User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Using Wearable Technology to Record Surgical Videos
Safe and efficient advanced surgical skill training is of tremendous importance. With the recent increase in Internet use for medical education, there has been a concomitant increase in video recording of surgical procedures and techniques. Surgical recordings have been used in a variety of ways—as live webcasts for remote participants, as “coaching” opportunities for surgeons evaluating their own performance in the operating room, and even as informational resources for patients about to undergo the same surgery.
Surgical multimedia is being delivered through several different outlets. Many academic conferences and meetings showcase videos of different procedures, and several subspecialty societies (eg, Arthroscopy Association of North America) house archives of technical videos for viewing by members. In addition, the VuMedi website offers videos and allows members to comment on them and interact with the videographers. Surgeons are even posting technique videos on YouTube and other public websites.
A large proportion of surgical multimedia is recorded with conventional high-definition video cameras.1 Besides being able to experience a case at any time and from outside the operating room, the audience can watch from numerous vantage points, angles, and zoom levels. Also, surgeons’ narration can be valuable in helping the audience follow along with the case.
Recording surgical multimedia historically required tight coordination and precise planning by surgeon and videographer. However, innovations in wearable technology now allow surgeons to literally wear video cameras and record procedures as they perform them, in real time—to act as both surgeon and videographer.
Two such products are Google Glass (Google, Mountain View, California) and GoPro Hero (GoPro, San Mateo, California), both of which allow surgeons to record exactly what they see during procedures (Figure 1). Using a wearable technology for surgical multimedia creation requires a deep familiarity with its capabilities and limitations. In this article, we summarize these products’ similarities and differences and provide a technical overview for using wearable technologies in surgical multimedia creation.
1. Choosing a device
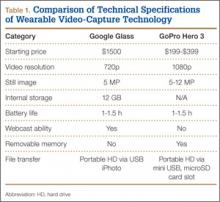
When purchasing either wearable device, several factors must be considered, including budget, possible uses outside the operating room, and possible limitations of the technology (Table 1). At this time, Google Glass is significantly more expensive than GoPro Hero. The Google Glass base unit costs $1500, and the GoPro Hero 3 model costs approximately $200 (higher-priced Hero models are available). Both devices require accessories (eg, portable battery unit, dedicated hard drive).
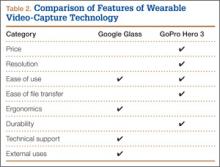
Device capabilities must also be considered (Table 2). Google Glass consists of both hardware and software. Users can record what is seen and heard through the lens and then use apps to create text and e-mail portals to online gaming, social media, and even golf-course GPS. The app market for Google Glass is nascent but undoubtedly will increase in volume and scope as more users adopt the technology (Google Glass comes with both Bluetooth and Wi-Fi and can function tethered through a smartphone). GoPro is mainly a hardware unit that can record in various settings (it is popular with athletes who want to capture and broadcast their participation in action sports). Newer GoPro Hero versions offer Wi-Fi, which allows streaming of video content to a smartphone or tablet through an app. Having clearly defined goals for a device—as they pertain to use outside the operating room, such as outdoor activities and underwater recording—may help the surgeon decide which product is more suitable. Last, it is important to consider limitations. Google Glass resolution is 720p (1280×720) for video and 5 MP for still images, and GoPro resolution can reach 1080p (1920×1080) for video and 5 MP for stills.
Both devices require purchase of accessories. An external USB battery pack is useful for both devices, as is a password-encrypted hard drive for media storage. Lenswear does not come with the base version of Google Glass and is purchased separately from the company. GoPro users buy micro SD cards (~$50 per 64-GB high-speed transfer card) for storage on the device and may buy lithium-ion batteries as an alternative to the external USB battery pack.
Author Update
In January 2015, Google announced that it was temporarily suspending its “Explorer” program, which allowed individual users to buy and test the device for personal use. However, Google is continuing its development of Glass with health care technology, among other areas of growth and development.2,3
2. Recording a successful surgical video
Unlike a camcorder, which typically is set on a tripod for conventional video recording of surgery, Google Glass and GoPro are intricately linked to the operator. Surgeons must be constantly aware of where they are during surgery and try not to let anything obstruct the camera’s view.
Before starting a case, the surgeon using either device must ensure that its battery is fully charged, as a full charge typically supports 1 hour of continuous recording (the Google Glass battery is a lithium-ion 670-mAh internal unit). A full charge should be enough to capture a short case. Newer GoPro models, with a battery listed at 1050 mAh, provide 1 to 2 hours of recording. When more than 1 hour is needed, an external USB battery pack can be used. This pack allows the device to remain plugged in throughout the case (the pack is kept in the surgeon’s back pocket). We recommend having an external battery pack that is at least 10,000 mAh (~$30 online retail), which easily provides 3+ hours of recording. Unfortunately, this arrangement can be cumbersome. Alternatively, with GoPro, additional batteries may be purchased, but the user needs to dismount the device in order to swap them in (may be difficult during surgery). With both units, partitioning a video into shorter segments conserves battery power and minimizes the risk of file corruption, which may occur if the battery dies or the device overheats.
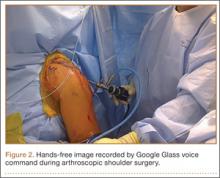
Google Glass users can bypass manual operation of the device by giving it voice commands (eg, start video, take still image). The exception is for recording video for more than 10 seconds (current default setting). Unfortunately, the surgeon must touch the device to start this recording, which means using extra gloves to preserve operating field sterility. Still images can be made through a combination of voice and head gestures and without manual intervention (Figure 2). Last, users must ensure that the device is not actively connected through Bluetooth to a mobile phone, as incoming calls, text messages, and e-mails may disrupt active recording and become a distraction. The connection can be deactivated by disabling Bluetooth on the host smartphone or by placing the phone into airplane mode and turning off Wi-Fi.
Google Glass users can see what is being recorded through the viewfinder prism, whereas GoPro requires precise framing of the video before recording. Framing is done by grossly aiming the device in the desired direction. However, there is no way to ensure exact aim during recording. If at any point during a case there is slight repositioning of the GoPro, there is a risk of recording the case out of the center of view. An important advantage to newer GoPro versions is the ability to control the device through a wireless remote that can be placed under the surgeon’s gown. The remote can be used to pause and resume recording, without changing gloves, as is done with Google Glass. Last, because the minimum viewing distance from the surgical field is usually 18 inches or more, typically there is no loss of focus or blurring of the image from short-distance recording on either device.
3. File management and playback
Before using wearable technology in the operating room, surgeons must become aware of its limitations with respect to file storage and playback. Google Glass has a usable memory of about 12 GB (1 hour of video may require 1.5-2.0 GB). Conversely, GoPro’s capacity is defined by the micro SD card used. Therefore, the Google Glass hard drive must be regularly maintained well before being brought into the operating room, whereas recording can be extended (with respect to memory) for the GoPro if the media card is large enough.
Both devices allow for wired file transfer, which may be done with Windows Explorer (PC) or iPhoto (Macintosh). However, Google Glass also allows for wireless transfer, through portable storage supported by Google. Although this type of file transfer may be convenient for short, everyday clips made outside the operating room, it is prohibitive for surgical media, mainly because of patient privacy concerns. With wireless transfer to a nonsecure cloud platform, there is a risk of breach of patient confidentiality. We therefore recommend against using wireless upload when producing surgical multimedia, as patient identifiers are likely to be included in the recorded audio or video contents. Conversely, with GoPro, the micro SD card can be used as a portable hard drive to transfer files to a laptop or media reader, obviating the need for wired or wireless transmission. Last, when using traditional wire transfer or memory card to upload to a hard drive, users must ensure that the drive complies with patient privacy laws and regulations.
4. Privacy and patient consent
As mentioned, great care must be taken to ensure that patient privacy laws are followed. This is especially relevant with content uploaded to online cloud storage, as with Google Glass. The upload may occur automatically if the unit is connected to a Wi-Fi hotspot. In addition, when using surgical media for a real-time webcast for education or demonstration purposes, surgeons must ensure that no protected health information is broadcast and that the patient and the surgical team are aware of the webcast and its purposes.
Before using wearable technology during patient care, patient consent must be obtained. Surgeons should ask the patient to consent to video recording of surgery or an encounter (eg, clinic visit) for education purposes. Our institution’s consent form includes a section for this particular type of consent. If an institution’s form lacks such a section, surgeons should consult their risk management department to ensure there is a proper avenue for obtaining patient consent to record the procedure or encounter. A separate, dedicated media consent form may be required. Last, whoever operates a wearable device should be careful to use the device only during encounters that have received explicit recording consent—as opposed to wearing the device in the hallways or elsewhere in the hospital, where protected health information might be inadvertently recorded.4
5. Putting it all to use
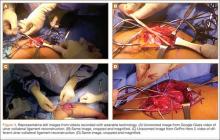
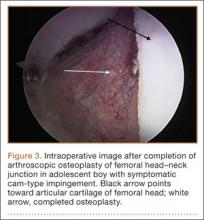
After successful recording of surgery, an effort should be made to produce a high-quality video for education or demonstration purposes. Unfortunately, there is no built-in optical zooming with Google Glass or GoPro, and recording segments in which surgeons focus on detailed anatomy (with high-quality zoom) may prove difficult. Online descriptions of do-it-yourself modifications to place zoom capability on GoPro devices may be useful in surgical video recording, particularly for small surgical fields (hand or foot surgery). In addition, footage may be zoomed in on during postprocessing (Figure 3), though some resolution will be lost in the editing.
There is no practical way to incorporate Google Glass or GoPro while using surgical loupes or a surgical microscope. As a result, videos recorded with wearable technology may not reach the minimum resolution needed for useful surgical technique videos, as these traditionally are produced on high-definition camcorders with optical zoom, allowing detailed viewing of anatomical structures without resolution loss through digital zoom or postprocessing editing.
There has been tremendous benefit in incorporating wearable technology into our practice. Videos made with Google Glass and GoPro have been successfully used for surgical preparation and training, allowing orthopedic surgical residents to rehearse surgery before participating in it. Alternatively, having used Google Glass or GoPro to record a case, residents have then been able to review each surgical step on video—thereby reinforcing their knowledge of the steps, techniques, pearls, and pitfalls before performing the surgery again. Footage from surgeries recorded with Google Glass and GoPro has also been shown at weekly technique-focused conferences, allowing surgeons to analyze particular steps and highlight applicable learning points. Last, attending surgeons in our practice have used wearable technology in “coaching” mode, either reviewing case footage to identify areas for improvement or sharing footage with senior surgeons in order to elicit feedback and suggestions for possible improvement.
As new iterations of wearable video technology come to market, with advancements in both hardware and software, surgeons may be able to enhance education and teaching through seamless recording of surgical procedures. Use of wearable technology may also begin to extend beyond the operating room—to outpatient settings, such as preoperative and postoperative physical examinations. The latest versions of Google Glass and GoPro Hero allow surgeons to record surgical procedures with relative ease, without the personnel, equipment, and coordination required for traditional surgical videography.
Video 1. Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video 2. Distal biceps repair performed by Dr. Makhni and Dr. Jobin, filmed by Dr. Makhni using Google Glass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
1. Leahy M. Creating a good surgical technique video. AAOS Now. 2010;4(11). http://www.aaos.org/news/aaosnow/nov10/clinical4.asp. Accessed February 15, 2015.
2. Google Glass sales halted but firm says kit is not dead. BBC News website. http://www.bbc.com/news/technology-30831128. Published January 15, 2015. Accessed February 18, 2015.
3. Metz C. Sorry, but Google Glass isn’t anywhere close to dead. Wired website. http://www.wired.com/2015/02/sorry-google-glass-isnt-anywhere-close-dead/. Published February 8, 2015. Accessed February 18, 2015.
4. Peregrin T. Surgeons see future applications for Google Glass. Bull Am Coll Surg. 2014;99(7):9-16. http://bulletin.facs.org/2014/07/surgeons-see-future-applications-for-google-glass/#.U8SLKZaJAyZ.twitter. Accessed February 15, 2015.
Safe and efficient advanced surgical skill training is of tremendous importance. With the recent increase in Internet use for medical education, there has been a concomitant increase in video recording of surgical procedures and techniques. Surgical recordings have been used in a variety of ways—as live webcasts for remote participants, as “coaching” opportunities for surgeons evaluating their own performance in the operating room, and even as informational resources for patients about to undergo the same surgery.
Surgical multimedia is being delivered through several different outlets. Many academic conferences and meetings showcase videos of different procedures, and several subspecialty societies (eg, Arthroscopy Association of North America) house archives of technical videos for viewing by members. In addition, the VuMedi website offers videos and allows members to comment on them and interact with the videographers. Surgeons are even posting technique videos on YouTube and other public websites.
A large proportion of surgical multimedia is recorded with conventional high-definition video cameras.1 Besides being able to experience a case at any time and from outside the operating room, the audience can watch from numerous vantage points, angles, and zoom levels. Also, surgeons’ narration can be valuable in helping the audience follow along with the case.
Recording surgical multimedia historically required tight coordination and precise planning by surgeon and videographer. However, innovations in wearable technology now allow surgeons to literally wear video cameras and record procedures as they perform them, in real time—to act as both surgeon and videographer.
Two such products are Google Glass (Google, Mountain View, California) and GoPro Hero (GoPro, San Mateo, California), both of which allow surgeons to record exactly what they see during procedures (Figure 1). Using a wearable technology for surgical multimedia creation requires a deep familiarity with its capabilities and limitations. In this article, we summarize these products’ similarities and differences and provide a technical overview for using wearable technologies in surgical multimedia creation.
1. Choosing a device
When purchasing either wearable device, several factors must be considered, including budget, possible uses outside the operating room, and possible limitations of the technology (Table 1). At this time, Google Glass is significantly more expensive than GoPro Hero. The Google Glass base unit costs $1500, and the GoPro Hero 3 model costs approximately $200 (higher-priced Hero models are available). Both devices require accessories (eg, portable battery unit, dedicated hard drive).
Device capabilities must also be considered (Table 2). Google Glass consists of both hardware and software. Users can record what is seen and heard through the lens and then use apps to create text and e-mail portals to online gaming, social media, and even golf-course GPS. The app market for Google Glass is nascent but undoubtedly will increase in volume and scope as more users adopt the technology (Google Glass comes with both Bluetooth and Wi-Fi and can function tethered through a smartphone). GoPro is mainly a hardware unit that can record in various settings (it is popular with athletes who want to capture and broadcast their participation in action sports). Newer GoPro Hero versions offer Wi-Fi, which allows streaming of video content to a smartphone or tablet through an app. Having clearly defined goals for a device—as they pertain to use outside the operating room, such as outdoor activities and underwater recording—may help the surgeon decide which product is more suitable. Last, it is important to consider limitations. Google Glass resolution is 720p (1280×720) for video and 5 MP for still images, and GoPro resolution can reach 1080p (1920×1080) for video and 5 MP for stills.
Both devices require purchase of accessories. An external USB battery pack is useful for both devices, as is a password-encrypted hard drive for media storage. Lenswear does not come with the base version of Google Glass and is purchased separately from the company. GoPro users buy micro SD cards (~$50 per 64-GB high-speed transfer card) for storage on the device and may buy lithium-ion batteries as an alternative to the external USB battery pack.
Author Update
In January 2015, Google announced that it was temporarily suspending its “Explorer” program, which allowed individual users to buy and test the device for personal use. However, Google is continuing its development of Glass with health care technology, among other areas of growth and development.2,3
2. Recording a successful surgical video
Unlike a camcorder, which typically is set on a tripod for conventional video recording of surgery, Google Glass and GoPro are intricately linked to the operator. Surgeons must be constantly aware of where they are during surgery and try not to let anything obstruct the camera’s view.
Before starting a case, the surgeon using either device must ensure that its battery is fully charged, as a full charge typically supports 1 hour of continuous recording (the Google Glass battery is a lithium-ion 670-mAh internal unit). A full charge should be enough to capture a short case. Newer GoPro models, with a battery listed at 1050 mAh, provide 1 to 2 hours of recording. When more than 1 hour is needed, an external USB battery pack can be used. This pack allows the device to remain plugged in throughout the case (the pack is kept in the surgeon’s back pocket). We recommend having an external battery pack that is at least 10,000 mAh (~$30 online retail), which easily provides 3+ hours of recording. Unfortunately, this arrangement can be cumbersome. Alternatively, with GoPro, additional batteries may be purchased, but the user needs to dismount the device in order to swap them in (may be difficult during surgery). With both units, partitioning a video into shorter segments conserves battery power and minimizes the risk of file corruption, which may occur if the battery dies or the device overheats.
Google Glass users can bypass manual operation of the device by giving it voice commands (eg, start video, take still image). The exception is for recording video for more than 10 seconds (current default setting). Unfortunately, the surgeon must touch the device to start this recording, which means using extra gloves to preserve operating field sterility. Still images can be made through a combination of voice and head gestures and without manual intervention (Figure 2). Last, users must ensure that the device is not actively connected through Bluetooth to a mobile phone, as incoming calls, text messages, and e-mails may disrupt active recording and become a distraction. The connection can be deactivated by disabling Bluetooth on the host smartphone or by placing the phone into airplane mode and turning off Wi-Fi.
Google Glass users can see what is being recorded through the viewfinder prism, whereas GoPro requires precise framing of the video before recording. Framing is done by grossly aiming the device in the desired direction. However, there is no way to ensure exact aim during recording. If at any point during a case there is slight repositioning of the GoPro, there is a risk of recording the case out of the center of view. An important advantage to newer GoPro versions is the ability to control the device through a wireless remote that can be placed under the surgeon’s gown. The remote can be used to pause and resume recording, without changing gloves, as is done with Google Glass. Last, because the minimum viewing distance from the surgical field is usually 18 inches or more, typically there is no loss of focus or blurring of the image from short-distance recording on either device.
3. File management and playback
Before using wearable technology in the operating room, surgeons must become aware of its limitations with respect to file storage and playback. Google Glass has a usable memory of about 12 GB (1 hour of video may require 1.5-2.0 GB). Conversely, GoPro’s capacity is defined by the micro SD card used. Therefore, the Google Glass hard drive must be regularly maintained well before being brought into the operating room, whereas recording can be extended (with respect to memory) for the GoPro if the media card is large enough.
Both devices allow for wired file transfer, which may be done with Windows Explorer (PC) or iPhoto (Macintosh). However, Google Glass also allows for wireless transfer, through portable storage supported by Google. Although this type of file transfer may be convenient for short, everyday clips made outside the operating room, it is prohibitive for surgical media, mainly because of patient privacy concerns. With wireless transfer to a nonsecure cloud platform, there is a risk of breach of patient confidentiality. We therefore recommend against using wireless upload when producing surgical multimedia, as patient identifiers are likely to be included in the recorded audio or video contents. Conversely, with GoPro, the micro SD card can be used as a portable hard drive to transfer files to a laptop or media reader, obviating the need for wired or wireless transmission. Last, when using traditional wire transfer or memory card to upload to a hard drive, users must ensure that the drive complies with patient privacy laws and regulations.
4. Privacy and patient consent
As mentioned, great care must be taken to ensure that patient privacy laws are followed. This is especially relevant with content uploaded to online cloud storage, as with Google Glass. The upload may occur automatically if the unit is connected to a Wi-Fi hotspot. In addition, when using surgical media for a real-time webcast for education or demonstration purposes, surgeons must ensure that no protected health information is broadcast and that the patient and the surgical team are aware of the webcast and its purposes.
Before using wearable technology during patient care, patient consent must be obtained. Surgeons should ask the patient to consent to video recording of surgery or an encounter (eg, clinic visit) for education purposes. Our institution’s consent form includes a section for this particular type of consent. If an institution’s form lacks such a section, surgeons should consult their risk management department to ensure there is a proper avenue for obtaining patient consent to record the procedure or encounter. A separate, dedicated media consent form may be required. Last, whoever operates a wearable device should be careful to use the device only during encounters that have received explicit recording consent—as opposed to wearing the device in the hallways or elsewhere in the hospital, where protected health information might be inadvertently recorded.4
5. Putting it all to use
After successful recording of surgery, an effort should be made to produce a high-quality video for education or demonstration purposes. Unfortunately, there is no built-in optical zooming with Google Glass or GoPro, and recording segments in which surgeons focus on detailed anatomy (with high-quality zoom) may prove difficult. Online descriptions of do-it-yourself modifications to place zoom capability on GoPro devices may be useful in surgical video recording, particularly for small surgical fields (hand or foot surgery). In addition, footage may be zoomed in on during postprocessing (Figure 3), though some resolution will be lost in the editing.
There is no practical way to incorporate Google Glass or GoPro while using surgical loupes or a surgical microscope. As a result, videos recorded with wearable technology may not reach the minimum resolution needed for useful surgical technique videos, as these traditionally are produced on high-definition camcorders with optical zoom, allowing detailed viewing of anatomical structures without resolution loss through digital zoom or postprocessing editing.
There has been tremendous benefit in incorporating wearable technology into our practice. Videos made with Google Glass and GoPro have been successfully used for surgical preparation and training, allowing orthopedic surgical residents to rehearse surgery before participating in it. Alternatively, having used Google Glass or GoPro to record a case, residents have then been able to review each surgical step on video—thereby reinforcing their knowledge of the steps, techniques, pearls, and pitfalls before performing the surgery again. Footage from surgeries recorded with Google Glass and GoPro has also been shown at weekly technique-focused conferences, allowing surgeons to analyze particular steps and highlight applicable learning points. Last, attending surgeons in our practice have used wearable technology in “coaching” mode, either reviewing case footage to identify areas for improvement or sharing footage with senior surgeons in order to elicit feedback and suggestions for possible improvement.
As new iterations of wearable video technology come to market, with advancements in both hardware and software, surgeons may be able to enhance education and teaching through seamless recording of surgical procedures. Use of wearable technology may also begin to extend beyond the operating room—to outpatient settings, such as preoperative and postoperative physical examinations. The latest versions of Google Glass and GoPro Hero allow surgeons to record surgical procedures with relative ease, without the personnel, equipment, and coordination required for traditional surgical videography.
Video 1. Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video 2. Distal biceps repair performed by Dr. Makhni and Dr. Jobin, filmed by Dr. Makhni using Google Glass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Safe and efficient advanced surgical skill training is of tremendous importance. With the recent increase in Internet use for medical education, there has been a concomitant increase in video recording of surgical procedures and techniques. Surgical recordings have been used in a variety of ways—as live webcasts for remote participants, as “coaching” opportunities for surgeons evaluating their own performance in the operating room, and even as informational resources for patients about to undergo the same surgery.
Surgical multimedia is being delivered through several different outlets. Many academic conferences and meetings showcase videos of different procedures, and several subspecialty societies (eg, Arthroscopy Association of North America) house archives of technical videos for viewing by members. In addition, the VuMedi website offers videos and allows members to comment on them and interact with the videographers. Surgeons are even posting technique videos on YouTube and other public websites.
A large proportion of surgical multimedia is recorded with conventional high-definition video cameras.1 Besides being able to experience a case at any time and from outside the operating room, the audience can watch from numerous vantage points, angles, and zoom levels. Also, surgeons’ narration can be valuable in helping the audience follow along with the case.
Recording surgical multimedia historically required tight coordination and precise planning by surgeon and videographer. However, innovations in wearable technology now allow surgeons to literally wear video cameras and record procedures as they perform them, in real time—to act as both surgeon and videographer.
Two such products are Google Glass (Google, Mountain View, California) and GoPro Hero (GoPro, San Mateo, California), both of which allow surgeons to record exactly what they see during procedures (Figure 1). Using a wearable technology for surgical multimedia creation requires a deep familiarity with its capabilities and limitations. In this article, we summarize these products’ similarities and differences and provide a technical overview for using wearable technologies in surgical multimedia creation.
1. Choosing a device
When purchasing either wearable device, several factors must be considered, including budget, possible uses outside the operating room, and possible limitations of the technology (Table 1). At this time, Google Glass is significantly more expensive than GoPro Hero. The Google Glass base unit costs $1500, and the GoPro Hero 3 model costs approximately $200 (higher-priced Hero models are available). Both devices require accessories (eg, portable battery unit, dedicated hard drive).
Device capabilities must also be considered (Table 2). Google Glass consists of both hardware and software. Users can record what is seen and heard through the lens and then use apps to create text and e-mail portals to online gaming, social media, and even golf-course GPS. The app market for Google Glass is nascent but undoubtedly will increase in volume and scope as more users adopt the technology (Google Glass comes with both Bluetooth and Wi-Fi and can function tethered through a smartphone). GoPro is mainly a hardware unit that can record in various settings (it is popular with athletes who want to capture and broadcast their participation in action sports). Newer GoPro Hero versions offer Wi-Fi, which allows streaming of video content to a smartphone or tablet through an app. Having clearly defined goals for a device—as they pertain to use outside the operating room, such as outdoor activities and underwater recording—may help the surgeon decide which product is more suitable. Last, it is important to consider limitations. Google Glass resolution is 720p (1280×720) for video and 5 MP for still images, and GoPro resolution can reach 1080p (1920×1080) for video and 5 MP for stills.
Both devices require purchase of accessories. An external USB battery pack is useful for both devices, as is a password-encrypted hard drive for media storage. Lenswear does not come with the base version of Google Glass and is purchased separately from the company. GoPro users buy micro SD cards (~$50 per 64-GB high-speed transfer card) for storage on the device and may buy lithium-ion batteries as an alternative to the external USB battery pack.
Author Update
In January 2015, Google announced that it was temporarily suspending its “Explorer” program, which allowed individual users to buy and test the device for personal use. However, Google is continuing its development of Glass with health care technology, among other areas of growth and development.2,3
2. Recording a successful surgical video
Unlike a camcorder, which typically is set on a tripod for conventional video recording of surgery, Google Glass and GoPro are intricately linked to the operator. Surgeons must be constantly aware of where they are during surgery and try not to let anything obstruct the camera’s view.
Before starting a case, the surgeon using either device must ensure that its battery is fully charged, as a full charge typically supports 1 hour of continuous recording (the Google Glass battery is a lithium-ion 670-mAh internal unit). A full charge should be enough to capture a short case. Newer GoPro models, with a battery listed at 1050 mAh, provide 1 to 2 hours of recording. When more than 1 hour is needed, an external USB battery pack can be used. This pack allows the device to remain plugged in throughout the case (the pack is kept in the surgeon’s back pocket). We recommend having an external battery pack that is at least 10,000 mAh (~$30 online retail), which easily provides 3+ hours of recording. Unfortunately, this arrangement can be cumbersome. Alternatively, with GoPro, additional batteries may be purchased, but the user needs to dismount the device in order to swap them in (may be difficult during surgery). With both units, partitioning a video into shorter segments conserves battery power and minimizes the risk of file corruption, which may occur if the battery dies or the device overheats.
Google Glass users can bypass manual operation of the device by giving it voice commands (eg, start video, take still image). The exception is for recording video for more than 10 seconds (current default setting). Unfortunately, the surgeon must touch the device to start this recording, which means using extra gloves to preserve operating field sterility. Still images can be made through a combination of voice and head gestures and without manual intervention (Figure 2). Last, users must ensure that the device is not actively connected through Bluetooth to a mobile phone, as incoming calls, text messages, and e-mails may disrupt active recording and become a distraction. The connection can be deactivated by disabling Bluetooth on the host smartphone or by placing the phone into airplane mode and turning off Wi-Fi.
Google Glass users can see what is being recorded through the viewfinder prism, whereas GoPro requires precise framing of the video before recording. Framing is done by grossly aiming the device in the desired direction. However, there is no way to ensure exact aim during recording. If at any point during a case there is slight repositioning of the GoPro, there is a risk of recording the case out of the center of view. An important advantage to newer GoPro versions is the ability to control the device through a wireless remote that can be placed under the surgeon’s gown. The remote can be used to pause and resume recording, without changing gloves, as is done with Google Glass. Last, because the minimum viewing distance from the surgical field is usually 18 inches or more, typically there is no loss of focus or blurring of the image from short-distance recording on either device.
3. File management and playback
Before using wearable technology in the operating room, surgeons must become aware of its limitations with respect to file storage and playback. Google Glass has a usable memory of about 12 GB (1 hour of video may require 1.5-2.0 GB). Conversely, GoPro’s capacity is defined by the micro SD card used. Therefore, the Google Glass hard drive must be regularly maintained well before being brought into the operating room, whereas recording can be extended (with respect to memory) for the GoPro if the media card is large enough.
Both devices allow for wired file transfer, which may be done with Windows Explorer (PC) or iPhoto (Macintosh). However, Google Glass also allows for wireless transfer, through portable storage supported by Google. Although this type of file transfer may be convenient for short, everyday clips made outside the operating room, it is prohibitive for surgical media, mainly because of patient privacy concerns. With wireless transfer to a nonsecure cloud platform, there is a risk of breach of patient confidentiality. We therefore recommend against using wireless upload when producing surgical multimedia, as patient identifiers are likely to be included in the recorded audio or video contents. Conversely, with GoPro, the micro SD card can be used as a portable hard drive to transfer files to a laptop or media reader, obviating the need for wired or wireless transmission. Last, when using traditional wire transfer or memory card to upload to a hard drive, users must ensure that the drive complies with patient privacy laws and regulations.
4. Privacy and patient consent
As mentioned, great care must be taken to ensure that patient privacy laws are followed. This is especially relevant with content uploaded to online cloud storage, as with Google Glass. The upload may occur automatically if the unit is connected to a Wi-Fi hotspot. In addition, when using surgical media for a real-time webcast for education or demonstration purposes, surgeons must ensure that no protected health information is broadcast and that the patient and the surgical team are aware of the webcast and its purposes.
Before using wearable technology during patient care, patient consent must be obtained. Surgeons should ask the patient to consent to video recording of surgery or an encounter (eg, clinic visit) for education purposes. Our institution’s consent form includes a section for this particular type of consent. If an institution’s form lacks such a section, surgeons should consult their risk management department to ensure there is a proper avenue for obtaining patient consent to record the procedure or encounter. A separate, dedicated media consent form may be required. Last, whoever operates a wearable device should be careful to use the device only during encounters that have received explicit recording consent—as opposed to wearing the device in the hallways or elsewhere in the hospital, where protected health information might be inadvertently recorded.4
5. Putting it all to use
After successful recording of surgery, an effort should be made to produce a high-quality video for education or demonstration purposes. Unfortunately, there is no built-in optical zooming with Google Glass or GoPro, and recording segments in which surgeons focus on detailed anatomy (with high-quality zoom) may prove difficult. Online descriptions of do-it-yourself modifications to place zoom capability on GoPro devices may be useful in surgical video recording, particularly for small surgical fields (hand or foot surgery). In addition, footage may be zoomed in on during postprocessing (Figure 3), though some resolution will be lost in the editing.
There is no practical way to incorporate Google Glass or GoPro while using surgical loupes or a surgical microscope. As a result, videos recorded with wearable technology may not reach the minimum resolution needed for useful surgical technique videos, as these traditionally are produced on high-definition camcorders with optical zoom, allowing detailed viewing of anatomical structures without resolution loss through digital zoom or postprocessing editing.
There has been tremendous benefit in incorporating wearable technology into our practice. Videos made with Google Glass and GoPro have been successfully used for surgical preparation and training, allowing orthopedic surgical residents to rehearse surgery before participating in it. Alternatively, having used Google Glass or GoPro to record a case, residents have then been able to review each surgical step on video—thereby reinforcing their knowledge of the steps, techniques, pearls, and pitfalls before performing the surgery again. Footage from surgeries recorded with Google Glass and GoPro has also been shown at weekly technique-focused conferences, allowing surgeons to analyze particular steps and highlight applicable learning points. Last, attending surgeons in our practice have used wearable technology in “coaching” mode, either reviewing case footage to identify areas for improvement or sharing footage with senior surgeons in order to elicit feedback and suggestions for possible improvement.
As new iterations of wearable video technology come to market, with advancements in both hardware and software, surgeons may be able to enhance education and teaching through seamless recording of surgical procedures. Use of wearable technology may also begin to extend beyond the operating room—to outpatient settings, such as preoperative and postoperative physical examinations. The latest versions of Google Glass and GoPro Hero allow surgeons to record surgical procedures with relative ease, without the personnel, equipment, and coordination required for traditional surgical videography.
Video 1. Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video 2. Distal biceps repair performed by Dr. Makhni and Dr. Jobin, filmed by Dr. Makhni using Google Glass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
1. Leahy M. Creating a good surgical technique video. AAOS Now. 2010;4(11). http://www.aaos.org/news/aaosnow/nov10/clinical4.asp. Accessed February 15, 2015.
2. Google Glass sales halted but firm says kit is not dead. BBC News website. http://www.bbc.com/news/technology-30831128. Published January 15, 2015. Accessed February 18, 2015.
3. Metz C. Sorry, but Google Glass isn’t anywhere close to dead. Wired website. http://www.wired.com/2015/02/sorry-google-glass-isnt-anywhere-close-dead/. Published February 8, 2015. Accessed February 18, 2015.
4. Peregrin T. Surgeons see future applications for Google Glass. Bull Am Coll Surg. 2014;99(7):9-16. http://bulletin.facs.org/2014/07/surgeons-see-future-applications-for-google-glass/#.U8SLKZaJAyZ.twitter. Accessed February 15, 2015.
1. Leahy M. Creating a good surgical technique video. AAOS Now. 2010;4(11). http://www.aaos.org/news/aaosnow/nov10/clinical4.asp. Accessed February 15, 2015.
2. Google Glass sales halted but firm says kit is not dead. BBC News website. http://www.bbc.com/news/technology-30831128. Published January 15, 2015. Accessed February 18, 2015.
3. Metz C. Sorry, but Google Glass isn’t anywhere close to dead. Wired website. http://www.wired.com/2015/02/sorry-google-glass-isnt-anywhere-close-dead/. Published February 8, 2015. Accessed February 18, 2015.
4. Peregrin T. Surgeons see future applications for Google Glass. Bull Am Coll Surg. 2014;99(7):9-16. http://bulletin.facs.org/2014/07/surgeons-see-future-applications-for-google-glass/#.U8SLKZaJAyZ.twitter. Accessed February 15, 2015.
Extensor Pollicis Longus Ruptures in Distal Radius Fractures: Clinical and Cadaveric Studies With a New Therapeutic Intervention
Distal radius fractures are among the most common upper extremity injuries. A Swedish study noted that 75% of forearm fractures involve the distal radius.1 Extensor pollicis longus (EPL) ruptures are a well-documented complication (0.3% incidence2) of distal radius fractures.
The first description of EPL ruptures is attributed to Duplay in 1876 and was termed drummer boy’s palsy.3 Spontaneous EPL ruptures are often described in the setting of acute or chronic tenosynovitis.4,5 Beginning in the early 1930s, multiple case reports began to connect distal radius fractures with EPL ruptures.6 Although EPL ruptures are rare, their consequences are substantial and typically necessitate reconstructive procedures. Extensor indicis proprius (EIP)-to-EPL tendon transfer has become a common surgical treatment for this complication. Increasing our knowledge of several characteristics associated with this complication may help clinically in preventing EPL ruptures.
Multiple studies have indicated that EPL ruptures occur more often in nondisplaced fractures and often occur between 6 and 8 weeks after injury.2,5,7,8 Several factors are implicated in the etiology of EPL ruptures in distal radius fractures. The classic 1979 study by Engkvist and Lundborg9 showed that the EPL tendon has an area of poor vascularity around the Lister tubercle. Explorations of nondisplaced distal radius fractures have shown an intact extensor retinaculum that allows the tendon to continue to travel through an enclosed space.4,5,7 In the setting of distal radius fracture, hematoma may contribute to tendon ruptures secondary to increased pressure within an intact third dorsal compartment, which further compromises vascularity in this region of the EPL tendon.
Recognition and prevention of an impending EPL rupture may help avoid the significant consequences of this complication. Decompression and release of the third dorsal compartment have been described as constituting a prophylactic surgical option.10,11 Early thumb range of motion is also advocated to help prevent EPL rupture.9 However, results reported in the literature are inconclusive as to the effectiveness of these or indeed any preventive procedures. Dr. Lourie uses a novel technique that involves aspiration of the third dorsal compartment in patients with clinical symptoms associated with impending EPL rupture. Needle decompression, a less invasive option, can be quickly performed in an office, and it is hypothesized that removal of the hematoma may prevent EPL ruptures.
In the present study, we retrospectively reviewed Dr. Lourie’s records of patients with EPL ruptures in association with distal radius fractures to help delineate which radiographic and clinical characteristics identify patients at risk for these ruptures. A cadaveric model of a nondisplaced distal radius fracture was then created in order to simulate a change in third compartment pressures before and after needle decompression. We present preliminary outcomes on a case series of 4 patients who underwent aspiration of the third compartment and who were thought to be at risk for EPL rupture.
Materials and Methods
Institutional review board approval was obtained for this study. From Dr. Lourie’s records, 19 patients treated between 1998 and 2009 were identified as having confirmed or clinically impending EPL ruptures in association with nonoperative treatment of distal radius fractures. Prodromal symptoms that were used to diagnose impending EPL ruptures included pain with resisted active EPL extension, pain with passive flexion of the thumb interphalangeal joint, and localized swelling over the third dorsal compartment of the wrist.5,7,10,12 Eleven patients had complete radiographs, which were reviewed for radiographic characteristics. Posteroanterior (PA) and lateral radiographs of the injured wrist were reviewed. On the PA radiographs, fraction location was measured from the tip of the radial styloid using a line perpendicular to the radial shaft. Fractures were also evaluated for displacement and intra-articular involvement.
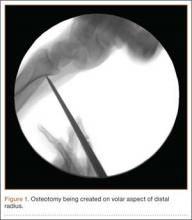
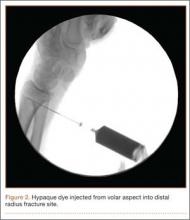
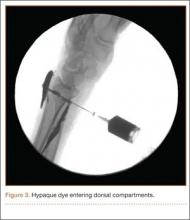
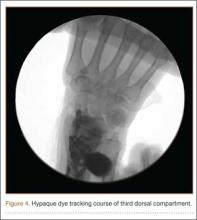
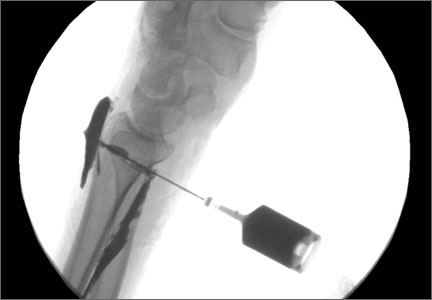
A cadaveric model was developed to evaluate compartment pressures in the EPL sheath in a simulated distal radius fracture. Six fresh-frozen cadaveric forearms were used after being thawed at room temperature. The cadavers were radiographically evaluated to determine that there was no evidence of prior fracture. A Stryker compartment pressure monitoring system (Stryker, Kalamazoo, Michigan) was used to take initial pressure readings in the third dorsal compartment slightly ulnar to the Lister tubercle (preinjection readings). A limited volar approach was then created. Under fluoroscopy, a half-inch osteotome was used to make an extra-articular fracture line in the distal radius, in the region of the Lister tubercle. The osteotomy was a mean of 1.2 cm from the distal aspect of the radius. The osteotomy site was then injected from the volar aspect with 5 mL of radiopaque (Hypaque) dye (Figure 1). Fluid extravasation into the third dorsal compartment was visualized under fluoroscopy (Figures 2–4). The monitor was then reinserted into the EPL sheath, and once again pressures were measured (postinjection readings). An 18-gauge needle was then used to aspirate the compartment just ulnar to the Lister tubercle. Compartment pressures were measured a final time (postaspiration readings). For all readings, 3 pressure measurements were recorded and then averaged. Pressure measurements were compared using t test.
In the office, the third dorsal compartment was aspirated after skin preparation with povidone-iodine. The Lister tubercle is typically palpable along the dorsal distal radius and is aligned with the cleft between the index and long fingers. Aspiration with an 18-gauge needle is performed just ulnar to the Lister tubercle in the EPL sheath, and hematoma is evacuated. The patient is then placed back into a long-arm cast or splint per the clinical situation.
Results
Patient age ranged from 17 to 81 years. Eight (1 male, 7 female) patients sustained an EPL rupture a mean of 46 days after initial trauma (range, 21-118 days). Two patients were treated with a prophylactic EPL transposition secondary to clinically apparent impending rupture, and 4 were treated with prophylactic needle decompression of the third compartment. Ruptures were treated with EIP-to-EPL transfers.
As in other studies, each patient’s radiographs showed a nondisplaced fracture and a transverse fracture line. Six patients also had a longitudinal, intra-articular fracture line that exited in a common spot between the scaphoid and the lunate facet.
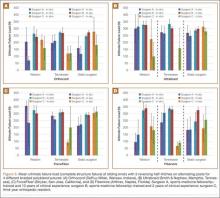
Results in our cadaveric model were consistent with those in in vitro decompression of the third dorsal compartment (Table 1). In the cadaver model, mean (SD) initial third dorsal compartment pressure was 0.77 (0.88) mm Hg. Mean (SD) pressure after osteotomy and Hypaque injection was 25.5 (11.11) mm Hg. After simulated therapeutic aspiration, mean (SD) pressure decreased to 1.61 (1.40) mm Hg. Mean change in pressure from after injection to after aspiration was 23.89 mm Hg (P = .000388) (Table 2).
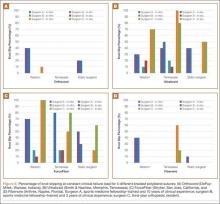
Information from other studies and from Dr. Lourie’s experience was used to identify patients at significant risk for EPL ruptures in association with distal radius fractures. Four patients in Dr. Lourie’s practice between 2004 and 2009 had characteristic findings, including a nondisplaced distal radius fracture, localized swelling over the third dorsal compartment, and pain with resisted active EPL extension. Prophylactic aspiration and hematoma evacuation were performed in this series, yielding a mean hematoma amount of 2 mL (Table 3).
For all 4 patients, aspirations were performed within 2 weeks of injury. Subjectively, these patients described almost immediate pain relief and less discomfort with EPL motion after aspiration. Three of the 4 reported sustained pain relief on close follow-up 7 and 14 days after aspiration. The fourth patient continued to have pain over the third dorsal compartment, though she described it as significantly improved. Her initial fracture contained about 50% dorsal comminution, and she began to have a significant callus response. After 2 months of continued symptoms, and out of concern about consequences of an impending rupture, open decompression and transposition of the EPL were performed. In follow-up over 29 months, this patient continued to do well and had full EPL function. The 3 patients treated with aspiration alone have not had an EPL rupture (range of follow-up, 29-89 months).
Discussion
Distal radius fractures are very common injuries, and treating physicians must attempt to prevent possible complications. EPL tendon ruptures continue to be rare events (incidence, <1%) in association with distal radius fractures. Although statistics vary, studies have found a higher incidence in nondisplaced (vs displaced) distal radius fractures.5,7,10 Ruptures in nondisplaced fractures occur within 2 weeks to 3 years after injury but typically an average of 6 weeks after injury.2,4,7-9 Prodromal symptoms often include tenderness and swelling around the dorsal distal radius region around the Lister tubercle.7,11,12 Patients may complain of pain with active thumb extension or passive thumb range of motion.11 Rupture is indicated by an inability to actively extend the thumb.
Studies have shown that the tendon rupture site is around the Lister tubercle.7 No single cause for EPL ruptures has been confirmed, and the etiology is likely a mix of factors in relation to the clinical situation. Two theories have been espoused for the relation between EPL ruptures and distal radius fractures. The mechanical theory involves a prominent spicule of bone abrading the tendon and subsequently causing rupture.5,9 This seems less likely for nondisplaced fractures. The vascular theory centers on a watershed region of the EPL tendon around the Lister tubercle. Studies have found microangiographic evidence of a 5-mm portion of tendon around the Lister tubercle that has no mesotenon and poor vascularity.7,9 The tendon in this section may be reliant on synovial diffusion for nutrition,7 but hematoma may displace synovial fluid, interfering with tendon nutrition.
Researchers have studied the third dorsal compartment in patients with impending or established ruptures. In a series by Hirasawa and colleagues,7 11 patients with a nondisplaced fracture and a tendon rupture had an intact retinaculum and smooth bony surfaces on the dorsal radius. Periosteal hypertrophy and narrowing of the third compartment were noted. In another series, Helal and colleagues5 reported on 16 patients (nondisplaced and displaced fractures) who had possible EPL ruptures over a 4-year period. In all cases, the extensor retinaculum was intact. Likewise, the 7 patients with EPL ruptures in a series by Bonatz and colleagues4 had an intact extensor retinaculum. On exploration, Bunata10 noted fluid collections, including hematomas, within the sheath, as well as a lack of bony prominences. Simpson12 explored 2 cases of blunt trauma, no fracture, and subsequent EPL rupture. Clinically these 2 patients had swelling in the region of the Lister tubercle, and surgically they were found to have a distended, blood-filled sheath. These ruptures may correlate with nondisplaced distal radius fractures and provide further evidence supporting the vascular theory of ruptures. The combination of intact compartment and volume overload presents a situation akin to compartment syndrome. Acute compartment decompression with needle evacuation would theoretically relieve the vascular insult.
In the study by Helal and colleagues,5 Hypaque injections were given to patients with nondisplaced distal radius fractures. The dye remained in the third dorsal compartment, which implies an intact closed space. That study used a cadaveric model as well, with distal radius osteotomies performed to simulate a nondisplaced distal radius fracture. The authors noted an intact extensor retinaculum in their model. Our cadaver model is similar, except we measured pressures in the third compartment; our model indicated increased compartment pressures within the EPL sheath. Subsequent aspiration in our cadaveric study led to lower pressures in the third dorsal compartment. This cadaveric model implies that needle decompression of the third dorsal compartment may be beneficial in the setting of nondisplaced distal radius fractures and symptoms indicating a compromised EPL.
Splinting is an important factor that may help prevent EPL tendon ruptures after distal radius fractures. Synovial diffusion may be the primary mechanism for delivering nutrition to the EPL tendon.7 A splint that allows thumb metacarpophalangeal and interphalangeal flexion may provide the EPL motion needed for effective synovial nutritional pathways.9 Prodromal symptoms of tendon rupture should then be carefully monitored.
Conclusion
Results of our retrospective review are consistent with previous results elucidating the risk factors for EPL ruptures in association with distal radius fractures. In our patients who sustained EPL ruptures, findings included nondisplaced fractures, about 50% with an intra-articular component. Clinical findings included localized swelling over the third dorsal compartment, pain with resisted active EPL extension, and pain with passive flexion of the thumb interphalangeal joint. The cadaveric portion of this study indicated a significant change in pressure in the third dorsal compartment after aspiration. Preliminary outcomes in this 4-patient series are no EPL ruptures after prophylactic aspiration. Hematoma evacuation after nondisplaced distal radius fractures may become a useful addition to the surgeon’s armamentarium. Studies are needed to determine if needle aspiration of the third dorsal compartment can become an office-based procedure with value in preventing EPL ruptures in the appropriate clinical situation.
1. Alffram PA, Bauer GC. Epidemiology of fractures of the forearm. A biomechanical investigation of bone strength. J Bone Joint Surg Am. 1962;44:105-114.
2. Hove LM. Delayed rupture of the thumb extensor tendon. A 5-year study of 18 consecutive cases. Acta Orthop Scand. 1994;65(2):199-203.
3. Duplay. Rupture sous-cutanee du tendon du long extenseur du pouce, au niveau de la tabatiere anatomique. Bull Et Mem de la Soc de Chir de Paris. 1876.
4. Bonatz E, Kramer TD, Masear VR. Rupture of the extensor pollicis longus tendon. Am J Orthop. 1996;25(2):118-122.
5. Helal B, Chen SC, Iwegbu G. Rupture of the extensor pollicis longus tendon in undisplaced Colles’ type of fracture. Hand. 1982;14(1):41-47.
6. McMaster PE. Late ruptures of extensor and flexor pollicis longus tendons following Colles’ fracture. J Bone Joint Surg Am. 1932;14:93-101.
7. Hirasawa Y, Katsumi Y, Akiyoshi T, Tamai K, Tokioka T. Clinical and microangiographic studies on rupture of the E.P.L. tendon after distal radius fractures. J Hand Surg Br. 1990;15(1):51-57.
8. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: a study of aetiological factors. Scand J Plast Reconstruct Surg Hand Surg. 2004;38(1):32-35.
9. Engkvist O, Lundborg G. Rupture of the extensor pollicis longus tendon after fracture of the lower end of the radius—a clinical and microangiographic study. Hand. 1979;11(1):76-86.
10. Bunata RE. Impending rupture of the extensor pollicis longus tendon after a minimally displaced Colles fracture. A case report. J Bone Joint Surg Am. 1983;65(3):401-402.
11. Skoff HD. Postfracture extensor pollicis longus tenosynovitis and tendon rupture: a scientific study and personal series. Am J Orthop. 2003;32(5):245-247.
12. Simpson RG. Delayed rupture of extensor pollicis longus tendon following closed injury. Hand. 1977;9(2):160-161.
Distal radius fractures are among the most common upper extremity injuries. A Swedish study noted that 75% of forearm fractures involve the distal radius.1 Extensor pollicis longus (EPL) ruptures are a well-documented complication (0.3% incidence2) of distal radius fractures.
The first description of EPL ruptures is attributed to Duplay in 1876 and was termed drummer boy’s palsy.3 Spontaneous EPL ruptures are often described in the setting of acute or chronic tenosynovitis.4,5 Beginning in the early 1930s, multiple case reports began to connect distal radius fractures with EPL ruptures.6 Although EPL ruptures are rare, their consequences are substantial and typically necessitate reconstructive procedures. Extensor indicis proprius (EIP)-to-EPL tendon transfer has become a common surgical treatment for this complication. Increasing our knowledge of several characteristics associated with this complication may help clinically in preventing EPL ruptures.
Multiple studies have indicated that EPL ruptures occur more often in nondisplaced fractures and often occur between 6 and 8 weeks after injury.2,5,7,8 Several factors are implicated in the etiology of EPL ruptures in distal radius fractures. The classic 1979 study by Engkvist and Lundborg9 showed that the EPL tendon has an area of poor vascularity around the Lister tubercle. Explorations of nondisplaced distal radius fractures have shown an intact extensor retinaculum that allows the tendon to continue to travel through an enclosed space.4,5,7 In the setting of distal radius fracture, hematoma may contribute to tendon ruptures secondary to increased pressure within an intact third dorsal compartment, which further compromises vascularity in this region of the EPL tendon.
Recognition and prevention of an impending EPL rupture may help avoid the significant consequences of this complication. Decompression and release of the third dorsal compartment have been described as constituting a prophylactic surgical option.10,11 Early thumb range of motion is also advocated to help prevent EPL rupture.9 However, results reported in the literature are inconclusive as to the effectiveness of these or indeed any preventive procedures. Dr. Lourie uses a novel technique that involves aspiration of the third dorsal compartment in patients with clinical symptoms associated with impending EPL rupture. Needle decompression, a less invasive option, can be quickly performed in an office, and it is hypothesized that removal of the hematoma may prevent EPL ruptures.
In the present study, we retrospectively reviewed Dr. Lourie’s records of patients with EPL ruptures in association with distal radius fractures to help delineate which radiographic and clinical characteristics identify patients at risk for these ruptures. A cadaveric model of a nondisplaced distal radius fracture was then created in order to simulate a change in third compartment pressures before and after needle decompression. We present preliminary outcomes on a case series of 4 patients who underwent aspiration of the third compartment and who were thought to be at risk for EPL rupture.
Materials and Methods
Institutional review board approval was obtained for this study. From Dr. Lourie’s records, 19 patients treated between 1998 and 2009 were identified as having confirmed or clinically impending EPL ruptures in association with nonoperative treatment of distal radius fractures. Prodromal symptoms that were used to diagnose impending EPL ruptures included pain with resisted active EPL extension, pain with passive flexion of the thumb interphalangeal joint, and localized swelling over the third dorsal compartment of the wrist.5,7,10,12 Eleven patients had complete radiographs, which were reviewed for radiographic characteristics. Posteroanterior (PA) and lateral radiographs of the injured wrist were reviewed. On the PA radiographs, fraction location was measured from the tip of the radial styloid using a line perpendicular to the radial shaft. Fractures were also evaluated for displacement and intra-articular involvement.
A cadaveric model was developed to evaluate compartment pressures in the EPL sheath in a simulated distal radius fracture. Six fresh-frozen cadaveric forearms were used after being thawed at room temperature. The cadavers were radiographically evaluated to determine that there was no evidence of prior fracture. A Stryker compartment pressure monitoring system (Stryker, Kalamazoo, Michigan) was used to take initial pressure readings in the third dorsal compartment slightly ulnar to the Lister tubercle (preinjection readings). A limited volar approach was then created. Under fluoroscopy, a half-inch osteotome was used to make an extra-articular fracture line in the distal radius, in the region of the Lister tubercle. The osteotomy was a mean of 1.2 cm from the distal aspect of the radius. The osteotomy site was then injected from the volar aspect with 5 mL of radiopaque (Hypaque) dye (Figure 1). Fluid extravasation into the third dorsal compartment was visualized under fluoroscopy (Figures 2–4). The monitor was then reinserted into the EPL sheath, and once again pressures were measured (postinjection readings). An 18-gauge needle was then used to aspirate the compartment just ulnar to the Lister tubercle. Compartment pressures were measured a final time (postaspiration readings). For all readings, 3 pressure measurements were recorded and then averaged. Pressure measurements were compared using t test.
In the office, the third dorsal compartment was aspirated after skin preparation with povidone-iodine. The Lister tubercle is typically palpable along the dorsal distal radius and is aligned with the cleft between the index and long fingers. Aspiration with an 18-gauge needle is performed just ulnar to the Lister tubercle in the EPL sheath, and hematoma is evacuated. The patient is then placed back into a long-arm cast or splint per the clinical situation.
Results
Patient age ranged from 17 to 81 years. Eight (1 male, 7 female) patients sustained an EPL rupture a mean of 46 days after initial trauma (range, 21-118 days). Two patients were treated with a prophylactic EPL transposition secondary to clinically apparent impending rupture, and 4 were treated with prophylactic needle decompression of the third compartment. Ruptures were treated with EIP-to-EPL transfers.
As in other studies, each patient’s radiographs showed a nondisplaced fracture and a transverse fracture line. Six patients also had a longitudinal, intra-articular fracture line that exited in a common spot between the scaphoid and the lunate facet.
Results in our cadaveric model were consistent with those in in vitro decompression of the third dorsal compartment (Table 1). In the cadaver model, mean (SD) initial third dorsal compartment pressure was 0.77 (0.88) mm Hg. Mean (SD) pressure after osteotomy and Hypaque injection was 25.5 (11.11) mm Hg. After simulated therapeutic aspiration, mean (SD) pressure decreased to 1.61 (1.40) mm Hg. Mean change in pressure from after injection to after aspiration was 23.89 mm Hg (P = .000388) (Table 2).
Information from other studies and from Dr. Lourie’s experience was used to identify patients at significant risk for EPL ruptures in association with distal radius fractures. Four patients in Dr. Lourie’s practice between 2004 and 2009 had characteristic findings, including a nondisplaced distal radius fracture, localized swelling over the third dorsal compartment, and pain with resisted active EPL extension. Prophylactic aspiration and hematoma evacuation were performed in this series, yielding a mean hematoma amount of 2 mL (Table 3).
For all 4 patients, aspirations were performed within 2 weeks of injury. Subjectively, these patients described almost immediate pain relief and less discomfort with EPL motion after aspiration. Three of the 4 reported sustained pain relief on close follow-up 7 and 14 days after aspiration. The fourth patient continued to have pain over the third dorsal compartment, though she described it as significantly improved. Her initial fracture contained about 50% dorsal comminution, and she began to have a significant callus response. After 2 months of continued symptoms, and out of concern about consequences of an impending rupture, open decompression and transposition of the EPL were performed. In follow-up over 29 months, this patient continued to do well and had full EPL function. The 3 patients treated with aspiration alone have not had an EPL rupture (range of follow-up, 29-89 months).
Discussion
Distal radius fractures are very common injuries, and treating physicians must attempt to prevent possible complications. EPL tendon ruptures continue to be rare events (incidence, <1%) in association with distal radius fractures. Although statistics vary, studies have found a higher incidence in nondisplaced (vs displaced) distal radius fractures.5,7,10 Ruptures in nondisplaced fractures occur within 2 weeks to 3 years after injury but typically an average of 6 weeks after injury.2,4,7-9 Prodromal symptoms often include tenderness and swelling around the dorsal distal radius region around the Lister tubercle.7,11,12 Patients may complain of pain with active thumb extension or passive thumb range of motion.11 Rupture is indicated by an inability to actively extend the thumb.
Studies have shown that the tendon rupture site is around the Lister tubercle.7 No single cause for EPL ruptures has been confirmed, and the etiology is likely a mix of factors in relation to the clinical situation. Two theories have been espoused for the relation between EPL ruptures and distal radius fractures. The mechanical theory involves a prominent spicule of bone abrading the tendon and subsequently causing rupture.5,9 This seems less likely for nondisplaced fractures. The vascular theory centers on a watershed region of the EPL tendon around the Lister tubercle. Studies have found microangiographic evidence of a 5-mm portion of tendon around the Lister tubercle that has no mesotenon and poor vascularity.7,9 The tendon in this section may be reliant on synovial diffusion for nutrition,7 but hematoma may displace synovial fluid, interfering with tendon nutrition.
Researchers have studied the third dorsal compartment in patients with impending or established ruptures. In a series by Hirasawa and colleagues,7 11 patients with a nondisplaced fracture and a tendon rupture had an intact retinaculum and smooth bony surfaces on the dorsal radius. Periosteal hypertrophy and narrowing of the third compartment were noted. In another series, Helal and colleagues5 reported on 16 patients (nondisplaced and displaced fractures) who had possible EPL ruptures over a 4-year period. In all cases, the extensor retinaculum was intact. Likewise, the 7 patients with EPL ruptures in a series by Bonatz and colleagues4 had an intact extensor retinaculum. On exploration, Bunata10 noted fluid collections, including hematomas, within the sheath, as well as a lack of bony prominences. Simpson12 explored 2 cases of blunt trauma, no fracture, and subsequent EPL rupture. Clinically these 2 patients had swelling in the region of the Lister tubercle, and surgically they were found to have a distended, blood-filled sheath. These ruptures may correlate with nondisplaced distal radius fractures and provide further evidence supporting the vascular theory of ruptures. The combination of intact compartment and volume overload presents a situation akin to compartment syndrome. Acute compartment decompression with needle evacuation would theoretically relieve the vascular insult.
In the study by Helal and colleagues,5 Hypaque injections were given to patients with nondisplaced distal radius fractures. The dye remained in the third dorsal compartment, which implies an intact closed space. That study used a cadaveric model as well, with distal radius osteotomies performed to simulate a nondisplaced distal radius fracture. The authors noted an intact extensor retinaculum in their model. Our cadaver model is similar, except we measured pressures in the third compartment; our model indicated increased compartment pressures within the EPL sheath. Subsequent aspiration in our cadaveric study led to lower pressures in the third dorsal compartment. This cadaveric model implies that needle decompression of the third dorsal compartment may be beneficial in the setting of nondisplaced distal radius fractures and symptoms indicating a compromised EPL.
Splinting is an important factor that may help prevent EPL tendon ruptures after distal radius fractures. Synovial diffusion may be the primary mechanism for delivering nutrition to the EPL tendon.7 A splint that allows thumb metacarpophalangeal and interphalangeal flexion may provide the EPL motion needed for effective synovial nutritional pathways.9 Prodromal symptoms of tendon rupture should then be carefully monitored.
Conclusion
Results of our retrospective review are consistent with previous results elucidating the risk factors for EPL ruptures in association with distal radius fractures. In our patients who sustained EPL ruptures, findings included nondisplaced fractures, about 50% with an intra-articular component. Clinical findings included localized swelling over the third dorsal compartment, pain with resisted active EPL extension, and pain with passive flexion of the thumb interphalangeal joint. The cadaveric portion of this study indicated a significant change in pressure in the third dorsal compartment after aspiration. Preliminary outcomes in this 4-patient series are no EPL ruptures after prophylactic aspiration. Hematoma evacuation after nondisplaced distal radius fractures may become a useful addition to the surgeon’s armamentarium. Studies are needed to determine if needle aspiration of the third dorsal compartment can become an office-based procedure with value in preventing EPL ruptures in the appropriate clinical situation.
Distal radius fractures are among the most common upper extremity injuries. A Swedish study noted that 75% of forearm fractures involve the distal radius.1 Extensor pollicis longus (EPL) ruptures are a well-documented complication (0.3% incidence2) of distal radius fractures.
The first description of EPL ruptures is attributed to Duplay in 1876 and was termed drummer boy’s palsy.3 Spontaneous EPL ruptures are often described in the setting of acute or chronic tenosynovitis.4,5 Beginning in the early 1930s, multiple case reports began to connect distal radius fractures with EPL ruptures.6 Although EPL ruptures are rare, their consequences are substantial and typically necessitate reconstructive procedures. Extensor indicis proprius (EIP)-to-EPL tendon transfer has become a common surgical treatment for this complication. Increasing our knowledge of several characteristics associated with this complication may help clinically in preventing EPL ruptures.
Multiple studies have indicated that EPL ruptures occur more often in nondisplaced fractures and often occur between 6 and 8 weeks after injury.2,5,7,8 Several factors are implicated in the etiology of EPL ruptures in distal radius fractures. The classic 1979 study by Engkvist and Lundborg9 showed that the EPL tendon has an area of poor vascularity around the Lister tubercle. Explorations of nondisplaced distal radius fractures have shown an intact extensor retinaculum that allows the tendon to continue to travel through an enclosed space.4,5,7 In the setting of distal radius fracture, hematoma may contribute to tendon ruptures secondary to increased pressure within an intact third dorsal compartment, which further compromises vascularity in this region of the EPL tendon.
Recognition and prevention of an impending EPL rupture may help avoid the significant consequences of this complication. Decompression and release of the third dorsal compartment have been described as constituting a prophylactic surgical option.10,11 Early thumb range of motion is also advocated to help prevent EPL rupture.9 However, results reported in the literature are inconclusive as to the effectiveness of these or indeed any preventive procedures. Dr. Lourie uses a novel technique that involves aspiration of the third dorsal compartment in patients with clinical symptoms associated with impending EPL rupture. Needle decompression, a less invasive option, can be quickly performed in an office, and it is hypothesized that removal of the hematoma may prevent EPL ruptures.
In the present study, we retrospectively reviewed Dr. Lourie’s records of patients with EPL ruptures in association with distal radius fractures to help delineate which radiographic and clinical characteristics identify patients at risk for these ruptures. A cadaveric model of a nondisplaced distal radius fracture was then created in order to simulate a change in third compartment pressures before and after needle decompression. We present preliminary outcomes on a case series of 4 patients who underwent aspiration of the third compartment and who were thought to be at risk for EPL rupture.
Materials and Methods
Institutional review board approval was obtained for this study. From Dr. Lourie’s records, 19 patients treated between 1998 and 2009 were identified as having confirmed or clinically impending EPL ruptures in association with nonoperative treatment of distal radius fractures. Prodromal symptoms that were used to diagnose impending EPL ruptures included pain with resisted active EPL extension, pain with passive flexion of the thumb interphalangeal joint, and localized swelling over the third dorsal compartment of the wrist.5,7,10,12 Eleven patients had complete radiographs, which were reviewed for radiographic characteristics. Posteroanterior (PA) and lateral radiographs of the injured wrist were reviewed. On the PA radiographs, fraction location was measured from the tip of the radial styloid using a line perpendicular to the radial shaft. Fractures were also evaluated for displacement and intra-articular involvement.
A cadaveric model was developed to evaluate compartment pressures in the EPL sheath in a simulated distal radius fracture. Six fresh-frozen cadaveric forearms were used after being thawed at room temperature. The cadavers were radiographically evaluated to determine that there was no evidence of prior fracture. A Stryker compartment pressure monitoring system (Stryker, Kalamazoo, Michigan) was used to take initial pressure readings in the third dorsal compartment slightly ulnar to the Lister tubercle (preinjection readings). A limited volar approach was then created. Under fluoroscopy, a half-inch osteotome was used to make an extra-articular fracture line in the distal radius, in the region of the Lister tubercle. The osteotomy was a mean of 1.2 cm from the distal aspect of the radius. The osteotomy site was then injected from the volar aspect with 5 mL of radiopaque (Hypaque) dye (Figure 1). Fluid extravasation into the third dorsal compartment was visualized under fluoroscopy (Figures 2–4). The monitor was then reinserted into the EPL sheath, and once again pressures were measured (postinjection readings). An 18-gauge needle was then used to aspirate the compartment just ulnar to the Lister tubercle. Compartment pressures were measured a final time (postaspiration readings). For all readings, 3 pressure measurements were recorded and then averaged. Pressure measurements were compared using t test.
In the office, the third dorsal compartment was aspirated after skin preparation with povidone-iodine. The Lister tubercle is typically palpable along the dorsal distal radius and is aligned with the cleft between the index and long fingers. Aspiration with an 18-gauge needle is performed just ulnar to the Lister tubercle in the EPL sheath, and hematoma is evacuated. The patient is then placed back into a long-arm cast or splint per the clinical situation.
Results
Patient age ranged from 17 to 81 years. Eight (1 male, 7 female) patients sustained an EPL rupture a mean of 46 days after initial trauma (range, 21-118 days). Two patients were treated with a prophylactic EPL transposition secondary to clinically apparent impending rupture, and 4 were treated with prophylactic needle decompression of the third compartment. Ruptures were treated with EIP-to-EPL transfers.
As in other studies, each patient’s radiographs showed a nondisplaced fracture and a transverse fracture line. Six patients also had a longitudinal, intra-articular fracture line that exited in a common spot between the scaphoid and the lunate facet.
Results in our cadaveric model were consistent with those in in vitro decompression of the third dorsal compartment (Table 1). In the cadaver model, mean (SD) initial third dorsal compartment pressure was 0.77 (0.88) mm Hg. Mean (SD) pressure after osteotomy and Hypaque injection was 25.5 (11.11) mm Hg. After simulated therapeutic aspiration, mean (SD) pressure decreased to 1.61 (1.40) mm Hg. Mean change in pressure from after injection to after aspiration was 23.89 mm Hg (P = .000388) (Table 2).
Information from other studies and from Dr. Lourie’s experience was used to identify patients at significant risk for EPL ruptures in association with distal radius fractures. Four patients in Dr. Lourie’s practice between 2004 and 2009 had characteristic findings, including a nondisplaced distal radius fracture, localized swelling over the third dorsal compartment, and pain with resisted active EPL extension. Prophylactic aspiration and hematoma evacuation were performed in this series, yielding a mean hematoma amount of 2 mL (Table 3).
For all 4 patients, aspirations were performed within 2 weeks of injury. Subjectively, these patients described almost immediate pain relief and less discomfort with EPL motion after aspiration. Three of the 4 reported sustained pain relief on close follow-up 7 and 14 days after aspiration. The fourth patient continued to have pain over the third dorsal compartment, though she described it as significantly improved. Her initial fracture contained about 50% dorsal comminution, and she began to have a significant callus response. After 2 months of continued symptoms, and out of concern about consequences of an impending rupture, open decompression and transposition of the EPL were performed. In follow-up over 29 months, this patient continued to do well and had full EPL function. The 3 patients treated with aspiration alone have not had an EPL rupture (range of follow-up, 29-89 months).
Discussion
Distal radius fractures are very common injuries, and treating physicians must attempt to prevent possible complications. EPL tendon ruptures continue to be rare events (incidence, <1%) in association with distal radius fractures. Although statistics vary, studies have found a higher incidence in nondisplaced (vs displaced) distal radius fractures.5,7,10 Ruptures in nondisplaced fractures occur within 2 weeks to 3 years after injury but typically an average of 6 weeks after injury.2,4,7-9 Prodromal symptoms often include tenderness and swelling around the dorsal distal radius region around the Lister tubercle.7,11,12 Patients may complain of pain with active thumb extension or passive thumb range of motion.11 Rupture is indicated by an inability to actively extend the thumb.
Studies have shown that the tendon rupture site is around the Lister tubercle.7 No single cause for EPL ruptures has been confirmed, and the etiology is likely a mix of factors in relation to the clinical situation. Two theories have been espoused for the relation between EPL ruptures and distal radius fractures. The mechanical theory involves a prominent spicule of bone abrading the tendon and subsequently causing rupture.5,9 This seems less likely for nondisplaced fractures. The vascular theory centers on a watershed region of the EPL tendon around the Lister tubercle. Studies have found microangiographic evidence of a 5-mm portion of tendon around the Lister tubercle that has no mesotenon and poor vascularity.7,9 The tendon in this section may be reliant on synovial diffusion for nutrition,7 but hematoma may displace synovial fluid, interfering with tendon nutrition.
Researchers have studied the third dorsal compartment in patients with impending or established ruptures. In a series by Hirasawa and colleagues,7 11 patients with a nondisplaced fracture and a tendon rupture had an intact retinaculum and smooth bony surfaces on the dorsal radius. Periosteal hypertrophy and narrowing of the third compartment were noted. In another series, Helal and colleagues5 reported on 16 patients (nondisplaced and displaced fractures) who had possible EPL ruptures over a 4-year period. In all cases, the extensor retinaculum was intact. Likewise, the 7 patients with EPL ruptures in a series by Bonatz and colleagues4 had an intact extensor retinaculum. On exploration, Bunata10 noted fluid collections, including hematomas, within the sheath, as well as a lack of bony prominences. Simpson12 explored 2 cases of blunt trauma, no fracture, and subsequent EPL rupture. Clinically these 2 patients had swelling in the region of the Lister tubercle, and surgically they were found to have a distended, blood-filled sheath. These ruptures may correlate with nondisplaced distal radius fractures and provide further evidence supporting the vascular theory of ruptures. The combination of intact compartment and volume overload presents a situation akin to compartment syndrome. Acute compartment decompression with needle evacuation would theoretically relieve the vascular insult.
In the study by Helal and colleagues,5 Hypaque injections were given to patients with nondisplaced distal radius fractures. The dye remained in the third dorsal compartment, which implies an intact closed space. That study used a cadaveric model as well, with distal radius osteotomies performed to simulate a nondisplaced distal radius fracture. The authors noted an intact extensor retinaculum in their model. Our cadaver model is similar, except we measured pressures in the third compartment; our model indicated increased compartment pressures within the EPL sheath. Subsequent aspiration in our cadaveric study led to lower pressures in the third dorsal compartment. This cadaveric model implies that needle decompression of the third dorsal compartment may be beneficial in the setting of nondisplaced distal radius fractures and symptoms indicating a compromised EPL.
Splinting is an important factor that may help prevent EPL tendon ruptures after distal radius fractures. Synovial diffusion may be the primary mechanism for delivering nutrition to the EPL tendon.7 A splint that allows thumb metacarpophalangeal and interphalangeal flexion may provide the EPL motion needed for effective synovial nutritional pathways.9 Prodromal symptoms of tendon rupture should then be carefully monitored.
Conclusion
Results of our retrospective review are consistent with previous results elucidating the risk factors for EPL ruptures in association with distal radius fractures. In our patients who sustained EPL ruptures, findings included nondisplaced fractures, about 50% with an intra-articular component. Clinical findings included localized swelling over the third dorsal compartment, pain with resisted active EPL extension, and pain with passive flexion of the thumb interphalangeal joint. The cadaveric portion of this study indicated a significant change in pressure in the third dorsal compartment after aspiration. Preliminary outcomes in this 4-patient series are no EPL ruptures after prophylactic aspiration. Hematoma evacuation after nondisplaced distal radius fractures may become a useful addition to the surgeon’s armamentarium. Studies are needed to determine if needle aspiration of the third dorsal compartment can become an office-based procedure with value in preventing EPL ruptures in the appropriate clinical situation.
1. Alffram PA, Bauer GC. Epidemiology of fractures of the forearm. A biomechanical investigation of bone strength. J Bone Joint Surg Am. 1962;44:105-114.
2. Hove LM. Delayed rupture of the thumb extensor tendon. A 5-year study of 18 consecutive cases. Acta Orthop Scand. 1994;65(2):199-203.
3. Duplay. Rupture sous-cutanee du tendon du long extenseur du pouce, au niveau de la tabatiere anatomique. Bull Et Mem de la Soc de Chir de Paris. 1876.
4. Bonatz E, Kramer TD, Masear VR. Rupture of the extensor pollicis longus tendon. Am J Orthop. 1996;25(2):118-122.
5. Helal B, Chen SC, Iwegbu G. Rupture of the extensor pollicis longus tendon in undisplaced Colles’ type of fracture. Hand. 1982;14(1):41-47.
6. McMaster PE. Late ruptures of extensor and flexor pollicis longus tendons following Colles’ fracture. J Bone Joint Surg Am. 1932;14:93-101.
7. Hirasawa Y, Katsumi Y, Akiyoshi T, Tamai K, Tokioka T. Clinical and microangiographic studies on rupture of the E.P.L. tendon after distal radius fractures. J Hand Surg Br. 1990;15(1):51-57.
8. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: a study of aetiological factors. Scand J Plast Reconstruct Surg Hand Surg. 2004;38(1):32-35.
9. Engkvist O, Lundborg G. Rupture of the extensor pollicis longus tendon after fracture of the lower end of the radius—a clinical and microangiographic study. Hand. 1979;11(1):76-86.
10. Bunata RE. Impending rupture of the extensor pollicis longus tendon after a minimally displaced Colles fracture. A case report. J Bone Joint Surg Am. 1983;65(3):401-402.
11. Skoff HD. Postfracture extensor pollicis longus tenosynovitis and tendon rupture: a scientific study and personal series. Am J Orthop. 2003;32(5):245-247.
12. Simpson RG. Delayed rupture of extensor pollicis longus tendon following closed injury. Hand. 1977;9(2):160-161.
1. Alffram PA, Bauer GC. Epidemiology of fractures of the forearm. A biomechanical investigation of bone strength. J Bone Joint Surg Am. 1962;44:105-114.
2. Hove LM. Delayed rupture of the thumb extensor tendon. A 5-year study of 18 consecutive cases. Acta Orthop Scand. 1994;65(2):199-203.
3. Duplay. Rupture sous-cutanee du tendon du long extenseur du pouce, au niveau de la tabatiere anatomique. Bull Et Mem de la Soc de Chir de Paris. 1876.
4. Bonatz E, Kramer TD, Masear VR. Rupture of the extensor pollicis longus tendon. Am J Orthop. 1996;25(2):118-122.
5. Helal B, Chen SC, Iwegbu G. Rupture of the extensor pollicis longus tendon in undisplaced Colles’ type of fracture. Hand. 1982;14(1):41-47.
6. McMaster PE. Late ruptures of extensor and flexor pollicis longus tendons following Colles’ fracture. J Bone Joint Surg Am. 1932;14:93-101.
7. Hirasawa Y, Katsumi Y, Akiyoshi T, Tamai K, Tokioka T. Clinical and microangiographic studies on rupture of the E.P.L. tendon after distal radius fractures. J Hand Surg Br. 1990;15(1):51-57.
8. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: a study of aetiological factors. Scand J Plast Reconstruct Surg Hand Surg. 2004;38(1):32-35.
9. Engkvist O, Lundborg G. Rupture of the extensor pollicis longus tendon after fracture of the lower end of the radius—a clinical and microangiographic study. Hand. 1979;11(1):76-86.
10. Bunata RE. Impending rupture of the extensor pollicis longus tendon after a minimally displaced Colles fracture. A case report. J Bone Joint Surg Am. 1983;65(3):401-402.
11. Skoff HD. Postfracture extensor pollicis longus tenosynovitis and tendon rupture: a scientific study and personal series. Am J Orthop. 2003;32(5):245-247.
12. Simpson RG. Delayed rupture of extensor pollicis longus tendon following closed injury. Hand. 1977;9(2):160-161.
The Importance of Sex of Patient in the Management of Femoroacetabular Impingement
Femoroacetabular impingement (FAI), a recently described hip condition in adolescents and young adults, results from abnormal physical contact between the proximal femur and the acetabulum.1 FAI is usually characterized by the site of the predominant morphologic abnormality—proximal femur (cam-type FAI), acetabulum (pincer-type FAI), or both (mixed impingement). Cam-type FAI is typified by the aspherical extension of the articular surface at the anterosuperior head–neck junction of the proximal femur with loss of the normal offset. With hip motion, especially in the maximal ranges of flexion and internal rotation, the aspherical proximal femur repeatedly contacts the anterosuperior acetabulum, damaging the chondrolabral junction and ultimately the labrum itself. In pincer-type impingement, femoral head overcoverage caused by acetabular retroversion and/or coxa profunda directly damages the anterior labrum when the acetabular rim contacts the proximal femur during physiologic motion. “Contrecoup” injury of the posterior-inferior acetabular cartilage may also occur. Over time, recurrent microtrauma to the acetabular cartilage and/or labrum may lead to degenerative changes of the hip and ultimately to premature osteoarthritis.1,2
Patients with FAI typically present with groin pain that may be activity-related or that may occur with prolonged sitting with the hip in a flexed position. Physical examination findings suggestive of FAI include decreased passive internal hip rotation and reproducible pain with adduction and internal rotation of the flexed hip—the impingement sign, or the flexion, adduction, and internal rotation (FADIR) test.3 Diagnostic imaging evaluation initially includes radiographs of the pelvis and hips. These radiographs may show a “pistol-grip” deformity and/or decreased head–neck offset (as determined by increased alpha angle) in the setting of cam-type impingement (Figure 1).4 Pincer-type impingement may be associated with a crossover sign, coxa profunda, and an increased center-edge angle (CEA). Advanced imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI) arthrogram, and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), are commonly used to better delineate bony deformity and concomitant injuries of the labrum and cartilage (Figure 2).
Treatment for FAI often consists initially of activity modification, use of anti-inflammatory medications, and physical therapy. Intra-articular corticosteroid injections may be used both diagnostically and therapeutically. When nonsurgical measures fail to adequately relieve symptoms, surgery may be warranted. Whether performed open or arthroscopically, surgery is directed first at correcting the underlying osseous abnormality—performing an osteoplasty of the proximal femur to remove the cam lesion, performing an acetabular osteoplasty (“rim-trimming”) to address a focal pincer lesion, and/or performing a periacetabular osteotomy to decrease global acetabular overcoverage (Figure 3).5
Sex-Based Differences in FAI Incidence
Traditionally, it was thought that cam-type impingement occurred predominantly in young, athletic males, whereas pincer-type impingement resulting from acetabular overcoverage occurred primarily in females during their fourth decade. However, our understanding of the sex-based differences in the incidence and presentation of FAI has evolved, and it is now clear that the interplay of sex, radiographic signs of impingement, and development of symptoms requiring treatment is more complex.
In recent large population-based studies, investigators have attempted to better characterize the sex-based differences in the incidence of osseous FAI deformity. Gosvig and colleagues2 examined radiographic and questionnaire outcomes of 3620 patients (age range, 21-90 years) and found that males were more likely than females to have a pistol-grip deformity of the hip (19.6% vs 5.2%); that deep acetabular sockets were common in both sexes (15.2% vs 19.4%); and that the presence of pistol-grip deformity or deep socket was significantly associated with development of osteoarthritis, independent of sex.
In a study of 2081 asymptomatic patients (mean age, 18.6 years), Laborie and colleagues4 reported similar radiographic findings. Males were significantly more likely than females to have a cam-type deformity, as evidenced by pistol-grip deformity, focal prominence of the femoral neck, and/or flattening of the lateral aspect of the femoral head. Males were also more likely than females to have a pincer deformity, though radiographic signs of pincer deformity—a crossover sign, excessive acetabular coverage (defined by increased CEA), and a posterior wall sign—were common in both sexes, occurring in 16.6% of females and 34.3% of males. Bilateral findings of FAI-associated deformity were also more common in males than in females, both for cam-type deformity (24.7% vs 6.3%) and pincer-type deformity (21.7% vs 9.7%).
Sex-Based Differences in FAI Presentation
In males and females, the clinical presentation of FAI is similar—insidious onset of deep groin pain, often exacerbated with activity, and physical examination findings of decreased hip motion (particularly internal rotation) and a positive impingement test.3 Nevertheless, the sexes’ clinical presentation differs in several ways. Specifically, in a study using 3-dimensional CT to assess bony deformity in both symptomatic and asymptomatic patients, Beaulé and colleagues6 reported that alpha angles were significantly higher in symptomatic males than in symptomatic females (73.3° vs 58.7°). Hetsroni and colleagues7 recently reported similar results in a study of 217 symptomatic young adults treated arthroscopically for hip pain. Preoperative CT showed that alpha angles were significantly larger in males than in females (63.6° vs 47.8°). The authors postulated that females may be more likely to be symptomatic in the setting of smaller cam lesions because of the increased peak hip flexion and frontal plane motion commonly demonstrated by females during drop landings in sport. The authors further hypothesized that sex differences in muscle mass (which contributes to dynamic hip stability) and ligamentous laxity (a component of static hip stability) may result in larger physiologic ranges of motion for many females. As a result, bony impingement may occur in the setting of smaller anatomical lesions in females. The authors further noted that, compared with their male counterparts, females being treated for symptomatic FAI had significantly more femoral and acetabular anteversion.
Another male–female presentation difference involves symptom bilaterality. Specifically, males are significantly more likely than females to have symptomatic FAI involving both hips. In a recent study of 646 patients who underwent hip arthroscopy for symptomatic FAI during a 2-year period, Klingenstein and colleagues8 found that females constituted 48.2% of unilateral arthroscopy patients but only 34.8% of bilateral arthroscopy patients. The odds ratio of males treated for both hips, compared with females, was 1.7 (95% confidence interval, 1.16–2.54).
Last, it has been reported that, on clinical presentation, hip function scores are significantly lower in females than in males. In a recent study of 612 cases of symptomatic FAI treated with hip arthroscopy, Malviya and colleagues9 found that females had significantly lower quality-of-life scores both before and after surgery. Hetsroni and colleagues7 reported similar findings, with females having significantly lower preoperative modified Harris Hip Scores and lower Hip Outcome Scores in the domains of Activities of Daily Living and Sports.
Sex-Based Differences in FAI Treatment
and Outcomes
Surgical treatment of FAI is focused on identifying the source of hip pain and dysfunction—be it osseous lesion, labral tearing, chondral injury, or iliopsoas tendonitis—and treating it accordingly, regardless of sex. Most studies of this approach find consistent improvement in the short-term and midterm outcome scores for a majority of patients. However, relatively few studies have focused specifically on sex in determining the percentage of patients who require surgical treatment, in deciding the type of surgery that should be performed, or in measuring surgical outcomes in patients with symptomatic FAI.
In their review of 23 studies of FAI surgery, Ng and colleagues10 found that, of 970 patients, 608 (62.7%) were male and 362 (37.3%) were female. Similarly higher rates for males were previously published.5,11 More recently, Clohisy and colleagues12 reported on the descriptive epidemiology of patients having surgery for FAI at 8 different medical centers in North America. Fifty-five percent of the hips surgically treated for symptomatic FAI were females’. The authors speculated that this unexpectedly high rate could have resulted from US and Canadian female athletes’ increasingly higher level of sports participation. The results of this study, one of the largest examining the rate of surgery for males and females with FAI, suggest that females are more likely to have surgery for symptomatic FAI despite being less likely to have radiographic evidence of impingement. Our understanding of this phenomenon continues to advance.
In a recent prospective study, Krych and colleagues13 evaluated the clinical outcomes of FAI surgeries (labral débridement, labral repair) in an all-female patient cohort. Female patients with symptomatic FAI were randomized to undergo either labral débridement or labral repair. There were clinical improvements in both groups, but, compared with labral débridement patients, labral repair patients had more significantly improved Hip Outcome Scores in the domains of Activities of Daily Living and Sports, as well as better subjective outcomes. Although the study did not compare female patients with male patients, it does provide evidence that female patients specifically may benefit more from labral repair than from labral débridement alone.
With respect to different surgical treatments for male and female patients, Hetsroni and colleagues7 introduced the idea of sex-specific treatment when they noted more hip anteversion in their study’s female patients than in its male patients. They suggested that, because the anterosuperior acetabulum is subjected to a high amount of stress during weight-bearing and gait, this area in females with suspected pincer lesions should be rim-trimmed judiciously to avoid increasing the stress and perhaps even hastening the development of degenerative disease. Last, though several authors have noted that hip function scores are lower in females than in males on presentation, it has also been reported that females demonstrate more improvement in functional scores after surgery.9 This may be important information to discuss during preoperative counseling about expected goals and outcomes.
Conclusion
Femoroacetabular impingement is a common clinical entity that affects both males and females. However, sexual dimorphism in FAI incidence, presentation, treatment, and outcomes has recently been described in the literature (Table). Being aware of these sex-based differences and tailoring patient evaluation and management accordingly will likely result in optimal outcomes for each person who presents with symptomatic FAI.
1. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop. 2003;(417):112-120.
2. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
3. Philippon MJ, Maxwell RB, Johnston TL, Schenker M, Briggs KK. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):1041-1047.
4. Laborie LB, Lehmann TG, Engesaeter IO, Eastwood DM, Engesaeter LB, Rosendahl K. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology. 2011;260(2):494-502.
5. Clohisy JC, St John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clin Orthop. 2010;468(2):555-564.
6. Beaulé PE, Zaragoza E, Motamedi K, Copelan N, Dorey FJ. Three-dimensional computed tomography of the hip in the assessment of femoroacetabular impingement. J Orthop Res. 2005;23(6):1286-1292.
7. Hetsroni I, Dela Torre K, Duke G, Lyman S, Kelly BT. Sex differences of hip morphology in young adults with hip pain and labral tears. Arthroscopy. 2013;29(1):54-63.
8. Klingenstein GG, Zbeda RM, Bedi A, Magennis E, Kelly BT. Prevalence and preoperative demographic and radiographic predictors of bilateral femoroacetabular impingement. Am J Sports Med. 2013;41(4):762-768.
9. Malviya A, Stafford GH, Villar RN. Impact of arthroscopy of the hip for femoroacetabular impingement on quality of life at a mean follow-up of 3.2 years. J Bone Joint Surg Br. 2012;94(4):466-470.
10. Ng VY, Arora N, Best TM, Pan X, Ellis TJ. Efficacy of surgery for femoroacetabular impingement: a systematic review. Am J Sports Med. 2010;38(11):2337-2345.
11. Matsuda DK, Carlisle JC, Arthurs SC, Wierks CH, Philippon MJ. Comparative systematic review of the open dislocation, mini-open, and arthroscopic surgeries for femoroacetabular impingement. Arthroscopy. 2011;27(2):252-269.
12. Clohisy JC, Baca G, Beaule PE, et al. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
13. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
Femoroacetabular impingement (FAI), a recently described hip condition in adolescents and young adults, results from abnormal physical contact between the proximal femur and the acetabulum.1 FAI is usually characterized by the site of the predominant morphologic abnormality—proximal femur (cam-type FAI), acetabulum (pincer-type FAI), or both (mixed impingement). Cam-type FAI is typified by the aspherical extension of the articular surface at the anterosuperior head–neck junction of the proximal femur with loss of the normal offset. With hip motion, especially in the maximal ranges of flexion and internal rotation, the aspherical proximal femur repeatedly contacts the anterosuperior acetabulum, damaging the chondrolabral junction and ultimately the labrum itself. In pincer-type impingement, femoral head overcoverage caused by acetabular retroversion and/or coxa profunda directly damages the anterior labrum when the acetabular rim contacts the proximal femur during physiologic motion. “Contrecoup” injury of the posterior-inferior acetabular cartilage may also occur. Over time, recurrent microtrauma to the acetabular cartilage and/or labrum may lead to degenerative changes of the hip and ultimately to premature osteoarthritis.1,2
Patients with FAI typically present with groin pain that may be activity-related or that may occur with prolonged sitting with the hip in a flexed position. Physical examination findings suggestive of FAI include decreased passive internal hip rotation and reproducible pain with adduction and internal rotation of the flexed hip—the impingement sign, or the flexion, adduction, and internal rotation (FADIR) test.3 Diagnostic imaging evaluation initially includes radiographs of the pelvis and hips. These radiographs may show a “pistol-grip” deformity and/or decreased head–neck offset (as determined by increased alpha angle) in the setting of cam-type impingement (Figure 1).4 Pincer-type impingement may be associated with a crossover sign, coxa profunda, and an increased center-edge angle (CEA). Advanced imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI) arthrogram, and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), are commonly used to better delineate bony deformity and concomitant injuries of the labrum and cartilage (Figure 2).
Treatment for FAI often consists initially of activity modification, use of anti-inflammatory medications, and physical therapy. Intra-articular corticosteroid injections may be used both diagnostically and therapeutically. When nonsurgical measures fail to adequately relieve symptoms, surgery may be warranted. Whether performed open or arthroscopically, surgery is directed first at correcting the underlying osseous abnormality—performing an osteoplasty of the proximal femur to remove the cam lesion, performing an acetabular osteoplasty (“rim-trimming”) to address a focal pincer lesion, and/or performing a periacetabular osteotomy to decrease global acetabular overcoverage (Figure 3).5
Sex-Based Differences in FAI Incidence
Traditionally, it was thought that cam-type impingement occurred predominantly in young, athletic males, whereas pincer-type impingement resulting from acetabular overcoverage occurred primarily in females during their fourth decade. However, our understanding of the sex-based differences in the incidence and presentation of FAI has evolved, and it is now clear that the interplay of sex, radiographic signs of impingement, and development of symptoms requiring treatment is more complex.
In recent large population-based studies, investigators have attempted to better characterize the sex-based differences in the incidence of osseous FAI deformity. Gosvig and colleagues2 examined radiographic and questionnaire outcomes of 3620 patients (age range, 21-90 years) and found that males were more likely than females to have a pistol-grip deformity of the hip (19.6% vs 5.2%); that deep acetabular sockets were common in both sexes (15.2% vs 19.4%); and that the presence of pistol-grip deformity or deep socket was significantly associated with development of osteoarthritis, independent of sex.
In a study of 2081 asymptomatic patients (mean age, 18.6 years), Laborie and colleagues4 reported similar radiographic findings. Males were significantly more likely than females to have a cam-type deformity, as evidenced by pistol-grip deformity, focal prominence of the femoral neck, and/or flattening of the lateral aspect of the femoral head. Males were also more likely than females to have a pincer deformity, though radiographic signs of pincer deformity—a crossover sign, excessive acetabular coverage (defined by increased CEA), and a posterior wall sign—were common in both sexes, occurring in 16.6% of females and 34.3% of males. Bilateral findings of FAI-associated deformity were also more common in males than in females, both for cam-type deformity (24.7% vs 6.3%) and pincer-type deformity (21.7% vs 9.7%).
Sex-Based Differences in FAI Presentation
In males and females, the clinical presentation of FAI is similar—insidious onset of deep groin pain, often exacerbated with activity, and physical examination findings of decreased hip motion (particularly internal rotation) and a positive impingement test.3 Nevertheless, the sexes’ clinical presentation differs in several ways. Specifically, in a study using 3-dimensional CT to assess bony deformity in both symptomatic and asymptomatic patients, Beaulé and colleagues6 reported that alpha angles were significantly higher in symptomatic males than in symptomatic females (73.3° vs 58.7°). Hetsroni and colleagues7 recently reported similar results in a study of 217 symptomatic young adults treated arthroscopically for hip pain. Preoperative CT showed that alpha angles were significantly larger in males than in females (63.6° vs 47.8°). The authors postulated that females may be more likely to be symptomatic in the setting of smaller cam lesions because of the increased peak hip flexion and frontal plane motion commonly demonstrated by females during drop landings in sport. The authors further hypothesized that sex differences in muscle mass (which contributes to dynamic hip stability) and ligamentous laxity (a component of static hip stability) may result in larger physiologic ranges of motion for many females. As a result, bony impingement may occur in the setting of smaller anatomical lesions in females. The authors further noted that, compared with their male counterparts, females being treated for symptomatic FAI had significantly more femoral and acetabular anteversion.
Another male–female presentation difference involves symptom bilaterality. Specifically, males are significantly more likely than females to have symptomatic FAI involving both hips. In a recent study of 646 patients who underwent hip arthroscopy for symptomatic FAI during a 2-year period, Klingenstein and colleagues8 found that females constituted 48.2% of unilateral arthroscopy patients but only 34.8% of bilateral arthroscopy patients. The odds ratio of males treated for both hips, compared with females, was 1.7 (95% confidence interval, 1.16–2.54).
Last, it has been reported that, on clinical presentation, hip function scores are significantly lower in females than in males. In a recent study of 612 cases of symptomatic FAI treated with hip arthroscopy, Malviya and colleagues9 found that females had significantly lower quality-of-life scores both before and after surgery. Hetsroni and colleagues7 reported similar findings, with females having significantly lower preoperative modified Harris Hip Scores and lower Hip Outcome Scores in the domains of Activities of Daily Living and Sports.
Sex-Based Differences in FAI Treatment
and Outcomes
Surgical treatment of FAI is focused on identifying the source of hip pain and dysfunction—be it osseous lesion, labral tearing, chondral injury, or iliopsoas tendonitis—and treating it accordingly, regardless of sex. Most studies of this approach find consistent improvement in the short-term and midterm outcome scores for a majority of patients. However, relatively few studies have focused specifically on sex in determining the percentage of patients who require surgical treatment, in deciding the type of surgery that should be performed, or in measuring surgical outcomes in patients with symptomatic FAI.
In their review of 23 studies of FAI surgery, Ng and colleagues10 found that, of 970 patients, 608 (62.7%) were male and 362 (37.3%) were female. Similarly higher rates for males were previously published.5,11 More recently, Clohisy and colleagues12 reported on the descriptive epidemiology of patients having surgery for FAI at 8 different medical centers in North America. Fifty-five percent of the hips surgically treated for symptomatic FAI were females’. The authors speculated that this unexpectedly high rate could have resulted from US and Canadian female athletes’ increasingly higher level of sports participation. The results of this study, one of the largest examining the rate of surgery for males and females with FAI, suggest that females are more likely to have surgery for symptomatic FAI despite being less likely to have radiographic evidence of impingement. Our understanding of this phenomenon continues to advance.
In a recent prospective study, Krych and colleagues13 evaluated the clinical outcomes of FAI surgeries (labral débridement, labral repair) in an all-female patient cohort. Female patients with symptomatic FAI were randomized to undergo either labral débridement or labral repair. There were clinical improvements in both groups, but, compared with labral débridement patients, labral repair patients had more significantly improved Hip Outcome Scores in the domains of Activities of Daily Living and Sports, as well as better subjective outcomes. Although the study did not compare female patients with male patients, it does provide evidence that female patients specifically may benefit more from labral repair than from labral débridement alone.
With respect to different surgical treatments for male and female patients, Hetsroni and colleagues7 introduced the idea of sex-specific treatment when they noted more hip anteversion in their study’s female patients than in its male patients. They suggested that, because the anterosuperior acetabulum is subjected to a high amount of stress during weight-bearing and gait, this area in females with suspected pincer lesions should be rim-trimmed judiciously to avoid increasing the stress and perhaps even hastening the development of degenerative disease. Last, though several authors have noted that hip function scores are lower in females than in males on presentation, it has also been reported that females demonstrate more improvement in functional scores after surgery.9 This may be important information to discuss during preoperative counseling about expected goals and outcomes.
Conclusion
Femoroacetabular impingement is a common clinical entity that affects both males and females. However, sexual dimorphism in FAI incidence, presentation, treatment, and outcomes has recently been described in the literature (Table). Being aware of these sex-based differences and tailoring patient evaluation and management accordingly will likely result in optimal outcomes for each person who presents with symptomatic FAI.
Femoroacetabular impingement (FAI), a recently described hip condition in adolescents and young adults, results from abnormal physical contact between the proximal femur and the acetabulum.1 FAI is usually characterized by the site of the predominant morphologic abnormality—proximal femur (cam-type FAI), acetabulum (pincer-type FAI), or both (mixed impingement). Cam-type FAI is typified by the aspherical extension of the articular surface at the anterosuperior head–neck junction of the proximal femur with loss of the normal offset. With hip motion, especially in the maximal ranges of flexion and internal rotation, the aspherical proximal femur repeatedly contacts the anterosuperior acetabulum, damaging the chondrolabral junction and ultimately the labrum itself. In pincer-type impingement, femoral head overcoverage caused by acetabular retroversion and/or coxa profunda directly damages the anterior labrum when the acetabular rim contacts the proximal femur during physiologic motion. “Contrecoup” injury of the posterior-inferior acetabular cartilage may also occur. Over time, recurrent microtrauma to the acetabular cartilage and/or labrum may lead to degenerative changes of the hip and ultimately to premature osteoarthritis.1,2
Patients with FAI typically present with groin pain that may be activity-related or that may occur with prolonged sitting with the hip in a flexed position. Physical examination findings suggestive of FAI include decreased passive internal hip rotation and reproducible pain with adduction and internal rotation of the flexed hip—the impingement sign, or the flexion, adduction, and internal rotation (FADIR) test.3 Diagnostic imaging evaluation initially includes radiographs of the pelvis and hips. These radiographs may show a “pistol-grip” deformity and/or decreased head–neck offset (as determined by increased alpha angle) in the setting of cam-type impingement (Figure 1).4 Pincer-type impingement may be associated with a crossover sign, coxa profunda, and an increased center-edge angle (CEA). Advanced imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI) arthrogram, and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), are commonly used to better delineate bony deformity and concomitant injuries of the labrum and cartilage (Figure 2).
Treatment for FAI often consists initially of activity modification, use of anti-inflammatory medications, and physical therapy. Intra-articular corticosteroid injections may be used both diagnostically and therapeutically. When nonsurgical measures fail to adequately relieve symptoms, surgery may be warranted. Whether performed open or arthroscopically, surgery is directed first at correcting the underlying osseous abnormality—performing an osteoplasty of the proximal femur to remove the cam lesion, performing an acetabular osteoplasty (“rim-trimming”) to address a focal pincer lesion, and/or performing a periacetabular osteotomy to decrease global acetabular overcoverage (Figure 3).5
Sex-Based Differences in FAI Incidence
Traditionally, it was thought that cam-type impingement occurred predominantly in young, athletic males, whereas pincer-type impingement resulting from acetabular overcoverage occurred primarily in females during their fourth decade. However, our understanding of the sex-based differences in the incidence and presentation of FAI has evolved, and it is now clear that the interplay of sex, radiographic signs of impingement, and development of symptoms requiring treatment is more complex.
In recent large population-based studies, investigators have attempted to better characterize the sex-based differences in the incidence of osseous FAI deformity. Gosvig and colleagues2 examined radiographic and questionnaire outcomes of 3620 patients (age range, 21-90 years) and found that males were more likely than females to have a pistol-grip deformity of the hip (19.6% vs 5.2%); that deep acetabular sockets were common in both sexes (15.2% vs 19.4%); and that the presence of pistol-grip deformity or deep socket was significantly associated with development of osteoarthritis, independent of sex.
In a study of 2081 asymptomatic patients (mean age, 18.6 years), Laborie and colleagues4 reported similar radiographic findings. Males were significantly more likely than females to have a cam-type deformity, as evidenced by pistol-grip deformity, focal prominence of the femoral neck, and/or flattening of the lateral aspect of the femoral head. Males were also more likely than females to have a pincer deformity, though radiographic signs of pincer deformity—a crossover sign, excessive acetabular coverage (defined by increased CEA), and a posterior wall sign—were common in both sexes, occurring in 16.6% of females and 34.3% of males. Bilateral findings of FAI-associated deformity were also more common in males than in females, both for cam-type deformity (24.7% vs 6.3%) and pincer-type deformity (21.7% vs 9.7%).
Sex-Based Differences in FAI Presentation
In males and females, the clinical presentation of FAI is similar—insidious onset of deep groin pain, often exacerbated with activity, and physical examination findings of decreased hip motion (particularly internal rotation) and a positive impingement test.3 Nevertheless, the sexes’ clinical presentation differs in several ways. Specifically, in a study using 3-dimensional CT to assess bony deformity in both symptomatic and asymptomatic patients, Beaulé and colleagues6 reported that alpha angles were significantly higher in symptomatic males than in symptomatic females (73.3° vs 58.7°). Hetsroni and colleagues7 recently reported similar results in a study of 217 symptomatic young adults treated arthroscopically for hip pain. Preoperative CT showed that alpha angles were significantly larger in males than in females (63.6° vs 47.8°). The authors postulated that females may be more likely to be symptomatic in the setting of smaller cam lesions because of the increased peak hip flexion and frontal plane motion commonly demonstrated by females during drop landings in sport. The authors further hypothesized that sex differences in muscle mass (which contributes to dynamic hip stability) and ligamentous laxity (a component of static hip stability) may result in larger physiologic ranges of motion for many females. As a result, bony impingement may occur in the setting of smaller anatomical lesions in females. The authors further noted that, compared with their male counterparts, females being treated for symptomatic FAI had significantly more femoral and acetabular anteversion.
Another male–female presentation difference involves symptom bilaterality. Specifically, males are significantly more likely than females to have symptomatic FAI involving both hips. In a recent study of 646 patients who underwent hip arthroscopy for symptomatic FAI during a 2-year period, Klingenstein and colleagues8 found that females constituted 48.2% of unilateral arthroscopy patients but only 34.8% of bilateral arthroscopy patients. The odds ratio of males treated for both hips, compared with females, was 1.7 (95% confidence interval, 1.16–2.54).
Last, it has been reported that, on clinical presentation, hip function scores are significantly lower in females than in males. In a recent study of 612 cases of symptomatic FAI treated with hip arthroscopy, Malviya and colleagues9 found that females had significantly lower quality-of-life scores both before and after surgery. Hetsroni and colleagues7 reported similar findings, with females having significantly lower preoperative modified Harris Hip Scores and lower Hip Outcome Scores in the domains of Activities of Daily Living and Sports.
Sex-Based Differences in FAI Treatment
and Outcomes
Surgical treatment of FAI is focused on identifying the source of hip pain and dysfunction—be it osseous lesion, labral tearing, chondral injury, or iliopsoas tendonitis—and treating it accordingly, regardless of sex. Most studies of this approach find consistent improvement in the short-term and midterm outcome scores for a majority of patients. However, relatively few studies have focused specifically on sex in determining the percentage of patients who require surgical treatment, in deciding the type of surgery that should be performed, or in measuring surgical outcomes in patients with symptomatic FAI.
In their review of 23 studies of FAI surgery, Ng and colleagues10 found that, of 970 patients, 608 (62.7%) were male and 362 (37.3%) were female. Similarly higher rates for males were previously published.5,11 More recently, Clohisy and colleagues12 reported on the descriptive epidemiology of patients having surgery for FAI at 8 different medical centers in North America. Fifty-five percent of the hips surgically treated for symptomatic FAI were females’. The authors speculated that this unexpectedly high rate could have resulted from US and Canadian female athletes’ increasingly higher level of sports participation. The results of this study, one of the largest examining the rate of surgery for males and females with FAI, suggest that females are more likely to have surgery for symptomatic FAI despite being less likely to have radiographic evidence of impingement. Our understanding of this phenomenon continues to advance.
In a recent prospective study, Krych and colleagues13 evaluated the clinical outcomes of FAI surgeries (labral débridement, labral repair) in an all-female patient cohort. Female patients with symptomatic FAI were randomized to undergo either labral débridement or labral repair. There were clinical improvements in both groups, but, compared with labral débridement patients, labral repair patients had more significantly improved Hip Outcome Scores in the domains of Activities of Daily Living and Sports, as well as better subjective outcomes. Although the study did not compare female patients with male patients, it does provide evidence that female patients specifically may benefit more from labral repair than from labral débridement alone.
With respect to different surgical treatments for male and female patients, Hetsroni and colleagues7 introduced the idea of sex-specific treatment when they noted more hip anteversion in their study’s female patients than in its male patients. They suggested that, because the anterosuperior acetabulum is subjected to a high amount of stress during weight-bearing and gait, this area in females with suspected pincer lesions should be rim-trimmed judiciously to avoid increasing the stress and perhaps even hastening the development of degenerative disease. Last, though several authors have noted that hip function scores are lower in females than in males on presentation, it has also been reported that females demonstrate more improvement in functional scores after surgery.9 This may be important information to discuss during preoperative counseling about expected goals and outcomes.
Conclusion
Femoroacetabular impingement is a common clinical entity that affects both males and females. However, sexual dimorphism in FAI incidence, presentation, treatment, and outcomes has recently been described in the literature (Table). Being aware of these sex-based differences and tailoring patient evaluation and management accordingly will likely result in optimal outcomes for each person who presents with symptomatic FAI.
1. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop. 2003;(417):112-120.
2. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
3. Philippon MJ, Maxwell RB, Johnston TL, Schenker M, Briggs KK. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):1041-1047.
4. Laborie LB, Lehmann TG, Engesaeter IO, Eastwood DM, Engesaeter LB, Rosendahl K. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology. 2011;260(2):494-502.
5. Clohisy JC, St John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clin Orthop. 2010;468(2):555-564.
6. Beaulé PE, Zaragoza E, Motamedi K, Copelan N, Dorey FJ. Three-dimensional computed tomography of the hip in the assessment of femoroacetabular impingement. J Orthop Res. 2005;23(6):1286-1292.
7. Hetsroni I, Dela Torre K, Duke G, Lyman S, Kelly BT. Sex differences of hip morphology in young adults with hip pain and labral tears. Arthroscopy. 2013;29(1):54-63.
8. Klingenstein GG, Zbeda RM, Bedi A, Magennis E, Kelly BT. Prevalence and preoperative demographic and radiographic predictors of bilateral femoroacetabular impingement. Am J Sports Med. 2013;41(4):762-768.
9. Malviya A, Stafford GH, Villar RN. Impact of arthroscopy of the hip for femoroacetabular impingement on quality of life at a mean follow-up of 3.2 years. J Bone Joint Surg Br. 2012;94(4):466-470.
10. Ng VY, Arora N, Best TM, Pan X, Ellis TJ. Efficacy of surgery for femoroacetabular impingement: a systematic review. Am J Sports Med. 2010;38(11):2337-2345.
11. Matsuda DK, Carlisle JC, Arthurs SC, Wierks CH, Philippon MJ. Comparative systematic review of the open dislocation, mini-open, and arthroscopic surgeries for femoroacetabular impingement. Arthroscopy. 2011;27(2):252-269.
12. Clohisy JC, Baca G, Beaule PE, et al. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
13. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
1. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop. 2003;(417):112-120.
2. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
3. Philippon MJ, Maxwell RB, Johnston TL, Schenker M, Briggs KK. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):1041-1047.
4. Laborie LB, Lehmann TG, Engesaeter IO, Eastwood DM, Engesaeter LB, Rosendahl K. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology. 2011;260(2):494-502.
5. Clohisy JC, St John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clin Orthop. 2010;468(2):555-564.
6. Beaulé PE, Zaragoza E, Motamedi K, Copelan N, Dorey FJ. Three-dimensional computed tomography of the hip in the assessment of femoroacetabular impingement. J Orthop Res. 2005;23(6):1286-1292.
7. Hetsroni I, Dela Torre K, Duke G, Lyman S, Kelly BT. Sex differences of hip morphology in young adults with hip pain and labral tears. Arthroscopy. 2013;29(1):54-63.
8. Klingenstein GG, Zbeda RM, Bedi A, Magennis E, Kelly BT. Prevalence and preoperative demographic and radiographic predictors of bilateral femoroacetabular impingement. Am J Sports Med. 2013;41(4):762-768.
9. Malviya A, Stafford GH, Villar RN. Impact of arthroscopy of the hip for femoroacetabular impingement on quality of life at a mean follow-up of 3.2 years. J Bone Joint Surg Br. 2012;94(4):466-470.
10. Ng VY, Arora N, Best TM, Pan X, Ellis TJ. Efficacy of surgery for femoroacetabular impingement: a systematic review. Am J Sports Med. 2010;38(11):2337-2345.
11. Matsuda DK, Carlisle JC, Arthurs SC, Wierks CH, Philippon MJ. Comparative systematic review of the open dislocation, mini-open, and arthroscopic surgeries for femoroacetabular impingement. Arthroscopy. 2011;27(2):252-269.
12. Clohisy JC, Baca G, Beaule PE, et al. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
13. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
The Value of National and Hospital Registries
Following Dr. Sarmiento’s commentary, “Orthopedic Registries: Second Thoughts,” we agree that it is important and appropriate to question the value of any new additions to the orthopedic field, and registries are no exception. We thank Dr. Sarmiento for his comments on the viability of registries and the need for continued critical evaluation. Before joint registries, however, we had to rely on small-cohort analyses to assess outcomes and complications. Now, national and hospital registries, specifically joint registries, may be an invaluable source of information for orthopedic surgeons, patients, health care administrators, regulators, and implant suppliers.1,2
Contrary to Dr. Sarmiento’s belief that registry data results are likely to have been reported in the literature, it is difficult to refute the value of recent years’ registry data in helping surgeons shape their practice. For example, according to Lewallen and Etkin,3 the National Joint Registry of England and Wales information has provided orthopedic surgeons with crucial findings regarding the outcomes of metal-on-metal hip arthroplasties. Using the England and Wales registry data from more than 400,000 primary total hip arthroplasties, Smith and colleagues4 noted that metal-on-metal stemmed articulations led to poor implant survival, particularly in young women with large-diameter heads, and indicated these articulations should not be used. Australian registry data on metal-on-metal devices and reports of failure rates up to 11%5 led one manufacturer to recall its implants.6 In addition, the Norwegian Arthroplasty Register evaluated survival rates and reasons for revision for 7 types of cemented primary total knee arthroplasty (TKA) between 1994 and 2009.7 Data on more than 17,000 primary TKAs allowed Plate and colleagues8 to confidently determine that aseptic loosening was related to certain TKA designs. Using registry information, they identified patients at risk for dislocation in total hip arthroplasty and concluded that large-diameter femoral head articulations could reduce dislocation rates.
Obtaining such large cohorts of patients in individual studies is not only difficult but highly unlikely. Unlike registry data, these studies are often impractical in evaluating factors of low incidence, such as revision rates, as it is often difficult to find significant differences in small populations.9 Furthermore, these controlled trials homogenize patients—using exclusion and inclusion criteria to eliminate potential confounders—and thus poorly represent the heterogeneity of a typical hospital’s patient population.10 Although the literature may indeed have alluded to such complications, only a database as extensive as a registry can allow us to fully comprehend the outcomes of particular implants and devices.
Dr. Sarmiento points to the AO Swiss Fracture Registry as being of little benefit and raises the concern that the American Joint Replacement Registry (AJRR) may follow with the same results. However, realizing a registry’s benefits may take time and the gradual accumulation of data. Supporting this, Hübschle and colleagues11 recently used AO Swiss Fracture Registry data to validate use of balloon kyphoplasty for vertebral compression fractures and concluded that the technique is safe and effective in reducing pain—thus possibly providing the federal office with the evidence needed for reimbursement for this intervention. Therefore, this registry is now providing useful information.
We can never truly know the veracity of participating surgeons, but it is naïve to assume that this issue arises only vis-à-vis registries. If we were to debate the ethical and professional standards of colleagues in our field, such questions could extend to all studies performed, even peer-reviewed studies. Therefore, we do not think this is reason to exclude the patient data and outcomes found in registries. We must emphasize that ultimately registry data are often most useful in highlighting trends and determining triggers for further study rather than in arriving at conclusions.1 In particular, registry data may be used in cohort studies that evaluate the risk factors for and incidence of certain outcomes. Focused higher-level interventional studies can then follow the trends observed.1 However, registry data are also valuable on their own, when higher-level, randomized controlled trials may be impractical or unethical.12
Dr. Sarmiento refers to corrupt relationships between companies and orthopedists as “representing a widespread loss of professionalism in our ranks.” Despite a US Justice Department investigation into these relationships, only a few doctors were found to have had inappropriate relationships.13 In addition, the investigation and prosecution of companies led to an agreement requiring federal monitoring and new corporate compliance procedures, which should ensure stricter adherence to regulations.14 We do not believe this should undermine the value of registries and the work that has been contributed by thousands of surgeons hoping to improve the field of orthopedics. In addition, concerns about the influence of well-known individuals may be better directed at individual institution–based research, particularly as these specific authors also often have conflicts of interest that may skew the presentation of results. The strength of registry data is in providing collective data and large samples from a multitude of surgeons rather than from just high-volume surgeons, and therefore registry data provide a better overall picture of patients and their procedures.15 Furthermore, trends observed in national registries in countries such as New Zealand16 may aid in effectively reducing the revision rate, possibly up to 10%.17 If a US national joint registry is marginally as effective, then we may see considerable savings for our health care services.17,18
We wholeheartedly agree that a yearly review of registries may be constructive. Dr. Sarmiento suggests an annual publication summarizing peer-reviewed articles and the opportunity for orthopedists to decide for themselves what treatments to choose based on reports from independent investigators. Although this sounds feasible, it would be difficult to decide which articles should be selected as pertinent for this type of publication. Any selection would be biased, and not all studies with high-level evidence are necessarily important or relevant. Therefore, selecting what is most appropriate to cite is not without its difficulties. We appreciate that there are problems in standardizing data reporting among registries. However, to improve interregistry collaboration, the US Food and Drug Administration is sponsoring the International Consortium of Orthopaedic Registries (ICOR) to facilitate data presentation.19 ICOR aims to increase cooperation, standardize analyses, and improve reporting, which will only strengthen the data available to us. Such efforts will ultimately enhance coordination and international collaboration among registries.15 In addition, incorporating patient-reported outcomes into our national registry will aid in quantifying arthroplasty outcomes from the patient’s perspective and will continue to improve total joint arthroplasties.20
Overall, this debate is useful and highly relevant in highlighting potential issues with registries. Although registries are not without their flaws, like all aspects of orthopedics they are ever evolving, and they must be continually modified and improved. However, disregard for the potential value of AJRR, which has benefits for orthopedists and patients alike, is premature. Once again, we thank Dr. Sarmiento for starting this discussion, which will allow us to continue to evaluate and improve our registries.
1. Konan S, Haddad FS. Joint registries: a Ptolemaic model of data interpretation? Bone Joint J Br. 2013;95(12):1585-1586.
2. Banerjee S, Cafri G, Isaacs AJ, et al. A distributed health data network analysis of survival outcomes: the International Consortium of Orthopaedic Registries perspective. J Bone Joint Surg Am. 2014;96(suppl 1):7-11.
3. Lewallen DG, Etkin CD. The need for a national total joint registry. Orthop Nurs. 2013;32(1):4-5.
4. Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW; National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379(9822):1199-1204.
5. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
6. Hug KT, Watters TS, Vail TP, Bolognesi MP. The withdrawn ASR™ THA and hip resurfacing systems: how have our patients fared over 1 to 6 years? Clin Orthop. 2013;471(2):430-438.
7. Gøthesen O, Espehaug B, Havelin L, et al. Survival rates and causes of revision in cemented primary total knee replacement: a report from the Norwegian Arthroplasty Register 1994–2009. Bone Joint J Br. 2013;95(5):636-642.
8. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5:553.
9. Daruwalla ZJ, Wong KL, Pillay KR, Leong KM, Murphy DP. Does ageing Singapore need an electronic database of hip fracture patients? The value and role of a national joint registry and an electronic database of intertrochanteric and femoral neck fractures. Singapore Med J. 2014;55(5):287-288.
10. Rasmussen JV, Olsen BS, Fevang BT, et al. A review of national shoulder and elbow joint replacement registries. J Shoulder Elbow Surg. 2012;21(10):1328-1335.
11. Hübschle L, Borgström F, Olafsson G, et al. Real-life results of balloon kyphoplasty for vertebral compression fractures from the SWISSspine registry. Spine J. 2014;14(9):2063-2077.
12. Ahn H, Court-Brown CM, McQueen MM, Schemitsch EH. The use of hospital registries in orthopaedic surgery. J Bone Joint Surg Am. 2009;91(suppl 3):68-72.
13. Youngstrom N. Swept up in major medical device case, physician pays $650,000 to settle kickback charges. AIS Health Business Daily. May 3, 2010.
14. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed February 19, 2015.
15. Namba RS, Inacio MC, Paxton EW, Robertsson O, Graves SE. The role of registry data in the evaluation of mobile-bearing total knee arthroplasty. J Bone Joint Surg Am. 2011;93(suppl 3):48-50.
16. Insull PJ, Cobbett H, Frampton CM, Munro JT. The use of a lipped acetabular liner decreases the rate of revision for instability after total hip replacement: a study using data from the New Zealand Joint Registry. Bone Joint J Br. 2014;96(7):884-888.
17. Rankin EA. AJRR: becoming a national US joint registry. Orthopedics. 2013;36(3):175-176.
18. American Joint Replacement Registry website. https://teamwork.aaos.org/ajrr/SitePages/About%20Us.aspx. Accessed February 19, 2015.
19. International Consortium of Orthopaedic Registries website. http://www.icor-initiative.org. Accessed February 19, 2015.
20. Franklin PD, Harrold L, Ayers DC. Incorporating patient-reported outcomes in total joint arthroplasty registries: challenges and opportunities. Clin Orthop. 2013;471(11):3482-3488.
Following Dr. Sarmiento’s commentary, “Orthopedic Registries: Second Thoughts,” we agree that it is important and appropriate to question the value of any new additions to the orthopedic field, and registries are no exception. We thank Dr. Sarmiento for his comments on the viability of registries and the need for continued critical evaluation. Before joint registries, however, we had to rely on small-cohort analyses to assess outcomes and complications. Now, national and hospital registries, specifically joint registries, may be an invaluable source of information for orthopedic surgeons, patients, health care administrators, regulators, and implant suppliers.1,2
Contrary to Dr. Sarmiento’s belief that registry data results are likely to have been reported in the literature, it is difficult to refute the value of recent years’ registry data in helping surgeons shape their practice. For example, according to Lewallen and Etkin,3 the National Joint Registry of England and Wales information has provided orthopedic surgeons with crucial findings regarding the outcomes of metal-on-metal hip arthroplasties. Using the England and Wales registry data from more than 400,000 primary total hip arthroplasties, Smith and colleagues4 noted that metal-on-metal stemmed articulations led to poor implant survival, particularly in young women with large-diameter heads, and indicated these articulations should not be used. Australian registry data on metal-on-metal devices and reports of failure rates up to 11%5 led one manufacturer to recall its implants.6 In addition, the Norwegian Arthroplasty Register evaluated survival rates and reasons for revision for 7 types of cemented primary total knee arthroplasty (TKA) between 1994 and 2009.7 Data on more than 17,000 primary TKAs allowed Plate and colleagues8 to confidently determine that aseptic loosening was related to certain TKA designs. Using registry information, they identified patients at risk for dislocation in total hip arthroplasty and concluded that large-diameter femoral head articulations could reduce dislocation rates.
Obtaining such large cohorts of patients in individual studies is not only difficult but highly unlikely. Unlike registry data, these studies are often impractical in evaluating factors of low incidence, such as revision rates, as it is often difficult to find significant differences in small populations.9 Furthermore, these controlled trials homogenize patients—using exclusion and inclusion criteria to eliminate potential confounders—and thus poorly represent the heterogeneity of a typical hospital’s patient population.10 Although the literature may indeed have alluded to such complications, only a database as extensive as a registry can allow us to fully comprehend the outcomes of particular implants and devices.
Dr. Sarmiento points to the AO Swiss Fracture Registry as being of little benefit and raises the concern that the American Joint Replacement Registry (AJRR) may follow with the same results. However, realizing a registry’s benefits may take time and the gradual accumulation of data. Supporting this, Hübschle and colleagues11 recently used AO Swiss Fracture Registry data to validate use of balloon kyphoplasty for vertebral compression fractures and concluded that the technique is safe and effective in reducing pain—thus possibly providing the federal office with the evidence needed for reimbursement for this intervention. Therefore, this registry is now providing useful information.
We can never truly know the veracity of participating surgeons, but it is naïve to assume that this issue arises only vis-à-vis registries. If we were to debate the ethical and professional standards of colleagues in our field, such questions could extend to all studies performed, even peer-reviewed studies. Therefore, we do not think this is reason to exclude the patient data and outcomes found in registries. We must emphasize that ultimately registry data are often most useful in highlighting trends and determining triggers for further study rather than in arriving at conclusions.1 In particular, registry data may be used in cohort studies that evaluate the risk factors for and incidence of certain outcomes. Focused higher-level interventional studies can then follow the trends observed.1 However, registry data are also valuable on their own, when higher-level, randomized controlled trials may be impractical or unethical.12
Dr. Sarmiento refers to corrupt relationships between companies and orthopedists as “representing a widespread loss of professionalism in our ranks.” Despite a US Justice Department investigation into these relationships, only a few doctors were found to have had inappropriate relationships.13 In addition, the investigation and prosecution of companies led to an agreement requiring federal monitoring and new corporate compliance procedures, which should ensure stricter adherence to regulations.14 We do not believe this should undermine the value of registries and the work that has been contributed by thousands of surgeons hoping to improve the field of orthopedics. In addition, concerns about the influence of well-known individuals may be better directed at individual institution–based research, particularly as these specific authors also often have conflicts of interest that may skew the presentation of results. The strength of registry data is in providing collective data and large samples from a multitude of surgeons rather than from just high-volume surgeons, and therefore registry data provide a better overall picture of patients and their procedures.15 Furthermore, trends observed in national registries in countries such as New Zealand16 may aid in effectively reducing the revision rate, possibly up to 10%.17 If a US national joint registry is marginally as effective, then we may see considerable savings for our health care services.17,18
We wholeheartedly agree that a yearly review of registries may be constructive. Dr. Sarmiento suggests an annual publication summarizing peer-reviewed articles and the opportunity for orthopedists to decide for themselves what treatments to choose based on reports from independent investigators. Although this sounds feasible, it would be difficult to decide which articles should be selected as pertinent for this type of publication. Any selection would be biased, and not all studies with high-level evidence are necessarily important or relevant. Therefore, selecting what is most appropriate to cite is not without its difficulties. We appreciate that there are problems in standardizing data reporting among registries. However, to improve interregistry collaboration, the US Food and Drug Administration is sponsoring the International Consortium of Orthopaedic Registries (ICOR) to facilitate data presentation.19 ICOR aims to increase cooperation, standardize analyses, and improve reporting, which will only strengthen the data available to us. Such efforts will ultimately enhance coordination and international collaboration among registries.15 In addition, incorporating patient-reported outcomes into our national registry will aid in quantifying arthroplasty outcomes from the patient’s perspective and will continue to improve total joint arthroplasties.20
Overall, this debate is useful and highly relevant in highlighting potential issues with registries. Although registries are not without their flaws, like all aspects of orthopedics they are ever evolving, and they must be continually modified and improved. However, disregard for the potential value of AJRR, which has benefits for orthopedists and patients alike, is premature. Once again, we thank Dr. Sarmiento for starting this discussion, which will allow us to continue to evaluate and improve our registries.
Following Dr. Sarmiento’s commentary, “Orthopedic Registries: Second Thoughts,” we agree that it is important and appropriate to question the value of any new additions to the orthopedic field, and registries are no exception. We thank Dr. Sarmiento for his comments on the viability of registries and the need for continued critical evaluation. Before joint registries, however, we had to rely on small-cohort analyses to assess outcomes and complications. Now, national and hospital registries, specifically joint registries, may be an invaluable source of information for orthopedic surgeons, patients, health care administrators, regulators, and implant suppliers.1,2
Contrary to Dr. Sarmiento’s belief that registry data results are likely to have been reported in the literature, it is difficult to refute the value of recent years’ registry data in helping surgeons shape their practice. For example, according to Lewallen and Etkin,3 the National Joint Registry of England and Wales information has provided orthopedic surgeons with crucial findings regarding the outcomes of metal-on-metal hip arthroplasties. Using the England and Wales registry data from more than 400,000 primary total hip arthroplasties, Smith and colleagues4 noted that metal-on-metal stemmed articulations led to poor implant survival, particularly in young women with large-diameter heads, and indicated these articulations should not be used. Australian registry data on metal-on-metal devices and reports of failure rates up to 11%5 led one manufacturer to recall its implants.6 In addition, the Norwegian Arthroplasty Register evaluated survival rates and reasons for revision for 7 types of cemented primary total knee arthroplasty (TKA) between 1994 and 2009.7 Data on more than 17,000 primary TKAs allowed Plate and colleagues8 to confidently determine that aseptic loosening was related to certain TKA designs. Using registry information, they identified patients at risk for dislocation in total hip arthroplasty and concluded that large-diameter femoral head articulations could reduce dislocation rates.
Obtaining such large cohorts of patients in individual studies is not only difficult but highly unlikely. Unlike registry data, these studies are often impractical in evaluating factors of low incidence, such as revision rates, as it is often difficult to find significant differences in small populations.9 Furthermore, these controlled trials homogenize patients—using exclusion and inclusion criteria to eliminate potential confounders—and thus poorly represent the heterogeneity of a typical hospital’s patient population.10 Although the literature may indeed have alluded to such complications, only a database as extensive as a registry can allow us to fully comprehend the outcomes of particular implants and devices.
Dr. Sarmiento points to the AO Swiss Fracture Registry as being of little benefit and raises the concern that the American Joint Replacement Registry (AJRR) may follow with the same results. However, realizing a registry’s benefits may take time and the gradual accumulation of data. Supporting this, Hübschle and colleagues11 recently used AO Swiss Fracture Registry data to validate use of balloon kyphoplasty for vertebral compression fractures and concluded that the technique is safe and effective in reducing pain—thus possibly providing the federal office with the evidence needed for reimbursement for this intervention. Therefore, this registry is now providing useful information.
We can never truly know the veracity of participating surgeons, but it is naïve to assume that this issue arises only vis-à-vis registries. If we were to debate the ethical and professional standards of colleagues in our field, such questions could extend to all studies performed, even peer-reviewed studies. Therefore, we do not think this is reason to exclude the patient data and outcomes found in registries. We must emphasize that ultimately registry data are often most useful in highlighting trends and determining triggers for further study rather than in arriving at conclusions.1 In particular, registry data may be used in cohort studies that evaluate the risk factors for and incidence of certain outcomes. Focused higher-level interventional studies can then follow the trends observed.1 However, registry data are also valuable on their own, when higher-level, randomized controlled trials may be impractical or unethical.12
Dr. Sarmiento refers to corrupt relationships between companies and orthopedists as “representing a widespread loss of professionalism in our ranks.” Despite a US Justice Department investigation into these relationships, only a few doctors were found to have had inappropriate relationships.13 In addition, the investigation and prosecution of companies led to an agreement requiring federal monitoring and new corporate compliance procedures, which should ensure stricter adherence to regulations.14 We do not believe this should undermine the value of registries and the work that has been contributed by thousands of surgeons hoping to improve the field of orthopedics. In addition, concerns about the influence of well-known individuals may be better directed at individual institution–based research, particularly as these specific authors also often have conflicts of interest that may skew the presentation of results. The strength of registry data is in providing collective data and large samples from a multitude of surgeons rather than from just high-volume surgeons, and therefore registry data provide a better overall picture of patients and their procedures.15 Furthermore, trends observed in national registries in countries such as New Zealand16 may aid in effectively reducing the revision rate, possibly up to 10%.17 If a US national joint registry is marginally as effective, then we may see considerable savings for our health care services.17,18
We wholeheartedly agree that a yearly review of registries may be constructive. Dr. Sarmiento suggests an annual publication summarizing peer-reviewed articles and the opportunity for orthopedists to decide for themselves what treatments to choose based on reports from independent investigators. Although this sounds feasible, it would be difficult to decide which articles should be selected as pertinent for this type of publication. Any selection would be biased, and not all studies with high-level evidence are necessarily important or relevant. Therefore, selecting what is most appropriate to cite is not without its difficulties. We appreciate that there are problems in standardizing data reporting among registries. However, to improve interregistry collaboration, the US Food and Drug Administration is sponsoring the International Consortium of Orthopaedic Registries (ICOR) to facilitate data presentation.19 ICOR aims to increase cooperation, standardize analyses, and improve reporting, which will only strengthen the data available to us. Such efforts will ultimately enhance coordination and international collaboration among registries.15 In addition, incorporating patient-reported outcomes into our national registry will aid in quantifying arthroplasty outcomes from the patient’s perspective and will continue to improve total joint arthroplasties.20
Overall, this debate is useful and highly relevant in highlighting potential issues with registries. Although registries are not without their flaws, like all aspects of orthopedics they are ever evolving, and they must be continually modified and improved. However, disregard for the potential value of AJRR, which has benefits for orthopedists and patients alike, is premature. Once again, we thank Dr. Sarmiento for starting this discussion, which will allow us to continue to evaluate and improve our registries.
1. Konan S, Haddad FS. Joint registries: a Ptolemaic model of data interpretation? Bone Joint J Br. 2013;95(12):1585-1586.
2. Banerjee S, Cafri G, Isaacs AJ, et al. A distributed health data network analysis of survival outcomes: the International Consortium of Orthopaedic Registries perspective. J Bone Joint Surg Am. 2014;96(suppl 1):7-11.
3. Lewallen DG, Etkin CD. The need for a national total joint registry. Orthop Nurs. 2013;32(1):4-5.
4. Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW; National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379(9822):1199-1204.
5. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
6. Hug KT, Watters TS, Vail TP, Bolognesi MP. The withdrawn ASR™ THA and hip resurfacing systems: how have our patients fared over 1 to 6 years? Clin Orthop. 2013;471(2):430-438.
7. Gøthesen O, Espehaug B, Havelin L, et al. Survival rates and causes of revision in cemented primary total knee replacement: a report from the Norwegian Arthroplasty Register 1994–2009. Bone Joint J Br. 2013;95(5):636-642.
8. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5:553.
9. Daruwalla ZJ, Wong KL, Pillay KR, Leong KM, Murphy DP. Does ageing Singapore need an electronic database of hip fracture patients? The value and role of a national joint registry and an electronic database of intertrochanteric and femoral neck fractures. Singapore Med J. 2014;55(5):287-288.
10. Rasmussen JV, Olsen BS, Fevang BT, et al. A review of national shoulder and elbow joint replacement registries. J Shoulder Elbow Surg. 2012;21(10):1328-1335.
11. Hübschle L, Borgström F, Olafsson G, et al. Real-life results of balloon kyphoplasty for vertebral compression fractures from the SWISSspine registry. Spine J. 2014;14(9):2063-2077.
12. Ahn H, Court-Brown CM, McQueen MM, Schemitsch EH. The use of hospital registries in orthopaedic surgery. J Bone Joint Surg Am. 2009;91(suppl 3):68-72.
13. Youngstrom N. Swept up in major medical device case, physician pays $650,000 to settle kickback charges. AIS Health Business Daily. May 3, 2010.
14. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed February 19, 2015.
15. Namba RS, Inacio MC, Paxton EW, Robertsson O, Graves SE. The role of registry data in the evaluation of mobile-bearing total knee arthroplasty. J Bone Joint Surg Am. 2011;93(suppl 3):48-50.
16. Insull PJ, Cobbett H, Frampton CM, Munro JT. The use of a lipped acetabular liner decreases the rate of revision for instability after total hip replacement: a study using data from the New Zealand Joint Registry. Bone Joint J Br. 2014;96(7):884-888.
17. Rankin EA. AJRR: becoming a national US joint registry. Orthopedics. 2013;36(3):175-176.
18. American Joint Replacement Registry website. https://teamwork.aaos.org/ajrr/SitePages/About%20Us.aspx. Accessed February 19, 2015.
19. International Consortium of Orthopaedic Registries website. http://www.icor-initiative.org. Accessed February 19, 2015.
20. Franklin PD, Harrold L, Ayers DC. Incorporating patient-reported outcomes in total joint arthroplasty registries: challenges and opportunities. Clin Orthop. 2013;471(11):3482-3488.
1. Konan S, Haddad FS. Joint registries: a Ptolemaic model of data interpretation? Bone Joint J Br. 2013;95(12):1585-1586.
2. Banerjee S, Cafri G, Isaacs AJ, et al. A distributed health data network analysis of survival outcomes: the International Consortium of Orthopaedic Registries perspective. J Bone Joint Surg Am. 2014;96(suppl 1):7-11.
3. Lewallen DG, Etkin CD. The need for a national total joint registry. Orthop Nurs. 2013;32(1):4-5.
4. Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW; National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379(9822):1199-1204.
5. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
6. Hug KT, Watters TS, Vail TP, Bolognesi MP. The withdrawn ASR™ THA and hip resurfacing systems: how have our patients fared over 1 to 6 years? Clin Orthop. 2013;471(2):430-438.
7. Gøthesen O, Espehaug B, Havelin L, et al. Survival rates and causes of revision in cemented primary total knee replacement: a report from the Norwegian Arthroplasty Register 1994–2009. Bone Joint J Br. 2013;95(5):636-642.
8. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5:553.
9. Daruwalla ZJ, Wong KL, Pillay KR, Leong KM, Murphy DP. Does ageing Singapore need an electronic database of hip fracture patients? The value and role of a national joint registry and an electronic database of intertrochanteric and femoral neck fractures. Singapore Med J. 2014;55(5):287-288.
10. Rasmussen JV, Olsen BS, Fevang BT, et al. A review of national shoulder and elbow joint replacement registries. J Shoulder Elbow Surg. 2012;21(10):1328-1335.
11. Hübschle L, Borgström F, Olafsson G, et al. Real-life results of balloon kyphoplasty for vertebral compression fractures from the SWISSspine registry. Spine J. 2014;14(9):2063-2077.
12. Ahn H, Court-Brown CM, McQueen MM, Schemitsch EH. The use of hospital registries in orthopaedic surgery. J Bone Joint Surg Am. 2009;91(suppl 3):68-72.
13. Youngstrom N. Swept up in major medical device case, physician pays $650,000 to settle kickback charges. AIS Health Business Daily. May 3, 2010.
14. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed February 19, 2015.
15. Namba RS, Inacio MC, Paxton EW, Robertsson O, Graves SE. The role of registry data in the evaluation of mobile-bearing total knee arthroplasty. J Bone Joint Surg Am. 2011;93(suppl 3):48-50.
16. Insull PJ, Cobbett H, Frampton CM, Munro JT. The use of a lipped acetabular liner decreases the rate of revision for instability after total hip replacement: a study using data from the New Zealand Joint Registry. Bone Joint J Br. 2014;96(7):884-888.
17. Rankin EA. AJRR: becoming a national US joint registry. Orthopedics. 2013;36(3):175-176.
18. American Joint Replacement Registry website. https://teamwork.aaos.org/ajrr/SitePages/About%20Us.aspx. Accessed February 19, 2015.
19. International Consortium of Orthopaedic Registries website. http://www.icor-initiative.org. Accessed February 19, 2015.
20. Franklin PD, Harrold L, Ayers DC. Incorporating patient-reported outcomes in total joint arthroplasty registries: challenges and opportunities. Clin Orthop. 2013;471(11):3482-3488.
Orthopedic Registries: Second Thoughts
Many assume that the American Joint Replacement Registry (AJRR) is moving forward as originally planned. No one has reported any obstacles that may cast doubt on its continued progress.
Despite the enthusiasm for AJRR, we must be realistic and admit that the project may not in the final analysis bring about its anticipated results. Therefore, periodic sober assessments of its course should be carried out, as they might result in identifying possible flaws and strengths. It is imperative to continue to express doubts regarding the true long-term value of this registry.
Much of the original support for an ongoing registry came from the example provided by the Swedish national registry. The Scandinavian registry had been said to dramatically reduce the number of complications and halve the revision rate for total hip arthroplasties. We need to question the claim that this reduction was solely the result of information produced by the registry. It is hard to believe that the literature had failed to report on those complications long before the registry publicized its findings.
As we take a fresh look at AJRR, it is perhaps wise to keep in mind the history of the AO Swiss Fracture Registry, founded by Maurice Müller and heavily subsidized by industry. Apparently, after gathering millions of pieces of information, primarily about equipment used for fracture fixation, the Swiss registry has failed to produce the greater benefits it had expected. Given the similarities between the Swiss Fracture Registry and AJRR, it is logical to assume that the latter may suffer the same fate.
I base my concerns on factors that, carefully analyzed, might be important in determining the future of AJRR. One major consideration is the difficulty in guaranteeing the veracity of data submitted—a factor shared by all registries.1 To assume that all participating surgeons adhere to high ethical and professional standards is naïve. Some surgeons who stand to make large profits from their ownership of implants or equipment are submitting false and erroneous information. Other unscrupulous orthopedists are receiving large kickbacks for helping the industry market its implants. These people will be tempted to embellish and falsify information about successes and failures and submit it to the registry.1-3
Militating against the “guaranteed success” of AJRR is this tainted relationship between the implant manufacturing industry and some members of the orthopedics community. A 2002–2006 investigation by the US Justice Department found egregious unethical transgressions and corrupt relationships between 5 companies and hundreds of orthopedists—representing a widespread loss of professionalism in our ranks.4 More recently, the Centers for Medicare & Medicaid Services5 disclosed that, in the last 5 months of 2013, $3.5 billion were paid by medical device companies to doctors and leading hospitals. As stated in a newspaper article, “‘Open Payments does not identify which financial relationships … could cause conflicts of interest,’ said Shantanu Agrawal, the agency official overseeing the project. ‘It simply makes the data available to the public.’”6 Further, “an initial Associated Press analysis found that orthopedists, cardiologists and adult medicine specialists were among the likeliest to receive payments from drug and device companies. Most of the contributions came in the form of cash payments, followed by in-kind gifts and services, and stock options.”6
This official government revelation is disturbing. Although the number of people who are deliberately committing clear infractions may be small, some of these people are likely well-known, and their influence should not be underestimated, particularly with regard to AJRR publications. Some in the orthopedic community do not question the accuracy of these publications but accept their conclusions as fact, and such may be the case with orthopedic guidelines.7
Given these concerns and the facts of the situation, can AJRR solve real problems that traditional systems have so far failed to solve? We have enough journals and scientific meetings informing us of the failures and successes of implants. I suspect it is wrong to believe that the AJRR data on 1 million patients’ arthroplasties are necessarily superior to the data from a 20,000-patient registry. Such an erroneous conclusion ignores the fact that, with clinical issues such as the one currently being addressed by AJRR, having a larger registry and more patients does not necessarily imply more meaningful information. In addition, follow-ups longer than those used with traditional methods are not possible—death will continue to intervene. No matter how many patients are included in the system, the maximum follow-up will forever remain the same.
Financing of AJRR is expensive, time-consuming, and likely to be terminated if clear evidence of the true value of the registry is not provided within the next few years. In light of such an outcome, we should replace the current system with a more effective mechanism. For example, we could produce an annual publication that summarizes the peer-reviewed articles published on joint replacement, with an emphasis on controversial topics. Orthopedic fellows, rather than readily accepting AJRR findings and recommendations, will instead be able to decide for themselves what treatment to use for each particular patient and situation, based on information provided by a number of independent investigators.
Meaningful progress in managing clinical conditions, such as the ones we are discussing, is achieved not by expanding the size of a registry but by being committed as individuals to making improvements. A cursory glance at the history of hip arthroplasty easily proves the point. Registries, guidelines, and other popular systems sometimes inadvertently create an environment that inhibits independent thinking. When powerful nonmedical economic and political bodies become involved in medical issues in order to ensure their continued profit, our autonomy is lost or compromised in major ways. Such scenarios must be avoided as forcefully as possible.8
Questioning the future of AJRR does not derive from rigid thinking or from a lack of awareness or understanding of the registry’s nature, procedures, benefits, goals, or highly altruistic and noble origins. However, pointing out a lack of evidence of success is not a crime. It is incumbent on us to look at this area and others with open minds while recognizing that honest and sincere scrutiny often helps make a better future a reality. The United States is working to achieve major goals for health care—access for all, lower costs, and fewer abuses of the system. Our involvement is a mandate to be followed enthusiastically.
1. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone Joint Surg Br. 2005;87(11):1452-1453.
2. Callahan D. False Hopes: Overcoming the Obstacles to a Sustainable, Affordable Medicine. New Brunswick, NJ: Rutgers University Press; 1999.
3. Relman AS. A Second Opinion: Rescuing America’s Healthcare: A Plan for Universal Coverage Serving Patients Over Profit. New York: Public Affairs; 2007.
4. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed February 19, 2015.
5. CMS makes first wave of drug & device company payments to teaching hospitals and physicians public [press release]. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2014-Press-releases-items/2014-09-30.html. Published September 30, 2014. Accessed February 19, 2015.
6. Alonso-Zaldivar R, Gillum J. Drug, device firms paid $3.5B to care providers. The Big Story. Associated Press website. http://bigstory.ap.org/article/c80ae51828a0497e87beda7f9ff60ac8/govt-reveal-drug-company-payments-doctors. Published September 30, 2014. Accessed February 19, 2015.
7. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
8. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222.
Many assume that the American Joint Replacement Registry (AJRR) is moving forward as originally planned. No one has reported any obstacles that may cast doubt on its continued progress.
Despite the enthusiasm for AJRR, we must be realistic and admit that the project may not in the final analysis bring about its anticipated results. Therefore, periodic sober assessments of its course should be carried out, as they might result in identifying possible flaws and strengths. It is imperative to continue to express doubts regarding the true long-term value of this registry.
Much of the original support for an ongoing registry came from the example provided by the Swedish national registry. The Scandinavian registry had been said to dramatically reduce the number of complications and halve the revision rate for total hip arthroplasties. We need to question the claim that this reduction was solely the result of information produced by the registry. It is hard to believe that the literature had failed to report on those complications long before the registry publicized its findings.
As we take a fresh look at AJRR, it is perhaps wise to keep in mind the history of the AO Swiss Fracture Registry, founded by Maurice Müller and heavily subsidized by industry. Apparently, after gathering millions of pieces of information, primarily about equipment used for fracture fixation, the Swiss registry has failed to produce the greater benefits it had expected. Given the similarities between the Swiss Fracture Registry and AJRR, it is logical to assume that the latter may suffer the same fate.
I base my concerns on factors that, carefully analyzed, might be important in determining the future of AJRR. One major consideration is the difficulty in guaranteeing the veracity of data submitted—a factor shared by all registries.1 To assume that all participating surgeons adhere to high ethical and professional standards is naïve. Some surgeons who stand to make large profits from their ownership of implants or equipment are submitting false and erroneous information. Other unscrupulous orthopedists are receiving large kickbacks for helping the industry market its implants. These people will be tempted to embellish and falsify information about successes and failures and submit it to the registry.1-3
Militating against the “guaranteed success” of AJRR is this tainted relationship between the implant manufacturing industry and some members of the orthopedics community. A 2002–2006 investigation by the US Justice Department found egregious unethical transgressions and corrupt relationships between 5 companies and hundreds of orthopedists—representing a widespread loss of professionalism in our ranks.4 More recently, the Centers for Medicare & Medicaid Services5 disclosed that, in the last 5 months of 2013, $3.5 billion were paid by medical device companies to doctors and leading hospitals. As stated in a newspaper article, “‘Open Payments does not identify which financial relationships … could cause conflicts of interest,’ said Shantanu Agrawal, the agency official overseeing the project. ‘It simply makes the data available to the public.’”6 Further, “an initial Associated Press analysis found that orthopedists, cardiologists and adult medicine specialists were among the likeliest to receive payments from drug and device companies. Most of the contributions came in the form of cash payments, followed by in-kind gifts and services, and stock options.”6
This official government revelation is disturbing. Although the number of people who are deliberately committing clear infractions may be small, some of these people are likely well-known, and their influence should not be underestimated, particularly with regard to AJRR publications. Some in the orthopedic community do not question the accuracy of these publications but accept their conclusions as fact, and such may be the case with orthopedic guidelines.7
Given these concerns and the facts of the situation, can AJRR solve real problems that traditional systems have so far failed to solve? We have enough journals and scientific meetings informing us of the failures and successes of implants. I suspect it is wrong to believe that the AJRR data on 1 million patients’ arthroplasties are necessarily superior to the data from a 20,000-patient registry. Such an erroneous conclusion ignores the fact that, with clinical issues such as the one currently being addressed by AJRR, having a larger registry and more patients does not necessarily imply more meaningful information. In addition, follow-ups longer than those used with traditional methods are not possible—death will continue to intervene. No matter how many patients are included in the system, the maximum follow-up will forever remain the same.
Financing of AJRR is expensive, time-consuming, and likely to be terminated if clear evidence of the true value of the registry is not provided within the next few years. In light of such an outcome, we should replace the current system with a more effective mechanism. For example, we could produce an annual publication that summarizes the peer-reviewed articles published on joint replacement, with an emphasis on controversial topics. Orthopedic fellows, rather than readily accepting AJRR findings and recommendations, will instead be able to decide for themselves what treatment to use for each particular patient and situation, based on information provided by a number of independent investigators.
Meaningful progress in managing clinical conditions, such as the ones we are discussing, is achieved not by expanding the size of a registry but by being committed as individuals to making improvements. A cursory glance at the history of hip arthroplasty easily proves the point. Registries, guidelines, and other popular systems sometimes inadvertently create an environment that inhibits independent thinking. When powerful nonmedical economic and political bodies become involved in medical issues in order to ensure their continued profit, our autonomy is lost or compromised in major ways. Such scenarios must be avoided as forcefully as possible.8
Questioning the future of AJRR does not derive from rigid thinking or from a lack of awareness or understanding of the registry’s nature, procedures, benefits, goals, or highly altruistic and noble origins. However, pointing out a lack of evidence of success is not a crime. It is incumbent on us to look at this area and others with open minds while recognizing that honest and sincere scrutiny often helps make a better future a reality. The United States is working to achieve major goals for health care—access for all, lower costs, and fewer abuses of the system. Our involvement is a mandate to be followed enthusiastically.
Many assume that the American Joint Replacement Registry (AJRR) is moving forward as originally planned. No one has reported any obstacles that may cast doubt on its continued progress.
Despite the enthusiasm for AJRR, we must be realistic and admit that the project may not in the final analysis bring about its anticipated results. Therefore, periodic sober assessments of its course should be carried out, as they might result in identifying possible flaws and strengths. It is imperative to continue to express doubts regarding the true long-term value of this registry.
Much of the original support for an ongoing registry came from the example provided by the Swedish national registry. The Scandinavian registry had been said to dramatically reduce the number of complications and halve the revision rate for total hip arthroplasties. We need to question the claim that this reduction was solely the result of information produced by the registry. It is hard to believe that the literature had failed to report on those complications long before the registry publicized its findings.
As we take a fresh look at AJRR, it is perhaps wise to keep in mind the history of the AO Swiss Fracture Registry, founded by Maurice Müller and heavily subsidized by industry. Apparently, after gathering millions of pieces of information, primarily about equipment used for fracture fixation, the Swiss registry has failed to produce the greater benefits it had expected. Given the similarities between the Swiss Fracture Registry and AJRR, it is logical to assume that the latter may suffer the same fate.
I base my concerns on factors that, carefully analyzed, might be important in determining the future of AJRR. One major consideration is the difficulty in guaranteeing the veracity of data submitted—a factor shared by all registries.1 To assume that all participating surgeons adhere to high ethical and professional standards is naïve. Some surgeons who stand to make large profits from their ownership of implants or equipment are submitting false and erroneous information. Other unscrupulous orthopedists are receiving large kickbacks for helping the industry market its implants. These people will be tempted to embellish and falsify information about successes and failures and submit it to the registry.1-3
Militating against the “guaranteed success” of AJRR is this tainted relationship between the implant manufacturing industry and some members of the orthopedics community. A 2002–2006 investigation by the US Justice Department found egregious unethical transgressions and corrupt relationships between 5 companies and hundreds of orthopedists—representing a widespread loss of professionalism in our ranks.4 More recently, the Centers for Medicare & Medicaid Services5 disclosed that, in the last 5 months of 2013, $3.5 billion were paid by medical device companies to doctors and leading hospitals. As stated in a newspaper article, “‘Open Payments does not identify which financial relationships … could cause conflicts of interest,’ said Shantanu Agrawal, the agency official overseeing the project. ‘It simply makes the data available to the public.’”6 Further, “an initial Associated Press analysis found that orthopedists, cardiologists and adult medicine specialists were among the likeliest to receive payments from drug and device companies. Most of the contributions came in the form of cash payments, followed by in-kind gifts and services, and stock options.”6
This official government revelation is disturbing. Although the number of people who are deliberately committing clear infractions may be small, some of these people are likely well-known, and their influence should not be underestimated, particularly with regard to AJRR publications. Some in the orthopedic community do not question the accuracy of these publications but accept their conclusions as fact, and such may be the case with orthopedic guidelines.7
Given these concerns and the facts of the situation, can AJRR solve real problems that traditional systems have so far failed to solve? We have enough journals and scientific meetings informing us of the failures and successes of implants. I suspect it is wrong to believe that the AJRR data on 1 million patients’ arthroplasties are necessarily superior to the data from a 20,000-patient registry. Such an erroneous conclusion ignores the fact that, with clinical issues such as the one currently being addressed by AJRR, having a larger registry and more patients does not necessarily imply more meaningful information. In addition, follow-ups longer than those used with traditional methods are not possible—death will continue to intervene. No matter how many patients are included in the system, the maximum follow-up will forever remain the same.
Financing of AJRR is expensive, time-consuming, and likely to be terminated if clear evidence of the true value of the registry is not provided within the next few years. In light of such an outcome, we should replace the current system with a more effective mechanism. For example, we could produce an annual publication that summarizes the peer-reviewed articles published on joint replacement, with an emphasis on controversial topics. Orthopedic fellows, rather than readily accepting AJRR findings and recommendations, will instead be able to decide for themselves what treatment to use for each particular patient and situation, based on information provided by a number of independent investigators.
Meaningful progress in managing clinical conditions, such as the ones we are discussing, is achieved not by expanding the size of a registry but by being committed as individuals to making improvements. A cursory glance at the history of hip arthroplasty easily proves the point. Registries, guidelines, and other popular systems sometimes inadvertently create an environment that inhibits independent thinking. When powerful nonmedical economic and political bodies become involved in medical issues in order to ensure their continued profit, our autonomy is lost or compromised in major ways. Such scenarios must be avoided as forcefully as possible.8
Questioning the future of AJRR does not derive from rigid thinking or from a lack of awareness or understanding of the registry’s nature, procedures, benefits, goals, or highly altruistic and noble origins. However, pointing out a lack of evidence of success is not a crime. It is incumbent on us to look at this area and others with open minds while recognizing that honest and sincere scrutiny often helps make a better future a reality. The United States is working to achieve major goals for health care—access for all, lower costs, and fewer abuses of the system. Our involvement is a mandate to be followed enthusiastically.
1. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone Joint Surg Br. 2005;87(11):1452-1453.
2. Callahan D. False Hopes: Overcoming the Obstacles to a Sustainable, Affordable Medicine. New Brunswick, NJ: Rutgers University Press; 1999.
3. Relman AS. A Second Opinion: Rescuing America’s Healthcare: A Plan for Universal Coverage Serving Patients Over Profit. New York: Public Affairs; 2007.
4. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed February 19, 2015.
5. CMS makes first wave of drug & device company payments to teaching hospitals and physicians public [press release]. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2014-Press-releases-items/2014-09-30.html. Published September 30, 2014. Accessed February 19, 2015.
6. Alonso-Zaldivar R, Gillum J. Drug, device firms paid $3.5B to care providers. The Big Story. Associated Press website. http://bigstory.ap.org/article/c80ae51828a0497e87beda7f9ff60ac8/govt-reveal-drug-company-payments-doctors. Published September 30, 2014. Accessed February 19, 2015.
7. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
8. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222.
1. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone Joint Surg Br. 2005;87(11):1452-1453.
2. Callahan D. False Hopes: Overcoming the Obstacles to a Sustainable, Affordable Medicine. New Brunswick, NJ: Rutgers University Press; 1999.
3. Relman AS. A Second Opinion: Rescuing America’s Healthcare: A Plan for Universal Coverage Serving Patients Over Profit. New York: Public Affairs; 2007.
4. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed February 19, 2015.
5. CMS makes first wave of drug & device company payments to teaching hospitals and physicians public [press release]. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2014-Press-releases-items/2014-09-30.html. Published September 30, 2014. Accessed February 19, 2015.
6. Alonso-Zaldivar R, Gillum J. Drug, device firms paid $3.5B to care providers. The Big Story. Associated Press website. http://bigstory.ap.org/article/c80ae51828a0497e87beda7f9ff60ac8/govt-reveal-drug-company-payments-doctors. Published September 30, 2014. Accessed February 19, 2015.
7. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
8. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222.
We Can Do Better for Our Veterans’ Health Care
The mission statement of the US Department of Veterans Affairs (VA) reiterates Abraham Lincoln’s promise, “to care for him who shall have borne the battle, and for his widow, and his orphan,” by serving the men and women who are American veterans.1 Robert A. McDonald is the current Secretary of Veterans Affairs. He was recently appointed after the scheduling scandal at the VA. He was the previous Chairman and Chief Executive Officer of Procter & Gamble and is a graduate of West Point. Mr. McDonald has recently been criticized for some public misstatements after only being on the job for a few months.2,3 His staff includes a wide variety of numerous secretaries, deputies, councils, and assistant associates. The budget for 2016 that was recently submitted was $169 billion.4 The scandalous scheduling fiasco in the entire VA system blatantly shows the neglect of our servicemen and servicewomen and is a permanent blemish on a government-run system. Despite claims of numerous firings, only 8 people have been dismissed out of an employee pool of over 300,000.3
I have been a volunteer physician for just under 40 years at the VA Hospital in La Jolla, California, which is also an associated teaching hospital for the University of California, San Diego. Many of my reflections are from personal experience. I am also a veteran. I have a deep affection for our veterans and their families, and write this column in the hope of some possible change in their care. The physicians and health care providers in this system are dedicated and professional individuals caught up in a tangled bureaucratic web that, in most cases, handcuffs the delivery of the health care that our veterans deserve.
When one goes to the VA website, it appears that there are a myriad of services available, but, as with all government agencies, more time is spent in the creation of the website and structure of the agency than is spent in servicing the patient. Picture trying to get your health care through the local Department of Motor Vehicles office. The VA system is a huge bureaucratic overregulated agency currently out of control and lacking efficiency. From the clinic to the operating room, the process is frustrating to all involved. There are clinics staffed with medical doctors, nurse practitioners, physician assistants, medical students, residents, and fellows. Generally, they can only process 10 to 12 patients per half-day clinic because of the endless paperwork and regulatory requirements.
The operating rooms have been a formidable frustration to the surgeon. It is routine for a 7:30 am case to start at 9 am and then be followed by a 2½-hour turnover time until the second case could be scheduled. Cases cannot be scheduled that could potentially start after 3 pm. Most data would probably suggest that the operating room efficiency in terms of numbers of cases is approximately 50% to 60% of what can be done in the private environment. Staffing for all facets of the hospital operation is about double what is necessary in the outside world. Physicians must take tests on a very frequent basis on subjects that are totally unrelated to health care. Examinations on American history, electrical safety, and sexual harassment in the workplace are commonplace topics. These tests must be taken and passed in order to maintain one’s privileges at the hospital.
Is there an answer to this government-run system? Perhaps. Here is a potential solution. Over a 5-year period, divest all VA facilities, sell or rent them, and sell or rent the land. Use the proceeds, in combination with the normal budget for the VA, to create a private health care system. Veterans and their families would then receive a veteran-based private policy that would have no deductibles or copays and would allow them to seek medical care from any provider. For more complex situations such as quadriplegia, posttraumatic stress disorder (PTSD), or complex amputations, private entities would bid on a local basis, assuming they pass a strict credentialing process. These private entities would be required to pay strict attention to protocol, deliver prompt service, and produce outcomes that are acceptable in the medical workplace. The newly created system would be run by a private board composed of retired military, business executives, and entrepreneurs with no political affiliations. The trust fund would not be susceptible to any other allocation other than the medical care of veterans.
I have seen far too many spouses and families of deployed servicemen and servicewomen whose care has been neglected while their spouses are serving in a foreign land. There are far too many homeless veterans that are in need of psychiatric care and suffering from PTSD. It is estimated that 11% of the current homeless population are veterans.5 Their housing needs have been completely neglected. These are not acceptable statistics. The government now provides some burial services and headstones for our deceased veterans instead of delivering the health care for them and their families while they are still alive.
1. Mission, vision, core values & goals. US Department of Veterans Affairs website. http://www.va.gov/about_va/mission.asp. Updated April 1, 2014. Accessed March 6, 2015.
2. VA Secretary apologizes for “misspeaking” about Special Forces service. Fox News Insider website. http://insider.foxnews.com/2015/02/24/va-secretary-robert-mcdonald-apologizes-misspeaking-about-special-forces-service. Published February 24, 2015. Accessed March 6, 2015.
3. Lee MYH. No, the VA has not fired 60 people for manipulating wait-time data. Washington Post website. http://www.washingtonpost.com/blogs/fact-checker/wp/2015/02/18/no-the-va-has-not-fired-60-people-for-manipulating-wait-time-data. Published February 18, 2015. Accessed March 6, 2015.
4. Annual budget submission. US Department of Veterans Affairs website. http://www.va.gov/budget/products.asp. Updated February 3, 2015. Accessed March 6, 2015.
5. Henry M, Cortes A, Shivji A, Buck K; US Department of Housing and Urban Development, Office of Community Planning and Development. The 2014 Annual Homeless Assessment Report (AHAR) to Congress, October 2014: Part 1, Point-in-Time Estimates of Homelessness. HUD Exchange website. https://www.hudexchange.info/resources/documents/2014-AHAR-Part1.pdf. Published December 2014. Accessed March 6, 2015.
The mission statement of the US Department of Veterans Affairs (VA) reiterates Abraham Lincoln’s promise, “to care for him who shall have borne the battle, and for his widow, and his orphan,” by serving the men and women who are American veterans.1 Robert A. McDonald is the current Secretary of Veterans Affairs. He was recently appointed after the scheduling scandal at the VA. He was the previous Chairman and Chief Executive Officer of Procter & Gamble and is a graduate of West Point. Mr. McDonald has recently been criticized for some public misstatements after only being on the job for a few months.2,3 His staff includes a wide variety of numerous secretaries, deputies, councils, and assistant associates. The budget for 2016 that was recently submitted was $169 billion.4 The scandalous scheduling fiasco in the entire VA system blatantly shows the neglect of our servicemen and servicewomen and is a permanent blemish on a government-run system. Despite claims of numerous firings, only 8 people have been dismissed out of an employee pool of over 300,000.3
I have been a volunteer physician for just under 40 years at the VA Hospital in La Jolla, California, which is also an associated teaching hospital for the University of California, San Diego. Many of my reflections are from personal experience. I am also a veteran. I have a deep affection for our veterans and their families, and write this column in the hope of some possible change in their care. The physicians and health care providers in this system are dedicated and professional individuals caught up in a tangled bureaucratic web that, in most cases, handcuffs the delivery of the health care that our veterans deserve.
When one goes to the VA website, it appears that there are a myriad of services available, but, as with all government agencies, more time is spent in the creation of the website and structure of the agency than is spent in servicing the patient. Picture trying to get your health care through the local Department of Motor Vehicles office. The VA system is a huge bureaucratic overregulated agency currently out of control and lacking efficiency. From the clinic to the operating room, the process is frustrating to all involved. There are clinics staffed with medical doctors, nurse practitioners, physician assistants, medical students, residents, and fellows. Generally, they can only process 10 to 12 patients per half-day clinic because of the endless paperwork and regulatory requirements.
The operating rooms have been a formidable frustration to the surgeon. It is routine for a 7:30 am case to start at 9 am and then be followed by a 2½-hour turnover time until the second case could be scheduled. Cases cannot be scheduled that could potentially start after 3 pm. Most data would probably suggest that the operating room efficiency in terms of numbers of cases is approximately 50% to 60% of what can be done in the private environment. Staffing for all facets of the hospital operation is about double what is necessary in the outside world. Physicians must take tests on a very frequent basis on subjects that are totally unrelated to health care. Examinations on American history, electrical safety, and sexual harassment in the workplace are commonplace topics. These tests must be taken and passed in order to maintain one’s privileges at the hospital.
Is there an answer to this government-run system? Perhaps. Here is a potential solution. Over a 5-year period, divest all VA facilities, sell or rent them, and sell or rent the land. Use the proceeds, in combination with the normal budget for the VA, to create a private health care system. Veterans and their families would then receive a veteran-based private policy that would have no deductibles or copays and would allow them to seek medical care from any provider. For more complex situations such as quadriplegia, posttraumatic stress disorder (PTSD), or complex amputations, private entities would bid on a local basis, assuming they pass a strict credentialing process. These private entities would be required to pay strict attention to protocol, deliver prompt service, and produce outcomes that are acceptable in the medical workplace. The newly created system would be run by a private board composed of retired military, business executives, and entrepreneurs with no political affiliations. The trust fund would not be susceptible to any other allocation other than the medical care of veterans.
I have seen far too many spouses and families of deployed servicemen and servicewomen whose care has been neglected while their spouses are serving in a foreign land. There are far too many homeless veterans that are in need of psychiatric care and suffering from PTSD. It is estimated that 11% of the current homeless population are veterans.5 Their housing needs have been completely neglected. These are not acceptable statistics. The government now provides some burial services and headstones for our deceased veterans instead of delivering the health care for them and their families while they are still alive.
The mission statement of the US Department of Veterans Affairs (VA) reiterates Abraham Lincoln’s promise, “to care for him who shall have borne the battle, and for his widow, and his orphan,” by serving the men and women who are American veterans.1 Robert A. McDonald is the current Secretary of Veterans Affairs. He was recently appointed after the scheduling scandal at the VA. He was the previous Chairman and Chief Executive Officer of Procter & Gamble and is a graduate of West Point. Mr. McDonald has recently been criticized for some public misstatements after only being on the job for a few months.2,3 His staff includes a wide variety of numerous secretaries, deputies, councils, and assistant associates. The budget for 2016 that was recently submitted was $169 billion.4 The scandalous scheduling fiasco in the entire VA system blatantly shows the neglect of our servicemen and servicewomen and is a permanent blemish on a government-run system. Despite claims of numerous firings, only 8 people have been dismissed out of an employee pool of over 300,000.3
I have been a volunteer physician for just under 40 years at the VA Hospital in La Jolla, California, which is also an associated teaching hospital for the University of California, San Diego. Many of my reflections are from personal experience. I am also a veteran. I have a deep affection for our veterans and their families, and write this column in the hope of some possible change in their care. The physicians and health care providers in this system are dedicated and professional individuals caught up in a tangled bureaucratic web that, in most cases, handcuffs the delivery of the health care that our veterans deserve.
When one goes to the VA website, it appears that there are a myriad of services available, but, as with all government agencies, more time is spent in the creation of the website and structure of the agency than is spent in servicing the patient. Picture trying to get your health care through the local Department of Motor Vehicles office. The VA system is a huge bureaucratic overregulated agency currently out of control and lacking efficiency. From the clinic to the operating room, the process is frustrating to all involved. There are clinics staffed with medical doctors, nurse practitioners, physician assistants, medical students, residents, and fellows. Generally, they can only process 10 to 12 patients per half-day clinic because of the endless paperwork and regulatory requirements.
The operating rooms have been a formidable frustration to the surgeon. It is routine for a 7:30 am case to start at 9 am and then be followed by a 2½-hour turnover time until the second case could be scheduled. Cases cannot be scheduled that could potentially start after 3 pm. Most data would probably suggest that the operating room efficiency in terms of numbers of cases is approximately 50% to 60% of what can be done in the private environment. Staffing for all facets of the hospital operation is about double what is necessary in the outside world. Physicians must take tests on a very frequent basis on subjects that are totally unrelated to health care. Examinations on American history, electrical safety, and sexual harassment in the workplace are commonplace topics. These tests must be taken and passed in order to maintain one’s privileges at the hospital.
Is there an answer to this government-run system? Perhaps. Here is a potential solution. Over a 5-year period, divest all VA facilities, sell or rent them, and sell or rent the land. Use the proceeds, in combination with the normal budget for the VA, to create a private health care system. Veterans and their families would then receive a veteran-based private policy that would have no deductibles or copays and would allow them to seek medical care from any provider. For more complex situations such as quadriplegia, posttraumatic stress disorder (PTSD), or complex amputations, private entities would bid on a local basis, assuming they pass a strict credentialing process. These private entities would be required to pay strict attention to protocol, deliver prompt service, and produce outcomes that are acceptable in the medical workplace. The newly created system would be run by a private board composed of retired military, business executives, and entrepreneurs with no political affiliations. The trust fund would not be susceptible to any other allocation other than the medical care of veterans.
I have seen far too many spouses and families of deployed servicemen and servicewomen whose care has been neglected while their spouses are serving in a foreign land. There are far too many homeless veterans that are in need of psychiatric care and suffering from PTSD. It is estimated that 11% of the current homeless population are veterans.5 Their housing needs have been completely neglected. These are not acceptable statistics. The government now provides some burial services and headstones for our deceased veterans instead of delivering the health care for them and their families while they are still alive.
1. Mission, vision, core values & goals. US Department of Veterans Affairs website. http://www.va.gov/about_va/mission.asp. Updated April 1, 2014. Accessed March 6, 2015.
2. VA Secretary apologizes for “misspeaking” about Special Forces service. Fox News Insider website. http://insider.foxnews.com/2015/02/24/va-secretary-robert-mcdonald-apologizes-misspeaking-about-special-forces-service. Published February 24, 2015. Accessed March 6, 2015.
3. Lee MYH. No, the VA has not fired 60 people for manipulating wait-time data. Washington Post website. http://www.washingtonpost.com/blogs/fact-checker/wp/2015/02/18/no-the-va-has-not-fired-60-people-for-manipulating-wait-time-data. Published February 18, 2015. Accessed March 6, 2015.
4. Annual budget submission. US Department of Veterans Affairs website. http://www.va.gov/budget/products.asp. Updated February 3, 2015. Accessed March 6, 2015.
5. Henry M, Cortes A, Shivji A, Buck K; US Department of Housing and Urban Development, Office of Community Planning and Development. The 2014 Annual Homeless Assessment Report (AHAR) to Congress, October 2014: Part 1, Point-in-Time Estimates of Homelessness. HUD Exchange website. https://www.hudexchange.info/resources/documents/2014-AHAR-Part1.pdf. Published December 2014. Accessed March 6, 2015.
1. Mission, vision, core values & goals. US Department of Veterans Affairs website. http://www.va.gov/about_va/mission.asp. Updated April 1, 2014. Accessed March 6, 2015.
2. VA Secretary apologizes for “misspeaking” about Special Forces service. Fox News Insider website. http://insider.foxnews.com/2015/02/24/va-secretary-robert-mcdonald-apologizes-misspeaking-about-special-forces-service. Published February 24, 2015. Accessed March 6, 2015.
3. Lee MYH. No, the VA has not fired 60 people for manipulating wait-time data. Washington Post website. http://www.washingtonpost.com/blogs/fact-checker/wp/2015/02/18/no-the-va-has-not-fired-60-people-for-manipulating-wait-time-data. Published February 18, 2015. Accessed March 6, 2015.
4. Annual budget submission. US Department of Veterans Affairs website. http://www.va.gov/budget/products.asp. Updated February 3, 2015. Accessed March 6, 2015.
5. Henry M, Cortes A, Shivji A, Buck K; US Department of Housing and Urban Development, Office of Community Planning and Development. The 2014 Annual Homeless Assessment Report (AHAR) to Congress, October 2014: Part 1, Point-in-Time Estimates of Homelessness. HUD Exchange website. https://www.hudexchange.info/resources/documents/2014-AHAR-Part1.pdf. Published December 2014. Accessed March 6, 2015.
Greater Auricular Nerve Palsy After Arthroscopic Anterior-Inferior and Posterior-Inferior Labral Tear Repair Using Beach-Chair Positioning and a Standard Universal Headrest
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
In Vitro and In Situ Characterization of Arthroscopic Loop Security and Knot Security of Braided Polyblend Sutures: A Biomechanical Study
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
Open-surgery knot tying is easily learned and performed, but knot tying during arthroscopic procedures can be both challenging and frustrating. According to Burkhart and colleagues,1,2 knot security is defined as the effectiveness of the knot in resisting slippage when load is applied, whereas loop security is the effectiveness in maintaining a tight suture loop while a knot is being tied. Arthroscopic knots commonly begin with an initial slipknot locked in place with a series of half-hitches. During arthroscopic surgery, the surgeon usually must tie an arthroscopic knot to obtain secure tissue fixation, an essential component of soft-tissue repair. A secure knot provides optimal tissue apposition for healing, which will ultimately improve functional outcome. For a knot to be effective, it must have both knot security and loop security. Knot security depends on knot configuration, the coefficient of friction, ductility, handling properties, solubility and diameter of suture material, internal interference, slack between throws, and surgeon experience. Tissue fluid and tissue reaction to suture material may affect knot and loop security.
The ideal knot would be easy to tie and reproducible and would not slip or stretch before tissue is healed. The ideal suture material should provide adequate strength to hold soft tissue in an anatomically correct position until healing can occur. It should also be easily and efficiently manipulated by arthroscopic means when tissues are being secured with knots and secure suture loops. Studies have been conducted to evaluate the security of knots tied with arthroscopic techniques, knot configurations, and suture materials, and these investigations have often evaluated knot performance under single load-to-failure (LTF) test scenarios and cyclic loading in vitro (dry environment) in a room-temperature environment.2-10 To our knowledge, few if any attempts have been made to simulate in situ conditions at body temperature when testing knot security. The fluid environment and the temperature could potentially affect the effectiveness of knots, as knot security depends on friction, internal interference, and slack between throws.1
We conducted a study to evaluate biomechanical performance (knot security, loop security) during destructive testing of several different suture materials with various arthroscopic knot configurations. The study was performed under in vitro (dry environment) and in situ (wet environment) conditions by surgeons with different levels of experience.
Materials and Methods
This investigation was conducted at the Orthopaedic Research Institute at Via Christi Health in Wichita, Kansas. The study compared 4 different suture materials tied with 3 different commonly used arthroscopic knots by 3 surgeons with different levels of experience. The 4 types of braided polyblend polyethylene sutures were Fiberwire (Arthrex, Naples, Florida), ForceFiber (Stryker, San Jose, California), Orthocord (DePuy-Mitek, Warsaw, Indiana), and Ultrabraid (Smith & Nephew, Memphis, Tennessee). Each suture material was tied with 3 arthroscopic knots—static surgeon’s knot, Weston knot,11 Tennessee slider12—and a series of 3 reversing half-hitches on alternating posts (RHAPs) (Figure 1). These knots were chosen based on studies showing they have a higher maximum force to failure when combined with 3 RHAPs.1,2,5,9,13-17
We evaluated performer variability with the help of 3 investigator-surgeons who differed in their level of experience tying arthroscopic knots. This experience was defined on the basis of total number of arthroscopies performed—one of the most important factors predicting basic arthroscopic skills. Our surgeon A was a sports medicine fellowship–trained surgeon with 10 years of experience and a significant number of arthroscopies performed annually (350); surgeon B was a sports medicine fellowship–trained surgeon with 3 years of experience and an annual arthroscopy volume of more than 250 procedures; and surgeon C was a third-year orthopedic resident with about 100 arthroscopies performed.
All knots were tied on a standardized post 30 mm in circumference, which provided a consistent starting circumference for each knot and replicated the suture loop created during arthroscopic rotator cuff repair. All knots were tied using standard arthroscopic techniques, with a standard knot pusher and a modified arthroscopic cannula, in a dry environment (Figure 2). Servohydraulic materials testing system instruments (model 810; MTS Systems, Eden Prairie, Minnesota) were used to test the knot security and loop security of each combination of knots and suture types. Two round hooks (diameter, 3.9 mm) were attached to the actuator and the load cell (Figure 3). Loops were preloaded to 6 N to avoid potential errors caused by slack in the loops or by stretching of suture materials and to provide a well-defined starting point for data recording.
LTF testing was performed for both in vitro and in situ conditions using 10 samples of each suture–knot configuration for each mechanical testing. Each type of testing was conducted for a total of 240 suture–knot combinations per investigator. For the in vitro condition, each suture loop was initiated with 5 preconditioning loading cycles, from 6 N to 30 N at 1 Hz. The load was then applied continuously at a crosshead speed of 1 mm/s until “clinical failure” (3 mm crosshead displacement). We used this criterion for clinical failure, as studies have indicated that 3 mm is the point at which tissue apposition is lost.15,18-21 After the crosshead reached the 3-mm displacement, the loads (under load control) were held for 5 minutes at maximum load, and then load was applied continuously at a crosshead speed of 1 mm/s until complete structure failure. Load and displacement data were collected at a frequency of 20 Hz.
For the in situ condition, the same test parameters were used, except that each combination of the suture loop was preloaded to 6 N and soaked in physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing in an effort to simulate the aqueous medium in vivo after surgery. The in situ tests were performed under physiologic solution maintained at 37°C to approximate postoperative physical conditions.
Statistical Analysis
Means and standard deviations of the knot security and loop security achieved by the surgeons (different experience levels) were calculated for each test configuration and each test condition. These values were used to determine the statistical relevance of the difference in arthroscopic loop security and knot security in each configuration. One-way analysis of variance (ANOVA) performed with SPSS Version 19.0 software (SPSS, Chicago, Illinois) with the least significant difference (LSD) multiple comparisons post hoc analysis was used to determine if any observed differences between the types of braided polyblend sutures, the types of sliding knots, the test conditions (in vitro, in situ), and the levels of surgeon experience were significant for each knot configuration. The level of significance of differences was set at P < .001.
Results
Figure 4 shows the mean maximum clinical failure load (3 mm of displacement) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. In the comparison of biomechanical performance (knot and loop security) under in vitro and in situ conditions, no significant difference was detected when Ultrabraid suture material was used, regardless of surgeon experience, for all knot configurations. For surgeon B, there was no significant difference between in vitro and in situ conditions for any knot configurations or suture materials. When Orthocord suture material was used, Weston knots tied by surgeon A, and static surgeon’s knots by surgeons A and C, resulted in a significant difference between the in vitro and in situ conditions. When ForceFiber suture material was used, only Weston knots and Tennessee slider knots by surgeon A had a significant difference between in vitro and in situ conditions. Weston knots by surgeon A exhibited a significant difference between in vitro and in situ conditions, except when Ultrabraid suture material was used.
Surgeon C’s Tennessee slider knots with all polyblend sutures showed significantly lower loads at clinical failure compared with all the other knot configurations and with knots tied by the other 2 surgeons under both in vitro and in situ conditions. Overall, knots tied by surgeon B had higher clinical failure load than knots tied by the other 2 surgeons.
Figure 5 shows the mean ultimate failure load (complete structural failure) of different arthroscopic knot configurations for different braided polyblend sutures by surgeons of different levels of experience. Knots tied with Orthocord suture material had the overall lower ultimate failure load compared with other suture materials, whereas knots tied with Ultrabraid suture material had the overall highest ultimate failure load. However, the ultimate failure loads for all the knots tied using any suture material, regardless of surgeon experience, were more than 61 N, which is the estimated minimum required ultimate load per suture during a maximum muscle contraction.1
Figure 6 shows the percentage of knot slipping at constant clinical failure load. Orthocord and Fiberwire suture materials had the lowest incidence of knot slippage. Surgeon C had complete knot slippage at constant clinical failure load using ForceFiber with the Weston knot and Ultrabraid with the Tennessee slider knot. When using Ultrabraid or ForceFiber, surgeons A and C had at least 2 knots slip for all knot configurations.
Discussion
Optimization of knot security for any given knot configuration, suture material, and surgeon experience level during arthroscopic knot tying is crucial.1-10 Our study results showed that, under single LTF test scenarios, there was a significant difference between in vitro and in situ conditions with respect to both knot configuration and surgeon experience level, except when Ultrabraid suture material was used. Arthroscopic sliding knots are lockable or nonlockable.7,12 With lockable sliding knots, slippage may be prevented by tensioning the wrapping limb, which distorts the post in the distal part of the knot, resulting in a kink in the post, thereby increasing the internal interference that increases the resistance of the knot from backing off. With nonlockable sliding knots, slippage may be prevented by the tight grip of the wrappings around the initial post.7 The static surgeon’s knot and the Tennessee slider knot are nonlockable, whereas the Weston knot is a distal lockable sliding knot. Compared with nonlockable sliding knots, lockable sliding knots cause less suture loop enlargement. In 1976, Tera and Aberg22 studied the strength of knotted thread for 12 different types of suture knots combined with 11 types of suture material. They conducted their study 1 week after suture material was inserted into the subcutaneous tissue of rabbits. Their results show a greater propensity for certain suture materials to slip when tested in an aqueous environment. In 1998, Babetty and colleagues23 used Wistar rats to compare the in vivo strength, knot efficiency, and knot security of 4 types of sliding knots and to assess tissue reaction as a result of knot configuration, knot volume, and suture size. They found that 4/0 knots lost more strength than 2/0 knots did, and they concluded that the tissue response to all the knots, except 2/0 nylon, was similar. They indicated that the inflammatory sheath volume varied with knot volume, suture size, and knot configuration. Our results agree with observations that exposure to an aqueous environment alters the force to clinical failure of comparable suture and knot configurations.
In addition, our findings indicate that surgeon familiarity with certain knots has a major effect on knot security. The difference in our 3 surgeons’ levels of familiarity with certain knots was somewhat minimized by the knot tying they practiced before submitting knots for testing. The findings contrast with those of Milia and colleagues,24 who conducted a biomechanical study to determine the effect of experience level on knot security. They compared an experienced arthroscopic shoulder surgeon with a junior-level orthopedic resident surgeon and concluded that experience did not affect knot security. However, the knots in their study were tied by hand, not through an arthroscopic cannula with instruments. Our findings suggest that both experienced and less experienced orthopedic residents should be encouraged to practice arthroscopic knot tying in a nonsurgical environment in order to become comfortable tying arthroscopic knots.
Braided nonabsorbable polyester suture traditionally has been found to be stronger than monofilament absorbable polydioxanone (PDS) and to have less slippage potential.8,9,25 Several studies have determined that the braided polyblend sutures now commonly used for arthroscopic knots have better strength profiles over more traditional materials.12,26,27 Orthocord has a dyed absorbable core (PDS, 68%), an undyed nonabsorbable ultrahigh-molecular-weight polyethylene (UHMWPE, 32%) sleeve, and a polyglactin coating.9,10 Both Ultrabraid and ForceFiber are made with braided UHMWPE and have just a few variations in weave patterns. Fiberwire has a multifiber UHMWPE core covered with braided polyester suture material. Several biomechanical studies25,26,28 have evaluated different arthroscopic sliding knot configurations with different suture materials, and all concluded that a surgeon who is choosing an arthroscopic repair technique should know the differences in suture materials and the knot strengths afforded by different knot configurations, as suture material is an important aspect of loop security. Our findings agree with their findings, that suture materials have a major effect on knot security, even with a series of 3 RHAPs, as in theory the RHAPs should minimize suture friction, internal interference, and slack between knot loops—emphasizing the effect of material selection. Furthermore, our findings also indicated that suture materials with a core in their design (Fiberwire, Orthocord) tend to have the lowest incidence of knot slippage. We had suspected that suture surface characteristics and suture construction could be important factors in knot slippage.
Our experimental design had its limitations. First, although we simulated factors such as temperature, plasma environment, and surgeon experience, tying a knot on a standardized post (30 mm in circumference) differed from what is typically done clinically. Second, the metal hooks used in this study were not compressible and did not interpose in the substance of the knot as soft tissue does in the clinical setting. Third, knots were tied with no tension against the sutures, whereas clinically knots are tied under tension as tissues are pulled together in reconstructions. Fourth, it was assumed that soaking in a physiologic solution bath (human blood plasma) at 37°C (body temperature) for 24 hours before testing was sufficient to simulate the aqueous medium in vivo after surgery, but these parameters may not represent conditions in a patient who has just undergone an arthroscopic shoulder repair and adheres to a passive motion protocol. Fifth, there was no blinding of knot type, and there was no randomization of tying order or testing order. Sixth, only a single LTF test was performed, and incremental cyclic loading can be more useful, as it has long been recognized as a leading source of failure in orthopedic repairs.
Conclusion
These study results advance our overall understanding of the biomechanics of the different knot configurations and loop security levels of the different braided polyblend sutures used in arthroscopic procedures through LTF in both in vitro and in situ conditions. Overall, no suture material was superior to any other in a fluid environment, as the combination of aqueous environment and surgeon level of experience with arthroscopic knot tying has a major effect on knot security under single LTF test scenarios. However, our data showed that Ultrabraid suture material had no effect on knot effectiveness over the fluid environment and the temperature. Furthermore, the study showed that the Tennessee slider knot had the steepest learning curve. This study may provide an alternative arthroscopic knots option for soft-tissue repair in which use of certain suture materials is limited.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
1. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202-207.
2. Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Loop security as a determinant of tissue fixation security. Arthroscopy. 1998;14(7):773-776.
3. Elkousy H, Hammerman SM, Edwards TB, et al. The arthroscopic square knot: a biomechanical comparison with open and arthroscopic knots. Arthroscopy. 2006;22(7):736-741.
4. Elkousy HA, Sekiya JK, Stabile KJ, McMahon PJ. A biomechanical comparison of arthroscopic sliding and sliding-locking knots. Arthroscopy. 2005;21(2):204-210.
5. Ilahi OA, Younas SA, Alexander J, Noble PC. Cyclic testing of arthroscopic knot security. Arthroscopy. 2004;20(1):62-68.
6. Loutzenheiser TD, Harryman DT 2nd, Ziegler DW, Yung SW. Optimizing arthroscopic knots using braided or monofilament suture. Arthroscopy. 1998;14(1):57-65.
7. Chan KC, Burkhart SS, Thiagarajan P, Goh JC. Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy. 2001;17(7):752-759.
8. Lee TQ, Matsuura PA, Fogolin RP, Lin AC, Kim D, McMahon PJ. Arthroscopic suture tying: a comparison of knot types and suture materials. Arthroscopy. 2001;17(4):348-352.
9. Mishra DK, Cannon WD Jr, Lucas DJ, Belzer JP. Elongation of arthroscopically tied knots. Am J Sports Med. 1997;25(1):113-117.
10. Kim SH, Ha KI, Kim SH, Kim JS. Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot. Arthroscopy. 2001;17(8):850-855.
11. Weston PV. A new clinch knot. Obstet Gynecol. 1991;78(1):144-147.
12. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
13. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
14. De Beer JF, van Rooyen K, Boezaart AP. Nicky’s knot—a new slip knot for arthroscopic surgery. Arthroscopy. 1998;14(1):109-110.
15. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
16. Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Fatigue properties of suture anchors in anterior shoulder reconstructions: Mitek GII. Arthroscopy. 1996;12(6):687-693.
17. Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy. 2009;25(2):192-199.
18. Richmond JC. A comparison of ultrasonic suture welding and traditional knot tying. Am J Sports Med. 200;29(3):297-299.
19. James JD, Wu MM, Batra EK, Rodeheaver GT, Edlich RF. Technical considerations in manual and instrument tying techniques. J Emerg Med. 1992;10(4):469-480.
20. Batra EK, Franz DA, Towler MA, et al. Influence of emergency physician’s tying technique on knot security. J Emerg Med. 1992;10(3):309-316.
21. Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop. 2010;39(12):569-576.
22. Tera H, Aberg C. The strength of suture knots after one week in vivo. Acta Chir Scand. 1976;142(4):301-307.
23. Babetty Z, Sümer A, Altintaş S, Ergüney S, Göksel S. Changes in knot-holding capacity of sliding knots in vivo and tissue reaction. Arch Surg. 1998;133(7):727-734.
24. Milia MJ, Peindl RD, Connor PM. Arthroscopic knot tying: the role of instrumentation in achieving knot security. Arthroscopy. 2005;21(1):69-76.
25. Lieurance RK, Pflaster DS, Abbott D, Nottage WM. Failure characteristics of various arthroscopically tied knots. Clin Orthop. 2003;(408):311-318.
26. Abbi G, Espinoza L, Odell T, Mahar A, Pedowitz R. Evaluation of 5 knots and 2 suture materials for arthroscopic rotator cuff repair: very strong sutures can still slip. Arthroscopy. 2006;22(1):38-43.
27. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146-1153.
28. Mahar AT, Moezzi DM, Serra-Hsu F, Pedowitz RA. Comparison and performance characteristics of 3 different knots when tied with 2 suture materials used for shoulder arthroscopy. Arthroscopy. 2006;22(6):614.e1-e2.
Arthroscopic Anterior Cruciate Ligament Reconstruction Using a Flexible Guide Pin With a Rigid Reamer
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
Anterior cruciate ligament (ACL) injuries are common, and arthroscopic ACL reconstruction is a routine procedure. Successful ACL reconstruction requires correct placement of the graft within the anatomical insertion of the native ACL.1-3 Errors in surgical technique—specifically, improper femoral tunnel placement—are the most common cause of graft failure in patients who present with recurrent instability after ACL reconstruction.4 There has been much emphasis on placing the tunnel more centrally in the ACL footprint as well as in a more horizontal position, which is thought to provide better rotational control and anterior-to-posterior translational stability.5-7
Two common techniques for creating the femoral tunnel, transtibial and anteromedial drilling, have their unique limitations. Transtibial drilling can place the tunnel high in the notch, resulting in nonanatomical, vertical graft placement.8,9 This technique can be modified to obtain a more anatomical tunnel, but the risk is the tunnel will be short and close to the joint line.10 To avoid these difficulties, surgeons began using an anteromedial portal.11,12 Although anteromedial drilling places the tunnel in a more anatomical position, it too has drawbacks, including the need to hyperflex the knee, a short tunnel, damage to articular cartilage, proximity to neurovascular structures, and difficulty in visualization during drilling.13-16
Femoral tunnel drilling techniques using flexible guide pins and reamers have been developed to address the limitations of rigid instruments. When we first started using flexible instruments through anteromedial portals, there were multiple incidents of reamer breakage during drilling. We therefore developed a technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position. The patient described in this article provided written informed consent for print and electronic publication of this report.
Technique
We begin with our standard arthroscopic portals, including superolateral outflow, lateral parapatellar, and medial parapatellar portals. The medial parapatellar portal is placed under direct visualization with insertion of an 18-gauge spinal needle, ensuring the trajectory reaches the anatomical location of the native ACL on the lateral femoral condyle (LFC). The ACL stump is débrided with a shaver and a radiofrequency ablator, leaving a remnant of tissue to assist with tunnel placement. We do not routinely perform a notchplasty unless there is a concern about possible graft impingement, or the notch is abnormally small. The anatomical footprint is marked with a small awl (Figure 1), and the arthroscope is moved into the anteromedial portal to confirm anatomical placement of the awl mark (Figure 2).
With the knee flexed to 100° to 110°, a flexible 2.7-mm nitonol guide pin (Smith & Nephew, Memphis, Tennessee) is placed freehand through the anteromedial portal into the anatomical footprint of the ACL, marked by the awl, and is passed through the femur before exiting the lateral skin. In most cases, we prefer freehand placement of the awl and pin; however, a femoral drill guide may be used to place the pin into the anatomical footprint of the ACL (Figure 3). The flexible pin allows for knee hyperflexion, clearance of the medial femoral condyle, central placement of the pin between the footprints of the anteromedial and posterolateral bundles for anatomical single-bundle reconstruction, and drilling of a long tunnel (average, 35-40 mm). The pin has a black laser marking that should be placed at the edge of the articular surface of the LFC to ensure appropriate depth of insertion (Figure 4).
A small incision is then made around the guide wire on the lateral thigh, and an outside-in depth gauge is used to obtain an accurate length for the femoral tunnel. The gauge must abut the femoral cortex for accurate assessment of tunnel length. We use an Endobutton (Smith & Nephew) for fixation of the graft in the tunnel. The measured length of the tunnel is used to select an Endobutton of appropriate size and the proper reaming depth for suspension. We routinely use a 10- or 15-mm Endobutton, which provides an average 20 to 25 mm of graft inside the bony tunnel. The knee may then be relaxed to a normal resting flexion angle off the side of the bed, and the arthroscope is inserted into a medial portal or an accessory anteromedial portal to ensure anatomical placement of the pin. Using a flexible guide pin allows the knee to be relatively extended, providing good visualization of overall positioning in relation to the posterior wall of the LFC, whereas keeping the knee in a flexed position (as with a rigid guide pin) can often compromise this visualization.
Using a solid reamer corresponding to the size of the graft, we drill over the guide pin to the appropriate depth, again with the knee hyperflexed (Figure 5), making sure not to breach the lateral femoral cortex, which would compromise fixation with the Endobutton. After drilling with the rigid reamer is completed, placement of the tunnel in an anatomical position is again confirmed with the knee in the normal resting flexion angle (Figure 6). Once the tibial tunnel is drilled at the anatomical footprint, the graft is passed with the proper-length Endobutton and is fixed on the tibial side with a bioabsorbable interference screw 1 to 2 mm larger than the soft-tissue graft and tibial tunnel size. The knee is flexed to 30° while the tibial screw is placed. Graft tension and impingement are then checked (Figure 7). Postoperative anteroposterior and lateral radiographs of the knee may be obtained to confirm anatomical placement of the tunnels as well as proper positioning of the Endobutton (Figures 8A, 8B).
Discussion
Successful ACL reconstruction depends heavily on anatomical tunnel positioning. Failure to place the femoral tunnel in the anatomical footprint of the native ACL results in incomplete restoration of knee kinematics, rotational instability, and graft failure.1-7 Two common techniques for creating this tunnel, transtibial and anteromedial drilling, can reliably place it in an anatomical position. Each technique, however, has limitations. Transtibial drilling can place the tunnel too vertical and high in the notch, or produce a short tibial tunnel close to the joint line.8-10 Anteromedial drilling requires knee hyperflexion, risks damaging the articular cartilage and nearby neurovascular structures, and makes visualization difficult.13-16
One option for addressing some of the difficulties and limitations with anteromedial drilling is to use flexible guide pins and reamers, as first introduced by Cain and Clancy.1 In a cadaveric study, Silver and colleagues17 demonstrated that interosseous tunnels drilled with flexible guide pins were on average more than 6 mm longer than those drilled with rigid pins and consistently were 40 mm or longer. In addition, all tunnels drilled with flexible guide pins were on average 42.3 mm away from the peroneal nerve and 26.1 mm away from the femoral origin of the lateral collateral ligament—safe distances.
Steiner and Smart18 compared flexible and rigid instruments used to drill transtibial and anteromedial (without hyperflexion) anatomical femoral tunnels in ACL reconstruction in cadaveric knees. Although transtibial drilling with flexible pins produced anatomical tunnels, the tunnels were shorter, and the pins exited more posterior in comparison with anteromedial drilling with flexible pins. Transtibial tunnels drilled with rigid pins were nonanatomical and exited more superior and anterior on the femur, resulting in longer tunnels. Anteromedial tunnels drilled with rigid and flexible pins were placed anatomically, but flexible pins produced longer tunnels, did not require hyperflexion (120°), could easily be placed with the knee in 90° of flexion, and did not violate the posterior femoral cortex.
Five times in our early experience with flexible guide pins and reamers, the reamer broke when LFC reaming was initiated. In each case, the broken reamer was retrieved. However, these complications resulted in increased surgical time and cost. In addition, an unretrievable reamer could have caused further injury and suboptimal outcomes. We subsequently developed an anteromedial technique that uses a flexible guide pin with a rigid reamer to place the femoral tunnel in an anatomical position (Figure 9). The flexible pin provides consistent placement of anatomical tunnels averaging 35 to 40 mm in length. Use of the flexible pin does not require constant hyperflexion of the knee, and it allows for better visualization of the posterior wall of the LFC, ensures anatomical graft placement, and decreases the risk of damaging articular cartilage and causing neurovascular injury. Use of the rigid reamer negates the risks and additional costs associated with reamer breakage. It is unclear why 5 flexible reamers broke during our early use of flexible guide pins and reamers, but it is possible that, because of the patients’ anatomy, placement of the pin in the correct anatomical position in the ACL footprint put a significant amount of abnormal stress on the reamer during tunnel reaming, leading to breakage and failure.
A short femoral tunnel is a common complication of using an anteromedial portal for tunnel drilling.13-16 With the technique we have been using, tunnel lengths average 35 to 40 mm. To address the occasional shorter tunnel, we use Endobutton Direct (Smith & Nephew), which allows for direct fixation of the graft on the button, maximizing the amount of graft in the femoral tunnel and minimizing graft–tunnel length mismatch. In the event there is a lateral wall breach during overdrilling with the reamer, the femoral graft may be secured with screw and post, with interference screw, or with the larger Xtendobuton (Smith & Nephew).
We have successfully used this technique with bone–patellar tendon–bone (BPTB) and hamstring autografts, as well as allografts. Complications, such as graft–tunnel length mismatch, have been uncommon, but, when using BPTB grafts, passing the bone block into the femoral tunnel can be difficult because of the sharp turn required.
Conclusion
Successful ACL reconstruction depends heavily on placement of the graft within the anatomical insertion of the native ACL. With the development of techniques that use flexible guide pins and reamers, it has become possible to place longer anatomical femoral tunnels without the need for hyperflexion. Use of a flexible guide pin with a rigid reamer allows placement of longer anatomical tunnels through an anteromedial portal, reduces time spent with the knee in hyperflexion, provides better viewing, poses less risk of damage to the articular cartilage and neurovascular structures, and at a lower cost with less risk of reamer breakage. In addition, this technique can be used with a variety of graft options, including BPTB grafts, hamstring autografts, and allografts.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
1. Cain EL Jr, Clancy WG Jr. Anatomic endoscopic anterior cruciate ligament reconstruction with patella tendon autograft. Orthop Clin North Am. 2002;33(4):717-725.
2. Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am. 2006;88(suppl 4):2-10.
3. Christel P, Sahasrabudhe A, Basdekis G. Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy. 2008;24(10):1146-1151.
4. Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34(1):79-98.
5. Miller CD, Gerdeman AC, Hart JM, et al. A comparison of 2 drilling techniques on the femoral tunnel for anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(3):372-379.
6. Seon JK, Park SJ, Lee KB, Seo HY, Kim MS, Song EK. In vivo stability and clinical comparison of anterior cruciate ligament reconstruction using low or high femoral tunnel positions. Am J Sports Med. 2011;39(1):127-133.
7. Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912-1919.
8. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
9. Tompkins M, Milewski MD, Brockmeier SF, Gaskin CM, Hart JM, Miller MD. Anatomic femoral tunnel drilling in anterior cruciate ligament reconstruction: use of an accessory medial portal versus traditional transtibial drilling. Am J Sports Med. 2012;40(6):1313-1321.
10. Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708-1715.
11. Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113-115.
12. Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25(1):95-101.
13. Basdekis G, Abisafi C, Christel P. Influence of knee flexion angle on femoral tunnel characteristics when drilled through the anteromedial portal during anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(4):459-464.
14. Zantop T, Haase AK, Fu FH, Petersen W. Potential risk of cartilage damage in double bundle ACL reconstruction: impact of knee flexion angle and portal location on the femoral PL bundle tunnel. Arch Orthop Trauma Surg. 2008;128(5):509-513.
15. Farrow LD, Parker RD. The relationship of lateral anatomic structures to exiting guide pins during femoral tunnel preparation utilizing an accessory medial portal. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):747-753.
16. Nakamura M, Deie M, Shibuya H, et al. Potential risks of femoral tunnel drilling through the far anteromedial portal: a cadaveric study. Arthroscopy. 2009;25(5):481-487.
17. Silver AG, Kaar SG, Grisell MK, Reagan JM, Farrow LD. Comparison between rigid and flexible systems for drilling the femoral tunnel through an anteromedial portal in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(6):790-795.
18. Steiner ME, Smart LR. Flexible instruments outperform rigid instruments to place anatomic anterior cruciate ligament femoral tunnels without hyperflexion. Arthroscopy. 2012;28(6):835-843.
Is Hemolysis a Clinical Marker of Propionibacterium acnes Orthopedic Infection or a Phylogenetic Marker?
Letter to the Editor
Is Hemolysis a Clinical Marker of Propionibacterium acnes Orthopedic Infection or a Phylogenetic Marker?
We read with great interest the study by Nodzo and colleagues in the May 2014 issue of The American Journal of Orthopedics on hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection.1 We agree with the authors that determining if a P acnes culture is a true infection or a contaminant remains a challenge. Although P acnes is described as a commensal bacterium with a low pathogenicity, its involvement has been reported in many clinical entities, especially device-related infections.2P acnes is usually the cause of delayed infections occurring 3 to 24 months or more after prosthesis placement. The rate of P acnes involvement, probably underestimated, is about 10%.3 Although this bacterium was considered to be a contaminant, several virulence factors have been recently identified: putative hemolysins or cytotoxins (CAMP factors, hemolysin III) and enzymes putatively involved in degrading host tissue or molecules (GehA lipase, lysophospholipase, hyaluronate lyase, endoglycoceramidase, etc).4
Interestingly, Nodzo and colleagues revealed that 13 out of 22 P acnes strains were hemolytic and, among them, 10 were considered as definite infections, including 3 with only 1 positive sample. The authors could not identify a statistically significant trend, probably because their study was underpowered due to the size of this case series, as discussed by the authors. Nevertheless, the hemolytic activity of the strains was investigated in the 1980s by adding different concentrations of blood obtained from rabbits, sheep, or humans.5 The hemolytic activity was recorded as positive when a clear, colorless zone around the colonies appeared or weak when slight and incomplete hemolysis under the colonies was found.5 Depending on the erythrocyte origin, differences in the lytic action of hemolysin or cytotoxin may indicate the existence of various enzymes. These enzymes could have different levels of production and provide a distinct hemolytic profile. This hemolytic activity observation could also be correlated to the genetic background of the isolates.
In fact, from a genetic and epidemiological point of view, the sequence analysis of recA gene distinguished 2 distinct lineages of P acnes: types I and II.4 The association of some strains with specific clinical presentations was also demonstrated. Later, McDowell and colleagues6 reported 5 main phylogenetically distinct groups: IA, IB, IC, II, and III. It would have been interesting to know the phylogenetic groups of the strains tested in the study by Nodzo and coauthors, especially as Sampedro and colleagues7 recently reported more phylogenetic groups IA and IB among P acnes strains involved in bone and joint infections. Both of these phylotypes are hemolytic, unlike phylotypes II and III, less often encountered in this clinical entity as reported recently.8 We agree with the authors that hemolytic behavior may be one of the key factors in the variability in the pathogenicity of P acnes strains, suggesting that some strains could be more aggressive than others during deep infection. Another feature is likely the biofilm-production ability of the strains.9,10
According to our experience, the hemolysis behavior was slightly different depending on which blood agar plates were used to detect hemolytic properties. We have selected 8 isolates or reference ATCC strains from different phylotypes. Each isolate was seeded on 5 different blood agar plates with erythrocyte from various origins (Table). We can confirm that only strains belonging to IA and IB phylotypes were hemolytic, with different behavior as previously reported (Figure).8 Similarly, within IA phylotype strains, the hemolytic property could be different suggesting a difference in the genetic background. However, as the genes encoding all 5 CAMP factors are present in all P acnes groups studied by Valanne and colleagues11 (IA, IB, and II), observed differences reflected different levels of expression rather than missing genes. Moreover, when camp2 or camp4 genes were deleted, the ∆camp2 but not the ∆camp4 mutant exhibited reduced hemolytic activity with sheep erythrocytes, indicating that CAMP factor 2 seems to be the major active cohemolytic factor, but in an IA phylotype P acnes genetic background.12
To conclude, the link between hemolysis and P acnes deep infection remains controversial and complex. The phenotypic differences observed between strains from various types reflect deeper differences in their phylogeny. The hemolytic ability raises the possibility that strains may also display a specific behavior according to their type and variation in their expression of putative virulence factors, including hemolysin, cytotoxin, or lipase. Further studies are clearly needed to better understand the virulence and phylogeny of P acnes strains in order to distinguish contamination from bone infection.
Stéphane Corvec, PharmD, PhD, Jérémy Luchetta, MSc, and Guillaume Ghislain Aubin, PharmD
Nantes University Hospital, Microbiology Laboratory, Nantes, France
Authors’ Response
Corvec and colleagues wrote an interesting summary and make excellent points about the role of hemolysis in Propionibacterium acnes. P acnes upper extremity infection has become an increasingly recognized problem, and determining whether a P acnes culture represents a true infection or a contaminant is still a challenge. We performed this study in hopes of finding an easily usable characteristic of P acnes that would assist the clinician in identifying P acnes strains as true infections rather than contaminants.
Certain pathogenic characteristics of P acnes have been identified, but the clinical implications of this bacterium are still being evaluated. We recognize that the hemolysis phenotype is a characteristic, and may not be the main pathogenic feature, of certain phylotypes of P acnes. It is possible the hemolytic strains in our study were from the IA and IB phylotypes, but, unfortunately, we did not specifically evaluate for phylogeny in our study. This would have correlated well with the work of Sampedro and colleagues,1 which suggested most deep bone and joint infections occur with type IA and IB P acnes phylotypes. Although less common in orthopedic infections, the type II and III phylotypes of P acnes are also capable of causing deep infection, and may not cause a hemolytic reaction on blood agar, which may be why we had some patients classified as a definite infection that did not have a hemolytic strain of P acnes. It is also possible a hemolytic strain may truly be a contaminant, but we did not observe this in our small case series. A larger series may help elucidate this finding, but the majority of truly infected patients in our case series had a hemolytic P acnes phenotype.
The type of blood agar used could have also influenced our results, as noted in the Table in Corvec and colleagues’ letter. We observed the most robust hemolysis on brucella blood agar, and limited hemolysis on CDC (Centers for Disease Control and Prevention) anaerobe blood agar; however, we did not evaluate multiple different blood agar preparations, which could have identified more hemolytic strains.
In our study, the presence of hemolysis was helpful in determining whether or not a true infection existed, but the absence of the hemolytic phenotype did not offer much additional information. The hemolytic phenotype may be a potential marker for those strains that are more aggressive and possibly represent the IA and IB phylotypes, which, as previously stated, are more commonly found in deep bone and joint infections.1 Hemolysis may serve as a surrogate marker for determining these phylotypes since determining phylogeny in a hospital laboratory is burdensome and not possible in most institutions.
In summary, we agree the hemolytic phenotype is commonly observed in certain P acnes phylotypes, and that not all upper extremity orthopedic P acnes infections will have a hemolytic finding. The genetic differences in P acnes strains are complex, and finding a marker of truly pathogenic strains has yet to be established. Larger studies evaluating the clinical outcomes and laboratory findings of patients with and without hemolytic strains of P acnes and evaluating which blood agar is the best at identifying the hemolytic phenotype may be beneficial. Identifying or combining multiple clinical and microbe-specific characteristics may also help guide treatment recommendations when a positive P acnes culture is identified.
Scott R. Nodzo, MD
John K. Crane, MD, PhD
Thomas R. Duquin, MD
Department of Orthopedics
University at Buffalo
Buffalo, NY
Letter to the Editor
1. Nodzo SR, Hohman DW, Crane JK, Duquin TR. Hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection. Am J Orthop. 2014;43(5):E93-E97.
2. Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. BioMed Res Int. 2013;2013:804391.
3. Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012;35(10):923-934.
4. Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Médecine Mal Infect. 2014;44(6):241-250.
5. Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6(6):555-558.
6. McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57(Pt 2):218-224.
7. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
8. Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PloS One. 2010;5(8):e12277.
9. Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885-1891.
10. Holmberg A, Lood R, Mörgelin M, et al. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin Microbiol Infect. 2009;15(8):787-795.
11. Valanne S, McDowell A, Ramage G, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiol. 2005;151(Pt 5):1369-1379.
12. Sörensen M, Mak TN, Hurwitz R, et al. Mutagenesis of Propionibacterium acnes and analysis of two CAMP factor knock-out mutants. J Microbiol Methods. 2010;83(2):211-216.
Authors' Response Reference
1. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
Letter to the Editor
Is Hemolysis a Clinical Marker of Propionibacterium acnes Orthopedic Infection or a Phylogenetic Marker?
We read with great interest the study by Nodzo and colleagues in the May 2014 issue of The American Journal of Orthopedics on hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection.1 We agree with the authors that determining if a P acnes culture is a true infection or a contaminant remains a challenge. Although P acnes is described as a commensal bacterium with a low pathogenicity, its involvement has been reported in many clinical entities, especially device-related infections.2P acnes is usually the cause of delayed infections occurring 3 to 24 months or more after prosthesis placement. The rate of P acnes involvement, probably underestimated, is about 10%.3 Although this bacterium was considered to be a contaminant, several virulence factors have been recently identified: putative hemolysins or cytotoxins (CAMP factors, hemolysin III) and enzymes putatively involved in degrading host tissue or molecules (GehA lipase, lysophospholipase, hyaluronate lyase, endoglycoceramidase, etc).4
Interestingly, Nodzo and colleagues revealed that 13 out of 22 P acnes strains were hemolytic and, among them, 10 were considered as definite infections, including 3 with only 1 positive sample. The authors could not identify a statistically significant trend, probably because their study was underpowered due to the size of this case series, as discussed by the authors. Nevertheless, the hemolytic activity of the strains was investigated in the 1980s by adding different concentrations of blood obtained from rabbits, sheep, or humans.5 The hemolytic activity was recorded as positive when a clear, colorless zone around the colonies appeared or weak when slight and incomplete hemolysis under the colonies was found.5 Depending on the erythrocyte origin, differences in the lytic action of hemolysin or cytotoxin may indicate the existence of various enzymes. These enzymes could have different levels of production and provide a distinct hemolytic profile. This hemolytic activity observation could also be correlated to the genetic background of the isolates.
In fact, from a genetic and epidemiological point of view, the sequence analysis of recA gene distinguished 2 distinct lineages of P acnes: types I and II.4 The association of some strains with specific clinical presentations was also demonstrated. Later, McDowell and colleagues6 reported 5 main phylogenetically distinct groups: IA, IB, IC, II, and III. It would have been interesting to know the phylogenetic groups of the strains tested in the study by Nodzo and coauthors, especially as Sampedro and colleagues7 recently reported more phylogenetic groups IA and IB among P acnes strains involved in bone and joint infections. Both of these phylotypes are hemolytic, unlike phylotypes II and III, less often encountered in this clinical entity as reported recently.8 We agree with the authors that hemolytic behavior may be one of the key factors in the variability in the pathogenicity of P acnes strains, suggesting that some strains could be more aggressive than others during deep infection. Another feature is likely the biofilm-production ability of the strains.9,10
According to our experience, the hemolysis behavior was slightly different depending on which blood agar plates were used to detect hemolytic properties. We have selected 8 isolates or reference ATCC strains from different phylotypes. Each isolate was seeded on 5 different blood agar plates with erythrocyte from various origins (Table). We can confirm that only strains belonging to IA and IB phylotypes were hemolytic, with different behavior as previously reported (Figure).8 Similarly, within IA phylotype strains, the hemolytic property could be different suggesting a difference in the genetic background. However, as the genes encoding all 5 CAMP factors are present in all P acnes groups studied by Valanne and colleagues11 (IA, IB, and II), observed differences reflected different levels of expression rather than missing genes. Moreover, when camp2 or camp4 genes were deleted, the ∆camp2 but not the ∆camp4 mutant exhibited reduced hemolytic activity with sheep erythrocytes, indicating that CAMP factor 2 seems to be the major active cohemolytic factor, but in an IA phylotype P acnes genetic background.12
To conclude, the link between hemolysis and P acnes deep infection remains controversial and complex. The phenotypic differences observed between strains from various types reflect deeper differences in their phylogeny. The hemolytic ability raises the possibility that strains may also display a specific behavior according to their type and variation in their expression of putative virulence factors, including hemolysin, cytotoxin, or lipase. Further studies are clearly needed to better understand the virulence and phylogeny of P acnes strains in order to distinguish contamination from bone infection.
Stéphane Corvec, PharmD, PhD, Jérémy Luchetta, MSc, and Guillaume Ghislain Aubin, PharmD
Nantes University Hospital, Microbiology Laboratory, Nantes, France
Authors’ Response
Corvec and colleagues wrote an interesting summary and make excellent points about the role of hemolysis in Propionibacterium acnes. P acnes upper extremity infection has become an increasingly recognized problem, and determining whether a P acnes culture represents a true infection or a contaminant is still a challenge. We performed this study in hopes of finding an easily usable characteristic of P acnes that would assist the clinician in identifying P acnes strains as true infections rather than contaminants.
Certain pathogenic characteristics of P acnes have been identified, but the clinical implications of this bacterium are still being evaluated. We recognize that the hemolysis phenotype is a characteristic, and may not be the main pathogenic feature, of certain phylotypes of P acnes. It is possible the hemolytic strains in our study were from the IA and IB phylotypes, but, unfortunately, we did not specifically evaluate for phylogeny in our study. This would have correlated well with the work of Sampedro and colleagues,1 which suggested most deep bone and joint infections occur with type IA and IB P acnes phylotypes. Although less common in orthopedic infections, the type II and III phylotypes of P acnes are also capable of causing deep infection, and may not cause a hemolytic reaction on blood agar, which may be why we had some patients classified as a definite infection that did not have a hemolytic strain of P acnes. It is also possible a hemolytic strain may truly be a contaminant, but we did not observe this in our small case series. A larger series may help elucidate this finding, but the majority of truly infected patients in our case series had a hemolytic P acnes phenotype.
The type of blood agar used could have also influenced our results, as noted in the Table in Corvec and colleagues’ letter. We observed the most robust hemolysis on brucella blood agar, and limited hemolysis on CDC (Centers for Disease Control and Prevention) anaerobe blood agar; however, we did not evaluate multiple different blood agar preparations, which could have identified more hemolytic strains.
In our study, the presence of hemolysis was helpful in determining whether or not a true infection existed, but the absence of the hemolytic phenotype did not offer much additional information. The hemolytic phenotype may be a potential marker for those strains that are more aggressive and possibly represent the IA and IB phylotypes, which, as previously stated, are more commonly found in deep bone and joint infections.1 Hemolysis may serve as a surrogate marker for determining these phylotypes since determining phylogeny in a hospital laboratory is burdensome and not possible in most institutions.
In summary, we agree the hemolytic phenotype is commonly observed in certain P acnes phylotypes, and that not all upper extremity orthopedic P acnes infections will have a hemolytic finding. The genetic differences in P acnes strains are complex, and finding a marker of truly pathogenic strains has yet to be established. Larger studies evaluating the clinical outcomes and laboratory findings of patients with and without hemolytic strains of P acnes and evaluating which blood agar is the best at identifying the hemolytic phenotype may be beneficial. Identifying or combining multiple clinical and microbe-specific characteristics may also help guide treatment recommendations when a positive P acnes culture is identified.
Scott R. Nodzo, MD
John K. Crane, MD, PhD
Thomas R. Duquin, MD
Department of Orthopedics
University at Buffalo
Buffalo, NY
Letter to the Editor
Is Hemolysis a Clinical Marker of Propionibacterium acnes Orthopedic Infection or a Phylogenetic Marker?
We read with great interest the study by Nodzo and colleagues in the May 2014 issue of The American Journal of Orthopedics on hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection.1 We agree with the authors that determining if a P acnes culture is a true infection or a contaminant remains a challenge. Although P acnes is described as a commensal bacterium with a low pathogenicity, its involvement has been reported in many clinical entities, especially device-related infections.2P acnes is usually the cause of delayed infections occurring 3 to 24 months or more after prosthesis placement. The rate of P acnes involvement, probably underestimated, is about 10%.3 Although this bacterium was considered to be a contaminant, several virulence factors have been recently identified: putative hemolysins or cytotoxins (CAMP factors, hemolysin III) and enzymes putatively involved in degrading host tissue or molecules (GehA lipase, lysophospholipase, hyaluronate lyase, endoglycoceramidase, etc).4
Interestingly, Nodzo and colleagues revealed that 13 out of 22 P acnes strains were hemolytic and, among them, 10 were considered as definite infections, including 3 with only 1 positive sample. The authors could not identify a statistically significant trend, probably because their study was underpowered due to the size of this case series, as discussed by the authors. Nevertheless, the hemolytic activity of the strains was investigated in the 1980s by adding different concentrations of blood obtained from rabbits, sheep, or humans.5 The hemolytic activity was recorded as positive when a clear, colorless zone around the colonies appeared or weak when slight and incomplete hemolysis under the colonies was found.5 Depending on the erythrocyte origin, differences in the lytic action of hemolysin or cytotoxin may indicate the existence of various enzymes. These enzymes could have different levels of production and provide a distinct hemolytic profile. This hemolytic activity observation could also be correlated to the genetic background of the isolates.
In fact, from a genetic and epidemiological point of view, the sequence analysis of recA gene distinguished 2 distinct lineages of P acnes: types I and II.4 The association of some strains with specific clinical presentations was also demonstrated. Later, McDowell and colleagues6 reported 5 main phylogenetically distinct groups: IA, IB, IC, II, and III. It would have been interesting to know the phylogenetic groups of the strains tested in the study by Nodzo and coauthors, especially as Sampedro and colleagues7 recently reported more phylogenetic groups IA and IB among P acnes strains involved in bone and joint infections. Both of these phylotypes are hemolytic, unlike phylotypes II and III, less often encountered in this clinical entity as reported recently.8 We agree with the authors that hemolytic behavior may be one of the key factors in the variability in the pathogenicity of P acnes strains, suggesting that some strains could be more aggressive than others during deep infection. Another feature is likely the biofilm-production ability of the strains.9,10
According to our experience, the hemolysis behavior was slightly different depending on which blood agar plates were used to detect hemolytic properties. We have selected 8 isolates or reference ATCC strains from different phylotypes. Each isolate was seeded on 5 different blood agar plates with erythrocyte from various origins (Table). We can confirm that only strains belonging to IA and IB phylotypes were hemolytic, with different behavior as previously reported (Figure).8 Similarly, within IA phylotype strains, the hemolytic property could be different suggesting a difference in the genetic background. However, as the genes encoding all 5 CAMP factors are present in all P acnes groups studied by Valanne and colleagues11 (IA, IB, and II), observed differences reflected different levels of expression rather than missing genes. Moreover, when camp2 or camp4 genes were deleted, the ∆camp2 but not the ∆camp4 mutant exhibited reduced hemolytic activity with sheep erythrocytes, indicating that CAMP factor 2 seems to be the major active cohemolytic factor, but in an IA phylotype P acnes genetic background.12
To conclude, the link between hemolysis and P acnes deep infection remains controversial and complex. The phenotypic differences observed between strains from various types reflect deeper differences in their phylogeny. The hemolytic ability raises the possibility that strains may also display a specific behavior according to their type and variation in their expression of putative virulence factors, including hemolysin, cytotoxin, or lipase. Further studies are clearly needed to better understand the virulence and phylogeny of P acnes strains in order to distinguish contamination from bone infection.
Stéphane Corvec, PharmD, PhD, Jérémy Luchetta, MSc, and Guillaume Ghislain Aubin, PharmD
Nantes University Hospital, Microbiology Laboratory, Nantes, France
Authors’ Response
Corvec and colleagues wrote an interesting summary and make excellent points about the role of hemolysis in Propionibacterium acnes. P acnes upper extremity infection has become an increasingly recognized problem, and determining whether a P acnes culture represents a true infection or a contaminant is still a challenge. We performed this study in hopes of finding an easily usable characteristic of P acnes that would assist the clinician in identifying P acnes strains as true infections rather than contaminants.
Certain pathogenic characteristics of P acnes have been identified, but the clinical implications of this bacterium are still being evaluated. We recognize that the hemolysis phenotype is a characteristic, and may not be the main pathogenic feature, of certain phylotypes of P acnes. It is possible the hemolytic strains in our study were from the IA and IB phylotypes, but, unfortunately, we did not specifically evaluate for phylogeny in our study. This would have correlated well with the work of Sampedro and colleagues,1 which suggested most deep bone and joint infections occur with type IA and IB P acnes phylotypes. Although less common in orthopedic infections, the type II and III phylotypes of P acnes are also capable of causing deep infection, and may not cause a hemolytic reaction on blood agar, which may be why we had some patients classified as a definite infection that did not have a hemolytic strain of P acnes. It is also possible a hemolytic strain may truly be a contaminant, but we did not observe this in our small case series. A larger series may help elucidate this finding, but the majority of truly infected patients in our case series had a hemolytic P acnes phenotype.
The type of blood agar used could have also influenced our results, as noted in the Table in Corvec and colleagues’ letter. We observed the most robust hemolysis on brucella blood agar, and limited hemolysis on CDC (Centers for Disease Control and Prevention) anaerobe blood agar; however, we did not evaluate multiple different blood agar preparations, which could have identified more hemolytic strains.
In our study, the presence of hemolysis was helpful in determining whether or not a true infection existed, but the absence of the hemolytic phenotype did not offer much additional information. The hemolytic phenotype may be a potential marker for those strains that are more aggressive and possibly represent the IA and IB phylotypes, which, as previously stated, are more commonly found in deep bone and joint infections.1 Hemolysis may serve as a surrogate marker for determining these phylotypes since determining phylogeny in a hospital laboratory is burdensome and not possible in most institutions.
In summary, we agree the hemolytic phenotype is commonly observed in certain P acnes phylotypes, and that not all upper extremity orthopedic P acnes infections will have a hemolytic finding. The genetic differences in P acnes strains are complex, and finding a marker of truly pathogenic strains has yet to be established. Larger studies evaluating the clinical outcomes and laboratory findings of patients with and without hemolytic strains of P acnes and evaluating which blood agar is the best at identifying the hemolytic phenotype may be beneficial. Identifying or combining multiple clinical and microbe-specific characteristics may also help guide treatment recommendations when a positive P acnes culture is identified.
Scott R. Nodzo, MD
John K. Crane, MD, PhD
Thomas R. Duquin, MD
Department of Orthopedics
University at Buffalo
Buffalo, NY
Letter to the Editor
1. Nodzo SR, Hohman DW, Crane JK, Duquin TR. Hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection. Am J Orthop. 2014;43(5):E93-E97.
2. Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. BioMed Res Int. 2013;2013:804391.
3. Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012;35(10):923-934.
4. Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Médecine Mal Infect. 2014;44(6):241-250.
5. Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6(6):555-558.
6. McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57(Pt 2):218-224.
7. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
8. Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PloS One. 2010;5(8):e12277.
9. Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885-1891.
10. Holmberg A, Lood R, Mörgelin M, et al. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin Microbiol Infect. 2009;15(8):787-795.
11. Valanne S, McDowell A, Ramage G, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiol. 2005;151(Pt 5):1369-1379.
12. Sörensen M, Mak TN, Hurwitz R, et al. Mutagenesis of Propionibacterium acnes and analysis of two CAMP factor knock-out mutants. J Microbiol Methods. 2010;83(2):211-216.
Authors' Response Reference
1. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
Letter to the Editor
1. Nodzo SR, Hohman DW, Crane JK, Duquin TR. Hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection. Am J Orthop. 2014;43(5):E93-E97.
2. Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. BioMed Res Int. 2013;2013:804391.
3. Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012;35(10):923-934.
4. Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Médecine Mal Infect. 2014;44(6):241-250.
5. Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6(6):555-558.
6. McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57(Pt 2):218-224.
7. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.
8. Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PloS One. 2010;5(8):e12277.
9. Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885-1891.
10. Holmberg A, Lood R, Mörgelin M, et al. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin Microbiol Infect. 2009;15(8):787-795.
11. Valanne S, McDowell A, Ramage G, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiol. 2005;151(Pt 5):1369-1379.
12. Sörensen M, Mak TN, Hurwitz R, et al. Mutagenesis of Propionibacterium acnes and analysis of two CAMP factor knock-out mutants. J Microbiol Methods. 2010;83(2):211-216.
Authors' Response Reference
1. Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138-145.