User login
Eyelid Dermatitis: Common Patterns and Contact Allergens
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
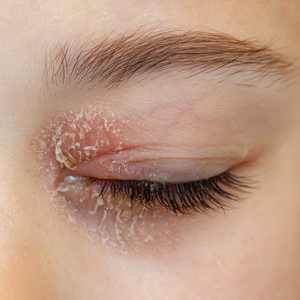
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.
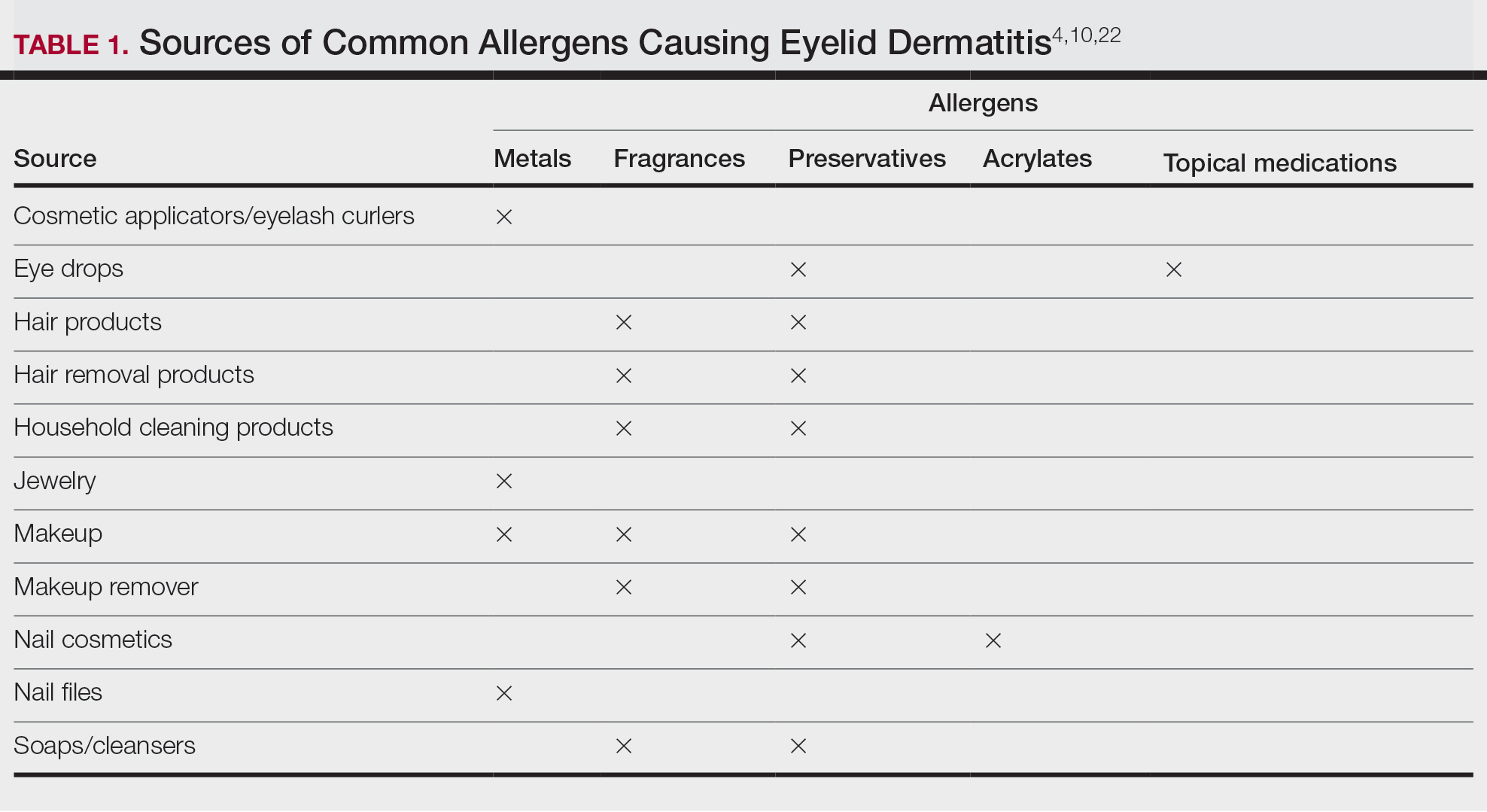
Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46
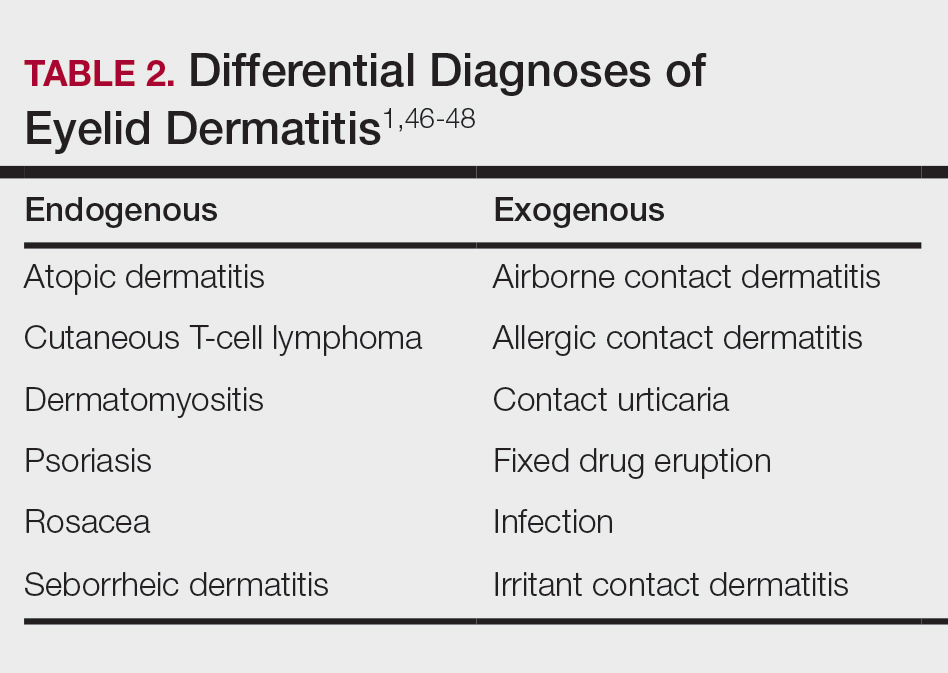
Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Practice Points
- Eyelid dermatitis is a common dermatologic concern representing a broad range of inflammatory dermatoses, most often caused by allergic contact dermatitis (ACD).
- The most common contact allergens associated with eyelid dermatitis are metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications, which may be found in a variety of sources, including cosmetics, ophthalmic medications, nail lacquers, and jewelry.
- Eyelid ACD is diagnosed via patch testing, and management involves strict allergen avoidance.
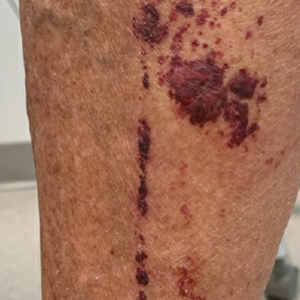
Purpuric Lesions on the Leg
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.
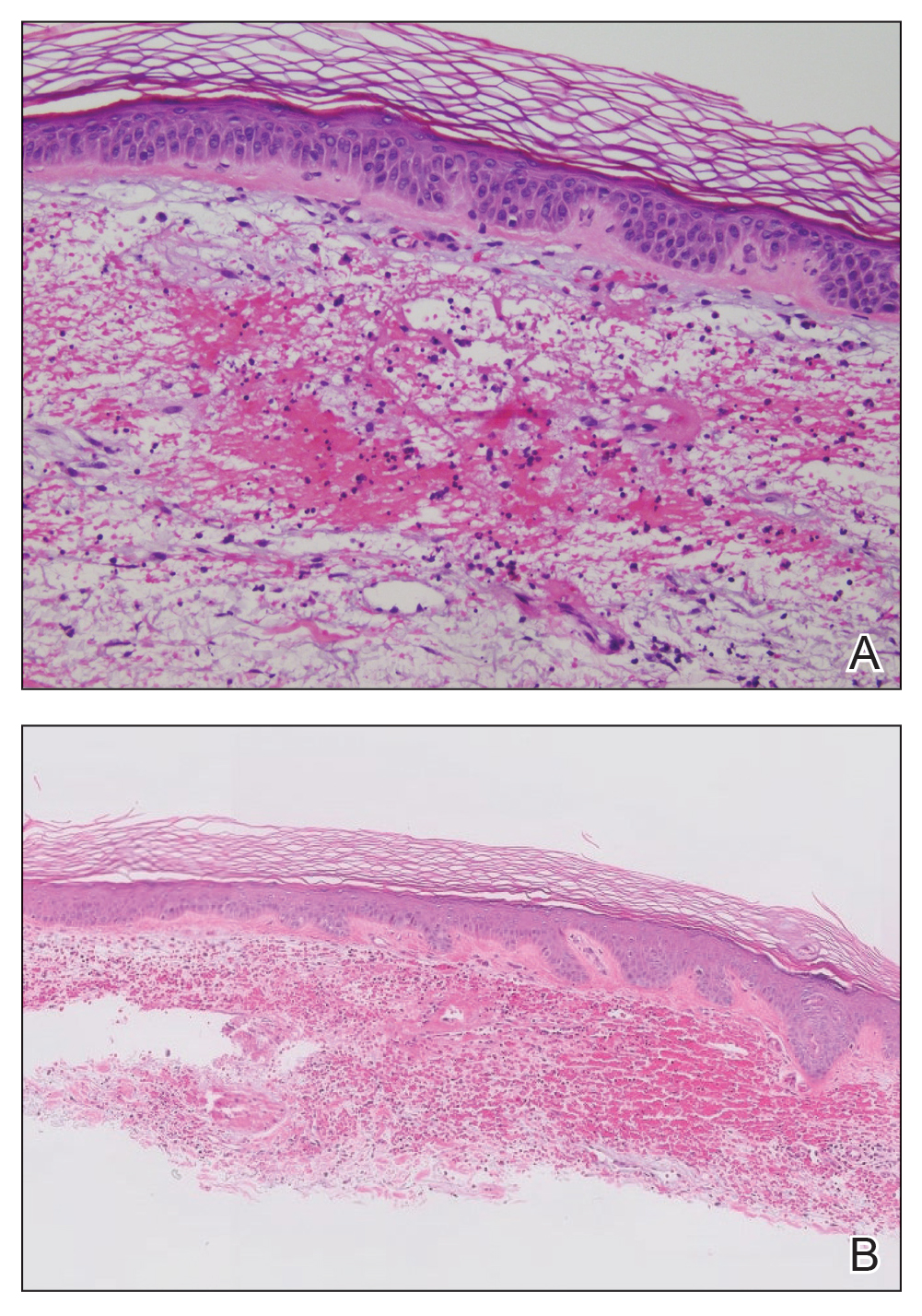
Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1
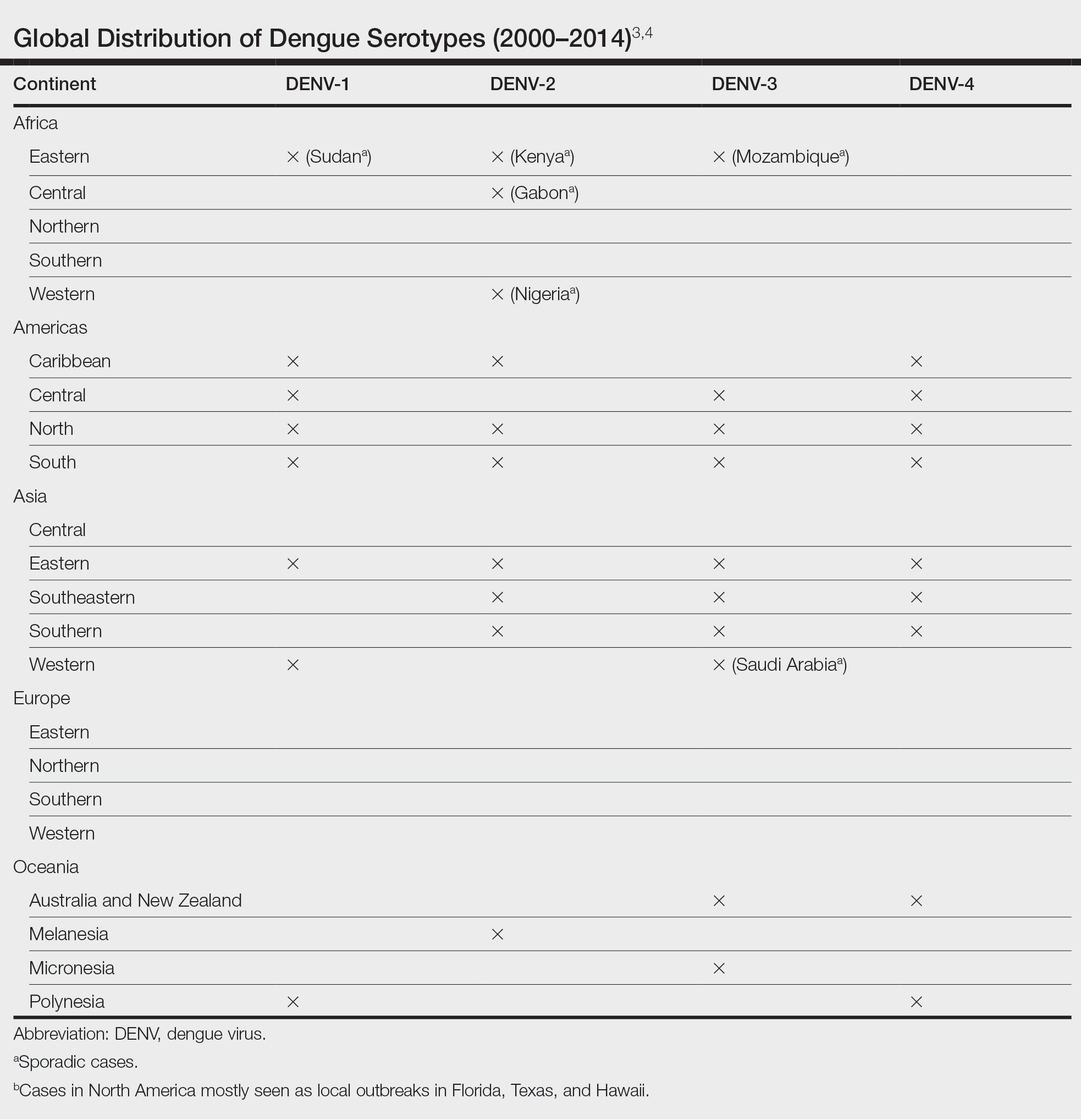
Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
A 74-year-old woman who frequently traveled abroad presented to the dermatology department with retiform purpura of the lower leg along with gastrointestinal cramps, fatigue, and myalgia. The patient reported that the symptoms had started 10 days after returning from a recent trip to Africa.
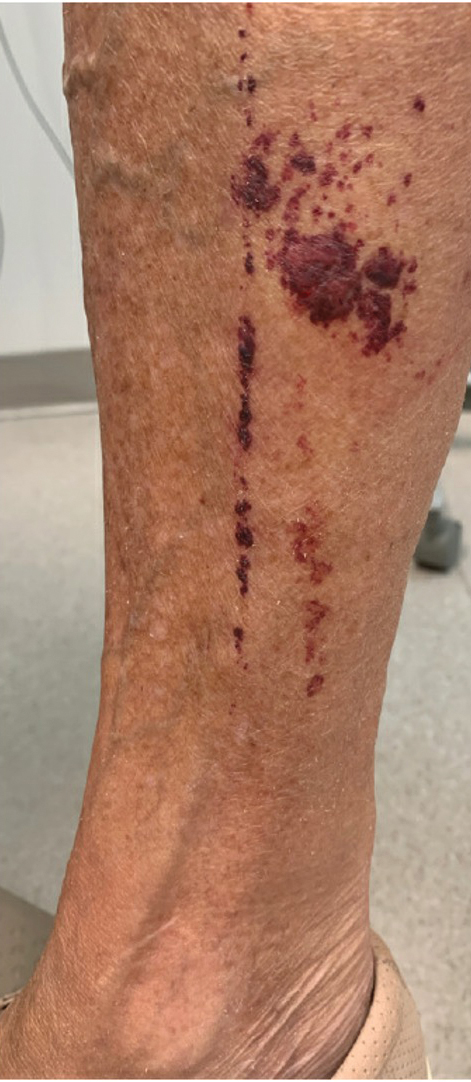
Achieving personalized outcomes in metastatic pancreatic adenocarcinoma: Treating with proactive AE management in mind
Developed under the direction and sponsorship of Ipsen Biopharmaceuticals
Metastatic pancreatic cancer is a difficult-to-treat cancer associated with poor outcomes. Moreover, it is known to cause challenging symptoms, which are oftentimes compounded by treatment. As more therapeutic options become available, oncologists can start to consider survival outcomes and the tolerability profile when making treatment recommendations.
Until recently, first-line options for patients with metastatic pancreatic adenocarcinoma (mPDAC) were limited to the following therapies: (1) gemcitabine plus nab-paclitaxel (GEM+Nab-P) and (2) fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX). In February 2024, the U.S. Food and Drug Administration approved ONIVYDE® (irinotecan liposome injection), a topoisomerase inhibitor, in combination with oxaliplatin, fluorouracil and leucovorin, for the first-line treatment of adults patients with metastatic pancreatic adenocarcinoma based on results of the Phase 3 NAPOLI 3 trial.
ONIVYDE® (irinotecan liposome injection) is indicated, in combination with oxaliplatin, fluorouracil, and leucovorin, for the first-line treatment of adult patients with metastatic pancreatic adenocarcinoma. ONIVYDE is indicated, in combination with fluorouracil and leucovorin, for the treatment of adult patients with metastatic pancreatic adenocarcinoma after disease progression following gemcitabine-based therapy. Limitations of Use: ONIVYDE is not indicated as a single agent for the treatment of patients with metastatic pancreatic adenocarcinoma. WARNING: SEVERE NEUTROPENIA AND SEVERE DIARRHEA Neutropenia
Diarrhea
|
"We are excited by the FDA-approval to have another treatment option for patients with newly diagnosed mPDAC,” said Maen Abdelrahim, MD, PhD, PharmB, Section Chief of Gastrointestinal Medical Oncology, Dr. Mary and Ron Neal Cancer Center, Houston Methodist Hospital. “The liposomal formulation of ONIVYDE encapsulates irinotecan in a lipid bilayer, allowing it to remain in circulation for longer than free irinotecan. When compared with free irinotecan, a pre-clinical study showed higher intratumoral levels of irinotecan and its active metabolite SN-38 can be achieved and sustained.”
The importance of proactive AE management and patient education
The ONIVYDE regimen has demonstrated improvements across key outcome measures like overall survival (OS) and progression-free survival (PFS) compared to the previous standard of care, GEM+Nab-P. It also showed higher numerical overall response rates (ORR) vs. GEM+Nab-P. However, when treating patients, it’s equally critical to consider the impact of treatment on day-to-day life and manageability of adverse events. Diarrhea was the most commonly observed adverse event for all grades in the NAPOLI 3 trial. If left unmanaged, diarrhea can be a serious adverse event, but if proactively managed through nutrition (patients should stop lactose-containing products, eat a low-fat diet and maintain hydration during treatment with ONIVYDE) and/or through administration of anti-diarrhetic (atropine for early-onset diarrhea, or loperamide for late-onset diarrhea), the adverse event may be managed and it may allow a patient to continue treatment.
Direct and ongoing conversation with patients about what to expect from treatment, specifically outlining potential adverse events and proactive ways to manage them, is essential to ensure patients speak up about symptoms as they experience them. In this way, early management of adverse events may potentially help the patient stay on therapy. “Including a discussion of the potential adverse events and toxicity for each treatment option is good clinical practice. We use a patient-centered approach that incorporates the input of the patient and their family for mPDAC treatment and the management of side effects. This approach can help empower the patient and their family to become active members of their cancer care team.”
A study was published in 2024 in Journal of Clinical Oncology, that described the reasons for non-treatment in a real-world cohort of patients with pancreatic cancer. The results showed that even among patients with mPDAC, 43.8% did not receive any cancer-directed treatment. The primary reason to decline treatment was due to patient/family preference even when treatment was recommended by their clinician. This further demonstrates the importance of educating patients about what to expect from treatment to make an informed decision.
NAPOLI 3 Results
NAPOLI 3 demonstrated superior efficacy of the ONIVYDE regimen over GEM+Nab-P as a therapeutic option for treatment-naïve patients with mPDAC. A total of 770 patients from 187 worldwide community and academic centers were randomized to receive either the ONIVYDE regimen (NALIRIFOX; n=383) or GEM+Nab-P (n=387). The primary outcome was overall survival (OS). Secondary outcomes included progression-free survival (PFS) and overall response rate (ORR), which were tested in a hierarchical approach if OS was significant.
Results of the study showed that the median OS was 11.1 months (95% confidence interval (CI) (10.0, 12.1)) in the ONIVYDE group and 9.2 months (95% CI (8.3, 10.6)) in the GEM+Nab-P group (hazard ratio (HR) 0.84 [95% CI 0.71–0.99]; p=0.0403; Table 1 and Figure 1). The median PFS was 7.4 months (95% CI (6.0, 7.7)) in the ONIVYDE group and 5.6 months (95% CI (5.3, 5.8)) in the GEM+Nab-P group (HR 0.70 [95% CI 0.59–0.85]; p=0.0001; Table 1). The ORR was 41.8% (36.8%-46.9%; 95% CI) for the ONIVYDE group versus 36.2% (31.4%-41.2%; 95% CI) for patients treated with GEM+Nab-P group (Table 1).
It should be noted that serious adverse reactions occurred in 54% of patients who received ONIVYDE in combination with oxaliplatin, fluorouracil and leucovorin. ONIVYDE has black box warnings for severe neutropenia and severe diarrhea: ONIVYDE should be withheld when the absolute neutrophil count is below 1500/mm3 or when neutropenic fever is present. In addition, ONIVYDE should be withheld with grade 2-4 diarrhea. Do not administer to patients with obstructive bowel disease.
The impact of the first-line ONIVYDE regimen
“Typically, fewer than 15% of our patients present with resectable disease; most have advanced or regional disease with a very low rate of 5-year survival. The meaningful clinical improvements in OS and PFS with the ONIVYDE regimen over the currently approved GEM+Nab-P regimen adds an exciting option for treating first-line mPDAC, helping to provide more time for our patients. And, by educating patients about what to expect from treatment, we can help them feel empowered to speak up and raise concerns.”
Table 1. Efficacy Results of All Randomized Patients in NAPOLI 3
| NALIRIFOX* (N=383) | Gem+NabP (N=387) |
Overall Survival | ||
Number of Deaths, n (%) | 259 (68) | 285 (74) |
Median Overall Survival (months) | 11.1 | 9.2 |
(95% CI) | (10.0, 12.1) | (8.3, 10.6) |
Hazard Ratio (95% CI) * | 0.84 (0.71, 0.99) | |
p-value † | 0.0403 | |
Progression-Free Survival | ||
Death or Progression, n (%) | 249 (65) | 259 (67) |
Median Progression-Free Survival (months) | 7.4 | 5.6 |
(95% CI) | (6.0, 7.7) | (5.3, 5.8) |
Hazard Ratio (95% CI) * | 0.70 (0.59, 0.85) | |
P-value † | 0.0001 | |
Objective Response Rate # | ||
ORR (95% CI) | 41.8 (36.8, 46.9) | 36.2 (31.4, 41.2) |
CR, n (%) | 1 (0.3) | 1 (0.3) |
PR, n (%) | 159 (41.5) | 139 (35.9) |
* NALIRIFOX= ONIVYDE+oxaliplatin/5-fluorouracil/leucovorin; Gem+NabP=gemcitabine+nab-paclitaxel; CI=confidence interval
** Based on the stratified Cox proportional hazard model; stratified by ECOG PS (0 vs. 1), region (North America vs. East Asia vs. Rest of the world), and liver metastases (yes vs. no) per interaction web response system
† Based on stratified log-rank test.
# ORR result was not statistically significant.

IMPORTANT SAFETY INFORMATION AND INDICATIONS
WARNING: SEVERE NEUTROPENIA and SEVERE DIARRHEA See full prescribing information for complete boxed warning Neutropenia
Diarrhea
|
CONTRAINDICATIONS
ONIVYDE is contraindicated in patients who have experienced a severe hypersensitivity reaction or anaphylaxis to ONIVYDE or irinotecan HCl.
WARNINGS AND PRECAUTIONS
Severe Neutropenia: See Boxed WARNING. In NAPOLI 3, Grade 3 and 4 neutropenia occurred in 26% of patients receiving ONIVYDE in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) and fatal neutropenic fever in 0.3% of patients. In NAPOLI 3, the incidence of Grade 3 or 4 neutropenia was similar among Asian patients [6 of 20 (30%)] compared to White patients [76 of 289 (26%)]. Neutropenic fever/neutropenic sepsis was reported in 5% of Asian patients (1 of 20) compared to 2.3% of White patients (7 of 306). In NAPOLI-1, Grade 3 and 4 neutropenia occurred in 20% of patients receiving ONIVYDE in combination with fluorouracil and leucovorin (ONIVYDE/FU/LV). Neutropenic sepsis occurred in 3% and fatal neutropenic sepsis in 0.8%. In NAPOLI-1, the incidence of Grade 3 or 4 neutropenia was higher among Asian patients [18 of 33 (55%)] compared to White patients [13 of 73 (18%)]. Neutropenic fever/neutropenic sepsis was reported in 6% of Asian patients compared to 1% of White patients.
Monitor complete blood cell counts on Days 1 and 8 of every cycle and more frequently if clinically indicated. Withhold ONIVYDE if the absolute neutrophil count (ANC) is below 1500/mm3 or if neutropenic fever occurs. Resume ONIVYDE when the ANC is 1500/mm3 or above. Reduce ONIVYDE dose for Grade 3-4 neutropenia or neutropenic fever following recovery in subsequent cycles.
Severe Diarrhea: See Boxed WARNING. In NAPOLI 3, Grade 3 and 4 diarrhea (early-onset [within 24 hours of chemotherapy] and late-onset [more than 24 hours following chemotherapy]) occurred in 20% receiving NALIRIFOX. In NAPOLI-1, Grade 3 or 4 diarrhea occurred in 13% receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 late-onset diarrhea was 9% in patients receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 early-onset diarrhea was 3% in patients receiving ONIVYDE/FU/LV.
To reduce the risk of severe diarrhea, patients should stop lactose-containing products, eat a low-fat diet, and maintain hydration during treatment with ONIVYDE. Withhold ONIVYDE for Grade 2-4 diarrhea. Local institutional guidelines should be followed for the treatment of diarrhea that does not improve within 48 hours and may include the addition of diphenoxylate hydrochloride plus atropine sulfate or octreotide. Following recovery to Grade 1 diarrhea, resume ONIVYDE at a reduced dose.
Interstitial Lung Disease (ILD): Irinotecan HCl can cause severe and fatal ILD. Patients with risk factors should be closely monitored for respiratory symptoms before and during ONIVYDE therapy. Withhold ONIVYDE in patients with new or progressive dyspnea, cough, and fever, pending diagnostic evaluation. Discontinue ONIVYDE in patients with a confirmed diagnosis of ILD.
Severe Hypersensitivity Reaction: Irinotecan, including ONIVYDE, can cause severe hypersensitivity reactions, including anaphylactic reactions. Permanently discontinue ONIVYDE in patients who experience a severe hypersensitivity reaction.
Embryo-Fetal Toxicity: ONIVYDE can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during and for 7 months after the last dose of ONIVYDE treatment.
ADVERSE REACTIONS FOR NALIRIFOX
- The most common adverse reactions (≥20%) of NALIRIFOX were diarrhea (72%), fatigue (62%), nausea (59%), vomiting (40%), decreased appetite (37%), abdominal pain (35%), mucosal inflammation (28%), constipation (25%), and weight decreased (22%).
- Permanent discontinuation of ONIVYDE due to an adverse reaction occurred in 17% of patients. Adverse reactions that resulted in permanent discontinuation of ONIVYDE in ≥1% of patients included neutropenia, thrombocytopenia, diarrhea, fatigue, infections, and cerebrovascular accident.
- Dosage reduction of ONIVYDE due to an adverse reaction occurred in 52% of patients. Adverse reactions that required dosage reduction in ≥1% of patients included anemia, decreased appetite, diarrhea, fatigue, febrile neutropenia, hypokalemia, liver function test abnormalities, nausea, mucosal inflammation, neutropenia, peripheral neuropathy, vomiting, thrombocytopenia, and weight decreased.
- Dosage interruptions of ONIVYDE due to an adverse reaction occurred in 1.9% of patients. Adverse reactions which required dosage interruption in ≥0.5% of patients included hypersensitivity and infusion-related reaction.
- The most common laboratory abnormalities (≥10% Grade 3 or 4) were decreased neutrophils (26%), decreased potassium (22%), decreased lymphocytes (11%), and decreased hemoglobin (10%).
ADVERSE REACTIONS FOR ONIVYDE/FU/LV
- The most common adverse reactions (≥20%) were diarrhea (59%), fatigue/asthenia (56%), vomiting (52%), nausea (51%), decreased appetite (44%), stomatitis (32%), and pyrexia (23%).
- Adverse reactions led to permanent discontinuation of ONIVYDE in 11% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions resulting in discontinuation of ONIVYDE were diarrhea, vomiting, and sepsis.
- Dose reductions of ONIVYDE for adverse reactions occurred in 33% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring dose reductions were neutropenia, diarrhea, nausea, and anemia.
- ONIVYDE was withheld or delayed for adverse reactions in 62% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring interruption or delays were neutropenia, diarrhea, fatigue, vomiting, and thrombocytopenia.
- The most common severe laboratory abnormalities (≥10% Grade 3 or 4) were lymphopenia and neutropenia.
Postmarketing Experience: Immune system disorders: Hypersensitivity (including anaphylactic reaction and angioedema).
DRUG INTERACTIONS
- Avoid the use of strong CYP3A4 inducers, if possible, and substitute non-enzyme inducing therapies ≥2 weeks prior to initiation of ONIVYDE.
- Avoid the use of strong CYP3A4 or UGT1A1 inhibitors, if possible, and discontinue strong CYP3A4 inhibitors ≥1 week prior to starting therapy.
USE IN SPECIFIC POPULATIONS
- Pregnancy and Reproductive Potential: See WARNINGS & PRECAUTIONS. Advise males with female partners of reproductive potential to use condoms during and for 4 months after the last dose of ONIVYDE treatment.
- Lactation: Advise nursing women not to breastfeed during and for 1 month after the last dose of ONIVYDE treatment.
To report SUSPECTED ADVERSE REACTIONS, contact Ipsen Biopharmaceuticals, Inc. at 1-855-463-5127 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including BOXED WARNING, for ONIVYDE.
ONIVYDE is a registered trademark of Ipsen Biopharm Ltd.
©2024 Ipsen Biopharmaceuticals, Inc. All rights reserved. August 2024 ONV-US-005210
Developed under the direction and sponsorship of Ipsen Biopharmaceuticals
Metastatic pancreatic cancer is a difficult-to-treat cancer associated with poor outcomes. Moreover, it is known to cause challenging symptoms, which are oftentimes compounded by treatment. As more therapeutic options become available, oncologists can start to consider survival outcomes and the tolerability profile when making treatment recommendations.
Until recently, first-line options for patients with metastatic pancreatic adenocarcinoma (mPDAC) were limited to the following therapies: (1) gemcitabine plus nab-paclitaxel (GEM+Nab-P) and (2) fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX). In February 2024, the U.S. Food and Drug Administration approved ONIVYDE® (irinotecan liposome injection), a topoisomerase inhibitor, in combination with oxaliplatin, fluorouracil and leucovorin, for the first-line treatment of adults patients with metastatic pancreatic adenocarcinoma based on results of the Phase 3 NAPOLI 3 trial.
ONIVYDE® (irinotecan liposome injection) is indicated, in combination with oxaliplatin, fluorouracil, and leucovorin, for the first-line treatment of adult patients with metastatic pancreatic adenocarcinoma. ONIVYDE is indicated, in combination with fluorouracil and leucovorin, for the treatment of adult patients with metastatic pancreatic adenocarcinoma after disease progression following gemcitabine-based therapy. Limitations of Use: ONIVYDE is not indicated as a single agent for the treatment of patients with metastatic pancreatic adenocarcinoma. WARNING: SEVERE NEUTROPENIA AND SEVERE DIARRHEA Neutropenia
Diarrhea
|
"We are excited by the FDA-approval to have another treatment option for patients with newly diagnosed mPDAC,” said Maen Abdelrahim, MD, PhD, PharmB, Section Chief of Gastrointestinal Medical Oncology, Dr. Mary and Ron Neal Cancer Center, Houston Methodist Hospital. “The liposomal formulation of ONIVYDE encapsulates irinotecan in a lipid bilayer, allowing it to remain in circulation for longer than free irinotecan. When compared with free irinotecan, a pre-clinical study showed higher intratumoral levels of irinotecan and its active metabolite SN-38 can be achieved and sustained.”
The importance of proactive AE management and patient education
The ONIVYDE regimen has demonstrated improvements across key outcome measures like overall survival (OS) and progression-free survival (PFS) compared to the previous standard of care, GEM+Nab-P. It also showed higher numerical overall response rates (ORR) vs. GEM+Nab-P. However, when treating patients, it’s equally critical to consider the impact of treatment on day-to-day life and manageability of adverse events. Diarrhea was the most commonly observed adverse event for all grades in the NAPOLI 3 trial. If left unmanaged, diarrhea can be a serious adverse event, but if proactively managed through nutrition (patients should stop lactose-containing products, eat a low-fat diet and maintain hydration during treatment with ONIVYDE) and/or through administration of anti-diarrhetic (atropine for early-onset diarrhea, or loperamide for late-onset diarrhea), the adverse event may be managed and it may allow a patient to continue treatment.
Direct and ongoing conversation with patients about what to expect from treatment, specifically outlining potential adverse events and proactive ways to manage them, is essential to ensure patients speak up about symptoms as they experience them. In this way, early management of adverse events may potentially help the patient stay on therapy. “Including a discussion of the potential adverse events and toxicity for each treatment option is good clinical practice. We use a patient-centered approach that incorporates the input of the patient and their family for mPDAC treatment and the management of side effects. This approach can help empower the patient and their family to become active members of their cancer care team.”
A study was published in 2024 in Journal of Clinical Oncology, that described the reasons for non-treatment in a real-world cohort of patients with pancreatic cancer. The results showed that even among patients with mPDAC, 43.8% did not receive any cancer-directed treatment. The primary reason to decline treatment was due to patient/family preference even when treatment was recommended by their clinician. This further demonstrates the importance of educating patients about what to expect from treatment to make an informed decision.
NAPOLI 3 Results
NAPOLI 3 demonstrated superior efficacy of the ONIVYDE regimen over GEM+Nab-P as a therapeutic option for treatment-naïve patients with mPDAC. A total of 770 patients from 187 worldwide community and academic centers were randomized to receive either the ONIVYDE regimen (NALIRIFOX; n=383) or GEM+Nab-P (n=387). The primary outcome was overall survival (OS). Secondary outcomes included progression-free survival (PFS) and overall response rate (ORR), which were tested in a hierarchical approach if OS was significant.
Results of the study showed that the median OS was 11.1 months (95% confidence interval (CI) (10.0, 12.1)) in the ONIVYDE group and 9.2 months (95% CI (8.3, 10.6)) in the GEM+Nab-P group (hazard ratio (HR) 0.84 [95% CI 0.71–0.99]; p=0.0403; Table 1 and Figure 1). The median PFS was 7.4 months (95% CI (6.0, 7.7)) in the ONIVYDE group and 5.6 months (95% CI (5.3, 5.8)) in the GEM+Nab-P group (HR 0.70 [95% CI 0.59–0.85]; p=0.0001; Table 1). The ORR was 41.8% (36.8%-46.9%; 95% CI) for the ONIVYDE group versus 36.2% (31.4%-41.2%; 95% CI) for patients treated with GEM+Nab-P group (Table 1).
It should be noted that serious adverse reactions occurred in 54% of patients who received ONIVYDE in combination with oxaliplatin, fluorouracil and leucovorin. ONIVYDE has black box warnings for severe neutropenia and severe diarrhea: ONIVYDE should be withheld when the absolute neutrophil count is below 1500/mm3 or when neutropenic fever is present. In addition, ONIVYDE should be withheld with grade 2-4 diarrhea. Do not administer to patients with obstructive bowel disease.
The impact of the first-line ONIVYDE regimen
“Typically, fewer than 15% of our patients present with resectable disease; most have advanced or regional disease with a very low rate of 5-year survival. The meaningful clinical improvements in OS and PFS with the ONIVYDE regimen over the currently approved GEM+Nab-P regimen adds an exciting option for treating first-line mPDAC, helping to provide more time for our patients. And, by educating patients about what to expect from treatment, we can help them feel empowered to speak up and raise concerns.”
Table 1. Efficacy Results of All Randomized Patients in NAPOLI 3
| NALIRIFOX* (N=383) | Gem+NabP (N=387) |
Overall Survival | ||
Number of Deaths, n (%) | 259 (68) | 285 (74) |
Median Overall Survival (months) | 11.1 | 9.2 |
(95% CI) | (10.0, 12.1) | (8.3, 10.6) |
Hazard Ratio (95% CI) * | 0.84 (0.71, 0.99) | |
p-value † | 0.0403 | |
Progression-Free Survival | ||
Death or Progression, n (%) | 249 (65) | 259 (67) |
Median Progression-Free Survival (months) | 7.4 | 5.6 |
(95% CI) | (6.0, 7.7) | (5.3, 5.8) |
Hazard Ratio (95% CI) * | 0.70 (0.59, 0.85) | |
P-value † | 0.0001 | |
Objective Response Rate # | ||
ORR (95% CI) | 41.8 (36.8, 46.9) | 36.2 (31.4, 41.2) |
CR, n (%) | 1 (0.3) | 1 (0.3) |
PR, n (%) | 159 (41.5) | 139 (35.9) |
* NALIRIFOX= ONIVYDE+oxaliplatin/5-fluorouracil/leucovorin; Gem+NabP=gemcitabine+nab-paclitaxel; CI=confidence interval
** Based on the stratified Cox proportional hazard model; stratified by ECOG PS (0 vs. 1), region (North America vs. East Asia vs. Rest of the world), and liver metastases (yes vs. no) per interaction web response system
† Based on stratified log-rank test.
# ORR result was not statistically significant.

IMPORTANT SAFETY INFORMATION AND INDICATIONS
WARNING: SEVERE NEUTROPENIA and SEVERE DIARRHEA See full prescribing information for complete boxed warning Neutropenia
Diarrhea
|
CONTRAINDICATIONS
ONIVYDE is contraindicated in patients who have experienced a severe hypersensitivity reaction or anaphylaxis to ONIVYDE or irinotecan HCl.
WARNINGS AND PRECAUTIONS
Severe Neutropenia: See Boxed WARNING. In NAPOLI 3, Grade 3 and 4 neutropenia occurred in 26% of patients receiving ONIVYDE in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) and fatal neutropenic fever in 0.3% of patients. In NAPOLI 3, the incidence of Grade 3 or 4 neutropenia was similar among Asian patients [6 of 20 (30%)] compared to White patients [76 of 289 (26%)]. Neutropenic fever/neutropenic sepsis was reported in 5% of Asian patients (1 of 20) compared to 2.3% of White patients (7 of 306). In NAPOLI-1, Grade 3 and 4 neutropenia occurred in 20% of patients receiving ONIVYDE in combination with fluorouracil and leucovorin (ONIVYDE/FU/LV). Neutropenic sepsis occurred in 3% and fatal neutropenic sepsis in 0.8%. In NAPOLI-1, the incidence of Grade 3 or 4 neutropenia was higher among Asian patients [18 of 33 (55%)] compared to White patients [13 of 73 (18%)]. Neutropenic fever/neutropenic sepsis was reported in 6% of Asian patients compared to 1% of White patients.
Monitor complete blood cell counts on Days 1 and 8 of every cycle and more frequently if clinically indicated. Withhold ONIVYDE if the absolute neutrophil count (ANC) is below 1500/mm3 or if neutropenic fever occurs. Resume ONIVYDE when the ANC is 1500/mm3 or above. Reduce ONIVYDE dose for Grade 3-4 neutropenia or neutropenic fever following recovery in subsequent cycles.
Severe Diarrhea: See Boxed WARNING. In NAPOLI 3, Grade 3 and 4 diarrhea (early-onset [within 24 hours of chemotherapy] and late-onset [more than 24 hours following chemotherapy]) occurred in 20% receiving NALIRIFOX. In NAPOLI-1, Grade 3 or 4 diarrhea occurred in 13% receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 late-onset diarrhea was 9% in patients receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 early-onset diarrhea was 3% in patients receiving ONIVYDE/FU/LV.
To reduce the risk of severe diarrhea, patients should stop lactose-containing products, eat a low-fat diet, and maintain hydration during treatment with ONIVYDE. Withhold ONIVYDE for Grade 2-4 diarrhea. Local institutional guidelines should be followed for the treatment of diarrhea that does not improve within 48 hours and may include the addition of diphenoxylate hydrochloride plus atropine sulfate or octreotide. Following recovery to Grade 1 diarrhea, resume ONIVYDE at a reduced dose.
Interstitial Lung Disease (ILD): Irinotecan HCl can cause severe and fatal ILD. Patients with risk factors should be closely monitored for respiratory symptoms before and during ONIVYDE therapy. Withhold ONIVYDE in patients with new or progressive dyspnea, cough, and fever, pending diagnostic evaluation. Discontinue ONIVYDE in patients with a confirmed diagnosis of ILD.
Severe Hypersensitivity Reaction: Irinotecan, including ONIVYDE, can cause severe hypersensitivity reactions, including anaphylactic reactions. Permanently discontinue ONIVYDE in patients who experience a severe hypersensitivity reaction.
Embryo-Fetal Toxicity: ONIVYDE can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during and for 7 months after the last dose of ONIVYDE treatment.
ADVERSE REACTIONS FOR NALIRIFOX
- The most common adverse reactions (≥20%) of NALIRIFOX were diarrhea (72%), fatigue (62%), nausea (59%), vomiting (40%), decreased appetite (37%), abdominal pain (35%), mucosal inflammation (28%), constipation (25%), and weight decreased (22%).
- Permanent discontinuation of ONIVYDE due to an adverse reaction occurred in 17% of patients. Adverse reactions that resulted in permanent discontinuation of ONIVYDE in ≥1% of patients included neutropenia, thrombocytopenia, diarrhea, fatigue, infections, and cerebrovascular accident.
- Dosage reduction of ONIVYDE due to an adverse reaction occurred in 52% of patients. Adverse reactions that required dosage reduction in ≥1% of patients included anemia, decreased appetite, diarrhea, fatigue, febrile neutropenia, hypokalemia, liver function test abnormalities, nausea, mucosal inflammation, neutropenia, peripheral neuropathy, vomiting, thrombocytopenia, and weight decreased.
- Dosage interruptions of ONIVYDE due to an adverse reaction occurred in 1.9% of patients. Adverse reactions which required dosage interruption in ≥0.5% of patients included hypersensitivity and infusion-related reaction.
- The most common laboratory abnormalities (≥10% Grade 3 or 4) were decreased neutrophils (26%), decreased potassium (22%), decreased lymphocytes (11%), and decreased hemoglobin (10%).
ADVERSE REACTIONS FOR ONIVYDE/FU/LV
- The most common adverse reactions (≥20%) were diarrhea (59%), fatigue/asthenia (56%), vomiting (52%), nausea (51%), decreased appetite (44%), stomatitis (32%), and pyrexia (23%).
- Adverse reactions led to permanent discontinuation of ONIVYDE in 11% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions resulting in discontinuation of ONIVYDE were diarrhea, vomiting, and sepsis.
- Dose reductions of ONIVYDE for adverse reactions occurred in 33% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring dose reductions were neutropenia, diarrhea, nausea, and anemia.
- ONIVYDE was withheld or delayed for adverse reactions in 62% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring interruption or delays were neutropenia, diarrhea, fatigue, vomiting, and thrombocytopenia.
- The most common severe laboratory abnormalities (≥10% Grade 3 or 4) were lymphopenia and neutropenia.
Postmarketing Experience: Immune system disorders: Hypersensitivity (including anaphylactic reaction and angioedema).
DRUG INTERACTIONS
- Avoid the use of strong CYP3A4 inducers, if possible, and substitute non-enzyme inducing therapies ≥2 weeks prior to initiation of ONIVYDE.
- Avoid the use of strong CYP3A4 or UGT1A1 inhibitors, if possible, and discontinue strong CYP3A4 inhibitors ≥1 week prior to starting therapy.
USE IN SPECIFIC POPULATIONS
- Pregnancy and Reproductive Potential: See WARNINGS & PRECAUTIONS. Advise males with female partners of reproductive potential to use condoms during and for 4 months after the last dose of ONIVYDE treatment.
- Lactation: Advise nursing women not to breastfeed during and for 1 month after the last dose of ONIVYDE treatment.
To report SUSPECTED ADVERSE REACTIONS, contact Ipsen Biopharmaceuticals, Inc. at 1-855-463-5127 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including BOXED WARNING, for ONIVYDE.
ONIVYDE is a registered trademark of Ipsen Biopharm Ltd.
©2024 Ipsen Biopharmaceuticals, Inc. All rights reserved. August 2024 ONV-US-005210
Developed under the direction and sponsorship of Ipsen Biopharmaceuticals
Metastatic pancreatic cancer is a difficult-to-treat cancer associated with poor outcomes. Moreover, it is known to cause challenging symptoms, which are oftentimes compounded by treatment. As more therapeutic options become available, oncologists can start to consider survival outcomes and the tolerability profile when making treatment recommendations.
Until recently, first-line options for patients with metastatic pancreatic adenocarcinoma (mPDAC) were limited to the following therapies: (1) gemcitabine plus nab-paclitaxel (GEM+Nab-P) and (2) fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX). In February 2024, the U.S. Food and Drug Administration approved ONIVYDE® (irinotecan liposome injection), a topoisomerase inhibitor, in combination with oxaliplatin, fluorouracil and leucovorin, for the first-line treatment of adults patients with metastatic pancreatic adenocarcinoma based on results of the Phase 3 NAPOLI 3 trial.
ONIVYDE® (irinotecan liposome injection) is indicated, in combination with oxaliplatin, fluorouracil, and leucovorin, for the first-line treatment of adult patients with metastatic pancreatic adenocarcinoma. ONIVYDE is indicated, in combination with fluorouracil and leucovorin, for the treatment of adult patients with metastatic pancreatic adenocarcinoma after disease progression following gemcitabine-based therapy. Limitations of Use: ONIVYDE is not indicated as a single agent for the treatment of patients with metastatic pancreatic adenocarcinoma. WARNING: SEVERE NEUTROPENIA AND SEVERE DIARRHEA Neutropenia
Diarrhea
|
"We are excited by the FDA-approval to have another treatment option for patients with newly diagnosed mPDAC,” said Maen Abdelrahim, MD, PhD, PharmB, Section Chief of Gastrointestinal Medical Oncology, Dr. Mary and Ron Neal Cancer Center, Houston Methodist Hospital. “The liposomal formulation of ONIVYDE encapsulates irinotecan in a lipid bilayer, allowing it to remain in circulation for longer than free irinotecan. When compared with free irinotecan, a pre-clinical study showed higher intratumoral levels of irinotecan and its active metabolite SN-38 can be achieved and sustained.”
The importance of proactive AE management and patient education
The ONIVYDE regimen has demonstrated improvements across key outcome measures like overall survival (OS) and progression-free survival (PFS) compared to the previous standard of care, GEM+Nab-P. It also showed higher numerical overall response rates (ORR) vs. GEM+Nab-P. However, when treating patients, it’s equally critical to consider the impact of treatment on day-to-day life and manageability of adverse events. Diarrhea was the most commonly observed adverse event for all grades in the NAPOLI 3 trial. If left unmanaged, diarrhea can be a serious adverse event, but if proactively managed through nutrition (patients should stop lactose-containing products, eat a low-fat diet and maintain hydration during treatment with ONIVYDE) and/or through administration of anti-diarrhetic (atropine for early-onset diarrhea, or loperamide for late-onset diarrhea), the adverse event may be managed and it may allow a patient to continue treatment.
Direct and ongoing conversation with patients about what to expect from treatment, specifically outlining potential adverse events and proactive ways to manage them, is essential to ensure patients speak up about symptoms as they experience them. In this way, early management of adverse events may potentially help the patient stay on therapy. “Including a discussion of the potential adverse events and toxicity for each treatment option is good clinical practice. We use a patient-centered approach that incorporates the input of the patient and their family for mPDAC treatment and the management of side effects. This approach can help empower the patient and their family to become active members of their cancer care team.”
A study was published in 2024 in Journal of Clinical Oncology, that described the reasons for non-treatment in a real-world cohort of patients with pancreatic cancer. The results showed that even among patients with mPDAC, 43.8% did not receive any cancer-directed treatment. The primary reason to decline treatment was due to patient/family preference even when treatment was recommended by their clinician. This further demonstrates the importance of educating patients about what to expect from treatment to make an informed decision.
NAPOLI 3 Results
NAPOLI 3 demonstrated superior efficacy of the ONIVYDE regimen over GEM+Nab-P as a therapeutic option for treatment-naïve patients with mPDAC. A total of 770 patients from 187 worldwide community and academic centers were randomized to receive either the ONIVYDE regimen (NALIRIFOX; n=383) or GEM+Nab-P (n=387). The primary outcome was overall survival (OS). Secondary outcomes included progression-free survival (PFS) and overall response rate (ORR), which were tested in a hierarchical approach if OS was significant.
Results of the study showed that the median OS was 11.1 months (95% confidence interval (CI) (10.0, 12.1)) in the ONIVYDE group and 9.2 months (95% CI (8.3, 10.6)) in the GEM+Nab-P group (hazard ratio (HR) 0.84 [95% CI 0.71–0.99]; p=0.0403; Table 1 and Figure 1). The median PFS was 7.4 months (95% CI (6.0, 7.7)) in the ONIVYDE group and 5.6 months (95% CI (5.3, 5.8)) in the GEM+Nab-P group (HR 0.70 [95% CI 0.59–0.85]; p=0.0001; Table 1). The ORR was 41.8% (36.8%-46.9%; 95% CI) for the ONIVYDE group versus 36.2% (31.4%-41.2%; 95% CI) for patients treated with GEM+Nab-P group (Table 1).
It should be noted that serious adverse reactions occurred in 54% of patients who received ONIVYDE in combination with oxaliplatin, fluorouracil and leucovorin. ONIVYDE has black box warnings for severe neutropenia and severe diarrhea: ONIVYDE should be withheld when the absolute neutrophil count is below 1500/mm3 or when neutropenic fever is present. In addition, ONIVYDE should be withheld with grade 2-4 diarrhea. Do not administer to patients with obstructive bowel disease.
The impact of the first-line ONIVYDE regimen
“Typically, fewer than 15% of our patients present with resectable disease; most have advanced or regional disease with a very low rate of 5-year survival. The meaningful clinical improvements in OS and PFS with the ONIVYDE regimen over the currently approved GEM+Nab-P regimen adds an exciting option for treating first-line mPDAC, helping to provide more time for our patients. And, by educating patients about what to expect from treatment, we can help them feel empowered to speak up and raise concerns.”
Table 1. Efficacy Results of All Randomized Patients in NAPOLI 3
| NALIRIFOX* (N=383) | Gem+NabP (N=387) |
Overall Survival | ||
Number of Deaths, n (%) | 259 (68) | 285 (74) |
Median Overall Survival (months) | 11.1 | 9.2 |
(95% CI) | (10.0, 12.1) | (8.3, 10.6) |
Hazard Ratio (95% CI) * | 0.84 (0.71, 0.99) | |
p-value † | 0.0403 | |
Progression-Free Survival | ||
Death or Progression, n (%) | 249 (65) | 259 (67) |
Median Progression-Free Survival (months) | 7.4 | 5.6 |
(95% CI) | (6.0, 7.7) | (5.3, 5.8) |
Hazard Ratio (95% CI) * | 0.70 (0.59, 0.85) | |
P-value † | 0.0001 | |
Objective Response Rate # | ||
ORR (95% CI) | 41.8 (36.8, 46.9) | 36.2 (31.4, 41.2) |
CR, n (%) | 1 (0.3) | 1 (0.3) |
PR, n (%) | 159 (41.5) | 139 (35.9) |
* NALIRIFOX= ONIVYDE+oxaliplatin/5-fluorouracil/leucovorin; Gem+NabP=gemcitabine+nab-paclitaxel; CI=confidence interval
** Based on the stratified Cox proportional hazard model; stratified by ECOG PS (0 vs. 1), region (North America vs. East Asia vs. Rest of the world), and liver metastases (yes vs. no) per interaction web response system
† Based on stratified log-rank test.
# ORR result was not statistically significant.

IMPORTANT SAFETY INFORMATION AND INDICATIONS
WARNING: SEVERE NEUTROPENIA and SEVERE DIARRHEA See full prescribing information for complete boxed warning Neutropenia
Diarrhea
|
CONTRAINDICATIONS
ONIVYDE is contraindicated in patients who have experienced a severe hypersensitivity reaction or anaphylaxis to ONIVYDE or irinotecan HCl.
WARNINGS AND PRECAUTIONS
Severe Neutropenia: See Boxed WARNING. In NAPOLI 3, Grade 3 and 4 neutropenia occurred in 26% of patients receiving ONIVYDE in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) and fatal neutropenic fever in 0.3% of patients. In NAPOLI 3, the incidence of Grade 3 or 4 neutropenia was similar among Asian patients [6 of 20 (30%)] compared to White patients [76 of 289 (26%)]. Neutropenic fever/neutropenic sepsis was reported in 5% of Asian patients (1 of 20) compared to 2.3% of White patients (7 of 306). In NAPOLI-1, Grade 3 and 4 neutropenia occurred in 20% of patients receiving ONIVYDE in combination with fluorouracil and leucovorin (ONIVYDE/FU/LV). Neutropenic sepsis occurred in 3% and fatal neutropenic sepsis in 0.8%. In NAPOLI-1, the incidence of Grade 3 or 4 neutropenia was higher among Asian patients [18 of 33 (55%)] compared to White patients [13 of 73 (18%)]. Neutropenic fever/neutropenic sepsis was reported in 6% of Asian patients compared to 1% of White patients.
Monitor complete blood cell counts on Days 1 and 8 of every cycle and more frequently if clinically indicated. Withhold ONIVYDE if the absolute neutrophil count (ANC) is below 1500/mm3 or if neutropenic fever occurs. Resume ONIVYDE when the ANC is 1500/mm3 or above. Reduce ONIVYDE dose for Grade 3-4 neutropenia or neutropenic fever following recovery in subsequent cycles.
Severe Diarrhea: See Boxed WARNING. In NAPOLI 3, Grade 3 and 4 diarrhea (early-onset [within 24 hours of chemotherapy] and late-onset [more than 24 hours following chemotherapy]) occurred in 20% receiving NALIRIFOX. In NAPOLI-1, Grade 3 or 4 diarrhea occurred in 13% receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 late-onset diarrhea was 9% in patients receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 early-onset diarrhea was 3% in patients receiving ONIVYDE/FU/LV.
To reduce the risk of severe diarrhea, patients should stop lactose-containing products, eat a low-fat diet, and maintain hydration during treatment with ONIVYDE. Withhold ONIVYDE for Grade 2-4 diarrhea. Local institutional guidelines should be followed for the treatment of diarrhea that does not improve within 48 hours and may include the addition of diphenoxylate hydrochloride plus atropine sulfate or octreotide. Following recovery to Grade 1 diarrhea, resume ONIVYDE at a reduced dose.
Interstitial Lung Disease (ILD): Irinotecan HCl can cause severe and fatal ILD. Patients with risk factors should be closely monitored for respiratory symptoms before and during ONIVYDE therapy. Withhold ONIVYDE in patients with new or progressive dyspnea, cough, and fever, pending diagnostic evaluation. Discontinue ONIVYDE in patients with a confirmed diagnosis of ILD.
Severe Hypersensitivity Reaction: Irinotecan, including ONIVYDE, can cause severe hypersensitivity reactions, including anaphylactic reactions. Permanently discontinue ONIVYDE in patients who experience a severe hypersensitivity reaction.
Embryo-Fetal Toxicity: ONIVYDE can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during and for 7 months after the last dose of ONIVYDE treatment.
ADVERSE REACTIONS FOR NALIRIFOX
- The most common adverse reactions (≥20%) of NALIRIFOX were diarrhea (72%), fatigue (62%), nausea (59%), vomiting (40%), decreased appetite (37%), abdominal pain (35%), mucosal inflammation (28%), constipation (25%), and weight decreased (22%).
- Permanent discontinuation of ONIVYDE due to an adverse reaction occurred in 17% of patients. Adverse reactions that resulted in permanent discontinuation of ONIVYDE in ≥1% of patients included neutropenia, thrombocytopenia, diarrhea, fatigue, infections, and cerebrovascular accident.
- Dosage reduction of ONIVYDE due to an adverse reaction occurred in 52% of patients. Adverse reactions that required dosage reduction in ≥1% of patients included anemia, decreased appetite, diarrhea, fatigue, febrile neutropenia, hypokalemia, liver function test abnormalities, nausea, mucosal inflammation, neutropenia, peripheral neuropathy, vomiting, thrombocytopenia, and weight decreased.
- Dosage interruptions of ONIVYDE due to an adverse reaction occurred in 1.9% of patients. Adverse reactions which required dosage interruption in ≥0.5% of patients included hypersensitivity and infusion-related reaction.
- The most common laboratory abnormalities (≥10% Grade 3 or 4) were decreased neutrophils (26%), decreased potassium (22%), decreased lymphocytes (11%), and decreased hemoglobin (10%).
ADVERSE REACTIONS FOR ONIVYDE/FU/LV
- The most common adverse reactions (≥20%) were diarrhea (59%), fatigue/asthenia (56%), vomiting (52%), nausea (51%), decreased appetite (44%), stomatitis (32%), and pyrexia (23%).
- Adverse reactions led to permanent discontinuation of ONIVYDE in 11% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions resulting in discontinuation of ONIVYDE were diarrhea, vomiting, and sepsis.
- Dose reductions of ONIVYDE for adverse reactions occurred in 33% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring dose reductions were neutropenia, diarrhea, nausea, and anemia.
- ONIVYDE was withheld or delayed for adverse reactions in 62% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring interruption or delays were neutropenia, diarrhea, fatigue, vomiting, and thrombocytopenia.
- The most common severe laboratory abnormalities (≥10% Grade 3 or 4) were lymphopenia and neutropenia.
Postmarketing Experience: Immune system disorders: Hypersensitivity (including anaphylactic reaction and angioedema).
DRUG INTERACTIONS
- Avoid the use of strong CYP3A4 inducers, if possible, and substitute non-enzyme inducing therapies ≥2 weeks prior to initiation of ONIVYDE.
- Avoid the use of strong CYP3A4 or UGT1A1 inhibitors, if possible, and discontinue strong CYP3A4 inhibitors ≥1 week prior to starting therapy.
USE IN SPECIFIC POPULATIONS
- Pregnancy and Reproductive Potential: See WARNINGS & PRECAUTIONS. Advise males with female partners of reproductive potential to use condoms during and for 4 months after the last dose of ONIVYDE treatment.
- Lactation: Advise nursing women not to breastfeed during and for 1 month after the last dose of ONIVYDE treatment.
To report SUSPECTED ADVERSE REACTIONS, contact Ipsen Biopharmaceuticals, Inc. at 1-855-463-5127 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including BOXED WARNING, for ONIVYDE.
ONIVYDE is a registered trademark of Ipsen Biopharm Ltd.
©2024 Ipsen Biopharmaceuticals, Inc. All rights reserved. August 2024 ONV-US-005210
Commentary: Migraine and Comorbidities, October 2024
Neck pain is commonly associated with headaches, especially with migraine headaches. This is well recognized, and the symptom of neck pain occurring during headache episodes or even independently of headache episodes is at least partially related to pain sensitivity.1 While neck pain is often considered a part of the migraine experience, it's not commonly thought of as a disabling symptom. However, neck pain can be a major aspect of migraine disability.
A systematic review published in August 2024 in the journal Cephalalgia described neck pain disability as a part of migraine. The authors used 33 clinic-based studies that utilized either the Neck Disability Index (NDI) or the Numeric Pain Rating Scale (NPRS) to define the severity of neck pain disability. They concluded that individuals with migraine had higher NDI and NPRS scores than patients with tension-type headaches and patients without headaches. According to the NDI scoring system, 0–4 points indicate no disability, 5–14 points indicate mild disability, 15–24 points indicate moderate disability, 25–34 points indicate severe disability, and ≥ 35 points indicate complete disability. The authors reported that the mean NDI score for patients with migraine was 16.2, which was approximately 12 points higher than for healthy headache-free control participants.2 This brings to light an issue that can substantially affect patients' quality of life. Patients who have neck pain with migraine may need focused attention to that symptom, in addition to overall migraine therapy, and it is important to ask migraine patients about the degree to which neck pain affects their life. In fact, many patients might not even realize that their neck pain is associated with their migraines.
Cardiovascular disease is another comorbidity that has been inconsistently associated with migraine. A study published in Headache: The Journal of Headache and Face Pain in August 2024 used data from a Danish population-based cohort longitudinal study that included over 140,000 women. The authors reported that migraine was associated with a risk for major adverse cardiovascular and cerebrovascular events in women aged ≤ 60 years.3
This link has been noted previously, although the studies have been inconsistent regarding how strong the link is, any specific causality, and whether there is a link at all. Potential causes for the possible associations have been attributed to "endothelial dysfunction, hypercoagulability, platelet aggregation, vasospasm, cardiovascular risk factors, paradoxical embolism, spreading depolarization, shared genetic risk, use of non-steroidal anti-inflammatory drugs, and immobilization."4
Of note, there has also been documentation of a possible negative correlation between migraine and cardiovascular disease. Another article, from The Journal of Headache and Pain, published in August 2024, used data from 873,341 and 554,569 individuals, respectively, in two meta-analyses. The authors reported a potential protective effect of migraine on coronary artery disease and ischemic stroke, and a potential protective effect of coronary atherosclerosis and myocardial infarction on migraine.5
A possible explanation for the conflicting results could lie in heterogeneity of migraine. For example, vestibular migraine is associated with many comorbidities, including anxiety disorders or depressive disorders, sleep disorders, persistent postural-perceptual dizziness, and Meniere disease.6 Given the serious consequences of cardiovascular disease, screening for risk factors could be beneficial for preventing adverse health outcomes for migraine patients. Eventually, further research may reveal more specific correlations between comorbidities and migraine subtypes, rather than generalizing comorbidities to all migraine types.
Sources
- Al-Khazali HM, Krøll LS, Ashina H, et al. Neck pain and headache: Pathophysiology, treatments and future directions. Musculoskelet Sci Pract. 2023;66:102804. Source
- Al-Khazali HM, Al-Sayegh Z, Younis S, et al. Systematic review and meta-analysis of Neck Disability Index and Numeric Pain Rating Scale in patients with migraine and tension-type headache. Cephalalgia. 2024;44:3331024241274266. Source
- Fuglsang CH, Pedersen L, Schmidt M, Vandenbroucke JP, Bøtker HE, Sørensen HT. The combined impact of migraine and gestational diabetes on long-term risk of premature myocardial infarction and stroke: A population-based cohort study. Headache. 2024 Aug 28. Source
- Agostoni EC, Longoni M. Migraine and cerebrovascular disease: still a dangerous connection? Neurol Sci. 2018;39(Suppl 1):33-37. Source
- Duan X, Du X, Zheng G, et al. Causality between migraine and cardiovascular disease: a bidirectional Mendelian randomization study. J Headache Pain. 2024;25:130. Source
- Ma YM, Zhang DP, Zhang HL, et al. Why is vestibular migraine associated with many comorbidities? J Neurol. 2024 Sept 20. Source
Neck pain is commonly associated with headaches, especially with migraine headaches. This is well recognized, and the symptom of neck pain occurring during headache episodes or even independently of headache episodes is at least partially related to pain sensitivity.1 While neck pain is often considered a part of the migraine experience, it's not commonly thought of as a disabling symptom. However, neck pain can be a major aspect of migraine disability.
A systematic review published in August 2024 in the journal Cephalalgia described neck pain disability as a part of migraine. The authors used 33 clinic-based studies that utilized either the Neck Disability Index (NDI) or the Numeric Pain Rating Scale (NPRS) to define the severity of neck pain disability. They concluded that individuals with migraine had higher NDI and NPRS scores than patients with tension-type headaches and patients without headaches. According to the NDI scoring system, 0–4 points indicate no disability, 5–14 points indicate mild disability, 15–24 points indicate moderate disability, 25–34 points indicate severe disability, and ≥ 35 points indicate complete disability. The authors reported that the mean NDI score for patients with migraine was 16.2, which was approximately 12 points higher than for healthy headache-free control participants.2 This brings to light an issue that can substantially affect patients' quality of life. Patients who have neck pain with migraine may need focused attention to that symptom, in addition to overall migraine therapy, and it is important to ask migraine patients about the degree to which neck pain affects their life. In fact, many patients might not even realize that their neck pain is associated with their migraines.
Cardiovascular disease is another comorbidity that has been inconsistently associated with migraine. A study published in Headache: The Journal of Headache and Face Pain in August 2024 used data from a Danish population-based cohort longitudinal study that included over 140,000 women. The authors reported that migraine was associated with a risk for major adverse cardiovascular and cerebrovascular events in women aged ≤ 60 years.3
This link has been noted previously, although the studies have been inconsistent regarding how strong the link is, any specific causality, and whether there is a link at all. Potential causes for the possible associations have been attributed to "endothelial dysfunction, hypercoagulability, platelet aggregation, vasospasm, cardiovascular risk factors, paradoxical embolism, spreading depolarization, shared genetic risk, use of non-steroidal anti-inflammatory drugs, and immobilization."4
Of note, there has also been documentation of a possible negative correlation between migraine and cardiovascular disease. Another article, from The Journal of Headache and Pain, published in August 2024, used data from 873,341 and 554,569 individuals, respectively, in two meta-analyses. The authors reported a potential protective effect of migraine on coronary artery disease and ischemic stroke, and a potential protective effect of coronary atherosclerosis and myocardial infarction on migraine.5
A possible explanation for the conflicting results could lie in heterogeneity of migraine. For example, vestibular migraine is associated with many comorbidities, including anxiety disorders or depressive disorders, sleep disorders, persistent postural-perceptual dizziness, and Meniere disease.6 Given the serious consequences of cardiovascular disease, screening for risk factors could be beneficial for preventing adverse health outcomes for migraine patients. Eventually, further research may reveal more specific correlations between comorbidities and migraine subtypes, rather than generalizing comorbidities to all migraine types.
Sources
- Al-Khazali HM, Krøll LS, Ashina H, et al. Neck pain and headache: Pathophysiology, treatments and future directions. Musculoskelet Sci Pract. 2023;66:102804. Source
- Al-Khazali HM, Al-Sayegh Z, Younis S, et al. Systematic review and meta-analysis of Neck Disability Index and Numeric Pain Rating Scale in patients with migraine and tension-type headache. Cephalalgia. 2024;44:3331024241274266. Source
- Fuglsang CH, Pedersen L, Schmidt M, Vandenbroucke JP, Bøtker HE, Sørensen HT. The combined impact of migraine and gestational diabetes on long-term risk of premature myocardial infarction and stroke: A population-based cohort study. Headache. 2024 Aug 28. Source
- Agostoni EC, Longoni M. Migraine and cerebrovascular disease: still a dangerous connection? Neurol Sci. 2018;39(Suppl 1):33-37. Source
- Duan X, Du X, Zheng G, et al. Causality between migraine and cardiovascular disease: a bidirectional Mendelian randomization study. J Headache Pain. 2024;25:130. Source
- Ma YM, Zhang DP, Zhang HL, et al. Why is vestibular migraine associated with many comorbidities? J Neurol. 2024 Sept 20. Source
Neck pain is commonly associated with headaches, especially with migraine headaches. This is well recognized, and the symptom of neck pain occurring during headache episodes or even independently of headache episodes is at least partially related to pain sensitivity.1 While neck pain is often considered a part of the migraine experience, it's not commonly thought of as a disabling symptom. However, neck pain can be a major aspect of migraine disability.
A systematic review published in August 2024 in the journal Cephalalgia described neck pain disability as a part of migraine. The authors used 33 clinic-based studies that utilized either the Neck Disability Index (NDI) or the Numeric Pain Rating Scale (NPRS) to define the severity of neck pain disability. They concluded that individuals with migraine had higher NDI and NPRS scores than patients with tension-type headaches and patients without headaches. According to the NDI scoring system, 0–4 points indicate no disability, 5–14 points indicate mild disability, 15–24 points indicate moderate disability, 25–34 points indicate severe disability, and ≥ 35 points indicate complete disability. The authors reported that the mean NDI score for patients with migraine was 16.2, which was approximately 12 points higher than for healthy headache-free control participants.2 This brings to light an issue that can substantially affect patients' quality of life. Patients who have neck pain with migraine may need focused attention to that symptom, in addition to overall migraine therapy, and it is important to ask migraine patients about the degree to which neck pain affects their life. In fact, many patients might not even realize that their neck pain is associated with their migraines.
Cardiovascular disease is another comorbidity that has been inconsistently associated with migraine. A study published in Headache: The Journal of Headache and Face Pain in August 2024 used data from a Danish population-based cohort longitudinal study that included over 140,000 women. The authors reported that migraine was associated with a risk for major adverse cardiovascular and cerebrovascular events in women aged ≤ 60 years.3
This link has been noted previously, although the studies have been inconsistent regarding how strong the link is, any specific causality, and whether there is a link at all. Potential causes for the possible associations have been attributed to "endothelial dysfunction, hypercoagulability, platelet aggregation, vasospasm, cardiovascular risk factors, paradoxical embolism, spreading depolarization, shared genetic risk, use of non-steroidal anti-inflammatory drugs, and immobilization."4
Of note, there has also been documentation of a possible negative correlation between migraine and cardiovascular disease. Another article, from The Journal of Headache and Pain, published in August 2024, used data from 873,341 and 554,569 individuals, respectively, in two meta-analyses. The authors reported a potential protective effect of migraine on coronary artery disease and ischemic stroke, and a potential protective effect of coronary atherosclerosis and myocardial infarction on migraine.5
A possible explanation for the conflicting results could lie in heterogeneity of migraine. For example, vestibular migraine is associated with many comorbidities, including anxiety disorders or depressive disorders, sleep disorders, persistent postural-perceptual dizziness, and Meniere disease.6 Given the serious consequences of cardiovascular disease, screening for risk factors could be beneficial for preventing adverse health outcomes for migraine patients. Eventually, further research may reveal more specific correlations between comorbidities and migraine subtypes, rather than generalizing comorbidities to all migraine types.
Sources
- Al-Khazali HM, Krøll LS, Ashina H, et al. Neck pain and headache: Pathophysiology, treatments and future directions. Musculoskelet Sci Pract. 2023;66:102804. Source
- Al-Khazali HM, Al-Sayegh Z, Younis S, et al. Systematic review and meta-analysis of Neck Disability Index and Numeric Pain Rating Scale in patients with migraine and tension-type headache. Cephalalgia. 2024;44:3331024241274266. Source
- Fuglsang CH, Pedersen L, Schmidt M, Vandenbroucke JP, Bøtker HE, Sørensen HT. The combined impact of migraine and gestational diabetes on long-term risk of premature myocardial infarction and stroke: A population-based cohort study. Headache. 2024 Aug 28. Source
- Agostoni EC, Longoni M. Migraine and cerebrovascular disease: still a dangerous connection? Neurol Sci. 2018;39(Suppl 1):33-37. Source
- Duan X, Du X, Zheng G, et al. Causality between migraine and cardiovascular disease: a bidirectional Mendelian randomization study. J Headache Pain. 2024;25:130. Source
- Ma YM, Zhang DP, Zhang HL, et al. Why is vestibular migraine associated with many comorbidities? J Neurol. 2024 Sept 20. Source
Head and Neck Cancer: Should Patients Get PEG Access Prior to Therapy? VA pilot study could help clinicians make better-informed decisions to head off malnutrition
Research conducted at the US Department of Veterans Affairs (VA) could offer crucial insight into the hotly debated question of whether patients with head and neck cancer should have access to percutaneous endoscopic gastrostomy (PEG) before they develop malnutrition.
While no definitive conclusions can be drawn until a complete study is performed, early findings of a pilot trial are intriguing, said advanced practice oncology dietitian Katherine Petersen, MS, RDN, CSO, of the Phoenix VA Health Care System, who spoke in an interview with Federal Practitioner and at the annual meeting of the Association of VA Hematology/Oncology.
So far, the 12 patients with head and neck cancer who agreed to the placement of prophylactic feeding tubes prior to chemoradiation have had worse outcomes in some areas compared to the 9 patients who had tubes inserted when clinically indicated and the 12 who didn't need feeding tubes.
Petersen cautioned that the study is small and underpowered at this point. Still, she noted, "We're seeing a hint of exactly the opposite of what I expected. Those who get a tube prophylactically are doing worse than those who are getting it reactively or not at all, If that's the case, that's a really important outcome."
As Petersen explained, the placement of PEG feeding tubes is a hot topic in head and neck cancer care. Malnutrition affects about 80% of these patients and can contribute to mortality, raising the question of whether they should have access to feeding tubes placed prior to treatment in case enteral nutrition is needed.
In some patients with head and neck cancer, malnutrition may arise when tumors block food intake or prevent patients from swallowing. "But in my clinical experience, most often it's from the adverse effects of radiation and chemotherapy. Radiation creates burns inside their throat that make it hard to swallow. Or they have taste changes or really dry mouth," Petersen said.
"On top of these problems, chemotherapy can cause nausea and vomiting," she said. Placing feeding tube access may seem like a smart strategy to head off malnutrition as soon as it occurs. But, as Petersen noted, feeding tube use can lead to dependency as patients lose their ability to swallow. "There's a theory that if we give people feeding tubes, they'll go with the easier route of using a feeding tube and not keep swallowing. Then those swallowing muscles would weaken, and patients would end up permanently on a feeding tube."
In 2020, a retrospective VA study linked feeding tube dependence to lower overall survival in head and neck cancer patients. There are also risks to feeding tube placement, such as infection, pain, leakage, and inflammation.
But what if feeding tube valves are inserted prophylactically so they can be used for nutrition if needed? "We just haven't had any prospective studies to get to the heart of the matter and answer the question," she said. "It's hard to recruit. How do you convince somebody to randomly be assigned to have a hole poked in their stomach?"
For the new pilot study, researchers in Phoenix decided not to randomize patients. Instead, they asked them whether they'd accept the placement of feeding tube valves on a prophylactic basis.
Thirty-six veterans enrolled in 3 years, 33% of those were eligible. Twelve have died, 1 withdrew, and 2 were lost to follow-up.
Those in the prophylactic group had worse physical function and muscle strength over time, while those who received feeding tubes when needed had more adverse events.
Why might some outcomes be worse for patients who chose the prophylactic approach? "The answer is unclear," Petersen said. "Although one possibility is that those patients had higher-risk tumors and were more clued into their own risk."
"The goal now is to get funding for an expanded, multicenter study within the VA," Petersen said. The big question that she hopes to answer is: Does a prophylactic approach work? "Does it make a difference for patients in terms of how quickly they go back to living a full, meaningful life and be able to do all the things that they normally would do?"
A complete study would likely last 7 years, but helpful results may come earlier. "We are starting to see significant differences in terms of our main outcomes of physical function," Petersen said. "We only need 1 to 2 years of data for each patient to get to the heart of that."
The study is not funded, and Petersen reported no disclosures.
Research conducted at the US Department of Veterans Affairs (VA) could offer crucial insight into the hotly debated question of whether patients with head and neck cancer should have access to percutaneous endoscopic gastrostomy (PEG) before they develop malnutrition.
While no definitive conclusions can be drawn until a complete study is performed, early findings of a pilot trial are intriguing, said advanced practice oncology dietitian Katherine Petersen, MS, RDN, CSO, of the Phoenix VA Health Care System, who spoke in an interview with Federal Practitioner and at the annual meeting of the Association of VA Hematology/Oncology.
So far, the 12 patients with head and neck cancer who agreed to the placement of prophylactic feeding tubes prior to chemoradiation have had worse outcomes in some areas compared to the 9 patients who had tubes inserted when clinically indicated and the 12 who didn't need feeding tubes.
Petersen cautioned that the study is small and underpowered at this point. Still, she noted, "We're seeing a hint of exactly the opposite of what I expected. Those who get a tube prophylactically are doing worse than those who are getting it reactively or not at all, If that's the case, that's a really important outcome."
As Petersen explained, the placement of PEG feeding tubes is a hot topic in head and neck cancer care. Malnutrition affects about 80% of these patients and can contribute to mortality, raising the question of whether they should have access to feeding tubes placed prior to treatment in case enteral nutrition is needed.
In some patients with head and neck cancer, malnutrition may arise when tumors block food intake or prevent patients from swallowing. "But in my clinical experience, most often it's from the adverse effects of radiation and chemotherapy. Radiation creates burns inside their throat that make it hard to swallow. Or they have taste changes or really dry mouth," Petersen said.
"On top of these problems, chemotherapy can cause nausea and vomiting," she said. Placing feeding tube access may seem like a smart strategy to head off malnutrition as soon as it occurs. But, as Petersen noted, feeding tube use can lead to dependency as patients lose their ability to swallow. "There's a theory that if we give people feeding tubes, they'll go with the easier route of using a feeding tube and not keep swallowing. Then those swallowing muscles would weaken, and patients would end up permanently on a feeding tube."
In 2020, a retrospective VA study linked feeding tube dependence to lower overall survival in head and neck cancer patients. There are also risks to feeding tube placement, such as infection, pain, leakage, and inflammation.
But what if feeding tube valves are inserted prophylactically so they can be used for nutrition if needed? "We just haven't had any prospective studies to get to the heart of the matter and answer the question," she said. "It's hard to recruit. How do you convince somebody to randomly be assigned to have a hole poked in their stomach?"
For the new pilot study, researchers in Phoenix decided not to randomize patients. Instead, they asked them whether they'd accept the placement of feeding tube valves on a prophylactic basis.
Thirty-six veterans enrolled in 3 years, 33% of those were eligible. Twelve have died, 1 withdrew, and 2 were lost to follow-up.
Those in the prophylactic group had worse physical function and muscle strength over time, while those who received feeding tubes when needed had more adverse events.
Why might some outcomes be worse for patients who chose the prophylactic approach? "The answer is unclear," Petersen said. "Although one possibility is that those patients had higher-risk tumors and were more clued into their own risk."
"The goal now is to get funding for an expanded, multicenter study within the VA," Petersen said. The big question that she hopes to answer is: Does a prophylactic approach work? "Does it make a difference for patients in terms of how quickly they go back to living a full, meaningful life and be able to do all the things that they normally would do?"
A complete study would likely last 7 years, but helpful results may come earlier. "We are starting to see significant differences in terms of our main outcomes of physical function," Petersen said. "We only need 1 to 2 years of data for each patient to get to the heart of that."
The study is not funded, and Petersen reported no disclosures.
Research conducted at the US Department of Veterans Affairs (VA) could offer crucial insight into the hotly debated question of whether patients with head and neck cancer should have access to percutaneous endoscopic gastrostomy (PEG) before they develop malnutrition.
While no definitive conclusions can be drawn until a complete study is performed, early findings of a pilot trial are intriguing, said advanced practice oncology dietitian Katherine Petersen, MS, RDN, CSO, of the Phoenix VA Health Care System, who spoke in an interview with Federal Practitioner and at the annual meeting of the Association of VA Hematology/Oncology.
So far, the 12 patients with head and neck cancer who agreed to the placement of prophylactic feeding tubes prior to chemoradiation have had worse outcomes in some areas compared to the 9 patients who had tubes inserted when clinically indicated and the 12 who didn't need feeding tubes.
Petersen cautioned that the study is small and underpowered at this point. Still, she noted, "We're seeing a hint of exactly the opposite of what I expected. Those who get a tube prophylactically are doing worse than those who are getting it reactively or not at all, If that's the case, that's a really important outcome."
As Petersen explained, the placement of PEG feeding tubes is a hot topic in head and neck cancer care. Malnutrition affects about 80% of these patients and can contribute to mortality, raising the question of whether they should have access to feeding tubes placed prior to treatment in case enteral nutrition is needed.
In some patients with head and neck cancer, malnutrition may arise when tumors block food intake or prevent patients from swallowing. "But in my clinical experience, most often it's from the adverse effects of radiation and chemotherapy. Radiation creates burns inside their throat that make it hard to swallow. Or they have taste changes or really dry mouth," Petersen said.
"On top of these problems, chemotherapy can cause nausea and vomiting," she said. Placing feeding tube access may seem like a smart strategy to head off malnutrition as soon as it occurs. But, as Petersen noted, feeding tube use can lead to dependency as patients lose their ability to swallow. "There's a theory that if we give people feeding tubes, they'll go with the easier route of using a feeding tube and not keep swallowing. Then those swallowing muscles would weaken, and patients would end up permanently on a feeding tube."
In 2020, a retrospective VA study linked feeding tube dependence to lower overall survival in head and neck cancer patients. There are also risks to feeding tube placement, such as infection, pain, leakage, and inflammation.
But what if feeding tube valves are inserted prophylactically so they can be used for nutrition if needed? "We just haven't had any prospective studies to get to the heart of the matter and answer the question," she said. "It's hard to recruit. How do you convince somebody to randomly be assigned to have a hole poked in their stomach?"
For the new pilot study, researchers in Phoenix decided not to randomize patients. Instead, they asked them whether they'd accept the placement of feeding tube valves on a prophylactic basis.
Thirty-six veterans enrolled in 3 years, 33% of those were eligible. Twelve have died, 1 withdrew, and 2 were lost to follow-up.
Those in the prophylactic group had worse physical function and muscle strength over time, while those who received feeding tubes when needed had more adverse events.
Why might some outcomes be worse for patients who chose the prophylactic approach? "The answer is unclear," Petersen said. "Although one possibility is that those patients had higher-risk tumors and were more clued into their own risk."
"The goal now is to get funding for an expanded, multicenter study within the VA," Petersen said. The big question that she hopes to answer is: Does a prophylactic approach work? "Does it make a difference for patients in terms of how quickly they go back to living a full, meaningful life and be able to do all the things that they normally would do?"
A complete study would likely last 7 years, but helpful results may come earlier. "We are starting to see significant differences in terms of our main outcomes of physical function," Petersen said. "We only need 1 to 2 years of data for each patient to get to the heart of that."
The study is not funded, and Petersen reported no disclosures.
Commentary: PsA Targeted Therapy Trials, October 2024
Matching adjusted indirect comparison is one method of evaluating comparative effectiveness. To compare the efficacy between bimekizumab, an interleukin (IL) 17A/F inhibitor and risankizumab, an IL-23 inhibitor, Mease et al conducted such a study using data from four phase 3 trials (BE OPTIMAL, BE COMPLETE, KEEPsAKE-1, and KEEPsAKE-2) involving patients who were biologic-naive or inadequate responders to tumour necrosis factor (TNF) inhibitors who received bimekizumab (n = 698) or risankizumab (n = 589).1
At week 52, bimekizumab led to a higher likelihood of achieving a ≥ 70% improvement in the American College of Rheumatology (ACR) response in patients who were biologic-naive and TNF inhibitor inadequate responders (TNFi-IR), compared with risankizumab. Bimekizumab also had greater odds of achieving minimal disease activity in patients who were TNFi-IR. Thus, bimekizumab may be superior to risankizumab for treating those with PsA. Randomized controlled head-to-head clinical trials are required to confirm these findings.
In regard to long-term safety and efficacy of bimekizumab, Mease et al reported that bimekizumab demonstrated consistent safety and sustained efficacy for up to 2 years in patients with PsA.2 In this open-label extension (BE VITAL) of two phase 3 trials that included biologic-naive (n = 852) and TNFi-IR (n = 400) patients with PsA who were randomly assigned to receive bimekizumab, placebo with crossover to bimekizumab at week 16, or adalimumab followed by bimekizumab at week 52, no new safety signals were noted from weeks 52 to 104,. SARS-CoV-2 infection was the most common treatment-emergent adverse event. Approximately 50% of biologic-naive and TNFi-IR patients maintained a 50% or greater improvement in the ACR response.
Guselkumab, another IL-23 inhibitor, has proven efficacy in treating PsA. Curtis et al investigated the impact of early achievement of improvement with guselkumab and longer-term outcomes.3 This was a post hoc analysis of two phase 3 trials, DISCOVER-1 and DISCOVER-2, which included 1120 patients with active PsA who received guselkumab every 4 or 8 weeks (Q4W) or placebo with a crossover to guselkumab Q4W at week 24. The study demonstrated that guselkumab led to early achievement of minimal clinically important improvement (MCII) in clinical disease activity index for PsA (cDAPSA), with higher response rates at week 4 compared with placebo. Moreover, achieving early MCII in cDAPSA was associated with sustained disease control at weeks 24 and 52. Thus, guselkumab treatment achieved MCII in cDAPSA after the first dose and sustained disease control for up to 1 year. Early treatment response and a proven safety record make guselkumab an attractive treatment option for PsA.
PsA clinical trials mostly include patients with polyarthritis. Little is known about treatment efficacy for oligoarticular PsA. To address this gap in knowledge, Gossec et al reported the results of the phase 4 FOREMOST trial that included 308 patients with early (symptom duration 5 years or less) targeted therapy–naive oligoarticular PsA and were randomly assigned to receive apremilast (n = 203) or placebo (n = 105).4 At week 16, a higher proportion of patients receiving apremilast achieved minimal disease activity (joints response) compared with those receiving placebo. No new safety signals were reported. Apremilast is thus efficacious in treating early oligoarticular PsA as well as polyarticular PsA and psoriasis. Similar studies with other targeted therapies will help clinicians better manage early oligoarticular PsA.
References
- Mease PJ, Warren RB, Nash P, et al. Comparative effectiveness of bimekizumab and risankizumab in patients with psoriatic arthritis at 52 weeks assessed using a matching-adjusted indirect comparison. Rheumatol Ther. 2024 Aug 9. Source
- Mease PJ, Merola JF, Tanaka Y, et al. Safety and efficacy of bimekizumab in patients with psoriatic arthritis: 2-year results from two phase 3 studies. Rheumatol Ther. 2024 Aug 31. Source
- Curtis JR, et al. Early improvements with guselkumab associate with sustained control of psoriatic arthritis: post hoc analyses of two phase 3 trials. Rheumatol Ther. 2024 Sep 11. Source
- Gossec L, Coates LC, Gladman DD, et al. Treatment of early oligoarticular psoriatic arthritis with apremilast: primary outcomes at week 16 from the FOREMOST randomised controlled trial. Ann Rheum Dis. 2024 Sep 16:ard-2024-225833. Source
Matching adjusted indirect comparison is one method of evaluating comparative effectiveness. To compare the efficacy between bimekizumab, an interleukin (IL) 17A/F inhibitor and risankizumab, an IL-23 inhibitor, Mease et al conducted such a study using data from four phase 3 trials (BE OPTIMAL, BE COMPLETE, KEEPsAKE-1, and KEEPsAKE-2) involving patients who were biologic-naive or inadequate responders to tumour necrosis factor (TNF) inhibitors who received bimekizumab (n = 698) or risankizumab (n = 589).1
At week 52, bimekizumab led to a higher likelihood of achieving a ≥ 70% improvement in the American College of Rheumatology (ACR) response in patients who were biologic-naive and TNF inhibitor inadequate responders (TNFi-IR), compared with risankizumab. Bimekizumab also had greater odds of achieving minimal disease activity in patients who were TNFi-IR. Thus, bimekizumab may be superior to risankizumab for treating those with PsA. Randomized controlled head-to-head clinical trials are required to confirm these findings.
In regard to long-term safety and efficacy of bimekizumab, Mease et al reported that bimekizumab demonstrated consistent safety and sustained efficacy for up to 2 years in patients with PsA.2 In this open-label extension (BE VITAL) of two phase 3 trials that included biologic-naive (n = 852) and TNFi-IR (n = 400) patients with PsA who were randomly assigned to receive bimekizumab, placebo with crossover to bimekizumab at week 16, or adalimumab followed by bimekizumab at week 52, no new safety signals were noted from weeks 52 to 104,. SARS-CoV-2 infection was the most common treatment-emergent adverse event. Approximately 50% of biologic-naive and TNFi-IR patients maintained a 50% or greater improvement in the ACR response.
Guselkumab, another IL-23 inhibitor, has proven efficacy in treating PsA. Curtis et al investigated the impact of early achievement of improvement with guselkumab and longer-term outcomes.3 This was a post hoc analysis of two phase 3 trials, DISCOVER-1 and DISCOVER-2, which included 1120 patients with active PsA who received guselkumab every 4 or 8 weeks (Q4W) or placebo with a crossover to guselkumab Q4W at week 24. The study demonstrated that guselkumab led to early achievement of minimal clinically important improvement (MCII) in clinical disease activity index for PsA (cDAPSA), with higher response rates at week 4 compared with placebo. Moreover, achieving early MCII in cDAPSA was associated with sustained disease control at weeks 24 and 52. Thus, guselkumab treatment achieved MCII in cDAPSA after the first dose and sustained disease control for up to 1 year. Early treatment response and a proven safety record make guselkumab an attractive treatment option for PsA.
PsA clinical trials mostly include patients with polyarthritis. Little is known about treatment efficacy for oligoarticular PsA. To address this gap in knowledge, Gossec et al reported the results of the phase 4 FOREMOST trial that included 308 patients with early (symptom duration 5 years or less) targeted therapy–naive oligoarticular PsA and were randomly assigned to receive apremilast (n = 203) or placebo (n = 105).4 At week 16, a higher proportion of patients receiving apremilast achieved minimal disease activity (joints response) compared with those receiving placebo. No new safety signals were reported. Apremilast is thus efficacious in treating early oligoarticular PsA as well as polyarticular PsA and psoriasis. Similar studies with other targeted therapies will help clinicians better manage early oligoarticular PsA.
References
- Mease PJ, Warren RB, Nash P, et al. Comparative effectiveness of bimekizumab and risankizumab in patients with psoriatic arthritis at 52 weeks assessed using a matching-adjusted indirect comparison. Rheumatol Ther. 2024 Aug 9. Source
- Mease PJ, Merola JF, Tanaka Y, et al. Safety and efficacy of bimekizumab in patients with psoriatic arthritis: 2-year results from two phase 3 studies. Rheumatol Ther. 2024 Aug 31. Source
- Curtis JR, et al. Early improvements with guselkumab associate with sustained control of psoriatic arthritis: post hoc analyses of two phase 3 trials. Rheumatol Ther. 2024 Sep 11. Source
- Gossec L, Coates LC, Gladman DD, et al. Treatment of early oligoarticular psoriatic arthritis with apremilast: primary outcomes at week 16 from the FOREMOST randomised controlled trial. Ann Rheum Dis. 2024 Sep 16:ard-2024-225833. Source
Matching adjusted indirect comparison is one method of evaluating comparative effectiveness. To compare the efficacy between bimekizumab, an interleukin (IL) 17A/F inhibitor and risankizumab, an IL-23 inhibitor, Mease et al conducted such a study using data from four phase 3 trials (BE OPTIMAL, BE COMPLETE, KEEPsAKE-1, and KEEPsAKE-2) involving patients who were biologic-naive or inadequate responders to tumour necrosis factor (TNF) inhibitors who received bimekizumab (n = 698) or risankizumab (n = 589).1
At week 52, bimekizumab led to a higher likelihood of achieving a ≥ 70% improvement in the American College of Rheumatology (ACR) response in patients who were biologic-naive and TNF inhibitor inadequate responders (TNFi-IR), compared with risankizumab. Bimekizumab also had greater odds of achieving minimal disease activity in patients who were TNFi-IR. Thus, bimekizumab may be superior to risankizumab for treating those with PsA. Randomized controlled head-to-head clinical trials are required to confirm these findings.
In regard to long-term safety and efficacy of bimekizumab, Mease et al reported that bimekizumab demonstrated consistent safety and sustained efficacy for up to 2 years in patients with PsA.2 In this open-label extension (BE VITAL) of two phase 3 trials that included biologic-naive (n = 852) and TNFi-IR (n = 400) patients with PsA who were randomly assigned to receive bimekizumab, placebo with crossover to bimekizumab at week 16, or adalimumab followed by bimekizumab at week 52, no new safety signals were noted from weeks 52 to 104,. SARS-CoV-2 infection was the most common treatment-emergent adverse event. Approximately 50% of biologic-naive and TNFi-IR patients maintained a 50% or greater improvement in the ACR response.
Guselkumab, another IL-23 inhibitor, has proven efficacy in treating PsA. Curtis et al investigated the impact of early achievement of improvement with guselkumab and longer-term outcomes.3 This was a post hoc analysis of two phase 3 trials, DISCOVER-1 and DISCOVER-2, which included 1120 patients with active PsA who received guselkumab every 4 or 8 weeks (Q4W) or placebo with a crossover to guselkumab Q4W at week 24. The study demonstrated that guselkumab led to early achievement of minimal clinically important improvement (MCII) in clinical disease activity index for PsA (cDAPSA), with higher response rates at week 4 compared with placebo. Moreover, achieving early MCII in cDAPSA was associated with sustained disease control at weeks 24 and 52. Thus, guselkumab treatment achieved MCII in cDAPSA after the first dose and sustained disease control for up to 1 year. Early treatment response and a proven safety record make guselkumab an attractive treatment option for PsA.
PsA clinical trials mostly include patients with polyarthritis. Little is known about treatment efficacy for oligoarticular PsA. To address this gap in knowledge, Gossec et al reported the results of the phase 4 FOREMOST trial that included 308 patients with early (symptom duration 5 years or less) targeted therapy–naive oligoarticular PsA and were randomly assigned to receive apremilast (n = 203) or placebo (n = 105).4 At week 16, a higher proportion of patients receiving apremilast achieved minimal disease activity (joints response) compared with those receiving placebo. No new safety signals were reported. Apremilast is thus efficacious in treating early oligoarticular PsA as well as polyarticular PsA and psoriasis. Similar studies with other targeted therapies will help clinicians better manage early oligoarticular PsA.
References
- Mease PJ, Warren RB, Nash P, et al. Comparative effectiveness of bimekizumab and risankizumab in patients with psoriatic arthritis at 52 weeks assessed using a matching-adjusted indirect comparison. Rheumatol Ther. 2024 Aug 9. Source
- Mease PJ, Merola JF, Tanaka Y, et al. Safety and efficacy of bimekizumab in patients with psoriatic arthritis: 2-year results from two phase 3 studies. Rheumatol Ther. 2024 Aug 31. Source
- Curtis JR, et al. Early improvements with guselkumab associate with sustained control of psoriatic arthritis: post hoc analyses of two phase 3 trials. Rheumatol Ther. 2024 Sep 11. Source
- Gossec L, Coates LC, Gladman DD, et al. Treatment of early oligoarticular psoriatic arthritis with apremilast: primary outcomes at week 16 from the FOREMOST randomised controlled trial. Ann Rheum Dis. 2024 Sep 16:ard-2024-225833. Source
A Rare Case of a Splenic Abscess as the Origin of Illness in Exudative Pleural Effusion
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6
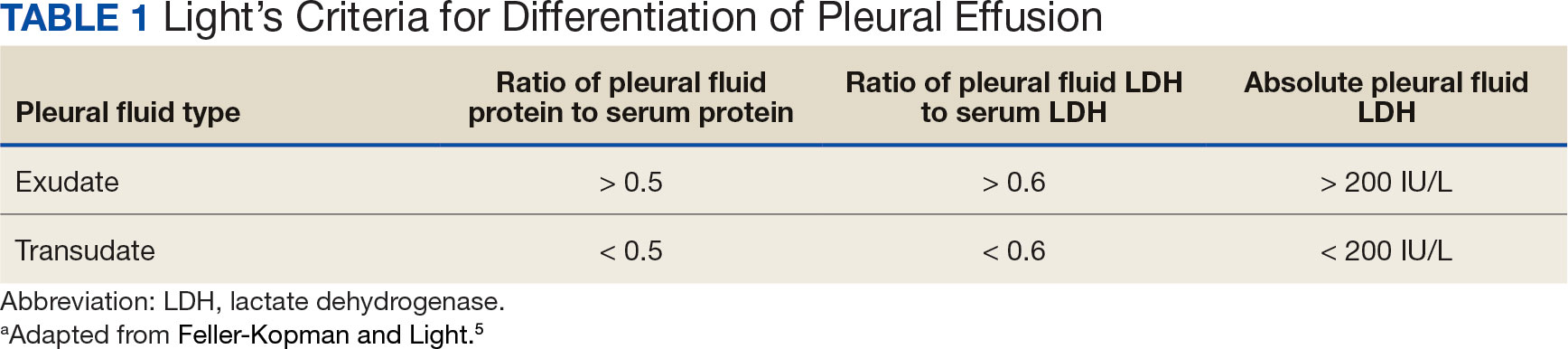
A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).
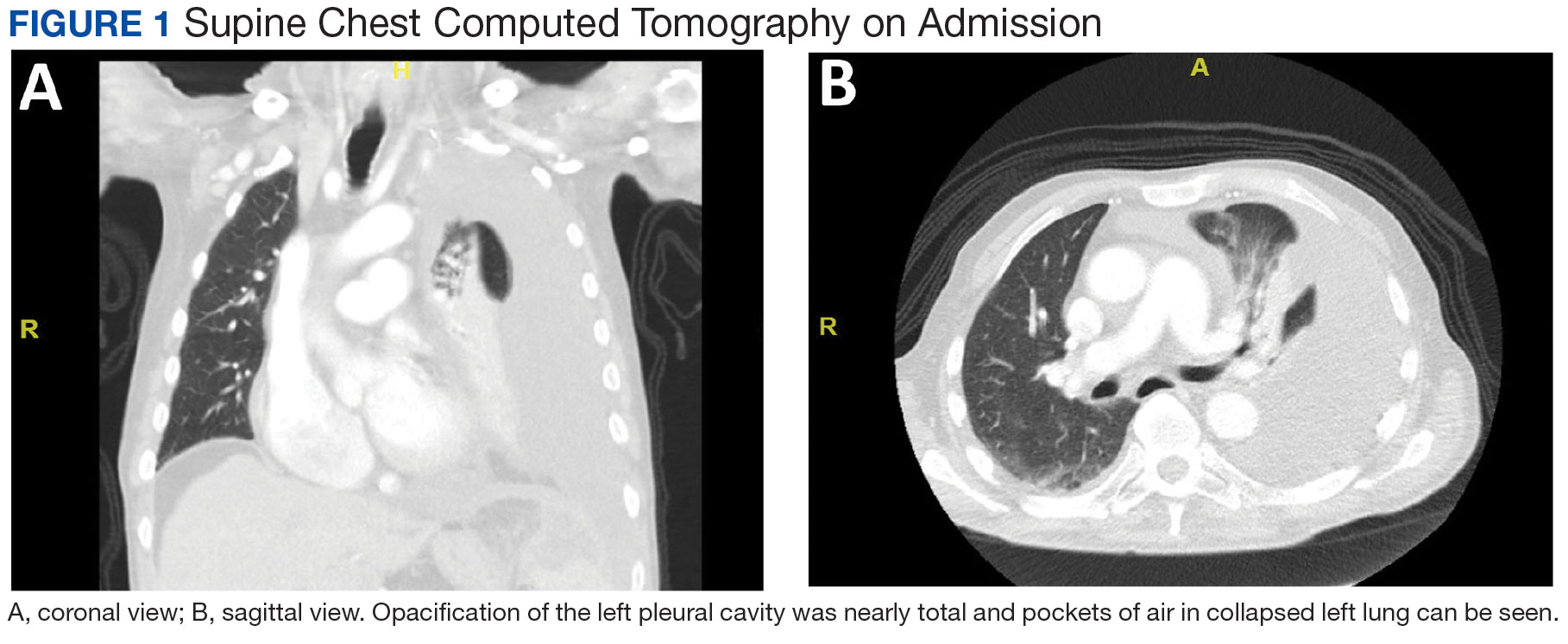
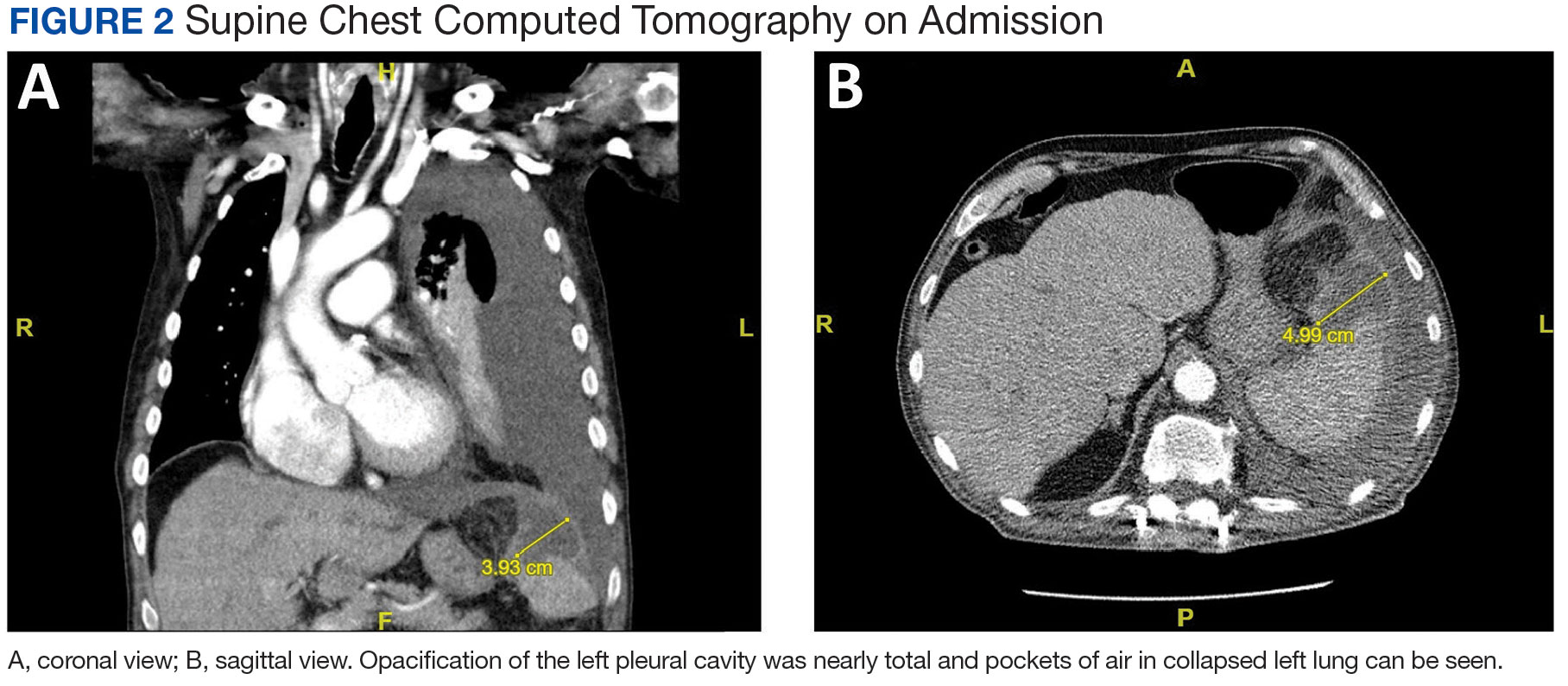
Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.
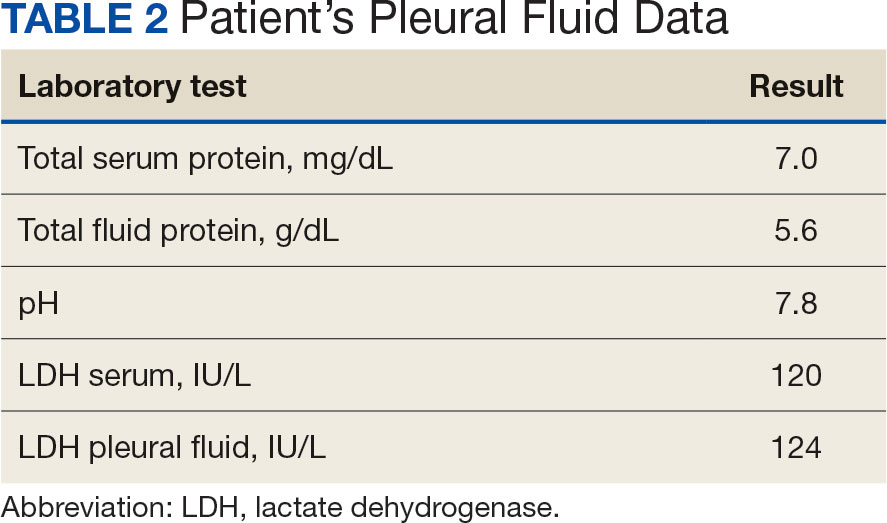
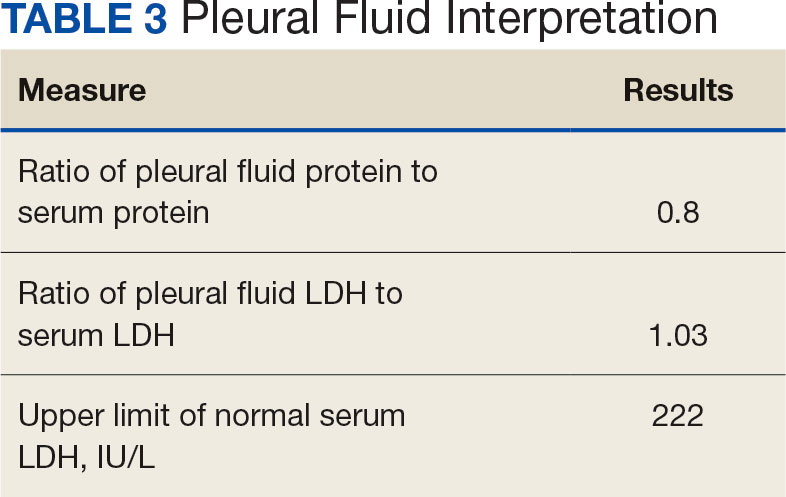
The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6

A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).


Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.


The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6

A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).


Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.


The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
Inspection of Deep Tumor Margins for Accurate Cutaneous Squamous Cell Carcinoma Staging
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.
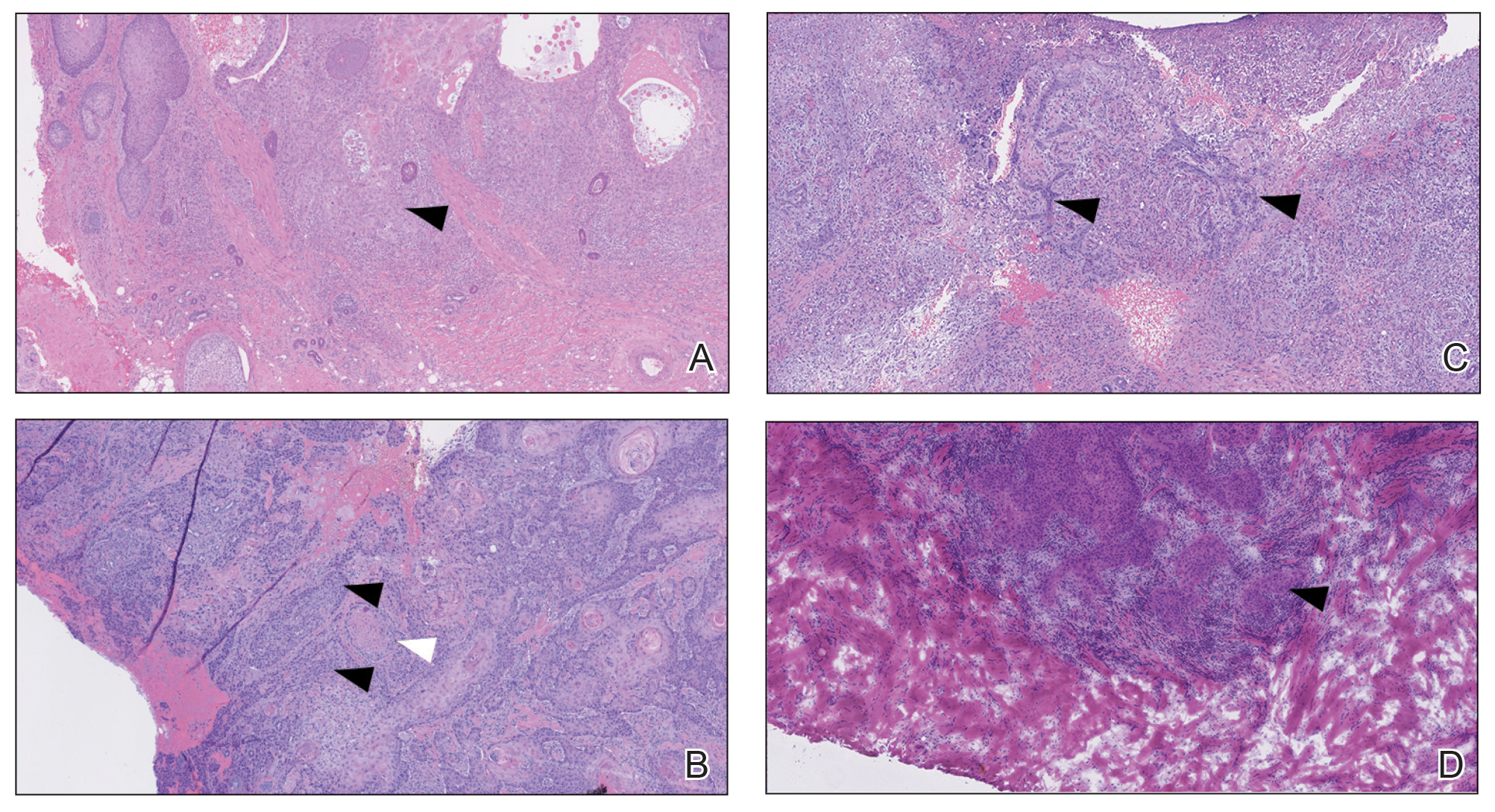
A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.
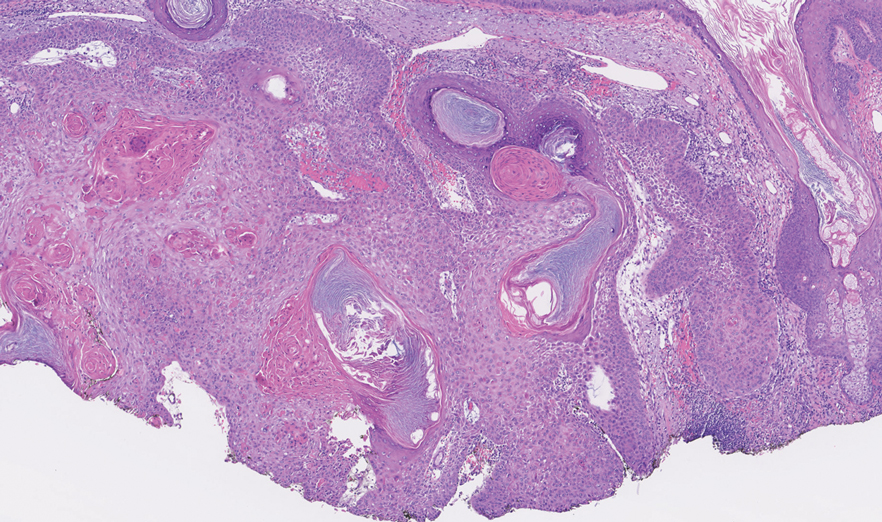
Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.

A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.

Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.

A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.

Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
Practice Points
- A proportion of cutaneous squamous cell carcinomas are upgraded from the initial biopsy during Mohs micrographic surgery due to evidence of perineural invasion, bony invasion, or lesser differentiation noted on Mohs stages or debulk analysis.
- Thorough inspection of the deep tumor margins may be required for accurate tumor staging and evaluation of metastatic risk. Cells at the deep margin of the tumor may demonstrate poorer differentiation and/or other higher-risk tumor features than those closer to the surface.
- Tumor staging may be incomplete until the deep margins are assessed to find the most dysplastic and likely clinically relevant cells, which may be missed without evaluation of the debulked tumor.
Reflectance Confocal Microscopy as a Diagnostic Aid in Allergic Contact Dermatitis to Mango Sap
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.
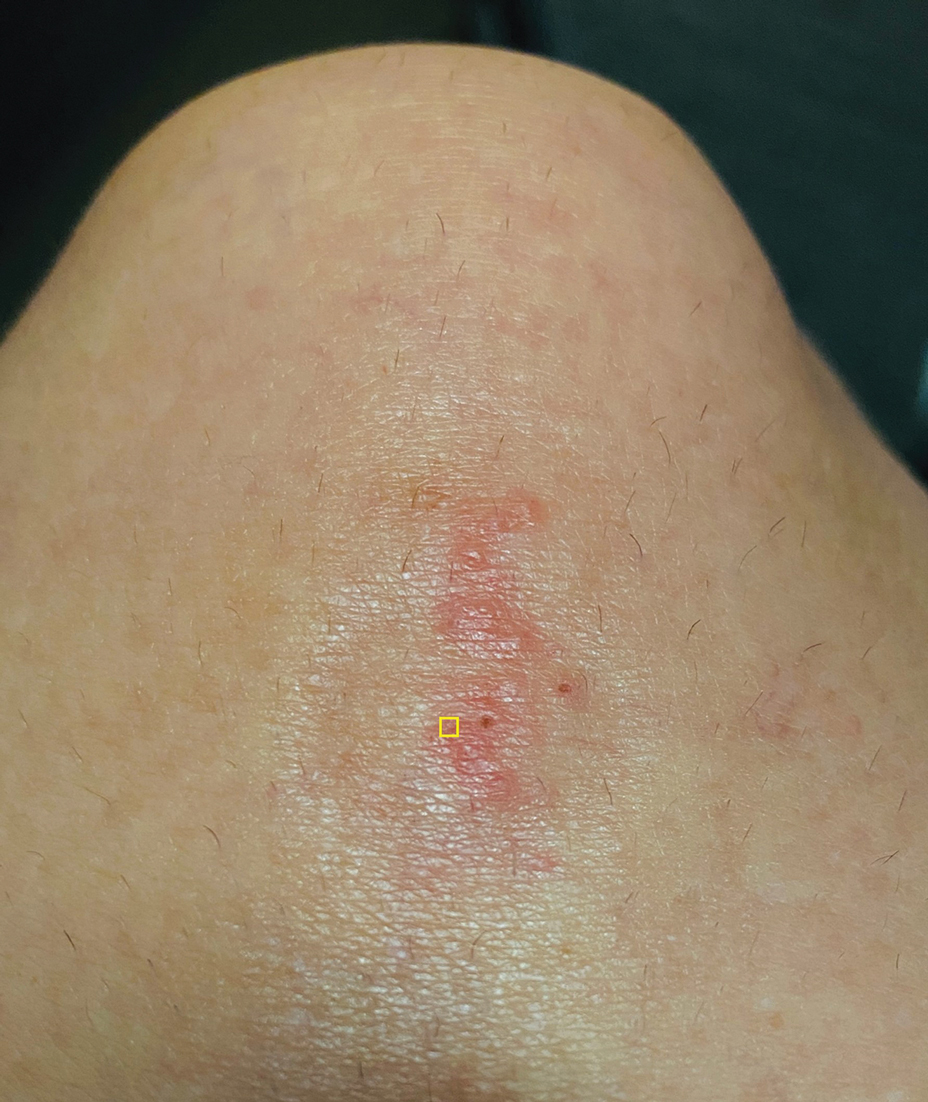
The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.
Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
Practice Points
- Contact with mango tree sap can induce allergic contact dermatitis.
- Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that can provide real-time in vivo visualization of affected skin in contact dermatitis.
- Predominant findings of contact dermatitis under RCM include disruption of the stratum corneum; parakeratosis; vesiculation; spongiosis; and exocytosis, vasodilation, and intercellular edema more specific to the allergic subtype.
Transient Eruption of Verrucous Keratoses During Encorafenib Therapy: Adverse Event or Paraneoplastic Phenomenon?
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.
Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.

Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.

Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
Practice Points
- Verrucous keratoses are common cutaneous adverse events (AEs) associated with BRAF inhibitor therapy.
- Verrucous papules may be a paraneoplastic phenomenon and can be differentiated from a treatment-related AE based on the timing and progression in relation to tumor burden.
- Although treatment of particularly bothersome lesions with cryotherapy may be warranted, verrucous papules secondary to BRAF inhibitor therapy may resolve spontaneously.