User login
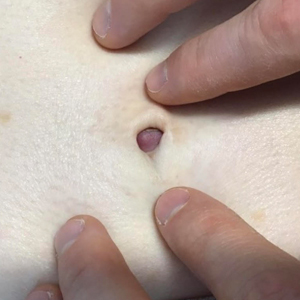
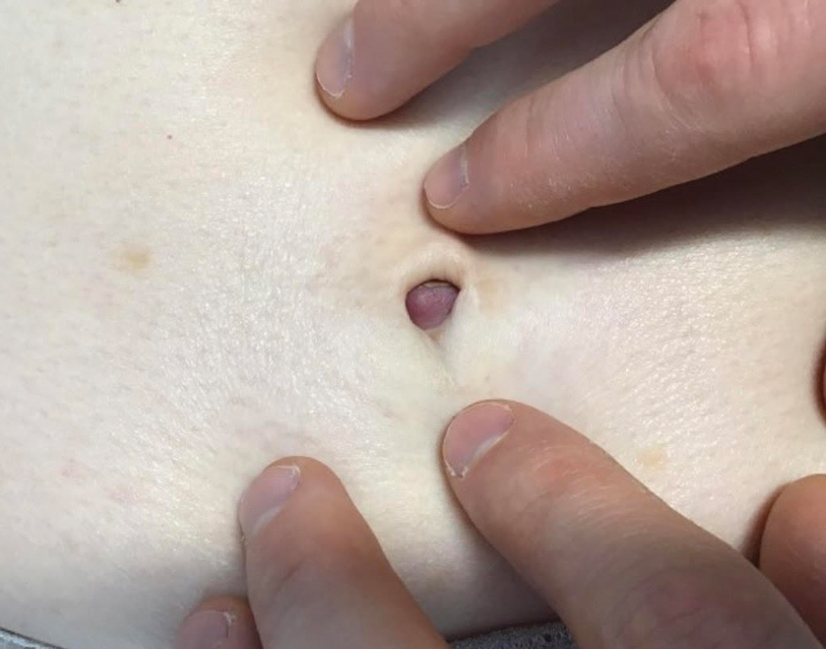
Asymptomatic Umbilical Nodule
The Diagnosis: Sister Mary Joseph Nodule
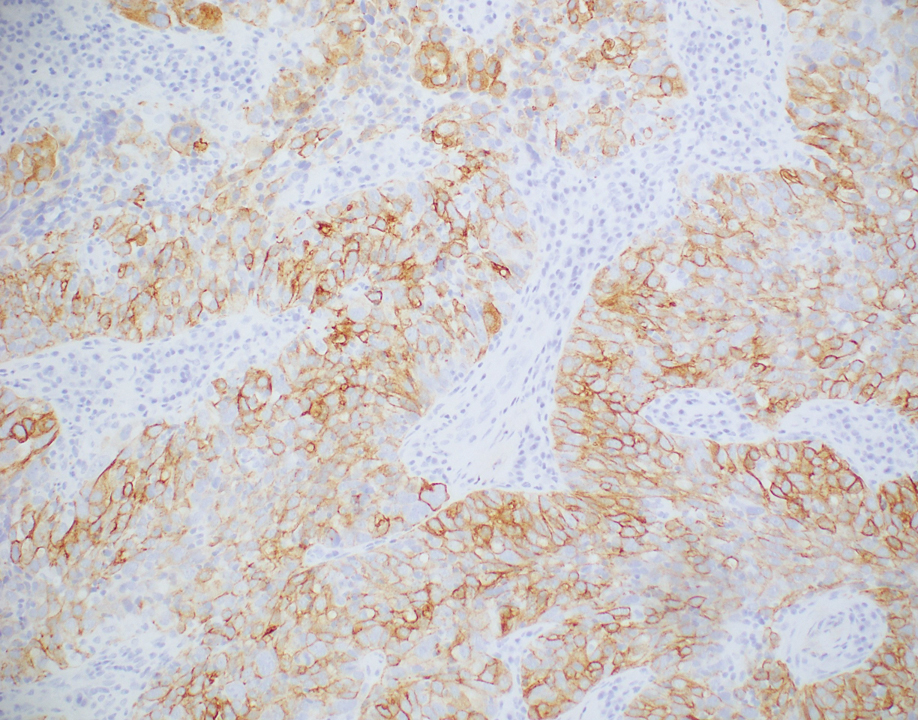
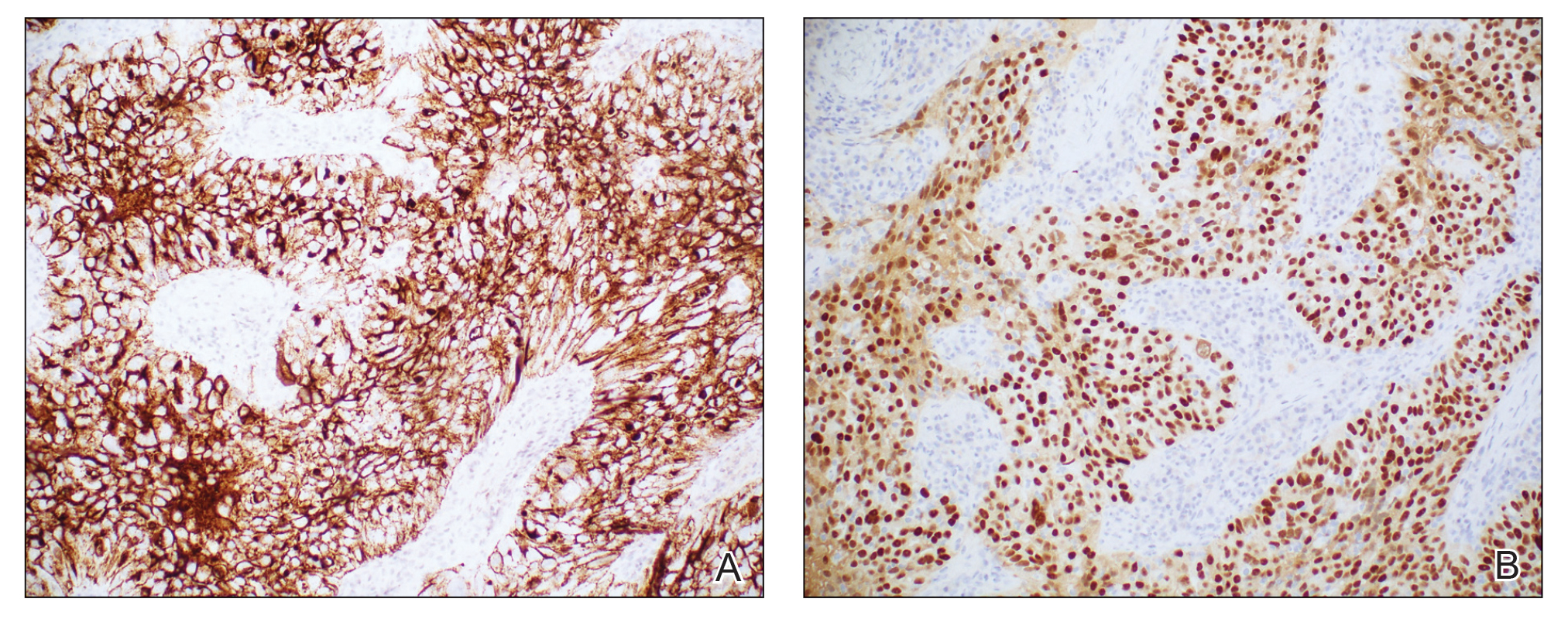
Histopathologic analysis of the biopsy specimen revealed a dense infiltrate of large, hyperchromatic, mucin-producing cells exhibiting varying degrees of nuclear pleomorphism (Figure 1). Immunohistochemical (IHC) staining was negative for cytokeratin (CK) 20; however, CK7 was found positive (Figure 2), which confirmed the presence of a metastatic adenocarcinoma, consistent with the clinical diagnosis of a Sister Mary Joseph nodule (SMJN). Subsequent IHC workup to determine the site of origin revealed densely positive expression of both cancer antigen 125 and paired homeobox gene 8 (PAX-8)(Figure 3), consistent with primary ovarian disease. Furthermore, expression of estrogen receptor and p53 both were positive within the nuclei, illustrating an aberrant expression pattern. On the other hand, cancer antigen 19-9, caudal-type homeobox 2, gross cystic disease fluid protein 15, and mammaglobin were all determined negative, thus leading to the pathologic diagnosis of a metastatic ovarian adenocarcinoma. Additional workup with computed tomography of the abdomen and pelvis highlighted a large left ovarian mass with multiple omental nodules as well as enlarged retroperitoneal and pelvic lymph nodes.

The SMJN is a rare presentation of internal malignancy that appears as a nodule that metastasizes to the umbilicus. It may be ulcerated or necrotic and is seen in up to 10% of patients with cutaneous metastases from internal malignancy.1 These nodules are named after Sister Mary Joseph, the surgical assistant of Dr. William Mayo who first described the relationship between umbilical nodules seen in patients with gastrointestinal and genitourinary cancer. The most common underlying malignancies include primary gastrointestinal and gynecologic adenocarcinomas. In a retrospective study of 34 patients by Chalya et al,2 the stomach was found to be the most common primary site (41.1%). The presence of an SMJN affords a poor prognosis, with a mean overall survival of 11 months from the time of diagnosis.3 The mechanism of disease dissemination remains unknown but is thought to occur through lymphovascular invasion of tumor cells and spread via the umbilical ligament.1,4
Merkel cell carcinoma is a cutaneous neuroendocrine tumor that most commonly presents in elderly patients as red-violet nodules or plaques. Although Merkel cell carcinoma most frequently is encountered on sun-exposed skin, they also can arise on the trunk and abdomen. Positive IHC staining for CK20 would be expected; however, it was negative in our case.5
Cutaneous endometriosis is a rare disease presentation and most commonly occurs as a secondary process due to surgical inoculation of the abdominal wall. Primary cutaneous endometriosis in which there is no history of abdominal surgery less frequently is encountered. Patients typically will report pain and cyclical bleeding with menses. Pathology demonstrates ectopic endometrial tissue with glands and uterine myxoid stroma.6
Amelanotic melanoma is an uncommon subtype of malignant melanoma that presents as nonpigmented nodules that have a propensity to ulcerate and bleed. Furthermore, the umbilicus is an exceedingly rare location for primary melanoma. However, one report does exist, and amelanotic melanoma should be considered in the differential for patients with umbilical nodules.7
Dermoid cysts are benign congenital lesions that typically present as a painless, slow-growing, and wellcircumscribed nodule, as similarly experienced by our patient. They most commonly are found on the testicles and ovaries but also are known to arise in embryologic fusion planes, and reports of umbilical lesions exist.8 Dermoid cysts are diagnosed based on histopathology, supporting the need for a biopsy to distinguish a malignant process from benign lesions.9
- Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph’s nodule at a university teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Leyrat B, Bernadach M, Ginzac A, et al. Sister Mary Joseph nodules: a case report about a rare location of skin metastasis. Case Rep Oncol. 2021;14:664-670.
- Yendluri V, Centeno B, Springett GM. Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case report and update of the literature. Pancreas. 2007;34:161-164.
- Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
- Bittar PG, Hryneewycz KT, Bryant EA. Primary cutaneous endometriosis presenting as an umbilical nodule. JAMA Dermatol. 2021;157:1227.
- Kovitwanichkanont T, Joseph S, Yip L. Hidden in plain sight: umbilical melanoma [published online January 28, 2020]. Med J Aust. 2020;212:154-155.e1.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Akinci O, Turker C, Erturk MS, et al. Umbilical dermoid cyst: a rare case. Cerrahpasa Med J. 2020;44:51-53.
The Diagnosis: Sister Mary Joseph Nodule
Histopathologic analysis of the biopsy specimen revealed a dense infiltrate of large, hyperchromatic, mucin-producing cells exhibiting varying degrees of nuclear pleomorphism (Figure 1). Immunohistochemical (IHC) staining was negative for cytokeratin (CK) 20; however, CK7 was found positive (Figure 2), which confirmed the presence of a metastatic adenocarcinoma, consistent with the clinical diagnosis of a Sister Mary Joseph nodule (SMJN). Subsequent IHC workup to determine the site of origin revealed densely positive expression of both cancer antigen 125 and paired homeobox gene 8 (PAX-8)(Figure 3), consistent with primary ovarian disease. Furthermore, expression of estrogen receptor and p53 both were positive within the nuclei, illustrating an aberrant expression pattern. On the other hand, cancer antigen 19-9, caudal-type homeobox 2, gross cystic disease fluid protein 15, and mammaglobin were all determined negative, thus leading to the pathologic diagnosis of a metastatic ovarian adenocarcinoma. Additional workup with computed tomography of the abdomen and pelvis highlighted a large left ovarian mass with multiple omental nodules as well as enlarged retroperitoneal and pelvic lymph nodes.

The SMJN is a rare presentation of internal malignancy that appears as a nodule that metastasizes to the umbilicus. It may be ulcerated or necrotic and is seen in up to 10% of patients with cutaneous metastases from internal malignancy.1 These nodules are named after Sister Mary Joseph, the surgical assistant of Dr. William Mayo who first described the relationship between umbilical nodules seen in patients with gastrointestinal and genitourinary cancer. The most common underlying malignancies include primary gastrointestinal and gynecologic adenocarcinomas. In a retrospective study of 34 patients by Chalya et al,2 the stomach was found to be the most common primary site (41.1%). The presence of an SMJN affords a poor prognosis, with a mean overall survival of 11 months from the time of diagnosis.3 The mechanism of disease dissemination remains unknown but is thought to occur through lymphovascular invasion of tumor cells and spread via the umbilical ligament.1,4

Merkel cell carcinoma is a cutaneous neuroendocrine tumor that most commonly presents in elderly patients as red-violet nodules or plaques. Although Merkel cell carcinoma most frequently is encountered on sun-exposed skin, they also can arise on the trunk and abdomen. Positive IHC staining for CK20 would be expected; however, it was negative in our case.5

Cutaneous endometriosis is a rare disease presentation and most commonly occurs as a secondary process due to surgical inoculation of the abdominal wall. Primary cutaneous endometriosis in which there is no history of abdominal surgery less frequently is encountered. Patients typically will report pain and cyclical bleeding with menses. Pathology demonstrates ectopic endometrial tissue with glands and uterine myxoid stroma.6
Amelanotic melanoma is an uncommon subtype of malignant melanoma that presents as nonpigmented nodules that have a propensity to ulcerate and bleed. Furthermore, the umbilicus is an exceedingly rare location for primary melanoma. However, one report does exist, and amelanotic melanoma should be considered in the differential for patients with umbilical nodules.7
Dermoid cysts are benign congenital lesions that typically present as a painless, slow-growing, and wellcircumscribed nodule, as similarly experienced by our patient. They most commonly are found on the testicles and ovaries but also are known to arise in embryologic fusion planes, and reports of umbilical lesions exist.8 Dermoid cysts are diagnosed based on histopathology, supporting the need for a biopsy to distinguish a malignant process from benign lesions.9
The Diagnosis: Sister Mary Joseph Nodule
Histopathologic analysis of the biopsy specimen revealed a dense infiltrate of large, hyperchromatic, mucin-producing cells exhibiting varying degrees of nuclear pleomorphism (Figure 1). Immunohistochemical (IHC) staining was negative for cytokeratin (CK) 20; however, CK7 was found positive (Figure 2), which confirmed the presence of a metastatic adenocarcinoma, consistent with the clinical diagnosis of a Sister Mary Joseph nodule (SMJN). Subsequent IHC workup to determine the site of origin revealed densely positive expression of both cancer antigen 125 and paired homeobox gene 8 (PAX-8)(Figure 3), consistent with primary ovarian disease. Furthermore, expression of estrogen receptor and p53 both were positive within the nuclei, illustrating an aberrant expression pattern. On the other hand, cancer antigen 19-9, caudal-type homeobox 2, gross cystic disease fluid protein 15, and mammaglobin were all determined negative, thus leading to the pathologic diagnosis of a metastatic ovarian adenocarcinoma. Additional workup with computed tomography of the abdomen and pelvis highlighted a large left ovarian mass with multiple omental nodules as well as enlarged retroperitoneal and pelvic lymph nodes.

The SMJN is a rare presentation of internal malignancy that appears as a nodule that metastasizes to the umbilicus. It may be ulcerated or necrotic and is seen in up to 10% of patients with cutaneous metastases from internal malignancy.1 These nodules are named after Sister Mary Joseph, the surgical assistant of Dr. William Mayo who first described the relationship between umbilical nodules seen in patients with gastrointestinal and genitourinary cancer. The most common underlying malignancies include primary gastrointestinal and gynecologic adenocarcinomas. In a retrospective study of 34 patients by Chalya et al,2 the stomach was found to be the most common primary site (41.1%). The presence of an SMJN affords a poor prognosis, with a mean overall survival of 11 months from the time of diagnosis.3 The mechanism of disease dissemination remains unknown but is thought to occur through lymphovascular invasion of tumor cells and spread via the umbilical ligament.1,4

Merkel cell carcinoma is a cutaneous neuroendocrine tumor that most commonly presents in elderly patients as red-violet nodules or plaques. Although Merkel cell carcinoma most frequently is encountered on sun-exposed skin, they also can arise on the trunk and abdomen. Positive IHC staining for CK20 would be expected; however, it was negative in our case.5

Cutaneous endometriosis is a rare disease presentation and most commonly occurs as a secondary process due to surgical inoculation of the abdominal wall. Primary cutaneous endometriosis in which there is no history of abdominal surgery less frequently is encountered. Patients typically will report pain and cyclical bleeding with menses. Pathology demonstrates ectopic endometrial tissue with glands and uterine myxoid stroma.6
Amelanotic melanoma is an uncommon subtype of malignant melanoma that presents as nonpigmented nodules that have a propensity to ulcerate and bleed. Furthermore, the umbilicus is an exceedingly rare location for primary melanoma. However, one report does exist, and amelanotic melanoma should be considered in the differential for patients with umbilical nodules.7
Dermoid cysts are benign congenital lesions that typically present as a painless, slow-growing, and wellcircumscribed nodule, as similarly experienced by our patient. They most commonly are found on the testicles and ovaries but also are known to arise in embryologic fusion planes, and reports of umbilical lesions exist.8 Dermoid cysts are diagnosed based on histopathology, supporting the need for a biopsy to distinguish a malignant process from benign lesions.9
- Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph’s nodule at a university teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Leyrat B, Bernadach M, Ginzac A, et al. Sister Mary Joseph nodules: a case report about a rare location of skin metastasis. Case Rep Oncol. 2021;14:664-670.
- Yendluri V, Centeno B, Springett GM. Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case report and update of the literature. Pancreas. 2007;34:161-164.
- Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
- Bittar PG, Hryneewycz KT, Bryant EA. Primary cutaneous endometriosis presenting as an umbilical nodule. JAMA Dermatol. 2021;157:1227.
- Kovitwanichkanont T, Joseph S, Yip L. Hidden in plain sight: umbilical melanoma [published online January 28, 2020]. Med J Aust. 2020;212:154-155.e1.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Akinci O, Turker C, Erturk MS, et al. Umbilical dermoid cyst: a rare case. Cerrahpasa Med J. 2020;44:51-53.
- Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph’s nodule at a university teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Leyrat B, Bernadach M, Ginzac A, et al. Sister Mary Joseph nodules: a case report about a rare location of skin metastasis. Case Rep Oncol. 2021;14:664-670.
- Yendluri V, Centeno B, Springett GM. Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case report and update of the literature. Pancreas. 2007;34:161-164.
- Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
- Bittar PG, Hryneewycz KT, Bryant EA. Primary cutaneous endometriosis presenting as an umbilical nodule. JAMA Dermatol. 2021;157:1227.
- Kovitwanichkanont T, Joseph S, Yip L. Hidden in plain sight: umbilical melanoma [published online January 28, 2020]. Med J Aust. 2020;212:154-155.e1.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Akinci O, Turker C, Erturk MS, et al. Umbilical dermoid cyst: a rare case. Cerrahpasa Med J. 2020;44:51-53.
A 64-year-old woman with no notable medical history was referred to our dermatology clinic with an intermittent eczematous rash around the eyelids of 3 months’ duration. While performing a total-body skin examination, a firm pink nodule with a smooth surface incidentally was discovered on the umbilicus. The patient was uncertain when the lesion first appeared and denied any associated symptoms including pain and bleeding. Additionally, a lymph node examination revealed right inguinal lymphadenopathy. Upon further questioning, she reported worsening muscle weakness, fatigue, night sweats, and an unintentional weight loss of 10 pounds. A 6-mm punch biopsy of the umbilical lesion was obtained for routine histopathology.
Preoperative D-dimer level is an independent prognostic factor for gastric cancer after radical resection
Key clinical point: Preoperative elevated plasma D-dimer levels serve as an independent risk factor for poorer long-term survival outcomes in patients who have undergone curative surgery for gastric cancer.
Major finding: Multivariate analysis revealed elevated D-dimer levels to be independently associated with shorter overall survival (adjusted hazard ratio [aHR] 1.633; P = .003) and disease-free survival (aHR 1.58; P = .005).
Study details: Findings are from a retrospective study that included 903 patients with gastric cancer who underwent radical gastrectomy.
Disclosures: This study was sponsored by the National Natural Science Foundation of China, among others. The authors declared no conflicts of interest.
Source: Zhang X et al. D-dimer, a predictor of bad outcome in gastric cancer patients undergoing radical resection. Sci Rep. 2022;12:16432 (Sep 30). Doi: 10.1038/s41598-022-16582-9
Key clinical point: Preoperative elevated plasma D-dimer levels serve as an independent risk factor for poorer long-term survival outcomes in patients who have undergone curative surgery for gastric cancer.
Major finding: Multivariate analysis revealed elevated D-dimer levels to be independently associated with shorter overall survival (adjusted hazard ratio [aHR] 1.633; P = .003) and disease-free survival (aHR 1.58; P = .005).
Study details: Findings are from a retrospective study that included 903 patients with gastric cancer who underwent radical gastrectomy.
Disclosures: This study was sponsored by the National Natural Science Foundation of China, among others. The authors declared no conflicts of interest.
Source: Zhang X et al. D-dimer, a predictor of bad outcome in gastric cancer patients undergoing radical resection. Sci Rep. 2022;12:16432 (Sep 30). Doi: 10.1038/s41598-022-16582-9
Key clinical point: Preoperative elevated plasma D-dimer levels serve as an independent risk factor for poorer long-term survival outcomes in patients who have undergone curative surgery for gastric cancer.
Major finding: Multivariate analysis revealed elevated D-dimer levels to be independently associated with shorter overall survival (adjusted hazard ratio [aHR] 1.633; P = .003) and disease-free survival (aHR 1.58; P = .005).
Study details: Findings are from a retrospective study that included 903 patients with gastric cancer who underwent radical gastrectomy.
Disclosures: This study was sponsored by the National Natural Science Foundation of China, among others. The authors declared no conflicts of interest.
Source: Zhang X et al. D-dimer, a predictor of bad outcome in gastric cancer patients undergoing radical resection. Sci Rep. 2022;12:16432 (Sep 30). Doi: 10.1038/s41598-022-16582-9
Helicobacter pylori infection may predict a good response to immunotherapy in gastric cancer
Key clinical point: Helicobacter pylori (HP) infection is associated with the tumor expression of programmed death ligand-1 (PD-L1) in patients with gastric cancer.
Major finding: HP infection was significantly associated with the tumor expression of PD-L1 in patients with gastric cancer (odds ratio 1.90; P < .001), with the association not being significantly affected by the sample size, evaluation methods for PD-L1 expression, or quality score (all P > .05).
Study details: This meta-analysis of 10 observational studies investigated the association between HP infection and the tumor expression of PD-L1 in 1870 patients with gastric cancer.
Disclosures: This study was sponsored by the Natural Science Foundation of the Anhui Higher Education Institutions of China and others. The authors declared no conflicts of interest.
Source: Zhu Y et al. Helicobacter pylori infection and PD-L1 expression in gastric cancer: A meta-analysis. Eur J Clin Invest. 2022:e13880 (Sep 27). Doi: 10.1111/eci.13880
Key clinical point: Helicobacter pylori (HP) infection is associated with the tumor expression of programmed death ligand-1 (PD-L1) in patients with gastric cancer.
Major finding: HP infection was significantly associated with the tumor expression of PD-L1 in patients with gastric cancer (odds ratio 1.90; P < .001), with the association not being significantly affected by the sample size, evaluation methods for PD-L1 expression, or quality score (all P > .05).
Study details: This meta-analysis of 10 observational studies investigated the association between HP infection and the tumor expression of PD-L1 in 1870 patients with gastric cancer.
Disclosures: This study was sponsored by the Natural Science Foundation of the Anhui Higher Education Institutions of China and others. The authors declared no conflicts of interest.
Source: Zhu Y et al. Helicobacter pylori infection and PD-L1 expression in gastric cancer: A meta-analysis. Eur J Clin Invest. 2022:e13880 (Sep 27). Doi: 10.1111/eci.13880
Key clinical point: Helicobacter pylori (HP) infection is associated with the tumor expression of programmed death ligand-1 (PD-L1) in patients with gastric cancer.
Major finding: HP infection was significantly associated with the tumor expression of PD-L1 in patients with gastric cancer (odds ratio 1.90; P < .001), with the association not being significantly affected by the sample size, evaluation methods for PD-L1 expression, or quality score (all P > .05).
Study details: This meta-analysis of 10 observational studies investigated the association between HP infection and the tumor expression of PD-L1 in 1870 patients with gastric cancer.
Disclosures: This study was sponsored by the Natural Science Foundation of the Anhui Higher Education Institutions of China and others. The authors declared no conflicts of interest.
Source: Zhu Y et al. Helicobacter pylori infection and PD-L1 expression in gastric cancer: A meta-analysis. Eur J Clin Invest. 2022:e13880 (Sep 27). Doi: 10.1111/eci.13880
Early gastric cancer: Outcomes of pylorus-preserving vs conventional distal gastrectomy
Key clinical point: In patients with early gastric cancer (EGC), pylorus-preserving gastrectomy (PPG) vs conventional distal gastrectomy (CDG) results in the harvest of fewer lymph nodes at stations 5, 6, 9, and 11p but similar survival outcomes.
Major finding: Patients who underwent PPG vs CDG had significantly lower numbers of lymph nodes harvested at stations 5, 6, 9, and 11p (weighted mean difference, −3.09; P < .001) but similar overall survival (hazard ratio [HR] 0.63; P = .852) and recurrence-free survival (HR 0.29; P = .900).
Study details: This was a meta-analysis of 16 studies including 4500 patients with EGC who had undergone PPG or CDG with lymph node dissection.
Disclosures: This study was sponsored by the Peking University People’s Hospital Research and Development Fund. The authors declared no conflicts of interest.
Source: Hou S et al. Pathological and oncological outcomes of pylorus-preserving versus conventional distal gastrectomy in early gastric cancer: A systematic review and meta-analysis. World J Surg Oncol. 2022;20:308 (Sep 24). Doi: 10.1186/s12957-022-02766-0
Key clinical point: In patients with early gastric cancer (EGC), pylorus-preserving gastrectomy (PPG) vs conventional distal gastrectomy (CDG) results in the harvest of fewer lymph nodes at stations 5, 6, 9, and 11p but similar survival outcomes.
Major finding: Patients who underwent PPG vs CDG had significantly lower numbers of lymph nodes harvested at stations 5, 6, 9, and 11p (weighted mean difference, −3.09; P < .001) but similar overall survival (hazard ratio [HR] 0.63; P = .852) and recurrence-free survival (HR 0.29; P = .900).
Study details: This was a meta-analysis of 16 studies including 4500 patients with EGC who had undergone PPG or CDG with lymph node dissection.
Disclosures: This study was sponsored by the Peking University People’s Hospital Research and Development Fund. The authors declared no conflicts of interest.
Source: Hou S et al. Pathological and oncological outcomes of pylorus-preserving versus conventional distal gastrectomy in early gastric cancer: A systematic review and meta-analysis. World J Surg Oncol. 2022;20:308 (Sep 24). Doi: 10.1186/s12957-022-02766-0
Key clinical point: In patients with early gastric cancer (EGC), pylorus-preserving gastrectomy (PPG) vs conventional distal gastrectomy (CDG) results in the harvest of fewer lymph nodes at stations 5, 6, 9, and 11p but similar survival outcomes.
Major finding: Patients who underwent PPG vs CDG had significantly lower numbers of lymph nodes harvested at stations 5, 6, 9, and 11p (weighted mean difference, −3.09; P < .001) but similar overall survival (hazard ratio [HR] 0.63; P = .852) and recurrence-free survival (HR 0.29; P = .900).
Study details: This was a meta-analysis of 16 studies including 4500 patients with EGC who had undergone PPG or CDG with lymph node dissection.
Disclosures: This study was sponsored by the Peking University People’s Hospital Research and Development Fund. The authors declared no conflicts of interest.
Source: Hou S et al. Pathological and oncological outcomes of pylorus-preserving versus conventional distal gastrectomy in early gastric cancer: A systematic review and meta-analysis. World J Surg Oncol. 2022;20:308 (Sep 24). Doi: 10.1186/s12957-022-02766-0
Risk factors for delayed gastric emptying following gastrectomy for gastric cancer
Key clinical point: The female sex, distal gastric tumors, and diabetes are risk factors for delayed gastric emptying (DGE) in patients who have undergone gastrectomy for gastric cancer.
Major finding: Multivariate analysis revealed female sex (adjusted odds ratio [aOR] 2.47; P = .037), diabetes (aOR 2.44; P = .041), and distal gastric tumors (aOR 2.59; P = .033) as independent risk factors for DGE.
Study details: This retrospective study included 412 patients with gastric cancer who underwent distal gastrectomy and thereafter did (n = 27) or did not (n = 385) experience DGE.
Disclosures: No information on funding sources was provided. The author declared no conflicts of interest.
Source: Mukoyama T et al. Assessment of risk factors for delayed gastric emptying after distal gastrectomy for gastric cancer. Sci Rep. 2022;12:15903 (Sep 23). Doi: 10.1038/s41598-022-20151-5
Key clinical point: The female sex, distal gastric tumors, and diabetes are risk factors for delayed gastric emptying (DGE) in patients who have undergone gastrectomy for gastric cancer.
Major finding: Multivariate analysis revealed female sex (adjusted odds ratio [aOR] 2.47; P = .037), diabetes (aOR 2.44; P = .041), and distal gastric tumors (aOR 2.59; P = .033) as independent risk factors for DGE.
Study details: This retrospective study included 412 patients with gastric cancer who underwent distal gastrectomy and thereafter did (n = 27) or did not (n = 385) experience DGE.
Disclosures: No information on funding sources was provided. The author declared no conflicts of interest.
Source: Mukoyama T et al. Assessment of risk factors for delayed gastric emptying after distal gastrectomy for gastric cancer. Sci Rep. 2022;12:15903 (Sep 23). Doi: 10.1038/s41598-022-20151-5
Key clinical point: The female sex, distal gastric tumors, and diabetes are risk factors for delayed gastric emptying (DGE) in patients who have undergone gastrectomy for gastric cancer.
Major finding: Multivariate analysis revealed female sex (adjusted odds ratio [aOR] 2.47; P = .037), diabetes (aOR 2.44; P = .041), and distal gastric tumors (aOR 2.59; P = .033) as independent risk factors for DGE.
Study details: This retrospective study included 412 patients with gastric cancer who underwent distal gastrectomy and thereafter did (n = 27) or did not (n = 385) experience DGE.
Disclosures: No information on funding sources was provided. The author declared no conflicts of interest.
Source: Mukoyama T et al. Assessment of risk factors for delayed gastric emptying after distal gastrectomy for gastric cancer. Sci Rep. 2022;12:15903 (Sep 23). Doi: 10.1038/s41598-022-20151-5
Laparoscopic gastrectomy for gastric cancer: Postoperative NSAID use requires caution
Key clinical point: Postoperative nonsteroidal anti-inflammatory drug (NSAID) use in intravenous patient-controlled analgesia (IV-PCA) is associated with an increased risk for anastomotic leakage, duodenal stump leakage, intra-abdominal bleeding, and intra-abdominal inflammation in patients who undergo laparoscopic gastrectomy for gastric cancer.
Major finding: The NSAID vs non-NSAID group had a significantly higher incidence rate of anastomotic leakage (2.4% vs 0.7%; P = .002), duodenal stump leakage (1.8% vs 0.6%; P = .007), intra-abdominal bleeding (2.1% vs 0.7%; P = .005), and intra-abdominal abscess (1.5% vs 0.4%; P = .008).
Study details: This single-center retrospective study included 2150 patients with gastric cancer who underwent went laparoscopic gastrectomy and thereafter did (n = 935) or did not (n = 1,215) receive NSAID in IV-PCA.
Disclosures: This study was sponsored by the Korean Gastric Cancer Association. The authors declared no conflicts of interest.
Source: Kim SJ et al. Impact of postoperative NSAIDs (IV-PCA) use on short-term outcomes after laparoscopic gastrectomy for the patients of gastric cancer. Surg Endosc. 2022 (Sep 21). Doi: 10.1007/s00464-022-09600-4
Key clinical point: Postoperative nonsteroidal anti-inflammatory drug (NSAID) use in intravenous patient-controlled analgesia (IV-PCA) is associated with an increased risk for anastomotic leakage, duodenal stump leakage, intra-abdominal bleeding, and intra-abdominal inflammation in patients who undergo laparoscopic gastrectomy for gastric cancer.
Major finding: The NSAID vs non-NSAID group had a significantly higher incidence rate of anastomotic leakage (2.4% vs 0.7%; P = .002), duodenal stump leakage (1.8% vs 0.6%; P = .007), intra-abdominal bleeding (2.1% vs 0.7%; P = .005), and intra-abdominal abscess (1.5% vs 0.4%; P = .008).
Study details: This single-center retrospective study included 2150 patients with gastric cancer who underwent went laparoscopic gastrectomy and thereafter did (n = 935) or did not (n = 1,215) receive NSAID in IV-PCA.
Disclosures: This study was sponsored by the Korean Gastric Cancer Association. The authors declared no conflicts of interest.
Source: Kim SJ et al. Impact of postoperative NSAIDs (IV-PCA) use on short-term outcomes after laparoscopic gastrectomy for the patients of gastric cancer. Surg Endosc. 2022 (Sep 21). Doi: 10.1007/s00464-022-09600-4
Key clinical point: Postoperative nonsteroidal anti-inflammatory drug (NSAID) use in intravenous patient-controlled analgesia (IV-PCA) is associated with an increased risk for anastomotic leakage, duodenal stump leakage, intra-abdominal bleeding, and intra-abdominal inflammation in patients who undergo laparoscopic gastrectomy for gastric cancer.
Major finding: The NSAID vs non-NSAID group had a significantly higher incidence rate of anastomotic leakage (2.4% vs 0.7%; P = .002), duodenal stump leakage (1.8% vs 0.6%; P = .007), intra-abdominal bleeding (2.1% vs 0.7%; P = .005), and intra-abdominal abscess (1.5% vs 0.4%; P = .008).
Study details: This single-center retrospective study included 2150 patients with gastric cancer who underwent went laparoscopic gastrectomy and thereafter did (n = 935) or did not (n = 1,215) receive NSAID in IV-PCA.
Disclosures: This study was sponsored by the Korean Gastric Cancer Association. The authors declared no conflicts of interest.
Source: Kim SJ et al. Impact of postoperative NSAIDs (IV-PCA) use on short-term outcomes after laparoscopic gastrectomy for the patients of gastric cancer. Surg Endosc. 2022 (Sep 21). Doi: 10.1007/s00464-022-09600-4
Early gastric cancer: Bleeding risk after ESD similar between patients with surgically altered and whole stomach
Key clinical point: The risk for bleeding after endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) is not significantly different between patients with surgically altered stomach and whole stomach.
Major finding: Patients with surgically altered vs whole stomach did not have a significant difference in the risk for bleeding after ESD (adjusted odds ratio 1.37; 95% CI 0.87-2.17).
Study details: This subanalysis of a multicenter retrospective study included 10,765 patients who underwent ESD for EGC, of which 445 had surgically altered stomach and 10,320 had whole stomach.
Disclosures: This study was partially supported by the Japanese Foundation for Research and Promotion of Endoscopy Grant. M Fujishiro declared receiving lecture honoraria from various sources.
Source: Odagiri H et al. Bleeding following endoscopic submucosal dissection for early gastric cancer in surgically altered stomach. Digestion. 2022 (Oct 4). Doi: 10.1159/000526865
Key clinical point: The risk for bleeding after endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) is not significantly different between patients with surgically altered stomach and whole stomach.
Major finding: Patients with surgically altered vs whole stomach did not have a significant difference in the risk for bleeding after ESD (adjusted odds ratio 1.37; 95% CI 0.87-2.17).
Study details: This subanalysis of a multicenter retrospective study included 10,765 patients who underwent ESD for EGC, of which 445 had surgically altered stomach and 10,320 had whole stomach.
Disclosures: This study was partially supported by the Japanese Foundation for Research and Promotion of Endoscopy Grant. M Fujishiro declared receiving lecture honoraria from various sources.
Source: Odagiri H et al. Bleeding following endoscopic submucosal dissection for early gastric cancer in surgically altered stomach. Digestion. 2022 (Oct 4). Doi: 10.1159/000526865
Key clinical point: The risk for bleeding after endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) is not significantly different between patients with surgically altered stomach and whole stomach.
Major finding: Patients with surgically altered vs whole stomach did not have a significant difference in the risk for bleeding after ESD (adjusted odds ratio 1.37; 95% CI 0.87-2.17).
Study details: This subanalysis of a multicenter retrospective study included 10,765 patients who underwent ESD for EGC, of which 445 had surgically altered stomach and 10,320 had whole stomach.
Disclosures: This study was partially supported by the Japanese Foundation for Research and Promotion of Endoscopy Grant. M Fujishiro declared receiving lecture honoraria from various sources.
Source: Odagiri H et al. Bleeding following endoscopic submucosal dissection for early gastric cancer in surgically altered stomach. Digestion. 2022 (Oct 4). Doi: 10.1159/000526865
Gastric cancer: Neoadjuvant treatment status should not guide the extent of lymphadenectomy
Key clinical point: Routine D2-lymphadenectomy should be performed during total and distal gastrectomy in patients with gastric cancer even after administering neoadjuvant chemotherapy (NAC).
Major finding: cT2, cT3, and cT4 stage tumors metastasized to all individual lymph node (LN) stations (1-9, 11, and 12a). Patients who did vs did not receive NAC had a numerically lower incidence of metastases in almost all stations (54% vs 63%) but a similar distribution of LN metastases over the different stations.
Study details: This side-study of the LOGICA trial included 212 patients with resectable gastric cancer who underwent total or distal D2-gastrectomy with en-bloc D2-lymphadenectomy combined with total omentectomy, of which 158 received NAC and 120 had LN metastases.
Disclosures: The LOGICA trial was sponsored by ZonMw (The Netherlands Organization for Health Research and Development); this side-study received no funding. Some authors declared serving as consultants or advisors for or receiving research funding and travel or accommodation fees and expenses from various sources.
Source: de Jongh C et al and the LOGICA Study Group. Pattern of lymph node metastases in gastric cancer: A side-study of the multicenter LOGICA-trial. Gastric Cancer. 2022 (Sep 14). Doi: 10.1007/s10120-022-01329-2
Key clinical point: Routine D2-lymphadenectomy should be performed during total and distal gastrectomy in patients with gastric cancer even after administering neoadjuvant chemotherapy (NAC).
Major finding: cT2, cT3, and cT4 stage tumors metastasized to all individual lymph node (LN) stations (1-9, 11, and 12a). Patients who did vs did not receive NAC had a numerically lower incidence of metastases in almost all stations (54% vs 63%) but a similar distribution of LN metastases over the different stations.
Study details: This side-study of the LOGICA trial included 212 patients with resectable gastric cancer who underwent total or distal D2-gastrectomy with en-bloc D2-lymphadenectomy combined with total omentectomy, of which 158 received NAC and 120 had LN metastases.
Disclosures: The LOGICA trial was sponsored by ZonMw (The Netherlands Organization for Health Research and Development); this side-study received no funding. Some authors declared serving as consultants or advisors for or receiving research funding and travel or accommodation fees and expenses from various sources.
Source: de Jongh C et al and the LOGICA Study Group. Pattern of lymph node metastases in gastric cancer: A side-study of the multicenter LOGICA-trial. Gastric Cancer. 2022 (Sep 14). Doi: 10.1007/s10120-022-01329-2
Key clinical point: Routine D2-lymphadenectomy should be performed during total and distal gastrectomy in patients with gastric cancer even after administering neoadjuvant chemotherapy (NAC).
Major finding: cT2, cT3, and cT4 stage tumors metastasized to all individual lymph node (LN) stations (1-9, 11, and 12a). Patients who did vs did not receive NAC had a numerically lower incidence of metastases in almost all stations (54% vs 63%) but a similar distribution of LN metastases over the different stations.
Study details: This side-study of the LOGICA trial included 212 patients with resectable gastric cancer who underwent total or distal D2-gastrectomy with en-bloc D2-lymphadenectomy combined with total omentectomy, of which 158 received NAC and 120 had LN metastases.
Disclosures: The LOGICA trial was sponsored by ZonMw (The Netherlands Organization for Health Research and Development); this side-study received no funding. Some authors declared serving as consultants or advisors for or receiving research funding and travel or accommodation fees and expenses from various sources.
Source: de Jongh C et al and the LOGICA Study Group. Pattern of lymph node metastases in gastric cancer: A side-study of the multicenter LOGICA-trial. Gastric Cancer. 2022 (Sep 14). Doi: 10.1007/s10120-022-01329-2
Multimodal treatment of gastric cancer affects outcomes in a stage‐specific manner
Key clinical point: Among patients with nonmetastatic gastric cancer who received both surgery and neoadjuvant chemotherapy (neoadj) or adjuvant chemotherapy (adj), those with stage III disease benefited from neoadj, whereas those with stage I disease benefited from upfront surgery followed by adj.
Major finding: Overall survival with surgery + neoadj vs surgery + adj was worse in patients with stage I disease (hazard ratio [HR] 1.186, 95% CI 1.004-1.402), comparable in those with stage II disease (HR 0.98; 95% CI 0.91-1.07), and significantly improved in those with stage III disease (HR 0.78; 95% CI 0.69-0.90).
Study details: This retrospective study included 11,984 patients with resectable gastric cancer (stage I 15%; stage II 67%; stage III 18%) who underwent surgery and chemotherapy treatment either before or after surgery.
Disclosures: No information on funding was available. The authors declared no conflicts of interest.
Source: Ramos-Santillan V et al. The order of surgery and chemotherapy matters: Multimodality therapy and stage-specific differences in survival in gastric cancer. J Surg Oncol. 2022 (Oct 4). Doi: 10.1002/jso.27110
Key clinical point: Among patients with nonmetastatic gastric cancer who received both surgery and neoadjuvant chemotherapy (neoadj) or adjuvant chemotherapy (adj), those with stage III disease benefited from neoadj, whereas those with stage I disease benefited from upfront surgery followed by adj.
Major finding: Overall survival with surgery + neoadj vs surgery + adj was worse in patients with stage I disease (hazard ratio [HR] 1.186, 95% CI 1.004-1.402), comparable in those with stage II disease (HR 0.98; 95% CI 0.91-1.07), and significantly improved in those with stage III disease (HR 0.78; 95% CI 0.69-0.90).
Study details: This retrospective study included 11,984 patients with resectable gastric cancer (stage I 15%; stage II 67%; stage III 18%) who underwent surgery and chemotherapy treatment either before or after surgery.
Disclosures: No information on funding was available. The authors declared no conflicts of interest.
Source: Ramos-Santillan V et al. The order of surgery and chemotherapy matters: Multimodality therapy and stage-specific differences in survival in gastric cancer. J Surg Oncol. 2022 (Oct 4). Doi: 10.1002/jso.27110
Key clinical point: Among patients with nonmetastatic gastric cancer who received both surgery and neoadjuvant chemotherapy (neoadj) or adjuvant chemotherapy (adj), those with stage III disease benefited from neoadj, whereas those with stage I disease benefited from upfront surgery followed by adj.
Major finding: Overall survival with surgery + neoadj vs surgery + adj was worse in patients with stage I disease (hazard ratio [HR] 1.186, 95% CI 1.004-1.402), comparable in those with stage II disease (HR 0.98; 95% CI 0.91-1.07), and significantly improved in those with stage III disease (HR 0.78; 95% CI 0.69-0.90).
Study details: This retrospective study included 11,984 patients with resectable gastric cancer (stage I 15%; stage II 67%; stage III 18%) who underwent surgery and chemotherapy treatment either before or after surgery.
Disclosures: No information on funding was available. The authors declared no conflicts of interest.
Source: Ramos-Santillan V et al. The order of surgery and chemotherapy matters: Multimodality therapy and stage-specific differences in survival in gastric cancer. J Surg Oncol. 2022 (Oct 4). Doi: 10.1002/jso.27110
Robotic-assisted distal gastrectomy: A feasible treatment option for advanced gastric cancer
Key clinical point: Compared with laparoscopic-assisted distal gastrectomy (LADG), robotic-assisted distal gastrectomy (RADG) results in less operative blood loss, more retrieved lymph nodes (LN), and similar complication rates and oncological outcomes in advanced gastric cancer.
Major finding: Patients who underwent RADG vs LADG had lower operative blood loss (139.3 ± 97.8 vs 167.3 ± 134.2 mL; P < .001); higher retrieved LN number (31.4 ± 12.1 vs 29.4 ± 12.3; P = .015); and similar overall complication rate (13.7% vs 16.6%; P = .242), 3-year overall survival rate (75.5% vs 73.1%; P = .471), and 3-year disease-free survival rate (72.9% vs 71.4%; P = .763).
Study details: Findings are from a retrospective study that propensity score-matched patients with advanced gastric cancer who underwent RADG (n = 410) with those who underwent LADG (n = 410).
Disclosures: This study was sponsored by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Gao G et al. Surgical and oncological outcomes of robotic- versus laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy for advanced gastric cancer: A propensity score-matched analysis of 1164 patients. World J Surg Oncol. 2022;20:315 (Sep 28). Doi: 10.1186/s12957-022-02778-w
Key clinical point: Compared with laparoscopic-assisted distal gastrectomy (LADG), robotic-assisted distal gastrectomy (RADG) results in less operative blood loss, more retrieved lymph nodes (LN), and similar complication rates and oncological outcomes in advanced gastric cancer.
Major finding: Patients who underwent RADG vs LADG had lower operative blood loss (139.3 ± 97.8 vs 167.3 ± 134.2 mL; P < .001); higher retrieved LN number (31.4 ± 12.1 vs 29.4 ± 12.3; P = .015); and similar overall complication rate (13.7% vs 16.6%; P = .242), 3-year overall survival rate (75.5% vs 73.1%; P = .471), and 3-year disease-free survival rate (72.9% vs 71.4%; P = .763).
Study details: Findings are from a retrospective study that propensity score-matched patients with advanced gastric cancer who underwent RADG (n = 410) with those who underwent LADG (n = 410).
Disclosures: This study was sponsored by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Gao G et al. Surgical and oncological outcomes of robotic- versus laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy for advanced gastric cancer: A propensity score-matched analysis of 1164 patients. World J Surg Oncol. 2022;20:315 (Sep 28). Doi: 10.1186/s12957-022-02778-w
Key clinical point: Compared with laparoscopic-assisted distal gastrectomy (LADG), robotic-assisted distal gastrectomy (RADG) results in less operative blood loss, more retrieved lymph nodes (LN), and similar complication rates and oncological outcomes in advanced gastric cancer.
Major finding: Patients who underwent RADG vs LADG had lower operative blood loss (139.3 ± 97.8 vs 167.3 ± 134.2 mL; P < .001); higher retrieved LN number (31.4 ± 12.1 vs 29.4 ± 12.3; P = .015); and similar overall complication rate (13.7% vs 16.6%; P = .242), 3-year overall survival rate (75.5% vs 73.1%; P = .471), and 3-year disease-free survival rate (72.9% vs 71.4%; P = .763).
Study details: Findings are from a retrospective study that propensity score-matched patients with advanced gastric cancer who underwent RADG (n = 410) with those who underwent LADG (n = 410).
Disclosures: This study was sponsored by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Gao G et al. Surgical and oncological outcomes of robotic- versus laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy for advanced gastric cancer: A propensity score-matched analysis of 1164 patients. World J Surg Oncol. 2022;20:315 (Sep 28). Doi: 10.1186/s12957-022-02778-w