User login
Leveraging the Million Veteran Program Infrastructure and Data for a Rapid Research Response to COVID-19
The Million Veteran Program (MVP) was launched in 2011 by the US Department of Veterans Affairs (VA) to enroll at least 1 million veterans in a longitudinal cohort to better understand how genes, lifestyle, military experience, and environmental exposures interact to influence health and illness and ultimately enable precision health care. The MVP has established a national, centralized infrastructure for recruitment and enrollment, biospecimen and data collection and storage, data generation and curation, and secure data access. When the COVID-19 pandemic hit in 2020, the MVP was leveraged to support research utilizing the following key infrastructure components: (1) MVP recruitment and enrollment platform to provide support for COVID-19 vaccine and treatment trials and to collect COVID-19 data from MVP participants; (2) using MVP Phenomics for COVID-19 research data cleaning and curation, assisting with the development of a VA Severity Index for COVID-19, and forming 6 scientific working groups to coordinate COVID-19 research questions; and (3) the VA/MVP and US Department of Energy (DOE) partnership to assist in responding to COVID-19 research questions identified by the US Food and Drug Administration (FDA). This article describes these infrastructure components in more detail and highlights key findings from the MVP COVID-19 research efforts.
MVP Infrastructure
The Veterans Health Administration (VHA) Office of Research and Development (ORD) oversaw efforts to develop the VA Coronavirus Research Volunteer List (the COVID-19 registry). To support the registry, the MVP leveraged its infrastructure to facilitate a rapid response. The MVP is designed as a full-service and centralized recruitment and enrollment platform. This includes MVP office oversight; MVP coordinating centers that manage the centralized platform; an information center that handles inbound and outbound calls; an informatics system built for recruitment and enrollment monitoring and tracking; and a network of more than 70 participating MVP sites with dedicated staff to conduct recruitment and enrollment activities. The MVP used its informatics infrastructure to support secure data storage for the registry volunteer information. MVP coordinating center staff worked with the COVID-19 registry to invite > 125,000 MVP participants from approximately 20 MVP sites. Additionally, MVP information center staff made > 4000 calls to prospective registry volunteers. This work resulted in 1300 volunteers agreeing to be
New Data Collection
The MVP protocol was approved by the VA Central Institutional Review Board (IRB) in 2011. As part of initial enrollment in MVP, participants consented to recontact for additional self-report information along with access to their electronic health record (EHR). This allows for the linkage of EHR and survey response data, thus providing a comprehensive understanding of health history before and after a self-reported COVID-19 diagnosis. Between May 2020 and September 2021, the MVP COVID-19 survey was distributed to existing MVP participants via mail, telephone, and email with the ability to complete the survey by paper and pencil or through the MVP online system. Dissemination of the survey was approved by the VA Central IRB in 2020, with nearly 730,000 eligible MVP participants contacted. As of June 2022, 255,737 MVP participants (35% of the eligible cohort) had completed the survey; 86% completed a paper survey while 14% completed it online. Respondents were primarily older (≥ 65 years); 90% were male; close to 7% reported Hispanic ethnicity, and 11% reported Black race.
Findings from this survey provide insight into pandemic behaviors not consistently captured in EHRs, such as psychosocial aspects, including social and emotional support, loss of tangible and intangible resources, as well as COVID-19–related behaviors, such as social distancing and self-protective practices.1 MVP COVID-19 survey data combined with veteran EHRs, responses to other MVP surveys, and genetic data enable MVP researchers to better understand epidemiological, clinical, and psychosocial aspects of the disease. Future COVID-19 studies may use self-reported survey responses to enrich understanding about the effects of the disease on a veteran’s daily life, and possibly validate existing EHR COVID-19 diagnoses and hospitalization findings. This comprehensive data resource provides a unique opportunity to identify new targets for disease prevention, treatment, and management with an emphasis on individual variability in genes, environment, and lifestyle.
COVID-19 Research
In early 2020, the burden of COVID-19 on the US was unprecedented, and little was known about risk factors for severe COVID-19 and deaths. The MVP Phenomics team quickly responded with a large-scale phenome-wide association study (PheWAS) of >
To broaden disease progression data curation and fit the specific needs of the VA, we operationalized and validated the World Health Organization clinical severity scale and used VA EHR data to create the VA Severity Index for COVID-19 (VASIC).3 The VASIC category is now part of the MVP core data repository, where volumes of data from multiple activities are integrated through an automated process to create monthly research-ready data cubes. These activities include extensive data curation, mapping, phenotyping, and adjudication that are performed to curate oxygen supplementation status and other procedures related to treatment that are processed and understood in real time. The data cubes were provisioned to MVP COVID-19 researchers. In addition, the VASIC scale variable is now integrated within the larger VA system for all researchers to use as part of its wider COVID-19 initiative. The VA Centralized Interactive Phenomics Resource (CIPHER) phenomics library now hosts the details of VASIC, codes, metadata, and related COVID-19 data products for all VA communities. In partnership with CIPHER and other internal and external COVID-19 initiatives, the MVP continues to play an integral part for the VA and beyond in the development of a phenomics algorithm for long COVID, or post-acute COVID-19 syndrome (PACS).
Host Genetics in COVID-19
As the SARS-CoV-2 virus continued to spread globally, it became clear that the symptoms and severity of infection experienced by patients varied across a broad spectrum, from being asymptomatic carriers to experiencing severe symptoms in 1 or more organ systems in the body, resulting in death. This variability suggested that host genetics and other host factors may play a role in determining the severity of COVID-19 infection. The MVP dataset, with genetic and health information on > 600,000 MVP participants, provided an ideal dataset to explore host contributions to COVID-19.
In late spring 2020, the MVP executive committee issued a call to the MVP research community to propose study aims around the COVID-19 pandemic that could leverage the phenotypic and genetic data and resources. The MVP quickly formed 6 rapid-response scientific working groups. Their mission was to cultivate collaboration and inclusivity and to coordinate COVID-19 research questions. A steering committee composed of the MVP executive committee, staff from computational environments, working group cochairs, and an administrator, who was responsible for daily oversight of the working groups. In addition, the ORD COVID-19 steering committee reviewed and approved research activities to ensure scientific rigor, as well as alignment with overall ongoing research activities.
The MVP COVID-19 working groups included dozens of researchers who used MVP data to identify disease mechanisms; understand the impact of host genetics on susceptibility, morbidity, and mortality; and identify potential targets for treatments and therapies. The working groups were further supported by MVP analysts to work cross-functionally on genomics, phenomics, statistical genetics, and PheWAS. Each working group chair was responsible for prioritizing concepts and moving them forward in coordination with the MVP and ORD COVID-19 steering committees. An overview of the MVP COVID-19 working groups follows (Table).4-9
Druggable genome. This working group researched drug-repurposing opportunities to prevent severe COVID-19, defined as hospitalization with oxygen therapy (high flow), intubation, mechanical ventilation, vasopressors, dialysis, or death from COVID-19; and prevent complications in patients hospitalized by COVID-19.
Pharmacogenomics. This working group focused on 2 main aims: the impact of apolipoprotein L1 risk variants on acute kidney injury (AKI) and death in Black veterans with COVID-19; and pharmacogenetic analysis of remdesivir-induced liver chemistry abnormalities.
Disease mechanisms. Understanding the underlying pathways and mechanisms behind COVID-19 has been a difficult but important challenge overall in the scientific community. This working group investigated specific genetic markers and effects on COVID-19, including polygenic predisposition to venous thromboembolism associated with increased COVID-19 susceptibility; renal comorbidities and new AKI and unfavorable outcomes among COVID-19–positive sickle cell trait carriers; and mucin 5B, oligomeric mucus/gel-forming gene polymorphism, and protective effects in COVID-19 infection.
Genomics for risk prediction, polygenic risk scores, and mendelian randomization. Risk prediction for COVID-19 has been widely studied mostly aiming at comorbidities and preexisting conditions. The MVP cohort provided a unique opportunity to understand how genetic information can enhance our understanding of COVID-19 risk. This working group focused on: (1) ABO blood group typing and the protective effects of the O blood group on COVID-19 infection; (2) polygenic risk scores and COVID-19 outcomes; (3) human leukocyte antigen typing and COVID-19 outcomes; and (4) a transcriptome-wide association study of COVID-19–positive MVP participants.
Genome-Wide Association Study (GWAS) and Downstream Analysis. This working group performed GWAS of the main COVID-19 outcomes. Results from GWAS unveiled new genetic loci to suggest further investigation on these candidate genes. The results were used by other MVP COVID-19 working groups for their activities. The results also contributed to external collaborations, such as the COVID-19 Host Genetics Initiative.
COVID-19–Related PheWAS. This working group focused on understanding the potential clinical significance of genetic variants associated with susceptibility to, or outcomes of, COVID-19 infection. They worked to identify traits that share genetic variants associated with severe COVID-19 from the Host Genetics Initiative. The group also studied the phenotypic consequences of acquired mosaic chromosomal alterations with early data linking to COVID-19 susceptibility.
COVID-19 Research Partnerships
In 2016, the VA and DOE formed an interagency partnership known as Computational Health Analytics for Medical Precision to Improve Outcomes Now (CHAMPION) to demonstrate the power of combining the VA EHR system, MVP genetic data, and clinical research expertise with DOE high-performance computing infrastructure and artificial intelligence expertise. The VA EHR captures longitudinal care information on veterans with records that go back decades. Furthermore, the VA covers the costs of medications and
The DOE Oak Ridge National Laboratory (ORNL) in Tennessee securely maintains this rich database for the VA. The ORNL Summit supercomputer can complete trillions of calculations per second to provide critical and timely analyses, applying the most advanced and powerful artificial intelligence methods, which would not be possible in more conventional research settings. CHAMPION taught the VA and DOE how to bring their disparate research cultures together for innovative collaborative investigation. Moreover, this collaboration produced a cadre of VA and DOE scientists familiar with VA patient data and experienced in conducting joint research successfully and integrating omics data with clinical data for a better mechanistic understanding. Because of this preexisting collaboration between the VA and DOE, interagency teams were prepared at the start of the COVID-19 pandemic.10-15
Other recently completed studies have developed and validated short-term mortality indices in individuals with COVID-19 based on their preexisting conditions, assessed the generalizability of VA COVID-19 experiences to the US population, and evaluated the effectiveness of hydroxychloroquine with and without azithromycin in VA patients with COVID-19.12,15 A recent study demonstrated the benefit of prophylactic anticoagulation at initial hospitalization.14
The VA also provided the FDA with daily reports on aggregate VA COVID-19 cases and their distribution across the VA system, demographics of VA patients with COVID-19, and analyses of predictive models for positive test results and death. The VA regularly sent the FDA aggregated data showing patterns of medication use and retrospective analyses of the effectiveness of certain medications (including remdesivir and some antithrombotic agents). The FDA used these data along with other data to understand the scope of the pandemic and to predict drug shortages or needs for additional medical equipment, including ventilators.
Limitations
For the most part, MVP infrastructure and partnerships were efficiently leveraged to significantly advance our understanding of the biological basis of COVID-19 and to develop treatments and vaccines. However, there were a few limitations that may have slowed timely and optimal outcomes. An issue not limited to the MVP or VA was the continual evolution of the pandemic and its response. This included evolving definitions of disease, symptomatology, testing, vaccines, and public health recommendations. Keeping pace with the emerging knowledge from these domains was a struggle for the entire scientific community. A more discrete limitation was the number of participants in the MVP with positive COVID-19 test results and positive symptoms; however, this was mitigated by partnering with other groups like the COVID-19 Host Genetics Initiative to increase study participant numbers. Finally, there were logistical and regulatory challenges associated with coordination of national clinical trial recruitment across a VA system with > 100 discrete hospitals.
Conclusions
Having a centralized infrastructure for recruitment and enrollment, including a national research volunteer registry, information center, research staff, and coordinating centers, can allow for expedited enrollment in vaccine and treatment trials in the face of future public health emergencies. VA assets, including its rich EHR and MVP, the world’s largest genomic cohort, have contributed to improving our understanding and management of COVID-19.
1. Whitbourne SB, Nguyen XT, Song RJ, et al. Million Veteran Program’s response to COVID-19: survey development and preliminary findings. PLoS One. 2022;17(4):e0266381. doi:10.1371/journal.pone.0266381
2. Song RJ, Ho YL, Schubert P, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi:10.1371/journal.pone.0251651
3. Galloway A, Park Y, Tanukonda V, et al. Impact of COVID-19 severity on long-term events in US veterans using the Veterans Affairs Severity Index for COVID-19 (VASIC). J Infect Dis. 2022;226(12):2113-2117. doi:10.1093/infdis/jiac182
4. Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668-676. doi:10.0138/s41591-021-01310-z
5. Hung AM, Sha SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386-395. doi:10.1001/jamainternmed.2021.8538
6. Verma A, Huffman JE, Gao L, et al. Association of kidney comorbidities and acute kidney failure with unfavorable outcomes after COVID-19 in individuals with the sickle cell trait. JAMA Intern Med. 2022;182(8):796-804. doi:10.1001/jamainternmed.2022.2141
7. Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18(4):e1010113. doi:10.1371/journal.pgen.1010113
8. Peloso GM, Tcheandjieu C, McGeary JE, et al. Genetic loci associated with COVID-19 positivity and hospitalization in White, Black, and Hispanic Veterans of the VA Million Veteran Program. Front Genetic. 2022;12:777076. doi:10.3389/fgene.2021.777076
9. Verma A, Minnier J, Wan ES, et al. A MUC5B gene polymorphism, rs35705950-T confers protective effects against COVID-19 hospitalization but not severe disease or mortality. Am J Respir Crit Care Med. 2022;182(8):796-804. doi:10.1164/rccm.202109-2166OC
10. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;e59177. doi:10.7554/eLife.59177
11. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. doi:10.1371/journal.pmed.1003379
12. King JT, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
13. Joubert W, Weighill D, Kainer D, et al. Attacking the opioid epidemic: determining the epistatic and pleiotropic genetic architectures for chronic pain and opioid addiction. SC18: International Conference for High Performance Computing, Networking, Storage and Analysis. Dallas, TX, USA, 2018:717-730. doi:10.1109/SC.2018.00060
14. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
15. Gerlovin H, Posner DC, Ho YL, et al. Pharmacoepidemiology, machine learning, and COVID-19: an intent-to-treat analysis of hydroxychloroquine, with or without Azithromycin, and COVID-19 outcomes among hospitalized US Veterans. Am J Epidemiol. 2021;190(11): 2405-2419. doi:10.1093/aje/kwab183
The Million Veteran Program (MVP) was launched in 2011 by the US Department of Veterans Affairs (VA) to enroll at least 1 million veterans in a longitudinal cohort to better understand how genes, lifestyle, military experience, and environmental exposures interact to influence health and illness and ultimately enable precision health care. The MVP has established a national, centralized infrastructure for recruitment and enrollment, biospecimen and data collection and storage, data generation and curation, and secure data access. When the COVID-19 pandemic hit in 2020, the MVP was leveraged to support research utilizing the following key infrastructure components: (1) MVP recruitment and enrollment platform to provide support for COVID-19 vaccine and treatment trials and to collect COVID-19 data from MVP participants; (2) using MVP Phenomics for COVID-19 research data cleaning and curation, assisting with the development of a VA Severity Index for COVID-19, and forming 6 scientific working groups to coordinate COVID-19 research questions; and (3) the VA/MVP and US Department of Energy (DOE) partnership to assist in responding to COVID-19 research questions identified by the US Food and Drug Administration (FDA). This article describes these infrastructure components in more detail and highlights key findings from the MVP COVID-19 research efforts.
MVP Infrastructure
The Veterans Health Administration (VHA) Office of Research and Development (ORD) oversaw efforts to develop the VA Coronavirus Research Volunteer List (the COVID-19 registry). To support the registry, the MVP leveraged its infrastructure to facilitate a rapid response. The MVP is designed as a full-service and centralized recruitment and enrollment platform. This includes MVP office oversight; MVP coordinating centers that manage the centralized platform; an information center that handles inbound and outbound calls; an informatics system built for recruitment and enrollment monitoring and tracking; and a network of more than 70 participating MVP sites with dedicated staff to conduct recruitment and enrollment activities. The MVP used its informatics infrastructure to support secure data storage for the registry volunteer information. MVP coordinating center staff worked with the COVID-19 registry to invite > 125,000 MVP participants from approximately 20 MVP sites. Additionally, MVP information center staff made > 4000 calls to prospective registry volunteers. This work resulted in 1300 volunteers agreeing to be
New Data Collection
The MVP protocol was approved by the VA Central Institutional Review Board (IRB) in 2011. As part of initial enrollment in MVP, participants consented to recontact for additional self-report information along with access to their electronic health record (EHR). This allows for the linkage of EHR and survey response data, thus providing a comprehensive understanding of health history before and after a self-reported COVID-19 diagnosis. Between May 2020 and September 2021, the MVP COVID-19 survey was distributed to existing MVP participants via mail, telephone, and email with the ability to complete the survey by paper and pencil or through the MVP online system. Dissemination of the survey was approved by the VA Central IRB in 2020, with nearly 730,000 eligible MVP participants contacted. As of June 2022, 255,737 MVP participants (35% of the eligible cohort) had completed the survey; 86% completed a paper survey while 14% completed it online. Respondents were primarily older (≥ 65 years); 90% were male; close to 7% reported Hispanic ethnicity, and 11% reported Black race.
Findings from this survey provide insight into pandemic behaviors not consistently captured in EHRs, such as psychosocial aspects, including social and emotional support, loss of tangible and intangible resources, as well as COVID-19–related behaviors, such as social distancing and self-protective practices.1 MVP COVID-19 survey data combined with veteran EHRs, responses to other MVP surveys, and genetic data enable MVP researchers to better understand epidemiological, clinical, and psychosocial aspects of the disease. Future COVID-19 studies may use self-reported survey responses to enrich understanding about the effects of the disease on a veteran’s daily life, and possibly validate existing EHR COVID-19 diagnoses and hospitalization findings. This comprehensive data resource provides a unique opportunity to identify new targets for disease prevention, treatment, and management with an emphasis on individual variability in genes, environment, and lifestyle.
COVID-19 Research
In early 2020, the burden of COVID-19 on the US was unprecedented, and little was known about risk factors for severe COVID-19 and deaths. The MVP Phenomics team quickly responded with a large-scale phenome-wide association study (PheWAS) of >
To broaden disease progression data curation and fit the specific needs of the VA, we operationalized and validated the World Health Organization clinical severity scale and used VA EHR data to create the VA Severity Index for COVID-19 (VASIC).3 The VASIC category is now part of the MVP core data repository, where volumes of data from multiple activities are integrated through an automated process to create monthly research-ready data cubes. These activities include extensive data curation, mapping, phenotyping, and adjudication that are performed to curate oxygen supplementation status and other procedures related to treatment that are processed and understood in real time. The data cubes were provisioned to MVP COVID-19 researchers. In addition, the VASIC scale variable is now integrated within the larger VA system for all researchers to use as part of its wider COVID-19 initiative. The VA Centralized Interactive Phenomics Resource (CIPHER) phenomics library now hosts the details of VASIC, codes, metadata, and related COVID-19 data products for all VA communities. In partnership with CIPHER and other internal and external COVID-19 initiatives, the MVP continues to play an integral part for the VA and beyond in the development of a phenomics algorithm for long COVID, or post-acute COVID-19 syndrome (PACS).
Host Genetics in COVID-19
As the SARS-CoV-2 virus continued to spread globally, it became clear that the symptoms and severity of infection experienced by patients varied across a broad spectrum, from being asymptomatic carriers to experiencing severe symptoms in 1 or more organ systems in the body, resulting in death. This variability suggested that host genetics and other host factors may play a role in determining the severity of COVID-19 infection. The MVP dataset, with genetic and health information on > 600,000 MVP participants, provided an ideal dataset to explore host contributions to COVID-19.
In late spring 2020, the MVP executive committee issued a call to the MVP research community to propose study aims around the COVID-19 pandemic that could leverage the phenotypic and genetic data and resources. The MVP quickly formed 6 rapid-response scientific working groups. Their mission was to cultivate collaboration and inclusivity and to coordinate COVID-19 research questions. A steering committee composed of the MVP executive committee, staff from computational environments, working group cochairs, and an administrator, who was responsible for daily oversight of the working groups. In addition, the ORD COVID-19 steering committee reviewed and approved research activities to ensure scientific rigor, as well as alignment with overall ongoing research activities.
The MVP COVID-19 working groups included dozens of researchers who used MVP data to identify disease mechanisms; understand the impact of host genetics on susceptibility, morbidity, and mortality; and identify potential targets for treatments and therapies. The working groups were further supported by MVP analysts to work cross-functionally on genomics, phenomics, statistical genetics, and PheWAS. Each working group chair was responsible for prioritizing concepts and moving them forward in coordination with the MVP and ORD COVID-19 steering committees. An overview of the MVP COVID-19 working groups follows (Table).4-9
Druggable genome. This working group researched drug-repurposing opportunities to prevent severe COVID-19, defined as hospitalization with oxygen therapy (high flow), intubation, mechanical ventilation, vasopressors, dialysis, or death from COVID-19; and prevent complications in patients hospitalized by COVID-19.
Pharmacogenomics. This working group focused on 2 main aims: the impact of apolipoprotein L1 risk variants on acute kidney injury (AKI) and death in Black veterans with COVID-19; and pharmacogenetic analysis of remdesivir-induced liver chemistry abnormalities.
Disease mechanisms. Understanding the underlying pathways and mechanisms behind COVID-19 has been a difficult but important challenge overall in the scientific community. This working group investigated specific genetic markers and effects on COVID-19, including polygenic predisposition to venous thromboembolism associated with increased COVID-19 susceptibility; renal comorbidities and new AKI and unfavorable outcomes among COVID-19–positive sickle cell trait carriers; and mucin 5B, oligomeric mucus/gel-forming gene polymorphism, and protective effects in COVID-19 infection.
Genomics for risk prediction, polygenic risk scores, and mendelian randomization. Risk prediction for COVID-19 has been widely studied mostly aiming at comorbidities and preexisting conditions. The MVP cohort provided a unique opportunity to understand how genetic information can enhance our understanding of COVID-19 risk. This working group focused on: (1) ABO blood group typing and the protective effects of the O blood group on COVID-19 infection; (2) polygenic risk scores and COVID-19 outcomes; (3) human leukocyte antigen typing and COVID-19 outcomes; and (4) a transcriptome-wide association study of COVID-19–positive MVP participants.
Genome-Wide Association Study (GWAS) and Downstream Analysis. This working group performed GWAS of the main COVID-19 outcomes. Results from GWAS unveiled new genetic loci to suggest further investigation on these candidate genes. The results were used by other MVP COVID-19 working groups for their activities. The results also contributed to external collaborations, such as the COVID-19 Host Genetics Initiative.
COVID-19–Related PheWAS. This working group focused on understanding the potential clinical significance of genetic variants associated with susceptibility to, or outcomes of, COVID-19 infection. They worked to identify traits that share genetic variants associated with severe COVID-19 from the Host Genetics Initiative. The group also studied the phenotypic consequences of acquired mosaic chromosomal alterations with early data linking to COVID-19 susceptibility.
COVID-19 Research Partnerships
In 2016, the VA and DOE formed an interagency partnership known as Computational Health Analytics for Medical Precision to Improve Outcomes Now (CHAMPION) to demonstrate the power of combining the VA EHR system, MVP genetic data, and clinical research expertise with DOE high-performance computing infrastructure and artificial intelligence expertise. The VA EHR captures longitudinal care information on veterans with records that go back decades. Furthermore, the VA covers the costs of medications and
The DOE Oak Ridge National Laboratory (ORNL) in Tennessee securely maintains this rich database for the VA. The ORNL Summit supercomputer can complete trillions of calculations per second to provide critical and timely analyses, applying the most advanced and powerful artificial intelligence methods, which would not be possible in more conventional research settings. CHAMPION taught the VA and DOE how to bring their disparate research cultures together for innovative collaborative investigation. Moreover, this collaboration produced a cadre of VA and DOE scientists familiar with VA patient data and experienced in conducting joint research successfully and integrating omics data with clinical data for a better mechanistic understanding. Because of this preexisting collaboration between the VA and DOE, interagency teams were prepared at the start of the COVID-19 pandemic.10-15
Other recently completed studies have developed and validated short-term mortality indices in individuals with COVID-19 based on their preexisting conditions, assessed the generalizability of VA COVID-19 experiences to the US population, and evaluated the effectiveness of hydroxychloroquine with and without azithromycin in VA patients with COVID-19.12,15 A recent study demonstrated the benefit of prophylactic anticoagulation at initial hospitalization.14
The VA also provided the FDA with daily reports on aggregate VA COVID-19 cases and their distribution across the VA system, demographics of VA patients with COVID-19, and analyses of predictive models for positive test results and death. The VA regularly sent the FDA aggregated data showing patterns of medication use and retrospective analyses of the effectiveness of certain medications (including remdesivir and some antithrombotic agents). The FDA used these data along with other data to understand the scope of the pandemic and to predict drug shortages or needs for additional medical equipment, including ventilators.
Limitations
For the most part, MVP infrastructure and partnerships were efficiently leveraged to significantly advance our understanding of the biological basis of COVID-19 and to develop treatments and vaccines. However, there were a few limitations that may have slowed timely and optimal outcomes. An issue not limited to the MVP or VA was the continual evolution of the pandemic and its response. This included evolving definitions of disease, symptomatology, testing, vaccines, and public health recommendations. Keeping pace with the emerging knowledge from these domains was a struggle for the entire scientific community. A more discrete limitation was the number of participants in the MVP with positive COVID-19 test results and positive symptoms; however, this was mitigated by partnering with other groups like the COVID-19 Host Genetics Initiative to increase study participant numbers. Finally, there were logistical and regulatory challenges associated with coordination of national clinical trial recruitment across a VA system with > 100 discrete hospitals.
Conclusions
Having a centralized infrastructure for recruitment and enrollment, including a national research volunteer registry, information center, research staff, and coordinating centers, can allow for expedited enrollment in vaccine and treatment trials in the face of future public health emergencies. VA assets, including its rich EHR and MVP, the world’s largest genomic cohort, have contributed to improving our understanding and management of COVID-19.
The Million Veteran Program (MVP) was launched in 2011 by the US Department of Veterans Affairs (VA) to enroll at least 1 million veterans in a longitudinal cohort to better understand how genes, lifestyle, military experience, and environmental exposures interact to influence health and illness and ultimately enable precision health care. The MVP has established a national, centralized infrastructure for recruitment and enrollment, biospecimen and data collection and storage, data generation and curation, and secure data access. When the COVID-19 pandemic hit in 2020, the MVP was leveraged to support research utilizing the following key infrastructure components: (1) MVP recruitment and enrollment platform to provide support for COVID-19 vaccine and treatment trials and to collect COVID-19 data from MVP participants; (2) using MVP Phenomics for COVID-19 research data cleaning and curation, assisting with the development of a VA Severity Index for COVID-19, and forming 6 scientific working groups to coordinate COVID-19 research questions; and (3) the VA/MVP and US Department of Energy (DOE) partnership to assist in responding to COVID-19 research questions identified by the US Food and Drug Administration (FDA). This article describes these infrastructure components in more detail and highlights key findings from the MVP COVID-19 research efforts.
MVP Infrastructure
The Veterans Health Administration (VHA) Office of Research and Development (ORD) oversaw efforts to develop the VA Coronavirus Research Volunteer List (the COVID-19 registry). To support the registry, the MVP leveraged its infrastructure to facilitate a rapid response. The MVP is designed as a full-service and centralized recruitment and enrollment platform. This includes MVP office oversight; MVP coordinating centers that manage the centralized platform; an information center that handles inbound and outbound calls; an informatics system built for recruitment and enrollment monitoring and tracking; and a network of more than 70 participating MVP sites with dedicated staff to conduct recruitment and enrollment activities. The MVP used its informatics infrastructure to support secure data storage for the registry volunteer information. MVP coordinating center staff worked with the COVID-19 registry to invite > 125,000 MVP participants from approximately 20 MVP sites. Additionally, MVP information center staff made > 4000 calls to prospective registry volunteers. This work resulted in 1300 volunteers agreeing to be
New Data Collection
The MVP protocol was approved by the VA Central Institutional Review Board (IRB) in 2011. As part of initial enrollment in MVP, participants consented to recontact for additional self-report information along with access to their electronic health record (EHR). This allows for the linkage of EHR and survey response data, thus providing a comprehensive understanding of health history before and after a self-reported COVID-19 diagnosis. Between May 2020 and September 2021, the MVP COVID-19 survey was distributed to existing MVP participants via mail, telephone, and email with the ability to complete the survey by paper and pencil or through the MVP online system. Dissemination of the survey was approved by the VA Central IRB in 2020, with nearly 730,000 eligible MVP participants contacted. As of June 2022, 255,737 MVP participants (35% of the eligible cohort) had completed the survey; 86% completed a paper survey while 14% completed it online. Respondents were primarily older (≥ 65 years); 90% were male; close to 7% reported Hispanic ethnicity, and 11% reported Black race.
Findings from this survey provide insight into pandemic behaviors not consistently captured in EHRs, such as psychosocial aspects, including social and emotional support, loss of tangible and intangible resources, as well as COVID-19–related behaviors, such as social distancing and self-protective practices.1 MVP COVID-19 survey data combined with veteran EHRs, responses to other MVP surveys, and genetic data enable MVP researchers to better understand epidemiological, clinical, and psychosocial aspects of the disease. Future COVID-19 studies may use self-reported survey responses to enrich understanding about the effects of the disease on a veteran’s daily life, and possibly validate existing EHR COVID-19 diagnoses and hospitalization findings. This comprehensive data resource provides a unique opportunity to identify new targets for disease prevention, treatment, and management with an emphasis on individual variability in genes, environment, and lifestyle.
COVID-19 Research
In early 2020, the burden of COVID-19 on the US was unprecedented, and little was known about risk factors for severe COVID-19 and deaths. The MVP Phenomics team quickly responded with a large-scale phenome-wide association study (PheWAS) of >
To broaden disease progression data curation and fit the specific needs of the VA, we operationalized and validated the World Health Organization clinical severity scale and used VA EHR data to create the VA Severity Index for COVID-19 (VASIC).3 The VASIC category is now part of the MVP core data repository, where volumes of data from multiple activities are integrated through an automated process to create monthly research-ready data cubes. These activities include extensive data curation, mapping, phenotyping, and adjudication that are performed to curate oxygen supplementation status and other procedures related to treatment that are processed and understood in real time. The data cubes were provisioned to MVP COVID-19 researchers. In addition, the VASIC scale variable is now integrated within the larger VA system for all researchers to use as part of its wider COVID-19 initiative. The VA Centralized Interactive Phenomics Resource (CIPHER) phenomics library now hosts the details of VASIC, codes, metadata, and related COVID-19 data products for all VA communities. In partnership with CIPHER and other internal and external COVID-19 initiatives, the MVP continues to play an integral part for the VA and beyond in the development of a phenomics algorithm for long COVID, or post-acute COVID-19 syndrome (PACS).
Host Genetics in COVID-19
As the SARS-CoV-2 virus continued to spread globally, it became clear that the symptoms and severity of infection experienced by patients varied across a broad spectrum, from being asymptomatic carriers to experiencing severe symptoms in 1 or more organ systems in the body, resulting in death. This variability suggested that host genetics and other host factors may play a role in determining the severity of COVID-19 infection. The MVP dataset, with genetic and health information on > 600,000 MVP participants, provided an ideal dataset to explore host contributions to COVID-19.
In late spring 2020, the MVP executive committee issued a call to the MVP research community to propose study aims around the COVID-19 pandemic that could leverage the phenotypic and genetic data and resources. The MVP quickly formed 6 rapid-response scientific working groups. Their mission was to cultivate collaboration and inclusivity and to coordinate COVID-19 research questions. A steering committee composed of the MVP executive committee, staff from computational environments, working group cochairs, and an administrator, who was responsible for daily oversight of the working groups. In addition, the ORD COVID-19 steering committee reviewed and approved research activities to ensure scientific rigor, as well as alignment with overall ongoing research activities.
The MVP COVID-19 working groups included dozens of researchers who used MVP data to identify disease mechanisms; understand the impact of host genetics on susceptibility, morbidity, and mortality; and identify potential targets for treatments and therapies. The working groups were further supported by MVP analysts to work cross-functionally on genomics, phenomics, statistical genetics, and PheWAS. Each working group chair was responsible for prioritizing concepts and moving them forward in coordination with the MVP and ORD COVID-19 steering committees. An overview of the MVP COVID-19 working groups follows (Table).4-9
Druggable genome. This working group researched drug-repurposing opportunities to prevent severe COVID-19, defined as hospitalization with oxygen therapy (high flow), intubation, mechanical ventilation, vasopressors, dialysis, or death from COVID-19; and prevent complications in patients hospitalized by COVID-19.
Pharmacogenomics. This working group focused on 2 main aims: the impact of apolipoprotein L1 risk variants on acute kidney injury (AKI) and death in Black veterans with COVID-19; and pharmacogenetic analysis of remdesivir-induced liver chemistry abnormalities.
Disease mechanisms. Understanding the underlying pathways and mechanisms behind COVID-19 has been a difficult but important challenge overall in the scientific community. This working group investigated specific genetic markers and effects on COVID-19, including polygenic predisposition to venous thromboembolism associated with increased COVID-19 susceptibility; renal comorbidities and new AKI and unfavorable outcomes among COVID-19–positive sickle cell trait carriers; and mucin 5B, oligomeric mucus/gel-forming gene polymorphism, and protective effects in COVID-19 infection.
Genomics for risk prediction, polygenic risk scores, and mendelian randomization. Risk prediction for COVID-19 has been widely studied mostly aiming at comorbidities and preexisting conditions. The MVP cohort provided a unique opportunity to understand how genetic information can enhance our understanding of COVID-19 risk. This working group focused on: (1) ABO blood group typing and the protective effects of the O blood group on COVID-19 infection; (2) polygenic risk scores and COVID-19 outcomes; (3) human leukocyte antigen typing and COVID-19 outcomes; and (4) a transcriptome-wide association study of COVID-19–positive MVP participants.
Genome-Wide Association Study (GWAS) and Downstream Analysis. This working group performed GWAS of the main COVID-19 outcomes. Results from GWAS unveiled new genetic loci to suggest further investigation on these candidate genes. The results were used by other MVP COVID-19 working groups for their activities. The results also contributed to external collaborations, such as the COVID-19 Host Genetics Initiative.
COVID-19–Related PheWAS. This working group focused on understanding the potential clinical significance of genetic variants associated with susceptibility to, or outcomes of, COVID-19 infection. They worked to identify traits that share genetic variants associated with severe COVID-19 from the Host Genetics Initiative. The group also studied the phenotypic consequences of acquired mosaic chromosomal alterations with early data linking to COVID-19 susceptibility.
COVID-19 Research Partnerships
In 2016, the VA and DOE formed an interagency partnership known as Computational Health Analytics for Medical Precision to Improve Outcomes Now (CHAMPION) to demonstrate the power of combining the VA EHR system, MVP genetic data, and clinical research expertise with DOE high-performance computing infrastructure and artificial intelligence expertise. The VA EHR captures longitudinal care information on veterans with records that go back decades. Furthermore, the VA covers the costs of medications and
The DOE Oak Ridge National Laboratory (ORNL) in Tennessee securely maintains this rich database for the VA. The ORNL Summit supercomputer can complete trillions of calculations per second to provide critical and timely analyses, applying the most advanced and powerful artificial intelligence methods, which would not be possible in more conventional research settings. CHAMPION taught the VA and DOE how to bring their disparate research cultures together for innovative collaborative investigation. Moreover, this collaboration produced a cadre of VA and DOE scientists familiar with VA patient data and experienced in conducting joint research successfully and integrating omics data with clinical data for a better mechanistic understanding. Because of this preexisting collaboration between the VA and DOE, interagency teams were prepared at the start of the COVID-19 pandemic.10-15
Other recently completed studies have developed and validated short-term mortality indices in individuals with COVID-19 based on their preexisting conditions, assessed the generalizability of VA COVID-19 experiences to the US population, and evaluated the effectiveness of hydroxychloroquine with and without azithromycin in VA patients with COVID-19.12,15 A recent study demonstrated the benefit of prophylactic anticoagulation at initial hospitalization.14
The VA also provided the FDA with daily reports on aggregate VA COVID-19 cases and their distribution across the VA system, demographics of VA patients with COVID-19, and analyses of predictive models for positive test results and death. The VA regularly sent the FDA aggregated data showing patterns of medication use and retrospective analyses of the effectiveness of certain medications (including remdesivir and some antithrombotic agents). The FDA used these data along with other data to understand the scope of the pandemic and to predict drug shortages or needs for additional medical equipment, including ventilators.
Limitations
For the most part, MVP infrastructure and partnerships were efficiently leveraged to significantly advance our understanding of the biological basis of COVID-19 and to develop treatments and vaccines. However, there were a few limitations that may have slowed timely and optimal outcomes. An issue not limited to the MVP or VA was the continual evolution of the pandemic and its response. This included evolving definitions of disease, symptomatology, testing, vaccines, and public health recommendations. Keeping pace with the emerging knowledge from these domains was a struggle for the entire scientific community. A more discrete limitation was the number of participants in the MVP with positive COVID-19 test results and positive symptoms; however, this was mitigated by partnering with other groups like the COVID-19 Host Genetics Initiative to increase study participant numbers. Finally, there were logistical and regulatory challenges associated with coordination of national clinical trial recruitment across a VA system with > 100 discrete hospitals.
Conclusions
Having a centralized infrastructure for recruitment and enrollment, including a national research volunteer registry, information center, research staff, and coordinating centers, can allow for expedited enrollment in vaccine and treatment trials in the face of future public health emergencies. VA assets, including its rich EHR and MVP, the world’s largest genomic cohort, have contributed to improving our understanding and management of COVID-19.
1. Whitbourne SB, Nguyen XT, Song RJ, et al. Million Veteran Program’s response to COVID-19: survey development and preliminary findings. PLoS One. 2022;17(4):e0266381. doi:10.1371/journal.pone.0266381
2. Song RJ, Ho YL, Schubert P, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi:10.1371/journal.pone.0251651
3. Galloway A, Park Y, Tanukonda V, et al. Impact of COVID-19 severity on long-term events in US veterans using the Veterans Affairs Severity Index for COVID-19 (VASIC). J Infect Dis. 2022;226(12):2113-2117. doi:10.1093/infdis/jiac182
4. Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668-676. doi:10.0138/s41591-021-01310-z
5. Hung AM, Sha SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386-395. doi:10.1001/jamainternmed.2021.8538
6. Verma A, Huffman JE, Gao L, et al. Association of kidney comorbidities and acute kidney failure with unfavorable outcomes after COVID-19 in individuals with the sickle cell trait. JAMA Intern Med. 2022;182(8):796-804. doi:10.1001/jamainternmed.2022.2141
7. Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18(4):e1010113. doi:10.1371/journal.pgen.1010113
8. Peloso GM, Tcheandjieu C, McGeary JE, et al. Genetic loci associated with COVID-19 positivity and hospitalization in White, Black, and Hispanic Veterans of the VA Million Veteran Program. Front Genetic. 2022;12:777076. doi:10.3389/fgene.2021.777076
9. Verma A, Minnier J, Wan ES, et al. A MUC5B gene polymorphism, rs35705950-T confers protective effects against COVID-19 hospitalization but not severe disease or mortality. Am J Respir Crit Care Med. 2022;182(8):796-804. doi:10.1164/rccm.202109-2166OC
10. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;e59177. doi:10.7554/eLife.59177
11. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. doi:10.1371/journal.pmed.1003379
12. King JT, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
13. Joubert W, Weighill D, Kainer D, et al. Attacking the opioid epidemic: determining the epistatic and pleiotropic genetic architectures for chronic pain and opioid addiction. SC18: International Conference for High Performance Computing, Networking, Storage and Analysis. Dallas, TX, USA, 2018:717-730. doi:10.1109/SC.2018.00060
14. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
15. Gerlovin H, Posner DC, Ho YL, et al. Pharmacoepidemiology, machine learning, and COVID-19: an intent-to-treat analysis of hydroxychloroquine, with or without Azithromycin, and COVID-19 outcomes among hospitalized US Veterans. Am J Epidemiol. 2021;190(11): 2405-2419. doi:10.1093/aje/kwab183
1. Whitbourne SB, Nguyen XT, Song RJ, et al. Million Veteran Program’s response to COVID-19: survey development and preliminary findings. PLoS One. 2022;17(4):e0266381. doi:10.1371/journal.pone.0266381
2. Song RJ, Ho YL, Schubert P, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi:10.1371/journal.pone.0251651
3. Galloway A, Park Y, Tanukonda V, et al. Impact of COVID-19 severity on long-term events in US veterans using the Veterans Affairs Severity Index for COVID-19 (VASIC). J Infect Dis. 2022;226(12):2113-2117. doi:10.1093/infdis/jiac182
4. Gaziano L, Giambartolomei C, Pereira AC, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27(4):668-676. doi:10.0138/s41591-021-01310-z
5. Hung AM, Sha SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386-395. doi:10.1001/jamainternmed.2021.8538
6. Verma A, Huffman JE, Gao L, et al. Association of kidney comorbidities and acute kidney failure with unfavorable outcomes after COVID-19 in individuals with the sickle cell trait. JAMA Intern Med. 2022;182(8):796-804. doi:10.1001/jamainternmed.2022.2141
7. Verma A, Tsao NL, Thomann LO, et al. A phenome-wide association study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18(4):e1010113. doi:10.1371/journal.pgen.1010113
8. Peloso GM, Tcheandjieu C, McGeary JE, et al. Genetic loci associated with COVID-19 positivity and hospitalization in White, Black, and Hispanic Veterans of the VA Million Veteran Program. Front Genetic. 2022;12:777076. doi:10.3389/fgene.2021.777076
9. Verma A, Minnier J, Wan ES, et al. A MUC5B gene polymorphism, rs35705950-T confers protective effects against COVID-19 hospitalization but not severe disease or mortality. Am J Respir Crit Care Med. 2022;182(8):796-804. doi:10.1164/rccm.202109-2166OC
10. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;e59177. doi:10.7554/eLife.59177
11. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020;17(9):e1003379. doi:10.1371/journal.pmed.1003379
12. King JT, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
13. Joubert W, Weighill D, Kainer D, et al. Attacking the opioid epidemic: determining the epistatic and pleiotropic genetic architectures for chronic pain and opioid addiction. SC18: International Conference for High Performance Computing, Networking, Storage and Analysis. Dallas, TX, USA, 2018:717-730. doi:10.1109/SC.2018.00060
14. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. BMJ. 2021;372:n311. doi:10.1136/bmj.n311
15. Gerlovin H, Posner DC, Ho YL, et al. Pharmacoepidemiology, machine learning, and COVID-19: an intent-to-treat analysis of hydroxychloroquine, with or without Azithromycin, and COVID-19 outcomes among hospitalized US Veterans. Am J Epidemiol. 2021;190(11): 2405-2419. doi:10.1093/aje/kwab183
VA Lessons From Partnering in COVID-19 Clinical Trials
The US Department of Veterans Affairs (VA), through its Office of Research and Development (ORD), supports an extensive and experienced clinical research enterprise, including the first multisite trials in the US.1 These resources contribute to the ORD support for the largest US integrated health care system, with a primary focus on the care and well-being of veterans. While the history of VA research has facilitated the creation of an experienced and organized research enterprise, the COVID-19 pandemic challenged VA to contribute even more significantly. These challenges became pronounced given the urgency associated with standing up VA sites for both therapeutic and vaccine trials.
VA Clinical Research Enterprise
The VA recognized an early need for an organized research response not only to address operational challenges resulting from COVID-19 but also ensure that the agency would be ready to support new scientific efforts focused specifically on the virus and related outcomes.2 As a result, the ORD took decisive action first by establishing itself as a central headquarters for VA COVID-19 research activities, and second, by leveraging existing resources, initiatives, and infrastructure to develop new mechanisms that would ensure that the VA was well positioned to develop or participate in research endeavors being driven by the VA as well federal, industry, and non-VA partners.
Prior to the pandemic, the ORD, through its Cooperative Studies Program (CSP), had strategies to address challenges associated with clinical trial startup and improved efficient conduct.3 For example, the VA Network of Dedicated Enrollment Sites (NODES) is a consortium of 23 VA medical centers (VAMCs) dedicated to rapid startup and recruitment into VA-sponsored clinical trials. NODES provides site-level expertise on clinical trial management, including troubleshooting challenges that may occur during clinical research execution.4 Another initiative, Access to Clinical Trials (ACT) for Veterans, engaged industry, academic, patient advocacy, and other partners to identify potential regulatory and operational hurdles to efficient startup activities specific to externally sponsored multisite clinical trials. Under ACT for Veterans, stakeholders emphasized the importance of developing a single VA point of contact for external partners to work with to more efficiently understand and navigate the VA system. In turn, such a resource could be designed to facilitate substantive research and long-term relationships with compatible external partners. Targeted to launch in April 2020, the Partnered Research Program (PRP) was expedited to respond to the pandemic.
During the pandemic, new VA efforts included the creation of the VA CoronavirUs Research and Efficacy Studies (VA CURES) network, initially established as a clinical trial master protocol framework to support and maximize VA-funded COVID-19 trial efficiency.5 VA CURES joined the consortium of trials networks funded by the National Heart, Lung, and Blood Institute. It began treatment trials under Accelerating COVID-19 Therapeutic Interventions and Vaccination (ACTIV), specifically ACTIV-4. The VA also partnered with the National Institutes of Allergy and Infectious Diseases (NIAID) by organizing the VA International Coordinating Center (VA ICC) for other ACTIV trials (ACTIV-2 and -3). When approached to startup studies that included veterans and the VA health care system, these capabilities comprised the VA research response.
A Need for a New Approach
As the impact of the pandemic expanded and the need for effective treatments and vaccines grew, national calls were made to assess the capabilities and readiness of available clinical trials networks. Additionally, the US Department of Health and Human Services Biomedical Advanced Research and Development Authority, ACTIV, NIAID Division of Clinical Research and Division of AIDS, and many pharmaceutical companies were starting to roll out trials of new therapeutics and vaccines. These groups approached the VA to help evaluate the safety and efficacy of several therapeutics and vaccines because they recognized several advantages of the VA enterprise, including its position as the nation’s largest integrated health care system, its diverse patient population, and its expertise in conducting clinical trials.
Although the VA was well positioned as an important player in a collaborative investigational approach to COVID-19 research, these trials required startup approaches that were significantly different from those it had employed in traditional, prepandemic, clinical research. Despite the VA being a single federal agency, each VAMC conducting research establishes its own practices to address both operational and regulatory requirements. This structure results in individual units that operate under different standard operating procedures. Efforts must be taken centrally to organize them into a singular network for the entire health care system. During a national crisis, when there was a need for rapid trial startup to answer safety and efficacy questions and participate under a common approach to protocol execution, this variability was neither manageable nor acceptable. Additionally, the intense resource demands associated with such research, coupled with frequent reporting requirements by VA leaders, Congress, and the White House, required that VAMCs function more like a single unit. Therefore, the ORD needed to develop VAMCs’ abilities to work collectively toward a common goal, share knowledge and experience, and capitalize on potential efficiencies concerning legal, regulatory, and operational processes.
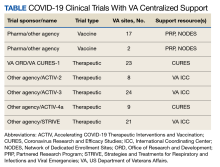
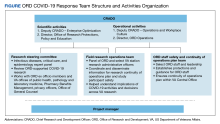
Beginning August 2020, 39 VAMCs joined 7 large-scale collaborative COVID-19 therapeutic and vaccine trials. Through its COVID-19 Research Response Team, the ORD identified, engaged, and directed appropriate resources to support the VAMC under a centralized framework for study management (Table). Centralized management not only afforded VAMCs the opportunity to work more collectively and efficiently but also provided an important advantage by enabling the VA to collect and organize its experiences (and on occasion data) to provide a base for continual learning and improvement efforts. While others have described efforts undertaken across networks to advance learning health systems, the VA’s national scope and integration of research and clinical care allow greater opportunities to learn in a practical setting.6
Challenges and Best Practices
Using surveys, webinars, interviews, and observation from site and VA Central Office personnel, the ORD identified specific variables that prevented the VAMCs from quickly starting up as a clinical trial site. We also documented strategies, solutions, and recommendations for improving startup time lines. These were organized into 8 categories: (1) site infrastructure needs and capabilities; (2) study management roles and responsibilities; (3) educational resources and training; (4) local review requirements and procedures; (5) study design demands; (6) contracting and budgeting; (7) central-level systems and processes; and (8) communication between external partners and within the VA.
Site Infrastructure Needs and Capabilities
A primary impediment to rapid study startup was a lack of basic infrastructure, including staff, space, and the agility necessary for the changing demands of high-priority, high-enrolling trials. This observation is not unique to the VA.7 Initially, certain facilities located in hot spots where COVID-19 was more prevalent became high-interest targets for study placement, despite varying degrees of available research infrastructure. Furthermore, pandemic shutdowns and quarantines permitted fewer employees onsite. This resulted in inadequate staffing in personnel needed to support required startup activities and those needed to handle the high volume of study participants who were being recruited, screened, enrolled, and followed. Additionally, as clinical care needs and infection control practices were prioritized, clinical research space was often appropriated for these needs, making it difficult to find the space to conduct trials. Lastly, supply chain issues also posed unique challenges, sometimes making it difficult for participating VAMCs to obtain needed materials, such as IV solution bags of specific sizes and contents, safety injection needles, and IV line filters.
The VA was able to use central purchasing/contracting at coordinating centers or the VA Central Office to support investigators and assist with finding supplies and clinical research space. VAMCs with research operating budgets to cover startup costs were better positioned to handle funding delays. During the pandemic, the ORD further contracted to supply administrative support to research offices to address regulatory and other requirements needed for startup activities. The ability to expand such central contracts to procure clinical research staff and outpatient clinical research space may also prove useful in meeting key needs at a site.
Management Roles and Responsibilities
Ambiguous and variable roles and responsibilities among the various partners and stakeholders represented a challenge given the large-scale, national, or international operations involved in the trials. VA attempts to operate uniformly were further limited given that each sponsor or group had preferred methods for operating and/or organizing work under urgent time lines. For example, one trial involved a coordinating center, a contract research organization, and federal partners that each worked with individual sites. Consequently, VA study teams would receive messages that were conflicting or unclear.
The VA learned that studies need a single “source of truth” and/or central command structure in times of urgency. To mitigate conflicting messages, vaccine trials relied on a clearinghouse through the PRP to interpret requirements or work on behalf of all sites before key actions were taken. For studies with the NIAID, the VA relied on experienced staff at the CSP coordinating center at the Perry Point, Maryland, VAMC before beginning. This approach especially helped with the challenges of understaffing and sites’ lack of familiarity with complex platform trial designs and already-established network practices within the ACTIV-2 and ACTIV-3 studies.
Educational Resources and Training
Since VA participation in externally sponsored, multisite clinical trials traditionally relies on an individual VAMC study team and its local resources, transitioning to centralized approaches for COVID-19 multisite studies created barriers. Many VAMCs were unfamiliar with newer capabilities for more rapid regulatory reviews and approvals involving commercial institutional review boards (IRBs) and central VA information security and privacy reviews. While tools and resources were available to facilitate these processes, real-time use had not been fully tested. As a result, everyone had to learn as they went along.
The simultaneous establishment of workflows required the ORD to centralize operations and provide training and guidance to field personnel. Although many principal investigators and clinical research coordinators had trial experience, training required unlearning previous understandings of requirements to meet urgent time lines. ORD enterprise road maps, central tools, and training materials also were made available on a study-by-study basis. Open communication was vital to train on central study materials while opportunities to discuss, question, and share experiences and ideas were promoted. The ORD also sent regular emails to prepare for upcoming work and/or raise awareness of identified challenges.
Local Review Requirements/Procedures
The clinical trials were impacted by varying VAMC review requirements and approval processes. Although VA policy defines standard requirements, the timing and procedures are left to the individual facility to determine any local factors to accommodate and/or resource availability. While such an approach is well understood within the VA, external sponsors were not as familiar and assumed a more uniform approach across all sites. In response, some VAMCs established ad hoc research and development committee review procedures, allowing study teams to obtain the necessary reviews in a timely fashion. However, not all VAMCs had the infrastructure (especially when clinical personnel had been redeployed to other priorities) to respond with such agility. One critical role of the VA Central Office coordinating entities was to communicate and manage external sponsor and group expectations surrounding individual site review time lines. However, establishing policies and procedures that focus on streamlining local review processes helped to broadly mitigate the COVID-19 trial challenges.
Study Design Demands
The design of COVID-19 studies combined with the uncertainty of the pandemic required rapid protocol changes and adaptations that were often difficult to deliver. The multinetwork trials that the VA collaborated on were platform or master protocol designs. These designs emphasized overall goals (eg, treating patients requiring intensive care unit care). However, because this trial strategy also introduces complexities that may impact review and execution among those unfamiliar with it, there is a need for increased discussion and understanding of this methodology.8 For example, there can be shared control groups, reliance on specific criteria for halting because of safety or futility concerns, or continuation and expansion applied through an external review board. Delays may arise when changes to study protocols occur rapidly or frequently and necessitate new regulatory reviews, negotiation of new agreements, modifications to contracts, changes to entry criteria, etc.
While the VA has adopted a quality by design framework, VA investigators noted many missed opportunities related to looking at outcomes with new diagnostics, studies of serology, outcomes related to vaccinations, and understanding the natural history of disease in these trials.9 The limited opportunities for investigator input suggested that the advantages offered by platform designs were not maximized during pandemic-focused urgencies. It was unclear whether this barrier was created by a general lack of awareness by sponsors or a lack of opportunities. At the very least, quality by design approaches may help avoid redundancies in documentation or study processes at the central and site levels.
Contracting and Budgeting
Given external sponsorship of COVID-19 trials, efficient contracting and budgeting were critical for a rapid start up. The variability of processes associated with these trials created several challenges that were compounded by issues, such as site sub-agreements and budget documents that did not always go to the correct groups and individuals. Furthermore, the VA’s ability to use contracted resources (eg, tents, trailers, personnel) that external sponsors had built into their contracts was more difficult for VA as a federal agency governed by other statues and policies. This also put VAMCs at a disadvantage from a timing perspective, as the VA often required additional time to find equivalent solutions that met federal regulations.
Although the VA was able to establish contract solutions to some issues, time was still lost while working to secure initial funding. Additionally, for needs such as home phlebotomy—commended for convenience to veterans and research staff—and engaging a specialized research team in the Office of General Counsel, early awareness of protocol needs and sponsor solutions could allow VA to pursue alternatives sooner.
Central-Level Systems and Processes
Not all challenges were at the VAMC level. As the ORD explored solutions, it learned that various tools and study platforms were available but not considered. Applications, such as eConsent, and file-sharing platforms that met existing information security and privacy requirements were needed but had to comply with the Privacy Act of 1974, Federal Information Security Modernization Act, and other requirements. Using sponsor-provided devices, such as drug temperature monitoring equipment, required additional review to ensure that they met system requirements for a national health care system. In addition, the VA uses a clinical trials management review system; however, its implementation was new at the time these trials began. Furthermore, the system engaged with some commercial IRBs but not all. This resulted in additional delays as VAMCs and central resources worked to familiarize themselves with the system and procedures.
The ability to work collaboratively across the VA includes having a framework in which key startup processes are standardized. This allows for efficiency and minimizes variability. Also, all stakeholders should understand the importance of holding discussions to identify appropriate solutions, guidance, and instruction. Finally, the VA must strive to be more nimble when adapting technological, regulatory, and financial processes.
Internal and External Communication
The value of communication—both internal and external—cannot be understated. Minimizing confusion, managing expectations, and ensuring consistent messaging were essential for rapid trial execution. Despite being the second largest federal agency, the VA did not have a seat at the study leadership table for several protocols. When it joined later, several study aspects were set and/or difficult to revise. Challenges affecting time and securing resources have been noted. The ability to plan and then share expectations and responsibilities across and within the respective participating organizations early in the process was perhaps the single factor that was most addressable. The VA enterprise organization and integration with other units could accentuate key communications that would be essential in time-sensitive activities.
VA as a Partner for Future Research
Before the pandemic, the VA had already undertaken a path to enhance its ability to partner as part of the national biomedical research enterprise. The need for COVID-19 therapeutic and vaccine trials accelerated opportunities to plan and develop processes and capabilities to advance this path. As a key strength for VA scientific activities, clinical trials represent a primary medium by which to develop its partnerships. Learning and development have become part of a culture that expedites opportunities for veterans who actively seek ways to contribute to medical knowledge and treatments for their peers and the nation.
CONCLUSIONS
Challenges associated with rapid startup and completion of clinical trials have been discussed for some time. During the pandemic, needs and barriers were magnified because of the heightened urgency for evidence-based therapeutics and vaccines. While the VA faced similar problems as well as those specific to it as a health care system, it had the opportunity to learn and more systematically implement solutions to help in its partnered efforts.10 As an enterprise, the VA hopes to apply lessons learned, strategies, and best practices to further its goals to enhance veteran access to clinical trials and respond to any future need to quickly establish evidence bases in pandemics and other health emergencies that warrant the rapid implementation of research.
Acknowledgments
The activities reported here were supported by the US Department of Veterans Affairs, Office of Research and Development.
1. Hays MT; US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. A historical look at the establishment of the Department of Veterans Affairs Research & Development Program. Accessed August 28, 2023. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
2. Garcia AP, Huang GD, Arnheim L, Ramoni R, Clancy C. The VA research enterprise: a platform for national partnerships toward evidence building and scientific innovation. Fed Pract. 2023;40(suppl 5):S12-S17. doi:10.12788/fp.0425
3. Johnston SC, Lewis-Hall F, Bajpai A, et al. It’s time to harmonize clinical trial site standards. NAM Perspectives. October 9, 2017. Accessed August 28, 2023. https://nam.edu/wp-content/uploads/2017/10/Its-Time-to-Harmonize-Clinical-Trial-Site-1.pdf
4. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
5. US Food and Drug Administration. Master protocols: efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry. March 2022. Accessed August 23, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and
6. IOM Roundtable on Value & Science-Driven Care; Institute of Medicine. Continuous learning and improvement in health care. In: Integrating Research and Practice: Health System Leaders Working Toward High-Value Care: Workshop Summary. National Academies Press (US); 2015:chap 2. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK284654 7. Institute of Medicine (US). Building an infrastructure to support clinical trials. In: Envisioning a Transformed Clinical Trials Enterprise in the United States. National Academies Press (US); 2012:chap 5. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK114656
8. Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ. An overview of platform trials with a checklist for clinical readers. J Clin Epidemiol. 2020;125:1-8. doi:10.1016/j.jclinepi.2020.04.025
9. Meeker-O’Connell A, Glessner C, Behm M, et al. Enhancing clinical evidence by proactively building quality into clinical trials. Clin Trials. 2016;13(4):439-444. doi:10.1177/1740774516643491
10. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
The US Department of Veterans Affairs (VA), through its Office of Research and Development (ORD), supports an extensive and experienced clinical research enterprise, including the first multisite trials in the US.1 These resources contribute to the ORD support for the largest US integrated health care system, with a primary focus on the care and well-being of veterans. While the history of VA research has facilitated the creation of an experienced and organized research enterprise, the COVID-19 pandemic challenged VA to contribute even more significantly. These challenges became pronounced given the urgency associated with standing up VA sites for both therapeutic and vaccine trials.
VA Clinical Research Enterprise
The VA recognized an early need for an organized research response not only to address operational challenges resulting from COVID-19 but also ensure that the agency would be ready to support new scientific efforts focused specifically on the virus and related outcomes.2 As a result, the ORD took decisive action first by establishing itself as a central headquarters for VA COVID-19 research activities, and second, by leveraging existing resources, initiatives, and infrastructure to develop new mechanisms that would ensure that the VA was well positioned to develop or participate in research endeavors being driven by the VA as well federal, industry, and non-VA partners.
Prior to the pandemic, the ORD, through its Cooperative Studies Program (CSP), had strategies to address challenges associated with clinical trial startup and improved efficient conduct.3 For example, the VA Network of Dedicated Enrollment Sites (NODES) is a consortium of 23 VA medical centers (VAMCs) dedicated to rapid startup and recruitment into VA-sponsored clinical trials. NODES provides site-level expertise on clinical trial management, including troubleshooting challenges that may occur during clinical research execution.4 Another initiative, Access to Clinical Trials (ACT) for Veterans, engaged industry, academic, patient advocacy, and other partners to identify potential regulatory and operational hurdles to efficient startup activities specific to externally sponsored multisite clinical trials. Under ACT for Veterans, stakeholders emphasized the importance of developing a single VA point of contact for external partners to work with to more efficiently understand and navigate the VA system. In turn, such a resource could be designed to facilitate substantive research and long-term relationships with compatible external partners. Targeted to launch in April 2020, the Partnered Research Program (PRP) was expedited to respond to the pandemic.
During the pandemic, new VA efforts included the creation of the VA CoronavirUs Research and Efficacy Studies (VA CURES) network, initially established as a clinical trial master protocol framework to support and maximize VA-funded COVID-19 trial efficiency.5 VA CURES joined the consortium of trials networks funded by the National Heart, Lung, and Blood Institute. It began treatment trials under Accelerating COVID-19 Therapeutic Interventions and Vaccination (ACTIV), specifically ACTIV-4. The VA also partnered with the National Institutes of Allergy and Infectious Diseases (NIAID) by organizing the VA International Coordinating Center (VA ICC) for other ACTIV trials (ACTIV-2 and -3). When approached to startup studies that included veterans and the VA health care system, these capabilities comprised the VA research response.
A Need for a New Approach
As the impact of the pandemic expanded and the need for effective treatments and vaccines grew, national calls were made to assess the capabilities and readiness of available clinical trials networks. Additionally, the US Department of Health and Human Services Biomedical Advanced Research and Development Authority, ACTIV, NIAID Division of Clinical Research and Division of AIDS, and many pharmaceutical companies were starting to roll out trials of new therapeutics and vaccines. These groups approached the VA to help evaluate the safety and efficacy of several therapeutics and vaccines because they recognized several advantages of the VA enterprise, including its position as the nation’s largest integrated health care system, its diverse patient population, and its expertise in conducting clinical trials.
Although the VA was well positioned as an important player in a collaborative investigational approach to COVID-19 research, these trials required startup approaches that were significantly different from those it had employed in traditional, prepandemic, clinical research. Despite the VA being a single federal agency, each VAMC conducting research establishes its own practices to address both operational and regulatory requirements. This structure results in individual units that operate under different standard operating procedures. Efforts must be taken centrally to organize them into a singular network for the entire health care system. During a national crisis, when there was a need for rapid trial startup to answer safety and efficacy questions and participate under a common approach to protocol execution, this variability was neither manageable nor acceptable. Additionally, the intense resource demands associated with such research, coupled with frequent reporting requirements by VA leaders, Congress, and the White House, required that VAMCs function more like a single unit. Therefore, the ORD needed to develop VAMCs’ abilities to work collectively toward a common goal, share knowledge and experience, and capitalize on potential efficiencies concerning legal, regulatory, and operational processes.
Beginning August 2020, 39 VAMCs joined 7 large-scale collaborative COVID-19 therapeutic and vaccine trials. Through its COVID-19 Research Response Team, the ORD identified, engaged, and directed appropriate resources to support the VAMC under a centralized framework for study management (Table). Centralized management not only afforded VAMCs the opportunity to work more collectively and efficiently but also provided an important advantage by enabling the VA to collect and organize its experiences (and on occasion data) to provide a base for continual learning and improvement efforts. While others have described efforts undertaken across networks to advance learning health systems, the VA’s national scope and integration of research and clinical care allow greater opportunities to learn in a practical setting.6
Challenges and Best Practices
Using surveys, webinars, interviews, and observation from site and VA Central Office personnel, the ORD identified specific variables that prevented the VAMCs from quickly starting up as a clinical trial site. We also documented strategies, solutions, and recommendations for improving startup time lines. These were organized into 8 categories: (1) site infrastructure needs and capabilities; (2) study management roles and responsibilities; (3) educational resources and training; (4) local review requirements and procedures; (5) study design demands; (6) contracting and budgeting; (7) central-level systems and processes; and (8) communication between external partners and within the VA.
Site Infrastructure Needs and Capabilities
A primary impediment to rapid study startup was a lack of basic infrastructure, including staff, space, and the agility necessary for the changing demands of high-priority, high-enrolling trials. This observation is not unique to the VA.7 Initially, certain facilities located in hot spots where COVID-19 was more prevalent became high-interest targets for study placement, despite varying degrees of available research infrastructure. Furthermore, pandemic shutdowns and quarantines permitted fewer employees onsite. This resulted in inadequate staffing in personnel needed to support required startup activities and those needed to handle the high volume of study participants who were being recruited, screened, enrolled, and followed. Additionally, as clinical care needs and infection control practices were prioritized, clinical research space was often appropriated for these needs, making it difficult to find the space to conduct trials. Lastly, supply chain issues also posed unique challenges, sometimes making it difficult for participating VAMCs to obtain needed materials, such as IV solution bags of specific sizes and contents, safety injection needles, and IV line filters.
The VA was able to use central purchasing/contracting at coordinating centers or the VA Central Office to support investigators and assist with finding supplies and clinical research space. VAMCs with research operating budgets to cover startup costs were better positioned to handle funding delays. During the pandemic, the ORD further contracted to supply administrative support to research offices to address regulatory and other requirements needed for startup activities. The ability to expand such central contracts to procure clinical research staff and outpatient clinical research space may also prove useful in meeting key needs at a site.
Management Roles and Responsibilities
Ambiguous and variable roles and responsibilities among the various partners and stakeholders represented a challenge given the large-scale, national, or international operations involved in the trials. VA attempts to operate uniformly were further limited given that each sponsor or group had preferred methods for operating and/or organizing work under urgent time lines. For example, one trial involved a coordinating center, a contract research organization, and federal partners that each worked with individual sites. Consequently, VA study teams would receive messages that were conflicting or unclear.
The VA learned that studies need a single “source of truth” and/or central command structure in times of urgency. To mitigate conflicting messages, vaccine trials relied on a clearinghouse through the PRP to interpret requirements or work on behalf of all sites before key actions were taken. For studies with the NIAID, the VA relied on experienced staff at the CSP coordinating center at the Perry Point, Maryland, VAMC before beginning. This approach especially helped with the challenges of understaffing and sites’ lack of familiarity with complex platform trial designs and already-established network practices within the ACTIV-2 and ACTIV-3 studies.
Educational Resources and Training
Since VA participation in externally sponsored, multisite clinical trials traditionally relies on an individual VAMC study team and its local resources, transitioning to centralized approaches for COVID-19 multisite studies created barriers. Many VAMCs were unfamiliar with newer capabilities for more rapid regulatory reviews and approvals involving commercial institutional review boards (IRBs) and central VA information security and privacy reviews. While tools and resources were available to facilitate these processes, real-time use had not been fully tested. As a result, everyone had to learn as they went along.
The simultaneous establishment of workflows required the ORD to centralize operations and provide training and guidance to field personnel. Although many principal investigators and clinical research coordinators had trial experience, training required unlearning previous understandings of requirements to meet urgent time lines. ORD enterprise road maps, central tools, and training materials also were made available on a study-by-study basis. Open communication was vital to train on central study materials while opportunities to discuss, question, and share experiences and ideas were promoted. The ORD also sent regular emails to prepare for upcoming work and/or raise awareness of identified challenges.
Local Review Requirements/Procedures
The clinical trials were impacted by varying VAMC review requirements and approval processes. Although VA policy defines standard requirements, the timing and procedures are left to the individual facility to determine any local factors to accommodate and/or resource availability. While such an approach is well understood within the VA, external sponsors were not as familiar and assumed a more uniform approach across all sites. In response, some VAMCs established ad hoc research and development committee review procedures, allowing study teams to obtain the necessary reviews in a timely fashion. However, not all VAMCs had the infrastructure (especially when clinical personnel had been redeployed to other priorities) to respond with such agility. One critical role of the VA Central Office coordinating entities was to communicate and manage external sponsor and group expectations surrounding individual site review time lines. However, establishing policies and procedures that focus on streamlining local review processes helped to broadly mitigate the COVID-19 trial challenges.
Study Design Demands
The design of COVID-19 studies combined with the uncertainty of the pandemic required rapid protocol changes and adaptations that were often difficult to deliver. The multinetwork trials that the VA collaborated on were platform or master protocol designs. These designs emphasized overall goals (eg, treating patients requiring intensive care unit care). However, because this trial strategy also introduces complexities that may impact review and execution among those unfamiliar with it, there is a need for increased discussion and understanding of this methodology.8 For example, there can be shared control groups, reliance on specific criteria for halting because of safety or futility concerns, or continuation and expansion applied through an external review board. Delays may arise when changes to study protocols occur rapidly or frequently and necessitate new regulatory reviews, negotiation of new agreements, modifications to contracts, changes to entry criteria, etc.
While the VA has adopted a quality by design framework, VA investigators noted many missed opportunities related to looking at outcomes with new diagnostics, studies of serology, outcomes related to vaccinations, and understanding the natural history of disease in these trials.9 The limited opportunities for investigator input suggested that the advantages offered by platform designs were not maximized during pandemic-focused urgencies. It was unclear whether this barrier was created by a general lack of awareness by sponsors or a lack of opportunities. At the very least, quality by design approaches may help avoid redundancies in documentation or study processes at the central and site levels.
Contracting and Budgeting
Given external sponsorship of COVID-19 trials, efficient contracting and budgeting were critical for a rapid start up. The variability of processes associated with these trials created several challenges that were compounded by issues, such as site sub-agreements and budget documents that did not always go to the correct groups and individuals. Furthermore, the VA’s ability to use contracted resources (eg, tents, trailers, personnel) that external sponsors had built into their contracts was more difficult for VA as a federal agency governed by other statues and policies. This also put VAMCs at a disadvantage from a timing perspective, as the VA often required additional time to find equivalent solutions that met federal regulations.
Although the VA was able to establish contract solutions to some issues, time was still lost while working to secure initial funding. Additionally, for needs such as home phlebotomy—commended for convenience to veterans and research staff—and engaging a specialized research team in the Office of General Counsel, early awareness of protocol needs and sponsor solutions could allow VA to pursue alternatives sooner.
Central-Level Systems and Processes
Not all challenges were at the VAMC level. As the ORD explored solutions, it learned that various tools and study platforms were available but not considered. Applications, such as eConsent, and file-sharing platforms that met existing information security and privacy requirements were needed but had to comply with the Privacy Act of 1974, Federal Information Security Modernization Act, and other requirements. Using sponsor-provided devices, such as drug temperature monitoring equipment, required additional review to ensure that they met system requirements for a national health care system. In addition, the VA uses a clinical trials management review system; however, its implementation was new at the time these trials began. Furthermore, the system engaged with some commercial IRBs but not all. This resulted in additional delays as VAMCs and central resources worked to familiarize themselves with the system and procedures.
The ability to work collaboratively across the VA includes having a framework in which key startup processes are standardized. This allows for efficiency and minimizes variability. Also, all stakeholders should understand the importance of holding discussions to identify appropriate solutions, guidance, and instruction. Finally, the VA must strive to be more nimble when adapting technological, regulatory, and financial processes.
Internal and External Communication
The value of communication—both internal and external—cannot be understated. Minimizing confusion, managing expectations, and ensuring consistent messaging were essential for rapid trial execution. Despite being the second largest federal agency, the VA did not have a seat at the study leadership table for several protocols. When it joined later, several study aspects were set and/or difficult to revise. Challenges affecting time and securing resources have been noted. The ability to plan and then share expectations and responsibilities across and within the respective participating organizations early in the process was perhaps the single factor that was most addressable. The VA enterprise organization and integration with other units could accentuate key communications that would be essential in time-sensitive activities.
VA as a Partner for Future Research
Before the pandemic, the VA had already undertaken a path to enhance its ability to partner as part of the national biomedical research enterprise. The need for COVID-19 therapeutic and vaccine trials accelerated opportunities to plan and develop processes and capabilities to advance this path. As a key strength for VA scientific activities, clinical trials represent a primary medium by which to develop its partnerships. Learning and development have become part of a culture that expedites opportunities for veterans who actively seek ways to contribute to medical knowledge and treatments for their peers and the nation.
CONCLUSIONS
Challenges associated with rapid startup and completion of clinical trials have been discussed for some time. During the pandemic, needs and barriers were magnified because of the heightened urgency for evidence-based therapeutics and vaccines. While the VA faced similar problems as well as those specific to it as a health care system, it had the opportunity to learn and more systematically implement solutions to help in its partnered efforts.10 As an enterprise, the VA hopes to apply lessons learned, strategies, and best practices to further its goals to enhance veteran access to clinical trials and respond to any future need to quickly establish evidence bases in pandemics and other health emergencies that warrant the rapid implementation of research.
Acknowledgments
The activities reported here were supported by the US Department of Veterans Affairs, Office of Research and Development.
The US Department of Veterans Affairs (VA), through its Office of Research and Development (ORD), supports an extensive and experienced clinical research enterprise, including the first multisite trials in the US.1 These resources contribute to the ORD support for the largest US integrated health care system, with a primary focus on the care and well-being of veterans. While the history of VA research has facilitated the creation of an experienced and organized research enterprise, the COVID-19 pandemic challenged VA to contribute even more significantly. These challenges became pronounced given the urgency associated with standing up VA sites for both therapeutic and vaccine trials.
VA Clinical Research Enterprise
The VA recognized an early need for an organized research response not only to address operational challenges resulting from COVID-19 but also ensure that the agency would be ready to support new scientific efforts focused specifically on the virus and related outcomes.2 As a result, the ORD took decisive action first by establishing itself as a central headquarters for VA COVID-19 research activities, and second, by leveraging existing resources, initiatives, and infrastructure to develop new mechanisms that would ensure that the VA was well positioned to develop or participate in research endeavors being driven by the VA as well federal, industry, and non-VA partners.
Prior to the pandemic, the ORD, through its Cooperative Studies Program (CSP), had strategies to address challenges associated with clinical trial startup and improved efficient conduct.3 For example, the VA Network of Dedicated Enrollment Sites (NODES) is a consortium of 23 VA medical centers (VAMCs) dedicated to rapid startup and recruitment into VA-sponsored clinical trials. NODES provides site-level expertise on clinical trial management, including troubleshooting challenges that may occur during clinical research execution.4 Another initiative, Access to Clinical Trials (ACT) for Veterans, engaged industry, academic, patient advocacy, and other partners to identify potential regulatory and operational hurdles to efficient startup activities specific to externally sponsored multisite clinical trials. Under ACT for Veterans, stakeholders emphasized the importance of developing a single VA point of contact for external partners to work with to more efficiently understand and navigate the VA system. In turn, such a resource could be designed to facilitate substantive research and long-term relationships with compatible external partners. Targeted to launch in April 2020, the Partnered Research Program (PRP) was expedited to respond to the pandemic.
During the pandemic, new VA efforts included the creation of the VA CoronavirUs Research and Efficacy Studies (VA CURES) network, initially established as a clinical trial master protocol framework to support and maximize VA-funded COVID-19 trial efficiency.5 VA CURES joined the consortium of trials networks funded by the National Heart, Lung, and Blood Institute. It began treatment trials under Accelerating COVID-19 Therapeutic Interventions and Vaccination (ACTIV), specifically ACTIV-4. The VA also partnered with the National Institutes of Allergy and Infectious Diseases (NIAID) by organizing the VA International Coordinating Center (VA ICC) for other ACTIV trials (ACTIV-2 and -3). When approached to startup studies that included veterans and the VA health care system, these capabilities comprised the VA research response.
A Need for a New Approach
As the impact of the pandemic expanded and the need for effective treatments and vaccines grew, national calls were made to assess the capabilities and readiness of available clinical trials networks. Additionally, the US Department of Health and Human Services Biomedical Advanced Research and Development Authority, ACTIV, NIAID Division of Clinical Research and Division of AIDS, and many pharmaceutical companies were starting to roll out trials of new therapeutics and vaccines. These groups approached the VA to help evaluate the safety and efficacy of several therapeutics and vaccines because they recognized several advantages of the VA enterprise, including its position as the nation’s largest integrated health care system, its diverse patient population, and its expertise in conducting clinical trials.
Although the VA was well positioned as an important player in a collaborative investigational approach to COVID-19 research, these trials required startup approaches that were significantly different from those it had employed in traditional, prepandemic, clinical research. Despite the VA being a single federal agency, each VAMC conducting research establishes its own practices to address both operational and regulatory requirements. This structure results in individual units that operate under different standard operating procedures. Efforts must be taken centrally to organize them into a singular network for the entire health care system. During a national crisis, when there was a need for rapid trial startup to answer safety and efficacy questions and participate under a common approach to protocol execution, this variability was neither manageable nor acceptable. Additionally, the intense resource demands associated with such research, coupled with frequent reporting requirements by VA leaders, Congress, and the White House, required that VAMCs function more like a single unit. Therefore, the ORD needed to develop VAMCs’ abilities to work collectively toward a common goal, share knowledge and experience, and capitalize on potential efficiencies concerning legal, regulatory, and operational processes.
Beginning August 2020, 39 VAMCs joined 7 large-scale collaborative COVID-19 therapeutic and vaccine trials. Through its COVID-19 Research Response Team, the ORD identified, engaged, and directed appropriate resources to support the VAMC under a centralized framework for study management (Table). Centralized management not only afforded VAMCs the opportunity to work more collectively and efficiently but also provided an important advantage by enabling the VA to collect and organize its experiences (and on occasion data) to provide a base for continual learning and improvement efforts. While others have described efforts undertaken across networks to advance learning health systems, the VA’s national scope and integration of research and clinical care allow greater opportunities to learn in a practical setting.6
Challenges and Best Practices
Using surveys, webinars, interviews, and observation from site and VA Central Office personnel, the ORD identified specific variables that prevented the VAMCs from quickly starting up as a clinical trial site. We also documented strategies, solutions, and recommendations for improving startup time lines. These were organized into 8 categories: (1) site infrastructure needs and capabilities; (2) study management roles and responsibilities; (3) educational resources and training; (4) local review requirements and procedures; (5) study design demands; (6) contracting and budgeting; (7) central-level systems and processes; and (8) communication between external partners and within the VA.
Site Infrastructure Needs and Capabilities
A primary impediment to rapid study startup was a lack of basic infrastructure, including staff, space, and the agility necessary for the changing demands of high-priority, high-enrolling trials. This observation is not unique to the VA.7 Initially, certain facilities located in hot spots where COVID-19 was more prevalent became high-interest targets for study placement, despite varying degrees of available research infrastructure. Furthermore, pandemic shutdowns and quarantines permitted fewer employees onsite. This resulted in inadequate staffing in personnel needed to support required startup activities and those needed to handle the high volume of study participants who were being recruited, screened, enrolled, and followed. Additionally, as clinical care needs and infection control practices were prioritized, clinical research space was often appropriated for these needs, making it difficult to find the space to conduct trials. Lastly, supply chain issues also posed unique challenges, sometimes making it difficult for participating VAMCs to obtain needed materials, such as IV solution bags of specific sizes and contents, safety injection needles, and IV line filters.
The VA was able to use central purchasing/contracting at coordinating centers or the VA Central Office to support investigators and assist with finding supplies and clinical research space. VAMCs with research operating budgets to cover startup costs were better positioned to handle funding delays. During the pandemic, the ORD further contracted to supply administrative support to research offices to address regulatory and other requirements needed for startup activities. The ability to expand such central contracts to procure clinical research staff and outpatient clinical research space may also prove useful in meeting key needs at a site.
Management Roles and Responsibilities
Ambiguous and variable roles and responsibilities among the various partners and stakeholders represented a challenge given the large-scale, national, or international operations involved in the trials. VA attempts to operate uniformly were further limited given that each sponsor or group had preferred methods for operating and/or organizing work under urgent time lines. For example, one trial involved a coordinating center, a contract research organization, and federal partners that each worked with individual sites. Consequently, VA study teams would receive messages that were conflicting or unclear.
The VA learned that studies need a single “source of truth” and/or central command structure in times of urgency. To mitigate conflicting messages, vaccine trials relied on a clearinghouse through the PRP to interpret requirements or work on behalf of all sites before key actions were taken. For studies with the NIAID, the VA relied on experienced staff at the CSP coordinating center at the Perry Point, Maryland, VAMC before beginning. This approach especially helped with the challenges of understaffing and sites’ lack of familiarity with complex platform trial designs and already-established network practices within the ACTIV-2 and ACTIV-3 studies.
Educational Resources and Training
Since VA participation in externally sponsored, multisite clinical trials traditionally relies on an individual VAMC study team and its local resources, transitioning to centralized approaches for COVID-19 multisite studies created barriers. Many VAMCs were unfamiliar with newer capabilities for more rapid regulatory reviews and approvals involving commercial institutional review boards (IRBs) and central VA information security and privacy reviews. While tools and resources were available to facilitate these processes, real-time use had not been fully tested. As a result, everyone had to learn as they went along.
The simultaneous establishment of workflows required the ORD to centralize operations and provide training and guidance to field personnel. Although many principal investigators and clinical research coordinators had trial experience, training required unlearning previous understandings of requirements to meet urgent time lines. ORD enterprise road maps, central tools, and training materials also were made available on a study-by-study basis. Open communication was vital to train on central study materials while opportunities to discuss, question, and share experiences and ideas were promoted. The ORD also sent regular emails to prepare for upcoming work and/or raise awareness of identified challenges.
Local Review Requirements/Procedures
The clinical trials were impacted by varying VAMC review requirements and approval processes. Although VA policy defines standard requirements, the timing and procedures are left to the individual facility to determine any local factors to accommodate and/or resource availability. While such an approach is well understood within the VA, external sponsors were not as familiar and assumed a more uniform approach across all sites. In response, some VAMCs established ad hoc research and development committee review procedures, allowing study teams to obtain the necessary reviews in a timely fashion. However, not all VAMCs had the infrastructure (especially when clinical personnel had been redeployed to other priorities) to respond with such agility. One critical role of the VA Central Office coordinating entities was to communicate and manage external sponsor and group expectations surrounding individual site review time lines. However, establishing policies and procedures that focus on streamlining local review processes helped to broadly mitigate the COVID-19 trial challenges.
Study Design Demands
The design of COVID-19 studies combined with the uncertainty of the pandemic required rapid protocol changes and adaptations that were often difficult to deliver. The multinetwork trials that the VA collaborated on were platform or master protocol designs. These designs emphasized overall goals (eg, treating patients requiring intensive care unit care). However, because this trial strategy also introduces complexities that may impact review and execution among those unfamiliar with it, there is a need for increased discussion and understanding of this methodology.8 For example, there can be shared control groups, reliance on specific criteria for halting because of safety or futility concerns, or continuation and expansion applied through an external review board. Delays may arise when changes to study protocols occur rapidly or frequently and necessitate new regulatory reviews, negotiation of new agreements, modifications to contracts, changes to entry criteria, etc.
While the VA has adopted a quality by design framework, VA investigators noted many missed opportunities related to looking at outcomes with new diagnostics, studies of serology, outcomes related to vaccinations, and understanding the natural history of disease in these trials.9 The limited opportunities for investigator input suggested that the advantages offered by platform designs were not maximized during pandemic-focused urgencies. It was unclear whether this barrier was created by a general lack of awareness by sponsors or a lack of opportunities. At the very least, quality by design approaches may help avoid redundancies in documentation or study processes at the central and site levels.
Contracting and Budgeting
Given external sponsorship of COVID-19 trials, efficient contracting and budgeting were critical for a rapid start up. The variability of processes associated with these trials created several challenges that were compounded by issues, such as site sub-agreements and budget documents that did not always go to the correct groups and individuals. Furthermore, the VA’s ability to use contracted resources (eg, tents, trailers, personnel) that external sponsors had built into their contracts was more difficult for VA as a federal agency governed by other statues and policies. This also put VAMCs at a disadvantage from a timing perspective, as the VA often required additional time to find equivalent solutions that met federal regulations.
Although the VA was able to establish contract solutions to some issues, time was still lost while working to secure initial funding. Additionally, for needs such as home phlebotomy—commended for convenience to veterans and research staff—and engaging a specialized research team in the Office of General Counsel, early awareness of protocol needs and sponsor solutions could allow VA to pursue alternatives sooner.
Central-Level Systems and Processes
Not all challenges were at the VAMC level. As the ORD explored solutions, it learned that various tools and study platforms were available but not considered. Applications, such as eConsent, and file-sharing platforms that met existing information security and privacy requirements were needed but had to comply with the Privacy Act of 1974, Federal Information Security Modernization Act, and other requirements. Using sponsor-provided devices, such as drug temperature monitoring equipment, required additional review to ensure that they met system requirements for a national health care system. In addition, the VA uses a clinical trials management review system; however, its implementation was new at the time these trials began. Furthermore, the system engaged with some commercial IRBs but not all. This resulted in additional delays as VAMCs and central resources worked to familiarize themselves with the system and procedures.
The ability to work collaboratively across the VA includes having a framework in which key startup processes are standardized. This allows for efficiency and minimizes variability. Also, all stakeholders should understand the importance of holding discussions to identify appropriate solutions, guidance, and instruction. Finally, the VA must strive to be more nimble when adapting technological, regulatory, and financial processes.
Internal and External Communication
The value of communication—both internal and external—cannot be understated. Minimizing confusion, managing expectations, and ensuring consistent messaging were essential for rapid trial execution. Despite being the second largest federal agency, the VA did not have a seat at the study leadership table for several protocols. When it joined later, several study aspects were set and/or difficult to revise. Challenges affecting time and securing resources have been noted. The ability to plan and then share expectations and responsibilities across and within the respective participating organizations early in the process was perhaps the single factor that was most addressable. The VA enterprise organization and integration with other units could accentuate key communications that would be essential in time-sensitive activities.
VA as a Partner for Future Research
Before the pandemic, the VA had already undertaken a path to enhance its ability to partner as part of the national biomedical research enterprise. The need for COVID-19 therapeutic and vaccine trials accelerated opportunities to plan and develop processes and capabilities to advance this path. As a key strength for VA scientific activities, clinical trials represent a primary medium by which to develop its partnerships. Learning and development have become part of a culture that expedites opportunities for veterans who actively seek ways to contribute to medical knowledge and treatments for their peers and the nation.
CONCLUSIONS
Challenges associated with rapid startup and completion of clinical trials have been discussed for some time. During the pandemic, needs and barriers were magnified because of the heightened urgency for evidence-based therapeutics and vaccines. While the VA faced similar problems as well as those specific to it as a health care system, it had the opportunity to learn and more systematically implement solutions to help in its partnered efforts.10 As an enterprise, the VA hopes to apply lessons learned, strategies, and best practices to further its goals to enhance veteran access to clinical trials and respond to any future need to quickly establish evidence bases in pandemics and other health emergencies that warrant the rapid implementation of research.
Acknowledgments
The activities reported here were supported by the US Department of Veterans Affairs, Office of Research and Development.
1. Hays MT; US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. A historical look at the establishment of the Department of Veterans Affairs Research & Development Program. Accessed August 28, 2023. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
2. Garcia AP, Huang GD, Arnheim L, Ramoni R, Clancy C. The VA research enterprise: a platform for national partnerships toward evidence building and scientific innovation. Fed Pract. 2023;40(suppl 5):S12-S17. doi:10.12788/fp.0425
3. Johnston SC, Lewis-Hall F, Bajpai A, et al. It’s time to harmonize clinical trial site standards. NAM Perspectives. October 9, 2017. Accessed August 28, 2023. https://nam.edu/wp-content/uploads/2017/10/Its-Time-to-Harmonize-Clinical-Trial-Site-1.pdf
4. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
5. US Food and Drug Administration. Master protocols: efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry. March 2022. Accessed August 23, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and
6. IOM Roundtable on Value & Science-Driven Care; Institute of Medicine. Continuous learning and improvement in health care. In: Integrating Research and Practice: Health System Leaders Working Toward High-Value Care: Workshop Summary. National Academies Press (US); 2015:chap 2. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK284654 7. Institute of Medicine (US). Building an infrastructure to support clinical trials. In: Envisioning a Transformed Clinical Trials Enterprise in the United States. National Academies Press (US); 2012:chap 5. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK114656
8. Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ. An overview of platform trials with a checklist for clinical readers. J Clin Epidemiol. 2020;125:1-8. doi:10.1016/j.jclinepi.2020.04.025
9. Meeker-O’Connell A, Glessner C, Behm M, et al. Enhancing clinical evidence by proactively building quality into clinical trials. Clin Trials. 2016;13(4):439-444. doi:10.1177/1740774516643491
10. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
1. Hays MT; US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. A historical look at the establishment of the Department of Veterans Affairs Research & Development Program. Accessed August 28, 2023. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
2. Garcia AP, Huang GD, Arnheim L, Ramoni R, Clancy C. The VA research enterprise: a platform for national partnerships toward evidence building and scientific innovation. Fed Pract. 2023;40(suppl 5):S12-S17. doi:10.12788/fp.0425
3. Johnston SC, Lewis-Hall F, Bajpai A, et al. It’s time to harmonize clinical trial site standards. NAM Perspectives. October 9, 2017. Accessed August 28, 2023. https://nam.edu/wp-content/uploads/2017/10/Its-Time-to-Harmonize-Clinical-Trial-Site-1.pdf
4. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
5. US Food and Drug Administration. Master protocols: efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry. March 2022. Accessed August 23, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and
6. IOM Roundtable on Value & Science-Driven Care; Institute of Medicine. Continuous learning and improvement in health care. In: Integrating Research and Practice: Health System Leaders Working Toward High-Value Care: Workshop Summary. National Academies Press (US); 2015:chap 2. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK284654 7. Institute of Medicine (US). Building an infrastructure to support clinical trials. In: Envisioning a Transformed Clinical Trials Enterprise in the United States. National Academies Press (US); 2012:chap 5. Accessed August 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK114656
8. Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ. An overview of platform trials with a checklist for clinical readers. J Clin Epidemiol. 2020;125:1-8. doi:10.1016/j.jclinepi.2020.04.025
9. Meeker-O’Connell A, Glessner C, Behm M, et al. Enhancing clinical evidence by proactively building quality into clinical trials. Clin Trials. 2016;13(4):439-444. doi:10.1177/1740774516643491
10. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
The VA Research Enterprise: A Platform for National Partnerships Toward Evidence Building and Scientific Innovation
The US Department of Veterans Affairs (VA) plays a substantial role in the nation’s public health through the Veterans Health Administration (VHA). Its statutory missions of teaching, clinical care, and research enable it to serve a foundational role in the US biomedical enterprise.1 Throughout its extensive network of VA medical centers (VAMCs) and partnering academic affiliates, thousands of clinicians and researchers have been trained to improve the lives of veterans and benefit the lives of all Americans. In supporting the largest US integrated health care system, the VA also has numerous capabilities and resources that distinctively position it to produce scientific and clinical results specifically within the context of providing care. The VA has formed partnerships with other federal agencies, industry, and nonprofit entities. Its ability to be a nexus of health care and practice, scientific discovery, and innovative ways to integrate shared interests in these areas have led to many transformative endeavors that save lives and improve the quality of care for veterans and the public.
The COVID-19 pandemic triggered another mission: service in times of national emergency. Known as the Fourth Mission, the VA rapidly shifted to highlight how its health care and research enterprises could apply strengths in a unique, coordinated manner. While the Fourth Mission is typically considered in the context of clinical care, the VA’s movement toward greater integration facilitated the role of research as a key component in efforts under a learning health care model.2
VA Office of Research and Development
Within the VHA, the Office of Research and Development (ORD) develops research policy and oversees interdisciplinary efforts focused on generating evidence to improve veteran health.3 These activities span at least 100 of 171 VAMCs and include thousands of investigators and staff across all major health research disciplines. Many of these investigators are also clinicians who provide patient care and are experts in the prevention, diagnosis, and treatment of diseases and disorders affecting veterans.
The ORD has invested in a range of scientific, operational, regulatory, and technological assets and infrastructure as part of its enterprise. These strengths come from a nearly 100-year history originating as part of a set of hospital-based medical studies. This established the model for a culture of cooperative research within the VA and with external groups who benefit from the VA’s foundational role in multisite clinical trials.2,4,5 Today, the VA prioritizes bench-to-bedside research covering a broad spectrum of investigations, which are integrated with clinical operations and systems that deliver care.3 The VA supports an extensive range of work that covers core areas in preclinical and clinical studies to health services research, rehabilitation and implementation science, establishing expertise in genomic and data sciences, and more recent activities in artificial intelligence.
In 2017, the ORD began a focused strategy to transform into a national enterprise that capitalized on its place within the VA and its particular ability to translate and implement scientific findings into real impact for veteran health and care through 5 initiatives: (1) enhancing veteran access to high-quality clinical trials; (2) increasing the substantial real-world impact of VA Research; (3) putting VA data to work for veteran health; (4) promoting diversity, equity, and inclusion within our sphere of influence; and (5) building community through research. These activities are interrelated and, where possible, the ORD works with other VA clinical and operational offices to accomplish multiple goals and coordinate within the health care system. As such, the VA continually seeks to increase efficiencies and improve abilities that provide veterans with best-in-class health care. While still in its early stages, this strategy and its initiatives established a path for the ORD response to the pandemic.
Within 2 weeks of the World Health Organization and the US declaring a COVID-19 pandemic, the ORD began to address the developing needs and challenges of the yet unknown emerging public health threat. This included outreach to and contact from federal, academic, and industry partners. At the same time, the ORD maintained its focus and energy to support its ongoing veteran-centric research portfolio and VHA health care system needs across its broad scope of activities.
This article discusses how the pandemic accelerated the VA’s research enterprise strategy and enacted a response, highlighting the advantages and strengths of this direction. We demonstrate how this evolving strategy enabled the VA to quickly leverage partnerships during a health emergency. While the ORD and VA Research have been used interchangeably, we will attempt to distinguish between the office that serves as headquarters for the national enterprise—the ORD—and the components of that enterprise composed of scientific personnel, equipment, operational units, and partners—VA Research. Finally, we present lessons from this experience toward a broader, post–COVID-19, enterprise-wide approach that the VA has for providing evidence-based care. These experiences may enrich our understanding of postpandemic future research opportunities with the VA as a leader and partner who leverages its commitment to veterans to improve the nation’s health.
ORGANIZING THE VA COVID-19 RESEARCH RESPONSE
VA Research seeks to internally standardize and integrate collaborations with clinical and operational partners throughout the agency. When possible, it seeks to streamline partnership efforts involving external groups less familiar with how the VA operates or its policies, as well as its capabilities. This need was more obvious during the pandemic, and the ORD assembled its COVID-19 response quickly.6
In early January 2020, VA offices, including the ORD, were carefully observing COVID-19. On March 4, 2020, a week before the World Health Organization declared COVID-19 a pandemic, the ORD and its National Research Advisory Council arranged a briefing from VA public health leaders to deal with reported cases of COVID-19 and VA plans. Immediately afterward, the ORD Chief Research and Development Officer gathered a team of experts in clinical research, infectious disease, and public health to strategize a broader research enterprise approach to the pandemic. This group quickly framed 3 key targets: (1) identify critical research questions to prioritize; (2) provide operational guidance to the research community; and (3) uphold VA research staff safety. This discussion led to the creation of a larger ORD COVID-19 Research Response Team that managed activities within this scope. This team included other ORD leaders and staff with operational, scientific, and regulatory expertise charged with enterprise-level planning and execution for all research activities addressing or affected by the pandemic (Figure).
Effective and timely communication was chief among key ORD responsibilities. On March 19, 2020, the Response Team informed the VA Research community about ORD plans for organizing the VA COVID-19 research response.7 It also mobilized VA research programs and investigators to support an enterprise approach that would be coordinated centrally. We achieved communication goals by developing a dedicated website, which provided a means to distribute up-to-date notices and guidance, answer frequently asked questions, and alert investigators about research opportunities. The site enabled the field to report on its efforts, which enhanced leadership and community awareness. A working group of ORD and field personnel managed communications. Given the volume of existing non–COVID-19 research, we established a research continuity of operations plan to provide guidelines for study participant and research staff safety. The ORD issued an unprecedented full-stop administrative hold on in-person research activities after the global announcement of the pandemic. This policy provided formal protections for research programs to safeguard staff and research participants and to determine appropriate alternatives to conduct research activities within necessary social distancing, safety, and other clinical care parameters. It also aligned with guidance and requirements that local VAMCs issued for their operations and care priorities.
The Response Team also established a scientific steering committee of VA infectious disease, critical care, informatics, and epidemiology experts to prioritize research questions, identify research opportunities, and evaluate proposals using a modified expeditious scientific review process. This group also minimized duplicate scientific efforts that might be expected from a large pool of investigators simultaneously pursuing similar research questions. Committee recommendations set up a portfolio that included basic science efforts in diagnostics, clinical trials, population studies, and research infrastructure.
Leveraging Existing Infrastructure
Besides quickly organizing a central touchpoint for the VA COVID-19 research response, the ORD capitalized on its extensive nationwide infrastructure. One key component was the Cooperative Studies Program (CSP); the longstanding VA clinical research enterprise that supports the planning and conduct of large multicenter clinical trials and epidemiological studies. The CSP includes experts at 5 data and statistical coordinating centers, a clinical research pharmacy coordinating center, and 4 epidemiological resource centers.8 CSP studies provide definitive evidence for clinical practice and care of veterans and the nation. CSP’s CONFIRM trial (CSP 577) is the largest VA interventional study with > 50,000 veterans.9 CONFIRM followed the Trial of Varicella Zoster Vaccine for the Prevention of Herpes Zoster and Its Complications (CSP 403), which involved > 38,000 participants to evaluate a vaccine to reduce the burden of illness-associated herpes zoster (shingles). In the study, the vaccine markedly reduced the shingles burden of illness among older adults.10 These studies highlight the CSP cohort development ability as evidenced by the Million Veteran Program.11
VA Research, particularly through the CSP, contributed to multiple federal actions for COVID-19. The CSP had already established partnerships with federal and industry groups in multisite clinical trials and observational studies. During COVID-19, the ORD established a COVID-19 clinical trial master protocol framework: the VA CoronavirUs Research & Efficacy Studies network.9 The CSP also supported studies by the Coronavirus Prevention Network, the National Institute of Allergy and Infectious Disease (NIAID), and the US Food and Drug Administration (FDA). As such, the VA could translate requirements in working with an industry sponsor on the rapid execution of studies within a federal health care system. Much of the success arose when there was either earlier engagement in planning and/or existing familiarity among parties with operational and regulatory requirements.
Before the pandemic, the ORD had also been working on various external partnerships to increase opportunities for veterans in clinical trial participation, particularly for cancer, which Caroff and colleagues discuss further.12 A newly emerging Partnered Research Program (PRP) offered a strategy for participation in the major COVID-19 vaccine efficacy clinical trials. VA Research, through PRP and CSP, rapidly engaged others and managed critical communication (Table 1). In quickly pivoting to COVID-19 clinical studies, the VA also used the Networks of Dedicated Enrollment Sites (NODES), its site-based, CSP-supported infrastructure of existing investigators and coordinators with clinical, operational, and regulatory proficiency for large trials.13,14 Together, the CSP and PRP solidified the VA’s scientific, operational, and regulatory support basis for working with industry partners and federal agencies to conduct therapeutic and vaccine trials.
Speed, Knowledge, and Safety
The scope of VA Research partnerships covers several goals but can be broadly categorized in the following ways: research aimed at evaluating the efficacy of new treatments; development of infrastructure to facilitate more rapid and innovative approaches to research; and building connections within the health care system to take an enterprise approach to research.
Activities are not limited to COVID-19. The VA partners with federal entities on research primarily through interagency agreements whose authorities are derived from the Economy Act (31 USC § 1535). For industry and nonfederal groups, the VA enters into Cooperative Research and Development Agreements that are rooted in the Federal Technology Transfer Act (15 USC § 3710). Although the VA has experience in each of these processes, COVID-19 prompted many groups, existing partners and new ones, to engage with the VA. Consequently, the ORD needed to quickly understand the complexities of how to handle such engagements on a larger scale. The VA Research enterprise strategy also focused on facilitating these processes.
As part of VA integration goals, ORD leaders engaged VA clinical leaders, especially in Public Health, Preventive Medicine, Pharmacy Benefits Management, and Pathology and Laboratory services. The ORD also worked closely with operational leaders, including those responsible for the Veterans Integrated Service Networks and VAMC chiefs of staff and network chief medical officers. The ORD’s familiarity with coordinating complex activities for research further helped to organize nonresearch responses for clinical needs and resources to support the VA COVID-19 response. The Office of the Under Secretary for Health recognized VA Research’s critical role as part of the VA health care system. In turn, it served as a major champion to drive success among the active research efforts, especially the partnered efforts, responding to COVID-19. Continuously communicating support and offering resources for the agency’s overall COVID-19 response reinforced the positive impact of VA Research that extended beyond its traditional roles. That is, the research component of VHA was highlighted as an integral part of the COVID-19 response along with its clinical operations. This integrated approach was perhaps best demonstrated in a VHA-wide push to start and conduct the national vaccine efficacy trials.
Other COVID-19 research supported by the ORD included participation in the Mayo Clinic–led convalescent plasma expanded access treatment protocol, which had emerged as a potential therapeutic option.15 The ORD provided centralized regulatory support to nearly 100 VAMCs, helping to reduce inconsistencies in protocol approval processes for what was hoped to be a promising treatment for COVID-19.16 This rapid approach to address a real-time treatment option demonstrated the VA Research capability for swift mobilization in an emergency.
The ORD also coordinated with other federal agencies. For example, it collaborated with the US Department of Defense to begin a parallel observational study on COVID-19 infections and potential severe outcomes. The study enrolled > 3000 veterans who are being followed for up to 2 years to better understand the natural history and course of COVID-19.17 Other interagency efforts focused on vaccine and therapeutic trials, including Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) with the National Institutes of Health. In these activities, VA Research helped increase recruitment, particularly of a more diverse patient population, in helping to assess promising treatments.10
Motivated by its expanding portfolio of COVID-19 intervention studies, the VA also created a COVID-19 research registry for all VA investigators. This registry included almost 59,000 veterans who indicated a willingness to volunteer for clinical studies. This registry exemplified a long-standing tradition of veterans willing to serve their nation again in a time of need. Iaquinto and colleagues showcased how VHA programs (eg, Office of Healthcare Innovation and Learning) collaborated by expediting a study on 3D-printed swabs to address supply chain shortages. The study, which involved the FDA, showed that the printed swabs were as effective as commercially available ones.18 It provided evidence supporting the production and dissemination of a greater number of testing swabs to the public while also reducing the cost and time requirements (Table 2).
Altogether, these collaborative efforts advanced a transformative approach within the VA that was already happening but was accelerated by the pandemic. Such activities enabled greater understanding throughout the VA for how research is not merely complementary but an integrated part of how veterans receive health care. By giving opportunities to veterans to participate in studies, especially clinical studies, the VA created a path in which such expectations, understanding, and operations were more fluid.
Future Directions
The VA continues to work for veterans by emphasizing its strategic goals and strengths in clinical, data science, and other pioneering activities at an enterprise level to provide the highest quality evidence for care. These capabilities perpetuate a scientific and learning environment that also builds toward the future by giving junior investigators and others opportunities to work within a national health care setting. In turn, this provides a more focused perspective on endeavors that align with the VA mission through ORD-supported career development, merit review (independent investigator submissions), and CSP.19 Preclinical, health services, genomic, and implementation research were given insights into more effective operational and methodological partnerships to help inform the health care system. The pandemic also served to strengthen our ability to mobilize and prepare even faster for emergencies and other potential disease outbreaks, including newer pandemic concerns (eg, mpox, Ebola) from research and public health perspectives.
Conclusions
Throughout its 100-year history, VA Research has been a critical, enduring institution within the national medical landscape. The ability to collaborate with partners has helped us to design and create even better processes, optimize and maximize our infrastructure, and learn more about common research interests that can be even more responsive to national health care needs. As an enterprise, VA Research also aims to continually learn and expand on these valuable lessons gained from internal, interagency, and industry collaborations to effectively meet and exceed our mission to serve our veterans.
Acknowledgments
The authors acknowledge Daphne Swancutt for her contribution as copywriter for this manuscript.
1. US Department of Veterans Affairs. Functional organization manual: description of organization, structure, missions, functions, tasks, and authorities. Version 6. 2020. Accessed September 11, 2023. https://www.va.gov/VA-Functional-Organization-Manual-2020-4.pdf
2. Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. Published 2022 Aug 16. doi:10.1002/lrh2.10333
3. O’Leary TJ, Dominitz JA, Chang KM. Veterans Affairs office of research and development: research programs and emerging opportunities in digestive diseases research. Gastroenterology. 2015;149(7):1652-1661. doi:10.1053/j.gastro.2015.10.021
4. Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veterans Administration and armed forces of the U.S.A. An account of the evolving education of the physician in clinical pharmacology. Bibl Tuberc. 1960;15:1-68.
5. Hays MT; Veterans Health Administration. A historical look at the establishment of the Department of Veterans Affairs research & development program. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
6. US Department of Veterans Affairs, Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report – annex a. May 10, 2021. Accessed September 11, 2023. https://www.va.gov/health/docs/VHA-COVID-19-Response-2021.pdf
7. US Department of Veterans Affairs, Veterans Health Administration. ORD Research Response to COVID-19. US Department of Veterans Affairs. Updated March 24, 2020. Accessed September 11, 2023. www.research.va.gov/programs/orppe/education/webinars/orppe-031920.cfm
8. Burnaska DR, Huang GD, O’Leary TJ. Clinical trials proposed for the VA cooperative studies program: success rates and factors impacting approval. Contemp Clin Trials Commun. 2021;23:100811. Published 2021 Jul 9. doi:10.1016/j.conctc.2021.100811
9. US Department of Veterans Affairs. VA CoronavirUs Research & Efficacy Studies (VA CURES). Updated January 6, 2022. Accessed September 11, 2023. https://www.research.va.gov/services/csrd/va_cures/default.cfm
10. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:10.1056/NEJMoa051016
11. Whitbourne SB, Moser J, Cho K, et al. Leveraging the Million Veteran Program infrastructure and data for a rapid research response to COVID-19. Fed Pract. 2023;40(suppl 5):S23-S28. doi:10.12788/fp.0416
12. Caroff K, Davey V, Smyth M, et al. VA lessons from partnering in COVID-19 clinical trials. Fed Pract. 2023;40(suppl 5): S18-S22. doi:10.12788/fp.0415
13. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ network of dedicated enrollment sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
14. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
15. Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. ClinicalTrials.gov identifier: NCT04338360. April 8, 2020. Updated September 2, 2020. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT04338360
16. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-4797. doi:10.1172/JCI140200
17. Lee JS, Smith NL. Epidemiology, immunology and clinical characteristics of COVID-19 (EPIC3). ClinicalTrials.gov identifier: NCT05764083. March 10, 2023. Updated August 1, 2023. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT05764083
18. Iaquinto J, Ripley B, Dorn PA. How VA innovative partnerships and health care system can respond to national needs: NOSE trial example. Fed Pract. 2023;40(suppl 5):S52-S56. doi:10.12788/fp.0418
19. US Department of Veterans Affairs. Health Services Research & Development research career development program. Updated March 4, 2021. Accessed September 11, 2023. https://hsrd.research.va.gov/cdp/
The US Department of Veterans Affairs (VA) plays a substantial role in the nation’s public health through the Veterans Health Administration (VHA). Its statutory missions of teaching, clinical care, and research enable it to serve a foundational role in the US biomedical enterprise.1 Throughout its extensive network of VA medical centers (VAMCs) and partnering academic affiliates, thousands of clinicians and researchers have been trained to improve the lives of veterans and benefit the lives of all Americans. In supporting the largest US integrated health care system, the VA also has numerous capabilities and resources that distinctively position it to produce scientific and clinical results specifically within the context of providing care. The VA has formed partnerships with other federal agencies, industry, and nonprofit entities. Its ability to be a nexus of health care and practice, scientific discovery, and innovative ways to integrate shared interests in these areas have led to many transformative endeavors that save lives and improve the quality of care for veterans and the public.
The COVID-19 pandemic triggered another mission: service in times of national emergency. Known as the Fourth Mission, the VA rapidly shifted to highlight how its health care and research enterprises could apply strengths in a unique, coordinated manner. While the Fourth Mission is typically considered in the context of clinical care, the VA’s movement toward greater integration facilitated the role of research as a key component in efforts under a learning health care model.2
VA Office of Research and Development
Within the VHA, the Office of Research and Development (ORD) develops research policy and oversees interdisciplinary efforts focused on generating evidence to improve veteran health.3 These activities span at least 100 of 171 VAMCs and include thousands of investigators and staff across all major health research disciplines. Many of these investigators are also clinicians who provide patient care and are experts in the prevention, diagnosis, and treatment of diseases and disorders affecting veterans.
The ORD has invested in a range of scientific, operational, regulatory, and technological assets and infrastructure as part of its enterprise. These strengths come from a nearly 100-year history originating as part of a set of hospital-based medical studies. This established the model for a culture of cooperative research within the VA and with external groups who benefit from the VA’s foundational role in multisite clinical trials.2,4,5 Today, the VA prioritizes bench-to-bedside research covering a broad spectrum of investigations, which are integrated with clinical operations and systems that deliver care.3 The VA supports an extensive range of work that covers core areas in preclinical and clinical studies to health services research, rehabilitation and implementation science, establishing expertise in genomic and data sciences, and more recent activities in artificial intelligence.
In 2017, the ORD began a focused strategy to transform into a national enterprise that capitalized on its place within the VA and its particular ability to translate and implement scientific findings into real impact for veteran health and care through 5 initiatives: (1) enhancing veteran access to high-quality clinical trials; (2) increasing the substantial real-world impact of VA Research; (3) putting VA data to work for veteran health; (4) promoting diversity, equity, and inclusion within our sphere of influence; and (5) building community through research. These activities are interrelated and, where possible, the ORD works with other VA clinical and operational offices to accomplish multiple goals and coordinate within the health care system. As such, the VA continually seeks to increase efficiencies and improve abilities that provide veterans with best-in-class health care. While still in its early stages, this strategy and its initiatives established a path for the ORD response to the pandemic.
Within 2 weeks of the World Health Organization and the US declaring a COVID-19 pandemic, the ORD began to address the developing needs and challenges of the yet unknown emerging public health threat. This included outreach to and contact from federal, academic, and industry partners. At the same time, the ORD maintained its focus and energy to support its ongoing veteran-centric research portfolio and VHA health care system needs across its broad scope of activities.
This article discusses how the pandemic accelerated the VA’s research enterprise strategy and enacted a response, highlighting the advantages and strengths of this direction. We demonstrate how this evolving strategy enabled the VA to quickly leverage partnerships during a health emergency. While the ORD and VA Research have been used interchangeably, we will attempt to distinguish between the office that serves as headquarters for the national enterprise—the ORD—and the components of that enterprise composed of scientific personnel, equipment, operational units, and partners—VA Research. Finally, we present lessons from this experience toward a broader, post–COVID-19, enterprise-wide approach that the VA has for providing evidence-based care. These experiences may enrich our understanding of postpandemic future research opportunities with the VA as a leader and partner who leverages its commitment to veterans to improve the nation’s health.
ORGANIZING THE VA COVID-19 RESEARCH RESPONSE
VA Research seeks to internally standardize and integrate collaborations with clinical and operational partners throughout the agency. When possible, it seeks to streamline partnership efforts involving external groups less familiar with how the VA operates or its policies, as well as its capabilities. This need was more obvious during the pandemic, and the ORD assembled its COVID-19 response quickly.6
In early January 2020, VA offices, including the ORD, were carefully observing COVID-19. On March 4, 2020, a week before the World Health Organization declared COVID-19 a pandemic, the ORD and its National Research Advisory Council arranged a briefing from VA public health leaders to deal with reported cases of COVID-19 and VA plans. Immediately afterward, the ORD Chief Research and Development Officer gathered a team of experts in clinical research, infectious disease, and public health to strategize a broader research enterprise approach to the pandemic. This group quickly framed 3 key targets: (1) identify critical research questions to prioritize; (2) provide operational guidance to the research community; and (3) uphold VA research staff safety. This discussion led to the creation of a larger ORD COVID-19 Research Response Team that managed activities within this scope. This team included other ORD leaders and staff with operational, scientific, and regulatory expertise charged with enterprise-level planning and execution for all research activities addressing or affected by the pandemic (Figure).
Effective and timely communication was chief among key ORD responsibilities. On March 19, 2020, the Response Team informed the VA Research community about ORD plans for organizing the VA COVID-19 research response.7 It also mobilized VA research programs and investigators to support an enterprise approach that would be coordinated centrally. We achieved communication goals by developing a dedicated website, which provided a means to distribute up-to-date notices and guidance, answer frequently asked questions, and alert investigators about research opportunities. The site enabled the field to report on its efforts, which enhanced leadership and community awareness. A working group of ORD and field personnel managed communications. Given the volume of existing non–COVID-19 research, we established a research continuity of operations plan to provide guidelines for study participant and research staff safety. The ORD issued an unprecedented full-stop administrative hold on in-person research activities after the global announcement of the pandemic. This policy provided formal protections for research programs to safeguard staff and research participants and to determine appropriate alternatives to conduct research activities within necessary social distancing, safety, and other clinical care parameters. It also aligned with guidance and requirements that local VAMCs issued for their operations and care priorities.
The Response Team also established a scientific steering committee of VA infectious disease, critical care, informatics, and epidemiology experts to prioritize research questions, identify research opportunities, and evaluate proposals using a modified expeditious scientific review process. This group also minimized duplicate scientific efforts that might be expected from a large pool of investigators simultaneously pursuing similar research questions. Committee recommendations set up a portfolio that included basic science efforts in diagnostics, clinical trials, population studies, and research infrastructure.
Leveraging Existing Infrastructure
Besides quickly organizing a central touchpoint for the VA COVID-19 research response, the ORD capitalized on its extensive nationwide infrastructure. One key component was the Cooperative Studies Program (CSP); the longstanding VA clinical research enterprise that supports the planning and conduct of large multicenter clinical trials and epidemiological studies. The CSP includes experts at 5 data and statistical coordinating centers, a clinical research pharmacy coordinating center, and 4 epidemiological resource centers.8 CSP studies provide definitive evidence for clinical practice and care of veterans and the nation. CSP’s CONFIRM trial (CSP 577) is the largest VA interventional study with > 50,000 veterans.9 CONFIRM followed the Trial of Varicella Zoster Vaccine for the Prevention of Herpes Zoster and Its Complications (CSP 403), which involved > 38,000 participants to evaluate a vaccine to reduce the burden of illness-associated herpes zoster (shingles). In the study, the vaccine markedly reduced the shingles burden of illness among older adults.10 These studies highlight the CSP cohort development ability as evidenced by the Million Veteran Program.11
VA Research, particularly through the CSP, contributed to multiple federal actions for COVID-19. The CSP had already established partnerships with federal and industry groups in multisite clinical trials and observational studies. During COVID-19, the ORD established a COVID-19 clinical trial master protocol framework: the VA CoronavirUs Research & Efficacy Studies network.9 The CSP also supported studies by the Coronavirus Prevention Network, the National Institute of Allergy and Infectious Disease (NIAID), and the US Food and Drug Administration (FDA). As such, the VA could translate requirements in working with an industry sponsor on the rapid execution of studies within a federal health care system. Much of the success arose when there was either earlier engagement in planning and/or existing familiarity among parties with operational and regulatory requirements.
Before the pandemic, the ORD had also been working on various external partnerships to increase opportunities for veterans in clinical trial participation, particularly for cancer, which Caroff and colleagues discuss further.12 A newly emerging Partnered Research Program (PRP) offered a strategy for participation in the major COVID-19 vaccine efficacy clinical trials. VA Research, through PRP and CSP, rapidly engaged others and managed critical communication (Table 1). In quickly pivoting to COVID-19 clinical studies, the VA also used the Networks of Dedicated Enrollment Sites (NODES), its site-based, CSP-supported infrastructure of existing investigators and coordinators with clinical, operational, and regulatory proficiency for large trials.13,14 Together, the CSP and PRP solidified the VA’s scientific, operational, and regulatory support basis for working with industry partners and federal agencies to conduct therapeutic and vaccine trials.
Speed, Knowledge, and Safety
The scope of VA Research partnerships covers several goals but can be broadly categorized in the following ways: research aimed at evaluating the efficacy of new treatments; development of infrastructure to facilitate more rapid and innovative approaches to research; and building connections within the health care system to take an enterprise approach to research.
Activities are not limited to COVID-19. The VA partners with federal entities on research primarily through interagency agreements whose authorities are derived from the Economy Act (31 USC § 1535). For industry and nonfederal groups, the VA enters into Cooperative Research and Development Agreements that are rooted in the Federal Technology Transfer Act (15 USC § 3710). Although the VA has experience in each of these processes, COVID-19 prompted many groups, existing partners and new ones, to engage with the VA. Consequently, the ORD needed to quickly understand the complexities of how to handle such engagements on a larger scale. The VA Research enterprise strategy also focused on facilitating these processes.
As part of VA integration goals, ORD leaders engaged VA clinical leaders, especially in Public Health, Preventive Medicine, Pharmacy Benefits Management, and Pathology and Laboratory services. The ORD also worked closely with operational leaders, including those responsible for the Veterans Integrated Service Networks and VAMC chiefs of staff and network chief medical officers. The ORD’s familiarity with coordinating complex activities for research further helped to organize nonresearch responses for clinical needs and resources to support the VA COVID-19 response. The Office of the Under Secretary for Health recognized VA Research’s critical role as part of the VA health care system. In turn, it served as a major champion to drive success among the active research efforts, especially the partnered efforts, responding to COVID-19. Continuously communicating support and offering resources for the agency’s overall COVID-19 response reinforced the positive impact of VA Research that extended beyond its traditional roles. That is, the research component of VHA was highlighted as an integral part of the COVID-19 response along with its clinical operations. This integrated approach was perhaps best demonstrated in a VHA-wide push to start and conduct the national vaccine efficacy trials.
Other COVID-19 research supported by the ORD included participation in the Mayo Clinic–led convalescent plasma expanded access treatment protocol, which had emerged as a potential therapeutic option.15 The ORD provided centralized regulatory support to nearly 100 VAMCs, helping to reduce inconsistencies in protocol approval processes for what was hoped to be a promising treatment for COVID-19.16 This rapid approach to address a real-time treatment option demonstrated the VA Research capability for swift mobilization in an emergency.
The ORD also coordinated with other federal agencies. For example, it collaborated with the US Department of Defense to begin a parallel observational study on COVID-19 infections and potential severe outcomes. The study enrolled > 3000 veterans who are being followed for up to 2 years to better understand the natural history and course of COVID-19.17 Other interagency efforts focused on vaccine and therapeutic trials, including Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) with the National Institutes of Health. In these activities, VA Research helped increase recruitment, particularly of a more diverse patient population, in helping to assess promising treatments.10
Motivated by its expanding portfolio of COVID-19 intervention studies, the VA also created a COVID-19 research registry for all VA investigators. This registry included almost 59,000 veterans who indicated a willingness to volunteer for clinical studies. This registry exemplified a long-standing tradition of veterans willing to serve their nation again in a time of need. Iaquinto and colleagues showcased how VHA programs (eg, Office of Healthcare Innovation and Learning) collaborated by expediting a study on 3D-printed swabs to address supply chain shortages. The study, which involved the FDA, showed that the printed swabs were as effective as commercially available ones.18 It provided evidence supporting the production and dissemination of a greater number of testing swabs to the public while also reducing the cost and time requirements (Table 2).
Altogether, these collaborative efforts advanced a transformative approach within the VA that was already happening but was accelerated by the pandemic. Such activities enabled greater understanding throughout the VA for how research is not merely complementary but an integrated part of how veterans receive health care. By giving opportunities to veterans to participate in studies, especially clinical studies, the VA created a path in which such expectations, understanding, and operations were more fluid.
Future Directions
The VA continues to work for veterans by emphasizing its strategic goals and strengths in clinical, data science, and other pioneering activities at an enterprise level to provide the highest quality evidence for care. These capabilities perpetuate a scientific and learning environment that also builds toward the future by giving junior investigators and others opportunities to work within a national health care setting. In turn, this provides a more focused perspective on endeavors that align with the VA mission through ORD-supported career development, merit review (independent investigator submissions), and CSP.19 Preclinical, health services, genomic, and implementation research were given insights into more effective operational and methodological partnerships to help inform the health care system. The pandemic also served to strengthen our ability to mobilize and prepare even faster for emergencies and other potential disease outbreaks, including newer pandemic concerns (eg, mpox, Ebola) from research and public health perspectives.
Conclusions
Throughout its 100-year history, VA Research has been a critical, enduring institution within the national medical landscape. The ability to collaborate with partners has helped us to design and create even better processes, optimize and maximize our infrastructure, and learn more about common research interests that can be even more responsive to national health care needs. As an enterprise, VA Research also aims to continually learn and expand on these valuable lessons gained from internal, interagency, and industry collaborations to effectively meet and exceed our mission to serve our veterans.
Acknowledgments
The authors acknowledge Daphne Swancutt for her contribution as copywriter for this manuscript.
The US Department of Veterans Affairs (VA) plays a substantial role in the nation’s public health through the Veterans Health Administration (VHA). Its statutory missions of teaching, clinical care, and research enable it to serve a foundational role in the US biomedical enterprise.1 Throughout its extensive network of VA medical centers (VAMCs) and partnering academic affiliates, thousands of clinicians and researchers have been trained to improve the lives of veterans and benefit the lives of all Americans. In supporting the largest US integrated health care system, the VA also has numerous capabilities and resources that distinctively position it to produce scientific and clinical results specifically within the context of providing care. The VA has formed partnerships with other federal agencies, industry, and nonprofit entities. Its ability to be a nexus of health care and practice, scientific discovery, and innovative ways to integrate shared interests in these areas have led to many transformative endeavors that save lives and improve the quality of care for veterans and the public.
The COVID-19 pandemic triggered another mission: service in times of national emergency. Known as the Fourth Mission, the VA rapidly shifted to highlight how its health care and research enterprises could apply strengths in a unique, coordinated manner. While the Fourth Mission is typically considered in the context of clinical care, the VA’s movement toward greater integration facilitated the role of research as a key component in efforts under a learning health care model.2
VA Office of Research and Development
Within the VHA, the Office of Research and Development (ORD) develops research policy and oversees interdisciplinary efforts focused on generating evidence to improve veteran health.3 These activities span at least 100 of 171 VAMCs and include thousands of investigators and staff across all major health research disciplines. Many of these investigators are also clinicians who provide patient care and are experts in the prevention, diagnosis, and treatment of diseases and disorders affecting veterans.
The ORD has invested in a range of scientific, operational, regulatory, and technological assets and infrastructure as part of its enterprise. These strengths come from a nearly 100-year history originating as part of a set of hospital-based medical studies. This established the model for a culture of cooperative research within the VA and with external groups who benefit from the VA’s foundational role in multisite clinical trials.2,4,5 Today, the VA prioritizes bench-to-bedside research covering a broad spectrum of investigations, which are integrated with clinical operations and systems that deliver care.3 The VA supports an extensive range of work that covers core areas in preclinical and clinical studies to health services research, rehabilitation and implementation science, establishing expertise in genomic and data sciences, and more recent activities in artificial intelligence.
In 2017, the ORD began a focused strategy to transform into a national enterprise that capitalized on its place within the VA and its particular ability to translate and implement scientific findings into real impact for veteran health and care through 5 initiatives: (1) enhancing veteran access to high-quality clinical trials; (2) increasing the substantial real-world impact of VA Research; (3) putting VA data to work for veteran health; (4) promoting diversity, equity, and inclusion within our sphere of influence; and (5) building community through research. These activities are interrelated and, where possible, the ORD works with other VA clinical and operational offices to accomplish multiple goals and coordinate within the health care system. As such, the VA continually seeks to increase efficiencies and improve abilities that provide veterans with best-in-class health care. While still in its early stages, this strategy and its initiatives established a path for the ORD response to the pandemic.
Within 2 weeks of the World Health Organization and the US declaring a COVID-19 pandemic, the ORD began to address the developing needs and challenges of the yet unknown emerging public health threat. This included outreach to and contact from federal, academic, and industry partners. At the same time, the ORD maintained its focus and energy to support its ongoing veteran-centric research portfolio and VHA health care system needs across its broad scope of activities.
This article discusses how the pandemic accelerated the VA’s research enterprise strategy and enacted a response, highlighting the advantages and strengths of this direction. We demonstrate how this evolving strategy enabled the VA to quickly leverage partnerships during a health emergency. While the ORD and VA Research have been used interchangeably, we will attempt to distinguish between the office that serves as headquarters for the national enterprise—the ORD—and the components of that enterprise composed of scientific personnel, equipment, operational units, and partners—VA Research. Finally, we present lessons from this experience toward a broader, post–COVID-19, enterprise-wide approach that the VA has for providing evidence-based care. These experiences may enrich our understanding of postpandemic future research opportunities with the VA as a leader and partner who leverages its commitment to veterans to improve the nation’s health.
ORGANIZING THE VA COVID-19 RESEARCH RESPONSE
VA Research seeks to internally standardize and integrate collaborations with clinical and operational partners throughout the agency. When possible, it seeks to streamline partnership efforts involving external groups less familiar with how the VA operates or its policies, as well as its capabilities. This need was more obvious during the pandemic, and the ORD assembled its COVID-19 response quickly.6
In early January 2020, VA offices, including the ORD, were carefully observing COVID-19. On March 4, 2020, a week before the World Health Organization declared COVID-19 a pandemic, the ORD and its National Research Advisory Council arranged a briefing from VA public health leaders to deal with reported cases of COVID-19 and VA plans. Immediately afterward, the ORD Chief Research and Development Officer gathered a team of experts in clinical research, infectious disease, and public health to strategize a broader research enterprise approach to the pandemic. This group quickly framed 3 key targets: (1) identify critical research questions to prioritize; (2) provide operational guidance to the research community; and (3) uphold VA research staff safety. This discussion led to the creation of a larger ORD COVID-19 Research Response Team that managed activities within this scope. This team included other ORD leaders and staff with operational, scientific, and regulatory expertise charged with enterprise-level planning and execution for all research activities addressing or affected by the pandemic (Figure).
Effective and timely communication was chief among key ORD responsibilities. On March 19, 2020, the Response Team informed the VA Research community about ORD plans for organizing the VA COVID-19 research response.7 It also mobilized VA research programs and investigators to support an enterprise approach that would be coordinated centrally. We achieved communication goals by developing a dedicated website, which provided a means to distribute up-to-date notices and guidance, answer frequently asked questions, and alert investigators about research opportunities. The site enabled the field to report on its efforts, which enhanced leadership and community awareness. A working group of ORD and field personnel managed communications. Given the volume of existing non–COVID-19 research, we established a research continuity of operations plan to provide guidelines for study participant and research staff safety. The ORD issued an unprecedented full-stop administrative hold on in-person research activities after the global announcement of the pandemic. This policy provided formal protections for research programs to safeguard staff and research participants and to determine appropriate alternatives to conduct research activities within necessary social distancing, safety, and other clinical care parameters. It also aligned with guidance and requirements that local VAMCs issued for their operations and care priorities.
The Response Team also established a scientific steering committee of VA infectious disease, critical care, informatics, and epidemiology experts to prioritize research questions, identify research opportunities, and evaluate proposals using a modified expeditious scientific review process. This group also minimized duplicate scientific efforts that might be expected from a large pool of investigators simultaneously pursuing similar research questions. Committee recommendations set up a portfolio that included basic science efforts in diagnostics, clinical trials, population studies, and research infrastructure.
Leveraging Existing Infrastructure
Besides quickly organizing a central touchpoint for the VA COVID-19 research response, the ORD capitalized on its extensive nationwide infrastructure. One key component was the Cooperative Studies Program (CSP); the longstanding VA clinical research enterprise that supports the planning and conduct of large multicenter clinical trials and epidemiological studies. The CSP includes experts at 5 data and statistical coordinating centers, a clinical research pharmacy coordinating center, and 4 epidemiological resource centers.8 CSP studies provide definitive evidence for clinical practice and care of veterans and the nation. CSP’s CONFIRM trial (CSP 577) is the largest VA interventional study with > 50,000 veterans.9 CONFIRM followed the Trial of Varicella Zoster Vaccine for the Prevention of Herpes Zoster and Its Complications (CSP 403), which involved > 38,000 participants to evaluate a vaccine to reduce the burden of illness-associated herpes zoster (shingles). In the study, the vaccine markedly reduced the shingles burden of illness among older adults.10 These studies highlight the CSP cohort development ability as evidenced by the Million Veteran Program.11
VA Research, particularly through the CSP, contributed to multiple federal actions for COVID-19. The CSP had already established partnerships with federal and industry groups in multisite clinical trials and observational studies. During COVID-19, the ORD established a COVID-19 clinical trial master protocol framework: the VA CoronavirUs Research & Efficacy Studies network.9 The CSP also supported studies by the Coronavirus Prevention Network, the National Institute of Allergy and Infectious Disease (NIAID), and the US Food and Drug Administration (FDA). As such, the VA could translate requirements in working with an industry sponsor on the rapid execution of studies within a federal health care system. Much of the success arose when there was either earlier engagement in planning and/or existing familiarity among parties with operational and regulatory requirements.
Before the pandemic, the ORD had also been working on various external partnerships to increase opportunities for veterans in clinical trial participation, particularly for cancer, which Caroff and colleagues discuss further.12 A newly emerging Partnered Research Program (PRP) offered a strategy for participation in the major COVID-19 vaccine efficacy clinical trials. VA Research, through PRP and CSP, rapidly engaged others and managed critical communication (Table 1). In quickly pivoting to COVID-19 clinical studies, the VA also used the Networks of Dedicated Enrollment Sites (NODES), its site-based, CSP-supported infrastructure of existing investigators and coordinators with clinical, operational, and regulatory proficiency for large trials.13,14 Together, the CSP and PRP solidified the VA’s scientific, operational, and regulatory support basis for working with industry partners and federal agencies to conduct therapeutic and vaccine trials.
Speed, Knowledge, and Safety
The scope of VA Research partnerships covers several goals but can be broadly categorized in the following ways: research aimed at evaluating the efficacy of new treatments; development of infrastructure to facilitate more rapid and innovative approaches to research; and building connections within the health care system to take an enterprise approach to research.
Activities are not limited to COVID-19. The VA partners with federal entities on research primarily through interagency agreements whose authorities are derived from the Economy Act (31 USC § 1535). For industry and nonfederal groups, the VA enters into Cooperative Research and Development Agreements that are rooted in the Federal Technology Transfer Act (15 USC § 3710). Although the VA has experience in each of these processes, COVID-19 prompted many groups, existing partners and new ones, to engage with the VA. Consequently, the ORD needed to quickly understand the complexities of how to handle such engagements on a larger scale. The VA Research enterprise strategy also focused on facilitating these processes.
As part of VA integration goals, ORD leaders engaged VA clinical leaders, especially in Public Health, Preventive Medicine, Pharmacy Benefits Management, and Pathology and Laboratory services. The ORD also worked closely with operational leaders, including those responsible for the Veterans Integrated Service Networks and VAMC chiefs of staff and network chief medical officers. The ORD’s familiarity with coordinating complex activities for research further helped to organize nonresearch responses for clinical needs and resources to support the VA COVID-19 response. The Office of the Under Secretary for Health recognized VA Research’s critical role as part of the VA health care system. In turn, it served as a major champion to drive success among the active research efforts, especially the partnered efforts, responding to COVID-19. Continuously communicating support and offering resources for the agency’s overall COVID-19 response reinforced the positive impact of VA Research that extended beyond its traditional roles. That is, the research component of VHA was highlighted as an integral part of the COVID-19 response along with its clinical operations. This integrated approach was perhaps best demonstrated in a VHA-wide push to start and conduct the national vaccine efficacy trials.
Other COVID-19 research supported by the ORD included participation in the Mayo Clinic–led convalescent plasma expanded access treatment protocol, which had emerged as a potential therapeutic option.15 The ORD provided centralized regulatory support to nearly 100 VAMCs, helping to reduce inconsistencies in protocol approval processes for what was hoped to be a promising treatment for COVID-19.16 This rapid approach to address a real-time treatment option demonstrated the VA Research capability for swift mobilization in an emergency.
The ORD also coordinated with other federal agencies. For example, it collaborated with the US Department of Defense to begin a parallel observational study on COVID-19 infections and potential severe outcomes. The study enrolled > 3000 veterans who are being followed for up to 2 years to better understand the natural history and course of COVID-19.17 Other interagency efforts focused on vaccine and therapeutic trials, including Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) with the National Institutes of Health. In these activities, VA Research helped increase recruitment, particularly of a more diverse patient population, in helping to assess promising treatments.10
Motivated by its expanding portfolio of COVID-19 intervention studies, the VA also created a COVID-19 research registry for all VA investigators. This registry included almost 59,000 veterans who indicated a willingness to volunteer for clinical studies. This registry exemplified a long-standing tradition of veterans willing to serve their nation again in a time of need. Iaquinto and colleagues showcased how VHA programs (eg, Office of Healthcare Innovation and Learning) collaborated by expediting a study on 3D-printed swabs to address supply chain shortages. The study, which involved the FDA, showed that the printed swabs were as effective as commercially available ones.18 It provided evidence supporting the production and dissemination of a greater number of testing swabs to the public while also reducing the cost and time requirements (Table 2).
Altogether, these collaborative efforts advanced a transformative approach within the VA that was already happening but was accelerated by the pandemic. Such activities enabled greater understanding throughout the VA for how research is not merely complementary but an integrated part of how veterans receive health care. By giving opportunities to veterans to participate in studies, especially clinical studies, the VA created a path in which such expectations, understanding, and operations were more fluid.
Future Directions
The VA continues to work for veterans by emphasizing its strategic goals and strengths in clinical, data science, and other pioneering activities at an enterprise level to provide the highest quality evidence for care. These capabilities perpetuate a scientific and learning environment that also builds toward the future by giving junior investigators and others opportunities to work within a national health care setting. In turn, this provides a more focused perspective on endeavors that align with the VA mission through ORD-supported career development, merit review (independent investigator submissions), and CSP.19 Preclinical, health services, genomic, and implementation research were given insights into more effective operational and methodological partnerships to help inform the health care system. The pandemic also served to strengthen our ability to mobilize and prepare even faster for emergencies and other potential disease outbreaks, including newer pandemic concerns (eg, mpox, Ebola) from research and public health perspectives.
Conclusions
Throughout its 100-year history, VA Research has been a critical, enduring institution within the national medical landscape. The ability to collaborate with partners has helped us to design and create even better processes, optimize and maximize our infrastructure, and learn more about common research interests that can be even more responsive to national health care needs. As an enterprise, VA Research also aims to continually learn and expand on these valuable lessons gained from internal, interagency, and industry collaborations to effectively meet and exceed our mission to serve our veterans.
Acknowledgments
The authors acknowledge Daphne Swancutt for her contribution as copywriter for this manuscript.
1. US Department of Veterans Affairs. Functional organization manual: description of organization, structure, missions, functions, tasks, and authorities. Version 6. 2020. Accessed September 11, 2023. https://www.va.gov/VA-Functional-Organization-Manual-2020-4.pdf
2. Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. Published 2022 Aug 16. doi:10.1002/lrh2.10333
3. O’Leary TJ, Dominitz JA, Chang KM. Veterans Affairs office of research and development: research programs and emerging opportunities in digestive diseases research. Gastroenterology. 2015;149(7):1652-1661. doi:10.1053/j.gastro.2015.10.021
4. Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veterans Administration and armed forces of the U.S.A. An account of the evolving education of the physician in clinical pharmacology. Bibl Tuberc. 1960;15:1-68.
5. Hays MT; Veterans Health Administration. A historical look at the establishment of the Department of Veterans Affairs research & development program. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
6. US Department of Veterans Affairs, Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report – annex a. May 10, 2021. Accessed September 11, 2023. https://www.va.gov/health/docs/VHA-COVID-19-Response-2021.pdf
7. US Department of Veterans Affairs, Veterans Health Administration. ORD Research Response to COVID-19. US Department of Veterans Affairs. Updated March 24, 2020. Accessed September 11, 2023. www.research.va.gov/programs/orppe/education/webinars/orppe-031920.cfm
8. Burnaska DR, Huang GD, O’Leary TJ. Clinical trials proposed for the VA cooperative studies program: success rates and factors impacting approval. Contemp Clin Trials Commun. 2021;23:100811. Published 2021 Jul 9. doi:10.1016/j.conctc.2021.100811
9. US Department of Veterans Affairs. VA CoronavirUs Research & Efficacy Studies (VA CURES). Updated January 6, 2022. Accessed September 11, 2023. https://www.research.va.gov/services/csrd/va_cures/default.cfm
10. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:10.1056/NEJMoa051016
11. Whitbourne SB, Moser J, Cho K, et al. Leveraging the Million Veteran Program infrastructure and data for a rapid research response to COVID-19. Fed Pract. 2023;40(suppl 5):S23-S28. doi:10.12788/fp.0416
12. Caroff K, Davey V, Smyth M, et al. VA lessons from partnering in COVID-19 clinical trials. Fed Pract. 2023;40(suppl 5): S18-S22. doi:10.12788/fp.0415
13. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ network of dedicated enrollment sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
14. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
15. Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. ClinicalTrials.gov identifier: NCT04338360. April 8, 2020. Updated September 2, 2020. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT04338360
16. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-4797. doi:10.1172/JCI140200
17. Lee JS, Smith NL. Epidemiology, immunology and clinical characteristics of COVID-19 (EPIC3). ClinicalTrials.gov identifier: NCT05764083. March 10, 2023. Updated August 1, 2023. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT05764083
18. Iaquinto J, Ripley B, Dorn PA. How VA innovative partnerships and health care system can respond to national needs: NOSE trial example. Fed Pract. 2023;40(suppl 5):S52-S56. doi:10.12788/fp.0418
19. US Department of Veterans Affairs. Health Services Research & Development research career development program. Updated March 4, 2021. Accessed September 11, 2023. https://hsrd.research.va.gov/cdp/
1. US Department of Veterans Affairs. Functional organization manual: description of organization, structure, missions, functions, tasks, and authorities. Version 6. 2020. Accessed September 11, 2023. https://www.va.gov/VA-Functional-Organization-Manual-2020-4.pdf
2. Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. Published 2022 Aug 16. doi:10.1002/lrh2.10333
3. O’Leary TJ, Dominitz JA, Chang KM. Veterans Affairs office of research and development: research programs and emerging opportunities in digestive diseases research. Gastroenterology. 2015;149(7):1652-1661. doi:10.1053/j.gastro.2015.10.021
4. Tucker WB. The evolution of the cooperative studies in the chemotherapy of tuberculosis of the Veterans Administration and armed forces of the U.S.A. An account of the evolving education of the physician in clinical pharmacology. Bibl Tuberc. 1960;15:1-68.
5. Hays MT; Veterans Health Administration. A historical look at the establishment of the Department of Veterans Affairs research & development program. https://www.research.va.gov/pubs/docs/ORD-85yrHistory.pdf
6. US Department of Veterans Affairs, Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report – annex a. May 10, 2021. Accessed September 11, 2023. https://www.va.gov/health/docs/VHA-COVID-19-Response-2021.pdf
7. US Department of Veterans Affairs, Veterans Health Administration. ORD Research Response to COVID-19. US Department of Veterans Affairs. Updated March 24, 2020. Accessed September 11, 2023. www.research.va.gov/programs/orppe/education/webinars/orppe-031920.cfm
8. Burnaska DR, Huang GD, O’Leary TJ. Clinical trials proposed for the VA cooperative studies program: success rates and factors impacting approval. Contemp Clin Trials Commun. 2021;23:100811. Published 2021 Jul 9. doi:10.1016/j.conctc.2021.100811
9. US Department of Veterans Affairs. VA CoronavirUs Research & Efficacy Studies (VA CURES). Updated January 6, 2022. Accessed September 11, 2023. https://www.research.va.gov/services/csrd/va_cures/default.cfm
10. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. doi:10.1056/NEJMoa051016
11. Whitbourne SB, Moser J, Cho K, et al. Leveraging the Million Veteran Program infrastructure and data for a rapid research response to COVID-19. Fed Pract. 2023;40(suppl 5):S23-S28. doi:10.12788/fp.0416
12. Caroff K, Davey V, Smyth M, et al. VA lessons from partnering in COVID-19 clinical trials. Fed Pract. 2023;40(suppl 5): S18-S22. doi:10.12788/fp.0415
13. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: the Department of Veterans Affairs’ network of dedicated enrollment sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
14. McClure J, Asghar A, Krajec A, et al. Clinical trial facilitators: a novel approach to support the execution of clinical research at the study site level. Contemp Clin Trials Commun. 2023;33:101106. doi:10.1016/j.conctc.2023.101106
15. Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. ClinicalTrials.gov identifier: NCT04338360. April 8, 2020. Updated September 2, 2020. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT04338360
16. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-4797. doi:10.1172/JCI140200
17. Lee JS, Smith NL. Epidemiology, immunology and clinical characteristics of COVID-19 (EPIC3). ClinicalTrials.gov identifier: NCT05764083. March 10, 2023. Updated August 1, 2023. Accessed September 11, 2023. https://clinicaltrials.gov/ct2/show/NCT05764083
18. Iaquinto J, Ripley B, Dorn PA. How VA innovative partnerships and health care system can respond to national needs: NOSE trial example. Fed Pract. 2023;40(suppl 5):S52-S56. doi:10.12788/fp.0418
19. US Department of Veterans Affairs. Health Services Research & Development research career development program. Updated March 4, 2021. Accessed September 11, 2023. https://hsrd.research.va.gov/cdp/
Introduction
Bad times have a scientific value. These are occasions a good learner would not miss.
Ralph Waldo Emerson
Like the flip of a light switch, the world in March 2020 went into lockdown. Suddenly the novel coronavirus disease (COVID-19) was ever-present and everywhere. At a time when very little was certain, scientific inquiry—along with its related skills and disciplines—offered a much-needed pathway for navigating the virus’s myriad unknowns.
From the pandemic’s onset, the Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) made singular contributions to the advancement and acceleration of national and international research activity. This special issue of Federal Practitioner demonstrates how the VHA, through its Office of Research and Development (ORD), took advantage of its newly deployed enterprise strategy to meet the unprecedented demands of this public health emergency.
Launched in 2017, the ORD enterprise strategy enabled the VHA not only to capitalize on existing collaborations—both internal and external—but also move swiftly in forging new ones. Additionally, the strategy was key to leveraging unique VHA assets as the nation’s largest integrated health care system, including: (1) nationwide clinical trials infrastructure, including its longstanding Cooperative Studies Program; (2) a tightly integrated system of clinical care and research that serves as a ready platform for big data science, the world’s largest genomic database, and emergent capabilities; and (3) an established innovation ecosystem that worked with VA research to address rapidly changing circumstances.
In The VA Research Enterprise (p. S12), Garcia and colleagues demonstrate how the VHA pandemic response “arose from an enterprise strategy that was already in motion and aimed at identifying needs for supporting the clinical care mission, more rapidly leveraging resources, and coordinating research across the national VA health care system.” Thus, the VHA took a “model for a culture of cooperative research within the VA and with external groups” and translated it beyond the scope of clinical trials, which had been its foundation.
Led by Chief Research and Development Officer Rachel Ramoni, DMD, ScD, this strategy forged 121 VA medical centers conducting research into an integrated enterprise that could respond to needs for scientific evidence in a coordinated fashion, thereby translating research into practice for real impact on veterans. This approach built on relationships with not only scientific communities but also clinical and operational partners working within the VA to address the immediate pandemic-related needs.
In tandem with its physical infrastructure, the VA’s longstanding network of collaborators, physical infrastructure, and ability to develop new partnerships became drivers of success. Because of previous, ongoing, multisite clinical trials and observational studies, the VA had already partnered with numerous federal government agencies and industry groups and was able to quickly set up a VA COVID-19 clinical trial master protocol framework called the CURES (VA Coronavirus Research and Efficacy studies) network. The ORD enterprise strategy is noted by several other authors, including Caroff and colleagues, who show how the VA efforts to broaden partnerships prepandemic were critical to its participation in 7 large-scale COVID-19 therapeutic and vaccine trials (p. S18).
Similarly, in discussing the VA Million Veteran Program (MVP), Whitbourne and colleagues (p. S23) demonstrate how the VA research strategy and infrastructure were key to leveraging “unique MVP and VA electronic health record data to drive rapid scientific discovery and inform clinical operations.”
Launched in 2011, the MVP is one of the world’s largest genomic cohorts, with more than 985,000 veterans enrolled. MVP developers had the prescience to foresee how a robust genomic database could inform public health emergencies. Whitbourne and colleagues show the many ways the MVP facilitated the VHA COVID-19 response. By extending the MVP centralized recruitment and enrollment infrastructure, an ORD COVID-19 volunteer registry successfully registered 50,000 veterans interested in volunteering for clinical trials.
This tight integration between research and clinical care is one of the VHA’s greatest assets as a health care system. More than 60% of VA researchers are also clinicians who provide direct patient care. This enables VA physician-researchers to learn directly from veteran patients and quickly translate new findings into improved care. It also supported numerous capabilities that played a key role during the pandemic.
For example, in the article VA Big Data Science (p. S39), Young-Xu and colleagues note that the VA use of health care data proved medical research could be performed “quickly and judiciously.” Foundational to this research was a data sharing framework, electronic health record, and VA Corporate Data Warehouse that were accessible to all VA researchers. Researchers had access to clinical data and patient health records that allowed them to perform targeted, time-sensitive research. By building a cohort of 1,363,180 veterans who received ≥ 1 vaccine dose by March 7, 2021, VA researchers added significantly to our understanding of the real-world COVID-19 vaccine clinical performance.
In addition to leveraging existing capabilities, VHA clinicians and researchers created new ones in response. Krishnan and colleagues discuss the launch of 2 clinical and research consortiums focused on COVID-19 genomic surveillance (p. S44). SeqFORCE positioned the VHA to rapidly detect emergent variants and better inform the care of patients with COVID-19. SeqCURE focused on the broader study and trends of variants through sequencing.
The tightly integrated nature of VA care also supported the creation of a large-scale biorepository of specimens with accompanying clinical data to advance research and improve diagnostic and therapeutic research. Epstein and colleagues share the developmental history of the VA SHIELD biorepository, its structure, and its current and future contributions to research science (p. S48).
Finally, the same forward-learning culture which gave rise to the ORD enterprise strategy also resulted in an innovation ecosystem that was well established prior to March 2020. Now a firmly established portfolio within the VHA Office of Healthcare Innovation and Learning (OHIL), the VHA Innovation Ecosystem engages frontline clinicians in reimagining veteran health care. Iaquinto and colleagues discuss how the ecosystem’s preexisting partnerships were critical to addressing shortages in personal protective equipment and other vital resources (p. S52). The OHIL provided the quality system and manufacturing oversight and delivery of swabs for testing, while the ORD furnished research infrastructure and human subjects oversight. Together, these offices not only addressed the shortage by producing swabs but also validated the swabs’ safety and efficacy in the clinical setting.
The articles in this special issue chronicle how the VA quickly mobilized its considerable enterprise-wide resources—especially during the pandemic’s acute phases—to contribute to timely veteran, national, and global evidence about what interventions were effective, what factors were associated with better care and outcomes, and how to flip the switch back to a nonemergency response. As Emerson might have observed, the scientific value of these recent “bad times” did not go unnoticed by VHA learners. In addition to catalyzing opportunities that accelerated the VHA enterprise strategy, the pandemic strengthened existing partnerships, led to new ones, and yielded lessons learned. With variants of the virus continuing to circulate, the VHA continues to harness the lessons learned from the emergency response perspective of the pandemic in order to effectively meet and exceed our mission to serve veterans.
The 35 authors whose work is featured in this issue—and their 3665 colleagues across the VHA research enterprise—offer testament not only to the power of scientific inquiry but of dedication to the mission by the individuals whose lives and families were also impacted by the pandemic.
VA Research continues working to unravel the ongoing impact of COVID-19. As the nation observes an increase in cases again, the VA is ready and well positioned to help lead and address needs for this and other public health crises.
Acknowledgments
This special issue is dedicated to Mitchell (Mitch) Mirkin and his enduring legacy at VA Research, helping to make the contributions of VA Research known as broadly as possible. A superb writer and “editor’s editor,” Mitch had an outstanding ability to translate complex scientific findings into layman’s terms. From the start of the pandemic to his unexpected passing in 2022, Mitch was Acting Director of VA Research Communications. He was a key member of the VA Office of Research and Development COVID-19 research response team. His contributions included his work leading to the generation of this Issue.
Bad times have a scientific value. These are occasions a good learner would not miss.
Ralph Waldo Emerson
Like the flip of a light switch, the world in March 2020 went into lockdown. Suddenly the novel coronavirus disease (COVID-19) was ever-present and everywhere. At a time when very little was certain, scientific inquiry—along with its related skills and disciplines—offered a much-needed pathway for navigating the virus’s myriad unknowns.
From the pandemic’s onset, the Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) made singular contributions to the advancement and acceleration of national and international research activity. This special issue of Federal Practitioner demonstrates how the VHA, through its Office of Research and Development (ORD), took advantage of its newly deployed enterprise strategy to meet the unprecedented demands of this public health emergency.
Launched in 2017, the ORD enterprise strategy enabled the VHA not only to capitalize on existing collaborations—both internal and external—but also move swiftly in forging new ones. Additionally, the strategy was key to leveraging unique VHA assets as the nation’s largest integrated health care system, including: (1) nationwide clinical trials infrastructure, including its longstanding Cooperative Studies Program; (2) a tightly integrated system of clinical care and research that serves as a ready platform for big data science, the world’s largest genomic database, and emergent capabilities; and (3) an established innovation ecosystem that worked with VA research to address rapidly changing circumstances.
In The VA Research Enterprise (p. S12), Garcia and colleagues demonstrate how the VHA pandemic response “arose from an enterprise strategy that was already in motion and aimed at identifying needs for supporting the clinical care mission, more rapidly leveraging resources, and coordinating research across the national VA health care system.” Thus, the VHA took a “model for a culture of cooperative research within the VA and with external groups” and translated it beyond the scope of clinical trials, which had been its foundation.
Led by Chief Research and Development Officer Rachel Ramoni, DMD, ScD, this strategy forged 121 VA medical centers conducting research into an integrated enterprise that could respond to needs for scientific evidence in a coordinated fashion, thereby translating research into practice for real impact on veterans. This approach built on relationships with not only scientific communities but also clinical and operational partners working within the VA to address the immediate pandemic-related needs.
In tandem with its physical infrastructure, the VA’s longstanding network of collaborators, physical infrastructure, and ability to develop new partnerships became drivers of success. Because of previous, ongoing, multisite clinical trials and observational studies, the VA had already partnered with numerous federal government agencies and industry groups and was able to quickly set up a VA COVID-19 clinical trial master protocol framework called the CURES (VA Coronavirus Research and Efficacy studies) network. The ORD enterprise strategy is noted by several other authors, including Caroff and colleagues, who show how the VA efforts to broaden partnerships prepandemic were critical to its participation in 7 large-scale COVID-19 therapeutic and vaccine trials (p. S18).
Similarly, in discussing the VA Million Veteran Program (MVP), Whitbourne and colleagues (p. S23) demonstrate how the VA research strategy and infrastructure were key to leveraging “unique MVP and VA electronic health record data to drive rapid scientific discovery and inform clinical operations.”
Launched in 2011, the MVP is one of the world’s largest genomic cohorts, with more than 985,000 veterans enrolled. MVP developers had the prescience to foresee how a robust genomic database could inform public health emergencies. Whitbourne and colleagues show the many ways the MVP facilitated the VHA COVID-19 response. By extending the MVP centralized recruitment and enrollment infrastructure, an ORD COVID-19 volunteer registry successfully registered 50,000 veterans interested in volunteering for clinical trials.
This tight integration between research and clinical care is one of the VHA’s greatest assets as a health care system. More than 60% of VA researchers are also clinicians who provide direct patient care. This enables VA physician-researchers to learn directly from veteran patients and quickly translate new findings into improved care. It also supported numerous capabilities that played a key role during the pandemic.
For example, in the article VA Big Data Science (p. S39), Young-Xu and colleagues note that the VA use of health care data proved medical research could be performed “quickly and judiciously.” Foundational to this research was a data sharing framework, electronic health record, and VA Corporate Data Warehouse that were accessible to all VA researchers. Researchers had access to clinical data and patient health records that allowed them to perform targeted, time-sensitive research. By building a cohort of 1,363,180 veterans who received ≥ 1 vaccine dose by March 7, 2021, VA researchers added significantly to our understanding of the real-world COVID-19 vaccine clinical performance.
In addition to leveraging existing capabilities, VHA clinicians and researchers created new ones in response. Krishnan and colleagues discuss the launch of 2 clinical and research consortiums focused on COVID-19 genomic surveillance (p. S44). SeqFORCE positioned the VHA to rapidly detect emergent variants and better inform the care of patients with COVID-19. SeqCURE focused on the broader study and trends of variants through sequencing.
The tightly integrated nature of VA care also supported the creation of a large-scale biorepository of specimens with accompanying clinical data to advance research and improve diagnostic and therapeutic research. Epstein and colleagues share the developmental history of the VA SHIELD biorepository, its structure, and its current and future contributions to research science (p. S48).
Finally, the same forward-learning culture which gave rise to the ORD enterprise strategy also resulted in an innovation ecosystem that was well established prior to March 2020. Now a firmly established portfolio within the VHA Office of Healthcare Innovation and Learning (OHIL), the VHA Innovation Ecosystem engages frontline clinicians in reimagining veteran health care. Iaquinto and colleagues discuss how the ecosystem’s preexisting partnerships were critical to addressing shortages in personal protective equipment and other vital resources (p. S52). The OHIL provided the quality system and manufacturing oversight and delivery of swabs for testing, while the ORD furnished research infrastructure and human subjects oversight. Together, these offices not only addressed the shortage by producing swabs but also validated the swabs’ safety and efficacy in the clinical setting.
The articles in this special issue chronicle how the VA quickly mobilized its considerable enterprise-wide resources—especially during the pandemic’s acute phases—to contribute to timely veteran, national, and global evidence about what interventions were effective, what factors were associated with better care and outcomes, and how to flip the switch back to a nonemergency response. As Emerson might have observed, the scientific value of these recent “bad times” did not go unnoticed by VHA learners. In addition to catalyzing opportunities that accelerated the VHA enterprise strategy, the pandemic strengthened existing partnerships, led to new ones, and yielded lessons learned. With variants of the virus continuing to circulate, the VHA continues to harness the lessons learned from the emergency response perspective of the pandemic in order to effectively meet and exceed our mission to serve veterans.
The 35 authors whose work is featured in this issue—and their 3665 colleagues across the VHA research enterprise—offer testament not only to the power of scientific inquiry but of dedication to the mission by the individuals whose lives and families were also impacted by the pandemic.
VA Research continues working to unravel the ongoing impact of COVID-19. As the nation observes an increase in cases again, the VA is ready and well positioned to help lead and address needs for this and other public health crises.
Acknowledgments
This special issue is dedicated to Mitchell (Mitch) Mirkin and his enduring legacy at VA Research, helping to make the contributions of VA Research known as broadly as possible. A superb writer and “editor’s editor,” Mitch had an outstanding ability to translate complex scientific findings into layman’s terms. From the start of the pandemic to his unexpected passing in 2022, Mitch was Acting Director of VA Research Communications. He was a key member of the VA Office of Research and Development COVID-19 research response team. His contributions included his work leading to the generation of this Issue.
Bad times have a scientific value. These are occasions a good learner would not miss.
Ralph Waldo Emerson
Like the flip of a light switch, the world in March 2020 went into lockdown. Suddenly the novel coronavirus disease (COVID-19) was ever-present and everywhere. At a time when very little was certain, scientific inquiry—along with its related skills and disciplines—offered a much-needed pathway for navigating the virus’s myriad unknowns.
From the pandemic’s onset, the Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) made singular contributions to the advancement and acceleration of national and international research activity. This special issue of Federal Practitioner demonstrates how the VHA, through its Office of Research and Development (ORD), took advantage of its newly deployed enterprise strategy to meet the unprecedented demands of this public health emergency.
Launched in 2017, the ORD enterprise strategy enabled the VHA not only to capitalize on existing collaborations—both internal and external—but also move swiftly in forging new ones. Additionally, the strategy was key to leveraging unique VHA assets as the nation’s largest integrated health care system, including: (1) nationwide clinical trials infrastructure, including its longstanding Cooperative Studies Program; (2) a tightly integrated system of clinical care and research that serves as a ready platform for big data science, the world’s largest genomic database, and emergent capabilities; and (3) an established innovation ecosystem that worked with VA research to address rapidly changing circumstances.
In The VA Research Enterprise (p. S12), Garcia and colleagues demonstrate how the VHA pandemic response “arose from an enterprise strategy that was already in motion and aimed at identifying needs for supporting the clinical care mission, more rapidly leveraging resources, and coordinating research across the national VA health care system.” Thus, the VHA took a “model for a culture of cooperative research within the VA and with external groups” and translated it beyond the scope of clinical trials, which had been its foundation.
Led by Chief Research and Development Officer Rachel Ramoni, DMD, ScD, this strategy forged 121 VA medical centers conducting research into an integrated enterprise that could respond to needs for scientific evidence in a coordinated fashion, thereby translating research into practice for real impact on veterans. This approach built on relationships with not only scientific communities but also clinical and operational partners working within the VA to address the immediate pandemic-related needs.
In tandem with its physical infrastructure, the VA’s longstanding network of collaborators, physical infrastructure, and ability to develop new partnerships became drivers of success. Because of previous, ongoing, multisite clinical trials and observational studies, the VA had already partnered with numerous federal government agencies and industry groups and was able to quickly set up a VA COVID-19 clinical trial master protocol framework called the CURES (VA Coronavirus Research and Efficacy studies) network. The ORD enterprise strategy is noted by several other authors, including Caroff and colleagues, who show how the VA efforts to broaden partnerships prepandemic were critical to its participation in 7 large-scale COVID-19 therapeutic and vaccine trials (p. S18).
Similarly, in discussing the VA Million Veteran Program (MVP), Whitbourne and colleagues (p. S23) demonstrate how the VA research strategy and infrastructure were key to leveraging “unique MVP and VA electronic health record data to drive rapid scientific discovery and inform clinical operations.”
Launched in 2011, the MVP is one of the world’s largest genomic cohorts, with more than 985,000 veterans enrolled. MVP developers had the prescience to foresee how a robust genomic database could inform public health emergencies. Whitbourne and colleagues show the many ways the MVP facilitated the VHA COVID-19 response. By extending the MVP centralized recruitment and enrollment infrastructure, an ORD COVID-19 volunteer registry successfully registered 50,000 veterans interested in volunteering for clinical trials.
This tight integration between research and clinical care is one of the VHA’s greatest assets as a health care system. More than 60% of VA researchers are also clinicians who provide direct patient care. This enables VA physician-researchers to learn directly from veteran patients and quickly translate new findings into improved care. It also supported numerous capabilities that played a key role during the pandemic.
For example, in the article VA Big Data Science (p. S39), Young-Xu and colleagues note that the VA use of health care data proved medical research could be performed “quickly and judiciously.” Foundational to this research was a data sharing framework, electronic health record, and VA Corporate Data Warehouse that were accessible to all VA researchers. Researchers had access to clinical data and patient health records that allowed them to perform targeted, time-sensitive research. By building a cohort of 1,363,180 veterans who received ≥ 1 vaccine dose by March 7, 2021, VA researchers added significantly to our understanding of the real-world COVID-19 vaccine clinical performance.
In addition to leveraging existing capabilities, VHA clinicians and researchers created new ones in response. Krishnan and colleagues discuss the launch of 2 clinical and research consortiums focused on COVID-19 genomic surveillance (p. S44). SeqFORCE positioned the VHA to rapidly detect emergent variants and better inform the care of patients with COVID-19. SeqCURE focused on the broader study and trends of variants through sequencing.
The tightly integrated nature of VA care also supported the creation of a large-scale biorepository of specimens with accompanying clinical data to advance research and improve diagnostic and therapeutic research. Epstein and colleagues share the developmental history of the VA SHIELD biorepository, its structure, and its current and future contributions to research science (p. S48).
Finally, the same forward-learning culture which gave rise to the ORD enterprise strategy also resulted in an innovation ecosystem that was well established prior to March 2020. Now a firmly established portfolio within the VHA Office of Healthcare Innovation and Learning (OHIL), the VHA Innovation Ecosystem engages frontline clinicians in reimagining veteran health care. Iaquinto and colleagues discuss how the ecosystem’s preexisting partnerships were critical to addressing shortages in personal protective equipment and other vital resources (p. S52). The OHIL provided the quality system and manufacturing oversight and delivery of swabs for testing, while the ORD furnished research infrastructure and human subjects oversight. Together, these offices not only addressed the shortage by producing swabs but also validated the swabs’ safety and efficacy in the clinical setting.
The articles in this special issue chronicle how the VA quickly mobilized its considerable enterprise-wide resources—especially during the pandemic’s acute phases—to contribute to timely veteran, national, and global evidence about what interventions were effective, what factors were associated with better care and outcomes, and how to flip the switch back to a nonemergency response. As Emerson might have observed, the scientific value of these recent “bad times” did not go unnoticed by VHA learners. In addition to catalyzing opportunities that accelerated the VHA enterprise strategy, the pandemic strengthened existing partnerships, led to new ones, and yielded lessons learned. With variants of the virus continuing to circulate, the VHA continues to harness the lessons learned from the emergency response perspective of the pandemic in order to effectively meet and exceed our mission to serve veterans.
The 35 authors whose work is featured in this issue—and their 3665 colleagues across the VHA research enterprise—offer testament not only to the power of scientific inquiry but of dedication to the mission by the individuals whose lives and families were also impacted by the pandemic.
VA Research continues working to unravel the ongoing impact of COVID-19. As the nation observes an increase in cases again, the VA is ready and well positioned to help lead and address needs for this and other public health crises.
Acknowledgments
This special issue is dedicated to Mitchell (Mitch) Mirkin and his enduring legacy at VA Research, helping to make the contributions of VA Research known as broadly as possible. A superb writer and “editor’s editor,” Mitch had an outstanding ability to translate complex scientific findings into layman’s terms. From the start of the pandemic to his unexpected passing in 2022, Mitch was Acting Director of VA Research Communications. He was a key member of the VA Office of Research and Development COVID-19 research response team. His contributions included his work leading to the generation of this Issue.
Foreword: VA Research and COVID-19
Sylvester Norman, a 67-year-old Coast Guard veteran and retired day-care worker from Nashville, Tennessee, volunteered to participate in the US Department of Veterans Affairs (VA) Million Veteran Program (MVP). He and all 4 of his brothers had experienced kidney illness. During the pandemic, Adriana Hung, MD, MPH, an MVP researcher and associate professor of nephrology at Vanderbilt University, noticed that a disproportionate number of Black patients hospitalized with COVID-19 were dying of acute kidney failure. Dr. Hung used data from Norman and other Black veterans provided through the MVP to identify genetic variations in the APOL1 gene linked to kidney disease found in 1 of every 8 people of African descent. Her research proved that a COVID-19 viral infection can trigger these genes and drive a patient’s kidneys to go into failure. Thanks to her research and volunteers like Norman, a new drug targeting APOL1 may soon receive approval from the US Food and Drug Administration (FDA).
This is only one example of the life-saving work conducted by the Veterans Health Administration (VHA) during the pandemic. On January 21, 2020, 1 day after the first confirmed COVID-19 case in the US, the VHA quickly activated its Emergency Management Coordination Cell (EMCC) under a unified command structure with round-the-clock operations to track the evolving risk and plan a response to this once-in-a-century pandemic. A few months later, and before the US declared COVID-19 a pandemic, the VHA research program sprang into action, preparing its community of investigators to address the emerging needs and challenges of the COVID-19 public health crisis. Three years later, although the federal COVID-19 public emergency is declared over, the VHA remains diligent in observing trends and conducting necessary research on the disease as case numbers rise and fall across time.
This special issue of Federal Practitioner showcases the many ways that the VHA successfully leveraged and rapidly mobilized its research enterprise capabilities as part of the national response to COVID-19 and continues to work in this area. As the virus rapidly spread across the country, the VHA research program, overseen by the Office of Research and Development (ORD) and in partnership with other VHA offices, demonstrated the strength and agility that come from being part of a nationwide integrated health care system.
Historically, the VHA has been one of the nation’s leaders in translating medical breakthroughs to the treatment and care of veterans and the nation. Today, the VHA ensures that veterans have increased access to innovative health care solutions by promoting new medical research initiatives, training health care professionals, and developing community partnerships.
As this special issue of Federal Practitioner demonstrates, the VHA’s extraordinary research response to the COVID-19 pandemic was shaped by its ongoing transformation to a full-scale research enterprise; diversity of partnerships with academia, other federal agencies, and industry; extensive infrastructure for funding and quickly ramping up multisite clinical trials; and longstanding partnership with veterans, who volunteer to serve their country twice—first in uniform, and later by volunteering to participate in VA research.
By leveraging these and other assets, VHA investigators have conducted > 900 COVID-19 research projects across 83 VA medical centers, with nearly 3000 VA-affiliated papers published by mid-2023. We have also become a leader in long COVID, generating notable findings using our electronic health record data and filling in the picture with studies that include interviews with thousands of patients, examinations of blood markers, and exploration of the role of genetics. Along the way, the VA collaborated with federal partners, such as the US Department of Defense, by funding a longitudinal research cohort in which 2800 veterans are enrolled. Through this joint effort, researchers will learn more about the natural history and outcomes among veterans affected by COVID-19. This work continues as part of the VA commitment to the health and care of these veterans and nation as a whole.
Additionally, by partnering with veterans, the VA established a research volunteer registry. More than 58,000 veterans volunteered to be contacted to participate in studies if they were eligible. This effort was critical to the VA’s ability to contribute to the vaccine and other therapeutic trials that were seeking approval from the FDA for broader public use. This volunteerism by these veterans showed the nation that the VA is a valuable partner in times of need.
The VA research program remains tightly focused on understanding the long-term impacts of COVID-19. At the same time, the VA is committed to using lessons learned during the crisis in addressing high priorities in veterans’ health care. Among those priorities is fulfilling our mission under the Sergeant First Class Heath Robinson Honoring Our Promise to Address Comprehensive Toxics (PACT) Act of 2022 to improve care for veterans with military environmental exposures. Over the next few years, VA researchers will analyze health care and epidemiologic data to improve the identification and treatment of medical conditions potentially associated with toxic exposures. This work will include analyses of health trends of post-9/11 veterans, cancer rates among veterans, toxic exposure and mental health outcomes, and the health effects of jet fuels.
Our research program also will support the VA priority of hiring faster and more competitively. With many of the 3700 VA-funded principal investigators also serving as faculty at top universities, VA research programs position us to recruit the best and brightest professionals on the cutting edge of health care. These efforts work hand in hand with the clinical training the VA provides to 113,000 health professions trainees, creating a pipeline of clinicians and physician-researchers for the future. Further, these partnerships strengthen the VA’s ability to expand access by connecting veterans to the best, immediate care.
Finally, VA research will continue to be critical to our top clinical priority of preventing veteran suicide. This area of VA research covers a wide and critically important set of topics, such as the use of predictive modeling to determine veterans most at risk as well as studies on substance use disorders and suicidal ideation, among others.
The impressive collection of articles in this special issue provides a snapshot of the large-scale, all-hands approach the VHA adopted during the COVID-19 public health crisis. I am extremely proud of the work you are about to read.
Sylvester Norman, a 67-year-old Coast Guard veteran and retired day-care worker from Nashville, Tennessee, volunteered to participate in the US Department of Veterans Affairs (VA) Million Veteran Program (MVP). He and all 4 of his brothers had experienced kidney illness. During the pandemic, Adriana Hung, MD, MPH, an MVP researcher and associate professor of nephrology at Vanderbilt University, noticed that a disproportionate number of Black patients hospitalized with COVID-19 were dying of acute kidney failure. Dr. Hung used data from Norman and other Black veterans provided through the MVP to identify genetic variations in the APOL1 gene linked to kidney disease found in 1 of every 8 people of African descent. Her research proved that a COVID-19 viral infection can trigger these genes and drive a patient’s kidneys to go into failure. Thanks to her research and volunteers like Norman, a new drug targeting APOL1 may soon receive approval from the US Food and Drug Administration (FDA).
This is only one example of the life-saving work conducted by the Veterans Health Administration (VHA) during the pandemic. On January 21, 2020, 1 day after the first confirmed COVID-19 case in the US, the VHA quickly activated its Emergency Management Coordination Cell (EMCC) under a unified command structure with round-the-clock operations to track the evolving risk and plan a response to this once-in-a-century pandemic. A few months later, and before the US declared COVID-19 a pandemic, the VHA research program sprang into action, preparing its community of investigators to address the emerging needs and challenges of the COVID-19 public health crisis. Three years later, although the federal COVID-19 public emergency is declared over, the VHA remains diligent in observing trends and conducting necessary research on the disease as case numbers rise and fall across time.
This special issue of Federal Practitioner showcases the many ways that the VHA successfully leveraged and rapidly mobilized its research enterprise capabilities as part of the national response to COVID-19 and continues to work in this area. As the virus rapidly spread across the country, the VHA research program, overseen by the Office of Research and Development (ORD) and in partnership with other VHA offices, demonstrated the strength and agility that come from being part of a nationwide integrated health care system.
Historically, the VHA has been one of the nation’s leaders in translating medical breakthroughs to the treatment and care of veterans and the nation. Today, the VHA ensures that veterans have increased access to innovative health care solutions by promoting new medical research initiatives, training health care professionals, and developing community partnerships.
As this special issue of Federal Practitioner demonstrates, the VHA’s extraordinary research response to the COVID-19 pandemic was shaped by its ongoing transformation to a full-scale research enterprise; diversity of partnerships with academia, other federal agencies, and industry; extensive infrastructure for funding and quickly ramping up multisite clinical trials; and longstanding partnership with veterans, who volunteer to serve their country twice—first in uniform, and later by volunteering to participate in VA research.
By leveraging these and other assets, VHA investigators have conducted > 900 COVID-19 research projects across 83 VA medical centers, with nearly 3000 VA-affiliated papers published by mid-2023. We have also become a leader in long COVID, generating notable findings using our electronic health record data and filling in the picture with studies that include interviews with thousands of patients, examinations of blood markers, and exploration of the role of genetics. Along the way, the VA collaborated with federal partners, such as the US Department of Defense, by funding a longitudinal research cohort in which 2800 veterans are enrolled. Through this joint effort, researchers will learn more about the natural history and outcomes among veterans affected by COVID-19. This work continues as part of the VA commitment to the health and care of these veterans and nation as a whole.
Additionally, by partnering with veterans, the VA established a research volunteer registry. More than 58,000 veterans volunteered to be contacted to participate in studies if they were eligible. This effort was critical to the VA’s ability to contribute to the vaccine and other therapeutic trials that were seeking approval from the FDA for broader public use. This volunteerism by these veterans showed the nation that the VA is a valuable partner in times of need.
The VA research program remains tightly focused on understanding the long-term impacts of COVID-19. At the same time, the VA is committed to using lessons learned during the crisis in addressing high priorities in veterans’ health care. Among those priorities is fulfilling our mission under the Sergeant First Class Heath Robinson Honoring Our Promise to Address Comprehensive Toxics (PACT) Act of 2022 to improve care for veterans with military environmental exposures. Over the next few years, VA researchers will analyze health care and epidemiologic data to improve the identification and treatment of medical conditions potentially associated with toxic exposures. This work will include analyses of health trends of post-9/11 veterans, cancer rates among veterans, toxic exposure and mental health outcomes, and the health effects of jet fuels.
Our research program also will support the VA priority of hiring faster and more competitively. With many of the 3700 VA-funded principal investigators also serving as faculty at top universities, VA research programs position us to recruit the best and brightest professionals on the cutting edge of health care. These efforts work hand in hand with the clinical training the VA provides to 113,000 health professions trainees, creating a pipeline of clinicians and physician-researchers for the future. Further, these partnerships strengthen the VA’s ability to expand access by connecting veterans to the best, immediate care.
Finally, VA research will continue to be critical to our top clinical priority of preventing veteran suicide. This area of VA research covers a wide and critically important set of topics, such as the use of predictive modeling to determine veterans most at risk as well as studies on substance use disorders and suicidal ideation, among others.
The impressive collection of articles in this special issue provides a snapshot of the large-scale, all-hands approach the VHA adopted during the COVID-19 public health crisis. I am extremely proud of the work you are about to read.
Sylvester Norman, a 67-year-old Coast Guard veteran and retired day-care worker from Nashville, Tennessee, volunteered to participate in the US Department of Veterans Affairs (VA) Million Veteran Program (MVP). He and all 4 of his brothers had experienced kidney illness. During the pandemic, Adriana Hung, MD, MPH, an MVP researcher and associate professor of nephrology at Vanderbilt University, noticed that a disproportionate number of Black patients hospitalized with COVID-19 were dying of acute kidney failure. Dr. Hung used data from Norman and other Black veterans provided through the MVP to identify genetic variations in the APOL1 gene linked to kidney disease found in 1 of every 8 people of African descent. Her research proved that a COVID-19 viral infection can trigger these genes and drive a patient’s kidneys to go into failure. Thanks to her research and volunteers like Norman, a new drug targeting APOL1 may soon receive approval from the US Food and Drug Administration (FDA).
This is only one example of the life-saving work conducted by the Veterans Health Administration (VHA) during the pandemic. On January 21, 2020, 1 day after the first confirmed COVID-19 case in the US, the VHA quickly activated its Emergency Management Coordination Cell (EMCC) under a unified command structure with round-the-clock operations to track the evolving risk and plan a response to this once-in-a-century pandemic. A few months later, and before the US declared COVID-19 a pandemic, the VHA research program sprang into action, preparing its community of investigators to address the emerging needs and challenges of the COVID-19 public health crisis. Three years later, although the federal COVID-19 public emergency is declared over, the VHA remains diligent in observing trends and conducting necessary research on the disease as case numbers rise and fall across time.
This special issue of Federal Practitioner showcases the many ways that the VHA successfully leveraged and rapidly mobilized its research enterprise capabilities as part of the national response to COVID-19 and continues to work in this area. As the virus rapidly spread across the country, the VHA research program, overseen by the Office of Research and Development (ORD) and in partnership with other VHA offices, demonstrated the strength and agility that come from being part of a nationwide integrated health care system.
Historically, the VHA has been one of the nation’s leaders in translating medical breakthroughs to the treatment and care of veterans and the nation. Today, the VHA ensures that veterans have increased access to innovative health care solutions by promoting new medical research initiatives, training health care professionals, and developing community partnerships.
As this special issue of Federal Practitioner demonstrates, the VHA’s extraordinary research response to the COVID-19 pandemic was shaped by its ongoing transformation to a full-scale research enterprise; diversity of partnerships with academia, other federal agencies, and industry; extensive infrastructure for funding and quickly ramping up multisite clinical trials; and longstanding partnership with veterans, who volunteer to serve their country twice—first in uniform, and later by volunteering to participate in VA research.
By leveraging these and other assets, VHA investigators have conducted > 900 COVID-19 research projects across 83 VA medical centers, with nearly 3000 VA-affiliated papers published by mid-2023. We have also become a leader in long COVID, generating notable findings using our electronic health record data and filling in the picture with studies that include interviews with thousands of patients, examinations of blood markers, and exploration of the role of genetics. Along the way, the VA collaborated with federal partners, such as the US Department of Defense, by funding a longitudinal research cohort in which 2800 veterans are enrolled. Through this joint effort, researchers will learn more about the natural history and outcomes among veterans affected by COVID-19. This work continues as part of the VA commitment to the health and care of these veterans and nation as a whole.
Additionally, by partnering with veterans, the VA established a research volunteer registry. More than 58,000 veterans volunteered to be contacted to participate in studies if they were eligible. This effort was critical to the VA’s ability to contribute to the vaccine and other therapeutic trials that were seeking approval from the FDA for broader public use. This volunteerism by these veterans showed the nation that the VA is a valuable partner in times of need.
The VA research program remains tightly focused on understanding the long-term impacts of COVID-19. At the same time, the VA is committed to using lessons learned during the crisis in addressing high priorities in veterans’ health care. Among those priorities is fulfilling our mission under the Sergeant First Class Heath Robinson Honoring Our Promise to Address Comprehensive Toxics (PACT) Act of 2022 to improve care for veterans with military environmental exposures. Over the next few years, VA researchers will analyze health care and epidemiologic data to improve the identification and treatment of medical conditions potentially associated with toxic exposures. This work will include analyses of health trends of post-9/11 veterans, cancer rates among veterans, toxic exposure and mental health outcomes, and the health effects of jet fuels.
Our research program also will support the VA priority of hiring faster and more competitively. With many of the 3700 VA-funded principal investigators also serving as faculty at top universities, VA research programs position us to recruit the best and brightest professionals on the cutting edge of health care. These efforts work hand in hand with the clinical training the VA provides to 113,000 health professions trainees, creating a pipeline of clinicians and physician-researchers for the future. Further, these partnerships strengthen the VA’s ability to expand access by connecting veterans to the best, immediate care.
Finally, VA research will continue to be critical to our top clinical priority of preventing veteran suicide. This area of VA research covers a wide and critically important set of topics, such as the use of predictive modeling to determine veterans most at risk as well as studies on substance use disorders and suicidal ideation, among others.
The impressive collection of articles in this special issue provides a snapshot of the large-scale, all-hands approach the VHA adopted during the COVID-19 public health crisis. I am extremely proud of the work you are about to read.
Meta-analysis evaluates conventional treatments for RA
Key clinical point: Methotrexate remains the anchor untargeted conventional treatment for rheumatoid arthritis (RA); however, several alternatives are now available in case of suboptimal outcomes or unacceptable adverse events with methotrexate.
Major finding: Methotrexate reduced the imputed tender joint count (TJCi) by 5.18 joints (95% credible interval [CrI] 4.07-6.28 joints) compared with placebo. Cyclophosphamide fared better than methotrexate in terms of TJCi reduction (6.08 joints; 95% CrI 0.44-11.66 joints), but glucocorticoids (−2.54 joints; 95% CrI −5.16 to 0.08 joints) and the remaining drugs showed similar or lower reductions in the TJCi.
Study details: Findings are from a network meta-analysis of 29 interventions investigated in 132 randomized clinical trials including 13,260 patients with RA who were randomly assigned to receive conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids, placebo, or a pharmacologic non-disease-modifying comparator.
Disclosures: This study was funded by grants from the Danish Regions Medicine Fund and other sources. The authors declared no conflicts of interest.
Source: Guski LS et al. Monotreatment with conventional antirheumatic drugs or glucocorticoids in rheumatoid arthritis: A network meta-analysis. JAMA Netw Open. 2023;6(10):e2335950 (Oct 6). doi: 10.1001/jamanetworkopen.2023.35950
Key clinical point: Methotrexate remains the anchor untargeted conventional treatment for rheumatoid arthritis (RA); however, several alternatives are now available in case of suboptimal outcomes or unacceptable adverse events with methotrexate.
Major finding: Methotrexate reduced the imputed tender joint count (TJCi) by 5.18 joints (95% credible interval [CrI] 4.07-6.28 joints) compared with placebo. Cyclophosphamide fared better than methotrexate in terms of TJCi reduction (6.08 joints; 95% CrI 0.44-11.66 joints), but glucocorticoids (−2.54 joints; 95% CrI −5.16 to 0.08 joints) and the remaining drugs showed similar or lower reductions in the TJCi.
Study details: Findings are from a network meta-analysis of 29 interventions investigated in 132 randomized clinical trials including 13,260 patients with RA who were randomly assigned to receive conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids, placebo, or a pharmacologic non-disease-modifying comparator.
Disclosures: This study was funded by grants from the Danish Regions Medicine Fund and other sources. The authors declared no conflicts of interest.
Source: Guski LS et al. Monotreatment with conventional antirheumatic drugs or glucocorticoids in rheumatoid arthritis: A network meta-analysis. JAMA Netw Open. 2023;6(10):e2335950 (Oct 6). doi: 10.1001/jamanetworkopen.2023.35950
Key clinical point: Methotrexate remains the anchor untargeted conventional treatment for rheumatoid arthritis (RA); however, several alternatives are now available in case of suboptimal outcomes or unacceptable adverse events with methotrexate.
Major finding: Methotrexate reduced the imputed tender joint count (TJCi) by 5.18 joints (95% credible interval [CrI] 4.07-6.28 joints) compared with placebo. Cyclophosphamide fared better than methotrexate in terms of TJCi reduction (6.08 joints; 95% CrI 0.44-11.66 joints), but glucocorticoids (−2.54 joints; 95% CrI −5.16 to 0.08 joints) and the remaining drugs showed similar or lower reductions in the TJCi.
Study details: Findings are from a network meta-analysis of 29 interventions investigated in 132 randomized clinical trials including 13,260 patients with RA who were randomly assigned to receive conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids, placebo, or a pharmacologic non-disease-modifying comparator.
Disclosures: This study was funded by grants from the Danish Regions Medicine Fund and other sources. The authors declared no conflicts of interest.
Source: Guski LS et al. Monotreatment with conventional antirheumatic drugs or glucocorticoids in rheumatoid arthritis: A network meta-analysis. JAMA Netw Open. 2023;6(10):e2335950 (Oct 6). doi: 10.1001/jamanetworkopen.2023.35950
Cardiovascular risk linked with JAKi and bDMARD use in RA
Key clinical point: The risk for cardiovascular events was similar with Janus kinase inhibitors (JAKi) and biologic disease-modifying antirheumatic drugs (bDMARD) in patients with rheumatoid arthritis (RA), although the risk may be slightly higher in elderly patients.
Major finding: JAKi vs bDMARD were not associated with a significantly different risk for cardiovascular events (adjusted incidence rate ratio [aIRR] 1.01; P = .965), but with a trend for a higher cardiovascular risk among patients > 65 years old (aIRR 1.24; 95% CI 0.80-1.91) and a lower risk among patients < 65 years old (aIRR 0.70; 95% CI 0.39-1.28).
Study details: Findings are from a retrospective inception cohort study including 15,191 patients with RA from the IQVIA’s Real-World Data Longitudinal Prescription database who had started a new bDMARD or JAKi.
Disclosures: This study was supported by an unrestricted educational grant from Pfizer BV. Two authors declared receiving past grants to the institution from various sources, including Pfizer. The other authors declared no conflicts of interest.
Source: Popa CD et al. Therapy with JAK inhibitors or bDMARDs and the risk of cardiovascular events in the Dutch rheumatoid arthritis population. Rheumatology (Oxford). 2023 (Oct 5). doi: 10.1093/rheumatology/kead531
Key clinical point: The risk for cardiovascular events was similar with Janus kinase inhibitors (JAKi) and biologic disease-modifying antirheumatic drugs (bDMARD) in patients with rheumatoid arthritis (RA), although the risk may be slightly higher in elderly patients.
Major finding: JAKi vs bDMARD were not associated with a significantly different risk for cardiovascular events (adjusted incidence rate ratio [aIRR] 1.01; P = .965), but with a trend for a higher cardiovascular risk among patients > 65 years old (aIRR 1.24; 95% CI 0.80-1.91) and a lower risk among patients < 65 years old (aIRR 0.70; 95% CI 0.39-1.28).
Study details: Findings are from a retrospective inception cohort study including 15,191 patients with RA from the IQVIA’s Real-World Data Longitudinal Prescription database who had started a new bDMARD or JAKi.
Disclosures: This study was supported by an unrestricted educational grant from Pfizer BV. Two authors declared receiving past grants to the institution from various sources, including Pfizer. The other authors declared no conflicts of interest.
Source: Popa CD et al. Therapy with JAK inhibitors or bDMARDs and the risk of cardiovascular events in the Dutch rheumatoid arthritis population. Rheumatology (Oxford). 2023 (Oct 5). doi: 10.1093/rheumatology/kead531
Key clinical point: The risk for cardiovascular events was similar with Janus kinase inhibitors (JAKi) and biologic disease-modifying antirheumatic drugs (bDMARD) in patients with rheumatoid arthritis (RA), although the risk may be slightly higher in elderly patients.
Major finding: JAKi vs bDMARD were not associated with a significantly different risk for cardiovascular events (adjusted incidence rate ratio [aIRR] 1.01; P = .965), but with a trend for a higher cardiovascular risk among patients > 65 years old (aIRR 1.24; 95% CI 0.80-1.91) and a lower risk among patients < 65 years old (aIRR 0.70; 95% CI 0.39-1.28).
Study details: Findings are from a retrospective inception cohort study including 15,191 patients with RA from the IQVIA’s Real-World Data Longitudinal Prescription database who had started a new bDMARD or JAKi.
Disclosures: This study was supported by an unrestricted educational grant from Pfizer BV. Two authors declared receiving past grants to the institution from various sources, including Pfizer. The other authors declared no conflicts of interest.
Source: Popa CD et al. Therapy with JAK inhibitors or bDMARDs and the risk of cardiovascular events in the Dutch rheumatoid arthritis population. Rheumatology (Oxford). 2023 (Oct 5). doi: 10.1093/rheumatology/kead531
Deciphering difficult-to-treat RA in patients receiving b/tsDMARD
Key clinical point: Patients with difficult-to-treat (D2T) rheumatoid arthritis (RA) had a higher disease activity despite treatment with biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) and demonstrated higher withdrawal rates due to inefficacy.
Major finding: Patients with vs without D2T RA had a higher Simplified Disease Activity Index (SDAI; P = .003) and higher withdrawal rates for b/tsDMARD due to inefficacy (P < .001). Higher SDAI (adjusted odds ratio [aOR] 1.06; P = .014), longer disease duration (aOR 1.06; P < .001), and lower prior use of methotrexate (aOR 0.44; P = .008), sulfasalazine (aOR 0.59; P = .003), and leflunomide (aOR 0.67; P = .013) were associated with D2T RA.
Study details: Findings are from a retrospective study including 2321 patients with RA from the Korean College of Rheumatology Biologics Registry who initiated or switched to b/tsDMARD, of which 271 patients had D2T RA.
Disclosures: This study was supported by a grant from the Yuhan Corporation, Seoul. The authors declared no conflicts of interest.
Source: Jung J-Y et al. Unveiling difficult-to-treat rheumatoid arthritis: Long-term impact of biologic or targeted synthetic DMARDs from the KOBIO registry. Arthritis Res Ther. 2023;25:174 (Sep 19). doi: 10.1186/s13075-023-03165-w
Key clinical point: Patients with difficult-to-treat (D2T) rheumatoid arthritis (RA) had a higher disease activity despite treatment with biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) and demonstrated higher withdrawal rates due to inefficacy.
Major finding: Patients with vs without D2T RA had a higher Simplified Disease Activity Index (SDAI; P = .003) and higher withdrawal rates for b/tsDMARD due to inefficacy (P < .001). Higher SDAI (adjusted odds ratio [aOR] 1.06; P = .014), longer disease duration (aOR 1.06; P < .001), and lower prior use of methotrexate (aOR 0.44; P = .008), sulfasalazine (aOR 0.59; P = .003), and leflunomide (aOR 0.67; P = .013) were associated with D2T RA.
Study details: Findings are from a retrospective study including 2321 patients with RA from the Korean College of Rheumatology Biologics Registry who initiated or switched to b/tsDMARD, of which 271 patients had D2T RA.
Disclosures: This study was supported by a grant from the Yuhan Corporation, Seoul. The authors declared no conflicts of interest.
Source: Jung J-Y et al. Unveiling difficult-to-treat rheumatoid arthritis: Long-term impact of biologic or targeted synthetic DMARDs from the KOBIO registry. Arthritis Res Ther. 2023;25:174 (Sep 19). doi: 10.1186/s13075-023-03165-w
Key clinical point: Patients with difficult-to-treat (D2T) rheumatoid arthritis (RA) had a higher disease activity despite treatment with biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) and demonstrated higher withdrawal rates due to inefficacy.
Major finding: Patients with vs without D2T RA had a higher Simplified Disease Activity Index (SDAI; P = .003) and higher withdrawal rates for b/tsDMARD due to inefficacy (P < .001). Higher SDAI (adjusted odds ratio [aOR] 1.06; P = .014), longer disease duration (aOR 1.06; P < .001), and lower prior use of methotrexate (aOR 0.44; P = .008), sulfasalazine (aOR 0.59; P = .003), and leflunomide (aOR 0.67; P = .013) were associated with D2T RA.
Study details: Findings are from a retrospective study including 2321 patients with RA from the Korean College of Rheumatology Biologics Registry who initiated or switched to b/tsDMARD, of which 271 patients had D2T RA.
Disclosures: This study was supported by a grant from the Yuhan Corporation, Seoul. The authors declared no conflicts of interest.
Source: Jung J-Y et al. Unveiling difficult-to-treat rheumatoid arthritis: Long-term impact of biologic or targeted synthetic DMARDs from the KOBIO registry. Arthritis Res Ther. 2023;25:174 (Sep 19). doi: 10.1186/s13075-023-03165-w
Differential joint-specific treatment response to tofacitinib and methotrexate in RA
Key clinical point: Methotrexate-naive patients with rheumatoid arthritis (RA) showed varied joint-specific clinical responses to tofacitinib and methotrexate monotherapies, with those receiving methotrexate demonstrating more radiographic progression in the foot joints despite improved clinical response.
Major finding: At 12 months, tofacitinib vs methotrexate improved the clinical response in most tender and swollen joints, except some foot joints. Methotrexate improved the clinical response in most foot joints; however, radiographic progression was significantly worse with methotrexate vs tofacitinib (P < .05).
Study details: Findings are from a post hoc analysis of the phase 3 ORAL Start trial including 956 methotrexate-naive patients with RA who were randomly assigned to receive monotherapy with 5 mg (n = 373) or 10 mg (n = 397) tofacitinib or methotrexate (n = 186).
Disclosures: This study was sponsored by Pfizer, Inc. Four authors declared being current or former employees or shareholders of Pfizer or Syneos Health. Some authors declared receiving grants, honoraria, or research funding or having other ties with various sources, including Pfizer.
Source: Ciurea A et al. Joint-level responses to tofacitinib and methotrexate: A post hoc analysis of data from ORAL Start. Arthritis Res Ther. 2023;25:185 (Sep 29). doi: 10.1186/s13075-023-03144-1
Key clinical point: Methotrexate-naive patients with rheumatoid arthritis (RA) showed varied joint-specific clinical responses to tofacitinib and methotrexate monotherapies, with those receiving methotrexate demonstrating more radiographic progression in the foot joints despite improved clinical response.
Major finding: At 12 months, tofacitinib vs methotrexate improved the clinical response in most tender and swollen joints, except some foot joints. Methotrexate improved the clinical response in most foot joints; however, radiographic progression was significantly worse with methotrexate vs tofacitinib (P < .05).
Study details: Findings are from a post hoc analysis of the phase 3 ORAL Start trial including 956 methotrexate-naive patients with RA who were randomly assigned to receive monotherapy with 5 mg (n = 373) or 10 mg (n = 397) tofacitinib or methotrexate (n = 186).
Disclosures: This study was sponsored by Pfizer, Inc. Four authors declared being current or former employees or shareholders of Pfizer or Syneos Health. Some authors declared receiving grants, honoraria, or research funding or having other ties with various sources, including Pfizer.
Source: Ciurea A et al. Joint-level responses to tofacitinib and methotrexate: A post hoc analysis of data from ORAL Start. Arthritis Res Ther. 2023;25:185 (Sep 29). doi: 10.1186/s13075-023-03144-1
Key clinical point: Methotrexate-naive patients with rheumatoid arthritis (RA) showed varied joint-specific clinical responses to tofacitinib and methotrexate monotherapies, with those receiving methotrexate demonstrating more radiographic progression in the foot joints despite improved clinical response.
Major finding: At 12 months, tofacitinib vs methotrexate improved the clinical response in most tender and swollen joints, except some foot joints. Methotrexate improved the clinical response in most foot joints; however, radiographic progression was significantly worse with methotrexate vs tofacitinib (P < .05).
Study details: Findings are from a post hoc analysis of the phase 3 ORAL Start trial including 956 methotrexate-naive patients with RA who were randomly assigned to receive monotherapy with 5 mg (n = 373) or 10 mg (n = 397) tofacitinib or methotrexate (n = 186).
Disclosures: This study was sponsored by Pfizer, Inc. Four authors declared being current or former employees or shareholders of Pfizer or Syneos Health. Some authors declared receiving grants, honoraria, or research funding or having other ties with various sources, including Pfizer.
Source: Ciurea A et al. Joint-level responses to tofacitinib and methotrexate: A post hoc analysis of data from ORAL Start. Arthritis Res Ther. 2023;25:185 (Sep 29). doi: 10.1186/s13075-023-03144-1
Risk factors for radiographic progression in bDMARD-treated RA
Key clinical point: Younger age, higher disease activity, prevalent erosions, and monotherapy were significant risk factors for the development of new bone erosions in biologic disease-modifying antirheumatic drug (bDMARD)-treated patients with rheumatoid arthritis (RA).
Major finding: Risk of developing new bone erosions increased with younger age (adjusted odds ratio [aOR] 0.970; P < .001), higher Disease Activity Scores for 28 Joints-C-Reactive Protein (aOR per point increase 5.349; P < .001), presence of erosions at baseline (aOR 7.820; P < .001), and conventional DMARD-naive status (aOR 2.068; P = .033).
Study details: Findings are from a retrospective analysis of prospectively collected data of 578 patients with RA who started bDMARD treatment.
Disclosures: This study did not receive any funding. G Adami, D Gatti, and M Rossini declared receiving personal fees or serving as a consultant or speaker for various sources. The other authors declared no conflicts of interest.
Source: Adami G et al. Factors associated with radiographic progression in rheumatoid arthritis starting biological diseases modifying anti-rheumatic drugs (bDMARDs). Ther Adv Musculoskelet Dis. 2023;15:1759720X231174534 (Sep 28). doi: 10.1177/1759720X231174534
Key clinical point: Younger age, higher disease activity, prevalent erosions, and monotherapy were significant risk factors for the development of new bone erosions in biologic disease-modifying antirheumatic drug (bDMARD)-treated patients with rheumatoid arthritis (RA).
Major finding: Risk of developing new bone erosions increased with younger age (adjusted odds ratio [aOR] 0.970; P < .001), higher Disease Activity Scores for 28 Joints-C-Reactive Protein (aOR per point increase 5.349; P < .001), presence of erosions at baseline (aOR 7.820; P < .001), and conventional DMARD-naive status (aOR 2.068; P = .033).
Study details: Findings are from a retrospective analysis of prospectively collected data of 578 patients with RA who started bDMARD treatment.
Disclosures: This study did not receive any funding. G Adami, D Gatti, and M Rossini declared receiving personal fees or serving as a consultant or speaker for various sources. The other authors declared no conflicts of interest.
Source: Adami G et al. Factors associated with radiographic progression in rheumatoid arthritis starting biological diseases modifying anti-rheumatic drugs (bDMARDs). Ther Adv Musculoskelet Dis. 2023;15:1759720X231174534 (Sep 28). doi: 10.1177/1759720X231174534
Key clinical point: Younger age, higher disease activity, prevalent erosions, and monotherapy were significant risk factors for the development of new bone erosions in biologic disease-modifying antirheumatic drug (bDMARD)-treated patients with rheumatoid arthritis (RA).
Major finding: Risk of developing new bone erosions increased with younger age (adjusted odds ratio [aOR] 0.970; P < .001), higher Disease Activity Scores for 28 Joints-C-Reactive Protein (aOR per point increase 5.349; P < .001), presence of erosions at baseline (aOR 7.820; P < .001), and conventional DMARD-naive status (aOR 2.068; P = .033).
Study details: Findings are from a retrospective analysis of prospectively collected data of 578 patients with RA who started bDMARD treatment.
Disclosures: This study did not receive any funding. G Adami, D Gatti, and M Rossini declared receiving personal fees or serving as a consultant or speaker for various sources. The other authors declared no conflicts of interest.
Source: Adami G et al. Factors associated with radiographic progression in rheumatoid arthritis starting biological diseases modifying anti-rheumatic drugs (bDMARDs). Ther Adv Musculoskelet Dis. 2023;15:1759720X231174534 (Sep 28). doi: 10.1177/1759720X231174534