User login
How useful is CRP as a marker for assessing disease activity in PsA?
Key clinical point: Levels of C-reactive protein (CRP), a classic inflammatory marker, do not adequately reflect the level of disease activity (DA) in patients with psoriatic arthritis (PsA).
Major finding: CRP status was only associated with the erythrocyte sedimentation rate (odds ratio 1.04; P = .005) and not with any DA-related parameter. Among patients with normal CRP levels, a substantial proportion (45.9%) of patients were in non-minimal DA state, with 21.2% having moderate DA and 5.9% having high DA.
Study details: Findings are from a cross-sectional study including 128 patients with PsA, of which 66.4% had normal CRP levels (≤0.5 mg/dL) and the remaining had high CRP levels (>0.5 mg/dL).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Gialouri CG et al. Normal C-reactive protein in active psoriatic arthritis: Results from real-world clinical practice. Ther Adv Musculoskelet Dis. 2022 (Sep 5). Doi: 10.1177/1759720X221122417
Key clinical point: Levels of C-reactive protein (CRP), a classic inflammatory marker, do not adequately reflect the level of disease activity (DA) in patients with psoriatic arthritis (PsA).
Major finding: CRP status was only associated with the erythrocyte sedimentation rate (odds ratio 1.04; P = .005) and not with any DA-related parameter. Among patients with normal CRP levels, a substantial proportion (45.9%) of patients were in non-minimal DA state, with 21.2% having moderate DA and 5.9% having high DA.
Study details: Findings are from a cross-sectional study including 128 patients with PsA, of which 66.4% had normal CRP levels (≤0.5 mg/dL) and the remaining had high CRP levels (>0.5 mg/dL).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Gialouri CG et al. Normal C-reactive protein in active psoriatic arthritis: Results from real-world clinical practice. Ther Adv Musculoskelet Dis. 2022 (Sep 5). Doi: 10.1177/1759720X221122417
Key clinical point: Levels of C-reactive protein (CRP), a classic inflammatory marker, do not adequately reflect the level of disease activity (DA) in patients with psoriatic arthritis (PsA).
Major finding: CRP status was only associated with the erythrocyte sedimentation rate (odds ratio 1.04; P = .005) and not with any DA-related parameter. Among patients with normal CRP levels, a substantial proportion (45.9%) of patients were in non-minimal DA state, with 21.2% having moderate DA and 5.9% having high DA.
Study details: Findings are from a cross-sectional study including 128 patients with PsA, of which 66.4% had normal CRP levels (≤0.5 mg/dL) and the remaining had high CRP levels (>0.5 mg/dL).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Gialouri CG et al. Normal C-reactive protein in active psoriatic arthritis: Results from real-world clinical practice. Ther Adv Musculoskelet Dis. 2022 (Sep 5). Doi: 10.1177/1759720X221122417
How useful is CRP as a marker for assessing disease activity in PsA?
Key clinical point: Levels of C-reactive protein (CRP), a classic inflammatory marker, do not adequately reflect the level of disease activity (DA) in patients with psoriatic arthritis (PsA).
Major finding: CRP status was only associated with the erythrocyte sedimentation rate (odds ratio 1.04; P = .005) and not with any DA-related parameter. Among patients with normal CRP levels, a substantial proportion (45.9%) of patients were in non-minimal DA state, with 21.2% having moderate DA and 5.9% having high DA.
Study details: Findings are from a cross-sectional study including 128 patients with PsA, of which 66.4% had normal CRP levels (≤0.5mg/dL) and the remaining had high CRP levels (>0.5mg/dL).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Gialouri CG et al. Normal C-reactive protein in active psoriatic arthritis: Results from real-world clinical practice. Ther Adv Musculoskelet Dis. 2022 (Sep 5). Doi: 10.1177/1759720X221122417
Key clinical point: Levels of C-reactive protein (CRP), a classic inflammatory marker, do not adequately reflect the level of disease activity (DA) in patients with psoriatic arthritis (PsA).
Major finding: CRP status was only associated with the erythrocyte sedimentation rate (odds ratio 1.04; P = .005) and not with any DA-related parameter. Among patients with normal CRP levels, a substantial proportion (45.9%) of patients were in non-minimal DA state, with 21.2% having moderate DA and 5.9% having high DA.
Study details: Findings are from a cross-sectional study including 128 patients with PsA, of which 66.4% had normal CRP levels (≤0.5mg/dL) and the remaining had high CRP levels (>0.5mg/dL).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Gialouri CG et al. Normal C-reactive protein in active psoriatic arthritis: Results from real-world clinical practice. Ther Adv Musculoskelet Dis. 2022 (Sep 5). Doi: 10.1177/1759720X221122417
Key clinical point: Levels of C-reactive protein (CRP), a classic inflammatory marker, do not adequately reflect the level of disease activity (DA) in patients with psoriatic arthritis (PsA).
Major finding: CRP status was only associated with the erythrocyte sedimentation rate (odds ratio 1.04; P = .005) and not with any DA-related parameter. Among patients with normal CRP levels, a substantial proportion (45.9%) of patients were in non-minimal DA state, with 21.2% having moderate DA and 5.9% having high DA.
Study details: Findings are from a cross-sectional study including 128 patients with PsA, of which 66.4% had normal CRP levels (≤0.5mg/dL) and the remaining had high CRP levels (>0.5mg/dL).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Gialouri CG et al. Normal C-reactive protein in active psoriatic arthritis: Results from real-world clinical practice. Ther Adv Musculoskelet Dis. 2022 (Sep 5). Doi: 10.1177/1759720X221122417
Rapid and sustained improvement in PsA with ustekinumab
Key clinical point: Patients with active psoriatic arthritis (PsA) who received ustekinumab for 160 weeks showed rapid and sustained improvements in the signs and symptoms of PsA.
Major finding: A decrease in both tender joint count (TJC; 8.0 to 5.8) and swollen joint count (SJC; 4.5 to 3.1) was observed from baseline to week 4, with further decrease until week 28. Improvements were sustained up to week 160 (TJC 1.0; SJC 0.4). Minimal disease activity was achieved by 31.9% of patients at week 28 and 33.6% of patients at week 52.
Study details: Findings are from the prospective, non-interventional SUSTAIN study including 129 patients with active PsA and a previous inadequate response to disease-modifying antirheumatic drugs who were followed up to week 160.
Disclosures: The SUSTAIN study was funded by Janssen-Cilag GmbH. Three authors declared being employees of Janssen-Cilag GmbH and holding stocks in Johnson and Johnson, the parent company of Janssen. The other authors declared no conflicts of interest.
Source: Wendler J et al. Ustekinumab is rapid-acting and is an effective long-term treatment for patients with active psoriatic arthritis: Real-world evidence from the non-interventional SUSTAIN study. Rheumatol Ther. 2022 (Sep 6). Doi: 10.1007/s40744-022-00484-3
Key clinical point: Patients with active psoriatic arthritis (PsA) who received ustekinumab for 160 weeks showed rapid and sustained improvements in the signs and symptoms of PsA.
Major finding: A decrease in both tender joint count (TJC; 8.0 to 5.8) and swollen joint count (SJC; 4.5 to 3.1) was observed from baseline to week 4, with further decrease until week 28. Improvements were sustained up to week 160 (TJC 1.0; SJC 0.4). Minimal disease activity was achieved by 31.9% of patients at week 28 and 33.6% of patients at week 52.
Study details: Findings are from the prospective, non-interventional SUSTAIN study including 129 patients with active PsA and a previous inadequate response to disease-modifying antirheumatic drugs who were followed up to week 160.
Disclosures: The SUSTAIN study was funded by Janssen-Cilag GmbH. Three authors declared being employees of Janssen-Cilag GmbH and holding stocks in Johnson and Johnson, the parent company of Janssen. The other authors declared no conflicts of interest.
Source: Wendler J et al. Ustekinumab is rapid-acting and is an effective long-term treatment for patients with active psoriatic arthritis: Real-world evidence from the non-interventional SUSTAIN study. Rheumatol Ther. 2022 (Sep 6). Doi: 10.1007/s40744-022-00484-3
Key clinical point: Patients with active psoriatic arthritis (PsA) who received ustekinumab for 160 weeks showed rapid and sustained improvements in the signs and symptoms of PsA.
Major finding: A decrease in both tender joint count (TJC; 8.0 to 5.8) and swollen joint count (SJC; 4.5 to 3.1) was observed from baseline to week 4, with further decrease until week 28. Improvements were sustained up to week 160 (TJC 1.0; SJC 0.4). Minimal disease activity was achieved by 31.9% of patients at week 28 and 33.6% of patients at week 52.
Study details: Findings are from the prospective, non-interventional SUSTAIN study including 129 patients with active PsA and a previous inadequate response to disease-modifying antirheumatic drugs who were followed up to week 160.
Disclosures: The SUSTAIN study was funded by Janssen-Cilag GmbH. Three authors declared being employees of Janssen-Cilag GmbH and holding stocks in Johnson and Johnson, the parent company of Janssen. The other authors declared no conflicts of interest.
Source: Wendler J et al. Ustekinumab is rapid-acting and is an effective long-term treatment for patients with active psoriatic arthritis: Real-world evidence from the non-interventional SUSTAIN study. Rheumatol Ther. 2022 (Sep 6). Doi: 10.1007/s40744-022-00484-3
Isolated axial PsA and eventual development of peripheral disease
Key clinical point: Patients with isolated axial psoriatic arthritis (PsA) had a better functional status than those with both axial and peripheral disease. Human leucocyte antigen (HLA)-B*27 positivity was associated with isolated axial PsA and predicted the development of peripheral disease over time.
Major finding: Compared with patients with concomitant axial and peripheral disease, HLA-B*27 positivity (odds ratio [OR] 25.00; P < .003) and lower Health Assessment Questionnaire scores (OR 0.004; P < .01) were associated with isolated axial disease. HLA-B*27 positivity predicted the development of peripheral PsA in patients with isolated axial PsA (hazard ratio 7.544; P < .006).
Study details: Findings are from a longitudinal study including 1688 patients with ankylosing spondylitis and psoriasis and 1576 patients with PsA, of which 32 had isolated axial disease and 463 had axial with peripheral disease.
Disclosures: The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. The authors declared no conflicts of interest.
Source: Kwok TSH et al. Isolated axial disease in psoriatic arthritis and ankylosing spondylitis with psoriasis. Ann Rheum Dis. 2022 (Aug 16). Doi: 10.1136/ard-2022-222537
Key clinical point: Patients with isolated axial psoriatic arthritis (PsA) had a better functional status than those with both axial and peripheral disease. Human leucocyte antigen (HLA)-B*27 positivity was associated with isolated axial PsA and predicted the development of peripheral disease over time.
Major finding: Compared with patients with concomitant axial and peripheral disease, HLA-B*27 positivity (odds ratio [OR] 25.00; P < .003) and lower Health Assessment Questionnaire scores (OR 0.004; P < .01) were associated with isolated axial disease. HLA-B*27 positivity predicted the development of peripheral PsA in patients with isolated axial PsA (hazard ratio 7.544; P < .006).
Study details: Findings are from a longitudinal study including 1688 patients with ankylosing spondylitis and psoriasis and 1576 patients with PsA, of which 32 had isolated axial disease and 463 had axial with peripheral disease.
Disclosures: The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. The authors declared no conflicts of interest.
Source: Kwok TSH et al. Isolated axial disease in psoriatic arthritis and ankylosing spondylitis with psoriasis. Ann Rheum Dis. 2022 (Aug 16). Doi: 10.1136/ard-2022-222537
Key clinical point: Patients with isolated axial psoriatic arthritis (PsA) had a better functional status than those with both axial and peripheral disease. Human leucocyte antigen (HLA)-B*27 positivity was associated with isolated axial PsA and predicted the development of peripheral disease over time.
Major finding: Compared with patients with concomitant axial and peripheral disease, HLA-B*27 positivity (odds ratio [OR] 25.00; P < .003) and lower Health Assessment Questionnaire scores (OR 0.004; P < .01) were associated with isolated axial disease. HLA-B*27 positivity predicted the development of peripheral PsA in patients with isolated axial PsA (hazard ratio 7.544; P < .006).
Study details: Findings are from a longitudinal study including 1688 patients with ankylosing spondylitis and psoriasis and 1576 patients with PsA, of which 32 had isolated axial disease and 463 had axial with peripheral disease.
Disclosures: The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. The authors declared no conflicts of interest.
Source: Kwok TSH et al. Isolated axial disease in psoriatic arthritis and ankylosing spondylitis with psoriasis. Ann Rheum Dis. 2022 (Aug 16). Doi: 10.1136/ard-2022-222537
Secukinumab delays disease flare in juvenile PsA
Key clinical point: Secukinumab significantly delayed disease flare compared with placebo and showed a consistent safety profile in patients with juvenile psoriatic arthritis (JPsA).
Major finding: Secukinumab vs placebo was associated with a significant delay in disease flare (hazard ratio 0.15; P < .001) and a higher proportion of patients achieving juvenile idiopathic arthritis American College of Rheumatology 30 response at week 104 (86.7% vs 62.5%). No new safety concerns were observed.
Study details: Findings are from a treatment-withdrawal, phase 3 study including 86 biologic-naive patients with active enthesitis-related arthritis (n = 52) or JPsA (n = 34) who were randomly assigned to receive secukinumab or placebo for up to 104 weeks.
Disclosures: This study was sponsored by Novartis Pharma AG. Four authors declared being employees and shareholders of Novartis. The other authors reported ties with several sources, including Novartis.
Source: Brunner HI et al on behalf of Paediatric Rheumatology INternational Trials Organization (PRINTO) and Pediatric Rheumatology Collaborative Study Group (PRCSG). Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: A randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann Rheum Dis. 2022 (Aug 12). Doi: 10.1136/ard-2022-222849
Key clinical point: Secukinumab significantly delayed disease flare compared with placebo and showed a consistent safety profile in patients with juvenile psoriatic arthritis (JPsA).
Major finding: Secukinumab vs placebo was associated with a significant delay in disease flare (hazard ratio 0.15; P < .001) and a higher proportion of patients achieving juvenile idiopathic arthritis American College of Rheumatology 30 response at week 104 (86.7% vs 62.5%). No new safety concerns were observed.
Study details: Findings are from a treatment-withdrawal, phase 3 study including 86 biologic-naive patients with active enthesitis-related arthritis (n = 52) or JPsA (n = 34) who were randomly assigned to receive secukinumab or placebo for up to 104 weeks.
Disclosures: This study was sponsored by Novartis Pharma AG. Four authors declared being employees and shareholders of Novartis. The other authors reported ties with several sources, including Novartis.
Source: Brunner HI et al on behalf of Paediatric Rheumatology INternational Trials Organization (PRINTO) and Pediatric Rheumatology Collaborative Study Group (PRCSG). Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: A randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann Rheum Dis. 2022 (Aug 12). Doi: 10.1136/ard-2022-222849
Key clinical point: Secukinumab significantly delayed disease flare compared with placebo and showed a consistent safety profile in patients with juvenile psoriatic arthritis (JPsA).
Major finding: Secukinumab vs placebo was associated with a significant delay in disease flare (hazard ratio 0.15; P < .001) and a higher proportion of patients achieving juvenile idiopathic arthritis American College of Rheumatology 30 response at week 104 (86.7% vs 62.5%). No new safety concerns were observed.
Study details: Findings are from a treatment-withdrawal, phase 3 study including 86 biologic-naive patients with active enthesitis-related arthritis (n = 52) or JPsA (n = 34) who were randomly assigned to receive secukinumab or placebo for up to 104 weeks.
Disclosures: This study was sponsored by Novartis Pharma AG. Four authors declared being employees and shareholders of Novartis. The other authors reported ties with several sources, including Novartis.
Source: Brunner HI et al on behalf of Paediatric Rheumatology INternational Trials Organization (PRINTO) and Pediatric Rheumatology Collaborative Study Group (PRCSG). Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: A randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann Rheum Dis. 2022 (Aug 12). Doi: 10.1136/ard-2022-222849
Improving Inpatient COVID-19 Vaccination Rates Among Adult Patients at a Tertiary Academic Medical Center
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.
In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
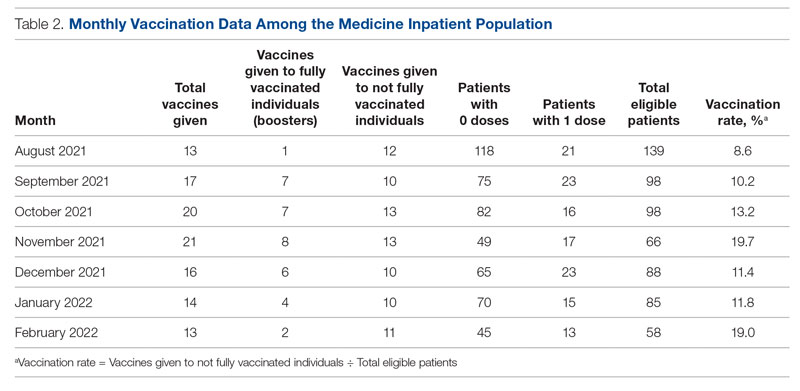
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.
Results
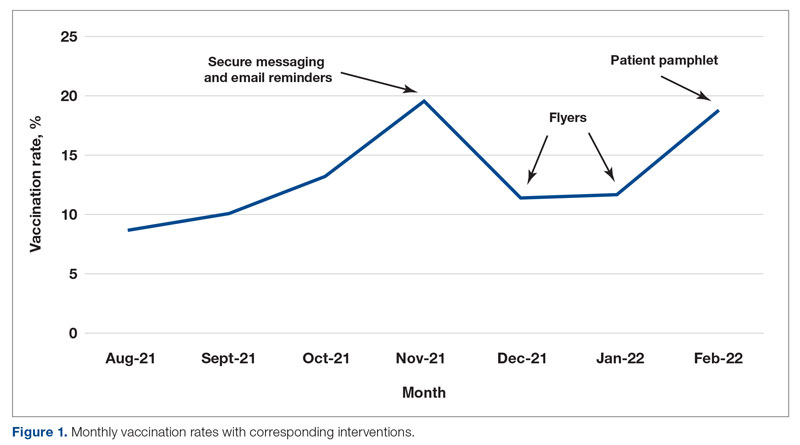
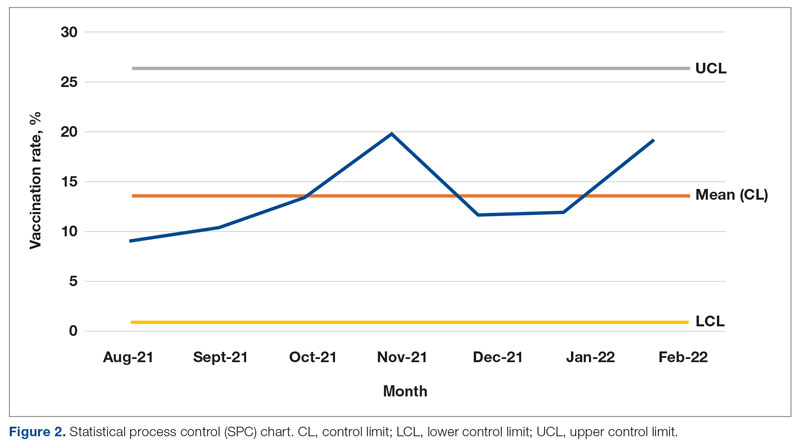
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).
Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
Diabetes Population Health Innovations in the Age of COVID-19: Insights From the T1D Exchange Quality Improvement Collaborative
From the T1D Exchange, Boston, MA (Ann Mungmode, Nicole Rioles, Jesse Cases, Dr. Ebekozien); The Leona M. and Harry B. Hemsley Charitable Trust, New York, NY (Laurel Koester); and the University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien).
Abstract
There have been remarkable innovations in diabetes management since the start of the COVID-19 pandemic, but these groundbreaking innovations are drawing limited focus as the field focuses on the adverse impact of the pandemic on patients with diabetes. This article reviews select population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of the T1D Exchange Quality Improvement Collaborative, a learning health network that focuses on improving care and outcomes for individuals with type 1 diabetes (T1D). Such innovations include expanded telemedicine access, collection of real-world data, machine learning and artificial intelligence, and new diabetes medications and devices. In addition, multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and advocacy efforts for specific populations have been successful. Looking to the future, work is required to explore additional health equity successes that do not further exacerbate inequities and to look for additional innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Keywords: type 1 diabetes, learning health network, continuous glucose monitoring, health equity
One in 10 people in the United States has diabetes.1 Diabetes is the nation’s second leading cause of death, costing the US health system more than $300 billion annually.2 The COVID-19 pandemic presented additional health burdens for people living with diabetes. For example, preexisting diabetes was identified as a risk factor for COVID-19–associated morbidity and mortality.3,4 Over the past 2 years, there have been remarkable innovations in diabetes management, including stem cell therapy and new medication options. Additionally, improved technology solutions have aided in diabetes management through continuous glucose monitors (CGM), smart insulin pens, advanced hybrid closed-loop systems, and continuous subcutaneous insulin injections.5,6 Unfortunately, these groundbreaking innovations are drawing limited focus, as the field is rightfully focused on the adverse impact of the pandemic on patients with diabetes.
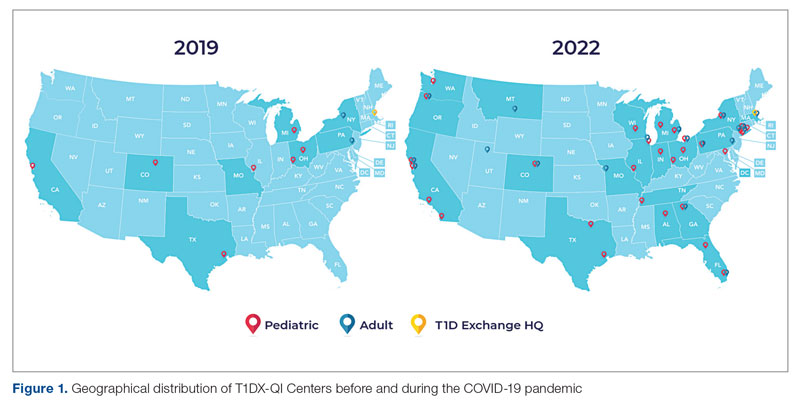
Learning health networks like the T1D Exchange Quality Improvement Collaborative (T1DX-QI) have implemented some of these innovative solutions to improve care for people with diabetes.7 T1DX-QI has more than 50 data-sharing endocrinology centers that care for over 75,000 people with diabetes across the United States (Figure 1). Centers participating in the T1DX-QI use quality improvement (QI) and implementation science methods to quickly translate research into evidence-based clinical practice. T1DX-QI leads diabetes population health and health system research and supports widespread transferability across health care organizations through regular collaborative calls, conferences, and case study documentation.8
In this review, we summarize impactful population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of T1DX-QI (see Figure 2 for relevant definitions). This review is limited in scope and is not meant to be an exhaustive list of innovations. The review also reflects significant changes from the perspective of academic diabetes centers, which may not apply to rural or primary care diabetes practices.
Methods
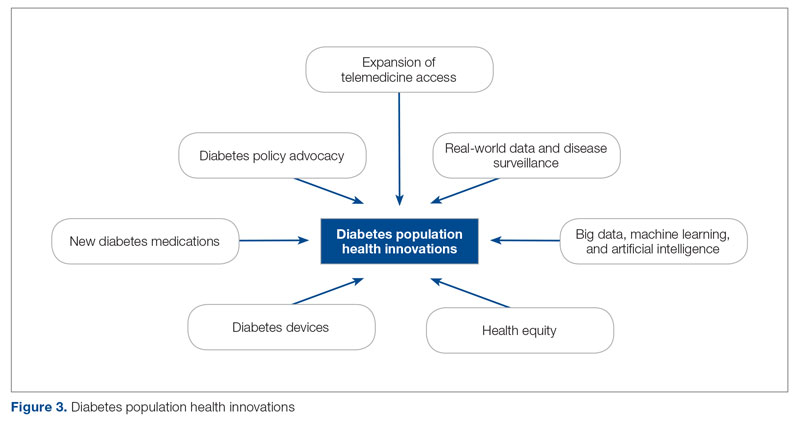
The first (A.M.), second (H.H.), and senior (O.E.) authors conducted a scoping review of published literature using terms related to diabetes, population health, and innovation on PubMed Central and Google Scholar for the period March 2020 to June 2022. To complement the review, A.M. and O.E. also reviewed abstracts from presentations at major international diabetes conferences, including the American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), the T1DX-QI Learning Session Conference, and the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 to 2022 conferences.9-14 The authors also searched FDA.gov and ClinicalTrials.gov for relevant insights. A.M. and O.E. sorted the reviewed literature into major themes (Figure 3) from the population health improvement perspective of the T1DX-QI.
Population Health Innovations in Diabetes Management
Expansion of Telemedicine Access
Telemedicine is cost-effective for patients with diabetes,15 including those with complex cases.16 Before the COVID-19 pandemic, telemedicine and virtual care were rare in diabetes management. However, the pandemic offered a new opportunity to expand the practice of telemedicine in diabetes management. A study from the T1DX-QI showed that telemedicine visits grew from comprising <1% of visits pre-pandemic (December 2019) to 95.2% during the pandemic (August 2020).17 Additional studies, like those conducted by Phillip et al,18 confirmed the noninferiority of telemedicine practice for patients with diabetes.Telemedicine was also found to be an effective strategy to educate patients on the use of diabetes technologies.19
Real-World Data and Disease Surveillance
As the COVID-19 pandemic exacerbated outcomes for people with type 1 diabetes (T1D), a need arose to understand the immediate effects of the pandemic on people with T1D through real-world data and disease surveillance. In April 2020, the T1DX-QI initiated a multicenter surveillance study to collect data and analyze the impact of COVID-19 on people with T1D. The existing health collaborative served as a springboard for robust surveillance study, documenting numerous works on the effects of COVID-19.3,4,20-28 Other investigators also embraced the power of real-world surveillance and real-world data.29,30
Big Data, Machine Learning, and Artificial Intelligence
The past 2 years have seen a shift toward embracing the incredible opportunity to tap the large volume of data generated from routine care for practical insights.31 In particular, researchers have demonstrated the widespread application of machine learning and artificial intelligence to improve diabetes management.32 The T1DX-QI also harnessed the growing power of big data by expanding the functionality of innovative benchmarking software. The T1DX QI Portal uses electronic medical record data of diabetes patients for clinic-to-clinic benchmarking and data analysis, using business intelligence solutions.33
Health Equity
While inequities across various health outcomes have been well documented for years,34 the COVID-19 pandemic further exaggerated racial/ethnic health inequities in T1D.23,35 In response, several organizations have outlined specific strategies to address these health inequities. Emboldened by the pandemic, the T1DX-QI announced a multipronged approach to address health inequities among patients with T1D through the Health Equity Advancement Lab (HEAL).36 One of HEAL’s main components is using real-world data to champion population-level insights and demonstrate progress in QI efforts.
Multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and these studies are expanding our understanding of the chasm.37 There have also been innovative solutions to addressing these inequities, with multiple studies published over the past 2 years.38 A source of inequity among patients with T1D is the lack of representation of racial/ethnic minorities with T1D in clinical trials.39 The T1DX-QI suggests that the equity-adapted framework for QI can be applied by research leaders to support trial diversity and representation, ensuring future device innovations are meaningful for all people with T1D.40
Diabetes Devices
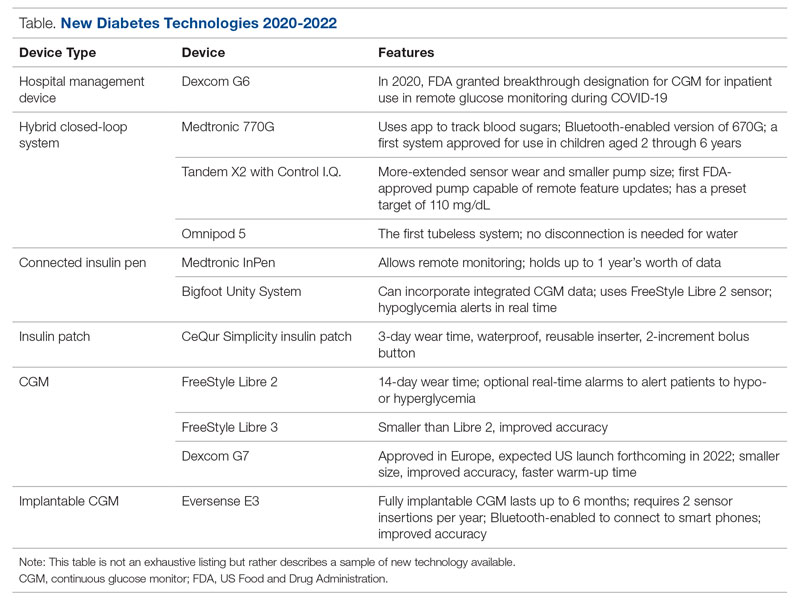
Glucose monitoring and insulin therapy are vital tools to support individuals living with T1D, and devices such as CGM and insulin pumps have become the standard of care for diabetes management (Table).41 Innovations in diabetes technology and device access are imperative for a chronic disease with no cure.
The COVID-19 pandemic created an opportunity to increase access to diabetes devices in inpatient settings. In 2020, the US Food and Drug Administration expanded the use of CGM to support remote monitoring of patients in inpatient hospital settings, simultaneously supporting the glucose monitoring needs of patients with T1D and reducing COVID-19 transmission through reduced patient-clinician contact.42 This effort has been expanded and will continue in 2022 and beyond,43 and aligns with the growing consensus that supports patients wearing both CGMs and insulin pumps in ambulatory settings to improve patient health outcomes.44
Since 2020, innovations in diabetes technology have improved and increased the variety of options available to people with T1D and made them easier to use (Table). New, advanced hybrid closed-loop systems have progressed to offer Bluetooth features, including automatic software upgrades, tubeless systems, and the ability to allow parents to use their smartphones to bolus for children.45-47 The next big step in insulin delivery innovation is the release of functioning, fully closed loop systems, of which several are currently in clinical trials.48 These systems support reduced hypoglycemia and improved time in range.49
Additional innovations in insulin delivery have improved the user experience and expanded therapeutic options, including a variety of smart insulin pens complete with dosing logs50,51 and even a patch to deliver insulin without the burden of injections.52 As barriers to diabetes technology persist,53 innovations in alternate insulin delivery provide people with T1D more options to align with their personal access and technology preferences.
Innovations in CGM address cited barriers to their use, including size or overall wear.53-55 CGMs released in the past few years are smaller in physical size, have longer durations of time between changings, are more accurate, and do not require calibrations for accuracy.
New Diabetes Medications
Many new medications and therapeutic advances have become available in the past 2 years.56 Additionally, more medications are being tested as adjunct therapies to support glycemic management in patients with T1D, including metformin, sodium-glucose cotransporter 1 and 2 inhibitors, pramlintide, glucagon-like polypeptide-1 analogs, and glucagon receptor agonists.57 Other recent advances include stem cell replacement therapy for patients with T1D.58 The ultra-long-acting biosimilar insulins are one medical innovation that has been stalled, rather than propelled, during the COVID-19 pandemic.59
Diabetes Policy Advocacy
People with T1D require insulin to survive. The cost of insulin has increased in recent years, with some studies citing a 64% to 100% increase in the past decade.60,61 In fact, 1 in 4 insulin users report that cost has impacted their insulin use, including rationing their insulin.62 Lockdowns during the COVID-19 pandemic stressed US families financially, increasing the urgency for insulin cost caps.
Although the COVID-19 pandemic halted national conversations on drug financing,63 advocacy efforts have succeeded for specific populations. The new Medicare Part D Senior Savings Model will cap the cost of insulin at $35 for a 30-day supply,64 and 20 states passed legislation capping insulin pricing.62 Efforts to codify national cost caps are under debate, including the passage of the Affordable Insulin Now Act, which passed the House in March 2022 and is currently under review in the Senate.65
Perspective: The Role of Private Philanthropy in Supporting Population Health Innovations
Funders and industry partners play a crucial role in leading and supporting innovations that improve the lives of people with T1D and reduce society’s costs of living with the disease. Data infrastructure is critical to supporting population health. While building the data infrastructure to support population health is both time- and resource-intensive, private foundations such as Helmsley are uniquely positioned—and have a responsibility—to take large, informed risks to help reach all communities with T1D.
The T1DX-QI is the largest source of population health data on T1D in the United States and is becoming the premiere data authority on its incidence, prevalence, and outcomes. The T1DX-QI enables a robust understanding of T1D-related health trends at the population level, as well as trends among clinics and providers. Pilot centers in the T1DX-QI have reported reductions in patients’ A1c and acute diabetes-related events, as well as improvements in device usage and depression screening. The ability to capture changes speaks to the promise and power of these data to demonstrate the clinical impact of QI interventions and to support the spread of best practices and learnings across health systems.
Additional philanthropic efforts have supported innovation in the last 2 years. For example, the JDRF, a nonprofit philanthropic equity firm, has supported efforts in developing artificial pancreas systems and cell therapies currently in clinical trials like teplizumab, a drug that has demonstrated delayed onset of T1D through JDRF’s T1D Fund.66 Industry partners also have an opportunity for significant influence in this area, as they continue to fund meaningful projects to advance care for people with T1D.67
Conclusion
We are optimistic that the innovations summarized here describe a shift in the tide of equitable T1D outcomes; however, future work is required to explore additional health equity successes that do not further exacerbate inequities. We also see further opportunities for innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Corresponding author: Ann Mungmode, MPH, T1D Exchange, 11 Avenue de Lafayette, Boston, MA 02111; Email: [email protected]
Disclosures: Dr. Ebekozien serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for the Medtronic Advisory Board and received research grants from Medtronic Diabetes, Eli Lilly, and Dexcom.
Funding: The T1DX-QI is funded by The Leona M. and Harry B. Hemsley Charitable Trust.
1. Centers for Disease Control and Prevention. National diabetes statistics report. Accessed August 30, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
2. Centers for Disease Control and Prevention. Diabetes fast facts. Accessed August 30, 2022. www.cdc.gov/diabetes/basics/quick-facts.html
3. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2020;106(2):e936-e942. doi:10.1210/clinem/dgaa825
4. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
5. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. doi:10.17925/ee.2019.15.2.70
6. Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. doi:10.1530/eje-20-0377
7. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. doi:10.2337/cd19-0032
8. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):256-263. doi:10.2337/cd21-0029
9. ATTD 2021 invited speaker abstracts. Diabetes Technol Ther. 2021;23(S2):A1-A206. doi:10.1089/dia.2021.2525.abstracts
10. Rompicherla SN, Edelen N, Gallagher R, et al. Children and adolescent patients with pre-existing type 1 diabetes and additional comorbidities have an increased risk of hospitalization from COVID-19; data from the T1D Exchange COVID Registry. Pediatr Diabetes. 2021;22(S30):3-32. doi:10.1111/pedi.13268
11. Abstracts for the T1D Exchange QI Collaborative (T1DX-QI) Learning Session 2021. November 8-9, 2021. J Diabetes. 2021;13(S1):3-17. doi:10.1111/1753-0407.13227
12. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes conference 27-30 April 2022. Barcelona and online. Diabetes Technol Ther. 2022;24(S1):A1-A237. doi:10.1089/dia.2022.2525.abstracts
13. Ebekozien ON, Kamboj N, Odugbesan MK, et al. Inequities in glycemic outcomes for patients with type 1 diabetes: six-year (2016-2021) longitudinal follow-up by race and ethnicity of 36,390 patients in the T1DX-QI Collaborative. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-167-OR
14. Narayan KA, Noor M, Rompicherla N, et al. No BMI increase during the COVID-pandemic in children and adults with T1D in three continents: joint analysis of ADDN, T1DX, and DPV registries. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-269-OR
15. Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018;20(7):492-500. doi:10.1089/dia.2018.0098
16. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi:10.1007/s11892-018-1015-3
17. Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642-651. doi:10.1089/dia.2021.0080
18. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now–recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. doi:10.1089/dia.2020.0375
19. Garg SK, Rodriguez E. COVID‐19 pandemic and diabetes care. Diabetes Technol Ther. 2022;24(S1):S2-S20. doi:10.1089/dia.2022.2501
20. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407.13141
21. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2020;106(4):1755-1762. doi:10.1210/clinem/dgaa920
22. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
23. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
24. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;107(2):410-418. doi:10.1210/clinem/dgab668
25. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2021 Oct 9. [Epub ahead of print] doi:10.1177/19322968211049783
26. Gallagher MP, Rompicherla S, Ebekozien O, et al. Differences in COVID-19 outcomes among patients with type 1 diabetes: first vs later surges. J Clin Outcomes Manage. 2022;29(1):27-31. doi:10.12788/jcom.0084
27. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23(4):433-438. doi:10.1111/pedi.13328
28. Lavik AR, Ebekozien O, Noor N, et al. Trends in type 1 diabetic ketoacidosis during COVID-19 surges at 7 US centers: highest burden on non-Hispanic Black patients. J Clin Endocrinol Metab. 2022;107(7):1948-1955. doi:10.1210/clinem/dgac158
29. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther. 2021;23(S1):S1-S7. doi:10.1089/dia.2020.0649
30. Nwosu BU, Al-Halbouni L, Parajuli S, et al. COVID-19 pandemic and pediatric type 1 diabetes: no significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne). 2021;12:703905. doi:10.3389/fendo.2021.703905
31. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi:10.1016/j.amjmed.2020.03.033
32. Nomura A, Noguchi M, Kometani M, et al. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21(12):61. doi:10.1007/s11892-021-01423-2
33. Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health using the T1D Exchange Quality Improvement Portal. Clin Diabetes. Forthcoming 2022.
34. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—past, current, and future directions. N Engl J Med. 2021;384(18):1681-1684. doi:10.1056/NEJMp2008628
35. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. doi:10.2337/cd21-0028
36. Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes. 2022;14(1):79-82. doi:10.1111/1753-0407.13235
37. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D Exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022 Jun 13. [Epub ahead of print] doi:10.1089/dia.2022.0042
38. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi:10.1089/dia.2021.0511
39. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121-e123. doi:10.2337/dc20-3063
40. Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304-312. doi:10.2237/dsi22-0002
41. Zhang J, Xu J, Lim J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv Healthc Mater. 2021;10(17):e2100194. doi:10.1002/adhm.202100194
42. FDA expands remote patient monitoring in hospitals for people with diabetes during COVID-19; manufacturers donate CGM supplies. News release. April 21, 2020. Accessed August 30, 2022. https://www.diabetes.org/newsroom/press-releases/2020/fda-remote-patient-monitoring-cgm
43. Campbell P. FDA grants Dexcom CGM breakthrough designation for in-hospital use. March 2, 2022. Accessed August 30, 2022. https://www.endocrinologynetwork.com/view/fda-grants-dexcom-cgm-breakthrough-designation-for-in-hospital-use
44. Yeh T, Yeung M, Mendelsohn Curanaj FA. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr Diab Rep. 2021;21(2):7. doi:10.1007/s11892-021-01375-7
45. Medtronic announces FDA approval for MiniMed 770G insulin pump system. News release. September 21, 2020. Accessed August 30, 2022. https://bit.ly/3TyEna4
46. Tandem Diabetes Care announces commercial launch of the t:slim X2 insulin pump with Control-IQ technology in the United States. News release. January 15, 2020. Accessed August 30, 2022. https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-commercial-launch-tslim-x2-0
47. Garza M, Gutow H, Mahoney K. Omnipod 5 cleared by the FDA. Updated August 22, 2022. Accessed August 30, 2022.https://diatribe.org/omnipod-5-approved-fda
48. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi:10.1016/s2589-7500(21)00218-1
49. Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118-e119. doi:10.2337/dc22-0329
50. Medtronic launches InPen with real-time Guardian Connect CGM data--the first integrated smart insulin pen for people with diabetes on MDI. News release. November 12, 2020. Accessed August 30, 2022. https://bit.ly/3CTSWPL
51. Bigfoot Biomedical receives FDA clearance for Bigfoot Unity Diabetes Management System, featuring first-of-its-kind smart pen caps for insulin pens used to treat type 1 and type 2 diabetes. News release. May 10, 2021. Accessed August 30, 2022. https://bit.ly/3BeyoAh
52. Vieira G. All about the CeQur Simplicity insulin patch. Updated May 24, 2022. Accessed August 30, 2022. https://beyondtype1.org/cequr-simplicity-insulin-patch/.
53. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi:10.1089/dia.2019.0509
54. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. doi:10.1089/dia.2019.0142
55. Dexcom G7 Release Delayed Until Late 2022. News release. August 8, 2022. Accessed September 7, 2022. https://diatribe.org/dexcom-g7-release-delayed-until-late-2022
56. Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64(5):1059-1065. doi:10.1007/s00125-021-05396-5
57. Garg SK, Rodriguez E, Shah VN, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2022;24(S1):S190-S208. doi:10.1089/dia.2022.2513
58. Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64(5):1030-1036. doi:10.1007/s00125-020-05367-2
59. Danne T, Heinemann L, Bolinder J. New insulins, biosimilars, and insulin therapy. Diabetes Technol Ther. 2022;24(S1):S35-S57. doi:10.1089/dia.2022.2503
60. Kenney J. Insulin copay caps–a path to affordability. July 6, 2021. Accessed August 30, 2022.https://diatribechange.org/news/insulin-copay-caps-path-affordability
61. Glied SA, Zhu B. Not so sweet: insulin affordability over time. September 25, 2020. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/sep/not-so-sweet-insulin-affordability-over-time
62. American Diabetes Association. Insulin and drug affordability. Accessed August 30, 2022. https://www.diabetes.org/advocacy/insulin-and-drug-affordability
63. Sullivan P. Chances for drug pricing, surprise billing action fade until November. March 24, 2020. Accessed August 30, 2022. https://thehill.com/policy/healthcare/489334-chances-for-drug-pricing-surprise-billing-action-fade-until-november/
64. Brown TD. How Medicare’s new Senior Savings Model makes insulin more affordable. June 4, 2020. Accessed August 30, 2022. https://www.diabetes.org/blog/how-medicares-new-senior-savings-model-makes-insulin-more-affordable
65. American Diabetes Association. ADA applauds the U.S. House of Representatives passage of the Affordable Insulin Now Act. News release. April 1, 2022. https://www.diabetes.org/newsroom/official-statement/2022/ada-applauds-us-house-of-representatives-passage-of-the-affordable-insulin-now-act
66. JDRF. Driving T1D cures during challenging times. 2022.
67. Medtronic announces ongoing initiatives to address health equity for people of color living with diabetes. News release. April 7, 2021. Access August 30, 2022. https://bit.ly/3KGTOZU
From the T1D Exchange, Boston, MA (Ann Mungmode, Nicole Rioles, Jesse Cases, Dr. Ebekozien); The Leona M. and Harry B. Hemsley Charitable Trust, New York, NY (Laurel Koester); and the University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien).
Abstract
There have been remarkable innovations in diabetes management since the start of the COVID-19 pandemic, but these groundbreaking innovations are drawing limited focus as the field focuses on the adverse impact of the pandemic on patients with diabetes. This article reviews select population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of the T1D Exchange Quality Improvement Collaborative, a learning health network that focuses on improving care and outcomes for individuals with type 1 diabetes (T1D). Such innovations include expanded telemedicine access, collection of real-world data, machine learning and artificial intelligence, and new diabetes medications and devices. In addition, multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and advocacy efforts for specific populations have been successful. Looking to the future, work is required to explore additional health equity successes that do not further exacerbate inequities and to look for additional innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Keywords: type 1 diabetes, learning health network, continuous glucose monitoring, health equity
One in 10 people in the United States has diabetes.1 Diabetes is the nation’s second leading cause of death, costing the US health system more than $300 billion annually.2 The COVID-19 pandemic presented additional health burdens for people living with diabetes. For example, preexisting diabetes was identified as a risk factor for COVID-19–associated morbidity and mortality.3,4 Over the past 2 years, there have been remarkable innovations in diabetes management, including stem cell therapy and new medication options. Additionally, improved technology solutions have aided in diabetes management through continuous glucose monitors (CGM), smart insulin pens, advanced hybrid closed-loop systems, and continuous subcutaneous insulin injections.5,6 Unfortunately, these groundbreaking innovations are drawing limited focus, as the field is rightfully focused on the adverse impact of the pandemic on patients with diabetes.

Learning health networks like the T1D Exchange Quality Improvement Collaborative (T1DX-QI) have implemented some of these innovative solutions to improve care for people with diabetes.7 T1DX-QI has more than 50 data-sharing endocrinology centers that care for over 75,000 people with diabetes across the United States (Figure 1). Centers participating in the T1DX-QI use quality improvement (QI) and implementation science methods to quickly translate research into evidence-based clinical practice. T1DX-QI leads diabetes population health and health system research and supports widespread transferability across health care organizations through regular collaborative calls, conferences, and case study documentation.8
In this review, we summarize impactful population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of T1DX-QI (see Figure 2 for relevant definitions). This review is limited in scope and is not meant to be an exhaustive list of innovations. The review also reflects significant changes from the perspective of academic diabetes centers, which may not apply to rural or primary care diabetes practices.
Methods
The first (A.M.), second (H.H.), and senior (O.E.) authors conducted a scoping review of published literature using terms related to diabetes, population health, and innovation on PubMed Central and Google Scholar for the period March 2020 to June 2022. To complement the review, A.M. and O.E. also reviewed abstracts from presentations at major international diabetes conferences, including the American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), the T1DX-QI Learning Session Conference, and the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 to 2022 conferences.9-14 The authors also searched FDA.gov and ClinicalTrials.gov for relevant insights. A.M. and O.E. sorted the reviewed literature into major themes (Figure 3) from the population health improvement perspective of the T1DX-QI.

Population Health Innovations in Diabetes Management
Expansion of Telemedicine Access
Telemedicine is cost-effective for patients with diabetes,15 including those with complex cases.16 Before the COVID-19 pandemic, telemedicine and virtual care were rare in diabetes management. However, the pandemic offered a new opportunity to expand the practice of telemedicine in diabetes management. A study from the T1DX-QI showed that telemedicine visits grew from comprising <1% of visits pre-pandemic (December 2019) to 95.2% during the pandemic (August 2020).17 Additional studies, like those conducted by Phillip et al,18 confirmed the noninferiority of telemedicine practice for patients with diabetes.Telemedicine was also found to be an effective strategy to educate patients on the use of diabetes technologies.19
Real-World Data and Disease Surveillance
As the COVID-19 pandemic exacerbated outcomes for people with type 1 diabetes (T1D), a need arose to understand the immediate effects of the pandemic on people with T1D through real-world data and disease surveillance. In April 2020, the T1DX-QI initiated a multicenter surveillance study to collect data and analyze the impact of COVID-19 on people with T1D. The existing health collaborative served as a springboard for robust surveillance study, documenting numerous works on the effects of COVID-19.3,4,20-28 Other investigators also embraced the power of real-world surveillance and real-world data.29,30
Big Data, Machine Learning, and Artificial Intelligence
The past 2 years have seen a shift toward embracing the incredible opportunity to tap the large volume of data generated from routine care for practical insights.31 In particular, researchers have demonstrated the widespread application of machine learning and artificial intelligence to improve diabetes management.32 The T1DX-QI also harnessed the growing power of big data by expanding the functionality of innovative benchmarking software. The T1DX QI Portal uses electronic medical record data of diabetes patients for clinic-to-clinic benchmarking and data analysis, using business intelligence solutions.33
Health Equity
While inequities across various health outcomes have been well documented for years,34 the COVID-19 pandemic further exaggerated racial/ethnic health inequities in T1D.23,35 In response, several organizations have outlined specific strategies to address these health inequities. Emboldened by the pandemic, the T1DX-QI announced a multipronged approach to address health inequities among patients with T1D through the Health Equity Advancement Lab (HEAL).36 One of HEAL’s main components is using real-world data to champion population-level insights and demonstrate progress in QI efforts.
Multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and these studies are expanding our understanding of the chasm.37 There have also been innovative solutions to addressing these inequities, with multiple studies published over the past 2 years.38 A source of inequity among patients with T1D is the lack of representation of racial/ethnic minorities with T1D in clinical trials.39 The T1DX-QI suggests that the equity-adapted framework for QI can be applied by research leaders to support trial diversity and representation, ensuring future device innovations are meaningful for all people with T1D.40
Diabetes Devices
Glucose monitoring and insulin therapy are vital tools to support individuals living with T1D, and devices such as CGM and insulin pumps have become the standard of care for diabetes management (Table).41 Innovations in diabetes technology and device access are imperative for a chronic disease with no cure.

The COVID-19 pandemic created an opportunity to increase access to diabetes devices in inpatient settings. In 2020, the US Food and Drug Administration expanded the use of CGM to support remote monitoring of patients in inpatient hospital settings, simultaneously supporting the glucose monitoring needs of patients with T1D and reducing COVID-19 transmission through reduced patient-clinician contact.42 This effort has been expanded and will continue in 2022 and beyond,43 and aligns with the growing consensus that supports patients wearing both CGMs and insulin pumps in ambulatory settings to improve patient health outcomes.44
Since 2020, innovations in diabetes technology have improved and increased the variety of options available to people with T1D and made them easier to use (Table). New, advanced hybrid closed-loop systems have progressed to offer Bluetooth features, including automatic software upgrades, tubeless systems, and the ability to allow parents to use their smartphones to bolus for children.45-47 The next big step in insulin delivery innovation is the release of functioning, fully closed loop systems, of which several are currently in clinical trials.48 These systems support reduced hypoglycemia and improved time in range.49
Additional innovations in insulin delivery have improved the user experience and expanded therapeutic options, including a variety of smart insulin pens complete with dosing logs50,51 and even a patch to deliver insulin without the burden of injections.52 As barriers to diabetes technology persist,53 innovations in alternate insulin delivery provide people with T1D more options to align with their personal access and technology preferences.
Innovations in CGM address cited barriers to their use, including size or overall wear.53-55 CGMs released in the past few years are smaller in physical size, have longer durations of time between changings, are more accurate, and do not require calibrations for accuracy.
New Diabetes Medications
Many new medications and therapeutic advances have become available in the past 2 years.56 Additionally, more medications are being tested as adjunct therapies to support glycemic management in patients with T1D, including metformin, sodium-glucose cotransporter 1 and 2 inhibitors, pramlintide, glucagon-like polypeptide-1 analogs, and glucagon receptor agonists.57 Other recent advances include stem cell replacement therapy for patients with T1D.58 The ultra-long-acting biosimilar insulins are one medical innovation that has been stalled, rather than propelled, during the COVID-19 pandemic.59
Diabetes Policy Advocacy
People with T1D require insulin to survive. The cost of insulin has increased in recent years, with some studies citing a 64% to 100% increase in the past decade.60,61 In fact, 1 in 4 insulin users report that cost has impacted their insulin use, including rationing their insulin.62 Lockdowns during the COVID-19 pandemic stressed US families financially, increasing the urgency for insulin cost caps.
Although the COVID-19 pandemic halted national conversations on drug financing,63 advocacy efforts have succeeded for specific populations. The new Medicare Part D Senior Savings Model will cap the cost of insulin at $35 for a 30-day supply,64 and 20 states passed legislation capping insulin pricing.62 Efforts to codify national cost caps are under debate, including the passage of the Affordable Insulin Now Act, which passed the House in March 2022 and is currently under review in the Senate.65
Perspective: The Role of Private Philanthropy in Supporting Population Health Innovations
Funders and industry partners play a crucial role in leading and supporting innovations that improve the lives of people with T1D and reduce society’s costs of living with the disease. Data infrastructure is critical to supporting population health. While building the data infrastructure to support population health is both time- and resource-intensive, private foundations such as Helmsley are uniquely positioned—and have a responsibility—to take large, informed risks to help reach all communities with T1D.
The T1DX-QI is the largest source of population health data on T1D in the United States and is becoming the premiere data authority on its incidence, prevalence, and outcomes. The T1DX-QI enables a robust understanding of T1D-related health trends at the population level, as well as trends among clinics and providers. Pilot centers in the T1DX-QI have reported reductions in patients’ A1c and acute diabetes-related events, as well as improvements in device usage and depression screening. The ability to capture changes speaks to the promise and power of these data to demonstrate the clinical impact of QI interventions and to support the spread of best practices and learnings across health systems.
Additional philanthropic efforts have supported innovation in the last 2 years. For example, the JDRF, a nonprofit philanthropic equity firm, has supported efforts in developing artificial pancreas systems and cell therapies currently in clinical trials like teplizumab, a drug that has demonstrated delayed onset of T1D through JDRF’s T1D Fund.66 Industry partners also have an opportunity for significant influence in this area, as they continue to fund meaningful projects to advance care for people with T1D.67
Conclusion
We are optimistic that the innovations summarized here describe a shift in the tide of equitable T1D outcomes; however, future work is required to explore additional health equity successes that do not further exacerbate inequities. We also see further opportunities for innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Corresponding author: Ann Mungmode, MPH, T1D Exchange, 11 Avenue de Lafayette, Boston, MA 02111; Email: [email protected]
Disclosures: Dr. Ebekozien serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for the Medtronic Advisory Board and received research grants from Medtronic Diabetes, Eli Lilly, and Dexcom.
Funding: The T1DX-QI is funded by The Leona M. and Harry B. Hemsley Charitable Trust.
From the T1D Exchange, Boston, MA (Ann Mungmode, Nicole Rioles, Jesse Cases, Dr. Ebekozien); The Leona M. and Harry B. Hemsley Charitable Trust, New York, NY (Laurel Koester); and the University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien).
Abstract
There have been remarkable innovations in diabetes management since the start of the COVID-19 pandemic, but these groundbreaking innovations are drawing limited focus as the field focuses on the adverse impact of the pandemic on patients with diabetes. This article reviews select population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of the T1D Exchange Quality Improvement Collaborative, a learning health network that focuses on improving care and outcomes for individuals with type 1 diabetes (T1D). Such innovations include expanded telemedicine access, collection of real-world data, machine learning and artificial intelligence, and new diabetes medications and devices. In addition, multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and advocacy efforts for specific populations have been successful. Looking to the future, work is required to explore additional health equity successes that do not further exacerbate inequities and to look for additional innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Keywords: type 1 diabetes, learning health network, continuous glucose monitoring, health equity
One in 10 people in the United States has diabetes.1 Diabetes is the nation’s second leading cause of death, costing the US health system more than $300 billion annually.2 The COVID-19 pandemic presented additional health burdens for people living with diabetes. For example, preexisting diabetes was identified as a risk factor for COVID-19–associated morbidity and mortality.3,4 Over the past 2 years, there have been remarkable innovations in diabetes management, including stem cell therapy and new medication options. Additionally, improved technology solutions have aided in diabetes management through continuous glucose monitors (CGM), smart insulin pens, advanced hybrid closed-loop systems, and continuous subcutaneous insulin injections.5,6 Unfortunately, these groundbreaking innovations are drawing limited focus, as the field is rightfully focused on the adverse impact of the pandemic on patients with diabetes.

Learning health networks like the T1D Exchange Quality Improvement Collaborative (T1DX-QI) have implemented some of these innovative solutions to improve care for people with diabetes.7 T1DX-QI has more than 50 data-sharing endocrinology centers that care for over 75,000 people with diabetes across the United States (Figure 1). Centers participating in the T1DX-QI use quality improvement (QI) and implementation science methods to quickly translate research into evidence-based clinical practice. T1DX-QI leads diabetes population health and health system research and supports widespread transferability across health care organizations through regular collaborative calls, conferences, and case study documentation.8
In this review, we summarize impactful population health innovations in diabetes management that have become available over the past 2 years of the COVID-19 pandemic from the perspective of T1DX-QI (see Figure 2 for relevant definitions). This review is limited in scope and is not meant to be an exhaustive list of innovations. The review also reflects significant changes from the perspective of academic diabetes centers, which may not apply to rural or primary care diabetes practices.
Methods
The first (A.M.), second (H.H.), and senior (O.E.) authors conducted a scoping review of published literature using terms related to diabetes, population health, and innovation on PubMed Central and Google Scholar for the period March 2020 to June 2022. To complement the review, A.M. and O.E. also reviewed abstracts from presentations at major international diabetes conferences, including the American Diabetes Association (ADA), the International Society for Pediatric and Adolescent Diabetes (ISPAD), the T1DX-QI Learning Session Conference, and the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 to 2022 conferences.9-14 The authors also searched FDA.gov and ClinicalTrials.gov for relevant insights. A.M. and O.E. sorted the reviewed literature into major themes (Figure 3) from the population health improvement perspective of the T1DX-QI.

Population Health Innovations in Diabetes Management
Expansion of Telemedicine Access
Telemedicine is cost-effective for patients with diabetes,15 including those with complex cases.16 Before the COVID-19 pandemic, telemedicine and virtual care were rare in diabetes management. However, the pandemic offered a new opportunity to expand the practice of telemedicine in diabetes management. A study from the T1DX-QI showed that telemedicine visits grew from comprising <1% of visits pre-pandemic (December 2019) to 95.2% during the pandemic (August 2020).17 Additional studies, like those conducted by Phillip et al,18 confirmed the noninferiority of telemedicine practice for patients with diabetes.Telemedicine was also found to be an effective strategy to educate patients on the use of diabetes technologies.19
Real-World Data and Disease Surveillance
As the COVID-19 pandemic exacerbated outcomes for people with type 1 diabetes (T1D), a need arose to understand the immediate effects of the pandemic on people with T1D through real-world data and disease surveillance. In April 2020, the T1DX-QI initiated a multicenter surveillance study to collect data and analyze the impact of COVID-19 on people with T1D. The existing health collaborative served as a springboard for robust surveillance study, documenting numerous works on the effects of COVID-19.3,4,20-28 Other investigators also embraced the power of real-world surveillance and real-world data.29,30
Big Data, Machine Learning, and Artificial Intelligence
The past 2 years have seen a shift toward embracing the incredible opportunity to tap the large volume of data generated from routine care for practical insights.31 In particular, researchers have demonstrated the widespread application of machine learning and artificial intelligence to improve diabetes management.32 The T1DX-QI also harnessed the growing power of big data by expanding the functionality of innovative benchmarking software. The T1DX QI Portal uses electronic medical record data of diabetes patients for clinic-to-clinic benchmarking and data analysis, using business intelligence solutions.33
Health Equity
While inequities across various health outcomes have been well documented for years,34 the COVID-19 pandemic further exaggerated racial/ethnic health inequities in T1D.23,35 In response, several organizations have outlined specific strategies to address these health inequities. Emboldened by the pandemic, the T1DX-QI announced a multipronged approach to address health inequities among patients with T1D through the Health Equity Advancement Lab (HEAL).36 One of HEAL’s main components is using real-world data to champion population-level insights and demonstrate progress in QI efforts.
Multiple innovative studies have been undertaken to explore contributors to health inequities in diabetes, and these studies are expanding our understanding of the chasm.37 There have also been innovative solutions to addressing these inequities, with multiple studies published over the past 2 years.38 A source of inequity among patients with T1D is the lack of representation of racial/ethnic minorities with T1D in clinical trials.39 The T1DX-QI suggests that the equity-adapted framework for QI can be applied by research leaders to support trial diversity and representation, ensuring future device innovations are meaningful for all people with T1D.40
Diabetes Devices
Glucose monitoring and insulin therapy are vital tools to support individuals living with T1D, and devices such as CGM and insulin pumps have become the standard of care for diabetes management (Table).41 Innovations in diabetes technology and device access are imperative for a chronic disease with no cure.

The COVID-19 pandemic created an opportunity to increase access to diabetes devices in inpatient settings. In 2020, the US Food and Drug Administration expanded the use of CGM to support remote monitoring of patients in inpatient hospital settings, simultaneously supporting the glucose monitoring needs of patients with T1D and reducing COVID-19 transmission through reduced patient-clinician contact.42 This effort has been expanded and will continue in 2022 and beyond,43 and aligns with the growing consensus that supports patients wearing both CGMs and insulin pumps in ambulatory settings to improve patient health outcomes.44
Since 2020, innovations in diabetes technology have improved and increased the variety of options available to people with T1D and made them easier to use (Table). New, advanced hybrid closed-loop systems have progressed to offer Bluetooth features, including automatic software upgrades, tubeless systems, and the ability to allow parents to use their smartphones to bolus for children.45-47 The next big step in insulin delivery innovation is the release of functioning, fully closed loop systems, of which several are currently in clinical trials.48 These systems support reduced hypoglycemia and improved time in range.49
Additional innovations in insulin delivery have improved the user experience and expanded therapeutic options, including a variety of smart insulin pens complete with dosing logs50,51 and even a patch to deliver insulin without the burden of injections.52 As barriers to diabetes technology persist,53 innovations in alternate insulin delivery provide people with T1D more options to align with their personal access and technology preferences.
Innovations in CGM address cited barriers to their use, including size or overall wear.53-55 CGMs released in the past few years are smaller in physical size, have longer durations of time between changings, are more accurate, and do not require calibrations for accuracy.
New Diabetes Medications
Many new medications and therapeutic advances have become available in the past 2 years.56 Additionally, more medications are being tested as adjunct therapies to support glycemic management in patients with T1D, including metformin, sodium-glucose cotransporter 1 and 2 inhibitors, pramlintide, glucagon-like polypeptide-1 analogs, and glucagon receptor agonists.57 Other recent advances include stem cell replacement therapy for patients with T1D.58 The ultra-long-acting biosimilar insulins are one medical innovation that has been stalled, rather than propelled, during the COVID-19 pandemic.59
Diabetes Policy Advocacy
People with T1D require insulin to survive. The cost of insulin has increased in recent years, with some studies citing a 64% to 100% increase in the past decade.60,61 In fact, 1 in 4 insulin users report that cost has impacted their insulin use, including rationing their insulin.62 Lockdowns during the COVID-19 pandemic stressed US families financially, increasing the urgency for insulin cost caps.
Although the COVID-19 pandemic halted national conversations on drug financing,63 advocacy efforts have succeeded for specific populations. The new Medicare Part D Senior Savings Model will cap the cost of insulin at $35 for a 30-day supply,64 and 20 states passed legislation capping insulin pricing.62 Efforts to codify national cost caps are under debate, including the passage of the Affordable Insulin Now Act, which passed the House in March 2022 and is currently under review in the Senate.65
Perspective: The Role of Private Philanthropy in Supporting Population Health Innovations
Funders and industry partners play a crucial role in leading and supporting innovations that improve the lives of people with T1D and reduce society’s costs of living with the disease. Data infrastructure is critical to supporting population health. While building the data infrastructure to support population health is both time- and resource-intensive, private foundations such as Helmsley are uniquely positioned—and have a responsibility—to take large, informed risks to help reach all communities with T1D.
The T1DX-QI is the largest source of population health data on T1D in the United States and is becoming the premiere data authority on its incidence, prevalence, and outcomes. The T1DX-QI enables a robust understanding of T1D-related health trends at the population level, as well as trends among clinics and providers. Pilot centers in the T1DX-QI have reported reductions in patients’ A1c and acute diabetes-related events, as well as improvements in device usage and depression screening. The ability to capture changes speaks to the promise and power of these data to demonstrate the clinical impact of QI interventions and to support the spread of best practices and learnings across health systems.
Additional philanthropic efforts have supported innovation in the last 2 years. For example, the JDRF, a nonprofit philanthropic equity firm, has supported efforts in developing artificial pancreas systems and cell therapies currently in clinical trials like teplizumab, a drug that has demonstrated delayed onset of T1D through JDRF’s T1D Fund.66 Industry partners also have an opportunity for significant influence in this area, as they continue to fund meaningful projects to advance care for people with T1D.67
Conclusion
We are optimistic that the innovations summarized here describe a shift in the tide of equitable T1D outcomes; however, future work is required to explore additional health equity successes that do not further exacerbate inequities. We also see further opportunities for innovative ways to engage people with T1D in their health care through conversations on social determinants of health and societal structures.
Corresponding author: Ann Mungmode, MPH, T1D Exchange, 11 Avenue de Lafayette, Boston, MA 02111; Email: [email protected]
Disclosures: Dr. Ebekozien serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for the Medtronic Advisory Board and received research grants from Medtronic Diabetes, Eli Lilly, and Dexcom.
Funding: The T1DX-QI is funded by The Leona M. and Harry B. Hemsley Charitable Trust.
1. Centers for Disease Control and Prevention. National diabetes statistics report. Accessed August 30, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
2. Centers for Disease Control and Prevention. Diabetes fast facts. Accessed August 30, 2022. www.cdc.gov/diabetes/basics/quick-facts.html
3. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2020;106(2):e936-e942. doi:10.1210/clinem/dgaa825
4. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
5. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. doi:10.17925/ee.2019.15.2.70
6. Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. doi:10.1530/eje-20-0377
7. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. doi:10.2337/cd19-0032
8. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):256-263. doi:10.2337/cd21-0029
9. ATTD 2021 invited speaker abstracts. Diabetes Technol Ther. 2021;23(S2):A1-A206. doi:10.1089/dia.2021.2525.abstracts
10. Rompicherla SN, Edelen N, Gallagher R, et al. Children and adolescent patients with pre-existing type 1 diabetes and additional comorbidities have an increased risk of hospitalization from COVID-19; data from the T1D Exchange COVID Registry. Pediatr Diabetes. 2021;22(S30):3-32. doi:10.1111/pedi.13268
11. Abstracts for the T1D Exchange QI Collaborative (T1DX-QI) Learning Session 2021. November 8-9, 2021. J Diabetes. 2021;13(S1):3-17. doi:10.1111/1753-0407.13227
12. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes conference 27-30 April 2022. Barcelona and online. Diabetes Technol Ther. 2022;24(S1):A1-A237. doi:10.1089/dia.2022.2525.abstracts
13. Ebekozien ON, Kamboj N, Odugbesan MK, et al. Inequities in glycemic outcomes for patients with type 1 diabetes: six-year (2016-2021) longitudinal follow-up by race and ethnicity of 36,390 patients in the T1DX-QI Collaborative. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-167-OR
14. Narayan KA, Noor M, Rompicherla N, et al. No BMI increase during the COVID-pandemic in children and adults with T1D in three continents: joint analysis of ADDN, T1DX, and DPV registries. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-269-OR
15. Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018;20(7):492-500. doi:10.1089/dia.2018.0098
16. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi:10.1007/s11892-018-1015-3
17. Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642-651. doi:10.1089/dia.2021.0080
18. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now–recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. doi:10.1089/dia.2020.0375
19. Garg SK, Rodriguez E. COVID‐19 pandemic and diabetes care. Diabetes Technol Ther. 2022;24(S1):S2-S20. doi:10.1089/dia.2022.2501
20. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407.13141
21. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2020;106(4):1755-1762. doi:10.1210/clinem/dgaa920
22. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
23. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
24. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;107(2):410-418. doi:10.1210/clinem/dgab668
25. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2021 Oct 9. [Epub ahead of print] doi:10.1177/19322968211049783
26. Gallagher MP, Rompicherla S, Ebekozien O, et al. Differences in COVID-19 outcomes among patients with type 1 diabetes: first vs later surges. J Clin Outcomes Manage. 2022;29(1):27-31. doi:10.12788/jcom.0084
27. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23(4):433-438. doi:10.1111/pedi.13328
28. Lavik AR, Ebekozien O, Noor N, et al. Trends in type 1 diabetic ketoacidosis during COVID-19 surges at 7 US centers: highest burden on non-Hispanic Black patients. J Clin Endocrinol Metab. 2022;107(7):1948-1955. doi:10.1210/clinem/dgac158
29. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther. 2021;23(S1):S1-S7. doi:10.1089/dia.2020.0649
30. Nwosu BU, Al-Halbouni L, Parajuli S, et al. COVID-19 pandemic and pediatric type 1 diabetes: no significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne). 2021;12:703905. doi:10.3389/fendo.2021.703905
31. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi:10.1016/j.amjmed.2020.03.033
32. Nomura A, Noguchi M, Kometani M, et al. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21(12):61. doi:10.1007/s11892-021-01423-2
33. Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health using the T1D Exchange Quality Improvement Portal. Clin Diabetes. Forthcoming 2022.
34. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—past, current, and future directions. N Engl J Med. 2021;384(18):1681-1684. doi:10.1056/NEJMp2008628
35. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. doi:10.2337/cd21-0028
36. Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes. 2022;14(1):79-82. doi:10.1111/1753-0407.13235
37. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D Exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022 Jun 13. [Epub ahead of print] doi:10.1089/dia.2022.0042
38. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi:10.1089/dia.2021.0511
39. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121-e123. doi:10.2337/dc20-3063
40. Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304-312. doi:10.2237/dsi22-0002
41. Zhang J, Xu J, Lim J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv Healthc Mater. 2021;10(17):e2100194. doi:10.1002/adhm.202100194
42. FDA expands remote patient monitoring in hospitals for people with diabetes during COVID-19; manufacturers donate CGM supplies. News release. April 21, 2020. Accessed August 30, 2022. https://www.diabetes.org/newsroom/press-releases/2020/fda-remote-patient-monitoring-cgm
43. Campbell P. FDA grants Dexcom CGM breakthrough designation for in-hospital use. March 2, 2022. Accessed August 30, 2022. https://www.endocrinologynetwork.com/view/fda-grants-dexcom-cgm-breakthrough-designation-for-in-hospital-use
44. Yeh T, Yeung M, Mendelsohn Curanaj FA. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr Diab Rep. 2021;21(2):7. doi:10.1007/s11892-021-01375-7
45. Medtronic announces FDA approval for MiniMed 770G insulin pump system. News release. September 21, 2020. Accessed August 30, 2022. https://bit.ly/3TyEna4
46. Tandem Diabetes Care announces commercial launch of the t:slim X2 insulin pump with Control-IQ technology in the United States. News release. January 15, 2020. Accessed August 30, 2022. https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-commercial-launch-tslim-x2-0
47. Garza M, Gutow H, Mahoney K. Omnipod 5 cleared by the FDA. Updated August 22, 2022. Accessed August 30, 2022.https://diatribe.org/omnipod-5-approved-fda
48. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi:10.1016/s2589-7500(21)00218-1
49. Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118-e119. doi:10.2337/dc22-0329
50. Medtronic launches InPen with real-time Guardian Connect CGM data--the first integrated smart insulin pen for people with diabetes on MDI. News release. November 12, 2020. Accessed August 30, 2022. https://bit.ly/3CTSWPL
51. Bigfoot Biomedical receives FDA clearance for Bigfoot Unity Diabetes Management System, featuring first-of-its-kind smart pen caps for insulin pens used to treat type 1 and type 2 diabetes. News release. May 10, 2021. Accessed August 30, 2022. https://bit.ly/3BeyoAh
52. Vieira G. All about the CeQur Simplicity insulin patch. Updated May 24, 2022. Accessed August 30, 2022. https://beyondtype1.org/cequr-simplicity-insulin-patch/.
53. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi:10.1089/dia.2019.0509
54. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. doi:10.1089/dia.2019.0142
55. Dexcom G7 Release Delayed Until Late 2022. News release. August 8, 2022. Accessed September 7, 2022. https://diatribe.org/dexcom-g7-release-delayed-until-late-2022
56. Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64(5):1059-1065. doi:10.1007/s00125-021-05396-5
57. Garg SK, Rodriguez E, Shah VN, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2022;24(S1):S190-S208. doi:10.1089/dia.2022.2513
58. Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64(5):1030-1036. doi:10.1007/s00125-020-05367-2
59. Danne T, Heinemann L, Bolinder J. New insulins, biosimilars, and insulin therapy. Diabetes Technol Ther. 2022;24(S1):S35-S57. doi:10.1089/dia.2022.2503
60. Kenney J. Insulin copay caps–a path to affordability. July 6, 2021. Accessed August 30, 2022.https://diatribechange.org/news/insulin-copay-caps-path-affordability
61. Glied SA, Zhu B. Not so sweet: insulin affordability over time. September 25, 2020. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/sep/not-so-sweet-insulin-affordability-over-time
62. American Diabetes Association. Insulin and drug affordability. Accessed August 30, 2022. https://www.diabetes.org/advocacy/insulin-and-drug-affordability
63. Sullivan P. Chances for drug pricing, surprise billing action fade until November. March 24, 2020. Accessed August 30, 2022. https://thehill.com/policy/healthcare/489334-chances-for-drug-pricing-surprise-billing-action-fade-until-november/
64. Brown TD. How Medicare’s new Senior Savings Model makes insulin more affordable. June 4, 2020. Accessed August 30, 2022. https://www.diabetes.org/blog/how-medicares-new-senior-savings-model-makes-insulin-more-affordable
65. American Diabetes Association. ADA applauds the U.S. House of Representatives passage of the Affordable Insulin Now Act. News release. April 1, 2022. https://www.diabetes.org/newsroom/official-statement/2022/ada-applauds-us-house-of-representatives-passage-of-the-affordable-insulin-now-act
66. JDRF. Driving T1D cures during challenging times. 2022.
67. Medtronic announces ongoing initiatives to address health equity for people of color living with diabetes. News release. April 7, 2021. Access August 30, 2022. https://bit.ly/3KGTOZU
1. Centers for Disease Control and Prevention. National diabetes statistics report. Accessed August 30, 2022. www.cdc.gov/diabetes/data/statistics-report/index.html
2. Centers for Disease Control and Prevention. Diabetes fast facts. Accessed August 30, 2022. www.cdc.gov/diabetes/basics/quick-facts.html
3. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2020;106(2):e936-e942. doi:10.1210/clinem/dgaa825
4. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
5. Zimmerman C, Albanese-O’Neill A, Haller MJ. Advances in type 1 diabetes technology over the last decade. Eur Endocrinol. 2019;15(2):70-76. doi:10.17925/ee.2019.15.2.70
6. Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID-19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183(2):G67-G77. doi:10.1530/eje-20-0377
7. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. doi:10.2337/cd19-0032
8. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):256-263. doi:10.2337/cd21-0029
9. ATTD 2021 invited speaker abstracts. Diabetes Technol Ther. 2021;23(S2):A1-A206. doi:10.1089/dia.2021.2525.abstracts
10. Rompicherla SN, Edelen N, Gallagher R, et al. Children and adolescent patients with pre-existing type 1 diabetes and additional comorbidities have an increased risk of hospitalization from COVID-19; data from the T1D Exchange COVID Registry. Pediatr Diabetes. 2021;22(S30):3-32. doi:10.1111/pedi.13268
11. Abstracts for the T1D Exchange QI Collaborative (T1DX-QI) Learning Session 2021. November 8-9, 2021. J Diabetes. 2021;13(S1):3-17. doi:10.1111/1753-0407.13227
12. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes conference 27-30 April 2022. Barcelona and online. Diabetes Technol Ther. 2022;24(S1):A1-A237. doi:10.1089/dia.2022.2525.abstracts
13. Ebekozien ON, Kamboj N, Odugbesan MK, et al. Inequities in glycemic outcomes for patients with type 1 diabetes: six-year (2016-2021) longitudinal follow-up by race and ethnicity of 36,390 patients in the T1DX-QI Collaborative. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-167-OR
14. Narayan KA, Noor M, Rompicherla N, et al. No BMI increase during the COVID-pandemic in children and adults with T1D in three continents: joint analysis of ADDN, T1DX, and DPV registries. Diabetes. 2022;71(suppl 1). doi:10.2337/db22-269-OR
15. Lee JY, Lee SWH. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther. 2018;20(7):492-500. doi:10.1089/dia.2018.0098
16. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi:10.1007/s11892-018-1015-3
17. Lee JM, Carlson E, Albanese-O’Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642-651. doi:10.1089/dia.2021.0080
18. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now–recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. doi:10.1089/dia.2020.0375
19. Garg SK, Rodriguez E. COVID‐19 pandemic and diabetes care. Diabetes Technol Ther. 2022;24(S1):S2-S20. doi:10.1089/dia.2022.2501
20. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407.13141
21. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2020;106(4):1755-1762. doi:10.1210/clinem/dgaa920
22. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
23. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
24. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;107(2):410-418. doi:10.1210/clinem/dgab668
25. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D Exchange real-world observational study. J Diabetes Sci Technol. 2021 Oct 9. [Epub ahead of print] doi:10.1177/19322968211049783
26. Gallagher MP, Rompicherla S, Ebekozien O, et al. Differences in COVID-19 outcomes among patients with type 1 diabetes: first vs later surges. J Clin Outcomes Manage. 2022;29(1):27-31. doi:10.12788/jcom.0084
27. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. 2022;23(4):433-438. doi:10.1111/pedi.13328
28. Lavik AR, Ebekozien O, Noor N, et al. Trends in type 1 diabetic ketoacidosis during COVID-19 surges at 7 US centers: highest burden on non-Hispanic Black patients. J Clin Endocrinol Metab. 2022;107(7):1948-1955. doi:10.1210/clinem/dgac158
29. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther. 2021;23(S1):S1-S7. doi:10.1089/dia.2020.0649
30. Nwosu BU, Al-Halbouni L, Parajuli S, et al. COVID-19 pandemic and pediatric type 1 diabetes: no significant change in glycemic control during the pandemic lockdown of 2020. Front Endocrinol (Lausanne). 2021;12:703905. doi:10.3389/fendo.2021.703905
31. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi:10.1016/j.amjmed.2020.03.033
32. Nomura A, Noguchi M, Kometani M, et al. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21(12):61. doi:10.1007/s11892-021-01423-2
33. Mungmode A, Noor N, Weinstock RS, et al. Making diabetes electronic medical record data actionable: promoting benchmarking and population health using the T1D Exchange Quality Improvement Portal. Clin Diabetes. Forthcoming 2022.
34. Lavizzo-Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities—past, current, and future directions. N Engl J Med. 2021;384(18):1681-1684. doi:10.1056/NEJMp2008628
35. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes. 2021;39(3):278-283. doi:10.2337/cd21-0028
36. Ebekozien O, Mungmode A, Odugbesan O, et al. Addressing type 1 diabetes health inequities in the United States: approaches from the T1D Exchange QI Collaborative. J Diabetes. 2022;14(1):79-82. doi:10.1111/1753-0407.13235
37. Odugbesan O, Addala A, Nelson G, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: insights from T1D Exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther. 2022 Jun 13. [Epub ahead of print] doi:10.1089/dia.2022.0042
38. Schmitt J, Fogle K, Scott ML, Iyer P. Improving equitable access to continuous glucose monitors for Alabama’s children with type 1 diabetes: a quality improvement project. Diabetes Technol Ther. 2022;24(7):481-491. doi:10.1089/dia.2021.0511
39. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121-e123. doi:10.2337/dc20-3063
40. Ebekozien O, Mungmode A, Buckingham D, et al. Achieving equity in diabetes research: borrowing from the field of quality improvement using a practical framework and improvement tools. Diabetes Spectr. 2022;35(3):304-312. doi:10.2237/dsi22-0002
41. Zhang J, Xu J, Lim J, et al. Wearable glucose monitoring and implantable drug delivery systems for diabetes management. Adv Healthc Mater. 2021;10(17):e2100194. doi:10.1002/adhm.202100194
42. FDA expands remote patient monitoring in hospitals for people with diabetes during COVID-19; manufacturers donate CGM supplies. News release. April 21, 2020. Accessed August 30, 2022. https://www.diabetes.org/newsroom/press-releases/2020/fda-remote-patient-monitoring-cgm
43. Campbell P. FDA grants Dexcom CGM breakthrough designation for in-hospital use. March 2, 2022. Accessed August 30, 2022. https://www.endocrinologynetwork.com/view/fda-grants-dexcom-cgm-breakthrough-designation-for-in-hospital-use
44. Yeh T, Yeung M, Mendelsohn Curanaj FA. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr Diab Rep. 2021;21(2):7. doi:10.1007/s11892-021-01375-7
45. Medtronic announces FDA approval for MiniMed 770G insulin pump system. News release. September 21, 2020. Accessed August 30, 2022. https://bit.ly/3TyEna4
46. Tandem Diabetes Care announces commercial launch of the t:slim X2 insulin pump with Control-IQ technology in the United States. News release. January 15, 2020. Accessed August 30, 2022. https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-commercial-launch-tslim-x2-0
47. Garza M, Gutow H, Mahoney K. Omnipod 5 cleared by the FDA. Updated August 22, 2022. Accessed August 30, 2022.https://diatribe.org/omnipod-5-approved-fda
48. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi:10.1016/s2589-7500(21)00218-1
49. Noor N, Kamboj MK, Triolo T, et al. Hybrid closed-loop systems and glycemic outcomes in children and adults with type 1 diabetes: real-world evidence from a U.S.-based multicenter collaborative. Diabetes Care. 2022;45(8):e118-e119. doi:10.2337/dc22-0329
50. Medtronic launches InPen with real-time Guardian Connect CGM data--the first integrated smart insulin pen for people with diabetes on MDI. News release. November 12, 2020. Accessed August 30, 2022. https://bit.ly/3CTSWPL
51. Bigfoot Biomedical receives FDA clearance for Bigfoot Unity Diabetes Management System, featuring first-of-its-kind smart pen caps for insulin pens used to treat type 1 and type 2 diabetes. News release. May 10, 2021. Accessed August 30, 2022. https://bit.ly/3BeyoAh
52. Vieira G. All about the CeQur Simplicity insulin patch. Updated May 24, 2022. Accessed August 30, 2022. https://beyondtype1.org/cequr-simplicity-insulin-patch/.
53. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi:10.1089/dia.2019.0509
54. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. doi:10.1089/dia.2019.0142
55. Dexcom G7 Release Delayed Until Late 2022. News release. August 8, 2022. Accessed September 7, 2022. https://diatribe.org/dexcom-g7-release-delayed-until-late-2022
56. Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64(5):1059-1065. doi:10.1007/s00125-021-05396-5
57. Garg SK, Rodriguez E, Shah VN, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2022;24(S1):S190-S208. doi:10.1089/dia.2022.2513
58. Melton D. The promise of stem cell-derived islet replacement therapy. Diabetologia. 2021;64(5):1030-1036. doi:10.1007/s00125-020-05367-2
59. Danne T, Heinemann L, Bolinder J. New insulins, biosimilars, and insulin therapy. Diabetes Technol Ther. 2022;24(S1):S35-S57. doi:10.1089/dia.2022.2503
60. Kenney J. Insulin copay caps–a path to affordability. July 6, 2021. Accessed August 30, 2022.https://diatribechange.org/news/insulin-copay-caps-path-affordability
61. Glied SA, Zhu B. Not so sweet: insulin affordability over time. September 25, 2020. Accessed August 30, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/sep/not-so-sweet-insulin-affordability-over-time
62. American Diabetes Association. Insulin and drug affordability. Accessed August 30, 2022. https://www.diabetes.org/advocacy/insulin-and-drug-affordability
63. Sullivan P. Chances for drug pricing, surprise billing action fade until November. March 24, 2020. Accessed August 30, 2022. https://thehill.com/policy/healthcare/489334-chances-for-drug-pricing-surprise-billing-action-fade-until-november/
64. Brown TD. How Medicare’s new Senior Savings Model makes insulin more affordable. June 4, 2020. Accessed August 30, 2022. https://www.diabetes.org/blog/how-medicares-new-senior-savings-model-makes-insulin-more-affordable
65. American Diabetes Association. ADA applauds the U.S. House of Representatives passage of the Affordable Insulin Now Act. News release. April 1, 2022. https://www.diabetes.org/newsroom/official-statement/2022/ada-applauds-us-house-of-representatives-passage-of-the-affordable-insulin-now-act
66. JDRF. Driving T1D cures during challenging times. 2022.
67. Medtronic announces ongoing initiatives to address health equity for people of color living with diabetes. News release. April 7, 2021. Access August 30, 2022. https://bit.ly/3KGTOZU
Deprescribing in Older Adults in Community and Nursing Home Settings
Study 1 Overview (Bayliss et al)
Objective: To examine the effect of a deprescribing educational intervention on medication use in older adults with cognitive impairment.
Design: This was a pragmatic, cluster randomized trial conducted in 8 primary care clinics that are part of a nonprofit health care system.
Setting and participants: The primary care clinic populations ranged from 170 to 1125 patients per clinic. The primary care clinics were randomly assigned to intervention or control using a uniform distribution in blocks by clinic size. Eligibility criteria for participants at those practices included age 65 years or older; health plan enrollment at least 1 year prior to intervention; diagnosis of Alzheimer disease and related dementia (ADRD) or mild cognitive impairment (MCI) by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code or from problem list; 1 or more chronic conditions from those in the Chronic Conditions Warehouse; and 5 or more long-term medications. Those who scheduled a visit at their primary care clinic in advance were eligible for the intervention. Primary care clinicians in intervention clinics were eligible to receive the clinician portion of the intervention. A total of 1433 participants were enrolled in the intervention group, and 1579 participants were enrolled in the control group.
Intervention: The intervention included 2 components: a patient and family component with materials mailed in advance of their primary care visits and a clinician component comprising monthly educational materials on deprescribing and notification in the electronic health record about visits with patient participants. The patient and family component consisted of a brochure titled “Managing Medication” and a questionnaire on attitudes toward deprescribing intended to educate patients and family about deprescribing. Clinicians at intervention clinics received an educational presentation at a monthly clinician meeting as well as tip sheets and a poster on deprescribing topics, and they also were notified of upcoming appointments with patients who received the patient component of the intervention. For the control group, patients and family did not receive any materials, and clinicians did not receive intervention materials or notification of participants enrolled in the trial. Usual care in both intervention and control groups included medication reconciliation and electronic health record alerts for potentially high-risk medications.
Main outcome measures: The primary outcomes of the study were the number of long-term medications per individual and the proportion of patients prescribed 1 or more potentially inappropriate medications. Outcome measurements were extracted from the electronic clinical data, and outcomes were assessed at 6 months, which involved comparing counts of medications at baseline with medications at 6 months. Long-term medications were defined as medications that are prescribed for 28 days or more based on pharmacy dispensing data. Potentially inappropriate medications (PIMs) were defined using the Beers list of medications to avoid in those with cognitive impairment and opioid medications. Analyses were conducted as intention to treat.
Main results: In the intervention group and control group, 56.2% and 54.4% of participants were women, and the mean age was 80.1 years (SD, 7.2) and 79.9 years (SD, 7.5), respectively. At baseline, the mean number of long-term medications was 7.0 (SD, 2.1) in the intervention group and 7.0 (SD, 2.2) in the control group. The proportion of patients taking any PIMs was 30.5% in the intervention group and 29.6% in the control group. At 6 months, the mean number of long-term medications was 6.4 in the intervention group and 6.5 in the control group, with an adjusted difference of –0.1 (95% CI, –0.2 to 0.04; P = .14); the proportion of patients with any PIMs was 17.8% in the intervention group and 20.9% in the control group, with an adjusted difference of –3.2% (95% CI, –6.2 to 0.4; P = .08). Preplanned analyses to examine subgroup differences for those with a higher number of medications (7+ vs 5 or 6 medications) did not find different effects of the intervention.
Conclusion: This educational intervention on deprescribing did not result in reductions in the number of medications or the use of PIMs in patients with cognitive impairment.
Study 2 Overview (Gedde et al)
Objective: To examine the effect of a deprescribing intervention (COSMOS) on medication use for nursing home residents.
Design: This was a randomized clinical trial.
Setting and participants: This trial was conducted in 67 units in 33 nursing homes in Norway. Participants were nursing home residents recruited from August 2014 to March 2015. Inclusion criteria included adults aged 65 years and older with at least 2 years of residency in nursing homes. Exclusion criteria included diagnosis of schizophrenia and a life expectancy of 6 months or less. Participants were followed for 4 months; participants were considered lost to follow-up if they died or moved from the nursing home unit. The analyses were per protocol and did not include those lost to follow-up or those who did not undergo a medication review in the intervention group. A total of 217 and 211 residents were included in the intervention and control groups, respectively.
Intervention: The intervention contained 5 components: communication and advance care planning, systematic pain management, medication reviews with collegial mentoring, organization of activities adjusted to needs and preferences, and safety. For medication review, the nursing home physician reviewed medications together with a nurse and study physicians who provided mentoring. The medication review involved a structured process that used assessment tools for behavioral and psychological symptoms of dementia (BPSD), activities of daily living (ADL), pain, cognitive status, well-being and quality of life, and clinical metrics of blood pressure, pulse, and body mass index. The study utilized the START/STOPP criteria1 for medication use in addition to a list of medications with anticholinergic properties for the medication review. In addition, drug interactions were documented through a drug interaction database; the team also incorporated patient wishes and concerns in the medication reviews. The nursing home physician made final decisions on medications. For the control group, nursing home residents received usual care without this intervention.
Main outcome measures: The primary outcome of the study was the mean change in the number of prescribed psychotropic medications, both regularly scheduled and total medications (which also included on-demand drugs) received at 4 months when compared to baseline. Psychotropic medications included antipsychotics, anxiolytics, hypnotics or sedatives, antidepressants, and antidementia drugs. Secondary outcomes included mean changes in BPSD using the Neuropsychiatric Inventory-Nursing home version (NPI-NH) and the Cornell Scale for Depression for Dementia (CSDD) and ADL using the Physical Self Maintenance Scale (PSMS).
Main results: In both the intervention and control groups, 76% of participants were women, and mean age was 86.3 years (SD, 7.95) in the intervention group and 86.6 years (SD, 7.21) in the control group. At baseline, the mean number of total medications was 10.9 (SD, 4.6) in the intervention group and 10.9 (SD, 4.7) in the control group, and the mean number of psychotropic medications was 2.2 (SD, 1.6) and 2.2 (SD, 1.7) in the intervention and control groups, respectively. At 4 months, the mean change from baseline of total psychotropic medications was –0.34 in the intervention group and 0.01 in the control group (P < .001), and the mean change of regularly scheduled psychotropic medications was –0.21 in the intervention group and 0.02 in the control group (P < .001). Measures of BPSD and depression did not differ between intervention and control groups, and ADL showed a small improvement in the intervention group.
Conclusion: This intervention reduced the use of psychotropic medications in nursing home residents without worsening BPSD or depression and may have yielded improvements in ADL.
Commentary
Polypharmacy is common among older adults, as many of them have multiple chronic conditions and often take multiple medications for managing them. Polypharmacy increases the risk of drug interactions and adverse effects from medications; older adults who are frail and/or who have cognitive impairment are especially at risk. Reducing medication use, especially medications likely to cause adverse effects such as those with anticholinergic properties, has the potential to yield beneficial effects while reducing the burden of taking medications. A large randomized trial found that a pharmacist-led education intervention can be effective in reducing PIM use in community-dwelling older adults,2 and that targeting patient motivation and capacity to deprescribe could be effective.3 This study by Bayliss and colleagues (Study 1), however, fell short of the effects seen in the earlier D-PRESCRIBE trial. One of the reasons for these findings may be that the clinician portion of the intervention was less intensive than that used in the earlier trial; specifically, in the present study, clinicians were not provided with or expected to utilize tools for structured medication review or deprescribing. Although the intervention primes the patient and family for discussions around deprescribing through the use of a brochure and questionnaire, the clinician portion of the intervention was less structured. Another example of an effective intervention that provided a more structured deprescribing intervention beyond education of clinicians utilized electronic decision-support to assist with deprescribing.4
The findings from the Gedde et al study (Study 2) are comparable to those of prior studies in the nursing home population,5 where participants are likely to take a large number of medications, including psychotropic medications, and are more likely to be frail. However, Gedde and colleagues employed a bundled intervention6 that included other components besides medication review, and thus it is unclear whether the effect on ADL can be attributed to the deprescribing of medications alone. Gedde et al’s finding that deprescribing can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression is an important contribution to our knowledge about polypharmacy and deprescribing in older patients. Thus, nursing home residents, their families, and clinicians could expect that the deprescribing of psychotropic medications does not lead to worsening symptoms. Of note, the clinician portion of the intervention in the Gedde et al study was quite structured, and this structure may have contributed to the observed effects.
Applications for Clinical Practice and System Implementation
Both studies add to the literature on deprescribing and may offer options for researchers and clinicians who are considering potential components of an effective deprescribing intervention. Patient activation for deprescribing via the methods used in these 2 studies may help to prime patients for conversations about deprescribing; however, as shown by the Bayliss et al study, a more structured approach to clinical encounters may be needed when deprescribing, such as the use of tools in the electronic health record, in order to reduce the use of medication deemed unnecessary or potentially harmful. Further studies should examine the effect of deprescribing on medication use, but perhaps even more importantly, how deprescribing impacts patient outcomes both in terms of risks and benefits.
Practice Points
- A more structured approach to clinical encounters (eg, the use of tools in the electronic health record) may be needed when deprescribing unnecessary or potentially harmful medications in older patients in community settings.
- In the nursing home setting, structured deprescribing intervention can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression.
–William W. Hung, MD, MPH
1. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218. doi:10.1093/ageing/afu145
2. Martin P, Tamblyn R, Benedetti A, et al. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
3. Martin P, Tannenbaum C. A realist evaluation of patients’ decisions to deprescribe in the EMPOWER trial. BMJ Open. 2017;7(4):e015959. doi:10.1136/bmjopen-2017-015959
4. Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369:m1822. doi:10.1136/bmj.m1822
5. Fournier A, Anrys P, Beuscart JB, et al. Use and deprescribing of potentially inappropriate medications in frail nursing home residents. Drugs Aging. 2020;37(12):917-924. doi:10.1007/s40266-020-00805-7
6. Husebø BS, Ballard C, Aarsland D, et al. The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS). J Am Med Dir Assoc. 2019;20(3):330-339. doi:10.1016/j.jamda.2018.11.006
Study 1 Overview (Bayliss et al)
Objective: To examine the effect of a deprescribing educational intervention on medication use in older adults with cognitive impairment.
Design: This was a pragmatic, cluster randomized trial conducted in 8 primary care clinics that are part of a nonprofit health care system.
Setting and participants: The primary care clinic populations ranged from 170 to 1125 patients per clinic. The primary care clinics were randomly assigned to intervention or control using a uniform distribution in blocks by clinic size. Eligibility criteria for participants at those practices included age 65 years or older; health plan enrollment at least 1 year prior to intervention; diagnosis of Alzheimer disease and related dementia (ADRD) or mild cognitive impairment (MCI) by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code or from problem list; 1 or more chronic conditions from those in the Chronic Conditions Warehouse; and 5 or more long-term medications. Those who scheduled a visit at their primary care clinic in advance were eligible for the intervention. Primary care clinicians in intervention clinics were eligible to receive the clinician portion of the intervention. A total of 1433 participants were enrolled in the intervention group, and 1579 participants were enrolled in the control group.
Intervention: The intervention included 2 components: a patient and family component with materials mailed in advance of their primary care visits and a clinician component comprising monthly educational materials on deprescribing and notification in the electronic health record about visits with patient participants. The patient and family component consisted of a brochure titled “Managing Medication” and a questionnaire on attitudes toward deprescribing intended to educate patients and family about deprescribing. Clinicians at intervention clinics received an educational presentation at a monthly clinician meeting as well as tip sheets and a poster on deprescribing topics, and they also were notified of upcoming appointments with patients who received the patient component of the intervention. For the control group, patients and family did not receive any materials, and clinicians did not receive intervention materials or notification of participants enrolled in the trial. Usual care in both intervention and control groups included medication reconciliation and electronic health record alerts for potentially high-risk medications.
Main outcome measures: The primary outcomes of the study were the number of long-term medications per individual and the proportion of patients prescribed 1 or more potentially inappropriate medications. Outcome measurements were extracted from the electronic clinical data, and outcomes were assessed at 6 months, which involved comparing counts of medications at baseline with medications at 6 months. Long-term medications were defined as medications that are prescribed for 28 days or more based on pharmacy dispensing data. Potentially inappropriate medications (PIMs) were defined using the Beers list of medications to avoid in those with cognitive impairment and opioid medications. Analyses were conducted as intention to treat.
Main results: In the intervention group and control group, 56.2% and 54.4% of participants were women, and the mean age was 80.1 years (SD, 7.2) and 79.9 years (SD, 7.5), respectively. At baseline, the mean number of long-term medications was 7.0 (SD, 2.1) in the intervention group and 7.0 (SD, 2.2) in the control group. The proportion of patients taking any PIMs was 30.5% in the intervention group and 29.6% in the control group. At 6 months, the mean number of long-term medications was 6.4 in the intervention group and 6.5 in the control group, with an adjusted difference of –0.1 (95% CI, –0.2 to 0.04; P = .14); the proportion of patients with any PIMs was 17.8% in the intervention group and 20.9% in the control group, with an adjusted difference of –3.2% (95% CI, –6.2 to 0.4; P = .08). Preplanned analyses to examine subgroup differences for those with a higher number of medications (7+ vs 5 or 6 medications) did not find different effects of the intervention.
Conclusion: This educational intervention on deprescribing did not result in reductions in the number of medications or the use of PIMs in patients with cognitive impairment.
Study 2 Overview (Gedde et al)
Objective: To examine the effect of a deprescribing intervention (COSMOS) on medication use for nursing home residents.
Design: This was a randomized clinical trial.
Setting and participants: This trial was conducted in 67 units in 33 nursing homes in Norway. Participants were nursing home residents recruited from August 2014 to March 2015. Inclusion criteria included adults aged 65 years and older with at least 2 years of residency in nursing homes. Exclusion criteria included diagnosis of schizophrenia and a life expectancy of 6 months or less. Participants were followed for 4 months; participants were considered lost to follow-up if they died or moved from the nursing home unit. The analyses were per protocol and did not include those lost to follow-up or those who did not undergo a medication review in the intervention group. A total of 217 and 211 residents were included in the intervention and control groups, respectively.
Intervention: The intervention contained 5 components: communication and advance care planning, systematic pain management, medication reviews with collegial mentoring, organization of activities adjusted to needs and preferences, and safety. For medication review, the nursing home physician reviewed medications together with a nurse and study physicians who provided mentoring. The medication review involved a structured process that used assessment tools for behavioral and psychological symptoms of dementia (BPSD), activities of daily living (ADL), pain, cognitive status, well-being and quality of life, and clinical metrics of blood pressure, pulse, and body mass index. The study utilized the START/STOPP criteria1 for medication use in addition to a list of medications with anticholinergic properties for the medication review. In addition, drug interactions were documented through a drug interaction database; the team also incorporated patient wishes and concerns in the medication reviews. The nursing home physician made final decisions on medications. For the control group, nursing home residents received usual care without this intervention.
Main outcome measures: The primary outcome of the study was the mean change in the number of prescribed psychotropic medications, both regularly scheduled and total medications (which also included on-demand drugs) received at 4 months when compared to baseline. Psychotropic medications included antipsychotics, anxiolytics, hypnotics or sedatives, antidepressants, and antidementia drugs. Secondary outcomes included mean changes in BPSD using the Neuropsychiatric Inventory-Nursing home version (NPI-NH) and the Cornell Scale for Depression for Dementia (CSDD) and ADL using the Physical Self Maintenance Scale (PSMS).
Main results: In both the intervention and control groups, 76% of participants were women, and mean age was 86.3 years (SD, 7.95) in the intervention group and 86.6 years (SD, 7.21) in the control group. At baseline, the mean number of total medications was 10.9 (SD, 4.6) in the intervention group and 10.9 (SD, 4.7) in the control group, and the mean number of psychotropic medications was 2.2 (SD, 1.6) and 2.2 (SD, 1.7) in the intervention and control groups, respectively. At 4 months, the mean change from baseline of total psychotropic medications was –0.34 in the intervention group and 0.01 in the control group (P < .001), and the mean change of regularly scheduled psychotropic medications was –0.21 in the intervention group and 0.02 in the control group (P < .001). Measures of BPSD and depression did not differ between intervention and control groups, and ADL showed a small improvement in the intervention group.
Conclusion: This intervention reduced the use of psychotropic medications in nursing home residents without worsening BPSD or depression and may have yielded improvements in ADL.
Commentary
Polypharmacy is common among older adults, as many of them have multiple chronic conditions and often take multiple medications for managing them. Polypharmacy increases the risk of drug interactions and adverse effects from medications; older adults who are frail and/or who have cognitive impairment are especially at risk. Reducing medication use, especially medications likely to cause adverse effects such as those with anticholinergic properties, has the potential to yield beneficial effects while reducing the burden of taking medications. A large randomized trial found that a pharmacist-led education intervention can be effective in reducing PIM use in community-dwelling older adults,2 and that targeting patient motivation and capacity to deprescribe could be effective.3 This study by Bayliss and colleagues (Study 1), however, fell short of the effects seen in the earlier D-PRESCRIBE trial. One of the reasons for these findings may be that the clinician portion of the intervention was less intensive than that used in the earlier trial; specifically, in the present study, clinicians were not provided with or expected to utilize tools for structured medication review or deprescribing. Although the intervention primes the patient and family for discussions around deprescribing through the use of a brochure and questionnaire, the clinician portion of the intervention was less structured. Another example of an effective intervention that provided a more structured deprescribing intervention beyond education of clinicians utilized electronic decision-support to assist with deprescribing.4
The findings from the Gedde et al study (Study 2) are comparable to those of prior studies in the nursing home population,5 where participants are likely to take a large number of medications, including psychotropic medications, and are more likely to be frail. However, Gedde and colleagues employed a bundled intervention6 that included other components besides medication review, and thus it is unclear whether the effect on ADL can be attributed to the deprescribing of medications alone. Gedde et al’s finding that deprescribing can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression is an important contribution to our knowledge about polypharmacy and deprescribing in older patients. Thus, nursing home residents, their families, and clinicians could expect that the deprescribing of psychotropic medications does not lead to worsening symptoms. Of note, the clinician portion of the intervention in the Gedde et al study was quite structured, and this structure may have contributed to the observed effects.
Applications for Clinical Practice and System Implementation
Both studies add to the literature on deprescribing and may offer options for researchers and clinicians who are considering potential components of an effective deprescribing intervention. Patient activation for deprescribing via the methods used in these 2 studies may help to prime patients for conversations about deprescribing; however, as shown by the Bayliss et al study, a more structured approach to clinical encounters may be needed when deprescribing, such as the use of tools in the electronic health record, in order to reduce the use of medication deemed unnecessary or potentially harmful. Further studies should examine the effect of deprescribing on medication use, but perhaps even more importantly, how deprescribing impacts patient outcomes both in terms of risks and benefits.
Practice Points
- A more structured approach to clinical encounters (eg, the use of tools in the electronic health record) may be needed when deprescribing unnecessary or potentially harmful medications in older patients in community settings.
- In the nursing home setting, structured deprescribing intervention can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression.
–William W. Hung, MD, MPH
Study 1 Overview (Bayliss et al)
Objective: To examine the effect of a deprescribing educational intervention on medication use in older adults with cognitive impairment.
Design: This was a pragmatic, cluster randomized trial conducted in 8 primary care clinics that are part of a nonprofit health care system.
Setting and participants: The primary care clinic populations ranged from 170 to 1125 patients per clinic. The primary care clinics were randomly assigned to intervention or control using a uniform distribution in blocks by clinic size. Eligibility criteria for participants at those practices included age 65 years or older; health plan enrollment at least 1 year prior to intervention; diagnosis of Alzheimer disease and related dementia (ADRD) or mild cognitive impairment (MCI) by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code or from problem list; 1 or more chronic conditions from those in the Chronic Conditions Warehouse; and 5 or more long-term medications. Those who scheduled a visit at their primary care clinic in advance were eligible for the intervention. Primary care clinicians in intervention clinics were eligible to receive the clinician portion of the intervention. A total of 1433 participants were enrolled in the intervention group, and 1579 participants were enrolled in the control group.
Intervention: The intervention included 2 components: a patient and family component with materials mailed in advance of their primary care visits and a clinician component comprising monthly educational materials on deprescribing and notification in the electronic health record about visits with patient participants. The patient and family component consisted of a brochure titled “Managing Medication” and a questionnaire on attitudes toward deprescribing intended to educate patients and family about deprescribing. Clinicians at intervention clinics received an educational presentation at a monthly clinician meeting as well as tip sheets and a poster on deprescribing topics, and they also were notified of upcoming appointments with patients who received the patient component of the intervention. For the control group, patients and family did not receive any materials, and clinicians did not receive intervention materials or notification of participants enrolled in the trial. Usual care in both intervention and control groups included medication reconciliation and electronic health record alerts for potentially high-risk medications.
Main outcome measures: The primary outcomes of the study were the number of long-term medications per individual and the proportion of patients prescribed 1 or more potentially inappropriate medications. Outcome measurements were extracted from the electronic clinical data, and outcomes were assessed at 6 months, which involved comparing counts of medications at baseline with medications at 6 months. Long-term medications were defined as medications that are prescribed for 28 days or more based on pharmacy dispensing data. Potentially inappropriate medications (PIMs) were defined using the Beers list of medications to avoid in those with cognitive impairment and opioid medications. Analyses were conducted as intention to treat.
Main results: In the intervention group and control group, 56.2% and 54.4% of participants were women, and the mean age was 80.1 years (SD, 7.2) and 79.9 years (SD, 7.5), respectively. At baseline, the mean number of long-term medications was 7.0 (SD, 2.1) in the intervention group and 7.0 (SD, 2.2) in the control group. The proportion of patients taking any PIMs was 30.5% in the intervention group and 29.6% in the control group. At 6 months, the mean number of long-term medications was 6.4 in the intervention group and 6.5 in the control group, with an adjusted difference of –0.1 (95% CI, –0.2 to 0.04; P = .14); the proportion of patients with any PIMs was 17.8% in the intervention group and 20.9% in the control group, with an adjusted difference of –3.2% (95% CI, –6.2 to 0.4; P = .08). Preplanned analyses to examine subgroup differences for those with a higher number of medications (7+ vs 5 or 6 medications) did not find different effects of the intervention.
Conclusion: This educational intervention on deprescribing did not result in reductions in the number of medications or the use of PIMs in patients with cognitive impairment.
Study 2 Overview (Gedde et al)
Objective: To examine the effect of a deprescribing intervention (COSMOS) on medication use for nursing home residents.
Design: This was a randomized clinical trial.
Setting and participants: This trial was conducted in 67 units in 33 nursing homes in Norway. Participants were nursing home residents recruited from August 2014 to March 2015. Inclusion criteria included adults aged 65 years and older with at least 2 years of residency in nursing homes. Exclusion criteria included diagnosis of schizophrenia and a life expectancy of 6 months or less. Participants were followed for 4 months; participants were considered lost to follow-up if they died or moved from the nursing home unit. The analyses were per protocol and did not include those lost to follow-up or those who did not undergo a medication review in the intervention group. A total of 217 and 211 residents were included in the intervention and control groups, respectively.
Intervention: The intervention contained 5 components: communication and advance care planning, systematic pain management, medication reviews with collegial mentoring, organization of activities adjusted to needs and preferences, and safety. For medication review, the nursing home physician reviewed medications together with a nurse and study physicians who provided mentoring. The medication review involved a structured process that used assessment tools for behavioral and psychological symptoms of dementia (BPSD), activities of daily living (ADL), pain, cognitive status, well-being and quality of life, and clinical metrics of blood pressure, pulse, and body mass index. The study utilized the START/STOPP criteria1 for medication use in addition to a list of medications with anticholinergic properties for the medication review. In addition, drug interactions were documented through a drug interaction database; the team also incorporated patient wishes and concerns in the medication reviews. The nursing home physician made final decisions on medications. For the control group, nursing home residents received usual care without this intervention.
Main outcome measures: The primary outcome of the study was the mean change in the number of prescribed psychotropic medications, both regularly scheduled and total medications (which also included on-demand drugs) received at 4 months when compared to baseline. Psychotropic medications included antipsychotics, anxiolytics, hypnotics or sedatives, antidepressants, and antidementia drugs. Secondary outcomes included mean changes in BPSD using the Neuropsychiatric Inventory-Nursing home version (NPI-NH) and the Cornell Scale for Depression for Dementia (CSDD) and ADL using the Physical Self Maintenance Scale (PSMS).
Main results: In both the intervention and control groups, 76% of participants were women, and mean age was 86.3 years (SD, 7.95) in the intervention group and 86.6 years (SD, 7.21) in the control group. At baseline, the mean number of total medications was 10.9 (SD, 4.6) in the intervention group and 10.9 (SD, 4.7) in the control group, and the mean number of psychotropic medications was 2.2 (SD, 1.6) and 2.2 (SD, 1.7) in the intervention and control groups, respectively. At 4 months, the mean change from baseline of total psychotropic medications was –0.34 in the intervention group and 0.01 in the control group (P < .001), and the mean change of regularly scheduled psychotropic medications was –0.21 in the intervention group and 0.02 in the control group (P < .001). Measures of BPSD and depression did not differ between intervention and control groups, and ADL showed a small improvement in the intervention group.
Conclusion: This intervention reduced the use of psychotropic medications in nursing home residents without worsening BPSD or depression and may have yielded improvements in ADL.
Commentary
Polypharmacy is common among older adults, as many of them have multiple chronic conditions and often take multiple medications for managing them. Polypharmacy increases the risk of drug interactions and adverse effects from medications; older adults who are frail and/or who have cognitive impairment are especially at risk. Reducing medication use, especially medications likely to cause adverse effects such as those with anticholinergic properties, has the potential to yield beneficial effects while reducing the burden of taking medications. A large randomized trial found that a pharmacist-led education intervention can be effective in reducing PIM use in community-dwelling older adults,2 and that targeting patient motivation and capacity to deprescribe could be effective.3 This study by Bayliss and colleagues (Study 1), however, fell short of the effects seen in the earlier D-PRESCRIBE trial. One of the reasons for these findings may be that the clinician portion of the intervention was less intensive than that used in the earlier trial; specifically, in the present study, clinicians were not provided with or expected to utilize tools for structured medication review or deprescribing. Although the intervention primes the patient and family for discussions around deprescribing through the use of a brochure and questionnaire, the clinician portion of the intervention was less structured. Another example of an effective intervention that provided a more structured deprescribing intervention beyond education of clinicians utilized electronic decision-support to assist with deprescribing.4
The findings from the Gedde et al study (Study 2) are comparable to those of prior studies in the nursing home population,5 where participants are likely to take a large number of medications, including psychotropic medications, and are more likely to be frail. However, Gedde and colleagues employed a bundled intervention6 that included other components besides medication review, and thus it is unclear whether the effect on ADL can be attributed to the deprescribing of medications alone. Gedde et al’s finding that deprescribing can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression is an important contribution to our knowledge about polypharmacy and deprescribing in older patients. Thus, nursing home residents, their families, and clinicians could expect that the deprescribing of psychotropic medications does not lead to worsening symptoms. Of note, the clinician portion of the intervention in the Gedde et al study was quite structured, and this structure may have contributed to the observed effects.
Applications for Clinical Practice and System Implementation
Both studies add to the literature on deprescribing and may offer options for researchers and clinicians who are considering potential components of an effective deprescribing intervention. Patient activation for deprescribing via the methods used in these 2 studies may help to prime patients for conversations about deprescribing; however, as shown by the Bayliss et al study, a more structured approach to clinical encounters may be needed when deprescribing, such as the use of tools in the electronic health record, in order to reduce the use of medication deemed unnecessary or potentially harmful. Further studies should examine the effect of deprescribing on medication use, but perhaps even more importantly, how deprescribing impacts patient outcomes both in terms of risks and benefits.
Practice Points
- A more structured approach to clinical encounters (eg, the use of tools in the electronic health record) may be needed when deprescribing unnecessary or potentially harmful medications in older patients in community settings.
- In the nursing home setting, structured deprescribing intervention can reduce the use of psychotropic medications while not leading to differences in behavioral and psychologic symptoms or depression.
–William W. Hung, MD, MPH
1. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218. doi:10.1093/ageing/afu145
2. Martin P, Tamblyn R, Benedetti A, et al. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
3. Martin P, Tannenbaum C. A realist evaluation of patients’ decisions to deprescribe in the EMPOWER trial. BMJ Open. 2017;7(4):e015959. doi:10.1136/bmjopen-2017-015959
4. Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369:m1822. doi:10.1136/bmj.m1822
5. Fournier A, Anrys P, Beuscart JB, et al. Use and deprescribing of potentially inappropriate medications in frail nursing home residents. Drugs Aging. 2020;37(12):917-924. doi:10.1007/s40266-020-00805-7
6. Husebø BS, Ballard C, Aarsland D, et al. The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS). J Am Med Dir Assoc. 2019;20(3):330-339. doi:10.1016/j.jamda.2018.11.006
1. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218. doi:10.1093/ageing/afu145
2. Martin P, Tamblyn R, Benedetti A, et al. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
3. Martin P, Tannenbaum C. A realist evaluation of patients’ decisions to deprescribe in the EMPOWER trial. BMJ Open. 2017;7(4):e015959. doi:10.1136/bmjopen-2017-015959
4. Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369:m1822. doi:10.1136/bmj.m1822
5. Fournier A, Anrys P, Beuscart JB, et al. Use and deprescribing of potentially inappropriate medications in frail nursing home residents. Drugs Aging. 2020;37(12):917-924. doi:10.1007/s40266-020-00805-7
6. Husebø BS, Ballard C, Aarsland D, et al. The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS). J Am Med Dir Assoc. 2019;20(3):330-339. doi:10.1016/j.jamda.2018.11.006
Abbreviated Delirium Screening Instruments: Plausible Tool to Improve Delirium Detection in Hospitalized Older Patients
Study 1 Overview (Oberhaus et al)
Objective: To compare the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) to the long-form Confusion Assessment Method (CAM) in detecting postoperative delirium.
Design: Prospective concurrent comparison of 3D-CAM and CAM evaluations in a cohort of postoperative geriatric patients.
Setting and participants: Eligible participants were patients aged 60 years or older undergoing major elective surgery at Barnes Jewish Hospital (St. Louis, Missouri) who were enrolled in ongoing clinical trials (PODCAST, ENGAGES, SATISFY-SOS) between 2015 and 2018. Surgeries were at least 2 hours in length and required general anesthesia, planned extubation, and a minimum 2-day hospital stay. Investigators were extensively trained in administering 3D-CAM and CAM instruments. Participants were evaluated 2 hours after the end of anesthesia care on the day of surgery, then daily until follow-up was completed per clinical trial protocol or until the participant was determined by CAM to be nondelirious for 3 consecutive days. For each evaluation, both 3D-CAM and CAM assessors approached the participant together, but the evaluation was conducted such that the 3D-CAM assessor was masked to the additional questions ascertained by the long-form CAM assessment. The 3D-CAM or CAM assessor independently scored their respective assessments blinded to the results of the other assessor.
Main outcome measures: Participants were concurrently evaluated for postoperative delirium by both 3D-CAM and long-form CAM assessments. Comparisons between 3D-CAM and CAM scores were made using Cohen κ with repeated measures, generalized linear mixed-effects model, and Bland-Altman analysis.
Main results: Sixteen raters performed 471 concurrent 3D-CAM and CAM assessments in 299 participants (mean [SD] age, 69 [6.5] years). Of these participants, 152 (50.8%) were men, 263 (88.0%) were White, and 211 (70.6%) underwent noncardiac surgery. Both instruments showed good intraclass correlation (0.98 for 3D-CAM, 0.84 for CAM) with good overall agreement (Cohen κ = 0.71; 95% CI, 0.58-0.83). The mixed-effects model indicated a significant disagreement between the 3D-CAM and CAM assessments (estimated difference in fixed effect, –0.68; 95% CI, –1.32 to –0.05; P = .04). The Bland-Altman analysis showed that the probability of a delirium diagnosis with the 3D-CAM was more than twice that with the CAM (probability ratio, 2.78; 95% CI, 2.44-3.23).
Conclusion: The high degree of agreement between 3D-CAM and long-form CAM assessments suggests that the former may be a pragmatic and easy-to-administer clinical tool to screen for postoperative delirium in vulnerable older surgical patients.
Study 2 Overview (Shenkin et al)
Objective: To assess the accuracy of the 4 ‘A’s Test (4AT) for delirium detection in the medical inpatient setting and to compare the 4AT to the CAM.
Design: Prospective randomized diagnostic test accuracy study.
Setting and participants: This study was conducted in emergency departments and acute medical wards at 3 UK sites (Edinburgh, Bradford, and Sheffield) and enrolled acute medical patients aged 70 years or older without acute life-threatening illnesses and/or coma. Assessors administering the delirium evaluation were nurses or graduate clinical research associates who underwent systematic training in delirium and delirium assessment. Additional training was provided to those administering the CAM but not to those administering the 4AT as the latter is designed to be administered without special training. First, all participants underwent a reference standard delirium assessment using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria to derive a final definitive diagnosis of delirium via expert consensus (1 psychiatrist and 2 geriatricians). Then, the participants were randomized to either the 4AT or the comparator CAM group using computer-generated pseudo-random numbers, stratified by study site, with block allocation. All assessments were performed by pairs of independent assessors blinded to the results of the other assessment.
Main outcome measures: All participants were evaluated by the reference standard (DSM-IV criteria for delirium) and by either 4AT or CAM instruments for delirium. The accuracy of the 4AT instrument was evaluated by comparing its positive and negative predictive values, sensitivity, and specificity to the reference standard and analyzed via the area under the receiver operating characteristic curve. The diagnostic accuracy of 4AT, compared to the CAM, was evaluated by comparing positive and negative predictive values, sensitivity, and specificity using Fisher’s exact test. The overall performance of 4AT and CAM was summarized using Youden’s Index and the diagnostic odds ratio of sensitivity to specificity.
Results: All 843 individuals enrolled in the study were randomized and 785 were included in the analysis (23 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome). Of the participants analyzed, the mean age was 81.4 [6.4] years, and 12.1% (95/785) had delirium by reference standard assessment, 14.3% (56/392) by 4AT, and 4.7% (18/384) by CAM. The 4AT group had an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.96), a sensitivity of 76% (95% CI, 61%-87%), and a specificity of 94% (95% CI, 92%-97%). In comparison, the CAM group had a sensitivity of 40% (95% CI, 26%-57%) and a specificity of 100% (95% CI, 98%-100%).
Conclusions: The 4AT is a pragmatic screening test for delirium in a medical space that does not require special training to administer. The use of this instrument may help to improve delirium detection as a part of routine clinical care in hospitalized older adults.
Commentary
Delirium is an acute confusional state marked by fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is exceedingly common in older patients in both surgical and medical settings and is associated with increased morbidity, mortality, hospital length of stay, institutionalization, and health care costs. Delirium is frequently underdiagnosed in the hospitalized setting, perhaps due to a combination of its waxing and waning nature and a lack of pragmatic and easily implementable screening tools that can be readily administered by clinicians and nonclinicians alike.1 While the CAM is a well-validated instrument to diagnose delirium, it requires specific training in the rating of each of the cardinal features ascertained through a brief cognitive assessment and takes 5 to 10 minutes to complete. Taken together, given the high patient load for clinicians in the hospital setting, the validation and application of brief delirium screening instruments that can be reliably administered by nonphysicians and nonclinicians may enhance delirium detection in vulnerable patients and consequently improve their outcomes.
In Study 1, Oberhaus et al approach the challenge of underdiagnosing delirium in the postoperative setting by investigating whether the widely accepted long-form CAM and an abbreviated 3-minute version, the 3D-CAM, provide similar delirium detection in older surgical patients. The authors found that both instruments were reliable tests individually (high interrater reliability) and had good overall agreement. However, the 3D-CAM was more likely to yield a positive diagnosis of delirium compared to the long-form CAM, consistent with its purpose as a screening tool with a high sensitivity. It is important to emphasize that the 3D-CAM takes less time to administer, but also requires less extensive training and clinical knowledge than the long-form CAM. Therefore, this instrument meets the prerequisite of a brief screening test that can be rapidly administered by nonclinicians, and if affirmative, followed by a more extensive confirmatory test performed by a clinician. Limitations of this study include a lack of a reference standard structured interview conducted by a physician-rater to better determine the true diagnostic accuracy of both 3D-CAM and CAM assessments, and the use of convenience sampling at a single center, which reduces the generalizability of its findings.
In a similar vein, Shenkin et al in Study 2 attempt to evaluate the utility of the 4AT instrument in diagnosing delirium in older medical inpatients by testing the diagnostic accuracy of the 4AT against a reference standard (ie, DSM-IV–based evaluation by physicians) as well as comparing it to CAM. The 4AT takes less time (~2 minutes) and requires less knowledge and training to administer as compared to the CAM. The study showed that the abbreviated 4AT, compared to CAM, had a higher sensitivity (76% vs 40%) and lower specificity (94% vs 100%) in delirium detection. Thus, akin to the application of 3D-CAM in the postoperative setting, 4AT possesses key characteristics of a brief delirium screening test for older patients in the acute medical setting. In contrast to the Oberhaus et al study, a major strength of this study was the utilization of a reference standard that was validated by expert consensus. This allowed the 4AT and CAM assessments to be compared to a more objective standard, thereby directly testing their diagnostic performance in detecting delirium.
Application for Clinical Practice and System Implementation
The findings from both Study 1 and 2 suggest that using an abbreviated delirium instrument in both surgical and acute medical settings may provide a pragmatic and sensitive method to detect delirium in older patients. The brevity of administration of 3D-CAM (~3 minutes) and 4AT (~2 minutes), combined with their higher sensitivity for detecting delirium compared to CAM, make these instruments potentially effective rapid screening tests for delirium in hospitalized older patients. Importantly, the utilization of such instruments might be a feasible way to mitigate the issue of underdiagnosing delirium in the hospital.
Several additional aspects of these abbreviated delirium instruments increase their suitability for clinical application. Specifically, the 3D-CAM and 4AT require less extensive training and clinical knowledge to both administer and interpret the results than the CAM.2 For instance, a multistage, multiday training for CAM is a key factor in maintaining its diagnostic accuracy.3,4 In contrast, the 3D-CAM requires only a 1- to 2-hour training session, and the 4AT can be administered by a nonclinician without the need for instrument-specific training. Thus, implementation of these instruments can be particularly pragmatic in clinical settings in which the staff involved in delirium screening cannot undergo the substantial training required to administer CAM. Moreover, these abbreviated tests enable nonphysician care team members to assume the role of delirium screener in the hospital. Taken together, the adoption of these abbreviated instruments may facilitate brief screenings of delirium in older patients by caregivers who see them most often—nurses and certified nursing assistants—thereby improving early detection and prevention of delirium-related complications in the hospital.
The feasibility of using abbreviated delirium screening instruments in the hospital setting raises a system implementation question—if these instruments are designed to be administered by those with limited to no training, could nonclinicians, such as hospital volunteers, effectively take on delirium screening roles in the hospital? If volunteers are able to take on this role, the integration of hospital volunteers into the clinical team can greatly expand the capacity for delirium screening in the hospital setting. Further research is warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Practice Points
- Abbreviated delirium screening tools such as 3D-CAM and 4AT may be pragmatic instruments to improve delirium detection in surgical and hospitalized older patients, respectively.
- Further studies are warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Jared Doan, BS, and Fred Ko, MD
Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
2. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi:10.7326/M14-0865
3. Green JR, Smith J, Teale E, et al. Use of the confusion assessment method in multicentre delirium trials: training and standardisation. BMC Geriatr. 2019;19(1):107. doi:10.1186/s12877-019-1129-8
4. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. Am Geriatr Soc. 2008;56(5):823-830. doi:10.1111/j.1532-5415.2008.01674.x
Study 1 Overview (Oberhaus et al)
Objective: To compare the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) to the long-form Confusion Assessment Method (CAM) in detecting postoperative delirium.
Design: Prospective concurrent comparison of 3D-CAM and CAM evaluations in a cohort of postoperative geriatric patients.
Setting and participants: Eligible participants were patients aged 60 years or older undergoing major elective surgery at Barnes Jewish Hospital (St. Louis, Missouri) who were enrolled in ongoing clinical trials (PODCAST, ENGAGES, SATISFY-SOS) between 2015 and 2018. Surgeries were at least 2 hours in length and required general anesthesia, planned extubation, and a minimum 2-day hospital stay. Investigators were extensively trained in administering 3D-CAM and CAM instruments. Participants were evaluated 2 hours after the end of anesthesia care on the day of surgery, then daily until follow-up was completed per clinical trial protocol or until the participant was determined by CAM to be nondelirious for 3 consecutive days. For each evaluation, both 3D-CAM and CAM assessors approached the participant together, but the evaluation was conducted such that the 3D-CAM assessor was masked to the additional questions ascertained by the long-form CAM assessment. The 3D-CAM or CAM assessor independently scored their respective assessments blinded to the results of the other assessor.
Main outcome measures: Participants were concurrently evaluated for postoperative delirium by both 3D-CAM and long-form CAM assessments. Comparisons between 3D-CAM and CAM scores were made using Cohen κ with repeated measures, generalized linear mixed-effects model, and Bland-Altman analysis.
Main results: Sixteen raters performed 471 concurrent 3D-CAM and CAM assessments in 299 participants (mean [SD] age, 69 [6.5] years). Of these participants, 152 (50.8%) were men, 263 (88.0%) were White, and 211 (70.6%) underwent noncardiac surgery. Both instruments showed good intraclass correlation (0.98 for 3D-CAM, 0.84 for CAM) with good overall agreement (Cohen κ = 0.71; 95% CI, 0.58-0.83). The mixed-effects model indicated a significant disagreement between the 3D-CAM and CAM assessments (estimated difference in fixed effect, –0.68; 95% CI, –1.32 to –0.05; P = .04). The Bland-Altman analysis showed that the probability of a delirium diagnosis with the 3D-CAM was more than twice that with the CAM (probability ratio, 2.78; 95% CI, 2.44-3.23).
Conclusion: The high degree of agreement between 3D-CAM and long-form CAM assessments suggests that the former may be a pragmatic and easy-to-administer clinical tool to screen for postoperative delirium in vulnerable older surgical patients.
Study 2 Overview (Shenkin et al)
Objective: To assess the accuracy of the 4 ‘A’s Test (4AT) for delirium detection in the medical inpatient setting and to compare the 4AT to the CAM.
Design: Prospective randomized diagnostic test accuracy study.
Setting and participants: This study was conducted in emergency departments and acute medical wards at 3 UK sites (Edinburgh, Bradford, and Sheffield) and enrolled acute medical patients aged 70 years or older without acute life-threatening illnesses and/or coma. Assessors administering the delirium evaluation were nurses or graduate clinical research associates who underwent systematic training in delirium and delirium assessment. Additional training was provided to those administering the CAM but not to those administering the 4AT as the latter is designed to be administered without special training. First, all participants underwent a reference standard delirium assessment using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria to derive a final definitive diagnosis of delirium via expert consensus (1 psychiatrist and 2 geriatricians). Then, the participants were randomized to either the 4AT or the comparator CAM group using computer-generated pseudo-random numbers, stratified by study site, with block allocation. All assessments were performed by pairs of independent assessors blinded to the results of the other assessment.
Main outcome measures: All participants were evaluated by the reference standard (DSM-IV criteria for delirium) and by either 4AT or CAM instruments for delirium. The accuracy of the 4AT instrument was evaluated by comparing its positive and negative predictive values, sensitivity, and specificity to the reference standard and analyzed via the area under the receiver operating characteristic curve. The diagnostic accuracy of 4AT, compared to the CAM, was evaluated by comparing positive and negative predictive values, sensitivity, and specificity using Fisher’s exact test. The overall performance of 4AT and CAM was summarized using Youden’s Index and the diagnostic odds ratio of sensitivity to specificity.
Results: All 843 individuals enrolled in the study were randomized and 785 were included in the analysis (23 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome). Of the participants analyzed, the mean age was 81.4 [6.4] years, and 12.1% (95/785) had delirium by reference standard assessment, 14.3% (56/392) by 4AT, and 4.7% (18/384) by CAM. The 4AT group had an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.96), a sensitivity of 76% (95% CI, 61%-87%), and a specificity of 94% (95% CI, 92%-97%). In comparison, the CAM group had a sensitivity of 40% (95% CI, 26%-57%) and a specificity of 100% (95% CI, 98%-100%).
Conclusions: The 4AT is a pragmatic screening test for delirium in a medical space that does not require special training to administer. The use of this instrument may help to improve delirium detection as a part of routine clinical care in hospitalized older adults.
Commentary
Delirium is an acute confusional state marked by fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is exceedingly common in older patients in both surgical and medical settings and is associated with increased morbidity, mortality, hospital length of stay, institutionalization, and health care costs. Delirium is frequently underdiagnosed in the hospitalized setting, perhaps due to a combination of its waxing and waning nature and a lack of pragmatic and easily implementable screening tools that can be readily administered by clinicians and nonclinicians alike.1 While the CAM is a well-validated instrument to diagnose delirium, it requires specific training in the rating of each of the cardinal features ascertained through a brief cognitive assessment and takes 5 to 10 minutes to complete. Taken together, given the high patient load for clinicians in the hospital setting, the validation and application of brief delirium screening instruments that can be reliably administered by nonphysicians and nonclinicians may enhance delirium detection in vulnerable patients and consequently improve their outcomes.
In Study 1, Oberhaus et al approach the challenge of underdiagnosing delirium in the postoperative setting by investigating whether the widely accepted long-form CAM and an abbreviated 3-minute version, the 3D-CAM, provide similar delirium detection in older surgical patients. The authors found that both instruments were reliable tests individually (high interrater reliability) and had good overall agreement. However, the 3D-CAM was more likely to yield a positive diagnosis of delirium compared to the long-form CAM, consistent with its purpose as a screening tool with a high sensitivity. It is important to emphasize that the 3D-CAM takes less time to administer, but also requires less extensive training and clinical knowledge than the long-form CAM. Therefore, this instrument meets the prerequisite of a brief screening test that can be rapidly administered by nonclinicians, and if affirmative, followed by a more extensive confirmatory test performed by a clinician. Limitations of this study include a lack of a reference standard structured interview conducted by a physician-rater to better determine the true diagnostic accuracy of both 3D-CAM and CAM assessments, and the use of convenience sampling at a single center, which reduces the generalizability of its findings.
In a similar vein, Shenkin et al in Study 2 attempt to evaluate the utility of the 4AT instrument in diagnosing delirium in older medical inpatients by testing the diagnostic accuracy of the 4AT against a reference standard (ie, DSM-IV–based evaluation by physicians) as well as comparing it to CAM. The 4AT takes less time (~2 minutes) and requires less knowledge and training to administer as compared to the CAM. The study showed that the abbreviated 4AT, compared to CAM, had a higher sensitivity (76% vs 40%) and lower specificity (94% vs 100%) in delirium detection. Thus, akin to the application of 3D-CAM in the postoperative setting, 4AT possesses key characteristics of a brief delirium screening test for older patients in the acute medical setting. In contrast to the Oberhaus et al study, a major strength of this study was the utilization of a reference standard that was validated by expert consensus. This allowed the 4AT and CAM assessments to be compared to a more objective standard, thereby directly testing their diagnostic performance in detecting delirium.
Application for Clinical Practice and System Implementation
The findings from both Study 1 and 2 suggest that using an abbreviated delirium instrument in both surgical and acute medical settings may provide a pragmatic and sensitive method to detect delirium in older patients. The brevity of administration of 3D-CAM (~3 minutes) and 4AT (~2 minutes), combined with their higher sensitivity for detecting delirium compared to CAM, make these instruments potentially effective rapid screening tests for delirium in hospitalized older patients. Importantly, the utilization of such instruments might be a feasible way to mitigate the issue of underdiagnosing delirium in the hospital.
Several additional aspects of these abbreviated delirium instruments increase their suitability for clinical application. Specifically, the 3D-CAM and 4AT require less extensive training and clinical knowledge to both administer and interpret the results than the CAM.2 For instance, a multistage, multiday training for CAM is a key factor in maintaining its diagnostic accuracy.3,4 In contrast, the 3D-CAM requires only a 1- to 2-hour training session, and the 4AT can be administered by a nonclinician without the need for instrument-specific training. Thus, implementation of these instruments can be particularly pragmatic in clinical settings in which the staff involved in delirium screening cannot undergo the substantial training required to administer CAM. Moreover, these abbreviated tests enable nonphysician care team members to assume the role of delirium screener in the hospital. Taken together, the adoption of these abbreviated instruments may facilitate brief screenings of delirium in older patients by caregivers who see them most often—nurses and certified nursing assistants—thereby improving early detection and prevention of delirium-related complications in the hospital.
The feasibility of using abbreviated delirium screening instruments in the hospital setting raises a system implementation question—if these instruments are designed to be administered by those with limited to no training, could nonclinicians, such as hospital volunteers, effectively take on delirium screening roles in the hospital? If volunteers are able to take on this role, the integration of hospital volunteers into the clinical team can greatly expand the capacity for delirium screening in the hospital setting. Further research is warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Practice Points
- Abbreviated delirium screening tools such as 3D-CAM and 4AT may be pragmatic instruments to improve delirium detection in surgical and hospitalized older patients, respectively.
- Further studies are warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Jared Doan, BS, and Fred Ko, MD
Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai
Study 1 Overview (Oberhaus et al)
Objective: To compare the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) to the long-form Confusion Assessment Method (CAM) in detecting postoperative delirium.
Design: Prospective concurrent comparison of 3D-CAM and CAM evaluations in a cohort of postoperative geriatric patients.
Setting and participants: Eligible participants were patients aged 60 years or older undergoing major elective surgery at Barnes Jewish Hospital (St. Louis, Missouri) who were enrolled in ongoing clinical trials (PODCAST, ENGAGES, SATISFY-SOS) between 2015 and 2018. Surgeries were at least 2 hours in length and required general anesthesia, planned extubation, and a minimum 2-day hospital stay. Investigators were extensively trained in administering 3D-CAM and CAM instruments. Participants were evaluated 2 hours after the end of anesthesia care on the day of surgery, then daily until follow-up was completed per clinical trial protocol or until the participant was determined by CAM to be nondelirious for 3 consecutive days. For each evaluation, both 3D-CAM and CAM assessors approached the participant together, but the evaluation was conducted such that the 3D-CAM assessor was masked to the additional questions ascertained by the long-form CAM assessment. The 3D-CAM or CAM assessor independently scored their respective assessments blinded to the results of the other assessor.
Main outcome measures: Participants were concurrently evaluated for postoperative delirium by both 3D-CAM and long-form CAM assessments. Comparisons between 3D-CAM and CAM scores were made using Cohen κ with repeated measures, generalized linear mixed-effects model, and Bland-Altman analysis.
Main results: Sixteen raters performed 471 concurrent 3D-CAM and CAM assessments in 299 participants (mean [SD] age, 69 [6.5] years). Of these participants, 152 (50.8%) were men, 263 (88.0%) were White, and 211 (70.6%) underwent noncardiac surgery. Both instruments showed good intraclass correlation (0.98 for 3D-CAM, 0.84 for CAM) with good overall agreement (Cohen κ = 0.71; 95% CI, 0.58-0.83). The mixed-effects model indicated a significant disagreement between the 3D-CAM and CAM assessments (estimated difference in fixed effect, –0.68; 95% CI, –1.32 to –0.05; P = .04). The Bland-Altman analysis showed that the probability of a delirium diagnosis with the 3D-CAM was more than twice that with the CAM (probability ratio, 2.78; 95% CI, 2.44-3.23).
Conclusion: The high degree of agreement between 3D-CAM and long-form CAM assessments suggests that the former may be a pragmatic and easy-to-administer clinical tool to screen for postoperative delirium in vulnerable older surgical patients.
Study 2 Overview (Shenkin et al)
Objective: To assess the accuracy of the 4 ‘A’s Test (4AT) for delirium detection in the medical inpatient setting and to compare the 4AT to the CAM.
Design: Prospective randomized diagnostic test accuracy study.
Setting and participants: This study was conducted in emergency departments and acute medical wards at 3 UK sites (Edinburgh, Bradford, and Sheffield) and enrolled acute medical patients aged 70 years or older without acute life-threatening illnesses and/or coma. Assessors administering the delirium evaluation were nurses or graduate clinical research associates who underwent systematic training in delirium and delirium assessment. Additional training was provided to those administering the CAM but not to those administering the 4AT as the latter is designed to be administered without special training. First, all participants underwent a reference standard delirium assessment using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria to derive a final definitive diagnosis of delirium via expert consensus (1 psychiatrist and 2 geriatricians). Then, the participants were randomized to either the 4AT or the comparator CAM group using computer-generated pseudo-random numbers, stratified by study site, with block allocation. All assessments were performed by pairs of independent assessors blinded to the results of the other assessment.
Main outcome measures: All participants were evaluated by the reference standard (DSM-IV criteria for delirium) and by either 4AT or CAM instruments for delirium. The accuracy of the 4AT instrument was evaluated by comparing its positive and negative predictive values, sensitivity, and specificity to the reference standard and analyzed via the area under the receiver operating characteristic curve. The diagnostic accuracy of 4AT, compared to the CAM, was evaluated by comparing positive and negative predictive values, sensitivity, and specificity using Fisher’s exact test. The overall performance of 4AT and CAM was summarized using Youden’s Index and the diagnostic odds ratio of sensitivity to specificity.
Results: All 843 individuals enrolled in the study were randomized and 785 were included in the analysis (23 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome). Of the participants analyzed, the mean age was 81.4 [6.4] years, and 12.1% (95/785) had delirium by reference standard assessment, 14.3% (56/392) by 4AT, and 4.7% (18/384) by CAM. The 4AT group had an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.96), a sensitivity of 76% (95% CI, 61%-87%), and a specificity of 94% (95% CI, 92%-97%). In comparison, the CAM group had a sensitivity of 40% (95% CI, 26%-57%) and a specificity of 100% (95% CI, 98%-100%).
Conclusions: The 4AT is a pragmatic screening test for delirium in a medical space that does not require special training to administer. The use of this instrument may help to improve delirium detection as a part of routine clinical care in hospitalized older adults.
Commentary
Delirium is an acute confusional state marked by fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is exceedingly common in older patients in both surgical and medical settings and is associated with increased morbidity, mortality, hospital length of stay, institutionalization, and health care costs. Delirium is frequently underdiagnosed in the hospitalized setting, perhaps due to a combination of its waxing and waning nature and a lack of pragmatic and easily implementable screening tools that can be readily administered by clinicians and nonclinicians alike.1 While the CAM is a well-validated instrument to diagnose delirium, it requires specific training in the rating of each of the cardinal features ascertained through a brief cognitive assessment and takes 5 to 10 minutes to complete. Taken together, given the high patient load for clinicians in the hospital setting, the validation and application of brief delirium screening instruments that can be reliably administered by nonphysicians and nonclinicians may enhance delirium detection in vulnerable patients and consequently improve their outcomes.
In Study 1, Oberhaus et al approach the challenge of underdiagnosing delirium in the postoperative setting by investigating whether the widely accepted long-form CAM and an abbreviated 3-minute version, the 3D-CAM, provide similar delirium detection in older surgical patients. The authors found that both instruments were reliable tests individually (high interrater reliability) and had good overall agreement. However, the 3D-CAM was more likely to yield a positive diagnosis of delirium compared to the long-form CAM, consistent with its purpose as a screening tool with a high sensitivity. It is important to emphasize that the 3D-CAM takes less time to administer, but also requires less extensive training and clinical knowledge than the long-form CAM. Therefore, this instrument meets the prerequisite of a brief screening test that can be rapidly administered by nonclinicians, and if affirmative, followed by a more extensive confirmatory test performed by a clinician. Limitations of this study include a lack of a reference standard structured interview conducted by a physician-rater to better determine the true diagnostic accuracy of both 3D-CAM and CAM assessments, and the use of convenience sampling at a single center, which reduces the generalizability of its findings.
In a similar vein, Shenkin et al in Study 2 attempt to evaluate the utility of the 4AT instrument in diagnosing delirium in older medical inpatients by testing the diagnostic accuracy of the 4AT against a reference standard (ie, DSM-IV–based evaluation by physicians) as well as comparing it to CAM. The 4AT takes less time (~2 minutes) and requires less knowledge and training to administer as compared to the CAM. The study showed that the abbreviated 4AT, compared to CAM, had a higher sensitivity (76% vs 40%) and lower specificity (94% vs 100%) in delirium detection. Thus, akin to the application of 3D-CAM in the postoperative setting, 4AT possesses key characteristics of a brief delirium screening test for older patients in the acute medical setting. In contrast to the Oberhaus et al study, a major strength of this study was the utilization of a reference standard that was validated by expert consensus. This allowed the 4AT and CAM assessments to be compared to a more objective standard, thereby directly testing their diagnostic performance in detecting delirium.
Application for Clinical Practice and System Implementation
The findings from both Study 1 and 2 suggest that using an abbreviated delirium instrument in both surgical and acute medical settings may provide a pragmatic and sensitive method to detect delirium in older patients. The brevity of administration of 3D-CAM (~3 minutes) and 4AT (~2 minutes), combined with their higher sensitivity for detecting delirium compared to CAM, make these instruments potentially effective rapid screening tests for delirium in hospitalized older patients. Importantly, the utilization of such instruments might be a feasible way to mitigate the issue of underdiagnosing delirium in the hospital.
Several additional aspects of these abbreviated delirium instruments increase their suitability for clinical application. Specifically, the 3D-CAM and 4AT require less extensive training and clinical knowledge to both administer and interpret the results than the CAM.2 For instance, a multistage, multiday training for CAM is a key factor in maintaining its diagnostic accuracy.3,4 In contrast, the 3D-CAM requires only a 1- to 2-hour training session, and the 4AT can be administered by a nonclinician without the need for instrument-specific training. Thus, implementation of these instruments can be particularly pragmatic in clinical settings in which the staff involved in delirium screening cannot undergo the substantial training required to administer CAM. Moreover, these abbreviated tests enable nonphysician care team members to assume the role of delirium screener in the hospital. Taken together, the adoption of these abbreviated instruments may facilitate brief screenings of delirium in older patients by caregivers who see them most often—nurses and certified nursing assistants—thereby improving early detection and prevention of delirium-related complications in the hospital.
The feasibility of using abbreviated delirium screening instruments in the hospital setting raises a system implementation question—if these instruments are designed to be administered by those with limited to no training, could nonclinicians, such as hospital volunteers, effectively take on delirium screening roles in the hospital? If volunteers are able to take on this role, the integration of hospital volunteers into the clinical team can greatly expand the capacity for delirium screening in the hospital setting. Further research is warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Practice Points
- Abbreviated delirium screening tools such as 3D-CAM and 4AT may be pragmatic instruments to improve delirium detection in surgical and hospitalized older patients, respectively.
- Further studies are warranted to validate the diagnostic accuracy of 3D-CAM and 4AT by nonclinician administrators in order to more broadly adopt this approach to delirium screening.
Jared Doan, BS, and Fred Ko, MD
Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
2. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi:10.7326/M14-0865
3. Green JR, Smith J, Teale E, et al. Use of the confusion assessment method in multicentre delirium trials: training and standardisation. BMC Geriatr. 2019;19(1):107. doi:10.1186/s12877-019-1129-8
4. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. Am Geriatr Soc. 2008;56(5):823-830. doi:10.1111/j.1532-5415.2008.01674.x
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
2. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi:10.7326/M14-0865
3. Green JR, Smith J, Teale E, et al. Use of the confusion assessment method in multicentre delirium trials: training and standardisation. BMC Geriatr. 2019;19(1):107. doi:10.1186/s12877-019-1129-8
4. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. Am Geriatr Soc. 2008;56(5):823-830. doi:10.1111/j.1532-5415.2008.01674.x
VA Launches Virtual Tumor Board
SAN DIEGO – The US Department of Veterans Affairs (VA) TeleOncology program has rolled out a virtual tumor board that brings medical professionals together to offer insight and guidance about challenging hematology cases. Over the past 6 months the board has held 10 sessions and reviewed about 20 cases. A small survey found that participants think the meetings are beneficial.
“Virtual tumor boards help to connect experts across the country to leverage the expertise within the VA,” he-matologist/oncologist Thomas Rodgers, MD, of the Duke Cancer Institute and Durham Veterans Affairs Medical Center, told Federal Practitioner in an interview. He is the lead author of a poster about the program that was pre-sented here at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
As Dr. Rodgers noted, tumor boards are already in place at some VA centers. However, “they are not available at every VA and often are not set up to cover every cancer type.”
The VA National TeleOncology program created the virtual tumor board program as part of its mission to ex-tend hematology/oncology services across the system. “Cancer care has become increasingly complex. Beyond ad-vancing therapeutics, patient care often involves multiple specialties and medical disciplines,” Dr. Rodgers said. “A tumor board offers a forum for these specialists to communicate with each other in real time, not only to help estab-lish the correct diagnosis and stage of cancer but also to form a consensus on the most fitting treatment option. Think of it as getting all of the people involved in a person’s care in the same room.”
Currently, he said, the virtual tumor boards cover patients with malignant hematology diagnoses such as leuke-mia, multiple myeloma, and lymphomas. “We welcome submissions. If a provider is interested in submitting a case, they can email us and will be provided with a short intake form. Once submitted, we will collect necessary imaging and pathology for review. The provider will then present the patient case on the day of the tumor board.”
Typically, more than 30 medical professionals participate in the virtual tumor boards, Dr. Rodgers said, repre-senting medical oncology/hematology, pathology, radiology, palliative care, pharmacy, social work, and die-tary/nutrition.
According to the poster presented at AVAHO, 9 participants responded to a survey after 4 tumor board sessions. All found the boards to be beneficial or somewhat beneficial, and 55% reported that they were “highly applicable” to their practice.
Pathologist Claudio A. Mosse, MD, PhD, of Vanderbilt University Medical Center and VA Tennessee Valley Healthcare System, praised the virtual tumor board program. “It’s been incredibly useful from my end as a pathologist as it shows me which diagnoses are most challenging for my colleagues,” Dr. Mosse said in an inter-view. “Reviewing and then presenting these challenging cases forces me to go into the published literature to come to a unitary diagnosis based on the patient history, radiology, various laboratory tests, and the biopsy I was asked to review.”
He added that “as a pathologist, I learn so much from the hematologists as they discuss the possible therapeutic options, and that strengthens my ability as a pathologist because I have to understand how one diagnosis versus an-other affects their therapeutic decision tree.”
What’s next for the virtual tumor board program? The next step is to expand to solid tumors, said VA Pittsburgh Healthcare System hematologist/oncologist Vida Almario Passero, MD, MBA, chief medical officer of National TeleOncology, in an interview.
No disclosures were reported.
SAN DIEGO – The US Department of Veterans Affairs (VA) TeleOncology program has rolled out a virtual tumor board that brings medical professionals together to offer insight and guidance about challenging hematology cases. Over the past 6 months the board has held 10 sessions and reviewed about 20 cases. A small survey found that participants think the meetings are beneficial.
“Virtual tumor boards help to connect experts across the country to leverage the expertise within the VA,” he-matologist/oncologist Thomas Rodgers, MD, of the Duke Cancer Institute and Durham Veterans Affairs Medical Center, told Federal Practitioner in an interview. He is the lead author of a poster about the program that was pre-sented here at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
As Dr. Rodgers noted, tumor boards are already in place at some VA centers. However, “they are not available at every VA and often are not set up to cover every cancer type.”
The VA National TeleOncology program created the virtual tumor board program as part of its mission to ex-tend hematology/oncology services across the system. “Cancer care has become increasingly complex. Beyond ad-vancing therapeutics, patient care often involves multiple specialties and medical disciplines,” Dr. Rodgers said. “A tumor board offers a forum for these specialists to communicate with each other in real time, not only to help estab-lish the correct diagnosis and stage of cancer but also to form a consensus on the most fitting treatment option. Think of it as getting all of the people involved in a person’s care in the same room.”
Currently, he said, the virtual tumor boards cover patients with malignant hematology diagnoses such as leuke-mia, multiple myeloma, and lymphomas. “We welcome submissions. If a provider is interested in submitting a case, they can email us and will be provided with a short intake form. Once submitted, we will collect necessary imaging and pathology for review. The provider will then present the patient case on the day of the tumor board.”
Typically, more than 30 medical professionals participate in the virtual tumor boards, Dr. Rodgers said, repre-senting medical oncology/hematology, pathology, radiology, palliative care, pharmacy, social work, and die-tary/nutrition.
According to the poster presented at AVAHO, 9 participants responded to a survey after 4 tumor board sessions. All found the boards to be beneficial or somewhat beneficial, and 55% reported that they were “highly applicable” to their practice.
Pathologist Claudio A. Mosse, MD, PhD, of Vanderbilt University Medical Center and VA Tennessee Valley Healthcare System, praised the virtual tumor board program. “It’s been incredibly useful from my end as a pathologist as it shows me which diagnoses are most challenging for my colleagues,” Dr. Mosse said in an inter-view. “Reviewing and then presenting these challenging cases forces me to go into the published literature to come to a unitary diagnosis based on the patient history, radiology, various laboratory tests, and the biopsy I was asked to review.”
He added that “as a pathologist, I learn so much from the hematologists as they discuss the possible therapeutic options, and that strengthens my ability as a pathologist because I have to understand how one diagnosis versus an-other affects their therapeutic decision tree.”
What’s next for the virtual tumor board program? The next step is to expand to solid tumors, said VA Pittsburgh Healthcare System hematologist/oncologist Vida Almario Passero, MD, MBA, chief medical officer of National TeleOncology, in an interview.
No disclosures were reported.
SAN DIEGO – The US Department of Veterans Affairs (VA) TeleOncology program has rolled out a virtual tumor board that brings medical professionals together to offer insight and guidance about challenging hematology cases. Over the past 6 months the board has held 10 sessions and reviewed about 20 cases. A small survey found that participants think the meetings are beneficial.
“Virtual tumor boards help to connect experts across the country to leverage the expertise within the VA,” he-matologist/oncologist Thomas Rodgers, MD, of the Duke Cancer Institute and Durham Veterans Affairs Medical Center, told Federal Practitioner in an interview. He is the lead author of a poster about the program that was pre-sented here at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
As Dr. Rodgers noted, tumor boards are already in place at some VA centers. However, “they are not available at every VA and often are not set up to cover every cancer type.”
The VA National TeleOncology program created the virtual tumor board program as part of its mission to ex-tend hematology/oncology services across the system. “Cancer care has become increasingly complex. Beyond ad-vancing therapeutics, patient care often involves multiple specialties and medical disciplines,” Dr. Rodgers said. “A tumor board offers a forum for these specialists to communicate with each other in real time, not only to help estab-lish the correct diagnosis and stage of cancer but also to form a consensus on the most fitting treatment option. Think of it as getting all of the people involved in a person’s care in the same room.”
Currently, he said, the virtual tumor boards cover patients with malignant hematology diagnoses such as leuke-mia, multiple myeloma, and lymphomas. “We welcome submissions. If a provider is interested in submitting a case, they can email us and will be provided with a short intake form. Once submitted, we will collect necessary imaging and pathology for review. The provider will then present the patient case on the day of the tumor board.”
Typically, more than 30 medical professionals participate in the virtual tumor boards, Dr. Rodgers said, repre-senting medical oncology/hematology, pathology, radiology, palliative care, pharmacy, social work, and die-tary/nutrition.
According to the poster presented at AVAHO, 9 participants responded to a survey after 4 tumor board sessions. All found the boards to be beneficial or somewhat beneficial, and 55% reported that they were “highly applicable” to their practice.
Pathologist Claudio A. Mosse, MD, PhD, of Vanderbilt University Medical Center and VA Tennessee Valley Healthcare System, praised the virtual tumor board program. “It’s been incredibly useful from my end as a pathologist as it shows me which diagnoses are most challenging for my colleagues,” Dr. Mosse said in an inter-view. “Reviewing and then presenting these challenging cases forces me to go into the published literature to come to a unitary diagnosis based on the patient history, radiology, various laboratory tests, and the biopsy I was asked to review.”
He added that “as a pathologist, I learn so much from the hematologists as they discuss the possible therapeutic options, and that strengthens my ability as a pathologist because I have to understand how one diagnosis versus an-other affects their therapeutic decision tree.”
What’s next for the virtual tumor board program? The next step is to expand to solid tumors, said VA Pittsburgh Healthcare System hematologist/oncologist Vida Almario Passero, MD, MBA, chief medical officer of National TeleOncology, in an interview.
No disclosures were reported.
