User login
How to place an IUD with minimal patient discomfort
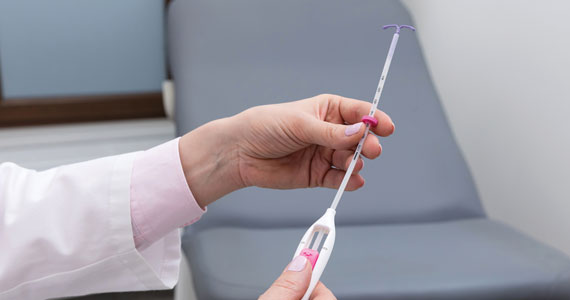
CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
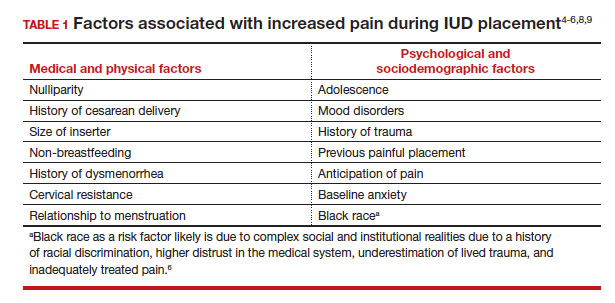
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8
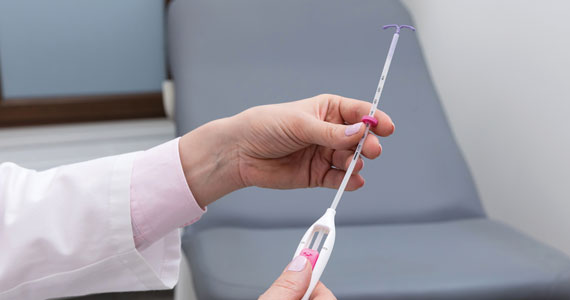
It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
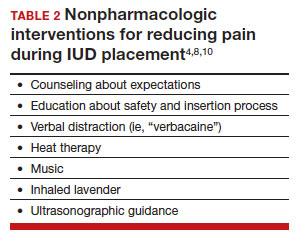
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10
Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
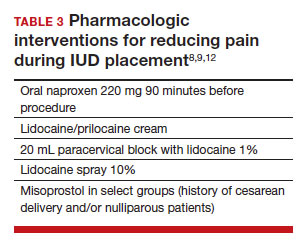
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).
Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
Atopic dermatitis increases the risk for food allergy, food sensitivity, challenge-proven food allergy, and vice versa
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk of developing food allergy (FA), food sensitivity (FS), and challenge-proven food allergy (CPFA), and vice versa.
Major finding: AD was significantly associated with an increased risk for FS (pooled odds ratio [pOR] 4.17; 95% CI 3.03-5.75), FA (pOR 3.91; 95% CI 3.44-4.45), and CPFA (pOR 4.99; 95% CI 2.20-11.35). A significantly increased risk for AD was observed in individuals with FS (pOR 3.92; 95% CI,2.88-5.33), FA (pOR 4.06; 95% CI 3.54-4.65), and CPFA (pOR 4.93; 95% CI 2.74-8.84).
Study details: Findings are from a meta-analysis of 465 studies involving patients with AD (n = 225,568); reference individuals without AD (n = 1,128,322); individuals with FS, FA, and CPFA (n = 1,357,793); and reference individuals without FS, FA, and CPFA (n = 1,244,596).
Disclosures: This study did not receive any funding. Some authors declared serving as advisors, speakers, or consultants for or receiving speaking or consulting fees from various sources.
Source: Christensen MO et al. Prevalence of and association between atopic dermatitis and food sensitivity, food allergy and challenge-proven food allergy: A systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023 (Jan 25). Doi: 10.1111/jdv.18919
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk of developing food allergy (FA), food sensitivity (FS), and challenge-proven food allergy (CPFA), and vice versa.
Major finding: AD was significantly associated with an increased risk for FS (pooled odds ratio [pOR] 4.17; 95% CI 3.03-5.75), FA (pOR 3.91; 95% CI 3.44-4.45), and CPFA (pOR 4.99; 95% CI 2.20-11.35). A significantly increased risk for AD was observed in individuals with FS (pOR 3.92; 95% CI,2.88-5.33), FA (pOR 4.06; 95% CI 3.54-4.65), and CPFA (pOR 4.93; 95% CI 2.74-8.84).
Study details: Findings are from a meta-analysis of 465 studies involving patients with AD (n = 225,568); reference individuals without AD (n = 1,128,322); individuals with FS, FA, and CPFA (n = 1,357,793); and reference individuals without FS, FA, and CPFA (n = 1,244,596).
Disclosures: This study did not receive any funding. Some authors declared serving as advisors, speakers, or consultants for or receiving speaking or consulting fees from various sources.
Source: Christensen MO et al. Prevalence of and association between atopic dermatitis and food sensitivity, food allergy and challenge-proven food allergy: A systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023 (Jan 25). Doi: 10.1111/jdv.18919
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk of developing food allergy (FA), food sensitivity (FS), and challenge-proven food allergy (CPFA), and vice versa.
Major finding: AD was significantly associated with an increased risk for FS (pooled odds ratio [pOR] 4.17; 95% CI 3.03-5.75), FA (pOR 3.91; 95% CI 3.44-4.45), and CPFA (pOR 4.99; 95% CI 2.20-11.35). A significantly increased risk for AD was observed in individuals with FS (pOR 3.92; 95% CI,2.88-5.33), FA (pOR 4.06; 95% CI 3.54-4.65), and CPFA (pOR 4.93; 95% CI 2.74-8.84).
Study details: Findings are from a meta-analysis of 465 studies involving patients with AD (n = 225,568); reference individuals without AD (n = 1,128,322); individuals with FS, FA, and CPFA (n = 1,357,793); and reference individuals without FS, FA, and CPFA (n = 1,244,596).
Disclosures: This study did not receive any funding. Some authors declared serving as advisors, speakers, or consultants for or receiving speaking or consulting fees from various sources.
Source: Christensen MO et al. Prevalence of and association between atopic dermatitis and food sensitivity, food allergy and challenge-proven food allergy: A systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023 (Jan 25). Doi: 10.1111/jdv.18919
Atopic dermatitis: Association of itch with patient- vs physician-reported outcomes
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), itch was more closely associated with patient-reported outcome measures than with objective assessments by the physician.
Major finding: Itch severity had a weak positive correlation with objective Scoring Atopic Dermatitis (rho (ρ) 0.44; 95% CI 0.39-0.48), Eczema Area and Severity Index (ρ 0.41; 95% CI 0.36-0.46), and Investigator Global Assessment (ρ 0.46; 95% CI 0.42-0.51) scores, but a strong positive correlation with Patient Global Assessment score (ρ 0.68; 95% CI 0.65-0.71), Patient-Oriented Eczema Measure sum score (ρ 0.66; 95% CI 0.63-0.69), and Dermatology Life Quality Index score (ρ 0.61; 95% CI 0.57-0.65).
Study details: This study analyzed the data of 1134 adult patients with moderate-to-severe AD from the multicenter prospective TREATgermany registry, of which 1121 had provided data on their itch.
Disclosures: TREATgermany is sponsored by AbbVie Deutschland GmbH & Co. KG, Galderma S.A., and others. Some authors reported ties with various organizations, including TREATgermany sponsors.
Source: Weisshaar E et al. Itching in atopic dermatitis: Patient- and physician-reported outcomes in the German atopic dermatitis registry TREATgermany. Acta Derm Venereol. 2023;103:adv00854 (Jan 23). Doi: 10.2340/actadv.v103.4426
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), itch was more closely associated with patient-reported outcome measures than with objective assessments by the physician.
Major finding: Itch severity had a weak positive correlation with objective Scoring Atopic Dermatitis (rho (ρ) 0.44; 95% CI 0.39-0.48), Eczema Area and Severity Index (ρ 0.41; 95% CI 0.36-0.46), and Investigator Global Assessment (ρ 0.46; 95% CI 0.42-0.51) scores, but a strong positive correlation with Patient Global Assessment score (ρ 0.68; 95% CI 0.65-0.71), Patient-Oriented Eczema Measure sum score (ρ 0.66; 95% CI 0.63-0.69), and Dermatology Life Quality Index score (ρ 0.61; 95% CI 0.57-0.65).
Study details: This study analyzed the data of 1134 adult patients with moderate-to-severe AD from the multicenter prospective TREATgermany registry, of which 1121 had provided data on their itch.
Disclosures: TREATgermany is sponsored by AbbVie Deutschland GmbH & Co. KG, Galderma S.A., and others. Some authors reported ties with various organizations, including TREATgermany sponsors.
Source: Weisshaar E et al. Itching in atopic dermatitis: Patient- and physician-reported outcomes in the German atopic dermatitis registry TREATgermany. Acta Derm Venereol. 2023;103:adv00854 (Jan 23). Doi: 10.2340/actadv.v103.4426
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), itch was more closely associated with patient-reported outcome measures than with objective assessments by the physician.
Major finding: Itch severity had a weak positive correlation with objective Scoring Atopic Dermatitis (rho (ρ) 0.44; 95% CI 0.39-0.48), Eczema Area and Severity Index (ρ 0.41; 95% CI 0.36-0.46), and Investigator Global Assessment (ρ 0.46; 95% CI 0.42-0.51) scores, but a strong positive correlation with Patient Global Assessment score (ρ 0.68; 95% CI 0.65-0.71), Patient-Oriented Eczema Measure sum score (ρ 0.66; 95% CI 0.63-0.69), and Dermatology Life Quality Index score (ρ 0.61; 95% CI 0.57-0.65).
Study details: This study analyzed the data of 1134 adult patients with moderate-to-severe AD from the multicenter prospective TREATgermany registry, of which 1121 had provided data on their itch.
Disclosures: TREATgermany is sponsored by AbbVie Deutschland GmbH & Co. KG, Galderma S.A., and others. Some authors reported ties with various organizations, including TREATgermany sponsors.
Source: Weisshaar E et al. Itching in atopic dermatitis: Patient- and physician-reported outcomes in the German atopic dermatitis registry TREATgermany. Acta Derm Venereol. 2023;103:adv00854 (Jan 23). Doi: 10.2340/actadv.v103.4426
IgE levels may guide diagnosis and management in children hospitalized for atopic dermatitis exacerbation
Key clinical point: Children who were hospitalized for atopic dermatitis (AD) exacerbation had significantly higher serum total IgE levels than those hospitalized for AD-associated infectious complications.
Major finding: Children with AD exacerbation vs an infectious complication had significantly higher mean serum total IgE levels (9603 ± 15,873 vs 3167 ± 5486 kU/L; P = .029). The likelihood of AD exacerbation vs an infectious complication was significantly greater in children with an age-adjusted IgE level (serum total IgE level/maximum reference IgE value for their age) of >4.
Study details: This retrospective chart review study included 68 children hospitalized for AD exacerbations (n = 34) or AD-associated infectious complications (n = 34) over a 17-year period.
Disclosures: This study did not report the source of funding. PY Ong declared serving as a consultant for and receiving research funding from various organizations.
Source: Atwal S, Ong PY. Elevated serum total IgE is associated with eczema exacerbation in children hospitalized for atopic dermatitis. Pediatr Dermatol. 2023 (Jan 19). Doi: 10.1111/pde.15245
Key clinical point: Children who were hospitalized for atopic dermatitis (AD) exacerbation had significantly higher serum total IgE levels than those hospitalized for AD-associated infectious complications.
Major finding: Children with AD exacerbation vs an infectious complication had significantly higher mean serum total IgE levels (9603 ± 15,873 vs 3167 ± 5486 kU/L; P = .029). The likelihood of AD exacerbation vs an infectious complication was significantly greater in children with an age-adjusted IgE level (serum total IgE level/maximum reference IgE value for their age) of >4.
Study details: This retrospective chart review study included 68 children hospitalized for AD exacerbations (n = 34) or AD-associated infectious complications (n = 34) over a 17-year period.
Disclosures: This study did not report the source of funding. PY Ong declared serving as a consultant for and receiving research funding from various organizations.
Source: Atwal S, Ong PY. Elevated serum total IgE is associated with eczema exacerbation in children hospitalized for atopic dermatitis. Pediatr Dermatol. 2023 (Jan 19). Doi: 10.1111/pde.15245
Key clinical point: Children who were hospitalized for atopic dermatitis (AD) exacerbation had significantly higher serum total IgE levels than those hospitalized for AD-associated infectious complications.
Major finding: Children with AD exacerbation vs an infectious complication had significantly higher mean serum total IgE levels (9603 ± 15,873 vs 3167 ± 5486 kU/L; P = .029). The likelihood of AD exacerbation vs an infectious complication was significantly greater in children with an age-adjusted IgE level (serum total IgE level/maximum reference IgE value for their age) of >4.
Study details: This retrospective chart review study included 68 children hospitalized for AD exacerbations (n = 34) or AD-associated infectious complications (n = 34) over a 17-year period.
Disclosures: This study did not report the source of funding. PY Ong declared serving as a consultant for and receiving research funding from various organizations.
Source: Atwal S, Ong PY. Elevated serum total IgE is associated with eczema exacerbation in children hospitalized for atopic dermatitis. Pediatr Dermatol. 2023 (Jan 19). Doi: 10.1111/pde.15245
Dupilumab offers long-term drug survival in moderate-to-severe atopic dermatitis in a real-world setting
Key clinical point: Dupilumab demonstrated good 4-year drug survival in patients with moderate-to-severe atopic dermatitis (AD); however, early-onset AD (at <18 years of age) was a risk factor for a shorter drug survival.
Major finding: The 1-, 2-, 3-, and 4-year overall dupilumab drug survival rates were 90.5%, 82.9%, 78.8%, and 76.4%, respectively. Early onset of AD may serve as a significant predictor of shorter overall drug survival (hazard ratio, 1.32; P = .04).
Study details: This real-world prospective cohort study included 363 patients with moderate-to-severe AD who had received dupilumab for ≥4 weeks.
Disclosures: This study did not receive any funding. Some authors declared serving as consultants and/or speakers for various organizations.
Source: Pezzolo E et al. Long-term drug survival of dupilumab and associated predictors in moderate to severe atopic dermatitis: A real-world prospective cohort study. J Eur Acad Dermatol Venereol. 2023 (Jan 20). Doi: 10.1111/jdv.18889
Key clinical point: Dupilumab demonstrated good 4-year drug survival in patients with moderate-to-severe atopic dermatitis (AD); however, early-onset AD (at <18 years of age) was a risk factor for a shorter drug survival.
Major finding: The 1-, 2-, 3-, and 4-year overall dupilumab drug survival rates were 90.5%, 82.9%, 78.8%, and 76.4%, respectively. Early onset of AD may serve as a significant predictor of shorter overall drug survival (hazard ratio, 1.32; P = .04).
Study details: This real-world prospective cohort study included 363 patients with moderate-to-severe AD who had received dupilumab for ≥4 weeks.
Disclosures: This study did not receive any funding. Some authors declared serving as consultants and/or speakers for various organizations.
Source: Pezzolo E et al. Long-term drug survival of dupilumab and associated predictors in moderate to severe atopic dermatitis: A real-world prospective cohort study. J Eur Acad Dermatol Venereol. 2023 (Jan 20). Doi: 10.1111/jdv.18889
Key clinical point: Dupilumab demonstrated good 4-year drug survival in patients with moderate-to-severe atopic dermatitis (AD); however, early-onset AD (at <18 years of age) was a risk factor for a shorter drug survival.
Major finding: The 1-, 2-, 3-, and 4-year overall dupilumab drug survival rates were 90.5%, 82.9%, 78.8%, and 76.4%, respectively. Early onset of AD may serve as a significant predictor of shorter overall drug survival (hazard ratio, 1.32; P = .04).
Study details: This real-world prospective cohort study included 363 patients with moderate-to-severe AD who had received dupilumab for ≥4 weeks.
Disclosures: This study did not receive any funding. Some authors declared serving as consultants and/or speakers for various organizations.
Source: Pezzolo E et al. Long-term drug survival of dupilumab and associated predictors in moderate to severe atopic dermatitis: A real-world prospective cohort study. J Eur Acad Dermatol Venereol. 2023 (Jan 20). Doi: 10.1111/jdv.18889
A probiotic reduces disease severity in children and adolescents with atopic dermatitis
Key clinical point: Coadjuvant treatment with a specific probiotic preparation reduced disease severity in children and adolescents with atopic dermatitis (AD), as evidenced by a decrease in Scoring of Atopic Dermatitis (SCORAD) and Investigator’s Global Assessment (IGA) scores.
Major finding: At 12 weeks, patients receiving the probiotic preparation vs placebo had a significantly higher rate of achieving at least a 1-point improvement in IGA score (90.5% vs 56.7%; P < .002) and lower SCORAD score (13.52 vs 18.96; P = .041).
Study details: This study included 70 patients aged 4-17 years with AD who were randomly assigned to receive the probiotic preparation (containing Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus casei; n = 35) or placebo (n = 35) daily for 12 weeks.
Disclosures: This study was funded by Biopolis SL. The authors declared no conflicts of interest.
Source: Feíto-Rodríguez M et al. Randomised double blind placebo controlled clinical trial to evaluate the effect of a mixture of probiotic strains on symptom severity and the use of corticosteroids in children and adolescents with atopic dermatitis. Clin Exp Dermatol. 2023 (Jan 13). Doi: 10.1093/ced/llad007
Key clinical point: Coadjuvant treatment with a specific probiotic preparation reduced disease severity in children and adolescents with atopic dermatitis (AD), as evidenced by a decrease in Scoring of Atopic Dermatitis (SCORAD) and Investigator’s Global Assessment (IGA) scores.
Major finding: At 12 weeks, patients receiving the probiotic preparation vs placebo had a significantly higher rate of achieving at least a 1-point improvement in IGA score (90.5% vs 56.7%; P < .002) and lower SCORAD score (13.52 vs 18.96; P = .041).
Study details: This study included 70 patients aged 4-17 years with AD who were randomly assigned to receive the probiotic preparation (containing Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus casei; n = 35) or placebo (n = 35) daily for 12 weeks.
Disclosures: This study was funded by Biopolis SL. The authors declared no conflicts of interest.
Source: Feíto-Rodríguez M et al. Randomised double blind placebo controlled clinical trial to evaluate the effect of a mixture of probiotic strains on symptom severity and the use of corticosteroids in children and adolescents with atopic dermatitis. Clin Exp Dermatol. 2023 (Jan 13). Doi: 10.1093/ced/llad007
Key clinical point: Coadjuvant treatment with a specific probiotic preparation reduced disease severity in children and adolescents with atopic dermatitis (AD), as evidenced by a decrease in Scoring of Atopic Dermatitis (SCORAD) and Investigator’s Global Assessment (IGA) scores.
Major finding: At 12 weeks, patients receiving the probiotic preparation vs placebo had a significantly higher rate of achieving at least a 1-point improvement in IGA score (90.5% vs 56.7%; P < .002) and lower SCORAD score (13.52 vs 18.96; P = .041).
Study details: This study included 70 patients aged 4-17 years with AD who were randomly assigned to receive the probiotic preparation (containing Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus casei; n = 35) or placebo (n = 35) daily for 12 weeks.
Disclosures: This study was funded by Biopolis SL. The authors declared no conflicts of interest.
Source: Feíto-Rodríguez M et al. Randomised double blind placebo controlled clinical trial to evaluate the effect of a mixture of probiotic strains on symptom severity and the use of corticosteroids in children and adolescents with atopic dermatitis. Clin Exp Dermatol. 2023 (Jan 13). Doi: 10.1093/ced/llad007
Atopic dermatitis with hand eczema: Upadacitinib is safe and effective in daily practice
Key clinical point: Upadacitinib can serve as an effective treatment option for atopic dermatitis (AD) and concomitant hand eczema (HE) in daily practice.
Major finding: At week 16, the mean Eczema Area and Severity Index (EASI) score decreased significantly from 17.2 (at baseline) to 4.8 (P < .001) and 50.0%, 40.6%, 59.3%, and 74.1% of patients achieved EASI-75, an Investigator’s Global Assessment score of (almost) clear, Hand Eczema Severity Index-75, and a score of (almost) clear on the photographic guide, respectively. Adverse events were generally mild in severity.
Study details: This multicenter prospective observational study included 38 patients with AD from the BioDay registry who received once-daily upadacitinib over 16 weeks, of which 32 patients had concomitant HE.
Disclosures: The BioDay registry is funded by Sanofi/Regeneron and others. Some authors declared serving as advisors, speakers, or consultants for or receiving consulting fees from various sources, including the registry funders.
Source: Kamphuis E, Loman L, et al. Experiences from daily practice of upadacitinib treatment on atopic dermatitis with a focus on hand eczema: Results from the BioDay registry. Contact Dermatitis. 2023 (Jan 9). Doi: 10.1111/cod.14276
Key clinical point: Upadacitinib can serve as an effective treatment option for atopic dermatitis (AD) and concomitant hand eczema (HE) in daily practice.
Major finding: At week 16, the mean Eczema Area and Severity Index (EASI) score decreased significantly from 17.2 (at baseline) to 4.8 (P < .001) and 50.0%, 40.6%, 59.3%, and 74.1% of patients achieved EASI-75, an Investigator’s Global Assessment score of (almost) clear, Hand Eczema Severity Index-75, and a score of (almost) clear on the photographic guide, respectively. Adverse events were generally mild in severity.
Study details: This multicenter prospective observational study included 38 patients with AD from the BioDay registry who received once-daily upadacitinib over 16 weeks, of which 32 patients had concomitant HE.
Disclosures: The BioDay registry is funded by Sanofi/Regeneron and others. Some authors declared serving as advisors, speakers, or consultants for or receiving consulting fees from various sources, including the registry funders.
Source: Kamphuis E, Loman L, et al. Experiences from daily practice of upadacitinib treatment on atopic dermatitis with a focus on hand eczema: Results from the BioDay registry. Contact Dermatitis. 2023 (Jan 9). Doi: 10.1111/cod.14276
Key clinical point: Upadacitinib can serve as an effective treatment option for atopic dermatitis (AD) and concomitant hand eczema (HE) in daily practice.
Major finding: At week 16, the mean Eczema Area and Severity Index (EASI) score decreased significantly from 17.2 (at baseline) to 4.8 (P < .001) and 50.0%, 40.6%, 59.3%, and 74.1% of patients achieved EASI-75, an Investigator’s Global Assessment score of (almost) clear, Hand Eczema Severity Index-75, and a score of (almost) clear on the photographic guide, respectively. Adverse events were generally mild in severity.
Study details: This multicenter prospective observational study included 38 patients with AD from the BioDay registry who received once-daily upadacitinib over 16 weeks, of which 32 patients had concomitant HE.
Disclosures: The BioDay registry is funded by Sanofi/Regeneron and others. Some authors declared serving as advisors, speakers, or consultants for or receiving consulting fees from various sources, including the registry funders.
Source: Kamphuis E, Loman L, et al. Experiences from daily practice of upadacitinib treatment on atopic dermatitis with a focus on hand eczema: Results from the BioDay registry. Contact Dermatitis. 2023 (Jan 9). Doi: 10.1111/cod.14276
Dupilumab a favorable treatment option for moderate-to-severe atopic dermatitis
Key clinical point: Dupilumab provides rapid improvement in atopic dermatitis (AD) signs and symptoms and is well tolerated in patients with moderate-to-severe AD in a real-world setting.
Major finding: At week 12, the percentages of patients who achieved ≥75% improvement in the Eczema Area and Severity Index, an Investigator’s Global Assessment score of 0/1 with a ≥2-point reduction from baseline, and a ≥4-point decrease in itch-numerical rating scale score were 59.4%, 33.0%, and 57.0%, respectively. Adverse event rates were lower than those reported in previous phase 3 trials.
Study details: Findings are from a 12-week analysis of the multicenter prospective real-life study PROLEAD including 828 dupilumab-naive adult patients with moderate-to-severe AD who received dupilumab.
Disclosures: This study was funded by Sanofi. Some authors reported ties with various organizations, including Sanofi. Three authors declared being employees of or holding stock or stock options in Sanofi.
Source: Augustin M et al. Dupilumab demonstrates rapid onset of action in improving signs, symptoms and quality of life in adults with atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Feb 4). Doi: 10.1007/s13555-023-00894-3
Key clinical point: Dupilumab provides rapid improvement in atopic dermatitis (AD) signs and symptoms and is well tolerated in patients with moderate-to-severe AD in a real-world setting.
Major finding: At week 12, the percentages of patients who achieved ≥75% improvement in the Eczema Area and Severity Index, an Investigator’s Global Assessment score of 0/1 with a ≥2-point reduction from baseline, and a ≥4-point decrease in itch-numerical rating scale score were 59.4%, 33.0%, and 57.0%, respectively. Adverse event rates were lower than those reported in previous phase 3 trials.
Study details: Findings are from a 12-week analysis of the multicenter prospective real-life study PROLEAD including 828 dupilumab-naive adult patients with moderate-to-severe AD who received dupilumab.
Disclosures: This study was funded by Sanofi. Some authors reported ties with various organizations, including Sanofi. Three authors declared being employees of or holding stock or stock options in Sanofi.
Source: Augustin M et al. Dupilumab demonstrates rapid onset of action in improving signs, symptoms and quality of life in adults with atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Feb 4). Doi: 10.1007/s13555-023-00894-3
Key clinical point: Dupilumab provides rapid improvement in atopic dermatitis (AD) signs and symptoms and is well tolerated in patients with moderate-to-severe AD in a real-world setting.
Major finding: At week 12, the percentages of patients who achieved ≥75% improvement in the Eczema Area and Severity Index, an Investigator’s Global Assessment score of 0/1 with a ≥2-point reduction from baseline, and a ≥4-point decrease in itch-numerical rating scale score were 59.4%, 33.0%, and 57.0%, respectively. Adverse event rates were lower than those reported in previous phase 3 trials.
Study details: Findings are from a 12-week analysis of the multicenter prospective real-life study PROLEAD including 828 dupilumab-naive adult patients with moderate-to-severe AD who received dupilumab.
Disclosures: This study was funded by Sanofi. Some authors reported ties with various organizations, including Sanofi. Three authors declared being employees of or holding stock or stock options in Sanofi.
Source: Augustin M et al. Dupilumab demonstrates rapid onset of action in improving signs, symptoms and quality of life in adults with atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Feb 4). Doi: 10.1007/s13555-023-00894-3
Tralokinumab counters difficult-to-treat moderate-to-severe atopic dermatitis
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Tralokinumab counters difficult-to-treat moderate-to-severe atopic dermatitis
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038