.I. Shows Promise Assisting Physicians,” by Cade Metz, The New York Times, Feb. 11, 2019). I ended my column with the hopeful assumption that despite incredible advances in A.I., the practice of medicine always would include a human element. However, I left unexplained exactly how physicians would fit into the post-A.I. revolution. In the weeks since I submitted that column, I have been searching for roles that might remain for physicians after A.I. has snatched their bread and butter of diagnosis and management.
I easily can envision a system in which the patient enters her chief complaint and current symptoms into her smartphone or tablet. Using its database of the patient’s past, family, and social history, the system generates a list of laboratory and imaging studies, some of which the patient may be able to submit directly from her handheld device. For example, the system may be able to use the patient’s phone to “examine” her. The A.I. system then generates a diagnosis.
If the diagnosed condition and management is simple and straightforward, such as a rash, the information could be communicated to the patient directly, with a short paragraph of explanation and list of persistent symptoms that would indicate that the condition was not improving as expected. A contact dermatitis comes to mind here.
However, suppose the A.I. system determines that the patient has a 90% chance of having stage IV pancreatic cancer, with a life expectancy of 6 months. Is this the kind of information you would like to learn about yourself by clicking “Your Diagnosis” box on your phone while you were having lunch with a friend? Obviously, a diagnosis of this severity should be communicated human to human, even though it was generated by a highly accurate computer system. And this communication would best be done in the form of a dialogue with someone who knows the patient and has some understanding of how she might understand and cope with the information. In the absence of a prior relationship, the dialogue should occur in real time and face to face at a minimum. I guess we have to acknowledge that FaceTime or Skype might be acceptable here.
Fortunately, stage IV cancers are rare, but there are a bazillion other conditions that, while not serious, require a nuanced explanation as part of a successful management plan that takes into account the patient’s level of anxiety and cognitive abilities. A boilerplate paragraph or two spit out by an A.I. system isn’t good health care. Although I know many physicians do rely on printed handouts for conditions they feel is a no-brainer.
The bottom line is that even when a machine may be better than we are at making some diagnoses, there always will be a role for a human to help other humans understand and cope with those diagnoses. At this point, physicians would appear be the obvious choice to fill that role. How we will get reimbursed for our communication skills is unclear.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Sleeping poorly may mean itching more
Study results showing an association between active atopic dermatitis (AD) and poor sleep quality were published in JAMA Pediatrics by a group of dermatologists at the University of California, San Francisco (JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025). The data on the sleep quality and quantity of nearly 14,000 children were collected over span of 11 years. Of these children, slightly fewer than 5,000 met the researchers’ definition of atopic dermatitis.
Although the sleep duration of children with and without AD was not statistically different, the reports of poor sleep quality and sleep disturbances by children with AD were dramatically more frequent – a nearly 50% higher chance of having more sleep-quality disturbances. In addition, children with more severe active disease were even more likely to report poor sleep quality – almost 80%.
I suspect that you’re not surprised by these findings. You have probably heard numerous tales of poor sleep from families who have children with AD. It just makes sense that a child whose skin is dry and itchy will have trouble sleeping. I’m sure you have struggled to help parents be more diligent about applying moisturizing creams and lotions, and have been aggressive with steroid creams during flare-ups. You may have added sleep onset-promoting antihistamines when topical treatments haven’t been as effective as you had hoped.
Has your working assumption always been that if you can get the child’s skin settled down, the itching will improve and the child will have an easier time falling asleep? But have you ever considered flipping the equation over and tried to be more aggressive in managing the child’s sleep problems?
Like many other folks with psoriasis, I have noticed that my itching is worse when I am tired, and particularly worse in that evil interval between crawling into bed and falling asleep. As the grandparent of a child with AD, I have observed a similar phenomenon. While I am not going to claim that sleep deprivation causes psoriasis or AD, I think that we need to consider the association between poor sleep quality and itching as a feedback loop that must be interrupted. This means that in addition to recommending topicals and moisturizing strategies, we must learn more about our patients’ sleep habits and suggest appropriate sleep hygiene practices.
Many parents aren’t aware of the cruel paradox that an overtired child is more likely to have trouble falling asleep. Has the child been allowed to give up his nap prematurely? Is bedtime at an appropriate hour, and does it consist of a limited number of sleep-promoting rituals? Is the bedroom dark enough, cool enough, and free of electronic distractions?
Providing effective counseling on sleep hygiene is time consuming and requires that you have first convinced the parents that the child’s itching is being aggravated by his sleep deprivation and not just the other way around. Successful management may require a close working relationship between the child’s pediatrician and his dermatologist, with both physicians reinforcing each other’s message that atopic dermatitis isn’t just skin deep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired?: The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
Study results showing an association between active atopic dermatitis (AD) and poor sleep quality were published in JAMA Pediatrics by a group of dermatologists at the University of California, San Francisco (JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025). The data on the sleep quality and quantity of nearly 14,000 children were collected over span of 11 years. Of these children, slightly fewer than 5,000 met the researchers’ definition of atopic dermatitis.
Although the sleep duration of children with and without AD was not statistically different, the reports of poor sleep quality and sleep disturbances by children with AD were dramatically more frequent – a nearly 50% higher chance of having more sleep-quality disturbances. In addition, children with more severe active disease were even more likely to report poor sleep quality – almost 80%.
I suspect that you’re not surprised by these findings. You have probably heard numerous tales of poor sleep from families who have children with AD. It just makes sense that a child whose skin is dry and itchy will have trouble sleeping. I’m sure you have struggled to help parents be more diligent about applying moisturizing creams and lotions, and have been aggressive with steroid creams during flare-ups. You may have added sleep onset-promoting antihistamines when topical treatments haven’t been as effective as you had hoped.
Has your working assumption always been that if you can get the child’s skin settled down, the itching will improve and the child will have an easier time falling asleep? But have you ever considered flipping the equation over and tried to be more aggressive in managing the child’s sleep problems?
Like many other folks with psoriasis, I have noticed that my itching is worse when I am tired, and particularly worse in that evil interval between crawling into bed and falling asleep. As the grandparent of a child with AD, I have observed a similar phenomenon. While I am not going to claim that sleep deprivation causes psoriasis or AD, I think that we need to consider the association between poor sleep quality and itching as a feedback loop that must be interrupted. This means that in addition to recommending topicals and moisturizing strategies, we must learn more about our patients’ sleep habits and suggest appropriate sleep hygiene practices.
Many parents aren’t aware of the cruel paradox that an overtired child is more likely to have trouble falling asleep. Has the child been allowed to give up his nap prematurely? Is bedtime at an appropriate hour, and does it consist of a limited number of sleep-promoting rituals? Is the bedroom dark enough, cool enough, and free of electronic distractions?
Providing effective counseling on sleep hygiene is time consuming and requires that you have first convinced the parents that the child’s itching is being aggravated by his sleep deprivation and not just the other way around. Successful management may require a close working relationship between the child’s pediatrician and his dermatologist, with both physicians reinforcing each other’s message that atopic dermatitis isn’t just skin deep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired?: The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
Study results showing an association between active atopic dermatitis (AD) and poor sleep quality were published in JAMA Pediatrics by a group of dermatologists at the University of California, San Francisco (JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025). The data on the sleep quality and quantity of nearly 14,000 children were collected over span of 11 years. Of these children, slightly fewer than 5,000 met the researchers’ definition of atopic dermatitis.
Although the sleep duration of children with and without AD was not statistically different, the reports of poor sleep quality and sleep disturbances by children with AD were dramatically more frequent – a nearly 50% higher chance of having more sleep-quality disturbances. In addition, children with more severe active disease were even more likely to report poor sleep quality – almost 80%.
I suspect that you’re not surprised by these findings. You have probably heard numerous tales of poor sleep from families who have children with AD. It just makes sense that a child whose skin is dry and itchy will have trouble sleeping. I’m sure you have struggled to help parents be more diligent about applying moisturizing creams and lotions, and have been aggressive with steroid creams during flare-ups. You may have added sleep onset-promoting antihistamines when topical treatments haven’t been as effective as you had hoped.
Has your working assumption always been that if you can get the child’s skin settled down, the itching will improve and the child will have an easier time falling asleep? But have you ever considered flipping the equation over and tried to be more aggressive in managing the child’s sleep problems?
Like many other folks with psoriasis, I have noticed that my itching is worse when I am tired, and particularly worse in that evil interval between crawling into bed and falling asleep. As the grandparent of a child with AD, I have observed a similar phenomenon. While I am not going to claim that sleep deprivation causes psoriasis or AD, I think that we need to consider the association between poor sleep quality and itching as a feedback loop that must be interrupted. This means that in addition to recommending topicals and moisturizing strategies, we must learn more about our patients’ sleep habits and suggest appropriate sleep hygiene practices.
Many parents aren’t aware of the cruel paradox that an overtired child is more likely to have trouble falling asleep. Has the child been allowed to give up his nap prematurely? Is bedtime at an appropriate hour, and does it consist of a limited number of sleep-promoting rituals? Is the bedroom dark enough, cool enough, and free of electronic distractions?
Providing effective counseling on sleep hygiene is time consuming and requires that you have first convinced the parents that the child’s itching is being aggravated by his sleep deprivation and not just the other way around. Successful management may require a close working relationship between the child’s pediatrician and his dermatologist, with both physicians reinforcing each other’s message that atopic dermatitis isn’t just skin deep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired?: The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
Implementation of a population-based cirrhosis identification and management system
Cirrhosis-related morbidity and mortality is potentially preventable. Antiviral treatment in patients with cirrhosis-related to hepatitis C virus (HCV) or hepatitis B virus can prevent complications.1-3 Beta-blockers and endoscopic treatments of esophageal varices are effective in primary prophylaxis of variceal hemorrhage.4 Surveillance for hepatocellular cancer is associated with increased detection of early-stage cancer and improved survival.5 However, many patients with cirrhosis are either not diagnosed in a primary care setting, or even when diagnosed, not seen or referred to specialty clinics to receive disease-specific care,6 and thus remain at high risk for complications.
Our goal was to implement a population-based cirrhosis identification and management system (P-CIMS) to allow identification of all patients with potential cirrhosis in the health care system and to facilitate their linkage to specialty liver care. We describe the implementation of P-CIMS at a large Veterans Health Administration (VHA) hospital and present initial results about its impact on patient care.
P-CIMS Intervention
P-CIMS is a multicomponent intervention that includes a secure web-based tracking system, standardized communication templates, and care coordination protocols.
Web-based tracking system
An interdisciplinary team of clinicians, programmers, and informatics experts developed the P-CIMS software program by extending an existing comprehensive care tracking system.7 The P-CIMS program (referred to as cirrhosis tracker) extracts information from VHA’s national corporate data warehouse. VHA corporate data warehouse includes diagnosis codes, laboratory test results, vital status, and pharmacy data for each encounter in the VA since October 1999. We designed the cirrhosis tracker program to identify patients who had outpatient or inpatient encounters in the last 3 years with either at least 1 cirrhosis diagnosis (defined as any instance of previously validated International Classification of Diseases-9 and -10 codes)8; or possible cirrhosis (defined as either aspartate aminotransferase to platelet ratio index greater than 2.0 or Fibrosis-4 above 3.24 in patients with active HCV infection9 [defined based on positive HCV RNA or genotype test results]).
The user interface of the cirrhosis tracker is designed for easy patient lookup with live links to patient information extracted from the corporate data warehouse (recent laboratory test results, recent imaging studies, and appointments). The tracker also includes free-text fields that store follow-up information and alerting functions that remind the end user when to follow up with a patient. Supplementary Figure 1 shows screen-shots from the program.
We refined the program through an iterative process to ensure accuracy and completeness of data. Each data element (e.g., cirrhosis diagnosis, laboratory tests, clinic appointments) was validated using the full electronic medical record as the reference standard; this process occurred over a period of 9 months. The program can run to update patient data on a daily basis.
Standardized communication templates and care coordination protocols
Our interdisciplinary team created chart review note templates for use in the VHA electronic medical record to verify diagnosis of cirrhosis and to facilitate accurate communication with primary care providers (PCPs) and other specialty clinicians. We also designed standard patient letters to communicate the recommendations with patients. We established protocols for initial clinical reviews, patient outreach, scheduling, and follow-ups. These care coordination protocols were modified in an iterative manner during the implementation phase of P-CIMS.
Setting and patients
Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston provides care to more than 111,000 veterans, including more than 3,800 patients with cirrhosis. At the time of P-CIMS implementation, there were three hepatologists and four advanced practice providers (APP) who provided liver-related care at the MEDVAMC.
The primary goal of the initial phase of implementation was to link patients with cirrhosis to regular liver-related care. Thus, the sample was limited to patients who did not have ongoing specialty care (i.e., no liver clinic visits in the last 6 months, including patients who were never seen in liver clinics).
Implementation strategy
We used implementation facilitation (IF), an evidence-based strategy, to implement P-CIMS.10 The IF team included facilitators (F.K., D.S.), local champions (S.M., K.H.), and technical support personnel (e.g., tracker programmers). Core components of IF were leadership engagement, creation of and regular engagement with a local stakeholder group of clinicians, educational outreach to clinicians and support staff, and problem solving. The IF activities took part in two phases: preimplementation and implementation.
Preimplementation phase
We interviewed key stakeholders to identify facilitators and barriers to P-CIMS implementation. One of the implementation facilitators (F.K.) obtained facility and clinical section’s leadership support, engaged key stakeholders, and devised a local implementation plan. Stakeholders included leadership in several disciplines: hepatology, infectious diseases, and primary care. We developed a map of clinical workflow processes to describe optimal integration of P-CIMS into existing workflow (Supplementary Figure 2).
Implementation phase
The facilitators met regularly (biweekly for the first year) with the stakeholder group including local champions and clinical staff. One of the facilitators (D.S.) served as the liaison between the P-CIMS team (F.K., A.M., R.M., T.T.) and the clinic staff to ensure that no patients were getting missed and to follow through on patient referrals to care. The programmers troubleshot technical issues that arose, and both facilitators worked with clinical staff to modify workflow as needed. At the start of IF, the facilitator conducted an initial round of trainings through in-person training or with the use of screen-sharing software. The impact of P-CIMS on patient care was tracked and feedback was provided to clinical staff on a quarterly basis.
Implementation results: Linkage to liver specialty care
P-CIMS was successfully implemented at the MEDVAMC. Patient data were first extracted in October 2015 with five updates through March 2017. In total, four APP, one MD, and the facilitator used the cirrhosis tracker on a regular basis. The clinical team (APP) conducted the initial review, triage, and outreach. It took on average 7 minutes (range, 2–20 minutes) for the initial review and outreach. The APPs entered each follow-up reminder in the tracker. For example, if they negotiated a liver clinic appointment with the patient, then they entered a reminder to follow up with the date by which this step (patient seen in liver clinic) should be completed. The tracker has a built-in alerting function. The implementation team was notified (via the tracker) when these tasks were due to ensure timely receipt of recommended care processes.
We identified 2,065 patients who met the case definition of cirrhosis (diagnosed and potentially undiagnosed) and were not in regular liver care. Based on initial review, 1,507 patients had an indication to be seen in the liver clinic. Among the remaining 558, the most common reasons for not requiring liver clinic follow-up were: being seen in other facilities (138 in other VHA and 51 in outside hospitals), followed in other specialty clinics (e.g., liver transplant or infectious disease, n = 93), or absence of cirrhosis based on initial review (n = 165) (see Figure 1 for other reasons).
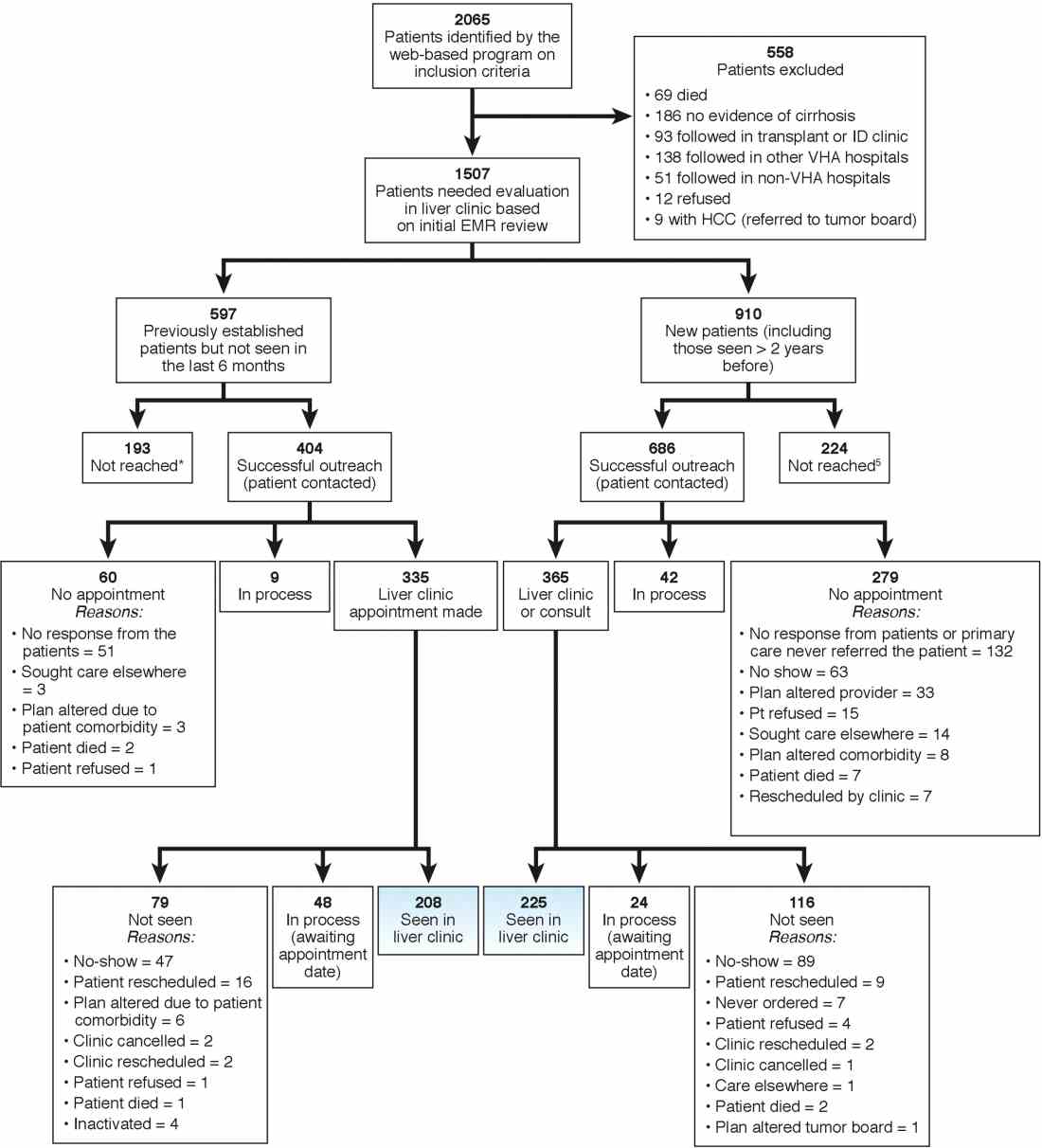
We used two different strategies to reach out to the patients. Of the 1,507 patients, 597 were previously seen in the liver clinics but were lost to follow-up. These patients were contacted directly by the liver clinic staff. The other 910 patients with cirrhosis (of 1,507) had never been seen in the ambulatory liver clinics (n = 559) or were seen more than 2 years before the implementation of cirrhosis tracker (n = 351). These patients were reached through their PCPs. We used standard electronic medical record templates to request PCP’s assistance in reviewing patients' records and submitting a liver consultation after they discussed the need for liver evaluation with the patient.
Of the 597 patients who were previously seen but lost to follow-up, we successfully contacted 404 (67.7%) patients via telephone and/or letters (for the latter, success was defined when patients called back); of these 335 (82.9%) patients had clinic appointments scheduled. In total, 208 (51.5% of 404; 34.8% of 597) patients were subsequently seen in the liver clinics during a median of 12-month follow-up. As shown in Figure 1, the most common reasons for inability to successfully link patients to the clinic were at the patient level, including no show, cancellation, and noninterest in seeking liver care. It took on average 1.5 attempts (range, 1–4) to link 214 patients to the liver clinic.
Of the other 910 patients with cirrhosis, 686 (75.4%) were successfully contacted; and of these 365 (53.2%) patients had liver clinic appointments scheduled. In total, 225 (61.7% of 365; 24.7% of 910) patients were seen in the liver clinics during a median of 12-month follow-up. The reasons underlying inability to link patients to liver specialty clinics are listed in Figure 1 and included shortfalls at the PCP and the patient levels. It took on average 2.4 attempts (range, 1–5) to link 225 patients to the liver clinic.
A total of 124 patients were initiated on direct-acting antiviral agents for HCV treatment and 18 new hepatocellular carcinoma cases were diagnosed as part of P-CIMS.
Discussion and future directions
We learned several lessons during this initiative. First, it was critical to allow time to iteratively revise the cirrhosis tracker program, with input from key stakeholders, including clinician end users. For example, based on feedback, the program was modified to exclude patients who had died or those who were seeking primary care at other VHA facilities. Second, merely having a program that accurately identifies patients with cirrhosis is not the same as knowing how to get organizations and providers to use it. We found that it was critical to involve local leadership and key stakeholders in the preimplementation phase to foster active ownership of P-CIMS and to encourage the rise of natural champions. Additionally, we focused on integrating P-CIMS in the existing workflow. We also had to be cognizant of the needs of patients, such as potential problems with communication relating to notification and appointments for evaluation. Third, several elements at the facility level played a key role in the successful implementation of P-CIMS, including the culture of the facility (commitment to quality improvement); leadership engagement; and perceived need for and relative priority of identifying and managing patients with cirrhosis, especially those with chronic HCV. We also had strong buy-in from the VHA National Program Office tasked with improving care for those with liver disease, which provided support for development of the cirrhosis tracker.
Overall, our early results show that about 30% of patients with cirrhosis without ongoing linkage to liver care were seen in the liver specialty clinics because of P-CIMS. This proportion should be interpreted in the context of the patient population and setting. Cirrhosis disproportionately affects vulnerable patients, including those who are impoverished, homeless, and with drug- and alcohol-related problems; a complex population who often have difficulty staying in care. Most patients in our sample had no linkage with specialty care. It is plausible that some patients with cirrhosis would have been seen in the liver clinics, regardless of P-CIMS. However, we expect this proportion would have been substantially lower than the 30% observed with P-CIMS.
We found several barriers to successful linkage and identified possible solutions. Our results suggest that a direct outreach to patients (without going through PCP) may result in fewer failures to linkage. In total, about 35% of patients who were contacted directly by the liver clinic met the endpoint compared with about 25% of patients who were contacted via their PCP. Future iterations of P-CIMS will rely on direct outreach for most patients. We also found that many patients were unable to keep scheduled appointments; some of this was because of inability to come on specific days and times. Open-access clinics may be one way to accommodate these high-risk patients. Although a full cost-effectiveness analysis is beyond the scope of this report, annual cost of maintaining P-CIMS was less than $100,000 (facilitator and programming support), which is equivalent to antiviral treatment cost of four to five HCV patients, suggesting that P-CIMS (with ability to reach out to hundreds of patients) may indeed be cost effective (if not cost saving).
In summary, we built and successfully implemented a population-based health management system with a structured care coordination strategy to facilitate identification and linkage to care of patients with cirrhosis. Our initial results suggest modest success in managing a complex population who often have difficulty staying in care. The next steps include comparing the rates of linkage to specialty care with rates in comparable facilities that did not use the tracker; broadening the scope to ensure patients are retained in care and receive guideline-concordant care over time. We will share these results in a subsequent manuscript. To our knowledge, cirrhosis tracker is the first informatics tool that leverages data from the electronic medical records with other tools and strategies to improve quality of cirrhosis care. We believe that the lessons that we learned can also help inform efforts to design programs that encourage use of administrative data–based risk screeners to identify patients with other chronic conditions who are at risk for suboptimal outcomes.
References
1. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-16.
2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996-1005.
3. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-31.
4. Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-8.
5. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099-106.
6. Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-7.
7. Taddei T, Hunnibell L, DeLorenzo A, et al. EMR-linked cancer tracker facilitates lung and liver cancer care. J Clin Oncol. 2012;30:77.
8. Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-82.
9. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-20.
10. Kirchner JE, Ritchie MJ, Pitcock JA, et al. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 2014;29:904-12.
Dr. Kanwal is professor of medicine, chief of gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Mapakshi is a fellow in gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Centerof Excellence, Michael E. DeBakey VA Medical Center; Ms. Smith is project manager at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Taddei is director of the HCC Initiative, VA Connecticut Healthcare System, associate professor of medicine, digestive diseases, Yale University School of Medicine, director, liver cancer team, Smilow Cancer Hospital at Yale New Haven Hospital; Dr. Hussain is assistant professor, Baylor College of Medicine, Michael E. DeBakey VA Medical Center; Ms. Madu is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Ms. Duong is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Dr. White is assistant professor of medicine, health services research, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Cao is a statistical analyst at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Mehta is in Health Services Research at the VA Connecticut Healthcare System, Yale University School of Medicine, New Haven; Dr. El-Serag is Chairman and Professor Margaret M. and Albert B. Alkek, department of medicine, Baylor College of Medicine, Houston; Dr. Asch is chief of health service research, director of HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System , Palo Alto, Calif., professor of medicine, primary care and population health, Stanford, Calif.; Dr. Midboe is co-implementation research coordinator, HIV/Hepatitis QUERI, director VA patient safety center of inquiry, HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System, Palo Alto, Calif. The authors disclose no conflicts. This material is based on work supported by Department of Veterans Affairs, QUERI Program, QUE 15-284, VA HIV, Hepatitis C, and Related Conditions Program, and VA National Center for Patient Safety. The work is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413); Michael E. DeBakey VA Medical Center, Houston, Tex.; and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Cirrhosis-related morbidity and mortality is potentially preventable. Antiviral treatment in patients with cirrhosis-related to hepatitis C virus (HCV) or hepatitis B virus can prevent complications.1-3 Beta-blockers and endoscopic treatments of esophageal varices are effective in primary prophylaxis of variceal hemorrhage.4 Surveillance for hepatocellular cancer is associated with increased detection of early-stage cancer and improved survival.5 However, many patients with cirrhosis are either not diagnosed in a primary care setting, or even when diagnosed, not seen or referred to specialty clinics to receive disease-specific care,6 and thus remain at high risk for complications.
Our goal was to implement a population-based cirrhosis identification and management system (P-CIMS) to allow identification of all patients with potential cirrhosis in the health care system and to facilitate their linkage to specialty liver care. We describe the implementation of P-CIMS at a large Veterans Health Administration (VHA) hospital and present initial results about its impact on patient care.
P-CIMS Intervention
P-CIMS is a multicomponent intervention that includes a secure web-based tracking system, standardized communication templates, and care coordination protocols.
Web-based tracking system
An interdisciplinary team of clinicians, programmers, and informatics experts developed the P-CIMS software program by extending an existing comprehensive care tracking system.7 The P-CIMS program (referred to as cirrhosis tracker) extracts information from VHA’s national corporate data warehouse. VHA corporate data warehouse includes diagnosis codes, laboratory test results, vital status, and pharmacy data for each encounter in the VA since October 1999. We designed the cirrhosis tracker program to identify patients who had outpatient or inpatient encounters in the last 3 years with either at least 1 cirrhosis diagnosis (defined as any instance of previously validated International Classification of Diseases-9 and -10 codes)8; or possible cirrhosis (defined as either aspartate aminotransferase to platelet ratio index greater than 2.0 or Fibrosis-4 above 3.24 in patients with active HCV infection9 [defined based on positive HCV RNA or genotype test results]).
The user interface of the cirrhosis tracker is designed for easy patient lookup with live links to patient information extracted from the corporate data warehouse (recent laboratory test results, recent imaging studies, and appointments). The tracker also includes free-text fields that store follow-up information and alerting functions that remind the end user when to follow up with a patient. Supplementary Figure 1 shows screen-shots from the program.
We refined the program through an iterative process to ensure accuracy and completeness of data. Each data element (e.g., cirrhosis diagnosis, laboratory tests, clinic appointments) was validated using the full electronic medical record as the reference standard; this process occurred over a period of 9 months. The program can run to update patient data on a daily basis.
Standardized communication templates and care coordination protocols
Our interdisciplinary team created chart review note templates for use in the VHA electronic medical record to verify diagnosis of cirrhosis and to facilitate accurate communication with primary care providers (PCPs) and other specialty clinicians. We also designed standard patient letters to communicate the recommendations with patients. We established protocols for initial clinical reviews, patient outreach, scheduling, and follow-ups. These care coordination protocols were modified in an iterative manner during the implementation phase of P-CIMS.
Setting and patients
Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston provides care to more than 111,000 veterans, including more than 3,800 patients with cirrhosis. At the time of P-CIMS implementation, there were three hepatologists and four advanced practice providers (APP) who provided liver-related care at the MEDVAMC.
The primary goal of the initial phase of implementation was to link patients with cirrhosis to regular liver-related care. Thus, the sample was limited to patients who did not have ongoing specialty care (i.e., no liver clinic visits in the last 6 months, including patients who were never seen in liver clinics).
Implementation strategy
We used implementation facilitation (IF), an evidence-based strategy, to implement P-CIMS.10 The IF team included facilitators (F.K., D.S.), local champions (S.M., K.H.), and technical support personnel (e.g., tracker programmers). Core components of IF were leadership engagement, creation of and regular engagement with a local stakeholder group of clinicians, educational outreach to clinicians and support staff, and problem solving. The IF activities took part in two phases: preimplementation and implementation.
Preimplementation phase
We interviewed key stakeholders to identify facilitators and barriers to P-CIMS implementation. One of the implementation facilitators (F.K.) obtained facility and clinical section’s leadership support, engaged key stakeholders, and devised a local implementation plan. Stakeholders included leadership in several disciplines: hepatology, infectious diseases, and primary care. We developed a map of clinical workflow processes to describe optimal integration of P-CIMS into existing workflow (Supplementary Figure 2).
Implementation phase
The facilitators met regularly (biweekly for the first year) with the stakeholder group including local champions and clinical staff. One of the facilitators (D.S.) served as the liaison between the P-CIMS team (F.K., A.M., R.M., T.T.) and the clinic staff to ensure that no patients were getting missed and to follow through on patient referrals to care. The programmers troubleshot technical issues that arose, and both facilitators worked with clinical staff to modify workflow as needed. At the start of IF, the facilitator conducted an initial round of trainings through in-person training or with the use of screen-sharing software. The impact of P-CIMS on patient care was tracked and feedback was provided to clinical staff on a quarterly basis.
Implementation results: Linkage to liver specialty care
P-CIMS was successfully implemented at the MEDVAMC. Patient data were first extracted in October 2015 with five updates through March 2017. In total, four APP, one MD, and the facilitator used the cirrhosis tracker on a regular basis. The clinical team (APP) conducted the initial review, triage, and outreach. It took on average 7 minutes (range, 2–20 minutes) for the initial review and outreach. The APPs entered each follow-up reminder in the tracker. For example, if they negotiated a liver clinic appointment with the patient, then they entered a reminder to follow up with the date by which this step (patient seen in liver clinic) should be completed. The tracker has a built-in alerting function. The implementation team was notified (via the tracker) when these tasks were due to ensure timely receipt of recommended care processes.
We identified 2,065 patients who met the case definition of cirrhosis (diagnosed and potentially undiagnosed) and were not in regular liver care. Based on initial review, 1,507 patients had an indication to be seen in the liver clinic. Among the remaining 558, the most common reasons for not requiring liver clinic follow-up were: being seen in other facilities (138 in other VHA and 51 in outside hospitals), followed in other specialty clinics (e.g., liver transplant or infectious disease, n = 93), or absence of cirrhosis based on initial review (n = 165) (see Figure 1 for other reasons).

We used two different strategies to reach out to the patients. Of the 1,507 patients, 597 were previously seen in the liver clinics but were lost to follow-up. These patients were contacted directly by the liver clinic staff. The other 910 patients with cirrhosis (of 1,507) had never been seen in the ambulatory liver clinics (n = 559) or were seen more than 2 years before the implementation of cirrhosis tracker (n = 351). These patients were reached through their PCPs. We used standard electronic medical record templates to request PCP’s assistance in reviewing patients' records and submitting a liver consultation after they discussed the need for liver evaluation with the patient.
Of the 597 patients who were previously seen but lost to follow-up, we successfully contacted 404 (67.7%) patients via telephone and/or letters (for the latter, success was defined when patients called back); of these 335 (82.9%) patients had clinic appointments scheduled. In total, 208 (51.5% of 404; 34.8% of 597) patients were subsequently seen in the liver clinics during a median of 12-month follow-up. As shown in Figure 1, the most common reasons for inability to successfully link patients to the clinic were at the patient level, including no show, cancellation, and noninterest in seeking liver care. It took on average 1.5 attempts (range, 1–4) to link 214 patients to the liver clinic.
Of the other 910 patients with cirrhosis, 686 (75.4%) were successfully contacted; and of these 365 (53.2%) patients had liver clinic appointments scheduled. In total, 225 (61.7% of 365; 24.7% of 910) patients were seen in the liver clinics during a median of 12-month follow-up. The reasons underlying inability to link patients to liver specialty clinics are listed in Figure 1 and included shortfalls at the PCP and the patient levels. It took on average 2.4 attempts (range, 1–5) to link 225 patients to the liver clinic.
A total of 124 patients were initiated on direct-acting antiviral agents for HCV treatment and 18 new hepatocellular carcinoma cases were diagnosed as part of P-CIMS.
Discussion and future directions
We learned several lessons during this initiative. First, it was critical to allow time to iteratively revise the cirrhosis tracker program, with input from key stakeholders, including clinician end users. For example, based on feedback, the program was modified to exclude patients who had died or those who were seeking primary care at other VHA facilities. Second, merely having a program that accurately identifies patients with cirrhosis is not the same as knowing how to get organizations and providers to use it. We found that it was critical to involve local leadership and key stakeholders in the preimplementation phase to foster active ownership of P-CIMS and to encourage the rise of natural champions. Additionally, we focused on integrating P-CIMS in the existing workflow. We also had to be cognizant of the needs of patients, such as potential problems with communication relating to notification and appointments for evaluation. Third, several elements at the facility level played a key role in the successful implementation of P-CIMS, including the culture of the facility (commitment to quality improvement); leadership engagement; and perceived need for and relative priority of identifying and managing patients with cirrhosis, especially those with chronic HCV. We also had strong buy-in from the VHA National Program Office tasked with improving care for those with liver disease, which provided support for development of the cirrhosis tracker.
Overall, our early results show that about 30% of patients with cirrhosis without ongoing linkage to liver care were seen in the liver specialty clinics because of P-CIMS. This proportion should be interpreted in the context of the patient population and setting. Cirrhosis disproportionately affects vulnerable patients, including those who are impoverished, homeless, and with drug- and alcohol-related problems; a complex population who often have difficulty staying in care. Most patients in our sample had no linkage with specialty care. It is plausible that some patients with cirrhosis would have been seen in the liver clinics, regardless of P-CIMS. However, we expect this proportion would have been substantially lower than the 30% observed with P-CIMS.
We found several barriers to successful linkage and identified possible solutions. Our results suggest that a direct outreach to patients (without going through PCP) may result in fewer failures to linkage. In total, about 35% of patients who were contacted directly by the liver clinic met the endpoint compared with about 25% of patients who were contacted via their PCP. Future iterations of P-CIMS will rely on direct outreach for most patients. We also found that many patients were unable to keep scheduled appointments; some of this was because of inability to come on specific days and times. Open-access clinics may be one way to accommodate these high-risk patients. Although a full cost-effectiveness analysis is beyond the scope of this report, annual cost of maintaining P-CIMS was less than $100,000 (facilitator and programming support), which is equivalent to antiviral treatment cost of four to five HCV patients, suggesting that P-CIMS (with ability to reach out to hundreds of patients) may indeed be cost effective (if not cost saving).
In summary, we built and successfully implemented a population-based health management system with a structured care coordination strategy to facilitate identification and linkage to care of patients with cirrhosis. Our initial results suggest modest success in managing a complex population who often have difficulty staying in care. The next steps include comparing the rates of linkage to specialty care with rates in comparable facilities that did not use the tracker; broadening the scope to ensure patients are retained in care and receive guideline-concordant care over time. We will share these results in a subsequent manuscript. To our knowledge, cirrhosis tracker is the first informatics tool that leverages data from the electronic medical records with other tools and strategies to improve quality of cirrhosis care. We believe that the lessons that we learned can also help inform efforts to design programs that encourage use of administrative data–based risk screeners to identify patients with other chronic conditions who are at risk for suboptimal outcomes.
References
1. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-16.
2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996-1005.
3. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-31.
4. Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-8.
5. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099-106.
6. Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-7.
7. Taddei T, Hunnibell L, DeLorenzo A, et al. EMR-linked cancer tracker facilitates lung and liver cancer care. J Clin Oncol. 2012;30:77.
8. Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-82.
9. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-20.
10. Kirchner JE, Ritchie MJ, Pitcock JA, et al. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 2014;29:904-12.
Dr. Kanwal is professor of medicine, chief of gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Mapakshi is a fellow in gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Centerof Excellence, Michael E. DeBakey VA Medical Center; Ms. Smith is project manager at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Taddei is director of the HCC Initiative, VA Connecticut Healthcare System, associate professor of medicine, digestive diseases, Yale University School of Medicine, director, liver cancer team, Smilow Cancer Hospital at Yale New Haven Hospital; Dr. Hussain is assistant professor, Baylor College of Medicine, Michael E. DeBakey VA Medical Center; Ms. Madu is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Ms. Duong is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Dr. White is assistant professor of medicine, health services research, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Cao is a statistical analyst at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Mehta is in Health Services Research at the VA Connecticut Healthcare System, Yale University School of Medicine, New Haven; Dr. El-Serag is Chairman and Professor Margaret M. and Albert B. Alkek, department of medicine, Baylor College of Medicine, Houston; Dr. Asch is chief of health service research, director of HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System , Palo Alto, Calif., professor of medicine, primary care and population health, Stanford, Calif.; Dr. Midboe is co-implementation research coordinator, HIV/Hepatitis QUERI, director VA patient safety center of inquiry, HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System, Palo Alto, Calif. The authors disclose no conflicts. This material is based on work supported by Department of Veterans Affairs, QUERI Program, QUE 15-284, VA HIV, Hepatitis C, and Related Conditions Program, and VA National Center for Patient Safety. The work is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413); Michael E. DeBakey VA Medical Center, Houston, Tex.; and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Cirrhosis-related morbidity and mortality is potentially preventable. Antiviral treatment in patients with cirrhosis-related to hepatitis C virus (HCV) or hepatitis B virus can prevent complications.1-3 Beta-blockers and endoscopic treatments of esophageal varices are effective in primary prophylaxis of variceal hemorrhage.4 Surveillance for hepatocellular cancer is associated with increased detection of early-stage cancer and improved survival.5 However, many patients with cirrhosis are either not diagnosed in a primary care setting, or even when diagnosed, not seen or referred to specialty clinics to receive disease-specific care,6 and thus remain at high risk for complications.
Our goal was to implement a population-based cirrhosis identification and management system (P-CIMS) to allow identification of all patients with potential cirrhosis in the health care system and to facilitate their linkage to specialty liver care. We describe the implementation of P-CIMS at a large Veterans Health Administration (VHA) hospital and present initial results about its impact on patient care.
P-CIMS Intervention
P-CIMS is a multicomponent intervention that includes a secure web-based tracking system, standardized communication templates, and care coordination protocols.
Web-based tracking system
An interdisciplinary team of clinicians, programmers, and informatics experts developed the P-CIMS software program by extending an existing comprehensive care tracking system.7 The P-CIMS program (referred to as cirrhosis tracker) extracts information from VHA’s national corporate data warehouse. VHA corporate data warehouse includes diagnosis codes, laboratory test results, vital status, and pharmacy data for each encounter in the VA since October 1999. We designed the cirrhosis tracker program to identify patients who had outpatient or inpatient encounters in the last 3 years with either at least 1 cirrhosis diagnosis (defined as any instance of previously validated International Classification of Diseases-9 and -10 codes)8; or possible cirrhosis (defined as either aspartate aminotransferase to platelet ratio index greater than 2.0 or Fibrosis-4 above 3.24 in patients with active HCV infection9 [defined based on positive HCV RNA or genotype test results]).
The user interface of the cirrhosis tracker is designed for easy patient lookup with live links to patient information extracted from the corporate data warehouse (recent laboratory test results, recent imaging studies, and appointments). The tracker also includes free-text fields that store follow-up information and alerting functions that remind the end user when to follow up with a patient. Supplementary Figure 1 shows screen-shots from the program.
We refined the program through an iterative process to ensure accuracy and completeness of data. Each data element (e.g., cirrhosis diagnosis, laboratory tests, clinic appointments) was validated using the full electronic medical record as the reference standard; this process occurred over a period of 9 months. The program can run to update patient data on a daily basis.
Standardized communication templates and care coordination protocols
Our interdisciplinary team created chart review note templates for use in the VHA electronic medical record to verify diagnosis of cirrhosis and to facilitate accurate communication with primary care providers (PCPs) and other specialty clinicians. We also designed standard patient letters to communicate the recommendations with patients. We established protocols for initial clinical reviews, patient outreach, scheduling, and follow-ups. These care coordination protocols were modified in an iterative manner during the implementation phase of P-CIMS.
Setting and patients
Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston provides care to more than 111,000 veterans, including more than 3,800 patients with cirrhosis. At the time of P-CIMS implementation, there were three hepatologists and four advanced practice providers (APP) who provided liver-related care at the MEDVAMC.
The primary goal of the initial phase of implementation was to link patients with cirrhosis to regular liver-related care. Thus, the sample was limited to patients who did not have ongoing specialty care (i.e., no liver clinic visits in the last 6 months, including patients who were never seen in liver clinics).
Implementation strategy
We used implementation facilitation (IF), an evidence-based strategy, to implement P-CIMS.10 The IF team included facilitators (F.K., D.S.), local champions (S.M., K.H.), and technical support personnel (e.g., tracker programmers). Core components of IF were leadership engagement, creation of and regular engagement with a local stakeholder group of clinicians, educational outreach to clinicians and support staff, and problem solving. The IF activities took part in two phases: preimplementation and implementation.
Preimplementation phase
We interviewed key stakeholders to identify facilitators and barriers to P-CIMS implementation. One of the implementation facilitators (F.K.) obtained facility and clinical section’s leadership support, engaged key stakeholders, and devised a local implementation plan. Stakeholders included leadership in several disciplines: hepatology, infectious diseases, and primary care. We developed a map of clinical workflow processes to describe optimal integration of P-CIMS into existing workflow (Supplementary Figure 2).
Implementation phase
The facilitators met regularly (biweekly for the first year) with the stakeholder group including local champions and clinical staff. One of the facilitators (D.S.) served as the liaison between the P-CIMS team (F.K., A.M., R.M., T.T.) and the clinic staff to ensure that no patients were getting missed and to follow through on patient referrals to care. The programmers troubleshot technical issues that arose, and both facilitators worked with clinical staff to modify workflow as needed. At the start of IF, the facilitator conducted an initial round of trainings through in-person training or with the use of screen-sharing software. The impact of P-CIMS on patient care was tracked and feedback was provided to clinical staff on a quarterly basis.
Implementation results: Linkage to liver specialty care
P-CIMS was successfully implemented at the MEDVAMC. Patient data were first extracted in October 2015 with five updates through March 2017. In total, four APP, one MD, and the facilitator used the cirrhosis tracker on a regular basis. The clinical team (APP) conducted the initial review, triage, and outreach. It took on average 7 minutes (range, 2–20 minutes) for the initial review and outreach. The APPs entered each follow-up reminder in the tracker. For example, if they negotiated a liver clinic appointment with the patient, then they entered a reminder to follow up with the date by which this step (patient seen in liver clinic) should be completed. The tracker has a built-in alerting function. The implementation team was notified (via the tracker) when these tasks were due to ensure timely receipt of recommended care processes.
We identified 2,065 patients who met the case definition of cirrhosis (diagnosed and potentially undiagnosed) and were not in regular liver care. Based on initial review, 1,507 patients had an indication to be seen in the liver clinic. Among the remaining 558, the most common reasons for not requiring liver clinic follow-up were: being seen in other facilities (138 in other VHA and 51 in outside hospitals), followed in other specialty clinics (e.g., liver transplant or infectious disease, n = 93), or absence of cirrhosis based on initial review (n = 165) (see Figure 1 for other reasons).

We used two different strategies to reach out to the patients. Of the 1,507 patients, 597 were previously seen in the liver clinics but were lost to follow-up. These patients were contacted directly by the liver clinic staff. The other 910 patients with cirrhosis (of 1,507) had never been seen in the ambulatory liver clinics (n = 559) or were seen more than 2 years before the implementation of cirrhosis tracker (n = 351). These patients were reached through their PCPs. We used standard electronic medical record templates to request PCP’s assistance in reviewing patients' records and submitting a liver consultation after they discussed the need for liver evaluation with the patient.
Of the 597 patients who were previously seen but lost to follow-up, we successfully contacted 404 (67.7%) patients via telephone and/or letters (for the latter, success was defined when patients called back); of these 335 (82.9%) patients had clinic appointments scheduled. In total, 208 (51.5% of 404; 34.8% of 597) patients were subsequently seen in the liver clinics during a median of 12-month follow-up. As shown in Figure 1, the most common reasons for inability to successfully link patients to the clinic were at the patient level, including no show, cancellation, and noninterest in seeking liver care. It took on average 1.5 attempts (range, 1–4) to link 214 patients to the liver clinic.
Of the other 910 patients with cirrhosis, 686 (75.4%) were successfully contacted; and of these 365 (53.2%) patients had liver clinic appointments scheduled. In total, 225 (61.7% of 365; 24.7% of 910) patients were seen in the liver clinics during a median of 12-month follow-up. The reasons underlying inability to link patients to liver specialty clinics are listed in Figure 1 and included shortfalls at the PCP and the patient levels. It took on average 2.4 attempts (range, 1–5) to link 225 patients to the liver clinic.
A total of 124 patients were initiated on direct-acting antiviral agents for HCV treatment and 18 new hepatocellular carcinoma cases were diagnosed as part of P-CIMS.
Discussion and future directions
We learned several lessons during this initiative. First, it was critical to allow time to iteratively revise the cirrhosis tracker program, with input from key stakeholders, including clinician end users. For example, based on feedback, the program was modified to exclude patients who had died or those who were seeking primary care at other VHA facilities. Second, merely having a program that accurately identifies patients with cirrhosis is not the same as knowing how to get organizations and providers to use it. We found that it was critical to involve local leadership and key stakeholders in the preimplementation phase to foster active ownership of P-CIMS and to encourage the rise of natural champions. Additionally, we focused on integrating P-CIMS in the existing workflow. We also had to be cognizant of the needs of patients, such as potential problems with communication relating to notification and appointments for evaluation. Third, several elements at the facility level played a key role in the successful implementation of P-CIMS, including the culture of the facility (commitment to quality improvement); leadership engagement; and perceived need for and relative priority of identifying and managing patients with cirrhosis, especially those with chronic HCV. We also had strong buy-in from the VHA National Program Office tasked with improving care for those with liver disease, which provided support for development of the cirrhosis tracker.
Overall, our early results show that about 30% of patients with cirrhosis without ongoing linkage to liver care were seen in the liver specialty clinics because of P-CIMS. This proportion should be interpreted in the context of the patient population and setting. Cirrhosis disproportionately affects vulnerable patients, including those who are impoverished, homeless, and with drug- and alcohol-related problems; a complex population who often have difficulty staying in care. Most patients in our sample had no linkage with specialty care. It is plausible that some patients with cirrhosis would have been seen in the liver clinics, regardless of P-CIMS. However, we expect this proportion would have been substantially lower than the 30% observed with P-CIMS.
We found several barriers to successful linkage and identified possible solutions. Our results suggest that a direct outreach to patients (without going through PCP) may result in fewer failures to linkage. In total, about 35% of patients who were contacted directly by the liver clinic met the endpoint compared with about 25% of patients who were contacted via their PCP. Future iterations of P-CIMS will rely on direct outreach for most patients. We also found that many patients were unable to keep scheduled appointments; some of this was because of inability to come on specific days and times. Open-access clinics may be one way to accommodate these high-risk patients. Although a full cost-effectiveness analysis is beyond the scope of this report, annual cost of maintaining P-CIMS was less than $100,000 (facilitator and programming support), which is equivalent to antiviral treatment cost of four to five HCV patients, suggesting that P-CIMS (with ability to reach out to hundreds of patients) may indeed be cost effective (if not cost saving).
In summary, we built and successfully implemented a population-based health management system with a structured care coordination strategy to facilitate identification and linkage to care of patients with cirrhosis. Our initial results suggest modest success in managing a complex population who often have difficulty staying in care. The next steps include comparing the rates of linkage to specialty care with rates in comparable facilities that did not use the tracker; broadening the scope to ensure patients are retained in care and receive guideline-concordant care over time. We will share these results in a subsequent manuscript. To our knowledge, cirrhosis tracker is the first informatics tool that leverages data from the electronic medical records with other tools and strategies to improve quality of cirrhosis care. We believe that the lessons that we learned can also help inform efforts to design programs that encourage use of administrative data–based risk screeners to identify patients with other chronic conditions who are at risk for suboptimal outcomes.
References
1. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-16.
2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996-1005.
3. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-31.
4. Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-8.
5. Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130:1099-106.
6. Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-7.
7. Taddei T, Hunnibell L, DeLorenzo A, et al. EMR-linked cancer tracker facilitates lung and liver cancer care. J Clin Oncol. 2012;30:77.
8. Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-82.
9. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807-20.
10. Kirchner JE, Ritchie MJ, Pitcock JA, et al. Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 2014;29:904-12.
Dr. Kanwal is professor of medicine, chief of gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Mapakshi is a fellow in gastroenterology and hepatology, Baylor College of Medicine, Houston Veterans Affairs HSR&D Centerof Excellence, Michael E. DeBakey VA Medical Center; Ms. Smith is project manager at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Dr. Taddei is director of the HCC Initiative, VA Connecticut Healthcare System, associate professor of medicine, digestive diseases, Yale University School of Medicine, director, liver cancer team, Smilow Cancer Hospital at Yale New Haven Hospital; Dr. Hussain is assistant professor, Baylor College of Medicine, Michael E. DeBakey VA Medical Center; Ms. Madu is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Ms. Duong is in gastroenterology and hepatology, Michael E. DeBakey VA Medical Center; Dr. White is assistant professor of medicine, health services research, Baylor College of Medicine, Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Cao is a statistical analyst at Houston Veterans Affairs HSR&D Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center; Ms. Mehta is in Health Services Research at the VA Connecticut Healthcare System, Yale University School of Medicine, New Haven; Dr. El-Serag is Chairman and Professor Margaret M. and Albert B. Alkek, department of medicine, Baylor College of Medicine, Houston; Dr. Asch is chief of health service research, director of HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System , Palo Alto, Calif., professor of medicine, primary care and population health, Stanford, Calif.; Dr. Midboe is co-implementation research coordinator, HIV/Hepatitis QUERI, director VA patient safety center of inquiry, HSR&D Center for Innovation to Implementation, VA Palo Alto Health Care System, Palo Alto, Calif. The authors disclose no conflicts. This material is based on work supported by Department of Veterans Affairs, QUERI Program, QUE 15-284, VA HIV, Hepatitis C, and Related Conditions Program, and VA National Center for Patient Safety. The work is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413); Michael E. DeBakey VA Medical Center, Houston, Tex.; and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Performance-based pay linked to anxiety, depression
A study from researchers at Washington University in St. Louis and Aarhus University in Denmark charts the relationship of payment based on work done and employee mental health. According to phys.org, an aggregator of science, research, and technology news, performance-based pay is in place in 70% of U.S. companies. This means that employee income is based on a combination of bonuses, commission, profit sharing, and individual/team incentives, rather than guaranteed salaries. For some employees, performance-based pay can prove lucrative. But for others, such systems can lead to poor mental health.
The study, published in the Academy of Management Discoveries, charted the use of prescription medications for anxiety and depression by nearly 319,000 employees at about 1,300 companies in Denmark (2019 Feb 26. doi: 10.5465/amd.2018.0007). Those in lower-paid positions and older employees were most vulnerable.
“Basically, older workers seem to be driving all of this effect,” said coauthor Lamar Pierce, PhD, professor of organization and strategy, and associate dean at Washington University. “One, it’s harder for them to move, so they have less labor mobility. And, two, they have less flexibility: Learning new roles, adapting to change, they have more fully formed preferences at this point.”
A gender link also was evident; women were more likely to leave companies that adopted a pay-for-performance system. “Our study expands existing work by showing that the mental health costs of performance-based pay can be severe enough to necessitate pharmaceutical treatment,” the authors wrote.
Once a firm switched to the pay-for-performance system, the number of employees using anxiety and depression medications, which included Xanax and Zoloft, increased by 5.7%. The actual number of affected employees is almost certainly much higher, according to Dr. Pierce. Projecting the data to the United States, Dr. Pierce and his coauthor, Michael S. Dahl, PhD, estimated that 100,000 Americans could be affected.
“These types of mental health problems are incredibly costly to both the individual and firm. If this is reflective of a broader increase in stress and depression in employees, the costs are very high,” added Dr. Pierce. The study highlights the broader health and wellness implications of the companies’ compensation policies, he said. phys.org.
More and more people in the United States with severe mental illness and addictions reportedly are homeless, particularly in the Pacific Northwest and on the West Coast. Legislation aimed at addressing that problem is under discussion by Washington state lawmakers and appears to have broad support. The bill, which would authorize creation of a teaching hospital with 150 beds for people with mental illness, garnered unanimous support in the state’s House of Representatives and now has passed a Senate committee.
“The need for mental health care across our state has outgrown our facilities and our supply of trained health care professionals,” said State Sen. Annette Cleveland, chair of the Senate Health & Long Term Care Committee. “This important facility will address those needs head-on by expanding our physical capacity, enlarging our skilled workforce and increasing access through the use of telehealth services. The establishment of this dedicated behavioral health facility at the University of Washington would be the first of its kind in the nation.”
Jürgen Unützer, MD, MPH told KOMO News that the facility would be accredited and modern. “We would have a facility that’s from 2021 that’s state of the art, that’s approved, that’s a safe, welcoming environment where people would take their family members and say ‘this is a place that can give me some hope,’ ” said Dr. Unützer, who chairs the department of psychiatry and behavioral services at the University of Washington, Seattle.
The legislation, H.B. 1593, is part of efforts by Gov. Jay Inslee to tackle mental health issues in the state. The state’s aging mental health infrastructure has been losing federal funding, and patient safety issues have been identified at state-run mental health hospitals. KOMO News.
The New York Police Department recently reported a near-doubling of 911 calls by people the city refers to as “emotionally disturbed persons” over the past decade. Encounters between police and people in need reportedly have resulted in the deaths of 14 people over the last 3 years.
“There is a serious problem in New York City in the manner in which the NYPD interacts with mentally ill people,” said attorney Sanford Rubenstein, who is representing seven families whose family members with mental illness have been shot by police since 2016. “The training of police officers with regard to that interaction is limited and the number of patrol officers who have been trained is small. That is unacceptable.”
The problem was recognized years ago, and a plan was put in place by the city to provide mental health training to every police officer. Flash ahead 4 years and less than one-third of the police force has received any mental health training – just 11,970 of the 36,753 uniformed police officers. What’s more, teams of mental health workers and police that were formed 3 years ago to help intervene in responses to emotionally disturbed people have not been brought into the loop of the 911 system. The result has been 911 responses by officers not trained to deal with such situations and without the support of those who could help. The number of 911 calls related to emotionally disturbed people rose from just over 91,000 in 2009 to nearly 180,000 in 2018, averaging almost 500 every day. The calls are disproportionately from predominantly black and Hispanic precincts.
A 2014 announcement of “diversion centers,” where people with emotional disturbances could be brought by police instead of ferrying them to hospitals or jail, has failed to materialize. “[The problem] is overwhelming in the neighborhoods that I represent,” said Bronx council member Ritchie J. Torres. “Whether it be Tremont or Fordham – you can feel it and see it on the ground. ... You see chemically addicted, mentally ill people, who either are languishing on the street or being cycled in and out of the criminal justice system. And I’m wondering to myself, there has to be a better approach. This is insane.” New York Magazine.
Iowa Gov. Kim Reynolds has signed a comprehensive bill that, among other things, aims to make sweeping changes in the state’s mental health system. The new law also requires suicide prevention training for school personnel in the state. “This legislation was pushed over the finish line by individuals and families who knew firsthand the importance of having a robust mental health system,” Gov. Reynolds said. Critics contend that the legislation does not go far enough in several respects, including specifying where funding will come from and ensuring full access to care. Meanwhile, the governor announced plans to sign an executive order “establishing a platform to begin developing a children’s mental health system.” Des Moines Register.
Emergency room staff at AdventHealth hospitals in Orlando and neighboring Kissimmee, Fla., will begin assessing the mental health of patients as part of a pilot project with the University of Central Florida, according to reporting by 90.7 WMFE, a National Public Radio affiliate in central Florida. “How do we start providing that preventive care like we would with a typical chest pain patient? The same type of health care probably doesn’t apply to those patients with that mental health disorder,” said Robert Geissler, director of emergency services at AdventHealth Kissimmee. “And that’s why we’re trying to change the landscape with this particular project.” Similar programs in Michigan and Tennessee have helped curb suicides and lowered costs associated with mental health–related emergency care. As part of the AdventHealth program in Florida, emergency room staff will ask patients about feelings of hopelessness or despair as part of routine assessments. Patients deemed at high risk of suicide will be paired with counselors for the next 3 months, with daily calls and possibly house visits. Other mental health care resources in the community will be enlisted. 90.7 WMFE.
A study from researchers at Washington University in St. Louis and Aarhus University in Denmark charts the relationship of payment based on work done and employee mental health. According to phys.org, an aggregator of science, research, and technology news, performance-based pay is in place in 70% of U.S. companies. This means that employee income is based on a combination of bonuses, commission, profit sharing, and individual/team incentives, rather than guaranteed salaries. For some employees, performance-based pay can prove lucrative. But for others, such systems can lead to poor mental health.
The study, published in the Academy of Management Discoveries, charted the use of prescription medications for anxiety and depression by nearly 319,000 employees at about 1,300 companies in Denmark (2019 Feb 26. doi: 10.5465/amd.2018.0007). Those in lower-paid positions and older employees were most vulnerable.
“Basically, older workers seem to be driving all of this effect,” said coauthor Lamar Pierce, PhD, professor of organization and strategy, and associate dean at Washington University. “One, it’s harder for them to move, so they have less labor mobility. And, two, they have less flexibility: Learning new roles, adapting to change, they have more fully formed preferences at this point.”
A gender link also was evident; women were more likely to leave companies that adopted a pay-for-performance system. “Our study expands existing work by showing that the mental health costs of performance-based pay can be severe enough to necessitate pharmaceutical treatment,” the authors wrote.
Once a firm switched to the pay-for-performance system, the number of employees using anxiety and depression medications, which included Xanax and Zoloft, increased by 5.7%. The actual number of affected employees is almost certainly much higher, according to Dr. Pierce. Projecting the data to the United States, Dr. Pierce and his coauthor, Michael S. Dahl, PhD, estimated that 100,000 Americans could be affected.
“These types of mental health problems are incredibly costly to both the individual and firm. If this is reflective of a broader increase in stress and depression in employees, the costs are very high,” added Dr. Pierce. The study highlights the broader health and wellness implications of the companies’ compensation policies, he said. phys.org.
More and more people in the United States with severe mental illness and addictions reportedly are homeless, particularly in the Pacific Northwest and on the West Coast. Legislation aimed at addressing that problem is under discussion by Washington state lawmakers and appears to have broad support. The bill, which would authorize creation of a teaching hospital with 150 beds for people with mental illness, garnered unanimous support in the state’s House of Representatives and now has passed a Senate committee.
“The need for mental health care across our state has outgrown our facilities and our supply of trained health care professionals,” said State Sen. Annette Cleveland, chair of the Senate Health & Long Term Care Committee. “This important facility will address those needs head-on by expanding our physical capacity, enlarging our skilled workforce and increasing access through the use of telehealth services. The establishment of this dedicated behavioral health facility at the University of Washington would be the first of its kind in the nation.”
Jürgen Unützer, MD, MPH told KOMO News that the facility would be accredited and modern. “We would have a facility that’s from 2021 that’s state of the art, that’s approved, that’s a safe, welcoming environment where people would take their family members and say ‘this is a place that can give me some hope,’ ” said Dr. Unützer, who chairs the department of psychiatry and behavioral services at the University of Washington, Seattle.
The legislation, H.B. 1593, is part of efforts by Gov. Jay Inslee to tackle mental health issues in the state. The state’s aging mental health infrastructure has been losing federal funding, and patient safety issues have been identified at state-run mental health hospitals. KOMO News.
The New York Police Department recently reported a near-doubling of 911 calls by people the city refers to as “emotionally disturbed persons” over the past decade. Encounters between police and people in need reportedly have resulted in the deaths of 14 people over the last 3 years.
“There is a serious problem in New York City in the manner in which the NYPD interacts with mentally ill people,” said attorney Sanford Rubenstein, who is representing seven families whose family members with mental illness have been shot by police since 2016. “The training of police officers with regard to that interaction is limited and the number of patrol officers who have been trained is small. That is unacceptable.”
The problem was recognized years ago, and a plan was put in place by the city to provide mental health training to every police officer. Flash ahead 4 years and less than one-third of the police force has received any mental health training – just 11,970 of the 36,753 uniformed police officers. What’s more, teams of mental health workers and police that were formed 3 years ago to help intervene in responses to emotionally disturbed people have not been brought into the loop of the 911 system. The result has been 911 responses by officers not trained to deal with such situations and without the support of those who could help. The number of 911 calls related to emotionally disturbed people rose from just over 91,000 in 2009 to nearly 180,000 in 2018, averaging almost 500 every day. The calls are disproportionately from predominantly black and Hispanic precincts.
A 2014 announcement of “diversion centers,” where people with emotional disturbances could be brought by police instead of ferrying them to hospitals or jail, has failed to materialize. “[The problem] is overwhelming in the neighborhoods that I represent,” said Bronx council member Ritchie J. Torres. “Whether it be Tremont or Fordham – you can feel it and see it on the ground. ... You see chemically addicted, mentally ill people, who either are languishing on the street or being cycled in and out of the criminal justice system. And I’m wondering to myself, there has to be a better approach. This is insane.” New York Magazine.
Iowa Gov. Kim Reynolds has signed a comprehensive bill that, among other things, aims to make sweeping changes in the state’s mental health system. The new law also requires suicide prevention training for school personnel in the state. “This legislation was pushed over the finish line by individuals and families who knew firsthand the importance of having a robust mental health system,” Gov. Reynolds said. Critics contend that the legislation does not go far enough in several respects, including specifying where funding will come from and ensuring full access to care. Meanwhile, the governor announced plans to sign an executive order “establishing a platform to begin developing a children’s mental health system.” Des Moines Register.
Emergency room staff at AdventHealth hospitals in Orlando and neighboring Kissimmee, Fla., will begin assessing the mental health of patients as part of a pilot project with the University of Central Florida, according to reporting by 90.7 WMFE, a National Public Radio affiliate in central Florida. “How do we start providing that preventive care like we would with a typical chest pain patient? The same type of health care probably doesn’t apply to those patients with that mental health disorder,” said Robert Geissler, director of emergency services at AdventHealth Kissimmee. “And that’s why we’re trying to change the landscape with this particular project.” Similar programs in Michigan and Tennessee have helped curb suicides and lowered costs associated with mental health–related emergency care. As part of the AdventHealth program in Florida, emergency room staff will ask patients about feelings of hopelessness or despair as part of routine assessments. Patients deemed at high risk of suicide will be paired with counselors for the next 3 months, with daily calls and possibly house visits. Other mental health care resources in the community will be enlisted. 90.7 WMFE.
A study from researchers at Washington University in St. Louis and Aarhus University in Denmark charts the relationship of payment based on work done and employee mental health. According to phys.org, an aggregator of science, research, and technology news, performance-based pay is in place in 70% of U.S. companies. This means that employee income is based on a combination of bonuses, commission, profit sharing, and individual/team incentives, rather than guaranteed salaries. For some employees, performance-based pay can prove lucrative. But for others, such systems can lead to poor mental health.
The study, published in the Academy of Management Discoveries, charted the use of prescription medications for anxiety and depression by nearly 319,000 employees at about 1,300 companies in Denmark (2019 Feb 26. doi: 10.5465/amd.2018.0007). Those in lower-paid positions and older employees were most vulnerable.
“Basically, older workers seem to be driving all of this effect,” said coauthor Lamar Pierce, PhD, professor of organization and strategy, and associate dean at Washington University. “One, it’s harder for them to move, so they have less labor mobility. And, two, they have less flexibility: Learning new roles, adapting to change, they have more fully formed preferences at this point.”
A gender link also was evident; women were more likely to leave companies that adopted a pay-for-performance system. “Our study expands existing work by showing that the mental health costs of performance-based pay can be severe enough to necessitate pharmaceutical treatment,” the authors wrote.
Once a firm switched to the pay-for-performance system, the number of employees using anxiety and depression medications, which included Xanax and Zoloft, increased by 5.7%. The actual number of affected employees is almost certainly much higher, according to Dr. Pierce. Projecting the data to the United States, Dr. Pierce and his coauthor, Michael S. Dahl, PhD, estimated that 100,000 Americans could be affected.
“These types of mental health problems are incredibly costly to both the individual and firm. If this is reflective of a broader increase in stress and depression in employees, the costs are very high,” added Dr. Pierce. The study highlights the broader health and wellness implications of the companies’ compensation policies, he said. phys.org.
More and more people in the United States with severe mental illness and addictions reportedly are homeless, particularly in the Pacific Northwest and on the West Coast. Legislation aimed at addressing that problem is under discussion by Washington state lawmakers and appears to have broad support. The bill, which would authorize creation of a teaching hospital with 150 beds for people with mental illness, garnered unanimous support in the state’s House of Representatives and now has passed a Senate committee.
“The need for mental health care across our state has outgrown our facilities and our supply of trained health care professionals,” said State Sen. Annette Cleveland, chair of the Senate Health & Long Term Care Committee. “This important facility will address those needs head-on by expanding our physical capacity, enlarging our skilled workforce and increasing access through the use of telehealth services. The establishment of this dedicated behavioral health facility at the University of Washington would be the first of its kind in the nation.”
Jürgen Unützer, MD, MPH told KOMO News that the facility would be accredited and modern. “We would have a facility that’s from 2021 that’s state of the art, that’s approved, that’s a safe, welcoming environment where people would take their family members and say ‘this is a place that can give me some hope,’ ” said Dr. Unützer, who chairs the department of psychiatry and behavioral services at the University of Washington, Seattle.
The legislation, H.B. 1593, is part of efforts by Gov. Jay Inslee to tackle mental health issues in the state. The state’s aging mental health infrastructure has been losing federal funding, and patient safety issues have been identified at state-run mental health hospitals. KOMO News.
The New York Police Department recently reported a near-doubling of 911 calls by people the city refers to as “emotionally disturbed persons” over the past decade. Encounters between police and people in need reportedly have resulted in the deaths of 14 people over the last 3 years.
“There is a serious problem in New York City in the manner in which the NYPD interacts with mentally ill people,” said attorney Sanford Rubenstein, who is representing seven families whose family members with mental illness have been shot by police since 2016. “The training of police officers with regard to that interaction is limited and the number of patrol officers who have been trained is small. That is unacceptable.”
The problem was recognized years ago, and a plan was put in place by the city to provide mental health training to every police officer. Flash ahead 4 years and less than one-third of the police force has received any mental health training – just 11,970 of the 36,753 uniformed police officers. What’s more, teams of mental health workers and police that were formed 3 years ago to help intervene in responses to emotionally disturbed people have not been brought into the loop of the 911 system. The result has been 911 responses by officers not trained to deal with such situations and without the support of those who could help. The number of 911 calls related to emotionally disturbed people rose from just over 91,000 in 2009 to nearly 180,000 in 2018, averaging almost 500 every day. The calls are disproportionately from predominantly black and Hispanic precincts.
A 2014 announcement of “diversion centers,” where people with emotional disturbances could be brought by police instead of ferrying them to hospitals or jail, has failed to materialize. “[The problem] is overwhelming in the neighborhoods that I represent,” said Bronx council member Ritchie J. Torres. “Whether it be Tremont or Fordham – you can feel it and see it on the ground. ... You see chemically addicted, mentally ill people, who either are languishing on the street or being cycled in and out of the criminal justice system. And I’m wondering to myself, there has to be a better approach. This is insane.” New York Magazine.
Iowa Gov. Kim Reynolds has signed a comprehensive bill that, among other things, aims to make sweeping changes in the state’s mental health system. The new law also requires suicide prevention training for school personnel in the state. “This legislation was pushed over the finish line by individuals and families who knew firsthand the importance of having a robust mental health system,” Gov. Reynolds said. Critics contend that the legislation does not go far enough in several respects, including specifying where funding will come from and ensuring full access to care. Meanwhile, the governor announced plans to sign an executive order “establishing a platform to begin developing a children’s mental health system.” Des Moines Register.
Emergency room staff at AdventHealth hospitals in Orlando and neighboring Kissimmee, Fla., will begin assessing the mental health of patients as part of a pilot project with the University of Central Florida, according to reporting by 90.7 WMFE, a National Public Radio affiliate in central Florida. “How do we start providing that preventive care like we would with a typical chest pain patient? The same type of health care probably doesn’t apply to those patients with that mental health disorder,” said Robert Geissler, director of emergency services at AdventHealth Kissimmee. “And that’s why we’re trying to change the landscape with this particular project.” Similar programs in Michigan and Tennessee have helped curb suicides and lowered costs associated with mental health–related emergency care. As part of the AdventHealth program in Florida, emergency room staff will ask patients about feelings of hopelessness or despair as part of routine assessments. Patients deemed at high risk of suicide will be paired with counselors for the next 3 months, with daily calls and possibly house visits. Other mental health care resources in the community will be enlisted. 90.7 WMFE.
Electronic health records and the lost power of prose
“Don’t tell me the moon is shining; show me the glint of light on broken glass,” Anton Chekhov
In March 2006, four programmers turned entrepreneurs launched Twitter. This revolutionary tool experienced a monumental growth in scale over the next 10 years from a handful of users sharing a few thousand messages (known as “tweets”) each day to a global social network of over 300 million users valued at over $25 billion dollars. In fact, on Election Day 2016, Twitter was the No. 1 source of breaking news1, and it has been used as a launchpad for everything from social activism to national revolutions.
When Twitter was first conceived, it was designed to operate through wireless phone carriers’ SMS messaging functionality (aka “via text message”). SMS messages are limited to just 160 characters, so Twitter’s creators decided to restrict tweets to 140 characters, allowing 20 characters for a username. This decision created a necessity for communication efficiency that harks back to the days of the telegraph. From the liberal use of contractions and abbreviations to the tireless search for the shortest synonyms possible, Twitter users have employed countless techniques to enable them to say more with less. While clever and creative, this extreme verbal austerity has pervaded other media as well, becoming the hallmark literary style of the current generation.
Contemporaneous with the Twitter revolution, the medical field has allowed technology to dramatically change its style of communication as well, but in the opposite way. We have become far less efficient in our use of words, yet we seem to be doing a really poor job of expressing ourselves.
Saying less with more
I was once asked to provide expert testimony in a medical malpractice lawsuit. Working in support of the defense, I endured question after question from the plaintiff’s legal team as they picked apart every aspect of the case. Of particular interest was the physician’s documentation. Sadly – yet perhaps unsurprisingly – it was poor. The defendant had clearly used an EHR template and clicked checkboxes to create his note, documenting history, physical exam, assessment, and plan without having typed a single word. While adequate for billing purposes, the note was missing any narrative that could communicate the story of what had transpired during the patient’s visit. Sure, the presenting symptoms and vital signs were there, but the no description of the patient’s appearance had been recorded? What had the physician been thinking? What unspoken messages had led the physician to make the decisions he had made?
Like Twitter, the dawn of EHRs created an entirely new form of communication, but instead of limiting the content of physicians’ notes it expanded it. Objectively, this has made for more complete notes. Subjectively, this has led to notes packed with data, yet devoid of meaningful narrative. While handwritten notes from the previous generation were brief, they included the most important elements of the patient’s history and often the physician’s thought process in forming the differential. The electronically generated notes of today are quite the opposite; they are dense, yet far from illuminating. A clinician referring back to the record might have tremendous difficulty discerning salient features amidst all of the “note bloat.”This puts the patient (and the provider, as in the case above) at risk. Details may be present, but the diagnosis will be missed without the story that ties them all together.
Writing a new chapter
Physicians hoping to create meaningful notes are often stymied by the technology at their disposal or the demands placed on their time. These issues, combined with an ever-growing number of regulatory requirements, are what led to the decay of narrative in the first place. As a result, doctors are looking for alternative ways to buck the trend and bring patients’ stories back to their medical records. These methods are often expensive or involved, but in many cases they dramatically improve quality and efficiency.
An example of a tool that allows doctors to achieve these goals is speech recognition technology. Instead of typing or clicking, physicians dictate into the EHR, creating notes that are typically richer and more akin to a story than a list of symptoms or data points. When voice-to-text is properly deployed and utilized, documentation improves along with efficiency. Alternately, many providers are now employing scribes to accompany them in the exam room and complete the medical record. Taking this step leads to more descriptive notes, better productivity, and happier providers. The use of scribes also seems to result in happier patients, who report better therapeutic interactions when their doctors aren’t typing or staring at a computer screen.
The above-mentioned methods for recording information about a patient during a visit may be too expensive or complicated for some providers, but there are other simple techniques that can be used without incurring additional cost or resources. Previsit planning is one such possibility. By reviewing patient charts in advance of appointments, physicians can look over results, identify preventive health gaps, and anticipate follow-up needs and medication refills. They can then create skeleton notes and prepopulate orders to reduce the documentation burden during the visit. While time consuming at first, physicians have reported this practice actually saves time in the long run and allows them to focus on recording the patient narrative during the visit.
Another strategy is even more simple in concept, though may seem counter-intuitive at first: get better acquainted with the electronic records system. That is, take the time to really learn and understand the tools designed to improve productivity that are available in your EHR, then use them judiciously; take advantage of templates and macros when they’ll make you more efficient yet won’t inhibit your ability to tell the patient’s story; embrace optimization but don’t compromise on narrative. By carefully choosing your words, you’ll paint a clearer picture of every patient and enable safer and more personalized care.
Reference
1. “For Election Day Influence, Twitter Ruled Social Media” New York Times. Nov. 8, 2016.
“Don’t tell me the moon is shining; show me the glint of light on broken glass,” Anton Chekhov
In March 2006, four programmers turned entrepreneurs launched Twitter. This revolutionary tool experienced a monumental growth in scale over the next 10 years from a handful of users sharing a few thousand messages (known as “tweets”) each day to a global social network of over 300 million users valued at over $25 billion dollars. In fact, on Election Day 2016, Twitter was the No. 1 source of breaking news1, and it has been used as a launchpad for everything from social activism to national revolutions.
When Twitter was first conceived, it was designed to operate through wireless phone carriers’ SMS messaging functionality (aka “via text message”). SMS messages are limited to just 160 characters, so Twitter’s creators decided to restrict tweets to 140 characters, allowing 20 characters for a username. This decision created a necessity for communication efficiency that harks back to the days of the telegraph. From the liberal use of contractions and abbreviations to the tireless search for the shortest synonyms possible, Twitter users have employed countless techniques to enable them to say more with less. While clever and creative, this extreme verbal austerity has pervaded other media as well, becoming the hallmark literary style of the current generation.
Contemporaneous with the Twitter revolution, the medical field has allowed technology to dramatically change its style of communication as well, but in the opposite way. We have become far less efficient in our use of words, yet we seem to be doing a really poor job of expressing ourselves.
Saying less with more
I was once asked to provide expert testimony in a medical malpractice lawsuit. Working in support of the defense, I endured question after question from the plaintiff’s legal team as they picked apart every aspect of the case. Of particular interest was the physician’s documentation. Sadly – yet perhaps unsurprisingly – it was poor. The defendant had clearly used an EHR template and clicked checkboxes to create his note, documenting history, physical exam, assessment, and plan without having typed a single word. While adequate for billing purposes, the note was missing any narrative that could communicate the story of what had transpired during the patient’s visit. Sure, the presenting symptoms and vital signs were there, but the no description of the patient’s appearance had been recorded? What had the physician been thinking? What unspoken messages had led the physician to make the decisions he had made?
Like Twitter, the dawn of EHRs created an entirely new form of communication, but instead of limiting the content of physicians’ notes it expanded it. Objectively, this has made for more complete notes. Subjectively, this has led to notes packed with data, yet devoid of meaningful narrative. While handwritten notes from the previous generation were brief, they included the most important elements of the patient’s history and often the physician’s thought process in forming the differential. The electronically generated notes of today are quite the opposite; they are dense, yet far from illuminating. A clinician referring back to the record might have tremendous difficulty discerning salient features amidst all of the “note bloat.”This puts the patient (and the provider, as in the case above) at risk. Details may be present, but the diagnosis will be missed without the story that ties them all together.
Writing a new chapter
Physicians hoping to create meaningful notes are often stymied by the technology at their disposal or the demands placed on their time. These issues, combined with an ever-growing number of regulatory requirements, are what led to the decay of narrative in the first place. As a result, doctors are looking for alternative ways to buck the trend and bring patients’ stories back to their medical records. These methods are often expensive or involved, but in many cases they dramatically improve quality and efficiency.
An example of a tool that allows doctors to achieve these goals is speech recognition technology. Instead of typing or clicking, physicians dictate into the EHR, creating notes that are typically richer and more akin to a story than a list of symptoms or data points. When voice-to-text is properly deployed and utilized, documentation improves along with efficiency. Alternately, many providers are now employing scribes to accompany them in the exam room and complete the medical record. Taking this step leads to more descriptive notes, better productivity, and happier providers. The use of scribes also seems to result in happier patients, who report better therapeutic interactions when their doctors aren’t typing or staring at a computer screen.
The above-mentioned methods for recording information about a patient during a visit may be too expensive or complicated for some providers, but there are other simple techniques that can be used without incurring additional cost or resources. Previsit planning is one such possibility. By reviewing patient charts in advance of appointments, physicians can look over results, identify preventive health gaps, and anticipate follow-up needs and medication refills. They can then create skeleton notes and prepopulate orders to reduce the documentation burden during the visit. While time consuming at first, physicians have reported this practice actually saves time in the long run and allows them to focus on recording the patient narrative during the visit.
Another strategy is even more simple in concept, though may seem counter-intuitive at first: get better acquainted with the electronic records system. That is, take the time to really learn and understand the tools designed to improve productivity that are available in your EHR, then use them judiciously; take advantage of templates and macros when they’ll make you more efficient yet won’t inhibit your ability to tell the patient’s story; embrace optimization but don’t compromise on narrative. By carefully choosing your words, you’ll paint a clearer picture of every patient and enable safer and more personalized care.
Reference
1. “For Election Day Influence, Twitter Ruled Social Media” New York Times. Nov. 8, 2016.
“Don’t tell me the moon is shining; show me the glint of light on broken glass,” Anton Chekhov
In March 2006, four programmers turned entrepreneurs launched Twitter. This revolutionary tool experienced a monumental growth in scale over the next 10 years from a handful of users sharing a few thousand messages (known as “tweets”) each day to a global social network of over 300 million users valued at over $25 billion dollars. In fact, on Election Day 2016, Twitter was the No. 1 source of breaking news1, and it has been used as a launchpad for everything from social activism to national revolutions.
When Twitter was first conceived, it was designed to operate through wireless phone carriers’ SMS messaging functionality (aka “via text message”). SMS messages are limited to just 160 characters, so Twitter’s creators decided to restrict tweets to 140 characters, allowing 20 characters for a username. This decision created a necessity for communication efficiency that harks back to the days of the telegraph. From the liberal use of contractions and abbreviations to the tireless search for the shortest synonyms possible, Twitter users have employed countless techniques to enable them to say more with less. While clever and creative, this extreme verbal austerity has pervaded other media as well, becoming the hallmark literary style of the current generation.
Contemporaneous with the Twitter revolution, the medical field has allowed technology to dramatically change its style of communication as well, but in the opposite way. We have become far less efficient in our use of words, yet we seem to be doing a really poor job of expressing ourselves.
Saying less with more
I was once asked to provide expert testimony in a medical malpractice lawsuit. Working in support of the defense, I endured question after question from the plaintiff’s legal team as they picked apart every aspect of the case. Of particular interest was the physician’s documentation. Sadly – yet perhaps unsurprisingly – it was poor. The defendant had clearly used an EHR template and clicked checkboxes to create his note, documenting history, physical exam, assessment, and plan without having typed a single word. While adequate for billing purposes, the note was missing any narrative that could communicate the story of what had transpired during the patient’s visit. Sure, the presenting symptoms and vital signs were there, but the no description of the patient’s appearance had been recorded? What had the physician been thinking? What unspoken messages had led the physician to make the decisions he had made?
Like Twitter, the dawn of EHRs created an entirely new form of communication, but instead of limiting the content of physicians’ notes it expanded it. Objectively, this has made for more complete notes. Subjectively, this has led to notes packed with data, yet devoid of meaningful narrative. While handwritten notes from the previous generation were brief, they included the most important elements of the patient’s history and often the physician’s thought process in forming the differential. The electronically generated notes of today are quite the opposite; they are dense, yet far from illuminating. A clinician referring back to the record might have tremendous difficulty discerning salient features amidst all of the “note bloat.”This puts the patient (and the provider, as in the case above) at risk. Details may be present, but the diagnosis will be missed without the story that ties them all together.
Writing a new chapter
Physicians hoping to create meaningful notes are often stymied by the technology at their disposal or the demands placed on their time. These issues, combined with an ever-growing number of regulatory requirements, are what led to the decay of narrative in the first place. As a result, doctors are looking for alternative ways to buck the trend and bring patients’ stories back to their medical records. These methods are often expensive or involved, but in many cases they dramatically improve quality and efficiency.
An example of a tool that allows doctors to achieve these goals is speech recognition technology. Instead of typing or clicking, physicians dictate into the EHR, creating notes that are typically richer and more akin to a story than a list of symptoms or data points. When voice-to-text is properly deployed and utilized, documentation improves along with efficiency. Alternately, many providers are now employing scribes to accompany them in the exam room and complete the medical record. Taking this step leads to more descriptive notes, better productivity, and happier providers. The use of scribes also seems to result in happier patients, who report better therapeutic interactions when their doctors aren’t typing or staring at a computer screen.
The above-mentioned methods for recording information about a patient during a visit may be too expensive or complicated for some providers, but there are other simple techniques that can be used without incurring additional cost or resources. Previsit planning is one such possibility. By reviewing patient charts in advance of appointments, physicians can look over results, identify preventive health gaps, and anticipate follow-up needs and medication refills. They can then create skeleton notes and prepopulate orders to reduce the documentation burden during the visit. While time consuming at first, physicians have reported this practice actually saves time in the long run and allows them to focus on recording the patient narrative during the visit.
Another strategy is even more simple in concept, though may seem counter-intuitive at first: get better acquainted with the electronic records system. That is, take the time to really learn and understand the tools designed to improve productivity that are available in your EHR, then use them judiciously; take advantage of templates and macros when they’ll make you more efficient yet won’t inhibit your ability to tell the patient’s story; embrace optimization but don’t compromise on narrative. By carefully choosing your words, you’ll paint a clearer picture of every patient and enable safer and more personalized care.
Reference
1. “For Election Day Influence, Twitter Ruled Social Media” New York Times. Nov. 8, 2016.
Who’s increasing health care costs? Not us!
Determining who is responsible for the increase in health care cost in the United States has seemingly limitless possibilities. There are enough culprits to go around, but a recent analysis points a finger at hospital-based care (Health Aff. 2019 Feb;38[2]:184-9).
The authors of the paper found that, during the period from 2007 to 2014, inpatient hospital care for surgical procedures increased 42% and outpatient hospital care increased 25%. In the same period, physician care increased only 6%. Much of this increase in hospital costs was associated with hospital consolidations and mergers.
We have been led to believe that hospital mergers will cut costs by eliminating duplication of both physical and personnel overhead costs. In fact that doesn’t seem to happen. It appears that hospital mergers were associated with increased per patient costs and is a result of decrease in competition in local health care markets. This observation has been made in the past (Am Econ Rev. 2015 Jan;105:172-203), and was reiterated by the most recent report. If there were decreases in overhead observed in the mergers, they were not passed on to the patients or insurers.
There was a time when community hospitals, large and small, were run by community leaders and local doctors, often under the aegis of religious and social groups. I can remember the medical and community leadership in Utica, N.Y., where I grew up and where I worked in a hospital as a summer intern. Their goal was to provide quality health care. The financial success or failures of the hospitals were the responsibility of the local community, and the profits and losses were kept at a minimum.
Fast forward to the 21st century and health care in general, and hospital care in particular, has become a “cash cow.” Community leadership has been minimized, and where it exists, it is under constant pressure to make a profit. Hospital mergers, arranged under the guise of economy of size, are now controlled by hedge funds and large health care corporations.
The community board of trustees has been replaced by investors, whose main concern is the return on their investments regardless of quality of care or need. If those profits fail to materialize, the hospitals are taken over by another investor group. So much for quality. As the corporations grow, they buy up the competition, particularly small community hospitals leaving many, particularly in rural America, without medical support.
There seems to be little recourse to consumers or insurers to mitigate this process. Investors have a right to a return on their investment, but until we have governmental control of competition, that incentive remains. We can see similar price increases in the pharmaceutical marketplace, where Congress has limited competition to preserve the drug monopoly. Americans will be asked to pay more to maintain a system that is inherently on the road to bankruptcy and fails to provide either quality or fair drug and hospital charges.
But what do we care, we can afford it.
Dr. Goldstein is professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit.
Determining who is responsible for the increase in health care cost in the United States has seemingly limitless possibilities. There are enough culprits to go around, but a recent analysis points a finger at hospital-based care (Health Aff. 2019 Feb;38[2]:184-9).
The authors of the paper found that, during the period from 2007 to 2014, inpatient hospital care for surgical procedures increased 42% and outpatient hospital care increased 25%. In the same period, physician care increased only 6%. Much of this increase in hospital costs was associated with hospital consolidations and mergers.
We have been led to believe that hospital mergers will cut costs by eliminating duplication of both physical and personnel overhead costs. In fact that doesn’t seem to happen. It appears that hospital mergers were associated with increased per patient costs and is a result of decrease in competition in local health care markets. This observation has been made in the past (Am Econ Rev. 2015 Jan;105:172-203), and was reiterated by the most recent report. If there were decreases in overhead observed in the mergers, they were not passed on to the patients or insurers.
There was a time when community hospitals, large and small, were run by community leaders and local doctors, often under the aegis of religious and social groups. I can remember the medical and community leadership in Utica, N.Y., where I grew up and where I worked in a hospital as a summer intern. Their goal was to provide quality health care. The financial success or failures of the hospitals were the responsibility of the local community, and the profits and losses were kept at a minimum.
Fast forward to the 21st century and health care in general, and hospital care in particular, has become a “cash cow.” Community leadership has been minimized, and where it exists, it is under constant pressure to make a profit. Hospital mergers, arranged under the guise of economy of size, are now controlled by hedge funds and large health care corporations.
The community board of trustees has been replaced by investors, whose main concern is the return on their investments regardless of quality of care or need. If those profits fail to materialize, the hospitals are taken over by another investor group. So much for quality. As the corporations grow, they buy up the competition, particularly small community hospitals leaving many, particularly in rural America, without medical support.
There seems to be little recourse to consumers or insurers to mitigate this process. Investors have a right to a return on their investment, but until we have governmental control of competition, that incentive remains. We can see similar price increases in the pharmaceutical marketplace, where Congress has limited competition to preserve the drug monopoly. Americans will be asked to pay more to maintain a system that is inherently on the road to bankruptcy and fails to provide either quality or fair drug and hospital charges.
But what do we care, we can afford it.
Dr. Goldstein is professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit.
Determining who is responsible for the increase in health care cost in the United States has seemingly limitless possibilities. There are enough culprits to go around, but a recent analysis points a finger at hospital-based care (Health Aff. 2019 Feb;38[2]:184-9).
The authors of the paper found that, during the period from 2007 to 2014, inpatient hospital care for surgical procedures increased 42% and outpatient hospital care increased 25%. In the same period, physician care increased only 6%. Much of this increase in hospital costs was associated with hospital consolidations and mergers.
We have been led to believe that hospital mergers will cut costs by eliminating duplication of both physical and personnel overhead costs. In fact that doesn’t seem to happen. It appears that hospital mergers were associated with increased per patient costs and is a result of decrease in competition in local health care markets. This observation has been made in the past (Am Econ Rev. 2015 Jan;105:172-203), and was reiterated by the most recent report. If there were decreases in overhead observed in the mergers, they were not passed on to the patients or insurers.
There was a time when community hospitals, large and small, were run by community leaders and local doctors, often under the aegis of religious and social groups. I can remember the medical and community leadership in Utica, N.Y., where I grew up and where I worked in a hospital as a summer intern. Their goal was to provide quality health care. The financial success or failures of the hospitals were the responsibility of the local community, and the profits and losses were kept at a minimum.
Fast forward to the 21st century and health care in general, and hospital care in particular, has become a “cash cow.” Community leadership has been minimized, and where it exists, it is under constant pressure to make a profit. Hospital mergers, arranged under the guise of economy of size, are now controlled by hedge funds and large health care corporations.
The community board of trustees has been replaced by investors, whose main concern is the return on their investments regardless of quality of care or need. If those profits fail to materialize, the hospitals are taken over by another investor group. So much for quality. As the corporations grow, they buy up the competition, particularly small community hospitals leaving many, particularly in rural America, without medical support.
There seems to be little recourse to consumers or insurers to mitigate this process. Investors have a right to a return on their investment, but until we have governmental control of competition, that incentive remains. We can see similar price increases in the pharmaceutical marketplace, where Congress has limited competition to preserve the drug monopoly. Americans will be asked to pay more to maintain a system that is inherently on the road to bankruptcy and fails to provide either quality or fair drug and hospital charges.
But what do we care, we can afford it.
Dr. Goldstein is professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit.
The 39th ASLMS meeting is now underway
WASHINGTON – At the annual meeting of the American Academy of Dermatology, the taking place March 27-31, 2019, in Denver.

“ASLMS is always an amazing meeting, and it’s a unique meeting,” said past president Mathew Avram, MD, director of the Dermatology Laser & Cosmetic Center at Massachusetts General Hospital, Boston. “At its core, it’s a scientific meeting ... you can take things back to your practice that change the practice of medicine.”
Current ASLMS president Eric Bernstein, MD, of Main Line Center for Laser Surgery, Ardmore, Pa., pointed out that, in addition to doctors and other health care practitioners, other available and accessible attendees include the engineers who build the lasers. And this year, injectables are being incorporated into the program.
MDedge reporter Doug Brunk will be reporting from the meeting.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, the taking place March 27-31, 2019, in Denver.

“ASLMS is always an amazing meeting, and it’s a unique meeting,” said past president Mathew Avram, MD, director of the Dermatology Laser & Cosmetic Center at Massachusetts General Hospital, Boston. “At its core, it’s a scientific meeting ... you can take things back to your practice that change the practice of medicine.”
Current ASLMS president Eric Bernstein, MD, of Main Line Center for Laser Surgery, Ardmore, Pa., pointed out that, in addition to doctors and other health care practitioners, other available and accessible attendees include the engineers who build the lasers. And this year, injectables are being incorporated into the program.
MDedge reporter Doug Brunk will be reporting from the meeting.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, the taking place March 27-31, 2019, in Denver.

“ASLMS is always an amazing meeting, and it’s a unique meeting,” said past president Mathew Avram, MD, director of the Dermatology Laser & Cosmetic Center at Massachusetts General Hospital, Boston. “At its core, it’s a scientific meeting ... you can take things back to your practice that change the practice of medicine.”
Current ASLMS president Eric Bernstein, MD, of Main Line Center for Laser Surgery, Ardmore, Pa., pointed out that, in addition to doctors and other health care practitioners, other available and accessible attendees include the engineers who build the lasers. And this year, injectables are being incorporated into the program.
MDedge reporter Doug Brunk will be reporting from the meeting.
REPORTING FROM AAD 2019
Cellulitis pearls
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
The paclitaxel paradox
As medical editor of Vascular Specialist, it has always been my hope to use our excellent reporters and rapid production schedule to keep readers abreast of the latest news in vascular surgery. While my colleagues at the Journal of Vascular Surgery publish studies that will drive treatment, my goal is to drive discussion.
With topics like burnout, workforce shortages, and electronic medical records, I feel we have been successful. The downside of staying current is we sometimes find ourselves publishing contradictory stories. This has been the case with paclitaxel. Let’s take a break from the fray and review where we are, and where we might go from here.
In 2012, the Zilver PTX became the first drug-eluting stent (DES) to gain Food and Drug Administration approval for the treatment of peripheral vascular disease. Two years later, the FDA approved the Lutonix 035 as the first drug-coated balloon (DCB) for use in the femoral-popliteal arteries. The Lutonix would also gain a second indication for failing dialysis fistulas. Medtronic and Spectranetics received authorizations for their DCBs in 2015 and 2017, respectively.
While the safety of paclitaxel-coated devices in the coronary system had previously been called into question, the drug was generally considered safe and effective in the peripheral arterial system. The controversy began in December 2018, when Katsanos et al.1 published a meta-analysis of 28 randomized, controlled trials (RCTs) investigating paclitaxel-coated devices in the femoral-popliteal arteries. While all-cause patient mortality was similar at 1 year between paclitaxel-coated devices and controls (2.3% in each), at 2 years the risk of death was significantly higher in those treated with paclitaxel (7.2% vs. 3.8%). The 5-year data were available for three trials where there was a continued significantly increased risk of mortality with paclitaxel (14.7% vs. 8.1%).
Opposition to these findings was prompt from both physicians and industry. Weaknesses of the analysis, both perceived and real, were hammered. The meta-analysis did not include individual patient data, and the actual cause of death was unknown in most of the included trials. The study was not adequately powered to eliminate the risk of type 1 error when comparing mortality after 2 years. Individuals assigned to the control group may have received paclitaxel treatment at some point in their follow-up. The DCB and DES treatment groups were combined. The methods employed by the authors, however, stood up reasonably well to scrutiny.
On Jan. 17, 2019, the FDA issued their first response stating, “the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”2
Later that month, Peter Schneider, MD, and associates published a patient-level meta-analysis in the Journal of the American College of Cardiology.3 The study included 1,980 patients and found no statistically significant difference in all-cause mortality between DCB (9.3%) and percutaneous transluminal angioplasty (PTA) (11.2%) through 5 years. Shortly after that, however, a correction was issued.
On Feb. 15, 2019, Medtronic reported an error in the 2- and 3-year follow-up periods for the IN.PACT Global postmarket study. The company stated, “Due to a programming error, mortality data were inadvertently omitted from the summary tables included in the statistical analysis.” The mortality in the DCB cohort was corrected from 9.30% to 15.12%. The authors stated that this new mortality rate was still not significantly higher than the PTA group (P = .09).4
Less than 1 week later, another device company issued a correction. And once again, the error had been made in favor of the paclitaxel-treated group. In 2016, the 5-year data from Cook Medical’s Zilver PTX trial were published in Circulation. The study reported a mortality of 10.2% in the DES group and 16.9% in the PTA cohort. Regrettably, these numbers were reversed and significantly higher in the paclitaxel-treated group (16.9% vs. 10.2%, P = .03).5
On Feb. 12, 2019, another response to the Katsanos meta-analysis was published in JAMA Cardiology.6 In this study, Secemsky et al. analyzed patient-level data from a Medicare database. The authors reported finding no evidence of paclitaxel- related deaths in 16,560 patients. Unfortunately, the mean follow-up time was only 389 days, which may have been insufficient to detect the late mortality reported in the Katsanos meta-analysis.
On March 15, 2019, the FDA issued a second statement, this time with a much stronger tone.7 The agency reported an ongoing analysis of the long-term survival data from the pivotal randomized trials. In the three studies with 5-year data available, each showed a significantly higher mortality in the paclitaxel group.
When pooled, there were 975 patients, and the risk of death was 20.1% in the paclitaxel group versus 13.4 % in the controls. The FDA recommended discussing the increased risk of mortality with all patients receiving paclitaxel therapy as part of the informed consent process. They also stated that for most patients alternative options should generally be used until additional analysis of the mortality risk is performed.
Industry bristled at this new, strongly worded statement. Becton Dickinson, makers of the Lutonix balloon, asserted that the FDA recommendation was based on “a limited review of data from less than 1,000 patients.”8 The company noted that its LEVANT 2 trial did not see a signal of increased mortality at 5 years. Although they did acknowledge that, among the randomized patients, there was a significantly higher mortality at 5 years for those treated with paclitaxel.
How do we make sense of this? Pac-litaxel is a cytotoxic drug. Its pharmacokinetics vary significantly based on the preparation and administration. The FDA label for the injectable form (Taxol) warns of anaphylaxis and severe hypersensitivity reactions, but there is no mention of long-term mortality. In the coronary vessels, paclitaxel-coated devices have been associated with myocardial infarction and death. Obviously it is easy to comprehend how local vessel effects in the coronary system can lead to increased mortality. The pathway is less clear with femoral-popliteal interventions. If the association of paclitaxel with death is truly causation there must be some systemic effects. The dose delivered with femoral- popliteal interventions is much higher than that seen with coronary devices.
The mortality may be associated with the platform used or even the formulation (crystalline formularies have a longer half-life). Could it be something more benign? Paclitaxel-treated patients see less recurrence of their femoral-popliteal disease. Are the control group patients with more recurrences seeing their interventionalist more often and therefore receiving more frequent reminders to comply with medical therapy?
At this point, we have few answers. After an all-day town hall at the recent Cardiovascular Research Technologies conference,9 one moderator said, “I came in with uncertainty and now I’m going away with uncertainty, but we made tremendous progress.” His comoderator added, “I know I don’t know.” Well then, glad we cleared that up!
In any event, changes are coming. The BASIL-3 trial has suspended recruitment. Physicians using paclitaxel-coated devices are now advised by the FDA to inform patients of the increased risk of death and to use alternatives in most cases. Therefore, if you employ these devices routinely in the femoral-popliteal vessels you are seemingly doing so in opposition to the recommendations of the FDA. Legal peril may follow.
The time for nitpicking the Katsanos analysis has ended. Our industry partners must be compelled to supply the data and finances needed to settle this issue. The signal seems real and it is time to find answers. Research initiatives are underway through the SVS, the VIVA group, the UK Medicines and Healthcare Products Regulatory Agency, and the FDA.
Going forward, the SVS has formed a Paclitaxel Safety Task Force under the leadership of President-elect Kim Hodgson. Their mission is to facilitate the performance and interpretation of an Individual Patient Data meta-analysis using patient-level RCT data from industry partners. The task force states: “We remain troubled by the recent reports of reanalysis of existing datasets, pooled analyses of RCTs, and other ‘series’, as we believe that the findings of these statistically inferior analyses bring no additional clarity, cannot be relied upon for guidance, and distract us from the analysis that needs to be performed.”
References
1. J Am Heart Assoc. 2018 Dec 18;7(24):e011245.
2. www.fda.gov/medicaldevices/safety/letterstohealthcareproviders/ucm629589.htm.
3. J Am Coll Cardiol. Jan 2019. doi: 10.1016/j.jacc.2019.01.013.
4. Circulation. 2019;139:e42.
5. https://evtoday.com/2019/02/20/zilver-ptx-trial-5-year-mortality-data-corrected-in-circulation.
6. JAMA Cardiol. 2019 Feb 12. doi:10.1001/jamacardio.2019.0325.
7. www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm.
8. www.med-technews.com/news/bud-defends-safety-of-drug-coated-device-following-fda-warnin/.
9. www.crtonline.org/news-detail/paclitaxel-device-safety-thoroughly-discussed-at-c.
As medical editor of Vascular Specialist, it has always been my hope to use our excellent reporters and rapid production schedule to keep readers abreast of the latest news in vascular surgery. While my colleagues at the Journal of Vascular Surgery publish studies that will drive treatment, my goal is to drive discussion.
With topics like burnout, workforce shortages, and electronic medical records, I feel we have been successful. The downside of staying current is we sometimes find ourselves publishing contradictory stories. This has been the case with paclitaxel. Let’s take a break from the fray and review where we are, and where we might go from here.
In 2012, the Zilver PTX became the first drug-eluting stent (DES) to gain Food and Drug Administration approval for the treatment of peripheral vascular disease. Two years later, the FDA approved the Lutonix 035 as the first drug-coated balloon (DCB) for use in the femoral-popliteal arteries. The Lutonix would also gain a second indication for failing dialysis fistulas. Medtronic and Spectranetics received authorizations for their DCBs in 2015 and 2017, respectively.
While the safety of paclitaxel-coated devices in the coronary system had previously been called into question, the drug was generally considered safe and effective in the peripheral arterial system. The controversy began in December 2018, when Katsanos et al.1 published a meta-analysis of 28 randomized, controlled trials (RCTs) investigating paclitaxel-coated devices in the femoral-popliteal arteries. While all-cause patient mortality was similar at 1 year between paclitaxel-coated devices and controls (2.3% in each), at 2 years the risk of death was significantly higher in those treated with paclitaxel (7.2% vs. 3.8%). The 5-year data were available for three trials where there was a continued significantly increased risk of mortality with paclitaxel (14.7% vs. 8.1%).
Opposition to these findings was prompt from both physicians and industry. Weaknesses of the analysis, both perceived and real, were hammered. The meta-analysis did not include individual patient data, and the actual cause of death was unknown in most of the included trials. The study was not adequately powered to eliminate the risk of type 1 error when comparing mortality after 2 years. Individuals assigned to the control group may have received paclitaxel treatment at some point in their follow-up. The DCB and DES treatment groups were combined. The methods employed by the authors, however, stood up reasonably well to scrutiny.
On Jan. 17, 2019, the FDA issued their first response stating, “the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”2
Later that month, Peter Schneider, MD, and associates published a patient-level meta-analysis in the Journal of the American College of Cardiology.3 The study included 1,980 patients and found no statistically significant difference in all-cause mortality between DCB (9.3%) and percutaneous transluminal angioplasty (PTA) (11.2%) through 5 years. Shortly after that, however, a correction was issued.
On Feb. 15, 2019, Medtronic reported an error in the 2- and 3-year follow-up periods for the IN.PACT Global postmarket study. The company stated, “Due to a programming error, mortality data were inadvertently omitted from the summary tables included in the statistical analysis.” The mortality in the DCB cohort was corrected from 9.30% to 15.12%. The authors stated that this new mortality rate was still not significantly higher than the PTA group (P = .09).4
Less than 1 week later, another device company issued a correction. And once again, the error had been made in favor of the paclitaxel-treated group. In 2016, the 5-year data from Cook Medical’s Zilver PTX trial were published in Circulation. The study reported a mortality of 10.2% in the DES group and 16.9% in the PTA cohort. Regrettably, these numbers were reversed and significantly higher in the paclitaxel-treated group (16.9% vs. 10.2%, P = .03).5
On Feb. 12, 2019, another response to the Katsanos meta-analysis was published in JAMA Cardiology.6 In this study, Secemsky et al. analyzed patient-level data from a Medicare database. The authors reported finding no evidence of paclitaxel- related deaths in 16,560 patients. Unfortunately, the mean follow-up time was only 389 days, which may have been insufficient to detect the late mortality reported in the Katsanos meta-analysis.
On March 15, 2019, the FDA issued a second statement, this time with a much stronger tone.7 The agency reported an ongoing analysis of the long-term survival data from the pivotal randomized trials. In the three studies with 5-year data available, each showed a significantly higher mortality in the paclitaxel group.
When pooled, there were 975 patients, and the risk of death was 20.1% in the paclitaxel group versus 13.4 % in the controls. The FDA recommended discussing the increased risk of mortality with all patients receiving paclitaxel therapy as part of the informed consent process. They also stated that for most patients alternative options should generally be used until additional analysis of the mortality risk is performed.
Industry bristled at this new, strongly worded statement. Becton Dickinson, makers of the Lutonix balloon, asserted that the FDA recommendation was based on “a limited review of data from less than 1,000 patients.”8 The company noted that its LEVANT 2 trial did not see a signal of increased mortality at 5 years. Although they did acknowledge that, among the randomized patients, there was a significantly higher mortality at 5 years for those treated with paclitaxel.
How do we make sense of this? Pac-litaxel is a cytotoxic drug. Its pharmacokinetics vary significantly based on the preparation and administration. The FDA label for the injectable form (Taxol) warns of anaphylaxis and severe hypersensitivity reactions, but there is no mention of long-term mortality. In the coronary vessels, paclitaxel-coated devices have been associated with myocardial infarction and death. Obviously it is easy to comprehend how local vessel effects in the coronary system can lead to increased mortality. The pathway is less clear with femoral-popliteal interventions. If the association of paclitaxel with death is truly causation there must be some systemic effects. The dose delivered with femoral- popliteal interventions is much higher than that seen with coronary devices.
The mortality may be associated with the platform used or even the formulation (crystalline formularies have a longer half-life). Could it be something more benign? Paclitaxel-treated patients see less recurrence of their femoral-popliteal disease. Are the control group patients with more recurrences seeing their interventionalist more often and therefore receiving more frequent reminders to comply with medical therapy?
At this point, we have few answers. After an all-day town hall at the recent Cardiovascular Research Technologies conference,9 one moderator said, “I came in with uncertainty and now I’m going away with uncertainty, but we made tremendous progress.” His comoderator added, “I know I don’t know.” Well then, glad we cleared that up!
In any event, changes are coming. The BASIL-3 trial has suspended recruitment. Physicians using paclitaxel-coated devices are now advised by the FDA to inform patients of the increased risk of death and to use alternatives in most cases. Therefore, if you employ these devices routinely in the femoral-popliteal vessels you are seemingly doing so in opposition to the recommendations of the FDA. Legal peril may follow.
The time for nitpicking the Katsanos analysis has ended. Our industry partners must be compelled to supply the data and finances needed to settle this issue. The signal seems real and it is time to find answers. Research initiatives are underway through the SVS, the VIVA group, the UK Medicines and Healthcare Products Regulatory Agency, and the FDA.
Going forward, the SVS has formed a Paclitaxel Safety Task Force under the leadership of President-elect Kim Hodgson. Their mission is to facilitate the performance and interpretation of an Individual Patient Data meta-analysis using patient-level RCT data from industry partners. The task force states: “We remain troubled by the recent reports of reanalysis of existing datasets, pooled analyses of RCTs, and other ‘series’, as we believe that the findings of these statistically inferior analyses bring no additional clarity, cannot be relied upon for guidance, and distract us from the analysis that needs to be performed.”
References
1. J Am Heart Assoc. 2018 Dec 18;7(24):e011245.
2. www.fda.gov/medicaldevices/safety/letterstohealthcareproviders/ucm629589.htm.
3. J Am Coll Cardiol. Jan 2019. doi: 10.1016/j.jacc.2019.01.013.
4. Circulation. 2019;139:e42.
5. https://evtoday.com/2019/02/20/zilver-ptx-trial-5-year-mortality-data-corrected-in-circulation.
6. JAMA Cardiol. 2019 Feb 12. doi:10.1001/jamacardio.2019.0325.
7. www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm.
8. www.med-technews.com/news/bud-defends-safety-of-drug-coated-device-following-fda-warnin/.
9. www.crtonline.org/news-detail/paclitaxel-device-safety-thoroughly-discussed-at-c.
As medical editor of Vascular Specialist, it has always been my hope to use our excellent reporters and rapid production schedule to keep readers abreast of the latest news in vascular surgery. While my colleagues at the Journal of Vascular Surgery publish studies that will drive treatment, my goal is to drive discussion.
With topics like burnout, workforce shortages, and electronic medical records, I feel we have been successful. The downside of staying current is we sometimes find ourselves publishing contradictory stories. This has been the case with paclitaxel. Let’s take a break from the fray and review where we are, and where we might go from here.
In 2012, the Zilver PTX became the first drug-eluting stent (DES) to gain Food and Drug Administration approval for the treatment of peripheral vascular disease. Two years later, the FDA approved the Lutonix 035 as the first drug-coated balloon (DCB) for use in the femoral-popliteal arteries. The Lutonix would also gain a second indication for failing dialysis fistulas. Medtronic and Spectranetics received authorizations for their DCBs in 2015 and 2017, respectively.
While the safety of paclitaxel-coated devices in the coronary system had previously been called into question, the drug was generally considered safe and effective in the peripheral arterial system. The controversy began in December 2018, when Katsanos et al.1 published a meta-analysis of 28 randomized, controlled trials (RCTs) investigating paclitaxel-coated devices in the femoral-popliteal arteries. While all-cause patient mortality was similar at 1 year between paclitaxel-coated devices and controls (2.3% in each), at 2 years the risk of death was significantly higher in those treated with paclitaxel (7.2% vs. 3.8%). The 5-year data were available for three trials where there was a continued significantly increased risk of mortality with paclitaxel (14.7% vs. 8.1%).
Opposition to these findings was prompt from both physicians and industry. Weaknesses of the analysis, both perceived and real, were hammered. The meta-analysis did not include individual patient data, and the actual cause of death was unknown in most of the included trials. The study was not adequately powered to eliminate the risk of type 1 error when comparing mortality after 2 years. Individuals assigned to the control group may have received paclitaxel treatment at some point in their follow-up. The DCB and DES treatment groups were combined. The methods employed by the authors, however, stood up reasonably well to scrutiny.
On Jan. 17, 2019, the FDA issued their first response stating, “the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”2
Later that month, Peter Schneider, MD, and associates published a patient-level meta-analysis in the Journal of the American College of Cardiology.3 The study included 1,980 patients and found no statistically significant difference in all-cause mortality between DCB (9.3%) and percutaneous transluminal angioplasty (PTA) (11.2%) through 5 years. Shortly after that, however, a correction was issued.
On Feb. 15, 2019, Medtronic reported an error in the 2- and 3-year follow-up periods for the IN.PACT Global postmarket study. The company stated, “Due to a programming error, mortality data were inadvertently omitted from the summary tables included in the statistical analysis.” The mortality in the DCB cohort was corrected from 9.30% to 15.12%. The authors stated that this new mortality rate was still not significantly higher than the PTA group (P = .09).4
Less than 1 week later, another device company issued a correction. And once again, the error had been made in favor of the paclitaxel-treated group. In 2016, the 5-year data from Cook Medical’s Zilver PTX trial were published in Circulation. The study reported a mortality of 10.2% in the DES group and 16.9% in the PTA cohort. Regrettably, these numbers were reversed and significantly higher in the paclitaxel-treated group (16.9% vs. 10.2%, P = .03).5
On Feb. 12, 2019, another response to the Katsanos meta-analysis was published in JAMA Cardiology.6 In this study, Secemsky et al. analyzed patient-level data from a Medicare database. The authors reported finding no evidence of paclitaxel- related deaths in 16,560 patients. Unfortunately, the mean follow-up time was only 389 days, which may have been insufficient to detect the late mortality reported in the Katsanos meta-analysis.
On March 15, 2019, the FDA issued a second statement, this time with a much stronger tone.7 The agency reported an ongoing analysis of the long-term survival data from the pivotal randomized trials. In the three studies with 5-year data available, each showed a significantly higher mortality in the paclitaxel group.
When pooled, there were 975 patients, and the risk of death was 20.1% in the paclitaxel group versus 13.4 % in the controls. The FDA recommended discussing the increased risk of mortality with all patients receiving paclitaxel therapy as part of the informed consent process. They also stated that for most patients alternative options should generally be used until additional analysis of the mortality risk is performed.
Industry bristled at this new, strongly worded statement. Becton Dickinson, makers of the Lutonix balloon, asserted that the FDA recommendation was based on “a limited review of data from less than 1,000 patients.”8 The company noted that its LEVANT 2 trial did not see a signal of increased mortality at 5 years. Although they did acknowledge that, among the randomized patients, there was a significantly higher mortality at 5 years for those treated with paclitaxel.
How do we make sense of this? Pac-litaxel is a cytotoxic drug. Its pharmacokinetics vary significantly based on the preparation and administration. The FDA label for the injectable form (Taxol) warns of anaphylaxis and severe hypersensitivity reactions, but there is no mention of long-term mortality. In the coronary vessels, paclitaxel-coated devices have been associated with myocardial infarction and death. Obviously it is easy to comprehend how local vessel effects in the coronary system can lead to increased mortality. The pathway is less clear with femoral-popliteal interventions. If the association of paclitaxel with death is truly causation there must be some systemic effects. The dose delivered with femoral- popliteal interventions is much higher than that seen with coronary devices.
The mortality may be associated with the platform used or even the formulation (crystalline formularies have a longer half-life). Could it be something more benign? Paclitaxel-treated patients see less recurrence of their femoral-popliteal disease. Are the control group patients with more recurrences seeing their interventionalist more often and therefore receiving more frequent reminders to comply with medical therapy?
At this point, we have few answers. After an all-day town hall at the recent Cardiovascular Research Technologies conference,9 one moderator said, “I came in with uncertainty and now I’m going away with uncertainty, but we made tremendous progress.” His comoderator added, “I know I don’t know.” Well then, glad we cleared that up!
In any event, changes are coming. The BASIL-3 trial has suspended recruitment. Physicians using paclitaxel-coated devices are now advised by the FDA to inform patients of the increased risk of death and to use alternatives in most cases. Therefore, if you employ these devices routinely in the femoral-popliteal vessels you are seemingly doing so in opposition to the recommendations of the FDA. Legal peril may follow.
The time for nitpicking the Katsanos analysis has ended. Our industry partners must be compelled to supply the data and finances needed to settle this issue. The signal seems real and it is time to find answers. Research initiatives are underway through the SVS, the VIVA group, the UK Medicines and Healthcare Products Regulatory Agency, and the FDA.
Going forward, the SVS has formed a Paclitaxel Safety Task Force under the leadership of President-elect Kim Hodgson. Their mission is to facilitate the performance and interpretation of an Individual Patient Data meta-analysis using patient-level RCT data from industry partners. The task force states: “We remain troubled by the recent reports of reanalysis of existing datasets, pooled analyses of RCTs, and other ‘series’, as we believe that the findings of these statistically inferior analyses bring no additional clarity, cannot be relied upon for guidance, and distract us from the analysis that needs to be performed.”
References
1. J Am Heart Assoc. 2018 Dec 18;7(24):e011245.
2. www.fda.gov/medicaldevices/safety/letterstohealthcareproviders/ucm629589.htm.
3. J Am Coll Cardiol. Jan 2019. doi: 10.1016/j.jacc.2019.01.013.
4. Circulation. 2019;139:e42.
5. https://evtoday.com/2019/02/20/zilver-ptx-trial-5-year-mortality-data-corrected-in-circulation.
6. JAMA Cardiol. 2019 Feb 12. doi:10.1001/jamacardio.2019.0325.
7. www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm.
8. www.med-technews.com/news/bud-defends-safety-of-drug-coated-device-following-fda-warnin/.
9. www.crtonline.org/news-detail/paclitaxel-device-safety-thoroughly-discussed-at-c.