User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Biomechanical Evaluation of Proximally Placed Femoral Less-Invasive Stabilization System Plates
Several surgical options are available for treatment of supracondylar and intercondylar distal femur fractures, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 33. Preserving the osseous blood supply via indirect reduction techniques has been shown to increase union rates without the need for bone grafting.1,2 The Less-Invasive Stabilization System (LISS) made by Synthes (Paoli, Pennsylvania) melds minimally invasive internal fixation with multiple fixed-angle distal screws. It allows for submuscular placement, percutaneous unicortical screws in the diaphysis, and preservation of the metaphyseal fracture soft-tissue envelope.3
Proper lateral placement of the plate on the femur proximally can be difficult. Kregor and colleagues3 noted that 6% of cases did not have ideal placement on the lateral shaft of the femur when the 13-hole LISS plate was used. They advocated making a small incision at the proximal end of the LISS plate to aid in proper lateral placement. Kolb and colleagues4 noted that 2 of 31 patients had a “cutting out” of the proximal screws on LISS plates with anterior placement on the femur that eventually required repeat surgery in order to heal. This malpositioned plate was present at the end of the operation. These authors also recommended a proximal incision to avoid the issue. Schütz and colleagues5 noted that there were 4 cases of implant loosening among 107 distal femur fractures treated with LISS plating and that the unicortical screws in the diaphysis had loosened. They suggested anterior placement of the plate as a possible reason for fixation failure.
Although several studies have noted proximal screw pull-out, and proximal anterior malposition in the sagittal plane of the LISS plate has been suggested as a possible cause, we found no studies comparing incorrect proximal positioning on the femoral shaft with correct lateral placement of the LISS plate. Therefore, we used a previously established biomechanical model to compare LISS plates proximally placed either too anterior or too posterior to the direct lateral position on the femoral shaft. The constructs were tested in axial, torsional, and cyclical axial modes to assess plastic and total deformation, stiffness, and fixation failure.
Materials and Methods
Using fourth-generation femoral synthetic composite bones (Sawbones; Pacific Research Laboratories, Vashon, Washington) and a 13-hole Synthes femoral LISS plate, we made 3 groups of 9 specimens each, for a total of 27 femurs. The number of specimens was based on a power assessment in a study by Khalafi and colleagues.8 Several studies have validated use of Sawbones instead of cadavers in biomechanical testing to prevent variability.6-9 Proximal fixation was achieved with 5 unicortical screws (26 mm long) at screw holes 13, 11, 9, 7, and 4. All distal screw holes were filled for distal fixation with 75-mm-long screws to achieve bicortical fixation.
After application of the LISS plate, an AO/OTA 33-A3 fracture model was created in each specimen. A 1-cm gap was made 6 cm proximal to the intercondylar notch to create an unstable distal femur fracture pattern. In the method described by Zlowodzki and colleagues,10 an additional 3-cm cut was made diagonally in the medial cortex to prevent contact of the bone during mechanical testing.
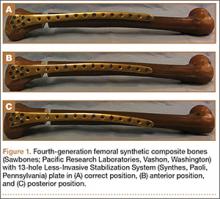
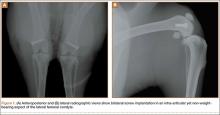
Three different plate positions were used. The correct group was placed directly laterally proximally (Figure 1A). One incorrect group was plated with the proximal aspect of the plate 1 cm anterior (anterior group) (Figure 1B), and another incorrect group was plated with the proximal aspect of the plate 1 cm posterior (posterior group) (Figure 1C). Anterior or posterior plate placement resulted in some of the proximal screws having a more tangential placement, with fewer screws engaged compared with the properly placed plate.
The distal and proximal ends of each specimen were held to simulate the mechanical axis of the femur. This design was based on a model by Cordey and colleagues.11 A materials testing system (MTS, Minneapolis, Minnesota) was used for mechanical testing of the model.
Based on the protocol of Khalafi and colleagues,8 the models were tested in axial, torsional, and cyclical axial modes (Figures 2, 3). Axial loading consisted of a preload of 100 N followed by a compressive loading rate of 100 mm per minute in a displacement control mode. Testing was considered completed when 1 of 3 events occurred: 500 N was reached, the medial fracture gap closed, or fixation was lost. Torsional loading involved a preload of 5 Nm and subsequent torqueing at 20° per minute up to 20 Nm or loss of fixation or screw pull-out.8 Cyclical axial loading was based on protocols described by Marti and colleagues2 and Zlowodzki and colleagues.10 The initial load was 10 cycles of 300 N. Each subsequent load increment was increased by 100 N up to 1000 N, providing 10-second rest increments. This loading was conducted in a displacement control mode at 0.75 mm per second. Testing was aborted on fixation loss or complete closure of the medial fracture gap.
After testing was completed, statistically significant between-groups differences in plastic deformation and axial and torsional stiffness were determined by performing a Tukey-Kramer honestly significant difference test. Significance was set at P ≤ .05.
Results
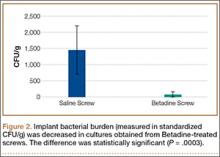
During axial loading, there was no visual loss of fixation or change in displacement of the fracture gap for any group, and there was no screw cut-out or pull-out from the cortex during testing. In 1 plate in the posterior group, the most proximal screw made only loose contact with the cortex at only the distal portion of the screw. There was no significant difference (P = .9762) in stiffness in axial loading between the anterior group and the correct group. There was a significant (P = .0261) 16.4% increase in stiffness in the posterior group compared with the correct group (Table).
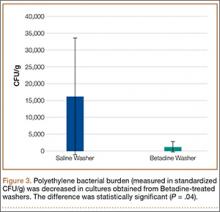
There was no screw cut-out, fixation failure, or change in displacement of the fracture gap for any group during torsional loading. There was a statistically significant (P = .0062) 12% increase in mean torsional stiffness in the anterior group compared with the correct group. There was no statistically significant difference (P = .1623) between the posterior group and the correct group (Table).
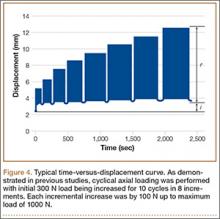
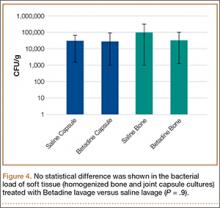
For cyclical axial testing, total deformation and plastic deformation were obtained by determining displacement under the initial 100 N load in the static/resting state. That number was then subtracted from maximum displacement, the peak value on the time-versus-displacement graph, to obtain the value for total deformation. Plastic deformation was calculated by subtracting initial displacement from final displacement in the static/resting state. The static/resting state is represented by the dips in displacement after each cycle on the time-versus-displacement graph (Figure 4).
There was a statistically significant (P = .0207) 14% increase in total deformation of the anteriorly positioned plate compared with the correctly positioned plate. There was no statistically significant difference in total deformation between the posteriorly placed plates and the correctly placed plates (Table).
There was no significant difference in plastic deformation between any of the groups in this study. There was no screw cut-out or fixation loss in any group to suggest a clinically relevant difference based on proximal placement of the LISS plate.
Discussion
In evaluating the stability of various constructs for fixation of distal femur fractures, the literature is consistent in reporting stiffness as the key factor. Stiffness is determined most often in terms of motion at the fracture site, as measured by displacement under axial and torsional loads.2,8,10,13 The LISS plate, which acts essentially as an “internal fixator” with proximal unicortical fixed-angle locking screws, has been shown to be comparable to other established methods of fixation.10,12 Zlowodzki and colleagues10 reported that the LISS plate had a higher load to failure when compared with angled blade plating and intramedullary nailing. Their study used fresh-frozen cadaver specimens from patients 70 years old or older. They concluded that, for distal femur fractures in osteoporotic bone, the LISS plate provided improved distal fixation.
In the present study, the posteriorly placed LISS plate outperformed the correctly placed plate in axial stiffness by 16.4%. However, there was no statistically significant difference in torsional stiffness and cyclical axial loading. This result is difficult to explain given that there was no screw cut-out or fixation loss for any of the constructs. Theoretically, with less proximal screw purchase in the posteriorly placed plate, the overall construct should be more susceptible to screw cut-out and fixation loss resulting in less axial stiffness overall.
Khalafi and colleagues8 created a distal femur fracture model using Sawbones with a 1-cm fracture gap. Using the 9-hole LISS plate for fixation, they tested this construct under axial, torsional, and cyclical axial loads. They tested 2 groups of 9 femurs. For group 1, the LISS plate was placed in the correct position on the distal femur, with the proximal end in the correct position on the femoral shaft. In group 2, the LISS plate was rotated 1 cm anteriorly. They found that axial stiffness (N/mm) was 21.5% greater in the correctly positioned plate. The anteriorly positioned group demonstrated 55% more irreversible or plastic deformation. The authors concluded that correct positioning of the femoral LISS plate provided improved mechanical stability.
Overall, our study results did not agree with those of Khalafi and colleagues8 in terms of the mechanical stability of a malpositioned LISS plate. Our construct showed a significant increase in torsional stiffness in the anteriorly placed plate. However, our construct also showed a significant increase in total deformation in cyclical axial loading in the anteriorly placed plate. There was no increased plastic deformation in either of the incorrectly placed groups in our study. The difference in results between studies can best be explained by the difference in plate lengths. We used a 13-hole plate, and Khalafi and colleagues8 used a 9-hole plate. Our theory is that the longer plate provided more resistance to relatively minor variations in plate position at the proximal end and thus resulted in less change in stiffness and stability around the fracture site.
Our model differed from that used in other biomechanical studies using Sawbones to simulate distal femur fractures in that it used the entire femur, including the proximal portion.8,13 This setup theoretically resulted in a more anatomical weight distribution compared with other models, in which the proximal portion of the femur was potted in polymethylmethacrylate. This difference in weight distribution could explain the variation in our results compared with other biomechanical studies. In addition, with use of different boundary conditions, the distal femur had unconstrained distal motion similar to the native environment of the femur.
This study had several limitations. First is its relatively low power (9 femurs per group). Although groups of 9 specimens in 2 groups were used in the study by Khalafi and colleagues8, testing a larger number of femurs could potentially identify more subtle differences between the 3 groups in our study. Second, given that femoral LISS plates come in different lengths, this study could be expanded to include the other plate sizes, as plate length could potentially play a role in stability at the fracture site. Third, though this Sawbones model has consistently reproduced the stability characteristics of human bone without variation between specimens, an osteoporotic model could be explored, as the femoral LISS plate is often used in osteoporotic fractures.7,14
Conclusion
Overall, our study results showed that 1-cm variations, anterior or posterior, had little effect on axial or torsional stiffness or plastic deformation under cyclical axial loading. Although these data can be promising for clinical application, the anterior placement of the LISS plate noted in failed fixation in other studies necessitates cautious interpretation of this study. Our use of a 13-hole (longer) plate, versus the 9-hole plate used in other studies, could explain the lack of variation between the 2 groups as well as the stability and tolerance of inappropriate placement. An osteoporotic model could help clinicians further discern the importance of accurate proximal placement of the femoral LISS plate.
1. Bolhofner BR, Carmen B, Clifford P. The results of open reduction and internal fixation of distal femur fractures using a biologic (indirect) reduction technique. J Orthop Trauma. 1996;10(6):372-377.
2. Marti A, Frankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma. 2001;15(7):482-487.
3. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system. J Orthop Trauma. 2004;18(8):509-520.
4. Kolb W, Guhlmann H, Windisch C, Marx F, Kolb K, Koller H. Fixation of distal femoral fractures with the less invasive stabilization system: a minimally invasive treatment with locked fixed-angle screws. J Trauma. 2008;65(6):1425-1434.
5. Schütz M, Müller M, Krettek C, et al. Minimally invasive fracture stabilization of distal femoral fractures with the LISS: a prospective multicenter study. Results of a clinical study with special emphasis on difficult cases. Injury. 2001;32(suppl 3):SC55-SC63.
6. Chong AC, Friis EA, Ballard GP, Czuwala PJ, Cooke FW. Fatigue performance of composite analogue femur constructs under high activity loading. Ann Biomed Eng. 2007;35(7):1196-1205.
7. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
8. Khalafi A, Curtiss S, Hazelwood S, Wolinsky P. The effect of plate rotation on the stiffness of femoral LISS: a mechanical study. J Orthop Trauma. 2006;20(8):542-546.
9. Zdero R, Olsen M, Bougherara H, Schemitsch EH. Cancellous bone screw purchase: a comparison of synthetic femurs, human femurs, and finite element analysis. Proc Inst Mech Eng H. 2008;222(8):1175-1183.
10. Zlowodzki M, Williamson S, Cole PA, Zardiackas LD, Kregor PJ. Biomechanical evaluation of the less invasive stabilization system, angled blade plate, and retrograde intramedullary nail for the internal fixation of distal femur fractures. J Orthop Trauma. 2004;18(8):494-502.
11. Cordey J, Borgeaud M, Frankle, M, Harder Y, Martinet O. Loading model for the human femur taking the tension band effect of the ilio-tibial tract into account. Injury. 1999;30(suppl 1):A26-A30.
12. Weight M, Collinge C. Early results of the less invasive stabilization system for mechanically unstable fractures of the distal femur (AO/OTA types A2, A3, C2, and C3). J Orthop Trauma. 2004;18(8):503-508.
13. Otto RJ, Moed BR, Bledsoe JG. Biomechanical comparison of polyaxial-type locking plates and a fixed-angle locking plate for internal fixation of distal femur fractures. J Orthop Trauma. 2009;23(9):645–652.
14. Wong M, Leung F, Chow SP. Treatment of distal femoral fractures in the elderly using a less-invasive plating technique. Int Orthop. 2005;29(2):
117-120.
Several surgical options are available for treatment of supracondylar and intercondylar distal femur fractures, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 33. Preserving the osseous blood supply via indirect reduction techniques has been shown to increase union rates without the need for bone grafting.1,2 The Less-Invasive Stabilization System (LISS) made by Synthes (Paoli, Pennsylvania) melds minimally invasive internal fixation with multiple fixed-angle distal screws. It allows for submuscular placement, percutaneous unicortical screws in the diaphysis, and preservation of the metaphyseal fracture soft-tissue envelope.3
Proper lateral placement of the plate on the femur proximally can be difficult. Kregor and colleagues3 noted that 6% of cases did not have ideal placement on the lateral shaft of the femur when the 13-hole LISS plate was used. They advocated making a small incision at the proximal end of the LISS plate to aid in proper lateral placement. Kolb and colleagues4 noted that 2 of 31 patients had a “cutting out” of the proximal screws on LISS plates with anterior placement on the femur that eventually required repeat surgery in order to heal. This malpositioned plate was present at the end of the operation. These authors also recommended a proximal incision to avoid the issue. Schütz and colleagues5 noted that there were 4 cases of implant loosening among 107 distal femur fractures treated with LISS plating and that the unicortical screws in the diaphysis had loosened. They suggested anterior placement of the plate as a possible reason for fixation failure.
Although several studies have noted proximal screw pull-out, and proximal anterior malposition in the sagittal plane of the LISS plate has been suggested as a possible cause, we found no studies comparing incorrect proximal positioning on the femoral shaft with correct lateral placement of the LISS plate. Therefore, we used a previously established biomechanical model to compare LISS plates proximally placed either too anterior or too posterior to the direct lateral position on the femoral shaft. The constructs were tested in axial, torsional, and cyclical axial modes to assess plastic and total deformation, stiffness, and fixation failure.
Materials and Methods
Using fourth-generation femoral synthetic composite bones (Sawbones; Pacific Research Laboratories, Vashon, Washington) and a 13-hole Synthes femoral LISS plate, we made 3 groups of 9 specimens each, for a total of 27 femurs. The number of specimens was based on a power assessment in a study by Khalafi and colleagues.8 Several studies have validated use of Sawbones instead of cadavers in biomechanical testing to prevent variability.6-9 Proximal fixation was achieved with 5 unicortical screws (26 mm long) at screw holes 13, 11, 9, 7, and 4. All distal screw holes were filled for distal fixation with 75-mm-long screws to achieve bicortical fixation.
After application of the LISS plate, an AO/OTA 33-A3 fracture model was created in each specimen. A 1-cm gap was made 6 cm proximal to the intercondylar notch to create an unstable distal femur fracture pattern. In the method described by Zlowodzki and colleagues,10 an additional 3-cm cut was made diagonally in the medial cortex to prevent contact of the bone during mechanical testing.
Three different plate positions were used. The correct group was placed directly laterally proximally (Figure 1A). One incorrect group was plated with the proximal aspect of the plate 1 cm anterior (anterior group) (Figure 1B), and another incorrect group was plated with the proximal aspect of the plate 1 cm posterior (posterior group) (Figure 1C). Anterior or posterior plate placement resulted in some of the proximal screws having a more tangential placement, with fewer screws engaged compared with the properly placed plate.
The distal and proximal ends of each specimen were held to simulate the mechanical axis of the femur. This design was based on a model by Cordey and colleagues.11 A materials testing system (MTS, Minneapolis, Minnesota) was used for mechanical testing of the model.
Based on the protocol of Khalafi and colleagues,8 the models were tested in axial, torsional, and cyclical axial modes (Figures 2, 3). Axial loading consisted of a preload of 100 N followed by a compressive loading rate of 100 mm per minute in a displacement control mode. Testing was considered completed when 1 of 3 events occurred: 500 N was reached, the medial fracture gap closed, or fixation was lost. Torsional loading involved a preload of 5 Nm and subsequent torqueing at 20° per minute up to 20 Nm or loss of fixation or screw pull-out.8 Cyclical axial loading was based on protocols described by Marti and colleagues2 and Zlowodzki and colleagues.10 The initial load was 10 cycles of 300 N. Each subsequent load increment was increased by 100 N up to 1000 N, providing 10-second rest increments. This loading was conducted in a displacement control mode at 0.75 mm per second. Testing was aborted on fixation loss or complete closure of the medial fracture gap.
After testing was completed, statistically significant between-groups differences in plastic deformation and axial and torsional stiffness were determined by performing a Tukey-Kramer honestly significant difference test. Significance was set at P ≤ .05.
Results
During axial loading, there was no visual loss of fixation or change in displacement of the fracture gap for any group, and there was no screw cut-out or pull-out from the cortex during testing. In 1 plate in the posterior group, the most proximal screw made only loose contact with the cortex at only the distal portion of the screw. There was no significant difference (P = .9762) in stiffness in axial loading between the anterior group and the correct group. There was a significant (P = .0261) 16.4% increase in stiffness in the posterior group compared with the correct group (Table).
There was no screw cut-out, fixation failure, or change in displacement of the fracture gap for any group during torsional loading. There was a statistically significant (P = .0062) 12% increase in mean torsional stiffness in the anterior group compared with the correct group. There was no statistically significant difference (P = .1623) between the posterior group and the correct group (Table).
For cyclical axial testing, total deformation and plastic deformation were obtained by determining displacement under the initial 100 N load in the static/resting state. That number was then subtracted from maximum displacement, the peak value on the time-versus-displacement graph, to obtain the value for total deformation. Plastic deformation was calculated by subtracting initial displacement from final displacement in the static/resting state. The static/resting state is represented by the dips in displacement after each cycle on the time-versus-displacement graph (Figure 4).
There was a statistically significant (P = .0207) 14% increase in total deformation of the anteriorly positioned plate compared with the correctly positioned plate. There was no statistically significant difference in total deformation between the posteriorly placed plates and the correctly placed plates (Table).
There was no significant difference in plastic deformation between any of the groups in this study. There was no screw cut-out or fixation loss in any group to suggest a clinically relevant difference based on proximal placement of the LISS plate.
Discussion
In evaluating the stability of various constructs for fixation of distal femur fractures, the literature is consistent in reporting stiffness as the key factor. Stiffness is determined most often in terms of motion at the fracture site, as measured by displacement under axial and torsional loads.2,8,10,13 The LISS plate, which acts essentially as an “internal fixator” with proximal unicortical fixed-angle locking screws, has been shown to be comparable to other established methods of fixation.10,12 Zlowodzki and colleagues10 reported that the LISS plate had a higher load to failure when compared with angled blade plating and intramedullary nailing. Their study used fresh-frozen cadaver specimens from patients 70 years old or older. They concluded that, for distal femur fractures in osteoporotic bone, the LISS plate provided improved distal fixation.
In the present study, the posteriorly placed LISS plate outperformed the correctly placed plate in axial stiffness by 16.4%. However, there was no statistically significant difference in torsional stiffness and cyclical axial loading. This result is difficult to explain given that there was no screw cut-out or fixation loss for any of the constructs. Theoretically, with less proximal screw purchase in the posteriorly placed plate, the overall construct should be more susceptible to screw cut-out and fixation loss resulting in less axial stiffness overall.
Khalafi and colleagues8 created a distal femur fracture model using Sawbones with a 1-cm fracture gap. Using the 9-hole LISS plate for fixation, they tested this construct under axial, torsional, and cyclical axial loads. They tested 2 groups of 9 femurs. For group 1, the LISS plate was placed in the correct position on the distal femur, with the proximal end in the correct position on the femoral shaft. In group 2, the LISS plate was rotated 1 cm anteriorly. They found that axial stiffness (N/mm) was 21.5% greater in the correctly positioned plate. The anteriorly positioned group demonstrated 55% more irreversible or plastic deformation. The authors concluded that correct positioning of the femoral LISS plate provided improved mechanical stability.
Overall, our study results did not agree with those of Khalafi and colleagues8 in terms of the mechanical stability of a malpositioned LISS plate. Our construct showed a significant increase in torsional stiffness in the anteriorly placed plate. However, our construct also showed a significant increase in total deformation in cyclical axial loading in the anteriorly placed plate. There was no increased plastic deformation in either of the incorrectly placed groups in our study. The difference in results between studies can best be explained by the difference in plate lengths. We used a 13-hole plate, and Khalafi and colleagues8 used a 9-hole plate. Our theory is that the longer plate provided more resistance to relatively minor variations in plate position at the proximal end and thus resulted in less change in stiffness and stability around the fracture site.
Our model differed from that used in other biomechanical studies using Sawbones to simulate distal femur fractures in that it used the entire femur, including the proximal portion.8,13 This setup theoretically resulted in a more anatomical weight distribution compared with other models, in which the proximal portion of the femur was potted in polymethylmethacrylate. This difference in weight distribution could explain the variation in our results compared with other biomechanical studies. In addition, with use of different boundary conditions, the distal femur had unconstrained distal motion similar to the native environment of the femur.
This study had several limitations. First is its relatively low power (9 femurs per group). Although groups of 9 specimens in 2 groups were used in the study by Khalafi and colleagues8, testing a larger number of femurs could potentially identify more subtle differences between the 3 groups in our study. Second, given that femoral LISS plates come in different lengths, this study could be expanded to include the other plate sizes, as plate length could potentially play a role in stability at the fracture site. Third, though this Sawbones model has consistently reproduced the stability characteristics of human bone without variation between specimens, an osteoporotic model could be explored, as the femoral LISS plate is often used in osteoporotic fractures.7,14
Conclusion
Overall, our study results showed that 1-cm variations, anterior or posterior, had little effect on axial or torsional stiffness or plastic deformation under cyclical axial loading. Although these data can be promising for clinical application, the anterior placement of the LISS plate noted in failed fixation in other studies necessitates cautious interpretation of this study. Our use of a 13-hole (longer) plate, versus the 9-hole plate used in other studies, could explain the lack of variation between the 2 groups as well as the stability and tolerance of inappropriate placement. An osteoporotic model could help clinicians further discern the importance of accurate proximal placement of the femoral LISS plate.
Several surgical options are available for treatment of supracondylar and intercondylar distal femur fractures, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 33. Preserving the osseous blood supply via indirect reduction techniques has been shown to increase union rates without the need for bone grafting.1,2 The Less-Invasive Stabilization System (LISS) made by Synthes (Paoli, Pennsylvania) melds minimally invasive internal fixation with multiple fixed-angle distal screws. It allows for submuscular placement, percutaneous unicortical screws in the diaphysis, and preservation of the metaphyseal fracture soft-tissue envelope.3
Proper lateral placement of the plate on the femur proximally can be difficult. Kregor and colleagues3 noted that 6% of cases did not have ideal placement on the lateral shaft of the femur when the 13-hole LISS plate was used. They advocated making a small incision at the proximal end of the LISS plate to aid in proper lateral placement. Kolb and colleagues4 noted that 2 of 31 patients had a “cutting out” of the proximal screws on LISS plates with anterior placement on the femur that eventually required repeat surgery in order to heal. This malpositioned plate was present at the end of the operation. These authors also recommended a proximal incision to avoid the issue. Schütz and colleagues5 noted that there were 4 cases of implant loosening among 107 distal femur fractures treated with LISS plating and that the unicortical screws in the diaphysis had loosened. They suggested anterior placement of the plate as a possible reason for fixation failure.
Although several studies have noted proximal screw pull-out, and proximal anterior malposition in the sagittal plane of the LISS plate has been suggested as a possible cause, we found no studies comparing incorrect proximal positioning on the femoral shaft with correct lateral placement of the LISS plate. Therefore, we used a previously established biomechanical model to compare LISS plates proximally placed either too anterior or too posterior to the direct lateral position on the femoral shaft. The constructs were tested in axial, torsional, and cyclical axial modes to assess plastic and total deformation, stiffness, and fixation failure.
Materials and Methods
Using fourth-generation femoral synthetic composite bones (Sawbones; Pacific Research Laboratories, Vashon, Washington) and a 13-hole Synthes femoral LISS plate, we made 3 groups of 9 specimens each, for a total of 27 femurs. The number of specimens was based on a power assessment in a study by Khalafi and colleagues.8 Several studies have validated use of Sawbones instead of cadavers in biomechanical testing to prevent variability.6-9 Proximal fixation was achieved with 5 unicortical screws (26 mm long) at screw holes 13, 11, 9, 7, and 4. All distal screw holes were filled for distal fixation with 75-mm-long screws to achieve bicortical fixation.
After application of the LISS plate, an AO/OTA 33-A3 fracture model was created in each specimen. A 1-cm gap was made 6 cm proximal to the intercondylar notch to create an unstable distal femur fracture pattern. In the method described by Zlowodzki and colleagues,10 an additional 3-cm cut was made diagonally in the medial cortex to prevent contact of the bone during mechanical testing.
Three different plate positions were used. The correct group was placed directly laterally proximally (Figure 1A). One incorrect group was plated with the proximal aspect of the plate 1 cm anterior (anterior group) (Figure 1B), and another incorrect group was plated with the proximal aspect of the plate 1 cm posterior (posterior group) (Figure 1C). Anterior or posterior plate placement resulted in some of the proximal screws having a more tangential placement, with fewer screws engaged compared with the properly placed plate.
The distal and proximal ends of each specimen were held to simulate the mechanical axis of the femur. This design was based on a model by Cordey and colleagues.11 A materials testing system (MTS, Minneapolis, Minnesota) was used for mechanical testing of the model.
Based on the protocol of Khalafi and colleagues,8 the models were tested in axial, torsional, and cyclical axial modes (Figures 2, 3). Axial loading consisted of a preload of 100 N followed by a compressive loading rate of 100 mm per minute in a displacement control mode. Testing was considered completed when 1 of 3 events occurred: 500 N was reached, the medial fracture gap closed, or fixation was lost. Torsional loading involved a preload of 5 Nm and subsequent torqueing at 20° per minute up to 20 Nm or loss of fixation or screw pull-out.8 Cyclical axial loading was based on protocols described by Marti and colleagues2 and Zlowodzki and colleagues.10 The initial load was 10 cycles of 300 N. Each subsequent load increment was increased by 100 N up to 1000 N, providing 10-second rest increments. This loading was conducted in a displacement control mode at 0.75 mm per second. Testing was aborted on fixation loss or complete closure of the medial fracture gap.
After testing was completed, statistically significant between-groups differences in plastic deformation and axial and torsional stiffness were determined by performing a Tukey-Kramer honestly significant difference test. Significance was set at P ≤ .05.
Results
During axial loading, there was no visual loss of fixation or change in displacement of the fracture gap for any group, and there was no screw cut-out or pull-out from the cortex during testing. In 1 plate in the posterior group, the most proximal screw made only loose contact with the cortex at only the distal portion of the screw. There was no significant difference (P = .9762) in stiffness in axial loading between the anterior group and the correct group. There was a significant (P = .0261) 16.4% increase in stiffness in the posterior group compared with the correct group (Table).
There was no screw cut-out, fixation failure, or change in displacement of the fracture gap for any group during torsional loading. There was a statistically significant (P = .0062) 12% increase in mean torsional stiffness in the anterior group compared with the correct group. There was no statistically significant difference (P = .1623) between the posterior group and the correct group (Table).
For cyclical axial testing, total deformation and plastic deformation were obtained by determining displacement under the initial 100 N load in the static/resting state. That number was then subtracted from maximum displacement, the peak value on the time-versus-displacement graph, to obtain the value for total deformation. Plastic deformation was calculated by subtracting initial displacement from final displacement in the static/resting state. The static/resting state is represented by the dips in displacement after each cycle on the time-versus-displacement graph (Figure 4).
There was a statistically significant (P = .0207) 14% increase in total deformation of the anteriorly positioned plate compared with the correctly positioned plate. There was no statistically significant difference in total deformation between the posteriorly placed plates and the correctly placed plates (Table).
There was no significant difference in plastic deformation between any of the groups in this study. There was no screw cut-out or fixation loss in any group to suggest a clinically relevant difference based on proximal placement of the LISS plate.
Discussion
In evaluating the stability of various constructs for fixation of distal femur fractures, the literature is consistent in reporting stiffness as the key factor. Stiffness is determined most often in terms of motion at the fracture site, as measured by displacement under axial and torsional loads.2,8,10,13 The LISS plate, which acts essentially as an “internal fixator” with proximal unicortical fixed-angle locking screws, has been shown to be comparable to other established methods of fixation.10,12 Zlowodzki and colleagues10 reported that the LISS plate had a higher load to failure when compared with angled blade plating and intramedullary nailing. Their study used fresh-frozen cadaver specimens from patients 70 years old or older. They concluded that, for distal femur fractures in osteoporotic bone, the LISS plate provided improved distal fixation.
In the present study, the posteriorly placed LISS plate outperformed the correctly placed plate in axial stiffness by 16.4%. However, there was no statistically significant difference in torsional stiffness and cyclical axial loading. This result is difficult to explain given that there was no screw cut-out or fixation loss for any of the constructs. Theoretically, with less proximal screw purchase in the posteriorly placed plate, the overall construct should be more susceptible to screw cut-out and fixation loss resulting in less axial stiffness overall.
Khalafi and colleagues8 created a distal femur fracture model using Sawbones with a 1-cm fracture gap. Using the 9-hole LISS plate for fixation, they tested this construct under axial, torsional, and cyclical axial loads. They tested 2 groups of 9 femurs. For group 1, the LISS plate was placed in the correct position on the distal femur, with the proximal end in the correct position on the femoral shaft. In group 2, the LISS plate was rotated 1 cm anteriorly. They found that axial stiffness (N/mm) was 21.5% greater in the correctly positioned plate. The anteriorly positioned group demonstrated 55% more irreversible or plastic deformation. The authors concluded that correct positioning of the femoral LISS plate provided improved mechanical stability.
Overall, our study results did not agree with those of Khalafi and colleagues8 in terms of the mechanical stability of a malpositioned LISS plate. Our construct showed a significant increase in torsional stiffness in the anteriorly placed plate. However, our construct also showed a significant increase in total deformation in cyclical axial loading in the anteriorly placed plate. There was no increased plastic deformation in either of the incorrectly placed groups in our study. The difference in results between studies can best be explained by the difference in plate lengths. We used a 13-hole plate, and Khalafi and colleagues8 used a 9-hole plate. Our theory is that the longer plate provided more resistance to relatively minor variations in plate position at the proximal end and thus resulted in less change in stiffness and stability around the fracture site.
Our model differed from that used in other biomechanical studies using Sawbones to simulate distal femur fractures in that it used the entire femur, including the proximal portion.8,13 This setup theoretically resulted in a more anatomical weight distribution compared with other models, in which the proximal portion of the femur was potted in polymethylmethacrylate. This difference in weight distribution could explain the variation in our results compared with other biomechanical studies. In addition, with use of different boundary conditions, the distal femur had unconstrained distal motion similar to the native environment of the femur.
This study had several limitations. First is its relatively low power (9 femurs per group). Although groups of 9 specimens in 2 groups were used in the study by Khalafi and colleagues8, testing a larger number of femurs could potentially identify more subtle differences between the 3 groups in our study. Second, given that femoral LISS plates come in different lengths, this study could be expanded to include the other plate sizes, as plate length could potentially play a role in stability at the fracture site. Third, though this Sawbones model has consistently reproduced the stability characteristics of human bone without variation between specimens, an osteoporotic model could be explored, as the femoral LISS plate is often used in osteoporotic fractures.7,14
Conclusion
Overall, our study results showed that 1-cm variations, anterior or posterior, had little effect on axial or torsional stiffness or plastic deformation under cyclical axial loading. Although these data can be promising for clinical application, the anterior placement of the LISS plate noted in failed fixation in other studies necessitates cautious interpretation of this study. Our use of a 13-hole (longer) plate, versus the 9-hole plate used in other studies, could explain the lack of variation between the 2 groups as well as the stability and tolerance of inappropriate placement. An osteoporotic model could help clinicians further discern the importance of accurate proximal placement of the femoral LISS plate.
1. Bolhofner BR, Carmen B, Clifford P. The results of open reduction and internal fixation of distal femur fractures using a biologic (indirect) reduction technique. J Orthop Trauma. 1996;10(6):372-377.
2. Marti A, Frankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma. 2001;15(7):482-487.
3. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system. J Orthop Trauma. 2004;18(8):509-520.
4. Kolb W, Guhlmann H, Windisch C, Marx F, Kolb K, Koller H. Fixation of distal femoral fractures with the less invasive stabilization system: a minimally invasive treatment with locked fixed-angle screws. J Trauma. 2008;65(6):1425-1434.
5. Schütz M, Müller M, Krettek C, et al. Minimally invasive fracture stabilization of distal femoral fractures with the LISS: a prospective multicenter study. Results of a clinical study with special emphasis on difficult cases. Injury. 2001;32(suppl 3):SC55-SC63.
6. Chong AC, Friis EA, Ballard GP, Czuwala PJ, Cooke FW. Fatigue performance of composite analogue femur constructs under high activity loading. Ann Biomed Eng. 2007;35(7):1196-1205.
7. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
8. Khalafi A, Curtiss S, Hazelwood S, Wolinsky P. The effect of plate rotation on the stiffness of femoral LISS: a mechanical study. J Orthop Trauma. 2006;20(8):542-546.
9. Zdero R, Olsen M, Bougherara H, Schemitsch EH. Cancellous bone screw purchase: a comparison of synthetic femurs, human femurs, and finite element analysis. Proc Inst Mech Eng H. 2008;222(8):1175-1183.
10. Zlowodzki M, Williamson S, Cole PA, Zardiackas LD, Kregor PJ. Biomechanical evaluation of the less invasive stabilization system, angled blade plate, and retrograde intramedullary nail for the internal fixation of distal femur fractures. J Orthop Trauma. 2004;18(8):494-502.
11. Cordey J, Borgeaud M, Frankle, M, Harder Y, Martinet O. Loading model for the human femur taking the tension band effect of the ilio-tibial tract into account. Injury. 1999;30(suppl 1):A26-A30.
12. Weight M, Collinge C. Early results of the less invasive stabilization system for mechanically unstable fractures of the distal femur (AO/OTA types A2, A3, C2, and C3). J Orthop Trauma. 2004;18(8):503-508.
13. Otto RJ, Moed BR, Bledsoe JG. Biomechanical comparison of polyaxial-type locking plates and a fixed-angle locking plate for internal fixation of distal femur fractures. J Orthop Trauma. 2009;23(9):645–652.
14. Wong M, Leung F, Chow SP. Treatment of distal femoral fractures in the elderly using a less-invasive plating technique. Int Orthop. 2005;29(2):
117-120.
1. Bolhofner BR, Carmen B, Clifford P. The results of open reduction and internal fixation of distal femur fractures using a biologic (indirect) reduction technique. J Orthop Trauma. 1996;10(6):372-377.
2. Marti A, Frankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma. 2001;15(7):482-487.
3. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system. J Orthop Trauma. 2004;18(8):509-520.
4. Kolb W, Guhlmann H, Windisch C, Marx F, Kolb K, Koller H. Fixation of distal femoral fractures with the less invasive stabilization system: a minimally invasive treatment with locked fixed-angle screws. J Trauma. 2008;65(6):1425-1434.
5. Schütz M, Müller M, Krettek C, et al. Minimally invasive fracture stabilization of distal femoral fractures with the LISS: a prospective multicenter study. Results of a clinical study with special emphasis on difficult cases. Injury. 2001;32(suppl 3):SC55-SC63.
6. Chong AC, Friis EA, Ballard GP, Czuwala PJ, Cooke FW. Fatigue performance of composite analogue femur constructs under high activity loading. Ann Biomed Eng. 2007;35(7):1196-1205.
7. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
8. Khalafi A, Curtiss S, Hazelwood S, Wolinsky P. The effect of plate rotation on the stiffness of femoral LISS: a mechanical study. J Orthop Trauma. 2006;20(8):542-546.
9. Zdero R, Olsen M, Bougherara H, Schemitsch EH. Cancellous bone screw purchase: a comparison of synthetic femurs, human femurs, and finite element analysis. Proc Inst Mech Eng H. 2008;222(8):1175-1183.
10. Zlowodzki M, Williamson S, Cole PA, Zardiackas LD, Kregor PJ. Biomechanical evaluation of the less invasive stabilization system, angled blade plate, and retrograde intramedullary nail for the internal fixation of distal femur fractures. J Orthop Trauma. 2004;18(8):494-502.
11. Cordey J, Borgeaud M, Frankle, M, Harder Y, Martinet O. Loading model for the human femur taking the tension band effect of the ilio-tibial tract into account. Injury. 1999;30(suppl 1):A26-A30.
12. Weight M, Collinge C. Early results of the less invasive stabilization system for mechanically unstable fractures of the distal femur (AO/OTA types A2, A3, C2, and C3). J Orthop Trauma. 2004;18(8):503-508.
13. Otto RJ, Moed BR, Bledsoe JG. Biomechanical comparison of polyaxial-type locking plates and a fixed-angle locking plate for internal fixation of distal femur fractures. J Orthop Trauma. 2009;23(9):645–652.
14. Wong M, Leung F, Chow SP. Treatment of distal femoral fractures in the elderly using a less-invasive plating technique. Int Orthop. 2005;29(2):
117-120.
Antibiotic Cement-Coated Plates for Management of Infected Fractures
Deep infection in the presence of an implant after open reduction and internal fixation (ORIF) is usually treated with removal of the implant, serial débridement procedures, lavage, intravenously administered antibiotics, and, in some cases, placement of antibiotic-impregnated beads. If infection occurs during the early stages of bone healing, stabilization of the fractures might be compromised after removal of the implant. Although antibiotic-impregnated beads offer local delivery of antibiotics, they do not provide structural support of the fracture site. The beads often are difficult to remove after in-growth of granulation tissue. In areas of subcutaneous bone, an antibiotic bead pouch might be preferred to an open wound. Published research regarding the use of antibiotic-coated plates during the acute or chronic stages of infection is scarce. Plates offer the versatility of fracture stabilization, and the addition of antibiotic cement to the plates might aid in eradication of infection without necessitating a second surgery for removal. The patients provided written informed consent for print and electronic publication of these case reports.
Technique
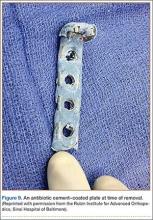
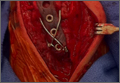
After removal of implants, we perform débridement of the soft tissues with a hydroscalpel (Versajet; Smith & Nephew, London, United Kingdom), mechanical débridement of bone, and curettage with high speed burr. The wound is then irrigated with pulse pressure lavage and a minimum of 3 L sterile normal saline. The extremity is re-prepped and re-draped; the entire surgical team’s gowns and gloves are changed; and new instrumentation, including cautery and suction equipment, is used. The cement is prepared with tobramycin (3.6 g) and vancomycin (1 g) per 40-g bag of cement. The plate is placed in silicon tubing, and the antibiotic-prepared cement is injected into the tubing and molded until dry. Care is taken to mold the locations of the screw holes by making incisions in the tubing at the appropriate locations. Screws are placed through the screw holes to ensure locking capability, and Kirschner wires are placed through temporary fixation holes (Figure 1). Once dry, the screws and wires are removed from the plate, and the cement-coated plate is removed from the tubing. The antibiotic-coated plate is applied to the fracture or osteotomy site and is seated with screws as appropriate (Figure 2). The wound is closed primarily. Wound drains or vacuum-assisted closure devices are not routinely used unless there is high risk for hematoma formation. The authors prefer to have high local concentrations of antibiotic in the surrounding tissues and wound.
Clinical Series
Case 1
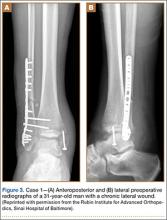
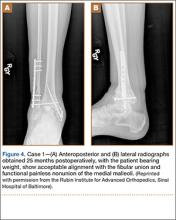
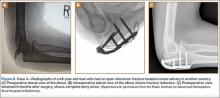
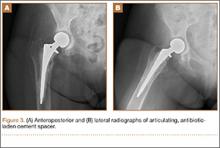
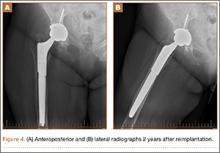
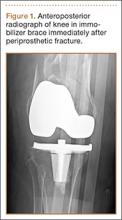
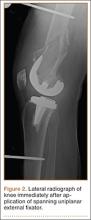
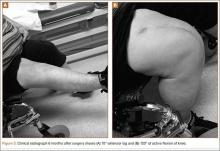
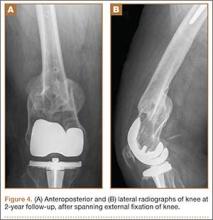
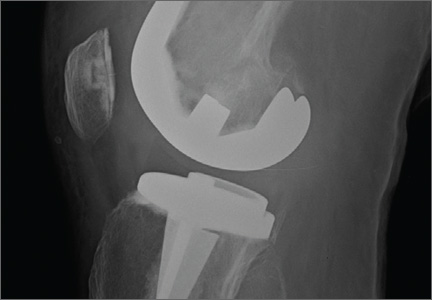
A 31-year-old man fell from a ladder and sustained a bimalleolar ankle fracture-dislocation that was treated with ORIF. Three weeks after initial injury, the patient presented with an infected lateral wound with purulent discharge. He was taken to the operating room for initial débridement, irrigation, and fracture stabilization with an antibiotic-coated plate and tension-band wiring of the medial malleoli. He was discharged from the hospital on day 4 after admission. Cultures of the wound grew beta-hemolytic strep group G and coagulase-negative staphylococci in broth that was sensitive to oxacillin, vancomycin, and gentamycin. The patient was treated with a 6-week regimen of Unasyn (Roerig, New York, New York), developed bony union, and has been free of clinical signs of infection for 2 years (Figures 3, 4).
Case 2
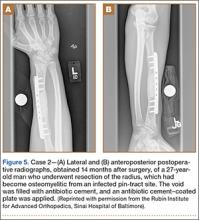
A 27-year-old male carpenter fell from a height of 12 feet and sustained a fracture of the distal radius that was treated with external fixation. The proximal pin site became clinically infected and subsequently developed osteomyelitis. The patient had a draining wound with a fracture for 2 months. He underwent débridement with partial resection of the radius and placement of an antibiotic cement–coated plate and calcium phosphate bone-void filler impregnated with antibiotics. Pathology specimens were positive for osteomyelitis, and bone cultures showed methicillin-sensitive Staphylococcus aureus (MSSA). He received intravenously administered antibiotic therapy for 6 weeks after surgery. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 5A, 5B).
Case 3
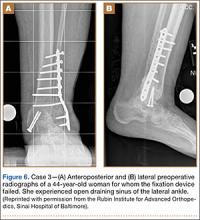
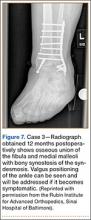
A 44-year-old woman with insulin-dependent diabetes mellitus and venous stasis sustained a trimalleolar ankle fracture after a low-energy fall that was initially treated with ORIF. She underwent revision ORIF to treat a malunion 3 months after initial treatment. At 8 months, the patient developed a draining sinus communicating with the plate. Computed tomography revealed nonunion and indicated infection. The patient underwent resection of the osteomyelitis and repair of the fibular nonunion with an antibiotic-coated plate. Tissue cultures were positive for coagulase-negative staphylococcus, and pathology specimens were positive for osteomyelitis. She received postoperative antibiotics intravenously and 6 weeks of antibiotic therapy after discharge from the hospital. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 6, 7).
Case 4
A 48-year-old man sustained an open olecranon fracture in another country. The fracture was initially treated with 1 dose of intravenously administered antibiotics and 5 days of orally administered antibiotics. The patient returned to the United States and was treated with intravenously administered antibiotics for cellulitis of the elbow for 11 days before referral to our institution, where he underwent ORIF with placement of an antibiotic-coated plate and tension-band wiring. Soft-tissue and bone cultures had no growth. He received intravenously administered antibiotics for 6 weeks. At 5 months postoperatively, the plate was removed because of pain. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 8A-8C).
Discussion
Acute infections of fractures have recently been treated with success by Berkes and colleagues,1 who reported a 71% union rate achieved with operative débridement, antibiotic suppression, and retention of fixation until fracture union occurs. The study by Berkes and colleagues1 had a small patient population, and larger cohorts are needed to show more reliable results; however, this treatment maintains structural support for the fracture during healing but requires multiple trips to the operating room for débridements as well as the use of systemic intravenous antibiotic therapy.
A technique that was developed by the primary author (Janet D. Conway, MD) and has not been described in the literature allows for use of antibiotic cement–coated plates to treat early postoperative infections and osteomyelitic nonunions. This approach permits fracture stabilization and local delivery of high concentrations of broad-spectrum antibiotics and can reduce the number of débridement procedures required in the operating room. We present a technique that includes the use of antibiotic cement–coated plates to treat early postoperative infections associated with fractures and nonunions in order to provide eradication of infection and bony stabilization.
Our approach parallels the current theory that treating infection at a site of union is preferable to treating infection at a site of nonunion.1 Fixation devices should remain in place until osseous union is achieved. With the addition of antibiotics to the plate, removal might not be necessary unless a device is loose, nonfunctional, or, ultimately, causing pain. Other options, such as external fixation, can be burdensome to patients and can be associated with other risks. One of our 4 patients required fixation removal because of pain at the elbow; however, even noncoated olecranon plates typically are removed because of pain after fracture healing. Antibiotic cement adds bulk to the construct and can become very prominent in areas of little soft-tissue coverage (Figure 9).
Studies, assessing variables that correlate with higher likelihood of failure for primary repairs, have shown that open fracture, use of an intramedullary nail, and smoking are the highest risk factors for infected nonunion.1−4 Among our 4 patients, 3 were smokers and 1 originally had an open fracture. Smokers have been found to have a 37% higher nonunion rate and are 2 times more likely to develop wound infection and osteomyelitis.1,5 More than 60% of the time, infections are caused by S aureus or coagulase-negative staphylococci.1,5,6 In our study population, 3 of the 4 patients had coagulase-negative staphylococci grow in the cultures. Implants infected with S aureus or Candida require surgical removal. Those with less virulent coagulase-negative staphylococci might not necessitate removal; however, our population had had antibiotic therapy and continued draining sinus.5 Rightmire and colleagues7 reported that those who develop infection earlier than 16 weeks postoperatively have a 68% success rate and that smoking is a major risk factor for infection. Development of Pseudomonas in the wound has been shown to have a positive correlation with amputation.1,2 Infection with Pseudomonas, smoking, and involvement of the femur, tibia, ankle, or foot tended to result in failure.1,2 Being clinically free of signs of infection after 3 months offers a 50% cure rate, with 78% at 6 months and 95% after 1 year.2
When determining an antibiotic to use with the polymethylmethacrylate (PMMA) cement, many factors must be considered, including spectrum, heat stability, and elution characteristics.8 A synergistic effect has been seen with combinations of antibiotics (eg, vancomycin and tobramycin used together). Vancomycin concentrations increased by 103% and tobramycin by 68% when used together compared with their elution rates when used alone, showing passive opportunism.9 This will, in essence, increase concentrations of antibiotics at the site locally, which will increase the bacteriocidal potential but also create a larger antimicrobial spectrum.9
The authors used Cobalt Bone Cement (Biomet Orthopedics, Inc, Warsaw, Indiana) which been shown to have higher elution properties than Simplex P Bone Cement (Stryker, Kalamazoo, Michigan).3,10 The majority of elution occurs in the first 3 to 5 days but can continue for weeks after implantation. We place the cement on the plate allowing for its retention, hoping to eliminate a second surgery for removal.8 We recommend 3.6 g of tobramycin, and 1 g of vancomycin per 40-g bag of PMMA.3 This dose has been shown to be safe in respect to renal toxicity, plus the entire dose is not administered in a single setting because only a small portion of the cement is used when coating the plate. We close all wounds primarily, and do not regularly use drains or vacuum-assisted closures to help prevent a decrease in the local concentration of the antibiotics.11
Broad-spectrum antibiotics are used to coat the plate in order to cover as many microbial organisms as possible without knowing the final offending organism. In our experience, this current technique provides antibiotic delivery with bony stability, therefore eliminating the need for multiple sequential surgical procedures. This difficult patient problem does not occur with enough frequency to warrant a large randomized clinical trial. However, this technique has been effective in these cases and may be useful to orthopedic surgeons in the future.
Conclusion
Based on our experience, early aggressive débridement, coupled with broad-spectrum antibiotic cement–coated plate insertion, provides fracture stability and helps eradicate the infection with 1 surgical procedure.
1. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823-828.
2. Tice AD, Hoaglund PA, Shoultz, DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261-1268.
3. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417-427.
4. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
5. Liporace FA, Yoon RS, Frank MA, et al. Use of an “antibiotic plate” for infected periprosthetic fracture in total hip arthroplasty. J Orthop Trauma. 2012;26(3):e18-e23.
6. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429.
7. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466(2):466-472.
8. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244-252.
9. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944.
10. Greene N, Holtom PD, Warren CA, et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201-205.
11. Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother. 2012;46(7-8):929-934.
Deep infection in the presence of an implant after open reduction and internal fixation (ORIF) is usually treated with removal of the implant, serial débridement procedures, lavage, intravenously administered antibiotics, and, in some cases, placement of antibiotic-impregnated beads. If infection occurs during the early stages of bone healing, stabilization of the fractures might be compromised after removal of the implant. Although antibiotic-impregnated beads offer local delivery of antibiotics, they do not provide structural support of the fracture site. The beads often are difficult to remove after in-growth of granulation tissue. In areas of subcutaneous bone, an antibiotic bead pouch might be preferred to an open wound. Published research regarding the use of antibiotic-coated plates during the acute or chronic stages of infection is scarce. Plates offer the versatility of fracture stabilization, and the addition of antibiotic cement to the plates might aid in eradication of infection without necessitating a second surgery for removal. The patients provided written informed consent for print and electronic publication of these case reports.
Technique
After removal of implants, we perform débridement of the soft tissues with a hydroscalpel (Versajet; Smith & Nephew, London, United Kingdom), mechanical débridement of bone, and curettage with high speed burr. The wound is then irrigated with pulse pressure lavage and a minimum of 3 L sterile normal saline. The extremity is re-prepped and re-draped; the entire surgical team’s gowns and gloves are changed; and new instrumentation, including cautery and suction equipment, is used. The cement is prepared with tobramycin (3.6 g) and vancomycin (1 g) per 40-g bag of cement. The plate is placed in silicon tubing, and the antibiotic-prepared cement is injected into the tubing and molded until dry. Care is taken to mold the locations of the screw holes by making incisions in the tubing at the appropriate locations. Screws are placed through the screw holes to ensure locking capability, and Kirschner wires are placed through temporary fixation holes (Figure 1). Once dry, the screws and wires are removed from the plate, and the cement-coated plate is removed from the tubing. The antibiotic-coated plate is applied to the fracture or osteotomy site and is seated with screws as appropriate (Figure 2). The wound is closed primarily. Wound drains or vacuum-assisted closure devices are not routinely used unless there is high risk for hematoma formation. The authors prefer to have high local concentrations of antibiotic in the surrounding tissues and wound.
Clinical Series
Case 1
A 31-year-old man fell from a ladder and sustained a bimalleolar ankle fracture-dislocation that was treated with ORIF. Three weeks after initial injury, the patient presented with an infected lateral wound with purulent discharge. He was taken to the operating room for initial débridement, irrigation, and fracture stabilization with an antibiotic-coated plate and tension-band wiring of the medial malleoli. He was discharged from the hospital on day 4 after admission. Cultures of the wound grew beta-hemolytic strep group G and coagulase-negative staphylococci in broth that was sensitive to oxacillin, vancomycin, and gentamycin. The patient was treated with a 6-week regimen of Unasyn (Roerig, New York, New York), developed bony union, and has been free of clinical signs of infection for 2 years (Figures 3, 4).
Case 2
A 27-year-old male carpenter fell from a height of 12 feet and sustained a fracture of the distal radius that was treated with external fixation. The proximal pin site became clinically infected and subsequently developed osteomyelitis. The patient had a draining wound with a fracture for 2 months. He underwent débridement with partial resection of the radius and placement of an antibiotic cement–coated plate and calcium phosphate bone-void filler impregnated with antibiotics. Pathology specimens were positive for osteomyelitis, and bone cultures showed methicillin-sensitive Staphylococcus aureus (MSSA). He received intravenously administered antibiotic therapy for 6 weeks after surgery. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 5A, 5B).
Case 3
A 44-year-old woman with insulin-dependent diabetes mellitus and venous stasis sustained a trimalleolar ankle fracture after a low-energy fall that was initially treated with ORIF. She underwent revision ORIF to treat a malunion 3 months after initial treatment. At 8 months, the patient developed a draining sinus communicating with the plate. Computed tomography revealed nonunion and indicated infection. The patient underwent resection of the osteomyelitis and repair of the fibular nonunion with an antibiotic-coated plate. Tissue cultures were positive for coagulase-negative staphylococcus, and pathology specimens were positive for osteomyelitis. She received postoperative antibiotics intravenously and 6 weeks of antibiotic therapy after discharge from the hospital. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 6, 7).
Case 4
A 48-year-old man sustained an open olecranon fracture in another country. The fracture was initially treated with 1 dose of intravenously administered antibiotics and 5 days of orally administered antibiotics. The patient returned to the United States and was treated with intravenously administered antibiotics for cellulitis of the elbow for 11 days before referral to our institution, where he underwent ORIF with placement of an antibiotic-coated plate and tension-band wiring. Soft-tissue and bone cultures had no growth. He received intravenously administered antibiotics for 6 weeks. At 5 months postoperatively, the plate was removed because of pain. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 8A-8C).
Discussion
Acute infections of fractures have recently been treated with success by Berkes and colleagues,1 who reported a 71% union rate achieved with operative débridement, antibiotic suppression, and retention of fixation until fracture union occurs. The study by Berkes and colleagues1 had a small patient population, and larger cohorts are needed to show more reliable results; however, this treatment maintains structural support for the fracture during healing but requires multiple trips to the operating room for débridements as well as the use of systemic intravenous antibiotic therapy.
A technique that was developed by the primary author (Janet D. Conway, MD) and has not been described in the literature allows for use of antibiotic cement–coated plates to treat early postoperative infections and osteomyelitic nonunions. This approach permits fracture stabilization and local delivery of high concentrations of broad-spectrum antibiotics and can reduce the number of débridement procedures required in the operating room. We present a technique that includes the use of antibiotic cement–coated plates to treat early postoperative infections associated with fractures and nonunions in order to provide eradication of infection and bony stabilization.
Our approach parallels the current theory that treating infection at a site of union is preferable to treating infection at a site of nonunion.1 Fixation devices should remain in place until osseous union is achieved. With the addition of antibiotics to the plate, removal might not be necessary unless a device is loose, nonfunctional, or, ultimately, causing pain. Other options, such as external fixation, can be burdensome to patients and can be associated with other risks. One of our 4 patients required fixation removal because of pain at the elbow; however, even noncoated olecranon plates typically are removed because of pain after fracture healing. Antibiotic cement adds bulk to the construct and can become very prominent in areas of little soft-tissue coverage (Figure 9).
Studies, assessing variables that correlate with higher likelihood of failure for primary repairs, have shown that open fracture, use of an intramedullary nail, and smoking are the highest risk factors for infected nonunion.1−4 Among our 4 patients, 3 were smokers and 1 originally had an open fracture. Smokers have been found to have a 37% higher nonunion rate and are 2 times more likely to develop wound infection and osteomyelitis.1,5 More than 60% of the time, infections are caused by S aureus or coagulase-negative staphylococci.1,5,6 In our study population, 3 of the 4 patients had coagulase-negative staphylococci grow in the cultures. Implants infected with S aureus or Candida require surgical removal. Those with less virulent coagulase-negative staphylococci might not necessitate removal; however, our population had had antibiotic therapy and continued draining sinus.5 Rightmire and colleagues7 reported that those who develop infection earlier than 16 weeks postoperatively have a 68% success rate and that smoking is a major risk factor for infection. Development of Pseudomonas in the wound has been shown to have a positive correlation with amputation.1,2 Infection with Pseudomonas, smoking, and involvement of the femur, tibia, ankle, or foot tended to result in failure.1,2 Being clinically free of signs of infection after 3 months offers a 50% cure rate, with 78% at 6 months and 95% after 1 year.2
When determining an antibiotic to use with the polymethylmethacrylate (PMMA) cement, many factors must be considered, including spectrum, heat stability, and elution characteristics.8 A synergistic effect has been seen with combinations of antibiotics (eg, vancomycin and tobramycin used together). Vancomycin concentrations increased by 103% and tobramycin by 68% when used together compared with their elution rates when used alone, showing passive opportunism.9 This will, in essence, increase concentrations of antibiotics at the site locally, which will increase the bacteriocidal potential but also create a larger antimicrobial spectrum.9
The authors used Cobalt Bone Cement (Biomet Orthopedics, Inc, Warsaw, Indiana) which been shown to have higher elution properties than Simplex P Bone Cement (Stryker, Kalamazoo, Michigan).3,10 The majority of elution occurs in the first 3 to 5 days but can continue for weeks after implantation. We place the cement on the plate allowing for its retention, hoping to eliminate a second surgery for removal.8 We recommend 3.6 g of tobramycin, and 1 g of vancomycin per 40-g bag of PMMA.3 This dose has been shown to be safe in respect to renal toxicity, plus the entire dose is not administered in a single setting because only a small portion of the cement is used when coating the plate. We close all wounds primarily, and do not regularly use drains or vacuum-assisted closures to help prevent a decrease in the local concentration of the antibiotics.11
Broad-spectrum antibiotics are used to coat the plate in order to cover as many microbial organisms as possible without knowing the final offending organism. In our experience, this current technique provides antibiotic delivery with bony stability, therefore eliminating the need for multiple sequential surgical procedures. This difficult patient problem does not occur with enough frequency to warrant a large randomized clinical trial. However, this technique has been effective in these cases and may be useful to orthopedic surgeons in the future.
Conclusion
Based on our experience, early aggressive débridement, coupled with broad-spectrum antibiotic cement–coated plate insertion, provides fracture stability and helps eradicate the infection with 1 surgical procedure.
Deep infection in the presence of an implant after open reduction and internal fixation (ORIF) is usually treated with removal of the implant, serial débridement procedures, lavage, intravenously administered antibiotics, and, in some cases, placement of antibiotic-impregnated beads. If infection occurs during the early stages of bone healing, stabilization of the fractures might be compromised after removal of the implant. Although antibiotic-impregnated beads offer local delivery of antibiotics, they do not provide structural support of the fracture site. The beads often are difficult to remove after in-growth of granulation tissue. In areas of subcutaneous bone, an antibiotic bead pouch might be preferred to an open wound. Published research regarding the use of antibiotic-coated plates during the acute or chronic stages of infection is scarce. Plates offer the versatility of fracture stabilization, and the addition of antibiotic cement to the plates might aid in eradication of infection without necessitating a second surgery for removal. The patients provided written informed consent for print and electronic publication of these case reports.
Technique
After removal of implants, we perform débridement of the soft tissues with a hydroscalpel (Versajet; Smith & Nephew, London, United Kingdom), mechanical débridement of bone, and curettage with high speed burr. The wound is then irrigated with pulse pressure lavage and a minimum of 3 L sterile normal saline. The extremity is re-prepped and re-draped; the entire surgical team’s gowns and gloves are changed; and new instrumentation, including cautery and suction equipment, is used. The cement is prepared with tobramycin (3.6 g) and vancomycin (1 g) per 40-g bag of cement. The plate is placed in silicon tubing, and the antibiotic-prepared cement is injected into the tubing and molded until dry. Care is taken to mold the locations of the screw holes by making incisions in the tubing at the appropriate locations. Screws are placed through the screw holes to ensure locking capability, and Kirschner wires are placed through temporary fixation holes (Figure 1). Once dry, the screws and wires are removed from the plate, and the cement-coated plate is removed from the tubing. The antibiotic-coated plate is applied to the fracture or osteotomy site and is seated with screws as appropriate (Figure 2). The wound is closed primarily. Wound drains or vacuum-assisted closure devices are not routinely used unless there is high risk for hematoma formation. The authors prefer to have high local concentrations of antibiotic in the surrounding tissues and wound.
Clinical Series
Case 1
A 31-year-old man fell from a ladder and sustained a bimalleolar ankle fracture-dislocation that was treated with ORIF. Three weeks after initial injury, the patient presented with an infected lateral wound with purulent discharge. He was taken to the operating room for initial débridement, irrigation, and fracture stabilization with an antibiotic-coated plate and tension-band wiring of the medial malleoli. He was discharged from the hospital on day 4 after admission. Cultures of the wound grew beta-hemolytic strep group G and coagulase-negative staphylococci in broth that was sensitive to oxacillin, vancomycin, and gentamycin. The patient was treated with a 6-week regimen of Unasyn (Roerig, New York, New York), developed bony union, and has been free of clinical signs of infection for 2 years (Figures 3, 4).
Case 2
A 27-year-old male carpenter fell from a height of 12 feet and sustained a fracture of the distal radius that was treated with external fixation. The proximal pin site became clinically infected and subsequently developed osteomyelitis. The patient had a draining wound with a fracture for 2 months. He underwent débridement with partial resection of the radius and placement of an antibiotic cement–coated plate and calcium phosphate bone-void filler impregnated with antibiotics. Pathology specimens were positive for osteomyelitis, and bone cultures showed methicillin-sensitive Staphylococcus aureus (MSSA). He received intravenously administered antibiotic therapy for 6 weeks after surgery. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 5A, 5B).
Case 3
A 44-year-old woman with insulin-dependent diabetes mellitus and venous stasis sustained a trimalleolar ankle fracture after a low-energy fall that was initially treated with ORIF. She underwent revision ORIF to treat a malunion 3 months after initial treatment. At 8 months, the patient developed a draining sinus communicating with the plate. Computed tomography revealed nonunion and indicated infection. The patient underwent resection of the osteomyelitis and repair of the fibular nonunion with an antibiotic-coated plate. Tissue cultures were positive for coagulase-negative staphylococcus, and pathology specimens were positive for osteomyelitis. She received postoperative antibiotics intravenously and 6 weeks of antibiotic therapy after discharge from the hospital. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 6, 7).
Case 4
A 48-year-old man sustained an open olecranon fracture in another country. The fracture was initially treated with 1 dose of intravenously administered antibiotics and 5 days of orally administered antibiotics. The patient returned to the United States and was treated with intravenously administered antibiotics for cellulitis of the elbow for 11 days before referral to our institution, where he underwent ORIF with placement of an antibiotic-coated plate and tension-band wiring. Soft-tissue and bone cultures had no growth. He received intravenously administered antibiotics for 6 weeks. At 5 months postoperatively, the plate was removed because of pain. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 8A-8C).
Discussion
Acute infections of fractures have recently been treated with success by Berkes and colleagues,1 who reported a 71% union rate achieved with operative débridement, antibiotic suppression, and retention of fixation until fracture union occurs. The study by Berkes and colleagues1 had a small patient population, and larger cohorts are needed to show more reliable results; however, this treatment maintains structural support for the fracture during healing but requires multiple trips to the operating room for débridements as well as the use of systemic intravenous antibiotic therapy.
A technique that was developed by the primary author (Janet D. Conway, MD) and has not been described in the literature allows for use of antibiotic cement–coated plates to treat early postoperative infections and osteomyelitic nonunions. This approach permits fracture stabilization and local delivery of high concentrations of broad-spectrum antibiotics and can reduce the number of débridement procedures required in the operating room. We present a technique that includes the use of antibiotic cement–coated plates to treat early postoperative infections associated with fractures and nonunions in order to provide eradication of infection and bony stabilization.
Our approach parallels the current theory that treating infection at a site of union is preferable to treating infection at a site of nonunion.1 Fixation devices should remain in place until osseous union is achieved. With the addition of antibiotics to the plate, removal might not be necessary unless a device is loose, nonfunctional, or, ultimately, causing pain. Other options, such as external fixation, can be burdensome to patients and can be associated with other risks. One of our 4 patients required fixation removal because of pain at the elbow; however, even noncoated olecranon plates typically are removed because of pain after fracture healing. Antibiotic cement adds bulk to the construct and can become very prominent in areas of little soft-tissue coverage (Figure 9).
Studies, assessing variables that correlate with higher likelihood of failure for primary repairs, have shown that open fracture, use of an intramedullary nail, and smoking are the highest risk factors for infected nonunion.1−4 Among our 4 patients, 3 were smokers and 1 originally had an open fracture. Smokers have been found to have a 37% higher nonunion rate and are 2 times more likely to develop wound infection and osteomyelitis.1,5 More than 60% of the time, infections are caused by S aureus or coagulase-negative staphylococci.1,5,6 In our study population, 3 of the 4 patients had coagulase-negative staphylococci grow in the cultures. Implants infected with S aureus or Candida require surgical removal. Those with less virulent coagulase-negative staphylococci might not necessitate removal; however, our population had had antibiotic therapy and continued draining sinus.5 Rightmire and colleagues7 reported that those who develop infection earlier than 16 weeks postoperatively have a 68% success rate and that smoking is a major risk factor for infection. Development of Pseudomonas in the wound has been shown to have a positive correlation with amputation.1,2 Infection with Pseudomonas, smoking, and involvement of the femur, tibia, ankle, or foot tended to result in failure.1,2 Being clinically free of signs of infection after 3 months offers a 50% cure rate, with 78% at 6 months and 95% after 1 year.2
When determining an antibiotic to use with the polymethylmethacrylate (PMMA) cement, many factors must be considered, including spectrum, heat stability, and elution characteristics.8 A synergistic effect has been seen with combinations of antibiotics (eg, vancomycin and tobramycin used together). Vancomycin concentrations increased by 103% and tobramycin by 68% when used together compared with their elution rates when used alone, showing passive opportunism.9 This will, in essence, increase concentrations of antibiotics at the site locally, which will increase the bacteriocidal potential but also create a larger antimicrobial spectrum.9
The authors used Cobalt Bone Cement (Biomet Orthopedics, Inc, Warsaw, Indiana) which been shown to have higher elution properties than Simplex P Bone Cement (Stryker, Kalamazoo, Michigan).3,10 The majority of elution occurs in the first 3 to 5 days but can continue for weeks after implantation. We place the cement on the plate allowing for its retention, hoping to eliminate a second surgery for removal.8 We recommend 3.6 g of tobramycin, and 1 g of vancomycin per 40-g bag of PMMA.3 This dose has been shown to be safe in respect to renal toxicity, plus the entire dose is not administered in a single setting because only a small portion of the cement is used when coating the plate. We close all wounds primarily, and do not regularly use drains or vacuum-assisted closures to help prevent a decrease in the local concentration of the antibiotics.11
Broad-spectrum antibiotics are used to coat the plate in order to cover as many microbial organisms as possible without knowing the final offending organism. In our experience, this current technique provides antibiotic delivery with bony stability, therefore eliminating the need for multiple sequential surgical procedures. This difficult patient problem does not occur with enough frequency to warrant a large randomized clinical trial. However, this technique has been effective in these cases and may be useful to orthopedic surgeons in the future.
Conclusion
Based on our experience, early aggressive débridement, coupled with broad-spectrum antibiotic cement–coated plate insertion, provides fracture stability and helps eradicate the infection with 1 surgical procedure.
1. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823-828.
2. Tice AD, Hoaglund PA, Shoultz, DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261-1268.
3. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417-427.
4. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
5. Liporace FA, Yoon RS, Frank MA, et al. Use of an “antibiotic plate” for infected periprosthetic fracture in total hip arthroplasty. J Orthop Trauma. 2012;26(3):e18-e23.
6. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429.
7. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466(2):466-472.
8. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244-252.
9. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944.
10. Greene N, Holtom PD, Warren CA, et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201-205.
11. Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother. 2012;46(7-8):929-934.
1. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823-828.
2. Tice AD, Hoaglund PA, Shoultz, DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261-1268.
3. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417-427.
4. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
5. Liporace FA, Yoon RS, Frank MA, et al. Use of an “antibiotic plate” for infected periprosthetic fracture in total hip arthroplasty. J Orthop Trauma. 2012;26(3):e18-e23.
6. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429.
7. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466(2):466-472.
8. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244-252.
9. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944.
10. Greene N, Holtom PD, Warren CA, et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201-205.
11. Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother. 2012;46(7-8):929-934.
Mycobacterium bovis Infection of Total Knee Arthroplasty After Bacillus Calmette-Guérin Therapy for Bladder Cancer
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
Failure of Total Hip Arthroplasty Secondary to Infection Caused by Brucella abortus and the Risk of Transmission to Operative Staff
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.
Discussion
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts. Transmission can occur via breaks in the skin in direct contact, through the ingestion of unpasteurized dairy products or raw meat, or through ingestion of aerosolized bacteria. Transmission via aerosolization has been described during medical procedures.
Brucella is endemic in India, Middle Eastern and Mediterranean countries, Central Asia, and South America. Brucella species are gram-negative coccobacilli that are capable of surviving within phagocytic cells, making antibiotic treatment difficult. Brucellosis is a febrile illness that occurs after a 1- to 3-week incubation period and is often accompanied by headache, arthralgias, and hepatosplenomegaly. Osteoarticular infection is the most common complication, occurring in 10% to 85% of cases and usually involves the sacroiliac joint and the large joints of the lower extremity. Spondylitis, bursitis, tenosynovitis, endocarditis, colitis, meningitis, and osteomyelitis have also been described.7,14-17
As mentioned previously, 18 cases of infected THAs and total knee arthroplasties (TKAs) in 16 patients were identified in the English literature: 9 THAs and 9 TKAs.1-12 With the exception of 1 case reported in Texas, all others were from the Middle East or the Mediterranean region. In these patients, symptom onset occurred from 2 months to 14 years from the time of the index surgery, and symptom duration ranged from 1 month to 2 years prior to presentation. The exposure was not reported in 2 cases, but the remaining patients either ingested unpasteurized dairy products or worked closely with livestock. Laboratory evaluation revealed elevated ESR or CRP in 8 cases. In 7 cases, no laboratory results were reported, although 1 had a draining sinus. In 1 case, the ESR was normal, but a bone scan was positive. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (one aspirate yielded Acinetobacter baumanii). Only 3 cases reported a time-to-culture positivity (1 “prolonged” and 2 took 7 days).
Eight cases presented with loose components, while 1 case was not reported, and the remaining were presumed to be well-fixed. In cases that were identified as loose, 5 underwent a 2-stage revision and 2 underwent a 1-stage revision (in one of the 1-stage revisions, the infection was identified only after the revision from intra-operative cultures). Of those with well-fixed components, 7 patients with 9 infected joints (including the case where no preoperative description of the components was reported) were treated with oral antibiotics only (range, 6 weeks to 26 months) and 1 with irrigation and débridement and oral antibiotics. Among those treated only with antibiotics, there were 2 failures (2 joints) leading to revision surgery. The other 5 cases were reportedly doing well between 8 months and 5 years after treatment. There were no reports of transmission to hospital or laboratory personnel in any of these cases nor were there reports of precautions to limit exposure for operating room staff or hospital personnel.
Failure of TKA or THA secondary to periprosthetic infection by Brucella species is rare, and this represents only the second reported case in the United States.4 This case highlights several important principles. Maintaining a high level of suspicion for infection in cases of failed joint arthroplasty is important. In addition, as more international travel occurs and patients are seen from areas where Brucella is endemic, the possibility of this infectious etiology should be considered. Based on reported cases, patients will usually have elevated ESR or CRP; all (except 2 cases in which no exposure was reported) had known exposure to unpasteurized dairy products or livestock. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (1 aspirate yielded Acinetobacter baumanii). In this case, ESR and CRP were elevated, and infection was suspected but joint aspiration was negative. The initial aspiration was cultured for 5 days and previous data, as well as that presented here, suggest that prolonged culture may provide diagnostic value.18 The patient had resided in an endemic area and had exposure to unpasteurized dairy products, but Brucella infection was not considered and, therefore, no precautions were taken.
Of the reported cases, only 1 met major criteria for periprosthetic joint infection (draining sinus) while 10 of the remaining 15 cases were positive for minor criteria of periprosthetic joint infection (elevated ESR or CRP, or positive culture from joint aspiration).19 Unfortunately, the available case reports did not detail the extent to which preoperative periprosthetic joint infection could be established based on minor criteria for periprosthetic joint infection (elevated joint synovial white blood cell count or neutrophil percentage, intra-articular purulence, or elevated neutrophil count on periprosthetic tissue histologic analysis).19
Periprosthetic joint infection by Brucella species is so rare that specific recommendations for this infectious etiology based on 18 reported cases would be overreaching. However, Brucella should be considered when evaluating a potentially infected joint replacement where the possibility of exposure exists (eg, travel to or previous residence in endemic areas, close contact with livestock, or ingestion of unpasteurized dairy products in endemic regions), with the potential for transmission to operating room and hospital personnel also considered. If there is concern about Brucella involvement, tissue and fluid specimens should be labeled so that laboratory personnel can take appropriate precautions. Brucella can be cultured using routine techniques on standard, nonselective media, but the culture time-to-growth may be prolonged. Culture plates should be held for 14 days before reporting no growth of Brucella if it is suspected; the New Mexico Department of Health Microbiology Laboratory holds routine cultures for 1 week after a report of no growth. Thus, a suspicion of Brucella should be communicated in order for culture time to be adjusted if the holding of culture plates after an initial report of no growth is not standard practice. If operative intervention is planned and brucellosis is known, personnel should be notified of the possibility of exposure and appropriate measures taken (ie, wearing N-95 respiratory masks during the procedure and considering other methods of irrigation less likely to aerosolize particulates). It is not known if preoperative antibiotic therapy can sufficiently lower the bacterial load to make aerosolization less likely. If brucellosis is suspected but not identified preoperatively, wearing N-95 respiratory masks should be considered during any open procedures.
Conclusion
In cases of Brucella infection and loose components, 1- or 2-stage revision with appropriate antibiotic therapy is indicated. (There is not enough data to recommend either 1- or 2-stage revision.) Several reports comment on the ability to treat periprosthetic joint infection in the setting of well-fixed components with antibiotic therapy alone. While this appears to have been successful in 7 of 9 infected joints reported in the literature, length of follow-up ranged from 8 months to 5 years, with no report of length of follow-up in some cases. Antibiotic therapy duration ranged from 6 weeks to 26 months, and the antibiotic treatment involved combination therapy with multiple agents reported but, most commonly, doxycycline, rifampin, and streptomycin. With 2 of 9 (22%) joints failing antibiotic therapy alone and those reported to be successful having relatively short-term follow-up, this treatment strategy should be approached with caution.
1. Agarwal S, Kadhi SK, Rooney RJ. Brucellosis complicating bilateral total knee arthroplasty. Clin Orthop. 1991;267:179-181.
2. Cairó M, Calbo E, Gomez L, et al. Foreign-body osteoarticular infection by Brucella melitensis: A report of three cases. J Bone Joint Surg Am. 2006; 88(1):202-204.
3. Erdogan H, Cakmak G, Erdogan A, Arslan H. Brucella melitensis infection in total knee arthroplasty: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):908-910.
4. Jones RE, Berryhill WH, Smith J, Hofman A, Rogers D. Secondary infection of a total hip replacement with Brucella abortus. Orthopedics. 1983; 6(2):184-186.
5. Kasim RA, Araj GF, Afeiche NE, Tabbarah ZA. Brucella infection in total hip replacement: case report and review of the literature. Scand J Infect Dis. 2004;36(1):65-67.
6. Malizos KN, Makris CA, Soucacos PN. Total knee arthroplasties infected by Brucella melitensis: a case report. Am J Orthop. 1997;26(4):283-285.
7. Ortega-Andreu M, Rodriguez-Merchan EC, Aguera-Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty. 2002;17(3):384-387.
8. Orti A, Alcala R, Navarro V, et al. Brucellar arthritis in a total knee replacement. Eur J Clin Microbiol Infect Dis. 1997;16(11):843-845.
9. Ruiz-Iban MA, Crespo P, Diaz-Peletier R, Rozado AM, Lopez-Pardo A. Total hip arthroplasty infected by Brucella: a report of two cases. J Orthop Surg (Hong Kong). 2006;14(1):99-103.
10. Tassinari E, Di Motta D, Giardina F, Traina F, Fine MD, Toni A. Brucella infection in total knee arthroplasty. Case report and revision of the literature. Chir Organi Mov. 2008;92(1):55-59.
11. Tena D, Romanillos O, Rodriguez-Zapata M, et al. Prosthetic hip infection due to Brucella melitensis: case report and literature review. Diagn Microbiol Infect Dis. 2007;58(4):481-485.
12. Weil Y, Mattan Y, Liebergall M, Rahav G. Brucella prosthetic joint infection: a report of 3 cases and a review of the literature. Clin Infect Dis. 2003;36(7):e81-e86.
13. Brucellosis. Centers for Disease Control and Prevention website. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/brucellosis/recommendations.html. Updated November 12, 2012. Accessed December 22, 2014.
14. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-786.
15. Khateeb MI, Araj GF, Majeed SA, Lulu AR. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis. 1990;49(12):994-998.
16. Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1-4):19-30.
17. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
18. Schafer P, Fink B, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Disease. 2008;47(11):1403-1409.
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.
Discussion
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts. Transmission can occur via breaks in the skin in direct contact, through the ingestion of unpasteurized dairy products or raw meat, or through ingestion of aerosolized bacteria. Transmission via aerosolization has been described during medical procedures.
Brucella is endemic in India, Middle Eastern and Mediterranean countries, Central Asia, and South America. Brucella species are gram-negative coccobacilli that are capable of surviving within phagocytic cells, making antibiotic treatment difficult. Brucellosis is a febrile illness that occurs after a 1- to 3-week incubation period and is often accompanied by headache, arthralgias, and hepatosplenomegaly. Osteoarticular infection is the most common complication, occurring in 10% to 85% of cases and usually involves the sacroiliac joint and the large joints of the lower extremity. Spondylitis, bursitis, tenosynovitis, endocarditis, colitis, meningitis, and osteomyelitis have also been described.7,14-17
As mentioned previously, 18 cases of infected THAs and total knee arthroplasties (TKAs) in 16 patients were identified in the English literature: 9 THAs and 9 TKAs.1-12 With the exception of 1 case reported in Texas, all others were from the Middle East or the Mediterranean region. In these patients, symptom onset occurred from 2 months to 14 years from the time of the index surgery, and symptom duration ranged from 1 month to 2 years prior to presentation. The exposure was not reported in 2 cases, but the remaining patients either ingested unpasteurized dairy products or worked closely with livestock. Laboratory evaluation revealed elevated ESR or CRP in 8 cases. In 7 cases, no laboratory results were reported, although 1 had a draining sinus. In 1 case, the ESR was normal, but a bone scan was positive. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (one aspirate yielded Acinetobacter baumanii). Only 3 cases reported a time-to-culture positivity (1 “prolonged” and 2 took 7 days).
Eight cases presented with loose components, while 1 case was not reported, and the remaining were presumed to be well-fixed. In cases that were identified as loose, 5 underwent a 2-stage revision and 2 underwent a 1-stage revision (in one of the 1-stage revisions, the infection was identified only after the revision from intra-operative cultures). Of those with well-fixed components, 7 patients with 9 infected joints (including the case where no preoperative description of the components was reported) were treated with oral antibiotics only (range, 6 weeks to 26 months) and 1 with irrigation and débridement and oral antibiotics. Among those treated only with antibiotics, there were 2 failures (2 joints) leading to revision surgery. The other 5 cases were reportedly doing well between 8 months and 5 years after treatment. There were no reports of transmission to hospital or laboratory personnel in any of these cases nor were there reports of precautions to limit exposure for operating room staff or hospital personnel.
Failure of TKA or THA secondary to periprosthetic infection by Brucella species is rare, and this represents only the second reported case in the United States.4 This case highlights several important principles. Maintaining a high level of suspicion for infection in cases of failed joint arthroplasty is important. In addition, as more international travel occurs and patients are seen from areas where Brucella is endemic, the possibility of this infectious etiology should be considered. Based on reported cases, patients will usually have elevated ESR or CRP; all (except 2 cases in which no exposure was reported) had known exposure to unpasteurized dairy products or livestock. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (1 aspirate yielded Acinetobacter baumanii). In this case, ESR and CRP were elevated, and infection was suspected but joint aspiration was negative. The initial aspiration was cultured for 5 days and previous data, as well as that presented here, suggest that prolonged culture may provide diagnostic value.18 The patient had resided in an endemic area and had exposure to unpasteurized dairy products, but Brucella infection was not considered and, therefore, no precautions were taken.
Of the reported cases, only 1 met major criteria for periprosthetic joint infection (draining sinus) while 10 of the remaining 15 cases were positive for minor criteria of periprosthetic joint infection (elevated ESR or CRP, or positive culture from joint aspiration).19 Unfortunately, the available case reports did not detail the extent to which preoperative periprosthetic joint infection could be established based on minor criteria for periprosthetic joint infection (elevated joint synovial white blood cell count or neutrophil percentage, intra-articular purulence, or elevated neutrophil count on periprosthetic tissue histologic analysis).19
Periprosthetic joint infection by Brucella species is so rare that specific recommendations for this infectious etiology based on 18 reported cases would be overreaching. However, Brucella should be considered when evaluating a potentially infected joint replacement where the possibility of exposure exists (eg, travel to or previous residence in endemic areas, close contact with livestock, or ingestion of unpasteurized dairy products in endemic regions), with the potential for transmission to operating room and hospital personnel also considered. If there is concern about Brucella involvement, tissue and fluid specimens should be labeled so that laboratory personnel can take appropriate precautions. Brucella can be cultured using routine techniques on standard, nonselective media, but the culture time-to-growth may be prolonged. Culture plates should be held for 14 days before reporting no growth of Brucella if it is suspected; the New Mexico Department of Health Microbiology Laboratory holds routine cultures for 1 week after a report of no growth. Thus, a suspicion of Brucella should be communicated in order for culture time to be adjusted if the holding of culture plates after an initial report of no growth is not standard practice. If operative intervention is planned and brucellosis is known, personnel should be notified of the possibility of exposure and appropriate measures taken (ie, wearing N-95 respiratory masks during the procedure and considering other methods of irrigation less likely to aerosolize particulates). It is not known if preoperative antibiotic therapy can sufficiently lower the bacterial load to make aerosolization less likely. If brucellosis is suspected but not identified preoperatively, wearing N-95 respiratory masks should be considered during any open procedures.
Conclusion
In cases of Brucella infection and loose components, 1- or 2-stage revision with appropriate antibiotic therapy is indicated. (There is not enough data to recommend either 1- or 2-stage revision.) Several reports comment on the ability to treat periprosthetic joint infection in the setting of well-fixed components with antibiotic therapy alone. While this appears to have been successful in 7 of 9 infected joints reported in the literature, length of follow-up ranged from 8 months to 5 years, with no report of length of follow-up in some cases. Antibiotic therapy duration ranged from 6 weeks to 26 months, and the antibiotic treatment involved combination therapy with multiple agents reported but, most commonly, doxycycline, rifampin, and streptomycin. With 2 of 9 (22%) joints failing antibiotic therapy alone and those reported to be successful having relatively short-term follow-up, this treatment strategy should be approached with caution.
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.
Discussion
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts. Transmission can occur via breaks in the skin in direct contact, through the ingestion of unpasteurized dairy products or raw meat, or through ingestion of aerosolized bacteria. Transmission via aerosolization has been described during medical procedures.
Brucella is endemic in India, Middle Eastern and Mediterranean countries, Central Asia, and South America. Brucella species are gram-negative coccobacilli that are capable of surviving within phagocytic cells, making antibiotic treatment difficult. Brucellosis is a febrile illness that occurs after a 1- to 3-week incubation period and is often accompanied by headache, arthralgias, and hepatosplenomegaly. Osteoarticular infection is the most common complication, occurring in 10% to 85% of cases and usually involves the sacroiliac joint and the large joints of the lower extremity. Spondylitis, bursitis, tenosynovitis, endocarditis, colitis, meningitis, and osteomyelitis have also been described.7,14-17
As mentioned previously, 18 cases of infected THAs and total knee arthroplasties (TKAs) in 16 patients were identified in the English literature: 9 THAs and 9 TKAs.1-12 With the exception of 1 case reported in Texas, all others were from the Middle East or the Mediterranean region. In these patients, symptom onset occurred from 2 months to 14 years from the time of the index surgery, and symptom duration ranged from 1 month to 2 years prior to presentation. The exposure was not reported in 2 cases, but the remaining patients either ingested unpasteurized dairy products or worked closely with livestock. Laboratory evaluation revealed elevated ESR or CRP in 8 cases. In 7 cases, no laboratory results were reported, although 1 had a draining sinus. In 1 case, the ESR was normal, but a bone scan was positive. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (one aspirate yielded Acinetobacter baumanii). Only 3 cases reported a time-to-culture positivity (1 “prolonged” and 2 took 7 days).
Eight cases presented with loose components, while 1 case was not reported, and the remaining were presumed to be well-fixed. In cases that were identified as loose, 5 underwent a 2-stage revision and 2 underwent a 1-stage revision (in one of the 1-stage revisions, the infection was identified only after the revision from intra-operative cultures). Of those with well-fixed components, 7 patients with 9 infected joints (including the case where no preoperative description of the components was reported) were treated with oral antibiotics only (range, 6 weeks to 26 months) and 1 with irrigation and débridement and oral antibiotics. Among those treated only with antibiotics, there were 2 failures (2 joints) leading to revision surgery. The other 5 cases were reportedly doing well between 8 months and 5 years after treatment. There were no reports of transmission to hospital or laboratory personnel in any of these cases nor were there reports of precautions to limit exposure for operating room staff or hospital personnel.
Failure of TKA or THA secondary to periprosthetic infection by Brucella species is rare, and this represents only the second reported case in the United States.4 This case highlights several important principles. Maintaining a high level of suspicion for infection in cases of failed joint arthroplasty is important. In addition, as more international travel occurs and patients are seen from areas where Brucella is endemic, the possibility of this infectious etiology should be considered. Based on reported cases, patients will usually have elevated ESR or CRP; all (except 2 cases in which no exposure was reported) had known exposure to unpasteurized dairy products or livestock. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (1 aspirate yielded Acinetobacter baumanii). In this case, ESR and CRP were elevated, and infection was suspected but joint aspiration was negative. The initial aspiration was cultured for 5 days and previous data, as well as that presented here, suggest that prolonged culture may provide diagnostic value.18 The patient had resided in an endemic area and had exposure to unpasteurized dairy products, but Brucella infection was not considered and, therefore, no precautions were taken.
Of the reported cases, only 1 met major criteria for periprosthetic joint infection (draining sinus) while 10 of the remaining 15 cases were positive for minor criteria of periprosthetic joint infection (elevated ESR or CRP, or positive culture from joint aspiration).19 Unfortunately, the available case reports did not detail the extent to which preoperative periprosthetic joint infection could be established based on minor criteria for periprosthetic joint infection (elevated joint synovial white blood cell count or neutrophil percentage, intra-articular purulence, or elevated neutrophil count on periprosthetic tissue histologic analysis).19
Periprosthetic joint infection by Brucella species is so rare that specific recommendations for this infectious etiology based on 18 reported cases would be overreaching. However, Brucella should be considered when evaluating a potentially infected joint replacement where the possibility of exposure exists (eg, travel to or previous residence in endemic areas, close contact with livestock, or ingestion of unpasteurized dairy products in endemic regions), with the potential for transmission to operating room and hospital personnel also considered. If there is concern about Brucella involvement, tissue and fluid specimens should be labeled so that laboratory personnel can take appropriate precautions. Brucella can be cultured using routine techniques on standard, nonselective media, but the culture time-to-growth may be prolonged. Culture plates should be held for 14 days before reporting no growth of Brucella if it is suspected; the New Mexico Department of Health Microbiology Laboratory holds routine cultures for 1 week after a report of no growth. Thus, a suspicion of Brucella should be communicated in order for culture time to be adjusted if the holding of culture plates after an initial report of no growth is not standard practice. If operative intervention is planned and brucellosis is known, personnel should be notified of the possibility of exposure and appropriate measures taken (ie, wearing N-95 respiratory masks during the procedure and considering other methods of irrigation less likely to aerosolize particulates). It is not known if preoperative antibiotic therapy can sufficiently lower the bacterial load to make aerosolization less likely. If brucellosis is suspected but not identified preoperatively, wearing N-95 respiratory masks should be considered during any open procedures.
Conclusion
In cases of Brucella infection and loose components, 1- or 2-stage revision with appropriate antibiotic therapy is indicated. (There is not enough data to recommend either 1- or 2-stage revision.) Several reports comment on the ability to treat periprosthetic joint infection in the setting of well-fixed components with antibiotic therapy alone. While this appears to have been successful in 7 of 9 infected joints reported in the literature, length of follow-up ranged from 8 months to 5 years, with no report of length of follow-up in some cases. Antibiotic therapy duration ranged from 6 weeks to 26 months, and the antibiotic treatment involved combination therapy with multiple agents reported but, most commonly, doxycycline, rifampin, and streptomycin. With 2 of 9 (22%) joints failing antibiotic therapy alone and those reported to be successful having relatively short-term follow-up, this treatment strategy should be approached with caution.
1. Agarwal S, Kadhi SK, Rooney RJ. Brucellosis complicating bilateral total knee arthroplasty. Clin Orthop. 1991;267:179-181.
2. Cairó M, Calbo E, Gomez L, et al. Foreign-body osteoarticular infection by Brucella melitensis: A report of three cases. J Bone Joint Surg Am. 2006; 88(1):202-204.
3. Erdogan H, Cakmak G, Erdogan A, Arslan H. Brucella melitensis infection in total knee arthroplasty: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):908-910.
4. Jones RE, Berryhill WH, Smith J, Hofman A, Rogers D. Secondary infection of a total hip replacement with Brucella abortus. Orthopedics. 1983; 6(2):184-186.
5. Kasim RA, Araj GF, Afeiche NE, Tabbarah ZA. Brucella infection in total hip replacement: case report and review of the literature. Scand J Infect Dis. 2004;36(1):65-67.
6. Malizos KN, Makris CA, Soucacos PN. Total knee arthroplasties infected by Brucella melitensis: a case report. Am J Orthop. 1997;26(4):283-285.
7. Ortega-Andreu M, Rodriguez-Merchan EC, Aguera-Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty. 2002;17(3):384-387.
8. Orti A, Alcala R, Navarro V, et al. Brucellar arthritis in a total knee replacement. Eur J Clin Microbiol Infect Dis. 1997;16(11):843-845.
9. Ruiz-Iban MA, Crespo P, Diaz-Peletier R, Rozado AM, Lopez-Pardo A. Total hip arthroplasty infected by Brucella: a report of two cases. J Orthop Surg (Hong Kong). 2006;14(1):99-103.
10. Tassinari E, Di Motta D, Giardina F, Traina F, Fine MD, Toni A. Brucella infection in total knee arthroplasty. Case report and revision of the literature. Chir Organi Mov. 2008;92(1):55-59.
11. Tena D, Romanillos O, Rodriguez-Zapata M, et al. Prosthetic hip infection due to Brucella melitensis: case report and literature review. Diagn Microbiol Infect Dis. 2007;58(4):481-485.
12. Weil Y, Mattan Y, Liebergall M, Rahav G. Brucella prosthetic joint infection: a report of 3 cases and a review of the literature. Clin Infect Dis. 2003;36(7):e81-e86.
13. Brucellosis. Centers for Disease Control and Prevention website. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/brucellosis/recommendations.html. Updated November 12, 2012. Accessed December 22, 2014.
14. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-786.
15. Khateeb MI, Araj GF, Majeed SA, Lulu AR. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis. 1990;49(12):994-998.
16. Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1-4):19-30.
17. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
18. Schafer P, Fink B, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Disease. 2008;47(11):1403-1409.
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
1. Agarwal S, Kadhi SK, Rooney RJ. Brucellosis complicating bilateral total knee arthroplasty. Clin Orthop. 1991;267:179-181.
2. Cairó M, Calbo E, Gomez L, et al. Foreign-body osteoarticular infection by Brucella melitensis: A report of three cases. J Bone Joint Surg Am. 2006; 88(1):202-204.
3. Erdogan H, Cakmak G, Erdogan A, Arslan H. Brucella melitensis infection in total knee arthroplasty: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):908-910.
4. Jones RE, Berryhill WH, Smith J, Hofman A, Rogers D. Secondary infection of a total hip replacement with Brucella abortus. Orthopedics. 1983; 6(2):184-186.
5. Kasim RA, Araj GF, Afeiche NE, Tabbarah ZA. Brucella infection in total hip replacement: case report and review of the literature. Scand J Infect Dis. 2004;36(1):65-67.
6. Malizos KN, Makris CA, Soucacos PN. Total knee arthroplasties infected by Brucella melitensis: a case report. Am J Orthop. 1997;26(4):283-285.
7. Ortega-Andreu M, Rodriguez-Merchan EC, Aguera-Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty. 2002;17(3):384-387.
8. Orti A, Alcala R, Navarro V, et al. Brucellar arthritis in a total knee replacement. Eur J Clin Microbiol Infect Dis. 1997;16(11):843-845.
9. Ruiz-Iban MA, Crespo P, Diaz-Peletier R, Rozado AM, Lopez-Pardo A. Total hip arthroplasty infected by Brucella: a report of two cases. J Orthop Surg (Hong Kong). 2006;14(1):99-103.
10. Tassinari E, Di Motta D, Giardina F, Traina F, Fine MD, Toni A. Brucella infection in total knee arthroplasty. Case report and revision of the literature. Chir Organi Mov. 2008;92(1):55-59.
11. Tena D, Romanillos O, Rodriguez-Zapata M, et al. Prosthetic hip infection due to Brucella melitensis: case report and literature review. Diagn Microbiol Infect Dis. 2007;58(4):481-485.
12. Weil Y, Mattan Y, Liebergall M, Rahav G. Brucella prosthetic joint infection: a report of 3 cases and a review of the literature. Clin Infect Dis. 2003;36(7):e81-e86.
13. Brucellosis. Centers for Disease Control and Prevention website. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/brucellosis/recommendations.html. Updated November 12, 2012. Accessed December 22, 2014.
14. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-786.
15. Khateeb MI, Araj GF, Majeed SA, Lulu AR. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis. 1990;49(12):994-998.
16. Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1-4):19-30.
17. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
18. Schafer P, Fink B, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Disease. 2008;47(11):1403-1409.
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
Dilute Betadine Lavage Reduces Implant-Related Bacterial Burden in a Rabbit Knee Prosthetic Infection Model
Surgical site infection after arthroplasty causes substantial morbidity and potential mortality. Prosthetic joint infection (PJI) ranges from simple superficial wound infection and cellulitis to deep subfascial infection that involves the prosthesis. Consistent use of prophylactic antibiotics has reduced postoperative hip and knee arthroplasty infections to rates of 0.25% to 2%.1-4 Treatment of a patient with PJI commonly includes hospitalization, long-term intravenously administered antibiotics, resection arthroplasty, and staged reimplantation. The estimated cost of interventions reaches tens of millions of dollars annually in the United States and does not include the costs of psychosocial effects on patients and their families.5,6
Betadine (povidone-iodine) is a widely used antiseptic for skin and mucous membrane wounds and has been shown to be effective for the prevention of PJI.7 Dilute Betadine solution has been proposed as an aid in treatment of PJI.8 At a minimum concentration of 5%, cytotoxicity has been observed in chicken tibia osteoblasts.9 A balance of the bactericidal and cytotoxic activities of Betadine, while maintaining its efficacy against resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), is optimized at dilutions between 0.5% and 4%.10-14 We hypothesized that a dilute Betadine lavage of 3.5% would achieve a significant decrease in bacterial counts compared with an isolated saline lavage in an in vivo knee PJI model.
Materials and Methods
Animal Protocol
All surgical procedures were conducted according to the protocol approved by our institutional animal care and use committee. Using a power analysis and data obtained at our institution, we determined that 12 was the minimum number of animals needed to reach significance set at P < .05 and assuming a 50% decrease in colony-forming units (CFU) (SigmaStat Version 2.03; Aspire Software International, Ashburn, Virginia). Eight New Zealand White rabbits were used in our study; because significance was reached early, 12 were not needed. The average weight of the rabbits was 3.5 kg (weight range, 3.2-4.1 kg). All rabbits completed 1 week of acclimation before surgery.
Bacteria Preparation
A broth culture of methicillin-sensitive S aureus (MSSA) (ATCC 25923) was prepared 1 day before surgery. The bacteria were suspended in 5 mL of Trypticase Soy Broth (Becton Dickinson & Co, Franklin Lakes, New Jersey) and incubated at 37°C in a shaking incubator for 16 hours. The next day, the culture was centrifuged and irrigated twice with normal saline to remove the broth and prevent further growth. The bacteria were reconstituted in normal saline, and the concentration was standardized using a turbidity meter (LaMotte 2020e; LaMotte Co, Chestertown, Maryland), which correlated with 106 CFU/100 µl plated on trypticase soy agar plates with 10% sheep blood (Fisher Scientific, Pittsburgh, Pennsylvania).
Surgical and Postoperative Procedures
Our procedure was based on the New Zealand White rabbit knee PJI model.15 General anesthesia was induced with ketamine and xylazine, and maintained with isoflurane inhalation via a nose cone mask. Rabbits were positioned supine, and bilateral knees were shaved, prepped, and draped in a sterile fashion.
A 2-cm longitudinal incision was made over the lateral knee, and arthrotomy was performed, exposing the lateral collateral ligament attachment at the lateral femoral condyle. Using a 4-mm drill bit, a defect was drilled obliquely into the lateral femoral condyle, anterior to the lateral collateral ligament attachment. This produced a defect in the non-weight-bearing, nonarticulating portion of the knee. A fully threaded 4×14-mm stainless steel screw (Synthes, West Chester, Pennsylvania) with a U-shaped ultrahigh-molecular-weight polyethylene washer (Synthes) was inserted into the defect. The joint capsule was closed with a running 3-0 Vicryl suture (Ethicon, Somerville, New Jersey). The knee joint was inoculated with 100 µL of the S aureus preparation using a 22-gauge needle. The skin was closed with a 4-0 Biosyn suture (Ethicon). The procedure was repeated on the contralateral knee (Figures 1A, 1B).
Seven days after the initial surgery, the rabbits were returned to the operating room and were anesthetized, positioned, and prepped for surgery as detailed above. Ceftriaxone (20 mg/kg of body weight) was intravenously administered to all rabbits for the treatment procedure. For each rabbit, a control knee and an experimental knee were randomly assigned. A longitudinal incision was made, exposing the previously placed implants. The screw was loosened slightly to remove the U-shaped polyethylene washer. Each knee then underwent lavage 2 times, for 90 seconds each time, with 3.5% dilute Betadine solution (experimental knee) or with normal saline (control knee). Because Pseudomonas contamination has been reported with povidone-iodine taken from unsterilized bottles,16,17 packets of sterilized povidone-iodine (Aplicare; Clorox, Oakland, California) were used. After the irrigation was complete, a new sterile polyethylene washer was placed and the screw was tightened. The wound closure was repeated as detailed above.
Postoperative analgesia was provided based on a standard institutional animal care and use committee protocol. Rabbits were permitted full cage activity and nutrition ad libitum. Wound healing, body weight, and signs of distress were monitored daily.
Outcome Measures
Seven days after surgery, the rabbits were euthanized with administration of phenobarbital (100 mg/kg of body weight). Arterial blood samples were obtained from the auricular vein to ensure that the rabbits were not systemically infected. Using a sterile technique, the screw, polyethylene washer, lateral femoral condyle bone from the defect, and joint capsule were cultured. Harvested bone and soft tissues were weighed and immediately homogenized (PowerGen Model 35 Handheld Homogenizer; Thermo Fisher Scientific, Inc, Waltham, Massachusetts). Implants were sonicated (UBATH-Y; World Precision Instruments, Inc, Sarasota, Florida) in cold saline to obtain a sensitive culture.18
Bacterial quantification was determined by using trypticase soy agar plates after 24 hours of growth. Final CFU were calculated after serial dilutions and were standardized per gram of biopsied tissues.19 Members of the team were blinded to the treatment type.
Statistical Analysis
Statistical differences in mean bacterial burden were calculated independently for lateral condyle bone, joint capsule, polyethylene, and screws by conducting a Student t test.
Results
Treatment effect was higher than expected, and the study was terminated after 8 animals completed the protocol. All 8 rabbits tolerated the procedures well and were appropriately monitored during the postoperative period. No animals had signs of systemic infection or positive blood culture. All local cultures for screw, polyethylene washer, lateral femoral condyle defect, and joint capsule were positive.
Statistically significant decreases were shown in the bacterial burden of the Betadine-irrigated screws and the Betadine-irrigated polyethylene washers compared with the saline-irrigated controls. Betadine-irrigated screws grew an average of 7.16 × 101 CFU of S aureus/g, whereas screws from control knees grew an average of 1.45 × 103 CFU/g (P = .0003) (Figure 2). Betadine-treated washers grew an average of 1.28 × 103 CFU/g compared with 1.62 × 104 CFU/g for control washers (P =. 04) (Figure 3).
A trend toward decreased bacterial counts was shown in Betadine-treated soft tissues compared with saline-treated soft tissues, but the difference did not reach statistical significance (P = .9). Biopsied joint capsule from knees treated with Betadine grew an average of 2.84 × 104 CFU/g compared with an average of 3.16 × 104 CFU/g in control-rabbit knees (Figure 4). Cultured lateral condyle from Betadine-treated knees had an average bacterial load of 3.22 × 104 CFU/g compared with an average bacterial load of 1 × 105 CFU/g in control knees (Figure 4).
Discussion
Knees irrigated with Betadine showed a significant (P = .0003) decrease in metal implant–related S aureus bacterial counts by 20-fold and a significant (P < .05) decrease in polyethylene implant–related counts by more than 10-fold. This arthroplasty model used Betadine lavage as a treatment adjunct with intravenously administered antibiotics and polyethylene exchange. Our 1-week interval after the index procedure classifies the infection as an acute postoperative arthroplasty infection (occurring less than 4 weeks postoperatively).
The gold standard treatment for these infections is irrigation and débridement with component retention.18 The success rate has been reported to be as high as 71%20 but was closer to 44% in a study by Fridkin and colleagues,21 especially with more virulent bacteria. Staphylococcal species, higher American Society of Anesthesiologists scores, and frank pus around the prosthesis were markers of débridement failure in a recent study by Azzam and colleagues.18
The majority of postoperative joint arthroplasty infections are caused by S aureus, and the incidence of MRSA bacteria continues to rise.22 Community-acquired MRSA is increasing at an alarming rate and is now the predominant organism in skin and soft-tissue infections.23 Organism resistance also occurs at a cellular level by the formation of a glycocalyx layer, or biofilm. This layer assists in changing the phenotypic properties of the organism and decreases the efficacy of antibiotics.24 The self-produced layer of extracellular matrices, deoxyribonucleic acid, and polysaccharides attaches to inert material, preventing phagocytic action by neutrophils. In addition to antibacterial activity, povidone-iodine has antibiofilm activity against Staphylococcal species.25 The active ingredient targets the gene that produces biofilm. This correlates to our study in which the largest decrease in bacterial counts was noted on the implants.
The use of Betadine lavage has shown some promise in vivo as well. A prospective randomized controlled trial26 used 3.5% Betadine irrigation to prevent spine infection. No infections occurred in the Betadine group compared with a deep-infection rate of 2.9% in the control group. Brown and colleagues8 reviewed 1862 hip and knee arthroplasty cases before the use of Betadine lavage and 688 cases after the use of Betadine lavage and found a decrease in infection rate, from 0.97% to 0.15%. S aureus caused 13 of the 18 infections in the control group. These studies8,26 used Betadine lavage for prophylaxis and prevention of deep spine and arthroplasty infection. Betadine lavage as a treatment adjunct for acute arthroplasty infection has not been studied clinically. It has the potential to increase isolated incision and débridement success and to improve component survivorship.
Our arthroplasty model mimics an intra-articular environment and accounts for an implant–polyethylene interface.15 Limitations of our study include the use of MSSA as opposed to MRSA. However, povidone-iodine has the same effects on both MSSA and MRSA.12 We also treated our postoperative infection with 1 dose of antibiotics and not a course, although it should be noted that the single dose of ceftriaxone allowed us to isolate the independent effect of the Betadine lavage. A baseline level of infection severity could have been measured with cultures obtained at the time of irrigation and débridement. Also, a decrease in CFU does not directly correlate to a clinically significant outcome, such as a defined surgical site infection requiring intervention. Nevertheless, it is noteworthy that the decrease in bacterial counts on the stainless steel screws and polyethylene washers were maintained 1 week after the Betadine lavage.
Conclusion
Dilute Betadine lavage is a simple and inexpensive adjunct for the treatment of acute postoperative arthroplasty infection and may increase the rate of component retention. Additionally, the bactericidal and antibiofilm activities of Betadine may improve the effectiveness of systemic antibiotics. Further clinical investigation is warranted.
1. Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72(6):878-883.
2. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
3. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
4. Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87(6):1222-1228.
5. Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics. 2010;33(9):659.
6. Poultsides LA, Liaropoulos LL, Malizos KN. The socioeconomic impact of musculoskeletal infections. J Bone Joint Surg Am. 2010;92(11):e13.
7. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
8. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
9. Kaysinger KK, Nicholson NC, Ramp WK, Kellam JF. Toxic effects of wound irrigation solutions on cultured tibiae and osteoblasts. J Orthop Trauma. 1995;9(4):303-311.
10. Haley CE, Marling-Cason M, Smith JW, Luby JP, Mackowiak PA. Bactericidal activity of antiseptics against methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;21(6):991-992.
11. Lacey RW, Catto A. Action of povidone-iodine against methicillin-sensitive and -resistant cultures of Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S78-S83.
12. McLure AR, Gordon J. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1992;21(4):291-299.
13. Suzuki J, Komatsuzawa H, Kozai K, Nagasaka N. In vitro susceptibility of Staphylococcus aureus including MRSA to four disinfectants. ASDC J Dent Child. 1997;64(4):260-263.
14. Yasuda T, Yoshimura S, Katsuno Y, et al. Comparison of bactericidal activities of various disinfectants against methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S66-S69.
15. Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TE. A novel total knee arthroplasty infection model in rabbits. J Orthop Res. 2005;23(5):1100-1104.
16. Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop. 1991;273:113-118.
17. Mont MA, Waldman B, Banerjee C, Pacheco IH, Hungerford DS. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12(4):426-433.
18. Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25(7):1022-1027.
19. Stall AC, Becker E, Ludwig SC, Gelb D, Poelstra KA. Reduction of postoperative spinal implant infection using gentamicin microspheres. Spine (Phila Pa 1976). 2009;34(5):479-483.
20. Hota B, Ellenbogen C, Hayden MK, Aroutcheva A, Rice TW, Weinstein RA. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med. 2007;167(10):1026-1033.
21. Fridkin SK, Hageman JC, Morrison M, et al, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436-1444.
22. Hosman AH, van der Mei HC, Bulstra SK, Busscher HJ, Neut D. Metal-on-metal bearings in total hip arthroplasties: influence of cobalt and chromium ions on bacterial growth and biofilm formation. J Biomed Mater Res A. 2009;88(3):711-716.
23. Oduwole KO, Glynn AA, Molony DC, et al. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J Orthop Res. 2010;28(9):1252-1256.
24. Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976). 2005;30(15):1689-1693.
25. Anderson RL, Vess RW, Panlilio AL, Favero MS. Prolonged survival of Pseudomonas cepacia in commercially manufactured povidone-iodine. Appl Environ Microbiol. 1990;56(11):3598-3600.
26. Panlilio AL, Beck-Sague CM, Siegel JD, et al. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin Infect Dis. 1992;14(5):1078-1083.
Surgical site infection after arthroplasty causes substantial morbidity and potential mortality. Prosthetic joint infection (PJI) ranges from simple superficial wound infection and cellulitis to deep subfascial infection that involves the prosthesis. Consistent use of prophylactic antibiotics has reduced postoperative hip and knee arthroplasty infections to rates of 0.25% to 2%.1-4 Treatment of a patient with PJI commonly includes hospitalization, long-term intravenously administered antibiotics, resection arthroplasty, and staged reimplantation. The estimated cost of interventions reaches tens of millions of dollars annually in the United States and does not include the costs of psychosocial effects on patients and their families.5,6
Betadine (povidone-iodine) is a widely used antiseptic for skin and mucous membrane wounds and has been shown to be effective for the prevention of PJI.7 Dilute Betadine solution has been proposed as an aid in treatment of PJI.8 At a minimum concentration of 5%, cytotoxicity has been observed in chicken tibia osteoblasts.9 A balance of the bactericidal and cytotoxic activities of Betadine, while maintaining its efficacy against resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), is optimized at dilutions between 0.5% and 4%.10-14 We hypothesized that a dilute Betadine lavage of 3.5% would achieve a significant decrease in bacterial counts compared with an isolated saline lavage in an in vivo knee PJI model.
Materials and Methods
Animal Protocol
All surgical procedures were conducted according to the protocol approved by our institutional animal care and use committee. Using a power analysis and data obtained at our institution, we determined that 12 was the minimum number of animals needed to reach significance set at P < .05 and assuming a 50% decrease in colony-forming units (CFU) (SigmaStat Version 2.03; Aspire Software International, Ashburn, Virginia). Eight New Zealand White rabbits were used in our study; because significance was reached early, 12 were not needed. The average weight of the rabbits was 3.5 kg (weight range, 3.2-4.1 kg). All rabbits completed 1 week of acclimation before surgery.
Bacteria Preparation
A broth culture of methicillin-sensitive S aureus (MSSA) (ATCC 25923) was prepared 1 day before surgery. The bacteria were suspended in 5 mL of Trypticase Soy Broth (Becton Dickinson & Co, Franklin Lakes, New Jersey) and incubated at 37°C in a shaking incubator for 16 hours. The next day, the culture was centrifuged and irrigated twice with normal saline to remove the broth and prevent further growth. The bacteria were reconstituted in normal saline, and the concentration was standardized using a turbidity meter (LaMotte 2020e; LaMotte Co, Chestertown, Maryland), which correlated with 106 CFU/100 µl plated on trypticase soy agar plates with 10% sheep blood (Fisher Scientific, Pittsburgh, Pennsylvania).
Surgical and Postoperative Procedures
Our procedure was based on the New Zealand White rabbit knee PJI model.15 General anesthesia was induced with ketamine and xylazine, and maintained with isoflurane inhalation via a nose cone mask. Rabbits were positioned supine, and bilateral knees were shaved, prepped, and draped in a sterile fashion.
A 2-cm longitudinal incision was made over the lateral knee, and arthrotomy was performed, exposing the lateral collateral ligament attachment at the lateral femoral condyle. Using a 4-mm drill bit, a defect was drilled obliquely into the lateral femoral condyle, anterior to the lateral collateral ligament attachment. This produced a defect in the non-weight-bearing, nonarticulating portion of the knee. A fully threaded 4×14-mm stainless steel screw (Synthes, West Chester, Pennsylvania) with a U-shaped ultrahigh-molecular-weight polyethylene washer (Synthes) was inserted into the defect. The joint capsule was closed with a running 3-0 Vicryl suture (Ethicon, Somerville, New Jersey). The knee joint was inoculated with 100 µL of the S aureus preparation using a 22-gauge needle. The skin was closed with a 4-0 Biosyn suture (Ethicon). The procedure was repeated on the contralateral knee (Figures 1A, 1B).
Seven days after the initial surgery, the rabbits were returned to the operating room and were anesthetized, positioned, and prepped for surgery as detailed above. Ceftriaxone (20 mg/kg of body weight) was intravenously administered to all rabbits for the treatment procedure. For each rabbit, a control knee and an experimental knee were randomly assigned. A longitudinal incision was made, exposing the previously placed implants. The screw was loosened slightly to remove the U-shaped polyethylene washer. Each knee then underwent lavage 2 times, for 90 seconds each time, with 3.5% dilute Betadine solution (experimental knee) or with normal saline (control knee). Because Pseudomonas contamination has been reported with povidone-iodine taken from unsterilized bottles,16,17 packets of sterilized povidone-iodine (Aplicare; Clorox, Oakland, California) were used. After the irrigation was complete, a new sterile polyethylene washer was placed and the screw was tightened. The wound closure was repeated as detailed above.
Postoperative analgesia was provided based on a standard institutional animal care and use committee protocol. Rabbits were permitted full cage activity and nutrition ad libitum. Wound healing, body weight, and signs of distress were monitored daily.
Outcome Measures
Seven days after surgery, the rabbits were euthanized with administration of phenobarbital (100 mg/kg of body weight). Arterial blood samples were obtained from the auricular vein to ensure that the rabbits were not systemically infected. Using a sterile technique, the screw, polyethylene washer, lateral femoral condyle bone from the defect, and joint capsule were cultured. Harvested bone and soft tissues were weighed and immediately homogenized (PowerGen Model 35 Handheld Homogenizer; Thermo Fisher Scientific, Inc, Waltham, Massachusetts). Implants were sonicated (UBATH-Y; World Precision Instruments, Inc, Sarasota, Florida) in cold saline to obtain a sensitive culture.18
Bacterial quantification was determined by using trypticase soy agar plates after 24 hours of growth. Final CFU were calculated after serial dilutions and were standardized per gram of biopsied tissues.19 Members of the team were blinded to the treatment type.
Statistical Analysis
Statistical differences in mean bacterial burden were calculated independently for lateral condyle bone, joint capsule, polyethylene, and screws by conducting a Student t test.
Results
Treatment effect was higher than expected, and the study was terminated after 8 animals completed the protocol. All 8 rabbits tolerated the procedures well and were appropriately monitored during the postoperative period. No animals had signs of systemic infection or positive blood culture. All local cultures for screw, polyethylene washer, lateral femoral condyle defect, and joint capsule were positive.
Statistically significant decreases were shown in the bacterial burden of the Betadine-irrigated screws and the Betadine-irrigated polyethylene washers compared with the saline-irrigated controls. Betadine-irrigated screws grew an average of 7.16 × 101 CFU of S aureus/g, whereas screws from control knees grew an average of 1.45 × 103 CFU/g (P = .0003) (Figure 2). Betadine-treated washers grew an average of 1.28 × 103 CFU/g compared with 1.62 × 104 CFU/g for control washers (P =. 04) (Figure 3).
A trend toward decreased bacterial counts was shown in Betadine-treated soft tissues compared with saline-treated soft tissues, but the difference did not reach statistical significance (P = .9). Biopsied joint capsule from knees treated with Betadine grew an average of 2.84 × 104 CFU/g compared with an average of 3.16 × 104 CFU/g in control-rabbit knees (Figure 4). Cultured lateral condyle from Betadine-treated knees had an average bacterial load of 3.22 × 104 CFU/g compared with an average bacterial load of 1 × 105 CFU/g in control knees (Figure 4).
Discussion
Knees irrigated with Betadine showed a significant (P = .0003) decrease in metal implant–related S aureus bacterial counts by 20-fold and a significant (P < .05) decrease in polyethylene implant–related counts by more than 10-fold. This arthroplasty model used Betadine lavage as a treatment adjunct with intravenously administered antibiotics and polyethylene exchange. Our 1-week interval after the index procedure classifies the infection as an acute postoperative arthroplasty infection (occurring less than 4 weeks postoperatively).
The gold standard treatment for these infections is irrigation and débridement with component retention.18 The success rate has been reported to be as high as 71%20 but was closer to 44% in a study by Fridkin and colleagues,21 especially with more virulent bacteria. Staphylococcal species, higher American Society of Anesthesiologists scores, and frank pus around the prosthesis were markers of débridement failure in a recent study by Azzam and colleagues.18
The majority of postoperative joint arthroplasty infections are caused by S aureus, and the incidence of MRSA bacteria continues to rise.22 Community-acquired MRSA is increasing at an alarming rate and is now the predominant organism in skin and soft-tissue infections.23 Organism resistance also occurs at a cellular level by the formation of a glycocalyx layer, or biofilm. This layer assists in changing the phenotypic properties of the organism and decreases the efficacy of antibiotics.24 The self-produced layer of extracellular matrices, deoxyribonucleic acid, and polysaccharides attaches to inert material, preventing phagocytic action by neutrophils. In addition to antibacterial activity, povidone-iodine has antibiofilm activity against Staphylococcal species.25 The active ingredient targets the gene that produces biofilm. This correlates to our study in which the largest decrease in bacterial counts was noted on the implants.
The use of Betadine lavage has shown some promise in vivo as well. A prospective randomized controlled trial26 used 3.5% Betadine irrigation to prevent spine infection. No infections occurred in the Betadine group compared with a deep-infection rate of 2.9% in the control group. Brown and colleagues8 reviewed 1862 hip and knee arthroplasty cases before the use of Betadine lavage and 688 cases after the use of Betadine lavage and found a decrease in infection rate, from 0.97% to 0.15%. S aureus caused 13 of the 18 infections in the control group. These studies8,26 used Betadine lavage for prophylaxis and prevention of deep spine and arthroplasty infection. Betadine lavage as a treatment adjunct for acute arthroplasty infection has not been studied clinically. It has the potential to increase isolated incision and débridement success and to improve component survivorship.
Our arthroplasty model mimics an intra-articular environment and accounts for an implant–polyethylene interface.15 Limitations of our study include the use of MSSA as opposed to MRSA. However, povidone-iodine has the same effects on both MSSA and MRSA.12 We also treated our postoperative infection with 1 dose of antibiotics and not a course, although it should be noted that the single dose of ceftriaxone allowed us to isolate the independent effect of the Betadine lavage. A baseline level of infection severity could have been measured with cultures obtained at the time of irrigation and débridement. Also, a decrease in CFU does not directly correlate to a clinically significant outcome, such as a defined surgical site infection requiring intervention. Nevertheless, it is noteworthy that the decrease in bacterial counts on the stainless steel screws and polyethylene washers were maintained 1 week after the Betadine lavage.
Conclusion
Dilute Betadine lavage is a simple and inexpensive adjunct for the treatment of acute postoperative arthroplasty infection and may increase the rate of component retention. Additionally, the bactericidal and antibiofilm activities of Betadine may improve the effectiveness of systemic antibiotics. Further clinical investigation is warranted.
Surgical site infection after arthroplasty causes substantial morbidity and potential mortality. Prosthetic joint infection (PJI) ranges from simple superficial wound infection and cellulitis to deep subfascial infection that involves the prosthesis. Consistent use of prophylactic antibiotics has reduced postoperative hip and knee arthroplasty infections to rates of 0.25% to 2%.1-4 Treatment of a patient with PJI commonly includes hospitalization, long-term intravenously administered antibiotics, resection arthroplasty, and staged reimplantation. The estimated cost of interventions reaches tens of millions of dollars annually in the United States and does not include the costs of psychosocial effects on patients and their families.5,6
Betadine (povidone-iodine) is a widely used antiseptic for skin and mucous membrane wounds and has been shown to be effective for the prevention of PJI.7 Dilute Betadine solution has been proposed as an aid in treatment of PJI.8 At a minimum concentration of 5%, cytotoxicity has been observed in chicken tibia osteoblasts.9 A balance of the bactericidal and cytotoxic activities of Betadine, while maintaining its efficacy against resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), is optimized at dilutions between 0.5% and 4%.10-14 We hypothesized that a dilute Betadine lavage of 3.5% would achieve a significant decrease in bacterial counts compared with an isolated saline lavage in an in vivo knee PJI model.
Materials and Methods
Animal Protocol
All surgical procedures were conducted according to the protocol approved by our institutional animal care and use committee. Using a power analysis and data obtained at our institution, we determined that 12 was the minimum number of animals needed to reach significance set at P < .05 and assuming a 50% decrease in colony-forming units (CFU) (SigmaStat Version 2.03; Aspire Software International, Ashburn, Virginia). Eight New Zealand White rabbits were used in our study; because significance was reached early, 12 were not needed. The average weight of the rabbits was 3.5 kg (weight range, 3.2-4.1 kg). All rabbits completed 1 week of acclimation before surgery.
Bacteria Preparation
A broth culture of methicillin-sensitive S aureus (MSSA) (ATCC 25923) was prepared 1 day before surgery. The bacteria were suspended in 5 mL of Trypticase Soy Broth (Becton Dickinson & Co, Franklin Lakes, New Jersey) and incubated at 37°C in a shaking incubator for 16 hours. The next day, the culture was centrifuged and irrigated twice with normal saline to remove the broth and prevent further growth. The bacteria were reconstituted in normal saline, and the concentration was standardized using a turbidity meter (LaMotte 2020e; LaMotte Co, Chestertown, Maryland), which correlated with 106 CFU/100 µl plated on trypticase soy agar plates with 10% sheep blood (Fisher Scientific, Pittsburgh, Pennsylvania).
Surgical and Postoperative Procedures
Our procedure was based on the New Zealand White rabbit knee PJI model.15 General anesthesia was induced with ketamine and xylazine, and maintained with isoflurane inhalation via a nose cone mask. Rabbits were positioned supine, and bilateral knees were shaved, prepped, and draped in a sterile fashion.
A 2-cm longitudinal incision was made over the lateral knee, and arthrotomy was performed, exposing the lateral collateral ligament attachment at the lateral femoral condyle. Using a 4-mm drill bit, a defect was drilled obliquely into the lateral femoral condyle, anterior to the lateral collateral ligament attachment. This produced a defect in the non-weight-bearing, nonarticulating portion of the knee. A fully threaded 4×14-mm stainless steel screw (Synthes, West Chester, Pennsylvania) with a U-shaped ultrahigh-molecular-weight polyethylene washer (Synthes) was inserted into the defect. The joint capsule was closed with a running 3-0 Vicryl suture (Ethicon, Somerville, New Jersey). The knee joint was inoculated with 100 µL of the S aureus preparation using a 22-gauge needle. The skin was closed with a 4-0 Biosyn suture (Ethicon). The procedure was repeated on the contralateral knee (Figures 1A, 1B).
Seven days after the initial surgery, the rabbits were returned to the operating room and were anesthetized, positioned, and prepped for surgery as detailed above. Ceftriaxone (20 mg/kg of body weight) was intravenously administered to all rabbits for the treatment procedure. For each rabbit, a control knee and an experimental knee were randomly assigned. A longitudinal incision was made, exposing the previously placed implants. The screw was loosened slightly to remove the U-shaped polyethylene washer. Each knee then underwent lavage 2 times, for 90 seconds each time, with 3.5% dilute Betadine solution (experimental knee) or with normal saline (control knee). Because Pseudomonas contamination has been reported with povidone-iodine taken from unsterilized bottles,16,17 packets of sterilized povidone-iodine (Aplicare; Clorox, Oakland, California) were used. After the irrigation was complete, a new sterile polyethylene washer was placed and the screw was tightened. The wound closure was repeated as detailed above.
Postoperative analgesia was provided based on a standard institutional animal care and use committee protocol. Rabbits were permitted full cage activity and nutrition ad libitum. Wound healing, body weight, and signs of distress were monitored daily.
Outcome Measures
Seven days after surgery, the rabbits were euthanized with administration of phenobarbital (100 mg/kg of body weight). Arterial blood samples were obtained from the auricular vein to ensure that the rabbits were not systemically infected. Using a sterile technique, the screw, polyethylene washer, lateral femoral condyle bone from the defect, and joint capsule were cultured. Harvested bone and soft tissues were weighed and immediately homogenized (PowerGen Model 35 Handheld Homogenizer; Thermo Fisher Scientific, Inc, Waltham, Massachusetts). Implants were sonicated (UBATH-Y; World Precision Instruments, Inc, Sarasota, Florida) in cold saline to obtain a sensitive culture.18
Bacterial quantification was determined by using trypticase soy agar plates after 24 hours of growth. Final CFU were calculated after serial dilutions and were standardized per gram of biopsied tissues.19 Members of the team were blinded to the treatment type.
Statistical Analysis
Statistical differences in mean bacterial burden were calculated independently for lateral condyle bone, joint capsule, polyethylene, and screws by conducting a Student t test.
Results
Treatment effect was higher than expected, and the study was terminated after 8 animals completed the protocol. All 8 rabbits tolerated the procedures well and were appropriately monitored during the postoperative period. No animals had signs of systemic infection or positive blood culture. All local cultures for screw, polyethylene washer, lateral femoral condyle defect, and joint capsule were positive.
Statistically significant decreases were shown in the bacterial burden of the Betadine-irrigated screws and the Betadine-irrigated polyethylene washers compared with the saline-irrigated controls. Betadine-irrigated screws grew an average of 7.16 × 101 CFU of S aureus/g, whereas screws from control knees grew an average of 1.45 × 103 CFU/g (P = .0003) (Figure 2). Betadine-treated washers grew an average of 1.28 × 103 CFU/g compared with 1.62 × 104 CFU/g for control washers (P =. 04) (Figure 3).
A trend toward decreased bacterial counts was shown in Betadine-treated soft tissues compared with saline-treated soft tissues, but the difference did not reach statistical significance (P = .9). Biopsied joint capsule from knees treated with Betadine grew an average of 2.84 × 104 CFU/g compared with an average of 3.16 × 104 CFU/g in control-rabbit knees (Figure 4). Cultured lateral condyle from Betadine-treated knees had an average bacterial load of 3.22 × 104 CFU/g compared with an average bacterial load of 1 × 105 CFU/g in control knees (Figure 4).
Discussion
Knees irrigated with Betadine showed a significant (P = .0003) decrease in metal implant–related S aureus bacterial counts by 20-fold and a significant (P < .05) decrease in polyethylene implant–related counts by more than 10-fold. This arthroplasty model used Betadine lavage as a treatment adjunct with intravenously administered antibiotics and polyethylene exchange. Our 1-week interval after the index procedure classifies the infection as an acute postoperative arthroplasty infection (occurring less than 4 weeks postoperatively).
The gold standard treatment for these infections is irrigation and débridement with component retention.18 The success rate has been reported to be as high as 71%20 but was closer to 44% in a study by Fridkin and colleagues,21 especially with more virulent bacteria. Staphylococcal species, higher American Society of Anesthesiologists scores, and frank pus around the prosthesis were markers of débridement failure in a recent study by Azzam and colleagues.18
The majority of postoperative joint arthroplasty infections are caused by S aureus, and the incidence of MRSA bacteria continues to rise.22 Community-acquired MRSA is increasing at an alarming rate and is now the predominant organism in skin and soft-tissue infections.23 Organism resistance also occurs at a cellular level by the formation of a glycocalyx layer, or biofilm. This layer assists in changing the phenotypic properties of the organism and decreases the efficacy of antibiotics.24 The self-produced layer of extracellular matrices, deoxyribonucleic acid, and polysaccharides attaches to inert material, preventing phagocytic action by neutrophils. In addition to antibacterial activity, povidone-iodine has antibiofilm activity against Staphylococcal species.25 The active ingredient targets the gene that produces biofilm. This correlates to our study in which the largest decrease in bacterial counts was noted on the implants.
The use of Betadine lavage has shown some promise in vivo as well. A prospective randomized controlled trial26 used 3.5% Betadine irrigation to prevent spine infection. No infections occurred in the Betadine group compared with a deep-infection rate of 2.9% in the control group. Brown and colleagues8 reviewed 1862 hip and knee arthroplasty cases before the use of Betadine lavage and 688 cases after the use of Betadine lavage and found a decrease in infection rate, from 0.97% to 0.15%. S aureus caused 13 of the 18 infections in the control group. These studies8,26 used Betadine lavage for prophylaxis and prevention of deep spine and arthroplasty infection. Betadine lavage as a treatment adjunct for acute arthroplasty infection has not been studied clinically. It has the potential to increase isolated incision and débridement success and to improve component survivorship.
Our arthroplasty model mimics an intra-articular environment and accounts for an implant–polyethylene interface.15 Limitations of our study include the use of MSSA as opposed to MRSA. However, povidone-iodine has the same effects on both MSSA and MRSA.12 We also treated our postoperative infection with 1 dose of antibiotics and not a course, although it should be noted that the single dose of ceftriaxone allowed us to isolate the independent effect of the Betadine lavage. A baseline level of infection severity could have been measured with cultures obtained at the time of irrigation and débridement. Also, a decrease in CFU does not directly correlate to a clinically significant outcome, such as a defined surgical site infection requiring intervention. Nevertheless, it is noteworthy that the decrease in bacterial counts on the stainless steel screws and polyethylene washers were maintained 1 week after the Betadine lavage.
Conclusion
Dilute Betadine lavage is a simple and inexpensive adjunct for the treatment of acute postoperative arthroplasty infection and may increase the rate of component retention. Additionally, the bactericidal and antibiofilm activities of Betadine may improve the effectiveness of systemic antibiotics. Further clinical investigation is warranted.
1. Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72(6):878-883.
2. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
3. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
4. Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87(6):1222-1228.
5. Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics. 2010;33(9):659.
6. Poultsides LA, Liaropoulos LL, Malizos KN. The socioeconomic impact of musculoskeletal infections. J Bone Joint Surg Am. 2010;92(11):e13.
7. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
8. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
9. Kaysinger KK, Nicholson NC, Ramp WK, Kellam JF. Toxic effects of wound irrigation solutions on cultured tibiae and osteoblasts. J Orthop Trauma. 1995;9(4):303-311.
10. Haley CE, Marling-Cason M, Smith JW, Luby JP, Mackowiak PA. Bactericidal activity of antiseptics against methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;21(6):991-992.
11. Lacey RW, Catto A. Action of povidone-iodine against methicillin-sensitive and -resistant cultures of Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S78-S83.
12. McLure AR, Gordon J. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1992;21(4):291-299.
13. Suzuki J, Komatsuzawa H, Kozai K, Nagasaka N. In vitro susceptibility of Staphylococcus aureus including MRSA to four disinfectants. ASDC J Dent Child. 1997;64(4):260-263.
14. Yasuda T, Yoshimura S, Katsuno Y, et al. Comparison of bactericidal activities of various disinfectants against methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S66-S69.
15. Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TE. A novel total knee arthroplasty infection model in rabbits. J Orthop Res. 2005;23(5):1100-1104.
16. Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop. 1991;273:113-118.
17. Mont MA, Waldman B, Banerjee C, Pacheco IH, Hungerford DS. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12(4):426-433.
18. Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25(7):1022-1027.
19. Stall AC, Becker E, Ludwig SC, Gelb D, Poelstra KA. Reduction of postoperative spinal implant infection using gentamicin microspheres. Spine (Phila Pa 1976). 2009;34(5):479-483.
20. Hota B, Ellenbogen C, Hayden MK, Aroutcheva A, Rice TW, Weinstein RA. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med. 2007;167(10):1026-1033.
21. Fridkin SK, Hageman JC, Morrison M, et al, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436-1444.
22. Hosman AH, van der Mei HC, Bulstra SK, Busscher HJ, Neut D. Metal-on-metal bearings in total hip arthroplasties: influence of cobalt and chromium ions on bacterial growth and biofilm formation. J Biomed Mater Res A. 2009;88(3):711-716.
23. Oduwole KO, Glynn AA, Molony DC, et al. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J Orthop Res. 2010;28(9):1252-1256.
24. Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976). 2005;30(15):1689-1693.
25. Anderson RL, Vess RW, Panlilio AL, Favero MS. Prolonged survival of Pseudomonas cepacia in commercially manufactured povidone-iodine. Appl Environ Microbiol. 1990;56(11):3598-3600.
26. Panlilio AL, Beck-Sague CM, Siegel JD, et al. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin Infect Dis. 1992;14(5):1078-1083.
1. Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72(6):878-883.
2. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
3. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
4. Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87(6):1222-1228.
5. Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics. 2010;33(9):659.
6. Poultsides LA, Liaropoulos LL, Malizos KN. The socioeconomic impact of musculoskeletal infections. J Bone Joint Surg Am. 2010;92(11):e13.
7. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
8. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
9. Kaysinger KK, Nicholson NC, Ramp WK, Kellam JF. Toxic effects of wound irrigation solutions on cultured tibiae and osteoblasts. J Orthop Trauma. 1995;9(4):303-311.
10. Haley CE, Marling-Cason M, Smith JW, Luby JP, Mackowiak PA. Bactericidal activity of antiseptics against methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;21(6):991-992.
11. Lacey RW, Catto A. Action of povidone-iodine against methicillin-sensitive and -resistant cultures of Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S78-S83.
12. McLure AR, Gordon J. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1992;21(4):291-299.
13. Suzuki J, Komatsuzawa H, Kozai K, Nagasaka N. In vitro susceptibility of Staphylococcus aureus including MRSA to four disinfectants. ASDC J Dent Child. 1997;64(4):260-263.
14. Yasuda T, Yoshimura S, Katsuno Y, et al. Comparison of bactericidal activities of various disinfectants against methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Postgrad Med J. 1993;69(3 suppl):S66-S69.
15. Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TE. A novel total knee arthroplasty infection model in rabbits. J Orthop Res. 2005;23(5):1100-1104.
16. Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop. 1991;273:113-118.
17. Mont MA, Waldman B, Banerjee C, Pacheco IH, Hungerford DS. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12(4):426-433.
18. Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25(7):1022-1027.
19. Stall AC, Becker E, Ludwig SC, Gelb D, Poelstra KA. Reduction of postoperative spinal implant infection using gentamicin microspheres. Spine (Phila Pa 1976). 2009;34(5):479-483.
20. Hota B, Ellenbogen C, Hayden MK, Aroutcheva A, Rice TW, Weinstein RA. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med. 2007;167(10):1026-1033.
21. Fridkin SK, Hageman JC, Morrison M, et al, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436-1444.
22. Hosman AH, van der Mei HC, Bulstra SK, Busscher HJ, Neut D. Metal-on-metal bearings in total hip arthroplasties: influence of cobalt and chromium ions on bacterial growth and biofilm formation. J Biomed Mater Res A. 2009;88(3):711-716.
23. Oduwole KO, Glynn AA, Molony DC, et al. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J Orthop Res. 2010;28(9):1252-1256.
24. Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976). 2005;30(15):1689-1693.
25. Anderson RL, Vess RW, Panlilio AL, Favero MS. Prolonged survival of Pseudomonas cepacia in commercially manufactured povidone-iodine. Appl Environ Microbiol. 1990;56(11):3598-3600.
26. Panlilio AL, Beck-Sague CM, Siegel JD, et al. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin Infect Dis. 1992;14(5):1078-1083.
Meniscus Regenerated With 3D-Printed Implant in Animal Study
Researchers have honed in on a way to replace the meniscus, using a personalized 3D-printed implant in sheep, according to a study published online December 10, 2014, in Science Translational Medicine. The therapy, successfully tested in sheep, could provide the first effective and long-lasting repair of damaged menisci.
“At present, there’s little that orthopedists can do to regenerate a torn knee meniscus,” said lead study author Jeremy Mao, DDS, PhD, the Edwin S. Robinson Professor of Dentistry in Orthopedic Surgery at Columbia University Medical Center in New York. “In contrast, we’re jumpstarting the process within the body, using factors that promote endogenous stem cells for tissue regeneration.”
This process was tested on 11 sheep. Knees of sheep were chosen because of their close resemblance to the human knee. In this study, the sheep were randomized to have part of their knee meniscus replaced with a protein-infused 3D scaffold or a 3D scaffold without protein.
Dr. Mao’s approach starts with MRI scans of the intact meniscus in the undamaged knee, which is then converted into a 3D image. Data from the image are then used to drive a 3D printer, which produces a scaffold in the exact shape of the meniscus, down to a resolution of 10 microns. The scaffold is infused with connective growth factor (CTGF) and transforming growth factor β3 (TGFβ3). Dr. Mao’s team found that sequential delivery of these two proteins attracts existing stem cells from the body and induces them to form meniscal tissue.
After three months, treated animals were walking normally. In a postmortem analysis, the researchers found that the regenerated meniscus in the treatment group had structural and mechanical properties very similar to those of natural meniscus. In sheep, the meniscus regenerates at a rate of about 4 to 6 weeks. Eventually, the scaffold dissolves and is eliminated by the body.
Suggested Reading
Lee CH, Rodeo SA, Fortier LA, et al. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med. 2014 Dec 10;6(266):266ra171.
Researchers have honed in on a way to replace the meniscus, using a personalized 3D-printed implant in sheep, according to a study published online December 10, 2014, in Science Translational Medicine. The therapy, successfully tested in sheep, could provide the first effective and long-lasting repair of damaged menisci.
“At present, there’s little that orthopedists can do to regenerate a torn knee meniscus,” said lead study author Jeremy Mao, DDS, PhD, the Edwin S. Robinson Professor of Dentistry in Orthopedic Surgery at Columbia University Medical Center in New York. “In contrast, we’re jumpstarting the process within the body, using factors that promote endogenous stem cells for tissue regeneration.”
This process was tested on 11 sheep. Knees of sheep were chosen because of their close resemblance to the human knee. In this study, the sheep were randomized to have part of their knee meniscus replaced with a protein-infused 3D scaffold or a 3D scaffold without protein.
Dr. Mao’s approach starts with MRI scans of the intact meniscus in the undamaged knee, which is then converted into a 3D image. Data from the image are then used to drive a 3D printer, which produces a scaffold in the exact shape of the meniscus, down to a resolution of 10 microns. The scaffold is infused with connective growth factor (CTGF) and transforming growth factor β3 (TGFβ3). Dr. Mao’s team found that sequential delivery of these two proteins attracts existing stem cells from the body and induces them to form meniscal tissue.
After three months, treated animals were walking normally. In a postmortem analysis, the researchers found that the regenerated meniscus in the treatment group had structural and mechanical properties very similar to those of natural meniscus. In sheep, the meniscus regenerates at a rate of about 4 to 6 weeks. Eventually, the scaffold dissolves and is eliminated by the body.
Researchers have honed in on a way to replace the meniscus, using a personalized 3D-printed implant in sheep, according to a study published online December 10, 2014, in Science Translational Medicine. The therapy, successfully tested in sheep, could provide the first effective and long-lasting repair of damaged menisci.
“At present, there’s little that orthopedists can do to regenerate a torn knee meniscus,” said lead study author Jeremy Mao, DDS, PhD, the Edwin S. Robinson Professor of Dentistry in Orthopedic Surgery at Columbia University Medical Center in New York. “In contrast, we’re jumpstarting the process within the body, using factors that promote endogenous stem cells for tissue regeneration.”
This process was tested on 11 sheep. Knees of sheep were chosen because of their close resemblance to the human knee. In this study, the sheep were randomized to have part of their knee meniscus replaced with a protein-infused 3D scaffold or a 3D scaffold without protein.
Dr. Mao’s approach starts with MRI scans of the intact meniscus in the undamaged knee, which is then converted into a 3D image. Data from the image are then used to drive a 3D printer, which produces a scaffold in the exact shape of the meniscus, down to a resolution of 10 microns. The scaffold is infused with connective growth factor (CTGF) and transforming growth factor β3 (TGFβ3). Dr. Mao’s team found that sequential delivery of these two proteins attracts existing stem cells from the body and induces them to form meniscal tissue.
After three months, treated animals were walking normally. In a postmortem analysis, the researchers found that the regenerated meniscus in the treatment group had structural and mechanical properties very similar to those of natural meniscus. In sheep, the meniscus regenerates at a rate of about 4 to 6 weeks. Eventually, the scaffold dissolves and is eliminated by the body.
Suggested Reading
Lee CH, Rodeo SA, Fortier LA, et al. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med. 2014 Dec 10;6(266):266ra171.
Suggested Reading
Lee CH, Rodeo SA, Fortier LA, et al. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med. 2014 Dec 10;6(266):266ra171.
An Additional Option for Pain Control Following Knee Replacement Surgery
DALLAS—Injecting long-acting liposomal bupivacaine into the tissue surrounding the knee during knee replacement surgery may provide a faster receovery and higher patient satisfaction, according to a report presented at the recent American Association for Hip and Knee Surgeons meeting.
“The pain scores for this injection technique averaged about 3/10, which is similar to the pain scores seen with our traditional method,” said Jason Davis, MD, a Henry Ford West Bloomfield Hospital joint replacement surgeon and senior study author. “Patients had pain relief for up to 2 days after surgery and better knee function compared with the traditional method.”
In the study, 216 patients were evaluated for pain control the first 2 days after surgery from October 2012 to September 2013. Half of the patients received the traditional pain control method with continuous femoral nerve blockade, in which common numbing medicine is injected into the groin area, blunting the main nerve down the front of the knee—a method that calls for a pain pump to extend pain control for two days but causes some leg weakness.
“Pain control came at the price of weakness and made patients somewhat tentative when walking during their hospital stay,” stated Dr. Davis.
The other half of patients received the liposomal bupivacaine injection at the site of the surgery. With this method, Dr. Davis says many patients were able to walk comfortably within hours after surgery.
Dr. Davis said that the injection around the knee itself “optimizes pain control early on” without the side effects of the traditional technique. “Function-wise, it was a lot easier for patients to move around more confidently,” Dr. Davis stated.
“In the past decade, we’ve made major advancements in pain control for knee replacement surgery. This option is a promising, viable one for our patients,” Dr. Davis said.
DALLAS—Injecting long-acting liposomal bupivacaine into the tissue surrounding the knee during knee replacement surgery may provide a faster receovery and higher patient satisfaction, according to a report presented at the recent American Association for Hip and Knee Surgeons meeting.
“The pain scores for this injection technique averaged about 3/10, which is similar to the pain scores seen with our traditional method,” said Jason Davis, MD, a Henry Ford West Bloomfield Hospital joint replacement surgeon and senior study author. “Patients had pain relief for up to 2 days after surgery and better knee function compared with the traditional method.”
In the study, 216 patients were evaluated for pain control the first 2 days after surgery from October 2012 to September 2013. Half of the patients received the traditional pain control method with continuous femoral nerve blockade, in which common numbing medicine is injected into the groin area, blunting the main nerve down the front of the knee—a method that calls for a pain pump to extend pain control for two days but causes some leg weakness.
“Pain control came at the price of weakness and made patients somewhat tentative when walking during their hospital stay,” stated Dr. Davis.
The other half of patients received the liposomal bupivacaine injection at the site of the surgery. With this method, Dr. Davis says many patients were able to walk comfortably within hours after surgery.
Dr. Davis said that the injection around the knee itself “optimizes pain control early on” without the side effects of the traditional technique. “Function-wise, it was a lot easier for patients to move around more confidently,” Dr. Davis stated.
“In the past decade, we’ve made major advancements in pain control for knee replacement surgery. This option is a promising, viable one for our patients,” Dr. Davis said.
DALLAS—Injecting long-acting liposomal bupivacaine into the tissue surrounding the knee during knee replacement surgery may provide a faster receovery and higher patient satisfaction, according to a report presented at the recent American Association for Hip and Knee Surgeons meeting.
“The pain scores for this injection technique averaged about 3/10, which is similar to the pain scores seen with our traditional method,” said Jason Davis, MD, a Henry Ford West Bloomfield Hospital joint replacement surgeon and senior study author. “Patients had pain relief for up to 2 days after surgery and better knee function compared with the traditional method.”
In the study, 216 patients were evaluated for pain control the first 2 days after surgery from October 2012 to September 2013. Half of the patients received the traditional pain control method with continuous femoral nerve blockade, in which common numbing medicine is injected into the groin area, blunting the main nerve down the front of the knee—a method that calls for a pain pump to extend pain control for two days but causes some leg weakness.
“Pain control came at the price of weakness and made patients somewhat tentative when walking during their hospital stay,” stated Dr. Davis.
The other half of patients received the liposomal bupivacaine injection at the site of the surgery. With this method, Dr. Davis says many patients were able to walk comfortably within hours after surgery.
Dr. Davis said that the injection around the knee itself “optimizes pain control early on” without the side effects of the traditional technique. “Function-wise, it was a lot easier for patients to move around more confidently,” Dr. Davis stated.
“In the past decade, we’ve made major advancements in pain control for knee replacement surgery. This option is a promising, viable one for our patients,” Dr. Davis said.
Cohort Study Reveals Link Between Menopausal Symptoms and Bone Health
Women with moderate to severe vasomotor symptoms (VMS) have lower bone mineral density and increased hip fracture rates, according to a study published online ahead of print December 18, 2014, in Journal of Clinical Endocrinology & Metabolism.
“This is the first large cohort study to examine the relationship between menopausal symptoms and bone health in menopausal women,” said lead author, Carolyn J. Crandall, MD, MS, of the David Geffen School of Medicine at the University of California, Los Angeles.
Data were examined from 23,573 participants in the Women’s Health Initiative (WHI) Clinical Trial. The participants were women between the ages of 50 and 79. This study, which was conducted at 40 clinical centers across the country, tracked women’s annual visits for 8 years on average.
Participants were asked about their menopausal symptoms, including hot flashes and night sweats, during their initial visit. WHI participants were monitored for fractures during the follow-up period. Among the study participants, 4,867 had their bone mineral density measured as part of a sub-study.
The analysis found that women who reported having moderate to severe hot flashes when they entered the study were more likely to fracture a hip during the follow-up period than were women who showed no menopausal symptoms. In addition, after researchers adjusted for age, body mass index, and demographic factors, they found that women who had moderate to severe menopausal symptoms had lower bone mass density at the neck and spine during the follow-up period than women with no symptoms.
“Our findings suggest that women who exhibit moderate or severe menopausal symptoms are more likely to have issues with bone health than their peers,” said Dr. Crandall. “Improved understanding would help clinicians advise women on how to better prevent osteoporosis and other bone conditions. Women who have hot flashes and want to protect their bones may benefit from healthy lifestyle habits such as avoiding smoking and excessive alcohol consumption, exercising, and getting sufficient calcium and vitamin D,” said Dr. Crandall.
Suggested Reading
Crandall CJ, Aragaki A, Cauley JA, et al. Associations of menopausal vasomotor symptoms with fracture incidence. J Clin Endocrinol Metab. 2014 Dec 18 [Epub ahead of print].
Women with moderate to severe vasomotor symptoms (VMS) have lower bone mineral density and increased hip fracture rates, according to a study published online ahead of print December 18, 2014, in Journal of Clinical Endocrinology & Metabolism.
“This is the first large cohort study to examine the relationship between menopausal symptoms and bone health in menopausal women,” said lead author, Carolyn J. Crandall, MD, MS, of the David Geffen School of Medicine at the University of California, Los Angeles.
Data were examined from 23,573 participants in the Women’s Health Initiative (WHI) Clinical Trial. The participants were women between the ages of 50 and 79. This study, which was conducted at 40 clinical centers across the country, tracked women’s annual visits for 8 years on average.
Participants were asked about their menopausal symptoms, including hot flashes and night sweats, during their initial visit. WHI participants were monitored for fractures during the follow-up period. Among the study participants, 4,867 had their bone mineral density measured as part of a sub-study.
The analysis found that women who reported having moderate to severe hot flashes when they entered the study were more likely to fracture a hip during the follow-up period than were women who showed no menopausal symptoms. In addition, after researchers adjusted for age, body mass index, and demographic factors, they found that women who had moderate to severe menopausal symptoms had lower bone mass density at the neck and spine during the follow-up period than women with no symptoms.
“Our findings suggest that women who exhibit moderate or severe menopausal symptoms are more likely to have issues with bone health than their peers,” said Dr. Crandall. “Improved understanding would help clinicians advise women on how to better prevent osteoporosis and other bone conditions. Women who have hot flashes and want to protect their bones may benefit from healthy lifestyle habits such as avoiding smoking and excessive alcohol consumption, exercising, and getting sufficient calcium and vitamin D,” said Dr. Crandall.
Women with moderate to severe vasomotor symptoms (VMS) have lower bone mineral density and increased hip fracture rates, according to a study published online ahead of print December 18, 2014, in Journal of Clinical Endocrinology & Metabolism.
“This is the first large cohort study to examine the relationship between menopausal symptoms and bone health in menopausal women,” said lead author, Carolyn J. Crandall, MD, MS, of the David Geffen School of Medicine at the University of California, Los Angeles.
Data were examined from 23,573 participants in the Women’s Health Initiative (WHI) Clinical Trial. The participants were women between the ages of 50 and 79. This study, which was conducted at 40 clinical centers across the country, tracked women’s annual visits for 8 years on average.
Participants were asked about their menopausal symptoms, including hot flashes and night sweats, during their initial visit. WHI participants were monitored for fractures during the follow-up period. Among the study participants, 4,867 had their bone mineral density measured as part of a sub-study.
The analysis found that women who reported having moderate to severe hot flashes when they entered the study were more likely to fracture a hip during the follow-up period than were women who showed no menopausal symptoms. In addition, after researchers adjusted for age, body mass index, and demographic factors, they found that women who had moderate to severe menopausal symptoms had lower bone mass density at the neck and spine during the follow-up period than women with no symptoms.
“Our findings suggest that women who exhibit moderate or severe menopausal symptoms are more likely to have issues with bone health than their peers,” said Dr. Crandall. “Improved understanding would help clinicians advise women on how to better prevent osteoporosis and other bone conditions. Women who have hot flashes and want to protect their bones may benefit from healthy lifestyle habits such as avoiding smoking and excessive alcohol consumption, exercising, and getting sufficient calcium and vitamin D,” said Dr. Crandall.
Suggested Reading
Crandall CJ, Aragaki A, Cauley JA, et al. Associations of menopausal vasomotor symptoms with fracture incidence. J Clin Endocrinol Metab. 2014 Dec 18 [Epub ahead of print].
Suggested Reading
Crandall CJ, Aragaki A, Cauley JA, et al. Associations of menopausal vasomotor symptoms with fracture incidence. J Clin Endocrinol Metab. 2014 Dec 18 [Epub ahead of print].
Should Men Receiving Androgen Deprivation Therapy Also Receive Bone-Strengthening Drugs?
Although some guidelines recommend use of bisphosphonates for men on androgen deprivation therapy (ADT), a study published in the December 3, 2014, JAMA reports that prescriptions for these drugs remain low, even for those men at high risk of subsequent fractures.
“Although the optimal rate of bisphosphonate use in men on ADT is unknown, it is reasonable that most men with prior osteoporosis or fracture should be taking a bisphosphonate or other effective bone medication,” stated Husayn Gulamhusein, BHSc, of the University Health Network in Toronto, and his research colleagues.
Using administrative databases at the Institute for Clinical Evaluative Sciences and the Ontario Cancer Registry, Mr. Gulamhusein and his team examined rates of bisphosphonate prescriptions in men initiating ADT in Ontario between 1995 and 2012. The study group included men ages 66 or older who were starting ADT for prostate cancer, who had undergone surgical removal of one or both testicles, or who received at least 6 months of continuous medical ADT and survived at least one year after ADT initiation.
Bisphosphonate claims within 12 months of ADT initiation were captured through drug database claims. Bisphosphonate prescription was examined for three groups: all nonusers of bisphosphonates, those with prior osteoporosis, and those with prior fragility fracture.
A total of 35,487 men with prostate cancer who began ADT during the study period were identified. Bisphosphonate claims among all nonusers increased from 0.35 per 100 persons in 1995 to 1997 to 3.40 per 100 persons in 2010 to 2012. Rates remained low, even among those with prior osteoporosis or fragility fracture. Among all three groups, peak bisphosphonate claims occurred between 2007 to 2009, with a high of 11.89 per 100 persons in those with prior osteoporosis.
Mr. Gulamhusein and his research team speculate that the decrease in bisphosphonate prescriptions after 2009 may be partly due to recent negative media attention regarding the association of bisphosphonates with rare osteonecrosis of the jaw and atypical femoral fractures. “This is appropriate for groups at low risk for fractures, but the decrease in use for high-risk patients is troubling,” the study authors wrote.
Suggested Reading
Gulamhusein H, Yun L, Cheung AM, et al. Bisphosphonate prescriptions in men with androgen deprivation therapy use. JAMA. 2014;312(21):2285-2286.
Although some guidelines recommend use of bisphosphonates for men on androgen deprivation therapy (ADT), a study published in the December 3, 2014, JAMA reports that prescriptions for these drugs remain low, even for those men at high risk of subsequent fractures.
“Although the optimal rate of bisphosphonate use in men on ADT is unknown, it is reasonable that most men with prior osteoporosis or fracture should be taking a bisphosphonate or other effective bone medication,” stated Husayn Gulamhusein, BHSc, of the University Health Network in Toronto, and his research colleagues.
Using administrative databases at the Institute for Clinical Evaluative Sciences and the Ontario Cancer Registry, Mr. Gulamhusein and his team examined rates of bisphosphonate prescriptions in men initiating ADT in Ontario between 1995 and 2012. The study group included men ages 66 or older who were starting ADT for prostate cancer, who had undergone surgical removal of one or both testicles, or who received at least 6 months of continuous medical ADT and survived at least one year after ADT initiation.
Bisphosphonate claims within 12 months of ADT initiation were captured through drug database claims. Bisphosphonate prescription was examined for three groups: all nonusers of bisphosphonates, those with prior osteoporosis, and those with prior fragility fracture.
A total of 35,487 men with prostate cancer who began ADT during the study period were identified. Bisphosphonate claims among all nonusers increased from 0.35 per 100 persons in 1995 to 1997 to 3.40 per 100 persons in 2010 to 2012. Rates remained low, even among those with prior osteoporosis or fragility fracture. Among all three groups, peak bisphosphonate claims occurred between 2007 to 2009, with a high of 11.89 per 100 persons in those with prior osteoporosis.
Mr. Gulamhusein and his research team speculate that the decrease in bisphosphonate prescriptions after 2009 may be partly due to recent negative media attention regarding the association of bisphosphonates with rare osteonecrosis of the jaw and atypical femoral fractures. “This is appropriate for groups at low risk for fractures, but the decrease in use for high-risk patients is troubling,” the study authors wrote.
Although some guidelines recommend use of bisphosphonates for men on androgen deprivation therapy (ADT), a study published in the December 3, 2014, JAMA reports that prescriptions for these drugs remain low, even for those men at high risk of subsequent fractures.
“Although the optimal rate of bisphosphonate use in men on ADT is unknown, it is reasonable that most men with prior osteoporosis or fracture should be taking a bisphosphonate or other effective bone medication,” stated Husayn Gulamhusein, BHSc, of the University Health Network in Toronto, and his research colleagues.
Using administrative databases at the Institute for Clinical Evaluative Sciences and the Ontario Cancer Registry, Mr. Gulamhusein and his team examined rates of bisphosphonate prescriptions in men initiating ADT in Ontario between 1995 and 2012. The study group included men ages 66 or older who were starting ADT for prostate cancer, who had undergone surgical removal of one or both testicles, or who received at least 6 months of continuous medical ADT and survived at least one year after ADT initiation.
Bisphosphonate claims within 12 months of ADT initiation were captured through drug database claims. Bisphosphonate prescription was examined for three groups: all nonusers of bisphosphonates, those with prior osteoporosis, and those with prior fragility fracture.
A total of 35,487 men with prostate cancer who began ADT during the study period were identified. Bisphosphonate claims among all nonusers increased from 0.35 per 100 persons in 1995 to 1997 to 3.40 per 100 persons in 2010 to 2012. Rates remained low, even among those with prior osteoporosis or fragility fracture. Among all three groups, peak bisphosphonate claims occurred between 2007 to 2009, with a high of 11.89 per 100 persons in those with prior osteoporosis.
Mr. Gulamhusein and his research team speculate that the decrease in bisphosphonate prescriptions after 2009 may be partly due to recent negative media attention regarding the association of bisphosphonates with rare osteonecrosis of the jaw and atypical femoral fractures. “This is appropriate for groups at low risk for fractures, but the decrease in use for high-risk patients is troubling,” the study authors wrote.
Suggested Reading
Gulamhusein H, Yun L, Cheung AM, et al. Bisphosphonate prescriptions in men with androgen deprivation therapy use. JAMA. 2014;312(21):2285-2286.
Suggested Reading
Gulamhusein H, Yun L, Cheung AM, et al. Bisphosphonate prescriptions in men with androgen deprivation therapy use. JAMA. 2014;312(21):2285-2286.
Periprosthetic Supracondylar Femur Fracture Treated With Spanning External Fixation
The incidence of periprosthetic supracondylar fractures of the femur after total knee arthroplasty (TKA) ranges from 0.6% to 2.5%.1 Treatment of periprosthetic fractures is often complicated by advanced patient age and osteoporosis, which frequently accompanies these fractures. Management of a periprosthetic fracture depends on the relation between the fracture site and the prosthesis, displacement of the prosthesis, integrity of the fixation of the prosthesis, extent of the bone loss caused by fracture comminution or preexisting osteolysis, general health of the patient, and surgeon expertise.2,3 The aim is to achieve fracture union around a stable, well-aligned arthroplasty with preserved or restored bone stock and therefore to return the patient to previous level of function. Although nonoperative treatments have been shown to be successful,4,5 in the great majority of cases surgical treatment is advised for these fractures.6-10 In cases in which bone stock is adequate for fixation rather than replacement of the distal femur, 2 modalities are commonly used: retrograde intramedullary nailing and locking plates. Each has its drawbacks and advantages.11,12
Although external fixation has been used in the treatment of distal femoral fractures,13 it is seldom considered in the treatment of periprosthetic fractures. Several authors have described cases that used external fixators, occasionally spanning the knee. The specific types of external fixators discussed in the literature have included ring fixators,14-17 hybrid fixators,18 and uniplanar nonspanning fixators14,19 (Table). Use of a simple anterior spanning external fixator in treating a periprosthetic femoral fracture has received little attention in the literature.
The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 54-year-old woman with previous total hip arthroplasty (THA) and ipsilateral TKA tripped on a carpet and sustained a comminuted fracture of the distal femur just above the TKA prosthesis (Figure 1). She was a Jehovah’s Witness and thus refused all blood products. She had an extensive history of osteoporosis, morbid obesity (5 feet tall, 250 pounds; body mass index, 49), diabetes, and rheumatoid arthritis. Evaluation by the internal medicine service revealed severe coronary artery disease on a stress thallium test and anemia with hematocrit of 24%. Given the patient’s medical comorbidities and religious status, and the location of the comminuted distal femur fracture, several treatment options were considered. First was nonoperative treatment with a cast or cast-brace (hinged cast). Because of her body habitus, however, we thought she would very likely experience skin complications, inadequate immobilization of the bone, and significant discomfort. Ultimately, use of a spanning external fixator was chosen as the safest course, given the significant medical risks accompanying a more extensive surgical reconstruction. With the spanning external fixator, the main risks were the inability to fully control fracture alignment and the potential introduction of infection into the functional THA. We thought that, by limiting the amount of time in the fixator and managing the pin site aggressively, we could minimize the risk for infection in this setting.
The procedure was performed with the patient under general anesthesia. During surgery, a lateral image of the femur was used to identify the distal end of the THA prosthesis. A level was marked 2 to 3 cm distal to the tip of this prosthesis, and another about 1 cm above the fracture (noted to be above the most proximal extent of the knee joint). These planned pin-entry sites were prepared from an anterior approach with incisions (using a No. 11 blade) of about 1 cm each. Blunt dissection was carried down to the femur. Each planned pin site was predrilled with a 3.5-mm drill; then, a 5-mm Shanz pin was placed. This process was repeated immediately distal to the tibial component and at the junction of the mid and distal thirds of the tibia (Figure 2). The preliminary external fixator frame was then applied. Once the reduction was satisfactory in the anteroposterior and lateral planes, the fixator clamps were tightened. A second row of bars was then incorporated.
Six weeks after surgery, radiographs showed early callus formation. Removing the external fixator and examining the knee under anesthesia confirmed there was no significant motion through the fracture site. A cast-brace (fiberglass thigh segment, fiberglass lower leg cast with hinged knee segment) was then applied. We remained concerned about skin complications but were encouraged by the early healing achieved with the fixator. The patient was started on a physical therapy program of gait training with a walker and toe-touch weight-bearing on the injured extremity. She also started a limited lower-extremity strengthening program. Three months after surgery, she was tolerating weight-bearing on the injured extremity with no pain. At 6 months, knee radiographs showed fracture consolidation with active range of motion of 10° to 120° and no pain (Figures 3A, 3B). Distal sensation, motor function, and vascular examination were normal. Two years after surgery, radiographs of the right knee showed minor malalignment in the coronal and sagittal planes (Figures 4A, 4B) and complete consolidation of the fracture.
Discussion
Periprosthetic fractures of the femur after TKA often occur in the setting of osteopenia, and some are associated with concurrent implant loosening. In most cases, these fractures require surgical stabilization. Nevertheless, the goals of treatment are to obtain and maintain anatomical alignment and stability to allow early range of motion. Nonoperative options include skeletal traction, cast, pins and plaster, and cast-brace.3-5,20 Operative options include intramedullary fixation,12,21 stabilization with various plates,21-23 revision knee arthroplasty, and arthrodesis.1 Treatment selection should be based on patient health, fracture displacement, comminution, osteopenia severity, and status of the prosthetic components.
The present case exemplifies some of the highest degrees of medical and surgical risk factors in people with a periprosthetic femoral fracture after TKA. Patients with rheumatoid arthritis, patients having corticosteroid treatment, patients of advanced age, and female patients are all at higher risk for supracondylar femoral fracture.9 Our patient had these risk factors on a background of anemia and extensive coronary artery disease. Given her past medical history and refusal of blood products out of religious belief, we thought she was too high risk for extensive surgical treatment for her fracture. In addition, she was not an ideal candidate for nonoperative treatment, as a periprosthetic fracture typically is treated with surgical revision or open reduction and internal fixation. Therefore, we selected an unconventional treatment modality, typically used as a temporizing measure in severe fractures around the knee—a spanning external fixator worn for 6 weeks and a cast-brace for an additional 6 weeks. This led to successful clinical and radiographic outcomes. We consider spanning external fixation a viable option for periprosthetic fractures after TKA in morbidly obese patients with relatively well-aligned fractures and extremely high risk for medical complications associated with traditional open surgery.
1. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
2. Su ET, Kubiak EN, Dewal H, Hiebert R, Di Cesare PE. A proposed classification of supracondylar femur fractures above total knee arthroplasties. J Arthroplasty. 2006;21(3):405-408.
3. Kim KI, Egol KA, Hozack WJ, Parvizi J. Periprosthetic fractures after total knee arthroplasties. Clin Orthop. 2006;(446):167-175.
4. Sochart DH, Hardinge K. Nonsurgical management of supracondylar fracture above total knee arthroplasty. Still the nineties option. J Arthroplasty. 1997;12(7):830-834.
5. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
6. Frigg R, Appenzeller A, Christensen R, Frenk A, Gilbert S, Schavan R. The development of the distal femur less invasive stabilization system (LISS). Injury. 2001;32(suppl 3):SC24-SC31.
7. Goesling T, Frenk A, Appenzeller A, Garapati R, Marti A, Krettek C. LISS PLT: design, mechanical and biomechanical characteristics. Injury. 2003;34(suppl 1):A11-A15.
8. Huang HT, Huang PJ, Su JY, Lin SY. Indirect reduction and bridge plating of supracondylar fractures of the femur. Injury. 2003;34(2):135-140.
9. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
10. Jamali AA, Lee MA, Donthineni R, Meehan JP. Minimally invasive management of a floating prosthesis injury with locking plates. J Arthroplasty. 2007;22(6):928-933.
11. Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. J Arthroplasty. 2002;17(7):876-881.
12. Firoozbakhsh K, Behzadi K, DeCoster TA, Moneim MS, Naraghi FF. Mechanics of retrograde nail versus plate fixation for supracondylar femur fractures. J Orthop Trauma. 1995;9(2):152-157.
13. Arazi M, Memik R, Ogun TC, Yel M. Ilizarov external fixation for severely comminuted supracondylar and intercondylar fractures of the distal femur. J Bone Joint Surg Br. 2001;83(5):663-667.
14. Pleva L, Sir M, Madeja R. Our experiences with the treatment of periprosthetic fractures of femur. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):75-79.
15. Simon RG, Brinker MR. Use of Ilizarov external fixation for a periprosthetic supracondylar femur fracture. J Arthroplasty. 1999;14(1):118-121.
16. Hurson C, Synnott K, McCormack D. Above-knee Ilizarov external fixation for early periprosthetic supracondylar femoral fracture—a case report. Knee. 2005;12(2):145-147.
17. Beris AE, Lykissas MG, Sioros V, Mavrodontidis AN, Korompilias AV. Femoral periprosthetic fracture in osteoporotic bone after a total knee replacement: treatment with Ilizarov external fixation. J Arthroplasty. 2010;25(7):1168.e9-e12.
18. Pafilas D, Kourtzis N. Hybrid external fixation as a new treatment method for periprosthetic femoral fracture. A case report. J Bone Joint Surg Am. 2006;88(1):188-192.
19. Merkel KD, Johnson EW Jr. Supracondylar fracture of the femur after total knee arthroplasty. J Bone Joint Surg Am. 1986;68(1):29-43.
20. Cordeiro EN, Costa RC, Carazzato JG, Silva Jdos S. Periprosthetic fractures in patients with total knee arthroplasties. Clin Orthop. 1990;(252):182-189.
21. Riemer BL, Butterfield SL, Burke CJ 3rd, Mathews D. Immediate plate fixation of highly comminuted femoral diaphyseal fractures in blunt polytrauma patients. Orthopedics. 1992;15(8):907-916.
22. Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the less invasive stabilization system (L.I.S.S.). Injury. 2001;32(suppl 3):SC64-SC75.
23. Althausen PL, Lee MA, Finkemeier CG, Meehan JP, Rodrigo JJ. Operative stabilization of supracondylar femur fractures above total knee arthroplasty: a comparison of four treatment methods. J Arthroplasty. 2003;18(7):834-839.
The incidence of periprosthetic supracondylar fractures of the femur after total knee arthroplasty (TKA) ranges from 0.6% to 2.5%.1 Treatment of periprosthetic fractures is often complicated by advanced patient age and osteoporosis, which frequently accompanies these fractures. Management of a periprosthetic fracture depends on the relation between the fracture site and the prosthesis, displacement of the prosthesis, integrity of the fixation of the prosthesis, extent of the bone loss caused by fracture comminution or preexisting osteolysis, general health of the patient, and surgeon expertise.2,3 The aim is to achieve fracture union around a stable, well-aligned arthroplasty with preserved or restored bone stock and therefore to return the patient to previous level of function. Although nonoperative treatments have been shown to be successful,4,5 in the great majority of cases surgical treatment is advised for these fractures.6-10 In cases in which bone stock is adequate for fixation rather than replacement of the distal femur, 2 modalities are commonly used: retrograde intramedullary nailing and locking plates. Each has its drawbacks and advantages.11,12
Although external fixation has been used in the treatment of distal femoral fractures,13 it is seldom considered in the treatment of periprosthetic fractures. Several authors have described cases that used external fixators, occasionally spanning the knee. The specific types of external fixators discussed in the literature have included ring fixators,14-17 hybrid fixators,18 and uniplanar nonspanning fixators14,19 (Table). Use of a simple anterior spanning external fixator in treating a periprosthetic femoral fracture has received little attention in the literature.
The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 54-year-old woman with previous total hip arthroplasty (THA) and ipsilateral TKA tripped on a carpet and sustained a comminuted fracture of the distal femur just above the TKA prosthesis (Figure 1). She was a Jehovah’s Witness and thus refused all blood products. She had an extensive history of osteoporosis, morbid obesity (5 feet tall, 250 pounds; body mass index, 49), diabetes, and rheumatoid arthritis. Evaluation by the internal medicine service revealed severe coronary artery disease on a stress thallium test and anemia with hematocrit of 24%. Given the patient’s medical comorbidities and religious status, and the location of the comminuted distal femur fracture, several treatment options were considered. First was nonoperative treatment with a cast or cast-brace (hinged cast). Because of her body habitus, however, we thought she would very likely experience skin complications, inadequate immobilization of the bone, and significant discomfort. Ultimately, use of a spanning external fixator was chosen as the safest course, given the significant medical risks accompanying a more extensive surgical reconstruction. With the spanning external fixator, the main risks were the inability to fully control fracture alignment and the potential introduction of infection into the functional THA. We thought that, by limiting the amount of time in the fixator and managing the pin site aggressively, we could minimize the risk for infection in this setting.
The procedure was performed with the patient under general anesthesia. During surgery, a lateral image of the femur was used to identify the distal end of the THA prosthesis. A level was marked 2 to 3 cm distal to the tip of this prosthesis, and another about 1 cm above the fracture (noted to be above the most proximal extent of the knee joint). These planned pin-entry sites were prepared from an anterior approach with incisions (using a No. 11 blade) of about 1 cm each. Blunt dissection was carried down to the femur. Each planned pin site was predrilled with a 3.5-mm drill; then, a 5-mm Shanz pin was placed. This process was repeated immediately distal to the tibial component and at the junction of the mid and distal thirds of the tibia (Figure 2). The preliminary external fixator frame was then applied. Once the reduction was satisfactory in the anteroposterior and lateral planes, the fixator clamps were tightened. A second row of bars was then incorporated.
Six weeks after surgery, radiographs showed early callus formation. Removing the external fixator and examining the knee under anesthesia confirmed there was no significant motion through the fracture site. A cast-brace (fiberglass thigh segment, fiberglass lower leg cast with hinged knee segment) was then applied. We remained concerned about skin complications but were encouraged by the early healing achieved with the fixator. The patient was started on a physical therapy program of gait training with a walker and toe-touch weight-bearing on the injured extremity. She also started a limited lower-extremity strengthening program. Three months after surgery, she was tolerating weight-bearing on the injured extremity with no pain. At 6 months, knee radiographs showed fracture consolidation with active range of motion of 10° to 120° and no pain (Figures 3A, 3B). Distal sensation, motor function, and vascular examination were normal. Two years after surgery, radiographs of the right knee showed minor malalignment in the coronal and sagittal planes (Figures 4A, 4B) and complete consolidation of the fracture.
Discussion
Periprosthetic fractures of the femur after TKA often occur in the setting of osteopenia, and some are associated with concurrent implant loosening. In most cases, these fractures require surgical stabilization. Nevertheless, the goals of treatment are to obtain and maintain anatomical alignment and stability to allow early range of motion. Nonoperative options include skeletal traction, cast, pins and plaster, and cast-brace.3-5,20 Operative options include intramedullary fixation,12,21 stabilization with various plates,21-23 revision knee arthroplasty, and arthrodesis.1 Treatment selection should be based on patient health, fracture displacement, comminution, osteopenia severity, and status of the prosthetic components.
The present case exemplifies some of the highest degrees of medical and surgical risk factors in people with a periprosthetic femoral fracture after TKA. Patients with rheumatoid arthritis, patients having corticosteroid treatment, patients of advanced age, and female patients are all at higher risk for supracondylar femoral fracture.9 Our patient had these risk factors on a background of anemia and extensive coronary artery disease. Given her past medical history and refusal of blood products out of religious belief, we thought she was too high risk for extensive surgical treatment for her fracture. In addition, she was not an ideal candidate for nonoperative treatment, as a periprosthetic fracture typically is treated with surgical revision or open reduction and internal fixation. Therefore, we selected an unconventional treatment modality, typically used as a temporizing measure in severe fractures around the knee—a spanning external fixator worn for 6 weeks and a cast-brace for an additional 6 weeks. This led to successful clinical and radiographic outcomes. We consider spanning external fixation a viable option for periprosthetic fractures after TKA in morbidly obese patients with relatively well-aligned fractures and extremely high risk for medical complications associated with traditional open surgery.
The incidence of periprosthetic supracondylar fractures of the femur after total knee arthroplasty (TKA) ranges from 0.6% to 2.5%.1 Treatment of periprosthetic fractures is often complicated by advanced patient age and osteoporosis, which frequently accompanies these fractures. Management of a periprosthetic fracture depends on the relation between the fracture site and the prosthesis, displacement of the prosthesis, integrity of the fixation of the prosthesis, extent of the bone loss caused by fracture comminution or preexisting osteolysis, general health of the patient, and surgeon expertise.2,3 The aim is to achieve fracture union around a stable, well-aligned arthroplasty with preserved or restored bone stock and therefore to return the patient to previous level of function. Although nonoperative treatments have been shown to be successful,4,5 in the great majority of cases surgical treatment is advised for these fractures.6-10 In cases in which bone stock is adequate for fixation rather than replacement of the distal femur, 2 modalities are commonly used: retrograde intramedullary nailing and locking plates. Each has its drawbacks and advantages.11,12
Although external fixation has been used in the treatment of distal femoral fractures,13 it is seldom considered in the treatment of periprosthetic fractures. Several authors have described cases that used external fixators, occasionally spanning the knee. The specific types of external fixators discussed in the literature have included ring fixators,14-17 hybrid fixators,18 and uniplanar nonspanning fixators14,19 (Table). Use of a simple anterior spanning external fixator in treating a periprosthetic femoral fracture has received little attention in the literature.
The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 54-year-old woman with previous total hip arthroplasty (THA) and ipsilateral TKA tripped on a carpet and sustained a comminuted fracture of the distal femur just above the TKA prosthesis (Figure 1). She was a Jehovah’s Witness and thus refused all blood products. She had an extensive history of osteoporosis, morbid obesity (5 feet tall, 250 pounds; body mass index, 49), diabetes, and rheumatoid arthritis. Evaluation by the internal medicine service revealed severe coronary artery disease on a stress thallium test and anemia with hematocrit of 24%. Given the patient’s medical comorbidities and religious status, and the location of the comminuted distal femur fracture, several treatment options were considered. First was nonoperative treatment with a cast or cast-brace (hinged cast). Because of her body habitus, however, we thought she would very likely experience skin complications, inadequate immobilization of the bone, and significant discomfort. Ultimately, use of a spanning external fixator was chosen as the safest course, given the significant medical risks accompanying a more extensive surgical reconstruction. With the spanning external fixator, the main risks were the inability to fully control fracture alignment and the potential introduction of infection into the functional THA. We thought that, by limiting the amount of time in the fixator and managing the pin site aggressively, we could minimize the risk for infection in this setting.
The procedure was performed with the patient under general anesthesia. During surgery, a lateral image of the femur was used to identify the distal end of the THA prosthesis. A level was marked 2 to 3 cm distal to the tip of this prosthesis, and another about 1 cm above the fracture (noted to be above the most proximal extent of the knee joint). These planned pin-entry sites were prepared from an anterior approach with incisions (using a No. 11 blade) of about 1 cm each. Blunt dissection was carried down to the femur. Each planned pin site was predrilled with a 3.5-mm drill; then, a 5-mm Shanz pin was placed. This process was repeated immediately distal to the tibial component and at the junction of the mid and distal thirds of the tibia (Figure 2). The preliminary external fixator frame was then applied. Once the reduction was satisfactory in the anteroposterior and lateral planes, the fixator clamps were tightened. A second row of bars was then incorporated.
Six weeks after surgery, radiographs showed early callus formation. Removing the external fixator and examining the knee under anesthesia confirmed there was no significant motion through the fracture site. A cast-brace (fiberglass thigh segment, fiberglass lower leg cast with hinged knee segment) was then applied. We remained concerned about skin complications but were encouraged by the early healing achieved with the fixator. The patient was started on a physical therapy program of gait training with a walker and toe-touch weight-bearing on the injured extremity. She also started a limited lower-extremity strengthening program. Three months after surgery, she was tolerating weight-bearing on the injured extremity with no pain. At 6 months, knee radiographs showed fracture consolidation with active range of motion of 10° to 120° and no pain (Figures 3A, 3B). Distal sensation, motor function, and vascular examination were normal. Two years after surgery, radiographs of the right knee showed minor malalignment in the coronal and sagittal planes (Figures 4A, 4B) and complete consolidation of the fracture.
Discussion
Periprosthetic fractures of the femur after TKA often occur in the setting of osteopenia, and some are associated with concurrent implant loosening. In most cases, these fractures require surgical stabilization. Nevertheless, the goals of treatment are to obtain and maintain anatomical alignment and stability to allow early range of motion. Nonoperative options include skeletal traction, cast, pins and plaster, and cast-brace.3-5,20 Operative options include intramedullary fixation,12,21 stabilization with various plates,21-23 revision knee arthroplasty, and arthrodesis.1 Treatment selection should be based on patient health, fracture displacement, comminution, osteopenia severity, and status of the prosthetic components.
The present case exemplifies some of the highest degrees of medical and surgical risk factors in people with a periprosthetic femoral fracture after TKA. Patients with rheumatoid arthritis, patients having corticosteroid treatment, patients of advanced age, and female patients are all at higher risk for supracondylar femoral fracture.9 Our patient had these risk factors on a background of anemia and extensive coronary artery disease. Given her past medical history and refusal of blood products out of religious belief, we thought she was too high risk for extensive surgical treatment for her fracture. In addition, she was not an ideal candidate for nonoperative treatment, as a periprosthetic fracture typically is treated with surgical revision or open reduction and internal fixation. Therefore, we selected an unconventional treatment modality, typically used as a temporizing measure in severe fractures around the knee—a spanning external fixator worn for 6 weeks and a cast-brace for an additional 6 weeks. This led to successful clinical and radiographic outcomes. We consider spanning external fixation a viable option for periprosthetic fractures after TKA in morbidly obese patients with relatively well-aligned fractures and extremely high risk for medical complications associated with traditional open surgery.
1. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
2. Su ET, Kubiak EN, Dewal H, Hiebert R, Di Cesare PE. A proposed classification of supracondylar femur fractures above total knee arthroplasties. J Arthroplasty. 2006;21(3):405-408.
3. Kim KI, Egol KA, Hozack WJ, Parvizi J. Periprosthetic fractures after total knee arthroplasties. Clin Orthop. 2006;(446):167-175.
4. Sochart DH, Hardinge K. Nonsurgical management of supracondylar fracture above total knee arthroplasty. Still the nineties option. J Arthroplasty. 1997;12(7):830-834.
5. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
6. Frigg R, Appenzeller A, Christensen R, Frenk A, Gilbert S, Schavan R. The development of the distal femur less invasive stabilization system (LISS). Injury. 2001;32(suppl 3):SC24-SC31.
7. Goesling T, Frenk A, Appenzeller A, Garapati R, Marti A, Krettek C. LISS PLT: design, mechanical and biomechanical characteristics. Injury. 2003;34(suppl 1):A11-A15.
8. Huang HT, Huang PJ, Su JY, Lin SY. Indirect reduction and bridge plating of supracondylar fractures of the femur. Injury. 2003;34(2):135-140.
9. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
10. Jamali AA, Lee MA, Donthineni R, Meehan JP. Minimally invasive management of a floating prosthesis injury with locking plates. J Arthroplasty. 2007;22(6):928-933.
11. Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. J Arthroplasty. 2002;17(7):876-881.
12. Firoozbakhsh K, Behzadi K, DeCoster TA, Moneim MS, Naraghi FF. Mechanics of retrograde nail versus plate fixation for supracondylar femur fractures. J Orthop Trauma. 1995;9(2):152-157.
13. Arazi M, Memik R, Ogun TC, Yel M. Ilizarov external fixation for severely comminuted supracondylar and intercondylar fractures of the distal femur. J Bone Joint Surg Br. 2001;83(5):663-667.
14. Pleva L, Sir M, Madeja R. Our experiences with the treatment of periprosthetic fractures of femur. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):75-79.
15. Simon RG, Brinker MR. Use of Ilizarov external fixation for a periprosthetic supracondylar femur fracture. J Arthroplasty. 1999;14(1):118-121.
16. Hurson C, Synnott K, McCormack D. Above-knee Ilizarov external fixation for early periprosthetic supracondylar femoral fracture—a case report. Knee. 2005;12(2):145-147.
17. Beris AE, Lykissas MG, Sioros V, Mavrodontidis AN, Korompilias AV. Femoral periprosthetic fracture in osteoporotic bone after a total knee replacement: treatment with Ilizarov external fixation. J Arthroplasty. 2010;25(7):1168.e9-e12.
18. Pafilas D, Kourtzis N. Hybrid external fixation as a new treatment method for periprosthetic femoral fracture. A case report. J Bone Joint Surg Am. 2006;88(1):188-192.
19. Merkel KD, Johnson EW Jr. Supracondylar fracture of the femur after total knee arthroplasty. J Bone Joint Surg Am. 1986;68(1):29-43.
20. Cordeiro EN, Costa RC, Carazzato JG, Silva Jdos S. Periprosthetic fractures in patients with total knee arthroplasties. Clin Orthop. 1990;(252):182-189.
21. Riemer BL, Butterfield SL, Burke CJ 3rd, Mathews D. Immediate plate fixation of highly comminuted femoral diaphyseal fractures in blunt polytrauma patients. Orthopedics. 1992;15(8):907-916.
22. Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the less invasive stabilization system (L.I.S.S.). Injury. 2001;32(suppl 3):SC64-SC75.
23. Althausen PL, Lee MA, Finkemeier CG, Meehan JP, Rodrigo JJ. Operative stabilization of supracondylar femur fractures above total knee arthroplasty: a comparison of four treatment methods. J Arthroplasty. 2003;18(7):834-839.
1. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
2. Su ET, Kubiak EN, Dewal H, Hiebert R, Di Cesare PE. A proposed classification of supracondylar femur fractures above total knee arthroplasties. J Arthroplasty. 2006;21(3):405-408.
3. Kim KI, Egol KA, Hozack WJ, Parvizi J. Periprosthetic fractures after total knee arthroplasties. Clin Orthop. 2006;(446):167-175.
4. Sochart DH, Hardinge K. Nonsurgical management of supracondylar fracture above total knee arthroplasty. Still the nineties option. J Arthroplasty. 1997;12(7):830-834.
5. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
6. Frigg R, Appenzeller A, Christensen R, Frenk A, Gilbert S, Schavan R. The development of the distal femur less invasive stabilization system (LISS). Injury. 2001;32(suppl 3):SC24-SC31.
7. Goesling T, Frenk A, Appenzeller A, Garapati R, Marti A, Krettek C. LISS PLT: design, mechanical and biomechanical characteristics. Injury. 2003;34(suppl 1):A11-A15.
8. Huang HT, Huang PJ, Su JY, Lin SY. Indirect reduction and bridge plating of supracondylar fractures of the femur. Injury. 2003;34(2):135-140.
9. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
10. Jamali AA, Lee MA, Donthineni R, Meehan JP. Minimally invasive management of a floating prosthesis injury with locking plates. J Arthroplasty. 2007;22(6):928-933.
11. Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. J Arthroplasty. 2002;17(7):876-881.
12. Firoozbakhsh K, Behzadi K, DeCoster TA, Moneim MS, Naraghi FF. Mechanics of retrograde nail versus plate fixation for supracondylar femur fractures. J Orthop Trauma. 1995;9(2):152-157.
13. Arazi M, Memik R, Ogun TC, Yel M. Ilizarov external fixation for severely comminuted supracondylar and intercondylar fractures of the distal femur. J Bone Joint Surg Br. 2001;83(5):663-667.
14. Pleva L, Sir M, Madeja R. Our experiences with the treatment of periprosthetic fractures of femur. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):75-79.
15. Simon RG, Brinker MR. Use of Ilizarov external fixation for a periprosthetic supracondylar femur fracture. J Arthroplasty. 1999;14(1):118-121.
16. Hurson C, Synnott K, McCormack D. Above-knee Ilizarov external fixation for early periprosthetic supracondylar femoral fracture—a case report. Knee. 2005;12(2):145-147.
17. Beris AE, Lykissas MG, Sioros V, Mavrodontidis AN, Korompilias AV. Femoral periprosthetic fracture in osteoporotic bone after a total knee replacement: treatment with Ilizarov external fixation. J Arthroplasty. 2010;25(7):1168.e9-e12.
18. Pafilas D, Kourtzis N. Hybrid external fixation as a new treatment method for periprosthetic femoral fracture. A case report. J Bone Joint Surg Am. 2006;88(1):188-192.
19. Merkel KD, Johnson EW Jr. Supracondylar fracture of the femur after total knee arthroplasty. J Bone Joint Surg Am. 1986;68(1):29-43.
20. Cordeiro EN, Costa RC, Carazzato JG, Silva Jdos S. Periprosthetic fractures in patients with total knee arthroplasties. Clin Orthop. 1990;(252):182-189.
21. Riemer BL, Butterfield SL, Burke CJ 3rd, Mathews D. Immediate plate fixation of highly comminuted femoral diaphyseal fractures in blunt polytrauma patients. Orthopedics. 1992;15(8):907-916.
22. Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the less invasive stabilization system (L.I.S.S.). Injury. 2001;32(suppl 3):SC64-SC75.
23. Althausen PL, Lee MA, Finkemeier CG, Meehan JP, Rodrigo JJ. Operative stabilization of supracondylar femur fractures above total knee arthroplasty: a comparison of four treatment methods. J Arthroplasty. 2003;18(7):834-839.