User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Arthroscopic Posterior-Inferior Capsular Release in the Treatment of Overhead Athletes
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
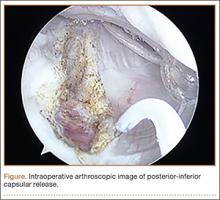
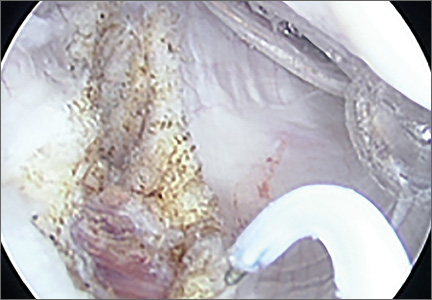
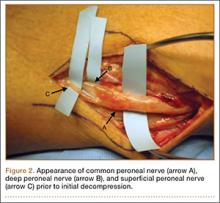
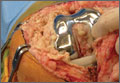
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
Percutaneous Fixation of Hypertrophic Nonunion of the Inferior Pubic Ramus: A Report of Two Cases and Surgical Technique
Fractures of the superior and inferior pelvic rami are common in pelvic ring injuries.1 These fractures are routinely treated successfully without surgery.2 When the pelvic ring is injured, and ramus fracture or fractures represent a point of instability, surgical fixation can be performed to impart stability and reduce discomfort.3 Patients with pubic ramus fracture(s) have overall greater long-term morbidity and mortality.4 Operative stabilization of the superior pubic ramus can be achieved with open reduction and internal fixation, external fixation, and percutaneous medullary screw fixation.5-8 Inferior ramus fractures are seldom treated directly and acutely with operative reduction and fixation, as the mechanical advantage inferior ramus fixation provides is unknown.
Persistent nonunion of the pelvic ring can cause pain and disability and make reconstruction increasingly difficult.9 Open and percutaneous fixation techniques have been used to address symptomatic nonunions of the superior pubic ramus.9,10 There is limited evidence supporting surgical fixation of the inferior ramus. Open surgical fixation for symptomatic nonunions of the inferior ramus has been described.11,12 The inferior ramus has an osseous fixation pathway (OFP) amenable to percutaneous screw placement.13 Placement of a percutaneous screw in the inferior ramus requires use of preoperative computed tomography (CT) and is technically demanding. Surgeons must understand use of intraoperative fluoroscopy to ensure that the screw is contained within bone and crosses the intended zone of nonunion.
In this article, we report 2 cases of adults with symptomatic hypertrophic nonunions of the inferior ramus, treated with percutaneous screw fixation. Both patients presented with focal groin pain and activity limitations. Each had concurrent ipsilateral hypertrophic nonunions of the superior ramus, treated with percutaneous antegrade intramedullary stabilization. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
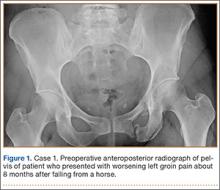
A 45-year-old woman fell from a horse about 8 months before presenting to the orthopedic outpatient clinic. Pelvic radiographs obtained after the fall were negative for fracture, but subsequent pelvic magnetic resonance imaging led to the diagnoses of minimally displaced left superior and inferior pubic ramus fractures and associated right-sided sacral ala fracture. The patient was treated with protected weight-bearing according to symptoms, but increasing activity-related pain and discomfort in the left groin persisted for months after injury. These symptoms were treated with analgesic medication, physical therapy, and chiropractic manipulation. Repeat imaging showed hypertrophic nonunions of the left superior and inferior pubic rami (Figure 1). Findings of the serologic testing performed for infection and metabolic deficiencies were normal at that time. The patient was referred for surgical consultation.
On evaluation, she reported constant pain in the left groin with ambulation. Specifically, squatting, pushing and pulling activities were extremely uncomfortable. She had been unable to return to work either full-time or part-time. On physical examination, she walked with an antalgic gait with a decreased stance phase of the left lower extremity. She had tenderness to palpation medial to the hip joint without evidence of hernia or lymphadenopathy. The pelvis was stable to manual compression testing.
Pelvic CT showed the nonunion site and the osteology of the inferior and superior pubic ramus of the pelvis, as well as minimal displacement and good alignment of the rami.
The patient was placed supine on a flat radiolucent table (Mizuho OSI, Union City, California). Preoperative cephalosporin antibiotics were administered. After induction of general anesthesia, the lumbosacral spine was elevated under 2 folded blankets. Arms were abducted to allow for pelvic imaging, and all bony prominences were padded. A urinary catheter was inserted aseptically to decompress the bladder. The entire abdomen and bilateral flanks were shaved, prepared, and draped in usual sterile fashion. A partially threaded cannulated screw was placed using a percutaneous antegrade technique to address the hypertrophic superior ramus nonunion.
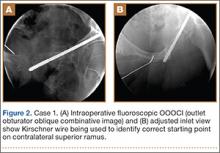
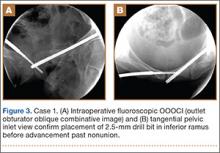
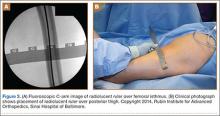
A C-arm fluoroscopy unit (Ziehm, Orlando, Florida) was positioned on the injured side. The surgeon stood on the contralateral side. A pelvic OOOCI (outlet obturator oblique combinative image) of the symphysis pubis was obtained. This view defined the medial and lateral extents of the inferior ramus. A 0.062-in smooth Kirschner wire was used to percutaneously locate an ideal starting point on the cranial aspect of the contralateral superior pubic ramus. The starting point was adjusted on this view until an ideal intended trajectory into the contralateral (affected) inferior pubic ramus was visualized (Figure 2A).
The C-arm beam was then oriented to an “excessive” pelvic inlet view tangential to the posterior cortical surface of the affected inferior pubic ramus (Figure 2B). The tip of the wire was then adjusted to position and aim it slightly anterior to the posterior cortical surface of the affected inferior ramus. The wire was advanced into the bone about 1 cm, and the location and direction of the wire were reconfirmed as accurate.
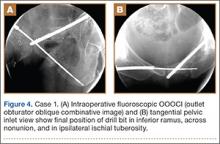
A vertical skin incision was then made around the wire, and the 4.5-mm cannulated drill was placed over the wire. A soft-tissue protective drill sleeve and oscillating technique were used to protect the soft-tissue anatomy. The trajectory of the drill was again confirmed on pelvic OOOCI and advanced into the bone. The intended path of the drill was from the cranial-medial symphyseal cortex of the contralateral superior ramus, through the symphysis pubis obliquely, and then into the medullary canal of the affected inferior ramus. Frequent biplanar fluoroscopic imaging followed this progression of the drill to the nonunion site. The cannulated drill was then removed and exchanged for a calibrated extra-long 2.5-mm drill bit, placed through the soft-tissue drill sleeve and into the glide hole created by the 4.5-mm cannulated drill. The C-arm unit ensured accurate positioning of the 2.5-mm drill on both pelvic OOOCI and “excessive” inlet view before advancement (Figures 3A, 3B). The 2.5-mm drill was advanced caudally, laterally, and anteriorly in the ramus, past the nonunion site, and then was stopped before it exited the cortex of the ischial tuberosity (Figures 4A, 4B).
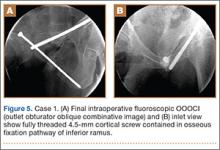
The depth of the drill bit was assessed with a known-length protective drill sleeve and calibrated drill. Alternatively, depth can be assessed with another same-length calibrated drill bit positioned adjacent to the inserted drill bit. A fully threaded, blunt-tipped 4.5-mm cortical screw was then placed through the glide hole. Both fluoroscopic views were used to confirm that the screw followed the same trajectory as the drill. Finally, the screw was again checked on biplanar fluoroscopy to confirm it had remained in the OFP of the inferior ramus (Figures 5A, 5B).
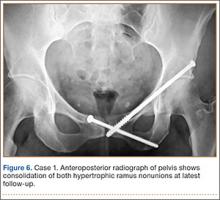
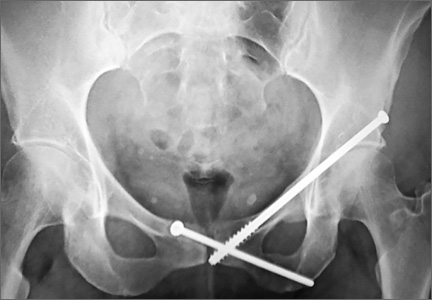
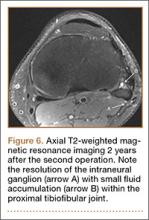
Postoperative pelvic CT confirmed position and length of the screws. The patient was allowed weight-of-limb weight-bearing on her affected side after surgery. She was discharged the first day after surgery and allowed use of oral analgesics. Six weeks after surgery, pelvic radiographs showed partial healing, and she reported symptom relief. Resistive strengthening exercises were instituted, and progressive weight-bearing proceeded to full weight-bearing over the next 6 weeks. The patient reported almost complete relief of pain by 3 months, and she was able to return to work and daily activities without medication. Radiographs showed consolidation of the fractures. She was essentially symptom-free 17 months after surgery (Figure 6).
Case 2
An obese 51-year-old woman presented to the orthopedic clinic with a 6-month history of left groin pain that worsened with ambulation. She did not recall a specific injury but acknowledged a history of previous falls. Past medical history was significant for ulcerative colitis/irritable bowel syndrome and degenerative disease in the lumbar spine and right ankle. Previous pelvic radiographs showed no evidence of fracture or abnormality, but radiographs obtained before evaluation in the clinic showed hypertrophic nonunion of the left superior and inferior pubic ramus.
The patient had pain deep in the left groin with weight-bearing. On physical examination, she denied pain with log roll of the left hip or resisted straight leg raise. The pelvis was stable to manual compression. There was no sign of hernia or lymphadenopathy in the region of the left groin.
The patient had obtained a technetium-99 nuclear medicine scan of the pelvis in addition to standard preoperative CT of the nonunion area. The nuclear medicine scan showed uptake in the area of the superior and inferior ramus, and CT confirmed presence of a superior and inferior ramus that would accommodate a medullary screw.
The patient was taken to the operating room, where percutaneous fixation of the left superior and inferior ramus was performed (as described above). The patient was discharged on postoperative day 2 and followed the same weight-bearing protocol that the first patient used.
At 6 weeks, the patient returned to clinic with improved comfort. At 3 months, she denied left groin pain and was limited in activity only by preexisting arthrosis in the left ankle and lumbar spine. She was using a walker only for long distances and was symptom-free 13 months after surgery.
Discussion
Acute surgical fixation of the inferior ramus is seldom performed. The anatomical location of the inferior ramus and the lack of defined criteria for fixation often leave the inferior ramus ignored, unreduced, and without stabilization. In the setting of symptomatic nonunion, open stabilization has been used.11,12 Plate fixation after open débridement of an inferior ramus nonunion requires more extensive dissection and may increase the risk for perioperative infection and hardware prominence compared with an intramedullary implant.14 If plate prominence becomes symptomatic, the plate must be removed in a second surgical procedure. Percutaneous medullary screw fixation avoids the risks of surgical soft-tissue dissection and placement of a surface implant on the bone and reduces the need for a second surgical procedure to remove bothersome hardware. Percutaneous pelvic fixation has been well described and shown to provide stability to the pelvis. It can also be used to treat hypertrophic nonunions of the pelvis when mechanical stability is required for healing.13
In the cases reported here, inferior ramus stabilization was combined with intramedullary fixation of the superior ramus. As each patient had deep groin pain that could not be localized to either ramus, both rami were stabilized after close assessment on preoperative CT. Solitary fixation of the superior ramus may or may not provide stability sufficient for inferior ramus union and should be performed when the OFP of the inferior ramus is unavailable.
The anatomy of the inferior ramus must be carefully reviewed before surgery, as it is seldom encountered in open and percutaneous orthopedic pelvic surgery. The inferior ramus extends from the symphysis pubis to the ischial tuberosity. The ramus is wider medially and thinner laterally near the obturator foramen. The anterior surface of the ramus is flat and concave, whereas the posterior surface is flat and convex. The anatomy of the inferior ramus varies somewhat, and any distortion (eg, fracture, nonunion) of the OFP can render it incapable of accommodating screw fixation.13
Percutaneous placement of a medullary screw in the inferior ramus requires an understanding of the fluoroscopy required. Challenges, including body habitus and unique osseous anatomy, must be recognized. Soft tissues must be protected with a drill sleeve during preparation of the screw pathway, and care must be taken to avoid placing the screw beyond the cortex of the ischial tuberosity. A prominent screw tip can irritate the patient in the hamstrings or while sitting.
Intramedullary screw fixation of the inferior ramus is a technically demanding surgical procedure. Meticulous evaluation of preoperative radiographic studies must accompany strict attention to surgical detail. A misplaced or malpositioned drill bit or screw can injure surrounding neurovascular structures. A screw that does not cross the fracture or is not in the OFP of the inferior ramus will be ineffective and
potentially dangerous.
Conclusion
We have presented a technique for percutaneous screw placement in the inferior ramus. This technique requires an understanding of the anatomy of the inferior ramus and of the intraoperative fluoroscopy required for screw placement. We have used this technique to successfully treat symptomatic hypertrophic nonunions of the inferior ramus that require skeletal stability for healing.
1. Hill RM, Robinson CM, Keating JF. Fractures of the pubic rami. Epidemiology and five-year survival. J Bone Joint Surg Br. 2001;83(8):1141-1144.
2. Matta JM, Dickson KF, Markovich GD. Surgical treatment of pelvic nonunions and malunions. Clin Orthop. 1996;(329):199-206.
3. Barei DP, Shafer BL, Beingessner DM, Gardner MJ, Nork SE, Routt ML. The impact of open reduction internal fixation on acute pain management in unstable pelvic ring injuries. J Trauma. 2010;68(4):949-953.
4. van Dijk WA, Poeze M, van Helden SH, Brink PR, Verbruggen JP. Ten-year mortality among hospitalised patients with fractures of the pubic rami. Injury. 2010;41(4):411-414.
5. Simonian PT, Routt ML Jr, Harrington RM, Tencer AF. Internal fixation of the unstable anterior pelvic ring: a biomechanical comparison of standard plating techniques and the retrograde medullary superior pubic ramus screw. J Orthop Trauma. 1994;8(6):476-482.
6. Routt ML Jr, Simonian PT, Grujic L. The retrograde medullary superior pubic ramus screw for the treatment of anterior pelvic ring disruptions: a new technique. J Orthop Trauma. 1995;9(1):35-44.
7. Matta JM. Indications for anterior fixation of pelvic fractures. Clin Orthop. 1996;(329):88-96.
8. Routt ML Jr, Nork SE, Mills WJ. Percutaneous fixation of pelvic ring disruptions. Clin Orthop. 2000;(375):15-29.
9. Gautier E, Rommens PM, Matta JM. Late reconstruction after pelvic ring injuries. Injury. 1996;27(suppl 2):B39-B46.
10. Altman GT, Altman DT, Routt ML Jr. Symptomatic hypertrophic pubic ramus nonunion treated with a retrograde medullary screw. J Orthop Trauma. 2000;14(8):582-585.
11. Archdeacon MT, Kuhlman G, Kazemi N. Fellow’s Corner: grand rounds from the University of Cincinnati Medical Center—painful superior and inferior pubic rami nonunion. J Orthop Trauma. 2010;24(11):e109-e112.
12. Schofer M, Illian C, Fuchs-Winkelmann S, Kortmann HR. Pseudoarthrosis of anterior pelvic ring fracture [in German]. Unfallchirurg. 2008;111(4):264, 266-267.
13. Bishop JA, Routt ML Jr. Osseous fixation pathways in pelvic and acetabular fracture surgery: osteology, radiology, and clinical applications. J Trauma Acute Care Surg. 2012;72(6):1502-1509.
14. Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000;8(5):285-291.
Fractures of the superior and inferior pelvic rami are common in pelvic ring injuries.1 These fractures are routinely treated successfully without surgery.2 When the pelvic ring is injured, and ramus fracture or fractures represent a point of instability, surgical fixation can be performed to impart stability and reduce discomfort.3 Patients with pubic ramus fracture(s) have overall greater long-term morbidity and mortality.4 Operative stabilization of the superior pubic ramus can be achieved with open reduction and internal fixation, external fixation, and percutaneous medullary screw fixation.5-8 Inferior ramus fractures are seldom treated directly and acutely with operative reduction and fixation, as the mechanical advantage inferior ramus fixation provides is unknown.
Persistent nonunion of the pelvic ring can cause pain and disability and make reconstruction increasingly difficult.9 Open and percutaneous fixation techniques have been used to address symptomatic nonunions of the superior pubic ramus.9,10 There is limited evidence supporting surgical fixation of the inferior ramus. Open surgical fixation for symptomatic nonunions of the inferior ramus has been described.11,12 The inferior ramus has an osseous fixation pathway (OFP) amenable to percutaneous screw placement.13 Placement of a percutaneous screw in the inferior ramus requires use of preoperative computed tomography (CT) and is technically demanding. Surgeons must understand use of intraoperative fluoroscopy to ensure that the screw is contained within bone and crosses the intended zone of nonunion.
In this article, we report 2 cases of adults with symptomatic hypertrophic nonunions of the inferior ramus, treated with percutaneous screw fixation. Both patients presented with focal groin pain and activity limitations. Each had concurrent ipsilateral hypertrophic nonunions of the superior ramus, treated with percutaneous antegrade intramedullary stabilization. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 45-year-old woman fell from a horse about 8 months before presenting to the orthopedic outpatient clinic. Pelvic radiographs obtained after the fall were negative for fracture, but subsequent pelvic magnetic resonance imaging led to the diagnoses of minimally displaced left superior and inferior pubic ramus fractures and associated right-sided sacral ala fracture. The patient was treated with protected weight-bearing according to symptoms, but increasing activity-related pain and discomfort in the left groin persisted for months after injury. These symptoms were treated with analgesic medication, physical therapy, and chiropractic manipulation. Repeat imaging showed hypertrophic nonunions of the left superior and inferior pubic rami (Figure 1). Findings of the serologic testing performed for infection and metabolic deficiencies were normal at that time. The patient was referred for surgical consultation.
On evaluation, she reported constant pain in the left groin with ambulation. Specifically, squatting, pushing and pulling activities were extremely uncomfortable. She had been unable to return to work either full-time or part-time. On physical examination, she walked with an antalgic gait with a decreased stance phase of the left lower extremity. She had tenderness to palpation medial to the hip joint without evidence of hernia or lymphadenopathy. The pelvis was stable to manual compression testing.
Pelvic CT showed the nonunion site and the osteology of the inferior and superior pubic ramus of the pelvis, as well as minimal displacement and good alignment of the rami.
The patient was placed supine on a flat radiolucent table (Mizuho OSI, Union City, California). Preoperative cephalosporin antibiotics were administered. After induction of general anesthesia, the lumbosacral spine was elevated under 2 folded blankets. Arms were abducted to allow for pelvic imaging, and all bony prominences were padded. A urinary catheter was inserted aseptically to decompress the bladder. The entire abdomen and bilateral flanks were shaved, prepared, and draped in usual sterile fashion. A partially threaded cannulated screw was placed using a percutaneous antegrade technique to address the hypertrophic superior ramus nonunion.
A C-arm fluoroscopy unit (Ziehm, Orlando, Florida) was positioned on the injured side. The surgeon stood on the contralateral side. A pelvic OOOCI (outlet obturator oblique combinative image) of the symphysis pubis was obtained. This view defined the medial and lateral extents of the inferior ramus. A 0.062-in smooth Kirschner wire was used to percutaneously locate an ideal starting point on the cranial aspect of the contralateral superior pubic ramus. The starting point was adjusted on this view until an ideal intended trajectory into the contralateral (affected) inferior pubic ramus was visualized (Figure 2A).
The C-arm beam was then oriented to an “excessive” pelvic inlet view tangential to the posterior cortical surface of the affected inferior pubic ramus (Figure 2B). The tip of the wire was then adjusted to position and aim it slightly anterior to the posterior cortical surface of the affected inferior ramus. The wire was advanced into the bone about 1 cm, and the location and direction of the wire were reconfirmed as accurate.
A vertical skin incision was then made around the wire, and the 4.5-mm cannulated drill was placed over the wire. A soft-tissue protective drill sleeve and oscillating technique were used to protect the soft-tissue anatomy. The trajectory of the drill was again confirmed on pelvic OOOCI and advanced into the bone. The intended path of the drill was from the cranial-medial symphyseal cortex of the contralateral superior ramus, through the symphysis pubis obliquely, and then into the medullary canal of the affected inferior ramus. Frequent biplanar fluoroscopic imaging followed this progression of the drill to the nonunion site. The cannulated drill was then removed and exchanged for a calibrated extra-long 2.5-mm drill bit, placed through the soft-tissue drill sleeve and into the glide hole created by the 4.5-mm cannulated drill. The C-arm unit ensured accurate positioning of the 2.5-mm drill on both pelvic OOOCI and “excessive” inlet view before advancement (Figures 3A, 3B). The 2.5-mm drill was advanced caudally, laterally, and anteriorly in the ramus, past the nonunion site, and then was stopped before it exited the cortex of the ischial tuberosity (Figures 4A, 4B).
The depth of the drill bit was assessed with a known-length protective drill sleeve and calibrated drill. Alternatively, depth can be assessed with another same-length calibrated drill bit positioned adjacent to the inserted drill bit. A fully threaded, blunt-tipped 4.5-mm cortical screw was then placed through the glide hole. Both fluoroscopic views were used to confirm that the screw followed the same trajectory as the drill. Finally, the screw was again checked on biplanar fluoroscopy to confirm it had remained in the OFP of the inferior ramus (Figures 5A, 5B).
Postoperative pelvic CT confirmed position and length of the screws. The patient was allowed weight-of-limb weight-bearing on her affected side after surgery. She was discharged the first day after surgery and allowed use of oral analgesics. Six weeks after surgery, pelvic radiographs showed partial healing, and she reported symptom relief. Resistive strengthening exercises were instituted, and progressive weight-bearing proceeded to full weight-bearing over the next 6 weeks. The patient reported almost complete relief of pain by 3 months, and she was able to return to work and daily activities without medication. Radiographs showed consolidation of the fractures. She was essentially symptom-free 17 months after surgery (Figure 6).
Case 2
An obese 51-year-old woman presented to the orthopedic clinic with a 6-month history of left groin pain that worsened with ambulation. She did not recall a specific injury but acknowledged a history of previous falls. Past medical history was significant for ulcerative colitis/irritable bowel syndrome and degenerative disease in the lumbar spine and right ankle. Previous pelvic radiographs showed no evidence of fracture or abnormality, but radiographs obtained before evaluation in the clinic showed hypertrophic nonunion of the left superior and inferior pubic ramus.
The patient had pain deep in the left groin with weight-bearing. On physical examination, she denied pain with log roll of the left hip or resisted straight leg raise. The pelvis was stable to manual compression. There was no sign of hernia or lymphadenopathy in the region of the left groin.
The patient had obtained a technetium-99 nuclear medicine scan of the pelvis in addition to standard preoperative CT of the nonunion area. The nuclear medicine scan showed uptake in the area of the superior and inferior ramus, and CT confirmed presence of a superior and inferior ramus that would accommodate a medullary screw.
The patient was taken to the operating room, where percutaneous fixation of the left superior and inferior ramus was performed (as described above). The patient was discharged on postoperative day 2 and followed the same weight-bearing protocol that the first patient used.
At 6 weeks, the patient returned to clinic with improved comfort. At 3 months, she denied left groin pain and was limited in activity only by preexisting arthrosis in the left ankle and lumbar spine. She was using a walker only for long distances and was symptom-free 13 months after surgery.
Discussion
Acute surgical fixation of the inferior ramus is seldom performed. The anatomical location of the inferior ramus and the lack of defined criteria for fixation often leave the inferior ramus ignored, unreduced, and without stabilization. In the setting of symptomatic nonunion, open stabilization has been used.11,12 Plate fixation after open débridement of an inferior ramus nonunion requires more extensive dissection and may increase the risk for perioperative infection and hardware prominence compared with an intramedullary implant.14 If plate prominence becomes symptomatic, the plate must be removed in a second surgical procedure. Percutaneous medullary screw fixation avoids the risks of surgical soft-tissue dissection and placement of a surface implant on the bone and reduces the need for a second surgical procedure to remove bothersome hardware. Percutaneous pelvic fixation has been well described and shown to provide stability to the pelvis. It can also be used to treat hypertrophic nonunions of the pelvis when mechanical stability is required for healing.13
In the cases reported here, inferior ramus stabilization was combined with intramedullary fixation of the superior ramus. As each patient had deep groin pain that could not be localized to either ramus, both rami were stabilized after close assessment on preoperative CT. Solitary fixation of the superior ramus may or may not provide stability sufficient for inferior ramus union and should be performed when the OFP of the inferior ramus is unavailable.
The anatomy of the inferior ramus must be carefully reviewed before surgery, as it is seldom encountered in open and percutaneous orthopedic pelvic surgery. The inferior ramus extends from the symphysis pubis to the ischial tuberosity. The ramus is wider medially and thinner laterally near the obturator foramen. The anterior surface of the ramus is flat and concave, whereas the posterior surface is flat and convex. The anatomy of the inferior ramus varies somewhat, and any distortion (eg, fracture, nonunion) of the OFP can render it incapable of accommodating screw fixation.13
Percutaneous placement of a medullary screw in the inferior ramus requires an understanding of the fluoroscopy required. Challenges, including body habitus and unique osseous anatomy, must be recognized. Soft tissues must be protected with a drill sleeve during preparation of the screw pathway, and care must be taken to avoid placing the screw beyond the cortex of the ischial tuberosity. A prominent screw tip can irritate the patient in the hamstrings or while sitting.
Intramedullary screw fixation of the inferior ramus is a technically demanding surgical procedure. Meticulous evaluation of preoperative radiographic studies must accompany strict attention to surgical detail. A misplaced or malpositioned drill bit or screw can injure surrounding neurovascular structures. A screw that does not cross the fracture or is not in the OFP of the inferior ramus will be ineffective and
potentially dangerous.
Conclusion
We have presented a technique for percutaneous screw placement in the inferior ramus. This technique requires an understanding of the anatomy of the inferior ramus and of the intraoperative fluoroscopy required for screw placement. We have used this technique to successfully treat symptomatic hypertrophic nonunions of the inferior ramus that require skeletal stability for healing.
Fractures of the superior and inferior pelvic rami are common in pelvic ring injuries.1 These fractures are routinely treated successfully without surgery.2 When the pelvic ring is injured, and ramus fracture or fractures represent a point of instability, surgical fixation can be performed to impart stability and reduce discomfort.3 Patients with pubic ramus fracture(s) have overall greater long-term morbidity and mortality.4 Operative stabilization of the superior pubic ramus can be achieved with open reduction and internal fixation, external fixation, and percutaneous medullary screw fixation.5-8 Inferior ramus fractures are seldom treated directly and acutely with operative reduction and fixation, as the mechanical advantage inferior ramus fixation provides is unknown.
Persistent nonunion of the pelvic ring can cause pain and disability and make reconstruction increasingly difficult.9 Open and percutaneous fixation techniques have been used to address symptomatic nonunions of the superior pubic ramus.9,10 There is limited evidence supporting surgical fixation of the inferior ramus. Open surgical fixation for symptomatic nonunions of the inferior ramus has been described.11,12 The inferior ramus has an osseous fixation pathway (OFP) amenable to percutaneous screw placement.13 Placement of a percutaneous screw in the inferior ramus requires use of preoperative computed tomography (CT) and is technically demanding. Surgeons must understand use of intraoperative fluoroscopy to ensure that the screw is contained within bone and crosses the intended zone of nonunion.
In this article, we report 2 cases of adults with symptomatic hypertrophic nonunions of the inferior ramus, treated with percutaneous screw fixation. Both patients presented with focal groin pain and activity limitations. Each had concurrent ipsilateral hypertrophic nonunions of the superior ramus, treated with percutaneous antegrade intramedullary stabilization. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 45-year-old woman fell from a horse about 8 months before presenting to the orthopedic outpatient clinic. Pelvic radiographs obtained after the fall were negative for fracture, but subsequent pelvic magnetic resonance imaging led to the diagnoses of minimally displaced left superior and inferior pubic ramus fractures and associated right-sided sacral ala fracture. The patient was treated with protected weight-bearing according to symptoms, but increasing activity-related pain and discomfort in the left groin persisted for months after injury. These symptoms were treated with analgesic medication, physical therapy, and chiropractic manipulation. Repeat imaging showed hypertrophic nonunions of the left superior and inferior pubic rami (Figure 1). Findings of the serologic testing performed for infection and metabolic deficiencies were normal at that time. The patient was referred for surgical consultation.
On evaluation, she reported constant pain in the left groin with ambulation. Specifically, squatting, pushing and pulling activities were extremely uncomfortable. She had been unable to return to work either full-time or part-time. On physical examination, she walked with an antalgic gait with a decreased stance phase of the left lower extremity. She had tenderness to palpation medial to the hip joint without evidence of hernia or lymphadenopathy. The pelvis was stable to manual compression testing.
Pelvic CT showed the nonunion site and the osteology of the inferior and superior pubic ramus of the pelvis, as well as minimal displacement and good alignment of the rami.
The patient was placed supine on a flat radiolucent table (Mizuho OSI, Union City, California). Preoperative cephalosporin antibiotics were administered. After induction of general anesthesia, the lumbosacral spine was elevated under 2 folded blankets. Arms were abducted to allow for pelvic imaging, and all bony prominences were padded. A urinary catheter was inserted aseptically to decompress the bladder. The entire abdomen and bilateral flanks were shaved, prepared, and draped in usual sterile fashion. A partially threaded cannulated screw was placed using a percutaneous antegrade technique to address the hypertrophic superior ramus nonunion.
A C-arm fluoroscopy unit (Ziehm, Orlando, Florida) was positioned on the injured side. The surgeon stood on the contralateral side. A pelvic OOOCI (outlet obturator oblique combinative image) of the symphysis pubis was obtained. This view defined the medial and lateral extents of the inferior ramus. A 0.062-in smooth Kirschner wire was used to percutaneously locate an ideal starting point on the cranial aspect of the contralateral superior pubic ramus. The starting point was adjusted on this view until an ideal intended trajectory into the contralateral (affected) inferior pubic ramus was visualized (Figure 2A).
The C-arm beam was then oriented to an “excessive” pelvic inlet view tangential to the posterior cortical surface of the affected inferior pubic ramus (Figure 2B). The tip of the wire was then adjusted to position and aim it slightly anterior to the posterior cortical surface of the affected inferior ramus. The wire was advanced into the bone about 1 cm, and the location and direction of the wire were reconfirmed as accurate.
A vertical skin incision was then made around the wire, and the 4.5-mm cannulated drill was placed over the wire. A soft-tissue protective drill sleeve and oscillating technique were used to protect the soft-tissue anatomy. The trajectory of the drill was again confirmed on pelvic OOOCI and advanced into the bone. The intended path of the drill was from the cranial-medial symphyseal cortex of the contralateral superior ramus, through the symphysis pubis obliquely, and then into the medullary canal of the affected inferior ramus. Frequent biplanar fluoroscopic imaging followed this progression of the drill to the nonunion site. The cannulated drill was then removed and exchanged for a calibrated extra-long 2.5-mm drill bit, placed through the soft-tissue drill sleeve and into the glide hole created by the 4.5-mm cannulated drill. The C-arm unit ensured accurate positioning of the 2.5-mm drill on both pelvic OOOCI and “excessive” inlet view before advancement (Figures 3A, 3B). The 2.5-mm drill was advanced caudally, laterally, and anteriorly in the ramus, past the nonunion site, and then was stopped before it exited the cortex of the ischial tuberosity (Figures 4A, 4B).
The depth of the drill bit was assessed with a known-length protective drill sleeve and calibrated drill. Alternatively, depth can be assessed with another same-length calibrated drill bit positioned adjacent to the inserted drill bit. A fully threaded, blunt-tipped 4.5-mm cortical screw was then placed through the glide hole. Both fluoroscopic views were used to confirm that the screw followed the same trajectory as the drill. Finally, the screw was again checked on biplanar fluoroscopy to confirm it had remained in the OFP of the inferior ramus (Figures 5A, 5B).
Postoperative pelvic CT confirmed position and length of the screws. The patient was allowed weight-of-limb weight-bearing on her affected side after surgery. She was discharged the first day after surgery and allowed use of oral analgesics. Six weeks after surgery, pelvic radiographs showed partial healing, and she reported symptom relief. Resistive strengthening exercises were instituted, and progressive weight-bearing proceeded to full weight-bearing over the next 6 weeks. The patient reported almost complete relief of pain by 3 months, and she was able to return to work and daily activities without medication. Radiographs showed consolidation of the fractures. She was essentially symptom-free 17 months after surgery (Figure 6).
Case 2
An obese 51-year-old woman presented to the orthopedic clinic with a 6-month history of left groin pain that worsened with ambulation. She did not recall a specific injury but acknowledged a history of previous falls. Past medical history was significant for ulcerative colitis/irritable bowel syndrome and degenerative disease in the lumbar spine and right ankle. Previous pelvic radiographs showed no evidence of fracture or abnormality, but radiographs obtained before evaluation in the clinic showed hypertrophic nonunion of the left superior and inferior pubic ramus.
The patient had pain deep in the left groin with weight-bearing. On physical examination, she denied pain with log roll of the left hip or resisted straight leg raise. The pelvis was stable to manual compression. There was no sign of hernia or lymphadenopathy in the region of the left groin.
The patient had obtained a technetium-99 nuclear medicine scan of the pelvis in addition to standard preoperative CT of the nonunion area. The nuclear medicine scan showed uptake in the area of the superior and inferior ramus, and CT confirmed presence of a superior and inferior ramus that would accommodate a medullary screw.
The patient was taken to the operating room, where percutaneous fixation of the left superior and inferior ramus was performed (as described above). The patient was discharged on postoperative day 2 and followed the same weight-bearing protocol that the first patient used.
At 6 weeks, the patient returned to clinic with improved comfort. At 3 months, she denied left groin pain and was limited in activity only by preexisting arthrosis in the left ankle and lumbar spine. She was using a walker only for long distances and was symptom-free 13 months after surgery.
Discussion
Acute surgical fixation of the inferior ramus is seldom performed. The anatomical location of the inferior ramus and the lack of defined criteria for fixation often leave the inferior ramus ignored, unreduced, and without stabilization. In the setting of symptomatic nonunion, open stabilization has been used.11,12 Plate fixation after open débridement of an inferior ramus nonunion requires more extensive dissection and may increase the risk for perioperative infection and hardware prominence compared with an intramedullary implant.14 If plate prominence becomes symptomatic, the plate must be removed in a second surgical procedure. Percutaneous medullary screw fixation avoids the risks of surgical soft-tissue dissection and placement of a surface implant on the bone and reduces the need for a second surgical procedure to remove bothersome hardware. Percutaneous pelvic fixation has been well described and shown to provide stability to the pelvis. It can also be used to treat hypertrophic nonunions of the pelvis when mechanical stability is required for healing.13
In the cases reported here, inferior ramus stabilization was combined with intramedullary fixation of the superior ramus. As each patient had deep groin pain that could not be localized to either ramus, both rami were stabilized after close assessment on preoperative CT. Solitary fixation of the superior ramus may or may not provide stability sufficient for inferior ramus union and should be performed when the OFP of the inferior ramus is unavailable.
The anatomy of the inferior ramus must be carefully reviewed before surgery, as it is seldom encountered in open and percutaneous orthopedic pelvic surgery. The inferior ramus extends from the symphysis pubis to the ischial tuberosity. The ramus is wider medially and thinner laterally near the obturator foramen. The anterior surface of the ramus is flat and concave, whereas the posterior surface is flat and convex. The anatomy of the inferior ramus varies somewhat, and any distortion (eg, fracture, nonunion) of the OFP can render it incapable of accommodating screw fixation.13
Percutaneous placement of a medullary screw in the inferior ramus requires an understanding of the fluoroscopy required. Challenges, including body habitus and unique osseous anatomy, must be recognized. Soft tissues must be protected with a drill sleeve during preparation of the screw pathway, and care must be taken to avoid placing the screw beyond the cortex of the ischial tuberosity. A prominent screw tip can irritate the patient in the hamstrings or while sitting.
Intramedullary screw fixation of the inferior ramus is a technically demanding surgical procedure. Meticulous evaluation of preoperative radiographic studies must accompany strict attention to surgical detail. A misplaced or malpositioned drill bit or screw can injure surrounding neurovascular structures. A screw that does not cross the fracture or is not in the OFP of the inferior ramus will be ineffective and
potentially dangerous.
Conclusion
We have presented a technique for percutaneous screw placement in the inferior ramus. This technique requires an understanding of the anatomy of the inferior ramus and of the intraoperative fluoroscopy required for screw placement. We have used this technique to successfully treat symptomatic hypertrophic nonunions of the inferior ramus that require skeletal stability for healing.
1. Hill RM, Robinson CM, Keating JF. Fractures of the pubic rami. Epidemiology and five-year survival. J Bone Joint Surg Br. 2001;83(8):1141-1144.
2. Matta JM, Dickson KF, Markovich GD. Surgical treatment of pelvic nonunions and malunions. Clin Orthop. 1996;(329):199-206.
3. Barei DP, Shafer BL, Beingessner DM, Gardner MJ, Nork SE, Routt ML. The impact of open reduction internal fixation on acute pain management in unstable pelvic ring injuries. J Trauma. 2010;68(4):949-953.
4. van Dijk WA, Poeze M, van Helden SH, Brink PR, Verbruggen JP. Ten-year mortality among hospitalised patients with fractures of the pubic rami. Injury. 2010;41(4):411-414.
5. Simonian PT, Routt ML Jr, Harrington RM, Tencer AF. Internal fixation of the unstable anterior pelvic ring: a biomechanical comparison of standard plating techniques and the retrograde medullary superior pubic ramus screw. J Orthop Trauma. 1994;8(6):476-482.
6. Routt ML Jr, Simonian PT, Grujic L. The retrograde medullary superior pubic ramus screw for the treatment of anterior pelvic ring disruptions: a new technique. J Orthop Trauma. 1995;9(1):35-44.
7. Matta JM. Indications for anterior fixation of pelvic fractures. Clin Orthop. 1996;(329):88-96.
8. Routt ML Jr, Nork SE, Mills WJ. Percutaneous fixation of pelvic ring disruptions. Clin Orthop. 2000;(375):15-29.
9. Gautier E, Rommens PM, Matta JM. Late reconstruction after pelvic ring injuries. Injury. 1996;27(suppl 2):B39-B46.
10. Altman GT, Altman DT, Routt ML Jr. Symptomatic hypertrophic pubic ramus nonunion treated with a retrograde medullary screw. J Orthop Trauma. 2000;14(8):582-585.
11. Archdeacon MT, Kuhlman G, Kazemi N. Fellow’s Corner: grand rounds from the University of Cincinnati Medical Center—painful superior and inferior pubic rami nonunion. J Orthop Trauma. 2010;24(11):e109-e112.
12. Schofer M, Illian C, Fuchs-Winkelmann S, Kortmann HR. Pseudoarthrosis of anterior pelvic ring fracture [in German]. Unfallchirurg. 2008;111(4):264, 266-267.
13. Bishop JA, Routt ML Jr. Osseous fixation pathways in pelvic and acetabular fracture surgery: osteology, radiology, and clinical applications. J Trauma Acute Care Surg. 2012;72(6):1502-1509.
14. Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000;8(5):285-291.
1. Hill RM, Robinson CM, Keating JF. Fractures of the pubic rami. Epidemiology and five-year survival. J Bone Joint Surg Br. 2001;83(8):1141-1144.
2. Matta JM, Dickson KF, Markovich GD. Surgical treatment of pelvic nonunions and malunions. Clin Orthop. 1996;(329):199-206.
3. Barei DP, Shafer BL, Beingessner DM, Gardner MJ, Nork SE, Routt ML. The impact of open reduction internal fixation on acute pain management in unstable pelvic ring injuries. J Trauma. 2010;68(4):949-953.
4. van Dijk WA, Poeze M, van Helden SH, Brink PR, Verbruggen JP. Ten-year mortality among hospitalised patients with fractures of the pubic rami. Injury. 2010;41(4):411-414.
5. Simonian PT, Routt ML Jr, Harrington RM, Tencer AF. Internal fixation of the unstable anterior pelvic ring: a biomechanical comparison of standard plating techniques and the retrograde medullary superior pubic ramus screw. J Orthop Trauma. 1994;8(6):476-482.
6. Routt ML Jr, Simonian PT, Grujic L. The retrograde medullary superior pubic ramus screw for the treatment of anterior pelvic ring disruptions: a new technique. J Orthop Trauma. 1995;9(1):35-44.
7. Matta JM. Indications for anterior fixation of pelvic fractures. Clin Orthop. 1996;(329):88-96.
8. Routt ML Jr, Nork SE, Mills WJ. Percutaneous fixation of pelvic ring disruptions. Clin Orthop. 2000;(375):15-29.
9. Gautier E, Rommens PM, Matta JM. Late reconstruction after pelvic ring injuries. Injury. 1996;27(suppl 2):B39-B46.
10. Altman GT, Altman DT, Routt ML Jr. Symptomatic hypertrophic pubic ramus nonunion treated with a retrograde medullary screw. J Orthop Trauma. 2000;14(8):582-585.
11. Archdeacon MT, Kuhlman G, Kazemi N. Fellow’s Corner: grand rounds from the University of Cincinnati Medical Center—painful superior and inferior pubic rami nonunion. J Orthop Trauma. 2010;24(11):e109-e112.
12. Schofer M, Illian C, Fuchs-Winkelmann S, Kortmann HR. Pseudoarthrosis of anterior pelvic ring fracture [in German]. Unfallchirurg. 2008;111(4):264, 266-267.
13. Bishop JA, Routt ML Jr. Osseous fixation pathways in pelvic and acetabular fracture surgery: osteology, radiology, and clinical applications. J Trauma Acute Care Surg. 2012;72(6):1502-1509.
14. Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000;8(5):285-291.
Long-Term Outcomes of Allograft Reconstruction of the Anterior Cruciate Ligament
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Enhancement of Acute Tendon Repair Using Chitosan Matrix
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
Retrograde Reamer/Irrigator/Aspirator Technique for Autologous Bone Graft Harvesting With the Patient in the Prone Position
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
Emerging Biologics in Orthopedics
The discipline of orthopedic medicine and surgery has dramatically advanced over the last several decades. Improved understanding of biomechanics, tissue healing, and the pathogenesis of musculoskeletal diseases has allowed us to make significant progress in the diagnosis, treatment, and rehabilitation of our patients. Despite these advancements, there is still much to be learned, especially in the field of orthobiologics and regenerative medicine. As our understanding of existing technologies, such as bone marrow aspirate, platelet-rich plasma, and adult stem cells, continues to evolve, even newer biologic treatment options are being developed. This issue of The American Journal of Orthopedics focuses on emerging biologics across the spectrum of orthopedic care.
In this issue, on pages 202-205, Mansour and Conway describe a new prone retrograde technique for obtaining bone graft using the Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania). While iliac crest bone graft has been the gold standard for many years, use of the RIA system to obtain bone graft has been studied and has been shown to have decreased morbidity when compared with iliac crest harvest.1 Additionally, intramedullary bone graft from the femur appears to be just as concentrated with biologically active bone marrow as iliac crest harvest.2 This new technique allows increased efficiency, especially for surgeries that are done in the prone position.
Melamed and colleagues examine a new biologic to augment repair of rotator cuff tears (see pages 212-216). Chitosan, a linear polysaccharide, has been shown to help with soft-tissue healing. Although in the past its use has been limited secondary to problems with the compound precipitating at physiologic pH, new formulations mitigate that problem. In the authors’ animal model of acute supraspinatus repair, the use of chitosan gel increased the number of fibroblasts and the amount of repair tissue when compared with untreated controls. Additionally, the experimental group showed a decreased inflammatory response when compared with the control group. This is very exciting research as the biologic enhancement of rotator cuff tendon healing could potentially help decrease the rate of rotator cuff repair failure.
Lenehan and colleagues analyze the long-term outcomes of anterior cruciate ligament reconstruction in a cohort of patients studied over an 8-year period (see pages 217-222). During this period, 99 patients were reconstructed with allograft tissue and 24 with autograft. Their analysis, like other recently published work, shows that the rates of revision were much higher for patients under 25 years of age who were reconstructed using allograft tissue. The rate of revision for NCAA (National Collegiate Athletic Association) Division I athletes reconstructed with allograft tissue was found to be 62%, while the revision rate for all patients under the age of 25 years who received an allograft was found to be 20.5%. Clearly, there is still a great deal to learn about the biology of graft incorporation and healing, especially as it relates to allograft tissue.
These 3 articles exemplify the breadth of orthopedic biologics and their potential role in orthopedic surgery. Through efforts of investigators highlighted in this journal and in others, biologics will become better understood and more widely used when appropriate, leading to improved patient outcomes.
1. Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116-S120.
2. van der Bel R, Blokhuis TJ. Increased osteogenic capacity of Reamer/Irrigator/Aspirator derived mesenchymal stem cells. Injury. 2014;45(12):2060-2064.
The discipline of orthopedic medicine and surgery has dramatically advanced over the last several decades. Improved understanding of biomechanics, tissue healing, and the pathogenesis of musculoskeletal diseases has allowed us to make significant progress in the diagnosis, treatment, and rehabilitation of our patients. Despite these advancements, there is still much to be learned, especially in the field of orthobiologics and regenerative medicine. As our understanding of existing technologies, such as bone marrow aspirate, platelet-rich plasma, and adult stem cells, continues to evolve, even newer biologic treatment options are being developed. This issue of The American Journal of Orthopedics focuses on emerging biologics across the spectrum of orthopedic care.
In this issue, on pages 202-205, Mansour and Conway describe a new prone retrograde technique for obtaining bone graft using the Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania). While iliac crest bone graft has been the gold standard for many years, use of the RIA system to obtain bone graft has been studied and has been shown to have decreased morbidity when compared with iliac crest harvest.1 Additionally, intramedullary bone graft from the femur appears to be just as concentrated with biologically active bone marrow as iliac crest harvest.2 This new technique allows increased efficiency, especially for surgeries that are done in the prone position.
Melamed and colleagues examine a new biologic to augment repair of rotator cuff tears (see pages 212-216). Chitosan, a linear polysaccharide, has been shown to help with soft-tissue healing. Although in the past its use has been limited secondary to problems with the compound precipitating at physiologic pH, new formulations mitigate that problem. In the authors’ animal model of acute supraspinatus repair, the use of chitosan gel increased the number of fibroblasts and the amount of repair tissue when compared with untreated controls. Additionally, the experimental group showed a decreased inflammatory response when compared with the control group. This is very exciting research as the biologic enhancement of rotator cuff tendon healing could potentially help decrease the rate of rotator cuff repair failure.
Lenehan and colleagues analyze the long-term outcomes of anterior cruciate ligament reconstruction in a cohort of patients studied over an 8-year period (see pages 217-222). During this period, 99 patients were reconstructed with allograft tissue and 24 with autograft. Their analysis, like other recently published work, shows that the rates of revision were much higher for patients under 25 years of age who were reconstructed using allograft tissue. The rate of revision for NCAA (National Collegiate Athletic Association) Division I athletes reconstructed with allograft tissue was found to be 62%, while the revision rate for all patients under the age of 25 years who received an allograft was found to be 20.5%. Clearly, there is still a great deal to learn about the biology of graft incorporation and healing, especially as it relates to allograft tissue.
These 3 articles exemplify the breadth of orthopedic biologics and their potential role in orthopedic surgery. Through efforts of investigators highlighted in this journal and in others, biologics will become better understood and more widely used when appropriate, leading to improved patient outcomes.
The discipline of orthopedic medicine and surgery has dramatically advanced over the last several decades. Improved understanding of biomechanics, tissue healing, and the pathogenesis of musculoskeletal diseases has allowed us to make significant progress in the diagnosis, treatment, and rehabilitation of our patients. Despite these advancements, there is still much to be learned, especially in the field of orthobiologics and regenerative medicine. As our understanding of existing technologies, such as bone marrow aspirate, platelet-rich plasma, and adult stem cells, continues to evolve, even newer biologic treatment options are being developed. This issue of The American Journal of Orthopedics focuses on emerging biologics across the spectrum of orthopedic care.
In this issue, on pages 202-205, Mansour and Conway describe a new prone retrograde technique for obtaining bone graft using the Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania). While iliac crest bone graft has been the gold standard for many years, use of the RIA system to obtain bone graft has been studied and has been shown to have decreased morbidity when compared with iliac crest harvest.1 Additionally, intramedullary bone graft from the femur appears to be just as concentrated with biologically active bone marrow as iliac crest harvest.2 This new technique allows increased efficiency, especially for surgeries that are done in the prone position.
Melamed and colleagues examine a new biologic to augment repair of rotator cuff tears (see pages 212-216). Chitosan, a linear polysaccharide, has been shown to help with soft-tissue healing. Although in the past its use has been limited secondary to problems with the compound precipitating at physiologic pH, new formulations mitigate that problem. In the authors’ animal model of acute supraspinatus repair, the use of chitosan gel increased the number of fibroblasts and the amount of repair tissue when compared with untreated controls. Additionally, the experimental group showed a decreased inflammatory response when compared with the control group. This is very exciting research as the biologic enhancement of rotator cuff tendon healing could potentially help decrease the rate of rotator cuff repair failure.
Lenehan and colleagues analyze the long-term outcomes of anterior cruciate ligament reconstruction in a cohort of patients studied over an 8-year period (see pages 217-222). During this period, 99 patients were reconstructed with allograft tissue and 24 with autograft. Their analysis, like other recently published work, shows that the rates of revision were much higher for patients under 25 years of age who were reconstructed using allograft tissue. The rate of revision for NCAA (National Collegiate Athletic Association) Division I athletes reconstructed with allograft tissue was found to be 62%, while the revision rate for all patients under the age of 25 years who received an allograft was found to be 20.5%. Clearly, there is still a great deal to learn about the biology of graft incorporation and healing, especially as it relates to allograft tissue.
These 3 articles exemplify the breadth of orthopedic biologics and their potential role in orthopedic surgery. Through efforts of investigators highlighted in this journal and in others, biologics will become better understood and more widely used when appropriate, leading to improved patient outcomes.
1. Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116-S120.
2. van der Bel R, Blokhuis TJ. Increased osteogenic capacity of Reamer/Irrigator/Aspirator derived mesenchymal stem cells. Injury. 2014;45(12):2060-2064.
1. Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116-S120.
2. van der Bel R, Blokhuis TJ. Increased osteogenic capacity of Reamer/Irrigator/Aspirator derived mesenchymal stem cells. Injury. 2014;45(12):2060-2064.
Successful Surgical Treatment of an Intraneural Ganglion of the Common Peroneal Nerve
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
Failure of Artelon Interposition Arthroplasty After Partial Trapeziectomy: A Case Report With Histologic and Immunohistochemical Analysis
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
Massive Baker Cyst Resulting in Tibial Nerve Compression Neuropathy Secondary to Polyethylene Wear Disease
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
Comparison of Locked Plate Fixation and Nonoperative Management for Displaced Proximal Humerus Fractures in Elderly Patients
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.