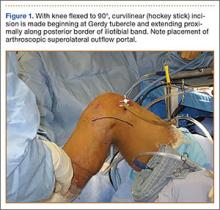
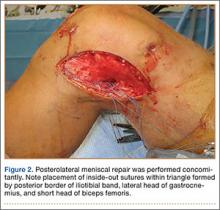
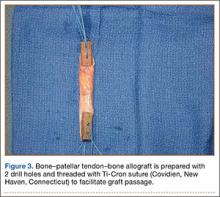
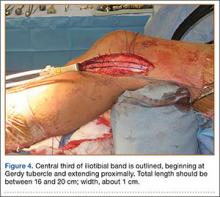
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Lumbar Degenerative Disc Disease and Tibiotalar Joint Arthritis: A 710-Specimen Postmortem Study
Osteoarthritis is the most common joint disorder, resulting in significant morbidity and disability. The worldwide prevalence of osteoarthritis was estimated at more than 151 million people, according to data published in 2004.1 In the United States, almost 27 million adults age 25 years and older suffer from clinically apparent disease.2 The spine is one of the most commonly affected joints of arthritis, and idiopathic low back pain is the most frequent complaint in the adult population.3 In adults with low back pain, evidence of lumbar intervertebral disc degeneration is often found on radiography.4 In 1 study, evidence of disc degeneration was found in 90% of adults age 50 to 59 years.5
Degenerative spinal disease most commonly affects the lumbar spine due to its high degree of mobility and weight-loading.6,7 Clinical8,9 and experimental studies10 have suggested that the degenerative changes in the lumbar spine begin in the intervertebral discs. Degenerative disc disease (DDD) results from a continuum of dehydration, degradation, and remodeling of the intervertebral discs and neighboring vertebrae to accommodate the changes in physical loading.11-13 This results in disc-space narrowing, disc bulging and herniation, vertebral rim osteophyte formation, and endplate sclerosis.7,14 Symptomatic neural compression may occur, often manifested by localized lower back and extremity pain, as well as sensory loss and weakness of the lower extremities.15-17 Changes in posture and gait may result because of altered sensation, and the consequent abnormal force transmission may predispose joints to accelerated wear and arthrosis.15,18
Numerous studies have delineated the association between lumbar spinal disorders and lower extremity arthrosis. Of note, research has demonstrated that hip and/or knee pathology and gait alteration may promote low back pain and lumbar disc degeneration.19-21 Although spinal abnormalities, such as scoliosis, may predispose an individual to accelerated hip degeneration,20 no studies have investigated the relationship between lumbar DDD and ankle osteoarthritis.
Ankle arthritis differs from hip and knee arthritis demographically, occurring approximately 9 times less frequently.21 The ankle joint is subjected to more weight-bearing force per square centimeter and is more commonly injured than any other joint in the body.21 Trauma and/or abnormal ankle mechanics are the most common causes of degenerative ankle arthritis.22 Other potential causes include inflammatory arthropathies, neuropathic arthropathy, infection, and tumor. The purpose of this study was to determine if a relationship exists between ankle arthrosis and lumbar disc degeneration, and to delineate if one may promote the onset or progression of the other.
Materials and Methods
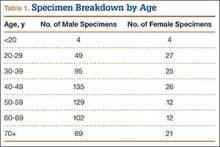
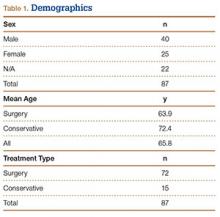
We randomly chose 710 cadaveric specimens from the Hamann-Todd Osteological Collection in Cleveland, Ohio. The Hamann-Todd Collection contains skeletal remains from more than 3000 individuals who died in Cleveland, Ohio between 1893 and 1938. The cohort for this study included 583 male and 127 female cadavers, ranging in age from 17 to 105 years at the time of death. Table 1 shows the breakdown of these specimens according to age group; of the 710 specimens, 306 were of African American ancestry, and 404 were Caucasian.
Lumbar DDD was graded at each lumbar spinal level by a single examiner using the Eubanks modification23 of the Kettler and Wilke classification of vertebral endplate osteophytosis24:
Grade 0: normal vertebral endplates;
Grade 1: mild arthrosis, with evidence of osteophytic reaction involving up to 50% of the vertebral endplates;
Grade 2: moderate arthrosis, with evidence of osteophytic reaction involving 50% to 100% of the vertebral endplates;
Grade 3: severe arthrosis, with evidence of osteophytic reaction involving 100% of the vertebral endplates. Osteophytes are hypertrophic and bridging the joint space (Figure 1);
Grade 4: complete ankylosis.
Tibiotalar joint osteoarthritis was evaluated by a single examiner using a modification of the Kellgren-Lawrence classification4 for knee osteoarthritis:
Grade 0: no discernable wear/osteophytes;
Grade 1: 1-mm osteophyte(s) and/or <25% surface wear;
Grade 2: 1- to 2-mm osteophyte(s) and/or 25% to 50% joint surface;
Grade 3: 2- to 3-mm osteophyte(s) and/or >50% joint surface (Figure 2);
Grade 4: multiple large osteophytes and/or definite bony end deformity.
Statistical analysis was performed on the compiled data using Stata software (StataCorp, College Station, Texas). Linear and logistic regression analyses correcting for confounding factors of age, sex, race, and height were performed using a standard P-value cutoff (P < .05) and 95% confidence interval to determine statistical significance.
Results
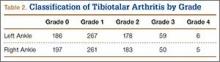
Patients were considered to have osteoarthritis of the tibiotalar joint if either of the extremities measured grade 1 or higher. Of the 710 specimens selected, 14 specimens did not have adequate bone available for bilateral tibiotalar joint measurement, either from extensive bone degradation or amputation. Of the remaining 696 specimens, 586 had some degree of tibiotalar osteoarthritis present (Table 2). Regression analysis showed a significant positive association between right- and left-ankle osteoarthritis (coefficient: 0.491, P < .01). Tibiotalar joint arthritis was classified as severe if either extremity had arthrosis of grade 3 or higher. Of the 586 specimens that had tibiotalar joint arthritis, only 16% (97 specimens) had severe tibiotalar joint arthritis.
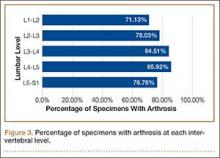
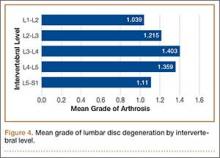
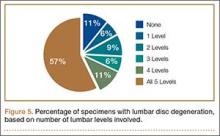
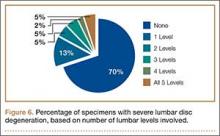
Data regarding lumbar disc degeneration were available for 516 of the 710 specimens selected, 443 of which showed some disc degeneration. Disc degeneration was most prevalent and significant at the L4-L5 and L3-L4 intervertebral levels (Figures 3, 4). Of these 516 specimens, 30 had degeneration at 1 level, 47 specimens had degeneration at 2 levels, 29 specimens had degeneration at 3 levels, 52 had degeneration at 4 levels, and 285 specimens had degeneration at all 5 lumbar levels. The majority of specimens were found to have some degree of degeneration at all 5 lumbar spinal levels (Figure 5). Severe lumbar DDD was defined as grade 3 or higher osteoarthritis present in at least 1 of the 5 lumbar levels. Of the 516 specimens that showed some degree of disc degeneration, 152 were classified as severe. When stratified by number of spinal levels, only 30% of specimens were found to have evidence of severe arthrosis, the majority of which was located at only 1 lumbar segment (Figure 6).
Linear regression analysis of the data showed a statistically significant positive association between lumbar disc degeneration and tibiotalar osteoarthritis (coefficient: 0.844, P < .01), even when correcting for confounding factors, such as age, sex, and race (coefficient: 0.331, P < .01).
Additional analysis of the data demonstrated that tibiotalar joint arthritis remained significantly associated with lumbar DDD across each lumbar level: L1-L2 (coefficient: 0.269, P < .01), L2-L3 (coefficient: 0.283, P < .01), L3-L4 (coefficient: 0.299, P < .01), L4-L5 (coefficient: 0.240, P < .02), L5-S1 (coefficient: 0.167, P < .05).
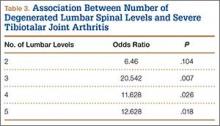
The presence of 3 or more levels of lumbar DDD significantly increased the possibility of developing severe tibiotalar joint arthritis. Lumbar DDD that encompassed 3 levels showed the highest odds for development of severe tibiotalar joint arthritis with an odds ratio (OR) of 20.542 (Table 3).
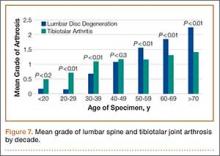
When subjects were compared by decade, the mean grade of tibiotalar joint arthritis was significantly higher than lumbar DDD in specimens who died in their 20s and 30s. This difference was insignificant in the fourth decade, and thereafter the mean value of lumbar DDD surpassed that of tibiotalar joint arthritis (Figure 7).
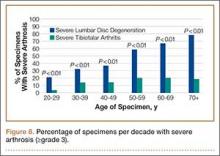
In contrast, severe lumbar DDD was more prevalent than severe tibiotalar joint arthritis in individuals age 20 years or older (Figure 8). There were no specimens under age 20 years with severe lumbar DDD or severe tibiotalar joint arthritis.
Logistic regression showed that individuals with severe lumbar disc degeneration had significantly higher odds of developing severe ankle arthritis (OR: 1.93, P < .05). Similarly, individuals with severe tibiotalar joint arthritis were just as likely to develop severe lumbar DDD with an OR of 1.97 (P < .05).
Discussion
Multiple joint involvement in osteoarthritis is well established with a wide range of evidence linking lower extremity joint pathology and lumbar spinal disease. In 1983, Offierski and MacNab20 were the first to describe hip-spine syndrome. In the next year, a study by Sponseller and colleagues25 of pediatric patients after hip arthrodesis further substantiated the association between spine and extremity disease, and demonstrated a continued cause and effect relationship after surgery.
Lumbar spinal degeneration has also been correlated with knee osteoarthritis. Tsuji and colleagues26 reported that degenerative changes in spinal alignment result in increased thigh muscle tension and knee flexion. Furthermore, in their radiographic analysis of 682 individuals, Horvath and colleagues27 also showed that individuals with spinal degeneration had a higher prevalence of knee and hip osteoarthritis.
One might hypothesize from this evidence that lumbar spinal degeneration and ankle arthritis would also be interrelated, given their interconnected role in lower extremity force transmission. Surprisingly, the literature correlating lumbar degeneration and lower extremity osteoarthritis has overlooked this association and has focused solely on the hip and knee. To our knowledge, this study is the first to identify a statistically significant association between tibiotalar joint osteoarthritis and lumbar disc degeneration.
The literature supported analysis of our data. Miller and colleagues28 evaluated disc degeneration in 600 autopsy specimens using the Nachemson29 grading system. This system categorizes disc degeneration into 4 grades based on macroscopic appearance. Miller and colleagues28 reported evidence of degenerative changes as early as the second decade of life, primarily involving the L3–L4 and L4–L5 levels. Of note, the Nachemson29 classification system includes only evidence of marginal osteophytes in grade 4 disease, which was not identified by Miller and colleagues28 until the fourth decade. These results were similar to those in our study, in which the L3-L4 and L4-L5 intervertebral levels were most commonly affected. However, in our study, significant degenerative changes were found in the third decade of life.
In addition, the percentage of specimens with severe disc degeneration increased with each decade (Figure 8). A substantial amount of histologic evidence demonstrates the progression of disc degeneration with age. With increased age, there is a gradual decrease in the osmotic swelling of intervertebral discs30 and a 2-fold decrease in disc hydration between adolescence and the eighth decade.31 Furthermore, the nucleus pulposus undergoes progressive fibrosis,32,33 with a 5-fold decrease in the fixed-charge density of nucleus glycosaminoglycans,34 and a 2-fold increase in intervertebral disc creep while under compression after age 30 years.35
While analyzing our findings, we had difficulty in determining which pathologic condition debuts and, subsequently, affects the other. According to our results, the mean grade of tibiotalar joint arthritis was higher than that of DDD in specimens through the third and fourth decades of life (Figure 7). After the age of 50 years, the mean grade of DDD surpasses that of tibiotalar arthritis. This may be initially interpreted that development of tibiotalar joint arthritis precedes lumbar disc degeneration. Ankle osteoarthritis is relatively rare, and given that the vast majority of ankle osteoarthritis is secondary to trauma,22 we would expect to see a higher incidence of ankle osteoarthritis in a younger, more active cohort. In addition, given our finding that ankle arthritis is related to lumbar disc degeneration, one could speculate that tibiotalar arthritis at a young age predisposes an individual to developing lumbar degeneration later in life.
However, this conclusion is inherently flawed; closer examination of the data revealed that the mean grade of tibiotalar arthritis and DDD in the third and fourth decades is relatively low, between grade 0 and grade 1 (Figure 7). Therefore, it is difficult to arrive at a conclusion when comparing such small values. Second, we must remember that we are comparing an average value of disc degeneration across all lumbar levels. When a specimen has only 1 disc that is severely degenerated, this value is averaged across all 5 lumbar levels and, thus, the overall mean grade of arthrosis is significantly diminished.
In fact, data from previous studies concur with the second argument. Upper-level lumbar disc degeneration is relatively rare and the vast majority of patients with disc degeneration present with significant disease in only 1 or 2 discs.36,37 Analysis of the specimens in this study revealed bony evidence of disc degeneration present at all 5 lumbar levels in over half of the specimens examined (57%). However, the majority of specimens in this cohort exhibit only low-grade degeneration. When specimens were analyzed for severe arthrosis (grade 3 and higher), nearly half of the specimens were found to have severe disease involving only 1 intervertebral disc (Figure 6). Data from Miller and colleagues28 and the present study show that the upper lumbar levels were relatively spared; the L3-L4 and L4-L5 lumbar levels showed the highest prevalence and severity of degenerative change.
To address this issue, we evaluated the percentage of specimens per decade with severe arthrosis (grade 3 and higher) of at least 1 lumbar intervertebral disc and 1 tibiotalar joint. Severe lumbar disc degeneration was found to be more prevalent than severe ankle arthritis in individuals age 20 years or older (Figure 8). Therefore, we postulate that significant degenerative changes in the lumbar spine precede the development of severe ankle arthritis.
One can further speculate that sequelae from lumbar disc degeneration may lead to the development of tibiotalar arthritis, given our finding that severe lumbar degeneration predisposes an individual to the development of ankle arthritis. Because significant lumbar disc degeneration has long been known to result in both spinal nerve and cord compression, we hypothesize that this resultant neurocompression promotes altered gait and translation of atypical forces to the ankle and foot, thus predisposing to the onset and/or progression of osteoarthritis. In support of this hypothesis, Morag and colleagues15 demonstrated that neurologic compression produced an altered posture and gait because of lost motor function and afferent proprioceptive sensation. This form of neurologic compromise may exert atypical forces upon the foot and ankle, predisposing the joint to accelerated wear and primary arthrosis.
In addition, DDD involving 3 or more lumbar intervertebral levels was found to significantly increase the likelihood of the subject having severe tibiotalar joint arthritis. Provided that lumbar disc degeneration typically involves significant degeneration at 1 level, we assume that significant arthrosis at 3 or more levels correlates to an overall more severe DDD with a higher corresponding likelihood of neural compression. However, compression of peripheral lower extremity nerves has been shown to result in neuropathic arthropathy akin to the diabetic Charcot foot.38 This could be a possible mechanism of accelerated ankle arthritis, but this study did not examine soft-tissue disease nor take into account other medical comorbidities of each specimen, including genetic predispositions towards osteoarthritis.
It should be noted that the aforementioned causative relationship between lumbar disc degeneration and tibiotalar arthritis is speculative and cannot be demonstrated definitively by this investigation. We acknowledge limitations of this study and the need for further research of the possible causative mechanism(s) of accelerated ankle arthrosis secondary to lumbar spinal disease. Ideally, the questions posed by our report would be answered via a large prospective cohort study that utilized both serial imaging and autopsy analysis. Unfortunately, this form of study is logistically and financially difficult to perform.
This was a retrospective cadaveric study in which determination of arthrosis severity was based solely on bony evidence. Therefore, the role of soft-tissue disease in the pathogenesis of arthrosis of the lumbar spine and tibiotalar joint could not be assessed, nor could definitive associations to clinically symptomatic disease. We made the assumption that progression of bone degeneration in both the lumbar spine and tibiotalar joint corresponded equally to the associated soft-tissue changes. Given this assumption, we cannot definitively conclude that degeneration of the lumbar spine precedes that of the ankle, because the absence of magnetic resonance imaging or fresh autopsy specimens in our study misses the early degenerative changes in the discs that precede the bony alteration measured in our study. Furthermore, readers should note that since this study compared only bone morphology, no emphasis was placed on clinical manifestation of lumbar disc degeneration or tibiotalar joint arthritis. As mentioned earlier, radiologic evidence of disc degeneration was found in 90% of adults age 50 to 59 years, according to a study by Hult5; however, it is important to note that not all individuals studied were symptomatic clinically. Unfortunately, medical records were not available for the bony specimens, and clinical correlations could not be assessed during this investigation.
Furthermore, no special attention was given to other pathologic conditions observed during specimen measurement. The presence of diseases, such as osteoporosis, spondylolysis, or previous traumatic injury, may have had implications in the resultant joint degeneration. Finally, the evaluation of arthrosis was performed subjectively without measuring reliability. However, the present analysis includes a large sample, each joint type was reviewed by a single examiner, and used a classification system that was modeled on a validated grading system. Ideally, multiple individuals should have been used for each type of measurement, with subsequent analysis of intraobserver and interobserver reliability.
Conclusion
Based on our study of a large population of adult skeletal specimens, we ascertained that lumbar intervertebral disc degeneration and tibiotalar osteoarthritis are associated. The prevalence of severe lumbar disc degeneration was higher than that of tibiotalar joint arthritis in individuals age 20 years or older. This may suggest that gait changes from disc degeneration or neural compression in the lumbar spine may play a role in the development of ankle osteoarthritis. Additionally, subjects with severe disc degeneration were twice as likely to develop significant tibiotalar osteoarthritis. This must be considered in the differential when treating patients with degenerative changes of the lumbar spine and leg pain.
1. Mathers C, Fat DM, Boerma JT, for the World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization, 2008.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Kelsey JL, Githens PB, White AA, et al. An epidemiological study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral disc. J Orthop Res. 1984;2(1):61-66.
4. Kellgren JH, Lawrence JS. Osteoarthrosis and disc degeneration in an urban population. Ann Rheum Dis. 1958;17(4):388-397.
5. Hult L. Cervical, dorsal and lumbar spinal syndromes; a field investigation of a non-selected material of 1200 workers in different occupations with special reference to disc degeneration and so-called muscular rheumatism. Acta Orthop Scand Suppl. 1954;17:65-73.
6. Hirsch C. The reaction of intervertebral discs to compression forces. J Bone Joint Surg Am. 1955;37(6):1188-1196.
7. Videman T, Nurminen M, Troup JD. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation and physical loading. Spine. 1990;15(8):728-740.
8. Butler D, Trafimow JH, Andersson GB, McNeil TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111-113.
9. Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
10. Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24(1):12-21.
11. Eisenstein S, Roberts S. The physiology of the disc and its clinical relevance. J Bone Joint Surg Br. 2003;85(5):633-636.
12. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304.
13. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487-499.
14. Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3-9.
15. Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson GB. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829-833.
16. Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-118.
17. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
18. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30(11):1171-1186.
19. McGregor AH, Hukins DW. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22(4):219-222.
20. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321.
21. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85(5):923-936.
22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop. 2009;467(7):1800-1806.
23. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop. 2007;464:184-189.
24. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
25. Sponseller PD, McBeath AA, Perpich M. Hip arthrodesis in young patients. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(6):853-859.
26. Tsuji T, Matsuyama Y, Goto M, et al. Knee-spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci. 2002;7(5):519-523.
27. Horvath G, Koroknai G, Acs B, Than P, Illés T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34(8):1245-1249.
28. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173-178.
29. Nachemson A. Lumbar intradiscal pressure: experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104.
30. Kraemer J. Pressure-dependent fluid shifts in the intervertebral disc. Orthop Clin North Am. 1977;8(1):211-216.
31. Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145-157.
32. Coventry MB, Ghromley RK, Kernohan JW. The intervertebral disc, its macroscopic anatomy and pathology: Part III. Pathologic changes in the intervertebral disc. J Bone Joint Surg Br. 1945;27:460-474.
33. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
34. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673(4):443-453.
35. Koeller W, Muehlhaus S, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech. 1986;19(10):807-816.
36. Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar herniations. Case reports. Orthopaedics. 1989;12(2):275-278.
37. Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
38. Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
Osteoarthritis is the most common joint disorder, resulting in significant morbidity and disability. The worldwide prevalence of osteoarthritis was estimated at more than 151 million people, according to data published in 2004.1 In the United States, almost 27 million adults age 25 years and older suffer from clinically apparent disease.2 The spine is one of the most commonly affected joints of arthritis, and idiopathic low back pain is the most frequent complaint in the adult population.3 In adults with low back pain, evidence of lumbar intervertebral disc degeneration is often found on radiography.4 In 1 study, evidence of disc degeneration was found in 90% of adults age 50 to 59 years.5
Degenerative spinal disease most commonly affects the lumbar spine due to its high degree of mobility and weight-loading.6,7 Clinical8,9 and experimental studies10 have suggested that the degenerative changes in the lumbar spine begin in the intervertebral discs. Degenerative disc disease (DDD) results from a continuum of dehydration, degradation, and remodeling of the intervertebral discs and neighboring vertebrae to accommodate the changes in physical loading.11-13 This results in disc-space narrowing, disc bulging and herniation, vertebral rim osteophyte formation, and endplate sclerosis.7,14 Symptomatic neural compression may occur, often manifested by localized lower back and extremity pain, as well as sensory loss and weakness of the lower extremities.15-17 Changes in posture and gait may result because of altered sensation, and the consequent abnormal force transmission may predispose joints to accelerated wear and arthrosis.15,18
Numerous studies have delineated the association between lumbar spinal disorders and lower extremity arthrosis. Of note, research has demonstrated that hip and/or knee pathology and gait alteration may promote low back pain and lumbar disc degeneration.19-21 Although spinal abnormalities, such as scoliosis, may predispose an individual to accelerated hip degeneration,20 no studies have investigated the relationship between lumbar DDD and ankle osteoarthritis.
Ankle arthritis differs from hip and knee arthritis demographically, occurring approximately 9 times less frequently.21 The ankle joint is subjected to more weight-bearing force per square centimeter and is more commonly injured than any other joint in the body.21 Trauma and/or abnormal ankle mechanics are the most common causes of degenerative ankle arthritis.22 Other potential causes include inflammatory arthropathies, neuropathic arthropathy, infection, and tumor. The purpose of this study was to determine if a relationship exists between ankle arthrosis and lumbar disc degeneration, and to delineate if one may promote the onset or progression of the other.
Materials and Methods
We randomly chose 710 cadaveric specimens from the Hamann-Todd Osteological Collection in Cleveland, Ohio. The Hamann-Todd Collection contains skeletal remains from more than 3000 individuals who died in Cleveland, Ohio between 1893 and 1938. The cohort for this study included 583 male and 127 female cadavers, ranging in age from 17 to 105 years at the time of death. Table 1 shows the breakdown of these specimens according to age group; of the 710 specimens, 306 were of African American ancestry, and 404 were Caucasian.
Lumbar DDD was graded at each lumbar spinal level by a single examiner using the Eubanks modification23 of the Kettler and Wilke classification of vertebral endplate osteophytosis24:
Grade 0: normal vertebral endplates;
Grade 1: mild arthrosis, with evidence of osteophytic reaction involving up to 50% of the vertebral endplates;
Grade 2: moderate arthrosis, with evidence of osteophytic reaction involving 50% to 100% of the vertebral endplates;
Grade 3: severe arthrosis, with evidence of osteophytic reaction involving 100% of the vertebral endplates. Osteophytes are hypertrophic and bridging the joint space (Figure 1);
Grade 4: complete ankylosis.
Tibiotalar joint osteoarthritis was evaluated by a single examiner using a modification of the Kellgren-Lawrence classification4 for knee osteoarthritis:
Grade 0: no discernable wear/osteophytes;
Grade 1: 1-mm osteophyte(s) and/or <25% surface wear;
Grade 2: 1- to 2-mm osteophyte(s) and/or 25% to 50% joint surface;
Grade 3: 2- to 3-mm osteophyte(s) and/or >50% joint surface (Figure 2);
Grade 4: multiple large osteophytes and/or definite bony end deformity.
Statistical analysis was performed on the compiled data using Stata software (StataCorp, College Station, Texas). Linear and logistic regression analyses correcting for confounding factors of age, sex, race, and height were performed using a standard P-value cutoff (P < .05) and 95% confidence interval to determine statistical significance.
Results
Patients were considered to have osteoarthritis of the tibiotalar joint if either of the extremities measured grade 1 or higher. Of the 710 specimens selected, 14 specimens did not have adequate bone available for bilateral tibiotalar joint measurement, either from extensive bone degradation or amputation. Of the remaining 696 specimens, 586 had some degree of tibiotalar osteoarthritis present (Table 2). Regression analysis showed a significant positive association between right- and left-ankle osteoarthritis (coefficient: 0.491, P < .01). Tibiotalar joint arthritis was classified as severe if either extremity had arthrosis of grade 3 or higher. Of the 586 specimens that had tibiotalar joint arthritis, only 16% (97 specimens) had severe tibiotalar joint arthritis.
Data regarding lumbar disc degeneration were available for 516 of the 710 specimens selected, 443 of which showed some disc degeneration. Disc degeneration was most prevalent and significant at the L4-L5 and L3-L4 intervertebral levels (Figures 3, 4). Of these 516 specimens, 30 had degeneration at 1 level, 47 specimens had degeneration at 2 levels, 29 specimens had degeneration at 3 levels, 52 had degeneration at 4 levels, and 285 specimens had degeneration at all 5 lumbar levels. The majority of specimens were found to have some degree of degeneration at all 5 lumbar spinal levels (Figure 5). Severe lumbar DDD was defined as grade 3 or higher osteoarthritis present in at least 1 of the 5 lumbar levels. Of the 516 specimens that showed some degree of disc degeneration, 152 were classified as severe. When stratified by number of spinal levels, only 30% of specimens were found to have evidence of severe arthrosis, the majority of which was located at only 1 lumbar segment (Figure 6).
Linear regression analysis of the data showed a statistically significant positive association between lumbar disc degeneration and tibiotalar osteoarthritis (coefficient: 0.844, P < .01), even when correcting for confounding factors, such as age, sex, and race (coefficient: 0.331, P < .01).
Additional analysis of the data demonstrated that tibiotalar joint arthritis remained significantly associated with lumbar DDD across each lumbar level: L1-L2 (coefficient: 0.269, P < .01), L2-L3 (coefficient: 0.283, P < .01), L3-L4 (coefficient: 0.299, P < .01), L4-L5 (coefficient: 0.240, P < .02), L5-S1 (coefficient: 0.167, P < .05).
The presence of 3 or more levels of lumbar DDD significantly increased the possibility of developing severe tibiotalar joint arthritis. Lumbar DDD that encompassed 3 levels showed the highest odds for development of severe tibiotalar joint arthritis with an odds ratio (OR) of 20.542 (Table 3).
When subjects were compared by decade, the mean grade of tibiotalar joint arthritis was significantly higher than lumbar DDD in specimens who died in their 20s and 30s. This difference was insignificant in the fourth decade, and thereafter the mean value of lumbar DDD surpassed that of tibiotalar joint arthritis (Figure 7).
In contrast, severe lumbar DDD was more prevalent than severe tibiotalar joint arthritis in individuals age 20 years or older (Figure 8). There were no specimens under age 20 years with severe lumbar DDD or severe tibiotalar joint arthritis.
Logistic regression showed that individuals with severe lumbar disc degeneration had significantly higher odds of developing severe ankle arthritis (OR: 1.93, P < .05). Similarly, individuals with severe tibiotalar joint arthritis were just as likely to develop severe lumbar DDD with an OR of 1.97 (P < .05).
Discussion
Multiple joint involvement in osteoarthritis is well established with a wide range of evidence linking lower extremity joint pathology and lumbar spinal disease. In 1983, Offierski and MacNab20 were the first to describe hip-spine syndrome. In the next year, a study by Sponseller and colleagues25 of pediatric patients after hip arthrodesis further substantiated the association between spine and extremity disease, and demonstrated a continued cause and effect relationship after surgery.
Lumbar spinal degeneration has also been correlated with knee osteoarthritis. Tsuji and colleagues26 reported that degenerative changes in spinal alignment result in increased thigh muscle tension and knee flexion. Furthermore, in their radiographic analysis of 682 individuals, Horvath and colleagues27 also showed that individuals with spinal degeneration had a higher prevalence of knee and hip osteoarthritis.
One might hypothesize from this evidence that lumbar spinal degeneration and ankle arthritis would also be interrelated, given their interconnected role in lower extremity force transmission. Surprisingly, the literature correlating lumbar degeneration and lower extremity osteoarthritis has overlooked this association and has focused solely on the hip and knee. To our knowledge, this study is the first to identify a statistically significant association between tibiotalar joint osteoarthritis and lumbar disc degeneration.
The literature supported analysis of our data. Miller and colleagues28 evaluated disc degeneration in 600 autopsy specimens using the Nachemson29 grading system. This system categorizes disc degeneration into 4 grades based on macroscopic appearance. Miller and colleagues28 reported evidence of degenerative changes as early as the second decade of life, primarily involving the L3–L4 and L4–L5 levels. Of note, the Nachemson29 classification system includes only evidence of marginal osteophytes in grade 4 disease, which was not identified by Miller and colleagues28 until the fourth decade. These results were similar to those in our study, in which the L3-L4 and L4-L5 intervertebral levels were most commonly affected. However, in our study, significant degenerative changes were found in the third decade of life.
In addition, the percentage of specimens with severe disc degeneration increased with each decade (Figure 8). A substantial amount of histologic evidence demonstrates the progression of disc degeneration with age. With increased age, there is a gradual decrease in the osmotic swelling of intervertebral discs30 and a 2-fold decrease in disc hydration between adolescence and the eighth decade.31 Furthermore, the nucleus pulposus undergoes progressive fibrosis,32,33 with a 5-fold decrease in the fixed-charge density of nucleus glycosaminoglycans,34 and a 2-fold increase in intervertebral disc creep while under compression after age 30 years.35
While analyzing our findings, we had difficulty in determining which pathologic condition debuts and, subsequently, affects the other. According to our results, the mean grade of tibiotalar joint arthritis was higher than that of DDD in specimens through the third and fourth decades of life (Figure 7). After the age of 50 years, the mean grade of DDD surpasses that of tibiotalar arthritis. This may be initially interpreted that development of tibiotalar joint arthritis precedes lumbar disc degeneration. Ankle osteoarthritis is relatively rare, and given that the vast majority of ankle osteoarthritis is secondary to trauma,22 we would expect to see a higher incidence of ankle osteoarthritis in a younger, more active cohort. In addition, given our finding that ankle arthritis is related to lumbar disc degeneration, one could speculate that tibiotalar arthritis at a young age predisposes an individual to developing lumbar degeneration later in life.
However, this conclusion is inherently flawed; closer examination of the data revealed that the mean grade of tibiotalar arthritis and DDD in the third and fourth decades is relatively low, between grade 0 and grade 1 (Figure 7). Therefore, it is difficult to arrive at a conclusion when comparing such small values. Second, we must remember that we are comparing an average value of disc degeneration across all lumbar levels. When a specimen has only 1 disc that is severely degenerated, this value is averaged across all 5 lumbar levels and, thus, the overall mean grade of arthrosis is significantly diminished.
In fact, data from previous studies concur with the second argument. Upper-level lumbar disc degeneration is relatively rare and the vast majority of patients with disc degeneration present with significant disease in only 1 or 2 discs.36,37 Analysis of the specimens in this study revealed bony evidence of disc degeneration present at all 5 lumbar levels in over half of the specimens examined (57%). However, the majority of specimens in this cohort exhibit only low-grade degeneration. When specimens were analyzed for severe arthrosis (grade 3 and higher), nearly half of the specimens were found to have severe disease involving only 1 intervertebral disc (Figure 6). Data from Miller and colleagues28 and the present study show that the upper lumbar levels were relatively spared; the L3-L4 and L4-L5 lumbar levels showed the highest prevalence and severity of degenerative change.
To address this issue, we evaluated the percentage of specimens per decade with severe arthrosis (grade 3 and higher) of at least 1 lumbar intervertebral disc and 1 tibiotalar joint. Severe lumbar disc degeneration was found to be more prevalent than severe ankle arthritis in individuals age 20 years or older (Figure 8). Therefore, we postulate that significant degenerative changes in the lumbar spine precede the development of severe ankle arthritis.
One can further speculate that sequelae from lumbar disc degeneration may lead to the development of tibiotalar arthritis, given our finding that severe lumbar degeneration predisposes an individual to the development of ankle arthritis. Because significant lumbar disc degeneration has long been known to result in both spinal nerve and cord compression, we hypothesize that this resultant neurocompression promotes altered gait and translation of atypical forces to the ankle and foot, thus predisposing to the onset and/or progression of osteoarthritis. In support of this hypothesis, Morag and colleagues15 demonstrated that neurologic compression produced an altered posture and gait because of lost motor function and afferent proprioceptive sensation. This form of neurologic compromise may exert atypical forces upon the foot and ankle, predisposing the joint to accelerated wear and primary arthrosis.
In addition, DDD involving 3 or more lumbar intervertebral levels was found to significantly increase the likelihood of the subject having severe tibiotalar joint arthritis. Provided that lumbar disc degeneration typically involves significant degeneration at 1 level, we assume that significant arthrosis at 3 or more levels correlates to an overall more severe DDD with a higher corresponding likelihood of neural compression. However, compression of peripheral lower extremity nerves has been shown to result in neuropathic arthropathy akin to the diabetic Charcot foot.38 This could be a possible mechanism of accelerated ankle arthritis, but this study did not examine soft-tissue disease nor take into account other medical comorbidities of each specimen, including genetic predispositions towards osteoarthritis.
It should be noted that the aforementioned causative relationship between lumbar disc degeneration and tibiotalar arthritis is speculative and cannot be demonstrated definitively by this investigation. We acknowledge limitations of this study and the need for further research of the possible causative mechanism(s) of accelerated ankle arthrosis secondary to lumbar spinal disease. Ideally, the questions posed by our report would be answered via a large prospective cohort study that utilized both serial imaging and autopsy analysis. Unfortunately, this form of study is logistically and financially difficult to perform.
This was a retrospective cadaveric study in which determination of arthrosis severity was based solely on bony evidence. Therefore, the role of soft-tissue disease in the pathogenesis of arthrosis of the lumbar spine and tibiotalar joint could not be assessed, nor could definitive associations to clinically symptomatic disease. We made the assumption that progression of bone degeneration in both the lumbar spine and tibiotalar joint corresponded equally to the associated soft-tissue changes. Given this assumption, we cannot definitively conclude that degeneration of the lumbar spine precedes that of the ankle, because the absence of magnetic resonance imaging or fresh autopsy specimens in our study misses the early degenerative changes in the discs that precede the bony alteration measured in our study. Furthermore, readers should note that since this study compared only bone morphology, no emphasis was placed on clinical manifestation of lumbar disc degeneration or tibiotalar joint arthritis. As mentioned earlier, radiologic evidence of disc degeneration was found in 90% of adults age 50 to 59 years, according to a study by Hult5; however, it is important to note that not all individuals studied were symptomatic clinically. Unfortunately, medical records were not available for the bony specimens, and clinical correlations could not be assessed during this investigation.
Furthermore, no special attention was given to other pathologic conditions observed during specimen measurement. The presence of diseases, such as osteoporosis, spondylolysis, or previous traumatic injury, may have had implications in the resultant joint degeneration. Finally, the evaluation of arthrosis was performed subjectively without measuring reliability. However, the present analysis includes a large sample, each joint type was reviewed by a single examiner, and used a classification system that was modeled on a validated grading system. Ideally, multiple individuals should have been used for each type of measurement, with subsequent analysis of intraobserver and interobserver reliability.
Conclusion
Based on our study of a large population of adult skeletal specimens, we ascertained that lumbar intervertebral disc degeneration and tibiotalar osteoarthritis are associated. The prevalence of severe lumbar disc degeneration was higher than that of tibiotalar joint arthritis in individuals age 20 years or older. This may suggest that gait changes from disc degeneration or neural compression in the lumbar spine may play a role in the development of ankle osteoarthritis. Additionally, subjects with severe disc degeneration were twice as likely to develop significant tibiotalar osteoarthritis. This must be considered in the differential when treating patients with degenerative changes of the lumbar spine and leg pain.
Osteoarthritis is the most common joint disorder, resulting in significant morbidity and disability. The worldwide prevalence of osteoarthritis was estimated at more than 151 million people, according to data published in 2004.1 In the United States, almost 27 million adults age 25 years and older suffer from clinically apparent disease.2 The spine is one of the most commonly affected joints of arthritis, and idiopathic low back pain is the most frequent complaint in the adult population.3 In adults with low back pain, evidence of lumbar intervertebral disc degeneration is often found on radiography.4 In 1 study, evidence of disc degeneration was found in 90% of adults age 50 to 59 years.5
Degenerative spinal disease most commonly affects the lumbar spine due to its high degree of mobility and weight-loading.6,7 Clinical8,9 and experimental studies10 have suggested that the degenerative changes in the lumbar spine begin in the intervertebral discs. Degenerative disc disease (DDD) results from a continuum of dehydration, degradation, and remodeling of the intervertebral discs and neighboring vertebrae to accommodate the changes in physical loading.11-13 This results in disc-space narrowing, disc bulging and herniation, vertebral rim osteophyte formation, and endplate sclerosis.7,14 Symptomatic neural compression may occur, often manifested by localized lower back and extremity pain, as well as sensory loss and weakness of the lower extremities.15-17 Changes in posture and gait may result because of altered sensation, and the consequent abnormal force transmission may predispose joints to accelerated wear and arthrosis.15,18
Numerous studies have delineated the association between lumbar spinal disorders and lower extremity arthrosis. Of note, research has demonstrated that hip and/or knee pathology and gait alteration may promote low back pain and lumbar disc degeneration.19-21 Although spinal abnormalities, such as scoliosis, may predispose an individual to accelerated hip degeneration,20 no studies have investigated the relationship between lumbar DDD and ankle osteoarthritis.
Ankle arthritis differs from hip and knee arthritis demographically, occurring approximately 9 times less frequently.21 The ankle joint is subjected to more weight-bearing force per square centimeter and is more commonly injured than any other joint in the body.21 Trauma and/or abnormal ankle mechanics are the most common causes of degenerative ankle arthritis.22 Other potential causes include inflammatory arthropathies, neuropathic arthropathy, infection, and tumor. The purpose of this study was to determine if a relationship exists between ankle arthrosis and lumbar disc degeneration, and to delineate if one may promote the onset or progression of the other.
Materials and Methods
We randomly chose 710 cadaveric specimens from the Hamann-Todd Osteological Collection in Cleveland, Ohio. The Hamann-Todd Collection contains skeletal remains from more than 3000 individuals who died in Cleveland, Ohio between 1893 and 1938. The cohort for this study included 583 male and 127 female cadavers, ranging in age from 17 to 105 years at the time of death. Table 1 shows the breakdown of these specimens according to age group; of the 710 specimens, 306 were of African American ancestry, and 404 were Caucasian.
Lumbar DDD was graded at each lumbar spinal level by a single examiner using the Eubanks modification23 of the Kettler and Wilke classification of vertebral endplate osteophytosis24:
Grade 0: normal vertebral endplates;
Grade 1: mild arthrosis, with evidence of osteophytic reaction involving up to 50% of the vertebral endplates;
Grade 2: moderate arthrosis, with evidence of osteophytic reaction involving 50% to 100% of the vertebral endplates;
Grade 3: severe arthrosis, with evidence of osteophytic reaction involving 100% of the vertebral endplates. Osteophytes are hypertrophic and bridging the joint space (Figure 1);
Grade 4: complete ankylosis.
Tibiotalar joint osteoarthritis was evaluated by a single examiner using a modification of the Kellgren-Lawrence classification4 for knee osteoarthritis:
Grade 0: no discernable wear/osteophytes;
Grade 1: 1-mm osteophyte(s) and/or <25% surface wear;
Grade 2: 1- to 2-mm osteophyte(s) and/or 25% to 50% joint surface;
Grade 3: 2- to 3-mm osteophyte(s) and/or >50% joint surface (Figure 2);
Grade 4: multiple large osteophytes and/or definite bony end deformity.
Statistical analysis was performed on the compiled data using Stata software (StataCorp, College Station, Texas). Linear and logistic regression analyses correcting for confounding factors of age, sex, race, and height were performed using a standard P-value cutoff (P < .05) and 95% confidence interval to determine statistical significance.
Results
Patients were considered to have osteoarthritis of the tibiotalar joint if either of the extremities measured grade 1 or higher. Of the 710 specimens selected, 14 specimens did not have adequate bone available for bilateral tibiotalar joint measurement, either from extensive bone degradation or amputation. Of the remaining 696 specimens, 586 had some degree of tibiotalar osteoarthritis present (Table 2). Regression analysis showed a significant positive association between right- and left-ankle osteoarthritis (coefficient: 0.491, P < .01). Tibiotalar joint arthritis was classified as severe if either extremity had arthrosis of grade 3 or higher. Of the 586 specimens that had tibiotalar joint arthritis, only 16% (97 specimens) had severe tibiotalar joint arthritis.
Data regarding lumbar disc degeneration were available for 516 of the 710 specimens selected, 443 of which showed some disc degeneration. Disc degeneration was most prevalent and significant at the L4-L5 and L3-L4 intervertebral levels (Figures 3, 4). Of these 516 specimens, 30 had degeneration at 1 level, 47 specimens had degeneration at 2 levels, 29 specimens had degeneration at 3 levels, 52 had degeneration at 4 levels, and 285 specimens had degeneration at all 5 lumbar levels. The majority of specimens were found to have some degree of degeneration at all 5 lumbar spinal levels (Figure 5). Severe lumbar DDD was defined as grade 3 or higher osteoarthritis present in at least 1 of the 5 lumbar levels. Of the 516 specimens that showed some degree of disc degeneration, 152 were classified as severe. When stratified by number of spinal levels, only 30% of specimens were found to have evidence of severe arthrosis, the majority of which was located at only 1 lumbar segment (Figure 6).
Linear regression analysis of the data showed a statistically significant positive association between lumbar disc degeneration and tibiotalar osteoarthritis (coefficient: 0.844, P < .01), even when correcting for confounding factors, such as age, sex, and race (coefficient: 0.331, P < .01).
Additional analysis of the data demonstrated that tibiotalar joint arthritis remained significantly associated with lumbar DDD across each lumbar level: L1-L2 (coefficient: 0.269, P < .01), L2-L3 (coefficient: 0.283, P < .01), L3-L4 (coefficient: 0.299, P < .01), L4-L5 (coefficient: 0.240, P < .02), L5-S1 (coefficient: 0.167, P < .05).
The presence of 3 or more levels of lumbar DDD significantly increased the possibility of developing severe tibiotalar joint arthritis. Lumbar DDD that encompassed 3 levels showed the highest odds for development of severe tibiotalar joint arthritis with an odds ratio (OR) of 20.542 (Table 3).
When subjects were compared by decade, the mean grade of tibiotalar joint arthritis was significantly higher than lumbar DDD in specimens who died in their 20s and 30s. This difference was insignificant in the fourth decade, and thereafter the mean value of lumbar DDD surpassed that of tibiotalar joint arthritis (Figure 7).
In contrast, severe lumbar DDD was more prevalent than severe tibiotalar joint arthritis in individuals age 20 years or older (Figure 8). There were no specimens under age 20 years with severe lumbar DDD or severe tibiotalar joint arthritis.
Logistic regression showed that individuals with severe lumbar disc degeneration had significantly higher odds of developing severe ankle arthritis (OR: 1.93, P < .05). Similarly, individuals with severe tibiotalar joint arthritis were just as likely to develop severe lumbar DDD with an OR of 1.97 (P < .05).
Discussion
Multiple joint involvement in osteoarthritis is well established with a wide range of evidence linking lower extremity joint pathology and lumbar spinal disease. In 1983, Offierski and MacNab20 were the first to describe hip-spine syndrome. In the next year, a study by Sponseller and colleagues25 of pediatric patients after hip arthrodesis further substantiated the association between spine and extremity disease, and demonstrated a continued cause and effect relationship after surgery.
Lumbar spinal degeneration has also been correlated with knee osteoarthritis. Tsuji and colleagues26 reported that degenerative changes in spinal alignment result in increased thigh muscle tension and knee flexion. Furthermore, in their radiographic analysis of 682 individuals, Horvath and colleagues27 also showed that individuals with spinal degeneration had a higher prevalence of knee and hip osteoarthritis.
One might hypothesize from this evidence that lumbar spinal degeneration and ankle arthritis would also be interrelated, given their interconnected role in lower extremity force transmission. Surprisingly, the literature correlating lumbar degeneration and lower extremity osteoarthritis has overlooked this association and has focused solely on the hip and knee. To our knowledge, this study is the first to identify a statistically significant association between tibiotalar joint osteoarthritis and lumbar disc degeneration.
The literature supported analysis of our data. Miller and colleagues28 evaluated disc degeneration in 600 autopsy specimens using the Nachemson29 grading system. This system categorizes disc degeneration into 4 grades based on macroscopic appearance. Miller and colleagues28 reported evidence of degenerative changes as early as the second decade of life, primarily involving the L3–L4 and L4–L5 levels. Of note, the Nachemson29 classification system includes only evidence of marginal osteophytes in grade 4 disease, which was not identified by Miller and colleagues28 until the fourth decade. These results were similar to those in our study, in which the L3-L4 and L4-L5 intervertebral levels were most commonly affected. However, in our study, significant degenerative changes were found in the third decade of life.
In addition, the percentage of specimens with severe disc degeneration increased with each decade (Figure 8). A substantial amount of histologic evidence demonstrates the progression of disc degeneration with age. With increased age, there is a gradual decrease in the osmotic swelling of intervertebral discs30 and a 2-fold decrease in disc hydration between adolescence and the eighth decade.31 Furthermore, the nucleus pulposus undergoes progressive fibrosis,32,33 with a 5-fold decrease in the fixed-charge density of nucleus glycosaminoglycans,34 and a 2-fold increase in intervertebral disc creep while under compression after age 30 years.35
While analyzing our findings, we had difficulty in determining which pathologic condition debuts and, subsequently, affects the other. According to our results, the mean grade of tibiotalar joint arthritis was higher than that of DDD in specimens through the third and fourth decades of life (Figure 7). After the age of 50 years, the mean grade of DDD surpasses that of tibiotalar arthritis. This may be initially interpreted that development of tibiotalar joint arthritis precedes lumbar disc degeneration. Ankle osteoarthritis is relatively rare, and given that the vast majority of ankle osteoarthritis is secondary to trauma,22 we would expect to see a higher incidence of ankle osteoarthritis in a younger, more active cohort. In addition, given our finding that ankle arthritis is related to lumbar disc degeneration, one could speculate that tibiotalar arthritis at a young age predisposes an individual to developing lumbar degeneration later in life.
However, this conclusion is inherently flawed; closer examination of the data revealed that the mean grade of tibiotalar arthritis and DDD in the third and fourth decades is relatively low, between grade 0 and grade 1 (Figure 7). Therefore, it is difficult to arrive at a conclusion when comparing such small values. Second, we must remember that we are comparing an average value of disc degeneration across all lumbar levels. When a specimen has only 1 disc that is severely degenerated, this value is averaged across all 5 lumbar levels and, thus, the overall mean grade of arthrosis is significantly diminished.
In fact, data from previous studies concur with the second argument. Upper-level lumbar disc degeneration is relatively rare and the vast majority of patients with disc degeneration present with significant disease in only 1 or 2 discs.36,37 Analysis of the specimens in this study revealed bony evidence of disc degeneration present at all 5 lumbar levels in over half of the specimens examined (57%). However, the majority of specimens in this cohort exhibit only low-grade degeneration. When specimens were analyzed for severe arthrosis (grade 3 and higher), nearly half of the specimens were found to have severe disease involving only 1 intervertebral disc (Figure 6). Data from Miller and colleagues28 and the present study show that the upper lumbar levels were relatively spared; the L3-L4 and L4-L5 lumbar levels showed the highest prevalence and severity of degenerative change.
To address this issue, we evaluated the percentage of specimens per decade with severe arthrosis (grade 3 and higher) of at least 1 lumbar intervertebral disc and 1 tibiotalar joint. Severe lumbar disc degeneration was found to be more prevalent than severe ankle arthritis in individuals age 20 years or older (Figure 8). Therefore, we postulate that significant degenerative changes in the lumbar spine precede the development of severe ankle arthritis.
One can further speculate that sequelae from lumbar disc degeneration may lead to the development of tibiotalar arthritis, given our finding that severe lumbar degeneration predisposes an individual to the development of ankle arthritis. Because significant lumbar disc degeneration has long been known to result in both spinal nerve and cord compression, we hypothesize that this resultant neurocompression promotes altered gait and translation of atypical forces to the ankle and foot, thus predisposing to the onset and/or progression of osteoarthritis. In support of this hypothesis, Morag and colleagues15 demonstrated that neurologic compression produced an altered posture and gait because of lost motor function and afferent proprioceptive sensation. This form of neurologic compromise may exert atypical forces upon the foot and ankle, predisposing the joint to accelerated wear and primary arthrosis.
In addition, DDD involving 3 or more lumbar intervertebral levels was found to significantly increase the likelihood of the subject having severe tibiotalar joint arthritis. Provided that lumbar disc degeneration typically involves significant degeneration at 1 level, we assume that significant arthrosis at 3 or more levels correlates to an overall more severe DDD with a higher corresponding likelihood of neural compression. However, compression of peripheral lower extremity nerves has been shown to result in neuropathic arthropathy akin to the diabetic Charcot foot.38 This could be a possible mechanism of accelerated ankle arthritis, but this study did not examine soft-tissue disease nor take into account other medical comorbidities of each specimen, including genetic predispositions towards osteoarthritis.
It should be noted that the aforementioned causative relationship between lumbar disc degeneration and tibiotalar arthritis is speculative and cannot be demonstrated definitively by this investigation. We acknowledge limitations of this study and the need for further research of the possible causative mechanism(s) of accelerated ankle arthrosis secondary to lumbar spinal disease. Ideally, the questions posed by our report would be answered via a large prospective cohort study that utilized both serial imaging and autopsy analysis. Unfortunately, this form of study is logistically and financially difficult to perform.
This was a retrospective cadaveric study in which determination of arthrosis severity was based solely on bony evidence. Therefore, the role of soft-tissue disease in the pathogenesis of arthrosis of the lumbar spine and tibiotalar joint could not be assessed, nor could definitive associations to clinically symptomatic disease. We made the assumption that progression of bone degeneration in both the lumbar spine and tibiotalar joint corresponded equally to the associated soft-tissue changes. Given this assumption, we cannot definitively conclude that degeneration of the lumbar spine precedes that of the ankle, because the absence of magnetic resonance imaging or fresh autopsy specimens in our study misses the early degenerative changes in the discs that precede the bony alteration measured in our study. Furthermore, readers should note that since this study compared only bone morphology, no emphasis was placed on clinical manifestation of lumbar disc degeneration or tibiotalar joint arthritis. As mentioned earlier, radiologic evidence of disc degeneration was found in 90% of adults age 50 to 59 years, according to a study by Hult5; however, it is important to note that not all individuals studied were symptomatic clinically. Unfortunately, medical records were not available for the bony specimens, and clinical correlations could not be assessed during this investigation.
Furthermore, no special attention was given to other pathologic conditions observed during specimen measurement. The presence of diseases, such as osteoporosis, spondylolysis, or previous traumatic injury, may have had implications in the resultant joint degeneration. Finally, the evaluation of arthrosis was performed subjectively without measuring reliability. However, the present analysis includes a large sample, each joint type was reviewed by a single examiner, and used a classification system that was modeled on a validated grading system. Ideally, multiple individuals should have been used for each type of measurement, with subsequent analysis of intraobserver and interobserver reliability.
Conclusion
Based on our study of a large population of adult skeletal specimens, we ascertained that lumbar intervertebral disc degeneration and tibiotalar osteoarthritis are associated. The prevalence of severe lumbar disc degeneration was higher than that of tibiotalar joint arthritis in individuals age 20 years or older. This may suggest that gait changes from disc degeneration or neural compression in the lumbar spine may play a role in the development of ankle osteoarthritis. Additionally, subjects with severe disc degeneration were twice as likely to develop significant tibiotalar osteoarthritis. This must be considered in the differential when treating patients with degenerative changes of the lumbar spine and leg pain.
1. Mathers C, Fat DM, Boerma JT, for the World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization, 2008.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Kelsey JL, Githens PB, White AA, et al. An epidemiological study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral disc. J Orthop Res. 1984;2(1):61-66.
4. Kellgren JH, Lawrence JS. Osteoarthrosis and disc degeneration in an urban population. Ann Rheum Dis. 1958;17(4):388-397.
5. Hult L. Cervical, dorsal and lumbar spinal syndromes; a field investigation of a non-selected material of 1200 workers in different occupations with special reference to disc degeneration and so-called muscular rheumatism. Acta Orthop Scand Suppl. 1954;17:65-73.
6. Hirsch C. The reaction of intervertebral discs to compression forces. J Bone Joint Surg Am. 1955;37(6):1188-1196.
7. Videman T, Nurminen M, Troup JD. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation and physical loading. Spine. 1990;15(8):728-740.
8. Butler D, Trafimow JH, Andersson GB, McNeil TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111-113.
9. Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
10. Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24(1):12-21.
11. Eisenstein S, Roberts S. The physiology of the disc and its clinical relevance. J Bone Joint Surg Br. 2003;85(5):633-636.
12. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304.
13. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487-499.
14. Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3-9.
15. Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson GB. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829-833.
16. Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-118.
17. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
18. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30(11):1171-1186.
19. McGregor AH, Hukins DW. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22(4):219-222.
20. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321.
21. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85(5):923-936.
22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop. 2009;467(7):1800-1806.
23. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop. 2007;464:184-189.
24. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
25. Sponseller PD, McBeath AA, Perpich M. Hip arthrodesis in young patients. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(6):853-859.
26. Tsuji T, Matsuyama Y, Goto M, et al. Knee-spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci. 2002;7(5):519-523.
27. Horvath G, Koroknai G, Acs B, Than P, Illés T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34(8):1245-1249.
28. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173-178.
29. Nachemson A. Lumbar intradiscal pressure: experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104.
30. Kraemer J. Pressure-dependent fluid shifts in the intervertebral disc. Orthop Clin North Am. 1977;8(1):211-216.
31. Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145-157.
32. Coventry MB, Ghromley RK, Kernohan JW. The intervertebral disc, its macroscopic anatomy and pathology: Part III. Pathologic changes in the intervertebral disc. J Bone Joint Surg Br. 1945;27:460-474.
33. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
34. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673(4):443-453.
35. Koeller W, Muehlhaus S, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech. 1986;19(10):807-816.
36. Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar herniations. Case reports. Orthopaedics. 1989;12(2):275-278.
37. Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
38. Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
1. Mathers C, Fat DM, Boerma JT, for the World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization, 2008.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Kelsey JL, Githens PB, White AA, et al. An epidemiological study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral disc. J Orthop Res. 1984;2(1):61-66.
4. Kellgren JH, Lawrence JS. Osteoarthrosis and disc degeneration in an urban population. Ann Rheum Dis. 1958;17(4):388-397.
5. Hult L. Cervical, dorsal and lumbar spinal syndromes; a field investigation of a non-selected material of 1200 workers in different occupations with special reference to disc degeneration and so-called muscular rheumatism. Acta Orthop Scand Suppl. 1954;17:65-73.
6. Hirsch C. The reaction of intervertebral discs to compression forces. J Bone Joint Surg Am. 1955;37(6):1188-1196.
7. Videman T, Nurminen M, Troup JD. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation and physical loading. Spine. 1990;15(8):728-740.
8. Butler D, Trafimow JH, Andersson GB, McNeil TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111-113.
9. Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
10. Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24(1):12-21.
11. Eisenstein S, Roberts S. The physiology of the disc and its clinical relevance. J Bone Joint Surg Br. 2003;85(5):633-636.
12. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304.
13. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487-499.
14. Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3-9.
15. Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson GB. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829-833.
16. Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-118.
17. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
18. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30(11):1171-1186.
19. McGregor AH, Hukins DW. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22(4):219-222.
20. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321.
21. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85(5):923-936.
22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop. 2009;467(7):1800-1806.
23. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop. 2007;464:184-189.
24. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
25. Sponseller PD, McBeath AA, Perpich M. Hip arthrodesis in young patients. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(6):853-859.
26. Tsuji T, Matsuyama Y, Goto M, et al. Knee-spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci. 2002;7(5):519-523.
27. Horvath G, Koroknai G, Acs B, Than P, Illés T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34(8):1245-1249.
28. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173-178.
29. Nachemson A. Lumbar intradiscal pressure: experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104.
30. Kraemer J. Pressure-dependent fluid shifts in the intervertebral disc. Orthop Clin North Am. 1977;8(1):211-216.
31. Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145-157.
32. Coventry MB, Ghromley RK, Kernohan JW. The intervertebral disc, its macroscopic anatomy and pathology: Part III. Pathologic changes in the intervertebral disc. J Bone Joint Surg Br. 1945;27:460-474.
33. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
34. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673(4):443-453.
35. Koeller W, Muehlhaus S, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech. 1986;19(10):807-816.
36. Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar herniations. Case reports. Orthopaedics. 1989;12(2):275-278.
37. Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
38. Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
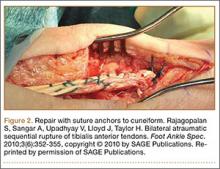
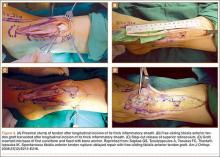
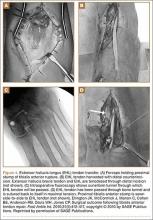
A Systematic Review of Tibialis Anterior Tendon Rupture Treatments and Outcomes
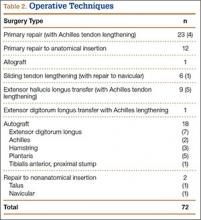
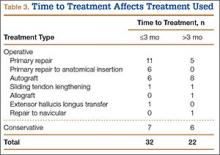
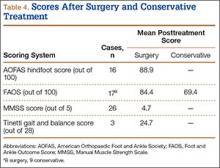
Subcutaneous rupture of the tibialis anterior (TA) tendon has been reported predominantly in case reports and small case series because of the relative rarity of the injury. Unlike traumatic lacerations or open injuries to the tendon, subcutaneous injuries often go unnoticed by patients because of compensation by surrounding dorsiflexors of the foot and toes—namely, the extensor hallucis longus (EHL) and the extensor digitorum longus (EDL).1 This can delay presentation to an orthopedic surgeon and lead to difficulties in treatment, such as allograft or autograft being required if primary repair is no longer possible. Case reports and series have described treatment methods as well as anecdotal evidence of outcomes after operative repair or conservative treatment, but there have been no comprehensive systematic reviews of outcomes after various types of treatment. Authors have come to conclusions about expected outcomes based on patient age, time to treatment, treatment used, and other variables, but no reviews have examined these variables across multiple studies. Given the low level of the evidence presented in most of these reports, it is difficult to perform a meta-analysis of the data.
Instead, we systematically reviewed 87 cases from all pertinent studies and examined commonly reported data, such as patient age, time to treatment, treatment used, and outcome. Using the PICO (population, intervention, comparison, outcome) model for systematic reviews, we looked at patients who had closed, spontaneous, complete rupture of the TA tendon and underwent operative repair or conservative treatment of the injury. Outcomes surveyed included successful operative repair or conservative treatment, as measured by objective systems, such as MMSS (Manual Muscle Strength Scale) score, AOFAS (American Orthopaedic Foot and Ankle Society) hindfoot score, and FAOS (Foot and Ankle Outcome Score) testing, or by subjective description of posttreatment outcome.
We intend this review to serve as a guide for surgeons who find themselves treating a ruptured TA tendon, a relatively rare injury. They will be able to select the operative technique or conservative treatment that best matches the patient’s needs, based on comparison with previous case studies.
Materials and Methods
The cases reviewed for this study were found through a comprehensive PubMed search and an independent review of references cited in similar articles. Articles included were published between 1975 and 2012, inclusive. The latest search was performed on March 22, 2013. The search criteria were tibialis anterior [Title/Abstract] OR anterior tibial [Title/Abstract] AND rupture [Title/Abstract]) AND surgery. Only English-language articles, or articles already translated into English, were included. Eligible studies described cases of closed tendon rupture. No traumatic lacerations or open ruptures were included. If a study described both open and subcutaneous ruptures, only the subcutaneous cases were included. Further, partial ruptures were not included. In addition, ruptures caused directly by a known comorbid condition—for example, a rupture caused by a gouty tophaceous deposit at the site of rupture2—were not included. Data were extracted from publications independently and analyzed in a Microsoft Excel workbook (Microsoft, Redmond, Washington). Variables examined included patient age and sex, side involved, time to treatment, mechanism of injury, defect size, predisposing comorbidities, surgery or conservative treatment, type of operative repair (if applicable), graft used (if applicable), pretreatment function (by independent scoring system, if applicable), and posttreatment function. These variables were not necessarily reported in all the studies.
A potential bias exists in our PubMed search. As the query was specific for studies that included operative repair of a ruptured TA tendon, case studies that involved only conservative treatment were excluded. However, the primary goal of this review was to compare operative possibilities and the patient characteristics and outcomes associated with these surgeries.
Results
Figure 1 shows the criteria used to select eligible papers for review. Twenty-three papers matched the criteria.3-25 Data were independently extracted from these papers, as described in the Methods section. Again, not all variables were reported by all authors. Sammarco and colleagues21 reported time to treatment as a mean for 2 groups: 8 cases defined as “early” treatment (mean time to treatment, 0.625 months) and 11 defined as “late” treatment (mean time to treatment, 10.7 months). These mean times were therefore used independently for each case in calculating mean time to treatment for this systematic review.
Table 1 lists the demographics. There were 40 male and 25 female patients, and 22 cases in which sex was not specified. Mean age was 63.9 years (surgery group), 72.4 years (conservative treatment group), and 65.8 years (overall). Of the 87 patients, 72 underwent surgery, and 15 were treated with conservative measures.
Table 2 lists the operative techniques identified. Of the 72 surgeries, 23 were primary repairs, 12 were primary repairs of the anatomical insertion, and 18 involved use of autograft.
Time to treatment was available for 54 of the 87 cases (Table 3). Primary repair was most often performed in cases in which the injury was less than 3 months old, and autograft was most often used in cases in which the injury occurred more than 3 months before presentation.
Posttreatment outcome scores were available for 59 cases. Only 3 authors reported preoperative scores.5,21,24 None of the authors who used conservative treatment measures reported pretreatment scores. Scores used included the MMSS score (26 cases), the AOFAS hindfoot score (16 cases),26 the FAOS (17 cases),27 and the Tinetti gait and balance score (3 cases; the author also used the MMSS score).28Table 4 lists the mean posttreatment scores for patients who underwent surgery and patients treated conservatively. AOFAS, MMSS, and Tinetti scores and FAOS were used by authors presenting operative treatment outcomes. Only posttreatment FAOS was available for both surgery (84.4/100) and conservative treatment (69.4/100).
Discussion
Closed rupture of the TA tendon is a relatively rare entity occurring mostly in older patients without any history of acute, traumatic injury. Some patients, however, recall a particular moment of rupture, often accompanied immediately by pain and swelling, which eventually resolve. Later sequelae include footdrop with associated steppage gait and a palpable mass on the dorsal aspect of the ankle.3,21 Chronic TA tendon rupture can also lead to clawing of the toes as the other foot extensors (EHL, EDL) overcompensate. Cohen and Gordon1 described the case of a patient who ruptured a TA tendon 25 years earlier and then, in the absence of operative repair, developed hypertrophy of the EHL and the EDL. This extensor substitution led to hammer toes and plantar prominence of the metatarsal heads, ultimately leading to moderate pain and a neuroma. Although this particular outcome is likely rare, the more common sequelae of footdrop, flatfoot, Achilles tendon contracture, and compromised gait are reason enough to consider operative repair for any ruptured TA tendon.
Most previous studies of TA tendon rupture were case reports and case studies. In the largest series, Sammarco and colleagues21 described 19 cases of closed rupture. These included 3 traumatic cases, 1 by blunt trauma to the tendon and 2 of open laceration, all treated surgically with various methods. Unfortunately, these 3 traumatic cases were not separated in the authors’ analysis and therefore had to be included in this systematic review. Including them here did not compromise our goals in this review, which included examining typical patient demographics and the most common methods of operative repair.
Conservative measures remain a treatment possibility for some patients. We found that patients treated with conservative measures historically have been older (mean age, 72.4 years) than patients treated surgically (mean age, 63.9 years). However, advanced age itself is not a contraindication for operative repair of a TA tendon rupture, and authors have described positive outcomes for active, elderly (>70 years) patients who wanted to maintain their activity level and therefore opted for operative repair.7,8,10,13,16,24 Ouzounian and Anderson18 described functional limitations (eg, persistent footdrop, slapfoot gait, limitations in walking) after conservative treatment with an ankle-foot orthosis. Operative repair offers the chance for better functional outcome for patients who are surgical candidates and lead even a mildly active lifestyle.
Of operative repair methods, primary repair is used most often. This technique, however, must be allowed by the gap between the 2 ruptured ends after débridement of any necrotic tissue. If the distal stump is not viable, primary repair of the proximal stump to the native anatomical insertion is feasible. Figure 2, reprinted from a case report by Rajagopalan and colleagues,19 shows a ligament–osseous reattachment of the proximal stump using suture anchors to the medial cuneiform. Both primary repair and repair to the anatomical insertion can be augmented with Achilles tendon lengthening if needed to achieve balance between flexor and extensor functions of the ankle.
If the gap between the 2 stumps cannot be covered by the native tendon, then autograft, another surgical technique with positive outcomes, can be used. The most popular autograft sites historically have been the EDL, Achilles, and plantaris tendons. In addition, Goehring and Liakos9 described 3 cases of good results with semitendinosus autograft. Sapkas and colleagues22 used a free-sliding TA graft harvested from the healthy tissue of the proximal tendon stump. Their technique is depicted in Figure 3. Sliding tendon lengthening, well described by Trout and colleagues24 in a case study, is feasible for use of the native tendon when there is a gap to bridge between the 2 stumps of ruptured tendon. EHL or EDL transfer with or without Achilles lengthening is another option, albeit historically less often used.6,7 This technique is depicted in Figure 4, reprinted from a case series by Ellington and colleagues,7 who used EHL transfer with and without Achilles tendon lengthening in 9 cases.
Last, less popular techniques have included repair to sites other than the medial cuneiform, including the neck of the talus and the navicular bone.10,13 An Achilles tendon allograft was used in a case described by Aderinto and Gross3 to repair a ruptured tendon found incidentally on preoperative examination for a scheduled knee arthroplasty. The patient had a postoperative MMSS score of 4/5.
Overall, primary repair is clearly preferred, but successful outcomes can be achieved by other means. As Table 3 shows, primary repair is more often used for ruptures less than 3 months old, and autograft for older ruptures. Although which operative technique to use can be decided after necrotic tissue is débrided, surgeons should try to ascertain age of injury ahead of time so that, going into surgery, they will have a better idea of the feasibility of primary repair.
Posttreatment ankle scores were not widely available. As Table 4 indicates, only FAOS was used for the conservative treatment cases. However, raw mean FAOS and raw mean AOFAS hindfoot, MMSS, and Tinetti scores showed that good outcomes and high scores can be achieved with surgery. Further, the mean FAOS reported by Gwynne-Jones and colleagues10 and Markarian and colleagues13 showed a clinically significant difference between surgery and conservative treatment. DiDomenico and colleagues,5 Sammarco and colleagues,21 and Trout and colleagues24 were the only authors who reported pretreatment and posttreatment scores.
We intend this systematic review of the literature on closed TA rupture to serve as a guide for surgeons who find themselves treating this relatively rare injury, which often presents with only a chief complaint of the foot catching while walking. Overall, the literature shows that operative repair provides very good outcomes for many patients. Patients who are surgical candidates and amenable to surgery can be counseled that operative repair leads to fewer sequelae, such as persistent footdrop and flatfooted gait, with a strong likelihood of return to baseline activity status. Patients who are not surgical candidates or are strongly against surgery can be offered conservative treatment with an ankle-foot orthosis or physical therapy, but they should also be counseled that persistent gait abnormalities and weakness in dorsiflexion are likely outcomes. Surgeons must also consider age of injury (time from probable rupture to presentation), estimating a particular moment of rupture if unknown by the patient. They can then gauge the feasibility of primary repair and, during surgery, decide which technique (primary repair, tendon transfer, autograft, or other technique) will produce the best results. They can also use scores such as the FAOS and the AOFAS hindfoot, MMSS, and Tinetti scores to compare preoperative and postoperative function, though subjective reports of return to previous activity can also serve as markers of successful repair.
This review highlights the need for further study regarding the treatment of TA ruptures. Larger, randomized studies with validated scoring systems for preoperative and postoperative function would offer more insight onto the best treatment options for these complex injuries.
1. Cohen DA, Gordon DH. The long-term effects of an untreated tibialis anterior tendon rupture. J Am Podiatr Med Assoc. 1999;89(3):149-152.
2. Jerome JTJ, Varghese M, Sankaran B, Thomas S, Thirumagal SK. Tibialis anterior tendon rupture in gout—case report and literature review. Foot Ankle Surg. 2008;14(3):166-169.
3. Aderinto J, Gross A. Delayed repair of tibialis anterior tendon rupture with Achilles tendon allograft. J Foot Ankle Surg. 2011;50(3):340-342.
4. Constantinou M, Wilson A. Traumatic tear of tibialis anterior during a Gaelic football game: a case report. Br J Sports Med. 2004;38(6):e30.
5. DiDomenico LA, Williams K, Petrolla AF. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Foot Ankle Surg. 2008;47(5):463-467.
6. Dooley BJ, Kudelka P, Menelaus MB. Subcutaneous rupture of the tendon of tibialis anterior. J Bone Joint Surg Br. 1980;62(4):471-472.
7. Ellington JK, McCormick J, Marion C, et al. Surgical outcome following tibialis anterior tendon repair. Foot Ankle Int. 2010;31(5):412-417.
8. ElMaraghy A, Devereaux MW. Bone tunnel fixation for repair of tibialis anterior tendon rupture. Foot Ankle Surg. 2010;16(2):e47-e50.
9. Goehring M, Liakos P. Long-term outcomes following anterior tibialis tendon reconstruction with hamstring autograft in a series of 3 cases. J Foot Ankle Surg. 2009;48(2):196-202.
10. Gwynne-Jones D, Garneti N, Wyatt M. Closed tibialis anterior tendon rupture: a case series. Foot Ankle Int. 2009;30(8):758-762.
11. Kashyap S, Prince R. Spontaneous rupture of the tibialis anterior tendon. A case report. Clin Orthop. 1987;(216):159-161.
12. Kausch T, Rütt J. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch Orthop Trauma Surg. 1998;117(4-5):290-293.
13. Markarian GG, Kelikian AS, Brage M, Trainor T, Dias L. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle Int. 1998;19(12):792-802.
14. Meyn MA Jr. Closed rupture of the anterior tibial tendon. A case report and review of the literature. Clin Orthop. 1975;(113):154-157.
15. Miller RR, Mahan KT. Closed rupture of the anterior tibial tendon. A case report. J Am Podiatr Med Assoc. 1998;88(8):394-399.
16. Neumayer F, Djembi YR, Gerin A, Masquelet AC. Closed rupture of the tibialis anterior tendon: a report of 2 cases. J Foot Ankle Surg. 2009;48(4):457-461.
17. Otte S, Klinger HM, Lorenz F, Haerer T. Operative treatment in case of a closed rupture of the anterior tibial tendon. Arch Orthop Trauma Surg. 2002;122(3):188-190.
18. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int. 1995;16(7):406-410.
19. Rajagopalan S, Sangar A, Upadhyay V, Lloyd J, Taylor H. Bilateral atraumatic sequential rupture of tibialis anterior tendons. Foot Ankle Spec. 2010;3(6):352-355.
20. Rimoldi RL, Oberlander MA, Waldrop JI, Hunter SC. Acute rupture of the tibialis anterior tendon: a case report. Foot Ankle. 1991;12(3):176-177.
21. Sammarco VJ, Sammarco GJ, Henning C, Chaim S. Surgical repair of acute and chronic tibialis anterior tendon ruptures. J Bone Joint Surg Am. 2009;91(2):325-332.
22. Sapkas GS, Tzoutzopoulos A, Tsoukas FC, Triantafillopoulos IK. Spontaneous tibialis anterior tendon rupture: delayed repair with free-sliding tibialis anterior tendon graft. Am J Orthop. 2008;37(12):E213-E216.
23. Stuart MJ. Traumatic disruption of the anterior tibial tendon while cross-country skiing. A case report. Clin Orthop. 1992;(281):193-194.
24. Trout BM, Hosey G, Wertheimer SJ. Rupture of the tibialis anterior tendon. J Foot Ankle Surg. 2000;39(1):54-58.
25. Van Acker G, Pingen F, Luitse J, Goslings C. Rupture of the tibialis anterior tendon. Acta Orthop Belg. 2006;72(1):105-107.
26. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353.
27. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794.
28. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429-434.
Subcutaneous rupture of the tibialis anterior (TA) tendon has been reported predominantly in case reports and small case series because of the relative rarity of the injury. Unlike traumatic lacerations or open injuries to the tendon, subcutaneous injuries often go unnoticed by patients because of compensation by surrounding dorsiflexors of the foot and toes—namely, the extensor hallucis longus (EHL) and the extensor digitorum longus (EDL).1 This can delay presentation to an orthopedic surgeon and lead to difficulties in treatment, such as allograft or autograft being required if primary repair is no longer possible. Case reports and series have described treatment methods as well as anecdotal evidence of outcomes after operative repair or conservative treatment, but there have been no comprehensive systematic reviews of outcomes after various types of treatment. Authors have come to conclusions about expected outcomes based on patient age, time to treatment, treatment used, and other variables, but no reviews have examined these variables across multiple studies. Given the low level of the evidence presented in most of these reports, it is difficult to perform a meta-analysis of the data.
Instead, we systematically reviewed 87 cases from all pertinent studies and examined commonly reported data, such as patient age, time to treatment, treatment used, and outcome. Using the PICO (population, intervention, comparison, outcome) model for systematic reviews, we looked at patients who had closed, spontaneous, complete rupture of the TA tendon and underwent operative repair or conservative treatment of the injury. Outcomes surveyed included successful operative repair or conservative treatment, as measured by objective systems, such as MMSS (Manual Muscle Strength Scale) score, AOFAS (American Orthopaedic Foot and Ankle Society) hindfoot score, and FAOS (Foot and Ankle Outcome Score) testing, or by subjective description of posttreatment outcome.
We intend this review to serve as a guide for surgeons who find themselves treating a ruptured TA tendon, a relatively rare injury. They will be able to select the operative technique or conservative treatment that best matches the patient’s needs, based on comparison with previous case studies.
Materials and Methods
The cases reviewed for this study were found through a comprehensive PubMed search and an independent review of references cited in similar articles. Articles included were published between 1975 and 2012, inclusive. The latest search was performed on March 22, 2013. The search criteria were tibialis anterior [Title/Abstract] OR anterior tibial [Title/Abstract] AND rupture [Title/Abstract]) AND surgery. Only English-language articles, or articles already translated into English, were included. Eligible studies described cases of closed tendon rupture. No traumatic lacerations or open ruptures were included. If a study described both open and subcutaneous ruptures, only the subcutaneous cases were included. Further, partial ruptures were not included. In addition, ruptures caused directly by a known comorbid condition—for example, a rupture caused by a gouty tophaceous deposit at the site of rupture2—were not included. Data were extracted from publications independently and analyzed in a Microsoft Excel workbook (Microsoft, Redmond, Washington). Variables examined included patient age and sex, side involved, time to treatment, mechanism of injury, defect size, predisposing comorbidities, surgery or conservative treatment, type of operative repair (if applicable), graft used (if applicable), pretreatment function (by independent scoring system, if applicable), and posttreatment function. These variables were not necessarily reported in all the studies.
A potential bias exists in our PubMed search. As the query was specific for studies that included operative repair of a ruptured TA tendon, case studies that involved only conservative treatment were excluded. However, the primary goal of this review was to compare operative possibilities and the patient characteristics and outcomes associated with these surgeries.
Results
Figure 1 shows the criteria used to select eligible papers for review. Twenty-three papers matched the criteria.3-25 Data were independently extracted from these papers, as described in the Methods section. Again, not all variables were reported by all authors. Sammarco and colleagues21 reported time to treatment as a mean for 2 groups: 8 cases defined as “early” treatment (mean time to treatment, 0.625 months) and 11 defined as “late” treatment (mean time to treatment, 10.7 months). These mean times were therefore used independently for each case in calculating mean time to treatment for this systematic review.
Table 1 lists the demographics. There were 40 male and 25 female patients, and 22 cases in which sex was not specified. Mean age was 63.9 years (surgery group), 72.4 years (conservative treatment group), and 65.8 years (overall). Of the 87 patients, 72 underwent surgery, and 15 were treated with conservative measures.
Table 2 lists the operative techniques identified. Of the 72 surgeries, 23 were primary repairs, 12 were primary repairs of the anatomical insertion, and 18 involved use of autograft.
Time to treatment was available for 54 of the 87 cases (Table 3). Primary repair was most often performed in cases in which the injury was less than 3 months old, and autograft was most often used in cases in which the injury occurred more than 3 months before presentation.
Posttreatment outcome scores were available for 59 cases. Only 3 authors reported preoperative scores.5,21,24 None of the authors who used conservative treatment measures reported pretreatment scores. Scores used included the MMSS score (26 cases), the AOFAS hindfoot score (16 cases),26 the FAOS (17 cases),27 and the Tinetti gait and balance score (3 cases; the author also used the MMSS score).28Table 4 lists the mean posttreatment scores for patients who underwent surgery and patients treated conservatively. AOFAS, MMSS, and Tinetti scores and FAOS were used by authors presenting operative treatment outcomes. Only posttreatment FAOS was available for both surgery (84.4/100) and conservative treatment (69.4/100).
Discussion
Closed rupture of the TA tendon is a relatively rare entity occurring mostly in older patients without any history of acute, traumatic injury. Some patients, however, recall a particular moment of rupture, often accompanied immediately by pain and swelling, which eventually resolve. Later sequelae include footdrop with associated steppage gait and a palpable mass on the dorsal aspect of the ankle.3,21 Chronic TA tendon rupture can also lead to clawing of the toes as the other foot extensors (EHL, EDL) overcompensate. Cohen and Gordon1 described the case of a patient who ruptured a TA tendon 25 years earlier and then, in the absence of operative repair, developed hypertrophy of the EHL and the EDL. This extensor substitution led to hammer toes and plantar prominence of the metatarsal heads, ultimately leading to moderate pain and a neuroma. Although this particular outcome is likely rare, the more common sequelae of footdrop, flatfoot, Achilles tendon contracture, and compromised gait are reason enough to consider operative repair for any ruptured TA tendon.
Most previous studies of TA tendon rupture were case reports and case studies. In the largest series, Sammarco and colleagues21 described 19 cases of closed rupture. These included 3 traumatic cases, 1 by blunt trauma to the tendon and 2 of open laceration, all treated surgically with various methods. Unfortunately, these 3 traumatic cases were not separated in the authors’ analysis and therefore had to be included in this systematic review. Including them here did not compromise our goals in this review, which included examining typical patient demographics and the most common methods of operative repair.
Conservative measures remain a treatment possibility for some patients. We found that patients treated with conservative measures historically have been older (mean age, 72.4 years) than patients treated surgically (mean age, 63.9 years). However, advanced age itself is not a contraindication for operative repair of a TA tendon rupture, and authors have described positive outcomes for active, elderly (>70 years) patients who wanted to maintain their activity level and therefore opted for operative repair.7,8,10,13,16,24 Ouzounian and Anderson18 described functional limitations (eg, persistent footdrop, slapfoot gait, limitations in walking) after conservative treatment with an ankle-foot orthosis. Operative repair offers the chance for better functional outcome for patients who are surgical candidates and lead even a mildly active lifestyle.
Of operative repair methods, primary repair is used most often. This technique, however, must be allowed by the gap between the 2 ruptured ends after débridement of any necrotic tissue. If the distal stump is not viable, primary repair of the proximal stump to the native anatomical insertion is feasible. Figure 2, reprinted from a case report by Rajagopalan and colleagues,19 shows a ligament–osseous reattachment of the proximal stump using suture anchors to the medial cuneiform. Both primary repair and repair to the anatomical insertion can be augmented with Achilles tendon lengthening if needed to achieve balance between flexor and extensor functions of the ankle.
If the gap between the 2 stumps cannot be covered by the native tendon, then autograft, another surgical technique with positive outcomes, can be used. The most popular autograft sites historically have been the EDL, Achilles, and plantaris tendons. In addition, Goehring and Liakos9 described 3 cases of good results with semitendinosus autograft. Sapkas and colleagues22 used a free-sliding TA graft harvested from the healthy tissue of the proximal tendon stump. Their technique is depicted in Figure 3. Sliding tendon lengthening, well described by Trout and colleagues24 in a case study, is feasible for use of the native tendon when there is a gap to bridge between the 2 stumps of ruptured tendon. EHL or EDL transfer with or without Achilles lengthening is another option, albeit historically less often used.6,7 This technique is depicted in Figure 4, reprinted from a case series by Ellington and colleagues,7 who used EHL transfer with and without Achilles tendon lengthening in 9 cases.
Last, less popular techniques have included repair to sites other than the medial cuneiform, including the neck of the talus and the navicular bone.10,13 An Achilles tendon allograft was used in a case described by Aderinto and Gross3 to repair a ruptured tendon found incidentally on preoperative examination for a scheduled knee arthroplasty. The patient had a postoperative MMSS score of 4/5.
Overall, primary repair is clearly preferred, but successful outcomes can be achieved by other means. As Table 3 shows, primary repair is more often used for ruptures less than 3 months old, and autograft for older ruptures. Although which operative technique to use can be decided after necrotic tissue is débrided, surgeons should try to ascertain age of injury ahead of time so that, going into surgery, they will have a better idea of the feasibility of primary repair.
Posttreatment ankle scores were not widely available. As Table 4 indicates, only FAOS was used for the conservative treatment cases. However, raw mean FAOS and raw mean AOFAS hindfoot, MMSS, and Tinetti scores showed that good outcomes and high scores can be achieved with surgery. Further, the mean FAOS reported by Gwynne-Jones and colleagues10 and Markarian and colleagues13 showed a clinically significant difference between surgery and conservative treatment. DiDomenico and colleagues,5 Sammarco and colleagues,21 and Trout and colleagues24 were the only authors who reported pretreatment and posttreatment scores.
We intend this systematic review of the literature on closed TA rupture to serve as a guide for surgeons who find themselves treating this relatively rare injury, which often presents with only a chief complaint of the foot catching while walking. Overall, the literature shows that operative repair provides very good outcomes for many patients. Patients who are surgical candidates and amenable to surgery can be counseled that operative repair leads to fewer sequelae, such as persistent footdrop and flatfooted gait, with a strong likelihood of return to baseline activity status. Patients who are not surgical candidates or are strongly against surgery can be offered conservative treatment with an ankle-foot orthosis or physical therapy, but they should also be counseled that persistent gait abnormalities and weakness in dorsiflexion are likely outcomes. Surgeons must also consider age of injury (time from probable rupture to presentation), estimating a particular moment of rupture if unknown by the patient. They can then gauge the feasibility of primary repair and, during surgery, decide which technique (primary repair, tendon transfer, autograft, or other technique) will produce the best results. They can also use scores such as the FAOS and the AOFAS hindfoot, MMSS, and Tinetti scores to compare preoperative and postoperative function, though subjective reports of return to previous activity can also serve as markers of successful repair.
This review highlights the need for further study regarding the treatment of TA ruptures. Larger, randomized studies with validated scoring systems for preoperative and postoperative function would offer more insight onto the best treatment options for these complex injuries.
Subcutaneous rupture of the tibialis anterior (TA) tendon has been reported predominantly in case reports and small case series because of the relative rarity of the injury. Unlike traumatic lacerations or open injuries to the tendon, subcutaneous injuries often go unnoticed by patients because of compensation by surrounding dorsiflexors of the foot and toes—namely, the extensor hallucis longus (EHL) and the extensor digitorum longus (EDL).1 This can delay presentation to an orthopedic surgeon and lead to difficulties in treatment, such as allograft or autograft being required if primary repair is no longer possible. Case reports and series have described treatment methods as well as anecdotal evidence of outcomes after operative repair or conservative treatment, but there have been no comprehensive systematic reviews of outcomes after various types of treatment. Authors have come to conclusions about expected outcomes based on patient age, time to treatment, treatment used, and other variables, but no reviews have examined these variables across multiple studies. Given the low level of the evidence presented in most of these reports, it is difficult to perform a meta-analysis of the data.
Instead, we systematically reviewed 87 cases from all pertinent studies and examined commonly reported data, such as patient age, time to treatment, treatment used, and outcome. Using the PICO (population, intervention, comparison, outcome) model for systematic reviews, we looked at patients who had closed, spontaneous, complete rupture of the TA tendon and underwent operative repair or conservative treatment of the injury. Outcomes surveyed included successful operative repair or conservative treatment, as measured by objective systems, such as MMSS (Manual Muscle Strength Scale) score, AOFAS (American Orthopaedic Foot and Ankle Society) hindfoot score, and FAOS (Foot and Ankle Outcome Score) testing, or by subjective description of posttreatment outcome.
We intend this review to serve as a guide for surgeons who find themselves treating a ruptured TA tendon, a relatively rare injury. They will be able to select the operative technique or conservative treatment that best matches the patient’s needs, based on comparison with previous case studies.
Materials and Methods
The cases reviewed for this study were found through a comprehensive PubMed search and an independent review of references cited in similar articles. Articles included were published between 1975 and 2012, inclusive. The latest search was performed on March 22, 2013. The search criteria were tibialis anterior [Title/Abstract] OR anterior tibial [Title/Abstract] AND rupture [Title/Abstract]) AND surgery. Only English-language articles, or articles already translated into English, were included. Eligible studies described cases of closed tendon rupture. No traumatic lacerations or open ruptures were included. If a study described both open and subcutaneous ruptures, only the subcutaneous cases were included. Further, partial ruptures were not included. In addition, ruptures caused directly by a known comorbid condition—for example, a rupture caused by a gouty tophaceous deposit at the site of rupture2—were not included. Data were extracted from publications independently and analyzed in a Microsoft Excel workbook (Microsoft, Redmond, Washington). Variables examined included patient age and sex, side involved, time to treatment, mechanism of injury, defect size, predisposing comorbidities, surgery or conservative treatment, type of operative repair (if applicable), graft used (if applicable), pretreatment function (by independent scoring system, if applicable), and posttreatment function. These variables were not necessarily reported in all the studies.
A potential bias exists in our PubMed search. As the query was specific for studies that included operative repair of a ruptured TA tendon, case studies that involved only conservative treatment were excluded. However, the primary goal of this review was to compare operative possibilities and the patient characteristics and outcomes associated with these surgeries.
Results
Figure 1 shows the criteria used to select eligible papers for review. Twenty-three papers matched the criteria.3-25 Data were independently extracted from these papers, as described in the Methods section. Again, not all variables were reported by all authors. Sammarco and colleagues21 reported time to treatment as a mean for 2 groups: 8 cases defined as “early” treatment (mean time to treatment, 0.625 months) and 11 defined as “late” treatment (mean time to treatment, 10.7 months). These mean times were therefore used independently for each case in calculating mean time to treatment for this systematic review.
Table 1 lists the demographics. There were 40 male and 25 female patients, and 22 cases in which sex was not specified. Mean age was 63.9 years (surgery group), 72.4 years (conservative treatment group), and 65.8 years (overall). Of the 87 patients, 72 underwent surgery, and 15 were treated with conservative measures.
Table 2 lists the operative techniques identified. Of the 72 surgeries, 23 were primary repairs, 12 were primary repairs of the anatomical insertion, and 18 involved use of autograft.
Time to treatment was available for 54 of the 87 cases (Table 3). Primary repair was most often performed in cases in which the injury was less than 3 months old, and autograft was most often used in cases in which the injury occurred more than 3 months before presentation.
Posttreatment outcome scores were available for 59 cases. Only 3 authors reported preoperative scores.5,21,24 None of the authors who used conservative treatment measures reported pretreatment scores. Scores used included the MMSS score (26 cases), the AOFAS hindfoot score (16 cases),26 the FAOS (17 cases),27 and the Tinetti gait and balance score (3 cases; the author also used the MMSS score).28Table 4 lists the mean posttreatment scores for patients who underwent surgery and patients treated conservatively. AOFAS, MMSS, and Tinetti scores and FAOS were used by authors presenting operative treatment outcomes. Only posttreatment FAOS was available for both surgery (84.4/100) and conservative treatment (69.4/100).
Discussion
Closed rupture of the TA tendon is a relatively rare entity occurring mostly in older patients without any history of acute, traumatic injury. Some patients, however, recall a particular moment of rupture, often accompanied immediately by pain and swelling, which eventually resolve. Later sequelae include footdrop with associated steppage gait and a palpable mass on the dorsal aspect of the ankle.3,21 Chronic TA tendon rupture can also lead to clawing of the toes as the other foot extensors (EHL, EDL) overcompensate. Cohen and Gordon1 described the case of a patient who ruptured a TA tendon 25 years earlier and then, in the absence of operative repair, developed hypertrophy of the EHL and the EDL. This extensor substitution led to hammer toes and plantar prominence of the metatarsal heads, ultimately leading to moderate pain and a neuroma. Although this particular outcome is likely rare, the more common sequelae of footdrop, flatfoot, Achilles tendon contracture, and compromised gait are reason enough to consider operative repair for any ruptured TA tendon.
Most previous studies of TA tendon rupture were case reports and case studies. In the largest series, Sammarco and colleagues21 described 19 cases of closed rupture. These included 3 traumatic cases, 1 by blunt trauma to the tendon and 2 of open laceration, all treated surgically with various methods. Unfortunately, these 3 traumatic cases were not separated in the authors’ analysis and therefore had to be included in this systematic review. Including them here did not compromise our goals in this review, which included examining typical patient demographics and the most common methods of operative repair.
Conservative measures remain a treatment possibility for some patients. We found that patients treated with conservative measures historically have been older (mean age, 72.4 years) than patients treated surgically (mean age, 63.9 years). However, advanced age itself is not a contraindication for operative repair of a TA tendon rupture, and authors have described positive outcomes for active, elderly (>70 years) patients who wanted to maintain their activity level and therefore opted for operative repair.7,8,10,13,16,24 Ouzounian and Anderson18 described functional limitations (eg, persistent footdrop, slapfoot gait, limitations in walking) after conservative treatment with an ankle-foot orthosis. Operative repair offers the chance for better functional outcome for patients who are surgical candidates and lead even a mildly active lifestyle.
Of operative repair methods, primary repair is used most often. This technique, however, must be allowed by the gap between the 2 ruptured ends after débridement of any necrotic tissue. If the distal stump is not viable, primary repair of the proximal stump to the native anatomical insertion is feasible. Figure 2, reprinted from a case report by Rajagopalan and colleagues,19 shows a ligament–osseous reattachment of the proximal stump using suture anchors to the medial cuneiform. Both primary repair and repair to the anatomical insertion can be augmented with Achilles tendon lengthening if needed to achieve balance between flexor and extensor functions of the ankle.
If the gap between the 2 stumps cannot be covered by the native tendon, then autograft, another surgical technique with positive outcomes, can be used. The most popular autograft sites historically have been the EDL, Achilles, and plantaris tendons. In addition, Goehring and Liakos9 described 3 cases of good results with semitendinosus autograft. Sapkas and colleagues22 used a free-sliding TA graft harvested from the healthy tissue of the proximal tendon stump. Their technique is depicted in Figure 3. Sliding tendon lengthening, well described by Trout and colleagues24 in a case study, is feasible for use of the native tendon when there is a gap to bridge between the 2 stumps of ruptured tendon. EHL or EDL transfer with or without Achilles lengthening is another option, albeit historically less often used.6,7 This technique is depicted in Figure 4, reprinted from a case series by Ellington and colleagues,7 who used EHL transfer with and without Achilles tendon lengthening in 9 cases.
Last, less popular techniques have included repair to sites other than the medial cuneiform, including the neck of the talus and the navicular bone.10,13 An Achilles tendon allograft was used in a case described by Aderinto and Gross3 to repair a ruptured tendon found incidentally on preoperative examination for a scheduled knee arthroplasty. The patient had a postoperative MMSS score of 4/5.
Overall, primary repair is clearly preferred, but successful outcomes can be achieved by other means. As Table 3 shows, primary repair is more often used for ruptures less than 3 months old, and autograft for older ruptures. Although which operative technique to use can be decided after necrotic tissue is débrided, surgeons should try to ascertain age of injury ahead of time so that, going into surgery, they will have a better idea of the feasibility of primary repair.
Posttreatment ankle scores were not widely available. As Table 4 indicates, only FAOS was used for the conservative treatment cases. However, raw mean FAOS and raw mean AOFAS hindfoot, MMSS, and Tinetti scores showed that good outcomes and high scores can be achieved with surgery. Further, the mean FAOS reported by Gwynne-Jones and colleagues10 and Markarian and colleagues13 showed a clinically significant difference between surgery and conservative treatment. DiDomenico and colleagues,5 Sammarco and colleagues,21 and Trout and colleagues24 were the only authors who reported pretreatment and posttreatment scores.
We intend this systematic review of the literature on closed TA rupture to serve as a guide for surgeons who find themselves treating this relatively rare injury, which often presents with only a chief complaint of the foot catching while walking. Overall, the literature shows that operative repair provides very good outcomes for many patients. Patients who are surgical candidates and amenable to surgery can be counseled that operative repair leads to fewer sequelae, such as persistent footdrop and flatfooted gait, with a strong likelihood of return to baseline activity status. Patients who are not surgical candidates or are strongly against surgery can be offered conservative treatment with an ankle-foot orthosis or physical therapy, but they should also be counseled that persistent gait abnormalities and weakness in dorsiflexion are likely outcomes. Surgeons must also consider age of injury (time from probable rupture to presentation), estimating a particular moment of rupture if unknown by the patient. They can then gauge the feasibility of primary repair and, during surgery, decide which technique (primary repair, tendon transfer, autograft, or other technique) will produce the best results. They can also use scores such as the FAOS and the AOFAS hindfoot, MMSS, and Tinetti scores to compare preoperative and postoperative function, though subjective reports of return to previous activity can also serve as markers of successful repair.
This review highlights the need for further study regarding the treatment of TA ruptures. Larger, randomized studies with validated scoring systems for preoperative and postoperative function would offer more insight onto the best treatment options for these complex injuries.
1. Cohen DA, Gordon DH. The long-term effects of an untreated tibialis anterior tendon rupture. J Am Podiatr Med Assoc. 1999;89(3):149-152.
2. Jerome JTJ, Varghese M, Sankaran B, Thomas S, Thirumagal SK. Tibialis anterior tendon rupture in gout—case report and literature review. Foot Ankle Surg. 2008;14(3):166-169.
3. Aderinto J, Gross A. Delayed repair of tibialis anterior tendon rupture with Achilles tendon allograft. J Foot Ankle Surg. 2011;50(3):340-342.
4. Constantinou M, Wilson A. Traumatic tear of tibialis anterior during a Gaelic football game: a case report. Br J Sports Med. 2004;38(6):e30.
5. DiDomenico LA, Williams K, Petrolla AF. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Foot Ankle Surg. 2008;47(5):463-467.
6. Dooley BJ, Kudelka P, Menelaus MB. Subcutaneous rupture of the tendon of tibialis anterior. J Bone Joint Surg Br. 1980;62(4):471-472.
7. Ellington JK, McCormick J, Marion C, et al. Surgical outcome following tibialis anterior tendon repair. Foot Ankle Int. 2010;31(5):412-417.
8. ElMaraghy A, Devereaux MW. Bone tunnel fixation for repair of tibialis anterior tendon rupture. Foot Ankle Surg. 2010;16(2):e47-e50.
9. Goehring M, Liakos P. Long-term outcomes following anterior tibialis tendon reconstruction with hamstring autograft in a series of 3 cases. J Foot Ankle Surg. 2009;48(2):196-202.
10. Gwynne-Jones D, Garneti N, Wyatt M. Closed tibialis anterior tendon rupture: a case series. Foot Ankle Int. 2009;30(8):758-762.
11. Kashyap S, Prince R. Spontaneous rupture of the tibialis anterior tendon. A case report. Clin Orthop. 1987;(216):159-161.
12. Kausch T, Rütt J. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch Orthop Trauma Surg. 1998;117(4-5):290-293.
13. Markarian GG, Kelikian AS, Brage M, Trainor T, Dias L. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle Int. 1998;19(12):792-802.
14. Meyn MA Jr. Closed rupture of the anterior tibial tendon. A case report and review of the literature. Clin Orthop. 1975;(113):154-157.
15. Miller RR, Mahan KT. Closed rupture of the anterior tibial tendon. A case report. J Am Podiatr Med Assoc. 1998;88(8):394-399.
16. Neumayer F, Djembi YR, Gerin A, Masquelet AC. Closed rupture of the tibialis anterior tendon: a report of 2 cases. J Foot Ankle Surg. 2009;48(4):457-461.
17. Otte S, Klinger HM, Lorenz F, Haerer T. Operative treatment in case of a closed rupture of the anterior tibial tendon. Arch Orthop Trauma Surg. 2002;122(3):188-190.
18. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int. 1995;16(7):406-410.
19. Rajagopalan S, Sangar A, Upadhyay V, Lloyd J, Taylor H. Bilateral atraumatic sequential rupture of tibialis anterior tendons. Foot Ankle Spec. 2010;3(6):352-355.
20. Rimoldi RL, Oberlander MA, Waldrop JI, Hunter SC. Acute rupture of the tibialis anterior tendon: a case report. Foot Ankle. 1991;12(3):176-177.
21. Sammarco VJ, Sammarco GJ, Henning C, Chaim S. Surgical repair of acute and chronic tibialis anterior tendon ruptures. J Bone Joint Surg Am. 2009;91(2):325-332.
22. Sapkas GS, Tzoutzopoulos A, Tsoukas FC, Triantafillopoulos IK. Spontaneous tibialis anterior tendon rupture: delayed repair with free-sliding tibialis anterior tendon graft. Am J Orthop. 2008;37(12):E213-E216.
23. Stuart MJ. Traumatic disruption of the anterior tibial tendon while cross-country skiing. A case report. Clin Orthop. 1992;(281):193-194.
24. Trout BM, Hosey G, Wertheimer SJ. Rupture of the tibialis anterior tendon. J Foot Ankle Surg. 2000;39(1):54-58.
25. Van Acker G, Pingen F, Luitse J, Goslings C. Rupture of the tibialis anterior tendon. Acta Orthop Belg. 2006;72(1):105-107.
26. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353.
27. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794.
28. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429-434.
1. Cohen DA, Gordon DH. The long-term effects of an untreated tibialis anterior tendon rupture. J Am Podiatr Med Assoc. 1999;89(3):149-152.
2. Jerome JTJ, Varghese M, Sankaran B, Thomas S, Thirumagal SK. Tibialis anterior tendon rupture in gout—case report and literature review. Foot Ankle Surg. 2008;14(3):166-169.
3. Aderinto J, Gross A. Delayed repair of tibialis anterior tendon rupture with Achilles tendon allograft. J Foot Ankle Surg. 2011;50(3):340-342.
4. Constantinou M, Wilson A. Traumatic tear of tibialis anterior during a Gaelic football game: a case report. Br J Sports Med. 2004;38(6):e30.
5. DiDomenico LA, Williams K, Petrolla AF. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Foot Ankle Surg. 2008;47(5):463-467.
6. Dooley BJ, Kudelka P, Menelaus MB. Subcutaneous rupture of the tendon of tibialis anterior. J Bone Joint Surg Br. 1980;62(4):471-472.
7. Ellington JK, McCormick J, Marion C, et al. Surgical outcome following tibialis anterior tendon repair. Foot Ankle Int. 2010;31(5):412-417.
8. ElMaraghy A, Devereaux MW. Bone tunnel fixation for repair of tibialis anterior tendon rupture. Foot Ankle Surg. 2010;16(2):e47-e50.
9. Goehring M, Liakos P. Long-term outcomes following anterior tibialis tendon reconstruction with hamstring autograft in a series of 3 cases. J Foot Ankle Surg. 2009;48(2):196-202.
10. Gwynne-Jones D, Garneti N, Wyatt M. Closed tibialis anterior tendon rupture: a case series. Foot Ankle Int. 2009;30(8):758-762.
11. Kashyap S, Prince R. Spontaneous rupture of the tibialis anterior tendon. A case report. Clin Orthop. 1987;(216):159-161.
12. Kausch T, Rütt J. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch Orthop Trauma Surg. 1998;117(4-5):290-293.
13. Markarian GG, Kelikian AS, Brage M, Trainor T, Dias L. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle Int. 1998;19(12):792-802.
14. Meyn MA Jr. Closed rupture of the anterior tibial tendon. A case report and review of the literature. Clin Orthop. 1975;(113):154-157.
15. Miller RR, Mahan KT. Closed rupture of the anterior tibial tendon. A case report. J Am Podiatr Med Assoc. 1998;88(8):394-399.
16. Neumayer F, Djembi YR, Gerin A, Masquelet AC. Closed rupture of the tibialis anterior tendon: a report of 2 cases. J Foot Ankle Surg. 2009;48(4):457-461.
17. Otte S, Klinger HM, Lorenz F, Haerer T. Operative treatment in case of a closed rupture of the anterior tibial tendon. Arch Orthop Trauma Surg. 2002;122(3):188-190.
18. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int. 1995;16(7):406-410.
19. Rajagopalan S, Sangar A, Upadhyay V, Lloyd J, Taylor H. Bilateral atraumatic sequential rupture of tibialis anterior tendons. Foot Ankle Spec. 2010;3(6):352-355.
20. Rimoldi RL, Oberlander MA, Waldrop JI, Hunter SC. Acute rupture of the tibialis anterior tendon: a case report. Foot Ankle. 1991;12(3):176-177.
21. Sammarco VJ, Sammarco GJ, Henning C, Chaim S. Surgical repair of acute and chronic tibialis anterior tendon ruptures. J Bone Joint Surg Am. 2009;91(2):325-332.
22. Sapkas GS, Tzoutzopoulos A, Tsoukas FC, Triantafillopoulos IK. Spontaneous tibialis anterior tendon rupture: delayed repair with free-sliding tibialis anterior tendon graft. Am J Orthop. 2008;37(12):E213-E216.
23. Stuart MJ. Traumatic disruption of the anterior tibial tendon while cross-country skiing. A case report. Clin Orthop. 1992;(281):193-194.
24. Trout BM, Hosey G, Wertheimer SJ. Rupture of the tibialis anterior tendon. J Foot Ankle Surg. 2000;39(1):54-58.
25. Van Acker G, Pingen F, Luitse J, Goslings C. Rupture of the tibialis anterior tendon. Acta Orthop Belg. 2006;72(1):105-107.
26. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353.
27. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794.
28. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429-434.
Revision Anterior Cruciate Ligament Reconstruction With Bone–Patellar Tendon–Bone Allograft and Extra-Articular Iliotibial Band Tenodesis
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
Child Pedestrians More Likely to Be Struck By Motor Vehicles in the Spring Months, While Unsupervised, Near Schools and Bus Stops
LAS VEGAS—Most child pedestrian injuries involving a motor vehicle occurred while children were unsupervised, near schools and bus stops, and in the spring months during the afternoon and evening hours, according to research presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
Pedestrian injuries are among the leading causes of pediatric deaths in the United States. In 2012, 557 child and young adult pedestrians younger than 20 years were killed by motor vehicles in the US and 22,000 were injured, according to the National Highway Traffic Safety Administration. Nearly three-fourths (73%) of pedestrian fatalities occur in urban settings.
In this study, researchers reviewed electronic medical records of 100 child pedestrian emergency department visits at St. Christopher’s Hospital for Children in Philadelphia from January 1 to December 21, 2012, including ambulance dispatch data, patient demographics, procedure(s), diagnoses, and length of stay. First responder narratives provided accident scene descriptions, including the individuals who were present at the time of the accident and the type of intersection or property where the injuries occurred. Google Maps were used to identify the accident site, injury clusters, and specific street locations.
The patients included 79 boys and 21 girls with an average age of 8 years. Sixty-one percent of patients were evaluated in the emergency department only, or were admitted for less than 24 hours, while 39 patients were admitted for 24 hours or more with a mean length of stay of 1.98 days. Eleven patients were admitted to the Intensive Care Unit (ICU) for at least 1 day. Among the other findings:
• At the time of the trauma, 40% of the children were accompanied to the emergency department by a parent or guardian, 34% by friends or peers, 13% by older siblings, and 13% were alone.
• Most injuries occurred around the time of school dismissal and during evening hours: 29% of injuries occurred between 2 PM and 5 PM, and 42% between 5 PM and 9 PM.
• The greatest number of injuries occurred during the month of June (13%) followed by the other spring months.
• Of the 44 cases with enough accident scene information to perform a detailed analysis, 70% (31) of the children were injured mid-block, and 18% (8) at a crosswalk. Nearly 10% (5) were struck on private property, a sidewalk, or in a parking lot.
• Injury clusters were identified near schools and public bus stops used by students for transportation to and from school.
“Accidents most frequently occurred when no parental supervision was present from the time of school dismissal until the early evening hours, and were most often located mid-block,” said orthopedic surgery resident and lead study author Alexa J. Karkenny, MD. “Injuries peaked during the warm months and clustered both near schools and bus stops located near schools.
“Keeping these spatial, temporal, and behavioral predictors of pediatric orthopedic trauma in mind, we can help guide prevention strategies in urban settings,” said Dr. Karkenny. In the emergency department, “knowledge of the high-risk injuries in this subset of patients can help the trauma team to prioritize patient evaluations, which is especially important in complicated cases involving multiple injuries.”
Injury prevention efforts should focus on improved supervision at school dismissal and public transportation safety near school zones, the study authors concluded.
LAS VEGAS—Most child pedestrian injuries involving a motor vehicle occurred while children were unsupervised, near schools and bus stops, and in the spring months during the afternoon and evening hours, according to research presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
Pedestrian injuries are among the leading causes of pediatric deaths in the United States. In 2012, 557 child and young adult pedestrians younger than 20 years were killed by motor vehicles in the US and 22,000 were injured, according to the National Highway Traffic Safety Administration. Nearly three-fourths (73%) of pedestrian fatalities occur in urban settings.
In this study, researchers reviewed electronic medical records of 100 child pedestrian emergency department visits at St. Christopher’s Hospital for Children in Philadelphia from January 1 to December 21, 2012, including ambulance dispatch data, patient demographics, procedure(s), diagnoses, and length of stay. First responder narratives provided accident scene descriptions, including the individuals who were present at the time of the accident and the type of intersection or property where the injuries occurred. Google Maps were used to identify the accident site, injury clusters, and specific street locations.
The patients included 79 boys and 21 girls with an average age of 8 years. Sixty-one percent of patients were evaluated in the emergency department only, or were admitted for less than 24 hours, while 39 patients were admitted for 24 hours or more with a mean length of stay of 1.98 days. Eleven patients were admitted to the Intensive Care Unit (ICU) for at least 1 day. Among the other findings:
• At the time of the trauma, 40% of the children were accompanied to the emergency department by a parent or guardian, 34% by friends or peers, 13% by older siblings, and 13% were alone.
• Most injuries occurred around the time of school dismissal and during evening hours: 29% of injuries occurred between 2 PM and 5 PM, and 42% between 5 PM and 9 PM.
• The greatest number of injuries occurred during the month of June (13%) followed by the other spring months.
• Of the 44 cases with enough accident scene information to perform a detailed analysis, 70% (31) of the children were injured mid-block, and 18% (8) at a crosswalk. Nearly 10% (5) were struck on private property, a sidewalk, or in a parking lot.
• Injury clusters were identified near schools and public bus stops used by students for transportation to and from school.
“Accidents most frequently occurred when no parental supervision was present from the time of school dismissal until the early evening hours, and were most often located mid-block,” said orthopedic surgery resident and lead study author Alexa J. Karkenny, MD. “Injuries peaked during the warm months and clustered both near schools and bus stops located near schools.
“Keeping these spatial, temporal, and behavioral predictors of pediatric orthopedic trauma in mind, we can help guide prevention strategies in urban settings,” said Dr. Karkenny. In the emergency department, “knowledge of the high-risk injuries in this subset of patients can help the trauma team to prioritize patient evaluations, which is especially important in complicated cases involving multiple injuries.”
Injury prevention efforts should focus on improved supervision at school dismissal and public transportation safety near school zones, the study authors concluded.
LAS VEGAS—Most child pedestrian injuries involving a motor vehicle occurred while children were unsupervised, near schools and bus stops, and in the spring months during the afternoon and evening hours, according to research presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
Pedestrian injuries are among the leading causes of pediatric deaths in the United States. In 2012, 557 child and young adult pedestrians younger than 20 years were killed by motor vehicles in the US and 22,000 were injured, according to the National Highway Traffic Safety Administration. Nearly three-fourths (73%) of pedestrian fatalities occur in urban settings.
In this study, researchers reviewed electronic medical records of 100 child pedestrian emergency department visits at St. Christopher’s Hospital for Children in Philadelphia from January 1 to December 21, 2012, including ambulance dispatch data, patient demographics, procedure(s), diagnoses, and length of stay. First responder narratives provided accident scene descriptions, including the individuals who were present at the time of the accident and the type of intersection or property where the injuries occurred. Google Maps were used to identify the accident site, injury clusters, and specific street locations.
The patients included 79 boys and 21 girls with an average age of 8 years. Sixty-one percent of patients were evaluated in the emergency department only, or were admitted for less than 24 hours, while 39 patients were admitted for 24 hours or more with a mean length of stay of 1.98 days. Eleven patients were admitted to the Intensive Care Unit (ICU) for at least 1 day. Among the other findings:
• At the time of the trauma, 40% of the children were accompanied to the emergency department by a parent or guardian, 34% by friends or peers, 13% by older siblings, and 13% were alone.
• Most injuries occurred around the time of school dismissal and during evening hours: 29% of injuries occurred between 2 PM and 5 PM, and 42% between 5 PM and 9 PM.
• The greatest number of injuries occurred during the month of June (13%) followed by the other spring months.
• Of the 44 cases with enough accident scene information to perform a detailed analysis, 70% (31) of the children were injured mid-block, and 18% (8) at a crosswalk. Nearly 10% (5) were struck on private property, a sidewalk, or in a parking lot.
• Injury clusters were identified near schools and public bus stops used by students for transportation to and from school.
“Accidents most frequently occurred when no parental supervision was present from the time of school dismissal until the early evening hours, and were most often located mid-block,” said orthopedic surgery resident and lead study author Alexa J. Karkenny, MD. “Injuries peaked during the warm months and clustered both near schools and bus stops located near schools.
“Keeping these spatial, temporal, and behavioral predictors of pediatric orthopedic trauma in mind, we can help guide prevention strategies in urban settings,” said Dr. Karkenny. In the emergency department, “knowledge of the high-risk injuries in this subset of patients can help the trauma team to prioritize patient evaluations, which is especially important in complicated cases involving multiple injuries.”
Injury prevention efforts should focus on improved supervision at school dismissal and public transportation safety near school zones, the study authors concluded.
More Than One-Third of Division I College Athletes May Have Low Vitamin D Levels
LAS VEGAS—A new study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) found that more than one-third of elite, Division I college athletes may have low levels of vitamin D, which aids the absorption of calcium. Male, black, and Hispanic athletes are at greatest risk, researchers reported.
“Although multiple studies have demonstrated a high prevalence of vitamin D insufficiency across various populations, there is a paucity of data regarding elite level athletes,” said orthopedic surgeon and lead study author Diego Villacis, MD, Administrative Chief Resident at the University of Southern California. “Recent studies also have demonstrated that vitamin D levels have a direct relationship with muscle power, force, velocity, and optimal bone mass.”
In this study, which appeared in the February 2014 online issue of Sports Health, researchers measured the serum 25-hydroxyvitamin D (serum 25) levels of 223 athletes (121 men and 102 women) between June 2012 and August 2012. The mean serum 25 level for the athletes, enrolled in a broad range of indoor and outdoor sports, was 40.1 ±14.9 ng/mL (≥32 ng/mL is considered normal; 20 to <32 ng/mL, insufficient; and <20 ng/mL, deficient). Overall, 66.4% of participants had sufficient vitamin D levels and 33.6% had insufficient or deficient levels.
Men were 2.8 times more likely to have an abnormal vitamin D level, according to the results, and athletes with darker skin tones also faced a “much higher risk” for insufficient vitamin D. Black athletes were 19.1 times more likely to have abnormal vitamin D levels compared to white athletes, and Hispanics, 6.1 times more likely.
“Our study demonstrated abnormal vitamin D levels in nearly one out of three elite NCAA Division I athletes tested,” said Dr. Villacis. “Although there is much more work to be done, our results open the possibility for improved performance and most importantly decreased risk of injury with correction of vitamin D levels. This may potentially be achieved simply and safely through modification of diet, sunlight exposure, and vitamin D supplementation.”
LAS VEGAS—A new study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) found that more than one-third of elite, Division I college athletes may have low levels of vitamin D, which aids the absorption of calcium. Male, black, and Hispanic athletes are at greatest risk, researchers reported.
“Although multiple studies have demonstrated a high prevalence of vitamin D insufficiency across various populations, there is a paucity of data regarding elite level athletes,” said orthopedic surgeon and lead study author Diego Villacis, MD, Administrative Chief Resident at the University of Southern California. “Recent studies also have demonstrated that vitamin D levels have a direct relationship with muscle power, force, velocity, and optimal bone mass.”
In this study, which appeared in the February 2014 online issue of Sports Health, researchers measured the serum 25-hydroxyvitamin D (serum 25) levels of 223 athletes (121 men and 102 women) between June 2012 and August 2012. The mean serum 25 level for the athletes, enrolled in a broad range of indoor and outdoor sports, was 40.1 ±14.9 ng/mL (≥32 ng/mL is considered normal; 20 to <32 ng/mL, insufficient; and <20 ng/mL, deficient). Overall, 66.4% of participants had sufficient vitamin D levels and 33.6% had insufficient or deficient levels.
Men were 2.8 times more likely to have an abnormal vitamin D level, according to the results, and athletes with darker skin tones also faced a “much higher risk” for insufficient vitamin D. Black athletes were 19.1 times more likely to have abnormal vitamin D levels compared to white athletes, and Hispanics, 6.1 times more likely.
“Our study demonstrated abnormal vitamin D levels in nearly one out of three elite NCAA Division I athletes tested,” said Dr. Villacis. “Although there is much more work to be done, our results open the possibility for improved performance and most importantly decreased risk of injury with correction of vitamin D levels. This may potentially be achieved simply and safely through modification of diet, sunlight exposure, and vitamin D supplementation.”
LAS VEGAS—A new study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) found that more than one-third of elite, Division I college athletes may have low levels of vitamin D, which aids the absorption of calcium. Male, black, and Hispanic athletes are at greatest risk, researchers reported.
“Although multiple studies have demonstrated a high prevalence of vitamin D insufficiency across various populations, there is a paucity of data regarding elite level athletes,” said orthopedic surgeon and lead study author Diego Villacis, MD, Administrative Chief Resident at the University of Southern California. “Recent studies also have demonstrated that vitamin D levels have a direct relationship with muscle power, force, velocity, and optimal bone mass.”
In this study, which appeared in the February 2014 online issue of Sports Health, researchers measured the serum 25-hydroxyvitamin D (serum 25) levels of 223 athletes (121 men and 102 women) between June 2012 and August 2012. The mean serum 25 level for the athletes, enrolled in a broad range of indoor and outdoor sports, was 40.1 ±14.9 ng/mL (≥32 ng/mL is considered normal; 20 to <32 ng/mL, insufficient; and <20 ng/mL, deficient). Overall, 66.4% of participants had sufficient vitamin D levels and 33.6% had insufficient or deficient levels.
Men were 2.8 times more likely to have an abnormal vitamin D level, according to the results, and athletes with darker skin tones also faced a “much higher risk” for insufficient vitamin D. Black athletes were 19.1 times more likely to have abnormal vitamin D levels compared to white athletes, and Hispanics, 6.1 times more likely.
“Our study demonstrated abnormal vitamin D levels in nearly one out of three elite NCAA Division I athletes tested,” said Dr. Villacis. “Although there is much more work to be done, our results open the possibility for improved performance and most importantly decreased risk of injury with correction of vitamin D levels. This may potentially be achieved simply and safely through modification of diet, sunlight exposure, and vitamin D supplementation.”
Women Fare Better Than Men Following Total Knee, Hip Replacement
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
Black, Hispanic Patients More Likely to Be Readmitted to the Hospital Within 30 Days Following Hip or Knee Replacement Surgery
LAS VEGAS—Black and Hispanic patients were 62% and 50%, respectively, more likely to be readmitted to the hospital within 30 days after total joint replacement (TJR) surgery compared to white patients, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). In addition, Medicaid patients were 40% more likely to be readmitted to the hospital than patients with private insurance. Poorer outcomes, due in part to patient comorbidities, may reflect limited access to primary care, insufficient patient-doctor communication, researchers suggest.
Disparities in the provision of health care services have long been documented, including that black patients utilize hip and total knee replacement at rates nearly 40% less than white patients, despite having comparable or higher rates of osteoarthritis.
In this study, researchers analyzed 5 years of data—demographic (including race/ethnicity), clinical, and billing—on nearly 53,000 patients admitted to Connecticut hospitals for TJR from 2008 to 2012. The average patient age was 67 years, and the vast majority of patients were white (87%), covered by Medicare (56.7%), and female (61%).
The overall 30-day readmission rate for patients was 5.2%. The most common reasons for readmission were postoperative infection (8%), infection and inflammatory reaction due to internal joint prosthesis (6%), hematoma complications during a procedure (3%), and dislocation of a prosthetic joint (3%). Among the other study findings:
• Readmission rates were 83.5 per thousand for black patients, 78.9 for Hispanic patients, and 53.3 for white patients.
• Longer length of hospital stay was significantly associated with increased odds of readmission.
• When controlling for comorbidities and type of insurance coverage, the readmission rate for Hispanic patients dropped 44%, and for black patients, 38%. Black patients remained significantly more likely than white patients to be readmitted following surgery, after controlling for comorbidities.
• Patients covered by Medicare were 30% more likely to be readmitted within 30 days following discharge compared to patients covered by private insurance, and Medicaid patients were 40% more likely.
Recent research using national data on Medicare suggests that community-based factors, such as availability of general practitioners in the area, may be as or more important than hospital factors in determining readmission rates, and that patients may have few options other than hospital care for both urgent and non-urgent conditions related to their surgery or other conditions.
“Using an all-payer database, our study shows that black patients who undergo total knee replacement may have poorer outcomes,” said lead study author and orthopedic surgeon Courtland Lewis, MD. “After controlling for two key variables implicated in race and ethnic disparities in hospital readmission—preoperative comorbidities and type of insurance coverage—black patients still have a 35% higher likelihood of all-cause, 30-day readmission compared to white patients.
“Our ongoing research in this area is focused on other factors, such as the patient’s connection to primary care and patient-provider communication, that may explain this troubling finding,” said Dr. Lewis.
LAS VEGAS—Black and Hispanic patients were 62% and 50%, respectively, more likely to be readmitted to the hospital within 30 days after total joint replacement (TJR) surgery compared to white patients, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). In addition, Medicaid patients were 40% more likely to be readmitted to the hospital than patients with private insurance. Poorer outcomes, due in part to patient comorbidities, may reflect limited access to primary care, insufficient patient-doctor communication, researchers suggest.
Disparities in the provision of health care services have long been documented, including that black patients utilize hip and total knee replacement at rates nearly 40% less than white patients, despite having comparable or higher rates of osteoarthritis.
In this study, researchers analyzed 5 years of data—demographic (including race/ethnicity), clinical, and billing—on nearly 53,000 patients admitted to Connecticut hospitals for TJR from 2008 to 2012. The average patient age was 67 years, and the vast majority of patients were white (87%), covered by Medicare (56.7%), and female (61%).
The overall 30-day readmission rate for patients was 5.2%. The most common reasons for readmission were postoperative infection (8%), infection and inflammatory reaction due to internal joint prosthesis (6%), hematoma complications during a procedure (3%), and dislocation of a prosthetic joint (3%). Among the other study findings:
• Readmission rates were 83.5 per thousand for black patients, 78.9 for Hispanic patients, and 53.3 for white patients.
• Longer length of hospital stay was significantly associated with increased odds of readmission.
• When controlling for comorbidities and type of insurance coverage, the readmission rate for Hispanic patients dropped 44%, and for black patients, 38%. Black patients remained significantly more likely than white patients to be readmitted following surgery, after controlling for comorbidities.
• Patients covered by Medicare were 30% more likely to be readmitted within 30 days following discharge compared to patients covered by private insurance, and Medicaid patients were 40% more likely.
Recent research using national data on Medicare suggests that community-based factors, such as availability of general practitioners in the area, may be as or more important than hospital factors in determining readmission rates, and that patients may have few options other than hospital care for both urgent and non-urgent conditions related to their surgery or other conditions.
“Using an all-payer database, our study shows that black patients who undergo total knee replacement may have poorer outcomes,” said lead study author and orthopedic surgeon Courtland Lewis, MD. “After controlling for two key variables implicated in race and ethnic disparities in hospital readmission—preoperative comorbidities and type of insurance coverage—black patients still have a 35% higher likelihood of all-cause, 30-day readmission compared to white patients.
“Our ongoing research in this area is focused on other factors, such as the patient’s connection to primary care and patient-provider communication, that may explain this troubling finding,” said Dr. Lewis.
LAS VEGAS—Black and Hispanic patients were 62% and 50%, respectively, more likely to be readmitted to the hospital within 30 days after total joint replacement (TJR) surgery compared to white patients, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). In addition, Medicaid patients were 40% more likely to be readmitted to the hospital than patients with private insurance. Poorer outcomes, due in part to patient comorbidities, may reflect limited access to primary care, insufficient patient-doctor communication, researchers suggest.
Disparities in the provision of health care services have long been documented, including that black patients utilize hip and total knee replacement at rates nearly 40% less than white patients, despite having comparable or higher rates of osteoarthritis.
In this study, researchers analyzed 5 years of data—demographic (including race/ethnicity), clinical, and billing—on nearly 53,000 patients admitted to Connecticut hospitals for TJR from 2008 to 2012. The average patient age was 67 years, and the vast majority of patients were white (87%), covered by Medicare (56.7%), and female (61%).
The overall 30-day readmission rate for patients was 5.2%. The most common reasons for readmission were postoperative infection (8%), infection and inflammatory reaction due to internal joint prosthesis (6%), hematoma complications during a procedure (3%), and dislocation of a prosthetic joint (3%). Among the other study findings:
• Readmission rates were 83.5 per thousand for black patients, 78.9 for Hispanic patients, and 53.3 for white patients.
• Longer length of hospital stay was significantly associated with increased odds of readmission.
• When controlling for comorbidities and type of insurance coverage, the readmission rate for Hispanic patients dropped 44%, and for black patients, 38%. Black patients remained significantly more likely than white patients to be readmitted following surgery, after controlling for comorbidities.
• Patients covered by Medicare were 30% more likely to be readmitted within 30 days following discharge compared to patients covered by private insurance, and Medicaid patients were 40% more likely.
Recent research using national data on Medicare suggests that community-based factors, such as availability of general practitioners in the area, may be as or more important than hospital factors in determining readmission rates, and that patients may have few options other than hospital care for both urgent and non-urgent conditions related to their surgery or other conditions.
“Using an all-payer database, our study shows that black patients who undergo total knee replacement may have poorer outcomes,” said lead study author and orthopedic surgeon Courtland Lewis, MD. “After controlling for two key variables implicated in race and ethnic disparities in hospital readmission—preoperative comorbidities and type of insurance coverage—black patients still have a 35% higher likelihood of all-cause, 30-day readmission compared to white patients.
“Our ongoing research in this area is focused on other factors, such as the patient’s connection to primary care and patient-provider communication, that may explain this troubling finding,” said Dr. Lewis.
Stem Cells May Significantly Improve Tendon Healing, Reduce Retear Risk in Rotator Cuff Surgery
LAS VEGAS—An injection of a patient’s bone marrow stem cells during rotator cuff surgery significantly improved healing and tendon durability, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
The French study, of which a portion appeared in the September 2014 issue of International Orthopaedics, included 90 patients who underwent rotator cuff surgery. Forty-five of the patients received injections of bone marrow concentrate (BMC) mesenchymal stem cells (MSCs) at the surgical site, and 45 had their rotator cuff repaired or reattached without MSCs. Researchers tried to make the 2 groups as equivalent as possible based on rotator cuff tear size, tendon rupture location, dominate shoulder, gender, and age.
Patient ultrasound images were obtained each month following surgery for 24 months. In addition, magnetic resonance imaging was obtained of patient shoulders at 3 and 6 months following surgery, and at 1 year, 2 years, and 10 years following surgery.
At 6 months, all 45 of the patients who received MSCs had healed rotator cuff tendons, compared to 30 (67%) of the patients who did not receive MSCs. The use of BMC also prevented further ruptures or retears. At 10 years after surgery, intact rotator cuffs were found in 39 (87%) of the MSC patients, but just 20 (44%) of the non-MSC patients.
In addition, “some retears or new tears occurred after 1 year,” said lead study author Philippe Hernigou, MD, an orthopedic surgeon at the University of Paris. “These retears were more frequently associated with the control group patients who were not treated with MSCs.
“While the risk of a retear after arthroscopic repair of the rotator cuff has been well documented, publications with long-term follow-up (more than 3 years) are relatively limited,” said Dr. Hernigou. “Many patients undergoing rotator cuff repair surgery show advanced degeneration of the tendons, which are thinner and atrophic, probably explaining why negative results are so often reported in the literature, with frequent post-operative complications, especially retear. Observations in the MSC treatment group support the potential that MSC treatment has both a short-term and long-term benefit in reducing the rate of tendon retear.”
LAS VEGAS—An injection of a patient’s bone marrow stem cells during rotator cuff surgery significantly improved healing and tendon durability, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
The French study, of which a portion appeared in the September 2014 issue of International Orthopaedics, included 90 patients who underwent rotator cuff surgery. Forty-five of the patients received injections of bone marrow concentrate (BMC) mesenchymal stem cells (MSCs) at the surgical site, and 45 had their rotator cuff repaired or reattached without MSCs. Researchers tried to make the 2 groups as equivalent as possible based on rotator cuff tear size, tendon rupture location, dominate shoulder, gender, and age.
Patient ultrasound images were obtained each month following surgery for 24 months. In addition, magnetic resonance imaging was obtained of patient shoulders at 3 and 6 months following surgery, and at 1 year, 2 years, and 10 years following surgery.
At 6 months, all 45 of the patients who received MSCs had healed rotator cuff tendons, compared to 30 (67%) of the patients who did not receive MSCs. The use of BMC also prevented further ruptures or retears. At 10 years after surgery, intact rotator cuffs were found in 39 (87%) of the MSC patients, but just 20 (44%) of the non-MSC patients.
In addition, “some retears or new tears occurred after 1 year,” said lead study author Philippe Hernigou, MD, an orthopedic surgeon at the University of Paris. “These retears were more frequently associated with the control group patients who were not treated with MSCs.
“While the risk of a retear after arthroscopic repair of the rotator cuff has been well documented, publications with long-term follow-up (more than 3 years) are relatively limited,” said Dr. Hernigou. “Many patients undergoing rotator cuff repair surgery show advanced degeneration of the tendons, which are thinner and atrophic, probably explaining why negative results are so often reported in the literature, with frequent post-operative complications, especially retear. Observations in the MSC treatment group support the potential that MSC treatment has both a short-term and long-term benefit in reducing the rate of tendon retear.”
LAS VEGAS—An injection of a patient’s bone marrow stem cells during rotator cuff surgery significantly improved healing and tendon durability, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
The French study, of which a portion appeared in the September 2014 issue of International Orthopaedics, included 90 patients who underwent rotator cuff surgery. Forty-five of the patients received injections of bone marrow concentrate (BMC) mesenchymal stem cells (MSCs) at the surgical site, and 45 had their rotator cuff repaired or reattached without MSCs. Researchers tried to make the 2 groups as equivalent as possible based on rotator cuff tear size, tendon rupture location, dominate shoulder, gender, and age.
Patient ultrasound images were obtained each month following surgery for 24 months. In addition, magnetic resonance imaging was obtained of patient shoulders at 3 and 6 months following surgery, and at 1 year, 2 years, and 10 years following surgery.
At 6 months, all 45 of the patients who received MSCs had healed rotator cuff tendons, compared to 30 (67%) of the patients who did not receive MSCs. The use of BMC also prevented further ruptures or retears. At 10 years after surgery, intact rotator cuffs were found in 39 (87%) of the MSC patients, but just 20 (44%) of the non-MSC patients.
In addition, “some retears or new tears occurred after 1 year,” said lead study author Philippe Hernigou, MD, an orthopedic surgeon at the University of Paris. “These retears were more frequently associated with the control group patients who were not treated with MSCs.
“While the risk of a retear after arthroscopic repair of the rotator cuff has been well documented, publications with long-term follow-up (more than 3 years) are relatively limited,” said Dr. Hernigou. “Many patients undergoing rotator cuff repair surgery show advanced degeneration of the tendons, which are thinner and atrophic, probably explaining why negative results are so often reported in the literature, with frequent post-operative complications, especially retear. Observations in the MSC treatment group support the potential that MSC treatment has both a short-term and long-term benefit in reducing the rate of tendon retear.”
Hip Replacements in Middle-Age Nearly Double From 2002-2011, Outpacing Growth in Elderly Population
LAS VEGAS—The number of total hip replacements (THRs) nearly doubled among middle-age patients between 2002 and 2011, primarily due to the expansion of the middle-age population in the United States, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). Continued growth in utilization of hip replacement surgery in patients ages 45 to 64 years, an increase in revision surgeries for this population as they age, and a nearly 30% decline in the number of surgeons who perform THR could have significant implications for future health care costs, THR demand, and access, researchers said.
The researchers used the Nationwide Inpatient Sample (NIS) to identify primary THRs performed between 2002 and 2011 in patients ages 45 to 64 years, as well as related hospital charges. Population data and projections were obtained from the US Census Bureau and surgeon workforce estimates from the AAOS.
In 2011, 42.3% of THRs were performed in patients ages 45 to 64 years compared to 33.9% in 2002. Utilization of THR in this age group increased 89.2% from 2002 to 2011, from approximately 68,000 THRs in 2002 to 128,000 THRs in 2011. The overall population increased 21.3%. In addition, the authors found that:
• Growth of THR utilization in the 45- to 64-year-old age group grew 2.4 times faster than it did in the Medicare-aged population (age > 65 years).
• A rise in the prevalence of obesity, a known risk factor for hip osteoarthritis, among middle-age Americans was not significantly associated with increased THR utilization.
• Mean hospital charges in the THR 45- to 64-year-old age group declined 5.7% from 2002 to 2011, and declined 2.5% in the Medicare population (age > 65 years).
• Mean physician reimbursement per THR, in 2011 US dollars, declined 26.2% over the same period.
• Concurrently, the number of physicians reporting that they performed THR surgeries declined 28.2%.
“The purpose of this study was to identify potential drivers of THR utilization in the middle-age patient segment,” said lead study author Alexander S. McLawhorn, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York City. “Our multivariable statistical model suggested that the observed growth was best explained by an expansion of the middle-age population in the US. This particular age group is projected to continue expanding, and as such the demand for THR in this active group of patients will likely continue to rise as well. Our results underscore concerns about consumption of premium-priced implants in younger patients and the future revision burden this trend implies in the face of a dwindling number of physicians who specialize in hip arthroplasty surgery.”
LAS VEGAS—The number of total hip replacements (THRs) nearly doubled among middle-age patients between 2002 and 2011, primarily due to the expansion of the middle-age population in the United States, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). Continued growth in utilization of hip replacement surgery in patients ages 45 to 64 years, an increase in revision surgeries for this population as they age, and a nearly 30% decline in the number of surgeons who perform THR could have significant implications for future health care costs, THR demand, and access, researchers said.
The researchers used the Nationwide Inpatient Sample (NIS) to identify primary THRs performed between 2002 and 2011 in patients ages 45 to 64 years, as well as related hospital charges. Population data and projections were obtained from the US Census Bureau and surgeon workforce estimates from the AAOS.
In 2011, 42.3% of THRs were performed in patients ages 45 to 64 years compared to 33.9% in 2002. Utilization of THR in this age group increased 89.2% from 2002 to 2011, from approximately 68,000 THRs in 2002 to 128,000 THRs in 2011. The overall population increased 21.3%. In addition, the authors found that:
• Growth of THR utilization in the 45- to 64-year-old age group grew 2.4 times faster than it did in the Medicare-aged population (age > 65 years).
• A rise in the prevalence of obesity, a known risk factor for hip osteoarthritis, among middle-age Americans was not significantly associated with increased THR utilization.
• Mean hospital charges in the THR 45- to 64-year-old age group declined 5.7% from 2002 to 2011, and declined 2.5% in the Medicare population (age > 65 years).
• Mean physician reimbursement per THR, in 2011 US dollars, declined 26.2% over the same period.
• Concurrently, the number of physicians reporting that they performed THR surgeries declined 28.2%.
“The purpose of this study was to identify potential drivers of THR utilization in the middle-age patient segment,” said lead study author Alexander S. McLawhorn, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York City. “Our multivariable statistical model suggested that the observed growth was best explained by an expansion of the middle-age population in the US. This particular age group is projected to continue expanding, and as such the demand for THR in this active group of patients will likely continue to rise as well. Our results underscore concerns about consumption of premium-priced implants in younger patients and the future revision burden this trend implies in the face of a dwindling number of physicians who specialize in hip arthroplasty surgery.”
LAS VEGAS—The number of total hip replacements (THRs) nearly doubled among middle-age patients between 2002 and 2011, primarily due to the expansion of the middle-age population in the United States, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). Continued growth in utilization of hip replacement surgery in patients ages 45 to 64 years, an increase in revision surgeries for this population as they age, and a nearly 30% decline in the number of surgeons who perform THR could have significant implications for future health care costs, THR demand, and access, researchers said.
The researchers used the Nationwide Inpatient Sample (NIS) to identify primary THRs performed between 2002 and 2011 in patients ages 45 to 64 years, as well as related hospital charges. Population data and projections were obtained from the US Census Bureau and surgeon workforce estimates from the AAOS.
In 2011, 42.3% of THRs were performed in patients ages 45 to 64 years compared to 33.9% in 2002. Utilization of THR in this age group increased 89.2% from 2002 to 2011, from approximately 68,000 THRs in 2002 to 128,000 THRs in 2011. The overall population increased 21.3%. In addition, the authors found that:
• Growth of THR utilization in the 45- to 64-year-old age group grew 2.4 times faster than it did in the Medicare-aged population (age > 65 years).
• A rise in the prevalence of obesity, a known risk factor for hip osteoarthritis, among middle-age Americans was not significantly associated with increased THR utilization.
• Mean hospital charges in the THR 45- to 64-year-old age group declined 5.7% from 2002 to 2011, and declined 2.5% in the Medicare population (age > 65 years).
• Mean physician reimbursement per THR, in 2011 US dollars, declined 26.2% over the same period.
• Concurrently, the number of physicians reporting that they performed THR surgeries declined 28.2%.
“The purpose of this study was to identify potential drivers of THR utilization in the middle-age patient segment,” said lead study author Alexander S. McLawhorn, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York City. “Our multivariable statistical model suggested that the observed growth was best explained by an expansion of the middle-age population in the US. This particular age group is projected to continue expanding, and as such the demand for THR in this active group of patients will likely continue to rise as well. Our results underscore concerns about consumption of premium-priced implants in younger patients and the future revision burden this trend implies in the face of a dwindling number of physicians who specialize in hip arthroplasty surgery.”
Study Identifies Low Back Pain Risk Factors
LAS VEGAS—Nicotine dependence, obesity, alcohol abuse, and depressive disorders are risk factors for low back pain, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). Monitoring and counseling at-risk patients may prevent and minimize pain and improve quality of life, researchers said.
According to the U.S. Centers for Disease Control and Prevention’s (CDC) 2012 National Health Survey, nearly one-third of American adults reported that they had experienced low back pain during the previous 3 months. Determining modifiable risk factors for low back pain could help avoid or diminish the financial and emotional costs of this condition.
Researchers reviewed electronic records of more than 26 million patients from 13 health care systems across the United States, including 1.2 million patients diagnosed with low back pain (approximately 4.54% of the patient records).
The review found that 19.3% of the patients diagnosed with a depressive disorder reported lower back pain, as did 16.75% of patients diagnosed as obese (BMI > 30kg/m²), 16.53% of the patients diagnosed with nicotine dependence, and 14.66% with reported alcohol abuse. Patients with nicotine dependence, obesity, depressive disorders, and alcohol abuse had statistically significant relative risks of 4.489, 6.007, 5.511, and 3.326 for low back pain, respectively, when compared to other patients.
“This study used an electronic health care database to identify modifiable risk factors—obesity, depressive disorders, alcohol and tobacco use—in patients with low back pain,” said lead study author and orthopedic surgeon Scott Shemory, MD. “The findings will allow physicians to better counsel and more closely follow their high-risk patients.”
LAS VEGAS—Nicotine dependence, obesity, alcohol abuse, and depressive disorders are risk factors for low back pain, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). Monitoring and counseling at-risk patients may prevent and minimize pain and improve quality of life, researchers said.
According to the U.S. Centers for Disease Control and Prevention’s (CDC) 2012 National Health Survey, nearly one-third of American adults reported that they had experienced low back pain during the previous 3 months. Determining modifiable risk factors for low back pain could help avoid or diminish the financial and emotional costs of this condition.
Researchers reviewed electronic records of more than 26 million patients from 13 health care systems across the United States, including 1.2 million patients diagnosed with low back pain (approximately 4.54% of the patient records).
The review found that 19.3% of the patients diagnosed with a depressive disorder reported lower back pain, as did 16.75% of patients diagnosed as obese (BMI > 30kg/m²), 16.53% of the patients diagnosed with nicotine dependence, and 14.66% with reported alcohol abuse. Patients with nicotine dependence, obesity, depressive disorders, and alcohol abuse had statistically significant relative risks of 4.489, 6.007, 5.511, and 3.326 for low back pain, respectively, when compared to other patients.
“This study used an electronic health care database to identify modifiable risk factors—obesity, depressive disorders, alcohol and tobacco use—in patients with low back pain,” said lead study author and orthopedic surgeon Scott Shemory, MD. “The findings will allow physicians to better counsel and more closely follow their high-risk patients.”
LAS VEGAS—Nicotine dependence, obesity, alcohol abuse, and depressive disorders are risk factors for low back pain, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). Monitoring and counseling at-risk patients may prevent and minimize pain and improve quality of life, researchers said.
According to the U.S. Centers for Disease Control and Prevention’s (CDC) 2012 National Health Survey, nearly one-third of American adults reported that they had experienced low back pain during the previous 3 months. Determining modifiable risk factors for low back pain could help avoid or diminish the financial and emotional costs of this condition.
Researchers reviewed electronic records of more than 26 million patients from 13 health care systems across the United States, including 1.2 million patients diagnosed with low back pain (approximately 4.54% of the patient records).
The review found that 19.3% of the patients diagnosed with a depressive disorder reported lower back pain, as did 16.75% of patients diagnosed as obese (BMI > 30kg/m²), 16.53% of the patients diagnosed with nicotine dependence, and 14.66% with reported alcohol abuse. Patients with nicotine dependence, obesity, depressive disorders, and alcohol abuse had statistically significant relative risks of 4.489, 6.007, 5.511, and 3.326 for low back pain, respectively, when compared to other patients.
“This study used an electronic health care database to identify modifiable risk factors—obesity, depressive disorders, alcohol and tobacco use—in patients with low back pain,” said lead study author and orthopedic surgeon Scott Shemory, MD. “The findings will allow physicians to better counsel and more closely follow their high-risk patients.”