User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Well-Leg Positioning on a Fracture Table: Using a Pillow Sling
The development of acute compartment syndrome in lower legs placed in the lithotomy position is a rare complication reported within various surgical subspecialties, including general surgery, gynecology, and urology.1-5 Although it is reported in arthroscopic knee cases, the more frequent occurrence in orthopedics, based on available case reports, appears to involve the well (uninjured or contralateral) leg placed in the hemilithotomy position on the fracture table.6-9
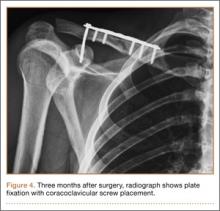
Prior studies have found significantly elevated lower leg compartment pressures in legs placed in the lithotomy position. Chase and colleagues10 measured the anterior compartment pressures in 16 limbs placed in the lithotomy position. They found minor elevations after initial lithotomy positioning, but gradual increases over time, with an average elevation to 30 mm Hg and maximum of 70 mm Hg. Similarly, Meyer and colleagues11 recorded the lower leg pressures in 8 healthy volunteers positioned on a fracture table. Changing from the supine position to the lithotomy position significantly increased the intramuscular pressure in the anterior compartment (from 11.6 to 19.4 mm Hg) and in the lateral compartment (from 13.0 to 25.8 mm Hg).
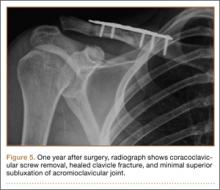
Along with increased intramuscular pressures, local hypotension occurs in lower legs placed in the lithotomy position. Mean diastolic blood pressure in the ankle was 63.9 mm Hg in the leg placed in the supine position as opposed to 34.6 mm Hg in the same leg placed in the lithotomy position.10 This finding is not unexpected, given that local arteriolar pressure decreases by 0.78 mm Hg for every 1.0 cm of elevation.12-14 Furthermore, some “kinking” of either femoral vessels at the hip or popliteal vessels at the knee may also occur.15
For prevention of these problems, the well leg can be placed in a position of slight hip extension and full knee extension on the fracture table—the so-called scissored position. This position is commonly achieved with an additional traction boot and support bar connected to the well leg. However, this additional setup can make positioning the C-arm machine difficult; there is obstruction by the additional support bars and the leg itself. In addition, the uninjured extremity may be placed into positions that cause unnecessarily high stresses across the joints and can potentially lead to iatrogenic injury and pain.
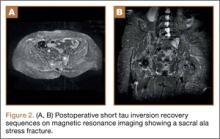
Risk of fracture in the well leg results from the C-arm machine abutting the well leg when swinging through to obtain a lateral image. This problem is overcome by securing the well leg to the fracture table’s longitudinal support bar using a pillow sling, thereby reducing the risks of compartment syndrome, allowing the uninjured limb to be in a relaxed position, and allowing good fluoroscopic images to easily be obtained. This brief report is an introduction to this positioning method.
Surgical Technique
The patient is intubated and anesthetized on the hospital bed before being transferred to the fracture table. On the fracture table, the operative leg is placed in a boot traction device in the standard fashion. The perineal post is then inserted, and the patient is pulled caudally on the bed so that the post is appropriately positioned for countertraction.
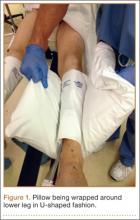
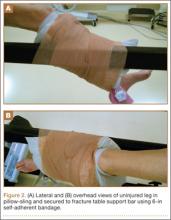
With an assistant holding the well leg, the distal flat-top table extension is removed. With a calf or foot sequential compression device still in place, a pillow enclosed in a pillowcase is wrapped around the lower leg and ankle in a U-shaped fashion using the longitudinal length of the pillow (Figure 1). The pillow-wrapped leg is then placed against the side (not the top) of the table’s support bar and secured in place using a 6-in self-adherent compression bandage (eg, Coban; 3M, St. Paul, Minnesota), wrapped circumferentially around both the pillow and the support post (Figures 2A, 2B). Although an Ace wrap may be more readily accessible, we have found it to slowly loosen and/or migrate, thus potentially changing the leg position throughout the case.
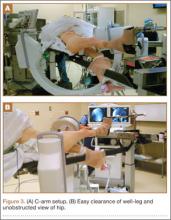
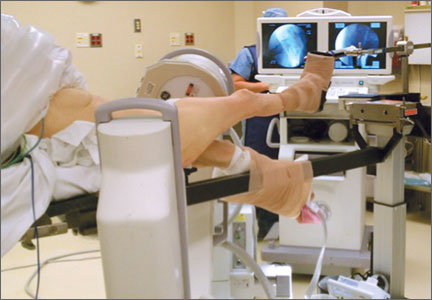
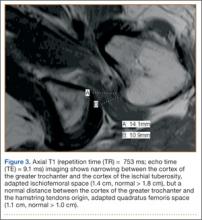
As shown in Figure 3, the C-arm machine can then be positioned in an oblique fashion relative to the bed with an unobstructed view of the hip. The C-arm can also be repositioned perpendicular to the injured limb, and unobstructed images can be obtained of the entire length of the femur. This quick and efficient setup of the well leg allows for an optimal amount of relaxed hip and knee extension, and limb adduction to midline along the table’s support bar, to permit lateral fluoroscopic imaging of the injured limb without overlap of the well leg or interference with C-arm positioning.
Results
For more than 2 years, Dr. Mir has used the pillow-sling technique for placement of the well leg in the scissored position on the fracture table in all patients. Between September 2010 and January 2013, he applied the technique 93 times, with the procedures listed as follows with their Current Procedural Terminology (CPT) codes: 14 cases of percutaneous fixation of femoral neck fracture (CPT 27235), 8 cases of treatment of intertrochanteric or subtrochanteric fracture with plate/screw type implant (CPT 27244), 34 cases of treatment of intertrochanteric or subtrochanteric fracture with intramedullary implant (CPT 27245), and 37 cases of treatment of femoral shaft fracture with intramedullary implant (CPT 27506).
With respect to compartment syndrome, there were no intraoperative or postoperative complications. Furthermore, no patients complained of pain in the well leg immediately after surgery or at subsequent follow-ups. No difficulty was encountered with intraoperative C-arm imaging of the injured limb at the hip or along the length of the femur in the lateral or anteroposterior planes. The well leg did not have to be repositioned in any cases to achieve adequate imaging of the hip and femur.
Discussion
Although rare, acute compartment syndrome remains a potential yet avoidable complication of the lithotomy position. Some surgeons avoid this setup of the well leg on the fracture table and instead use a scissored position for the uninjured limb.
In this report, we presented a safe and efficient technique for placing the well leg in a scissored position on the fracture table using a pillow and a self-adherent compression bandage. We did not compare the pillow-sling with other well-leg positioning techniques but instead described a reproducible technique that we have used effectively and successfully, even with multiple morbidly obese patients who met the weight limits for the fracture table.
In addition, even with consistent use of this pillow-sling technique at our high-volume trauma center, there have been no complications, such as compartment syndrome, well-leg pain, or difficulty in intraoperative imaging of the injured limb. The pillow-sling is a safe and expedient alternative technique for well-leg positioning on the fracture table, and it can be easily reproduced by other surgeons.
1. Leff RG, Shapiro SR. Lower extremity complications of the lithotomy position: prevention and management. J Urol. 1979;122(1):138-139.
2. Lydon JC, Spielman FJ. Bilateral compartment syndrome following prolonged surgery in the lithotomy position. Anesthesiology. 1984;60(3):236-238.
3. Kubiak R, Wilcox DT, Spitz L, Kiely EM. Neurovascular morbidity from the lithotomy position. J Pediatr Surg. 1998;33(12):1808-1810.
4. Cohen SA, Hurt WG. Compartment syndrome associated with lithotomy position and intermittent compression stockings. Obstet Gynecol. 2001;97(5 pt 2):832-833.
5. Moses TA, Kreder KJ, Thrasher JB. Compartment syndrome: an unusual complication of the lithotomy position. Urology. 1994;43(5):746-747.
6. Chung JH, Ahn KR, Park JH, et al. Lower leg compartment syndrome following prolonged orthopedic surgery in the lithotomy position –A case report–. Korean J Anesthesiol. 2010;59(suppl):S49-S52.
7. Tan V, Pepe MD, Glaser DL, Seldes RM, Heppenstall RB, Esterhai JL Jr. Well-leg compartment pressures during hemilithotomy position for fracture fixation. J Orthop Trauma. 2000;14(3):157-161.
8. Anglen J, Banovetz J. Compartment syndrome in the well leg resulting from fracture-table positioning. Clin Orthop. 1994;(301):239-242.
9. Mathews PV, Perry JJ, Murray PC. Compartment syndrome of the well leg as a result of the hemilithotomy position: a report of two cases and review of literature. J Orthop Trauma. 2001;15(8):580-583.
10. Chase J, Harford F, Pinzur MS, Zussman M. Intraoperative lower extremity compartment pressures in lithotomy-positioned patients. Dis Colon Rectum. 2000;43(5):678-680.
11. Meyer RS, White KK, Smith JM, Groppo ER, Mubarak SJ, Hargens AR. Intramuscular and blood pressures in legs positioned in the hemilithotomy position: clarification of risk factors for well-leg acute compartment syndrome. J Bone Joint Surg Am. 2002;84(10):1829-1835.
12. Enderby GE. Postural ischaemia and blood-pressure. Lancet. 1954;266(6804):185-187.
13. Matsen FA 3rd, Mayo KA, Krugmire RB Jr, Sheridan GW, Kraft GH. A model compartmental syndrome in man with particular reference to the quantification of nerve function. J Bone Joint Surg Am. 1977;59(5):648-653.
14. Peters P, Baker SR, Leopold PW, Taub NA, Burnand KG. Compartment syndrome following prolonged pelvic surgery. Br J Surg. 1994;81(8):1128-1131.
15. Gershuni DH, Yaru NC, Hargens AR, Lieber RL, O’Hara RC, Akeson WH. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66(9):1415-1420.
The development of acute compartment syndrome in lower legs placed in the lithotomy position is a rare complication reported within various surgical subspecialties, including general surgery, gynecology, and urology.1-5 Although it is reported in arthroscopic knee cases, the more frequent occurrence in orthopedics, based on available case reports, appears to involve the well (uninjured or contralateral) leg placed in the hemilithotomy position on the fracture table.6-9
Prior studies have found significantly elevated lower leg compartment pressures in legs placed in the lithotomy position. Chase and colleagues10 measured the anterior compartment pressures in 16 limbs placed in the lithotomy position. They found minor elevations after initial lithotomy positioning, but gradual increases over time, with an average elevation to 30 mm Hg and maximum of 70 mm Hg. Similarly, Meyer and colleagues11 recorded the lower leg pressures in 8 healthy volunteers positioned on a fracture table. Changing from the supine position to the lithotomy position significantly increased the intramuscular pressure in the anterior compartment (from 11.6 to 19.4 mm Hg) and in the lateral compartment (from 13.0 to 25.8 mm Hg).
Along with increased intramuscular pressures, local hypotension occurs in lower legs placed in the lithotomy position. Mean diastolic blood pressure in the ankle was 63.9 mm Hg in the leg placed in the supine position as opposed to 34.6 mm Hg in the same leg placed in the lithotomy position.10 This finding is not unexpected, given that local arteriolar pressure decreases by 0.78 mm Hg for every 1.0 cm of elevation.12-14 Furthermore, some “kinking” of either femoral vessels at the hip or popliteal vessels at the knee may also occur.15
For prevention of these problems, the well leg can be placed in a position of slight hip extension and full knee extension on the fracture table—the so-called scissored position. This position is commonly achieved with an additional traction boot and support bar connected to the well leg. However, this additional setup can make positioning the C-arm machine difficult; there is obstruction by the additional support bars and the leg itself. In addition, the uninjured extremity may be placed into positions that cause unnecessarily high stresses across the joints and can potentially lead to iatrogenic injury and pain.
Risk of fracture in the well leg results from the C-arm machine abutting the well leg when swinging through to obtain a lateral image. This problem is overcome by securing the well leg to the fracture table’s longitudinal support bar using a pillow sling, thereby reducing the risks of compartment syndrome, allowing the uninjured limb to be in a relaxed position, and allowing good fluoroscopic images to easily be obtained. This brief report is an introduction to this positioning method.
Surgical Technique
The patient is intubated and anesthetized on the hospital bed before being transferred to the fracture table. On the fracture table, the operative leg is placed in a boot traction device in the standard fashion. The perineal post is then inserted, and the patient is pulled caudally on the bed so that the post is appropriately positioned for countertraction.
With an assistant holding the well leg, the distal flat-top table extension is removed. With a calf or foot sequential compression device still in place, a pillow enclosed in a pillowcase is wrapped around the lower leg and ankle in a U-shaped fashion using the longitudinal length of the pillow (Figure 1). The pillow-wrapped leg is then placed against the side (not the top) of the table’s support bar and secured in place using a 6-in self-adherent compression bandage (eg, Coban; 3M, St. Paul, Minnesota), wrapped circumferentially around both the pillow and the support post (Figures 2A, 2B). Although an Ace wrap may be more readily accessible, we have found it to slowly loosen and/or migrate, thus potentially changing the leg position throughout the case.
As shown in Figure 3, the C-arm machine can then be positioned in an oblique fashion relative to the bed with an unobstructed view of the hip. The C-arm can also be repositioned perpendicular to the injured limb, and unobstructed images can be obtained of the entire length of the femur. This quick and efficient setup of the well leg allows for an optimal amount of relaxed hip and knee extension, and limb adduction to midline along the table’s support bar, to permit lateral fluoroscopic imaging of the injured limb without overlap of the well leg or interference with C-arm positioning.
Results
For more than 2 years, Dr. Mir has used the pillow-sling technique for placement of the well leg in the scissored position on the fracture table in all patients. Between September 2010 and January 2013, he applied the technique 93 times, with the procedures listed as follows with their Current Procedural Terminology (CPT) codes: 14 cases of percutaneous fixation of femoral neck fracture (CPT 27235), 8 cases of treatment of intertrochanteric or subtrochanteric fracture with plate/screw type implant (CPT 27244), 34 cases of treatment of intertrochanteric or subtrochanteric fracture with intramedullary implant (CPT 27245), and 37 cases of treatment of femoral shaft fracture with intramedullary implant (CPT 27506).
With respect to compartment syndrome, there were no intraoperative or postoperative complications. Furthermore, no patients complained of pain in the well leg immediately after surgery or at subsequent follow-ups. No difficulty was encountered with intraoperative C-arm imaging of the injured limb at the hip or along the length of the femur in the lateral or anteroposterior planes. The well leg did not have to be repositioned in any cases to achieve adequate imaging of the hip and femur.
Discussion
Although rare, acute compartment syndrome remains a potential yet avoidable complication of the lithotomy position. Some surgeons avoid this setup of the well leg on the fracture table and instead use a scissored position for the uninjured limb.
In this report, we presented a safe and efficient technique for placing the well leg in a scissored position on the fracture table using a pillow and a self-adherent compression bandage. We did not compare the pillow-sling with other well-leg positioning techniques but instead described a reproducible technique that we have used effectively and successfully, even with multiple morbidly obese patients who met the weight limits for the fracture table.
In addition, even with consistent use of this pillow-sling technique at our high-volume trauma center, there have been no complications, such as compartment syndrome, well-leg pain, or difficulty in intraoperative imaging of the injured limb. The pillow-sling is a safe and expedient alternative technique for well-leg positioning on the fracture table, and it can be easily reproduced by other surgeons.
The development of acute compartment syndrome in lower legs placed in the lithotomy position is a rare complication reported within various surgical subspecialties, including general surgery, gynecology, and urology.1-5 Although it is reported in arthroscopic knee cases, the more frequent occurrence in orthopedics, based on available case reports, appears to involve the well (uninjured or contralateral) leg placed in the hemilithotomy position on the fracture table.6-9
Prior studies have found significantly elevated lower leg compartment pressures in legs placed in the lithotomy position. Chase and colleagues10 measured the anterior compartment pressures in 16 limbs placed in the lithotomy position. They found minor elevations after initial lithotomy positioning, but gradual increases over time, with an average elevation to 30 mm Hg and maximum of 70 mm Hg. Similarly, Meyer and colleagues11 recorded the lower leg pressures in 8 healthy volunteers positioned on a fracture table. Changing from the supine position to the lithotomy position significantly increased the intramuscular pressure in the anterior compartment (from 11.6 to 19.4 mm Hg) and in the lateral compartment (from 13.0 to 25.8 mm Hg).
Along with increased intramuscular pressures, local hypotension occurs in lower legs placed in the lithotomy position. Mean diastolic blood pressure in the ankle was 63.9 mm Hg in the leg placed in the supine position as opposed to 34.6 mm Hg in the same leg placed in the lithotomy position.10 This finding is not unexpected, given that local arteriolar pressure decreases by 0.78 mm Hg for every 1.0 cm of elevation.12-14 Furthermore, some “kinking” of either femoral vessels at the hip or popliteal vessels at the knee may also occur.15
For prevention of these problems, the well leg can be placed in a position of slight hip extension and full knee extension on the fracture table—the so-called scissored position. This position is commonly achieved with an additional traction boot and support bar connected to the well leg. However, this additional setup can make positioning the C-arm machine difficult; there is obstruction by the additional support bars and the leg itself. In addition, the uninjured extremity may be placed into positions that cause unnecessarily high stresses across the joints and can potentially lead to iatrogenic injury and pain.
Risk of fracture in the well leg results from the C-arm machine abutting the well leg when swinging through to obtain a lateral image. This problem is overcome by securing the well leg to the fracture table’s longitudinal support bar using a pillow sling, thereby reducing the risks of compartment syndrome, allowing the uninjured limb to be in a relaxed position, and allowing good fluoroscopic images to easily be obtained. This brief report is an introduction to this positioning method.
Surgical Technique
The patient is intubated and anesthetized on the hospital bed before being transferred to the fracture table. On the fracture table, the operative leg is placed in a boot traction device in the standard fashion. The perineal post is then inserted, and the patient is pulled caudally on the bed so that the post is appropriately positioned for countertraction.
With an assistant holding the well leg, the distal flat-top table extension is removed. With a calf or foot sequential compression device still in place, a pillow enclosed in a pillowcase is wrapped around the lower leg and ankle in a U-shaped fashion using the longitudinal length of the pillow (Figure 1). The pillow-wrapped leg is then placed against the side (not the top) of the table’s support bar and secured in place using a 6-in self-adherent compression bandage (eg, Coban; 3M, St. Paul, Minnesota), wrapped circumferentially around both the pillow and the support post (Figures 2A, 2B). Although an Ace wrap may be more readily accessible, we have found it to slowly loosen and/or migrate, thus potentially changing the leg position throughout the case.
As shown in Figure 3, the C-arm machine can then be positioned in an oblique fashion relative to the bed with an unobstructed view of the hip. The C-arm can also be repositioned perpendicular to the injured limb, and unobstructed images can be obtained of the entire length of the femur. This quick and efficient setup of the well leg allows for an optimal amount of relaxed hip and knee extension, and limb adduction to midline along the table’s support bar, to permit lateral fluoroscopic imaging of the injured limb without overlap of the well leg or interference with C-arm positioning.
Results
For more than 2 years, Dr. Mir has used the pillow-sling technique for placement of the well leg in the scissored position on the fracture table in all patients. Between September 2010 and January 2013, he applied the technique 93 times, with the procedures listed as follows with their Current Procedural Terminology (CPT) codes: 14 cases of percutaneous fixation of femoral neck fracture (CPT 27235), 8 cases of treatment of intertrochanteric or subtrochanteric fracture with plate/screw type implant (CPT 27244), 34 cases of treatment of intertrochanteric or subtrochanteric fracture with intramedullary implant (CPT 27245), and 37 cases of treatment of femoral shaft fracture with intramedullary implant (CPT 27506).
With respect to compartment syndrome, there were no intraoperative or postoperative complications. Furthermore, no patients complained of pain in the well leg immediately after surgery or at subsequent follow-ups. No difficulty was encountered with intraoperative C-arm imaging of the injured limb at the hip or along the length of the femur in the lateral or anteroposterior planes. The well leg did not have to be repositioned in any cases to achieve adequate imaging of the hip and femur.
Discussion
Although rare, acute compartment syndrome remains a potential yet avoidable complication of the lithotomy position. Some surgeons avoid this setup of the well leg on the fracture table and instead use a scissored position for the uninjured limb.
In this report, we presented a safe and efficient technique for placing the well leg in a scissored position on the fracture table using a pillow and a self-adherent compression bandage. We did not compare the pillow-sling with other well-leg positioning techniques but instead described a reproducible technique that we have used effectively and successfully, even with multiple morbidly obese patients who met the weight limits for the fracture table.
In addition, even with consistent use of this pillow-sling technique at our high-volume trauma center, there have been no complications, such as compartment syndrome, well-leg pain, or difficulty in intraoperative imaging of the injured limb. The pillow-sling is a safe and expedient alternative technique for well-leg positioning on the fracture table, and it can be easily reproduced by other surgeons.
1. Leff RG, Shapiro SR. Lower extremity complications of the lithotomy position: prevention and management. J Urol. 1979;122(1):138-139.
2. Lydon JC, Spielman FJ. Bilateral compartment syndrome following prolonged surgery in the lithotomy position. Anesthesiology. 1984;60(3):236-238.
3. Kubiak R, Wilcox DT, Spitz L, Kiely EM. Neurovascular morbidity from the lithotomy position. J Pediatr Surg. 1998;33(12):1808-1810.
4. Cohen SA, Hurt WG. Compartment syndrome associated with lithotomy position and intermittent compression stockings. Obstet Gynecol. 2001;97(5 pt 2):832-833.
5. Moses TA, Kreder KJ, Thrasher JB. Compartment syndrome: an unusual complication of the lithotomy position. Urology. 1994;43(5):746-747.
6. Chung JH, Ahn KR, Park JH, et al. Lower leg compartment syndrome following prolonged orthopedic surgery in the lithotomy position –A case report–. Korean J Anesthesiol. 2010;59(suppl):S49-S52.
7. Tan V, Pepe MD, Glaser DL, Seldes RM, Heppenstall RB, Esterhai JL Jr. Well-leg compartment pressures during hemilithotomy position for fracture fixation. J Orthop Trauma. 2000;14(3):157-161.
8. Anglen J, Banovetz J. Compartment syndrome in the well leg resulting from fracture-table positioning. Clin Orthop. 1994;(301):239-242.
9. Mathews PV, Perry JJ, Murray PC. Compartment syndrome of the well leg as a result of the hemilithotomy position: a report of two cases and review of literature. J Orthop Trauma. 2001;15(8):580-583.
10. Chase J, Harford F, Pinzur MS, Zussman M. Intraoperative lower extremity compartment pressures in lithotomy-positioned patients. Dis Colon Rectum. 2000;43(5):678-680.
11. Meyer RS, White KK, Smith JM, Groppo ER, Mubarak SJ, Hargens AR. Intramuscular and blood pressures in legs positioned in the hemilithotomy position: clarification of risk factors for well-leg acute compartment syndrome. J Bone Joint Surg Am. 2002;84(10):1829-1835.
12. Enderby GE. Postural ischaemia and blood-pressure. Lancet. 1954;266(6804):185-187.
13. Matsen FA 3rd, Mayo KA, Krugmire RB Jr, Sheridan GW, Kraft GH. A model compartmental syndrome in man with particular reference to the quantification of nerve function. J Bone Joint Surg Am. 1977;59(5):648-653.
14. Peters P, Baker SR, Leopold PW, Taub NA, Burnand KG. Compartment syndrome following prolonged pelvic surgery. Br J Surg. 1994;81(8):1128-1131.
15. Gershuni DH, Yaru NC, Hargens AR, Lieber RL, O’Hara RC, Akeson WH. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66(9):1415-1420.
1. Leff RG, Shapiro SR. Lower extremity complications of the lithotomy position: prevention and management. J Urol. 1979;122(1):138-139.
2. Lydon JC, Spielman FJ. Bilateral compartment syndrome following prolonged surgery in the lithotomy position. Anesthesiology. 1984;60(3):236-238.
3. Kubiak R, Wilcox DT, Spitz L, Kiely EM. Neurovascular morbidity from the lithotomy position. J Pediatr Surg. 1998;33(12):1808-1810.
4. Cohen SA, Hurt WG. Compartment syndrome associated with lithotomy position and intermittent compression stockings. Obstet Gynecol. 2001;97(5 pt 2):832-833.
5. Moses TA, Kreder KJ, Thrasher JB. Compartment syndrome: an unusual complication of the lithotomy position. Urology. 1994;43(5):746-747.
6. Chung JH, Ahn KR, Park JH, et al. Lower leg compartment syndrome following prolonged orthopedic surgery in the lithotomy position –A case report–. Korean J Anesthesiol. 2010;59(suppl):S49-S52.
7. Tan V, Pepe MD, Glaser DL, Seldes RM, Heppenstall RB, Esterhai JL Jr. Well-leg compartment pressures during hemilithotomy position for fracture fixation. J Orthop Trauma. 2000;14(3):157-161.
8. Anglen J, Banovetz J. Compartment syndrome in the well leg resulting from fracture-table positioning. Clin Orthop. 1994;(301):239-242.
9. Mathews PV, Perry JJ, Murray PC. Compartment syndrome of the well leg as a result of the hemilithotomy position: a report of two cases and review of literature. J Orthop Trauma. 2001;15(8):580-583.
10. Chase J, Harford F, Pinzur MS, Zussman M. Intraoperative lower extremity compartment pressures in lithotomy-positioned patients. Dis Colon Rectum. 2000;43(5):678-680.
11. Meyer RS, White KK, Smith JM, Groppo ER, Mubarak SJ, Hargens AR. Intramuscular and blood pressures in legs positioned in the hemilithotomy position: clarification of risk factors for well-leg acute compartment syndrome. J Bone Joint Surg Am. 2002;84(10):1829-1835.
12. Enderby GE. Postural ischaemia and blood-pressure. Lancet. 1954;266(6804):185-187.
13. Matsen FA 3rd, Mayo KA, Krugmire RB Jr, Sheridan GW, Kraft GH. A model compartmental syndrome in man with particular reference to the quantification of nerve function. J Bone Joint Surg Am. 1977;59(5):648-653.
14. Peters P, Baker SR, Leopold PW, Taub NA, Burnand KG. Compartment syndrome following prolonged pelvic surgery. Br J Surg. 1994;81(8):1128-1131.
15. Gershuni DH, Yaru NC, Hargens AR, Lieber RL, O’Hara RC, Akeson WH. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66(9):1415-1420.
Increased Incidence of Patella Baja After Total Knee Arthroplasty Revision for Infection
Patellar height may be important in determining function after total knee arthroplasty (TKA). By altering patellofemoral joint mechanics, patella baja may cause several functional issues after TKA.1-8 Patella baja leads to decreased range of motion (ROM) affecting both extension and flexion.5,8,9 Deep flexion can be restricted in TKA patients with patella baja because of tracking limitations associated with an inferiorly displaced patella. As the knee is brought into flexion, the patella can impinge on the anterior aspect of the tibial polyethylene or the tibial tray—presenting a true block to flexion and potentially altering wear.1,10
Another functional issue with patella baja is loss of strength in the extensor mechanism. The patella serves as a fulcrum for the extensor muscles of the knee. When positioned properly and functioning properly, the patella increases the extensor forces generated. When the patella is positioned in baja, the knee generates decreased extensor mechanism force.6,7 This can result in a lag, with the patient being unable to fully extend the knee. Extension-dependent activities are impaired. Patients with weak extensor function can experience poor function with stair climbing, rising from a chair, and exiting an automobile. The improper function and scarring of the patella can result in increased anterior knee pain and worse functional outcome scores after TKAs.3,9
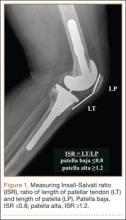
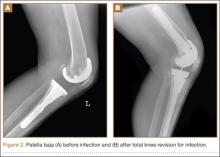
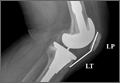
An abnormally positioned patella can either result from or lead to increased scarring in the knee.9,11 Patellar height is often measured with the Insall-Salvati ratio (ISR), which is the patella tendon length (measurement of the tendon from the tibial tubercle to the inferior pole of the patella) divided by the patellar length (longest measured dimension of the patella) (Figure 1).12 Patella baja is defined as an ISR of less than 0.8. Other indices that reference off the tibial plateau (Blackburne-Peel ratio, Canton-Deschamps ratio) reflect an elevation of the joint line, or pseudobaja, and are unreliable for analysis of patella baja after TKA.13
Postoperative patella baja has been reported in 10% to 34% of primary TKAs.4,7 Inferior positioning of the patella and scarring can cause intraoperative difficulty with exposure and may complicate outcomes.9,13 The exposure scar is often larger in TKA revisions for infection compared with primary TKAs.
We conducted a study to compare the incidence of patella baja in noninfected and infected TKA revisions. We hypothesized that, compared with noninfected knees, infected knees treated with nonarticulating spacers would have a higher incidence of patella baja both before and after surgery secondary to more inflammation, immobilization, and related scarring.
Materials and Methods
We conducted a retrospective case–cohort study of 148 consecutive TKA revisions. All TKA revisions were performed between 2003 and 2009 using a mobile-bearing revision system from a single manufacturer. All surgeries were done at a single institution by the 2 senior surgeons. The surgical approach was a standard medial parapatellar approach without patellar eversion. Our institutional review board approved the study and waived the requirement for informed consent, as this was a retrospective study of existing medical records that posed no more than minimal risk to patients.
To properly evaluate patellar height, orthopedic specialty–trained radiologic technicians obtained preoperative and postoperative weight-bearing radiographs using a standardized lateral radiograph in clinic. Two blinded investigators measured ISR radiographically both before surgery (preexplant for septic revisions) and at latest follow-up (postreplant for septic revisions). Patients with inadequate films and/or patellectomies were excluded, along with patients who had less than 6 months of postoperative follow-up.
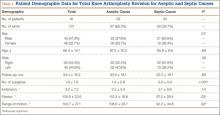
Ninety-one patients (101 TKAs) met the study inclusion criteria. Two groups of cases were compared: aseptic revisions (n = 67) and septic revisions (n = 34). Reasons for aseptic revisions included implant loosening (24/67, 35.8%), instability (12/67, 17.9%), pain (12/67, 17.9%), lysis (5/67, 7.5%), stiffness (3/67, 4.5%), and malrotation (2/67, 3.0%). Infection was determined by Musculoskeletal Infection Society criteria, as documented by positive aspirations and/or intraoperative tissue cultures taken at prosthesis explantation, elevated white blood cell count in the aspirate, elevated percentage of polymorphonuclear (PMN) cells in the aspirate, gross purulence, presence of chronic draining sinus, or histologic analysis revealing acute inflammation with more than 5 PMN cells per high power field.14,15
All infected TKAs were treated with 2-stage revisions. The standard of care at our institution through this series was to use a nonarticulating spacer for the treatment of infection. Weight-bearing status varied by extent of bone damage. Six weeks of culture-specific intravenous antibiotics were administered with assistance from an infectious disease consultant. Reimplantation was performed when clinical and laboratory criteria for resolution of infection were met—specifically, when erythrocyte sedimentation rate was less than 30 mm/h, C-reactive protein level was less than 10 mg/L, and aspirates were culture-negative. Mean (range) follow-up was 33.9 (6.2-75.7) months for aseptic revisions and 32.3 (7.5-94.2) months for septic revisions. Radiographic follow-up was performed at each visit, with weight-bearing anteroposterior and posteroanterior views, along with a lateral knee radiograph. At final follow-up, ROM was recorded by the senior attending evaluating the patient.
Categorical variables were statistically analyzed with χ2 tests, and continuous variables were analyzed with Student t test, analysis of variance, and univariate analysis of covariance (ANCOVA). Statistical significance was set at P < .05. Intrarater reliability was measured with the intraclass correlation coefficient (ICC). All statistical analysis was performed with Predictive Analytics SoftWare Statistics Version 20.0 (SPSS, Chicago, Illinois).
Results
Ninety-one consecutive patients (43 men, 48 women) were included in this study. Mean (SD) age was 66.4 (10.1) years. Mean (SD) preoperative ISR in septic and aseptic cases was 0.94 (0.25) for men and 1.02 (0.23) for women (P = .10). Mean postoperative ISR in septic and aseptic cases was 0.84 (0.27) for men and 0.99 (0.23) for women (P = .004). There was a sex difference between septic and aseptic revisions. There were 22 men and 36 women in the aseptic group and 21 men and 12 women in the septic group (P = .01). Men were more likely than women to have septic revisions and patella baja. Table 1 compares the patient demographics of the 2 patient populations. Mean (SD) number of surgeries, including irrigation and débridement procedures before reimplantation, was larger for septic revisions, 2.9 (0.9), than for aseptic revisions, 1.4 (0.8) (P < .001).
Infection was the most common reason for revision and accounted for 33.7% (34/101) of all revisions. Noninfectious indications, in declining order of frequency, included loosening (23.8%, 24/101), instability (11.9%, 12/101), pain (11.9%, 12/101), osteolysis (5.0%, 5/101), polyethylene wear (5.0%, 5/101), failed unicompartmental knee (4.0%, 4/101), stiffness (3.0%, 3/101), and patellar problems (2.0%, 2/101) (Table 2). ISR decreased significantly only in infected revisions. It is important to note that there was not a high incidence of stiffness or patellofemoral failure in revision patients before surgery.
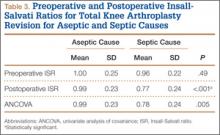
Mean (SD) ISR did not differ between groups before surgery, 1.00 (0.25) for aseptic and 0.96 (0.22) for septic (P = .49), but differed significantly after surgery, 0.99 (0.23) for aseptic and 0.77 (0.24) for septic (P < .001) (Figure 2). The univariate ANCOVA also demonstrated a postoperative difference between groups when taking the preoperative ratio into account: 0.99 (0.23) for aseptic and 0.78 (0.24) for septic (P = .005) (Table 3). Before surgery, 22.4% and 23.9% of the aseptic and septic groups, respectively, had patella baja (P = .58). After surgery, 17.6% and 58.8% of the aseptic and septic groups had patella baja (P = .001) (Table 4). The ICC for preoperative ISR was 0.94, and the ICC for postoperative ISR was 0.96, which indicates excellent agreement of measurements between the 2 blinded investigators.
ROM differed between septic and aseptic groups owing to the difference in postoperative flexion. Mean (SD) postoperative extension was 2.2° (5.4°) for the aseptic group and 5.1° (9.8°) for the septic group—not significantly different (P = .13). Mean (SD) postoperative flexion was 110.2° (18.8°) for the aseptic group and 97.2° (29.4°) for the septic group—significantly different (P = .02). The groups differed significantly (P = .02) in mean (SD) ROM: 108.0° (20.7°) for aseptic and 92.2° (34.6°) for septic (Table 1). ROM was also significantly associated with patella baja (P = .04), as patients with ISR of less than 0.8 had mean (SD) postoperative ROM of 95.1° (31.6°), and patients without patella baja had mean (SD) postoperative ROM of 106.8° (23.6°).
For the septic group, mean (SD) time between first and second stages was 13.0 (8.3) weeks (range, 1-44.3 weeks). Mean (SD) timing of spacer placement was not statistically significantly different (P = .90) between patients who had patella baja, 12.9 (8.8) weeks, and patients who did not have patella baja, 13.2 (7.8) weeks.
Discussion
This study demonstrated that TKAs done for septic reasons resulted in a higher incidence of patella baja and decreased ROM. Incidence of patella baja was higher both before and after revision in septic TKAs than in aseptic TKAs, proving the hypothesis under study. Prerevision incidence was not significantly different, but there was a trend that could not be ignored. This may suggest that there is already an ongoing process in the infected knee that contributes to patella baja; the precise etiology remains unclear and is likely multifactorial. For example, scar formation may be increased in patients with chronic infection, predisposing to patella baja. This assertion is indirectly supported by a recent study from our institution revealing longer average surgical time in septic versus aseptic knee revisions; the difference was thought to reflect increased scar-tissue formation.16 That study also found that patients who underwent septic revisions had significantly more surgical procedures than patients who underwent aseptic revisions. Repetitive surgeries—specifically, repetitive arthrotomies during irrigation and débridement before reimplantation—lead to increased scar formation, which may contribute to preoperative and postoperative patella baja. This may be reflected in the findings that ROM was decreased in patients in the septic group versus patients in the aseptic group and that ROM was decreased in patients with patella baja. In addition, our study found that male patients were more likely to undergo TKA revision for septic reasons and to develop postoperative patella baja. This finding contrasts with that of a study5 that compared preoperative and postoperative ISR in primary TKA and found that women were more likely than men to have patella baja. Although women are more likely to undergo TKA revision,17 men may be more susceptible to infection and subsequent patella baja.
The higher postoperative rate of patella baja in the septic group became statistically significant even when preoperative incidence was considered. This may have been caused by infection-related scarring and by prolonged immobilization of septic knees with use of nonarticulating antibiotic spacers. By keeping these knees immobile with a nonarticulating spacer for a prolonged period in the healing phase of the infection, scar tissue may mature and form over the time between stages. A comparable example may be high tibial osteotomies, in which a high incidence of patella baja has been partly attributed to prolonged casting.11 Future work comparing the results of articulating and nonarticulating spacers will help to determine if immobilization contributes to patella baja in infected TKAs.
There are several limitations to our study. Patient outcome questionnaires were not used, and they would have allowed for the assessment of physical outcomes and emotional satisfaction by comparing outcomes between patients with and without patella baja and comparing septic and aseptic TKAs. In addition, there was no standard method for quantifying difficulty of revision, which would have enabled us to compare difficulty of revision in patients with patella baja.
Conclusion
This study identified a high rate of patella baja and decreased ROM in TKA revisions, particularly infected revisions treated with a nonarticulating spacer. It is important to determine if there are functional consequences. Further investigation is needed regarding the cause, prevention, and management of this potentially debilitating outcome after revision TKA.
1. Aglietti P, Buzzi R, Gaudenzi A. Patello-femoral functional results and complications with the posterior stabilised total condylar knee prosthesis. J Arthroplasty. 1988;3(1):17-25.
2. Fern ED, Winson IG, Getty CJM. Anterior knee pain in rheumatoid patients after total knee replacement: possible selection criteria for patellar resurfacing. J Bone Joint Surg Br. 1992;74(5):745-748.
3. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Surg Surg Am. 1986;68(7):1035-1040.
4. Floren M, Davis J, Peterson MG, Laskin RS. A mini-midvastus capsular approach with patellar displacement decreases the prevalence of patellar baja. J Arthroplasty. 2007;22(6 Suppl 2):51-57.
5. Meneghini RM, Ritter MA, Pierson JL, Meding JB, Berend ME, Faris PM. The effect of the Insall-Salvati ratio on outcome after total knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):116-120.
6. Singerman R, Davy DT, Goldberg VM. Effects of patella alta and patella infera on patellofemoral contact forces. J Biomech. 1994;27(8):1059-1065.
7. Van Eijden TM, Kouwenhoven E, Weijs WA. Mechanics of the patellar articulation: effects of patellar ligament length studied with a mathematical model. Acta Orthop Scand. 1987;58(5):560-566.
8. Weale AE, Murray DW, Newman JH, Ackroyd CE. The length of the patellar tendon after unicompartmental and total knee replacement. J Bone Joint Surg Br. 1999;81(5):790-795.
9. Chonko DJ, Lombardi AV Jr, Berend KR. Patella baja and total knee arthroplasty (TKA): etiology, diagnosis, and management. Surg Technol Int. 2004;12:231-238.
10. Cameron HU, Jung YB. Patella baja complicating total knee arthroplasty. A report of two cases. J Arthroplasty. 1988;3(2):177-180.
11. Scuderi GR, Windsor RE, Insall JN. Observations on patellar height after proximal tibial osteotomy. J Bone Joint Surg Am. 1989;71(2):245-248.
12. Insall JN, Salvati E. Patella position in the normal knee joint. Radiology. 1971;101(1):101-104.
13. Grelsamer RP. Patella baja after total knee arthroplasty: is it really patella baja? J Arthroplasty. 2002;17(1):66-69.
14. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
15. Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty. 2011;26(8):1136-1138.
16. Laudermilch DJ, Fedorka CJ, Heyl A, Rao N, McGough RL. Outcomes of revision total knee arthroplasty after methicillin-resistant Staphylococcus aureus infection. Clin Orthop. 2010;468(8):2067-2073.
17. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop. 2010;468(1):45-51.
Patellar height may be important in determining function after total knee arthroplasty (TKA). By altering patellofemoral joint mechanics, patella baja may cause several functional issues after TKA.1-8 Patella baja leads to decreased range of motion (ROM) affecting both extension and flexion.5,8,9 Deep flexion can be restricted in TKA patients with patella baja because of tracking limitations associated with an inferiorly displaced patella. As the knee is brought into flexion, the patella can impinge on the anterior aspect of the tibial polyethylene or the tibial tray—presenting a true block to flexion and potentially altering wear.1,10
Another functional issue with patella baja is loss of strength in the extensor mechanism. The patella serves as a fulcrum for the extensor muscles of the knee. When positioned properly and functioning properly, the patella increases the extensor forces generated. When the patella is positioned in baja, the knee generates decreased extensor mechanism force.6,7 This can result in a lag, with the patient being unable to fully extend the knee. Extension-dependent activities are impaired. Patients with weak extensor function can experience poor function with stair climbing, rising from a chair, and exiting an automobile. The improper function and scarring of the patella can result in increased anterior knee pain and worse functional outcome scores after TKAs.3,9
An abnormally positioned patella can either result from or lead to increased scarring in the knee.9,11 Patellar height is often measured with the Insall-Salvati ratio (ISR), which is the patella tendon length (measurement of the tendon from the tibial tubercle to the inferior pole of the patella) divided by the patellar length (longest measured dimension of the patella) (Figure 1).12 Patella baja is defined as an ISR of less than 0.8. Other indices that reference off the tibial plateau (Blackburne-Peel ratio, Canton-Deschamps ratio) reflect an elevation of the joint line, or pseudobaja, and are unreliable for analysis of patella baja after TKA.13
Postoperative patella baja has been reported in 10% to 34% of primary TKAs.4,7 Inferior positioning of the patella and scarring can cause intraoperative difficulty with exposure and may complicate outcomes.9,13 The exposure scar is often larger in TKA revisions for infection compared with primary TKAs.
We conducted a study to compare the incidence of patella baja in noninfected and infected TKA revisions. We hypothesized that, compared with noninfected knees, infected knees treated with nonarticulating spacers would have a higher incidence of patella baja both before and after surgery secondary to more inflammation, immobilization, and related scarring.
Materials and Methods
We conducted a retrospective case–cohort study of 148 consecutive TKA revisions. All TKA revisions were performed between 2003 and 2009 using a mobile-bearing revision system from a single manufacturer. All surgeries were done at a single institution by the 2 senior surgeons. The surgical approach was a standard medial parapatellar approach without patellar eversion. Our institutional review board approved the study and waived the requirement for informed consent, as this was a retrospective study of existing medical records that posed no more than minimal risk to patients.
To properly evaluate patellar height, orthopedic specialty–trained radiologic technicians obtained preoperative and postoperative weight-bearing radiographs using a standardized lateral radiograph in clinic. Two blinded investigators measured ISR radiographically both before surgery (preexplant for septic revisions) and at latest follow-up (postreplant for septic revisions). Patients with inadequate films and/or patellectomies were excluded, along with patients who had less than 6 months of postoperative follow-up.
Ninety-one patients (101 TKAs) met the study inclusion criteria. Two groups of cases were compared: aseptic revisions (n = 67) and septic revisions (n = 34). Reasons for aseptic revisions included implant loosening (24/67, 35.8%), instability (12/67, 17.9%), pain (12/67, 17.9%), lysis (5/67, 7.5%), stiffness (3/67, 4.5%), and malrotation (2/67, 3.0%). Infection was determined by Musculoskeletal Infection Society criteria, as documented by positive aspirations and/or intraoperative tissue cultures taken at prosthesis explantation, elevated white blood cell count in the aspirate, elevated percentage of polymorphonuclear (PMN) cells in the aspirate, gross purulence, presence of chronic draining sinus, or histologic analysis revealing acute inflammation with more than 5 PMN cells per high power field.14,15
All infected TKAs were treated with 2-stage revisions. The standard of care at our institution through this series was to use a nonarticulating spacer for the treatment of infection. Weight-bearing status varied by extent of bone damage. Six weeks of culture-specific intravenous antibiotics were administered with assistance from an infectious disease consultant. Reimplantation was performed when clinical and laboratory criteria for resolution of infection were met—specifically, when erythrocyte sedimentation rate was less than 30 mm/h, C-reactive protein level was less than 10 mg/L, and aspirates were culture-negative. Mean (range) follow-up was 33.9 (6.2-75.7) months for aseptic revisions and 32.3 (7.5-94.2) months for septic revisions. Radiographic follow-up was performed at each visit, with weight-bearing anteroposterior and posteroanterior views, along with a lateral knee radiograph. At final follow-up, ROM was recorded by the senior attending evaluating the patient.
Categorical variables were statistically analyzed with χ2 tests, and continuous variables were analyzed with Student t test, analysis of variance, and univariate analysis of covariance (ANCOVA). Statistical significance was set at P < .05. Intrarater reliability was measured with the intraclass correlation coefficient (ICC). All statistical analysis was performed with Predictive Analytics SoftWare Statistics Version 20.0 (SPSS, Chicago, Illinois).
Results
Ninety-one consecutive patients (43 men, 48 women) were included in this study. Mean (SD) age was 66.4 (10.1) years. Mean (SD) preoperative ISR in septic and aseptic cases was 0.94 (0.25) for men and 1.02 (0.23) for women (P = .10). Mean postoperative ISR in septic and aseptic cases was 0.84 (0.27) for men and 0.99 (0.23) for women (P = .004). There was a sex difference between septic and aseptic revisions. There were 22 men and 36 women in the aseptic group and 21 men and 12 women in the septic group (P = .01). Men were more likely than women to have septic revisions and patella baja. Table 1 compares the patient demographics of the 2 patient populations. Mean (SD) number of surgeries, including irrigation and débridement procedures before reimplantation, was larger for septic revisions, 2.9 (0.9), than for aseptic revisions, 1.4 (0.8) (P < .001).
Infection was the most common reason for revision and accounted for 33.7% (34/101) of all revisions. Noninfectious indications, in declining order of frequency, included loosening (23.8%, 24/101), instability (11.9%, 12/101), pain (11.9%, 12/101), osteolysis (5.0%, 5/101), polyethylene wear (5.0%, 5/101), failed unicompartmental knee (4.0%, 4/101), stiffness (3.0%, 3/101), and patellar problems (2.0%, 2/101) (Table 2). ISR decreased significantly only in infected revisions. It is important to note that there was not a high incidence of stiffness or patellofemoral failure in revision patients before surgery.
Mean (SD) ISR did not differ between groups before surgery, 1.00 (0.25) for aseptic and 0.96 (0.22) for septic (P = .49), but differed significantly after surgery, 0.99 (0.23) for aseptic and 0.77 (0.24) for septic (P < .001) (Figure 2). The univariate ANCOVA also demonstrated a postoperative difference between groups when taking the preoperative ratio into account: 0.99 (0.23) for aseptic and 0.78 (0.24) for septic (P = .005) (Table 3). Before surgery, 22.4% and 23.9% of the aseptic and septic groups, respectively, had patella baja (P = .58). After surgery, 17.6% and 58.8% of the aseptic and septic groups had patella baja (P = .001) (Table 4). The ICC for preoperative ISR was 0.94, and the ICC for postoperative ISR was 0.96, which indicates excellent agreement of measurements between the 2 blinded investigators.
ROM differed between septic and aseptic groups owing to the difference in postoperative flexion. Mean (SD) postoperative extension was 2.2° (5.4°) for the aseptic group and 5.1° (9.8°) for the septic group—not significantly different (P = .13). Mean (SD) postoperative flexion was 110.2° (18.8°) for the aseptic group and 97.2° (29.4°) for the septic group—significantly different (P = .02). The groups differed significantly (P = .02) in mean (SD) ROM: 108.0° (20.7°) for aseptic and 92.2° (34.6°) for septic (Table 1). ROM was also significantly associated with patella baja (P = .04), as patients with ISR of less than 0.8 had mean (SD) postoperative ROM of 95.1° (31.6°), and patients without patella baja had mean (SD) postoperative ROM of 106.8° (23.6°).
For the septic group, mean (SD) time between first and second stages was 13.0 (8.3) weeks (range, 1-44.3 weeks). Mean (SD) timing of spacer placement was not statistically significantly different (P = .90) between patients who had patella baja, 12.9 (8.8) weeks, and patients who did not have patella baja, 13.2 (7.8) weeks.
Discussion
This study demonstrated that TKAs done for septic reasons resulted in a higher incidence of patella baja and decreased ROM. Incidence of patella baja was higher both before and after revision in septic TKAs than in aseptic TKAs, proving the hypothesis under study. Prerevision incidence was not significantly different, but there was a trend that could not be ignored. This may suggest that there is already an ongoing process in the infected knee that contributes to patella baja; the precise etiology remains unclear and is likely multifactorial. For example, scar formation may be increased in patients with chronic infection, predisposing to patella baja. This assertion is indirectly supported by a recent study from our institution revealing longer average surgical time in septic versus aseptic knee revisions; the difference was thought to reflect increased scar-tissue formation.16 That study also found that patients who underwent septic revisions had significantly more surgical procedures than patients who underwent aseptic revisions. Repetitive surgeries—specifically, repetitive arthrotomies during irrigation and débridement before reimplantation—lead to increased scar formation, which may contribute to preoperative and postoperative patella baja. This may be reflected in the findings that ROM was decreased in patients in the septic group versus patients in the aseptic group and that ROM was decreased in patients with patella baja. In addition, our study found that male patients were more likely to undergo TKA revision for septic reasons and to develop postoperative patella baja. This finding contrasts with that of a study5 that compared preoperative and postoperative ISR in primary TKA and found that women were more likely than men to have patella baja. Although women are more likely to undergo TKA revision,17 men may be more susceptible to infection and subsequent patella baja.
The higher postoperative rate of patella baja in the septic group became statistically significant even when preoperative incidence was considered. This may have been caused by infection-related scarring and by prolonged immobilization of septic knees with use of nonarticulating antibiotic spacers. By keeping these knees immobile with a nonarticulating spacer for a prolonged period in the healing phase of the infection, scar tissue may mature and form over the time between stages. A comparable example may be high tibial osteotomies, in which a high incidence of patella baja has been partly attributed to prolonged casting.11 Future work comparing the results of articulating and nonarticulating spacers will help to determine if immobilization contributes to patella baja in infected TKAs.
There are several limitations to our study. Patient outcome questionnaires were not used, and they would have allowed for the assessment of physical outcomes and emotional satisfaction by comparing outcomes between patients with and without patella baja and comparing septic and aseptic TKAs. In addition, there was no standard method for quantifying difficulty of revision, which would have enabled us to compare difficulty of revision in patients with patella baja.
Conclusion
This study identified a high rate of patella baja and decreased ROM in TKA revisions, particularly infected revisions treated with a nonarticulating spacer. It is important to determine if there are functional consequences. Further investigation is needed regarding the cause, prevention, and management of this potentially debilitating outcome after revision TKA.
Patellar height may be important in determining function after total knee arthroplasty (TKA). By altering patellofemoral joint mechanics, patella baja may cause several functional issues after TKA.1-8 Patella baja leads to decreased range of motion (ROM) affecting both extension and flexion.5,8,9 Deep flexion can be restricted in TKA patients with patella baja because of tracking limitations associated with an inferiorly displaced patella. As the knee is brought into flexion, the patella can impinge on the anterior aspect of the tibial polyethylene or the tibial tray—presenting a true block to flexion and potentially altering wear.1,10
Another functional issue with patella baja is loss of strength in the extensor mechanism. The patella serves as a fulcrum for the extensor muscles of the knee. When positioned properly and functioning properly, the patella increases the extensor forces generated. When the patella is positioned in baja, the knee generates decreased extensor mechanism force.6,7 This can result in a lag, with the patient being unable to fully extend the knee. Extension-dependent activities are impaired. Patients with weak extensor function can experience poor function with stair climbing, rising from a chair, and exiting an automobile. The improper function and scarring of the patella can result in increased anterior knee pain and worse functional outcome scores after TKAs.3,9
An abnormally positioned patella can either result from or lead to increased scarring in the knee.9,11 Patellar height is often measured with the Insall-Salvati ratio (ISR), which is the patella tendon length (measurement of the tendon from the tibial tubercle to the inferior pole of the patella) divided by the patellar length (longest measured dimension of the patella) (Figure 1).12 Patella baja is defined as an ISR of less than 0.8. Other indices that reference off the tibial plateau (Blackburne-Peel ratio, Canton-Deschamps ratio) reflect an elevation of the joint line, or pseudobaja, and are unreliable for analysis of patella baja after TKA.13
Postoperative patella baja has been reported in 10% to 34% of primary TKAs.4,7 Inferior positioning of the patella and scarring can cause intraoperative difficulty with exposure and may complicate outcomes.9,13 The exposure scar is often larger in TKA revisions for infection compared with primary TKAs.
We conducted a study to compare the incidence of patella baja in noninfected and infected TKA revisions. We hypothesized that, compared with noninfected knees, infected knees treated with nonarticulating spacers would have a higher incidence of patella baja both before and after surgery secondary to more inflammation, immobilization, and related scarring.
Materials and Methods
We conducted a retrospective case–cohort study of 148 consecutive TKA revisions. All TKA revisions were performed between 2003 and 2009 using a mobile-bearing revision system from a single manufacturer. All surgeries were done at a single institution by the 2 senior surgeons. The surgical approach was a standard medial parapatellar approach without patellar eversion. Our institutional review board approved the study and waived the requirement for informed consent, as this was a retrospective study of existing medical records that posed no more than minimal risk to patients.
To properly evaluate patellar height, orthopedic specialty–trained radiologic technicians obtained preoperative and postoperative weight-bearing radiographs using a standardized lateral radiograph in clinic. Two blinded investigators measured ISR radiographically both before surgery (preexplant for septic revisions) and at latest follow-up (postreplant for septic revisions). Patients with inadequate films and/or patellectomies were excluded, along with patients who had less than 6 months of postoperative follow-up.
Ninety-one patients (101 TKAs) met the study inclusion criteria. Two groups of cases were compared: aseptic revisions (n = 67) and septic revisions (n = 34). Reasons for aseptic revisions included implant loosening (24/67, 35.8%), instability (12/67, 17.9%), pain (12/67, 17.9%), lysis (5/67, 7.5%), stiffness (3/67, 4.5%), and malrotation (2/67, 3.0%). Infection was determined by Musculoskeletal Infection Society criteria, as documented by positive aspirations and/or intraoperative tissue cultures taken at prosthesis explantation, elevated white blood cell count in the aspirate, elevated percentage of polymorphonuclear (PMN) cells in the aspirate, gross purulence, presence of chronic draining sinus, or histologic analysis revealing acute inflammation with more than 5 PMN cells per high power field.14,15
All infected TKAs were treated with 2-stage revisions. The standard of care at our institution through this series was to use a nonarticulating spacer for the treatment of infection. Weight-bearing status varied by extent of bone damage. Six weeks of culture-specific intravenous antibiotics were administered with assistance from an infectious disease consultant. Reimplantation was performed when clinical and laboratory criteria for resolution of infection were met—specifically, when erythrocyte sedimentation rate was less than 30 mm/h, C-reactive protein level was less than 10 mg/L, and aspirates were culture-negative. Mean (range) follow-up was 33.9 (6.2-75.7) months for aseptic revisions and 32.3 (7.5-94.2) months for septic revisions. Radiographic follow-up was performed at each visit, with weight-bearing anteroposterior and posteroanterior views, along with a lateral knee radiograph. At final follow-up, ROM was recorded by the senior attending evaluating the patient.
Categorical variables were statistically analyzed with χ2 tests, and continuous variables were analyzed with Student t test, analysis of variance, and univariate analysis of covariance (ANCOVA). Statistical significance was set at P < .05. Intrarater reliability was measured with the intraclass correlation coefficient (ICC). All statistical analysis was performed with Predictive Analytics SoftWare Statistics Version 20.0 (SPSS, Chicago, Illinois).
Results
Ninety-one consecutive patients (43 men, 48 women) were included in this study. Mean (SD) age was 66.4 (10.1) years. Mean (SD) preoperative ISR in septic and aseptic cases was 0.94 (0.25) for men and 1.02 (0.23) for women (P = .10). Mean postoperative ISR in septic and aseptic cases was 0.84 (0.27) for men and 0.99 (0.23) for women (P = .004). There was a sex difference between septic and aseptic revisions. There were 22 men and 36 women in the aseptic group and 21 men and 12 women in the septic group (P = .01). Men were more likely than women to have septic revisions and patella baja. Table 1 compares the patient demographics of the 2 patient populations. Mean (SD) number of surgeries, including irrigation and débridement procedures before reimplantation, was larger for septic revisions, 2.9 (0.9), than for aseptic revisions, 1.4 (0.8) (P < .001).
Infection was the most common reason for revision and accounted for 33.7% (34/101) of all revisions. Noninfectious indications, in declining order of frequency, included loosening (23.8%, 24/101), instability (11.9%, 12/101), pain (11.9%, 12/101), osteolysis (5.0%, 5/101), polyethylene wear (5.0%, 5/101), failed unicompartmental knee (4.0%, 4/101), stiffness (3.0%, 3/101), and patellar problems (2.0%, 2/101) (Table 2). ISR decreased significantly only in infected revisions. It is important to note that there was not a high incidence of stiffness or patellofemoral failure in revision patients before surgery.
Mean (SD) ISR did not differ between groups before surgery, 1.00 (0.25) for aseptic and 0.96 (0.22) for septic (P = .49), but differed significantly after surgery, 0.99 (0.23) for aseptic and 0.77 (0.24) for septic (P < .001) (Figure 2). The univariate ANCOVA also demonstrated a postoperative difference between groups when taking the preoperative ratio into account: 0.99 (0.23) for aseptic and 0.78 (0.24) for septic (P = .005) (Table 3). Before surgery, 22.4% and 23.9% of the aseptic and septic groups, respectively, had patella baja (P = .58). After surgery, 17.6% and 58.8% of the aseptic and septic groups had patella baja (P = .001) (Table 4). The ICC for preoperative ISR was 0.94, and the ICC for postoperative ISR was 0.96, which indicates excellent agreement of measurements between the 2 blinded investigators.
ROM differed between septic and aseptic groups owing to the difference in postoperative flexion. Mean (SD) postoperative extension was 2.2° (5.4°) for the aseptic group and 5.1° (9.8°) for the septic group—not significantly different (P = .13). Mean (SD) postoperative flexion was 110.2° (18.8°) for the aseptic group and 97.2° (29.4°) for the septic group—significantly different (P = .02). The groups differed significantly (P = .02) in mean (SD) ROM: 108.0° (20.7°) for aseptic and 92.2° (34.6°) for septic (Table 1). ROM was also significantly associated with patella baja (P = .04), as patients with ISR of less than 0.8 had mean (SD) postoperative ROM of 95.1° (31.6°), and patients without patella baja had mean (SD) postoperative ROM of 106.8° (23.6°).
For the septic group, mean (SD) time between first and second stages was 13.0 (8.3) weeks (range, 1-44.3 weeks). Mean (SD) timing of spacer placement was not statistically significantly different (P = .90) between patients who had patella baja, 12.9 (8.8) weeks, and patients who did not have patella baja, 13.2 (7.8) weeks.
Discussion
This study demonstrated that TKAs done for septic reasons resulted in a higher incidence of patella baja and decreased ROM. Incidence of patella baja was higher both before and after revision in septic TKAs than in aseptic TKAs, proving the hypothesis under study. Prerevision incidence was not significantly different, but there was a trend that could not be ignored. This may suggest that there is already an ongoing process in the infected knee that contributes to patella baja; the precise etiology remains unclear and is likely multifactorial. For example, scar formation may be increased in patients with chronic infection, predisposing to patella baja. This assertion is indirectly supported by a recent study from our institution revealing longer average surgical time in septic versus aseptic knee revisions; the difference was thought to reflect increased scar-tissue formation.16 That study also found that patients who underwent septic revisions had significantly more surgical procedures than patients who underwent aseptic revisions. Repetitive surgeries—specifically, repetitive arthrotomies during irrigation and débridement before reimplantation—lead to increased scar formation, which may contribute to preoperative and postoperative patella baja. This may be reflected in the findings that ROM was decreased in patients in the septic group versus patients in the aseptic group and that ROM was decreased in patients with patella baja. In addition, our study found that male patients were more likely to undergo TKA revision for septic reasons and to develop postoperative patella baja. This finding contrasts with that of a study5 that compared preoperative and postoperative ISR in primary TKA and found that women were more likely than men to have patella baja. Although women are more likely to undergo TKA revision,17 men may be more susceptible to infection and subsequent patella baja.
The higher postoperative rate of patella baja in the septic group became statistically significant even when preoperative incidence was considered. This may have been caused by infection-related scarring and by prolonged immobilization of septic knees with use of nonarticulating antibiotic spacers. By keeping these knees immobile with a nonarticulating spacer for a prolonged period in the healing phase of the infection, scar tissue may mature and form over the time between stages. A comparable example may be high tibial osteotomies, in which a high incidence of patella baja has been partly attributed to prolonged casting.11 Future work comparing the results of articulating and nonarticulating spacers will help to determine if immobilization contributes to patella baja in infected TKAs.
There are several limitations to our study. Patient outcome questionnaires were not used, and they would have allowed for the assessment of physical outcomes and emotional satisfaction by comparing outcomes between patients with and without patella baja and comparing septic and aseptic TKAs. In addition, there was no standard method for quantifying difficulty of revision, which would have enabled us to compare difficulty of revision in patients with patella baja.
Conclusion
This study identified a high rate of patella baja and decreased ROM in TKA revisions, particularly infected revisions treated with a nonarticulating spacer. It is important to determine if there are functional consequences. Further investigation is needed regarding the cause, prevention, and management of this potentially debilitating outcome after revision TKA.
1. Aglietti P, Buzzi R, Gaudenzi A. Patello-femoral functional results and complications with the posterior stabilised total condylar knee prosthesis. J Arthroplasty. 1988;3(1):17-25.
2. Fern ED, Winson IG, Getty CJM. Anterior knee pain in rheumatoid patients after total knee replacement: possible selection criteria for patellar resurfacing. J Bone Joint Surg Br. 1992;74(5):745-748.
3. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Surg Surg Am. 1986;68(7):1035-1040.
4. Floren M, Davis J, Peterson MG, Laskin RS. A mini-midvastus capsular approach with patellar displacement decreases the prevalence of patellar baja. J Arthroplasty. 2007;22(6 Suppl 2):51-57.
5. Meneghini RM, Ritter MA, Pierson JL, Meding JB, Berend ME, Faris PM. The effect of the Insall-Salvati ratio on outcome after total knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):116-120.
6. Singerman R, Davy DT, Goldberg VM. Effects of patella alta and patella infera on patellofemoral contact forces. J Biomech. 1994;27(8):1059-1065.
7. Van Eijden TM, Kouwenhoven E, Weijs WA. Mechanics of the patellar articulation: effects of patellar ligament length studied with a mathematical model. Acta Orthop Scand. 1987;58(5):560-566.
8. Weale AE, Murray DW, Newman JH, Ackroyd CE. The length of the patellar tendon after unicompartmental and total knee replacement. J Bone Joint Surg Br. 1999;81(5):790-795.
9. Chonko DJ, Lombardi AV Jr, Berend KR. Patella baja and total knee arthroplasty (TKA): etiology, diagnosis, and management. Surg Technol Int. 2004;12:231-238.
10. Cameron HU, Jung YB. Patella baja complicating total knee arthroplasty. A report of two cases. J Arthroplasty. 1988;3(2):177-180.
11. Scuderi GR, Windsor RE, Insall JN. Observations on patellar height after proximal tibial osteotomy. J Bone Joint Surg Am. 1989;71(2):245-248.
12. Insall JN, Salvati E. Patella position in the normal knee joint. Radiology. 1971;101(1):101-104.
13. Grelsamer RP. Patella baja after total knee arthroplasty: is it really patella baja? J Arthroplasty. 2002;17(1):66-69.
14. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
15. Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty. 2011;26(8):1136-1138.
16. Laudermilch DJ, Fedorka CJ, Heyl A, Rao N, McGough RL. Outcomes of revision total knee arthroplasty after methicillin-resistant Staphylococcus aureus infection. Clin Orthop. 2010;468(8):2067-2073.
17. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop. 2010;468(1):45-51.
1. Aglietti P, Buzzi R, Gaudenzi A. Patello-femoral functional results and complications with the posterior stabilised total condylar knee prosthesis. J Arthroplasty. 1988;3(1):17-25.
2. Fern ED, Winson IG, Getty CJM. Anterior knee pain in rheumatoid patients after total knee replacement: possible selection criteria for patellar resurfacing. J Bone Joint Surg Br. 1992;74(5):745-748.
3. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Surg Surg Am. 1986;68(7):1035-1040.
4. Floren M, Davis J, Peterson MG, Laskin RS. A mini-midvastus capsular approach with patellar displacement decreases the prevalence of patellar baja. J Arthroplasty. 2007;22(6 Suppl 2):51-57.
5. Meneghini RM, Ritter MA, Pierson JL, Meding JB, Berend ME, Faris PM. The effect of the Insall-Salvati ratio on outcome after total knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):116-120.
6. Singerman R, Davy DT, Goldberg VM. Effects of patella alta and patella infera on patellofemoral contact forces. J Biomech. 1994;27(8):1059-1065.
7. Van Eijden TM, Kouwenhoven E, Weijs WA. Mechanics of the patellar articulation: effects of patellar ligament length studied with a mathematical model. Acta Orthop Scand. 1987;58(5):560-566.
8. Weale AE, Murray DW, Newman JH, Ackroyd CE. The length of the patellar tendon after unicompartmental and total knee replacement. J Bone Joint Surg Br. 1999;81(5):790-795.
9. Chonko DJ, Lombardi AV Jr, Berend KR. Patella baja and total knee arthroplasty (TKA): etiology, diagnosis, and management. Surg Technol Int. 2004;12:231-238.
10. Cameron HU, Jung YB. Patella baja complicating total knee arthroplasty. A report of two cases. J Arthroplasty. 1988;3(2):177-180.
11. Scuderi GR, Windsor RE, Insall JN. Observations on patellar height after proximal tibial osteotomy. J Bone Joint Surg Am. 1989;71(2):245-248.
12. Insall JN, Salvati E. Patella position in the normal knee joint. Radiology. 1971;101(1):101-104.
13. Grelsamer RP. Patella baja after total knee arthroplasty: is it really patella baja? J Arthroplasty. 2002;17(1):66-69.
14. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
15. Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty. 2011;26(8):1136-1138.
16. Laudermilch DJ, Fedorka CJ, Heyl A, Rao N, McGough RL. Outcomes of revision total knee arthroplasty after methicillin-resistant Staphylococcus aureus infection. Clin Orthop. 2010;468(8):2067-2073.
17. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop. 2010;468(1):45-51.
Midfoot Sprains in the National Football League
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
Ischiofemoral Impingement and the Utility of Full-Range-of-Motion Magnetic Resonance Imaging in Its Detection
With the first cases described in 1977, ischiofemoral impingement (IFI) is a relatively recently discovered and less known potential cause of hip pain caused by compression on the quadratus femoris muscle (QFM).1-10 These first patients, who were treated with surgical excision of the lesser trochanter, experienced symptom improvement in all 3 cases.5,7 The most widely accepted diagnostic criteria use a combination of clinical and imaging findings.1-10 Criteria most often cited in the literature include isolated edema-like signal in the QFM on magnetic resonance imaging (MRI) and ipsilateral hip pain without a known cause, such as recent trauma or infection.4,5 All studies describe QFM compression occurring as the muscle passes between the lesser trochanter of the femur and the origin of the ischial tuberosity/hamstring tendons.1-10
Several authors have sought to improve diagnostic accuracy by providing various measurements to quantify the probability of impingement.5,7,9 Although groups have proposed different thresholds, our institution currently uses values reported by Tosun and colleagues5 because theirs is the most robust sample size to date and included 50 patients with IFI.7,9 Although 5 different measurements were proposed, 2 are more commonly cited. The first is the ischiofemoral space (IFS), which is the most narrow distance between the cortex of the lesser trochanter and the cortex of the ischial tuberosity. This space should normally be greater than 1.8 cm.5 The second measurement is called the quadratus femoris space (QFS) and is the most narrow distance between the hamstring tendons and either the iliopsoas tendon or the cortex of the lesser trochanter. The QFS should normally be greater than 1.0 cm.5 However, because these measurements may depend on the hip position during imaging, full-range-of-motion (FROM) MRI may increase diagnostic yield. At our institution, patients are usually imaged supine in neutral position (with respect to internal or external rotation).
In this article, we briefly review IFI, provide an example of how FROM MRI can improve diagnostic accuracy, describe our FROM protocol, and propose an expanded definition of the impingement criteria. The patient provided written informed consent for print and electronic publication of the case details and images.
Full–Range-of-Motion MRI Technique
A 58-year-old woman with no surgical history or diagnosed inflammatory arthropathy presented to the department of physical medicine and rehabilitation with left-buttock pain radiating down the left thigh. Despite nonsurgical management with nonsteroidal anti-inflammatory medication, exercise therapy, use of a transcutaneous electrical nerve stimulator unit, and oral corticosteroid therapy, the pain continued. The patient was referred for MRI, and routine static imaging of the pelvis was performed. Although edema-like signal was present in both QFMs (Figure 1), left more than right, the measurement of the QFS and IFS did not meet all criteria for narrowing as described in previous studies. On the symptomatic left side, the IFS measured 1.5 cm and the QFS measured 1.4 cm (Figure 2). On the same side, the distance between the cortex of the greater trochanter and the cortex of the ischial tuberosity, proposed adapted IFS, measured 1.4 cm, and the distance between the cortex of the greater trochanter and the hamstring tendons origin, proposed adapted QFS, measured 1.1 cm (Figure 3). However, because of the isolated QFM edema, refractory buttock and thigh pain, and exclusion of other diagnoses (such as labral tear, bone marrow edema/stress reaction in the hip, or MRI findings of sciatic neuropathy), we determined that the patient needed evaluation of the QFS and the IFS through a full range of motion. The patient returned for the FROM MRI 16 days after the initial static MRI.
Our FROM MRI was performed on a Magnetom Skyra 3 Tesla magnet (Seimens Healthcare Global, Munich, Germany), using a body array 18-channel coil and a table spine coil. In a supine position, the patient’s imaging started with the hip in extension, adduction, and approximately 20º of internal rotation. During imaging acquisition, the patient was maintained in adduction and extension while the hip was passively externally rotated (Figure 3). A technologist assisted the patient in maintaining the position through a 60º arc of external rotation, while an axial-gradient echo sequence was used to obtain sequential images through the entire arc. Selected parameters are listed in the Table. Acquisition of the arc of motion in the axial plane requires approximately 3 minutes per hip to generate between 8 and 10 images.
With the patient’s hip in internal rotation, narrowing between the ischium or hamstring tendons and the lesser trochanter did not meet all of the criteria described by Tosun and colleagues5 or Torriani and colleagues.7 However, when the patient shifted into external rotation, the distance between the ischial tuberosity and the greater trochanter, and between the hamstring tendons origin and the greater trochanter, significantly narrowed. The adapted IFS decreased from 3.4 cm to 1.5 cm, and the adapted QFS decreased from 3.2 cm to 0.9 cm, accounting for a 54% and 72% reduction of the adapted IFS and QFS, respectively, with maximum external rotation (Figures 4, 5).
Discussion
While femoroacetabular impingement is a widely recognized and sometimes surgically treated syndrome, IFI may be overlooked as a cause of hip pain. Although IFI is traditionally described as mass effect on the QFM by the ischium/hamstring tendons origin and the lesser trochanter, we propose expansion of this criteria to include narrowing resulting from the greater trochanter in external rotation as a potential source of impingement. By use of FROM MRI, we adapted measurements previously described for IFI to evaluate for compression of the QFM by adjacent osseous and tendinous structures throughout the full range of internal/external hip rotation. In this case, FROM imaging provided evidence of possible anatomical narrowing caused by the greater trochanter, in addition to that caused by the lesser trochanter. Given that impingement may be caused by either the greater or lesser trochanters, it is prudent to perform FROM MRI in evaluating patients with suspected IFI. If FROM imaging is not feasible, static imaging in both maximal internal and external rotation may allow for better assessment. There have been no large studies conducted to assess the normal interval between the ischial tuberosity/hamstring origins and the greater trochanter.
The purpose of this report is to call attention to a source of impingement that may be undetected with static MRI, possibly leading to a missed diagnosis. While we believe this to be the first reported example of impingement involving the greater trochanter, larger studies should be conducted to explore this possible source of impingement. Information about the incidence of greater trochanteric impingement could lead to changes in our understanding of this syndrome and its management.
1. Lee S, Kim I, Lee SM, Lee J. Ischiofemoral impingement syndrome. Ann Rehabil Med. 2013;37(1):143-146.
2. Sussman WI, Han E, Schuenke MD. Quantitative assessment of the ischiofemoral space and evidence of degenerative changes in the quadratus femoris muscle. Surg Radiol Anat. 2013;35(4):273-281.
3. López-Sánchez MC, Armesto Pérez V, Montero Furelos LÁ, Vázquez-Rodríguez TR, Calvo Arrojo G, Díaz Román TM. Ischiofemoral impingement: hip pain of infrequent cause. Ischiofemoral impingement: hip pain of infrequent cause. Rheumatol Clin. 2013;9(3):186-187.
4. Viala P, Vanel D, Larbi A, Cyteval C, Laredo JD. Bilateral ischiofemoral impingement in a patient with hereditary multiple exostoses. Skeletal Radiol. 2012;41(12):1637-1640.
5. Tosun O, Algin O, Yalcin N, Cay N, Ocakoglu G, Karaoglanoglu M. Ischiofemoral impingement: evaluation with new MRI parameters and assessment of their reliability. Skeletal Radiol. 2012;41(5):575-587.
6. Ali AM, Whitwell D, Ostlere SJ. Case report: imaging and surgical treatment of a snapping hip due to ischiofemoral impingement. Skeletal Radiol. 2011;40(5):653-656.
7. Torriani M, Souto SC, Thomas BJ, Ouellette H, Bredella MA. Ischiofemoral impingement syndrome: an entity with hip pain and abnormalities of the quadratus femoris muscle. AJR Am J Roentgenol. 2009;193(1):186-190.
8. Ali AM, Teh J, Whitwell D, Ostlere S. Ischiofemoral impingement: a retrospective analysis of cases in a specialist orthopaedic centre over a four-year period. Hip Int. 2013;3(23):263-268.
9. Sussman WI, Han E, Schuenke MD. Quantitative assessment of the ischiofemoral space and evidence of degenerative changes in the quadratus femoris muscle. Surg Radiol Anat. 2013;35(4):273-281.
10. Kassarjian A. Signal abnormalities in the quadratus femoris muscle: tear or impingement? AJR Am J Roentgenol. 2008;190(6):W379.
With the first cases described in 1977, ischiofemoral impingement (IFI) is a relatively recently discovered and less known potential cause of hip pain caused by compression on the quadratus femoris muscle (QFM).1-10 These first patients, who were treated with surgical excision of the lesser trochanter, experienced symptom improvement in all 3 cases.5,7 The most widely accepted diagnostic criteria use a combination of clinical and imaging findings.1-10 Criteria most often cited in the literature include isolated edema-like signal in the QFM on magnetic resonance imaging (MRI) and ipsilateral hip pain without a known cause, such as recent trauma or infection.4,5 All studies describe QFM compression occurring as the muscle passes between the lesser trochanter of the femur and the origin of the ischial tuberosity/hamstring tendons.1-10
Several authors have sought to improve diagnostic accuracy by providing various measurements to quantify the probability of impingement.5,7,9 Although groups have proposed different thresholds, our institution currently uses values reported by Tosun and colleagues5 because theirs is the most robust sample size to date and included 50 patients with IFI.7,9 Although 5 different measurements were proposed, 2 are more commonly cited. The first is the ischiofemoral space (IFS), which is the most narrow distance between the cortex of the lesser trochanter and the cortex of the ischial tuberosity. This space should normally be greater than 1.8 cm.5 The second measurement is called the quadratus femoris space (QFS) and is the most narrow distance between the hamstring tendons and either the iliopsoas tendon or the cortex of the lesser trochanter. The QFS should normally be greater than 1.0 cm.5 However, because these measurements may depend on the hip position during imaging, full-range-of-motion (FROM) MRI may increase diagnostic yield. At our institution, patients are usually imaged supine in neutral position (with respect to internal or external rotation).
In this article, we briefly review IFI, provide an example of how FROM MRI can improve diagnostic accuracy, describe our FROM protocol, and propose an expanded definition of the impingement criteria. The patient provided written informed consent for print and electronic publication of the case details and images.
Full–Range-of-Motion MRI Technique
A 58-year-old woman with no surgical history or diagnosed inflammatory arthropathy presented to the department of physical medicine and rehabilitation with left-buttock pain radiating down the left thigh. Despite nonsurgical management with nonsteroidal anti-inflammatory medication, exercise therapy, use of a transcutaneous electrical nerve stimulator unit, and oral corticosteroid therapy, the pain continued. The patient was referred for MRI, and routine static imaging of the pelvis was performed. Although edema-like signal was present in both QFMs (Figure 1), left more than right, the measurement of the QFS and IFS did not meet all criteria for narrowing as described in previous studies. On the symptomatic left side, the IFS measured 1.5 cm and the QFS measured 1.4 cm (Figure 2). On the same side, the distance between the cortex of the greater trochanter and the cortex of the ischial tuberosity, proposed adapted IFS, measured 1.4 cm, and the distance between the cortex of the greater trochanter and the hamstring tendons origin, proposed adapted QFS, measured 1.1 cm (Figure 3). However, because of the isolated QFM edema, refractory buttock and thigh pain, and exclusion of other diagnoses (such as labral tear, bone marrow edema/stress reaction in the hip, or MRI findings of sciatic neuropathy), we determined that the patient needed evaluation of the QFS and the IFS through a full range of motion. The patient returned for the FROM MRI 16 days after the initial static MRI.
Our FROM MRI was performed on a Magnetom Skyra 3 Tesla magnet (Seimens Healthcare Global, Munich, Germany), using a body array 18-channel coil and a table spine coil. In a supine position, the patient’s imaging started with the hip in extension, adduction, and approximately 20º of internal rotation. During imaging acquisition, the patient was maintained in adduction and extension while the hip was passively externally rotated (Figure 3). A technologist assisted the patient in maintaining the position through a 60º arc of external rotation, while an axial-gradient echo sequence was used to obtain sequential images through the entire arc. Selected parameters are listed in the Table. Acquisition of the arc of motion in the axial plane requires approximately 3 minutes per hip to generate between 8 and 10 images.
With the patient’s hip in internal rotation, narrowing between the ischium or hamstring tendons and the lesser trochanter did not meet all of the criteria described by Tosun and colleagues5 or Torriani and colleagues.7 However, when the patient shifted into external rotation, the distance between the ischial tuberosity and the greater trochanter, and between the hamstring tendons origin and the greater trochanter, significantly narrowed. The adapted IFS decreased from 3.4 cm to 1.5 cm, and the adapted QFS decreased from 3.2 cm to 0.9 cm, accounting for a 54% and 72% reduction of the adapted IFS and QFS, respectively, with maximum external rotation (Figures 4, 5).
Discussion
While femoroacetabular impingement is a widely recognized and sometimes surgically treated syndrome, IFI may be overlooked as a cause of hip pain. Although IFI is traditionally described as mass effect on the QFM by the ischium/hamstring tendons origin and the lesser trochanter, we propose expansion of this criteria to include narrowing resulting from the greater trochanter in external rotation as a potential source of impingement. By use of FROM MRI, we adapted measurements previously described for IFI to evaluate for compression of the QFM by adjacent osseous and tendinous structures throughout the full range of internal/external hip rotation. In this case, FROM imaging provided evidence of possible anatomical narrowing caused by the greater trochanter, in addition to that caused by the lesser trochanter. Given that impingement may be caused by either the greater or lesser trochanters, it is prudent to perform FROM MRI in evaluating patients with suspected IFI. If FROM imaging is not feasible, static imaging in both maximal internal and external rotation may allow for better assessment. There have been no large studies conducted to assess the normal interval between the ischial tuberosity/hamstring origins and the greater trochanter.
The purpose of this report is to call attention to a source of impingement that may be undetected with static MRI, possibly leading to a missed diagnosis. While we believe this to be the first reported example of impingement involving the greater trochanter, larger studies should be conducted to explore this possible source of impingement. Information about the incidence of greater trochanteric impingement could lead to changes in our understanding of this syndrome and its management.
With the first cases described in 1977, ischiofemoral impingement (IFI) is a relatively recently discovered and less known potential cause of hip pain caused by compression on the quadratus femoris muscle (QFM).1-10 These first patients, who were treated with surgical excision of the lesser trochanter, experienced symptom improvement in all 3 cases.5,7 The most widely accepted diagnostic criteria use a combination of clinical and imaging findings.1-10 Criteria most often cited in the literature include isolated edema-like signal in the QFM on magnetic resonance imaging (MRI) and ipsilateral hip pain without a known cause, such as recent trauma or infection.4,5 All studies describe QFM compression occurring as the muscle passes between the lesser trochanter of the femur and the origin of the ischial tuberosity/hamstring tendons.1-10
Several authors have sought to improve diagnostic accuracy by providing various measurements to quantify the probability of impingement.5,7,9 Although groups have proposed different thresholds, our institution currently uses values reported by Tosun and colleagues5 because theirs is the most robust sample size to date and included 50 patients with IFI.7,9 Although 5 different measurements were proposed, 2 are more commonly cited. The first is the ischiofemoral space (IFS), which is the most narrow distance between the cortex of the lesser trochanter and the cortex of the ischial tuberosity. This space should normally be greater than 1.8 cm.5 The second measurement is called the quadratus femoris space (QFS) and is the most narrow distance between the hamstring tendons and either the iliopsoas tendon or the cortex of the lesser trochanter. The QFS should normally be greater than 1.0 cm.5 However, because these measurements may depend on the hip position during imaging, full-range-of-motion (FROM) MRI may increase diagnostic yield. At our institution, patients are usually imaged supine in neutral position (with respect to internal or external rotation).
In this article, we briefly review IFI, provide an example of how FROM MRI can improve diagnostic accuracy, describe our FROM protocol, and propose an expanded definition of the impingement criteria. The patient provided written informed consent for print and electronic publication of the case details and images.
Full–Range-of-Motion MRI Technique
A 58-year-old woman with no surgical history or diagnosed inflammatory arthropathy presented to the department of physical medicine and rehabilitation with left-buttock pain radiating down the left thigh. Despite nonsurgical management with nonsteroidal anti-inflammatory medication, exercise therapy, use of a transcutaneous electrical nerve stimulator unit, and oral corticosteroid therapy, the pain continued. The patient was referred for MRI, and routine static imaging of the pelvis was performed. Although edema-like signal was present in both QFMs (Figure 1), left more than right, the measurement of the QFS and IFS did not meet all criteria for narrowing as described in previous studies. On the symptomatic left side, the IFS measured 1.5 cm and the QFS measured 1.4 cm (Figure 2). On the same side, the distance between the cortex of the greater trochanter and the cortex of the ischial tuberosity, proposed adapted IFS, measured 1.4 cm, and the distance between the cortex of the greater trochanter and the hamstring tendons origin, proposed adapted QFS, measured 1.1 cm (Figure 3). However, because of the isolated QFM edema, refractory buttock and thigh pain, and exclusion of other diagnoses (such as labral tear, bone marrow edema/stress reaction in the hip, or MRI findings of sciatic neuropathy), we determined that the patient needed evaluation of the QFS and the IFS through a full range of motion. The patient returned for the FROM MRI 16 days after the initial static MRI.
Our FROM MRI was performed on a Magnetom Skyra 3 Tesla magnet (Seimens Healthcare Global, Munich, Germany), using a body array 18-channel coil and a table spine coil. In a supine position, the patient’s imaging started with the hip in extension, adduction, and approximately 20º of internal rotation. During imaging acquisition, the patient was maintained in adduction and extension while the hip was passively externally rotated (Figure 3). A technologist assisted the patient in maintaining the position through a 60º arc of external rotation, while an axial-gradient echo sequence was used to obtain sequential images through the entire arc. Selected parameters are listed in the Table. Acquisition of the arc of motion in the axial plane requires approximately 3 minutes per hip to generate between 8 and 10 images.
With the patient’s hip in internal rotation, narrowing between the ischium or hamstring tendons and the lesser trochanter did not meet all of the criteria described by Tosun and colleagues5 or Torriani and colleagues.7 However, when the patient shifted into external rotation, the distance between the ischial tuberosity and the greater trochanter, and between the hamstring tendons origin and the greater trochanter, significantly narrowed. The adapted IFS decreased from 3.4 cm to 1.5 cm, and the adapted QFS decreased from 3.2 cm to 0.9 cm, accounting for a 54% and 72% reduction of the adapted IFS and QFS, respectively, with maximum external rotation (Figures 4, 5).
Discussion
While femoroacetabular impingement is a widely recognized and sometimes surgically treated syndrome, IFI may be overlooked as a cause of hip pain. Although IFI is traditionally described as mass effect on the QFM by the ischium/hamstring tendons origin and the lesser trochanter, we propose expansion of this criteria to include narrowing resulting from the greater trochanter in external rotation as a potential source of impingement. By use of FROM MRI, we adapted measurements previously described for IFI to evaluate for compression of the QFM by adjacent osseous and tendinous structures throughout the full range of internal/external hip rotation. In this case, FROM imaging provided evidence of possible anatomical narrowing caused by the greater trochanter, in addition to that caused by the lesser trochanter. Given that impingement may be caused by either the greater or lesser trochanters, it is prudent to perform FROM MRI in evaluating patients with suspected IFI. If FROM imaging is not feasible, static imaging in both maximal internal and external rotation may allow for better assessment. There have been no large studies conducted to assess the normal interval between the ischial tuberosity/hamstring origins and the greater trochanter.
The purpose of this report is to call attention to a source of impingement that may be undetected with static MRI, possibly leading to a missed diagnosis. While we believe this to be the first reported example of impingement involving the greater trochanter, larger studies should be conducted to explore this possible source of impingement. Information about the incidence of greater trochanteric impingement could lead to changes in our understanding of this syndrome and its management.
1. Lee S, Kim I, Lee SM, Lee J. Ischiofemoral impingement syndrome. Ann Rehabil Med. 2013;37(1):143-146.
2. Sussman WI, Han E, Schuenke MD. Quantitative assessment of the ischiofemoral space and evidence of degenerative changes in the quadratus femoris muscle. Surg Radiol Anat. 2013;35(4):273-281.
3. López-Sánchez MC, Armesto Pérez V, Montero Furelos LÁ, Vázquez-Rodríguez TR, Calvo Arrojo G, Díaz Román TM. Ischiofemoral impingement: hip pain of infrequent cause. Ischiofemoral impingement: hip pain of infrequent cause. Rheumatol Clin. 2013;9(3):186-187.
4. Viala P, Vanel D, Larbi A, Cyteval C, Laredo JD. Bilateral ischiofemoral impingement in a patient with hereditary multiple exostoses. Skeletal Radiol. 2012;41(12):1637-1640.
5. Tosun O, Algin O, Yalcin N, Cay N, Ocakoglu G, Karaoglanoglu M. Ischiofemoral impingement: evaluation with new MRI parameters and assessment of their reliability. Skeletal Radiol. 2012;41(5):575-587.
6. Ali AM, Whitwell D, Ostlere SJ. Case report: imaging and surgical treatment of a snapping hip due to ischiofemoral impingement. Skeletal Radiol. 2011;40(5):653-656.
7. Torriani M, Souto SC, Thomas BJ, Ouellette H, Bredella MA. Ischiofemoral impingement syndrome: an entity with hip pain and abnormalities of the quadratus femoris muscle. AJR Am J Roentgenol. 2009;193(1):186-190.
8. Ali AM, Teh J, Whitwell D, Ostlere S. Ischiofemoral impingement: a retrospective analysis of cases in a specialist orthopaedic centre over a four-year period. Hip Int. 2013;3(23):263-268.
9. Sussman WI, Han E, Schuenke MD. Quantitative assessment of the ischiofemoral space and evidence of degenerative changes in the quadratus femoris muscle. Surg Radiol Anat. 2013;35(4):273-281.
10. Kassarjian A. Signal abnormalities in the quadratus femoris muscle: tear or impingement? AJR Am J Roentgenol. 2008;190(6):W379.
1. Lee S, Kim I, Lee SM, Lee J. Ischiofemoral impingement syndrome. Ann Rehabil Med. 2013;37(1):143-146.
2. Sussman WI, Han E, Schuenke MD. Quantitative assessment of the ischiofemoral space and evidence of degenerative changes in the quadratus femoris muscle. Surg Radiol Anat. 2013;35(4):273-281.
3. López-Sánchez MC, Armesto Pérez V, Montero Furelos LÁ, Vázquez-Rodríguez TR, Calvo Arrojo G, Díaz Román TM. Ischiofemoral impingement: hip pain of infrequent cause. Ischiofemoral impingement: hip pain of infrequent cause. Rheumatol Clin. 2013;9(3):186-187.
4. Viala P, Vanel D, Larbi A, Cyteval C, Laredo JD. Bilateral ischiofemoral impingement in a patient with hereditary multiple exostoses. Skeletal Radiol. 2012;41(12):1637-1640.
5. Tosun O, Algin O, Yalcin N, Cay N, Ocakoglu G, Karaoglanoglu M. Ischiofemoral impingement: evaluation with new MRI parameters and assessment of their reliability. Skeletal Radiol. 2012;41(5):575-587.
6. Ali AM, Whitwell D, Ostlere SJ. Case report: imaging and surgical treatment of a snapping hip due to ischiofemoral impingement. Skeletal Radiol. 2011;40(5):653-656.
7. Torriani M, Souto SC, Thomas BJ, Ouellette H, Bredella MA. Ischiofemoral impingement syndrome: an entity with hip pain and abnormalities of the quadratus femoris muscle. AJR Am J Roentgenol. 2009;193(1):186-190.
8. Ali AM, Teh J, Whitwell D, Ostlere S. Ischiofemoral impingement: a retrospective analysis of cases in a specialist orthopaedic centre over a four-year period. Hip Int. 2013;3(23):263-268.
9. Sussman WI, Han E, Schuenke MD. Quantitative assessment of the ischiofemoral space and evidence of degenerative changes in the quadratus femoris muscle. Surg Radiol Anat. 2013;35(4):273-281.
10. Kassarjian A. Signal abnormalities in the quadratus femoris muscle: tear or impingement? AJR Am J Roentgenol. 2008;190(6):W379.
Patient Safety: Innovation and Critical Thinking
Preventable medical errors rank as the third most common cause of death in the United States after heart disease and cancer.1 They are responsible for 400,000 deaths each year (over 1095 per day) and another 10,000 serious complications resulting from medical errors each day.1 That is the equivalent of two 747 airliner midair crashes per day. The economic cost to our nation is $1 trillion per year.1
On July 17, 2014, the US Senate Subcommittee on Primary Health and Aging met to address this crisis. Participants included senators and John James, PhD, Founder, Patient Safety America, Houston, Texas; Ashish Jha, MD, MPH, Professor of Health Policy and Management, Harvard School of Public Health, Boston, Massachusetts; Tejal Gandhi, MD, MPH, President, National Patient Safety Foundation, and Associate Professor of Medicine, Harvard Medical School, Boston, Massachusetts; Peter Pronovost, MD, PhD, Senior Vice President for Patient Safety and Quality, and Director of the Armstrong Institute for Patient Safety and Quality, Johns Hopkins Medicine, Baltimore, Maryland; Joanne Disch, PhD, RN, Professor ad Honorem, University of Minnesota School of Nursing, Minneapolis, Minnesota; and Lisa McGiffert, Director, Safe Patient Project, Consumers Union, Austin, Texas. While each speaker suggested various strategies for improving patient safety, they all agreed that information technology is not living up to our expectations for meeting this need. They also agreed that health care has become increasingly “high tech and low touch,” and, as a result, the medical community is leveraging neither technology nor the knowledge accrued from individual patient/physician interactions to improve patient safety and outcomes.1
Last year my mother had a spinal fusion. The surgery was a success by all measures. Two days after she was discharged home, she became weak and was unable to walk. She went to the emergency room, where it was noted that she was severely hyponatremic, weak, and experiencing severe back pain. For the next 36 hours she was not seen by a physician or physician assistant (PA), as the PA who admitted her to the hospital had not notified the “team” that she was admitted. My father, who is a vascular surgeon, notified her spine surgeon, who came to see her. Her hyponatremia was markedly worse, and she was transferred to the intensive care unit (ICU). She continued to decline and was started on hypertonic intravenous (IV) saline. Over the next several days her hyponatremia improved, and she was transferred out of the ICU but continued to have pain. The spine surgeon examined her several times, and imaging showed no evidence of epidural bleeding, infection, or misplaced hardware.
Over the next several days, I was informed by family members that the nurses were “keeping the pain in check” with IV narcotics and that my mom was heavily sedated most of the time. My dad later informed me that she had a foot drop on the left, and the next day another family member told me the foot drop was on the right. My dad and stepbrother each assured me that they were right. When my mom could talk, she told me how weak she was and that sometimes it was her right leg and other times her left. She was seen by a neurologist on 6 out of the next 10 days and underwent 3 computed tomography scans and magnetic resonance imaging, and the neurologist assured us that she had not had a stroke. On a Friday evening, I called my mom, who was progressively short of breath, and she told me that she felt weaker and weaker each day. The “foot drop,” which was now bilateral according to the neurologist, was from “not using it while she was in the ICU.”
My mom, who is an artist, commented that she was having trouble using her hands now and unable to hold a cup. I called the physician on call, who assured me that she was taking care of my mom’s blood pressure (which was labile for the first time ever; she had no history of hypertension) and her pain score was a 5. I explained that I knew that she was not “looking to play mystery diagnosis with an orthopedic surgeon 500 miles away, but I think my mom has Guillain-Barré syndrome.” Fortunately, the doctor said, “Oh my god, I think you’re right.” Monday morning, her diagnosis was confirmed and she has made a remarkable recovery. So how is it that she could be seen by a neurologist and a team of nurses, doctors, therapists, and resident staff and no one made a diagnosis? Certainly contributing factors include a system of multiple medical teams with frequent turnovers and a desire to consult others but no real “quarterback” who was looking at the overall care in a responsible and critical way. A thorough history and physical examination, rather than a multitude of expensive and unnecessary imaging studies, could certainly have led to a quicker diagnosis and avoidance of a protracted hospital stay and rehabilitation.
To be sure, there are many factors that lead to delays in diagnosis. The reliance on advanced imaging, the lack of a simple physical examination, and the lack of critical thinking played prominently in the failure to make a diagnosis in my mom’s case. Some would argue that we need information technology (IT) systems that will allow us to better diagnose and treat patients. They believe that with electronic medical records (EMRs) data points will be entered and a diagnosis will be made. Major corporations like IBM and GE are working to make this a reality. Although Watson (the artificially intelligent computer system created by IBM) may be able to win on Jeopardy and may move the needle forward to improving patient care, 2 things are certain: (1) Appropriate data will need to be input by people, and (2) without critical thinking, the appropriate data can’t be entered or interpreted correctly.
The fact remains that EMR has fallen short of expectations. We have more data at our fingertips but this has not translated into a significant improvement in patient safety. The human factor remains critical. Even though industry and health care workers strive to innovate and merge technological advances with improved patient outcomes, technology will continue to fall short of expectations without the input of critical thinking. There are things that computers and technological advances can do that people can’t, and there are things that people can do that computers can’t.
We cannot become a profession reliant on technology to substitute for critical thinking, and we cannot become a profession that doesn’t recognize what technology can bring to us and our patients. Like a railroad track that needs 2 parallel tracks to move trains, we must continue to build on 2 tracks: innovation and critical thinking. ◾
Reference
1. McCann E. Deaths by medical mistakes hit records. Healthcare IT News. http://www.healthcareitnews.com/news/deaths-by-medical-mistakes-hit-records. Published July 18, 2014. Accessed November 17, 2014.
Preventable medical errors rank as the third most common cause of death in the United States after heart disease and cancer.1 They are responsible for 400,000 deaths each year (over 1095 per day) and another 10,000 serious complications resulting from medical errors each day.1 That is the equivalent of two 747 airliner midair crashes per day. The economic cost to our nation is $1 trillion per year.1
On July 17, 2014, the US Senate Subcommittee on Primary Health and Aging met to address this crisis. Participants included senators and John James, PhD, Founder, Patient Safety America, Houston, Texas; Ashish Jha, MD, MPH, Professor of Health Policy and Management, Harvard School of Public Health, Boston, Massachusetts; Tejal Gandhi, MD, MPH, President, National Patient Safety Foundation, and Associate Professor of Medicine, Harvard Medical School, Boston, Massachusetts; Peter Pronovost, MD, PhD, Senior Vice President for Patient Safety and Quality, and Director of the Armstrong Institute for Patient Safety and Quality, Johns Hopkins Medicine, Baltimore, Maryland; Joanne Disch, PhD, RN, Professor ad Honorem, University of Minnesota School of Nursing, Minneapolis, Minnesota; and Lisa McGiffert, Director, Safe Patient Project, Consumers Union, Austin, Texas. While each speaker suggested various strategies for improving patient safety, they all agreed that information technology is not living up to our expectations for meeting this need. They also agreed that health care has become increasingly “high tech and low touch,” and, as a result, the medical community is leveraging neither technology nor the knowledge accrued from individual patient/physician interactions to improve patient safety and outcomes.1
Last year my mother had a spinal fusion. The surgery was a success by all measures. Two days after she was discharged home, she became weak and was unable to walk. She went to the emergency room, where it was noted that she was severely hyponatremic, weak, and experiencing severe back pain. For the next 36 hours she was not seen by a physician or physician assistant (PA), as the PA who admitted her to the hospital had not notified the “team” that she was admitted. My father, who is a vascular surgeon, notified her spine surgeon, who came to see her. Her hyponatremia was markedly worse, and she was transferred to the intensive care unit (ICU). She continued to decline and was started on hypertonic intravenous (IV) saline. Over the next several days her hyponatremia improved, and she was transferred out of the ICU but continued to have pain. The spine surgeon examined her several times, and imaging showed no evidence of epidural bleeding, infection, or misplaced hardware.
Over the next several days, I was informed by family members that the nurses were “keeping the pain in check” with IV narcotics and that my mom was heavily sedated most of the time. My dad later informed me that she had a foot drop on the left, and the next day another family member told me the foot drop was on the right. My dad and stepbrother each assured me that they were right. When my mom could talk, she told me how weak she was and that sometimes it was her right leg and other times her left. She was seen by a neurologist on 6 out of the next 10 days and underwent 3 computed tomography scans and magnetic resonance imaging, and the neurologist assured us that she had not had a stroke. On a Friday evening, I called my mom, who was progressively short of breath, and she told me that she felt weaker and weaker each day. The “foot drop,” which was now bilateral according to the neurologist, was from “not using it while she was in the ICU.”
My mom, who is an artist, commented that she was having trouble using her hands now and unable to hold a cup. I called the physician on call, who assured me that she was taking care of my mom’s blood pressure (which was labile for the first time ever; she had no history of hypertension) and her pain score was a 5. I explained that I knew that she was not “looking to play mystery diagnosis with an orthopedic surgeon 500 miles away, but I think my mom has Guillain-Barré syndrome.” Fortunately, the doctor said, “Oh my god, I think you’re right.” Monday morning, her diagnosis was confirmed and she has made a remarkable recovery. So how is it that she could be seen by a neurologist and a team of nurses, doctors, therapists, and resident staff and no one made a diagnosis? Certainly contributing factors include a system of multiple medical teams with frequent turnovers and a desire to consult others but no real “quarterback” who was looking at the overall care in a responsible and critical way. A thorough history and physical examination, rather than a multitude of expensive and unnecessary imaging studies, could certainly have led to a quicker diagnosis and avoidance of a protracted hospital stay and rehabilitation.
To be sure, there are many factors that lead to delays in diagnosis. The reliance on advanced imaging, the lack of a simple physical examination, and the lack of critical thinking played prominently in the failure to make a diagnosis in my mom’s case. Some would argue that we need information technology (IT) systems that will allow us to better diagnose and treat patients. They believe that with electronic medical records (EMRs) data points will be entered and a diagnosis will be made. Major corporations like IBM and GE are working to make this a reality. Although Watson (the artificially intelligent computer system created by IBM) may be able to win on Jeopardy and may move the needle forward to improving patient care, 2 things are certain: (1) Appropriate data will need to be input by people, and (2) without critical thinking, the appropriate data can’t be entered or interpreted correctly.
The fact remains that EMR has fallen short of expectations. We have more data at our fingertips but this has not translated into a significant improvement in patient safety. The human factor remains critical. Even though industry and health care workers strive to innovate and merge technological advances with improved patient outcomes, technology will continue to fall short of expectations without the input of critical thinking. There are things that computers and technological advances can do that people can’t, and there are things that people can do that computers can’t.
We cannot become a profession reliant on technology to substitute for critical thinking, and we cannot become a profession that doesn’t recognize what technology can bring to us and our patients. Like a railroad track that needs 2 parallel tracks to move trains, we must continue to build on 2 tracks: innovation and critical thinking. ◾
Preventable medical errors rank as the third most common cause of death in the United States after heart disease and cancer.1 They are responsible for 400,000 deaths each year (over 1095 per day) and another 10,000 serious complications resulting from medical errors each day.1 That is the equivalent of two 747 airliner midair crashes per day. The economic cost to our nation is $1 trillion per year.1
On July 17, 2014, the US Senate Subcommittee on Primary Health and Aging met to address this crisis. Participants included senators and John James, PhD, Founder, Patient Safety America, Houston, Texas; Ashish Jha, MD, MPH, Professor of Health Policy and Management, Harvard School of Public Health, Boston, Massachusetts; Tejal Gandhi, MD, MPH, President, National Patient Safety Foundation, and Associate Professor of Medicine, Harvard Medical School, Boston, Massachusetts; Peter Pronovost, MD, PhD, Senior Vice President for Patient Safety and Quality, and Director of the Armstrong Institute for Patient Safety and Quality, Johns Hopkins Medicine, Baltimore, Maryland; Joanne Disch, PhD, RN, Professor ad Honorem, University of Minnesota School of Nursing, Minneapolis, Minnesota; and Lisa McGiffert, Director, Safe Patient Project, Consumers Union, Austin, Texas. While each speaker suggested various strategies for improving patient safety, they all agreed that information technology is not living up to our expectations for meeting this need. They also agreed that health care has become increasingly “high tech and low touch,” and, as a result, the medical community is leveraging neither technology nor the knowledge accrued from individual patient/physician interactions to improve patient safety and outcomes.1
Last year my mother had a spinal fusion. The surgery was a success by all measures. Two days after she was discharged home, she became weak and was unable to walk. She went to the emergency room, where it was noted that she was severely hyponatremic, weak, and experiencing severe back pain. For the next 36 hours she was not seen by a physician or physician assistant (PA), as the PA who admitted her to the hospital had not notified the “team” that she was admitted. My father, who is a vascular surgeon, notified her spine surgeon, who came to see her. Her hyponatremia was markedly worse, and she was transferred to the intensive care unit (ICU). She continued to decline and was started on hypertonic intravenous (IV) saline. Over the next several days her hyponatremia improved, and she was transferred out of the ICU but continued to have pain. The spine surgeon examined her several times, and imaging showed no evidence of epidural bleeding, infection, or misplaced hardware.
Over the next several days, I was informed by family members that the nurses were “keeping the pain in check” with IV narcotics and that my mom was heavily sedated most of the time. My dad later informed me that she had a foot drop on the left, and the next day another family member told me the foot drop was on the right. My dad and stepbrother each assured me that they were right. When my mom could talk, she told me how weak she was and that sometimes it was her right leg and other times her left. She was seen by a neurologist on 6 out of the next 10 days and underwent 3 computed tomography scans and magnetic resonance imaging, and the neurologist assured us that she had not had a stroke. On a Friday evening, I called my mom, who was progressively short of breath, and she told me that she felt weaker and weaker each day. The “foot drop,” which was now bilateral according to the neurologist, was from “not using it while she was in the ICU.”
My mom, who is an artist, commented that she was having trouble using her hands now and unable to hold a cup. I called the physician on call, who assured me that she was taking care of my mom’s blood pressure (which was labile for the first time ever; she had no history of hypertension) and her pain score was a 5. I explained that I knew that she was not “looking to play mystery diagnosis with an orthopedic surgeon 500 miles away, but I think my mom has Guillain-Barré syndrome.” Fortunately, the doctor said, “Oh my god, I think you’re right.” Monday morning, her diagnosis was confirmed and she has made a remarkable recovery. So how is it that she could be seen by a neurologist and a team of nurses, doctors, therapists, and resident staff and no one made a diagnosis? Certainly contributing factors include a system of multiple medical teams with frequent turnovers and a desire to consult others but no real “quarterback” who was looking at the overall care in a responsible and critical way. A thorough history and physical examination, rather than a multitude of expensive and unnecessary imaging studies, could certainly have led to a quicker diagnosis and avoidance of a protracted hospital stay and rehabilitation.
To be sure, there are many factors that lead to delays in diagnosis. The reliance on advanced imaging, the lack of a simple physical examination, and the lack of critical thinking played prominently in the failure to make a diagnosis in my mom’s case. Some would argue that we need information technology (IT) systems that will allow us to better diagnose and treat patients. They believe that with electronic medical records (EMRs) data points will be entered and a diagnosis will be made. Major corporations like IBM and GE are working to make this a reality. Although Watson (the artificially intelligent computer system created by IBM) may be able to win on Jeopardy and may move the needle forward to improving patient care, 2 things are certain: (1) Appropriate data will need to be input by people, and (2) without critical thinking, the appropriate data can’t be entered or interpreted correctly.
The fact remains that EMR has fallen short of expectations. We have more data at our fingertips but this has not translated into a significant improvement in patient safety. The human factor remains critical. Even though industry and health care workers strive to innovate and merge technological advances with improved patient outcomes, technology will continue to fall short of expectations without the input of critical thinking. There are things that computers and technological advances can do that people can’t, and there are things that people can do that computers can’t.
We cannot become a profession reliant on technology to substitute for critical thinking, and we cannot become a profession that doesn’t recognize what technology can bring to us and our patients. Like a railroad track that needs 2 parallel tracks to move trains, we must continue to build on 2 tracks: innovation and critical thinking. ◾
Reference
1. McCann E. Deaths by medical mistakes hit records. Healthcare IT News. http://www.healthcareitnews.com/news/deaths-by-medical-mistakes-hit-records. Published July 18, 2014. Accessed November 17, 2014.
Reference
1. McCann E. Deaths by medical mistakes hit records. Healthcare IT News. http://www.healthcareitnews.com/news/deaths-by-medical-mistakes-hit-records. Published July 18, 2014. Accessed November 17, 2014.
Large Solitary Glomus Tumor of the Wrist Involving the Radial Artery
Glomus tumors are neoplasms that originate from normal glomus bodies in the skin and are most commonly found in the subungual areas of the digits.1 Glomus bodies are neuromyoarterial structures in the reticular dermis that serve as specialized arteriovenous anastomoses. These bodies contain afferent arterioles and efferent veins with multiple connections, and glomus cells have contractile properties because of their similarity to smooth muscle cells.1,2 Glomus bodies help regulate blood flow and temperature of the skin and are found in their largest concentration in the fingertips, palms of the hands, and soles of the feet.3,4
Glomus tumors represent hyperplastic glomus bodies and make up 1% to 4.5% of upper extremity neoplasms, with approximately 75% in the hand and 50% in the subungual area.1,5,6 These tumors can also present in multiple locations at once and can occur in atypical and ectopic locations.3 Although generally benign, glomus tumors can also exhibit malignant and metastatic potential in rare cases.7,8 They can also be locally aggressive with bony destruction of the distal phalynx.2,9,10 Tumors typically present as painful solitary soft-tissue lesions that are exquisitely tender to palpation, dark red-purple or bluish, and hypersensitive to cold.5,10 Van Geertruyden and colleagues10 reported that the diagnosis of glomus tumor can be made clinically in 90% of cases. However, glomus tumors can easily be mistaken for other lesions, such as hemangiomas, angiomas, neuromas, neurofibromas, lipomas, and ganglion cysts. An inaccurate or incomplete workup can result in persistent pain and symptoms along with intraoperative complications.3 Magnetic resonance imaging (MRI), the most sensitive imaging modality for detecting glomus tumors of the hand, can assist in the workup.3,11,12
Extradigital glomus tumors are difficult to diagnose because of their rarity and unspecific symptoms and presentation.13 Misdiagnosis and delayed diagnosis can result in significant chronic pain, disuse syndromes, and disability.1,10 Correct diagnosis and surgical resection are generally curative with complete resolution of symptoms.
In this article, we report a case of a large atypical glomus tumor that occurred on the wrist and involved the radial artery. This tumor was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 63-year-old man presented to clinic with an extremely tender soft-tissue mass on his nondominant, left wrist. The mass had been increasing in size for a year. It was painless at rest but very painful to light palpation, with referred pain proximally up to the shoulder.
The patient did not recall any traumatic or inciting event, had not undergone any prior workup or treatment for symptoms, and had no history of masses elsewhere on the body. Past medical history was significant for type 2 diabetes and colon and prostate cancer, which had been treated with chemotherapy and was now in remission.
Physical examination revealed a 2×2.5-cm well-circumscribed soft-tissue mass on the volar-radial aspect of the left wrist proximal to the thenar eminence and radial to the flexor carpi radialis tendon (Figure 1). The mass was soft, mobile, and nonfluctuant and did not transilluminate. The overlying skin was normal in color and appearance—no discoloration, erythema, wounds, or drainage. The radial artery was palpable, and the mass did not pulsate or have a bruit. The patient had normal wrist range of motion limited by pain on compression of the mass with motor and sensation intact throughout the hand. Plain radiographs of the wrist showed no bony pathology or involvement from the mass. A soft-tissue shadow was visible around the wrist without calcifications. A wrist MRI was performed to better evaluate the mass, and the T2-weighted images showed a heterogeneous subcutaneous mass adjacent to the radial artery with increased signal intensity from surrounding feeding vessels (Figure 2).
Given the clinical and imaging findings, there was concern for a possible vascular tumor. Therefore, excisional biopsy was recommended over needle biopsy because of the bleeding risk. With the patient under general anesthesia, and a tourniquet used without exsanguination, a Brunner-type zigzag incision was made centered over the mass with elevated skin flaps. The 2.7×2.6×1.1-cm mass was superficial and involved the radial artery (Figure 3). After the radial artery was dissected proximally and distally, 2 perforating vessels were found entering the mass. These vessels were ligated, which allowed the mass to be peeled completely off the artery. Histology with hematoxylin-eosin staining showed solid sheets of uniform round cells with interspersed capillaries and centrally placed nuclei without evidence of malignancy (Figure 4).
The tourniquet was released before skin closure, and adequate hemostasis was obtained. The wound was closed, and the patient was placed in a volar wrist splint for immobilization. Pain relief after excision of the mass was immediate, and the postoperative course uneventful. After surgery, immunohistochemistry of the mass showed minimal mitotic activity, with a positive immunoperoxidase stain for smooth muscle actin confirming a diagnosis of glomus tumor (Figure 5). At 3-year follow-up, the patient had no pain, symptoms, or tumor recurrence.
Discussion
Glomus tumors are an established cause of pain in the subungual areas of the hand; numerous cases have been reported.1,5,10,14 However, extradigital glomus tumors, particularly those involving the wrist, are rare, and only a few have been described. Given the lack of consistent findings and presentations, diagnosis is difficult. Case series have documented an overall 2:1 female-to-male predominance of glomus tumors,6 but extradigital tumors are more common in men (4.6:1 male-to-female ratio).3 Extradigital glomus tumors are commonly diagnosed between ages 40 and 80 years. Classic symptoms of subungual tumors include pain, localized tenderness, and cold hypersensitivity,1,10 but symptoms are much more variable with extradigital locations. Previous trauma or injury to the lesion area is reported in 20% to 30% of cases before symptom onset.3,15 Intravascular locations of glomus tumors are extremely rare; only 4 cases of tumors involving venous structures have been reported.16-19 In the present case, the patient’s main complaints were pain and localized tenderness associated with a progressively increasing mass without any history of trauma. The large size of his mass (~2.5 cm in diameter) on examination was unique, as was involvement of the radial artery.
Misdiagnosis and delayed diagnosis of extradigital glomus tumors are common, and symptoms such as chronic pain typically persist for 7 to 11 years before the correct diagnosis is made.1,10 On average, 2.5 physician consultants (including psychiatrists) evaluate the patient before glomus tumor is identified.10 There are other reports of atypical or ectopic glomus tumors taking 5 to 25 years to be diagnosed.20-22 The differential diagnosis for glomus tumors includes hemangiomas, cellular or cavernous hemangiomas, vascular tumors, neuromas, neurofibromas, lipomas, paragangliomas, ganglion cysts, pigmented nevi, Pacinian corpuscle hyperplasia, and foreign bodies. A key element of clinical diagnosis is the disproportionate amount of pain and localized tenderness caused by the lesion relative to its size. The hypersensitivity of this tumor is thought to result from enlargement of the tumor and impingement on nearby Pacinian corpuscles, nerve endings in the skin that are responsible for sensitivity to vibration and pressure.2,9
Plain radiographs can be useful in detecting glomus tumors of the hand but are less helpful with extradigital tumors, with identification rates of 24% in certain series.3 MRI is the most sensitive imaging modality for diagnosing glomus tumors of the hand; a detection rate of 80% to 100% has been reported in various case series.3,11,12 Specificity of MRI for glomus tumors has been reported at 50%.11,23 Placement of a radiographic marker directly over the area of most pain can assist in tumor localization.3 Glomus tumors typically have decreased signal intensity on T1-weighted images and increased intensity on T2-weighted images, but signal patterns are variable and particularly difficult to differentiate with small tumors. MRI is useful in the setting of recurrent glomus tumors, where incomplete excision is possible. In 24 cases of continued pain after glomus tumor excision, Theumann and colleagues24 used MRI to identify a nodule consistent with recurrent glomus tumor in all patients. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) can also help diagnose glomus tumors while providing valuable information regarding size and location for surgical planning.25,26 With MRA, it is crucial to evaluate the arterial or arteriovenous phase of imaging, as the glomus tumor is richly vascularized and shows contrast enhancement after intravenous injection of gadolinium.27 Angiography, ultrasonography, thermography, and scintigraphy have all been used to diagnose glomus tumors but have shown limited utility and accuracy.11
Treatment of glomus tumors is complete surgical excision because of their relatively small size and subcutaneous location. Resection success rates are consistently higher than 95%, with resolution of all symptoms.1,10,14 Local recurrence of tumors after excision occurs in 1% to 33% of cases, depending on series, and may be immediate or delayed, with immediate recurrence commonly caused by inadequate excision.1,10,15,28 Delayed recurrence is less common and presents several years after excision, typically with a new growth near the previous excision.10 Recurrence years after surgery may also represent multiple tumors unrecognized during initial workup and can be treated with repeat excision or radiotherapy.
Robert and colleagues29 recently reported the case of a glomus tumor, on the dorsal aspect of the wrist, discovered incidentally in a 71-year-old patient and treated with surgical excision. Several years earlier, Chim and colleagues30 described a similar case, of a large wrist glomus tumor worked up with MRI. In a retrospective review of all extradigital glomus tumors seen over a 20-year period, Schiefer and colleagues3 reported 4 glomus tumors of the wrist out of 56 tumors total. The most common sites were forearm (11 cases) and knee (10 cases), and the majority of patients presented with pain and localized tenderness. Mean tumor size was 0.66 cm (range, 0.1-0.3 cm), with 77% of tumors less than 1 cm. Our patient’s 2.7×2.6×1.1-cm tumor was large for a glomus tumor. Its involvement with the radial artery feeding vessels likely contributed to its large and progressively increasing size. It is worth noting that, in the series by Schiefer and colleagues,3 the only patient with symptoms persisting after excision had a large (3 cm in diameter) deep tumor of the foot; the entire tumor was removed, and there was no recurrence by 10-year follow-up. Folpe and colleagues7 suggested that deep tumors larger than 2 cm should be at higher suspicion for malignancy. Joseph and Posner21 reported 3 cases of glomus tumors, on the ulnar side of the wrist, diagnosed with help of a provocative test using ethyl chloride spray.
Conclusion
Overall, glomus tumors are rare and challenging to diagnosis and should be in the differential in any symptomatic patient with a painful soft-tissue mass of the wrist. Advanced imaging studies, such as MRI, can assist in localization, diagnosis, and preoperative planning. Histology and immunohistochemistry are essential to differentiate glomus tumor from other vascular tumors, and complete excision is necessary to prevent local recurrence.
1. Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54(4):691-703.
2. Riddell DH, Martin RS. Glomus tumor of unusual size; case report. Ann Surg. 1951;133(3):401-403.
3. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006;81(10):1337-1344.
4. Tuncali D, Yilmaz AC, Terzioglu A, Aslan G. Multiple occurrences of different histologic types of the glomus tumor. J Hand Surg Am. 2005;30(1):161-164.
5. Greene RG. Soft tissue tumors of the hand and wrist. A 10 year survey. J Med Soc N J. 1964;61:495-498.
6. Maxwell GP, Curtis RM, Wilgis EF. Multiple digital glomus tumors. J Hand Surg Am. 1979;4(4):363-367.
7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
8. De Chiara A, Apice G, Mori S, et al. Malignant glomus tumour: a case report and review of the literature. Sarcoma. 2003;7(2):87-91.
9. Riveros M, Pack GT. The glomus tumor; report of 20 cases. Ann Surg. 1951;133(3):394-400.
10. Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21(2):257-260.
11. Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005;30(5):535-540.
12. Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507-515.
13. Heys SD, Brittenden J, Atkinson P, Eremin O. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79(4):345-347.
14. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27(3):229-231.
15. Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg Am. 1977;2(4):261-265.
16. Beham A, Fletcher CD. Intravascular glomus tumour: a previously undescribed phenomenon. Virchows Arch A Pathol Anat Histopathol. 1991;418(2):175-177.
17. Googe PB, Griffin WC. Intravenous glomus tumor of the forearm. J Cutan Pathol. 1993;20(4):359-363.
18. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008;36(6):369-370.
19. Acebo E, Val-Bernal JF, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24(6):384-389.
20. Ghaly RF, Ring AM. Supraclavicular glomus tumor, 20 year history of undiagnosed shoulder pain: a case report. Pain. 1999;83(2):379-382.
21. Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8(6):918-920.
22. Abou Jaoude JF, Roula Farah A, Sargi Z, Khairallah S, Fakih C. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000;19(4):243-252.
23. Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg Am. 1990;15(3):507-509.
24. Theumann NH, Goettmann S, Le Viet D, et al. Recurrent glomus tumors of fingertips: MR imaging evaluation. Radiology. 2002;223(1):143-151.
25. Boudghene FP, Gouny P, Tassart M, Callard P, Le Breton C, Vayssairat M. Subungual glomus tumor: combined use of MRI and three-dimensional contrast MR angiography. J Magn Reson Imaging. 1998;8(6):1326-1328.
26. Van Ruyssevelt CE, Vranckx P. Subungual glomus tumor: emphasis on MR angiography. AJR Am J Roentgenol. 2004;182(1):263-264.
27. Connell DA, Koulouris G, Thorn DA, Potter HG. Contrast-enhanced MR angiography of the hand. Radiographics. 2002;22(3):583-599.
28. Varian JP, Cleak DK. Glomus tumours in the hand. Hand. 1980;12(3):293-299.
29. Robert G, Sawaya E, Pelissier P. Glomus tumor of the dorsal aspect of the wrist: a case report [in French]. Chir Main. 2012;31(4):214-216.
30. Chim H, Lahiri A, Chew WY. Atypical glomus tumour of the wrist: a case report. Hand Surg. 2009;14(2-3):121-123.
Glomus tumors are neoplasms that originate from normal glomus bodies in the skin and are most commonly found in the subungual areas of the digits.1 Glomus bodies are neuromyoarterial structures in the reticular dermis that serve as specialized arteriovenous anastomoses. These bodies contain afferent arterioles and efferent veins with multiple connections, and glomus cells have contractile properties because of their similarity to smooth muscle cells.1,2 Glomus bodies help regulate blood flow and temperature of the skin and are found in their largest concentration in the fingertips, palms of the hands, and soles of the feet.3,4
Glomus tumors represent hyperplastic glomus bodies and make up 1% to 4.5% of upper extremity neoplasms, with approximately 75% in the hand and 50% in the subungual area.1,5,6 These tumors can also present in multiple locations at once and can occur in atypical and ectopic locations.3 Although generally benign, glomus tumors can also exhibit malignant and metastatic potential in rare cases.7,8 They can also be locally aggressive with bony destruction of the distal phalynx.2,9,10 Tumors typically present as painful solitary soft-tissue lesions that are exquisitely tender to palpation, dark red-purple or bluish, and hypersensitive to cold.5,10 Van Geertruyden and colleagues10 reported that the diagnosis of glomus tumor can be made clinically in 90% of cases. However, glomus tumors can easily be mistaken for other lesions, such as hemangiomas, angiomas, neuromas, neurofibromas, lipomas, and ganglion cysts. An inaccurate or incomplete workup can result in persistent pain and symptoms along with intraoperative complications.3 Magnetic resonance imaging (MRI), the most sensitive imaging modality for detecting glomus tumors of the hand, can assist in the workup.3,11,12
Extradigital glomus tumors are difficult to diagnose because of their rarity and unspecific symptoms and presentation.13 Misdiagnosis and delayed diagnosis can result in significant chronic pain, disuse syndromes, and disability.1,10 Correct diagnosis and surgical resection are generally curative with complete resolution of symptoms.
In this article, we report a case of a large atypical glomus tumor that occurred on the wrist and involved the radial artery. This tumor was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 63-year-old man presented to clinic with an extremely tender soft-tissue mass on his nondominant, left wrist. The mass had been increasing in size for a year. It was painless at rest but very painful to light palpation, with referred pain proximally up to the shoulder.
The patient did not recall any traumatic or inciting event, had not undergone any prior workup or treatment for symptoms, and had no history of masses elsewhere on the body. Past medical history was significant for type 2 diabetes and colon and prostate cancer, which had been treated with chemotherapy and was now in remission.
Physical examination revealed a 2×2.5-cm well-circumscribed soft-tissue mass on the volar-radial aspect of the left wrist proximal to the thenar eminence and radial to the flexor carpi radialis tendon (Figure 1). The mass was soft, mobile, and nonfluctuant and did not transilluminate. The overlying skin was normal in color and appearance—no discoloration, erythema, wounds, or drainage. The radial artery was palpable, and the mass did not pulsate or have a bruit. The patient had normal wrist range of motion limited by pain on compression of the mass with motor and sensation intact throughout the hand. Plain radiographs of the wrist showed no bony pathology or involvement from the mass. A soft-tissue shadow was visible around the wrist without calcifications. A wrist MRI was performed to better evaluate the mass, and the T2-weighted images showed a heterogeneous subcutaneous mass adjacent to the radial artery with increased signal intensity from surrounding feeding vessels (Figure 2).
Given the clinical and imaging findings, there was concern for a possible vascular tumor. Therefore, excisional biopsy was recommended over needle biopsy because of the bleeding risk. With the patient under general anesthesia, and a tourniquet used without exsanguination, a Brunner-type zigzag incision was made centered over the mass with elevated skin flaps. The 2.7×2.6×1.1-cm mass was superficial and involved the radial artery (Figure 3). After the radial artery was dissected proximally and distally, 2 perforating vessels were found entering the mass. These vessels were ligated, which allowed the mass to be peeled completely off the artery. Histology with hematoxylin-eosin staining showed solid sheets of uniform round cells with interspersed capillaries and centrally placed nuclei without evidence of malignancy (Figure 4).
The tourniquet was released before skin closure, and adequate hemostasis was obtained. The wound was closed, and the patient was placed in a volar wrist splint for immobilization. Pain relief after excision of the mass was immediate, and the postoperative course uneventful. After surgery, immunohistochemistry of the mass showed minimal mitotic activity, with a positive immunoperoxidase stain for smooth muscle actin confirming a diagnosis of glomus tumor (Figure 5). At 3-year follow-up, the patient had no pain, symptoms, or tumor recurrence.
Discussion
Glomus tumors are an established cause of pain in the subungual areas of the hand; numerous cases have been reported.1,5,10,14 However, extradigital glomus tumors, particularly those involving the wrist, are rare, and only a few have been described. Given the lack of consistent findings and presentations, diagnosis is difficult. Case series have documented an overall 2:1 female-to-male predominance of glomus tumors,6 but extradigital tumors are more common in men (4.6:1 male-to-female ratio).3 Extradigital glomus tumors are commonly diagnosed between ages 40 and 80 years. Classic symptoms of subungual tumors include pain, localized tenderness, and cold hypersensitivity,1,10 but symptoms are much more variable with extradigital locations. Previous trauma or injury to the lesion area is reported in 20% to 30% of cases before symptom onset.3,15 Intravascular locations of glomus tumors are extremely rare; only 4 cases of tumors involving venous structures have been reported.16-19 In the present case, the patient’s main complaints were pain and localized tenderness associated with a progressively increasing mass without any history of trauma. The large size of his mass (~2.5 cm in diameter) on examination was unique, as was involvement of the radial artery.
Misdiagnosis and delayed diagnosis of extradigital glomus tumors are common, and symptoms such as chronic pain typically persist for 7 to 11 years before the correct diagnosis is made.1,10 On average, 2.5 physician consultants (including psychiatrists) evaluate the patient before glomus tumor is identified.10 There are other reports of atypical or ectopic glomus tumors taking 5 to 25 years to be diagnosed.20-22 The differential diagnosis for glomus tumors includes hemangiomas, cellular or cavernous hemangiomas, vascular tumors, neuromas, neurofibromas, lipomas, paragangliomas, ganglion cysts, pigmented nevi, Pacinian corpuscle hyperplasia, and foreign bodies. A key element of clinical diagnosis is the disproportionate amount of pain and localized tenderness caused by the lesion relative to its size. The hypersensitivity of this tumor is thought to result from enlargement of the tumor and impingement on nearby Pacinian corpuscles, nerve endings in the skin that are responsible for sensitivity to vibration and pressure.2,9
Plain radiographs can be useful in detecting glomus tumors of the hand but are less helpful with extradigital tumors, with identification rates of 24% in certain series.3 MRI is the most sensitive imaging modality for diagnosing glomus tumors of the hand; a detection rate of 80% to 100% has been reported in various case series.3,11,12 Specificity of MRI for glomus tumors has been reported at 50%.11,23 Placement of a radiographic marker directly over the area of most pain can assist in tumor localization.3 Glomus tumors typically have decreased signal intensity on T1-weighted images and increased intensity on T2-weighted images, but signal patterns are variable and particularly difficult to differentiate with small tumors. MRI is useful in the setting of recurrent glomus tumors, where incomplete excision is possible. In 24 cases of continued pain after glomus tumor excision, Theumann and colleagues24 used MRI to identify a nodule consistent with recurrent glomus tumor in all patients. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) can also help diagnose glomus tumors while providing valuable information regarding size and location for surgical planning.25,26 With MRA, it is crucial to evaluate the arterial or arteriovenous phase of imaging, as the glomus tumor is richly vascularized and shows contrast enhancement after intravenous injection of gadolinium.27 Angiography, ultrasonography, thermography, and scintigraphy have all been used to diagnose glomus tumors but have shown limited utility and accuracy.11
Treatment of glomus tumors is complete surgical excision because of their relatively small size and subcutaneous location. Resection success rates are consistently higher than 95%, with resolution of all symptoms.1,10,14 Local recurrence of tumors after excision occurs in 1% to 33% of cases, depending on series, and may be immediate or delayed, with immediate recurrence commonly caused by inadequate excision.1,10,15,28 Delayed recurrence is less common and presents several years after excision, typically with a new growth near the previous excision.10 Recurrence years after surgery may also represent multiple tumors unrecognized during initial workup and can be treated with repeat excision or radiotherapy.
Robert and colleagues29 recently reported the case of a glomus tumor, on the dorsal aspect of the wrist, discovered incidentally in a 71-year-old patient and treated with surgical excision. Several years earlier, Chim and colleagues30 described a similar case, of a large wrist glomus tumor worked up with MRI. In a retrospective review of all extradigital glomus tumors seen over a 20-year period, Schiefer and colleagues3 reported 4 glomus tumors of the wrist out of 56 tumors total. The most common sites were forearm (11 cases) and knee (10 cases), and the majority of patients presented with pain and localized tenderness. Mean tumor size was 0.66 cm (range, 0.1-0.3 cm), with 77% of tumors less than 1 cm. Our patient’s 2.7×2.6×1.1-cm tumor was large for a glomus tumor. Its involvement with the radial artery feeding vessels likely contributed to its large and progressively increasing size. It is worth noting that, in the series by Schiefer and colleagues,3 the only patient with symptoms persisting after excision had a large (3 cm in diameter) deep tumor of the foot; the entire tumor was removed, and there was no recurrence by 10-year follow-up. Folpe and colleagues7 suggested that deep tumors larger than 2 cm should be at higher suspicion for malignancy. Joseph and Posner21 reported 3 cases of glomus tumors, on the ulnar side of the wrist, diagnosed with help of a provocative test using ethyl chloride spray.
Conclusion
Overall, glomus tumors are rare and challenging to diagnosis and should be in the differential in any symptomatic patient with a painful soft-tissue mass of the wrist. Advanced imaging studies, such as MRI, can assist in localization, diagnosis, and preoperative planning. Histology and immunohistochemistry are essential to differentiate glomus tumor from other vascular tumors, and complete excision is necessary to prevent local recurrence.
Glomus tumors are neoplasms that originate from normal glomus bodies in the skin and are most commonly found in the subungual areas of the digits.1 Glomus bodies are neuromyoarterial structures in the reticular dermis that serve as specialized arteriovenous anastomoses. These bodies contain afferent arterioles and efferent veins with multiple connections, and glomus cells have contractile properties because of their similarity to smooth muscle cells.1,2 Glomus bodies help regulate blood flow and temperature of the skin and are found in their largest concentration in the fingertips, palms of the hands, and soles of the feet.3,4
Glomus tumors represent hyperplastic glomus bodies and make up 1% to 4.5% of upper extremity neoplasms, with approximately 75% in the hand and 50% in the subungual area.1,5,6 These tumors can also present in multiple locations at once and can occur in atypical and ectopic locations.3 Although generally benign, glomus tumors can also exhibit malignant and metastatic potential in rare cases.7,8 They can also be locally aggressive with bony destruction of the distal phalynx.2,9,10 Tumors typically present as painful solitary soft-tissue lesions that are exquisitely tender to palpation, dark red-purple or bluish, and hypersensitive to cold.5,10 Van Geertruyden and colleagues10 reported that the diagnosis of glomus tumor can be made clinically in 90% of cases. However, glomus tumors can easily be mistaken for other lesions, such as hemangiomas, angiomas, neuromas, neurofibromas, lipomas, and ganglion cysts. An inaccurate or incomplete workup can result in persistent pain and symptoms along with intraoperative complications.3 Magnetic resonance imaging (MRI), the most sensitive imaging modality for detecting glomus tumors of the hand, can assist in the workup.3,11,12
Extradigital glomus tumors are difficult to diagnose because of their rarity and unspecific symptoms and presentation.13 Misdiagnosis and delayed diagnosis can result in significant chronic pain, disuse syndromes, and disability.1,10 Correct diagnosis and surgical resection are generally curative with complete resolution of symptoms.
In this article, we report a case of a large atypical glomus tumor that occurred on the wrist and involved the radial artery. This tumor was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 63-year-old man presented to clinic with an extremely tender soft-tissue mass on his nondominant, left wrist. The mass had been increasing in size for a year. It was painless at rest but very painful to light palpation, with referred pain proximally up to the shoulder.
The patient did not recall any traumatic or inciting event, had not undergone any prior workup or treatment for symptoms, and had no history of masses elsewhere on the body. Past medical history was significant for type 2 diabetes and colon and prostate cancer, which had been treated with chemotherapy and was now in remission.
Physical examination revealed a 2×2.5-cm well-circumscribed soft-tissue mass on the volar-radial aspect of the left wrist proximal to the thenar eminence and radial to the flexor carpi radialis tendon (Figure 1). The mass was soft, mobile, and nonfluctuant and did not transilluminate. The overlying skin was normal in color and appearance—no discoloration, erythema, wounds, or drainage. The radial artery was palpable, and the mass did not pulsate or have a bruit. The patient had normal wrist range of motion limited by pain on compression of the mass with motor and sensation intact throughout the hand. Plain radiographs of the wrist showed no bony pathology or involvement from the mass. A soft-tissue shadow was visible around the wrist without calcifications. A wrist MRI was performed to better evaluate the mass, and the T2-weighted images showed a heterogeneous subcutaneous mass adjacent to the radial artery with increased signal intensity from surrounding feeding vessels (Figure 2).
Given the clinical and imaging findings, there was concern for a possible vascular tumor. Therefore, excisional biopsy was recommended over needle biopsy because of the bleeding risk. With the patient under general anesthesia, and a tourniquet used without exsanguination, a Brunner-type zigzag incision was made centered over the mass with elevated skin flaps. The 2.7×2.6×1.1-cm mass was superficial and involved the radial artery (Figure 3). After the radial artery was dissected proximally and distally, 2 perforating vessels were found entering the mass. These vessels were ligated, which allowed the mass to be peeled completely off the artery. Histology with hematoxylin-eosin staining showed solid sheets of uniform round cells with interspersed capillaries and centrally placed nuclei without evidence of malignancy (Figure 4).
The tourniquet was released before skin closure, and adequate hemostasis was obtained. The wound was closed, and the patient was placed in a volar wrist splint for immobilization. Pain relief after excision of the mass was immediate, and the postoperative course uneventful. After surgery, immunohistochemistry of the mass showed minimal mitotic activity, with a positive immunoperoxidase stain for smooth muscle actin confirming a diagnosis of glomus tumor (Figure 5). At 3-year follow-up, the patient had no pain, symptoms, or tumor recurrence.
Discussion
Glomus tumors are an established cause of pain in the subungual areas of the hand; numerous cases have been reported.1,5,10,14 However, extradigital glomus tumors, particularly those involving the wrist, are rare, and only a few have been described. Given the lack of consistent findings and presentations, diagnosis is difficult. Case series have documented an overall 2:1 female-to-male predominance of glomus tumors,6 but extradigital tumors are more common in men (4.6:1 male-to-female ratio).3 Extradigital glomus tumors are commonly diagnosed between ages 40 and 80 years. Classic symptoms of subungual tumors include pain, localized tenderness, and cold hypersensitivity,1,10 but symptoms are much more variable with extradigital locations. Previous trauma or injury to the lesion area is reported in 20% to 30% of cases before symptom onset.3,15 Intravascular locations of glomus tumors are extremely rare; only 4 cases of tumors involving venous structures have been reported.16-19 In the present case, the patient’s main complaints were pain and localized tenderness associated with a progressively increasing mass without any history of trauma. The large size of his mass (~2.5 cm in diameter) on examination was unique, as was involvement of the radial artery.
Misdiagnosis and delayed diagnosis of extradigital glomus tumors are common, and symptoms such as chronic pain typically persist for 7 to 11 years before the correct diagnosis is made.1,10 On average, 2.5 physician consultants (including psychiatrists) evaluate the patient before glomus tumor is identified.10 There are other reports of atypical or ectopic glomus tumors taking 5 to 25 years to be diagnosed.20-22 The differential diagnosis for glomus tumors includes hemangiomas, cellular or cavernous hemangiomas, vascular tumors, neuromas, neurofibromas, lipomas, paragangliomas, ganglion cysts, pigmented nevi, Pacinian corpuscle hyperplasia, and foreign bodies. A key element of clinical diagnosis is the disproportionate amount of pain and localized tenderness caused by the lesion relative to its size. The hypersensitivity of this tumor is thought to result from enlargement of the tumor and impingement on nearby Pacinian corpuscles, nerve endings in the skin that are responsible for sensitivity to vibration and pressure.2,9
Plain radiographs can be useful in detecting glomus tumors of the hand but are less helpful with extradigital tumors, with identification rates of 24% in certain series.3 MRI is the most sensitive imaging modality for diagnosing glomus tumors of the hand; a detection rate of 80% to 100% has been reported in various case series.3,11,12 Specificity of MRI for glomus tumors has been reported at 50%.11,23 Placement of a radiographic marker directly over the area of most pain can assist in tumor localization.3 Glomus tumors typically have decreased signal intensity on T1-weighted images and increased intensity on T2-weighted images, but signal patterns are variable and particularly difficult to differentiate with small tumors. MRI is useful in the setting of recurrent glomus tumors, where incomplete excision is possible. In 24 cases of continued pain after glomus tumor excision, Theumann and colleagues24 used MRI to identify a nodule consistent with recurrent glomus tumor in all patients. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) can also help diagnose glomus tumors while providing valuable information regarding size and location for surgical planning.25,26 With MRA, it is crucial to evaluate the arterial or arteriovenous phase of imaging, as the glomus tumor is richly vascularized and shows contrast enhancement after intravenous injection of gadolinium.27 Angiography, ultrasonography, thermography, and scintigraphy have all been used to diagnose glomus tumors but have shown limited utility and accuracy.11
Treatment of glomus tumors is complete surgical excision because of their relatively small size and subcutaneous location. Resection success rates are consistently higher than 95%, with resolution of all symptoms.1,10,14 Local recurrence of tumors after excision occurs in 1% to 33% of cases, depending on series, and may be immediate or delayed, with immediate recurrence commonly caused by inadequate excision.1,10,15,28 Delayed recurrence is less common and presents several years after excision, typically with a new growth near the previous excision.10 Recurrence years after surgery may also represent multiple tumors unrecognized during initial workup and can be treated with repeat excision or radiotherapy.
Robert and colleagues29 recently reported the case of a glomus tumor, on the dorsal aspect of the wrist, discovered incidentally in a 71-year-old patient and treated with surgical excision. Several years earlier, Chim and colleagues30 described a similar case, of a large wrist glomus tumor worked up with MRI. In a retrospective review of all extradigital glomus tumors seen over a 20-year period, Schiefer and colleagues3 reported 4 glomus tumors of the wrist out of 56 tumors total. The most common sites were forearm (11 cases) and knee (10 cases), and the majority of patients presented with pain and localized tenderness. Mean tumor size was 0.66 cm (range, 0.1-0.3 cm), with 77% of tumors less than 1 cm. Our patient’s 2.7×2.6×1.1-cm tumor was large for a glomus tumor. Its involvement with the radial artery feeding vessels likely contributed to its large and progressively increasing size. It is worth noting that, in the series by Schiefer and colleagues,3 the only patient with symptoms persisting after excision had a large (3 cm in diameter) deep tumor of the foot; the entire tumor was removed, and there was no recurrence by 10-year follow-up. Folpe and colleagues7 suggested that deep tumors larger than 2 cm should be at higher suspicion for malignancy. Joseph and Posner21 reported 3 cases of glomus tumors, on the ulnar side of the wrist, diagnosed with help of a provocative test using ethyl chloride spray.
Conclusion
Overall, glomus tumors are rare and challenging to diagnosis and should be in the differential in any symptomatic patient with a painful soft-tissue mass of the wrist. Advanced imaging studies, such as MRI, can assist in localization, diagnosis, and preoperative planning. Histology and immunohistochemistry are essential to differentiate glomus tumor from other vascular tumors, and complete excision is necessary to prevent local recurrence.
1. Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54(4):691-703.
2. Riddell DH, Martin RS. Glomus tumor of unusual size; case report. Ann Surg. 1951;133(3):401-403.
3. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006;81(10):1337-1344.
4. Tuncali D, Yilmaz AC, Terzioglu A, Aslan G. Multiple occurrences of different histologic types of the glomus tumor. J Hand Surg Am. 2005;30(1):161-164.
5. Greene RG. Soft tissue tumors of the hand and wrist. A 10 year survey. J Med Soc N J. 1964;61:495-498.
6. Maxwell GP, Curtis RM, Wilgis EF. Multiple digital glomus tumors. J Hand Surg Am. 1979;4(4):363-367.
7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
8. De Chiara A, Apice G, Mori S, et al. Malignant glomus tumour: a case report and review of the literature. Sarcoma. 2003;7(2):87-91.
9. Riveros M, Pack GT. The glomus tumor; report of 20 cases. Ann Surg. 1951;133(3):394-400.
10. Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21(2):257-260.
11. Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005;30(5):535-540.
12. Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507-515.
13. Heys SD, Brittenden J, Atkinson P, Eremin O. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79(4):345-347.
14. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27(3):229-231.
15. Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg Am. 1977;2(4):261-265.
16. Beham A, Fletcher CD. Intravascular glomus tumour: a previously undescribed phenomenon. Virchows Arch A Pathol Anat Histopathol. 1991;418(2):175-177.
17. Googe PB, Griffin WC. Intravenous glomus tumor of the forearm. J Cutan Pathol. 1993;20(4):359-363.
18. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008;36(6):369-370.
19. Acebo E, Val-Bernal JF, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24(6):384-389.
20. Ghaly RF, Ring AM. Supraclavicular glomus tumor, 20 year history of undiagnosed shoulder pain: a case report. Pain. 1999;83(2):379-382.
21. Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8(6):918-920.
22. Abou Jaoude JF, Roula Farah A, Sargi Z, Khairallah S, Fakih C. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000;19(4):243-252.
23. Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg Am. 1990;15(3):507-509.
24. Theumann NH, Goettmann S, Le Viet D, et al. Recurrent glomus tumors of fingertips: MR imaging evaluation. Radiology. 2002;223(1):143-151.
25. Boudghene FP, Gouny P, Tassart M, Callard P, Le Breton C, Vayssairat M. Subungual glomus tumor: combined use of MRI and three-dimensional contrast MR angiography. J Magn Reson Imaging. 1998;8(6):1326-1328.
26. Van Ruyssevelt CE, Vranckx P. Subungual glomus tumor: emphasis on MR angiography. AJR Am J Roentgenol. 2004;182(1):263-264.
27. Connell DA, Koulouris G, Thorn DA, Potter HG. Contrast-enhanced MR angiography of the hand. Radiographics. 2002;22(3):583-599.
28. Varian JP, Cleak DK. Glomus tumours in the hand. Hand. 1980;12(3):293-299.
29. Robert G, Sawaya E, Pelissier P. Glomus tumor of the dorsal aspect of the wrist: a case report [in French]. Chir Main. 2012;31(4):214-216.
30. Chim H, Lahiri A, Chew WY. Atypical glomus tumour of the wrist: a case report. Hand Surg. 2009;14(2-3):121-123.
1. Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54(4):691-703.
2. Riddell DH, Martin RS. Glomus tumor of unusual size; case report. Ann Surg. 1951;133(3):401-403.
3. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006;81(10):1337-1344.
4. Tuncali D, Yilmaz AC, Terzioglu A, Aslan G. Multiple occurrences of different histologic types of the glomus tumor. J Hand Surg Am. 2005;30(1):161-164.
5. Greene RG. Soft tissue tumors of the hand and wrist. A 10 year survey. J Med Soc N J. 1964;61:495-498.
6. Maxwell GP, Curtis RM, Wilgis EF. Multiple digital glomus tumors. J Hand Surg Am. 1979;4(4):363-367.
7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
8. De Chiara A, Apice G, Mori S, et al. Malignant glomus tumour: a case report and review of the literature. Sarcoma. 2003;7(2):87-91.
9. Riveros M, Pack GT. The glomus tumor; report of 20 cases. Ann Surg. 1951;133(3):394-400.
10. Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21(2):257-260.
11. Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005;30(5):535-540.
12. Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507-515.
13. Heys SD, Brittenden J, Atkinson P, Eremin O. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79(4):345-347.
14. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27(3):229-231.
15. Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg Am. 1977;2(4):261-265.
16. Beham A, Fletcher CD. Intravascular glomus tumour: a previously undescribed phenomenon. Virchows Arch A Pathol Anat Histopathol. 1991;418(2):175-177.
17. Googe PB, Griffin WC. Intravenous glomus tumor of the forearm. J Cutan Pathol. 1993;20(4):359-363.
18. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008;36(6):369-370.
19. Acebo E, Val-Bernal JF, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24(6):384-389.
20. Ghaly RF, Ring AM. Supraclavicular glomus tumor, 20 year history of undiagnosed shoulder pain: a case report. Pain. 1999;83(2):379-382.
21. Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8(6):918-920.
22. Abou Jaoude JF, Roula Farah A, Sargi Z, Khairallah S, Fakih C. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000;19(4):243-252.
23. Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg Am. 1990;15(3):507-509.
24. Theumann NH, Goettmann S, Le Viet D, et al. Recurrent glomus tumors of fingertips: MR imaging evaluation. Radiology. 2002;223(1):143-151.
25. Boudghene FP, Gouny P, Tassart M, Callard P, Le Breton C, Vayssairat M. Subungual glomus tumor: combined use of MRI and three-dimensional contrast MR angiography. J Magn Reson Imaging. 1998;8(6):1326-1328.
26. Van Ruyssevelt CE, Vranckx P. Subungual glomus tumor: emphasis on MR angiography. AJR Am J Roentgenol. 2004;182(1):263-264.
27. Connell DA, Koulouris G, Thorn DA, Potter HG. Contrast-enhanced MR angiography of the hand. Radiographics. 2002;22(3):583-599.
28. Varian JP, Cleak DK. Glomus tumours in the hand. Hand. 1980;12(3):293-299.
29. Robert G, Sawaya E, Pelissier P. Glomus tumor of the dorsal aspect of the wrist: a case report [in French]. Chir Main. 2012;31(4):214-216.
30. Chim H, Lahiri A, Chew WY. Atypical glomus tumour of the wrist: a case report. Hand Surg. 2009;14(2-3):121-123.
Osteoid Osteomas of the Foot and Ankle: A Study of Patients Over a 20-Year Period
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
Neonatal Physeal Separation of Distal Humerus During Cesarean Section
Physeal separation of the distal humerus in a newborn is a rare and severe injury that requires immediate treatment. This fracture was reported as an extremely rare complication of cesarean section.1 The correct diagnosis can be established by clinical and radiologic findings. However, this injury can be easily overlooked and misdiagnosed. Presentation often involves swelling, tenderness, and agitation with movement of the elbow.
We report a case in which neonatal physeal separation of the distal humerus occurred during cesarean section. The diagnosis was based on clinical and radiologic/arthrographic findings and treated with closed reduction and percutaneous fixation. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A full-term (40-week gestation) male neonate weighing 3690 g was born through cesarean section at the mother’s request. Apgar score was 9 at 1 minute and 10 at 5 minutes. The vertex position of the fetus was confirmed with preoperative ultrasonography. This was the mother’s first pregnancy and an in vitro fertilization. On his second day of life, the patient was referred to the orthopedic department for evaluation of local swelling and diminished spontaneous motion of the right elbow.
Examination revealed local tenderness and swelling in the anterior and lateral aspects of the elbow. Passive elbow range of motion (ROM) caused agitation, and elbow instability was present. A complete neurovascular examination was performed, and neurovascular injury and compartment syndrome were ruled out. Hematologic workup showed no signs of septic arthritis. Radiographs showed posteromedial displacement of the humeroulnar joint. The patient was placed in a long-arm splint, and no reduction was attempted initially.
The patient was taken to the operating room the same day. With the patient under general anesthesia, an arthrogram of the right elbow was obtained. It showed posteromedial displacement of the distal humeral epiphysis (Figure 1A). Closed reduction was performed, and the quality of the reduction was confirmed by intraoperative imaging. Percutaneously, a single 2-mm Kirschner wire (K-wire) was placed in an oblique fashion from the inferolateral aspect of the distal fragment to the contralateral metaphysis of the humerus (Figure 1B). The patient was put in a long-arm splint with the elbow flexed at 90° and the forearm in midpronation.
Follow-up visits were scheduled for 1 week, 3 weeks, and 5 weeks after surgery. Three weeks after surgery, callus formation was confirmed, and the K-wire was removed. Five weeks after surgery, the long-arm splint was removed.
At 6-month follow-up, the patient was pain-free and had full elbow ROM, and radiographs (Figures 2A, 2B) confirmed anatomical restoration of the fracture.
Discussion
Madsen2 reported the incidence of birth-related long-bone fractures, including fractures of the humerus, the femur, and the tibia (< 0.1%). According to that review, only 1 of 105,119 patients sustained traumatic physeal separation of the distal humerus.
Different mechanisms have been described for this rare fracture. As the physeal region is the weakest part of the distal humerus, it is prone to injury by rotational shear forces,3,4 hyperextension of the elbow, or a backward thrust on the forearm with the elbow flexed.5 Excessive traction applied during cesarean delivery might cause physeal separation, which was the possible cause in the present case. Most patients have a complicated birth history.
This injury should be suspected in an irritable newborn with swelling, tenderness, and reduced mobility of the upper extremity. Osteomyelitis and septic arthritis should be considered in the differential diagnosis. Brachial plexus injury and dislocation of the elbow joint should also be kept in mind. Child abuse and metabolic bone diseases (eg, osteogenesis imperfecta) should also be considered.
Anteroposterior and lateral plain radiographs of the elbow usually establish the diagnosis. Alteration of humeroulnar alignment and displacement of the proximal forearm are the key points leading to the diagnosis.
The cartilaginous part of the distal humerus and humeroulnar alignment can be demonstrated by ultrasonography.6 Magnetic resonance imaging (MRI) can be helpful in diagnosis but is seldom required,7 and the sedation or general anesthesia used is a disadvantage. Arthrography is useful not only in diagnosis but in determining the quality of the reduction.8 An arthrogram may show that open reduction is unnecessary.
Treatment differs widely. In neonates, who have a tremendous healing capability, this fracture almost always heals uneventfully. An effective treatment method is closed reduction and cast immobilization. However, valgus malalignment and limited elbow ROM were noted in 5% of the patients treated with this method.4
Jacobsen and colleagues4 reported on 6 neonates who sustained traumatic separation of the distal epiphysis of the humerus at birth and who were treated with casting with or without closed reduction. The authors described good results. One patient had varus malalignment, which was attributed to fragment internal rotation caused by rotational instability.
As our patient’s instability was noted during surgery, we performed percutaneous pinning after arthrography-assisted closed reduction. We considered using 2 lateral pins for fixation, but, after the first pin was placed, fluoroscopic stress testing with the patient under anesthesia demonstrated adequate stability. A second, smaller pin could have been used to control rotation, if needed. Medial pin placement that avoids the ulnar nerve is difficult in the newborn elbow; medial pins should probably be avoided in the newborn, if possible.
Early diagnosis and treatment are essential. Late diagnosis was reported to lead to complications such as varus deformity and restriction of joint ROM.4
Our patient healed without any complications and achieved full ROM. Long-term follow-up is needed to diagnose any physeal bar that might lead to secondary deformities.
Conclusion
Cesarean section is reported to reduce birth complications, but it might cause fractures of the femur and humerus.1 Avoiding application of excessive traction to the forearm can prevent physeal separation of the distal humerus. This entity should be kept in mind as a potential complication of cesarean section. Arthrography is helpful in treatment and may help avoid unnecessary open reduction.
1. Sabat D, Maini L, Gautam VK. Neonatal separation of distal humeral epiphysis during caesarean section: a case report. J Orthop Surg (Hong Kong). 2011;19(3):376-378.
2. Madsen ET. Fractures of the extremities in the newborn. Acta Obstet Gynecol Scand. 1955;34(1):41-74.
3. Peterson HA. Physeal fractures. In: Morrey BF, ed. The Elbow and Its Disorders. Philadelphia, PA: Saunders; 1985:222-236.
4. Jacobsen S, Hansson G, Nathorst-Westfelt J. Traumatic separation of the distal epiphysis of the humerus sustained at birth. J Bone Joint Surg Br. 2009;91(6):797-802.
5. Siffert RS. Displacement of distal humeral epiphysis in the newborn. J Bone Joint Surg Am. 1963;45(1):165-169.
6. Davidson RS, Markowitz RI, Dormans J, Drummond DS. Ultrasonographic evaluation of the elbow in infants and young children after suspected trauma. J Bone Joint Surg Am. 1994;76(12):1804-1813.
7. Sawant MR, Narayanan S, O‘Neill K, Hudson I. Distal humeral epiphysis fracture separation in neonates—diagnosis using MRI scan. Injury. 2002;33(2):179-181.
8. Akbarnia BA, Silberstein MJ, Rende RJ, Graviss ER, Luisiri A. Arthrography in the diagnosis of fractures of the distal end of the humerus in infants. J Bone Joint Surg Am. 1986;68(4):599-602.
Physeal separation of the distal humerus in a newborn is a rare and severe injury that requires immediate treatment. This fracture was reported as an extremely rare complication of cesarean section.1 The correct diagnosis can be established by clinical and radiologic findings. However, this injury can be easily overlooked and misdiagnosed. Presentation often involves swelling, tenderness, and agitation with movement of the elbow.
We report a case in which neonatal physeal separation of the distal humerus occurred during cesarean section. The diagnosis was based on clinical and radiologic/arthrographic findings and treated with closed reduction and percutaneous fixation. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A full-term (40-week gestation) male neonate weighing 3690 g was born through cesarean section at the mother’s request. Apgar score was 9 at 1 minute and 10 at 5 minutes. The vertex position of the fetus was confirmed with preoperative ultrasonography. This was the mother’s first pregnancy and an in vitro fertilization. On his second day of life, the patient was referred to the orthopedic department for evaluation of local swelling and diminished spontaneous motion of the right elbow.
Examination revealed local tenderness and swelling in the anterior and lateral aspects of the elbow. Passive elbow range of motion (ROM) caused agitation, and elbow instability was present. A complete neurovascular examination was performed, and neurovascular injury and compartment syndrome were ruled out. Hematologic workup showed no signs of septic arthritis. Radiographs showed posteromedial displacement of the humeroulnar joint. The patient was placed in a long-arm splint, and no reduction was attempted initially.
The patient was taken to the operating room the same day. With the patient under general anesthesia, an arthrogram of the right elbow was obtained. It showed posteromedial displacement of the distal humeral epiphysis (Figure 1A). Closed reduction was performed, and the quality of the reduction was confirmed by intraoperative imaging. Percutaneously, a single 2-mm Kirschner wire (K-wire) was placed in an oblique fashion from the inferolateral aspect of the distal fragment to the contralateral metaphysis of the humerus (Figure 1B). The patient was put in a long-arm splint with the elbow flexed at 90° and the forearm in midpronation.
Follow-up visits were scheduled for 1 week, 3 weeks, and 5 weeks after surgery. Three weeks after surgery, callus formation was confirmed, and the K-wire was removed. Five weeks after surgery, the long-arm splint was removed.
At 6-month follow-up, the patient was pain-free and had full elbow ROM, and radiographs (Figures 2A, 2B) confirmed anatomical restoration of the fracture.
Discussion
Madsen2 reported the incidence of birth-related long-bone fractures, including fractures of the humerus, the femur, and the tibia (< 0.1%). According to that review, only 1 of 105,119 patients sustained traumatic physeal separation of the distal humerus.
Different mechanisms have been described for this rare fracture. As the physeal region is the weakest part of the distal humerus, it is prone to injury by rotational shear forces,3,4 hyperextension of the elbow, or a backward thrust on the forearm with the elbow flexed.5 Excessive traction applied during cesarean delivery might cause physeal separation, which was the possible cause in the present case. Most patients have a complicated birth history.
This injury should be suspected in an irritable newborn with swelling, tenderness, and reduced mobility of the upper extremity. Osteomyelitis and septic arthritis should be considered in the differential diagnosis. Brachial plexus injury and dislocation of the elbow joint should also be kept in mind. Child abuse and metabolic bone diseases (eg, osteogenesis imperfecta) should also be considered.
Anteroposterior and lateral plain radiographs of the elbow usually establish the diagnosis. Alteration of humeroulnar alignment and displacement of the proximal forearm are the key points leading to the diagnosis.
The cartilaginous part of the distal humerus and humeroulnar alignment can be demonstrated by ultrasonography.6 Magnetic resonance imaging (MRI) can be helpful in diagnosis but is seldom required,7 and the sedation or general anesthesia used is a disadvantage. Arthrography is useful not only in diagnosis but in determining the quality of the reduction.8 An arthrogram may show that open reduction is unnecessary.
Treatment differs widely. In neonates, who have a tremendous healing capability, this fracture almost always heals uneventfully. An effective treatment method is closed reduction and cast immobilization. However, valgus malalignment and limited elbow ROM were noted in 5% of the patients treated with this method.4
Jacobsen and colleagues4 reported on 6 neonates who sustained traumatic separation of the distal epiphysis of the humerus at birth and who were treated with casting with or without closed reduction. The authors described good results. One patient had varus malalignment, which was attributed to fragment internal rotation caused by rotational instability.
As our patient’s instability was noted during surgery, we performed percutaneous pinning after arthrography-assisted closed reduction. We considered using 2 lateral pins for fixation, but, after the first pin was placed, fluoroscopic stress testing with the patient under anesthesia demonstrated adequate stability. A second, smaller pin could have been used to control rotation, if needed. Medial pin placement that avoids the ulnar nerve is difficult in the newborn elbow; medial pins should probably be avoided in the newborn, if possible.
Early diagnosis and treatment are essential. Late diagnosis was reported to lead to complications such as varus deformity and restriction of joint ROM.4
Our patient healed without any complications and achieved full ROM. Long-term follow-up is needed to diagnose any physeal bar that might lead to secondary deformities.
Conclusion
Cesarean section is reported to reduce birth complications, but it might cause fractures of the femur and humerus.1 Avoiding application of excessive traction to the forearm can prevent physeal separation of the distal humerus. This entity should be kept in mind as a potential complication of cesarean section. Arthrography is helpful in treatment and may help avoid unnecessary open reduction.
Physeal separation of the distal humerus in a newborn is a rare and severe injury that requires immediate treatment. This fracture was reported as an extremely rare complication of cesarean section.1 The correct diagnosis can be established by clinical and radiologic findings. However, this injury can be easily overlooked and misdiagnosed. Presentation often involves swelling, tenderness, and agitation with movement of the elbow.
We report a case in which neonatal physeal separation of the distal humerus occurred during cesarean section. The diagnosis was based on clinical and radiologic/arthrographic findings and treated with closed reduction and percutaneous fixation. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A full-term (40-week gestation) male neonate weighing 3690 g was born through cesarean section at the mother’s request. Apgar score was 9 at 1 minute and 10 at 5 minutes. The vertex position of the fetus was confirmed with preoperative ultrasonography. This was the mother’s first pregnancy and an in vitro fertilization. On his second day of life, the patient was referred to the orthopedic department for evaluation of local swelling and diminished spontaneous motion of the right elbow.
Examination revealed local tenderness and swelling in the anterior and lateral aspects of the elbow. Passive elbow range of motion (ROM) caused agitation, and elbow instability was present. A complete neurovascular examination was performed, and neurovascular injury and compartment syndrome were ruled out. Hematologic workup showed no signs of septic arthritis. Radiographs showed posteromedial displacement of the humeroulnar joint. The patient was placed in a long-arm splint, and no reduction was attempted initially.
The patient was taken to the operating room the same day. With the patient under general anesthesia, an arthrogram of the right elbow was obtained. It showed posteromedial displacement of the distal humeral epiphysis (Figure 1A). Closed reduction was performed, and the quality of the reduction was confirmed by intraoperative imaging. Percutaneously, a single 2-mm Kirschner wire (K-wire) was placed in an oblique fashion from the inferolateral aspect of the distal fragment to the contralateral metaphysis of the humerus (Figure 1B). The patient was put in a long-arm splint with the elbow flexed at 90° and the forearm in midpronation.
Follow-up visits were scheduled for 1 week, 3 weeks, and 5 weeks after surgery. Three weeks after surgery, callus formation was confirmed, and the K-wire was removed. Five weeks after surgery, the long-arm splint was removed.
At 6-month follow-up, the patient was pain-free and had full elbow ROM, and radiographs (Figures 2A, 2B) confirmed anatomical restoration of the fracture.
Discussion
Madsen2 reported the incidence of birth-related long-bone fractures, including fractures of the humerus, the femur, and the tibia (< 0.1%). According to that review, only 1 of 105,119 patients sustained traumatic physeal separation of the distal humerus.
Different mechanisms have been described for this rare fracture. As the physeal region is the weakest part of the distal humerus, it is prone to injury by rotational shear forces,3,4 hyperextension of the elbow, or a backward thrust on the forearm with the elbow flexed.5 Excessive traction applied during cesarean delivery might cause physeal separation, which was the possible cause in the present case. Most patients have a complicated birth history.
This injury should be suspected in an irritable newborn with swelling, tenderness, and reduced mobility of the upper extremity. Osteomyelitis and septic arthritis should be considered in the differential diagnosis. Brachial plexus injury and dislocation of the elbow joint should also be kept in mind. Child abuse and metabolic bone diseases (eg, osteogenesis imperfecta) should also be considered.
Anteroposterior and lateral plain radiographs of the elbow usually establish the diagnosis. Alteration of humeroulnar alignment and displacement of the proximal forearm are the key points leading to the diagnosis.
The cartilaginous part of the distal humerus and humeroulnar alignment can be demonstrated by ultrasonography.6 Magnetic resonance imaging (MRI) can be helpful in diagnosis but is seldom required,7 and the sedation or general anesthesia used is a disadvantage. Arthrography is useful not only in diagnosis but in determining the quality of the reduction.8 An arthrogram may show that open reduction is unnecessary.
Treatment differs widely. In neonates, who have a tremendous healing capability, this fracture almost always heals uneventfully. An effective treatment method is closed reduction and cast immobilization. However, valgus malalignment and limited elbow ROM were noted in 5% of the patients treated with this method.4
Jacobsen and colleagues4 reported on 6 neonates who sustained traumatic separation of the distal epiphysis of the humerus at birth and who were treated with casting with or without closed reduction. The authors described good results. One patient had varus malalignment, which was attributed to fragment internal rotation caused by rotational instability.
As our patient’s instability was noted during surgery, we performed percutaneous pinning after arthrography-assisted closed reduction. We considered using 2 lateral pins for fixation, but, after the first pin was placed, fluoroscopic stress testing with the patient under anesthesia demonstrated adequate stability. A second, smaller pin could have been used to control rotation, if needed. Medial pin placement that avoids the ulnar nerve is difficult in the newborn elbow; medial pins should probably be avoided in the newborn, if possible.
Early diagnosis and treatment are essential. Late diagnosis was reported to lead to complications such as varus deformity and restriction of joint ROM.4
Our patient healed without any complications and achieved full ROM. Long-term follow-up is needed to diagnose any physeal bar that might lead to secondary deformities.
Conclusion
Cesarean section is reported to reduce birth complications, but it might cause fractures of the femur and humerus.1 Avoiding application of excessive traction to the forearm can prevent physeal separation of the distal humerus. This entity should be kept in mind as a potential complication of cesarean section. Arthrography is helpful in treatment and may help avoid unnecessary open reduction.
1. Sabat D, Maini L, Gautam VK. Neonatal separation of distal humeral epiphysis during caesarean section: a case report. J Orthop Surg (Hong Kong). 2011;19(3):376-378.
2. Madsen ET. Fractures of the extremities in the newborn. Acta Obstet Gynecol Scand. 1955;34(1):41-74.
3. Peterson HA. Physeal fractures. In: Morrey BF, ed. The Elbow and Its Disorders. Philadelphia, PA: Saunders; 1985:222-236.
4. Jacobsen S, Hansson G, Nathorst-Westfelt J. Traumatic separation of the distal epiphysis of the humerus sustained at birth. J Bone Joint Surg Br. 2009;91(6):797-802.
5. Siffert RS. Displacement of distal humeral epiphysis in the newborn. J Bone Joint Surg Am. 1963;45(1):165-169.
6. Davidson RS, Markowitz RI, Dormans J, Drummond DS. Ultrasonographic evaluation of the elbow in infants and young children after suspected trauma. J Bone Joint Surg Am. 1994;76(12):1804-1813.
7. Sawant MR, Narayanan S, O‘Neill K, Hudson I. Distal humeral epiphysis fracture separation in neonates—diagnosis using MRI scan. Injury. 2002;33(2):179-181.
8. Akbarnia BA, Silberstein MJ, Rende RJ, Graviss ER, Luisiri A. Arthrography in the diagnosis of fractures of the distal end of the humerus in infants. J Bone Joint Surg Am. 1986;68(4):599-602.
1. Sabat D, Maini L, Gautam VK. Neonatal separation of distal humeral epiphysis during caesarean section: a case report. J Orthop Surg (Hong Kong). 2011;19(3):376-378.
2. Madsen ET. Fractures of the extremities in the newborn. Acta Obstet Gynecol Scand. 1955;34(1):41-74.
3. Peterson HA. Physeal fractures. In: Morrey BF, ed. The Elbow and Its Disorders. Philadelphia, PA: Saunders; 1985:222-236.
4. Jacobsen S, Hansson G, Nathorst-Westfelt J. Traumatic separation of the distal epiphysis of the humerus sustained at birth. J Bone Joint Surg Br. 2009;91(6):797-802.
5. Siffert RS. Displacement of distal humeral epiphysis in the newborn. J Bone Joint Surg Am. 1963;45(1):165-169.
6. Davidson RS, Markowitz RI, Dormans J, Drummond DS. Ultrasonographic evaluation of the elbow in infants and young children after suspected trauma. J Bone Joint Surg Am. 1994;76(12):1804-1813.
7. Sawant MR, Narayanan S, O‘Neill K, Hudson I. Distal humeral epiphysis fracture separation in neonates—diagnosis using MRI scan. Injury. 2002;33(2):179-181.
8. Akbarnia BA, Silberstein MJ, Rende RJ, Graviss ER, Luisiri A. Arthrography in the diagnosis of fractures of the distal end of the humerus in infants. J Bone Joint Surg Am. 1986;68(4):599-602.
Concurrent Treatment of a Middle-Third Clavicle Fracture and Type IV Acromioclavicular Dislocation
Acromioclavicular (AC) dislocations and displaced fractures of the middle third of the clavicle rarely occur together. Isolated AC joint separation is often treated nonoperatively with internal coracoclavicular (CC) fixation or reconstruction considered for type IV-VI AC dislocations and some type III injuries.1 Isolated clavicle fractures traditionally have been treated nonoperatively. The current trend is toward internal fixation for displaced and shortened fractures.2 There have been only a handful of reports of concomitant AC dislocation and midshaft clavicle fracture.3-6 Previous treatments have included nonoperative treatment, AC fixation, or internal fixation of the clavicle with ligamentous reconstruction.
We present a previously undescribed technique for internal fixation of this rare shoulder injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
While driving an all-terrain vehicle, a healthy 19-year-old right-hand–dominant man hit a bridge and sustained direct impact to his right shoulder. He presented to the emergency department complaining of right shoulder pain and deformity without skin disruption, vascular insufficiency, or neurologic symptoms. Anteroposterior (AP) radiograph showed an oblique, displaced, middle-third clavicle shaft fracture (Figure 1). An associated type IV AC dislocation was confirmed on axillary radiograph (Figure 2) and on an axial cut from a trauma chest computed tomography (CT) scan (Figure 3). The patient was discharged home from the trauma service the next day with a sling for comfort and plans for delayed, elective operative fixation 1 week later.
The patient was placed in a beach-chair position. Through a longitudinal incision extending laterally over the AC joint, the clavicle was exposed for fracture reduction, with care taken to retain soft-tissue attachments. The distal clavicle was buttonholed posteriorly through the trapezius muscle and fascia. The distal fracture fragment was devoid of any remaining CC ligamentous attachment. After satisfactory reduction, a low-profile precontoured clavicle plate (Superior Midshaft Clavicle Plate; Acumed, Hillsboro, Oregon) was placed superiorly; the fracture was compressed through the plate and internally fixed with three 3.5-mm bicortical screws on both sides of the fracture. Approximately 5 mm of the distal clavicle was resected at the AC joint to facilitate adequate AC and CC reduction without disruption of the clavicle fracture. With an adequate CC reduction, a 3.5-mm fully threaded cortical screw was placed through the most distal hole in the clavicle plate, clavicle, and coracoid.
After surgery, the patient was placed into an ARC shoulder immobilizer (Bledsoe, Grand Prairie, Texas) for 6 weeks, removing the immobilizer only for elbow and wrist range of motion (ROM) exercises. Radiographs at 3-month follow-up (Figure 4) showed a healed fracture with no loss of AC or CC reduction. Three months after surgery, another procedure was performed to remove the CC screw. One year after the initial surgery, the patient complained of intermittent soreness over the lateral shoulder but was not limited in his activities and was back to performing manual labor without difficulty. He had full ROM in forward flexion, abduction, internal rotation, and external rotation without weakness, tenderness, or any neurovascular deficit. After CC screw removal, no deformity returned at the shoulder. Radiographs showed a healed fracture with minimal superior subluxation at the AC joint without significant change from the 3-month follow-up (Figure 5).
Discussion
The combined injury pattern of a type IV AC dislocation and a displaced middle-third clavicle shaft fracture is rare. The usual mechanism of injury, as seen in the present case, is a direct blow to the shoulder at the tip of the acromion, though indirect forces from a fall on an outstretched hand are also described.7 Disruption of the CC ligaments with AC separation likely dissipates the stress necessary to create a clavicle fracture in most cases,1 explaining the rarity of this injury. It is imperative to evaluate patients for injury to both the osseous and ligamentous structures.
Previous case reports of concomitant AC separation and midshaft clavicle fracture have described a variety of treatment options, but to date our case represents the only episode in which both the clavicle fracture and the AC joint were treated with open reduction and internal fixation (ORIF). Wurtz and colleagues5 reported on a series of 4 patients with AC disruption and middle-third clavicle fracture. Three of the 4 patients had type IV AC separation; all 3 were treated, 2 acutely and 1 chronically, with open reduction of the AC and CC joints; 2 of these patients had CC screw fixation only after reduction, and the third had 2 Steinmann pins placed across the AC joint without CC screw fixation. All hardware was removed after 12 weeks. The fourth patient had a type II AC dislocation and was treated with closed reduction of the clavicle with no intervention for the AC joint. None of the clavicle fractures in this series were treated with internal fixation. All patients had full and pain-free ROM at 1- to 3-year follow-up.
Juhn and Simonian3 reported on a case of type VI separation with greenstick midshaft clavicle fracture in a hockey player seen 7 days after injury. The patient described some tingling in the upper extremity and had shoulder pain on initial presentation but was noted to have minimal displacement of both the AC joint and the midshaft clavicle fracture. Both injuries were treated nonsurgically with good outcome, and the patient returned to full activity (including hockey) within 14 weeks after injury.
Lancourt4 described the case of a patient with a type V AC dislocation and a displaced midshaft clavicle fracture. The AC joint was treated with Steinmann pin fixation, and the clavicle fracture was treated nonoperatively. The author cited high complication rates of plate fixation for clavicle fractures as the reason for not performing the additional procedure. The pins were removed 8 weeks after surgery. At 3-year follow-up, the patient had good radiographic and clinical outcome.
Yeh and colleagues6 described a patient who sustained a displaced midshaft clavicle fracture and a type IV AC dislocation in a fall from a horse. The patient underwent ORIF of the clavicle fracture with plate fixation. After the procedure, the AC joint was still unstable intraoperatively, and the AC and CC ligaments were reconstructed with semitendinosus allograft. The patient had full and painless ROM at 1-year follow-up.
The present case report serves as a reminder to obtain adequate shoulder radiographs when evaluating “just another clavicle fracture.” The radiographs should include a good axillary view to ensure there is not an associated AC dislocation. Increasingly, some authors have been advocating internal fixation for clavicle fractures, with reports of improved functional outcomes, improved cosmesis, and increased union rates.2 Indications for operative fixation include shortening and 100% displacement,8 and relative indications include open fractures.1 Operative fixation is perhaps more important for younger, athletic, and manual-labor populations. The trend in treatment of clavicle fractures toward operative fixation lends itself well to ORIF of the AC and CC joints; hence, a modern treatment for this rarely described combination injury should include internal plate fixation of the clavicle in addition to CC fixation. This additional procedure requires little extra time and energy in an operative scenario already requiring anesthesia, with easy insertion of the CC screw through the clavicle plate. Use of a CC screw obviates any potential risks associated with use of allograft tissue, and there is no anticipated difficulty with screw removal at 12 weeks.
Alternative options for AC stability include CC reconstruction with ligamentous allograft, ligamentous autograft, or suture/tightrope techniques. A noted advantage of these alternative techniques is less need to return to the operating room for the hardware removal that is recommended with CC screw fixation. However, these procedures potentially increase surgical exposure and operating time. In addition, screw fixation minimizes the possibility of donor-site morbidity from autograft transfers and potential complications from allograft tissue.
Hook plate fixation of the AC joint has also been described. In a recent case report of a similar injury pattern, plate fixation of the clavicle with simultaneous hook plate fixation of the AC joint was described.9 The patient did well but required removal of hardware of the hook plate and the clavicle plate 1 and 3 years after surgery, respectively. Although screw fixation is biomechanically stronger, debate persists about the clinical importance of this increase in strength.1 In the setting of plate fixation for the clavicle, these alternative AC fixations would require technique adjustments, including length of grafts and/or sutures, and raise concerns regarding interaction of the metal with the fixation material.
Critical evaluation of our technique revealed a lucency larger than the screw (Figure 5). However, the screw was not clinically loose at removal. This potential complication, in combination with the bent screw (Figure 4) before removal, highlights the concern for screw breakage with this technique, given the increased construct stiffness caused by the added plate.
Conclusion
As in the other reports mentioned, our patient had an excellent clinical and radiographic outcome. It could be inferred that, if fixation for isolated clavicle fractures demonstrates improved function, better outcomes would be seen for higher-energy fractures associated with AC dislocation. Given the current trend toward surgical fixation for certain clavicle fractures, we recommend that clavicle fractures associated with type IV AC dislocation be treated with ORIF of both injuries.
1. Ring D, Jupiter J. Injuries to the shoulder girdle. In: Browner, BD. Skeletal Trauma. Philadelphia, PA: Elsevier Health Sciences; 2008:1755-1778.
2. Altamimi SA, McKee MD; Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 1):1-8.
3. Juhn MS, Simonian PT. Type VI acromioclavicular separation with middle-third clavicle fracture in an ice hockey player. Clin J Sports Med. 2002;12(5):315-317.
4. Lancourt JE. Acromioclavicular dislocation with adjacent clavicular fracture in a horseback rider. A case report. Am J Sports Med. 1990;18(3):321-322.
5. Wurtz LD, Lyons FA, Rockwood CA Jr. Fracture of the middle third of the clavicle and dislocation of the acromioclavicular joint. A report of four cases. J Bone Joint Surg Am. 1992;74(1):133-137.
6. Yeh PC, Miller SR, Cunningham JG, Sethi PM. Midshaft clavicle fracture and acromioclavicular dislocation: a case report of a rare injury. J Shoulder Elbow Surg. 2009;18(5):e1-e4.
7. Stanley D, Trowbridge EA, Norris SH. The mechanism of clavicular fracture. A clinical and biomechanical analysis. J Bone Joint Surg Br. 1988;70(3):461-464.
8. Kim W, McKee MD. Management of acute clavicle fractures. Orthop Clin North Am. 2008;39(4):491-505.
9. Woolf SK, Valentine BJ, Barfield WR, Hartsock LA. Middle-third clavicle fracture with associated type IV acromioclavicular separation: case report and literature review. J Surg Orthop Adv. 2013;22(2):183-186.
Acromioclavicular (AC) dislocations and displaced fractures of the middle third of the clavicle rarely occur together. Isolated AC joint separation is often treated nonoperatively with internal coracoclavicular (CC) fixation or reconstruction considered for type IV-VI AC dislocations and some type III injuries.1 Isolated clavicle fractures traditionally have been treated nonoperatively. The current trend is toward internal fixation for displaced and shortened fractures.2 There have been only a handful of reports of concomitant AC dislocation and midshaft clavicle fracture.3-6 Previous treatments have included nonoperative treatment, AC fixation, or internal fixation of the clavicle with ligamentous reconstruction.
We present a previously undescribed technique for internal fixation of this rare shoulder injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
While driving an all-terrain vehicle, a healthy 19-year-old right-hand–dominant man hit a bridge and sustained direct impact to his right shoulder. He presented to the emergency department complaining of right shoulder pain and deformity without skin disruption, vascular insufficiency, or neurologic symptoms. Anteroposterior (AP) radiograph showed an oblique, displaced, middle-third clavicle shaft fracture (Figure 1). An associated type IV AC dislocation was confirmed on axillary radiograph (Figure 2) and on an axial cut from a trauma chest computed tomography (CT) scan (Figure 3). The patient was discharged home from the trauma service the next day with a sling for comfort and plans for delayed, elective operative fixation 1 week later.
The patient was placed in a beach-chair position. Through a longitudinal incision extending laterally over the AC joint, the clavicle was exposed for fracture reduction, with care taken to retain soft-tissue attachments. The distal clavicle was buttonholed posteriorly through the trapezius muscle and fascia. The distal fracture fragment was devoid of any remaining CC ligamentous attachment. After satisfactory reduction, a low-profile precontoured clavicle plate (Superior Midshaft Clavicle Plate; Acumed, Hillsboro, Oregon) was placed superiorly; the fracture was compressed through the plate and internally fixed with three 3.5-mm bicortical screws on both sides of the fracture. Approximately 5 mm of the distal clavicle was resected at the AC joint to facilitate adequate AC and CC reduction without disruption of the clavicle fracture. With an adequate CC reduction, a 3.5-mm fully threaded cortical screw was placed through the most distal hole in the clavicle plate, clavicle, and coracoid.
After surgery, the patient was placed into an ARC shoulder immobilizer (Bledsoe, Grand Prairie, Texas) for 6 weeks, removing the immobilizer only for elbow and wrist range of motion (ROM) exercises. Radiographs at 3-month follow-up (Figure 4) showed a healed fracture with no loss of AC or CC reduction. Three months after surgery, another procedure was performed to remove the CC screw. One year after the initial surgery, the patient complained of intermittent soreness over the lateral shoulder but was not limited in his activities and was back to performing manual labor without difficulty. He had full ROM in forward flexion, abduction, internal rotation, and external rotation without weakness, tenderness, or any neurovascular deficit. After CC screw removal, no deformity returned at the shoulder. Radiographs showed a healed fracture with minimal superior subluxation at the AC joint without significant change from the 3-month follow-up (Figure 5).
Discussion
The combined injury pattern of a type IV AC dislocation and a displaced middle-third clavicle shaft fracture is rare. The usual mechanism of injury, as seen in the present case, is a direct blow to the shoulder at the tip of the acromion, though indirect forces from a fall on an outstretched hand are also described.7 Disruption of the CC ligaments with AC separation likely dissipates the stress necessary to create a clavicle fracture in most cases,1 explaining the rarity of this injury. It is imperative to evaluate patients for injury to both the osseous and ligamentous structures.
Previous case reports of concomitant AC separation and midshaft clavicle fracture have described a variety of treatment options, but to date our case represents the only episode in which both the clavicle fracture and the AC joint were treated with open reduction and internal fixation (ORIF). Wurtz and colleagues5 reported on a series of 4 patients with AC disruption and middle-third clavicle fracture. Three of the 4 patients had type IV AC separation; all 3 were treated, 2 acutely and 1 chronically, with open reduction of the AC and CC joints; 2 of these patients had CC screw fixation only after reduction, and the third had 2 Steinmann pins placed across the AC joint without CC screw fixation. All hardware was removed after 12 weeks. The fourth patient had a type II AC dislocation and was treated with closed reduction of the clavicle with no intervention for the AC joint. None of the clavicle fractures in this series were treated with internal fixation. All patients had full and pain-free ROM at 1- to 3-year follow-up.
Juhn and Simonian3 reported on a case of type VI separation with greenstick midshaft clavicle fracture in a hockey player seen 7 days after injury. The patient described some tingling in the upper extremity and had shoulder pain on initial presentation but was noted to have minimal displacement of both the AC joint and the midshaft clavicle fracture. Both injuries were treated nonsurgically with good outcome, and the patient returned to full activity (including hockey) within 14 weeks after injury.
Lancourt4 described the case of a patient with a type V AC dislocation and a displaced midshaft clavicle fracture. The AC joint was treated with Steinmann pin fixation, and the clavicle fracture was treated nonoperatively. The author cited high complication rates of plate fixation for clavicle fractures as the reason for not performing the additional procedure. The pins were removed 8 weeks after surgery. At 3-year follow-up, the patient had good radiographic and clinical outcome.
Yeh and colleagues6 described a patient who sustained a displaced midshaft clavicle fracture and a type IV AC dislocation in a fall from a horse. The patient underwent ORIF of the clavicle fracture with plate fixation. After the procedure, the AC joint was still unstable intraoperatively, and the AC and CC ligaments were reconstructed with semitendinosus allograft. The patient had full and painless ROM at 1-year follow-up.
The present case report serves as a reminder to obtain adequate shoulder radiographs when evaluating “just another clavicle fracture.” The radiographs should include a good axillary view to ensure there is not an associated AC dislocation. Increasingly, some authors have been advocating internal fixation for clavicle fractures, with reports of improved functional outcomes, improved cosmesis, and increased union rates.2 Indications for operative fixation include shortening and 100% displacement,8 and relative indications include open fractures.1 Operative fixation is perhaps more important for younger, athletic, and manual-labor populations. The trend in treatment of clavicle fractures toward operative fixation lends itself well to ORIF of the AC and CC joints; hence, a modern treatment for this rarely described combination injury should include internal plate fixation of the clavicle in addition to CC fixation. This additional procedure requires little extra time and energy in an operative scenario already requiring anesthesia, with easy insertion of the CC screw through the clavicle plate. Use of a CC screw obviates any potential risks associated with use of allograft tissue, and there is no anticipated difficulty with screw removal at 12 weeks.
Alternative options for AC stability include CC reconstruction with ligamentous allograft, ligamentous autograft, or suture/tightrope techniques. A noted advantage of these alternative techniques is less need to return to the operating room for the hardware removal that is recommended with CC screw fixation. However, these procedures potentially increase surgical exposure and operating time. In addition, screw fixation minimizes the possibility of donor-site morbidity from autograft transfers and potential complications from allograft tissue.
Hook plate fixation of the AC joint has also been described. In a recent case report of a similar injury pattern, plate fixation of the clavicle with simultaneous hook plate fixation of the AC joint was described.9 The patient did well but required removal of hardware of the hook plate and the clavicle plate 1 and 3 years after surgery, respectively. Although screw fixation is biomechanically stronger, debate persists about the clinical importance of this increase in strength.1 In the setting of plate fixation for the clavicle, these alternative AC fixations would require technique adjustments, including length of grafts and/or sutures, and raise concerns regarding interaction of the metal with the fixation material.
Critical evaluation of our technique revealed a lucency larger than the screw (Figure 5). However, the screw was not clinically loose at removal. This potential complication, in combination with the bent screw (Figure 4) before removal, highlights the concern for screw breakage with this technique, given the increased construct stiffness caused by the added plate.
Conclusion
As in the other reports mentioned, our patient had an excellent clinical and radiographic outcome. It could be inferred that, if fixation for isolated clavicle fractures demonstrates improved function, better outcomes would be seen for higher-energy fractures associated with AC dislocation. Given the current trend toward surgical fixation for certain clavicle fractures, we recommend that clavicle fractures associated with type IV AC dislocation be treated with ORIF of both injuries.
Acromioclavicular (AC) dislocations and displaced fractures of the middle third of the clavicle rarely occur together. Isolated AC joint separation is often treated nonoperatively with internal coracoclavicular (CC) fixation or reconstruction considered for type IV-VI AC dislocations and some type III injuries.1 Isolated clavicle fractures traditionally have been treated nonoperatively. The current trend is toward internal fixation for displaced and shortened fractures.2 There have been only a handful of reports of concomitant AC dislocation and midshaft clavicle fracture.3-6 Previous treatments have included nonoperative treatment, AC fixation, or internal fixation of the clavicle with ligamentous reconstruction.
We present a previously undescribed technique for internal fixation of this rare shoulder injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
While driving an all-terrain vehicle, a healthy 19-year-old right-hand–dominant man hit a bridge and sustained direct impact to his right shoulder. He presented to the emergency department complaining of right shoulder pain and deformity without skin disruption, vascular insufficiency, or neurologic symptoms. Anteroposterior (AP) radiograph showed an oblique, displaced, middle-third clavicle shaft fracture (Figure 1). An associated type IV AC dislocation was confirmed on axillary radiograph (Figure 2) and on an axial cut from a trauma chest computed tomography (CT) scan (Figure 3). The patient was discharged home from the trauma service the next day with a sling for comfort and plans for delayed, elective operative fixation 1 week later.
The patient was placed in a beach-chair position. Through a longitudinal incision extending laterally over the AC joint, the clavicle was exposed for fracture reduction, with care taken to retain soft-tissue attachments. The distal clavicle was buttonholed posteriorly through the trapezius muscle and fascia. The distal fracture fragment was devoid of any remaining CC ligamentous attachment. After satisfactory reduction, a low-profile precontoured clavicle plate (Superior Midshaft Clavicle Plate; Acumed, Hillsboro, Oregon) was placed superiorly; the fracture was compressed through the plate and internally fixed with three 3.5-mm bicortical screws on both sides of the fracture. Approximately 5 mm of the distal clavicle was resected at the AC joint to facilitate adequate AC and CC reduction without disruption of the clavicle fracture. With an adequate CC reduction, a 3.5-mm fully threaded cortical screw was placed through the most distal hole in the clavicle plate, clavicle, and coracoid.
After surgery, the patient was placed into an ARC shoulder immobilizer (Bledsoe, Grand Prairie, Texas) for 6 weeks, removing the immobilizer only for elbow and wrist range of motion (ROM) exercises. Radiographs at 3-month follow-up (Figure 4) showed a healed fracture with no loss of AC or CC reduction. Three months after surgery, another procedure was performed to remove the CC screw. One year after the initial surgery, the patient complained of intermittent soreness over the lateral shoulder but was not limited in his activities and was back to performing manual labor without difficulty. He had full ROM in forward flexion, abduction, internal rotation, and external rotation without weakness, tenderness, or any neurovascular deficit. After CC screw removal, no deformity returned at the shoulder. Radiographs showed a healed fracture with minimal superior subluxation at the AC joint without significant change from the 3-month follow-up (Figure 5).
Discussion
The combined injury pattern of a type IV AC dislocation and a displaced middle-third clavicle shaft fracture is rare. The usual mechanism of injury, as seen in the present case, is a direct blow to the shoulder at the tip of the acromion, though indirect forces from a fall on an outstretched hand are also described.7 Disruption of the CC ligaments with AC separation likely dissipates the stress necessary to create a clavicle fracture in most cases,1 explaining the rarity of this injury. It is imperative to evaluate patients for injury to both the osseous and ligamentous structures.
Previous case reports of concomitant AC separation and midshaft clavicle fracture have described a variety of treatment options, but to date our case represents the only episode in which both the clavicle fracture and the AC joint were treated with open reduction and internal fixation (ORIF). Wurtz and colleagues5 reported on a series of 4 patients with AC disruption and middle-third clavicle fracture. Three of the 4 patients had type IV AC separation; all 3 were treated, 2 acutely and 1 chronically, with open reduction of the AC and CC joints; 2 of these patients had CC screw fixation only after reduction, and the third had 2 Steinmann pins placed across the AC joint without CC screw fixation. All hardware was removed after 12 weeks. The fourth patient had a type II AC dislocation and was treated with closed reduction of the clavicle with no intervention for the AC joint. None of the clavicle fractures in this series were treated with internal fixation. All patients had full and pain-free ROM at 1- to 3-year follow-up.
Juhn and Simonian3 reported on a case of type VI separation with greenstick midshaft clavicle fracture in a hockey player seen 7 days after injury. The patient described some tingling in the upper extremity and had shoulder pain on initial presentation but was noted to have minimal displacement of both the AC joint and the midshaft clavicle fracture. Both injuries were treated nonsurgically with good outcome, and the patient returned to full activity (including hockey) within 14 weeks after injury.
Lancourt4 described the case of a patient with a type V AC dislocation and a displaced midshaft clavicle fracture. The AC joint was treated with Steinmann pin fixation, and the clavicle fracture was treated nonoperatively. The author cited high complication rates of plate fixation for clavicle fractures as the reason for not performing the additional procedure. The pins were removed 8 weeks after surgery. At 3-year follow-up, the patient had good radiographic and clinical outcome.
Yeh and colleagues6 described a patient who sustained a displaced midshaft clavicle fracture and a type IV AC dislocation in a fall from a horse. The patient underwent ORIF of the clavicle fracture with plate fixation. After the procedure, the AC joint was still unstable intraoperatively, and the AC and CC ligaments were reconstructed with semitendinosus allograft. The patient had full and painless ROM at 1-year follow-up.
The present case report serves as a reminder to obtain adequate shoulder radiographs when evaluating “just another clavicle fracture.” The radiographs should include a good axillary view to ensure there is not an associated AC dislocation. Increasingly, some authors have been advocating internal fixation for clavicle fractures, with reports of improved functional outcomes, improved cosmesis, and increased union rates.2 Indications for operative fixation include shortening and 100% displacement,8 and relative indications include open fractures.1 Operative fixation is perhaps more important for younger, athletic, and manual-labor populations. The trend in treatment of clavicle fractures toward operative fixation lends itself well to ORIF of the AC and CC joints; hence, a modern treatment for this rarely described combination injury should include internal plate fixation of the clavicle in addition to CC fixation. This additional procedure requires little extra time and energy in an operative scenario already requiring anesthesia, with easy insertion of the CC screw through the clavicle plate. Use of a CC screw obviates any potential risks associated with use of allograft tissue, and there is no anticipated difficulty with screw removal at 12 weeks.
Alternative options for AC stability include CC reconstruction with ligamentous allograft, ligamentous autograft, or suture/tightrope techniques. A noted advantage of these alternative techniques is less need to return to the operating room for the hardware removal that is recommended with CC screw fixation. However, these procedures potentially increase surgical exposure and operating time. In addition, screw fixation minimizes the possibility of donor-site morbidity from autograft transfers and potential complications from allograft tissue.
Hook plate fixation of the AC joint has also been described. In a recent case report of a similar injury pattern, plate fixation of the clavicle with simultaneous hook plate fixation of the AC joint was described.9 The patient did well but required removal of hardware of the hook plate and the clavicle plate 1 and 3 years after surgery, respectively. Although screw fixation is biomechanically stronger, debate persists about the clinical importance of this increase in strength.1 In the setting of plate fixation for the clavicle, these alternative AC fixations would require technique adjustments, including length of grafts and/or sutures, and raise concerns regarding interaction of the metal with the fixation material.
Critical evaluation of our technique revealed a lucency larger than the screw (Figure 5). However, the screw was not clinically loose at removal. This potential complication, in combination with the bent screw (Figure 4) before removal, highlights the concern for screw breakage with this technique, given the increased construct stiffness caused by the added plate.
Conclusion
As in the other reports mentioned, our patient had an excellent clinical and radiographic outcome. It could be inferred that, if fixation for isolated clavicle fractures demonstrates improved function, better outcomes would be seen for higher-energy fractures associated with AC dislocation. Given the current trend toward surgical fixation for certain clavicle fractures, we recommend that clavicle fractures associated with type IV AC dislocation be treated with ORIF of both injuries.
1. Ring D, Jupiter J. Injuries to the shoulder girdle. In: Browner, BD. Skeletal Trauma. Philadelphia, PA: Elsevier Health Sciences; 2008:1755-1778.
2. Altamimi SA, McKee MD; Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 1):1-8.
3. Juhn MS, Simonian PT. Type VI acromioclavicular separation with middle-third clavicle fracture in an ice hockey player. Clin J Sports Med. 2002;12(5):315-317.
4. Lancourt JE. Acromioclavicular dislocation with adjacent clavicular fracture in a horseback rider. A case report. Am J Sports Med. 1990;18(3):321-322.
5. Wurtz LD, Lyons FA, Rockwood CA Jr. Fracture of the middle third of the clavicle and dislocation of the acromioclavicular joint. A report of four cases. J Bone Joint Surg Am. 1992;74(1):133-137.
6. Yeh PC, Miller SR, Cunningham JG, Sethi PM. Midshaft clavicle fracture and acromioclavicular dislocation: a case report of a rare injury. J Shoulder Elbow Surg. 2009;18(5):e1-e4.
7. Stanley D, Trowbridge EA, Norris SH. The mechanism of clavicular fracture. A clinical and biomechanical analysis. J Bone Joint Surg Br. 1988;70(3):461-464.
8. Kim W, McKee MD. Management of acute clavicle fractures. Orthop Clin North Am. 2008;39(4):491-505.
9. Woolf SK, Valentine BJ, Barfield WR, Hartsock LA. Middle-third clavicle fracture with associated type IV acromioclavicular separation: case report and literature review. J Surg Orthop Adv. 2013;22(2):183-186.
1. Ring D, Jupiter J. Injuries to the shoulder girdle. In: Browner, BD. Skeletal Trauma. Philadelphia, PA: Elsevier Health Sciences; 2008:1755-1778.
2. Altamimi SA, McKee MD; Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 1):1-8.
3. Juhn MS, Simonian PT. Type VI acromioclavicular separation with middle-third clavicle fracture in an ice hockey player. Clin J Sports Med. 2002;12(5):315-317.
4. Lancourt JE. Acromioclavicular dislocation with adjacent clavicular fracture in a horseback rider. A case report. Am J Sports Med. 1990;18(3):321-322.
5. Wurtz LD, Lyons FA, Rockwood CA Jr. Fracture of the middle third of the clavicle and dislocation of the acromioclavicular joint. A report of four cases. J Bone Joint Surg Am. 1992;74(1):133-137.
6. Yeh PC, Miller SR, Cunningham JG, Sethi PM. Midshaft clavicle fracture and acromioclavicular dislocation: a case report of a rare injury. J Shoulder Elbow Surg. 2009;18(5):e1-e4.
7. Stanley D, Trowbridge EA, Norris SH. The mechanism of clavicular fracture. A clinical and biomechanical analysis. J Bone Joint Surg Br. 1988;70(3):461-464.
8. Kim W, McKee MD. Management of acute clavicle fractures. Orthop Clin North Am. 2008;39(4):491-505.
9. Woolf SK, Valentine BJ, Barfield WR, Hartsock LA. Middle-third clavicle fracture with associated type IV acromioclavicular separation: case report and literature review. J Surg Orthop Adv. 2013;22(2):183-186.
Sacral Insufficiency Fracture After Partial Sacrectomy
Chordomas persist as one of the rarer malignancies, accounting for approximately 1% to 4% of primary bone cancers.1 When chordomas occur, these tumors localize predominantly in the sacrococcygeal region.2 In addition to the urgency for addressing a relatively fast-growing tumor, the anatomical complexity of this area complicates the potential treatments. Furthermore, because of the lack of definitive symptoms, diagnosis is often difficult and typically occurs later in the disease progression.3 An aggressive treatment approach is often warranted because of the biologically aggressive nature of this disease. Full or partial sacrectomy is often the only option that offers the possibility of a long-term cure.4 A sacrectomy is a destructive procedure that can lead to mechanical instability depending on the extent of the surgical resection. When the entire sacrum is removed, there is an obvious need for lumbar-pelvic fixation; however, traditionally, partial sacrectomy procedures have been successfully performed without the need for instrumentation.3,4
This report describes the case of a patient with a noninstrumented sacrectomy procedure distal to the S2 foramen that resulted in an insufficiency fracture. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman presented with severe lower back pain of a month’s duration. Her pain was localized to the coccyx area and did not radiate to the lower legs. Although the pain could not be elicited by palpation, pain occurred when sitting and increased when standing for prolonged periods. Three weeks prior to the patient’s initial office visit, she noticed transient constipation and urinary retention. She denied any fever, chills, nausea, vomiting, unexplained weight loss, weight gain, and abdominal pain. There were no motor deficits in the lower limbs. Sensation was intact in the lower limbs except for the posterior aspect of the left leg down to the popliteal fossa, where light touch perception was absent. She recalled the loss of sensation in this area 20 years earlier, and it had neither progressed nor abated since then. She had a history of osteoarthritis and had been diagnosed with degenerative disc disease 20 years ago.
A radiographic review of her lumbar spine showed significant spinal stenosis and degenerative disease of the lumbar spine on non–contrast-enhanced magnetic resonance imaging (MRI). The MRI also revealed a large, soft-tissue mass at the S3-S4 level, eroding most of the S3 vertebral body and extending into the S4 vertebral body. The MRI images used for this analysis were insufficient in providing a complete portrayal of the entire mass. Because of these uncertainties, contrast-enhanced and non–contrast-enhanced pelvic MRIs were taken. The MRI analyses identified a mass density replacing the lower sacrum and upper coccyx that was bright in intensity on T2 and dim on T1 sequences. Sagittal imaging measurements were 5.9×2.5 cm and 4.4 cm right-to-left on coronal imaging. The mass extended beyond the involved sacrococcygeal segments and dorsally beyond the normal cortical margin of the sacrum and coccyx (Figures 1A, 1B). Next, a computer tomographic–guided needle biopsy through a posterior paraspinal approach was obtained. The biopsy consisted of fragments of a malignant neoplasm consistent with physaliferous cells. The specimen was positive for pankeratin, keratin AE1/AE3, epithelial membrane antigen, and S100 protein. This supported a diagnosis of a sacral chordoma. An en bloc sacrectomy at S2; lumbar laminectomy at L5, S1, and S2; and thecal sac transection at the S3 nerve roots were planned.
Surgical Procedure
The patient was placed in the prone position after a colostomy and harvesting of a rectus flap in the supine position. A midline incision was made from the spinous process of L5 down through the tip of the coccyx, and soft tissues were elevated while maintaining hemostasis. The most distal part of the coccyx was transected, and using a combination of electrocautery and paraspinal elevators, rectal and peritoneal tissues were elevated off the ventral component of the coccyx until a hand could easily reach the bifurcation of the iliac vessels. Electrocautery transected paraspinal muscles at the S1 and S2 levels while the more cranial paraspinal musculature was elevated to allow for a laminectomy. The spinous processes were removed from L5 and the sacrum with a Leksell rongeur. A high-speed burr thinned the dorsal lamina components of L5, S1, and the leading edge of S2. The L5, S1, and S2 nerve roots were identified. The gluteal muscles were elevated and the sacral coccygeal ligaments were transected. After identifying the sciatic notches, the S2 nerves exiting the foramen were identified, followed out through the sciatic notch, and a wire was passed through this region. Three 2-0 silk ties were applied to the exposed portion of the S3 and S4 nerve roots, and the nerves were transected because they were integrally involved with the tumor. Using a series of high-speed burrs and osteotomes, lateral cuts were made through the sciatic notch. The sacrum was osteotomized at the S2 sacral foramen through the anterior component with an osteotome, while a hand protected the ventral structures. The remaining parts of the S3 and S4 dorsal nerve roots were transected. An incision through the peritoneum was made to access the rectus flap, and a plastic surgeon closed the wounds and secured the flap.
Postoperative Course
The patient’s final pathology confirmed a chordoma with negative margins. Postoperatively, the rectus flap became ischemic and a wound infection developed. It was irrigated, débrided, and treated with vacuum-assisted closure (VAC), in addition to perioperative antibiotic administration. An abdominal computed tomography (CT) scan did not show any fistula, and her wound remained healthy, pink, and viable as her VAC was changed every 3 days. Because the patient’s nutritional status was compromised, she started nutritional supplements in addition to a regular diet. Physical therapy was prescribed and the patient began bladder training with self-catheterization after a failed voiding trial attempt. After 2 months of convalescence, the patient had mobilized well and had progressed to walking without an ambulatory aide.
At her third postoperative month, the patient noted new onset of extreme pain in the groin and left thigh regions. The patient was examined and appeared to have a stable neurological exam. She had reproducible pain with a FABER (Flexion, Abduction, External Rotation, and Extension) test. MRI showed increased signal on short tau inversion recovery (STIR) sequences and T2-weighted images that was consistent with a left sacral ala stress fracture with a vertically oriented fracture line (Figures 2A, 2B). The patient was asked to begin utilizing a walker for ambulatory assistance, but her weight-bearing status was not changed. Over the course of 3 months, the patient noted a resolution of her pain. All postoperative MRI images confirmed the patient to be disease-free; and in addition, all of her follow-up radiographs showed a stable pelvic ring (Figures 3A, 3B). At her 2-year follow-up, the patient remained disease- and pain-free.
Discussion
Full discussions of the mechanical considerations of a partial sacrectomy have been described previously5-8; however, surgeons typically consider the need for lumbar-pelvic stabilization when the surgical resection requires a violation of the S1 body. Approximately two-thirds of sacral tumors occur at or below the level of the S2 body.8 These lesions of the caudal sacrum can sometimes be effectively resected with transverse partial sacrectomy. Great care is taken to resect only the portion of the sacrum necessary for local disease control, sparing as much of the sacroiliac joint and as many of the lumbosacral nerve roots as possible.
Under normal conditions, the sacroiliac articulation is stabilized by both its geometric interface and its extraordinarily strong ligaments. This spatial arrangement conveys stability primarily against caudal migration of the sacrum. The sacroiliac, sacrotuberous, sacrospinous, and lumbosacral ligaments, which are among the strongest ligaments in the body, primarily act to provide stability to the pelvic ring by preventing diastasis. The combination of these factors renders the spinopelvic segment especially stable. Previously, 2 biomechanical studies that specifically looked at extreme loading patterns to better understand the need for lumbar-pelvic instrumentation predicted a fracture pattern when there was an inability of the base of the sacral ala to resist shear.8,9 This is precisely where our patient’s insufficiency fracture occurred.
To our knowledge, this is the first reported in vivo evidence of this fracture pattern. While this patient’s potential history of osteoporosis may have elevated or contributed to her risk for fracture, her preoperative bone densitometry, with T scores of -1.0 on the left and right femur necks and 0.8 on her L1-L4 anteroposterior spine, would argue against this risk factor. None of these values represent a truly osteoporotic patient. It would appear that our patient sustained the fracture pattern predicted by Hugate and colleagues.8
The edema seen on the MRI most likely represents a fracture; however, sacroiliitis and infection are also potential diagnoses. Because there was no tumor in this region on the preoperative scans, we thought that a residual tumor was unlikely. The signal changes seen on T2 MRI sequences represent edema. The use of a bone scan that detects healing bone may have been a useful additional study to confirm this fracture as opposed to sacroiliitis. A CT scan would have been a potentially useful study to provide detail of fracture displacement and the overall fracture pattern. Standing plain radiographs are best for viewing fracture displacement with weight-bearing.
Surgeons contemplating performing partial sacrectomies should bear in mind that, even with preservation of the S1 body, a potential for fracture exists as evidenced by our patient. In our opinion, this patient did not require instrumentation but a more gradual rehabilitation program.
1. Varga PP, Lazary A. Chordoma of the sacrum: “en bloc” total sacrectomy and lumbopelvic reconstruction. Eur Spine. 2010;19(6):1039-1040.
2. Heffelfinger MJ, Dahlin DC, MacCarty CS, Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973; 32(2):410-420.
3. Varga PP, Bors I, Lazary A. Sacral tumors and management. Orthop Clin North Am. 2009;40(1):105-123.
4. Puri A, Agarwal MG, Shah M, et al. Decision making in primary sacral tumors. Spine J. 2009;9(5):396-403.
5. Cheng L, Yu Y, Zhu R, et al. Structural stability of different reconstruction techniques following total sacrectomy: a biomechanical study. Clin Biomech (Bristol, Avon). 2011;26 (10):977-981.
6. Yu BS, Zhuang XM, Li ZM, et al. Biomechanical effects of the extent of sacrectomy on the stability of lumbo-iliac reconstruction using iliac screw techniques: What level of sacrectomy requires the bilateral dual iliac screw technique? Clin Biomech (Bristol, Avon). 2010;25(9):867-872.
7. Yu B, Zheng Z, Zhuang X, et al. Biomechanical effects of transverse partial sacrectomy on the sacroiliac joints: an in vitro human cadaveric investigation of the borderline of sacroiliac joint instability. Spine (Phila Pa 1976). 2009;34(13):1370-1375.
8. Hugate RR Jr, Dickey ID, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop. 2006;450:82-88.
9. Gunterberg B, Romanus B, Stener B. Pelvic strength after major amputation of the sacrum. An experimental study. Acta Orthop Scand. 1976; 47(6):635-642.
Chordomas persist as one of the rarer malignancies, accounting for approximately 1% to 4% of primary bone cancers.1 When chordomas occur, these tumors localize predominantly in the sacrococcygeal region.2 In addition to the urgency for addressing a relatively fast-growing tumor, the anatomical complexity of this area complicates the potential treatments. Furthermore, because of the lack of definitive symptoms, diagnosis is often difficult and typically occurs later in the disease progression.3 An aggressive treatment approach is often warranted because of the biologically aggressive nature of this disease. Full or partial sacrectomy is often the only option that offers the possibility of a long-term cure.4 A sacrectomy is a destructive procedure that can lead to mechanical instability depending on the extent of the surgical resection. When the entire sacrum is removed, there is an obvious need for lumbar-pelvic fixation; however, traditionally, partial sacrectomy procedures have been successfully performed without the need for instrumentation.3,4
This report describes the case of a patient with a noninstrumented sacrectomy procedure distal to the S2 foramen that resulted in an insufficiency fracture. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman presented with severe lower back pain of a month’s duration. Her pain was localized to the coccyx area and did not radiate to the lower legs. Although the pain could not be elicited by palpation, pain occurred when sitting and increased when standing for prolonged periods. Three weeks prior to the patient’s initial office visit, she noticed transient constipation and urinary retention. She denied any fever, chills, nausea, vomiting, unexplained weight loss, weight gain, and abdominal pain. There were no motor deficits in the lower limbs. Sensation was intact in the lower limbs except for the posterior aspect of the left leg down to the popliteal fossa, where light touch perception was absent. She recalled the loss of sensation in this area 20 years earlier, and it had neither progressed nor abated since then. She had a history of osteoarthritis and had been diagnosed with degenerative disc disease 20 years ago.
A radiographic review of her lumbar spine showed significant spinal stenosis and degenerative disease of the lumbar spine on non–contrast-enhanced magnetic resonance imaging (MRI). The MRI also revealed a large, soft-tissue mass at the S3-S4 level, eroding most of the S3 vertebral body and extending into the S4 vertebral body. The MRI images used for this analysis were insufficient in providing a complete portrayal of the entire mass. Because of these uncertainties, contrast-enhanced and non–contrast-enhanced pelvic MRIs were taken. The MRI analyses identified a mass density replacing the lower sacrum and upper coccyx that was bright in intensity on T2 and dim on T1 sequences. Sagittal imaging measurements were 5.9×2.5 cm and 4.4 cm right-to-left on coronal imaging. The mass extended beyond the involved sacrococcygeal segments and dorsally beyond the normal cortical margin of the sacrum and coccyx (Figures 1A, 1B). Next, a computer tomographic–guided needle biopsy through a posterior paraspinal approach was obtained. The biopsy consisted of fragments of a malignant neoplasm consistent with physaliferous cells. The specimen was positive for pankeratin, keratin AE1/AE3, epithelial membrane antigen, and S100 protein. This supported a diagnosis of a sacral chordoma. An en bloc sacrectomy at S2; lumbar laminectomy at L5, S1, and S2; and thecal sac transection at the S3 nerve roots were planned.
Surgical Procedure
The patient was placed in the prone position after a colostomy and harvesting of a rectus flap in the supine position. A midline incision was made from the spinous process of L5 down through the tip of the coccyx, and soft tissues were elevated while maintaining hemostasis. The most distal part of the coccyx was transected, and using a combination of electrocautery and paraspinal elevators, rectal and peritoneal tissues were elevated off the ventral component of the coccyx until a hand could easily reach the bifurcation of the iliac vessels. Electrocautery transected paraspinal muscles at the S1 and S2 levels while the more cranial paraspinal musculature was elevated to allow for a laminectomy. The spinous processes were removed from L5 and the sacrum with a Leksell rongeur. A high-speed burr thinned the dorsal lamina components of L5, S1, and the leading edge of S2. The L5, S1, and S2 nerve roots were identified. The gluteal muscles were elevated and the sacral coccygeal ligaments were transected. After identifying the sciatic notches, the S2 nerves exiting the foramen were identified, followed out through the sciatic notch, and a wire was passed through this region. Three 2-0 silk ties were applied to the exposed portion of the S3 and S4 nerve roots, and the nerves were transected because they were integrally involved with the tumor. Using a series of high-speed burrs and osteotomes, lateral cuts were made through the sciatic notch. The sacrum was osteotomized at the S2 sacral foramen through the anterior component with an osteotome, while a hand protected the ventral structures. The remaining parts of the S3 and S4 dorsal nerve roots were transected. An incision through the peritoneum was made to access the rectus flap, and a plastic surgeon closed the wounds and secured the flap.
Postoperative Course
The patient’s final pathology confirmed a chordoma with negative margins. Postoperatively, the rectus flap became ischemic and a wound infection developed. It was irrigated, débrided, and treated with vacuum-assisted closure (VAC), in addition to perioperative antibiotic administration. An abdominal computed tomography (CT) scan did not show any fistula, and her wound remained healthy, pink, and viable as her VAC was changed every 3 days. Because the patient’s nutritional status was compromised, she started nutritional supplements in addition to a regular diet. Physical therapy was prescribed and the patient began bladder training with self-catheterization after a failed voiding trial attempt. After 2 months of convalescence, the patient had mobilized well and had progressed to walking without an ambulatory aide.
At her third postoperative month, the patient noted new onset of extreme pain in the groin and left thigh regions. The patient was examined and appeared to have a stable neurological exam. She had reproducible pain with a FABER (Flexion, Abduction, External Rotation, and Extension) test. MRI showed increased signal on short tau inversion recovery (STIR) sequences and T2-weighted images that was consistent with a left sacral ala stress fracture with a vertically oriented fracture line (Figures 2A, 2B). The patient was asked to begin utilizing a walker for ambulatory assistance, but her weight-bearing status was not changed. Over the course of 3 months, the patient noted a resolution of her pain. All postoperative MRI images confirmed the patient to be disease-free; and in addition, all of her follow-up radiographs showed a stable pelvic ring (Figures 3A, 3B). At her 2-year follow-up, the patient remained disease- and pain-free.
Discussion
Full discussions of the mechanical considerations of a partial sacrectomy have been described previously5-8; however, surgeons typically consider the need for lumbar-pelvic stabilization when the surgical resection requires a violation of the S1 body. Approximately two-thirds of sacral tumors occur at or below the level of the S2 body.8 These lesions of the caudal sacrum can sometimes be effectively resected with transverse partial sacrectomy. Great care is taken to resect only the portion of the sacrum necessary for local disease control, sparing as much of the sacroiliac joint and as many of the lumbosacral nerve roots as possible.
Under normal conditions, the sacroiliac articulation is stabilized by both its geometric interface and its extraordinarily strong ligaments. This spatial arrangement conveys stability primarily against caudal migration of the sacrum. The sacroiliac, sacrotuberous, sacrospinous, and lumbosacral ligaments, which are among the strongest ligaments in the body, primarily act to provide stability to the pelvic ring by preventing diastasis. The combination of these factors renders the spinopelvic segment especially stable. Previously, 2 biomechanical studies that specifically looked at extreme loading patterns to better understand the need for lumbar-pelvic instrumentation predicted a fracture pattern when there was an inability of the base of the sacral ala to resist shear.8,9 This is precisely where our patient’s insufficiency fracture occurred.
To our knowledge, this is the first reported in vivo evidence of this fracture pattern. While this patient’s potential history of osteoporosis may have elevated or contributed to her risk for fracture, her preoperative bone densitometry, with T scores of -1.0 on the left and right femur necks and 0.8 on her L1-L4 anteroposterior spine, would argue against this risk factor. None of these values represent a truly osteoporotic patient. It would appear that our patient sustained the fracture pattern predicted by Hugate and colleagues.8
The edema seen on the MRI most likely represents a fracture; however, sacroiliitis and infection are also potential diagnoses. Because there was no tumor in this region on the preoperative scans, we thought that a residual tumor was unlikely. The signal changes seen on T2 MRI sequences represent edema. The use of a bone scan that detects healing bone may have been a useful additional study to confirm this fracture as opposed to sacroiliitis. A CT scan would have been a potentially useful study to provide detail of fracture displacement and the overall fracture pattern. Standing plain radiographs are best for viewing fracture displacement with weight-bearing.
Surgeons contemplating performing partial sacrectomies should bear in mind that, even with preservation of the S1 body, a potential for fracture exists as evidenced by our patient. In our opinion, this patient did not require instrumentation but a more gradual rehabilitation program.
Chordomas persist as one of the rarer malignancies, accounting for approximately 1% to 4% of primary bone cancers.1 When chordomas occur, these tumors localize predominantly in the sacrococcygeal region.2 In addition to the urgency for addressing a relatively fast-growing tumor, the anatomical complexity of this area complicates the potential treatments. Furthermore, because of the lack of definitive symptoms, diagnosis is often difficult and typically occurs later in the disease progression.3 An aggressive treatment approach is often warranted because of the biologically aggressive nature of this disease. Full or partial sacrectomy is often the only option that offers the possibility of a long-term cure.4 A sacrectomy is a destructive procedure that can lead to mechanical instability depending on the extent of the surgical resection. When the entire sacrum is removed, there is an obvious need for lumbar-pelvic fixation; however, traditionally, partial sacrectomy procedures have been successfully performed without the need for instrumentation.3,4
This report describes the case of a patient with a noninstrumented sacrectomy procedure distal to the S2 foramen that resulted in an insufficiency fracture. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman presented with severe lower back pain of a month’s duration. Her pain was localized to the coccyx area and did not radiate to the lower legs. Although the pain could not be elicited by palpation, pain occurred when sitting and increased when standing for prolonged periods. Three weeks prior to the patient’s initial office visit, she noticed transient constipation and urinary retention. She denied any fever, chills, nausea, vomiting, unexplained weight loss, weight gain, and abdominal pain. There were no motor deficits in the lower limbs. Sensation was intact in the lower limbs except for the posterior aspect of the left leg down to the popliteal fossa, where light touch perception was absent. She recalled the loss of sensation in this area 20 years earlier, and it had neither progressed nor abated since then. She had a history of osteoarthritis and had been diagnosed with degenerative disc disease 20 years ago.
A radiographic review of her lumbar spine showed significant spinal stenosis and degenerative disease of the lumbar spine on non–contrast-enhanced magnetic resonance imaging (MRI). The MRI also revealed a large, soft-tissue mass at the S3-S4 level, eroding most of the S3 vertebral body and extending into the S4 vertebral body. The MRI images used for this analysis were insufficient in providing a complete portrayal of the entire mass. Because of these uncertainties, contrast-enhanced and non–contrast-enhanced pelvic MRIs were taken. The MRI analyses identified a mass density replacing the lower sacrum and upper coccyx that was bright in intensity on T2 and dim on T1 sequences. Sagittal imaging measurements were 5.9×2.5 cm and 4.4 cm right-to-left on coronal imaging. The mass extended beyond the involved sacrococcygeal segments and dorsally beyond the normal cortical margin of the sacrum and coccyx (Figures 1A, 1B). Next, a computer tomographic–guided needle biopsy through a posterior paraspinal approach was obtained. The biopsy consisted of fragments of a malignant neoplasm consistent with physaliferous cells. The specimen was positive for pankeratin, keratin AE1/AE3, epithelial membrane antigen, and S100 protein. This supported a diagnosis of a sacral chordoma. An en bloc sacrectomy at S2; lumbar laminectomy at L5, S1, and S2; and thecal sac transection at the S3 nerve roots were planned.
Surgical Procedure
The patient was placed in the prone position after a colostomy and harvesting of a rectus flap in the supine position. A midline incision was made from the spinous process of L5 down through the tip of the coccyx, and soft tissues were elevated while maintaining hemostasis. The most distal part of the coccyx was transected, and using a combination of electrocautery and paraspinal elevators, rectal and peritoneal tissues were elevated off the ventral component of the coccyx until a hand could easily reach the bifurcation of the iliac vessels. Electrocautery transected paraspinal muscles at the S1 and S2 levels while the more cranial paraspinal musculature was elevated to allow for a laminectomy. The spinous processes were removed from L5 and the sacrum with a Leksell rongeur. A high-speed burr thinned the dorsal lamina components of L5, S1, and the leading edge of S2. The L5, S1, and S2 nerve roots were identified. The gluteal muscles were elevated and the sacral coccygeal ligaments were transected. After identifying the sciatic notches, the S2 nerves exiting the foramen were identified, followed out through the sciatic notch, and a wire was passed through this region. Three 2-0 silk ties were applied to the exposed portion of the S3 and S4 nerve roots, and the nerves were transected because they were integrally involved with the tumor. Using a series of high-speed burrs and osteotomes, lateral cuts were made through the sciatic notch. The sacrum was osteotomized at the S2 sacral foramen through the anterior component with an osteotome, while a hand protected the ventral structures. The remaining parts of the S3 and S4 dorsal nerve roots were transected. An incision through the peritoneum was made to access the rectus flap, and a plastic surgeon closed the wounds and secured the flap.
Postoperative Course
The patient’s final pathology confirmed a chordoma with negative margins. Postoperatively, the rectus flap became ischemic and a wound infection developed. It was irrigated, débrided, and treated with vacuum-assisted closure (VAC), in addition to perioperative antibiotic administration. An abdominal computed tomography (CT) scan did not show any fistula, and her wound remained healthy, pink, and viable as her VAC was changed every 3 days. Because the patient’s nutritional status was compromised, she started nutritional supplements in addition to a regular diet. Physical therapy was prescribed and the patient began bladder training with self-catheterization after a failed voiding trial attempt. After 2 months of convalescence, the patient had mobilized well and had progressed to walking without an ambulatory aide.
At her third postoperative month, the patient noted new onset of extreme pain in the groin and left thigh regions. The patient was examined and appeared to have a stable neurological exam. She had reproducible pain with a FABER (Flexion, Abduction, External Rotation, and Extension) test. MRI showed increased signal on short tau inversion recovery (STIR) sequences and T2-weighted images that was consistent with a left sacral ala stress fracture with a vertically oriented fracture line (Figures 2A, 2B). The patient was asked to begin utilizing a walker for ambulatory assistance, but her weight-bearing status was not changed. Over the course of 3 months, the patient noted a resolution of her pain. All postoperative MRI images confirmed the patient to be disease-free; and in addition, all of her follow-up radiographs showed a stable pelvic ring (Figures 3A, 3B). At her 2-year follow-up, the patient remained disease- and pain-free.
Discussion
Full discussions of the mechanical considerations of a partial sacrectomy have been described previously5-8; however, surgeons typically consider the need for lumbar-pelvic stabilization when the surgical resection requires a violation of the S1 body. Approximately two-thirds of sacral tumors occur at or below the level of the S2 body.8 These lesions of the caudal sacrum can sometimes be effectively resected with transverse partial sacrectomy. Great care is taken to resect only the portion of the sacrum necessary for local disease control, sparing as much of the sacroiliac joint and as many of the lumbosacral nerve roots as possible.
Under normal conditions, the sacroiliac articulation is stabilized by both its geometric interface and its extraordinarily strong ligaments. This spatial arrangement conveys stability primarily against caudal migration of the sacrum. The sacroiliac, sacrotuberous, sacrospinous, and lumbosacral ligaments, which are among the strongest ligaments in the body, primarily act to provide stability to the pelvic ring by preventing diastasis. The combination of these factors renders the spinopelvic segment especially stable. Previously, 2 biomechanical studies that specifically looked at extreme loading patterns to better understand the need for lumbar-pelvic instrumentation predicted a fracture pattern when there was an inability of the base of the sacral ala to resist shear.8,9 This is precisely where our patient’s insufficiency fracture occurred.
To our knowledge, this is the first reported in vivo evidence of this fracture pattern. While this patient’s potential history of osteoporosis may have elevated or contributed to her risk for fracture, her preoperative bone densitometry, with T scores of -1.0 on the left and right femur necks and 0.8 on her L1-L4 anteroposterior spine, would argue against this risk factor. None of these values represent a truly osteoporotic patient. It would appear that our patient sustained the fracture pattern predicted by Hugate and colleagues.8
The edema seen on the MRI most likely represents a fracture; however, sacroiliitis and infection are also potential diagnoses. Because there was no tumor in this region on the preoperative scans, we thought that a residual tumor was unlikely. The signal changes seen on T2 MRI sequences represent edema. The use of a bone scan that detects healing bone may have been a useful additional study to confirm this fracture as opposed to sacroiliitis. A CT scan would have been a potentially useful study to provide detail of fracture displacement and the overall fracture pattern. Standing plain radiographs are best for viewing fracture displacement with weight-bearing.
Surgeons contemplating performing partial sacrectomies should bear in mind that, even with preservation of the S1 body, a potential for fracture exists as evidenced by our patient. In our opinion, this patient did not require instrumentation but a more gradual rehabilitation program.
1. Varga PP, Lazary A. Chordoma of the sacrum: “en bloc” total sacrectomy and lumbopelvic reconstruction. Eur Spine. 2010;19(6):1039-1040.
2. Heffelfinger MJ, Dahlin DC, MacCarty CS, Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973; 32(2):410-420.
3. Varga PP, Bors I, Lazary A. Sacral tumors and management. Orthop Clin North Am. 2009;40(1):105-123.
4. Puri A, Agarwal MG, Shah M, et al. Decision making in primary sacral tumors. Spine J. 2009;9(5):396-403.
5. Cheng L, Yu Y, Zhu R, et al. Structural stability of different reconstruction techniques following total sacrectomy: a biomechanical study. Clin Biomech (Bristol, Avon). 2011;26 (10):977-981.
6. Yu BS, Zhuang XM, Li ZM, et al. Biomechanical effects of the extent of sacrectomy on the stability of lumbo-iliac reconstruction using iliac screw techniques: What level of sacrectomy requires the bilateral dual iliac screw technique? Clin Biomech (Bristol, Avon). 2010;25(9):867-872.
7. Yu B, Zheng Z, Zhuang X, et al. Biomechanical effects of transverse partial sacrectomy on the sacroiliac joints: an in vitro human cadaveric investigation of the borderline of sacroiliac joint instability. Spine (Phila Pa 1976). 2009;34(13):1370-1375.
8. Hugate RR Jr, Dickey ID, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop. 2006;450:82-88.
9. Gunterberg B, Romanus B, Stener B. Pelvic strength after major amputation of the sacrum. An experimental study. Acta Orthop Scand. 1976; 47(6):635-642.
1. Varga PP, Lazary A. Chordoma of the sacrum: “en bloc” total sacrectomy and lumbopelvic reconstruction. Eur Spine. 2010;19(6):1039-1040.
2. Heffelfinger MJ, Dahlin DC, MacCarty CS, Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973; 32(2):410-420.
3. Varga PP, Bors I, Lazary A. Sacral tumors and management. Orthop Clin North Am. 2009;40(1):105-123.
4. Puri A, Agarwal MG, Shah M, et al. Decision making in primary sacral tumors. Spine J. 2009;9(5):396-403.
5. Cheng L, Yu Y, Zhu R, et al. Structural stability of different reconstruction techniques following total sacrectomy: a biomechanical study. Clin Biomech (Bristol, Avon). 2011;26 (10):977-981.
6. Yu BS, Zhuang XM, Li ZM, et al. Biomechanical effects of the extent of sacrectomy on the stability of lumbo-iliac reconstruction using iliac screw techniques: What level of sacrectomy requires the bilateral dual iliac screw technique? Clin Biomech (Bristol, Avon). 2010;25(9):867-872.
7. Yu B, Zheng Z, Zhuang X, et al. Biomechanical effects of transverse partial sacrectomy on the sacroiliac joints: an in vitro human cadaveric investigation of the borderline of sacroiliac joint instability. Spine (Phila Pa 1976). 2009;34(13):1370-1375.
8. Hugate RR Jr, Dickey ID, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop. 2006;450:82-88.
9. Gunterberg B, Romanus B, Stener B. Pelvic strength after major amputation of the sacrum. An experimental study. Acta Orthop Scand. 1976; 47(6):635-642.