User login
Put down the electronics after a concussion?
ILLUSTRATIVE CASE
A 17-year-old high school football player presents to the emergency department (ED) after a helmet-to-helmet tackle in a game earlier that day. After the tackle, he experienced immediate confusion. Once he returned to his feet, he felt dizzy and nauseated and began to develop a headache. When his symptoms failed to resolve within a few hours, his mother brought him to the hospital for an evaluation. In the ED, he receives a diagnosis of concussion, and his mother asks for recommendations on how he can recover as quickly as possible.
Traumatic brain injuries account for an estimated 2.5 million ED visits annually in the United States.2 Concussions are the most common form of traumatic brain injury, with adolescents contributing to the highest incidence of concussions.3,4 An estimated 1.6 to 3.8 million people experience a sports-related concussion annually.5
Time to recovery is a clinical endpoint that matters greatly to our young, physically active patients, who are often eager to return to their daily activities as soon as possible. Guidelines frequently recommend cognitive and physical rest for 24 to 48 hours immediately following a concussion, but the use of screens during this cognitive rest period remains uncertain.6,7 International guidelines and the Centers for Disease Control and Prevention recommend symptom-limited activities—including screen time—during the initial period of a concussion.6,7 Although this gradual approach is standard of care, it has been unclear if abstaining completely from certain activities during the initial days of a concussion has any impact on recovery time.
Recent studies have examined physical activity to clarify the optimal timing of physical rest after a concussion. Among adolescents with concussions, strict rest for 5 days does not appear to improve symptoms compared with rest for 1 to 2 days.8 Additionally, physical activity within 7 days of acute head injury may help reduce symptoms and prevent postconcussive symptoms.9,10
This same level of clarity has been lacking for cognitive rest and screen time. The use of screens is a part of most patients’ daily activities, particularly among adolescents and young adults. One report found that students ages 8 to 18 years engage in approximately 7 hours of daily screen time, excluding that related to
STUDY SUMMARY
Symptom duration was significantly reduced by cutting screen time
This single-site, parallel-design, randomized clinical trial examined the effectiveness of limiting screen time exposure within the first 48 hours after a concussion in reducing the time to resolution of concussive symptoms in 125 patients. 1 Patients were included if they were 12 to 25 years old (mean age, 17 years) and presented within 24 hours of sustaining a concussion (as defined on the Acute Concussion Evaluation–Emergency Department tool) to the pediatric or adult ED at a US tertiary medical center.
Patients were randomized to either engage in screen time as tolerated or to abstain from screen time for 48 hours following their injury. Screen modalities included television, phones, video games, and computers/tablets. The Post-Concussive Symptom Scale (PCSS; 0-132) was used to characterize 22 symptoms from 0 (absent) to 6 (severe) daily for 10 days. Patients also self-reported the amount of screen time they engaged in during Days 1 to 3 of the study period and completed an activity survey on Days 4 to 10. Among the participants, 76% completed the PCSS form until symptom resolution or until Day 10 (the end of the study period).
Continue to: The primary outcome...
The primary outcome was days to resolution of concussive symptoms, defined as a PCSS score ≤ 3. The median baseline PCSS score was 21 in the screen time–permitted group and 24.5 in the screen time–abstinent group. The screen time–permitted group reported a median screen time of 630 minutes during the intervention period, compared with 130 minutes in the screen time–abstinent group, and was less likely to recover during the study period than the screen time–abstinent group (hazard ratio = 0.51; 95% CI, 0.29-0.90). The screen time–permitted group had a significantly longer median recovery time compared with the screen time–abstinent group (8.0 vs 3.5 days; P = .03).
WHAT'S NEW?
Exploring the role of screen time during the cognitive rest period
This study provides evidence supporting the recommendation that adolescent and young adult patients abstain from screen time in the first 48 hours following a concussion to decrease time to symptom resolution, thus shortening the timeline to return to their usual daily activities.
CAVEATS
Self-reporting of data may introduce bias
This study used a self-reporting method to collect data, which could have resulted in underreporting or overreporting of screen time and potentially introduced recall and reporting bias. The screen time–abstinent group did not completely abstain from all screen time, with a self-reported average of 5 to 10 minutes of daily screen time to complete the required research surveys, so it is not immediately clear what extent of abstinence vs significant screen time reduction led to the clinical endpoints observed. Furthermore, this study did not ask patients to differentiate between active screen time (eg, texting and gaming) and passive screen time (eg, watching videos), which may differentially impact symptom resolution.
CHALLENGES TO IMPLEMENTATION
Turning off the ever-present screen may present obstacles
This intervention is easy to recommend, with few barriers to implementation. It’s worth noting that screens are often used in a patient’s school or job, and 48 hours of abstinence from these activities is a difficult ask when much of our society’s education, entertainment, and productivity revolve around the use of technology. When appropriate, a shared decision-making discussion between patient and physician should center on the idea that 48 hours of screen time abstinence could be well worth the increased likelihood of total recovery at Day 10, as opposed to the risk for persistent and prolonged symptoms that interfere with the patient’s lifestyle.
1. Macnow T, Curran T, Tolliday C, et al. Effect of screen time on recovery from concussion: a randomized clinical trial. JAMA Pediatr. 2021;175:1124-1131. doi: 10.1001/jamapediat rics.2021.2782
2. Taylor CA, Bell JM, Breiding MJ, et al. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1-16. doi: 10.15585/mmwr.ss6609a1
3. Vos PE, Battistin L, Birbamer G, et al; European Federation of Neurological Societies. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207-219. doi: 10.1046/j.1468-1331.2002.00407.x
4. Zhang AL, Sing DC, Rugg CM, et al. The rise of concussions in the adolescent population. Orthop J Sports Med. 2016;4:2325967116662458. doi: 10.1177/2325967116662458
5. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709-735. doi: 10.1097/NEN.0b013e3181a9d503
6. McCrory P, Meeuwisse W, Dvorák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi: 10.1136/bjsports-2017-097699
7. Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172:e182853. doi: 10.1001/jamapediat rics.2018.2853
8. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:213-223. doi: 10.1542/peds.2014-0966
9. Grool AM, Aglipay M, Momoli F, et al; Pediatric Emergency Research Canada (PERC) Concussion Team. Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316:2504-2514. doi: 10.1001/jama.2016.17396
10. Lal A, Kolakowsky-Hayner SA, Ghajar J, et al. The effect of physical exercise after a concussion: a systematic review and meta-analysis. Am J Sports Med. 2018;46:743-752. doi: 10.1177/0363546517706137
11. Rideout V, Peebles A, Mann S, et al. The Common Sense Census: Media Use by Tweens and Teens, 2021. Common Sense Media; 2022. Accessed December 28, 2022. www.commonsensemedia.org/sites/default/files/research/report/8-18-census-integrated-report-final-web_0.pdf
ILLUSTRATIVE CASE
A 17-year-old high school football player presents to the emergency department (ED) after a helmet-to-helmet tackle in a game earlier that day. After the tackle, he experienced immediate confusion. Once he returned to his feet, he felt dizzy and nauseated and began to develop a headache. When his symptoms failed to resolve within a few hours, his mother brought him to the hospital for an evaluation. In the ED, he receives a diagnosis of concussion, and his mother asks for recommendations on how he can recover as quickly as possible.
Traumatic brain injuries account for an estimated 2.5 million ED visits annually in the United States.2 Concussions are the most common form of traumatic brain injury, with adolescents contributing to the highest incidence of concussions.3,4 An estimated 1.6 to 3.8 million people experience a sports-related concussion annually.5
Time to recovery is a clinical endpoint that matters greatly to our young, physically active patients, who are often eager to return to their daily activities as soon as possible. Guidelines frequently recommend cognitive and physical rest for 24 to 48 hours immediately following a concussion, but the use of screens during this cognitive rest period remains uncertain.6,7 International guidelines and the Centers for Disease Control and Prevention recommend symptom-limited activities—including screen time—during the initial period of a concussion.6,7 Although this gradual approach is standard of care, it has been unclear if abstaining completely from certain activities during the initial days of a concussion has any impact on recovery time.
Recent studies have examined physical activity to clarify the optimal timing of physical rest after a concussion. Among adolescents with concussions, strict rest for 5 days does not appear to improve symptoms compared with rest for 1 to 2 days.8 Additionally, physical activity within 7 days of acute head injury may help reduce symptoms and prevent postconcussive symptoms.9,10
This same level of clarity has been lacking for cognitive rest and screen time. The use of screens is a part of most patients’ daily activities, particularly among adolescents and young adults. One report found that students ages 8 to 18 years engage in approximately 7 hours of daily screen time, excluding that related to
STUDY SUMMARY
Symptom duration was significantly reduced by cutting screen time
This single-site, parallel-design, randomized clinical trial examined the effectiveness of limiting screen time exposure within the first 48 hours after a concussion in reducing the time to resolution of concussive symptoms in 125 patients. 1 Patients were included if they were 12 to 25 years old (mean age, 17 years) and presented within 24 hours of sustaining a concussion (as defined on the Acute Concussion Evaluation–Emergency Department tool) to the pediatric or adult ED at a US tertiary medical center.
Patients were randomized to either engage in screen time as tolerated or to abstain from screen time for 48 hours following their injury. Screen modalities included television, phones, video games, and computers/tablets. The Post-Concussive Symptom Scale (PCSS; 0-132) was used to characterize 22 symptoms from 0 (absent) to 6 (severe) daily for 10 days. Patients also self-reported the amount of screen time they engaged in during Days 1 to 3 of the study period and completed an activity survey on Days 4 to 10. Among the participants, 76% completed the PCSS form until symptom resolution or until Day 10 (the end of the study period).
Continue to: The primary outcome...
The primary outcome was days to resolution of concussive symptoms, defined as a PCSS score ≤ 3. The median baseline PCSS score was 21 in the screen time–permitted group and 24.5 in the screen time–abstinent group. The screen time–permitted group reported a median screen time of 630 minutes during the intervention period, compared with 130 minutes in the screen time–abstinent group, and was less likely to recover during the study period than the screen time–abstinent group (hazard ratio = 0.51; 95% CI, 0.29-0.90). The screen time–permitted group had a significantly longer median recovery time compared with the screen time–abstinent group (8.0 vs 3.5 days; P = .03).
WHAT'S NEW?
Exploring the role of screen time during the cognitive rest period
This study provides evidence supporting the recommendation that adolescent and young adult patients abstain from screen time in the first 48 hours following a concussion to decrease time to symptom resolution, thus shortening the timeline to return to their usual daily activities.
CAVEATS
Self-reporting of data may introduce bias
This study used a self-reporting method to collect data, which could have resulted in underreporting or overreporting of screen time and potentially introduced recall and reporting bias. The screen time–abstinent group did not completely abstain from all screen time, with a self-reported average of 5 to 10 minutes of daily screen time to complete the required research surveys, so it is not immediately clear what extent of abstinence vs significant screen time reduction led to the clinical endpoints observed. Furthermore, this study did not ask patients to differentiate between active screen time (eg, texting and gaming) and passive screen time (eg, watching videos), which may differentially impact symptom resolution.
CHALLENGES TO IMPLEMENTATION
Turning off the ever-present screen may present obstacles
This intervention is easy to recommend, with few barriers to implementation. It’s worth noting that screens are often used in a patient’s school or job, and 48 hours of abstinence from these activities is a difficult ask when much of our society’s education, entertainment, and productivity revolve around the use of technology. When appropriate, a shared decision-making discussion between patient and physician should center on the idea that 48 hours of screen time abstinence could be well worth the increased likelihood of total recovery at Day 10, as opposed to the risk for persistent and prolonged symptoms that interfere with the patient’s lifestyle.
ILLUSTRATIVE CASE
A 17-year-old high school football player presents to the emergency department (ED) after a helmet-to-helmet tackle in a game earlier that day. After the tackle, he experienced immediate confusion. Once he returned to his feet, he felt dizzy and nauseated and began to develop a headache. When his symptoms failed to resolve within a few hours, his mother brought him to the hospital for an evaluation. In the ED, he receives a diagnosis of concussion, and his mother asks for recommendations on how he can recover as quickly as possible.
Traumatic brain injuries account for an estimated 2.5 million ED visits annually in the United States.2 Concussions are the most common form of traumatic brain injury, with adolescents contributing to the highest incidence of concussions.3,4 An estimated 1.6 to 3.8 million people experience a sports-related concussion annually.5
Time to recovery is a clinical endpoint that matters greatly to our young, physically active patients, who are often eager to return to their daily activities as soon as possible. Guidelines frequently recommend cognitive and physical rest for 24 to 48 hours immediately following a concussion, but the use of screens during this cognitive rest period remains uncertain.6,7 International guidelines and the Centers for Disease Control and Prevention recommend symptom-limited activities—including screen time—during the initial period of a concussion.6,7 Although this gradual approach is standard of care, it has been unclear if abstaining completely from certain activities during the initial days of a concussion has any impact on recovery time.
Recent studies have examined physical activity to clarify the optimal timing of physical rest after a concussion. Among adolescents with concussions, strict rest for 5 days does not appear to improve symptoms compared with rest for 1 to 2 days.8 Additionally, physical activity within 7 days of acute head injury may help reduce symptoms and prevent postconcussive symptoms.9,10
This same level of clarity has been lacking for cognitive rest and screen time. The use of screens is a part of most patients’ daily activities, particularly among adolescents and young adults. One report found that students ages 8 to 18 years engage in approximately 7 hours of daily screen time, excluding that related to
STUDY SUMMARY
Symptom duration was significantly reduced by cutting screen time
This single-site, parallel-design, randomized clinical trial examined the effectiveness of limiting screen time exposure within the first 48 hours after a concussion in reducing the time to resolution of concussive symptoms in 125 patients. 1 Patients were included if they were 12 to 25 years old (mean age, 17 years) and presented within 24 hours of sustaining a concussion (as defined on the Acute Concussion Evaluation–Emergency Department tool) to the pediatric or adult ED at a US tertiary medical center.
Patients were randomized to either engage in screen time as tolerated or to abstain from screen time for 48 hours following their injury. Screen modalities included television, phones, video games, and computers/tablets. The Post-Concussive Symptom Scale (PCSS; 0-132) was used to characterize 22 symptoms from 0 (absent) to 6 (severe) daily for 10 days. Patients also self-reported the amount of screen time they engaged in during Days 1 to 3 of the study period and completed an activity survey on Days 4 to 10. Among the participants, 76% completed the PCSS form until symptom resolution or until Day 10 (the end of the study period).
Continue to: The primary outcome...
The primary outcome was days to resolution of concussive symptoms, defined as a PCSS score ≤ 3. The median baseline PCSS score was 21 in the screen time–permitted group and 24.5 in the screen time–abstinent group. The screen time–permitted group reported a median screen time of 630 minutes during the intervention period, compared with 130 minutes in the screen time–abstinent group, and was less likely to recover during the study period than the screen time–abstinent group (hazard ratio = 0.51; 95% CI, 0.29-0.90). The screen time–permitted group had a significantly longer median recovery time compared with the screen time–abstinent group (8.0 vs 3.5 days; P = .03).
WHAT'S NEW?
Exploring the role of screen time during the cognitive rest period
This study provides evidence supporting the recommendation that adolescent and young adult patients abstain from screen time in the first 48 hours following a concussion to decrease time to symptom resolution, thus shortening the timeline to return to their usual daily activities.
CAVEATS
Self-reporting of data may introduce bias
This study used a self-reporting method to collect data, which could have resulted in underreporting or overreporting of screen time and potentially introduced recall and reporting bias. The screen time–abstinent group did not completely abstain from all screen time, with a self-reported average of 5 to 10 minutes of daily screen time to complete the required research surveys, so it is not immediately clear what extent of abstinence vs significant screen time reduction led to the clinical endpoints observed. Furthermore, this study did not ask patients to differentiate between active screen time (eg, texting and gaming) and passive screen time (eg, watching videos), which may differentially impact symptom resolution.
CHALLENGES TO IMPLEMENTATION
Turning off the ever-present screen may present obstacles
This intervention is easy to recommend, with few barriers to implementation. It’s worth noting that screens are often used in a patient’s school or job, and 48 hours of abstinence from these activities is a difficult ask when much of our society’s education, entertainment, and productivity revolve around the use of technology. When appropriate, a shared decision-making discussion between patient and physician should center on the idea that 48 hours of screen time abstinence could be well worth the increased likelihood of total recovery at Day 10, as opposed to the risk for persistent and prolonged symptoms that interfere with the patient’s lifestyle.
1. Macnow T, Curran T, Tolliday C, et al. Effect of screen time on recovery from concussion: a randomized clinical trial. JAMA Pediatr. 2021;175:1124-1131. doi: 10.1001/jamapediat rics.2021.2782
2. Taylor CA, Bell JM, Breiding MJ, et al. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1-16. doi: 10.15585/mmwr.ss6609a1
3. Vos PE, Battistin L, Birbamer G, et al; European Federation of Neurological Societies. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207-219. doi: 10.1046/j.1468-1331.2002.00407.x
4. Zhang AL, Sing DC, Rugg CM, et al. The rise of concussions in the adolescent population. Orthop J Sports Med. 2016;4:2325967116662458. doi: 10.1177/2325967116662458
5. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709-735. doi: 10.1097/NEN.0b013e3181a9d503
6. McCrory P, Meeuwisse W, Dvorák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi: 10.1136/bjsports-2017-097699
7. Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172:e182853. doi: 10.1001/jamapediat rics.2018.2853
8. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:213-223. doi: 10.1542/peds.2014-0966
9. Grool AM, Aglipay M, Momoli F, et al; Pediatric Emergency Research Canada (PERC) Concussion Team. Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316:2504-2514. doi: 10.1001/jama.2016.17396
10. Lal A, Kolakowsky-Hayner SA, Ghajar J, et al. The effect of physical exercise after a concussion: a systematic review and meta-analysis. Am J Sports Med. 2018;46:743-752. doi: 10.1177/0363546517706137
11. Rideout V, Peebles A, Mann S, et al. The Common Sense Census: Media Use by Tweens and Teens, 2021. Common Sense Media; 2022. Accessed December 28, 2022. www.commonsensemedia.org/sites/default/files/research/report/8-18-census-integrated-report-final-web_0.pdf
1. Macnow T, Curran T, Tolliday C, et al. Effect of screen time on recovery from concussion: a randomized clinical trial. JAMA Pediatr. 2021;175:1124-1131. doi: 10.1001/jamapediat rics.2021.2782
2. Taylor CA, Bell JM, Breiding MJ, et al. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1-16. doi: 10.15585/mmwr.ss6609a1
3. Vos PE, Battistin L, Birbamer G, et al; European Federation of Neurological Societies. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207-219. doi: 10.1046/j.1468-1331.2002.00407.x
4. Zhang AL, Sing DC, Rugg CM, et al. The rise of concussions in the adolescent population. Orthop J Sports Med. 2016;4:2325967116662458. doi: 10.1177/2325967116662458
5. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709-735. doi: 10.1097/NEN.0b013e3181a9d503
6. McCrory P, Meeuwisse W, Dvorák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi: 10.1136/bjsports-2017-097699
7. Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172:e182853. doi: 10.1001/jamapediat rics.2018.2853
8. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:213-223. doi: 10.1542/peds.2014-0966
9. Grool AM, Aglipay M, Momoli F, et al; Pediatric Emergency Research Canada (PERC) Concussion Team. Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316:2504-2514. doi: 10.1001/jama.2016.17396
10. Lal A, Kolakowsky-Hayner SA, Ghajar J, et al. The effect of physical exercise after a concussion: a systematic review and meta-analysis. Am J Sports Med. 2018;46:743-752. doi: 10.1177/0363546517706137
11. Rideout V, Peebles A, Mann S, et al. The Common Sense Census: Media Use by Tweens and Teens, 2021. Common Sense Media; 2022. Accessed December 28, 2022. www.commonsensemedia.org/sites/default/files/research/report/8-18-census-integrated-report-final-web_0.pdf
PRACTICE CHANGER
Advise your teenaged and young adult patients with concussion to avoid electronic screens in the first 48 hours after a concussion to minimize time to symptom resolution.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized clinical trial.1
Macnow T, Curran T, Tolliday C, et al. Effect of screen time on recovery from concussion: a randomized clinical trial. JAMA Pediatr. 2021;175:1124-1131. doi: 10.1001/jamapediatrics.2021.2782
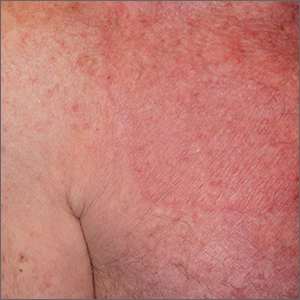
Circular patch on chest
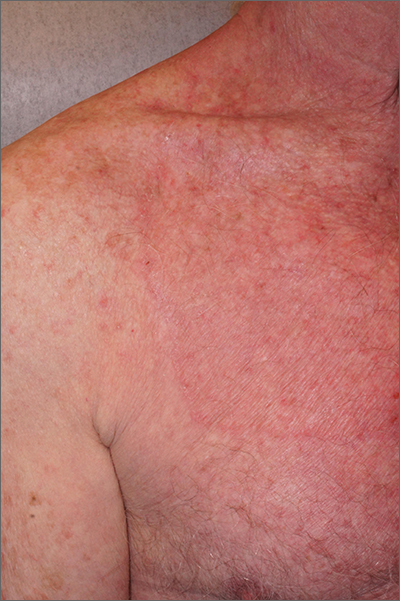
A skin scraping and potassium hydroxide (KOH) prep confirmed the presence of branching hyphae, consistent with tinea corporis. The large size of this plaque could have easily made this diagnosis more difficult. When tinea corporis is suspected, look at the edge of the plaque; there is often thin scale and sometimes small pustules corresponding to follicular involvement.
Commonly called by the misnomer “ringworm,” tinea corporis is a skin infection caused by a wide variety of dermatophytes and affects all ages, sexes, and skin types. Trichophyton, Microsporum, and Epidermophyton species are frequently isolated.1 Patients with atopic dermatitis or weakened immunity may be more susceptible to more frequent or long-lasting episodes. Diabetes may have contributed to the extent of the disease in this case.
Patients with tinea corporis present with one or several annular patches to plaques that grow in size. When the source of contagion is an animal, inflammation can be dramatic. In the case above, there was minimal to no itching and the patient didn’t notice the rash; thus, it was able to enlarge for months.
Treatment options include systemic and topical antifungal therapy. Consideration should be given to the severity of the disease and causal organism. Azoles, terbinafine, and ciclopirox are common treatment options. Topical therapy with an appropriately selected antifungal for 1 to 6 weeks, based on clinical response, is safe and effective. It is important to consider other foci of infection, including the feet and hands. More extensive disease may be treated with oral therapy such as terbinafine, fluconazole, or itraconazole.
Because of the extent of the disease and the challenge of effective coverage with topical therapy, this patient was treated with oral terbinafine 250 mg daily for 3 weeks. The plaque cleared completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi: 10.7573/dic.2020-5-6

A skin scraping and potassium hydroxide (KOH) prep confirmed the presence of branching hyphae, consistent with tinea corporis. The large size of this plaque could have easily made this diagnosis more difficult. When tinea corporis is suspected, look at the edge of the plaque; there is often thin scale and sometimes small pustules corresponding to follicular involvement.
Commonly called by the misnomer “ringworm,” tinea corporis is a skin infection caused by a wide variety of dermatophytes and affects all ages, sexes, and skin types. Trichophyton, Microsporum, and Epidermophyton species are frequently isolated.1 Patients with atopic dermatitis or weakened immunity may be more susceptible to more frequent or long-lasting episodes. Diabetes may have contributed to the extent of the disease in this case.
Patients with tinea corporis present with one or several annular patches to plaques that grow in size. When the source of contagion is an animal, inflammation can be dramatic. In the case above, there was minimal to no itching and the patient didn’t notice the rash; thus, it was able to enlarge for months.
Treatment options include systemic and topical antifungal therapy. Consideration should be given to the severity of the disease and causal organism. Azoles, terbinafine, and ciclopirox are common treatment options. Topical therapy with an appropriately selected antifungal for 1 to 6 weeks, based on clinical response, is safe and effective. It is important to consider other foci of infection, including the feet and hands. More extensive disease may be treated with oral therapy such as terbinafine, fluconazole, or itraconazole.
Because of the extent of the disease and the challenge of effective coverage with topical therapy, this patient was treated with oral terbinafine 250 mg daily for 3 weeks. The plaque cleared completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A skin scraping and potassium hydroxide (KOH) prep confirmed the presence of branching hyphae, consistent with tinea corporis. The large size of this plaque could have easily made this diagnosis more difficult. When tinea corporis is suspected, look at the edge of the plaque; there is often thin scale and sometimes small pustules corresponding to follicular involvement.
Commonly called by the misnomer “ringworm,” tinea corporis is a skin infection caused by a wide variety of dermatophytes and affects all ages, sexes, and skin types. Trichophyton, Microsporum, and Epidermophyton species are frequently isolated.1 Patients with atopic dermatitis or weakened immunity may be more susceptible to more frequent or long-lasting episodes. Diabetes may have contributed to the extent of the disease in this case.
Patients with tinea corporis present with one or several annular patches to plaques that grow in size. When the source of contagion is an animal, inflammation can be dramatic. In the case above, there was minimal to no itching and the patient didn’t notice the rash; thus, it was able to enlarge for months.
Treatment options include systemic and topical antifungal therapy. Consideration should be given to the severity of the disease and causal organism. Azoles, terbinafine, and ciclopirox are common treatment options. Topical therapy with an appropriately selected antifungal for 1 to 6 weeks, based on clinical response, is safe and effective. It is important to consider other foci of infection, including the feet and hands. More extensive disease may be treated with oral therapy such as terbinafine, fluconazole, or itraconazole.
Because of the extent of the disease and the challenge of effective coverage with topical therapy, this patient was treated with oral terbinafine 250 mg daily for 3 weeks. The plaque cleared completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi: 10.7573/dic.2020-5-6
1. Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi: 10.7573/dic.2020-5-6
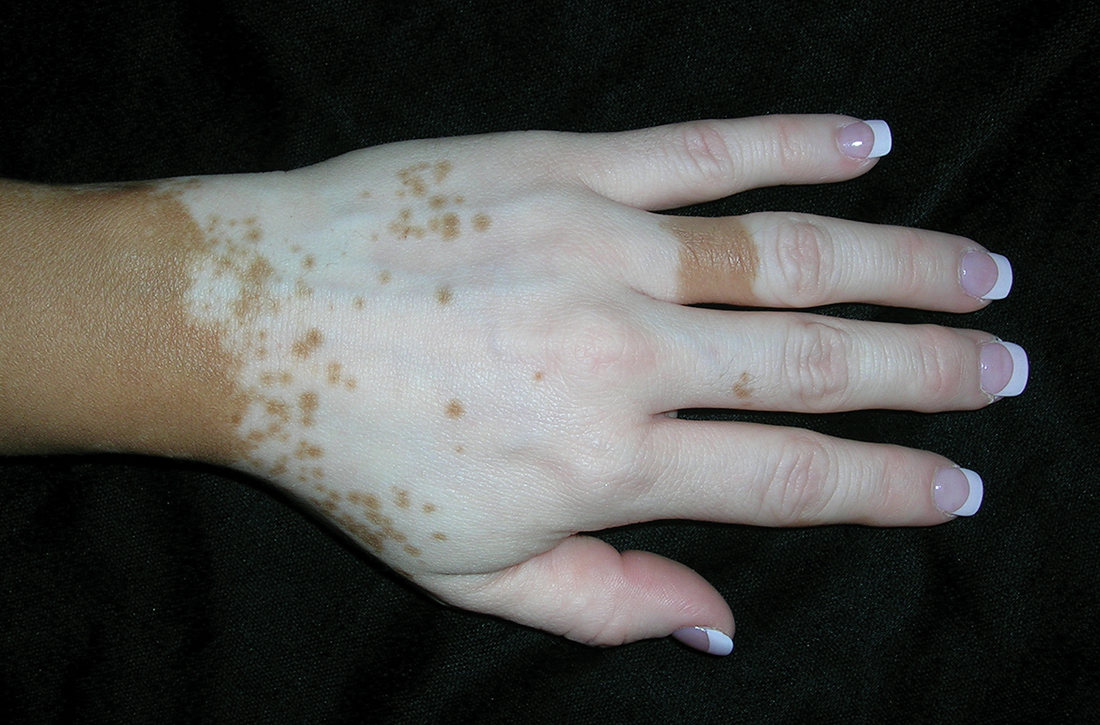
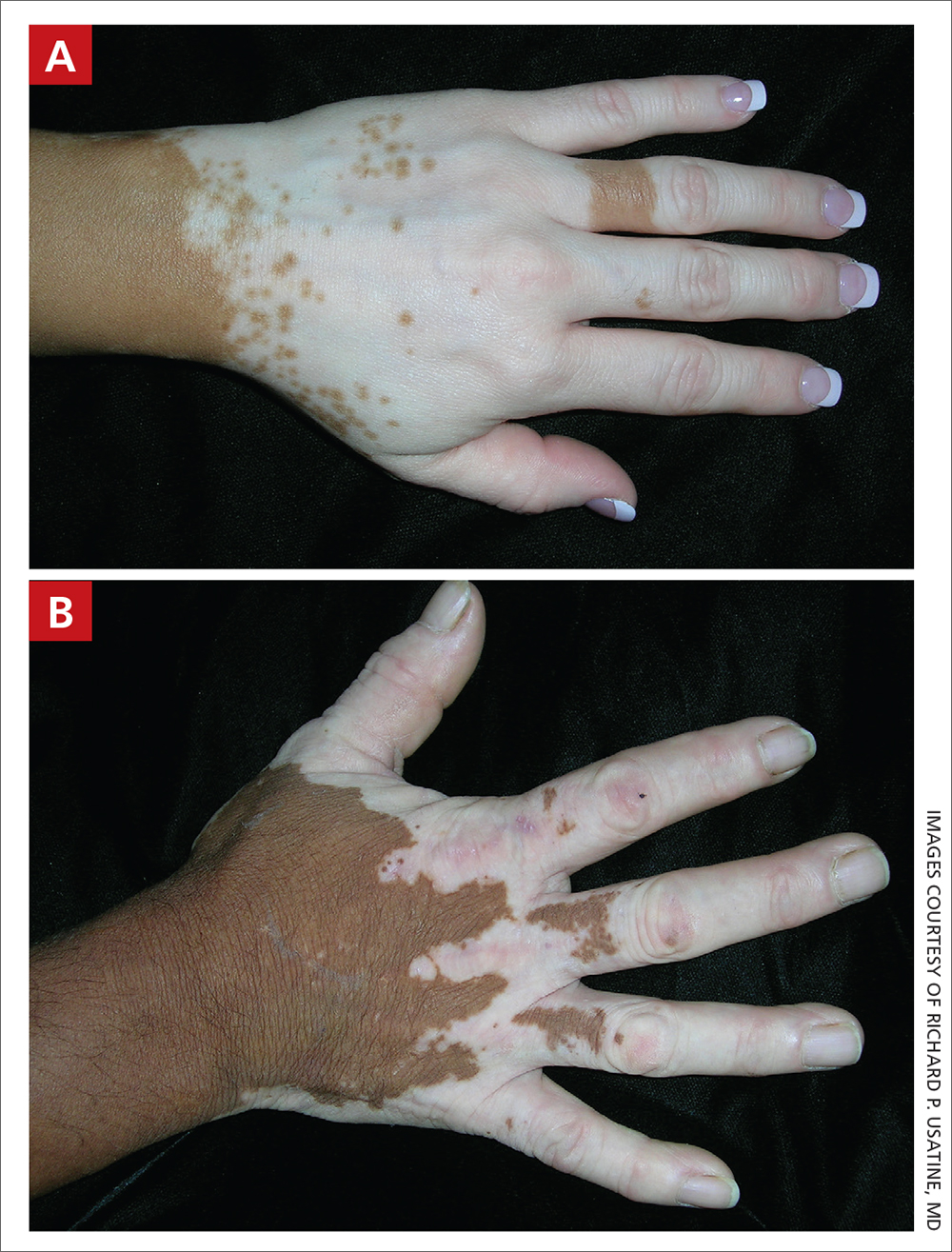
Vitiligo
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband ultraviolet B (UVB) treatment.
B Vitiligo on the hand in a young Hispanic male.
Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1
Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (FIGURES A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when making the diagnosis is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Continue to: Treatment of vitiligo...
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients ages 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase–signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
FDA approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
1. Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi: 10.1016/j.det. 2016.11.014
2. Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi: 10.1016/j.jaad.2010.11.061
3. van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi: 10.1016/j.det.2016.11.005
4. Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi: 10.1016/j.ijwd.2017.11.005
5. Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review. Am J Clin Dermatol. 2021; 22:757-774. doi: 10.1007/s40257-021-00631-6
6. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical- treatment-addressing-repigmentation-vitiligo-patients-aged- 12-and-older
7. Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color. Pediatr Dermatol. 2021;38(suppl 2):79-85. doi: 10.1111/ pde.14714
8. Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. www.drugs.com/priceguide/opzelura
9. Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
10. Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. doi: 10.25251/skin.6.3.6
11. Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi: 10.2147/CCID.S185798
12. Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. www.census.gov/library/publications/2021/demo/p60-273.html
13. Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi: 10.1002/14651858.CD003263.pub4
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband ultraviolet B (UVB) treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1
Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (FIGURES A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when making the diagnosis is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Continue to: Treatment of vitiligo...
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients ages 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase–signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
FDA approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband ultraviolet B (UVB) treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1
Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (FIGURES A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when making the diagnosis is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Continue to: Treatment of vitiligo...
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients ages 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase–signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
FDA approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
1. Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi: 10.1016/j.det. 2016.11.014
2. Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi: 10.1016/j.jaad.2010.11.061
3. van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi: 10.1016/j.det.2016.11.005
4. Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi: 10.1016/j.ijwd.2017.11.005
5. Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review. Am J Clin Dermatol. 2021; 22:757-774. doi: 10.1007/s40257-021-00631-6
6. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical- treatment-addressing-repigmentation-vitiligo-patients-aged- 12-and-older
7. Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color. Pediatr Dermatol. 2021;38(suppl 2):79-85. doi: 10.1111/ pde.14714
8. Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. www.drugs.com/priceguide/opzelura
9. Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
10. Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. doi: 10.25251/skin.6.3.6
11. Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi: 10.2147/CCID.S185798
12. Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. www.census.gov/library/publications/2021/demo/p60-273.html
13. Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi: 10.1002/14651858.CD003263.pub4
1. Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi: 10.1016/j.det. 2016.11.014
2. Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi: 10.1016/j.jaad.2010.11.061
3. van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi: 10.1016/j.det.2016.11.005
4. Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi: 10.1016/j.ijwd.2017.11.005
5. Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review. Am J Clin Dermatol. 2021; 22:757-774. doi: 10.1007/s40257-021-00631-6
6. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical- treatment-addressing-repigmentation-vitiligo-patients-aged- 12-and-older
7. Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color. Pediatr Dermatol. 2021;38(suppl 2):79-85. doi: 10.1111/ pde.14714
8. Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. www.drugs.com/priceguide/opzelura
9. Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
10. Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. doi: 10.25251/skin.6.3.6
11. Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi: 10.2147/CCID.S185798
12. Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. www.census.gov/library/publications/2021/demo/p60-273.html
13. Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi: 10.1002/14651858.CD003263.pub4
Dissociating Fibroepithelioma of Pinkus From Internal Malignancy: A Single-Center Retrospective Study
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
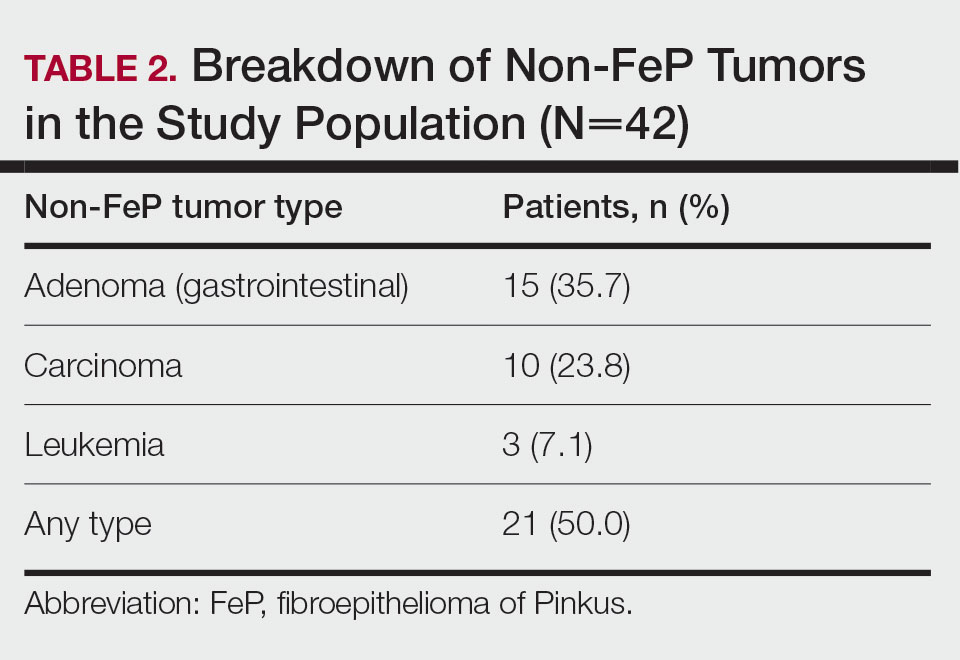
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).
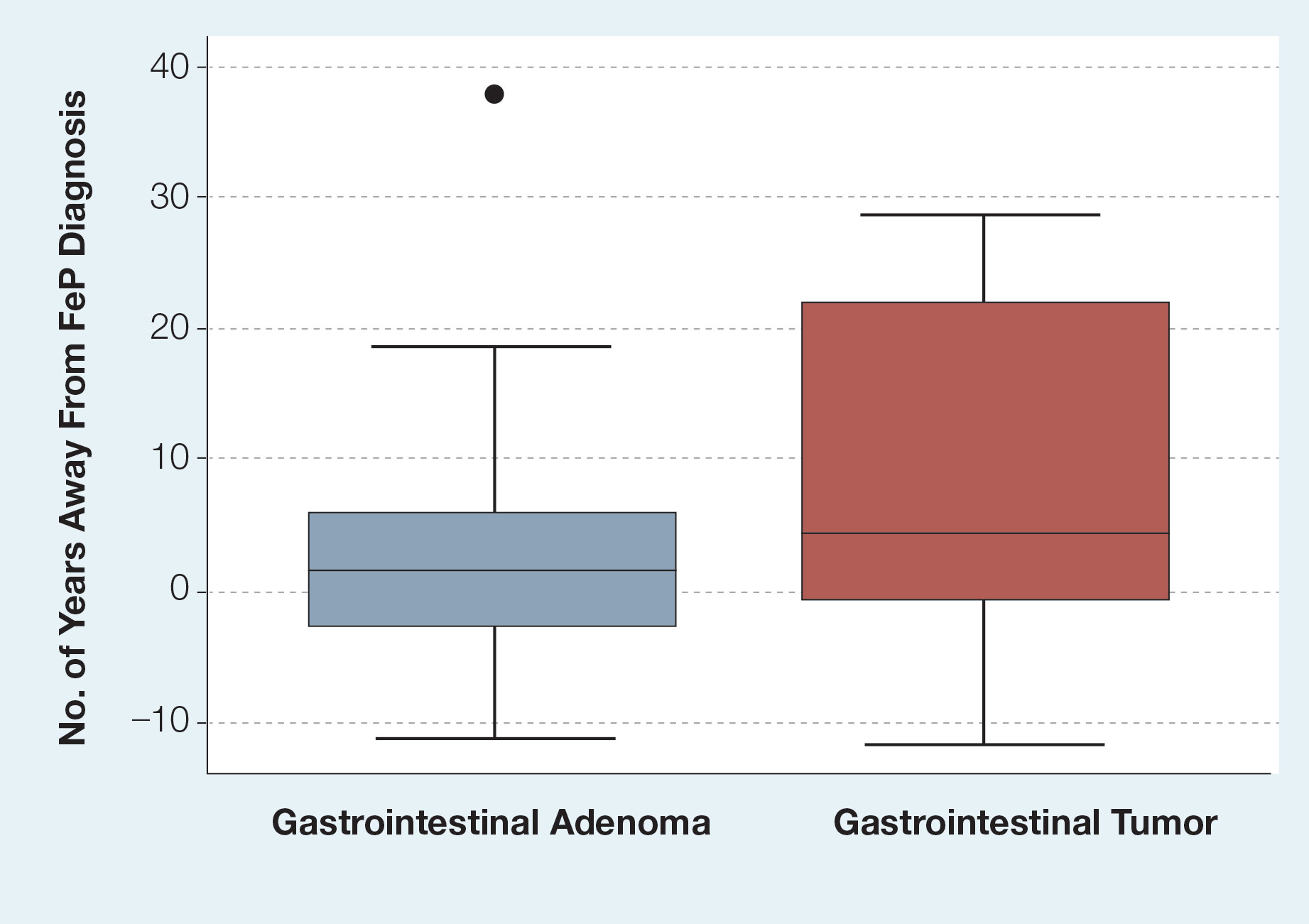
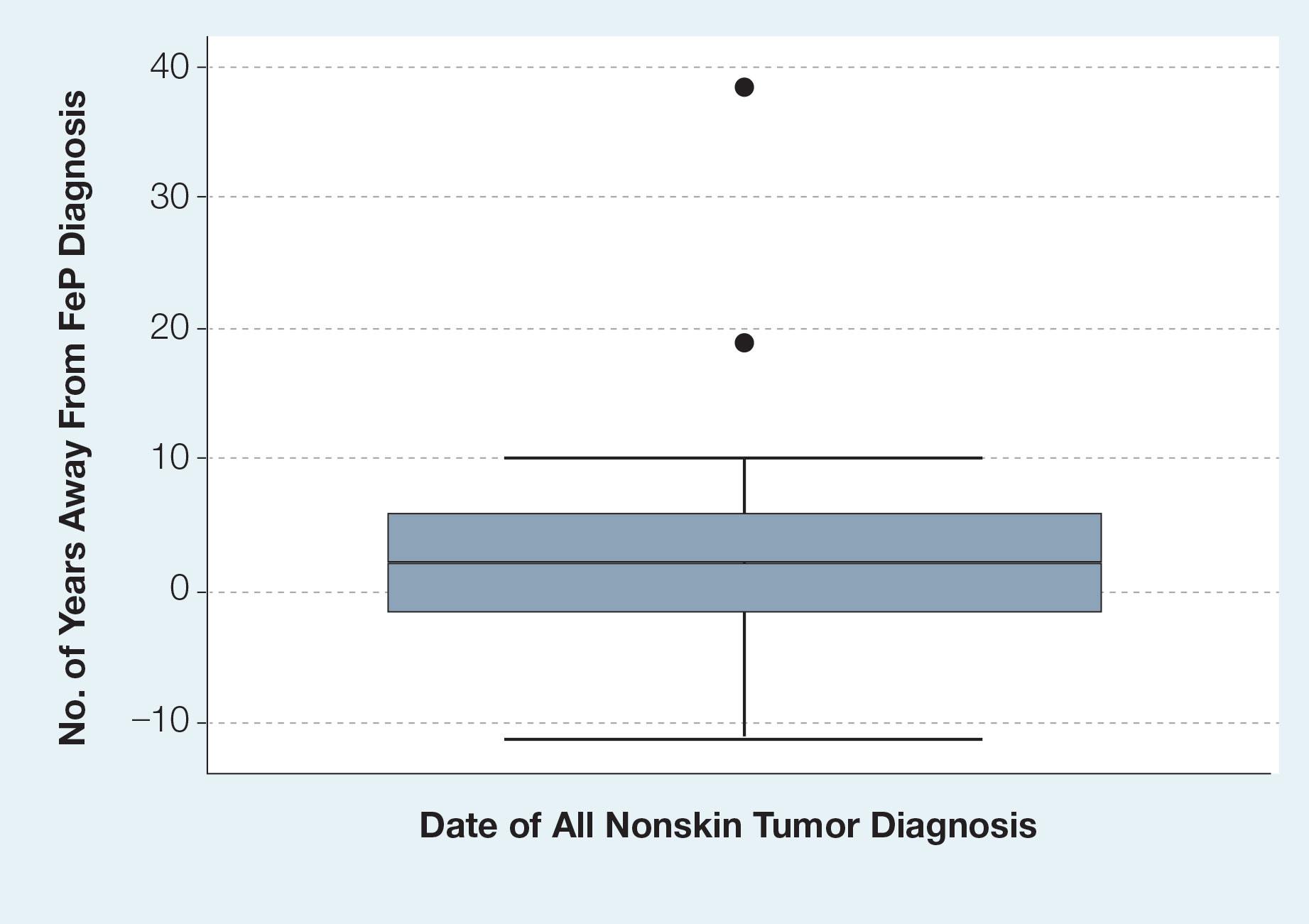
Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.
Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.
Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
PRACTICE POINTS
- Dermatologic reactions may be the initial presentation of an internal malignancy.
- Fibroepithelioma of Pinkus is considered on the spectrum between adnexal neoplasms and a nonaggressive variant of basal cell carcinoma (BCC).
- Fibroepithelioma of Pinkus should be managed similar to nonaggressive variants of BCC such as nodular BCC.
- Fibroepithelioma of Pinkus is not associated with internal malignancy.
Chronic Ulcerative Lesion
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.
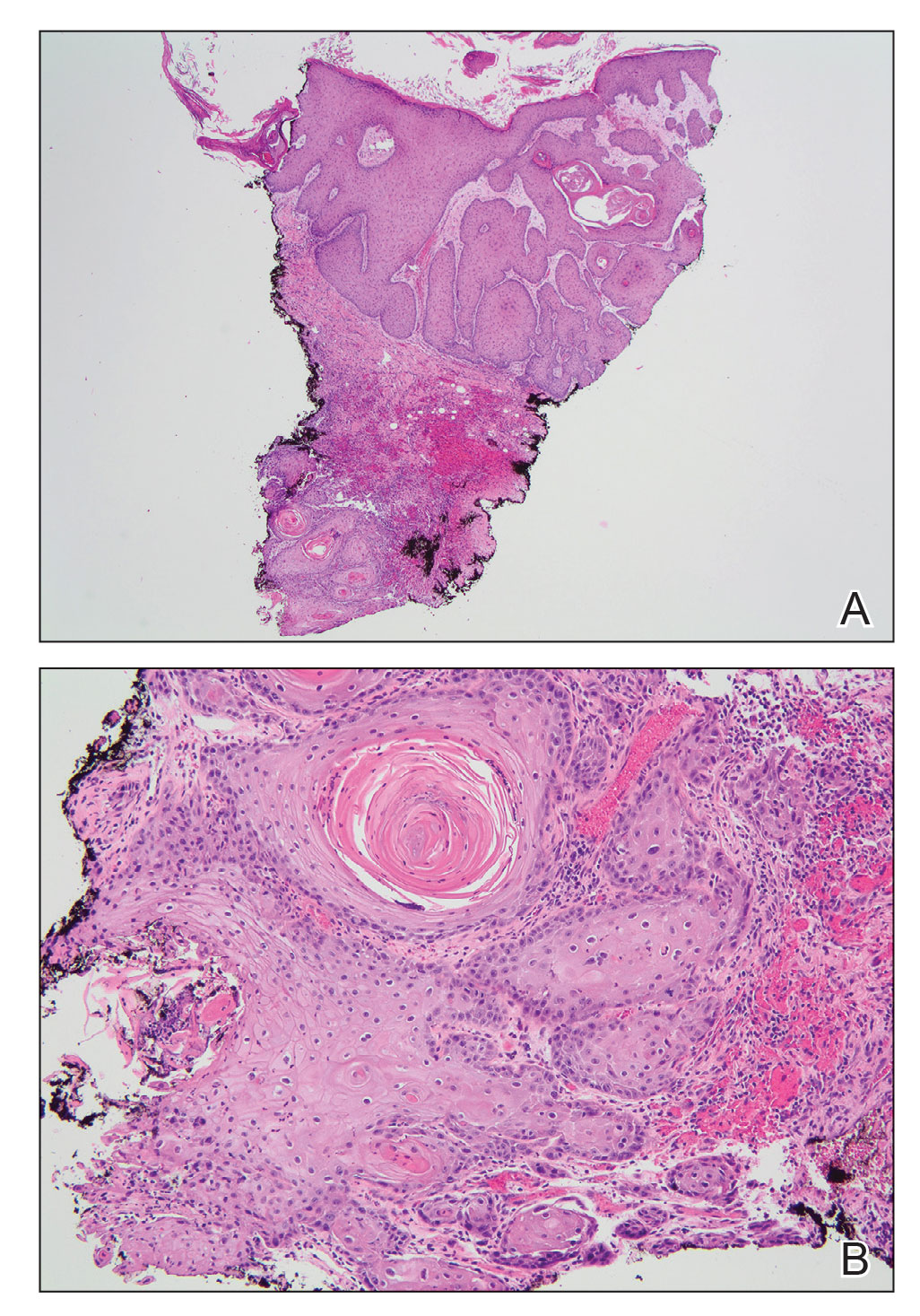
Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
A 46-year-old man with a history of a left leg burn during childhood that was unsuccessfully treated with multiple skin grafts presented as a hospital follow-up for outpatient management of an ulcer. The patient had an ulcer that gradually increased in size over 7 years. Over the course of 2 weeks prior to the hospital presentation, he noted increased pain and severe difficulty with ambulation but remained afebrile without other systemic symptoms. Prior to the outpatient follow-up, he had been admitted to the hospital where he underwent imaging, laboratory studies, and skin biopsy, as well as treatment with empiric vancomycin. Physical examination revealed a large undermined ulcer with an elevated peripheral margin and crusting on the left lower leg with surrounding chronic scarring.
Cutaneous T-Cell Lymphoma Treatment: Case Series of Combination Therapy With Intralesional Injections of 5-Fluorouracil and Topical Imiquimod
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.
Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea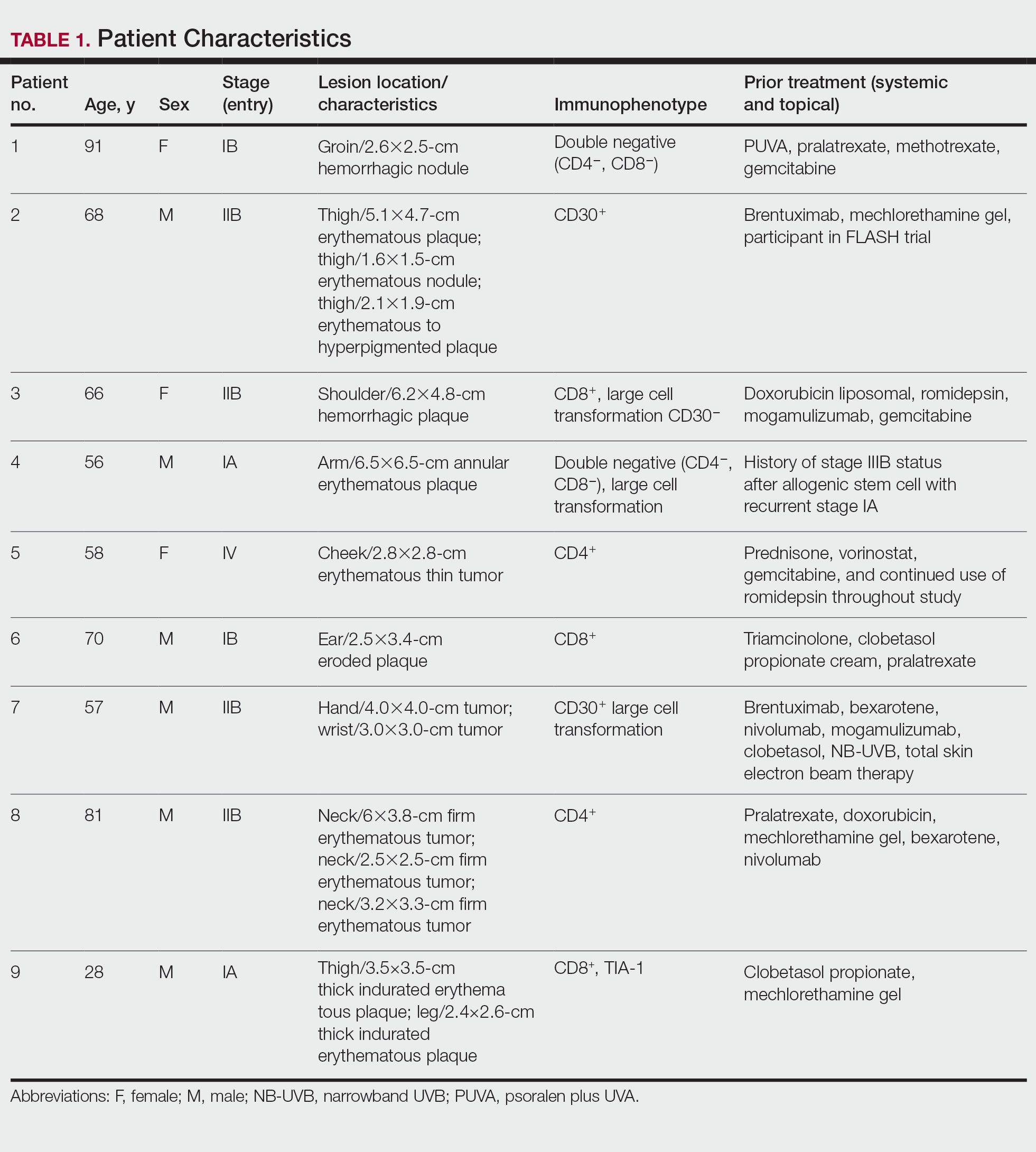
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.
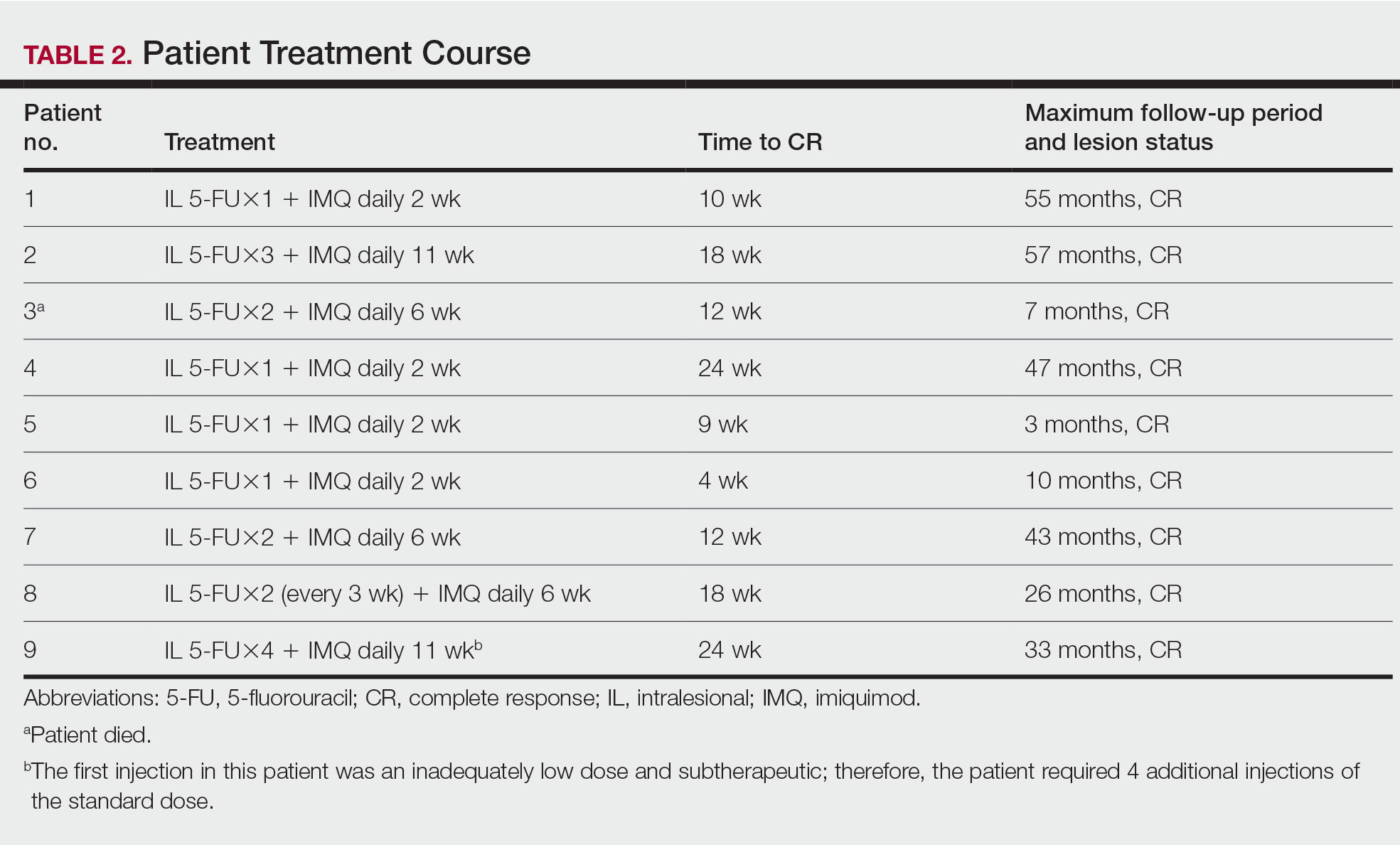
Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.
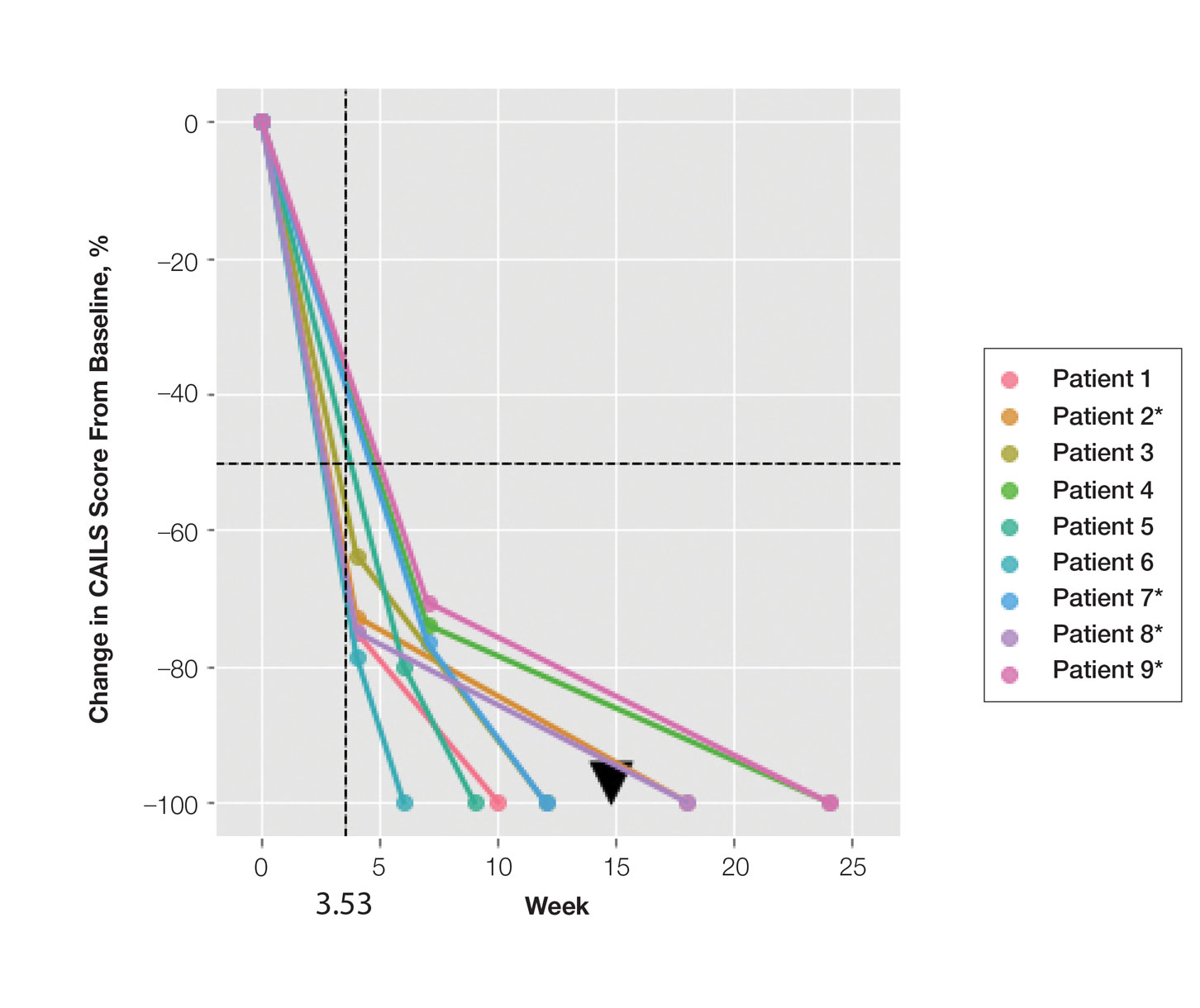
Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.
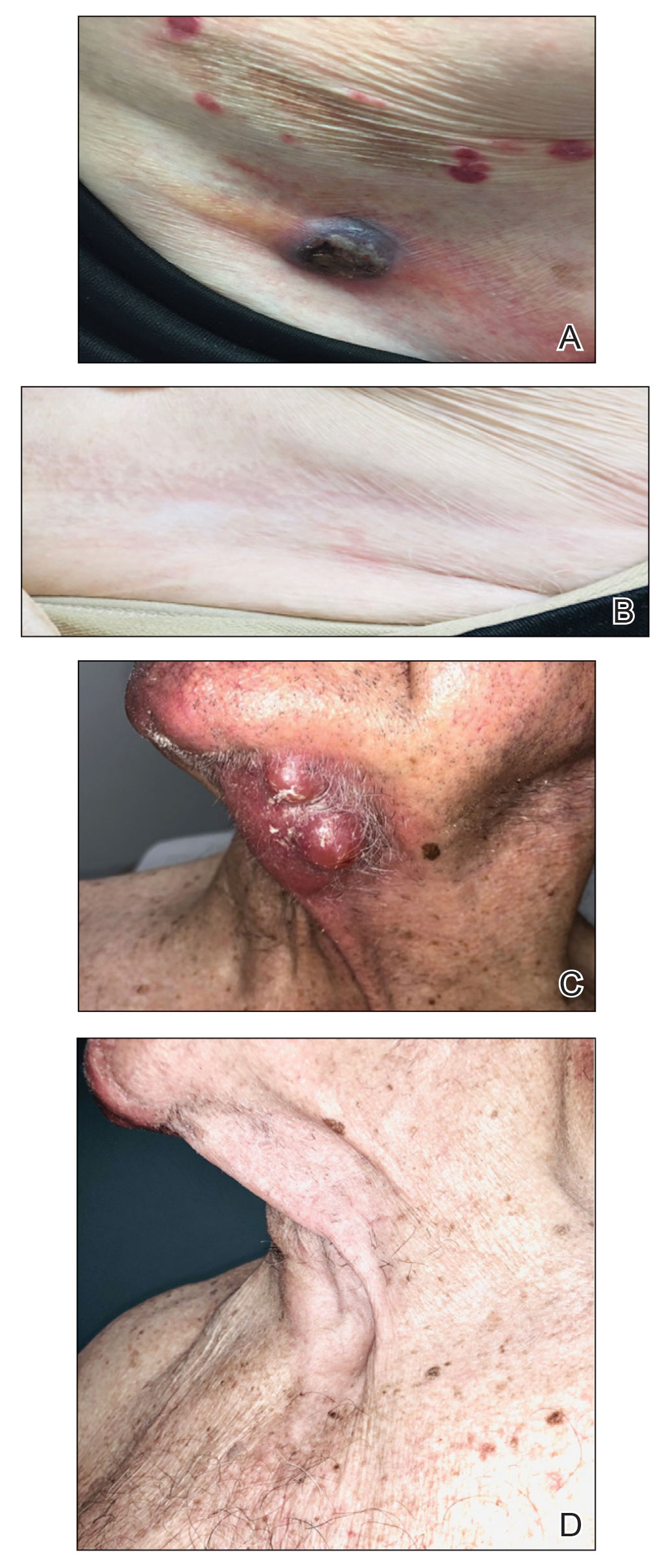
Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
PRACTICE POINTS
- Cutaneous T-cell lymphoma (CTCL) is a chronic lymphoma affecting the skin with limited durable effective skin-directed therapies.
- Combination intralesional 5-fluorouracil and topical imiquimod is a well-tolerated, fast, convenient, and durable therapy for recalcitrant thick plaques and tumors of CTCL.
- This regimen may be utilized as monotherapy or as the skin-directed component of combination therapy based on disease stage.
IL-6Ri shows the greatest benefit in improving systemic inflammation and hemoglobin in RA
Key clinical point: Continuous 6-month therapy with interleukin-6 receptor inhibitors (IL-6Ri) vs tumor necrosis factor inhibitors (TNFi) or Janus kinase inhibitors (JAKi) demonstrated greater improvements in hemoglobin and C-reactive protein (CRP) levels regardless of baseline levels in patients with rheumatoid arthritis (RA).
Major finding: Six months of continuous therapy with IL-6Ri vs TNFi and JAKi led to significantly greater improvements in hemoglobin levels (adjusted odds ratios for achieving normal hemoglobin levels 3.15 and 3.85, respectively; both P < .001) and greater reductions in CRP levels (P < .01) regardless of baseline levels.
Study details: The data come from an analysis of 2772 patients with RA who received continuous TNFi, IL-6Ri, or JAKi treatment for ≥6 months.
Disclosures: This study was funded by Sanofi, and the RA registry was sponsored by CorEvitas, LLC. Six authors declared being current or former employees of, consultants for, or holding shares or stocks or stock options in Sanofi or CorEvitas LLC.
Source: Padula AS et al. The effect of targeted rheumatoid arthritis therapeutics on systemic inflammation and anemia: Analysis of data from the CorEvitas RA registry. Arthritis Res Ther. 2022;24:276 (Dec 21). Doi: 10.1186/s13075-022-02955-y
Key clinical point: Continuous 6-month therapy with interleukin-6 receptor inhibitors (IL-6Ri) vs tumor necrosis factor inhibitors (TNFi) or Janus kinase inhibitors (JAKi) demonstrated greater improvements in hemoglobin and C-reactive protein (CRP) levels regardless of baseline levels in patients with rheumatoid arthritis (RA).
Major finding: Six months of continuous therapy with IL-6Ri vs TNFi and JAKi led to significantly greater improvements in hemoglobin levels (adjusted odds ratios for achieving normal hemoglobin levels 3.15 and 3.85, respectively; both P < .001) and greater reductions in CRP levels (P < .01) regardless of baseline levels.
Study details: The data come from an analysis of 2772 patients with RA who received continuous TNFi, IL-6Ri, or JAKi treatment for ≥6 months.
Disclosures: This study was funded by Sanofi, and the RA registry was sponsored by CorEvitas, LLC. Six authors declared being current or former employees of, consultants for, or holding shares or stocks or stock options in Sanofi or CorEvitas LLC.
Source: Padula AS et al. The effect of targeted rheumatoid arthritis therapeutics on systemic inflammation and anemia: Analysis of data from the CorEvitas RA registry. Arthritis Res Ther. 2022;24:276 (Dec 21). Doi: 10.1186/s13075-022-02955-y
Key clinical point: Continuous 6-month therapy with interleukin-6 receptor inhibitors (IL-6Ri) vs tumor necrosis factor inhibitors (TNFi) or Janus kinase inhibitors (JAKi) demonstrated greater improvements in hemoglobin and C-reactive protein (CRP) levels regardless of baseline levels in patients with rheumatoid arthritis (RA).
Major finding: Six months of continuous therapy with IL-6Ri vs TNFi and JAKi led to significantly greater improvements in hemoglobin levels (adjusted odds ratios for achieving normal hemoglobin levels 3.15 and 3.85, respectively; both P < .001) and greater reductions in CRP levels (P < .01) regardless of baseline levels.
Study details: The data come from an analysis of 2772 patients with RA who received continuous TNFi, IL-6Ri, or JAKi treatment for ≥6 months.
Disclosures: This study was funded by Sanofi, and the RA registry was sponsored by CorEvitas, LLC. Six authors declared being current or former employees of, consultants for, or holding shares or stocks or stock options in Sanofi or CorEvitas LLC.
Source: Padula AS et al. The effect of targeted rheumatoid arthritis therapeutics on systemic inflammation and anemia: Analysis of data from the CorEvitas RA registry. Arthritis Res Ther. 2022;24:276 (Dec 21). Doi: 10.1186/s13075-022-02955-y
Improved efficacy with subcutaneous vs intravenous infliximab in RA
Key clinical point: Subcutaneous vs intravenous infliximab demonstrated improved efficacy in patients with rheumatoid arthritis (RA) who were inadequate responders to methotrexate (methotrexate-IR).
Major finding: At week 30, subcutaneous vs intravenous infliximab led to significantly lower Disease Activity Scores in 28 joints-C-reactive protein (DAS28-CRP; mean 3.07 vs 3.58; P = .0001) and significantly higher proportion of patients achieving DAS28-CRP low disease activity and remission (53.3% vs 38.5%; P = .0062), with no significant between-group difference after the switch to subcutaneous infliximab.
Study details: This post hoc analysis of a phase 3 trial included 339 patients with active RA who were methotrexate-IR and were randomly assigned to receive subcutaneous or intravenous infliximab; patients assigned to receive intravenous infliximab switched to subcutaneous infliximab from week 30 to 54.
Disclosures: This study was supported by Celltrion Healthcare Co., Ltd. Five authors declared being full-time employees of or receiving personal fees for advisory board and speaker’s bureau and research grants from Celltrion outside this work. Several authors reported ties with other various sources.
Source: Constantin A et al. Efficacy of subcutaneous vs intravenous infliximab in rheumatoid arthritis: A post-hoc analysis of a randomised phase III trial. Rheumatology (Oxford). 2022 (Dec 19). Doi: 10.1093/rheumatology/keac689
Key clinical point: Subcutaneous vs intravenous infliximab demonstrated improved efficacy in patients with rheumatoid arthritis (RA) who were inadequate responders to methotrexate (methotrexate-IR).
Major finding: At week 30, subcutaneous vs intravenous infliximab led to significantly lower Disease Activity Scores in 28 joints-C-reactive protein (DAS28-CRP; mean 3.07 vs 3.58; P = .0001) and significantly higher proportion of patients achieving DAS28-CRP low disease activity and remission (53.3% vs 38.5%; P = .0062), with no significant between-group difference after the switch to subcutaneous infliximab.
Study details: This post hoc analysis of a phase 3 trial included 339 patients with active RA who were methotrexate-IR and were randomly assigned to receive subcutaneous or intravenous infliximab; patients assigned to receive intravenous infliximab switched to subcutaneous infliximab from week 30 to 54.
Disclosures: This study was supported by Celltrion Healthcare Co., Ltd. Five authors declared being full-time employees of or receiving personal fees for advisory board and speaker’s bureau and research grants from Celltrion outside this work. Several authors reported ties with other various sources.
Source: Constantin A et al. Efficacy of subcutaneous vs intravenous infliximab in rheumatoid arthritis: A post-hoc analysis of a randomised phase III trial. Rheumatology (Oxford). 2022 (Dec 19). Doi: 10.1093/rheumatology/keac689
Key clinical point: Subcutaneous vs intravenous infliximab demonstrated improved efficacy in patients with rheumatoid arthritis (RA) who were inadequate responders to methotrexate (methotrexate-IR).
Major finding: At week 30, subcutaneous vs intravenous infliximab led to significantly lower Disease Activity Scores in 28 joints-C-reactive protein (DAS28-CRP; mean 3.07 vs 3.58; P = .0001) and significantly higher proportion of patients achieving DAS28-CRP low disease activity and remission (53.3% vs 38.5%; P = .0062), with no significant between-group difference after the switch to subcutaneous infliximab.
Study details: This post hoc analysis of a phase 3 trial included 339 patients with active RA who were methotrexate-IR and were randomly assigned to receive subcutaneous or intravenous infliximab; patients assigned to receive intravenous infliximab switched to subcutaneous infliximab from week 30 to 54.
Disclosures: This study was supported by Celltrion Healthcare Co., Ltd. Five authors declared being full-time employees of or receiving personal fees for advisory board and speaker’s bureau and research grants from Celltrion outside this work. Several authors reported ties with other various sources.
Source: Constantin A et al. Efficacy of subcutaneous vs intravenous infliximab in rheumatoid arthritis: A post-hoc analysis of a randomised phase III trial. Rheumatology (Oxford). 2022 (Dec 19). Doi: 10.1093/rheumatology/keac689
TNFi raises the risk for septic arthritis in seropositive RA
Key clinical point: Tumor necrosis factor inhibitors (TNFi) increased the risk for septic arthritis in patients with seropositive rheumatoid arthritis (RA), with higher incidences within 1 year of initiating TNFi.
Major finding: Patients with seropositive RA treated with infliximab (adjusted hazard ratio [aHR] 2.37), etanercept (aHR 1.82), or adalimumab/golimumab (aHR 1.82; all P < .01) were prone to develop septic arthritis, with the incidence being higher within 1 year of initiating TNFi (incidence rate/1000 person-year 25.51).
Study details: This retrospective study included 145,129 patients with new-onset seropositive RA or ankylosing spondylitis, of which 1170 patients developed septic arthritis.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Kim HW et al. Incidence of septic arthritis in patients with ankylosing spondylitis and seropositive rheumatoid arthritis following TNF-inhibitor therapy. Rheumatology (Oxford). 2022 (Dec 23). Doi: 10.1093/rheumatology/keac721
Key clinical point: Tumor necrosis factor inhibitors (TNFi) increased the risk for septic arthritis in patients with seropositive rheumatoid arthritis (RA), with higher incidences within 1 year of initiating TNFi.
Major finding: Patients with seropositive RA treated with infliximab (adjusted hazard ratio [aHR] 2.37), etanercept (aHR 1.82), or adalimumab/golimumab (aHR 1.82; all P < .01) were prone to develop septic arthritis, with the incidence being higher within 1 year of initiating TNFi (incidence rate/1000 person-year 25.51).
Study details: This retrospective study included 145,129 patients with new-onset seropositive RA or ankylosing spondylitis, of which 1170 patients developed septic arthritis.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Kim HW et al. Incidence of septic arthritis in patients with ankylosing spondylitis and seropositive rheumatoid arthritis following TNF-inhibitor therapy. Rheumatology (Oxford). 2022 (Dec 23). Doi: 10.1093/rheumatology/keac721
Key clinical point: Tumor necrosis factor inhibitors (TNFi) increased the risk for septic arthritis in patients with seropositive rheumatoid arthritis (RA), with higher incidences within 1 year of initiating TNFi.
Major finding: Patients with seropositive RA treated with infliximab (adjusted hazard ratio [aHR] 2.37), etanercept (aHR 1.82), or adalimumab/golimumab (aHR 1.82; all P < .01) were prone to develop septic arthritis, with the incidence being higher within 1 year of initiating TNFi (incidence rate/1000 person-year 25.51).
Study details: This retrospective study included 145,129 patients with new-onset seropositive RA or ankylosing spondylitis, of which 1170 patients developed septic arthritis.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Kim HW et al. Incidence of septic arthritis in patients with ankylosing spondylitis and seropositive rheumatoid arthritis following TNF-inhibitor therapy. Rheumatology (Oxford). 2022 (Dec 23). Doi: 10.1093/rheumatology/keac721
Frequent joint inflammation increases local joint damage progression in early RA
Key clinical point: Cumulative local joint inflammation over time was significantly associated with radiographic joint damage progression in the same joint in patients with early rheumatoid arthritis (RA) who were treated to a target disease activity score (DAS) of ≤2.4 for 10 years.
Major finding: Cumulative joint swelling was positively associated with local joint damage progression in the same joint (β 0.14; 95% CI 0.13-0.15). Each additional visit for joint swelling increased the joint damage score by a 0.13 unit and frequency of joint swelling in same vs other joints better predicted local joint damage progression (P < .001).
Study details: This post hoc analysis of the BeSt study included 473 patients with early RA who were randomly assigned to receive sequential monotherapy, step-up combination therapy, or initial combination therapy with methotrexate with or without sulfasalazine+prednisone or infliximab, with treatment intensification every 3 months until DAS ≤2.4 was achieved.
Disclosures: The BeSt study received funding from the Dutch College of Health Insurances and others. No competing interests were declared.
Source: Heckert SL et al. Frequency of joint inflammation is associated with local joint damage progression in rheumatoid arthritis despite long-term targeted treatment. RMD Open. 2023;9(1):e002552 (Jan 6). Doi: 10.1136/rmdopen-2022-002552
Key clinical point: Cumulative local joint inflammation over time was significantly associated with radiographic joint damage progression in the same joint in patients with early rheumatoid arthritis (RA) who were treated to a target disease activity score (DAS) of ≤2.4 for 10 years.
Major finding: Cumulative joint swelling was positively associated with local joint damage progression in the same joint (β 0.14; 95% CI 0.13-0.15). Each additional visit for joint swelling increased the joint damage score by a 0.13 unit and frequency of joint swelling in same vs other joints better predicted local joint damage progression (P < .001).
Study details: This post hoc analysis of the BeSt study included 473 patients with early RA who were randomly assigned to receive sequential monotherapy, step-up combination therapy, or initial combination therapy with methotrexate with or without sulfasalazine+prednisone or infliximab, with treatment intensification every 3 months until DAS ≤2.4 was achieved.
Disclosures: The BeSt study received funding from the Dutch College of Health Insurances and others. No competing interests were declared.
Source: Heckert SL et al. Frequency of joint inflammation is associated with local joint damage progression in rheumatoid arthritis despite long-term targeted treatment. RMD Open. 2023;9(1):e002552 (Jan 6). Doi: 10.1136/rmdopen-2022-002552
Key clinical point: Cumulative local joint inflammation over time was significantly associated with radiographic joint damage progression in the same joint in patients with early rheumatoid arthritis (RA) who were treated to a target disease activity score (DAS) of ≤2.4 for 10 years.
Major finding: Cumulative joint swelling was positively associated with local joint damage progression in the same joint (β 0.14; 95% CI 0.13-0.15). Each additional visit for joint swelling increased the joint damage score by a 0.13 unit and frequency of joint swelling in same vs other joints better predicted local joint damage progression (P < .001).
Study details: This post hoc analysis of the BeSt study included 473 patients with early RA who were randomly assigned to receive sequential monotherapy, step-up combination therapy, or initial combination therapy with methotrexate with or without sulfasalazine+prednisone or infliximab, with treatment intensification every 3 months until DAS ≤2.4 was achieved.
Disclosures: The BeSt study received funding from the Dutch College of Health Insurances and others. No competing interests were declared.
Source: Heckert SL et al. Frequency of joint inflammation is associated with local joint damage progression in rheumatoid arthritis despite long-term targeted treatment. RMD Open. 2023;9(1):e002552 (Jan 6). Doi: 10.1136/rmdopen-2022-002552