User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Surgery for Blastomycosis of the Spine
Blastomycosis is a rare fungal infection that primarily produces acute lung infections but may on occasion disseminate to multiple sites, including the skin, bone, central nervous system (CNS), and oropharynx.1-30 In the case of a primary infection of the lung, if there is a high index of suspicion and a thorough diagnostic workup, the diagnosis can be made from sputum or bronchoscopy.24 Patients present with acute pneumonia that either resolves spontaneously or proceeds to chronic pneumonia with extrapulmonary spread to multiple organs, including the spine. Once vertebral involvement occurs, an untreated infection may result in vertebral body destruction and paraspinal and epidural abscess formation followed by neurologic injury and loss of structural integrity of the spine.11,13,17,23,27,29
In this article, we present a case of blastomycosis of the vertebral body and provide a detailed review of the literature concerning this extremely rare infection of the spine. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
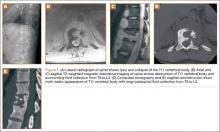
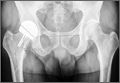
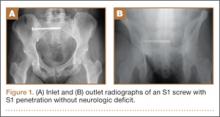
A 30-year-old African American man with known pulmonary blastomycosis, for which he had been treated with oral itraconazole 200 mg twice daily for 6 months, was admitted to the hospital with a 2-month history of mild thoracolumbar back pain. He reported transient numbness and tingling in the lower extremities but no weakness. He denied weight loss, fatigue, appetite loss, and significant night pain. On physical examination, he was alert and oriented, well nourished, and in no acute distress. Percussion revealed limited range of motion and pain. Further examination of the spine demonstrated no spasm, swelling, erythema, or drainage. The lower extremities had intact sensation, motor strength, reflexes, and pulses, and clonus was absent. White blood cell count was 8100 cells/μL (normal), erythrocyte sedimentation rate was 77 mm/h (normal range, 0-20 mm/h), and C-reactive protein level was 57.2 mg/L (normal, ≤ 10 mg/L). The patient was HIV-negative. Chest radiographs were normal except for a small pleural effusion. Radiographs showed a destructive lesion of T11 with an extensive paravertebral and retropleural abscess tracking a spinal level above and below with extension into the spinal canal (Figure 1).
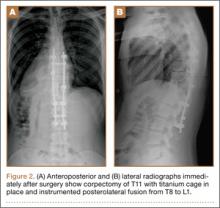
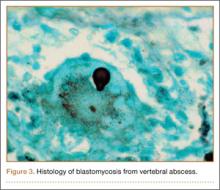
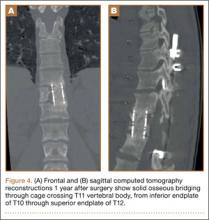
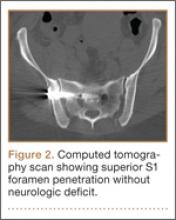
As the patient had signs of spinal cord compression, he was taken to surgery for incision and drainage and culture procurement and corpectomy of T11 with autogenous rib graft. One week later, he was stabilized with posterior fusion and instrumentation (Figure 2). Gram stain of the specimen demonstrated broad-based budding yeast forms 15 to 20 micrometers in size, consistent with blastomycosis. Cultures were positive for Blastomyces dermatitidis. Histopathologic slides (Figure 3) of the surgical pathology specimen showed granulomatous inflammation. Oral itraconazole 200 mg twice daily was continued, as it has been found to be efficacious in treating immunocompetent patients with blastomycosis17 and is considered the medication of choice for non–life-threatening, non-CNS blastomycosis. (Intravenous amphotericin B was ruled out because of its known serious side effects, such as bone marrow suppression and renal function impairment10; itraconazole was the better alternative.) The patient was placed in a thoracolumbar orthosis and discharged. As the effect of presence of instrumentation in the setting of a fungal infection is unknown, it was deemed prudent to maintain the patient on chronic antifungal suppression. One year after surgery, computed tomography (CT) showed solid osseous bridging through the cage crossing the T11 vertebral body, from the inferior endplate of T10 through the superior endplate of T12 (Figure 4). In addition, there had been no recurrence of the spinal infection, and the patient was neurologically intact and doing well.
Discussion
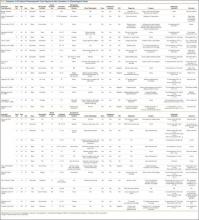
North American blastomycosis (B dermatitidis) is a ubiquitous dimorphic fungus that occurs worldwide and on occasion causes serious infections in humans.9,23,26,29 It was first characterized in 1894 by Gilchrist and Stokes (Gilchrist disease) when they recovered the fungus from the lung tissue of a patient.3 In North America, blastomycosis infections occur from central Canada to the Gulf Coast to east of the Mississippi River.2,5,7,8,13,14,17,21,22,24,27,29 Additional cases of the disease have been reported in Africa,9,16,23,28 Asia,12,19 and South America7,8 (Table [on pages E270-E271]). Recent epidemiologic studies have linked transmission of the disease to bodies of water and have questioned previous reports of male predominance and racial preference for African Americans (Table).
Blastomycosis is acquired when inhaled fungus (airborne conidia spores) causes a primary pulmonary infection or, rarely, when there is direct inoculation through the skin. The differential diagnosis includes neoplasm, tuberculosis, actinomycosis, bacterial infections, cryptococcosis, and coccidioidomycosis.3,9,12,20,25,31 Blastomycosis occurs in adults and children.1-30 The rate of mortality is much higher in immunocompromised patients. Initial symptoms include fever, chills, fatigue, malaise, myalgia, arthalgia, weight loss, and stigmata of chronic disease.1-30 Acute pulmonary infection with blastomycosis generally resolves spontaneously but may progress to acute respiratory distress syndrome, which has a mortality rate of 50% to 89%.19 With systemic dissemination, the infection may spread to other organs11—there is a particular predilection for the skin9,20,29—and to the long bones7,16 and the oropharynx.16,26,28
In 50% to 64% of cases, bone involvement may be the first disease manifestation.6,7,16,22 Osseous involvement with blastomycosis most commonly affects the long bones15 but may include the vertebrae,1-29 the ribs,26 and the carpal or tarsal bones.7,16 The most common vertebral involvement occurs in the thoracic or lumbar spine1,2,7-9,11-14,17,19,21-24,26 and typically results in destruction of the body, development of a paraspinal abscess, and potential extension into the spinal canal, causing an epidural abscess and development of chronic draining cutaneous sinuses.2,7,9,11-13,16,17,19,22,23,26,28,29 In the present case, we do not know whether the vertebral body was involved before the patient presented with mid-thoracolumbar back pain. There may have been bony involvement during initial presentation.
Diagnosis is often difficult because of a low index of suspicion, leading to a significant delay in treatment. Primary pulmonary infections are successfully diagnosed 86% of the time from sputum and 92% of the time from bronchoscopy.19 Once the infection involves the spine, plain radiographs, CT, and magnetic resonance imaging (MRI) can be used to identify not only the bony involvement but also any adjacent soft-tissue extension.13 The radiographic findings, typical of tuberculosis or a neoplasm, include disc space narrowing, vertebral body destruction and collapse, late segmental kyphotic deformity, and development of a psoas abscess or a retropleural abscess.7,26 Such abscesses lend themselves well to fine-needle aspiration,7,8,11,13,14,17,19,26 which, when combined with CT and MRI guidance, reliably assists in the diagnosis of blastomycosis.1,13,17 If fine-needle aspiration fails, then open biopsy and surgical débridement specimens may be effective in the diagnosis.2,9,12,21,22,27
The mortality rate for systemic blastomycosis exceeded 90% before the development of antifungal medications, and these medications remain the primary treatment for most initial infections.15 For severe infections in critically ill patients and for patients with CNS involvement, amphotericin B has been effective, with cure rates approaching 97%.17 Itraconazole, which is well tolerated, has replaced ketoconazole as the preferred long-term oral treatment for blastomycosis. Cure rates for itraconazole approach 90% when treatment is instituted over 2 years in a compliant patient.10,19,20 Nonsurgical (antifungal) treatment for blastomycosis of the spine has also proved successful in neurologically intact patients.7,9,11,26,28
A case involving the spine and requiring surgical drainage was first reported in 19085; since then, only a few more cases have been reported.1,2,5,7-9,11-14,16,17,19,21-24,26-29 Thus, the literature includes very little information that can be used to establish indications for surgery for a blastomycotic infection of the spine. However, there is enough evidence to establish that surgery is indicated for patients who have a known blastomycosis infection and are developing neurologic or structural loss of integrity of the spinal column or have an abscess that requires drainage and débridement.
Our patient had been on long-term antifungal treatment but nevertheless developed a destructive spinal lesion with a concurrent epidural and retropleural abscess. Given his risk of pathologic fracture, we performed anterior débridement and stabilization followed by posterior fusion and instrumentation. We are unaware of any other cases in which an anterior titanium cage was combined with rib autograft after anterior débridement and vertebrectomy combined with posterior instrumentation for blastomycosis. This technique proved very useful, as it allowed for immediate stabilization of the spine. Therefore, the treatment goal is similar to that for any destructive infection that fails medical treatment: preservation of neurologic function, stabilization of spinal vertebrae, débridement of abscess cavity, and definitive culture procurement.
Conclusion
Although there is little reported information regarding surgical indications for blastomycotic vertebral osteomyelitis that has failed medical management—in patients with a destructive lesion and compromise of both the spinal canal and the integrity of the vertebral column—anterior débridement and stabilization followed by posterior fusion and instrumentation are useful in preventing vertebral collapse, further canal compromise, and possible cord injury.
1. Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M. Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases [published correction appears in Acta Cytol. 2006;50(5):600]. Acta Cytol. 2006;50(4):364-371.
2. Arvin MC, Gehring RL, Crecelius JL, Curfman MF. Man with progressive lower back pain. Indiana Med. 1991;84(8):554-556.
3. Baylin GJ, Wear JM. Blastomycosis and actinomycosis of the spine. Am J Roentgenol Radium Ther Nucl Med. 1953;69(3):395-398.
4. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-40.
5. Brewer GE, Wood FC. XII. Blastomycosis of the spine: double lesion: two operations: recovery. Ann Surg. 1908;48(6):889-896.
6. Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologica. 1989;107(1):25-32.
7. Challapalli M, Cunningham DG. North American blastomycosis of the vertebrae in an adolescent. Clin Infect Dis. 1996;23(4):853-854.
8. Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. Vertebral North American blastomycosis. Surg Neurol. 1980;13(4):311-312.
9. Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26(2):203-206.
10. Goodman LS, Brunton LL, Chabner B, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical; 2011.
11. Gottlieb JR, Eismont FJ. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am. 2006;88(4):854-856.
12. Güler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
13. Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7(5):416-421.
14. Hardjasudarma M, Willis B, Black-Payne C, Edwards R. Pediatric spinal blastomycosis: case report. Neurosurgery. 1995;37(3):534-536.
15. Jahangir AA, Heck RK. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop. 2010;39(3):E22-E24.
16. Koen AF, Blumberg LH. North American blastomycosis in South Africa simulating tuberculosis. Clin Radiol. 1999;54(4):260-262.
17. Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis. 1994;26(1):111-115.
18. MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am. 1990;72(6):860-864.
19. Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. Nonsurgical treatment of lumbosacral blastomycosis involving L2–S1: a case report. Spine. 2008;33(13):E442-E446.
20. McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30(2):227-239.
21. Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097-1101.
22. Muñiz AE, Evans T. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med. 2000;19(3):245-248.
23. Osmond JD, Schweitzer G, Dunbar JM, Villet W. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45(16):431-434.
24. Parr AM, Fewer D. Intramedullary blastomycosis in a child: case report. Can J Neurol Sci. 2004;31(2):282-285.
25. Rein MF, Fischetti JL, Sande MA. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis. 1974;109(2):286-289.
26. Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
27. Titrud LA. Blastomycosis of the cervical spine. Minn Med. 1975;58(10):729-732.
28. Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop. 1972;52(4):467-479.
29. Voris HC, Greenwood RC. Blastomycosis of the spine with invasion of the spinal canal. Proc Inst Med Chic. 1947;16(17):463.
30. Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47(3):169-200.
31. Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Pyogenic spondylodiskitis: a radiologic/pathologic and culture correlation study. Arch Pathol Lab Med. 2000;124(5):712-716.
Blastomycosis is a rare fungal infection that primarily produces acute lung infections but may on occasion disseminate to multiple sites, including the skin, bone, central nervous system (CNS), and oropharynx.1-30 In the case of a primary infection of the lung, if there is a high index of suspicion and a thorough diagnostic workup, the diagnosis can be made from sputum or bronchoscopy.24 Patients present with acute pneumonia that either resolves spontaneously or proceeds to chronic pneumonia with extrapulmonary spread to multiple organs, including the spine. Once vertebral involvement occurs, an untreated infection may result in vertebral body destruction and paraspinal and epidural abscess formation followed by neurologic injury and loss of structural integrity of the spine.11,13,17,23,27,29
In this article, we present a case of blastomycosis of the vertebral body and provide a detailed review of the literature concerning this extremely rare infection of the spine. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old African American man with known pulmonary blastomycosis, for which he had been treated with oral itraconazole 200 mg twice daily for 6 months, was admitted to the hospital with a 2-month history of mild thoracolumbar back pain. He reported transient numbness and tingling in the lower extremities but no weakness. He denied weight loss, fatigue, appetite loss, and significant night pain. On physical examination, he was alert and oriented, well nourished, and in no acute distress. Percussion revealed limited range of motion and pain. Further examination of the spine demonstrated no spasm, swelling, erythema, or drainage. The lower extremities had intact sensation, motor strength, reflexes, and pulses, and clonus was absent. White blood cell count was 8100 cells/μL (normal), erythrocyte sedimentation rate was 77 mm/h (normal range, 0-20 mm/h), and C-reactive protein level was 57.2 mg/L (normal, ≤ 10 mg/L). The patient was HIV-negative. Chest radiographs were normal except for a small pleural effusion. Radiographs showed a destructive lesion of T11 with an extensive paravertebral and retropleural abscess tracking a spinal level above and below with extension into the spinal canal (Figure 1).
As the patient had signs of spinal cord compression, he was taken to surgery for incision and drainage and culture procurement and corpectomy of T11 with autogenous rib graft. One week later, he was stabilized with posterior fusion and instrumentation (Figure 2). Gram stain of the specimen demonstrated broad-based budding yeast forms 15 to 20 micrometers in size, consistent with blastomycosis. Cultures were positive for Blastomyces dermatitidis. Histopathologic slides (Figure 3) of the surgical pathology specimen showed granulomatous inflammation. Oral itraconazole 200 mg twice daily was continued, as it has been found to be efficacious in treating immunocompetent patients with blastomycosis17 and is considered the medication of choice for non–life-threatening, non-CNS blastomycosis. (Intravenous amphotericin B was ruled out because of its known serious side effects, such as bone marrow suppression and renal function impairment10; itraconazole was the better alternative.) The patient was placed in a thoracolumbar orthosis and discharged. As the effect of presence of instrumentation in the setting of a fungal infection is unknown, it was deemed prudent to maintain the patient on chronic antifungal suppression. One year after surgery, computed tomography (CT) showed solid osseous bridging through the cage crossing the T11 vertebral body, from the inferior endplate of T10 through the superior endplate of T12 (Figure 4). In addition, there had been no recurrence of the spinal infection, and the patient was neurologically intact and doing well.
Discussion
North American blastomycosis (B dermatitidis) is a ubiquitous dimorphic fungus that occurs worldwide and on occasion causes serious infections in humans.9,23,26,29 It was first characterized in 1894 by Gilchrist and Stokes (Gilchrist disease) when they recovered the fungus from the lung tissue of a patient.3 In North America, blastomycosis infections occur from central Canada to the Gulf Coast to east of the Mississippi River.2,5,7,8,13,14,17,21,22,24,27,29 Additional cases of the disease have been reported in Africa,9,16,23,28 Asia,12,19 and South America7,8 (Table [on pages E270-E271]). Recent epidemiologic studies have linked transmission of the disease to bodies of water and have questioned previous reports of male predominance and racial preference for African Americans (Table).
Blastomycosis is acquired when inhaled fungus (airborne conidia spores) causes a primary pulmonary infection or, rarely, when there is direct inoculation through the skin. The differential diagnosis includes neoplasm, tuberculosis, actinomycosis, bacterial infections, cryptococcosis, and coccidioidomycosis.3,9,12,20,25,31 Blastomycosis occurs in adults and children.1-30 The rate of mortality is much higher in immunocompromised patients. Initial symptoms include fever, chills, fatigue, malaise, myalgia, arthalgia, weight loss, and stigmata of chronic disease.1-30 Acute pulmonary infection with blastomycosis generally resolves spontaneously but may progress to acute respiratory distress syndrome, which has a mortality rate of 50% to 89%.19 With systemic dissemination, the infection may spread to other organs11—there is a particular predilection for the skin9,20,29—and to the long bones7,16 and the oropharynx.16,26,28
In 50% to 64% of cases, bone involvement may be the first disease manifestation.6,7,16,22 Osseous involvement with blastomycosis most commonly affects the long bones15 but may include the vertebrae,1-29 the ribs,26 and the carpal or tarsal bones.7,16 The most common vertebral involvement occurs in the thoracic or lumbar spine1,2,7-9,11-14,17,19,21-24,26 and typically results in destruction of the body, development of a paraspinal abscess, and potential extension into the spinal canal, causing an epidural abscess and development of chronic draining cutaneous sinuses.2,7,9,11-13,16,17,19,22,23,26,28,29 In the present case, we do not know whether the vertebral body was involved before the patient presented with mid-thoracolumbar back pain. There may have been bony involvement during initial presentation.
Diagnosis is often difficult because of a low index of suspicion, leading to a significant delay in treatment. Primary pulmonary infections are successfully diagnosed 86% of the time from sputum and 92% of the time from bronchoscopy.19 Once the infection involves the spine, plain radiographs, CT, and magnetic resonance imaging (MRI) can be used to identify not only the bony involvement but also any adjacent soft-tissue extension.13 The radiographic findings, typical of tuberculosis or a neoplasm, include disc space narrowing, vertebral body destruction and collapse, late segmental kyphotic deformity, and development of a psoas abscess or a retropleural abscess.7,26 Such abscesses lend themselves well to fine-needle aspiration,7,8,11,13,14,17,19,26 which, when combined with CT and MRI guidance, reliably assists in the diagnosis of blastomycosis.1,13,17 If fine-needle aspiration fails, then open biopsy and surgical débridement specimens may be effective in the diagnosis.2,9,12,21,22,27
The mortality rate for systemic blastomycosis exceeded 90% before the development of antifungal medications, and these medications remain the primary treatment for most initial infections.15 For severe infections in critically ill patients and for patients with CNS involvement, amphotericin B has been effective, with cure rates approaching 97%.17 Itraconazole, which is well tolerated, has replaced ketoconazole as the preferred long-term oral treatment for blastomycosis. Cure rates for itraconazole approach 90% when treatment is instituted over 2 years in a compliant patient.10,19,20 Nonsurgical (antifungal) treatment for blastomycosis of the spine has also proved successful in neurologically intact patients.7,9,11,26,28
A case involving the spine and requiring surgical drainage was first reported in 19085; since then, only a few more cases have been reported.1,2,5,7-9,11-14,16,17,19,21-24,26-29 Thus, the literature includes very little information that can be used to establish indications for surgery for a blastomycotic infection of the spine. However, there is enough evidence to establish that surgery is indicated for patients who have a known blastomycosis infection and are developing neurologic or structural loss of integrity of the spinal column or have an abscess that requires drainage and débridement.
Our patient had been on long-term antifungal treatment but nevertheless developed a destructive spinal lesion with a concurrent epidural and retropleural abscess. Given his risk of pathologic fracture, we performed anterior débridement and stabilization followed by posterior fusion and instrumentation. We are unaware of any other cases in which an anterior titanium cage was combined with rib autograft after anterior débridement and vertebrectomy combined with posterior instrumentation for blastomycosis. This technique proved very useful, as it allowed for immediate stabilization of the spine. Therefore, the treatment goal is similar to that for any destructive infection that fails medical treatment: preservation of neurologic function, stabilization of spinal vertebrae, débridement of abscess cavity, and definitive culture procurement.
Conclusion
Although there is little reported information regarding surgical indications for blastomycotic vertebral osteomyelitis that has failed medical management—in patients with a destructive lesion and compromise of both the spinal canal and the integrity of the vertebral column—anterior débridement and stabilization followed by posterior fusion and instrumentation are useful in preventing vertebral collapse, further canal compromise, and possible cord injury.
Blastomycosis is a rare fungal infection that primarily produces acute lung infections but may on occasion disseminate to multiple sites, including the skin, bone, central nervous system (CNS), and oropharynx.1-30 In the case of a primary infection of the lung, if there is a high index of suspicion and a thorough diagnostic workup, the diagnosis can be made from sputum or bronchoscopy.24 Patients present with acute pneumonia that either resolves spontaneously or proceeds to chronic pneumonia with extrapulmonary spread to multiple organs, including the spine. Once vertebral involvement occurs, an untreated infection may result in vertebral body destruction and paraspinal and epidural abscess formation followed by neurologic injury and loss of structural integrity of the spine.11,13,17,23,27,29
In this article, we present a case of blastomycosis of the vertebral body and provide a detailed review of the literature concerning this extremely rare infection of the spine. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old African American man with known pulmonary blastomycosis, for which he had been treated with oral itraconazole 200 mg twice daily for 6 months, was admitted to the hospital with a 2-month history of mild thoracolumbar back pain. He reported transient numbness and tingling in the lower extremities but no weakness. He denied weight loss, fatigue, appetite loss, and significant night pain. On physical examination, he was alert and oriented, well nourished, and in no acute distress. Percussion revealed limited range of motion and pain. Further examination of the spine demonstrated no spasm, swelling, erythema, or drainage. The lower extremities had intact sensation, motor strength, reflexes, and pulses, and clonus was absent. White blood cell count was 8100 cells/μL (normal), erythrocyte sedimentation rate was 77 mm/h (normal range, 0-20 mm/h), and C-reactive protein level was 57.2 mg/L (normal, ≤ 10 mg/L). The patient was HIV-negative. Chest radiographs were normal except for a small pleural effusion. Radiographs showed a destructive lesion of T11 with an extensive paravertebral and retropleural abscess tracking a spinal level above and below with extension into the spinal canal (Figure 1).
As the patient had signs of spinal cord compression, he was taken to surgery for incision and drainage and culture procurement and corpectomy of T11 with autogenous rib graft. One week later, he was stabilized with posterior fusion and instrumentation (Figure 2). Gram stain of the specimen demonstrated broad-based budding yeast forms 15 to 20 micrometers in size, consistent with blastomycosis. Cultures were positive for Blastomyces dermatitidis. Histopathologic slides (Figure 3) of the surgical pathology specimen showed granulomatous inflammation. Oral itraconazole 200 mg twice daily was continued, as it has been found to be efficacious in treating immunocompetent patients with blastomycosis17 and is considered the medication of choice for non–life-threatening, non-CNS blastomycosis. (Intravenous amphotericin B was ruled out because of its known serious side effects, such as bone marrow suppression and renal function impairment10; itraconazole was the better alternative.) The patient was placed in a thoracolumbar orthosis and discharged. As the effect of presence of instrumentation in the setting of a fungal infection is unknown, it was deemed prudent to maintain the patient on chronic antifungal suppression. One year after surgery, computed tomography (CT) showed solid osseous bridging through the cage crossing the T11 vertebral body, from the inferior endplate of T10 through the superior endplate of T12 (Figure 4). In addition, there had been no recurrence of the spinal infection, and the patient was neurologically intact and doing well.
Discussion
North American blastomycosis (B dermatitidis) is a ubiquitous dimorphic fungus that occurs worldwide and on occasion causes serious infections in humans.9,23,26,29 It was first characterized in 1894 by Gilchrist and Stokes (Gilchrist disease) when they recovered the fungus from the lung tissue of a patient.3 In North America, blastomycosis infections occur from central Canada to the Gulf Coast to east of the Mississippi River.2,5,7,8,13,14,17,21,22,24,27,29 Additional cases of the disease have been reported in Africa,9,16,23,28 Asia,12,19 and South America7,8 (Table [on pages E270-E271]). Recent epidemiologic studies have linked transmission of the disease to bodies of water and have questioned previous reports of male predominance and racial preference for African Americans (Table).
Blastomycosis is acquired when inhaled fungus (airborne conidia spores) causes a primary pulmonary infection or, rarely, when there is direct inoculation through the skin. The differential diagnosis includes neoplasm, tuberculosis, actinomycosis, bacterial infections, cryptococcosis, and coccidioidomycosis.3,9,12,20,25,31 Blastomycosis occurs in adults and children.1-30 The rate of mortality is much higher in immunocompromised patients. Initial symptoms include fever, chills, fatigue, malaise, myalgia, arthalgia, weight loss, and stigmata of chronic disease.1-30 Acute pulmonary infection with blastomycosis generally resolves spontaneously but may progress to acute respiratory distress syndrome, which has a mortality rate of 50% to 89%.19 With systemic dissemination, the infection may spread to other organs11—there is a particular predilection for the skin9,20,29—and to the long bones7,16 and the oropharynx.16,26,28
In 50% to 64% of cases, bone involvement may be the first disease manifestation.6,7,16,22 Osseous involvement with blastomycosis most commonly affects the long bones15 but may include the vertebrae,1-29 the ribs,26 and the carpal or tarsal bones.7,16 The most common vertebral involvement occurs in the thoracic or lumbar spine1,2,7-9,11-14,17,19,21-24,26 and typically results in destruction of the body, development of a paraspinal abscess, and potential extension into the spinal canal, causing an epidural abscess and development of chronic draining cutaneous sinuses.2,7,9,11-13,16,17,19,22,23,26,28,29 In the present case, we do not know whether the vertebral body was involved before the patient presented with mid-thoracolumbar back pain. There may have been bony involvement during initial presentation.
Diagnosis is often difficult because of a low index of suspicion, leading to a significant delay in treatment. Primary pulmonary infections are successfully diagnosed 86% of the time from sputum and 92% of the time from bronchoscopy.19 Once the infection involves the spine, plain radiographs, CT, and magnetic resonance imaging (MRI) can be used to identify not only the bony involvement but also any adjacent soft-tissue extension.13 The radiographic findings, typical of tuberculosis or a neoplasm, include disc space narrowing, vertebral body destruction and collapse, late segmental kyphotic deformity, and development of a psoas abscess or a retropleural abscess.7,26 Such abscesses lend themselves well to fine-needle aspiration,7,8,11,13,14,17,19,26 which, when combined with CT and MRI guidance, reliably assists in the diagnosis of blastomycosis.1,13,17 If fine-needle aspiration fails, then open biopsy and surgical débridement specimens may be effective in the diagnosis.2,9,12,21,22,27
The mortality rate for systemic blastomycosis exceeded 90% before the development of antifungal medications, and these medications remain the primary treatment for most initial infections.15 For severe infections in critically ill patients and for patients with CNS involvement, amphotericin B has been effective, with cure rates approaching 97%.17 Itraconazole, which is well tolerated, has replaced ketoconazole as the preferred long-term oral treatment for blastomycosis. Cure rates for itraconazole approach 90% when treatment is instituted over 2 years in a compliant patient.10,19,20 Nonsurgical (antifungal) treatment for blastomycosis of the spine has also proved successful in neurologically intact patients.7,9,11,26,28
A case involving the spine and requiring surgical drainage was first reported in 19085; since then, only a few more cases have been reported.1,2,5,7-9,11-14,16,17,19,21-24,26-29 Thus, the literature includes very little information that can be used to establish indications for surgery for a blastomycotic infection of the spine. However, there is enough evidence to establish that surgery is indicated for patients who have a known blastomycosis infection and are developing neurologic or structural loss of integrity of the spinal column or have an abscess that requires drainage and débridement.
Our patient had been on long-term antifungal treatment but nevertheless developed a destructive spinal lesion with a concurrent epidural and retropleural abscess. Given his risk of pathologic fracture, we performed anterior débridement and stabilization followed by posterior fusion and instrumentation. We are unaware of any other cases in which an anterior titanium cage was combined with rib autograft after anterior débridement and vertebrectomy combined with posterior instrumentation for blastomycosis. This technique proved very useful, as it allowed for immediate stabilization of the spine. Therefore, the treatment goal is similar to that for any destructive infection that fails medical treatment: preservation of neurologic function, stabilization of spinal vertebrae, débridement of abscess cavity, and definitive culture procurement.
Conclusion
Although there is little reported information regarding surgical indications for blastomycotic vertebral osteomyelitis that has failed medical management—in patients with a destructive lesion and compromise of both the spinal canal and the integrity of the vertebral column—anterior débridement and stabilization followed by posterior fusion and instrumentation are useful in preventing vertebral collapse, further canal compromise, and possible cord injury.
1. Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M. Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases [published correction appears in Acta Cytol. 2006;50(5):600]. Acta Cytol. 2006;50(4):364-371.
2. Arvin MC, Gehring RL, Crecelius JL, Curfman MF. Man with progressive lower back pain. Indiana Med. 1991;84(8):554-556.
3. Baylin GJ, Wear JM. Blastomycosis and actinomycosis of the spine. Am J Roentgenol Radium Ther Nucl Med. 1953;69(3):395-398.
4. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-40.
5. Brewer GE, Wood FC. XII. Blastomycosis of the spine: double lesion: two operations: recovery. Ann Surg. 1908;48(6):889-896.
6. Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologica. 1989;107(1):25-32.
7. Challapalli M, Cunningham DG. North American blastomycosis of the vertebrae in an adolescent. Clin Infect Dis. 1996;23(4):853-854.
8. Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. Vertebral North American blastomycosis. Surg Neurol. 1980;13(4):311-312.
9. Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26(2):203-206.
10. Goodman LS, Brunton LL, Chabner B, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical; 2011.
11. Gottlieb JR, Eismont FJ. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am. 2006;88(4):854-856.
12. Güler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
13. Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7(5):416-421.
14. Hardjasudarma M, Willis B, Black-Payne C, Edwards R. Pediatric spinal blastomycosis: case report. Neurosurgery. 1995;37(3):534-536.
15. Jahangir AA, Heck RK. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop. 2010;39(3):E22-E24.
16. Koen AF, Blumberg LH. North American blastomycosis in South Africa simulating tuberculosis. Clin Radiol. 1999;54(4):260-262.
17. Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis. 1994;26(1):111-115.
18. MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am. 1990;72(6):860-864.
19. Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. Nonsurgical treatment of lumbosacral blastomycosis involving L2–S1: a case report. Spine. 2008;33(13):E442-E446.
20. McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30(2):227-239.
21. Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097-1101.
22. Muñiz AE, Evans T. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med. 2000;19(3):245-248.
23. Osmond JD, Schweitzer G, Dunbar JM, Villet W. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45(16):431-434.
24. Parr AM, Fewer D. Intramedullary blastomycosis in a child: case report. Can J Neurol Sci. 2004;31(2):282-285.
25. Rein MF, Fischetti JL, Sande MA. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis. 1974;109(2):286-289.
26. Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
27. Titrud LA. Blastomycosis of the cervical spine. Minn Med. 1975;58(10):729-732.
28. Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop. 1972;52(4):467-479.
29. Voris HC, Greenwood RC. Blastomycosis of the spine with invasion of the spinal canal. Proc Inst Med Chic. 1947;16(17):463.
30. Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47(3):169-200.
31. Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Pyogenic spondylodiskitis: a radiologic/pathologic and culture correlation study. Arch Pathol Lab Med. 2000;124(5):712-716.
1. Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M. Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases [published correction appears in Acta Cytol. 2006;50(5):600]. Acta Cytol. 2006;50(4):364-371.
2. Arvin MC, Gehring RL, Crecelius JL, Curfman MF. Man with progressive lower back pain. Indiana Med. 1991;84(8):554-556.
3. Baylin GJ, Wear JM. Blastomycosis and actinomycosis of the spine. Am J Roentgenol Radium Ther Nucl Med. 1953;69(3):395-398.
4. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-40.
5. Brewer GE, Wood FC. XII. Blastomycosis of the spine: double lesion: two operations: recovery. Ann Surg. 1908;48(6):889-896.
6. Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologica. 1989;107(1):25-32.
7. Challapalli M, Cunningham DG. North American blastomycosis of the vertebrae in an adolescent. Clin Infect Dis. 1996;23(4):853-854.
8. Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. Vertebral North American blastomycosis. Surg Neurol. 1980;13(4):311-312.
9. Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26(2):203-206.
10. Goodman LS, Brunton LL, Chabner B, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical; 2011.
11. Gottlieb JR, Eismont FJ. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am. 2006;88(4):854-856.
12. Güler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
13. Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7(5):416-421.
14. Hardjasudarma M, Willis B, Black-Payne C, Edwards R. Pediatric spinal blastomycosis: case report. Neurosurgery. 1995;37(3):534-536.
15. Jahangir AA, Heck RK. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop. 2010;39(3):E22-E24.
16. Koen AF, Blumberg LH. North American blastomycosis in South Africa simulating tuberculosis. Clin Radiol. 1999;54(4):260-262.
17. Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis. 1994;26(1):111-115.
18. MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am. 1990;72(6):860-864.
19. Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. Nonsurgical treatment of lumbosacral blastomycosis involving L2–S1: a case report. Spine. 2008;33(13):E442-E446.
20. McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30(2):227-239.
21. Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097-1101.
22. Muñiz AE, Evans T. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med. 2000;19(3):245-248.
23. Osmond JD, Schweitzer G, Dunbar JM, Villet W. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45(16):431-434.
24. Parr AM, Fewer D. Intramedullary blastomycosis in a child: case report. Can J Neurol Sci. 2004;31(2):282-285.
25. Rein MF, Fischetti JL, Sande MA. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis. 1974;109(2):286-289.
26. Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
27. Titrud LA. Blastomycosis of the cervical spine. Minn Med. 1975;58(10):729-732.
28. Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop. 1972;52(4):467-479.
29. Voris HC, Greenwood RC. Blastomycosis of the spine with invasion of the spinal canal. Proc Inst Med Chic. 1947;16(17):463.
30. Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47(3):169-200.
31. Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Pyogenic spondylodiskitis: a radiologic/pathologic and culture correlation study. Arch Pathol Lab Med. 2000;124(5):712-716.
Improving Visual Estimates of Cervical Spine Range of Motion
Assessment of cervical spine range of motion (ROM) is an integral aspect of the physical examination for cervical conditions,1-3 surgical outcomes,4 and functional impairment.1 In fact, the emphasis being placed on such functional measures before and after treatments is increasing.4,5
Cervical spine range of motion is routinely used as an outcome measure in clinical studies.6-8 Underscoring the importance of defining cervical spine ROM, studies have found it to be a preoperative predictor of outcomes of anterior cervical surgery,9 and other studies have suggested it is a determinant of athletes’ return to play.10
Spinal ROM measurements can be used to determine the degree of disability experienced by a patient with a spinal condition as defined in the Guides to the Evaluation of Permanent Impairment by the American Medical Association (AMA).1 In the medicolegal realm, ROM measurements made by clinicians can influence the dollar amounts of awards in legal claims, and, according to the AMA guides, the difference in cervical spine ROM between normality and disability or impairment can be as little as 5°.
Although cervical spine ROM is routinely assessed and documented in clinical practice, no universal protocol exists for its evaluation.11,12 In fact, considerable inter-examiner variation in visual estimates of ROM has been found,13-16 and significant inaccuracies have been reported.17,18
Goniometers have been shown to be reliable and highly accurate, with low inter-examiner and intra-examiner variability.5,19-21 Nevertheless, logistics22 and costs21 generally limit their being accepted in routine clinical practice. Among many methods available for assessing ROM, visual estimation is the least reliable or accurate,23 but it is the quickest and least expensive and is recommended in textbooks that describe the spinal-specific physical examination.24 Despite the superiority of goniometers in measuring ROM, these significant barriers have limited their use in clinical practice. When assessing cervical spine ROM, most clinicians prefer visual estimates over goniometers.
We conducted a study to determine whether training could improve the accuracy of visual estimates. We compared the accuracy of visual estimates of cervical spine ROM with that of a radiographically validated electrogoniometer and then investigated whether accuracy and reliability of visual estimates could be improved with a session of instruction and demonstration. Assessments of accuracy were made immediately after and 1 month after this training session.
Materials and Methods
Assessments Made Before Training
This study was approved by our institution’s human investigation committee and was conducted in accordance with the ethical standards of that committee.
Cervical spine ROM was assessed by 8 examiners (2 attending spine surgeons, 4 orthopedic residents, 2 medical students). They were informed they would be participating in a study evaluating visual estimates of motion but were given no other information prior to the study.
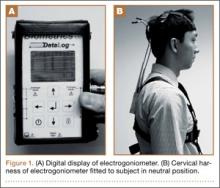
Four healthy volunteer subjects (examiners who rotated through the role) were assessed. No subject reported any ongoing neck or spine discomfort or had had any previous spinal surgery. One at a time, subjects were fitted with a cervical harness electrogoniometer capable of measuring angulation of the cervical spine to the nearest degree (modified electrogoniometer, torsiometer, and display from Biometrics, Gwent, UK; Figures 1A, 1B). This electrogoniometer has been shown to have a mean (SD) error of 2.3° (2.6°) relative to radiographic assessments.8
With the electrogoniometer fitted, each subject was instructed to sit upright in a chair with his back to the backrest and his head neutrally positioned. The electrogoniometer was then zeroed, and the subject proceeded with 5 series of flexion-extension, left and right lateral bending, and left and right rotation movements. The subject was instructed to make 1 movement in full motion in each direction and the other 4 movements in less than full motion to yield a variety of excursions for assessment. Each subject was instructed to pause at the apex of each motion. During these pauses, the examiners recorded their visual estimates of movement in each direction while the investigator recorded degrees of motion (displayed by the electrogoniometer) in flexion-extension, lateral bending, and rotation (Figures 2A–2D). The electrogoniometer display was not visible to subjects or examiners.
A total of 840 independent visual estimates of 120 distinct movements were recorded.
Training, and Assessments Made Immediately Thereafter
After the first round of visual estimates, the 8 examiners were verbally instructed in cervical spine ROM assessment and were asked to observe 1 subject, fitted with the electrogoniometer, demonstrating partial and full cervical motions while the investigator announced the electrogoniometric measurements. The motions demonstrated included 15°, 30°, and the extremes of cervical spine ROM in each of 6 directions from neutral.
After this training session, each of the 4 subjects from the first round of assessments was again fitted with the harness electrogoniometer and instructed to repeat the movements in turn while examiners visually estimated cervical spine ROM and independently recorded their estimates. Meanwhile, the investigator recorded the degree of motion during each movement (as measured by the electrogoniometer). Again, a total of 840 independent visual estimates of 120 distinct movements were recorded.
Assessments Made 1 Month After Training
One month after the training session, the examiners and the investigator reconvened to assess the same 4 subjects using a procedure for simultaneous visual estimation and electrogoniometric measurement identical to that used 1 month earlier. No additional training was given. Again, 840 independent visual estimates of 120 distinct movements were recorded.
Data Analysis
The reliabilities of visual estimates were analyzed by calculating the intraclass coefficients (ICCs) using random-effect 1-way analyses of variance. By convention, ICCs of < 0.2, 0.2 to 0.39, 0.4 to 0.59, 0.6 to 0.8, and > 0.8 correspond to poor, fair, moderate, substantial, and perfect reliability, respectively.25
We compared the visual estimates and electrogoniometric measurements made for 3 planes of motion (flexion-extension, lateral bending, axial rotation) before, immediately after, and 1 month after training and drew trend lines generated by linear regression relative to a line of perfect correlation.
Mean errors in examiners’ visual estimates (relative to electrogoniometric measurements) made before, immediately after, and 1 month after training were calculated. Paired Student t tests were then used to compare the mean errors before training with the mean errors immediately after and 1 month after training.
All analyses were performed with SPSS for Windows 16.0 (SPSS, Chicago, Illinois).
Results
Inter-examiner reliability of the visual estimates in all planes of motion ranged from 0.51 to 0.79 (suggestive of moderate to substantial reliability). For reference, standard goniometers measuring knee ROM have inter-examiner ICCs of 0.89 to 0.9826 (suggestive of perfect reliability). The ICCs before, immediately after, and 1 month after training were not significantly different.
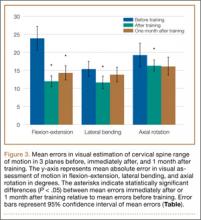
As expected, there were significant errors in visual estimates of cervical spine ROM in all planes. Initial errors in visual estimates (relative to electrogoniometric measurements) were 23.9° (flexion-extension), 15.5° (lateral bending), and 19.3° (axial rotation) (Table, Figure 3).
Immediately after training, mean errors in visual estimates decreased to 12.0° (flexion-extension), 11.7° (lateral bending), and 16.4° (axial rotation) (Table, Figure 3). In all 3 planes of cervical motion, these improvements were statistically significant.
One month after training, mean errors in visual estimates were 14.4° (flexion-extension), 13.9° (lateral bending), and 16.2° (axial rotation) (Table, Figure 3). Only the improvement in the estimate of flexion-extension (the direction of the largest error initially) remained statistically significant—a 39.7% decrease in error.
We also considered how errors varied with degree of motion observed. In flexion-extension, the tendency to overestimate at larger degrees of motion was not apparent after training, and 1 month after training we found a tendency to underestimate at smaller degrees of motion (Figure 4A). The tendency to overestimate lateral bending before training did not persist immediately after or 1 month after training (Figure 4B). Estimates of axial rotation correlated well with goniometer measurements before training and were also well correlated immediately after and 1 month after training (Figure 4C).
Discussion
Visual estimation of spinal motion is unreliable and inaccurate, but its widespread use in clinical practice continues. Goniometers are far more accurate and reliable but are seldom used. We investigated whether a training session featuring verbal instruction and demonstration with an electrogoniometer could improve visual estimates and whether potential improvement in visual estimates would remain 1 month after training.
Widely variable ICCs (0.42-0.90) have been reported for visual estimates of cervical spine ROM.17,18,22 Our findings on the reliability of these estimates are consistent with the literature.
We recorded the greatest initial error in estimates of motion in flexion-extension. Previous studies have also found the greatest error and least reliability in visual estimates in this plane.14,15,18 Visual estimation may be more difficult in flexion-extension because the shoulders cannot be used as landmarks, whereas they serve as approximate 90° reference points during estimation of lateral bending and axial rotation. Demonstration of 15°, 30° and the extremes of ROM during the training session may have provided alternative reference points during visual estimation after training—decreasing the error to within the range found in other planes of motion.
Initial errors in visual estimates were 23.9° (flexion-extension), 15.5° (lateral bending), and 19.3° (axial rotation). Based on normative cervical spine ROM in a healthy population— 126° ± 12° for flexion-extension, 86° ± 5° for lateral bending, 151° ± 23° for axial rotation22—the errors we identified are 18.9% of the normal range of flexion-extension, 18.0% of lateral bending, and 12.8% of axial rotation.
Training clearly improved the accuracy of visual estimates of cervical spine ROM. Estimates were statistically improved for all planes immediately after training and remained significantly improved for flexion-extension (the plane of largest error initially) 1 month after training. Before training, mean errors varied across planes. Training normalized mean errors to about 15°, and this effect lasted in flexion-extension, lateral bending, and axial rotation (Figures 4A–4C). Of note, before training these percentage errors increased with increased motion from neutral in the flexion-extension and lateral bending planes. At full ROM, percentage errors in estimates were greater. After training, percentage errors did not increase appreciably with increasing motion.
Readers will naturally reflect on the clinical significance of the motion assessment improvements demonstrated after the training session described in this study. We must be aware that functional assessments are increasingly being emphasized in the clinical arena—with respect to clinical conditions, surgical outcomes, and functional impairments. We highlight a point made earlier: A difference of only 5° can affect impairment ratings in the medicolegal realm.1 In estimating flexion-extension motion, lasting improvements of almost 10° were demonstrated and maintained 1 month after the training session described in this study.
Nevertheless, mean errors in visual estimation remained at about 15° in all planes of motion, despite our modest improvements. This finding raises the question of whether visually estimated ROM should be pertinent to assessments of impairment and disability. Although visual estimates of ROM may have more utility as a screening test for impairment and disability, fine differences in ROM simply cannot be reliably assessed by visual estimation.
This study has limitations. First, it was conducted at a single institution where the evaluators received most of their training. Their skill in visually estimating cervical spine ROM may not be generalizable to a larger population of spine specialists who are practicing at other institutions and may have different training backgrounds.
Second, only healthy subjects were assessed. Some studies of cervical spine ROM have shown better reliability in symptomatic subjects relative to asymptomatic subjects.13,14 To attempt to overcome this limitation, we assessed many different excursions of motion that were often not to the extremes of motion.
Third, the “gold standard” we used for motion assessment was an electrogoniometer, which has some inherent error (previously validated mean [SD] error of 2.3° [2.6°] relative to radiographs8). Although obtaining radiographs of each movement would have more closely resembled the gold standard, the radiation dose associated with such a study is prohibitive.
Last, the assessors included medical students. The medical students’ estimates, however, tended to be more accurate than the residents’ or attending surgeons’ (though the difference was not statistically significant). This tendency may reflect the medical students’ closer attention to detail. Clearly, including medical students in the study did not negatively affect the accuracy of the estimates or the validity of our findings.
Conclusion
Despite its limitations, visual assessment of cervical spine motion remains the gold standard in clinical practice and is routinely recorded and reported. Mean errors ranged from 15.5° to 23.9°, depending on plane of motion being assessed, but these improved after a training session.
Visual estimates of motion in flexion-extension were most improved by training, as the initial errors in this plane were the largest. Statistically significant improvement of about 10° remained for flexion-extension motion estimates 1 month after training.
During a time when we are increasingly emphasizing functional outcomes, such a degree of improvement could be of clinical significance. Our study results support a call for more formalized training of ROM assessment, but clinicians should also be aware of the limitations of visual estimates of cervical spine ROM, and our study results support scrutiny of visual assessment of ROM as a criterion for diagnosing permanent impairment or disability.
1. Rondinelli RD, Genovese E, Brigham CR; American Medical Association. Guides to the Evaluation of Permanent Impairment. 6th ed. Chicago, IL: American Medical Association; 2008.
2. Hall TM, Briffa K, Hopper D, Robinson K. Comparative analysis and diagnostic accuracy of the cervical flexion-rotation test. J Headache Pain. 2010;11(5):391-397.
3. De Hertogh WJ, Vaes PH, Vijverman V, De Cordt A, Duquet W. The clinical examination of neck pain patients: the validity of a group of tests. Man Ther. 2007;12(1):50-55.
4. Koller H, Resch H, Acosta F, et al. Assessment of two measurement techniques of cervical spine and C1–C2 rotation in the outcome research of axis fractures: a morphometrical analysis using dynamic computed tomography scanning. Spine. 2010;35(3):286-290.
5. Garrett TR, Youdas JW, Madson TJ. Reliability of measuring forward head posture in a clinical setting. J Orthop Sports Phys Ther. 1993;17(3):155-160.
6. Pearcy MJ, Tibrewal SB. Axial rotation and lateral bending in the normal lumbar spine measured by three-dimensional radiography. Spine. 1984;9(6):582-587.
7. Hayes MA, Howard TC, Gruel CR, Kopta JA. Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals. Spine. 1989;14(3):327-331.
8. Bible JE, Biswas D, Miller CP, Whang PG, Grauer JN. Normal functional range of motion of the cervical spine during 15 activities of daily living. J Spinal Disord Tech. 2010;23(1):15-21.
9. Penning L. Normal movements of the cervical spine. AJR Am J Roentgenol. 1978;130(2):317-326.
10. Mayer TG, Tencer AF, Kristoferson S, Mooney V. Use of noninvasive techniques for quantification of spinal range-of-motion in normal subjects and chronic low-back dysfunction patients. Spine. 1984;9(6):588-595.
11. Williams MA, McCarthy CJ, Chorti A, Cooke MW, Gates S. A systematic review of reliability and validity studies of methods for measuring active and passive cervical range of motion. J Manipulative Physiol Ther. 2010;33(2):138-155.
12. Schaufele MK, Boden SD. Physical function measurements in neck pain. Phys Med Rehabil Clin North Am. 2003;14(3):569-588.
13. Fjellner A, Bexander C, Faleij R, Strender LE. Interexaminer reliability in physical examination of the cervical spine. J Manipulative Physiol Ther. 1999;22(8):511-516.
14. Nilsson N, Christensen HW, Hartvigsen J. The interexaminer reliability of measuring passive cervical range of motion, revisited. J Manipulative Physiol Ther. 1996;19(5):302-305.
15. Pool JJ, Hoving JL, de Vet HC, van Mameren H, Bouter LM. The interexaminer reproducibility of physical examination of the cervical spine. J Manipulative Physiol Ther. 2004;27(2):84-90.
16. Strender LE, Lundin M, Nell K. Interexaminer reliability in physical examination of the neck. J Manipulative Physiol Ther. 1997;20(8):516-520.
17. Youdas JW, Carey JR, Garrett TR. Reliability of measurements of cervical spine range of motion—comparison of three methods. Phys Ther. 1991;71(2):98-104.
18. Whitcroft KL, Massouh L, Amirfeyz R, Bannister G. Comparison of methods of measuring active cervical range of motion. Spine. 2010;35(19):E976-E980.
19. de Koning CH, van den Heuvel SP, Staal JB, Smits-Engelsman BC, Hendriks EJ. Clinimetric evaluation of active range of motion measures in patients with non-specific neck pain: a systematic review. Eur Spine J. 2008;17(7):905-921.
20. Christensen HW, Nilsson N. The reliability of measuring active and passive cervical range of motion: an observer-blinded and randomized repeated-measures design. J Manipulative Physiol Ther. 1998;21(5):341-347.
21. Florêncio LL, Pereira PA, Silva ER, Pegoretti KS, Gonçalves MC, Bevilaqua-Grossi D. Agreement and reliability of two non-invasive methods for assessing cervical range of motion among young adults. Rev Bras Fisioter. 2010;14(2):175-181.
22. Lea RD, Gerhardt JJ. Range-of-motion measurements. J Bone Joint Surg Am. 1995;77(5):784-798.
23. Youdas JW, Carey JR, Garrett TR. Reliability of measurements of cervical spine range of motion—comparison of three methods. Phys Ther. 1991;71(2):98-104.
24. Greene WB, Netter FH. Netter’s Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2006.
25. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428.
26. Brosseau L, Balmer S, Tousignant M, et al. Intra- and intertester reliability and criterion validity of the parallelogram and universal goniometers for measuring maximum active knee flexion and extension of patients with knee restrictions. Arch Phys Med Rehabil. 2001;82(3):396-402.
Assessment of cervical spine range of motion (ROM) is an integral aspect of the physical examination for cervical conditions,1-3 surgical outcomes,4 and functional impairment.1 In fact, the emphasis being placed on such functional measures before and after treatments is increasing.4,5
Cervical spine range of motion is routinely used as an outcome measure in clinical studies.6-8 Underscoring the importance of defining cervical spine ROM, studies have found it to be a preoperative predictor of outcomes of anterior cervical surgery,9 and other studies have suggested it is a determinant of athletes’ return to play.10
Spinal ROM measurements can be used to determine the degree of disability experienced by a patient with a spinal condition as defined in the Guides to the Evaluation of Permanent Impairment by the American Medical Association (AMA).1 In the medicolegal realm, ROM measurements made by clinicians can influence the dollar amounts of awards in legal claims, and, according to the AMA guides, the difference in cervical spine ROM between normality and disability or impairment can be as little as 5°.
Although cervical spine ROM is routinely assessed and documented in clinical practice, no universal protocol exists for its evaluation.11,12 In fact, considerable inter-examiner variation in visual estimates of ROM has been found,13-16 and significant inaccuracies have been reported.17,18
Goniometers have been shown to be reliable and highly accurate, with low inter-examiner and intra-examiner variability.5,19-21 Nevertheless, logistics22 and costs21 generally limit their being accepted in routine clinical practice. Among many methods available for assessing ROM, visual estimation is the least reliable or accurate,23 but it is the quickest and least expensive and is recommended in textbooks that describe the spinal-specific physical examination.24 Despite the superiority of goniometers in measuring ROM, these significant barriers have limited their use in clinical practice. When assessing cervical spine ROM, most clinicians prefer visual estimates over goniometers.
We conducted a study to determine whether training could improve the accuracy of visual estimates. We compared the accuracy of visual estimates of cervical spine ROM with that of a radiographically validated electrogoniometer and then investigated whether accuracy and reliability of visual estimates could be improved with a session of instruction and demonstration. Assessments of accuracy were made immediately after and 1 month after this training session.
Materials and Methods
Assessments Made Before Training
This study was approved by our institution’s human investigation committee and was conducted in accordance with the ethical standards of that committee.
Cervical spine ROM was assessed by 8 examiners (2 attending spine surgeons, 4 orthopedic residents, 2 medical students). They were informed they would be participating in a study evaluating visual estimates of motion but were given no other information prior to the study.
Four healthy volunteer subjects (examiners who rotated through the role) were assessed. No subject reported any ongoing neck or spine discomfort or had had any previous spinal surgery. One at a time, subjects were fitted with a cervical harness electrogoniometer capable of measuring angulation of the cervical spine to the nearest degree (modified electrogoniometer, torsiometer, and display from Biometrics, Gwent, UK; Figures 1A, 1B). This electrogoniometer has been shown to have a mean (SD) error of 2.3° (2.6°) relative to radiographic assessments.8
With the electrogoniometer fitted, each subject was instructed to sit upright in a chair with his back to the backrest and his head neutrally positioned. The electrogoniometer was then zeroed, and the subject proceeded with 5 series of flexion-extension, left and right lateral bending, and left and right rotation movements. The subject was instructed to make 1 movement in full motion in each direction and the other 4 movements in less than full motion to yield a variety of excursions for assessment. Each subject was instructed to pause at the apex of each motion. During these pauses, the examiners recorded their visual estimates of movement in each direction while the investigator recorded degrees of motion (displayed by the electrogoniometer) in flexion-extension, lateral bending, and rotation (Figures 2A–2D). The electrogoniometer display was not visible to subjects or examiners.
A total of 840 independent visual estimates of 120 distinct movements were recorded.
Training, and Assessments Made Immediately Thereafter
After the first round of visual estimates, the 8 examiners were verbally instructed in cervical spine ROM assessment and were asked to observe 1 subject, fitted with the electrogoniometer, demonstrating partial and full cervical motions while the investigator announced the electrogoniometric measurements. The motions demonstrated included 15°, 30°, and the extremes of cervical spine ROM in each of 6 directions from neutral.
After this training session, each of the 4 subjects from the first round of assessments was again fitted with the harness electrogoniometer and instructed to repeat the movements in turn while examiners visually estimated cervical spine ROM and independently recorded their estimates. Meanwhile, the investigator recorded the degree of motion during each movement (as measured by the electrogoniometer). Again, a total of 840 independent visual estimates of 120 distinct movements were recorded.
Assessments Made 1 Month After Training
One month after the training session, the examiners and the investigator reconvened to assess the same 4 subjects using a procedure for simultaneous visual estimation and electrogoniometric measurement identical to that used 1 month earlier. No additional training was given. Again, 840 independent visual estimates of 120 distinct movements were recorded.
Data Analysis
The reliabilities of visual estimates were analyzed by calculating the intraclass coefficients (ICCs) using random-effect 1-way analyses of variance. By convention, ICCs of < 0.2, 0.2 to 0.39, 0.4 to 0.59, 0.6 to 0.8, and > 0.8 correspond to poor, fair, moderate, substantial, and perfect reliability, respectively.25
We compared the visual estimates and electrogoniometric measurements made for 3 planes of motion (flexion-extension, lateral bending, axial rotation) before, immediately after, and 1 month after training and drew trend lines generated by linear regression relative to a line of perfect correlation.
Mean errors in examiners’ visual estimates (relative to electrogoniometric measurements) made before, immediately after, and 1 month after training were calculated. Paired Student t tests were then used to compare the mean errors before training with the mean errors immediately after and 1 month after training.
All analyses were performed with SPSS for Windows 16.0 (SPSS, Chicago, Illinois).
Results
Inter-examiner reliability of the visual estimates in all planes of motion ranged from 0.51 to 0.79 (suggestive of moderate to substantial reliability). For reference, standard goniometers measuring knee ROM have inter-examiner ICCs of 0.89 to 0.9826 (suggestive of perfect reliability). The ICCs before, immediately after, and 1 month after training were not significantly different.
As expected, there were significant errors in visual estimates of cervical spine ROM in all planes. Initial errors in visual estimates (relative to electrogoniometric measurements) were 23.9° (flexion-extension), 15.5° (lateral bending), and 19.3° (axial rotation) (Table, Figure 3).
Immediately after training, mean errors in visual estimates decreased to 12.0° (flexion-extension), 11.7° (lateral bending), and 16.4° (axial rotation) (Table, Figure 3). In all 3 planes of cervical motion, these improvements were statistically significant.
One month after training, mean errors in visual estimates were 14.4° (flexion-extension), 13.9° (lateral bending), and 16.2° (axial rotation) (Table, Figure 3). Only the improvement in the estimate of flexion-extension (the direction of the largest error initially) remained statistically significant—a 39.7% decrease in error.
We also considered how errors varied with degree of motion observed. In flexion-extension, the tendency to overestimate at larger degrees of motion was not apparent after training, and 1 month after training we found a tendency to underestimate at smaller degrees of motion (Figure 4A). The tendency to overestimate lateral bending before training did not persist immediately after or 1 month after training (Figure 4B). Estimates of axial rotation correlated well with goniometer measurements before training and were also well correlated immediately after and 1 month after training (Figure 4C).
Discussion
Visual estimation of spinal motion is unreliable and inaccurate, but its widespread use in clinical practice continues. Goniometers are far more accurate and reliable but are seldom used. We investigated whether a training session featuring verbal instruction and demonstration with an electrogoniometer could improve visual estimates and whether potential improvement in visual estimates would remain 1 month after training.
Widely variable ICCs (0.42-0.90) have been reported for visual estimates of cervical spine ROM.17,18,22 Our findings on the reliability of these estimates are consistent with the literature.
We recorded the greatest initial error in estimates of motion in flexion-extension. Previous studies have also found the greatest error and least reliability in visual estimates in this plane.14,15,18 Visual estimation may be more difficult in flexion-extension because the shoulders cannot be used as landmarks, whereas they serve as approximate 90° reference points during estimation of lateral bending and axial rotation. Demonstration of 15°, 30° and the extremes of ROM during the training session may have provided alternative reference points during visual estimation after training—decreasing the error to within the range found in other planes of motion.
Initial errors in visual estimates were 23.9° (flexion-extension), 15.5° (lateral bending), and 19.3° (axial rotation). Based on normative cervical spine ROM in a healthy population— 126° ± 12° for flexion-extension, 86° ± 5° for lateral bending, 151° ± 23° for axial rotation22—the errors we identified are 18.9% of the normal range of flexion-extension, 18.0% of lateral bending, and 12.8% of axial rotation.
Training clearly improved the accuracy of visual estimates of cervical spine ROM. Estimates were statistically improved for all planes immediately after training and remained significantly improved for flexion-extension (the plane of largest error initially) 1 month after training. Before training, mean errors varied across planes. Training normalized mean errors to about 15°, and this effect lasted in flexion-extension, lateral bending, and axial rotation (Figures 4A–4C). Of note, before training these percentage errors increased with increased motion from neutral in the flexion-extension and lateral bending planes. At full ROM, percentage errors in estimates were greater. After training, percentage errors did not increase appreciably with increasing motion.
Readers will naturally reflect on the clinical significance of the motion assessment improvements demonstrated after the training session described in this study. We must be aware that functional assessments are increasingly being emphasized in the clinical arena—with respect to clinical conditions, surgical outcomes, and functional impairments. We highlight a point made earlier: A difference of only 5° can affect impairment ratings in the medicolegal realm.1 In estimating flexion-extension motion, lasting improvements of almost 10° were demonstrated and maintained 1 month after the training session described in this study.
Nevertheless, mean errors in visual estimation remained at about 15° in all planes of motion, despite our modest improvements. This finding raises the question of whether visually estimated ROM should be pertinent to assessments of impairment and disability. Although visual estimates of ROM may have more utility as a screening test for impairment and disability, fine differences in ROM simply cannot be reliably assessed by visual estimation.
This study has limitations. First, it was conducted at a single institution where the evaluators received most of their training. Their skill in visually estimating cervical spine ROM may not be generalizable to a larger population of spine specialists who are practicing at other institutions and may have different training backgrounds.
Second, only healthy subjects were assessed. Some studies of cervical spine ROM have shown better reliability in symptomatic subjects relative to asymptomatic subjects.13,14 To attempt to overcome this limitation, we assessed many different excursions of motion that were often not to the extremes of motion.
Third, the “gold standard” we used for motion assessment was an electrogoniometer, which has some inherent error (previously validated mean [SD] error of 2.3° [2.6°] relative to radiographs8). Although obtaining radiographs of each movement would have more closely resembled the gold standard, the radiation dose associated with such a study is prohibitive.
Last, the assessors included medical students. The medical students’ estimates, however, tended to be more accurate than the residents’ or attending surgeons’ (though the difference was not statistically significant). This tendency may reflect the medical students’ closer attention to detail. Clearly, including medical students in the study did not negatively affect the accuracy of the estimates or the validity of our findings.
Conclusion
Despite its limitations, visual assessment of cervical spine motion remains the gold standard in clinical practice and is routinely recorded and reported. Mean errors ranged from 15.5° to 23.9°, depending on plane of motion being assessed, but these improved after a training session.
Visual estimates of motion in flexion-extension were most improved by training, as the initial errors in this plane were the largest. Statistically significant improvement of about 10° remained for flexion-extension motion estimates 1 month after training.
During a time when we are increasingly emphasizing functional outcomes, such a degree of improvement could be of clinical significance. Our study results support a call for more formalized training of ROM assessment, but clinicians should also be aware of the limitations of visual estimates of cervical spine ROM, and our study results support scrutiny of visual assessment of ROM as a criterion for diagnosing permanent impairment or disability.
Assessment of cervical spine range of motion (ROM) is an integral aspect of the physical examination for cervical conditions,1-3 surgical outcomes,4 and functional impairment.1 In fact, the emphasis being placed on such functional measures before and after treatments is increasing.4,5
Cervical spine range of motion is routinely used as an outcome measure in clinical studies.6-8 Underscoring the importance of defining cervical spine ROM, studies have found it to be a preoperative predictor of outcomes of anterior cervical surgery,9 and other studies have suggested it is a determinant of athletes’ return to play.10
Spinal ROM measurements can be used to determine the degree of disability experienced by a patient with a spinal condition as defined in the Guides to the Evaluation of Permanent Impairment by the American Medical Association (AMA).1 In the medicolegal realm, ROM measurements made by clinicians can influence the dollar amounts of awards in legal claims, and, according to the AMA guides, the difference in cervical spine ROM between normality and disability or impairment can be as little as 5°.
Although cervical spine ROM is routinely assessed and documented in clinical practice, no universal protocol exists for its evaluation.11,12 In fact, considerable inter-examiner variation in visual estimates of ROM has been found,13-16 and significant inaccuracies have been reported.17,18
Goniometers have been shown to be reliable and highly accurate, with low inter-examiner and intra-examiner variability.5,19-21 Nevertheless, logistics22 and costs21 generally limit their being accepted in routine clinical practice. Among many methods available for assessing ROM, visual estimation is the least reliable or accurate,23 but it is the quickest and least expensive and is recommended in textbooks that describe the spinal-specific physical examination.24 Despite the superiority of goniometers in measuring ROM, these significant barriers have limited their use in clinical practice. When assessing cervical spine ROM, most clinicians prefer visual estimates over goniometers.
We conducted a study to determine whether training could improve the accuracy of visual estimates. We compared the accuracy of visual estimates of cervical spine ROM with that of a radiographically validated electrogoniometer and then investigated whether accuracy and reliability of visual estimates could be improved with a session of instruction and demonstration. Assessments of accuracy were made immediately after and 1 month after this training session.
Materials and Methods
Assessments Made Before Training
This study was approved by our institution’s human investigation committee and was conducted in accordance with the ethical standards of that committee.
Cervical spine ROM was assessed by 8 examiners (2 attending spine surgeons, 4 orthopedic residents, 2 medical students). They were informed they would be participating in a study evaluating visual estimates of motion but were given no other information prior to the study.
Four healthy volunteer subjects (examiners who rotated through the role) were assessed. No subject reported any ongoing neck or spine discomfort or had had any previous spinal surgery. One at a time, subjects were fitted with a cervical harness electrogoniometer capable of measuring angulation of the cervical spine to the nearest degree (modified electrogoniometer, torsiometer, and display from Biometrics, Gwent, UK; Figures 1A, 1B). This electrogoniometer has been shown to have a mean (SD) error of 2.3° (2.6°) relative to radiographic assessments.8
With the electrogoniometer fitted, each subject was instructed to sit upright in a chair with his back to the backrest and his head neutrally positioned. The electrogoniometer was then zeroed, and the subject proceeded with 5 series of flexion-extension, left and right lateral bending, and left and right rotation movements. The subject was instructed to make 1 movement in full motion in each direction and the other 4 movements in less than full motion to yield a variety of excursions for assessment. Each subject was instructed to pause at the apex of each motion. During these pauses, the examiners recorded their visual estimates of movement in each direction while the investigator recorded degrees of motion (displayed by the electrogoniometer) in flexion-extension, lateral bending, and rotation (Figures 2A–2D). The electrogoniometer display was not visible to subjects or examiners.
A total of 840 independent visual estimates of 120 distinct movements were recorded.
Training, and Assessments Made Immediately Thereafter
After the first round of visual estimates, the 8 examiners were verbally instructed in cervical spine ROM assessment and were asked to observe 1 subject, fitted with the electrogoniometer, demonstrating partial and full cervical motions while the investigator announced the electrogoniometric measurements. The motions demonstrated included 15°, 30°, and the extremes of cervical spine ROM in each of 6 directions from neutral.
After this training session, each of the 4 subjects from the first round of assessments was again fitted with the harness electrogoniometer and instructed to repeat the movements in turn while examiners visually estimated cervical spine ROM and independently recorded their estimates. Meanwhile, the investigator recorded the degree of motion during each movement (as measured by the electrogoniometer). Again, a total of 840 independent visual estimates of 120 distinct movements were recorded.
Assessments Made 1 Month After Training
One month after the training session, the examiners and the investigator reconvened to assess the same 4 subjects using a procedure for simultaneous visual estimation and electrogoniometric measurement identical to that used 1 month earlier. No additional training was given. Again, 840 independent visual estimates of 120 distinct movements were recorded.
Data Analysis
The reliabilities of visual estimates were analyzed by calculating the intraclass coefficients (ICCs) using random-effect 1-way analyses of variance. By convention, ICCs of < 0.2, 0.2 to 0.39, 0.4 to 0.59, 0.6 to 0.8, and > 0.8 correspond to poor, fair, moderate, substantial, and perfect reliability, respectively.25
We compared the visual estimates and electrogoniometric measurements made for 3 planes of motion (flexion-extension, lateral bending, axial rotation) before, immediately after, and 1 month after training and drew trend lines generated by linear regression relative to a line of perfect correlation.
Mean errors in examiners’ visual estimates (relative to electrogoniometric measurements) made before, immediately after, and 1 month after training were calculated. Paired Student t tests were then used to compare the mean errors before training with the mean errors immediately after and 1 month after training.
All analyses were performed with SPSS for Windows 16.0 (SPSS, Chicago, Illinois).
Results
Inter-examiner reliability of the visual estimates in all planes of motion ranged from 0.51 to 0.79 (suggestive of moderate to substantial reliability). For reference, standard goniometers measuring knee ROM have inter-examiner ICCs of 0.89 to 0.9826 (suggestive of perfect reliability). The ICCs before, immediately after, and 1 month after training were not significantly different.
As expected, there were significant errors in visual estimates of cervical spine ROM in all planes. Initial errors in visual estimates (relative to electrogoniometric measurements) were 23.9° (flexion-extension), 15.5° (lateral bending), and 19.3° (axial rotation) (Table, Figure 3).
Immediately after training, mean errors in visual estimates decreased to 12.0° (flexion-extension), 11.7° (lateral bending), and 16.4° (axial rotation) (Table, Figure 3). In all 3 planes of cervical motion, these improvements were statistically significant.
One month after training, mean errors in visual estimates were 14.4° (flexion-extension), 13.9° (lateral bending), and 16.2° (axial rotation) (Table, Figure 3). Only the improvement in the estimate of flexion-extension (the direction of the largest error initially) remained statistically significant—a 39.7% decrease in error.
We also considered how errors varied with degree of motion observed. In flexion-extension, the tendency to overestimate at larger degrees of motion was not apparent after training, and 1 month after training we found a tendency to underestimate at smaller degrees of motion (Figure 4A). The tendency to overestimate lateral bending before training did not persist immediately after or 1 month after training (Figure 4B). Estimates of axial rotation correlated well with goniometer measurements before training and were also well correlated immediately after and 1 month after training (Figure 4C).
Discussion
Visual estimation of spinal motion is unreliable and inaccurate, but its widespread use in clinical practice continues. Goniometers are far more accurate and reliable but are seldom used. We investigated whether a training session featuring verbal instruction and demonstration with an electrogoniometer could improve visual estimates and whether potential improvement in visual estimates would remain 1 month after training.
Widely variable ICCs (0.42-0.90) have been reported for visual estimates of cervical spine ROM.17,18,22 Our findings on the reliability of these estimates are consistent with the literature.
We recorded the greatest initial error in estimates of motion in flexion-extension. Previous studies have also found the greatest error and least reliability in visual estimates in this plane.14,15,18 Visual estimation may be more difficult in flexion-extension because the shoulders cannot be used as landmarks, whereas they serve as approximate 90° reference points during estimation of lateral bending and axial rotation. Demonstration of 15°, 30° and the extremes of ROM during the training session may have provided alternative reference points during visual estimation after training—decreasing the error to within the range found in other planes of motion.
Initial errors in visual estimates were 23.9° (flexion-extension), 15.5° (lateral bending), and 19.3° (axial rotation). Based on normative cervical spine ROM in a healthy population— 126° ± 12° for flexion-extension, 86° ± 5° for lateral bending, 151° ± 23° for axial rotation22—the errors we identified are 18.9% of the normal range of flexion-extension, 18.0% of lateral bending, and 12.8% of axial rotation.
Training clearly improved the accuracy of visual estimates of cervical spine ROM. Estimates were statistically improved for all planes immediately after training and remained significantly improved for flexion-extension (the plane of largest error initially) 1 month after training. Before training, mean errors varied across planes. Training normalized mean errors to about 15°, and this effect lasted in flexion-extension, lateral bending, and axial rotation (Figures 4A–4C). Of note, before training these percentage errors increased with increased motion from neutral in the flexion-extension and lateral bending planes. At full ROM, percentage errors in estimates were greater. After training, percentage errors did not increase appreciably with increasing motion.
Readers will naturally reflect on the clinical significance of the motion assessment improvements demonstrated after the training session described in this study. We must be aware that functional assessments are increasingly being emphasized in the clinical arena—with respect to clinical conditions, surgical outcomes, and functional impairments. We highlight a point made earlier: A difference of only 5° can affect impairment ratings in the medicolegal realm.1 In estimating flexion-extension motion, lasting improvements of almost 10° were demonstrated and maintained 1 month after the training session described in this study.
Nevertheless, mean errors in visual estimation remained at about 15° in all planes of motion, despite our modest improvements. This finding raises the question of whether visually estimated ROM should be pertinent to assessments of impairment and disability. Although visual estimates of ROM may have more utility as a screening test for impairment and disability, fine differences in ROM simply cannot be reliably assessed by visual estimation.
This study has limitations. First, it was conducted at a single institution where the evaluators received most of their training. Their skill in visually estimating cervical spine ROM may not be generalizable to a larger population of spine specialists who are practicing at other institutions and may have different training backgrounds.
Second, only healthy subjects were assessed. Some studies of cervical spine ROM have shown better reliability in symptomatic subjects relative to asymptomatic subjects.13,14 To attempt to overcome this limitation, we assessed many different excursions of motion that were often not to the extremes of motion.
Third, the “gold standard” we used for motion assessment was an electrogoniometer, which has some inherent error (previously validated mean [SD] error of 2.3° [2.6°] relative to radiographs8). Although obtaining radiographs of each movement would have more closely resembled the gold standard, the radiation dose associated with such a study is prohibitive.
Last, the assessors included medical students. The medical students’ estimates, however, tended to be more accurate than the residents’ or attending surgeons’ (though the difference was not statistically significant). This tendency may reflect the medical students’ closer attention to detail. Clearly, including medical students in the study did not negatively affect the accuracy of the estimates or the validity of our findings.
Conclusion
Despite its limitations, visual assessment of cervical spine motion remains the gold standard in clinical practice and is routinely recorded and reported. Mean errors ranged from 15.5° to 23.9°, depending on plane of motion being assessed, but these improved after a training session.
Visual estimates of motion in flexion-extension were most improved by training, as the initial errors in this plane were the largest. Statistically significant improvement of about 10° remained for flexion-extension motion estimates 1 month after training.
During a time when we are increasingly emphasizing functional outcomes, such a degree of improvement could be of clinical significance. Our study results support a call for more formalized training of ROM assessment, but clinicians should also be aware of the limitations of visual estimates of cervical spine ROM, and our study results support scrutiny of visual assessment of ROM as a criterion for diagnosing permanent impairment or disability.
1. Rondinelli RD, Genovese E, Brigham CR; American Medical Association. Guides to the Evaluation of Permanent Impairment. 6th ed. Chicago, IL: American Medical Association; 2008.
2. Hall TM, Briffa K, Hopper D, Robinson K. Comparative analysis and diagnostic accuracy of the cervical flexion-rotation test. J Headache Pain. 2010;11(5):391-397.
3. De Hertogh WJ, Vaes PH, Vijverman V, De Cordt A, Duquet W. The clinical examination of neck pain patients: the validity of a group of tests. Man Ther. 2007;12(1):50-55.
4. Koller H, Resch H, Acosta F, et al. Assessment of two measurement techniques of cervical spine and C1–C2 rotation in the outcome research of axis fractures: a morphometrical analysis using dynamic computed tomography scanning. Spine. 2010;35(3):286-290.
5. Garrett TR, Youdas JW, Madson TJ. Reliability of measuring forward head posture in a clinical setting. J Orthop Sports Phys Ther. 1993;17(3):155-160.
6. Pearcy MJ, Tibrewal SB. Axial rotation and lateral bending in the normal lumbar spine measured by three-dimensional radiography. Spine. 1984;9(6):582-587.
7. Hayes MA, Howard TC, Gruel CR, Kopta JA. Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals. Spine. 1989;14(3):327-331.
8. Bible JE, Biswas D, Miller CP, Whang PG, Grauer JN. Normal functional range of motion of the cervical spine during 15 activities of daily living. J Spinal Disord Tech. 2010;23(1):15-21.
9. Penning L. Normal movements of the cervical spine. AJR Am J Roentgenol. 1978;130(2):317-326.
10. Mayer TG, Tencer AF, Kristoferson S, Mooney V. Use of noninvasive techniques for quantification of spinal range-of-motion in normal subjects and chronic low-back dysfunction patients. Spine. 1984;9(6):588-595.
11. Williams MA, McCarthy CJ, Chorti A, Cooke MW, Gates S. A systematic review of reliability and validity studies of methods for measuring active and passive cervical range of motion. J Manipulative Physiol Ther. 2010;33(2):138-155.
12. Schaufele MK, Boden SD. Physical function measurements in neck pain. Phys Med Rehabil Clin North Am. 2003;14(3):569-588.
13. Fjellner A, Bexander C, Faleij R, Strender LE. Interexaminer reliability in physical examination of the cervical spine. J Manipulative Physiol Ther. 1999;22(8):511-516.
14. Nilsson N, Christensen HW, Hartvigsen J. The interexaminer reliability of measuring passive cervical range of motion, revisited. J Manipulative Physiol Ther. 1996;19(5):302-305.
15. Pool JJ, Hoving JL, de Vet HC, van Mameren H, Bouter LM. The interexaminer reproducibility of physical examination of the cervical spine. J Manipulative Physiol Ther. 2004;27(2):84-90.
16. Strender LE, Lundin M, Nell K. Interexaminer reliability in physical examination of the neck. J Manipulative Physiol Ther. 1997;20(8):516-520.
17. Youdas JW, Carey JR, Garrett TR. Reliability of measurements of cervical spine range of motion—comparison of three methods. Phys Ther. 1991;71(2):98-104.
18. Whitcroft KL, Massouh L, Amirfeyz R, Bannister G. Comparison of methods of measuring active cervical range of motion. Spine. 2010;35(19):E976-E980.
19. de Koning CH, van den Heuvel SP, Staal JB, Smits-Engelsman BC, Hendriks EJ. Clinimetric evaluation of active range of motion measures in patients with non-specific neck pain: a systematic review. Eur Spine J. 2008;17(7):905-921.
20. Christensen HW, Nilsson N. The reliability of measuring active and passive cervical range of motion: an observer-blinded and randomized repeated-measures design. J Manipulative Physiol Ther. 1998;21(5):341-347.
21. Florêncio LL, Pereira PA, Silva ER, Pegoretti KS, Gonçalves MC, Bevilaqua-Grossi D. Agreement and reliability of two non-invasive methods for assessing cervical range of motion among young adults. Rev Bras Fisioter. 2010;14(2):175-181.
22. Lea RD, Gerhardt JJ. Range-of-motion measurements. J Bone Joint Surg Am. 1995;77(5):784-798.
23. Youdas JW, Carey JR, Garrett TR. Reliability of measurements of cervical spine range of motion—comparison of three methods. Phys Ther. 1991;71(2):98-104.
24. Greene WB, Netter FH. Netter’s Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2006.
25. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428.
26. Brosseau L, Balmer S, Tousignant M, et al. Intra- and intertester reliability and criterion validity of the parallelogram and universal goniometers for measuring maximum active knee flexion and extension of patients with knee restrictions. Arch Phys Med Rehabil. 2001;82(3):396-402.
1. Rondinelli RD, Genovese E, Brigham CR; American Medical Association. Guides to the Evaluation of Permanent Impairment. 6th ed. Chicago, IL: American Medical Association; 2008.
2. Hall TM, Briffa K, Hopper D, Robinson K. Comparative analysis and diagnostic accuracy of the cervical flexion-rotation test. J Headache Pain. 2010;11(5):391-397.
3. De Hertogh WJ, Vaes PH, Vijverman V, De Cordt A, Duquet W. The clinical examination of neck pain patients: the validity of a group of tests. Man Ther. 2007;12(1):50-55.
4. Koller H, Resch H, Acosta F, et al. Assessment of two measurement techniques of cervical spine and C1–C2 rotation in the outcome research of axis fractures: a morphometrical analysis using dynamic computed tomography scanning. Spine. 2010;35(3):286-290.
5. Garrett TR, Youdas JW, Madson TJ. Reliability of measuring forward head posture in a clinical setting. J Orthop Sports Phys Ther. 1993;17(3):155-160.
6. Pearcy MJ, Tibrewal SB. Axial rotation and lateral bending in the normal lumbar spine measured by three-dimensional radiography. Spine. 1984;9(6):582-587.
7. Hayes MA, Howard TC, Gruel CR, Kopta JA. Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals. Spine. 1989;14(3):327-331.
8. Bible JE, Biswas D, Miller CP, Whang PG, Grauer JN. Normal functional range of motion of the cervical spine during 15 activities of daily living. J Spinal Disord Tech. 2010;23(1):15-21.
9. Penning L. Normal movements of the cervical spine. AJR Am J Roentgenol. 1978;130(2):317-326.
10. Mayer TG, Tencer AF, Kristoferson S, Mooney V. Use of noninvasive techniques for quantification of spinal range-of-motion in normal subjects and chronic low-back dysfunction patients. Spine. 1984;9(6):588-595.
11. Williams MA, McCarthy CJ, Chorti A, Cooke MW, Gates S. A systematic review of reliability and validity studies of methods for measuring active and passive cervical range of motion. J Manipulative Physiol Ther. 2010;33(2):138-155.
12. Schaufele MK, Boden SD. Physical function measurements in neck pain. Phys Med Rehabil Clin North Am. 2003;14(3):569-588.
13. Fjellner A, Bexander C, Faleij R, Strender LE. Interexaminer reliability in physical examination of the cervical spine. J Manipulative Physiol Ther. 1999;22(8):511-516.
14. Nilsson N, Christensen HW, Hartvigsen J. The interexaminer reliability of measuring passive cervical range of motion, revisited. J Manipulative Physiol Ther. 1996;19(5):302-305.
15. Pool JJ, Hoving JL, de Vet HC, van Mameren H, Bouter LM. The interexaminer reproducibility of physical examination of the cervical spine. J Manipulative Physiol Ther. 2004;27(2):84-90.
16. Strender LE, Lundin M, Nell K. Interexaminer reliability in physical examination of the neck. J Manipulative Physiol Ther. 1997;20(8):516-520.
17. Youdas JW, Carey JR, Garrett TR. Reliability of measurements of cervical spine range of motion—comparison of three methods. Phys Ther. 1991;71(2):98-104.
18. Whitcroft KL, Massouh L, Amirfeyz R, Bannister G. Comparison of methods of measuring active cervical range of motion. Spine. 2010;35(19):E976-E980.
19. de Koning CH, van den Heuvel SP, Staal JB, Smits-Engelsman BC, Hendriks EJ. Clinimetric evaluation of active range of motion measures in patients with non-specific neck pain: a systematic review. Eur Spine J. 2008;17(7):905-921.
20. Christensen HW, Nilsson N. The reliability of measuring active and passive cervical range of motion: an observer-blinded and randomized repeated-measures design. J Manipulative Physiol Ther. 1998;21(5):341-347.
21. Florêncio LL, Pereira PA, Silva ER, Pegoretti KS, Gonçalves MC, Bevilaqua-Grossi D. Agreement and reliability of two non-invasive methods for assessing cervical range of motion among young adults. Rev Bras Fisioter. 2010;14(2):175-181.
22. Lea RD, Gerhardt JJ. Range-of-motion measurements. J Bone Joint Surg Am. 1995;77(5):784-798.
23. Youdas JW, Carey JR, Garrett TR. Reliability of measurements of cervical spine range of motion—comparison of three methods. Phys Ther. 1991;71(2):98-104.
24. Greene WB, Netter FH. Netter’s Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2006.
25. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428.
26. Brosseau L, Balmer S, Tousignant M, et al. Intra- and intertester reliability and criterion validity of the parallelogram and universal goniometers for measuring maximum active knee flexion and extension of patients with knee restrictions. Arch Phys Med Rehabil. 2001;82(3):396-402.
Does a Prior Hip Arthroscopy Affect Clinical Outcomes in Metal-on-Metal Hip Resurfacing Arthroplasty?
Metal-on-metal hip resurfacing arthroplasty (HRA) remains an alternative to total hip arthroplasty (THA) in appropriately selected, younger, active adults with degenerative hip disease.1-4 While concerns remain regarding the potential for adverse local tissue reactions from wear of the metal-on-metal bearing surface,5-8 10-year data from the Australian Orthopaedic Association National Joint Replacement Registry Annual Report9 showed a revision rate of only 6.3% when the Birmingham Hip Resurfacing (BHR) System was used (Smith & Nephew Inc, Memphis, Tennessee).In addition, in an independent review of 230 consecutive BHRs at a mean follow-up of 10.4 years, Coulter and colleagues10 showed encouraging clinical results, with a mean Oxford Hip Score of 45.0 and a mean University of California at Los Angeles (UCLA) activity score of 7.4.
Similar to the prior increase in popularity of HRA, hip arthroscopy has also become much more commonplace, and its indications continue to evolve.11 Hip arthroscopy has been used in the native hip joint to manage femoroacetabular impingement, labral tears, and iliopsoas tendinopathy, among other conditions.12 In addition, the use of hip arthroscopy has not been limited to the native hip but also has increased as a diagnostic and therapeutic procedure after hip arthroplasties. Bajwa and Villar12 found hip arthroscopy to be diagnostic in 23 of 24 patients who underwent the procedure after a hip arthroplasty, concluding that arthroscopy is a useful adjunct in the diagnosis of symptomatic arthroplasties.
Therefore, hip arthroscopy has been shown to be an effective modality to treat pathology in both the native hip and after hip arthroplasties. However, the effect of a prior hip arthroscopy on the outcome of a subsequent metal-on-metal HRA has not been determined. Piedade and colleagues13 showed a prior knee arthroscopy to increase the risk of postoperative complications and subsequent revision after total knee arthroplasty. Complications included reflex sympathetic dystrophy, undiagnosed pain, infection, stiffness, and component loosening. A prior osteochondroplasty at the femoral head-neck junction could increase the risk of femoral neck fracture after a subsequent HRA. Thus, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy and to compare these results with a cohort of patients who received an HRA with no prior hip surgeries. Our hypothesis is that a prior hip arthroscopy will lead to inferior outcomes in patients undergoing HRA.
Materials and Methods
This study is a retrospective, case-control study using a 1:2 matching analysis. Dr. Su performed all HRAs, which were enrolled in an institutional review board–approved arthroplasty registry. All HRAs were performed using the BHR System.
The surgical technique for hip resurfacing arthroplasty has been described.1 All procedures were performed via a posterior approach with the patient in the lateral decubitus position. All patients received a hybrid metal-on-metal hip resurfacing, with an uncemented acetabular component and cemented femoral component. Intraoperative anesthesia for all patients was performed via a combined spinal-epidural anesthetic, and an epidural patient-controlled analgesic was used for the first day postoperatively, followed by a transition to oral analgesics. The sizes of the acetabular and femoral components were recorded for each hip resurfacing. Postoperatively, intermittent pneumatic compression devices were placed upon arrival in the recovery room, and active ankle flexion and extension exercises were initiated immediately after the patient’s neurologic function returned.14 Aspirin was used for chemical deep venous thrombosis prophylaxis in all patients postoperatively for a period of 6 weeks. Full weight-bearing, with the use of crutches for assistance with balance, was permitted immediately. Crutches were used for a period of 3 weeks prior to being discontinued.
From a database of 1357 HRAs (all BHR implants) performed between June 2006 and June 2012, 51 patients were identified who received an HRA after a prior hip arthroscopy. Eight patients were excluded because they did not possess adequate clinical documentation or were lost to follow-up. In the remaining 43 patients, there were 32 men and 11 women (21 right hips, 22 left hips), which formed the arthroscopy cohort. Two patients had a history of multiple hip arthroscopies (1 patient with 2 prior procedures, 1 patient with 3 prior procedures). The mean (SD) time from the most recent hip arthroscopy to the HRA was 2.5 (2.5) years. Table 1 presents a summary of the hip arthroscopy procedures (including only the most recent hip arthroscopy procedure in those with multiple arthroscopies).
Patient demographic variables (age, body mass index [BMI]) were recorded preoperatively, along with the Harris Hip Score (HHS),15 UCLA activity score,16 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score,17 and preoperative hip range of motion (flexion, extension, abduction, adduction, internal rotation, and external rotation). The same clinical indices were assessed postoperatively along with the Short Form-12 (SF-12) Health Survey Score,18 at the 6-week, 3-month, 6-month, 1-year, and most recent follow-up visits.
Radiographic assessment consisted of a low anteroposterior (AP) pelvic radiograph (with the radiographic beam centered on the pubic symphysis) and a cross-table lateral radiograph obtained at the most recent follow-up visit. Both the acetabular component abduction relative to the inter-teardrop line, and the angle between the femoral stem and the anatomic axis of the femoral shaft (stem-shaft angle) were measured on AP radiographs.19,20 Acetabular component anteversion was measured on the cross-table lateral radiographs as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the long axis plane of the body (Figures A, B).21
The same registry database was used to identify patients who received an HRA without a prior history of arthroscopy or hip surgery. A 1:2 matching analysis for those patients with a prior hip arthroscopy to those without a prior hip arthroscopy was performed to formulate a control group (control cohort) of 86 patients. Each patient in the arthroscopy cohort was matched with 2 patients in the control cohort based on the following parameters: age (± 6 years), sex (same), BMI (± 4 kg/m2), femoral head size (± 4 mm), and preoperative HHS and WOMAC scores (± 7 points). In the event an arthroscopy patient matched to 2 or more control patients, the patients who minimized the least squared error among the matching variables were selected.
Statistical Analysis
All data were collected and analyzed using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Statistical comparisons between the 2 cohorts regarding demographic variables, clinical outcomes, and radiographic alignment were performed using an unpaired, Student 2-tailed t test, with statistical significance set at P ≤ .05.
Results
A comparison of the results of the 1:2 matching analysis between the arthroscopy and control cohorts is presented in Table 2. There was no significant difference in the preoperative age, BMI, femoral head size, HHS, or WOMAC score between the 2 cohorts. However, the control cohort did show a more severe, preoperative flexion contracture (as expressed by a decreased amount of extension) and a decreased amount of preoperative abduction (Table 3). The preoperative UCLA activity score was also decreased in the control cohort, but this was not statistically significant.
The mean (SD) follow-up was 2.0 (1.0) years in the arthroscopy cohort and 2.1 (1.1) years in the control cohort. There was no significant difference in radiographic alignment between the 2 cohorts. The stem-shaft angle was 139.3° (SD, 5.4°) in the arthroscopy cohort (vs 138.3° [SD, 5.5°] in the control cohort; P = .3), the acetabular abduction was 43.9° (SD, 5.8°) in the arthroscopy cohort (vs 42.9° [SD, 6.1°] in the control cohort; P = .4), and the acetabular anteversion was 21.1° (SD, 7.5°) in the arthroscopy cohort (vs 20.8° [SD, 7.1°] in the control cohort; P = .8).
At 6-week follow-up, the arthroscopy cohort showed a significantly decreased WOMAC score compared with the control cohort (72.9 [SD, 15.5] vs 80.5 [SD, 11.8], respectively; P = .05). In addition, there was a trend towards a decreased SF-12 mental component score in the arthroscopy cohort (52.2 [SD, 9.3] vs 56.5 [SD, 7.8] in the control cohort; P = .06). However, none of the remaining clinical indices showed a significant difference between the 2 cohorts, and there was no difference in range of motion between the 2 cohorts at the 6-week follow-up visit (Table 4).
In addition, at 3-month follow-up, no statistically significant differences were seen between the 2 cohorts for any of the clinical indices or range of motion values. Both groups continued to improve rapidly, with HHS of 96.9 (SD, 3.5) in the arthroscopy cohort and 95.5 (SD, 6.6) in the control cohort, and WOMAC scores of 88.7 (SD, 10.2) and 89.5 (SD, 9.8), respectively (Table 5). Similarly, at the 6-month and 1-year follow-up intervals, the 2 cohorts showed continued improvement in their clinical measures, with no statistically significant differences between the 2 cohorts (Tables 6, 7).
At the most recent follow-up visit, more than 1 year after surgery, the HHS was 99.5 (SD, 1.3) in the arthroscopy cohort and 99.2 (SD, 9.7) in the control cohort (P = .9), and the WOMAC score was 93.5 (SD, 11.3) and 92.4 (SD, 12.2), respectively (P = .8). No significant perioperative complications were seen in the arthroscopy cohort. In the arthroscopy cohort, 1 patient was diagnosed with a deep venous thrombosis 2 weeks after the procedure and was placed on low-molecular-weight heparin and coumadin for treatment. A second patient in the arthroscopy cohort had continued serosanguinous drainage for 4 days postoperatively, which resolved with continued compressive dressings. To date, no patients in the arthroscopy or control cohorts have required a second operation or revision of their components.
Discussion
Given the increasing prevalence of hip arthroscopies to treat multiple disorders of the native joint, it is important to assess the potential consequences of these procedures on future arthroplasties. Piedade and colleagues,13 in a retrospective review of 1474 primary total knee arthroplasties, showed a prior bony procedure (high tibial osteotomy, tibial plateau fracture, patellar realignment) to be predictive of decreased range of motion postoperatively. In addition, a prior knee arthroscopy was associated with a higher rate of postoperative complications, with 30% of the complications requiring a reoperation, and 8.3% of the complications requiring a revision total knee arthroplasty. Kaplan-Meier survival curves showed a survival rate of only 86.8% in those patients with a prior knee arthroscopy (vs 98.1% in those without a prior knee surgery).22 Therefore, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy. After the initial 6-week follow-up visit, no significant difference was seen in the functional outcomes between those patients with or without a history of prior hip arthroscopy who received an HRA.
After analysis of patient outcomes using multiple clinical measurement tools, at 6-week, 3-month, 6-month, 1-year, and most recent follow-up intervals, the only significant difference between the 2 cohorts was the WOMAC score at 6-week follow-up. Interestingly, there was no significant difference seen in the other clinical assessments, including the SF-12 score, HHS, range of motion, or UCLA activity score (although this did trend towards significance). This can be explained by the difference in both the mode of administration and various metrics assessed by these instruments. In comparison to the HHS evaluation, the patient completes the WOMAC (rather than the clinician) and also provides a more detailed assessment of symptoms, pain, stiffness, and activities of daily living.17 Therefore, this study suggests that patients with a prior hip arthroscopy may require more time to return to their activities of daily living after an HRA. However, whether the statistically significant difference between the 2 scores translates into a clinically significant difference can be questioned.
The clinical outcomes of this series of patients were excellent at the short-term follow-up, and both groups achieved clinical results comparable to prior reported results of HRA.1,10,23,24 However, despite these results, there are several limitations to this study. First, longer-term follow-up is required to determine if any significant differences (such as aseptic loosening, infection, and prosthesis survival) are associated with a prior hip arthroscopy. In addition, this study included a relatively small cohort of patients who had a prior hip arthroscopy. However, a relatively large, single-surgeon database of 1357 HRAs was reviewed, with only 51 cases being reported (3.7%). With the increasing popularity of hip arthroscopy, the number of patients presenting for HRA will likely continue to increase. However, despite these limitations, this study shows that a prior hip arthroscopy does not appear to affect the short-term, clinical outcomes of a metal-on-metal HRA.
1. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty. Surgical Technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):234-249.
2. Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86(2):177-184.
3. Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88(5):592-600.
4. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
5. Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92(16):2663-2671.
6. Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study.
J Bone Joint Surg Br. 2007;89(2):169-173.
7. deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92(12):1642-1647.
8. Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-450.
9. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. Adelaide: Australian Orthopaedic Association; 2011. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2011. Accessed September 16, 2014.
10. Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg Br. 2012;94(3):315-321.
11. McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop. 2011;469(2):362-371.
12. Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93(7):890-896.
13. Piedade SR, Pinaroli A, Servien E, Neyret P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2009;17(4):328-333.
14. Gonzalez Della Valle A, Serota A, Go G, et al. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop. 2006;(444):146-153.
15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
16. Kershaw CJ, Atkins RM, Dodd CA, Bulstrode CJ. Revision total hip arthroplasty for aseptic failure. A review of 276 cases. J Bone Joint Surg Br. 1991;73(4):564-568.
17. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473-2476.
18. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
19. Clark JM, Freeman MA, Witham D. The relationship of neck orientation to the shape of the proximal femur. J Arthroplasty. 1987;2(2):99-109.
20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
21. Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop. 1995;(316):106-111.
22. Piedade SR, Pinaroli A, Servien E, Neyret P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2737-2743.
23. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86(1):28-39.
24. Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br. 2008;90(4):436-441.
Metal-on-metal hip resurfacing arthroplasty (HRA) remains an alternative to total hip arthroplasty (THA) in appropriately selected, younger, active adults with degenerative hip disease.1-4 While concerns remain regarding the potential for adverse local tissue reactions from wear of the metal-on-metal bearing surface,5-8 10-year data from the Australian Orthopaedic Association National Joint Replacement Registry Annual Report9 showed a revision rate of only 6.3% when the Birmingham Hip Resurfacing (BHR) System was used (Smith & Nephew Inc, Memphis, Tennessee).In addition, in an independent review of 230 consecutive BHRs at a mean follow-up of 10.4 years, Coulter and colleagues10 showed encouraging clinical results, with a mean Oxford Hip Score of 45.0 and a mean University of California at Los Angeles (UCLA) activity score of 7.4.
Similar to the prior increase in popularity of HRA, hip arthroscopy has also become much more commonplace, and its indications continue to evolve.11 Hip arthroscopy has been used in the native hip joint to manage femoroacetabular impingement, labral tears, and iliopsoas tendinopathy, among other conditions.12 In addition, the use of hip arthroscopy has not been limited to the native hip but also has increased as a diagnostic and therapeutic procedure after hip arthroplasties. Bajwa and Villar12 found hip arthroscopy to be diagnostic in 23 of 24 patients who underwent the procedure after a hip arthroplasty, concluding that arthroscopy is a useful adjunct in the diagnosis of symptomatic arthroplasties.
Therefore, hip arthroscopy has been shown to be an effective modality to treat pathology in both the native hip and after hip arthroplasties. However, the effect of a prior hip arthroscopy on the outcome of a subsequent metal-on-metal HRA has not been determined. Piedade and colleagues13 showed a prior knee arthroscopy to increase the risk of postoperative complications and subsequent revision after total knee arthroplasty. Complications included reflex sympathetic dystrophy, undiagnosed pain, infection, stiffness, and component loosening. A prior osteochondroplasty at the femoral head-neck junction could increase the risk of femoral neck fracture after a subsequent HRA. Thus, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy and to compare these results with a cohort of patients who received an HRA with no prior hip surgeries. Our hypothesis is that a prior hip arthroscopy will lead to inferior outcomes in patients undergoing HRA.
Materials and Methods
This study is a retrospective, case-control study using a 1:2 matching analysis. Dr. Su performed all HRAs, which were enrolled in an institutional review board–approved arthroplasty registry. All HRAs were performed using the BHR System.
The surgical technique for hip resurfacing arthroplasty has been described.1 All procedures were performed via a posterior approach with the patient in the lateral decubitus position. All patients received a hybrid metal-on-metal hip resurfacing, with an uncemented acetabular component and cemented femoral component. Intraoperative anesthesia for all patients was performed via a combined spinal-epidural anesthetic, and an epidural patient-controlled analgesic was used for the first day postoperatively, followed by a transition to oral analgesics. The sizes of the acetabular and femoral components were recorded for each hip resurfacing. Postoperatively, intermittent pneumatic compression devices were placed upon arrival in the recovery room, and active ankle flexion and extension exercises were initiated immediately after the patient’s neurologic function returned.14 Aspirin was used for chemical deep venous thrombosis prophylaxis in all patients postoperatively for a period of 6 weeks. Full weight-bearing, with the use of crutches for assistance with balance, was permitted immediately. Crutches were used for a period of 3 weeks prior to being discontinued.
From a database of 1357 HRAs (all BHR implants) performed between June 2006 and June 2012, 51 patients were identified who received an HRA after a prior hip arthroscopy. Eight patients were excluded because they did not possess adequate clinical documentation or were lost to follow-up. In the remaining 43 patients, there were 32 men and 11 women (21 right hips, 22 left hips), which formed the arthroscopy cohort. Two patients had a history of multiple hip arthroscopies (1 patient with 2 prior procedures, 1 patient with 3 prior procedures). The mean (SD) time from the most recent hip arthroscopy to the HRA was 2.5 (2.5) years. Table 1 presents a summary of the hip arthroscopy procedures (including only the most recent hip arthroscopy procedure in those with multiple arthroscopies).
Patient demographic variables (age, body mass index [BMI]) were recorded preoperatively, along with the Harris Hip Score (HHS),15 UCLA activity score,16 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score,17 and preoperative hip range of motion (flexion, extension, abduction, adduction, internal rotation, and external rotation). The same clinical indices were assessed postoperatively along with the Short Form-12 (SF-12) Health Survey Score,18 at the 6-week, 3-month, 6-month, 1-year, and most recent follow-up visits.
Radiographic assessment consisted of a low anteroposterior (AP) pelvic radiograph (with the radiographic beam centered on the pubic symphysis) and a cross-table lateral radiograph obtained at the most recent follow-up visit. Both the acetabular component abduction relative to the inter-teardrop line, and the angle between the femoral stem and the anatomic axis of the femoral shaft (stem-shaft angle) were measured on AP radiographs.19,20 Acetabular component anteversion was measured on the cross-table lateral radiographs as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the long axis plane of the body (Figures A, B).21
The same registry database was used to identify patients who received an HRA without a prior history of arthroscopy or hip surgery. A 1:2 matching analysis for those patients with a prior hip arthroscopy to those without a prior hip arthroscopy was performed to formulate a control group (control cohort) of 86 patients. Each patient in the arthroscopy cohort was matched with 2 patients in the control cohort based on the following parameters: age (± 6 years), sex (same), BMI (± 4 kg/m2), femoral head size (± 4 mm), and preoperative HHS and WOMAC scores (± 7 points). In the event an arthroscopy patient matched to 2 or more control patients, the patients who minimized the least squared error among the matching variables were selected.
Statistical Analysis
All data were collected and analyzed using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Statistical comparisons between the 2 cohorts regarding demographic variables, clinical outcomes, and radiographic alignment were performed using an unpaired, Student 2-tailed t test, with statistical significance set at P ≤ .05.
Results
A comparison of the results of the 1:2 matching analysis between the arthroscopy and control cohorts is presented in Table 2. There was no significant difference in the preoperative age, BMI, femoral head size, HHS, or WOMAC score between the 2 cohorts. However, the control cohort did show a more severe, preoperative flexion contracture (as expressed by a decreased amount of extension) and a decreased amount of preoperative abduction (Table 3). The preoperative UCLA activity score was also decreased in the control cohort, but this was not statistically significant.
The mean (SD) follow-up was 2.0 (1.0) years in the arthroscopy cohort and 2.1 (1.1) years in the control cohort. There was no significant difference in radiographic alignment between the 2 cohorts. The stem-shaft angle was 139.3° (SD, 5.4°) in the arthroscopy cohort (vs 138.3° [SD, 5.5°] in the control cohort; P = .3), the acetabular abduction was 43.9° (SD, 5.8°) in the arthroscopy cohort (vs 42.9° [SD, 6.1°] in the control cohort; P = .4), and the acetabular anteversion was 21.1° (SD, 7.5°) in the arthroscopy cohort (vs 20.8° [SD, 7.1°] in the control cohort; P = .8).
At 6-week follow-up, the arthroscopy cohort showed a significantly decreased WOMAC score compared with the control cohort (72.9 [SD, 15.5] vs 80.5 [SD, 11.8], respectively; P = .05). In addition, there was a trend towards a decreased SF-12 mental component score in the arthroscopy cohort (52.2 [SD, 9.3] vs 56.5 [SD, 7.8] in the control cohort; P = .06). However, none of the remaining clinical indices showed a significant difference between the 2 cohorts, and there was no difference in range of motion between the 2 cohorts at the 6-week follow-up visit (Table 4).
In addition, at 3-month follow-up, no statistically significant differences were seen between the 2 cohorts for any of the clinical indices or range of motion values. Both groups continued to improve rapidly, with HHS of 96.9 (SD, 3.5) in the arthroscopy cohort and 95.5 (SD, 6.6) in the control cohort, and WOMAC scores of 88.7 (SD, 10.2) and 89.5 (SD, 9.8), respectively (Table 5). Similarly, at the 6-month and 1-year follow-up intervals, the 2 cohorts showed continued improvement in their clinical measures, with no statistically significant differences between the 2 cohorts (Tables 6, 7).
At the most recent follow-up visit, more than 1 year after surgery, the HHS was 99.5 (SD, 1.3) in the arthroscopy cohort and 99.2 (SD, 9.7) in the control cohort (P = .9), and the WOMAC score was 93.5 (SD, 11.3) and 92.4 (SD, 12.2), respectively (P = .8). No significant perioperative complications were seen in the arthroscopy cohort. In the arthroscopy cohort, 1 patient was diagnosed with a deep venous thrombosis 2 weeks after the procedure and was placed on low-molecular-weight heparin and coumadin for treatment. A second patient in the arthroscopy cohort had continued serosanguinous drainage for 4 days postoperatively, which resolved with continued compressive dressings. To date, no patients in the arthroscopy or control cohorts have required a second operation or revision of their components.
Discussion
Given the increasing prevalence of hip arthroscopies to treat multiple disorders of the native joint, it is important to assess the potential consequences of these procedures on future arthroplasties. Piedade and colleagues,13 in a retrospective review of 1474 primary total knee arthroplasties, showed a prior bony procedure (high tibial osteotomy, tibial plateau fracture, patellar realignment) to be predictive of decreased range of motion postoperatively. In addition, a prior knee arthroscopy was associated with a higher rate of postoperative complications, with 30% of the complications requiring a reoperation, and 8.3% of the complications requiring a revision total knee arthroplasty. Kaplan-Meier survival curves showed a survival rate of only 86.8% in those patients with a prior knee arthroscopy (vs 98.1% in those without a prior knee surgery).22 Therefore, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy. After the initial 6-week follow-up visit, no significant difference was seen in the functional outcomes between those patients with or without a history of prior hip arthroscopy who received an HRA.
After analysis of patient outcomes using multiple clinical measurement tools, at 6-week, 3-month, 6-month, 1-year, and most recent follow-up intervals, the only significant difference between the 2 cohorts was the WOMAC score at 6-week follow-up. Interestingly, there was no significant difference seen in the other clinical assessments, including the SF-12 score, HHS, range of motion, or UCLA activity score (although this did trend towards significance). This can be explained by the difference in both the mode of administration and various metrics assessed by these instruments. In comparison to the HHS evaluation, the patient completes the WOMAC (rather than the clinician) and also provides a more detailed assessment of symptoms, pain, stiffness, and activities of daily living.17 Therefore, this study suggests that patients with a prior hip arthroscopy may require more time to return to their activities of daily living after an HRA. However, whether the statistically significant difference between the 2 scores translates into a clinically significant difference can be questioned.
The clinical outcomes of this series of patients were excellent at the short-term follow-up, and both groups achieved clinical results comparable to prior reported results of HRA.1,10,23,24 However, despite these results, there are several limitations to this study. First, longer-term follow-up is required to determine if any significant differences (such as aseptic loosening, infection, and prosthesis survival) are associated with a prior hip arthroscopy. In addition, this study included a relatively small cohort of patients who had a prior hip arthroscopy. However, a relatively large, single-surgeon database of 1357 HRAs was reviewed, with only 51 cases being reported (3.7%). With the increasing popularity of hip arthroscopy, the number of patients presenting for HRA will likely continue to increase. However, despite these limitations, this study shows that a prior hip arthroscopy does not appear to affect the short-term, clinical outcomes of a metal-on-metal HRA.
Metal-on-metal hip resurfacing arthroplasty (HRA) remains an alternative to total hip arthroplasty (THA) in appropriately selected, younger, active adults with degenerative hip disease.1-4 While concerns remain regarding the potential for adverse local tissue reactions from wear of the metal-on-metal bearing surface,5-8 10-year data from the Australian Orthopaedic Association National Joint Replacement Registry Annual Report9 showed a revision rate of only 6.3% when the Birmingham Hip Resurfacing (BHR) System was used (Smith & Nephew Inc, Memphis, Tennessee).In addition, in an independent review of 230 consecutive BHRs at a mean follow-up of 10.4 years, Coulter and colleagues10 showed encouraging clinical results, with a mean Oxford Hip Score of 45.0 and a mean University of California at Los Angeles (UCLA) activity score of 7.4.
Similar to the prior increase in popularity of HRA, hip arthroscopy has also become much more commonplace, and its indications continue to evolve.11 Hip arthroscopy has been used in the native hip joint to manage femoroacetabular impingement, labral tears, and iliopsoas tendinopathy, among other conditions.12 In addition, the use of hip arthroscopy has not been limited to the native hip but also has increased as a diagnostic and therapeutic procedure after hip arthroplasties. Bajwa and Villar12 found hip arthroscopy to be diagnostic in 23 of 24 patients who underwent the procedure after a hip arthroplasty, concluding that arthroscopy is a useful adjunct in the diagnosis of symptomatic arthroplasties.
Therefore, hip arthroscopy has been shown to be an effective modality to treat pathology in both the native hip and after hip arthroplasties. However, the effect of a prior hip arthroscopy on the outcome of a subsequent metal-on-metal HRA has not been determined. Piedade and colleagues13 showed a prior knee arthroscopy to increase the risk of postoperative complications and subsequent revision after total knee arthroplasty. Complications included reflex sympathetic dystrophy, undiagnosed pain, infection, stiffness, and component loosening. A prior osteochondroplasty at the femoral head-neck junction could increase the risk of femoral neck fracture after a subsequent HRA. Thus, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy and to compare these results with a cohort of patients who received an HRA with no prior hip surgeries. Our hypothesis is that a prior hip arthroscopy will lead to inferior outcomes in patients undergoing HRA.
Materials and Methods
This study is a retrospective, case-control study using a 1:2 matching analysis. Dr. Su performed all HRAs, which were enrolled in an institutional review board–approved arthroplasty registry. All HRAs were performed using the BHR System.
The surgical technique for hip resurfacing arthroplasty has been described.1 All procedures were performed via a posterior approach with the patient in the lateral decubitus position. All patients received a hybrid metal-on-metal hip resurfacing, with an uncemented acetabular component and cemented femoral component. Intraoperative anesthesia for all patients was performed via a combined spinal-epidural anesthetic, and an epidural patient-controlled analgesic was used for the first day postoperatively, followed by a transition to oral analgesics. The sizes of the acetabular and femoral components were recorded for each hip resurfacing. Postoperatively, intermittent pneumatic compression devices were placed upon arrival in the recovery room, and active ankle flexion and extension exercises were initiated immediately after the patient’s neurologic function returned.14 Aspirin was used for chemical deep venous thrombosis prophylaxis in all patients postoperatively for a period of 6 weeks. Full weight-bearing, with the use of crutches for assistance with balance, was permitted immediately. Crutches were used for a period of 3 weeks prior to being discontinued.
From a database of 1357 HRAs (all BHR implants) performed between June 2006 and June 2012, 51 patients were identified who received an HRA after a prior hip arthroscopy. Eight patients were excluded because they did not possess adequate clinical documentation or were lost to follow-up. In the remaining 43 patients, there were 32 men and 11 women (21 right hips, 22 left hips), which formed the arthroscopy cohort. Two patients had a history of multiple hip arthroscopies (1 patient with 2 prior procedures, 1 patient with 3 prior procedures). The mean (SD) time from the most recent hip arthroscopy to the HRA was 2.5 (2.5) years. Table 1 presents a summary of the hip arthroscopy procedures (including only the most recent hip arthroscopy procedure in those with multiple arthroscopies).
Patient demographic variables (age, body mass index [BMI]) were recorded preoperatively, along with the Harris Hip Score (HHS),15 UCLA activity score,16 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score,17 and preoperative hip range of motion (flexion, extension, abduction, adduction, internal rotation, and external rotation). The same clinical indices were assessed postoperatively along with the Short Form-12 (SF-12) Health Survey Score,18 at the 6-week, 3-month, 6-month, 1-year, and most recent follow-up visits.
Radiographic assessment consisted of a low anteroposterior (AP) pelvic radiograph (with the radiographic beam centered on the pubic symphysis) and a cross-table lateral radiograph obtained at the most recent follow-up visit. Both the acetabular component abduction relative to the inter-teardrop line, and the angle between the femoral stem and the anatomic axis of the femoral shaft (stem-shaft angle) were measured on AP radiographs.19,20 Acetabular component anteversion was measured on the cross-table lateral radiographs as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the long axis plane of the body (Figures A, B).21
The same registry database was used to identify patients who received an HRA without a prior history of arthroscopy or hip surgery. A 1:2 matching analysis for those patients with a prior hip arthroscopy to those without a prior hip arthroscopy was performed to formulate a control group (control cohort) of 86 patients. Each patient in the arthroscopy cohort was matched with 2 patients in the control cohort based on the following parameters: age (± 6 years), sex (same), BMI (± 4 kg/m2), femoral head size (± 4 mm), and preoperative HHS and WOMAC scores (± 7 points). In the event an arthroscopy patient matched to 2 or more control patients, the patients who minimized the least squared error among the matching variables were selected.
Statistical Analysis
All data were collected and analyzed using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Statistical comparisons between the 2 cohorts regarding demographic variables, clinical outcomes, and radiographic alignment were performed using an unpaired, Student 2-tailed t test, with statistical significance set at P ≤ .05.
Results
A comparison of the results of the 1:2 matching analysis between the arthroscopy and control cohorts is presented in Table 2. There was no significant difference in the preoperative age, BMI, femoral head size, HHS, or WOMAC score between the 2 cohorts. However, the control cohort did show a more severe, preoperative flexion contracture (as expressed by a decreased amount of extension) and a decreased amount of preoperative abduction (Table 3). The preoperative UCLA activity score was also decreased in the control cohort, but this was not statistically significant.
The mean (SD) follow-up was 2.0 (1.0) years in the arthroscopy cohort and 2.1 (1.1) years in the control cohort. There was no significant difference in radiographic alignment between the 2 cohorts. The stem-shaft angle was 139.3° (SD, 5.4°) in the arthroscopy cohort (vs 138.3° [SD, 5.5°] in the control cohort; P = .3), the acetabular abduction was 43.9° (SD, 5.8°) in the arthroscopy cohort (vs 42.9° [SD, 6.1°] in the control cohort; P = .4), and the acetabular anteversion was 21.1° (SD, 7.5°) in the arthroscopy cohort (vs 20.8° [SD, 7.1°] in the control cohort; P = .8).
At 6-week follow-up, the arthroscopy cohort showed a significantly decreased WOMAC score compared with the control cohort (72.9 [SD, 15.5] vs 80.5 [SD, 11.8], respectively; P = .05). In addition, there was a trend towards a decreased SF-12 mental component score in the arthroscopy cohort (52.2 [SD, 9.3] vs 56.5 [SD, 7.8] in the control cohort; P = .06). However, none of the remaining clinical indices showed a significant difference between the 2 cohorts, and there was no difference in range of motion between the 2 cohorts at the 6-week follow-up visit (Table 4).
In addition, at 3-month follow-up, no statistically significant differences were seen between the 2 cohorts for any of the clinical indices or range of motion values. Both groups continued to improve rapidly, with HHS of 96.9 (SD, 3.5) in the arthroscopy cohort and 95.5 (SD, 6.6) in the control cohort, and WOMAC scores of 88.7 (SD, 10.2) and 89.5 (SD, 9.8), respectively (Table 5). Similarly, at the 6-month and 1-year follow-up intervals, the 2 cohorts showed continued improvement in their clinical measures, with no statistically significant differences between the 2 cohorts (Tables 6, 7).
At the most recent follow-up visit, more than 1 year after surgery, the HHS was 99.5 (SD, 1.3) in the arthroscopy cohort and 99.2 (SD, 9.7) in the control cohort (P = .9), and the WOMAC score was 93.5 (SD, 11.3) and 92.4 (SD, 12.2), respectively (P = .8). No significant perioperative complications were seen in the arthroscopy cohort. In the arthroscopy cohort, 1 patient was diagnosed with a deep venous thrombosis 2 weeks after the procedure and was placed on low-molecular-weight heparin and coumadin for treatment. A second patient in the arthroscopy cohort had continued serosanguinous drainage for 4 days postoperatively, which resolved with continued compressive dressings. To date, no patients in the arthroscopy or control cohorts have required a second operation or revision of their components.
Discussion
Given the increasing prevalence of hip arthroscopies to treat multiple disorders of the native joint, it is important to assess the potential consequences of these procedures on future arthroplasties. Piedade and colleagues,13 in a retrospective review of 1474 primary total knee arthroplasties, showed a prior bony procedure (high tibial osteotomy, tibial plateau fracture, patellar realignment) to be predictive of decreased range of motion postoperatively. In addition, a prior knee arthroscopy was associated with a higher rate of postoperative complications, with 30% of the complications requiring a reoperation, and 8.3% of the complications requiring a revision total knee arthroplasty. Kaplan-Meier survival curves showed a survival rate of only 86.8% in those patients with a prior knee arthroscopy (vs 98.1% in those without a prior knee surgery).22 Therefore, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy. After the initial 6-week follow-up visit, no significant difference was seen in the functional outcomes between those patients with or without a history of prior hip arthroscopy who received an HRA.
After analysis of patient outcomes using multiple clinical measurement tools, at 6-week, 3-month, 6-month, 1-year, and most recent follow-up intervals, the only significant difference between the 2 cohorts was the WOMAC score at 6-week follow-up. Interestingly, there was no significant difference seen in the other clinical assessments, including the SF-12 score, HHS, range of motion, or UCLA activity score (although this did trend towards significance). This can be explained by the difference in both the mode of administration and various metrics assessed by these instruments. In comparison to the HHS evaluation, the patient completes the WOMAC (rather than the clinician) and also provides a more detailed assessment of symptoms, pain, stiffness, and activities of daily living.17 Therefore, this study suggests that patients with a prior hip arthroscopy may require more time to return to their activities of daily living after an HRA. However, whether the statistically significant difference between the 2 scores translates into a clinically significant difference can be questioned.
The clinical outcomes of this series of patients were excellent at the short-term follow-up, and both groups achieved clinical results comparable to prior reported results of HRA.1,10,23,24 However, despite these results, there are several limitations to this study. First, longer-term follow-up is required to determine if any significant differences (such as aseptic loosening, infection, and prosthesis survival) are associated with a prior hip arthroscopy. In addition, this study included a relatively small cohort of patients who had a prior hip arthroscopy. However, a relatively large, single-surgeon database of 1357 HRAs was reviewed, with only 51 cases being reported (3.7%). With the increasing popularity of hip arthroscopy, the number of patients presenting for HRA will likely continue to increase. However, despite these limitations, this study shows that a prior hip arthroscopy does not appear to affect the short-term, clinical outcomes of a metal-on-metal HRA.
1. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty. Surgical Technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):234-249.
2. Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86(2):177-184.
3. Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88(5):592-600.
4. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
5. Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92(16):2663-2671.
6. Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study.
J Bone Joint Surg Br. 2007;89(2):169-173.
7. deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92(12):1642-1647.
8. Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-450.
9. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. Adelaide: Australian Orthopaedic Association; 2011. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2011. Accessed September 16, 2014.
10. Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg Br. 2012;94(3):315-321.
11. McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop. 2011;469(2):362-371.
12. Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93(7):890-896.
13. Piedade SR, Pinaroli A, Servien E, Neyret P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2009;17(4):328-333.
14. Gonzalez Della Valle A, Serota A, Go G, et al. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop. 2006;(444):146-153.
15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
16. Kershaw CJ, Atkins RM, Dodd CA, Bulstrode CJ. Revision total hip arthroplasty for aseptic failure. A review of 276 cases. J Bone Joint Surg Br. 1991;73(4):564-568.
17. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473-2476.
18. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
19. Clark JM, Freeman MA, Witham D. The relationship of neck orientation to the shape of the proximal femur. J Arthroplasty. 1987;2(2):99-109.
20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
21. Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop. 1995;(316):106-111.
22. Piedade SR, Pinaroli A, Servien E, Neyret P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2737-2743.
23. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86(1):28-39.
24. Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br. 2008;90(4):436-441.
1. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty. Surgical Technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):234-249.
2. Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86(2):177-184.
3. Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88(5):592-600.
4. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
5. Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92(16):2663-2671.
6. Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study.
J Bone Joint Surg Br. 2007;89(2):169-173.
7. deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92(12):1642-1647.
8. Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-450.
9. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. Adelaide: Australian Orthopaedic Association; 2011. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2011. Accessed September 16, 2014.
10. Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg Br. 2012;94(3):315-321.
11. McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop. 2011;469(2):362-371.
12. Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93(7):890-896.
13. Piedade SR, Pinaroli A, Servien E, Neyret P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2009;17(4):328-333.
14. Gonzalez Della Valle A, Serota A, Go G, et al. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop. 2006;(444):146-153.
15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
16. Kershaw CJ, Atkins RM, Dodd CA, Bulstrode CJ. Revision total hip arthroplasty for aseptic failure. A review of 276 cases. J Bone Joint Surg Br. 1991;73(4):564-568.
17. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473-2476.
18. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
19. Clark JM, Freeman MA, Witham D. The relationship of neck orientation to the shape of the proximal femur. J Arthroplasty. 1987;2(2):99-109.
20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
21. Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop. 1995;(316):106-111.
22. Piedade SR, Pinaroli A, Servien E, Neyret P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2737-2743.
23. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86(1):28-39.
24. Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br. 2008;90(4):436-441.
Pilot Study for an Orthopedic Surgical Training Laboratory for Basic Motor Skills
For the resident, the surgical residency is physically, emotionally, and intellectually demanding, requiring longitudinally concentrated effort. Although education of orthopedic surgeons necessarily occurs within the context of the health care delivery system, vital lessons also are taught in laboratories, skill stations, and surgical simulators. Before practice-based learning can take place, residents must gain experience and demonstrate growth in surgical skills, including decision-making and technical skills. These skill sets are difficult to systematically teach and objectively analyze.
The most effective way to teach and assess a resident’s knowledge of musculoskeletal medicine remains unclear at this point. Much of the current literature addresses the issue at the medical student level.1-7 Some studies have shown the effectiveness of surgical training programs, both cadaveric and computer-based simulators, in teaching various surgical skill sets.8-14 The orthopedic literature has seen a boom in surgical simulators aimed at the upper-level resident. Many of the topics involve use of arthroscopic simulators.15-19 Evidence suggests that simulators can discriminate between novice and expert users, but discrimination between novice and intermediate trainees in surgical education should be paramount.20
The American Board of Orthopaedic Surgery (ABOS) and the orthopedic Residency Review Committee (RRC) recommended new requirements for structured motor skills training in basic orthopedic surgery education,21 which were approved by the Accreditation Council for Graduate Medical Education (ACGME) board of directors and went into effect on July 1, 2013. In response to the new ACGME guidelines, our institution created a skills laboratory devoted to surgical simulation. Our focus in implementing this surgical skills simulation was junior-level, specifically postgraduate year 1 to 3 (PGY-1 to PGY-3), orthopedic residents. Our first goal was to set up a series of surgical training stations to educate junior-level residents in 4 core areas: handling and comfort with basic power equipment, casting/splinting, suturing, and surgical instrument identification. A secondary goal was to objectively evaluate the residents through written examinations (presession–postsession) and a novel ankle fracture model (pre–post).
Materials and Methods
Institutional review board approval was obtained before beginning the investigation.
Written Examination
We created a multiple-choice 25-question written examination (Appendix) and administered it to 11 junior residents before and after they participated in the training. This examination assessed their knowledge base of basic orthopedic tenets, including basic bone healing, basic fracture repair (Arbeitsgemeinschaft für Osteosynthesefragen [AO] principles22), suturing, surgical instrument identification, casting/splinting, and elementary implant-design rationale.
Evaluator Scorecard
We created an evaluation scorecard (Figure 1) and had 2 faculty members and 2 senior-level residents complete it independently. Junior residents were evaluated on a sawbones lateral malleolar ankle fracture model at 2 time points. As with the written examinations, the junior residents completed the fracture model both before and immediately after the multiple skill sessions. Each of the 15 data points was scored from 1 to 4, for a total of 60 points.
Facility for Surgical Training Session
Our Clinical Skills Education and Assessment Center houses small-group interactive laboratories for administration, debriefing, and assessment of simulations with the latest in audiovisual equipment. Five stations were created: hands-on introduction to surgical power equipment using sawbones, wood, and polyvinylchloride (PVC) pipe; hands-on introduction to casting and splinting; hands-on introduction to suturing; hands-on interaction with surgical scrub technician assisting with instrument identification; and didactic PowerPoint (Microsoft, Redmond, Washington) presentation focusing on core trauma competencies, basic orthopedic design rationale, and basic bone biology.
Development of Surgical Skills Training Session
Multiple faculty members and senior-level residents collaborated to create the skill stations (Figure 2), which were designed based on ACGME recommendations and on weaknesses our program had seen in junior-level residents. We devoted an afternoon to this session, excusing our program’s junior residents from clinical responsibilities. Four PGY-1, 5 PGY-2, and 2 PGY-3 residents participated. (Four of our 15 junior residents were unable to attend because of clinical responsibilities.) The afternoon started by dividing the 11 junior residents into 2 groups. Before the session, while one group performed the ankle fracture model and was being evaluated, the other took the written examination. This closely timed portion was allotted only 20 minutes. Then residents were divided into 5 groups of 2 or 3 and were rotated through all 5 stations. Forty minutes were allotted for each station. Residents were not evaluated during this portion. The stations were intended solely for education, and each station was staffed by a faculty member and/or senior-level resident.
Cordless reciprocating saws and drills were purchased to introduce and refine junior residents’ motor skills. Sawbones, 2×4-in sections of wood, and PVC pipe were used in the training. Emphasis was placed on tactile feel and feedback with both sawing and drilling. For the casting and splinting session, we used 4-in fiberglass, 4-in plaster rolls, and cotton soft roll to demonstrate a multitude of common casts and splints (Figure 3). Casts included short- and long-arm casts and short-leg casts. Splinting included coaptation, sugar tong, and ulnar gutter splints for the upper extremity and a short-leg posterior splint for the lower extremity.
The didactic PowerPoint presentation drew largely from content in chapters of the book AO Principles of Fracture Management.22 Content included condensed, to-the-point high-yield summaries of AO tenets, basic bone healing and biology, and orthopedic implant-design rationale focused on these elementary principles:
◾ Basic screw design, including cortical, cancellous, and locking screw designs.
◾ Evolution of plate osteosynthesis to currently used locking compression plate.
◾ Locking plate principles.
◾ Lag technique.
◾ Plate use: compression mode, neutralization, bridging, buttress, anti-glide.
The suturing portion was performed with thawed ham hocks (Figure 4). This model replicates live tissue layers and allows a layered closure technique as a training tool. Both 0 and 2-0 absorbable suture were available for a layered, deep fascial closure; also available was 2-0 nonabsorbable nylon for the skin. Staple guns were available, as were basic surgical instruments, including quality needle drivers, Adson forceps, and suture scissors. The knots demonstrated included simple, horizontal mattress, vertical mattress, and tension-relieving. One- and 2-hand tying and instrument tying were reinforced.
The final session consisted of surgical instrument identification. A certified orthopedic scrub technician participated. On site were multiple trays, including a basic bone set, a hand-and-foot set, small and large fragment sets, and a hip set. A detailed review of each set was led by the surgical technician. This review was followed by a question-and-answer session with the junior residents. After the session, we ended with the written examination and the ankle fracture model.
Statistical Methods
We report presession and postsession means, modes, and medians as measures of score-central tendencies. Our small sample size makes the assumption of Gaussian distribution tenuous and more susceptible to outliers. Therefore, in addition to reporting means, we include medians and modes to more accurately account for outliers. Moreover, the κ statistic is a robust measure of interrater agreement for 2 or more groups. We report κ statistics to determine the interrater reliability of 4 independent observers.
Results
Written Examination
Eleven residents (PGY-1 to PGY-3) completed the examination (Table 1). For the entire group, mean (SD) presession percentile was 87.3 (10.4), median was 88, and mode was 96; mean (SD) was 80 (12.6) for PGY-1, 89.6 (6.7) for PGY-2, and 96 (5.7) for PGY-3. For the entire group, mean (SD) postsession percentile was 92 (8.4), median was 96, and mode was 96; mean (SD) was 85 (10.5) for PGY-1, 96 (4) for PGY-2, and 96 (0) for PGY-3 (Table 2).
There was a significant presession–postsession difference in scores among all test takers, regardless of training level (P = .019). The PGY-1 level did not reach statistical significance in improvement from presession to postsession (P = .080); the PGY-2 level also did not reach statistical significance in improvement (P = .099); the PGY-3 level did not have enough participants to calculate a P value based on a paired Student t test.
Ankle Fracture Model
Actual percentile scores are listed in Table 3. For the entire group, mean (SD) overall presession percentile was 68.6 (13.9), median was 67, and mode was 67; mean (SD) was 58.8 (9.8) for PGY-1, 76.1 (13.6) for PGY-2, and 69.5 (9.8) for PGY-3. For the entire group, mean (SD) postsession percentile was 95.2 (5.2), median was 97, and mode was 97; mean (SD) was 91.8 (6.3) for PGY-1, 97.1 (3.5) for PGY-2, and 97.3 (2.4) for PGY-3.
There was a large and significant presession–postsession difference in scores among all test takers, regardless of training level (P = .03). Each group reached statistical significance in improvement from presession to postsession: PGY-1 (P = .04), PGY-2 (P = .01), and PGY-3 (P = .03).
For κ calculations, we adjusted all scores to ordinal data and thus used a standard grading system:
Score Grade
90–100 A
80–89 B
70–79 C
60–69 D
0–59 F
For the presession fracture model, the κ among the 4 independent observational scorers was 0.1148 (Table 4), which is poor based on κ scoring criteria and which we attribute to the particularly harsh grading by 1 observational scorer (faculty 1) relative to the other scorers’. Examination of the κ scores of faculty 1 and faculty 2 indicated only 9.09% agreement. Conversely, the κ among resident scorers agreed 54.55% of the time. Removing faculty 1 as an outlier raised the κ score dramatically, to 0.3125 (fair interobserver agreement).
For the postsession fracture model, the κ among the 4 independent observational scorers improved only marginally, to 0.1156 (still poor), again attributed to a difference in severity of grading: faculty 1 (harsh) versus faculty 2 (relatively kind). Examination of the κ scores of faculty 1 and faculty 2 revealed 72.73% agreement; residents agreed 81.82% of the time.
Discussion
The importance of surgical skill development in resident education is emphasized in the ACGME Core Competencies.23 The ACGME instructed all programs to require residents to gain competency in 6 areas: patient care, interpersonal and communication skills, medical knowledge, professionalism, practice-based learning and systems-based practice. Although many surgeon educators and residents are focused on these 6 Core Competencies, current standards do not require surgical skills laboratory training and simply require residents to log cases into the ACGME website. Minimal case number recommendations are in place for graduating senior residents, but these numbers are based on averages with no strong scientific basis.
Although sweeping changes in orthopedic residency training went into effect July 1, 2013, this system remains untested and may offer room for improvement. One change is the restructuring of the PGY-1 internship. A basic surgical skills curriculum must include goals, objectives, and assessment metrics; skills used in the initial management of injured patients, including splinting, casting, application of traction devices, and other types of immobilization; and basic operative skills, including soft-tissue management, suturing, bone management, arthroscopy, fluoroscopy, and use of basic orthopedic equipment.21
Orthopedic program directors and residents were recently surveyed regarding the current state of orthopedic motor skills training.24 Three key findings deserve emphasis: There is a lack of objective criteria for evaluating resident performance in the skills laboratory; most program directors who have a laboratory do not understand the associated costs; and the most significant issue for program directors is the financial challenge of operating a motor skills laboratory. The survey findings strongly suggest that proposed changes in skills training should be accompanied by careful cost analysis before widespread implementation.
Although various online demonstrations of entire surgeries are available, as are textbooks describing a generalized approach to musculoskeletal surgery, we assume that, as laid out in the Core Competencies, residents are fine-tuning their surgical skills by actively participating in operating rooms under direct observation of attending physicians. To our knowledge, however, there are no data regarding how often this happens in the operative setting, where volume and efficiency are becoming increasingly scrutinized. There has been much concern over how hour restrictions will affect residents’ total operative experience.25,26 Finally, we have no means to objectively evaluate residents’ surgical skills on graduation.
Other programs have implemented surgical skill simulators, but an orthopedics-specific surgical skills laboratory, to our knowledge, has been discussed in only 1 study.21 Results from randomized controlled trials reported in the general surgery literature have proved simulation-based training leads to detectable benefits for learners in clinical settings.27-29 Over the past decade, some alternative surgical skills training methods have been adopted in orthopedic surgery as well. These methods include hands-on training in specifically designed surgical skills laboratories using cadaver models or synthetic bones; software tools; and computerized simulators. In recent years, numerous studies reported in the orthopedic literature have examined arthroscopic simulators in residency training.18-20,30-34 However, these studies are arguably more specific to sports subspecialties and thus more pertinent to upper-level trainees.
Our study results showed that surgical skills laboratory training should be a required aspect of our residents’ training. Although less of a dramatic improvement was noted in the written examination component of the laboratory, the overall knowledge base improved (Table 3). This was especially evident at the PGY-1 level, where written examination scores increased from a presession median of 80% to a postsession median of 85%. A larger degree of improvement was found with the ankle fracture model, and there was statistical improvement at all training levels, from PGY-1 to PGY-3. Previous work has shown that intensive laboratory-based training can be effective, particularly for first-year residents. Sonnadara and colleagues35 demonstrated that a 30-day intensive surgical skills course effectively helped first-year orthopedic residents develop targeted basic surgical skills. In a follow-up study, Sonnadara and colleagues36 demonstrated that a surgical skills course completed at the beginning of a residency was effective in teaching targeted technical skills, and that skills taught in this manner can have excellent retention rates.
There are limitations inherent in our skills course. The κ agreement in the ankle fracture model was low before and after administration, which we attribute to 1 observer outlier. This could be amended by removing outliers and further objectifying and simplifying the scoring system (A–F). Right now, we do not have enough data to determine whether the scores actually improve significantly through the training years or whether they will correlate with operating room experience. Our study had no control. For future investigations, we are considering having general orthopedic surgeons from the community perform the same scenarios and be graded with the same checklists as a control. Implementation, however, may be a challenge. Both our written examination and our ankle fracture model checklist have not been validated—this is one of our next steps. The point system used to score the ankle fracture model was subjectively developed and would benefit from further standardization before drawing conclusions about true validity.
Conclusion
Orthopedic residency programs, like programs in other surgical specialties, are increasingly focused on teaching and documenting the learning of core competencies, even as work-hour restrictions and demands for clinical efficiency limit the amount of time residents spend in the operating room. We have demonstrated the potential value of an intensive laboratory in improving junior-level residents’ basic surgical skills and knowledge. We will continue to refine our methods, with a goal being to create reproducible models that could be adapted by other orthopedic residency programs and by other surgical educators.
1. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
2. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
3. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
4. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
5. Corbett EC Jr, Elnicki DM, Conaway MR. When should students learn essential physical examination skills? Views of internal medicine clerkship directors in North America. Acad Med. 2008;83(1):96-99.
6. Coady DA, Walker DJ, Kay LJ. Teaching medical students musculoskeletal examination skills: identifying barriers to learning and ways of overcoming them. Scand J Rheumatol. 2004;33(1):47-51.
7. Saleh K, Messner R, Axtell S, Harris I, Mahowald ML. Development and evaluation of an integrated musculoskeletal disease course for medical students. J Bone Joint Surg Am. 2004;86(8):1653-1658.
8. van Empel PJ, Verdam MG, Huirne JA, Bonjer HJ, Meijerink WJ, Scheele F. Open knot-tying skills: resident skills assessed. J Obstet Gynaecol Res. 2013;39(5):1030-1036.
9. Barrier BF, Thompson AB, McCullough MW, Occhino JA. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7(6):374-379.
10. Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19(2):124-129.
11. Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(4):767-774.
12. Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58(2):524-528.
13. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013;74(2):664-670.
14. Sanfey HA, Dunnington GL. Basic surgical skills testing for junior residents: current views of general surgery program directors. J Am Coll Surg. 2011;212(3):406-412.
15. Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97.
16. Jackson WF, Khan T, Alvand A, et al. Learning and retaining simulated arthroscopic meniscal repair skills. J Bone Joint Surg Am. 2012;94(17):e132.
17. Pernar LI, Smink DS, Hicks G, Peyre SE. Residents can successfully teach basic surgical skills in the simulation center. J Surg Educ. 2012;69(5):617-622.
18. Tuijthof GJ, Visser P, Sierevelt IN, Van Dijk CN, Kerkhoffs GM. Does perception of usefulness of arthroscopic simulators differ with levels of experience? Clin Orthop. 2011;469(6):1701-1708.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Slade Shantz JA, Leiter JR, Gottschalk T, MacDonald PB. The internal validity of arthroscopic simulators and their effectiveness in arthroscopic education. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):33-40.
21. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747-757.
22. Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. Stuttgart, Germany: Thieme; 2007.
23. Chen CL, Chen WC, Chiang JH, Ho CF. Interscapular hibernoma: case report and literature review. Kaohsiung J Med Sci. 2011;27(8):348-352.
24. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
25. Baskies MA, Ruchelsman DE, Capeci CM, Zuckerman JD, Egol KA. Operative experience in an orthopaedic surgery residency program: the effect of work-hour restrictions. J Bone Joint Surg Am. 2008;90(4):924-927.
26. Pappas AJ, Teague DC. The impact of the Accreditation Council for Graduate Medical Education work-hour regulations on the surgical experience of orthopaedic surgery residents. J Bone Joint Surg Am. 2007;89(4):904-909.
27. Palter VN, Grantcharov T, Harvey A, Macrae HM. Ex vivo technical skills training transfers to the operating room and enhances cognitive learning: a randomized controlled trial. Ann Surg. 2011;253(5):886-889.
28. Franzeck FM, Rosenthal R, Muller MK, et al. Prospective randomized controlled trial of simulator-based versus traditional in-surgery laparoscopic camera navigation training. Surg Endosc. 2012;26(1):235-241.
29. Zendejas B, Cook DA, Bingener J, et al. Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg. 2011;254(3):502-509.
30. Hui Y, Safir O, Dubrowski A, Carnahan H. What skills should simulation training in arthroscopy teach residents? A focus on resident input. Int J Comput Assist Radiol Surg. 2013;8(6):945-953.
31. Butler A, Olson T, Koehler R, Nicandri G. Do the skills acquired by novice surgeons using anatomic dry models transfer effectively to the task of diagnostic knee arthroscopy performed on cadaveric specimens? J Bone Joint Surg Am. 2013;95(3):e15(1-8).
32. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
33. Elliott MJ, Caprise PA, Henning AE, Kurtz CA, Sekiya JK. Diagnostic knee arthroscopy: a pilot study to evaluate surgical skills. Arthroscopy. 2012;28(2):218-224.
34. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop. 2011;82(1):90-95.
35. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
36. Sonnadara RR, Garbedian S, Safir O, et al. Orthopaedic boot camp II: examining the retention rates of an intensive surgical skills course. Surgery. 2012;151(6):803-807.
For the resident, the surgical residency is physically, emotionally, and intellectually demanding, requiring longitudinally concentrated effort. Although education of orthopedic surgeons necessarily occurs within the context of the health care delivery system, vital lessons also are taught in laboratories, skill stations, and surgical simulators. Before practice-based learning can take place, residents must gain experience and demonstrate growth in surgical skills, including decision-making and technical skills. These skill sets are difficult to systematically teach and objectively analyze.
The most effective way to teach and assess a resident’s knowledge of musculoskeletal medicine remains unclear at this point. Much of the current literature addresses the issue at the medical student level.1-7 Some studies have shown the effectiveness of surgical training programs, both cadaveric and computer-based simulators, in teaching various surgical skill sets.8-14 The orthopedic literature has seen a boom in surgical simulators aimed at the upper-level resident. Many of the topics involve use of arthroscopic simulators.15-19 Evidence suggests that simulators can discriminate between novice and expert users, but discrimination between novice and intermediate trainees in surgical education should be paramount.20
The American Board of Orthopaedic Surgery (ABOS) and the orthopedic Residency Review Committee (RRC) recommended new requirements for structured motor skills training in basic orthopedic surgery education,21 which were approved by the Accreditation Council for Graduate Medical Education (ACGME) board of directors and went into effect on July 1, 2013. In response to the new ACGME guidelines, our institution created a skills laboratory devoted to surgical simulation. Our focus in implementing this surgical skills simulation was junior-level, specifically postgraduate year 1 to 3 (PGY-1 to PGY-3), orthopedic residents. Our first goal was to set up a series of surgical training stations to educate junior-level residents in 4 core areas: handling and comfort with basic power equipment, casting/splinting, suturing, and surgical instrument identification. A secondary goal was to objectively evaluate the residents through written examinations (presession–postsession) and a novel ankle fracture model (pre–post).
Materials and Methods
Institutional review board approval was obtained before beginning the investigation.
Written Examination
We created a multiple-choice 25-question written examination (Appendix) and administered it to 11 junior residents before and after they participated in the training. This examination assessed their knowledge base of basic orthopedic tenets, including basic bone healing, basic fracture repair (Arbeitsgemeinschaft für Osteosynthesefragen [AO] principles22), suturing, surgical instrument identification, casting/splinting, and elementary implant-design rationale.
Evaluator Scorecard
We created an evaluation scorecard (Figure 1) and had 2 faculty members and 2 senior-level residents complete it independently. Junior residents were evaluated on a sawbones lateral malleolar ankle fracture model at 2 time points. As with the written examinations, the junior residents completed the fracture model both before and immediately after the multiple skill sessions. Each of the 15 data points was scored from 1 to 4, for a total of 60 points.
Facility for Surgical Training Session
Our Clinical Skills Education and Assessment Center houses small-group interactive laboratories for administration, debriefing, and assessment of simulations with the latest in audiovisual equipment. Five stations were created: hands-on introduction to surgical power equipment using sawbones, wood, and polyvinylchloride (PVC) pipe; hands-on introduction to casting and splinting; hands-on introduction to suturing; hands-on interaction with surgical scrub technician assisting with instrument identification; and didactic PowerPoint (Microsoft, Redmond, Washington) presentation focusing on core trauma competencies, basic orthopedic design rationale, and basic bone biology.
Development of Surgical Skills Training Session
Multiple faculty members and senior-level residents collaborated to create the skill stations (Figure 2), which were designed based on ACGME recommendations and on weaknesses our program had seen in junior-level residents. We devoted an afternoon to this session, excusing our program’s junior residents from clinical responsibilities. Four PGY-1, 5 PGY-2, and 2 PGY-3 residents participated. (Four of our 15 junior residents were unable to attend because of clinical responsibilities.) The afternoon started by dividing the 11 junior residents into 2 groups. Before the session, while one group performed the ankle fracture model and was being evaluated, the other took the written examination. This closely timed portion was allotted only 20 minutes. Then residents were divided into 5 groups of 2 or 3 and were rotated through all 5 stations. Forty minutes were allotted for each station. Residents were not evaluated during this portion. The stations were intended solely for education, and each station was staffed by a faculty member and/or senior-level resident.
Cordless reciprocating saws and drills were purchased to introduce and refine junior residents’ motor skills. Sawbones, 2×4-in sections of wood, and PVC pipe were used in the training. Emphasis was placed on tactile feel and feedback with both sawing and drilling. For the casting and splinting session, we used 4-in fiberglass, 4-in plaster rolls, and cotton soft roll to demonstrate a multitude of common casts and splints (Figure 3). Casts included short- and long-arm casts and short-leg casts. Splinting included coaptation, sugar tong, and ulnar gutter splints for the upper extremity and a short-leg posterior splint for the lower extremity.
The didactic PowerPoint presentation drew largely from content in chapters of the book AO Principles of Fracture Management.22 Content included condensed, to-the-point high-yield summaries of AO tenets, basic bone healing and biology, and orthopedic implant-design rationale focused on these elementary principles:
◾ Basic screw design, including cortical, cancellous, and locking screw designs.
◾ Evolution of plate osteosynthesis to currently used locking compression plate.
◾ Locking plate principles.
◾ Lag technique.
◾ Plate use: compression mode, neutralization, bridging, buttress, anti-glide.
The suturing portion was performed with thawed ham hocks (Figure 4). This model replicates live tissue layers and allows a layered closure technique as a training tool. Both 0 and 2-0 absorbable suture were available for a layered, deep fascial closure; also available was 2-0 nonabsorbable nylon for the skin. Staple guns were available, as were basic surgical instruments, including quality needle drivers, Adson forceps, and suture scissors. The knots demonstrated included simple, horizontal mattress, vertical mattress, and tension-relieving. One- and 2-hand tying and instrument tying were reinforced.
The final session consisted of surgical instrument identification. A certified orthopedic scrub technician participated. On site were multiple trays, including a basic bone set, a hand-and-foot set, small and large fragment sets, and a hip set. A detailed review of each set was led by the surgical technician. This review was followed by a question-and-answer session with the junior residents. After the session, we ended with the written examination and the ankle fracture model.
Statistical Methods
We report presession and postsession means, modes, and medians as measures of score-central tendencies. Our small sample size makes the assumption of Gaussian distribution tenuous and more susceptible to outliers. Therefore, in addition to reporting means, we include medians and modes to more accurately account for outliers. Moreover, the κ statistic is a robust measure of interrater agreement for 2 or more groups. We report κ statistics to determine the interrater reliability of 4 independent observers.
Results
Written Examination
Eleven residents (PGY-1 to PGY-3) completed the examination (Table 1). For the entire group, mean (SD) presession percentile was 87.3 (10.4), median was 88, and mode was 96; mean (SD) was 80 (12.6) for PGY-1, 89.6 (6.7) for PGY-2, and 96 (5.7) for PGY-3. For the entire group, mean (SD) postsession percentile was 92 (8.4), median was 96, and mode was 96; mean (SD) was 85 (10.5) for PGY-1, 96 (4) for PGY-2, and 96 (0) for PGY-3 (Table 2).
There was a significant presession–postsession difference in scores among all test takers, regardless of training level (P = .019). The PGY-1 level did not reach statistical significance in improvement from presession to postsession (P = .080); the PGY-2 level also did not reach statistical significance in improvement (P = .099); the PGY-3 level did not have enough participants to calculate a P value based on a paired Student t test.
Ankle Fracture Model
Actual percentile scores are listed in Table 3. For the entire group, mean (SD) overall presession percentile was 68.6 (13.9), median was 67, and mode was 67; mean (SD) was 58.8 (9.8) for PGY-1, 76.1 (13.6) for PGY-2, and 69.5 (9.8) for PGY-3. For the entire group, mean (SD) postsession percentile was 95.2 (5.2), median was 97, and mode was 97; mean (SD) was 91.8 (6.3) for PGY-1, 97.1 (3.5) for PGY-2, and 97.3 (2.4) for PGY-3.
There was a large and significant presession–postsession difference in scores among all test takers, regardless of training level (P = .03). Each group reached statistical significance in improvement from presession to postsession: PGY-1 (P = .04), PGY-2 (P = .01), and PGY-3 (P = .03).
For κ calculations, we adjusted all scores to ordinal data and thus used a standard grading system:
Score Grade
90–100 A
80–89 B
70–79 C
60–69 D
0–59 F
For the presession fracture model, the κ among the 4 independent observational scorers was 0.1148 (Table 4), which is poor based on κ scoring criteria and which we attribute to the particularly harsh grading by 1 observational scorer (faculty 1) relative to the other scorers’. Examination of the κ scores of faculty 1 and faculty 2 indicated only 9.09% agreement. Conversely, the κ among resident scorers agreed 54.55% of the time. Removing faculty 1 as an outlier raised the κ score dramatically, to 0.3125 (fair interobserver agreement).
For the postsession fracture model, the κ among the 4 independent observational scorers improved only marginally, to 0.1156 (still poor), again attributed to a difference in severity of grading: faculty 1 (harsh) versus faculty 2 (relatively kind). Examination of the κ scores of faculty 1 and faculty 2 revealed 72.73% agreement; residents agreed 81.82% of the time.
Discussion
The importance of surgical skill development in resident education is emphasized in the ACGME Core Competencies.23 The ACGME instructed all programs to require residents to gain competency in 6 areas: patient care, interpersonal and communication skills, medical knowledge, professionalism, practice-based learning and systems-based practice. Although many surgeon educators and residents are focused on these 6 Core Competencies, current standards do not require surgical skills laboratory training and simply require residents to log cases into the ACGME website. Minimal case number recommendations are in place for graduating senior residents, but these numbers are based on averages with no strong scientific basis.
Although sweeping changes in orthopedic residency training went into effect July 1, 2013, this system remains untested and may offer room for improvement. One change is the restructuring of the PGY-1 internship. A basic surgical skills curriculum must include goals, objectives, and assessment metrics; skills used in the initial management of injured patients, including splinting, casting, application of traction devices, and other types of immobilization; and basic operative skills, including soft-tissue management, suturing, bone management, arthroscopy, fluoroscopy, and use of basic orthopedic equipment.21
Orthopedic program directors and residents were recently surveyed regarding the current state of orthopedic motor skills training.24 Three key findings deserve emphasis: There is a lack of objective criteria for evaluating resident performance in the skills laboratory; most program directors who have a laboratory do not understand the associated costs; and the most significant issue for program directors is the financial challenge of operating a motor skills laboratory. The survey findings strongly suggest that proposed changes in skills training should be accompanied by careful cost analysis before widespread implementation.
Although various online demonstrations of entire surgeries are available, as are textbooks describing a generalized approach to musculoskeletal surgery, we assume that, as laid out in the Core Competencies, residents are fine-tuning their surgical skills by actively participating in operating rooms under direct observation of attending physicians. To our knowledge, however, there are no data regarding how often this happens in the operative setting, where volume and efficiency are becoming increasingly scrutinized. There has been much concern over how hour restrictions will affect residents’ total operative experience.25,26 Finally, we have no means to objectively evaluate residents’ surgical skills on graduation.
Other programs have implemented surgical skill simulators, but an orthopedics-specific surgical skills laboratory, to our knowledge, has been discussed in only 1 study.21 Results from randomized controlled trials reported in the general surgery literature have proved simulation-based training leads to detectable benefits for learners in clinical settings.27-29 Over the past decade, some alternative surgical skills training methods have been adopted in orthopedic surgery as well. These methods include hands-on training in specifically designed surgical skills laboratories using cadaver models or synthetic bones; software tools; and computerized simulators. In recent years, numerous studies reported in the orthopedic literature have examined arthroscopic simulators in residency training.18-20,30-34 However, these studies are arguably more specific to sports subspecialties and thus more pertinent to upper-level trainees.
Our study results showed that surgical skills laboratory training should be a required aspect of our residents’ training. Although less of a dramatic improvement was noted in the written examination component of the laboratory, the overall knowledge base improved (Table 3). This was especially evident at the PGY-1 level, where written examination scores increased from a presession median of 80% to a postsession median of 85%. A larger degree of improvement was found with the ankle fracture model, and there was statistical improvement at all training levels, from PGY-1 to PGY-3. Previous work has shown that intensive laboratory-based training can be effective, particularly for first-year residents. Sonnadara and colleagues35 demonstrated that a 30-day intensive surgical skills course effectively helped first-year orthopedic residents develop targeted basic surgical skills. In a follow-up study, Sonnadara and colleagues36 demonstrated that a surgical skills course completed at the beginning of a residency was effective in teaching targeted technical skills, and that skills taught in this manner can have excellent retention rates.
There are limitations inherent in our skills course. The κ agreement in the ankle fracture model was low before and after administration, which we attribute to 1 observer outlier. This could be amended by removing outliers and further objectifying and simplifying the scoring system (A–F). Right now, we do not have enough data to determine whether the scores actually improve significantly through the training years or whether they will correlate with operating room experience. Our study had no control. For future investigations, we are considering having general orthopedic surgeons from the community perform the same scenarios and be graded with the same checklists as a control. Implementation, however, may be a challenge. Both our written examination and our ankle fracture model checklist have not been validated—this is one of our next steps. The point system used to score the ankle fracture model was subjectively developed and would benefit from further standardization before drawing conclusions about true validity.
Conclusion
Orthopedic residency programs, like programs in other surgical specialties, are increasingly focused on teaching and documenting the learning of core competencies, even as work-hour restrictions and demands for clinical efficiency limit the amount of time residents spend in the operating room. We have demonstrated the potential value of an intensive laboratory in improving junior-level residents’ basic surgical skills and knowledge. We will continue to refine our methods, with a goal being to create reproducible models that could be adapted by other orthopedic residency programs and by other surgical educators.
For the resident, the surgical residency is physically, emotionally, and intellectually demanding, requiring longitudinally concentrated effort. Although education of orthopedic surgeons necessarily occurs within the context of the health care delivery system, vital lessons also are taught in laboratories, skill stations, and surgical simulators. Before practice-based learning can take place, residents must gain experience and demonstrate growth in surgical skills, including decision-making and technical skills. These skill sets are difficult to systematically teach and objectively analyze.
The most effective way to teach and assess a resident’s knowledge of musculoskeletal medicine remains unclear at this point. Much of the current literature addresses the issue at the medical student level.1-7 Some studies have shown the effectiveness of surgical training programs, both cadaveric and computer-based simulators, in teaching various surgical skill sets.8-14 The orthopedic literature has seen a boom in surgical simulators aimed at the upper-level resident. Many of the topics involve use of arthroscopic simulators.15-19 Evidence suggests that simulators can discriminate between novice and expert users, but discrimination between novice and intermediate trainees in surgical education should be paramount.20
The American Board of Orthopaedic Surgery (ABOS) and the orthopedic Residency Review Committee (RRC) recommended new requirements for structured motor skills training in basic orthopedic surgery education,21 which were approved by the Accreditation Council for Graduate Medical Education (ACGME) board of directors and went into effect on July 1, 2013. In response to the new ACGME guidelines, our institution created a skills laboratory devoted to surgical simulation. Our focus in implementing this surgical skills simulation was junior-level, specifically postgraduate year 1 to 3 (PGY-1 to PGY-3), orthopedic residents. Our first goal was to set up a series of surgical training stations to educate junior-level residents in 4 core areas: handling and comfort with basic power equipment, casting/splinting, suturing, and surgical instrument identification. A secondary goal was to objectively evaluate the residents through written examinations (presession–postsession) and a novel ankle fracture model (pre–post).
Materials and Methods
Institutional review board approval was obtained before beginning the investigation.
Written Examination
We created a multiple-choice 25-question written examination (Appendix) and administered it to 11 junior residents before and after they participated in the training. This examination assessed their knowledge base of basic orthopedic tenets, including basic bone healing, basic fracture repair (Arbeitsgemeinschaft für Osteosynthesefragen [AO] principles22), suturing, surgical instrument identification, casting/splinting, and elementary implant-design rationale.
Evaluator Scorecard
We created an evaluation scorecard (Figure 1) and had 2 faculty members and 2 senior-level residents complete it independently. Junior residents were evaluated on a sawbones lateral malleolar ankle fracture model at 2 time points. As with the written examinations, the junior residents completed the fracture model both before and immediately after the multiple skill sessions. Each of the 15 data points was scored from 1 to 4, for a total of 60 points.
Facility for Surgical Training Session
Our Clinical Skills Education and Assessment Center houses small-group interactive laboratories for administration, debriefing, and assessment of simulations with the latest in audiovisual equipment. Five stations were created: hands-on introduction to surgical power equipment using sawbones, wood, and polyvinylchloride (PVC) pipe; hands-on introduction to casting and splinting; hands-on introduction to suturing; hands-on interaction with surgical scrub technician assisting with instrument identification; and didactic PowerPoint (Microsoft, Redmond, Washington) presentation focusing on core trauma competencies, basic orthopedic design rationale, and basic bone biology.
Development of Surgical Skills Training Session
Multiple faculty members and senior-level residents collaborated to create the skill stations (Figure 2), which were designed based on ACGME recommendations and on weaknesses our program had seen in junior-level residents. We devoted an afternoon to this session, excusing our program’s junior residents from clinical responsibilities. Four PGY-1, 5 PGY-2, and 2 PGY-3 residents participated. (Four of our 15 junior residents were unable to attend because of clinical responsibilities.) The afternoon started by dividing the 11 junior residents into 2 groups. Before the session, while one group performed the ankle fracture model and was being evaluated, the other took the written examination. This closely timed portion was allotted only 20 minutes. Then residents were divided into 5 groups of 2 or 3 and were rotated through all 5 stations. Forty minutes were allotted for each station. Residents were not evaluated during this portion. The stations were intended solely for education, and each station was staffed by a faculty member and/or senior-level resident.
Cordless reciprocating saws and drills were purchased to introduce and refine junior residents’ motor skills. Sawbones, 2×4-in sections of wood, and PVC pipe were used in the training. Emphasis was placed on tactile feel and feedback with both sawing and drilling. For the casting and splinting session, we used 4-in fiberglass, 4-in plaster rolls, and cotton soft roll to demonstrate a multitude of common casts and splints (Figure 3). Casts included short- and long-arm casts and short-leg casts. Splinting included coaptation, sugar tong, and ulnar gutter splints for the upper extremity and a short-leg posterior splint for the lower extremity.
The didactic PowerPoint presentation drew largely from content in chapters of the book AO Principles of Fracture Management.22 Content included condensed, to-the-point high-yield summaries of AO tenets, basic bone healing and biology, and orthopedic implant-design rationale focused on these elementary principles:
◾ Basic screw design, including cortical, cancellous, and locking screw designs.
◾ Evolution of plate osteosynthesis to currently used locking compression plate.
◾ Locking plate principles.
◾ Lag technique.
◾ Plate use: compression mode, neutralization, bridging, buttress, anti-glide.
The suturing portion was performed with thawed ham hocks (Figure 4). This model replicates live tissue layers and allows a layered closure technique as a training tool. Both 0 and 2-0 absorbable suture were available for a layered, deep fascial closure; also available was 2-0 nonabsorbable nylon for the skin. Staple guns were available, as were basic surgical instruments, including quality needle drivers, Adson forceps, and suture scissors. The knots demonstrated included simple, horizontal mattress, vertical mattress, and tension-relieving. One- and 2-hand tying and instrument tying were reinforced.
The final session consisted of surgical instrument identification. A certified orthopedic scrub technician participated. On site were multiple trays, including a basic bone set, a hand-and-foot set, small and large fragment sets, and a hip set. A detailed review of each set was led by the surgical technician. This review was followed by a question-and-answer session with the junior residents. After the session, we ended with the written examination and the ankle fracture model.
Statistical Methods
We report presession and postsession means, modes, and medians as measures of score-central tendencies. Our small sample size makes the assumption of Gaussian distribution tenuous and more susceptible to outliers. Therefore, in addition to reporting means, we include medians and modes to more accurately account for outliers. Moreover, the κ statistic is a robust measure of interrater agreement for 2 or more groups. We report κ statistics to determine the interrater reliability of 4 independent observers.
Results
Written Examination
Eleven residents (PGY-1 to PGY-3) completed the examination (Table 1). For the entire group, mean (SD) presession percentile was 87.3 (10.4), median was 88, and mode was 96; mean (SD) was 80 (12.6) for PGY-1, 89.6 (6.7) for PGY-2, and 96 (5.7) for PGY-3. For the entire group, mean (SD) postsession percentile was 92 (8.4), median was 96, and mode was 96; mean (SD) was 85 (10.5) for PGY-1, 96 (4) for PGY-2, and 96 (0) for PGY-3 (Table 2).
There was a significant presession–postsession difference in scores among all test takers, regardless of training level (P = .019). The PGY-1 level did not reach statistical significance in improvement from presession to postsession (P = .080); the PGY-2 level also did not reach statistical significance in improvement (P = .099); the PGY-3 level did not have enough participants to calculate a P value based on a paired Student t test.
Ankle Fracture Model
Actual percentile scores are listed in Table 3. For the entire group, mean (SD) overall presession percentile was 68.6 (13.9), median was 67, and mode was 67; mean (SD) was 58.8 (9.8) for PGY-1, 76.1 (13.6) for PGY-2, and 69.5 (9.8) for PGY-3. For the entire group, mean (SD) postsession percentile was 95.2 (5.2), median was 97, and mode was 97; mean (SD) was 91.8 (6.3) for PGY-1, 97.1 (3.5) for PGY-2, and 97.3 (2.4) for PGY-3.
There was a large and significant presession–postsession difference in scores among all test takers, regardless of training level (P = .03). Each group reached statistical significance in improvement from presession to postsession: PGY-1 (P = .04), PGY-2 (P = .01), and PGY-3 (P = .03).
For κ calculations, we adjusted all scores to ordinal data and thus used a standard grading system:
Score Grade
90–100 A
80–89 B
70–79 C
60–69 D
0–59 F
For the presession fracture model, the κ among the 4 independent observational scorers was 0.1148 (Table 4), which is poor based on κ scoring criteria and which we attribute to the particularly harsh grading by 1 observational scorer (faculty 1) relative to the other scorers’. Examination of the κ scores of faculty 1 and faculty 2 indicated only 9.09% agreement. Conversely, the κ among resident scorers agreed 54.55% of the time. Removing faculty 1 as an outlier raised the κ score dramatically, to 0.3125 (fair interobserver agreement).
For the postsession fracture model, the κ among the 4 independent observational scorers improved only marginally, to 0.1156 (still poor), again attributed to a difference in severity of grading: faculty 1 (harsh) versus faculty 2 (relatively kind). Examination of the κ scores of faculty 1 and faculty 2 revealed 72.73% agreement; residents agreed 81.82% of the time.
Discussion
The importance of surgical skill development in resident education is emphasized in the ACGME Core Competencies.23 The ACGME instructed all programs to require residents to gain competency in 6 areas: patient care, interpersonal and communication skills, medical knowledge, professionalism, practice-based learning and systems-based practice. Although many surgeon educators and residents are focused on these 6 Core Competencies, current standards do not require surgical skills laboratory training and simply require residents to log cases into the ACGME website. Minimal case number recommendations are in place for graduating senior residents, but these numbers are based on averages with no strong scientific basis.
Although sweeping changes in orthopedic residency training went into effect July 1, 2013, this system remains untested and may offer room for improvement. One change is the restructuring of the PGY-1 internship. A basic surgical skills curriculum must include goals, objectives, and assessment metrics; skills used in the initial management of injured patients, including splinting, casting, application of traction devices, and other types of immobilization; and basic operative skills, including soft-tissue management, suturing, bone management, arthroscopy, fluoroscopy, and use of basic orthopedic equipment.21
Orthopedic program directors and residents were recently surveyed regarding the current state of orthopedic motor skills training.24 Three key findings deserve emphasis: There is a lack of objective criteria for evaluating resident performance in the skills laboratory; most program directors who have a laboratory do not understand the associated costs; and the most significant issue for program directors is the financial challenge of operating a motor skills laboratory. The survey findings strongly suggest that proposed changes in skills training should be accompanied by careful cost analysis before widespread implementation.
Although various online demonstrations of entire surgeries are available, as are textbooks describing a generalized approach to musculoskeletal surgery, we assume that, as laid out in the Core Competencies, residents are fine-tuning their surgical skills by actively participating in operating rooms under direct observation of attending physicians. To our knowledge, however, there are no data regarding how often this happens in the operative setting, where volume and efficiency are becoming increasingly scrutinized. There has been much concern over how hour restrictions will affect residents’ total operative experience.25,26 Finally, we have no means to objectively evaluate residents’ surgical skills on graduation.
Other programs have implemented surgical skill simulators, but an orthopedics-specific surgical skills laboratory, to our knowledge, has been discussed in only 1 study.21 Results from randomized controlled trials reported in the general surgery literature have proved simulation-based training leads to detectable benefits for learners in clinical settings.27-29 Over the past decade, some alternative surgical skills training methods have been adopted in orthopedic surgery as well. These methods include hands-on training in specifically designed surgical skills laboratories using cadaver models or synthetic bones; software tools; and computerized simulators. In recent years, numerous studies reported in the orthopedic literature have examined arthroscopic simulators in residency training.18-20,30-34 However, these studies are arguably more specific to sports subspecialties and thus more pertinent to upper-level trainees.
Our study results showed that surgical skills laboratory training should be a required aspect of our residents’ training. Although less of a dramatic improvement was noted in the written examination component of the laboratory, the overall knowledge base improved (Table 3). This was especially evident at the PGY-1 level, where written examination scores increased from a presession median of 80% to a postsession median of 85%. A larger degree of improvement was found with the ankle fracture model, and there was statistical improvement at all training levels, from PGY-1 to PGY-3. Previous work has shown that intensive laboratory-based training can be effective, particularly for first-year residents. Sonnadara and colleagues35 demonstrated that a 30-day intensive surgical skills course effectively helped first-year orthopedic residents develop targeted basic surgical skills. In a follow-up study, Sonnadara and colleagues36 demonstrated that a surgical skills course completed at the beginning of a residency was effective in teaching targeted technical skills, and that skills taught in this manner can have excellent retention rates.
There are limitations inherent in our skills course. The κ agreement in the ankle fracture model was low before and after administration, which we attribute to 1 observer outlier. This could be amended by removing outliers and further objectifying and simplifying the scoring system (A–F). Right now, we do not have enough data to determine whether the scores actually improve significantly through the training years or whether they will correlate with operating room experience. Our study had no control. For future investigations, we are considering having general orthopedic surgeons from the community perform the same scenarios and be graded with the same checklists as a control. Implementation, however, may be a challenge. Both our written examination and our ankle fracture model checklist have not been validated—this is one of our next steps. The point system used to score the ankle fracture model was subjectively developed and would benefit from further standardization before drawing conclusions about true validity.
Conclusion
Orthopedic residency programs, like programs in other surgical specialties, are increasingly focused on teaching and documenting the learning of core competencies, even as work-hour restrictions and demands for clinical efficiency limit the amount of time residents spend in the operating room. We have demonstrated the potential value of an intensive laboratory in improving junior-level residents’ basic surgical skills and knowledge. We will continue to refine our methods, with a goal being to create reproducible models that could be adapted by other orthopedic residency programs and by other surgical educators.
1. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
2. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
3. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
4. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
5. Corbett EC Jr, Elnicki DM, Conaway MR. When should students learn essential physical examination skills? Views of internal medicine clerkship directors in North America. Acad Med. 2008;83(1):96-99.
6. Coady DA, Walker DJ, Kay LJ. Teaching medical students musculoskeletal examination skills: identifying barriers to learning and ways of overcoming them. Scand J Rheumatol. 2004;33(1):47-51.
7. Saleh K, Messner R, Axtell S, Harris I, Mahowald ML. Development and evaluation of an integrated musculoskeletal disease course for medical students. J Bone Joint Surg Am. 2004;86(8):1653-1658.
8. van Empel PJ, Verdam MG, Huirne JA, Bonjer HJ, Meijerink WJ, Scheele F. Open knot-tying skills: resident skills assessed. J Obstet Gynaecol Res. 2013;39(5):1030-1036.
9. Barrier BF, Thompson AB, McCullough MW, Occhino JA. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7(6):374-379.
10. Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19(2):124-129.
11. Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(4):767-774.
12. Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58(2):524-528.
13. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013;74(2):664-670.
14. Sanfey HA, Dunnington GL. Basic surgical skills testing for junior residents: current views of general surgery program directors. J Am Coll Surg. 2011;212(3):406-412.
15. Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97.
16. Jackson WF, Khan T, Alvand A, et al. Learning and retaining simulated arthroscopic meniscal repair skills. J Bone Joint Surg Am. 2012;94(17):e132.
17. Pernar LI, Smink DS, Hicks G, Peyre SE. Residents can successfully teach basic surgical skills in the simulation center. J Surg Educ. 2012;69(5):617-622.
18. Tuijthof GJ, Visser P, Sierevelt IN, Van Dijk CN, Kerkhoffs GM. Does perception of usefulness of arthroscopic simulators differ with levels of experience? Clin Orthop. 2011;469(6):1701-1708.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Slade Shantz JA, Leiter JR, Gottschalk T, MacDonald PB. The internal validity of arthroscopic simulators and their effectiveness in arthroscopic education. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):33-40.
21. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747-757.
22. Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. Stuttgart, Germany: Thieme; 2007.
23. Chen CL, Chen WC, Chiang JH, Ho CF. Interscapular hibernoma: case report and literature review. Kaohsiung J Med Sci. 2011;27(8):348-352.
24. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
25. Baskies MA, Ruchelsman DE, Capeci CM, Zuckerman JD, Egol KA. Operative experience in an orthopaedic surgery residency program: the effect of work-hour restrictions. J Bone Joint Surg Am. 2008;90(4):924-927.
26. Pappas AJ, Teague DC. The impact of the Accreditation Council for Graduate Medical Education work-hour regulations on the surgical experience of orthopaedic surgery residents. J Bone Joint Surg Am. 2007;89(4):904-909.
27. Palter VN, Grantcharov T, Harvey A, Macrae HM. Ex vivo technical skills training transfers to the operating room and enhances cognitive learning: a randomized controlled trial. Ann Surg. 2011;253(5):886-889.
28. Franzeck FM, Rosenthal R, Muller MK, et al. Prospective randomized controlled trial of simulator-based versus traditional in-surgery laparoscopic camera navigation training. Surg Endosc. 2012;26(1):235-241.
29. Zendejas B, Cook DA, Bingener J, et al. Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg. 2011;254(3):502-509.
30. Hui Y, Safir O, Dubrowski A, Carnahan H. What skills should simulation training in arthroscopy teach residents? A focus on resident input. Int J Comput Assist Radiol Surg. 2013;8(6):945-953.
31. Butler A, Olson T, Koehler R, Nicandri G. Do the skills acquired by novice surgeons using anatomic dry models transfer effectively to the task of diagnostic knee arthroscopy performed on cadaveric specimens? J Bone Joint Surg Am. 2013;95(3):e15(1-8).
32. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
33. Elliott MJ, Caprise PA, Henning AE, Kurtz CA, Sekiya JK. Diagnostic knee arthroscopy: a pilot study to evaluate surgical skills. Arthroscopy. 2012;28(2):218-224.
34. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop. 2011;82(1):90-95.
35. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
36. Sonnadara RR, Garbedian S, Safir O, et al. Orthopaedic boot camp II: examining the retention rates of an intensive surgical skills course. Surgery. 2012;151(6):803-807.
1. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
2. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
3. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
4. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
5. Corbett EC Jr, Elnicki DM, Conaway MR. When should students learn essential physical examination skills? Views of internal medicine clerkship directors in North America. Acad Med. 2008;83(1):96-99.
6. Coady DA, Walker DJ, Kay LJ. Teaching medical students musculoskeletal examination skills: identifying barriers to learning and ways of overcoming them. Scand J Rheumatol. 2004;33(1):47-51.
7. Saleh K, Messner R, Axtell S, Harris I, Mahowald ML. Development and evaluation of an integrated musculoskeletal disease course for medical students. J Bone Joint Surg Am. 2004;86(8):1653-1658.
8. van Empel PJ, Verdam MG, Huirne JA, Bonjer HJ, Meijerink WJ, Scheele F. Open knot-tying skills: resident skills assessed. J Obstet Gynaecol Res. 2013;39(5):1030-1036.
9. Barrier BF, Thompson AB, McCullough MW, Occhino JA. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7(6):374-379.
10. Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19(2):124-129.
11. Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(4):767-774.
12. Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58(2):524-528.
13. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013;74(2):664-670.
14. Sanfey HA, Dunnington GL. Basic surgical skills testing for junior residents: current views of general surgery program directors. J Am Coll Surg. 2011;212(3):406-412.
15. Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97.
16. Jackson WF, Khan T, Alvand A, et al. Learning and retaining simulated arthroscopic meniscal repair skills. J Bone Joint Surg Am. 2012;94(17):e132.
17. Pernar LI, Smink DS, Hicks G, Peyre SE. Residents can successfully teach basic surgical skills in the simulation center. J Surg Educ. 2012;69(5):617-622.
18. Tuijthof GJ, Visser P, Sierevelt IN, Van Dijk CN, Kerkhoffs GM. Does perception of usefulness of arthroscopic simulators differ with levels of experience? Clin Orthop. 2011;469(6):1701-1708.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Slade Shantz JA, Leiter JR, Gottschalk T, MacDonald PB. The internal validity of arthroscopic simulators and their effectiveness in arthroscopic education. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):33-40.
21. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747-757.
22. Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. Stuttgart, Germany: Thieme; 2007.
23. Chen CL, Chen WC, Chiang JH, Ho CF. Interscapular hibernoma: case report and literature review. Kaohsiung J Med Sci. 2011;27(8):348-352.
24. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
25. Baskies MA, Ruchelsman DE, Capeci CM, Zuckerman JD, Egol KA. Operative experience in an orthopaedic surgery residency program: the effect of work-hour restrictions. J Bone Joint Surg Am. 2008;90(4):924-927.
26. Pappas AJ, Teague DC. The impact of the Accreditation Council for Graduate Medical Education work-hour regulations on the surgical experience of orthopaedic surgery residents. J Bone Joint Surg Am. 2007;89(4):904-909.
27. Palter VN, Grantcharov T, Harvey A, Macrae HM. Ex vivo technical skills training transfers to the operating room and enhances cognitive learning: a randomized controlled trial. Ann Surg. 2011;253(5):886-889.
28. Franzeck FM, Rosenthal R, Muller MK, et al. Prospective randomized controlled trial of simulator-based versus traditional in-surgery laparoscopic camera navigation training. Surg Endosc. 2012;26(1):235-241.
29. Zendejas B, Cook DA, Bingener J, et al. Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg. 2011;254(3):502-509.
30. Hui Y, Safir O, Dubrowski A, Carnahan H. What skills should simulation training in arthroscopy teach residents? A focus on resident input. Int J Comput Assist Radiol Surg. 2013;8(6):945-953.
31. Butler A, Olson T, Koehler R, Nicandri G. Do the skills acquired by novice surgeons using anatomic dry models transfer effectively to the task of diagnostic knee arthroscopy performed on cadaveric specimens? J Bone Joint Surg Am. 2013;95(3):e15(1-8).
32. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
33. Elliott MJ, Caprise PA, Henning AE, Kurtz CA, Sekiya JK. Diagnostic knee arthroscopy: a pilot study to evaluate surgical skills. Arthroscopy. 2012;28(2):218-224.
34. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop. 2011;82(1):90-95.
35. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
36. Sonnadara RR, Garbedian S, Safir O, et al. Orthopaedic boot camp II: examining the retention rates of an intensive surgical skills course. Surgery. 2012;151(6):803-807.
Universal Hepatitis C Screening and Surgeon Safety
Letter to the Editor
Universal Hepatitis C Screening
and Surgeon Safety
I read with interest the article “Risk of Hepatitis C Virus Exposure in Orthopedic Surgery: Is Universal Screening Needed?” by Dr. DelSole and colleagues (Am J Orthop. 2014;43(6):E117-E123). The authors make a compelling case for universal hepatitis C screening, and I agree that this program may have significant benefits, including risk stratification for elective surgery and identification of patients for antiviral treatment. However, based largely on personal experiences, I disagree that preoperative knowledge of the patient’s seropositive status will improve surgeon safety.
The authors list a number of interventions to decrease the risk of exposure, such as the use of Kevlar gloves, double gloving, eye protection, and others. However, perhaps the largest barrier to their use is their perceived inconvenience. Surgeons often cite decreased dexterity and sensation in their opposition to double or thick gloves, for instance. My concern is that with the advent of universal screening, many surgeons will abandon their universal approach and only wear Kevlar or double gloves on infected cases.
I have observed first-hand such a policy this year, when I spent 3 months in orthopedic centers in Russia. In Russia, all patients undergo routine preoperative testing for both hepatitis C virus and human immunodeficiency virus (HIV). While the policy of standard precautions exists, the majority of surgeons used single gloves and did not use eye protection for most cases, but took additional measures when operating on seropositive patients. Paradoxically, I witnessed numerous needle sticks during those cases, precisely because the surgeons and staff were not comfortable with wearing double gloves or hands-free passing of sharp instruments. Even goggles often ended up being removed during the cases, because the surgeons were not accustomed to using them.
Like any surgical skill, standard precautions require repetition and practice. Therefore, I am concerned that with adoption of universal screening in the United States, we will become complacent and accustomed to unsafe practices, which may paradoxically increase the risk of operating on infected patients.
Igor Immerman, MD
Pleasanton, CA
Author’s Disclosure Statement: The author reports no actual or potential conflict of interest in relation to this letter.
Author’s Response
Edward M. DelSole, MD
We appreciate the response of the reader. This is indeed an interesting paradox—that an effort to improve safety in the operating room might yield the opposite effect owing to a heightened sense of protection, which induces a laxity in adhering to standard precautions. This possibility does create a real concern for the safety of the surgical team. Although we do recommend preoperative screening for hepatitis C virus (HCV), our intent was not in any way to diminish the need for adhering to standard precautions.
The foundation of standard precautions rests upon the universal assumption of infectivity of blood exposures—this is why the precautions are “standard” and should be adhered to during all operative procedures.
First and foremost, identification of patients with hepatitis C infection offers the chance for referral for care, which in many cases is curative thanks to recent advances in hepatitis C therapy. Whether or not the patient accepts the referral for hepatitis C treatment should not, as we emphasize in our paper, affect the plan for surgery.
Secondly, a negative preoperative test should in no way alter normal intraoperative safety practices. The test results could be falsely negative due to human or mechanical error. In addition, the patient could have other transmissible diseases such as HIV, hepatitis B, or other less common yet transmissible viral infections. Importantly, given the historical narratives of the HIV and HCV epidemics, we as surgeons hold the responsibility to never be complacent about the next “unknown” novel viral agent that has yet to reveal itself.
Thirdly, perceived inconvenience of protective equipment is largely a matter of training. At our institution residents infrequently wear single layers of gloves, masks without eye protection, body suits for arthroplasty, lead vests without thyroid shields, etc. We suspect similar practice occurs at other teaching hospitals as well. As today’s residents learn the craft in an environment of heightened protection, it is our hope that they will carry these good habits into their own practice as orthopedic surgeons and that patient outcomes will not differ.
The goal of screening is to create an improved environment of safety and health awareness for the patient and the surgical team, the foundation of which is standard precautions in the operating room. We advocate strongly that standard precautions continue to be the basis of intraoperative safety and that they be used for all patients indiscriminately.
Author’s Disclosure Statement: The author reports no actual or potential conflict of interest in relation to this letter.
The journal welcomes Letters to the Editor. Letters are not peer reviewed. Opinions expressed in letters published here do not necessarily reflect those of the editorial board or the publishing company and its employees.
Letter to the Editor
Universal Hepatitis C Screening
and Surgeon Safety
I read with interest the article “Risk of Hepatitis C Virus Exposure in Orthopedic Surgery: Is Universal Screening Needed?” by Dr. DelSole and colleagues (Am J Orthop. 2014;43(6):E117-E123). The authors make a compelling case for universal hepatitis C screening, and I agree that this program may have significant benefits, including risk stratification for elective surgery and identification of patients for antiviral treatment. However, based largely on personal experiences, I disagree that preoperative knowledge of the patient’s seropositive status will improve surgeon safety.
The authors list a number of interventions to decrease the risk of exposure, such as the use of Kevlar gloves, double gloving, eye protection, and others. However, perhaps the largest barrier to their use is their perceived inconvenience. Surgeons often cite decreased dexterity and sensation in their opposition to double or thick gloves, for instance. My concern is that with the advent of universal screening, many surgeons will abandon their universal approach and only wear Kevlar or double gloves on infected cases.
I have observed first-hand such a policy this year, when I spent 3 months in orthopedic centers in Russia. In Russia, all patients undergo routine preoperative testing for both hepatitis C virus and human immunodeficiency virus (HIV). While the policy of standard precautions exists, the majority of surgeons used single gloves and did not use eye protection for most cases, but took additional measures when operating on seropositive patients. Paradoxically, I witnessed numerous needle sticks during those cases, precisely because the surgeons and staff were not comfortable with wearing double gloves or hands-free passing of sharp instruments. Even goggles often ended up being removed during the cases, because the surgeons were not accustomed to using them.
Like any surgical skill, standard precautions require repetition and practice. Therefore, I am concerned that with adoption of universal screening in the United States, we will become complacent and accustomed to unsafe practices, which may paradoxically increase the risk of operating on infected patients.
Igor Immerman, MD
Pleasanton, CA
Author’s Disclosure Statement: The author reports no actual or potential conflict of interest in relation to this letter.
Author’s Response
Edward M. DelSole, MD
We appreciate the response of the reader. This is indeed an interesting paradox—that an effort to improve safety in the operating room might yield the opposite effect owing to a heightened sense of protection, which induces a laxity in adhering to standard precautions. This possibility does create a real concern for the safety of the surgical team. Although we do recommend preoperative screening for hepatitis C virus (HCV), our intent was not in any way to diminish the need for adhering to standard precautions.
The foundation of standard precautions rests upon the universal assumption of infectivity of blood exposures—this is why the precautions are “standard” and should be adhered to during all operative procedures.
First and foremost, identification of patients with hepatitis C infection offers the chance for referral for care, which in many cases is curative thanks to recent advances in hepatitis C therapy. Whether or not the patient accepts the referral for hepatitis C treatment should not, as we emphasize in our paper, affect the plan for surgery.
Secondly, a negative preoperative test should in no way alter normal intraoperative safety practices. The test results could be falsely negative due to human or mechanical error. In addition, the patient could have other transmissible diseases such as HIV, hepatitis B, or other less common yet transmissible viral infections. Importantly, given the historical narratives of the HIV and HCV epidemics, we as surgeons hold the responsibility to never be complacent about the next “unknown” novel viral agent that has yet to reveal itself.
Thirdly, perceived inconvenience of protective equipment is largely a matter of training. At our institution residents infrequently wear single layers of gloves, masks without eye protection, body suits for arthroplasty, lead vests without thyroid shields, etc. We suspect similar practice occurs at other teaching hospitals as well. As today’s residents learn the craft in an environment of heightened protection, it is our hope that they will carry these good habits into their own practice as orthopedic surgeons and that patient outcomes will not differ.
The goal of screening is to create an improved environment of safety and health awareness for the patient and the surgical team, the foundation of which is standard precautions in the operating room. We advocate strongly that standard precautions continue to be the basis of intraoperative safety and that they be used for all patients indiscriminately.
Author’s Disclosure Statement: The author reports no actual or potential conflict of interest in relation to this letter.
The journal welcomes Letters to the Editor. Letters are not peer reviewed. Opinions expressed in letters published here do not necessarily reflect those of the editorial board or the publishing company and its employees.
Letter to the Editor
Universal Hepatitis C Screening
and Surgeon Safety
I read with interest the article “Risk of Hepatitis C Virus Exposure in Orthopedic Surgery: Is Universal Screening Needed?” by Dr. DelSole and colleagues (Am J Orthop. 2014;43(6):E117-E123). The authors make a compelling case for universal hepatitis C screening, and I agree that this program may have significant benefits, including risk stratification for elective surgery and identification of patients for antiviral treatment. However, based largely on personal experiences, I disagree that preoperative knowledge of the patient’s seropositive status will improve surgeon safety.
The authors list a number of interventions to decrease the risk of exposure, such as the use of Kevlar gloves, double gloving, eye protection, and others. However, perhaps the largest barrier to their use is their perceived inconvenience. Surgeons often cite decreased dexterity and sensation in their opposition to double or thick gloves, for instance. My concern is that with the advent of universal screening, many surgeons will abandon their universal approach and only wear Kevlar or double gloves on infected cases.
I have observed first-hand such a policy this year, when I spent 3 months in orthopedic centers in Russia. In Russia, all patients undergo routine preoperative testing for both hepatitis C virus and human immunodeficiency virus (HIV). While the policy of standard precautions exists, the majority of surgeons used single gloves and did not use eye protection for most cases, but took additional measures when operating on seropositive patients. Paradoxically, I witnessed numerous needle sticks during those cases, precisely because the surgeons and staff were not comfortable with wearing double gloves or hands-free passing of sharp instruments. Even goggles often ended up being removed during the cases, because the surgeons were not accustomed to using them.
Like any surgical skill, standard precautions require repetition and practice. Therefore, I am concerned that with adoption of universal screening in the United States, we will become complacent and accustomed to unsafe practices, which may paradoxically increase the risk of operating on infected patients.
Igor Immerman, MD
Pleasanton, CA
Author’s Disclosure Statement: The author reports no actual or potential conflict of interest in relation to this letter.
Author’s Response
Edward M. DelSole, MD
We appreciate the response of the reader. This is indeed an interesting paradox—that an effort to improve safety in the operating room might yield the opposite effect owing to a heightened sense of protection, which induces a laxity in adhering to standard precautions. This possibility does create a real concern for the safety of the surgical team. Although we do recommend preoperative screening for hepatitis C virus (HCV), our intent was not in any way to diminish the need for adhering to standard precautions.
The foundation of standard precautions rests upon the universal assumption of infectivity of blood exposures—this is why the precautions are “standard” and should be adhered to during all operative procedures.
First and foremost, identification of patients with hepatitis C infection offers the chance for referral for care, which in many cases is curative thanks to recent advances in hepatitis C therapy. Whether or not the patient accepts the referral for hepatitis C treatment should not, as we emphasize in our paper, affect the plan for surgery.
Secondly, a negative preoperative test should in no way alter normal intraoperative safety practices. The test results could be falsely negative due to human or mechanical error. In addition, the patient could have other transmissible diseases such as HIV, hepatitis B, or other less common yet transmissible viral infections. Importantly, given the historical narratives of the HIV and HCV epidemics, we as surgeons hold the responsibility to never be complacent about the next “unknown” novel viral agent that has yet to reveal itself.
Thirdly, perceived inconvenience of protective equipment is largely a matter of training. At our institution residents infrequently wear single layers of gloves, masks without eye protection, body suits for arthroplasty, lead vests without thyroid shields, etc. We suspect similar practice occurs at other teaching hospitals as well. As today’s residents learn the craft in an environment of heightened protection, it is our hope that they will carry these good habits into their own practice as orthopedic surgeons and that patient outcomes will not differ.
The goal of screening is to create an improved environment of safety and health awareness for the patient and the surgical team, the foundation of which is standard precautions in the operating room. We advocate strongly that standard precautions continue to be the basis of intraoperative safety and that they be used for all patients indiscriminately.
Author’s Disclosure Statement: The author reports no actual or potential conflict of interest in relation to this letter.
The journal welcomes Letters to the Editor. Letters are not peer reviewed. Opinions expressed in letters published here do not necessarily reflect those of the editorial board or the publishing company and its employees.
To Outsource or Not to Outsource Your Physical Therapy Service Line Management?
You currently offer a physical therapy (PT) service line but feel like it could be doing better, or you are thinking of adding PT services and are not sure where to begin. Either way, the thought of your patients and bottom line benefiting from PT services within your practice but without you having to manage another service line is appealing. Although related to orthopedic surgery and an obvious ancillary service, PT is a different type of practice that requires active management of the professionals, revenue cycle, operations, regulatory requirements, and changing coding and reimbursement protocols. There are more and more companies nationwide that claim a mastery of the PT management niche and would be more than happy to shoulder your burden and share in your profits. Whether you already have PT services in your practice or are looking to add them, proceed with care and caution as you consider partnering with a PT management company.
Drawing on my involvement with both successful PT management–orthopedic practice partnerships and the arduous, expensive demise of one, this article offers my recommendations for determining if a PT management partnership is best for your practice. Many of the contracts last for several years and include clauses that are extremely binding even after the contract conclusion. As with any decision to outsource, the decision to outsource PT services deserves a comprehensive request for proposal (RFP) to multiple candidate companies, a thorough vetting and decision process that includes all partners or an executive committee/board, and legal review of the contract by an experienced health care attorney representing the practice.
Request for Proposal
Begin the RFP with a reference to your website and a brief description of the practice and current PT services. For example, state that you don’t have PT and are looking to start it, or, if your practice offers PT, include a description of the number of locations, square footage allocation, and number of full-time equivalent (FTE) physical therapists, occupational therapists, physical therapy assistants, and support staff of the existing program. Also mention whether the billing is done within the practice or is outsourced.
Next, you want to learn about the company. Ask about its number of years in PT management and the history of the company. Ask them to provide the previous business names and an overview of the history of ownership. You want to know this because the national PT management community is relatively small and includes frequent acquisitions and mergers. Just as important as the company’s history is any future plans it has to acquire or be acquired. This is all part of knowing who you may be partnering with.
You will want to know how many other practices the company provides PT/occupational therapy (OT) management services for, in what states those practices are located, and a breakdown of specialties. The number of physician providers the company represents along with the answers to the other questions will give you an idea of its size, experience, location, alignment, and focus. Some of these companies manage PT in family practices. You will want to know how many orthopedic practices the company partners with. If your practice has subspecialties, ask about its experience with subspecialty orthopedics.
Ask for the company’s website so that you can get an idea of the company and its mission, vision, and values. Even though you will likely be interacting with regional representatives, you will be signing the contract with the company leadership, so be sure to learn—either from the company’s website or its response to the RFP—about the individuals leading the organization and any parent company. Ask for a list of officers, board of directors, managers, and perhaps an organization chart with titles and names. Because you are looking to partner with the company, it might be good to ask for managerial turnover rates in the last few years. PT is a professional health care service, so take note of the number of licensed therapists the company has in leadership positions. If you were partnering with a medical organization, you would certainly want physicians in leadership positions, and this is no different. Determine if the company is publicly traded or privately held. You may also consider requesting the company’s last few annual reports to learn more.
Exclusivity
Some PT management companies have their own facilities and are partnering with practices in the same market despite the seemingly inherent conflict. As part of your RFP, ask the company to include the names of practices, their specialties, and site locations where it already has a presence as well as a list of its own (independent of a physician practice) locations in the state or region. Future plans are important here too; if the company does not currently have a presence in the region, what commitment will it make to exclusivity or noncompete?
Human Resources
In many cases, the PT management company actually employs the entire staff for the service line. If you already have PT in your practice, this means the employees will transition from your employment to another company. After the contract is signed, some of the employees will get paychecks and benefits from your practice and be subject to your practice’s policies, while others will not. Having 2 different employers in the same practice can have an impact on employee morale and satisfaction. Find out during the proposal process if the company’s typical model is based on the company’s or the practice’s employment of the professional and support staff.
Obtain the following information from the management company if it is to be the employer:
1. Titles and compensation ranges for each position
2. Description of all bonuses and incentive structures
3. Paid time off (PTO) policies and accrual rates
4. Paid holidays
5. Details of employee benefits, such as employee out-of-pocket premiums for individual and family health care coverage, availability of vision and dental coverage, and investment and match for retirement or 401(k) plans.
6. Human resources (HR) support. Describe how employees get needed support from human resources, such as questions about benefits, FMLA (Family Medical Leave Act), HR policies, and other issues.
7. Results of any employee satisfaction surveys completed in the last 2 years
8. Nonrecruitment. In what ways will the professional staff’s careers be affected by this partnership? Will the professional staff have to sign employment agreements? If so, please provide a sample. Will there be covenants not to compete for the professional staff?
9. Provide a schematic on how you typically cover professional staff vacation and family leave.
10. Does your organization provide accredited continuing education for professional staff or do you send staff to state and national meetings and courses to maintain their CEU (continuing education units) requirement?
11. Do you provide annual regulatory training, such as HIPAA (Health Insurance Portability and Accountability Act) training, for all staff or is the practice responsible for that?
12. Professional liability coverage for the therapists.
Initially, the company may say they are open to doing it either way and you may proceed with the process without answers to these questions. If at any point, you explore a situation in which the employer is not the practice, you must get answers to these questions before agreeing to anything. Number 8 above is extremely important. Many PT management companies will include a nonrecruitment clause in their contracts so that neither party can recruit therapists from the engagement. Depending on how the clause is written, you may not be able to legally hire therapists who were with you for years before the management agreement at the conclusion of the contract. Individual therapists can find themselves very limited in where they can work in the community depending on a covenant not to compete. And while covenants not to compete and nonrecruitment clauses may be generalized as “indefensible,” it is expensive, time-consuming, and exhausting to get one rendered as such after the fact.
Operations
Operational compatibility is essential to a smooth transition or start-up. As part of your RFP, let the candidate companies know which electronic medical record (EMR) and electronic practice management (EPM) systems you use and ask about their experience level with those. Ask if it is expected that PT will operate on the practice’s EMR and EPM systems or if the company will be having PT operate on separate systems. If the company will be implementing different systems, find out which one and then do an accounting of the costs for interface, training, and other compatibility elements.
Inquire as to each candidate company’s standard operations policies and procedures as well as the operational and productivity standards the company maintains. For example, ask for a range of how many patients a physical therapist should be able to see (including documentation) in an 8-hour day? If you already have a PT service line, compare the company’s productivity standards to what your therapists are currently doing. Of the many enhancements a therapy management company can bring, an increase in productivity is essential. Request a description of productivity incentive programs for the professional staff so that you can determine if you are comfortable with them. By the same token, ask if the company has a comprehensive compliance program including procedures and policies.
Any outsourcing agreements will be subject to operational and contractual compliance elements such as HIPAA, business associates agreement, and all other applicable regulations through state, federal, and payor entities. Ask each candidate company about its compliance program.
Revenue Cycle
Some therapy management companies will leave the billing and collections to the practice, while others have their own operations. As part of the RFP, ask the company which way it is done in its standard model. If the company manages the revenue cycle, ask which key revenue cycle performance indicators it monitors, how it calculates them, and with what frequency it tracks and reports them. Ask how the company has approached a revenue cycle performance improvement effort and what successes it has had. And definitely ask about the emphasis, training, and performance standards on point-of-service (POS) collections. In PT, successful, service-oriented POS collections are essential to cash flow and patient satisfaction. Typically, there is a copayment for each visit, and patients come 3 times or more per week. When patients pay the copayment before each visit, it feels manageable to them. If they get a bill for 6 copayments 2 weeks into PT, they often get angry and do not see the value of the therapy.
Seek specifics on the elements of the company’s performance improvement. A number of companies will default to providing incentive bonuses for POS collections and other revenue cycle improvements. Incentive bonuses for collections can compromise the coding and billing integrity of the practice and are guaranteed to cause discontent among support staff. Everyone who works hard should be recognized, not just the staff in a position to collect money. On top of this, if the PT reception staff are getting paid POS collections bonuses and the practice reception staff are not, a managerial dichotomy ensues.
Accounting and Financial Reporting
In your RFP, ask about the accounting and financial model that each candidate company most often uses. Inquire as to which—the practice or the management company—is responsible for monthly, quarterly, and annual accounting and financial reporting and what typical monthly reconcilement process the company recommends. Include the following requests in your RFP:
1. Please provide samples of the monthly financials produced or preferred.
2. Describe your annual expense and revenue budgeting and approval processes.
3. Please address how you recommend handling the purchase of new equipment and supplies as well as the handling of existing equipment and supplies.
4. Will you rent our current space? Will you look to move the PT department into an alternate space in the future? If so, where?
General
Make some general inquiries that will help you get to know each organization and determine which one may be the best fit for a long, committed relationship with your practice. Find out how often the organization will have corporate representatives in the practice and at physician board meetings. Inquire as to the types of referral reports they generate and share with the practice. Request the names and contact information of 3 to 5 orthopedic surgery practice managers or physician leaders whom you can contact as references for the company.
If you have an existing PT service line, ask the company how it proposes to enhance the services, quality, and bottom line. What value will the company’s management services add to an existing program?
Get to know the organization by asking how many of its partnering practices have terminated their agreements with the management company and if it has any current or past litigation with partner practices. These are detailed, binding contracts with the potential for a lot of money. When the relationships or even the local markets change, suits are filed.
As part of the RFP, inquire as to the standard proposed model for income distribution between the practice and the management company.
Conclusion
If your RFP covers all of the inquiries discussed in this article, it will be necessarily comprehensive. Send it to several companies with a clear indication of the response deadline and the contact person for the response and for any questions they may have. The contact person is typically the practice manager or executive administrator. Individual physicians in your group may have relationships with local representatives of a PT management company, and it can put them in an awkward position during the proposal submission and evaluation process.
Some companies may not respond to an RFP this comprehensive, which provides an unequivocal answer that they are not qualified to be your practice’s partner. Compare the responses you receive and set up presentations or conference calls for those companies whose proposals warrant it.
Hire your own health care attorney to review any and all contracts before signing. The HR support, exclusivity, income distribution models, compliance, and duration of these contracts must be approved by an experienced attorney that advocates for the practice alone.
You currently offer a physical therapy (PT) service line but feel like it could be doing better, or you are thinking of adding PT services and are not sure where to begin. Either way, the thought of your patients and bottom line benefiting from PT services within your practice but without you having to manage another service line is appealing. Although related to orthopedic surgery and an obvious ancillary service, PT is a different type of practice that requires active management of the professionals, revenue cycle, operations, regulatory requirements, and changing coding and reimbursement protocols. There are more and more companies nationwide that claim a mastery of the PT management niche and would be more than happy to shoulder your burden and share in your profits. Whether you already have PT services in your practice or are looking to add them, proceed with care and caution as you consider partnering with a PT management company.
Drawing on my involvement with both successful PT management–orthopedic practice partnerships and the arduous, expensive demise of one, this article offers my recommendations for determining if a PT management partnership is best for your practice. Many of the contracts last for several years and include clauses that are extremely binding even after the contract conclusion. As with any decision to outsource, the decision to outsource PT services deserves a comprehensive request for proposal (RFP) to multiple candidate companies, a thorough vetting and decision process that includes all partners or an executive committee/board, and legal review of the contract by an experienced health care attorney representing the practice.
Request for Proposal
Begin the RFP with a reference to your website and a brief description of the practice and current PT services. For example, state that you don’t have PT and are looking to start it, or, if your practice offers PT, include a description of the number of locations, square footage allocation, and number of full-time equivalent (FTE) physical therapists, occupational therapists, physical therapy assistants, and support staff of the existing program. Also mention whether the billing is done within the practice or is outsourced.
Next, you want to learn about the company. Ask about its number of years in PT management and the history of the company. Ask them to provide the previous business names and an overview of the history of ownership. You want to know this because the national PT management community is relatively small and includes frequent acquisitions and mergers. Just as important as the company’s history is any future plans it has to acquire or be acquired. This is all part of knowing who you may be partnering with.
You will want to know how many other practices the company provides PT/occupational therapy (OT) management services for, in what states those practices are located, and a breakdown of specialties. The number of physician providers the company represents along with the answers to the other questions will give you an idea of its size, experience, location, alignment, and focus. Some of these companies manage PT in family practices. You will want to know how many orthopedic practices the company partners with. If your practice has subspecialties, ask about its experience with subspecialty orthopedics.
Ask for the company’s website so that you can get an idea of the company and its mission, vision, and values. Even though you will likely be interacting with regional representatives, you will be signing the contract with the company leadership, so be sure to learn—either from the company’s website or its response to the RFP—about the individuals leading the organization and any parent company. Ask for a list of officers, board of directors, managers, and perhaps an organization chart with titles and names. Because you are looking to partner with the company, it might be good to ask for managerial turnover rates in the last few years. PT is a professional health care service, so take note of the number of licensed therapists the company has in leadership positions. If you were partnering with a medical organization, you would certainly want physicians in leadership positions, and this is no different. Determine if the company is publicly traded or privately held. You may also consider requesting the company’s last few annual reports to learn more.
Exclusivity
Some PT management companies have their own facilities and are partnering with practices in the same market despite the seemingly inherent conflict. As part of your RFP, ask the company to include the names of practices, their specialties, and site locations where it already has a presence as well as a list of its own (independent of a physician practice) locations in the state or region. Future plans are important here too; if the company does not currently have a presence in the region, what commitment will it make to exclusivity or noncompete?
Human Resources
In many cases, the PT management company actually employs the entire staff for the service line. If you already have PT in your practice, this means the employees will transition from your employment to another company. After the contract is signed, some of the employees will get paychecks and benefits from your practice and be subject to your practice’s policies, while others will not. Having 2 different employers in the same practice can have an impact on employee morale and satisfaction. Find out during the proposal process if the company’s typical model is based on the company’s or the practice’s employment of the professional and support staff.
Obtain the following information from the management company if it is to be the employer:
1. Titles and compensation ranges for each position
2. Description of all bonuses and incentive structures
3. Paid time off (PTO) policies and accrual rates
4. Paid holidays
5. Details of employee benefits, such as employee out-of-pocket premiums for individual and family health care coverage, availability of vision and dental coverage, and investment and match for retirement or 401(k) plans.
6. Human resources (HR) support. Describe how employees get needed support from human resources, such as questions about benefits, FMLA (Family Medical Leave Act), HR policies, and other issues.
7. Results of any employee satisfaction surveys completed in the last 2 years
8. Nonrecruitment. In what ways will the professional staff’s careers be affected by this partnership? Will the professional staff have to sign employment agreements? If so, please provide a sample. Will there be covenants not to compete for the professional staff?
9. Provide a schematic on how you typically cover professional staff vacation and family leave.
10. Does your organization provide accredited continuing education for professional staff or do you send staff to state and national meetings and courses to maintain their CEU (continuing education units) requirement?
11. Do you provide annual regulatory training, such as HIPAA (Health Insurance Portability and Accountability Act) training, for all staff or is the practice responsible for that?
12. Professional liability coverage for the therapists.
Initially, the company may say they are open to doing it either way and you may proceed with the process without answers to these questions. If at any point, you explore a situation in which the employer is not the practice, you must get answers to these questions before agreeing to anything. Number 8 above is extremely important. Many PT management companies will include a nonrecruitment clause in their contracts so that neither party can recruit therapists from the engagement. Depending on how the clause is written, you may not be able to legally hire therapists who were with you for years before the management agreement at the conclusion of the contract. Individual therapists can find themselves very limited in where they can work in the community depending on a covenant not to compete. And while covenants not to compete and nonrecruitment clauses may be generalized as “indefensible,” it is expensive, time-consuming, and exhausting to get one rendered as such after the fact.
Operations
Operational compatibility is essential to a smooth transition or start-up. As part of your RFP, let the candidate companies know which electronic medical record (EMR) and electronic practice management (EPM) systems you use and ask about their experience level with those. Ask if it is expected that PT will operate on the practice’s EMR and EPM systems or if the company will be having PT operate on separate systems. If the company will be implementing different systems, find out which one and then do an accounting of the costs for interface, training, and other compatibility elements.
Inquire as to each candidate company’s standard operations policies and procedures as well as the operational and productivity standards the company maintains. For example, ask for a range of how many patients a physical therapist should be able to see (including documentation) in an 8-hour day? If you already have a PT service line, compare the company’s productivity standards to what your therapists are currently doing. Of the many enhancements a therapy management company can bring, an increase in productivity is essential. Request a description of productivity incentive programs for the professional staff so that you can determine if you are comfortable with them. By the same token, ask if the company has a comprehensive compliance program including procedures and policies.
Any outsourcing agreements will be subject to operational and contractual compliance elements such as HIPAA, business associates agreement, and all other applicable regulations through state, federal, and payor entities. Ask each candidate company about its compliance program.
Revenue Cycle
Some therapy management companies will leave the billing and collections to the practice, while others have their own operations. As part of the RFP, ask the company which way it is done in its standard model. If the company manages the revenue cycle, ask which key revenue cycle performance indicators it monitors, how it calculates them, and with what frequency it tracks and reports them. Ask how the company has approached a revenue cycle performance improvement effort and what successes it has had. And definitely ask about the emphasis, training, and performance standards on point-of-service (POS) collections. In PT, successful, service-oriented POS collections are essential to cash flow and patient satisfaction. Typically, there is a copayment for each visit, and patients come 3 times or more per week. When patients pay the copayment before each visit, it feels manageable to them. If they get a bill for 6 copayments 2 weeks into PT, they often get angry and do not see the value of the therapy.
Seek specifics on the elements of the company’s performance improvement. A number of companies will default to providing incentive bonuses for POS collections and other revenue cycle improvements. Incentive bonuses for collections can compromise the coding and billing integrity of the practice and are guaranteed to cause discontent among support staff. Everyone who works hard should be recognized, not just the staff in a position to collect money. On top of this, if the PT reception staff are getting paid POS collections bonuses and the practice reception staff are not, a managerial dichotomy ensues.
Accounting and Financial Reporting
In your RFP, ask about the accounting and financial model that each candidate company most often uses. Inquire as to which—the practice or the management company—is responsible for monthly, quarterly, and annual accounting and financial reporting and what typical monthly reconcilement process the company recommends. Include the following requests in your RFP:
1. Please provide samples of the monthly financials produced or preferred.
2. Describe your annual expense and revenue budgeting and approval processes.
3. Please address how you recommend handling the purchase of new equipment and supplies as well as the handling of existing equipment and supplies.
4. Will you rent our current space? Will you look to move the PT department into an alternate space in the future? If so, where?
General
Make some general inquiries that will help you get to know each organization and determine which one may be the best fit for a long, committed relationship with your practice. Find out how often the organization will have corporate representatives in the practice and at physician board meetings. Inquire as to the types of referral reports they generate and share with the practice. Request the names and contact information of 3 to 5 orthopedic surgery practice managers or physician leaders whom you can contact as references for the company.
If you have an existing PT service line, ask the company how it proposes to enhance the services, quality, and bottom line. What value will the company’s management services add to an existing program?
Get to know the organization by asking how many of its partnering practices have terminated their agreements with the management company and if it has any current or past litigation with partner practices. These are detailed, binding contracts with the potential for a lot of money. When the relationships or even the local markets change, suits are filed.
As part of the RFP, inquire as to the standard proposed model for income distribution between the practice and the management company.
Conclusion
If your RFP covers all of the inquiries discussed in this article, it will be necessarily comprehensive. Send it to several companies with a clear indication of the response deadline and the contact person for the response and for any questions they may have. The contact person is typically the practice manager or executive administrator. Individual physicians in your group may have relationships with local representatives of a PT management company, and it can put them in an awkward position during the proposal submission and evaluation process.
Some companies may not respond to an RFP this comprehensive, which provides an unequivocal answer that they are not qualified to be your practice’s partner. Compare the responses you receive and set up presentations or conference calls for those companies whose proposals warrant it.
Hire your own health care attorney to review any and all contracts before signing. The HR support, exclusivity, income distribution models, compliance, and duration of these contracts must be approved by an experienced attorney that advocates for the practice alone.
You currently offer a physical therapy (PT) service line but feel like it could be doing better, or you are thinking of adding PT services and are not sure where to begin. Either way, the thought of your patients and bottom line benefiting from PT services within your practice but without you having to manage another service line is appealing. Although related to orthopedic surgery and an obvious ancillary service, PT is a different type of practice that requires active management of the professionals, revenue cycle, operations, regulatory requirements, and changing coding and reimbursement protocols. There are more and more companies nationwide that claim a mastery of the PT management niche and would be more than happy to shoulder your burden and share in your profits. Whether you already have PT services in your practice or are looking to add them, proceed with care and caution as you consider partnering with a PT management company.
Drawing on my involvement with both successful PT management–orthopedic practice partnerships and the arduous, expensive demise of one, this article offers my recommendations for determining if a PT management partnership is best for your practice. Many of the contracts last for several years and include clauses that are extremely binding even after the contract conclusion. As with any decision to outsource, the decision to outsource PT services deserves a comprehensive request for proposal (RFP) to multiple candidate companies, a thorough vetting and decision process that includes all partners or an executive committee/board, and legal review of the contract by an experienced health care attorney representing the practice.
Request for Proposal
Begin the RFP with a reference to your website and a brief description of the practice and current PT services. For example, state that you don’t have PT and are looking to start it, or, if your practice offers PT, include a description of the number of locations, square footage allocation, and number of full-time equivalent (FTE) physical therapists, occupational therapists, physical therapy assistants, and support staff of the existing program. Also mention whether the billing is done within the practice or is outsourced.
Next, you want to learn about the company. Ask about its number of years in PT management and the history of the company. Ask them to provide the previous business names and an overview of the history of ownership. You want to know this because the national PT management community is relatively small and includes frequent acquisitions and mergers. Just as important as the company’s history is any future plans it has to acquire or be acquired. This is all part of knowing who you may be partnering with.
You will want to know how many other practices the company provides PT/occupational therapy (OT) management services for, in what states those practices are located, and a breakdown of specialties. The number of physician providers the company represents along with the answers to the other questions will give you an idea of its size, experience, location, alignment, and focus. Some of these companies manage PT in family practices. You will want to know how many orthopedic practices the company partners with. If your practice has subspecialties, ask about its experience with subspecialty orthopedics.
Ask for the company’s website so that you can get an idea of the company and its mission, vision, and values. Even though you will likely be interacting with regional representatives, you will be signing the contract with the company leadership, so be sure to learn—either from the company’s website or its response to the RFP—about the individuals leading the organization and any parent company. Ask for a list of officers, board of directors, managers, and perhaps an organization chart with titles and names. Because you are looking to partner with the company, it might be good to ask for managerial turnover rates in the last few years. PT is a professional health care service, so take note of the number of licensed therapists the company has in leadership positions. If you were partnering with a medical organization, you would certainly want physicians in leadership positions, and this is no different. Determine if the company is publicly traded or privately held. You may also consider requesting the company’s last few annual reports to learn more.
Exclusivity
Some PT management companies have their own facilities and are partnering with practices in the same market despite the seemingly inherent conflict. As part of your RFP, ask the company to include the names of practices, their specialties, and site locations where it already has a presence as well as a list of its own (independent of a physician practice) locations in the state or region. Future plans are important here too; if the company does not currently have a presence in the region, what commitment will it make to exclusivity or noncompete?
Human Resources
In many cases, the PT management company actually employs the entire staff for the service line. If you already have PT in your practice, this means the employees will transition from your employment to another company. After the contract is signed, some of the employees will get paychecks and benefits from your practice and be subject to your practice’s policies, while others will not. Having 2 different employers in the same practice can have an impact on employee morale and satisfaction. Find out during the proposal process if the company’s typical model is based on the company’s or the practice’s employment of the professional and support staff.
Obtain the following information from the management company if it is to be the employer:
1. Titles and compensation ranges for each position
2. Description of all bonuses and incentive structures
3. Paid time off (PTO) policies and accrual rates
4. Paid holidays
5. Details of employee benefits, such as employee out-of-pocket premiums for individual and family health care coverage, availability of vision and dental coverage, and investment and match for retirement or 401(k) plans.
6. Human resources (HR) support. Describe how employees get needed support from human resources, such as questions about benefits, FMLA (Family Medical Leave Act), HR policies, and other issues.
7. Results of any employee satisfaction surveys completed in the last 2 years
8. Nonrecruitment. In what ways will the professional staff’s careers be affected by this partnership? Will the professional staff have to sign employment agreements? If so, please provide a sample. Will there be covenants not to compete for the professional staff?
9. Provide a schematic on how you typically cover professional staff vacation and family leave.
10. Does your organization provide accredited continuing education for professional staff or do you send staff to state and national meetings and courses to maintain their CEU (continuing education units) requirement?
11. Do you provide annual regulatory training, such as HIPAA (Health Insurance Portability and Accountability Act) training, for all staff or is the practice responsible for that?
12. Professional liability coverage for the therapists.
Initially, the company may say they are open to doing it either way and you may proceed with the process without answers to these questions. If at any point, you explore a situation in which the employer is not the practice, you must get answers to these questions before agreeing to anything. Number 8 above is extremely important. Many PT management companies will include a nonrecruitment clause in their contracts so that neither party can recruit therapists from the engagement. Depending on how the clause is written, you may not be able to legally hire therapists who were with you for years before the management agreement at the conclusion of the contract. Individual therapists can find themselves very limited in where they can work in the community depending on a covenant not to compete. And while covenants not to compete and nonrecruitment clauses may be generalized as “indefensible,” it is expensive, time-consuming, and exhausting to get one rendered as such after the fact.
Operations
Operational compatibility is essential to a smooth transition or start-up. As part of your RFP, let the candidate companies know which electronic medical record (EMR) and electronic practice management (EPM) systems you use and ask about their experience level with those. Ask if it is expected that PT will operate on the practice’s EMR and EPM systems or if the company will be having PT operate on separate systems. If the company will be implementing different systems, find out which one and then do an accounting of the costs for interface, training, and other compatibility elements.
Inquire as to each candidate company’s standard operations policies and procedures as well as the operational and productivity standards the company maintains. For example, ask for a range of how many patients a physical therapist should be able to see (including documentation) in an 8-hour day? If you already have a PT service line, compare the company’s productivity standards to what your therapists are currently doing. Of the many enhancements a therapy management company can bring, an increase in productivity is essential. Request a description of productivity incentive programs for the professional staff so that you can determine if you are comfortable with them. By the same token, ask if the company has a comprehensive compliance program including procedures and policies.
Any outsourcing agreements will be subject to operational and contractual compliance elements such as HIPAA, business associates agreement, and all other applicable regulations through state, federal, and payor entities. Ask each candidate company about its compliance program.
Revenue Cycle
Some therapy management companies will leave the billing and collections to the practice, while others have their own operations. As part of the RFP, ask the company which way it is done in its standard model. If the company manages the revenue cycle, ask which key revenue cycle performance indicators it monitors, how it calculates them, and with what frequency it tracks and reports them. Ask how the company has approached a revenue cycle performance improvement effort and what successes it has had. And definitely ask about the emphasis, training, and performance standards on point-of-service (POS) collections. In PT, successful, service-oriented POS collections are essential to cash flow and patient satisfaction. Typically, there is a copayment for each visit, and patients come 3 times or more per week. When patients pay the copayment before each visit, it feels manageable to them. If they get a bill for 6 copayments 2 weeks into PT, they often get angry and do not see the value of the therapy.
Seek specifics on the elements of the company’s performance improvement. A number of companies will default to providing incentive bonuses for POS collections and other revenue cycle improvements. Incentive bonuses for collections can compromise the coding and billing integrity of the practice and are guaranteed to cause discontent among support staff. Everyone who works hard should be recognized, not just the staff in a position to collect money. On top of this, if the PT reception staff are getting paid POS collections bonuses and the practice reception staff are not, a managerial dichotomy ensues.
Accounting and Financial Reporting
In your RFP, ask about the accounting and financial model that each candidate company most often uses. Inquire as to which—the practice or the management company—is responsible for monthly, quarterly, and annual accounting and financial reporting and what typical monthly reconcilement process the company recommends. Include the following requests in your RFP:
1. Please provide samples of the monthly financials produced or preferred.
2. Describe your annual expense and revenue budgeting and approval processes.
3. Please address how you recommend handling the purchase of new equipment and supplies as well as the handling of existing equipment and supplies.
4. Will you rent our current space? Will you look to move the PT department into an alternate space in the future? If so, where?
General
Make some general inquiries that will help you get to know each organization and determine which one may be the best fit for a long, committed relationship with your practice. Find out how often the organization will have corporate representatives in the practice and at physician board meetings. Inquire as to the types of referral reports they generate and share with the practice. Request the names and contact information of 3 to 5 orthopedic surgery practice managers or physician leaders whom you can contact as references for the company.
If you have an existing PT service line, ask the company how it proposes to enhance the services, quality, and bottom line. What value will the company’s management services add to an existing program?
Get to know the organization by asking how many of its partnering practices have terminated their agreements with the management company and if it has any current or past litigation with partner practices. These are detailed, binding contracts with the potential for a lot of money. When the relationships or even the local markets change, suits are filed.
As part of the RFP, inquire as to the standard proposed model for income distribution between the practice and the management company.
Conclusion
If your RFP covers all of the inquiries discussed in this article, it will be necessarily comprehensive. Send it to several companies with a clear indication of the response deadline and the contact person for the response and for any questions they may have. The contact person is typically the practice manager or executive administrator. Individual physicians in your group may have relationships with local representatives of a PT management company, and it can put them in an awkward position during the proposal submission and evaluation process.
Some companies may not respond to an RFP this comprehensive, which provides an unequivocal answer that they are not qualified to be your practice’s partner. Compare the responses you receive and set up presentations or conference calls for those companies whose proposals warrant it.
Hire your own health care attorney to review any and all contracts before signing. The HR support, exclusivity, income distribution models, compliance, and duration of these contracts must be approved by an experienced attorney that advocates for the practice alone.
Application of Epoxy Resin to a Solid-Foam Pelvic Model: Creating a Dry-Erase Pelvis
The value of preoperative planning and templating has been well-established in fracture surgery.1,2 Traditionally, this involves writing a surgical tactic and tracing the radiographs on paper to plan the ultimate reduction and implant placement.1 The advent of sophisticated computer programs has allowed electronic preoperative planning in trauma and arthroplasty surgery.3,4 Software for computer templating of acetabular fractures is available.5 Still, the renderings generated in these exercises are 2-dimensional, and their quality is somewhat dependent on the surgeon’s artistic ability. Ultimately, drawing a fracture is meant to help the surgeon understand its 3-dimensional (3-D) characteristics. This can be difficult working in 2 dimensions especially for bones, such as the pelvis, with complex 3-D structures. A useful alternative is to draw the fracture on a plastic-bone model.
We plan all acetabular fracture surgeries on 3-D models (plastic bones). These models are commonly provided to residents and fellows through educational courses or can be purchased online. Residents, fellows, and staff have their own models for planning, and we typically keep several models in the operating room for teaching before the surgery. Although these models are ideal for visualizing the bony anatomy, they are less than ideal for drawing fracture lines. Ink pens do not leave lines, and lines from markers and pencils cannot be easily erased. After a few planning sessions, the models typically look like a city map, making it difficult to tell the current fracture from those previously evaluated.
Here we describe a technique for turning standard plastic models into white boards so that lines can be drawn clearly with a marker and easily erased. To facilitate the correction of errors and reuse for future cases, we coat pelvic models with dry-erase epoxy resin. Although there is a commercially available product that has similar capabilities, our technique creates a significantly less expensive model that will likely be appealing to residents and fellows.
Technique
Throughout the process of creating the pelvic model, it is important to work in a well-ventilated area. Gloves should be worn at all times. The working surface should be protected with an impervious plastic sheet to avoid primer or epoxy soaking through.
In creating our dry-erase pelvic models, we use the Sawbones large male solid-foam pelvic model (Figure 1; Model 1301, Pacific Research Laboratories, Vashon, Washington). These models are often available to residents and fellows after Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) trauma courses or resident educational sessions. Alternatively, they can be purchased online.
We sand the model to smooth out surface irregularities and to prepare it to accept primer. First, use 100-grit and then 220-grit sandpaper to create the optimum surface. We recommend suspending the pelvis from string by placing an eyelet screw into the top of the sacrum or looping a string through 1 of the sacral foramen while priming, painting, and curing. To prime the pelvic model, we have used KILZ original spray primer (Masterchem Industries, Imperial, Missouri). It is important that the entire model be well coated with primer because the epoxy resin will not adhere to unprimed plastic-foam surface. Take care to apply an even coat and to avoid drip formation.
Once the primer is completely dry, apply the dry-erase epoxy resin (Rust-Oleum, Vernon Hills, Illinois). Mix the 2 parts of the epoxy resin and apply to the pelvic model with a foam brush. It is important to cover the entire surface of the model with enough epoxy to give a smooth, even finish. This requires 2 to 3 coats applied approximately 30 minutes apart (the epoxy will remain wet but will take additional coats well).
Once the final coat has been applied, the model takes about 48 hours to cure. Hang in a dry, well-ventilated location until it is fully cured. Then, use a dry-erase marker to trace fracture lines and the planned location of plates and screws (Figure 2).
An alternative to creating a dry-erase pelvis is to create a blackboard pelvis. Use Chalkboard Spray (Rust-Oleum) to create a surface that will accept white and colored chalk. The application of this product is much easier than the dry-erase epoxy, because it can be applied in a similar fashion as the spray primer. This creates a black model that can be marked with chalk. However, we have found these models to be less useful than the dry-erase versions, because chalk leaves less precise lines and is harder to remove from the model.
Once created, the pelvic model can be used intraoperatively to help understand fracture reduction and to facilitate the precontouring of pelvic and acetabular plates. We place the model in a sterile radiographic cassette bag (Figure 3). This gives the operating team access to the model and is useful in teaching anatomy, and particularly, screw placement. Further, the model allows an assistant to precontour plates to the model during the exposure portion of the case (Figure 4). While the precontoured plate is not always a perfect fit, it can usually be adjusted easily to fit the unique anatomy of the patient.
Discussion
Understanding the complex anatomy of pelvic and acetabular fractures can be challenging. We use models in the teaching of anatomy, in the interpretation of radiographs and computed tomography (CT) scans, and in preoperative planning. Fracture lines are traced on the pelvic model based on radiographs and/or CT scans and then compared with 3-D reconstruction images and, eventually, with operative findings. The use of our dry-erase models allows for easy correction of mistakes and reuse for further cases.
We have found dry-erase pelvic models to be an invaluable tool for resident and fellow education. While conventional 2-dimensional planning is adequate for most long-bone and periarticular fractures, the creation of these 3-D planning tools is useful in understanding the anatomy and surgical treatment of pelvic and acetabular fractures.
1. Reudi TP, Buckley R, Moran C. AO Principles of Fracture Management. New York, NY: Thieme; 2007.
2. Mast J, Jakob R, Ganz R. Planning & Reduction Techniques in Fracture Surgery. Berlin, Germany: Springer-Verlag; 2006.
3. Pilson HT, Reddix RN Jr, Mutty CE, Webb LX. The long lost art of preoperative planning—resurrected? Orthopedics. 2008;31(12):1238.
4. Unnanuntana A, Wagner D, Goodman SB. The accuracy of preoperative templating in cementless total hip arthroplasty. J Arthroplasty. 2009;24(2):180-186.
5. Reddix RN Jr, Webb LX. Computer-assisted preoperative planning in the surgical treatment of acetabular fractures. J Surg Orthop Adv. 2007;16(3):138-143.
The value of preoperative planning and templating has been well-established in fracture surgery.1,2 Traditionally, this involves writing a surgical tactic and tracing the radiographs on paper to plan the ultimate reduction and implant placement.1 The advent of sophisticated computer programs has allowed electronic preoperative planning in trauma and arthroplasty surgery.3,4 Software for computer templating of acetabular fractures is available.5 Still, the renderings generated in these exercises are 2-dimensional, and their quality is somewhat dependent on the surgeon’s artistic ability. Ultimately, drawing a fracture is meant to help the surgeon understand its 3-dimensional (3-D) characteristics. This can be difficult working in 2 dimensions especially for bones, such as the pelvis, with complex 3-D structures. A useful alternative is to draw the fracture on a plastic-bone model.
We plan all acetabular fracture surgeries on 3-D models (plastic bones). These models are commonly provided to residents and fellows through educational courses or can be purchased online. Residents, fellows, and staff have their own models for planning, and we typically keep several models in the operating room for teaching before the surgery. Although these models are ideal for visualizing the bony anatomy, they are less than ideal for drawing fracture lines. Ink pens do not leave lines, and lines from markers and pencils cannot be easily erased. After a few planning sessions, the models typically look like a city map, making it difficult to tell the current fracture from those previously evaluated.
Here we describe a technique for turning standard plastic models into white boards so that lines can be drawn clearly with a marker and easily erased. To facilitate the correction of errors and reuse for future cases, we coat pelvic models with dry-erase epoxy resin. Although there is a commercially available product that has similar capabilities, our technique creates a significantly less expensive model that will likely be appealing to residents and fellows.
Technique
Throughout the process of creating the pelvic model, it is important to work in a well-ventilated area. Gloves should be worn at all times. The working surface should be protected with an impervious plastic sheet to avoid primer or epoxy soaking through.
In creating our dry-erase pelvic models, we use the Sawbones large male solid-foam pelvic model (Figure 1; Model 1301, Pacific Research Laboratories, Vashon, Washington). These models are often available to residents and fellows after Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) trauma courses or resident educational sessions. Alternatively, they can be purchased online.
We sand the model to smooth out surface irregularities and to prepare it to accept primer. First, use 100-grit and then 220-grit sandpaper to create the optimum surface. We recommend suspending the pelvis from string by placing an eyelet screw into the top of the sacrum or looping a string through 1 of the sacral foramen while priming, painting, and curing. To prime the pelvic model, we have used KILZ original spray primer (Masterchem Industries, Imperial, Missouri). It is important that the entire model be well coated with primer because the epoxy resin will not adhere to unprimed plastic-foam surface. Take care to apply an even coat and to avoid drip formation.
Once the primer is completely dry, apply the dry-erase epoxy resin (Rust-Oleum, Vernon Hills, Illinois). Mix the 2 parts of the epoxy resin and apply to the pelvic model with a foam brush. It is important to cover the entire surface of the model with enough epoxy to give a smooth, even finish. This requires 2 to 3 coats applied approximately 30 minutes apart (the epoxy will remain wet but will take additional coats well).
Once the final coat has been applied, the model takes about 48 hours to cure. Hang in a dry, well-ventilated location until it is fully cured. Then, use a dry-erase marker to trace fracture lines and the planned location of plates and screws (Figure 2).
An alternative to creating a dry-erase pelvis is to create a blackboard pelvis. Use Chalkboard Spray (Rust-Oleum) to create a surface that will accept white and colored chalk. The application of this product is much easier than the dry-erase epoxy, because it can be applied in a similar fashion as the spray primer. This creates a black model that can be marked with chalk. However, we have found these models to be less useful than the dry-erase versions, because chalk leaves less precise lines and is harder to remove from the model.
Once created, the pelvic model can be used intraoperatively to help understand fracture reduction and to facilitate the precontouring of pelvic and acetabular plates. We place the model in a sterile radiographic cassette bag (Figure 3). This gives the operating team access to the model and is useful in teaching anatomy, and particularly, screw placement. Further, the model allows an assistant to precontour plates to the model during the exposure portion of the case (Figure 4). While the precontoured plate is not always a perfect fit, it can usually be adjusted easily to fit the unique anatomy of the patient.
Discussion
Understanding the complex anatomy of pelvic and acetabular fractures can be challenging. We use models in the teaching of anatomy, in the interpretation of radiographs and computed tomography (CT) scans, and in preoperative planning. Fracture lines are traced on the pelvic model based on radiographs and/or CT scans and then compared with 3-D reconstruction images and, eventually, with operative findings. The use of our dry-erase models allows for easy correction of mistakes and reuse for further cases.
We have found dry-erase pelvic models to be an invaluable tool for resident and fellow education. While conventional 2-dimensional planning is adequate for most long-bone and periarticular fractures, the creation of these 3-D planning tools is useful in understanding the anatomy and surgical treatment of pelvic and acetabular fractures.
The value of preoperative planning and templating has been well-established in fracture surgery.1,2 Traditionally, this involves writing a surgical tactic and tracing the radiographs on paper to plan the ultimate reduction and implant placement.1 The advent of sophisticated computer programs has allowed electronic preoperative planning in trauma and arthroplasty surgery.3,4 Software for computer templating of acetabular fractures is available.5 Still, the renderings generated in these exercises are 2-dimensional, and their quality is somewhat dependent on the surgeon’s artistic ability. Ultimately, drawing a fracture is meant to help the surgeon understand its 3-dimensional (3-D) characteristics. This can be difficult working in 2 dimensions especially for bones, such as the pelvis, with complex 3-D structures. A useful alternative is to draw the fracture on a plastic-bone model.
We plan all acetabular fracture surgeries on 3-D models (plastic bones). These models are commonly provided to residents and fellows through educational courses or can be purchased online. Residents, fellows, and staff have their own models for planning, and we typically keep several models in the operating room for teaching before the surgery. Although these models are ideal for visualizing the bony anatomy, they are less than ideal for drawing fracture lines. Ink pens do not leave lines, and lines from markers and pencils cannot be easily erased. After a few planning sessions, the models typically look like a city map, making it difficult to tell the current fracture from those previously evaluated.
Here we describe a technique for turning standard plastic models into white boards so that lines can be drawn clearly with a marker and easily erased. To facilitate the correction of errors and reuse for future cases, we coat pelvic models with dry-erase epoxy resin. Although there is a commercially available product that has similar capabilities, our technique creates a significantly less expensive model that will likely be appealing to residents and fellows.
Technique
Throughout the process of creating the pelvic model, it is important to work in a well-ventilated area. Gloves should be worn at all times. The working surface should be protected with an impervious plastic sheet to avoid primer or epoxy soaking through.
In creating our dry-erase pelvic models, we use the Sawbones large male solid-foam pelvic model (Figure 1; Model 1301, Pacific Research Laboratories, Vashon, Washington). These models are often available to residents and fellows after Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) trauma courses or resident educational sessions. Alternatively, they can be purchased online.
We sand the model to smooth out surface irregularities and to prepare it to accept primer. First, use 100-grit and then 220-grit sandpaper to create the optimum surface. We recommend suspending the pelvis from string by placing an eyelet screw into the top of the sacrum or looping a string through 1 of the sacral foramen while priming, painting, and curing. To prime the pelvic model, we have used KILZ original spray primer (Masterchem Industries, Imperial, Missouri). It is important that the entire model be well coated with primer because the epoxy resin will not adhere to unprimed plastic-foam surface. Take care to apply an even coat and to avoid drip formation.
Once the primer is completely dry, apply the dry-erase epoxy resin (Rust-Oleum, Vernon Hills, Illinois). Mix the 2 parts of the epoxy resin and apply to the pelvic model with a foam brush. It is important to cover the entire surface of the model with enough epoxy to give a smooth, even finish. This requires 2 to 3 coats applied approximately 30 minutes apart (the epoxy will remain wet but will take additional coats well).
Once the final coat has been applied, the model takes about 48 hours to cure. Hang in a dry, well-ventilated location until it is fully cured. Then, use a dry-erase marker to trace fracture lines and the planned location of plates and screws (Figure 2).
An alternative to creating a dry-erase pelvis is to create a blackboard pelvis. Use Chalkboard Spray (Rust-Oleum) to create a surface that will accept white and colored chalk. The application of this product is much easier than the dry-erase epoxy, because it can be applied in a similar fashion as the spray primer. This creates a black model that can be marked with chalk. However, we have found these models to be less useful than the dry-erase versions, because chalk leaves less precise lines and is harder to remove from the model.
Once created, the pelvic model can be used intraoperatively to help understand fracture reduction and to facilitate the precontouring of pelvic and acetabular plates. We place the model in a sterile radiographic cassette bag (Figure 3). This gives the operating team access to the model and is useful in teaching anatomy, and particularly, screw placement. Further, the model allows an assistant to precontour plates to the model during the exposure portion of the case (Figure 4). While the precontoured plate is not always a perfect fit, it can usually be adjusted easily to fit the unique anatomy of the patient.
Discussion
Understanding the complex anatomy of pelvic and acetabular fractures can be challenging. We use models in the teaching of anatomy, in the interpretation of radiographs and computed tomography (CT) scans, and in preoperative planning. Fracture lines are traced on the pelvic model based on radiographs and/or CT scans and then compared with 3-D reconstruction images and, eventually, with operative findings. The use of our dry-erase models allows for easy correction of mistakes and reuse for further cases.
We have found dry-erase pelvic models to be an invaluable tool for resident and fellow education. While conventional 2-dimensional planning is adequate for most long-bone and periarticular fractures, the creation of these 3-D planning tools is useful in understanding the anatomy and surgical treatment of pelvic and acetabular fractures.
1. Reudi TP, Buckley R, Moran C. AO Principles of Fracture Management. New York, NY: Thieme; 2007.
2. Mast J, Jakob R, Ganz R. Planning & Reduction Techniques in Fracture Surgery. Berlin, Germany: Springer-Verlag; 2006.
3. Pilson HT, Reddix RN Jr, Mutty CE, Webb LX. The long lost art of preoperative planning—resurrected? Orthopedics. 2008;31(12):1238.
4. Unnanuntana A, Wagner D, Goodman SB. The accuracy of preoperative templating in cementless total hip arthroplasty. J Arthroplasty. 2009;24(2):180-186.
5. Reddix RN Jr, Webb LX. Computer-assisted preoperative planning in the surgical treatment of acetabular fractures. J Surg Orthop Adv. 2007;16(3):138-143.
1. Reudi TP, Buckley R, Moran C. AO Principles of Fracture Management. New York, NY: Thieme; 2007.
2. Mast J, Jakob R, Ganz R. Planning & Reduction Techniques in Fracture Surgery. Berlin, Germany: Springer-Verlag; 2006.
3. Pilson HT, Reddix RN Jr, Mutty CE, Webb LX. The long lost art of preoperative planning—resurrected? Orthopedics. 2008;31(12):1238.
4. Unnanuntana A, Wagner D, Goodman SB. The accuracy of preoperative templating in cementless total hip arthroplasty. J Arthroplasty. 2009;24(2):180-186.
5. Reddix RN Jr, Webb LX. Computer-assisted preoperative planning in the surgical treatment of acetabular fractures. J Surg Orthop Adv. 2007;16(3):138-143.
The Role of Computed Tomography for Postoperative Evaluation of Percutaneous Sacroiliac Screw Fixation and Description of a “Safe Zone”
Pelvic injuries account for 3% of all skeletal fractures.1 Injury to the sacroiliac (SI) joint is frequently associated with unstable pelvic ring fractures, which are potentially life-threatening injuries. Surgical fixation of these injuries is preferred to nonoperative treatment given the potential for improved reduction and early mobilization and weight-bearing, thereby decreasing perioperative morbidity and improving functional outcome.2
The classic method of surgical fixation of the SI joint consisted of open reduction and internal fixation. This method carried a substantial risk for large dissection, iatrogenic nerve injury, and increased blood loss to the already traumatized patient.3 Percutaneous fixation allows for a shorter operating time, decreased soft-tissue stripping, and decreased blood loss compared with a traditional open procedure.4 However, posterior pelvic anatomy is complex and variable, and reports have found screw misplacements as high as 24%5 and neurologic complication rates up to 18%.6-9
Various imaging modalities, including fluoroscopy,5 computed tomography (CT),6-7 fluoroscopic CT, and computer-assisted techniques5,9 have been used to achieve proper screw placement. Conventional fluoroscopy is the standard for intraoperative screw placement. However, acceptable reduction of the SI joint and proper implantation of the screws without perforation of the neural foramina is challenging, especially when coupled with difficulties of fluoroscopic imaging and variations in pelvic anatomy.
Sacral dysplasia has been reported to occur in up to 20% to 40% of the population and has significant implications in patients indicated for iliosacral screw placement.10 Incorrect placement of iliosacral screws may result in iatrogenic neurovascular complications.11-13 Malpositioned screws using fluoroscopic guidance have been reported in 2% to 15% of patients with an incidence of neurologic compromise between 0.5% and 7.7%. As little as 4° of misdirection can result in damage to neurovascular structures.14
At our institution, we routinely obtained postoperative CT to evaluate the placement of SI screws. The objective of this retrospective study is to evaluate the rate of revision surgery of percutaneous SI screw fixation, to determine whether CT is an accurate tool for evaluation of the reduction and the need for revision surgery, and to decide if any violation of the neural foramina is safe.
Materials and Methods
After institutional review board approval, we retrospectively reviewed and evaluated medical records and radiographs of all patients who sustained unstable pelvic ring fractures between July 1, 2005, and June 30, 2010. We identified all patients who were treated with closed reductions and percutaneous iliosacral screw fixation, according to the method described by Routt in 1995.4 We excluded all pelvic fractures in patients who underwent open reduction for the posterior injury or did not have percutaneous SI screws placed, those with spinal injury, and those without follow-up. Of the 46 patients who met the inclusion criteria were 26 men and 20 women with a mean age of 42 years (range, 16 to 73 years). Motor vehicle accidents accounted for 13 cases; 19 were crush injuries and 14 were falls from height. Seventeen patients (37%) met the radiographic criteria for sacral dysmorphism. Forty-two of the 46 patients were polytrauma patients with associated musculoskeletal injuries and/or abdominal, chest, or head injuries.
Six patients presented with some neurologic deficit at the time of injury; all fractures were closed. The initial imaging study included plain anteroposterior (AP), inlet, and outlet radiographs of the pelvis and a pelvic CT scan. Using the classification of Young and Burgess,15 there were 3 vertical shear injuries, 13 lateral compression–type injuries, 17 anterior-posterior–type injuries, 7 sacral fractures, and 6 combination- or unclassifiable-type pelvic injuries. Of the sacral fractures, there were 3 Denis zone 1, 3 Denis zone 2, and 1 Denis zone 3.
The pelvic CT scan included the entire pelvis from the ilium to the ischial tuberosities. Each scan consisted of either a 5.0-mm or a 2.5-mm sequential axial image. A picture archiving and communication system (PACS) workstation using Centricity version 2.1 (GE Medical Systems, Waukesha, Wisconsin) was used to analyze each scan with a bone algorithm. On PACS, each initial displacement was characterized by the amount of SI joint widening at the level of the S1 and was measured using digital calipers.
Surgery
Mean time to surgery was 4 days (range, 2 to 15 days) after the injury. A total of 51 SI screws were implanted in 46 patients. We achieved closed reduction of the posterior pelvic ring by various techniques, including compression with percutaneous partially threaded screw fixation. In the cases in which the posterior ring lesion was associated with a pure pubic symphysis disruption, the anterior pelvis was initially reduced and stabilized with small-fragment plate fixation (Synthes, Inc, Paoli, Pennsylvania). The posterior complex was stabilized with 1 screw in 41 patients, 2 cases required a transiliac screw, and 2 screws (S1 and S2) were placed in each of the remaining 3 cases. Definitive stabilization of the posterior pelvis was achieved with percutaneous, partially threaded 7.3- or 7.5-mm–diameter cannulated screws (Synthes, Inc, and Zimmer Inc, Warsaw, Indiana, respectively) in 42 fractures and 6.5-mm screws (Synthes, Inc) in 4 fractures. In 11 cases where the fracture was through the sacrum, fully threaded cannulated screws were used to avoid compression. Screw insertion was performed under fluoroscopic guidance with inlet, outlet, and lateral sacral views. One of 2 fellowship-trained trauma surgeons performed the surgeries. Rehabilitation plans were customized to each patient based on concomitant injuries.
Postoperative Assessment
AP, lateral sacral, and inlet and outlet postoperative radiographs were taken in all cases within 24 hours after surgery. Pelvic CT was also obtained within 24 hours of surgery to review reduction and screw placement.
Using the measurement tool on the PACS system, we measured the penetration of the screw into the foramen. Screws were graded as intraosseous (completely contained within the sacral bone), skived (less than 2 mm of partial penetration into the S1 foramen), or extruded (the screw not contained by the bone). Screw penetration of the S1 was evaluated on the radiographic images as well as the axial images of the CT scans.
After surgery, the senior orthopedic resident and attending surgeon performed and documented detailed neurologic evaluations. They reviewed the medical record for neurologic deficit following surgical fixation.
Results
The mean follow-up time was 12 months (range, 8 months to 2 years). Two patients expired secondary to associated injuries. There were no early deaths related to the pelvic surgery. Stable fixation, including bone or ligamentous healing, as well as full weight-bearing status, was noted in every case. No case exhibited loss of reduction or implant failure or infection.
According to Matta’s criteria of anatomic reduction within 1 cm, all patients were found to have satisfactory reductions.7 Six of 46 patients had documented preoperative neurologic deficits. After percutaneous screw fixation, 10 of 46 patients had postoperative neurologic deficit, 2 of which were unchanged from preoperative evaluation. Of the 8 patients with new/altered postoperative neurologic deficit, CT showed neural foramen penetration greater than 2.1 mm in only 2 patients. Both patients underwent screw revision, resulting in improved neurologic deficit. The remaining 4 patients did not have foramen penetration and improved their neurologic function over the course of 2 weeks with return to presurgical status by 6 weeks without necessitating screw removal.
Twenty-three of the 51 screws (45%) had some violation of the S1 foramen on the CT. There were 17 patients with dysmorphic sacrums in which 21 S1 screws were placed. Eleven of 21 (52%) screws showed some penetration of the S1 foramen on CT. There were 29 patients with normal sacral morphology in which 30 S1 screws were placed. Twelve of 30 (40%) screws penetrated the S1 foramen. All violations were in the superior one-third position of the foramen. Two of 46 (4%; 1 with dysmorphism, 1 without) had a new neurologic deficit associated with the surgery (Table). CT showed sacral foramen penetration, and both screws were revised with a better neurologic examination.
High-resolution CTs were obtained in 32 patients, while 14 patients underwent the standard 5.0-mm–cut CTs. Of the 32 patients in which a 2.5-mm high-resolution CT was obtained, 20 (62.5%) had evidence of screw penetration (Figures 1, 2). All violations of the S1 neural foramen were in the superior portion of the foramen.
When compared with patients who had a 5.0-mm CT, the patients who underwent a high-resolution CT were more likely to show neural foramen penetration (P = .3). The average screw penetration into the S1 neural foramen measured 3.3 mm (range, 1.6-5.7 mm) in dysmorphic sacrum and 2.7 mm (range, 1.4-7 mm) in normal sacrum. However, in our study, any foramen penetration of less than 2.1 mm on CT did not result in neurologic deficit.
Discussion
Pelvic fractures are fairly common and represent approximately 5% of all trauma admissions and 3% of all skeletal fractures nationwide.1 The current treatment for SI disruption is either nonoperative or operative. Surgical fixation is technically demanding and surgeons often need a long learning curve to acquire the demanding technique because of the limitations of radiographic visualization of the relevant landmarks.16
Letournel17 developed the technique for iliosacral screw fixation for the treatment of posterior pelvic ring injuries, where 1 or 2 large screws (6.5-7.3 mm in diameter) are inserted under fluoroscopic guidance through the ilium, across the SI articulation, and into the superior sacral vertebral bodies using percutaneous techniques. Currently, the standard procedure to accomplish the percutaneous placement of iliosacral screws derives mainly from the technique described by Matta with the C-arm fluoroscopy visualizing the pelvis in 3 views: strict AP, inlet, and outlet views.7
Routt and colleagues4 recommend a strict lateral view of the sacrum, particularly when crossing the narrow zone of the sacral alar. They reported high union rates and accurate placement of the screws.4 There are limitations to the use of biplanar fluoroscopy because the intraoperative images are not orthogonal, with the average arc (67º) between the ideal inlet and outlet. However, because of the variability in sacral anatomy, CT guidance was recommended by others.2,6,8,18 Operating in a CT suite had other complications. Misinterpretation of CT led to “in-out-in” screws, which resulted in neurapraxia.
In our study, we used the technique described by Matta and colleagues for placement of the screws and performed a postoperative CT to evaluate screw placement and to assess pelvic reduction.7 We had a high penetration rate using CT, which increased with better resolution, even though none of the radiographs showed any obvious evidence of misplacement of the screws. Ebraheim and colleagues6 described the relationship of the S1 nerve root in its neural foramen and found it to be approximately 8.7 mm inferior and 7.8 mm medial to the starting point for a pedicle screw. Given these numbers, it is possible that a large amount of skiving can be tolerated contingent on an adequate reduction of the SI joint.
Because of our high rates of skiving and low rates of neurologic deficit, a new “safe zone” for screw insertion can be expanded to include skiving of the S1 neural foramen up to 3 mm without fear of nerve root injury. However, drilling and screw insertion at higher speeds can also cause neurologic injury secondary to thermal injury or soft tissue being caught up in a rotating drill/screw.
Evaluation of placement of percutaneous SI screw placement in our study resulted in neural foramen penetration in 43% of SI screws, which is higher than other studies.14,19,20 Our study showed that screw penetration up to 2 mm does not correlate with neurologic deficit. Iatrogenic neurologic deficit secondary to perforation of the foramina occurred in only 1 patient. Penetration of the foramina in all cases was in the superior portion of the foramen. We propose that there is a safe zone within the S1 neural foramen, and small amounts of penetration in the superior one-third of the foramen on axial CT images do not correlate with neurologic deficit. This potential safe zone is predicated on adequate reduction of the SI joint.
Neural foramen penetration shown on postoperative CT does not necessarily correlate with neurologic deficit. A postoperative CT is not indicated unless there are findings of a postoperative nerve injury. Our ideal screw placement skives the superior S1 foramen allowing for a larger screw diameter in a safe zone.
CT-guided placement has been proposed; however, concerns about radiation exposure, cost, and feasibility with similar outcomes compared with fluoroscopic-guided screw placement has resulted in its falling out of favor.
Iatrogenic nerve injuries are reported to occur in 0% to 6% of all percutaneous SI screw placement.14,21 Risk factors for iatrogenic nerve injury while using fluoroscopic guidance include sacral morphologic abnormalities, presence of intestinal gas, or contrast.22 Although these may be minimized with proper use of fluoroscopy, obtaining anatomic reduction as well as a thorough understanding of the pelvic morphology, the surgeon must be prepared to obtain further studies, such as a CT scan, if there is postoperative neurologic deficit.
Based on our findings, we do not routinely obtain a postoperative CT for SI screw placement, unless there is concern for malreduction or there is neurologic deficit. We also believe that up to 2 mm of foramen penetration is safe and does not result in neurologic deficit.
1. Failinger MS, McGanity PL. Unstable fractures of the pelvic ring. J Bone and Joint Surg Am. 1992;74(5):781-791.
2. Smith HE, Yuan PS, Sasso R, Papadopolous S, Vaccaro AR. An evaluation of image-guided technologies in the placement of percutaneous iliosacral screws. Spine (Phila Pa 1976). 2006;31(2):234-238.
3. Judet R, Judet J, Letournel E. Fractures of the acetabulum: classification and surgical approaches for open reduction. Preliminary report. J Bone Joint Surg Am. 1964;46(16):1615-1646.
4. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
5. Tonetti J, Carrat L, Blendea S, et al. Clinical results of percutaneous pelvic surgery. Computer assisted surgery using ultrasound compared to standard fluoroscopy. Comput Aided Surg. 2001;6(4):204-211.
6. Ebraheim NA, Coombs R, Jackson WT, Rusin JJ. Percutaneous computed tomography-guided stabilization of posterior pelvic fractures. Clin Orthop. 1994;(307):222-228.
7. Keating JF, Werier J, Blachut P, et al. Early fixation of the vertically unstable pelvis: the role of iliosacral screw fixation of the posterior lesion. J Orthop Trauma. 1999;13(2):107-113.
8. Webb LX, de Araujo W, Donofrio P, et al. Electromyography monitoring for percutaneous placement of iliosacral screws. J Orthop Trauma. 2000;14(4):245-254.
9. Barrick EF, O’Mara JW, Lane HE 3rd. Iliosacral screw insertion using computer-assisted CT image guidance: a laboratory study. Comput Aided Surg. 1998;3(6):289-296.
10. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
11. Altman DT, Jones CB, Routt ML Jr. Superior gluteal artery injury during iliosacral screw placement. J Orthop Trauma. 1999;13(3):220-227.
12. Stephen DJ. Pseudoaneurysm of the superior gluteal arterial system: an unusual cause of pain after a pelvic fracture. J Trauma. 1997;43(1):146-149.
13. Stöckle U, König B, Hofstetter R, Nolte LP, Haas NP. [Navigation assisted by image conversion. An experimental study on pelvic screw fixation]
[in German]. Unfallchirurg. 2001;104(3):215-220.
14. Templeman D, Schmidt A, Freese J, Weisman I, et al. Proximity of iliosacral screws to neurovascular structures after internal fixation. Clin Orthop. 1996;(329):194-198.
15. Young JW, Burgess AR, Brumback RJ, Poka A. Pelvic fractures: value of plain radiography in early assessment and management. Radiology. 1986;160(2):445-451.
16. Graves ML, Routt ML Jr. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Trauma. 2011;7(1):204-208.
17. Letournel E. Pelvic fractures. Injury. 1978;10(2):145-148.
18. Blake-Toker AM, Hawkins L, Nadalo L, et al. CT guided percutaneous fixation of sacroiliac fractures in trauma patients. J Trauma. 2001;51(6):1117-1121.
19. Hinsche AF, Giannoudis PV, Smith RM. Fluoroscopy-based multiplanar image guidance for insertion of sacroiliac screws. Clin Orthop. 2002;(395):135-144.
20. van den Bosch EW, van Zwienen CM, van Vugt AB. Fluoroscopic positioning of sacroiliac screws in 88 patients. J Trauma. 2002;53(1):44-48.
21. Cole JD, Blum DA, Ansel LJ. Outcome after fixation of unstable posterior pelvic ring injuries. Clin Orthop. 1996;(329):160-179.
22. Routt ML Jr, Simonian PT. Closed reduction and percutaneous skeletal fixation of sacral fractures. Clin Orthop. 1996;(329):121-128.
Pelvic injuries account for 3% of all skeletal fractures.1 Injury to the sacroiliac (SI) joint is frequently associated with unstable pelvic ring fractures, which are potentially life-threatening injuries. Surgical fixation of these injuries is preferred to nonoperative treatment given the potential for improved reduction and early mobilization and weight-bearing, thereby decreasing perioperative morbidity and improving functional outcome.2
The classic method of surgical fixation of the SI joint consisted of open reduction and internal fixation. This method carried a substantial risk for large dissection, iatrogenic nerve injury, and increased blood loss to the already traumatized patient.3 Percutaneous fixation allows for a shorter operating time, decreased soft-tissue stripping, and decreased blood loss compared with a traditional open procedure.4 However, posterior pelvic anatomy is complex and variable, and reports have found screw misplacements as high as 24%5 and neurologic complication rates up to 18%.6-9
Various imaging modalities, including fluoroscopy,5 computed tomography (CT),6-7 fluoroscopic CT, and computer-assisted techniques5,9 have been used to achieve proper screw placement. Conventional fluoroscopy is the standard for intraoperative screw placement. However, acceptable reduction of the SI joint and proper implantation of the screws without perforation of the neural foramina is challenging, especially when coupled with difficulties of fluoroscopic imaging and variations in pelvic anatomy.
Sacral dysplasia has been reported to occur in up to 20% to 40% of the population and has significant implications in patients indicated for iliosacral screw placement.10 Incorrect placement of iliosacral screws may result in iatrogenic neurovascular complications.11-13 Malpositioned screws using fluoroscopic guidance have been reported in 2% to 15% of patients with an incidence of neurologic compromise between 0.5% and 7.7%. As little as 4° of misdirection can result in damage to neurovascular structures.14
At our institution, we routinely obtained postoperative CT to evaluate the placement of SI screws. The objective of this retrospective study is to evaluate the rate of revision surgery of percutaneous SI screw fixation, to determine whether CT is an accurate tool for evaluation of the reduction and the need for revision surgery, and to decide if any violation of the neural foramina is safe.
Materials and Methods
After institutional review board approval, we retrospectively reviewed and evaluated medical records and radiographs of all patients who sustained unstable pelvic ring fractures between July 1, 2005, and June 30, 2010. We identified all patients who were treated with closed reductions and percutaneous iliosacral screw fixation, according to the method described by Routt in 1995.4 We excluded all pelvic fractures in patients who underwent open reduction for the posterior injury or did not have percutaneous SI screws placed, those with spinal injury, and those without follow-up. Of the 46 patients who met the inclusion criteria were 26 men and 20 women with a mean age of 42 years (range, 16 to 73 years). Motor vehicle accidents accounted for 13 cases; 19 were crush injuries and 14 were falls from height. Seventeen patients (37%) met the radiographic criteria for sacral dysmorphism. Forty-two of the 46 patients were polytrauma patients with associated musculoskeletal injuries and/or abdominal, chest, or head injuries.
Six patients presented with some neurologic deficit at the time of injury; all fractures were closed. The initial imaging study included plain anteroposterior (AP), inlet, and outlet radiographs of the pelvis and a pelvic CT scan. Using the classification of Young and Burgess,15 there were 3 vertical shear injuries, 13 lateral compression–type injuries, 17 anterior-posterior–type injuries, 7 sacral fractures, and 6 combination- or unclassifiable-type pelvic injuries. Of the sacral fractures, there were 3 Denis zone 1, 3 Denis zone 2, and 1 Denis zone 3.
The pelvic CT scan included the entire pelvis from the ilium to the ischial tuberosities. Each scan consisted of either a 5.0-mm or a 2.5-mm sequential axial image. A picture archiving and communication system (PACS) workstation using Centricity version 2.1 (GE Medical Systems, Waukesha, Wisconsin) was used to analyze each scan with a bone algorithm. On PACS, each initial displacement was characterized by the amount of SI joint widening at the level of the S1 and was measured using digital calipers.
Surgery
Mean time to surgery was 4 days (range, 2 to 15 days) after the injury. A total of 51 SI screws were implanted in 46 patients. We achieved closed reduction of the posterior pelvic ring by various techniques, including compression with percutaneous partially threaded screw fixation. In the cases in which the posterior ring lesion was associated with a pure pubic symphysis disruption, the anterior pelvis was initially reduced and stabilized with small-fragment plate fixation (Synthes, Inc, Paoli, Pennsylvania). The posterior complex was stabilized with 1 screw in 41 patients, 2 cases required a transiliac screw, and 2 screws (S1 and S2) were placed in each of the remaining 3 cases. Definitive stabilization of the posterior pelvis was achieved with percutaneous, partially threaded 7.3- or 7.5-mm–diameter cannulated screws (Synthes, Inc, and Zimmer Inc, Warsaw, Indiana, respectively) in 42 fractures and 6.5-mm screws (Synthes, Inc) in 4 fractures. In 11 cases where the fracture was through the sacrum, fully threaded cannulated screws were used to avoid compression. Screw insertion was performed under fluoroscopic guidance with inlet, outlet, and lateral sacral views. One of 2 fellowship-trained trauma surgeons performed the surgeries. Rehabilitation plans were customized to each patient based on concomitant injuries.
Postoperative Assessment
AP, lateral sacral, and inlet and outlet postoperative radiographs were taken in all cases within 24 hours after surgery. Pelvic CT was also obtained within 24 hours of surgery to review reduction and screw placement.
Using the measurement tool on the PACS system, we measured the penetration of the screw into the foramen. Screws were graded as intraosseous (completely contained within the sacral bone), skived (less than 2 mm of partial penetration into the S1 foramen), or extruded (the screw not contained by the bone). Screw penetration of the S1 was evaluated on the radiographic images as well as the axial images of the CT scans.
After surgery, the senior orthopedic resident and attending surgeon performed and documented detailed neurologic evaluations. They reviewed the medical record for neurologic deficit following surgical fixation.
Results
The mean follow-up time was 12 months (range, 8 months to 2 years). Two patients expired secondary to associated injuries. There were no early deaths related to the pelvic surgery. Stable fixation, including bone or ligamentous healing, as well as full weight-bearing status, was noted in every case. No case exhibited loss of reduction or implant failure or infection.
According to Matta’s criteria of anatomic reduction within 1 cm, all patients were found to have satisfactory reductions.7 Six of 46 patients had documented preoperative neurologic deficits. After percutaneous screw fixation, 10 of 46 patients had postoperative neurologic deficit, 2 of which were unchanged from preoperative evaluation. Of the 8 patients with new/altered postoperative neurologic deficit, CT showed neural foramen penetration greater than 2.1 mm in only 2 patients. Both patients underwent screw revision, resulting in improved neurologic deficit. The remaining 4 patients did not have foramen penetration and improved their neurologic function over the course of 2 weeks with return to presurgical status by 6 weeks without necessitating screw removal.
Twenty-three of the 51 screws (45%) had some violation of the S1 foramen on the CT. There were 17 patients with dysmorphic sacrums in which 21 S1 screws were placed. Eleven of 21 (52%) screws showed some penetration of the S1 foramen on CT. There were 29 patients with normal sacral morphology in which 30 S1 screws were placed. Twelve of 30 (40%) screws penetrated the S1 foramen. All violations were in the superior one-third position of the foramen. Two of 46 (4%; 1 with dysmorphism, 1 without) had a new neurologic deficit associated with the surgery (Table). CT showed sacral foramen penetration, and both screws were revised with a better neurologic examination.
High-resolution CTs were obtained in 32 patients, while 14 patients underwent the standard 5.0-mm–cut CTs. Of the 32 patients in which a 2.5-mm high-resolution CT was obtained, 20 (62.5%) had evidence of screw penetration (Figures 1, 2). All violations of the S1 neural foramen were in the superior portion of the foramen.
When compared with patients who had a 5.0-mm CT, the patients who underwent a high-resolution CT were more likely to show neural foramen penetration (P = .3). The average screw penetration into the S1 neural foramen measured 3.3 mm (range, 1.6-5.7 mm) in dysmorphic sacrum and 2.7 mm (range, 1.4-7 mm) in normal sacrum. However, in our study, any foramen penetration of less than 2.1 mm on CT did not result in neurologic deficit.
Discussion
Pelvic fractures are fairly common and represent approximately 5% of all trauma admissions and 3% of all skeletal fractures nationwide.1 The current treatment for SI disruption is either nonoperative or operative. Surgical fixation is technically demanding and surgeons often need a long learning curve to acquire the demanding technique because of the limitations of radiographic visualization of the relevant landmarks.16
Letournel17 developed the technique for iliosacral screw fixation for the treatment of posterior pelvic ring injuries, where 1 or 2 large screws (6.5-7.3 mm in diameter) are inserted under fluoroscopic guidance through the ilium, across the SI articulation, and into the superior sacral vertebral bodies using percutaneous techniques. Currently, the standard procedure to accomplish the percutaneous placement of iliosacral screws derives mainly from the technique described by Matta with the C-arm fluoroscopy visualizing the pelvis in 3 views: strict AP, inlet, and outlet views.7
Routt and colleagues4 recommend a strict lateral view of the sacrum, particularly when crossing the narrow zone of the sacral alar. They reported high union rates and accurate placement of the screws.4 There are limitations to the use of biplanar fluoroscopy because the intraoperative images are not orthogonal, with the average arc (67º) between the ideal inlet and outlet. However, because of the variability in sacral anatomy, CT guidance was recommended by others.2,6,8,18 Operating in a CT suite had other complications. Misinterpretation of CT led to “in-out-in” screws, which resulted in neurapraxia.
In our study, we used the technique described by Matta and colleagues for placement of the screws and performed a postoperative CT to evaluate screw placement and to assess pelvic reduction.7 We had a high penetration rate using CT, which increased with better resolution, even though none of the radiographs showed any obvious evidence of misplacement of the screws. Ebraheim and colleagues6 described the relationship of the S1 nerve root in its neural foramen and found it to be approximately 8.7 mm inferior and 7.8 mm medial to the starting point for a pedicle screw. Given these numbers, it is possible that a large amount of skiving can be tolerated contingent on an adequate reduction of the SI joint.
Because of our high rates of skiving and low rates of neurologic deficit, a new “safe zone” for screw insertion can be expanded to include skiving of the S1 neural foramen up to 3 mm without fear of nerve root injury. However, drilling and screw insertion at higher speeds can also cause neurologic injury secondary to thermal injury or soft tissue being caught up in a rotating drill/screw.
Evaluation of placement of percutaneous SI screw placement in our study resulted in neural foramen penetration in 43% of SI screws, which is higher than other studies.14,19,20 Our study showed that screw penetration up to 2 mm does not correlate with neurologic deficit. Iatrogenic neurologic deficit secondary to perforation of the foramina occurred in only 1 patient. Penetration of the foramina in all cases was in the superior portion of the foramen. We propose that there is a safe zone within the S1 neural foramen, and small amounts of penetration in the superior one-third of the foramen on axial CT images do not correlate with neurologic deficit. This potential safe zone is predicated on adequate reduction of the SI joint.
Neural foramen penetration shown on postoperative CT does not necessarily correlate with neurologic deficit. A postoperative CT is not indicated unless there are findings of a postoperative nerve injury. Our ideal screw placement skives the superior S1 foramen allowing for a larger screw diameter in a safe zone.
CT-guided placement has been proposed; however, concerns about radiation exposure, cost, and feasibility with similar outcomes compared with fluoroscopic-guided screw placement has resulted in its falling out of favor.
Iatrogenic nerve injuries are reported to occur in 0% to 6% of all percutaneous SI screw placement.14,21 Risk factors for iatrogenic nerve injury while using fluoroscopic guidance include sacral morphologic abnormalities, presence of intestinal gas, or contrast.22 Although these may be minimized with proper use of fluoroscopy, obtaining anatomic reduction as well as a thorough understanding of the pelvic morphology, the surgeon must be prepared to obtain further studies, such as a CT scan, if there is postoperative neurologic deficit.
Based on our findings, we do not routinely obtain a postoperative CT for SI screw placement, unless there is concern for malreduction or there is neurologic deficit. We also believe that up to 2 mm of foramen penetration is safe and does not result in neurologic deficit.
Pelvic injuries account for 3% of all skeletal fractures.1 Injury to the sacroiliac (SI) joint is frequently associated with unstable pelvic ring fractures, which are potentially life-threatening injuries. Surgical fixation of these injuries is preferred to nonoperative treatment given the potential for improved reduction and early mobilization and weight-bearing, thereby decreasing perioperative morbidity and improving functional outcome.2
The classic method of surgical fixation of the SI joint consisted of open reduction and internal fixation. This method carried a substantial risk for large dissection, iatrogenic nerve injury, and increased blood loss to the already traumatized patient.3 Percutaneous fixation allows for a shorter operating time, decreased soft-tissue stripping, and decreased blood loss compared with a traditional open procedure.4 However, posterior pelvic anatomy is complex and variable, and reports have found screw misplacements as high as 24%5 and neurologic complication rates up to 18%.6-9
Various imaging modalities, including fluoroscopy,5 computed tomography (CT),6-7 fluoroscopic CT, and computer-assisted techniques5,9 have been used to achieve proper screw placement. Conventional fluoroscopy is the standard for intraoperative screw placement. However, acceptable reduction of the SI joint and proper implantation of the screws without perforation of the neural foramina is challenging, especially when coupled with difficulties of fluoroscopic imaging and variations in pelvic anatomy.
Sacral dysplasia has been reported to occur in up to 20% to 40% of the population and has significant implications in patients indicated for iliosacral screw placement.10 Incorrect placement of iliosacral screws may result in iatrogenic neurovascular complications.11-13 Malpositioned screws using fluoroscopic guidance have been reported in 2% to 15% of patients with an incidence of neurologic compromise between 0.5% and 7.7%. As little as 4° of misdirection can result in damage to neurovascular structures.14
At our institution, we routinely obtained postoperative CT to evaluate the placement of SI screws. The objective of this retrospective study is to evaluate the rate of revision surgery of percutaneous SI screw fixation, to determine whether CT is an accurate tool for evaluation of the reduction and the need for revision surgery, and to decide if any violation of the neural foramina is safe.
Materials and Methods
After institutional review board approval, we retrospectively reviewed and evaluated medical records and radiographs of all patients who sustained unstable pelvic ring fractures between July 1, 2005, and June 30, 2010. We identified all patients who were treated with closed reductions and percutaneous iliosacral screw fixation, according to the method described by Routt in 1995.4 We excluded all pelvic fractures in patients who underwent open reduction for the posterior injury or did not have percutaneous SI screws placed, those with spinal injury, and those without follow-up. Of the 46 patients who met the inclusion criteria were 26 men and 20 women with a mean age of 42 years (range, 16 to 73 years). Motor vehicle accidents accounted for 13 cases; 19 were crush injuries and 14 were falls from height. Seventeen patients (37%) met the radiographic criteria for sacral dysmorphism. Forty-two of the 46 patients were polytrauma patients with associated musculoskeletal injuries and/or abdominal, chest, or head injuries.
Six patients presented with some neurologic deficit at the time of injury; all fractures were closed. The initial imaging study included plain anteroposterior (AP), inlet, and outlet radiographs of the pelvis and a pelvic CT scan. Using the classification of Young and Burgess,15 there were 3 vertical shear injuries, 13 lateral compression–type injuries, 17 anterior-posterior–type injuries, 7 sacral fractures, and 6 combination- or unclassifiable-type pelvic injuries. Of the sacral fractures, there were 3 Denis zone 1, 3 Denis zone 2, and 1 Denis zone 3.
The pelvic CT scan included the entire pelvis from the ilium to the ischial tuberosities. Each scan consisted of either a 5.0-mm or a 2.5-mm sequential axial image. A picture archiving and communication system (PACS) workstation using Centricity version 2.1 (GE Medical Systems, Waukesha, Wisconsin) was used to analyze each scan with a bone algorithm. On PACS, each initial displacement was characterized by the amount of SI joint widening at the level of the S1 and was measured using digital calipers.
Surgery
Mean time to surgery was 4 days (range, 2 to 15 days) after the injury. A total of 51 SI screws were implanted in 46 patients. We achieved closed reduction of the posterior pelvic ring by various techniques, including compression with percutaneous partially threaded screw fixation. In the cases in which the posterior ring lesion was associated with a pure pubic symphysis disruption, the anterior pelvis was initially reduced and stabilized with small-fragment plate fixation (Synthes, Inc, Paoli, Pennsylvania). The posterior complex was stabilized with 1 screw in 41 patients, 2 cases required a transiliac screw, and 2 screws (S1 and S2) were placed in each of the remaining 3 cases. Definitive stabilization of the posterior pelvis was achieved with percutaneous, partially threaded 7.3- or 7.5-mm–diameter cannulated screws (Synthes, Inc, and Zimmer Inc, Warsaw, Indiana, respectively) in 42 fractures and 6.5-mm screws (Synthes, Inc) in 4 fractures. In 11 cases where the fracture was through the sacrum, fully threaded cannulated screws were used to avoid compression. Screw insertion was performed under fluoroscopic guidance with inlet, outlet, and lateral sacral views. One of 2 fellowship-trained trauma surgeons performed the surgeries. Rehabilitation plans were customized to each patient based on concomitant injuries.
Postoperative Assessment
AP, lateral sacral, and inlet and outlet postoperative radiographs were taken in all cases within 24 hours after surgery. Pelvic CT was also obtained within 24 hours of surgery to review reduction and screw placement.
Using the measurement tool on the PACS system, we measured the penetration of the screw into the foramen. Screws were graded as intraosseous (completely contained within the sacral bone), skived (less than 2 mm of partial penetration into the S1 foramen), or extruded (the screw not contained by the bone). Screw penetration of the S1 was evaluated on the radiographic images as well as the axial images of the CT scans.
After surgery, the senior orthopedic resident and attending surgeon performed and documented detailed neurologic evaluations. They reviewed the medical record for neurologic deficit following surgical fixation.
Results
The mean follow-up time was 12 months (range, 8 months to 2 years). Two patients expired secondary to associated injuries. There were no early deaths related to the pelvic surgery. Stable fixation, including bone or ligamentous healing, as well as full weight-bearing status, was noted in every case. No case exhibited loss of reduction or implant failure or infection.
According to Matta’s criteria of anatomic reduction within 1 cm, all patients were found to have satisfactory reductions.7 Six of 46 patients had documented preoperative neurologic deficits. After percutaneous screw fixation, 10 of 46 patients had postoperative neurologic deficit, 2 of which were unchanged from preoperative evaluation. Of the 8 patients with new/altered postoperative neurologic deficit, CT showed neural foramen penetration greater than 2.1 mm in only 2 patients. Both patients underwent screw revision, resulting in improved neurologic deficit. The remaining 4 patients did not have foramen penetration and improved their neurologic function over the course of 2 weeks with return to presurgical status by 6 weeks without necessitating screw removal.
Twenty-three of the 51 screws (45%) had some violation of the S1 foramen on the CT. There were 17 patients with dysmorphic sacrums in which 21 S1 screws were placed. Eleven of 21 (52%) screws showed some penetration of the S1 foramen on CT. There were 29 patients with normal sacral morphology in which 30 S1 screws were placed. Twelve of 30 (40%) screws penetrated the S1 foramen. All violations were in the superior one-third position of the foramen. Two of 46 (4%; 1 with dysmorphism, 1 without) had a new neurologic deficit associated with the surgery (Table). CT showed sacral foramen penetration, and both screws were revised with a better neurologic examination.
High-resolution CTs were obtained in 32 patients, while 14 patients underwent the standard 5.0-mm–cut CTs. Of the 32 patients in which a 2.5-mm high-resolution CT was obtained, 20 (62.5%) had evidence of screw penetration (Figures 1, 2). All violations of the S1 neural foramen were in the superior portion of the foramen.
When compared with patients who had a 5.0-mm CT, the patients who underwent a high-resolution CT were more likely to show neural foramen penetration (P = .3). The average screw penetration into the S1 neural foramen measured 3.3 mm (range, 1.6-5.7 mm) in dysmorphic sacrum and 2.7 mm (range, 1.4-7 mm) in normal sacrum. However, in our study, any foramen penetration of less than 2.1 mm on CT did not result in neurologic deficit.
Discussion
Pelvic fractures are fairly common and represent approximately 5% of all trauma admissions and 3% of all skeletal fractures nationwide.1 The current treatment for SI disruption is either nonoperative or operative. Surgical fixation is technically demanding and surgeons often need a long learning curve to acquire the demanding technique because of the limitations of radiographic visualization of the relevant landmarks.16
Letournel17 developed the technique for iliosacral screw fixation for the treatment of posterior pelvic ring injuries, where 1 or 2 large screws (6.5-7.3 mm in diameter) are inserted under fluoroscopic guidance through the ilium, across the SI articulation, and into the superior sacral vertebral bodies using percutaneous techniques. Currently, the standard procedure to accomplish the percutaneous placement of iliosacral screws derives mainly from the technique described by Matta with the C-arm fluoroscopy visualizing the pelvis in 3 views: strict AP, inlet, and outlet views.7
Routt and colleagues4 recommend a strict lateral view of the sacrum, particularly when crossing the narrow zone of the sacral alar. They reported high union rates and accurate placement of the screws.4 There are limitations to the use of biplanar fluoroscopy because the intraoperative images are not orthogonal, with the average arc (67º) between the ideal inlet and outlet. However, because of the variability in sacral anatomy, CT guidance was recommended by others.2,6,8,18 Operating in a CT suite had other complications. Misinterpretation of CT led to “in-out-in” screws, which resulted in neurapraxia.
In our study, we used the technique described by Matta and colleagues for placement of the screws and performed a postoperative CT to evaluate screw placement and to assess pelvic reduction.7 We had a high penetration rate using CT, which increased with better resolution, even though none of the radiographs showed any obvious evidence of misplacement of the screws. Ebraheim and colleagues6 described the relationship of the S1 nerve root in its neural foramen and found it to be approximately 8.7 mm inferior and 7.8 mm medial to the starting point for a pedicle screw. Given these numbers, it is possible that a large amount of skiving can be tolerated contingent on an adequate reduction of the SI joint.
Because of our high rates of skiving and low rates of neurologic deficit, a new “safe zone” for screw insertion can be expanded to include skiving of the S1 neural foramen up to 3 mm without fear of nerve root injury. However, drilling and screw insertion at higher speeds can also cause neurologic injury secondary to thermal injury or soft tissue being caught up in a rotating drill/screw.
Evaluation of placement of percutaneous SI screw placement in our study resulted in neural foramen penetration in 43% of SI screws, which is higher than other studies.14,19,20 Our study showed that screw penetration up to 2 mm does not correlate with neurologic deficit. Iatrogenic neurologic deficit secondary to perforation of the foramina occurred in only 1 patient. Penetration of the foramina in all cases was in the superior portion of the foramen. We propose that there is a safe zone within the S1 neural foramen, and small amounts of penetration in the superior one-third of the foramen on axial CT images do not correlate with neurologic deficit. This potential safe zone is predicated on adequate reduction of the SI joint.
Neural foramen penetration shown on postoperative CT does not necessarily correlate with neurologic deficit. A postoperative CT is not indicated unless there are findings of a postoperative nerve injury. Our ideal screw placement skives the superior S1 foramen allowing for a larger screw diameter in a safe zone.
CT-guided placement has been proposed; however, concerns about radiation exposure, cost, and feasibility with similar outcomes compared with fluoroscopic-guided screw placement has resulted in its falling out of favor.
Iatrogenic nerve injuries are reported to occur in 0% to 6% of all percutaneous SI screw placement.14,21 Risk factors for iatrogenic nerve injury while using fluoroscopic guidance include sacral morphologic abnormalities, presence of intestinal gas, or contrast.22 Although these may be minimized with proper use of fluoroscopy, obtaining anatomic reduction as well as a thorough understanding of the pelvic morphology, the surgeon must be prepared to obtain further studies, such as a CT scan, if there is postoperative neurologic deficit.
Based on our findings, we do not routinely obtain a postoperative CT for SI screw placement, unless there is concern for malreduction or there is neurologic deficit. We also believe that up to 2 mm of foramen penetration is safe and does not result in neurologic deficit.
1. Failinger MS, McGanity PL. Unstable fractures of the pelvic ring. J Bone and Joint Surg Am. 1992;74(5):781-791.
2. Smith HE, Yuan PS, Sasso R, Papadopolous S, Vaccaro AR. An evaluation of image-guided technologies in the placement of percutaneous iliosacral screws. Spine (Phila Pa 1976). 2006;31(2):234-238.
3. Judet R, Judet J, Letournel E. Fractures of the acetabulum: classification and surgical approaches for open reduction. Preliminary report. J Bone Joint Surg Am. 1964;46(16):1615-1646.
4. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
5. Tonetti J, Carrat L, Blendea S, et al. Clinical results of percutaneous pelvic surgery. Computer assisted surgery using ultrasound compared to standard fluoroscopy. Comput Aided Surg. 2001;6(4):204-211.
6. Ebraheim NA, Coombs R, Jackson WT, Rusin JJ. Percutaneous computed tomography-guided stabilization of posterior pelvic fractures. Clin Orthop. 1994;(307):222-228.
7. Keating JF, Werier J, Blachut P, et al. Early fixation of the vertically unstable pelvis: the role of iliosacral screw fixation of the posterior lesion. J Orthop Trauma. 1999;13(2):107-113.
8. Webb LX, de Araujo W, Donofrio P, et al. Electromyography monitoring for percutaneous placement of iliosacral screws. J Orthop Trauma. 2000;14(4):245-254.
9. Barrick EF, O’Mara JW, Lane HE 3rd. Iliosacral screw insertion using computer-assisted CT image guidance: a laboratory study. Comput Aided Surg. 1998;3(6):289-296.
10. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
11. Altman DT, Jones CB, Routt ML Jr. Superior gluteal artery injury during iliosacral screw placement. J Orthop Trauma. 1999;13(3):220-227.
12. Stephen DJ. Pseudoaneurysm of the superior gluteal arterial system: an unusual cause of pain after a pelvic fracture. J Trauma. 1997;43(1):146-149.
13. Stöckle U, König B, Hofstetter R, Nolte LP, Haas NP. [Navigation assisted by image conversion. An experimental study on pelvic screw fixation]
[in German]. Unfallchirurg. 2001;104(3):215-220.
14. Templeman D, Schmidt A, Freese J, Weisman I, et al. Proximity of iliosacral screws to neurovascular structures after internal fixation. Clin Orthop. 1996;(329):194-198.
15. Young JW, Burgess AR, Brumback RJ, Poka A. Pelvic fractures: value of plain radiography in early assessment and management. Radiology. 1986;160(2):445-451.
16. Graves ML, Routt ML Jr. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Trauma. 2011;7(1):204-208.
17. Letournel E. Pelvic fractures. Injury. 1978;10(2):145-148.
18. Blake-Toker AM, Hawkins L, Nadalo L, et al. CT guided percutaneous fixation of sacroiliac fractures in trauma patients. J Trauma. 2001;51(6):1117-1121.
19. Hinsche AF, Giannoudis PV, Smith RM. Fluoroscopy-based multiplanar image guidance for insertion of sacroiliac screws. Clin Orthop. 2002;(395):135-144.
20. van den Bosch EW, van Zwienen CM, van Vugt AB. Fluoroscopic positioning of sacroiliac screws in 88 patients. J Trauma. 2002;53(1):44-48.
21. Cole JD, Blum DA, Ansel LJ. Outcome after fixation of unstable posterior pelvic ring injuries. Clin Orthop. 1996;(329):160-179.
22. Routt ML Jr, Simonian PT. Closed reduction and percutaneous skeletal fixation of sacral fractures. Clin Orthop. 1996;(329):121-128.
1. Failinger MS, McGanity PL. Unstable fractures of the pelvic ring. J Bone and Joint Surg Am. 1992;74(5):781-791.
2. Smith HE, Yuan PS, Sasso R, Papadopolous S, Vaccaro AR. An evaluation of image-guided technologies in the placement of percutaneous iliosacral screws. Spine (Phila Pa 1976). 2006;31(2):234-238.
3. Judet R, Judet J, Letournel E. Fractures of the acetabulum: classification and surgical approaches for open reduction. Preliminary report. J Bone Joint Surg Am. 1964;46(16):1615-1646.
4. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
5. Tonetti J, Carrat L, Blendea S, et al. Clinical results of percutaneous pelvic surgery. Computer assisted surgery using ultrasound compared to standard fluoroscopy. Comput Aided Surg. 2001;6(4):204-211.
6. Ebraheim NA, Coombs R, Jackson WT, Rusin JJ. Percutaneous computed tomography-guided stabilization of posterior pelvic fractures. Clin Orthop. 1994;(307):222-228.
7. Keating JF, Werier J, Blachut P, et al. Early fixation of the vertically unstable pelvis: the role of iliosacral screw fixation of the posterior lesion. J Orthop Trauma. 1999;13(2):107-113.
8. Webb LX, de Araujo W, Donofrio P, et al. Electromyography monitoring for percutaneous placement of iliosacral screws. J Orthop Trauma. 2000;14(4):245-254.
9. Barrick EF, O’Mara JW, Lane HE 3rd. Iliosacral screw insertion using computer-assisted CT image guidance: a laboratory study. Comput Aided Surg. 1998;3(6):289-296.
10. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
11. Altman DT, Jones CB, Routt ML Jr. Superior gluteal artery injury during iliosacral screw placement. J Orthop Trauma. 1999;13(3):220-227.
12. Stephen DJ. Pseudoaneurysm of the superior gluteal arterial system: an unusual cause of pain after a pelvic fracture. J Trauma. 1997;43(1):146-149.
13. Stöckle U, König B, Hofstetter R, Nolte LP, Haas NP. [Navigation assisted by image conversion. An experimental study on pelvic screw fixation]
[in German]. Unfallchirurg. 2001;104(3):215-220.
14. Templeman D, Schmidt A, Freese J, Weisman I, et al. Proximity of iliosacral screws to neurovascular structures after internal fixation. Clin Orthop. 1996;(329):194-198.
15. Young JW, Burgess AR, Brumback RJ, Poka A. Pelvic fractures: value of plain radiography in early assessment and management. Radiology. 1986;160(2):445-451.
16. Graves ML, Routt ML Jr. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Trauma. 2011;7(1):204-208.
17. Letournel E. Pelvic fractures. Injury. 1978;10(2):145-148.
18. Blake-Toker AM, Hawkins L, Nadalo L, et al. CT guided percutaneous fixation of sacroiliac fractures in trauma patients. J Trauma. 2001;51(6):1117-1121.
19. Hinsche AF, Giannoudis PV, Smith RM. Fluoroscopy-based multiplanar image guidance for insertion of sacroiliac screws. Clin Orthop. 2002;(395):135-144.
20. van den Bosch EW, van Zwienen CM, van Vugt AB. Fluoroscopic positioning of sacroiliac screws in 88 patients. J Trauma. 2002;53(1):44-48.
21. Cole JD, Blum DA, Ansel LJ. Outcome after fixation of unstable posterior pelvic ring injuries. Clin Orthop. 1996;(329):160-179.
22. Routt ML Jr, Simonian PT. Closed reduction and percutaneous skeletal fixation of sacral fractures. Clin Orthop. 1996;(329):121-128.
Large-Diameter Femoral Heads in Total Hip Arthroplasty: An Evidence-Based Review
A common cause for total hip arthroplasty (THA) revision is joint instability.1,2 The reported incidence of dislocation in primary THA ranges from 0.4% to 5.8%,3-5 but this rate increases after revision surgery.1,3-8 Use of large-diameter femoral heads has been proposed to decrease the risks for instability and to improve impingement-free range of motion (ROM).
The biomechanical rationale for using large-diameter femoral heads is that they must travel farther before subluxation or dislocation occurs (jump distance). Despite these benefits, there were initial concerns that catastrophic failure and high levels of volumetric wear would occur if these heads were used with conventional polyethylene liners. These concerns led to the development of alternative bearing surfaces, particularly metal-on-metal bearings, which offered theoretical benefits of large-diameter articulations that improved stability while purportedly being highly wear-resistant.9-11 However, concerns about adverse local soft-tissue reactions and high blood concentrations of metal ions tempered the initial enthusiasm for metal bearings.12-16 Fortunately, highly cross-linked polyethylene and fourth-generation ceramic bearing surfaces, with improved toughness and better wear properties, may allow use of large-diameter heads without the need for metal-on-metal bearings.17,18
In this article, we review the concepts and principles behind use of large-diameter ceramic or cobalt-chromium femoral heads on polyethylene-bearing surfaces in THA with particular attention to biomechanics, early concerns about polyethylene wear and rim fractures, recent improvements in material properties of polyethylene and ceramic bearings, dislocation rates, and clinical and functional outcomes.
Definitions
For this review, we define large-diameter femoral heads as having diameters of 36 mm or more and conventional or small-diameter femoral heads as having diameters between 22 and 32 mm.
Biomechanics
Head–Neck Ratio, Impingement-Free ROM, and Jump Distance
Several implant design principles have been proposed to reduce the risks for impingement and dislocation. Of these, large femoral head diameters have been extensively studied.19,20 It is well known that impingement of the femoral neck on the cup edge promotes edge loading and higher wear rates. In addition, impingement increases the tendency of the head to sublux from the acetabulum. One strategy for avoiding this component-to-component impingement is to increase the head–neck ratio (HNR), the ratio of the femoral head to the neck diameter. Biomechanically, increased HNRs lead to delayed contact between the femoral neck and the acetabular liner.21,22 Therefore, with large femoral heads, which have large HNRs, impingement occurs later and at larger ROMs—compared with small-diameter femoral heads, which have lower HNRs and are more prone to early impingement and subluxation (Figure 1).23-26
In a cadaveric study of 6 hips, Bartz and colleagues23 reported a significantly higher preimpingement ROM when the prosthetic head size increased from 22 mm to 28 mm (P < .05). They found a change from prosthetic to osseous impingement when the head size increased from 22 mm to 32 mm. Similar results were observed in a computer simulation model by Cinotti and colleagues,27 who demonstrated that increasing the femoral head size from 28 mm to 38 mm resulted in a 5° improvement in ROM. However, the largest gains were observed when the heads with the smallest diameters were upsized; ROM improved only marginally when femoral head size was further increased from 32 mm to 38 mm. The primary reason for the lack of expected improvement in ROM with head sizes of more than 32 mm is often bone-on-bone impingement. Burroughs and colleagues28 demonstrated that the 38-mm and 44-mm heads virtually eliminated component-to-component impingement except in extremes of external rotation. However, there were no differences in ROM between 38-mm and 44-mm heads because of osseous impingement. In addition, large heads are less likely to sublux or dislocate, as they need to travel farther before reaching the edge of the acetabular cup before dislocation. This is known as the jump distance, and it corresponds to the depth of the acetabular shell, which in turn equates with the radius of the femoral head (Figures 2A, 2B). For this reason, the larger the femoral head diameter, the farther the jump distance and, correspondingly, the lower the risk for dislocation.29
Elevated liners historically were used to increase the jump distance for dislocation.30 These liners, however, can increase impingement at the extremes of motion.31 Some of these problems can be avoided with use of larger heads, which have increased jump distances without additional risks for impingement. Moreover, large heads create a suction effect that provides passive resistance to dislocation.32 With head diameters beyond 38 mm, impingement-free ROM often plateaus. However, the jump distance required for dislocations to occur continues to increase as femoral head diameters increase in size. Thus, patients may experience fewer motion benefits but continue to benefit from overall stability with femoral head sizes increasing beyond 38 mm.
Current evidence suggests there may be substantial benefits toward improved stability from increasing head diameters from 22 mm to 38 mm because of the increase in jump distances and improvements in prosthetic impingement-free ROM. However, there may be little gain in ROM from increasing the head diameters beyond these dimensions because of the potential risks of bony impingement. Nevertheless, there may be some additional benefits toward stability from improvement in jump distances with incremental head sizes
beyond 38 mm.29,33,34
Finite Element Analysis Studies
Finite element analysis of large-diameter heads in THA has shown that, at optimal cup inclination (45°), most stresses occur on the articular surface of the liner. However, these stresses remain well below the yield strength of the polyethylene liners.29 With increasing abduction angles, the stress concentration increases substantially because of the decreased contact surface area. At these angles, the point of maximum contact moves toward the rim of the polyethylene liner, which can lead to rim fractures or failure of locking mechanisms.29,35,36
Early Concerns With Large-Diameter Femoral Heads: Wear, Liner Failure, and Fracture of Ceramic Components
Use of small-diameter femoral heads started with the first report by Charnley37 of “low frictional torque arthroplasty.” Charnley initially considered a 41.5-mm femoral head, but he thought it would increase risks for acetabular loosening from high frictional torque generated by the large head, and he switched to a small-diameter (22.5 mm) design. One of the tradeoffs with smaller diameter heads was decreased jump height in addition to increased linear wear.
Large femoral heads used with cemented polyethylene acetabular components historically have been associated with increased rates of volumetric wear but low rates of linear wear, which potentially may increase the risk for osteolysis.38-40 However, newer highly cross-linked polyethylene liners have shown improved in vitro and in vivo volumetric wear characteristics and potentially lower linear wear rates compared with earlier designs (Table 1).28,41-43
Another concern about earlier generations of large femoral heads was the risk for catastrophic liner failure on conventional polyethylene. This was originally reported by Berry and colleagues,47 who described wear-through and failure in patients with thin (< 5 mm) acetabular cups. However, these concerns have been largely addressed by the development of highly cross-linked polyethylene, which has improved wear characteristics and fatigue resistance.48
Recent Improvements in Material Properties of Polyethylene and Ceramic Bearings
The development of highly cross-linked polyethylene and fourth-generation ceramics has renewed interest in large-diameter bearings in THA. These bearing surfaces improve wear, enhance material properties, and have superior oxidation resistance.42,48-53
We now briefly describe the methods used to improve the material properties of polyethylene and ceramics. Studies have shown that increasing the radiation dose (up to 200 kGy) increases cross-linking and causes an inverse exponential decrease in polyethylene wear.28,41,48-51 However, increasing radiation doses also increases production of free radicals, which diminish the material strength of these polyethylenes. The current generation of highly cross-linked polyethylene liners is produced through a variety of manufacturing strategies to improve cross-linking and reduce wear. These strategies include differential radiation doses (50-100 kGy), techniques (electron beam, radiation), and thermal treatments (melting, annealing). Moreover, to enhance the material properties and reduce the incidence of rim cracking and delamination, authors have proposed using vitamin E supplementation to minimize the amount of subsurface oxidation that occurs as an inevitable consequence of free radical formation during fabrication.54,55 A terminal sterilization process (eg, gas plasma, ethylene oxide, or gamma sterilization in nitrogen) is needed to make commercial, highly cross-linked polyethylene.52,53
Fourth-generation ceramics manufactured with nano-sized yttria-stabilized tetragonal zirconia particles in a stable alumina matrix have more fracture toughness and improved wear characteristics.54,55 In addition, oxide additives (eg, chromium oxide, strontium oxide) improve hardness and dissipate energy by deflecting cracks to prevent their propagation.56 Moreover, the smaller grain sizes of fourth-generation ceramic bearings compared with third-generation designs (0.8 µm vs 1-5 µm) cause less disruption of the fluid film layer, which ultimately results in improved wear performance.57
Multiple studies have found reduced wear rates with metal and ceramic large heads coupled with highly cross-linked polyethylene-bearings (Table 2).17,41,50,58 Bragdon and colleagues,58 using radiostereometric analysis in 25 patients, found no significant differences in mean head penetration rates between 36-mm and 28-mm cobalt-chromium (Co-Cr) heads articulating with highly cross-linked polyethylene cups at a mean follow-up of 3 years (0.035 mm/y vs 0.046 mm/y; P = .11). Geller and colleagues,64 in their study of 42 patients with large-diameter (> 32 mm) Co-Cr femoral heads, found low mean (SD) linear wear rates of 0.06 (0.41) mm/y at a mean follow-up of 3 years. D’Antonio and colleagues,65 in a multicenter study, reported low average linear wear (0.015 mm/y) and volumetric wear (12.1 mm3/y) over 5 years using sequentially annealed cross-linked polyethylene. In vitro reports suggest that large-diameter ceramic heads may have lower wear properties than Co-Cr heads do. Galvin and colleagues,66 in an in vitro hip simulator study, found that large-diameter ceramic heads on highly cross-linked ultrahigh-molecular-weight polyethylene had 40% reductions in steady-state wear rates compared with Co-Cr heads on highly cross-linked bearings (4.7 vs 8.1 mm3/million cycles; P < 0.01).
Dislocation Rates
Several patient, surgeon, and implant factors affect the rate of dislocations after THA. Multiple implant options utilize the biomechanical advantage that large-diameter heads have in improving stability. Various alternatives include use of constrained tripolar heads, dual-mobility bearings, and conventional large-diameter heads with standard liners.67-69
Large-Diameter Heads
Despite the biomechanical advantages of large-diameter metal-on-polyethylene bearings, prior studies have questioned use of these bearings because of risks for increased wear and rim failures. However, the improved wear properties of highly cross-linked polyethylene, elaborated earlier, have led to a reappraisal of this option (Table 2).4,70 Howie and colleagues,71 in a randomized control trial of 644 patients, also found significantly lower rates of dislocation after primary THA with 36-mm heads compared with 28-mm heads (1.3% vs 5.4%; P = .012); in addition, fewer dislocations occurred with 36-mm heads than with 28-mm heads (4.9% vs 12.2%; P = .27) in a series of 44 patients in revision settings. Similarly, in a study conducted with 39,271 Medicare patients between 1998 and 2007, Malkani and colleagues72 found a decrease in the dislocation rate, from 4.21% to 2.14%, with use of large-diameter femoral heads. These results have been confirmed by several other authors.34,66,73,74 Similar results were observed in 65,992 patients in the Australian National Joint Replacement Registry by Conroy and colleagues,75 who reported a significant decrease in the risk for dislocation with large heads (≥ 30 mm) compared with 22-mm heads (relative risk, 1.0 vs 3.1; P ≤ .001).
Few studies have analyzed the role of large-diameter femoral heads in the presence of compromised soft tissues around the hip. Kung and Ries,76 evaluating the influence of large-diameter heads in the presence and absence of a deficient abductor mechanism, demonstrated statistically significant reductions in rates of dislocation after 230 revision THAs when the abductor mechanism was intact with use of 36-mm heads compared with 28-mm heads (12.7% vs 0%; P = .015). With abductor deficiency, though, the positive effect of large heads in reducing dislocation rates was substantially reduced and was similar to that of small heads (P = .74).76
Large heads considerably improve overall stability and lower dislocation rates in THA. With the development of newer ceramics and highly cross-linked polyethylenes, the wear rates reported in multiple studies appear to be less concerning.
Constrained Tripolar Heads
Tripolar heads have been proposed as treatment options for improving stability in patients with chronic and recurrent instability after THA. The tripolar implant consists of a metal head that snap-fits into a polyethylene liner with a polished Co-Cr backing. This bipolar head articulates with a polyethylene bearing that is press-fitted onto a metal acetabular shell and constrained by a metal ring snapped to the outer polyethylene bearing. The bipolar component behaves as a large-diameter femoral head, and the metal ring provides additional restraint, further improving stability.
Williams and colleagues77 performed a systematic review and reported on the outcomes of constrained tripolar liners in 1199 hips at a mean follow-up of 4 years (range, 2-10 years). The mean dislocation rate was 10%, and the mean rate of revision surgery unrelated to instability was 4%. In a study of 43 hips at a mean follow-up of 4 years (range, 2-9 years), Zywiel and colleagues78 reported on the clinical and radiographic outcomes of tripolar constrained liners. Their study group had a mean Harris Hip Score (HHS) of 82 points (range, 38-100 points) and overall survival of 91%, with no evidence of radiographic loosening during follow-up. Despite the improvements in stability with constrained tripolar liners, some authors have reported multiple mechanisms of failure with these devices.79-81 In a study of 43 failed constrained tripolar liners with a mean time to failure of about 2 years, Guyen and colleagues79 identified 5 different failure modes (types 1-5) involving all 4 interfaces in these components.
Encouraging outcomes have been reported at midterm follow-up with tripolar constrained liners. However, concerns about failure at the interfaces suggest that use of these components should be restricted to patients with deficient abductor mechanisms or neuromuscular compromise, low-demand elderly patients, and salvage cases of recurrent dislocations.79
Dual-Mobility Bearings
For more than 20 years, different dual-mobility bearings have been used for difficult acetabular reconstructive scenarios and prevention of instability.82,83 Dual-mobility cups provide constructs that snap-fit a small-diameter femoral head within a large polyethylene insert that articulates with a fixed metal shell. This effectively increases the functional head diameter.
Various authors have reported excellent survivorship rates (92%-99%) and low dislocation rates for these bearings at 5- to 10-year follow-up.82,84-90 Philippot and colleagues,86 in a recent study of 438 hips with dual-mobility cups, reported excellent survivorship (96%) and no early or late instability within a 15-year follow-up. Bouchet and colleagues69 compared dual-mobility bearings (105 hips) with conventional metal-on-polythene bearings (108 hips) and found significantly (P < .05) lower dislocation rates for the dual-mobility implants at a minimum 1-year follow-up. The French Society of Orthopaedics and Traumatology performed a multicenter analysis of 3473 hips with dual-mobility cups implanted in France between January 1998 and December 2003.87 During a mean follow-up of 7 years (range, 5-11 years), there were 15 dislocations (0.43%), 14 of which occurred early, within 3 months of implantation (0.4%). Aseptic implant survivorship was 95% at 10-year follow-up.
Use of these bearings has recently increased in the United States. Short-term and midterm follow-up data show low rates of dislocation and wear. Long-term data are to come.
Clinical and Functional Outcomes of Large-Diameter Femoral Heads
There is a paucity of long-term outcomes data on use of large-diameter heads with highly cross-linked polyethylene bearings. Short-term and midterm clinical results appear to be excellent, with low rates of wear, osteolysis, and aseptic loosening.28,41,73,89-92
Plate and colleagues91 compared the effects of large-diameter (≥ 36 mm) and small-diameter (26 mm, 28 mm) metal heads on highly cross-linked polyethylene bearings. At a mean follow-up of 5 years (range, 4-8.4 years), the large-head cohort had a mean HHS of 90 points (range, 50-100 points) and no dislocations or radiographic evidence of stem or cup loosening. Similarly, Meftah and colleagues93 reported 100% stem survivorship and excellent clinical outcomes—a mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) score of 30 points—for 72 hips with use of large ceramic heads (≥ 32 mm) on highly cross-linked polyethylene at a mean follow-up of 3 years. Gagala and colleagues94 reported excellent clinical and radiographic outcomes in 50 hips (18 ceramic on ceramic, 32 ceramic on polyethylene; 36-mm heads) at a mean follow-up of 3.5 years. Mean HHS was 94 points, and there was no evidence of liner fractures, aseptic loosening, or osteolysis.
In summary, large-diameter femoral heads in THA have become increasingly popular because of improvements in the material properties and wear characteristics of highly cross-linked polyethylene and fourth-generation ceramics. Despite the potential advantages of large heads in preventing dislocations, the basic surgical tenets of placing the acetabular component in appropriate alignment remain firmly established. Implants with functionally large heads (eg, dual-mobility bearings, constrained tripolar liners) may play an important role in patients at high risk for dislocation—particularly elderly patients with poor neuromuscular muscle coordination or deficient abductors, trauma patients, and patients with prior dislocations. Short-term and midterm results are excellent; rates of wear, aseptic loosening, and osteolysis are low. However, long-term outcomes data are needed to support widespread use of large heads in younger and more active patients.
1. Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop. 1983;(173):151-158.
2. Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop. 1998;(355):144-151.
3. Turner RS. Postoperative total hip prosthetic femoral head dislocations. Incidence, etiologic factors, and management. Clin Orthop. 1994;(301):196-204.
4. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295-1306.
5. Etienne A, Cupic Z, Charnley J. Postoperative dislocation after Charnley low-friction arthroplasty. Clin Orthop. 1978;(132):19-23.
6. Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169-178.
7. Joshi A, Lee CM, Markovic L, Vlatis G, Murphy JC. Prognosis of dislocation after total hip arthroplasty. J Arthroplasty. 1998;13(1):17-21.
8. Lindberg HO, Carlsson AS, Gentz CF, Pettersson H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop Scand. 1982;53(6):947-952.
9. Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90(10):1278-1283.
10. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841-848.
11. Leslie I, Williams S, Brown C, et al. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements—an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87(1):163-172.
12. Verhaar JA. The hard lesson of metal-on-metal hip implants [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(42):A5564.
13. Fabi D, Levine B, Paprosky W, et al. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35(7):e1009-e1016.
14. Heneghan C, Langton D, Thompson M. Ongoing problems with metal-on-metal hip implants. BMJ. 2012;344:e1349.
15. Lee RK, Nevelos J, Vigdorchik J, Markel DC. Bearing surfaces for hip arthroplasty—is metal-on-metal a passing fancy? Surg Technol Int. 2012;22:243-249.
16. Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(10):1844-1849.
17. Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results.
J Bone Joint Surg Am. 2001;83(11):1688-1694.
18. Callaghan JJ, Liu SS. Ceramic on crosslinked polyethylene in total hip replacement: any better than metal on crosslinked polyethylene? Iowa Orthop J. 2009;29:1-4.
19. Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
20. Krushell RJ, Burke DW, Harris WH. Elevated-rim acetabular components. Effect on range of motion and stability in total hip arthroplasty. J Arthroplasty. 1991;6(suppl):S53-S58.
21. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23(2):237-248.
22. Morrey BF. Dislocation. In: Morrey BF, ed. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991:851-865.
23. Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82(9):1300-1307.
24. Nicholas RM, Orr JF, Mollan RA, Calderwood JW, Nixon JR, Watson P. Dislocation of total hip replacements. A comparative study of standard, long posterior wall and augmented acetabular components. J Bone Joint Surg Br. 1990;72(3):418-422.
25. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
26. Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, orientation and component interaction in dislocation of the total hip prosthesis. Acta Radiol. 1988;29(4):441-444.
27. Cinotti G, Lucioli N, Malagoli A, Calderoli C, Cassese F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int Orthop. 2011;35(3):317-323.
28. Burroughs BR, Rubash HE, Harris WH. Femoral head sizes larger than 32 mm against highly cross-linked polyethylene. Clin Orthop. 2002;(405):150-157.
29. Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don‘t do. Clin Orthop. 2004;(429):102-107.
30. Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979.
31. Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15(3):305-313.
32. Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140-144.
33. Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop. 2002;(405):46-53.
34. Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84(2):256-263.
35. Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27(6):917-925.
36. Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146-154.
37. Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop. 1970;(72):7-21.
38. Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75(2):254-258.
39. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72(4):518-528.
40. Ma SM, Kabo JM, Amstutz HC. Frictional torque in surface and conventional hip replacement. J Bone Joint Surg Am. 1983;65(3):366-370.
41. Muratoglu OK, Bragdon CR, O‘Connor D, et al. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 suppl 1):24-30.
42. Thomas GER, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
43. Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop. 2010;468(12):3228-3233.
44. Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Malchau H. The 2012 John Charnley Award: clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop. 2013;471(2):393-402.
45. Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop. 2009;467(12):3290-3296.
46. Sychterz CJ, Engh CA Jr, Young AM, Hopper RH Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82(7):948-951.
47. Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components.
J Bone Joint Surg Br. 1994;76(4):575-578.
48. McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157-167.
49. Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol Int. 1998;31(1-3):17-33.
50. Estok DM 2nd, Burroughs BR, Muratoglu OK, Harris WH. Comparison of hip simulator wear of 2 different highly cross-linked ultra high molecular weight polyethylene acetabular components using both 32- and 38-mm femoral heads. J Arthroplasty. 2007;22(4):581-589.
51. Muratoglu OK, Bragdon CR, O‘Connor DO, et al. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20(16):1463-1470.
52. Harris WH, Muratoglu OK. A review of current cross-linked polyethylenes used in total joint arthroplasty. Clin Orthop. 2005;(430):46-52.
53. Muratoglu OK, Bragdon CR, O‘Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16(2):149-160.
54. Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. Hip Int. 2007;17(1):21-30.
55. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. Biomed Res Int. 2013;2013:157247.
56. Cai YZ, Yan SG. Development of ceramic-on-ceramic implants for total hip arthroplasty. Orthop Surg. 2010;2(3):175-181.
57. Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66(2):567-573.
58. Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125-129.
59. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop. 2011;469(6):1547-1553.
60. Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop. 2013;471(2):439-443.
61. Cai P, Hu Y, Xie J. Large-diameter Delta ceramic-on-ceramic versus common-sized ceramic-on-polyethylene bearings in THA. Orthopedics. 2012;35(9):e1307-e1313.
62. Park KS, Yoon TR, Hwang SY, Lee KB. Modified minimally invasive two-incision total hip arthroplasty using large diameter femoral head. Indian
J Orthop. 2012;46(1):29-35.
63. Garbuz DS, Masri BA, Duncan CP, et al. The Frank Stinchfield Award: dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop. 2012;470(2):351-356.
64. Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop. 2006;(447):53-59.
65. D’Antonio JA, Capello WN, Ramakrishnan R. Second-generation annealed highly cross-linked polyethylene exhibits low wear. Clin Orthop. 2012;470(6):1696-1704.
66. Galvin AL, Jennings LM, Tipper JL, Ingham E, Fisher J. Wear and creep of highly crosslinked polyethylene against cobalt chrome and ceramic femoral heads. Proc Inst Mech Eng H. 2010;224(10):1175-1183.
67. Skeels MD, Berend KR, Lombardi AV Jr. The dislocator, early and late: the role of large heads. Orthopedics. 2009;32(9).
68. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
69. Bouchet R, Mercier N, Saragaglia D. Posterior approach and dislocation rate: a 213 total hip replacements case–control study comparing the dual mobility cup with a conventional 28-mm metal head/polyethylene prosthesis. Orthop Traumatol Surg Res. 2011;97(1):2-7.
70. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63(2):214-218.
71. Howie DW, Holubowycz OT, Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095-1102.
72. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
73. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
74. Cho MR, Lee HS, Lee SW, Choi CH, Kim SK, Ko SB. Results after total hip arthroplasty with a large head and bipolar arthroplasty in patients with displaced femoral neck fractures. J Arthroplasty. 2011;26(6):893-896.
75. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty.
J Arthroplasty. 2008;23(6):867-872.
76. Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop. 2007;(465):170-174.
77. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
78. Zywiel MG, Mustafa LH, Bonutti PM, Mont MA. Are abductor muscle quality and previous revision surgery predictors of constrained liner failure in hip arthroplasty? Int Orthop. 2011;35(6):797-802.
79. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
80. Banks LN, McElwain JP. An unusual mode of failure of a tripolar constrained acetabular liner: a case report. Arch Orthop Trauma Surg. 2010;130(4):503-505.
81. Robertson WJ, Mattern CJ, Hur J, Su EP, Pellicci PM. Failure mechanisms and closed reduction of a constrained tripolar acetabular liner.
J Arthroplasty. 2009;24(2):322.e5-e11.
82. Aubriot JH, Lesimple P, Leclercq S. Study of Bousquet‘s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component). Average 5-year follow-up [in French]. Acta Orthop Belg. 1993;59(suppl 1):267-271.
83. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
84. Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8(3):251-256.
85. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop. 2008;466(2):389-395.
86. Philippot R, Farizon F, Camilleri JP, et al. Survival of dual mobility socket with a mean 17 years follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(1):43-48.
87. Adam P, Philippe R, Ehlinger M, et al. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly.
A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop Traumatol Surg Res. 2012;98(3):296-300.
88. Fessy MH. La double mobilité [Dual mobility]. Revue de Chirurgie Orthopédique et Traumatologique. 2010;96(7):891-898.
89. Mont MA, Issa K, Naziri Q, Harwin SF, Delanois RE, Johnson AJ. The use of dual-mobility bearings in difficult hip arthroplasty reconstructive cases. Surg Technol Int. 2011;21:234-240.
90. Sayeed SA, Mont MA, Costa CR, et al. Early outcomes of sequentially cross-linked thin polyethylene liners with large diameter femoral heads in total hip arthroplasty. Bull NYU Hosp Jt Dis. 2011;69(suppl 1):S90-S94.
91. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
92. Sato T, Nakashima Y, Akiyama M, et al. Wear resistant performance of highly cross-linked and annealed ultra-high molecular weight polyethylene against ceramic heads in total hip arthroplasty. J Orthop Res. 2012;30(12):2031-2037.
93. Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34(6):133.
94. Gagala J, Mazurkiewicz T, Dajewski Z. Large diameter femoral heads in primary alumina/alumina and XSPE/alumina total hip arthroplasty.
A follow-up study of 50 hips after average 40 months and review of literature [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):14-20.
A common cause for total hip arthroplasty (THA) revision is joint instability.1,2 The reported incidence of dislocation in primary THA ranges from 0.4% to 5.8%,3-5 but this rate increases after revision surgery.1,3-8 Use of large-diameter femoral heads has been proposed to decrease the risks for instability and to improve impingement-free range of motion (ROM).
The biomechanical rationale for using large-diameter femoral heads is that they must travel farther before subluxation or dislocation occurs (jump distance). Despite these benefits, there were initial concerns that catastrophic failure and high levels of volumetric wear would occur if these heads were used with conventional polyethylene liners. These concerns led to the development of alternative bearing surfaces, particularly metal-on-metal bearings, which offered theoretical benefits of large-diameter articulations that improved stability while purportedly being highly wear-resistant.9-11 However, concerns about adverse local soft-tissue reactions and high blood concentrations of metal ions tempered the initial enthusiasm for metal bearings.12-16 Fortunately, highly cross-linked polyethylene and fourth-generation ceramic bearing surfaces, with improved toughness and better wear properties, may allow use of large-diameter heads without the need for metal-on-metal bearings.17,18
In this article, we review the concepts and principles behind use of large-diameter ceramic or cobalt-chromium femoral heads on polyethylene-bearing surfaces in THA with particular attention to biomechanics, early concerns about polyethylene wear and rim fractures, recent improvements in material properties of polyethylene and ceramic bearings, dislocation rates, and clinical and functional outcomes.
Definitions
For this review, we define large-diameter femoral heads as having diameters of 36 mm or more and conventional or small-diameter femoral heads as having diameters between 22 and 32 mm.
Biomechanics
Head–Neck Ratio, Impingement-Free ROM, and Jump Distance
Several implant design principles have been proposed to reduce the risks for impingement and dislocation. Of these, large femoral head diameters have been extensively studied.19,20 It is well known that impingement of the femoral neck on the cup edge promotes edge loading and higher wear rates. In addition, impingement increases the tendency of the head to sublux from the acetabulum. One strategy for avoiding this component-to-component impingement is to increase the head–neck ratio (HNR), the ratio of the femoral head to the neck diameter. Biomechanically, increased HNRs lead to delayed contact between the femoral neck and the acetabular liner.21,22 Therefore, with large femoral heads, which have large HNRs, impingement occurs later and at larger ROMs—compared with small-diameter femoral heads, which have lower HNRs and are more prone to early impingement and subluxation (Figure 1).23-26
In a cadaveric study of 6 hips, Bartz and colleagues23 reported a significantly higher preimpingement ROM when the prosthetic head size increased from 22 mm to 28 mm (P < .05). They found a change from prosthetic to osseous impingement when the head size increased from 22 mm to 32 mm. Similar results were observed in a computer simulation model by Cinotti and colleagues,27 who demonstrated that increasing the femoral head size from 28 mm to 38 mm resulted in a 5° improvement in ROM. However, the largest gains were observed when the heads with the smallest diameters were upsized; ROM improved only marginally when femoral head size was further increased from 32 mm to 38 mm. The primary reason for the lack of expected improvement in ROM with head sizes of more than 32 mm is often bone-on-bone impingement. Burroughs and colleagues28 demonstrated that the 38-mm and 44-mm heads virtually eliminated component-to-component impingement except in extremes of external rotation. However, there were no differences in ROM between 38-mm and 44-mm heads because of osseous impingement. In addition, large heads are less likely to sublux or dislocate, as they need to travel farther before reaching the edge of the acetabular cup before dislocation. This is known as the jump distance, and it corresponds to the depth of the acetabular shell, which in turn equates with the radius of the femoral head (Figures 2A, 2B). For this reason, the larger the femoral head diameter, the farther the jump distance and, correspondingly, the lower the risk for dislocation.29
Elevated liners historically were used to increase the jump distance for dislocation.30 These liners, however, can increase impingement at the extremes of motion.31 Some of these problems can be avoided with use of larger heads, which have increased jump distances without additional risks for impingement. Moreover, large heads create a suction effect that provides passive resistance to dislocation.32 With head diameters beyond 38 mm, impingement-free ROM often plateaus. However, the jump distance required for dislocations to occur continues to increase as femoral head diameters increase in size. Thus, patients may experience fewer motion benefits but continue to benefit from overall stability with femoral head sizes increasing beyond 38 mm.
Current evidence suggests there may be substantial benefits toward improved stability from increasing head diameters from 22 mm to 38 mm because of the increase in jump distances and improvements in prosthetic impingement-free ROM. However, there may be little gain in ROM from increasing the head diameters beyond these dimensions because of the potential risks of bony impingement. Nevertheless, there may be some additional benefits toward stability from improvement in jump distances with incremental head sizes
beyond 38 mm.29,33,34
Finite Element Analysis Studies
Finite element analysis of large-diameter heads in THA has shown that, at optimal cup inclination (45°), most stresses occur on the articular surface of the liner. However, these stresses remain well below the yield strength of the polyethylene liners.29 With increasing abduction angles, the stress concentration increases substantially because of the decreased contact surface area. At these angles, the point of maximum contact moves toward the rim of the polyethylene liner, which can lead to rim fractures or failure of locking mechanisms.29,35,36
Early Concerns With Large-Diameter Femoral Heads: Wear, Liner Failure, and Fracture of Ceramic Components
Use of small-diameter femoral heads started with the first report by Charnley37 of “low frictional torque arthroplasty.” Charnley initially considered a 41.5-mm femoral head, but he thought it would increase risks for acetabular loosening from high frictional torque generated by the large head, and he switched to a small-diameter (22.5 mm) design. One of the tradeoffs with smaller diameter heads was decreased jump height in addition to increased linear wear.
Large femoral heads used with cemented polyethylene acetabular components historically have been associated with increased rates of volumetric wear but low rates of linear wear, which potentially may increase the risk for osteolysis.38-40 However, newer highly cross-linked polyethylene liners have shown improved in vitro and in vivo volumetric wear characteristics and potentially lower linear wear rates compared with earlier designs (Table 1).28,41-43
Another concern about earlier generations of large femoral heads was the risk for catastrophic liner failure on conventional polyethylene. This was originally reported by Berry and colleagues,47 who described wear-through and failure in patients with thin (< 5 mm) acetabular cups. However, these concerns have been largely addressed by the development of highly cross-linked polyethylene, which has improved wear characteristics and fatigue resistance.48
Recent Improvements in Material Properties of Polyethylene and Ceramic Bearings
The development of highly cross-linked polyethylene and fourth-generation ceramics has renewed interest in large-diameter bearings in THA. These bearing surfaces improve wear, enhance material properties, and have superior oxidation resistance.42,48-53
We now briefly describe the methods used to improve the material properties of polyethylene and ceramics. Studies have shown that increasing the radiation dose (up to 200 kGy) increases cross-linking and causes an inverse exponential decrease in polyethylene wear.28,41,48-51 However, increasing radiation doses also increases production of free radicals, which diminish the material strength of these polyethylenes. The current generation of highly cross-linked polyethylene liners is produced through a variety of manufacturing strategies to improve cross-linking and reduce wear. These strategies include differential radiation doses (50-100 kGy), techniques (electron beam, radiation), and thermal treatments (melting, annealing). Moreover, to enhance the material properties and reduce the incidence of rim cracking and delamination, authors have proposed using vitamin E supplementation to minimize the amount of subsurface oxidation that occurs as an inevitable consequence of free radical formation during fabrication.54,55 A terminal sterilization process (eg, gas plasma, ethylene oxide, or gamma sterilization in nitrogen) is needed to make commercial, highly cross-linked polyethylene.52,53
Fourth-generation ceramics manufactured with nano-sized yttria-stabilized tetragonal zirconia particles in a stable alumina matrix have more fracture toughness and improved wear characteristics.54,55 In addition, oxide additives (eg, chromium oxide, strontium oxide) improve hardness and dissipate energy by deflecting cracks to prevent their propagation.56 Moreover, the smaller grain sizes of fourth-generation ceramic bearings compared with third-generation designs (0.8 µm vs 1-5 µm) cause less disruption of the fluid film layer, which ultimately results in improved wear performance.57
Multiple studies have found reduced wear rates with metal and ceramic large heads coupled with highly cross-linked polyethylene-bearings (Table 2).17,41,50,58 Bragdon and colleagues,58 using radiostereometric analysis in 25 patients, found no significant differences in mean head penetration rates between 36-mm and 28-mm cobalt-chromium (Co-Cr) heads articulating with highly cross-linked polyethylene cups at a mean follow-up of 3 years (0.035 mm/y vs 0.046 mm/y; P = .11). Geller and colleagues,64 in their study of 42 patients with large-diameter (> 32 mm) Co-Cr femoral heads, found low mean (SD) linear wear rates of 0.06 (0.41) mm/y at a mean follow-up of 3 years. D’Antonio and colleagues,65 in a multicenter study, reported low average linear wear (0.015 mm/y) and volumetric wear (12.1 mm3/y) over 5 years using sequentially annealed cross-linked polyethylene. In vitro reports suggest that large-diameter ceramic heads may have lower wear properties than Co-Cr heads do. Galvin and colleagues,66 in an in vitro hip simulator study, found that large-diameter ceramic heads on highly cross-linked ultrahigh-molecular-weight polyethylene had 40% reductions in steady-state wear rates compared with Co-Cr heads on highly cross-linked bearings (4.7 vs 8.1 mm3/million cycles; P < 0.01).
Dislocation Rates
Several patient, surgeon, and implant factors affect the rate of dislocations after THA. Multiple implant options utilize the biomechanical advantage that large-diameter heads have in improving stability. Various alternatives include use of constrained tripolar heads, dual-mobility bearings, and conventional large-diameter heads with standard liners.67-69
Large-Diameter Heads
Despite the biomechanical advantages of large-diameter metal-on-polyethylene bearings, prior studies have questioned use of these bearings because of risks for increased wear and rim failures. However, the improved wear properties of highly cross-linked polyethylene, elaborated earlier, have led to a reappraisal of this option (Table 2).4,70 Howie and colleagues,71 in a randomized control trial of 644 patients, also found significantly lower rates of dislocation after primary THA with 36-mm heads compared with 28-mm heads (1.3% vs 5.4%; P = .012); in addition, fewer dislocations occurred with 36-mm heads than with 28-mm heads (4.9% vs 12.2%; P = .27) in a series of 44 patients in revision settings. Similarly, in a study conducted with 39,271 Medicare patients between 1998 and 2007, Malkani and colleagues72 found a decrease in the dislocation rate, from 4.21% to 2.14%, with use of large-diameter femoral heads. These results have been confirmed by several other authors.34,66,73,74 Similar results were observed in 65,992 patients in the Australian National Joint Replacement Registry by Conroy and colleagues,75 who reported a significant decrease in the risk for dislocation with large heads (≥ 30 mm) compared with 22-mm heads (relative risk, 1.0 vs 3.1; P ≤ .001).
Few studies have analyzed the role of large-diameter femoral heads in the presence of compromised soft tissues around the hip. Kung and Ries,76 evaluating the influence of large-diameter heads in the presence and absence of a deficient abductor mechanism, demonstrated statistically significant reductions in rates of dislocation after 230 revision THAs when the abductor mechanism was intact with use of 36-mm heads compared with 28-mm heads (12.7% vs 0%; P = .015). With abductor deficiency, though, the positive effect of large heads in reducing dislocation rates was substantially reduced and was similar to that of small heads (P = .74).76
Large heads considerably improve overall stability and lower dislocation rates in THA. With the development of newer ceramics and highly cross-linked polyethylenes, the wear rates reported in multiple studies appear to be less concerning.
Constrained Tripolar Heads
Tripolar heads have been proposed as treatment options for improving stability in patients with chronic and recurrent instability after THA. The tripolar implant consists of a metal head that snap-fits into a polyethylene liner with a polished Co-Cr backing. This bipolar head articulates with a polyethylene bearing that is press-fitted onto a metal acetabular shell and constrained by a metal ring snapped to the outer polyethylene bearing. The bipolar component behaves as a large-diameter femoral head, and the metal ring provides additional restraint, further improving stability.
Williams and colleagues77 performed a systematic review and reported on the outcomes of constrained tripolar liners in 1199 hips at a mean follow-up of 4 years (range, 2-10 years). The mean dislocation rate was 10%, and the mean rate of revision surgery unrelated to instability was 4%. In a study of 43 hips at a mean follow-up of 4 years (range, 2-9 years), Zywiel and colleagues78 reported on the clinical and radiographic outcomes of tripolar constrained liners. Their study group had a mean Harris Hip Score (HHS) of 82 points (range, 38-100 points) and overall survival of 91%, with no evidence of radiographic loosening during follow-up. Despite the improvements in stability with constrained tripolar liners, some authors have reported multiple mechanisms of failure with these devices.79-81 In a study of 43 failed constrained tripolar liners with a mean time to failure of about 2 years, Guyen and colleagues79 identified 5 different failure modes (types 1-5) involving all 4 interfaces in these components.
Encouraging outcomes have been reported at midterm follow-up with tripolar constrained liners. However, concerns about failure at the interfaces suggest that use of these components should be restricted to patients with deficient abductor mechanisms or neuromuscular compromise, low-demand elderly patients, and salvage cases of recurrent dislocations.79
Dual-Mobility Bearings
For more than 20 years, different dual-mobility bearings have been used for difficult acetabular reconstructive scenarios and prevention of instability.82,83 Dual-mobility cups provide constructs that snap-fit a small-diameter femoral head within a large polyethylene insert that articulates with a fixed metal shell. This effectively increases the functional head diameter.
Various authors have reported excellent survivorship rates (92%-99%) and low dislocation rates for these bearings at 5- to 10-year follow-up.82,84-90 Philippot and colleagues,86 in a recent study of 438 hips with dual-mobility cups, reported excellent survivorship (96%) and no early or late instability within a 15-year follow-up. Bouchet and colleagues69 compared dual-mobility bearings (105 hips) with conventional metal-on-polythene bearings (108 hips) and found significantly (P < .05) lower dislocation rates for the dual-mobility implants at a minimum 1-year follow-up. The French Society of Orthopaedics and Traumatology performed a multicenter analysis of 3473 hips with dual-mobility cups implanted in France between January 1998 and December 2003.87 During a mean follow-up of 7 years (range, 5-11 years), there were 15 dislocations (0.43%), 14 of which occurred early, within 3 months of implantation (0.4%). Aseptic implant survivorship was 95% at 10-year follow-up.
Use of these bearings has recently increased in the United States. Short-term and midterm follow-up data show low rates of dislocation and wear. Long-term data are to come.
Clinical and Functional Outcomes of Large-Diameter Femoral Heads
There is a paucity of long-term outcomes data on use of large-diameter heads with highly cross-linked polyethylene bearings. Short-term and midterm clinical results appear to be excellent, with low rates of wear, osteolysis, and aseptic loosening.28,41,73,89-92
Plate and colleagues91 compared the effects of large-diameter (≥ 36 mm) and small-diameter (26 mm, 28 mm) metal heads on highly cross-linked polyethylene bearings. At a mean follow-up of 5 years (range, 4-8.4 years), the large-head cohort had a mean HHS of 90 points (range, 50-100 points) and no dislocations or radiographic evidence of stem or cup loosening. Similarly, Meftah and colleagues93 reported 100% stem survivorship and excellent clinical outcomes—a mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) score of 30 points—for 72 hips with use of large ceramic heads (≥ 32 mm) on highly cross-linked polyethylene at a mean follow-up of 3 years. Gagala and colleagues94 reported excellent clinical and radiographic outcomes in 50 hips (18 ceramic on ceramic, 32 ceramic on polyethylene; 36-mm heads) at a mean follow-up of 3.5 years. Mean HHS was 94 points, and there was no evidence of liner fractures, aseptic loosening, or osteolysis.
In summary, large-diameter femoral heads in THA have become increasingly popular because of improvements in the material properties and wear characteristics of highly cross-linked polyethylene and fourth-generation ceramics. Despite the potential advantages of large heads in preventing dislocations, the basic surgical tenets of placing the acetabular component in appropriate alignment remain firmly established. Implants with functionally large heads (eg, dual-mobility bearings, constrained tripolar liners) may play an important role in patients at high risk for dislocation—particularly elderly patients with poor neuromuscular muscle coordination or deficient abductors, trauma patients, and patients with prior dislocations. Short-term and midterm results are excellent; rates of wear, aseptic loosening, and osteolysis are low. However, long-term outcomes data are needed to support widespread use of large heads in younger and more active patients.
A common cause for total hip arthroplasty (THA) revision is joint instability.1,2 The reported incidence of dislocation in primary THA ranges from 0.4% to 5.8%,3-5 but this rate increases after revision surgery.1,3-8 Use of large-diameter femoral heads has been proposed to decrease the risks for instability and to improve impingement-free range of motion (ROM).
The biomechanical rationale for using large-diameter femoral heads is that they must travel farther before subluxation or dislocation occurs (jump distance). Despite these benefits, there were initial concerns that catastrophic failure and high levels of volumetric wear would occur if these heads were used with conventional polyethylene liners. These concerns led to the development of alternative bearing surfaces, particularly metal-on-metal bearings, which offered theoretical benefits of large-diameter articulations that improved stability while purportedly being highly wear-resistant.9-11 However, concerns about adverse local soft-tissue reactions and high blood concentrations of metal ions tempered the initial enthusiasm for metal bearings.12-16 Fortunately, highly cross-linked polyethylene and fourth-generation ceramic bearing surfaces, with improved toughness and better wear properties, may allow use of large-diameter heads without the need for metal-on-metal bearings.17,18
In this article, we review the concepts and principles behind use of large-diameter ceramic or cobalt-chromium femoral heads on polyethylene-bearing surfaces in THA with particular attention to biomechanics, early concerns about polyethylene wear and rim fractures, recent improvements in material properties of polyethylene and ceramic bearings, dislocation rates, and clinical and functional outcomes.
Definitions
For this review, we define large-diameter femoral heads as having diameters of 36 mm or more and conventional or small-diameter femoral heads as having diameters between 22 and 32 mm.
Biomechanics
Head–Neck Ratio, Impingement-Free ROM, and Jump Distance
Several implant design principles have been proposed to reduce the risks for impingement and dislocation. Of these, large femoral head diameters have been extensively studied.19,20 It is well known that impingement of the femoral neck on the cup edge promotes edge loading and higher wear rates. In addition, impingement increases the tendency of the head to sublux from the acetabulum. One strategy for avoiding this component-to-component impingement is to increase the head–neck ratio (HNR), the ratio of the femoral head to the neck diameter. Biomechanically, increased HNRs lead to delayed contact between the femoral neck and the acetabular liner.21,22 Therefore, with large femoral heads, which have large HNRs, impingement occurs later and at larger ROMs—compared with small-diameter femoral heads, which have lower HNRs and are more prone to early impingement and subluxation (Figure 1).23-26
In a cadaveric study of 6 hips, Bartz and colleagues23 reported a significantly higher preimpingement ROM when the prosthetic head size increased from 22 mm to 28 mm (P < .05). They found a change from prosthetic to osseous impingement when the head size increased from 22 mm to 32 mm. Similar results were observed in a computer simulation model by Cinotti and colleagues,27 who demonstrated that increasing the femoral head size from 28 mm to 38 mm resulted in a 5° improvement in ROM. However, the largest gains were observed when the heads with the smallest diameters were upsized; ROM improved only marginally when femoral head size was further increased from 32 mm to 38 mm. The primary reason for the lack of expected improvement in ROM with head sizes of more than 32 mm is often bone-on-bone impingement. Burroughs and colleagues28 demonstrated that the 38-mm and 44-mm heads virtually eliminated component-to-component impingement except in extremes of external rotation. However, there were no differences in ROM between 38-mm and 44-mm heads because of osseous impingement. In addition, large heads are less likely to sublux or dislocate, as they need to travel farther before reaching the edge of the acetabular cup before dislocation. This is known as the jump distance, and it corresponds to the depth of the acetabular shell, which in turn equates with the radius of the femoral head (Figures 2A, 2B). For this reason, the larger the femoral head diameter, the farther the jump distance and, correspondingly, the lower the risk for dislocation.29
Elevated liners historically were used to increase the jump distance for dislocation.30 These liners, however, can increase impingement at the extremes of motion.31 Some of these problems can be avoided with use of larger heads, which have increased jump distances without additional risks for impingement. Moreover, large heads create a suction effect that provides passive resistance to dislocation.32 With head diameters beyond 38 mm, impingement-free ROM often plateaus. However, the jump distance required for dislocations to occur continues to increase as femoral head diameters increase in size. Thus, patients may experience fewer motion benefits but continue to benefit from overall stability with femoral head sizes increasing beyond 38 mm.
Current evidence suggests there may be substantial benefits toward improved stability from increasing head diameters from 22 mm to 38 mm because of the increase in jump distances and improvements in prosthetic impingement-free ROM. However, there may be little gain in ROM from increasing the head diameters beyond these dimensions because of the potential risks of bony impingement. Nevertheless, there may be some additional benefits toward stability from improvement in jump distances with incremental head sizes
beyond 38 mm.29,33,34
Finite Element Analysis Studies
Finite element analysis of large-diameter heads in THA has shown that, at optimal cup inclination (45°), most stresses occur on the articular surface of the liner. However, these stresses remain well below the yield strength of the polyethylene liners.29 With increasing abduction angles, the stress concentration increases substantially because of the decreased contact surface area. At these angles, the point of maximum contact moves toward the rim of the polyethylene liner, which can lead to rim fractures or failure of locking mechanisms.29,35,36
Early Concerns With Large-Diameter Femoral Heads: Wear, Liner Failure, and Fracture of Ceramic Components
Use of small-diameter femoral heads started with the first report by Charnley37 of “low frictional torque arthroplasty.” Charnley initially considered a 41.5-mm femoral head, but he thought it would increase risks for acetabular loosening from high frictional torque generated by the large head, and he switched to a small-diameter (22.5 mm) design. One of the tradeoffs with smaller diameter heads was decreased jump height in addition to increased linear wear.
Large femoral heads used with cemented polyethylene acetabular components historically have been associated with increased rates of volumetric wear but low rates of linear wear, which potentially may increase the risk for osteolysis.38-40 However, newer highly cross-linked polyethylene liners have shown improved in vitro and in vivo volumetric wear characteristics and potentially lower linear wear rates compared with earlier designs (Table 1).28,41-43
Another concern about earlier generations of large femoral heads was the risk for catastrophic liner failure on conventional polyethylene. This was originally reported by Berry and colleagues,47 who described wear-through and failure in patients with thin (< 5 mm) acetabular cups. However, these concerns have been largely addressed by the development of highly cross-linked polyethylene, which has improved wear characteristics and fatigue resistance.48
Recent Improvements in Material Properties of Polyethylene and Ceramic Bearings
The development of highly cross-linked polyethylene and fourth-generation ceramics has renewed interest in large-diameter bearings in THA. These bearing surfaces improve wear, enhance material properties, and have superior oxidation resistance.42,48-53
We now briefly describe the methods used to improve the material properties of polyethylene and ceramics. Studies have shown that increasing the radiation dose (up to 200 kGy) increases cross-linking and causes an inverse exponential decrease in polyethylene wear.28,41,48-51 However, increasing radiation doses also increases production of free radicals, which diminish the material strength of these polyethylenes. The current generation of highly cross-linked polyethylene liners is produced through a variety of manufacturing strategies to improve cross-linking and reduce wear. These strategies include differential radiation doses (50-100 kGy), techniques (electron beam, radiation), and thermal treatments (melting, annealing). Moreover, to enhance the material properties and reduce the incidence of rim cracking and delamination, authors have proposed using vitamin E supplementation to minimize the amount of subsurface oxidation that occurs as an inevitable consequence of free radical formation during fabrication.54,55 A terminal sterilization process (eg, gas plasma, ethylene oxide, or gamma sterilization in nitrogen) is needed to make commercial, highly cross-linked polyethylene.52,53
Fourth-generation ceramics manufactured with nano-sized yttria-stabilized tetragonal zirconia particles in a stable alumina matrix have more fracture toughness and improved wear characteristics.54,55 In addition, oxide additives (eg, chromium oxide, strontium oxide) improve hardness and dissipate energy by deflecting cracks to prevent their propagation.56 Moreover, the smaller grain sizes of fourth-generation ceramic bearings compared with third-generation designs (0.8 µm vs 1-5 µm) cause less disruption of the fluid film layer, which ultimately results in improved wear performance.57
Multiple studies have found reduced wear rates with metal and ceramic large heads coupled with highly cross-linked polyethylene-bearings (Table 2).17,41,50,58 Bragdon and colleagues,58 using radiostereometric analysis in 25 patients, found no significant differences in mean head penetration rates between 36-mm and 28-mm cobalt-chromium (Co-Cr) heads articulating with highly cross-linked polyethylene cups at a mean follow-up of 3 years (0.035 mm/y vs 0.046 mm/y; P = .11). Geller and colleagues,64 in their study of 42 patients with large-diameter (> 32 mm) Co-Cr femoral heads, found low mean (SD) linear wear rates of 0.06 (0.41) mm/y at a mean follow-up of 3 years. D’Antonio and colleagues,65 in a multicenter study, reported low average linear wear (0.015 mm/y) and volumetric wear (12.1 mm3/y) over 5 years using sequentially annealed cross-linked polyethylene. In vitro reports suggest that large-diameter ceramic heads may have lower wear properties than Co-Cr heads do. Galvin and colleagues,66 in an in vitro hip simulator study, found that large-diameter ceramic heads on highly cross-linked ultrahigh-molecular-weight polyethylene had 40% reductions in steady-state wear rates compared with Co-Cr heads on highly cross-linked bearings (4.7 vs 8.1 mm3/million cycles; P < 0.01).
Dislocation Rates
Several patient, surgeon, and implant factors affect the rate of dislocations after THA. Multiple implant options utilize the biomechanical advantage that large-diameter heads have in improving stability. Various alternatives include use of constrained tripolar heads, dual-mobility bearings, and conventional large-diameter heads with standard liners.67-69
Large-Diameter Heads
Despite the biomechanical advantages of large-diameter metal-on-polyethylene bearings, prior studies have questioned use of these bearings because of risks for increased wear and rim failures. However, the improved wear properties of highly cross-linked polyethylene, elaborated earlier, have led to a reappraisal of this option (Table 2).4,70 Howie and colleagues,71 in a randomized control trial of 644 patients, also found significantly lower rates of dislocation after primary THA with 36-mm heads compared with 28-mm heads (1.3% vs 5.4%; P = .012); in addition, fewer dislocations occurred with 36-mm heads than with 28-mm heads (4.9% vs 12.2%; P = .27) in a series of 44 patients in revision settings. Similarly, in a study conducted with 39,271 Medicare patients between 1998 and 2007, Malkani and colleagues72 found a decrease in the dislocation rate, from 4.21% to 2.14%, with use of large-diameter femoral heads. These results have been confirmed by several other authors.34,66,73,74 Similar results were observed in 65,992 patients in the Australian National Joint Replacement Registry by Conroy and colleagues,75 who reported a significant decrease in the risk for dislocation with large heads (≥ 30 mm) compared with 22-mm heads (relative risk, 1.0 vs 3.1; P ≤ .001).
Few studies have analyzed the role of large-diameter femoral heads in the presence of compromised soft tissues around the hip. Kung and Ries,76 evaluating the influence of large-diameter heads in the presence and absence of a deficient abductor mechanism, demonstrated statistically significant reductions in rates of dislocation after 230 revision THAs when the abductor mechanism was intact with use of 36-mm heads compared with 28-mm heads (12.7% vs 0%; P = .015). With abductor deficiency, though, the positive effect of large heads in reducing dislocation rates was substantially reduced and was similar to that of small heads (P = .74).76
Large heads considerably improve overall stability and lower dislocation rates in THA. With the development of newer ceramics and highly cross-linked polyethylenes, the wear rates reported in multiple studies appear to be less concerning.
Constrained Tripolar Heads
Tripolar heads have been proposed as treatment options for improving stability in patients with chronic and recurrent instability after THA. The tripolar implant consists of a metal head that snap-fits into a polyethylene liner with a polished Co-Cr backing. This bipolar head articulates with a polyethylene bearing that is press-fitted onto a metal acetabular shell and constrained by a metal ring snapped to the outer polyethylene bearing. The bipolar component behaves as a large-diameter femoral head, and the metal ring provides additional restraint, further improving stability.
Williams and colleagues77 performed a systematic review and reported on the outcomes of constrained tripolar liners in 1199 hips at a mean follow-up of 4 years (range, 2-10 years). The mean dislocation rate was 10%, and the mean rate of revision surgery unrelated to instability was 4%. In a study of 43 hips at a mean follow-up of 4 years (range, 2-9 years), Zywiel and colleagues78 reported on the clinical and radiographic outcomes of tripolar constrained liners. Their study group had a mean Harris Hip Score (HHS) of 82 points (range, 38-100 points) and overall survival of 91%, with no evidence of radiographic loosening during follow-up. Despite the improvements in stability with constrained tripolar liners, some authors have reported multiple mechanisms of failure with these devices.79-81 In a study of 43 failed constrained tripolar liners with a mean time to failure of about 2 years, Guyen and colleagues79 identified 5 different failure modes (types 1-5) involving all 4 interfaces in these components.
Encouraging outcomes have been reported at midterm follow-up with tripolar constrained liners. However, concerns about failure at the interfaces suggest that use of these components should be restricted to patients with deficient abductor mechanisms or neuromuscular compromise, low-demand elderly patients, and salvage cases of recurrent dislocations.79
Dual-Mobility Bearings
For more than 20 years, different dual-mobility bearings have been used for difficult acetabular reconstructive scenarios and prevention of instability.82,83 Dual-mobility cups provide constructs that snap-fit a small-diameter femoral head within a large polyethylene insert that articulates with a fixed metal shell. This effectively increases the functional head diameter.
Various authors have reported excellent survivorship rates (92%-99%) and low dislocation rates for these bearings at 5- to 10-year follow-up.82,84-90 Philippot and colleagues,86 in a recent study of 438 hips with dual-mobility cups, reported excellent survivorship (96%) and no early or late instability within a 15-year follow-up. Bouchet and colleagues69 compared dual-mobility bearings (105 hips) with conventional metal-on-polythene bearings (108 hips) and found significantly (P < .05) lower dislocation rates for the dual-mobility implants at a minimum 1-year follow-up. The French Society of Orthopaedics and Traumatology performed a multicenter analysis of 3473 hips with dual-mobility cups implanted in France between January 1998 and December 2003.87 During a mean follow-up of 7 years (range, 5-11 years), there were 15 dislocations (0.43%), 14 of which occurred early, within 3 months of implantation (0.4%). Aseptic implant survivorship was 95% at 10-year follow-up.
Use of these bearings has recently increased in the United States. Short-term and midterm follow-up data show low rates of dislocation and wear. Long-term data are to come.
Clinical and Functional Outcomes of Large-Diameter Femoral Heads
There is a paucity of long-term outcomes data on use of large-diameter heads with highly cross-linked polyethylene bearings. Short-term and midterm clinical results appear to be excellent, with low rates of wear, osteolysis, and aseptic loosening.28,41,73,89-92
Plate and colleagues91 compared the effects of large-diameter (≥ 36 mm) and small-diameter (26 mm, 28 mm) metal heads on highly cross-linked polyethylene bearings. At a mean follow-up of 5 years (range, 4-8.4 years), the large-head cohort had a mean HHS of 90 points (range, 50-100 points) and no dislocations or radiographic evidence of stem or cup loosening. Similarly, Meftah and colleagues93 reported 100% stem survivorship and excellent clinical outcomes—a mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) score of 30 points—for 72 hips with use of large ceramic heads (≥ 32 mm) on highly cross-linked polyethylene at a mean follow-up of 3 years. Gagala and colleagues94 reported excellent clinical and radiographic outcomes in 50 hips (18 ceramic on ceramic, 32 ceramic on polyethylene; 36-mm heads) at a mean follow-up of 3.5 years. Mean HHS was 94 points, and there was no evidence of liner fractures, aseptic loosening, or osteolysis.
In summary, large-diameter femoral heads in THA have become increasingly popular because of improvements in the material properties and wear characteristics of highly cross-linked polyethylene and fourth-generation ceramics. Despite the potential advantages of large heads in preventing dislocations, the basic surgical tenets of placing the acetabular component in appropriate alignment remain firmly established. Implants with functionally large heads (eg, dual-mobility bearings, constrained tripolar liners) may play an important role in patients at high risk for dislocation—particularly elderly patients with poor neuromuscular muscle coordination or deficient abductors, trauma patients, and patients with prior dislocations. Short-term and midterm results are excellent; rates of wear, aseptic loosening, and osteolysis are low. However, long-term outcomes data are needed to support widespread use of large heads in younger and more active patients.
1. Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop. 1983;(173):151-158.
2. Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop. 1998;(355):144-151.
3. Turner RS. Postoperative total hip prosthetic femoral head dislocations. Incidence, etiologic factors, and management. Clin Orthop. 1994;(301):196-204.
4. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295-1306.
5. Etienne A, Cupic Z, Charnley J. Postoperative dislocation after Charnley low-friction arthroplasty. Clin Orthop. 1978;(132):19-23.
6. Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169-178.
7. Joshi A, Lee CM, Markovic L, Vlatis G, Murphy JC. Prognosis of dislocation after total hip arthroplasty. J Arthroplasty. 1998;13(1):17-21.
8. Lindberg HO, Carlsson AS, Gentz CF, Pettersson H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop Scand. 1982;53(6):947-952.
9. Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90(10):1278-1283.
10. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841-848.
11. Leslie I, Williams S, Brown C, et al. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements—an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87(1):163-172.
12. Verhaar JA. The hard lesson of metal-on-metal hip implants [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(42):A5564.
13. Fabi D, Levine B, Paprosky W, et al. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35(7):e1009-e1016.
14. Heneghan C, Langton D, Thompson M. Ongoing problems with metal-on-metal hip implants. BMJ. 2012;344:e1349.
15. Lee RK, Nevelos J, Vigdorchik J, Markel DC. Bearing surfaces for hip arthroplasty—is metal-on-metal a passing fancy? Surg Technol Int. 2012;22:243-249.
16. Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(10):1844-1849.
17. Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results.
J Bone Joint Surg Am. 2001;83(11):1688-1694.
18. Callaghan JJ, Liu SS. Ceramic on crosslinked polyethylene in total hip replacement: any better than metal on crosslinked polyethylene? Iowa Orthop J. 2009;29:1-4.
19. Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
20. Krushell RJ, Burke DW, Harris WH. Elevated-rim acetabular components. Effect on range of motion and stability in total hip arthroplasty. J Arthroplasty. 1991;6(suppl):S53-S58.
21. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23(2):237-248.
22. Morrey BF. Dislocation. In: Morrey BF, ed. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991:851-865.
23. Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82(9):1300-1307.
24. Nicholas RM, Orr JF, Mollan RA, Calderwood JW, Nixon JR, Watson P. Dislocation of total hip replacements. A comparative study of standard, long posterior wall and augmented acetabular components. J Bone Joint Surg Br. 1990;72(3):418-422.
25. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
26. Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, orientation and component interaction in dislocation of the total hip prosthesis. Acta Radiol. 1988;29(4):441-444.
27. Cinotti G, Lucioli N, Malagoli A, Calderoli C, Cassese F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int Orthop. 2011;35(3):317-323.
28. Burroughs BR, Rubash HE, Harris WH. Femoral head sizes larger than 32 mm against highly cross-linked polyethylene. Clin Orthop. 2002;(405):150-157.
29. Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don‘t do. Clin Orthop. 2004;(429):102-107.
30. Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979.
31. Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15(3):305-313.
32. Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140-144.
33. Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop. 2002;(405):46-53.
34. Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84(2):256-263.
35. Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27(6):917-925.
36. Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146-154.
37. Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop. 1970;(72):7-21.
38. Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75(2):254-258.
39. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72(4):518-528.
40. Ma SM, Kabo JM, Amstutz HC. Frictional torque in surface and conventional hip replacement. J Bone Joint Surg Am. 1983;65(3):366-370.
41. Muratoglu OK, Bragdon CR, O‘Connor D, et al. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 suppl 1):24-30.
42. Thomas GER, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
43. Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop. 2010;468(12):3228-3233.
44. Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Malchau H. The 2012 John Charnley Award: clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop. 2013;471(2):393-402.
45. Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop. 2009;467(12):3290-3296.
46. Sychterz CJ, Engh CA Jr, Young AM, Hopper RH Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82(7):948-951.
47. Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components.
J Bone Joint Surg Br. 1994;76(4):575-578.
48. McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157-167.
49. Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol Int. 1998;31(1-3):17-33.
50. Estok DM 2nd, Burroughs BR, Muratoglu OK, Harris WH. Comparison of hip simulator wear of 2 different highly cross-linked ultra high molecular weight polyethylene acetabular components using both 32- and 38-mm femoral heads. J Arthroplasty. 2007;22(4):581-589.
51. Muratoglu OK, Bragdon CR, O‘Connor DO, et al. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20(16):1463-1470.
52. Harris WH, Muratoglu OK. A review of current cross-linked polyethylenes used in total joint arthroplasty. Clin Orthop. 2005;(430):46-52.
53. Muratoglu OK, Bragdon CR, O‘Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16(2):149-160.
54. Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. Hip Int. 2007;17(1):21-30.
55. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. Biomed Res Int. 2013;2013:157247.
56. Cai YZ, Yan SG. Development of ceramic-on-ceramic implants for total hip arthroplasty. Orthop Surg. 2010;2(3):175-181.
57. Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66(2):567-573.
58. Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125-129.
59. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop. 2011;469(6):1547-1553.
60. Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop. 2013;471(2):439-443.
61. Cai P, Hu Y, Xie J. Large-diameter Delta ceramic-on-ceramic versus common-sized ceramic-on-polyethylene bearings in THA. Orthopedics. 2012;35(9):e1307-e1313.
62. Park KS, Yoon TR, Hwang SY, Lee KB. Modified minimally invasive two-incision total hip arthroplasty using large diameter femoral head. Indian
J Orthop. 2012;46(1):29-35.
63. Garbuz DS, Masri BA, Duncan CP, et al. The Frank Stinchfield Award: dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop. 2012;470(2):351-356.
64. Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop. 2006;(447):53-59.
65. D’Antonio JA, Capello WN, Ramakrishnan R. Second-generation annealed highly cross-linked polyethylene exhibits low wear. Clin Orthop. 2012;470(6):1696-1704.
66. Galvin AL, Jennings LM, Tipper JL, Ingham E, Fisher J. Wear and creep of highly crosslinked polyethylene against cobalt chrome and ceramic femoral heads. Proc Inst Mech Eng H. 2010;224(10):1175-1183.
67. Skeels MD, Berend KR, Lombardi AV Jr. The dislocator, early and late: the role of large heads. Orthopedics. 2009;32(9).
68. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
69. Bouchet R, Mercier N, Saragaglia D. Posterior approach and dislocation rate: a 213 total hip replacements case–control study comparing the dual mobility cup with a conventional 28-mm metal head/polyethylene prosthesis. Orthop Traumatol Surg Res. 2011;97(1):2-7.
70. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63(2):214-218.
71. Howie DW, Holubowycz OT, Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095-1102.
72. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
73. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
74. Cho MR, Lee HS, Lee SW, Choi CH, Kim SK, Ko SB. Results after total hip arthroplasty with a large head and bipolar arthroplasty in patients with displaced femoral neck fractures. J Arthroplasty. 2011;26(6):893-896.
75. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty.
J Arthroplasty. 2008;23(6):867-872.
76. Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop. 2007;(465):170-174.
77. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
78. Zywiel MG, Mustafa LH, Bonutti PM, Mont MA. Are abductor muscle quality and previous revision surgery predictors of constrained liner failure in hip arthroplasty? Int Orthop. 2011;35(6):797-802.
79. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
80. Banks LN, McElwain JP. An unusual mode of failure of a tripolar constrained acetabular liner: a case report. Arch Orthop Trauma Surg. 2010;130(4):503-505.
81. Robertson WJ, Mattern CJ, Hur J, Su EP, Pellicci PM. Failure mechanisms and closed reduction of a constrained tripolar acetabular liner.
J Arthroplasty. 2009;24(2):322.e5-e11.
82. Aubriot JH, Lesimple P, Leclercq S. Study of Bousquet‘s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component). Average 5-year follow-up [in French]. Acta Orthop Belg. 1993;59(suppl 1):267-271.
83. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
84. Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8(3):251-256.
85. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop. 2008;466(2):389-395.
86. Philippot R, Farizon F, Camilleri JP, et al. Survival of dual mobility socket with a mean 17 years follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(1):43-48.
87. Adam P, Philippe R, Ehlinger M, et al. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly.
A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop Traumatol Surg Res. 2012;98(3):296-300.
88. Fessy MH. La double mobilité [Dual mobility]. Revue de Chirurgie Orthopédique et Traumatologique. 2010;96(7):891-898.
89. Mont MA, Issa K, Naziri Q, Harwin SF, Delanois RE, Johnson AJ. The use of dual-mobility bearings in difficult hip arthroplasty reconstructive cases. Surg Technol Int. 2011;21:234-240.
90. Sayeed SA, Mont MA, Costa CR, et al. Early outcomes of sequentially cross-linked thin polyethylene liners with large diameter femoral heads in total hip arthroplasty. Bull NYU Hosp Jt Dis. 2011;69(suppl 1):S90-S94.
91. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
92. Sato T, Nakashima Y, Akiyama M, et al. Wear resistant performance of highly cross-linked and annealed ultra-high molecular weight polyethylene against ceramic heads in total hip arthroplasty. J Orthop Res. 2012;30(12):2031-2037.
93. Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34(6):133.
94. Gagala J, Mazurkiewicz T, Dajewski Z. Large diameter femoral heads in primary alumina/alumina and XSPE/alumina total hip arthroplasty.
A follow-up study of 50 hips after average 40 months and review of literature [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):14-20.
1. Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop. 1983;(173):151-158.
2. Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop. 1998;(355):144-151.
3. Turner RS. Postoperative total hip prosthetic femoral head dislocations. Incidence, etiologic factors, and management. Clin Orthop. 1994;(301):196-204.
4. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295-1306.
5. Etienne A, Cupic Z, Charnley J. Postoperative dislocation after Charnley low-friction arthroplasty. Clin Orthop. 1978;(132):19-23.
6. Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169-178.
7. Joshi A, Lee CM, Markovic L, Vlatis G, Murphy JC. Prognosis of dislocation after total hip arthroplasty. J Arthroplasty. 1998;13(1):17-21.
8. Lindberg HO, Carlsson AS, Gentz CF, Pettersson H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop Scand. 1982;53(6):947-952.
9. Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90(10):1278-1283.
10. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841-848.
11. Leslie I, Williams S, Brown C, et al. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements—an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87(1):163-172.
12. Verhaar JA. The hard lesson of metal-on-metal hip implants [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(42):A5564.
13. Fabi D, Levine B, Paprosky W, et al. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35(7):e1009-e1016.
14. Heneghan C, Langton D, Thompson M. Ongoing problems with metal-on-metal hip implants. BMJ. 2012;344:e1349.
15. Lee RK, Nevelos J, Vigdorchik J, Markel DC. Bearing surfaces for hip arthroplasty—is metal-on-metal a passing fancy? Surg Technol Int. 2012;22:243-249.
16. Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(10):1844-1849.
17. Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results.
J Bone Joint Surg Am. 2001;83(11):1688-1694.
18. Callaghan JJ, Liu SS. Ceramic on crosslinked polyethylene in total hip replacement: any better than metal on crosslinked polyethylene? Iowa Orthop J. 2009;29:1-4.
19. Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
20. Krushell RJ, Burke DW, Harris WH. Elevated-rim acetabular components. Effect on range of motion and stability in total hip arthroplasty. J Arthroplasty. 1991;6(suppl):S53-S58.
21. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23(2):237-248.
22. Morrey BF. Dislocation. In: Morrey BF, ed. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991:851-865.
23. Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82(9):1300-1307.
24. Nicholas RM, Orr JF, Mollan RA, Calderwood JW, Nixon JR, Watson P. Dislocation of total hip replacements. A comparative study of standard, long posterior wall and augmented acetabular components. J Bone Joint Surg Br. 1990;72(3):418-422.
25. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
26. Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, orientation and component interaction in dislocation of the total hip prosthesis. Acta Radiol. 1988;29(4):441-444.
27. Cinotti G, Lucioli N, Malagoli A, Calderoli C, Cassese F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int Orthop. 2011;35(3):317-323.
28. Burroughs BR, Rubash HE, Harris WH. Femoral head sizes larger than 32 mm against highly cross-linked polyethylene. Clin Orthop. 2002;(405):150-157.
29. Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don‘t do. Clin Orthop. 2004;(429):102-107.
30. Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979.
31. Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15(3):305-313.
32. Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140-144.
33. Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop. 2002;(405):46-53.
34. Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84(2):256-263.
35. Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27(6):917-925.
36. Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146-154.
37. Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop. 1970;(72):7-21.
38. Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75(2):254-258.
39. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72(4):518-528.
40. Ma SM, Kabo JM, Amstutz HC. Frictional torque in surface and conventional hip replacement. J Bone Joint Surg Am. 1983;65(3):366-370.
41. Muratoglu OK, Bragdon CR, O‘Connor D, et al. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 suppl 1):24-30.
42. Thomas GER, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
43. Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop. 2010;468(12):3228-3233.
44. Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Malchau H. The 2012 John Charnley Award: clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop. 2013;471(2):393-402.
45. Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop. 2009;467(12):3290-3296.
46. Sychterz CJ, Engh CA Jr, Young AM, Hopper RH Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82(7):948-951.
47. Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components.
J Bone Joint Surg Br. 1994;76(4):575-578.
48. McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157-167.
49. Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol Int. 1998;31(1-3):17-33.
50. Estok DM 2nd, Burroughs BR, Muratoglu OK, Harris WH. Comparison of hip simulator wear of 2 different highly cross-linked ultra high molecular weight polyethylene acetabular components using both 32- and 38-mm femoral heads. J Arthroplasty. 2007;22(4):581-589.
51. Muratoglu OK, Bragdon CR, O‘Connor DO, et al. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20(16):1463-1470.
52. Harris WH, Muratoglu OK. A review of current cross-linked polyethylenes used in total joint arthroplasty. Clin Orthop. 2005;(430):46-52.
53. Muratoglu OK, Bragdon CR, O‘Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16(2):149-160.
54. Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. Hip Int. 2007;17(1):21-30.
55. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. Biomed Res Int. 2013;2013:157247.
56. Cai YZ, Yan SG. Development of ceramic-on-ceramic implants for total hip arthroplasty. Orthop Surg. 2010;2(3):175-181.
57. Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66(2):567-573.
58. Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125-129.
59. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop. 2011;469(6):1547-1553.
60. Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop. 2013;471(2):439-443.
61. Cai P, Hu Y, Xie J. Large-diameter Delta ceramic-on-ceramic versus common-sized ceramic-on-polyethylene bearings in THA. Orthopedics. 2012;35(9):e1307-e1313.
62. Park KS, Yoon TR, Hwang SY, Lee KB. Modified minimally invasive two-incision total hip arthroplasty using large diameter femoral head. Indian
J Orthop. 2012;46(1):29-35.
63. Garbuz DS, Masri BA, Duncan CP, et al. The Frank Stinchfield Award: dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop. 2012;470(2):351-356.
64. Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop. 2006;(447):53-59.
65. D’Antonio JA, Capello WN, Ramakrishnan R. Second-generation annealed highly cross-linked polyethylene exhibits low wear. Clin Orthop. 2012;470(6):1696-1704.
66. Galvin AL, Jennings LM, Tipper JL, Ingham E, Fisher J. Wear and creep of highly crosslinked polyethylene against cobalt chrome and ceramic femoral heads. Proc Inst Mech Eng H. 2010;224(10):1175-1183.
67. Skeels MD, Berend KR, Lombardi AV Jr. The dislocator, early and late: the role of large heads. Orthopedics. 2009;32(9).
68. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
69. Bouchet R, Mercier N, Saragaglia D. Posterior approach and dislocation rate: a 213 total hip replacements case–control study comparing the dual mobility cup with a conventional 28-mm metal head/polyethylene prosthesis. Orthop Traumatol Surg Res. 2011;97(1):2-7.
70. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63(2):214-218.
71. Howie DW, Holubowycz OT, Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095-1102.
72. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
73. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
74. Cho MR, Lee HS, Lee SW, Choi CH, Kim SK, Ko SB. Results after total hip arthroplasty with a large head and bipolar arthroplasty in patients with displaced femoral neck fractures. J Arthroplasty. 2011;26(6):893-896.
75. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty.
J Arthroplasty. 2008;23(6):867-872.
76. Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop. 2007;(465):170-174.
77. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
78. Zywiel MG, Mustafa LH, Bonutti PM, Mont MA. Are abductor muscle quality and previous revision surgery predictors of constrained liner failure in hip arthroplasty? Int Orthop. 2011;35(6):797-802.
79. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
80. Banks LN, McElwain JP. An unusual mode of failure of a tripolar constrained acetabular liner: a case report. Arch Orthop Trauma Surg. 2010;130(4):503-505.
81. Robertson WJ, Mattern CJ, Hur J, Su EP, Pellicci PM. Failure mechanisms and closed reduction of a constrained tripolar acetabular liner.
J Arthroplasty. 2009;24(2):322.e5-e11.
82. Aubriot JH, Lesimple P, Leclercq S. Study of Bousquet‘s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component). Average 5-year follow-up [in French]. Acta Orthop Belg. 1993;59(suppl 1):267-271.
83. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
84. Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8(3):251-256.
85. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop. 2008;466(2):389-395.
86. Philippot R, Farizon F, Camilleri JP, et al. Survival of dual mobility socket with a mean 17 years follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(1):43-48.
87. Adam P, Philippe R, Ehlinger M, et al. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly.
A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop Traumatol Surg Res. 2012;98(3):296-300.
88. Fessy MH. La double mobilité [Dual mobility]. Revue de Chirurgie Orthopédique et Traumatologique. 2010;96(7):891-898.
89. Mont MA, Issa K, Naziri Q, Harwin SF, Delanois RE, Johnson AJ. The use of dual-mobility bearings in difficult hip arthroplasty reconstructive cases. Surg Technol Int. 2011;21:234-240.
90. Sayeed SA, Mont MA, Costa CR, et al. Early outcomes of sequentially cross-linked thin polyethylene liners with large diameter femoral heads in total hip arthroplasty. Bull NYU Hosp Jt Dis. 2011;69(suppl 1):S90-S94.
91. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
92. Sato T, Nakashima Y, Akiyama M, et al. Wear resistant performance of highly cross-linked and annealed ultra-high molecular weight polyethylene against ceramic heads in total hip arthroplasty. J Orthop Res. 2012;30(12):2031-2037.
93. Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34(6):133.
94. Gagala J, Mazurkiewicz T, Dajewski Z. Large diameter femoral heads in primary alumina/alumina and XSPE/alumina total hip arthroplasty.
A follow-up study of 50 hips after average 40 months and review of literature [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):14-20.
Crisis in Medicine: Have We Traded Technology for Our Six Senses?
Technology creates change, and change is moving fast and is relentless. Physicians, on the other hand, are generally slow to change. Wisely, we question change—we observe it, we study it, and we try to ensure our patients will benefit from it over time. Maybe as a result of this or as a consequence of our often myopic view of the world, we mistakenly let others lead the way and dictate how we must change and what our practices must absorb. We must turn this around and be the agents of change for our profession so we can appropriately use the available technology and create systems for managing the demands of a society that expects instant answers with fewer doctor resources devoted to the answer. The insurance industry is encouraging a wholesale dismantling of the classic patient visit to be replaced by nonphysician interactions, virtual diagnostics, and electronic medical records. We must not allow this and must ensure that we safeguard our profession by employing traditional skills, utilizing our 5 senses, and incorporating technology as a tool for better diagnosis and treatment but not as a substitute for the same.
Great doctors are often described as having a sixth sense—an intuition that guides them in diagnosing and treating patients. It is assumed, therefore, that the good doctor will have the benefit of 5 senses: sight, sound, touch, smell, and taste. Sound: What does the patient tell or neglect to tell the doctor? What sounds does a joint produce when it moves? Sight: How does the patient present? Are they weary from pain or chronic disease? Touch: What does the joint feel like? How does it move? What is the patient’s response to stabilization of a joint? Smell: Is there an odor that helps detect the presence of infection or decay? Is the patient coming into contact with a substance causing harm or preventing healing?
A good doctor must employ these senses first to understand the patient’s needs and then to treat the patient. The sixth sense is a gift, one that comes from years of experience, an attention to detail, and a commitment to the craft of medicine. A recent trend toward virtual medicine is a dangerous path that must be walked with care and discretion so that the 6 senses are maintained and nurtured. Technology must be used to enhance and not limit these senses. The patient cannot be reduced to a 2-dimensional version of his/herself so that the doctor’s powers of diagnosis and healing are similarly limited.
Change in the office has occurred with mandates for electronic medical records and work-hour restrictions for residents. Data do not support that either change has resulted in a net benefit to patients. We are mandated to invest scarce capital to support new technology, resulting in increased pressure to recoup investment. Where there is a cap on revenue, the only way to increase net profit is to increase volume and decrease services. Physician time is the variable and can be streamlined by performing video conferences or smartphone consultations. Change may bring higher order, as the English philosopher John Locke said, but it is time for all of us as physicians to step back and question that this type of change is the path we must take. An office with a schedule of 80 patients seen at 5-minute intervals by physician assistants has no place in medicine. The pressure imposed by the insurance industry or hospital administrators to meet quotas has gotten out of hand and the time is now to say with a strong but fair voice a resounding NO!
The office visit with a history and physical examination is the most exciting and effective time to meet, console, and relate to our patients. The use of the 5 senses is critical. We must not let technological advancements (eg, smartphones, the Internet, and electronic medical records) destroy what was created and taught to us all through our training. The reward that is accomplished by placing one’s hand on a patient’s knee to understand its warmth and swelling, the tactile feeling of a fluid wave, or performing carefully with compassion a provocative maneuver that gives by sight a grimace of discomfort can tell so much more than a status update on the phone. We must not allow ourselves to be replaced by ancillary services for so-called efficiency and cost saving. Rather, we must be innovative and sharp. We must find the way to use the wonders of the virtual world without giving up the human consult.
Imagine that you are able to travel to Iguazu Falls, South America, to see one of the wonders of our world. You sit in that life raft moving upstream to feel the heat from the water as it crushes the rocks below, and you feel the mist on your face. You see the majesty and hear the screams and breadth of excitement of those around you, while you listen to the deafening sounds created by this waterfall. Now imagine you are required to report on this same experience through a video or some form of technology that the world has convinced us is the best and far cheaper substitute. This is our electronic medical record. A tool we are forced to use, and while it has a purpose, it is a sterile tool that fails to provide information that will give clues to awaken the sixth sense. It is a checklist that could allow for completion of a task—like how to fix a leaky faucet.
How then do we accomplish walking the fine line of working with nonphysicians and technology and yet delivering pinnacle care? The answer isn’t simple but it must include education and a commitment to the profession. We must make the public aware that we are one of the few professions that dedicate our lives to others by promoting health and advancing research. My colleagues, the pendulum has swung too far; it is time to take back our great profession through education of ourselves and the public. While technology may help the world connect, it has a limited role unless we first use our 6 senses to help our patients. We must not submit to a compassionless and callous approach that is the inevitable outcome of virtual medicine done with speed. We must maintain our dignity and let the public understand how many years of sacrifice has taken place to earn a sixth sense and not allow a third party to take it away. We are the only source of protection for our patients and we need each one of our senses to perform this task.
Advancing research has been a cornerstone for the orthopedic surgeon. Position statements through meta-analyses and systematic reviews of the literature have recently been utilized with increasing frequency. Combining data of potentially flawed studies can often lead to erroneous conclusions and may stray away from best practices. Is this where we want evidence-based medicine to go? The end result is that decisions are made by insurance companies who rely on these flawed studies to force clinical decisions on the physician, as was most recently seen by the investigation of viscosupplementation for knee osteoarthritis.1
In a 2007 study published in JAMA (The Journal of the American Medical Association), only 62% of residents could appropriately interpret a P value.2 How can we expect young clinicians to evaluate, interpret, and apply the multitude of evidence in the literature to everyday practice? We must marry the use of best evidence with our expertise to make the most informed decision while managing the expectations of our patients. In order to achieve that balance, we must rely on our intuition, our sixth sense. There is too much patient individuality and complexity surrounding each individual’s situation for a one-size-fits-all approach and for wholesale reliance on research to address each unique situation.
If Nathan Davis in 1845 was able to convince the New York Medical Society to establish a nationwide professional association to assist in regulating the practice of medicine, then it is time for all of us to stand up and insist on a code of ethics that is unrelenting and uncompromising. Our wise leaders of the American Orthopaedic Association (AOA) who founded the formation of orthopedics in America knew guidelines were needed to “foster advances in the care of patients, improve the teaching of orthopaedic surgery in medical schools and formal orthopaedic training, and to promote orthopaedic surgery as a surgical discipline worldwide.”3 It is now our turn to renew the guidelines and encourage our leaders to help educate ourselves and patients as we work with technology and administrators, nurses and physician assistants to deliver pinnacle care. We must reform medical education and the practice of medicine so that technology is used as a companion but not a substitute for our 6 senses.
The next time a patient comes into the exam room, sit down, look the patient in the eye, listen, touch, console anxiety, make a human connection, and form a lasting relationship. By all means apologize to your patients as you fill out the electronic medical record and insurance forms. Discuss how we are in the same crisis together and ask for their help as they have come to you for yours.
1. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576.
2. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010-1022.
3. DeRosa GP. 75 Years of Doing the Right Thing: A History of the American Board of Orthopaedic Surgery. American Board of Orthopaedic Surgery; 2009.
Technology creates change, and change is moving fast and is relentless. Physicians, on the other hand, are generally slow to change. Wisely, we question change—we observe it, we study it, and we try to ensure our patients will benefit from it over time. Maybe as a result of this or as a consequence of our often myopic view of the world, we mistakenly let others lead the way and dictate how we must change and what our practices must absorb. We must turn this around and be the agents of change for our profession so we can appropriately use the available technology and create systems for managing the demands of a society that expects instant answers with fewer doctor resources devoted to the answer. The insurance industry is encouraging a wholesale dismantling of the classic patient visit to be replaced by nonphysician interactions, virtual diagnostics, and electronic medical records. We must not allow this and must ensure that we safeguard our profession by employing traditional skills, utilizing our 5 senses, and incorporating technology as a tool for better diagnosis and treatment but not as a substitute for the same.
Great doctors are often described as having a sixth sense—an intuition that guides them in diagnosing and treating patients. It is assumed, therefore, that the good doctor will have the benefit of 5 senses: sight, sound, touch, smell, and taste. Sound: What does the patient tell or neglect to tell the doctor? What sounds does a joint produce when it moves? Sight: How does the patient present? Are they weary from pain or chronic disease? Touch: What does the joint feel like? How does it move? What is the patient’s response to stabilization of a joint? Smell: Is there an odor that helps detect the presence of infection or decay? Is the patient coming into contact with a substance causing harm or preventing healing?
A good doctor must employ these senses first to understand the patient’s needs and then to treat the patient. The sixth sense is a gift, one that comes from years of experience, an attention to detail, and a commitment to the craft of medicine. A recent trend toward virtual medicine is a dangerous path that must be walked with care and discretion so that the 6 senses are maintained and nurtured. Technology must be used to enhance and not limit these senses. The patient cannot be reduced to a 2-dimensional version of his/herself so that the doctor’s powers of diagnosis and healing are similarly limited.
Change in the office has occurred with mandates for electronic medical records and work-hour restrictions for residents. Data do not support that either change has resulted in a net benefit to patients. We are mandated to invest scarce capital to support new technology, resulting in increased pressure to recoup investment. Where there is a cap on revenue, the only way to increase net profit is to increase volume and decrease services. Physician time is the variable and can be streamlined by performing video conferences or smartphone consultations. Change may bring higher order, as the English philosopher John Locke said, but it is time for all of us as physicians to step back and question that this type of change is the path we must take. An office with a schedule of 80 patients seen at 5-minute intervals by physician assistants has no place in medicine. The pressure imposed by the insurance industry or hospital administrators to meet quotas has gotten out of hand and the time is now to say with a strong but fair voice a resounding NO!
The office visit with a history and physical examination is the most exciting and effective time to meet, console, and relate to our patients. The use of the 5 senses is critical. We must not let technological advancements (eg, smartphones, the Internet, and electronic medical records) destroy what was created and taught to us all through our training. The reward that is accomplished by placing one’s hand on a patient’s knee to understand its warmth and swelling, the tactile feeling of a fluid wave, or performing carefully with compassion a provocative maneuver that gives by sight a grimace of discomfort can tell so much more than a status update on the phone. We must not allow ourselves to be replaced by ancillary services for so-called efficiency and cost saving. Rather, we must be innovative and sharp. We must find the way to use the wonders of the virtual world without giving up the human consult.
Imagine that you are able to travel to Iguazu Falls, South America, to see one of the wonders of our world. You sit in that life raft moving upstream to feel the heat from the water as it crushes the rocks below, and you feel the mist on your face. You see the majesty and hear the screams and breadth of excitement of those around you, while you listen to the deafening sounds created by this waterfall. Now imagine you are required to report on this same experience through a video or some form of technology that the world has convinced us is the best and far cheaper substitute. This is our electronic medical record. A tool we are forced to use, and while it has a purpose, it is a sterile tool that fails to provide information that will give clues to awaken the sixth sense. It is a checklist that could allow for completion of a task—like how to fix a leaky faucet.
How then do we accomplish walking the fine line of working with nonphysicians and technology and yet delivering pinnacle care? The answer isn’t simple but it must include education and a commitment to the profession. We must make the public aware that we are one of the few professions that dedicate our lives to others by promoting health and advancing research. My colleagues, the pendulum has swung too far; it is time to take back our great profession through education of ourselves and the public. While technology may help the world connect, it has a limited role unless we first use our 6 senses to help our patients. We must not submit to a compassionless and callous approach that is the inevitable outcome of virtual medicine done with speed. We must maintain our dignity and let the public understand how many years of sacrifice has taken place to earn a sixth sense and not allow a third party to take it away. We are the only source of protection for our patients and we need each one of our senses to perform this task.
Advancing research has been a cornerstone for the orthopedic surgeon. Position statements through meta-analyses and systematic reviews of the literature have recently been utilized with increasing frequency. Combining data of potentially flawed studies can often lead to erroneous conclusions and may stray away from best practices. Is this where we want evidence-based medicine to go? The end result is that decisions are made by insurance companies who rely on these flawed studies to force clinical decisions on the physician, as was most recently seen by the investigation of viscosupplementation for knee osteoarthritis.1
In a 2007 study published in JAMA (The Journal of the American Medical Association), only 62% of residents could appropriately interpret a P value.2 How can we expect young clinicians to evaluate, interpret, and apply the multitude of evidence in the literature to everyday practice? We must marry the use of best evidence with our expertise to make the most informed decision while managing the expectations of our patients. In order to achieve that balance, we must rely on our intuition, our sixth sense. There is too much patient individuality and complexity surrounding each individual’s situation for a one-size-fits-all approach and for wholesale reliance on research to address each unique situation.
If Nathan Davis in 1845 was able to convince the New York Medical Society to establish a nationwide professional association to assist in regulating the practice of medicine, then it is time for all of us to stand up and insist on a code of ethics that is unrelenting and uncompromising. Our wise leaders of the American Orthopaedic Association (AOA) who founded the formation of orthopedics in America knew guidelines were needed to “foster advances in the care of patients, improve the teaching of orthopaedic surgery in medical schools and formal orthopaedic training, and to promote orthopaedic surgery as a surgical discipline worldwide.”3 It is now our turn to renew the guidelines and encourage our leaders to help educate ourselves and patients as we work with technology and administrators, nurses and physician assistants to deliver pinnacle care. We must reform medical education and the practice of medicine so that technology is used as a companion but not a substitute for our 6 senses.
The next time a patient comes into the exam room, sit down, look the patient in the eye, listen, touch, console anxiety, make a human connection, and form a lasting relationship. By all means apologize to your patients as you fill out the electronic medical record and insurance forms. Discuss how we are in the same crisis together and ask for their help as they have come to you for yours.
Technology creates change, and change is moving fast and is relentless. Physicians, on the other hand, are generally slow to change. Wisely, we question change—we observe it, we study it, and we try to ensure our patients will benefit from it over time. Maybe as a result of this or as a consequence of our often myopic view of the world, we mistakenly let others lead the way and dictate how we must change and what our practices must absorb. We must turn this around and be the agents of change for our profession so we can appropriately use the available technology and create systems for managing the demands of a society that expects instant answers with fewer doctor resources devoted to the answer. The insurance industry is encouraging a wholesale dismantling of the classic patient visit to be replaced by nonphysician interactions, virtual diagnostics, and electronic medical records. We must not allow this and must ensure that we safeguard our profession by employing traditional skills, utilizing our 5 senses, and incorporating technology as a tool for better diagnosis and treatment but not as a substitute for the same.
Great doctors are often described as having a sixth sense—an intuition that guides them in diagnosing and treating patients. It is assumed, therefore, that the good doctor will have the benefit of 5 senses: sight, sound, touch, smell, and taste. Sound: What does the patient tell or neglect to tell the doctor? What sounds does a joint produce when it moves? Sight: How does the patient present? Are they weary from pain or chronic disease? Touch: What does the joint feel like? How does it move? What is the patient’s response to stabilization of a joint? Smell: Is there an odor that helps detect the presence of infection or decay? Is the patient coming into contact with a substance causing harm or preventing healing?
A good doctor must employ these senses first to understand the patient’s needs and then to treat the patient. The sixth sense is a gift, one that comes from years of experience, an attention to detail, and a commitment to the craft of medicine. A recent trend toward virtual medicine is a dangerous path that must be walked with care and discretion so that the 6 senses are maintained and nurtured. Technology must be used to enhance and not limit these senses. The patient cannot be reduced to a 2-dimensional version of his/herself so that the doctor’s powers of diagnosis and healing are similarly limited.
Change in the office has occurred with mandates for electronic medical records and work-hour restrictions for residents. Data do not support that either change has resulted in a net benefit to patients. We are mandated to invest scarce capital to support new technology, resulting in increased pressure to recoup investment. Where there is a cap on revenue, the only way to increase net profit is to increase volume and decrease services. Physician time is the variable and can be streamlined by performing video conferences or smartphone consultations. Change may bring higher order, as the English philosopher John Locke said, but it is time for all of us as physicians to step back and question that this type of change is the path we must take. An office with a schedule of 80 patients seen at 5-minute intervals by physician assistants has no place in medicine. The pressure imposed by the insurance industry or hospital administrators to meet quotas has gotten out of hand and the time is now to say with a strong but fair voice a resounding NO!
The office visit with a history and physical examination is the most exciting and effective time to meet, console, and relate to our patients. The use of the 5 senses is critical. We must not let technological advancements (eg, smartphones, the Internet, and electronic medical records) destroy what was created and taught to us all through our training. The reward that is accomplished by placing one’s hand on a patient’s knee to understand its warmth and swelling, the tactile feeling of a fluid wave, or performing carefully with compassion a provocative maneuver that gives by sight a grimace of discomfort can tell so much more than a status update on the phone. We must not allow ourselves to be replaced by ancillary services for so-called efficiency and cost saving. Rather, we must be innovative and sharp. We must find the way to use the wonders of the virtual world without giving up the human consult.
Imagine that you are able to travel to Iguazu Falls, South America, to see one of the wonders of our world. You sit in that life raft moving upstream to feel the heat from the water as it crushes the rocks below, and you feel the mist on your face. You see the majesty and hear the screams and breadth of excitement of those around you, while you listen to the deafening sounds created by this waterfall. Now imagine you are required to report on this same experience through a video or some form of technology that the world has convinced us is the best and far cheaper substitute. This is our electronic medical record. A tool we are forced to use, and while it has a purpose, it is a sterile tool that fails to provide information that will give clues to awaken the sixth sense. It is a checklist that could allow for completion of a task—like how to fix a leaky faucet.
How then do we accomplish walking the fine line of working with nonphysicians and technology and yet delivering pinnacle care? The answer isn’t simple but it must include education and a commitment to the profession. We must make the public aware that we are one of the few professions that dedicate our lives to others by promoting health and advancing research. My colleagues, the pendulum has swung too far; it is time to take back our great profession through education of ourselves and the public. While technology may help the world connect, it has a limited role unless we first use our 6 senses to help our patients. We must not submit to a compassionless and callous approach that is the inevitable outcome of virtual medicine done with speed. We must maintain our dignity and let the public understand how many years of sacrifice has taken place to earn a sixth sense and not allow a third party to take it away. We are the only source of protection for our patients and we need each one of our senses to perform this task.
Advancing research has been a cornerstone for the orthopedic surgeon. Position statements through meta-analyses and systematic reviews of the literature have recently been utilized with increasing frequency. Combining data of potentially flawed studies can often lead to erroneous conclusions and may stray away from best practices. Is this where we want evidence-based medicine to go? The end result is that decisions are made by insurance companies who rely on these flawed studies to force clinical decisions on the physician, as was most recently seen by the investigation of viscosupplementation for knee osteoarthritis.1
In a 2007 study published in JAMA (The Journal of the American Medical Association), only 62% of residents could appropriately interpret a P value.2 How can we expect young clinicians to evaluate, interpret, and apply the multitude of evidence in the literature to everyday practice? We must marry the use of best evidence with our expertise to make the most informed decision while managing the expectations of our patients. In order to achieve that balance, we must rely on our intuition, our sixth sense. There is too much patient individuality and complexity surrounding each individual’s situation for a one-size-fits-all approach and for wholesale reliance on research to address each unique situation.
If Nathan Davis in 1845 was able to convince the New York Medical Society to establish a nationwide professional association to assist in regulating the practice of medicine, then it is time for all of us to stand up and insist on a code of ethics that is unrelenting and uncompromising. Our wise leaders of the American Orthopaedic Association (AOA) who founded the formation of orthopedics in America knew guidelines were needed to “foster advances in the care of patients, improve the teaching of orthopaedic surgery in medical schools and formal orthopaedic training, and to promote orthopaedic surgery as a surgical discipline worldwide.”3 It is now our turn to renew the guidelines and encourage our leaders to help educate ourselves and patients as we work with technology and administrators, nurses and physician assistants to deliver pinnacle care. We must reform medical education and the practice of medicine so that technology is used as a companion but not a substitute for our 6 senses.
The next time a patient comes into the exam room, sit down, look the patient in the eye, listen, touch, console anxiety, make a human connection, and form a lasting relationship. By all means apologize to your patients as you fill out the electronic medical record and insurance forms. Discuss how we are in the same crisis together and ask for their help as they have come to you for yours.
1. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576.
2. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010-1022.
3. DeRosa GP. 75 Years of Doing the Right Thing: A History of the American Board of Orthopaedic Surgery. American Board of Orthopaedic Surgery; 2009.
1. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576.
2. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010-1022.
3. DeRosa GP. 75 Years of Doing the Right Thing: A History of the American Board of Orthopaedic Surgery. American Board of Orthopaedic Surgery; 2009.