User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Nanotechnology: Why Should We Care?
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
Intragrade Intramedullary Nailing of an Open Tibial Shaft Fracture in a Patient With Concomitant Ipsilateral Total Knee Arthroplasty
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
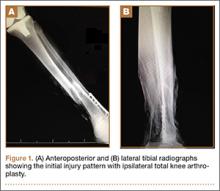
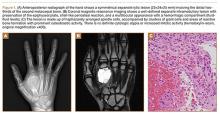
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
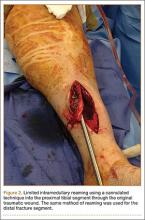
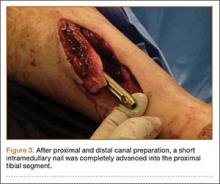
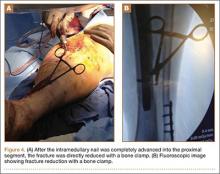
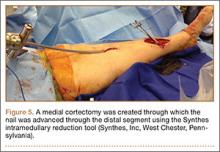
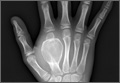
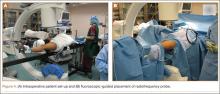
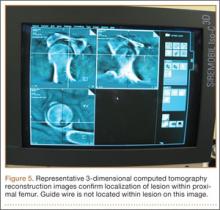
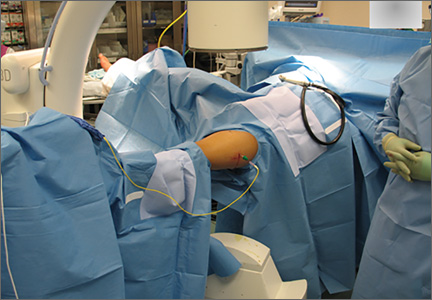
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
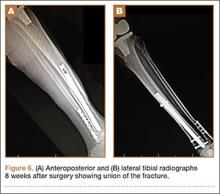
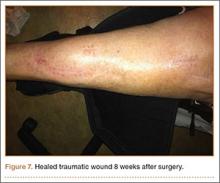
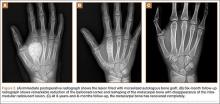
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
Glenoid Damage From Articular Protrusion of Metal Suture Anchor After Arthroscopic Rotator Cuff Repair
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Harrington Rod Revision After Failed Total Hip Arthroplasty Due to Missed Acetabular Metastasis
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
Atypical Presentation of Fat Embolism Syndrome After Gunshot Wound to the Foot
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
Aneurysmal Bone Cyst Involving the Metacarpal Bone in a Child
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Assessment of Medical School Musculoskeletal Education
A basic understanding of the clinical components of musculoskeletal medicine is necessary in many medical fields. Musculoskeletal injuries represent the second most common presentation in US emergency departments, account for 49 million visits to orthopedic surgeon offices, and cost the United States an estimated $950 billion annually.1-3 Despite the staggering need for competency in musculoskeletal medicine, Freedman and Bernstein4 in 1998 demonstrated that musculoskeletal knowledge is inadequate among medical school graduates.
In 2005, the United States Bone and Joint Decade (now the United States Bone and Joint Initiative) announced Project 100, which recommended that 100% of US medical schools begin requiring a musculoskeletal course by the end of the decade.5 This project was intended to increase musculoskeletal understanding among medical students. Optimal curriculum reform, however, remains controversial. Many studies have found that medical schools continue to provide inadequate musculoskeletal education.4,6-9 For example, an integrated musculoskeletal curriculum with increased lecture time was found to improve clinical confidence but not musculoskeletal knowledge.6 In contrast, a 2-week intensive musculoskeletal module and a 6-week course involving orthopedic resident and faculty education has been shown to improve understanding.10,11
We conducted a study to determine the adequacy of musculoskeletal knowledge in medical school students, to determine musculoskeletal competency after curriculum reform through increased lecture and laboratory time, and to draw conclusions about factors leading to increased musculoskeletal competency to guide future curriculum reforms. We hypothesized that musculoskeletal knowledge would increase as a result of increased lecture and laboratory time.
Materials and Methods
The Tufts University School of Medicine Institutional Review Board approved this study. The medical school curriculum at our institution was redesigned to include a dedicated musculoskeletal module to improve medical student education (Table). The study compared medical students given the old musculoskeletal module (premodule group) and those given the new musculoskeletal module (postmodule group). The premodule group received 8.5 hours of physical diagnosis skills training and 30 hours of clinical anatomy training (12 lecture hours, 18 laboratory hours), and the postmodule group received 10 hours of physical diagnosis training and 41.5 hours of clinical anatomy training (18.5 lecture hours, 23 laboratory hours).
The lecture material for the postmodule group covered all topics/questions addressed on the validated musculoskeletal adequacy assessment. The new module specifically added 6 hours of dedicated clinically based musculoskeletal lecture time during the anatomy course and concurrent with the physical diagnosis course. The topics covered with case-based scenarios were orthopedic emergencies, adult hip and back, knee, pediatric hip and back, elbow/wrist/hand, and shoulder. A validated musculoskeletal competency examination was given to the premodule group at the end of the musculoskeletal anatomy and physical diagnosis lectures. The postmodule group took the competency examination at the end of the “new” 6 hours of musculoskeletal education.
We used the Freedman and Bernstein4 basic competency examination for musculoskeletal education assessment to assess knowledge. This examination is the only validated tool for assessing basic clinical musculoskeletal knowledge. All students were also asked whether they had experience rotating in orthopedic surgery or in a related musculoskeletal field (rheumatology, physiatry, neurology) during the medical school “selective” period that allowed 1 afternoon per week of elective time. Duration of exposure was assessed. The examination was given to 109 students in the premodule group 1 year before the curriculum changes. The examination was then given to the students for the first 2 years after the curriculum changes were implemented, with 296 students in the postmodule group. All questions on the examination were addressed within the 6 lecture hours in the new module. A single reviewer anonymously scored the examinations according to the validated scoring system over a 2-week period.4 Independent t tests were used to compare examination scores between the premodule and postmodule groups. Statistical significance was set at P < .05. Equal variance between groups was not assumed.
Results
The examination was given to 405 medical students (109 premodule, 296 postmodule) Mean examination score was 40%, significantly below the recommended mean passing score of 73.1%.4 Only 1 student achieved a passing score. Independent t tests were used to compare examination scores of the premodule and postmodule groups. Mean (SD) scores were higher (P < .05) for the premodule group, 42.1 (13.6), than the postmodule group, 39.1 (11.4) (Figure 1).
Independent t tests were used to compare examination scores of students with either orthopedics or rheumatology experience and students without this experience. Mean (SD) scores were higher (P < .05) for students with experience, 44.4 (11.9), than students without experience, 39.6 (12.1) (Figure 2). Students with neurology or physiatry experience did not score significantly higher than students without this experience. Statistical significance was set as P < .05. SAS 9.0 software (SAS Institute, Cary, North Carolina) was used for statistical analysis.
Discussion
Our students’ mean examination score was significantly lower than the passing (competency) score of 73.1%. Although Project 100 acknowledged many medical schools for implementing a required musculoskeletal course, our study results showed that adequate competency as indicated by a passing score on the Freedman and Bernstein examination was not achieved in medical school despite devoted musculoskeletal lecture time. Our postmodule group had lower scores than our premodule group despite being exposed to more lecture and laboratory material that systematically addressed every examination question. Similarly, Day and colleagues6 found that medical students scored a mean of 45% on the Freedman and Bernstein examination despite increased lecture and laboratory time.6 Lecture and laboratory exposure does not result in long-term information retention. Medical school competency is not significantly higher than what Freedman and Bernstein4 found for musculoskeletal education almost a decade earlier.
Musculoskeletal medicine typically is not revisited during medical school unless a student opts for an elective with a musculoskeletal basis. This specialty differs from others, such as neurology, which requires a 1-month clinical rotation before graduation. As increasing lecture and laboratory time did little to increase competency, adding a required clinical rotation in a musculoskeletal field or integrated musculoskeletal modules for anatomy and clinical training may be the best option for educational reform.
Our study found significantly higher Freedman and Bernstein examination scores for students with orthopedic surgery or rheumatology experience than for students without this experience. First- and second-year medical students are allowed to do a “selective” rotation—1 afternoon a week in an elective rotation of their choice. Students with orthopedics or rheumatology experience in this setting tended to score higher on the examination, possibly a result of both exposure to and interest in musculoskeletal issues. Many other studies have found that clinical exposure within a musculoskeletal field resulted in significantly higher musculoskeletal knowledge.8,12-16 Skelley and colleagues12 found that musculoskeletal clinical exposure of as short as 15 days significantly increased understanding among medical students. Grunfeld and colleagues15 found that students interested in orthopedics also had significantly more musculoskeletal knowledge. Musculoskeletal clinical exposure should be considered in medical school reform. As coordinating a curriculum with orthopedic resident and faculty involvement can set up educational barriers at some medical schools, dedicated musculoskeletal modules with a mock clinical skills component may be a useful consideration, and these have been shown to improve musculoskeletal knowledge.10,11
There are limitations to our study. The musculoskeletal examination was not given to an equal number of medical students in each group. The study also did not control for attendance, and therefore some students who took the musculoskeletal examination may not have attended all musculoskeletal module lectures. The validated examination used for basic competency is another possible limitation. The Freedman and Bernstein examination is the only validated examination used for basic competency, but it may not accurately assess meaningful development of clinical skills applicable to a patient musculoskeletal setting. No studies have assessed the correlation between musculoskeletal competency based on the Freedman and Bernstein examination and patient outcomes.
We conclude that increasing dedicated musculoskeletal lecture hours does not improve musculoskeletal knowledge. Future considerations should include incorporating further hands-on training through clinical skills workshops or rotations in orthopedic surgery or rheumatology.
1. Centers for Disease Control and Prevention, National Center for Health Statistics, Ambulatory and Hospital Care Statistics Branch. National Hospital Ambulatory Medical Care Survey: 2009 outpatient department summary tables. http://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2009_opd_web_tables.pdf. Accessed January 8, 2015.
2. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008 Aug 6;(7):1-38.
3. United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Costs. Rosemont, IL: United States Bone and Joint Initiative; 2008. http://www.boneandjointburden.org. Accessed January 9, 2015.
4. Freedman KB, Bernstein J. The adequacy of medical school education in musculoskeletal medicine. J Bone Joint Surg Am. 1998;80(10):1421-1427.
5. United States Bone and Joint Initiative. Project 100—Undergraduate Musculoskeletal Education. Rosemont, IL: United States Bone and Joint Initiative; 2005. www.usbjd.org/projects/project_op.cfm?dirID=127. Accessed January 9, 2015.
6. Day CS, Ahn CS, Yeh AC, Tabrizi S. Early assessment of a new integrated preclinical musculoskeletal curriculum at a medical school. Am J Orthop. 2011;40(1):14-18.
7. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
8. Matzkin E, Smith EL, Freccero D, Richardson AB. Adequacy of education in musculoskeletal medicine. J Bone Joint Surg Am. 2005;87(2):310-314.
9. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
10. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
11. Queally JM, Cummins F, Brennan SA, Shelly MJ, O’Byrne JM. Assessment of a new undergraduate module in musculoskeletal medicine. J Bone Joint Surg Am. 2011;93(3):e9.
12. Skelley NW, Tanaka MJ, Skelley LM, LaPorte DM. Medical student musculoskeletal education: an institutional survey. J Bone Joint Surg Am. 2012;94(19):e146(1-7).
13. DiGiovanni BF, Chu JY, Mooney CJ, Lambert DR. Maturation of medical student musculoskeletal medicine knowledge and clinical confidence. Med Educ Online. 2012;17. doi:10.3402/meo.v17i0.17092. Epub 2012 Jul 23.
14. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
15. Grunfeld R, Banks S, Fox E, Levy BA, Craig C, Black K. An assessment of musculoskeletal knowledge in graduating medical and physician assistant students and implications for musculoskeletal care providers. J Bone Joint Surg Am. 2012;94(4):343-348.
16. Yeh AC, Franko O, Day CS. Impact of clinical electives and residency interest on medical students’ education in musculoskeletal medicine. J Bone Joint Surg Am. 2008;90(2):307-315.
A basic understanding of the clinical components of musculoskeletal medicine is necessary in many medical fields. Musculoskeletal injuries represent the second most common presentation in US emergency departments, account for 49 million visits to orthopedic surgeon offices, and cost the United States an estimated $950 billion annually.1-3 Despite the staggering need for competency in musculoskeletal medicine, Freedman and Bernstein4 in 1998 demonstrated that musculoskeletal knowledge is inadequate among medical school graduates.
In 2005, the United States Bone and Joint Decade (now the United States Bone and Joint Initiative) announced Project 100, which recommended that 100% of US medical schools begin requiring a musculoskeletal course by the end of the decade.5 This project was intended to increase musculoskeletal understanding among medical students. Optimal curriculum reform, however, remains controversial. Many studies have found that medical schools continue to provide inadequate musculoskeletal education.4,6-9 For example, an integrated musculoskeletal curriculum with increased lecture time was found to improve clinical confidence but not musculoskeletal knowledge.6 In contrast, a 2-week intensive musculoskeletal module and a 6-week course involving orthopedic resident and faculty education has been shown to improve understanding.10,11
We conducted a study to determine the adequacy of musculoskeletal knowledge in medical school students, to determine musculoskeletal competency after curriculum reform through increased lecture and laboratory time, and to draw conclusions about factors leading to increased musculoskeletal competency to guide future curriculum reforms. We hypothesized that musculoskeletal knowledge would increase as a result of increased lecture and laboratory time.
Materials and Methods
The Tufts University School of Medicine Institutional Review Board approved this study. The medical school curriculum at our institution was redesigned to include a dedicated musculoskeletal module to improve medical student education (Table). The study compared medical students given the old musculoskeletal module (premodule group) and those given the new musculoskeletal module (postmodule group). The premodule group received 8.5 hours of physical diagnosis skills training and 30 hours of clinical anatomy training (12 lecture hours, 18 laboratory hours), and the postmodule group received 10 hours of physical diagnosis training and 41.5 hours of clinical anatomy training (18.5 lecture hours, 23 laboratory hours).
The lecture material for the postmodule group covered all topics/questions addressed on the validated musculoskeletal adequacy assessment. The new module specifically added 6 hours of dedicated clinically based musculoskeletal lecture time during the anatomy course and concurrent with the physical diagnosis course. The topics covered with case-based scenarios were orthopedic emergencies, adult hip and back, knee, pediatric hip and back, elbow/wrist/hand, and shoulder. A validated musculoskeletal competency examination was given to the premodule group at the end of the musculoskeletal anatomy and physical diagnosis lectures. The postmodule group took the competency examination at the end of the “new” 6 hours of musculoskeletal education.
We used the Freedman and Bernstein4 basic competency examination for musculoskeletal education assessment to assess knowledge. This examination is the only validated tool for assessing basic clinical musculoskeletal knowledge. All students were also asked whether they had experience rotating in orthopedic surgery or in a related musculoskeletal field (rheumatology, physiatry, neurology) during the medical school “selective” period that allowed 1 afternoon per week of elective time. Duration of exposure was assessed. The examination was given to 109 students in the premodule group 1 year before the curriculum changes. The examination was then given to the students for the first 2 years after the curriculum changes were implemented, with 296 students in the postmodule group. All questions on the examination were addressed within the 6 lecture hours in the new module. A single reviewer anonymously scored the examinations according to the validated scoring system over a 2-week period.4 Independent t tests were used to compare examination scores between the premodule and postmodule groups. Statistical significance was set at P < .05. Equal variance between groups was not assumed.
Results
The examination was given to 405 medical students (109 premodule, 296 postmodule) Mean examination score was 40%, significantly below the recommended mean passing score of 73.1%.4 Only 1 student achieved a passing score. Independent t tests were used to compare examination scores of the premodule and postmodule groups. Mean (SD) scores were higher (P < .05) for the premodule group, 42.1 (13.6), than the postmodule group, 39.1 (11.4) (Figure 1).
Independent t tests were used to compare examination scores of students with either orthopedics or rheumatology experience and students without this experience. Mean (SD) scores were higher (P < .05) for students with experience, 44.4 (11.9), than students without experience, 39.6 (12.1) (Figure 2). Students with neurology or physiatry experience did not score significantly higher than students without this experience. Statistical significance was set as P < .05. SAS 9.0 software (SAS Institute, Cary, North Carolina) was used for statistical analysis.
Discussion
Our students’ mean examination score was significantly lower than the passing (competency) score of 73.1%. Although Project 100 acknowledged many medical schools for implementing a required musculoskeletal course, our study results showed that adequate competency as indicated by a passing score on the Freedman and Bernstein examination was not achieved in medical school despite devoted musculoskeletal lecture time. Our postmodule group had lower scores than our premodule group despite being exposed to more lecture and laboratory material that systematically addressed every examination question. Similarly, Day and colleagues6 found that medical students scored a mean of 45% on the Freedman and Bernstein examination despite increased lecture and laboratory time.6 Lecture and laboratory exposure does not result in long-term information retention. Medical school competency is not significantly higher than what Freedman and Bernstein4 found for musculoskeletal education almost a decade earlier.
Musculoskeletal medicine typically is not revisited during medical school unless a student opts for an elective with a musculoskeletal basis. This specialty differs from others, such as neurology, which requires a 1-month clinical rotation before graduation. As increasing lecture and laboratory time did little to increase competency, adding a required clinical rotation in a musculoskeletal field or integrated musculoskeletal modules for anatomy and clinical training may be the best option for educational reform.
Our study found significantly higher Freedman and Bernstein examination scores for students with orthopedic surgery or rheumatology experience than for students without this experience. First- and second-year medical students are allowed to do a “selective” rotation—1 afternoon a week in an elective rotation of their choice. Students with orthopedics or rheumatology experience in this setting tended to score higher on the examination, possibly a result of both exposure to and interest in musculoskeletal issues. Many other studies have found that clinical exposure within a musculoskeletal field resulted in significantly higher musculoskeletal knowledge.8,12-16 Skelley and colleagues12 found that musculoskeletal clinical exposure of as short as 15 days significantly increased understanding among medical students. Grunfeld and colleagues15 found that students interested in orthopedics also had significantly more musculoskeletal knowledge. Musculoskeletal clinical exposure should be considered in medical school reform. As coordinating a curriculum with orthopedic resident and faculty involvement can set up educational barriers at some medical schools, dedicated musculoskeletal modules with a mock clinical skills component may be a useful consideration, and these have been shown to improve musculoskeletal knowledge.10,11
There are limitations to our study. The musculoskeletal examination was not given to an equal number of medical students in each group. The study also did not control for attendance, and therefore some students who took the musculoskeletal examination may not have attended all musculoskeletal module lectures. The validated examination used for basic competency is another possible limitation. The Freedman and Bernstein examination is the only validated examination used for basic competency, but it may not accurately assess meaningful development of clinical skills applicable to a patient musculoskeletal setting. No studies have assessed the correlation between musculoskeletal competency based on the Freedman and Bernstein examination and patient outcomes.
We conclude that increasing dedicated musculoskeletal lecture hours does not improve musculoskeletal knowledge. Future considerations should include incorporating further hands-on training through clinical skills workshops or rotations in orthopedic surgery or rheumatology.
A basic understanding of the clinical components of musculoskeletal medicine is necessary in many medical fields. Musculoskeletal injuries represent the second most common presentation in US emergency departments, account for 49 million visits to orthopedic surgeon offices, and cost the United States an estimated $950 billion annually.1-3 Despite the staggering need for competency in musculoskeletal medicine, Freedman and Bernstein4 in 1998 demonstrated that musculoskeletal knowledge is inadequate among medical school graduates.
In 2005, the United States Bone and Joint Decade (now the United States Bone and Joint Initiative) announced Project 100, which recommended that 100% of US medical schools begin requiring a musculoskeletal course by the end of the decade.5 This project was intended to increase musculoskeletal understanding among medical students. Optimal curriculum reform, however, remains controversial. Many studies have found that medical schools continue to provide inadequate musculoskeletal education.4,6-9 For example, an integrated musculoskeletal curriculum with increased lecture time was found to improve clinical confidence but not musculoskeletal knowledge.6 In contrast, a 2-week intensive musculoskeletal module and a 6-week course involving orthopedic resident and faculty education has been shown to improve understanding.10,11
We conducted a study to determine the adequacy of musculoskeletal knowledge in medical school students, to determine musculoskeletal competency after curriculum reform through increased lecture and laboratory time, and to draw conclusions about factors leading to increased musculoskeletal competency to guide future curriculum reforms. We hypothesized that musculoskeletal knowledge would increase as a result of increased lecture and laboratory time.
Materials and Methods
The Tufts University School of Medicine Institutional Review Board approved this study. The medical school curriculum at our institution was redesigned to include a dedicated musculoskeletal module to improve medical student education (Table). The study compared medical students given the old musculoskeletal module (premodule group) and those given the new musculoskeletal module (postmodule group). The premodule group received 8.5 hours of physical diagnosis skills training and 30 hours of clinical anatomy training (12 lecture hours, 18 laboratory hours), and the postmodule group received 10 hours of physical diagnosis training and 41.5 hours of clinical anatomy training (18.5 lecture hours, 23 laboratory hours).
The lecture material for the postmodule group covered all topics/questions addressed on the validated musculoskeletal adequacy assessment. The new module specifically added 6 hours of dedicated clinically based musculoskeletal lecture time during the anatomy course and concurrent with the physical diagnosis course. The topics covered with case-based scenarios were orthopedic emergencies, adult hip and back, knee, pediatric hip and back, elbow/wrist/hand, and shoulder. A validated musculoskeletal competency examination was given to the premodule group at the end of the musculoskeletal anatomy and physical diagnosis lectures. The postmodule group took the competency examination at the end of the “new” 6 hours of musculoskeletal education.
We used the Freedman and Bernstein4 basic competency examination for musculoskeletal education assessment to assess knowledge. This examination is the only validated tool for assessing basic clinical musculoskeletal knowledge. All students were also asked whether they had experience rotating in orthopedic surgery or in a related musculoskeletal field (rheumatology, physiatry, neurology) during the medical school “selective” period that allowed 1 afternoon per week of elective time. Duration of exposure was assessed. The examination was given to 109 students in the premodule group 1 year before the curriculum changes. The examination was then given to the students for the first 2 years after the curriculum changes were implemented, with 296 students in the postmodule group. All questions on the examination were addressed within the 6 lecture hours in the new module. A single reviewer anonymously scored the examinations according to the validated scoring system over a 2-week period.4 Independent t tests were used to compare examination scores between the premodule and postmodule groups. Statistical significance was set at P < .05. Equal variance between groups was not assumed.
Results
The examination was given to 405 medical students (109 premodule, 296 postmodule) Mean examination score was 40%, significantly below the recommended mean passing score of 73.1%.4 Only 1 student achieved a passing score. Independent t tests were used to compare examination scores of the premodule and postmodule groups. Mean (SD) scores were higher (P < .05) for the premodule group, 42.1 (13.6), than the postmodule group, 39.1 (11.4) (Figure 1).
Independent t tests were used to compare examination scores of students with either orthopedics or rheumatology experience and students without this experience. Mean (SD) scores were higher (P < .05) for students with experience, 44.4 (11.9), than students without experience, 39.6 (12.1) (Figure 2). Students with neurology or physiatry experience did not score significantly higher than students without this experience. Statistical significance was set as P < .05. SAS 9.0 software (SAS Institute, Cary, North Carolina) was used for statistical analysis.
Discussion
Our students’ mean examination score was significantly lower than the passing (competency) score of 73.1%. Although Project 100 acknowledged many medical schools for implementing a required musculoskeletal course, our study results showed that adequate competency as indicated by a passing score on the Freedman and Bernstein examination was not achieved in medical school despite devoted musculoskeletal lecture time. Our postmodule group had lower scores than our premodule group despite being exposed to more lecture and laboratory material that systematically addressed every examination question. Similarly, Day and colleagues6 found that medical students scored a mean of 45% on the Freedman and Bernstein examination despite increased lecture and laboratory time.6 Lecture and laboratory exposure does not result in long-term information retention. Medical school competency is not significantly higher than what Freedman and Bernstein4 found for musculoskeletal education almost a decade earlier.
Musculoskeletal medicine typically is not revisited during medical school unless a student opts for an elective with a musculoskeletal basis. This specialty differs from others, such as neurology, which requires a 1-month clinical rotation before graduation. As increasing lecture and laboratory time did little to increase competency, adding a required clinical rotation in a musculoskeletal field or integrated musculoskeletal modules for anatomy and clinical training may be the best option for educational reform.
Our study found significantly higher Freedman and Bernstein examination scores for students with orthopedic surgery or rheumatology experience than for students without this experience. First- and second-year medical students are allowed to do a “selective” rotation—1 afternoon a week in an elective rotation of their choice. Students with orthopedics or rheumatology experience in this setting tended to score higher on the examination, possibly a result of both exposure to and interest in musculoskeletal issues. Many other studies have found that clinical exposure within a musculoskeletal field resulted in significantly higher musculoskeletal knowledge.8,12-16 Skelley and colleagues12 found that musculoskeletal clinical exposure of as short as 15 days significantly increased understanding among medical students. Grunfeld and colleagues15 found that students interested in orthopedics also had significantly more musculoskeletal knowledge. Musculoskeletal clinical exposure should be considered in medical school reform. As coordinating a curriculum with orthopedic resident and faculty involvement can set up educational barriers at some medical schools, dedicated musculoskeletal modules with a mock clinical skills component may be a useful consideration, and these have been shown to improve musculoskeletal knowledge.10,11
There are limitations to our study. The musculoskeletal examination was not given to an equal number of medical students in each group. The study also did not control for attendance, and therefore some students who took the musculoskeletal examination may not have attended all musculoskeletal module lectures. The validated examination used for basic competency is another possible limitation. The Freedman and Bernstein examination is the only validated examination used for basic competency, but it may not accurately assess meaningful development of clinical skills applicable to a patient musculoskeletal setting. No studies have assessed the correlation between musculoskeletal competency based on the Freedman and Bernstein examination and patient outcomes.
We conclude that increasing dedicated musculoskeletal lecture hours does not improve musculoskeletal knowledge. Future considerations should include incorporating further hands-on training through clinical skills workshops or rotations in orthopedic surgery or rheumatology.
1. Centers for Disease Control and Prevention, National Center for Health Statistics, Ambulatory and Hospital Care Statistics Branch. National Hospital Ambulatory Medical Care Survey: 2009 outpatient department summary tables. http://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2009_opd_web_tables.pdf. Accessed January 8, 2015.
2. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008 Aug 6;(7):1-38.
3. United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Costs. Rosemont, IL: United States Bone and Joint Initiative; 2008. http://www.boneandjointburden.org. Accessed January 9, 2015.
4. Freedman KB, Bernstein J. The adequacy of medical school education in musculoskeletal medicine. J Bone Joint Surg Am. 1998;80(10):1421-1427.
5. United States Bone and Joint Initiative. Project 100—Undergraduate Musculoskeletal Education. Rosemont, IL: United States Bone and Joint Initiative; 2005. www.usbjd.org/projects/project_op.cfm?dirID=127. Accessed January 9, 2015.
6. Day CS, Ahn CS, Yeh AC, Tabrizi S. Early assessment of a new integrated preclinical musculoskeletal curriculum at a medical school. Am J Orthop. 2011;40(1):14-18.
7. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
8. Matzkin E, Smith EL, Freccero D, Richardson AB. Adequacy of education in musculoskeletal medicine. J Bone Joint Surg Am. 2005;87(2):310-314.
9. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
10. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
11. Queally JM, Cummins F, Brennan SA, Shelly MJ, O’Byrne JM. Assessment of a new undergraduate module in musculoskeletal medicine. J Bone Joint Surg Am. 2011;93(3):e9.
12. Skelley NW, Tanaka MJ, Skelley LM, LaPorte DM. Medical student musculoskeletal education: an institutional survey. J Bone Joint Surg Am. 2012;94(19):e146(1-7).
13. DiGiovanni BF, Chu JY, Mooney CJ, Lambert DR. Maturation of medical student musculoskeletal medicine knowledge and clinical confidence. Med Educ Online. 2012;17. doi:10.3402/meo.v17i0.17092. Epub 2012 Jul 23.
14. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
15. Grunfeld R, Banks S, Fox E, Levy BA, Craig C, Black K. An assessment of musculoskeletal knowledge in graduating medical and physician assistant students and implications for musculoskeletal care providers. J Bone Joint Surg Am. 2012;94(4):343-348.
16. Yeh AC, Franko O, Day CS. Impact of clinical electives and residency interest on medical students’ education in musculoskeletal medicine. J Bone Joint Surg Am. 2008;90(2):307-315.
1. Centers for Disease Control and Prevention, National Center for Health Statistics, Ambulatory and Hospital Care Statistics Branch. National Hospital Ambulatory Medical Care Survey: 2009 outpatient department summary tables. http://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2009_opd_web_tables.pdf. Accessed January 8, 2015.
2. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008 Aug 6;(7):1-38.
3. United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Costs. Rosemont, IL: United States Bone and Joint Initiative; 2008. http://www.boneandjointburden.org. Accessed January 9, 2015.
4. Freedman KB, Bernstein J. The adequacy of medical school education in musculoskeletal medicine. J Bone Joint Surg Am. 1998;80(10):1421-1427.
5. United States Bone and Joint Initiative. Project 100—Undergraduate Musculoskeletal Education. Rosemont, IL: United States Bone and Joint Initiative; 2005. www.usbjd.org/projects/project_op.cfm?dirID=127. Accessed January 9, 2015.
6. Day CS, Ahn CS, Yeh AC, Tabrizi S. Early assessment of a new integrated preclinical musculoskeletal curriculum at a medical school. Am J Orthop. 2011;40(1):14-18.
7. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
8. Matzkin E, Smith EL, Freccero D, Richardson AB. Adequacy of education in musculoskeletal medicine. J Bone Joint Surg Am. 2005;87(2):310-314.
9. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
10. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
11. Queally JM, Cummins F, Brennan SA, Shelly MJ, O’Byrne JM. Assessment of a new undergraduate module in musculoskeletal medicine. J Bone Joint Surg Am. 2011;93(3):e9.
12. Skelley NW, Tanaka MJ, Skelley LM, LaPorte DM. Medical student musculoskeletal education: an institutional survey. J Bone Joint Surg Am. 2012;94(19):e146(1-7).
13. DiGiovanni BF, Chu JY, Mooney CJ, Lambert DR. Maturation of medical student musculoskeletal medicine knowledge and clinical confidence. Med Educ Online. 2012;17. doi:10.3402/meo.v17i0.17092. Epub 2012 Jul 23.
14. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
15. Grunfeld R, Banks S, Fox E, Levy BA, Craig C, Black K. An assessment of musculoskeletal knowledge in graduating medical and physician assistant students and implications for musculoskeletal care providers. J Bone Joint Surg Am. 2012;94(4):343-348.
16. Yeh AC, Franko O, Day CS. Impact of clinical electives and residency interest on medical students’ education in musculoskeletal medicine. J Bone Joint Surg Am. 2008;90(2):307-315.
Meaningful Use for Surgeons—It’s Not as Complicated as You Think
It’s spring. Have you started your Meaningful Use reporting yet? More important, have you begun reporting at all?
“Say the words Meaningful Use to most orthopedists, and they usually roll their eyes or shake their heads,” says Cheyenne Brinson, MBA, CPA, a KarenZupko & Associates consultant who has been advising surgical practices on Meaningful Use since the program’s inception. Although many orthopedists are successfully using certified electronic health records (EHRs) to e-prescribe and enter radiology and laboratory orders, Brinson says many other requirements are misunderstood and perceived as overly complex. In many cases, practices are doing more work than they need to in order to attest.
“It’s actually not that complicated to meet Meaningful Use requirements,” she says. “The trick is to zero in on what’s relevant only for surgeons. This isn’t crystal clear in the CMS [Centers for Medicare & Medicaid Services] documents, and it’s not the forte of most EHR vendors or trainers either.” In fact, in Brinson’s experience, most EHR trainers present Meaningful Use to every practice as if it were primary care. Yet, the requirements for surgeons are different for primary care and are, frankly, less involved.
That’s good news. Because if you didn’t attest for Meaningful Use in 2014, the first year that reporting was required, you’re automatically getting dinged 2% on your Medicare payments in 2015. So, it’s time to get organized and get moving to avoid further penalties.
Avoid These Four Common Faux Pas
Brinson says the Clinical Quality Measures (CQMs) are hands down the most misunderstood component of Meaningful Use. “When I explain Meaningful Use to surgeons, I can’t jump up and down and wave my hands in the air enough to call attention to this,” she quips.
At issue: There are 64 CQMs, but very few are applicable to surgeons. Yet, many surgeons think they have to perform them for Meaningful Use. Not so, says Brinson. “Surgeons have to report a CQM only if it’s clinically relevant. If none of the CQMs are clinically relevant in your practice, it’s okay to report a zero value if you have not actually performed it.”
Here’s how this plays out. In Stage 2, physicians must report 9 CQMs across 3 domains; Population/Public Health, Patient Safety, and Efficient Use of Healthcare Resources are examples of domains that are most applicable to orthopedists. “If you choose Low Back Pain: Use of Imaging Studies as one of these, it’s possible an orthopedist would have a numeric value to report,” Brinson says. “But if you also choose Use of High-Risk Medications in the Elderly, an orthopedist will probably report a zero value. And that’s totally acceptable. You will not be penalized for reporting zero.”
Another common misconception is around the Vital Signs and Smoking Status measures. “We have worked with surgical practices that think Meaningful Use is requiring them to collect vital signs and smoking status at every visit, even though they may not be clinically relevant,” says Brinson. Again, not true.
“Height and/or weight and blood pressure, as well as smoking status measures, need to be reported only once per patient during the reporting period,” Brinson clarifies. “So from a practical standpoint, most orthopedic practices can collect this data from new patients and then again as clinically necessary,” adding there are even exclusions for physicians who attest that either height and weight and/or blood pressure has no relevance to their scope of practice at all.
Brinson also sees practices do more work than they need to when it comes to Patient Care Reminders. She recently worked with a surgery practice that sent reminders for colonoscopies. “Not exactly clinically relevant,” she says, “and an unnecessary step for staff.” That’s because physicians aren’t required to send reminders that aren’t relevant to their specialty.
The Federal Register states, “An eligible provider (EP) should use clinically relevant information stored within the [EHR] to identify patients who should receive reminders…. The EP is best positioned to decide which information is clinically relevant for this purpose.”
“In orthopedics, clinically relevant reminders could be for an outside referral, a follow-up on an MRI or other test, or a reminder to schedule a postoperative appointment,” Brinson explains. “Work with your EHR vendor to create the reminders that are most appropriate for your patient base.”
The final faux pas that Brinson finds: “Meaningful Use requires you to report data for all patients, not just Medicare patients. That seems to be a point of confusion for many.”
Three Cheers for the Patient Portal Requirement
Stage 2 saw the addition of the Patient Portal Requirement, and Brinson suggests that the benefits of this tool go far beyond Meaningful Use. “Patient portals are essential to a modern practice,” she says. “Patients use them to complete a health history prior to their appointment, pay their bill, schedule follow-up appointments, and more.” Further, the patient portal facilitates another Meaningful Use Stage 2 requirement: secure electronic messaging with patients. For both Meaningful Use and risk management, moving away from e-mail and texting and toward secure/encrypted messaging is a must. The patient portal has this feature already built in, and all messages are stored securely and archived—which meets the HIPAA (Health Insurance Portability and Accountability Act) Omnibus requirements, too.
So if you’ve implemented a patient portal, that’s good for your practice and your patients on many levels. But there is a caveat about meeting the Meaningful Use requirement. “For this requirement, 5% of the unique patients seen during the reporting period must ‘view, download, or transmit to a third party their health information,’” Brinson explains. “So the onus is on your practice to ‘sell’ the benefits of the patient portal and get patients to use it so you can achieve the 5% threshold.”
Clinical Decision Support and Summaries
The requirements of Clinical Decision Support Interventions and Clinical Summaries may seem daunting, but, if you think beyond Meaningful Use for a moment, both facilitate better care.
Take Clinical Decision Support Interventions. What would be helpful for you to know about a patient before surgery? What information would enable you to deliver better care?
“One surgeon told me that a family history of malignant hyperthermia would mean the difference between performing the case in the operating room versus the ambulatory surgery center,” Brinson says. “This is a good example of an intervention that a surgeon would work with their EHR vendor to set up.”
The objective states that each intervention is to be an evidence-based decision-support intervention based on each one and at least one combination of the following data: problem list, medication list, medication allergy list, demographics, laboratory tests and values/results, and vital signs. “Stage 1 requires physicians to implement 1 Clinical Decision Support Intervention, and Stage 2 requires 5,” reminds Brinson.
And here’s all you need to know about Clinical Summaries. Although there are 20 specific required elements of a clinical summary, physicians themselves need to provide details only for clinical instructions and the care plan, including goals and instructions. Ancillary staff can populate the other elements.
Brinson points out that surgeons are not expected to provide a copy of the patient’s note, or to complete the note, before the patient checks out. The requirement under Stage 2 is that the clinical summary is provided to the patient within 1 business day. “From a practical standpoint, practices can print the clinical summary for patients at checkout. A well-done clinical summary is a practice efficiency tool as much as a clinical document. It can reduce phone calls from patients asking, ‘Now what did the doctor tell me to do?’”
Often Overlooked
There are requirements that, Brinson says, surgeons often gloss over: Protect Electronic Health Information and Text-Searchable Progress Notes.
“Stage 2 requires physicians to conduct a privacy risk analysis to protect electronic health information,” she explains. “Most EHR vendors don’t offer this as part of their product, so it’s frequently overlooked.” Such an analysis typically requires an outside vendor, but there are free, do-it-yourself tools available, such as the Privacy and Security Toolkit for Small Provider Organizations,* from the Healthcare Information and Management Systems Society (HIMSS).
The analysis should follow HIPAA guidelines, and the most intensive part of this requirement is to conduct or review a privacy risk analysis of the clinical technology. “You’ve also got to address data encryption and security in the EHR, and ensure HIPAA policies and procedures are in place,” Brinson states.
Text-Searchable Progress Notes are also a new requirement in Stage 2. All progress notes must be text searchable—practices can no longer include progress notes as scanned attachments. “That means no more PDFs,” Brinson says. “Surgeons can still dictate, but the dictation must be entered into the EHR in such a way that it’s searchable. In Stage 2, 30% of unique patients must have a minimum of 1 text-searchable electronic progress note created, edited, and signed in the EHR.”
Conclusion
Meaningful Use does not have to be cumbersome. Focus on what surgical practices need to know, and attestation won’t be as complicated as you think.
*http://www.himss.org/library/healthcare-privacy-security/small-provider-toolkit?navItemNumber=16493.
It’s spring. Have you started your Meaningful Use reporting yet? More important, have you begun reporting at all?
“Say the words Meaningful Use to most orthopedists, and they usually roll their eyes or shake their heads,” says Cheyenne Brinson, MBA, CPA, a KarenZupko & Associates consultant who has been advising surgical practices on Meaningful Use since the program’s inception. Although many orthopedists are successfully using certified electronic health records (EHRs) to e-prescribe and enter radiology and laboratory orders, Brinson says many other requirements are misunderstood and perceived as overly complex. In many cases, practices are doing more work than they need to in order to attest.
“It’s actually not that complicated to meet Meaningful Use requirements,” she says. “The trick is to zero in on what’s relevant only for surgeons. This isn’t crystal clear in the CMS [Centers for Medicare & Medicaid Services] documents, and it’s not the forte of most EHR vendors or trainers either.” In fact, in Brinson’s experience, most EHR trainers present Meaningful Use to every practice as if it were primary care. Yet, the requirements for surgeons are different for primary care and are, frankly, less involved.
That’s good news. Because if you didn’t attest for Meaningful Use in 2014, the first year that reporting was required, you’re automatically getting dinged 2% on your Medicare payments in 2015. So, it’s time to get organized and get moving to avoid further penalties.
Avoid These Four Common Faux Pas
Brinson says the Clinical Quality Measures (CQMs) are hands down the most misunderstood component of Meaningful Use. “When I explain Meaningful Use to surgeons, I can’t jump up and down and wave my hands in the air enough to call attention to this,” she quips.
At issue: There are 64 CQMs, but very few are applicable to surgeons. Yet, many surgeons think they have to perform them for Meaningful Use. Not so, says Brinson. “Surgeons have to report a CQM only if it’s clinically relevant. If none of the CQMs are clinically relevant in your practice, it’s okay to report a zero value if you have not actually performed it.”
Here’s how this plays out. In Stage 2, physicians must report 9 CQMs across 3 domains; Population/Public Health, Patient Safety, and Efficient Use of Healthcare Resources are examples of domains that are most applicable to orthopedists. “If you choose Low Back Pain: Use of Imaging Studies as one of these, it’s possible an orthopedist would have a numeric value to report,” Brinson says. “But if you also choose Use of High-Risk Medications in the Elderly, an orthopedist will probably report a zero value. And that’s totally acceptable. You will not be penalized for reporting zero.”
Another common misconception is around the Vital Signs and Smoking Status measures. “We have worked with surgical practices that think Meaningful Use is requiring them to collect vital signs and smoking status at every visit, even though they may not be clinically relevant,” says Brinson. Again, not true.
“Height and/or weight and blood pressure, as well as smoking status measures, need to be reported only once per patient during the reporting period,” Brinson clarifies. “So from a practical standpoint, most orthopedic practices can collect this data from new patients and then again as clinically necessary,” adding there are even exclusions for physicians who attest that either height and weight and/or blood pressure has no relevance to their scope of practice at all.
Brinson also sees practices do more work than they need to when it comes to Patient Care Reminders. She recently worked with a surgery practice that sent reminders for colonoscopies. “Not exactly clinically relevant,” she says, “and an unnecessary step for staff.” That’s because physicians aren’t required to send reminders that aren’t relevant to their specialty.
The Federal Register states, “An eligible provider (EP) should use clinically relevant information stored within the [EHR] to identify patients who should receive reminders…. The EP is best positioned to decide which information is clinically relevant for this purpose.”
“In orthopedics, clinically relevant reminders could be for an outside referral, a follow-up on an MRI or other test, or a reminder to schedule a postoperative appointment,” Brinson explains. “Work with your EHR vendor to create the reminders that are most appropriate for your patient base.”
The final faux pas that Brinson finds: “Meaningful Use requires you to report data for all patients, not just Medicare patients. That seems to be a point of confusion for many.”
Three Cheers for the Patient Portal Requirement
Stage 2 saw the addition of the Patient Portal Requirement, and Brinson suggests that the benefits of this tool go far beyond Meaningful Use. “Patient portals are essential to a modern practice,” she says. “Patients use them to complete a health history prior to their appointment, pay their bill, schedule follow-up appointments, and more.” Further, the patient portal facilitates another Meaningful Use Stage 2 requirement: secure electronic messaging with patients. For both Meaningful Use and risk management, moving away from e-mail and texting and toward secure/encrypted messaging is a must. The patient portal has this feature already built in, and all messages are stored securely and archived—which meets the HIPAA (Health Insurance Portability and Accountability Act) Omnibus requirements, too.
So if you’ve implemented a patient portal, that’s good for your practice and your patients on many levels. But there is a caveat about meeting the Meaningful Use requirement. “For this requirement, 5% of the unique patients seen during the reporting period must ‘view, download, or transmit to a third party their health information,’” Brinson explains. “So the onus is on your practice to ‘sell’ the benefits of the patient portal and get patients to use it so you can achieve the 5% threshold.”
Clinical Decision Support and Summaries
The requirements of Clinical Decision Support Interventions and Clinical Summaries may seem daunting, but, if you think beyond Meaningful Use for a moment, both facilitate better care.
Take Clinical Decision Support Interventions. What would be helpful for you to know about a patient before surgery? What information would enable you to deliver better care?
“One surgeon told me that a family history of malignant hyperthermia would mean the difference between performing the case in the operating room versus the ambulatory surgery center,” Brinson says. “This is a good example of an intervention that a surgeon would work with their EHR vendor to set up.”
The objective states that each intervention is to be an evidence-based decision-support intervention based on each one and at least one combination of the following data: problem list, medication list, medication allergy list, demographics, laboratory tests and values/results, and vital signs. “Stage 1 requires physicians to implement 1 Clinical Decision Support Intervention, and Stage 2 requires 5,” reminds Brinson.
And here’s all you need to know about Clinical Summaries. Although there are 20 specific required elements of a clinical summary, physicians themselves need to provide details only for clinical instructions and the care plan, including goals and instructions. Ancillary staff can populate the other elements.
Brinson points out that surgeons are not expected to provide a copy of the patient’s note, or to complete the note, before the patient checks out. The requirement under Stage 2 is that the clinical summary is provided to the patient within 1 business day. “From a practical standpoint, practices can print the clinical summary for patients at checkout. A well-done clinical summary is a practice efficiency tool as much as a clinical document. It can reduce phone calls from patients asking, ‘Now what did the doctor tell me to do?’”
Often Overlooked
There are requirements that, Brinson says, surgeons often gloss over: Protect Electronic Health Information and Text-Searchable Progress Notes.
“Stage 2 requires physicians to conduct a privacy risk analysis to protect electronic health information,” she explains. “Most EHR vendors don’t offer this as part of their product, so it’s frequently overlooked.” Such an analysis typically requires an outside vendor, but there are free, do-it-yourself tools available, such as the Privacy and Security Toolkit for Small Provider Organizations,* from the Healthcare Information and Management Systems Society (HIMSS).
The analysis should follow HIPAA guidelines, and the most intensive part of this requirement is to conduct or review a privacy risk analysis of the clinical technology. “You’ve also got to address data encryption and security in the EHR, and ensure HIPAA policies and procedures are in place,” Brinson states.
Text-Searchable Progress Notes are also a new requirement in Stage 2. All progress notes must be text searchable—practices can no longer include progress notes as scanned attachments. “That means no more PDFs,” Brinson says. “Surgeons can still dictate, but the dictation must be entered into the EHR in such a way that it’s searchable. In Stage 2, 30% of unique patients must have a minimum of 1 text-searchable electronic progress note created, edited, and signed in the EHR.”
Conclusion
Meaningful Use does not have to be cumbersome. Focus on what surgical practices need to know, and attestation won’t be as complicated as you think.
It’s spring. Have you started your Meaningful Use reporting yet? More important, have you begun reporting at all?
“Say the words Meaningful Use to most orthopedists, and they usually roll their eyes or shake their heads,” says Cheyenne Brinson, MBA, CPA, a KarenZupko & Associates consultant who has been advising surgical practices on Meaningful Use since the program’s inception. Although many orthopedists are successfully using certified electronic health records (EHRs) to e-prescribe and enter radiology and laboratory orders, Brinson says many other requirements are misunderstood and perceived as overly complex. In many cases, practices are doing more work than they need to in order to attest.
“It’s actually not that complicated to meet Meaningful Use requirements,” she says. “The trick is to zero in on what’s relevant only for surgeons. This isn’t crystal clear in the CMS [Centers for Medicare & Medicaid Services] documents, and it’s not the forte of most EHR vendors or trainers either.” In fact, in Brinson’s experience, most EHR trainers present Meaningful Use to every practice as if it were primary care. Yet, the requirements for surgeons are different for primary care and are, frankly, less involved.
That’s good news. Because if you didn’t attest for Meaningful Use in 2014, the first year that reporting was required, you’re automatically getting dinged 2% on your Medicare payments in 2015. So, it’s time to get organized and get moving to avoid further penalties.
Avoid These Four Common Faux Pas
Brinson says the Clinical Quality Measures (CQMs) are hands down the most misunderstood component of Meaningful Use. “When I explain Meaningful Use to surgeons, I can’t jump up and down and wave my hands in the air enough to call attention to this,” she quips.
At issue: There are 64 CQMs, but very few are applicable to surgeons. Yet, many surgeons think they have to perform them for Meaningful Use. Not so, says Brinson. “Surgeons have to report a CQM only if it’s clinically relevant. If none of the CQMs are clinically relevant in your practice, it’s okay to report a zero value if you have not actually performed it.”
Here’s how this plays out. In Stage 2, physicians must report 9 CQMs across 3 domains; Population/Public Health, Patient Safety, and Efficient Use of Healthcare Resources are examples of domains that are most applicable to orthopedists. “If you choose Low Back Pain: Use of Imaging Studies as one of these, it’s possible an orthopedist would have a numeric value to report,” Brinson says. “But if you also choose Use of High-Risk Medications in the Elderly, an orthopedist will probably report a zero value. And that’s totally acceptable. You will not be penalized for reporting zero.”
Another common misconception is around the Vital Signs and Smoking Status measures. “We have worked with surgical practices that think Meaningful Use is requiring them to collect vital signs and smoking status at every visit, even though they may not be clinically relevant,” says Brinson. Again, not true.
“Height and/or weight and blood pressure, as well as smoking status measures, need to be reported only once per patient during the reporting period,” Brinson clarifies. “So from a practical standpoint, most orthopedic practices can collect this data from new patients and then again as clinically necessary,” adding there are even exclusions for physicians who attest that either height and weight and/or blood pressure has no relevance to their scope of practice at all.
Brinson also sees practices do more work than they need to when it comes to Patient Care Reminders. She recently worked with a surgery practice that sent reminders for colonoscopies. “Not exactly clinically relevant,” she says, “and an unnecessary step for staff.” That’s because physicians aren’t required to send reminders that aren’t relevant to their specialty.
The Federal Register states, “An eligible provider (EP) should use clinically relevant information stored within the [EHR] to identify patients who should receive reminders…. The EP is best positioned to decide which information is clinically relevant for this purpose.”
“In orthopedics, clinically relevant reminders could be for an outside referral, a follow-up on an MRI or other test, or a reminder to schedule a postoperative appointment,” Brinson explains. “Work with your EHR vendor to create the reminders that are most appropriate for your patient base.”
The final faux pas that Brinson finds: “Meaningful Use requires you to report data for all patients, not just Medicare patients. That seems to be a point of confusion for many.”
Three Cheers for the Patient Portal Requirement
Stage 2 saw the addition of the Patient Portal Requirement, and Brinson suggests that the benefits of this tool go far beyond Meaningful Use. “Patient portals are essential to a modern practice,” she says. “Patients use them to complete a health history prior to their appointment, pay their bill, schedule follow-up appointments, and more.” Further, the patient portal facilitates another Meaningful Use Stage 2 requirement: secure electronic messaging with patients. For both Meaningful Use and risk management, moving away from e-mail and texting and toward secure/encrypted messaging is a must. The patient portal has this feature already built in, and all messages are stored securely and archived—which meets the HIPAA (Health Insurance Portability and Accountability Act) Omnibus requirements, too.
So if you’ve implemented a patient portal, that’s good for your practice and your patients on many levels. But there is a caveat about meeting the Meaningful Use requirement. “For this requirement, 5% of the unique patients seen during the reporting period must ‘view, download, or transmit to a third party their health information,’” Brinson explains. “So the onus is on your practice to ‘sell’ the benefits of the patient portal and get patients to use it so you can achieve the 5% threshold.”
Clinical Decision Support and Summaries
The requirements of Clinical Decision Support Interventions and Clinical Summaries may seem daunting, but, if you think beyond Meaningful Use for a moment, both facilitate better care.
Take Clinical Decision Support Interventions. What would be helpful for you to know about a patient before surgery? What information would enable you to deliver better care?
“One surgeon told me that a family history of malignant hyperthermia would mean the difference between performing the case in the operating room versus the ambulatory surgery center,” Brinson says. “This is a good example of an intervention that a surgeon would work with their EHR vendor to set up.”
The objective states that each intervention is to be an evidence-based decision-support intervention based on each one and at least one combination of the following data: problem list, medication list, medication allergy list, demographics, laboratory tests and values/results, and vital signs. “Stage 1 requires physicians to implement 1 Clinical Decision Support Intervention, and Stage 2 requires 5,” reminds Brinson.
And here’s all you need to know about Clinical Summaries. Although there are 20 specific required elements of a clinical summary, physicians themselves need to provide details only for clinical instructions and the care plan, including goals and instructions. Ancillary staff can populate the other elements.
Brinson points out that surgeons are not expected to provide a copy of the patient’s note, or to complete the note, before the patient checks out. The requirement under Stage 2 is that the clinical summary is provided to the patient within 1 business day. “From a practical standpoint, practices can print the clinical summary for patients at checkout. A well-done clinical summary is a practice efficiency tool as much as a clinical document. It can reduce phone calls from patients asking, ‘Now what did the doctor tell me to do?’”
Often Overlooked
There are requirements that, Brinson says, surgeons often gloss over: Protect Electronic Health Information and Text-Searchable Progress Notes.
“Stage 2 requires physicians to conduct a privacy risk analysis to protect electronic health information,” she explains. “Most EHR vendors don’t offer this as part of their product, so it’s frequently overlooked.” Such an analysis typically requires an outside vendor, but there are free, do-it-yourself tools available, such as the Privacy and Security Toolkit for Small Provider Organizations,* from the Healthcare Information and Management Systems Society (HIMSS).
The analysis should follow HIPAA guidelines, and the most intensive part of this requirement is to conduct or review a privacy risk analysis of the clinical technology. “You’ve also got to address data encryption and security in the EHR, and ensure HIPAA policies and procedures are in place,” Brinson states.
Text-Searchable Progress Notes are also a new requirement in Stage 2. All progress notes must be text searchable—practices can no longer include progress notes as scanned attachments. “That means no more PDFs,” Brinson says. “Surgeons can still dictate, but the dictation must be entered into the EHR in such a way that it’s searchable. In Stage 2, 30% of unique patients must have a minimum of 1 text-searchable electronic progress note created, edited, and signed in the EHR.”
Conclusion
Meaningful Use does not have to be cumbersome. Focus on what surgical practices need to know, and attestation won’t be as complicated as you think.
*http://www.himss.org/library/healthcare-privacy-security/small-provider-toolkit?navItemNumber=16493.
*http://www.himss.org/library/healthcare-privacy-security/small-provider-toolkit?navItemNumber=16493.
Wrisberg-Variant Discoid Lateral Meniscus: Current Concepts, Treatment Options, and Imaging Features With Emphasis on Dynamic Ultrasonography
First described by Young1 in 1889, discoid lateral meniscus covers a spectrum of meniscal disorders of varying morphology and stability. Determining the true incidence of discoid lateral menisci is difficult because of the large number of asymptomatic cases, though published estimates range from 1% to 17%2-4 of the population, with bilaterality occurring in up to 20%.5 The most commonly used classification system for discoid lateral menisci—reported by Watanabe and colleagues6—describes 3 types of meniscal pathology based on stability to probing and arthroscopic appearance. Type I is stable to probing, has normal tibial attachments, and is “block-shaped,” with increased thickness spanning the entire lateral tibial plateau. Type II is stable to probing and has normal tibial attachments as well, but covers less than 80% of the lateral tibial plateau. Type III (the Wrisberg variant) is unstable because it lacks a posterior meniscotibial (coronary) ligament and has only 1 posterior attachment, the posterior meniscofemoral ligament, or Wrisberg ligament. Wrisberg-variant discoid lateral menisci are rare; estimated incidence is 0.2%.7
Pathophysiology
The normal lateral meniscus, with its flat tibial and concave femoral surfaces, is crucial to load transmission across the knee joint.8 Embryologically differentiating from mesenchymal tissue within the limb bud during fetal development, a normal lateral meniscus never has a discoid shape.8-10 The implication, that discoid lateral menisci represent a congenital anomaly, is further supported by ultrastructural studies involving transmission electron microscopy. These studies have demonstrated that discoid menisci have fewer collagen fibers with a more disorganized course compared with normal menisci.11
With considerable variability, the average normal lateral meniscus is 12 mm wide and 4 mm thick.2 The blood supply to the lateral meniscus recedes during growth, with only the peripheral third remaining in adulthood8 and the inner two-thirds receiving nutrients by diffusion from the intra-articular fluid.5 In comparison, discoid lateral menisci often have poorer vascularity than normal menisci and therefore are more susceptible to tears.8,12,13
Ligamentous attachments to the lateral meniscus include the lateral meniscocapsular ligament, which attaches to the lateral joint capsule. In addition, 70% to 100% of people have accessory meniscofemoral ligaments, which insert anterior (ligament of Humphrey) or posterior (ligament of Wrisberg) to the posterior cruciate ligament.14 There are no ligamentous attachments at the popliteus hiatus or lateral collateral ligament, allowing for 9- to 11-mm excursion of the lateral meniscus during knee flexion and extension.3 Morphologically, the lack of a meniscotibial (coronary) ligament in the setting of a discoid lateral meniscus (Wrisberg variant) results in meniscal hypermobility. During knee range of motion, compressive forces between the femoral condyle and the tibial plateau spread through the peripheral portion of the meniscus and, without ligamentous attachments, allow it to displace anteriorly into the femoral intercondylar notch. This displacement results in impingement between the femur and the tibia15-18 and leads to the characteristic symptoms of “snapping knee syndrome.”10
Clinical Features
Snapping knee syndrome was first described by Kroiss19 in 1910.5 Multiple authors have described patients’ primary complaints as pain, swelling, locking, and a palpable or visible snap at terminal extension. Sudden movement of a soft-tissue structure across a bony prominence during a provocative maneuver is the source of the snapping. The syndrome has many etiologies. Extra-articular causes of lateral snapping knee syndrome include iliotibial band friction syndrome, soft-tissue tumors, hypermobile popliteus tendons, and abnormal anterior insertions of the biceps femoris tendons.20,21 Common intra-articular etiologies include ganglion, synovial, and parameniscal cysts; intra-articular loose bodies; lateral meniscal tears; and discoid lateral menisci.22 Patients with discoid lateral menisci often present with knee pain, popping, range-of-motion limitations, and snapping.23,24 However, the symptoms are quite variable and depend on type of discoid meniscus, presence of a tear, and stability of the rim.2,7,18
Obtaining a thorough history is essential in evaluating patients with suspected discoid lateral menisci. Physical examination should include evaluation of the lateral joint line for bulges, effusion, and tenderness. Patients may experience knee pain with flexion to 30° to 40° when varus or valgus stress (modified McMurray maneuver) is applied.10 In addition, a clunk may be appreciated with McMurray testing as a result of subluxation of the unstable lateral meniscus.10 The contralateral knee should be carefully evaluated, given the frequency of bilateral discoid menisci.10
The figure-4 test, a maneuver developed by LaPrade and Konowalchuk25 to detect peripheral meniscal tears or tears of the popliteomeniscal fascicles, is performed with the patient in the supine position, with the foot of the affected extremity placed on the contralateral knee. Normally, the popliteus tendon pulls the meniscus out of the joint when the knee is brought into the figure-4 position. However, without popliteomeniscal fascicles, the meniscus subluxes into the joint and becomes impinged. With the patient in the figure-4 position, reproduction of symptoms over the lateral joint line is a positive test and suggests peripheral meniscal tears and/or tears or absence of the popliteomeniscal fascicles.25
In the series reported by LaPrade and Konowalchuk,25 all of the patients who experienced symptoms during figure-4 testing were found, on arthroscopic examination, to have lateral meniscal hypermobility caused by tears of the popliteomeniscal fascicles. Despite the success of those authors in using the figure-4 technique for diagnosis, others have reported that the accuracy of the clinical examination (vs arthroscopy) in diagnosing Wrisberg-variant discoid lateral menisci ranges from 29% to 93%.5,26,27 This emphasizes the importance of diagnostic imaging in the work-up of patients with suspected Wrisberg-variant discoid lateral menisci.
Imaging Features
Radiography
In 1964, Picard and Constantin28 recommended that patients with suspected discoid lateral menisci undergo standard anteroposterior, lateral, tunnel, and skyline radiographs as part of the diagnostic work-up. In patients with discoid lateral menisci, plain film radiographs are often normal10 but may demonstrate lateral femoral condyle squaring, widening of the lateral joint line, lateral tibial plateau cupping, tibial eminence hypoplasia, and fibular head elevation.5,29 Plain radiography is unreliable, however, and patients often require advanced imaging, such as knee magnetic resonance imaging (MRI).10
Magnetic Resonance Imaging
Because it clearly depicts soft-tissue structures, MRI is widely used to diagnose musculoskeletal pathology in and around the knee. Criteria for the diagnosis of discoid menisci include meniscal width of 15 mm or more, ratio of minimum meniscal width to maximum tibial width on coronal slice of more than 20%, ratio of sum of width of both lateral horns to meniscal diameter (on sagittal slice showing maximum meniscal diameter) of more than 75%, and continuity of anterior and posterior horns on at least 3 consecutive sagittal slices (bow tie sign).5,30,31 Even in the presence of a tear, the described ratios have sensitivity and specificity of 95% and 97% in detecting discoid lateral menisci.30
However, the Wrisberg variant, which may consist of only a thickened portion of the posterior horn, is often more difficult to diagnose using these criteria and can even appear normal on MRI.26,32 In a series by Neuschwander and colleagues,7 none of the Wrisberg-variant menisci had a true discoid shape, suggesting that the size of the lateral meniscus may appear normal in affected patients. Appropriate positioning during MRI evaluation of patients suspected of having the Wrisberg variant was emphasized by Moser and colleagues,33 who described a case of discoid lateral meniscus not observable on initial MRI but visible on MRI performed with the affected knee extended in the locked position.
The unstable lateral meniscus may be seen subluxed anteriorly or laterally because of lack of posterior attachments. A deficiency of normal popliteomeniscal fascicles and coronary ligaments is represented by a high T2 signal interposed between the discoid lateral meniscus and the posterior joint capsule, simulating a vertical peripheral tear and suggesting presence of the Wrisberg variant (Figures 1A–1C). In addition, the posterior horn of the enlarged discoid lateral meniscus may connect to a prominent and thickened meniscofemoral ligament of Wrisberg. Despite these characteristic imaging features, some studies have found low sensitivity of MRI in the diagnosis of Wrisberg-variant discoid lateral menisci.26
Ultrasonography
There is a growing interest in using ultrasonography in the diagnosis of Wrisberg-variant discoid lateral menisci because of its availability, multiplanar capability, and lower cost compared with MRI. Ultrasonographic criteria for the diagnosis of discoid menisci include absence of normal triangular shape, presence of abnormally elongated and thickened meniscal tissue, and demonstration of a heterogeneous central pattern.5 Through use of a high-resolution probe, which better fits the anatomical concavity of the popliteal fossa, a positive predictive value of 95% and a negative predictive value of 100% have been reported for ultrasonography in the diagnosis of meniscal tears.34
Perhaps the main advantage of ultrasonography is the possibility of performing a dynamic study to evaluate the extrusion of the meniscus into the lateral gutter and to correlate this with knee snapping (Figures 2A, 2B).35 One technique for sonographic evaluation of a hypermobile lateral meniscus involves placing the patient supine with the high-resolution (9 or 12 MHz) linear transducer along the lateral knee joint line. The patient is then asked to place the foot of the affected extremity on the contralateral knee; the combination resembles the numeral 4 (figure-4 test) (Figures 3A, 3B). In a symptomatic patient, this results in clicking, snapping, and/or pain along the lateral joint line, and the lateral meniscus is noted sonographically to extrude into the lateral gutter (Figure 2B), either the result of torn popliteomeniscal fascicles or the increased meniscal mobility of Wrisberg variants.
The main drawback of ultrasonography is operator dependence. As clinicians become more familiar with ultrasonography, dynamic ultrasonography should be used for what is often a difficult diagnosis both clinically and with nondynamic imaging.
Management
The historical treatment for symptomatic discoid lateral menisci, open total meniscectomy,5,7,15,36 is no longer performed, as studies have shown it increases contact stresses proportional to the amount of meniscus removed, with up to a 235% increase after total meniscectomy,37 predisposing patients to early degenerative changes and osteoarthritis.38-41
With an appreciation of the role of menisci as load distributors and joint stabilizers in cartilage nutrition, current treatments aim to preserve as much stable meniscal tissue as possible.5 Surgical management of Wrisberg-variant discoid lateral menisci involves posterior stabilization with or without saucerization.7,33,42 The goal of arthroscopic saucerization is to preserve healthy tissue and create a stable remaining meniscus (6-8 mm in width)2,7,43,44 that provides adequate shock absorption without retearing.10 Wrisberg-variant discoid menisci can be stabilized with use of all-inside sutures from the meniscus to the joint capsule (Figures 4A–4F) when there is sufficient residual meniscus to allow for suture fixation to the posterior capsule after débridement. In contrast, some prefer an inside-out technique, as described by Neuschwander and colleagues,7 with inclusion of a mini-open approach. Any meniscal tears are addressed at time of surgery, either by partial meniscectomy or repair. Relative indications for meniscal repair include longitudinal, vertical, nondegenerative tears that are within 3 mm of the periphery (vascular zone) and are less than 3 cm in length.45 However, the majority of tears in adults are degenerative cleavage tears outside the vascular zone and therefore not amenable to repair.45,46 Before surgery, patients treated with stabilization with or without saucerization are prescribed partial weight-bearing in a hinged knee brace with gradual range of motion to 90° by 6 weeks and return to sports in 3 to 4 months.
Clinical Results
As has been consistently demonstrated, the long-term outcomes of total meniscectomy are poor function39,40,47 and radiographic evidence of lateral compartment arthritis.48 Patients who previously underwent total meniscectomy should be offered meniscal allograft transplantation, as it may offset the increased peak local contact pressures in the lateral compartment10 and improve function.49
With an appreciation for the importance of meniscus preservation, more recent studies have found encouraging results for arthroscopic saucerization and stabilization of Wrisberg-variant discoid lateral menisci. For example, Woods and Whelan44 reported excellent results in 75% of patients at 37.5-month follow-up after open repair of discoid lateral menisci lacking posterior attachments. In another study, by Neuschwander and colleagues,7 4 of 6 patients who underwent arthroscopic repair of unstable discoid lateral menisci without posterior coronary ligaments had excellent outcomes. Although these studies demonstrated symptom resolution and lack of radiographic evidence of degenerative changes at midterm follow-up,50 additional long-term studies should be performed to determine whether saucerization and stabilization prevent the onset of lateral compartment osteoarthritis.
Conclusion
Abnormally mobile discoid lateral menisci can result in painful lateral snapping knee syndromes but are often challenging to diagnose clinically and with traditional static imaging. Dynamic ultrasonography with provocative maneuvers can reveal lateral meniscal subluxation, which often cannot be appreciated on MRI, allowing for timely stabilization and symptom resolution.
1. Young RB. The external semilunar cartilage as a complete disc. In: Cleland J, Mackey JY, Young RB, eds. Memoirs and Memoranda in Anatomy. London, England: Williams & Norgate; 1889:179.
2. Jordan MR. Lateral meniscal variants: evaluation and treatment. J Am Acad Orthop Surg. 1996;4(4):191-200.
3. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176.
4. Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop. 1982;(167):19-28.
5. Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop. 2007;1(2):89-96.
6. Watanabe M, Takeda S, Ikeuchi H. Atlas of Arthroscopy. Tokyo, Japan: Igaku-Shoin; 1978.
7. Neuschwander DC, Drez D Jr, Finney TP. Lateral meniscal variant with absence of the posterior coronary ligament. J Bone Joint Surg Am. 1992;74(8):1186-1190.
8. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65(4):538-547.
9. Kaplan EB. Discoid lateral meniscus of the knee joint; nature, mechanism, and operative treatment. J Bone Joint Surg Am. 1957;39(1):77-87.
10. Kramer DE, Micheli LJ. Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg. 2009;17(11):698-707.
11. Atay OA, Pekmezci M, Doral MN, Sargon MF, Ayvaz M, Johnson DL. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35(3):475-478.
12. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
13. Good CR, Green DW, Griffith MH, Valen AW, Widmann RF, Rodeo SA. Arthroscopic treatment of symptomatic discoid meniscus in children: classification, technique, and results. Arthroscopy. 2007;23(2):157-163.
14. Harner CD, Xerogeanes JW, Livesay GA, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736-745.
15. Smillie IS. The congenital discoid meniscus. J Bone Joint Surg Br. 1948;30(4):671-682.
16. Yoo WJ, Choi IH, Chung CY, et al. Discoid lateral meniscus in children: limited knee extension and meniscal instability in the posterior segment. J Pediatr Orthop. 2008;28(5):544-548.
17. Simonian PT, Sussmann PS, Wickiewicz TL, et al. Popliteomeniscal fasciculi and the unstable lateral meniscus: clinical correlation and magnetic resonance diagnosis. Arthroscopy. 1997;13(5):590-596.
18. Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am. 1982;64(7):1068-1073.
19. Kroiss F. Die Verletzungen der Kniegelenkoszwischenknorpel und ihrer Verbindungen. Beitr Klin Chir. 1910;66:598-801.
20. Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop. 1992;(283):205-206.
21. Cooper DE. Snapping popliteus tendon syndrome. A cause of mechanical knee popping in athletes. Am J Sports Med. 1999;27(5):671-674.
22. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007;14(2):167-168.
23. Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5(1):52-56.
24. Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am. 1995;77(9):1357-1361.
25. LaPrade RF, Konowalchuk BK. Popliteomeniscal fascicle tears causing symptomatic lateral compartment knee pain: diagnosis by the figure-4 test and treatment by open repair. Am J Sports Med. 2005;33(8):1231-1236.
26. Kocher MS, DiCanzio J, Zurakowski D, Micheli LJ. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29(3):292-296.
27. Stanitski CL. Correlation of arthroscopic and clinical examinations with magnetic resonance imaging findings of injured knees in children and adolescents. Am J Sports Med. 1998;26(1):2-6.
28. Picard JJ, Constantin L. Radiological aspects of the discoid meniscus [in French]. J Radiol Electrol Med Nucl. 1964;45:839-841.
29. Kerr R. Radiologic case study. Discoid lateral meniscus. Orthopedics. 1986;9(8):1142, 1145-1147.
30. Samoto N, Kozuma M, Tokuhisa T, Kobayashi K. Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging. 2002;20(1):59-64.
31. Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology. 1989;173(2):351-354.
32. Singh K, Helms CA, Jacobs MT, Higgins LD. MRI appearance of Wrisberg variant of discoid lateral meniscus. AJR Am J Roentgenol. 2006;187(2):384-387.
33. Moser MW, Dugas J, Hartzell J, Thornton DD. A hypermobile Wrisberg variant lateral discoid meniscus seen on MRI. Clin Orthop. 2007;(456):264-267.
34. Najafi J, Bagheri S, Lahiji FA. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J Ultrasound Med. 2006;25(5):593-597.
35. Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142-150.
36. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
37. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270-275.
38. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
39. Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11(3):111-115.
40. Wroble RR, Henderson RC, Campion ER, el-Khoury GY, Albright JP. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop. 1992;(279):180-189.
41. Abdon P, Turner MS, Pettersson H, Lindstrand A, Stenstrom A, Swanson AJ. A long-term follow-up study of total meniscectomy in children. Clin Orthop. 1990;(257):166-170.
42. Rosenberg TD, Paulos LE, Parker RD, Harner CD, Gurley WD. Discoid lateral meniscus: case report of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy. 1987;3(4):277-282.
43. Fleissner PR, Eilert RE. Discoid lateral meniscus. Am J Knee Surg. 1999;12(2):125-131.
44. Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9(3):695-706.
45. Yue BW, Gupta AK, Moorman CT 3rd, Garrett WE, Helms CA. Wrisberg variant of the discoid lateral meniscus with flipped meniscal fragments simulating bucket-handle tear: MRI and arthroscopic correlation. Skeletal Radiol. 2011;40(8):1089-1094.
46. Weiss CB, Lundberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71(6):811-822.
47. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
48. Kim SJ, Chun YM, Jeong JH, Ryu SW, Oh KS, Lubis AM. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1315-1320.
49. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22(12):1344-1350.e1.
50. Ogut T, Kesmezacar H, Akgun I, Cansu E. Arthroscopic meniscectomy for discoid lateral meniscus in children and adolescents: 4.5 year follow-up. J Pediatr Orthop B. 2003;12(6):390-397.
First described by Young1 in 1889, discoid lateral meniscus covers a spectrum of meniscal disorders of varying morphology and stability. Determining the true incidence of discoid lateral menisci is difficult because of the large number of asymptomatic cases, though published estimates range from 1% to 17%2-4 of the population, with bilaterality occurring in up to 20%.5 The most commonly used classification system for discoid lateral menisci—reported by Watanabe and colleagues6—describes 3 types of meniscal pathology based on stability to probing and arthroscopic appearance. Type I is stable to probing, has normal tibial attachments, and is “block-shaped,” with increased thickness spanning the entire lateral tibial plateau. Type II is stable to probing and has normal tibial attachments as well, but covers less than 80% of the lateral tibial plateau. Type III (the Wrisberg variant) is unstable because it lacks a posterior meniscotibial (coronary) ligament and has only 1 posterior attachment, the posterior meniscofemoral ligament, or Wrisberg ligament. Wrisberg-variant discoid lateral menisci are rare; estimated incidence is 0.2%.7
Pathophysiology
The normal lateral meniscus, with its flat tibial and concave femoral surfaces, is crucial to load transmission across the knee joint.8 Embryologically differentiating from mesenchymal tissue within the limb bud during fetal development, a normal lateral meniscus never has a discoid shape.8-10 The implication, that discoid lateral menisci represent a congenital anomaly, is further supported by ultrastructural studies involving transmission electron microscopy. These studies have demonstrated that discoid menisci have fewer collagen fibers with a more disorganized course compared with normal menisci.11
With considerable variability, the average normal lateral meniscus is 12 mm wide and 4 mm thick.2 The blood supply to the lateral meniscus recedes during growth, with only the peripheral third remaining in adulthood8 and the inner two-thirds receiving nutrients by diffusion from the intra-articular fluid.5 In comparison, discoid lateral menisci often have poorer vascularity than normal menisci and therefore are more susceptible to tears.8,12,13
Ligamentous attachments to the lateral meniscus include the lateral meniscocapsular ligament, which attaches to the lateral joint capsule. In addition, 70% to 100% of people have accessory meniscofemoral ligaments, which insert anterior (ligament of Humphrey) or posterior (ligament of Wrisberg) to the posterior cruciate ligament.14 There are no ligamentous attachments at the popliteus hiatus or lateral collateral ligament, allowing for 9- to 11-mm excursion of the lateral meniscus during knee flexion and extension.3 Morphologically, the lack of a meniscotibial (coronary) ligament in the setting of a discoid lateral meniscus (Wrisberg variant) results in meniscal hypermobility. During knee range of motion, compressive forces between the femoral condyle and the tibial plateau spread through the peripheral portion of the meniscus and, without ligamentous attachments, allow it to displace anteriorly into the femoral intercondylar notch. This displacement results in impingement between the femur and the tibia15-18 and leads to the characteristic symptoms of “snapping knee syndrome.”10
Clinical Features
Snapping knee syndrome was first described by Kroiss19 in 1910.5 Multiple authors have described patients’ primary complaints as pain, swelling, locking, and a palpable or visible snap at terminal extension. Sudden movement of a soft-tissue structure across a bony prominence during a provocative maneuver is the source of the snapping. The syndrome has many etiologies. Extra-articular causes of lateral snapping knee syndrome include iliotibial band friction syndrome, soft-tissue tumors, hypermobile popliteus tendons, and abnormal anterior insertions of the biceps femoris tendons.20,21 Common intra-articular etiologies include ganglion, synovial, and parameniscal cysts; intra-articular loose bodies; lateral meniscal tears; and discoid lateral menisci.22 Patients with discoid lateral menisci often present with knee pain, popping, range-of-motion limitations, and snapping.23,24 However, the symptoms are quite variable and depend on type of discoid meniscus, presence of a tear, and stability of the rim.2,7,18
Obtaining a thorough history is essential in evaluating patients with suspected discoid lateral menisci. Physical examination should include evaluation of the lateral joint line for bulges, effusion, and tenderness. Patients may experience knee pain with flexion to 30° to 40° when varus or valgus stress (modified McMurray maneuver) is applied.10 In addition, a clunk may be appreciated with McMurray testing as a result of subluxation of the unstable lateral meniscus.10 The contralateral knee should be carefully evaluated, given the frequency of bilateral discoid menisci.10
The figure-4 test, a maneuver developed by LaPrade and Konowalchuk25 to detect peripheral meniscal tears or tears of the popliteomeniscal fascicles, is performed with the patient in the supine position, with the foot of the affected extremity placed on the contralateral knee. Normally, the popliteus tendon pulls the meniscus out of the joint when the knee is brought into the figure-4 position. However, without popliteomeniscal fascicles, the meniscus subluxes into the joint and becomes impinged. With the patient in the figure-4 position, reproduction of symptoms over the lateral joint line is a positive test and suggests peripheral meniscal tears and/or tears or absence of the popliteomeniscal fascicles.25
In the series reported by LaPrade and Konowalchuk,25 all of the patients who experienced symptoms during figure-4 testing were found, on arthroscopic examination, to have lateral meniscal hypermobility caused by tears of the popliteomeniscal fascicles. Despite the success of those authors in using the figure-4 technique for diagnosis, others have reported that the accuracy of the clinical examination (vs arthroscopy) in diagnosing Wrisberg-variant discoid lateral menisci ranges from 29% to 93%.5,26,27 This emphasizes the importance of diagnostic imaging in the work-up of patients with suspected Wrisberg-variant discoid lateral menisci.
Imaging Features
Radiography
In 1964, Picard and Constantin28 recommended that patients with suspected discoid lateral menisci undergo standard anteroposterior, lateral, tunnel, and skyline radiographs as part of the diagnostic work-up. In patients with discoid lateral menisci, plain film radiographs are often normal10 but may demonstrate lateral femoral condyle squaring, widening of the lateral joint line, lateral tibial plateau cupping, tibial eminence hypoplasia, and fibular head elevation.5,29 Plain radiography is unreliable, however, and patients often require advanced imaging, such as knee magnetic resonance imaging (MRI).10
Magnetic Resonance Imaging
Because it clearly depicts soft-tissue structures, MRI is widely used to diagnose musculoskeletal pathology in and around the knee. Criteria for the diagnosis of discoid menisci include meniscal width of 15 mm or more, ratio of minimum meniscal width to maximum tibial width on coronal slice of more than 20%, ratio of sum of width of both lateral horns to meniscal diameter (on sagittal slice showing maximum meniscal diameter) of more than 75%, and continuity of anterior and posterior horns on at least 3 consecutive sagittal slices (bow tie sign).5,30,31 Even in the presence of a tear, the described ratios have sensitivity and specificity of 95% and 97% in detecting discoid lateral menisci.30
However, the Wrisberg variant, which may consist of only a thickened portion of the posterior horn, is often more difficult to diagnose using these criteria and can even appear normal on MRI.26,32 In a series by Neuschwander and colleagues,7 none of the Wrisberg-variant menisci had a true discoid shape, suggesting that the size of the lateral meniscus may appear normal in affected patients. Appropriate positioning during MRI evaluation of patients suspected of having the Wrisberg variant was emphasized by Moser and colleagues,33 who described a case of discoid lateral meniscus not observable on initial MRI but visible on MRI performed with the affected knee extended in the locked position.
The unstable lateral meniscus may be seen subluxed anteriorly or laterally because of lack of posterior attachments. A deficiency of normal popliteomeniscal fascicles and coronary ligaments is represented by a high T2 signal interposed between the discoid lateral meniscus and the posterior joint capsule, simulating a vertical peripheral tear and suggesting presence of the Wrisberg variant (Figures 1A–1C). In addition, the posterior horn of the enlarged discoid lateral meniscus may connect to a prominent and thickened meniscofemoral ligament of Wrisberg. Despite these characteristic imaging features, some studies have found low sensitivity of MRI in the diagnosis of Wrisberg-variant discoid lateral menisci.26
Ultrasonography
There is a growing interest in using ultrasonography in the diagnosis of Wrisberg-variant discoid lateral menisci because of its availability, multiplanar capability, and lower cost compared with MRI. Ultrasonographic criteria for the diagnosis of discoid menisci include absence of normal triangular shape, presence of abnormally elongated and thickened meniscal tissue, and demonstration of a heterogeneous central pattern.5 Through use of a high-resolution probe, which better fits the anatomical concavity of the popliteal fossa, a positive predictive value of 95% and a negative predictive value of 100% have been reported for ultrasonography in the diagnosis of meniscal tears.34
Perhaps the main advantage of ultrasonography is the possibility of performing a dynamic study to evaluate the extrusion of the meniscus into the lateral gutter and to correlate this with knee snapping (Figures 2A, 2B).35 One technique for sonographic evaluation of a hypermobile lateral meniscus involves placing the patient supine with the high-resolution (9 or 12 MHz) linear transducer along the lateral knee joint line. The patient is then asked to place the foot of the affected extremity on the contralateral knee; the combination resembles the numeral 4 (figure-4 test) (Figures 3A, 3B). In a symptomatic patient, this results in clicking, snapping, and/or pain along the lateral joint line, and the lateral meniscus is noted sonographically to extrude into the lateral gutter (Figure 2B), either the result of torn popliteomeniscal fascicles or the increased meniscal mobility of Wrisberg variants.
The main drawback of ultrasonography is operator dependence. As clinicians become more familiar with ultrasonography, dynamic ultrasonography should be used for what is often a difficult diagnosis both clinically and with nondynamic imaging.
Management
The historical treatment for symptomatic discoid lateral menisci, open total meniscectomy,5,7,15,36 is no longer performed, as studies have shown it increases contact stresses proportional to the amount of meniscus removed, with up to a 235% increase after total meniscectomy,37 predisposing patients to early degenerative changes and osteoarthritis.38-41
With an appreciation of the role of menisci as load distributors and joint stabilizers in cartilage nutrition, current treatments aim to preserve as much stable meniscal tissue as possible.5 Surgical management of Wrisberg-variant discoid lateral menisci involves posterior stabilization with or without saucerization.7,33,42 The goal of arthroscopic saucerization is to preserve healthy tissue and create a stable remaining meniscus (6-8 mm in width)2,7,43,44 that provides adequate shock absorption without retearing.10 Wrisberg-variant discoid menisci can be stabilized with use of all-inside sutures from the meniscus to the joint capsule (Figures 4A–4F) when there is sufficient residual meniscus to allow for suture fixation to the posterior capsule after débridement. In contrast, some prefer an inside-out technique, as described by Neuschwander and colleagues,7 with inclusion of a mini-open approach. Any meniscal tears are addressed at time of surgery, either by partial meniscectomy or repair. Relative indications for meniscal repair include longitudinal, vertical, nondegenerative tears that are within 3 mm of the periphery (vascular zone) and are less than 3 cm in length.45 However, the majority of tears in adults are degenerative cleavage tears outside the vascular zone and therefore not amenable to repair.45,46 Before surgery, patients treated with stabilization with or without saucerization are prescribed partial weight-bearing in a hinged knee brace with gradual range of motion to 90° by 6 weeks and return to sports in 3 to 4 months.
Clinical Results
As has been consistently demonstrated, the long-term outcomes of total meniscectomy are poor function39,40,47 and radiographic evidence of lateral compartment arthritis.48 Patients who previously underwent total meniscectomy should be offered meniscal allograft transplantation, as it may offset the increased peak local contact pressures in the lateral compartment10 and improve function.49
With an appreciation for the importance of meniscus preservation, more recent studies have found encouraging results for arthroscopic saucerization and stabilization of Wrisberg-variant discoid lateral menisci. For example, Woods and Whelan44 reported excellent results in 75% of patients at 37.5-month follow-up after open repair of discoid lateral menisci lacking posterior attachments. In another study, by Neuschwander and colleagues,7 4 of 6 patients who underwent arthroscopic repair of unstable discoid lateral menisci without posterior coronary ligaments had excellent outcomes. Although these studies demonstrated symptom resolution and lack of radiographic evidence of degenerative changes at midterm follow-up,50 additional long-term studies should be performed to determine whether saucerization and stabilization prevent the onset of lateral compartment osteoarthritis.
Conclusion
Abnormally mobile discoid lateral menisci can result in painful lateral snapping knee syndromes but are often challenging to diagnose clinically and with traditional static imaging. Dynamic ultrasonography with provocative maneuvers can reveal lateral meniscal subluxation, which often cannot be appreciated on MRI, allowing for timely stabilization and symptom resolution.
First described by Young1 in 1889, discoid lateral meniscus covers a spectrum of meniscal disorders of varying morphology and stability. Determining the true incidence of discoid lateral menisci is difficult because of the large number of asymptomatic cases, though published estimates range from 1% to 17%2-4 of the population, with bilaterality occurring in up to 20%.5 The most commonly used classification system for discoid lateral menisci—reported by Watanabe and colleagues6—describes 3 types of meniscal pathology based on stability to probing and arthroscopic appearance. Type I is stable to probing, has normal tibial attachments, and is “block-shaped,” with increased thickness spanning the entire lateral tibial plateau. Type II is stable to probing and has normal tibial attachments as well, but covers less than 80% of the lateral tibial plateau. Type III (the Wrisberg variant) is unstable because it lacks a posterior meniscotibial (coronary) ligament and has only 1 posterior attachment, the posterior meniscofemoral ligament, or Wrisberg ligament. Wrisberg-variant discoid lateral menisci are rare; estimated incidence is 0.2%.7
Pathophysiology
The normal lateral meniscus, with its flat tibial and concave femoral surfaces, is crucial to load transmission across the knee joint.8 Embryologically differentiating from mesenchymal tissue within the limb bud during fetal development, a normal lateral meniscus never has a discoid shape.8-10 The implication, that discoid lateral menisci represent a congenital anomaly, is further supported by ultrastructural studies involving transmission electron microscopy. These studies have demonstrated that discoid menisci have fewer collagen fibers with a more disorganized course compared with normal menisci.11
With considerable variability, the average normal lateral meniscus is 12 mm wide and 4 mm thick.2 The blood supply to the lateral meniscus recedes during growth, with only the peripheral third remaining in adulthood8 and the inner two-thirds receiving nutrients by diffusion from the intra-articular fluid.5 In comparison, discoid lateral menisci often have poorer vascularity than normal menisci and therefore are more susceptible to tears.8,12,13
Ligamentous attachments to the lateral meniscus include the lateral meniscocapsular ligament, which attaches to the lateral joint capsule. In addition, 70% to 100% of people have accessory meniscofemoral ligaments, which insert anterior (ligament of Humphrey) or posterior (ligament of Wrisberg) to the posterior cruciate ligament.14 There are no ligamentous attachments at the popliteus hiatus or lateral collateral ligament, allowing for 9- to 11-mm excursion of the lateral meniscus during knee flexion and extension.3 Morphologically, the lack of a meniscotibial (coronary) ligament in the setting of a discoid lateral meniscus (Wrisberg variant) results in meniscal hypermobility. During knee range of motion, compressive forces between the femoral condyle and the tibial plateau spread through the peripheral portion of the meniscus and, without ligamentous attachments, allow it to displace anteriorly into the femoral intercondylar notch. This displacement results in impingement between the femur and the tibia15-18 and leads to the characteristic symptoms of “snapping knee syndrome.”10
Clinical Features
Snapping knee syndrome was first described by Kroiss19 in 1910.5 Multiple authors have described patients’ primary complaints as pain, swelling, locking, and a palpable or visible snap at terminal extension. Sudden movement of a soft-tissue structure across a bony prominence during a provocative maneuver is the source of the snapping. The syndrome has many etiologies. Extra-articular causes of lateral snapping knee syndrome include iliotibial band friction syndrome, soft-tissue tumors, hypermobile popliteus tendons, and abnormal anterior insertions of the biceps femoris tendons.20,21 Common intra-articular etiologies include ganglion, synovial, and parameniscal cysts; intra-articular loose bodies; lateral meniscal tears; and discoid lateral menisci.22 Patients with discoid lateral menisci often present with knee pain, popping, range-of-motion limitations, and snapping.23,24 However, the symptoms are quite variable and depend on type of discoid meniscus, presence of a tear, and stability of the rim.2,7,18
Obtaining a thorough history is essential in evaluating patients with suspected discoid lateral menisci. Physical examination should include evaluation of the lateral joint line for bulges, effusion, and tenderness. Patients may experience knee pain with flexion to 30° to 40° when varus or valgus stress (modified McMurray maneuver) is applied.10 In addition, a clunk may be appreciated with McMurray testing as a result of subluxation of the unstable lateral meniscus.10 The contralateral knee should be carefully evaluated, given the frequency of bilateral discoid menisci.10
The figure-4 test, a maneuver developed by LaPrade and Konowalchuk25 to detect peripheral meniscal tears or tears of the popliteomeniscal fascicles, is performed with the patient in the supine position, with the foot of the affected extremity placed on the contralateral knee. Normally, the popliteus tendon pulls the meniscus out of the joint when the knee is brought into the figure-4 position. However, without popliteomeniscal fascicles, the meniscus subluxes into the joint and becomes impinged. With the patient in the figure-4 position, reproduction of symptoms over the lateral joint line is a positive test and suggests peripheral meniscal tears and/or tears or absence of the popliteomeniscal fascicles.25
In the series reported by LaPrade and Konowalchuk,25 all of the patients who experienced symptoms during figure-4 testing were found, on arthroscopic examination, to have lateral meniscal hypermobility caused by tears of the popliteomeniscal fascicles. Despite the success of those authors in using the figure-4 technique for diagnosis, others have reported that the accuracy of the clinical examination (vs arthroscopy) in diagnosing Wrisberg-variant discoid lateral menisci ranges from 29% to 93%.5,26,27 This emphasizes the importance of diagnostic imaging in the work-up of patients with suspected Wrisberg-variant discoid lateral menisci.
Imaging Features
Radiography
In 1964, Picard and Constantin28 recommended that patients with suspected discoid lateral menisci undergo standard anteroposterior, lateral, tunnel, and skyline radiographs as part of the diagnostic work-up. In patients with discoid lateral menisci, plain film radiographs are often normal10 but may demonstrate lateral femoral condyle squaring, widening of the lateral joint line, lateral tibial plateau cupping, tibial eminence hypoplasia, and fibular head elevation.5,29 Plain radiography is unreliable, however, and patients often require advanced imaging, such as knee magnetic resonance imaging (MRI).10
Magnetic Resonance Imaging
Because it clearly depicts soft-tissue structures, MRI is widely used to diagnose musculoskeletal pathology in and around the knee. Criteria for the diagnosis of discoid menisci include meniscal width of 15 mm or more, ratio of minimum meniscal width to maximum tibial width on coronal slice of more than 20%, ratio of sum of width of both lateral horns to meniscal diameter (on sagittal slice showing maximum meniscal diameter) of more than 75%, and continuity of anterior and posterior horns on at least 3 consecutive sagittal slices (bow tie sign).5,30,31 Even in the presence of a tear, the described ratios have sensitivity and specificity of 95% and 97% in detecting discoid lateral menisci.30
However, the Wrisberg variant, which may consist of only a thickened portion of the posterior horn, is often more difficult to diagnose using these criteria and can even appear normal on MRI.26,32 In a series by Neuschwander and colleagues,7 none of the Wrisberg-variant menisci had a true discoid shape, suggesting that the size of the lateral meniscus may appear normal in affected patients. Appropriate positioning during MRI evaluation of patients suspected of having the Wrisberg variant was emphasized by Moser and colleagues,33 who described a case of discoid lateral meniscus not observable on initial MRI but visible on MRI performed with the affected knee extended in the locked position.
The unstable lateral meniscus may be seen subluxed anteriorly or laterally because of lack of posterior attachments. A deficiency of normal popliteomeniscal fascicles and coronary ligaments is represented by a high T2 signal interposed between the discoid lateral meniscus and the posterior joint capsule, simulating a vertical peripheral tear and suggesting presence of the Wrisberg variant (Figures 1A–1C). In addition, the posterior horn of the enlarged discoid lateral meniscus may connect to a prominent and thickened meniscofemoral ligament of Wrisberg. Despite these characteristic imaging features, some studies have found low sensitivity of MRI in the diagnosis of Wrisberg-variant discoid lateral menisci.26
Ultrasonography
There is a growing interest in using ultrasonography in the diagnosis of Wrisberg-variant discoid lateral menisci because of its availability, multiplanar capability, and lower cost compared with MRI. Ultrasonographic criteria for the diagnosis of discoid menisci include absence of normal triangular shape, presence of abnormally elongated and thickened meniscal tissue, and demonstration of a heterogeneous central pattern.5 Through use of a high-resolution probe, which better fits the anatomical concavity of the popliteal fossa, a positive predictive value of 95% and a negative predictive value of 100% have been reported for ultrasonography in the diagnosis of meniscal tears.34
Perhaps the main advantage of ultrasonography is the possibility of performing a dynamic study to evaluate the extrusion of the meniscus into the lateral gutter and to correlate this with knee snapping (Figures 2A, 2B).35 One technique for sonographic evaluation of a hypermobile lateral meniscus involves placing the patient supine with the high-resolution (9 or 12 MHz) linear transducer along the lateral knee joint line. The patient is then asked to place the foot of the affected extremity on the contralateral knee; the combination resembles the numeral 4 (figure-4 test) (Figures 3A, 3B). In a symptomatic patient, this results in clicking, snapping, and/or pain along the lateral joint line, and the lateral meniscus is noted sonographically to extrude into the lateral gutter (Figure 2B), either the result of torn popliteomeniscal fascicles or the increased meniscal mobility of Wrisberg variants.
The main drawback of ultrasonography is operator dependence. As clinicians become more familiar with ultrasonography, dynamic ultrasonography should be used for what is often a difficult diagnosis both clinically and with nondynamic imaging.
Management
The historical treatment for symptomatic discoid lateral menisci, open total meniscectomy,5,7,15,36 is no longer performed, as studies have shown it increases contact stresses proportional to the amount of meniscus removed, with up to a 235% increase after total meniscectomy,37 predisposing patients to early degenerative changes and osteoarthritis.38-41
With an appreciation of the role of menisci as load distributors and joint stabilizers in cartilage nutrition, current treatments aim to preserve as much stable meniscal tissue as possible.5 Surgical management of Wrisberg-variant discoid lateral menisci involves posterior stabilization with or without saucerization.7,33,42 The goal of arthroscopic saucerization is to preserve healthy tissue and create a stable remaining meniscus (6-8 mm in width)2,7,43,44 that provides adequate shock absorption without retearing.10 Wrisberg-variant discoid menisci can be stabilized with use of all-inside sutures from the meniscus to the joint capsule (Figures 4A–4F) when there is sufficient residual meniscus to allow for suture fixation to the posterior capsule after débridement. In contrast, some prefer an inside-out technique, as described by Neuschwander and colleagues,7 with inclusion of a mini-open approach. Any meniscal tears are addressed at time of surgery, either by partial meniscectomy or repair. Relative indications for meniscal repair include longitudinal, vertical, nondegenerative tears that are within 3 mm of the periphery (vascular zone) and are less than 3 cm in length.45 However, the majority of tears in adults are degenerative cleavage tears outside the vascular zone and therefore not amenable to repair.45,46 Before surgery, patients treated with stabilization with or without saucerization are prescribed partial weight-bearing in a hinged knee brace with gradual range of motion to 90° by 6 weeks and return to sports in 3 to 4 months.
Clinical Results
As has been consistently demonstrated, the long-term outcomes of total meniscectomy are poor function39,40,47 and radiographic evidence of lateral compartment arthritis.48 Patients who previously underwent total meniscectomy should be offered meniscal allograft transplantation, as it may offset the increased peak local contact pressures in the lateral compartment10 and improve function.49
With an appreciation for the importance of meniscus preservation, more recent studies have found encouraging results for arthroscopic saucerization and stabilization of Wrisberg-variant discoid lateral menisci. For example, Woods and Whelan44 reported excellent results in 75% of patients at 37.5-month follow-up after open repair of discoid lateral menisci lacking posterior attachments. In another study, by Neuschwander and colleagues,7 4 of 6 patients who underwent arthroscopic repair of unstable discoid lateral menisci without posterior coronary ligaments had excellent outcomes. Although these studies demonstrated symptom resolution and lack of radiographic evidence of degenerative changes at midterm follow-up,50 additional long-term studies should be performed to determine whether saucerization and stabilization prevent the onset of lateral compartment osteoarthritis.
Conclusion
Abnormally mobile discoid lateral menisci can result in painful lateral snapping knee syndromes but are often challenging to diagnose clinically and with traditional static imaging. Dynamic ultrasonography with provocative maneuvers can reveal lateral meniscal subluxation, which often cannot be appreciated on MRI, allowing for timely stabilization and symptom resolution.
1. Young RB. The external semilunar cartilage as a complete disc. In: Cleland J, Mackey JY, Young RB, eds. Memoirs and Memoranda in Anatomy. London, England: Williams & Norgate; 1889:179.
2. Jordan MR. Lateral meniscal variants: evaluation and treatment. J Am Acad Orthop Surg. 1996;4(4):191-200.
3. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176.
4. Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop. 1982;(167):19-28.
5. Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop. 2007;1(2):89-96.
6. Watanabe M, Takeda S, Ikeuchi H. Atlas of Arthroscopy. Tokyo, Japan: Igaku-Shoin; 1978.
7. Neuschwander DC, Drez D Jr, Finney TP. Lateral meniscal variant with absence of the posterior coronary ligament. J Bone Joint Surg Am. 1992;74(8):1186-1190.
8. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65(4):538-547.
9. Kaplan EB. Discoid lateral meniscus of the knee joint; nature, mechanism, and operative treatment. J Bone Joint Surg Am. 1957;39(1):77-87.
10. Kramer DE, Micheli LJ. Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg. 2009;17(11):698-707.
11. Atay OA, Pekmezci M, Doral MN, Sargon MF, Ayvaz M, Johnson DL. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35(3):475-478.
12. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
13. Good CR, Green DW, Griffith MH, Valen AW, Widmann RF, Rodeo SA. Arthroscopic treatment of symptomatic discoid meniscus in children: classification, technique, and results. Arthroscopy. 2007;23(2):157-163.
14. Harner CD, Xerogeanes JW, Livesay GA, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736-745.
15. Smillie IS. The congenital discoid meniscus. J Bone Joint Surg Br. 1948;30(4):671-682.
16. Yoo WJ, Choi IH, Chung CY, et al. Discoid lateral meniscus in children: limited knee extension and meniscal instability in the posterior segment. J Pediatr Orthop. 2008;28(5):544-548.
17. Simonian PT, Sussmann PS, Wickiewicz TL, et al. Popliteomeniscal fasciculi and the unstable lateral meniscus: clinical correlation and magnetic resonance diagnosis. Arthroscopy. 1997;13(5):590-596.
18. Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am. 1982;64(7):1068-1073.
19. Kroiss F. Die Verletzungen der Kniegelenkoszwischenknorpel und ihrer Verbindungen. Beitr Klin Chir. 1910;66:598-801.
20. Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop. 1992;(283):205-206.
21. Cooper DE. Snapping popliteus tendon syndrome. A cause of mechanical knee popping in athletes. Am J Sports Med. 1999;27(5):671-674.
22. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007;14(2):167-168.
23. Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5(1):52-56.
24. Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am. 1995;77(9):1357-1361.
25. LaPrade RF, Konowalchuk BK. Popliteomeniscal fascicle tears causing symptomatic lateral compartment knee pain: diagnosis by the figure-4 test and treatment by open repair. Am J Sports Med. 2005;33(8):1231-1236.
26. Kocher MS, DiCanzio J, Zurakowski D, Micheli LJ. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29(3):292-296.
27. Stanitski CL. Correlation of arthroscopic and clinical examinations with magnetic resonance imaging findings of injured knees in children and adolescents. Am J Sports Med. 1998;26(1):2-6.
28. Picard JJ, Constantin L. Radiological aspects of the discoid meniscus [in French]. J Radiol Electrol Med Nucl. 1964;45:839-841.
29. Kerr R. Radiologic case study. Discoid lateral meniscus. Orthopedics. 1986;9(8):1142, 1145-1147.
30. Samoto N, Kozuma M, Tokuhisa T, Kobayashi K. Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging. 2002;20(1):59-64.
31. Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology. 1989;173(2):351-354.
32. Singh K, Helms CA, Jacobs MT, Higgins LD. MRI appearance of Wrisberg variant of discoid lateral meniscus. AJR Am J Roentgenol. 2006;187(2):384-387.
33. Moser MW, Dugas J, Hartzell J, Thornton DD. A hypermobile Wrisberg variant lateral discoid meniscus seen on MRI. Clin Orthop. 2007;(456):264-267.
34. Najafi J, Bagheri S, Lahiji FA. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J Ultrasound Med. 2006;25(5):593-597.
35. Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142-150.
36. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
37. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270-275.
38. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
39. Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11(3):111-115.
40. Wroble RR, Henderson RC, Campion ER, el-Khoury GY, Albright JP. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop. 1992;(279):180-189.
41. Abdon P, Turner MS, Pettersson H, Lindstrand A, Stenstrom A, Swanson AJ. A long-term follow-up study of total meniscectomy in children. Clin Orthop. 1990;(257):166-170.
42. Rosenberg TD, Paulos LE, Parker RD, Harner CD, Gurley WD. Discoid lateral meniscus: case report of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy. 1987;3(4):277-282.
43. Fleissner PR, Eilert RE. Discoid lateral meniscus. Am J Knee Surg. 1999;12(2):125-131.
44. Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9(3):695-706.
45. Yue BW, Gupta AK, Moorman CT 3rd, Garrett WE, Helms CA. Wrisberg variant of the discoid lateral meniscus with flipped meniscal fragments simulating bucket-handle tear: MRI and arthroscopic correlation. Skeletal Radiol. 2011;40(8):1089-1094.
46. Weiss CB, Lundberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71(6):811-822.
47. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
48. Kim SJ, Chun YM, Jeong JH, Ryu SW, Oh KS, Lubis AM. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1315-1320.
49. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22(12):1344-1350.e1.
50. Ogut T, Kesmezacar H, Akgun I, Cansu E. Arthroscopic meniscectomy for discoid lateral meniscus in children and adolescents: 4.5 year follow-up. J Pediatr Orthop B. 2003;12(6):390-397.
1. Young RB. The external semilunar cartilage as a complete disc. In: Cleland J, Mackey JY, Young RB, eds. Memoirs and Memoranda in Anatomy. London, England: Williams & Norgate; 1889:179.
2. Jordan MR. Lateral meniscal variants: evaluation and treatment. J Am Acad Orthop Surg. 1996;4(4):191-200.
3. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176.
4. Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop. 1982;(167):19-28.
5. Yaniv M, Blumberg N. The discoid meniscus. J Child Orthop. 2007;1(2):89-96.
6. Watanabe M, Takeda S, Ikeuchi H. Atlas of Arthroscopy. Tokyo, Japan: Igaku-Shoin; 1978.
7. Neuschwander DC, Drez D Jr, Finney TP. Lateral meniscal variant with absence of the posterior coronary ligament. J Bone Joint Surg Am. 1992;74(8):1186-1190.
8. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65(4):538-547.
9. Kaplan EB. Discoid lateral meniscus of the knee joint; nature, mechanism, and operative treatment. J Bone Joint Surg Am. 1957;39(1):77-87.
10. Kramer DE, Micheli LJ. Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg. 2009;17(11):698-707.
11. Atay OA, Pekmezci M, Doral MN, Sargon MF, Ayvaz M, Johnson DL. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35(3):475-478.
12. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
13. Good CR, Green DW, Griffith MH, Valen AW, Widmann RF, Rodeo SA. Arthroscopic treatment of symptomatic discoid meniscus in children: classification, technique, and results. Arthroscopy. 2007;23(2):157-163.
14. Harner CD, Xerogeanes JW, Livesay GA, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736-745.
15. Smillie IS. The congenital discoid meniscus. J Bone Joint Surg Br. 1948;30(4):671-682.
16. Yoo WJ, Choi IH, Chung CY, et al. Discoid lateral meniscus in children: limited knee extension and meniscal instability in the posterior segment. J Pediatr Orthop. 2008;28(5):544-548.
17. Simonian PT, Sussmann PS, Wickiewicz TL, et al. Popliteomeniscal fasciculi and the unstable lateral meniscus: clinical correlation and magnetic resonance diagnosis. Arthroscopy. 1997;13(5):590-596.
18. Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am. 1982;64(7):1068-1073.
19. Kroiss F. Die Verletzungen der Kniegelenkoszwischenknorpel und ihrer Verbindungen. Beitr Klin Chir. 1910;66:598-801.
20. Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop. 1992;(283):205-206.
21. Cooper DE. Snapping popliteus tendon syndrome. A cause of mechanical knee popping in athletes. Am J Sports Med. 1999;27(5):671-674.
22. Liu PC, Chen CH, Huang HT, et al. Snapping knee symptoms caused by an intra-articular ganglion cyst. Knee. 2007;14(2):167-168.
23. Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5(1):52-56.
24. Washington ER 3rd, Root L, Liener UC. Discoid lateral meniscus in children. Long-term follow-up after excision. J Bone Joint Surg Am. 1995;77(9):1357-1361.
25. LaPrade RF, Konowalchuk BK. Popliteomeniscal fascicle tears causing symptomatic lateral compartment knee pain: diagnosis by the figure-4 test and treatment by open repair. Am J Sports Med. 2005;33(8):1231-1236.
26. Kocher MS, DiCanzio J, Zurakowski D, Micheli LJ. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29(3):292-296.
27. Stanitski CL. Correlation of arthroscopic and clinical examinations with magnetic resonance imaging findings of injured knees in children and adolescents. Am J Sports Med. 1998;26(1):2-6.
28. Picard JJ, Constantin L. Radiological aspects of the discoid meniscus [in French]. J Radiol Electrol Med Nucl. 1964;45:839-841.
29. Kerr R. Radiologic case study. Discoid lateral meniscus. Orthopedics. 1986;9(8):1142, 1145-1147.
30. Samoto N, Kozuma M, Tokuhisa T, Kobayashi K. Diagnosis of discoid lateral meniscus of the knee on MR imaging. Magn Reson Imaging. 2002;20(1):59-64.
31. Silverman JM, Mink JH, Deutsch AL. Discoid menisci of the knee: MR imaging appearance. Radiology. 1989;173(2):351-354.
32. Singh K, Helms CA, Jacobs MT, Higgins LD. MRI appearance of Wrisberg variant of discoid lateral meniscus. AJR Am J Roentgenol. 2006;187(2):384-387.
33. Moser MW, Dugas J, Hartzell J, Thornton DD. A hypermobile Wrisberg variant lateral discoid meniscus seen on MRI. Clin Orthop. 2007;(456):264-267.
34. Najafi J, Bagheri S, Lahiji FA. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J Ultrasound Med. 2006;25(5):593-597.
35. Marchand AJ, Proisy M, Ropars M, Cohen M, Duvauferrier R, Guillin R. Snapping knee: imaging findings with an emphasis on dynamic sonography. AJR Am J Roentgenol. 2012;199(1):142-150.
36. Nathan PA, Cole SC. Discoid meniscus. A clinical and pathologic study. Clin Orthop. 1969;(64):107-113.
37. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270-275.
38. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
39. Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11(3):111-115.
40. Wroble RR, Henderson RC, Campion ER, el-Khoury GY, Albright JP. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop. 1992;(279):180-189.
41. Abdon P, Turner MS, Pettersson H, Lindstrand A, Stenstrom A, Swanson AJ. A long-term follow-up study of total meniscectomy in children. Clin Orthop. 1990;(257):166-170.
42. Rosenberg TD, Paulos LE, Parker RD, Harner CD, Gurley WD. Discoid lateral meniscus: case report of arthroscopic attachment of a symptomatic Wrisberg-ligament type. Arthroscopy. 1987;3(4):277-282.
43. Fleissner PR, Eilert RE. Discoid lateral meniscus. Am J Knee Surg. 1999;12(2):125-131.
44. Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9(3):695-706.
45. Yue BW, Gupta AK, Moorman CT 3rd, Garrett WE, Helms CA. Wrisberg variant of the discoid lateral meniscus with flipped meniscal fragments simulating bucket-handle tear: MRI and arthroscopic correlation. Skeletal Radiol. 2011;40(8):1089-1094.
46. Weiss CB, Lundberg M, Hamberg P, DeHaven KE, Gillquist J. Non-operative treatment of meniscal tears. J Bone Joint Surg Am. 1989;71(6):811-822.
47. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
48. Kim SJ, Chun YM, Jeong JH, Ryu SW, Oh KS, Lubis AM. Effects of arthroscopic meniscectomy on the long-term prognosis for the discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2007;15(11):1315-1320.
49. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22(12):1344-1350.e1.
50. Ogut T, Kesmezacar H, Akgun I, Cansu E. Arthroscopic meniscectomy for discoid lateral meniscus in children and adolescents: 4.5 year follow-up. J Pediatr Orthop B. 2003;12(6):390-397.
Intraoperative Radiofrequency Ablation for Osteoid Osteoma
Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.
Follow-Up
We phoned all the patients to ask about symptom recurrence, outside treatment, and satisfaction with RFA and to obtain informed consent to participate in our study. Only 1 of the 28 patients could not be reached and was lost to follow-up. Mean follow-up at time of study completion was 31.1 months (range, 5.2-55.8 months).
The 27 patients were asked a series of questions about their treatment: Have you had any recurrence of symptoms following treatment for your OO? Have you received treatment elsewhere? Were you satisfied with your treatment? Would you have the procedure again if you had a recurrence of symptoms?
Primary success was defined as complete pain relief after initial RFA with no evidence of recurrence at time of final follow-up, and secondary success was defined as presence of recurrent symptoms after initial RFA with complete pain relief after a second procedure with no evidence of recurrence.
Results
All RFAs were technically successful with adequate localization of the tumor nidus and subsequent probe placement within the lesion. There were no intraoperative or postoperative complications. All 28 patients were discharged home the day of procedure. Twenty-six patients (92.8%) experienced complete pain relief after primary RFA, had no evidence of recurrence at final follow-up, and denied symptom recurrence at time of study completion.
The other 2 patients reported symptom recurrence after the index treatment (1 proximal femur lesion, 1 distal femur lesion). One of these patients did well initially but had a recurrence about 2 months after the primary RFA; a second RFA provided complete resolution of pain with no evidence of recurrence at time of study completion. In the other patient’s case, intermittent pain persisted for 2 weeks after the primary RFA, and evidence of recurrence was documented 3 months after surgery; a second RFA was performed shortly thereafter, but the patient was subsequently lost to follow-up.
At time of study completion, all 27 patients who had been contacted by phone denied seeking additional treatment elsewhere and stated they would have the procedure again if their symptoms ever recurred.
Discussion
Osteoid osteoma is one of the most common benign tumors of bone. Over the past 2 decades, percutaneous RFA, in comparison with open excision, has emerged as a safe and effective treatment option with minimal patient morbidity.9-11 RFA traditionally has been performed by radiologists under CT guidance in the radiology suite. However, now orthopedic surgeons can obtain advanced intraoperative imaging beyond standard fluoroscopy. The Siemens Siremobil ISO-C3D fluoroscopic C-arm is an innovative intraoperative imaging device that functions as a standard fluoroscope but also generates 3-D reconstructions of surgical anatomy. The isocentric design and integrated motor unit allow the C-arm to move through a 190º arc while centering its beam directly on the area of interest. This data set is transferred to a computer workstation, where it is reformatted so that CT-quality images are generated in axial, sagittal, and coronal planes. This acquisition process takes only minutes, and the multiplanar images produced may be simultaneously displayed and manipulated on the screen in real time.
One concern about this technology is the amount of radiation exposure for patients, surgeons, and operating room staff. The device measures only radiation time, and the amount of exposure during that time depends on the volume and density of the radiated body. We did not calculate the amount of exposure for this study. Mean exposure time was between 20 and 40 seconds, reflecting the number of attempts required to localize the lesion and the surgeon’s experience with the technique. Although the potential for increased exposure is a valid concern, previous studies using this technology have demonstrated that a similar average exposure time is equivalent to that of standard CT, and that use of the device, over conventional techniques, potentially can lead to decreased overall radiation exposure.12,13
This series demonstrated that OO can be safely and effectively treated with intraoperative percutaneous RFA by an orthopedic oncologist. Our success rate is very similar to rates reported in the radiology literature. Studies are needed to confirm the efficacy of this novel technique in comparison with what has been reported in that literature. Given these promising preliminary results, and the relative ease of use and minimal learning curve associated with this technology, all orthopedic oncologists should be able to offer this treatment for OO. Furthermore, this technique allows orthopedic oncologists to provide appropriate definitive treatment and care directly, rather than by referring patients to radiologists.
In the treatment of OO, we reserve RFA for lesions involving the long and short bones of the upper and lower extremities, as well as selected flat bones, such as those in the pelvis. Although percutaneous RFA of spinal lesions has been reported in the literature, we think these represent a relative contraindication for this technique; image resolution, in our opinion, is not high enough to justify risking injury to the nerves in the spinal canal, lateral recesses, and neural foramina. In addition, given the radiation exposure, we recommend caution when using this technique for a pelvic or proximal femoral lesion in a woman of childbearing age.
1. Gitelis S, Wilkins R, Conrad EU 2nd. Benign bone tumors. Instr Course Lect. 1996;45:425-424.
2. Schajowicz F. Bone forming tumors. In: Tumors and Tumorlike Lesions of Bone. 2nd ed. New York, NY: Springer-Verlag; 1994:36-62.
3. Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559-574.
4. Golding JS. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36(2):218-229.
5. Simm RJ. The natural history of osteoid osteoma. Aust N Z J Surg. 1975;45(4):412-415.
6. Sans N, Galy-Fourcade D, Assoun J, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212(3):687-692.
7. Skjeldal S, Lilleås F, Follerås G, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637-638.
8. Sanhaji L, Gharbaoui IS, Hassani RE, Chakir N, Jiddane M, Boukhrissi N. A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance [in French]. J Radiol. 1996;77(1):37-40.
9. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
10. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607-617.
11. Ruiz Santiago F, Castellano García Mdel M, Guzmán Álvarez L, Martínez Montes JL, Ruiz García M, Tristán Fernández JM. Percutaneous treatment of bone tumors by radiofrequency thermal ablation. Eur J Radiol. 2011;77(1):156-163.
12. Richter M, Geerling J, Zech S, Goesling T, Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-Arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259-266.
13. Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German]. Unfallchirurg. 2003;106(6):492-497.
Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.
Follow-Up
We phoned all the patients to ask about symptom recurrence, outside treatment, and satisfaction with RFA and to obtain informed consent to participate in our study. Only 1 of the 28 patients could not be reached and was lost to follow-up. Mean follow-up at time of study completion was 31.1 months (range, 5.2-55.8 months).
The 27 patients were asked a series of questions about their treatment: Have you had any recurrence of symptoms following treatment for your OO? Have you received treatment elsewhere? Were you satisfied with your treatment? Would you have the procedure again if you had a recurrence of symptoms?
Primary success was defined as complete pain relief after initial RFA with no evidence of recurrence at time of final follow-up, and secondary success was defined as presence of recurrent symptoms after initial RFA with complete pain relief after a second procedure with no evidence of recurrence.
Results
All RFAs were technically successful with adequate localization of the tumor nidus and subsequent probe placement within the lesion. There were no intraoperative or postoperative complications. All 28 patients were discharged home the day of procedure. Twenty-six patients (92.8%) experienced complete pain relief after primary RFA, had no evidence of recurrence at final follow-up, and denied symptom recurrence at time of study completion.
The other 2 patients reported symptom recurrence after the index treatment (1 proximal femur lesion, 1 distal femur lesion). One of these patients did well initially but had a recurrence about 2 months after the primary RFA; a second RFA provided complete resolution of pain with no evidence of recurrence at time of study completion. In the other patient’s case, intermittent pain persisted for 2 weeks after the primary RFA, and evidence of recurrence was documented 3 months after surgery; a second RFA was performed shortly thereafter, but the patient was subsequently lost to follow-up.
At time of study completion, all 27 patients who had been contacted by phone denied seeking additional treatment elsewhere and stated they would have the procedure again if their symptoms ever recurred.
Discussion
Osteoid osteoma is one of the most common benign tumors of bone. Over the past 2 decades, percutaneous RFA, in comparison with open excision, has emerged as a safe and effective treatment option with minimal patient morbidity.9-11 RFA traditionally has been performed by radiologists under CT guidance in the radiology suite. However, now orthopedic surgeons can obtain advanced intraoperative imaging beyond standard fluoroscopy. The Siemens Siremobil ISO-C3D fluoroscopic C-arm is an innovative intraoperative imaging device that functions as a standard fluoroscope but also generates 3-D reconstructions of surgical anatomy. The isocentric design and integrated motor unit allow the C-arm to move through a 190º arc while centering its beam directly on the area of interest. This data set is transferred to a computer workstation, where it is reformatted so that CT-quality images are generated in axial, sagittal, and coronal planes. This acquisition process takes only minutes, and the multiplanar images produced may be simultaneously displayed and manipulated on the screen in real time.
One concern about this technology is the amount of radiation exposure for patients, surgeons, and operating room staff. The device measures only radiation time, and the amount of exposure during that time depends on the volume and density of the radiated body. We did not calculate the amount of exposure for this study. Mean exposure time was between 20 and 40 seconds, reflecting the number of attempts required to localize the lesion and the surgeon’s experience with the technique. Although the potential for increased exposure is a valid concern, previous studies using this technology have demonstrated that a similar average exposure time is equivalent to that of standard CT, and that use of the device, over conventional techniques, potentially can lead to decreased overall radiation exposure.12,13
This series demonstrated that OO can be safely and effectively treated with intraoperative percutaneous RFA by an orthopedic oncologist. Our success rate is very similar to rates reported in the radiology literature. Studies are needed to confirm the efficacy of this novel technique in comparison with what has been reported in that literature. Given these promising preliminary results, and the relative ease of use and minimal learning curve associated with this technology, all orthopedic oncologists should be able to offer this treatment for OO. Furthermore, this technique allows orthopedic oncologists to provide appropriate definitive treatment and care directly, rather than by referring patients to radiologists.
In the treatment of OO, we reserve RFA for lesions involving the long and short bones of the upper and lower extremities, as well as selected flat bones, such as those in the pelvis. Although percutaneous RFA of spinal lesions has been reported in the literature, we think these represent a relative contraindication for this technique; image resolution, in our opinion, is not high enough to justify risking injury to the nerves in the spinal canal, lateral recesses, and neural foramina. In addition, given the radiation exposure, we recommend caution when using this technique for a pelvic or proximal femoral lesion in a woman of childbearing age.
Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.
Follow-Up
We phoned all the patients to ask about symptom recurrence, outside treatment, and satisfaction with RFA and to obtain informed consent to participate in our study. Only 1 of the 28 patients could not be reached and was lost to follow-up. Mean follow-up at time of study completion was 31.1 months (range, 5.2-55.8 months).
The 27 patients were asked a series of questions about their treatment: Have you had any recurrence of symptoms following treatment for your OO? Have you received treatment elsewhere? Were you satisfied with your treatment? Would you have the procedure again if you had a recurrence of symptoms?
Primary success was defined as complete pain relief after initial RFA with no evidence of recurrence at time of final follow-up, and secondary success was defined as presence of recurrent symptoms after initial RFA with complete pain relief after a second procedure with no evidence of recurrence.
Results
All RFAs were technically successful with adequate localization of the tumor nidus and subsequent probe placement within the lesion. There were no intraoperative or postoperative complications. All 28 patients were discharged home the day of procedure. Twenty-six patients (92.8%) experienced complete pain relief after primary RFA, had no evidence of recurrence at final follow-up, and denied symptom recurrence at time of study completion.
The other 2 patients reported symptom recurrence after the index treatment (1 proximal femur lesion, 1 distal femur lesion). One of these patients did well initially but had a recurrence about 2 months after the primary RFA; a second RFA provided complete resolution of pain with no evidence of recurrence at time of study completion. In the other patient’s case, intermittent pain persisted for 2 weeks after the primary RFA, and evidence of recurrence was documented 3 months after surgery; a second RFA was performed shortly thereafter, but the patient was subsequently lost to follow-up.
At time of study completion, all 27 patients who had been contacted by phone denied seeking additional treatment elsewhere and stated they would have the procedure again if their symptoms ever recurred.
Discussion
Osteoid osteoma is one of the most common benign tumors of bone. Over the past 2 decades, percutaneous RFA, in comparison with open excision, has emerged as a safe and effective treatment option with minimal patient morbidity.9-11 RFA traditionally has been performed by radiologists under CT guidance in the radiology suite. However, now orthopedic surgeons can obtain advanced intraoperative imaging beyond standard fluoroscopy. The Siemens Siremobil ISO-C3D fluoroscopic C-arm is an innovative intraoperative imaging device that functions as a standard fluoroscope but also generates 3-D reconstructions of surgical anatomy. The isocentric design and integrated motor unit allow the C-arm to move through a 190º arc while centering its beam directly on the area of interest. This data set is transferred to a computer workstation, where it is reformatted so that CT-quality images are generated in axial, sagittal, and coronal planes. This acquisition process takes only minutes, and the multiplanar images produced may be simultaneously displayed and manipulated on the screen in real time.
One concern about this technology is the amount of radiation exposure for patients, surgeons, and operating room staff. The device measures only radiation time, and the amount of exposure during that time depends on the volume and density of the radiated body. We did not calculate the amount of exposure for this study. Mean exposure time was between 20 and 40 seconds, reflecting the number of attempts required to localize the lesion and the surgeon’s experience with the technique. Although the potential for increased exposure is a valid concern, previous studies using this technology have demonstrated that a similar average exposure time is equivalent to that of standard CT, and that use of the device, over conventional techniques, potentially can lead to decreased overall radiation exposure.12,13
This series demonstrated that OO can be safely and effectively treated with intraoperative percutaneous RFA by an orthopedic oncologist. Our success rate is very similar to rates reported in the radiology literature. Studies are needed to confirm the efficacy of this novel technique in comparison with what has been reported in that literature. Given these promising preliminary results, and the relative ease of use and minimal learning curve associated with this technology, all orthopedic oncologists should be able to offer this treatment for OO. Furthermore, this technique allows orthopedic oncologists to provide appropriate definitive treatment and care directly, rather than by referring patients to radiologists.
In the treatment of OO, we reserve RFA for lesions involving the long and short bones of the upper and lower extremities, as well as selected flat bones, such as those in the pelvis. Although percutaneous RFA of spinal lesions has been reported in the literature, we think these represent a relative contraindication for this technique; image resolution, in our opinion, is not high enough to justify risking injury to the nerves in the spinal canal, lateral recesses, and neural foramina. In addition, given the radiation exposure, we recommend caution when using this technique for a pelvic or proximal femoral lesion in a woman of childbearing age.
1. Gitelis S, Wilkins R, Conrad EU 2nd. Benign bone tumors. Instr Course Lect. 1996;45:425-424.
2. Schajowicz F. Bone forming tumors. In: Tumors and Tumorlike Lesions of Bone. 2nd ed. New York, NY: Springer-Verlag; 1994:36-62.
3. Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559-574.
4. Golding JS. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36(2):218-229.
5. Simm RJ. The natural history of osteoid osteoma. Aust N Z J Surg. 1975;45(4):412-415.
6. Sans N, Galy-Fourcade D, Assoun J, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212(3):687-692.
7. Skjeldal S, Lilleås F, Follerås G, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637-638.
8. Sanhaji L, Gharbaoui IS, Hassani RE, Chakir N, Jiddane M, Boukhrissi N. A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance [in French]. J Radiol. 1996;77(1):37-40.
9. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
10. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607-617.
11. Ruiz Santiago F, Castellano García Mdel M, Guzmán Álvarez L, Martínez Montes JL, Ruiz García M, Tristán Fernández JM. Percutaneous treatment of bone tumors by radiofrequency thermal ablation. Eur J Radiol. 2011;77(1):156-163.
12. Richter M, Geerling J, Zech S, Goesling T, Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-Arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259-266.
13. Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German]. Unfallchirurg. 2003;106(6):492-497.
1. Gitelis S, Wilkins R, Conrad EU 2nd. Benign bone tumors. Instr Course Lect. 1996;45:425-424.
2. Schajowicz F. Bone forming tumors. In: Tumors and Tumorlike Lesions of Bone. 2nd ed. New York, NY: Springer-Verlag; 1994:36-62.
3. Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559-574.
4. Golding JS. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36(2):218-229.
5. Simm RJ. The natural history of osteoid osteoma. Aust N Z J Surg. 1975;45(4):412-415.
6. Sans N, Galy-Fourcade D, Assoun J, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212(3):687-692.
7. Skjeldal S, Lilleås F, Follerås G, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637-638.
8. Sanhaji L, Gharbaoui IS, Hassani RE, Chakir N, Jiddane M, Boukhrissi N. A new treatment of osteoid osteoma: percutaneous sclerosis with ethanol under scanner guidance [in French]. J Radiol. 1996;77(1):37-40.
9. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
10. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14(4):607-617.
11. Ruiz Santiago F, Castellano García Mdel M, Guzmán Álvarez L, Martínez Montes JL, Ruiz García M, Tristán Fernández JM. Percutaneous treatment of bone tumors by radiofrequency thermal ablation. Eur J Radiol. 2011;77(1):156-163.
12. Richter M, Geerling J, Zech S, Goesling T, Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-Arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259-266.
13. Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German]. Unfallchirurg. 2003;106(6):492-497.