User login
Adding ET to dual anti-HER2 targeted therapy beneficial in HER2+/HR+ metastatic BC
Key clinical point: In patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC), adding endocrine therapy (ET) to dual anti-HER2 targeted therapy after chemotherapy improved progression-free survival (PFS) without increasing the rate of adverse events.
Major finding: There was a significant improvement in 5-year PFS with vs without the addition of ET (hazard ratio 0.59; P = .031). Nausea and vomiting were more common in patients who did not vs did receive ET (21% vs 7%; P = .010).
Study details: This study analyzed the real-world data of 147 patients with HER2+/HR+ metastatic BC from a prospective registry who received first-line chemotherapy plus trastuzumab and pertuzumab with (n = 91) or without (n = 56) concurrent ET.
Disclosures: This study did not receive any funding. Some authors declared serving on the advisory board for or receiving research funding, speaker honoraria, or travel grants from several sources.
Source: Loft M et al. Addition of endocrine therapy to dual anti-HER2 targeted therapy in initial treatment of HER2 + /HR + metastatic breast cancer. Breast Cancer Res Treat. 2023;198:67-74 (Jan 9). Doi: 10.1007/s10549-022-06856-1
Key clinical point: In patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC), adding endocrine therapy (ET) to dual anti-HER2 targeted therapy after chemotherapy improved progression-free survival (PFS) without increasing the rate of adverse events.
Major finding: There was a significant improvement in 5-year PFS with vs without the addition of ET (hazard ratio 0.59; P = .031). Nausea and vomiting were more common in patients who did not vs did receive ET (21% vs 7%; P = .010).
Study details: This study analyzed the real-world data of 147 patients with HER2+/HR+ metastatic BC from a prospective registry who received first-line chemotherapy plus trastuzumab and pertuzumab with (n = 91) or without (n = 56) concurrent ET.
Disclosures: This study did not receive any funding. Some authors declared serving on the advisory board for or receiving research funding, speaker honoraria, or travel grants from several sources.
Source: Loft M et al. Addition of endocrine therapy to dual anti-HER2 targeted therapy in initial treatment of HER2 + /HR + metastatic breast cancer. Breast Cancer Res Treat. 2023;198:67-74 (Jan 9). Doi: 10.1007/s10549-022-06856-1
Key clinical point: In patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC), adding endocrine therapy (ET) to dual anti-HER2 targeted therapy after chemotherapy improved progression-free survival (PFS) without increasing the rate of adverse events.
Major finding: There was a significant improvement in 5-year PFS with vs without the addition of ET (hazard ratio 0.59; P = .031). Nausea and vomiting were more common in patients who did not vs did receive ET (21% vs 7%; P = .010).
Study details: This study analyzed the real-world data of 147 patients with HER2+/HR+ metastatic BC from a prospective registry who received first-line chemotherapy plus trastuzumab and pertuzumab with (n = 91) or without (n = 56) concurrent ET.
Disclosures: This study did not receive any funding. Some authors declared serving on the advisory board for or receiving research funding, speaker honoraria, or travel grants from several sources.
Source: Loft M et al. Addition of endocrine therapy to dual anti-HER2 targeted therapy in initial treatment of HER2 + /HR + metastatic breast cancer. Breast Cancer Res Treat. 2023;198:67-74 (Jan 9). Doi: 10.1007/s10549-022-06856-1
SPIO can help avoid upfront sentinel lymph node dissection in ductal carcinoma in situ of the breast
Key clinical point: Marking sentinel lymph nodes (SLN) with superparamagnetic iron oxide (SPIO) nanoparticles led to a substantial proportion of patients with ductal carcinoma in situ (DCIS) of the breast avoiding an upfront SLN dissection (SLND).
Major finding: Upfront SLND could be avoided in 78.3% of patients after marking SLN with SPIO nanoparticles. Among patients receiving delayed SLND, the detection rate was significantly better with SPIO vs radioisotope (99Tc), both with (98.2% vs 63.6%) and without the concomitant use of blue dye (92.7% vs 50.9%; both P < .001).
Study details: Findings are from a prospective, multicenter cohort study including 254 patients with DCIS.
Disclosures: This study was funded by the Uppsala University. One author declared serving on an advisory board and receiving institutional grants and speaker honoraria from several sources.
Source: Karakatsanis A et al. Delayed sentinel lymph node dissection in patients with a preoperative diagnosis of ductal cancer in situ by preoperative injection with superparamagnetic iron oxide (SPIO) nanoparticles: The SentiNot study. Ann Surg Oncol. 2023 (Jan 31). Doi: 10.1245/s10434-022-13064-0
Key clinical point: Marking sentinel lymph nodes (SLN) with superparamagnetic iron oxide (SPIO) nanoparticles led to a substantial proportion of patients with ductal carcinoma in situ (DCIS) of the breast avoiding an upfront SLN dissection (SLND).
Major finding: Upfront SLND could be avoided in 78.3% of patients after marking SLN with SPIO nanoparticles. Among patients receiving delayed SLND, the detection rate was significantly better with SPIO vs radioisotope (99Tc), both with (98.2% vs 63.6%) and without the concomitant use of blue dye (92.7% vs 50.9%; both P < .001).
Study details: Findings are from a prospective, multicenter cohort study including 254 patients with DCIS.
Disclosures: This study was funded by the Uppsala University. One author declared serving on an advisory board and receiving institutional grants and speaker honoraria from several sources.
Source: Karakatsanis A et al. Delayed sentinel lymph node dissection in patients with a preoperative diagnosis of ductal cancer in situ by preoperative injection with superparamagnetic iron oxide (SPIO) nanoparticles: The SentiNot study. Ann Surg Oncol. 2023 (Jan 31). Doi: 10.1245/s10434-022-13064-0
Key clinical point: Marking sentinel lymph nodes (SLN) with superparamagnetic iron oxide (SPIO) nanoparticles led to a substantial proportion of patients with ductal carcinoma in situ (DCIS) of the breast avoiding an upfront SLN dissection (SLND).
Major finding: Upfront SLND could be avoided in 78.3% of patients after marking SLN with SPIO nanoparticles. Among patients receiving delayed SLND, the detection rate was significantly better with SPIO vs radioisotope (99Tc), both with (98.2% vs 63.6%) and without the concomitant use of blue dye (92.7% vs 50.9%; both P < .001).
Study details: Findings are from a prospective, multicenter cohort study including 254 patients with DCIS.
Disclosures: This study was funded by the Uppsala University. One author declared serving on an advisory board and receiving institutional grants and speaker honoraria from several sources.
Source: Karakatsanis A et al. Delayed sentinel lymph node dissection in patients with a preoperative diagnosis of ductal cancer in situ by preoperative injection with superparamagnetic iron oxide (SPIO) nanoparticles: The SentiNot study. Ann Surg Oncol. 2023 (Jan 31). Doi: 10.1245/s10434-022-13064-0
Contralateral prophylactic mastectomy offers no survival advantage in TNBC
Key clinical point: Contralateral prophylactic mastectomy (CPM) did not offer any survival benefit to patients with triple-negative breast cancer (TNBC) irrespective of the presence of BRCA mutations.
Major finding: The 5-year overall survival did not significantly improve with vs without CPM in the entire population of patients with TNBC (P = .05) and in the subgroups of patients with (P = .35) and without (P = .12) BRCA mutations.
Study details: Findings are from a multi-institutional database study including 796 patients with TNBC, of which 15.5% of patients underwent CPM.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Fasano GA et al. Survival outcomes in women with unilateral, triple-negative, breast cancer correlated with contralateral prophylactic mastectomy. Ann Surg Oncol. 2023 (Jan 21). Doi: 10.1245/s10434-022-13056-0
Key clinical point: Contralateral prophylactic mastectomy (CPM) did not offer any survival benefit to patients with triple-negative breast cancer (TNBC) irrespective of the presence of BRCA mutations.
Major finding: The 5-year overall survival did not significantly improve with vs without CPM in the entire population of patients with TNBC (P = .05) and in the subgroups of patients with (P = .35) and without (P = .12) BRCA mutations.
Study details: Findings are from a multi-institutional database study including 796 patients with TNBC, of which 15.5% of patients underwent CPM.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Fasano GA et al. Survival outcomes in women with unilateral, triple-negative, breast cancer correlated with contralateral prophylactic mastectomy. Ann Surg Oncol. 2023 (Jan 21). Doi: 10.1245/s10434-022-13056-0
Key clinical point: Contralateral prophylactic mastectomy (CPM) did not offer any survival benefit to patients with triple-negative breast cancer (TNBC) irrespective of the presence of BRCA mutations.
Major finding: The 5-year overall survival did not significantly improve with vs without CPM in the entire population of patients with TNBC (P = .05) and in the subgroups of patients with (P = .35) and without (P = .12) BRCA mutations.
Study details: Findings are from a multi-institutional database study including 796 patients with TNBC, of which 15.5% of patients underwent CPM.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Fasano GA et al. Survival outcomes in women with unilateral, triple-negative, breast cancer correlated with contralateral prophylactic mastectomy. Ann Surg Oncol. 2023 (Jan 21). Doi: 10.1245/s10434-022-13056-0
Adjuvant AI yields better survival outcomes than tamoxifen or tamoxifen+AI in HR+/HER2+ BC
Key clinical point: Adjuvant endocrine therapy (ET) with an aromatase inhibitor (AI) was associated with better disease-free survival (DFS) than tamoxifen-only or tamoxifen+AI in patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor2-positive (HER2+) breast cancer (BC).
Major finding: Adjuvant ET with tamoxifen or tamoxifen+AI was associated with significantly worse DFS rates than AI in the entire population of women with HR+/HER2+ BC (hazard ratio 1.64; P = .025), with gonadotropin-releasing hormone being associated with improved DFS rates in premenopausal patients aged ≤45 years (hazard ratio 0.41; P = .023).
Study details: Findings are from a post hoc analysis of the ShortHER trial including 784 patients with HR+/HER2+ early BC who received adjuvant anthracycline/taxane-based chemotherapy plus trastuzumab.
Disclosures: This study was supported by Agenzia Italiana del Farmaco and other sources. Some authors declared receiving personal fees from several sources.
Source: Dieci MV et al. Type of adjuvant endocrine therapy and disease-free survival in patients with early HR-positive/HER2-positive BC: Analysis from the phase III randomized ShortHER trial. NPJ Breast Cancer. 2023;9(1):6 (Feb 4). Doi: 10.1038/s41523-023-00509-2
Key clinical point: Adjuvant endocrine therapy (ET) with an aromatase inhibitor (AI) was associated with better disease-free survival (DFS) than tamoxifen-only or tamoxifen+AI in patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor2-positive (HER2+) breast cancer (BC).
Major finding: Adjuvant ET with tamoxifen or tamoxifen+AI was associated with significantly worse DFS rates than AI in the entire population of women with HR+/HER2+ BC (hazard ratio 1.64; P = .025), with gonadotropin-releasing hormone being associated with improved DFS rates in premenopausal patients aged ≤45 years (hazard ratio 0.41; P = .023).
Study details: Findings are from a post hoc analysis of the ShortHER trial including 784 patients with HR+/HER2+ early BC who received adjuvant anthracycline/taxane-based chemotherapy plus trastuzumab.
Disclosures: This study was supported by Agenzia Italiana del Farmaco and other sources. Some authors declared receiving personal fees from several sources.
Source: Dieci MV et al. Type of adjuvant endocrine therapy and disease-free survival in patients with early HR-positive/HER2-positive BC: Analysis from the phase III randomized ShortHER trial. NPJ Breast Cancer. 2023;9(1):6 (Feb 4). Doi: 10.1038/s41523-023-00509-2
Key clinical point: Adjuvant endocrine therapy (ET) with an aromatase inhibitor (AI) was associated with better disease-free survival (DFS) than tamoxifen-only or tamoxifen+AI in patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor2-positive (HER2+) breast cancer (BC).
Major finding: Adjuvant ET with tamoxifen or tamoxifen+AI was associated with significantly worse DFS rates than AI in the entire population of women with HR+/HER2+ BC (hazard ratio 1.64; P = .025), with gonadotropin-releasing hormone being associated with improved DFS rates in premenopausal patients aged ≤45 years (hazard ratio 0.41; P = .023).
Study details: Findings are from a post hoc analysis of the ShortHER trial including 784 patients with HR+/HER2+ early BC who received adjuvant anthracycline/taxane-based chemotherapy plus trastuzumab.
Disclosures: This study was supported by Agenzia Italiana del Farmaco and other sources. Some authors declared receiving personal fees from several sources.
Source: Dieci MV et al. Type of adjuvant endocrine therapy and disease-free survival in patients with early HR-positive/HER2-positive BC: Analysis from the phase III randomized ShortHER trial. NPJ Breast Cancer. 2023;9(1):6 (Feb 4). Doi: 10.1038/s41523-023-00509-2
More on ‘intellectual constipation’ and more
More on ‘intellectual constipation’
Regarding your editorial, “From debate to stalemate and hate: An epidemic of intellectual constipation” (
Robert W. Pollack, MD, ABPN, DLFAPA
Fort Myers, Florida
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
More on SWOT analysis of psychiatry
I loved your recent analysis of psychiatry (“Contemporary psychiatry: A SWOT analysis,”
Paul R. Crosby, MD, MBA
President and CEO of the Frances and Craig Lindner Center of HOPE
Associate Professor and Vice Chair
Department of Psychiatry and Behavioral Neuroscience
University of Cincinnati College of Medicine
Cincinnati, Ohio
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
More on evolution’s triumphs and blunders
I enjoyed your editorial, “Is evolution’s greatest triumph its worst blunder?” (
Michele A. Packard, PhD
Boulder, Colorado
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
1. Kirsch A. The people cheering for humanity’s end. The Atlantic. January/February 2023.
More on ‘intellectual constipation’
Regarding your editorial, “From debate to stalemate and hate: An epidemic of intellectual constipation” (
Robert W. Pollack, MD, ABPN, DLFAPA
Fort Myers, Florida
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
More on SWOT analysis of psychiatry
I loved your recent analysis of psychiatry (“Contemporary psychiatry: A SWOT analysis,”
Paul R. Crosby, MD, MBA
President and CEO of the Frances and Craig Lindner Center of HOPE
Associate Professor and Vice Chair
Department of Psychiatry and Behavioral Neuroscience
University of Cincinnati College of Medicine
Cincinnati, Ohio
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
More on evolution’s triumphs and blunders
I enjoyed your editorial, “Is evolution’s greatest triumph its worst blunder?” (
Michele A. Packard, PhD
Boulder, Colorado
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
More on ‘intellectual constipation’
Regarding your editorial, “From debate to stalemate and hate: An epidemic of intellectual constipation” (
Robert W. Pollack, MD, ABPN, DLFAPA
Fort Myers, Florida
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
More on SWOT analysis of psychiatry
I loved your recent analysis of psychiatry (“Contemporary psychiatry: A SWOT analysis,”
Paul R. Crosby, MD, MBA
President and CEO of the Frances and Craig Lindner Center of HOPE
Associate Professor and Vice Chair
Department of Psychiatry and Behavioral Neuroscience
University of Cincinnati College of Medicine
Cincinnati, Ohio
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
More on evolution’s triumphs and blunders
I enjoyed your editorial, “Is evolution’s greatest triumph its worst blunder?” (
Michele A. Packard, PhD
Boulder, Colorado
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
1. Kirsch A. The people cheering for humanity’s end. The Atlantic. January/February 2023.
1. Kirsch A. The people cheering for humanity’s end. The Atlantic. January/February 2023.
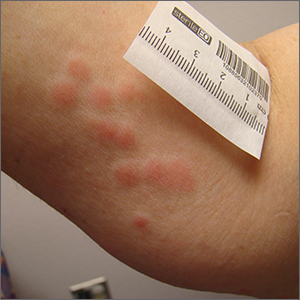
Clustered erythematous limb lesions
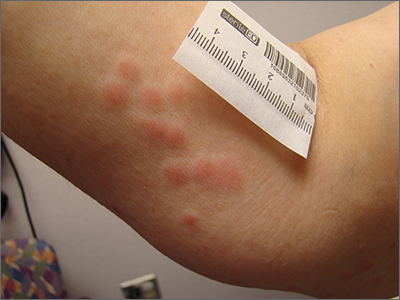
Clustered erythematous macular to papular lesions, especially those that stop at clothing lines, are a frequent manifestation of insect bites. In this case, the lesions lacked a central punctum that is common in many insect bites, so the most likely culprit was bed bugs. It is likely that this patient’s friend inadvertently brought the bed bugs into the home in her luggage or packed belongings. Over time, they spread around the home, causing the patient’s bites and inflammation. When questioned, the patient noted that she could actually see bugs around the couch in her home.
The scientific name for bed bugs is Cimex lectularis. Bed bugs require a blood meal from a host to survive, but they do not remain attached to the human body. Instead, they live in nearby fabrics. Bed bugs are visible to the naked eye when they are in the open, although they usually remain along the seams of fabric, edges of bedding, or in cracks and crevices. Often the feces of bed bugs will collect and be seen as dark spots or streaks on bedding.1
Treatment hinges on the eradication of the bed bugs. The erythematous itching lesions will resolve spontaneously over 1 to 2 weeks. Topical corticosteroids, including hydrocortisone, can be used as necessary to control the itching. Oral antihistamines can also help with itching.
Eradication of all the bed bugs in the home can be difficult and warrant professional extermination services. Washing clothing in hot water of at least 140 °F will kill the insects. Freezing items below –4 °F for at least 2 hours is also effective but may not be possible with home freezers.
It’s worth noting that resistance to insecticides has developed, making chemical eradication difficult. An alternative extermination protocol involves heating an entire home to the required temperatures to eradicate the infestation.1
This patient noted that she had already thrown away the couch, clothes, and bedding where she had seen the insects and had sprayed her apartment with insecticide. She was counseled to contact a professional exterminator to further evaluate the home for any additional areas of infestation and treat if any bed bugs were still in the home. She was also counseled to use loratadine 10 mg/d orally and topical 1% hydrocortisone ointment, as needed, for the itching and inflammation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Parola P, Izri A. Bedbugs. N Engl J Med. 2020;382:2230-2237. doi: 10.1056/NEJMcp1905840

Clustered erythematous macular to papular lesions, especially those that stop at clothing lines, are a frequent manifestation of insect bites. In this case, the lesions lacked a central punctum that is common in many insect bites, so the most likely culprit was bed bugs. It is likely that this patient’s friend inadvertently brought the bed bugs into the home in her luggage or packed belongings. Over time, they spread around the home, causing the patient’s bites and inflammation. When questioned, the patient noted that she could actually see bugs around the couch in her home.
The scientific name for bed bugs is Cimex lectularis. Bed bugs require a blood meal from a host to survive, but they do not remain attached to the human body. Instead, they live in nearby fabrics. Bed bugs are visible to the naked eye when they are in the open, although they usually remain along the seams of fabric, edges of bedding, or in cracks and crevices. Often the feces of bed bugs will collect and be seen as dark spots or streaks on bedding.1
Treatment hinges on the eradication of the bed bugs. The erythematous itching lesions will resolve spontaneously over 1 to 2 weeks. Topical corticosteroids, including hydrocortisone, can be used as necessary to control the itching. Oral antihistamines can also help with itching.
Eradication of all the bed bugs in the home can be difficult and warrant professional extermination services. Washing clothing in hot water of at least 140 °F will kill the insects. Freezing items below –4 °F for at least 2 hours is also effective but may not be possible with home freezers.
It’s worth noting that resistance to insecticides has developed, making chemical eradication difficult. An alternative extermination protocol involves heating an entire home to the required temperatures to eradicate the infestation.1
This patient noted that she had already thrown away the couch, clothes, and bedding where she had seen the insects and had sprayed her apartment with insecticide. She was counseled to contact a professional exterminator to further evaluate the home for any additional areas of infestation and treat if any bed bugs were still in the home. She was also counseled to use loratadine 10 mg/d orally and topical 1% hydrocortisone ointment, as needed, for the itching and inflammation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Clustered erythematous macular to papular lesions, especially those that stop at clothing lines, are a frequent manifestation of insect bites. In this case, the lesions lacked a central punctum that is common in many insect bites, so the most likely culprit was bed bugs. It is likely that this patient’s friend inadvertently brought the bed bugs into the home in her luggage or packed belongings. Over time, they spread around the home, causing the patient’s bites and inflammation. When questioned, the patient noted that she could actually see bugs around the couch in her home.
The scientific name for bed bugs is Cimex lectularis. Bed bugs require a blood meal from a host to survive, but they do not remain attached to the human body. Instead, they live in nearby fabrics. Bed bugs are visible to the naked eye when they are in the open, although they usually remain along the seams of fabric, edges of bedding, or in cracks and crevices. Often the feces of bed bugs will collect and be seen as dark spots or streaks on bedding.1
Treatment hinges on the eradication of the bed bugs. The erythematous itching lesions will resolve spontaneously over 1 to 2 weeks. Topical corticosteroids, including hydrocortisone, can be used as necessary to control the itching. Oral antihistamines can also help with itching.
Eradication of all the bed bugs in the home can be difficult and warrant professional extermination services. Washing clothing in hot water of at least 140 °F will kill the insects. Freezing items below –4 °F for at least 2 hours is also effective but may not be possible with home freezers.
It’s worth noting that resistance to insecticides has developed, making chemical eradication difficult. An alternative extermination protocol involves heating an entire home to the required temperatures to eradicate the infestation.1
This patient noted that she had already thrown away the couch, clothes, and bedding where she had seen the insects and had sprayed her apartment with insecticide. She was counseled to contact a professional exterminator to further evaluate the home for any additional areas of infestation and treat if any bed bugs were still in the home. She was also counseled to use loratadine 10 mg/d orally and topical 1% hydrocortisone ointment, as needed, for the itching and inflammation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Parola P, Izri A. Bedbugs. N Engl J Med. 2020;382:2230-2237. doi: 10.1056/NEJMcp1905840
1. Parola P, Izri A. Bedbugs. N Engl J Med. 2020;382:2230-2237. doi: 10.1056/NEJMcp1905840
VA Plans to Waive Health Care Copayments for American Indian Veterans
New VA rule proposes to eliminate many copays for Native American veteran.
The US Department of Veterans Affairs (VA) has proposed a new rule that would waive medical copayments incurred on or after January 5, 2022, for eligible American Indian and Alaska Native (AI/AN) veterans.
The policy is intended to encourage veterans to seek regular primary care treatment, the VA says. “It’s no mystery to a lot of people that health care is sometimes hard to come by in many Native American communities,” Travis Trueblood, director of the VA Office of Tribal Health, told reporters in January. “So, this effort by VA will enhance getting people into the facilities, helping them feel welcome and getting them to use those benefits that they've earned.”
Copayments for more than 3 visits to community-based urgent care in any calendar year would still be required. Follow-up care provided by a VA-authorized primary care provider would be exempt from copays. Members of federally recognized tribes are already exempt from copays at Indian Health Service clinics.
Eligibility may be based in part on documentation issued by AI/AN tribal governments to show tribal membership. The VA has proposed the documentation requirement “as this recognizes tribal sovereignty and promotes the Nation-to-Nation relationship that exists between the United States and tribal governments.” The requirement, the notice says, is consistent with the preferences of tribal leaders.
The regulation implements a requirement in the Johnny Isakson and David P. Roe, MD, Veterans Health Care and Benefits Improvement Act of 2020, which prohibited the VA from collecting copayments from AI/AN veterans for hospital care or medical services. Senator Jon Tester (D-MT), chair of the Senate Veterans’ Affairs Committee, and Senator Jerry Moran (R-KS) introduced legislation in 2020 to enact the new policy in January 2022 , which is why the rule is retroactive.
Congress passed the measure as part of a package of veterans’ legislation at the end of 2020, and then-President Donald Trump signed it into law in January 2021. Trueblood said the nature of the federal rulemaking process makes it hard to say exactly when the change will take effect, but that no veteran will be turned away from VA care for not making a copayment, even before the rule is finalized. The VA plans to reimburse eligible veterans who received care in the past year for copayment costs.
“I’m encouraged to see VA answering my call to implement the law and remove burdensome copayments for Native veterans accessing their earned health care,” said Tester in a press release. “The fact is Native veterans have bravely answered the call to duty for generations. And I’ll continue to hold VA accountable in delivering these veterans their long-overdue support.”
New VA rule proposes to eliminate many copays for Native American veteran.
The US Department of Veterans Affairs (VA) has proposed a new rule that would waive medical copayments incurred on or after January 5, 2022, for eligible American Indian and Alaska Native (AI/AN) veterans.
The policy is intended to encourage veterans to seek regular primary care treatment, the VA says. “It’s no mystery to a lot of people that health care is sometimes hard to come by in many Native American communities,” Travis Trueblood, director of the VA Office of Tribal Health, told reporters in January. “So, this effort by VA will enhance getting people into the facilities, helping them feel welcome and getting them to use those benefits that they've earned.”
Copayments for more than 3 visits to community-based urgent care in any calendar year would still be required. Follow-up care provided by a VA-authorized primary care provider would be exempt from copays. Members of federally recognized tribes are already exempt from copays at Indian Health Service clinics.
Eligibility may be based in part on documentation issued by AI/AN tribal governments to show tribal membership. The VA has proposed the documentation requirement “as this recognizes tribal sovereignty and promotes the Nation-to-Nation relationship that exists between the United States and tribal governments.” The requirement, the notice says, is consistent with the preferences of tribal leaders.
The regulation implements a requirement in the Johnny Isakson and David P. Roe, MD, Veterans Health Care and Benefits Improvement Act of 2020, which prohibited the VA from collecting copayments from AI/AN veterans for hospital care or medical services. Senator Jon Tester (D-MT), chair of the Senate Veterans’ Affairs Committee, and Senator Jerry Moran (R-KS) introduced legislation in 2020 to enact the new policy in January 2022 , which is why the rule is retroactive.
Congress passed the measure as part of a package of veterans’ legislation at the end of 2020, and then-President Donald Trump signed it into law in January 2021. Trueblood said the nature of the federal rulemaking process makes it hard to say exactly when the change will take effect, but that no veteran will be turned away from VA care for not making a copayment, even before the rule is finalized. The VA plans to reimburse eligible veterans who received care in the past year for copayment costs.
“I’m encouraged to see VA answering my call to implement the law and remove burdensome copayments for Native veterans accessing their earned health care,” said Tester in a press release. “The fact is Native veterans have bravely answered the call to duty for generations. And I’ll continue to hold VA accountable in delivering these veterans their long-overdue support.”
New VA rule proposes to eliminate many copays for Native American veteran.
The US Department of Veterans Affairs (VA) has proposed a new rule that would waive medical copayments incurred on or after January 5, 2022, for eligible American Indian and Alaska Native (AI/AN) veterans.
The policy is intended to encourage veterans to seek regular primary care treatment, the VA says. “It’s no mystery to a lot of people that health care is sometimes hard to come by in many Native American communities,” Travis Trueblood, director of the VA Office of Tribal Health, told reporters in January. “So, this effort by VA will enhance getting people into the facilities, helping them feel welcome and getting them to use those benefits that they've earned.”
Copayments for more than 3 visits to community-based urgent care in any calendar year would still be required. Follow-up care provided by a VA-authorized primary care provider would be exempt from copays. Members of federally recognized tribes are already exempt from copays at Indian Health Service clinics.
Eligibility may be based in part on documentation issued by AI/AN tribal governments to show tribal membership. The VA has proposed the documentation requirement “as this recognizes tribal sovereignty and promotes the Nation-to-Nation relationship that exists between the United States and tribal governments.” The requirement, the notice says, is consistent with the preferences of tribal leaders.
The regulation implements a requirement in the Johnny Isakson and David P. Roe, MD, Veterans Health Care and Benefits Improvement Act of 2020, which prohibited the VA from collecting copayments from AI/AN veterans for hospital care or medical services. Senator Jon Tester (D-MT), chair of the Senate Veterans’ Affairs Committee, and Senator Jerry Moran (R-KS) introduced legislation in 2020 to enact the new policy in January 2022 , which is why the rule is retroactive.
Congress passed the measure as part of a package of veterans’ legislation at the end of 2020, and then-President Donald Trump signed it into law in January 2021. Trueblood said the nature of the federal rulemaking process makes it hard to say exactly when the change will take effect, but that no veteran will be turned away from VA care for not making a copayment, even before the rule is finalized. The VA plans to reimburse eligible veterans who received care in the past year for copayment costs.
“I’m encouraged to see VA answering my call to implement the law and remove burdensome copayments for Native veterans accessing their earned health care,” said Tester in a press release. “The fact is Native veterans have bravely answered the call to duty for generations. And I’ll continue to hold VA accountable in delivering these veterans their long-overdue support.”
Decrease in cognitive functioning
The history and findings in this case are suggestive of late-onset Alzheimer's disease (AD).
AD is a neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. At least two thirds of cases of dementia in people ≥ 65 years of age are due to AD, making it the most common type of dementia. At present, there is no cure for AD, which is associated with a long preclinical stage and a progressive disease course. In the United States, AD is the sixth leading cause of death.
Individuals with AD develop amyloid plaques in the hippocampus and in other areas of the cerebral cortex. The symptoms of AD vary depending on the stage of the disease; however, in most patients with late-onset AD (≥ 65 years of age), the most common presenting symptom is episodic short-term memory loss, with relative sparing of long-term memory. Subsequently, patients may experience impairments in problem-solving, judgment, executive functioning, motivation, and organization. It is not uncommon for individuals with AD to lack insight into the impairments they are experiences, or even to deny deficits.
Neuropsychiatric symptoms, such as apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are common in the mid- to late stages of the disease. Patients may also experience difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, and sleep disturbances; develop extrapyramidal motor signs (eg, dystonia, akathisia, and parkinsonian symptoms) followed by difficulties with primitive reflexes and incontinence, and may ultimately become totally dependent on caregivers.
A thorough history and physical examination are essential for the diagnosis of AD. Because some patients may lack insight into their disease, it is vital to elicit a history from the patient's family and caregivers as well. Onset and early symptoms are important to note to aid in differentiating AD from other types of dementia. In most patients with late-onset AD, comprehensive clinical assessment can provide reasonable diagnostic certainty. This should include a detailed neurologic examination to rule out other conditions; most patients with AD will have a normal neurologic exam.
A mental status examination to evaluate concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning should also be conducted. Brief standard examinations, such the Mini-Mental State Examination, can be used for initial screening purposes, although they are less sensitive and specific than more comprehensive tests. Follow-up visits for patients diagnosed with AD should therefore include a full mental status examination to gauge disease progression as well as the development of neuropsychiatric symptoms.
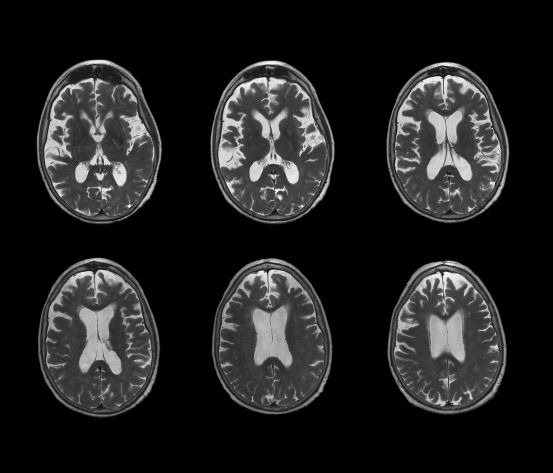
Brain imaging can be beneficial both for diagnosing AD and monitoring the disease's clinical course. MRI or CT of the brain can help eliminate alternate causes of dementia, such as stroke or tumors, from consideration. Dilated lateral ventricles and widened cortical sulci, particularly in the temporal area, are typical findings in AD.
The standard medical treatment for AD includes cholinesterase inhibitors (ChEIs) and a partial N-methyl-D-aspartate (NMDA) antagonist. Both US and European guidelines list ChEIs (donepezil, rivastigmine, galantamine, tacrine) as first-line pharmacotherapies for mild to moderate AD; however, these agents only show modest efficacy on cognitive deficits and nonsignificant efficacy on functional capacity in mild to moderate AD. Memantine, a partial NMDA antagonist, shows very limited efficacy on cognitive symptoms, with no improvement in functional domains. Newly approved anti-amyloid therapies include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may help to mitigate the secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and sleep disorders. Behavioral interventions (eg, patient-centered approaches and caregiver training) may be beneficial for managing the cognitive and behavioral manifestations of AD and are often combined with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise also be beneficial for brain health and delaying disease progression.
Numerous novel agents are under investigation for AD, including anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents (such as NMDA receptor modulators), and brain stimulation.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of late-onset Alzheimer's disease (AD).
AD is a neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. At least two thirds of cases of dementia in people ≥ 65 years of age are due to AD, making it the most common type of dementia. At present, there is no cure for AD, which is associated with a long preclinical stage and a progressive disease course. In the United States, AD is the sixth leading cause of death.
Individuals with AD develop amyloid plaques in the hippocampus and in other areas of the cerebral cortex. The symptoms of AD vary depending on the stage of the disease; however, in most patients with late-onset AD (≥ 65 years of age), the most common presenting symptom is episodic short-term memory loss, with relative sparing of long-term memory. Subsequently, patients may experience impairments in problem-solving, judgment, executive functioning, motivation, and organization. It is not uncommon for individuals with AD to lack insight into the impairments they are experiences, or even to deny deficits.
Neuropsychiatric symptoms, such as apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are common in the mid- to late stages of the disease. Patients may also experience difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, and sleep disturbances; develop extrapyramidal motor signs (eg, dystonia, akathisia, and parkinsonian symptoms) followed by difficulties with primitive reflexes and incontinence, and may ultimately become totally dependent on caregivers.
A thorough history and physical examination are essential for the diagnosis of AD. Because some patients may lack insight into their disease, it is vital to elicit a history from the patient's family and caregivers as well. Onset and early symptoms are important to note to aid in differentiating AD from other types of dementia. In most patients with late-onset AD, comprehensive clinical assessment can provide reasonable diagnostic certainty. This should include a detailed neurologic examination to rule out other conditions; most patients with AD will have a normal neurologic exam.
A mental status examination to evaluate concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning should also be conducted. Brief standard examinations, such the Mini-Mental State Examination, can be used for initial screening purposes, although they are less sensitive and specific than more comprehensive tests. Follow-up visits for patients diagnosed with AD should therefore include a full mental status examination to gauge disease progression as well as the development of neuropsychiatric symptoms.
Brain imaging can be beneficial both for diagnosing AD and monitoring the disease's clinical course. MRI or CT of the brain can help eliminate alternate causes of dementia, such as stroke or tumors, from consideration. Dilated lateral ventricles and widened cortical sulci, particularly in the temporal area, are typical findings in AD.
The standard medical treatment for AD includes cholinesterase inhibitors (ChEIs) and a partial N-methyl-D-aspartate (NMDA) antagonist. Both US and European guidelines list ChEIs (donepezil, rivastigmine, galantamine, tacrine) as first-line pharmacotherapies for mild to moderate AD; however, these agents only show modest efficacy on cognitive deficits and nonsignificant efficacy on functional capacity in mild to moderate AD. Memantine, a partial NMDA antagonist, shows very limited efficacy on cognitive symptoms, with no improvement in functional domains. Newly approved anti-amyloid therapies include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may help to mitigate the secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and sleep disorders. Behavioral interventions (eg, patient-centered approaches and caregiver training) may be beneficial for managing the cognitive and behavioral manifestations of AD and are often combined with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise also be beneficial for brain health and delaying disease progression.
Numerous novel agents are under investigation for AD, including anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents (such as NMDA receptor modulators), and brain stimulation.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of late-onset Alzheimer's disease (AD).
AD is a neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. At least two thirds of cases of dementia in people ≥ 65 years of age are due to AD, making it the most common type of dementia. At present, there is no cure for AD, which is associated with a long preclinical stage and a progressive disease course. In the United States, AD is the sixth leading cause of death.
Individuals with AD develop amyloid plaques in the hippocampus and in other areas of the cerebral cortex. The symptoms of AD vary depending on the stage of the disease; however, in most patients with late-onset AD (≥ 65 years of age), the most common presenting symptom is episodic short-term memory loss, with relative sparing of long-term memory. Subsequently, patients may experience impairments in problem-solving, judgment, executive functioning, motivation, and organization. It is not uncommon for individuals with AD to lack insight into the impairments they are experiences, or even to deny deficits.
Neuropsychiatric symptoms, such as apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are common in the mid- to late stages of the disease. Patients may also experience difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, and sleep disturbances; develop extrapyramidal motor signs (eg, dystonia, akathisia, and parkinsonian symptoms) followed by difficulties with primitive reflexes and incontinence, and may ultimately become totally dependent on caregivers.
A thorough history and physical examination are essential for the diagnosis of AD. Because some patients may lack insight into their disease, it is vital to elicit a history from the patient's family and caregivers as well. Onset and early symptoms are important to note to aid in differentiating AD from other types of dementia. In most patients with late-onset AD, comprehensive clinical assessment can provide reasonable diagnostic certainty. This should include a detailed neurologic examination to rule out other conditions; most patients with AD will have a normal neurologic exam.
A mental status examination to evaluate concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning should also be conducted. Brief standard examinations, such the Mini-Mental State Examination, can be used for initial screening purposes, although they are less sensitive and specific than more comprehensive tests. Follow-up visits for patients diagnosed with AD should therefore include a full mental status examination to gauge disease progression as well as the development of neuropsychiatric symptoms.
Brain imaging can be beneficial both for diagnosing AD and monitoring the disease's clinical course. MRI or CT of the brain can help eliminate alternate causes of dementia, such as stroke or tumors, from consideration. Dilated lateral ventricles and widened cortical sulci, particularly in the temporal area, are typical findings in AD.
The standard medical treatment for AD includes cholinesterase inhibitors (ChEIs) and a partial N-methyl-D-aspartate (NMDA) antagonist. Both US and European guidelines list ChEIs (donepezil, rivastigmine, galantamine, tacrine) as first-line pharmacotherapies for mild to moderate AD; however, these agents only show modest efficacy on cognitive deficits and nonsignificant efficacy on functional capacity in mild to moderate AD. Memantine, a partial NMDA antagonist, shows very limited efficacy on cognitive symptoms, with no improvement in functional domains. Newly approved anti-amyloid therapies include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may help to mitigate the secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and sleep disorders. Behavioral interventions (eg, patient-centered approaches and caregiver training) may be beneficial for managing the cognitive and behavioral manifestations of AD and are often combined with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise also be beneficial for brain health and delaying disease progression.
Numerous novel agents are under investigation for AD, including anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents (such as NMDA receptor modulators), and brain stimulation.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 79-year-old man presents to his primary care provider (PCP) for an annual examination. The patient is accompanied by his oldest daughter, with whom he has lived since the death of his spouse approximately 9 months earlier. During the examination, the patient's daughter expresses concern about her father's cognitive functioning. Specifically, she has observed him becoming increasingly forgetful since he moved in with her. She states he has repeatedly forgotten the names of her dogs and has forgotten food in the microwave or on the stove on several occasions. Recently, after leaving a restaurant, her father was unable to remember where he had parked his car, and she suspects he has gotten lost while driving to and from familiar places several times. When questioned, the patient denies impairment and states occasional memory loss is "just part of the aging process."
Neither the patient nor his daughter reports any difficulties with his ability to groom and dress himself. His medical history is notable for high cholesterol, which is managed with a statin. The patient is a former smoker (24 pack-years) and occasionally drinks alcohol. His current height and weight are 5 ft 11 in and 177 lb, respectively.
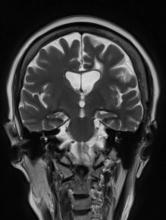
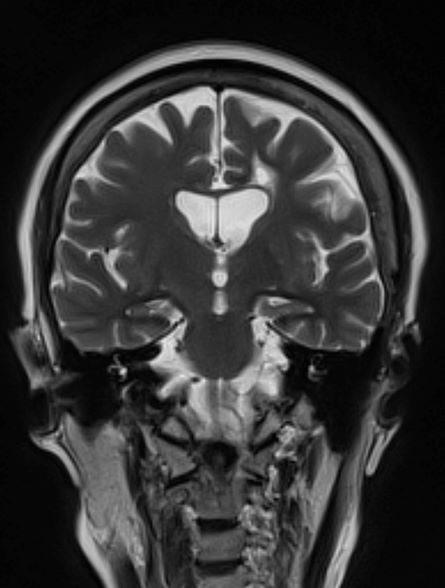
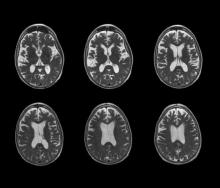
The patient appears well nourished and oriented to time and place, although he appears to have moderate difficulty hearing and questions sometimes need to be repeated to him. His blood pressure, pulse oximetry, and heart rate are within normal ranges. Laboratory tests are all within normal ranges. The patient scores 16 on the Mini-Mental State Examination. His PCP orders MRI, which reveals atrophy on both hippocampi.
Difficulty remembering words
The history and findings in this case are suggestive of early-onset Alzheimer's disease (AD) with aphasia.
AD is a neurodegenerative disorder characterized by cognitive and behavioral impairment that significantly interferes with a patient's social and occupational functioning. There is currently no cure for AD, which has a long preclinical period and a progressive course. Individuals with AD develop amyloid plaques in the hippocampus, a structure deep in the brain that helps to encode memories, and in other areas of the cerebral cortex that are involved in thinking and making decisions.
Patients with AD typically present with insidiously progressive memory loss; over the course of several years, other areas of cognition are impaired. Subsequent to memory loss, patients may also experience language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. In many patients, slowly progressive behavioral changes are also observed.
AD is most prevalent in individuals older than 65 years; however, early‐onset AD (in individuals aged 60 years or older) can also occur. Early-onset AD shares the same essential neuropathological characteristics (ie, amyloid plaques and neurofibrillary tangles) as late-onset (65 years or older) AD, but it differs in several ways. For example, memory loss is an extremely common presenting symptom in late-onset AD, whereas nonamnestic presentation (ie, language, visuospatial, or executive impairment) is very rare, occurring in only about 5% of cases. Conversely, nonamnestic presentations may occur in 30%-40% of patients with early-onset AD. Frequent nonamnestic cognitive manifestations in patients with early-onset AD are those seen in mild to moderate AD, including visual agnosia (55.1%), aphasia (57.9%), and behavioral changes (61.7%). In addition, several studies have suggested that early-onset AD may have a more aggressive course than late-onset AD does, including faster cognitive and functional decline.
Presently, only symptomatic therapies are available for AD. The standard medical treatment for AD includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Newly approved antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may be used to treat the secondary symptoms of AD (eg, depression, agitation, aggression, hallucinations, delusions, sleep disorders), which can be problematic. Behavioral interventions ranging from patient-centered approaches to caregiver training may also be used to help manage cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions, such as anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders and specific manifestations (eg, episodes of anger or rage). Routine physical activity and exercise may affect AD progression and may possibly exert a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of early-onset Alzheimer's disease (AD) with aphasia.
AD is a neurodegenerative disorder characterized by cognitive and behavioral impairment that significantly interferes with a patient's social and occupational functioning. There is currently no cure for AD, which has a long preclinical period and a progressive course. Individuals with AD develop amyloid plaques in the hippocampus, a structure deep in the brain that helps to encode memories, and in other areas of the cerebral cortex that are involved in thinking and making decisions.
Patients with AD typically present with insidiously progressive memory loss; over the course of several years, other areas of cognition are impaired. Subsequent to memory loss, patients may also experience language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. In many patients, slowly progressive behavioral changes are also observed.
AD is most prevalent in individuals older than 65 years; however, early‐onset AD (in individuals aged 60 years or older) can also occur. Early-onset AD shares the same essential neuropathological characteristics (ie, amyloid plaques and neurofibrillary tangles) as late-onset (65 years or older) AD, but it differs in several ways. For example, memory loss is an extremely common presenting symptom in late-onset AD, whereas nonamnestic presentation (ie, language, visuospatial, or executive impairment) is very rare, occurring in only about 5% of cases. Conversely, nonamnestic presentations may occur in 30%-40% of patients with early-onset AD. Frequent nonamnestic cognitive manifestations in patients with early-onset AD are those seen in mild to moderate AD, including visual agnosia (55.1%), aphasia (57.9%), and behavioral changes (61.7%). In addition, several studies have suggested that early-onset AD may have a more aggressive course than late-onset AD does, including faster cognitive and functional decline.
Presently, only symptomatic therapies are available for AD. The standard medical treatment for AD includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Newly approved antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may be used to treat the secondary symptoms of AD (eg, depression, agitation, aggression, hallucinations, delusions, sleep disorders), which can be problematic. Behavioral interventions ranging from patient-centered approaches to caregiver training may also be used to help manage cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions, such as anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders and specific manifestations (eg, episodes of anger or rage). Routine physical activity and exercise may affect AD progression and may possibly exert a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of early-onset Alzheimer's disease (AD) with aphasia.
AD is a neurodegenerative disorder characterized by cognitive and behavioral impairment that significantly interferes with a patient's social and occupational functioning. There is currently no cure for AD, which has a long preclinical period and a progressive course. Individuals with AD develop amyloid plaques in the hippocampus, a structure deep in the brain that helps to encode memories, and in other areas of the cerebral cortex that are involved in thinking and making decisions.
Patients with AD typically present with insidiously progressive memory loss; over the course of several years, other areas of cognition are impaired. Subsequent to memory loss, patients may also experience language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. In many patients, slowly progressive behavioral changes are also observed.
AD is most prevalent in individuals older than 65 years; however, early‐onset AD (in individuals aged 60 years or older) can also occur. Early-onset AD shares the same essential neuropathological characteristics (ie, amyloid plaques and neurofibrillary tangles) as late-onset (65 years or older) AD, but it differs in several ways. For example, memory loss is an extremely common presenting symptom in late-onset AD, whereas nonamnestic presentation (ie, language, visuospatial, or executive impairment) is very rare, occurring in only about 5% of cases. Conversely, nonamnestic presentations may occur in 30%-40% of patients with early-onset AD. Frequent nonamnestic cognitive manifestations in patients with early-onset AD are those seen in mild to moderate AD, including visual agnosia (55.1%), aphasia (57.9%), and behavioral changes (61.7%). In addition, several studies have suggested that early-onset AD may have a more aggressive course than late-onset AD does, including faster cognitive and functional decline.
Presently, only symptomatic therapies are available for AD. The standard medical treatment for AD includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Newly approved antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may be used to treat the secondary symptoms of AD (eg, depression, agitation, aggression, hallucinations, delusions, sleep disorders), which can be problematic. Behavioral interventions ranging from patient-centered approaches to caregiver training may also be used to help manage cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions, such as anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders and specific manifestations (eg, episodes of anger or rage). Routine physical activity and exercise may affect AD progression and may possibly exert a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 53-year-old woman, who is a high school mathematics teacher, presents with reports of progressively increasing cognitive impairments. Specifically, she notes increasing difficulty with remembering words as well as challenges with her executive functioning. She was recently reprimanded by her principal for missing several mandatory staff meetings and deadlines for submitting student grades. The patient states her symptoms began approximately 2 years ago. She initially attributed them to hormonal changes because of menopause but is becoming concerned about the impact they are having on her ability to function. She recently began experiencing difficulties with spatial perception, which resulted in her falling down the stairs of her home and spraining an ankle. The patient lives alone and has no children. Her medical history is unremarkable except for a motor vehicle accident 5 years earlier that resulted in her sustaining a concussion and a fractured wrist. She does not currently take any medications. There is no history of tobacco use or excessive alcohol consumption. Her current height and weight are 5 ft 3 in and 147 lb, respectively.
No abnormalities are noted on physical exam; the patient's blood pressure, pulse oximetry, and heart rate are within normal ranges. Laboratory tests are all within normal ranges, including thyroid-stimulating hormone and vitamin B12 levels. The patient scores 16 on the Montreal Cognitive Assessment test. Her clinician orders an MRI, which reveals deep indentations around the front and sides of the brain.
Trends in HPV vaccination among adults aged 27 to 45 years
In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?
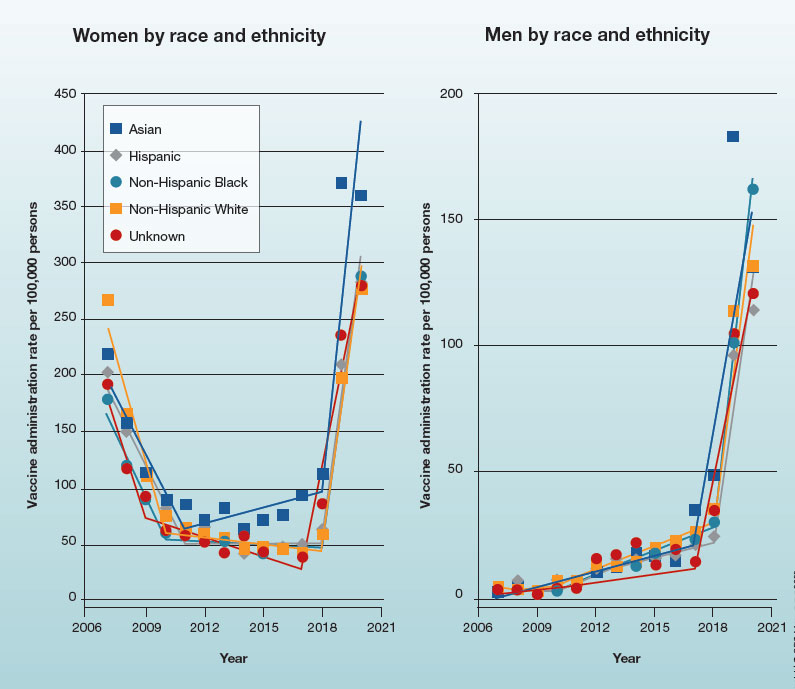
In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?

In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?