User login
Brexanolone approval ‘marks an important milestone’
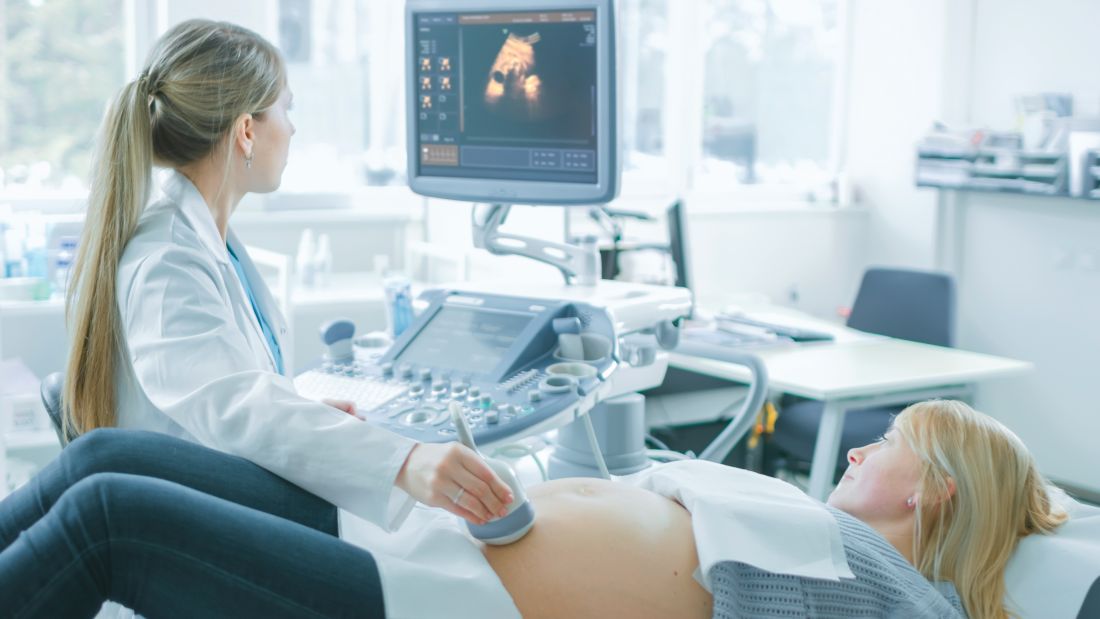
In March 2019, the Food and Drug Administration approved a novel medication, Zulresso (brexanolone), for the treatment of postpartum depression. Brexanolone is the first FDA-approved medication for the treatment of postpartum depression, a serious illness that affects nearly one in nine women soon after giving birth.1
Mothers with postpartum depression experience feelings of sadness, irritability, and anxiety, as well as isolation from their loved ones (including their new baby) and exhaustion. The feelings of sadness and anxiety can be extreme, and can interfere with a woman’s ability to care for herself or her family. In some cases, these symptoms can be life threatening. Indeed, the most common cause of maternal death after childbirth in the developed world is suicide.2 Because of the severity of the symptoms and their impact on the family, postpartum depression usually requires treatment.
Until now, there have been no drugs specifically approved to treat postpartum depression. Commonly, postpartum depression is treated with medications that previously were approved for the treatment of major depressive disorder, despite limited evidence documenting their efficacy for postpartum depression. Other putative treatment alternatives include psychotherapy, estrogen therapy, and neuromodulation, such as electroconvulsive therapy and repetitive transcranial magnetic stimulation. Each of these treatments can take weeks or longer to take effect, time that is of elevated importance given the rapidly developing mother-infant relationship in the early postpartum period. Brexanolone addresses both the issue of efficacy and speed of onset, representing a major step forward in the care of women suffering from postpartum depression.
Importantly, the approval of brexanolone marks an important milestone for the psychiatric research community in general and the National Institute of Mental Health in particular, as it represents a compelling example of successful bench-to-bedside translation of basic neuroscience findings to benefit patients. As we have noted elsewhere,3 the research underlying the discovery of endogenous neurosteroids and their role in modulating GABA receptors laid the foundation for the development of brexanolone, an intravenous formulation of the neurosteroid allopregnanolone. The recognition that allopregnanolone was a protective factor induced by stress, that it derived from progesterone, and that its peripheral blood levels were dramatically reduced in the early postpartum period led to the hypothesis that it might be useful as a treatment for postpartum depression.
Sage Therapeutics took on the task of testing this hypothesis, designing a program, in consultation with the FDA, to test the efficacy of allopregnanolone in women with postpartum depression in a series of randomized, placebo-controlled studies assessing brexanolone. 4,5 It is a significant accomplishment of Sage Therapeutics to not only successfully complete the therapeutic program of studies (given past experience with difficulties recruiting these women for placebo-controlled treatment trials) but as well to demonstrate a robust therapeutic effect.
Although the FDA’s approval of a new and novel treatment is exciting for many women, there are still limitations to the broader use of brexanolone. It is delivered intravenously, requires an overnight stay in a certified medical center, and is likely to be considerably expensive, according to early reports – potentially limiting the access to the treatment. There also are potentially serious side effects, such as sedation, dizziness, or sudden loss of consciousness. Nonetheless, this is a promising first step and hopefully will spur further efforts to identify and optimize additional strategies to treat postpartum depression. In fact, other formulations of allopregnanolone and novel analogs to treat postpartum depression already are under study, including some that are orally bioavailable.6,7,8
Several important questions remain to be answered about both brexanolone and postpartum depression: What is the underlying mechanism through which allopregnanolone acts in the brain and reduces depressive symptoms? Is the mechanism unique to postpartum women, or might brexanolone also be effective in nonreproductive depressions in women and men? What causes postpartum depression, and what are the risk factors involved for women who develop this serious condition? Future work will focus on these and other important questions to the benefit of women who have suffered with this condition.
The FDA approval of brexanolone represents the second approval in a month of a new antidepressant treatment targeting different molecules in the brain. In early March 2019, the agency approved Spravato (esketamine) nasal spray as a therapy for treatment-resistant depression. Like brexanolone, esketamine is a fast-acting antidepressant that works through a novel mechanism, completely different from other antidepressants. These new treatment approvals are encouraging, as there has been a paucity for many years in approving new effective treatments for mood disorders.
However, treatment development in psychiatry still has a long way to go and the full underlying neurobiology of mood disorders, including postpartum depression, remains poorly understood. Many challenges are ahead of us in our efforts to develop new treatments and increase our understanding of mental illnesses. Nevertheless, the approval of brexanolone is an important milestone, giving hope to the many women who suffer from postpartum depression, and paving the way for the development of additional novel and effective medications to treat this serious and sometimes life-threatening condition.
Dr. Gordon is the director of the National Institute of Mental Health (NIMH), the lead federal agency for research on mental disorders. He oversees an extensive research portfolio of basic and clinical research that seeks to transform the understanding and treatment of mental illnesses, paving the way for prevention, recovery, and cure. Dr. Hillefors works at the NIMH and oversees the Translational Therapeutics Program in the division of translational research, focusing on the development of novel treatments and biomarkers and early phase clinical trials. She received her MD and PhD in neuroscience at the Karolinska Institute, Sweden. Dr. Schmidt joined the NIMH in 1986 after completing his psychiatric residency at the University of Toronto. He is the chief of the Section on Behavioral Endocrinology, within the Intramural Research Program at the NIMH, where his laboratory studies the relationship between hormones, stress, and mood – particularly in the areas of postpartum depression, severe premenstrual dysphoria, and perimenopausal depression.
References
1. J Psychiatric Res. 2018 Sep;104:235-48.
2. Br J Psychiatry. 2003 Oct;183:279-81.
3. NIMH Director’s Messages. 2019 Mar 20.
4. Lancet. 2017 Jul 29;390(10093):480-9.
5. Lancet. 2018 Sep 22; 392(10152):1058-70.
6. Sage Therapeutics News. 2019 Jan 7.
7. Marinus Pharmaceuticals. 2017 Jun 27.
8. ClinicalTrials.gov Identifier: NCT03460756. 2019 Mar.
In March 2019, the Food and Drug Administration approved a novel medication, Zulresso (brexanolone), for the treatment of postpartum depression. Brexanolone is the first FDA-approved medication for the treatment of postpartum depression, a serious illness that affects nearly one in nine women soon after giving birth.1
Mothers with postpartum depression experience feelings of sadness, irritability, and anxiety, as well as isolation from their loved ones (including their new baby) and exhaustion. The feelings of sadness and anxiety can be extreme, and can interfere with a woman’s ability to care for herself or her family. In some cases, these symptoms can be life threatening. Indeed, the most common cause of maternal death after childbirth in the developed world is suicide.2 Because of the severity of the symptoms and their impact on the family, postpartum depression usually requires treatment.
Until now, there have been no drugs specifically approved to treat postpartum depression. Commonly, postpartum depression is treated with medications that previously were approved for the treatment of major depressive disorder, despite limited evidence documenting their efficacy for postpartum depression. Other putative treatment alternatives include psychotherapy, estrogen therapy, and neuromodulation, such as electroconvulsive therapy and repetitive transcranial magnetic stimulation. Each of these treatments can take weeks or longer to take effect, time that is of elevated importance given the rapidly developing mother-infant relationship in the early postpartum period. Brexanolone addresses both the issue of efficacy and speed of onset, representing a major step forward in the care of women suffering from postpartum depression.
Importantly, the approval of brexanolone marks an important milestone for the psychiatric research community in general and the National Institute of Mental Health in particular, as it represents a compelling example of successful bench-to-bedside translation of basic neuroscience findings to benefit patients. As we have noted elsewhere,3 the research underlying the discovery of endogenous neurosteroids and their role in modulating GABA receptors laid the foundation for the development of brexanolone, an intravenous formulation of the neurosteroid allopregnanolone. The recognition that allopregnanolone was a protective factor induced by stress, that it derived from progesterone, and that its peripheral blood levels were dramatically reduced in the early postpartum period led to the hypothesis that it might be useful as a treatment for postpartum depression.
Sage Therapeutics took on the task of testing this hypothesis, designing a program, in consultation with the FDA, to test the efficacy of allopregnanolone in women with postpartum depression in a series of randomized, placebo-controlled studies assessing brexanolone. 4,5 It is a significant accomplishment of Sage Therapeutics to not only successfully complete the therapeutic program of studies (given past experience with difficulties recruiting these women for placebo-controlled treatment trials) but as well to demonstrate a robust therapeutic effect.
Although the FDA’s approval of a new and novel treatment is exciting for many women, there are still limitations to the broader use of brexanolone. It is delivered intravenously, requires an overnight stay in a certified medical center, and is likely to be considerably expensive, according to early reports – potentially limiting the access to the treatment. There also are potentially serious side effects, such as sedation, dizziness, or sudden loss of consciousness. Nonetheless, this is a promising first step and hopefully will spur further efforts to identify and optimize additional strategies to treat postpartum depression. In fact, other formulations of allopregnanolone and novel analogs to treat postpartum depression already are under study, including some that are orally bioavailable.6,7,8
Several important questions remain to be answered about both brexanolone and postpartum depression: What is the underlying mechanism through which allopregnanolone acts in the brain and reduces depressive symptoms? Is the mechanism unique to postpartum women, or might brexanolone also be effective in nonreproductive depressions in women and men? What causes postpartum depression, and what are the risk factors involved for women who develop this serious condition? Future work will focus on these and other important questions to the benefit of women who have suffered with this condition.
The FDA approval of brexanolone represents the second approval in a month of a new antidepressant treatment targeting different molecules in the brain. In early March 2019, the agency approved Spravato (esketamine) nasal spray as a therapy for treatment-resistant depression. Like brexanolone, esketamine is a fast-acting antidepressant that works through a novel mechanism, completely different from other antidepressants. These new treatment approvals are encouraging, as there has been a paucity for many years in approving new effective treatments for mood disorders.
However, treatment development in psychiatry still has a long way to go and the full underlying neurobiology of mood disorders, including postpartum depression, remains poorly understood. Many challenges are ahead of us in our efforts to develop new treatments and increase our understanding of mental illnesses. Nevertheless, the approval of brexanolone is an important milestone, giving hope to the many women who suffer from postpartum depression, and paving the way for the development of additional novel and effective medications to treat this serious and sometimes life-threatening condition.
Dr. Gordon is the director of the National Institute of Mental Health (NIMH), the lead federal agency for research on mental disorders. He oversees an extensive research portfolio of basic and clinical research that seeks to transform the understanding and treatment of mental illnesses, paving the way for prevention, recovery, and cure. Dr. Hillefors works at the NIMH and oversees the Translational Therapeutics Program in the division of translational research, focusing on the development of novel treatments and biomarkers and early phase clinical trials. She received her MD and PhD in neuroscience at the Karolinska Institute, Sweden. Dr. Schmidt joined the NIMH in 1986 after completing his psychiatric residency at the University of Toronto. He is the chief of the Section on Behavioral Endocrinology, within the Intramural Research Program at the NIMH, where his laboratory studies the relationship between hormones, stress, and mood – particularly in the areas of postpartum depression, severe premenstrual dysphoria, and perimenopausal depression.
References
1. J Psychiatric Res. 2018 Sep;104:235-48.
2. Br J Psychiatry. 2003 Oct;183:279-81.
3. NIMH Director’s Messages. 2019 Mar 20.
4. Lancet. 2017 Jul 29;390(10093):480-9.
5. Lancet. 2018 Sep 22; 392(10152):1058-70.
6. Sage Therapeutics News. 2019 Jan 7.
7. Marinus Pharmaceuticals. 2017 Jun 27.
8. ClinicalTrials.gov Identifier: NCT03460756. 2019 Mar.
In March 2019, the Food and Drug Administration approved a novel medication, Zulresso (brexanolone), for the treatment of postpartum depression. Brexanolone is the first FDA-approved medication for the treatment of postpartum depression, a serious illness that affects nearly one in nine women soon after giving birth.1
Mothers with postpartum depression experience feelings of sadness, irritability, and anxiety, as well as isolation from their loved ones (including their new baby) and exhaustion. The feelings of sadness and anxiety can be extreme, and can interfere with a woman’s ability to care for herself or her family. In some cases, these symptoms can be life threatening. Indeed, the most common cause of maternal death after childbirth in the developed world is suicide.2 Because of the severity of the symptoms and their impact on the family, postpartum depression usually requires treatment.
Until now, there have been no drugs specifically approved to treat postpartum depression. Commonly, postpartum depression is treated with medications that previously were approved for the treatment of major depressive disorder, despite limited evidence documenting their efficacy for postpartum depression. Other putative treatment alternatives include psychotherapy, estrogen therapy, and neuromodulation, such as electroconvulsive therapy and repetitive transcranial magnetic stimulation. Each of these treatments can take weeks or longer to take effect, time that is of elevated importance given the rapidly developing mother-infant relationship in the early postpartum period. Brexanolone addresses both the issue of efficacy and speed of onset, representing a major step forward in the care of women suffering from postpartum depression.
Importantly, the approval of brexanolone marks an important milestone for the psychiatric research community in general and the National Institute of Mental Health in particular, as it represents a compelling example of successful bench-to-bedside translation of basic neuroscience findings to benefit patients. As we have noted elsewhere,3 the research underlying the discovery of endogenous neurosteroids and their role in modulating GABA receptors laid the foundation for the development of brexanolone, an intravenous formulation of the neurosteroid allopregnanolone. The recognition that allopregnanolone was a protective factor induced by stress, that it derived from progesterone, and that its peripheral blood levels were dramatically reduced in the early postpartum period led to the hypothesis that it might be useful as a treatment for postpartum depression.
Sage Therapeutics took on the task of testing this hypothesis, designing a program, in consultation with the FDA, to test the efficacy of allopregnanolone in women with postpartum depression in a series of randomized, placebo-controlled studies assessing brexanolone. 4,5 It is a significant accomplishment of Sage Therapeutics to not only successfully complete the therapeutic program of studies (given past experience with difficulties recruiting these women for placebo-controlled treatment trials) but as well to demonstrate a robust therapeutic effect.
Although the FDA’s approval of a new and novel treatment is exciting for many women, there are still limitations to the broader use of brexanolone. It is delivered intravenously, requires an overnight stay in a certified medical center, and is likely to be considerably expensive, according to early reports – potentially limiting the access to the treatment. There also are potentially serious side effects, such as sedation, dizziness, or sudden loss of consciousness. Nonetheless, this is a promising first step and hopefully will spur further efforts to identify and optimize additional strategies to treat postpartum depression. In fact, other formulations of allopregnanolone and novel analogs to treat postpartum depression already are under study, including some that are orally bioavailable.6,7,8
Several important questions remain to be answered about both brexanolone and postpartum depression: What is the underlying mechanism through which allopregnanolone acts in the brain and reduces depressive symptoms? Is the mechanism unique to postpartum women, or might brexanolone also be effective in nonreproductive depressions in women and men? What causes postpartum depression, and what are the risk factors involved for women who develop this serious condition? Future work will focus on these and other important questions to the benefit of women who have suffered with this condition.
The FDA approval of brexanolone represents the second approval in a month of a new antidepressant treatment targeting different molecules in the brain. In early March 2019, the agency approved Spravato (esketamine) nasal spray as a therapy for treatment-resistant depression. Like brexanolone, esketamine is a fast-acting antidepressant that works through a novel mechanism, completely different from other antidepressants. These new treatment approvals are encouraging, as there has been a paucity for many years in approving new effective treatments for mood disorders.
However, treatment development in psychiatry still has a long way to go and the full underlying neurobiology of mood disorders, including postpartum depression, remains poorly understood. Many challenges are ahead of us in our efforts to develop new treatments and increase our understanding of mental illnesses. Nevertheless, the approval of brexanolone is an important milestone, giving hope to the many women who suffer from postpartum depression, and paving the way for the development of additional novel and effective medications to treat this serious and sometimes life-threatening condition.
Dr. Gordon is the director of the National Institute of Mental Health (NIMH), the lead federal agency for research on mental disorders. He oversees an extensive research portfolio of basic and clinical research that seeks to transform the understanding and treatment of mental illnesses, paving the way for prevention, recovery, and cure. Dr. Hillefors works at the NIMH and oversees the Translational Therapeutics Program in the division of translational research, focusing on the development of novel treatments and biomarkers and early phase clinical trials. She received her MD and PhD in neuroscience at the Karolinska Institute, Sweden. Dr. Schmidt joined the NIMH in 1986 after completing his psychiatric residency at the University of Toronto. He is the chief of the Section on Behavioral Endocrinology, within the Intramural Research Program at the NIMH, where his laboratory studies the relationship between hormones, stress, and mood – particularly in the areas of postpartum depression, severe premenstrual dysphoria, and perimenopausal depression.
References
1. J Psychiatric Res. 2018 Sep;104:235-48.
2. Br J Psychiatry. 2003 Oct;183:279-81.
3. NIMH Director’s Messages. 2019 Mar 20.
4. Lancet. 2017 Jul 29;390(10093):480-9.
5. Lancet. 2018 Sep 22; 392(10152):1058-70.
6. Sage Therapeutics News. 2019 Jan 7.
7. Marinus Pharmaceuticals. 2017 Jun 27.
8. ClinicalTrials.gov Identifier: NCT03460756. 2019 Mar.
“I go by thickness”
One of our favorite comics was Steven Wright, who made it to the Tonight Show with Johnny Carson. (Google him.)
Wright’s offbeat humor was quirky and a bit philosophical, and was delivered in a deadpan, mumbled monotone. For instance:
When I got to school, the teacher said, “The socks you’re wearing don’t match. They’re two different colors.”
I said, “I go by thickness.”
That punchline goes pop in your head, like a shy little firecracker: How come it never occurred to me to look at it that way?
I thought of Steven Wright recently while I was enrolling Stacy, a 20-year-old, in the iPledge program for a planned course of isotretinoin. Stacy told me she is sexually active and has an IUD.
“When you start the medicine next month,” I told her, “you’ll need to pick a second form of contraception.”
Stacy looked bewildered. When I’ve made that statement to a thousand previous patients, none of them ever looked bewildered.
“I mean,” I said, “besides the IUD, you’ll need to use a second type of contraception, to be sure you don’t get pregnant. You could choose condoms, or one of the other types listed in the booklet I gave you.”
That didn’t seem to help. Stacey hemmed a bit. “Does that mean I have to tell you ... ?”
“Yes, you have to pick another form of birth control and tell me which one it is.”
“I have to tell you every time?”
My go-by-thickness moment – I finally got it. “NO,” I said. “You do NOT have to tell me which second contraceptive you use every time you have sex!”
Steven Wright would be proud of Stacy. Isotretinoin came out in 1982, but nobody ever thought of “choose a second type of contraception” that way before. Stacy goes by retail.
That case reminded of another out-of-left field question I heard for the first – and only – time almost 40 years ago. I had prescribed a cream for a young man.
“Can I get it refilled?” he asked.
“Sure,” I said.
“How do I refill it?” he asked.
“You take it back to the pharmacy, and they refill it for you,” I said.
“But how do they refill it?”
“You show them what you need, and they refill it.”
“But how?”
“Why do you keep asking me that?”
“The tube is going to be all scrunched up from my squeezing it,” he said. “How do they get the new cream back in?”
Well son of a gun, “refill” could mean that, couldn’t it? If you go by thickness.
In idle moments I like to let novel perspectives such as those roll around in my head. The other day I accompanied a relative to an emergency department. While waiting in triage for 5 hours, I looked up and saw a sign on the wall, in big, blue letters: “Support ED Research!”
That puzzled me. I know it can be an important problem, but why the dickens would someone come to an emergency department for erectile dysfunction?
I go by acronyms.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
One of our favorite comics was Steven Wright, who made it to the Tonight Show with Johnny Carson. (Google him.)
Wright’s offbeat humor was quirky and a bit philosophical, and was delivered in a deadpan, mumbled monotone. For instance:
When I got to school, the teacher said, “The socks you’re wearing don’t match. They’re two different colors.”
I said, “I go by thickness.”
That punchline goes pop in your head, like a shy little firecracker: How come it never occurred to me to look at it that way?
I thought of Steven Wright recently while I was enrolling Stacy, a 20-year-old, in the iPledge program for a planned course of isotretinoin. Stacy told me she is sexually active and has an IUD.
“When you start the medicine next month,” I told her, “you’ll need to pick a second form of contraception.”
Stacy looked bewildered. When I’ve made that statement to a thousand previous patients, none of them ever looked bewildered.
“I mean,” I said, “besides the IUD, you’ll need to use a second type of contraception, to be sure you don’t get pregnant. You could choose condoms, or one of the other types listed in the booklet I gave you.”
That didn’t seem to help. Stacey hemmed a bit. “Does that mean I have to tell you ... ?”
“Yes, you have to pick another form of birth control and tell me which one it is.”
“I have to tell you every time?”
My go-by-thickness moment – I finally got it. “NO,” I said. “You do NOT have to tell me which second contraceptive you use every time you have sex!”
Steven Wright would be proud of Stacy. Isotretinoin came out in 1982, but nobody ever thought of “choose a second type of contraception” that way before. Stacy goes by retail.
That case reminded of another out-of-left field question I heard for the first – and only – time almost 40 years ago. I had prescribed a cream for a young man.
“Can I get it refilled?” he asked.
“Sure,” I said.
“How do I refill it?” he asked.
“You take it back to the pharmacy, and they refill it for you,” I said.
“But how do they refill it?”
“You show them what you need, and they refill it.”
“But how?”
“Why do you keep asking me that?”
“The tube is going to be all scrunched up from my squeezing it,” he said. “How do they get the new cream back in?”
Well son of a gun, “refill” could mean that, couldn’t it? If you go by thickness.
In idle moments I like to let novel perspectives such as those roll around in my head. The other day I accompanied a relative to an emergency department. While waiting in triage for 5 hours, I looked up and saw a sign on the wall, in big, blue letters: “Support ED Research!”
That puzzled me. I know it can be an important problem, but why the dickens would someone come to an emergency department for erectile dysfunction?
I go by acronyms.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
One of our favorite comics was Steven Wright, who made it to the Tonight Show with Johnny Carson. (Google him.)
Wright’s offbeat humor was quirky and a bit philosophical, and was delivered in a deadpan, mumbled monotone. For instance:
When I got to school, the teacher said, “The socks you’re wearing don’t match. They’re two different colors.”
I said, “I go by thickness.”
That punchline goes pop in your head, like a shy little firecracker: How come it never occurred to me to look at it that way?
I thought of Steven Wright recently while I was enrolling Stacy, a 20-year-old, in the iPledge program for a planned course of isotretinoin. Stacy told me she is sexually active and has an IUD.
“When you start the medicine next month,” I told her, “you’ll need to pick a second form of contraception.”
Stacy looked bewildered. When I’ve made that statement to a thousand previous patients, none of them ever looked bewildered.
“I mean,” I said, “besides the IUD, you’ll need to use a second type of contraception, to be sure you don’t get pregnant. You could choose condoms, or one of the other types listed in the booklet I gave you.”
That didn’t seem to help. Stacey hemmed a bit. “Does that mean I have to tell you ... ?”
“Yes, you have to pick another form of birth control and tell me which one it is.”
“I have to tell you every time?”
My go-by-thickness moment – I finally got it. “NO,” I said. “You do NOT have to tell me which second contraceptive you use every time you have sex!”
Steven Wright would be proud of Stacy. Isotretinoin came out in 1982, but nobody ever thought of “choose a second type of contraception” that way before. Stacy goes by retail.
That case reminded of another out-of-left field question I heard for the first – and only – time almost 40 years ago. I had prescribed a cream for a young man.
“Can I get it refilled?” he asked.
“Sure,” I said.
“How do I refill it?” he asked.
“You take it back to the pharmacy, and they refill it for you,” I said.
“But how do they refill it?”
“You show them what you need, and they refill it.”
“But how?”
“Why do you keep asking me that?”
“The tube is going to be all scrunched up from my squeezing it,” he said. “How do they get the new cream back in?”
Well son of a gun, “refill” could mean that, couldn’t it? If you go by thickness.
In idle moments I like to let novel perspectives such as those roll around in my head. The other day I accompanied a relative to an emergency department. While waiting in triage for 5 hours, I looked up and saw a sign on the wall, in big, blue letters: “Support ED Research!”
That puzzled me. I know it can be an important problem, but why the dickens would someone come to an emergency department for erectile dysfunction?
I go by acronyms.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Q&A on LGBT Youth: Affirmation in your practice
In recent years, pediatricians have learned more about the diversity of gender identities and gender expressions that exist, but many think they have gaps in their knowledge that need to be filled in order to provide better care to these patients.
On April 3, Pediatric News hosted a Twitter question-and-answer session for the purpose of trying to help pediatricians close some of these gaps, including ones related to the medical and mental health issues particular to LGBT patients. During this session Pediatric News’ LGBT Youth Consult columnists, Gerald T. Montano, DO, MS, and Gayathri Chelvakumar, MD, MPH, responded to four questions about working with children and teens who are lesbian, gay, bisexual, transgender, or questioning (LGBTQ). Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Dr. Chelvakumar, MD, MPH, is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
The following is an edited version of the Q&A session.
Question 1: How do people become aware they are lesbian, gay, bisexual, transgender?
Dr. Montano: For many adolescents, the teenage years are the time that these identities are forming and that questions around sexuality and gender identity may arise. Check out #genderunicorn! It illustrates the spectrum of normal gender and sexual identities that are part of the human experience. Many LGBT people recall how they felt when they were younger but couldn’t find the right words until they were older (usually during adolescence).
Dr. Chelvakumar: Every person’s experience is unique. I have had patients tell me they knew who they were attracted to in kindergarten, and other patients who are still figuring it out in college. It is important to note that every experience is valid.
Question 2: How can we address some of the specific health concerns of LGBT youth?
Dr. Chelvakumar: We need to educate ourselves. The American Academy of Pediatrics has a great resource for this, “Ensuring Comprehensive Care and Support for Transgender and Gender Diverse Children and Adolescents (Pediatrics. 2019 Oct. doi: 10.1542/peds.2018-2162).
Dr. Chelvakumar: Some LGBTQ youth are at increased risk of depression, anxiety, and suicidality related to stigma and internal/external transphobia/homophobia, NOT their identities.
Dr. Montano: A lot of LGBT youth report that health care providers fixate on sexual activity, even though that was not the reason why they came to the clinic.
Dr. Chelvakumar: A lot of these youth feel that providers won’t understand their needs or may discriminate against them because of their LGBTQ identity. This is why it is important to make sure our health care spaces are welcoming to everyone.
Dr. Montano: The role of the psychiatrist is not to determine the person’s gender identity or sexual orientation; rather, they should focus on making others feel comfortable with themselves.
Question 3: How can we make our clinics a safe space for LGBT youth?
Dr. Chelvakumar: Ensure that ALL clinic staff receive training on culturally affirming care of LGBTQ people. The National LGBT Health Education Center (@LGBTHealthEdCtr) offers some of these resources.
Dr. Montano: Train everyone in the clinic to be sensitive and aware of the needs of LGBT patients; it only takes one person to make a clinical experience horrible for an LGBT person.
Dr. Chelvakumar: To me, culturally affirming care means being aware of the spectrum of identities and experiences of all of our patients/families and being respectful of these identities. I also like the term cultural humility – we must continually learn about these diverse experiences.
Dr. Montano: Remember that if they get upset, these children and teens are not mad at you, they are mad at the situation.
Dr. Chelvakumar: An easy way to show that a clinic is a safe space is to clearly display a nondiscrimination policy, such as “We serve and respect all patients regardless of gender identity, race, sexual orientation, religion, socioeconomic status ... ”
Dr. Montano: Not every LGBT youth will disclose their sexual orientation or gender identity to the provider, even if the clinic appears welcoming. I suggest you begin a conversation with a patient by telling the patient your own pronouns, opening the door to additional conversations.
Dr. Chelvakumar: Many organizations are moving to universally asking questions about gender identity and pronouns. In the pediatric population, this can be difficult given privacy/confidentiality concerns. I usually ask these questions when obtaining my sensitive sexual history.
Question 4: What is gender dysphoria, and how is it treated?
Dr. Montano: Gender dysphoria is the distress related to gender identity that does not match sex/gender assigned at birth. Treat the distress, not the identity. There are many ways to treat gender dysphoria. You can provide social support, use pubertal blockers to prevent the development of secondary sex characteristics during adolescence, and/or use hormones or surgery to develop characteristics of the affirmed gender. And there is no “right” treatment for gender dysphoria. Each treatment is tailored to the needs of the youth.
Dr. Chelvakumar: It is important to recognize that each person’s journey/transition is different. Our job as providers is to help them on the path that will help them live their lives as their authentic selves. This may or may not involve taking medication and/or undergoing surgery.
Dr. Montano: Providers should respect the wishes of those who want to affirm their gender identity that makes sense to the patient and not try to impose their own idea of what transitioning should “look like.”
Dr. Chelvakumar: There are many guidelines that exist for patients with gender dysphoria. The World Professional Association for Transgender Health, the Endocrine Society, and University of California, San Francisco’s Center of Excellence for Transgender Health are all excellent resources.
In recent years, pediatricians have learned more about the diversity of gender identities and gender expressions that exist, but many think they have gaps in their knowledge that need to be filled in order to provide better care to these patients.
On April 3, Pediatric News hosted a Twitter question-and-answer session for the purpose of trying to help pediatricians close some of these gaps, including ones related to the medical and mental health issues particular to LGBT patients. During this session Pediatric News’ LGBT Youth Consult columnists, Gerald T. Montano, DO, MS, and Gayathri Chelvakumar, MD, MPH, responded to four questions about working with children and teens who are lesbian, gay, bisexual, transgender, or questioning (LGBTQ). Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Dr. Chelvakumar, MD, MPH, is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
The following is an edited version of the Q&A session.
Question 1: How do people become aware they are lesbian, gay, bisexual, transgender?
Dr. Montano: For many adolescents, the teenage years are the time that these identities are forming and that questions around sexuality and gender identity may arise. Check out #genderunicorn! It illustrates the spectrum of normal gender and sexual identities that are part of the human experience. Many LGBT people recall how they felt when they were younger but couldn’t find the right words until they were older (usually during adolescence).
Dr. Chelvakumar: Every person’s experience is unique. I have had patients tell me they knew who they were attracted to in kindergarten, and other patients who are still figuring it out in college. It is important to note that every experience is valid.
Question 2: How can we address some of the specific health concerns of LGBT youth?
Dr. Chelvakumar: We need to educate ourselves. The American Academy of Pediatrics has a great resource for this, “Ensuring Comprehensive Care and Support for Transgender and Gender Diverse Children and Adolescents (Pediatrics. 2019 Oct. doi: 10.1542/peds.2018-2162).
Dr. Chelvakumar: Some LGBTQ youth are at increased risk of depression, anxiety, and suicidality related to stigma and internal/external transphobia/homophobia, NOT their identities.
Dr. Montano: A lot of LGBT youth report that health care providers fixate on sexual activity, even though that was not the reason why they came to the clinic.
Dr. Chelvakumar: A lot of these youth feel that providers won’t understand their needs or may discriminate against them because of their LGBTQ identity. This is why it is important to make sure our health care spaces are welcoming to everyone.
Dr. Montano: The role of the psychiatrist is not to determine the person’s gender identity or sexual orientation; rather, they should focus on making others feel comfortable with themselves.
Question 3: How can we make our clinics a safe space for LGBT youth?
Dr. Chelvakumar: Ensure that ALL clinic staff receive training on culturally affirming care of LGBTQ people. The National LGBT Health Education Center (@LGBTHealthEdCtr) offers some of these resources.
Dr. Montano: Train everyone in the clinic to be sensitive and aware of the needs of LGBT patients; it only takes one person to make a clinical experience horrible for an LGBT person.
Dr. Chelvakumar: To me, culturally affirming care means being aware of the spectrum of identities and experiences of all of our patients/families and being respectful of these identities. I also like the term cultural humility – we must continually learn about these diverse experiences.
Dr. Montano: Remember that if they get upset, these children and teens are not mad at you, they are mad at the situation.
Dr. Chelvakumar: An easy way to show that a clinic is a safe space is to clearly display a nondiscrimination policy, such as “We serve and respect all patients regardless of gender identity, race, sexual orientation, religion, socioeconomic status ... ”
Dr. Montano: Not every LGBT youth will disclose their sexual orientation or gender identity to the provider, even if the clinic appears welcoming. I suggest you begin a conversation with a patient by telling the patient your own pronouns, opening the door to additional conversations.
Dr. Chelvakumar: Many organizations are moving to universally asking questions about gender identity and pronouns. In the pediatric population, this can be difficult given privacy/confidentiality concerns. I usually ask these questions when obtaining my sensitive sexual history.
Question 4: What is gender dysphoria, and how is it treated?
Dr. Montano: Gender dysphoria is the distress related to gender identity that does not match sex/gender assigned at birth. Treat the distress, not the identity. There are many ways to treat gender dysphoria. You can provide social support, use pubertal blockers to prevent the development of secondary sex characteristics during adolescence, and/or use hormones or surgery to develop characteristics of the affirmed gender. And there is no “right” treatment for gender dysphoria. Each treatment is tailored to the needs of the youth.
Dr. Chelvakumar: It is important to recognize that each person’s journey/transition is different. Our job as providers is to help them on the path that will help them live their lives as their authentic selves. This may or may not involve taking medication and/or undergoing surgery.
Dr. Montano: Providers should respect the wishes of those who want to affirm their gender identity that makes sense to the patient and not try to impose their own idea of what transitioning should “look like.”
Dr. Chelvakumar: There are many guidelines that exist for patients with gender dysphoria. The World Professional Association for Transgender Health, the Endocrine Society, and University of California, San Francisco’s Center of Excellence for Transgender Health are all excellent resources.
In recent years, pediatricians have learned more about the diversity of gender identities and gender expressions that exist, but many think they have gaps in their knowledge that need to be filled in order to provide better care to these patients.
On April 3, Pediatric News hosted a Twitter question-and-answer session for the purpose of trying to help pediatricians close some of these gaps, including ones related to the medical and mental health issues particular to LGBT patients. During this session Pediatric News’ LGBT Youth Consult columnists, Gerald T. Montano, DO, MS, and Gayathri Chelvakumar, MD, MPH, responded to four questions about working with children and teens who are lesbian, gay, bisexual, transgender, or questioning (LGBTQ). Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Dr. Chelvakumar, MD, MPH, is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
The following is an edited version of the Q&A session.
Question 1: How do people become aware they are lesbian, gay, bisexual, transgender?
Dr. Montano: For many adolescents, the teenage years are the time that these identities are forming and that questions around sexuality and gender identity may arise. Check out #genderunicorn! It illustrates the spectrum of normal gender and sexual identities that are part of the human experience. Many LGBT people recall how they felt when they were younger but couldn’t find the right words until they were older (usually during adolescence).
Dr. Chelvakumar: Every person’s experience is unique. I have had patients tell me they knew who they were attracted to in kindergarten, and other patients who are still figuring it out in college. It is important to note that every experience is valid.
Question 2: How can we address some of the specific health concerns of LGBT youth?
Dr. Chelvakumar: We need to educate ourselves. The American Academy of Pediatrics has a great resource for this, “Ensuring Comprehensive Care and Support for Transgender and Gender Diverse Children and Adolescents (Pediatrics. 2019 Oct. doi: 10.1542/peds.2018-2162).
Dr. Chelvakumar: Some LGBTQ youth are at increased risk of depression, anxiety, and suicidality related to stigma and internal/external transphobia/homophobia, NOT their identities.
Dr. Montano: A lot of LGBT youth report that health care providers fixate on sexual activity, even though that was not the reason why they came to the clinic.
Dr. Chelvakumar: A lot of these youth feel that providers won’t understand their needs or may discriminate against them because of their LGBTQ identity. This is why it is important to make sure our health care spaces are welcoming to everyone.
Dr. Montano: The role of the psychiatrist is not to determine the person’s gender identity or sexual orientation; rather, they should focus on making others feel comfortable with themselves.
Question 3: How can we make our clinics a safe space for LGBT youth?
Dr. Chelvakumar: Ensure that ALL clinic staff receive training on culturally affirming care of LGBTQ people. The National LGBT Health Education Center (@LGBTHealthEdCtr) offers some of these resources.
Dr. Montano: Train everyone in the clinic to be sensitive and aware of the needs of LGBT patients; it only takes one person to make a clinical experience horrible for an LGBT person.
Dr. Chelvakumar: To me, culturally affirming care means being aware of the spectrum of identities and experiences of all of our patients/families and being respectful of these identities. I also like the term cultural humility – we must continually learn about these diverse experiences.
Dr. Montano: Remember that if they get upset, these children and teens are not mad at you, they are mad at the situation.
Dr. Chelvakumar: An easy way to show that a clinic is a safe space is to clearly display a nondiscrimination policy, such as “We serve and respect all patients regardless of gender identity, race, sexual orientation, religion, socioeconomic status ... ”
Dr. Montano: Not every LGBT youth will disclose their sexual orientation or gender identity to the provider, even if the clinic appears welcoming. I suggest you begin a conversation with a patient by telling the patient your own pronouns, opening the door to additional conversations.
Dr. Chelvakumar: Many organizations are moving to universally asking questions about gender identity and pronouns. In the pediatric population, this can be difficult given privacy/confidentiality concerns. I usually ask these questions when obtaining my sensitive sexual history.
Question 4: What is gender dysphoria, and how is it treated?
Dr. Montano: Gender dysphoria is the distress related to gender identity that does not match sex/gender assigned at birth. Treat the distress, not the identity. There are many ways to treat gender dysphoria. You can provide social support, use pubertal blockers to prevent the development of secondary sex characteristics during adolescence, and/or use hormones or surgery to develop characteristics of the affirmed gender. And there is no “right” treatment for gender dysphoria. Each treatment is tailored to the needs of the youth.
Dr. Chelvakumar: It is important to recognize that each person’s journey/transition is different. Our job as providers is to help them on the path that will help them live their lives as their authentic selves. This may or may not involve taking medication and/or undergoing surgery.
Dr. Montano: Providers should respect the wishes of those who want to affirm their gender identity that makes sense to the patient and not try to impose their own idea of what transitioning should “look like.”
Dr. Chelvakumar: There are many guidelines that exist for patients with gender dysphoria. The World Professional Association for Transgender Health, the Endocrine Society, and University of California, San Francisco’s Center of Excellence for Transgender Health are all excellent resources.
Powerful breast-implant testimony constrained by limited evidence
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
Survivors offer ‘mass shooting grief 101’
People coping with the aftermath of mass shootings might find some solace from Sandy and Lonnie Phillips. The couple, whose daughter was gunned down in the 2012 slaughter in an Aurora, Colo., movie theater, travels the country to help those whose pain is raw begin processing their grief. Mr. and Mrs. Phillips, driven by compassion, hope that sharing their experience will help others. They have started a nonprofit organization called Survivors Empowered to offer advice and kinship in the wake of mass shootings.
Emergency department visits can prove disorienting for patients with physical illnesses. But for those with mental illness, such visits can feel not only disorienting but disconcerting – particularly if sedation or restraints are involved. “If you are living with schizophrenia or bipolar disorder, that is a really tough way to begin that road to recovery,” said Jack Rozel, MD, president of the American Association for Emergency Psychiatry (AAEP), in an interview with CNN. At about 100 locations across the country, psychiatric EDs staffed by psychiatrists and other physicians, nurses, and social workers are triaging and treating patients with mental illness. The goal is treatment and either release or referral to more specialized care within 24 hours. The approach is the brainchild of Scott Zeller, MD, vice president of acute psychiatry at Vituity and a past president of the AAEP. The physician-led organization provides staffing and consulting services to medical centers nationwide. Dr. Zeller hatched the idea of a psychiatric ED while working as chief of psychiatric emergency services at John George Psychiatric Hospital in San Leandro, Calif. “We need to treat people at the emergency level of care,” he said. “The vast majority of psychiatric emergencies can be resolved in less than 24 hours.” Dr. Zeller took the reins in transforming the center from a traditional ward, including the use of restraints, to a setting more like a living room that was supportive rather than institutional. The results included improved patient outcomes and cost savings – in part because of the reduced time spent in the ED. CNN.
In Buffalo, N.Y., a new memorial to be installed in the Buffalo and Erie County Naval and Military Park will honor veterans who were lost to suicide. Ground was broken recently for the Battle Within Memorial. The 8-feet-tall sculpture will portray one soldier carrying the empty outline of another soldier as a metaphor for help between comrades and the losses that can be tied to the fallout from combat. “It’s a compelling piece of steel,” because it is designed in such a way that allows viewers to see through it, said Paul Marzello, the park’s president and chief executive officer, in an interview with WBFO, a National Public Radio affiliate in Buffalo. “By looking through it you’ll be able to look, as you see through it, someone’s soul.” Among veterans and active duty personnel, an estimated 20 commit suicide each day, according to the military publication Stars and Stripes. One hope of supporters of The Battle Within Foundation, which is leading the project, is that the memorial will inspire those experiencing psychic pain to seek help.“It is our sincerest hope that this monument will in some way help build public awareness of this ongoing tragedy, provide a lifeline for the suffering, and honor our heroes for their service, no matter where they died,” said Mark Donnelly, PhD, president of the Battle Within Foundation. The unveiling is scheduled for May 27, 2019. WBFO.
The field of psychiatry needs to do a better job of helping some patients come off of psychiatric drugs, said Allen Frances, MD, chairperson of DSM-IV task force, in an interview with the New Yorker. This process of removing drugs, called “deprescribing,” “requires a great deal more skill, time, commitment, and knowledge of the patient than prescribing does.” Another psychiatrist quoted in the article, Giovanni A. Fava, MD, said he has struggled to publish research looking at what happens to patients when they stop taking antidepressants. Yet another psychiatrist quoted in the article, Swapnil Gupta, MD, MBBS, said that, for many patients, coming off their medications “is a loss of identity, a different way of living. Suddenly, everything that you are doing is yours – and not necessarily your medication.” The article chronicles the experiences of Laura Delano, a woman diagnosed with bipolar disorder and prescribed valproic acid (Depakote) while in high school. Over the next several years, Ms. Delano found herself taking numerous medications and with a different diagnosis. Eventually, she removed herself from her many medications, started a blog, and launched a website called The Withdrawal Project. The New Yorker.
The National Council for Behavioral Health and Lady Gaga’s Born This Way Foundation have teamed up to develop a Teen Mental Health First Aid pilot program designed to “enhance the mental health of young people,” according to a report on Washington’s WTOP radio. Eight U.S. high schools have been chosen to participate in the program, which will teach students about mental illnesses and addictions. It also will help teenagers respond to friends who might be struggling with a problem with mental health or addiction. The Teen Mental Health First Aid program, a five-step action plan, was adapted from an evidence-based training program from Australia. Researchers at Johns Hopkins University, Baltimore, will evaluate the pilot program to assess its effectiveness. The National Alliance on Mental Illness has reported than 20% of American adults and the same percentage of U.S. teens are living with mental health challenges. After the pilot study results are analyzed, the training will be made available to the public. WTOP.
People coping with the aftermath of mass shootings might find some solace from Sandy and Lonnie Phillips. The couple, whose daughter was gunned down in the 2012 slaughter in an Aurora, Colo., movie theater, travels the country to help those whose pain is raw begin processing their grief. Mr. and Mrs. Phillips, driven by compassion, hope that sharing their experience will help others. They have started a nonprofit organization called Survivors Empowered to offer advice and kinship in the wake of mass shootings.
Emergency department visits can prove disorienting for patients with physical illnesses. But for those with mental illness, such visits can feel not only disorienting but disconcerting – particularly if sedation or restraints are involved. “If you are living with schizophrenia or bipolar disorder, that is a really tough way to begin that road to recovery,” said Jack Rozel, MD, president of the American Association for Emergency Psychiatry (AAEP), in an interview with CNN. At about 100 locations across the country, psychiatric EDs staffed by psychiatrists and other physicians, nurses, and social workers are triaging and treating patients with mental illness. The goal is treatment and either release or referral to more specialized care within 24 hours. The approach is the brainchild of Scott Zeller, MD, vice president of acute psychiatry at Vituity and a past president of the AAEP. The physician-led organization provides staffing and consulting services to medical centers nationwide. Dr. Zeller hatched the idea of a psychiatric ED while working as chief of psychiatric emergency services at John George Psychiatric Hospital in San Leandro, Calif. “We need to treat people at the emergency level of care,” he said. “The vast majority of psychiatric emergencies can be resolved in less than 24 hours.” Dr. Zeller took the reins in transforming the center from a traditional ward, including the use of restraints, to a setting more like a living room that was supportive rather than institutional. The results included improved patient outcomes and cost savings – in part because of the reduced time spent in the ED. CNN.
In Buffalo, N.Y., a new memorial to be installed in the Buffalo and Erie County Naval and Military Park will honor veterans who were lost to suicide. Ground was broken recently for the Battle Within Memorial. The 8-feet-tall sculpture will portray one soldier carrying the empty outline of another soldier as a metaphor for help between comrades and the losses that can be tied to the fallout from combat. “It’s a compelling piece of steel,” because it is designed in such a way that allows viewers to see through it, said Paul Marzello, the park’s president and chief executive officer, in an interview with WBFO, a National Public Radio affiliate in Buffalo. “By looking through it you’ll be able to look, as you see through it, someone’s soul.” Among veterans and active duty personnel, an estimated 20 commit suicide each day, according to the military publication Stars and Stripes. One hope of supporters of The Battle Within Foundation, which is leading the project, is that the memorial will inspire those experiencing psychic pain to seek help.“It is our sincerest hope that this monument will in some way help build public awareness of this ongoing tragedy, provide a lifeline for the suffering, and honor our heroes for their service, no matter where they died,” said Mark Donnelly, PhD, president of the Battle Within Foundation. The unveiling is scheduled for May 27, 2019. WBFO.
The field of psychiatry needs to do a better job of helping some patients come off of psychiatric drugs, said Allen Frances, MD, chairperson of DSM-IV task force, in an interview with the New Yorker. This process of removing drugs, called “deprescribing,” “requires a great deal more skill, time, commitment, and knowledge of the patient than prescribing does.” Another psychiatrist quoted in the article, Giovanni A. Fava, MD, said he has struggled to publish research looking at what happens to patients when they stop taking antidepressants. Yet another psychiatrist quoted in the article, Swapnil Gupta, MD, MBBS, said that, for many patients, coming off their medications “is a loss of identity, a different way of living. Suddenly, everything that you are doing is yours – and not necessarily your medication.” The article chronicles the experiences of Laura Delano, a woman diagnosed with bipolar disorder and prescribed valproic acid (Depakote) while in high school. Over the next several years, Ms. Delano found herself taking numerous medications and with a different diagnosis. Eventually, she removed herself from her many medications, started a blog, and launched a website called The Withdrawal Project. The New Yorker.
The National Council for Behavioral Health and Lady Gaga’s Born This Way Foundation have teamed up to develop a Teen Mental Health First Aid pilot program designed to “enhance the mental health of young people,” according to a report on Washington’s WTOP radio. Eight U.S. high schools have been chosen to participate in the program, which will teach students about mental illnesses and addictions. It also will help teenagers respond to friends who might be struggling with a problem with mental health or addiction. The Teen Mental Health First Aid program, a five-step action plan, was adapted from an evidence-based training program from Australia. Researchers at Johns Hopkins University, Baltimore, will evaluate the pilot program to assess its effectiveness. The National Alliance on Mental Illness has reported than 20% of American adults and the same percentage of U.S. teens are living with mental health challenges. After the pilot study results are analyzed, the training will be made available to the public. WTOP.
People coping with the aftermath of mass shootings might find some solace from Sandy and Lonnie Phillips. The couple, whose daughter was gunned down in the 2012 slaughter in an Aurora, Colo., movie theater, travels the country to help those whose pain is raw begin processing their grief. Mr. and Mrs. Phillips, driven by compassion, hope that sharing their experience will help others. They have started a nonprofit organization called Survivors Empowered to offer advice and kinship in the wake of mass shootings.
Emergency department visits can prove disorienting for patients with physical illnesses. But for those with mental illness, such visits can feel not only disorienting but disconcerting – particularly if sedation or restraints are involved. “If you are living with schizophrenia or bipolar disorder, that is a really tough way to begin that road to recovery,” said Jack Rozel, MD, president of the American Association for Emergency Psychiatry (AAEP), in an interview with CNN. At about 100 locations across the country, psychiatric EDs staffed by psychiatrists and other physicians, nurses, and social workers are triaging and treating patients with mental illness. The goal is treatment and either release or referral to more specialized care within 24 hours. The approach is the brainchild of Scott Zeller, MD, vice president of acute psychiatry at Vituity and a past president of the AAEP. The physician-led organization provides staffing and consulting services to medical centers nationwide. Dr. Zeller hatched the idea of a psychiatric ED while working as chief of psychiatric emergency services at John George Psychiatric Hospital in San Leandro, Calif. “We need to treat people at the emergency level of care,” he said. “The vast majority of psychiatric emergencies can be resolved in less than 24 hours.” Dr. Zeller took the reins in transforming the center from a traditional ward, including the use of restraints, to a setting more like a living room that was supportive rather than institutional. The results included improved patient outcomes and cost savings – in part because of the reduced time spent in the ED. CNN.
In Buffalo, N.Y., a new memorial to be installed in the Buffalo and Erie County Naval and Military Park will honor veterans who were lost to suicide. Ground was broken recently for the Battle Within Memorial. The 8-feet-tall sculpture will portray one soldier carrying the empty outline of another soldier as a metaphor for help between comrades and the losses that can be tied to the fallout from combat. “It’s a compelling piece of steel,” because it is designed in such a way that allows viewers to see through it, said Paul Marzello, the park’s president and chief executive officer, in an interview with WBFO, a National Public Radio affiliate in Buffalo. “By looking through it you’ll be able to look, as you see through it, someone’s soul.” Among veterans and active duty personnel, an estimated 20 commit suicide each day, according to the military publication Stars and Stripes. One hope of supporters of The Battle Within Foundation, which is leading the project, is that the memorial will inspire those experiencing psychic pain to seek help.“It is our sincerest hope that this monument will in some way help build public awareness of this ongoing tragedy, provide a lifeline for the suffering, and honor our heroes for their service, no matter where they died,” said Mark Donnelly, PhD, president of the Battle Within Foundation. The unveiling is scheduled for May 27, 2019. WBFO.
The field of psychiatry needs to do a better job of helping some patients come off of psychiatric drugs, said Allen Frances, MD, chairperson of DSM-IV task force, in an interview with the New Yorker. This process of removing drugs, called “deprescribing,” “requires a great deal more skill, time, commitment, and knowledge of the patient than prescribing does.” Another psychiatrist quoted in the article, Giovanni A. Fava, MD, said he has struggled to publish research looking at what happens to patients when they stop taking antidepressants. Yet another psychiatrist quoted in the article, Swapnil Gupta, MD, MBBS, said that, for many patients, coming off their medications “is a loss of identity, a different way of living. Suddenly, everything that you are doing is yours – and not necessarily your medication.” The article chronicles the experiences of Laura Delano, a woman diagnosed with bipolar disorder and prescribed valproic acid (Depakote) while in high school. Over the next several years, Ms. Delano found herself taking numerous medications and with a different diagnosis. Eventually, she removed herself from her many medications, started a blog, and launched a website called The Withdrawal Project. The New Yorker.
The National Council for Behavioral Health and Lady Gaga’s Born This Way Foundation have teamed up to develop a Teen Mental Health First Aid pilot program designed to “enhance the mental health of young people,” according to a report on Washington’s WTOP radio. Eight U.S. high schools have been chosen to participate in the program, which will teach students about mental illnesses and addictions. It also will help teenagers respond to friends who might be struggling with a problem with mental health or addiction. The Teen Mental Health First Aid program, a five-step action plan, was adapted from an evidence-based training program from Australia. Researchers at Johns Hopkins University, Baltimore, will evaluate the pilot program to assess its effectiveness. The National Alliance on Mental Illness has reported than 20% of American adults and the same percentage of U.S. teens are living with mental health challenges. After the pilot study results are analyzed, the training will be made available to the public. WTOP.
Prevention and Treatment of Traveler’s Diarrhea
Importance
The prevention and treatment of traveler’s diarrhea (TD) is a common reason that patients consult their physician prior to foreign travel. TD can result in lost time and opportunity, as well as overseas medical encounters and hospitalization. to providers regarding the use of antibiotic and nonantibiotic therapies for the prevention and treatment of TD.
Prophylaxis
The panel recommends that antimicrobial prophylaxis should not be used routinely in travelers, but it should be considered for travelers who are at high risk of health-related complications of TD (both strong recommendations, low/very low level of evidence [LOE]). High-risk individuals include those with a history of clinically significant long-term morbidity following an enteric infection or serious chronic illnesses that predisposes them for TD-related complications. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent TD (3, strong recommendation, high LOE). Studies show that a lower dose of 1.05 g/day is preventive, although it is unclear whether it is as effective as higher doses of 2.1 g/day or 4.2 g/day. When prophylaxis is indicated, travelers should be prescribed rifaximin (strong recommendation, moderate LOE) based on susceptibility of most enteric pathogens and the drug’s extremely favorable safety profile. Fluoroquinolones (FQ) are no longer recommended for prophylaxis (strong recommendation, low/very low LOE) because of neurologic and musculoskeletal side effects that may outweigh benefits, as well as emerging resistance of enteric pathogens (70%-80% in Campylobacter spp. from Nepal and Thailand and 65% in Enterotoxigenic Escherichia coli [ETEC] and Enteroaggregative E. coli [EAEC] in India).
Treatment
The following treatment recommendations are based on the classification of TD using functional effects of severity; therefore, the panel made new definitions for TD severity. This is a change from previous definitions that utilized a traditional frequency-based algorithm in order to tailor therapy for the individual. Individuals can be prescribed antibiotics and antimotility agents to take with them during travel, along with advice regarding how to judge when to use each agent.
Mild: diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Encourage supportive measures such as rehydration and nonantibiotic, antimotility drugs, such as loperamide or BSS (both strong recommendations, moderate LOE).
Moderate: diarrhea that is distressing or interferes with planned activities.
Antibiotics may be used (weak recommendation, moderate LOE) as early and effective treatment may mitigate the well-described chronic health consequences including irritable bowel syndrome. Three options exist. FQs may be used outside of Southeast and South Asia (strong recommendation, moderate LOE), but their potential for adverse effects and musculoskeletal consequences must be considered. Azithromycin may be used (strong recommendation, high LOE) because studies show no significant differences in efficacy between it and FQs, limited resistance to common TD pathogens (although concerns exist in Nepal), and good side effect profile. Another choice is rifaximin (weak recommendation, moderate LOE), although one should exercise caution for empirical therapy in regions in which being at high risk of invasive pathogens is anticipated.
Loperamide may be used as adjunctive therapy for moderate to severe TD (strong recommendation, high LOE) to add symptomatic relief with curative treatment or as monotherapy in moderate TD (strong recommendation, high LOE). This is specifically true in children aged 2-11 years, in whom loperamide is beneficial without causing severe side effects.
Severe: diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools).
Antibiotics should be used (strong recommendation, high LOE). Azithromycin is the preferred choice and is first-line for dysentery or febrile diarrhea (strong recommendation, moderate LOE) because of the likelihood of FQ-resistant bacteria being the cause of dysentery. FQs and rifaximin are also choices that can be used to treat severe, nondysenteric TD (both weak recommendations, moderate LOE).
Furthermore, single-dose antibiotics may be used to treat moderate or severe TD (strong recommendation, high LOE) because studies have shown equivalent efficacy for treatment of watery noninvasive diarrhea among FQs (3 days, single dose), azithromycin (3 days, single dose), and rifaximin (3 days, three times daily).
Persistent: diarrhea lasting longer than 2 weeks.
Functional bowel disease (FBD) may occur after bouts of TD and may meet Rome III or IV criteria for irritable bowel syndrome. Thus, in a traveler without pretravel GI disease, in whom the evaluation for microbial etiologies and underlying GI disease is negative, postinfectious FBD must be considered.
Follow-up and diagnostic testing
The panel recommends microbiological testing in returning travelers with severe or persistent symptoms, bloody/mucousy diarrhea, or in those who fail empiric therapy (strong recommendation, low/very low LOE). Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or nonmolecular tests have failed to establish a diagnosis. Furthermore, molecular testing may, in some cases, detect colonization rather than infection.
The bottom line
The expert panel made 20 graded recommendations to help guide the provider with nonantibiotic and antibiotic prophylaxis and treatment of TD. The main take-home points include:
- Prophylaxis should be considered only in high-risk groups; rifaximin is the first choice, and BSS is a second option.
- All travelers should be provided with loperamide and an antibiotic for self-treatment if needed.
- Mild diarrhea should be treated with increased fluid intake and loperamide or BSS.
- Moderate to severe diarrhea should be treated with single-dose antimicrobial therapy of FQ or azithromycin or with rifaximin dosing three times a day.
- Instead of antibiotics, loperamide may be considered as monotherapy for moderate diarrhea; loperamide can be used with antibiotics for both moderate and severe TD.
Dr. Shrestha is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) - Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Reference:
Importance
The prevention and treatment of traveler’s diarrhea (TD) is a common reason that patients consult their physician prior to foreign travel. TD can result in lost time and opportunity, as well as overseas medical encounters and hospitalization. to providers regarding the use of antibiotic and nonantibiotic therapies for the prevention and treatment of TD.
Prophylaxis
The panel recommends that antimicrobial prophylaxis should not be used routinely in travelers, but it should be considered for travelers who are at high risk of health-related complications of TD (both strong recommendations, low/very low level of evidence [LOE]). High-risk individuals include those with a history of clinically significant long-term morbidity following an enteric infection or serious chronic illnesses that predisposes them for TD-related complications. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent TD (3, strong recommendation, high LOE). Studies show that a lower dose of 1.05 g/day is preventive, although it is unclear whether it is as effective as higher doses of 2.1 g/day or 4.2 g/day. When prophylaxis is indicated, travelers should be prescribed rifaximin (strong recommendation, moderate LOE) based on susceptibility of most enteric pathogens and the drug’s extremely favorable safety profile. Fluoroquinolones (FQ) are no longer recommended for prophylaxis (strong recommendation, low/very low LOE) because of neurologic and musculoskeletal side effects that may outweigh benefits, as well as emerging resistance of enteric pathogens (70%-80% in Campylobacter spp. from Nepal and Thailand and 65% in Enterotoxigenic Escherichia coli [ETEC] and Enteroaggregative E. coli [EAEC] in India).
Treatment
The following treatment recommendations are based on the classification of TD using functional effects of severity; therefore, the panel made new definitions for TD severity. This is a change from previous definitions that utilized a traditional frequency-based algorithm in order to tailor therapy for the individual. Individuals can be prescribed antibiotics and antimotility agents to take with them during travel, along with advice regarding how to judge when to use each agent.
Mild: diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Encourage supportive measures such as rehydration and nonantibiotic, antimotility drugs, such as loperamide or BSS (both strong recommendations, moderate LOE).
Moderate: diarrhea that is distressing or interferes with planned activities.
Antibiotics may be used (weak recommendation, moderate LOE) as early and effective treatment may mitigate the well-described chronic health consequences including irritable bowel syndrome. Three options exist. FQs may be used outside of Southeast and South Asia (strong recommendation, moderate LOE), but their potential for adverse effects and musculoskeletal consequences must be considered. Azithromycin may be used (strong recommendation, high LOE) because studies show no significant differences in efficacy between it and FQs, limited resistance to common TD pathogens (although concerns exist in Nepal), and good side effect profile. Another choice is rifaximin (weak recommendation, moderate LOE), although one should exercise caution for empirical therapy in regions in which being at high risk of invasive pathogens is anticipated.
Loperamide may be used as adjunctive therapy for moderate to severe TD (strong recommendation, high LOE) to add symptomatic relief with curative treatment or as monotherapy in moderate TD (strong recommendation, high LOE). This is specifically true in children aged 2-11 years, in whom loperamide is beneficial without causing severe side effects.
Severe: diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools).
Antibiotics should be used (strong recommendation, high LOE). Azithromycin is the preferred choice and is first-line for dysentery or febrile diarrhea (strong recommendation, moderate LOE) because of the likelihood of FQ-resistant bacteria being the cause of dysentery. FQs and rifaximin are also choices that can be used to treat severe, nondysenteric TD (both weak recommendations, moderate LOE).
Furthermore, single-dose antibiotics may be used to treat moderate or severe TD (strong recommendation, high LOE) because studies have shown equivalent efficacy for treatment of watery noninvasive diarrhea among FQs (3 days, single dose), azithromycin (3 days, single dose), and rifaximin (3 days, three times daily).
Persistent: diarrhea lasting longer than 2 weeks.
Functional bowel disease (FBD) may occur after bouts of TD and may meet Rome III or IV criteria for irritable bowel syndrome. Thus, in a traveler without pretravel GI disease, in whom the evaluation for microbial etiologies and underlying GI disease is negative, postinfectious FBD must be considered.
Follow-up and diagnostic testing
The panel recommends microbiological testing in returning travelers with severe or persistent symptoms, bloody/mucousy diarrhea, or in those who fail empiric therapy (strong recommendation, low/very low LOE). Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or nonmolecular tests have failed to establish a diagnosis. Furthermore, molecular testing may, in some cases, detect colonization rather than infection.
The bottom line
The expert panel made 20 graded recommendations to help guide the provider with nonantibiotic and antibiotic prophylaxis and treatment of TD. The main take-home points include:
- Prophylaxis should be considered only in high-risk groups; rifaximin is the first choice, and BSS is a second option.
- All travelers should be provided with loperamide and an antibiotic for self-treatment if needed.
- Mild diarrhea should be treated with increased fluid intake and loperamide or BSS.
- Moderate to severe diarrhea should be treated with single-dose antimicrobial therapy of FQ or azithromycin or with rifaximin dosing three times a day.
- Instead of antibiotics, loperamide may be considered as monotherapy for moderate diarrhea; loperamide can be used with antibiotics for both moderate and severe TD.
Dr. Shrestha is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) - Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Reference:
Importance
The prevention and treatment of traveler’s diarrhea (TD) is a common reason that patients consult their physician prior to foreign travel. TD can result in lost time and opportunity, as well as overseas medical encounters and hospitalization. to providers regarding the use of antibiotic and nonantibiotic therapies for the prevention and treatment of TD.
Prophylaxis
The panel recommends that antimicrobial prophylaxis should not be used routinely in travelers, but it should be considered for travelers who are at high risk of health-related complications of TD (both strong recommendations, low/very low level of evidence [LOE]). High-risk individuals include those with a history of clinically significant long-term morbidity following an enteric infection or serious chronic illnesses that predisposes them for TD-related complications. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent TD (3, strong recommendation, high LOE). Studies show that a lower dose of 1.05 g/day is preventive, although it is unclear whether it is as effective as higher doses of 2.1 g/day or 4.2 g/day. When prophylaxis is indicated, travelers should be prescribed rifaximin (strong recommendation, moderate LOE) based on susceptibility of most enteric pathogens and the drug’s extremely favorable safety profile. Fluoroquinolones (FQ) are no longer recommended for prophylaxis (strong recommendation, low/very low LOE) because of neurologic and musculoskeletal side effects that may outweigh benefits, as well as emerging resistance of enteric pathogens (70%-80% in Campylobacter spp. from Nepal and Thailand and 65% in Enterotoxigenic Escherichia coli [ETEC] and Enteroaggregative E. coli [EAEC] in India).
Treatment
The following treatment recommendations are based on the classification of TD using functional effects of severity; therefore, the panel made new definitions for TD severity. This is a change from previous definitions that utilized a traditional frequency-based algorithm in order to tailor therapy for the individual. Individuals can be prescribed antibiotics and antimotility agents to take with them during travel, along with advice regarding how to judge when to use each agent.
Mild: diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Encourage supportive measures such as rehydration and nonantibiotic, antimotility drugs, such as loperamide or BSS (both strong recommendations, moderate LOE).
Moderate: diarrhea that is distressing or interferes with planned activities.
Antibiotics may be used (weak recommendation, moderate LOE) as early and effective treatment may mitigate the well-described chronic health consequences including irritable bowel syndrome. Three options exist. FQs may be used outside of Southeast and South Asia (strong recommendation, moderate LOE), but their potential for adverse effects and musculoskeletal consequences must be considered. Azithromycin may be used (strong recommendation, high LOE) because studies show no significant differences in efficacy between it and FQs, limited resistance to common TD pathogens (although concerns exist in Nepal), and good side effect profile. Another choice is rifaximin (weak recommendation, moderate LOE), although one should exercise caution for empirical therapy in regions in which being at high risk of invasive pathogens is anticipated.
Loperamide may be used as adjunctive therapy for moderate to severe TD (strong recommendation, high LOE) to add symptomatic relief with curative treatment or as monotherapy in moderate TD (strong recommendation, high LOE). This is specifically true in children aged 2-11 years, in whom loperamide is beneficial without causing severe side effects.
Severe: diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools).
Antibiotics should be used (strong recommendation, high LOE). Azithromycin is the preferred choice and is first-line for dysentery or febrile diarrhea (strong recommendation, moderate LOE) because of the likelihood of FQ-resistant bacteria being the cause of dysentery. FQs and rifaximin are also choices that can be used to treat severe, nondysenteric TD (both weak recommendations, moderate LOE).
Furthermore, single-dose antibiotics may be used to treat moderate or severe TD (strong recommendation, high LOE) because studies have shown equivalent efficacy for treatment of watery noninvasive diarrhea among FQs (3 days, single dose), azithromycin (3 days, single dose), and rifaximin (3 days, three times daily).
Persistent: diarrhea lasting longer than 2 weeks.
Functional bowel disease (FBD) may occur after bouts of TD and may meet Rome III or IV criteria for irritable bowel syndrome. Thus, in a traveler without pretravel GI disease, in whom the evaluation for microbial etiologies and underlying GI disease is negative, postinfectious FBD must be considered.
Follow-up and diagnostic testing
The panel recommends microbiological testing in returning travelers with severe or persistent symptoms, bloody/mucousy diarrhea, or in those who fail empiric therapy (strong recommendation, low/very low LOE). Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or nonmolecular tests have failed to establish a diagnosis. Furthermore, molecular testing may, in some cases, detect colonization rather than infection.
The bottom line
The expert panel made 20 graded recommendations to help guide the provider with nonantibiotic and antibiotic prophylaxis and treatment of TD. The main take-home points include:
- Prophylaxis should be considered only in high-risk groups; rifaximin is the first choice, and BSS is a second option.
- All travelers should be provided with loperamide and an antibiotic for self-treatment if needed.
- Mild diarrhea should be treated with increased fluid intake and loperamide or BSS.
- Moderate to severe diarrhea should be treated with single-dose antimicrobial therapy of FQ or azithromycin or with rifaximin dosing three times a day.
- Instead of antibiotics, loperamide may be considered as monotherapy for moderate diarrhea; loperamide can be used with antibiotics for both moderate and severe TD.
Dr. Shrestha is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) - Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Reference:
Doctor, will you please lie to me?
“Doctor, I have a question for you.”
“Yes?”
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
Mr. B thought he felt too well to have an aggressive cancer infiltrating his liver, abdominal wall, and lungs. He lived an active life, going to church, volunteering, and playing with his grandchildren. But the biopsy results were back. It wasn’t an infection. It wasn’t inflammation. It was stage 4 cancer.
And just like that, his life changed.
His prognosis was likely months, with a best case scenario of several years. But gone were the days of thinking 10, 20, and 30 years ahead.
Mr. B was a spiritual person. He told me this was God’s plan for him. He trusted God to get him through this, so he would not need chemotherapy.
Looking for hope in the face of terrible news is a common reaction. It’s natural. We tend to be optimists, and we look for a silver lining. We look for the “but.”
Patients and doctors alike do this.
“The cancer is metastatic ... but we have chemotherapy that can slow it down.”
“The doctor told me the median survival is 3 months ... but I plan to beat those odds.”
“Your mother is dying ... but we have good medicines to make her comfortable.”
It’s not a bad thing. It’s an adaptive mechanism and positive outlooks can even be associated with better medical outcomes. There’s almost always a “but” if you look hard enough. And in the face of life-changing news, we are incentivized to look really, really hard.
Yet Mr. B caught me off guard because he wasn’t asking me to maintain hope. He was pushing me one step further.
At one point in our conversation, he revealed this clearly. “Doctor, I don’t even care if it’s false hope – just tell me the cancer will disappear.”
Mr. B was asking me to lie to him.
The last thing I wanted was to take away hope, closing the door on a future that truly was clouded in uncertainty. But I also couldn’t look him in the eye and tell him his incurable disease was, in fact, curable.
That doesn’t mean there aren’t ways to phrase things kindly. Patients respond differently to “there’s a 90% chance of survival” and “there’s a 10% chance of death” even though the facts are identical.
When I sense a person like Mr. B is looking to me for hope, I try to frame that initial conversation in the style of the first statement. It’s not dishonest. With an already overwhelming piece of information, it’s choosing to share the most positive version of a narrative that can be told in many different ways and will evolve over time.
Moreover, being hopeful and realistic are not mutually exclusive. Just because someone is seeking the rare chance of a good outcome doesn’t mean he or she doesn’t understand the seriousness of the situation. It is possible to seek out experimental drugs while also considering hospice. It is possible to hope for a plan A while preparing for a plan B. Those are two compatible beliefs, and it’s OK – preferred, even – to hold them at once.
The challenge is avoiding getting pulled into an outlook that is not just positive, not just unlikely – but one with no basis in reality. There’s hope – and then there’s false hope. There’s a positive take – and then there’s lying. It can be a fine line, and with the intention of being kind it can be all too easy to “yes, but” our way into untruths.
The best we can do is set limits with compassion, and understand that telling the truth doesn’t always mean that someone hears it.
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
The chances of Mr. B’s cancer responding to chemotherapy were not good but hardly impossible. But without any therapy at all?
“I have not.”
“What about at other places ... in other states or countries. Have you ever heard of a person like me who was cured without treatment?”
“I have not.”
“I guess what I’m asking, Doctor, is ... have you ever seen where the cancer was there and then one day was not ... have you ever seen a miracle?”
Reaching. Grasping.
“I have not.”
“Well,” he said, “Maybe I will be the first.”
We look at each other, pondering this. In my silence is the answer.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
“Doctor, I have a question for you.”
“Yes?”
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
Mr. B thought he felt too well to have an aggressive cancer infiltrating his liver, abdominal wall, and lungs. He lived an active life, going to church, volunteering, and playing with his grandchildren. But the biopsy results were back. It wasn’t an infection. It wasn’t inflammation. It was stage 4 cancer.
And just like that, his life changed.
His prognosis was likely months, with a best case scenario of several years. But gone were the days of thinking 10, 20, and 30 years ahead.
Mr. B was a spiritual person. He told me this was God’s plan for him. He trusted God to get him through this, so he would not need chemotherapy.
Looking for hope in the face of terrible news is a common reaction. It’s natural. We tend to be optimists, and we look for a silver lining. We look for the “but.”
Patients and doctors alike do this.
“The cancer is metastatic ... but we have chemotherapy that can slow it down.”
“The doctor told me the median survival is 3 months ... but I plan to beat those odds.”
“Your mother is dying ... but we have good medicines to make her comfortable.”
It’s not a bad thing. It’s an adaptive mechanism and positive outlooks can even be associated with better medical outcomes. There’s almost always a “but” if you look hard enough. And in the face of life-changing news, we are incentivized to look really, really hard.
Yet Mr. B caught me off guard because he wasn’t asking me to maintain hope. He was pushing me one step further.
At one point in our conversation, he revealed this clearly. “Doctor, I don’t even care if it’s false hope – just tell me the cancer will disappear.”
Mr. B was asking me to lie to him.
The last thing I wanted was to take away hope, closing the door on a future that truly was clouded in uncertainty. But I also couldn’t look him in the eye and tell him his incurable disease was, in fact, curable.
That doesn’t mean there aren’t ways to phrase things kindly. Patients respond differently to “there’s a 90% chance of survival” and “there’s a 10% chance of death” even though the facts are identical.
When I sense a person like Mr. B is looking to me for hope, I try to frame that initial conversation in the style of the first statement. It’s not dishonest. With an already overwhelming piece of information, it’s choosing to share the most positive version of a narrative that can be told in many different ways and will evolve over time.
Moreover, being hopeful and realistic are not mutually exclusive. Just because someone is seeking the rare chance of a good outcome doesn’t mean he or she doesn’t understand the seriousness of the situation. It is possible to seek out experimental drugs while also considering hospice. It is possible to hope for a plan A while preparing for a plan B. Those are two compatible beliefs, and it’s OK – preferred, even – to hold them at once.
The challenge is avoiding getting pulled into an outlook that is not just positive, not just unlikely – but one with no basis in reality. There’s hope – and then there’s false hope. There’s a positive take – and then there’s lying. It can be a fine line, and with the intention of being kind it can be all too easy to “yes, but” our way into untruths.
The best we can do is set limits with compassion, and understand that telling the truth doesn’t always mean that someone hears it.
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
The chances of Mr. B’s cancer responding to chemotherapy were not good but hardly impossible. But without any therapy at all?
“I have not.”
“What about at other places ... in other states or countries. Have you ever heard of a person like me who was cured without treatment?”
“I have not.”
“I guess what I’m asking, Doctor, is ... have you ever seen where the cancer was there and then one day was not ... have you ever seen a miracle?”
Reaching. Grasping.
“I have not.”
“Well,” he said, “Maybe I will be the first.”
We look at each other, pondering this. In my silence is the answer.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
“Doctor, I have a question for you.”
“Yes?”
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
Mr. B thought he felt too well to have an aggressive cancer infiltrating his liver, abdominal wall, and lungs. He lived an active life, going to church, volunteering, and playing with his grandchildren. But the biopsy results were back. It wasn’t an infection. It wasn’t inflammation. It was stage 4 cancer.
And just like that, his life changed.
His prognosis was likely months, with a best case scenario of several years. But gone were the days of thinking 10, 20, and 30 years ahead.
Mr. B was a spiritual person. He told me this was God’s plan for him. He trusted God to get him through this, so he would not need chemotherapy.
Looking for hope in the face of terrible news is a common reaction. It’s natural. We tend to be optimists, and we look for a silver lining. We look for the “but.”
Patients and doctors alike do this.
“The cancer is metastatic ... but we have chemotherapy that can slow it down.”
“The doctor told me the median survival is 3 months ... but I plan to beat those odds.”
“Your mother is dying ... but we have good medicines to make her comfortable.”
It’s not a bad thing. It’s an adaptive mechanism and positive outlooks can even be associated with better medical outcomes. There’s almost always a “but” if you look hard enough. And in the face of life-changing news, we are incentivized to look really, really hard.
Yet Mr. B caught me off guard because he wasn’t asking me to maintain hope. He was pushing me one step further.
At one point in our conversation, he revealed this clearly. “Doctor, I don’t even care if it’s false hope – just tell me the cancer will disappear.”
Mr. B was asking me to lie to him.
The last thing I wanted was to take away hope, closing the door on a future that truly was clouded in uncertainty. But I also couldn’t look him in the eye and tell him his incurable disease was, in fact, curable.
That doesn’t mean there aren’t ways to phrase things kindly. Patients respond differently to “there’s a 90% chance of survival” and “there’s a 10% chance of death” even though the facts are identical.
When I sense a person like Mr. B is looking to me for hope, I try to frame that initial conversation in the style of the first statement. It’s not dishonest. With an already overwhelming piece of information, it’s choosing to share the most positive version of a narrative that can be told in many different ways and will evolve over time.
Moreover, being hopeful and realistic are not mutually exclusive. Just because someone is seeking the rare chance of a good outcome doesn’t mean he or she doesn’t understand the seriousness of the situation. It is possible to seek out experimental drugs while also considering hospice. It is possible to hope for a plan A while preparing for a plan B. Those are two compatible beliefs, and it’s OK – preferred, even – to hold them at once.
The challenge is avoiding getting pulled into an outlook that is not just positive, not just unlikely – but one with no basis in reality. There’s hope – and then there’s false hope. There’s a positive take – and then there’s lying. It can be a fine line, and with the intention of being kind it can be all too easy to “yes, but” our way into untruths.
The best we can do is set limits with compassion, and understand that telling the truth doesn’t always mean that someone hears it.
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
The chances of Mr. B’s cancer responding to chemotherapy were not good but hardly impossible. But without any therapy at all?
“I have not.”
“What about at other places ... in other states or countries. Have you ever heard of a person like me who was cured without treatment?”
“I have not.”
“I guess what I’m asking, Doctor, is ... have you ever seen where the cancer was there and then one day was not ... have you ever seen a miracle?”
Reaching. Grasping.
“I have not.”
“Well,” he said, “Maybe I will be the first.”
We look at each other, pondering this. In my silence is the answer.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Town and gown – the virtuous circle
I recall in college we used to talk a lot about the relationship between town and gown, that is, the town community and the academic community, and how they relate to each other. There should be a virtuous circle between dermatologists in their community and their academic medical center.
Academic medical centers are up to date and on the cutting edge of science and treatments – and happy to share with community dermatologists. This is where you will learn about teledermatology and how to use the latest biologics.
They provide educational opportunities such as grand rounds and visiting speakers. They allow community physicians to attend staff and resident lectures, bring their most challenging cases to grand rounds, and get feedback and suggestions, with everyone learning from the experience. They host dermatology events for all. They allow community physicians to staff resident clinics where both benefit from the interaction. The academic center provides a charity care network for the community – and usually provides the experts of last resort for difficult cases as they are often on the cutting edge of research and newest treatments.
Large multispecialty practices– and private equity groups – are not going to fill this role.
Dermatology is a highly isolated specialty and the interaction with others at the academic center may be the only physical link to the outside world of medicine. Having an academic dermatology program in your community is a serious asset that community dermatologists should appreciate and support.
However, as the gown supports the town, so should the town support the gown. There is a pervasive inaccuracy in the community that these academic dermatology departments are supported by government funds or a “state line” of support. This is incorrect. These departments support themselves with clinical work. In addition, the dean and/or the medical school takes a generous cut out of their profits, and the departments also must compete – furiously – for grants, of which the medical school immediately takes about 55% for indirect “overhead,” a frequent complaint of academic physicians.
If you practice near such an academic center and benefit from its virtuous circle, you can consider sending them some of your dermatopathology or Mohs, even if you are a large group and could handle them yourselves. You can join the local dermatologic society and bring cases to grand rounds. You can offer to teach the residents in their clinic, or in your office, and expose them to real life practice.
If you have the means, you could consider funding a lectureship or an endowed departmental chair. No matter the virtual content available online, the quality of learning one on one from another specialist cannot be beat. So make the effort to visit the gown from the town. You will be warmly welcomed and join a virtuous circle of lifelong learners.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
I recall in college we used to talk a lot about the relationship between town and gown, that is, the town community and the academic community, and how they relate to each other. There should be a virtuous circle between dermatologists in their community and their academic medical center.
Academic medical centers are up to date and on the cutting edge of science and treatments – and happy to share with community dermatologists. This is where you will learn about teledermatology and how to use the latest biologics.
They provide educational opportunities such as grand rounds and visiting speakers. They allow community physicians to attend staff and resident lectures, bring their most challenging cases to grand rounds, and get feedback and suggestions, with everyone learning from the experience. They host dermatology events for all. They allow community physicians to staff resident clinics where both benefit from the interaction. The academic center provides a charity care network for the community – and usually provides the experts of last resort for difficult cases as they are often on the cutting edge of research and newest treatments.
Large multispecialty practices– and private equity groups – are not going to fill this role.
Dermatology is a highly isolated specialty and the interaction with others at the academic center may be the only physical link to the outside world of medicine. Having an academic dermatology program in your community is a serious asset that community dermatologists should appreciate and support.
However, as the gown supports the town, so should the town support the gown. There is a pervasive inaccuracy in the community that these academic dermatology departments are supported by government funds or a “state line” of support. This is incorrect. These departments support themselves with clinical work. In addition, the dean and/or the medical school takes a generous cut out of their profits, and the departments also must compete – furiously – for grants, of which the medical school immediately takes about 55% for indirect “overhead,” a frequent complaint of academic physicians.
If you practice near such an academic center and benefit from its virtuous circle, you can consider sending them some of your dermatopathology or Mohs, even if you are a large group and could handle them yourselves. You can join the local dermatologic society and bring cases to grand rounds. You can offer to teach the residents in their clinic, or in your office, and expose them to real life practice.
If you have the means, you could consider funding a lectureship or an endowed departmental chair. No matter the virtual content available online, the quality of learning one on one from another specialist cannot be beat. So make the effort to visit the gown from the town. You will be warmly welcomed and join a virtuous circle of lifelong learners.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
I recall in college we used to talk a lot about the relationship between town and gown, that is, the town community and the academic community, and how they relate to each other. There should be a virtuous circle between dermatologists in their community and their academic medical center.
Academic medical centers are up to date and on the cutting edge of science and treatments – and happy to share with community dermatologists. This is where you will learn about teledermatology and how to use the latest biologics.
They provide educational opportunities such as grand rounds and visiting speakers. They allow community physicians to attend staff and resident lectures, bring their most challenging cases to grand rounds, and get feedback and suggestions, with everyone learning from the experience. They host dermatology events for all. They allow community physicians to staff resident clinics where both benefit from the interaction. The academic center provides a charity care network for the community – and usually provides the experts of last resort for difficult cases as they are often on the cutting edge of research and newest treatments.
Large multispecialty practices– and private equity groups – are not going to fill this role.
Dermatology is a highly isolated specialty and the interaction with others at the academic center may be the only physical link to the outside world of medicine. Having an academic dermatology program in your community is a serious asset that community dermatologists should appreciate and support.
However, as the gown supports the town, so should the town support the gown. There is a pervasive inaccuracy in the community that these academic dermatology departments are supported by government funds or a “state line” of support. This is incorrect. These departments support themselves with clinical work. In addition, the dean and/or the medical school takes a generous cut out of their profits, and the departments also must compete – furiously – for grants, of which the medical school immediately takes about 55% for indirect “overhead,” a frequent complaint of academic physicians.
If you practice near such an academic center and benefit from its virtuous circle, you can consider sending them some of your dermatopathology or Mohs, even if you are a large group and could handle them yourselves. You can join the local dermatologic society and bring cases to grand rounds. You can offer to teach the residents in their clinic, or in your office, and expose them to real life practice.
If you have the means, you could consider funding a lectureship or an endowed departmental chair. No matter the virtual content available online, the quality of learning one on one from another specialist cannot be beat. So make the effort to visit the gown from the town. You will be warmly welcomed and join a virtuous circle of lifelong learners.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Reframing the conversation around the ‘gender-reveal’ ultrasound
How revealing fetal anatomy, not gender, is inclusive
Every ob.gyn. expects that the topic of gender will come up at some point in a patient’s pregnancy. “When will I find out the gender?” asks the 24-year-old at her first prenatal visit. “We want the gender to be a surprise!” exclaims the couple at their anatomy scan for their second in vitro fertilization pregnancy. “Do you know what you’re having?” asks the obstetrician anticipating an imminent delivery.
The topic of gender is in fact so ingrained in our practice that we don’t bat an eye when approached with questions about fetal gender. But what exactly are we talking about when we discuss the gender of an unborn baby?
As we established in our previous column, gender identity is an internal experience of gender that one feels to be a part of oneself. So, then, what does an ultrasound actually reveal? Objectively, ultrasound can show the provider the presence or absence of a hyperechoic anatomical structure between the fetal legs that may become a penis, a vagina, or an ambiguous form of genitalia. While ultrasound is an incredible tool for anatomical and other forms of antenatal testing, ultrasound cannot detect identity characteristics because identities are, by definition, socially and internally experienced without respect to anatomy.
The distinction between gender identity and sex assigned at birth in discussions of antenatal ultrasonography is more than just a simple problem of semantics or vocabulary. To describe a fetus as a boy or a girl based on the presence/absence of a projection between the fetal legs seen on ultrasound is to reinforce the idea that gender identity and sex assigned at birth are equivalent. This conflation also erases nonbinary, genderqueer, and many other groups that identify with genders other than “boy” or “girl.” To be clear, unborn fetuses do not have a gender identity. Studies have shown that children begin to self-label their gender as early as 18-24 months of age, and similarly those who grow up to inhabit gender-nonconforming identities usually already are starting to show signs of their nonconformity starting at age 2 years.1 Some of the deepest traumas that trans and gender-nonconforming people experience are rated to the enforcement of unwritten gender laws during early childhood that are applied based on the sex assigned at birth.
Obstetricians can help to break the cycle of inappropriate gender assignment by correctly using the terms “gender” and “sex assigned at birth.” One opportunity for addressing patients’ questions about fetal gender might be to avoid the term “gender” altogether when discussing fetal sex assigned at birth, emphasizing instead what fetal ultrasound is actually able to do: Give us information about the appearance of external genitalia to help predict what sex will be assigned at birth.2 We have used this strategy when performing anatomy scans, and our experience has been that patients often will make their own assumptions about what it means to see certain external genitalia on ultrasound between the fetal legs. Motivated providers who want to go the extra mile may use a patient’s exclamation about their understanding of the fetus’s gender as an opportunity to educate the patient on the distinction between gender and sex assigned at birth, but even just smiling and moving onto the next part of the scan is an appropriate way of maintaining an atmosphere of inclusion and respect.
One of the roots of gender-based violence and gender dysphoria later in life is the conflation of gender and sex assigned at birth. While there is an entire social and political framework that enforces and polices gender after birth, the obstetrician can take steps to break the cycle starting before the birth has even occurred. Obstetricians are tasked with the unique challenge of providing care for the mother-fetus dyad, and much of the work is in setting up the fetus for the best possible life. Our scope of inclusion should be sufficiently wide to account for nonanatomical variations that could develop later in life in the infants we deliver.
Dr. Bahng is a PGY-1 resident physician in the gynecology & obstetrics residency program at Emory University, Atlanta. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner is an assistant professor at Emory University and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Bahng and Dr. Joyner reported no financial disclosures.
References
1. Horm Behav. 2013 Jul;64(2):288-97.
2. Obstet Gynecol Surv. 2009 Jan;64(1):50-7.
How revealing fetal anatomy, not gender, is inclusive
How revealing fetal anatomy, not gender, is inclusive
Every ob.gyn. expects that the topic of gender will come up at some point in a patient’s pregnancy. “When will I find out the gender?” asks the 24-year-old at her first prenatal visit. “We want the gender to be a surprise!” exclaims the couple at their anatomy scan for their second in vitro fertilization pregnancy. “Do you know what you’re having?” asks the obstetrician anticipating an imminent delivery.
The topic of gender is in fact so ingrained in our practice that we don’t bat an eye when approached with questions about fetal gender. But what exactly are we talking about when we discuss the gender of an unborn baby?
As we established in our previous column, gender identity is an internal experience of gender that one feels to be a part of oneself. So, then, what does an ultrasound actually reveal? Objectively, ultrasound can show the provider the presence or absence of a hyperechoic anatomical structure between the fetal legs that may become a penis, a vagina, or an ambiguous form of genitalia. While ultrasound is an incredible tool for anatomical and other forms of antenatal testing, ultrasound cannot detect identity characteristics because identities are, by definition, socially and internally experienced without respect to anatomy.
The distinction between gender identity and sex assigned at birth in discussions of antenatal ultrasonography is more than just a simple problem of semantics or vocabulary. To describe a fetus as a boy or a girl based on the presence/absence of a projection between the fetal legs seen on ultrasound is to reinforce the idea that gender identity and sex assigned at birth are equivalent. This conflation also erases nonbinary, genderqueer, and many other groups that identify with genders other than “boy” or “girl.” To be clear, unborn fetuses do not have a gender identity. Studies have shown that children begin to self-label their gender as early as 18-24 months of age, and similarly those who grow up to inhabit gender-nonconforming identities usually already are starting to show signs of their nonconformity starting at age 2 years.1 Some of the deepest traumas that trans and gender-nonconforming people experience are rated to the enforcement of unwritten gender laws during early childhood that are applied based on the sex assigned at birth.
Obstetricians can help to break the cycle of inappropriate gender assignment by correctly using the terms “gender” and “sex assigned at birth.” One opportunity for addressing patients’ questions about fetal gender might be to avoid the term “gender” altogether when discussing fetal sex assigned at birth, emphasizing instead what fetal ultrasound is actually able to do: Give us information about the appearance of external genitalia to help predict what sex will be assigned at birth.2 We have used this strategy when performing anatomy scans, and our experience has been that patients often will make their own assumptions about what it means to see certain external genitalia on ultrasound between the fetal legs. Motivated providers who want to go the extra mile may use a patient’s exclamation about their understanding of the fetus’s gender as an opportunity to educate the patient on the distinction between gender and sex assigned at birth, but even just smiling and moving onto the next part of the scan is an appropriate way of maintaining an atmosphere of inclusion and respect.
One of the roots of gender-based violence and gender dysphoria later in life is the conflation of gender and sex assigned at birth. While there is an entire social and political framework that enforces and polices gender after birth, the obstetrician can take steps to break the cycle starting before the birth has even occurred. Obstetricians are tasked with the unique challenge of providing care for the mother-fetus dyad, and much of the work is in setting up the fetus for the best possible life. Our scope of inclusion should be sufficiently wide to account for nonanatomical variations that could develop later in life in the infants we deliver.
Dr. Bahng is a PGY-1 resident physician in the gynecology & obstetrics residency program at Emory University, Atlanta. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner is an assistant professor at Emory University and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Bahng and Dr. Joyner reported no financial disclosures.
References
1. Horm Behav. 2013 Jul;64(2):288-97.
2. Obstet Gynecol Surv. 2009 Jan;64(1):50-7.
Every ob.gyn. expects that the topic of gender will come up at some point in a patient’s pregnancy. “When will I find out the gender?” asks the 24-year-old at her first prenatal visit. “We want the gender to be a surprise!” exclaims the couple at their anatomy scan for their second in vitro fertilization pregnancy. “Do you know what you’re having?” asks the obstetrician anticipating an imminent delivery.
The topic of gender is in fact so ingrained in our practice that we don’t bat an eye when approached with questions about fetal gender. But what exactly are we talking about when we discuss the gender of an unborn baby?
As we established in our previous column, gender identity is an internal experience of gender that one feels to be a part of oneself. So, then, what does an ultrasound actually reveal? Objectively, ultrasound can show the provider the presence or absence of a hyperechoic anatomical structure between the fetal legs that may become a penis, a vagina, or an ambiguous form of genitalia. While ultrasound is an incredible tool for anatomical and other forms of antenatal testing, ultrasound cannot detect identity characteristics because identities are, by definition, socially and internally experienced without respect to anatomy.
The distinction between gender identity and sex assigned at birth in discussions of antenatal ultrasonography is more than just a simple problem of semantics or vocabulary. To describe a fetus as a boy or a girl based on the presence/absence of a projection between the fetal legs seen on ultrasound is to reinforce the idea that gender identity and sex assigned at birth are equivalent. This conflation also erases nonbinary, genderqueer, and many other groups that identify with genders other than “boy” or “girl.” To be clear, unborn fetuses do not have a gender identity. Studies have shown that children begin to self-label their gender as early as 18-24 months of age, and similarly those who grow up to inhabit gender-nonconforming identities usually already are starting to show signs of their nonconformity starting at age 2 years.1 Some of the deepest traumas that trans and gender-nonconforming people experience are rated to the enforcement of unwritten gender laws during early childhood that are applied based on the sex assigned at birth.
Obstetricians can help to break the cycle of inappropriate gender assignment by correctly using the terms “gender” and “sex assigned at birth.” One opportunity for addressing patients’ questions about fetal gender might be to avoid the term “gender” altogether when discussing fetal sex assigned at birth, emphasizing instead what fetal ultrasound is actually able to do: Give us information about the appearance of external genitalia to help predict what sex will be assigned at birth.2 We have used this strategy when performing anatomy scans, and our experience has been that patients often will make their own assumptions about what it means to see certain external genitalia on ultrasound between the fetal legs. Motivated providers who want to go the extra mile may use a patient’s exclamation about their understanding of the fetus’s gender as an opportunity to educate the patient on the distinction between gender and sex assigned at birth, but even just smiling and moving onto the next part of the scan is an appropriate way of maintaining an atmosphere of inclusion and respect.
One of the roots of gender-based violence and gender dysphoria later in life is the conflation of gender and sex assigned at birth. While there is an entire social and political framework that enforces and polices gender after birth, the obstetrician can take steps to break the cycle starting before the birth has even occurred. Obstetricians are tasked with the unique challenge of providing care for the mother-fetus dyad, and much of the work is in setting up the fetus for the best possible life. Our scope of inclusion should be sufficiently wide to account for nonanatomical variations that could develop later in life in the infants we deliver.
Dr. Bahng is a PGY-1 resident physician in the gynecology & obstetrics residency program at Emory University, Atlanta. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner is an assistant professor at Emory University and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Bahng and Dr. Joyner reported no financial disclosures.
References
1. Horm Behav. 2013 Jul;64(2):288-97.
2. Obstet Gynecol Surv. 2009 Jan;64(1):50-7.
Lactobionic acid
Lactobionic acid (4-O-beta-galactopyranosyl-D-gluconic acid), a disaccharide formed from gluconic acid and galactose, has been established as a potent antioxidant well suited for use in solutions intended to preserve organs stored for transplantation.1,2 This polyhydroxy bionic acid is used as an excipient agent in some pharmaceutical products and has been the object of increasing interest and use in cosmetics and cosmeceuticals.3 It is included in skin care formulations for its strong humectant and antiaging effects.3,4 Lactobionic acid has been shown to suppress the synthesis of hydroxyl radicals by dint of iron-chelating activity and hinders the production of matrix metalloproteinases (MMPs), which promote photoaging.2,3,5 It may also present an advantage over the class of alpha-hydroxy acids used to treat photoaging by engendering less or no irritation, because of its larger molecular size and corresponding slower penetration rate.6 This column will focus on some recent research on the application of this strong antioxidant in dermatologic practice.
Lactobionic acid as an ingredient and vehicle
In 2010, Tasic-Kostov et al. compared the efficacy and irritation potential of lactobionic and glycolic acids (in gel and emulsion vehicles). In 77 healthy volunteers, the investigators found that , insofar as the former caused no irritation or skin barrier damage. In a second part to the study, they determined that efficacy of the acids was improved through the use of vehicles based on the natural emulsifier, alkyl polyglucoside (APG). They concluded that lactobionic acid in a 6% concentration in an APG vehicle warranted consideration as a low-molecular option in cosmeceutical products.6
In a subsequent study, the same team found supportive evidence that APG-based emulsions are safe cosmetic/dermopharmaceutical vehicles and carriers for extremely acidic and hygroscopic AHAs, particularly lactobionic acid. They did note, however, that lactobionic acid markedly affected the colloidal structure of the emulsion and fostered the development of lamellar structures, which could influence water distribution within the cream. They concluded, therefore, that such an emulsion, which was stabilized by lamellar liquid crystalline structures, would not be a viable carrier for the hygroscopic actives to achieve optimal moisturizing potential.7More recently, Tasic-Kostov et al. investigated the antioxidant and moisturizing traits of lactobionic acid in solution as well as in a natural APG emulsifier–based system using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging and lipid peroxidation inhibition assays. The researchers found that lactobionic acid exhibited suitable physical stability (though it exerted notable impact on the colloidal structure of the vehicle) as well as antioxidant activity in both formats, suggesting its application as a versatile cosmeceutical agent for treating photoaged skin.2
In 2017, Chaouat et al. found that lactobionic acid was a key component in a green microparticle carrier system for cosmetics also containing chitosan and linoleic acid (as the skin penetration–enhancing constituent). Chitosan and lactobionic acid made up the shell surrounding the linoleic acid core. The carrier system, in an aqueous solution, was found to be stable and able to encapsulate the hydrophobic skin lightener phenylethyl resorcinol.8
Potential in atopic dermatitis treatment
Using an oxazolone-induced, atopic dermatitis–like murine dermatitis model, Sakai et al. demonstrated in 2016 that the coapplication of a PAR2 inhibitor and lactobionic acid, which maintained stratum corneum acidity, could target skin barrier abnormality and allergic inflammation, the key mechanisms in atopic dermatitis etiology.9
Lactobionic acid in chemical peels
Early this year, Algiert-Zielinska et al. reported on the results of a split-face study with 20 white women in which the effects of a 20% lactobionic acid peel were compared with those of the 20% peel combined with aluminum oxide crystal microdermabrasion. Treatments were administered weekly over 6 weeks, with the peel alone performed on the left side and the combination therapy on the right. The combination was found to achieve a significantly higher hydration level as well as skin elasticity measurements. There were no statistically significant differences between the tested therapies in transepidermal water loss, which decreased for both approaches. Both the lactobionic acid peel and combination procedure delivered notable moisturizing effects.10
Previously, this team performed a comparative evaluation of the skin-moisturizing activities of lactobionic acid in 10% and 30% concentrations in 10 white subjects between 26 and 73 years old. In this split-face study, 10% lactobionic acid was applied on the left side and 30% on the right on a weekly basis through eight treatments. A 5% lactobionic acid cream was supplied for overnight use. Skin hydration levels were measured before each weekly treatment. Although any differences between cutaneous hydration between the lactobionic acid preparations could not be ascertained, the investigators identified a statistically significant enhancement of hydration levels for both concentrations after the full series of treatments. They concluded that lactobionic is a potent moisturizing compound.11The same authors also conducted a literature review on the moisturizing properties of lactobionic and lactic acids, noting that both acids are capable of binding copious amounts of water and display robust chelating characteristics, as well as antioxidant activity, by suppressing MMPs. The authors added that both act as strong moisturizing substances, helping to maintain epidermal barrier integrity, and are suitable for sensitive skin.3
Conclusion
Greater capacity to moisturize and deliver antiaging benefits while causing less or no irritation are desirable qualities in a dermatologic agent. Evidence is limited, but the data available seem to suggest that lactobionic acid exhibits such qualities in comparison to alpha-hydroxy acids. Much more research is needed, though, to determine the most appropriate ways to use this promising compound.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Annu Rev Med. 1995;46:235-47.
2. Int J Cosmet Sci. 2012 Oct;34(5):424-34.
3. Int J Dermatol. 2019 Mar;58(3):374-79.
4. Clin Dermatol. 2009 Sep-Oct;27(5):495-501.
5.The next generation hydroxy acids, in “Cosmeceuticals” (New York: Elsevier Saunders, 2005, pp. 205-11).
6. J Cosmet Dermatol. 2010 Mar;9(1):3-10.
7. Pharmazie. 2011 Nov;66(11):862-70.
8. J Microencapsul. 2017 Mar;34(2):162-70.
9. J Invest Dermatol. 2016 Feb;136(2):538-41.
10. J Cosmet Dermatol. 2019 Jan 20. doi: 10.1111/jocd.12859. [Epub ahead of print].
11. J Cosmet Dermatol. 2018 Dec;17(6):1096-1100.
Lactobionic acid (4-O-beta-galactopyranosyl-D-gluconic acid), a disaccharide formed from gluconic acid and galactose, has been established as a potent antioxidant well suited for use in solutions intended to preserve organs stored for transplantation.1,2 This polyhydroxy bionic acid is used as an excipient agent in some pharmaceutical products and has been the object of increasing interest and use in cosmetics and cosmeceuticals.3 It is included in skin care formulations for its strong humectant and antiaging effects.3,4 Lactobionic acid has been shown to suppress the synthesis of hydroxyl radicals by dint of iron-chelating activity and hinders the production of matrix metalloproteinases (MMPs), which promote photoaging.2,3,5 It may also present an advantage over the class of alpha-hydroxy acids used to treat photoaging by engendering less or no irritation, because of its larger molecular size and corresponding slower penetration rate.6 This column will focus on some recent research on the application of this strong antioxidant in dermatologic practice.
Lactobionic acid as an ingredient and vehicle
In 2010, Tasic-Kostov et al. compared the efficacy and irritation potential of lactobionic and glycolic acids (in gel and emulsion vehicles). In 77 healthy volunteers, the investigators found that , insofar as the former caused no irritation or skin barrier damage. In a second part to the study, they determined that efficacy of the acids was improved through the use of vehicles based on the natural emulsifier, alkyl polyglucoside (APG). They concluded that lactobionic acid in a 6% concentration in an APG vehicle warranted consideration as a low-molecular option in cosmeceutical products.6
In a subsequent study, the same team found supportive evidence that APG-based emulsions are safe cosmetic/dermopharmaceutical vehicles and carriers for extremely acidic and hygroscopic AHAs, particularly lactobionic acid. They did note, however, that lactobionic acid markedly affected the colloidal structure of the emulsion and fostered the development of lamellar structures, which could influence water distribution within the cream. They concluded, therefore, that such an emulsion, which was stabilized by lamellar liquid crystalline structures, would not be a viable carrier for the hygroscopic actives to achieve optimal moisturizing potential.7More recently, Tasic-Kostov et al. investigated the antioxidant and moisturizing traits of lactobionic acid in solution as well as in a natural APG emulsifier–based system using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging and lipid peroxidation inhibition assays. The researchers found that lactobionic acid exhibited suitable physical stability (though it exerted notable impact on the colloidal structure of the vehicle) as well as antioxidant activity in both formats, suggesting its application as a versatile cosmeceutical agent for treating photoaged skin.2
In 2017, Chaouat et al. found that lactobionic acid was a key component in a green microparticle carrier system for cosmetics also containing chitosan and linoleic acid (as the skin penetration–enhancing constituent). Chitosan and lactobionic acid made up the shell surrounding the linoleic acid core. The carrier system, in an aqueous solution, was found to be stable and able to encapsulate the hydrophobic skin lightener phenylethyl resorcinol.8
Potential in atopic dermatitis treatment
Using an oxazolone-induced, atopic dermatitis–like murine dermatitis model, Sakai et al. demonstrated in 2016 that the coapplication of a PAR2 inhibitor and lactobionic acid, which maintained stratum corneum acidity, could target skin barrier abnormality and allergic inflammation, the key mechanisms in atopic dermatitis etiology.9
Lactobionic acid in chemical peels
Early this year, Algiert-Zielinska et al. reported on the results of a split-face study with 20 white women in which the effects of a 20% lactobionic acid peel were compared with those of the 20% peel combined with aluminum oxide crystal microdermabrasion. Treatments were administered weekly over 6 weeks, with the peel alone performed on the left side and the combination therapy on the right. The combination was found to achieve a significantly higher hydration level as well as skin elasticity measurements. There were no statistically significant differences between the tested therapies in transepidermal water loss, which decreased for both approaches. Both the lactobionic acid peel and combination procedure delivered notable moisturizing effects.10
Previously, this team performed a comparative evaluation of the skin-moisturizing activities of lactobionic acid in 10% and 30% concentrations in 10 white subjects between 26 and 73 years old. In this split-face study, 10% lactobionic acid was applied on the left side and 30% on the right on a weekly basis through eight treatments. A 5% lactobionic acid cream was supplied for overnight use. Skin hydration levels were measured before each weekly treatment. Although any differences between cutaneous hydration between the lactobionic acid preparations could not be ascertained, the investigators identified a statistically significant enhancement of hydration levels for both concentrations after the full series of treatments. They concluded that lactobionic is a potent moisturizing compound.11The same authors also conducted a literature review on the moisturizing properties of lactobionic and lactic acids, noting that both acids are capable of binding copious amounts of water and display robust chelating characteristics, as well as antioxidant activity, by suppressing MMPs. The authors added that both act as strong moisturizing substances, helping to maintain epidermal barrier integrity, and are suitable for sensitive skin.3
Conclusion
Greater capacity to moisturize and deliver antiaging benefits while causing less or no irritation are desirable qualities in a dermatologic agent. Evidence is limited, but the data available seem to suggest that lactobionic acid exhibits such qualities in comparison to alpha-hydroxy acids. Much more research is needed, though, to determine the most appropriate ways to use this promising compound.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Annu Rev Med. 1995;46:235-47.
2. Int J Cosmet Sci. 2012 Oct;34(5):424-34.
3. Int J Dermatol. 2019 Mar;58(3):374-79.
4. Clin Dermatol. 2009 Sep-Oct;27(5):495-501.
5.The next generation hydroxy acids, in “Cosmeceuticals” (New York: Elsevier Saunders, 2005, pp. 205-11).
6. J Cosmet Dermatol. 2010 Mar;9(1):3-10.
7. Pharmazie. 2011 Nov;66(11):862-70.
8. J Microencapsul. 2017 Mar;34(2):162-70.
9. J Invest Dermatol. 2016 Feb;136(2):538-41.
10. J Cosmet Dermatol. 2019 Jan 20. doi: 10.1111/jocd.12859. [Epub ahead of print].
11. J Cosmet Dermatol. 2018 Dec;17(6):1096-1100.
Lactobionic acid (4-O-beta-galactopyranosyl-D-gluconic acid), a disaccharide formed from gluconic acid and galactose, has been established as a potent antioxidant well suited for use in solutions intended to preserve organs stored for transplantation.1,2 This polyhydroxy bionic acid is used as an excipient agent in some pharmaceutical products and has been the object of increasing interest and use in cosmetics and cosmeceuticals.3 It is included in skin care formulations for its strong humectant and antiaging effects.3,4 Lactobionic acid has been shown to suppress the synthesis of hydroxyl radicals by dint of iron-chelating activity and hinders the production of matrix metalloproteinases (MMPs), which promote photoaging.2,3,5 It may also present an advantage over the class of alpha-hydroxy acids used to treat photoaging by engendering less or no irritation, because of its larger molecular size and corresponding slower penetration rate.6 This column will focus on some recent research on the application of this strong antioxidant in dermatologic practice.
Lactobionic acid as an ingredient and vehicle
In 2010, Tasic-Kostov et al. compared the efficacy and irritation potential of lactobionic and glycolic acids (in gel and emulsion vehicles). In 77 healthy volunteers, the investigators found that , insofar as the former caused no irritation or skin barrier damage. In a second part to the study, they determined that efficacy of the acids was improved through the use of vehicles based on the natural emulsifier, alkyl polyglucoside (APG). They concluded that lactobionic acid in a 6% concentration in an APG vehicle warranted consideration as a low-molecular option in cosmeceutical products.6
In a subsequent study, the same team found supportive evidence that APG-based emulsions are safe cosmetic/dermopharmaceutical vehicles and carriers for extremely acidic and hygroscopic AHAs, particularly lactobionic acid. They did note, however, that lactobionic acid markedly affected the colloidal structure of the emulsion and fostered the development of lamellar structures, which could influence water distribution within the cream. They concluded, therefore, that such an emulsion, which was stabilized by lamellar liquid crystalline structures, would not be a viable carrier for the hygroscopic actives to achieve optimal moisturizing potential.7More recently, Tasic-Kostov et al. investigated the antioxidant and moisturizing traits of lactobionic acid in solution as well as in a natural APG emulsifier–based system using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging and lipid peroxidation inhibition assays. The researchers found that lactobionic acid exhibited suitable physical stability (though it exerted notable impact on the colloidal structure of the vehicle) as well as antioxidant activity in both formats, suggesting its application as a versatile cosmeceutical agent for treating photoaged skin.2
In 2017, Chaouat et al. found that lactobionic acid was a key component in a green microparticle carrier system for cosmetics also containing chitosan and linoleic acid (as the skin penetration–enhancing constituent). Chitosan and lactobionic acid made up the shell surrounding the linoleic acid core. The carrier system, in an aqueous solution, was found to be stable and able to encapsulate the hydrophobic skin lightener phenylethyl resorcinol.8
Potential in atopic dermatitis treatment
Using an oxazolone-induced, atopic dermatitis–like murine dermatitis model, Sakai et al. demonstrated in 2016 that the coapplication of a PAR2 inhibitor and lactobionic acid, which maintained stratum corneum acidity, could target skin barrier abnormality and allergic inflammation, the key mechanisms in atopic dermatitis etiology.9
Lactobionic acid in chemical peels
Early this year, Algiert-Zielinska et al. reported on the results of a split-face study with 20 white women in which the effects of a 20% lactobionic acid peel were compared with those of the 20% peel combined with aluminum oxide crystal microdermabrasion. Treatments were administered weekly over 6 weeks, with the peel alone performed on the left side and the combination therapy on the right. The combination was found to achieve a significantly higher hydration level as well as skin elasticity measurements. There were no statistically significant differences between the tested therapies in transepidermal water loss, which decreased for both approaches. Both the lactobionic acid peel and combination procedure delivered notable moisturizing effects.10
Previously, this team performed a comparative evaluation of the skin-moisturizing activities of lactobionic acid in 10% and 30% concentrations in 10 white subjects between 26 and 73 years old. In this split-face study, 10% lactobionic acid was applied on the left side and 30% on the right on a weekly basis through eight treatments. A 5% lactobionic acid cream was supplied for overnight use. Skin hydration levels were measured before each weekly treatment. Although any differences between cutaneous hydration between the lactobionic acid preparations could not be ascertained, the investigators identified a statistically significant enhancement of hydration levels for both concentrations after the full series of treatments. They concluded that lactobionic is a potent moisturizing compound.11The same authors also conducted a literature review on the moisturizing properties of lactobionic and lactic acids, noting that both acids are capable of binding copious amounts of water and display robust chelating characteristics, as well as antioxidant activity, by suppressing MMPs. The authors added that both act as strong moisturizing substances, helping to maintain epidermal barrier integrity, and are suitable for sensitive skin.3
Conclusion
Greater capacity to moisturize and deliver antiaging benefits while causing less or no irritation are desirable qualities in a dermatologic agent. Evidence is limited, but the data available seem to suggest that lactobionic acid exhibits such qualities in comparison to alpha-hydroxy acids. Much more research is needed, though, to determine the most appropriate ways to use this promising compound.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Annu Rev Med. 1995;46:235-47.
2. Int J Cosmet Sci. 2012 Oct;34(5):424-34.
3. Int J Dermatol. 2019 Mar;58(3):374-79.
4. Clin Dermatol. 2009 Sep-Oct;27(5):495-501.
5.The next generation hydroxy acids, in “Cosmeceuticals” (New York: Elsevier Saunders, 2005, pp. 205-11).
6. J Cosmet Dermatol. 2010 Mar;9(1):3-10.
7. Pharmazie. 2011 Nov;66(11):862-70.
8. J Microencapsul. 2017 Mar;34(2):162-70.
9. J Invest Dermatol. 2016 Feb;136(2):538-41.
10. J Cosmet Dermatol. 2019 Jan 20. doi: 10.1111/jocd.12859. [Epub ahead of print].
11. J Cosmet Dermatol. 2018 Dec;17(6):1096-1100.