User login
Commentary: Drug efficacy and comorbid factors in PsA, November 2022
The effectiveness and safety of advanced therapies for psoriatic arthritis (PsA) was a focus of many published studies last month. Janus kinase inhibitors (JAKi) are a recent class of drugs made available to treat PsA and related diseases, and several clinical trials have been published. Sarabia and colleagues reported the results of a meta-analysis of 15 randomized controlled trials including 6757 patients with psoriasis or PsA who received treatment with a JAKi or placebo. Their analyses revealed that treatment with JAKi vs placebo was associated with higher odds of achieving American College of Rheumatology 20 (ACR20) response (odds ratio [OR] 4.45; 95% CI 3.64-5.44), with similar outcomes observed with tofacitinib vs placebo (OR 2.96; 95% CI 2.01-4.35) and non-tofacitinib JAKi vs placebo (OR 5.41; 95% CI 3.95-7.40). Serious adverse event rates were low (1%-7% in the maximum-dose intervention group).
Interleukin-23i (guselkumab, tildrakizumab, or risankizumab) are another class of biologics recently approved for the treatment of PsA. Preliminary results from a real-world study demonstrate the efficacy of these drugs for PsA. In a retrospective observational study including 80 patients with psoriasis (22 with PsA) who received guselkumab, tildrakizumab, or risankizumab, Elgaard and colleagues demonstrated that 40.9% or 36.4% of the PsA patients achieved complete or partial remission, respectively, compared with only 18.2% of patients with no improvement.
Regarding drug safety, a recent study demonstrated low rates of opportunistic infections with biologic disease-modifying antirheumatic drugs (bDMARD) and targeted synthetic DMARD (tsDMARD). Vassilopoulos and colleagues conducted a meta-analysis of 47 randomized controlled trials and 26 follow-up extension studies that included patients with PsA who received at least one dose of a bDMARD or a tsDMARD (n = 11,790) or placebo (n = 6425) during the placebo-controlled period, and 17,197 patients who received at least one dose of a bDMARD or a tsDMARD in the long-term extension period.
The cumulative incidence of opportunistic infections was < 3% when stratified by the mechanism of action: JAKi (2.72%; 95% CI 1.05%-5.04%), anti-interleukin (IL)-17i (1.18%; 95% CI 0.60%-1.90%), anti-IL-23i (0.24%; 95% CI 0.04%-0.54%), and TNFi (0.01%; 95% CI 0.00%-0.21%). These results are consistent with my own observations in my clinic. Thus, currently available advanced therapies, including JAKi and IL-23i, are effective and safe for the management of patients with PsA when used as monotherapy with or without conventional synthetic DMARD (csDMARD). Ongoing studies on combination therapy will provide us with guidance on the efficacy and safety of combining these drugs for the treatment of resistant disease.
Many patients do not respond to treatment, however. Actionable risk factors for lack of response are of clinical interest. One such factor is obesity. In an observational study of 774 adult PsA patients who started their first b/tsDMARD, Vallejo-Yague and colleagues reported that the odds of achieving minimal disease activity (adjusted OR [aOR] 0.45; 95% CI 0.24-0.82) and Disease Activity Index for Psoriatic Arthritis (DAPSA)-remission (aOR 0.42; 95% CI 0.21-0.85) were lower in the obese vs normal-weight group within the first year. Thus, obese patients had ~50% lower likelihood of achieving a state of low disease activity. Comprehensive management of PsA must include management of obesity and other comorbid conditions to achieve optimal outcomes.
Finally, an interesting study by Freuer and colleagues used bidirectional two-sample Mendelian randomization in 12,882 patients with inflammatory bowel disease (IBD), 21,770 matched controls, 5621 patients with psoriasis, 2063 patients with PsA, and 252,323 controls. The study found that genetically predicted IBD was associated with a higher risk for PsA (pooled OR 1.11; P = .003) with the risk being majorly mediated by Crohn's disease (OR 1.12; P = .002) and not ulcerative colitis (P = .70). Thus, patients with Crohn's disease need to be carefully evaluated for the development of PsA.
The effectiveness and safety of advanced therapies for psoriatic arthritis (PsA) was a focus of many published studies last month. Janus kinase inhibitors (JAKi) are a recent class of drugs made available to treat PsA and related diseases, and several clinical trials have been published. Sarabia and colleagues reported the results of a meta-analysis of 15 randomized controlled trials including 6757 patients with psoriasis or PsA who received treatment with a JAKi or placebo. Their analyses revealed that treatment with JAKi vs placebo was associated with higher odds of achieving American College of Rheumatology 20 (ACR20) response (odds ratio [OR] 4.45; 95% CI 3.64-5.44), with similar outcomes observed with tofacitinib vs placebo (OR 2.96; 95% CI 2.01-4.35) and non-tofacitinib JAKi vs placebo (OR 5.41; 95% CI 3.95-7.40). Serious adverse event rates were low (1%-7% in the maximum-dose intervention group).
Interleukin-23i (guselkumab, tildrakizumab, or risankizumab) are another class of biologics recently approved for the treatment of PsA. Preliminary results from a real-world study demonstrate the efficacy of these drugs for PsA. In a retrospective observational study including 80 patients with psoriasis (22 with PsA) who received guselkumab, tildrakizumab, or risankizumab, Elgaard and colleagues demonstrated that 40.9% or 36.4% of the PsA patients achieved complete or partial remission, respectively, compared with only 18.2% of patients with no improvement.
Regarding drug safety, a recent study demonstrated low rates of opportunistic infections with biologic disease-modifying antirheumatic drugs (bDMARD) and targeted synthetic DMARD (tsDMARD). Vassilopoulos and colleagues conducted a meta-analysis of 47 randomized controlled trials and 26 follow-up extension studies that included patients with PsA who received at least one dose of a bDMARD or a tsDMARD (n = 11,790) or placebo (n = 6425) during the placebo-controlled period, and 17,197 patients who received at least one dose of a bDMARD or a tsDMARD in the long-term extension period.
The cumulative incidence of opportunistic infections was < 3% when stratified by the mechanism of action: JAKi (2.72%; 95% CI 1.05%-5.04%), anti-interleukin (IL)-17i (1.18%; 95% CI 0.60%-1.90%), anti-IL-23i (0.24%; 95% CI 0.04%-0.54%), and TNFi (0.01%; 95% CI 0.00%-0.21%). These results are consistent with my own observations in my clinic. Thus, currently available advanced therapies, including JAKi and IL-23i, are effective and safe for the management of patients with PsA when used as monotherapy with or without conventional synthetic DMARD (csDMARD). Ongoing studies on combination therapy will provide us with guidance on the efficacy and safety of combining these drugs for the treatment of resistant disease.
Many patients do not respond to treatment, however. Actionable risk factors for lack of response are of clinical interest. One such factor is obesity. In an observational study of 774 adult PsA patients who started their first b/tsDMARD, Vallejo-Yague and colleagues reported that the odds of achieving minimal disease activity (adjusted OR [aOR] 0.45; 95% CI 0.24-0.82) and Disease Activity Index for Psoriatic Arthritis (DAPSA)-remission (aOR 0.42; 95% CI 0.21-0.85) were lower in the obese vs normal-weight group within the first year. Thus, obese patients had ~50% lower likelihood of achieving a state of low disease activity. Comprehensive management of PsA must include management of obesity and other comorbid conditions to achieve optimal outcomes.
Finally, an interesting study by Freuer and colleagues used bidirectional two-sample Mendelian randomization in 12,882 patients with inflammatory bowel disease (IBD), 21,770 matched controls, 5621 patients with psoriasis, 2063 patients with PsA, and 252,323 controls. The study found that genetically predicted IBD was associated with a higher risk for PsA (pooled OR 1.11; P = .003) with the risk being majorly mediated by Crohn's disease (OR 1.12; P = .002) and not ulcerative colitis (P = .70). Thus, patients with Crohn's disease need to be carefully evaluated for the development of PsA.
The effectiveness and safety of advanced therapies for psoriatic arthritis (PsA) was a focus of many published studies last month. Janus kinase inhibitors (JAKi) are a recent class of drugs made available to treat PsA and related diseases, and several clinical trials have been published. Sarabia and colleagues reported the results of a meta-analysis of 15 randomized controlled trials including 6757 patients with psoriasis or PsA who received treatment with a JAKi or placebo. Their analyses revealed that treatment with JAKi vs placebo was associated with higher odds of achieving American College of Rheumatology 20 (ACR20) response (odds ratio [OR] 4.45; 95% CI 3.64-5.44), with similar outcomes observed with tofacitinib vs placebo (OR 2.96; 95% CI 2.01-4.35) and non-tofacitinib JAKi vs placebo (OR 5.41; 95% CI 3.95-7.40). Serious adverse event rates were low (1%-7% in the maximum-dose intervention group).
Interleukin-23i (guselkumab, tildrakizumab, or risankizumab) are another class of biologics recently approved for the treatment of PsA. Preliminary results from a real-world study demonstrate the efficacy of these drugs for PsA. In a retrospective observational study including 80 patients with psoriasis (22 with PsA) who received guselkumab, tildrakizumab, or risankizumab, Elgaard and colleagues demonstrated that 40.9% or 36.4% of the PsA patients achieved complete or partial remission, respectively, compared with only 18.2% of patients with no improvement.
Regarding drug safety, a recent study demonstrated low rates of opportunistic infections with biologic disease-modifying antirheumatic drugs (bDMARD) and targeted synthetic DMARD (tsDMARD). Vassilopoulos and colleagues conducted a meta-analysis of 47 randomized controlled trials and 26 follow-up extension studies that included patients with PsA who received at least one dose of a bDMARD or a tsDMARD (n = 11,790) or placebo (n = 6425) during the placebo-controlled period, and 17,197 patients who received at least one dose of a bDMARD or a tsDMARD in the long-term extension period.
The cumulative incidence of opportunistic infections was < 3% when stratified by the mechanism of action: JAKi (2.72%; 95% CI 1.05%-5.04%), anti-interleukin (IL)-17i (1.18%; 95% CI 0.60%-1.90%), anti-IL-23i (0.24%; 95% CI 0.04%-0.54%), and TNFi (0.01%; 95% CI 0.00%-0.21%). These results are consistent with my own observations in my clinic. Thus, currently available advanced therapies, including JAKi and IL-23i, are effective and safe for the management of patients with PsA when used as monotherapy with or without conventional synthetic DMARD (csDMARD). Ongoing studies on combination therapy will provide us with guidance on the efficacy and safety of combining these drugs for the treatment of resistant disease.
Many patients do not respond to treatment, however. Actionable risk factors for lack of response are of clinical interest. One such factor is obesity. In an observational study of 774 adult PsA patients who started their first b/tsDMARD, Vallejo-Yague and colleagues reported that the odds of achieving minimal disease activity (adjusted OR [aOR] 0.45; 95% CI 0.24-0.82) and Disease Activity Index for Psoriatic Arthritis (DAPSA)-remission (aOR 0.42; 95% CI 0.21-0.85) were lower in the obese vs normal-weight group within the first year. Thus, obese patients had ~50% lower likelihood of achieving a state of low disease activity. Comprehensive management of PsA must include management of obesity and other comorbid conditions to achieve optimal outcomes.
Finally, an interesting study by Freuer and colleagues used bidirectional two-sample Mendelian randomization in 12,882 patients with inflammatory bowel disease (IBD), 21,770 matched controls, 5621 patients with psoriasis, 2063 patients with PsA, and 252,323 controls. The study found that genetically predicted IBD was associated with a higher risk for PsA (pooled OR 1.11; P = .003) with the risk being majorly mediated by Crohn's disease (OR 1.12; P = .002) and not ulcerative colitis (P = .70). Thus, patients with Crohn's disease need to be carefully evaluated for the development of PsA.
Commentary: Renal Disease in Type 2 Diabetes, November 2022
Agents proven to reduce major kidney issues in type 2 diabetes include renin-angiotensin system blockers, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and nonsteroidal mineralocorticoid inhibitors, but there are few data on the renal effects of the glucagon-like peptide-1 (GLP-1)/glucose-dependent insulinotropic polypeptide (GIP) receptor agonist tirzepatide. In a post-hoc analysis of the SURPASS-4 trial, Heerspink and colleagues reported that tirzepatide slowed the rate of estimated glomerular filtration rate (eGFR) decline and reduced urine albumin-to-creatinine ratio (ACR) compared with insulin glargine U100. There was also a reduction (≥ 40% decline) in the composite kidney outcome of eGFR, end-stage kidney disease (ESKD), death due to kidney failure, and new-onset macroalbuminuria, and this was driven by the reduction in new-onset macroalbuminuria. Although this was a post-hoc, exploratory analysis, the benefit of tirzepatide on kidney effects suggests that this agent should be studied in type 2 diabetes patients at high risk for kidney disease progression to determine whether indeed there will be a kidney protective effect.
Diabetes is the leading cause of ESKD, and recognizing patients at high risk for progression to ESKD is paramount. Abnormal glycolipid metabolism contributes to the development and progression of diabetic kidney disease (DKD). Bile acids, by regulating glycolipid metabolism, may indirectly provide renoprotective effects. Xiao and colleagues have published a retrospective cohort study of 184 Chinese patients with type 2 diabetes and biopsy-proven DKD. They found that low levels of bile acids (≤2.8 mmol/L) were associated with an over fivefold risk for ESKD after adjusting for known factors associated with ESKD. This is the first study suggesting a link between low bile acid levels and adverse kidney outcomes in DKD, and it provides a rationale for studying bile acid analogs as therapeutic agents for the treatment of DKD.
SGLT2 inhibitors and GLP-1 receptor agonists have proven cardiorenal benefits in type 2 diabetes, and each is recommended in guidelines for patients at higher risk for cardiorenal complications. There are no head-to-head randomized trials of SGLT2 inhibitors vs GLP-1 receptor agonists, and studies suggesting an increased risk for lower-extremity amputation with SGLT2 inhibitors have shown inconsistent results. Lee and colleagues conducted a retrospective cohort study in Taiwan, and, after propensity score-matching patients with type 2 diabetes treated with SGLT inhibitors or GLP-1 receptor agonists, they found no significant difference in major adverse limb events between the two groups. Although limited by retrospective design, short follow-up, and a low number of events, this study suggests that SGLT2 inhibitors and GLP-1 receptor agonists should continue to be used as indicated and according to diabetes guidelines, with no difference in amputation rates between these two classes of antihyperglycemic agents.
Gastrointestinal adverse events are the most common side effects related to metformin use. Many clinicians choose an extended-release metformin preparation over immediate-release, believing that there may be better tolerability, but studies have shown inconsistent results. In a systematic review, meta-analysis, and meta-regression of randomized controlled trials, Nabrdalik and colleagues demonstrated an increased risk for abdominal pain, nausea, and diarrhea with metformin compared with other antidiabetic drugs or placebo, as well as a reduced risk for bloating and diarrhea with extended-release metformin compared with immediate-release formulations. These findings reinforce the practice for considering metformin extended-release over immediate-release formulations to reduce the chance of gastrointestinal side effects.
Agents proven to reduce major kidney issues in type 2 diabetes include renin-angiotensin system blockers, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and nonsteroidal mineralocorticoid inhibitors, but there are few data on the renal effects of the glucagon-like peptide-1 (GLP-1)/glucose-dependent insulinotropic polypeptide (GIP) receptor agonist tirzepatide. In a post-hoc analysis of the SURPASS-4 trial, Heerspink and colleagues reported that tirzepatide slowed the rate of estimated glomerular filtration rate (eGFR) decline and reduced urine albumin-to-creatinine ratio (ACR) compared with insulin glargine U100. There was also a reduction (≥ 40% decline) in the composite kidney outcome of eGFR, end-stage kidney disease (ESKD), death due to kidney failure, and new-onset macroalbuminuria, and this was driven by the reduction in new-onset macroalbuminuria. Although this was a post-hoc, exploratory analysis, the benefit of tirzepatide on kidney effects suggests that this agent should be studied in type 2 diabetes patients at high risk for kidney disease progression to determine whether indeed there will be a kidney protective effect.
Diabetes is the leading cause of ESKD, and recognizing patients at high risk for progression to ESKD is paramount. Abnormal glycolipid metabolism contributes to the development and progression of diabetic kidney disease (DKD). Bile acids, by regulating glycolipid metabolism, may indirectly provide renoprotective effects. Xiao and colleagues have published a retrospective cohort study of 184 Chinese patients with type 2 diabetes and biopsy-proven DKD. They found that low levels of bile acids (≤2.8 mmol/L) were associated with an over fivefold risk for ESKD after adjusting for known factors associated with ESKD. This is the first study suggesting a link between low bile acid levels and adverse kidney outcomes in DKD, and it provides a rationale for studying bile acid analogs as therapeutic agents for the treatment of DKD.
SGLT2 inhibitors and GLP-1 receptor agonists have proven cardiorenal benefits in type 2 diabetes, and each is recommended in guidelines for patients at higher risk for cardiorenal complications. There are no head-to-head randomized trials of SGLT2 inhibitors vs GLP-1 receptor agonists, and studies suggesting an increased risk for lower-extremity amputation with SGLT2 inhibitors have shown inconsistent results. Lee and colleagues conducted a retrospective cohort study in Taiwan, and, after propensity score-matching patients with type 2 diabetes treated with SGLT inhibitors or GLP-1 receptor agonists, they found no significant difference in major adverse limb events between the two groups. Although limited by retrospective design, short follow-up, and a low number of events, this study suggests that SGLT2 inhibitors and GLP-1 receptor agonists should continue to be used as indicated and according to diabetes guidelines, with no difference in amputation rates between these two classes of antihyperglycemic agents.
Gastrointestinal adverse events are the most common side effects related to metformin use. Many clinicians choose an extended-release metformin preparation over immediate-release, believing that there may be better tolerability, but studies have shown inconsistent results. In a systematic review, meta-analysis, and meta-regression of randomized controlled trials, Nabrdalik and colleagues demonstrated an increased risk for abdominal pain, nausea, and diarrhea with metformin compared with other antidiabetic drugs or placebo, as well as a reduced risk for bloating and diarrhea with extended-release metformin compared with immediate-release formulations. These findings reinforce the practice for considering metformin extended-release over immediate-release formulations to reduce the chance of gastrointestinal side effects.
Agents proven to reduce major kidney issues in type 2 diabetes include renin-angiotensin system blockers, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and nonsteroidal mineralocorticoid inhibitors, but there are few data on the renal effects of the glucagon-like peptide-1 (GLP-1)/glucose-dependent insulinotropic polypeptide (GIP) receptor agonist tirzepatide. In a post-hoc analysis of the SURPASS-4 trial, Heerspink and colleagues reported that tirzepatide slowed the rate of estimated glomerular filtration rate (eGFR) decline and reduced urine albumin-to-creatinine ratio (ACR) compared with insulin glargine U100. There was also a reduction (≥ 40% decline) in the composite kidney outcome of eGFR, end-stage kidney disease (ESKD), death due to kidney failure, and new-onset macroalbuminuria, and this was driven by the reduction in new-onset macroalbuminuria. Although this was a post-hoc, exploratory analysis, the benefit of tirzepatide on kidney effects suggests that this agent should be studied in type 2 diabetes patients at high risk for kidney disease progression to determine whether indeed there will be a kidney protective effect.
Diabetes is the leading cause of ESKD, and recognizing patients at high risk for progression to ESKD is paramount. Abnormal glycolipid metabolism contributes to the development and progression of diabetic kidney disease (DKD). Bile acids, by regulating glycolipid metabolism, may indirectly provide renoprotective effects. Xiao and colleagues have published a retrospective cohort study of 184 Chinese patients with type 2 diabetes and biopsy-proven DKD. They found that low levels of bile acids (≤2.8 mmol/L) were associated with an over fivefold risk for ESKD after adjusting for known factors associated with ESKD. This is the first study suggesting a link between low bile acid levels and adverse kidney outcomes in DKD, and it provides a rationale for studying bile acid analogs as therapeutic agents for the treatment of DKD.
SGLT2 inhibitors and GLP-1 receptor agonists have proven cardiorenal benefits in type 2 diabetes, and each is recommended in guidelines for patients at higher risk for cardiorenal complications. There are no head-to-head randomized trials of SGLT2 inhibitors vs GLP-1 receptor agonists, and studies suggesting an increased risk for lower-extremity amputation with SGLT2 inhibitors have shown inconsistent results. Lee and colleagues conducted a retrospective cohort study in Taiwan, and, after propensity score-matching patients with type 2 diabetes treated with SGLT inhibitors or GLP-1 receptor agonists, they found no significant difference in major adverse limb events between the two groups. Although limited by retrospective design, short follow-up, and a low number of events, this study suggests that SGLT2 inhibitors and GLP-1 receptor agonists should continue to be used as indicated and according to diabetes guidelines, with no difference in amputation rates between these two classes of antihyperglycemic agents.
Gastrointestinal adverse events are the most common side effects related to metformin use. Many clinicians choose an extended-release metformin preparation over immediate-release, believing that there may be better tolerability, but studies have shown inconsistent results. In a systematic review, meta-analysis, and meta-regression of randomized controlled trials, Nabrdalik and colleagues demonstrated an increased risk for abdominal pain, nausea, and diarrhea with metformin compared with other antidiabetic drugs or placebo, as well as a reduced risk for bloating and diarrhea with extended-release metformin compared with immediate-release formulations. These findings reinforce the practice for considering metformin extended-release over immediate-release formulations to reduce the chance of gastrointestinal side effects.
Commentary: Hypertension, morbidity in MTOP, and hypothyroidism risk in obstetric emergencies, November 2022
Richards and colleagues explored the effects of aspirin prophylaxis in women with chronic hypertension. They did not detect a lowered risk for preeclampsia but did note a significantly decreased risk for preterm birth in the aspirin group. This was a systematic review and meta-analysis of nine studies (including retrospective cohort and randomized controlled trials). The mixed quality of the source data did limit the meta-analysis. However, this finding suggests that further research is warranted, and we may have a new role for aspirin in helping to decrease preterm birth in women with chronic hypertension.
Cleary and colleagues investigated the use of 30 mg oral nifedipine ER given every 24 hours until delivery in patients with preE with SF. In this randomized, triple-blinded, placebo-controlled trial, 110 patients were randomly assigned to nifedipine treatment or placebo. The results suggest a role for this medication early in the treatment of preE with SF, as the treated patients were much less likely to require acute therapy for severe-range blood pressure. The researchers also noted a trend toward fewer cesarean deliveries (20.8% vs 34.7%) and lower neonatal intensive care unit admissions (29.1% vs 47.1%) in the nifedipine ER group. This favors the use of nifedipine ER in patients with preE with SF.
Stewart and colleagues examined the more common morbidities associated with MTOP after 20 weeks estimated gestational age using a 10-year retrospective cohort study involving 407 patients. They found that 99% of the women had a successful vaginal delivery; however, 25% had some morbidity. Additionally, 16% of the women needed manual removal of placental tissue, 11% had postpartum hemorrhage, and 1.3% experienced severe maternal morbidity (including amniotic fluid embolism), but no maternal deaths occurred. Increased surveillance for postpartum hemorrhage in this patient population should be considered.
Righini and colleagues provide reassurance regarding a commonly used test to rule out pulmonary embolism in pregnant women. They present ancillary data from a prospective management outcome study of 149 women who underwent CT pulmonary angiography testing in pregnancy. There have been concerns raised regarding potential harmful effects related to intravenous iodinated contrast agents on thyroid function. None of the infants born to these patients had evidence of neonatal hypothyroidism (assessed via thyroid-stimulating hormone measurements). This gives reassurance that the use of CT pulmonary angiography testing for pulmonary embolism in pregnancy is safe.
Richards and colleagues explored the effects of aspirin prophylaxis in women with chronic hypertension. They did not detect a lowered risk for preeclampsia but did note a significantly decreased risk for preterm birth in the aspirin group. This was a systematic review and meta-analysis of nine studies (including retrospective cohort and randomized controlled trials). The mixed quality of the source data did limit the meta-analysis. However, this finding suggests that further research is warranted, and we may have a new role for aspirin in helping to decrease preterm birth in women with chronic hypertension.
Cleary and colleagues investigated the use of 30 mg oral nifedipine ER given every 24 hours until delivery in patients with preE with SF. In this randomized, triple-blinded, placebo-controlled trial, 110 patients were randomly assigned to nifedipine treatment or placebo. The results suggest a role for this medication early in the treatment of preE with SF, as the treated patients were much less likely to require acute therapy for severe-range blood pressure. The researchers also noted a trend toward fewer cesarean deliveries (20.8% vs 34.7%) and lower neonatal intensive care unit admissions (29.1% vs 47.1%) in the nifedipine ER group. This favors the use of nifedipine ER in patients with preE with SF.
Stewart and colleagues examined the more common morbidities associated with MTOP after 20 weeks estimated gestational age using a 10-year retrospective cohort study involving 407 patients. They found that 99% of the women had a successful vaginal delivery; however, 25% had some morbidity. Additionally, 16% of the women needed manual removal of placental tissue, 11% had postpartum hemorrhage, and 1.3% experienced severe maternal morbidity (including amniotic fluid embolism), but no maternal deaths occurred. Increased surveillance for postpartum hemorrhage in this patient population should be considered.
Righini and colleagues provide reassurance regarding a commonly used test to rule out pulmonary embolism in pregnant women. They present ancillary data from a prospective management outcome study of 149 women who underwent CT pulmonary angiography testing in pregnancy. There have been concerns raised regarding potential harmful effects related to intravenous iodinated contrast agents on thyroid function. None of the infants born to these patients had evidence of neonatal hypothyroidism (assessed via thyroid-stimulating hormone measurements). This gives reassurance that the use of CT pulmonary angiography testing for pulmonary embolism in pregnancy is safe.
Richards and colleagues explored the effects of aspirin prophylaxis in women with chronic hypertension. They did not detect a lowered risk for preeclampsia but did note a significantly decreased risk for preterm birth in the aspirin group. This was a systematic review and meta-analysis of nine studies (including retrospective cohort and randomized controlled trials). The mixed quality of the source data did limit the meta-analysis. However, this finding suggests that further research is warranted, and we may have a new role for aspirin in helping to decrease preterm birth in women with chronic hypertension.
Cleary and colleagues investigated the use of 30 mg oral nifedipine ER given every 24 hours until delivery in patients with preE with SF. In this randomized, triple-blinded, placebo-controlled trial, 110 patients were randomly assigned to nifedipine treatment or placebo. The results suggest a role for this medication early in the treatment of preE with SF, as the treated patients were much less likely to require acute therapy for severe-range blood pressure. The researchers also noted a trend toward fewer cesarean deliveries (20.8% vs 34.7%) and lower neonatal intensive care unit admissions (29.1% vs 47.1%) in the nifedipine ER group. This favors the use of nifedipine ER in patients with preE with SF.
Stewart and colleagues examined the more common morbidities associated with MTOP after 20 weeks estimated gestational age using a 10-year retrospective cohort study involving 407 patients. They found that 99% of the women had a successful vaginal delivery; however, 25% had some morbidity. Additionally, 16% of the women needed manual removal of placental tissue, 11% had postpartum hemorrhage, and 1.3% experienced severe maternal morbidity (including amniotic fluid embolism), but no maternal deaths occurred. Increased surveillance for postpartum hemorrhage in this patient population should be considered.
Righini and colleagues provide reassurance regarding a commonly used test to rule out pulmonary embolism in pregnant women. They present ancillary data from a prospective management outcome study of 149 women who underwent CT pulmonary angiography testing in pregnancy. There have been concerns raised regarding potential harmful effects related to intravenous iodinated contrast agents on thyroid function. None of the infants born to these patients had evidence of neonatal hypothyroidism (assessed via thyroid-stimulating hormone measurements). This gives reassurance that the use of CT pulmonary angiography testing for pulmonary embolism in pregnancy is safe.
Commentary: Endocrine therapies and male breast cancer, November 2022
A total of 126 patients were included in the full analysis set; median progression-free survival (PFS) was 18.8 months, overall response rate was 64.3%, and the safety profile was similar to prior studies. The median PFS in this observational study was comparable to a median PFS of 16.9 months in the CLEOPTRA study among 88 patients with prior (neo)adjuvant trastuzumab. HELENA also demonstrated similar PFS results for the hormone receptor (HR)-negative and HR-positive (HR+) subgroups (19.4 months vs 18.2 months), as well as for patients with nonvisceral and visceral metastases (20.5 months vs 18.0 months). These findings provide further support for use of the THP regimen as first-line treatment in the real-world setting for patients with HER2+ MBC and prior receipt of trastuzumab.
Adjuvant endocrine therapy (ET) is associated with a survival benefit for early-stage HR+ breast cancer; however, the absolute degree of benefit depends on various clinicopathologic features.2 Although it generally has a manageable toxicity profile, some side effects carry more significant consequences (thromboembolism, endometrial carcinoma, osteoporosis), and some of the more common ones can affect routine quality of life (hot flashes, vaginal dryness, arthralgia).
A retrospective observational study including 5545 patients with pT1a-b estrogen receptor-positive (ER+) breast cancer demonstrated improvements in disease-free survival (DFS) and recurrence-free survival (RFS) among those who received ET vs those who did not receive ET after 5 and 7 years of follow-up (DFS: increases of 2.5% and 3.3%; RFS: increases of 1.9% and 4.3%) (Houvenaeghel et al). Among all patients, absence of ET was associated with decreased DFS (hazard ratio [HR] 1.275; P = .047) but no difference in RFS or OS. Patients with pT1a-b ER+ grade 2-3 tumors (n = 2363) experienced decreased DFS (HR 1.502, P = .049) without ET; however, those with pT1a-b ER+ grade 1 tumors did not experience a negative effect on DFS without ET.
These results provide further support for the survival improvements seen with adjuvant ET — although the relative benefit may be fairly modest — and that ET omission is a relevant consideration in patients with comorbidities or tolerance issues, particularly those with pT1a-b grade 1 tumors.
Advancements in breast cancer therapies have led to improvements in survival outcomes, and it is therefore increasingly essential to recognize risks for other cancer types in breast cancer survivors. Male breast cancer is rare, and although clinical management for the most part mirrors that of female breast cancer, it is important to be aware of potential differences in this population, including risks for subsequent non-breast primary cancers.3
A meta-analysis including eight retrospective cohort studies with male breast cancer survivors reported the standardized incidence ratio (SIR), which compares the incidence of non-breast second primary cancers (SPC) among men with first primary breast cancer vs the expected incidence of non-breast primary cancers in the general male population. The summary SIR estimate was 1.27 (95% CI 1.03-1.56), with increased risk for certain SPCs: colorectal (SIR 1.29; 95% CI 1.03-1.61), pancreatic (SIR 1.64; 95% CI 1.05-2.55), and thyroid (SIR 5.58; 95% CI 1.04-30.05) (Allen et al). Additionally, men diagnosed with breast cancer before 50 years of age were observed to have increased SPC risk compared with men who were older than 50 years at breast cancer onset (SIR 1.50 vs 1.14; P = .040).
This study highlights the importance of genetic assessment for men diagnosed with breast cancer, so they can be appropriately counseled on subsequent cancer risk. It also stimulates thinking regarding other potential contributing factors to the observed increased SPC risk among male breast cancer survivors, including the effect of various treatments, hormonal influences, and significant family history.
Studies have shown that older women derive a survival benefit with adjuvant chemotherapy; however, they may be at increased risk of experiencing toxicities owing to physical functioning and comorbidities.4 A comprehensive geriatric assessment is key, and it is also beneficial for identifying which patients have a higher likelihood of clinical decline after chemotherapy.
A prospective study including 295 robust women age ≥ 65 years with stage I-III breast cancer treated with chemotherapy showed that 26% had a chemotherapy-induced decline in frailty status; patients with high interleukin-6 (IL-6) and C-reactive protein (CRP) inflammatory markers before chemotherapy had a more than threefold odds of experiencing a chemotherapy-induced decline in frailty compared with those with low IL-6 and CRP (odds ratio 3.52; 95% CI 1.55-8.01; P = .003) (Ji et al).
These findings support the relationship between inflammation, aging, and chemotherapy-induced functional decline. Further research is warranted to identify whether there are specific drugs that are implicated, methods to enhance anti-inflammatory effects, and any downstream effect on breast cancer outcomes of these patients.
Additional References
- Swain SM, Miles D, Kim SB, et al; CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519-530. Doi: 10.1016/S1470-2045(19)30863-0
- Ma SJ, Oladeru OT, Singh AK. Association of endocrine therapy with overall survival in women with small, hormone receptor-positive, ERBB2-negative breast cancer. JAMA Netw Open. 2020;3:e2013973. Doi: 10.1001/jamanetworkopen.2020.13973
- Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: Insights and unexpected results. Breast Cancer Res Treat. 2017;161:575-586. Doi: 10.1007/s10549-016-4085-4
- Tamirisa N, Lin H, Shen Y, et al. Association of chemotherapy with survival in elderly patients with multiple comorbidities and estrogen receptor-positive, node-positive breast cancer. JAMA Oncol. 2020;6:1548-155 Doi: 10.1001/jamaoncol.2020.2388
A total of 126 patients were included in the full analysis set; median progression-free survival (PFS) was 18.8 months, overall response rate was 64.3%, and the safety profile was similar to prior studies. The median PFS in this observational study was comparable to a median PFS of 16.9 months in the CLEOPTRA study among 88 patients with prior (neo)adjuvant trastuzumab. HELENA also demonstrated similar PFS results for the hormone receptor (HR)-negative and HR-positive (HR+) subgroups (19.4 months vs 18.2 months), as well as for patients with nonvisceral and visceral metastases (20.5 months vs 18.0 months). These findings provide further support for use of the THP regimen as first-line treatment in the real-world setting for patients with HER2+ MBC and prior receipt of trastuzumab.
Adjuvant endocrine therapy (ET) is associated with a survival benefit for early-stage HR+ breast cancer; however, the absolute degree of benefit depends on various clinicopathologic features.2 Although it generally has a manageable toxicity profile, some side effects carry more significant consequences (thromboembolism, endometrial carcinoma, osteoporosis), and some of the more common ones can affect routine quality of life (hot flashes, vaginal dryness, arthralgia).
A retrospective observational study including 5545 patients with pT1a-b estrogen receptor-positive (ER+) breast cancer demonstrated improvements in disease-free survival (DFS) and recurrence-free survival (RFS) among those who received ET vs those who did not receive ET after 5 and 7 years of follow-up (DFS: increases of 2.5% and 3.3%; RFS: increases of 1.9% and 4.3%) (Houvenaeghel et al). Among all patients, absence of ET was associated with decreased DFS (hazard ratio [HR] 1.275; P = .047) but no difference in RFS or OS. Patients with pT1a-b ER+ grade 2-3 tumors (n = 2363) experienced decreased DFS (HR 1.502, P = .049) without ET; however, those with pT1a-b ER+ grade 1 tumors did not experience a negative effect on DFS without ET.
These results provide further support for the survival improvements seen with adjuvant ET — although the relative benefit may be fairly modest — and that ET omission is a relevant consideration in patients with comorbidities or tolerance issues, particularly those with pT1a-b grade 1 tumors.
Advancements in breast cancer therapies have led to improvements in survival outcomes, and it is therefore increasingly essential to recognize risks for other cancer types in breast cancer survivors. Male breast cancer is rare, and although clinical management for the most part mirrors that of female breast cancer, it is important to be aware of potential differences in this population, including risks for subsequent non-breast primary cancers.3
A meta-analysis including eight retrospective cohort studies with male breast cancer survivors reported the standardized incidence ratio (SIR), which compares the incidence of non-breast second primary cancers (SPC) among men with first primary breast cancer vs the expected incidence of non-breast primary cancers in the general male population. The summary SIR estimate was 1.27 (95% CI 1.03-1.56), with increased risk for certain SPCs: colorectal (SIR 1.29; 95% CI 1.03-1.61), pancreatic (SIR 1.64; 95% CI 1.05-2.55), and thyroid (SIR 5.58; 95% CI 1.04-30.05) (Allen et al). Additionally, men diagnosed with breast cancer before 50 years of age were observed to have increased SPC risk compared with men who were older than 50 years at breast cancer onset (SIR 1.50 vs 1.14; P = .040).
This study highlights the importance of genetic assessment for men diagnosed with breast cancer, so they can be appropriately counseled on subsequent cancer risk. It also stimulates thinking regarding other potential contributing factors to the observed increased SPC risk among male breast cancer survivors, including the effect of various treatments, hormonal influences, and significant family history.
Studies have shown that older women derive a survival benefit with adjuvant chemotherapy; however, they may be at increased risk of experiencing toxicities owing to physical functioning and comorbidities.4 A comprehensive geriatric assessment is key, and it is also beneficial for identifying which patients have a higher likelihood of clinical decline after chemotherapy.
A prospective study including 295 robust women age ≥ 65 years with stage I-III breast cancer treated with chemotherapy showed that 26% had a chemotherapy-induced decline in frailty status; patients with high interleukin-6 (IL-6) and C-reactive protein (CRP) inflammatory markers before chemotherapy had a more than threefold odds of experiencing a chemotherapy-induced decline in frailty compared with those with low IL-6 and CRP (odds ratio 3.52; 95% CI 1.55-8.01; P = .003) (Ji et al).
These findings support the relationship between inflammation, aging, and chemotherapy-induced functional decline. Further research is warranted to identify whether there are specific drugs that are implicated, methods to enhance anti-inflammatory effects, and any downstream effect on breast cancer outcomes of these patients.
Additional References
- Swain SM, Miles D, Kim SB, et al; CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519-530. Doi: 10.1016/S1470-2045(19)30863-0
- Ma SJ, Oladeru OT, Singh AK. Association of endocrine therapy with overall survival in women with small, hormone receptor-positive, ERBB2-negative breast cancer. JAMA Netw Open. 2020;3:e2013973. Doi: 10.1001/jamanetworkopen.2020.13973
- Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: Insights and unexpected results. Breast Cancer Res Treat. 2017;161:575-586. Doi: 10.1007/s10549-016-4085-4
- Tamirisa N, Lin H, Shen Y, et al. Association of chemotherapy with survival in elderly patients with multiple comorbidities and estrogen receptor-positive, node-positive breast cancer. JAMA Oncol. 2020;6:1548-155 Doi: 10.1001/jamaoncol.2020.2388
A total of 126 patients were included in the full analysis set; median progression-free survival (PFS) was 18.8 months, overall response rate was 64.3%, and the safety profile was similar to prior studies. The median PFS in this observational study was comparable to a median PFS of 16.9 months in the CLEOPTRA study among 88 patients with prior (neo)adjuvant trastuzumab. HELENA also demonstrated similar PFS results for the hormone receptor (HR)-negative and HR-positive (HR+) subgroups (19.4 months vs 18.2 months), as well as for patients with nonvisceral and visceral metastases (20.5 months vs 18.0 months). These findings provide further support for use of the THP regimen as first-line treatment in the real-world setting for patients with HER2+ MBC and prior receipt of trastuzumab.
Adjuvant endocrine therapy (ET) is associated with a survival benefit for early-stage HR+ breast cancer; however, the absolute degree of benefit depends on various clinicopathologic features.2 Although it generally has a manageable toxicity profile, some side effects carry more significant consequences (thromboembolism, endometrial carcinoma, osteoporosis), and some of the more common ones can affect routine quality of life (hot flashes, vaginal dryness, arthralgia).
A retrospective observational study including 5545 patients with pT1a-b estrogen receptor-positive (ER+) breast cancer demonstrated improvements in disease-free survival (DFS) and recurrence-free survival (RFS) among those who received ET vs those who did not receive ET after 5 and 7 years of follow-up (DFS: increases of 2.5% and 3.3%; RFS: increases of 1.9% and 4.3%) (Houvenaeghel et al). Among all patients, absence of ET was associated with decreased DFS (hazard ratio [HR] 1.275; P = .047) but no difference in RFS or OS. Patients with pT1a-b ER+ grade 2-3 tumors (n = 2363) experienced decreased DFS (HR 1.502, P = .049) without ET; however, those with pT1a-b ER+ grade 1 tumors did not experience a negative effect on DFS without ET.
These results provide further support for the survival improvements seen with adjuvant ET — although the relative benefit may be fairly modest — and that ET omission is a relevant consideration in patients with comorbidities or tolerance issues, particularly those with pT1a-b grade 1 tumors.
Advancements in breast cancer therapies have led to improvements in survival outcomes, and it is therefore increasingly essential to recognize risks for other cancer types in breast cancer survivors. Male breast cancer is rare, and although clinical management for the most part mirrors that of female breast cancer, it is important to be aware of potential differences in this population, including risks for subsequent non-breast primary cancers.3
A meta-analysis including eight retrospective cohort studies with male breast cancer survivors reported the standardized incidence ratio (SIR), which compares the incidence of non-breast second primary cancers (SPC) among men with first primary breast cancer vs the expected incidence of non-breast primary cancers in the general male population. The summary SIR estimate was 1.27 (95% CI 1.03-1.56), with increased risk for certain SPCs: colorectal (SIR 1.29; 95% CI 1.03-1.61), pancreatic (SIR 1.64; 95% CI 1.05-2.55), and thyroid (SIR 5.58; 95% CI 1.04-30.05) (Allen et al). Additionally, men diagnosed with breast cancer before 50 years of age were observed to have increased SPC risk compared with men who were older than 50 years at breast cancer onset (SIR 1.50 vs 1.14; P = .040).
This study highlights the importance of genetic assessment for men diagnosed with breast cancer, so they can be appropriately counseled on subsequent cancer risk. It also stimulates thinking regarding other potential contributing factors to the observed increased SPC risk among male breast cancer survivors, including the effect of various treatments, hormonal influences, and significant family history.
Studies have shown that older women derive a survival benefit with adjuvant chemotherapy; however, they may be at increased risk of experiencing toxicities owing to physical functioning and comorbidities.4 A comprehensive geriatric assessment is key, and it is also beneficial for identifying which patients have a higher likelihood of clinical decline after chemotherapy.
A prospective study including 295 robust women age ≥ 65 years with stage I-III breast cancer treated with chemotherapy showed that 26% had a chemotherapy-induced decline in frailty status; patients with high interleukin-6 (IL-6) and C-reactive protein (CRP) inflammatory markers before chemotherapy had a more than threefold odds of experiencing a chemotherapy-induced decline in frailty compared with those with low IL-6 and CRP (odds ratio 3.52; 95% CI 1.55-8.01; P = .003) (Ji et al).
These findings support the relationship between inflammation, aging, and chemotherapy-induced functional decline. Further research is warranted to identify whether there are specific drugs that are implicated, methods to enhance anti-inflammatory effects, and any downstream effect on breast cancer outcomes of these patients.
Additional References
- Swain SM, Miles D, Kim SB, et al; CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519-530. Doi: 10.1016/S1470-2045(19)30863-0
- Ma SJ, Oladeru OT, Singh AK. Association of endocrine therapy with overall survival in women with small, hormone receptor-positive, ERBB2-negative breast cancer. JAMA Netw Open. 2020;3:e2013973. Doi: 10.1001/jamanetworkopen.2020.13973
- Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: Insights and unexpected results. Breast Cancer Res Treat. 2017;161:575-586. Doi: 10.1007/s10549-016-4085-4
- Tamirisa N, Lin H, Shen Y, et al. Association of chemotherapy with survival in elderly patients with multiple comorbidities and estrogen receptor-positive, node-positive breast cancer. JAMA Oncol. 2020;6:1548-155 Doi: 10.1001/jamaoncol.2020.2388
Commentary: Complementary treatments for AD, November 2022
Still, some patients seek alternative or adjunctive treatment approaches, owing to a desire to identify the root cause of disease, their aversion toward Western medicine, or fear of adverse events. Yepes-Nuñez and colleagues performed a systematic review and meta-analysis including 23 studies of benefits and harms of allergen immunotherapy for AD. I had the privilege of participating in this study and can testify to the astronomical amount of work that went into comprehensively identifying all of the relevant studies and synthesizing the data. We found that adjunctive subcutaneous or sublingual allergen immunotherapy, particularly for house dust mites, led to modest but generally delayed improvements of AD severity, itch, and quality of life, and less definitive effects on sleep disturbance and AD flares. Overall, both were well tolerated, though subcutaneous immunotherapy was associated with more adverse events than sublingual immunotherapy. Allergen immunotherapy requires a significant investment of time by patients and was only modestly effective. Nevertheless, it may be a reasonable approach to consider in select patients with AD.
Benjamin Franklin famously stated that "an ounce of prevention is worth a pound of cure." Likewise, while successful treatment of AD is great, how can we advise patients and caregivers of children who are at high risk for AD? To answer this question, Voigt and Lele performed a systematic review and meta-analysis of 11 randomized controlled trials examining the efficacy of Lactobacillus rhamnosus at preventing AD in children when taken by mothers during pregnancy. They found that L. rhamnosus significantly reduced the risk of developing AD within 2 years, marginally significantly reduced risk at 4-5 years, and significantly reduced risk at 6-7 years, but no significant risk differences were observed at 10-11 years. The authors concluded that use of L. rhamnosus with or without other probiotics during pregnancy reduces the incidence of childhood AD at least up to age 7 years.
Wang and colleagues conducted an observational study of the relationship of home environment exposures with atopic disease, including AD, in 17,881 offspring from Iceland, Norway, Sweden, Denmark, and Estonia who had undergone two follow-up investigations every 10 years. They found that AD was associated with parent-reported visible mold and dampness/mold at home, living in an apartment, and living in newer buildings. Avoidance of these environmental exposures could possibly decrease the risk of developing AD, although future confirmatory studies are needed.
For each of these treatment/prevention approaches, the magnitude of benefit is not very large. Thus, these approaches do not replace our armamentarium of treatments and avoidance strategies for AD. Rather, they can be used complementarily as low-risk add-on interventions with a potential upside.
Still, some patients seek alternative or adjunctive treatment approaches, owing to a desire to identify the root cause of disease, their aversion toward Western medicine, or fear of adverse events. Yepes-Nuñez and colleagues performed a systematic review and meta-analysis including 23 studies of benefits and harms of allergen immunotherapy for AD. I had the privilege of participating in this study and can testify to the astronomical amount of work that went into comprehensively identifying all of the relevant studies and synthesizing the data. We found that adjunctive subcutaneous or sublingual allergen immunotherapy, particularly for house dust mites, led to modest but generally delayed improvements of AD severity, itch, and quality of life, and less definitive effects on sleep disturbance and AD flares. Overall, both were well tolerated, though subcutaneous immunotherapy was associated with more adverse events than sublingual immunotherapy. Allergen immunotherapy requires a significant investment of time by patients and was only modestly effective. Nevertheless, it may be a reasonable approach to consider in select patients with AD.
Benjamin Franklin famously stated that "an ounce of prevention is worth a pound of cure." Likewise, while successful treatment of AD is great, how can we advise patients and caregivers of children who are at high risk for AD? To answer this question, Voigt and Lele performed a systematic review and meta-analysis of 11 randomized controlled trials examining the efficacy of Lactobacillus rhamnosus at preventing AD in children when taken by mothers during pregnancy. They found that L. rhamnosus significantly reduced the risk of developing AD within 2 years, marginally significantly reduced risk at 4-5 years, and significantly reduced risk at 6-7 years, but no significant risk differences were observed at 10-11 years. The authors concluded that use of L. rhamnosus with or without other probiotics during pregnancy reduces the incidence of childhood AD at least up to age 7 years.
Wang and colleagues conducted an observational study of the relationship of home environment exposures with atopic disease, including AD, in 17,881 offspring from Iceland, Norway, Sweden, Denmark, and Estonia who had undergone two follow-up investigations every 10 years. They found that AD was associated with parent-reported visible mold and dampness/mold at home, living in an apartment, and living in newer buildings. Avoidance of these environmental exposures could possibly decrease the risk of developing AD, although future confirmatory studies are needed.
For each of these treatment/prevention approaches, the magnitude of benefit is not very large. Thus, these approaches do not replace our armamentarium of treatments and avoidance strategies for AD. Rather, they can be used complementarily as low-risk add-on interventions with a potential upside.
Still, some patients seek alternative or adjunctive treatment approaches, owing to a desire to identify the root cause of disease, their aversion toward Western medicine, or fear of adverse events. Yepes-Nuñez and colleagues performed a systematic review and meta-analysis including 23 studies of benefits and harms of allergen immunotherapy for AD. I had the privilege of participating in this study and can testify to the astronomical amount of work that went into comprehensively identifying all of the relevant studies and synthesizing the data. We found that adjunctive subcutaneous or sublingual allergen immunotherapy, particularly for house dust mites, led to modest but generally delayed improvements of AD severity, itch, and quality of life, and less definitive effects on sleep disturbance and AD flares. Overall, both were well tolerated, though subcutaneous immunotherapy was associated with more adverse events than sublingual immunotherapy. Allergen immunotherapy requires a significant investment of time by patients and was only modestly effective. Nevertheless, it may be a reasonable approach to consider in select patients with AD.
Benjamin Franklin famously stated that "an ounce of prevention is worth a pound of cure." Likewise, while successful treatment of AD is great, how can we advise patients and caregivers of children who are at high risk for AD? To answer this question, Voigt and Lele performed a systematic review and meta-analysis of 11 randomized controlled trials examining the efficacy of Lactobacillus rhamnosus at preventing AD in children when taken by mothers during pregnancy. They found that L. rhamnosus significantly reduced the risk of developing AD within 2 years, marginally significantly reduced risk at 4-5 years, and significantly reduced risk at 6-7 years, but no significant risk differences were observed at 10-11 years. The authors concluded that use of L. rhamnosus with or without other probiotics during pregnancy reduces the incidence of childhood AD at least up to age 7 years.
Wang and colleagues conducted an observational study of the relationship of home environment exposures with atopic disease, including AD, in 17,881 offspring from Iceland, Norway, Sweden, Denmark, and Estonia who had undergone two follow-up investigations every 10 years. They found that AD was associated with parent-reported visible mold and dampness/mold at home, living in an apartment, and living in newer buildings. Avoidance of these environmental exposures could possibly decrease the risk of developing AD, although future confirmatory studies are needed.
For each of these treatment/prevention approaches, the magnitude of benefit is not very large. Thus, these approaches do not replace our armamentarium of treatments and avoidance strategies for AD. Rather, they can be used complementarily as low-risk add-on interventions with a potential upside.
Commentary: Potential new treatments in gastroesophageal adenocarcinoma, November 2022
The phase 2 FIGHT trial1 evaluated the role of bemarituzumab, an anti-FGFR2 antibody, in combination with chemotherapy during first-line treatment of advanced gastroesophageal adenocarcinoma. The primary endpoint of this trial was progression-free survival (PFS). This trial enrolled 155 patients with upper gastrointestinal tumors with FGFR2b overexpression (defined as at least 2+ by immunohistochemistry) or amplification on next-generation sequencing. About 30% of patients with HER2 nonpositive tumors (ie, those that would not qualify for treatment with the targeted agent trastuzumab) were eligible for participation. In the FIGHT trial, patients were randomized in a 1:1 ratio to receive either standard chemotherapy (folinic acid, fluorouracil, and oxaliplatin [FOLFOX]) or chemotherapy plus bemarituzumab. Patients in the experimental group were allowed to receive one dose of standard FOLFOX chemotherapy while biomarker testing was ongoing.
With a median follow-up time of 10.9 moths, PFS was numerically prolonged in the bemarituzumab group (9.5 vs 7.4 months), but it did not reach statistical significance (P = .073). Overall survival (OS) was improved in the experimental group (not reached vs 12.9 months; P = .027). With a longer follow-up of 12.5 months, in post hoc exploratory analysis, OS was significantly longer in the experimental group (19.2 vs 13.5 months; hazard ratio 0.60, P = .027). The rate of serious adverse events was similar between the two groups. However, it is important to note ocular toxicities associated with bemarituzumab treatment. Corneal adverse events were seen in 67% of patients in the experimental group, with 24% of patients experiencing grade 3 events. Moreover, 26% of patients discontinued bemarituzumab because of corneal adverse events.
Overall, this phase 2 trial demonstrated that FGFR2b is emerging as an important biomarker and target in patients with advanced gastroesophageal adenocarcinoma. Ongoing phase 3 trials (FORTITUDE-101 with FOLFOX [NCT05052801] and FORTITUDE-102 with FOLFOX and nivolumab [NCT05111626]) hopefully will confirm the early results seen in the FIGHT trial. Awareness and early attention to treatment-associated toxicities will be critical for the potential future incorporation of bemarituzumab into clinical practice.
A study by Ramos‐Santillan and colleagues explored whether the order of treatment modalities matter in the management of early-stage gastric cancer. Typically, perioperative chemotherapy (both neoadjuvant and adjuvant) is used during treatment of early-stage gastric cancer, which is usually defined as at least cT2N0 or cTxN+ disease. In this study, multivariable Cox regression analyses were performed on propensity score-matched cohorts. The study analyzed outcomes of 11,984 patients who were identified using the US National Cancer Database and treated between 2005 and 2014. The results revealed that patients who had stage I disease had better outcomes with upfront resection followed by adjuvant therapy. Patients with stage III disease did better with a neoadjuvant approach, whereas patients with stage II disease had similar outcomes regardless of chemotherapy timing. This research has the limitations inherent to the retrospective nature of the analysis and lack of prospective enrollment and controls. However, it does suggest that there may be a fraction of patients who should be treated with upfront resection. For incorporation of this change into standard practice, the question of therapy sequencing should be answered in a randomized prospective trial that incorporates the most updated systemic therapy (fluorouracil, leucovorin, oxaliplatin, and docetaxel [FLOT]) into its design.
Chemotherapy continues to play a critical role during first-line treatment of advanced esophageal and gastric adenocarcinoma. Triple chemotherapy regimens have been known to have increased efficacy in this setting, but their use has been limited by associated toxicities. A study by Nguyen and colleagues evaluated the TCX regimen (paclitaxel, carboplatin, and capecitabine) during first-line treatment of advanced gastric cancer. This regimen is similar to other triple chemotherapy regimens, such as FLOT and DCF (docetaxel, cisplatin, and fluorouracil), which have proven activity in this disease. This prospective phase 2 trial enrolled 83 patients. The median PFS (9.3 months) and OS (17 months) compared favorably with historical references. The regimen had expected adverse events, with cytopenias and fatigue being the most frequently reported. On the basis of the reported safety and efficacy, TCX has potential to be used as a chemotherapy backbone in future trials, but larger trials are needed to confirm the phase 2 trial results.
References
Wainberg ZA, Enzinger PC, Kang YK, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022 Oct 13. Doi: 10.1016/S1470-2045(22)00603-9
The phase 2 FIGHT trial1 evaluated the role of bemarituzumab, an anti-FGFR2 antibody, in combination with chemotherapy during first-line treatment of advanced gastroesophageal adenocarcinoma. The primary endpoint of this trial was progression-free survival (PFS). This trial enrolled 155 patients with upper gastrointestinal tumors with FGFR2b overexpression (defined as at least 2+ by immunohistochemistry) or amplification on next-generation sequencing. About 30% of patients with HER2 nonpositive tumors (ie, those that would not qualify for treatment with the targeted agent trastuzumab) were eligible for participation. In the FIGHT trial, patients were randomized in a 1:1 ratio to receive either standard chemotherapy (folinic acid, fluorouracil, and oxaliplatin [FOLFOX]) or chemotherapy plus bemarituzumab. Patients in the experimental group were allowed to receive one dose of standard FOLFOX chemotherapy while biomarker testing was ongoing.
With a median follow-up time of 10.9 moths, PFS was numerically prolonged in the bemarituzumab group (9.5 vs 7.4 months), but it did not reach statistical significance (P = .073). Overall survival (OS) was improved in the experimental group (not reached vs 12.9 months; P = .027). With a longer follow-up of 12.5 months, in post hoc exploratory analysis, OS was significantly longer in the experimental group (19.2 vs 13.5 months; hazard ratio 0.60, P = .027). The rate of serious adverse events was similar between the two groups. However, it is important to note ocular toxicities associated with bemarituzumab treatment. Corneal adverse events were seen in 67% of patients in the experimental group, with 24% of patients experiencing grade 3 events. Moreover, 26% of patients discontinued bemarituzumab because of corneal adverse events.
Overall, this phase 2 trial demonstrated that FGFR2b is emerging as an important biomarker and target in patients with advanced gastroesophageal adenocarcinoma. Ongoing phase 3 trials (FORTITUDE-101 with FOLFOX [NCT05052801] and FORTITUDE-102 with FOLFOX and nivolumab [NCT05111626]) hopefully will confirm the early results seen in the FIGHT trial. Awareness and early attention to treatment-associated toxicities will be critical for the potential future incorporation of bemarituzumab into clinical practice.
A study by Ramos‐Santillan and colleagues explored whether the order of treatment modalities matter in the management of early-stage gastric cancer. Typically, perioperative chemotherapy (both neoadjuvant and adjuvant) is used during treatment of early-stage gastric cancer, which is usually defined as at least cT2N0 or cTxN+ disease. In this study, multivariable Cox regression analyses were performed on propensity score-matched cohorts. The study analyzed outcomes of 11,984 patients who were identified using the US National Cancer Database and treated between 2005 and 2014. The results revealed that patients who had stage I disease had better outcomes with upfront resection followed by adjuvant therapy. Patients with stage III disease did better with a neoadjuvant approach, whereas patients with stage II disease had similar outcomes regardless of chemotherapy timing. This research has the limitations inherent to the retrospective nature of the analysis and lack of prospective enrollment and controls. However, it does suggest that there may be a fraction of patients who should be treated with upfront resection. For incorporation of this change into standard practice, the question of therapy sequencing should be answered in a randomized prospective trial that incorporates the most updated systemic therapy (fluorouracil, leucovorin, oxaliplatin, and docetaxel [FLOT]) into its design.
Chemotherapy continues to play a critical role during first-line treatment of advanced esophageal and gastric adenocarcinoma. Triple chemotherapy regimens have been known to have increased efficacy in this setting, but their use has been limited by associated toxicities. A study by Nguyen and colleagues evaluated the TCX regimen (paclitaxel, carboplatin, and capecitabine) during first-line treatment of advanced gastric cancer. This regimen is similar to other triple chemotherapy regimens, such as FLOT and DCF (docetaxel, cisplatin, and fluorouracil), which have proven activity in this disease. This prospective phase 2 trial enrolled 83 patients. The median PFS (9.3 months) and OS (17 months) compared favorably with historical references. The regimen had expected adverse events, with cytopenias and fatigue being the most frequently reported. On the basis of the reported safety and efficacy, TCX has potential to be used as a chemotherapy backbone in future trials, but larger trials are needed to confirm the phase 2 trial results.
References
Wainberg ZA, Enzinger PC, Kang YK, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022 Oct 13. Doi: 10.1016/S1470-2045(22)00603-9
The phase 2 FIGHT trial1 evaluated the role of bemarituzumab, an anti-FGFR2 antibody, in combination with chemotherapy during first-line treatment of advanced gastroesophageal adenocarcinoma. The primary endpoint of this trial was progression-free survival (PFS). This trial enrolled 155 patients with upper gastrointestinal tumors with FGFR2b overexpression (defined as at least 2+ by immunohistochemistry) or amplification on next-generation sequencing. About 30% of patients with HER2 nonpositive tumors (ie, those that would not qualify for treatment with the targeted agent trastuzumab) were eligible for participation. In the FIGHT trial, patients were randomized in a 1:1 ratio to receive either standard chemotherapy (folinic acid, fluorouracil, and oxaliplatin [FOLFOX]) or chemotherapy plus bemarituzumab. Patients in the experimental group were allowed to receive one dose of standard FOLFOX chemotherapy while biomarker testing was ongoing.
With a median follow-up time of 10.9 moths, PFS was numerically prolonged in the bemarituzumab group (9.5 vs 7.4 months), but it did not reach statistical significance (P = .073). Overall survival (OS) was improved in the experimental group (not reached vs 12.9 months; P = .027). With a longer follow-up of 12.5 months, in post hoc exploratory analysis, OS was significantly longer in the experimental group (19.2 vs 13.5 months; hazard ratio 0.60, P = .027). The rate of serious adverse events was similar between the two groups. However, it is important to note ocular toxicities associated with bemarituzumab treatment. Corneal adverse events were seen in 67% of patients in the experimental group, with 24% of patients experiencing grade 3 events. Moreover, 26% of patients discontinued bemarituzumab because of corneal adverse events.
Overall, this phase 2 trial demonstrated that FGFR2b is emerging as an important biomarker and target in patients with advanced gastroesophageal adenocarcinoma. Ongoing phase 3 trials (FORTITUDE-101 with FOLFOX [NCT05052801] and FORTITUDE-102 with FOLFOX and nivolumab [NCT05111626]) hopefully will confirm the early results seen in the FIGHT trial. Awareness and early attention to treatment-associated toxicities will be critical for the potential future incorporation of bemarituzumab into clinical practice.
A study by Ramos‐Santillan and colleagues explored whether the order of treatment modalities matter in the management of early-stage gastric cancer. Typically, perioperative chemotherapy (both neoadjuvant and adjuvant) is used during treatment of early-stage gastric cancer, which is usually defined as at least cT2N0 or cTxN+ disease. In this study, multivariable Cox regression analyses were performed on propensity score-matched cohorts. The study analyzed outcomes of 11,984 patients who were identified using the US National Cancer Database and treated between 2005 and 2014. The results revealed that patients who had stage I disease had better outcomes with upfront resection followed by adjuvant therapy. Patients with stage III disease did better with a neoadjuvant approach, whereas patients with stage II disease had similar outcomes regardless of chemotherapy timing. This research has the limitations inherent to the retrospective nature of the analysis and lack of prospective enrollment and controls. However, it does suggest that there may be a fraction of patients who should be treated with upfront resection. For incorporation of this change into standard practice, the question of therapy sequencing should be answered in a randomized prospective trial that incorporates the most updated systemic therapy (fluorouracil, leucovorin, oxaliplatin, and docetaxel [FLOT]) into its design.
Chemotherapy continues to play a critical role during first-line treatment of advanced esophageal and gastric adenocarcinoma. Triple chemotherapy regimens have been known to have increased efficacy in this setting, but their use has been limited by associated toxicities. A study by Nguyen and colleagues evaluated the TCX regimen (paclitaxel, carboplatin, and capecitabine) during first-line treatment of advanced gastric cancer. This regimen is similar to other triple chemotherapy regimens, such as FLOT and DCF (docetaxel, cisplatin, and fluorouracil), which have proven activity in this disease. This prospective phase 2 trial enrolled 83 patients. The median PFS (9.3 months) and OS (17 months) compared favorably with historical references. The regimen had expected adverse events, with cytopenias and fatigue being the most frequently reported. On the basis of the reported safety and efficacy, TCX has potential to be used as a chemotherapy backbone in future trials, but larger trials are needed to confirm the phase 2 trial results.
References
Wainberg ZA, Enzinger PC, Kang YK, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022 Oct 13. Doi: 10.1016/S1470-2045(22)00603-9
Commentary: Chemoradiotherapy in CRC, November 2022
Once again, I have been given the distinct honor of analyzing two of the most provocative studies in colorectal cancer this month for Clinical Edge. The first study I will examine was done by Khamzina and colleagues and attempts to define the optimal time to perform surgery after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. In this retrospective analysis, 770 patients who received long-course chemoradiotherapy for rectal cancer followed by total mesorectal excision (TME) were analyzed by how long the interval was between completion of radiation and surgery. Patients were separated into two groups: 6-8 weeks (n = 502) vs >8 weeks (n = 268). Though the pathologic complete response rates and 5-year disease-free survival rates were not significantly different between the two groups, tumor regression grade was significantly better in the >8 weeks arm (P = .004). This result confirms many previous studies that demonstrate continued tumor shrinkage months after completion of chemoradiotherapy and may provide an explanation of why the OPRA trial demonstrated a higher TME-free rate in the chemoradiotherapy-then-chemotherapy arm than it did in the induction chemotherapy-then-chemoradiotherapy arm (53% vs 41%).
Schaefer and colleagues looked at the potential prognostic markers for efficacy of transarterial radioembolization (TARE) with 90Y resin microspheres in the treatment of liver-dominant metastatic colorectal cancer (mCRC). Their study evaluated 237 patients with liver-dominant mCRC from the prospective observational CIRSE Registry for SIR-Spheres Therapy (CIRT) study who were scheduled to receive TARE with 90Y resin microspheres. For these patients, the aspartate transaminase-to-platelet ratio index (APRI), international normalized ratio (INR), and albumin-bilirubin (ALBI) grade were measured prior to treatment to potentially detect values that might be associated with differential outcomes from TARE. An APRI > 0.40 independently predicted worse overall survival (OS) (hazard ratio [HR] 2.25; P < .0001), progression-free survival (PFS) (HR 1.42; P = .0416), and hepatic PFS (HR 1.50; P = .0207). The other independent predictors for worse OS and hepatic PFS were an INR value of < 1 (HR 1.66; P = .0091) and ALBI grade 3 (HR 5.29; P = .0075), respectively. It is very difficult to make much out of this study save to say that poorer liver function at baseline (at least with respect to APRI and ALBI) predicts worse outcomes after TARE, which is none too controversial an opinion. That said, APRI and ALBI may be able to provide an extra measure of granularity to determine who might be more of a marginal candidate for TARE than would categorization according to Child-Pugh score alone. Saving these patients from a potentially morbid procedure would be a significant benefit.
Once again, I have been given the distinct honor of analyzing two of the most provocative studies in colorectal cancer this month for Clinical Edge. The first study I will examine was done by Khamzina and colleagues and attempts to define the optimal time to perform surgery after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. In this retrospective analysis, 770 patients who received long-course chemoradiotherapy for rectal cancer followed by total mesorectal excision (TME) were analyzed by how long the interval was between completion of radiation and surgery. Patients were separated into two groups: 6-8 weeks (n = 502) vs >8 weeks (n = 268). Though the pathologic complete response rates and 5-year disease-free survival rates were not significantly different between the two groups, tumor regression grade was significantly better in the >8 weeks arm (P = .004). This result confirms many previous studies that demonstrate continued tumor shrinkage months after completion of chemoradiotherapy and may provide an explanation of why the OPRA trial demonstrated a higher TME-free rate in the chemoradiotherapy-then-chemotherapy arm than it did in the induction chemotherapy-then-chemoradiotherapy arm (53% vs 41%).
Schaefer and colleagues looked at the potential prognostic markers for efficacy of transarterial radioembolization (TARE) with 90Y resin microspheres in the treatment of liver-dominant metastatic colorectal cancer (mCRC). Their study evaluated 237 patients with liver-dominant mCRC from the prospective observational CIRSE Registry for SIR-Spheres Therapy (CIRT) study who were scheduled to receive TARE with 90Y resin microspheres. For these patients, the aspartate transaminase-to-platelet ratio index (APRI), international normalized ratio (INR), and albumin-bilirubin (ALBI) grade were measured prior to treatment to potentially detect values that might be associated with differential outcomes from TARE. An APRI > 0.40 independently predicted worse overall survival (OS) (hazard ratio [HR] 2.25; P < .0001), progression-free survival (PFS) (HR 1.42; P = .0416), and hepatic PFS (HR 1.50; P = .0207). The other independent predictors for worse OS and hepatic PFS were an INR value of < 1 (HR 1.66; P = .0091) and ALBI grade 3 (HR 5.29; P = .0075), respectively. It is very difficult to make much out of this study save to say that poorer liver function at baseline (at least with respect to APRI and ALBI) predicts worse outcomes after TARE, which is none too controversial an opinion. That said, APRI and ALBI may be able to provide an extra measure of granularity to determine who might be more of a marginal candidate for TARE than would categorization according to Child-Pugh score alone. Saving these patients from a potentially morbid procedure would be a significant benefit.
Once again, I have been given the distinct honor of analyzing two of the most provocative studies in colorectal cancer this month for Clinical Edge. The first study I will examine was done by Khamzina and colleagues and attempts to define the optimal time to perform surgery after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. In this retrospective analysis, 770 patients who received long-course chemoradiotherapy for rectal cancer followed by total mesorectal excision (TME) were analyzed by how long the interval was between completion of radiation and surgery. Patients were separated into two groups: 6-8 weeks (n = 502) vs >8 weeks (n = 268). Though the pathologic complete response rates and 5-year disease-free survival rates were not significantly different between the two groups, tumor regression grade was significantly better in the >8 weeks arm (P = .004). This result confirms many previous studies that demonstrate continued tumor shrinkage months after completion of chemoradiotherapy and may provide an explanation of why the OPRA trial demonstrated a higher TME-free rate in the chemoradiotherapy-then-chemotherapy arm than it did in the induction chemotherapy-then-chemoradiotherapy arm (53% vs 41%).
Schaefer and colleagues looked at the potential prognostic markers for efficacy of transarterial radioembolization (TARE) with 90Y resin microspheres in the treatment of liver-dominant metastatic colorectal cancer (mCRC). Their study evaluated 237 patients with liver-dominant mCRC from the prospective observational CIRSE Registry for SIR-Spheres Therapy (CIRT) study who were scheduled to receive TARE with 90Y resin microspheres. For these patients, the aspartate transaminase-to-platelet ratio index (APRI), international normalized ratio (INR), and albumin-bilirubin (ALBI) grade were measured prior to treatment to potentially detect values that might be associated with differential outcomes from TARE. An APRI > 0.40 independently predicted worse overall survival (OS) (hazard ratio [HR] 2.25; P < .0001), progression-free survival (PFS) (HR 1.42; P = .0416), and hepatic PFS (HR 1.50; P = .0207). The other independent predictors for worse OS and hepatic PFS were an INR value of < 1 (HR 1.66; P = .0091) and ALBI grade 3 (HR 5.29; P = .0075), respectively. It is very difficult to make much out of this study save to say that poorer liver function at baseline (at least with respect to APRI and ALBI) predicts worse outcomes after TARE, which is none too controversial an opinion. That said, APRI and ALBI may be able to provide an extra measure of granularity to determine who might be more of a marginal candidate for TARE than would categorization according to Child-Pugh score alone. Saving these patients from a potentially morbid procedure would be a significant benefit.
Commentary: COVID-19, Tenosynovitis, and RA, November 2022
Multiple studies have emphasized the potential for severe COVID-19 outcomes in patients with rheumatic disease, including patients with rheumatoid arthritis (RA). Because these studies often group together patients with different diseases, medications, and manifestations, differences in outcomes between patients with these conditions may be difficult to tease out.
Figueroa-Parra and colleagues performed a retrospective cohort study comparing people with RA who developed COVID-19 to those who did not have RA to examine the effect of RA characteristics, such as interstitial lung disease (ILD), serostatus, and bone erosions, on COVID-19 outcomes. Patients with RA, particularly those with seropositive RA, bone erosions, and RA-associated ILD, had approximately twofold (or higher) risk for severe COVID-19 outcomes, such as mortality or mechanical ventilation, than did those without RA. However, there was no difference in outcomes seen between patients with RA who were seropositive compared with those who were seronegative, with or without bone erosions, or with or without ILD. The mechanism by which RA phenotypes and their treatment affect this risk remains unclear.
Li and colleagues also looked at COVID-19 outcomes in patients with RA according to vaccination status using a UK primary care database. Among unvaccinated patients, the risk for SARS-CoV-2 infection and hospitalization or mortality because of COVID-19 were modestly higher in people with RA. Among vaccinated patients, there was no increased risk for breakthrough infection, COVID-19 hospitalization, or mortality observed in patients with RA over 3 or 6 months of follow-up, with a slight increase over 9 months of follow-up. Overall, both studies support prior research suggesting a higher risk for more severe COVID-19 in patients with RA, as well as potential mitigation with vaccination.
Predictors of RA course and severity are of great interest in determining the optimal therapy to reduce joint damage and prevent RA progression while also minimizing the adverse effects of treatment. Early disease course has been shown to be important in several studies. Giollo and colleagues compared patients with "difficult-to-treat RA," ie, RA that is resistant to multiple biologic disease-modifying antirheumatic drugs (bDMARD) or targeted synthetic DMARD (tsDMARD), with those without in an inception cohort study and found that early difficult management as well as delay of methotrexate initiation was associated with persistent inflammatory symptoms. This finding does not show a causative relationship between methotrexate and protection from the development of refractory RA but does lend support for early aggressive treatment in patients with a high inflammatory burden.
Conversely, Parisi and colleagues performed a subanalysis of the STARTER study of patients with RA in clinical remission to evaluate the impact of different therapies. The STARTER study had shown an association between ultrasound detection of tenosynovitis and RA flares. Of the more than 250 patients completing the study, ultrasound evidence of tenosynovitis was better controlled in patients on combination bDMARD and conventional synthetic DMARD (csDMARD) therapy than in those on csDMARDs monotherapy, with a trend toward reduction in flares in patients on combination therapy. Given the relatively small effect, it is not clear that combination therapy is associated with deeper remission, but, as suggested in prior studies, ultrasound evidence of tenosynovitis may be worthwhile considering prior to tapering therapy.
Multiple studies have emphasized the potential for severe COVID-19 outcomes in patients with rheumatic disease, including patients with rheumatoid arthritis (RA). Because these studies often group together patients with different diseases, medications, and manifestations, differences in outcomes between patients with these conditions may be difficult to tease out.
Figueroa-Parra and colleagues performed a retrospective cohort study comparing people with RA who developed COVID-19 to those who did not have RA to examine the effect of RA characteristics, such as interstitial lung disease (ILD), serostatus, and bone erosions, on COVID-19 outcomes. Patients with RA, particularly those with seropositive RA, bone erosions, and RA-associated ILD, had approximately twofold (or higher) risk for severe COVID-19 outcomes, such as mortality or mechanical ventilation, than did those without RA. However, there was no difference in outcomes seen between patients with RA who were seropositive compared with those who were seronegative, with or without bone erosions, or with or without ILD. The mechanism by which RA phenotypes and their treatment affect this risk remains unclear.
Li and colleagues also looked at COVID-19 outcomes in patients with RA according to vaccination status using a UK primary care database. Among unvaccinated patients, the risk for SARS-CoV-2 infection and hospitalization or mortality because of COVID-19 were modestly higher in people with RA. Among vaccinated patients, there was no increased risk for breakthrough infection, COVID-19 hospitalization, or mortality observed in patients with RA over 3 or 6 months of follow-up, with a slight increase over 9 months of follow-up. Overall, both studies support prior research suggesting a higher risk for more severe COVID-19 in patients with RA, as well as potential mitigation with vaccination.
Predictors of RA course and severity are of great interest in determining the optimal therapy to reduce joint damage and prevent RA progression while also minimizing the adverse effects of treatment. Early disease course has been shown to be important in several studies. Giollo and colleagues compared patients with "difficult-to-treat RA," ie, RA that is resistant to multiple biologic disease-modifying antirheumatic drugs (bDMARD) or targeted synthetic DMARD (tsDMARD), with those without in an inception cohort study and found that early difficult management as well as delay of methotrexate initiation was associated with persistent inflammatory symptoms. This finding does not show a causative relationship between methotrexate and protection from the development of refractory RA but does lend support for early aggressive treatment in patients with a high inflammatory burden.
Conversely, Parisi and colleagues performed a subanalysis of the STARTER study of patients with RA in clinical remission to evaluate the impact of different therapies. The STARTER study had shown an association between ultrasound detection of tenosynovitis and RA flares. Of the more than 250 patients completing the study, ultrasound evidence of tenosynovitis was better controlled in patients on combination bDMARD and conventional synthetic DMARD (csDMARD) therapy than in those on csDMARDs monotherapy, with a trend toward reduction in flares in patients on combination therapy. Given the relatively small effect, it is not clear that combination therapy is associated with deeper remission, but, as suggested in prior studies, ultrasound evidence of tenosynovitis may be worthwhile considering prior to tapering therapy.
Multiple studies have emphasized the potential for severe COVID-19 outcomes in patients with rheumatic disease, including patients with rheumatoid arthritis (RA). Because these studies often group together patients with different diseases, medications, and manifestations, differences in outcomes between patients with these conditions may be difficult to tease out.
Figueroa-Parra and colleagues performed a retrospective cohort study comparing people with RA who developed COVID-19 to those who did not have RA to examine the effect of RA characteristics, such as interstitial lung disease (ILD), serostatus, and bone erosions, on COVID-19 outcomes. Patients with RA, particularly those with seropositive RA, bone erosions, and RA-associated ILD, had approximately twofold (or higher) risk for severe COVID-19 outcomes, such as mortality or mechanical ventilation, than did those without RA. However, there was no difference in outcomes seen between patients with RA who were seropositive compared with those who were seronegative, with or without bone erosions, or with or without ILD. The mechanism by which RA phenotypes and their treatment affect this risk remains unclear.
Li and colleagues also looked at COVID-19 outcomes in patients with RA according to vaccination status using a UK primary care database. Among unvaccinated patients, the risk for SARS-CoV-2 infection and hospitalization or mortality because of COVID-19 were modestly higher in people with RA. Among vaccinated patients, there was no increased risk for breakthrough infection, COVID-19 hospitalization, or mortality observed in patients with RA over 3 or 6 months of follow-up, with a slight increase over 9 months of follow-up. Overall, both studies support prior research suggesting a higher risk for more severe COVID-19 in patients with RA, as well as potential mitigation with vaccination.
Predictors of RA course and severity are of great interest in determining the optimal therapy to reduce joint damage and prevent RA progression while also minimizing the adverse effects of treatment. Early disease course has been shown to be important in several studies. Giollo and colleagues compared patients with "difficult-to-treat RA," ie, RA that is resistant to multiple biologic disease-modifying antirheumatic drugs (bDMARD) or targeted synthetic DMARD (tsDMARD), with those without in an inception cohort study and found that early difficult management as well as delay of methotrexate initiation was associated with persistent inflammatory symptoms. This finding does not show a causative relationship between methotrexate and protection from the development of refractory RA but does lend support for early aggressive treatment in patients with a high inflammatory burden.
Conversely, Parisi and colleagues performed a subanalysis of the STARTER study of patients with RA in clinical remission to evaluate the impact of different therapies. The STARTER study had shown an association between ultrasound detection of tenosynovitis and RA flares. Of the more than 250 patients completing the study, ultrasound evidence of tenosynovitis was better controlled in patients on combination bDMARD and conventional synthetic DMARD (csDMARD) therapy than in those on csDMARDs monotherapy, with a trend toward reduction in flares in patients on combination therapy. Given the relatively small effect, it is not clear that combination therapy is associated with deeper remission, but, as suggested in prior studies, ultrasound evidence of tenosynovitis may be worthwhile considering prior to tapering therapy.
Tips on Better Patients Communication
SAN DIEGO—Don’t stand when you talk at bedside. Ditch the white gowns, turn away from your computers and pagers, and stop yourself from interrupting all the time.
These tips—and more—can help clinicians provide better and more effective care, said a colorectal surgeon who spoke about communication skills at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Research has suggested that nearly half of Americans don’t think their health care practitioners (HCPs) are compassionate, “and that’s really sad,” said Lorene Valdez-Boyle, MD, MS, surgery chief at the New Mexico VA Health Care Service.
To combat this perception, she said, HCPs can adopt multiple strategies as they work with veterans and their families. The goal, she said, is “to try to get them to trust you and want to be part of their treatment. This is how we're going to have better outcomes.”
Some strategies are simple. Dr. Valdez-Boyle, for example, doesn’t wear a white gown when she sees patients. “Obviously, they’re really gross,” she said. “But also, I want them to be comfortable with me. I sit down at their level, and we have a conversation. We talk about our dogs and we bond, because that’s going to help them trust me and want to work with me. I do that with families too. We joke, and we laugh.”
Sitting bedside instead of standing is important, she said, and a 2016 study backs up this idea. “It’s difficult when you’re running around or you want to get to the next one, and the patient just keeps talking,” she said. But research showed that “when the clinician sat, the patient felt like they listened more carefully, and they explained things in a better way that was much easier for them to understand. They definitely had an improved perception of their [clinician’s] communication skills.”
She highlighted another 2016 study that examined a Commit to Sit initiative in which nurses were urged to sit with patients during each shift. Nurse communication scores and overall patient experience scores went up.
The VA now has a Commit to Sit initiative, which urges clinicians to put away computers, smart phones, and pagers. “The patient feels that we’ve listened more intently to their concerns and care more about them as a patient,” Dr. Valdez-Boyle said. “We have an improved understanding of their health as a result of this. It allows the site employee to continue to be efficient while still delivering compassionate care and fosters trusted relationships in an empathetic and respectful manner.”
For more about the initiative, visit the VA PX SharePoint.
The VA, she said, also has a Take a Moment initiative that emphasizes eye contact, face-to-face interaction without electronics for at least the first 5 minutes of each visit, and seated conversations.
Dr. Valdez-Boyle also urged colleagues to pay attention to how often they interrupt. She pointed to a 2019 study that reported that patients had a median of 11 seconds—yes, seconds—to explain their problem in two-thirds of clinician encounters. “I think some of it is because we think we know what they're going to say.”
In the age of COVID-19, she suggested turning to fist or elbow bumps instead of handshakes. And she said, let patients wear street clothes when appropriate so they’re more comfortable.
In the big picture, she said, good communication and a commitment to shared decision making “really create a shared responsibility. They give your patients ownership over their disease and the ability to make the decisions with their team.
Dr. Valdez-Boyle reported no disclosures.
SAN DIEGO—Don’t stand when you talk at bedside. Ditch the white gowns, turn away from your computers and pagers, and stop yourself from interrupting all the time.
These tips—and more—can help clinicians provide better and more effective care, said a colorectal surgeon who spoke about communication skills at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Research has suggested that nearly half of Americans don’t think their health care practitioners (HCPs) are compassionate, “and that’s really sad,” said Lorene Valdez-Boyle, MD, MS, surgery chief at the New Mexico VA Health Care Service.
To combat this perception, she said, HCPs can adopt multiple strategies as they work with veterans and their families. The goal, she said, is “to try to get them to trust you and want to be part of their treatment. This is how we're going to have better outcomes.”
Some strategies are simple. Dr. Valdez-Boyle, for example, doesn’t wear a white gown when she sees patients. “Obviously, they’re really gross,” she said. “But also, I want them to be comfortable with me. I sit down at their level, and we have a conversation. We talk about our dogs and we bond, because that’s going to help them trust me and want to work with me. I do that with families too. We joke, and we laugh.”
Sitting bedside instead of standing is important, she said, and a 2016 study backs up this idea. “It’s difficult when you’re running around or you want to get to the next one, and the patient just keeps talking,” she said. But research showed that “when the clinician sat, the patient felt like they listened more carefully, and they explained things in a better way that was much easier for them to understand. They definitely had an improved perception of their [clinician’s] communication skills.”
She highlighted another 2016 study that examined a Commit to Sit initiative in which nurses were urged to sit with patients during each shift. Nurse communication scores and overall patient experience scores went up.
The VA now has a Commit to Sit initiative, which urges clinicians to put away computers, smart phones, and pagers. “The patient feels that we’ve listened more intently to their concerns and care more about them as a patient,” Dr. Valdez-Boyle said. “We have an improved understanding of their health as a result of this. It allows the site employee to continue to be efficient while still delivering compassionate care and fosters trusted relationships in an empathetic and respectful manner.”
For more about the initiative, visit the VA PX SharePoint.
The VA, she said, also has a Take a Moment initiative that emphasizes eye contact, face-to-face interaction without electronics for at least the first 5 minutes of each visit, and seated conversations.
Dr. Valdez-Boyle also urged colleagues to pay attention to how often they interrupt. She pointed to a 2019 study that reported that patients had a median of 11 seconds—yes, seconds—to explain their problem in two-thirds of clinician encounters. “I think some of it is because we think we know what they're going to say.”
In the age of COVID-19, she suggested turning to fist or elbow bumps instead of handshakes. And she said, let patients wear street clothes when appropriate so they’re more comfortable.
In the big picture, she said, good communication and a commitment to shared decision making “really create a shared responsibility. They give your patients ownership over their disease and the ability to make the decisions with their team.
Dr. Valdez-Boyle reported no disclosures.
SAN DIEGO—Don’t stand when you talk at bedside. Ditch the white gowns, turn away from your computers and pagers, and stop yourself from interrupting all the time.
These tips—and more—can help clinicians provide better and more effective care, said a colorectal surgeon who spoke about communication skills at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Research has suggested that nearly half of Americans don’t think their health care practitioners (HCPs) are compassionate, “and that’s really sad,” said Lorene Valdez-Boyle, MD, MS, surgery chief at the New Mexico VA Health Care Service.
To combat this perception, she said, HCPs can adopt multiple strategies as they work with veterans and their families. The goal, she said, is “to try to get them to trust you and want to be part of their treatment. This is how we're going to have better outcomes.”
Some strategies are simple. Dr. Valdez-Boyle, for example, doesn’t wear a white gown when she sees patients. “Obviously, they’re really gross,” she said. “But also, I want them to be comfortable with me. I sit down at their level, and we have a conversation. We talk about our dogs and we bond, because that’s going to help them trust me and want to work with me. I do that with families too. We joke, and we laugh.”
Sitting bedside instead of standing is important, she said, and a 2016 study backs up this idea. “It’s difficult when you’re running around or you want to get to the next one, and the patient just keeps talking,” she said. But research showed that “when the clinician sat, the patient felt like they listened more carefully, and they explained things in a better way that was much easier for them to understand. They definitely had an improved perception of their [clinician’s] communication skills.”
She highlighted another 2016 study that examined a Commit to Sit initiative in which nurses were urged to sit with patients during each shift. Nurse communication scores and overall patient experience scores went up.
The VA now has a Commit to Sit initiative, which urges clinicians to put away computers, smart phones, and pagers. “The patient feels that we’ve listened more intently to their concerns and care more about them as a patient,” Dr. Valdez-Boyle said. “We have an improved understanding of their health as a result of this. It allows the site employee to continue to be efficient while still delivering compassionate care and fosters trusted relationships in an empathetic and respectful manner.”
For more about the initiative, visit the VA PX SharePoint.
The VA, she said, also has a Take a Moment initiative that emphasizes eye contact, face-to-face interaction without electronics for at least the first 5 minutes of each visit, and seated conversations.
Dr. Valdez-Boyle also urged colleagues to pay attention to how often they interrupt. She pointed to a 2019 study that reported that patients had a median of 11 seconds—yes, seconds—to explain their problem in two-thirds of clinician encounters. “I think some of it is because we think we know what they're going to say.”
In the age of COVID-19, she suggested turning to fist or elbow bumps instead of handshakes. And she said, let patients wear street clothes when appropriate so they’re more comfortable.
In the big picture, she said, good communication and a commitment to shared decision making “really create a shared responsibility. They give your patients ownership over their disease and the ability to make the decisions with their team.
Dr. Valdez-Boyle reported no disclosures.
IgA Vasculitis in the Setting of Biologic Therapy for Psoriasis and Recurrent Cutaneous Methicillin-Resistant Staphylococcus aureus Colonization
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.
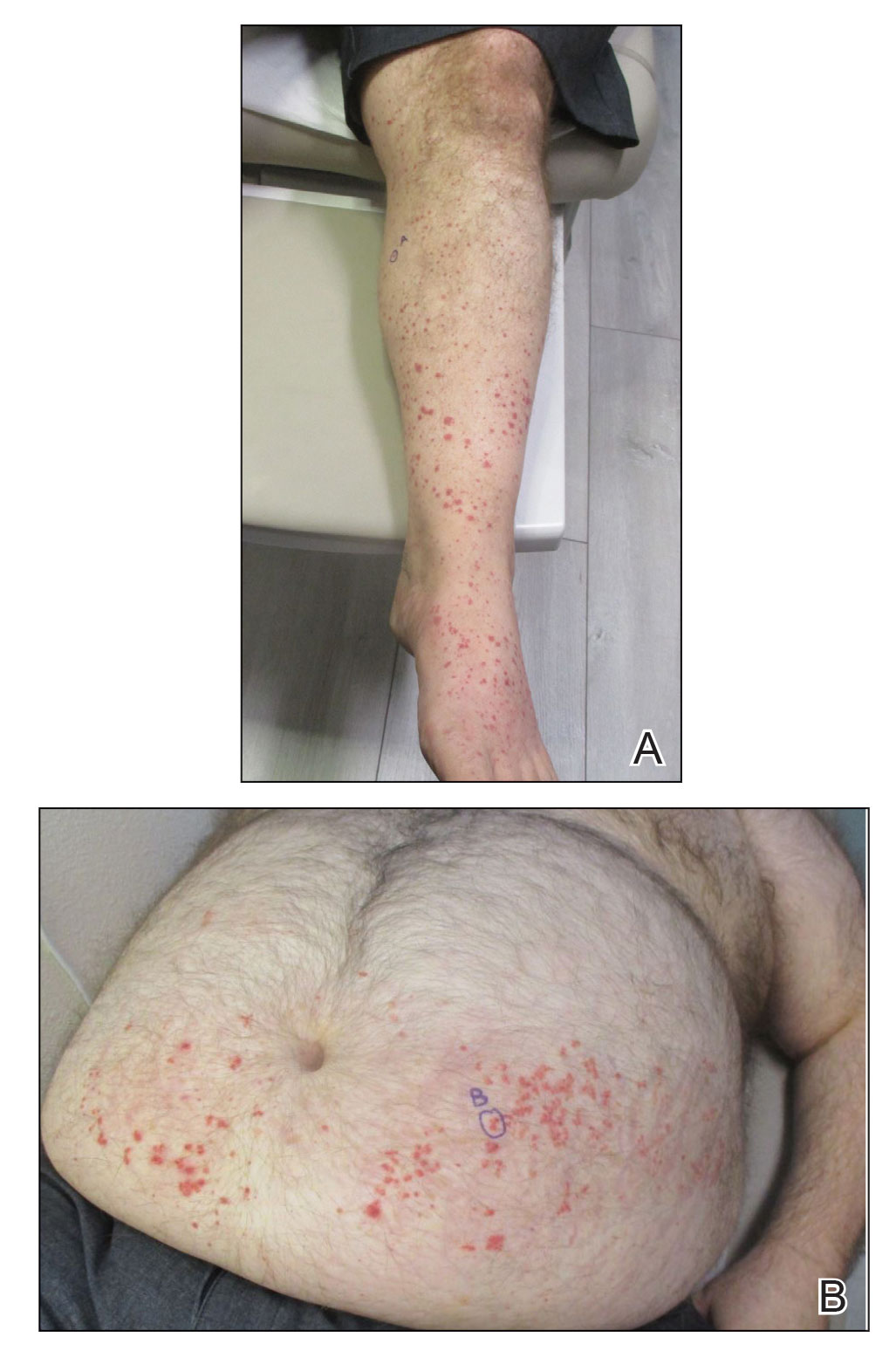
Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.
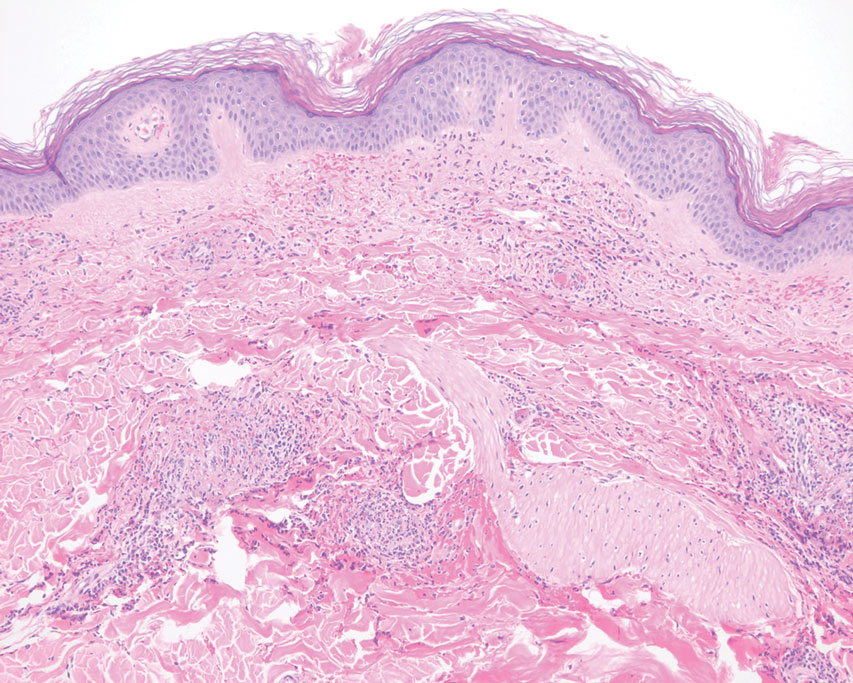
Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.
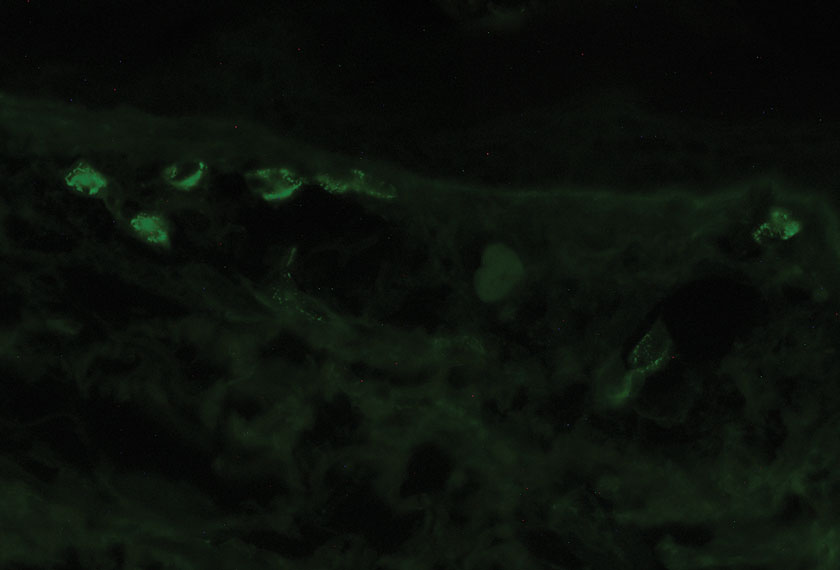
Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.
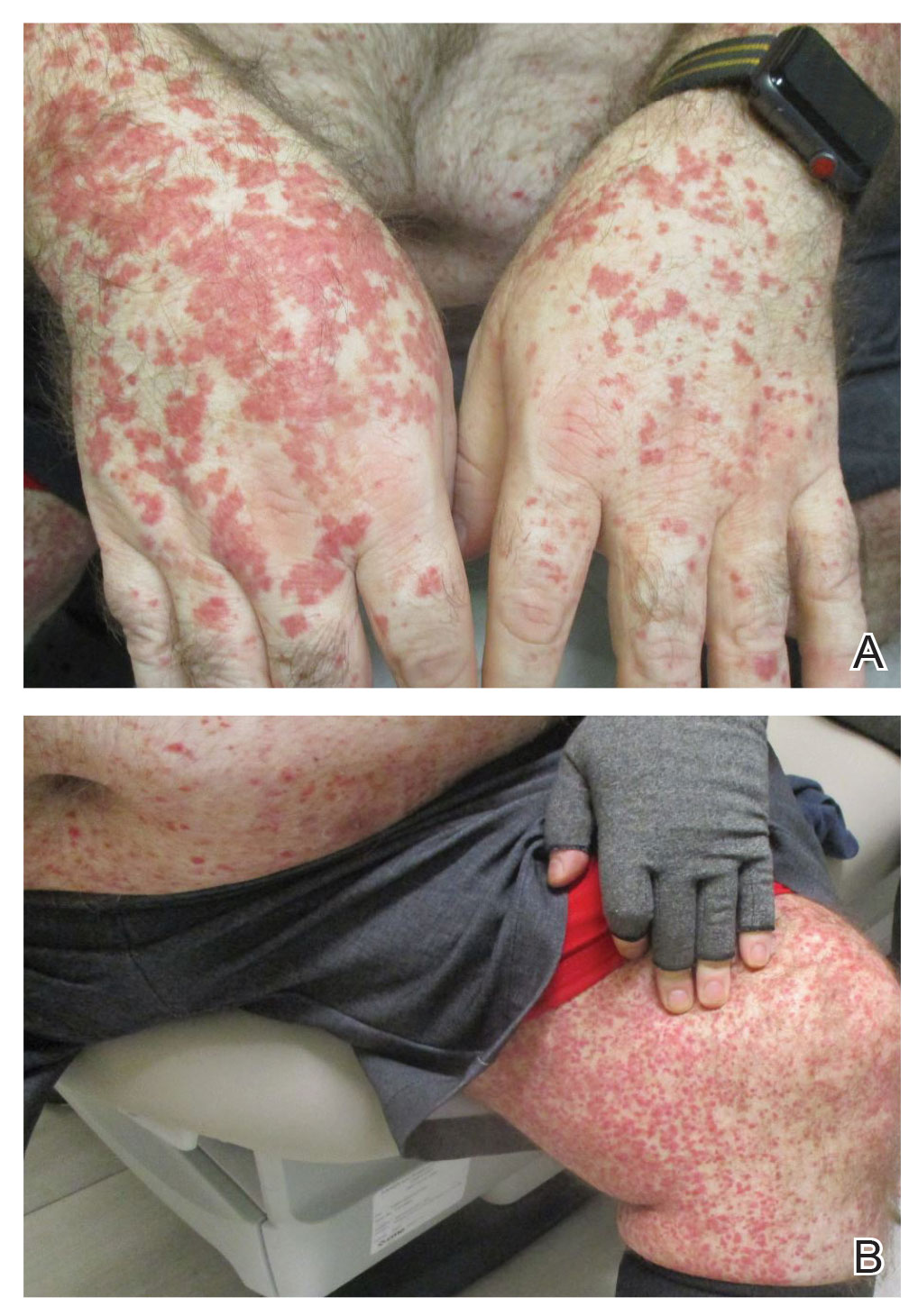
Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7
The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10
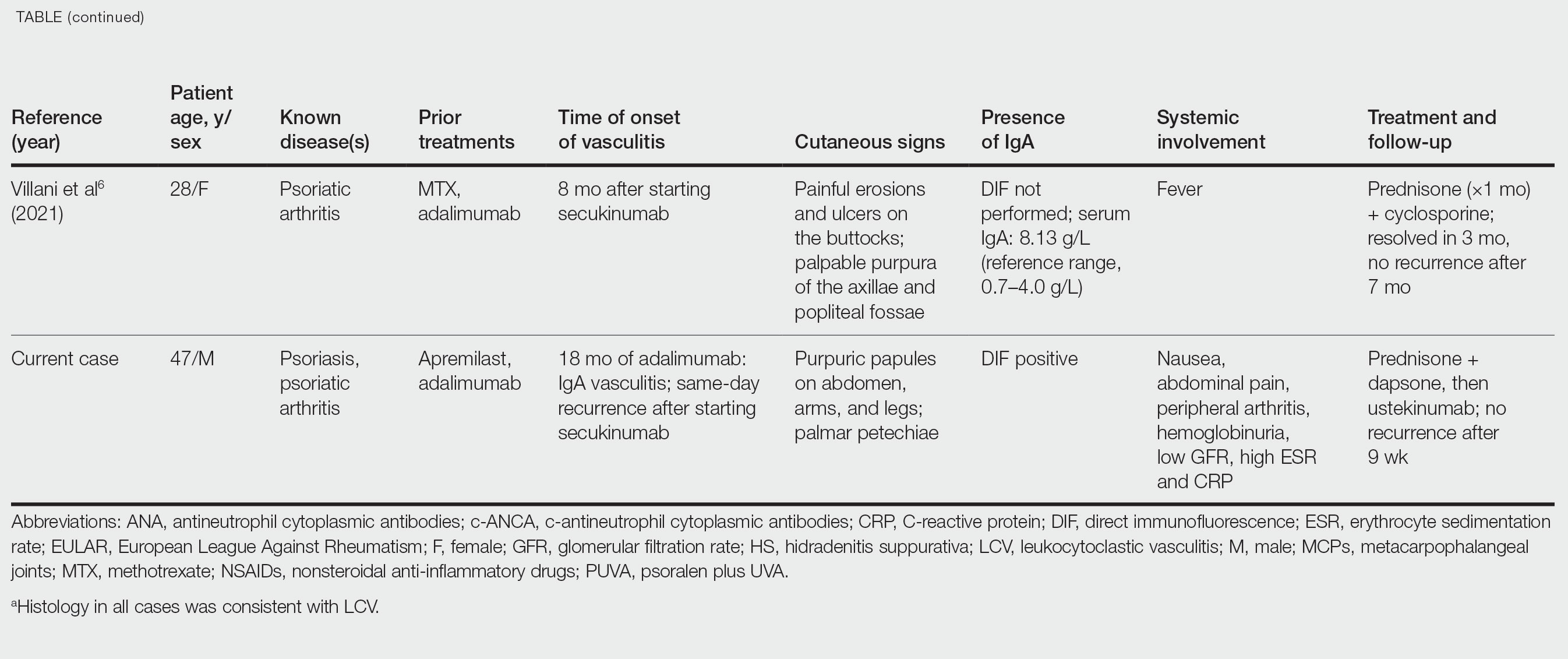
There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Practice Points
- Biologic medications including adalimumab and more rarely secukinumab may be associated with leukocytoclastic vasculitis; a smaller subset of patients may experience IgA vasculitis.
- The IL-23 blocker ustekinumab may represent an ideal therapeutic agent when secukinumabassociated vasculitis is suspected. Because IL-23 is the main driver and sustainer of TH17 cell differentiation, it may cease the main causative cytokine cascades “upstream.”