User login
Plaque With Central Ulceration on the Abdomen
Plaque With Central Ulceration on the Abdomen
THE DIAGNOSIS: Plaquelike Myofibroblastic Tumor
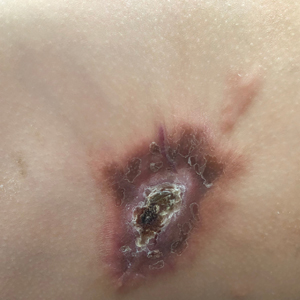
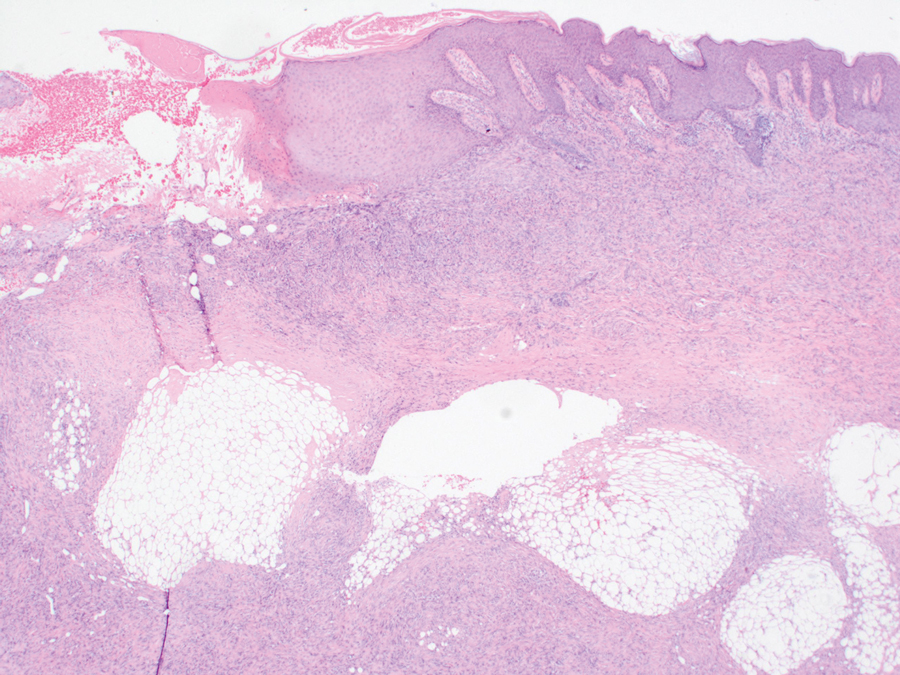
An incisional biopsy of the plaque demonstrated a hypercellular proliferation of bland spindle cells in the dermis that infiltrated the subcutis. The overlying epidermis was mildly acanthotic with both ulceration and follicular induction. There was trapping of individual adipocytes in a honeycomb pattern with foci of erythrocyte extravasation, microvesiculation, and widened fibrous septa (Figure 1). Immunohistochemistry was positive for vimentin, actin, and smooth muscle actin (SMA)(Figure 2A). Variable positivity for Factor XIIIa antibodies was noted. CD68 staining was focal positive, suggesting fibrohistiocytic lineage. Expression of CD31, CD34, S100, and anaplastic lymphoma kinase was negative, and Ki-67 was present in less than 10% of cells (Figure 2B).
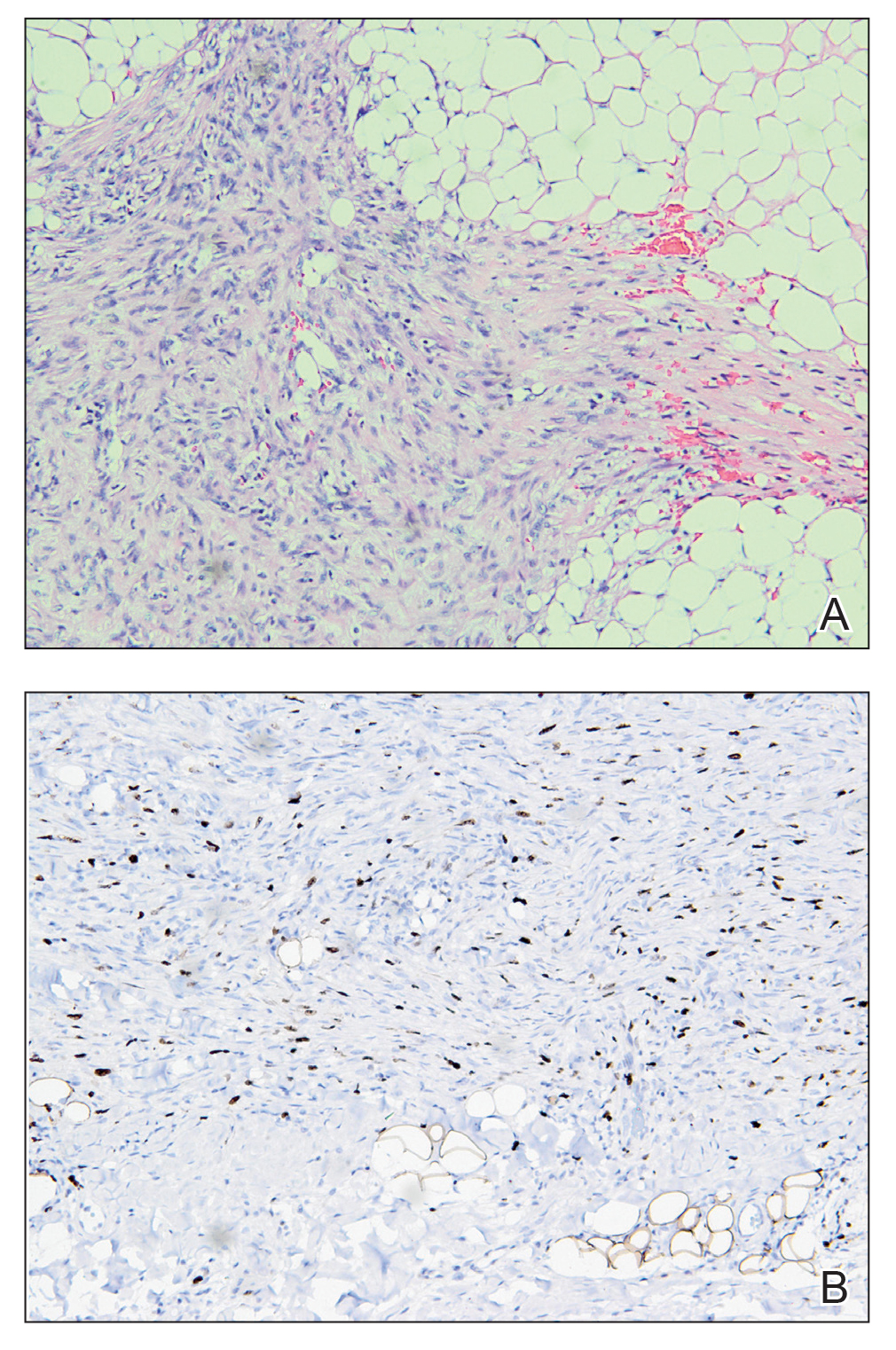
We reviewed the case in conjunction with a soft-tissue pathologist (Y.L.), and based on the clinical and immunophenotypic features, a diagnosis of plaquelike myofibroblastic tumor (PLMT) was made. The patient’s parents refused further treatment, and there was no sign of disease progression at 6-month follow-up.
Plaquelike myofibroblastic tumor is an unusual pediatric dermal tumor that was first described by Clarke et al1 in 2007. Clinical manifestation of PLMT on the right abdomen was unique in our patient, as the lesions typically present as indurated plaques on the lower back, but the central ulceration in our case resembled a report by Marqueling et al.2 Ulceration and induration of PLMT developing at 8 months of age can suggest an aggressive disease course corresponding with deep infiltration and is seen mostly in children.
The histopathologic features of PLMT include an acanthotic epidermis and follicular induction, which also are characteristic of dermatofibroma (DF). The proliferation of spindle cells extended deep into the fat with foci of erythrocyte extravasation and microvesiculation of the stroma similar to nodular fasciitis and proliferative fasciitis. The presentation of infiltrating and expanding fibrous septae and trapping of individual adipocytes in a honeycomb pattern is similar to dermatofibrosarcoma protuberans (DFSP). Most cases of PLMT are positive for SMA. Factor XIIIa typically is variably positive, and in one report, 31% (4/13) of cases showed positive staining for calponin.3 Rapid growth, ulceration, and recurrence emphasize that PLMT can be locally aggressive, similar to DFSP.4
The main differential diagnoses include DF and its variants, dermatomyofibroma, DFSP, and proliferative fasciitis.3,5 In the cases mentioned above, microscopic features were similar with a relatively well-circumscribed proliferation of spindle cells arranged in short fascicles through the entire reticular dermis, and the overlying epidermis was acanthotic.
Dermatofibroma commonly manifests in adults as a minor nodular lesion (commonly <1 cm), and usually is located on the legs. It has several clinical and histologic variants, including multiple clustered DF (MCDF)—a rare condition that has been reported in children and young adults and generally appears in the first and second decades of life. Of the reported cases of MCDF, immunohistochemical staining for SMA was performed in 8 cases. All these cases showed negative or minimal staining.3-5 Smooth muscle actin staining in DFs is negative, or weak and patchy, unlike in PLMT where it is diffuse, uniform, and strong.
Dermatofibrosarcoma protuberans typically occurs in young adults and manifests as dermal and subcutaneous nodular/multinodular or plaquelike masses, with rare congenital cases. Immunohistochemical staining for CD34, which typically is firmly and diffusely positive, is the most reliable marker of DFSP.6 Factor XIIIA in DFSP typically is negative for focal staining, mainly at periphery or in scattered dendritic cells. The prognosis of DFSP generally is excellent, with local recurrences in up to 30% of cases and extremely low metastatic potential (essentially only in cases with fibrosarcomatous transformation).6 Dermatomyofibroma is another rare benign dermal myofibroblastic tumor that typically manifests with indurated hyperpigmented or erythematous plaques or nodules on the shoulders and torso.6 This condition occurs mainly in adolescents and young adults, unlike PLMT. The most striking features of dermatomyofibroma are the horizontal orientation of the spindle cell nuclei and the pattern of the proliferation concerning the adnexal structures, especially hair follicles. The hair follicles have a normal appearance, and the proliferation extends up to each follicle, then continues to the other side without any displacement of the follicle. Tumor cells are variably positive for SMA in dermatomyofibromas and are negative for muscle-specific actin, desmin, S100, CD34, and Factor XIIIA.6
Immunohistochemistry can be very useful in differentiating PLMT from other conditions. Neoplastic cells stain positively for CD34 but not for Factor XIIIa and SMA in cases of DFSP. Dermatofibroma and its variants always present with collagen trapping at the periphery of the lesions and may demonstrate foamy macrophages, hemosiderin, or plasma cells FXIIIA(+), CD34(-), and variable SMA reactivity. This positivity usually is less prominent in DF than in PLMT. Neoplastic cells in dermatomyofibroma often stain positive for calponin, but only focally for SMA. The clinical features of dermatomyofibroma include early onset, large size, multiple nodules, and plaquelike morphology. Moulonguet et al4 hypothesized that, although MCDF and PLMT appear to show some distinctive clinical and histologic features, they also show similarities that could suggest they form part of the myofibroblastic spectrum. Furthermore, Moradi et al7 also considered them as part of the same disease spectrum because of their overlapping clinical, histologic, and immunohistochemical features.
The microscopic features in our case are notable, as the lesion demonstrated overlying acanthosis and follicular induction, resembling DF. The stroma contained microvesicular changes and erythrocyte extravasation, characteristic of nodular or proliferative fasciitis. Additionally, densely packed spindle cells infiltrated deep into the subcutaneous adipose tissue, similar to DFSP.2,3 Our findings expand on the reported histopathologic spectrum of this tumor to date.
- Clarke JT, Clarke LE, Miller C, et al. Plaque-like myofibroblastic tumor of infancy. Pediatr Dermatol. 2007;24:E83-E87. doi:10.1111 /j.1525-1470.2007.00449.x
- Marqueling AL, Dasher D, Friedlander SF, et al. Plaque-like myofibroblastic tumor: report of three cases. Pediatr Dermatol. 2013;30:600-607. doi:10.1111/pde.12185
- Sekar T, Mushtaq J, AlBadry W, et al. Plaque-like myofibroblastic tumor: a series of 2 cases of this unusual dermal tumor which occurs in infancy and early childhood. Pediatr Dev Pathol. 2018;21:444-448. doi: 10.1177/1093526617746807
- Moulonguet I, Biaggi A, Eschard C, et al. Plaque-like myofibroblastic tumor: report of 4 cases. Am J Dermatopathol. 2017;39:767-772. doi: 10.1097/DAD.0000000000000869
- Virdi A, Baraldi C, Barisani A, et al. Plaque-like myofibroblastic tumor, a rare entity of childhood: possible pitfalls in differential diagnosis. J Cutan Pathol. 2019;46:389-392. doi:10.1111/cup.13441
- Cassarino DS. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2021.
- Moradi S, Mnayer L, Earle J, et al. Plaque-like dermatofibroma: case report of a rare entity. Dermatopathology (Basel). 2021;8:337-341. doi:10.3390/dermatopathology8030038
THE DIAGNOSIS: Plaquelike Myofibroblastic Tumor
An incisional biopsy of the plaque demonstrated a hypercellular proliferation of bland spindle cells in the dermis that infiltrated the subcutis. The overlying epidermis was mildly acanthotic with both ulceration and follicular induction. There was trapping of individual adipocytes in a honeycomb pattern with foci of erythrocyte extravasation, microvesiculation, and widened fibrous septa (Figure 1). Immunohistochemistry was positive for vimentin, actin, and smooth muscle actin (SMA)(Figure 2A). Variable positivity for Factor XIIIa antibodies was noted. CD68 staining was focal positive, suggesting fibrohistiocytic lineage. Expression of CD31, CD34, S100, and anaplastic lymphoma kinase was negative, and Ki-67 was present in less than 10% of cells (Figure 2B).


We reviewed the case in conjunction with a soft-tissue pathologist (Y.L.), and based on the clinical and immunophenotypic features, a diagnosis of plaquelike myofibroblastic tumor (PLMT) was made. The patient’s parents refused further treatment, and there was no sign of disease progression at 6-month follow-up.
Plaquelike myofibroblastic tumor is an unusual pediatric dermal tumor that was first described by Clarke et al1 in 2007. Clinical manifestation of PLMT on the right abdomen was unique in our patient, as the lesions typically present as indurated plaques on the lower back, but the central ulceration in our case resembled a report by Marqueling et al.2 Ulceration and induration of PLMT developing at 8 months of age can suggest an aggressive disease course corresponding with deep infiltration and is seen mostly in children.
The histopathologic features of PLMT include an acanthotic epidermis and follicular induction, which also are characteristic of dermatofibroma (DF). The proliferation of spindle cells extended deep into the fat with foci of erythrocyte extravasation and microvesiculation of the stroma similar to nodular fasciitis and proliferative fasciitis. The presentation of infiltrating and expanding fibrous septae and trapping of individual adipocytes in a honeycomb pattern is similar to dermatofibrosarcoma protuberans (DFSP). Most cases of PLMT are positive for SMA. Factor XIIIa typically is variably positive, and in one report, 31% (4/13) of cases showed positive staining for calponin.3 Rapid growth, ulceration, and recurrence emphasize that PLMT can be locally aggressive, similar to DFSP.4
The main differential diagnoses include DF and its variants, dermatomyofibroma, DFSP, and proliferative fasciitis.3,5 In the cases mentioned above, microscopic features were similar with a relatively well-circumscribed proliferation of spindle cells arranged in short fascicles through the entire reticular dermis, and the overlying epidermis was acanthotic.
Dermatofibroma commonly manifests in adults as a minor nodular lesion (commonly <1 cm), and usually is located on the legs. It has several clinical and histologic variants, including multiple clustered DF (MCDF)—a rare condition that has been reported in children and young adults and generally appears in the first and second decades of life. Of the reported cases of MCDF, immunohistochemical staining for SMA was performed in 8 cases. All these cases showed negative or minimal staining.3-5 Smooth muscle actin staining in DFs is negative, or weak and patchy, unlike in PLMT where it is diffuse, uniform, and strong.
Dermatofibrosarcoma protuberans typically occurs in young adults and manifests as dermal and subcutaneous nodular/multinodular or plaquelike masses, with rare congenital cases. Immunohistochemical staining for CD34, which typically is firmly and diffusely positive, is the most reliable marker of DFSP.6 Factor XIIIA in DFSP typically is negative for focal staining, mainly at periphery or in scattered dendritic cells. The prognosis of DFSP generally is excellent, with local recurrences in up to 30% of cases and extremely low metastatic potential (essentially only in cases with fibrosarcomatous transformation).6 Dermatomyofibroma is another rare benign dermal myofibroblastic tumor that typically manifests with indurated hyperpigmented or erythematous plaques or nodules on the shoulders and torso.6 This condition occurs mainly in adolescents and young adults, unlike PLMT. The most striking features of dermatomyofibroma are the horizontal orientation of the spindle cell nuclei and the pattern of the proliferation concerning the adnexal structures, especially hair follicles. The hair follicles have a normal appearance, and the proliferation extends up to each follicle, then continues to the other side without any displacement of the follicle. Tumor cells are variably positive for SMA in dermatomyofibromas and are negative for muscle-specific actin, desmin, S100, CD34, and Factor XIIIA.6
Immunohistochemistry can be very useful in differentiating PLMT from other conditions. Neoplastic cells stain positively for CD34 but not for Factor XIIIa and SMA in cases of DFSP. Dermatofibroma and its variants always present with collagen trapping at the periphery of the lesions and may demonstrate foamy macrophages, hemosiderin, or plasma cells FXIIIA(+), CD34(-), and variable SMA reactivity. This positivity usually is less prominent in DF than in PLMT. Neoplastic cells in dermatomyofibroma often stain positive for calponin, but only focally for SMA. The clinical features of dermatomyofibroma include early onset, large size, multiple nodules, and plaquelike morphology. Moulonguet et al4 hypothesized that, although MCDF and PLMT appear to show some distinctive clinical and histologic features, they also show similarities that could suggest they form part of the myofibroblastic spectrum. Furthermore, Moradi et al7 also considered them as part of the same disease spectrum because of their overlapping clinical, histologic, and immunohistochemical features.
The microscopic features in our case are notable, as the lesion demonstrated overlying acanthosis and follicular induction, resembling DF. The stroma contained microvesicular changes and erythrocyte extravasation, characteristic of nodular or proliferative fasciitis. Additionally, densely packed spindle cells infiltrated deep into the subcutaneous adipose tissue, similar to DFSP.2,3 Our findings expand on the reported histopathologic spectrum of this tumor to date.
THE DIAGNOSIS: Plaquelike Myofibroblastic Tumor
An incisional biopsy of the plaque demonstrated a hypercellular proliferation of bland spindle cells in the dermis that infiltrated the subcutis. The overlying epidermis was mildly acanthotic with both ulceration and follicular induction. There was trapping of individual adipocytes in a honeycomb pattern with foci of erythrocyte extravasation, microvesiculation, and widened fibrous septa (Figure 1). Immunohistochemistry was positive for vimentin, actin, and smooth muscle actin (SMA)(Figure 2A). Variable positivity for Factor XIIIa antibodies was noted. CD68 staining was focal positive, suggesting fibrohistiocytic lineage. Expression of CD31, CD34, S100, and anaplastic lymphoma kinase was negative, and Ki-67 was present in less than 10% of cells (Figure 2B).


We reviewed the case in conjunction with a soft-tissue pathologist (Y.L.), and based on the clinical and immunophenotypic features, a diagnosis of plaquelike myofibroblastic tumor (PLMT) was made. The patient’s parents refused further treatment, and there was no sign of disease progression at 6-month follow-up.
Plaquelike myofibroblastic tumor is an unusual pediatric dermal tumor that was first described by Clarke et al1 in 2007. Clinical manifestation of PLMT on the right abdomen was unique in our patient, as the lesions typically present as indurated plaques on the lower back, but the central ulceration in our case resembled a report by Marqueling et al.2 Ulceration and induration of PLMT developing at 8 months of age can suggest an aggressive disease course corresponding with deep infiltration and is seen mostly in children.
The histopathologic features of PLMT include an acanthotic epidermis and follicular induction, which also are characteristic of dermatofibroma (DF). The proliferation of spindle cells extended deep into the fat with foci of erythrocyte extravasation and microvesiculation of the stroma similar to nodular fasciitis and proliferative fasciitis. The presentation of infiltrating and expanding fibrous septae and trapping of individual adipocytes in a honeycomb pattern is similar to dermatofibrosarcoma protuberans (DFSP). Most cases of PLMT are positive for SMA. Factor XIIIa typically is variably positive, and in one report, 31% (4/13) of cases showed positive staining for calponin.3 Rapid growth, ulceration, and recurrence emphasize that PLMT can be locally aggressive, similar to DFSP.4
The main differential diagnoses include DF and its variants, dermatomyofibroma, DFSP, and proliferative fasciitis.3,5 In the cases mentioned above, microscopic features were similar with a relatively well-circumscribed proliferation of spindle cells arranged in short fascicles through the entire reticular dermis, and the overlying epidermis was acanthotic.
Dermatofibroma commonly manifests in adults as a minor nodular lesion (commonly <1 cm), and usually is located on the legs. It has several clinical and histologic variants, including multiple clustered DF (MCDF)—a rare condition that has been reported in children and young adults and generally appears in the first and second decades of life. Of the reported cases of MCDF, immunohistochemical staining for SMA was performed in 8 cases. All these cases showed negative or minimal staining.3-5 Smooth muscle actin staining in DFs is negative, or weak and patchy, unlike in PLMT where it is diffuse, uniform, and strong.
Dermatofibrosarcoma protuberans typically occurs in young adults and manifests as dermal and subcutaneous nodular/multinodular or plaquelike masses, with rare congenital cases. Immunohistochemical staining for CD34, which typically is firmly and diffusely positive, is the most reliable marker of DFSP.6 Factor XIIIA in DFSP typically is negative for focal staining, mainly at periphery or in scattered dendritic cells. The prognosis of DFSP generally is excellent, with local recurrences in up to 30% of cases and extremely low metastatic potential (essentially only in cases with fibrosarcomatous transformation).6 Dermatomyofibroma is another rare benign dermal myofibroblastic tumor that typically manifests with indurated hyperpigmented or erythematous plaques or nodules on the shoulders and torso.6 This condition occurs mainly in adolescents and young adults, unlike PLMT. The most striking features of dermatomyofibroma are the horizontal orientation of the spindle cell nuclei and the pattern of the proliferation concerning the adnexal structures, especially hair follicles. The hair follicles have a normal appearance, and the proliferation extends up to each follicle, then continues to the other side without any displacement of the follicle. Tumor cells are variably positive for SMA in dermatomyofibromas and are negative for muscle-specific actin, desmin, S100, CD34, and Factor XIIIA.6
Immunohistochemistry can be very useful in differentiating PLMT from other conditions. Neoplastic cells stain positively for CD34 but not for Factor XIIIa and SMA in cases of DFSP. Dermatofibroma and its variants always present with collagen trapping at the periphery of the lesions and may demonstrate foamy macrophages, hemosiderin, or plasma cells FXIIIA(+), CD34(-), and variable SMA reactivity. This positivity usually is less prominent in DF than in PLMT. Neoplastic cells in dermatomyofibroma often stain positive for calponin, but only focally for SMA. The clinical features of dermatomyofibroma include early onset, large size, multiple nodules, and plaquelike morphology. Moulonguet et al4 hypothesized that, although MCDF and PLMT appear to show some distinctive clinical and histologic features, they also show similarities that could suggest they form part of the myofibroblastic spectrum. Furthermore, Moradi et al7 also considered them as part of the same disease spectrum because of their overlapping clinical, histologic, and immunohistochemical features.
The microscopic features in our case are notable, as the lesion demonstrated overlying acanthosis and follicular induction, resembling DF. The stroma contained microvesicular changes and erythrocyte extravasation, characteristic of nodular or proliferative fasciitis. Additionally, densely packed spindle cells infiltrated deep into the subcutaneous adipose tissue, similar to DFSP.2,3 Our findings expand on the reported histopathologic spectrum of this tumor to date.
- Clarke JT, Clarke LE, Miller C, et al. Plaque-like myofibroblastic tumor of infancy. Pediatr Dermatol. 2007;24:E83-E87. doi:10.1111 /j.1525-1470.2007.00449.x
- Marqueling AL, Dasher D, Friedlander SF, et al. Plaque-like myofibroblastic tumor: report of three cases. Pediatr Dermatol. 2013;30:600-607. doi:10.1111/pde.12185
- Sekar T, Mushtaq J, AlBadry W, et al. Plaque-like myofibroblastic tumor: a series of 2 cases of this unusual dermal tumor which occurs in infancy and early childhood. Pediatr Dev Pathol. 2018;21:444-448. doi: 10.1177/1093526617746807
- Moulonguet I, Biaggi A, Eschard C, et al. Plaque-like myofibroblastic tumor: report of 4 cases. Am J Dermatopathol. 2017;39:767-772. doi: 10.1097/DAD.0000000000000869
- Virdi A, Baraldi C, Barisani A, et al. Plaque-like myofibroblastic tumor, a rare entity of childhood: possible pitfalls in differential diagnosis. J Cutan Pathol. 2019;46:389-392. doi:10.1111/cup.13441
- Cassarino DS. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2021.
- Moradi S, Mnayer L, Earle J, et al. Plaque-like dermatofibroma: case report of a rare entity. Dermatopathology (Basel). 2021;8:337-341. doi:10.3390/dermatopathology8030038
- Clarke JT, Clarke LE, Miller C, et al. Plaque-like myofibroblastic tumor of infancy. Pediatr Dermatol. 2007;24:E83-E87. doi:10.1111 /j.1525-1470.2007.00449.x
- Marqueling AL, Dasher D, Friedlander SF, et al. Plaque-like myofibroblastic tumor: report of three cases. Pediatr Dermatol. 2013;30:600-607. doi:10.1111/pde.12185
- Sekar T, Mushtaq J, AlBadry W, et al. Plaque-like myofibroblastic tumor: a series of 2 cases of this unusual dermal tumor which occurs in infancy and early childhood. Pediatr Dev Pathol. 2018;21:444-448. doi: 10.1177/1093526617746807
- Moulonguet I, Biaggi A, Eschard C, et al. Plaque-like myofibroblastic tumor: report of 4 cases. Am J Dermatopathol. 2017;39:767-772. doi: 10.1097/DAD.0000000000000869
- Virdi A, Baraldi C, Barisani A, et al. Plaque-like myofibroblastic tumor, a rare entity of childhood: possible pitfalls in differential diagnosis. J Cutan Pathol. 2019;46:389-392. doi:10.1111/cup.13441
- Cassarino DS. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2021.
- Moradi S, Mnayer L, Earle J, et al. Plaque-like dermatofibroma: case report of a rare entity. Dermatopathology (Basel). 2021;8:337-341. doi:10.3390/dermatopathology8030038
Plaque With Central Ulceration on the Abdomen
Plaque With Central Ulceration on the Abdomen
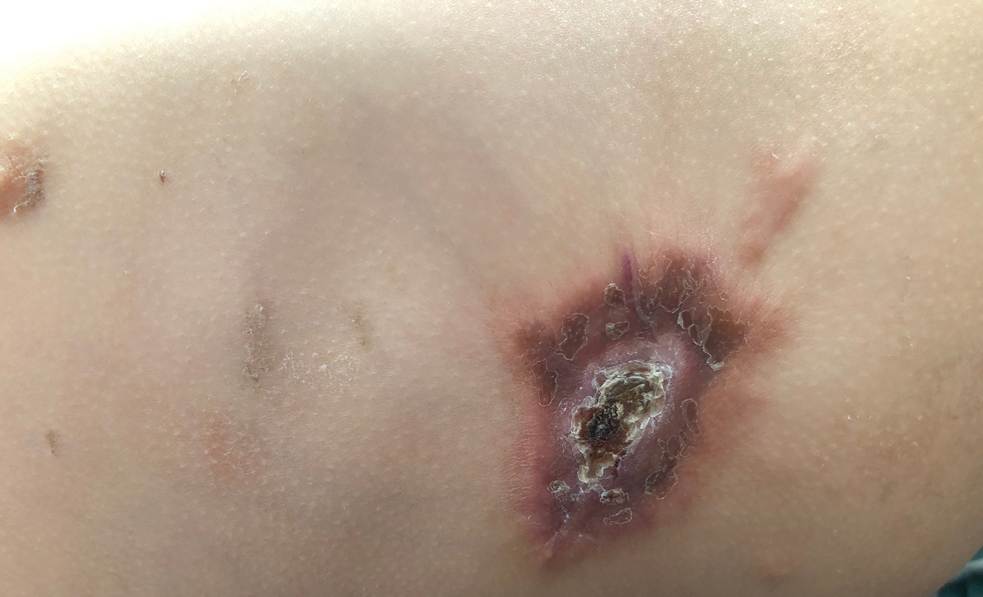
A 14-month-old girl presented to the dermatology department with a firm asymptomatic lesion on the abdomen of 6 months’ duration. The lesion started as a flesh-colored papule and developed slowly into an indurated plaque that darkened in color. The patient had no history of trauma to the area. Physical examination revealed a dark reddish–brown, indurated, irregularly shaped plaque with central ulceration and elevated borders on the right abdomen. The plaque measured 2×3 cm with a few smaller satellite nodules distributed along the periphery. Abdominal ultrasonography revealed a multinodular proliferation in the dermis and subcutis of the right abdomen.
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
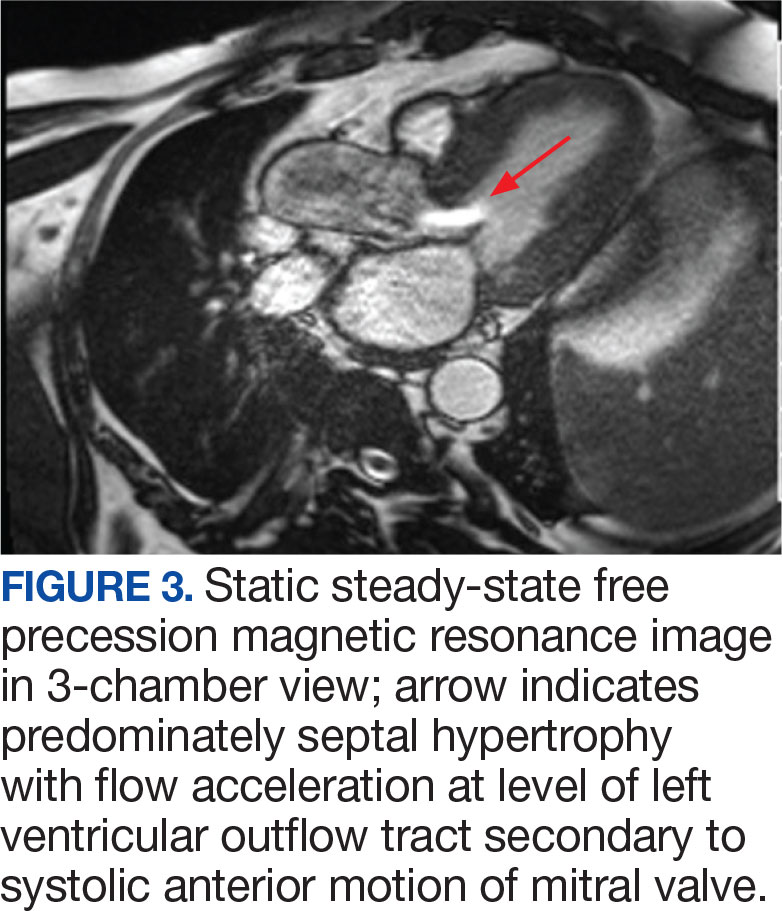
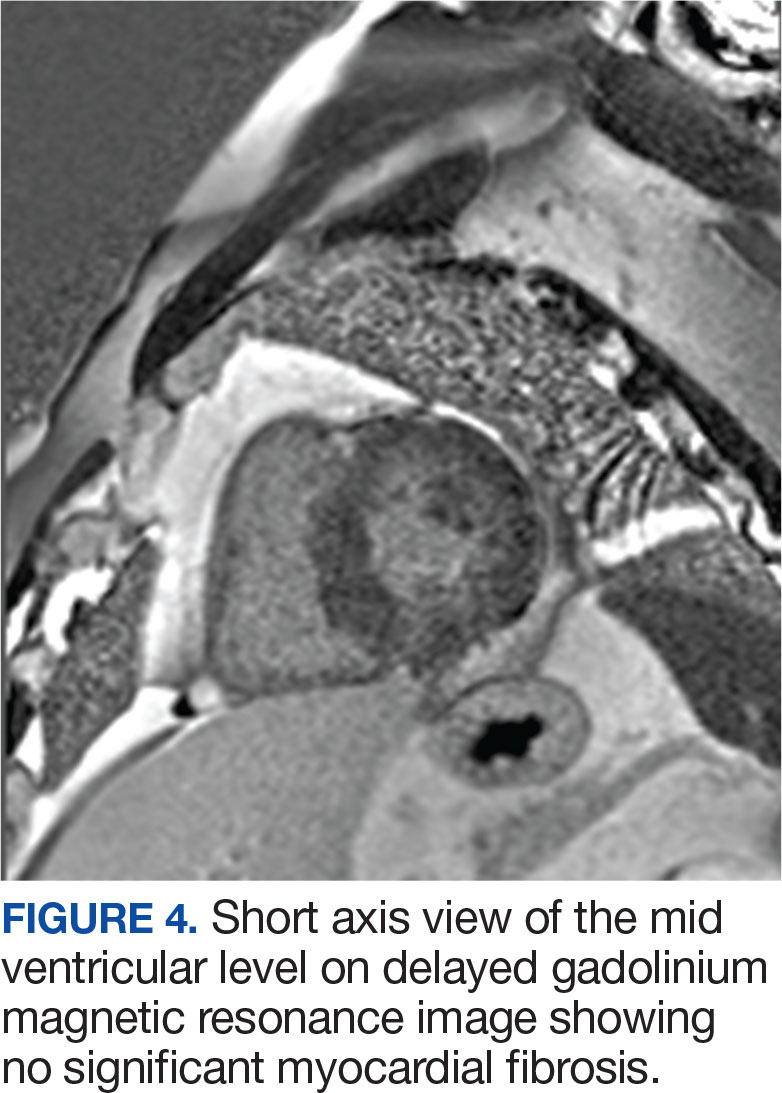
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.
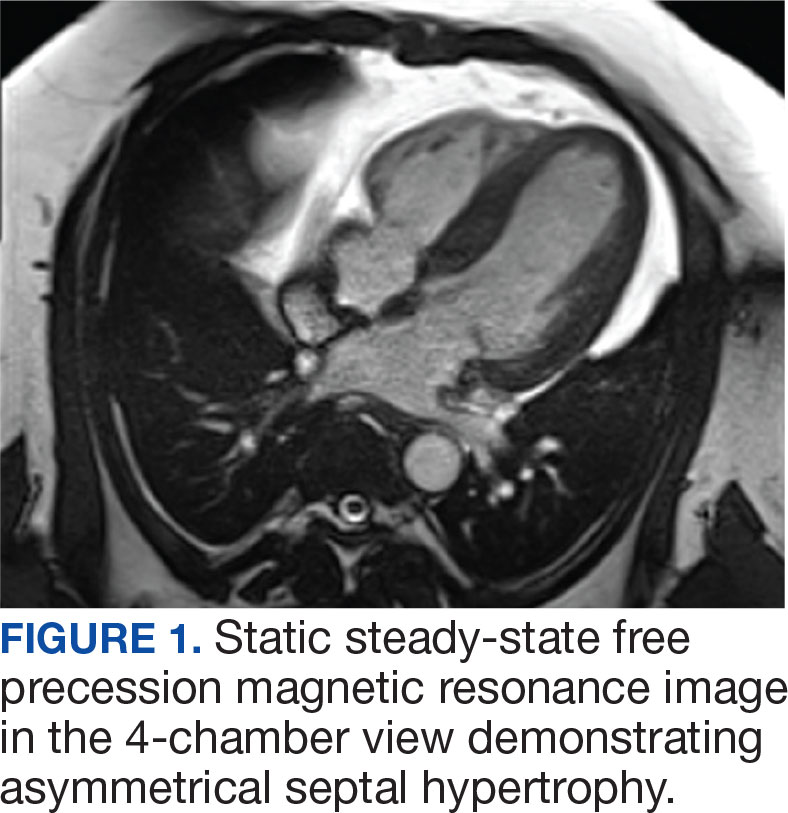
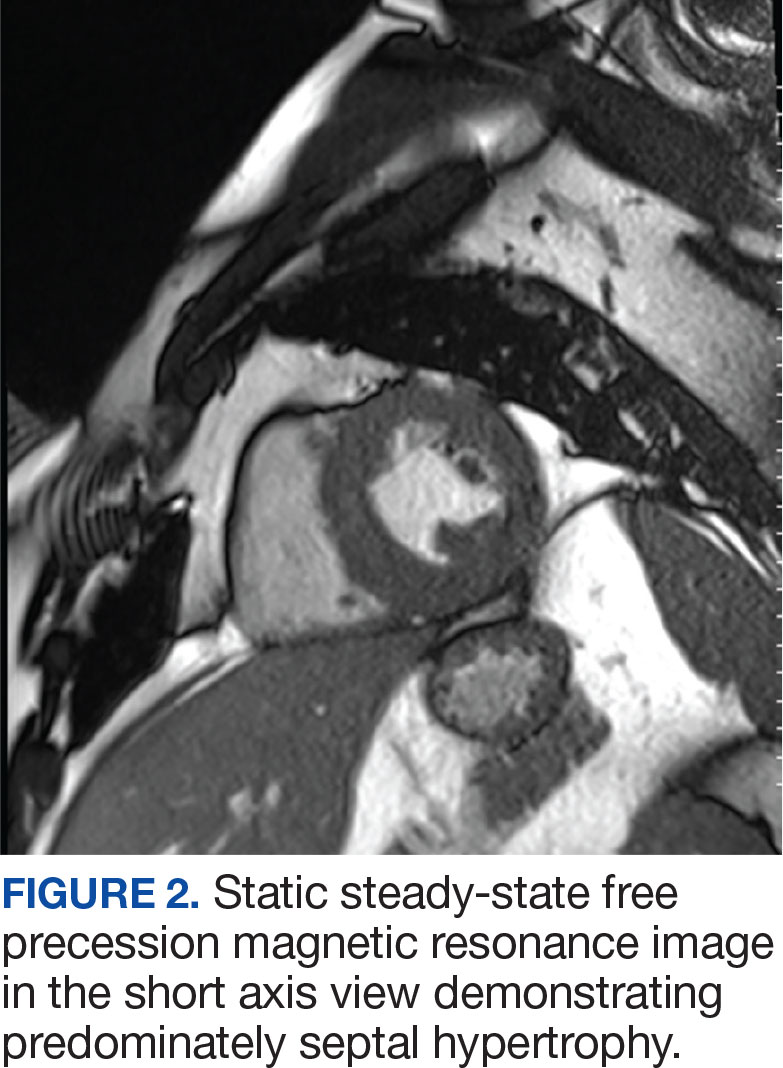
DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.
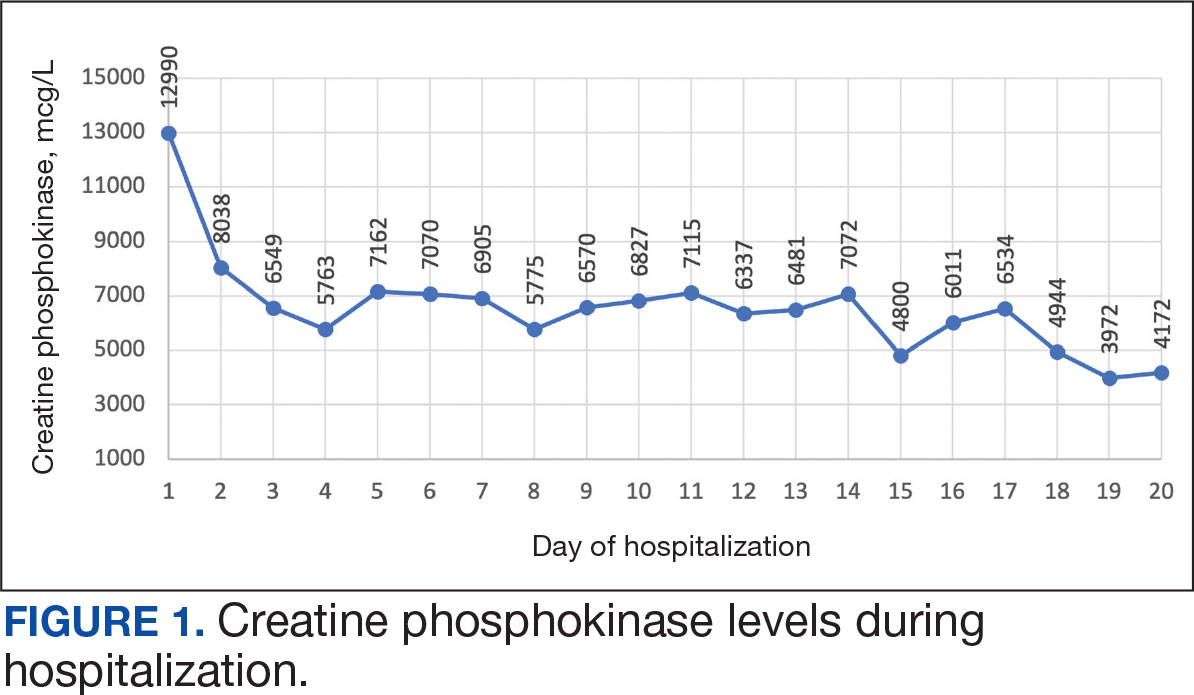
After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).
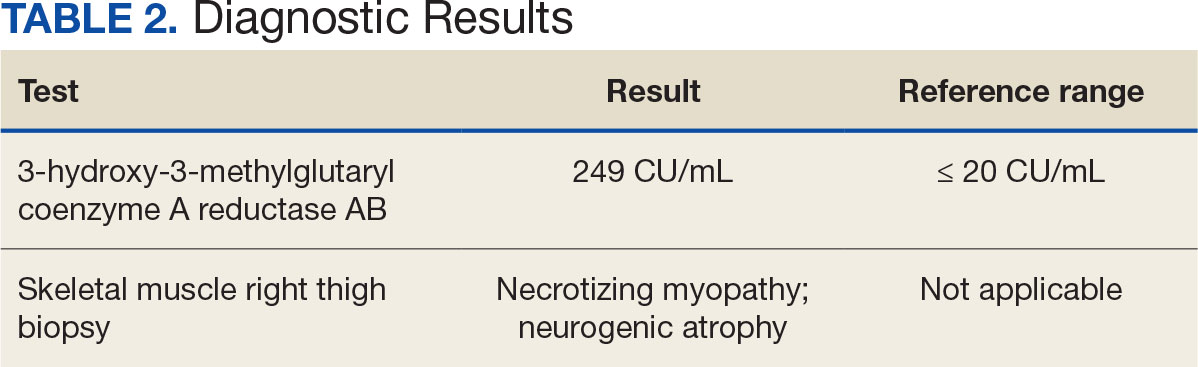
On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.
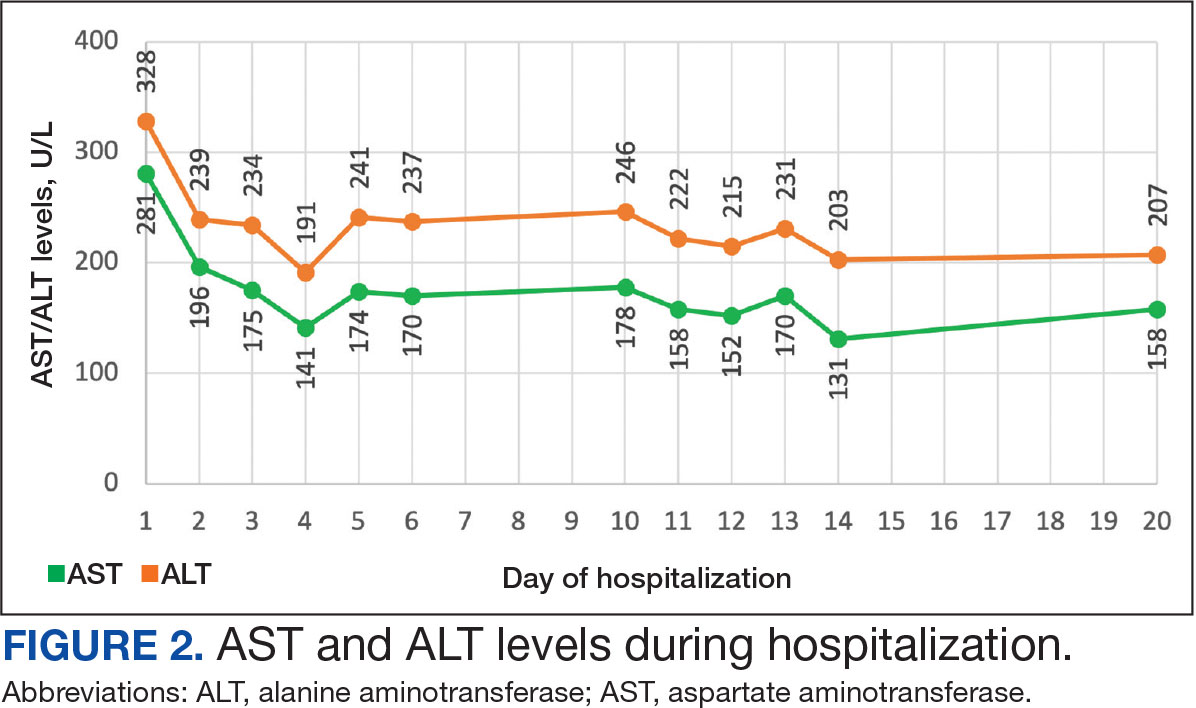
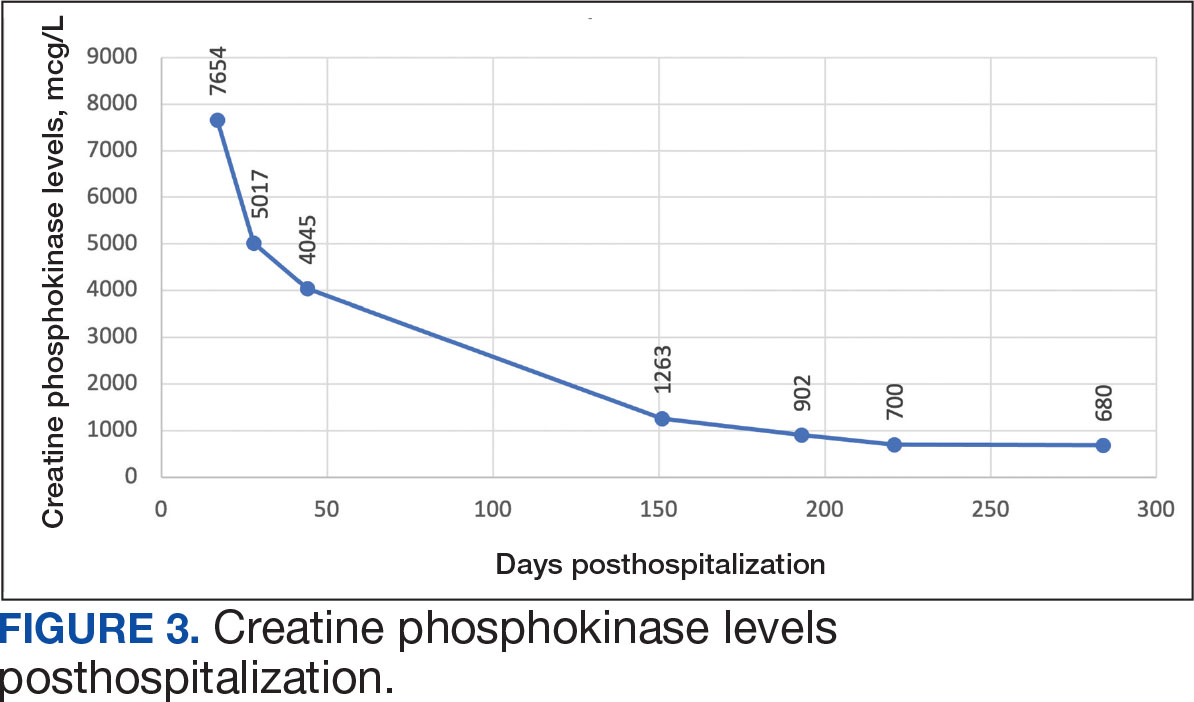
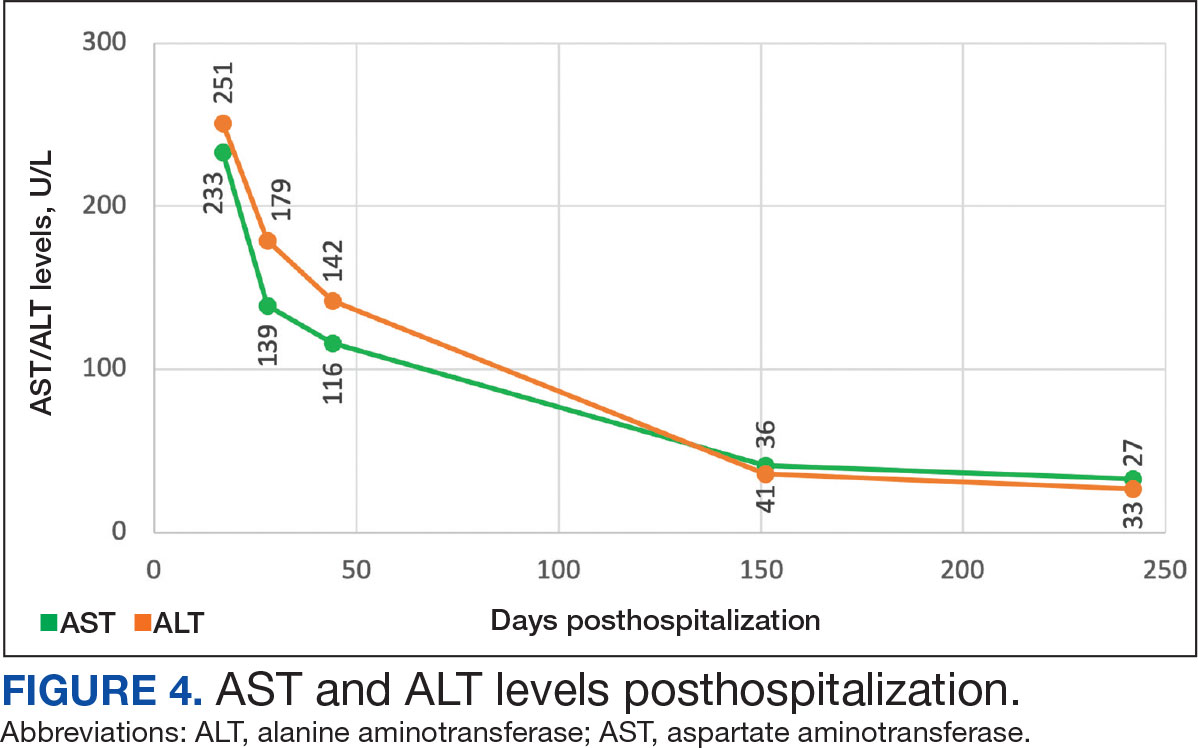
The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.

After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).

On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.



The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.

After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).

On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.



The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Scholarly Activity Among VA Podiatrists: A Cross-Sectional Study
Scholarly Activity Among VA Podiatrists: A Cross-Sectional Study
The US Department of Veterans Affairs (VA) delivers care to > 9 million veterans, including primary and specialty care.1 While clinical duties remain important across the health system, proposed productivity models have included clinician research activity, given that many hold roles in academia.2 Within this framework, research plays a pivotal role in advancing clinical practices and outcomes. Studies have found that physicians who participated in research report higher job satisfaction.3
As a specialty within the VA, podiatrists diagnose, treat, and prevent foot and ankle disorders. In addition to clinical practice, various scholarly activities are shared among these physicians.4 Reasons for scholarly pursuits among podiatrists vary, including participation in research for academic promotion or to establish expertise in a given area.4-7 Although research remains a component associated with promotion within the VA, little is known about the scholarly activity of VA podiatrists. Specifically, there remains a paucity of data concerning their expertise, as evidenced through peer-reviewed publications, among these physicians and surgeons. To date, no analysis of scholarly activity among VA podiatrists has been conducted.
The primary aim of this investigation was to describe the scholarly productivity among podiatrists employed by the VA through an analysis of the number of peer-reviewed publications and the respective h-index of each physician. The secondary aim of this investigation was to assess the effect of academic productivity on compensation. This study describes research activities pursued by VA physicians and provides the veteran patient population with the confidence that their foot health care remains in the hands of experts within the field.
MATERIALS AND METHODS
The Feds Data Center (www.fedsdatacenter.com) online database of employees was used to identify VA podiatrists on June 17, 2024. All GS-15 physicians and their respective salaries in fiscal year 2023 were recorded. Administratively determined employees, including residents, were excluded. The h-index and number of published documents from any point during a physician’s training or career were reported for each podiatrist using Scopus; podiatrists without an h-index or publication were excluded. 8 Among podiatrists with scholarly activity, this analysis collected academic appointment, sex, and region of practice.
Statistical Analysis
Descriptive statistics, presented as counts and frequencies, were used. The median and IQR were used to describe the number of publications and h-index due to their nonnormal distribution. A Kruskal-Wallis test was used to compare median publication counts and h-index values among for junior faculty (JF), which includes instructors and assistant professors; senior faculty (SF), which includes associate professors and professors; and those with no academic affiliation (NF). Salary was reported as mean (SD) as it remained normally distributed and was compared using analysis of variance with posthoc Tukey test to increase statistical power. Additionally, this analysis used linear regression to investigate the relationship between scholarly activity and salary. The threshold for statistical significance was set at P < .05.
RESULTS
Among 819 VA podiatrists, 150 were administratively determined and excluded, and 512 were excluded for no history of publications, leaving 157 eligible for analysis (Table). A statistically significant difference was found in median (IQR) publication count by faculty appointment. JF had 6.0 (9.5), SF had 12.5 (22.3), and NF had 1.0 (2.0) publication(s) (P < .001) (Figure 1A). There was a statistically significant difference in h-index by faculty appointment. The median (IQR) h-index for JF was 2.0 (3.5), for SF was 5.5 (4.25), and for NF was 1.0 (2.0) (P = .002) (Figure 1B). Salary was not significantly associated with publication count (P = .20) or h-index (P = .62) (Figure 2). No statistically significant difference was found between academic appointment and mean (SD) salary. JF had a median (IQR) salary of $224,063 (27,989), SF of $234,260 (42,963), and NF of $219,811 (P = .35).
(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.
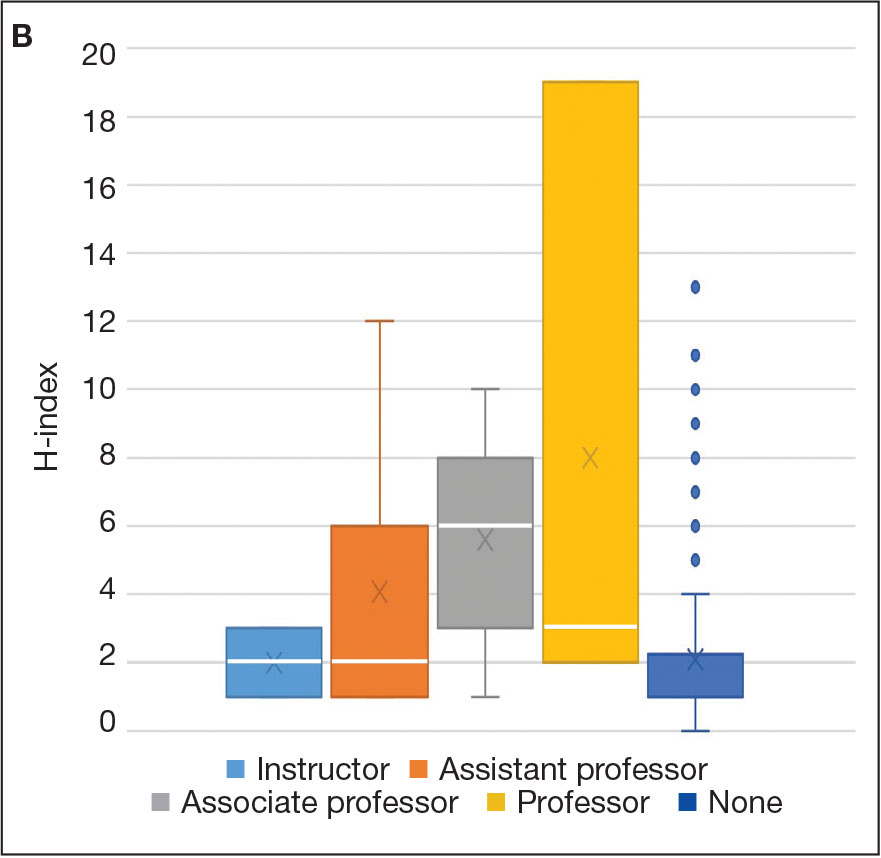
(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.
DISCUSSION
Focused on providing high-quality care, VA physicians use their expertise to practice comprehensive and specialized care.9,10 A cornerstone to this expertise is scholarly activity that contributes to the body of knowledge and, ultimately, the evidence-based medicine by which these physicians practice.11 With veterans considering VA care, it is important to highlight the commitment and dedication to the science and the practice of medicine. This analysis describes the scholarly activity of VA podiatrists and underscores the expertise veterans will receive for the diagnosis and treatment of their foot and ankle pathology.
were not part of an academic facility, a finding that may encourage further action to increase academic productivity in this specialty. For example, collaboration through academic affiliations has been seen throughout VA medical and surgical specialties and provides many benefits. Beginning with graduate medical education, the VA serves as a tremendous resource for resident training.12 Additionally, veterans who sought emergency care at the VA had a lower risk of death than those treated at non-VA hospitals.13 In podiatric medicine and surgery, scholarly activity has been linked to improved outcomes, particularly in the study of ulceration development and its role in either prolonging or preventing amputation.14
Beyond improving clinical outcomes and patient care, engagement in research and inquiry offers other benefits. A cross-sectional study of 7734 physicians within the VA found that research involvement was associated with more favorable job characteristics and job satisfaction perceptions. 3 While this analysis found that about 19% of podiatrists have published once in their career, it remains likely that more may continue to engage in research during their VA tenure. Although this finding shows that an appreciable number of VA podiatrists have published in their field of study, it also encourages departments to provide resources to engage in research. Similar to previous research among foot and ankle surgeons, this analysis also found an increase in publications and h-index as tenure increased.4 Unlike previous research, which found h-index and academic appointment to be contributors to VA dermatologists’ salaries, no significant difference in salary was found in this study associated with publications, h-index, or academic role.15 Although the increase was not statistically significant, salary tended to rise as these variables increased.
Limitations
This analysis was confined to the most recent year of available data, which may not fully capture the longitudinal academic contributions and trends of individual podiatrists. Academic productivity can fluctuate significantly over time due to various factors, including changes in research focus and administrative responsibilities. The study also relied on Scopus to identify and quantify academic productivity. This database may not include all publications relevant to podiatrists, particularly those in niche or nonindexed journals. Additionally, name variations and potential misspellings could lead to missing data for individual podiatrists’ publications. Furthermore, this study did not account for other significant contributors to salary and career advancement within the federal system. Factors such as clinical performance, administrative duties, patient satisfaction, and contributions to teaching and mentoring are critical elements that also influence career progression and compensation but were not captured in this analysis. The retrospective design of this study inherently limits the ability to establish causal relationships. While associations between academic productivity and certain outcomes may be identified, it is not possible to definitively determine the direction or causality of these relationships. Future research may examine how scholarly activity continues once a clinician is part of VA.
CONCLUSIONS
This study highlights the significant academic contributions of VA podiatrists to research and the medical literature. By fostering an active research environment, the VA can ensure veterans receive the highest quality of care from knowledgeable and expert clinicians. Future research should aim to provide a more comprehensive analysis, capturing long-term trends and considering all factors influencing career advancement in VA.
- Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
- Coleman DL, Moran E, Serfilippi D, et al. Measuring physicians’ productivity in a Veterans’ Affairs Medical Center. Acad Med. 2003;78(7):682-689. doi:10.1097/00001888-200307000-00007
- Mohr DC, Burgess JF Jr. Job characteristics and job satisfaction among physicians involved with research in the Veterans Health Administration. Acad Med. 2011;86(8):938-945. doi:10.1097/ACM.0b013e3182223b76
- Casciato DJ, Cravey KS, Barron IM. Scholarly productivity among academic foot and ankle surgeons affiliated with US podiatric medicine and surgery residency and fellowship training programs. J Foot Ankle Surg. 2021;60(6):1222-1226. doi:10.1053/j.jfas.2021.04.017
- Hyer CF, Casciato DJ, Rushing CJ, Schuberth JM. Incidence of scholarly publication by selected content experts presenting at national society foot and ankle meetings from 2016 to 2020. J Foot Ankle Surg. 2022;61(6):1317-1320. doi:10.1053/j.jfas.2022.04.011
- Casciato DJ, Thompson J, Yancovitz S, Chandra A, Prissel MA, Hyer CF. Research activity among foot and ankle surgery fellows: a systematic review. J Foot Ankle Surg. 2021;60(6):1227-1231. doi:10.1053/j.jfas.2021.04.018
- Casciato DJ, Thompson J, Hyer CF. Post-fellowship foot and ankle surgeon research productivity: a systematic review. J Foot Ankle Surg. 2022;61(4):896-899. doi:10.1053/j.jfas.2021.12.028
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA. 2005;102(46):16569-16572. doi:10.1073/pnas.0507655102
- US Department of Veterans Affairs. Veterans Health Administration. About VHA. Updated January 20, 2025. Accessed February 17, 2025. https://www.va.gov/health/aboutvha.asp
- US Department of Veterans Affairs. VHA National Center for Patient Safety. About Us. Updated November 29, 2023. Accessed February 17, 2025. https://www.patientsafety.va.gov/
- US Department of Veterans Affairs. VA/DoD Clinical Practice Guidelines. Updated February 7, 2025. Accessed February 17, 2025. https://www.healthquality.va.gov
- Ravin AG, Gottlieb NB, Wang HT, et al. Effect of the Veterans Affairs Medical System on plastic surgery residency training. Plast Reconstr Surg. 2006;117(2):656-660. doi:10.1097/01.prs.0000197216.95544.f7
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Gibson LW, Abbas A. Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2013;25(1):131-134. doi:10.1016/j.ccell.2012.11.004
- Do MH, Lipner SR. Contribution of gender on compensation of Veterans Affairs-affiliated dermatologists: a cross-sectional study. Int J Womens Dermatol. 2020;6(5):414-418. doi:10.1016/j.ijwd.2020.09.009
The US Department of Veterans Affairs (VA) delivers care to > 9 million veterans, including primary and specialty care.1 While clinical duties remain important across the health system, proposed productivity models have included clinician research activity, given that many hold roles in academia.2 Within this framework, research plays a pivotal role in advancing clinical practices and outcomes. Studies have found that physicians who participated in research report higher job satisfaction.3
As a specialty within the VA, podiatrists diagnose, treat, and prevent foot and ankle disorders. In addition to clinical practice, various scholarly activities are shared among these physicians.4 Reasons for scholarly pursuits among podiatrists vary, including participation in research for academic promotion or to establish expertise in a given area.4-7 Although research remains a component associated with promotion within the VA, little is known about the scholarly activity of VA podiatrists. Specifically, there remains a paucity of data concerning their expertise, as evidenced through peer-reviewed publications, among these physicians and surgeons. To date, no analysis of scholarly activity among VA podiatrists has been conducted.
The primary aim of this investigation was to describe the scholarly productivity among podiatrists employed by the VA through an analysis of the number of peer-reviewed publications and the respective h-index of each physician. The secondary aim of this investigation was to assess the effect of academic productivity on compensation. This study describes research activities pursued by VA physicians and provides the veteran patient population with the confidence that their foot health care remains in the hands of experts within the field.
MATERIALS AND METHODS
The Feds Data Center (www.fedsdatacenter.com) online database of employees was used to identify VA podiatrists on June 17, 2024. All GS-15 physicians and their respective salaries in fiscal year 2023 were recorded. Administratively determined employees, including residents, were excluded. The h-index and number of published documents from any point during a physician’s training or career were reported for each podiatrist using Scopus; podiatrists without an h-index or publication were excluded. 8 Among podiatrists with scholarly activity, this analysis collected academic appointment, sex, and region of practice.
Statistical Analysis
Descriptive statistics, presented as counts and frequencies, were used. The median and IQR were used to describe the number of publications and h-index due to their nonnormal distribution. A Kruskal-Wallis test was used to compare median publication counts and h-index values among for junior faculty (JF), which includes instructors and assistant professors; senior faculty (SF), which includes associate professors and professors; and those with no academic affiliation (NF). Salary was reported as mean (SD) as it remained normally distributed and was compared using analysis of variance with posthoc Tukey test to increase statistical power. Additionally, this analysis used linear regression to investigate the relationship between scholarly activity and salary. The threshold for statistical significance was set at P < .05.
RESULTS
Among 819 VA podiatrists, 150 were administratively determined and excluded, and 512 were excluded for no history of publications, leaving 157 eligible for analysis (Table). A statistically significant difference was found in median (IQR) publication count by faculty appointment. JF had 6.0 (9.5), SF had 12.5 (22.3), and NF had 1.0 (2.0) publication(s) (P < .001) (Figure 1A). There was a statistically significant difference in h-index by faculty appointment. The median (IQR) h-index for JF was 2.0 (3.5), for SF was 5.5 (4.25), and for NF was 1.0 (2.0) (P = .002) (Figure 1B). Salary was not significantly associated with publication count (P = .20) or h-index (P = .62) (Figure 2). No statistically significant difference was found between academic appointment and mean (SD) salary. JF had a median (IQR) salary of $224,063 (27,989), SF of $234,260 (42,963), and NF of $219,811 (P = .35).

(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.

(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.


DISCUSSION
Focused on providing high-quality care, VA physicians use their expertise to practice comprehensive and specialized care.9,10 A cornerstone to this expertise is scholarly activity that contributes to the body of knowledge and, ultimately, the evidence-based medicine by which these physicians practice.11 With veterans considering VA care, it is important to highlight the commitment and dedication to the science and the practice of medicine. This analysis describes the scholarly activity of VA podiatrists and underscores the expertise veterans will receive for the diagnosis and treatment of their foot and ankle pathology.
were not part of an academic facility, a finding that may encourage further action to increase academic productivity in this specialty. For example, collaboration through academic affiliations has been seen throughout VA medical and surgical specialties and provides many benefits. Beginning with graduate medical education, the VA serves as a tremendous resource for resident training.12 Additionally, veterans who sought emergency care at the VA had a lower risk of death than those treated at non-VA hospitals.13 In podiatric medicine and surgery, scholarly activity has been linked to improved outcomes, particularly in the study of ulceration development and its role in either prolonging or preventing amputation.14
Beyond improving clinical outcomes and patient care, engagement in research and inquiry offers other benefits. A cross-sectional study of 7734 physicians within the VA found that research involvement was associated with more favorable job characteristics and job satisfaction perceptions. 3 While this analysis found that about 19% of podiatrists have published once in their career, it remains likely that more may continue to engage in research during their VA tenure. Although this finding shows that an appreciable number of VA podiatrists have published in their field of study, it also encourages departments to provide resources to engage in research. Similar to previous research among foot and ankle surgeons, this analysis also found an increase in publications and h-index as tenure increased.4 Unlike previous research, which found h-index and academic appointment to be contributors to VA dermatologists’ salaries, no significant difference in salary was found in this study associated with publications, h-index, or academic role.15 Although the increase was not statistically significant, salary tended to rise as these variables increased.
Limitations
This analysis was confined to the most recent year of available data, which may not fully capture the longitudinal academic contributions and trends of individual podiatrists. Academic productivity can fluctuate significantly over time due to various factors, including changes in research focus and administrative responsibilities. The study also relied on Scopus to identify and quantify academic productivity. This database may not include all publications relevant to podiatrists, particularly those in niche or nonindexed journals. Additionally, name variations and potential misspellings could lead to missing data for individual podiatrists’ publications. Furthermore, this study did not account for other significant contributors to salary and career advancement within the federal system. Factors such as clinical performance, administrative duties, patient satisfaction, and contributions to teaching and mentoring are critical elements that also influence career progression and compensation but were not captured in this analysis. The retrospective design of this study inherently limits the ability to establish causal relationships. While associations between academic productivity and certain outcomes may be identified, it is not possible to definitively determine the direction or causality of these relationships. Future research may examine how scholarly activity continues once a clinician is part of VA.
CONCLUSIONS
This study highlights the significant academic contributions of VA podiatrists to research and the medical literature. By fostering an active research environment, the VA can ensure veterans receive the highest quality of care from knowledgeable and expert clinicians. Future research should aim to provide a more comprehensive analysis, capturing long-term trends and considering all factors influencing career advancement in VA.
The US Department of Veterans Affairs (VA) delivers care to > 9 million veterans, including primary and specialty care.1 While clinical duties remain important across the health system, proposed productivity models have included clinician research activity, given that many hold roles in academia.2 Within this framework, research plays a pivotal role in advancing clinical practices and outcomes. Studies have found that physicians who participated in research report higher job satisfaction.3
As a specialty within the VA, podiatrists diagnose, treat, and prevent foot and ankle disorders. In addition to clinical practice, various scholarly activities are shared among these physicians.4 Reasons for scholarly pursuits among podiatrists vary, including participation in research for academic promotion or to establish expertise in a given area.4-7 Although research remains a component associated with promotion within the VA, little is known about the scholarly activity of VA podiatrists. Specifically, there remains a paucity of data concerning their expertise, as evidenced through peer-reviewed publications, among these physicians and surgeons. To date, no analysis of scholarly activity among VA podiatrists has been conducted.
The primary aim of this investigation was to describe the scholarly productivity among podiatrists employed by the VA through an analysis of the number of peer-reviewed publications and the respective h-index of each physician. The secondary aim of this investigation was to assess the effect of academic productivity on compensation. This study describes research activities pursued by VA physicians and provides the veteran patient population with the confidence that their foot health care remains in the hands of experts within the field.
MATERIALS AND METHODS
The Feds Data Center (www.fedsdatacenter.com) online database of employees was used to identify VA podiatrists on June 17, 2024. All GS-15 physicians and their respective salaries in fiscal year 2023 were recorded. Administratively determined employees, including residents, were excluded. The h-index and number of published documents from any point during a physician’s training or career were reported for each podiatrist using Scopus; podiatrists without an h-index or publication were excluded. 8 Among podiatrists with scholarly activity, this analysis collected academic appointment, sex, and region of practice.
Statistical Analysis
Descriptive statistics, presented as counts and frequencies, were used. The median and IQR were used to describe the number of publications and h-index due to their nonnormal distribution. A Kruskal-Wallis test was used to compare median publication counts and h-index values among for junior faculty (JF), which includes instructors and assistant professors; senior faculty (SF), which includes associate professors and professors; and those with no academic affiliation (NF). Salary was reported as mean (SD) as it remained normally distributed and was compared using analysis of variance with posthoc Tukey test to increase statistical power. Additionally, this analysis used linear regression to investigate the relationship between scholarly activity and salary. The threshold for statistical significance was set at P < .05.
RESULTS
Among 819 VA podiatrists, 150 were administratively determined and excluded, and 512 were excluded for no history of publications, leaving 157 eligible for analysis (Table). A statistically significant difference was found in median (IQR) publication count by faculty appointment. JF had 6.0 (9.5), SF had 12.5 (22.3), and NF had 1.0 (2.0) publication(s) (P < .001) (Figure 1A). There was a statistically significant difference in h-index by faculty appointment. The median (IQR) h-index for JF was 2.0 (3.5), for SF was 5.5 (4.25), and for NF was 1.0 (2.0) (P = .002) (Figure 1B). Salary was not significantly associated with publication count (P = .20) or h-index (P = .62) (Figure 2). No statistically significant difference was found between academic appointment and mean (SD) salary. JF had a median (IQR) salary of $224,063 (27,989), SF of $234,260 (42,963), and NF of $219,811 (P = .35).

(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.

(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.


DISCUSSION
Focused on providing high-quality care, VA physicians use their expertise to practice comprehensive and specialized care.9,10 A cornerstone to this expertise is scholarly activity that contributes to the body of knowledge and, ultimately, the evidence-based medicine by which these physicians practice.11 With veterans considering VA care, it is important to highlight the commitment and dedication to the science and the practice of medicine. This analysis describes the scholarly activity of VA podiatrists and underscores the expertise veterans will receive for the diagnosis and treatment of their foot and ankle pathology.
were not part of an academic facility, a finding that may encourage further action to increase academic productivity in this specialty. For example, collaboration through academic affiliations has been seen throughout VA medical and surgical specialties and provides many benefits. Beginning with graduate medical education, the VA serves as a tremendous resource for resident training.12 Additionally, veterans who sought emergency care at the VA had a lower risk of death than those treated at non-VA hospitals.13 In podiatric medicine and surgery, scholarly activity has been linked to improved outcomes, particularly in the study of ulceration development and its role in either prolonging or preventing amputation.14
Beyond improving clinical outcomes and patient care, engagement in research and inquiry offers other benefits. A cross-sectional study of 7734 physicians within the VA found that research involvement was associated with more favorable job characteristics and job satisfaction perceptions. 3 While this analysis found that about 19% of podiatrists have published once in their career, it remains likely that more may continue to engage in research during their VA tenure. Although this finding shows that an appreciable number of VA podiatrists have published in their field of study, it also encourages departments to provide resources to engage in research. Similar to previous research among foot and ankle surgeons, this analysis also found an increase in publications and h-index as tenure increased.4 Unlike previous research, which found h-index and academic appointment to be contributors to VA dermatologists’ salaries, no significant difference in salary was found in this study associated with publications, h-index, or academic role.15 Although the increase was not statistically significant, salary tended to rise as these variables increased.
Limitations
This analysis was confined to the most recent year of available data, which may not fully capture the longitudinal academic contributions and trends of individual podiatrists. Academic productivity can fluctuate significantly over time due to various factors, including changes in research focus and administrative responsibilities. The study also relied on Scopus to identify and quantify academic productivity. This database may not include all publications relevant to podiatrists, particularly those in niche or nonindexed journals. Additionally, name variations and potential misspellings could lead to missing data for individual podiatrists’ publications. Furthermore, this study did not account for other significant contributors to salary and career advancement within the federal system. Factors such as clinical performance, administrative duties, patient satisfaction, and contributions to teaching and mentoring are critical elements that also influence career progression and compensation but were not captured in this analysis. The retrospective design of this study inherently limits the ability to establish causal relationships. While associations between academic productivity and certain outcomes may be identified, it is not possible to definitively determine the direction or causality of these relationships. Future research may examine how scholarly activity continues once a clinician is part of VA.
CONCLUSIONS
This study highlights the significant academic contributions of VA podiatrists to research and the medical literature. By fostering an active research environment, the VA can ensure veterans receive the highest quality of care from knowledgeable and expert clinicians. Future research should aim to provide a more comprehensive analysis, capturing long-term trends and considering all factors influencing career advancement in VA.
- Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
- Coleman DL, Moran E, Serfilippi D, et al. Measuring physicians’ productivity in a Veterans’ Affairs Medical Center. Acad Med. 2003;78(7):682-689. doi:10.1097/00001888-200307000-00007
- Mohr DC, Burgess JF Jr. Job characteristics and job satisfaction among physicians involved with research in the Veterans Health Administration. Acad Med. 2011;86(8):938-945. doi:10.1097/ACM.0b013e3182223b76
- Casciato DJ, Cravey KS, Barron IM. Scholarly productivity among academic foot and ankle surgeons affiliated with US podiatric medicine and surgery residency and fellowship training programs. J Foot Ankle Surg. 2021;60(6):1222-1226. doi:10.1053/j.jfas.2021.04.017
- Hyer CF, Casciato DJ, Rushing CJ, Schuberth JM. Incidence of scholarly publication by selected content experts presenting at national society foot and ankle meetings from 2016 to 2020. J Foot Ankle Surg. 2022;61(6):1317-1320. doi:10.1053/j.jfas.2022.04.011
- Casciato DJ, Thompson J, Yancovitz S, Chandra A, Prissel MA, Hyer CF. Research activity among foot and ankle surgery fellows: a systematic review. J Foot Ankle Surg. 2021;60(6):1227-1231. doi:10.1053/j.jfas.2021.04.018
- Casciato DJ, Thompson J, Hyer CF. Post-fellowship foot and ankle surgeon research productivity: a systematic review. J Foot Ankle Surg. 2022;61(4):896-899. doi:10.1053/j.jfas.2021.12.028
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA. 2005;102(46):16569-16572. doi:10.1073/pnas.0507655102
- US Department of Veterans Affairs. Veterans Health Administration. About VHA. Updated January 20, 2025. Accessed February 17, 2025. https://www.va.gov/health/aboutvha.asp
- US Department of Veterans Affairs. VHA National Center for Patient Safety. About Us. Updated November 29, 2023. Accessed February 17, 2025. https://www.patientsafety.va.gov/
- US Department of Veterans Affairs. VA/DoD Clinical Practice Guidelines. Updated February 7, 2025. Accessed February 17, 2025. https://www.healthquality.va.gov
- Ravin AG, Gottlieb NB, Wang HT, et al. Effect of the Veterans Affairs Medical System on plastic surgery residency training. Plast Reconstr Surg. 2006;117(2):656-660. doi:10.1097/01.prs.0000197216.95544.f7
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Gibson LW, Abbas A. Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2013;25(1):131-134. doi:10.1016/j.ccell.2012.11.004
- Do MH, Lipner SR. Contribution of gender on compensation of Veterans Affairs-affiliated dermatologists: a cross-sectional study. Int J Womens Dermatol. 2020;6(5):414-418. doi:10.1016/j.ijwd.2020.09.009
- Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
- Coleman DL, Moran E, Serfilippi D, et al. Measuring physicians’ productivity in a Veterans’ Affairs Medical Center. Acad Med. 2003;78(7):682-689. doi:10.1097/00001888-200307000-00007
- Mohr DC, Burgess JF Jr. Job characteristics and job satisfaction among physicians involved with research in the Veterans Health Administration. Acad Med. 2011;86(8):938-945. doi:10.1097/ACM.0b013e3182223b76
- Casciato DJ, Cravey KS, Barron IM. Scholarly productivity among academic foot and ankle surgeons affiliated with US podiatric medicine and surgery residency and fellowship training programs. J Foot Ankle Surg. 2021;60(6):1222-1226. doi:10.1053/j.jfas.2021.04.017
- Hyer CF, Casciato DJ, Rushing CJ, Schuberth JM. Incidence of scholarly publication by selected content experts presenting at national society foot and ankle meetings from 2016 to 2020. J Foot Ankle Surg. 2022;61(6):1317-1320. doi:10.1053/j.jfas.2022.04.011
- Casciato DJ, Thompson J, Yancovitz S, Chandra A, Prissel MA, Hyer CF. Research activity among foot and ankle surgery fellows: a systematic review. J Foot Ankle Surg. 2021;60(6):1227-1231. doi:10.1053/j.jfas.2021.04.018
- Casciato DJ, Thompson J, Hyer CF. Post-fellowship foot and ankle surgeon research productivity: a systematic review. J Foot Ankle Surg. 2022;61(4):896-899. doi:10.1053/j.jfas.2021.12.028
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA. 2005;102(46):16569-16572. doi:10.1073/pnas.0507655102
- US Department of Veterans Affairs. Veterans Health Administration. About VHA. Updated January 20, 2025. Accessed February 17, 2025. https://www.va.gov/health/aboutvha.asp
- US Department of Veterans Affairs. VHA National Center for Patient Safety. About Us. Updated November 29, 2023. Accessed February 17, 2025. https://www.patientsafety.va.gov/
- US Department of Veterans Affairs. VA/DoD Clinical Practice Guidelines. Updated February 7, 2025. Accessed February 17, 2025. https://www.healthquality.va.gov
- Ravin AG, Gottlieb NB, Wang HT, et al. Effect of the Veterans Affairs Medical System on plastic surgery residency training. Plast Reconstr Surg. 2006;117(2):656-660. doi:10.1097/01.prs.0000197216.95544.f7
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Gibson LW, Abbas A. Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2013;25(1):131-134. doi:10.1016/j.ccell.2012.11.004
- Do MH, Lipner SR. Contribution of gender on compensation of Veterans Affairs-affiliated dermatologists: a cross-sectional study. Int J Womens Dermatol. 2020;6(5):414-418. doi:10.1016/j.ijwd.2020.09.009
Scholarly Activity Among VA Podiatrists: A Cross-Sectional Study
Scholarly Activity Among VA Podiatrists: A Cross-Sectional Study
Stretcher vs Table for Operative Hand Surgery
Stretcher vs Table for Operative Hand Surgery
US Department of Veterans Affairs (VA) health care facilities have not recovered from staff shortages that occurred during the COVID-19 pandemic.1 Veterans Health Administration operating rooms (ORs) lost many valuable clinicians during the pandemic due to illness, relocation, burnout, and retirement, and remain below prepandemic levels. The staffing shortage has resulted in lost OR time, leading to longer wait times for surgery. In October 2021, the Malcom Randall VA Medical Center (MRVAMC) Plastic Surgery Service implemented a surgery-on-stretcher initiative, in which patients arriving in the OR remained on the stretcher throughout surgery rather than being transferred to the operating table. Avoiding patient transfers was identified as a strategy to increase the number of procedures performed while providing additional benefits to the patients and staff.
The intent of the surgery-on-stretcher initiative was to reduce OR turnover time and in-room time, decrease supply costs, and improve patient and staff safety. The objective of this study was to evaluate the new process in terms of time efficiency, cost savings, and safety.
METHODS
The University of Florida Institutional Review Board (IRB) and North Florida/South Georgia Veterans Health System Research and Development Committee (IRB.net) approved a retrospective chart review of hand surgery cases performed in the same OR by the same surgeon over 2 year-long periods: October 1, 2020, through September 30, 2021, when surgeries were performed on the operating table (Figure 1), and June 1, 2022, through May 31, 2023, when surgeries were performed on the stretcher (Figure 2). Time intervals were obtained from the Nurse Intraoperative Report found in the electronic medical record. They ranged from “patient in OR” to “operation begin,” “operation end” to “patient out OR,” and “patient out OR” to next “patient in OR.” The median time intervals were obtained for the 3 different time intervals in each study period and compared.
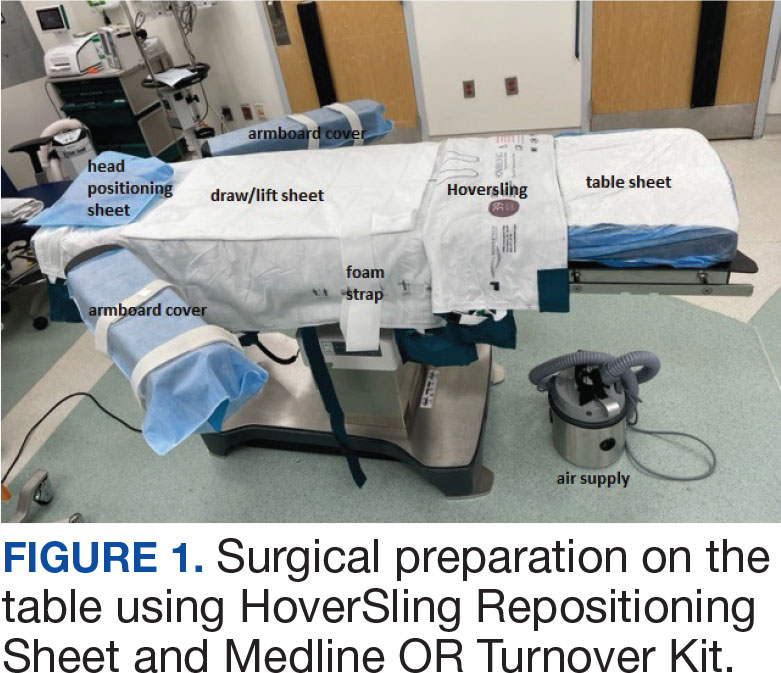

A Mann-Whitney U test was used to determine statistical significance between the groups. We queried the Patient Safety Manager (Jason Ringlehan, BSN, RN, oral communication, 2023) and the Employee Health Nurse (Ivan Cool, BSN, RN, oral communication, June 16, 2023) for reported patient or employee–patient transfer injuries. We requested Inventory Supply personnel to provide the cost of materials used in the transfer process. There was no cost for surgeries performed on the stretcher.
RESULTS
A total of 306 hand surgeries were performed on a table and 191 were performed on a stretcher during the study periods. The median patient in OR to operation begin time interval was 25 minutes for the table and 23 minutes for the stretcher. The median operation end to patient out OR time was 4 minutes for the table and 3 minutes for the stretcher. Time savings was statistically significant (P < .001) for both ends of the surgery. The median room turnover time was 27 minutes for both time periods and was not statistically significant (P = .70). There were no reported employee or patient injuries attributed to OR transfers during either time period. Supply cost savings was $111.28 per case when surgery was performed on the stretcher (Table).
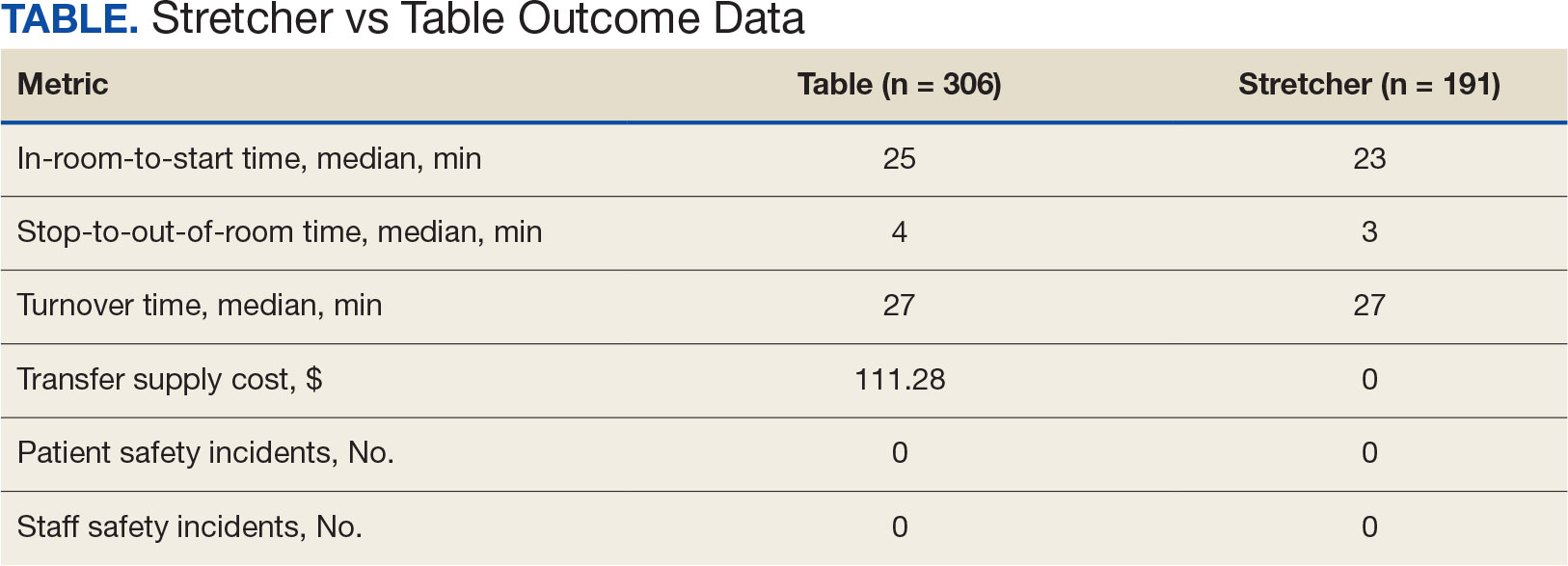
DISCUSSION
The new process of doing surgery on the stretcher was introduced to improve OR time efficiency. This improved efficiency has been reported in the hand surgery literature; however, the authors anticipated resistance to implementing a new process to seasoned OR staff.2,3 Once the idea was conceived, the plan was reviewed with the Anesthesia Service to confirm they had no safety concerns. The rest of the OR staff, including nurses and surgical technicians, agreed to participate. No resistance was encountered. The anesthesia, nursing, and scrub staff were happy to skip a potentially hazardous step at the beginning and end of each hand surgery case. The anesthesiologists communicated that the OR bed is preferred for intubating, but our hand surgeries are performed under local or regional block and intravenous sedation. The table was removed from the room to avoid any confusion with changes in staff during the day.
Compared with table use, surgery on the stretcher saved a median of 3 minutes of in-room time per case, with no significant difference in turnover time. The time savings reported here were consistent with what has been reported in other studies. Garras et al saved 7.5 minutes per case using a rolling hand table for their hand surgeries,2 while Gonzalez et al reported a 4-minute reduction per case when using a stretcher-based hand table for carpal tunnel and trigger finger surgeries.3 Lause et al found a 2-minute time savings at the start of their foot and ankle surgeries.4
Although 3 minutes per case may seem minimal, when applied to a conservative number of 5 hand cases twice a week, this time savings translates to an additional 15-minute nursing break each day, a 30-minute lunch break each week, and 26 extra hours each year. This efficiency can reduce direct costs in overtime. Consistently ending the day on time and allowing time for scheduled breaks can facilitate retention and improve morale in our current environment of chronically short-staffed surgical services. Recent literature estimates the cost of 1 OR minute to be about $36 to $46.5,6
Lateral transfers, in which a patient is moved horizontally, take place throughout the day in the OR and are a known risk factor for musculoskeletal disorders among the nursing staff. Contributing factors include patient obesity, environmental barriers in the OR, uneven patient weight distribution, and height differences among surgical team members. The Association of periOperative Registered Nurses recommends use of a lateral transfer device such as a friction-reducing sheet, slider board, or air-assisted device.7 The single-use Hover- Sling Repositioning Sheet is the transfer assist device used in our OR. It is an inflatable transfer mattress that reduces the amount of force used in patient transfer. The mattress is inflated with air from a small motor. While the HoverSling is inflated, escaping air from little holes on the underside of the mattress acts as a lubricant between the patient and transfer surface. This air reduces the force needed to move the patient.8
Patient transfers are a known risk for both patient and staff injuries.9,10 We suspected that not transferring our surgical patients between the stretcher and bed would improve patient and staff safety. A review of Patient Safety and Employee Health services found no reported patient or staff injuries during either timeframe. This finding led to the conclusion that effective safety precautions were already in place before the surgery-on-stretcher initiative. The MRVAMC routinely uses patient transfer equipment and the standard procedure in the OR is for 5 people to participate in 1 patient transfer between bed and table. The patient transfer device plus multiple staff involvement with patient transfers could explain the lack of patient and staff injury that predated the surgery-on-stretcher initiative and continued throughout the study period.
The inventory required to facilitate patient transfers at MRVAMC cost on average $111.28 per patient based on a search of the inventory database. This amount includes the HoverSling priced at $97 and the Medline OR Turnover Kit (table sheet, draw sheet, arm board covers, head positioning cover, and positioning foam strap) priced at $14.28. The Plastic Surgery Service routinely performs a minimum of 10 hand cases per week. If $111.28 per case is multiplied by the average of 10 cases each week over 52 weeks, the annualized savings could be about $57,866. This direct cost savings can potentially be applied to necessary equipment expenditures, educational training, or staff salaries.
Hand surgery literature has encouraged initiatives to reduce waste and develop more environmentally responsible practices.11-13 Eliminating the single-use patient transfer device and the turnover kit would avoid generating additional trash from the OR. Fewer sheets would have to be washed when patients stay on the same stretcher throughout their surgery day, which saves electricity and water.
Strengths and Limitations
A strength of this study is the consistency of the data, which were obtained from observing the same surgeon performing the same surgeries in the same OR. The data were logged into the electronic medical record in real time and easily accessible for data collection and comparison when reviewed retrospectively. A weakness of the study is the inconsistency in logging the in/out and start/end times by the OR circulating nurses who were involved in the patient transfers. The OR circulating nurses can vary from day to day, depending on the staffing assignments, which could affect the speed of each part of the procedure.
CONCLUSIONS
Hand surgery performed on the stretcher saves OR time and supply costs. This added efficiency translates to a savings of 26 hours of OR time and $57,866 in supply costs over the course of a year. Turnover time and staff and patient safety were not affected. This process can be introduced to other surgical specialties that do not need the accessories or various positions the OR table allows.
- Hersey LF. COVID-19 worsened staff shortages at veterans’ medical facilities, IG report finds. Stars and Stripes. October 13, 2023. Accessed February 28, 2025. https:// www.stripes.com/theaters/us/2023-10-13/veterans-affairs-health-care-staff-shortages-11695546.html
- Garras DN, Beredjiklian PK, Leinberry CF Jr. Operating on a stretcher: a cost analysis. J Hand Surg Am. 2011;36(12):2078-2079. doi:10.1016/j.jhsa.2011.09.006
- Gonzalez TA, Stanbury SJ, Mora AN, Floyd WE IV, Blazar PE, Earp BE. The effect of stretcher-based hand tables on operating room efficiency at an outpatient surgery center. Orthop J Harv Med Sch. 2017;18:20-24.
- Lause GE, Parker EB, Farid A, et al. Efficiency and perceived safety of foot and ankle procedures performed on the preoperative stretcher versus operating room table. J Perioper Pract. 2024;34(9):268-273. doi:10.1177/17504589231215939
- Childers CP, Maggard-Gibbons M. Understanding costs of care in the operating room. JAMA Surg. 2018;153(4):e176233. doi:10.1001/jamasurg.2017.6233
- Smith TS, Evans J, Moriel K, et al. Cost of operating room time is $46.04 dollars per minute. J Orthop Bus. 2022;2(4):10-13. doi:10.55576/job.v2i4.23
- Waters T, Baptiste A, Short M, Plante-Mallon L, Nelson A. AORN ergonomic tool 1: lateral transfer of a patient from a stretcher to an OR bed. AORN J. 2011;93(3):334-339. doi:10.1016/j.aorn.2010.08.025
- Barry J. The HoverMatt system for patient transfer: enhancing productivity, efficiency, and safety. J Nurs Adm. 2006;36(3):114-117. doi:10.1097/00005110-200603000-00003
- Apple B, Letvak S. Ergonomic challenges in the perioperative setting. AORN J. 2021;113(4):339-348. doi:10.1002/aorn.13345
- Tan J, Krishnan S, Vacanti JC, et al. Patient falls in the operating room setting: an analysis of reported safety events. J Healthc Risk Manag. 2022;42(1):9-14. doi:10.1002/jhrm.21503
- Van Demark RE Jr, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg Am. 2018;43(2):179-181. doi:10.1016/j.jhsa.2017.11.007
- Bravo D, Gaston RG, Melamed E. Environmentally responsible hand surgery: past, present, and future. J Hand Surg Am. 2020;45(5):444-448. doi:10.1016/j.jhsa.2019.10.031
- Tevlin R, Panton JA, Fox PM. Greening hand surgery: targeted measures to reduce waste in ambulatory trigger finger and carpal tunnel decompression. Hand (N Y). 2023;15589447231220412. doi:10.1177/15589447231220412
US Department of Veterans Affairs (VA) health care facilities have not recovered from staff shortages that occurred during the COVID-19 pandemic.1 Veterans Health Administration operating rooms (ORs) lost many valuable clinicians during the pandemic due to illness, relocation, burnout, and retirement, and remain below prepandemic levels. The staffing shortage has resulted in lost OR time, leading to longer wait times for surgery. In October 2021, the Malcom Randall VA Medical Center (MRVAMC) Plastic Surgery Service implemented a surgery-on-stretcher initiative, in which patients arriving in the OR remained on the stretcher throughout surgery rather than being transferred to the operating table. Avoiding patient transfers was identified as a strategy to increase the number of procedures performed while providing additional benefits to the patients and staff.
The intent of the surgery-on-stretcher initiative was to reduce OR turnover time and in-room time, decrease supply costs, and improve patient and staff safety. The objective of this study was to evaluate the new process in terms of time efficiency, cost savings, and safety.
METHODS
The University of Florida Institutional Review Board (IRB) and North Florida/South Georgia Veterans Health System Research and Development Committee (IRB.net) approved a retrospective chart review of hand surgery cases performed in the same OR by the same surgeon over 2 year-long periods: October 1, 2020, through September 30, 2021, when surgeries were performed on the operating table (Figure 1), and June 1, 2022, through May 31, 2023, when surgeries were performed on the stretcher (Figure 2). Time intervals were obtained from the Nurse Intraoperative Report found in the electronic medical record. They ranged from “patient in OR” to “operation begin,” “operation end” to “patient out OR,” and “patient out OR” to next “patient in OR.” The median time intervals were obtained for the 3 different time intervals in each study period and compared.


A Mann-Whitney U test was used to determine statistical significance between the groups. We queried the Patient Safety Manager (Jason Ringlehan, BSN, RN, oral communication, 2023) and the Employee Health Nurse (Ivan Cool, BSN, RN, oral communication, June 16, 2023) for reported patient or employee–patient transfer injuries. We requested Inventory Supply personnel to provide the cost of materials used in the transfer process. There was no cost for surgeries performed on the stretcher.
RESULTS
A total of 306 hand surgeries were performed on a table and 191 were performed on a stretcher during the study periods. The median patient in OR to operation begin time interval was 25 minutes for the table and 23 minutes for the stretcher. The median operation end to patient out OR time was 4 minutes for the table and 3 minutes for the stretcher. Time savings was statistically significant (P < .001) for both ends of the surgery. The median room turnover time was 27 minutes for both time periods and was not statistically significant (P = .70). There were no reported employee or patient injuries attributed to OR transfers during either time period. Supply cost savings was $111.28 per case when surgery was performed on the stretcher (Table).

DISCUSSION
The new process of doing surgery on the stretcher was introduced to improve OR time efficiency. This improved efficiency has been reported in the hand surgery literature; however, the authors anticipated resistance to implementing a new process to seasoned OR staff.2,3 Once the idea was conceived, the plan was reviewed with the Anesthesia Service to confirm they had no safety concerns. The rest of the OR staff, including nurses and surgical technicians, agreed to participate. No resistance was encountered. The anesthesia, nursing, and scrub staff were happy to skip a potentially hazardous step at the beginning and end of each hand surgery case. The anesthesiologists communicated that the OR bed is preferred for intubating, but our hand surgeries are performed under local or regional block and intravenous sedation. The table was removed from the room to avoid any confusion with changes in staff during the day.
Compared with table use, surgery on the stretcher saved a median of 3 minutes of in-room time per case, with no significant difference in turnover time. The time savings reported here were consistent with what has been reported in other studies. Garras et al saved 7.5 minutes per case using a rolling hand table for their hand surgeries,2 while Gonzalez et al reported a 4-minute reduction per case when using a stretcher-based hand table for carpal tunnel and trigger finger surgeries.3 Lause et al found a 2-minute time savings at the start of their foot and ankle surgeries.4
Although 3 minutes per case may seem minimal, when applied to a conservative number of 5 hand cases twice a week, this time savings translates to an additional 15-minute nursing break each day, a 30-minute lunch break each week, and 26 extra hours each year. This efficiency can reduce direct costs in overtime. Consistently ending the day on time and allowing time for scheduled breaks can facilitate retention and improve morale in our current environment of chronically short-staffed surgical services. Recent literature estimates the cost of 1 OR minute to be about $36 to $46.5,6
Lateral transfers, in which a patient is moved horizontally, take place throughout the day in the OR and are a known risk factor for musculoskeletal disorders among the nursing staff. Contributing factors include patient obesity, environmental barriers in the OR, uneven patient weight distribution, and height differences among surgical team members. The Association of periOperative Registered Nurses recommends use of a lateral transfer device such as a friction-reducing sheet, slider board, or air-assisted device.7 The single-use Hover- Sling Repositioning Sheet is the transfer assist device used in our OR. It is an inflatable transfer mattress that reduces the amount of force used in patient transfer. The mattress is inflated with air from a small motor. While the HoverSling is inflated, escaping air from little holes on the underside of the mattress acts as a lubricant between the patient and transfer surface. This air reduces the force needed to move the patient.8
Patient transfers are a known risk for both patient and staff injuries.9,10 We suspected that not transferring our surgical patients between the stretcher and bed would improve patient and staff safety. A review of Patient Safety and Employee Health services found no reported patient or staff injuries during either timeframe. This finding led to the conclusion that effective safety precautions were already in place before the surgery-on-stretcher initiative. The MRVAMC routinely uses patient transfer equipment and the standard procedure in the OR is for 5 people to participate in 1 patient transfer between bed and table. The patient transfer device plus multiple staff involvement with patient transfers could explain the lack of patient and staff injury that predated the surgery-on-stretcher initiative and continued throughout the study period.
The inventory required to facilitate patient transfers at MRVAMC cost on average $111.28 per patient based on a search of the inventory database. This amount includes the HoverSling priced at $97 and the Medline OR Turnover Kit (table sheet, draw sheet, arm board covers, head positioning cover, and positioning foam strap) priced at $14.28. The Plastic Surgery Service routinely performs a minimum of 10 hand cases per week. If $111.28 per case is multiplied by the average of 10 cases each week over 52 weeks, the annualized savings could be about $57,866. This direct cost savings can potentially be applied to necessary equipment expenditures, educational training, or staff salaries.
Hand surgery literature has encouraged initiatives to reduce waste and develop more environmentally responsible practices.11-13 Eliminating the single-use patient transfer device and the turnover kit would avoid generating additional trash from the OR. Fewer sheets would have to be washed when patients stay on the same stretcher throughout their surgery day, which saves electricity and water.
Strengths and Limitations
A strength of this study is the consistency of the data, which were obtained from observing the same surgeon performing the same surgeries in the same OR. The data were logged into the electronic medical record in real time and easily accessible for data collection and comparison when reviewed retrospectively. A weakness of the study is the inconsistency in logging the in/out and start/end times by the OR circulating nurses who were involved in the patient transfers. The OR circulating nurses can vary from day to day, depending on the staffing assignments, which could affect the speed of each part of the procedure.
CONCLUSIONS
Hand surgery performed on the stretcher saves OR time and supply costs. This added efficiency translates to a savings of 26 hours of OR time and $57,866 in supply costs over the course of a year. Turnover time and staff and patient safety were not affected. This process can be introduced to other surgical specialties that do not need the accessories or various positions the OR table allows.
US Department of Veterans Affairs (VA) health care facilities have not recovered from staff shortages that occurred during the COVID-19 pandemic.1 Veterans Health Administration operating rooms (ORs) lost many valuable clinicians during the pandemic due to illness, relocation, burnout, and retirement, and remain below prepandemic levels. The staffing shortage has resulted in lost OR time, leading to longer wait times for surgery. In October 2021, the Malcom Randall VA Medical Center (MRVAMC) Plastic Surgery Service implemented a surgery-on-stretcher initiative, in which patients arriving in the OR remained on the stretcher throughout surgery rather than being transferred to the operating table. Avoiding patient transfers was identified as a strategy to increase the number of procedures performed while providing additional benefits to the patients and staff.
The intent of the surgery-on-stretcher initiative was to reduce OR turnover time and in-room time, decrease supply costs, and improve patient and staff safety. The objective of this study was to evaluate the new process in terms of time efficiency, cost savings, and safety.
METHODS
The University of Florida Institutional Review Board (IRB) and North Florida/South Georgia Veterans Health System Research and Development Committee (IRB.net) approved a retrospective chart review of hand surgery cases performed in the same OR by the same surgeon over 2 year-long periods: October 1, 2020, through September 30, 2021, when surgeries were performed on the operating table (Figure 1), and June 1, 2022, through May 31, 2023, when surgeries were performed on the stretcher (Figure 2). Time intervals were obtained from the Nurse Intraoperative Report found in the electronic medical record. They ranged from “patient in OR” to “operation begin,” “operation end” to “patient out OR,” and “patient out OR” to next “patient in OR.” The median time intervals were obtained for the 3 different time intervals in each study period and compared.


A Mann-Whitney U test was used to determine statistical significance between the groups. We queried the Patient Safety Manager (Jason Ringlehan, BSN, RN, oral communication, 2023) and the Employee Health Nurse (Ivan Cool, BSN, RN, oral communication, June 16, 2023) for reported patient or employee–patient transfer injuries. We requested Inventory Supply personnel to provide the cost of materials used in the transfer process. There was no cost for surgeries performed on the stretcher.
RESULTS
A total of 306 hand surgeries were performed on a table and 191 were performed on a stretcher during the study periods. The median patient in OR to operation begin time interval was 25 minutes for the table and 23 minutes for the stretcher. The median operation end to patient out OR time was 4 minutes for the table and 3 minutes for the stretcher. Time savings was statistically significant (P < .001) for both ends of the surgery. The median room turnover time was 27 minutes for both time periods and was not statistically significant (P = .70). There were no reported employee or patient injuries attributed to OR transfers during either time period. Supply cost savings was $111.28 per case when surgery was performed on the stretcher (Table).

DISCUSSION
The new process of doing surgery on the stretcher was introduced to improve OR time efficiency. This improved efficiency has been reported in the hand surgery literature; however, the authors anticipated resistance to implementing a new process to seasoned OR staff.2,3 Once the idea was conceived, the plan was reviewed with the Anesthesia Service to confirm they had no safety concerns. The rest of the OR staff, including nurses and surgical technicians, agreed to participate. No resistance was encountered. The anesthesia, nursing, and scrub staff were happy to skip a potentially hazardous step at the beginning and end of each hand surgery case. The anesthesiologists communicated that the OR bed is preferred for intubating, but our hand surgeries are performed under local or regional block and intravenous sedation. The table was removed from the room to avoid any confusion with changes in staff during the day.
Compared with table use, surgery on the stretcher saved a median of 3 minutes of in-room time per case, with no significant difference in turnover time. The time savings reported here were consistent with what has been reported in other studies. Garras et al saved 7.5 minutes per case using a rolling hand table for their hand surgeries,2 while Gonzalez et al reported a 4-minute reduction per case when using a stretcher-based hand table for carpal tunnel and trigger finger surgeries.3 Lause et al found a 2-minute time savings at the start of their foot and ankle surgeries.4
Although 3 minutes per case may seem minimal, when applied to a conservative number of 5 hand cases twice a week, this time savings translates to an additional 15-minute nursing break each day, a 30-minute lunch break each week, and 26 extra hours each year. This efficiency can reduce direct costs in overtime. Consistently ending the day on time and allowing time for scheduled breaks can facilitate retention and improve morale in our current environment of chronically short-staffed surgical services. Recent literature estimates the cost of 1 OR minute to be about $36 to $46.5,6
Lateral transfers, in which a patient is moved horizontally, take place throughout the day in the OR and are a known risk factor for musculoskeletal disorders among the nursing staff. Contributing factors include patient obesity, environmental barriers in the OR, uneven patient weight distribution, and height differences among surgical team members. The Association of periOperative Registered Nurses recommends use of a lateral transfer device such as a friction-reducing sheet, slider board, or air-assisted device.7 The single-use Hover- Sling Repositioning Sheet is the transfer assist device used in our OR. It is an inflatable transfer mattress that reduces the amount of force used in patient transfer. The mattress is inflated with air from a small motor. While the HoverSling is inflated, escaping air from little holes on the underside of the mattress acts as a lubricant between the patient and transfer surface. This air reduces the force needed to move the patient.8
Patient transfers are a known risk for both patient and staff injuries.9,10 We suspected that not transferring our surgical patients between the stretcher and bed would improve patient and staff safety. A review of Patient Safety and Employee Health services found no reported patient or staff injuries during either timeframe. This finding led to the conclusion that effective safety precautions were already in place before the surgery-on-stretcher initiative. The MRVAMC routinely uses patient transfer equipment and the standard procedure in the OR is for 5 people to participate in 1 patient transfer between bed and table. The patient transfer device plus multiple staff involvement with patient transfers could explain the lack of patient and staff injury that predated the surgery-on-stretcher initiative and continued throughout the study period.
The inventory required to facilitate patient transfers at MRVAMC cost on average $111.28 per patient based on a search of the inventory database. This amount includes the HoverSling priced at $97 and the Medline OR Turnover Kit (table sheet, draw sheet, arm board covers, head positioning cover, and positioning foam strap) priced at $14.28. The Plastic Surgery Service routinely performs a minimum of 10 hand cases per week. If $111.28 per case is multiplied by the average of 10 cases each week over 52 weeks, the annualized savings could be about $57,866. This direct cost savings can potentially be applied to necessary equipment expenditures, educational training, or staff salaries.
Hand surgery literature has encouraged initiatives to reduce waste and develop more environmentally responsible practices.11-13 Eliminating the single-use patient transfer device and the turnover kit would avoid generating additional trash from the OR. Fewer sheets would have to be washed when patients stay on the same stretcher throughout their surgery day, which saves electricity and water.
Strengths and Limitations
A strength of this study is the consistency of the data, which were obtained from observing the same surgeon performing the same surgeries in the same OR. The data were logged into the electronic medical record in real time and easily accessible for data collection and comparison when reviewed retrospectively. A weakness of the study is the inconsistency in logging the in/out and start/end times by the OR circulating nurses who were involved in the patient transfers. The OR circulating nurses can vary from day to day, depending on the staffing assignments, which could affect the speed of each part of the procedure.
CONCLUSIONS
Hand surgery performed on the stretcher saves OR time and supply costs. This added efficiency translates to a savings of 26 hours of OR time and $57,866 in supply costs over the course of a year. Turnover time and staff and patient safety were not affected. This process can be introduced to other surgical specialties that do not need the accessories or various positions the OR table allows.
- Hersey LF. COVID-19 worsened staff shortages at veterans’ medical facilities, IG report finds. Stars and Stripes. October 13, 2023. Accessed February 28, 2025. https:// www.stripes.com/theaters/us/2023-10-13/veterans-affairs-health-care-staff-shortages-11695546.html
- Garras DN, Beredjiklian PK, Leinberry CF Jr. Operating on a stretcher: a cost analysis. J Hand Surg Am. 2011;36(12):2078-2079. doi:10.1016/j.jhsa.2011.09.006
- Gonzalez TA, Stanbury SJ, Mora AN, Floyd WE IV, Blazar PE, Earp BE. The effect of stretcher-based hand tables on operating room efficiency at an outpatient surgery center. Orthop J Harv Med Sch. 2017;18:20-24.
- Lause GE, Parker EB, Farid A, et al. Efficiency and perceived safety of foot and ankle procedures performed on the preoperative stretcher versus operating room table. J Perioper Pract. 2024;34(9):268-273. doi:10.1177/17504589231215939
- Childers CP, Maggard-Gibbons M. Understanding costs of care in the operating room. JAMA Surg. 2018;153(4):e176233. doi:10.1001/jamasurg.2017.6233
- Smith TS, Evans J, Moriel K, et al. Cost of operating room time is $46.04 dollars per minute. J Orthop Bus. 2022;2(4):10-13. doi:10.55576/job.v2i4.23
- Waters T, Baptiste A, Short M, Plante-Mallon L, Nelson A. AORN ergonomic tool 1: lateral transfer of a patient from a stretcher to an OR bed. AORN J. 2011;93(3):334-339. doi:10.1016/j.aorn.2010.08.025
- Barry J. The HoverMatt system for patient transfer: enhancing productivity, efficiency, and safety. J Nurs Adm. 2006;36(3):114-117. doi:10.1097/00005110-200603000-00003
- Apple B, Letvak S. Ergonomic challenges in the perioperative setting. AORN J. 2021;113(4):339-348. doi:10.1002/aorn.13345
- Tan J, Krishnan S, Vacanti JC, et al. Patient falls in the operating room setting: an analysis of reported safety events. J Healthc Risk Manag. 2022;42(1):9-14. doi:10.1002/jhrm.21503
- Van Demark RE Jr, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg Am. 2018;43(2):179-181. doi:10.1016/j.jhsa.2017.11.007
- Bravo D, Gaston RG, Melamed E. Environmentally responsible hand surgery: past, present, and future. J Hand Surg Am. 2020;45(5):444-448. doi:10.1016/j.jhsa.2019.10.031
- Tevlin R, Panton JA, Fox PM. Greening hand surgery: targeted measures to reduce waste in ambulatory trigger finger and carpal tunnel decompression. Hand (N Y). 2023;15589447231220412. doi:10.1177/15589447231220412
- Hersey LF. COVID-19 worsened staff shortages at veterans’ medical facilities, IG report finds. Stars and Stripes. October 13, 2023. Accessed February 28, 2025. https:// www.stripes.com/theaters/us/2023-10-13/veterans-affairs-health-care-staff-shortages-11695546.html
- Garras DN, Beredjiklian PK, Leinberry CF Jr. Operating on a stretcher: a cost analysis. J Hand Surg Am. 2011;36(12):2078-2079. doi:10.1016/j.jhsa.2011.09.006
- Gonzalez TA, Stanbury SJ, Mora AN, Floyd WE IV, Blazar PE, Earp BE. The effect of stretcher-based hand tables on operating room efficiency at an outpatient surgery center. Orthop J Harv Med Sch. 2017;18:20-24.
- Lause GE, Parker EB, Farid A, et al. Efficiency and perceived safety of foot and ankle procedures performed on the preoperative stretcher versus operating room table. J Perioper Pract. 2024;34(9):268-273. doi:10.1177/17504589231215939
- Childers CP, Maggard-Gibbons M. Understanding costs of care in the operating room. JAMA Surg. 2018;153(4):e176233. doi:10.1001/jamasurg.2017.6233
- Smith TS, Evans J, Moriel K, et al. Cost of operating room time is $46.04 dollars per minute. J Orthop Bus. 2022;2(4):10-13. doi:10.55576/job.v2i4.23
- Waters T, Baptiste A, Short M, Plante-Mallon L, Nelson A. AORN ergonomic tool 1: lateral transfer of a patient from a stretcher to an OR bed. AORN J. 2011;93(3):334-339. doi:10.1016/j.aorn.2010.08.025
- Barry J. The HoverMatt system for patient transfer: enhancing productivity, efficiency, and safety. J Nurs Adm. 2006;36(3):114-117. doi:10.1097/00005110-200603000-00003
- Apple B, Letvak S. Ergonomic challenges in the perioperative setting. AORN J. 2021;113(4):339-348. doi:10.1002/aorn.13345
- Tan J, Krishnan S, Vacanti JC, et al. Patient falls in the operating room setting: an analysis of reported safety events. J Healthc Risk Manag. 2022;42(1):9-14. doi:10.1002/jhrm.21503
- Van Demark RE Jr, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg Am. 2018;43(2):179-181. doi:10.1016/j.jhsa.2017.11.007
- Bravo D, Gaston RG, Melamed E. Environmentally responsible hand surgery: past, present, and future. J Hand Surg Am. 2020;45(5):444-448. doi:10.1016/j.jhsa.2019.10.031
- Tevlin R, Panton JA, Fox PM. Greening hand surgery: targeted measures to reduce waste in ambulatory trigger finger and carpal tunnel decompression. Hand (N Y). 2023;15589447231220412. doi:10.1177/15589447231220412
Stretcher vs Table for Operative Hand Surgery
Stretcher vs Table for Operative Hand Surgery
Achieving Psychological Safety in High Reliability Organizations
Achieving Psychological Safety in High Reliability Organizations
Worldwide, health care is becoming increasingly complex as a result of greater clinical workforce demands, expanded roles and responsibilities, health care system mergers, stakeholder calls for new capabilities, and digital transformation. 1,2These increasing demands has prompted many health care institutions to place greater focus on the psychological safety of their workforce, particularly in high reliability organizations (HROs). Building a robust foundation for high reliability in health care requires the presence of psychological safety—that is, staff members at all levels of the organization must feel comfortable speaking up when they have questions or concerns.3,4 Psychological safety can improve the safety and quality of patient care but has not reached its full potential in health care.5,6 However, there are strategies that promote the widespread implementation of psychological safety in health care organizations.3-6
PSYCHOLOGICAL SAFETY
The concept of psychological safety in organizational behavior originated in 1965 when Edgar Schein and Warren Bennis, leaders in organizational psychology and management, published their reflections on the importance of psychological safety in helping individuals feel secure in the work environment.5-7 Psychological safety in the workplace is foundational to staff members feeling comfortable asking questions or expressing concerns without fear of negative consequences.8,9 It supports both individual and team efforts to raise safety concerns and report near misses and adverse events so that similar events can be averted in the future.9 Patients aren’t the only ones who benefit; psychological safety has also been found to promote job satisfaction and employee well-being.10
THE VETERANS HEALTH ADMINISTRATION JOURNEY
Achieving psychological safety is by no means an easy or comfortable process. As with any organizational change, a multipronged approach offers the best chance of success.6,9 When the Veterans Health Administration (VHA) began its incremental, enterprise-wide journey to high reliability in 2019, 3 cohorts were identified. In February 2019, 18 US Department of Veterans Affairs (VA) medical centers (VAMCs) (cohort 1) began the process of becoming HROs. Cohort 2 followed in October 2020 and included 54 VAMC. Finally, in October 2021, 67 additional VAMCs (cohort 3) started the process.2 During cohort 2, the VA Providence Healthcare System (VAPHCS) decided to emphasize psychological safety at the start of the journey to becoming an HRO. This system is part of the VA New England Healthcare System (VISN 1), which includes VAMCs and clinics in Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont.11 Soon thereafter, the VA Bedford Healthcare System and the VA Connecticut Healthcare System adopted similar strategies. Since then, other VAMCs have also adopted this approach. These collective experiences identified 4 useful strategies for achieving psychological safety: leadership engagement, open communication, education and training, and accountability.
Leadership Engagement
Health care organization leaders play a critical role in making psychological safety happen—especially in complex and constantly changing environments, such as HROs.4 Leaders behaviors are consistently linked to the perception of psychological safety at the individual, team, and organizational levels.8 It is especially important to have leaders who recognize the views of individuals and team members and encourage staff participation in discussions to gain additional perspectives.7,8,12 Psychological safety can also be facilitated when leaders are visible, approachable, and communicative.4,7-9
Organizational practices, policies, and processes (eg, reporting adverse events without the fear of negative consequences) are also important ways that leaders can establish and sustain psychological safety. On a more granular level, leaders can enhance psychological safety by promoting and acknowledging individuals who speak up, regularly asking staff about safety concerns, highlighting “good catches” when harm is avoided, and using staff feedback to initiate improvements.4,7,13Finally, in the authors’ experience, psychological safety requires clear commitment from leaders at all levels of an organization. Communication should be bidirectional, and leaders should close the proverbial “loop” with feedback and timely follow-up. This encourages and reinforces staff engagement and speaking up behaviors.2,4,7,13
Open Communication
Promoting an environment of open communication, where all individuals and teams feel empowered to speak up with questions, concerns, and recommendations—regardless of position within the organization—is critical to psychological safety.4,6,9 Open communication is especially critical when processes and systems are constantly changing and advancing as a result of new information and technology.9 Promoting open, bidirectional communication during the delivery of patient care can be accomplished with huddles, tiered safety huddles, leader rounding for high reliability, and time-outs.2,4,6 These opportunities allow team members to discuss concerns, identify resources that support safe, high-quality care; reflect on successes and opportunities for improvement; and circle back on concerns.2,6 Open communication in psychologically safe environments empowers staff to raise patient care concerns and is instrumental for improving patient safety, increasing staff job satisfaction, and decreasing turnover.6,14
Education and Training
Education and training for all staff—from the frontline to the executive level—are essential to successfully implementing the principles and practices of psychological safety.5-7 VHA training covers many topics, including the origins, benefits, and implementation strategies of psychological safety (Table). Role-playing simulation is an effective teaching format, providing staff with opportunities to practice techniques for raising concerns or share feedback in a controlled environment.6 In addition, education should be ongoing; it helps leaders and staff members feel competent and confident when implementing psychological safety across the health care organization.6,10

Accountability
The final critical strategy for achieving psychological safety is accountability. It is the responsibility of all leadership—from senior leaders to clinical and nonclinical managers—to create a culture of shared accountability.5 But first, expectations must be set. Leadership must establish well-defined behavioral expectations that align with the organization’s values. Understanding behavioral expectations will help to ensure that employees know what achievement looks like, as well as how they are being held accountable for their individual actions.4,5,7 In practical terms, this means ensuring that staff members have the skills and resources to achieve goals and expectations, providing performance feedback in a timely manner, and including expectations in annual performance evaluations (as they are in the VHA).
Consistency is key. Accountability should be the expectation across all levels and services of the health care organization. No staff member should be exempt from promoting a psychologically safe work environment. Compliance with behavioral expectations should be monitored and if a person’s actions are not consistent with expectations, the situation will need to be addressed. Interventions will depend on the type, severity, and frequency of the problematic behaviors. Depending on an organization’s policies and practices, courses of action can range from feedback counseling to employment termination.5
A practical matter in ensuring accountability is implementing a psychologically safe process for reporting concerns. Staff members must feel comfortable reporting behavioral concerns without fear of retaliation, negative judgment, or consequences from peers and supervisors. One method for doing this is to create a confidential, centralized process for reporting concerns.5
First-Hand Results
VAPHCS has seen the results of implementing the strategies outlined here. For example, VAPHCS has observed a 45% increase in the use of the patient safety reporting system that logs medical errors and near-misses. In addition, there have been improvements in levels of psychological safety and patient safety reported in the annual VHA All Employee Survey, which is conducted annually to gauge workplace satisfaction, culture, climate, turnover, supervisory behaviors, and general workplace perceptions. VAPHCS has shown consistent improvements in 12 patient safety elements scored on a 5-point scale (1, very dissatisfied; 5, very satisfied) (Figure). Notably, employee ratings of error prevention discussed increased from 4.0 in 2022 to 4.3 in 2024. Data collection and analysis are ongoing; more comprehensive findings will be published in the future.
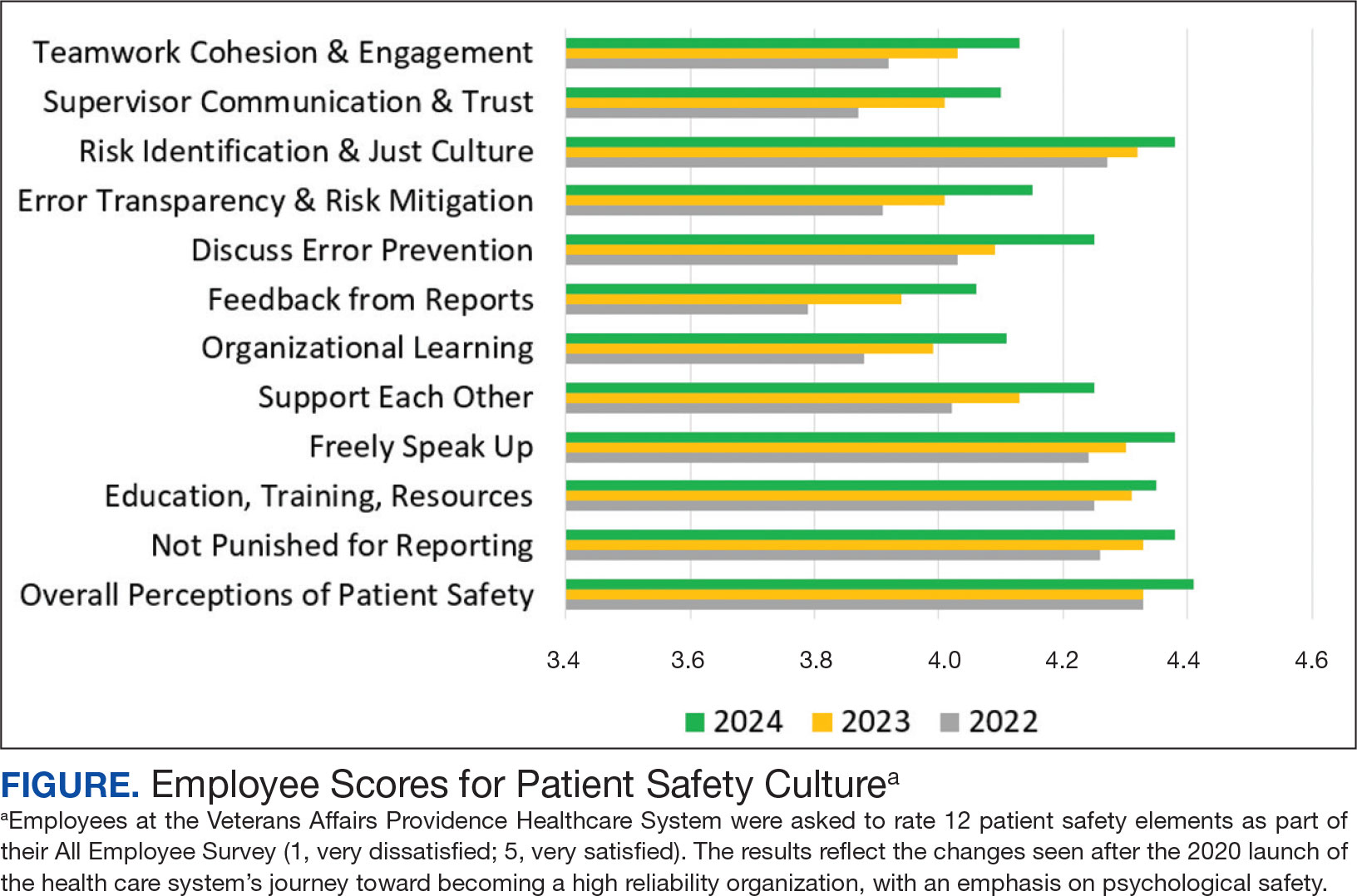
CONCLUSIONS
Health care organizations are increasingly recognizing the importance of psychologically safe workplaces in order to provide safe, high-quality patient care. Psychological safety is a critical tool for empowering staff to raise concerns, ask tough questions, challenge the status quo, and share new ideas for providing health care services. While psychological safety has been slowly adopted in health care, it’s clear that evidence-based strategies can make psychological safety a reality.
- Spanos S, Leask E, Patel R, Datyner M, Loh E, Braithwaite J. Healthcare leaders navigating complexity: A scoping review of key trends in future roles and competencies. BMC Med Educ. 2024;24(1):720. doi:10.1186/s12909-024-05689-4
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41(7):214-221. doi:10.12788/fp.0486
- Bransby DP, Kerrissey M, Edmondson AC. Paradise lost (and restored?): a study of psychological safety over time. Acad Manag Discov. Published online March 14, 2024. doi:10.5465/amd.2023.0084
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
- Jamal N, Young VN, Shapiro J, Brenner MJ, Schmalbach CE. Patient safety/quality improvement primer, part IV: Psychological safety-drivers to outcomes and well-being. Otolaryngol Head Neck Surg. 2023;168(4):881-888. doi:10.1177/01945998221126966
- Sarofim M. Psychological safety in medicine: What is it, and who cares? Med J Aust. 2024;220(8):398-399. doi:10.5694/mja2.52263
- Edmondson AC, Bransby DP. Psychological safety comes of age: Observed themes in an established literature. Annu Rev Organ Psychol Organ Behav. 2023;10:55-78. doi.org/10.1146/annurev-orgpsych-120920-055217
- Kumar S. Psychological safety: What it is, why teams need it, and how to make it flourish. Chest. 2024; 165(4):942-949. doi:10.1016/j.chest.2023.11.016
- Hallam KT, Popovic N, Karimi L. Identifying the key elements of psychologically safe workplaces in healthcare settings. Brain Sci. 2023;13(10):1450. doi:10.3390/brainsci13101450
- Grailey KE, Murray E, Reader T, Brett SJ. The presence and potential impact of psychological safety in the healthcare setting: an evidence synthesis. BMC Health Serv Res. 2021;21(1):773. doi:10.1186/s12913-021-06740-6
- US Department of Veterans Affairs. VISN 1: VA New England Healthcare System. Accessed March 25, 2025. https://department.va.gov/integrated-service-networks/visn-01
- Brimhall KC, Tsai CY, Eckardt R, Dionne S, Yang B, Sharp A. The effects of leadership for self-worth, inclusion, trust, and psychological safety on medical error reporting. Health Care Manage Rev. 2023;48(2):120-129. doi:10.1097/HMR.0000000000000358
- Adair KC, Heath A, Frye MA, et al. The Psychological Safety Scale of the Safety, Communication, Operational, Reliability, and Engagement (SCORE) Survey: a brief, diagnostic, and actionable metric for the ability to speak up in healthcare settings. J Patient Saf. 2022;18(6):513-520. doi:10.1097/PTS.0000000000001048
- Cho H, Steege LM, Arsenault Knudsen ÉN. Psychological safety, communication openness, nurse job outcomes, and patient safety in hospital nurses. Res Nurs Health. 2023;46(4):445-453.
- Practical Tool 2: 5 minute psychological safety audit. Accessed March 25, 2025. https://www.educationsupport.org.uk/media/jlnf3cju/practical-tool-2-psychological-safety-audit.pdf
Worldwide, health care is becoming increasingly complex as a result of greater clinical workforce demands, expanded roles and responsibilities, health care system mergers, stakeholder calls for new capabilities, and digital transformation. 1,2These increasing demands has prompted many health care institutions to place greater focus on the psychological safety of their workforce, particularly in high reliability organizations (HROs). Building a robust foundation for high reliability in health care requires the presence of psychological safety—that is, staff members at all levels of the organization must feel comfortable speaking up when they have questions or concerns.3,4 Psychological safety can improve the safety and quality of patient care but has not reached its full potential in health care.5,6 However, there are strategies that promote the widespread implementation of psychological safety in health care organizations.3-6
PSYCHOLOGICAL SAFETY
The concept of psychological safety in organizational behavior originated in 1965 when Edgar Schein and Warren Bennis, leaders in organizational psychology and management, published their reflections on the importance of psychological safety in helping individuals feel secure in the work environment.5-7 Psychological safety in the workplace is foundational to staff members feeling comfortable asking questions or expressing concerns without fear of negative consequences.8,9 It supports both individual and team efforts to raise safety concerns and report near misses and adverse events so that similar events can be averted in the future.9 Patients aren’t the only ones who benefit; psychological safety has also been found to promote job satisfaction and employee well-being.10
THE VETERANS HEALTH ADMINISTRATION JOURNEY
Achieving psychological safety is by no means an easy or comfortable process. As with any organizational change, a multipronged approach offers the best chance of success.6,9 When the Veterans Health Administration (VHA) began its incremental, enterprise-wide journey to high reliability in 2019, 3 cohorts were identified. In February 2019, 18 US Department of Veterans Affairs (VA) medical centers (VAMCs) (cohort 1) began the process of becoming HROs. Cohort 2 followed in October 2020 and included 54 VAMC. Finally, in October 2021, 67 additional VAMCs (cohort 3) started the process.2 During cohort 2, the VA Providence Healthcare System (VAPHCS) decided to emphasize psychological safety at the start of the journey to becoming an HRO. This system is part of the VA New England Healthcare System (VISN 1), which includes VAMCs and clinics in Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont.11 Soon thereafter, the VA Bedford Healthcare System and the VA Connecticut Healthcare System adopted similar strategies. Since then, other VAMCs have also adopted this approach. These collective experiences identified 4 useful strategies for achieving psychological safety: leadership engagement, open communication, education and training, and accountability.
Leadership Engagement
Health care organization leaders play a critical role in making psychological safety happen—especially in complex and constantly changing environments, such as HROs.4 Leaders behaviors are consistently linked to the perception of psychological safety at the individual, team, and organizational levels.8 It is especially important to have leaders who recognize the views of individuals and team members and encourage staff participation in discussions to gain additional perspectives.7,8,12 Psychological safety can also be facilitated when leaders are visible, approachable, and communicative.4,7-9
Organizational practices, policies, and processes (eg, reporting adverse events without the fear of negative consequences) are also important ways that leaders can establish and sustain psychological safety. On a more granular level, leaders can enhance psychological safety by promoting and acknowledging individuals who speak up, regularly asking staff about safety concerns, highlighting “good catches” when harm is avoided, and using staff feedback to initiate improvements.4,7,13Finally, in the authors’ experience, psychological safety requires clear commitment from leaders at all levels of an organization. Communication should be bidirectional, and leaders should close the proverbial “loop” with feedback and timely follow-up. This encourages and reinforces staff engagement and speaking up behaviors.2,4,7,13
Open Communication
Promoting an environment of open communication, where all individuals and teams feel empowered to speak up with questions, concerns, and recommendations—regardless of position within the organization—is critical to psychological safety.4,6,9 Open communication is especially critical when processes and systems are constantly changing and advancing as a result of new information and technology.9 Promoting open, bidirectional communication during the delivery of patient care can be accomplished with huddles, tiered safety huddles, leader rounding for high reliability, and time-outs.2,4,6 These opportunities allow team members to discuss concerns, identify resources that support safe, high-quality care; reflect on successes and opportunities for improvement; and circle back on concerns.2,6 Open communication in psychologically safe environments empowers staff to raise patient care concerns and is instrumental for improving patient safety, increasing staff job satisfaction, and decreasing turnover.6,14
Education and Training
Education and training for all staff—from the frontline to the executive level—are essential to successfully implementing the principles and practices of psychological safety.5-7 VHA training covers many topics, including the origins, benefits, and implementation strategies of psychological safety (Table). Role-playing simulation is an effective teaching format, providing staff with opportunities to practice techniques for raising concerns or share feedback in a controlled environment.6 In addition, education should be ongoing; it helps leaders and staff members feel competent and confident when implementing psychological safety across the health care organization.6,10

Accountability
The final critical strategy for achieving psychological safety is accountability. It is the responsibility of all leadership—from senior leaders to clinical and nonclinical managers—to create a culture of shared accountability.5 But first, expectations must be set. Leadership must establish well-defined behavioral expectations that align with the organization’s values. Understanding behavioral expectations will help to ensure that employees know what achievement looks like, as well as how they are being held accountable for their individual actions.4,5,7 In practical terms, this means ensuring that staff members have the skills and resources to achieve goals and expectations, providing performance feedback in a timely manner, and including expectations in annual performance evaluations (as they are in the VHA).
Consistency is key. Accountability should be the expectation across all levels and services of the health care organization. No staff member should be exempt from promoting a psychologically safe work environment. Compliance with behavioral expectations should be monitored and if a person’s actions are not consistent with expectations, the situation will need to be addressed. Interventions will depend on the type, severity, and frequency of the problematic behaviors. Depending on an organization’s policies and practices, courses of action can range from feedback counseling to employment termination.5
A practical matter in ensuring accountability is implementing a psychologically safe process for reporting concerns. Staff members must feel comfortable reporting behavioral concerns without fear of retaliation, negative judgment, or consequences from peers and supervisors. One method for doing this is to create a confidential, centralized process for reporting concerns.5
First-Hand Results
VAPHCS has seen the results of implementing the strategies outlined here. For example, VAPHCS has observed a 45% increase in the use of the patient safety reporting system that logs medical errors and near-misses. In addition, there have been improvements in levels of psychological safety and patient safety reported in the annual VHA All Employee Survey, which is conducted annually to gauge workplace satisfaction, culture, climate, turnover, supervisory behaviors, and general workplace perceptions. VAPHCS has shown consistent improvements in 12 patient safety elements scored on a 5-point scale (1, very dissatisfied; 5, very satisfied) (Figure). Notably, employee ratings of error prevention discussed increased from 4.0 in 2022 to 4.3 in 2024. Data collection and analysis are ongoing; more comprehensive findings will be published in the future.

CONCLUSIONS
Health care organizations are increasingly recognizing the importance of psychologically safe workplaces in order to provide safe, high-quality patient care. Psychological safety is a critical tool for empowering staff to raise concerns, ask tough questions, challenge the status quo, and share new ideas for providing health care services. While psychological safety has been slowly adopted in health care, it’s clear that evidence-based strategies can make psychological safety a reality.
Worldwide, health care is becoming increasingly complex as a result of greater clinical workforce demands, expanded roles and responsibilities, health care system mergers, stakeholder calls for new capabilities, and digital transformation. 1,2These increasing demands has prompted many health care institutions to place greater focus on the psychological safety of their workforce, particularly in high reliability organizations (HROs). Building a robust foundation for high reliability in health care requires the presence of psychological safety—that is, staff members at all levels of the organization must feel comfortable speaking up when they have questions or concerns.3,4 Psychological safety can improve the safety and quality of patient care but has not reached its full potential in health care.5,6 However, there are strategies that promote the widespread implementation of psychological safety in health care organizations.3-6
PSYCHOLOGICAL SAFETY
The concept of psychological safety in organizational behavior originated in 1965 when Edgar Schein and Warren Bennis, leaders in organizational psychology and management, published their reflections on the importance of psychological safety in helping individuals feel secure in the work environment.5-7 Psychological safety in the workplace is foundational to staff members feeling comfortable asking questions or expressing concerns without fear of negative consequences.8,9 It supports both individual and team efforts to raise safety concerns and report near misses and adverse events so that similar events can be averted in the future.9 Patients aren’t the only ones who benefit; psychological safety has also been found to promote job satisfaction and employee well-being.10
THE VETERANS HEALTH ADMINISTRATION JOURNEY
Achieving psychological safety is by no means an easy or comfortable process. As with any organizational change, a multipronged approach offers the best chance of success.6,9 When the Veterans Health Administration (VHA) began its incremental, enterprise-wide journey to high reliability in 2019, 3 cohorts were identified. In February 2019, 18 US Department of Veterans Affairs (VA) medical centers (VAMCs) (cohort 1) began the process of becoming HROs. Cohort 2 followed in October 2020 and included 54 VAMC. Finally, in October 2021, 67 additional VAMCs (cohort 3) started the process.2 During cohort 2, the VA Providence Healthcare System (VAPHCS) decided to emphasize psychological safety at the start of the journey to becoming an HRO. This system is part of the VA New England Healthcare System (VISN 1), which includes VAMCs and clinics in Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont.11 Soon thereafter, the VA Bedford Healthcare System and the VA Connecticut Healthcare System adopted similar strategies. Since then, other VAMCs have also adopted this approach. These collective experiences identified 4 useful strategies for achieving psychological safety: leadership engagement, open communication, education and training, and accountability.
Leadership Engagement
Health care organization leaders play a critical role in making psychological safety happen—especially in complex and constantly changing environments, such as HROs.4 Leaders behaviors are consistently linked to the perception of psychological safety at the individual, team, and organizational levels.8 It is especially important to have leaders who recognize the views of individuals and team members and encourage staff participation in discussions to gain additional perspectives.7,8,12 Psychological safety can also be facilitated when leaders are visible, approachable, and communicative.4,7-9
Organizational practices, policies, and processes (eg, reporting adverse events without the fear of negative consequences) are also important ways that leaders can establish and sustain psychological safety. On a more granular level, leaders can enhance psychological safety by promoting and acknowledging individuals who speak up, regularly asking staff about safety concerns, highlighting “good catches” when harm is avoided, and using staff feedback to initiate improvements.4,7,13Finally, in the authors’ experience, psychological safety requires clear commitment from leaders at all levels of an organization. Communication should be bidirectional, and leaders should close the proverbial “loop” with feedback and timely follow-up. This encourages and reinforces staff engagement and speaking up behaviors.2,4,7,13
Open Communication
Promoting an environment of open communication, where all individuals and teams feel empowered to speak up with questions, concerns, and recommendations—regardless of position within the organization—is critical to psychological safety.4,6,9 Open communication is especially critical when processes and systems are constantly changing and advancing as a result of new information and technology.9 Promoting open, bidirectional communication during the delivery of patient care can be accomplished with huddles, tiered safety huddles, leader rounding for high reliability, and time-outs.2,4,6 These opportunities allow team members to discuss concerns, identify resources that support safe, high-quality care; reflect on successes and opportunities for improvement; and circle back on concerns.2,6 Open communication in psychologically safe environments empowers staff to raise patient care concerns and is instrumental for improving patient safety, increasing staff job satisfaction, and decreasing turnover.6,14
Education and Training
Education and training for all staff—from the frontline to the executive level—are essential to successfully implementing the principles and practices of psychological safety.5-7 VHA training covers many topics, including the origins, benefits, and implementation strategies of psychological safety (Table). Role-playing simulation is an effective teaching format, providing staff with opportunities to practice techniques for raising concerns or share feedback in a controlled environment.6 In addition, education should be ongoing; it helps leaders and staff members feel competent and confident when implementing psychological safety across the health care organization.6,10

Accountability
The final critical strategy for achieving psychological safety is accountability. It is the responsibility of all leadership—from senior leaders to clinical and nonclinical managers—to create a culture of shared accountability.5 But first, expectations must be set. Leadership must establish well-defined behavioral expectations that align with the organization’s values. Understanding behavioral expectations will help to ensure that employees know what achievement looks like, as well as how they are being held accountable for their individual actions.4,5,7 In practical terms, this means ensuring that staff members have the skills and resources to achieve goals and expectations, providing performance feedback in a timely manner, and including expectations in annual performance evaluations (as they are in the VHA).
Consistency is key. Accountability should be the expectation across all levels and services of the health care organization. No staff member should be exempt from promoting a psychologically safe work environment. Compliance with behavioral expectations should be monitored and if a person’s actions are not consistent with expectations, the situation will need to be addressed. Interventions will depend on the type, severity, and frequency of the problematic behaviors. Depending on an organization’s policies and practices, courses of action can range from feedback counseling to employment termination.5
A practical matter in ensuring accountability is implementing a psychologically safe process for reporting concerns. Staff members must feel comfortable reporting behavioral concerns without fear of retaliation, negative judgment, or consequences from peers and supervisors. One method for doing this is to create a confidential, centralized process for reporting concerns.5
First-Hand Results
VAPHCS has seen the results of implementing the strategies outlined here. For example, VAPHCS has observed a 45% increase in the use of the patient safety reporting system that logs medical errors and near-misses. In addition, there have been improvements in levels of psychological safety and patient safety reported in the annual VHA All Employee Survey, which is conducted annually to gauge workplace satisfaction, culture, climate, turnover, supervisory behaviors, and general workplace perceptions. VAPHCS has shown consistent improvements in 12 patient safety elements scored on a 5-point scale (1, very dissatisfied; 5, very satisfied) (Figure). Notably, employee ratings of error prevention discussed increased from 4.0 in 2022 to 4.3 in 2024. Data collection and analysis are ongoing; more comprehensive findings will be published in the future.

CONCLUSIONS
Health care organizations are increasingly recognizing the importance of psychologically safe workplaces in order to provide safe, high-quality patient care. Psychological safety is a critical tool for empowering staff to raise concerns, ask tough questions, challenge the status quo, and share new ideas for providing health care services. While psychological safety has been slowly adopted in health care, it’s clear that evidence-based strategies can make psychological safety a reality.
- Spanos S, Leask E, Patel R, Datyner M, Loh E, Braithwaite J. Healthcare leaders navigating complexity: A scoping review of key trends in future roles and competencies. BMC Med Educ. 2024;24(1):720. doi:10.1186/s12909-024-05689-4
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41(7):214-221. doi:10.12788/fp.0486
- Bransby DP, Kerrissey M, Edmondson AC. Paradise lost (and restored?): a study of psychological safety over time. Acad Manag Discov. Published online March 14, 2024. doi:10.5465/amd.2023.0084
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
- Jamal N, Young VN, Shapiro J, Brenner MJ, Schmalbach CE. Patient safety/quality improvement primer, part IV: Psychological safety-drivers to outcomes and well-being. Otolaryngol Head Neck Surg. 2023;168(4):881-888. doi:10.1177/01945998221126966
- Sarofim M. Psychological safety in medicine: What is it, and who cares? Med J Aust. 2024;220(8):398-399. doi:10.5694/mja2.52263
- Edmondson AC, Bransby DP. Psychological safety comes of age: Observed themes in an established literature. Annu Rev Organ Psychol Organ Behav. 2023;10:55-78. doi.org/10.1146/annurev-orgpsych-120920-055217
- Kumar S. Psychological safety: What it is, why teams need it, and how to make it flourish. Chest. 2024; 165(4):942-949. doi:10.1016/j.chest.2023.11.016
- Hallam KT, Popovic N, Karimi L. Identifying the key elements of psychologically safe workplaces in healthcare settings. Brain Sci. 2023;13(10):1450. doi:10.3390/brainsci13101450
- Grailey KE, Murray E, Reader T, Brett SJ. The presence and potential impact of psychological safety in the healthcare setting: an evidence synthesis. BMC Health Serv Res. 2021;21(1):773. doi:10.1186/s12913-021-06740-6
- US Department of Veterans Affairs. VISN 1: VA New England Healthcare System. Accessed March 25, 2025. https://department.va.gov/integrated-service-networks/visn-01
- Brimhall KC, Tsai CY, Eckardt R, Dionne S, Yang B, Sharp A. The effects of leadership for self-worth, inclusion, trust, and psychological safety on medical error reporting. Health Care Manage Rev. 2023;48(2):120-129. doi:10.1097/HMR.0000000000000358
- Adair KC, Heath A, Frye MA, et al. The Psychological Safety Scale of the Safety, Communication, Operational, Reliability, and Engagement (SCORE) Survey: a brief, diagnostic, and actionable metric for the ability to speak up in healthcare settings. J Patient Saf. 2022;18(6):513-520. doi:10.1097/PTS.0000000000001048
- Cho H, Steege LM, Arsenault Knudsen ÉN. Psychological safety, communication openness, nurse job outcomes, and patient safety in hospital nurses. Res Nurs Health. 2023;46(4):445-453.
- Practical Tool 2: 5 minute psychological safety audit. Accessed March 25, 2025. https://www.educationsupport.org.uk/media/jlnf3cju/practical-tool-2-psychological-safety-audit.pdf
- Spanos S, Leask E, Patel R, Datyner M, Loh E, Braithwaite J. Healthcare leaders navigating complexity: A scoping review of key trends in future roles and competencies. BMC Med Educ. 2024;24(1):720. doi:10.1186/s12909-024-05689-4
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41(7):214-221. doi:10.12788/fp.0486
- Bransby DP, Kerrissey M, Edmondson AC. Paradise lost (and restored?): a study of psychological safety over time. Acad Manag Discov. Published online March 14, 2024. doi:10.5465/amd.2023.0084
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
- Jamal N, Young VN, Shapiro J, Brenner MJ, Schmalbach CE. Patient safety/quality improvement primer, part IV: Psychological safety-drivers to outcomes and well-being. Otolaryngol Head Neck Surg. 2023;168(4):881-888. doi:10.1177/01945998221126966
- Sarofim M. Psychological safety in medicine: What is it, and who cares? Med J Aust. 2024;220(8):398-399. doi:10.5694/mja2.52263
- Edmondson AC, Bransby DP. Psychological safety comes of age: Observed themes in an established literature. Annu Rev Organ Psychol Organ Behav. 2023;10:55-78. doi.org/10.1146/annurev-orgpsych-120920-055217
- Kumar S. Psychological safety: What it is, why teams need it, and how to make it flourish. Chest. 2024; 165(4):942-949. doi:10.1016/j.chest.2023.11.016
- Hallam KT, Popovic N, Karimi L. Identifying the key elements of psychologically safe workplaces in healthcare settings. Brain Sci. 2023;13(10):1450. doi:10.3390/brainsci13101450
- Grailey KE, Murray E, Reader T, Brett SJ. The presence and potential impact of psychological safety in the healthcare setting: an evidence synthesis. BMC Health Serv Res. 2021;21(1):773. doi:10.1186/s12913-021-06740-6
- US Department of Veterans Affairs. VISN 1: VA New England Healthcare System. Accessed March 25, 2025. https://department.va.gov/integrated-service-networks/visn-01
- Brimhall KC, Tsai CY, Eckardt R, Dionne S, Yang B, Sharp A. The effects of leadership for self-worth, inclusion, trust, and psychological safety on medical error reporting. Health Care Manage Rev. 2023;48(2):120-129. doi:10.1097/HMR.0000000000000358
- Adair KC, Heath A, Frye MA, et al. The Psychological Safety Scale of the Safety, Communication, Operational, Reliability, and Engagement (SCORE) Survey: a brief, diagnostic, and actionable metric for the ability to speak up in healthcare settings. J Patient Saf. 2022;18(6):513-520. doi:10.1097/PTS.0000000000001048
- Cho H, Steege LM, Arsenault Knudsen ÉN. Psychological safety, communication openness, nurse job outcomes, and patient safety in hospital nurses. Res Nurs Health. 2023;46(4):445-453.
- Practical Tool 2: 5 minute psychological safety audit. Accessed March 25, 2025. https://www.educationsupport.org.uk/media/jlnf3cju/practical-tool-2-psychological-safety-audit.pdf
Achieving Psychological Safety in High Reliability Organizations
Achieving Psychological Safety in High Reliability Organizations
The Cruelty of April: Suicide in Spring
The Cruelty of April: Suicide in Spring
April is the cruellest month, breeding
Lilacs out of the dead land, mixing
Memory and desire, stirring
Dull roots with spring rain.
T.S. Eliot1
The epigraph for this column is from The Waste Land, T.S. Eliot’s postmodern poem that, in part, reflects his experience of the destruction of an entire way of living and a generation of young men in the wake of the First World War. The terrible contemporary toll suicide has taken on veterans and the active-duty military makes it easy to forget that suicide is an inveterate and disturbing aftermath of all wars.2
There is a profound and elemental connection in the human mind between Spring and renewal. In almost every culture and religion, across nearly every historical epoch and location, Spring is associated with themes of growth, returning life, light, and hope. On a more prosaic modern level, almost all of us—us—especially those in Northern climates—look forward to warmer weather, more time spent outdoors, and the simple joys of seeing perennials return in the garden and birds nest in blooming trees.
It is a paradox of human life that suicide is more common in the season of rebirth than in the season of decline. The bare trees, freezing temperatures, and icy darkness that accompany winter in much of the world inherently lead us to contemplate our mortality. The counterintuitive finding that individuals, many of them veterans, take their own lives more often in Spring creates a cognitive dissonance to be explored in this editorial.
As a layperson, I too assumed there were more suicides in winter, especially around the holidays when the expectation of belonging, privilege, and pleasure painfully reminds the alienated, lonely, homeless, and ailing of all they lack and all they have lost. As a psychiatric intern, I anticipated that the inpatient US Department of Veterans Affairs (VA) ward where I was training would empty with the arrival of nicer weather. Instead, I was mystified when the opposite occurred and the unit was overflowing with manic and suicidal patients.
The Centers for Disease Control and Prevention National Center for Health Statistics ranked suicide by month from 1999 to 2010. Contrary to popular belief, more suicides occurred in late Spring and Summer than any other season.3 A 2023 study of systematic reviews of seasonal variation in mood disorders, suicide risk, and health care utilization found that suicide was 11% to 23% higher, suicide attempts resulting in emergency department visits showed an increase of 1.2% to 1.7%, and hospital admissions for mania rose 7.4% to 16.0% in Spring and Summer, compared with Fall and Winter.4 This general population finding is also seen in veteran and military cohorts. A recent study analyzed VA and US Department of Defense (DoD) data from 133,867 veteran suicides from 2001 to 2021. Results showed that veteran suicides were highest in Summer.5
The rise of suicide in the Spring was first observed in the 17th century and has been the object of scientific study for at least 3 decades. That research has produced several different hypotheses from a variety of disciplines, none of which are conclusive as of this writing. Cho and Lee note that the phrase “Spring fever” is a much more serious illness for those with a predisposition or diagnosis of unipolar or bipolar disorder than the quotidian irritant that afflicts those without affective disorders.6 In residency, I learned that longer exposure to light in Spring led to an imbalance in neurotransmitters that triggered manias. This is a simplistic version of the complex circadian interactions of temperature, climate, light, and other environmental variables causing dysregulation or misalignment of our natural biological cycles and those of nature proposed by chronobiologists.7
Sociological and criminal justice scholars underscore that an increase in temperature may exacerbate violent tendencies, especially in older males—a demographic profile more frequently found in veterans—and those already prone to acting out their frustrations with firearms.8 Psychologists have hypothesized that individuals with depressions persevere through Winter by telling themselves they will feel relief in the Spring. Too often the coming of Spring brings not reprieve but a deadly combination of deeper mental desperation coupled with the release from winter lassitude that energizes the now hopeless person to put ideation into action.4,9 The elevation of suicide rates in Spring is likely multidetermined with all these putative causes contributing in different variations to every individual who tragically dies by suicide.
Yet despite decades of public education, this dangerous fiction stubbornly persists in the educated public and even among many health care professionals, in part due to misguided media. For years, the Annenberg Public Policy Center (APPC) has made busting this myth of holiday suicides in the media an organizational initiative. A 2023 APPC survey found that 4 of 5 Americans picked December as the month when suicide rates were highest. The organization has been analyzing holiday—related media reports for decades; those results show some improvement, with the most recent analysis of media reports somewhat better and 40% communicating erroneous information. 10
APPC believes the opinion that suicide is more common around the holidays will persuade those struggling with an exacerbation of a mental health condition or an acute crisis to attempt or die by suicide, believing it to be a reasonable social response. While recognizing there is a real risk of such contagion behavior, I believe the reverse problem is more concerning. As I observed during my internship, the acceptance of the fiction that everyone is happy in Spring may even blind health care professionals from detecting clues that patients and even our loved ones are contemplating suicide. Our relief that Winter has passed and enjoyment of Spring activities can fool us into believing everyone else is also feeling fine and doing well and miss an opportunity to intervene and treat mania or depression to save a life—the medical manifestation of renewal.
- Elliot TS, North M. The Waste Land and Other Poems: A Norton Critical Edition. W.W. Norton & Company; 2022.
- Lester D. Suicide rates before, during, and after the world wars. Eur Psychiatry. 1994;9(5):262-264. doi:10.1017/S092493380000362X
- Centers for Disease Control Center and Prevention. National Center for Health Statistics. Fact or fiction: suicides increase during the holiday season and winter months. January 10, 2014. Accessed March 27, 2025. https://blogs.cdc.gov/nchs/2014/01/10/1121/
- Della DF, Allison S, Bidargaddi N, Wa SK, Bastiampillai T. An umbrella systematic review of seasonality in mood disorders and suicide risk: the impact on demand for primary behavioral health care and acute psychiatric services. Prim Care Companion CNS Disord. 2023;25(3):22r03395. doi:10.4088/PCC.22r03395
- Gold SA, Goodrich M, Morley SW, Stephens B, McCarthy JF. Temporal patterns of veteran suicide: variation by season, day of the week, and holidays. Suicide Life Threat Behav. 2025;55(2):e13148. doi:10.1111/sltb.13148
- Cho CH, Lee HJ. Why do mania and suicide occur most often in the Spring? Psychiatry Investig. 2018;15(3):232-234. doi:10.30773/pi.2017.12.20
- Postolache TT, Mortensen PB, Tonelli LH, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1-2):88-93. doi:10.1016/j.jad.2009.05.015
- Christodoulou C, Efstathiou V, Bouras G, Korkoliakou P, Lykouras L. Seasonal variation of suicide: a brief review. Encephalos. 2012;49:73-79.
- Shapiro M. Suicide rates spike in spring, not winter. Dome. May/June 2019. Accessed March 28, 2025. https://www.hopkinsmedicine.org/news/articles/2019/05/suicide-rates-spike-in-spring-not-winter
- Annenberg Public Policy Center. Suicides don’t spike around the holiday season, but Americans think they do. December 6, 2023. Accessed March 27, 2025. https:// www.asc.upenn.edu/news-events/news/suicides-dont-spike-around-holiday-season-americans-think-they-do
April is the cruellest month, breeding
Lilacs out of the dead land, mixing
Memory and desire, stirring
Dull roots with spring rain.
T.S. Eliot1
The epigraph for this column is from The Waste Land, T.S. Eliot’s postmodern poem that, in part, reflects his experience of the destruction of an entire way of living and a generation of young men in the wake of the First World War. The terrible contemporary toll suicide has taken on veterans and the active-duty military makes it easy to forget that suicide is an inveterate and disturbing aftermath of all wars.2
There is a profound and elemental connection in the human mind between Spring and renewal. In almost every culture and religion, across nearly every historical epoch and location, Spring is associated with themes of growth, returning life, light, and hope. On a more prosaic modern level, almost all of us—us—especially those in Northern climates—look forward to warmer weather, more time spent outdoors, and the simple joys of seeing perennials return in the garden and birds nest in blooming trees.
It is a paradox of human life that suicide is more common in the season of rebirth than in the season of decline. The bare trees, freezing temperatures, and icy darkness that accompany winter in much of the world inherently lead us to contemplate our mortality. The counterintuitive finding that individuals, many of them veterans, take their own lives more often in Spring creates a cognitive dissonance to be explored in this editorial.
As a layperson, I too assumed there were more suicides in winter, especially around the holidays when the expectation of belonging, privilege, and pleasure painfully reminds the alienated, lonely, homeless, and ailing of all they lack and all they have lost. As a psychiatric intern, I anticipated that the inpatient US Department of Veterans Affairs (VA) ward where I was training would empty with the arrival of nicer weather. Instead, I was mystified when the opposite occurred and the unit was overflowing with manic and suicidal patients.
The Centers for Disease Control and Prevention National Center for Health Statistics ranked suicide by month from 1999 to 2010. Contrary to popular belief, more suicides occurred in late Spring and Summer than any other season.3 A 2023 study of systematic reviews of seasonal variation in mood disorders, suicide risk, and health care utilization found that suicide was 11% to 23% higher, suicide attempts resulting in emergency department visits showed an increase of 1.2% to 1.7%, and hospital admissions for mania rose 7.4% to 16.0% in Spring and Summer, compared with Fall and Winter.4 This general population finding is also seen in veteran and military cohorts. A recent study analyzed VA and US Department of Defense (DoD) data from 133,867 veteran suicides from 2001 to 2021. Results showed that veteran suicides were highest in Summer.5
The rise of suicide in the Spring was first observed in the 17th century and has been the object of scientific study for at least 3 decades. That research has produced several different hypotheses from a variety of disciplines, none of which are conclusive as of this writing. Cho and Lee note that the phrase “Spring fever” is a much more serious illness for those with a predisposition or diagnosis of unipolar or bipolar disorder than the quotidian irritant that afflicts those without affective disorders.6 In residency, I learned that longer exposure to light in Spring led to an imbalance in neurotransmitters that triggered manias. This is a simplistic version of the complex circadian interactions of temperature, climate, light, and other environmental variables causing dysregulation or misalignment of our natural biological cycles and those of nature proposed by chronobiologists.7
Sociological and criminal justice scholars underscore that an increase in temperature may exacerbate violent tendencies, especially in older males—a demographic profile more frequently found in veterans—and those already prone to acting out their frustrations with firearms.8 Psychologists have hypothesized that individuals with depressions persevere through Winter by telling themselves they will feel relief in the Spring. Too often the coming of Spring brings not reprieve but a deadly combination of deeper mental desperation coupled with the release from winter lassitude that energizes the now hopeless person to put ideation into action.4,9 The elevation of suicide rates in Spring is likely multidetermined with all these putative causes contributing in different variations to every individual who tragically dies by suicide.
Yet despite decades of public education, this dangerous fiction stubbornly persists in the educated public and even among many health care professionals, in part due to misguided media. For years, the Annenberg Public Policy Center (APPC) has made busting this myth of holiday suicides in the media an organizational initiative. A 2023 APPC survey found that 4 of 5 Americans picked December as the month when suicide rates were highest. The organization has been analyzing holiday—related media reports for decades; those results show some improvement, with the most recent analysis of media reports somewhat better and 40% communicating erroneous information. 10
APPC believes the opinion that suicide is more common around the holidays will persuade those struggling with an exacerbation of a mental health condition or an acute crisis to attempt or die by suicide, believing it to be a reasonable social response. While recognizing there is a real risk of such contagion behavior, I believe the reverse problem is more concerning. As I observed during my internship, the acceptance of the fiction that everyone is happy in Spring may even blind health care professionals from detecting clues that patients and even our loved ones are contemplating suicide. Our relief that Winter has passed and enjoyment of Spring activities can fool us into believing everyone else is also feeling fine and doing well and miss an opportunity to intervene and treat mania or depression to save a life—the medical manifestation of renewal.
April is the cruellest month, breeding
Lilacs out of the dead land, mixing
Memory and desire, stirring
Dull roots with spring rain.
T.S. Eliot1
The epigraph for this column is from The Waste Land, T.S. Eliot’s postmodern poem that, in part, reflects his experience of the destruction of an entire way of living and a generation of young men in the wake of the First World War. The terrible contemporary toll suicide has taken on veterans and the active-duty military makes it easy to forget that suicide is an inveterate and disturbing aftermath of all wars.2
There is a profound and elemental connection in the human mind between Spring and renewal. In almost every culture and religion, across nearly every historical epoch and location, Spring is associated with themes of growth, returning life, light, and hope. On a more prosaic modern level, almost all of us—us—especially those in Northern climates—look forward to warmer weather, more time spent outdoors, and the simple joys of seeing perennials return in the garden and birds nest in blooming trees.
It is a paradox of human life that suicide is more common in the season of rebirth than in the season of decline. The bare trees, freezing temperatures, and icy darkness that accompany winter in much of the world inherently lead us to contemplate our mortality. The counterintuitive finding that individuals, many of them veterans, take their own lives more often in Spring creates a cognitive dissonance to be explored in this editorial.
As a layperson, I too assumed there were more suicides in winter, especially around the holidays when the expectation of belonging, privilege, and pleasure painfully reminds the alienated, lonely, homeless, and ailing of all they lack and all they have lost. As a psychiatric intern, I anticipated that the inpatient US Department of Veterans Affairs (VA) ward where I was training would empty with the arrival of nicer weather. Instead, I was mystified when the opposite occurred and the unit was overflowing with manic and suicidal patients.
The Centers for Disease Control and Prevention National Center for Health Statistics ranked suicide by month from 1999 to 2010. Contrary to popular belief, more suicides occurred in late Spring and Summer than any other season.3 A 2023 study of systematic reviews of seasonal variation in mood disorders, suicide risk, and health care utilization found that suicide was 11% to 23% higher, suicide attempts resulting in emergency department visits showed an increase of 1.2% to 1.7%, and hospital admissions for mania rose 7.4% to 16.0% in Spring and Summer, compared with Fall and Winter.4 This general population finding is also seen in veteran and military cohorts. A recent study analyzed VA and US Department of Defense (DoD) data from 133,867 veteran suicides from 2001 to 2021. Results showed that veteran suicides were highest in Summer.5
The rise of suicide in the Spring was first observed in the 17th century and has been the object of scientific study for at least 3 decades. That research has produced several different hypotheses from a variety of disciplines, none of which are conclusive as of this writing. Cho and Lee note that the phrase “Spring fever” is a much more serious illness for those with a predisposition or diagnosis of unipolar or bipolar disorder than the quotidian irritant that afflicts those without affective disorders.6 In residency, I learned that longer exposure to light in Spring led to an imbalance in neurotransmitters that triggered manias. This is a simplistic version of the complex circadian interactions of temperature, climate, light, and other environmental variables causing dysregulation or misalignment of our natural biological cycles and those of nature proposed by chronobiologists.7
Sociological and criminal justice scholars underscore that an increase in temperature may exacerbate violent tendencies, especially in older males—a demographic profile more frequently found in veterans—and those already prone to acting out their frustrations with firearms.8 Psychologists have hypothesized that individuals with depressions persevere through Winter by telling themselves they will feel relief in the Spring. Too often the coming of Spring brings not reprieve but a deadly combination of deeper mental desperation coupled with the release from winter lassitude that energizes the now hopeless person to put ideation into action.4,9 The elevation of suicide rates in Spring is likely multidetermined with all these putative causes contributing in different variations to every individual who tragically dies by suicide.
Yet despite decades of public education, this dangerous fiction stubbornly persists in the educated public and even among many health care professionals, in part due to misguided media. For years, the Annenberg Public Policy Center (APPC) has made busting this myth of holiday suicides in the media an organizational initiative. A 2023 APPC survey found that 4 of 5 Americans picked December as the month when suicide rates were highest. The organization has been analyzing holiday—related media reports for decades; those results show some improvement, with the most recent analysis of media reports somewhat better and 40% communicating erroneous information. 10
APPC believes the opinion that suicide is more common around the holidays will persuade those struggling with an exacerbation of a mental health condition or an acute crisis to attempt or die by suicide, believing it to be a reasonable social response. While recognizing there is a real risk of such contagion behavior, I believe the reverse problem is more concerning. As I observed during my internship, the acceptance of the fiction that everyone is happy in Spring may even blind health care professionals from detecting clues that patients and even our loved ones are contemplating suicide. Our relief that Winter has passed and enjoyment of Spring activities can fool us into believing everyone else is also feeling fine and doing well and miss an opportunity to intervene and treat mania or depression to save a life—the medical manifestation of renewal.
- Elliot TS, North M. The Waste Land and Other Poems: A Norton Critical Edition. W.W. Norton & Company; 2022.
- Lester D. Suicide rates before, during, and after the world wars. Eur Psychiatry. 1994;9(5):262-264. doi:10.1017/S092493380000362X
- Centers for Disease Control Center and Prevention. National Center for Health Statistics. Fact or fiction: suicides increase during the holiday season and winter months. January 10, 2014. Accessed March 27, 2025. https://blogs.cdc.gov/nchs/2014/01/10/1121/
- Della DF, Allison S, Bidargaddi N, Wa SK, Bastiampillai T. An umbrella systematic review of seasonality in mood disorders and suicide risk: the impact on demand for primary behavioral health care and acute psychiatric services. Prim Care Companion CNS Disord. 2023;25(3):22r03395. doi:10.4088/PCC.22r03395
- Gold SA, Goodrich M, Morley SW, Stephens B, McCarthy JF. Temporal patterns of veteran suicide: variation by season, day of the week, and holidays. Suicide Life Threat Behav. 2025;55(2):e13148. doi:10.1111/sltb.13148
- Cho CH, Lee HJ. Why do mania and suicide occur most often in the Spring? Psychiatry Investig. 2018;15(3):232-234. doi:10.30773/pi.2017.12.20
- Postolache TT, Mortensen PB, Tonelli LH, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1-2):88-93. doi:10.1016/j.jad.2009.05.015
- Christodoulou C, Efstathiou V, Bouras G, Korkoliakou P, Lykouras L. Seasonal variation of suicide: a brief review. Encephalos. 2012;49:73-79.
- Shapiro M. Suicide rates spike in spring, not winter. Dome. May/June 2019. Accessed March 28, 2025. https://www.hopkinsmedicine.org/news/articles/2019/05/suicide-rates-spike-in-spring-not-winter
- Annenberg Public Policy Center. Suicides don’t spike around the holiday season, but Americans think they do. December 6, 2023. Accessed March 27, 2025. https:// www.asc.upenn.edu/news-events/news/suicides-dont-spike-around-holiday-season-americans-think-they-do
- Elliot TS, North M. The Waste Land and Other Poems: A Norton Critical Edition. W.W. Norton & Company; 2022.
- Lester D. Suicide rates before, during, and after the world wars. Eur Psychiatry. 1994;9(5):262-264. doi:10.1017/S092493380000362X
- Centers for Disease Control Center and Prevention. National Center for Health Statistics. Fact or fiction: suicides increase during the holiday season and winter months. January 10, 2014. Accessed March 27, 2025. https://blogs.cdc.gov/nchs/2014/01/10/1121/
- Della DF, Allison S, Bidargaddi N, Wa SK, Bastiampillai T. An umbrella systematic review of seasonality in mood disorders and suicide risk: the impact on demand for primary behavioral health care and acute psychiatric services. Prim Care Companion CNS Disord. 2023;25(3):22r03395. doi:10.4088/PCC.22r03395
- Gold SA, Goodrich M, Morley SW, Stephens B, McCarthy JF. Temporal patterns of veteran suicide: variation by season, day of the week, and holidays. Suicide Life Threat Behav. 2025;55(2):e13148. doi:10.1111/sltb.13148
- Cho CH, Lee HJ. Why do mania and suicide occur most often in the Spring? Psychiatry Investig. 2018;15(3):232-234. doi:10.30773/pi.2017.12.20
- Postolache TT, Mortensen PB, Tonelli LH, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1-2):88-93. doi:10.1016/j.jad.2009.05.015
- Christodoulou C, Efstathiou V, Bouras G, Korkoliakou P, Lykouras L. Seasonal variation of suicide: a brief review. Encephalos. 2012;49:73-79.
- Shapiro M. Suicide rates spike in spring, not winter. Dome. May/June 2019. Accessed March 28, 2025. https://www.hopkinsmedicine.org/news/articles/2019/05/suicide-rates-spike-in-spring-not-winter
- Annenberg Public Policy Center. Suicides don’t spike around the holiday season, but Americans think they do. December 6, 2023. Accessed March 27, 2025. https:// www.asc.upenn.edu/news-events/news/suicides-dont-spike-around-holiday-season-americans-think-they-do
The Cruelty of April: Suicide in Spring
The Cruelty of April: Suicide in Spring
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
The yellow fly (Diachlorus ferrugatus) is a flying biting insect belonging to the order Diptera, family Tabanidae, which also includes deer flies (genus Chrysops) and horse flies (genus Tabanus).1 They are different from stinging insects of the order Hymenoptera (bees, wasps, yellow jackets, and hornets). As the name suggests, the yellow fly has a distinct yellow appearance, and adult yellow flies have a body length of approximately 1 cm.1,2 Distinguishing features of the yellow fly include prominently dark forelegs (the remaining legs are yellow), dark purple to black eyes with 2 fluorescent green lines, and a yellow abdomen with black hairs along the lateral regions and a broad central yellow stripe.1-3 Their wings have longitudinal black veins with clear spaces in between and a conspicuous brown patch at the apex (Figure 1A). In comparison, horse flies are darker and larger (Figure 1B), and deer flies are similar in shape but have stripes on the abdomen and thorax and mottled wings with dark patches near the apex (Figure 1C).1

The Tabanidae family comprises 4455 species belonging to 137 genera and is notorious for bites that result in localized pain, swelling, itching, and discomfort.4 While some Tabanidae species are mechanical or biologic vectors of pathogens (eg, Loa loa, equine infectious anemia virus, Trypanosoma species, cattle and sheep anthrax and tularemia), yellow flies do not appear to play a considerable role in disease transmission.4,5 Nonetheless, their bites can cause discomfort and create a nuisance for individuals residing within their distribution areas as well as for agricultural livestock, contributing to lower weight gain and milk production.1
Yellow flies are a commonly occurring species in the southeastern United States; their distribution spans several states, including New Jersey, Florida, and Texas.1,2 In Florida, specifically, yellow flies exhibit a seasonal pattern, with peak activity typically occurring from April through June.6-9 Activity levels are heightened around sunset as well as sunrise.1,9 Tabanids can be found in forests, parks, and gardens—particularly those that contain waterways such as freshwater lakes and streams—and typically stay near shaded woodlands that are prone to flooding.9
Tabanids go through the life cycle stages of egg, larva, pupa, and adult; the life cycle typically spans 1 year, with the adults living 30 to 60 days.1 Mating occurs soon after adults emerge from the pupal case in the soil.1,10 Females then are attracted to large dark moving objects and will feed on blood to develop eggs.2,10 Only female members of the Tabanidae family have modifications of the mouth parts that allow wounding of the skin (Figure 2). Their bites introduce saliva to the skin containing anticoagulants and other likely allergens. The tongue is used to lap between 20 to 600 microliters of blood.11 Males feed primarily on pollen and nectar.10 Most tabanid bites result in transient wheal-and-flare reactions, but some can result in more severe allergic reactions such as in our reported case.10 Rarely, anaphylactic reactions have been documented.10,12

Case Report
A 48-year-old man presented with swelling of the left hand following a yellow fly bite to the wrist 30 minutes prior while he worked outside at a ranch in central Florida (Figure 3). The patient was afebrile and reported no respiratory or gastrointestinal symptoms. The left hand and forearm were warm to the touch and appeared red and edematous (Figure 4). He was not tachycardic and did not appear to be in any distress. The patient reported that he had worked on the ranch for several years, and during that time had noted he was developing worsening localized reactions to yellow fly bites. He had visually identified the offending insect prior to the current presentation and had trapped some flies in previous incidents. Recently he had experienced rapid swelling at the bite sites but had never experienced respiratory difficulties or signs of systemic allergic reactions. He previously had used topical steroids when bites resulted in mild wheal-and-flare reactions, but he reported that these were no longer effective.

Management of the current bite reaction included oral prednisone tapered over 1 week from 40 mg to 10 mg daily as well as oral cetirizine 10 mg daily. Although bacterial cellulitis was considered in the differential diagnosis, no oral antibiotics were prescribed given the patient’s history of similar clinical presentations following yellow fly bites. His symptoms resolved within a few hours of his dose of prednisone. Incidentally, our patient has been able to control the progression of subsequent hypersensitivity reactions to yellow fly bites with a single 20-mg dose of prednisone administered at the onset of the bite.
Comment
In general, blood-feeding (hematophagous) insects rarely cause anaphylaxis and are more likely to cause cutaneous hypersensitivity reactions, possibly due to the small amount of antigen injected from a bite.13,14 The immediate wheal-and-flare reaction is an IgE-mediated type 1 immune reaction compared to a less common type 4 T-cell mediated delayed hypersensitivity reaction.14,15 There are many protein allergens in the saliva of biting insects that are not well characterized. Relevant allergens include a 69 kDa salivary gland protein as well as a Tab y 1 (anticoagulant), Tab y 2 (hyaluronidase), and Tab y 5 (antigen 5–related venom protein).11,15-17 Some of these proteins have structural homology between insects of different orders and can cause cross-reactivity in patients who also are allergic to Hymenoptera stings (wasp-horsefly syndrome).12,16
Our patient’s cutaneous reaction was localized and clinically manifested with rapidly progressive erythema and edema at the bite location. He did not exhibit signs of a systemic reaction such as angioedema, respiratory or gastrointestinal symptoms, tachycardia, or hypotension. Management of affected patients depends on the extent of the reaction and may include oral or parenteral antihistamines as well as oral steroids for more severe edema.11 Anaphylactic reactions generally respond to subcutaneous epinephrine.15 It would be prudent for patients with a relevant anaphylactic history to carry an autoinjectable epinephrine pen in case of difficulty breathing or general malaise following a bite. Besides avoidance of insect bites, personal protection methods include wearing long-sleeved shirts and pants and using insect repellents containing diethyl toluamide (DEET), citronella, or geraniol.1
At present, diagnosis of cutaneous reactions to yellow fly bites is best made based on the patient’s personal history.14 If the offending fly is trapped, it can be identified. As most patients cannot differentiate between insects, it may be helpful for dermatologists to know that a small amount of blood at the bite site is suggestive of a fly bite rather than a sting from a member of the order Hymenoptera. Currently, there are no consistently useful extracts for intradermal skin testing.11 Although there are several commercially available serum-specific IgE tests for suspected horse fly reactions, their usefulness is doubtful without further information on sensitivity and specificity as well as the allergen utilized.11,18,19 The use of allergen immunotherapy to induce hyposensitization in patients who experience cutaneous reactions is not standardized and poses some risks including severe allergic reactions requiring facilities for resuscitation, variability of response patterns, and supporting evidence is weak.11
Final Thoughts
Cutaneous reactions to yellow fly bites rarely are described in the dermatology literature. The salivary proteins implicated in inducing an allergic response and cross-reactivity of D ferrugatus with other biting and stinging insects as well as the natural course of immune reactions over time need to be further characterized.
- Squitier JM. Deer flies, yellow flies, and horse flies, Chrysops, Diachlorus and Tabanus spp. (Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN155
- Fairchild GB, Weems HB Jr, Fasulo TR. Yellow fly, Diachlorus ferrugatus (Fabricius)(Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN595
- Mullens BA. Horse flies and deer flies (Tabanidae). In: Mullen G, Durden L. Med Vet Entomol. Elsevier Science; 2009:327-344.
- Akhoundi M, Sereno D, Marteau A, et al. Who bites me? A tentative discriminative key to diagnose hematophagous ectoparasites biting using clinical manifestations. Diagnostics (Basel). 2020;10:308.
- Cheng TC. General Parasitology. 2nd ed. Elsevier Science; 2021:660.
- Wells K, Varnadoe C, Dorman D, et al. Survey of the distribution and seasonal activity of yellow flies (Diptera: Tabanidae) in Florida, USA. J Vector Ecol. 2019;44:235-242.
- Hribar LJ, Leppla NC, Beshear RJ, et al. Seasonal abundance of Diachlorus ferrugatus (Diptera: Tabanidae) in Monroe County, Florida. Florida Scientist. 2003;66:52-54.
- Fairchild GB, Weems HV. Diachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular. 1973;139.
- Cilek JE, Schreiber ET. Diel host-seeking activity of adult Diachlorus ferrugatus (F.) (Diptera: Tabanidae) in Northwestern Florida. J Entomol Sci. 1999;34:462-466.
- Sean S. Tabanids (horseflies). Dermatol Online J. 1999;5:6.
- Whyte AF, Popeseu FD, Carlson J. Tabanidae insect (horsefly and deerfly) allergy in humans: a review of the literature. Clin Exp Allergy. 2020;50:886-893.
- Buonomo A, Rizzi A, Aruanno A, et al. Anaphylaxis after horsefly sting: a strange case of wasp-horsefly syndrome. Postepi Dermatol Alergol. 2021;2:331-332.
- Freye HB, Litwin C. Coexistent anaphylaxis to Diptera and Hymenoptera. Ann Allergy Asthma Immunol. 1996 76:270-272.
- Hemmer W, Wantke F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol Select. 2020;4:97-104.
- Ewan PW. Allergy to insect stings: a review. J R Soc Med. 1985;78:234-239.
- Ma D, Li Y, Dong J, et al. Purification and characterization of two new allergens from the salivary glands of the horsefly Tabanus yao. Allergy. 2011;66:101-109.
- Hemmer W, Focke M, Vieluf D, et al. Anaphylaxis induced by horsefly bites: identification of a 69 kd IgE-binding protein from Chrysops spp. (Diptera: Tabanidae) by western blot analysis. J Allergy Clin Immunol. 1998;101:134-136.
- Mayo Clinic Laboratories. Test catalog: horse fly. Accessed March 11, 2025. https://www.mayocliniclabs.com/search?q=horse%20fly
- HealthLabs.com. Horsefly allergy test. Accessed March 11, 2025. https://www.healthlabs.com/horsefly-allergy-testing
The yellow fly (Diachlorus ferrugatus) is a flying biting insect belonging to the order Diptera, family Tabanidae, which also includes deer flies (genus Chrysops) and horse flies (genus Tabanus).1 They are different from stinging insects of the order Hymenoptera (bees, wasps, yellow jackets, and hornets). As the name suggests, the yellow fly has a distinct yellow appearance, and adult yellow flies have a body length of approximately 1 cm.1,2 Distinguishing features of the yellow fly include prominently dark forelegs (the remaining legs are yellow), dark purple to black eyes with 2 fluorescent green lines, and a yellow abdomen with black hairs along the lateral regions and a broad central yellow stripe.1-3 Their wings have longitudinal black veins with clear spaces in between and a conspicuous brown patch at the apex (Figure 1A). In comparison, horse flies are darker and larger (Figure 1B), and deer flies are similar in shape but have stripes on the abdomen and thorax and mottled wings with dark patches near the apex (Figure 1C).1

The Tabanidae family comprises 4455 species belonging to 137 genera and is notorious for bites that result in localized pain, swelling, itching, and discomfort.4 While some Tabanidae species are mechanical or biologic vectors of pathogens (eg, Loa loa, equine infectious anemia virus, Trypanosoma species, cattle and sheep anthrax and tularemia), yellow flies do not appear to play a considerable role in disease transmission.4,5 Nonetheless, their bites can cause discomfort and create a nuisance for individuals residing within their distribution areas as well as for agricultural livestock, contributing to lower weight gain and milk production.1
Yellow flies are a commonly occurring species in the southeastern United States; their distribution spans several states, including New Jersey, Florida, and Texas.1,2 In Florida, specifically, yellow flies exhibit a seasonal pattern, with peak activity typically occurring from April through June.6-9 Activity levels are heightened around sunset as well as sunrise.1,9 Tabanids can be found in forests, parks, and gardens—particularly those that contain waterways such as freshwater lakes and streams—and typically stay near shaded woodlands that are prone to flooding.9
Tabanids go through the life cycle stages of egg, larva, pupa, and adult; the life cycle typically spans 1 year, with the adults living 30 to 60 days.1 Mating occurs soon after adults emerge from the pupal case in the soil.1,10 Females then are attracted to large dark moving objects and will feed on blood to develop eggs.2,10 Only female members of the Tabanidae family have modifications of the mouth parts that allow wounding of the skin (Figure 2). Their bites introduce saliva to the skin containing anticoagulants and other likely allergens. The tongue is used to lap between 20 to 600 microliters of blood.11 Males feed primarily on pollen and nectar.10 Most tabanid bites result in transient wheal-and-flare reactions, but some can result in more severe allergic reactions such as in our reported case.10 Rarely, anaphylactic reactions have been documented.10,12

Case Report
A 48-year-old man presented with swelling of the left hand following a yellow fly bite to the wrist 30 minutes prior while he worked outside at a ranch in central Florida (Figure 3). The patient was afebrile and reported no respiratory or gastrointestinal symptoms. The left hand and forearm were warm to the touch and appeared red and edematous (Figure 4). He was not tachycardic and did not appear to be in any distress. The patient reported that he had worked on the ranch for several years, and during that time had noted he was developing worsening localized reactions to yellow fly bites. He had visually identified the offending insect prior to the current presentation and had trapped some flies in previous incidents. Recently he had experienced rapid swelling at the bite sites but had never experienced respiratory difficulties or signs of systemic allergic reactions. He previously had used topical steroids when bites resulted in mild wheal-and-flare reactions, but he reported that these were no longer effective.


Management of the current bite reaction included oral prednisone tapered over 1 week from 40 mg to 10 mg daily as well as oral cetirizine 10 mg daily. Although bacterial cellulitis was considered in the differential diagnosis, no oral antibiotics were prescribed given the patient’s history of similar clinical presentations following yellow fly bites. His symptoms resolved within a few hours of his dose of prednisone. Incidentally, our patient has been able to control the progression of subsequent hypersensitivity reactions to yellow fly bites with a single 20-mg dose of prednisone administered at the onset of the bite.
Comment
In general, blood-feeding (hematophagous) insects rarely cause anaphylaxis and are more likely to cause cutaneous hypersensitivity reactions, possibly due to the small amount of antigen injected from a bite.13,14 The immediate wheal-and-flare reaction is an IgE-mediated type 1 immune reaction compared to a less common type 4 T-cell mediated delayed hypersensitivity reaction.14,15 There are many protein allergens in the saliva of biting insects that are not well characterized. Relevant allergens include a 69 kDa salivary gland protein as well as a Tab y 1 (anticoagulant), Tab y 2 (hyaluronidase), and Tab y 5 (antigen 5–related venom protein).11,15-17 Some of these proteins have structural homology between insects of different orders and can cause cross-reactivity in patients who also are allergic to Hymenoptera stings (wasp-horsefly syndrome).12,16
Our patient’s cutaneous reaction was localized and clinically manifested with rapidly progressive erythema and edema at the bite location. He did not exhibit signs of a systemic reaction such as angioedema, respiratory or gastrointestinal symptoms, tachycardia, or hypotension. Management of affected patients depends on the extent of the reaction and may include oral or parenteral antihistamines as well as oral steroids for more severe edema.11 Anaphylactic reactions generally respond to subcutaneous epinephrine.15 It would be prudent for patients with a relevant anaphylactic history to carry an autoinjectable epinephrine pen in case of difficulty breathing or general malaise following a bite. Besides avoidance of insect bites, personal protection methods include wearing long-sleeved shirts and pants and using insect repellents containing diethyl toluamide (DEET), citronella, or geraniol.1
At present, diagnosis of cutaneous reactions to yellow fly bites is best made based on the patient’s personal history.14 If the offending fly is trapped, it can be identified. As most patients cannot differentiate between insects, it may be helpful for dermatologists to know that a small amount of blood at the bite site is suggestive of a fly bite rather than a sting from a member of the order Hymenoptera. Currently, there are no consistently useful extracts for intradermal skin testing.11 Although there are several commercially available serum-specific IgE tests for suspected horse fly reactions, their usefulness is doubtful without further information on sensitivity and specificity as well as the allergen utilized.11,18,19 The use of allergen immunotherapy to induce hyposensitization in patients who experience cutaneous reactions is not standardized and poses some risks including severe allergic reactions requiring facilities for resuscitation, variability of response patterns, and supporting evidence is weak.11
Final Thoughts
Cutaneous reactions to yellow fly bites rarely are described in the dermatology literature. The salivary proteins implicated in inducing an allergic response and cross-reactivity of D ferrugatus with other biting and stinging insects as well as the natural course of immune reactions over time need to be further characterized.
The yellow fly (Diachlorus ferrugatus) is a flying biting insect belonging to the order Diptera, family Tabanidae, which also includes deer flies (genus Chrysops) and horse flies (genus Tabanus).1 They are different from stinging insects of the order Hymenoptera (bees, wasps, yellow jackets, and hornets). As the name suggests, the yellow fly has a distinct yellow appearance, and adult yellow flies have a body length of approximately 1 cm.1,2 Distinguishing features of the yellow fly include prominently dark forelegs (the remaining legs are yellow), dark purple to black eyes with 2 fluorescent green lines, and a yellow abdomen with black hairs along the lateral regions and a broad central yellow stripe.1-3 Their wings have longitudinal black veins with clear spaces in between and a conspicuous brown patch at the apex (Figure 1A). In comparison, horse flies are darker and larger (Figure 1B), and deer flies are similar in shape but have stripes on the abdomen and thorax and mottled wings with dark patches near the apex (Figure 1C).1

The Tabanidae family comprises 4455 species belonging to 137 genera and is notorious for bites that result in localized pain, swelling, itching, and discomfort.4 While some Tabanidae species are mechanical or biologic vectors of pathogens (eg, Loa loa, equine infectious anemia virus, Trypanosoma species, cattle and sheep anthrax and tularemia), yellow flies do not appear to play a considerable role in disease transmission.4,5 Nonetheless, their bites can cause discomfort and create a nuisance for individuals residing within their distribution areas as well as for agricultural livestock, contributing to lower weight gain and milk production.1
Yellow flies are a commonly occurring species in the southeastern United States; their distribution spans several states, including New Jersey, Florida, and Texas.1,2 In Florida, specifically, yellow flies exhibit a seasonal pattern, with peak activity typically occurring from April through June.6-9 Activity levels are heightened around sunset as well as sunrise.1,9 Tabanids can be found in forests, parks, and gardens—particularly those that contain waterways such as freshwater lakes and streams—and typically stay near shaded woodlands that are prone to flooding.9
Tabanids go through the life cycle stages of egg, larva, pupa, and adult; the life cycle typically spans 1 year, with the adults living 30 to 60 days.1 Mating occurs soon after adults emerge from the pupal case in the soil.1,10 Females then are attracted to large dark moving objects and will feed on blood to develop eggs.2,10 Only female members of the Tabanidae family have modifications of the mouth parts that allow wounding of the skin (Figure 2). Their bites introduce saliva to the skin containing anticoagulants and other likely allergens. The tongue is used to lap between 20 to 600 microliters of blood.11 Males feed primarily on pollen and nectar.10 Most tabanid bites result in transient wheal-and-flare reactions, but some can result in more severe allergic reactions such as in our reported case.10 Rarely, anaphylactic reactions have been documented.10,12

Case Report
A 48-year-old man presented with swelling of the left hand following a yellow fly bite to the wrist 30 minutes prior while he worked outside at a ranch in central Florida (Figure 3). The patient was afebrile and reported no respiratory or gastrointestinal symptoms. The left hand and forearm were warm to the touch and appeared red and edematous (Figure 4). He was not tachycardic and did not appear to be in any distress. The patient reported that he had worked on the ranch for several years, and during that time had noted he was developing worsening localized reactions to yellow fly bites. He had visually identified the offending insect prior to the current presentation and had trapped some flies in previous incidents. Recently he had experienced rapid swelling at the bite sites but had never experienced respiratory difficulties or signs of systemic allergic reactions. He previously had used topical steroids when bites resulted in mild wheal-and-flare reactions, but he reported that these were no longer effective.


Management of the current bite reaction included oral prednisone tapered over 1 week from 40 mg to 10 mg daily as well as oral cetirizine 10 mg daily. Although bacterial cellulitis was considered in the differential diagnosis, no oral antibiotics were prescribed given the patient’s history of similar clinical presentations following yellow fly bites. His symptoms resolved within a few hours of his dose of prednisone. Incidentally, our patient has been able to control the progression of subsequent hypersensitivity reactions to yellow fly bites with a single 20-mg dose of prednisone administered at the onset of the bite.
Comment
In general, blood-feeding (hematophagous) insects rarely cause anaphylaxis and are more likely to cause cutaneous hypersensitivity reactions, possibly due to the small amount of antigen injected from a bite.13,14 The immediate wheal-and-flare reaction is an IgE-mediated type 1 immune reaction compared to a less common type 4 T-cell mediated delayed hypersensitivity reaction.14,15 There are many protein allergens in the saliva of biting insects that are not well characterized. Relevant allergens include a 69 kDa salivary gland protein as well as a Tab y 1 (anticoagulant), Tab y 2 (hyaluronidase), and Tab y 5 (antigen 5–related venom protein).11,15-17 Some of these proteins have structural homology between insects of different orders and can cause cross-reactivity in patients who also are allergic to Hymenoptera stings (wasp-horsefly syndrome).12,16
Our patient’s cutaneous reaction was localized and clinically manifested with rapidly progressive erythema and edema at the bite location. He did not exhibit signs of a systemic reaction such as angioedema, respiratory or gastrointestinal symptoms, tachycardia, or hypotension. Management of affected patients depends on the extent of the reaction and may include oral or parenteral antihistamines as well as oral steroids for more severe edema.11 Anaphylactic reactions generally respond to subcutaneous epinephrine.15 It would be prudent for patients with a relevant anaphylactic history to carry an autoinjectable epinephrine pen in case of difficulty breathing or general malaise following a bite. Besides avoidance of insect bites, personal protection methods include wearing long-sleeved shirts and pants and using insect repellents containing diethyl toluamide (DEET), citronella, or geraniol.1
At present, diagnosis of cutaneous reactions to yellow fly bites is best made based on the patient’s personal history.14 If the offending fly is trapped, it can be identified. As most patients cannot differentiate between insects, it may be helpful for dermatologists to know that a small amount of blood at the bite site is suggestive of a fly bite rather than a sting from a member of the order Hymenoptera. Currently, there are no consistently useful extracts for intradermal skin testing.11 Although there are several commercially available serum-specific IgE tests for suspected horse fly reactions, their usefulness is doubtful without further information on sensitivity and specificity as well as the allergen utilized.11,18,19 The use of allergen immunotherapy to induce hyposensitization in patients who experience cutaneous reactions is not standardized and poses some risks including severe allergic reactions requiring facilities for resuscitation, variability of response patterns, and supporting evidence is weak.11
Final Thoughts
Cutaneous reactions to yellow fly bites rarely are described in the dermatology literature. The salivary proteins implicated in inducing an allergic response and cross-reactivity of D ferrugatus with other biting and stinging insects as well as the natural course of immune reactions over time need to be further characterized.
- Squitier JM. Deer flies, yellow flies, and horse flies, Chrysops, Diachlorus and Tabanus spp. (Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN155
- Fairchild GB, Weems HB Jr, Fasulo TR. Yellow fly, Diachlorus ferrugatus (Fabricius)(Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN595
- Mullens BA. Horse flies and deer flies (Tabanidae). In: Mullen G, Durden L. Med Vet Entomol. Elsevier Science; 2009:327-344.
- Akhoundi M, Sereno D, Marteau A, et al. Who bites me? A tentative discriminative key to diagnose hematophagous ectoparasites biting using clinical manifestations. Diagnostics (Basel). 2020;10:308.
- Cheng TC. General Parasitology. 2nd ed. Elsevier Science; 2021:660.
- Wells K, Varnadoe C, Dorman D, et al. Survey of the distribution and seasonal activity of yellow flies (Diptera: Tabanidae) in Florida, USA. J Vector Ecol. 2019;44:235-242.
- Hribar LJ, Leppla NC, Beshear RJ, et al. Seasonal abundance of Diachlorus ferrugatus (Diptera: Tabanidae) in Monroe County, Florida. Florida Scientist. 2003;66:52-54.
- Fairchild GB, Weems HV. Diachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular. 1973;139.
- Cilek JE, Schreiber ET. Diel host-seeking activity of adult Diachlorus ferrugatus (F.) (Diptera: Tabanidae) in Northwestern Florida. J Entomol Sci. 1999;34:462-466.
- Sean S. Tabanids (horseflies). Dermatol Online J. 1999;5:6.
- Whyte AF, Popeseu FD, Carlson J. Tabanidae insect (horsefly and deerfly) allergy in humans: a review of the literature. Clin Exp Allergy. 2020;50:886-893.
- Buonomo A, Rizzi A, Aruanno A, et al. Anaphylaxis after horsefly sting: a strange case of wasp-horsefly syndrome. Postepi Dermatol Alergol. 2021;2:331-332.
- Freye HB, Litwin C. Coexistent anaphylaxis to Diptera and Hymenoptera. Ann Allergy Asthma Immunol. 1996 76:270-272.
- Hemmer W, Wantke F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol Select. 2020;4:97-104.
- Ewan PW. Allergy to insect stings: a review. J R Soc Med. 1985;78:234-239.
- Ma D, Li Y, Dong J, et al. Purification and characterization of two new allergens from the salivary glands of the horsefly Tabanus yao. Allergy. 2011;66:101-109.
- Hemmer W, Focke M, Vieluf D, et al. Anaphylaxis induced by horsefly bites: identification of a 69 kd IgE-binding protein from Chrysops spp. (Diptera: Tabanidae) by western blot analysis. J Allergy Clin Immunol. 1998;101:134-136.
- Mayo Clinic Laboratories. Test catalog: horse fly. Accessed March 11, 2025. https://www.mayocliniclabs.com/search?q=horse%20fly
- HealthLabs.com. Horsefly allergy test. Accessed March 11, 2025. https://www.healthlabs.com/horsefly-allergy-testing
- Squitier JM. Deer flies, yellow flies, and horse flies, Chrysops, Diachlorus and Tabanus spp. (Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN155
- Fairchild GB, Weems HB Jr, Fasulo TR. Yellow fly, Diachlorus ferrugatus (Fabricius)(Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN595
- Mullens BA. Horse flies and deer flies (Tabanidae). In: Mullen G, Durden L. Med Vet Entomol. Elsevier Science; 2009:327-344.
- Akhoundi M, Sereno D, Marteau A, et al. Who bites me? A tentative discriminative key to diagnose hematophagous ectoparasites biting using clinical manifestations. Diagnostics (Basel). 2020;10:308.
- Cheng TC. General Parasitology. 2nd ed. Elsevier Science; 2021:660.
- Wells K, Varnadoe C, Dorman D, et al. Survey of the distribution and seasonal activity of yellow flies (Diptera: Tabanidae) in Florida, USA. J Vector Ecol. 2019;44:235-242.
- Hribar LJ, Leppla NC, Beshear RJ, et al. Seasonal abundance of Diachlorus ferrugatus (Diptera: Tabanidae) in Monroe County, Florida. Florida Scientist. 2003;66:52-54.
- Fairchild GB, Weems HV. Diachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular. 1973;139.
- Cilek JE, Schreiber ET. Diel host-seeking activity of adult Diachlorus ferrugatus (F.) (Diptera: Tabanidae) in Northwestern Florida. J Entomol Sci. 1999;34:462-466.
- Sean S. Tabanids (horseflies). Dermatol Online J. 1999;5:6.
- Whyte AF, Popeseu FD, Carlson J. Tabanidae insect (horsefly and deerfly) allergy in humans: a review of the literature. Clin Exp Allergy. 2020;50:886-893.
- Buonomo A, Rizzi A, Aruanno A, et al. Anaphylaxis after horsefly sting: a strange case of wasp-horsefly syndrome. Postepi Dermatol Alergol. 2021;2:331-332.
- Freye HB, Litwin C. Coexistent anaphylaxis to Diptera and Hymenoptera. Ann Allergy Asthma Immunol. 1996 76:270-272.
- Hemmer W, Wantke F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol Select. 2020;4:97-104.
- Ewan PW. Allergy to insect stings: a review. J R Soc Med. 1985;78:234-239.
- Ma D, Li Y, Dong J, et al. Purification and characterization of two new allergens from the salivary glands of the horsefly Tabanus yao. Allergy. 2011;66:101-109.
- Hemmer W, Focke M, Vieluf D, et al. Anaphylaxis induced by horsefly bites: identification of a 69 kd IgE-binding protein from Chrysops spp. (Diptera: Tabanidae) by western blot analysis. J Allergy Clin Immunol. 1998;101:134-136.
- Mayo Clinic Laboratories. Test catalog: horse fly. Accessed March 11, 2025. https://www.mayocliniclabs.com/search?q=horse%20fly
- HealthLabs.com. Horsefly allergy test. Accessed March 11, 2025. https://www.healthlabs.com/horsefly-allergy-testing
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
PRACTICE POINTS
- Diachlorus ferrugatus, commonly known as the yellow fly, belongs to the Tabanidae family of insects that also includes deer flies and horse flies.
- The female yellow fly can instill a painful bite in humans and can cause local and systemic allergic reactions.
- Medical management of yellow fly bites is dictated by the severity of the reaction.
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15
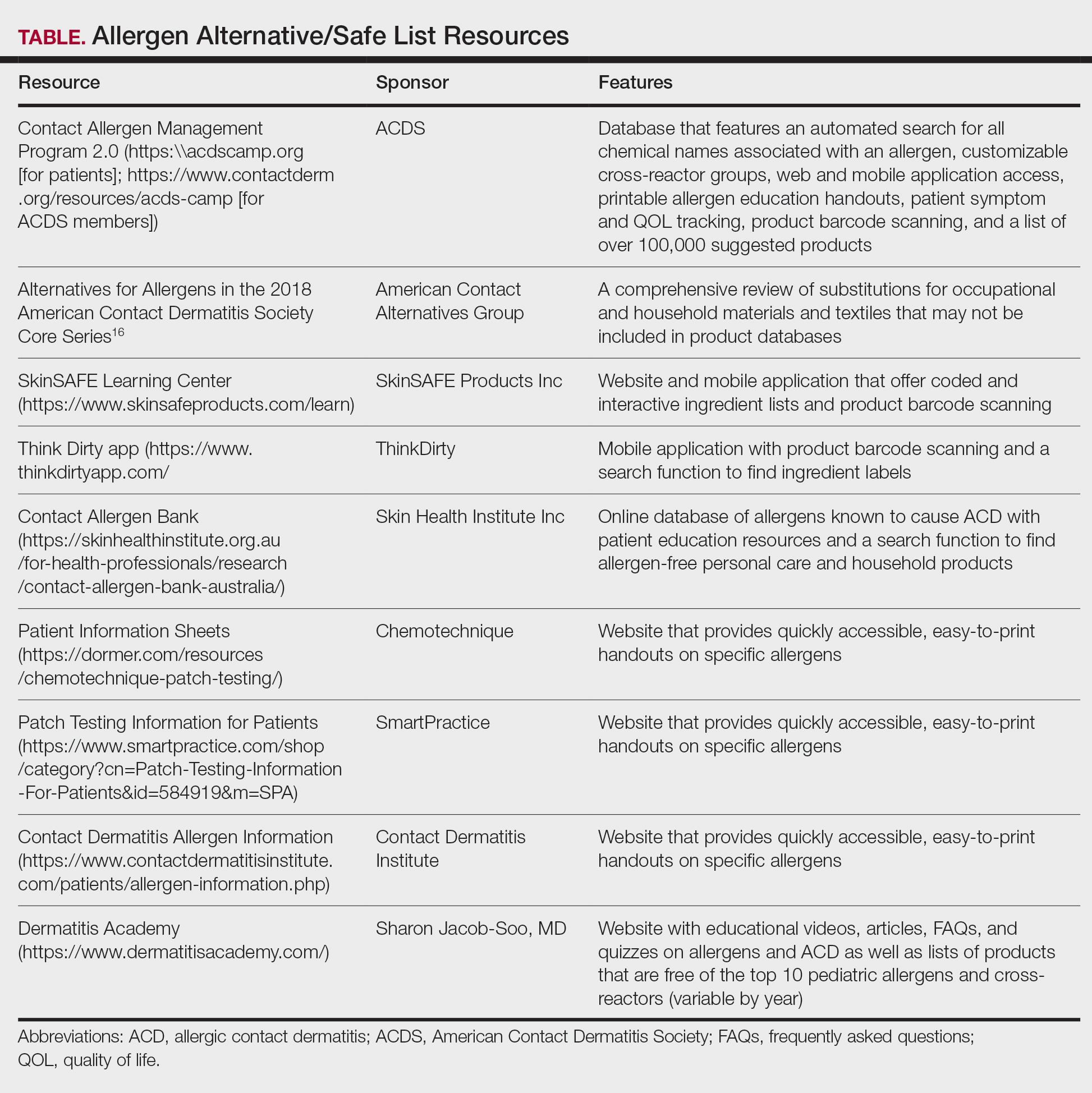
Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.
Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.
Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
PRACTICE POINTS
- Comprehensive patient education is critical for appropriate allergen avoidance after patch testing, and allergen databases and product safe lists are invaluable tools to complement clinical guidance.
- The updated Contact Allergen Management Program 2.0 offers an updated approach to patient guidance, including a database of more than 100,000 products and an easy-to-use platform to find safe, allergen-free products.
- Interactive learning resources, product pages, and quality-of-life tracking tools can help equip patients with information to encourage further autonomy in allergen avoidance.
Using Superficial Curettage to Diagnose Talon Noir
Using Superficial Curettage to Diagnose Talon Noir
Practice Gap
Brown macules on the feet can pose diagnostic challenges, often raising suspicion of acral melanoma. Talon noir, which is benign and self-resolving, is characterized by dark patches on the skin of the feet due to hemorrhage within the stratum corneum and commonly is observed in athletes who sustain repetitive foot trauma. In one study, nearly 50% (9/20) of talon noir cases initially were misdiagnosed as acral melanoma or melanocytic nevi.1 Accurate identification of talon noir is essential to prevent unnecessary interventions or delayed treatment of malignant lesions. Here, we describe a low-risk, cost-effective, and time-efficient diagnostic technique for talon noir using a disposable curette to potentially avoid more invasive procedures.
The Technique
A 34-year-old man presented to the dermatology department with a new brown macule on the second toe. The lesion had been present and stable for more than 4 months, showing no changes in shape or color. The patient reported that he was a frequent runner but did not recall any trauma to the toe, and he denied any associated pain, pruritus, or bleeding. Physical examination revealed a 6-mm dark-brown macule on the hyponychium of the left second toe, with numerous petechiae noted on dermoscopic examination. The findings were consistent with talon noir.
Given the clinical suspicion of talon noir, we used a 5-mm disposable curette to gently pare the superficial epidermis. The superficial curettage effectively removed the lesion, leaving behind a healthy epidermis with no pinpoint bleeding, which confirmed the diagnosis of talon noir (Figure). Pathologic changes from acral melanoma reside deeper than talon noir and consequently cannot be effectively removed by superficial curettage alone. Curettage acts as a curative technique for talon noir, but also as a low-risk, cost-effective, and time-efficient diagnostic technique to rule out insidious diagnoses, including acral melanoma.2 A follow-up examination performed several weeks later showed no pigmentation or recurrence of the lesion in our patient, further supporting the diagnosis of talon noir.
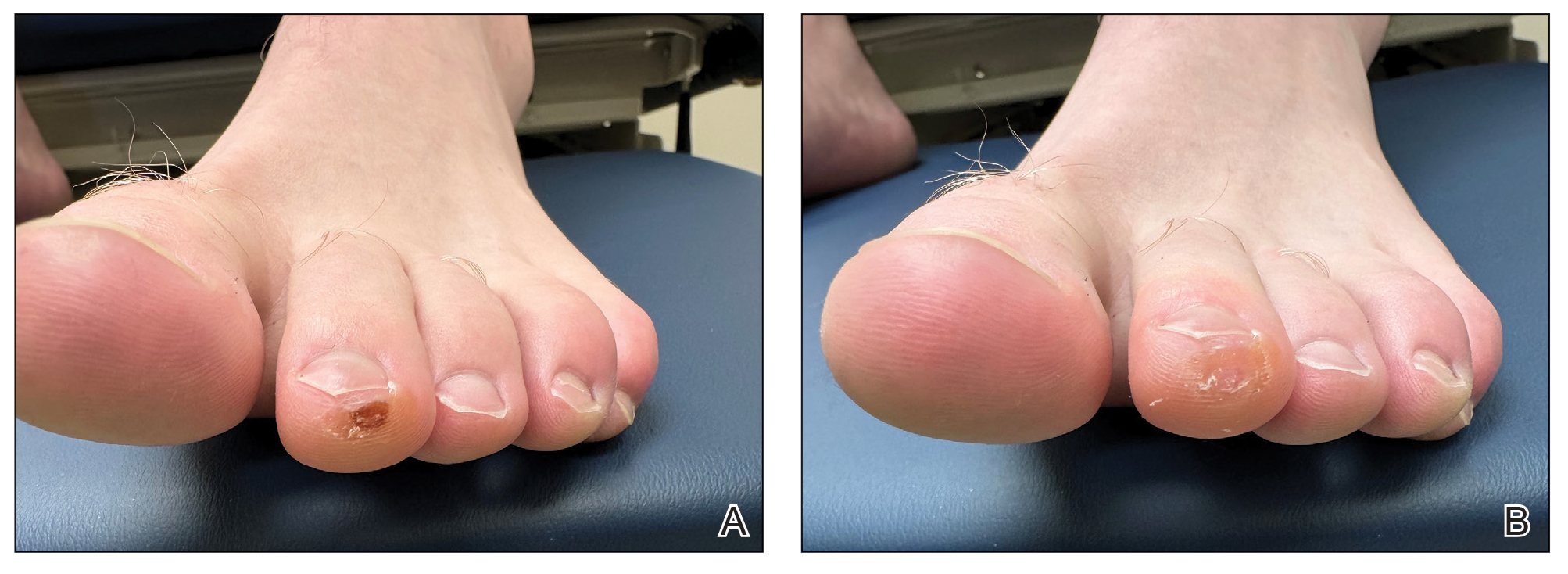
Practice Implications
Talon noir refers to localized accumulation of blood within the epidermis due to repetitive trauma, pressure, and shearing forces on the skin that results in pigmented macules.3-5 Repetitive trauma damages the microvasculature in areas of the skin with minimal subcutaneous adipose tissue.6 Talon noir also is known as subcorneal hematoma, intracorneal hematoma, black heel, hyperkeratosis hemorrhagica, and basketball heel.1,3 First described by Crissey and Peachey3 in 1961 as calcaneal petechiae, the condition was identified in basketball players with well-circumscribed, deep-red lesions on the posterior lateral heels, located between the Achilles tendon insertion and calcaneal fat pad.3 Subsequent reports have documented talon noir in athletes from a range of sports such as tennis and football, whose activities involve rapid directional changes and shearing forces on the feet.6 Similar lesions, termed tache noir, have been observed on the hands of athletes including gymnasts, weightlifters, golfers, and climbers due to repetitive hand trauma.6 Gross examination reveals blood collecting in the thickened stratum corneum.5
The cutaneous manifestations of talon noir can mimic acral melanoma, highlighting the need for dermatologists to understand its clinical, dermoscopic, and microscopic features. Poor patient recall can complicate diagnosis; for instance, in one study only 20% (4/20) of patients remembered the inciting trauma that caused the subcorneal hematomas.1 Balancing vigilance for melanoma with recognition of more benign conditions such as talon noir—particularly in younger active populations—is essential to minimize patient anxiety and avoid invasive procedures.
Further investigation is warranted in lesions that persist without obvious cause or in those that demonstrate concerning features such as extensive growth. One case of talon noir in a patient with diabetes required an excisional biopsy due to its atypical progression over 1 year with considerable hyperpigmentation and friability.7 Additional investigation such as dermoscopy may be required with paring of the skin to establish a diagnosis.1 Using a curette to pare the thickened stratum corneum, which has no nerve endings, does not require anesthetics.8 In talon noir, paring completely removes the lesion, leaving behind unaffected skin, while melanomas would retain their pigmentation due to melanin in the basal layer.2
Talon noir is a benign condition frequently misdiagnosed due to its resemblance to more serious pathologies such as melanoma. Awareness of its clinical and dermoscopic features can promote cost-effective care while reducing unnecessary procedures. Diagnostic paring of the skin with a curette offers a simple and reliable means of distinguishing talon noir from acral melanoma and other potential conditions.
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: dermoscopic diagnosis. North Clin Istanb. 2019;7:56-59. doi:10.14744/nci.2019.65481
- Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for a biopsy. Postgrad Med J. 2014;90:730-731. doi:10.1136/postgradmedj-2014-132996
- Crissey JT, Peachey JC. Calcaneal petechiae. Arch Dermatol. 1961;83:501. doi:10.1001/archderm.1961.01580090151017
- Martin SB, Lucas JK, Posa M, et al. Talon noir in a young baseball player: a case report. J Pediatr Health Care. 2021;35:235-238. doi:10.1016 /j.pedhc.2020.10.009
- Bolognia JL, Schaffer JV, Duncan KO, et al. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Emer J, Sivek R, Marciniak B. Sports dermatology: part 1 of 2 traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthetic Dermatol. 2015; 8:31-43.
- Choudhury S, Mandal A. Talon noir: a case report and literature review. Cureus. 2023;15:E35905. doi:10.7759/cureus.35905
- Oberdorfer KL, Farshchian M, Moossavi M. Paring of skin for superficially lodged foreign body removal. Cureus. 2023;15:E42396. doi:10.7759/cureus.42396
Practice Gap
Brown macules on the feet can pose diagnostic challenges, often raising suspicion of acral melanoma. Talon noir, which is benign and self-resolving, is characterized by dark patches on the skin of the feet due to hemorrhage within the stratum corneum and commonly is observed in athletes who sustain repetitive foot trauma. In one study, nearly 50% (9/20) of talon noir cases initially were misdiagnosed as acral melanoma or melanocytic nevi.1 Accurate identification of talon noir is essential to prevent unnecessary interventions or delayed treatment of malignant lesions. Here, we describe a low-risk, cost-effective, and time-efficient diagnostic technique for talon noir using a disposable curette to potentially avoid more invasive procedures.
The Technique
A 34-year-old man presented to the dermatology department with a new brown macule on the second toe. The lesion had been present and stable for more than 4 months, showing no changes in shape or color. The patient reported that he was a frequent runner but did not recall any trauma to the toe, and he denied any associated pain, pruritus, or bleeding. Physical examination revealed a 6-mm dark-brown macule on the hyponychium of the left second toe, with numerous petechiae noted on dermoscopic examination. The findings were consistent with talon noir.
Given the clinical suspicion of talon noir, we used a 5-mm disposable curette to gently pare the superficial epidermis. The superficial curettage effectively removed the lesion, leaving behind a healthy epidermis with no pinpoint bleeding, which confirmed the diagnosis of talon noir (Figure). Pathologic changes from acral melanoma reside deeper than talon noir and consequently cannot be effectively removed by superficial curettage alone. Curettage acts as a curative technique for talon noir, but also as a low-risk, cost-effective, and time-efficient diagnostic technique to rule out insidious diagnoses, including acral melanoma.2 A follow-up examination performed several weeks later showed no pigmentation or recurrence of the lesion in our patient, further supporting the diagnosis of talon noir.

Practice Implications
Talon noir refers to localized accumulation of blood within the epidermis due to repetitive trauma, pressure, and shearing forces on the skin that results in pigmented macules.3-5 Repetitive trauma damages the microvasculature in areas of the skin with minimal subcutaneous adipose tissue.6 Talon noir also is known as subcorneal hematoma, intracorneal hematoma, black heel, hyperkeratosis hemorrhagica, and basketball heel.1,3 First described by Crissey and Peachey3 in 1961 as calcaneal petechiae, the condition was identified in basketball players with well-circumscribed, deep-red lesions on the posterior lateral heels, located between the Achilles tendon insertion and calcaneal fat pad.3 Subsequent reports have documented talon noir in athletes from a range of sports such as tennis and football, whose activities involve rapid directional changes and shearing forces on the feet.6 Similar lesions, termed tache noir, have been observed on the hands of athletes including gymnasts, weightlifters, golfers, and climbers due to repetitive hand trauma.6 Gross examination reveals blood collecting in the thickened stratum corneum.5
The cutaneous manifestations of talon noir can mimic acral melanoma, highlighting the need for dermatologists to understand its clinical, dermoscopic, and microscopic features. Poor patient recall can complicate diagnosis; for instance, in one study only 20% (4/20) of patients remembered the inciting trauma that caused the subcorneal hematomas.1 Balancing vigilance for melanoma with recognition of more benign conditions such as talon noir—particularly in younger active populations—is essential to minimize patient anxiety and avoid invasive procedures.
Further investigation is warranted in lesions that persist without obvious cause or in those that demonstrate concerning features such as extensive growth. One case of talon noir in a patient with diabetes required an excisional biopsy due to its atypical progression over 1 year with considerable hyperpigmentation and friability.7 Additional investigation such as dermoscopy may be required with paring of the skin to establish a diagnosis.1 Using a curette to pare the thickened stratum corneum, which has no nerve endings, does not require anesthetics.8 In talon noir, paring completely removes the lesion, leaving behind unaffected skin, while melanomas would retain their pigmentation due to melanin in the basal layer.2
Talon noir is a benign condition frequently misdiagnosed due to its resemblance to more serious pathologies such as melanoma. Awareness of its clinical and dermoscopic features can promote cost-effective care while reducing unnecessary procedures. Diagnostic paring of the skin with a curette offers a simple and reliable means of distinguishing talon noir from acral melanoma and other potential conditions.
Practice Gap
Brown macules on the feet can pose diagnostic challenges, often raising suspicion of acral melanoma. Talon noir, which is benign and self-resolving, is characterized by dark patches on the skin of the feet due to hemorrhage within the stratum corneum and commonly is observed in athletes who sustain repetitive foot trauma. In one study, nearly 50% (9/20) of talon noir cases initially were misdiagnosed as acral melanoma or melanocytic nevi.1 Accurate identification of talon noir is essential to prevent unnecessary interventions or delayed treatment of malignant lesions. Here, we describe a low-risk, cost-effective, and time-efficient diagnostic technique for talon noir using a disposable curette to potentially avoid more invasive procedures.
The Technique
A 34-year-old man presented to the dermatology department with a new brown macule on the second toe. The lesion had been present and stable for more than 4 months, showing no changes in shape or color. The patient reported that he was a frequent runner but did not recall any trauma to the toe, and he denied any associated pain, pruritus, or bleeding. Physical examination revealed a 6-mm dark-brown macule on the hyponychium of the left second toe, with numerous petechiae noted on dermoscopic examination. The findings were consistent with talon noir.
Given the clinical suspicion of talon noir, we used a 5-mm disposable curette to gently pare the superficial epidermis. The superficial curettage effectively removed the lesion, leaving behind a healthy epidermis with no pinpoint bleeding, which confirmed the diagnosis of talon noir (Figure). Pathologic changes from acral melanoma reside deeper than talon noir and consequently cannot be effectively removed by superficial curettage alone. Curettage acts as a curative technique for talon noir, but also as a low-risk, cost-effective, and time-efficient diagnostic technique to rule out insidious diagnoses, including acral melanoma.2 A follow-up examination performed several weeks later showed no pigmentation or recurrence of the lesion in our patient, further supporting the diagnosis of talon noir.

Practice Implications
Talon noir refers to localized accumulation of blood within the epidermis due to repetitive trauma, pressure, and shearing forces on the skin that results in pigmented macules.3-5 Repetitive trauma damages the microvasculature in areas of the skin with minimal subcutaneous adipose tissue.6 Talon noir also is known as subcorneal hematoma, intracorneal hematoma, black heel, hyperkeratosis hemorrhagica, and basketball heel.1,3 First described by Crissey and Peachey3 in 1961 as calcaneal petechiae, the condition was identified in basketball players with well-circumscribed, deep-red lesions on the posterior lateral heels, located between the Achilles tendon insertion and calcaneal fat pad.3 Subsequent reports have documented talon noir in athletes from a range of sports such as tennis and football, whose activities involve rapid directional changes and shearing forces on the feet.6 Similar lesions, termed tache noir, have been observed on the hands of athletes including gymnasts, weightlifters, golfers, and climbers due to repetitive hand trauma.6 Gross examination reveals blood collecting in the thickened stratum corneum.5
The cutaneous manifestations of talon noir can mimic acral melanoma, highlighting the need for dermatologists to understand its clinical, dermoscopic, and microscopic features. Poor patient recall can complicate diagnosis; for instance, in one study only 20% (4/20) of patients remembered the inciting trauma that caused the subcorneal hematomas.1 Balancing vigilance for melanoma with recognition of more benign conditions such as talon noir—particularly in younger active populations—is essential to minimize patient anxiety and avoid invasive procedures.
Further investigation is warranted in lesions that persist without obvious cause or in those that demonstrate concerning features such as extensive growth. One case of talon noir in a patient with diabetes required an excisional biopsy due to its atypical progression over 1 year with considerable hyperpigmentation and friability.7 Additional investigation such as dermoscopy may be required with paring of the skin to establish a diagnosis.1 Using a curette to pare the thickened stratum corneum, which has no nerve endings, does not require anesthetics.8 In talon noir, paring completely removes the lesion, leaving behind unaffected skin, while melanomas would retain their pigmentation due to melanin in the basal layer.2
Talon noir is a benign condition frequently misdiagnosed due to its resemblance to more serious pathologies such as melanoma. Awareness of its clinical and dermoscopic features can promote cost-effective care while reducing unnecessary procedures. Diagnostic paring of the skin with a curette offers a simple and reliable means of distinguishing talon noir from acral melanoma and other potential conditions.
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: dermoscopic diagnosis. North Clin Istanb. 2019;7:56-59. doi:10.14744/nci.2019.65481
- Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for a biopsy. Postgrad Med J. 2014;90:730-731. doi:10.1136/postgradmedj-2014-132996
- Crissey JT, Peachey JC. Calcaneal petechiae. Arch Dermatol. 1961;83:501. doi:10.1001/archderm.1961.01580090151017
- Martin SB, Lucas JK, Posa M, et al. Talon noir in a young baseball player: a case report. J Pediatr Health Care. 2021;35:235-238. doi:10.1016 /j.pedhc.2020.10.009
- Bolognia JL, Schaffer JV, Duncan KO, et al. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Emer J, Sivek R, Marciniak B. Sports dermatology: part 1 of 2 traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthetic Dermatol. 2015; 8:31-43.
- Choudhury S, Mandal A. Talon noir: a case report and literature review. Cureus. 2023;15:E35905. doi:10.7759/cureus.35905
- Oberdorfer KL, Farshchian M, Moossavi M. Paring of skin for superficially lodged foreign body removal. Cureus. 2023;15:E42396. doi:10.7759/cureus.42396
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: dermoscopic diagnosis. North Clin Istanb. 2019;7:56-59. doi:10.14744/nci.2019.65481
- Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for a biopsy. Postgrad Med J. 2014;90:730-731. doi:10.1136/postgradmedj-2014-132996
- Crissey JT, Peachey JC. Calcaneal petechiae. Arch Dermatol. 1961;83:501. doi:10.1001/archderm.1961.01580090151017
- Martin SB, Lucas JK, Posa M, et al. Talon noir in a young baseball player: a case report. J Pediatr Health Care. 2021;35:235-238. doi:10.1016 /j.pedhc.2020.10.009
- Bolognia JL, Schaffer JV, Duncan KO, et al. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Emer J, Sivek R, Marciniak B. Sports dermatology: part 1 of 2 traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthetic Dermatol. 2015; 8:31-43.
- Choudhury S, Mandal A. Talon noir: a case report and literature review. Cureus. 2023;15:E35905. doi:10.7759/cureus.35905
- Oberdorfer KL, Farshchian M, Moossavi M. Paring of skin for superficially lodged foreign body removal. Cureus. 2023;15:E42396. doi:10.7759/cureus.42396
Using Superficial Curettage to Diagnose Talon Noir
Using Superficial Curettage to Diagnose Talon Noir