User login
Increased contralateral BC risk after adjuvant radiotherapy in germline-BRCA2 pathogenic variants
Key clinical point: Patients with germline (g) BRCA1/2-associated primary breast cancer (BC), particularly those with gBRCA2 pathogenic mutations, faced a moderately increased risk of developing contralateral breast cancer (CBC) after receiving adjuvant radiotherapy.
Major finding: The risk for invasive and in situ CBC increased by 44% in patients with gBRCA1/2 mutations who did vs did not receive radiotherapy (adjusted hazard ratio [HR] 1.44; 95% CI 1.12-1.86), with the risk being even more prominent in gBRCA2 pathogenic variant carriers (adjusted HR 1.77; 95% CI 1.13-2.77).
Study details: Findings are from an analysis including 3602 patients with gBRCA1/2-associated primary BC from the prospective international BRCA1/2 Carrier Cohort Study, of whom 64% of patients received adjuvant radiotherapy.
Disclosures: This study did not receive any specific external funding. DG Evans reported ties with AstraZeneca and AmGen, K Kast declared ties with Roche Pharma AG, and J Simard declared holding patents related to BRCA1 and BRCA2.
Source: van Barele M et al. Contralateral breast cancer risk in irradiated breast cancer patients with a germline-BRCA1/2 pathogenic variant. J Natl Cancer Inst. 2023 (Jun 27). Doi: 10.1093/jnci/djad116
Key clinical point: Patients with germline (g) BRCA1/2-associated primary breast cancer (BC), particularly those with gBRCA2 pathogenic mutations, faced a moderately increased risk of developing contralateral breast cancer (CBC) after receiving adjuvant radiotherapy.
Major finding: The risk for invasive and in situ CBC increased by 44% in patients with gBRCA1/2 mutations who did vs did not receive radiotherapy (adjusted hazard ratio [HR] 1.44; 95% CI 1.12-1.86), with the risk being even more prominent in gBRCA2 pathogenic variant carriers (adjusted HR 1.77; 95% CI 1.13-2.77).
Study details: Findings are from an analysis including 3602 patients with gBRCA1/2-associated primary BC from the prospective international BRCA1/2 Carrier Cohort Study, of whom 64% of patients received adjuvant radiotherapy.
Disclosures: This study did not receive any specific external funding. DG Evans reported ties with AstraZeneca and AmGen, K Kast declared ties with Roche Pharma AG, and J Simard declared holding patents related to BRCA1 and BRCA2.
Source: van Barele M et al. Contralateral breast cancer risk in irradiated breast cancer patients with a germline-BRCA1/2 pathogenic variant. J Natl Cancer Inst. 2023 (Jun 27). Doi: 10.1093/jnci/djad116
Key clinical point: Patients with germline (g) BRCA1/2-associated primary breast cancer (BC), particularly those with gBRCA2 pathogenic mutations, faced a moderately increased risk of developing contralateral breast cancer (CBC) after receiving adjuvant radiotherapy.
Major finding: The risk for invasive and in situ CBC increased by 44% in patients with gBRCA1/2 mutations who did vs did not receive radiotherapy (adjusted hazard ratio [HR] 1.44; 95% CI 1.12-1.86), with the risk being even more prominent in gBRCA2 pathogenic variant carriers (adjusted HR 1.77; 95% CI 1.13-2.77).
Study details: Findings are from an analysis including 3602 patients with gBRCA1/2-associated primary BC from the prospective international BRCA1/2 Carrier Cohort Study, of whom 64% of patients received adjuvant radiotherapy.
Disclosures: This study did not receive any specific external funding. DG Evans reported ties with AstraZeneca and AmGen, K Kast declared ties with Roche Pharma AG, and J Simard declared holding patents related to BRCA1 and BRCA2.
Source: van Barele M et al. Contralateral breast cancer risk in irradiated breast cancer patients with a germline-BRCA1/2 pathogenic variant. J Natl Cancer Inst. 2023 (Jun 27). Doi: 10.1093/jnci/djad116
Benefit of regional nodal irradiation remains questionable in HR+/ERBB2− node-positive BC
Key clinical point: Patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (ERBB2−, aka HER2-) breast cancer (BC) experienced very low locoregional recurrence (LRR) events and comparable invasive disease-free survival (iDFS) outcomes both with and without receiving regional nodal irradiation (RNI) after surgery.
Major finding: The cumulative incidence of LRR at 5 years was low among patients who underwent breast-conserving surgery and radiotherapy with RNI (0.85%), breast-conserving surgery and radiotherapy without RNI (0.55%), mastectomy followed by radiotherapy (0.11%), or mastectomy without radiotherapy (1.7%). Receiving RNI was not associated with better iDFS outcomes (P > .1 for both pre- and postmenopausal women).
Study details: Findings are from the secondary analysis of the SWOG S1007 trial including 4871 women with HR+/ERBB2− node-positive BC, of whom 2274 women received RNI.
Disclosures: This study was supported by the US National Institutes of Health (NIH) and other sources. Some authors declared serving as consultants or receiving grants and personal fees from various sources, including NIH.
Source: Jagsi R et al. Radiotherapy use and incidence of locoregional recurrence in patients with favorable-risk, node-positive breast cancer enrolled in the SWOG S1007 trial. JAMA Oncol. 2023 (Jul 6). Doi: 10.1001/jamaoncol.2023.1984
Key clinical point: Patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (ERBB2−, aka HER2-) breast cancer (BC) experienced very low locoregional recurrence (LRR) events and comparable invasive disease-free survival (iDFS) outcomes both with and without receiving regional nodal irradiation (RNI) after surgery.
Major finding: The cumulative incidence of LRR at 5 years was low among patients who underwent breast-conserving surgery and radiotherapy with RNI (0.85%), breast-conserving surgery and radiotherapy without RNI (0.55%), mastectomy followed by radiotherapy (0.11%), or mastectomy without radiotherapy (1.7%). Receiving RNI was not associated with better iDFS outcomes (P > .1 for both pre- and postmenopausal women).
Study details: Findings are from the secondary analysis of the SWOG S1007 trial including 4871 women with HR+/ERBB2− node-positive BC, of whom 2274 women received RNI.
Disclosures: This study was supported by the US National Institutes of Health (NIH) and other sources. Some authors declared serving as consultants or receiving grants and personal fees from various sources, including NIH.
Source: Jagsi R et al. Radiotherapy use and incidence of locoregional recurrence in patients with favorable-risk, node-positive breast cancer enrolled in the SWOG S1007 trial. JAMA Oncol. 2023 (Jul 6). Doi: 10.1001/jamaoncol.2023.1984
Key clinical point: Patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (ERBB2−, aka HER2-) breast cancer (BC) experienced very low locoregional recurrence (LRR) events and comparable invasive disease-free survival (iDFS) outcomes both with and without receiving regional nodal irradiation (RNI) after surgery.
Major finding: The cumulative incidence of LRR at 5 years was low among patients who underwent breast-conserving surgery and radiotherapy with RNI (0.85%), breast-conserving surgery and radiotherapy without RNI (0.55%), mastectomy followed by radiotherapy (0.11%), or mastectomy without radiotherapy (1.7%). Receiving RNI was not associated with better iDFS outcomes (P > .1 for both pre- and postmenopausal women).
Study details: Findings are from the secondary analysis of the SWOG S1007 trial including 4871 women with HR+/ERBB2− node-positive BC, of whom 2274 women received RNI.
Disclosures: This study was supported by the US National Institutes of Health (NIH) and other sources. Some authors declared serving as consultants or receiving grants and personal fees from various sources, including NIH.
Source: Jagsi R et al. Radiotherapy use and incidence of locoregional recurrence in patients with favorable-risk, node-positive breast cancer enrolled in the SWOG S1007 trial. JAMA Oncol. 2023 (Jul 6). Doi: 10.1001/jamaoncol.2023.1984
Biofeedback Training Is a Good Alternative to Preventive Medication for Migraine and Tension-Type Headache
What types of headache and other illnesses respond to biofeedback training?
Migraine affects about 12% of the US and most Western populations, and is 3 times more common in women than men. Migraine can be treated with a variety of strategies that include medications and nonpharmacologic therapies such as biofeedback, as well as other behavioral therapies and devices. Biofeedback refers to the use of instrumentation to monitor and display physiologic responses that the patient may not be aware of so that they can be “modified” in a more adaptive direction. Feedback gives immediate objective information and is usually combined with a relaxation-based therapy. The most common biofeedback treatments for migraine include feeding back muscle activity in the face and neck to help people relax contracted muscles and teach them a “low-arousal” response; they learn to increase finger temperature, which coincides with modifying the “stress” nervous system. Biofeedback has been shown to be helpful for migraine and tension-type headache. It is also helpful to decrease anxiety and stress levels and lower blood pressure.
Can patients use biofeedback treatments in conjunction with migraine medication?
Research has shown that migraine, in particular higher-frequency migraine, is best treated by a combination of medications and behavioral strategies. Biofeedback is a good option for use in conjunction with medication for migraine.
Who is trained to provide biofeedback treatments?
Biofeedback for migraine is something usually performed by psychologists and other professionals with specialized training. Many practitioners are certified by the Biofeedback Certification Institute of America (BCIA). It is important to find an experienced biofeedback practitioner who also has some expertise in treating headache disorders.
How are biofeedback treatments performed?
Biofeedback is a therapy that follows a learning model whereby the patient gradually learns a self-regulation skill that impacts their headaches.
Patients come to an office, typically once per week, where they are attached to instruments that measure physical responses associated with migraine. The patient typically sits in a comfortable chair or recliner, and numerous physiologic responses are monitored with surface electrodes.
When treating headache, sensors are typically placed on the head and neck to monitor muscle responses and a thermistor is placed on a finger to measure temperature. Feedback is given via visual cues (computer graphics) or a change in auditory tone as the patient is taught various relaxation techniques.
Patients use the feedback to learn a physiologic relaxation response that may be beneficial for their headache management. Most of the research on biofeedback is related to treatment to prevent migraine; however, these techniques can be helpful to use during an acute attack, ideally paired with an acute care migraine medication.
Can children with migraine have biofeedback treatments as well?
Most children typically have a lower frequency of migraine than adults, although some have frequent migraines with associated disability and are candidates for preventive medication (although no medications are yet approved for children by the US Food and Drug Association). Most children are good candidates for behavioral therapies such as biofeedback. There are many computer games utilized in biofeedback training that children easily learn to modify physical responses. There are some fairly recent data suggesting that behavioral treatments, some of which include biofeedback, are effective strategies for children and may be more effective than preventive medication.
What other types of illnesses could benefit from biofeedback training?
There are data showing that biofeedback therapy can be helpful for anxiety disorders, insomnia, and functional bowel disorders and may help in modifying high blood pressure. It can also be a very effective stress-management strategy.
Do you refer some of your patients with migraine to a psychologist for biofeedback treatments?
Yes, but it is always good for the referring physician to check on the credentials of the person performing the biofeedback treatment. Some headache specialists might do it themselves, but we do not. It takes a while—at least several sessions—and psychologists are better at it. Patients’ perspectives are also important. We communicate with the referred biofeedback treatment specialist to get more insight on the patient. For example, some patients are anxious and depressed, and they are not sleeping at night. The doctor we referred the patient to may recommend an antidepressant for the patient to help address those issues. It is a team effort.
What is the average cost per treatment?
The costs vary throughout the country and range from $75 to $400 per session. Insurance coverage varies.
How many times should a patient go in for treatment?
Most protocols are about 10 to 12 sessions, depending on patient response.
Have there been clinical trials on biofeedback treatments/devices?
There have been many clinical trials that have been positive, so there is good evidence that biofeedback can be an effective treatment for migraine and tension-type headache. There are many types of biofeedback devices that measure different modalities. The most common one used in migraine is measuring scalp muscle contraction with surface electromyography or measuring peripheral blood flow with a temperature gauge. The goals are to relax muscles and learn to increase finger temperature, which is related to decreased arousal of the sympathetic nervous system or stress system. Other modalities include learning to modulate brain waves (electroencephalography or neurofeedback) and certain cardiovascular measures that reduce the stress response by a different mechanism. The goal is for patients to learn a low arousal response that they can utilize in their natural environment. Certain breathing techniques and visualization exercises are also helpful, but biofeedback refers to using physiologic recording equipment to help learn to change physical responses related to headache disorder.
Over our years of experience, we have found that biofeedback can help a large percentage of our patients with migraine and tension-type headache, and it is associated with almost no adverse events.
What types of headache and other illnesses respond to biofeedback training?
Migraine affects about 12% of the US and most Western populations, and is 3 times more common in women than men. Migraine can be treated with a variety of strategies that include medications and nonpharmacologic therapies such as biofeedback, as well as other behavioral therapies and devices. Biofeedback refers to the use of instrumentation to monitor and display physiologic responses that the patient may not be aware of so that they can be “modified” in a more adaptive direction. Feedback gives immediate objective information and is usually combined with a relaxation-based therapy. The most common biofeedback treatments for migraine include feeding back muscle activity in the face and neck to help people relax contracted muscles and teach them a “low-arousal” response; they learn to increase finger temperature, which coincides with modifying the “stress” nervous system. Biofeedback has been shown to be helpful for migraine and tension-type headache. It is also helpful to decrease anxiety and stress levels and lower blood pressure.
Can patients use biofeedback treatments in conjunction with migraine medication?
Research has shown that migraine, in particular higher-frequency migraine, is best treated by a combination of medications and behavioral strategies. Biofeedback is a good option for use in conjunction with medication for migraine.
Who is trained to provide biofeedback treatments?
Biofeedback for migraine is something usually performed by psychologists and other professionals with specialized training. Many practitioners are certified by the Biofeedback Certification Institute of America (BCIA). It is important to find an experienced biofeedback practitioner who also has some expertise in treating headache disorders.
How are biofeedback treatments performed?
Biofeedback is a therapy that follows a learning model whereby the patient gradually learns a self-regulation skill that impacts their headaches.
Patients come to an office, typically once per week, where they are attached to instruments that measure physical responses associated with migraine. The patient typically sits in a comfortable chair or recliner, and numerous physiologic responses are monitored with surface electrodes.
When treating headache, sensors are typically placed on the head and neck to monitor muscle responses and a thermistor is placed on a finger to measure temperature. Feedback is given via visual cues (computer graphics) or a change in auditory tone as the patient is taught various relaxation techniques.
Patients use the feedback to learn a physiologic relaxation response that may be beneficial for their headache management. Most of the research on biofeedback is related to treatment to prevent migraine; however, these techniques can be helpful to use during an acute attack, ideally paired with an acute care migraine medication.
Can children with migraine have biofeedback treatments as well?
Most children typically have a lower frequency of migraine than adults, although some have frequent migraines with associated disability and are candidates for preventive medication (although no medications are yet approved for children by the US Food and Drug Association). Most children are good candidates for behavioral therapies such as biofeedback. There are many computer games utilized in biofeedback training that children easily learn to modify physical responses. There are some fairly recent data suggesting that behavioral treatments, some of which include biofeedback, are effective strategies for children and may be more effective than preventive medication.
What other types of illnesses could benefit from biofeedback training?
There are data showing that biofeedback therapy can be helpful for anxiety disorders, insomnia, and functional bowel disorders and may help in modifying high blood pressure. It can also be a very effective stress-management strategy.
Do you refer some of your patients with migraine to a psychologist for biofeedback treatments?
Yes, but it is always good for the referring physician to check on the credentials of the person performing the biofeedback treatment. Some headache specialists might do it themselves, but we do not. It takes a while—at least several sessions—and psychologists are better at it. Patients’ perspectives are also important. We communicate with the referred biofeedback treatment specialist to get more insight on the patient. For example, some patients are anxious and depressed, and they are not sleeping at night. The doctor we referred the patient to may recommend an antidepressant for the patient to help address those issues. It is a team effort.
What is the average cost per treatment?
The costs vary throughout the country and range from $75 to $400 per session. Insurance coverage varies.
How many times should a patient go in for treatment?
Most protocols are about 10 to 12 sessions, depending on patient response.
Have there been clinical trials on biofeedback treatments/devices?
There have been many clinical trials that have been positive, so there is good evidence that biofeedback can be an effective treatment for migraine and tension-type headache. There are many types of biofeedback devices that measure different modalities. The most common one used in migraine is measuring scalp muscle contraction with surface electromyography or measuring peripheral blood flow with a temperature gauge. The goals are to relax muscles and learn to increase finger temperature, which is related to decreased arousal of the sympathetic nervous system or stress system. Other modalities include learning to modulate brain waves (electroencephalography or neurofeedback) and certain cardiovascular measures that reduce the stress response by a different mechanism. The goal is for patients to learn a low arousal response that they can utilize in their natural environment. Certain breathing techniques and visualization exercises are also helpful, but biofeedback refers to using physiologic recording equipment to help learn to change physical responses related to headache disorder.
Over our years of experience, we have found that biofeedback can help a large percentage of our patients with migraine and tension-type headache, and it is associated with almost no adverse events.
What types of headache and other illnesses respond to biofeedback training?
Migraine affects about 12% of the US and most Western populations, and is 3 times more common in women than men. Migraine can be treated with a variety of strategies that include medications and nonpharmacologic therapies such as biofeedback, as well as other behavioral therapies and devices. Biofeedback refers to the use of instrumentation to monitor and display physiologic responses that the patient may not be aware of so that they can be “modified” in a more adaptive direction. Feedback gives immediate objective information and is usually combined with a relaxation-based therapy. The most common biofeedback treatments for migraine include feeding back muscle activity in the face and neck to help people relax contracted muscles and teach them a “low-arousal” response; they learn to increase finger temperature, which coincides with modifying the “stress” nervous system. Biofeedback has been shown to be helpful for migraine and tension-type headache. It is also helpful to decrease anxiety and stress levels and lower blood pressure.
Can patients use biofeedback treatments in conjunction with migraine medication?
Research has shown that migraine, in particular higher-frequency migraine, is best treated by a combination of medications and behavioral strategies. Biofeedback is a good option for use in conjunction with medication for migraine.
Who is trained to provide biofeedback treatments?
Biofeedback for migraine is something usually performed by psychologists and other professionals with specialized training. Many practitioners are certified by the Biofeedback Certification Institute of America (BCIA). It is important to find an experienced biofeedback practitioner who also has some expertise in treating headache disorders.
How are biofeedback treatments performed?
Biofeedback is a therapy that follows a learning model whereby the patient gradually learns a self-regulation skill that impacts their headaches.
Patients come to an office, typically once per week, where they are attached to instruments that measure physical responses associated with migraine. The patient typically sits in a comfortable chair or recliner, and numerous physiologic responses are monitored with surface electrodes.
When treating headache, sensors are typically placed on the head and neck to monitor muscle responses and a thermistor is placed on a finger to measure temperature. Feedback is given via visual cues (computer graphics) or a change in auditory tone as the patient is taught various relaxation techniques.
Patients use the feedback to learn a physiologic relaxation response that may be beneficial for their headache management. Most of the research on biofeedback is related to treatment to prevent migraine; however, these techniques can be helpful to use during an acute attack, ideally paired with an acute care migraine medication.
Can children with migraine have biofeedback treatments as well?
Most children typically have a lower frequency of migraine than adults, although some have frequent migraines with associated disability and are candidates for preventive medication (although no medications are yet approved for children by the US Food and Drug Association). Most children are good candidates for behavioral therapies such as biofeedback. There are many computer games utilized in biofeedback training that children easily learn to modify physical responses. There are some fairly recent data suggesting that behavioral treatments, some of which include biofeedback, are effective strategies for children and may be more effective than preventive medication.
What other types of illnesses could benefit from biofeedback training?
There are data showing that biofeedback therapy can be helpful for anxiety disorders, insomnia, and functional bowel disorders and may help in modifying high blood pressure. It can also be a very effective stress-management strategy.
Do you refer some of your patients with migraine to a psychologist for biofeedback treatments?
Yes, but it is always good for the referring physician to check on the credentials of the person performing the biofeedback treatment. Some headache specialists might do it themselves, but we do not. It takes a while—at least several sessions—and psychologists are better at it. Patients’ perspectives are also important. We communicate with the referred biofeedback treatment specialist to get more insight on the patient. For example, some patients are anxious and depressed, and they are not sleeping at night. The doctor we referred the patient to may recommend an antidepressant for the patient to help address those issues. It is a team effort.
What is the average cost per treatment?
The costs vary throughout the country and range from $75 to $400 per session. Insurance coverage varies.
How many times should a patient go in for treatment?
Most protocols are about 10 to 12 sessions, depending on patient response.
Have there been clinical trials on biofeedback treatments/devices?
There have been many clinical trials that have been positive, so there is good evidence that biofeedback can be an effective treatment for migraine and tension-type headache. There are many types of biofeedback devices that measure different modalities. The most common one used in migraine is measuring scalp muscle contraction with surface electromyography or measuring peripheral blood flow with a temperature gauge. The goals are to relax muscles and learn to increase finger temperature, which is related to decreased arousal of the sympathetic nervous system or stress system. Other modalities include learning to modulate brain waves (electroencephalography or neurofeedback) and certain cardiovascular measures that reduce the stress response by a different mechanism. The goal is for patients to learn a low arousal response that they can utilize in their natural environment. Certain breathing techniques and visualization exercises are also helpful, but biofeedback refers to using physiologic recording equipment to help learn to change physical responses related to headache disorder.
Over our years of experience, we have found that biofeedback can help a large percentage of our patients with migraine and tension-type headache, and it is associated with almost no adverse events.
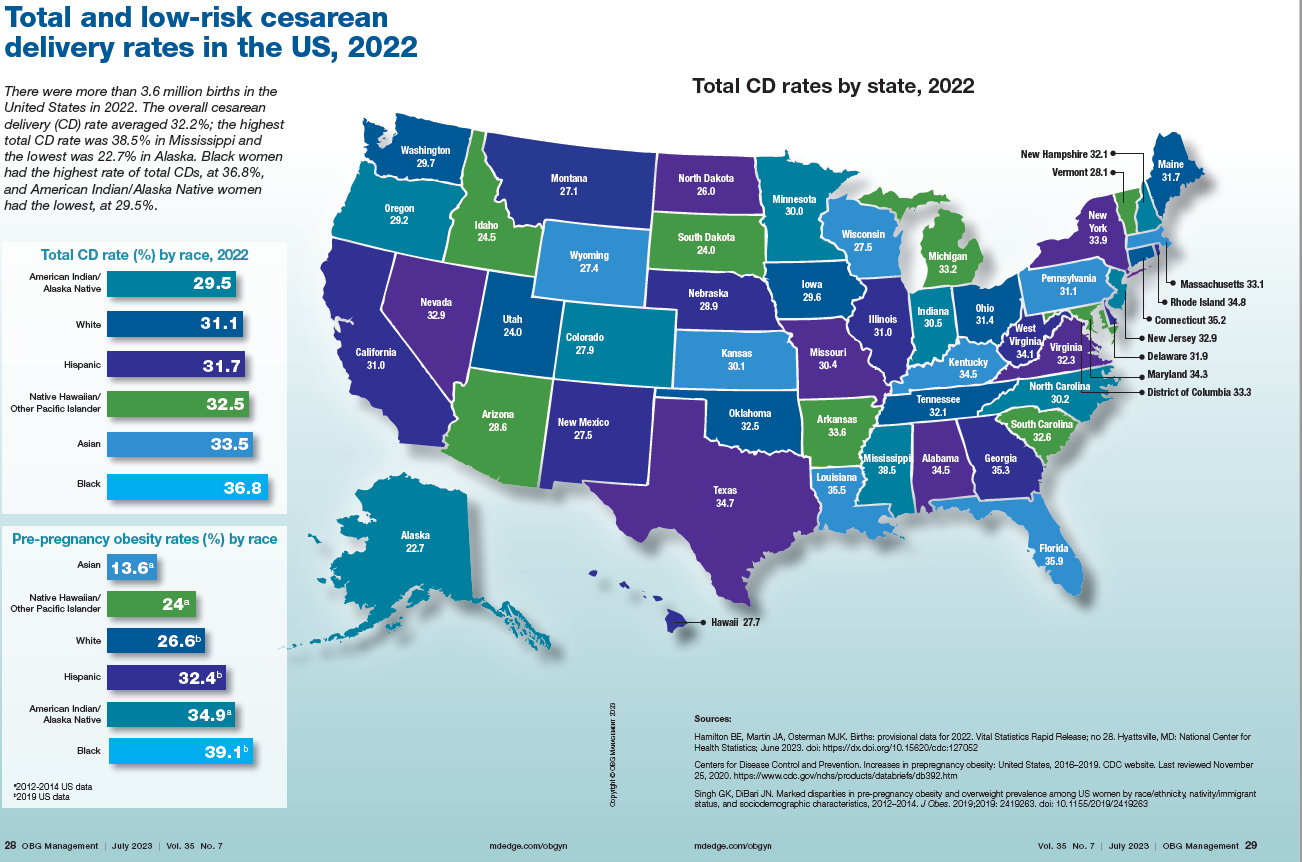
Total cesarean delivery rates in the US, 2022


News & Perspectives from Ob.Gyn. News
REPRODUCTIVE ROUNDS
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
Performed properly, office hysteroscopy can transform your practice by accurately, gently, and safely assessing the uterine cavity as well as assessing tubal patency.
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively. Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope. Further, infection rates and vasovagal events were far lower with hysteroscopy.
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity. Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
https://www.mdedge.com/obgyn/reproductive-rounds
FEATURE
Is ChatGPT a friend or foe of medical publishing?
Researchers may use artificial intelligence (AI) language models such as ChatGPT to write and revise scientific manuscripts, according to a new announcement from the International Committee of Medical Journal Editors. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said.
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
Continue to: FROM THE JOURNALS...
FROM THE JOURNALS
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
“Masking in interactions between patients and health care personnel should continue to receive serious consideration as a patient safety measure,” Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
https://www.mdedge.com/obgyn/covid-19-updates
CONFERENCE COVERAGE
A ‘one-stop shop’: New guidance on hormones and aging
A new statement from the Endocrine Society on hormones and aging highlights the differences between normal aging and disease, and when treatment is and isn’t appropriate.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men and diabetes treatment goals in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
REPRODUCTIVE ROUNDS
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
Performed properly, office hysteroscopy can transform your practice by accurately, gently, and safely assessing the uterine cavity as well as assessing tubal patency.
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively. Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope. Further, infection rates and vasovagal events were far lower with hysteroscopy.
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity. Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
https://www.mdedge.com/obgyn/reproductive-rounds
FEATURE
Is ChatGPT a friend or foe of medical publishing?
Researchers may use artificial intelligence (AI) language models such as ChatGPT to write and revise scientific manuscripts, according to a new announcement from the International Committee of Medical Journal Editors. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said.
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
Continue to: FROM THE JOURNALS...
FROM THE JOURNALS
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
“Masking in interactions between patients and health care personnel should continue to receive serious consideration as a patient safety measure,” Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
https://www.mdedge.com/obgyn/covid-19-updates
CONFERENCE COVERAGE
A ‘one-stop shop’: New guidance on hormones and aging
A new statement from the Endocrine Society on hormones and aging highlights the differences between normal aging and disease, and when treatment is and isn’t appropriate.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men and diabetes treatment goals in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
REPRODUCTIVE ROUNDS
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
Performed properly, office hysteroscopy can transform your practice by accurately, gently, and safely assessing the uterine cavity as well as assessing tubal patency.
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively. Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope. Further, infection rates and vasovagal events were far lower with hysteroscopy.
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity. Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
https://www.mdedge.com/obgyn/reproductive-rounds
FEATURE
Is ChatGPT a friend or foe of medical publishing?
Researchers may use artificial intelligence (AI) language models such as ChatGPT to write and revise scientific manuscripts, according to a new announcement from the International Committee of Medical Journal Editors. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said.
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
Continue to: FROM THE JOURNALS...
FROM THE JOURNALS
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
“Masking in interactions between patients and health care personnel should continue to receive serious consideration as a patient safety measure,” Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
https://www.mdedge.com/obgyn/covid-19-updates
CONFERENCE COVERAGE
A ‘one-stop shop’: New guidance on hormones and aging
A new statement from the Endocrine Society on hormones and aging highlights the differences between normal aging and disease, and when treatment is and isn’t appropriate.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men and diabetes treatment goals in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
Product updates and reviews
Product Update
Newly available single-use vaginal speculum
Ceek Women’s Health introduces the Nella single-use vaginal speculum for use during gynecologic examinations and procedures. Designed “by women for women, along with trusted clinicians to enhance patient comfort,” according to Ceek’s press release, the Nella speculum has a quiet operating mechanism, an LED light, and sidewall retractors. Its narrow shape allows for patient comfort and cervical visualization and because it is single use, it eliminates possibilities of cross contamination, according to the manufacturer. In addition, Ceek says it is an ergonomic tool, made from premium material, and is available in one size.
For more information, visit https://www.nellaspec.com

Product Update
Newly available single-use vaginal speculum
Ceek Women’s Health introduces the Nella single-use vaginal speculum for use during gynecologic examinations and procedures. Designed “by women for women, along with trusted clinicians to enhance patient comfort,” according to Ceek’s press release, the Nella speculum has a quiet operating mechanism, an LED light, and sidewall retractors. Its narrow shape allows for patient comfort and cervical visualization and because it is single use, it eliminates possibilities of cross contamination, according to the manufacturer. In addition, Ceek says it is an ergonomic tool, made from premium material, and is available in one size.
For more information, visit https://www.nellaspec.com

Product Update
Newly available single-use vaginal speculum
Ceek Women’s Health introduces the Nella single-use vaginal speculum for use during gynecologic examinations and procedures. Designed “by women for women, along with trusted clinicians to enhance patient comfort,” according to Ceek’s press release, the Nella speculum has a quiet operating mechanism, an LED light, and sidewall retractors. Its narrow shape allows for patient comfort and cervical visualization and because it is single use, it eliminates possibilities of cross contamination, according to the manufacturer. In addition, Ceek says it is an ergonomic tool, made from premium material, and is available in one size.
For more information, visit https://www.nellaspec.com

Meta-analysis identifies factors associated with increased risk for interstitial lung disease in RA
Key clinical point: Risk for interstitial lung disease (ILD) was higher among patients with rheumatoid arthritis (RA) who were older, had longer disease duration, were male, were positive for rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA), and had higher erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels.
Major finding: The factors associated with an increased risk for RA-ILD were older age (weighted mean difference [WMD] 5.77 years; P < .00001), longer RA duration (WMD 0.80 years; P = .02), male sex (pooled odds ratio [OR] 1.92; P < .00001), positive RF (OR 1.72; P < .00001), positive ACPA (OR 1.58; P < .00001), higher ESR level (WMD 7.41 mm/h; P = .005), and higher CRP level (WMD 4.98 mg/L; P = .02).
Study details: Findings are from a systematic review and meta-analysis of 15 retrospective cohort studies and seven observational studies including 1887 patients with RA-ILD and 8066 patients with RA without ILD.
Disclosures: The authors received no specific funding for this study and declared no conflicts of interest.
Source: Zhang M et al. Factors associated with interstitial lung disease in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2023;18(6):e0286191 (Jun 23). Doi: 10.1371/journal.pone.0286191
Key clinical point: Risk for interstitial lung disease (ILD) was higher among patients with rheumatoid arthritis (RA) who were older, had longer disease duration, were male, were positive for rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA), and had higher erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels.
Major finding: The factors associated with an increased risk for RA-ILD were older age (weighted mean difference [WMD] 5.77 years; P < .00001), longer RA duration (WMD 0.80 years; P = .02), male sex (pooled odds ratio [OR] 1.92; P < .00001), positive RF (OR 1.72; P < .00001), positive ACPA (OR 1.58; P < .00001), higher ESR level (WMD 7.41 mm/h; P = .005), and higher CRP level (WMD 4.98 mg/L; P = .02).
Study details: Findings are from a systematic review and meta-analysis of 15 retrospective cohort studies and seven observational studies including 1887 patients with RA-ILD and 8066 patients with RA without ILD.
Disclosures: The authors received no specific funding for this study and declared no conflicts of interest.
Source: Zhang M et al. Factors associated with interstitial lung disease in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2023;18(6):e0286191 (Jun 23). Doi: 10.1371/journal.pone.0286191
Key clinical point: Risk for interstitial lung disease (ILD) was higher among patients with rheumatoid arthritis (RA) who were older, had longer disease duration, were male, were positive for rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA), and had higher erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels.
Major finding: The factors associated with an increased risk for RA-ILD were older age (weighted mean difference [WMD] 5.77 years; P < .00001), longer RA duration (WMD 0.80 years; P = .02), male sex (pooled odds ratio [OR] 1.92; P < .00001), positive RF (OR 1.72; P < .00001), positive ACPA (OR 1.58; P < .00001), higher ESR level (WMD 7.41 mm/h; P = .005), and higher CRP level (WMD 4.98 mg/L; P = .02).
Study details: Findings are from a systematic review and meta-analysis of 15 retrospective cohort studies and seven observational studies including 1887 patients with RA-ILD and 8066 patients with RA without ILD.
Disclosures: The authors received no specific funding for this study and declared no conflicts of interest.
Source: Zhang M et al. Factors associated with interstitial lung disease in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2023;18(6):e0286191 (Jun 23). Doi: 10.1371/journal.pone.0286191
IL-6Ri and JAKi improve hemoglobin levels in patients with RA and anemi
Key clinical point: Interleukin-6 receptor inhibitors (IL-6Ri) effectively increased hemoglobin levels in patients with rheumatoid arthritis (RA) irrespective of their baseline levels, whereas Janus-kinase inhibitors (JAKi) improved hemoglobin levels in patients with RA and anemia and retained or decreased in those without anemia.
Major finding: From baseline to the 12-month follow-up, IL-6Ri increased hemoglobin levels in all patients with RA, irrespective of whether their baseline hemoglobin levels were low, intermediate, or high, and JAKi increased hemoglobin levels in those with RA and anemia, with levels remaining unaltered in those with intermediate hemoglobin levels and decreasing in those without anemia (all P < .001).
Study details: This study evaluated 2093 patients with RA from the ANSWER cohort who received biologic or targeted-synthetic disease-modifying antirheumatic drugs.
Disclosures: The ANSWER cohort study was supported by grants from 10 pharmaceutical companies, including AbbVie G.K., Asahi-Kasei Pharma, AYUMI Pharmaceutical Co., and others, and an information technology services company. Several authors declared receiving speaker fees, consulting fees, honoraria, or research grants from various sources.
Source: Nakayama Y et al. IL-6 inhibitors and JAK inhibitors as favourable treatment options for patients with anaemia and rheumatoid arthritis: ANSWER cohort study. Rheumatology (Oxford). 2023 (Jun 24). Doi: 10.1093/rheumatology/kead299
Key clinical point: Interleukin-6 receptor inhibitors (IL-6Ri) effectively increased hemoglobin levels in patients with rheumatoid arthritis (RA) irrespective of their baseline levels, whereas Janus-kinase inhibitors (JAKi) improved hemoglobin levels in patients with RA and anemia and retained or decreased in those without anemia.
Major finding: From baseline to the 12-month follow-up, IL-6Ri increased hemoglobin levels in all patients with RA, irrespective of whether their baseline hemoglobin levels were low, intermediate, or high, and JAKi increased hemoglobin levels in those with RA and anemia, with levels remaining unaltered in those with intermediate hemoglobin levels and decreasing in those without anemia (all P < .001).
Study details: This study evaluated 2093 patients with RA from the ANSWER cohort who received biologic or targeted-synthetic disease-modifying antirheumatic drugs.
Disclosures: The ANSWER cohort study was supported by grants from 10 pharmaceutical companies, including AbbVie G.K., Asahi-Kasei Pharma, AYUMI Pharmaceutical Co., and others, and an information technology services company. Several authors declared receiving speaker fees, consulting fees, honoraria, or research grants from various sources.
Source: Nakayama Y et al. IL-6 inhibitors and JAK inhibitors as favourable treatment options for patients with anaemia and rheumatoid arthritis: ANSWER cohort study. Rheumatology (Oxford). 2023 (Jun 24). Doi: 10.1093/rheumatology/kead299
Key clinical point: Interleukin-6 receptor inhibitors (IL-6Ri) effectively increased hemoglobin levels in patients with rheumatoid arthritis (RA) irrespective of their baseline levels, whereas Janus-kinase inhibitors (JAKi) improved hemoglobin levels in patients with RA and anemia and retained or decreased in those without anemia.
Major finding: From baseline to the 12-month follow-up, IL-6Ri increased hemoglobin levels in all patients with RA, irrespective of whether their baseline hemoglobin levels were low, intermediate, or high, and JAKi increased hemoglobin levels in those with RA and anemia, with levels remaining unaltered in those with intermediate hemoglobin levels and decreasing in those without anemia (all P < .001).
Study details: This study evaluated 2093 patients with RA from the ANSWER cohort who received biologic or targeted-synthetic disease-modifying antirheumatic drugs.
Disclosures: The ANSWER cohort study was supported by grants from 10 pharmaceutical companies, including AbbVie G.K., Asahi-Kasei Pharma, AYUMI Pharmaceutical Co., and others, and an information technology services company. Several authors declared receiving speaker fees, consulting fees, honoraria, or research grants from various sources.
Source: Nakayama Y et al. IL-6 inhibitors and JAK inhibitors as favourable treatment options for patients with anaemia and rheumatoid arthritis: ANSWER cohort study. Rheumatology (Oxford). 2023 (Jun 24). Doi: 10.1093/rheumatology/kead299
Mediterranean diet tied to reduced disease activity, disease impact, and functional disability in RA
Key clinical point: Higher adherence to the Mediterranean diet may help reduce disease impact, disease activity, and functional disability in patients with rheumatoid arthritis (RA).
Major finding: Patients with high vs low adherence to the Mediterranean diet had significantly lower Disease Activity Score of 28 Joints calculated with C-reactive protein (median 2.29 vs 3.27; P = .038), Health Assessment Questionnaire score (median 0.56 vs 1.00; P = .027), and Rheumatoid Arthritis Impact of Disease questionnaire score (median 3.51 vs 5.65; P = .032).
Study details: Findings are from a cross-sectional observational study including 120 patients with RA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Charneca S et al. The Mediterranean diet, and not dietary inflammatory index, is associated with rheumatoid arthritis disease activity, the impact of disease and functional disability. Eur J Nutr. 2023 (Jun 24). Doi: 10.1007/s00394-023-03196-8
Key clinical point: Higher adherence to the Mediterranean diet may help reduce disease impact, disease activity, and functional disability in patients with rheumatoid arthritis (RA).
Major finding: Patients with high vs low adherence to the Mediterranean diet had significantly lower Disease Activity Score of 28 Joints calculated with C-reactive protein (median 2.29 vs 3.27; P = .038), Health Assessment Questionnaire score (median 0.56 vs 1.00; P = .027), and Rheumatoid Arthritis Impact of Disease questionnaire score (median 3.51 vs 5.65; P = .032).
Study details: Findings are from a cross-sectional observational study including 120 patients with RA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Charneca S et al. The Mediterranean diet, and not dietary inflammatory index, is associated with rheumatoid arthritis disease activity, the impact of disease and functional disability. Eur J Nutr. 2023 (Jun 24). Doi: 10.1007/s00394-023-03196-8
Key clinical point: Higher adherence to the Mediterranean diet may help reduce disease impact, disease activity, and functional disability in patients with rheumatoid arthritis (RA).
Major finding: Patients with high vs low adherence to the Mediterranean diet had significantly lower Disease Activity Score of 28 Joints calculated with C-reactive protein (median 2.29 vs 3.27; P = .038), Health Assessment Questionnaire score (median 0.56 vs 1.00; P = .027), and Rheumatoid Arthritis Impact of Disease questionnaire score (median 3.51 vs 5.65; P = .032).
Study details: Findings are from a cross-sectional observational study including 120 patients with RA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Charneca S et al. The Mediterranean diet, and not dietary inflammatory index, is associated with rheumatoid arthritis disease activity, the impact of disease and functional disability. Eur J Nutr. 2023 (Jun 24). Doi: 10.1007/s00394-023-03196-8
Concomitant ILD negatively affects clinical remission in RA
Key clinical point: Concomitant interstitial lung disease (ILD) is a significant factor associated with failure to achieve clinical remission and an increased risk for unfavorable clinical events in patients with rheumatoid arthritis (RA).
Major finding: The presence of ILD was significantly associated with failure to achieve Disease Activity Score in 28 Joints remission (adjusted hazard ratio [aHR] 0.71; P = .002) and increased risk for death (aHR 3.24; P < .001), hospitalized infections (aHR 2.60; P < .001), major adverse cardiac events (aHR 3.40; P < .001), and lung cancer (aHR 16.0; P < .001).
Study details: This study analyzed the data of 1522 patients with RA (ILD group n = 287; non-ILD group n = 1235) from the observational IORRA cohort.
Disclosures: This study was supported by the Ministry of Health, Labour, and Welfare of Japan and other sources. Some authors declared serving as consultants for or receiving research grants, research funding, or lecture, speaker, or consulting fees from various sources.
Source: Sugano E et al. Impact of interstitial lung disease on clinical remission and unfavourable events of rheumatoid arthritis: Results from the IORRA cohort. Rheumatology (Oxford). 2023 (Jun 28). Doi: 10.1093/rheumatology/kead317
Key clinical point: Concomitant interstitial lung disease (ILD) is a significant factor associated with failure to achieve clinical remission and an increased risk for unfavorable clinical events in patients with rheumatoid arthritis (RA).
Major finding: The presence of ILD was significantly associated with failure to achieve Disease Activity Score in 28 Joints remission (adjusted hazard ratio [aHR] 0.71; P = .002) and increased risk for death (aHR 3.24; P < .001), hospitalized infections (aHR 2.60; P < .001), major adverse cardiac events (aHR 3.40; P < .001), and lung cancer (aHR 16.0; P < .001).
Study details: This study analyzed the data of 1522 patients with RA (ILD group n = 287; non-ILD group n = 1235) from the observational IORRA cohort.
Disclosures: This study was supported by the Ministry of Health, Labour, and Welfare of Japan and other sources. Some authors declared serving as consultants for or receiving research grants, research funding, or lecture, speaker, or consulting fees from various sources.
Source: Sugano E et al. Impact of interstitial lung disease on clinical remission and unfavourable events of rheumatoid arthritis: Results from the IORRA cohort. Rheumatology (Oxford). 2023 (Jun 28). Doi: 10.1093/rheumatology/kead317
Key clinical point: Concomitant interstitial lung disease (ILD) is a significant factor associated with failure to achieve clinical remission and an increased risk for unfavorable clinical events in patients with rheumatoid arthritis (RA).
Major finding: The presence of ILD was significantly associated with failure to achieve Disease Activity Score in 28 Joints remission (adjusted hazard ratio [aHR] 0.71; P = .002) and increased risk for death (aHR 3.24; P < .001), hospitalized infections (aHR 2.60; P < .001), major adverse cardiac events (aHR 3.40; P < .001), and lung cancer (aHR 16.0; P < .001).
Study details: This study analyzed the data of 1522 patients with RA (ILD group n = 287; non-ILD group n = 1235) from the observational IORRA cohort.
Disclosures: This study was supported by the Ministry of Health, Labour, and Welfare of Japan and other sources. Some authors declared serving as consultants for or receiving research grants, research funding, or lecture, speaker, or consulting fees from various sources.
Source: Sugano E et al. Impact of interstitial lung disease on clinical remission and unfavourable events of rheumatoid arthritis: Results from the IORRA cohort. Rheumatology (Oxford). 2023 (Jun 28). Doi: 10.1093/rheumatology/kead317


