User login
Colonoscopy screening leads to modest reduction in risk for CRC
Key clinical point: Participants invited to undergo a single screening colonoscopy had a modestly reduced risk for colorectal cancer (CRC) at 10 years than those who were assigned to no screening.
Major finding: At 10 years, the real-world risk for CRC was 18% lower among participants who were invited vs not invited to undergo screening colonoscopy (risk ratio 0.82; 95% CI 0.70-0.93), with the number needed to invite to undergo screening to prevent 1 case of CRC within 10 years being 455 (95% CI 270-1,429).
Study details: The findings are 10-year follow-up results of the NordICC trial including 84,585 participants who were randomly assigned to receive (invited group; n = 28,220) or not receive (usual-care group; n = 56,365) an invitation to undergo a single screening colonoscopy.
Disclosures: This study was funded by the Research Council of Norway, Nordic Cancer Union, and others. Some authors declared serving as expert witnesses or consultants for or receiving research support, speakers' fees, or consultancy fees from various sources.
Source: Bretthauer M et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556 (Oct 27). Doi: 10.1056/NEJMoa2208375
Key clinical point: Participants invited to undergo a single screening colonoscopy had a modestly reduced risk for colorectal cancer (CRC) at 10 years than those who were assigned to no screening.
Major finding: At 10 years, the real-world risk for CRC was 18% lower among participants who were invited vs not invited to undergo screening colonoscopy (risk ratio 0.82; 95% CI 0.70-0.93), with the number needed to invite to undergo screening to prevent 1 case of CRC within 10 years being 455 (95% CI 270-1,429).
Study details: The findings are 10-year follow-up results of the NordICC trial including 84,585 participants who were randomly assigned to receive (invited group; n = 28,220) or not receive (usual-care group; n = 56,365) an invitation to undergo a single screening colonoscopy.
Disclosures: This study was funded by the Research Council of Norway, Nordic Cancer Union, and others. Some authors declared serving as expert witnesses or consultants for or receiving research support, speakers' fees, or consultancy fees from various sources.
Source: Bretthauer M et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556 (Oct 27). Doi: 10.1056/NEJMoa2208375
Key clinical point: Participants invited to undergo a single screening colonoscopy had a modestly reduced risk for colorectal cancer (CRC) at 10 years than those who were assigned to no screening.
Major finding: At 10 years, the real-world risk for CRC was 18% lower among participants who were invited vs not invited to undergo screening colonoscopy (risk ratio 0.82; 95% CI 0.70-0.93), with the number needed to invite to undergo screening to prevent 1 case of CRC within 10 years being 455 (95% CI 270-1,429).
Study details: The findings are 10-year follow-up results of the NordICC trial including 84,585 participants who were randomly assigned to receive (invited group; n = 28,220) or not receive (usual-care group; n = 56,365) an invitation to undergo a single screening colonoscopy.
Disclosures: This study was funded by the Research Council of Norway, Nordic Cancer Union, and others. Some authors declared serving as expert witnesses or consultants for or receiving research support, speakers' fees, or consultancy fees from various sources.
Source: Bretthauer M et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556 (Oct 27). Doi: 10.1056/NEJMoa2208375
Diffuse Papular Eruption With Erosions and Ulcerations
The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
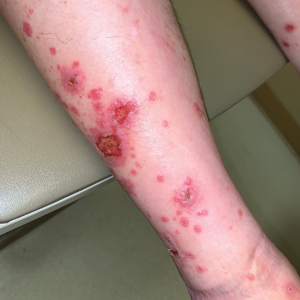
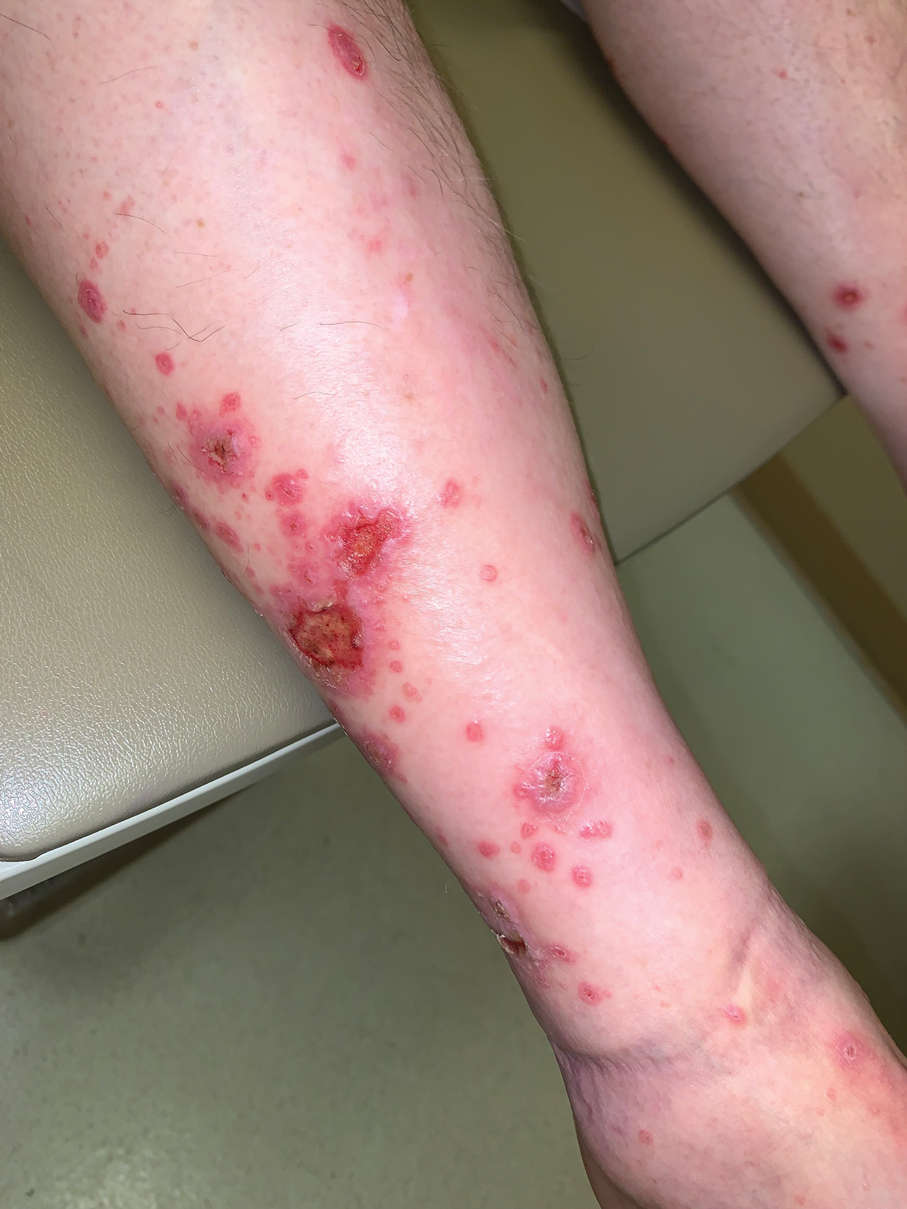
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
A 70-year-old man presented with a painful, pruritic, diffuse eruption on the trunk, legs, and arms of 2 months’ duration. He had a history of stage IV pleomorphic cell sarcoma of the retroperitoneum and was started on pembrolizumab therapy 6 weeks prior to the eruption. Physical examination revealed violaceous papules and plaques with shiny reticulated scaling as well as multiple scattered eroded papules and shallow ulcerations. The oral mucosa and genitals were spared. The patient endorsed blisters followed by open sores that were both itchy and painful. He denied self-infliction. Both the patient and his wife denied scratching. Two biopsies for direct immunofluorescence and histopathology were performed.
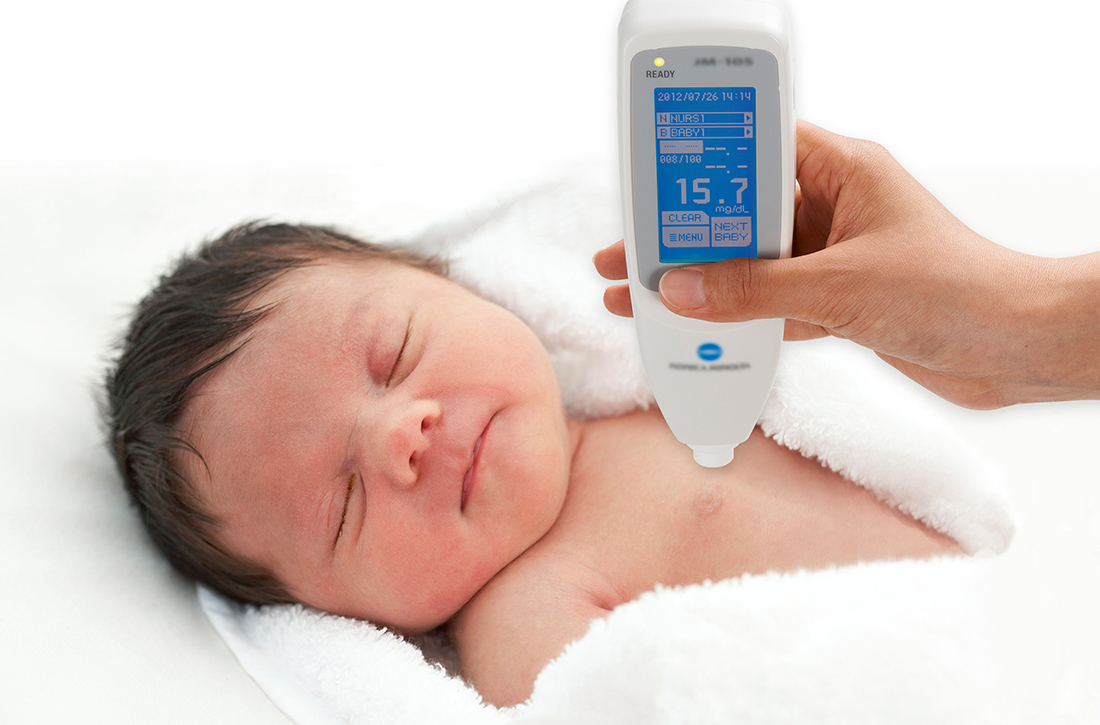
How accurate is transcutaneous bilirubin testing in newborns with darker skin tones?
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
EVIDENCE-BASED ANSWER:
Fairly accurate. Photometric transcutaneous bilirubin (TcB) testing may overestimate total serum bilirubin (TSB) in neonates with darker skin tones by a mean of 0.68 to > 2 mg/dL (strength of recommendation [SOR]: C, diagnostic cohort studies with differing reference standards).
Overall, TcB meters retain acceptable accuracy in infants of all skin tones across a range of bilirubin levels, despite being more likely to underestimate lighter skin tones and overestimate darker ones (SOR: C, diagnostic cohort studies with differing reference standards). It is unclear if the higher readings prompt an increase in blood draws or otherwise alter care.
26-year-old woman • nausea and vomiting • currently breastfeeding • ketogenic diet • Dx?
THE CASE
A 26-year-old woman presented to the emergency department (ED) with a history of nausea and vomiting for more than 24 hours. The vomiting began when she awoke to breastfeed her 3-month-old infant. She had been unable to eat or drink anything for about 16 hours.
She’d seen her primary care provider earlier in the day. Antiemetics were prescribed, but they did not provide relief. So 10 hours later, when her symptoms worsened, she presented to the ED.
Her medical history was notable for a body mass index of 26. The patient also reported positional back pain, but the review of systems was otherwise negative. The patient indicated that she’d been on a ketogenic diet for about 1 month, but she denied use of supplements.
Upon presentation to the ED, the patient was found to have a metabolic acidosis with a pH of 7.02 and an anion gap of 25. Her glucose level was 132 mg/dL, and she had a positive serum acetone and a beta-hydroxybutyrate level of 75 mg/dL (reference range, 0-2.8 mg/dL). Her salicylate testing was negative, and her lactate level was 1.4 mmol/L (reference range, 0.4-2.0 mmol/L).
THE DIAGNOSIS
This patient, with severe acidosis and an elevated anion gap, received a diagnosis of starvation ketoacidosis—specifically, lactation ketoacidosis. Other causes of elevated anion gap metabolic acidosis were ruled out, including salicylate overdose, lactic acidosis, diabetic ketoacidosis, and other ingestions. The elevated acetone and beta-hydroxybutyrate levels confirmed the diagnosis. The patient was treated with a bolus of 1 L normal saline with 5% dextrose (D5NS) in the ED and admitted.
DISCUSSION
Lactation ketoacidosis is a relatively uncommon condition, but reports have increased with the growing popularity of low-carbohydrate diets. The treatment approach has differed in previous reports in regard to insulin and bicarbonate use.1-9
The use of bicarbonate is controversial in diabetic ketoacidosis and unlikely to be helpful in lactation ketoacidosis, but it is something to consider when the patient’s pH is < 6.9. Insulin use is likely unnecessary for lactation ketoacidosis, as metabolic derangements have been corrected without intervention.
Continue to: With an increasing prevalence of cases...
With an increasing prevalence of cases, we suggest a conservative approach for treatment based on this case presentation and review of other presentations. Our patient responded rapidly to conservative treatment with intravenous (IV) fluids (D5NS), a liberalized diet, and electrolyte repletion (described in detail later).
Suggested management
Once other causes of a patient’s signs and symptoms are excluded and the diagnosis of lactation ketoacidosis is made, you’ll want to follow the initial set of lab work with the following: a venous blood gas, basic metabolic panel, and testing of magnesium and phosphorous levels every 8 hours after initial presentation, with repletion as indicated. Some patients may require more frequent monitoring based on repletion of electrolytes.
The patient will initially require IV fluid resuscitation; the initial fluid of choice would be D5NS. Patients will likely need no more than 2 L, but this will depend on the degree of hypovolemia.
Diet should be advanced as tolerated and include no restriction of carbohydrates.
Previous reports have varied regarding continuation of breastfeeding and pumping. In this case, the patient continued to breastfeed without any adverse effects. Continuation of breastfeeding is unlikely to cause harm in these circumstances, but severity of symptoms (pain, nausea, vomiting) or unresolved acidosis may require discontinuation.
Continue to: Discharge should be determined...
Discharge should be determined by resolution of symptoms and correction of metabolic derangements. In previous reports, discharge time varied from 48 hours up to 144 hours, with most patients discharged on Day 2 or 3. Pending clinical factors, discharge is likely appropriate between 36 to 72 hours from time of admission.
Our patient received an additional 1 L of D5NS for continued signs of dehydration during admission. Her pH and electrolyte levels were monitored every 8 hours, with repletion of electrolytes as needed. Her acidosis, nausea, vomiting, and pain resolved within 36 hours. The patient continued to breastfeed her infant throughout her stay. With resolution of symptoms and metabolic derangements, the patient was discharged about 36 hours after admission. She was advised to follow up with her primary care provider within 1 week after discharge.
THE TAKEAWAY
As the popularity of low-carbohydrate diets increases, patients should be educated about the warning signs of clinically significant ketoacidosis. This information is especially important for those who are lactating, as this metabolic state increases predilection to ketoacidosis. When cases do present, conservative management with IV fluids and a liberalized diet is likely to be an appropriate course of care for most patients.
CORRESPONDENCE
C.W. Ferguson, DO, Navy Medicine Readiness and Training Command, Camp Lejeune Family Medicine Residency, 100 Brewster Boulevard, Camp Lejeune, NC 28547; [email protected]
1. Al Alawi AM, Falhammar H. Lactation ketoacidosis: case presentation and literature review. BMJ Case Rep. 2018;2018:bcr2017223494. doi:10.1136/bcr-2017-223494
2. von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep. 2015;9:224. doi:10.1186/s13256-015-0709-2
3. Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J. 2015;14:117. doi:10.1186/s12937-015-0076-2
4. Azzam O, Prentice D. Lactation ketoacidosis: an easily missed diagnosis. Intern Med J. 2019;49:256‐259. doi:10.1111/imj.14207
5. Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus. 2009;2:278‐279. doi:10.1093/ndtplus/sfp052
6. Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med. 2008;35:385‐387. doi:10.1016/j.jemermed.2007.04.013
7. Szulewski A, Howes D, Morton AR. A severe case of iatrogenic lactation ketoacidosis. BMJ Case Rep. 2012;2012:bcr1220115409. doi:10.1136/bcr.12.2011.5409
8. Nnodum BN, Oduah E, Albert D, et al. Ketogenic diet-induced severe ketoacidosis in a lactating woman: a case report and review of the literature. Case Rep Nephrol. 2019;2019:1214208. doi:10.1155/2019/1214208
9. Gleeson S, Mulroy E, Clarke DE. Lactation ketoacidosis: an unusual entity and a review of the literature. Perm J. 2016;20:71‐73. doi:10.7812/TPP/15-097
THE CASE
A 26-year-old woman presented to the emergency department (ED) with a history of nausea and vomiting for more than 24 hours. The vomiting began when she awoke to breastfeed her 3-month-old infant. She had been unable to eat or drink anything for about 16 hours.
She’d seen her primary care provider earlier in the day. Antiemetics were prescribed, but they did not provide relief. So 10 hours later, when her symptoms worsened, she presented to the ED.
Her medical history was notable for a body mass index of 26. The patient also reported positional back pain, but the review of systems was otherwise negative. The patient indicated that she’d been on a ketogenic diet for about 1 month, but she denied use of supplements.
Upon presentation to the ED, the patient was found to have a metabolic acidosis with a pH of 7.02 and an anion gap of 25. Her glucose level was 132 mg/dL, and she had a positive serum acetone and a beta-hydroxybutyrate level of 75 mg/dL (reference range, 0-2.8 mg/dL). Her salicylate testing was negative, and her lactate level was 1.4 mmol/L (reference range, 0.4-2.0 mmol/L).
THE DIAGNOSIS
This patient, with severe acidosis and an elevated anion gap, received a diagnosis of starvation ketoacidosis—specifically, lactation ketoacidosis. Other causes of elevated anion gap metabolic acidosis were ruled out, including salicylate overdose, lactic acidosis, diabetic ketoacidosis, and other ingestions. The elevated acetone and beta-hydroxybutyrate levels confirmed the diagnosis. The patient was treated with a bolus of 1 L normal saline with 5% dextrose (D5NS) in the ED and admitted.
DISCUSSION
Lactation ketoacidosis is a relatively uncommon condition, but reports have increased with the growing popularity of low-carbohydrate diets. The treatment approach has differed in previous reports in regard to insulin and bicarbonate use.1-9
The use of bicarbonate is controversial in diabetic ketoacidosis and unlikely to be helpful in lactation ketoacidosis, but it is something to consider when the patient’s pH is < 6.9. Insulin use is likely unnecessary for lactation ketoacidosis, as metabolic derangements have been corrected without intervention.
Continue to: With an increasing prevalence of cases...
With an increasing prevalence of cases, we suggest a conservative approach for treatment based on this case presentation and review of other presentations. Our patient responded rapidly to conservative treatment with intravenous (IV) fluids (D5NS), a liberalized diet, and electrolyte repletion (described in detail later).
Suggested management
Once other causes of a patient’s signs and symptoms are excluded and the diagnosis of lactation ketoacidosis is made, you’ll want to follow the initial set of lab work with the following: a venous blood gas, basic metabolic panel, and testing of magnesium and phosphorous levels every 8 hours after initial presentation, with repletion as indicated. Some patients may require more frequent monitoring based on repletion of electrolytes.
The patient will initially require IV fluid resuscitation; the initial fluid of choice would be D5NS. Patients will likely need no more than 2 L, but this will depend on the degree of hypovolemia.
Diet should be advanced as tolerated and include no restriction of carbohydrates.
Previous reports have varied regarding continuation of breastfeeding and pumping. In this case, the patient continued to breastfeed without any adverse effects. Continuation of breastfeeding is unlikely to cause harm in these circumstances, but severity of symptoms (pain, nausea, vomiting) or unresolved acidosis may require discontinuation.
Continue to: Discharge should be determined...
Discharge should be determined by resolution of symptoms and correction of metabolic derangements. In previous reports, discharge time varied from 48 hours up to 144 hours, with most patients discharged on Day 2 or 3. Pending clinical factors, discharge is likely appropriate between 36 to 72 hours from time of admission.
Our patient received an additional 1 L of D5NS for continued signs of dehydration during admission. Her pH and electrolyte levels were monitored every 8 hours, with repletion of electrolytes as needed. Her acidosis, nausea, vomiting, and pain resolved within 36 hours. The patient continued to breastfeed her infant throughout her stay. With resolution of symptoms and metabolic derangements, the patient was discharged about 36 hours after admission. She was advised to follow up with her primary care provider within 1 week after discharge.
THE TAKEAWAY
As the popularity of low-carbohydrate diets increases, patients should be educated about the warning signs of clinically significant ketoacidosis. This information is especially important for those who are lactating, as this metabolic state increases predilection to ketoacidosis. When cases do present, conservative management with IV fluids and a liberalized diet is likely to be an appropriate course of care for most patients.
CORRESPONDENCE
C.W. Ferguson, DO, Navy Medicine Readiness and Training Command, Camp Lejeune Family Medicine Residency, 100 Brewster Boulevard, Camp Lejeune, NC 28547; [email protected]
THE CASE
A 26-year-old woman presented to the emergency department (ED) with a history of nausea and vomiting for more than 24 hours. The vomiting began when she awoke to breastfeed her 3-month-old infant. She had been unable to eat or drink anything for about 16 hours.
She’d seen her primary care provider earlier in the day. Antiemetics were prescribed, but they did not provide relief. So 10 hours later, when her symptoms worsened, she presented to the ED.
Her medical history was notable for a body mass index of 26. The patient also reported positional back pain, but the review of systems was otherwise negative. The patient indicated that she’d been on a ketogenic diet for about 1 month, but she denied use of supplements.
Upon presentation to the ED, the patient was found to have a metabolic acidosis with a pH of 7.02 and an anion gap of 25. Her glucose level was 132 mg/dL, and she had a positive serum acetone and a beta-hydroxybutyrate level of 75 mg/dL (reference range, 0-2.8 mg/dL). Her salicylate testing was negative, and her lactate level was 1.4 mmol/L (reference range, 0.4-2.0 mmol/L).
THE DIAGNOSIS
This patient, with severe acidosis and an elevated anion gap, received a diagnosis of starvation ketoacidosis—specifically, lactation ketoacidosis. Other causes of elevated anion gap metabolic acidosis were ruled out, including salicylate overdose, lactic acidosis, diabetic ketoacidosis, and other ingestions. The elevated acetone and beta-hydroxybutyrate levels confirmed the diagnosis. The patient was treated with a bolus of 1 L normal saline with 5% dextrose (D5NS) in the ED and admitted.
DISCUSSION
Lactation ketoacidosis is a relatively uncommon condition, but reports have increased with the growing popularity of low-carbohydrate diets. The treatment approach has differed in previous reports in regard to insulin and bicarbonate use.1-9
The use of bicarbonate is controversial in diabetic ketoacidosis and unlikely to be helpful in lactation ketoacidosis, but it is something to consider when the patient’s pH is < 6.9. Insulin use is likely unnecessary for lactation ketoacidosis, as metabolic derangements have been corrected without intervention.
Continue to: With an increasing prevalence of cases...
With an increasing prevalence of cases, we suggest a conservative approach for treatment based on this case presentation and review of other presentations. Our patient responded rapidly to conservative treatment with intravenous (IV) fluids (D5NS), a liberalized diet, and electrolyte repletion (described in detail later).
Suggested management
Once other causes of a patient’s signs and symptoms are excluded and the diagnosis of lactation ketoacidosis is made, you’ll want to follow the initial set of lab work with the following: a venous blood gas, basic metabolic panel, and testing of magnesium and phosphorous levels every 8 hours after initial presentation, with repletion as indicated. Some patients may require more frequent monitoring based on repletion of electrolytes.
The patient will initially require IV fluid resuscitation; the initial fluid of choice would be D5NS. Patients will likely need no more than 2 L, but this will depend on the degree of hypovolemia.
Diet should be advanced as tolerated and include no restriction of carbohydrates.
Previous reports have varied regarding continuation of breastfeeding and pumping. In this case, the patient continued to breastfeed without any adverse effects. Continuation of breastfeeding is unlikely to cause harm in these circumstances, but severity of symptoms (pain, nausea, vomiting) or unresolved acidosis may require discontinuation.
Continue to: Discharge should be determined...
Discharge should be determined by resolution of symptoms and correction of metabolic derangements. In previous reports, discharge time varied from 48 hours up to 144 hours, with most patients discharged on Day 2 or 3. Pending clinical factors, discharge is likely appropriate between 36 to 72 hours from time of admission.
Our patient received an additional 1 L of D5NS for continued signs of dehydration during admission. Her pH and electrolyte levels were monitored every 8 hours, with repletion of electrolytes as needed. Her acidosis, nausea, vomiting, and pain resolved within 36 hours. The patient continued to breastfeed her infant throughout her stay. With resolution of symptoms and metabolic derangements, the patient was discharged about 36 hours after admission. She was advised to follow up with her primary care provider within 1 week after discharge.
THE TAKEAWAY
As the popularity of low-carbohydrate diets increases, patients should be educated about the warning signs of clinically significant ketoacidosis. This information is especially important for those who are lactating, as this metabolic state increases predilection to ketoacidosis. When cases do present, conservative management with IV fluids and a liberalized diet is likely to be an appropriate course of care for most patients.
CORRESPONDENCE
C.W. Ferguson, DO, Navy Medicine Readiness and Training Command, Camp Lejeune Family Medicine Residency, 100 Brewster Boulevard, Camp Lejeune, NC 28547; [email protected]
1. Al Alawi AM, Falhammar H. Lactation ketoacidosis: case presentation and literature review. BMJ Case Rep. 2018;2018:bcr2017223494. doi:10.1136/bcr-2017-223494
2. von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep. 2015;9:224. doi:10.1186/s13256-015-0709-2
3. Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J. 2015;14:117. doi:10.1186/s12937-015-0076-2
4. Azzam O, Prentice D. Lactation ketoacidosis: an easily missed diagnosis. Intern Med J. 2019;49:256‐259. doi:10.1111/imj.14207
5. Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus. 2009;2:278‐279. doi:10.1093/ndtplus/sfp052
6. Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med. 2008;35:385‐387. doi:10.1016/j.jemermed.2007.04.013
7. Szulewski A, Howes D, Morton AR. A severe case of iatrogenic lactation ketoacidosis. BMJ Case Rep. 2012;2012:bcr1220115409. doi:10.1136/bcr.12.2011.5409
8. Nnodum BN, Oduah E, Albert D, et al. Ketogenic diet-induced severe ketoacidosis in a lactating woman: a case report and review of the literature. Case Rep Nephrol. 2019;2019:1214208. doi:10.1155/2019/1214208
9. Gleeson S, Mulroy E, Clarke DE. Lactation ketoacidosis: an unusual entity and a review of the literature. Perm J. 2016;20:71‐73. doi:10.7812/TPP/15-097
1. Al Alawi AM, Falhammar H. Lactation ketoacidosis: case presentation and literature review. BMJ Case Rep. 2018;2018:bcr2017223494. doi:10.1136/bcr-2017-223494
2. von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep. 2015;9:224. doi:10.1186/s13256-015-0709-2
3. Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J. 2015;14:117. doi:10.1186/s12937-015-0076-2
4. Azzam O, Prentice D. Lactation ketoacidosis: an easily missed diagnosis. Intern Med J. 2019;49:256‐259. doi:10.1111/imj.14207
5. Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus. 2009;2:278‐279. doi:10.1093/ndtplus/sfp052
6. Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med. 2008;35:385‐387. doi:10.1016/j.jemermed.2007.04.013
7. Szulewski A, Howes D, Morton AR. A severe case of iatrogenic lactation ketoacidosis. BMJ Case Rep. 2012;2012:bcr1220115409. doi:10.1136/bcr.12.2011.5409
8. Nnodum BN, Oduah E, Albert D, et al. Ketogenic diet-induced severe ketoacidosis in a lactating woman: a case report and review of the literature. Case Rep Nephrol. 2019;2019:1214208. doi:10.1155/2019/1214208
9. Gleeson S, Mulroy E, Clarke DE. Lactation ketoacidosis: an unusual entity and a review of the literature. Perm J. 2016;20:71‐73. doi:10.7812/TPP/15-097
An FP’s guide to identifying—and treating—postpartum depression
THE CASE
Alex T,* a 23-year-old first-time mom, presented to the family medicine office for her baby’s 2-week appointment. When asked how she was doing, she began to cry. She said, “I feel crazy” and indicated that she was feeling down and overwhelmed, and was struggling to bond with the baby. She filled out an Edinburgh Postnatal Depression Scale, a standard postpartum depression (PPD) screen; her score, 15 out of 30, was suggestive of depression. Ms. T had been coming to the practice for the past 3 years and had no significant physical or mental health history. She and the baby did not live with the baby’s father, and his degree of presence in their lives varied.
●
* The patient’s name has been changed to protect her identity.
PPD, traditionally defined as depression in the postpartum period for as long as a year after childbirth, is a common, underdiagnosed outcome of both normal and complicated pregnancies.1 Peripartum depression, which includes PPD and depression during pregnancy, occurs in approximately 10% of pregnancies.2,3 When depression first appears in the postpartum period, most women develop symptoms in the first month after delivery (54% of cases) or in the next 2 to 4 months (40%).4
The most significant risk factor for PPD is previous depression, peripartum or otherwise.1,4-6 Other common risk factors include major life events or stressors during or after pregnancy, domestic violence, poor social support, and preterm birth or an infant admission to the neonatal intensive care unit.1,7 Women with a self-perceived negative birth experience are also likely to experience PPD.8 PPD can be associated with significant morbidity and mortality, with suicide a more common cause of maternal mortality than either hemorrhage or hypertensive disorders of pregnancy.9
Early diagnosis and intervention are crucial to improving patient outcomes. Women with PPD initiate breastfeeding at lower rates and continue for shorter durations.10 PPD also affects maternal–infant bonding; may adversely affect an infant’s social, cognitive, and language development; and may lead to attachment disorders of infancy.11,12 In severe cases, it can lead to failure to thrive or infanticide.11
When to screen. The US Preventive Services Task Force (USPSTF) recommends clinicians screen for depression in pregnant and postpartum women (Grade Ba) and for women at increased risk, provide or refer to counseling interventions (Grade Ba).13,14 The American College of Obstetricians and Gynecologists (ACOG) recommends screening at least once in the postpartum period.15 Repeat screening at follow-up in the later postpartum period increases the likelihood of diagnosis.16 Screening for PPD as part of well-child care improves maternal outcomes, and the American Academy of Pediatrics recommends screening at the 1-, 2-, 4-, and 6-month visits.11,17 These screens are separately billable. Family physicians are uniquely suited to screening at both well-child and postpartum visits, as many women share a medical home with their child, and those who do not are equally willing to receive medical advice from their child’s physician.18
Continue to: Is it "the blues" or something else? Diagnosing PPD
Is it “the blues” or something else? Diagnosing PPD
Many new mothers experience postpartum blues, which manifest as tearfulness, insomnia, irritability, and anxiety. The postpartum blues, however, don’t meet the criteria for major depressive disorder and typically resolve within 14 days of delivery.19-21 On the other end of the spectrum is postpartum psychosis, which is severe and rare, and can also affect new mothers.
Screening for PPD. The most commonly used screening tool for PPD is the Edinburgh Postnatal Depression Scale (EPDS 10), a free 10-item instrument scored out of 30 possible points, with any score ≥ 13 suggesting PPD.22 The EPDS 10 has a sensitivity of 74% and specificity of 97% for the diagnosis of PPD.23 Other screening options include the Beck Depression Inventory II (BDI-II) and the Patient Health Questionnaire 9 (PHQ-9). The 21-item BDI-II takes longer to perform and is less sensitive (57%) than the EPDS.1 The PHQ-9, which asks about some symptoms common to the postpartum period (including sleep changes), is less specific than the EPDS (sensitivity, 75%; specificity, 90%).1 The EPDS also includes screening questions about anxiety.1
A positive depression screen, or any positive response to a question on suicidal ideation, should be followed up for confirmation using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for major depressive disorder with peripartum onset.24 Women with PPD should also be asked about current or prior symptoms of bipolar disorder or mania.25 Up to 67% of women with bipolar disorder may relapse postpartum, and they also have an elevated risk of postpartum psychosis.26 The Mood Disorder Questionnaire is a useful tool if a concern for bipolar depression arises.27
Refer any woman in whom bipolar depression is a concern to a clinician experienced with its management. The presence of auditory or visual hallucinations should also be assessed as indicators of postpartum psychosis. Active suicidal or homicidal ideation and postpartum psychosis all require emergent psychiatric care.21,22 Intimate partner violence may also exist or escalate in the postpartum period and may exacerbate PPD. Both ACOG and the USPSTF recommend screening postpartum women for intimate partner violence.28,29
Also consider possible medical causes of PPD symptoms. Hypothyroidism in the postpartum period may manifest with some similar symptoms to PPD and is commonly underdiagnosed.22,30 Women with postpartum anemia and low ferritin stores also have a higher likelihood of PPD (odds ratio, 1.7-4.64), and postpartum iron supplementation may reduce this risk (number needed to treat = 4 in at least 1 randomized controlled trial).31 When anemia is present, ensure that it is properly treated.
Continue to: Steps you can take to manage pPD
Steps you can take to manage pPD
Refer any woman who has PPD to a qualified therapist whenever possible. Generally, the psychological recommendations for treatment of PPD are very similar to recommendations for general treatment of depression. Psychotherapy on its own is considered a first-line treatment for mild-to-moderate PPD, and medication plus psychotherapy is considered first-line treatment for severe PPD.32 (Worth noting: It may also be useful to offer counseling to a patient who appears distressed, even if she does not fully meet all DSM-5 criteria.)
Of the psychotherapy options, cognitive behavioral therapy (CBT) is supported by the most evidence. There is also evidence for the use of interpersonal therapy (IPT), especially in higher socioeconomic status populations.33 Key therapeutic targets in IPT are increasing behavioral engagement (eg, reaching out to friends), decreasing negative self-talk (eg, “I am a bad mother”), and negotiating roles and support (eg, both mom’s and family members’ expectations of new motherhood). There is mixed evidence for recommending exercise as a treatment for PPD.32,34 However, as exercise is a low-risk intervention, you may choose to make that recommendation to patients. Additionally, including partners/support people in treatment/visits for PPD has been shown to increase positive outcomes.35
When medication is considered, selective serotonin reuptake inhibitors (SSRIs) are most commonly used. Research indicates that SSRIs are significantly more effective than placebo for treatment of women with PPD.36 Sertraline, in particular, has shown to be both effective in treating PPD and safe in lactation.37,38 Dosing and duration of therapy are equivalent to treatment of major depression outside the perinatal period. Consult a trusted source on medications in lactation before prescribing any antidepressant to a breastfeeding mother. One resource is the National Institutes of Health drugs and lactation database (LactMed; www.ncbi.nlm.nih.gov/books/NBK501922/), which provides detailed information on the levels of medications in breastmilk and their potential effects on an infant.
Women with severe, refractory PPD may require hospitalization. Additional treatment options for women with severe, refractory PPD include electroconvulsive therapy or the new medication brexanolone, which is administered as a 60-hour continuous infusion.39,40
THE CASE
Further conversation with Ms. T revealed that she met the criteria for PPD (major depressive disorder with peripartum onset). She denied suicidal or homicidal ideation and was not experiencing any symptoms of psychosis. A complete blood count was drawn and showed no anemia, and her thyroid-stimulating hormone level was within normal limits. She had a good support network at home, with both her mom and sister taking shifts to help her get some extra rest and allow her to attend medical appointments. She said there was no domestic violence.
Ms. T was introduced to the clinic’s embedded counselor, who scheduled a follow-up appointment within the week to start CBT. After a discussion of risks and benefits, Ms. T also started a low dose of sertraline once daily. At follow-up postpartum visits, she reported significant improvement in her mood. She and her physician decided to taper her SSRI medication at 3 months postpartum. Screens for depression at her infant’s 4- and 6-month well-child visits in the office were reassuringly negative.
a There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.
CORRESPONDENCE
Katherine Buck, PhD, JPS Family Health Center, 1500 South Main Street, 4th Floor, Fort Worth, TX 76110; [email protected]
1. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132:e208-e212. doi: 10.1097/AOG.0000000000002927
2. Banti S, Mauri M, Oppo A, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression–Research & Screening Unit study. Compr Psychiatry. 2011;52:343-351. doi: 10.1016/j.comppsych.2010.08.003
3. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164):1515-1520. doi: 10.1176/appi.ajp.2007.06111893
4. Altemus M, Neeb CC, Davis A, et al. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485-e1491. doi: 10.4088/JCP.12m07693
5. Wilson LM, Reid AJ, Midmer DK, et al. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ. 1996;154:785-799.
6. Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289-295. doi: 10.1016/j.genhosppsych.2004.02.006
7. Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275-285. doi: 10.1097/00006199-200109000-00004
8. Bell AF, E Andersson. The birth experience and women’s postnatal depression: a systematic review. Midwifery. 2016;39:112-123. doi: 10.1016/j.midw.2016.04.014
9. Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056-1063. doi: 10.1097/AOG.0b013e31823294da
10. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158. doi: 10.15585/mmwr.mm6606a1
11. Rafferty J, Mattson G, Earls MF, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183260. doi: 10.1542/peds.2018-3260
12. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592. doi: 10.1016/s0272-7358(98)00100-7
13. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. doi: 10.1001/jama.2019.0007
14. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380-387. doi: 10.1001/jama.2015.18392
15. ACOG. Screening for perinatal depression. 2018. Accessed October 5, 2022. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
16. Yawn BP, Bertram S, Kurland M, et al. Repeated depression screening during the first postpartum year. Ann Fam Med. 2015;13:228-234. doi: 10.1370/afm.1777
17. van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, et al. Post-up study: postpartum depression screening in well-child care and maternal outcomes. Pediatrics. 2017;140:e20170110. doi: 10.1542/peds.2017-0110
18. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network study. Ann Fam Med. 2016;14:350-355. doi: 10.1370/afm.1933
19. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34-40.
20. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097/AOG.0000000000002633
21. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
22. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. 2012;34:436-442. doi: 10.1016/S1701-2163(16)35240-9
23. Owara AH, Carabin H, Reese J, et al. Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: a meta-analysis. J Affect Disord. 2016;205:335-343. doi: 10.1016/j.jad.2016.08.014
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C.: 2013:160.
25. Mandelli L, Souery D, Bartova L, et al. Bipolar II disorder as a risk factor for postpartum depression. J Affect Disord. 2016;204:54-58. doi:10.1016/j.jad.2016.06.025
26. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020. doi: 10.1097/AOG.0b013e31816fd910
27. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875. doi: 10.1176/appi.ajp.157.11.1873
28. Curry SJ, Krist AH, Owens DK, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2018;320:1678-1687. doi: 10.1001/jama.2018.14741
29. ACOG Committee Opinion No. 518: Intimate partner violence. Obstet Gynecol. 2012;119:412-417. doi: 10.1097/AOG.0b013e318249ff74
30. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
31. Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol. 2019;40:19-28. doi: 10.1080/0167482X.2018.1427725
32. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82:926-933.
33. Nillni YI, Mehralizade A, Mayer L, et al. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev. 2018;66:136-148. doi: 10.1016/j.cpr.2018.06.004
34. Daley AJ, Macarthur C, Winter H. The role of exercise in treating postpartum depression: a review of the literature. J Midwifery Womens Health. 2007;52:56-62. doi: 10.1016/j.jmwh.2006.08.017
35. Misri S, Kostaras X, Fox D, et al. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry. 2000;45:554-558. doi: 10.1177/070674370004500607
36. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;CD002018. doi: 10.1002/14651858.CD002018.pub2
37. Pinheiro E, Bogen DL, Hoxha D, et al. Sertraline and breastfeeding: review and meta-analysis. Arch Women Ment Health. 2015;18:139-146. doi: 10.1007/s00737-015-0499-y
38. Hantsoo L, Ward-O’Brien D, Czarkowski KA, et al. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231:939-948. doi: 10.1007/s00213-013-3316-1
39. Rundgren S, Brus O, Båve U, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258-264. doi: 10.1016/j.jad.2018.04.043
40. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. doi: 10.1016/S0140-6736(18)31551-4
THE CASE
Alex T,* a 23-year-old first-time mom, presented to the family medicine office for her baby’s 2-week appointment. When asked how she was doing, she began to cry. She said, “I feel crazy” and indicated that she was feeling down and overwhelmed, and was struggling to bond with the baby. She filled out an Edinburgh Postnatal Depression Scale, a standard postpartum depression (PPD) screen; her score, 15 out of 30, was suggestive of depression. Ms. T had been coming to the practice for the past 3 years and had no significant physical or mental health history. She and the baby did not live with the baby’s father, and his degree of presence in their lives varied.
●
* The patient’s name has been changed to protect her identity.
PPD, traditionally defined as depression in the postpartum period for as long as a year after childbirth, is a common, underdiagnosed outcome of both normal and complicated pregnancies.1 Peripartum depression, which includes PPD and depression during pregnancy, occurs in approximately 10% of pregnancies.2,3 When depression first appears in the postpartum period, most women develop symptoms in the first month after delivery (54% of cases) or in the next 2 to 4 months (40%).4
The most significant risk factor for PPD is previous depression, peripartum or otherwise.1,4-6 Other common risk factors include major life events or stressors during or after pregnancy, domestic violence, poor social support, and preterm birth or an infant admission to the neonatal intensive care unit.1,7 Women with a self-perceived negative birth experience are also likely to experience PPD.8 PPD can be associated with significant morbidity and mortality, with suicide a more common cause of maternal mortality than either hemorrhage or hypertensive disorders of pregnancy.9
Early diagnosis and intervention are crucial to improving patient outcomes. Women with PPD initiate breastfeeding at lower rates and continue for shorter durations.10 PPD also affects maternal–infant bonding; may adversely affect an infant’s social, cognitive, and language development; and may lead to attachment disorders of infancy.11,12 In severe cases, it can lead to failure to thrive or infanticide.11
When to screen. The US Preventive Services Task Force (USPSTF) recommends clinicians screen for depression in pregnant and postpartum women (Grade Ba) and for women at increased risk, provide or refer to counseling interventions (Grade Ba).13,14 The American College of Obstetricians and Gynecologists (ACOG) recommends screening at least once in the postpartum period.15 Repeat screening at follow-up in the later postpartum period increases the likelihood of diagnosis.16 Screening for PPD as part of well-child care improves maternal outcomes, and the American Academy of Pediatrics recommends screening at the 1-, 2-, 4-, and 6-month visits.11,17 These screens are separately billable. Family physicians are uniquely suited to screening at both well-child and postpartum visits, as many women share a medical home with their child, and those who do not are equally willing to receive medical advice from their child’s physician.18
Continue to: Is it "the blues" or something else? Diagnosing PPD
Is it “the blues” or something else? Diagnosing PPD
Many new mothers experience postpartum blues, which manifest as tearfulness, insomnia, irritability, and anxiety. The postpartum blues, however, don’t meet the criteria for major depressive disorder and typically resolve within 14 days of delivery.19-21 On the other end of the spectrum is postpartum psychosis, which is severe and rare, and can also affect new mothers.
Screening for PPD. The most commonly used screening tool for PPD is the Edinburgh Postnatal Depression Scale (EPDS 10), a free 10-item instrument scored out of 30 possible points, with any score ≥ 13 suggesting PPD.22 The EPDS 10 has a sensitivity of 74% and specificity of 97% for the diagnosis of PPD.23 Other screening options include the Beck Depression Inventory II (BDI-II) and the Patient Health Questionnaire 9 (PHQ-9). The 21-item BDI-II takes longer to perform and is less sensitive (57%) than the EPDS.1 The PHQ-9, which asks about some symptoms common to the postpartum period (including sleep changes), is less specific than the EPDS (sensitivity, 75%; specificity, 90%).1 The EPDS also includes screening questions about anxiety.1
A positive depression screen, or any positive response to a question on suicidal ideation, should be followed up for confirmation using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for major depressive disorder with peripartum onset.24 Women with PPD should also be asked about current or prior symptoms of bipolar disorder or mania.25 Up to 67% of women with bipolar disorder may relapse postpartum, and they also have an elevated risk of postpartum psychosis.26 The Mood Disorder Questionnaire is a useful tool if a concern for bipolar depression arises.27
Refer any woman in whom bipolar depression is a concern to a clinician experienced with its management. The presence of auditory or visual hallucinations should also be assessed as indicators of postpartum psychosis. Active suicidal or homicidal ideation and postpartum psychosis all require emergent psychiatric care.21,22 Intimate partner violence may also exist or escalate in the postpartum period and may exacerbate PPD. Both ACOG and the USPSTF recommend screening postpartum women for intimate partner violence.28,29
Also consider possible medical causes of PPD symptoms. Hypothyroidism in the postpartum period may manifest with some similar symptoms to PPD and is commonly underdiagnosed.22,30 Women with postpartum anemia and low ferritin stores also have a higher likelihood of PPD (odds ratio, 1.7-4.64), and postpartum iron supplementation may reduce this risk (number needed to treat = 4 in at least 1 randomized controlled trial).31 When anemia is present, ensure that it is properly treated.
Continue to: Steps you can take to manage pPD
Steps you can take to manage pPD
Refer any woman who has PPD to a qualified therapist whenever possible. Generally, the psychological recommendations for treatment of PPD are very similar to recommendations for general treatment of depression. Psychotherapy on its own is considered a first-line treatment for mild-to-moderate PPD, and medication plus psychotherapy is considered first-line treatment for severe PPD.32 (Worth noting: It may also be useful to offer counseling to a patient who appears distressed, even if she does not fully meet all DSM-5 criteria.)
Of the psychotherapy options, cognitive behavioral therapy (CBT) is supported by the most evidence. There is also evidence for the use of interpersonal therapy (IPT), especially in higher socioeconomic status populations.33 Key therapeutic targets in IPT are increasing behavioral engagement (eg, reaching out to friends), decreasing negative self-talk (eg, “I am a bad mother”), and negotiating roles and support (eg, both mom’s and family members’ expectations of new motherhood). There is mixed evidence for recommending exercise as a treatment for PPD.32,34 However, as exercise is a low-risk intervention, you may choose to make that recommendation to patients. Additionally, including partners/support people in treatment/visits for PPD has been shown to increase positive outcomes.35
When medication is considered, selective serotonin reuptake inhibitors (SSRIs) are most commonly used. Research indicates that SSRIs are significantly more effective than placebo for treatment of women with PPD.36 Sertraline, in particular, has shown to be both effective in treating PPD and safe in lactation.37,38 Dosing and duration of therapy are equivalent to treatment of major depression outside the perinatal period. Consult a trusted source on medications in lactation before prescribing any antidepressant to a breastfeeding mother. One resource is the National Institutes of Health drugs and lactation database (LactMed; www.ncbi.nlm.nih.gov/books/NBK501922/), which provides detailed information on the levels of medications in breastmilk and their potential effects on an infant.
Women with severe, refractory PPD may require hospitalization. Additional treatment options for women with severe, refractory PPD include electroconvulsive therapy or the new medication brexanolone, which is administered as a 60-hour continuous infusion.39,40
THE CASE
Further conversation with Ms. T revealed that she met the criteria for PPD (major depressive disorder with peripartum onset). She denied suicidal or homicidal ideation and was not experiencing any symptoms of psychosis. A complete blood count was drawn and showed no anemia, and her thyroid-stimulating hormone level was within normal limits. She had a good support network at home, with both her mom and sister taking shifts to help her get some extra rest and allow her to attend medical appointments. She said there was no domestic violence.
Ms. T was introduced to the clinic’s embedded counselor, who scheduled a follow-up appointment within the week to start CBT. After a discussion of risks and benefits, Ms. T also started a low dose of sertraline once daily. At follow-up postpartum visits, she reported significant improvement in her mood. She and her physician decided to taper her SSRI medication at 3 months postpartum. Screens for depression at her infant’s 4- and 6-month well-child visits in the office were reassuringly negative.
a There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.
CORRESPONDENCE
Katherine Buck, PhD, JPS Family Health Center, 1500 South Main Street, 4th Floor, Fort Worth, TX 76110; [email protected]
THE CASE
Alex T,* a 23-year-old first-time mom, presented to the family medicine office for her baby’s 2-week appointment. When asked how she was doing, she began to cry. She said, “I feel crazy” and indicated that she was feeling down and overwhelmed, and was struggling to bond with the baby. She filled out an Edinburgh Postnatal Depression Scale, a standard postpartum depression (PPD) screen; her score, 15 out of 30, was suggestive of depression. Ms. T had been coming to the practice for the past 3 years and had no significant physical or mental health history. She and the baby did not live with the baby’s father, and his degree of presence in their lives varied.
●
* The patient’s name has been changed to protect her identity.
PPD, traditionally defined as depression in the postpartum period for as long as a year after childbirth, is a common, underdiagnosed outcome of both normal and complicated pregnancies.1 Peripartum depression, which includes PPD and depression during pregnancy, occurs in approximately 10% of pregnancies.2,3 When depression first appears in the postpartum period, most women develop symptoms in the first month after delivery (54% of cases) or in the next 2 to 4 months (40%).4
The most significant risk factor for PPD is previous depression, peripartum or otherwise.1,4-6 Other common risk factors include major life events or stressors during or after pregnancy, domestic violence, poor social support, and preterm birth or an infant admission to the neonatal intensive care unit.1,7 Women with a self-perceived negative birth experience are also likely to experience PPD.8 PPD can be associated with significant morbidity and mortality, with suicide a more common cause of maternal mortality than either hemorrhage or hypertensive disorders of pregnancy.9
Early diagnosis and intervention are crucial to improving patient outcomes. Women with PPD initiate breastfeeding at lower rates and continue for shorter durations.10 PPD also affects maternal–infant bonding; may adversely affect an infant’s social, cognitive, and language development; and may lead to attachment disorders of infancy.11,12 In severe cases, it can lead to failure to thrive or infanticide.11
When to screen. The US Preventive Services Task Force (USPSTF) recommends clinicians screen for depression in pregnant and postpartum women (Grade Ba) and for women at increased risk, provide or refer to counseling interventions (Grade Ba).13,14 The American College of Obstetricians and Gynecologists (ACOG) recommends screening at least once in the postpartum period.15 Repeat screening at follow-up in the later postpartum period increases the likelihood of diagnosis.16 Screening for PPD as part of well-child care improves maternal outcomes, and the American Academy of Pediatrics recommends screening at the 1-, 2-, 4-, and 6-month visits.11,17 These screens are separately billable. Family physicians are uniquely suited to screening at both well-child and postpartum visits, as many women share a medical home with their child, and those who do not are equally willing to receive medical advice from their child’s physician.18
Continue to: Is it "the blues" or something else? Diagnosing PPD
Is it “the blues” or something else? Diagnosing PPD
Many new mothers experience postpartum blues, which manifest as tearfulness, insomnia, irritability, and anxiety. The postpartum blues, however, don’t meet the criteria for major depressive disorder and typically resolve within 14 days of delivery.19-21 On the other end of the spectrum is postpartum psychosis, which is severe and rare, and can also affect new mothers.
Screening for PPD. The most commonly used screening tool for PPD is the Edinburgh Postnatal Depression Scale (EPDS 10), a free 10-item instrument scored out of 30 possible points, with any score ≥ 13 suggesting PPD.22 The EPDS 10 has a sensitivity of 74% and specificity of 97% for the diagnosis of PPD.23 Other screening options include the Beck Depression Inventory II (BDI-II) and the Patient Health Questionnaire 9 (PHQ-9). The 21-item BDI-II takes longer to perform and is less sensitive (57%) than the EPDS.1 The PHQ-9, which asks about some symptoms common to the postpartum period (including sleep changes), is less specific than the EPDS (sensitivity, 75%; specificity, 90%).1 The EPDS also includes screening questions about anxiety.1
A positive depression screen, or any positive response to a question on suicidal ideation, should be followed up for confirmation using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for major depressive disorder with peripartum onset.24 Women with PPD should also be asked about current or prior symptoms of bipolar disorder or mania.25 Up to 67% of women with bipolar disorder may relapse postpartum, and they also have an elevated risk of postpartum psychosis.26 The Mood Disorder Questionnaire is a useful tool if a concern for bipolar depression arises.27
Refer any woman in whom bipolar depression is a concern to a clinician experienced with its management. The presence of auditory or visual hallucinations should also be assessed as indicators of postpartum psychosis. Active suicidal or homicidal ideation and postpartum psychosis all require emergent psychiatric care.21,22 Intimate partner violence may also exist or escalate in the postpartum period and may exacerbate PPD. Both ACOG and the USPSTF recommend screening postpartum women for intimate partner violence.28,29
Also consider possible medical causes of PPD symptoms. Hypothyroidism in the postpartum period may manifest with some similar symptoms to PPD and is commonly underdiagnosed.22,30 Women with postpartum anemia and low ferritin stores also have a higher likelihood of PPD (odds ratio, 1.7-4.64), and postpartum iron supplementation may reduce this risk (number needed to treat = 4 in at least 1 randomized controlled trial).31 When anemia is present, ensure that it is properly treated.
Continue to: Steps you can take to manage pPD
Steps you can take to manage pPD
Refer any woman who has PPD to a qualified therapist whenever possible. Generally, the psychological recommendations for treatment of PPD are very similar to recommendations for general treatment of depression. Psychotherapy on its own is considered a first-line treatment for mild-to-moderate PPD, and medication plus psychotherapy is considered first-line treatment for severe PPD.32 (Worth noting: It may also be useful to offer counseling to a patient who appears distressed, even if she does not fully meet all DSM-5 criteria.)
Of the psychotherapy options, cognitive behavioral therapy (CBT) is supported by the most evidence. There is also evidence for the use of interpersonal therapy (IPT), especially in higher socioeconomic status populations.33 Key therapeutic targets in IPT are increasing behavioral engagement (eg, reaching out to friends), decreasing negative self-talk (eg, “I am a bad mother”), and negotiating roles and support (eg, both mom’s and family members’ expectations of new motherhood). There is mixed evidence for recommending exercise as a treatment for PPD.32,34 However, as exercise is a low-risk intervention, you may choose to make that recommendation to patients. Additionally, including partners/support people in treatment/visits for PPD has been shown to increase positive outcomes.35
When medication is considered, selective serotonin reuptake inhibitors (SSRIs) are most commonly used. Research indicates that SSRIs are significantly more effective than placebo for treatment of women with PPD.36 Sertraline, in particular, has shown to be both effective in treating PPD and safe in lactation.37,38 Dosing and duration of therapy are equivalent to treatment of major depression outside the perinatal period. Consult a trusted source on medications in lactation before prescribing any antidepressant to a breastfeeding mother. One resource is the National Institutes of Health drugs and lactation database (LactMed; www.ncbi.nlm.nih.gov/books/NBK501922/), which provides detailed information on the levels of medications in breastmilk and their potential effects on an infant.
Women with severe, refractory PPD may require hospitalization. Additional treatment options for women with severe, refractory PPD include electroconvulsive therapy or the new medication brexanolone, which is administered as a 60-hour continuous infusion.39,40
THE CASE
Further conversation with Ms. T revealed that she met the criteria for PPD (major depressive disorder with peripartum onset). She denied suicidal or homicidal ideation and was not experiencing any symptoms of psychosis. A complete blood count was drawn and showed no anemia, and her thyroid-stimulating hormone level was within normal limits. She had a good support network at home, with both her mom and sister taking shifts to help her get some extra rest and allow her to attend medical appointments. She said there was no domestic violence.
Ms. T was introduced to the clinic’s embedded counselor, who scheduled a follow-up appointment within the week to start CBT. After a discussion of risks and benefits, Ms. T also started a low dose of sertraline once daily. At follow-up postpartum visits, she reported significant improvement in her mood. She and her physician decided to taper her SSRI medication at 3 months postpartum. Screens for depression at her infant’s 4- and 6-month well-child visits in the office were reassuringly negative.
a There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.
CORRESPONDENCE
Katherine Buck, PhD, JPS Family Health Center, 1500 South Main Street, 4th Floor, Fort Worth, TX 76110; [email protected]
1. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132:e208-e212. doi: 10.1097/AOG.0000000000002927
2. Banti S, Mauri M, Oppo A, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression–Research & Screening Unit study. Compr Psychiatry. 2011;52:343-351. doi: 10.1016/j.comppsych.2010.08.003
3. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164):1515-1520. doi: 10.1176/appi.ajp.2007.06111893
4. Altemus M, Neeb CC, Davis A, et al. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485-e1491. doi: 10.4088/JCP.12m07693
5. Wilson LM, Reid AJ, Midmer DK, et al. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ. 1996;154:785-799.
6. Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289-295. doi: 10.1016/j.genhosppsych.2004.02.006
7. Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275-285. doi: 10.1097/00006199-200109000-00004
8. Bell AF, E Andersson. The birth experience and women’s postnatal depression: a systematic review. Midwifery. 2016;39:112-123. doi: 10.1016/j.midw.2016.04.014
9. Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056-1063. doi: 10.1097/AOG.0b013e31823294da
10. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158. doi: 10.15585/mmwr.mm6606a1
11. Rafferty J, Mattson G, Earls MF, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183260. doi: 10.1542/peds.2018-3260
12. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592. doi: 10.1016/s0272-7358(98)00100-7
13. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. doi: 10.1001/jama.2019.0007
14. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380-387. doi: 10.1001/jama.2015.18392
15. ACOG. Screening for perinatal depression. 2018. Accessed October 5, 2022. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
16. Yawn BP, Bertram S, Kurland M, et al. Repeated depression screening during the first postpartum year. Ann Fam Med. 2015;13:228-234. doi: 10.1370/afm.1777
17. van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, et al. Post-up study: postpartum depression screening in well-child care and maternal outcomes. Pediatrics. 2017;140:e20170110. doi: 10.1542/peds.2017-0110
18. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network study. Ann Fam Med. 2016;14:350-355. doi: 10.1370/afm.1933
19. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34-40.
20. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097/AOG.0000000000002633
21. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
22. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. 2012;34:436-442. doi: 10.1016/S1701-2163(16)35240-9
23. Owara AH, Carabin H, Reese J, et al. Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: a meta-analysis. J Affect Disord. 2016;205:335-343. doi: 10.1016/j.jad.2016.08.014
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C.: 2013:160.
25. Mandelli L, Souery D, Bartova L, et al. Bipolar II disorder as a risk factor for postpartum depression. J Affect Disord. 2016;204:54-58. doi:10.1016/j.jad.2016.06.025
26. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020. doi: 10.1097/AOG.0b013e31816fd910
27. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875. doi: 10.1176/appi.ajp.157.11.1873
28. Curry SJ, Krist AH, Owens DK, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2018;320:1678-1687. doi: 10.1001/jama.2018.14741
29. ACOG Committee Opinion No. 518: Intimate partner violence. Obstet Gynecol. 2012;119:412-417. doi: 10.1097/AOG.0b013e318249ff74
30. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
31. Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol. 2019;40:19-28. doi: 10.1080/0167482X.2018.1427725
32. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82:926-933.
33. Nillni YI, Mehralizade A, Mayer L, et al. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev. 2018;66:136-148. doi: 10.1016/j.cpr.2018.06.004
34. Daley AJ, Macarthur C, Winter H. The role of exercise in treating postpartum depression: a review of the literature. J Midwifery Womens Health. 2007;52:56-62. doi: 10.1016/j.jmwh.2006.08.017
35. Misri S, Kostaras X, Fox D, et al. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry. 2000;45:554-558. doi: 10.1177/070674370004500607
36. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;CD002018. doi: 10.1002/14651858.CD002018.pub2
37. Pinheiro E, Bogen DL, Hoxha D, et al. Sertraline and breastfeeding: review and meta-analysis. Arch Women Ment Health. 2015;18:139-146. doi: 10.1007/s00737-015-0499-y
38. Hantsoo L, Ward-O’Brien D, Czarkowski KA, et al. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231:939-948. doi: 10.1007/s00213-013-3316-1
39. Rundgren S, Brus O, Båve U, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258-264. doi: 10.1016/j.jad.2018.04.043
40. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. doi: 10.1016/S0140-6736(18)31551-4
1. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132:e208-e212. doi: 10.1097/AOG.0000000000002927
2. Banti S, Mauri M, Oppo A, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression–Research & Screening Unit study. Compr Psychiatry. 2011;52:343-351. doi: 10.1016/j.comppsych.2010.08.003
3. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164):1515-1520. doi: 10.1176/appi.ajp.2007.06111893
4. Altemus M, Neeb CC, Davis A, et al. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485-e1491. doi: 10.4088/JCP.12m07693
5. Wilson LM, Reid AJ, Midmer DK, et al. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ. 1996;154:785-799.
6. Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289-295. doi: 10.1016/j.genhosppsych.2004.02.006
7. Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275-285. doi: 10.1097/00006199-200109000-00004
8. Bell AF, E Andersson. The birth experience and women’s postnatal depression: a systematic review. Midwifery. 2016;39:112-123. doi: 10.1016/j.midw.2016.04.014
9. Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056-1063. doi: 10.1097/AOG.0b013e31823294da
10. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158. doi: 10.15585/mmwr.mm6606a1
11. Rafferty J, Mattson G, Earls MF, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183260. doi: 10.1542/peds.2018-3260
12. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592. doi: 10.1016/s0272-7358(98)00100-7
13. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. doi: 10.1001/jama.2019.0007
14. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380-387. doi: 10.1001/jama.2015.18392
15. ACOG. Screening for perinatal depression. 2018. Accessed October 5, 2022. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
16. Yawn BP, Bertram S, Kurland M, et al. Repeated depression screening during the first postpartum year. Ann Fam Med. 2015;13:228-234. doi: 10.1370/afm.1777
17. van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, et al. Post-up study: postpartum depression screening in well-child care and maternal outcomes. Pediatrics. 2017;140:e20170110. doi: 10.1542/peds.2017-0110
18. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network study. Ann Fam Med. 2016;14:350-355. doi: 10.1370/afm.1933
19. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34-40.
20. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097/AOG.0000000000002633
21. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
22. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. 2012;34:436-442. doi: 10.1016/S1701-2163(16)35240-9
23. Owara AH, Carabin H, Reese J, et al. Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: a meta-analysis. J Affect Disord. 2016;205:335-343. doi: 10.1016/j.jad.2016.08.014
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C.: 2013:160.
25. Mandelli L, Souery D, Bartova L, et al. Bipolar II disorder as a risk factor for postpartum depression. J Affect Disord. 2016;204:54-58. doi:10.1016/j.jad.2016.06.025
26. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020. doi: 10.1097/AOG.0b013e31816fd910
27. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875. doi: 10.1176/appi.ajp.157.11.1873
28. Curry SJ, Krist AH, Owens DK, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2018;320:1678-1687. doi: 10.1001/jama.2018.14741
29. ACOG Committee Opinion No. 518: Intimate partner violence. Obstet Gynecol. 2012;119:412-417. doi: 10.1097/AOG.0b013e318249ff74
30. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
31. Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol. 2019;40:19-28. doi: 10.1080/0167482X.2018.1427725
32. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82:926-933.
33. Nillni YI, Mehralizade A, Mayer L, et al. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev. 2018;66:136-148. doi: 10.1016/j.cpr.2018.06.004
34. Daley AJ, Macarthur C, Winter H. The role of exercise in treating postpartum depression: a review of the literature. J Midwifery Womens Health. 2007;52:56-62. doi: 10.1016/j.jmwh.2006.08.017
35. Misri S, Kostaras X, Fox D, et al. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry. 2000;45:554-558. doi: 10.1177/070674370004500607
36. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;CD002018. doi: 10.1002/14651858.CD002018.pub2
37. Pinheiro E, Bogen DL, Hoxha D, et al. Sertraline and breastfeeding: review and meta-analysis. Arch Women Ment Health. 2015;18:139-146. doi: 10.1007/s00737-015-0499-y
38. Hantsoo L, Ward-O’Brien D, Czarkowski KA, et al. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231:939-948. doi: 10.1007/s00213-013-3316-1
39. Rundgren S, Brus O, Båve U, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258-264. doi: 10.1016/j.jad.2018.04.043
40. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. doi: 10.1016/S0140-6736(18)31551-4
Asthma management: How the guidelines compare
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.
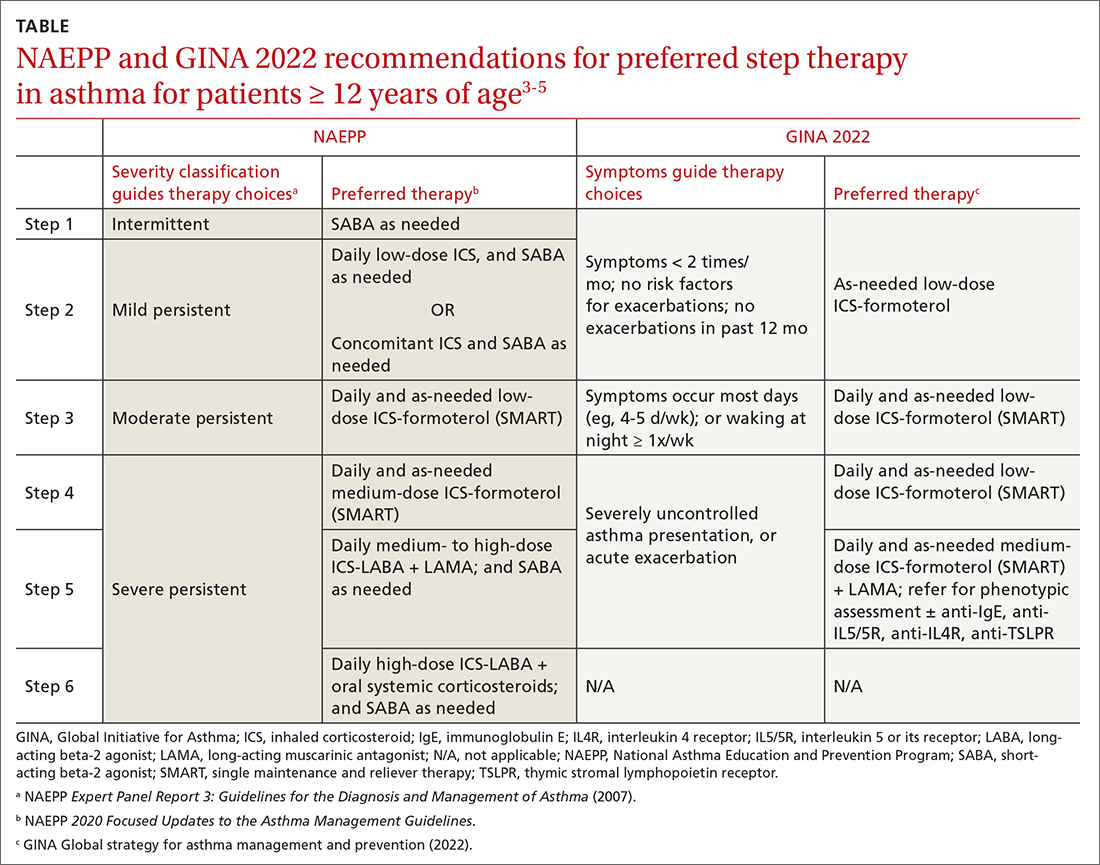
A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; [email protected]
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.

A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; [email protected]
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.

A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; [email protected]
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
PRACTICE RECOMMENDATIONS
› Consider early initiation of intermittent inhaled corticosteroid (ICS)- formoterol over a short-acting beta-2 agonist for reliever therapy. A
› Start prescribing single maintenance and reliever therapy (SMART) with ICS-formoterol to reduce exacerbation rates and simplify application. A
› Consider FeNO assessment when the diagnosis of asthma remains unclear despite history and spirometry findings. B
› Consider adding a longacting antimuscarinic agent to a medium- or high-dose ICS-LABA (long-acting beta-2 agonist) combination in uncontrolled asthma. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Dupilumab as a Therapeutic Approach in Alopecia Universalis
To the Editor:
Atopic diseases, specifically atopic dermatitis (AD) and alopecia areata (AA), are at the forefront of a new era in dermatology involving molecular-directed therapy. Dupilumab is one specific example, having received US Food and Drug Administration approval in March 2017 for the treatment of adults with moderate to severe AD.1 It currently is being investigated for use in pediatric AD. The most commonly reported side effects associated with the use of dupilumab include headaches, conjunctivitis, keratitis, blepharitis, nasopharyngitis, and injection-site reactions.2 We discuss a case of hair regrowth in a patient who was previously diagnosed with AA after treatment with dupilumab for refractory AD.
A 65-year-old White man presented with a history of AD since childhood. Additional medical history included hyperlipidemia; herpes simplex virus infection; asthma; and a diagnosis of AA 6 years prior, which eventually progressed to alopecia universalis. Physical examination demonstrated scattered erythematous lichenified plaques with excoriations involving the arms, legs, and trunk. The patient’s face and scalp were spared of lesions. Complete loss of body hair including the eyelashes and eyebrows also was noted, which was consistent with alopecia universalis.
The patient was started on dupilumab for refractory AD after multiple courses of topical and systemic steroids failed. Prior treatment for AD did not include immunosuppressive or light therapy. The standard dosage of dupilumab was administered, which consisted of a 600-mg subcutaneous loading dose, followed by 300 mg every 2 weeks. There was no concurrent topical corticosteroid or topical calcineurin inhibitor prescribed. After 1 month of treatment with dupilumab, near-complete resolution of the patient’s AD was noted, and after 10 months of treatment, the patient experienced regrowth of the eyelashes, terminal hairs of the beard area (Figure), and vellus hairs of the eyebrows. This hair regrowth persists today with continued dupilumab treatment, and the patient has experienced no additional side effects.

Multiple retrospective and meta-analysis studies have demonstrated a high occurrence of AD comorbid with AA, which strongly suggests a common pathogenesis.3,4 Atopic dermatitis is an inflammatory skin disease mediated by IL-4, IL-5, and IL-13 of the helper T-cell type 2 (TH2) pathway.1 Dupilumab is a human monoclonal antibody that binds to IL-4Rα, which also is found in IL-13 receptors. Dupilumab prevents TH2 pathway-related downstream signaling effects of both cytokines. Although this effect was originally utilized to suppress the TH2-mediated signaling in AD, our patient and others have demonstrated successful hair regrowth with dupilumab, which likely stems from a similar TH2-related antagonism in AA.5,6
The cause of AA is unknown, but IL-4 and IL-13 of the TH2 pathway have been implicated, which renders support for the therapeutic effect of dupilumab in the treatment of AA. Scalp samples of patients with AA have demonstrated upregulation of TH2, helper T-cell type 1 (TH1), and IL-23 cytokines, suggesting efficacy with the use of anti-TH2, anti-TH1, and anti–IL-23 therapies.7 Polymerase chain reaction testing performed on serum samples in patients with AA displayed marked elevation of TH2 cytokines, notably IL-13, which were reduced following intralesional corticosteroid treatment.8 It also has been demonstrated that multiple TH2-related genes contribute to the genetic susceptibility of developing AA, specifically IL-4 and IL-13.9,10
Prior case reports have shown contradicting effects (dupilumab-induced AA), which are speculated to be caused by a stronger TH1 response from TH2 suppression.11,12 In one report, dupilumab was initiated for AD refractory to multiple topical and oral interventions. New-onset hair loss to the scalp was noted after 18 weeks of therapy. Twenty-six weeks into therapy with dupilumab, full hair regrowth was then reported.11 Despite this report, our patient’s hair regrowth after the use of dupilumab for refractory AD further strengthens support for the use of dupilumab as a potential therapy for alopecia universalis and other lymphocyte-mediated hair loss conditions. However, a large disparity in response time and an overall slower progression of hair regrowth reported in our case separate it from other reports of rapid voluminous hair regrowth.5,6 Our findings support the potential use of dupilumab in the treatment of patients with AA.
- Shirly M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303-310.
- Andersen YMF, Egeberg A, Gislason GH, et al. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-280.e1.
- Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015;15:522-528.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154:1358-1360.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5:111-112.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Fuentes-Duculan J, Gulati N, Bonifacio KM, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25:282-286.
- Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192-2197.
- Kalkan G, Karakus N, Bas¸ Y, et al. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527:565-569.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-56.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-144.
To the Editor:
Atopic diseases, specifically atopic dermatitis (AD) and alopecia areata (AA), are at the forefront of a new era in dermatology involving molecular-directed therapy. Dupilumab is one specific example, having received US Food and Drug Administration approval in March 2017 for the treatment of adults with moderate to severe AD.1 It currently is being investigated for use in pediatric AD. The most commonly reported side effects associated with the use of dupilumab include headaches, conjunctivitis, keratitis, blepharitis, nasopharyngitis, and injection-site reactions.2 We discuss a case of hair regrowth in a patient who was previously diagnosed with AA after treatment with dupilumab for refractory AD.
A 65-year-old White man presented with a history of AD since childhood. Additional medical history included hyperlipidemia; herpes simplex virus infection; asthma; and a diagnosis of AA 6 years prior, which eventually progressed to alopecia universalis. Physical examination demonstrated scattered erythematous lichenified plaques with excoriations involving the arms, legs, and trunk. The patient’s face and scalp were spared of lesions. Complete loss of body hair including the eyelashes and eyebrows also was noted, which was consistent with alopecia universalis.
The patient was started on dupilumab for refractory AD after multiple courses of topical and systemic steroids failed. Prior treatment for AD did not include immunosuppressive or light therapy. The standard dosage of dupilumab was administered, which consisted of a 600-mg subcutaneous loading dose, followed by 300 mg every 2 weeks. There was no concurrent topical corticosteroid or topical calcineurin inhibitor prescribed. After 1 month of treatment with dupilumab, near-complete resolution of the patient’s AD was noted, and after 10 months of treatment, the patient experienced regrowth of the eyelashes, terminal hairs of the beard area (Figure), and vellus hairs of the eyebrows. This hair regrowth persists today with continued dupilumab treatment, and the patient has experienced no additional side effects.

Multiple retrospective and meta-analysis studies have demonstrated a high occurrence of AD comorbid with AA, which strongly suggests a common pathogenesis.3,4 Atopic dermatitis is an inflammatory skin disease mediated by IL-4, IL-5, and IL-13 of the helper T-cell type 2 (TH2) pathway.1 Dupilumab is a human monoclonal antibody that binds to IL-4Rα, which also is found in IL-13 receptors. Dupilumab prevents TH2 pathway-related downstream signaling effects of both cytokines. Although this effect was originally utilized to suppress the TH2-mediated signaling in AD, our patient and others have demonstrated successful hair regrowth with dupilumab, which likely stems from a similar TH2-related antagonism in AA.5,6
The cause of AA is unknown, but IL-4 and IL-13 of the TH2 pathway have been implicated, which renders support for the therapeutic effect of dupilumab in the treatment of AA. Scalp samples of patients with AA have demonstrated upregulation of TH2, helper T-cell type 1 (TH1), and IL-23 cytokines, suggesting efficacy with the use of anti-TH2, anti-TH1, and anti–IL-23 therapies.7 Polymerase chain reaction testing performed on serum samples in patients with AA displayed marked elevation of TH2 cytokines, notably IL-13, which were reduced following intralesional corticosteroid treatment.8 It also has been demonstrated that multiple TH2-related genes contribute to the genetic susceptibility of developing AA, specifically IL-4 and IL-13.9,10
Prior case reports have shown contradicting effects (dupilumab-induced AA), which are speculated to be caused by a stronger TH1 response from TH2 suppression.11,12 In one report, dupilumab was initiated for AD refractory to multiple topical and oral interventions. New-onset hair loss to the scalp was noted after 18 weeks of therapy. Twenty-six weeks into therapy with dupilumab, full hair regrowth was then reported.11 Despite this report, our patient’s hair regrowth after the use of dupilumab for refractory AD further strengthens support for the use of dupilumab as a potential therapy for alopecia universalis and other lymphocyte-mediated hair loss conditions. However, a large disparity in response time and an overall slower progression of hair regrowth reported in our case separate it from other reports of rapid voluminous hair regrowth.5,6 Our findings support the potential use of dupilumab in the treatment of patients with AA.
To the Editor:
Atopic diseases, specifically atopic dermatitis (AD) and alopecia areata (AA), are at the forefront of a new era in dermatology involving molecular-directed therapy. Dupilumab is one specific example, having received US Food and Drug Administration approval in March 2017 for the treatment of adults with moderate to severe AD.1 It currently is being investigated for use in pediatric AD. The most commonly reported side effects associated with the use of dupilumab include headaches, conjunctivitis, keratitis, blepharitis, nasopharyngitis, and injection-site reactions.2 We discuss a case of hair regrowth in a patient who was previously diagnosed with AA after treatment with dupilumab for refractory AD.
A 65-year-old White man presented with a history of AD since childhood. Additional medical history included hyperlipidemia; herpes simplex virus infection; asthma; and a diagnosis of AA 6 years prior, which eventually progressed to alopecia universalis. Physical examination demonstrated scattered erythematous lichenified plaques with excoriations involving the arms, legs, and trunk. The patient’s face and scalp were spared of lesions. Complete loss of body hair including the eyelashes and eyebrows also was noted, which was consistent with alopecia universalis.
The patient was started on dupilumab for refractory AD after multiple courses of topical and systemic steroids failed. Prior treatment for AD did not include immunosuppressive or light therapy. The standard dosage of dupilumab was administered, which consisted of a 600-mg subcutaneous loading dose, followed by 300 mg every 2 weeks. There was no concurrent topical corticosteroid or topical calcineurin inhibitor prescribed. After 1 month of treatment with dupilumab, near-complete resolution of the patient’s AD was noted, and after 10 months of treatment, the patient experienced regrowth of the eyelashes, terminal hairs of the beard area (Figure), and vellus hairs of the eyebrows. This hair regrowth persists today with continued dupilumab treatment, and the patient has experienced no additional side effects.

Multiple retrospective and meta-analysis studies have demonstrated a high occurrence of AD comorbid with AA, which strongly suggests a common pathogenesis.3,4 Atopic dermatitis is an inflammatory skin disease mediated by IL-4, IL-5, and IL-13 of the helper T-cell type 2 (TH2) pathway.1 Dupilumab is a human monoclonal antibody that binds to IL-4Rα, which also is found in IL-13 receptors. Dupilumab prevents TH2 pathway-related downstream signaling effects of both cytokines. Although this effect was originally utilized to suppress the TH2-mediated signaling in AD, our patient and others have demonstrated successful hair regrowth with dupilumab, which likely stems from a similar TH2-related antagonism in AA.5,6
The cause of AA is unknown, but IL-4 and IL-13 of the TH2 pathway have been implicated, which renders support for the therapeutic effect of dupilumab in the treatment of AA. Scalp samples of patients with AA have demonstrated upregulation of TH2, helper T-cell type 1 (TH1), and IL-23 cytokines, suggesting efficacy with the use of anti-TH2, anti-TH1, and anti–IL-23 therapies.7 Polymerase chain reaction testing performed on serum samples in patients with AA displayed marked elevation of TH2 cytokines, notably IL-13, which were reduced following intralesional corticosteroid treatment.8 It also has been demonstrated that multiple TH2-related genes contribute to the genetic susceptibility of developing AA, specifically IL-4 and IL-13.9,10
Prior case reports have shown contradicting effects (dupilumab-induced AA), which are speculated to be caused by a stronger TH1 response from TH2 suppression.11,12 In one report, dupilumab was initiated for AD refractory to multiple topical and oral interventions. New-onset hair loss to the scalp was noted after 18 weeks of therapy. Twenty-six weeks into therapy with dupilumab, full hair regrowth was then reported.11 Despite this report, our patient’s hair regrowth after the use of dupilumab for refractory AD further strengthens support for the use of dupilumab as a potential therapy for alopecia universalis and other lymphocyte-mediated hair loss conditions. However, a large disparity in response time and an overall slower progression of hair regrowth reported in our case separate it from other reports of rapid voluminous hair regrowth.5,6 Our findings support the potential use of dupilumab in the treatment of patients with AA.
- Shirly M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303-310.
- Andersen YMF, Egeberg A, Gislason GH, et al. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-280.e1.
- Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015;15:522-528.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154:1358-1360.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5:111-112.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Fuentes-Duculan J, Gulati N, Bonifacio KM, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25:282-286.
- Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192-2197.
- Kalkan G, Karakus N, Bas¸ Y, et al. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527:565-569.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-56.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-144.
- Shirly M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303-310.
- Andersen YMF, Egeberg A, Gislason GH, et al. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-280.e1.
- Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015;15:522-528.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154:1358-1360.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5:111-112.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Fuentes-Duculan J, Gulati N, Bonifacio KM, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25:282-286.
- Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192-2197.
- Kalkan G, Karakus N, Bas¸ Y, et al. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527:565-569.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-56.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-144.
Practice Points
- Practicing dermatologists should be aware of the shared pathophysiology of alopecia areata and atopic dermatitis and the relief patients with these conditions can experience when treated with dupilumab.
- As molecular-directed biologic therapies emerge in the marketplace, their potential for targeting one atopic disease may offer notable benefits for use in the treatment of other atopic diseases.
Pediatric Asthma
2022 Update on pelvic floor dysfunction
Knowledge of the latest evidence on the management of pelvic floor disorders is essential for all practicing ObGyns. In this Update, we review long-term outcomes for a polyacrylamide hydrogel urethral bulking agent for the treatment of stress urinary incontinence (SUI) that presents a viable alternative to the gold standard, midurethral sling. We review the new recommendations from the American Urogynecologic Society (AUGS) regarding the administration of anticholinergics, highlighting a paradigm shift in the management of overactive bladder (OAB). In addition, we present data on a proposed threshold glycosylated hemoglobin A1c (HbA1c) level for patients undergoing pelvic organ prolapse (POP) surgery that may help reduce the risk of perioperative complications. Finally, we consider new evidence on the long-term efficacy and safety of transvaginal mesh for repair of POP.
Periurethral injection with polyacrylamide hydrogel is a long-term durable and safe option for women with SUI
Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508. doi:10.1002/nau.24589.
Urethral bulking agents are a less invasive management option for women with SUI compared with the gold standard, midurethral sling. Treatment with a polyacrylamide hydrogel (PAHG; Bulkamid)—a nonparticulate hydrogel bulking agent—showed long-term efficacy and a favorable safety profile at 7 years’ follow-up.
Study details
Brosche and colleagues conducted a retrospective cohort study that included women with SUI or stress-predominant mixed urinary incontinence (MUI) who underwent transurethral PAHG injections for primary treatment of their incontinence symptoms. The study objective was to evaluate the long-term efficacy of PAHG based on patient satisfaction. Treatment safety was a secondary outcome.
Pad counts and validated questionnaires were used to determine treatment effectiveness. Additional data on reinjection rates, perioperative complications, and postoperative complications also were collected.
Long-term outcomes favorable
During the study time period, 1,200 patients were treated with PAHG, and 7-year data were available for 553 women. Of the 553 patients, 67% reported improvement or cure of their SUI symptoms when PAHG was performed as a primary procedure, consistent with previously published 12-month data. There were no perioperative complications. Postoperative complications were transient. Short-term subjective prolonged bladder emptying was the most common complication and occurred in 15% of patients.
PAHG injection is a durable and safe alternative for the treatment of stress urinary incontinence in women who are not candidates for or who decline treatment with alternative methods, such as a midurethral sling.
Continue to: New society guidance...
New society guidance on the use of anticholinergic medications for the treatment of OAB
AUGS Clinical Consensus Statement: Association of anticholinergic medication use and cognition in women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27:69-71. doi:10.1097/ SPV.0000000000001008.
In 2021, AUGS updated its consensus statement on the use of anticholinergic medications for the treatment of OAB. This action was in response to growing evidence that supports the association of anticholinergic medications with long-term cognitive adverse effects, including cognitive impairment, dementia, and Alzheimer disease.
Here, we summarize the most recent modifications, which differentiate the updated statement from the preceding consensus document published in 2017.
Updated AUGS recommendations
- If considering anticholinergic medications, counsel patients about the risk of cognitive adverse effects and weigh these risks against the potential benefits to their quality of life and overall health.
- Use the lowest possible dose when prescribing anticholinergics and consider alternatives such as β3 agonists (for example, mirabegron or vibegron).
- Avoid using anticholinergic medications in women older than age 70. However, if an anticholinergic must be used, consider a medication that has low potential to cross the blood-brain barrier (for example, trospium).
For patients who are unresponsive to behavioral therapies for OAB, medical management may be considered. However, the risks of anticholinergic medications may outweigh the benefits—especially for older women—and these medications should be prescribed with caution after discussing the potential cognitive adverse effects with patients. β3 agonists should be preferentially prescribed when appropriate. Consider referral to a urogynecologist for discussion of third-line therapies in patients who prefer to forego or may not be candidates for medical management of their OAB symptoms.
HbA1c levels > 8% may increase complications risk in urogyn surgery
Ringel NE, de Winter KL, Siddique M, et al. Surgical outcomes in urogynecology—assessment of perioperative and postoperative complications relative to preoperative hemoglobin A1c—a Fellows Pelvic Research Network study. Female Pelvic Med Reconstr Surg. 2022;28:7-13. doi:10.1097/ SPV.0000000000001057.
Diabetes mellitus is a known risk factor for complications following surgery. Adoption of an HbA1c level threshold for risk stratification before urogynecologic surgery may help improve patient outcomes.
Study details
Ringel and colleagues conducted a multicenter retrospective cohort study that included women with diabetes mellitus who underwent prolapse and/or SUI surgery between 2013 and 2018. The aim of the study was to identify a hemoglobin A1C threshold that would help predict increased risk for perioperative complications in women undergoing pelvic reconstructive surgery. Demographics, preoperative HbA1c levels, and surgical data were collected.
Complication risks correlated with higher HbA1c threshold
The study included 807 women with HbA1c values that ranged from 5% to 12%. The overall complication rate was 44%. Sensitivity analysis was performed to compare complication rates between patients with varying HbA1c levels and determine a threshold HbA1c value with the greatest difference in complication rates.
The authors concluded that women with an HbA1c level ≥ 8% showed the greatest increase of perioperative complications. Patients with an HbA1c ≥ 8%, compared with those who had an HbA1c < 8%, had a statistically significantly increased rate of overall (58% vs 42%, P = .002) and severe (27% vs 13%, P< .001) perioperative complications.
After multivariate logistic regression, the risk of overall complications remained elevated, with a 1.9-times higher risk of perioperative complications for women with an HbA1c ≥ 8%.
Women should be medically optimized before undergoing surgery and, while this study was restricted to urogynecologic surgery patients, it seems reasonable to assume that a similar HbA1c threshold would be beneficial for women undergoing other gynecologic procedures. Appropriately screening patients and referring them for early intervention with their primary care clinician or endocrinologist may improve surgical outcomes, especially in women with an HbA1c level > 8%.
Continue to: Success is similar for TV mesh and native tissue repair...
Success is similar for TV mesh and native tissue repair
Kahn B, Varner RE, Murphy M, et al. Transvaginal mesh compared with native tissue repair for pelvic organ prolapse. Obstet Gynecol. 2022;139:975-985. doi:10.1097/AOG.0000000000004794.
The distribution of vaginal mesh kits for the repair of POP was halted by the US Food and Drug Administration (FDA) in 2019. However, concerns have been raised about the measures used by the FDA to justify pulling these devices from the market. A cohort study compared 36-month outcomes between women who underwent prolapse repair with newer generation transvaginal mesh versus native tissue repair.
Study details
In a nonrandomized prospective multicenter cohort study, Kahn and colleagues compared outcomes in women with POP who underwent native tissue repair or transvaginal mesh repair with the Uphold LITE vaginal support system. The study’s objective was to compare the safety and efficacy of native tissue and transvaginal mesh prolapse repairs at 36 months postoperatively.
Treatment success was measured based on composite and individual measures of anatomic and subjective success, need for retreatment, and the occurrence of adverse events. Quality of life (QoL) measures also were obtained using validated questionnaires. Intention-to-treat and per-protocol analyses were performed.
Composite success rate was higher for mesh repair
A total of 710 patients were screened for eligibility (225 received transvaginal mesh and 485 received native tissue repair). Transvaginal mesh placement was found to be significantly superior to native tissue repair for composite success (84% vs 73%, P = .009) when prolapse within the hymen (that is, Ba and/or C < 0 on the Pelvic Organ Prolapse Quantification System) was used to define anatomic success.
Adverse events were similar between transvaginal mesh and native tissue repair groups, with most adverse events occurring within the first 6 months. The mesh exposure rate was 4.9%. Of the 13 incidents of mesh exposure, 4 patients required surgical intervention and 1 incident was considered a serious adverse event. QoL measures demonstrated improvement without any statistically significant differences between the treatment cohorts. ●
This study established the superiority and safety of newer generation transvaginal mesh used for the treatment of pelvic organ prolapse. Women who received newer generation transvaginal mesh can be reassured that the prolapse recurrence rates are low and that adverse events related to their mesh are rare—even when compared with those of native tissue repair. Patients also may be reassured that most adverse events would have occurred within 6 months of the initial prolapse repair surgery
Knowledge of the latest evidence on the management of pelvic floor disorders is essential for all practicing ObGyns. In this Update, we review long-term outcomes for a polyacrylamide hydrogel urethral bulking agent for the treatment of stress urinary incontinence (SUI) that presents a viable alternative to the gold standard, midurethral sling. We review the new recommendations from the American Urogynecologic Society (AUGS) regarding the administration of anticholinergics, highlighting a paradigm shift in the management of overactive bladder (OAB). In addition, we present data on a proposed threshold glycosylated hemoglobin A1c (HbA1c) level for patients undergoing pelvic organ prolapse (POP) surgery that may help reduce the risk of perioperative complications. Finally, we consider new evidence on the long-term efficacy and safety of transvaginal mesh for repair of POP.
Periurethral injection with polyacrylamide hydrogel is a long-term durable and safe option for women with SUI
Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508. doi:10.1002/nau.24589.
Urethral bulking agents are a less invasive management option for women with SUI compared with the gold standard, midurethral sling. Treatment with a polyacrylamide hydrogel (PAHG; Bulkamid)—a nonparticulate hydrogel bulking agent—showed long-term efficacy and a favorable safety profile at 7 years’ follow-up.
Study details
Brosche and colleagues conducted a retrospective cohort study that included women with SUI or stress-predominant mixed urinary incontinence (MUI) who underwent transurethral PAHG injections for primary treatment of their incontinence symptoms. The study objective was to evaluate the long-term efficacy of PAHG based on patient satisfaction. Treatment safety was a secondary outcome.
Pad counts and validated questionnaires were used to determine treatment effectiveness. Additional data on reinjection rates, perioperative complications, and postoperative complications also were collected.
Long-term outcomes favorable
During the study time period, 1,200 patients were treated with PAHG, and 7-year data were available for 553 women. Of the 553 patients, 67% reported improvement or cure of their SUI symptoms when PAHG was performed as a primary procedure, consistent with previously published 12-month data. There were no perioperative complications. Postoperative complications were transient. Short-term subjective prolonged bladder emptying was the most common complication and occurred in 15% of patients.
PAHG injection is a durable and safe alternative for the treatment of stress urinary incontinence in women who are not candidates for or who decline treatment with alternative methods, such as a midurethral sling.
Continue to: New society guidance...
New society guidance on the use of anticholinergic medications for the treatment of OAB
AUGS Clinical Consensus Statement: Association of anticholinergic medication use and cognition in women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27:69-71. doi:10.1097/ SPV.0000000000001008.
In 2021, AUGS updated its consensus statement on the use of anticholinergic medications for the treatment of OAB. This action was in response to growing evidence that supports the association of anticholinergic medications with long-term cognitive adverse effects, including cognitive impairment, dementia, and Alzheimer disease.
Here, we summarize the most recent modifications, which differentiate the updated statement from the preceding consensus document published in 2017.
Updated AUGS recommendations
- If considering anticholinergic medications, counsel patients about the risk of cognitive adverse effects and weigh these risks against the potential benefits to their quality of life and overall health.
- Use the lowest possible dose when prescribing anticholinergics and consider alternatives such as β3 agonists (for example, mirabegron or vibegron).
- Avoid using anticholinergic medications in women older than age 70. However, if an anticholinergic must be used, consider a medication that has low potential to cross the blood-brain barrier (for example, trospium).
For patients who are unresponsive to behavioral therapies for OAB, medical management may be considered. However, the risks of anticholinergic medications may outweigh the benefits—especially for older women—and these medications should be prescribed with caution after discussing the potential cognitive adverse effects with patients. β3 agonists should be preferentially prescribed when appropriate. Consider referral to a urogynecologist for discussion of third-line therapies in patients who prefer to forego or may not be candidates for medical management of their OAB symptoms.
HbA1c levels > 8% may increase complications risk in urogyn surgery
Ringel NE, de Winter KL, Siddique M, et al. Surgical outcomes in urogynecology—assessment of perioperative and postoperative complications relative to preoperative hemoglobin A1c—a Fellows Pelvic Research Network study. Female Pelvic Med Reconstr Surg. 2022;28:7-13. doi:10.1097/ SPV.0000000000001057.
Diabetes mellitus is a known risk factor for complications following surgery. Adoption of an HbA1c level threshold for risk stratification before urogynecologic surgery may help improve patient outcomes.
Study details
Ringel and colleagues conducted a multicenter retrospective cohort study that included women with diabetes mellitus who underwent prolapse and/or SUI surgery between 2013 and 2018. The aim of the study was to identify a hemoglobin A1C threshold that would help predict increased risk for perioperative complications in women undergoing pelvic reconstructive surgery. Demographics, preoperative HbA1c levels, and surgical data were collected.
Complication risks correlated with higher HbA1c threshold
The study included 807 women with HbA1c values that ranged from 5% to 12%. The overall complication rate was 44%. Sensitivity analysis was performed to compare complication rates between patients with varying HbA1c levels and determine a threshold HbA1c value with the greatest difference in complication rates.
The authors concluded that women with an HbA1c level ≥ 8% showed the greatest increase of perioperative complications. Patients with an HbA1c ≥ 8%, compared with those who had an HbA1c < 8%, had a statistically significantly increased rate of overall (58% vs 42%, P = .002) and severe (27% vs 13%, P< .001) perioperative complications.
After multivariate logistic regression, the risk of overall complications remained elevated, with a 1.9-times higher risk of perioperative complications for women with an HbA1c ≥ 8%.
Women should be medically optimized before undergoing surgery and, while this study was restricted to urogynecologic surgery patients, it seems reasonable to assume that a similar HbA1c threshold would be beneficial for women undergoing other gynecologic procedures. Appropriately screening patients and referring them for early intervention with their primary care clinician or endocrinologist may improve surgical outcomes, especially in women with an HbA1c level > 8%.
Continue to: Success is similar for TV mesh and native tissue repair...
Success is similar for TV mesh and native tissue repair
Kahn B, Varner RE, Murphy M, et al. Transvaginal mesh compared with native tissue repair for pelvic organ prolapse. Obstet Gynecol. 2022;139:975-985. doi:10.1097/AOG.0000000000004794.
The distribution of vaginal mesh kits for the repair of POP was halted by the US Food and Drug Administration (FDA) in 2019. However, concerns have been raised about the measures used by the FDA to justify pulling these devices from the market. A cohort study compared 36-month outcomes between women who underwent prolapse repair with newer generation transvaginal mesh versus native tissue repair.
Study details
In a nonrandomized prospective multicenter cohort study, Kahn and colleagues compared outcomes in women with POP who underwent native tissue repair or transvaginal mesh repair with the Uphold LITE vaginal support system. The study’s objective was to compare the safety and efficacy of native tissue and transvaginal mesh prolapse repairs at 36 months postoperatively.
Treatment success was measured based on composite and individual measures of anatomic and subjective success, need for retreatment, and the occurrence of adverse events. Quality of life (QoL) measures also were obtained using validated questionnaires. Intention-to-treat and per-protocol analyses were performed.
Composite success rate was higher for mesh repair
A total of 710 patients were screened for eligibility (225 received transvaginal mesh and 485 received native tissue repair). Transvaginal mesh placement was found to be significantly superior to native tissue repair for composite success (84% vs 73%, P = .009) when prolapse within the hymen (that is, Ba and/or C < 0 on the Pelvic Organ Prolapse Quantification System) was used to define anatomic success.
Adverse events were similar between transvaginal mesh and native tissue repair groups, with most adverse events occurring within the first 6 months. The mesh exposure rate was 4.9%. Of the 13 incidents of mesh exposure, 4 patients required surgical intervention and 1 incident was considered a serious adverse event. QoL measures demonstrated improvement without any statistically significant differences between the treatment cohorts. ●
This study established the superiority and safety of newer generation transvaginal mesh used for the treatment of pelvic organ prolapse. Women who received newer generation transvaginal mesh can be reassured that the prolapse recurrence rates are low and that adverse events related to their mesh are rare—even when compared with those of native tissue repair. Patients also may be reassured that most adverse events would have occurred within 6 months of the initial prolapse repair surgery
Knowledge of the latest evidence on the management of pelvic floor disorders is essential for all practicing ObGyns. In this Update, we review long-term outcomes for a polyacrylamide hydrogel urethral bulking agent for the treatment of stress urinary incontinence (SUI) that presents a viable alternative to the gold standard, midurethral sling. We review the new recommendations from the American Urogynecologic Society (AUGS) regarding the administration of anticholinergics, highlighting a paradigm shift in the management of overactive bladder (OAB). In addition, we present data on a proposed threshold glycosylated hemoglobin A1c (HbA1c) level for patients undergoing pelvic organ prolapse (POP) surgery that may help reduce the risk of perioperative complications. Finally, we consider new evidence on the long-term efficacy and safety of transvaginal mesh for repair of POP.
Periurethral injection with polyacrylamide hydrogel is a long-term durable and safe option for women with SUI
Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508. doi:10.1002/nau.24589.
Urethral bulking agents are a less invasive management option for women with SUI compared with the gold standard, midurethral sling. Treatment with a polyacrylamide hydrogel (PAHG; Bulkamid)—a nonparticulate hydrogel bulking agent—showed long-term efficacy and a favorable safety profile at 7 years’ follow-up.
Study details
Brosche and colleagues conducted a retrospective cohort study that included women with SUI or stress-predominant mixed urinary incontinence (MUI) who underwent transurethral PAHG injections for primary treatment of their incontinence symptoms. The study objective was to evaluate the long-term efficacy of PAHG based on patient satisfaction. Treatment safety was a secondary outcome.
Pad counts and validated questionnaires were used to determine treatment effectiveness. Additional data on reinjection rates, perioperative complications, and postoperative complications also were collected.
Long-term outcomes favorable
During the study time period, 1,200 patients were treated with PAHG, and 7-year data were available for 553 women. Of the 553 patients, 67% reported improvement or cure of their SUI symptoms when PAHG was performed as a primary procedure, consistent with previously published 12-month data. There were no perioperative complications. Postoperative complications were transient. Short-term subjective prolonged bladder emptying was the most common complication and occurred in 15% of patients.
PAHG injection is a durable and safe alternative for the treatment of stress urinary incontinence in women who are not candidates for or who decline treatment with alternative methods, such as a midurethral sling.
Continue to: New society guidance...
New society guidance on the use of anticholinergic medications for the treatment of OAB
AUGS Clinical Consensus Statement: Association of anticholinergic medication use and cognition in women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27:69-71. doi:10.1097/ SPV.0000000000001008.
In 2021, AUGS updated its consensus statement on the use of anticholinergic medications for the treatment of OAB. This action was in response to growing evidence that supports the association of anticholinergic medications with long-term cognitive adverse effects, including cognitive impairment, dementia, and Alzheimer disease.
Here, we summarize the most recent modifications, which differentiate the updated statement from the preceding consensus document published in 2017.
Updated AUGS recommendations
- If considering anticholinergic medications, counsel patients about the risk of cognitive adverse effects and weigh these risks against the potential benefits to their quality of life and overall health.
- Use the lowest possible dose when prescribing anticholinergics and consider alternatives such as β3 agonists (for example, mirabegron or vibegron).
- Avoid using anticholinergic medications in women older than age 70. However, if an anticholinergic must be used, consider a medication that has low potential to cross the blood-brain barrier (for example, trospium).
For patients who are unresponsive to behavioral therapies for OAB, medical management may be considered. However, the risks of anticholinergic medications may outweigh the benefits—especially for older women—and these medications should be prescribed with caution after discussing the potential cognitive adverse effects with patients. β3 agonists should be preferentially prescribed when appropriate. Consider referral to a urogynecologist for discussion of third-line therapies in patients who prefer to forego or may not be candidates for medical management of their OAB symptoms.
HbA1c levels > 8% may increase complications risk in urogyn surgery
Ringel NE, de Winter KL, Siddique M, et al. Surgical outcomes in urogynecology—assessment of perioperative and postoperative complications relative to preoperative hemoglobin A1c—a Fellows Pelvic Research Network study. Female Pelvic Med Reconstr Surg. 2022;28:7-13. doi:10.1097/ SPV.0000000000001057.
Diabetes mellitus is a known risk factor for complications following surgery. Adoption of an HbA1c level threshold for risk stratification before urogynecologic surgery may help improve patient outcomes.
Study details
Ringel and colleagues conducted a multicenter retrospective cohort study that included women with diabetes mellitus who underwent prolapse and/or SUI surgery between 2013 and 2018. The aim of the study was to identify a hemoglobin A1C threshold that would help predict increased risk for perioperative complications in women undergoing pelvic reconstructive surgery. Demographics, preoperative HbA1c levels, and surgical data were collected.
Complication risks correlated with higher HbA1c threshold
The study included 807 women with HbA1c values that ranged from 5% to 12%. The overall complication rate was 44%. Sensitivity analysis was performed to compare complication rates between patients with varying HbA1c levels and determine a threshold HbA1c value with the greatest difference in complication rates.
The authors concluded that women with an HbA1c level ≥ 8% showed the greatest increase of perioperative complications. Patients with an HbA1c ≥ 8%, compared with those who had an HbA1c < 8%, had a statistically significantly increased rate of overall (58% vs 42%, P = .002) and severe (27% vs 13%, P< .001) perioperative complications.
After multivariate logistic regression, the risk of overall complications remained elevated, with a 1.9-times higher risk of perioperative complications for women with an HbA1c ≥ 8%.
Women should be medically optimized before undergoing surgery and, while this study was restricted to urogynecologic surgery patients, it seems reasonable to assume that a similar HbA1c threshold would be beneficial for women undergoing other gynecologic procedures. Appropriately screening patients and referring them for early intervention with their primary care clinician or endocrinologist may improve surgical outcomes, especially in women with an HbA1c level > 8%.
Continue to: Success is similar for TV mesh and native tissue repair...
Success is similar for TV mesh and native tissue repair
Kahn B, Varner RE, Murphy M, et al. Transvaginal mesh compared with native tissue repair for pelvic organ prolapse. Obstet Gynecol. 2022;139:975-985. doi:10.1097/AOG.0000000000004794.
The distribution of vaginal mesh kits for the repair of POP was halted by the US Food and Drug Administration (FDA) in 2019. However, concerns have been raised about the measures used by the FDA to justify pulling these devices from the market. A cohort study compared 36-month outcomes between women who underwent prolapse repair with newer generation transvaginal mesh versus native tissue repair.
Study details
In a nonrandomized prospective multicenter cohort study, Kahn and colleagues compared outcomes in women with POP who underwent native tissue repair or transvaginal mesh repair with the Uphold LITE vaginal support system. The study’s objective was to compare the safety and efficacy of native tissue and transvaginal mesh prolapse repairs at 36 months postoperatively.
Treatment success was measured based on composite and individual measures of anatomic and subjective success, need for retreatment, and the occurrence of adverse events. Quality of life (QoL) measures also were obtained using validated questionnaires. Intention-to-treat and per-protocol analyses were performed.
Composite success rate was higher for mesh repair
A total of 710 patients were screened for eligibility (225 received transvaginal mesh and 485 received native tissue repair). Transvaginal mesh placement was found to be significantly superior to native tissue repair for composite success (84% vs 73%, P = .009) when prolapse within the hymen (that is, Ba and/or C < 0 on the Pelvic Organ Prolapse Quantification System) was used to define anatomic success.
Adverse events were similar between transvaginal mesh and native tissue repair groups, with most adverse events occurring within the first 6 months. The mesh exposure rate was 4.9%. Of the 13 incidents of mesh exposure, 4 patients required surgical intervention and 1 incident was considered a serious adverse event. QoL measures demonstrated improvement without any statistically significant differences between the treatment cohorts. ●
This study established the superiority and safety of newer generation transvaginal mesh used for the treatment of pelvic organ prolapse. Women who received newer generation transvaginal mesh can be reassured that the prolapse recurrence rates are low and that adverse events related to their mesh are rare—even when compared with those of native tissue repair. Patients also may be reassured that most adverse events would have occurred within 6 months of the initial prolapse repair surgery
Aspirin use confers multifaceted benefits in HCC
Key clinical point: The use of aspirin is independently associated with a reduced risk for hepatocellular carcinoma (HCC) incidence, recurrence, and mortality, but an increased risk for bleeding.
Major finding: Aspirin use was associated with a reduced incidence of HCC (hazard ratio [HR] 0.75; 95% CI 0.71-0.80), recurrence of HCC (HR 0.79; 95% CI 0.65-0.96), and HCC-related mortality (HR 0.64; 95% CI 0.44-0.94), but an increased risk for bleeding (HR 1.10; 95% CI 1.02-1.20).
Study details: This study meta-analyzed the data of 3,273,524 individuals from 30 studies on HCC incidence, HCC recurrence, or mortality.
Disclosures: This study did not report the source of funding. The authors declared no conflicts of interest.
Source: Ma S, Qu G et al. Does aspirin reduce the incidence, recurrence, and mortality of hepatocellular carcinoma? A GRADE-assessed systematic review and dose-response meta-analysis. Eur J Clin Pharmacol. 2022 (Nov 5). Doi: 10.1007/s00228-022-03414-y
Key clinical point: The use of aspirin is independently associated with a reduced risk for hepatocellular carcinoma (HCC) incidence, recurrence, and mortality, but an increased risk for bleeding.
Major finding: Aspirin use was associated with a reduced incidence of HCC (hazard ratio [HR] 0.75; 95% CI 0.71-0.80), recurrence of HCC (HR 0.79; 95% CI 0.65-0.96), and HCC-related mortality (HR 0.64; 95% CI 0.44-0.94), but an increased risk for bleeding (HR 1.10; 95% CI 1.02-1.20).
Study details: This study meta-analyzed the data of 3,273,524 individuals from 30 studies on HCC incidence, HCC recurrence, or mortality.
Disclosures: This study did not report the source of funding. The authors declared no conflicts of interest.
Source: Ma S, Qu G et al. Does aspirin reduce the incidence, recurrence, and mortality of hepatocellular carcinoma? A GRADE-assessed systematic review and dose-response meta-analysis. Eur J Clin Pharmacol. 2022 (Nov 5). Doi: 10.1007/s00228-022-03414-y
Key clinical point: The use of aspirin is independently associated with a reduced risk for hepatocellular carcinoma (HCC) incidence, recurrence, and mortality, but an increased risk for bleeding.
Major finding: Aspirin use was associated with a reduced incidence of HCC (hazard ratio [HR] 0.75; 95% CI 0.71-0.80), recurrence of HCC (HR 0.79; 95% CI 0.65-0.96), and HCC-related mortality (HR 0.64; 95% CI 0.44-0.94), but an increased risk for bleeding (HR 1.10; 95% CI 1.02-1.20).
Study details: This study meta-analyzed the data of 3,273,524 individuals from 30 studies on HCC incidence, HCC recurrence, or mortality.
Disclosures: This study did not report the source of funding. The authors declared no conflicts of interest.
Source: Ma S, Qu G et al. Does aspirin reduce the incidence, recurrence, and mortality of hepatocellular carcinoma? A GRADE-assessed systematic review and dose-response meta-analysis. Eur J Clin Pharmacol. 2022 (Nov 5). Doi: 10.1007/s00228-022-03414-y