User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Unstable Dorsal Proximal Interphalangeal Joint Fracture-Dislocations Treated With Extension-Block Pinning
The proximal interphalangeal (PIP) joint plays a crucial role in hand function, accounting for an estimated 85% of the motion required to grasp an object.1 The anatomy and biomechanics of the PIP joint, however, make it particularly prone to injury.2,3 Dorsal PIP fracture-dislocations represent a subset of PIP injuries that often require surgical intervention.2 The stability of these fracture-dislocations largely depends on the extent of articular involvement of the base of the middle phalanx. Fractures that involve less than 30% of the joint surface typically remain stable after reduction.2,4,5 In cases in which involvement ranges from 30% to 50%, PIP joint stability is more tenuous, and more joint flexion is required to maintain concentric reduction. Fractures that involve more than 50% of the articular surface are unstable and require operative intervention.2,5,6 Fractures that require more than 30° of flexion for reduction maintenance are generally considered unstable and may benefit from surgical intervention.2
The goals of treatment for this injury are to restore a stable, concentrically reduced joint and initiate early joint mobilization to prevent stiffness, pain, recurrent instability, and posttraumatic arthritis.3,7 Numerous surgical interventions for unstable PIP fracture-dislocations have been proposed, including open reduction and internal fixation (ORIF),8-10 extension-block pinning (EBP),11-13 dynamic external fixation,14-17 volar plate arthroplasty,18,19 and hemi-hamate resurfacing arthroplasty.20,21 Many of these techniques can be technically demanding and may require prolonged immobilization. EBP can be performed easily and efficiently and allows for early joint motion.
Extension-block pinning—placing a Kirschner wire (K-wire) into the head of the proximal phalanx at an angle that blocks PIP extension and prevents joint subluxation—was first described by Sugawa and colleagues12 in 1979. In a study by Inoue and Tamura,11 patients treated with EBP had a mean PIP range of motion (ROM) of 94° at a mean follow-up of 14 months. In a series of 3 case reports, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM in patients treated with EBP.
We conducted a study to expand on previous research on pain, function, and satisfaction outcomes in addition to ROM. We hypothesized that percutaneous EBP is an effective treatment for unstable dorsal PIP fracture-dislocations and has efficacy similar to that of more complex and technically demanding methods of treatment.
Materials and Methods
We retrospectively reviewed patient charts to identify candidates for this study. Inclusion criteria were unstable dorsal PIP fracture-dislocations treated with EBP and minimum 4-month follow-up. (Fracture-dislocations were deemed unstable if they involved at least 30% of the articular surface or required more than 30° of flexion for reduction maintenance.) Exclusion criteria were open injury, neurovascular or tendon injury, or any prior injury to the PIP joint.
Twelve patients (5 females, 7 males) treated over a 4-year period (2002–2006) met the inclusion criteria. Mean age was 30 years (range, 15-64 years). Each surgery was performed by Dr. Hagberg or Dr. Balk. Half the cases involved the dominant hand. Two small fingers, 4 ring fingers, 2 long fingers, and 4 index fingers were injured. The injuries were sustained in an all-terrain vehicle accident (n = 1), in falls (n = 2), while swimming (n = 1), or while playing softball (n = 3), football (n = 4), or soccer (n = 1). Mean time from injury to surgery was 7.5 days (range, 4-27 days). Extent of articular surface involvement of the base of the fractured middle phalanx was calculated using preoperatively obtained lateral radiographs.
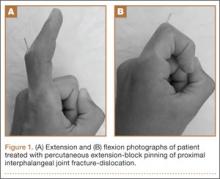
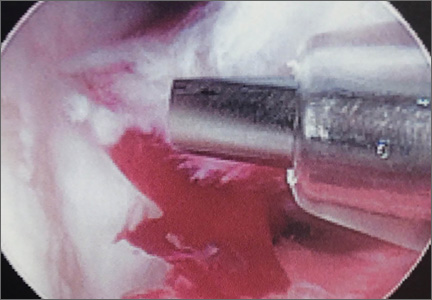
Surgical intervention was performed in a reproducible fashion. All patients were treated with closed reduction of the PIP joint under fluoroscopic guidance. Before pinning, joint stability was assessed fluoroscopically both at rest and through an arc of motion. A single smooth 0.045-in K-wire was then inserted percutaneously into the distal and dorsal aspects of the proximal phalanx in retrograde fashion (Figure 1). During wire insertion, the distal interphalangeal joint was flexed to relax the intrinsic mechanism, and the central slip tendon was pierced just proximal to its insertion. We have not noted significant adhesion formation about the central slip with this technique, likely because of limited tendon excursion in this location. Stable joint reduction was confirmed with fluoroscopy. No attempt was made to reduce the intra-articular fracture at the base of the middle phalanx.
A therapy program was initiated 2 to 9 days after surgery. At the first postoperative visit, patients were allowed to perform active ROM (AROM) with the pin in place (Figure 1). K-wires were removed a mean of 25 days (range, 17-31 days) after surgery. A static dorsal block splint was then applied, and patients were encouraged to remove it several times per day for AROM between 20° and full flexion until 6 weeks after surgery. At that time, formal occupational therapy was commenced for another 6 weeks. If there was residual flexion contracture of the PIP joint, dynamic extension splinting was initiated after fracture consolidation.
Mean follow-up was 35.5 months (range, 4-94 months). Postoperative anteroposterior and lateral radiographs were used to evaluate maintenance of joint congruity, fracture union, remodeling, and evidence of degenerative changes. At final follow-up, grip strength of injured and contralateral hands was measured with a dynamometer (Jamar; Patterson Medical, Warrenville, Illinois). AROM and passive ROM (PROM) of the PIP joint was documented at follow-up visits. In addition, patients rated their pain on a 0-to-10 visual analog scale (VAS), with 0 representing no pain and 10 representing excruciating pain. Patients also completed a questionnaire assessing satisfaction with surgical outcome. Physical function and disability were assessed with the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire. Any complications, including the need for further surgeries, were documented. Pearson correlation coefficients and Student t tests (with significance set at P < .05) were used to compare outcomes.
Results
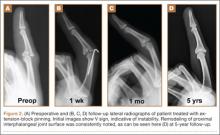
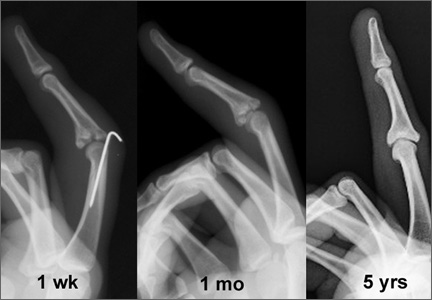
Radiographic reduction of joint dislocation was achieved and maintained in 11 of the 12 patients at a mean follow-up of 35.5 months (range, 4-94 months). Extent of joint surface involvement, based on preoperative lateral radiographs, averaged 43% (range, 25%-75%). Although no direct articular reduction was performed, remodeling of the joint surface was consistently noted at follow-up (Figure 2). Mild radiographic degenerative changes were noted at final follow-up in 4 patients, and moderate changes were noted in 1 patient. Radiographic union was achieved in all cases, and no pin-tract infections were noted.
Mean AROM of the PIP joint at final follow-up was 84° (range, 50°-110°), with patients lacking a mean of 7° of full extension and achieving mean flexion of 91°. Mean PROM was 93° (range, 75°-110°). There was no correlation between extent of articular surface involvement and ROM. Furthermore, no correlation was found between time from injury to surgery and ROM. Patients regained full grip strength in the operative hand. At final follow-up, mean grip strength was 79.4 pounds in the operative hand and 79.6 pounds in the contralateral hand, demonstrating equal grip strengths bilaterally.
Patients overall had very low levels of pain; mean VAS score was 0.64 (range, 0-3). Mean QuickDASH score was 5.7 (range, 0-30), suggesting minimal functional impairment. One patient developed a malunion of the middle phalanx fracture resulting in a rotational deformity and required corrective osteotomy. This patient’s VAS score (3) and QuickDASH score (30) were significantly higher than those of the other patients in the study. No other complications were noted by final follow-up.
A higher level of patient satisfaction was found to be directly related to length of follow-up (P < .05). Satisfaction was inversely related to higher VAS score (P < .05) and higher QuickDASH score (P < .001). Pain at work correlated with lower satisfaction level (P < .05). There was no correlation between patient satisfaction and AROM or PROM.
Discussion
The results of this study demonstrate the efficacy of EBP in the treatment of dorsal PIP joint fracture-dislocations. EBP maintained joint dislocation reduction and allowed for early mobilization, which resulted in good ROM, minimal pain, and good functional outcomes. Of note, postoperative patient satisfaction correlated with pain but not with ROM. It is possible that EBP yielded sufficient functional ROM in all patients such that improvement beyond this threshold did not lead to further improvement in satisfaction. Hume and colleagues23 found that mean PIP joint flexion of 60° is needed for activities of daily living. As mean PIP active flexion was 91° (range, 70°-105°) in the present study, it is possible that satisfaction did not correlate with ROM, as all 12 patients achieved active flexion of more than 60°. Despite the lack of correlation between ROM and satisfaction, early PIP joint mobilization is likely a key contributor to positive outcomes because of its significant role in cartilage healing.24
Postoperative ROM in the present study is consistent with that in other reports of patients with PIP joint fracture-dislocations treated with EBP.11,12,22 In a study by Inoue and Tamura,11 14 such patients had mean PIP ROM of 94° at a mean follow-up of 14 months. Viegas22 followed a series of 3 patients for a mean of 7 weeks. At final follow-up, their mean PIP arc of motion was 71°; they lacked 12° of full extension and achieved 83° of flexion. The larger PIP arc of motion (84°) found in the present study may be due to our significantly longer follow-up (35 months). Unlike us, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM. Our finding a lack of correlation may be a result of the significant amount of joint remodeling noted on follow-up radiographs.
Studies of transarticular pinning of PIP joints after dorsal PIP fracture-dislocations have reported outcomes similar to ours.25,26 Newington and colleagues25 evaluated 10 cases of transarticular pinning of the PIP joint and found mean arc of motion of 85° and equal grip strengths between injured and contralateral hands. In a series of 19 patients with PIP fracture-dislocations, Aladin and Davis26 noted similar outcomes of transarticular K-wire fixation and ORIF. In both of their treatment groups, however, there was evidence of PIP joint incongruity and subluxation. Of note, PIP arc motion was lower in their study than in ours.
Recent studies have evaluated unstable PIP fracture-dislocations treated with both EBP and percutaneous reduction and pinning with a second K-wire.13,27 At a mean follow-up of 18 months, Vitale and colleagues13 noted maintenance of concentric fracture reduction, good PIP ROM (mean range, 4°-93°), and low VAS and DASH scores (1.4 and 8, respectively). Waris and Alanen27 noted mean PIP AROM of 83° and low VAS and DASH scores (1 and 4, respectively). The EBP technique used in the present study did not involve percutaneous fracture reduction but achieved equally good ROM and VAS and QuickDASH scores.
Clinical outcomes of EBP of PIP joint fracture-dislocations are also comparable to outcomes of more complex treatment methods.8-10,15-19,21,26,28-33 Dynamic distraction external fixation has led to equally good ROM (mean AROM, 80°-85°15,16) and VAS scores, but with a higher incidence of pin-site infection.14-17 ORIF of the intra-articular middle phalanx fracture has the advantage of obtaining a direct anatomical reduction, but clinical outcomes are similar to those in the present study (mean AROM, 70°; 78% pain-free9), and flexion contractures have been noted.8-10 Furthermore, reduction of the fractured PIP joint articular surface has not been shown to be necessary for good outcomes.16,34 This may be explained in part by PIP joint remodeling, which has been routinely observed on long-term follow-up by the senior authors of the present study. Hemi-hamate autografting and volar plate arthroplasty are other options that have had promising results in the treatment of acute and chronic unstable PIP fracture-dislocations.18-21 However, the postoperative ROM (mean AROM, 61°-85°18,21), VAS scores, and patient satisfaction (91% very satisfied21) of these operations are similar to those of EBP in the present study and may not justify the longer operative times and technical challenges associated with these techniques.
We believe that our study group’s 1 complication, a malunion that was treated with corrective osteotomy, resulted from lack of appreciation of the degree of injury. The teenaged female patient’s index finger PIP joint had a rotational malalignment that was not appreciated before or during surgery. After pinning and after ROM was restored, the index finger was observed crossing over the middle finger with digital flexion. The patient returned to the operating room for corrective osteotomy.
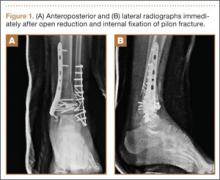
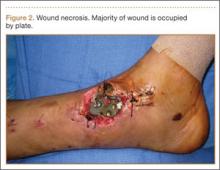
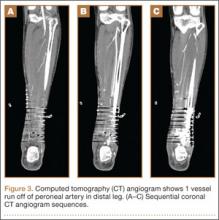
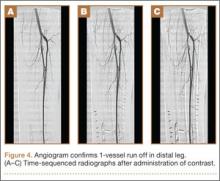
We recommend that surgeons assess alignment carefully, before and during surgery, when considering this technique. Although complications are rare, the technique is not for patients with rotational malalignment; ORIF may be more suitable in these cases. In addition, though EBP may be appropriate for pilon-type injuries, as it allows for early AROM, our procedure of choice for pilon fracture is dynamic external fixation, which in addition to allowing for AROM provides ligamentotaxis. In the event that a large volar articular fragment extends into the middle phalanx diaphysis, we typically proceed with ORIF through a volar shotgun approach. At our institution, injuries lasting more than 3 months are often treated with volar plate arthroplasty or hemi-hamate resurfacing. Finally, we believe that caution should be exercised when using this technique in patients with more than 50% articular involvement. In the present study, though we used this treatment in cases of up to 75% surface involvement, alternative techniques, such as hemi-hamate resurfacing arthroplasty, may provide a better volar bony buttress and limit the risk for recurrent instability. Despite its relative contraindications, our technique has been appropriate for more than 90% of the acute PIP fracture-dislocations we have seen.
This study expands on prior research by demonstrating good function, satisfaction, and pain outcomes of percutaneous EBP in the treatment of unstable dorsal PIP fracture-dislocations. In addition, this study demonstrated that the efficacy of EBP is similar to that of more complex and technically demanding methods of treatment. Our technique has the advantage of simplicity. It obviates the soft-tissue damage required for ORIF and more complex fixation techniques. Furthermore, use of this simple technique may save time and costs and lead to more reproducible outcomes.
One limitation of this study is its small sample size. It is possible that outcomes may have been different with a larger sample. Furthermore, we did not make a direct comparison with other treatment methods. To better determine the optimal treatment method for this fracture type, future studies should prospectively evaluate outcomes for multiple treatment modalities in a randomized fashion.
1. Leibovic SJ, Bowers WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10(2):169-178.
2. Kiefhaber TR, Stern PJ. Fracture dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1998;23(3):368-380.
3. Ng CY, Oliver CW. Fractures of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Br. 2009;91(6):705-712.
4. Isani A. Small joint injuries requiring surgical treatment. Orthop Clin North Am. 1986;17(3):407-419.
5. McElfresh EC, Dobyns JH, O’Brien ET. Management of fracture-dislocation of the proximal interphalangeal joints by extension-block splinting. J Bone Joint Surg Am. 1972;54(8):1705-1711.
6. Hastings H 2nd, Carroll C 4th. Treatment of closed articular fractures of the metacarpophalangeal and proximal interphalangeal joints. Hand Clin. 1988;4(3):503-527.
7. O’Rourke SK, Gaur S, Barton NJ. Long-term outcome of articular fractures of the phalanges: an eleven year follow up. J Hand Surg Br. 1989;14(2):183-193.
8. Grant I, Berger AC, Tham SK. Internal fixation of unstable fracture dislocations of the proximal interphalangeal joint. J Hand Surg Br. 2005;30(5):492-498.
9. Hamilton SC, Stern PJ, Fassler PR, Kiefhaber TR. Mini-screw fixation for the treatment of proximal interphalangeal joint dorsal fracture-dislocations. J Hand Surg Am. 2006;31(8):1349-1354.
10. Lee JY, Teoh LC. Dorsal fracture dislocations of the proximal interphalangeal joint treated by open reduction and interfragmentary screw fixation: indications, approaches and results. J Hand Surg Br. 2006;31(2):138-146.
11. Inoue G, Tamura Y. Treatment of fracture-dislocation of the proximal interphalangeal joint using extension-block Kirschner wire. Ann Chir Main Memb Super. 1991;10(6):564-568.
12. Sugawa I, Otani K, Kobayashi A. Treatment of fracture dislocation PIP-joint by Kirschner wire extension block method. Cent Jpn J Orthop Traumat. 1979;22:1409-1412.
13. Vitale MA, White NJ, Strauch RJ. A percutaneous technique to treat unstable dorsal fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2011;36(9):1453-1459.
14. Badia A, Riano F, Ravikoff J, Khouri R, Gonzalez-Hernandez E, Orbay JL. Dynamic intradigital external fixation for proximal interphalangeal joint fracture dislocations. J Hand Surg Am. 2005;30(1):154-160.
15. Ellis SJ, Cheng R, Prokopis P, et al. Treatment of proximal interphalangeal dorsal fracture-dislocation injuries with dynamic external fixation: a pins and rubber band system. J Hand Surg Am. 2007;32(8):1242-1250.
16. Morgan JP, Gordon DA, Klug MS, Perry PE, Barre PS. Dynamic digital traction for unstable comminuted intra-articular fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1995;20(4):565-573.
17. Ruland RT, Hogan CJ, Cannon DL, Slade JF. Use of dynamic distraction external fixation for unstable fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2008;33(1):19-25.
18. Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25(3):429-437.
19. Durham-Smith G, McCarten GM. Volar plate arthroplasty for closed proximal interphalangeal joint injuries. J Hand Surg Br. 1992;17(4):422-428.
20. Calfee RP, Kiefhaber TR, Sommerkamp TG, Stern PJ. Hemi-hamate arthroplasty provides functional reconstruction of acute and chronic proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2009;34(7):1232-1241.
21. Williams RM, Kiefhaber TR, Sommerkamp TG, Stern PJ. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg Am. 2003;28(5):856-865.
22. Viegas SF. Extension block pinning for proximal interphalangeal joint fracture dislocations: preliminary report of a new technique. J Hand Surg Am. 1992;17(5):896-901.
23. Hume MC, Gellman H, McKellop H, Brumfield RH Jr. Functional range of motion of the joints of the hand. J Hand Surg Am. 1990;15(2):240-243.
24. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10(2):211-220.
25. Newington DP, Davis TR, Barton NJ. The treatment of dorsal fracture-dislocation of the proximal interphalangeal joint by closed reduction and Kirschner wire fixation: a 16-year follow up. J Hand Surg Br. 2001;26(6):537-540.
26. Aladin A, Davis TR. Dorsal fracture-dislocation of the proximal interphalangeal joint: a comparative study of percutaneous Kirschner wire fixation versus open reduction and internal fixation. J Hand Surg Br. 2005;30(2):120-128.
27. Waris E, Alanen V. Percutaneous, intramedullary fracture reduction and extension block pinning for dorsal proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2010;35(12):2046-2052.
28. Bain GI, Mehta JA, Heptinstall RJ, Bria M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J Bone Joint Surg Br. 1998;80(6):1014-1019.
29. Eaton RG, Malerich MM. Volar plate arthroplasty of the proximal interphalangeal joint: a review of ten years’ experience. J Hand Surg Am. 1980;5(3):260-268.
30. Green A, Smith J, Redding M, Akelman E. Acute open reduction and rigid internal fixation of proximal interphalangeal joint fracture dislocation. J Hand Surg Am. 1992;17(3):512-517.
31. Inanami H, Ninomiya S, Okutsu I, Tarui T. Dynamic external finger fixator for fracture dislocation of the proximal interphalangeal joint. J Hand Surg Am. 1993;18(1):160-164.
32. Suzuki Y, Matsunaga T, Sato S, Yokoi T. The pins and rubbers traction system for treatment of comminuted intraarticular fractures and fracture-dislocations in the hand. J Hand Surg Br. 1994;19(1):98-107.
33. Weiss AP. Cerclage fixation for fracture dislocation of the proximal interphalangeal joint. Clin Orthop. 1996;(327):21-28.
34. Agee JM. Unstable fracture dislocations of the proximal interphalangeal joint. Treatment with the force couple splint. Clin Orthop. 1987;(214):101-112.
The proximal interphalangeal (PIP) joint plays a crucial role in hand function, accounting for an estimated 85% of the motion required to grasp an object.1 The anatomy and biomechanics of the PIP joint, however, make it particularly prone to injury.2,3 Dorsal PIP fracture-dislocations represent a subset of PIP injuries that often require surgical intervention.2 The stability of these fracture-dislocations largely depends on the extent of articular involvement of the base of the middle phalanx. Fractures that involve less than 30% of the joint surface typically remain stable after reduction.2,4,5 In cases in which involvement ranges from 30% to 50%, PIP joint stability is more tenuous, and more joint flexion is required to maintain concentric reduction. Fractures that involve more than 50% of the articular surface are unstable and require operative intervention.2,5,6 Fractures that require more than 30° of flexion for reduction maintenance are generally considered unstable and may benefit from surgical intervention.2
The goals of treatment for this injury are to restore a stable, concentrically reduced joint and initiate early joint mobilization to prevent stiffness, pain, recurrent instability, and posttraumatic arthritis.3,7 Numerous surgical interventions for unstable PIP fracture-dislocations have been proposed, including open reduction and internal fixation (ORIF),8-10 extension-block pinning (EBP),11-13 dynamic external fixation,14-17 volar plate arthroplasty,18,19 and hemi-hamate resurfacing arthroplasty.20,21 Many of these techniques can be technically demanding and may require prolonged immobilization. EBP can be performed easily and efficiently and allows for early joint motion.
Extension-block pinning—placing a Kirschner wire (K-wire) into the head of the proximal phalanx at an angle that blocks PIP extension and prevents joint subluxation—was first described by Sugawa and colleagues12 in 1979. In a study by Inoue and Tamura,11 patients treated with EBP had a mean PIP range of motion (ROM) of 94° at a mean follow-up of 14 months. In a series of 3 case reports, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM in patients treated with EBP.
We conducted a study to expand on previous research on pain, function, and satisfaction outcomes in addition to ROM. We hypothesized that percutaneous EBP is an effective treatment for unstable dorsal PIP fracture-dislocations and has efficacy similar to that of more complex and technically demanding methods of treatment.
Materials and Methods
We retrospectively reviewed patient charts to identify candidates for this study. Inclusion criteria were unstable dorsal PIP fracture-dislocations treated with EBP and minimum 4-month follow-up. (Fracture-dislocations were deemed unstable if they involved at least 30% of the articular surface or required more than 30° of flexion for reduction maintenance.) Exclusion criteria were open injury, neurovascular or tendon injury, or any prior injury to the PIP joint.
Twelve patients (5 females, 7 males) treated over a 4-year period (2002–2006) met the inclusion criteria. Mean age was 30 years (range, 15-64 years). Each surgery was performed by Dr. Hagberg or Dr. Balk. Half the cases involved the dominant hand. Two small fingers, 4 ring fingers, 2 long fingers, and 4 index fingers were injured. The injuries were sustained in an all-terrain vehicle accident (n = 1), in falls (n = 2), while swimming (n = 1), or while playing softball (n = 3), football (n = 4), or soccer (n = 1). Mean time from injury to surgery was 7.5 days (range, 4-27 days). Extent of articular surface involvement of the base of the fractured middle phalanx was calculated using preoperatively obtained lateral radiographs.
Surgical intervention was performed in a reproducible fashion. All patients were treated with closed reduction of the PIP joint under fluoroscopic guidance. Before pinning, joint stability was assessed fluoroscopically both at rest and through an arc of motion. A single smooth 0.045-in K-wire was then inserted percutaneously into the distal and dorsal aspects of the proximal phalanx in retrograde fashion (Figure 1). During wire insertion, the distal interphalangeal joint was flexed to relax the intrinsic mechanism, and the central slip tendon was pierced just proximal to its insertion. We have not noted significant adhesion formation about the central slip with this technique, likely because of limited tendon excursion in this location. Stable joint reduction was confirmed with fluoroscopy. No attempt was made to reduce the intra-articular fracture at the base of the middle phalanx.
A therapy program was initiated 2 to 9 days after surgery. At the first postoperative visit, patients were allowed to perform active ROM (AROM) with the pin in place (Figure 1). K-wires were removed a mean of 25 days (range, 17-31 days) after surgery. A static dorsal block splint was then applied, and patients were encouraged to remove it several times per day for AROM between 20° and full flexion until 6 weeks after surgery. At that time, formal occupational therapy was commenced for another 6 weeks. If there was residual flexion contracture of the PIP joint, dynamic extension splinting was initiated after fracture consolidation.
Mean follow-up was 35.5 months (range, 4-94 months). Postoperative anteroposterior and lateral radiographs were used to evaluate maintenance of joint congruity, fracture union, remodeling, and evidence of degenerative changes. At final follow-up, grip strength of injured and contralateral hands was measured with a dynamometer (Jamar; Patterson Medical, Warrenville, Illinois). AROM and passive ROM (PROM) of the PIP joint was documented at follow-up visits. In addition, patients rated their pain on a 0-to-10 visual analog scale (VAS), with 0 representing no pain and 10 representing excruciating pain. Patients also completed a questionnaire assessing satisfaction with surgical outcome. Physical function and disability were assessed with the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire. Any complications, including the need for further surgeries, were documented. Pearson correlation coefficients and Student t tests (with significance set at P < .05) were used to compare outcomes.
Results
Radiographic reduction of joint dislocation was achieved and maintained in 11 of the 12 patients at a mean follow-up of 35.5 months (range, 4-94 months). Extent of joint surface involvement, based on preoperative lateral radiographs, averaged 43% (range, 25%-75%). Although no direct articular reduction was performed, remodeling of the joint surface was consistently noted at follow-up (Figure 2). Mild radiographic degenerative changes were noted at final follow-up in 4 patients, and moderate changes were noted in 1 patient. Radiographic union was achieved in all cases, and no pin-tract infections were noted.
Mean AROM of the PIP joint at final follow-up was 84° (range, 50°-110°), with patients lacking a mean of 7° of full extension and achieving mean flexion of 91°. Mean PROM was 93° (range, 75°-110°). There was no correlation between extent of articular surface involvement and ROM. Furthermore, no correlation was found between time from injury to surgery and ROM. Patients regained full grip strength in the operative hand. At final follow-up, mean grip strength was 79.4 pounds in the operative hand and 79.6 pounds in the contralateral hand, demonstrating equal grip strengths bilaterally.
Patients overall had very low levels of pain; mean VAS score was 0.64 (range, 0-3). Mean QuickDASH score was 5.7 (range, 0-30), suggesting minimal functional impairment. One patient developed a malunion of the middle phalanx fracture resulting in a rotational deformity and required corrective osteotomy. This patient’s VAS score (3) and QuickDASH score (30) were significantly higher than those of the other patients in the study. No other complications were noted by final follow-up.
A higher level of patient satisfaction was found to be directly related to length of follow-up (P < .05). Satisfaction was inversely related to higher VAS score (P < .05) and higher QuickDASH score (P < .001). Pain at work correlated with lower satisfaction level (P < .05). There was no correlation between patient satisfaction and AROM or PROM.
Discussion
The results of this study demonstrate the efficacy of EBP in the treatment of dorsal PIP joint fracture-dislocations. EBP maintained joint dislocation reduction and allowed for early mobilization, which resulted in good ROM, minimal pain, and good functional outcomes. Of note, postoperative patient satisfaction correlated with pain but not with ROM. It is possible that EBP yielded sufficient functional ROM in all patients such that improvement beyond this threshold did not lead to further improvement in satisfaction. Hume and colleagues23 found that mean PIP joint flexion of 60° is needed for activities of daily living. As mean PIP active flexion was 91° (range, 70°-105°) in the present study, it is possible that satisfaction did not correlate with ROM, as all 12 patients achieved active flexion of more than 60°. Despite the lack of correlation between ROM and satisfaction, early PIP joint mobilization is likely a key contributor to positive outcomes because of its significant role in cartilage healing.24
Postoperative ROM in the present study is consistent with that in other reports of patients with PIP joint fracture-dislocations treated with EBP.11,12,22 In a study by Inoue and Tamura,11 14 such patients had mean PIP ROM of 94° at a mean follow-up of 14 months. Viegas22 followed a series of 3 patients for a mean of 7 weeks. At final follow-up, their mean PIP arc of motion was 71°; they lacked 12° of full extension and achieved 83° of flexion. The larger PIP arc of motion (84°) found in the present study may be due to our significantly longer follow-up (35 months). Unlike us, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM. Our finding a lack of correlation may be a result of the significant amount of joint remodeling noted on follow-up radiographs.
Studies of transarticular pinning of PIP joints after dorsal PIP fracture-dislocations have reported outcomes similar to ours.25,26 Newington and colleagues25 evaluated 10 cases of transarticular pinning of the PIP joint and found mean arc of motion of 85° and equal grip strengths between injured and contralateral hands. In a series of 19 patients with PIP fracture-dislocations, Aladin and Davis26 noted similar outcomes of transarticular K-wire fixation and ORIF. In both of their treatment groups, however, there was evidence of PIP joint incongruity and subluxation. Of note, PIP arc motion was lower in their study than in ours.
Recent studies have evaluated unstable PIP fracture-dislocations treated with both EBP and percutaneous reduction and pinning with a second K-wire.13,27 At a mean follow-up of 18 months, Vitale and colleagues13 noted maintenance of concentric fracture reduction, good PIP ROM (mean range, 4°-93°), and low VAS and DASH scores (1.4 and 8, respectively). Waris and Alanen27 noted mean PIP AROM of 83° and low VAS and DASH scores (1 and 4, respectively). The EBP technique used in the present study did not involve percutaneous fracture reduction but achieved equally good ROM and VAS and QuickDASH scores.
Clinical outcomes of EBP of PIP joint fracture-dislocations are also comparable to outcomes of more complex treatment methods.8-10,15-19,21,26,28-33 Dynamic distraction external fixation has led to equally good ROM (mean AROM, 80°-85°15,16) and VAS scores, but with a higher incidence of pin-site infection.14-17 ORIF of the intra-articular middle phalanx fracture has the advantage of obtaining a direct anatomical reduction, but clinical outcomes are similar to those in the present study (mean AROM, 70°; 78% pain-free9), and flexion contractures have been noted.8-10 Furthermore, reduction of the fractured PIP joint articular surface has not been shown to be necessary for good outcomes.16,34 This may be explained in part by PIP joint remodeling, which has been routinely observed on long-term follow-up by the senior authors of the present study. Hemi-hamate autografting and volar plate arthroplasty are other options that have had promising results in the treatment of acute and chronic unstable PIP fracture-dislocations.18-21 However, the postoperative ROM (mean AROM, 61°-85°18,21), VAS scores, and patient satisfaction (91% very satisfied21) of these operations are similar to those of EBP in the present study and may not justify the longer operative times and technical challenges associated with these techniques.
We believe that our study group’s 1 complication, a malunion that was treated with corrective osteotomy, resulted from lack of appreciation of the degree of injury. The teenaged female patient’s index finger PIP joint had a rotational malalignment that was not appreciated before or during surgery. After pinning and after ROM was restored, the index finger was observed crossing over the middle finger with digital flexion. The patient returned to the operating room for corrective osteotomy.
We recommend that surgeons assess alignment carefully, before and during surgery, when considering this technique. Although complications are rare, the technique is not for patients with rotational malalignment; ORIF may be more suitable in these cases. In addition, though EBP may be appropriate for pilon-type injuries, as it allows for early AROM, our procedure of choice for pilon fracture is dynamic external fixation, which in addition to allowing for AROM provides ligamentotaxis. In the event that a large volar articular fragment extends into the middle phalanx diaphysis, we typically proceed with ORIF through a volar shotgun approach. At our institution, injuries lasting more than 3 months are often treated with volar plate arthroplasty or hemi-hamate resurfacing. Finally, we believe that caution should be exercised when using this technique in patients with more than 50% articular involvement. In the present study, though we used this treatment in cases of up to 75% surface involvement, alternative techniques, such as hemi-hamate resurfacing arthroplasty, may provide a better volar bony buttress and limit the risk for recurrent instability. Despite its relative contraindications, our technique has been appropriate for more than 90% of the acute PIP fracture-dislocations we have seen.
This study expands on prior research by demonstrating good function, satisfaction, and pain outcomes of percutaneous EBP in the treatment of unstable dorsal PIP fracture-dislocations. In addition, this study demonstrated that the efficacy of EBP is similar to that of more complex and technically demanding methods of treatment. Our technique has the advantage of simplicity. It obviates the soft-tissue damage required for ORIF and more complex fixation techniques. Furthermore, use of this simple technique may save time and costs and lead to more reproducible outcomes.
One limitation of this study is its small sample size. It is possible that outcomes may have been different with a larger sample. Furthermore, we did not make a direct comparison with other treatment methods. To better determine the optimal treatment method for this fracture type, future studies should prospectively evaluate outcomes for multiple treatment modalities in a randomized fashion.
The proximal interphalangeal (PIP) joint plays a crucial role in hand function, accounting for an estimated 85% of the motion required to grasp an object.1 The anatomy and biomechanics of the PIP joint, however, make it particularly prone to injury.2,3 Dorsal PIP fracture-dislocations represent a subset of PIP injuries that often require surgical intervention.2 The stability of these fracture-dislocations largely depends on the extent of articular involvement of the base of the middle phalanx. Fractures that involve less than 30% of the joint surface typically remain stable after reduction.2,4,5 In cases in which involvement ranges from 30% to 50%, PIP joint stability is more tenuous, and more joint flexion is required to maintain concentric reduction. Fractures that involve more than 50% of the articular surface are unstable and require operative intervention.2,5,6 Fractures that require more than 30° of flexion for reduction maintenance are generally considered unstable and may benefit from surgical intervention.2
The goals of treatment for this injury are to restore a stable, concentrically reduced joint and initiate early joint mobilization to prevent stiffness, pain, recurrent instability, and posttraumatic arthritis.3,7 Numerous surgical interventions for unstable PIP fracture-dislocations have been proposed, including open reduction and internal fixation (ORIF),8-10 extension-block pinning (EBP),11-13 dynamic external fixation,14-17 volar plate arthroplasty,18,19 and hemi-hamate resurfacing arthroplasty.20,21 Many of these techniques can be technically demanding and may require prolonged immobilization. EBP can be performed easily and efficiently and allows for early joint motion.
Extension-block pinning—placing a Kirschner wire (K-wire) into the head of the proximal phalanx at an angle that blocks PIP extension and prevents joint subluxation—was first described by Sugawa and colleagues12 in 1979. In a study by Inoue and Tamura,11 patients treated with EBP had a mean PIP range of motion (ROM) of 94° at a mean follow-up of 14 months. In a series of 3 case reports, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM in patients treated with EBP.
We conducted a study to expand on previous research on pain, function, and satisfaction outcomes in addition to ROM. We hypothesized that percutaneous EBP is an effective treatment for unstable dorsal PIP fracture-dislocations and has efficacy similar to that of more complex and technically demanding methods of treatment.
Materials and Methods
We retrospectively reviewed patient charts to identify candidates for this study. Inclusion criteria were unstable dorsal PIP fracture-dislocations treated with EBP and minimum 4-month follow-up. (Fracture-dislocations were deemed unstable if they involved at least 30% of the articular surface or required more than 30° of flexion for reduction maintenance.) Exclusion criteria were open injury, neurovascular or tendon injury, or any prior injury to the PIP joint.
Twelve patients (5 females, 7 males) treated over a 4-year period (2002–2006) met the inclusion criteria. Mean age was 30 years (range, 15-64 years). Each surgery was performed by Dr. Hagberg or Dr. Balk. Half the cases involved the dominant hand. Two small fingers, 4 ring fingers, 2 long fingers, and 4 index fingers were injured. The injuries were sustained in an all-terrain vehicle accident (n = 1), in falls (n = 2), while swimming (n = 1), or while playing softball (n = 3), football (n = 4), or soccer (n = 1). Mean time from injury to surgery was 7.5 days (range, 4-27 days). Extent of articular surface involvement of the base of the fractured middle phalanx was calculated using preoperatively obtained lateral radiographs.
Surgical intervention was performed in a reproducible fashion. All patients were treated with closed reduction of the PIP joint under fluoroscopic guidance. Before pinning, joint stability was assessed fluoroscopically both at rest and through an arc of motion. A single smooth 0.045-in K-wire was then inserted percutaneously into the distal and dorsal aspects of the proximal phalanx in retrograde fashion (Figure 1). During wire insertion, the distal interphalangeal joint was flexed to relax the intrinsic mechanism, and the central slip tendon was pierced just proximal to its insertion. We have not noted significant adhesion formation about the central slip with this technique, likely because of limited tendon excursion in this location. Stable joint reduction was confirmed with fluoroscopy. No attempt was made to reduce the intra-articular fracture at the base of the middle phalanx.
A therapy program was initiated 2 to 9 days after surgery. At the first postoperative visit, patients were allowed to perform active ROM (AROM) with the pin in place (Figure 1). K-wires were removed a mean of 25 days (range, 17-31 days) after surgery. A static dorsal block splint was then applied, and patients were encouraged to remove it several times per day for AROM between 20° and full flexion until 6 weeks after surgery. At that time, formal occupational therapy was commenced for another 6 weeks. If there was residual flexion contracture of the PIP joint, dynamic extension splinting was initiated after fracture consolidation.
Mean follow-up was 35.5 months (range, 4-94 months). Postoperative anteroposterior and lateral radiographs were used to evaluate maintenance of joint congruity, fracture union, remodeling, and evidence of degenerative changes. At final follow-up, grip strength of injured and contralateral hands was measured with a dynamometer (Jamar; Patterson Medical, Warrenville, Illinois). AROM and passive ROM (PROM) of the PIP joint was documented at follow-up visits. In addition, patients rated their pain on a 0-to-10 visual analog scale (VAS), with 0 representing no pain and 10 representing excruciating pain. Patients also completed a questionnaire assessing satisfaction with surgical outcome. Physical function and disability were assessed with the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire. Any complications, including the need for further surgeries, were documented. Pearson correlation coefficients and Student t tests (with significance set at P < .05) were used to compare outcomes.
Results
Radiographic reduction of joint dislocation was achieved and maintained in 11 of the 12 patients at a mean follow-up of 35.5 months (range, 4-94 months). Extent of joint surface involvement, based on preoperative lateral radiographs, averaged 43% (range, 25%-75%). Although no direct articular reduction was performed, remodeling of the joint surface was consistently noted at follow-up (Figure 2). Mild radiographic degenerative changes were noted at final follow-up in 4 patients, and moderate changes were noted in 1 patient. Radiographic union was achieved in all cases, and no pin-tract infections were noted.
Mean AROM of the PIP joint at final follow-up was 84° (range, 50°-110°), with patients lacking a mean of 7° of full extension and achieving mean flexion of 91°. Mean PROM was 93° (range, 75°-110°). There was no correlation between extent of articular surface involvement and ROM. Furthermore, no correlation was found between time from injury to surgery and ROM. Patients regained full grip strength in the operative hand. At final follow-up, mean grip strength was 79.4 pounds in the operative hand and 79.6 pounds in the contralateral hand, demonstrating equal grip strengths bilaterally.
Patients overall had very low levels of pain; mean VAS score was 0.64 (range, 0-3). Mean QuickDASH score was 5.7 (range, 0-30), suggesting minimal functional impairment. One patient developed a malunion of the middle phalanx fracture resulting in a rotational deformity and required corrective osteotomy. This patient’s VAS score (3) and QuickDASH score (30) were significantly higher than those of the other patients in the study. No other complications were noted by final follow-up.
A higher level of patient satisfaction was found to be directly related to length of follow-up (P < .05). Satisfaction was inversely related to higher VAS score (P < .05) and higher QuickDASH score (P < .001). Pain at work correlated with lower satisfaction level (P < .05). There was no correlation between patient satisfaction and AROM or PROM.
Discussion
The results of this study demonstrate the efficacy of EBP in the treatment of dorsal PIP joint fracture-dislocations. EBP maintained joint dislocation reduction and allowed for early mobilization, which resulted in good ROM, minimal pain, and good functional outcomes. Of note, postoperative patient satisfaction correlated with pain but not with ROM. It is possible that EBP yielded sufficient functional ROM in all patients such that improvement beyond this threshold did not lead to further improvement in satisfaction. Hume and colleagues23 found that mean PIP joint flexion of 60° is needed for activities of daily living. As mean PIP active flexion was 91° (range, 70°-105°) in the present study, it is possible that satisfaction did not correlate with ROM, as all 12 patients achieved active flexion of more than 60°. Despite the lack of correlation between ROM and satisfaction, early PIP joint mobilization is likely a key contributor to positive outcomes because of its significant role in cartilage healing.24
Postoperative ROM in the present study is consistent with that in other reports of patients with PIP joint fracture-dislocations treated with EBP.11,12,22 In a study by Inoue and Tamura,11 14 such patients had mean PIP ROM of 94° at a mean follow-up of 14 months. Viegas22 followed a series of 3 patients for a mean of 7 weeks. At final follow-up, their mean PIP arc of motion was 71°; they lacked 12° of full extension and achieved 83° of flexion. The larger PIP arc of motion (84°) found in the present study may be due to our significantly longer follow-up (35 months). Unlike us, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM. Our finding a lack of correlation may be a result of the significant amount of joint remodeling noted on follow-up radiographs.
Studies of transarticular pinning of PIP joints after dorsal PIP fracture-dislocations have reported outcomes similar to ours.25,26 Newington and colleagues25 evaluated 10 cases of transarticular pinning of the PIP joint and found mean arc of motion of 85° and equal grip strengths between injured and contralateral hands. In a series of 19 patients with PIP fracture-dislocations, Aladin and Davis26 noted similar outcomes of transarticular K-wire fixation and ORIF. In both of their treatment groups, however, there was evidence of PIP joint incongruity and subluxation. Of note, PIP arc motion was lower in their study than in ours.
Recent studies have evaluated unstable PIP fracture-dislocations treated with both EBP and percutaneous reduction and pinning with a second K-wire.13,27 At a mean follow-up of 18 months, Vitale and colleagues13 noted maintenance of concentric fracture reduction, good PIP ROM (mean range, 4°-93°), and low VAS and DASH scores (1.4 and 8, respectively). Waris and Alanen27 noted mean PIP AROM of 83° and low VAS and DASH scores (1 and 4, respectively). The EBP technique used in the present study did not involve percutaneous fracture reduction but achieved equally good ROM and VAS and QuickDASH scores.
Clinical outcomes of EBP of PIP joint fracture-dislocations are also comparable to outcomes of more complex treatment methods.8-10,15-19,21,26,28-33 Dynamic distraction external fixation has led to equally good ROM (mean AROM, 80°-85°15,16) and VAS scores, but with a higher incidence of pin-site infection.14-17 ORIF of the intra-articular middle phalanx fracture has the advantage of obtaining a direct anatomical reduction, but clinical outcomes are similar to those in the present study (mean AROM, 70°; 78% pain-free9), and flexion contractures have been noted.8-10 Furthermore, reduction of the fractured PIP joint articular surface has not been shown to be necessary for good outcomes.16,34 This may be explained in part by PIP joint remodeling, which has been routinely observed on long-term follow-up by the senior authors of the present study. Hemi-hamate autografting and volar plate arthroplasty are other options that have had promising results in the treatment of acute and chronic unstable PIP fracture-dislocations.18-21 However, the postoperative ROM (mean AROM, 61°-85°18,21), VAS scores, and patient satisfaction (91% very satisfied21) of these operations are similar to those of EBP in the present study and may not justify the longer operative times and technical challenges associated with these techniques.
We believe that our study group’s 1 complication, a malunion that was treated with corrective osteotomy, resulted from lack of appreciation of the degree of injury. The teenaged female patient’s index finger PIP joint had a rotational malalignment that was not appreciated before or during surgery. After pinning and after ROM was restored, the index finger was observed crossing over the middle finger with digital flexion. The patient returned to the operating room for corrective osteotomy.
We recommend that surgeons assess alignment carefully, before and during surgery, when considering this technique. Although complications are rare, the technique is not for patients with rotational malalignment; ORIF may be more suitable in these cases. In addition, though EBP may be appropriate for pilon-type injuries, as it allows for early AROM, our procedure of choice for pilon fracture is dynamic external fixation, which in addition to allowing for AROM provides ligamentotaxis. In the event that a large volar articular fragment extends into the middle phalanx diaphysis, we typically proceed with ORIF through a volar shotgun approach. At our institution, injuries lasting more than 3 months are often treated with volar plate arthroplasty or hemi-hamate resurfacing. Finally, we believe that caution should be exercised when using this technique in patients with more than 50% articular involvement. In the present study, though we used this treatment in cases of up to 75% surface involvement, alternative techniques, such as hemi-hamate resurfacing arthroplasty, may provide a better volar bony buttress and limit the risk for recurrent instability. Despite its relative contraindications, our technique has been appropriate for more than 90% of the acute PIP fracture-dislocations we have seen.
This study expands on prior research by demonstrating good function, satisfaction, and pain outcomes of percutaneous EBP in the treatment of unstable dorsal PIP fracture-dislocations. In addition, this study demonstrated that the efficacy of EBP is similar to that of more complex and technically demanding methods of treatment. Our technique has the advantage of simplicity. It obviates the soft-tissue damage required for ORIF and more complex fixation techniques. Furthermore, use of this simple technique may save time and costs and lead to more reproducible outcomes.
One limitation of this study is its small sample size. It is possible that outcomes may have been different with a larger sample. Furthermore, we did not make a direct comparison with other treatment methods. To better determine the optimal treatment method for this fracture type, future studies should prospectively evaluate outcomes for multiple treatment modalities in a randomized fashion.
1. Leibovic SJ, Bowers WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10(2):169-178.
2. Kiefhaber TR, Stern PJ. Fracture dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1998;23(3):368-380.
3. Ng CY, Oliver CW. Fractures of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Br. 2009;91(6):705-712.
4. Isani A. Small joint injuries requiring surgical treatment. Orthop Clin North Am. 1986;17(3):407-419.
5. McElfresh EC, Dobyns JH, O’Brien ET. Management of fracture-dislocation of the proximal interphalangeal joints by extension-block splinting. J Bone Joint Surg Am. 1972;54(8):1705-1711.
6. Hastings H 2nd, Carroll C 4th. Treatment of closed articular fractures of the metacarpophalangeal and proximal interphalangeal joints. Hand Clin. 1988;4(3):503-527.
7. O’Rourke SK, Gaur S, Barton NJ. Long-term outcome of articular fractures of the phalanges: an eleven year follow up. J Hand Surg Br. 1989;14(2):183-193.
8. Grant I, Berger AC, Tham SK. Internal fixation of unstable fracture dislocations of the proximal interphalangeal joint. J Hand Surg Br. 2005;30(5):492-498.
9. Hamilton SC, Stern PJ, Fassler PR, Kiefhaber TR. Mini-screw fixation for the treatment of proximal interphalangeal joint dorsal fracture-dislocations. J Hand Surg Am. 2006;31(8):1349-1354.
10. Lee JY, Teoh LC. Dorsal fracture dislocations of the proximal interphalangeal joint treated by open reduction and interfragmentary screw fixation: indications, approaches and results. J Hand Surg Br. 2006;31(2):138-146.
11. Inoue G, Tamura Y. Treatment of fracture-dislocation of the proximal interphalangeal joint using extension-block Kirschner wire. Ann Chir Main Memb Super. 1991;10(6):564-568.
12. Sugawa I, Otani K, Kobayashi A. Treatment of fracture dislocation PIP-joint by Kirschner wire extension block method. Cent Jpn J Orthop Traumat. 1979;22:1409-1412.
13. Vitale MA, White NJ, Strauch RJ. A percutaneous technique to treat unstable dorsal fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2011;36(9):1453-1459.
14. Badia A, Riano F, Ravikoff J, Khouri R, Gonzalez-Hernandez E, Orbay JL. Dynamic intradigital external fixation for proximal interphalangeal joint fracture dislocations. J Hand Surg Am. 2005;30(1):154-160.
15. Ellis SJ, Cheng R, Prokopis P, et al. Treatment of proximal interphalangeal dorsal fracture-dislocation injuries with dynamic external fixation: a pins and rubber band system. J Hand Surg Am. 2007;32(8):1242-1250.
16. Morgan JP, Gordon DA, Klug MS, Perry PE, Barre PS. Dynamic digital traction for unstable comminuted intra-articular fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1995;20(4):565-573.
17. Ruland RT, Hogan CJ, Cannon DL, Slade JF. Use of dynamic distraction external fixation for unstable fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2008;33(1):19-25.
18. Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25(3):429-437.
19. Durham-Smith G, McCarten GM. Volar plate arthroplasty for closed proximal interphalangeal joint injuries. J Hand Surg Br. 1992;17(4):422-428.
20. Calfee RP, Kiefhaber TR, Sommerkamp TG, Stern PJ. Hemi-hamate arthroplasty provides functional reconstruction of acute and chronic proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2009;34(7):1232-1241.
21. Williams RM, Kiefhaber TR, Sommerkamp TG, Stern PJ. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg Am. 2003;28(5):856-865.
22. Viegas SF. Extension block pinning for proximal interphalangeal joint fracture dislocations: preliminary report of a new technique. J Hand Surg Am. 1992;17(5):896-901.
23. Hume MC, Gellman H, McKellop H, Brumfield RH Jr. Functional range of motion of the joints of the hand. J Hand Surg Am. 1990;15(2):240-243.
24. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10(2):211-220.
25. Newington DP, Davis TR, Barton NJ. The treatment of dorsal fracture-dislocation of the proximal interphalangeal joint by closed reduction and Kirschner wire fixation: a 16-year follow up. J Hand Surg Br. 2001;26(6):537-540.
26. Aladin A, Davis TR. Dorsal fracture-dislocation of the proximal interphalangeal joint: a comparative study of percutaneous Kirschner wire fixation versus open reduction and internal fixation. J Hand Surg Br. 2005;30(2):120-128.
27. Waris E, Alanen V. Percutaneous, intramedullary fracture reduction and extension block pinning for dorsal proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2010;35(12):2046-2052.
28. Bain GI, Mehta JA, Heptinstall RJ, Bria M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J Bone Joint Surg Br. 1998;80(6):1014-1019.
29. Eaton RG, Malerich MM. Volar plate arthroplasty of the proximal interphalangeal joint: a review of ten years’ experience. J Hand Surg Am. 1980;5(3):260-268.
30. Green A, Smith J, Redding M, Akelman E. Acute open reduction and rigid internal fixation of proximal interphalangeal joint fracture dislocation. J Hand Surg Am. 1992;17(3):512-517.
31. Inanami H, Ninomiya S, Okutsu I, Tarui T. Dynamic external finger fixator for fracture dislocation of the proximal interphalangeal joint. J Hand Surg Am. 1993;18(1):160-164.
32. Suzuki Y, Matsunaga T, Sato S, Yokoi T. The pins and rubbers traction system for treatment of comminuted intraarticular fractures and fracture-dislocations in the hand. J Hand Surg Br. 1994;19(1):98-107.
33. Weiss AP. Cerclage fixation for fracture dislocation of the proximal interphalangeal joint. Clin Orthop. 1996;(327):21-28.
34. Agee JM. Unstable fracture dislocations of the proximal interphalangeal joint. Treatment with the force couple splint. Clin Orthop. 1987;(214):101-112.
1. Leibovic SJ, Bowers WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10(2):169-178.
2. Kiefhaber TR, Stern PJ. Fracture dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1998;23(3):368-380.
3. Ng CY, Oliver CW. Fractures of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Br. 2009;91(6):705-712.
4. Isani A. Small joint injuries requiring surgical treatment. Orthop Clin North Am. 1986;17(3):407-419.
5. McElfresh EC, Dobyns JH, O’Brien ET. Management of fracture-dislocation of the proximal interphalangeal joints by extension-block splinting. J Bone Joint Surg Am. 1972;54(8):1705-1711.
6. Hastings H 2nd, Carroll C 4th. Treatment of closed articular fractures of the metacarpophalangeal and proximal interphalangeal joints. Hand Clin. 1988;4(3):503-527.
7. O’Rourke SK, Gaur S, Barton NJ. Long-term outcome of articular fractures of the phalanges: an eleven year follow up. J Hand Surg Br. 1989;14(2):183-193.
8. Grant I, Berger AC, Tham SK. Internal fixation of unstable fracture dislocations of the proximal interphalangeal joint. J Hand Surg Br. 2005;30(5):492-498.
9. Hamilton SC, Stern PJ, Fassler PR, Kiefhaber TR. Mini-screw fixation for the treatment of proximal interphalangeal joint dorsal fracture-dislocations. J Hand Surg Am. 2006;31(8):1349-1354.
10. Lee JY, Teoh LC. Dorsal fracture dislocations of the proximal interphalangeal joint treated by open reduction and interfragmentary screw fixation: indications, approaches and results. J Hand Surg Br. 2006;31(2):138-146.
11. Inoue G, Tamura Y. Treatment of fracture-dislocation of the proximal interphalangeal joint using extension-block Kirschner wire. Ann Chir Main Memb Super. 1991;10(6):564-568.
12. Sugawa I, Otani K, Kobayashi A. Treatment of fracture dislocation PIP-joint by Kirschner wire extension block method. Cent Jpn J Orthop Traumat. 1979;22:1409-1412.
13. Vitale MA, White NJ, Strauch RJ. A percutaneous technique to treat unstable dorsal fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2011;36(9):1453-1459.
14. Badia A, Riano F, Ravikoff J, Khouri R, Gonzalez-Hernandez E, Orbay JL. Dynamic intradigital external fixation for proximal interphalangeal joint fracture dislocations. J Hand Surg Am. 2005;30(1):154-160.
15. Ellis SJ, Cheng R, Prokopis P, et al. Treatment of proximal interphalangeal dorsal fracture-dislocation injuries with dynamic external fixation: a pins and rubber band system. J Hand Surg Am. 2007;32(8):1242-1250.
16. Morgan JP, Gordon DA, Klug MS, Perry PE, Barre PS. Dynamic digital traction for unstable comminuted intra-articular fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1995;20(4):565-573.
17. Ruland RT, Hogan CJ, Cannon DL, Slade JF. Use of dynamic distraction external fixation for unstable fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2008;33(1):19-25.
18. Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25(3):429-437.
19. Durham-Smith G, McCarten GM. Volar plate arthroplasty for closed proximal interphalangeal joint injuries. J Hand Surg Br. 1992;17(4):422-428.
20. Calfee RP, Kiefhaber TR, Sommerkamp TG, Stern PJ. Hemi-hamate arthroplasty provides functional reconstruction of acute and chronic proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2009;34(7):1232-1241.
21. Williams RM, Kiefhaber TR, Sommerkamp TG, Stern PJ. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg Am. 2003;28(5):856-865.
22. Viegas SF. Extension block pinning for proximal interphalangeal joint fracture dislocations: preliminary report of a new technique. J Hand Surg Am. 1992;17(5):896-901.
23. Hume MC, Gellman H, McKellop H, Brumfield RH Jr. Functional range of motion of the joints of the hand. J Hand Surg Am. 1990;15(2):240-243.
24. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10(2):211-220.
25. Newington DP, Davis TR, Barton NJ. The treatment of dorsal fracture-dislocation of the proximal interphalangeal joint by closed reduction and Kirschner wire fixation: a 16-year follow up. J Hand Surg Br. 2001;26(6):537-540.
26. Aladin A, Davis TR. Dorsal fracture-dislocation of the proximal interphalangeal joint: a comparative study of percutaneous Kirschner wire fixation versus open reduction and internal fixation. J Hand Surg Br. 2005;30(2):120-128.
27. Waris E, Alanen V. Percutaneous, intramedullary fracture reduction and extension block pinning for dorsal proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2010;35(12):2046-2052.
28. Bain GI, Mehta JA, Heptinstall RJ, Bria M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J Bone Joint Surg Br. 1998;80(6):1014-1019.
29. Eaton RG, Malerich MM. Volar plate arthroplasty of the proximal interphalangeal joint: a review of ten years’ experience. J Hand Surg Am. 1980;5(3):260-268.
30. Green A, Smith J, Redding M, Akelman E. Acute open reduction and rigid internal fixation of proximal interphalangeal joint fracture dislocation. J Hand Surg Am. 1992;17(3):512-517.
31. Inanami H, Ninomiya S, Okutsu I, Tarui T. Dynamic external finger fixator for fracture dislocation of the proximal interphalangeal joint. J Hand Surg Am. 1993;18(1):160-164.
32. Suzuki Y, Matsunaga T, Sato S, Yokoi T. The pins and rubbers traction system for treatment of comminuted intraarticular fractures and fracture-dislocations in the hand. J Hand Surg Br. 1994;19(1):98-107.
33. Weiss AP. Cerclage fixation for fracture dislocation of the proximal interphalangeal joint. Clin Orthop. 1996;(327):21-28.
34. Agee JM. Unstable fracture dislocations of the proximal interphalangeal joint. Treatment with the force couple splint. Clin Orthop. 1987;(214):101-112.
Potential Utility of Liposome Bupivacaine in Orthopedic Surgery
Approximately 5.5 million patients undergo orthopedic surgery in the United States each year, and more than 1 million of the procedures are total knee arthroplasty (TKA) or total hip arthroplasty.1 From its 2010 level, demand for joint arthroplasty is expected to double by 2020 and quadruple by 2030.2
About half the patients who have major joint arthroplasty experience severe postsurgical pain.3 Because postsurgical pain may persist for days or weeks, and inadequate treatment is associated with negative outcomes, achieving effective postsurgical analgesia is an important consideration.4-7 Complications of inadequate postsurgical pain management include thromboembolic or pulmonary complications, development of chronic pain, and decrements in health-related quality of life.4,8
In patients who have orthopedic surgery, the inability to adequately control postsurgical pain has been associated with increased hospital length of stay (LOS), delayed time to ambulation, and reduced capacity for exercise.9-12 A recent study involving 4709 patients who had hip or knee arthroplasty found that postsurgical pain relief was the second most highly correlated factor with respect to overall patient satisfaction (how well surgery met patient expectations was the most highly correlated factor),13 suggesting that postsurgical analgesia should be a focus of surgical practice.
A prolonged-release liposomal formulation of the local anesthetic bupivacaine is now available. Bupivacaine liposome injectable suspension (Exparel; Pacira Pharmaceuticals, Inc., Parsippany, New Jersey) is indicated for administration into the surgical site to produce postsurgical analgesia.14 In this article, we review evidence from clinical studies regarding the potential contribution of liposome bupivacaine to improving postsurgical pain management when used as part of a multimodal analgesic regimen in patients undergoing orthopedic surgery.
Postsurgical Pain Management in Orthopedic Surgery
Frequently Used Modalities
Analgesic modalities commonly used for perioperative pain management include central (eg, epidural),4,10,15,16 central regional (eg, neuraxial),4 peripheral regional (eg, peripheral nerve blocks, local/regional surgical site infiltration, intra-articular administration),4,10,15,17-25 and intravenous (IV) patient-controlled analgesia.4,10,25 These pharmacologic interventions may be augmented by nonpharmacologic modalities (eg, transcutaneous electrical nerve stimulation).26
Pharmacologic treatment options for perioperative pain management include opioids, local anesthetics, clonidine, ketamine, nonsteroidal anti-inflammatory drugs, acetaminophen, and calcium-channel blockers.4,26-28 In TKA, “drug cocktails” (eg, combinations of ropivacaine, ketorolac, epinephrine, and clonidine) for regional or intra-articular injection can also provide effective immediate postsurgical analgesia.25 Although opioids are the most commonly used analgesics for management of orthopedic perioperative pain,25 their use is often associated with adverse effects (AEs), including constipation or ileus, nausea, sedation, dizziness, pruritus, urinary retention, and respiratory depression.6
Multimodal Analgesic Regimens for Postsurgical Pain Management
Current American Society of Anesthesiologists guidelines endorse use of multimodal analgesia, whenever possible, to provide effective management of acute perioperative pain.4 Multimodal analgesia involves applying 2 or more agents with different mechanisms of action to achieve a synergistic effect, which allows each agent to be reduced in dose4,28 and thereby may limit the risk and severity of dose-related AEs.4,25,28
Multimodal analgesia aims to reduce the risk for opioid-related AEs (ORAEs) and the impact of opioids on postsurgical milestones (eg, ambulation, discharge) and may reduce opioid consumption, with attendant reductions in ORAE risk.29,30 Health economics studies have shown that postsurgical ORAEs are associated with increased hospital costs and LOS.6 In a study using a national hospital database, development of an ORAE (vs no ORAE) in postsurgical patients was associated with mean increases of about $4700 in hospital costs and 3.3 days in LOS.7 Reducing postsurgical opioid use may also help reduce the risk for opioid abuse, addiction, and diversion.31-33
One approach to reducing opioid use involves continuous or intermittent administration of local anesthetics by elastomeric pumps to extend duration of postsurgical analgesia.34-36 However, use of elastomeric pumps has been associated with risk for AEs, including tissue necrosis, sloughing, wound infection, and chondrolysis.37-40 In addition, AEs related to “dose dumping” (accidental delivery of excessive doses) have been reported.40-44 Key issues that may negatively affect rehabilitation after orthopedic surgery include consistency and accuracy of analgesic delivery and the potential for motor block–induced muscle weakness, which may lead to falls and constrain ambulation.45-47
Liposome Bupivacaine
Description
Drug Delivery Technology. Liposome bupivacaine incorporates DepoFoam drug delivery technology (Pacira Pharmaceuticals, Inc.) to facilitate prolonged release of bupivacaine. This technology is based on creation of multivesicular liposome particles (diameter, 10-30 µm) with multiple aqueous chambers.30,48 After administration into the surgical site, bupivacaine diffuses from chambers in the liposomal particles over time, providing analgesia and reduced opioid requirements for up to 72 hours.29,30
Indication, Mechanism of Action, Pharmacokinetics, and Dose/Administration. Liposome bupivacaine is indicated for single-dose administration into the surgical site to produce postsurgical analgesia in patients at least 18 years old.14 Like other local anesthetics, liposome bupivacaine is thought to exert its pharmacologic effects by interacting with voltage-gated Na+ channels on neural membranes to raise the threshold for electrical excitability, to slow nerve impulse propagation, and to reduce the rate of rise of the action potential.14,49
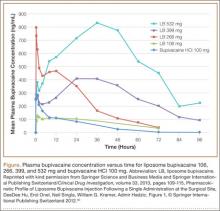
Liposome bupivacaine has dose-proportional pharmacokinetics.50 Presence of a small amount of extra-liposomal bupivacaine in the formulation leads to a bimodal pharmacokinetic profile, with an initial peak serum concentration about 1 hour after administration, followed by a second peak within 12 to 36 hours (Figure).50
Maximum amount of liposome bupivacaine approved for single administration is 266 mg (packaged as 20 mL of a 1.3% solution). However, product labeling includes safety data associated with doses of 532 mg or less.14 The appropriate volume to be used should be based on the amount required to cover the surgical area. Liposome bupivacaine may be expanded with preservative-free normal (0.9%) sterile saline to a total volume of 300 mL: 20 mL liposome bupivacaine plus 280 mL or less diluent, with final concentration of 0.89 mg/mL (1:14 by volume).14
A 25-gauge or larger bore needle should be used to slowly inject liposome bupivacaine into soft tissues of the surgical site, with frequent aspiration to check for blood to minimize risk for intravascular injection.14 Total volume used and fraction injected in specific regions of the surgical site depend on the procedure. For example, a TKA study used 266 mg diluted to a total volume of 60 mL, with 8 mL infiltrated to the area around the medial capsule, 8 mL around the lateral capsule, 12 mL around the posterior capsule, 8 mL around the peripatellar area, 12 mL into the capsulotomy incision, and 12 mL into the subcutaneous tissue on each side of the incision.51
Efficacy
Multiple Surgical Settings. The efficacy of liposome bupivacaine, either alone or as a component of a multimodal analgesic regimen, has been evaluated in a series of 10 phase 2 and 3 studies (8 active-controlled, 2 placebo-controlled) involving 823 patients undergoing TKA, bunionectomy, hemorrhoidectomy, inguinal hernia repair, or mammoplasty.52 Patients received a single liposome bupivacaine dose ranging from 66 to 532 mg.52
Combined analyses of efficacy data from these studies found that liposome bupivacaine–based multimodal analgesic regimens produced postsurgical analgesia for up to 72 hours, increased time to first use of opioid rescue medication after surgery, and reduced total amount of postsurgical opioid consumption versus placebo.52
Compared with standard of care, liposome bupivacaine has been shown to provide effective analgesia in open-label studies in patients undergoing open colectomy,53 laparoscopic colectomy,54 and ileostomy reversal,55,56 as reflected in assessments of postsurgical opioid consumption, LOS, and hospital costs. It has also been studied when administered by infiltration into the transversus abdominis plane (TAP) in patients having laparoscopic prostatectomy and open abdominal hernia repair.57,58
Orthopedic Surgery. In a phase 2 randomized, double-blind, dose-ranging study, TKA patients (N = 138) received bupivacaine HCl 150 mg or liposome bupivacaine 133, 266, 399, or 532 mg administered by local infiltration into the capsulotomy incision and on either side of the incision before wound closure.51 Postsurgical rescue analgesia was available to all patients. Cumulative pain intensity scores with activity (primary efficacy measure) were not statistically different between liposome bupivacaine groups and the bupivacaine HCl group through postoperative day 4. Mean scores in the liposome bupivacaine 266-, 399-, and 532-mg groups were numerically lower than for those treated with bupivacaine HCl on postoperative days 2 to 5, with all doses of liposome bupivacaine having a statistically significant lower pain score at rest on day 5. There were no statistically significant differences across treatment groups with respect to total amount of postsurgical opioids used.
In a phase 3 randomized, double-blind study of TKA patients (N = 245), liposome bupivacaine 532 mg administered into the surgical site was compared with bupivacaine HCl 200 mg for postsurgical analgesia.52 Rescue analgesia was available to all patients. No statistically significant between-group differences were found with respect to postsurgical cumulative pain scores through 72 hours (primary efficacy endpoint).
In a single-center retrospective TKA study, postsurgical outcomes in a patient cohort that received intraoperative periarticular infiltration with liposome bupivacaine 266 mg (n = 65) were compared with a cohort that received infiltration with a combination of ropivacaine 400 mg, morphine 5 mg, and epinephrine 0.4 mg (n = 85).59 Patient-reported postsurgical pain scores were similar in the 2 treatment groups during the first 24 hours after surgery and at discharge. Mean (SD) pain scores during hospitalization after the first 24 hours until discharge were significantly (P = .04) higher in the liposome bupivacaine group, 4.9 (1.4), than in the periarticular group, 4.4 (1.6). There was no significant difference between the 2 treatment groups in postsurgical opioid use. The study demonstrated no advantage to using liposome bupivacaine injections with respect to pain relief, but it was a retrospective review in which pain scores were obtained from electronic medical records. It is essential that liposome bupivacaine be compared with intra-articular injections in well-designed randomized trials.
Another single-center, matched-cohort TKA study (N = 200) compared a liposome bupivacaine regimen with femoral nerve block.60 Compared with patients who received femoral nerve block, patients who received liposome bupivacaine reported lower pain intensity scores after surgery and had shorter LOS, reduced costs, and improved knee flexion at follow-up.60
Results from 2 other studies were presented at the 2014 meeting of the American Academy of Orthopaedic Surgeons (AAOS). One was a single-center, matched-cohort TKA study (N = 72) comparing infiltration of a single dose of liposome bupivacaine into the surgical site with continuous femoral nerve block.61 The 2 treatment groups had similar mean postsurgical pain intensity scores on a 0-to-10 visual analog scale, 1.8 for liposome bupivacaine and 2.3 for continuous nerve block (P = NS), but total amount of postsurgical opioids (hydrocodone-equivalent milligrams) was significantly (P < .0001) less in the liposome bupivacaine group (82 vs 177 mg).
The other study presented at the AAOS meeting was a larger, prospective case–control study comparing outcomes between 1000 patients who had total joint arthroplasty (TJA) with liposome bupivacaine and 1000 control patients who had TJA without liposome bupivacaine.62 For the control and liposome bupivacaine cohorts, respectively, mean postsurgical pain intensity scores were 2.41 and 1.98 (P < .0001), mean LOS was 2.83 days and 2.66 days (P < .02), and incidence of falls was 1.0% and 0.2% (P = .02). Average per-patient costs were $1246 lower in the liposome bupivacaine cohort.
A pivotal phase 3 placebo-controlled study compared liposome bupivacaine 106 mg with placebo in patients undergoing bunionectomy (N = 193).5 Rescue medication was available to all patients. Cumulative pain scores were significantly (P = .0005) lower in the liposome bupivacaine group (125) than in the placebo group (146) through 24 hours after surgery (primary efficacy measure) and significantly (P = .0229) lower (197 vs 220) through 36 hours. Median time to first use of rescue opioids was delayed in favor of the liposome bupivacaine group (7.2 vs 4.3 hours; P < .0001). Mean total number of opioid tablets used within 24 hours after surgery was also significantly lower (3.8 vs 4.7; P = .008), and a larger percentage of patients in the liposome bupivacaine group avoided opioid use altogether through 24 hours (7% vs 1%; P = .04).
Efficacy data for liposome bupivacaine appear promising for relief of pain after joint arthroplasty and other orthopedic procedures but have their limitations. First, no randomized trials have compared liposome bupivacaine with locally injected pain medications (intra-articular injections in TKA or hip arthroplasty). As these injections are quite common now, such analyses are essential. Second, cost-effectiveness studies are needed for orthopedic procedures. Third, most of the published studies were sponsored by the manufacturer of liposome bupivacaine—a situation that raises questions about potential bias. Non-industry-sponsored randomized trials assessing efficacy, safety, and cost-effectiveness are needed.
Safety
Local anesthetics, including liposome bupivacaine, have the potential for central nervous system (CNS) or cardiac toxicity resulting from excessive systemic absorption or inadvertent IV administration.63 However, reported serious CNS or cardiac-related AEs are rare.63,64
AE Profile. Safety data from 10 phase 2 and 3 studies involving 823 patients who received liposome bupivacaine were evaluated.65 Of these patients, 545 received a dose of 266 mg or less (maximum dose approved by the US Food and Drug Administration [FDA]). Liposome bupivacaine was generally well tolerated. Reported AE incidence was 62% (liposome bupivacaine), 75% (bupivacaine HCl), and 43% (placebo). More than 90% of reported AEs were mild or moderate. The most frequently reported AEs were nausea, constipation, and vomiting (liposome bupivacaine, bupivacaine HCl) and nausea, dizziness, and vomiting (placebo).
Serious AEs were reported in 22 (2.7%) of the 823 patients in the liposome bupivacaine group, 24 (5.4%) of the 446 in the bupivacaine HCl group, and 2 (1.1%) of the 190 in the placebo group.65 None of the serious AEs in the liposome bupivacaine and placebo groups were considered treatment-related. Six patients in the bupivacaine HCl group had treatment-related serious AEs (hypoglycemia, arthrofibrosis, hemarthrosis, joint swelling, scar, knee arthroplasty).
Cardiac Safety. Possible cardiac effects associated with liposome bupivacaine were evaluated with data from studies conducted during the clinical development program.66 One hundred thirty-eight patients participated in the phase 2 safety and efficacy study in TKA. In these patients, a consistent change in mean heart rate (range, +12.2 to +16.5 beats per minute) was found across all liposome bupivacaine doses and with bupivacaine HCl. No clinically relevant changes from baseline in mean electrocardiographic parameters, including QTcF interval (QT interval adjusted using Fridericia’s correction formula), were found. In another analysis,67 liposome bupivacaine administered in a single subcutaneous dose (266, 399, 532, or 665 mg) to healthy volunteers did not prolong (vs placebo) QTc interval.
Wound Healing. The potential effects of liposome bupivacaine on wound healing were evaluated with results from 10 phase 2 and 3 studies.68 The assessments, which varied across studies, included clinicians’ overall satisfaction with patient wound healing, wound status assessment (categories included erythema, drainage, edema, and induration), and wound scarring (categories included pigmentation, height, pliability, and vascularity). Clinician-assessed scores reflected high satisfaction with wound healing overall. There were few statistically significant differences in wound status assessments between liposome bupivacaine and the comparators and no statistically significant differences in scarring between liposome bupivacaine and bupivacaine HCl.
The potential of liposome bupivacaine to have adverse intra-articular effects was assessed with drainage samples from patients (n = 23) who had TKA and received liposome bupivacaine (133, 266, 399, or 532 mg) or bupivacaine HCl (150 mg) by wound infiltration near the intra-articular space.51,65 Only small amounts of bupivacaine were present in drainage fluid collected for 12 hours after liposome bupivacaine administration, comparable to bupivacaine HCl administration.65 Currently, the product is not approved for intra-articular use.
Compatibility With Diluents, Other Medications, and Implant Materials
Liposome bupivacaine may be expanded up to a ratio of 1:14 by volume (to a final total volume of 300 mL or a concentration of 0.89 mg/mL) using preservative-free normal (0.9%) sterile saline for injection.14 It has also been shown in vitro to be compatible with lactated Ringer solution as a diluent.69
Liposome bupivacaine should not be admixed with other medications before administration.14 No formal drug–drug interaction studies have been conducted with liposome bupivacaine, but it has been shown in vitro to be compatible with epinephrine solutions, with certain anti-infective medications (eg, bacitracin, gentamicin, cefazolin, cefuroxime), with certain analgesics (eg, ketorolac, morphine), with an antihypertensive medication (clonidine), with an antihemorrhagic medication (tranexamic acid), and with certain corticosteroids (eg, methylprednisolone, triamcinolone acetonide). These medications may be coadministered in the same location as liposome bupivacaine.69
Topical antiseptics (eg, povidone iodine) may be used in surgical procedures involving liposome bupivacaine as long as they are not directly mixed with liposome bupivacaine and are allowed to dry before it is administered. If a topical antiseptic is used for wound irrigation, the wound should be rinsed clear before liposome bupivacaine administration.14,69
Liposome bupivacaine may be coadministered into the same surgical site immediately after bupivacaine HCl as long as the dose ratio of liposome bupivacaine to bupivacaine HCl is 2:1 or higher. Because of the prolonged-release pharmacokinetic profile of liposome bupivacaine and the potential for increased bupivacaine exposure, bupivacaine HCl should not be administered within 96 hours after administration of liposome bupivacaine.14,69
In vitro coincubation studies of liposome bupivacaine and other local anesthetics, including ropivacaine, lidocaine, and mepivacaine, have found rapid release of free bupivacaine from the liposome matrix. Therefore, after giving any of these other local anesthetics, surgeons should wait at least 20 minutes before administering liposome bupivacaine into the same area.14,69
In vitro studies have shown that liposome bupivacaine is compatible with a wide range of commonly used implant materials, including polypropylene, expanded polytetrafluoroethylene, stainless steel, titanium, and smooth- and textured-type silicone.69
Investigational Use and Ongoing Studies
A phase 2 randomized, double-masked, dose-escalating/deescalating study was conducted to evaluate the efficacy, safety, and pharmacokinetics of liposome bupivacaine (155, 199, or 310 mg) in comparison with bupivacaine HCl 125 mg for ankle nerve block in patients undergoing bunionectomy (N = 58).70 The study medication was injected into 3 sites to reach the posterior tibial, sural, deep peroneal, superficial peroneal, and saphenous nerves. Pharmacokinetic exposure was higher for liposome bupivacaine than for bupivacaine HCl, as reflected by a significantly greater area under the curve, lower Cmax (maximum serum concentration), and longer mean half-life. Mean pain intensity scores were lower in the bupivacaine HCl group than in each liposome bupivacaine group the first 12 hours after surgery. However, the liposome bupivacaine 310-mg group had similar or lower scores than the bupivacaine HCl group from 12 to 96 hours after surgery. The most common AEs in the liposome bupivacaine group were gastrointestinal and not treatment-related.70
The efficacy and safety of liposome bupivacaine, administered as a femoral nerve block for postsurgical analgesia, were assessed in a phase 2/3 manufacturer-sponsored, placebo-controlled, multicenter, randomized, double-blind 2-part study (NCT01683071)71 in 280 TKA patients.71,72 Part 2 of the study, comparing liposome bupivacaine 266 mg (n = 116) and placebo (n = 116), met its primary endpoint, demonstrating statistical significance in favor of liposome bupivacaine for cumulative pain scores over 72 hours (P < .0001), with decreased opioid use (P < .05) and a safety profile similar to that of placebo.72
Other ongoing investigator-sponsored studies in orthopedic populations include comparisons of liposome bupivacaine and bupivacaine HCl for ultrasound-guided periarticular hip infiltration in hip arthroplasty (NTC01917191),73 as femoral nerve block in TKA (NCT01977339),74 and as interscalene brachial plexus block in arthroscopic shoulder surgery (NCT01977352).75 The primary efficacy outcome measure in these studies was postsurgical opioid use.73-75
Health Economics
A series of phase 4 health economics studies was conducted for gastrointestinal surgeries, including open colectomy, laparoscopic colectomy, and ileostomy reversal.53-56,76 These studies, of similar design, showed that a liposome bupivacaine–based multimodal analgesic regimen was associated with reduced opioid use, shorter hospital LOS, and lower hospitalization costs in comparison with a traditional opioid-based regimen.53-56 Although pooled analysis of these studies showed a cost savings of more than $2000 per patient and an LOS decrease of 1.4 days,76 all were conducted in the gastrointestinal surgery setting. Studies are needed to fully assess the economic benefits associated with liposome bupivacaine in the orthopedic surgery setting.
Conclusion
Liposome bupivacaine represents a potentially important contributor to multimodal analgesic regimens used to manage postsurgical pain. Liposome bupivacaine has demonstrated efficacy in providing prolonged postsurgical analgesia and reducing postsurgical opioid use in most surgical settings studied. Additional data from health economics studies in gastrointestinal surgery suggest liposome bupivacaine–based multimodal analgesic regimens may also contribute to reductions in hospital LOS and hospitalization costs. Non-industry-sponsored trials are needed to answer these crucial questions in orthopedic surgery settings. Nevertheless, data on the safety and efficacy of liposome bupivacaine for postsurgical analgesia continue to accumulate, and liposome bupivacaine appears to be a feasible therapeutic option for managing postsurgical pain in orthopedic surgery.
1. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Accessed January 30, 2015.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Bonica JJ. Postoperative pain. In: Bonica JJ, ed. The Management of Pain. Malvern, PA: Lea & Febiger; 1990:461-480.
4. Apfelbaum JL, Ashburn MA, Connis RT, et al; American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
5. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28(9):776-788.
6. Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 pt 2):6S-11S.
7. Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27(1):62-70.
8. Wu CL, Naqibuddin M, Rowlingson AJ, Lietman SA, Jermyn RM, Fleisher LA. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97(4):1078-1085.
9. Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303-311.
10. Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91(1):8-15.
11. Capdevila X, Dadure C, Bringuier S, et al. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105(3):566-573.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):12-15.
13. Hamilton DF, Lane JV, Gaston P, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open. 2013;3(4):e002525.
14. Exparel [prescribing information]. Parsippany, NJ: Pacira Pharmaceuticals, Inc.; 2014.
15. DeWeese FT, Akbari Z, Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001;392(11):226-231.
16. Pati AB, Perme D, Trail M, Henry PK, Bryan WJ. Rehabilitation parameters in total knee replacement patients undergoing epidural vs. conventional analgesia. J Orthop Sports Phys Ther. 1994;19(2):88-92.
17. Browne C, Copp S, Reden L, Pulido P, Colwell C Jr. Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty. 2004;19(3):377-380.
18. Nechleba J, Rogers V, Cortina G, Cooney T. Continuous intra-articular infusion of bupivacaine for postoperative pain following total knee arthroplasty. J Knee Surg. 2005;18(3):197-202.
19. Campbell A, McCormick M, McKinlay K, Scott NB. Epidural vs. lumbar plexus infusions following total knee arthroplasty: randomized controlled trial. Eur J Anaesthesiol. 2008;25(6):502-507.
20. Serpell MG, Millar FA, Thomson MF. Comparison of lumbar plexus block versus conventional opioid analgesia after total knee replacement. Anaesthesia. 1991;46(4):275-277.
21. Lareau JM, Robbins CE, Talmo CT, Mehio AK, Puri L, Bono JV. Complications of femoral nerve blockade in total knee arthroplasty and strategies to reduce patient risk. J Arthroplasty. 2012;27(4):564-568.
22. Charous MT, Madison SJ, Suresh PJ, et al. Continuous femoral nerve blocks: varying local anesthetic delivery method (bolus versus basal) to minimize quadriceps motor block while maintaining sensory block. Anesthesiology. 2011;115(4):774-781.
23. Gottschalk A, Burmeister MA, Radtke P, et al. Continuous wound infiltration with ropivacaine reduces pain and analgesic requirement after shoulder surgery. Anesth Analg. 2003;97(4):1086-1091.
24. Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79(2):174-183.
25. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
26. White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94(3):577-585.
27. Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth. 1991;66(6):703-712.
28. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
29. Candiotti K. Liposomal bupivacaine: an innovative nonopioid local analgesic for the management of postsurgical pain. Pharmacotherapy. 2012;32(9 Pt 2):19S-26S.
30. Bergese SD, Onel E, Portillo J. Evaluation of DepoFoam® bupivacaine for the treatment of postsurgical pain. Pain Manag. 2011;1(6):539-547.
31. Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710-1714.
32. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
33. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
34. Ilfeld BM, Morey TE, Enneking FK. Delivery rate accuracy of portable, bolus-capable infusion pumps used for patient-controlled continuous regional analgesia. Reg Anesth Pain Med. 2003;28(1):17-23.
35. Ganapathy S, Amendola A, Lichfield R, Fowler PJ, Ling E. Elastomeric pumps for ambulatory patient controlled regional analgesia. Can J Anaesth. 2000;47(9):897-902.
36. Bray DA Jr, Nguyen J, Craig J, Cohen BE, Collins DR Jr. Efficacy of a local anesthetic pain pump in abdominoplasty. Plast Reconstr Surg. 2007;119(3):1054-1059.
37. Brown SL, Morrison AE. Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology. 2004;100(5):1305-1307.
38. Noyes FR, Fleckenstein CM, Barber-Westin SD. The development of postoperative knee chondrolysis after intra-articular pain pump infusion of an anesthetic medication: a series of twenty-one cases. J Bone Joint Surg Am. 2012;94(16):1448-1457.
39. Rapley JH, Beavis RC, Barber FA. Glenohumeral chondrolysis after shoulder arthroscopy associated with continuous bupivacaine infusion. Arthroscopy. 2009;25(12):1367-1373.
40. Institute for Safe Medication Practices. Process for handling elastomeric pain relief balls (ON-Q PainBuster and others) requires safety improvements. ISMP Medication Safety Alert. http://www.ismp.org/Newsletters/acutecare/articles/20090716.asp. Accessed January 30, 2015.
41. Pepin JL, Dasta JF, New M. Ensuring safe and economical use of elastomeric infusion devices. Am J Health Syst Pharm. 2011;68(24):2330-2331.
42. Birrer KL, Anderson RL, Liu-DeRyke X, Patel KR. Measures to improve safety of an elastomeric infusion system for pain management. Am J Health Syst Pharm. 2011;68(13):1251-1255.
43. Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg. 2005;100(6):1822-1833.
44. US Food and Drug Administration. Medical device recalls: I-Flow ON-Q Pump with ONDEMAND Bolus Button. http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm317826.htm. Accessed July 15, 2014.
45. Ilfeld BM, Morey TE, Enneking FK. Portable infusion pumps used for continuous regional analgesia: delivery rate accuracy and consistency. Reg Anesth Pain Med. 2003;28(5):424-432.
46. Ganapathy S. Wound/intra-articular infiltration or peripheral nerve blocks for orthopedic joint surgery: efficacy and safety issues. Curr Opin Anaesthesiol. 2012;25(5):615-620.
47. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554.
48. Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam™: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45(12):1153-1176.
49. Catterall WA, Mackie K. Local anesthetics. In: Gutstein HB, Akil H, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:565-582.
50. Hu D, Onel E, Singla N, Kramer WG, Hadzic A. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33(2):109-115.
51. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
52. Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107-116.
53. Cohen SM. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567-572.
54. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res. 2014;76:1-6.
55. Marcet JE, Nfonsam VN, Larach S. An extended paIn relief trial utilizing the infiltration of a long-acting Multivesicular liPosome foRmulation Of bupiVacaine, EXPAREL (IMPROVE): a Phase IV health economic trial in adult patients undergoing ileostomy reversal. J Pain Res. 2013;6:549-555.
56. Vogel JD. Liposome bupivacaine (EXPAREL®) for extended pain relief in patients undergoing ileostomy reversal at a single institution with a fast-track discharge protocol: an IMPROVE phase IV health economics trial. J Pain Res. 2013;6:605-610.
57. Sternlicht A, Shapiro M, Robelen G, Vellayappan U, Tuerk IA. Initial findings using EXPAREL® (bupivacaine liposome injectable suspension) via infiltration into the transversus abdominis plane (TAP) for postsurgical analgesia in robotic prostatectomy (RP). Abstract presented at: Annual Fall Pain Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine; November 15-18, 2012; Miami Beach, FL.
58. Feierman DE, Kronenfeld M, Gupta PM, Younger N, Logvinskiy E. Evaluation of Exparel® use via infiltration into the transversus abdominis plane for prolonged postoperative analgesia in subjects undergoing open abdominal hernia repair. Poster presented at: Annual Meeting of the International Anesthesia Research Society; May 4-7, 2013; San Diego, CA.
59. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
60. Broome B, Backlund I. Rapid recovery pain pathway for total knee arthroplasty results in improved pain management, decreased length of stay, and significant cost savings. Poster presented at: Annual Orthopedic and Spine Summit; September 18-20, 2013; San Antonio, TX.
61. Emerson RH, Barrington JW. Comparison of infiltration with long-acting bupivacaine to a femoral nerve catheter for total knee replacement. Abstract presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA. Abstract P124.
62. Barrington JW. Emerging data in the use of liposome bupivacaine: comparative review in 2,000 TJA patients. Oral presentation presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA.
63. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35(2):152-161.
64. D’Angelo R. Are the new local anesthetics worth their cost? Acta Anaesthesiol Scand. 2000;44(6):639-641.
65. Viscusi ER, Sinatra R, Onel E, Ramamoorthy SL. The safety of liposome bupivacaine, a novel local analgesic formulation. Clin J Pain. 2014;30(2):102-110.
66. Bergese SD, Onel E, Morren M, Morganroth J. Bupivacaine extended-release liposome injection exhibits a favorable cardiac safety profile. Reg Anesth Pain Med. 2012;37(2):145-151.
67. Naseem A, Harada T, Wang D, et al. Bupivacaine extended release liposome injection does not prolong QTc interval in a thorough QT/QTc study in healthy volunteers. J Clin Pharmacol. 2012;52(9):1441-1447.
68. Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35(3):312-320.
69. Kharitonov V. A review of the compatibility of liposome bupivacaine with other drug products and commonly used implant materials. Postgrad Med. 2014;126(1):129-138.
70. Ilfeld BM. Liposome bupivacaine in peripheral nerve blocks and epidural injections to manage postoperative pain. Expert Opin Pharmacother. 2013;14(17):2421-2431.
71. Femoral nerve block with liposome bupivacaine for postsurgical analgesia following total knee arthroplasty [NCT01683071]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01683071?term=NCT01683071%5C&rank=1. Accessed January 30, 2015.
72. Minkowitz H, Matthews A, Puckett C, Melson T. Liposome bupivacaine in femoral nerve block: initial results from a phase 2/3 pivotal study. Poster presented at: Annual Meeting of the American Society of Regional Anesthesia and Pain Medicine; April 3-6, 2014; Chicago, IL.
73. Ultrasound guided local infiltration analgesia for hip arthroscopy [NCT01907191]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01907191?term=NCT01907191&rank=1. Accessed January 30, 2015.
74. Efficacy of single injection femoral nerve block with liposomal bupivacaine for total knee arthroplasty [NCT01977339]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977339?term=NCT01977339&rank=1. Accessed January 30, 2015.
75. Efficacy of interscalene brachial plexus block with liposomal bupivacaine for arthroscopic shoulder surgery [NCT01977352]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977352?term=NCT01977352&rank=1. Accessed January 30, 2015.
76. Cohen SM, Vogel JD, Marcet JE, Candiotti K. Liposome bupivacaine for improvement in economic outcomes and opioid burden in GI surgery: IMPROVE study pooled analysis. J Pain Res. 2014;7:359-366.
Approximately 5.5 million patients undergo orthopedic surgery in the United States each year, and more than 1 million of the procedures are total knee arthroplasty (TKA) or total hip arthroplasty.1 From its 2010 level, demand for joint arthroplasty is expected to double by 2020 and quadruple by 2030.2
About half the patients who have major joint arthroplasty experience severe postsurgical pain.3 Because postsurgical pain may persist for days or weeks, and inadequate treatment is associated with negative outcomes, achieving effective postsurgical analgesia is an important consideration.4-7 Complications of inadequate postsurgical pain management include thromboembolic or pulmonary complications, development of chronic pain, and decrements in health-related quality of life.4,8
In patients who have orthopedic surgery, the inability to adequately control postsurgical pain has been associated with increased hospital length of stay (LOS), delayed time to ambulation, and reduced capacity for exercise.9-12 A recent study involving 4709 patients who had hip or knee arthroplasty found that postsurgical pain relief was the second most highly correlated factor with respect to overall patient satisfaction (how well surgery met patient expectations was the most highly correlated factor),13 suggesting that postsurgical analgesia should be a focus of surgical practice.
A prolonged-release liposomal formulation of the local anesthetic bupivacaine is now available. Bupivacaine liposome injectable suspension (Exparel; Pacira Pharmaceuticals, Inc., Parsippany, New Jersey) is indicated for administration into the surgical site to produce postsurgical analgesia.14 In this article, we review evidence from clinical studies regarding the potential contribution of liposome bupivacaine to improving postsurgical pain management when used as part of a multimodal analgesic regimen in patients undergoing orthopedic surgery.
Postsurgical Pain Management in Orthopedic Surgery
Frequently Used Modalities
Analgesic modalities commonly used for perioperative pain management include central (eg, epidural),4,10,15,16 central regional (eg, neuraxial),4 peripheral regional (eg, peripheral nerve blocks, local/regional surgical site infiltration, intra-articular administration),4,10,15,17-25 and intravenous (IV) patient-controlled analgesia.4,10,25 These pharmacologic interventions may be augmented by nonpharmacologic modalities (eg, transcutaneous electrical nerve stimulation).26
Pharmacologic treatment options for perioperative pain management include opioids, local anesthetics, clonidine, ketamine, nonsteroidal anti-inflammatory drugs, acetaminophen, and calcium-channel blockers.4,26-28 In TKA, “drug cocktails” (eg, combinations of ropivacaine, ketorolac, epinephrine, and clonidine) for regional or intra-articular injection can also provide effective immediate postsurgical analgesia.25 Although opioids are the most commonly used analgesics for management of orthopedic perioperative pain,25 their use is often associated with adverse effects (AEs), including constipation or ileus, nausea, sedation, dizziness, pruritus, urinary retention, and respiratory depression.6
Multimodal Analgesic Regimens for Postsurgical Pain Management
Current American Society of Anesthesiologists guidelines endorse use of multimodal analgesia, whenever possible, to provide effective management of acute perioperative pain.4 Multimodal analgesia involves applying 2 or more agents with different mechanisms of action to achieve a synergistic effect, which allows each agent to be reduced in dose4,28 and thereby may limit the risk and severity of dose-related AEs.4,25,28
Multimodal analgesia aims to reduce the risk for opioid-related AEs (ORAEs) and the impact of opioids on postsurgical milestones (eg, ambulation, discharge) and may reduce opioid consumption, with attendant reductions in ORAE risk.29,30 Health economics studies have shown that postsurgical ORAEs are associated with increased hospital costs and LOS.6 In a study using a national hospital database, development of an ORAE (vs no ORAE) in postsurgical patients was associated with mean increases of about $4700 in hospital costs and 3.3 days in LOS.7 Reducing postsurgical opioid use may also help reduce the risk for opioid abuse, addiction, and diversion.31-33
One approach to reducing opioid use involves continuous or intermittent administration of local anesthetics by elastomeric pumps to extend duration of postsurgical analgesia.34-36 However, use of elastomeric pumps has been associated with risk for AEs, including tissue necrosis, sloughing, wound infection, and chondrolysis.37-40 In addition, AEs related to “dose dumping” (accidental delivery of excessive doses) have been reported.40-44 Key issues that may negatively affect rehabilitation after orthopedic surgery include consistency and accuracy of analgesic delivery and the potential for motor block–induced muscle weakness, which may lead to falls and constrain ambulation.45-47
Liposome Bupivacaine
Description
Drug Delivery Technology. Liposome bupivacaine incorporates DepoFoam drug delivery technology (Pacira Pharmaceuticals, Inc.) to facilitate prolonged release of bupivacaine. This technology is based on creation of multivesicular liposome particles (diameter, 10-30 µm) with multiple aqueous chambers.30,48 After administration into the surgical site, bupivacaine diffuses from chambers in the liposomal particles over time, providing analgesia and reduced opioid requirements for up to 72 hours.29,30
Indication, Mechanism of Action, Pharmacokinetics, and Dose/Administration. Liposome bupivacaine is indicated for single-dose administration into the surgical site to produce postsurgical analgesia in patients at least 18 years old.14 Like other local anesthetics, liposome bupivacaine is thought to exert its pharmacologic effects by interacting with voltage-gated Na+ channels on neural membranes to raise the threshold for electrical excitability, to slow nerve impulse propagation, and to reduce the rate of rise of the action potential.14,49
Liposome bupivacaine has dose-proportional pharmacokinetics.50 Presence of a small amount of extra-liposomal bupivacaine in the formulation leads to a bimodal pharmacokinetic profile, with an initial peak serum concentration about 1 hour after administration, followed by a second peak within 12 to 36 hours (Figure).50
Maximum amount of liposome bupivacaine approved for single administration is 266 mg (packaged as 20 mL of a 1.3% solution). However, product labeling includes safety data associated with doses of 532 mg or less.14 The appropriate volume to be used should be based on the amount required to cover the surgical area. Liposome bupivacaine may be expanded with preservative-free normal (0.9%) sterile saline to a total volume of 300 mL: 20 mL liposome bupivacaine plus 280 mL or less diluent, with final concentration of 0.89 mg/mL (1:14 by volume).14
A 25-gauge or larger bore needle should be used to slowly inject liposome bupivacaine into soft tissues of the surgical site, with frequent aspiration to check for blood to minimize risk for intravascular injection.14 Total volume used and fraction injected in specific regions of the surgical site depend on the procedure. For example, a TKA study used 266 mg diluted to a total volume of 60 mL, with 8 mL infiltrated to the area around the medial capsule, 8 mL around the lateral capsule, 12 mL around the posterior capsule, 8 mL around the peripatellar area, 12 mL into the capsulotomy incision, and 12 mL into the subcutaneous tissue on each side of the incision.51
Efficacy
Multiple Surgical Settings. The efficacy of liposome bupivacaine, either alone or as a component of a multimodal analgesic regimen, has been evaluated in a series of 10 phase 2 and 3 studies (8 active-controlled, 2 placebo-controlled) involving 823 patients undergoing TKA, bunionectomy, hemorrhoidectomy, inguinal hernia repair, or mammoplasty.52 Patients received a single liposome bupivacaine dose ranging from 66 to 532 mg.52
Combined analyses of efficacy data from these studies found that liposome bupivacaine–based multimodal analgesic regimens produced postsurgical analgesia for up to 72 hours, increased time to first use of opioid rescue medication after surgery, and reduced total amount of postsurgical opioid consumption versus placebo.52
Compared with standard of care, liposome bupivacaine has been shown to provide effective analgesia in open-label studies in patients undergoing open colectomy,53 laparoscopic colectomy,54 and ileostomy reversal,55,56 as reflected in assessments of postsurgical opioid consumption, LOS, and hospital costs. It has also been studied when administered by infiltration into the transversus abdominis plane (TAP) in patients having laparoscopic prostatectomy and open abdominal hernia repair.57,58
Orthopedic Surgery. In a phase 2 randomized, double-blind, dose-ranging study, TKA patients (N = 138) received bupivacaine HCl 150 mg or liposome bupivacaine 133, 266, 399, or 532 mg administered by local infiltration into the capsulotomy incision and on either side of the incision before wound closure.51 Postsurgical rescue analgesia was available to all patients. Cumulative pain intensity scores with activity (primary efficacy measure) were not statistically different between liposome bupivacaine groups and the bupivacaine HCl group through postoperative day 4. Mean scores in the liposome bupivacaine 266-, 399-, and 532-mg groups were numerically lower than for those treated with bupivacaine HCl on postoperative days 2 to 5, with all doses of liposome bupivacaine having a statistically significant lower pain score at rest on day 5. There were no statistically significant differences across treatment groups with respect to total amount of postsurgical opioids used.
In a phase 3 randomized, double-blind study of TKA patients (N = 245), liposome bupivacaine 532 mg administered into the surgical site was compared with bupivacaine HCl 200 mg for postsurgical analgesia.52 Rescue analgesia was available to all patients. No statistically significant between-group differences were found with respect to postsurgical cumulative pain scores through 72 hours (primary efficacy endpoint).
In a single-center retrospective TKA study, postsurgical outcomes in a patient cohort that received intraoperative periarticular infiltration with liposome bupivacaine 266 mg (n = 65) were compared with a cohort that received infiltration with a combination of ropivacaine 400 mg, morphine 5 mg, and epinephrine 0.4 mg (n = 85).59 Patient-reported postsurgical pain scores were similar in the 2 treatment groups during the first 24 hours after surgery and at discharge. Mean (SD) pain scores during hospitalization after the first 24 hours until discharge were significantly (P = .04) higher in the liposome bupivacaine group, 4.9 (1.4), than in the periarticular group, 4.4 (1.6). There was no significant difference between the 2 treatment groups in postsurgical opioid use. The study demonstrated no advantage to using liposome bupivacaine injections with respect to pain relief, but it was a retrospective review in which pain scores were obtained from electronic medical records. It is essential that liposome bupivacaine be compared with intra-articular injections in well-designed randomized trials.
Another single-center, matched-cohort TKA study (N = 200) compared a liposome bupivacaine regimen with femoral nerve block.60 Compared with patients who received femoral nerve block, patients who received liposome bupivacaine reported lower pain intensity scores after surgery and had shorter LOS, reduced costs, and improved knee flexion at follow-up.60
Results from 2 other studies were presented at the 2014 meeting of the American Academy of Orthopaedic Surgeons (AAOS). One was a single-center, matched-cohort TKA study (N = 72) comparing infiltration of a single dose of liposome bupivacaine into the surgical site with continuous femoral nerve block.61 The 2 treatment groups had similar mean postsurgical pain intensity scores on a 0-to-10 visual analog scale, 1.8 for liposome bupivacaine and 2.3 for continuous nerve block (P = NS), but total amount of postsurgical opioids (hydrocodone-equivalent milligrams) was significantly (P < .0001) less in the liposome bupivacaine group (82 vs 177 mg).
The other study presented at the AAOS meeting was a larger, prospective case–control study comparing outcomes between 1000 patients who had total joint arthroplasty (TJA) with liposome bupivacaine and 1000 control patients who had TJA without liposome bupivacaine.62 For the control and liposome bupivacaine cohorts, respectively, mean postsurgical pain intensity scores were 2.41 and 1.98 (P < .0001), mean LOS was 2.83 days and 2.66 days (P < .02), and incidence of falls was 1.0% and 0.2% (P = .02). Average per-patient costs were $1246 lower in the liposome bupivacaine cohort.
A pivotal phase 3 placebo-controlled study compared liposome bupivacaine 106 mg with placebo in patients undergoing bunionectomy (N = 193).5 Rescue medication was available to all patients. Cumulative pain scores were significantly (P = .0005) lower in the liposome bupivacaine group (125) than in the placebo group (146) through 24 hours after surgery (primary efficacy measure) and significantly (P = .0229) lower (197 vs 220) through 36 hours. Median time to first use of rescue opioids was delayed in favor of the liposome bupivacaine group (7.2 vs 4.3 hours; P < .0001). Mean total number of opioid tablets used within 24 hours after surgery was also significantly lower (3.8 vs 4.7; P = .008), and a larger percentage of patients in the liposome bupivacaine group avoided opioid use altogether through 24 hours (7% vs 1%; P = .04).
Efficacy data for liposome bupivacaine appear promising for relief of pain after joint arthroplasty and other orthopedic procedures but have their limitations. First, no randomized trials have compared liposome bupivacaine with locally injected pain medications (intra-articular injections in TKA or hip arthroplasty). As these injections are quite common now, such analyses are essential. Second, cost-effectiveness studies are needed for orthopedic procedures. Third, most of the published studies were sponsored by the manufacturer of liposome bupivacaine—a situation that raises questions about potential bias. Non-industry-sponsored randomized trials assessing efficacy, safety, and cost-effectiveness are needed.
Safety
Local anesthetics, including liposome bupivacaine, have the potential for central nervous system (CNS) or cardiac toxicity resulting from excessive systemic absorption or inadvertent IV administration.63 However, reported serious CNS or cardiac-related AEs are rare.63,64
AE Profile. Safety data from 10 phase 2 and 3 studies involving 823 patients who received liposome bupivacaine were evaluated.65 Of these patients, 545 received a dose of 266 mg or less (maximum dose approved by the US Food and Drug Administration [FDA]). Liposome bupivacaine was generally well tolerated. Reported AE incidence was 62% (liposome bupivacaine), 75% (bupivacaine HCl), and 43% (placebo). More than 90% of reported AEs were mild or moderate. The most frequently reported AEs were nausea, constipation, and vomiting (liposome bupivacaine, bupivacaine HCl) and nausea, dizziness, and vomiting (placebo).
Serious AEs were reported in 22 (2.7%) of the 823 patients in the liposome bupivacaine group, 24 (5.4%) of the 446 in the bupivacaine HCl group, and 2 (1.1%) of the 190 in the placebo group.65 None of the serious AEs in the liposome bupivacaine and placebo groups were considered treatment-related. Six patients in the bupivacaine HCl group had treatment-related serious AEs (hypoglycemia, arthrofibrosis, hemarthrosis, joint swelling, scar, knee arthroplasty).
Cardiac Safety. Possible cardiac effects associated with liposome bupivacaine were evaluated with data from studies conducted during the clinical development program.66 One hundred thirty-eight patients participated in the phase 2 safety and efficacy study in TKA. In these patients, a consistent change in mean heart rate (range, +12.2 to +16.5 beats per minute) was found across all liposome bupivacaine doses and with bupivacaine HCl. No clinically relevant changes from baseline in mean electrocardiographic parameters, including QTcF interval (QT interval adjusted using Fridericia’s correction formula), were found. In another analysis,67 liposome bupivacaine administered in a single subcutaneous dose (266, 399, 532, or 665 mg) to healthy volunteers did not prolong (vs placebo) QTc interval.
Wound Healing. The potential effects of liposome bupivacaine on wound healing were evaluated with results from 10 phase 2 and 3 studies.68 The assessments, which varied across studies, included clinicians’ overall satisfaction with patient wound healing, wound status assessment (categories included erythema, drainage, edema, and induration), and wound scarring (categories included pigmentation, height, pliability, and vascularity). Clinician-assessed scores reflected high satisfaction with wound healing overall. There were few statistically significant differences in wound status assessments between liposome bupivacaine and the comparators and no statistically significant differences in scarring between liposome bupivacaine and bupivacaine HCl.
The potential of liposome bupivacaine to have adverse intra-articular effects was assessed with drainage samples from patients (n = 23) who had TKA and received liposome bupivacaine (133, 266, 399, or 532 mg) or bupivacaine HCl (150 mg) by wound infiltration near the intra-articular space.51,65 Only small amounts of bupivacaine were present in drainage fluid collected for 12 hours after liposome bupivacaine administration, comparable to bupivacaine HCl administration.65 Currently, the product is not approved for intra-articular use.
Compatibility With Diluents, Other Medications, and Implant Materials
Liposome bupivacaine may be expanded up to a ratio of 1:14 by volume (to a final total volume of 300 mL or a concentration of 0.89 mg/mL) using preservative-free normal (0.9%) sterile saline for injection.14 It has also been shown in vitro to be compatible with lactated Ringer solution as a diluent.69
Liposome bupivacaine should not be admixed with other medications before administration.14 No formal drug–drug interaction studies have been conducted with liposome bupivacaine, but it has been shown in vitro to be compatible with epinephrine solutions, with certain anti-infective medications (eg, bacitracin, gentamicin, cefazolin, cefuroxime), with certain analgesics (eg, ketorolac, morphine), with an antihypertensive medication (clonidine), with an antihemorrhagic medication (tranexamic acid), and with certain corticosteroids (eg, methylprednisolone, triamcinolone acetonide). These medications may be coadministered in the same location as liposome bupivacaine.69
Topical antiseptics (eg, povidone iodine) may be used in surgical procedures involving liposome bupivacaine as long as they are not directly mixed with liposome bupivacaine and are allowed to dry before it is administered. If a topical antiseptic is used for wound irrigation, the wound should be rinsed clear before liposome bupivacaine administration.14,69
Liposome bupivacaine may be coadministered into the same surgical site immediately after bupivacaine HCl as long as the dose ratio of liposome bupivacaine to bupivacaine HCl is 2:1 or higher. Because of the prolonged-release pharmacokinetic profile of liposome bupivacaine and the potential for increased bupivacaine exposure, bupivacaine HCl should not be administered within 96 hours after administration of liposome bupivacaine.14,69
In vitro coincubation studies of liposome bupivacaine and other local anesthetics, including ropivacaine, lidocaine, and mepivacaine, have found rapid release of free bupivacaine from the liposome matrix. Therefore, after giving any of these other local anesthetics, surgeons should wait at least 20 minutes before administering liposome bupivacaine into the same area.14,69
In vitro studies have shown that liposome bupivacaine is compatible with a wide range of commonly used implant materials, including polypropylene, expanded polytetrafluoroethylene, stainless steel, titanium, and smooth- and textured-type silicone.69
Investigational Use and Ongoing Studies
A phase 2 randomized, double-masked, dose-escalating/deescalating study was conducted to evaluate the efficacy, safety, and pharmacokinetics of liposome bupivacaine (155, 199, or 310 mg) in comparison with bupivacaine HCl 125 mg for ankle nerve block in patients undergoing bunionectomy (N = 58).70 The study medication was injected into 3 sites to reach the posterior tibial, sural, deep peroneal, superficial peroneal, and saphenous nerves. Pharmacokinetic exposure was higher for liposome bupivacaine than for bupivacaine HCl, as reflected by a significantly greater area under the curve, lower Cmax (maximum serum concentration), and longer mean half-life. Mean pain intensity scores were lower in the bupivacaine HCl group than in each liposome bupivacaine group the first 12 hours after surgery. However, the liposome bupivacaine 310-mg group had similar or lower scores than the bupivacaine HCl group from 12 to 96 hours after surgery. The most common AEs in the liposome bupivacaine group were gastrointestinal and not treatment-related.70
The efficacy and safety of liposome bupivacaine, administered as a femoral nerve block for postsurgical analgesia, were assessed in a phase 2/3 manufacturer-sponsored, placebo-controlled, multicenter, randomized, double-blind 2-part study (NCT01683071)71 in 280 TKA patients.71,72 Part 2 of the study, comparing liposome bupivacaine 266 mg (n = 116) and placebo (n = 116), met its primary endpoint, demonstrating statistical significance in favor of liposome bupivacaine for cumulative pain scores over 72 hours (P < .0001), with decreased opioid use (P < .05) and a safety profile similar to that of placebo.72
Other ongoing investigator-sponsored studies in orthopedic populations include comparisons of liposome bupivacaine and bupivacaine HCl for ultrasound-guided periarticular hip infiltration in hip arthroplasty (NTC01917191),73 as femoral nerve block in TKA (NCT01977339),74 and as interscalene brachial plexus block in arthroscopic shoulder surgery (NCT01977352).75 The primary efficacy outcome measure in these studies was postsurgical opioid use.73-75
Health Economics
A series of phase 4 health economics studies was conducted for gastrointestinal surgeries, including open colectomy, laparoscopic colectomy, and ileostomy reversal.53-56,76 These studies, of similar design, showed that a liposome bupivacaine–based multimodal analgesic regimen was associated with reduced opioid use, shorter hospital LOS, and lower hospitalization costs in comparison with a traditional opioid-based regimen.53-56 Although pooled analysis of these studies showed a cost savings of more than $2000 per patient and an LOS decrease of 1.4 days,76 all were conducted in the gastrointestinal surgery setting. Studies are needed to fully assess the economic benefits associated with liposome bupivacaine in the orthopedic surgery setting.
Conclusion
Liposome bupivacaine represents a potentially important contributor to multimodal analgesic regimens used to manage postsurgical pain. Liposome bupivacaine has demonstrated efficacy in providing prolonged postsurgical analgesia and reducing postsurgical opioid use in most surgical settings studied. Additional data from health economics studies in gastrointestinal surgery suggest liposome bupivacaine–based multimodal analgesic regimens may also contribute to reductions in hospital LOS and hospitalization costs. Non-industry-sponsored trials are needed to answer these crucial questions in orthopedic surgery settings. Nevertheless, data on the safety and efficacy of liposome bupivacaine for postsurgical analgesia continue to accumulate, and liposome bupivacaine appears to be a feasible therapeutic option for managing postsurgical pain in orthopedic surgery.
Approximately 5.5 million patients undergo orthopedic surgery in the United States each year, and more than 1 million of the procedures are total knee arthroplasty (TKA) or total hip arthroplasty.1 From its 2010 level, demand for joint arthroplasty is expected to double by 2020 and quadruple by 2030.2
About half the patients who have major joint arthroplasty experience severe postsurgical pain.3 Because postsurgical pain may persist for days or weeks, and inadequate treatment is associated with negative outcomes, achieving effective postsurgical analgesia is an important consideration.4-7 Complications of inadequate postsurgical pain management include thromboembolic or pulmonary complications, development of chronic pain, and decrements in health-related quality of life.4,8
In patients who have orthopedic surgery, the inability to adequately control postsurgical pain has been associated with increased hospital length of stay (LOS), delayed time to ambulation, and reduced capacity for exercise.9-12 A recent study involving 4709 patients who had hip or knee arthroplasty found that postsurgical pain relief was the second most highly correlated factor with respect to overall patient satisfaction (how well surgery met patient expectations was the most highly correlated factor),13 suggesting that postsurgical analgesia should be a focus of surgical practice.
A prolonged-release liposomal formulation of the local anesthetic bupivacaine is now available. Bupivacaine liposome injectable suspension (Exparel; Pacira Pharmaceuticals, Inc., Parsippany, New Jersey) is indicated for administration into the surgical site to produce postsurgical analgesia.14 In this article, we review evidence from clinical studies regarding the potential contribution of liposome bupivacaine to improving postsurgical pain management when used as part of a multimodal analgesic regimen in patients undergoing orthopedic surgery.
Postsurgical Pain Management in Orthopedic Surgery
Frequently Used Modalities
Analgesic modalities commonly used for perioperative pain management include central (eg, epidural),4,10,15,16 central regional (eg, neuraxial),4 peripheral regional (eg, peripheral nerve blocks, local/regional surgical site infiltration, intra-articular administration),4,10,15,17-25 and intravenous (IV) patient-controlled analgesia.4,10,25 These pharmacologic interventions may be augmented by nonpharmacologic modalities (eg, transcutaneous electrical nerve stimulation).26
Pharmacologic treatment options for perioperative pain management include opioids, local anesthetics, clonidine, ketamine, nonsteroidal anti-inflammatory drugs, acetaminophen, and calcium-channel blockers.4,26-28 In TKA, “drug cocktails” (eg, combinations of ropivacaine, ketorolac, epinephrine, and clonidine) for regional or intra-articular injection can also provide effective immediate postsurgical analgesia.25 Although opioids are the most commonly used analgesics for management of orthopedic perioperative pain,25 their use is often associated with adverse effects (AEs), including constipation or ileus, nausea, sedation, dizziness, pruritus, urinary retention, and respiratory depression.6
Multimodal Analgesic Regimens for Postsurgical Pain Management
Current American Society of Anesthesiologists guidelines endorse use of multimodal analgesia, whenever possible, to provide effective management of acute perioperative pain.4 Multimodal analgesia involves applying 2 or more agents with different mechanisms of action to achieve a synergistic effect, which allows each agent to be reduced in dose4,28 and thereby may limit the risk and severity of dose-related AEs.4,25,28
Multimodal analgesia aims to reduce the risk for opioid-related AEs (ORAEs) and the impact of opioids on postsurgical milestones (eg, ambulation, discharge) and may reduce opioid consumption, with attendant reductions in ORAE risk.29,30 Health economics studies have shown that postsurgical ORAEs are associated with increased hospital costs and LOS.6 In a study using a national hospital database, development of an ORAE (vs no ORAE) in postsurgical patients was associated with mean increases of about $4700 in hospital costs and 3.3 days in LOS.7 Reducing postsurgical opioid use may also help reduce the risk for opioid abuse, addiction, and diversion.31-33
One approach to reducing opioid use involves continuous or intermittent administration of local anesthetics by elastomeric pumps to extend duration of postsurgical analgesia.34-36 However, use of elastomeric pumps has been associated with risk for AEs, including tissue necrosis, sloughing, wound infection, and chondrolysis.37-40 In addition, AEs related to “dose dumping” (accidental delivery of excessive doses) have been reported.40-44 Key issues that may negatively affect rehabilitation after orthopedic surgery include consistency and accuracy of analgesic delivery and the potential for motor block–induced muscle weakness, which may lead to falls and constrain ambulation.45-47
Liposome Bupivacaine
Description
Drug Delivery Technology. Liposome bupivacaine incorporates DepoFoam drug delivery technology (Pacira Pharmaceuticals, Inc.) to facilitate prolonged release of bupivacaine. This technology is based on creation of multivesicular liposome particles (diameter, 10-30 µm) with multiple aqueous chambers.30,48 After administration into the surgical site, bupivacaine diffuses from chambers in the liposomal particles over time, providing analgesia and reduced opioid requirements for up to 72 hours.29,30
Indication, Mechanism of Action, Pharmacokinetics, and Dose/Administration. Liposome bupivacaine is indicated for single-dose administration into the surgical site to produce postsurgical analgesia in patients at least 18 years old.14 Like other local anesthetics, liposome bupivacaine is thought to exert its pharmacologic effects by interacting with voltage-gated Na+ channels on neural membranes to raise the threshold for electrical excitability, to slow nerve impulse propagation, and to reduce the rate of rise of the action potential.14,49
Liposome bupivacaine has dose-proportional pharmacokinetics.50 Presence of a small amount of extra-liposomal bupivacaine in the formulation leads to a bimodal pharmacokinetic profile, with an initial peak serum concentration about 1 hour after administration, followed by a second peak within 12 to 36 hours (Figure).50
Maximum amount of liposome bupivacaine approved for single administration is 266 mg (packaged as 20 mL of a 1.3% solution). However, product labeling includes safety data associated with doses of 532 mg or less.14 The appropriate volume to be used should be based on the amount required to cover the surgical area. Liposome bupivacaine may be expanded with preservative-free normal (0.9%) sterile saline to a total volume of 300 mL: 20 mL liposome bupivacaine plus 280 mL or less diluent, with final concentration of 0.89 mg/mL (1:14 by volume).14
A 25-gauge or larger bore needle should be used to slowly inject liposome bupivacaine into soft tissues of the surgical site, with frequent aspiration to check for blood to minimize risk for intravascular injection.14 Total volume used and fraction injected in specific regions of the surgical site depend on the procedure. For example, a TKA study used 266 mg diluted to a total volume of 60 mL, with 8 mL infiltrated to the area around the medial capsule, 8 mL around the lateral capsule, 12 mL around the posterior capsule, 8 mL around the peripatellar area, 12 mL into the capsulotomy incision, and 12 mL into the subcutaneous tissue on each side of the incision.51
Efficacy
Multiple Surgical Settings. The efficacy of liposome bupivacaine, either alone or as a component of a multimodal analgesic regimen, has been evaluated in a series of 10 phase 2 and 3 studies (8 active-controlled, 2 placebo-controlled) involving 823 patients undergoing TKA, bunionectomy, hemorrhoidectomy, inguinal hernia repair, or mammoplasty.52 Patients received a single liposome bupivacaine dose ranging from 66 to 532 mg.52
Combined analyses of efficacy data from these studies found that liposome bupivacaine–based multimodal analgesic regimens produced postsurgical analgesia for up to 72 hours, increased time to first use of opioid rescue medication after surgery, and reduced total amount of postsurgical opioid consumption versus placebo.52
Compared with standard of care, liposome bupivacaine has been shown to provide effective analgesia in open-label studies in patients undergoing open colectomy,53 laparoscopic colectomy,54 and ileostomy reversal,55,56 as reflected in assessments of postsurgical opioid consumption, LOS, and hospital costs. It has also been studied when administered by infiltration into the transversus abdominis plane (TAP) in patients having laparoscopic prostatectomy and open abdominal hernia repair.57,58
Orthopedic Surgery. In a phase 2 randomized, double-blind, dose-ranging study, TKA patients (N = 138) received bupivacaine HCl 150 mg or liposome bupivacaine 133, 266, 399, or 532 mg administered by local infiltration into the capsulotomy incision and on either side of the incision before wound closure.51 Postsurgical rescue analgesia was available to all patients. Cumulative pain intensity scores with activity (primary efficacy measure) were not statistically different between liposome bupivacaine groups and the bupivacaine HCl group through postoperative day 4. Mean scores in the liposome bupivacaine 266-, 399-, and 532-mg groups were numerically lower than for those treated with bupivacaine HCl on postoperative days 2 to 5, with all doses of liposome bupivacaine having a statistically significant lower pain score at rest on day 5. There were no statistically significant differences across treatment groups with respect to total amount of postsurgical opioids used.
In a phase 3 randomized, double-blind study of TKA patients (N = 245), liposome bupivacaine 532 mg administered into the surgical site was compared with bupivacaine HCl 200 mg for postsurgical analgesia.52 Rescue analgesia was available to all patients. No statistically significant between-group differences were found with respect to postsurgical cumulative pain scores through 72 hours (primary efficacy endpoint).
In a single-center retrospective TKA study, postsurgical outcomes in a patient cohort that received intraoperative periarticular infiltration with liposome bupivacaine 266 mg (n = 65) were compared with a cohort that received infiltration with a combination of ropivacaine 400 mg, morphine 5 mg, and epinephrine 0.4 mg (n = 85).59 Patient-reported postsurgical pain scores were similar in the 2 treatment groups during the first 24 hours after surgery and at discharge. Mean (SD) pain scores during hospitalization after the first 24 hours until discharge were significantly (P = .04) higher in the liposome bupivacaine group, 4.9 (1.4), than in the periarticular group, 4.4 (1.6). There was no significant difference between the 2 treatment groups in postsurgical opioid use. The study demonstrated no advantage to using liposome bupivacaine injections with respect to pain relief, but it was a retrospective review in which pain scores were obtained from electronic medical records. It is essential that liposome bupivacaine be compared with intra-articular injections in well-designed randomized trials.
Another single-center, matched-cohort TKA study (N = 200) compared a liposome bupivacaine regimen with femoral nerve block.60 Compared with patients who received femoral nerve block, patients who received liposome bupivacaine reported lower pain intensity scores after surgery and had shorter LOS, reduced costs, and improved knee flexion at follow-up.60
Results from 2 other studies were presented at the 2014 meeting of the American Academy of Orthopaedic Surgeons (AAOS). One was a single-center, matched-cohort TKA study (N = 72) comparing infiltration of a single dose of liposome bupivacaine into the surgical site with continuous femoral nerve block.61 The 2 treatment groups had similar mean postsurgical pain intensity scores on a 0-to-10 visual analog scale, 1.8 for liposome bupivacaine and 2.3 for continuous nerve block (P = NS), but total amount of postsurgical opioids (hydrocodone-equivalent milligrams) was significantly (P < .0001) less in the liposome bupivacaine group (82 vs 177 mg).
The other study presented at the AAOS meeting was a larger, prospective case–control study comparing outcomes between 1000 patients who had total joint arthroplasty (TJA) with liposome bupivacaine and 1000 control patients who had TJA without liposome bupivacaine.62 For the control and liposome bupivacaine cohorts, respectively, mean postsurgical pain intensity scores were 2.41 and 1.98 (P < .0001), mean LOS was 2.83 days and 2.66 days (P < .02), and incidence of falls was 1.0% and 0.2% (P = .02). Average per-patient costs were $1246 lower in the liposome bupivacaine cohort.
A pivotal phase 3 placebo-controlled study compared liposome bupivacaine 106 mg with placebo in patients undergoing bunionectomy (N = 193).5 Rescue medication was available to all patients. Cumulative pain scores were significantly (P = .0005) lower in the liposome bupivacaine group (125) than in the placebo group (146) through 24 hours after surgery (primary efficacy measure) and significantly (P = .0229) lower (197 vs 220) through 36 hours. Median time to first use of rescue opioids was delayed in favor of the liposome bupivacaine group (7.2 vs 4.3 hours; P < .0001). Mean total number of opioid tablets used within 24 hours after surgery was also significantly lower (3.8 vs 4.7; P = .008), and a larger percentage of patients in the liposome bupivacaine group avoided opioid use altogether through 24 hours (7% vs 1%; P = .04).
Efficacy data for liposome bupivacaine appear promising for relief of pain after joint arthroplasty and other orthopedic procedures but have their limitations. First, no randomized trials have compared liposome bupivacaine with locally injected pain medications (intra-articular injections in TKA or hip arthroplasty). As these injections are quite common now, such analyses are essential. Second, cost-effectiveness studies are needed for orthopedic procedures. Third, most of the published studies were sponsored by the manufacturer of liposome bupivacaine—a situation that raises questions about potential bias. Non-industry-sponsored randomized trials assessing efficacy, safety, and cost-effectiveness are needed.
Safety
Local anesthetics, including liposome bupivacaine, have the potential for central nervous system (CNS) or cardiac toxicity resulting from excessive systemic absorption or inadvertent IV administration.63 However, reported serious CNS or cardiac-related AEs are rare.63,64
AE Profile. Safety data from 10 phase 2 and 3 studies involving 823 patients who received liposome bupivacaine were evaluated.65 Of these patients, 545 received a dose of 266 mg or less (maximum dose approved by the US Food and Drug Administration [FDA]). Liposome bupivacaine was generally well tolerated. Reported AE incidence was 62% (liposome bupivacaine), 75% (bupivacaine HCl), and 43% (placebo). More than 90% of reported AEs were mild or moderate. The most frequently reported AEs were nausea, constipation, and vomiting (liposome bupivacaine, bupivacaine HCl) and nausea, dizziness, and vomiting (placebo).
Serious AEs were reported in 22 (2.7%) of the 823 patients in the liposome bupivacaine group, 24 (5.4%) of the 446 in the bupivacaine HCl group, and 2 (1.1%) of the 190 in the placebo group.65 None of the serious AEs in the liposome bupivacaine and placebo groups were considered treatment-related. Six patients in the bupivacaine HCl group had treatment-related serious AEs (hypoglycemia, arthrofibrosis, hemarthrosis, joint swelling, scar, knee arthroplasty).
Cardiac Safety. Possible cardiac effects associated with liposome bupivacaine were evaluated with data from studies conducted during the clinical development program.66 One hundred thirty-eight patients participated in the phase 2 safety and efficacy study in TKA. In these patients, a consistent change in mean heart rate (range, +12.2 to +16.5 beats per minute) was found across all liposome bupivacaine doses and with bupivacaine HCl. No clinically relevant changes from baseline in mean electrocardiographic parameters, including QTcF interval (QT interval adjusted using Fridericia’s correction formula), were found. In another analysis,67 liposome bupivacaine administered in a single subcutaneous dose (266, 399, 532, or 665 mg) to healthy volunteers did not prolong (vs placebo) QTc interval.
Wound Healing. The potential effects of liposome bupivacaine on wound healing were evaluated with results from 10 phase 2 and 3 studies.68 The assessments, which varied across studies, included clinicians’ overall satisfaction with patient wound healing, wound status assessment (categories included erythema, drainage, edema, and induration), and wound scarring (categories included pigmentation, height, pliability, and vascularity). Clinician-assessed scores reflected high satisfaction with wound healing overall. There were few statistically significant differences in wound status assessments between liposome bupivacaine and the comparators and no statistically significant differences in scarring between liposome bupivacaine and bupivacaine HCl.
The potential of liposome bupivacaine to have adverse intra-articular effects was assessed with drainage samples from patients (n = 23) who had TKA and received liposome bupivacaine (133, 266, 399, or 532 mg) or bupivacaine HCl (150 mg) by wound infiltration near the intra-articular space.51,65 Only small amounts of bupivacaine were present in drainage fluid collected for 12 hours after liposome bupivacaine administration, comparable to bupivacaine HCl administration.65 Currently, the product is not approved for intra-articular use.
Compatibility With Diluents, Other Medications, and Implant Materials
Liposome bupivacaine may be expanded up to a ratio of 1:14 by volume (to a final total volume of 300 mL or a concentration of 0.89 mg/mL) using preservative-free normal (0.9%) sterile saline for injection.14 It has also been shown in vitro to be compatible with lactated Ringer solution as a diluent.69
Liposome bupivacaine should not be admixed with other medications before administration.14 No formal drug–drug interaction studies have been conducted with liposome bupivacaine, but it has been shown in vitro to be compatible with epinephrine solutions, with certain anti-infective medications (eg, bacitracin, gentamicin, cefazolin, cefuroxime), with certain analgesics (eg, ketorolac, morphine), with an antihypertensive medication (clonidine), with an antihemorrhagic medication (tranexamic acid), and with certain corticosteroids (eg, methylprednisolone, triamcinolone acetonide). These medications may be coadministered in the same location as liposome bupivacaine.69
Topical antiseptics (eg, povidone iodine) may be used in surgical procedures involving liposome bupivacaine as long as they are not directly mixed with liposome bupivacaine and are allowed to dry before it is administered. If a topical antiseptic is used for wound irrigation, the wound should be rinsed clear before liposome bupivacaine administration.14,69
Liposome bupivacaine may be coadministered into the same surgical site immediately after bupivacaine HCl as long as the dose ratio of liposome bupivacaine to bupivacaine HCl is 2:1 or higher. Because of the prolonged-release pharmacokinetic profile of liposome bupivacaine and the potential for increased bupivacaine exposure, bupivacaine HCl should not be administered within 96 hours after administration of liposome bupivacaine.14,69
In vitro coincubation studies of liposome bupivacaine and other local anesthetics, including ropivacaine, lidocaine, and mepivacaine, have found rapid release of free bupivacaine from the liposome matrix. Therefore, after giving any of these other local anesthetics, surgeons should wait at least 20 minutes before administering liposome bupivacaine into the same area.14,69
In vitro studies have shown that liposome bupivacaine is compatible with a wide range of commonly used implant materials, including polypropylene, expanded polytetrafluoroethylene, stainless steel, titanium, and smooth- and textured-type silicone.69
Investigational Use and Ongoing Studies
A phase 2 randomized, double-masked, dose-escalating/deescalating study was conducted to evaluate the efficacy, safety, and pharmacokinetics of liposome bupivacaine (155, 199, or 310 mg) in comparison with bupivacaine HCl 125 mg for ankle nerve block in patients undergoing bunionectomy (N = 58).70 The study medication was injected into 3 sites to reach the posterior tibial, sural, deep peroneal, superficial peroneal, and saphenous nerves. Pharmacokinetic exposure was higher for liposome bupivacaine than for bupivacaine HCl, as reflected by a significantly greater area under the curve, lower Cmax (maximum serum concentration), and longer mean half-life. Mean pain intensity scores were lower in the bupivacaine HCl group than in each liposome bupivacaine group the first 12 hours after surgery. However, the liposome bupivacaine 310-mg group had similar or lower scores than the bupivacaine HCl group from 12 to 96 hours after surgery. The most common AEs in the liposome bupivacaine group were gastrointestinal and not treatment-related.70
The efficacy and safety of liposome bupivacaine, administered as a femoral nerve block for postsurgical analgesia, were assessed in a phase 2/3 manufacturer-sponsored, placebo-controlled, multicenter, randomized, double-blind 2-part study (NCT01683071)71 in 280 TKA patients.71,72 Part 2 of the study, comparing liposome bupivacaine 266 mg (n = 116) and placebo (n = 116), met its primary endpoint, demonstrating statistical significance in favor of liposome bupivacaine for cumulative pain scores over 72 hours (P < .0001), with decreased opioid use (P < .05) and a safety profile similar to that of placebo.72
Other ongoing investigator-sponsored studies in orthopedic populations include comparisons of liposome bupivacaine and bupivacaine HCl for ultrasound-guided periarticular hip infiltration in hip arthroplasty (NTC01917191),73 as femoral nerve block in TKA (NCT01977339),74 and as interscalene brachial plexus block in arthroscopic shoulder surgery (NCT01977352).75 The primary efficacy outcome measure in these studies was postsurgical opioid use.73-75
Health Economics
A series of phase 4 health economics studies was conducted for gastrointestinal surgeries, including open colectomy, laparoscopic colectomy, and ileostomy reversal.53-56,76 These studies, of similar design, showed that a liposome bupivacaine–based multimodal analgesic regimen was associated with reduced opioid use, shorter hospital LOS, and lower hospitalization costs in comparison with a traditional opioid-based regimen.53-56 Although pooled analysis of these studies showed a cost savings of more than $2000 per patient and an LOS decrease of 1.4 days,76 all were conducted in the gastrointestinal surgery setting. Studies are needed to fully assess the economic benefits associated with liposome bupivacaine in the orthopedic surgery setting.
Conclusion
Liposome bupivacaine represents a potentially important contributor to multimodal analgesic regimens used to manage postsurgical pain. Liposome bupivacaine has demonstrated efficacy in providing prolonged postsurgical analgesia and reducing postsurgical opioid use in most surgical settings studied. Additional data from health economics studies in gastrointestinal surgery suggest liposome bupivacaine–based multimodal analgesic regimens may also contribute to reductions in hospital LOS and hospitalization costs. Non-industry-sponsored trials are needed to answer these crucial questions in orthopedic surgery settings. Nevertheless, data on the safety and efficacy of liposome bupivacaine for postsurgical analgesia continue to accumulate, and liposome bupivacaine appears to be a feasible therapeutic option for managing postsurgical pain in orthopedic surgery.
1. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Accessed January 30, 2015.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Bonica JJ. Postoperative pain. In: Bonica JJ, ed. The Management of Pain. Malvern, PA: Lea & Febiger; 1990:461-480.
4. Apfelbaum JL, Ashburn MA, Connis RT, et al; American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
5. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28(9):776-788.
6. Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 pt 2):6S-11S.
7. Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27(1):62-70.
8. Wu CL, Naqibuddin M, Rowlingson AJ, Lietman SA, Jermyn RM, Fleisher LA. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97(4):1078-1085.
9. Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303-311.
10. Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91(1):8-15.
11. Capdevila X, Dadure C, Bringuier S, et al. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105(3):566-573.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):12-15.
13. Hamilton DF, Lane JV, Gaston P, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open. 2013;3(4):e002525.
14. Exparel [prescribing information]. Parsippany, NJ: Pacira Pharmaceuticals, Inc.; 2014.
15. DeWeese FT, Akbari Z, Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001;392(11):226-231.
16. Pati AB, Perme D, Trail M, Henry PK, Bryan WJ. Rehabilitation parameters in total knee replacement patients undergoing epidural vs. conventional analgesia. J Orthop Sports Phys Ther. 1994;19(2):88-92.
17. Browne C, Copp S, Reden L, Pulido P, Colwell C Jr. Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty. 2004;19(3):377-380.
18. Nechleba J, Rogers V, Cortina G, Cooney T. Continuous intra-articular infusion of bupivacaine for postoperative pain following total knee arthroplasty. J Knee Surg. 2005;18(3):197-202.
19. Campbell A, McCormick M, McKinlay K, Scott NB. Epidural vs. lumbar plexus infusions following total knee arthroplasty: randomized controlled trial. Eur J Anaesthesiol. 2008;25(6):502-507.
20. Serpell MG, Millar FA, Thomson MF. Comparison of lumbar plexus block versus conventional opioid analgesia after total knee replacement. Anaesthesia. 1991;46(4):275-277.
21. Lareau JM, Robbins CE, Talmo CT, Mehio AK, Puri L, Bono JV. Complications of femoral nerve blockade in total knee arthroplasty and strategies to reduce patient risk. J Arthroplasty. 2012;27(4):564-568.
22. Charous MT, Madison SJ, Suresh PJ, et al. Continuous femoral nerve blocks: varying local anesthetic delivery method (bolus versus basal) to minimize quadriceps motor block while maintaining sensory block. Anesthesiology. 2011;115(4):774-781.
23. Gottschalk A, Burmeister MA, Radtke P, et al. Continuous wound infiltration with ropivacaine reduces pain and analgesic requirement after shoulder surgery. Anesth Analg. 2003;97(4):1086-1091.
24. Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79(2):174-183.
25. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
26. White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94(3):577-585.
27. Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth. 1991;66(6):703-712.
28. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
29. Candiotti K. Liposomal bupivacaine: an innovative nonopioid local analgesic for the management of postsurgical pain. Pharmacotherapy. 2012;32(9 Pt 2):19S-26S.
30. Bergese SD, Onel E, Portillo J. Evaluation of DepoFoam® bupivacaine for the treatment of postsurgical pain. Pain Manag. 2011;1(6):539-547.
31. Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710-1714.
32. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
33. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
34. Ilfeld BM, Morey TE, Enneking FK. Delivery rate accuracy of portable, bolus-capable infusion pumps used for patient-controlled continuous regional analgesia. Reg Anesth Pain Med. 2003;28(1):17-23.
35. Ganapathy S, Amendola A, Lichfield R, Fowler PJ, Ling E. Elastomeric pumps for ambulatory patient controlled regional analgesia. Can J Anaesth. 2000;47(9):897-902.
36. Bray DA Jr, Nguyen J, Craig J, Cohen BE, Collins DR Jr. Efficacy of a local anesthetic pain pump in abdominoplasty. Plast Reconstr Surg. 2007;119(3):1054-1059.
37. Brown SL, Morrison AE. Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology. 2004;100(5):1305-1307.
38. Noyes FR, Fleckenstein CM, Barber-Westin SD. The development of postoperative knee chondrolysis after intra-articular pain pump infusion of an anesthetic medication: a series of twenty-one cases. J Bone Joint Surg Am. 2012;94(16):1448-1457.
39. Rapley JH, Beavis RC, Barber FA. Glenohumeral chondrolysis after shoulder arthroscopy associated with continuous bupivacaine infusion. Arthroscopy. 2009;25(12):1367-1373.
40. Institute for Safe Medication Practices. Process for handling elastomeric pain relief balls (ON-Q PainBuster and others) requires safety improvements. ISMP Medication Safety Alert. http://www.ismp.org/Newsletters/acutecare/articles/20090716.asp. Accessed January 30, 2015.
41. Pepin JL, Dasta JF, New M. Ensuring safe and economical use of elastomeric infusion devices. Am J Health Syst Pharm. 2011;68(24):2330-2331.
42. Birrer KL, Anderson RL, Liu-DeRyke X, Patel KR. Measures to improve safety of an elastomeric infusion system for pain management. Am J Health Syst Pharm. 2011;68(13):1251-1255.
43. Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg. 2005;100(6):1822-1833.
44. US Food and Drug Administration. Medical device recalls: I-Flow ON-Q Pump with ONDEMAND Bolus Button. http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm317826.htm. Accessed July 15, 2014.
45. Ilfeld BM, Morey TE, Enneking FK. Portable infusion pumps used for continuous regional analgesia: delivery rate accuracy and consistency. Reg Anesth Pain Med. 2003;28(5):424-432.
46. Ganapathy S. Wound/intra-articular infiltration or peripheral nerve blocks for orthopedic joint surgery: efficacy and safety issues. Curr Opin Anaesthesiol. 2012;25(5):615-620.
47. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554.
48. Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam™: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45(12):1153-1176.
49. Catterall WA, Mackie K. Local anesthetics. In: Gutstein HB, Akil H, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:565-582.
50. Hu D, Onel E, Singla N, Kramer WG, Hadzic A. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33(2):109-115.
51. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
52. Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107-116.
53. Cohen SM. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567-572.
54. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res. 2014;76:1-6.
55. Marcet JE, Nfonsam VN, Larach S. An extended paIn relief trial utilizing the infiltration of a long-acting Multivesicular liPosome foRmulation Of bupiVacaine, EXPAREL (IMPROVE): a Phase IV health economic trial in adult patients undergoing ileostomy reversal. J Pain Res. 2013;6:549-555.
56. Vogel JD. Liposome bupivacaine (EXPAREL®) for extended pain relief in patients undergoing ileostomy reversal at a single institution with a fast-track discharge protocol: an IMPROVE phase IV health economics trial. J Pain Res. 2013;6:605-610.
57. Sternlicht A, Shapiro M, Robelen G, Vellayappan U, Tuerk IA. Initial findings using EXPAREL® (bupivacaine liposome injectable suspension) via infiltration into the transversus abdominis plane (TAP) for postsurgical analgesia in robotic prostatectomy (RP). Abstract presented at: Annual Fall Pain Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine; November 15-18, 2012; Miami Beach, FL.
58. Feierman DE, Kronenfeld M, Gupta PM, Younger N, Logvinskiy E. Evaluation of Exparel® use via infiltration into the transversus abdominis plane for prolonged postoperative analgesia in subjects undergoing open abdominal hernia repair. Poster presented at: Annual Meeting of the International Anesthesia Research Society; May 4-7, 2013; San Diego, CA.
59. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
60. Broome B, Backlund I. Rapid recovery pain pathway for total knee arthroplasty results in improved pain management, decreased length of stay, and significant cost savings. Poster presented at: Annual Orthopedic and Spine Summit; September 18-20, 2013; San Antonio, TX.
61. Emerson RH, Barrington JW. Comparison of infiltration with long-acting bupivacaine to a femoral nerve catheter for total knee replacement. Abstract presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA. Abstract P124.
62. Barrington JW. Emerging data in the use of liposome bupivacaine: comparative review in 2,000 TJA patients. Oral presentation presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA.
63. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35(2):152-161.
64. D’Angelo R. Are the new local anesthetics worth their cost? Acta Anaesthesiol Scand. 2000;44(6):639-641.
65. Viscusi ER, Sinatra R, Onel E, Ramamoorthy SL. The safety of liposome bupivacaine, a novel local analgesic formulation. Clin J Pain. 2014;30(2):102-110.
66. Bergese SD, Onel E, Morren M, Morganroth J. Bupivacaine extended-release liposome injection exhibits a favorable cardiac safety profile. Reg Anesth Pain Med. 2012;37(2):145-151.
67. Naseem A, Harada T, Wang D, et al. Bupivacaine extended release liposome injection does not prolong QTc interval in a thorough QT/QTc study in healthy volunteers. J Clin Pharmacol. 2012;52(9):1441-1447.
68. Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35(3):312-320.
69. Kharitonov V. A review of the compatibility of liposome bupivacaine with other drug products and commonly used implant materials. Postgrad Med. 2014;126(1):129-138.
70. Ilfeld BM. Liposome bupivacaine in peripheral nerve blocks and epidural injections to manage postoperative pain. Expert Opin Pharmacother. 2013;14(17):2421-2431.
71. Femoral nerve block with liposome bupivacaine for postsurgical analgesia following total knee arthroplasty [NCT01683071]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01683071?term=NCT01683071%5C&rank=1. Accessed January 30, 2015.
72. Minkowitz H, Matthews A, Puckett C, Melson T. Liposome bupivacaine in femoral nerve block: initial results from a phase 2/3 pivotal study. Poster presented at: Annual Meeting of the American Society of Regional Anesthesia and Pain Medicine; April 3-6, 2014; Chicago, IL.
73. Ultrasound guided local infiltration analgesia for hip arthroscopy [NCT01907191]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01907191?term=NCT01907191&rank=1. Accessed January 30, 2015.
74. Efficacy of single injection femoral nerve block with liposomal bupivacaine for total knee arthroplasty [NCT01977339]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977339?term=NCT01977339&rank=1. Accessed January 30, 2015.
75. Efficacy of interscalene brachial plexus block with liposomal bupivacaine for arthroscopic shoulder surgery [NCT01977352]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977352?term=NCT01977352&rank=1. Accessed January 30, 2015.
76. Cohen SM, Vogel JD, Marcet JE, Candiotti K. Liposome bupivacaine for improvement in economic outcomes and opioid burden in GI surgery: IMPROVE study pooled analysis. J Pain Res. 2014;7:359-366.
1. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Accessed January 30, 2015.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Bonica JJ. Postoperative pain. In: Bonica JJ, ed. The Management of Pain. Malvern, PA: Lea & Febiger; 1990:461-480.
4. Apfelbaum JL, Ashburn MA, Connis RT, et al; American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
5. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28(9):776-788.
6. Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 pt 2):6S-11S.
7. Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27(1):62-70.
8. Wu CL, Naqibuddin M, Rowlingson AJ, Lietman SA, Jermyn RM, Fleisher LA. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97(4):1078-1085.
9. Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303-311.
10. Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91(1):8-15.
11. Capdevila X, Dadure C, Bringuier S, et al. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105(3):566-573.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):12-15.
13. Hamilton DF, Lane JV, Gaston P, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open. 2013;3(4):e002525.
14. Exparel [prescribing information]. Parsippany, NJ: Pacira Pharmaceuticals, Inc.; 2014.
15. DeWeese FT, Akbari Z, Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001;392(11):226-231.
16. Pati AB, Perme D, Trail M, Henry PK, Bryan WJ. Rehabilitation parameters in total knee replacement patients undergoing epidural vs. conventional analgesia. J Orthop Sports Phys Ther. 1994;19(2):88-92.
17. Browne C, Copp S, Reden L, Pulido P, Colwell C Jr. Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty. 2004;19(3):377-380.
18. Nechleba J, Rogers V, Cortina G, Cooney T. Continuous intra-articular infusion of bupivacaine for postoperative pain following total knee arthroplasty. J Knee Surg. 2005;18(3):197-202.
19. Campbell A, McCormick M, McKinlay K, Scott NB. Epidural vs. lumbar plexus infusions following total knee arthroplasty: randomized controlled trial. Eur J Anaesthesiol. 2008;25(6):502-507.
20. Serpell MG, Millar FA, Thomson MF. Comparison of lumbar plexus block versus conventional opioid analgesia after total knee replacement. Anaesthesia. 1991;46(4):275-277.
21. Lareau JM, Robbins CE, Talmo CT, Mehio AK, Puri L, Bono JV. Complications of femoral nerve blockade in total knee arthroplasty and strategies to reduce patient risk. J Arthroplasty. 2012;27(4):564-568.
22. Charous MT, Madison SJ, Suresh PJ, et al. Continuous femoral nerve blocks: varying local anesthetic delivery method (bolus versus basal) to minimize quadriceps motor block while maintaining sensory block. Anesthesiology. 2011;115(4):774-781.
23. Gottschalk A, Burmeister MA, Radtke P, et al. Continuous wound infiltration with ropivacaine reduces pain and analgesic requirement after shoulder surgery. Anesth Analg. 2003;97(4):1086-1091.
24. Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79(2):174-183.
25. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
26. White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94(3):577-585.
27. Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth. 1991;66(6):703-712.
28. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
29. Candiotti K. Liposomal bupivacaine: an innovative nonopioid local analgesic for the management of postsurgical pain. Pharmacotherapy. 2012;32(9 Pt 2):19S-26S.
30. Bergese SD, Onel E, Portillo J. Evaluation of DepoFoam® bupivacaine for the treatment of postsurgical pain. Pain Manag. 2011;1(6):539-547.
31. Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710-1714.
32. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
33. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
34. Ilfeld BM, Morey TE, Enneking FK. Delivery rate accuracy of portable, bolus-capable infusion pumps used for patient-controlled continuous regional analgesia. Reg Anesth Pain Med. 2003;28(1):17-23.
35. Ganapathy S, Amendola A, Lichfield R, Fowler PJ, Ling E. Elastomeric pumps for ambulatory patient controlled regional analgesia. Can J Anaesth. 2000;47(9):897-902.
36. Bray DA Jr, Nguyen J, Craig J, Cohen BE, Collins DR Jr. Efficacy of a local anesthetic pain pump in abdominoplasty. Plast Reconstr Surg. 2007;119(3):1054-1059.
37. Brown SL, Morrison AE. Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology. 2004;100(5):1305-1307.
38. Noyes FR, Fleckenstein CM, Barber-Westin SD. The development of postoperative knee chondrolysis after intra-articular pain pump infusion of an anesthetic medication: a series of twenty-one cases. J Bone Joint Surg Am. 2012;94(16):1448-1457.
39. Rapley JH, Beavis RC, Barber FA. Glenohumeral chondrolysis after shoulder arthroscopy associated with continuous bupivacaine infusion. Arthroscopy. 2009;25(12):1367-1373.
40. Institute for Safe Medication Practices. Process for handling elastomeric pain relief balls (ON-Q PainBuster and others) requires safety improvements. ISMP Medication Safety Alert. http://www.ismp.org/Newsletters/acutecare/articles/20090716.asp. Accessed January 30, 2015.
41. Pepin JL, Dasta JF, New M. Ensuring safe and economical use of elastomeric infusion devices. Am J Health Syst Pharm. 2011;68(24):2330-2331.
42. Birrer KL, Anderson RL, Liu-DeRyke X, Patel KR. Measures to improve safety of an elastomeric infusion system for pain management. Am J Health Syst Pharm. 2011;68(13):1251-1255.
43. Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg. 2005;100(6):1822-1833.
44. US Food and Drug Administration. Medical device recalls: I-Flow ON-Q Pump with ONDEMAND Bolus Button. http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm317826.htm. Accessed July 15, 2014.
45. Ilfeld BM, Morey TE, Enneking FK. Portable infusion pumps used for continuous regional analgesia: delivery rate accuracy and consistency. Reg Anesth Pain Med. 2003;28(5):424-432.
46. Ganapathy S. Wound/intra-articular infiltration or peripheral nerve blocks for orthopedic joint surgery: efficacy and safety issues. Curr Opin Anaesthesiol. 2012;25(5):615-620.
47. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554.
48. Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam™: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45(12):1153-1176.
49. Catterall WA, Mackie K. Local anesthetics. In: Gutstein HB, Akil H, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:565-582.
50. Hu D, Onel E, Singla N, Kramer WG, Hadzic A. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33(2):109-115.
51. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
52. Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107-116.
53. Cohen SM. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567-572.
54. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res. 2014;76:1-6.
55. Marcet JE, Nfonsam VN, Larach S. An extended paIn relief trial utilizing the infiltration of a long-acting Multivesicular liPosome foRmulation Of bupiVacaine, EXPAREL (IMPROVE): a Phase IV health economic trial in adult patients undergoing ileostomy reversal. J Pain Res. 2013;6:549-555.
56. Vogel JD. Liposome bupivacaine (EXPAREL®) for extended pain relief in patients undergoing ileostomy reversal at a single institution with a fast-track discharge protocol: an IMPROVE phase IV health economics trial. J Pain Res. 2013;6:605-610.
57. Sternlicht A, Shapiro M, Robelen G, Vellayappan U, Tuerk IA. Initial findings using EXPAREL® (bupivacaine liposome injectable suspension) via infiltration into the transversus abdominis plane (TAP) for postsurgical analgesia in robotic prostatectomy (RP). Abstract presented at: Annual Fall Pain Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine; November 15-18, 2012; Miami Beach, FL.
58. Feierman DE, Kronenfeld M, Gupta PM, Younger N, Logvinskiy E. Evaluation of Exparel® use via infiltration into the transversus abdominis plane for prolonged postoperative analgesia in subjects undergoing open abdominal hernia repair. Poster presented at: Annual Meeting of the International Anesthesia Research Society; May 4-7, 2013; San Diego, CA.
59. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
60. Broome B, Backlund I. Rapid recovery pain pathway for total knee arthroplasty results in improved pain management, decreased length of stay, and significant cost savings. Poster presented at: Annual Orthopedic and Spine Summit; September 18-20, 2013; San Antonio, TX.
61. Emerson RH, Barrington JW. Comparison of infiltration with long-acting bupivacaine to a femoral nerve catheter for total knee replacement. Abstract presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA. Abstract P124.
62. Barrington JW. Emerging data in the use of liposome bupivacaine: comparative review in 2,000 TJA patients. Oral presentation presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA.
63. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35(2):152-161.
64. D’Angelo R. Are the new local anesthetics worth their cost? Acta Anaesthesiol Scand. 2000;44(6):639-641.
65. Viscusi ER, Sinatra R, Onel E, Ramamoorthy SL. The safety of liposome bupivacaine, a novel local analgesic formulation. Clin J Pain. 2014;30(2):102-110.
66. Bergese SD, Onel E, Morren M, Morganroth J. Bupivacaine extended-release liposome injection exhibits a favorable cardiac safety profile. Reg Anesth Pain Med. 2012;37(2):145-151.
67. Naseem A, Harada T, Wang D, et al. Bupivacaine extended release liposome injection does not prolong QTc interval in a thorough QT/QTc study in healthy volunteers. J Clin Pharmacol. 2012;52(9):1441-1447.
68. Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35(3):312-320.
69. Kharitonov V. A review of the compatibility of liposome bupivacaine with other drug products and commonly used implant materials. Postgrad Med. 2014;126(1):129-138.
70. Ilfeld BM. Liposome bupivacaine in peripheral nerve blocks and epidural injections to manage postoperative pain. Expert Opin Pharmacother. 2013;14(17):2421-2431.
71. Femoral nerve block with liposome bupivacaine for postsurgical analgesia following total knee arthroplasty [NCT01683071]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01683071?term=NCT01683071%5C&rank=1. Accessed January 30, 2015.
72. Minkowitz H, Matthews A, Puckett C, Melson T. Liposome bupivacaine in femoral nerve block: initial results from a phase 2/3 pivotal study. Poster presented at: Annual Meeting of the American Society of Regional Anesthesia and Pain Medicine; April 3-6, 2014; Chicago, IL.
73. Ultrasound guided local infiltration analgesia for hip arthroscopy [NCT01907191]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01907191?term=NCT01907191&rank=1. Accessed January 30, 2015.
74. Efficacy of single injection femoral nerve block with liposomal bupivacaine for total knee arthroplasty [NCT01977339]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977339?term=NCT01977339&rank=1. Accessed January 30, 2015.
75. Efficacy of interscalene brachial plexus block with liposomal bupivacaine for arthroscopic shoulder surgery [NCT01977352]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977352?term=NCT01977352&rank=1. Accessed January 30, 2015.
76. Cohen SM, Vogel JD, Marcet JE, Candiotti K. Liposome bupivacaine for improvement in economic outcomes and opioid burden in GI surgery: IMPROVE study pooled analysis. J Pain Res. 2014;7:359-366.
Patient Satisfaction as a Metric for Quality
As orthopedic surgeons, we typically equate a quality outcome with the patient’s end result—resolved or diminished functional disability, fracture union, and/or pain relief, to name a few metrics. Although research has not identified a clear link between quality outcomes and patient satisfaction scores, patient satisfaction is increasingly used as a proxy for quality of care. It’s speculated that more personal care may result in better communication, more reasonable expectations, and more patient involvement, all of which may result in better quality of care. Regardless, it’s unclear whether satisfaction is an attribute of quality care or an indicator.
In a recent article in Modern Healthcare, Irwin Press,1 cofounder of Press Ganey, challenges any campaign to cast doubt on satisfaction as a relevant indicator of quality care: “It can be argued that diagnostic procedures, surgeries and therapies constitute treatment, but not care. Treatment alone isn’t care…. One is objective, involving highly standardized technical, mechanical or chemical interventions. The other is subjective, composed of behaviors, decisions and interactions of humans with idiosyncratic personalities, stresses, agendas and sensitivities.”
As surgeons, we understandably focus on objective treatment and outcome and may underappreciate the importance of the process—the experience of care. Wellness probably requires mastery of both. Indeed, just as a patient’s poor coping skills, depression, anxiety, and proclivity to catastrophize may compromise their recovery and self-reported assessments of outcome,2-5 so too do the qualitative components of our interaction with patients undoubtedly impact, not only their experience, but also their recovery. Patient self-efficacy (the feeling that they can do it), engagement (“activation”), compliance, and expectations all derive in part from the “Art” of our practice. Our “Heart” is as important to that Art, if not more so, than our “Head” (our intellect and knowledge). Whether we buy into this or not is a matter of personal opinion and experience, I suppose, but the reality is that the important singular metric of patient satisfaction is here to stay—patient satisfaction has become an important component of pay-for-performance metrics which expressly intend to reward quality over volume.
What does this mean for us? First, we need to adapt to the reality that the patient’s perception of their interaction with us impacts their experience and their level of satisfaction, and accept our role in their overall perception of quality. Being rewarded with a high satisfaction score is within our sphere of influence and requires more than just providing a good objective outcome. We might not revisit a restaurant with great food but lousy service and an underwhelming environment. We might also never eat at a place that was really nice inside and had great service, but which provided horrible food. So we must aspire to provide both objective quality outcomes and stellar patient care. As third-party payers increasingly follow the lead of the Centers for Medicare and Medicaid Services (CMS), the patient will not be at our table unless we both ask for feedback and respond to it. We all aspire to be great technicians and have a command of the knowledge base in our respective areas of practice. Some of us are privileged to have earned regional, national, or international reputations among our peers, but we all will be increasingly judged based on patient satisfaction with our care. This means that we must care about their experience and how they perceive our care: Do we spend enough time, listen attentively, answer questions, and explain the diagnosis and plan?
Just as we may hold our breath unknowingly during stressful situations when we are not mindful, so too might our “Heart” not be clearly evident in the complex health care environment today—too little time, too much paperwork, increasing patient demands. But practicing with heightened self-awareness, empathy, and unambiguous intention, and modeling our values during our interaction with our patients—“mindful practice”—is increasingly advocated as a necessary component to “best practice.” For truly rewarding practice, during which we can attain not only great results but also satisfied patients, we need to revisit why we do what we do, and rebalance our emphasis on what we do and how we do it. Mindful practice is both an objective and a strategy. It may require making structural adjustments to our practice, such as seeing fewer patients per hour, for, perhaps, an hour or two more in a day, completing some of our electronic medical record notes at day’s end, and maybe adding an extra clinic day every other week. We must also deliberately solicit feedback from our patients so that we can respond to any perceived room for improvement.
Thirteen years ago when I received my Master of Business Administration (MBA) degree, I felt that improving operational efficiency would enable me to do more in a day—and it did. But when patient satisfaction becomes the proxy for quality, sound business practice may not translate into sound clinical practice. After 21 years of practice, and deliberate attentiveness to patient feedback, I am increasingly aware that the Art of practice is as important as the Science—our Heart is as important as our Head. In this light, patient satisfaction is a very sound metric for quality.
1. Press I. Don’t downplay patient satisfaction. Modern Healthcare. http://www.modernhealthcare.com/article/20140322/MAGAZINE/303229940. Published March 22, 2014. Accessed January 7, 2015.
2. Cho CH, Seo HJ, Bae KC, Lee KJ, Hwang I, Warner JJ. The impact of depression and anxiety on self-assessed pain, disability, and quality of life in patients scheduled for rotator cuff repair. J Shoulder Elbow Surg. 2013;22(9):1160-1166.
3. Blackburn J, Qureshi A, Amirfeyz R, Bannister G. Does preoperative anxiety and depression predict satisfaction after total knee replacement? Knee. 2012;19(5):522-524.
4. Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14(7):397-405.
5. Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51-56.
As orthopedic surgeons, we typically equate a quality outcome with the patient’s end result—resolved or diminished functional disability, fracture union, and/or pain relief, to name a few metrics. Although research has not identified a clear link between quality outcomes and patient satisfaction scores, patient satisfaction is increasingly used as a proxy for quality of care. It’s speculated that more personal care may result in better communication, more reasonable expectations, and more patient involvement, all of which may result in better quality of care. Regardless, it’s unclear whether satisfaction is an attribute of quality care or an indicator.
In a recent article in Modern Healthcare, Irwin Press,1 cofounder of Press Ganey, challenges any campaign to cast doubt on satisfaction as a relevant indicator of quality care: “It can be argued that diagnostic procedures, surgeries and therapies constitute treatment, but not care. Treatment alone isn’t care…. One is objective, involving highly standardized technical, mechanical or chemical interventions. The other is subjective, composed of behaviors, decisions and interactions of humans with idiosyncratic personalities, stresses, agendas and sensitivities.”
As surgeons, we understandably focus on objective treatment and outcome and may underappreciate the importance of the process—the experience of care. Wellness probably requires mastery of both. Indeed, just as a patient’s poor coping skills, depression, anxiety, and proclivity to catastrophize may compromise their recovery and self-reported assessments of outcome,2-5 so too do the qualitative components of our interaction with patients undoubtedly impact, not only their experience, but also their recovery. Patient self-efficacy (the feeling that they can do it), engagement (“activation”), compliance, and expectations all derive in part from the “Art” of our practice. Our “Heart” is as important to that Art, if not more so, than our “Head” (our intellect and knowledge). Whether we buy into this or not is a matter of personal opinion and experience, I suppose, but the reality is that the important singular metric of patient satisfaction is here to stay—patient satisfaction has become an important component of pay-for-performance metrics which expressly intend to reward quality over volume.
What does this mean for us? First, we need to adapt to the reality that the patient’s perception of their interaction with us impacts their experience and their level of satisfaction, and accept our role in their overall perception of quality. Being rewarded with a high satisfaction score is within our sphere of influence and requires more than just providing a good objective outcome. We might not revisit a restaurant with great food but lousy service and an underwhelming environment. We might also never eat at a place that was really nice inside and had great service, but which provided horrible food. So we must aspire to provide both objective quality outcomes and stellar patient care. As third-party payers increasingly follow the lead of the Centers for Medicare and Medicaid Services (CMS), the patient will not be at our table unless we both ask for feedback and respond to it. We all aspire to be great technicians and have a command of the knowledge base in our respective areas of practice. Some of us are privileged to have earned regional, national, or international reputations among our peers, but we all will be increasingly judged based on patient satisfaction with our care. This means that we must care about their experience and how they perceive our care: Do we spend enough time, listen attentively, answer questions, and explain the diagnosis and plan?
Just as we may hold our breath unknowingly during stressful situations when we are not mindful, so too might our “Heart” not be clearly evident in the complex health care environment today—too little time, too much paperwork, increasing patient demands. But practicing with heightened self-awareness, empathy, and unambiguous intention, and modeling our values during our interaction with our patients—“mindful practice”—is increasingly advocated as a necessary component to “best practice.” For truly rewarding practice, during which we can attain not only great results but also satisfied patients, we need to revisit why we do what we do, and rebalance our emphasis on what we do and how we do it. Mindful practice is both an objective and a strategy. It may require making structural adjustments to our practice, such as seeing fewer patients per hour, for, perhaps, an hour or two more in a day, completing some of our electronic medical record notes at day’s end, and maybe adding an extra clinic day every other week. We must also deliberately solicit feedback from our patients so that we can respond to any perceived room for improvement.
Thirteen years ago when I received my Master of Business Administration (MBA) degree, I felt that improving operational efficiency would enable me to do more in a day—and it did. But when patient satisfaction becomes the proxy for quality, sound business practice may not translate into sound clinical practice. After 21 years of practice, and deliberate attentiveness to patient feedback, I am increasingly aware that the Art of practice is as important as the Science—our Heart is as important as our Head. In this light, patient satisfaction is a very sound metric for quality.
As orthopedic surgeons, we typically equate a quality outcome with the patient’s end result—resolved or diminished functional disability, fracture union, and/or pain relief, to name a few metrics. Although research has not identified a clear link between quality outcomes and patient satisfaction scores, patient satisfaction is increasingly used as a proxy for quality of care. It’s speculated that more personal care may result in better communication, more reasonable expectations, and more patient involvement, all of which may result in better quality of care. Regardless, it’s unclear whether satisfaction is an attribute of quality care or an indicator.
In a recent article in Modern Healthcare, Irwin Press,1 cofounder of Press Ganey, challenges any campaign to cast doubt on satisfaction as a relevant indicator of quality care: “It can be argued that diagnostic procedures, surgeries and therapies constitute treatment, but not care. Treatment alone isn’t care…. One is objective, involving highly standardized technical, mechanical or chemical interventions. The other is subjective, composed of behaviors, decisions and interactions of humans with idiosyncratic personalities, stresses, agendas and sensitivities.”
As surgeons, we understandably focus on objective treatment and outcome and may underappreciate the importance of the process—the experience of care. Wellness probably requires mastery of both. Indeed, just as a patient’s poor coping skills, depression, anxiety, and proclivity to catastrophize may compromise their recovery and self-reported assessments of outcome,2-5 so too do the qualitative components of our interaction with patients undoubtedly impact, not only their experience, but also their recovery. Patient self-efficacy (the feeling that they can do it), engagement (“activation”), compliance, and expectations all derive in part from the “Art” of our practice. Our “Heart” is as important to that Art, if not more so, than our “Head” (our intellect and knowledge). Whether we buy into this or not is a matter of personal opinion and experience, I suppose, but the reality is that the important singular metric of patient satisfaction is here to stay—patient satisfaction has become an important component of pay-for-performance metrics which expressly intend to reward quality over volume.
What does this mean for us? First, we need to adapt to the reality that the patient’s perception of their interaction with us impacts their experience and their level of satisfaction, and accept our role in their overall perception of quality. Being rewarded with a high satisfaction score is within our sphere of influence and requires more than just providing a good objective outcome. We might not revisit a restaurant with great food but lousy service and an underwhelming environment. We might also never eat at a place that was really nice inside and had great service, but which provided horrible food. So we must aspire to provide both objective quality outcomes and stellar patient care. As third-party payers increasingly follow the lead of the Centers for Medicare and Medicaid Services (CMS), the patient will not be at our table unless we both ask for feedback and respond to it. We all aspire to be great technicians and have a command of the knowledge base in our respective areas of practice. Some of us are privileged to have earned regional, national, or international reputations among our peers, but we all will be increasingly judged based on patient satisfaction with our care. This means that we must care about their experience and how they perceive our care: Do we spend enough time, listen attentively, answer questions, and explain the diagnosis and plan?
Just as we may hold our breath unknowingly during stressful situations when we are not mindful, so too might our “Heart” not be clearly evident in the complex health care environment today—too little time, too much paperwork, increasing patient demands. But practicing with heightened self-awareness, empathy, and unambiguous intention, and modeling our values during our interaction with our patients—“mindful practice”—is increasingly advocated as a necessary component to “best practice.” For truly rewarding practice, during which we can attain not only great results but also satisfied patients, we need to revisit why we do what we do, and rebalance our emphasis on what we do and how we do it. Mindful practice is both an objective and a strategy. It may require making structural adjustments to our practice, such as seeing fewer patients per hour, for, perhaps, an hour or two more in a day, completing some of our electronic medical record notes at day’s end, and maybe adding an extra clinic day every other week. We must also deliberately solicit feedback from our patients so that we can respond to any perceived room for improvement.
Thirteen years ago when I received my Master of Business Administration (MBA) degree, I felt that improving operational efficiency would enable me to do more in a day—and it did. But when patient satisfaction becomes the proxy for quality, sound business practice may not translate into sound clinical practice. After 21 years of practice, and deliberate attentiveness to patient feedback, I am increasingly aware that the Art of practice is as important as the Science—our Heart is as important as our Head. In this light, patient satisfaction is a very sound metric for quality.
1. Press I. Don’t downplay patient satisfaction. Modern Healthcare. http://www.modernhealthcare.com/article/20140322/MAGAZINE/303229940. Published March 22, 2014. Accessed January 7, 2015.
2. Cho CH, Seo HJ, Bae KC, Lee KJ, Hwang I, Warner JJ. The impact of depression and anxiety on self-assessed pain, disability, and quality of life in patients scheduled for rotator cuff repair. J Shoulder Elbow Surg. 2013;22(9):1160-1166.
3. Blackburn J, Qureshi A, Amirfeyz R, Bannister G. Does preoperative anxiety and depression predict satisfaction after total knee replacement? Knee. 2012;19(5):522-524.
4. Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14(7):397-405.
5. Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51-56.
1. Press I. Don’t downplay patient satisfaction. Modern Healthcare. http://www.modernhealthcare.com/article/20140322/MAGAZINE/303229940. Published March 22, 2014. Accessed January 7, 2015.
2. Cho CH, Seo HJ, Bae KC, Lee KJ, Hwang I, Warner JJ. The impact of depression and anxiety on self-assessed pain, disability, and quality of life in patients scheduled for rotator cuff repair. J Shoulder Elbow Surg. 2013;22(9):1160-1166.
3. Blackburn J, Qureshi A, Amirfeyz R, Bannister G. Does preoperative anxiety and depression predict satisfaction after total knee replacement? Knee. 2012;19(5):522-524.
4. Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14(7):397-405.
5. Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51-56.
Use of Cross-Leg Flap for Wound Complications Resulting From Open Pilon Fracture
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
Complications of Open Reduction and Internal Fixation of Ankle Fractures in Patients With Positive Urine Drug Screen
Open treatment of ankle fractures is one of the most common procedures performed by orthopedic surgeons.1 Among the younger patient population, ankle fractures represent a significant proportion of orthopedic injuries.2 The reported incidence of illicit drug and alcohol use in the urban trauma population ranges from 36% to 86%,2 and medical and anesthetic complications associated with illicit drug use have been well documented in surgical patients.2 However, patients with a recent history of drug abuse may be subject to a separate but related set of complications of open treatment of ankle fractures.
The perioperative complications associated with open treatment of ankle fractures in patients with diabetes mellitus have been well described.3-6 Similarly, previous studies have suggested that peripheral vascular disease, complicated diabetes, and smoking are risk factors for poor outcomes in patients who require open reduction and internal fixation (ORIF) in lower extremity trauma.7-9 However, there are few data on the complications specifically associated with illicit drug use and orthopedic surgery. Properly identifying these high-risk groups and being cognizant of commonly associated complications are likely important in ensuring proper perioperative care and may alter follow-up protocols in these patients.
We conducted a study to identify the complications associated with open treatment of ankle fractures in patients who tested positive for illicit drugs on urine drug screen (UDS). We hypothesized that patients who had a history of positive UDS and underwent ORIF of an ankle fracture would have a higher incidence of major and minor complications.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 142 patients who underwent open treatment of an ankle fracture between 2006 and 2010. Data sources included patient demographic information, radiographs, preoperative UDS, attending surgeons’ clinical office notes, and clinical laboratory data. Our institution’s standard protocol for ankle fractures was followed for all patients in the study. All patients were evaluated by an orthopedic physician, in either the emergency department or the office, during application of a well-padded Jones splint before surgery. Oral narcotic pain medication was routinely prescribed. All patients were seen, within 10 days of injury, for surgery planning. A board-certified orthopedic surgeon surgically stabilized the ankle fractures. The postoperative treatment regimen, per protocol, included non-weight-bearing in a padded Jones splint dressing; oral narcotic pain medication; physical therapy; and routine scheduled follow-up. In open fracture cases, patients were taken urgently to the operating room for irrigation and débridement with stabilization. Which treatment would be initially used—external fixation or ORIF—was determined on a case-by-case basis.
The sample consisted of adults (age, >18 years) who had undergone definitive ORIF of a lateral malleolar, bimalleolar, or trimalleolar ankle fracture during the study period. Polytrauma patients, patients with external fixation as definitive treatment, and patients with nonoperative treatment were excluded. Before surgical management, all patients were tested for recent illicit drug use by UDS (standard protocol at our institution). UDS, measured for cocaine, marijuana, PCP (phencyclidine), opiates, and barbiturates, was obtained in the office setting or emergency department or on day of surgery. The patients were divided into 2 groups, positive and negative UDS. Patients with documented receipt of narcotic pain medication before UDS were excluded.
The outcomes identified as dependent variables included nonunion, malunion, superficial or deep infection, amputation, delay in treatment, days to healing, repeat surgery, long-term bracing, and loss to follow-up. A nonunion was defined as lasting longer than 9 months and not showing radiographic signs of progression toward healing for 3 consecutive months. These complications were identified with use of attending surgeon clinical progress notes, laboratory values, radiographic parameters, and inpatient readmissions/surgeries associated with these outcomes. Nonunion, malunion, superficial or deep infection, and amputation were then grouped as major complications and analyzed as pooled major complications.
The Fisher exact test was used to analyze categorical variables with respect to UDS. The Wilcoxon rank sum test was used to determine statistical significance for continuous variables. Univariate logistic regression examined both continuous and categorical variables to evaluate predictors for a selected outcome. Statistical significance was set a priori at P ≤ .05, with significant factors indicating an increase (or decrease) in the outcome variable being tested.
Results
We retrospectively reviewed the cases of 142 patients. Table 1 lists the number of cases by fracture type. Bimalleolar fractures were most common, accounting for 99 (69.8%) of the 142 cases. Isolated lateral malleolar fractures accounted for 16 cases (11.2%), and trimalleolar fractures accounted for 27 cases (19%).
Twenty-five (18%) of the 142 patients tested positive for illicit drugs. Mean age was 45.2 years for positive UDS patients and 41.5 years for negative UDS patients. Open fracture cases represented 4.3% of negative UDS patients and 16% of positive UDS patients. Fifty-two percent of positive UDS patients and 32% of negative UDS patients were also tobacco users. These data were statistically significant (P = .003) There were no significant differences in age, sex, incidence of diabetes, incidence of open fracture, or time to surgery between the groups (Table 2).
Incidence of nonunion was higher in positive UDS patients (n = 5; P = .01), as was incidence of deep infection (n = 4; P = .05) (Table 3).
Mean time to radiographic healing was 50.7 days in negative UDS patients and 82.8 days in positive UDS patients (P > .99). Incidence of nonunion was 3.5% in negative UDS patients and 20% in positive UDS patients (P = .01). There were no malunions in negative UDS patients and 2 malunions in positive UDS patients. Incidence of deep infections was 2.5% in negative UDS patients and 16% in positive UDS patients (P = .04). No significant differences were found in incidence of malunions, superficial infections, amputations, need for repeat surgery, continued bracing, or loss to follow-up.
Major complications were defined as superficial or deep infections, amputations, malunions, and nonunions. The rate of major complication was significantly (P = .03) higher in positive UDS patients (24.24%) than in negative UDS patients (7.69%) (Table 4).
Discussion
In the present study, we retrospectively reviewed the cases of patients treated with ORIF for varying types of ankle fractures. Important major and minor complications were analyzed. The overall incidence of major complications in negative UDS patients was only 7.69%, consistent with previously reported results in patients with ankle fractures.6,10 However, a statistically significant (P = .03) increased incidence of major complications—an alarmingly high rate of almost 1 in 4—was found in positive UDS patients. Our results also demonstrated a significantly higher rate of nonunion and deep infection in positive UDS patients. Calculated odds ratios were 7.37 and 4.27 for nonunion and deep infection, respectively—arguably 2 of the most devastating postoperative complications in positive UDS patients.
Previous studies have found that open fractures, age, and medical comorbidities are significant predictors of short-term complications, such as wound healing, infection, persistent pain, and delayed union.3-6 Levy and colleagues11 examined the incidence of orthopedic trauma in positive UDS patients. These patients had orthopedic injuries that were more severe and required longer hospitalization. However, the study did not address patients with ankle fractures or the incidence of major complications. Diabetes and peripheral vascular disease are significant risk factors for many surgical procedures in orthopedic surgery.3,7-9,12,13 Tight glycemic control and optimization of medical comorbidities decrease postoperative complications.12,13 SooHoo and colleagues6 found that history of diabetes and history of peripheral vascular disease were significant predictors of short-term complications of mortality, infection, reoperation, and amputation. The rate of infection in the complicated diabetes group was statistically higher as well. The effect of illicit drug use was not analyzed in that study. We think the findings of the present study highlight the importance of screening for high-risk populations (eg, patients with diabetes, patients with peripheral vascular disease, drug abusers) before orthopedic surgery, especially during definitive treatment of ankle fracture.
Recently, Nåsell and colleagues10 found that a well-implemented smoking cessation program was associated with a statistically significant reduction in complications 6 and 12 weeks after surgery. The target treatment groups were patients who underwent major lower extremity and upper extremity orthopedic surgery. The most common surgery performed in the study was ORIF of ankle fractures. The authors concluded that a smoking cessation intervention program during the first 6 weeks after acute fracture surgery decreases the risk for postoperative complications. However, no recommendations were made for treating patients with other addictions, such as alcohol and illicit drug addictions.
To our knowledge, our study is the first to critically examine postoperative complications in ankle fracture patients with a history of illicit drug abuse as determined by preoperative positive UDS. These data suggest the importance of critically evaluating this patient population. The rates of deep infection, nonunion, and pooled major complications were all notable. Furthermore, compared with negative UDS patients, positive UDS patients were more than 7 times likely to develop a nonunion and more than 4 times likely to develop a deep infection. The reasons are likely multifactorial but may involve factors such as injury severity, poor nutrition, suboptimal living conditions, difficulty complying with weight-bearing restrictions, and, possibly, poor compliance with wound-care recommendations. Determining the influence of each factor was beyond the scope of this study. However, further investigation is warranted.
The difference in incidence of smoking between the 2 groups was statistically significant. As smoking has been well documented as contributing to poor wound and bone healing,14-16 it is likely to have been a contributory factor. However, nicotine levels are not routinely part of UDS, and people who quit smoking typically take 7 to 10 days to demonstrate a measurable drop in cotinine levels. On the other hand, screening for drugs takes only a few minutes and can provide useful information during the preoperative period. It was suggested that positive UDS patients were significantly likely to be tobacco users as well.
The 2 groups were not significantly different with respect to mean follow-up time or loss to follow-up. Although mean follow-up was longer in negative UDS patients, the standard deviation was large in both groups. Given the positive UDS patients’ higher incidence of deep infection and nonunion, both of which typically prolong the course of treatment, the results were likely deceptive. Patients with a history of illicit drug use have confounding variables (eg, psychiatric disorders, financial strife) that make treatment compliance and follow-up difficult.17
Some of the weaknesses of this study are inherent to its retrospective design and limited sample size. Furthermore, patient satisfaction scores and ankle-specific outcome measures, such as AOFAS (American Orthopaedic Foot and Ankle Society) scores, were not considered. Prospective collection of data that include patient satisfaction scores and ankle-specific outcome measures would be optimal. Our current recommendation is to obtain preoperative UDS and illicit drug use history for all trauma patients. In addition, operating surgeons should exercise caution when caring for patients who test positive for illicit drugs.
Conclusion
We evaluated the incidence of complications experienced by positive UDS patients undergoing surgical treatment of ankle fractures. It is well documented that illicit drug users who receive general anesthesia have complications. However, little is known about the untoward effects of illicit drugs on postoperative complications. Furthermore, the efficacy of drug cessation programs in minimizing these complications has not been fully explored.
In conclusion, similar to patients with diabetes, patients with a history of recent illicit drug use, as evidenced by preoperative positive UDS, are at increased risk for complications during treatment for ankle fracture. These data suggest that practicing orthopedists should be more vigilant when caring for ankle fracture patients with preoperative positive UDS.
1. Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77(1):142-152.
2. Culver JL, Walker JR. Anesthetic implications of illicit drug use. J Perianesth Nurs. 1999;14(2):82-90.
3. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32(1):113-133.
4. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2);288-293.
5. Clark RF, Harchelroad F. Toxicology screening of the trauma patient: a changing profile. Ann Emerg Med. 1991;20(2):151-153.
6. SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91(5):1042-1049.
7. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570-1578.
8. Egol KA, Tejwani NC, Walsh MG, Capla EL, Koval KJ. Predictors of short-term functional outcome following ankle fracture surgery. J Bone Joint Surg Am. 2006;88(5):974-979.
9. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus J Bone Joint Surg Br. 2005;87(4):489-495.
10. Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342.
11. Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21-27.
12. Flynn JM, Rodriguez-del Rio F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21(4):311-319.
13. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375-380.
14. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1-5.
15. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181.
16. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
17. Torrens M, Gilchrist G, Domingo-Salvany A; PsyCoBarcelona Group. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2010;113(2-3):147-156.
Open treatment of ankle fractures is one of the most common procedures performed by orthopedic surgeons.1 Among the younger patient population, ankle fractures represent a significant proportion of orthopedic injuries.2 The reported incidence of illicit drug and alcohol use in the urban trauma population ranges from 36% to 86%,2 and medical and anesthetic complications associated with illicit drug use have been well documented in surgical patients.2 However, patients with a recent history of drug abuse may be subject to a separate but related set of complications of open treatment of ankle fractures.
The perioperative complications associated with open treatment of ankle fractures in patients with diabetes mellitus have been well described.3-6 Similarly, previous studies have suggested that peripheral vascular disease, complicated diabetes, and smoking are risk factors for poor outcomes in patients who require open reduction and internal fixation (ORIF) in lower extremity trauma.7-9 However, there are few data on the complications specifically associated with illicit drug use and orthopedic surgery. Properly identifying these high-risk groups and being cognizant of commonly associated complications are likely important in ensuring proper perioperative care and may alter follow-up protocols in these patients.
We conducted a study to identify the complications associated with open treatment of ankle fractures in patients who tested positive for illicit drugs on urine drug screen (UDS). We hypothesized that patients who had a history of positive UDS and underwent ORIF of an ankle fracture would have a higher incidence of major and minor complications.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 142 patients who underwent open treatment of an ankle fracture between 2006 and 2010. Data sources included patient demographic information, radiographs, preoperative UDS, attending surgeons’ clinical office notes, and clinical laboratory data. Our institution’s standard protocol for ankle fractures was followed for all patients in the study. All patients were evaluated by an orthopedic physician, in either the emergency department or the office, during application of a well-padded Jones splint before surgery. Oral narcotic pain medication was routinely prescribed. All patients were seen, within 10 days of injury, for surgery planning. A board-certified orthopedic surgeon surgically stabilized the ankle fractures. The postoperative treatment regimen, per protocol, included non-weight-bearing in a padded Jones splint dressing; oral narcotic pain medication; physical therapy; and routine scheduled follow-up. In open fracture cases, patients were taken urgently to the operating room for irrigation and débridement with stabilization. Which treatment would be initially used—external fixation or ORIF—was determined on a case-by-case basis.
The sample consisted of adults (age, >18 years) who had undergone definitive ORIF of a lateral malleolar, bimalleolar, or trimalleolar ankle fracture during the study period. Polytrauma patients, patients with external fixation as definitive treatment, and patients with nonoperative treatment were excluded. Before surgical management, all patients were tested for recent illicit drug use by UDS (standard protocol at our institution). UDS, measured for cocaine, marijuana, PCP (phencyclidine), opiates, and barbiturates, was obtained in the office setting or emergency department or on day of surgery. The patients were divided into 2 groups, positive and negative UDS. Patients with documented receipt of narcotic pain medication before UDS were excluded.
The outcomes identified as dependent variables included nonunion, malunion, superficial or deep infection, amputation, delay in treatment, days to healing, repeat surgery, long-term bracing, and loss to follow-up. A nonunion was defined as lasting longer than 9 months and not showing radiographic signs of progression toward healing for 3 consecutive months. These complications were identified with use of attending surgeon clinical progress notes, laboratory values, radiographic parameters, and inpatient readmissions/surgeries associated with these outcomes. Nonunion, malunion, superficial or deep infection, and amputation were then grouped as major complications and analyzed as pooled major complications.
The Fisher exact test was used to analyze categorical variables with respect to UDS. The Wilcoxon rank sum test was used to determine statistical significance for continuous variables. Univariate logistic regression examined both continuous and categorical variables to evaluate predictors for a selected outcome. Statistical significance was set a priori at P ≤ .05, with significant factors indicating an increase (or decrease) in the outcome variable being tested.
Results
We retrospectively reviewed the cases of 142 patients. Table 1 lists the number of cases by fracture type. Bimalleolar fractures were most common, accounting for 99 (69.8%) of the 142 cases. Isolated lateral malleolar fractures accounted for 16 cases (11.2%), and trimalleolar fractures accounted for 27 cases (19%).
Twenty-five (18%) of the 142 patients tested positive for illicit drugs. Mean age was 45.2 years for positive UDS patients and 41.5 years for negative UDS patients. Open fracture cases represented 4.3% of negative UDS patients and 16% of positive UDS patients. Fifty-two percent of positive UDS patients and 32% of negative UDS patients were also tobacco users. These data were statistically significant (P = .003) There were no significant differences in age, sex, incidence of diabetes, incidence of open fracture, or time to surgery between the groups (Table 2).
Incidence of nonunion was higher in positive UDS patients (n = 5; P = .01), as was incidence of deep infection (n = 4; P = .05) (Table 3).
Mean time to radiographic healing was 50.7 days in negative UDS patients and 82.8 days in positive UDS patients (P > .99). Incidence of nonunion was 3.5% in negative UDS patients and 20% in positive UDS patients (P = .01). There were no malunions in negative UDS patients and 2 malunions in positive UDS patients. Incidence of deep infections was 2.5% in negative UDS patients and 16% in positive UDS patients (P = .04). No significant differences were found in incidence of malunions, superficial infections, amputations, need for repeat surgery, continued bracing, or loss to follow-up.
Major complications were defined as superficial or deep infections, amputations, malunions, and nonunions. The rate of major complication was significantly (P = .03) higher in positive UDS patients (24.24%) than in negative UDS patients (7.69%) (Table 4).
Discussion
In the present study, we retrospectively reviewed the cases of patients treated with ORIF for varying types of ankle fractures. Important major and minor complications were analyzed. The overall incidence of major complications in negative UDS patients was only 7.69%, consistent with previously reported results in patients with ankle fractures.6,10 However, a statistically significant (P = .03) increased incidence of major complications—an alarmingly high rate of almost 1 in 4—was found in positive UDS patients. Our results also demonstrated a significantly higher rate of nonunion and deep infection in positive UDS patients. Calculated odds ratios were 7.37 and 4.27 for nonunion and deep infection, respectively—arguably 2 of the most devastating postoperative complications in positive UDS patients.
Previous studies have found that open fractures, age, and medical comorbidities are significant predictors of short-term complications, such as wound healing, infection, persistent pain, and delayed union.3-6 Levy and colleagues11 examined the incidence of orthopedic trauma in positive UDS patients. These patients had orthopedic injuries that were more severe and required longer hospitalization. However, the study did not address patients with ankle fractures or the incidence of major complications. Diabetes and peripheral vascular disease are significant risk factors for many surgical procedures in orthopedic surgery.3,7-9,12,13 Tight glycemic control and optimization of medical comorbidities decrease postoperative complications.12,13 SooHoo and colleagues6 found that history of diabetes and history of peripheral vascular disease were significant predictors of short-term complications of mortality, infection, reoperation, and amputation. The rate of infection in the complicated diabetes group was statistically higher as well. The effect of illicit drug use was not analyzed in that study. We think the findings of the present study highlight the importance of screening for high-risk populations (eg, patients with diabetes, patients with peripheral vascular disease, drug abusers) before orthopedic surgery, especially during definitive treatment of ankle fracture.
Recently, Nåsell and colleagues10 found that a well-implemented smoking cessation program was associated with a statistically significant reduction in complications 6 and 12 weeks after surgery. The target treatment groups were patients who underwent major lower extremity and upper extremity orthopedic surgery. The most common surgery performed in the study was ORIF of ankle fractures. The authors concluded that a smoking cessation intervention program during the first 6 weeks after acute fracture surgery decreases the risk for postoperative complications. However, no recommendations were made for treating patients with other addictions, such as alcohol and illicit drug addictions.
To our knowledge, our study is the first to critically examine postoperative complications in ankle fracture patients with a history of illicit drug abuse as determined by preoperative positive UDS. These data suggest the importance of critically evaluating this patient population. The rates of deep infection, nonunion, and pooled major complications were all notable. Furthermore, compared with negative UDS patients, positive UDS patients were more than 7 times likely to develop a nonunion and more than 4 times likely to develop a deep infection. The reasons are likely multifactorial but may involve factors such as injury severity, poor nutrition, suboptimal living conditions, difficulty complying with weight-bearing restrictions, and, possibly, poor compliance with wound-care recommendations. Determining the influence of each factor was beyond the scope of this study. However, further investigation is warranted.
The difference in incidence of smoking between the 2 groups was statistically significant. As smoking has been well documented as contributing to poor wound and bone healing,14-16 it is likely to have been a contributory factor. However, nicotine levels are not routinely part of UDS, and people who quit smoking typically take 7 to 10 days to demonstrate a measurable drop in cotinine levels. On the other hand, screening for drugs takes only a few minutes and can provide useful information during the preoperative period. It was suggested that positive UDS patients were significantly likely to be tobacco users as well.
The 2 groups were not significantly different with respect to mean follow-up time or loss to follow-up. Although mean follow-up was longer in negative UDS patients, the standard deviation was large in both groups. Given the positive UDS patients’ higher incidence of deep infection and nonunion, both of which typically prolong the course of treatment, the results were likely deceptive. Patients with a history of illicit drug use have confounding variables (eg, psychiatric disorders, financial strife) that make treatment compliance and follow-up difficult.17
Some of the weaknesses of this study are inherent to its retrospective design and limited sample size. Furthermore, patient satisfaction scores and ankle-specific outcome measures, such as AOFAS (American Orthopaedic Foot and Ankle Society) scores, were not considered. Prospective collection of data that include patient satisfaction scores and ankle-specific outcome measures would be optimal. Our current recommendation is to obtain preoperative UDS and illicit drug use history for all trauma patients. In addition, operating surgeons should exercise caution when caring for patients who test positive for illicit drugs.
Conclusion
We evaluated the incidence of complications experienced by positive UDS patients undergoing surgical treatment of ankle fractures. It is well documented that illicit drug users who receive general anesthesia have complications. However, little is known about the untoward effects of illicit drugs on postoperative complications. Furthermore, the efficacy of drug cessation programs in minimizing these complications has not been fully explored.
In conclusion, similar to patients with diabetes, patients with a history of recent illicit drug use, as evidenced by preoperative positive UDS, are at increased risk for complications during treatment for ankle fracture. These data suggest that practicing orthopedists should be more vigilant when caring for ankle fracture patients with preoperative positive UDS.
Open treatment of ankle fractures is one of the most common procedures performed by orthopedic surgeons.1 Among the younger patient population, ankle fractures represent a significant proportion of orthopedic injuries.2 The reported incidence of illicit drug and alcohol use in the urban trauma population ranges from 36% to 86%,2 and medical and anesthetic complications associated with illicit drug use have been well documented in surgical patients.2 However, patients with a recent history of drug abuse may be subject to a separate but related set of complications of open treatment of ankle fractures.
The perioperative complications associated with open treatment of ankle fractures in patients with diabetes mellitus have been well described.3-6 Similarly, previous studies have suggested that peripheral vascular disease, complicated diabetes, and smoking are risk factors for poor outcomes in patients who require open reduction and internal fixation (ORIF) in lower extremity trauma.7-9 However, there are few data on the complications specifically associated with illicit drug use and orthopedic surgery. Properly identifying these high-risk groups and being cognizant of commonly associated complications are likely important in ensuring proper perioperative care and may alter follow-up protocols in these patients.
We conducted a study to identify the complications associated with open treatment of ankle fractures in patients who tested positive for illicit drugs on urine drug screen (UDS). We hypothesized that patients who had a history of positive UDS and underwent ORIF of an ankle fracture would have a higher incidence of major and minor complications.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 142 patients who underwent open treatment of an ankle fracture between 2006 and 2010. Data sources included patient demographic information, radiographs, preoperative UDS, attending surgeons’ clinical office notes, and clinical laboratory data. Our institution’s standard protocol for ankle fractures was followed for all patients in the study. All patients were evaluated by an orthopedic physician, in either the emergency department or the office, during application of a well-padded Jones splint before surgery. Oral narcotic pain medication was routinely prescribed. All patients were seen, within 10 days of injury, for surgery planning. A board-certified orthopedic surgeon surgically stabilized the ankle fractures. The postoperative treatment regimen, per protocol, included non-weight-bearing in a padded Jones splint dressing; oral narcotic pain medication; physical therapy; and routine scheduled follow-up. In open fracture cases, patients were taken urgently to the operating room for irrigation and débridement with stabilization. Which treatment would be initially used—external fixation or ORIF—was determined on a case-by-case basis.
The sample consisted of adults (age, >18 years) who had undergone definitive ORIF of a lateral malleolar, bimalleolar, or trimalleolar ankle fracture during the study period. Polytrauma patients, patients with external fixation as definitive treatment, and patients with nonoperative treatment were excluded. Before surgical management, all patients were tested for recent illicit drug use by UDS (standard protocol at our institution). UDS, measured for cocaine, marijuana, PCP (phencyclidine), opiates, and barbiturates, was obtained in the office setting or emergency department or on day of surgery. The patients were divided into 2 groups, positive and negative UDS. Patients with documented receipt of narcotic pain medication before UDS were excluded.
The outcomes identified as dependent variables included nonunion, malunion, superficial or deep infection, amputation, delay in treatment, days to healing, repeat surgery, long-term bracing, and loss to follow-up. A nonunion was defined as lasting longer than 9 months and not showing radiographic signs of progression toward healing for 3 consecutive months. These complications were identified with use of attending surgeon clinical progress notes, laboratory values, radiographic parameters, and inpatient readmissions/surgeries associated with these outcomes. Nonunion, malunion, superficial or deep infection, and amputation were then grouped as major complications and analyzed as pooled major complications.
The Fisher exact test was used to analyze categorical variables with respect to UDS. The Wilcoxon rank sum test was used to determine statistical significance for continuous variables. Univariate logistic regression examined both continuous and categorical variables to evaluate predictors for a selected outcome. Statistical significance was set a priori at P ≤ .05, with significant factors indicating an increase (or decrease) in the outcome variable being tested.
Results
We retrospectively reviewed the cases of 142 patients. Table 1 lists the number of cases by fracture type. Bimalleolar fractures were most common, accounting for 99 (69.8%) of the 142 cases. Isolated lateral malleolar fractures accounted for 16 cases (11.2%), and trimalleolar fractures accounted for 27 cases (19%).
Twenty-five (18%) of the 142 patients tested positive for illicit drugs. Mean age was 45.2 years for positive UDS patients and 41.5 years for negative UDS patients. Open fracture cases represented 4.3% of negative UDS patients and 16% of positive UDS patients. Fifty-two percent of positive UDS patients and 32% of negative UDS patients were also tobacco users. These data were statistically significant (P = .003) There were no significant differences in age, sex, incidence of diabetes, incidence of open fracture, or time to surgery between the groups (Table 2).
Incidence of nonunion was higher in positive UDS patients (n = 5; P = .01), as was incidence of deep infection (n = 4; P = .05) (Table 3).
Mean time to radiographic healing was 50.7 days in negative UDS patients and 82.8 days in positive UDS patients (P > .99). Incidence of nonunion was 3.5% in negative UDS patients and 20% in positive UDS patients (P = .01). There were no malunions in negative UDS patients and 2 malunions in positive UDS patients. Incidence of deep infections was 2.5% in negative UDS patients and 16% in positive UDS patients (P = .04). No significant differences were found in incidence of malunions, superficial infections, amputations, need for repeat surgery, continued bracing, or loss to follow-up.
Major complications were defined as superficial or deep infections, amputations, malunions, and nonunions. The rate of major complication was significantly (P = .03) higher in positive UDS patients (24.24%) than in negative UDS patients (7.69%) (Table 4).
Discussion
In the present study, we retrospectively reviewed the cases of patients treated with ORIF for varying types of ankle fractures. Important major and minor complications were analyzed. The overall incidence of major complications in negative UDS patients was only 7.69%, consistent with previously reported results in patients with ankle fractures.6,10 However, a statistically significant (P = .03) increased incidence of major complications—an alarmingly high rate of almost 1 in 4—was found in positive UDS patients. Our results also demonstrated a significantly higher rate of nonunion and deep infection in positive UDS patients. Calculated odds ratios were 7.37 and 4.27 for nonunion and deep infection, respectively—arguably 2 of the most devastating postoperative complications in positive UDS patients.
Previous studies have found that open fractures, age, and medical comorbidities are significant predictors of short-term complications, such as wound healing, infection, persistent pain, and delayed union.3-6 Levy and colleagues11 examined the incidence of orthopedic trauma in positive UDS patients. These patients had orthopedic injuries that were more severe and required longer hospitalization. However, the study did not address patients with ankle fractures or the incidence of major complications. Diabetes and peripheral vascular disease are significant risk factors for many surgical procedures in orthopedic surgery.3,7-9,12,13 Tight glycemic control and optimization of medical comorbidities decrease postoperative complications.12,13 SooHoo and colleagues6 found that history of diabetes and history of peripheral vascular disease were significant predictors of short-term complications of mortality, infection, reoperation, and amputation. The rate of infection in the complicated diabetes group was statistically higher as well. The effect of illicit drug use was not analyzed in that study. We think the findings of the present study highlight the importance of screening for high-risk populations (eg, patients with diabetes, patients with peripheral vascular disease, drug abusers) before orthopedic surgery, especially during definitive treatment of ankle fracture.
Recently, Nåsell and colleagues10 found that a well-implemented smoking cessation program was associated with a statistically significant reduction in complications 6 and 12 weeks after surgery. The target treatment groups were patients who underwent major lower extremity and upper extremity orthopedic surgery. The most common surgery performed in the study was ORIF of ankle fractures. The authors concluded that a smoking cessation intervention program during the first 6 weeks after acute fracture surgery decreases the risk for postoperative complications. However, no recommendations were made for treating patients with other addictions, such as alcohol and illicit drug addictions.
To our knowledge, our study is the first to critically examine postoperative complications in ankle fracture patients with a history of illicit drug abuse as determined by preoperative positive UDS. These data suggest the importance of critically evaluating this patient population. The rates of deep infection, nonunion, and pooled major complications were all notable. Furthermore, compared with negative UDS patients, positive UDS patients were more than 7 times likely to develop a nonunion and more than 4 times likely to develop a deep infection. The reasons are likely multifactorial but may involve factors such as injury severity, poor nutrition, suboptimal living conditions, difficulty complying with weight-bearing restrictions, and, possibly, poor compliance with wound-care recommendations. Determining the influence of each factor was beyond the scope of this study. However, further investigation is warranted.
The difference in incidence of smoking between the 2 groups was statistically significant. As smoking has been well documented as contributing to poor wound and bone healing,14-16 it is likely to have been a contributory factor. However, nicotine levels are not routinely part of UDS, and people who quit smoking typically take 7 to 10 days to demonstrate a measurable drop in cotinine levels. On the other hand, screening for drugs takes only a few minutes and can provide useful information during the preoperative period. It was suggested that positive UDS patients were significantly likely to be tobacco users as well.
The 2 groups were not significantly different with respect to mean follow-up time or loss to follow-up. Although mean follow-up was longer in negative UDS patients, the standard deviation was large in both groups. Given the positive UDS patients’ higher incidence of deep infection and nonunion, both of which typically prolong the course of treatment, the results were likely deceptive. Patients with a history of illicit drug use have confounding variables (eg, psychiatric disorders, financial strife) that make treatment compliance and follow-up difficult.17
Some of the weaknesses of this study are inherent to its retrospective design and limited sample size. Furthermore, patient satisfaction scores and ankle-specific outcome measures, such as AOFAS (American Orthopaedic Foot and Ankle Society) scores, were not considered. Prospective collection of data that include patient satisfaction scores and ankle-specific outcome measures would be optimal. Our current recommendation is to obtain preoperative UDS and illicit drug use history for all trauma patients. In addition, operating surgeons should exercise caution when caring for patients who test positive for illicit drugs.
Conclusion
We evaluated the incidence of complications experienced by positive UDS patients undergoing surgical treatment of ankle fractures. It is well documented that illicit drug users who receive general anesthesia have complications. However, little is known about the untoward effects of illicit drugs on postoperative complications. Furthermore, the efficacy of drug cessation programs in minimizing these complications has not been fully explored.
In conclusion, similar to patients with diabetes, patients with a history of recent illicit drug use, as evidenced by preoperative positive UDS, are at increased risk for complications during treatment for ankle fracture. These data suggest that practicing orthopedists should be more vigilant when caring for ankle fracture patients with preoperative positive UDS.
1. Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77(1):142-152.
2. Culver JL, Walker JR. Anesthetic implications of illicit drug use. J Perianesth Nurs. 1999;14(2):82-90.
3. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32(1):113-133.
4. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2);288-293.
5. Clark RF, Harchelroad F. Toxicology screening of the trauma patient: a changing profile. Ann Emerg Med. 1991;20(2):151-153.
6. SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91(5):1042-1049.
7. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570-1578.
8. Egol KA, Tejwani NC, Walsh MG, Capla EL, Koval KJ. Predictors of short-term functional outcome following ankle fracture surgery. J Bone Joint Surg Am. 2006;88(5):974-979.
9. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus J Bone Joint Surg Br. 2005;87(4):489-495.
10. Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342.
11. Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21-27.
12. Flynn JM, Rodriguez-del Rio F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21(4):311-319.
13. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375-380.
14. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1-5.
15. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181.
16. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
17. Torrens M, Gilchrist G, Domingo-Salvany A; PsyCoBarcelona Group. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2010;113(2-3):147-156.
1. Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77(1):142-152.
2. Culver JL, Walker JR. Anesthetic implications of illicit drug use. J Perianesth Nurs. 1999;14(2):82-90.
3. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32(1):113-133.
4. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2);288-293.
5. Clark RF, Harchelroad F. Toxicology screening of the trauma patient: a changing profile. Ann Emerg Med. 1991;20(2):151-153.
6. SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91(5):1042-1049.
7. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570-1578.
8. Egol KA, Tejwani NC, Walsh MG, Capla EL, Koval KJ. Predictors of short-term functional outcome following ankle fracture surgery. J Bone Joint Surg Am. 2006;88(5):974-979.
9. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus J Bone Joint Surg Br. 2005;87(4):489-495.
10. Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342.
11. Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21-27.
12. Flynn JM, Rodriguez-del Rio F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21(4):311-319.
13. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375-380.
14. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1-5.
15. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181.
16. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
17. Torrens M, Gilchrist G, Domingo-Salvany A; PsyCoBarcelona Group. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2010;113(2-3):147-156.
MOVES Study Touts Benefits of Glucosamine/Chondroitin for Knee Osteoarthritis
The combination of chondroitin sulfate plus glucosamine hydrochloride has comparable efficacy to the anti-inflammatory drug celecoxib after 6 months of treatment in severe osteoarthritis, according to a recent clinical trial published online ahead of print January 14 in Annals of the Rheumatic Diseases. Specifically, it was seen that the combination of these two drugs caused a clinically relevant reduction in pain, functional disability, stiffness, swelling, and joint effusion.
MOVES (Multicentre Osteoarthritis InterVEntion Trial with Sysadoa) is a multicenter, randomized, parallel-group, double-blind controlled clinical trial that enrolled 606 patients from at 42 centers in France, Germany, Poland, and Spain. The study was sponsored by Bioibérica, suppliers of the chondroitin sulfate used in Cosamin DS. The trial was developed as an extension of the GAIT (Glucosamine/Chondroitin Arthritis Intervention Trial) study, which showed that the combination of 1,500 mg per day of glucosamine hydrochloride and 1,200 mg per day of chondroitin sulfate was effective in a moderate to severe subgroup of patients.
Patients with primary knee osteoarthritis and moderate to severe pain were randomized to receive 1,200 mg of chondroitin sulfate (400 mg/d tid) plus 1,500 mg of glucosamine hydrochloride (500 mg/d tid) or celecoxib (200 mg) every day for 6 months.
After 6 months, patients experienced the following:
• A reduction in pain by 50.1%.
• A reduction in swelling by 53%.
• A reduction in stiffness by 46.9%.
• A reduction in functional disability by 45.5%.
• A reduction in joint effusion by 56%.
“This study confirms the efficacy of the combination of pharmaceutical-grade chondroitin sulfate and glucosamine [hydrochloride] in the long term and suggests that, considering its excellent safety profile, it may be a good alternative for patients with cardiovascular or gastrointestinal problems, for whom chronic treatment with NSAIDs cannot be recommended,” said lead author Marc C. Hochberg, MD, MPH, a Professor at the University of Maryland School of Medicine in Baltimore.
Suggested Reading
Hochberg MC, Martel-Pelletier J, Monfort J, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2015 Jan 14. [Epub ahead of print]
The combination of chondroitin sulfate plus glucosamine hydrochloride has comparable efficacy to the anti-inflammatory drug celecoxib after 6 months of treatment in severe osteoarthritis, according to a recent clinical trial published online ahead of print January 14 in Annals of the Rheumatic Diseases. Specifically, it was seen that the combination of these two drugs caused a clinically relevant reduction in pain, functional disability, stiffness, swelling, and joint effusion.
MOVES (Multicentre Osteoarthritis InterVEntion Trial with Sysadoa) is a multicenter, randomized, parallel-group, double-blind controlled clinical trial that enrolled 606 patients from at 42 centers in France, Germany, Poland, and Spain. The study was sponsored by Bioibérica, suppliers of the chondroitin sulfate used in Cosamin DS. The trial was developed as an extension of the GAIT (Glucosamine/Chondroitin Arthritis Intervention Trial) study, which showed that the combination of 1,500 mg per day of glucosamine hydrochloride and 1,200 mg per day of chondroitin sulfate was effective in a moderate to severe subgroup of patients.
Patients with primary knee osteoarthritis and moderate to severe pain were randomized to receive 1,200 mg of chondroitin sulfate (400 mg/d tid) plus 1,500 mg of glucosamine hydrochloride (500 mg/d tid) or celecoxib (200 mg) every day for 6 months.
After 6 months, patients experienced the following:
• A reduction in pain by 50.1%.
• A reduction in swelling by 53%.
• A reduction in stiffness by 46.9%.
• A reduction in functional disability by 45.5%.
• A reduction in joint effusion by 56%.
“This study confirms the efficacy of the combination of pharmaceutical-grade chondroitin sulfate and glucosamine [hydrochloride] in the long term and suggests that, considering its excellent safety profile, it may be a good alternative for patients with cardiovascular or gastrointestinal problems, for whom chronic treatment with NSAIDs cannot be recommended,” said lead author Marc C. Hochberg, MD, MPH, a Professor at the University of Maryland School of Medicine in Baltimore.
The combination of chondroitin sulfate plus glucosamine hydrochloride has comparable efficacy to the anti-inflammatory drug celecoxib after 6 months of treatment in severe osteoarthritis, according to a recent clinical trial published online ahead of print January 14 in Annals of the Rheumatic Diseases. Specifically, it was seen that the combination of these two drugs caused a clinically relevant reduction in pain, functional disability, stiffness, swelling, and joint effusion.
MOVES (Multicentre Osteoarthritis InterVEntion Trial with Sysadoa) is a multicenter, randomized, parallel-group, double-blind controlled clinical trial that enrolled 606 patients from at 42 centers in France, Germany, Poland, and Spain. The study was sponsored by Bioibérica, suppliers of the chondroitin sulfate used in Cosamin DS. The trial was developed as an extension of the GAIT (Glucosamine/Chondroitin Arthritis Intervention Trial) study, which showed that the combination of 1,500 mg per day of glucosamine hydrochloride and 1,200 mg per day of chondroitin sulfate was effective in a moderate to severe subgroup of patients.
Patients with primary knee osteoarthritis and moderate to severe pain were randomized to receive 1,200 mg of chondroitin sulfate (400 mg/d tid) plus 1,500 mg of glucosamine hydrochloride (500 mg/d tid) or celecoxib (200 mg) every day for 6 months.
After 6 months, patients experienced the following:
• A reduction in pain by 50.1%.
• A reduction in swelling by 53%.
• A reduction in stiffness by 46.9%.
• A reduction in functional disability by 45.5%.
• A reduction in joint effusion by 56%.
“This study confirms the efficacy of the combination of pharmaceutical-grade chondroitin sulfate and glucosamine [hydrochloride] in the long term and suggests that, considering its excellent safety profile, it may be a good alternative for patients with cardiovascular or gastrointestinal problems, for whom chronic treatment with NSAIDs cannot be recommended,” said lead author Marc C. Hochberg, MD, MPH, a Professor at the University of Maryland School of Medicine in Baltimore.
Suggested Reading
Hochberg MC, Martel-Pelletier J, Monfort J, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2015 Jan 14. [Epub ahead of print]
Suggested Reading
Hochberg MC, Martel-Pelletier J, Monfort J, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2015 Jan 14. [Epub ahead of print]
Fluorescent Probe May Detect Early to Moderate Osteoarthritis
A fluorescent probe may make it easier to diagnose and monitor osteoarthritis, according to a study published in the February issue of Arthritis & Rheumatology. Researchers found that a fluorescent probe tracked the development of osteoarthritis in male mice, brightening as the disease progressed. Their study is the first to demonstrate that near-infared fluorescence can be used to detect osteoarthritis changes over time.
“Patients are frequently in pain by the time osteoarthritis is diagnosed. The imaging tests most frequently used, x-rays, do not indicate the level of pain or allow us to directly see the amount of cartilage loss, which is a challenge for physicians and patients,” said lead author Averi A. Leahy, BA, an MD/PhD student in the medical scientist training program at Tufts University School of Medicine (TUSM) and the Sackler School of Graduate Biomedical Sciences at Tufts in Boston.
For this study, the right knees of 54 mice were affected by injury-induced osteoarthritis and served as the experimental group. The healthy left knees of the mice served as the control group.
Over a 2-month period, the researchers took images of each knee every 2 weeks to determine if the fluorescent probe emitted a signal. The signal became brighter in the injured right knee, at every examined time point, through the early to moderate stages of osteoarthritis. The probe emitted a lower signal in the healthy left knee, and did not increase significantly over time.
According to the researchers, the fluorescent probe made it easy to see the activities that lead to cartilage breakdown in the initial and moderate stages of osteoarthritis, which is necessary for early detection and adequate monitoring of the disease.
Senior author Li Zeng, PhD, an Associate Professor in the Department of Integrative Physiology and Pathobiology at TUSM and member of the cellular, molecular, and developmental biology program faculty at the Sackler School reported that the next step is to monitor the fluorescent probe over a longer period of time to determine whether the same results are produced during the late stages of osteoarthritis.
Suggested Reading
Leahy AA, Esfahani SA, Foote AT, et al. Analysis of the trajectory of osteoarthritis development in a mouse model by serial near-infrared fluorescence imaging of matrix metalloproteinase activities. Arthritis Rheumatol. 2015;67(2):442-453.
A fluorescent probe may make it easier to diagnose and monitor osteoarthritis, according to a study published in the February issue of Arthritis & Rheumatology. Researchers found that a fluorescent probe tracked the development of osteoarthritis in male mice, brightening as the disease progressed. Their study is the first to demonstrate that near-infared fluorescence can be used to detect osteoarthritis changes over time.
“Patients are frequently in pain by the time osteoarthritis is diagnosed. The imaging tests most frequently used, x-rays, do not indicate the level of pain or allow us to directly see the amount of cartilage loss, which is a challenge for physicians and patients,” said lead author Averi A. Leahy, BA, an MD/PhD student in the medical scientist training program at Tufts University School of Medicine (TUSM) and the Sackler School of Graduate Biomedical Sciences at Tufts in Boston.
For this study, the right knees of 54 mice were affected by injury-induced osteoarthritis and served as the experimental group. The healthy left knees of the mice served as the control group.
Over a 2-month period, the researchers took images of each knee every 2 weeks to determine if the fluorescent probe emitted a signal. The signal became brighter in the injured right knee, at every examined time point, through the early to moderate stages of osteoarthritis. The probe emitted a lower signal in the healthy left knee, and did not increase significantly over time.
According to the researchers, the fluorescent probe made it easy to see the activities that lead to cartilage breakdown in the initial and moderate stages of osteoarthritis, which is necessary for early detection and adequate monitoring of the disease.
Senior author Li Zeng, PhD, an Associate Professor in the Department of Integrative Physiology and Pathobiology at TUSM and member of the cellular, molecular, and developmental biology program faculty at the Sackler School reported that the next step is to monitor the fluorescent probe over a longer period of time to determine whether the same results are produced during the late stages of osteoarthritis.
A fluorescent probe may make it easier to diagnose and monitor osteoarthritis, according to a study published in the February issue of Arthritis & Rheumatology. Researchers found that a fluorescent probe tracked the development of osteoarthritis in male mice, brightening as the disease progressed. Their study is the first to demonstrate that near-infared fluorescence can be used to detect osteoarthritis changes over time.
“Patients are frequently in pain by the time osteoarthritis is diagnosed. The imaging tests most frequently used, x-rays, do not indicate the level of pain or allow us to directly see the amount of cartilage loss, which is a challenge for physicians and patients,” said lead author Averi A. Leahy, BA, an MD/PhD student in the medical scientist training program at Tufts University School of Medicine (TUSM) and the Sackler School of Graduate Biomedical Sciences at Tufts in Boston.
For this study, the right knees of 54 mice were affected by injury-induced osteoarthritis and served as the experimental group. The healthy left knees of the mice served as the control group.
Over a 2-month period, the researchers took images of each knee every 2 weeks to determine if the fluorescent probe emitted a signal. The signal became brighter in the injured right knee, at every examined time point, through the early to moderate stages of osteoarthritis. The probe emitted a lower signal in the healthy left knee, and did not increase significantly over time.
According to the researchers, the fluorescent probe made it easy to see the activities that lead to cartilage breakdown in the initial and moderate stages of osteoarthritis, which is necessary for early detection and adequate monitoring of the disease.
Senior author Li Zeng, PhD, an Associate Professor in the Department of Integrative Physiology and Pathobiology at TUSM and member of the cellular, molecular, and developmental biology program faculty at the Sackler School reported that the next step is to monitor the fluorescent probe over a longer period of time to determine whether the same results are produced during the late stages of osteoarthritis.
Suggested Reading
Leahy AA, Esfahani SA, Foote AT, et al. Analysis of the trajectory of osteoarthritis development in a mouse model by serial near-infrared fluorescence imaging of matrix metalloproteinase activities. Arthritis Rheumatol. 2015;67(2):442-453.
Suggested Reading
Leahy AA, Esfahani SA, Foote AT, et al. Analysis of the trajectory of osteoarthritis development in a mouse model by serial near-infrared fluorescence imaging of matrix metalloproteinase activities. Arthritis Rheumatol. 2015;67(2):442-453.
A Novel Treatment for Refractory Plantar Fasciitis
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
Low Back Pain Triggered by Fatigue, Distractions, and Awkward Positions
New research reveals the physical and psychosocial factors that significantly increase the risk of low back pain onset. The trial results, which were published online ahead of print February 9 in Arthritis Care & Research, show that being engaged in manual tasks involving awkward positions will increase the risk of low back pain by 8 times. People who are distracted during activities or fatigued also significantly increase their risk of acute low back pain.
“Understanding which risk factors contribute to back pain and controlling exposure to these risks is an important first step in prevention,” said Manuela Ferreira, PhD, Associate Professor at the George Institute for Global Health and Sydney Medical School at the University of Sydney in New South Wales, Australia. “Our study is the first to examine brief exposure to a range of modifiable triggers for an acute episode of low back pain.”
For this case-crossover study, Dr. Ferreira and colleagues recruited 999 participants from 300 primary care clinics in Sydney, Australia, who had an acute low back pain episode between October 2011 and November 2012. Participants were asked to report exposure to 12 physical or psychosocial factors in the 96 hours prior to the onset of back pain.
The study found that:
• The risk of a new episode of low back pain significantly increased due to a range of triggers, from an odds ratio of 2.7 for moderate to vigorous physical activity to 25.0 for distraction during an activity.
• Back pain risk was highest between 7:00 am and at noontime.
• Age moderated the effect of exposure to heavy loads, with an odds ratio for individuals age 20, 40, or 60 at 13.6, 6.0, and 2.7, respectively.
“Understanding which modifiable risk factors lead to low back pain is an important step toward controlling a condition that affects so many worldwide,” said Dr. Ferreira. “Our findings enhance knowledge of low back pain triggers and will assist the development of new prevention programs that can reduce suffering from this potentially disabling condition.”
Suggested Reading
Steffens D, Ferreira ML, Latimer J, et al. What triggers an episode of acute low back pain? a case-crossover study. Arthritis Care Res. 2015 Feb 9. [Epub ahead of print]
New research reveals the physical and psychosocial factors that significantly increase the risk of low back pain onset. The trial results, which were published online ahead of print February 9 in Arthritis Care & Research, show that being engaged in manual tasks involving awkward positions will increase the risk of low back pain by 8 times. People who are distracted during activities or fatigued also significantly increase their risk of acute low back pain.
“Understanding which risk factors contribute to back pain and controlling exposure to these risks is an important first step in prevention,” said Manuela Ferreira, PhD, Associate Professor at the George Institute for Global Health and Sydney Medical School at the University of Sydney in New South Wales, Australia. “Our study is the first to examine brief exposure to a range of modifiable triggers for an acute episode of low back pain.”
For this case-crossover study, Dr. Ferreira and colleagues recruited 999 participants from 300 primary care clinics in Sydney, Australia, who had an acute low back pain episode between October 2011 and November 2012. Participants were asked to report exposure to 12 physical or psychosocial factors in the 96 hours prior to the onset of back pain.
The study found that:
• The risk of a new episode of low back pain significantly increased due to a range of triggers, from an odds ratio of 2.7 for moderate to vigorous physical activity to 25.0 for distraction during an activity.
• Back pain risk was highest between 7:00 am and at noontime.
• Age moderated the effect of exposure to heavy loads, with an odds ratio for individuals age 20, 40, or 60 at 13.6, 6.0, and 2.7, respectively.
“Understanding which modifiable risk factors lead to low back pain is an important step toward controlling a condition that affects so many worldwide,” said Dr. Ferreira. “Our findings enhance knowledge of low back pain triggers and will assist the development of new prevention programs that can reduce suffering from this potentially disabling condition.”
New research reveals the physical and psychosocial factors that significantly increase the risk of low back pain onset. The trial results, which were published online ahead of print February 9 in Arthritis Care & Research, show that being engaged in manual tasks involving awkward positions will increase the risk of low back pain by 8 times. People who are distracted during activities or fatigued also significantly increase their risk of acute low back pain.
“Understanding which risk factors contribute to back pain and controlling exposure to these risks is an important first step in prevention,” said Manuela Ferreira, PhD, Associate Professor at the George Institute for Global Health and Sydney Medical School at the University of Sydney in New South Wales, Australia. “Our study is the first to examine brief exposure to a range of modifiable triggers for an acute episode of low back pain.”
For this case-crossover study, Dr. Ferreira and colleagues recruited 999 participants from 300 primary care clinics in Sydney, Australia, who had an acute low back pain episode between October 2011 and November 2012. Participants were asked to report exposure to 12 physical or psychosocial factors in the 96 hours prior to the onset of back pain.
The study found that:
• The risk of a new episode of low back pain significantly increased due to a range of triggers, from an odds ratio of 2.7 for moderate to vigorous physical activity to 25.0 for distraction during an activity.
• Back pain risk was highest between 7:00 am and at noontime.
• Age moderated the effect of exposure to heavy loads, with an odds ratio for individuals age 20, 40, or 60 at 13.6, 6.0, and 2.7, respectively.
“Understanding which modifiable risk factors lead to low back pain is an important step toward controlling a condition that affects so many worldwide,” said Dr. Ferreira. “Our findings enhance knowledge of low back pain triggers and will assist the development of new prevention programs that can reduce suffering from this potentially disabling condition.”
Suggested Reading
Steffens D, Ferreira ML, Latimer J, et al. What triggers an episode of acute low back pain? a case-crossover study. Arthritis Care Res. 2015 Feb 9. [Epub ahead of print]
Suggested Reading
Steffens D, Ferreira ML, Latimer J, et al. What triggers an episode of acute low back pain? a case-crossover study. Arthritis Care Res. 2015 Feb 9. [Epub ahead of print]
Cutaneous Burn Caused by Radiofrequency Ablation Probe During Shoulder Arthroscopy
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.